Abstract
Purpose
Poor semen quality is associated with reduced somatic health and increased cancer risk. Infertility and cancer are increasingly being linked by epidemiologists and basic scientists. We sought to identify semen parameters associated with an increased childhood cancer risk in the family members of subfertile men.
Materials and Methods
We performed a retrospective cohort study in men from the SHARE (Subfertility Heath and Assisted Reproduction) study who underwent semen analysis between 1994 and 2011. We used fertile population controls from the Utah Population Data Base. Our primary outcome was the risk of any childhood (18 years or younger) cancer in the siblings and cousins of men who underwent semen analysis compared to fertile, age matched controls. Cox proportional hazard regression models were used to test the association between semen quality and childhood cancer incidence.
Results
We selected 10,511 men with complete semen analysis and an equal number of fertile controls. These men had a total of 63,891 siblings and 327,753 cousins. A total of 170 and 958 childhood cancers were identified in siblings and cousins, respectively. The 3 most common cancers diagnosed in siblings were acute lymphoblastic leukemia in 37, brain cancer in 35 and Hodgkin lymphoma in 15. Oligozoospermia was associated with a twofold increased risk of any childhood cancer and a threefold increased risk of acute lymphoblastic leukemia in the siblings of subfertile men compared to fertile controls (HR 2.09, 95% CI 1.18–3.69 vs HR 3.07, 95% CI 1.11–8.46).
Conclusions
Siblings of men with oligozoospermia are at increased risk for any-site cancer and acute lymphoblastic leukemia. This suggests a shared genetic/epigenetic insult or an environmental exposure that merits further investigation.
Keywords: testis, oligospermia, siblings, neoplasms, precursor cell lymphoblastic leukemia-lymphoma
Cancer is the second most common cause of childhood death after trauma and leukemia is the most common subtype of the estimated 10,380 new cases each year.1 There is mounting evidence that a link exists between infertility and leukemia. A Danish population study showed that if a woman was evaluated for infertility, there was a significantly increased risk of leukemia developing in the offspring in childhood (HR 1.30, 95% CI 1.06–1.60).2 This increased risk was postulated to stem from drugs used for ovulation induction, such as clomiphene citrate, but male factors have not been investigated.3,4 Less is known about the association of male factor infertility and the risks of associated cancers in the offspring or family members.
Male factor infertility is extremely common with a self-reported 7.5% of American men having undergone SA at an assisted reproduction center.5 Of American couples 15% report infertility and 1.5% of children are born through ART.6 Defining the phenotype of male factor infertility is an active area of research and several studies demonstrated associations with an increased risk of cancers, obesity and poor overall health.7–11
With a known increased risk of cancers in men undergoing SA, we sought to identify semen parameters associated with an increased childhood cancer risk in the family members of subfertile men. Our goal was to explore the association between male infertility and childhood cancer risk in family members using the multigenerational UPDB. Understanding the familial risk of cancer in subfertile men will help elucidate biological and environmental mechanisms leading to infertility in men and the health of their family members.
Our primary objective was to characterize the male infertility phenotype based on childhood cancer risk in the siblings and cousins of men who underwent SA. We hypothesized that poor quality semen parameters are associated with an increased childhood cancer risk in the family members of men who undergo SA.
METHODS
Data
We used data compiled in the SHARE study, which combines medical, genealogical and administrative data with biospecimen data to create a unique resource for the evaluation of fertility and familial cancer history. This was then coupled to UPDB, a health data repository with more than 8 million individuals and 22 million records. Multiple epidemiological studies have used the complex pedigrees of the UPDB to identify and understand familial diseases.12–15
Cancer diagnoses were obtained from UPDB using linked data from the Utah Cancer Registry and Utah death certificates. This registry is a NCI (National Cancer Institute) SEER (Surveillance, Epidemiology and End Result) registry that has collected information on all cancer diagnoses from 1966 to 2012 for Utah residents. The study was approved by the University of Utah institutional review boards (www.research.utah.edu/rge/) No. IRB_00069711.
Study Design
We performed a retrospective cohort analysis of childhood cancer risk in siblings and cousins of men who underwent SA at the University of Utah Andrology Clinic from 1996 to 2011 and at the Intermountain® Healthcare Sunquest™ system from 2002 to 2011. Together these 2 andrology laboratories have captured approximately 90% of all semen analyses performed in Utah since 2004.
We identified 26,147 men in whom SA was performed during our study period. This cohort included all men evaluated at these 2 assisted reproductive technology centers. Thus, fertile men, infertile men and men with infertile female partners were included. Men presented for male factor infertility workup or as part of the evaluation for infertility of a couple. The sample of men with SA consisted of 12,889 men with complete information on first-degree relatives. Figure 1 lists inclusion and exclusion criteria.
Figure 1.

Fertile population controls were selected randomly without replacement from UPDB. Men evaluated at either infertility clinic were excluded from the pool of potential controls. Controls were required to be residents of Utah with adequate followup or familial data in UPDB. They were matched by age and birth year at a matching ratio of 1:1. We used birth certificate data to define fertile as having at least 1 naturally conceived child. We did not have SA data on the controls. These men did not have a cancer diagnosis at the time of SA in the matched subfertile male.
Siblings and cousins of men with SA and the respective relatives of the matched controls were selected from UPDB. This provided a total of 63,891 siblings and 327,753 cousins born after 1966. We chose to only look at the siblings and cousins of men who underwent semen analyses because the Utah Cancer Registry was founded in 1966 and we would not have had adequate followup for older family members. A total of 10,511 men with SA had at least 1 sibling and 10,300 had at least 1 cousin born after 1966. Tables 1 and 2 show descriptive statistics for these men. Childhood cancer was defined as a cancer diagnosis at age 18 years or less.
Table 1.
Cohort demographics
| Semen Analysis | No. Pts | Mean ± SD (range) | |
|---|---|---|---|
| + Sibling: | |||
| Birth yr | 10,511 | 1977.06 ± 6.18 | (1951–1992) |
| Death yr | 59 | 2009.24 ± 3.11 | (2000–2013) |
| Last yr known alive | 10,511 | 2012.62 ± 1.93 | (1997–2015) |
| 1st Yr semen analysis collected | 10,511 | 2007.02 ± 4.36 | (1996–2014) |
| No. semen analyses | 10,511 | 1.24 ± 0.63 | (1–10) |
| Age at semen analysis | 10,511 | 29.96 ± 4.77 | (21–55) |
| + 1st Cousin: | |||
| Birth yr | 10,300 | 1976.41 ± 6.86 | (1941–1992) |
| Death yr | 61 | 2009.26 ± 3.28 | (2000–2013) |
| Last yr known alive | 10,300 | 2012.59 ± 1.98 | (1996–2015) |
| 1st Yr semen analysis collected | 10,300 | 2006.77 ± 4.49 | (1996–2014) |
| No. semen analyses | 10,300 | 1.23 ± 0.61 | (1–10) |
| Age at semen analysis | 10,300 | 30.36 ± 5.25 | (21–67) |
Table 2.
Semen parameters of men with semen analysis
| No. Pts (%) | |
|---|---|
| Concentration: | |
| Azoospermia | 436 (4.15) |
| Oligozoospermia | 1,088 (10.35) |
| Normozoospermia | 5,452 (51.87) |
| Hyperzoospermia | 3,535 (33.63) |
| Count: | |
| Azoospermia | 436 (4.15) |
| Oligozoospermia | 1,042 (9.91) |
| Normozoospermia | 7,923 (75.38) |
| Hyperzoospermia | 1,110 (10.56) |
| Motility: | |
| Azoospermia | 436 (4.15) |
| Quartile 1 (0–49) | 2,370 (22.55) |
| Quartile 2 (50–59) | 2,110 (20.07) |
| Quartile 3 (60–69) | 2,340 (22.26) |
| Quartile 4 (70–100) | 3,255 (30.97) |
| Vitality: | |
| Azoospermia | 185 (3.87) |
| Quartile 1 (0–45) | 1,094 (22.87) |
| Quartile 2 (46–55) | 1,023 (21.39) |
| Quartile 3 (56–64.4) | 1,259 (26.32) |
| Quartile 4 (64.5–94) | 1,222 (25.55) |
| Morphology: | |
| Azoospermia | 185 (4.05) |
| Quartile 1 | 952 (20.82) |
| Quartile 2 | 1,143 (25.00) |
| Quartile 3 | 1,184 (25.90) |
| Quartile 4 | 1,108 (24.23) |
| Total motile count: | |
| Azoospermia | 185 (3.87) |
| Quartile 1 | 1,179 (24.65) |
| Quartile 2 | 1,068 (22.33) |
| Quartile 3 | 1,131 (23.65) |
| Quartile 4 | 1,220 (25.51) |
SAs were performed and processed based on the 2010 WHO guidelines.16 When men underwent more than 1 SA, the mean of each semen parameter was used for our study. Details regarding the subcategorization of semen parameters in this data set were previously published.17
Statistical Methods
Cox regression models were used to test the association between semen quality and childhood cancer incidence in siblings and cousins of men with SA and matched controls. The risk in relatives of men who underwent SA compared to relatives of controls was determined independently for each relation type (sibling and cousin). Analyses were done to compare any-site and site specific cancer risk separately in siblings and cousins of men seen at a fertility clinic to those in fertile population controls. We then investigated the association by semen parameter in separate models. To determine the risk of cancer in relatives of men with male factor infertility and men with normal semen parameters case-case analyses were also completed, in which relatives of men with abnormal semen parameters were compared to relatives of men with normal semen parameters.
All relatives of men who underwent SA and their fertile population controls were included in analyses. For example, for families containing brothers with semen analyses each man was included as a separate index case and the risk among relatives of each case was calculated separately. This approach has been shown to lead to unbiased estimates of risk.18 We used the Huber-White sandwich estimator of variance for clustered data corrected for the nonindependence of observations within families.19 Relatives of fertile men not presenting in a fertility clinic served as the reference group in all analyses except as noted.
Time was measured as years at risk. All models controlled for gender when the cancer was not gender specific, and birth year. Death date and the last known date of residing in Utah were used as the date of right censoring. Analyses were performed for all cancers combined and then for the most common subtypes of childhood cancer seen in our population, including ALL, brain cancer, Hodgkin lymphoma and bone/joint cancers.
RESULTS
Our subfertile cohort of 10,511 men had a total of 32,151 siblings and 152,015 cousins, while control men had a total of 31,740 siblings and 175,738 cousins who were included in the final analysis. There were no significant differences in average followup between the siblings and cousins of subfertile men and controls (224.9 vs 225.3 and 219.1 vs 218.3 months, respectively).
A total of 170 childhood cancers were identified in siblings and 958 were identified in cousins. A total of 95 childhood cancers were diagnosed in the siblings of men who underwent SA compared to 75 in the control group. The 3 most common cancers diagnosed in siblings were ALL in 37, brain cancer in 35 and Hodgkin lymphoma in 15. There were no differences in the number of cancers diagnosed in family members based on gender.
A total of 517 childhood cancers were diagnosed in the cousins of men who underwent SA compared to 441 in the control group. The 3 most common cancers diagnosed in cousins were ALL in 180, brain cancer in 156 and bone/joint cancers in 73.
Any-Site Cancer
The siblings of men with an oligozoospermic concentration were at nearly twofold increased risk for any-site cancer compared to the siblings of controls (HR 2.09, 95% CI 1.18–3.69, fig. 2). The siblings of men with an oligozoospermic count were also at an associated increased risk of any-site cancer compared to the siblings of controls (HR 1.90, 95% CI 1.03–3.48, fig. 3). All other semen parameters did not demonstrate a significant change in risk for any-site cancer in the siblings of men who underwent SA compared to fertile control siblings.
Figure 2.

Semen concentration and risk of any site cancer and ALL in siblings of men who underwent semen analysis compared to fertile controls.
Figure 3.
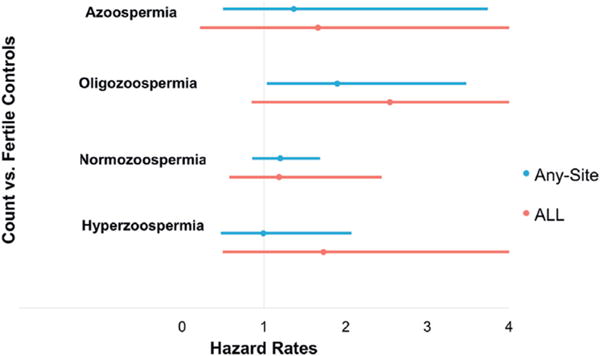
Semen count and risk of any site cancer and ALL in siblings of men.
Acute Lymphoblastic Leukemia
For the siblings of men with an oligozoospermic concentration there was a threefold increased risk of ALL compared to the siblings of controls (HR 3.07, 95% CI 1.11–8.46, table 3). The siblings of men with azoospermia or a normozoospermic concentration were not at increased risk (fig. 2). All other semen parameters did not demonstrate a significant change in the risk of ALL in the siblings of men who underwent SA.
Table 3.
Semen parameters and childhood cancer risk in siblings
| Model vs Fertile Controls | Sibling Childhood Ca HR (95% CI)
|
||
|---|---|---|---|
| All Site | Acute Lymphoblastic Lymphoma | Brain | |
| Concentration: | |||
| Azoospermia | 1.37 (0.50–3.74) | 1.66 (0.22–12.77) | 1.84 (0.24–14.24) |
| Oligozoospermia | 2.09 (1.18–3.69) | 3.07 (1.11–8.46) | 1.29 (0.29–5.83) |
| Normozoospermia | 1.15 (0.78–1.68) | 1.14 (0.51–2.53) | 1.40 (0.62–3.16) |
| Hyperzoospermia | 1.15 (0.74–1.78) | 1.27 (0.52–3.10) | 1.49 (0.59–3.78) |
| Count: | |||
| Azoospermia | 1.37 (0.50–3.74) | 1.66 (0.22–12.78) | 1.84 (0.24–14.23) |
| Oligozoospermia | 1.90 (1.03–3.48) | 2.54 (0.85–7.61) | 2.07 (0.57–7.43) |
| Normozoospermia | 1.20 (0.85–1.69) | 1.19 (0.58–2.44) | 1.44 (0.68–3.04) |
| Hyperzoospermia | 0.99 (0.47–2.07) | 1.73 (0.50–6.01) | 0.65 (0.08–5.07) |
| Motility: | |||
| Azoospermia | 1.36 (0.50–3.74) | 1.66 (0.22–12.77) | 1.84 (0.24–14.22) |
| Quartile 1 (0–49) | 0.95 (0.54–1.68) | 1.10 (0.36–3.32) | 0.64 (0.14–2.81) |
| Quartile 2 (50–59) | 1.33 (0.81–2.19) | 1.24 (0.40–3.82) | 2.20 (0.83–5.83) |
| Quartile 3 (60–69) | 1.68 (1.08–2.61) | 1.62 (0.64–4.12) | 2.58 (1.07–6.26) |
| Quartile 4 (70–100) | 1.11 (0.71–1.74) | 1.49 (0.62–3.60) | 0.78 (0.25–2.42) |
Brain Cancer
Brain cancer was the second most common childhood cancer in our cohort in siblings and cousins. The quality of semen parameters did not demonstrate a significant difference in childhood cancer risk for the siblings or cousins of men who underwent SA compared to fertile control family members.
Cousins
There was no significant difference in cancer risk (any-site or site specific) between the cousins of men who underwent SA and their respective controls based on semen parameters.
DISCUSSION
Using the SHARE semen analysis database, we identified an association between semen quality and childhood cancers in the family members of men with an oligozoospermic concentration and count. This suggests an inherent link between infertility and cancer risk. While other studies have shown the complexity of the infertility and health phenotype, to our knowledge this is the first study to investigate whether the quality of semen parameters in a man is associated with a childhood cancer risk in their family members.
The association between infertility and leukemia in the offspring has been identified in several large European population database studies. One of the larger studies used the Medical Birth Registry of Norway.20 That study showed that, although the overall cancer risk was no different, if a couple used ART, their offspring was at increased risk for leukemia. The French ESCALE Study (Étude Sur les Cancers et les Leucémies de l’Enfant) demonstrated an increased risk of ALL in the offspring of mothers who used ovulation induction but not in vitro fertilization or artificial insemination compared to fertile control children.4 Maternal factors, such as hormonal adjuncts during conception, specific ART therapies and subfertility, are all associated with leukemia risk in offspring.
We found an increased risk of leukemia in the siblings of men with an oligozoospermic concentration. This emphasizes the importance of understanding the impact of subfertility on the somatic health of their family members. Further, it poses the question of whether the linkage seen in these studies is, in fact, a result of familial genetic or environmental factors that predispose family members to cancer and infertility.
Our finding of an increased risk of leukemia in first-degree relatives is suggestive of a shared genetic insult or an environmental exposure that affects both fertility and leukemia risk. A meta-analysis of exposure and associated risks of leukemia demonstrated that occupational and household product exposure increased the relative risk of childhood leukemia.21 A shared environmental exposure at the household level is a possible explanation for the association of a higher cancer risk in the siblings of oligozoospermic men.
To further complicate this story, male infertility results from rare variants in 3,500 genes required for spermatogenesis and it is often a result of de novo mutations.22–24 This heterogeneity in male factor infertility results in the inability to identify a cause of infertility in 22% of cases.25 There is now evidence that parental health and exposures can influence the health of an offspring through epigenetic changes.26,27
Since the childhood cancer risk was increased in the siblings of oligozoospermic men, epigenetic changes in 1 parent or both parents is another possible mechanism. Similar to our prior work, we found this elevated cancer risk in oligozoospermic but not azoospermic men and their family members.17 A possible explanation for this is that the aberrant stem cells found in both these groups could theoretically represent a somatic stem cell dysregulation leading to cancer. However, the genetics underlying this are so disordered in the azoospermic group that they are nonfunctional and not associated with familial cancer risk.
There also may be a common mechanism between known molecular contributors to infertility, like DNA mismatch repair, and cancers such as ALL.28 Future studies investigating the health of the offspring of subfertile parents should not ignore the role of the father, and the broader genetic and environmental components that may lead to these observed associations.
Despite the interesting findings of this study, it is not without limitations.
1) We used a fertile population control group of men and their associated siblings/cousins to attempt to minimize the inherent shortcomings of this retrospective cohort study. 2) We did not have SA data on our fertile controls. 3) We did not have medical comorbidity indexes or smoking status for the men who underwent SA. In Utah the age adjusted rate of cancer at all sites in children younger than 15 years is similar to the rate in the United States (15.6 vs 16/100,000 person-years).29 We were unable to control for subject location in the state and environmental exposure is a potential confounder for this study but we plan to investigate this in future studies. Our database did not subcategorize each infertile male by infertility diagnosis, for example Klinefelter syndrome. This did not impact our goal of understanding how semen parameters influenced familial cancer risk but in future studies we will investigate associations of specific diagnoses and familial cancer risks. 4) This study only involved Utah residents and the state has less ethnic and racial diversity than other regions. 5) We do not know the proportion of female partners with diagnosed infertility. 6) There is a possible selection bias based on socioeconomic status because this cohort of men had the means to be evaluated as an individual or couple seeking infertility evaluation. It is important to note that, because childhood cancer is a rare event and our study was subject to these limitations, more research is needed to make a definitive causal statement about the observed associations.
The risk of leukemia associated with subfertility may be due to a genetic predisposition to infertility or to shared familial exposure. It may not be due to ART itself. This study has helped identify families with both an increased cancer risk and infertility. In the future we need to focus on identifying a common mechanism that impacts spermatogonial and hematopoietic stem cells. We also plan to investigate environmental factors that affect male infertility and cancer risk using residential proximity to known sites of pollution.
CONCLUSIONS
Oligozoospermia was associated with an increased risk of childhood cancer and ALL in the siblings of subfertile men who underwent SA compared to fertile controls. This suggests a shared genetic/epigenetic insult or an environmental exposure that merits further investigation.
Acknowledgments
Supported by Grant 1K12HD085852-01 (HAH).
Abbreviations and Acronyms
- ALL
acute lymphoblastic leukemia
- ART
assisted reproductive technology
- SA
semen analysis
- UPDB
Utah Population Database
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hargreave M, Jensen A, Deltour I, et al. Increased risk for cancer among offspring of women with fertility problems. Int J Cancer. 2013;133:1180. doi: 10.1002/ijc.28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreave M, Jensen A, Nielsen TSS, et al. Maternal use of fertility drugs and risk of cancer in children—a nationwide population-based cohort study in Denmark. Int J Cancer. 2015;136:1931. doi: 10.1002/ijc.29235. [DOI] [PubMed] [Google Scholar]
- 4.Rudant J, Amigou A, Orsi L, et al. Fertility treatments, congenital malformations, fetal loss, and childhood acute leukemia: the ESCALE study (SFCE) Pediatr Blood Cancer. 2013;60:301. doi: 10.1002/pbc.24192. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JE, Farr SL, Jamieson DJ, et al. Infertility services reported by men in the United States: national survey data. Fertil Steril. 2009;91:2466. doi: 10.1016/j.fertnstert.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2013 Assisted Reproductive Technology National Summary Report. Atlanta: United States Department of Health and Human Services; 2015. [Google Scholar]
- 7.Walsh TJ, Croughan MS, Schembri M, et al. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TJ, Croughan MS, Schembri M, et al. Infertile men may have increased risk for non-germ cell cancers: data from 51,318 infertile couples. J Urol. 2008;179:654. [Google Scholar]
- 9.Eisenberg ML, Park Y, Hollenbeck AR, et al. Fatherhood and the risk of cardiovascular mortality in the NIH-AARP Diet and Health Study. Hum Reprod. 2011;26:3479. doi: 10.1093/humrep/der305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salonia A, Matloob R, Gallina A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009;56:1025. doi: 10.1016/j.eururo.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley GM, Curtin K, Layfield L, et al. Increased melanoma risk in individuals with papillary thyroid carcinoma. JAMA Otolaryngology Head Neck Surg. 2014;140:423. doi: 10.1001/jamaoto.2014.78. [DOI] [PubMed] [Google Scholar]
- 13.Gibson SB, Figueroa KP, Bromberg MB, et al. Familial clustering of ALS in a population-based resource. Neurology. 2014;82:17. doi: 10.1212/01.wnl.0000438219.39061.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuohy TMF, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer. 2014;120:35. doi: 10.1002/cncr.28227. [DOI] [PubMed] [Google Scholar]
- 15.Kerber RA, O’Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th. Geneva: World Health Organization; 2010. [Google Scholar]
- 17.Hanson HA, Anderson RE, Aston KI, et al. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil Steril. 2016;105:322. doi: 10.1016/j.fertnstert.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 19.Jorde LB. Inbreeding in the Utah Mormons: an evaluation of estimates based on pedigrees, isonymy, and migration matrices. Ann Hum Genet. 1989;53:339. doi: 10.1111/j.1469-1809.1989.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 20.Reigstad MM, Larsen IK, Myklebust TÅ, et al. Risk of cancer in children conceived by assisted reproductive technology. Pediatrics. 2016;137:1. doi: 10.1542/peds.2015-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlos-Wallace FM, Zhang L, Smith MT, et al. Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol. 2016;183:1. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aston KI, Conrad DF. A review of genome-wide approaches to study the genetic basis for spermatogenic defects. Methods Mol Biol. 2013;927:397. doi: 10.1007/978-1-62703-038-0_34. [DOI] [PubMed] [Google Scholar]
- 24.O’Bryan MK, Grealy A, Stahl PJ, et al. Genetic variants in the ETV5 gene in fertile and infertile men with nonobstructive azoospermia associated with Sertoli cell-only syndrome. Fertil Steril. 2012;98:827. doi: 10.1016/j.fertnstert.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 26.Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;9:369. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji G, Long Y, Zhou Y, et al. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC Med. 2012;10:49. doi: 10.1186/1741-7015-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute Surveillance Epidemiology, and End Results Program. Cancer Statistics, U.S. Population Data—1969–2014. Revised March 2016. Available at http://www.seer.cancer.gov/popdata. Accessed September 18, 2016.


