Abstract
Pseudomonas aeruginosa is an opportunistic and frequently drug-resistant pulmonary pathogen especially in cystic fibrosis sufferers. Recently, the frog skin-derived antimicrobial peptide (AMP) Esc(1–21) and its diastereomer Esc(1–21)-1c were found to possess potent in vitro antipseudomonal activity. Here, they were first shown to preserve the barrier integrity of airway epithelial cells better than the human AMP LL-37. Furthermore, Esc(1–21)-1c was more efficacious than Esc(1–21) and LL-37 in protecting host from pulmonary bacterial infection after a single intra-tracheal instillation at a very low dosage of 0.1 mg/kg. The protection was evidenced by 2-log reduction of lung bacterial burden and was accompanied by less leukocytes recruitment and attenuated inflammatory response. In addition, the diastereomer was more efficient in reducing the systemic dissemination of bacterial cells. Importantly, in contrast to what reported for other AMPs, the peptide was administered at 2 hours after bacterial challenge to better reflect the real life infectious conditions. To the best of our knowledge, this is also the first study investigating the effect of AMPs on airway-epithelia associated genes upon administration to infected lungs. Overall, our data highly support advanced preclinical studies for the development of Esc(1–21)-1c as an efficacious therapeutic alternative against pulmonary P. aeruginosa infections.
Introduction
Multidrug-resistant (MDR) bacterial infections represent a serious life-threat causing almost 50,000 deaths per year in Europe and in the US; and this number is expected to grow up to tenfold by 2050, killing more than cancer1, 2. In particular, pulmonary infections due to the Gram-negative bacterium Pseudomonas aeruginosa remain one of the major cause of morbidity and mortality3, either in intensive care units or in ventilated patients, as well as in cystic fibrosis (CF) sufferers, complicating therapy in the CF airways4–7. In parallel, the decrease in the pharmaceutical industry research pipeline for novel antimicrobial agents during the last three decades has resulted in an urgent need for the discovery of new strategies to address the vital problems of infectious diseases8, 9. Naturally occurring antimicrobial peptides (AMPs) or their derivatives stand for an appealing source for the generation of new therapeutics9–16. AMPs are ubiquitous in nature and act as the first line of defence against invading microorganisms17, 18. Although human lung epithelial cells produce AMPs (e.g. defensins and the cathelicidins LL-37)19, 20, most of them are present at very low concentrations and are salt-sensitive in vitro making it difficult for them to be active in the abnormally low pH and high-salt environment existing at the apical side of CF epithelial cells21–23. A promising therapeutic approach to defeat P. aeruginosa lung infections would be to exogenously apply AMPs in the lung environment. We recently identified a short-sized (21 amino acids long) derivative of the frog-skin AMP esculentin-1a, named Esculentin-1a(1–21)NH2 [GIFSKLAGKKIKNLLISGLKG-NH2, Esc(1–21)]24, 25, with the following attractive features: (i) a potent and rapid killing kinetics against both planktonic and biofilms forms of P. aeruginosa strains, with a pronounced membrane-perturbing activity as a plausible mode of action26. This is a highly non-specific mechanism which limits the induction of resistance compared to the highly selective conventional antibiotics e.g. tobramycin and ciprofloxacin that would no longer be able to recognize their specific and single target, after mutation27; (ii) the ability to preserve antimicrobial activity at high ionic strength, in contrast with the majority of AMPs of mammalian origin28; (iii) the ability to detoxify, in vitro, P. aeruginosa lipopolysaccharide (LPS), by inhibiting the release of TNF-α from LPS-activated macrophages28.
In addition, a diastereomer of Esc(1–21), named Esc(1–21)-1c, containing only two d-amino acid substitutions at the C-terminal region (dLeu14 and dSer17) was lately synthesized and found to be significantly more stable than the corresponding all-L peptide and less susceptible to enzymatic degradation (i.e. human and bacterial elastases)29; less toxic towards mammalian cells; more active against P. aeruginosa biofilms and more efficient in stimulating bronchial cells migration and presumably in restoring the integrity of an injured bronchial epithelium, a property which is not shown by any traditional antibiotic28, 30.
Even though AMPs are under investigation as novel therapeutics to defeat microbial infections, only a few studies have been reported to date on their antibacterial activity in the lungs of animal models of P. aeruginosa pneumonia31, 32. Remarkably, as learned from literature, when locally applied via intra-nasal or intra-tracheal route in murine models of acute lung infection, they are generally administered after a short time interval (5–15 min) from bacterial infection33, 34. Furthermore, to the best of our knowledge, no studies have been accomplished by the effect(s) of AMPs on the airway epithelial gene expression after P. aeruginosa-induced respiratory infection.
Taking into account the attractive properties of the two esculentin-derived peptides, we first evaluated their outcome on the bronchial epithelium permeability followed by their therapeutic efficacy in murine models of acute Pseudomonas lung infections, upon intra-tracheal instillation. Their effects on the expression of inflammation associated genes were also investigated. Overall, the results of our experiments have pointed out a higher in vivo antipseudomonal activity of the diastereomer Esc(1–21)-1c than its all-l counterpart without provoking any noticeable inflammation or damage at the lung level. Importantly, our work is the first demonstration of a frog-skin derived AMP to significantly promote lung clearance of P. aeruginosa when administered in a single dose at 2 hours after infection. The designed diastereomer demonstrated a higher antibacterial activity than the mammalian AMP LL-37, as well as a comparable potency to that of the clinically used colistin peptide35, 36, whose antibacterial activity is exerted through binding to LPS, the major component of the outer membrane of Gram-negative bacteria.
Hence, our data imply the diastereomer Esc(1–21)-1c as an attractive peptide for the development of future therapy for effective treatments of Pseudomonas-induced lung infections.
Results
Effect of peptides on the lung epithelial tight junction permeability
It was previously demonstrated that Esc(1–21) has a strong in vitro antipseudomonal activity, with a minimal inhibitory concentration (MIC) of 4 μM26. Before performing in vivo studies, the effect of the two esculentin derivatives on the integrity of human airway epithelium was analyzed at three selected peptide concentrations by measuring the transepithelial electrical resistance (TEER) in polarized human primary bronchial cells. Furthermore, the human cathelicidin AMP LL-37 was used as a reference. As shown in Fig. 1, when all peptides were used at a concentration corresponding to 2 × MIC (8 μM), they did not affect the epithelium integrity. When tested at a higher concentration (i.e. 20 μM), LL-37 initially decreased TEER, but this value recovered and was close to the initial one after 10 hours. Differently, when used at the higher concentration of 64 μM, it exhibited significantly detrimental effect on disturbing the epithelial cell tight junction and its negative effect persisted for a long period of time (up-triangle, broken line). In comparison, only a mild decrease in the TEER was observed for 64 μM of Esc(1–21) within 24 h (upside down triangle, broken line). Interestingly, Esc(1-21)-1c treatment had no measureable difference on TEER and did not disturb the membrane integrity at the same 64 μM concentration (square).
Figure 1.
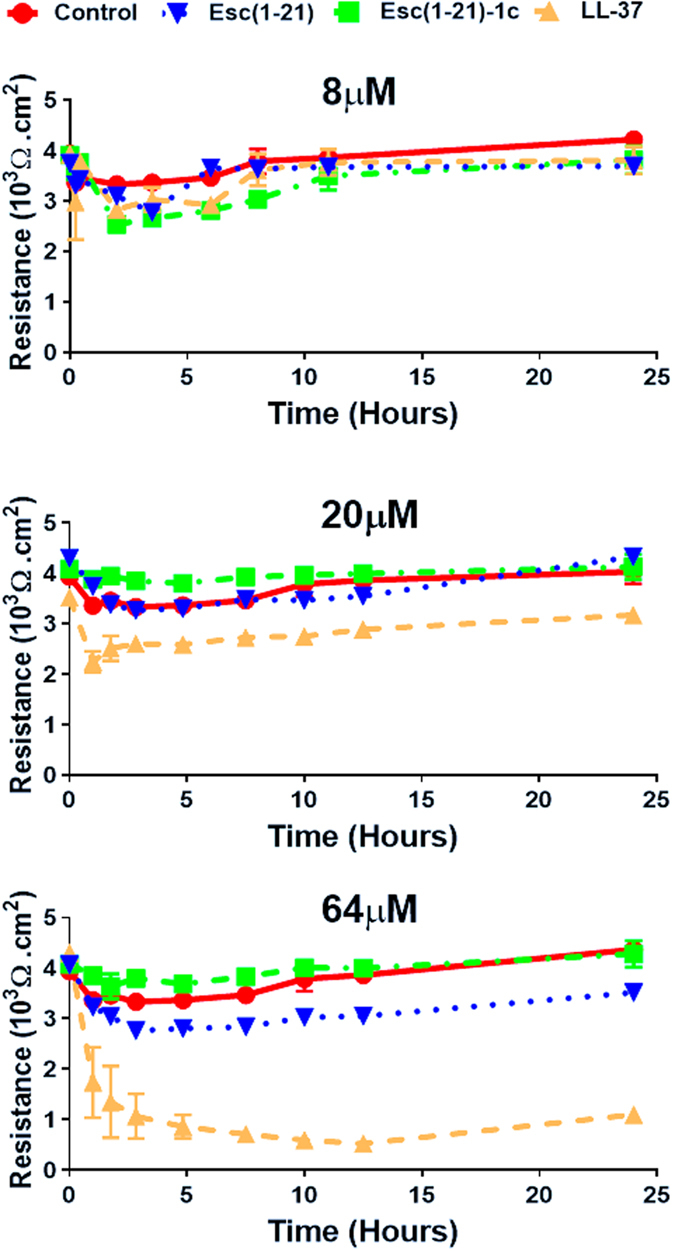
Effects of AMPs on the TEER of lung epithelial cells. Peptides in PBS were added to the apical compartment of the normal primary human bronchial epithelial cells maintained in ALI condition. TEER was measured at multiple time points (1, 2, 3, 5, 8, 10, 12 and 24 hours). Results are representative data from three independent experiments.
Effect of peptides on the lung inflammatory response and pulmonary toxicity
Prior to analyzing the in vivo antipseudomonal efficacy of exogenous AMPs, the ability of the selected peptides [i.e. Esc(1–21), Esc(1–21)-1c and LL-37] to elicit host immune response and eventually lead to pulmonary toxicity upon intra-tracheal delivery, was evaluated at 24 hours after their administration. The peptides were tested at a concentration of 20 μM (2 μg/mouse) and 64 μM (7 μg/mouse).
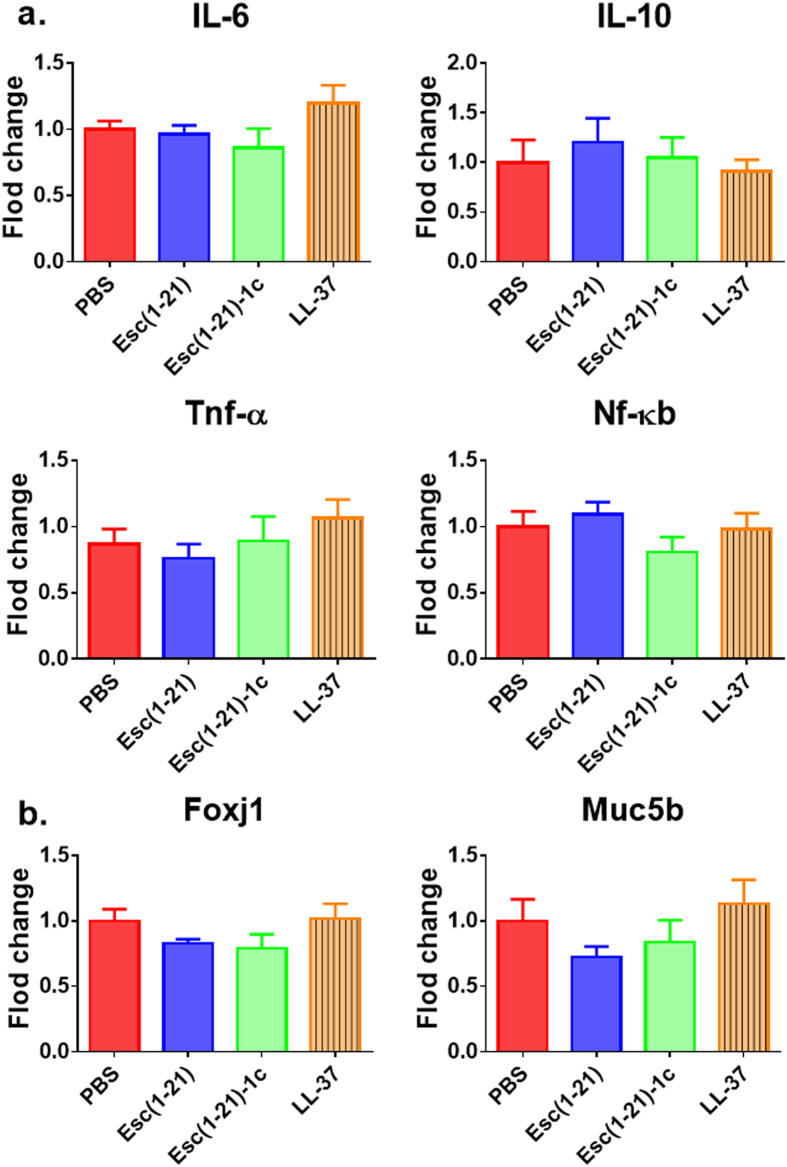
Based on the concentrations used for determining the epithelial membrane permeability (Fig. 1), 64 μM (7 μg/mouse, 0.35 mg/kg) of both Esc(1–21) and LL-37 stimulated the host immune system, causing a considerable increase in the number of neutrophils and macrophages in the bronchoalveolar lavage (BAL) compared to the amount found in the control animals receiving the vehicle phosphate buffered saline (PBS) (Fig. 2a, red bars). However, the lung inflammatory reaction was negligible when the peptides were used at 20 μM (2 μg/mouse, 0.1 mg/kg), as demonstrated by the invariant number of total immune cells in the BAL compared to PBS-treated mice (Fig. 2b). In parallel, no observable effects on inflammation-related genes expression (such as those encoding for the cytokines IL-6, IL-10 or the tumor necrosis factor-α TNFα, and NF-kB) were observed in the lungs of mice, at 24 hours after peptide treatment at a concentration of 0.1 mg/kg (Fig. 3a).
Figure 2.
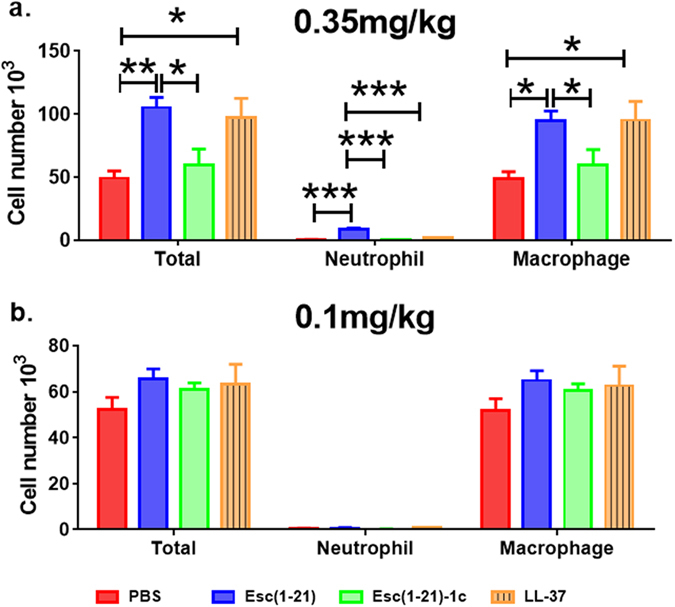
Effect of Esc(1–21), Esc(1–21)-1c and LL37 on the number of lung inflammatory cells in mice. The number of macrophages, neutrophils and total inflammatory cells in the BAL of mice was counted at 24 hours after peptide administration at 7 μg/mouse (0.35 mg/kg) (panel a) or 2 μg/mouse (0.1 mg/kg) (panel b). Results are mean ± SEM from three independent experiments; n = 4–6 mice for each group in each experiment. Following one-way analysis of variance (ANOVA), posthoc comparisons were made using the Dunnett’s multiple comparison test when the P-value was significant (p < 0.05). *p < 0.05, **p < 0.01, for peptide-treated mice versus PBS-receiving animals.
Figure 3.
Comparison among Esc(1–21), Esc(1–21)-1c and LL-37 on the in vivo gene expression in the lungs of mice. Effects of peptides on the expression of different inflammatory genes (panel a) or mucociliary-associated genes (panel b) in the lungs of mice after 24 hours from peptide administration at 0.1 mg/kg. AMPs treatments at 0.1 mg/kg do not noticeably affect the expression of inflammation and mucociliary clearance-related genes. Results are mean ± SEM from two independent experiments; n = 4–6 mice for each group in each experiment.
Furthermore, no detectable pulmonary toxicity was recorded for all peptides tested. In support of this, airway epithelial cell-associated genes expression including Foxj1 (ciliated cells) and Muc5b (mucous cells), having important roles in the lung mucociliary clearance37, was minimally affected (Fig. 3b).
Antimicrobial efficacy at 6 hours after bacterial infection
Since no unwanted harmful action at the lung level was pointed out when all the selected AMPs were administered at 0.1 mg/kg, their in vivo efficacy in treating P. aeruginosa-induced respiratory infection was then examined using a mouse model of acute lung infection. The peptides were intra-tracheally administered at 2 hours after bacterial challenge and the number of colony forming units (CFUs) in lung, BAL, and spleen was determined at 6 hours after bacterial instillation. As indicated in Fig. 4a, the total lung burden as shown in number of CFU (lung homogenate + BAL) was significantly reduced (90% reduction) after AMP treatment, compared to the number found in control animals that were infected but received only the vehicle PBS (red bars). Overall, Esc(1–21)-1c revealed to be the peptide with the best in vivo antimicrobial efficacy, probably due to its longer biostability29. In addition, the significant lowering in the number of bacteria in the spleen of Esc(1–21)-1c-treated mice compared to that of control animals or those treated with Esc(1–21) or LL-37 (Fig. 4a) indicated a substantial reduction in the systemic dissemination of bacterial cells and spread of infection. In parallel, the total number of leukocytes in the infected mouse lungs after AMPs treatment was investigated. As reported in Fig. 4b, in line with the diminished bacterial burden provoked by all peptides, a lower number of immune cells, especially neutrophils, was counted in comparison with PBS-treated infected animals, 4 hours after peptide instillation (e.g. 6 hours from infection).
Figure 4.
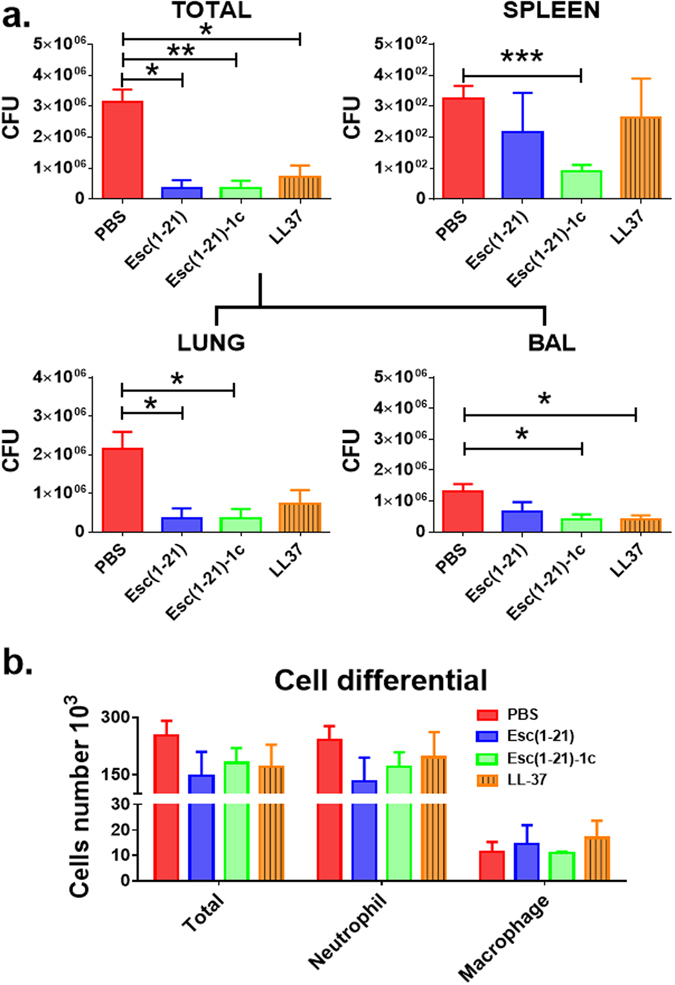
Comparison among Esc(1–21), Esc(1–21)-1c and LL-37 on the number of viable P. aeruginosa cells (CFU). CFU in mouse lungs (Total = lung homogenate + BAL) and spleens (panel a) as well as on the number of inflammatory cells in the BAL (panel b) of pulmonary-infected mice at 4 hours after peptide administration at 0.1 mg/kg were enumerated. Animals were intra-tracheally infected with 3 million PAO1 cells. The peptides were administered intra-tracheally, at 2 μg (0.1 mg/kg) at 2 hours after bacterial infection. The number of viable Pseudomonas cells in the lung and spleen as well as the number of inflammatory cells in the BAL were evaluated 6 hours after infection, as described in the Experimental section. Results are mean ± SEM from three independent experiments; n = 4–6 mice for each treatment group. Following one-way analysis of variance (ANOVA), posthoc comparisons were made using the Dunnett’s multiple comparison test when the P-value was significant (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001 for peptide-treated animals versus PBS-treated mice.
Antimicrobial efficacy at 24 hours after infection with a single or double AMP administration
Subsequently, the in vivo antipseudomonal activity of the diastereomer was further evaluated after a longer-term treatment (i.e. 24 hours from the bacterial administration). Excitingly, in this case a more noticeable antibacterial effectiveness than that from 4 hours AMP treatment was observed, with approximately 2-log reduction in the lung bacterial burden compared to the control PBS-treated infected mice (Fig. 5a). Interestingly, a second instillation of the diastereomer (0.1 mg/kg) at 12 hours after bacterial inoculation did not further enhance the therapeutic efficacy against P. aeruginosa-induced respiratory infection. Similarly, the second administration of this AMP did not further decrease the infection level of the spleen (Fig. 5a).
Figure 5.
Effect of single or double administration of Esc(1–21)-1c (panel a); LL-37 and colistin (panel b) on the number of viable P. aeruginosa. The effect of single or double dosage of Esc(1–21)-1c on total leukocytes was also investigated (panel c). Bacterial burden (CFU) in the lung and spleen of infected mice was determined at 24 hours after bacterial infection. Animals were intra-tracheally infected with PAO1 cells. The peptides were instilled intra-tracheally, at 2 μg (0.1 mg/kg) at 2 hours (for single administration) and at 2 hours and 12 hours (for double administration) after bacterial infection. The numbers of viable Pseudomonas cells in the lung and spleen as well as the number of inflammatory cells in the BAL were counted at 24 hours after the infection, as described above. Results are mean ± SEM from three independent experiments; n = 4–6 mice for each treatment group. Following one-way analysis of variance (ANOVA), posthoc comparisons were made using the Dunnett’s multiple comparison test when the P-value was significant (p < 0.05). *p < 0.05, **p < 0.01 for peptide-treated animals versus PBS-treated mice.
Importantly, the efficacy of Esc(1–21)-1c in reducing lung bacterial burden at 24 hours after Pseudomonas challenge revealed to be comparable (about 2-log reduction of lung burden) to that found for the last resort of antibiotics, i.e. colistin, in another set of experiments (Fig. 5b). In comparison, the human LL-37 almost completely lost its antimicrobial potency (Fig. 5b), presumably due to its higher susceptibility to proteolytic degradation29.
Effect of Esc(1–21)-1c on the number of inflammatory cells in the BAL at 24 hours after infection
In consistence with the described results of significantly lower bacterial burden in the lungs after treatment with Esc(1–21)-1c, a substantial decrease in the total number of inflammatory cells was also recorded in the BAL of infected mice after a longer-term treatment with a single or double dosage of the diastereomer (at 0.1 mg/kg) compared to the control infected animals receiving the vehicle PBS only (Fig. 5c).
The therapeutic efficacy of the diastereomer closely correlated with the diminished number of inflammatory cells in the BAL of the animals, even though the percentage of macrophages was slightly higher than that of controls. This very low percentage of macrophages within the total leukocytes presumably reflected the lower amount of bacterial burden and the minimally recruited neutrophils to the site of infection.
Pulmonary inflammation of infected lungs after treatment with Esc(1–21)-1c
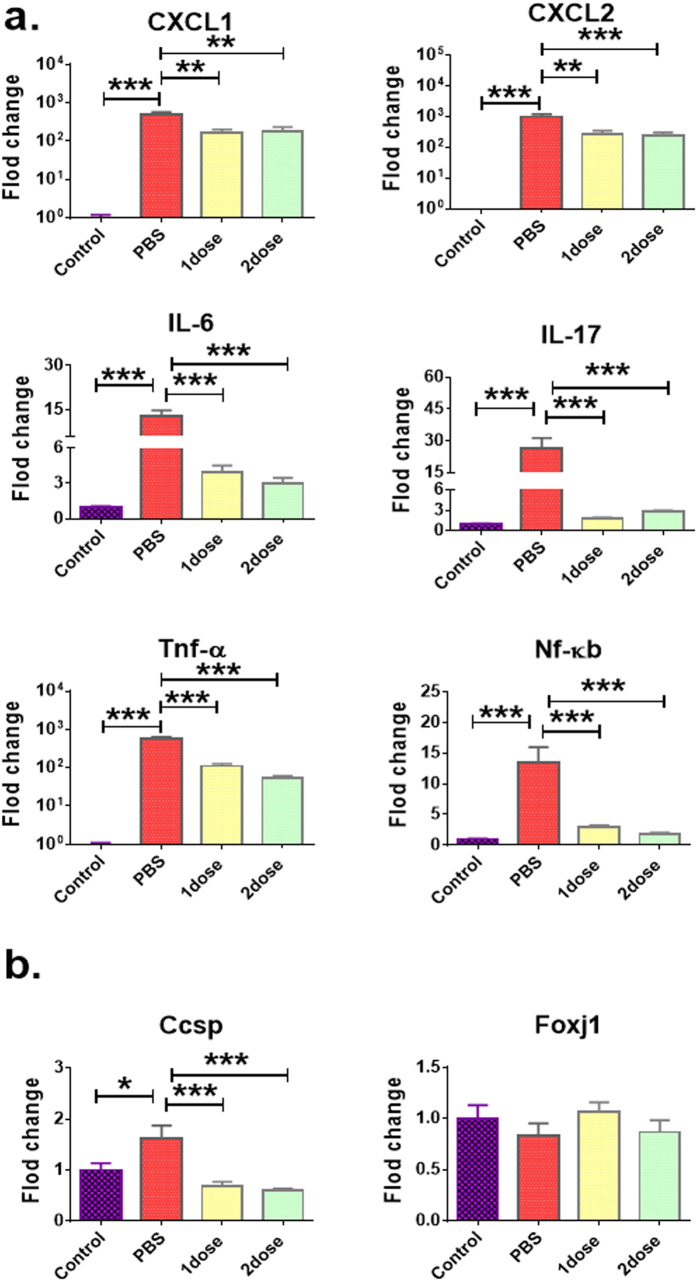
Compared to infected mice treated with PBS, a marked reduction in the expression level of pro-inflammatory chemokines (e.g. CXCL-1, CXCL-2), cytokines (i.e. IL-6 and IL-17), and other inflammatory-related genes (Tnf-α, Nf-κb) was detected in the lungs of mice at 24 hours after treatment with a single or a double dosage of Esc(1–21)-1c at 0.1 mg/kg (Fig. 6a). Indeed, the gene-expression level was similar to that of untreated non-infected mice (control, Fig. 6a). This suggests that therapeutic treatments with the diastereomer led to a substantially attenuated inflammatory response upon P. aeruginosa-induced respiratory infection. Furthermore, bacterial infection resulted in noticeable induction of airway epithelial gene Ccsp but only mild changes in Pseudomonas-induced lung infection, after administration of Esc(1–21)-1c (Fig. 6b). Ccsp gene encodes for Clara/Club cell secretory protein, and serves as a marker for assessing the cellular integrity and permeability of the lung epithelium that could be induced after epithelial injury38. Our results clearly supported that bacterial infection-induced lung injury was significantly less after one or two dosage of Esc(1–21)-1c administration. The gene expression difference in foxj1 among various treatments was not significant.
Figure 6.
Effect of Esc(1–21)-1c on the expression level of different inflammatory-related genes (panel a) and airway epithelial-associated genes (panel b). Gene expression in the lungs of P. aeruginosa infected mice after a single or a double peptide administration (at 0.1 mg/kg) was investigated at 24 hours after bacterial infection. Mice were intra-tracheally infected with 3 million PAO1 cells. The peptides were administered intra-tracheally at 2 μg (0.1 mg/kg) at 2 hours and 12 hours after the infection. After 24 hours from bacterial challenge, the RNA was extracted from the lung tissue and quantitative PCR was performed as described54. Results are mean ± SEM from three independent experiments; n = 4–6 mice for each treatment group. The t-test was used to compare the means of PBS-receiving non-infected mice (control) versus PBS-treated infected animals. One-way analysis of variance (ANOVA) was used peptide-treated infected mice and PBS-treated infected animals, posthoc comparisons were made using the Dunnett’s multiple comparison test when the P-value was significant (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001 for peptide-treated infected mice or PBS-receiving non-infected mice (control) versus PBS-treated infected animals.
The efficacy of Esc(1–21)-1c in alleviating respiratory Pseudomonal infection was further assessed by mouse lung histopathological evaluation. We observed the increased number of inflammatory cells after Pseudomonas infection in both airways and alveolar regions, but the increase was more prominent in the alveolar region (Fig. 7). There was clearly more neutrophilic infiltration in the alveoli of PBS-treated mice than in AMP-treated mice at 24 hours after infection (Fig. 7).
Figure 7.
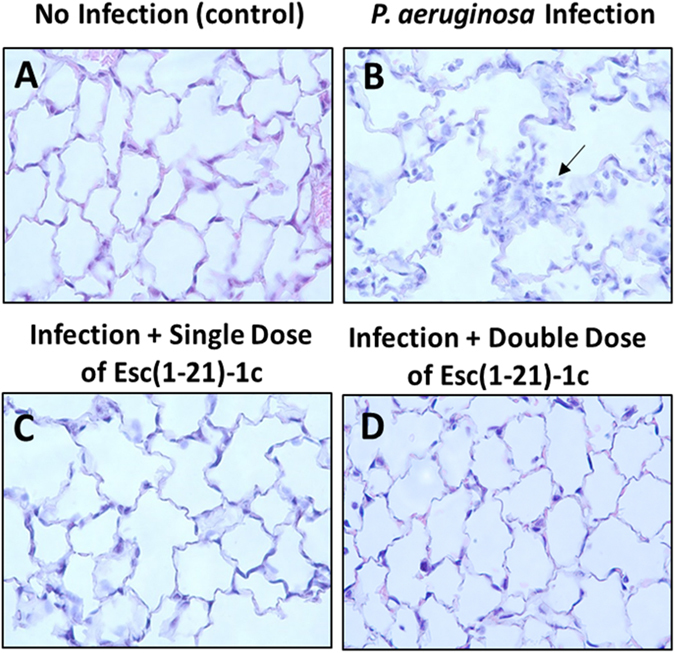
Histologic analysis of lung tissues with PAO1 challenge at 24 hours after bacterial infection. Mouse lung tissues were harvested, fixed and stained for histological evaluation without peptide treatment (B) or with a single (C) and double (D) administration of Esc(1–21)-1c at 0.1 mg/kg in comparison with non-infected and untreated samples. (A) Infected but untreated mice (B) exhibited more inflammation, airway lumen leukocyte accumulation (black arrow).
Discussion
Respiratory infection is the most common lung disease, especially in CF sufferers39, 40 and P. aeruginosa is the most predominant lung pathogen in the CF population where it drives to progressive loss of respiratory functions and shortened survival of these patients. This is mainly due to its increasing resistance to the available antibiotics and adaptation within the lung environment, favoring persistence and chronic lung colonization by P. aeruginosa 41, 42.
AMPs hold promise to act as lead compounds for the generation of novel therapeutic agents with fast and wide spectrum of activity, low capacity to induce resistance while displaying relevant immunomodulatory functions (e.g. LPS detoxification; wound-healing). However, a lot of AMPs loss their antimicrobial activity in biological environments and only a limited number of studies have been carried out to demonstrate the in vivo effect(s) of AMPs or derivatives in animal models of P. aeruginosa-induced lung infection.
We previously proved that by changing the configuration of only two amino acids in Esc(1–21), with the corresponding d-enantiomers, the resulting diastereomer Esc(1–21)-1c had a (i) significantly higher biostability, (ii) lower cytotoxicity, (iii) better antibiofilm activity, (iv) stronger ability in killing Pseudomonas internalized into bronchial cells expressing a copy of functional or mutated CF-transmembrane conductance regulator (AF508 CFTR) and (v) a more pronounced wound-healing activity in monolayers of epithelial cells29. All these findings contributed to make Esc(1–21)-1c a very attractive candidate for the development of new drugs against Pseudomonas-induced lung infections. Nevertheless, no evidences on its in vivo antimicrobial efficacy were previously reported. Here, for the first time we demonstrated a remarkable in vivo antipseudomonal activity of the diastereomer Esc(1–21)-1c after a single local instillation in the lungs, with a higher potency than the all-L parent peptide and the widely used and characterized human AMP LL-37. Our in vivo studies underlined that 4 hours peptide treatment is not an ideal time for an optimal evaluation of the in vivo antimicrobial effectiveness of esculentin-derived AMPs, which presumably relies on a direct bactericidal activity of the peptide. However, at this stage we cannot exclude that the respiratory epithelial cell-mediated immune modulation could also contribute to the overall host antimicrobial activity. The bacterial killing activity by Esc(1–21) and Esc(1–21)-1c would prevent inhaled/inoculated bacteria from propagate in mouse lung. Nonetheless, the bacterial infection-induced host innate immunity may be able to provide additional killing mechanism to control the severity of bacterial infection and to reduce inflammatory response. Importantly, we have found out that a single intra-tracheal instillation of Esc(1–21)-1c, at a very low peptide dosage i.e. 20 μM (0.1 mg/kg), is sufficient to cause approximately 2-log reduction in the lung Pseudomonas burden, within 24 hours from bacterial challenge. The data suggest that the diastereomer Esc(1–21)-1c likely has a sustained residence time in the lungs, due to its higher biostability and resistance to proteolytic degradation than Esc(1–21) and LL-37, according to our previously published data28, 29. Thus, it would continue to display microbicidal effects at longer-term. In comparison, the in vivo antipseudomonal activity of Esc(1–21)-1c resulted to be similar in bacterial reduction to that of colistin, which is extensively used in clinical practice43. Note however that colistin is a peptide which is active only against Gram-negative bacteria and it easily induces microbial resistance31, 44, 45, most likely because of modifications to the phosphate groups of lipid A and core oligosaccharide moieties of LPS, weakening colistin binding to it. Yet, colistin resistance in humans without prior exposure to this peptide has been recently disclosed; and this is an important concern to public health46.
Differently, the in vivo antibacterial efficacy of LL-37 was mostly lost at longer-term (Fig. 5b), probably due to its higher susceptibility to proteases29. This is in contrast with previous findings by Beagumont and colleagues showing that in a murine model of acute P. aeruginosa lung infection, LL-37 enhanced bacterial clearance in vivo only over 24 hours, by means of neutrophils recruitment, while no effect was obtained 6 hours after infection47. However, in their work, bacteria and peptide were co-administered via intranasal instillation rather than intra-tracheally. This latter administration route provides a more direct introduction of bacteria into the lungs and a more reproducible infection that can be more representative of pneumonia, generally leading to establishment of infection in the lower regions of the lungs48. In addition, intra-tracheal administration of a drug better mimics an aerosol-based therapy. Furthermore, immediate administration of LL-37 following bacterial inoculation as used in the paper47 did not allow sufficient time for the bacteria to adapt to the lung microenvironment before being exposed to LL-37. Note that this previously used experimental procedure could not provide a valuable indication of the antimicrobial effectiveness of AMPs in vivo. Thus, in this study, we purposely delayed the AMP administration at 2 hours after bacterial infection to better reflect the real life infectious conditions.
Finally in our hands, LL-37 negatively disturbed bronchial epithelial cell tight junction (Fig. 1). This could potentially result in elevated cytotoxicity to the lung epithelial cells and limit its practical use in exogenously supplying viable therapy for lung bacterial infection.
To the best of our knowledge, no indication on the effect of AMPs on the expression of airway epithelia-associated genes upon their administration to infected or non-infected lungs has been provided so far. This is a fundamental issue that deserves to be investigated in order to explore the clinical safety of a new drug. Indeed, besides experiencing the AMPs effect on the bacterial clearance, it is also meaningful to get insight into their potential beneficial/toxic effect on the lung epithelium, in vivo.
Our findings have emphasized that two amino acids substitution in Esc(1–21) with the corresponding d-enantiomers can reduce the peptide’s cytotoxicity and withstand the feasibility of using the diasteremer to alleviate pneumonia severity. Furthermore, we have also shown that in addition to having a therapeutic outcome, the diastereomer does not induce any undesirable side effect at the lung, but it is able to abate the inflammatory response upon bacterial infection, as corroborated by the results of immune cell differential counts, gene expression and lung histology analysis.
In summary, the results of our work suggest a strong therapeutic potential of diastereomer Esc(1–21)-1c in treating bacterial infection and concur to support further advanced preclinical studies aimed at developing it as a viable lead compound for the manufacture of new peptide-based formulation(s) for topical treatment of Pseudomonas lung infection.
Methods
Materials and chemicals
Synthetic Esc(1–21) and its diastereomer Esc(1–21)-1c (Table 1) were purchased from Chematek Spa (Milan, Italy). Briefly, each peptide was assembled by step-wise solid-phase synthesis using a standard F-moc strategy and purified via RP-HPLC to a purity of 98%, while the molecular mass was verified by mass spectrometry. Colistin sulphate was purchased from Sigma (St. Louis, MO) and the natural AMP human cathelicidin LL-37 (Table 1) was synthesized by Genscript (Piscataway, NJ). BronchialLife Epithelial Airway Medium was purchased from Lifeline Technology (Frederick, MD). B-ALI Bronchial Air Liquid Interface BulletKit was purchased from Lonza (Walkersville, MD). Eagle’s minimum essential medium (EMEM), was purchased from Sigma (St. Louis, MO), and tryptic soy broth (TSB) and trypitc soy agar (TSA) were purchased from MP Biomedicals (Santa Ana, CA).
Table 1.
Primary structure of the peptides under study.
| Peptide | Primary structurea |
|---|---|
| Esc(1–21) | GIFSKLAGKKIKNLLISGLKG-NH2 |
| Esc(1–21)-1c | GIFSKLAGKKIKNLLISGLKG-NH2 |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
a d-amino acids are in italics and bolded.
Bacteria
The Pseudomonas aeruginosa strain (PAO1, ATCC BAA-47) was used for all the experiments. For each experiment, an aliquot of bacteria was grown overnight at 37 °C in TSB with shaking at 225 rpm to achieve a stationary phase suspension. Afterwards, an aliquot was diluted 1:5 into fresh TSB and incubated at 37 °C for additional 2 h at 37 °C to reach an exponential growth phase. Bacterial cells were harvested by centrifugation at 1,500 × g for 10 min, washed twice and resuspended in PBS to adjust the concentration at 6 × 107 CFU/ml, for use in the experiments.
Primary bronchial epithelial cells
Fully differentiated primary human bronchial epithelial (HBE) cell cultures were derived from lungs removed at the time of lung transplantation from the Center for Organ Recovery and Education. Cells were prepared using previously described methods approved by the University of Pittsburgh IRB49, 50. Briefly, bronchi from the 2nd to 6th generations were collected, rinsed, and incubated overnight in EMEM at 4 °C. The bronchi were then digested in MEM containing protease XIV and DNase (0.2%). The epithelial cells were removed and collected by centrifugation and then resuspended in BronchiaLife medium and plated onto collagen-treated tissue culture flasks. When 80–90% confluence was reached, the passage 0 cells were trypsinized and seeded onto collagen-coated transwell permeable supports (Corning #3450, 105 cells/well). B-ALI Bronchial Air Liquid Interface Medium was replaced three times a week on both apical and basolateal sides of the permeable supports up to 8–10 days. Subsequently the apical medium was removed and the cultures were maintained at air liquid interface (ALI) to promote a further polarization and differentiation of the epithelium. The basolateral medium was changed twice weekly.
Animals
Wild-type C57BL/6J female mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in a specific pathogen-free status in a 12 hours light/dark cycle. All procedures were conducted using mice 6–8 weeks of age maintained in ventilated microisolator cages housed in an American Association for Accreditation of Laboratory Animal Care (AAALAC) approved animal housing facility. Protocols and studies involving animals were conducted in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh.
Lung epithelium permeability
Lung epithelial integrity in polarized primary bronchial cells was examined by measuring the TEER using an electronic resistance system (Millicell ERS-2, EMD Millipore). Ex vivo-differentiated lung epithelium was grown on ALI in 24-transwell plates (Corning, Tewksbury, MA) using BronchiaLife Epithelial Airway Medium in the basolateral compartment. Peptides dissolved in PBS at different concentrations were added onto the apical surface of the differentiated epithelial cells in a final volume of 100 μl. A sterile electrode was applied onto the apical side of the transwell insert containing the cultured epithelial cells with/without peptide treatments and the electrical resistance was measured three times for comparison at different time intervals.
Bronchoalveolar lavage and differential cell counts
Mice were anesthetized with inhalation of isoflurane and the peptide was intra-tracheally administered in 50 µl of PBS at 20 μM and 64 μM corresponding to a peptide dosage of 0.1 mg/kg and 0.35 mg/kg, respectively. Control mice were instilled with 50 µl of PBS without peptide. After 24 hours of treatment, mice were anesthetized with 2.5% tribromoethanol (Avertin). The trachea was cannulated, the lungs were lavaged twice using 1 ml saline, and the BAL samples collected as previously described51. The number of live immune cells in the BAL was determined by a Vision Cell Analyzer automatic cell counter (Nexcelom, Lawrence, MA). An additional aliquot was placed onto glass microscope slides (Shanon Cytospin; Thermo Fisher, Pittsburgh, PA), stained with Diff-Quick (Thermo Fisher Scientific, Waltham, MA); cell differential was determined microscopically. A total of 400 cells of every slide were counted at least twice independently for inflammatory cell differential counts. In other sets of experiments, cell differential counts were evaluated in the BAL of infected mice treated with or without peptides at 6 hours or 24 hours after peptide administration, as indicated.
Murine infection model
Mice were anesthetized with inhalation of isoflurane and instilled with PAO1 bacteria (sensitive to all peptides), through intra-tracheal inoculation of ~3 × 106 CFU per mouse in 50 µl PBS. Two hours after the bacterial infection, the peptide was intra-tracheally administered at desired dosage in 50 µl of PBS. Control mice were instilled with 50 µl of PBS without peptide. For repeated dosage experiments, the peptide was intra-tracheally instilled at 2 hours and 12 hours after bacterial infection.
Determination of bacterial burden
After 6 hours or 24 hours of bacterial instillation, the numbers of CFU in lungs, BAL and spleen were determined by serial dilution on TSB agar plates. Mice (5–6 mice/group) were anesthetized with 2.5% tribromoethanol. The trachea was cannulated, the lungs were lavaged twice using 1 ml saline, and the BAL samples collected. The left lung lobe was homogenized in 1 ml saline and placed on ice. Dilution of 100 μl of lung tissue homogenate or BAL was mixed with 900 μl PBS. Four serial 10-fold dilutions in saline were prepared and plated on TSB agar plates and incubated overnight at 37 °C, each dilution plated in triplicate, for counting.
Gene expression analysis by quantitative real-time PCR
The peptides’ effect on the expression of different inflammatory genes or airway epithelial cells-related genes after peptide administration at 0.1 mg/kg to non-infected or infected mice was determined, as indicated. Briefly, total mRNA was isolated from the right lung tissues using Trizol reagent. Quantitative PCR was performed using ABI 7900HT (Applied Biosystems, Foster City, CA) and primers of all genes tested including IL-6, IL-10, Tnf-α, Nf-κb, Muc5b, Foxj1. Validation tests were performed to confirm equivalent PCR efficiencies for the target genes. Test and calibrator lung RNAs were reverse transcribed using a high-capacity cDNA reverse transcription kit (Life Technologies) and PCR was amplified as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles; 95 °C for 15 s; 60 °C for 1 min. Three replicates were used to calculate the average cycle threshold for the transcript of interest and for a transcript for normalization (β-glucuronidase; Assays on Demand; Applied Biosystems). Relative mRNA abundance was calculated using the ΔΔ cycle threshold (Ct) method.
Lung histopathology
Lung tissues were harvested from mice at 24 hours after infection and compared with those without infection or after infection and peptide treatment (single or double administration at 0.1 mg/kg, as described above). Afterwards, they were fixed in situ with 4% paraformaldehyde for 10 minutes with the chest cavity open. The right lobe was embedded in paraffin and 5 μm sections were prepared. Sections were stained with hematoxylin and eosin, and histological evaluation was performed to examine bacterial infection-induced pathological severity. The stained lung sections were evaluated in a double-blind fashion under a light microscope, as described previously52, 53.
Data analysis
Data are expressed as mean ± SEM. Statistical comparisons between groups of mice were made using ANOVA, followed by Dunnett’s multiple comparison test or Tukey–Kramer test (one way ANOVA). A p value < 0.05 was considered to be statistically significant.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
This research was supported by the Italian Cystic Fibrosis Research Foundation (Project FFC#11/2014 adopted by FFC Delegations from Siena, Sondrio Valchiavenna, Cerea Il Sorriso di Jenny, and Pavia), grants from Sapienza University of Rome; NIH awards R01 HL-125128 and AI-133361, and a grant (CIA-123062) from Flight Attendant Medical Research Institute. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
C.C. performed the experiments and organized the data, M.L.M. and Y.P.D. designed and conceived the experiments, analyzed the data, and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Luisa Mangoni, Email: marialuisa.mangoni@uniroma1.it.
Y. Peter Di, Email: peterdi@pitt.edu.
References
- 1.Neil, J.O. Tackling drug-resistant infections globally: final report and recommendations https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (2016).
- 2.Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros. 2015;14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Parker D, Ahn D, Cohen T, Prince A. Innate Immune Signaling Activated by MDR Bacteria in the Airway. Physiol Rev. 2016;96:19–53. doi: 10.1152/physrev.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorde R, Pahissa A, Rello J. Management of refractory Pseudomonas aeruginosa infection in cystic fibrosis. Infect Drug Resist. 2011;4:31–41. doi: 10.2147/IDR.S16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciofu O, Tolker-Nielsen T, Jensen PO, Wang H, Hoiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnsholt T, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 8.Kosikowska P, Lesner A. Antimicrobial peptides (AMPs) as drug candidates: a patent review (2003–2015) Expert Opin Ther Pat. 2016;26:689–702. doi: 10.1080/13543776.2016.1176149. [DOI] [PubMed] [Google Scholar]
- 9.Mercer DK, O’Neil DA. Peptides as the next generation of anti-infectives. Future Med Chem. 2013;5:315–337. doi: 10.4155/fmc.12.213. [DOI] [PubMed] [Google Scholar]
- 10.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Findlay F, Proudfoot L, Stevens C, Barlow PG. Cationic host defense peptides; novel antimicrobial therapeutics against Category A pathogens and emerging infections. Pathog Glob Health. 2016;110:137–147. doi: 10.1080/20477724.2016.1195036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, et al. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob Agents Chemother. 2005;49:2921–2927. doi: 10.1128/AAC.49.7.2921-2927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangoni ML. Host-defense peptides: from biology to therapeutic strategies. Cell Mol Life Sci. 2011;68:2157–2159. doi: 10.1007/s00018-011-0709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today. 2013;18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 18.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 21.Mizgerd JP, Kobzik L, Warner AE, Brain JD. Effects of sodium concentration on human neutrophil bactericidal functions. Am J Physiol. 1995;269:L388–393. doi: 10.1152/ajplung.1995.269.3.L388. [DOI] [PubMed] [Google Scholar]
- 22.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman MJ, et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/S0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 24.Mangoni ML, Fiocco D, Mignogna G, Barra D, Simmaco M. Functional characterisation of the 1-18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides. 2003;24:1771–1777. doi: 10.1016/j.peptides.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Islas-Rodriguez AE, et al. Esculentin 1–21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J Pept Sci. 2009;15:607–614. doi: 10.1002/psc.1148. [DOI] [PubMed] [Google Scholar]
- 26.Luca V, Stringaro A, Colone M, Pini A, Mangoni ML. Esculentin(1–21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol Life Sci. 2013;70:2773–2786. doi: 10.1007/s00018-013-1291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangoni ML, Luca V, McDermott AM. Fighting microbial infections: A lesson from amphibian skin-derived esculentin-1 peptides. Peptides. 2015;71:286–295. doi: 10.1016/j.peptides.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Di Grazia A, et al. D-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1-21)NH2 is beneficial for its multiple functions. Amino Acids. 2015;47:2505–2519. doi: 10.1007/s00726-015-2041-y. [DOI] [PubMed] [Google Scholar]
- 29.Cappiello F, et al. Esculentin-1a-Derived peptides promote clearance of Pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: biochemical properties and a plausible mode of action. Antimicrob Agents Chemother. 2016;60:7252–7262. doi: 10.1128/AAC.00904-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Grazia A, et al. The frog skin-derived antimicrobial peptide esculentin-1a(1–21)NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PLoS One. 2015;10:e0128663. doi: 10.1371/journal.pone.0128663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunetti J, et al. In vitro and in vivo efficacy, toxicity, bio-distribution and resistance selection of a novel antibacterial drug candidate. Sci Rep. 2016;6:26077. doi: 10.1038/srep26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meunier D, et al. Carbapenem resistance mediated by blaOXA-181 in Pseudomonas aeruginosa. J Antimicrob Chemother. 2016;71:2056–2057. doi: 10.1093/jac/dkw087. [DOI] [PubMed] [Google Scholar]
- 33.Mardirossian M, et al. In vitro and in vivo evaluation of BMAP-derived peptides for the treatment of cystic fibrosis-related pulmonary infections. Amino Acids. 2016;48:2253–2260. doi: 10.1007/s00726-016-2266-4. [DOI] [PubMed] [Google Scholar]
- 34.Cigana C, et al. Efficacy of the novel antibiotic POL7001 in preclinical models of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 2016;60:4991–5000. doi: 10.1128/AAC.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 36.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013;1:e24997. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 39.Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 40.Talbot GH, et al. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability Task Force of the infectious diseases society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 41.Staudinger BJ, et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2014;189:812–824. doi: 10.1164/rccm.201312-2142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43:1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain A, et al. NDM carbapenemases in the United Kingdom: an analysis of the first 250 cases. J Antimicrob Chemother. 2014;69:1777–1784. doi: 10.1093/jac/dku084. [DOI] [PubMed] [Google Scholar]
- 44.Merlo CA, et al. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest. 2007;132:562–568. doi: 10.1378/chest.06-2888. [DOI] [PubMed] [Google Scholar]
- 45.Miller AK, et al. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother. 2011;55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olaitan AO, Morand S, Rolain JM. Emergence of colistin-resistant bacteria in humans without colistin usage: a new worry and cause for vigilance. Int J Antimicrob Agents. 2016;47:1–3. doi: 10.1016/j.ijantimicag.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Beaumont PE, et al. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One. 2014;9:e99029. doi: 10.1371/journal.pone.0099029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheah SE, et al. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother. 2015;70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, et al. SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection. Am J Pathol. 2013;182:1519–1531. doi: 10.1016/j.ajpath.2013.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, et al. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol. 2013;191:4259–4268. doi: 10.4049/jimmunol.1202340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di YP. Assessment of pathological and physiological changes in mouse lung through bronchoalveolar lavage. Methods Mol Biol. 2014;1105:33–42. doi: 10.1007/978-1-62703-739-6_3. [DOI] [PubMed] [Google Scholar]
- 52.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 53.Di YP, et al. Dual acute proinflammatory and antifibrotic pulmonary effects of short palate, lung, and nasal epithelium clone-1 after exposure to carbon nanotubes. Am J Respir Cell Mol Biol. 2013;49:759–767. doi: 10.1165/rcmb.2012-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukinskiene L, et al. Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J Immunol. 2011;187:382–390. doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.