Abstract
Objective
Pregnancy is accompanied by dramatic physiologic changes in maternal plasma proteins. Characterization of the maternal plasma proteome in normal pregnancy is an essential step for understanding changes to predict pregnancy outcome. The objective of this study was to describe maternal plasma proteins that change in abundance with advancing gestational age, and determine biological processes that are perturbed in normal pregnancy.
Materials and methods
A longitudinal study included 43 normal pregnancies that had a term delivery of an infant who was appropriate for gestational age (AGA) without maternal or neonatal complications. For each pregnancy, 3 to 6 maternal plasma samples (median=5,) were profiled to measure the abundance of 1,125 proteins using multiplex assays. Linear mixed effects models with polynomial splines were used to model protein abundance as a function of gestational age, and significance of the association was inferred via likelihood ratio tests. Proteins considered to be significantly changed were defined as having: 1) more than 1.5 fold change between 8 and 40 weeks of gestation; and 2) a false discovery rate (FDR) adjusted p-value <0.1. Gene ontology enrichment analysis was employed to identify biological processes over-represented among the proteins that changed with advancing gestation.
Results
1) Ten percent (112/1,125) of the profiled proteins changed in abundance as a function of gestational age; 2) of the 1,125 proteins analyzed Glypican-3, sialic acid-binding immunoglobulin-type lectins (Siglec)-6, placental growth factor (PlGF), C-C motif (CCL)-28, carbonic anhydrase 6, Prolactin (PRL), interleukin-1 receptor 4 (IL-1 R4), dual specificity mitogen-activated protein kinase 4 (MP2K4) and pregnancy-associated plasma protein-A (PAPP-A) had more than 5 fold change in abundance across gestation. These 9 proteins are known to be involved in a wide range of both physiologic and pathologic processes, such as growth regulation, embryogenesis, angiogenesis immunoregulation, inflammation etc.; and 3) biological processes associated with protein changes in normal pregnancy included defense response, defense response to bacteria, proteolysis and leukocyte migration (FDR=10%).
Conclusions
The plasma proteome of normal pregnancy demonstrates dramatic changes in both magnitude of changes and the fraction of the proteins involved. Such information is important to understand the physiology of pregnancy, development of biomarkers to differentiate normal vs. abnormal pregnancy, and determine the response to interventions.
Keywords: aptamer, biomarker, C-C motif (CCL)-28, carbonic anhydrase 6, dual specificity mitogen-activated protein kinase kinase 4 (MP2K4), high-throughput biology, glypican-3, placental growth factor (PlGF), prolactin (PRL), interleukin-1 receptor 4 (IL-1 R4), pregnancy-associated plasma protein A (PAPP-A), proteins, sialic acid-binding immunoglobulin-type lectins (Siglec)-6
Introduction
Viviparity requires major adaptations in the mother to sustain the establishment, development, growth, and eventual separation of a fetal semi-allograft at the time of parturition. Pregnancy in placental mammals is a unique biological phenomenon, perhaps unmatched in the history of evolutionary biology. Reproductive success depends on an intensive and rich dialogue among mother, placenta, and fetus. The adaptations required for pregnancy appear to involve virtually all maternal organs/systems, including the metabolic/endocrine (1–6), cardiovascular (7–12), respiratory (13), gastrointestinal (12, 14–21), hematologic (22, 23), and central nervous systems (24–26).
The study of the physiology of pregnancy has spanned decades, and has largely depended upon available technological capabilities to study parameters that change with gestational age. Such parameters have been studied with relatively simple methods, such as hormonal determinations (20, 21, 27), concentrations of nutrients/fuels (glucose(17–19), lipids(28–33), proteins(34–40), amino acids(41–43)), blood pressure (8, 44), cardiovascular function (9, 11), spirometry (13, 16), immunological assays (14, 24, 45), and different tests of central nervous system function (25, 26). These studies have been essential to understand changes in body composition (4, 18), physiologic adaptation (10, 13), pathophysiology of selected pregnancy complications (17, 46–48). One of the domains of interest that has proven to be extraordinarily successful is the study of changes in plasma protein concentrations in maternal blood. For example, the detection of human chorionic gonadotrophin (hCG) in human blood has allowed the early detection of pregnancy, even before the first signs of amenorrhea(56–58). Monitoring early pregnancy with serial determinations of hCG has also allowed identification of patients at risk for ectopic gestation(59–70), and other forms of pregnancy failure (60, 62, 63, 65, 67). In the case of ectopic pregnancy, this has allowed not only earlier diagnosis, but even the introduction of minimally invasive surgical techniques (e.g. laparoscopic resection and medical treatment) (71–79). The monitoring of gestational trophoblastic disease has also been dramatically changed with the use of serial hCG determinations in maternal serum/plasma (80, 81). However, these examples only represent the first of many diagnostic advances made possible by monitoring concentrations of a single protein during pregnancy.
The observation that a fetus with a neural tube defect had been born to a mother with an idiopathic elevation of maternal serum alpha fetoprotein eventually led to the discovery that such elevations could be used to screen for the presence of neural tube defects in the mid-trimester of pregnancy(56, 82–86), and continue to be the basis – more than 20 years later – for the biochemical screening of these congenital anomalies(84, 87–93). Further advances occurred with the introduction of the triple (followed by the quadruple) biochemical screening, including three proteins (alpha fetoprotein, hCG, inhibin), which were subsequently combined with a steroid hormone, estriol, to assess the likelihood of trisomies 18 and 21 at the end of the first trimester (88, 89, 93–100). Collectively, this evidence, which is now in routine clinical practice, provides compelling evidence that examination of the plasma/serum composition can provide important insight into the biology of pregnancy, fetal health and disease, and has changed the practice of obstetrics and medicine.
The most recent entry into the protein biomarker discovery panel is the determination of angiogenic/anti-angiogenic factors for the early prediction of preterm preeclampsia(47, 101–110), fetal death(111, 112), late preeclampsia(47, 101–110), small-for-gestational-age (SGA) (113), and maternal floor infarction(114). This set of biomarkers can now be readily determined using commercially-available tests, and has the potential to identify patients at risk for pregnancy complications in the long subclinical phase of the “great obstetrical syndromes”, and open the door for prevention, which has been a challenge in obstetrics for decades(105, 115–124).
High-dimensional biology techniques allow characterization of the genome (54, 55, 125, 126), transcriptome(55, 127–137), proteome(55, 125, 127, 138–148), lipidome(149–155), glucome(156, 157), metabolome(6, 55, 128, 158–163), and cytome(164–169). Major trust of the new biology and medicine has been to gather the information derived from these platforms to optimize diagnosis, prognosis, and treatment (170, 171). Although each of these techniques has a particular informational domain (DNA, RNA, proteins, metabolites, lipids, etc.), they can be used independently or in combination (172, 173). Proteins are considered to be important executors of biological functions, as they include enzymes (80), structural components of tissues/cells (174), the coagulation cascade (175, 176), and the inflammatory network (177).
There have been substantial attempts to characterize the human plasma proteome in non-pregnant subjects, which have been made possible by the development of sophisticated mass-spectrometry techniques, using a top-down approach (81, 140, 178). These efforts have been extended to the maternal plasma proteome and amniotic fluid(138–140, 145, 147, 148, 179–203); however, technological complexities continue to challenge the goal of developing a comprehensive map of the entire human plasma proteome. The total estimated number of proteins in the human body ranges from 20,000 to one million(78) – of these, plasma proteome studies have been able to detect and semi-quantify a number between 16,500–20,000 (79, 204–206). Immunoassays and related methods continue to be the “gold standard” for the sensitive determination of proteins in peripheral plasma, guide diagnosis, monitoring of disease, and treatment; in addition, they are more sensitive than 2D gels or mass spectrometry, and can detect analytes in very small quantities (below nM range). In addition, technologies like enzyme-linked immunosorbent assay (ELISA) require two antibodies to the same protein to elicit a signal. Such immunoassays cannot be multiplexed above a few tens of simultaneous measurement, largely due to the cross-reactivity of the secondary antibodies to surface-immobilized proteins (207–210).
To enlarge the number of proteins that can be detected simultaneously with a high degree of sensitivity and dynamic range, a new technology has been developed which is not based on conventional antigen/antibody reaction. The aptamer-based method uses single-strand DNA or RNA molecules that bind, with high affinity and specificity, to proteins, peptides, or other pre-defined molecules. Recent publications have emphasized the extraordinary potential of aptamer technology in biomarker discovery for cardiovascular disease (211),and other important biomedical disorders (212–218).
The objective of this study was to use aptamer-based technology to characterize the maternal plasma proteome of normal pregnancy as a function of gestational age. This study is important for the description of the simultaneous changes in plasma protein composition, and will serve as the basis to detect departures of abnormalities, before and during the time of diagnosis of major obstetrical complications.
Methods
Study design
We conducted a prospective longitudinal study that enrolled normal pregnant women attending the Center for Advanced Obstetrical Care and Research of the Perinatology Research Branch, NICHD, and the Detroit Medical Center / Wayne State University. A retrospective cohort study was conducted to include 43 who delivered at term, and each one of them had 3 to 6 plasma samples obtained during pregnancy, before the spontaneous onset of labor (median number of samples=5),]. Plasma samples were collected at the time of a prenatal visit, scheduled at four-week intervals from the first or early second trimester until delivery. Each patient had at least three samples collected during the following gestational age intervals (8-<16 weeks, 16-<24 weeks, 24-<28 weeks, 28-<32 weeks, 32-<37 weeks and >37 weeks). All patients had the placenta collected at the time of delivery, transported to the laboratory, and sectioned for histological examination, following criteria of the Society for Perinatal Pathology. Lesions were diagnosed using previously established criteria(71–73). Only patients without acute inflammatory lesions of the placenta were included in this study because of the potential to confound the relative abundance of the maternal plasma proteome. All patients provided written informed consent and the use of biological specimens, as well as clinical and ultrasound data for research purposes were approved by the Wayne State University and Institutional Review Boards of NICHD.
Proteomics technique
Maternal plasma protein abundance was determined using the SOMAmer (Slow Off-rate Modified Aptamers) platform and its reagents. This platform allows measurement of over 1,125 proteins in maternal plasma samples (210, 219, 220). Proteomics profiling was performed by Somalogic, Inc. (Boulder, CO).
The serum samples were diluted and then incubated with the respective SOMAmer mixes pre-immobilized onto streptavidin-coated beads. The beads were washed in order to remove all non-specifically bound proteins and other matrix constituents. Proteins that remained specifically bound to their cognate SOMAmer reagents were tagged using an NHS-biotin reagent. After the labeling reaction, the beads were exposed to an anionic competitor solution that prevents non-specific interactions from reforming after disruption.
Using this approach, pure cognate-SOMAmer complexes and unbound (free) SOMAmer reagents are released from the streptavidin beads using ultraviolet light that cleaves the photo-cleavable linker used to quantitate protein. The photo-cleavage eluate, which contains all SOMAmer reagents (some bound to a biotin-labeled protein and some free), was separated from the beads and then incubated with a second streptavidin-coated bead that binds the biotin-labeled proteins and the biotin-labeled protein-SOMAmer complexes. The free SOMAmer reagents were then removed during subsequent washing steps. In the final elution step, protein-bound SOMAmer reagents were released from their cognate proteins using denaturing conditions. These SOMAmer reagents were then quantified by hybridization to custom DNA microarrays. The Cyanine-3 signal from the SOMAmer reagent was detected on microarrays (210, 219, 220).
Statistical analysis
Demographics data analysis
Clinical characteristics of the patient population were summarized as median and inter-quartile ranges (IQR) for continuous variables, or percentages for categorical variables, using SPSS software (version 19; IBM Corporation, Armonk, NY)
Differential abundance analysis
Protein abundance expressed as fluorescence units was log (base 2) transformed to improve normality. Linear mixed-effects models with cubic splines (number of knots = 3) were used to model protein abundance as a function of gestational age using lme4 package (221) under the R statistical language and environment (www.r-project.org). Inference about statistical significance of associations was calculated using likelihood ratio tests between a model that included the gestational age terms (fixed and corresponding random effects) and a simpler random intercept linear mixed-effects model without gestational age terms.
Protein abundance was considered to have changed significantly with gestational age if it met the following criteria: 1) the magnitude of abundance of change was >1.5 fold between 8 and 40 weeks of gestation; and 2) false discovery rate (FDR) (222) adjusted p-values (q-values) <0.1. Similar or less stringent criteria have been extensively used in high-dimensional biology and have shown good validation by alternative techniques and/or in independent sets of samples(223–226).
Fold change was defined as the ratio of protein abundance (in relative fluorescence units) between the highest and the lowest mean value across gestational age. The median (50th quantile) and 10th and 90th quantiles of the protein abundance were also determined using quantile regression modeling (227, 228). In these models, the relationship between protein abundance was assumed to be linear within a narrow moving window of gestational age, and this allowed obtaining a non-linear smooth estimate of the quantiles as previously described (107).
Clustering proteins by average profile
To identify groups of proteins based on their pattern of change across gestation, we have used unsupervised hierarchical clustering. The input in this analysis was the mean protein abundance across gestation (longitudinal patterns) computed from linear mixed-effects models for each gestational week from 8 to 40 weeks. Average profiles were scaled between 0 and 1 prior to applying hierarchical clustering with Euclidean distance, so that proteins with similarity longitudinal patterns (e.g. monotonically increasing) are grouped together despite eventual differences in the magnitude of change (e.g. rate of increase) or overall protein abundance. With hierarchical clustering, each protein is considered a cluster by itself and iteratively, clusters are merged so that the distance between the farthest apart members of the clusters (complete linkage) is minimized. To determine the optimal number of clusters, we have used a goodness of clustering measure (the gap statistic) which compares the change in within-cluster dispersion (sum of squared distances between cluster members) with that expected under the null distribution (simulated by bootstrap)(229).
Gene ontology enrichment analysis
Having identified proteins which change in abundance as a function of gestational age, the next step was to gain an understanding of the potential functional roles of these proteins in human pregnancy. To accomplish this, we relied on the information deposited for the genes encoding for each protein, and which have been organized in publicly-available databases (i.e. gene ontology). We focused on the biological processes in which these proteins have been implicated. Each protein was mapped to an identifier in the Entrez gene database.(230) based on Somalogic, Inc. protein annotation system, and then to gene ontology (231). Biological processes over-represented among the proteins that changing in abundance with advancing gestational age. We used a Fisher’s exact test and odds ratios to estimate enrichment. Gene ontology terms with three or more hits and an adjusted p-value <0.1 were considered significantly enriched in gestational age modulated proteins.
Protein-protein interaction network analysis
To explore the potential impact of gestation on human plasma proteins not profiled in this study, we conducted an in-silico analysis for which we retrieved the known protein-protein map interactions from the publicly available Protein Reference Database (HPRD, release 9) (232) using the NCBI2R package. For each of the 1,125 proteins profiled, we determined the number of protein-protein interactions documented in the database. For visualization purposes, a graph was constructed linking proteins with known interactions with the 112 proteins changing with gestational age.
Results
Clinical characteristic of the study population
Forty-three women with normal pregnancies met the criteria for inclusion in this study. The median (interquartile range) maternal age was 25 (21–28) years old, and 88.4% of patients were of African-American origin by self-reporting. All patients delivered at term without any obstetrical complications and had neonates with an appropriate weight for gestational age(233). The median gestational age at delivery was 39.4 [interquatile range (IQR) 39.0–40.1].
Maternal Plasma Proteome in Normal Pregnancy Characterized by SomaSCAN
Ten percent (112/1,125) of the proteins profiled changed in abundance as a function of gestational age (fold change >1.5 and q-value <0.1) (see Table 2). Figure 1 shows longitudinal protein abundance for two selected proteins together with the fitted mean (by linear-mixed effects models) and median (by quantile regression) as a function of gestation. Similar plots for all 112 significant proteins are displayed in supp. File 1
Table 2.
List of proteins that change with gestational age in normal pregnancy. Somalogic identifiers and protein symbols and names are provided together with corresponding UniProt database identifiers and gene symbols. Fold change represents the ratio in protein abundance between the maximum and minimum average value across gestation. The protein trend types correspond to the clusters defined in Figure 1.
| Target | Target Full Name | UniProt | Gene Symbol | FC | p | q | Direction | Trend Type |
|---|---|---|---|---|---|---|---|---|
| B7-H2 | ICOS ligand | O75144 | ICOSLG | 1.84 | 0.0000 | 0.000 | Decreasing | Constant rate |
| BLC | C-X-C motif chemokine 13 | O43927 | CXCL13 | 1.70 | 0.0000 | 0.000 | Decreasing | Constant rate |
| CAMK2B | Calcium/calmodulin-dependent protein kinase type II subunit beta | Q13554 | CAMK2B | 1.53 | 0.0172 | 0.024 | Decreasing | Constant rate |
| CAMK2D | Calcium/calmodulin-dependent protein kinase type II subunit delta | Q13557 | CAMK2D | 1.56 | 0.0183 | 0.025 | Decreasing | Constant rate |
| Coagulation Factor XI | Coagulation Factor XI | P03951 | F11 | 1.72 | 0.0000 | 0.000 | Decreasing | Constant rate |
| Cyclophilin F | Peptidyl-prolyl cis-trans isomerase F, mitochondrial | P30405 | PPIF | 1.56 | 0.0471 | 0.059 | Decreasing | Constant rate |
| FYN | Tyrosine-protein kinase Fyn | P06241 | FYN | 1.70 | 0.0029 | 0.005 | Decreasing | Constant rate |
| HGFA | Hepatocyte growth factor activator | Q04756 | HGFAC | 1.91 | 0.0000 | 0.000 | Decreasing | Constant rate |
| HRG | Histidine-rich glycoprotein | P04196 | HRG | 3.26 | 0.0000 | 0.000 | Decreasing | Constant rate |
| IGFBP-4 | Insulin-like growth factor-binding protein 4 | P22692 | IGFBP4 | 1.61 | 0.0000 | 0.000 | Decreasing | Constant rate |
| Integrin a1b1 | Integrin alpha-I: beta-1 complex | P56199, P05556 | ITGA1 ITGB1 | 1.60 | 0.0012 | 0.002 | Decreasing | Constant rate |
| JAK2 | Tyrosine-protein kinase JAK2 | O60674 | JAK2 | 1.55 | 0.0004 | 0.001 | Decreasing | Constant rate |
| MMP-12 | Macrophage metalloelastase | P39900 | MMP12 | 3.38 | 0.0000 | 0.000 | Decreasing | Constant rate |
| MP2K4 | Dual specificity mitogen-activated protein kinase kinase 4 | P45985 | MAP2K4 | 5.23 | 0.0000 | 0.000 | Decreasing | Constant rate |
| PAK6 | Serine/threonine-protein kinase PAK 6 | Q9NQU5 | PAK6 | 1.54 | 0.0000 | 0.000 | Decreasing | Constant rate |
| SCF sR | Mast/stem cell growth factor receptor Kit | P10721 | KIT | 2.09 | 0.0000 | 0.000 | Decreasing | Constant rate |
| CTAP-III | Connective tissue-activating peptide III | P02775 | PPBP | 1.52 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| Endothelin-converting enzyme 1 | Endothelin-converting enzyme 1 | P42892 | ECE1 | 2.21 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| GCP-2 | C-X-C motif chemokine 6 | P80162 | CXCL6 | 1.55 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| Granulysin | Granulysin | P22749 | GNLY | 1.53 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| Haptoglobin, Mixed Type | Haptoglobin | P00738 | HP | 2.11 | 0.0004 | 0.001 | Decreasing | Decreasing rate |
| LEAP-1 | Hepcidin | P81172 | HAMP | 4.32 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| MMP-9 | Matrix metalloproteinase-9 | P14780 | MMP9 | 1.56 | 0.0652 | 0.079 | Decreasing | Decreasing rate |
| Myeloperoxidase | Myeloperoxidase | P05164 | MPO | 1.99 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| NAP-2 | Neutrophil-activating peptide 2 | P02775 | PPBP | 1.59 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| PF-4 | Platelet factor 4 | P02776 | PF4 | 2.09 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| PTK6 | Protein-tyrosine kinase 6 | Q13882 | PTK6 | 1.55 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| RAN | GTP-binding nuclear protein Ran | P62826 | RAN | 2.45 | 0.0002 | 0.000 | Decreasing | Decreasing rate |
| Renin | Renin | P00797 | REN | 1.69 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| SDF-1 | Stromal cell-derived factor 1 | P48061 | CXCL12 | 1.54 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| SRCN1 | Proto-oncogene tyrosine-protein kinase Src | P12931 | SRC | 1.54 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| TGM3 | Protein-glutamine gamma-glutamyltransferase E | Q08188 | TGM3 | 1.67 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| TSG-6 | Tumor necrosis factor-inducible gene 6 protein | P98066 | TNFAIP6 | 1.61 | 0.0000 | 0.000 | Decreasing | Decreasing rate |
| Angiopoietin-2 | Angiopoietin-2 | O15123 | ANGPT2 | 2.27 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| Carbonic anhydrase XIII | Carbonic anhydrase 13 | Q8N1Q1 | CA13 | 1.83 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| FCN1 | Ficolin-1 | O00602 | FCN1 | 1.71 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| Growth hormone receptor | Growth hormone receptor | P10912 | GHR | 1.89 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| MPIF-1 | C-C motif chemokine 23 | P55773 | CCL23 | 1.57 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| SAA | Serum amyloid A-1 protein | P0DJI8 | SAA1 | 2.04 | 0.0166 | 0.023 | Decreasing | Increasing rate |
| SLIK5 | SLIT and NTRK-like protein 5 | O94991 | SLITRK5 | 2.20 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| sRAGE | Advanced glycosylation end product-specific receptor, soluble | Q15109 | AGER | 1.54 | 0.0000 | 0.000 | Decreasing | Increasing rate |
| B7-H1 | Programmed cell death 1 ligand 1 | Q9NZQ7 | CD274 | 3.12 | 0.0000 | 0.000 | Increasing | Constant rate |
| BGN | Biglycan | P21810 | BGN | 2.62 | 0.0000 | 0.000 | Increasing | Constant rate |
| BMP-1 | Bone morphogenetic protein 1 | P13497 | BMP1 | 3.78 | 0.0000 | 0.000 | Increasing | Constant rate |
| Cathepsin A | Lysosomal protective protein | P10619 | CTSA | 1.66 | 0.0000 | 0.000 | Increasing | Constant rate |
| discoidin domain receptor 1 | Epithelial discoidin domain-containing receptor 1 | Q08345 | DDR1 | 3.48 | 0.0000 | 0.000 | Increasing | Constant rate |
| FGF23 | Fibroblast growth factor 23 | Q9GZV9 | FGF23 | 2.86 | 0.0000 | 0.000 | Increasing | Constant rate |
| FN1.3 | Fibronectin Fragment 3 | P02751 | FN1 | 1.66 | 0.0000 | 0.000 | Increasing | Constant rate |
| GNS | N-acetylglucosamine-6-sulfatase | P15586 | GNS | 1.74 | 0.0000 | 0.000 | Increasing | Constant rate |
| GRN | Granulins | P28799 | GRN | 2.69 | 0.0000 | 0.000 | Increasing | Constant rate |
| IDS | Iduronate 2-sulfatase | P22304 | IDS | 2.31 | 0.0000 | 0.000 | Increasing | Constant rate |
| IGFBP-2 | Insulin-like growth factor-binding protein 2 | P18065 | IGFBP2 | 2.01 | 0.0000 | 0.000 | Increasing | Constant rate |
| IL-1 sRI | Interleukin-1 receptor type 1 | P14778 | IL1R1 | 1.66 | 0.0000 | 0.000 | Increasing | Constant rate |
| IL-22BP | Interleukin-22 receptor subunit alpha-2 | Q969J5 | IL22RA2 | 2.04 | 0.0000 | 0.000 | Increasing | Constant rate |
| LG3BP | Galectin-3-binding protein | Q08380 | LGALS3BP | 1.61 | 0.0000 | 0.000 | Increasing | Constant rate |
| LRIG3 | Leucine-rich repeats and immunoglobulin-like domains protein 3 | Q6UXM1 | LRIG3 | 2.10 | 0.0000 | 0.000 | Increasing | Constant rate |
| M-CSF R | Macrophage colony-stimulating factor 1 receptor | P07333 | CSF1R | 3.40 | 0.0000 | 0.000 | Increasing | Constant rate |
| MIP-5 | C-C motif chemokine 15 | Q16663 | CCL15 | 2.01 | 0.0000 | 0.000 | Increasing | Constant rate |
| PRL | Prolactin | P01236 | PRL | 5.75 | 0.0000 | 0.000 | Increasing | Constant rate |
| Semaphorin-6A | Semaphorin-6A | Q9H2E6 | SEMA6A | 2.52 | 0.0000 | 0.000 | Increasing | Constant rate |
| TGF-b R III | Transforming growth factor beta receptor type 3 | Q03167 | TGFBR3 | 1.59 | 0.0000 | 0.000 | Increasing | Constant rate |
| TSP2 | Thrombospondin-2 | P35442 | THBS2 | 2.00 | 0.0000 | 0.000 | Increasing | Constant rate |
| VEGF sR3 | Vascular endothelial growth factor receptor 3 | P35916 | FLT4 | 1.75 | 0.0000 | 0.000 | Increasing | Constant rate |
| vWF | von Willebrand factor | P04275 | VWF | 2.49 | 0.0000 | 0.000 | Increasing | Constant rate |
| Aminoacylase-1 | Aminoacylase-1 | Q03154 | ACY1 | 1.67 | 0.0001 | 0.000 | Increasing | Decreasing rate |
| BOC | Brother of CDO | Q9BWV1 | BOC | 1.64 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| BSSP4 | Brain-specific serine protease 4 | Q9GZN4 | PRSS22 | 2.18 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Carbonic anhydrase 6 | Carbonic anhydrase 6 | P23280 | CA6 | 5.79 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| CATZ | Cathepsin Z | Q9UBR2 | CTSZ | 1.60 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| CCL28 | C-C motif chemokine 28 | Q9NRJ3 | CCL28 | 6.25 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Coagulation Factor VII | Coagulation Factor VII | P08709 | F7 | 1.59 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| ENPP7 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 7 | Q6UWV6 | ENPP7 | 1.84 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| EphA1 | Ephrin type-A receptor 1 | P21709 | EPHA1 | 2.12 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| EPHB2 | Ephrin type-B receptor 2 | P29323 | EPHB2 | 1.53 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Epo | Erythropoietin | P01588 | EPO | 1.59 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Glypican 3 | Glypican-3 | P51654 | GPC3 | 26.04 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| IGFBP-1 | Insulin-like growth factor-binding protein 1 | P08833 | IGFBP1 | 2.92 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| IL-17B | Interleukin-17B | Q9UHF5 | IL17B | 1.56 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Insulin | Insulin | P01308 | INS | 1.54 | 0.0274 | 0.037 | Increasing | Decreasing rate |
| Lactoferrin | Lactotransferrin | P02788 | LTF | 1.50 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| PAPP-A | Pappalysin-1 | Q13219 | PAPPA | 5.18 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 | Q8NBP7 | PCSK9 | 1.89 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| PIGR | Polymeric immunoglobulin receptor | P01833 | PIGR | 1.86 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| PlGF | Placenta growth factor | P49763 | PGF | 14.46 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| RET | Proto-oncogene tyrosine-protein kinase receptor Ret | P07949 | RET | 4.15 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| sCD163 | Scavenger receptor cysteine-rich type 1 protein M130 | Q86VB7 | CD163 | 1.57 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| SHBG | Sex hormone-binding globulin | P04278 | SHBG | 2.38 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| TECK | C-C motif chemokine 25 | O15444 | CCL25 | 2.02 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| TFF3 | Trefoil factor 3 | Q07654 | TFF3 | 4.31 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| TSH | Thyroid Stimulating Hormone | P01215 P01222 | CGA TSHB | 2.63 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| VEGF121 | Vascular endothelial growth factor A, isoform 121 | P15692 | VEGFA | 2.02 | 0.0000 | 0.000 | Increasing | Decreasing rate |
| Activin A | Inhibin beta A chain | P08476 | INHBA | 3.21 | 0.0000 | 0.000 | Increasing | Increasing rate |
| ADAM12 | Disintegrin and metalloproteinase domain-containing protein 12 | O43184 | ADAM12 | 1.74 | 0.0000 | 0.000 | Increasing | Increasing rate |
| Cystatin C | Cystatin-C | P01034 | CST3 | 1.98 | 0.0000 | 0.000 | Increasing | Increasing rate |
| EMAP-2 | Endothelial monocyte-activating polypeptide 2 | Q12904 | AIMP1 | 1.85 | 0.0000 | 0.000 | Increasing | Increasing rate |
| FABP | Fatty acid-binding protein, heart | P05413 | FABP3 | 1.58 | 0.0000 | 0.000 | Increasing | Increasing rate |
| FSTL3 | Follistatin-related protein 3 | O95633 | FSTL3 | 1.56 | 0.0000 | 0.000 | Increasing | Increasing rate |
| gpIIbIIIa | Integrin alpha-IIb: beta-3 complex | P08514 P05106 | ITGA2B ITGB3 | 1.79 | 0.0696 | 0.084 | Increasing | Increasing rate |
| GPNMB | Transmembrane glycoprotein NMB | Q14956 | GPNMB | 1.52 | 0.0000 | 0.000 | Increasing | Increasing rate |
| IGFBP-5 | Insulin-like growth factor-binding protein 5 | P24593 | IGFBP5 | 1.51 | 0.0000 | 0.000 | Increasing | Increasing rate |
| IL-1 R4 | Interleukin-1 receptor-like 1 | Q01638 | IL1RL1 | 5.49 | 0.0000 | 0.000 | Increasing | Increasing rate |
| MMP-1 | Interstitial collagenase | P03956 | MMP1 | 1.58 | 0.0000 | 0.000 | Increasing | Increasing rate |
| MMP-7 | Matrilysin | P09237 | MMP7 | 1.53 | 0.0000 | 0.000 | Increasing | Increasing rate |
| NET4 | Netrin-4 | Q9HB63 | NTN4 | 1.50 | 0.0000 | 0.000 | Increasing | Increasing rate |
| NID2 | Nidogen-2 | Q14112 | NID2 | 1.51 | 0.0000 | 0.000 | Increasing | Increasing rate |
| Nidogen | Nidogen-1 | P14543 | NID1 | 1.79 | 0.0000 | 0.000 | Increasing | Increasing rate |
| OLR1 | Oxidized low-density lipoprotein receptor 1 | P78380 | OLR1 | 2.65 | 0.0000 | 0.000 | Increasing | Increasing rate |
| OMD | Osteomodulin | Q99983 | OMD | 1.79 | 0.0000 | 0.000 | Increasing | Increasing rate |
| OX2G | OX-2 membrane glycoprotein | P41217 | CD200 | 2.60 | 0.0000 | 0.000 | Increasing | Increasing rate |
| PAI-1 | Plasminogen activator inhibitor 1 | P05121 | SERPINE1 | 2.00 | 0.0000 | 0.000 | Increasing | Increasing rate |
| Siglec-6 | Sialic acid-binding Ig-like lectin 6 | O43699 | SIGLEC6 | 16.92 | 0.0000 | 0.000 | Increasing | Increasing rate |
| uPA | Urokinase-type plasminogen activator | P00749 | PLAU | 2.18 | 0.0000 | 0.000 | Increasing | Increasing rate |
Figure 1.
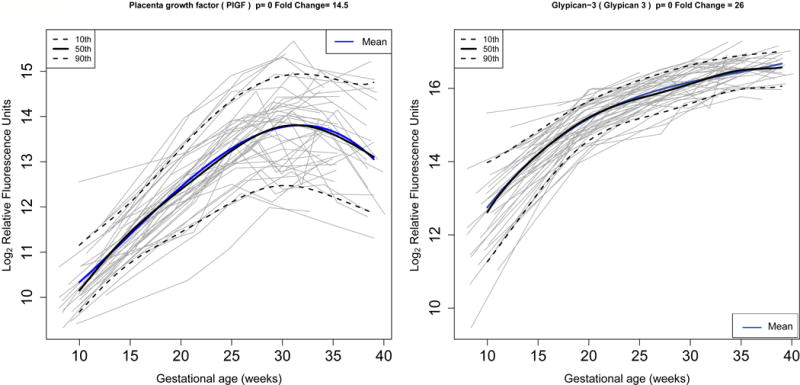
Longitudinal profiles of placenta growth factor (left) and glypican-3 (right) in normal pregnancy. Protein abundance in (log, base 2, of) relative fluorescence units is shown for each of the 43 patients (grey lines). Mean protein abundance estimated by linear mixed-effects models with cubic splines (thick blue line) as well as median level (thick black line) and 10th / 90th centiles computed by quantile regression are also shown. Fold change (FC) is computed as the ratio in abundance between the highest and lowest value of the mean abundance over gestation.
Thirty-six percent (41/112) of proteins decrease in abundance and 64% (71/112) increase with gestational age. Hierarchical cluster analysis of average protein profiles across gestation (see Figure 2) demonstrated among the increasing abundant proteins three patterns in the change of the relative fluorescence reading (increasing rate: n=21, constant rate: n=23, and decreasing rate: n=27). Similar patterns were observed among proteins with decreasing abundance during gestation (increasing rate: n=8, constant rate: n=16, and decreasing rate: n=17).
Figure 2.
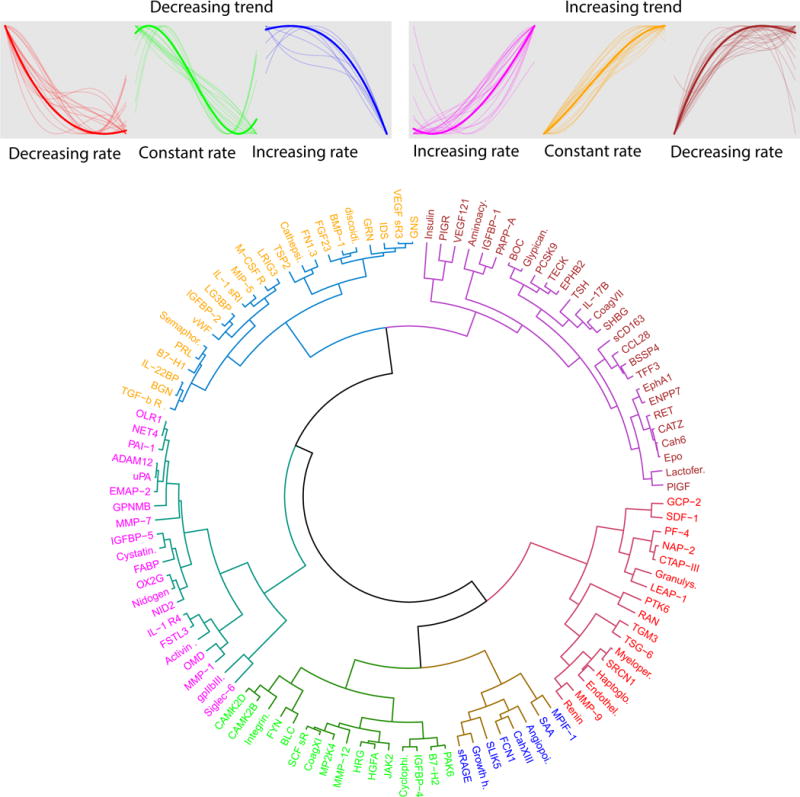
Clustering of maternal plasma average protein profiles. The figure shows three clusters of proteins with increasing overall trends (increasing rate: n=21, constant rate: n=23, and decreasing rate: n=27) and three clusters with decreasing overall trends (increasing rate: n=8, constant rate: n=16, and increasing rate: n=17).
The most highly modulated proteins (highest fold change) among the significant ones were: 1) placental growth factor (PlGF); 2) pregnancy-associated plasma protein A (PAPP-A) (>5 fold change in abundance across gestation,; 3) sialic acid-binding immunoglobulin-type lectins (Siglec)-6; 4) glypican-3; 5) C-C motif (CCL)-28; 6) carbonic anhydrase 6; 7) prolactin (PRL); 8) interleukin-1 receptor 4 (IL-1 R4); and 9) dual specificity mitogen-activated protein kinase kinase 4 (MP2K4)(see Figure 3).
Figure 3.
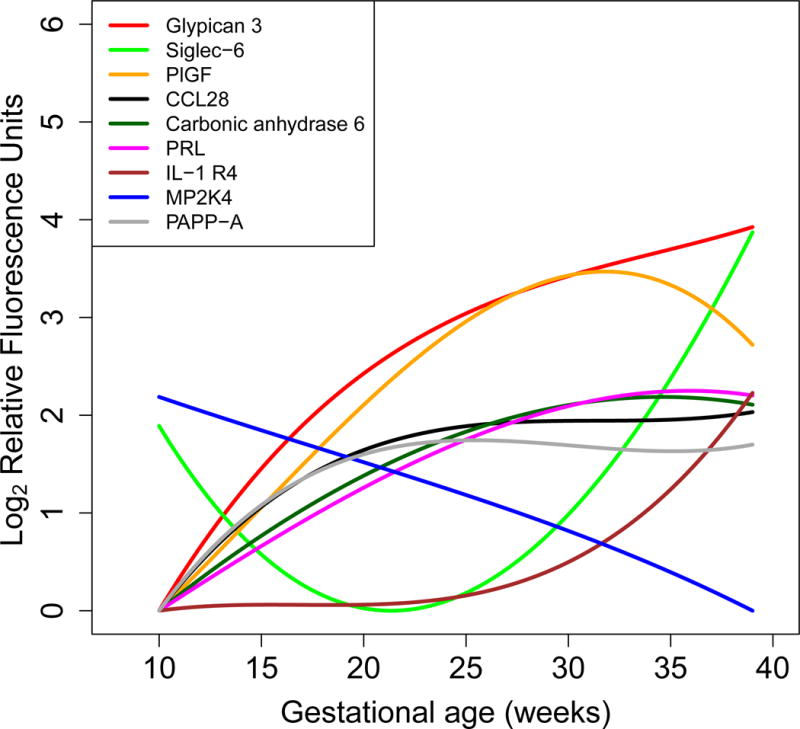
Maternal plasma average protein abundance for nine highest modulated proteins. The figure shows protein abundance in log (base 2) relative fluorescence units computed from linear mixed-effects models with cubic splines. Average profiles were shifted so that minimum expression corresponds to 0 for all preteins. Most changing nine proteins were (decreasing fold change order): Glypican-3, Siglec-6: Sialic acid-binding immunoglobulin-type lectins 6, PlGF: placental growth factor, CCL28: C-C motif 28, Carbonic anhydrase 6, PRL: Prolactin, IL-1 R4: Interleukin-1 receptor 4, MP2K4: Dual specificity mitogen-activated protein kinase kinase 4, PAPP-A: pregnancy-associated plasma protein A.
Biological Processes Modulated During Gestation
Gene ontology analysis of the corresponding proteins that changed with gestational age revealed fourteen biological processes that are impacted with advancing gestation. These biological processes included: general defense response, defense response to bacteria, defense response to fungi, germ cell migration, proteolysis and leukocyte migration (FDR=10%) (see Table 3). Out of the fourteen processes, defense response to fungus, germ cell migration, and defense response to bacterium had all decreased abundance of the protein associated with these biological processes with advancing gestation. In contrast, only the biological process of smooth muscle cell migration has an increase abundance of the proteins involved in it.
Table 3.
Biological processes enriched in proteins that change with gestational age. Odds ratios from a Fisher’s exact test and p-values are provided for all biological processes that had 3 or more significant proteins.
| Biological process | No. of Proteins |
Corresponding Genes Symbols | Odds Ratio |
p | Decreasing | Increasing |
|---|---|---|---|---|---|---|
| defense response to fungus | 4 | MPO;GNLY;HAMP;HRG | 12.4 | 0.003 | MPO;GNLY;HAMP;HRG | |
| defense response | 6 | MPO; CST3; INHBA; HP; TFF3; ICOSLG | 5.7 | 0.003 | MPO;HP;ICOSLG | CST3;INHBA;TFF3 |
| germ cell migration | 3 | KIT;ITGA1 ITGB1;CXCL12 | 27.7 | 0.004 | KIT;ITGA1 ITGB1;CXCL12 | |
| smooth muscle cell migration | 3 | ITGA2B ITGB3;DDR1;PLAU | 27.7 | 0.004 | ITGA2B ITGB3;DDR1;PLAU | |
| regulation of bone resorption | 3 | CSF1R;ITGA2B ITGB3;SRC | 27.7 | 0.004 | SRC | CSF1R;ITGA2B ITGB3 |
| negative regulation of leukocyte tethering or rolling | 3 | CCL25;CCL28;CXCL12 | 27.7 | 0.004 | CXCL12 | CCL25;CCL28 |
| proteolysis | 20 | F11;MMP9;LTF;MMP7;CTSA;F7; ACY1;BMP1;REN;ECE1;HGFAC;O LR1;PAPPA;PLAU;ADAM12;MMP 12;PRSS22;MMP1;CTSZ;PCSK9 | 2.1 | 0.006 | F11;MMP9;REN;ECE1;HGF AC;MMP12 | LTF;MMP7;CTSA;F7;ACY1;B MP1;OLR1;PAPPA;PLAU;A DAM12;PRSS22;MMP1;CTS Z;PCSK9 |
| cell chemotaxis | 8 | KIT;CCL25;PPBP;CCL28;CXCL6;CC L15;CXCL12;PPBP | 3.5 | 0.007 | KIT;PPBP;CXCL6;CXCL12;PP BP | CCL25;CCL28;CCL15 |
| leukocyte migration | 11 | MMP9;ANGPT2; CCL25;AIMP1;F N1;ITGA1 ITGB1;OLR1;ITGA2B ITGB3;FYN;MMP1;SRC | 2.7 | 0.008 | MMP9;ANGPT2;ITGA1 ITGB1;FYN;SRC | CCL25;AIMP1;FN1;OLR1;IT GA2B ITGB3;MMP1 |
| cellular response to hormone stimulus | 4 | IGFBP2;GHR;PGF;CGA TSHB | 7.4 | 0.008 | GHR | IGFBP2;PGF;CGA TSHB |
| macrophage differentiation | 3 | MMP9;CSF1R;VEGFA | 13.8 | 0.008 | MMP9 | CSF1R;VEGFA |
| cell-substrate adhesion | 3 | VWF;ITGA1 ITGB1;ITGA2B ITGB3 | 13.8 | 0.008 | ITGA1 ITGB1 | VWF;ITGA2B ITGB3 |
| mesodermal cell differentiation | 3 | INHBA;ITGA1 ITGB1;ITGA2B ITGB3 | 13.8 | 0.008 | ITGA1 ITGB1 | INHBA;ITGA2B ITGB3 |
| defense response to bacterium | 7 | PPBP;HP;GNLY;CXCL13;CXCL6;H AMP;PPBP | 3.7 | 0.008 | PPBP;HP;GNLY;CXCL13;CXC L6;HAMP;PPBP |
Protein-protein Interaction network
An analysis was performed to retrieve the known protein-protein interactions from the Human Protein Reference Database to assess the connections with proteins changing with gestational age of this study (see Figure 4). Proto-oncogene tyrosine-protein kinase Src (SRCN1) and Tyrosine-protein kinase Fyn (FYN) had the largest number of documented interactions (260 and 196, respectively) of all proteins changing during pregnancy suggesting that they have important roles in the interactome. Other well-known proteins such as MMP-9, VEGF and PlGF had 38, 26, and 5 documented interactions, respectively.
Figure 4.
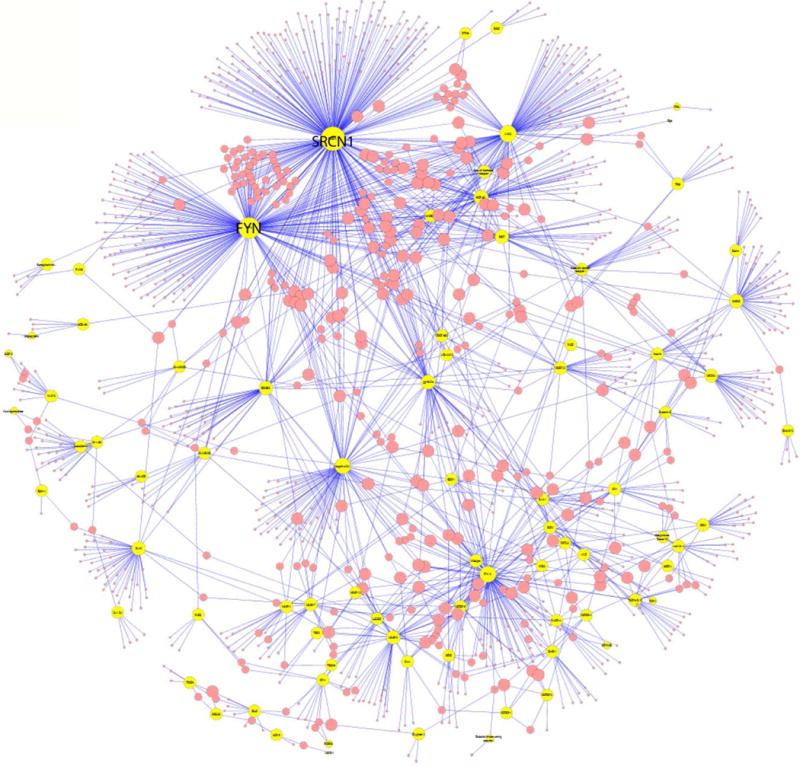
Protein-protein interaction network in normal pregnancy. Yellow circles represent proteins profiled that change with gestation while red circles represent proteins from the Human Protein Reference Database known to interact with these. The size of the circles is proportional with the number of interactions. Proto-oncogene tyrosine-protein kinase Src (SRCN1) and Tyrosine-protein kinase Fyn (FYN) had 260 and 196 interactions, respectively.
Discussion
Principal findings of the study
I) Ten percent (112/1,125) of the 1,125 proteins assessed in this study in maternal plasma change with advancing gestational age; II) the concentration of nine proteins [1) placental growth factor (PlGF); 2) pregnancy-associated plasma protein A (PAPP-A) 3) sialic acid-binding immunoglobulin-type lectins (Siglec)-6; 4) glypican-3; 5) C-C motif (CCL)-28; 6) carbonic anhydrase 6; 7) prolactin (PRL); 8) interleukin-1 receptor 4 (IL-1 R4); and 9) dual specificity mitogen-activated protein kinase kinase 4 (MP2K4)] increased dramatically in abundance (fold change >5); III) proteins demonstrated either increased or decreased in abundance with advancing gestational age following at least 3 distinct patters of change (increasing rate, constant rate, and decreasing rate of change in abundance); and IV) functional analysis revealed that the proteins that were identified as changing with gestational age belonged to the following biological processes (as determined by gene ontology-derived analysis methods): a) general defense response, b) defense response to bacteria, c) defense response to fungi, d) germ cell migration, e) proteolysis, and f) leukocyte migration. This communication reports changes in a large number of proteins in the maternal plasma proteome in normal pregnancy using a state-of-the-art proteomic multiplex platform. The results in normal pregnancy reported herein can serve as the basis for the identification of biomarkers for the prediction, monitoring of disease, and diagnosis of obstetrical disorders.
Meaning of the Study
The reasons to characterize the maternal plasma proteome in normal pregnancy have been outlined in the introduction of this manuscript, and the information derived from measuring protein concentrations has had great clinical value for the care of pregnant women.
We found nine proteins which showed a dramatic increase in concentration, defined as more than five-fold. The magnitude of change of Placental growth factor (PlGF) was 14.5 fold, and this protein plays an important role in the control of angiogenesis, which is a key process in placental development. Low concentrations of PlGF have already been reported in patients who subsequently developed early onset preeclampsia(104–107, 234–237), fetal death of unknown origin(111, 112), small-for-gestational-age infants (with and without Doppler abnormalities) (104–107), maternal floor infarction(109, 238, 239), mirror syndrome(114, 123, 240–244), and some forms of twin-to-twin transfusion syndrome(74). The identification of PlGF as a protein that undergoes dramatic changes in maternal plasma concentration in this study (and using current technologies) is reassuring, given its physiologic importance, as well as the prognostic value in measuring this concentration in maternal plasma to identify patients at risk for adverse pregnancy outcome.
The same applies to Pappalysin-1 (PAPP-A), one of the top nine proteins whose concentration increased more than five-fold in this study. PAPP-A is mainly produced by the placenta, and its concentration is low in the first trimester of pregnancies complicated by either chromosomal abnormalities (Trisomies 21, 18 and 13, and monosomy X) (93, 97–100, 245–248) or a subset destined to develop placenta-mediated obstetrical syndromes such as SGA and preeclampsia (119, 249–261) and preterm birth (262). In this study, we also report the longitudinal changes in the plasma concentrations of PAPP-A as a function of gestational age. The concentration of PAPP-A increased steadily until about 20 weeks of gestation, when it plateaued and remained relatively stable until term. Our observations are consistent with those of a previous report, and provide further strength to the validity of our findings (263).
Maternal plasma Siglec-6 and Glypican-3 were also among the top-ranked proteins which increase in concentration with advancing gestational age 17 and 26 fold, respectively (Table 2). Although these proteins were previously described in in vitro studies and non-pregnant subjects, there is no systematic study describing the changes in the concentrations of these proteins in maternal plasma in normal or abnormal pregnancy.
Sialic-acid-binding immunoglobulin-like lectins (Siglec)-6 is a CD33rSiglec that has been implicated in the regulation of two major biological functions: cell-to-cell interactions and regulation, through glycan recognition, of the innate and adaptive immune systems (264–267). In addition, this molecule can also serve as a leptin receptor (OB-BP1), which has high specificity and affinity to this molecule. Importantly, Siglec-6 is expressed only in cyto- and syncytiotrophoblasts of the human placenta (268–270), and this may explain how it gains access to the maternal circulation and increases with gestational age as the volume of the villous tree increases (270). A recent report has documented an increase in Siglec-6 expression in the placenta following spontaneous labor and delivery in comparison to those following elective cesarean section without labor; therefore, it has been proposed that this molecule may play a role in the process of parturition (270, 271). In addition, increased Siglec-6 trophoblast expression was reported in women with gestational trophoblastic disease and those with preterm preeclampsia (124, 269, 272–274). The findings in Siglec-6 reported herein provide further support that an increase in abundance of this protein, detected with current technology in the maternal circulation, may have biological and clinical implications.
There is a paucity of information about Glypican-3 in pregnancy. This protein is a heparan-sulfate proteoglycan which acts as a co-receptor for heparin-binding growth factors, such as insulin-like growth factor (275–281), which is expressed in the syncytiotrophoblast of term placentas, and has been reported to be downregulated in trophoblasts of patients with preeclampsia (282, 283). One study reported a link between Glypican-3 and one of the major anticoagulation proteins of the trophoblast-placenta protein 5/tissue factor pathway inhibitor 2: this anticoagulation protein is specific to pregnancy and has been implicated in the maintenance of placenta hemostasis; the placental expression of the latter is reduced in preeclampsia(282). Glypican-3 has been proposed to serve as the anchoring protein of placenta protein 5/tissue factor pathway inhibitor 2; and that the decrease in Glypican-3 in preeclampsia leads to the release of placenta protein 5/tissue factor pathway inhibitor 2 into the maternal circulation, promoting a higher concentration in this obstetrical syndrome. Our study reports the first changes in the concentrations of Glypican-3 in the maternal circulation, and it is noteworthy that this protein increases 26-fold during pregnancy in a pattern of decreasing slope (Figure 3) with advancing gestational age.
Several of the findings reported herein are consistent with those described in maternal plasma/serum for other proteins: this lends reassurance to the validity of our findings derived with the use of a novel aptamer-based technology. Specific examples include plasminogen activator inhibitor type 1 (PAI-1) (284), insulin-like growth factor binding protein (IGFBP)-1 (285–290), hepcidin (291) and thyroid stimulating hormone (292). The current study is the first to examine and analyze longitudinally the profile of the maternal plasma proteome of more than 1,000 proteins simultaneously using a high-dimensional biology platform.
Biological processes modulated during gestation
Gene ontology analysis revealed that the defense response, proteolysis and cellular response to hormone stimulus were the most enriched biological processes among proteins that changed with gestational age. The host defense response is a protective mechanism against pathogens and “danger signals” such as alarmins, and involves activation of both the innate and adaptive immune response (293–300). Tolerance of a semi-allograft (fetus and placenta) represents a major biological challenge imposed by viviparity. While the fundamental mechanisms responsible for this remain to be elucidated, a general proposal is that there is a down-regulation of the specific limb of the adaptive immune response aimed at paternal antigens, with an up-regulation of the innate immune response (116–118, 149–154, 301, 302). Down-regulation of specific immune responses includes not only paternal/fetal antigens, but also microbial products; therefore, the physiologic modulation of the immune response to enhance tolerance of the semi-allograft could expose the mother to infection, and the infectious process may be more severe (165, 169). Evidence in support of this is that patients with pyelonephritis during pregnancy are more likely to develop acute respiratory distress syndrome and other complications,(155–157, 166–168) and those with viral diseases during pregnancy (e.g. influenza, varicella, H1N1, SARS) are more likely to develop serious complications, and even die during pregnancy ((179–186, 188, 189). The production of antimicrobial peptides (AMP) is a mechanism to enhance host immunoprotection, and indeed, the production of a broad range of antimicrobial peptides (antibacterial and antiviral) is enhanced during pregnancy (303–308). Such products have been detected in both the amniotic cavity and systemic circulation ((187, 190, 309, 310).
The amniotic fluid is known to have anti-microbial properties (310–316) and this could be due to the presence of naturally occurring AMPs, such as bactericidal/permeability-increasing protein (307, 309), lysozyme (141, 317–320) lactoferrin (320–322), calprotectin (MRP8/14) (309), LL37 (319) and neutrophil defensins (305, 309, 310, 319, 323, 324). Moreover, we reported that increased AMP concentrations are associated with pregnancy complications such as intra-amniotic infection, preterm labor and preterm prelabor rupture of membranes (309, 310). The nature of the host immune response to microorganisms during pregnancy may not be uniform, and may be context-dependent. For example, there is some evidence that the host defense against fungi is not as robust as that against bacteria, and this may explain the increased predisposition to vaginal candidiasis during pregnancy ((191, 192, 325–327). In addition, patients who conceived with an intrauterine contraceptive device are particularly susceptible to infections with fungi in the amniotic cavity, and this may reflect unique features of the complex immune response to different microorganisms during pregnancy(193–202, 328).
One of the findings of this study is that proteins related to smooth muscle cell migration are increased in abundance in maternal plasma with advancing gestational age – this finding is entirely consistent with the fact that the uterus must grow during pregnancy to accommodate the increased size of the fetus, placenta, and amniotic fluid. Importantly, there has not been a good biomarker to monitor smooth muscle function in maternal plasma, and the findings in this study open the door for the identification of such proteins.
Protein-protein interaction network
Proteins function in concert as a part of larger protein complexes within a cell; therefore, an important aspect of proteomic analysis lies in the elucidation of interacting proteins and mapping the corresponding binding sites (329, 330). By identifying proteins interacting with those modulated during normal pregnancy, we can hypothesize on the importance of the change in protein abundance with gestation. These inferences rely on the basis of their degree (number of direct protein-protein interactions) in the interactome. Of note, the average number of interacting proteins in the Human Protein Reference Database is ~3.7 per protein (330). Proto-oncogene tyrosine-protein kinase Src (SRCN1) and Tyrosine-protein kinase Fyn (FYN) had 260 and 196 documented interactions in the Human Protein Reference Database; thus, these two proteins appear to play a central role in the interactome.
SRCN1 is part of the Src signaling system involved in cellular signaling of proteinase-activated receptors (PAR) 2, that is, a G-protein coupled receptor that modulates activation induces vascular endothelial growth factor receptor (VEGFR)-1 promoter activity and sVEGFR-1 release from endothelial cells. Given the importance of VEGFR-1 in the regulation of endothelial function during pregnancy and its pathogenesis in preeclampsia, SGA, fetal death, maternal floor infarction, and some forms of spontaneous preterm labor, our findings strengthen the case for the importance of discovery techniques in the elucidation of fundamental mechanisms of disease in obstetrics.
Tyrosine-protein kinase Fyn (FYN) is also a member of the Src family. In contrast to SRCN1, FYN is involved in immune system activity, especially in T-cell signaling and animal models, suggesting that it is associated with decreased fetal maternal tolerance through the regulation of Th17. In addition, animal experimentation has demonstrated that this protein is also involved in neuronal signaling, migration, and cortical development cell physiology (331, 332).
Strengths and Limitations
The major strength of this study is the characterization of the maternal plasma proteome in longitudinal samples of patients who had a normal pregnancy outcome defined by clinical, neonatal, and pathologic examination of the placenta. We have examined a large number of proteins simultaneously (over 1,000), using sensitive techniques which have been rigorously validated. This study represents the largest examination of the maternal plasma proteome during pregnancy. Another strength of this study is that we have identified proteins that have been found previously to be dramatically up-regulated during pregnancy, such as PlGF and PAPP-A, but also discovered novel proteins which were not known to be drastically changed in concentration. The identification of three broad patterns of protein changes with a large number of proteins is also important, as these observations were not known with specificity prior to this study.
One limitation of this study is that the concentrations are expressed in relative fluorescence units, rather than absolute concentrations, and hence batch effects do not allow a direct comparison of the normal pregnancy reference intervals estimated in this study with data obtained in future studies. However, departures of longitudinal patterns across gestation from the references intervals we have determined can enable discovery of biomarkers unhindered by possible experimental biases. Such discoveries can be followed by quantification by alternative protein measurement techniques such as immunoassays or mass-spectrometry-based assays. In addition, the large majority of the patients included in this study were of African American descent, and future studies will be required to determine whether the changes reported herein occur in populations of other ethnic groups.
Supplementary Material
Table 1. Clinical characteristics.
Demographic characteristics of the study population. Data is presented as number (%percentage) for categorical variables or median/Inter quartile Range (IQR) for continuous variables.
| Characteristics of the study population (n=43) | Median (IQR) or % (n) |
|---|---|
| Age (years) | 25 (21–28) |
| Prepregnant BMI (kg/m2) | 25.2 (21.3–30.6) |
| Nulliparity (%) | 27.9% (12) |
| Race (%) • African American • White • Other |
88.4% (38) 4.7% (2) 7.0% (3) |
| Gestational age at delivery (weeks) | 39.4 (39.0–40.1) |
| Route of delivery • Vaginal delivery • Cesarean delivery |
67.4% (29) 32.6% (14) |
| Birthweight (grams) | 3330 (3120–3545) |
Summary.
This is the first comprehensive study to characterize the longitudinal maternal plasma proteome in pregnancy with the use of novel technology. Since the proteomics technique used in this study provided protein abundance measurements expressed in relative fluorescence units, we could not derive references ranges of protein concentrations so that one can compare future data directly against these references. However, the patterns of change with gestation that we describe can still be useful in discovering disease markers in future studies provided that they deviate from the expected trajectory, regardless of the baseline concentration, which may be subject to experimental batch effects.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 12th World Congress of Perinatal Medicine, November3-6, 2015, Madrid, Spain, as an oral presentation.
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71(5 Suppl):1218S–25S. doi: 10.1093/ajcn/71.5.1218s. [DOI] [PubMed] [Google Scholar]
- 2.Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54(Suppl 1):S47–51. doi: 10.1038/sj.ejcn.1600984. [DOI] [PubMed] [Google Scholar]
- 3.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19(1):43–55. doi: 10.1385/ENDO:19:1:43. [DOI] [PubMed] [Google Scholar]
- 4.Lof M, Olausson H, Bostrom K, Janerot-Sjoberg B, Sohlstrom A, Forsum E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am J Clin Nutr. 2005;81(3):678–85. doi: 10.1093/ajcn/81.3.678. [DOI] [PubMed] [Google Scholar]
- 5.Luan H, Meng N, Liu P, Feng Q, Lin S, Fu J, et al. Pregnancy-induced metabolic phenotype variations in maternal plasma. J Proteome Res. 2014;13(3):1527–36. doi: 10.1021/pr401068k. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay KL, Hellmuth C, Uhl O, Buss C, Wadhwa PD, Koletzko B, et al. Longitudinal Metabolomic Profiling of Amino Acids and Lipids across Healthy Pregnancy. PLoS One. 2015;10(12):e0145794. doi: 10.1371/journal.pone.0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubler S, Damani PM, Pinto ER. Cardiac size and performance during pregnancy estimated with echocardiography. Am J Cardiol. 1977;40(4):534–40. doi: 10.1016/0002-9149(77)90068-6. [DOI] [PubMed] [Google Scholar]
- 8.Clapp JF, 3rd, Seaward BL, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. American journal of obstetrics and gynecology. 1988;159(6):1456–60. doi: 10.1016/0002-9378(88)90574-1. [DOI] [PubMed] [Google Scholar]
- 9.Clapp JF, 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469–73. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 10.Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I, Popescu BA, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5(3):289–97. doi: 10.1161/CIRCIMAGING.111.970012. [DOI] [PubMed] [Google Scholar]
- 11.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101(4):561–70. doi: 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32(1):1–13. vii. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Bonney EA. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet Gynecol Clin North Am. 2016;43(4):679–98. doi: 10.1016/j.ogc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlin A, Alfirevic Z. Physiological changes of pregnancy and monitoring. Best practice & research Clinical obstetrics & gynaecology. 2008;22(5):801–23. doi: 10.1016/j.bpobgyn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Rees GB, Broughton Pipkin F, Symonds EM, Patrick JM. A longitudinal study of respiratory changes in normal human pregnancy with cross-sectional data on subjects with pregnancy-induced hypertension. American journal of obstetrics and gynecology. 1990;162(3):826–30. doi: 10.1016/0002-9378(90)91018-8. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. American journal of obstetrics and gynecology. 1990;162(4):1008–14. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 18.Baeyens L, Hindi S, Sorenson RL, German MS. beta-Cell adaptation in pregnancy. Diabetes Obes Metab. 2016;18(Suppl 1):63–70. doi: 10.1111/dom.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. American journal of obstetrics and gynecology. 1999;180(4):903–16. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky D, Hobel CJ. Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17 alpha-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. American journal of obstetrics and gynecology. 1973;117(7):884–93. doi: 10.1016/0002-9378(73)90057-4. [DOI] [PubMed] [Google Scholar]
- 21.Frankenne F, Closset J, Gomez F, Scippo ML, Smal J, Hennen G. The physiology of growth hormones (GHs) in pregnant women and partial characterization of the placental GH variant. J Clin Endocrinol Metab. 1988;66(6):1171–80. doi: 10.1210/jcem-66-6-1171. [DOI] [PubMed] [Google Scholar]
- 22.Peck TM, Arias F. Hematologic changes associated with pregnancy. Clin Obstet Gynecol. 1979;22(4):785–98. doi: 10.1097/00003081-197912000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Letsky EA. Erythropoiesis in pregnancy. Journal of perinatal medicine. 1995;23(1–2):39–45. doi: 10.1515/jpme.1995.23.1-2.39. [DOI] [PubMed] [Google Scholar]
- 24.Fragiadakis GK, Baca QJ, Gherardini PF, Ganio EA, Gaudilliere DK, Tingle M, et al. Mapping the Fetomaternal Peripheral Immune System at Term Pregnancy. J Immunol. 2016;197(11):4482–92. doi: 10.4049/jimmunol.1601195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen H, Leach LS, Mackinnon A. Cognition in pregnancy and motherhood: prospective cohort study. Br J Psychiatry. 2010;196(2):126–32. doi: 10.1192/bjp.bp.109.068635. [DOI] [PubMed] [Google Scholar]
- 26.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29(8):793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 27.Feldt-Rasmussen U, Mathiesen ER. Endocrine disorders in pregnancy: physiological and hormonal aspects of pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25(6):875–84. doi: 10.1016/j.beem.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Edstrom K, Persson B, Cerasi E, Luft R. Patterns of free fatty acids, glycerol, D-beta-hydroxybutyrate and insulin in pregnant women and their newborn infants. Effects of a low and a high insulin response to glucose in the mothers. Acta obstetricia et gynecologica Scandinavica. 1975;54(4):347–56. doi: 10.3109/00016347509156766. [DOI] [PubMed] [Google Scholar]
- 29.Herrera E, Lasuncion MA, Gomez-Coronado D, Aranda P, Lopez-Luna P, Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. American journal of obstetrics and gynecology. 1988;158(6 Pt 2):1575–83. doi: 10.1016/0002-9378(88)90193-7. [DOI] [PubMed] [Google Scholar]
- 30.Kashyap ML, Sivasamboo R, Sothy SP, Cheah JS, Gartside PS. Carbohydrate and lipid metabolism during human labor: free fatty acids, glucose, insulin, and lactic acid metabolism during normal and oxytocin-induced labor for postmaturity. Metabolism: clinical and experimental. 1976;25(8):865–75. doi: 10.1016/0026-0495(76)90119-0. [DOI] [PubMed] [Google Scholar]
- 31.Knopp RH, Warth MR, Charles D, Childs M, Li JR, Mabuchi H, et al. Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes. Biology of the neonate. 1986;50(6):297–317. doi: 10.1159/000242614. [DOI] [PubMed] [Google Scholar]
- 32.Potter JM. Perinatal plasma lipid concentrations. Australian and New Zealand journal of medicine. 1977;7(2):155–60. doi: 10.1111/j.1445-5994.1977.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 33.Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. American journal of obstetrics and gynecology. 1979;133(2):165–70. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 34.Joseph JC, Baker C, Sprang ML, Bermes EW. Changes in plasma proteins during pregnancy. Annals of clinical and laboratory science. 1978;8(2):130–41. [PubMed] [Google Scholar]
- 35.Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, Pereira-Leite L, et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 2005;123(1):46–51. doi: 10.1016/j.ejogrb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Del Priore G, Chatterton R, Jr, Lee C, Silver R, Berg L, Lee MJ. Comparison of mononuclear cell proteins and plasma proteins before and during parturition by two dimensional electrophoresis. Journal of perinatal medicine. 1991;19(5):373–7. doi: 10.1515/jpme.1991.19.5.373. [DOI] [PubMed] [Google Scholar]
- 37.Malm J, Laurell M, Dahlback B. Changes in the plasma levels of vitamin K-dependent proteins C and S and of C4b-binding protein during pregnancy and oral contraception. British journal of haematology. 1988;68(4):437–43. doi: 10.1111/j.1365-2141.1988.tb04232.x. [DOI] [PubMed] [Google Scholar]
- 38.Walker MC, Smith GN, Perkins SL, Keely EJ, Garner PR. Changes in homocysteine levels during normal pregnancy. American journal of obstetrics and gynecology. 1999;180(3 Pt 1):660–4. doi: 10.1016/s0002-9378(99)70269-3. [DOI] [PubMed] [Google Scholar]
- 39.Whittaker PG, Lind T. The intravascular mass of albumin during human pregnancy: a serial study in normal and diabetic women. British journal of obstetrics and gynaecology. 1993;100(6):587–92. doi: 10.1111/j.1471-0528.1993.tb15315.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoneyama Y, Suzuki S, Sawa R, Takeuchi T, Kobayashi H, Takei R, et al. Changes in plasma adenosine concentrations during normal pregnancy. Gynecologic and obstetric investigation. 2000;50(3):145–8. doi: 10.1159/000010313. [DOI] [PubMed] [Google Scholar]
- 41.Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, et al. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. American journal of obstetrics and gynecology. 1996;174(5):1575–83. doi: 10.1016/s0002-9378(96)70609-9. [DOI] [PubMed] [Google Scholar]
- 42.Cox BD, Calame DP. Changes in plasma amino acid levels during the human menstrual cycle and in early pregnancy. A preliminary report. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1978;10(5):428–33. doi: 10.1055/s-0028-1093407. [DOI] [PubMed] [Google Scholar]
- 43.Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. American journal of obstetrics and gynecology. 1998;178(3):551–6. doi: 10.1016/s0002-9378(98)70437-5. [DOI] [PubMed] [Google Scholar]
- 44.Chesley LC. Blood pressure, edema and proteinuria in pregnancy. 9. Proposal for classification. Progress in clinical and biological research. 1976;7:249–68. [PubMed] [Google Scholar]
- 45.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625–33. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 46.Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstetrics and gynecology. 2007;109(1):168–80. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 47.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10(9):531–40. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Possomato-Vieira JS, Khalil RA. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv Pharmacol. 2016;77:361–431. doi: 10.1016/bs.apha.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Premawardhana LD, Parkes AB, Ammari F, John R, Darke C, Adams H, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab. 2000;85(1):71–5. doi: 10.1210/jcem.85.1.6227. [DOI] [PubMed] [Google Scholar]
- 50.Lonstein JS, Maguire J, Meinlschmidt G, Neumann ID. Emotion and mood adaptations in the peripartum female:complementary contributions of GABA and oxytocin. J Neuroendocrinol. 2014;26(10):649–64. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoekzema E, Barba-Muller E, Pozzobon C, Picado M, Lucco F, Garcia-Garcia D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2016 doi: 10.1038/nn.4458. [DOI] [PubMed] [Google Scholar]
- 52.Wilkins MR, Pasquali C, Appel RD, Ou K, Golaz O, Sanchez JC, et al. From proteins to proteomes: large scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology (N Y) 1996;14(1):61–5. doi: 10.1038/nbt0196-61. [DOI] [PubMed] [Google Scholar]
- 53.Romero R, Kuivaniemi H, Tromp G, Olson J. The design, execution, and interpretation of genetic association studies to decipher complex diseases. American journal of obstetrics and gynecology. 2002;187(5):1299–312. doi: 10.1067/mob.2002.128319. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. American journal of obstetrics and gynecology. 2006;195(2):360–3. doi: 10.1016/j.ajog.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 55.Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006;113(Suppl 3):118–35. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chard T. Pregnancy tests: a review. Human reproduction (Oxford, England) 1992;7(5):701–10. doi: 10.1093/oxfordjournals.humrep.a137722. [DOI] [PubMed] [Google Scholar]
- 57.Hinney B, Bertagnoli C, Tobler-Sommer M, Osmers R, Wuttke W, Kuhn W. Diagnosis of early ectopic pregnancy by measurement of the maternal serum to cul-de-sac fluid beta-hCG ratio. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1995;5(4):260–6. doi: 10.1046/j.1469-0705.1995.05040260.x. [DOI] [PubMed] [Google Scholar]
- 58.Nyberg DA, Filly RA, Mahony BS, Monroe S, Laing FC, Jeffrey RB., Jr Early gestation: correlation of HCG levels and sonographic identification. AJR American journal of roentgenology. 1985;144(5):951–4. doi: 10.2214/ajr.144.5.951. [DOI] [PubMed] [Google Scholar]
- 59.Enk L, Wikland M, Hammarberg K, Lindblom B. The value of endovaginal sonography and urinary human chorionic gonadotropin tests for differentiation between intrauterine and ectopic pregnancy. Journal of clinical ultrasound : JCU. 1990;18(2):73–8. doi: 10.1002/jcu.1870180202. [DOI] [PubMed] [Google Scholar]
- 60.Feldkamp CS, Pfeffer WH. The measurement of human chorionic gonadotropin for pregnancy testing. Henry Ford Hospital medical journal. 1982;30(4):207–13. [PubMed] [Google Scholar]
- 61.Jouppila P, Tapanainen J, Huhtaniemi I. Plasma hCG and ultrasound in suspected ectopic pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 1980;10(1):3–12. doi: 10.1016/0028-2243(80)90031-3. [DOI] [PubMed] [Google Scholar]
- 62.Landesman R, Saxena BB. Results of the first 1000 radioreceptorassays for the determination of human chorionic gonadotropin: a new, rapid, reliable, and sensitive pregnancy test. Fertility and sterility. 1976;27(4):357–68. [PubMed] [Google Scholar]
- 63.Mishell DR, Jr, Davajan V. Quantitative immunologic assay of human chorionic gonadotropin in normal and abnormal pregnancies. American journal of obstetrics and gynecology. 1966;96(2):231–9. doi: 10.1016/0002-9378(66)90320-6. [DOI] [PubMed] [Google Scholar]
- 64.Nyberg DA, Filly RA, Laing FC, Mack LA, Zarutskie PW. Ectopic pregnancy. Diagnosis by sonography correlated with quantitative HCG levels. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1987;6(3):145–50. doi: 10.7863/jum.1987.6.3.145. [DOI] [PubMed] [Google Scholar]
- 65.Pittaway DE, Wentz AC. Evaluation of early pregnancy by serial chorionic gonadotropin determinations: a comparison of methods by receiver operating characteristic curve analysis. Fertility and sterility. 1985;43(4):529–33. doi: 10.1016/s0015-0282(16)48492-x. [DOI] [PubMed] [Google Scholar]
- 66.Saxena BB, Landesman R. The use of a radioreceptorassay of human chorionic gonadotropin for the diagnosis and management of ectopic pregnancy. Fertility and sterility. 1975;26(5):397–404. doi: 10.1016/s0015-0282(16)41110-6. [DOI] [PubMed] [Google Scholar]
- 67.Steier JA, Bergsjo P, Myking OL. Human chorionic gonadotropin in maternal plasma after induced abortion, spontaneous abortion, and removed ectopic pregnancy. Obstetrics and gynecology. 1984;64(3):391–4. [PubMed] [Google Scholar]
- 68.Kadar N, DeCherney AH, Romero R. Receiver operating characteristic (ROC) curve analysis of the relative efficacy of single and serial chorionic gonadotropin determinations in the early diagnosis of ectopic pregnancy. Fertility and sterility. 1982;37(4):542–7. doi: 10.1016/s0015-0282(16)46163-7. [DOI] [PubMed] [Google Scholar]
- 69.Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstetrics and gynecology. 1981;58(2):156–61. [PubMed] [Google Scholar]
- 70.Kadar N, Taylor KJ, Rosenfield AT, Romero R. Combined use of serum HCG and sonography in the diagnosis of ectopic pregnancy. AJR American journal of roentgenology. 1983;141(3):609–15. doi: 10.2214/ajr.141.3.609. [DOI] [PubMed] [Google Scholar]
- 71.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7(3):237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 72.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 73.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation-a workshop report. Placenta. 2005;26(Suppl A):S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, et al. Twin-to-twin transfusion syndrome: an antiangiogenic state? American journal of obstetrics and gynecology. 2008;198(4):382.e1–8. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest. 2013;123(7):2775–7. doi: 10.1172/JCI70431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. American journal of obstetrics and gynecology. 2007;196(4):326.e1–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstetrics and gynecology. 2006;107(2 Pt 1):399–413. doi: 10.1097/01.AOG.0000198632.15229.be. [DOI] [PubMed] [Google Scholar]
- 78.Cox J, Mann M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu Rev Biochem. 2011;80:273–99. doi: 10.1146/annurev-biochem-061308-093216. [DOI] [PubMed] [Google Scholar]
- 79.Wilhelm M, Schlegl J, Hahne H, Gholami AM, Lieberenz M, Savitski MM, et al. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509(7502):582–7. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 80.Levitt M. Nature of the protein universe. Proc Natl Acad Sci U S A. 2009;106(27):11079–84. doi: 10.1073/pnas.0905029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han X, Aslanian A, Yates JR., 3rd Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12(5):483–90. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amniotic-fluid alpha-fetoprotein measurement in antenatal diagnosis of anencephaly and open spina bifida in early pregnancy. Second report of the U.K. Collaborative Study on Alpha-fetoprotein in Relation to Neural-tube Defects. Lancet (London, England) 1979;2(8144):651–62. [PubMed] [Google Scholar]
- 83.Bond EB, Thompson W, Elwood JH, Cran GW. Evaluation of measurement of maternal plasma alpha-fetoprotein levels as a screening test for fetal neural tube defects. British journal of obstetrics and gynaecology. 1977;84(8):574–7. doi: 10.1111/j.1471-0528.1977.tb12655.x. [DOI] [PubMed] [Google Scholar]
- 84.Merkatz IR, Nitowsky HM, Macri JN, Johnson WE. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. American journal of obstetrics and gynecology. 1984;148(7):886–94. doi: 10.1016/0002-9378(84)90530-1. [DOI] [PubMed] [Google Scholar]
- 85.Wald N, Barker S, Peto R, Brock DJ, Bonnar J. Maternal serum alpha-fetoprotein and previous neural tube defects. British journal of obstetrics and gynaecology. 1976;83(3):213–6. doi: 10.1111/j.1471-0528.1976.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 86.Weiss RR, Macri JN, Elligers K, Princler GL, McIntire R, Waldman TA. Amniotic fluid alpha-fetoprotein as a marker in prenatal diagnosis of neural tube defects. Obstetrics and gynecology. 1976;47(2):148–51. [PubMed] [Google Scholar]
- 87.Cuckle HS, Wald NJ, Lindenbaum RH. Maternal serum alpha-fetoprotein measurement: a screening test for Down syndrome. Lancet (London, England) 1984;1(8383):926–9. doi: 10.1016/s0140-6736(84)92389-4. [DOI] [PubMed] [Google Scholar]
- 88.Heyl PS, Miller W, Canick JA. Maternal serum screening for aneuploid pregnancy by alpha-fetoprotein, hCG, and unconjugated estriol. Obstetrics and gynecology. 1990;76(6):1025–31. [PubMed] [Google Scholar]
- 89.Crossley JA, Aitken DA, Connor JM. Prenatal screening for chromosome abnormalities using maternal serum chorionic gonadotrophin, alpha-fetoprotein, and age. Prenat Diagn. 1991;11(2):83–101. doi: 10.1002/pd.1970110204. [DOI] [PubMed] [Google Scholar]
- 90.Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS) Health Technol Assess. 2003;7(11):1–77. doi: 10.3310/hta7110. [DOI] [PubMed] [Google Scholar]
- 91.Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353(19):2001–11. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- 92.Bredaki FE, Wright D, Matos P, Syngelaki A, Nicolaides KH. First-trimester screening for trisomy 21 using alpha-fetoprotein. Fetal Diagn Ther. 2011;30(3):215–8. doi: 10.1159/000330198. [DOI] [PubMed] [Google Scholar]
- 93.Wright D, Syngelaki A, Bradbury I, Akolekar R, Nicolaides KH. First-trimester screening for trisomies 21, 18 and 13 by ultrasound and biochemical testing. Fetal Diagn Ther. 2014;35(2):118–26. doi: 10.1159/000357430. [DOI] [PubMed] [Google Scholar]
- 94.Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenat Diagn. 1987;7(9):623–30. doi: 10.1002/pd.1970070904. [DOI] [PubMed] [Google Scholar]
- 95.MacDonald ML, Wagner RM, Slotnick RN. Sensitivity and specificity of screening for Down syndrome with alpha-fetoprotein, hCG, unconjugated estriol, and maternal age. Obstetrics and gynecology. 1991;77(1):63–8. [PubMed] [Google Scholar]
- 96.Spencer K, Macri JN, Aitken DA, Connor JM. Free beta-hCG as first-trimester marker for fetal trisomy. Lancet (London, England) 1992;339(8807):1480. doi: 10.1016/0140-6736(92)92073-o. [DOI] [PubMed] [Google Scholar]
- 97.Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta-hCG and pregnancy-associated plasma protein-A. Human reproduction (Oxford, England) 2008;23(9):1968–75. doi: 10.1093/humrep/den224. [DOI] [PubMed] [Google Scholar]
- 98.Kagan KO, Anderson JM, Anwandter G, Neksasova K, Nicolaides KH. Screening for triploidy by the risk algorithms for trisomies 21, 18 and 13 at 11 weeks to 13 weeks and 6 days of gestation. Prenat Diagn. 2008;28(13):1209–13. doi: 10.1002/pd.2149. [DOI] [PubMed] [Google Scholar]
- 99.Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;31(5):493–502. doi: 10.1002/uog.5332. [DOI] [PubMed] [Google Scholar]
- 100.Wright D, Spencer K, Kagan KK, Torring N, Petersen OB, Christou A, et al. First-trimester combined screening for trisomy 21 at 7–14 weeks’ gestation. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010;36(4):404–11. doi: 10.1002/uog.7755. [DOI] [PubMed] [Google Scholar]
- 101.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 102.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 103.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 104.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22(11):1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24(10):1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. American journal of obstetrics and gynecology. 2013;208(4):287.e1–e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JM, Pappas A, Tarca AL, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27(2):132–44. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20(7):495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23(12):1384–99. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaiworapongsa T, Romero R, Whitten AE, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med. 2016;29(8):1214–28. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stepan H, Faber R. Cytomegalovirus-induced mirror syndrome associated with elevated levels of angiogenic factors. Obstetrics and gynecology. 2007;109(5):1205–6. doi: 10.1097/01.AOG.0000263776.46071.d1. author reply 6. [DOI] [PubMed] [Google Scholar]
- 115.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;32(6):732–9. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 116.Sacks G, Sargent I, Redman C. Innate immunity in pregnancy. Immunol Today. 2000;21(4):200–1. doi: 10.1016/s0167-5699(00)01615-7. [DOI] [PubMed] [Google Scholar]
- 117.Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta. 2001;22(6):550–9. doi: 10.1053/plac.2001.0686. [DOI] [PubMed] [Google Scholar]
- 118.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One. 2011;6(5):e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karagiannis G, Akolekar R, Sarquis R, Wright D, Nicolaides KH. Prediction of small-for-gestation neonates from biophysical and biochemical markers at 11–13 weeks. Fetal Diagn Ther. 2011;29(2):148–54. doi: 10.1159/000321694. [DOI] [PubMed] [Google Scholar]
- 120.Nucci M, Poon LC, Demirdjian G, Darbouret B, Nicolaides KH. Maternal serum placental growth factor (PlGF) isoforms 1 and 2 at 11–13 weeks’ gestation in normal and pathological pregnancies. Fetal Diagn Ther. 2014;36(2):106–16. doi: 10.1159/000357842. [DOI] [PubMed] [Google Scholar]
- 121.Dragan I, Wright D, Fiolna M, Leipold G, Nicolaides KH. Development of preeclampsia within four weeks of sFLT to PLGF ratio >38: Comparison of performance at 31–34 versus 35–37 weeks’ gestation. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2016 doi: 10.1002/uog.17310. [DOI] [PubMed] [Google Scholar]
- 122.Khalil A, Maiz N, Garcia-Mandujano R, Penco JM, Nicolaides KH. Longitudinal changes in maternal serum placental growth factor and soluble fms-like tyrosine kinase-1 in women at increased risk of pre-eclampsia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2016;47(3):324–31. doi: 10.1002/uog.15750. [DOI] [PubMed] [Google Scholar]
- 123.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19(10):607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varkonyi T, Nagy B, Fule T, Tarca AL, Karaszi K, Schonleber J, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32(Suppl):S21–9. doi: 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab. 2002;87(6):2431–4. doi: 10.1210/jcem.87.6.8689. [DOI] [PubMed] [Google Scholar]
- 126.Finishing the euchromatic sequence of the human genome. Nature. 2004;431(7011):931–45. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 127.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70(4):265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reshetova P, Smilde AK, van Kampen AH, Westerhuis JA. Use of prior knowledge for the analysis of high-throughput transcriptomics and metabolomics data. BMC Syst Biol. 2014;8(Suppl 2):S2. doi: 10.1186/1752-0509-8-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox B, Leavey K, Nosi U, Wong F, Kingdom J. Placental transcriptome in development and pathology: expression, function, and methods of analysis. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S138–51. doi: 10.1016/j.ajog.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 130.Edlow AG, Guedj F, Pennings JL, Sverdlov D, Neri C, Bianchi DW. Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. American journal of obstetrics and gynecology. 2016;214(5):623.e1–e10. doi: 10.1016/j.ajog.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edlow AG, Slonim DK, Wick HC, Hui L, Bianchi DW. The pathway not taken: understanding ‘omics data in the perinatal context. American journal of obstetrics and gynecology. 2015;213(1):59.e1–172. doi: 10.1016/j.ajog.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lassance L, Haghiac M, Leahy P, Basu S, Minium J, Zhou J, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. American journal of obstetrics and gynecology. 2015;212(5):647.e1–11. doi: 10.1016/j.ajog.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monk D. Genomic imprinting in the human placenta. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S152–62. doi: 10.1016/j.ajog.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 134.Pew BK, Harris RA, Sbrana E, Guaman MC, Shope C, Chen R, et al. Structural and transcriptomic response to antenatal corticosteroids in an Erk3-null mouse model of respiratory distress. American journal of obstetrics and gynecology. 2016;215(3):384.e1–e89. doi: 10.1016/j.ajog.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosa-e-Silva JC, Virginio LA, Jr, Meola J, Dentillo DB, Ferriani RA, Giuliatti S. A system for storing, retrieving, and comparing gene expression information of patients with endometriosis. American journal of obstetrics and gynecology. 2015;212(3):407. doi: 10.1016/j.ajog.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 136.Tain YL, Lee CT, Chan JY, Hsu CN. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal NG-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. American journal of obstetrics and gynecology. 2016;215(5):636.e1–e72. doi: 10.1016/j.ajog.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 137.Whitehead CL, McNamara H, Walker SP, Alexiadis M, Fuller PJ, Vickers DK, et al. Identifying late-onset fetal growth restriction by measuring circulating placental RNA in the maternal blood at 28 weeks’ gestation. American journal of obstetrics and gynecology. 2016;214(4):521.e1–8. doi: 10.1016/j.ajog.2016.01.191. [DOI] [PubMed] [Google Scholar]
- 138.Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21(10):697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21(6):367–88. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee DC, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteomics. 2011;74(6):817–28. doi: 10.1016/j.jprot.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang AH, Sun H, Yan GL, Han Y, Wang XJ. Serum proteomics in biomedical research: a systematic review. Appl Biochem Biotechnol. 2013;170(4):774–86. doi: 10.1007/s12010-013-0238-7. [DOI] [PubMed] [Google Scholar]
- 143.Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, et al. A proteome-scale map of the human interactome network. Cell. 2014;159(5):1212–26. doi: 10.1016/j.cell.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Menni C, Kiddle SJ, Mangino M, Vinuela A, Psatha M, Steves C, et al. Circulating Proteomic Signatures of Chronological Age. J Gerontol A Biol Sci Med Sci. 2015;70(7):809–16. doi: 10.1093/gerona/glu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lynch AM, Wagner BD, Deterding RR, Giclas PC, Gibbs RS, Janoff EN, et al. The relationship of circulating proteins in early pregnancy with preterm birth. American journal of obstetrics and gynecology. 2016;214(4):517.e1–8. doi: 10.1016/j.ajog.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. American journal of obstetrics and gynecology. 2015;212(4):487.e1–e11. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, et al. Predictors of response to 17-alpha hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. American journal of obstetrics and gynecology. 2016;214(3):376.e1–8. doi: 10.1016/j.ajog.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saade GR, Boggess KA, Sullivan SA, Markenson GR, Iams JD, Coonrod DV, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. American journal of obstetrics and gynecology. 2016;214(5):633.e1–e24. doi: 10.1016/j.ajog.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. American journal of obstetrics and gynecology. 2001;185(5):1124–9. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 150.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72(2):107–16. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. American journal of obstetrics and gynecology. 2002;187(4):889–93. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 152.Koga K, Izumi G, Mor G, Fujii T, Osuga Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol. 2014;72(2):192–205. doi: 10.1111/aji.12258. [DOI] [PubMed] [Google Scholar]
- 153.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bubeck RW. Acute pyelonephritis during pregnancy with anuria, septicemia and thrombocytopenia. Del Med J. 1968;40(5):143–7. [PubMed] [Google Scholar]
- 156.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstetrics and gynecology. 1973;42(1):112–7. [PubMed] [Google Scholar]
- 157.Dilworth EE, Ward JV. Bacteremic shock in pyelonephritis and criminal abortion. Obstetrics and gynecology. 1961;17:160–7. [PubMed] [Google Scholar]
- 158.Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23(12):1344–59. doi: 10.3109/14767058.2010.482618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wishart DS, Mandal R, Stanislaus A, Ramirez-Gaona M. Cancer Metabolomics and the Human Metabolome Database. Metabolites. 2016;6(1) doi: 10.3390/metabo6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bahado-Singh RO, Syngelaki A, Akolekar R, Mandal R, Bjondahl TC, Han B, et al. Validation of metabolomic models for prediction of early-onset preeclampsia. American journal of obstetrics and gynecology. 2015;213(4):530.e1–e10. doi: 10.1016/j.ajog.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 161.Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. American journal of obstetrics and gynecology. 2015;212(6):776.e1–e12. doi: 10.1016/j.ajog.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jelliffe-Pawlowski LL, Norton ME, Baer RJ, Santos N, Rutherford GW. Gestational dating by metabolic profile at birth: a California cohort study. American journal of obstetrics and gynecology. 2016;214(4):511.e1–13. doi: 10.1016/j.ajog.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pinto J, Almeida LM, Martins AS, Duarte D, Domingues MR, Barros AS, et al. Impact of fetal chromosomal disorders on maternal blood metabolome: toward new biomarkers? American journal of obstetrics and gynecology. 2015;213(6):841.e1–e15. doi: 10.1016/j.ajog.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 164.Ventura JE, Villa M, Mizraji R, Ferreiros R. Acute renal failure in pregnancy. Ren Fail. 1997;19(2):217–20. doi: 10.3109/08860229709026279. [DOI] [PubMed] [Google Scholar]
- 165.Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstetrics and gynecology. 1997;90(4 Pt 1):553–61. doi: 10.1016/s0029-7844(97)00352-9. [DOI] [PubMed] [Google Scholar]
- 166.Pruett K, Faro S. Pyelonephritis associated with respiratory distress. Obstetrics and gynecology. 1987;69(3 Pt 2):444–6. [PubMed] [Google Scholar]
- 167.Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. American journal of obstetrics and gynecology. 1987;156(4):797–807. doi: 10.1016/0002-9378(87)90335-8. [DOI] [PubMed] [Google Scholar]
- 168.Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8(2):353–73. doi: 10.1016/s0950-3552(05)80325-6. [DOI] [PubMed] [Google Scholar]
- 169.Catanzarite VA, Willms D. Adult respiratory distress syndrome in pregnancy: report of three cases and review of the literature. Obstet Gynecol Surv. 1997;52(6):381–92. doi: 10.1097/00006254-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 170.Bianchi DW. From prenatal genomic diagnosis to fetal personalized medicine: progress and challenges. Nat Med. 2012;18(7):1041–51. doi: 10.1038/nm.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Butte AJ. It takes a genome to understand a village: Population scale precision medicine. Proc Natl Acad Sci U S A. 2016;113(44):12344–6. doi: 10.1073/pnas.1615329113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Edlow AG, Slonim DK, Wick HC, Hui L, Bianchi DW. The pathway not taken: understanding ‘omics data in the perinatal context. American journal of obstetrics and gynecology. 2015;213(1):59.e1–172. doi: 10.1016/j.ajog.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gligorijevic V, Malod-Dognin N, Przulj N. Integrative methods for analyzing big data in precision medicine. Proteomics. 2016;16(5):741–58. doi: 10.1002/pmic.201500396. [DOI] [PubMed] [Google Scholar]
- 174.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–92. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Green D. Coagulation cascade. Hemodial Int. 2006;10(Suppl 2):S2–4. doi: 10.1111/j.1542-4758.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 176.Cimmino G, Ciccarelli G, Golino P. Role of Tissue Factor in the Coagulation Network. Semin Thromb Hemost. 2015;41(7):708–17. doi: 10.1055/s-0035-1564045. [DOI] [PubMed] [Google Scholar]
- 177.Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016;26(4):249–61. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 178.Tarca AL, Romero R, Draghici S. Analysis of microarray experiments of gene expression profiling. American journal of obstetrics and gynecology. 2006;195(2):373–88. doi: 10.1016/j.ajog.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei L, Sun S, Zhang J, Zhu H, Xu Y, Ma Q, et al. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS) Biochem Cell Biol. 2010;88(4):723–30. doi: 10.1139/O10-022. [DOI] [PubMed] [Google Scholar]
- 180.Phillips KP, O’Sullivan TL, Dow D, Amaratunga CA. Infectious respiratory disease outbreaks and pregnancy: occupational health and safety concerns of Canadian nurses. Prehosp Disaster Med. 2011;26(2):114–21. doi: 10.1017/S1049023X11000100. [DOI] [PubMed] [Google Scholar]
- 181.Wang G, Zhou Y, Gong S, Dong H, Wu G, Xiang X, et al. A PREGNANT WOMAN WITH AVIAN INFLUENZA A (H7N9) VIRUS PNEUMONIA AND ARDS MANAGED WITH EXTRACORPOREAL MEMBRANE OXYGENATION. Southeast Asian J Trop Med Public Health. 2015;46(3):444–8. [PubMed] [Google Scholar]
- 182.Maxwell C, McGeer A, Tai KF, Seimer A. SOGC Clinical Practice Guideline. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). No. 225, April 2009. Int J Gynaecol Obstet. 2009;107(1):82–6. doi: 10.1016/j.ijgo.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heron M. Deaths: Leading Causes for 2012. Natl Vital Stat Rep. 2015;64(10):1–93. [PubMed] [Google Scholar]
- 184.Stockman LJ, Lowther SA, Coy K, Saw J, Parashar UD. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10(9):1689–90. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.D’Mello T, Brammer L, Blanton L, Kniss K, Smith S, Mustaquim D, et al. Update: Influenza activity-United States, September 28, 2014-February 21, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(8):206–12. [PMC free article] [PubMed] [Google Scholar]
- 186.Gresh L, Kuan G, Sanchez N, Azziz-Baumgartner E, Ojeda S, Melendez M, et al. Burden of Influenza and Influenza-associated Pneumonia in the First Year of Life in a Prospective Cohort Study in Managua, Nicaragua. Pediatr Infect Dis J. 2016;35(2):152–6. doi: 10.1097/INF.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. American journal of obstetrics and gynecology. 1997;176(2):470–5. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 188.Chu HY, Katz J, Tielsch J, Khatry SK, Shrestha L, LeClerq SC, et al. Clinical Presentation and Birth Outcomes Associated with Respiratory Syncytial Virus Infection in Pregnancy. PLoS One. 2016;11(3):e0152015. doi: 10.1371/journal.pone.0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Acosta CD, Harrison DA, Rowan K, Lucas DN, Kurinczuk JJ, Knight M. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open. 2016;6(8):e012323. doi: 10.1136/bmjopen-2016-012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997;38(4):252–5. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 191.Us E, Cengiz SA. Prevalence and phenotypic evaluation of Candida dubliniensis in pregnant women with vulvovaginal candidosis in a university hospital in Ankara. Mycoses. 2007;50(1):13–20. doi: 10.1111/j.1439-0507.2006.01289.x. [DOI] [PubMed] [Google Scholar]
- 192.Audisio T, Penacino M, Cannistraci R, Bertolotto P. Detection of bacterial vaginosis, Trichomonas vaginalis infection, and vaginal Candida infection: a comparative study of methods of extracting exudates, with and without a speculum, during pregnancy. J Low Genit Tract Dis. 2005;9(4):213–5. doi: 10.1097/01.lgt.0000179859.29456.ba. [DOI] [PubMed] [Google Scholar]
- 193.Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD) Journal of perinatal medicine. 2010;38(1):45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chaim W, Mazor M, Meril T, Peleg R, Maor E. Late miscarriage and intraamniotic candidiasis in a woman with a retained intrauterine contraceptive device. Arch Gynecol Obstet. 1993;253(3):157–60. doi: 10.1007/BF02767335. [DOI] [PubMed] [Google Scholar]
- 195.Horn LC, Nenoff P, Ziegert M, Hockel M. Missed abortion complicated by Candida infection in a woman with rested IUD. Arch Gynecol Obstet. 2001;264(4):215–7. doi: 10.1007/s004040000117. [DOI] [PubMed] [Google Scholar]
- 196.Marelli G, Mariani A, Frigerio L, Leone E, Ferrari A. Fetal Candida infection associated with an intrauterine contraceptive device. European journal of obstetrics, gynecology, and reproductive biology. 1996;68(1–2):209–12. doi: 10.1016/0301-2115(96)02471-2. [DOI] [PubMed] [Google Scholar]
- 197.Smith CV, Horenstein J, Platt LD. Intraamniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: a case report. American journal of obstetrics and gynecology. 1988;159(1):123–4. doi: 10.1016/0002-9378(88)90505-4. [DOI] [PubMed] [Google Scholar]
- 198.Bider D, Ben-Rafael Z, Barkai G, Mashiach S. Intrauterine fetal death apparently due to Candida chorioamnionitis. Arch Gynecol Obstet. 1989;244(3):175–7. doi: 10.1007/BF00931296. [DOI] [PubMed] [Google Scholar]
- 199.Potasman I, Leibovitz Z, Sharf M. Candida sepsis in pregnancy and the postpartum period. Rev Infect Dis. 1991;13(1):146–9. doi: 10.1093/clinids/13.1.146. [DOI] [PubMed] [Google Scholar]
- 200.Chaim W, Mazor M, Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Arch Gynecol Obstet. 1992;251(1):9–15. doi: 10.1007/BF02718273. [DOI] [PubMed] [Google Scholar]
- 201.Spaun E, Klunder K. Candida chorioamnionitis and intra-uterine contraceptive device. Acta obstetricia et gynecologica Scandinavica. 1986;65(2):183–4. doi: 10.3109/00016348609158377. [DOI] [PubMed] [Google Scholar]
- 202.Delprado WJ, Baird PJ, Russell P. Placental candidiasis: report of three cases with a review of the literature. Pathology. 1982;14(2):191–5. doi: 10.3109/00313028209061293. [DOI] [PubMed] [Google Scholar]
- 203.Klein J, Buffin-Meyer B, Mullen W, Carty DM, Delles C, Vlahou A, et al. Clinical proteomics in obstetrics and neonatology. Expert Rev Proteomics. 2014;11(1):75–89. doi: 10.1586/14789450.2014.872564. [DOI] [PubMed] [Google Scholar]
- 204.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, et al. A draft map of the human proteome. Nature. 2014;509(7502):575–81. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kusebauch U, Campbell DS, Deutsch EW, Chu CS, Spicer DA, Brusniak MY, et al. Human SRMAtlas: A Resource of Targeted Assays to Quantify the Complete Human Proteome. Cell. 2016;166(3):766–78. doi: 10.1016/j.cell.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Omenn GS, Lane L, Lundberg EK, Beavis RC, Overall CM, Deutsch EW. Metrics for the Human Proteome Project 2016: Progress on Identifying and Characterizing the Human Proteome, Including Post-Translational Modifications. J Proteome Res. 2016;15(11):3951–60. doi: 10.1021/acs.jproteome.6b00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schweitzer B, Roberts S, Grimwade B, Shao W, Wang M, Fu Q, et al. Multiplexed protein profiling on microarrays by rolling-circle amplification. Nat Biotechnol. 2002;20(4):359–65. doi: 10.1038/nbt0402-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fredriksson S, Dixon W, Ji H, Koong AC, Mindrinos M, Davis RW. Multiplexed protein detection by proximity ligation for cancer biomarker validation. Nat Methods. 2007;4(4):327–9. doi: 10.1038/nmeth1020. [DOI] [PubMed] [Google Scholar]
- 209.Zichi D, Eaton B, Singer B, Gold L. Proteomics and diagnostics: Let’s Get Specific, again. Curr Opin Chem Biol. 2008;12(1):78–85. doi: 10.1016/j.cbpa.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 210.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12):e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–39. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ostroff RM, Bigbee WL, Franklin W, Gold L, Mehan M, Miller YE, et al. Unlocking biomarker discovery: large scale application of aptamer proteomic technology for early detection of lung cancer. PLoS One. 2010;5(12):e15003. doi: 10.1371/journal.pone.0015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ostroff RM, Mehan MR, Stewart A, Ayers D, Brody EN, Williams SA, et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS One. 2012;7(10):e46091. doi: 10.1371/journal.pone.0046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mehan MR, Ayers D, Thirstrup D, Xiong W, Ostroff RM, Brody EN, et al. Protein signature of lung cancer tissues. PLoS One. 2012;7(4):e35157. doi: 10.1371/journal.pone.0035157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mehan MR, Williams SA, Siegfried JM, Bigbee WL, Weissfeld JL, Wilson DO, et al. Validation of a blood protein signature for non-small cell lung cancer. Clin Proteomics. 2014;11(1):32. doi: 10.1186/1559-0275-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Webber J, Stone TC, Katilius E, Smith BC, Gordon B, Mason MD, et al. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol Cell Proteomics. 2014;13(4):1050–64. doi: 10.1074/mcp.M113.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112(23):7153–8. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Drolet DW, Green LS, Gold L, Janjic N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016 doi: 10.1089/nat.2015.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Davies DR, Gelinas AD, Zhang C, Rohloff JC, Carter JD, O’Connell D, et al. Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. Proc Natl Acad Sci U S A. 2012;109(49):19971–6. doi: 10.1073/pnas.1213933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.SomaLogic. SOMAmer Technical notes. http://www.somalogic.com/somalogic/media/Assets/PDFs/SSM-017-Rev-3-SOMAmer-Technical-Note-3-7-15.pdf.
- 221.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. 2014 http://arxivorg/abs/14065823.
- 222.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 223.Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006;7(1):55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- 224.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. Journal of perinatal medicine. 2010;38(6):617–43. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pappas A, Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JC, Bhatti G, et al. Transcriptomics of maternal and fetal membranes can discriminate between gestational-age matched preterm neonates with and without cognitive impairment diagnosed at 18–24 months. PLoS One. 2015;10(3):e0118573. doi: 10.1371/journal.pone.0118573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tarca AL, Lauria M, Unger M, Bilal E, Boue S, Kumar Dey K, et al. Strengths and limitations of microarray-based phenotype prediction: lessons learned from the IMPROVER Diagnostic Signature Challenge. Bioinformatics. 2013;29(22):2892–9. doi: 10.1093/bioinformatics/btt492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Koenker R. quantreg: Quantile Regression. R package 5.05 ed.
- 228.Koenker RW. Quantile Regression. Cambridge U. Press; 2005. [Google Scholar]
- 229.Tibshirani R, Guenther W, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J Royal Stat Soc B. 2001;63(2):411–23. [Google Scholar]
- 230.Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2005;33(Database issue):D54–8. doi: 10.1093/nar/gki031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Peri S, Navarro JD, Kristiansen TZ, Amanchy R, Surendranath V, Muthusamy B, et al. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004;32(Database issue):D497–501. doi: 10.1093/nar/gkh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 234.Baltajian K, Bajracharya S, Salahuddin S, Berg AH, Geahchan C, Wenger JB, et al. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. American journal of obstetrics and gynecology. 2016;215(1):89.e1–e10. doi: 10.1016/j.ajog.2016.01.168. [DOI] [PubMed] [Google Scholar]
- 235.Holme AM, Roland MC, Henriksen T, Michelsen TM. In vivo uteroplacental release of placental growth factor and soluble Fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies. American journal of obstetrics and gynecology. 2016;215(6):782.e1–e9. doi: 10.1016/j.ajog.2016.07.056. [DOI] [PubMed] [Google Scholar]
- 236.Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. American journal of obstetrics and gynecology. 2016;214(1):108.e1–e14. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang J, Pearl M, DeLorenze GN, Romero R, Dong Z, Jelliffe-Pawlowski L, et al. Racial-ethnic differences in midtrimester maternal serum levels of angiogenic and antiangiogenic factors. American journal of obstetrics and gynecology. 2016;215(3):359.e1–9. doi: 10.1016/j.ajog.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chaiworapongsa T, Romero R, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Segars JH, et al. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD) J Matern Fetal Neonatal Med. 2016;29(6):855–62. doi: 10.3109/14767058.2015.1022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, et al. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. American journal of obstetrics and gynecology. 2013;208(4):310.e1–e11. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bixel K, Silasi M, Zelop CM, Lim KH, Zsengeller Z, Stillman IE, et al. Placental origins of angiogenic dysfunction in mirror syndrome. Hypertens Pregnancy. 2012;31(2):211–7. doi: 10.3109/10641955.2011.638959. [DOI] [PubMed] [Google Scholar]
- 241.Fisher SJ. Why is placentation abnormal in preeclampsia? American journal of obstetrics and gynecology. 2015;213(4 Suppl):S115–22. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Graham N, Garrod A, Bullen P, Heazell AE. Placental expression of anti-angiogenic proteins in mirror syndrome: a case report. Placenta. 2012;33(6):528–31. doi: 10.1016/j.placenta.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 243.Llurba E, Marsal G, Sanchez O, Dominguez C, Alijotas-Reig J, Carreras E, et al. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012;40(3):367–9. doi: 10.1002/uog.10136. [DOI] [PubMed] [Google Scholar]
- 244.Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstetrics and gynecology. 2007;109(2 Pt2):549–52. doi: 10.1097/01.AOG.0000248538.03280.cf. [DOI] [PubMed] [Google Scholar]
- 245.Spencer K, Tul N, Nicolaides KH. Maternal serum free beta-hCG and PAPP-A in fetal sex chromosome defects in the first trimester. Prenat Diagn. 2000;20(5):390–4. doi: 10.1002/(sici)1097-0223(200005)20:5<390::aid-pd824>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 246.Colosi E, D’Ambrosio V, Periti E. FIRST TRIMESTER CONTINGENT SCREENING FOR TRISOMIES 21,18,13: IS THIS MODEL COST EFFICIENT AND FEASIBLE IN PUBBLIC HEALTH SYSTEM? J Matern Fetal Neonatal Med. 2016:1–13. doi: 10.1080/14767058.2016.1268593. [DOI] [PubMed] [Google Scholar]
- 247.Gupta S, Fox NS, Rebarber A, Saltzman DH, Klauser CK, Roman AS. Biochemical screening for aneuploidy in patients with donor oocyte pregnancies compared with autologous pregnancies. J Matern Fetal Neonatal Med. 2014;27(14):1418–21. doi: 10.3109/14767058.2013.866644. [DOI] [PubMed] [Google Scholar]
- 248.Wiechec M, Knafel A, Nocun A, Ludwin A, Ludwin I, Maczka M, et al. Screening for trisomy 18 using traditional combined screening vs. ultrasound-based protocol in tertiary center environment. J Matern Fetal Neonatal Med. 2016:1–6. doi: 10.1080/14767058.2016.1224837. [DOI] [PubMed] [Google Scholar]
- 249.Poon LC, Maiz N, Valencia C, Plasencia W, Nicolaides KH. First-trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009;33(1):23–33. doi: 10.1002/uog.6280. [DOI] [PubMed] [Google Scholar]
- 250.Poon LC, Syngelaki A, Akolekar R, Lai J, Nicolaides KH. Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther. 2013;33(1):16–27. doi: 10.1159/000341712. [DOI] [PubMed] [Google Scholar]
- 251.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 252.Conde-Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. American journal of obstetrics and gynecology. 2014;211(6):583–95. doi: 10.1016/j.ajog.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Han CS, Platt LD. Noninvasive prenatal testing: need for informed enthusiasm. American journal of obstetrics and gynecology. 2014;211(6):577–80. doi: 10.1016/j.ajog.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 254.Odibo AO. Pregnancy associated-plasma protein-A (PAPP-A) and alfa-fetoprotein (AFP) associated with placental abruption. American journal of obstetrics and gynecology. 2014;211(2):89–90. doi: 10.1016/j.ajog.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 255.O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. American journal of obstetrics and gynecology. 2016;214(1):103.e1–e12. doi: 10.1016/j.ajog.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 256.Abdel Moety GA, Almohamady M, Sherif NA, Raslana AN, Mohamed TF, El Moneam HM, et al. Could first-trimester assessment of placental functions predict preeclampsia and intrauterine growth restriction? A prospective cohort study. J Matern Fetal Neonatal Med. 2016;29(3):413–7. doi: 10.3109/14767058.2014.1002763. [DOI] [PubMed] [Google Scholar]
- 257.Dukhovny S, Zera C, Little SE, McElrath T, Wilkins-Haug L. Eliminating first trimester markers: will replacing PAPP-A and betahCG miss women at risk for small for gestational age? J Matern Fetal Neonatal Med. 2014;27(17):1761–4. doi: 10.3109/14767058.2013.879703. [DOI] [PubMed] [Google Scholar]
- 258.Duran M, Kosus A, Kosus N, Turhan NO. Relation between serum PAPP-A level and umbilical cord thickness during first trimester of pregnancy. J Matern Fetal Neonatal Med. 2014;27(4):385–7. doi: 10.3109/14767058.2013.818129. [DOI] [PubMed] [Google Scholar]
- 259.Krauskopf AL, Knippel AJ, Verde PE, Kozlowski P. Predicting SGA neonates using first-trimester screening: influence of previous pregnancy’s birthweight and PAPP-A MoM. J Matern Fetal Neonatal Med. 2016;29(18):2962–7. doi: 10.3109/14767058.2015.1109622. [DOI] [PubMed] [Google Scholar]
- 260.Kumar M, Singh S, Sharma K, Singh R, Ravi V, Bhattacharya J. Adverse fetal outcome: is first trimester ultrasound and Doppler better predictor than biomarkers? J Matern Fetal Neonatal Med. 2016:1–7. doi: 10.1080/14767058.2016.1214709. [DOI] [PubMed] [Google Scholar]
- 261.Moon M, Odibo A. First-trimester screening for preeclampsia: impact of maternal parity on modeling and screening effectiveness. J Matern Fetal Neonatal Med. 2015;28(17):2028–33. doi: 10.3109/14767058.2014.978758. [DOI] [PubMed] [Google Scholar]
- 262.Lynch AM, Wagner BD, Deterding RR, Giclas PC, Gibbs RS, Janoff EN, et al. The relationship of circulating proteins in early pregnancy with preterm birth. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wright D, Silva M, Papadopoulos S, Wright A, Nicolaides KH. Serum pregnancy-associated plasma protein-A in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015;46(1):42–50. doi: 10.1002/uog.14870. [DOI] [PubMed] [Google Scholar]
- 264.Crocker PR, Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001;22(6):337–42. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 265.von Gunten S, Simon HU. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 2006;20(6):601–5. doi: 10.1096/fj.05-5401hyp. [DOI] [PubMed] [Google Scholar]
- 266.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 267.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lam KK, Chiu PC, Lee CL, Pang RT, Leung CO, Koistinen H, et al. Glycodelin-A protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J Biol Chem. 2011;286(43):37118–27. doi: 10.1074/jbc.M111.233841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rumer KK, Post MD, Larivee RS, Zink M, Uyenishi J, Kramer A, et al. Siglec-6 is expressed in gestational trophoblastic disease and affects proliferation, apoptosis and invasion. Endocr Relat Cancer. 2012;19(6):827–40. doi: 10.1530/ERC-11-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17(9):922–31. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 271.Patel N, Brinkman-Van der Linden EC, Altmann SW, Gish K, Balasubramanian S, Timans JC, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274(32):22729–38. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 272.Rumer KK, Uyenishi J, Hoffman MC, Fisher BM, Winn VD. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod Sci. 2013;20(6):646–53. doi: 10.1177/1933719112461185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–62. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tsai S, Hardison NE, James AH, Motsinger-Reif AA, Bischoff SR, Thames BH, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32(2):175–82. doi: 10.1016/j.placenta.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64(4):841–8. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 276.De Cat B, David G. Developmental roles of the glypicans. Semin Cell Dev Biol. 2001;12(2):117–25. doi: 10.1006/scdb.2000.0240. [DOI] [PubMed] [Google Scholar]
- 277.Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573(3):241–6. doi: 10.1016/s0304-4165(02)00390-2. [DOI] [PubMed] [Google Scholar]
- 278.Fransson LA, Belting M, Cheng F, Jonsson M, Mani K, Sandgren S. Novel aspects of glypican glycobiology. Cell Mol Life Sci. 2004;61(9):1016–24. doi: 10.1007/s00018-004-3445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82(2):184–9. doi: 10.1016/j.yexmp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 280.Fico A, Maina F, Dono R. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci. 2011;68(6):923–9. doi: 10.1007/s00018-007-7471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280(10):2471–6. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 282.Ogawa M, Yanoma S, Nagashima Y, Okamoto N, Ishikawa H, Haruki A, et al. Paradoxical discrepancy between the serum level and the placental intensity of PP5/TFPI-2 in preeclampsia and/or intrauterine growth restriction: possible interaction and correlation with glypican-3 hold the key. Placenta. 2007;28(2–3):224–32. doi: 10.1016/j.placenta.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 283.Chui A, Murthi P, Brennecke SP, Ignjatovic V, Monagle PT, Said JM. The expression of placental proteoglycans in pre-eclampsia. Gynecologic and obstetric investigation. 2012;73(4):277–84. doi: 10.1159/000333262. [DOI] [PubMed] [Google Scholar]
- 284.Coolman M, de Groot CJ, Steegers EA, Geurts-Moespot A, Thomas CM, Steegers-Theunissen RP, et al. Concentrations of plasminogen activators and their inhibitors in blood preconceptionally, during and after pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 2006;128(1–2):22–8. doi: 10.1016/j.ejogrb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 285.Hills FA, English J, Chard T. Circulating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weight. J Endocrinol. 1996;148(2):303–9. doi: 10.1677/joe.0.1480303. [DOI] [PubMed] [Google Scholar]
- 286.Skjaerbaek C, Frystyk J, Orskov H, Flyvbjerg A. Free IGF-I, IGFBP-1, and the binary complex of IGFBP-1 and IGF-I are increased during human pregnancy. Horm Res. 2004;62(5):215–20. doi: 10.1159/000081246. [DOI] [PubMed] [Google Scholar]
- 287.Olausson H, Lof M, Brismar K, Lewitt M, Forsum E, Sohlstrom A. Longitudinal study of the maternal insulin-like growth factor system before, during and after pregnancy in relation to fetal and infant weight. Horm Res. 2008;69(2):99–106. doi: 10.1159/000111813. [DOI] [PubMed] [Google Scholar]
- 288.Monaghan JM, Godber IM, Lawson N, Kaur M, Wark G, Teale D, et al. Longitudinal changes of insulin-like growth factors and their binding proteins throughout normal pregnancy. Ann Clin Biochem. 2004;41(Pt 3):220–6. doi: 10.1258/000456304323019596. [DOI] [PubMed] [Google Scholar]
- 289.Hills FA, Iles RK, Sullivan MH. Differential proteolysis of insulin-like growth factor binding protein-1 (IGFBP-1) in pregnancy. Journal of perinatal medicine. 2013;41(3):241–9. doi: 10.1515/jpm-2012-0086. [DOI] [PubMed] [Google Scholar]
- 290.Lee SE, Han BD, Park IS, Romero R, Yoon BH. Evidence supporting proteolytic cleavage of insulin-like growth factor binding protein-1 (IGFBP-1) protein in amniotic fluid. Journal of perinatal medicine. 2008;36(4):316–23. doi: 10.1515/JPM.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med. 2013;51(7):1395–401. doi: 10.1515/cclm-2012-0576. [DOI] [PubMed] [Google Scholar]
- 292.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstetrics and gynecology. 2005;106(4):753–7. doi: 10.1097/01.AOG.0000175836.41390.73. [DOI] [PubMed] [Google Scholar]
- 293.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;(54 Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 294.Janeway C. Immunogenicity signals 1,2,3 … and 0. Immunol Today. 1989;10(9):283–6. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- 295.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 296.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 297.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 298.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 299.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 300.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323(5922):1683–4. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 301.Redman CW, Sargent IL. Immunological disorders of human pregnancy. Oxf Rev Reprod Biol. 1986;8:223–65. [PubMed] [Google Scholar]
- 302.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. American journal of obstetrics and gynecology. 1998;179(1):80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 303.Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347(15):1199–200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 304.Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43(2):300–4. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- 305.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 306.Yeaman MR, Yount NY. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5(9):727–40. doi: 10.1038/nrmicro1744. [DOI] [PubMed] [Google Scholar]
- 307.Frew L, Stock SJ. Antimicrobial peptides and pregnancy. Reproduction. 2011;141(6):725–35. doi: 10.1530/REP-10-0537. [DOI] [PubMed] [Google Scholar]
- 308.Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353–77. doi: 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 309.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 310.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20(1):15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. American journal of obstetrics and gynecology. 1970;106(1):59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 312.Thadepalli H, Appleman MD, Maidman JE, Arce JJ, Davidson EC., Jr Antimicrobial effect of amniotic fluid against anaerobic bacteria. American journal of obstetrics and gynecology. 1977;127(3):250–4. doi: 10.1016/0002-9378(77)90463-x. [DOI] [PubMed] [Google Scholar]
- 313.Tafari N, Ross SM, Naeye RL, Galask RP, Zaar B. Failure of bacterial growth inhibition by amniotic fluid. American journal of obstetrics and gynecology. 1977;128(2):187–9. doi: 10.1016/0002-9378(77)90685-8. [DOI] [PubMed] [Google Scholar]
- 314.Thadepalli H, Bach VT, Davidson EC., Jr Antimicrobial effect of amniotic fluid. Obstetrics and gynecology. 1978;52(2):198–204. [PubMed] [Google Scholar]
- 315.Appelbaum PC, Shulman G, Chambers NL, Simon NV, Granados JL, Fairbrother PF, et al. Studies on the growth-inhibiting property of amniotic fluids from two United States population groups. American journal of obstetrics and gynecology. 1980;137(5):579–82. doi: 10.1016/0002-9378(80)90699-7. [DOI] [PubMed] [Google Scholar]
- 316.Thadepalli H, Gangopadhyay PK, Maidman JE. Amniotic fluid analysis for antimicrobial factors. Int J Gynaecol Obstet. 1982;20(1):65–72. doi: 10.1016/0020-7292(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 317.Cherry SH, Filler M, Harvey H. Lysozyme content of amniotic fluid. American journal of obstetrics and gynecology. 1973;116(5):639–42. doi: 10.1016/s0002-9378(15)33127-6. [DOI] [PubMed] [Google Scholar]
- 318.Ford LC, DeLange RJ, Lebherz TB. Identification of a bactericidal factor (B-lysin) in amnionic fluid at 14 and 40 weeks’ gestation. American journal of obstetrics and gynecology. 1977;127(7):788–92. doi: 10.1016/0002-9378(77)90259-9. [DOI] [PubMed] [Google Scholar]
- 319.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53(2):211–6. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 320.Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. American journal of obstetrics and gynecology. 2004;191(6):2090–6. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 321.Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, et al. Amniotic fluid lactoferrin in intrauterine infection. Placenta. 1999;20(2–3):175–9. doi: 10.1053/plac.1998.0368. [DOI] [PubMed] [Google Scholar]
- 322.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. American journal of obstetrics and gynecology. 2000;183(4):904–10. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 323.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27(3):513–8. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 324.Marinoni E, Di Iorio R, Letizia C, Villaccio B, Alberini A, Cosmi EV. Amniotic fluid concentrations of adrenomedullin in preterm labor. Obstetrics and gynecology. 1999;93(6):964–7. doi: 10.1016/s0029-7844(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 325.Akinbiyi AA, Watson R, Feyi-Waboso P. Prevalence of Candida albicans and bacterial vaginosis in asymptomatic pregnant women in South Yorkshire, United Kingdom. Outcome of a prospective study. Arch Gynecol Obstet. 2008;278(5):463–6. doi: 10.1007/s00404-008-0593-8. [DOI] [PubMed] [Google Scholar]
- 326.Purtilo DT. Opportunistic mycotic infections in pregnant women. American journal of obstetrics and gynecology. 1975;122(5):607–10. doi: 10.1016/0002-9378(75)90058-7. [DOI] [PubMed] [Google Scholar]
- 327.Robinson SC, Nicholas WC, Lee DT, Wanklin JM, Zwicker B. The relationship of pregnancy, vaginal candidiasis and glucose metabolism. Can Med Assoc J. 1967;96(10):583–4. [PMC free article] [PubMed] [Google Scholar]
- 328.Brandsma MA, Braaksma JT, van der Harten JJ. Immature delivery after intrauterine Candida albicans infection. European journal of obstetrics, gynecology, and reproductive biology. 1975;5(6):331–5. doi: 10.1016/0028-2243(75)90062-3. [DOI] [PubMed] [Google Scholar]
- 329.Walhout AJ, Vidal M. Protein interaction maps for model organisms. Nat Rev Mol Cell Biol. 2001;2(1):55–62. doi: 10.1038/35048107. [DOI] [PubMed] [Google Scholar]
- 330.Peri S, Navarro JD, Amanchy R, Kristiansen TZ, Jonnalagadda CK, Surendranath V, et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13(10):2363–71. doi: 10.1101/gr.1680803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Knox R, Jiang X. Fyn in Neurodevelopment and Ischemic Brain Injury. Dev Neurosci. 2015;37(4–5):311–20. doi: 10.1159/000369995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr Med Chem. 2011;18(19):2921–42. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


