Abstract
Importance
Lithium is a first-line mood stabilizer for the treatment of bipolar affective disorder (BPAD). However, the efficacy of lithium varies widely, with a nonresponse rate of up to 30%. Biological response markers are lacking. Genetic factors are thought to mediate treatment response to lithium, and there is a previously reported genetic overlap between BPAD and schizophrenia (SCZ).
Objectives
To test whether a polygenic score for SCZ is associated with treatment response to lithium in BPAD and to explore the potential molecular underpinnings of this association.
Design, Setting, and Participants
A total of 2586 patients with BPAD who had undergone lithium treatment were genotyped and assessed for long-term response to treatment between 2008 and 2013. Weighted SCZ polygenic scores were computed at different P value thresholds using summary statistics from an international multicenter genome-wide association study (GWAS) of 36 989 individuals with SCZ and genotype data from patients with BPAD from the Consortium on Lithium Genetics. For functional exploration, a cross-trait meta-GWAS and pathway analysis was performed, combining GWAS summary statistics on SCZ and response to treatment with lithium. Data analysis was performed from September 2016 to February 2017.
Main Outcomes and Measures
Treatment response to lithium was defined on both the categorical and continuous scales using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with Bipolar Disorder score. The effect measures include odds ratios and the proportion of variance explained.
Results
Of the 2586 patients in the study (mean [SD] age, 47.2 [13.9] years), 1478 were women and 1108 were men. The polygenic score for SCZ was inversely associated with lithium treatment response in the categorical outcome, at a threshold P < 5 × 10−2. Patients with BPAD who had a low polygenic load for SCZ responded better to lithium, with odds ratios for lithium response ranging from 3.46 (95% CI, 1.42-8.41) at the first decile to 2.03 (95% CI, 0.86-4.81) at the ninth decile, compared with the patients in the 10th decile of SCZ risk. In the cross-trait meta-GWAS, 15 genetic loci that may have overlapping effects on lithium treatment response and susceptibility to SCZ were identified. Functional pathway and network analysis of these loci point to the HLA antigen complex and inflammatory cytokines.
Conclusions and Relevance
This study provides evidence for a negative association between high genetic loading for SCZ and poor response to lithium in patients with BPAD. These results suggest the potential for translational research aimed at personalized prescribing of lithium.
This genome-wide association study tests whether a polygenic score for schizophrenia is associated with treatment response to lithium in bipolar affective disorder and explores the potential molecular underpinnings of this association.
Key Points
Questions
Is a polygenic score for schizophrenia associated with response to lithium in patients with bipolar affective disorder, and, if so, what are the molecular drivers of this association?
Findings
This genome-wide association study found an inverse association between genetic loading for schizophrenia risk variants and response to lithium in patients with bipolar affective disorder. Genetic variants in the HLA antigen region and the antigen presentation pathway point to the molecular underpinnings of schizophrenia and lithium treatment response.
Meaning
For patients with bipolar affective disorder, assessment of a polygenic load for schizophrenia risk variants, in conjunction with clinical data, may assist in determining whether they would respond to lithium treatment.
Introduction
Bipolar affective disorder (BPAD) is a severe and often disabling psychiatric condition characterized by recurrent dysregulation of mood, with episodes of mania and depression. With an early onset and an estimated lifetime prevalence of 1% to 4.4%, BPAD is associated with high levels of personal impairment and high societal costs, accounting for 9.9 million years of life lived with disability worldwide, increased all-cause mortality, and risk of suicide. The possible causes of BPAD are complex, and both genetic and environmental factors contribute to its pathogenesis. The estimated heritability of BPAD ranges from 60% to 85%, and candidate gene and genome-wide association studies (GWASs) have successfully identified genetic loci implicated in the illness.
Lithium’s mood-stabilizing properties were discovered in 1949. It has retained a status as the criterion standard mood stabilizer, possessing unique protective effects against both manic and depressive episodes, as well as for suicide prevention. Consequently, lithium is recommended as first-line maintenance treatment for BPAD by several clinical practice guidelines. However, there is significant interindividual variation between those who do and those who do not respond to treatment with lithium. About 30% of patients are only partially responsive, and more than one-fourth show no clinical response. Although clinical studies report a combination of demographic and clinical characteristics as potential factors determining response to lithium treatment, genetic factors also appear to be highly involved. So far, 3 GWASs have successfully identified single-nucleotide polymorphisms (SNPs) associated with treatment response to lithium in BPAD pointing to different genetic loci.
To improve our understanding of the molecular mechanisms underlying the therapeutic effects of lithium, alternative genomic approaches that can complement GWASs deserve consideration. One such approach is polygenic analysis, which quantifies the combined effects of genetic variants across the whole genome on a given clinical outcome, computed as a weighted summation of effect sizes of multiple independent polymorphisms. An accurate and successful polygenic model may assist early screening for disease risk, clinical diagnosis, and the determination of treatment response and prognosis. In the present study, we aimed to investigate whether patients with BPAD who had a high genetic susceptibility for schizophrenia (SCZ), expressed by their SCZ polygenic score (PGS), would respond better or more poorly to lithium compared with patients with BPAD who had a low PGS for SCZ. In addition, we set out to explore the genetic and molecular underpinnings of any identified association between SCZ and treatment response to lithium.
Several previous observations motivated this approach. First, there is increasing evidence for a substantial genetic overlap between BPAD and SCZ. The Psychiatric Genomics Consortium (PGC; http://www.med.unc.edu/pgc/) estimated a shared genetic variation between BPAD and SCZ of approximately 68%, which is the highest among all pairs of psychiatric diagnoses, and several shared risk genes and shared biological pathways associated with both disorders have been identified. Second, despite these genetic and molecular commonalities, lithium is not an effective medication for people with SCZ, and increased SCZ trait loading in those with BPAD might be expected to be associated with poor treatment response to lithium. An earlier family study found an association between family history of SCZ and poor response to lithium. Third, during acute episodes of illness, BPAD and SCZ are often difficult to distinguish clinically because of overlapping psychotic symptoms such as hallucinations, delusions, and disorganization, as well as some common behavioral disturbances such as irritability or anger. Aiming to determine response to lithium, which could potentially confer advantages for patients and their treating physicians, we sought to evaluate the aggregated outcome of genome-wide SNPs for SCZ on treatment response to lithium in patients with BPAD using a PGS approach that was based on the results of the largest SCZ GWAS to date. Furthermore, to explore potential genetic and molecular drivers of any detected association, we carried out a cross-trait GWAS meta-analysis, combining the summary statistics from the largest available GWAS for both SCZ and response to lithium.
Methods
In the present study, conducted from 2008 to 2013, we first tested whether a PGS for SCZ is associated with treatment response to lithium in patients with BPAD; 2043 patients (79.0%) had BPAD type I and 543 (21.0%) had BPAD type II. In a second step, we applied a cross-trait GWAS meta-analysis approach to identify individual genetic variants shared between SCZ and treatment response to lithium. In a third step, we characterized the genetic variants identified in the second step and explored the shared biological pathways underlying genetic susceptibility to SCZ and treatment response in BPAD. We built the PGS using the discovery GWAS outcome estimates (logs of odds ratio [OR]) of 36 989 patients with SCZ and the targeted genetic data of 2586 patients from the International Consortium on Lithium Genetics (ConLi+Gen). The cross-trait meta-analysis and pathway analysis were based on GWAS summary statistics from GWASs of SCZ and treatment response to lithium from ConLi+Gen. Overlapping SNPs that met genome-wide significance in the meta-GWAS were subsequently analyzed for biological context using the Ingenuity Pathway Analysis platform (IPA; QIAGEN [http://www.ingenuity.com]). This study used consortium data through an international collaboration. The University of Heidelberg Ethics Committee provided central ethics approval for the consortium. Written consent was obtained from each patient according to the study protocols of the participating cohorts.
Target Outcome
Lithium treatment outcome was assessed using the Retrospective Criteria of Long-term Treatment Response in Research Subjects With Bipolar Disorder scale, also known as the ALDA scale. The ALDA scale quantifies symptom improvement over the course of treatment (A score; range, 0-10), which is then weighted against 5 criteria (B score) that assess confounding factors, each scored 0, 1, or 2. The total score is calculated by subtracting the total B score from the A score, with negative scores set to zero. We employed a categorical and a continuous outcome for response to lithium. The categorical (ie, good vs poor) response to lithium was defined based on the total score as a cutoff score of 7, in which patients with a total score of 7 or higher were categorized as responders. The ALDA score on subscale A was used as a continuous outcome after excluding individuals with a total B score greater than 4 or who had missing data on the totals of ALDA subscale A or B.
Polygenic Scoring
Quality-controlled SNPs were clumped for linkage disequilibrium based on GWAS association P value–informed clumping using r2 = 0.1 within a 250-kilobase (kb) window to create an SNP set in linkage equilibrium using PLINK software run on Linux (plink–clump-p1 1–clump-p2 1–clump-r2 0.1–clump-kb 250). Then, the SNPs up to 10 P value thresholds (<1 × 10−4, <1 × 10−3, <.01, <.05, <.1, <.20, <.30, <.40, <.50, and <1.0) were selected to compute the SCZ PGSs in the ConLi+Gen sample. A genome-wide weighted SCZ PGS for each participant was calculated at each P value threshold as the sum of independent SNPs genotype dosage (from 0 to 2) of the reference allele in the ConLi+Gen genotype data, multiplied by effect sizes on the SCZ GWAS for the reference allele, estimated as log (OR) divided by the total number of SNPs in each threshold.
Statistical Analyses
Statistical analysis was performed from September 2016 to February 2017. We applied PGS association analyses, cross-trait meta-GWAS, and IPA of the cross-trait findings.
PGS Association Analysis
Once the PGSs were constructed, the association of the PGSs at each threshold P value with treatment response to lithium was evaluated using regression models. While a binary logistic regression was implemented for the categorical outcome (response vs nonresponse), a linear regression was applied to treatment response to lithium on the continuous scale. Using the PGS at the most significant threshold (P < 5 × 10−2), we divided the study samples into 10 deciles, ranging from the lowest polygenic load (first decile) to the highest polygenic load (10th decile). We then compared patients with BPAD with a lower polygenic load (first to ninth deciles) for SCZ with patients with the highest polygenic load (10th decile) to quantify the association of SCZ polygenic load with lithium treatment outcomes.
To control for confounding factors, the PGS association analyses were adjusted for the covariates of age, sex, genotyping platforms, and 7 principal components. The analyses were performed using R (R Foundation for Statistical Computing) and PLINK, version 1.9, for Linux. The accuracy of determining factors and the percentage of variance in lithium response accounted for by the PGS at each P value threshold were estimated as the variance explained by the full model including each PGS and covariates minus the variance explained by the model including only covariates. Statistical significance was determined at P < .05 after adjusting for covariates.
Cross-trait Meta-analysis of GWASs
Biologically, a significantly associated PGS implies that genetic factors influencing the 2 traits are overlapping. Thus, further analyses were performed to identify genetic polymorphisms that are likely to increase the susceptibility to SCZ and also influence treatment response to lithium in patients with BPAD. We performed cross-trait meta-analyses by combining the summary statistics for GWAS on lithium response from the ConLi+Gen and GWAS on SCZ from the PGC. We applied both the O’Brien method and the direct linear combination of dependent test statistics approach, which are implemented in the C2+ eLX package (https://sites.google.com/site/multivariateyihsianghsu/). In brief, the O’Brien method and the direct linear combination of dependent test statistics approach combine univariate meta-GWAS summary statistics (β coefficients or z scores) at each SNP. Further details are available elsewhere.
Ingenuity Pathway Analysis
To characterize the potential biological significance of the SNPs discovered from the cross-trait meta-analyses, we performed analyses using IPA (eAppendix in the Supplement).
Results
Sample Characteristics
A total of 3193 patients with BPAD who had undergone lithium treatment and had available genotype and clinical data participated in the study. After quality control, 2586 patients remained for analysis, of whom 2366 were of European ancestry and the rest Asian. The mean (SD) age of all the patients combined was 47.2 (13.9) years and 1478 (57.2%) were female. A total of 704 patients (27.2%) had a good response to lithium treatment (ALDA scale score ≥7). The mean (SD) ALDA scale score for all participants was 4.1 (3.2) (Table 1).
Table 1. Characteristics of Patients With BPAD and Outcomes With Lithium Treatment .
| Characteristic | Categorical Outcomea (Good vs Poor Response) (n = 2586) |
Continuous Scaleb (ALDA Score on Subscale A) (n = 2244) |
|---|---|---|
| Responders, No. (%) | 704 (27.2) | NA |
| Age at interview, mean (SD), y | 47.2 (13.9) | 47.4 (13.9) |
| Female, No. (%) | 1478 (57.2) | 1291 (57.5) |
| ALDA scale A score, mean (SD) | 6.2 (3.0) | 6.3 (3.0) |
| ALDA scale total B, mean (SD) | 2.5 (1.7) | 2.1 (1.2) |
| ALDA scale total, mean (SD) | 4.1 (3.2) | 4.5 (3.1) |
Abbreviations: ALDA, Retrospective Criteria of Long-term Treatment Response in Research Subjects With Bipolar Disorder; BPAD, bipolar affective disorder; NA, not applicable.
Total ALDA scale score of 7 or higher was defined as good response.
Participants with total B score higher than 4 or who had missing data on the total scores on ALDA subscale A or B were excluded.
Association of SCZ PGS With Treatment Response to Lithium in Patients With BPAD
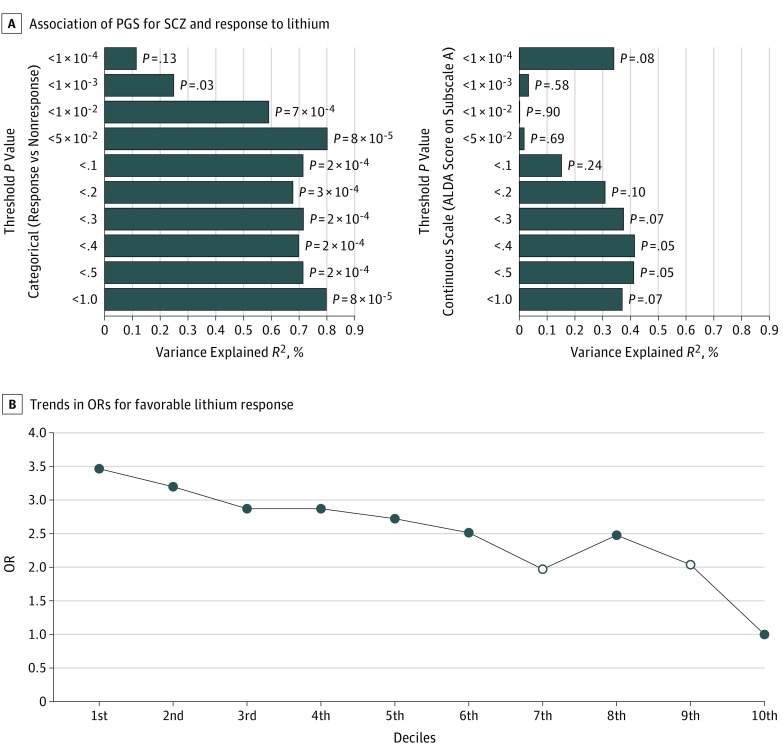
At the most significantly associated P value threshold (P < 5 × 10−2), the PGS for SCZ was strongly associated with lithium treatment response in BPAD for the categorical outcome on the ALDA scale (Figure, A), explaining 0.8% of the variance. For the continuous outcome (total score on the ALDA subscale A), the direction of association was congruent with the finding on the categorical outcome but was not statistically significant. As shown in eFigure 1 in the Supplement, the relationship between the PGS for SCZ and the total score on the ALDA subscale A deviates from linearity; thus, the continuous scale might be a less powerful and less suitable measure to represent treatment response to lithium in a linear model. The association results of the categorical and continuous outcomes at each threshold level are detailed in the Figure, A. At each threshold, a lower polygenic load for SCZ was associated with a favorable treatment response to lithium in patients with BPAD (Figure, B).
Figure. Polygenic Score (PGS) for Schizophrenia (SCZ) and Treatment Response to Lithium.
A, The association of PGS for SCZ and lithium treatment response defined as a categorical and continuous scale, at different SCZ genome-wide association study (GWAS) P value thresholds. The x-axis refers to the percentage of variance in treatment response to lithium accounted for by the PGSs of SCZ at a particular P value threshold. On the y-axis, plotted from top to bottom, are the GWAS P value thresholds used to group single-nucleotide polymorphisms for PGSs. On the right of each bar are the P values of the association between the PGS for SCZ and lithium treatment response. B, Trends in the odds ratios (ORs) for favorable treatment response to lithium for patients with BPAD in the low SCZ deciles (first to ninth) compared with patients in the highest SCZ PGS decile (10th), estimated at the most significant P value thresholds (P < 5 x 10−2) (n = 2586). The open circles on the line plot indicate that the association is not statistically significant at that particular decile. ALDA indicates Retrospective Criteria of Long-term Treatment Response in Research Subjects With Bipolar Disorder.
Table 2 shows the ORs for the association between treatment response to lithium in BPAD and SCZ PGS in deciles, comparing the response status of patients in the low polygenic load categories (first to ninth deciles) with the response status of patients in the highest polygenic load category for SCZ (10th decile). Patients with BPAD who carry a lower polygenic load for SCZ have higher odds of favorable treatment response to lithium compared with patients carrying a high polygenic load; the OR of favorable treatment response decreased as the genetic load for SCZ increased, ranging from an OR of 3.46 (95% CI, 1.42-8.41) at the first decile to an OR of 2.03 (95% CI, 0.86-4.81) at the ninth decile, compared with the reference SCZ PGS at the 10th decile (Table 2). There was a significant linear trend in the odds of treatment response to lithium across the deciles (Figure, B).
Table 2. Odds Ratios (ORs) of Favorable Treatment Response to Lithium in Patients With BPAD .
| SCZ PGS by Decile | Patients With BPAD (n = 2586) | ||
|---|---|---|---|
| R/N, No. | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| First (lowest score) | 83/175 | 1.97 (1.32-2.96) | 3.46 (1.42-8.41) |
| Second | 80/179 | 1.86 (1.24-2.79) | 3.19 (1.32-7.74) |
| Third | 78/180 | 1.80 (1.20-2.71) | 2.87 (1.18-6.95) |
| Fourth | 76/184 | 1.72 (1.14-2.59) | 2.86 (1.18-6.91) |
| Fifth | 76/180 | 1.76 (1.17-2.64) | 2.71 (1.12-6.55) |
| Sixth | 67/194 | 1.44 (0.95-2.18) | 2.50 (1.03-6.05) |
| Seventh | 58/200 | 1.21 (0.79-1.85) | 1.97 (0.81-4.79) |
| Eighth | 75/184 | 1.70 (1.13-2.55) | 2.47 (1.03-5.96) |
| Ninth | 61/198 | 1.28 (0.84-1.95) | 2.03 (0.86-4.81) |
| 10th (highest score)b | 50/208 | 1 [Reference] | 1 [Reference] |
Abbreviations: BPAD, bipolar affective disorder; N, nonresponders; PGS, polygenic score; R, responders; SCZ, schizophrenia.
Adjusted for age, sex, genotyping platform, and 7 principal components.
The reference decile (10th decile) is the PGS category with the highest polygenic load for SCZ at a threshold P < 5 × 10−2.
Cross-trait Meta-analysis of GWAS for Lithium Treatment Response in BPAD and of GWAS for SCZ
Subsequent to the PGS analysis, we performed an SNP-based cross-trait meta-analysis by combining the summary statistics for the GWASs on SCZ and treatment response to lithium in the categorical outcome and on SCZ and treatment response to lithium in the continuous outcome. This meta-analysis yielded 15 loci with P values below the genome-wide significance level (P < 5 × 10−8). The top 6 loci and closest genes were rs1611255 (HCG4 [HUGO Gene Nomenclature Committee 21241]), rs66486766 (ADAMTSL3 [OMIM 609199]), rs7405404 (ERCC4 [OMIM 133520), rs1611259 (HCG4), rs3919583 (CCNH [OMIM 601953]), and rs59724122 (EPHX2 [OMIM 132811) (Table 3 and eFigure 2A and B in the Supplement).
Table 3. Loci Resulting From Cross-trait Meta-analysis of GWAS on Lithium Treatment Response in Patients With BPAD and GWAS on SCZ .
| SNP | Chr | BP | Allele | P Value | Effect Directionc | Nearby Gene | |||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | Schizophreniaa | Lithiuma | Cross-traitb | |||||
| rs324899 | 5 | 87915582 | A | G | 5.82 × 10−7 | 4.63 × 10−3d | 2.28 × 10−8 | – to – | MEF2C |
| rs6942227 | 6 | 25177508 | A | G | 9.86 × 10−8 | 8.45 × 10−3d | 2.53 × 10−8 | + to – | CMAHP |
| rs142425863 | 6 | 29751753 | T | C | 2.50 × 10−10 | 9.92 × 10−3d | 5.13 × 10−11 | – to – | HCG4 |
| rs59724122 | 8 | 27424696 | T | C | 2.22 × 10−8 | 7.21 × 10−3d | 5.16 × 10−9 | – to + | EPHX2 |
| rs61123830 | 11 | 123392846 | A | G | 2.85 × 10−6 | 2.60 × 10−3d | 4.53 × 10−8 | – to – | GRAMD1B |
| rs7959663 | 12 | 109884367 | C | G | 4.74 × 10−5 | 2.06 × 10−4d | 2.79 × 10−8 | – to – | MYO1H |
| rs66486766 | 15 | 84806060 | A | G | 1.07 × 10−10 | 4.95 × 10−3d | 1.38 × 10−11 | – to – | ADAMTSL3 |
| rs7405404 | 16 | 13749859 | T | C | 3.93 × 10−10 | 5.27 × 10−3d | 4.62 × 10−11 | + to + | ERCC4 |
| rs6728642 | 2 | 97607071 | A | G | 1.10 × 10−4 | 1.34 × 10−4e | 4.81 × 10−8 | – to – | FAM178B |
| rs62200793 | 2 | 185750642 | T | C | 1.70 × 10−7 | 5.45 × 10−3e | 1.40 × 10−8 | + to + | ZNF804A |
| rs7588746 | 2 | 200986345 | A | G | 2.08 × 10−7 | 6.33 × 10−3e | 3.91 × 10−8 | + to – | MAIP1 |
| rs3919583 | 5 | 86947591 | A | C | 4.18 × 10−6 | 2.65 × 10−4e | 4.54 × 10−9 | – to – | CCNH |
| rs144373461 | 6 | 29751005 | A | C | 8.30 × 10−17 | 3.93 × 10−3e | 1.28 × 10−17 | – to – | HCG4 |
| rs209474 | 6 | 32924584 | A | G | 7.49 × 10−7 | 3.41 × 10−3e | 2.20 × 10−8 | – to – | HLA-DMA |
| rs1521470 | 7 | 45646852 | A | G | 2.41 × 10−6 | 3.92 × 10−4e | 3.23 × 10−8 | + to – | ADCY1 |
| rs79403677 | 14 | 35539131 | T | G | 2.91 × 10−7 | 2.04 × 10−3e | 1.92 × 10−8 | + to – | FAM177A1 |
Abbreviations: A1, effect allele; A2, another allele; BPAD, bipolar affective disorder; BP, position in base pairs at Human Genome Assembly build 37; Chr, chromosome; GWAS, genome-wide association study; SCZ, schizophrenia; SNP, single-nucleotide polymorphism; +, increased susceptibility to SCZ or positive effect on lithium response; –, decreased susceptibility to SCZ or negative effect on lithium response.
P < 1 × 10−2.
Cross-trait P < 5 × 10−8.
Effect direction is the effect of the SNPs on schizophrenia and treatment response to lithium oriented to the effect allele (A1). Nearest genes were based on The Reference Sequence genes (build 37).
Categorical.
Continuous.
To characterize the functional implications of these loci, we undertook IPA using query gene inputs generated from the results of the cross-trait and expression quantitative trait loci analyses (http://www.genenetwork.org/webqtl/main.py; http://www.braineac.org/; eTable 1 in the Supplement). The IPA found significantly represented canonical pathways, with the top 5 being antigen presentation pathway, OX40 signaling pathway, autoimmune thyroid disease signaling, Cdc42 signaling, and B-cell development (eTable 2 in the Supplement). These pathways were predominantly identified on the basis of several HLA antigen genes: HLA-A (OMIM 142800), HLA-DMA (OMIM 142855), HLA-DMB (OMIM 142856), HLA-DOB (OMIM 600629), HLA-DPB1 (OMIM 142858), HLA-F (OMIM 143110), HLA-G (OMIM 142871), PSMB9 (OMIM 177045), and TAP2 (OMIM 170261).
The IPA revealed 2 relevant functional networks (eTable 3 in the Supplement). As shown in eFigure 3A and B in the Supplement, the top 2 networks indicate that tumor necrosis factor (TNF), interleukin 4 (IL-4), and interferon-gamma (IFNγ) might represent important functional molecular nodes in the interaction between response to lithium and SCZ.
Discussion
The present study reports 2 main findings. First, using PGS, we demonstrate that there is an inverse association between genetic loading for SCZ risk variants and long-term therapeutic response to lithium in patients with BPAD on the categorical outcome of the ALDA scale. Second, we show in the cross-trait meta-GWAS and IPA that genetic variants in the HLA antigen region, the antigen presentation pathway, and inflammatory cytokines such as TNF, IL-4 and IFNγ could play a biological role in treatment response to lithium in BPAD.
These findings are consistent with previous clinical and epidemiologic studies of response to lithium. Lithium is not an effective medication for people with SCZ spectrum disorders. Moreover, lithium may be deleterious for patients with SCZ because of their greater liability to developing lithium-induced neurotoxic effects even at modest doses and blood levels. The severity of psychotic symptoms in patients with BPAD was found to be inversely associated with treatment response to lithium. Similarly, slow resolution of psychosis in response to lithium treatment during acute manic episodes has been shown to be associated with poorer overall response to the drug. Among patients with BPAD, those with a family history of SCZ show poorer response to lithium compared with those with a family history of BPAD. Our findings may provide insight into the genetic architecture underlying these clinical observations.
In the SCZ to lithium response cross-trait GWAS meta-analyses, 15 genetic loci located within protein-coding genes that appear to have overlapping outcomes on SCZ risk and treatment response to lithium in BPAD were identified. Only 1 of these genes, type 1 adenylyl cyclase (ADCY1 [OMIM 103072]), had previously been directly implicated in genetic studies of both SCZ and treatment response to lithium.
Both the most significant finding of the cross-trait GWAS and the SNPs from the post-GWAS functional analyses suggest that the HLA antigen system could be implicated in genetic susceptibility to SCZ and treatment response to lithium. The HLA antigen region is the most robust genetic finding in SCZ and could be marking a SCZ-type pathogenesis that is associated with nonresponse to lithium. Although the extensive linkage disequilibrium in the HLA antigen region, and the fact that non-HLA antigen genes are embedded within it, could compromise the biological precision of our pathway analysis, some previous studies have linked HLA antigen surface protein composition to responsiveness to lithium in patients with BPAD. Lithium exposure of human monocytes and mouse microglia in vitro resulted in an increased expression of complement component 3, an HLA antigen protein, which in turn was driven by the inhibition of glycogen synthase kinase-3. Inhibition of glycogen synthase kinase-3 is, to date, the most comprehensively documented molecular effect of lithium in neurons, glia, and peripheral immune cells. Whether these outcomes are in some way compromised by the decreased neuronal complement component 3 expression that is associated with SCZ risk variants in the HLA antigen region, and whether such mechanisms play a role in the clinical efficacy of lithium, needs to be explored in future studies.
Furthermore, network analyses of genes from our meta-GWAS findings implicated TNF, IL-4, and IFNγ as central functional nodes, suggesting that the negative interaction between response to lithium and genetic predisposition for SCZ could be mediated by mechanisms implicating these inflammatory cytokines; this finding is also supported by a growing body of evidence describing aberrant inflammatory processes in patients with a first episode of psychosis and SCZ. Previous studies have reported modulatory outcomes of lithium treatment on these cytokines and underscore the possibility that mechanisms involving inflammatory cytokines might play a role in mediating the therapeutic outcomes of lithium in patients with BPAD.
Our findings have important implications for the treatment of BPAD and for future research. We show for the first time, to our knowledge, that genetic characterization has the potential to aid the stratification of patients with BPAD into those who respond and those who do not respond to lithium, prior to initiation of treatment. Our study also supports the idea that responsiveness to lithium could represent a true psychiatric endophenotype beyond current nosologic descriptions. The findings underscore the importance of careful assessments of patients’ family psychiatric histories in the context of treatment selection. In schizoaffective disorder, which remains challenging clinically owing to a lack of specific effective treatments, determination of SCZ PGS might aid the choice of mood-stabilizing agents. To achieve full clinical translation, PGS analyses could be combined with other biological and clinical factors in prognostic algorithms.
Limitations
This study has some limitations. First, the polygenic load for SCZ accounted for only a modest percentage (approximately 1%) of the observed variation in lithium treatment response in patients with BPAD. Although this finding is in line with previous reports on the outcomes of PGSs on complex clinical phenotypes such as SCZ and BPAD, the significance of this finding at clinical and population levels needs to be further explored. Second, response to lithium in our study was assessed using the ALDA scale, which is a retrospective measure. To substantiate our findings further, prospective studies are required that can prospectively measure clinical responses to lithium. Third, while our strategy for exploring the biological context of our genetic findings can point toward avenues for future research, it is not designed to provide definitive mechanistic answers. Hypothesis-driven experiments are required to follow up on these leads.
Conclusions
We demonstrated for the first time that lower SCZ loading is associated with better response to lithium in patients with BPAD. Follow-up functional analyses implicate genes that code for the immune system, including the HLA antigen complex and inflammatory cytokines. For future clinical translation, a high genetic loading for SCZ risk variants could be used in conjunction with clinical parameters to determine the likelihood of nonresponse to lithium treatment in BPAD.
eAppendix. Methods
eTable 1. Combined list of eGenes and hGenes used as an input in the Ingenuity Pathway Analysis (IPA)
eTable 2. The top canonical signaling pathways enriched for genes identified in the cross-trait meta-analyses
eTable 3. Top IPA protein networks, molecules in network and top diseases functionally related to the network
eFigure 1. Scatter plot that assesses the linearity between the PGS for SCZ and total score on the ALDA subscale A
eFigure 2. Manhattan plot showing the result of cross-trait meta-analysis of GWASs on SCZ and the GWASs on lithium treatment response ALDA, highlighting the loci that showed genome-wide significance (orange), and the nearest genes (top)
eFigure 3. Top networks of molecules in IPA, in which TNFα, IL-4, and IFNγ represent the main functional nodes mediating the genetic interaction between lithium response and SCZ
eReferences
References
- 1.Merikangas KR, Jin R, He J-P, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari AJ, Stockings E, Khoo JP, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440-450. [DOI] [PubMed] [Google Scholar]
- 4.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561-1572. [DOI] [PubMed] [Google Scholar]
- 6.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C(1):48-58. [DOI] [PubMed] [Google Scholar]
- 7.Weber H, Kittel-Schneider S, Gessner A, et al. Cross-disorder analysis of bipolar risk genes: further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology. 2011;36(10):2076-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4 [published correction appears in Nat Genet. 2012;44(9):1072]. Nat Genet. 2011;43(10):977-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda M, Takahashi A, Kamatani Y, et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder [published online January 24, 2017]. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mühleisen TW, Leber M, Schulze TG, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. [DOI] [PubMed] [Google Scholar]
- 11.Hou L, Bergen SE, Akula N, et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum Mol Genet. 2016;25(15):3383-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cichon S, Mühleisen TW, Degenhardt FA, et al. ; Bipolar Disorder Genome Study (BiGS) Consortium . Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88(3):372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):349-352. [DOI] [PubMed] [Google Scholar]
- 14.Miura T, Noma H, Furukawa TA, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351-359. [DOI] [PubMed] [Google Scholar]
- 15.Malhi GS, Tanious M, Das P, Berk M. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. [DOI] [PubMed] [Google Scholar]
- 16.Malhi GS, Adams D, Berk M. Is lithium in a class of its own? a brief profile of its clinical use. Aust N Z J Psychiatry. 2009;43(12):1096-1104. [DOI] [PubMed] [Google Scholar]
- 17.Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104(3):163-172. [DOI] [PubMed] [Google Scholar]
- 18.National Collaborating Centre for Mental Health Bipolar Disorder: The Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. Leicester, England: British Psychological Society; 2006. [PubMed] [Google Scholar]
- 19.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1-44. [DOI] [PubMed] [Google Scholar]
- 20.Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087-1206. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou L, Heilbronner U, Degenhardt F, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet. 2016;387(10023):1085-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694. [DOI] [PubMed] [Google Scholar]
- 24.Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10):942-947. [DOI] [PubMed] [Google Scholar]
- 25.Higgins GA, Allyn-Feuer A, Barbour E, Athey BD. A glutamatergic network mediates lithium response in bipolar disorder as defined by epigenome pathway analysis. Pharmacogenomics. 2015;16(14):1547-1563. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Bergen SE, Di Florio A, et al. ; Members of the International Cohort Collection for Bipolar Disorder (ICCBD) . Genome-wide association study identifies SESTD1 as a novel risk gene for lithium-responsive bipolar disorder. Mol Psychiatry. 2016;21(9):1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CH, Lee CS, Lee MT, et al. ; Taiwan Bipolar Consortium . Variant GADL1 and response to lithium therapy in bipolar I disorder. N Engl J Med. 2014;370(2):119-128. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forstner AJ, Hecker J, Hofmann A, et al. Identification of shared risk loci and pathways for bipolar disorder and schizophrenia. PLoS One. 2017;12(2):e0171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Föcking M, Dicker P, English JA, Schubert KO, Dunn MJ, Cotter DR. Common proteomic changes in the hippocampus in schizophrenia and bipolar disorder and particular evidence for involvement of cornu ammonis regions 2 and 3. Arch Gen Psychiatry. 2011;68(5):477-488. [DOI] [PubMed] [Google Scholar]
- 32.Leucht S, Helfer B, Dold M, Kissling W, McGrath JJ. Lithium for schizophrenia. Cochrane Database Syst Rev. 2015;(10):CD003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grof P, Alda M, Grof E, Zvolsky P, Walsh M. Lithium response and genetics of affective disorders. J Affect Disord. 1994;32(2):85-95. [DOI] [PubMed] [Google Scholar]
- 34.Pearlson GD. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu Rev Clin Psychol. 2015;11:251-281. [DOI] [PubMed] [Google Scholar]
- 35.Sachs GS, Peters AT, Sylvia L, Grunze H. Polypharmacy and bipolar disorder: what’s personality got to do with it? Int J Neuropsychopharmacol. 2014;17(7):1053-1061. [DOI] [PubMed] [Google Scholar]
- 36.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze TG, Alda M, Adli M, et al. The International Consortium on Lithium Genetics (ConLiGen): an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology. 2010;62(1):72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy A, Alda M, Milin R, Grof P. A consecutive series of treated affected offspring of parents with bipolar disorder: is response associated with the clinical profile? Can J Psychiatry. 2007;52(6):369-376. [DOI] [PubMed] [Google Scholar]
- 39.Garnham J, Munro A, Slaney C, et al. Prophylactic treatment response in bipolar disorder: results of a naturalistic observation study. J Affect Disord. 2007;104(1-3):185-190. [DOI] [PubMed] [Google Scholar]
- 40.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Q, Wu H, Guo CY, Fox CS. Analyze multivariate phenotypes in genetic association studies by combining univariate association tests. Genet Epidemiol. 2010;34(5):444-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Q, Wang Y. Methods for analyzing multivariate phenotypes in genetic association studies. J Probab Stat. 2012;2012:652569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prien RJ. Lithium in the treatment of schizophrenia and schizoaffective disorders. Arch Gen Psychiatry. 1979;36(8 spec no):852-853. [DOI] [PubMed] [Google Scholar]
- 44.Shopsin B, Kim SS, Gershon S. A controlled study of lithium vs. chlorpromazine in acute schizophrenics. Br J Psychiatry. 1971;119(551):435-440. [DOI] [PubMed] [Google Scholar]
- 45.Silva LFAL, Loureiro JC, Franco SCR, et al. Assessing treatment response to prophylactic lithium use in patients with bipolar disorder. J Bras Psiquiatr. 2016;65(1):9-16. doi: 10.1590/0047-2085000000097 [DOI] [Google Scholar]
- 46.de Sousa RT, Busnello JV, Forlenza OV, et al. Early improvement of psychotic symptoms with lithium monotherapy as a predictor of later response in mania. J Psychiatr Res. 2012;46(12):1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry. 2015;20(6):661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goes FS, McGrath J, Avramopoulos D, et al. Genome-wide association study of schizophrenia in Ashkenazi Jews. Am J Med Genet B Neuropsychiatr Genet. 2015;168(8):649-659. [DOI] [PubMed] [Google Scholar]
- 49.Sekar A, Bialas AR, de Rivera H, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Vecchio M, Farzati B, Maj M, Minucci P, Guida L, Kemali D. Cell membrane predictors of response to lithium prophylaxis of affective disorders. Neuropsychobiology. 1981;7(5):243-247. [DOI] [PubMed] [Google Scholar]
- 51.Maj M, Del Vecchio M, Starace F, Pirozzi R, Kemali D. Prediction of affective psychoses response to lithium prophylaxis: the role of socio-demographic, clinical, psychological and biological variables. Acta Psychiatr Scand. 1984;69(1):37-44. [DOI] [PubMed] [Google Scholar]
- 52.Perris C, Strandman E, Wählby L. HL-A antigens and the response to prophylactic lithium. Neuropsychobiology. 1979;5(2):114-118. [DOI] [PubMed] [Google Scholar]
- 53.Yu Z, Ono C, Aiba S, et al. Therapeutic concentration of lithium stimulates complement C3 production in dendritic cells and microglia via GSK-3 inhibition. Glia. 2015;63(2):257-270. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4(2):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6(8):777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155(1-3):101-108. [DOI] [PubMed] [Google Scholar]
- 57.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137. [DOI] [PubMed] [Google Scholar]
- 59.Guloksuz S, Altinbas K, Aktas Cetin E, et al. Evidence for an association between tumor necrosis factor-alpha levels and lithium response. J Affect Disord. 2012;143(1-3):148-152. [DOI] [PubMed] [Google Scholar]
- 60.Giambelluca MS, Bertheau-Mailhot G, Laflamme C, Rollet-Labelle E, Servant MJ, Pouliot M. TNF-α expression in neutrophils and its regulation by glycogen synthase kinase-3: a potentiating role for lithium. FASEB J. 2014;28(8):3679-3690. [DOI] [PubMed] [Google Scholar]
- 61.Petersein C, Sack U, Mergl R, et al. Impact of lithium alone and in combination with antidepressants on cytokine production in vitro. J Neural Transm (Vienna). 2015;122(1):109-122. [DOI] [PubMed] [Google Scholar]
- 62.Rowse AL, Naves R, Cashman KS, et al. Lithium controls central nervous system autoimmunity through modulation of IFN-γ signaling. PLoS One. 2012;7(12):e52658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rapaport MH, Manji HK. The effects of lithium on ex vivo cytokine production. Biol Psychiatry. 2001;50(3):217-224. [DOI] [PubMed] [Google Scholar]
- 64.Boufidou F, Nikolaou C, Alevizos B, Liappas IA, Christodoulou GN. Cytokine production in bipolar affective disorder patients under lithium treatment. J Affect Disord. 2004;82(2):309-313. [DOI] [PubMed] [Google Scholar]
- 65.Guloksuz S, Cetin EA, Cetin T, Deniz G, Oral ET, Nutt DJ. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126(3):458-462. [DOI] [PubMed] [Google Scholar]
- 66.Mertens J, Wang QW, Kim Y, et al. ; Pharmacogenomics of Bipolar Disorder Study . Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder [published correction appears in Nature. 2016;530(7589):242]. Nature. 2015;527(7576):95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jäger M, Haack S, Becker T, Frasch K. Schizoaffective disorder—an ongoing challenge for psychiatric nosology. Eur Psychiatry. 2011;26(3):159-165. [DOI] [PubMed] [Google Scholar]
- 68.Purcell SM, Wray NR, Stone JL, et al. ; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. Combined list of eGenes and hGenes used as an input in the Ingenuity Pathway Analysis (IPA)
eTable 2. The top canonical signaling pathways enriched for genes identified in the cross-trait meta-analyses
eTable 3. Top IPA protein networks, molecules in network and top diseases functionally related to the network
eFigure 1. Scatter plot that assesses the linearity between the PGS for SCZ and total score on the ALDA subscale A
eFigure 2. Manhattan plot showing the result of cross-trait meta-analysis of GWASs on SCZ and the GWASs on lithium treatment response ALDA, highlighting the loci that showed genome-wide significance (orange), and the nearest genes (top)
eFigure 3. Top networks of molecules in IPA, in which TNFα, IL-4, and IFNγ represent the main functional nodes mediating the genetic interaction between lithium response and SCZ
eReferences