Abstract
Introduction:
As a consequence of a sessile lifestyle, plants often have to face a number of life threatening abiotic and biotic stresses. Plants counteract the stresses through morphological and physiological adaptations, which are imparted through flexible and well-coordinated network of signalling and effector molecules, where phytohormones play important role. Hormone synthesis, signal transduction, perception and cross-talks create a complex network. Omics approaches, which include transcriptomics, genomics, proteomics and metabolomics, have opened new paths to understand such complex networks.
Objective:
This review concentrates on the importance of phytohormones and enzymatic expressions under metal stressed conditions.
Conclusion:
This review sheds light on gene expressions involved in plant adaptive and defence responses during metal stress. It gives an insight of genomic approaches leading to identification and functional annotation of genes involved in phytohormone signal transduction and perception. Moreover, it also emphasizes on perception, signalling and cross-talks among various phytohormones and other signalling components viz., Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS).
Keywords: ABA, Auxins, Comparative genomics, Jasmonates, NADPH oxidase, Phytochelatins, Strigalactones
1. INTRODUCTION
Heavy metals are natural component of earth’s crust. However, their levels and availability have significantly increased in the past few decades. The anthropogenic activities like mining, smelting, industrial applications, use of excessive pesticides etc. as well as natural geogenic activities are the sources of heavy metal contamination [1, 2] (Table 1). Metals are non-biodegradable [3] and continuously change their forms into sulphates, carbonates, oxalates etc. In developing countries, where industrialization is at boom and environmental regulations are still in the phase of development, heavy metal pollution has spread vastly in surface and groundwater and soils [4, 5]. Hence, a number of heavy metals are entering into plant and human body owing to the phenomenon of bioaccumulation and bio-magnification, respectively in greater amounts than required or tolerable. Metals are of concern for humans since elements like Arsenic (As) and Cadmium (Cd) are toxic and Carcinogenic [6, 7].
Table 1. Sources of toxic heavy metals.
| Heavy Metal | Sources |
|---|---|
| Arsenic | Geogenic processes, thermal power plants, fuel burning |
| Chromium | Mining, Leather tanning, industrial coolants, chromium salt manufacturing |
| Cadmium | Waste batteries, e-waste, paint sludge, incinerations, Zinc smelting |
| Copper | Mining, electroplating, smelting processes |
| Mercury | Chlor-alkali plants, thermal power plants hospital wastes |
| Lead | Lead acid batteries, e-waste, paints, coal based thermal plants. |
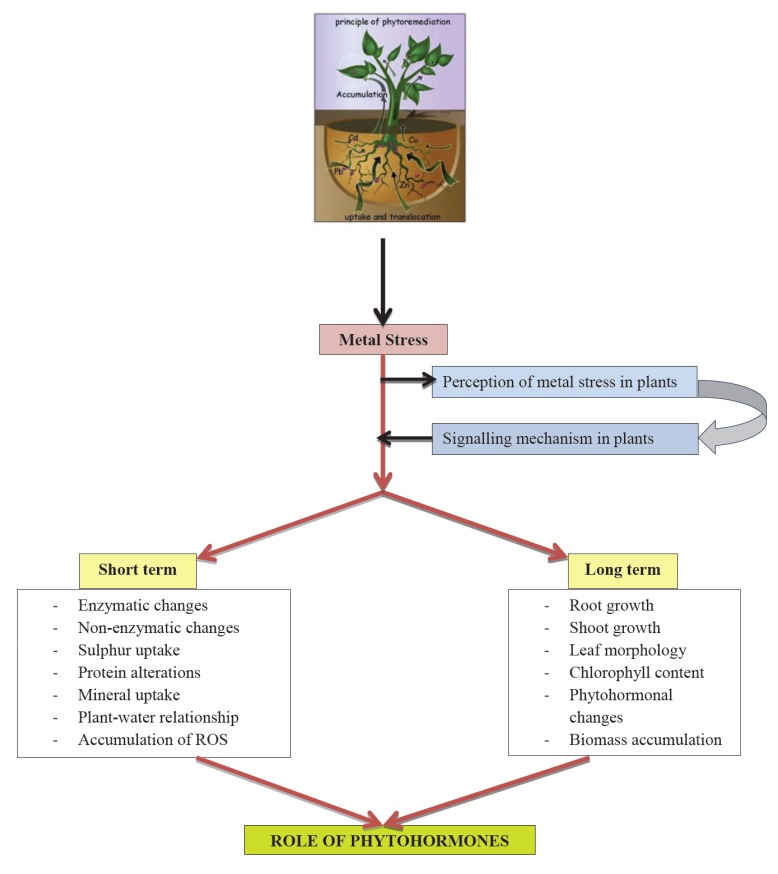
In the plants, toxic non-essential metals gain entry in competition with essential metals due to ionic mimicry [8, 9]. The influx of arsenate [As(V)] is driven by phosphate transporters [10], while arsenite [As(III)] is taken up by primarily via nodulin 26-like intrinsic proteins class of aquaglyceroporins [8, 11]. The uptake of Cd occurs via transporters of divalent cations like Iron (Fe2+), Calcium (Ca2+), Zinc (Zn2+), and Manganese (Mn2+) [12, 13]. The transport of essential metals takes place through specific transporters for the purpose viz., Copper Transporter (COPT) protein family for Copper (Cu) [14]. Non-essential metals as well as essential metals in more than required amounts affect plant growth and biomass accumulation. They also cause many deleterious effects on physiological processes of plants like mineral nutrient uptake, photosynthesis and plant-water relationships [15]. The increased production of reactive oxygen species (ROS), reactive nitrogen species (RNS) and altered redox and energetics status have been considered as the major causes of toxic impacts of metals [16]. Plants counteract the stress effects through multifaceted response. The antioxidant compounds and enzymes take a lead to keep ROS level under control. The alterations in redox state and energetics are controlled through changes in redox molecules like glutathione and ascorbate, utilization of alterative pathways for electron transport, and regulation of ATP metabolism [16, 17]. To avoid any mineral disturbances, transporters expression and functions may be regulated. Furthermore, plants adapt to continuing metal stress through modified root and shoot length, root hair development etc. These multifaceted responses are regulated through focal functioning of various phytohormones. The metal stress and its effects have been illustrated in Fig. (1) along with the possible roles of phytohormones in the regulation of these events.
Fig. (1).
Schematic representation of metal stress mediated response in plants.
The use of ‘omics’ approach to study the genes (genomics), proteins (proteomics) and metabolites (metabolomics) from different tissues and at various developmental stages plays a crucial role in understanding the effects of heavy metals on physiology, biochemistry and metabolism of plants. Genomics and functional genomics help in discovering genes and their respective functions and in heavy metal stress. These tools can help in identifying new genes, comparing them with already existing genes and even help in studying their roles in plant adaptive responses, which can be at physiological, biochemical and molecular level. Genomics studies can be further categorized in three sub-levels: Structural Genomics where genetic and physical mapping of genes are carried out, Functional Genomics represents analysis of genes’ functions and Comparative Genomics helps in comparing genomes across the species.
2. Role of phytohormones in plant defence systems under heavy metal stress
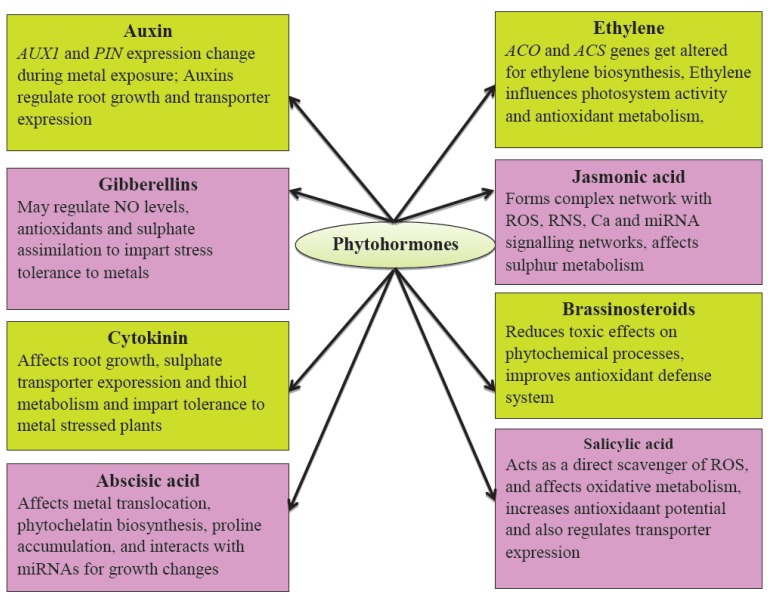
Phytohormones are complex organic compounds that regulate plant growth and development at different stages of their life cycle from seed germination to grain development. Plants produce a number of hormones like auxins, cytokinins, gibberellins, Abscisic Acid (ABA), Salicylic Acid (SA), ethylene, jasmonates, brassinosteroids, strigalactones and some low molecular weight peptides. A number of synthetic compounds are also available, which serve same functions as that of phytohormones, e.g., Indole-3-Acetic Acid (IAA) for auxins. Such synthetic compounds are known as Plant Growth Regulators (PGRs). Phytohormones are vital elements of the signal-transduction pathway and their presence may stimulate reactions that are signals or causative agents for stress responses [18, 19]. In the process of acclimatization to a long term metal stress, biosynthesis, transportation, distribution, and conjugation of plant hormones get altered [17, 20-23]. The importance of phytohormones has also been proved through the exogenous application of plant hormones that has resulted in increased stress tolerance against heavy metals [17, 23-32] (Fig. 2). In the following sections, the roles of various phytohormones are being discussed under separate subheadings.
Fig. (2).
Some specific roles of phytohormones in heavy metal stress condition.
2.1. Auxins
Auxins are a class of phytohormones involved in the regulation of growth and life cycle of plants. Auxin is synthesised in tissues like young leaves and cotyledons and is transported passively to root system via phloem. Auxins transportation represents the inverted fountain model [33]. It is also transported through active system also known as polar auxin transport (PAT), which is mediated by a special class of protein family ‘PIN Family’ [34]. There are two types of auxin receptors: ABP1 (Auxin Binding Protein 1) and TIRs/AFBs (Transport Inhibitor Response 1/ Auxin signalling F-Box) [35]. Another recently identified auxin receptor is F-box protein: SKP2A (S-Phase Kinase Associated Protein 2A) [36].
Roots are the first organs to come in contact with the stress and hence, roots are an important link in the stress perception and signalling. The changes in concentration and distribution pattern of auxins have been found in response to several heavy metals like Cd, Zn, Cu, and lead (Pb) [29]. Therefore, a direct involvement of auxins in metal stress signalling is suggested. Further, auxins play crucial role in growth of root tissues and may be indirectly involved in stress regulation by affecting the expression of transporters. In rice AUX1 mutants (OsAUX1), the Cd sensitivity has been found to increase along with reduced growth of primary and lateral roots and root hairs. The effects could be reversed by exogenous supply of auxin analogue suggesting important role of AUX1 in response to Cd stress [37]. In silico analysis shows that Os08g01480 which is a CYP450 like gene might help plants to combat environmental stress via modulation of auxin metabolism [38]. In Arabidopsis, Cd exposure has been found to affect expression of PINs and AUX1 and induce lateral root density. However, in aux1-7 and pin2-1 mutants, this effect was not seen. In addition to changes in transport of auxins, the level of IAA is also altered due to increased activity of IAA oxidase. Excess of Cu and aluminium (Al) was found to inhibit meristematic cell division and root elongation by altering the auxin transport and distribution through changes in the expression of PIN1 and, PIN2 and AUX1, respectively [39, 40]. An exogenous supply of IAA has been found to improve the growth of Brassica juncea under As stress and also to modulate the expression of miR167, miR319, and miR854, suggesting a protective role of IAA in enhancing As tolerance through interaction with miRNAs [17]. The exogenous application of auxin along with selenium (Se) has been found to further improve the As toxicity amelioration in rice as compared to that of Se alone [41]. In addition to protection of plants under stress, exogenous application of IAA has also been enhanced heavy metal accumulation in plants [42] and improved metal phytoextraction ability viz., for lead (Pb) in maize [43]. It can thus be concluded that, auxins play a crucial role in heavy metal stress mediated responses.
2.2. Cytokinins
Cytokinin is a N6-prenylated adenine derivative, which is involved in various developmental processes like cell division, nutrient metabolism, chloroplast development, nodulation, circadian rhythms [18, 44]. This hormone is usually synthesised in roots, young fruits and seeds. There are two major forms of cytokinins: isopentenyladenine (iP) and trans-zeatin [45]. They enter shoot system via xylem. They act as the negative regulators of lateral root formation by downregulating the expression of PIN genes [46]. The cumulative effect of auxin-cytokinin antagonistic behaviour determines the final root meristem and root growth. Cytokinin reduces the number of dividing cells and inhibits root growth by promoting cellular differentiation in the transition zone of root system [47].
A study by Srivastava et al. [48] proposed role of cytokinin in As stress in B. juncea. It was found that cytokinin response 1 (CRE1), a cytokinin receptor, was down-regulated in response to As that in turn induced the expression of Group 1 sulphate transporters. In a recent study on A. thaliana also, cytokinin depletion, induced by the expression of cytokinin oxidase/dehydrogease 1 (CKX1), was observed to initiate the activation of As tolerance mechanism leading to the accumulation of thiol compounds like Phytochelatins (PCs) [49]. During Cu stress, the inhibition of primary root growth has been linked to a significant increase in the amount of cytokinin [50]. Cd stress was responsible for reduced growth and cytokinin content in soybean [51]. By contrast, in other studies, the exogenous application of cytokinins has been reported to increase the stress-tolerance capacity of plants indicating a beneficial effect of cytokinins in the regulation of plants’ adaptation to environmental stresses. It has also been observed that Cd-treatment inhibited the growth rate, chlorophyll content, and net photosynthesis. However, the addition of kinetin reduced Cd-induced alterations in pea seedlings [52]. Many deleterious effects of heavy metals (Cd, Pb, and Cu) on growth of green algae, Chlorella vulgaris have been reported [53]. However, exogenous application of cytokinins alleviated stress symptoms by reducing metal absorption and stimulating the defense system. It has been reported that Pb treatment lowered the terminal electron transport activity by 25% in root tissues of Picea abies. Zeatin mitigated the Pb-induced inhibition of root growth and the ETS activity [54]. In a transgenic approach, tobacco plants expressing an isopentenyl transferase (ipt) gene having enhanced cytokinin accumulation were found to possess higher tolerance against Cu stress in comparison to non-transformed plants. This was accompanied by an increased expression of the metallothionein gene (MT-L2) [55]. The above studies hence propose that cytokinins also play a key role in response strategies adopted by plants against heavy metal stress.
2.3. Gibberellins
Gibberellic Acids (GA) were originally identified as a fungal toxin causing unusual shoot elongation of rice plants [56]. Gibberellins are a large family of tetracyclic diterpinoid plant growth substances associated with seed germination, leaf expansion, floral initiation, floral organ development and induction of some hydrolytic enzymes in the aleurone of cereal grains [57]. It has been reported that gibberellins also play a role in modulating source-sink relationships under environmental stresses [58]. A few studies also propose role of gibberellins under metal stresses as discussed below.
GA plays a crucial role in providing protection against Cd-Stress by diminishing the Cd-induced changes. Cd exposure can increase the expression of iron-regulated transporter 1 (IRT1), a transporter involved in Cd uptake and this up-regulation was suppressed by exogenous application of GA through reduction in Nitric Oxide (NO) levels in A. thaliana [32]. In wheat seedlings, Nickel (Ni) has been reported to decrease growth, chlorophyll content, and carbonic anhydrase activity by enhancing oxidative stress. However, these effects were reversed when seeds were soaked in a combination of GA and Ca due to enhanced antioxidant potential [59]. GA also ameliorated the toxic effects of Chromium (Cr) on growth and ammonium assimilation of pea seedlings by regulating oxidative stress and the antioxidant system [26]. GA alleviated Cd induced adverse effects on seed germination and growth of Brassica napus by regulating oxidative stress and ROS damage [60]. This hormone also abolished the detrimental effects of Cd and Pb by regulating the activities of proteases, catalases, and peroxidases in broad bean and lupin plants [61]. Khan and Lee [62] assessed endophyte-metal-plant interaction with Penicillium funiculosum-Cu-Glycine max and observed significant reversal of Cu toxicity in endophyte-inoculated plants with increased biomass and Cu accumulation. The positive effects of endophyte were ascribed to its potential of gibberelins secretion and to decline in stress-induced ABA levels. Similarly, Khan et al. [63] compared the effectiveness of endophyte P. janthinellum or GA3 application on aluminium (Al) tolerance of tomato plants and found similar potential of endophyte and exogenous GA3. For Arabidopsis, it has been demonstrated that the expression of adenosine-5’-phosphosulfate reductase (APR), the key enzyme of sulfate assimilation, is increased using GA signalling under stress, while other hormones do not show such an effect. This suggests GA mediated signalling may be utilized under metal stress to improve sulphur metabolism [25]. Through the above studies, it appears that gibberellins may regulate heavy metal stress in plants through alteration in ROS and RNS levels, expression of metal transporters as well as sulphur metabolism.
2.4. Abscisic Acid
Abscisic Acid (ABA) is a carotenoid derivative. It plays a specific role in abscission of leaves and fruits, control of seed dormancy, bud growth and in stress responses. It has well established role in drought stress [64]. An increase in ABA effects stomatal conductance. Basal level of this hormone is important for the plants but it may act as growth inhibitor when its levels increase [65]. ABA is synthesised in both leaves and roots. The movement of this hormone occurs both through xylem and phloem. The known carriers of ABA are ABCG40 (importer) and ABCG25 (exporter) (ATP-Binding Cassette G40 and G25), which are situated in plasma membrane [66, 67]. There are multiple receptors known for ABA perception. GTG1 and GTG2 (GPCR Type G Protein) are plasma membrane localised ABA receptors that function in seed germination process [68]. PYR/PYL (Pyrabactin Resistance/ Pyrabactin Resistance Like) are nucleocytoplasmic ABA receptors, which are involved in guard cell movement [69], while CHLH (Chelate H Subunit) is a chloroplast localised receptor and has same function as PYR receptors [70].
An elevated level of ABA has been reported under various stress conditions that plays an important role in adaptive stress responses but rather few reports are available in classifying its role in metal stress. In a study on potato (Solanum tuberosum) plants, it was found that Cd supply induced the expression of 9-cis-epoxycarotenoid dioxygenase 1 (NCED1) and ABA synthesis. This in turn led to increased synthesis of PCs through induced expression of phytochelatin synthase (PCS) gene. The role of ABA was further proved through the use of ABA biosynthesis inhibitor, fluridone (Flu), whose application abolished the Cd-induced responses and decreased ABA level [71]. Silicon mediated Cd toxicity amelioration in rice has been found to be associated to phytohormone changes including ABA, jasmonic acid and salicylic acid [72]. The role of ABA in Cu stress mediated accumulation of proline, an important osmolyte and ROS scavenger, has been suggested [73]. An elevated concentration of ABA has been reported under Zn and Pb stresses in germinating chickpea seeds [74]. ABA plays a significant role in Cr-stressed condition also and its efficiency increases with the cross talks among other phytohormones [75]. An increase in ABA synthesis under As stress has been found in B. juncea. The work suggested an interaction of ABA and miR159 to have a role in As stress responses through effects on other hormones [17]. In transcriptomic analysis in rice under arsenate and arsenite also, changes in ABA metabolism genes were noticed viz., [76]. The mechanistic investigation of ABA mediated metal-stress tolerance has been performed through exogenous supply of ABA to plants [77, 78]. In Pb-stressed Atractylodes macrocephala, positive effects of an optimum supply of ABA were associated to increased antioxidant enzyme activities and reduced oxidative stress [77]. In Populus x Canescens subjected to Zn stress, exogenous ABA supply was associated to an increase in endogenous ABA, GA and salicylic acid levels and increased foliar ascrobate. The ABA supply led to decline in Zn concentrations through down-regulation of genes involved in Zn uptake and detoxification viz., yellow stripe-like family protein 2 (YSL2) and plant cadmium resistance protein 2 (PCR2) [78].
2.5. Ethylene
Ethylene is gaseous plant hormone. The highest production of ethylene is reported in meristematic cells and in ripening and stressed tissues. This hormone is basically involved in fruit ripening, sex determination, and abscission [79]. Ethylene is strong inhibitor of root elongation and lateral root development but it stimulates root hair formation [80]. It also regulates auxin transport within the root tip [81]. Ethylene adversely affects the cell expansion capacities by increasing the formation of specific cell wall components [82]. Ethylene has five receptors ERT1 and ETR2 (Ethylene Response 1 and 2), ERS1, ERS2 (Ethylene Response Sensor 1&2), EIN4 (Ethylene Insensitive 4). These all are negative regulators of ethylene signalling pathway and are localized on endoplasmic reticulum membrane [83].
It has been reported that the level of endogenous ethylene increases in metal stress conditions [84]. Cd-stress can cause an up-regulation of ethylene responsive genes [85] as well as ethylene production in various plants like B. juncea [86, 87] and Hordeum vulgare [88]. Ni and Zn exposure also enhance ethylene levels in B. juncea [89]. Ethylene can reverse the Ni and Zn-mediated inhibition of photosynthesis by changing the activity of photosystem II in B. juncea [89] and can also influence antioxidant metabolism. In wheat plants, Cd stress amelioration was demonstrated through Se and S supplementation and the effects were associated to changes in ethylene levels. The application of ethylene biosynthesis inhibitor, aminoethoxyvinylglycine (AVG), reversed the positive effects of Se and S supplementation indicated an involvement of ethylene in Cd-stress resopnses [90]. High concentration of Cu induces up-regulation of 1-aminocyclopropane-1-carboxylate oxidase (ACO) genes that catalyzes the final step of ethylene formation in plants [91]. Ethylene also mediates the inhibition of root elongation in high Al-stress condition [92]. Cd-induced stress also up-regulates 1-aminocyclopropane-1-carboxylate synthase 2 (ACS2) and ACS6 genes which are members of the biosynthesis pathway of ethylene [93]. Sesbania drummondii, a Pb hyperaccumulator, also showed increased mRNA levels of the putative ACS/ACO gene upon exposure to lead [94]. Cd-tolerance in tolerant species of H. vulgare was also related to induction of ethylene signalling [95]. Hence, a number of studies reveal that ethylene is an important hormone involved in plants’ responses to several metal stresses in mediating physiological, biochemical and metabolic changes.
2.6. Jasmonates
Jasmonates are oxylipin signalling molecules that regulate wound responses, secondary metabolites production, and defence against abiotic and biotic stresses [96]. Jasmonic Acid (JA) is very effective against necrotrophic pathogens and herbivorous insects [97]. Chemically, these compounds are tri-unsaturated fatty acids that are released from plastids. The most active natural form of JA is JA-Ile (jasmonoyl-L-isoleucine). JA enhances auxin biosynthesis but conversely auxin attenuates JA signalling [96]. JA also inhibits primary root growth as it arrests cell division in mitosis stage [98]. The JA receptor COI1 (Coronatine Insensitive 1) is an F-box protein which binds with negative regulator of JA i.e. JAZs (Jasmonate Zim Domain) [99].
JAs can nullify the toxic effects of low concentration of Cu and Cd by inducing the accumulation of phytochelatins, glutathione and carotenoids [100, 101]. A complex network of JA along with ethylene, ROS, NO and Ca signalling systems was reported to mediate the Cd-stress responses in pea plants including the induction of pathogenesis-related and heat shock proteins [102]. Methyl jasmonate also plays role in Cu- mediated stress condition by regulating the oxidative stress mechanism in Phaseolus coccineus plants [103]. JA can modulate antioxidant mechanism of higher plants with tight regulation of biomembrane peroxidation making plants tolerant to toxic Ni [104]. It has also been reported that toxic effects of Cu are nullified by the JA-mediated enhanced accumulation of osmolytes like proline, glutathione, carotenoids and enhanced antioxidant enzymes [105] in pigeon pea. An involvement of jasmonates in the signalling of As-mediated stress has been proposed in B. juncea [48]. Jasmonates were proposed to sense the As stress through indirect perception of stress to sulfur metabolism. The proposition was further proved through monitoring of significant changes in JA and MeJA in As stressed B. juncea and by visualizing the improved growth and tolerance of plants by external supply of JA [17]. In transcriptomic analysis in rice under As(III) stress, genes involved in JA biosynthesis and JAZs were significantly up-regulated [106]. Conversely, JA was suggested to be involved in the toxic action of heavy metals rather than for the defence in a few studies [107]. Jasmonates appear to be involved in perceiving and signalling the metal stress to plants and to be associated in cross talks among phytohormones as well as ROS, RNS and Ca signalling networks.
2.7. Brassinosteroides
Brassinolide was the first isolated Brassinosteroid (BR) from Brassica napus and since then almost 50 natural analogues have been found in various plants species [108]. Chemically they are polyhydroxylated triterpenoids and are essential for plant growth. Their major functions include male fertility, vascular development, flowering time regulation and plant responses in light, temperature, salt and plant-pathogen interaction. BRs are found in young tissue, reproductive organs, seeds and fruits. They serve as local signal as they do not undergo long distance transport. There are three important receptor for brassinosteroides, which are localised on plasma membrane; BRI1 (Brassinosteroid Insensitive 1), BRL1 and BRL2 (BRI1-Like 1 and 2) [109].
The exogenous 24-Epibrassinolides (EBL) caused a significant reduction in Cr accumulation and improved the growth of seedlings by strengthening antioxidant defence system via up-regulation of gene expression in rice viz., superoxide dismutase (Mn-SOD, Cu/Zn-SOD), catalase (Cat A, Cat B), ascorbate peroxidase (APX) and glutathione reductase (GR) [110]. Further, it has been confirmed that BRs hasve a potential role in mitigating Zn oxide nanoparticles-induced toxicity in tomato seedlings [111]. In B. napus, BRs reduce the toxic effects of Cd by diminishing the damage on photochemical centres and by maintaining the activity of oxygen evolving complex and electron transport chain in a balanced state [112]. Cd stress condition triggers the activation of BRs signalling pathway in A. thaliana. The consequent high BR contents lead to hypersensitivity to Cd that in turn initiates the plant adaptive mechanism [113]. In another study, the exogenous foliar application of BRs on Raphanus sativa significantly reduced the stress caused due to Zn contamination [114]. An interactive effect of EBL and Se has been reported to reduce the Cu stress in B. juncea through altered proline metabolism and antioxidants [115]. Exogenous application of EBL also reduced Zn-induced toxicity in eggplant seedlings by regulating the glutathione-ascorbate dependent detoxification pathway [116]. Ni treatment also induces accumulation of BRs viz., 24-EBL, Castasterone, Dolicholide and Typhasterole in B. juncea [117]. As-induced changes in BRs like castasterone, teasterone, 24-epibrassinolide, and typhasterol, in B. juncea have also been reported [118]. A role of BRs in metal-stressed plants ranges from antioxidant and phytochelatin changes to biosorption and growth alterations [119].
2.8. Salicylic Acid
Salicylic Acid (SA) is a phenolic compound. It is synthesized from chorismate compound in chloroplast and from phenylalanine in the cytoplasm via different pathways [120, 121]. The main functions of SA are in regulation of respiration, seed germination and thermo-tolerance. It plays a vital role in response to plant pathogens and abiotic stresses [122, 123].
SA treatment stimulates signalling systems related to plant defence actions against Cd-induced oxidative stress [124]. It has been demonstrated that SA serves as a signalling molecule and hence, it has been used in maize seed priming to reduce the accumulation of Cr and to trigger up-regulation of antioxidants [125]. SA can also act as a direct scavenger of ROS species like hydroxyl radicals that are formed especially in metal stress conditions. The exogenous application of SA has significantly improved Cd-tolerance in Phaseolus aureus and Vicia sativa by increasing antioxidant enzyme pool of both apoplastic and symplastic compartments and consequently by decreasing H2O2 accumulation [126]. It has been reported that SA reduced Cd-uptake, improved photosynthetic efficiency and enhanced antioxidant activities in Cannabis sativa [127]. Exogenous application of SA was also found to revert growth and oxidative stress caused by As in rice and to also reduce As translocation to shoots through changes in antioxidants and expression of As transporter genes [128]. SA has also been suggested to mitigate chlorotic effects caused by Fe deficiency and also enhance Fe uptake and transport in Arachis hypogaea [129]. Thus, SA plays an important role in providing metal stress resistance to plants.
3. Cross-talk among phytohormones and reactive oxygen species
ROS production and dismutation are natural processes in plants owing to oxygen-driven life. However, this need to happen in a fine balanced state as whenever this balance is disturbed, ROS production may increase substantially leading to oxidative stress [16]. Heavy metals too cause stress to plants through changes in ROS levels. The ROS, activated by metal stress, can act as an intra and intercellular signalling molecule [130]. Pro-oxidant enzymes like glycolate oxidase and NADPH oxidase may also participate in metal induced ROS generation [131]. NADPH oxidase mediated ROS accumulation in apoplast induces activation of GA signalling pathway, which in turn triggers seed germination [132]. Further, interconnections of phytohormones with ROS are well discovered. Auxin-induced ROS are involved in cell wall loosening and cell elongation [133]. A decrease in endogenous auxin level increases plant tolerance to oxidative stress [134]. It has been demonstrated that NADPH oxidase activity is correlated with endogenous levels of BR in cucumber [135]. ABA-induced inhibition of primary root growth is related with the activation of NADPH oxidases and ROS accumulation [136]. Ethylene regulates stomatal closure via H2O2 production in A. thaliana [137]. SA modulates cellular redox homeostasis by inhibiting catalases in peroxisomes, which in turn increase ROS level that activate thiol signalling pathway [138, 139]. All SA mediated responses of ROS generation are independent of NADPH oxidases [140]. These studies indicate how phytohormones function through changes in ROS metabolism and vice versa. Thus, cross-talks among phytohormones with ROS might play important role in perception and signalling of metal stress and to help plants’ adapt to the stress.
4. Reactive nitrogen species and their role in metal stress
Various NO derived compounds, like nitroxyl anion, nitrosonium cation, higher oxides of nitrogen, S-nitrosothiols, and dinitrosyl iron complexes are collectively known as Reactive Nitrogen Species (RNS). These highly reactive species can cause cell injury and death by inducing nitrosative stress [141]. NO plays a crucial role in signalling mechanism and regulates plant responses during abiotic and biotic stresses. Further, this hydrophobic gas is important at molecular level too with protein modifications by binding to cysteine residues, heme or iron sulfur centers and tyrosine residue nitration [142]. NO also interacts with other signalling molecules and plant hormones. It activates H2O2, SA, ABA, JA and ethylene signalling pathways, which in turn increase the antioxidant activity and improve oxidative stress tolerance [143]. It has been demonstrated that ABA-H2O2-NO-MAPK (mitogen-activated protein kinase) together forms an antioxidant survival cycle [144].
It has been found that NO2--dependent NO production in Triticum aestivum roots increased under Cd stress [145]. Recently, a strong relationship has been established between ROS and RNS metabolism in two species of Brassica subjected to Zn stress. Oxidative components were the predominant players as compared to nitrosative stress [146]. ROS and RNS relationship have also been well studied in Arabidopsis where the alteration in their metabolism causes As-induced nitro-oxidative stress. In this context, As affects the activity of the two important enzymes involved in the glutathione metabolism (glutathione reductase and S-nitrosoglutathione reductase). This in turn decreases GSH and GSNO content and causes nitro-oxidative stress [147]. In a recent study conducted in model plant Arabidopsis, it has been demonstrated that Cd stress causes the over-production of peroxisomes, which are the endogenous source of peroxynitrite implicating a pivotal role of peroxisomes in response mechanism against heavy metal stress [148]. An interaction of NO with JA and ethylene as well as ROS has been proposed in Cd-stressed pea seedlings [99]. Hence, RNS metabolism appear to be an important determinant in the metal-stress perception and signalling [149] that needs to be investigated in more detail in future.
CONCLUSION
Metal stresses are one of the major culprits that limit the overall agricultural productivity. With the growing population, the need of the hour is to enhance food production even in adverse condition in order to satisfy the growing demand of food. In past few decades, there has been significant progress with the help of advancement in biotechnological approaches, molecular biology approaches and currently the ‘omics’ era. Various studies discussed above might not have included all the research conducted but provide conclusive evidences on the role of various phytohormones and plant growth regulators during metal stress along with cross-talks with ROS and RNS metabolisms. A lot of information about growth regulation by phytohormones is well known and hence it becomes apparent that phytohormones may also play crucial role in adaptive growth changes of plants during extended metal stress conditions. The future research needs to focus on integrating genomics, metabolomics and proteomics approaches to unravel integrative mechanism of metal-stress perception, signalling and responses.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
The research in SS lab is funded by University Grants Commission (UGC), Science & Engineering Research Board (SERB) and Banaras Hindu University (BHU), India.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.McBride M.B. Toxic metals in sewage sludge-amended soil: has proportion of beneficial use discounted the risks? Adv. Environ. Res. 2003;8:5–19. [Google Scholar]
- 2.Lone M.I., He Z., Stofella P.J., Yang X. Phytoremediation of heavy metal polluted soils and water progress and perspectives. J. Zhejiang Univ. Sci. B. 2008;9:210–220. doi: 10.1631/jzus.B0710633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh A., Prasad S.M. Reduction of heavy metal load in food chain: technology assessment. Rev. Environ. Sci. Biotechnol. 2011;10:199–214. [Google Scholar]
- 4.Dai J.Y., Chen L., Zhao J.F., Ma N. Characteristics of sewage sludge and distribution of heavy metal in plants with amendment of sewage sludge. J. Environ. Sci. (China) 2006;18:1094–1100. doi: 10.1016/s1001-0742(06)60045-4. [DOI] [PubMed] [Google Scholar]
- 5.Singh R.P., Agrawal M. Variation of heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol. Environ. Saf. 2010;73:632–641. doi: 10.1016/j.ecoenv.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 6.IARC International Agency for Research on Cancer. Beryllium, cadmium, mercury and exposure in glass manufacturing industry. IARC Monogr. Eval. Carcinog. Risks Hum. 1993;58:41–117. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao F.J., McGrath S.P., Meharg A. Arsenic as a food chain containment: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010;61:539–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 8.Verbruggen N., Hermans C., Schat H. Mechanisms to cope with arsenic and cadmium excess in Plants. Curr. Opin. Plant Biol. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Ye W.L., Wood B.A., Stoud J.L., Andralojc P.J., Raab A., McGrath S.P., Feldmann J., Zhao F.J. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol. 2010;154:1505–1513. doi: 10.1104/pp.110.163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z., Ren H., McGrath S.P., Wu P., Zhao F.J. Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol. 2011;157:1498–1508. doi: 10.1104/pp.111.178921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bienert G.P., Thorsen M., Schussler M.D., Nilsson H.R., Wagner A., Tamas M.J., Jahn T.P. A subgroup of plant aquaporins facilitates the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemen S. Toxic metal and accumulation, responses to exposure and mechanism of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Mendoza-Cozalt D.G., Jobe T.O., Hauser F., Schroeder J.I. Long distance transport, vacuolar sequestration, tolerance and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011;14:554–562. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancenon V., Puig S., Mira H., Thiele D.J., Penarrubia L. Identification of copper transporter family in Arabidopsis thaliana. Plant Mol. Biol. 2003;51:577–587. doi: 10.1023/a:1022345507112. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan P.M., Chen W. Arsenic toxicity: The effect on plant metabolism. Front. Plant Sci. 2012;3:182. doi: 10.3389/fphys.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava S., Suprasanna P., D’Souza S.F. Redox states and energetic equilibrium determine the magnitude of stress in Hydrilla verticillata upon exposure to arsenate. Protoplasma. 2011;248:805–816. doi: 10.1007/s00709-010-0256-z. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S., Srivastava A.K., Suprasanna P., D’Souza S.F. Identification and profiling of arsenic stress induced microRNA in Brassica juncea. J. Exp. Bot. 2013;64:303–315. doi: 10.1093/jxb/ers333. [DOI] [PubMed] [Google Scholar]
- 18.Argueso C.T., Raines T., Kieber J.J. Cytokinin signalling and transcriptional networks. Curr. Opin. Plant Biol. 2010;13:533–539. doi: 10.1016/j.pbi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Leyser Q. The power of auxin in plants. Plant Physiol. 2010;154:501–505. doi: 10.1104/pp.110.161323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du H., Wu N., Fu J., Wang S., Li X., Xiao J., Xiong L.A. GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012;63:6467–6480. doi: 10.1093/jxb/ers300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H., He L., Gu M. Interaction between nitric oxide and plant hormones in aluminium tolerance. Plant Signal. Behav. 2012;7:469–471. doi: 10.4161/psb.19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson S., Kudoyarova G.R., Veselov D.S., Arkhipova T.N., Davies W.J. Plant hormones interactions: innovative target for plant breeding and management. J. Exp. Bot. 2012;63:3499–3509. doi: 10.1093/jxb/ers148. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy A., Rathinasabapathi B. Auxin and its transport play role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ. 2013;36:1838–1849. doi: 10.1111/pce.12093. [DOI] [PubMed] [Google Scholar]
- 24.Quint M., Gray W.M. Auxin signalling. Curr. Opin. Plant Biol. 2006;9:448–453. doi: 10.1016/j.pbi.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koprivova A., North K.A., Kopriva S. Complex signalling network in regulation of adenosine-5′-phosphosulphate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008;146:1408–1420. doi: 10.1104/pp.107.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangwar S., Singh V.P., Srivastava P.K., Maurya J.N. Modification of chromium (VI) phytotoxicty by exogenous gibberellic acid application in Pisum sativa (L.) seedlings. Acta Physiol. Plant. 2011;33:1385–1397. [Google Scholar]
- 27.Gangwar S., Singh V.P., Prasad S.M., Maurya J.N. Differential responses of pea seedlings to IAA under manganese toxicity. Acta Physiol. Plant. 2011;33:451–462. [Google Scholar]
- 28.Rubio-Wilhelmi M.M., Sanchez-Rodriguez E., Rosales M.A., Begona B., Rios J.J., Romero L., Blumwald E., Ruiz J.M. Effects of cytokinin on oxidative stress in tobacco plants under nitrogen deficiency. Environ. Exp. Bot. 2011;72:167–173. [Google Scholar]
- 29.Luo Z.B., He J., Polle A., Rennenberg H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016;34:1131–1148. doi: 10.1016/j.biotechadv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Elobeid M., Gobel C., Feussner I., Polle A. Cadmium interferes with auxin physiology and lignification in Poplar. J. Exp. Bot. 2012;63:1413–1421. doi: 10.1093/jxb/err384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam Y.J., Tran L.S., Kojima M., Sakakibara H., Nishiyama R., Shin R. Regulatory roles of cytokinins and cytokinin signalling in response to potassium deficiency in Arabidopsis. PLoS One. 2012;7:e47797. doi: 10.1371/journal.pone.0047797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X.F., Jiang T., Wang Z.W., Lei G.J., Shi Y.Z., Li G.X. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012;239-240:302–307. doi: 10.1016/j.jhazmat.2012.08.077. [DOI] [PubMed] [Google Scholar]
- 33.Benkova E., Michiniewicz M., Sauer M., Teichmann T., Seifertova D., Jurgens G., Friml J. Local efflux dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 34.Kazan K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013;112:1655–1665. doi: 10.1093/aob/mct229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J.H., Yang Z.B. Is ABP1 an auxin receptor yet? Mol. Plant. 2011;4:635–640. doi: 10.1093/mp/ssr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurado S., Abraham Z., Manzano C., Lopez-Torrejon G., Pacios L.F., del Pozo J.C. The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell. 2010;22:3891–3904. doi: 10.1105/tpc.110.078972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C., Sun C., Shen C., Wang S., Liu F., Liu Y., Chen Y., Li C., Qian Q., Aryal B., Geisler M. Jiang de, A.; Qi, Y. The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J. 2015;83:818–830. doi: 10.1111/tpj.12929. [DOI] [PubMed] [Google Scholar]
- 38.Rai A., Singh R., Shirke P.A., Tripathi R.D., Trivedi P.K., Chakrabarty D. Expression of rice CYP450-like gene (Os08g01480) in Arabidopsis modulates regulatory network leading to heavy metal and other abiotic stress tolerance. PLoS One. 2015;10:e0138574. doi: 10.1371/journal.pone.0138574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H.M., Xu H.H., Liu W.C., Lu Y.T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 2013;54:766–778. doi: 10.1093/pcp/pct030. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y.F., Zhou G., Na X.F., Yang L., Nan W.B., Liu X., Zhang Y.Q., Li J.L., Bi Y.R. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 2013;170:965–975. doi: 10.1016/j.jplph.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Pandey C., Gupta M. Selenium and auxin mitigates arsenic stress in rice by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015;287:384–391. doi: 10.1016/j.jhazmat.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 42.Fassler E., Evengelou M.W., Robinson B.H., Schulin R. Effect of IAA on sunflower growth and heavy metal uptake in combination with ethylene diaminedisuccinic acid (EDDA). Chemosphere. 2010;80:901–907. doi: 10.1016/j.chemosphere.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 43.Hadi F., Bano A., Fuller M.P. The improved phytoextraction of lead and the growth of maize: The role of plant growth regulators and EDTA alone and in combination. Chemosphere. 2010;80:457–462. doi: 10.1016/j.chemosphere.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Perilli S., Moubayidin L., Sabatini S. The molecular basis of cytokinin function. Curr. Opin. Plant Biol. 2010;13:21–26. doi: 10.1016/j.pbi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Sakakibara H. Cytokinin: Activity, biosynthesis and translocation. Annu. Rev. Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- 46.Laplaze L., Benkova E., Casimiro I., Maes L., Vanneste S., Swarupm R., Weijers D., Calvo V., Parizot B., Herrera-Rodriguez M.B., Offringa R., Graham N., Doumas P., Friml J., Bogusz D., Beeckmann T., Benette M. Cytokinins act directly on lateral root founder cells to initiate root initiation. Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petricka J.J., Winter C.M., Benfey P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012;63:563–590. doi: 10.1146/annurev-arplant-042811-105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava S., Srivastava A.K., Suprasanna P., D’Souza S.F. Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J. Exp. Bot. 2009;60:3419–3431. doi: 10.1093/jxb/erp181. [DOI] [PubMed] [Google Scholar]
- 49.Mohan T.C., Castrillo G., Navarro C., Zarco-Fernandez S., Ramireddy E., Mateo C., Zanarreno A.M., Paz-Ares J., Munoz R., Garcia-Mina J.M., Hernandez L.E., Schmulling T., Leyva A. Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol. 2016;171:1418–1426. doi: 10.1104/pp.16.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lequeux H., Hermans C., Lutts S., Verbruggen N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010;48:673–682. doi: 10.1016/j.plaphy.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Hashem H.A. Cadmium toxicity induces lipid peroxidation and alters cytokine content and antioxidant enzymes activities in soybean. Botony. 2014;92:1–7. [Google Scholar]
- 52.Al-Hakimi A.M. Modification of cadmium toxicity in pea seedlings by kinetin. Plant Soil Environ. 2007;53:129–135. [Google Scholar]
- 53.Piotrowska-Niczyporuk A., Bajguz A., Zambrzycka E., Godlewska-ZylKiewicz B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga (Chlorella vulgaris). Plant Physiol. Biochem. 2012;52:52–65. doi: 10.1016/j.plaphy.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Vodnik D., Gaberscik A., Gogala N. Lead phtotoxicity in Norway spruce: the effects of lead and zeatin-riboside on root respiratory potential. Phyton. 1999;39:155–159. [Google Scholar]
- 55.Thomas J.C., Perron M., LaRosa P.C., Smigocki A.C. Cytokinin and the regulation of a tobacco metallothionein-like gene during copper stress. Physiol. Plant. 2005;123:262–271. [Google Scholar]
- 56.Hori S. Some observation on “Bakanae” disease of the rice plant. Memo. Agric. Res. Stn. 1898;12:110–119. [Google Scholar]
- 57.Matsuoka M. Gibberellin signalling: how do plant cells respond to GA signals? J. Plant Growth Regul. 2003;22:123–125. [Google Scholar]
- 58.Iqbal N., Nazar R., Khan M.I., Masood A., Khan N.A. Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions. Curr. Sci. 2011;100:998–1007. [Google Scholar]
- 59.Siddiqui M.H., Al-Whahibi M.H., Basalah M.O. Interactive effect of calcium and gibberellins on nickel tolerance in relation to antioxidant system in Triticum aestivum L. Protoplasma. 2011;248:503–511. doi: 10.1007/s00709-010-0197-6. [DOI] [PubMed] [Google Scholar]
- 60.Meng H., Hua S., Shamsi I.H., Jilani G., Li Y., Jiang L. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009;58:47–59. [Google Scholar]
- 61.Sharaf A.E., Farghal I.I., Sofy M.R. Role of gibberellic acid in abolishing the detrimental effects of cadmium and lead on the broad bean and lupin plants. Res. J. Agric. Biol. Sci. 2009;5:668–673. [Google Scholar]
- 62.Khan A.L., Lee I.J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013;13:86. doi: 10.1186/1471-2229-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan A.L., Waqas M., Hussain J., Al-Harrasi A., Hamayun M., Lee I.J. Phytohormones enabled endophytic fungal symbiosis improves aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J. Hazard. Mater. 2015;295:70–78. doi: 10.1016/j.jhazmat.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Holdsworth M.J., Bentsink L., Soppe W.J. Molecular network regulating Arabidopsis seed maturation after ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 65.Smith S., De Smet I. Root system architecture: Insight from Arabidopsis and cereal crops. Philos. Trans. R. Soc. Lond., B. 2012;367:1441–1452. doi: 10.1098/rstb.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang J., Hwang J.U., Lee M., Kim Y.Y., Assmann S.M., Martinoia E., Lee Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuromori T., Miyaji T., Yabuuchi H., Shimizu H., Sugimoto E., Kamiya A., Moriyama Y., Shinozaki K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey S., Nelson D.C., Assmann S.M. Two novel GPCR-type G-Proteins are abscisic acid receptor in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 69.Park S.Y., Fung P., Nishimura N., Jensen D.R., Fujii H., Zhao Y., Lumba S., Santiago J., Rodriguez A., Chow T.F., Alfred S.E., Bonetta D., Finkelstein R., Provart N.J., Desveaux D., Rodriguez P.L., McCourt P., Zhu J.K., Schroeder J.I., Volkman B.F., Cutler S.R. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen Y.Y., Wang X.F., Wu F.Q., Du S.Y., Cao Z., Shang Y., Wang X.L., Peng C.C., Yu X.C., Zhu S.Y. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- 71.Stroinski A., Gizewska K., Zielezinska M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013;57:121–127. [Google Scholar]
- 72.Kim Y.H., Khan A.L., Kim D.H., Lee S.Y., Kim K.M., Waqas M., Jung H.Y., Shin J.H., Kim J.G., Lee I.J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014;14:13. doi: 10.1186/1471-2229-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma S.S., Dietz K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- 74.Atici O., Agar G., Battal P. Changes in phytohormones contents in chickpea seeds germinating under lead or zinc stress. Biol. Plant. 2005;49:215–222. [Google Scholar]
- 75.Choudhary S.P., Kanwar M., Bhardwaj R., Yu J.Q., Tran L.S. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS One. 2012;7:e33210. doi: 10.1371/journal.pone.0033210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakrabarty D., Trivedi P.K., Mishra P., Tiwari M., Shri M., Shukla D., Kumar S., Rai A., Pandey A., Nigam D., Tripathi R.D., Tuli R. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009;74:688–702. doi: 10.1016/j.chemosphere.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 77.Wang J., Chen J., Pan K. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodesma crocephala Koidz under lead stress. Environ. Sci. Pollut. Res. Int. 2013;20:1441–1449. doi: 10.1007/s11356-012-1048-0. [DOI] [PubMed] [Google Scholar]
- 78.Shi W.G., Li H., Liu T.X., Polle A., Peng C.H., Luo Z.B. Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant Cell Environ. 2015;38:207–223. doi: 10.1111/pce.12434. [DOI] [PubMed] [Google Scholar]
- 79.Guo H., Ecker J.R. The ethylene signalling pathway: New insight. Curr. Opin. Plant Biol. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 80.Lewis D.R., Negi S., Sukumar P., Munday G.K. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011;138:3485–3495. doi: 10.1242/dev.065102. [DOI] [PubMed] [Google Scholar]
- 81.Swarup R., Perry P., Hagenbeek D., Van Der Staeten D., Beemster G.T., Sandberg G., Bhalerao R., Ljung K., Bennett M.J. Ethylene regulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markakis M.N., De Cnodder T., Lewandowski M., Simon D., Boron A., Balcerowicz D., Doubbo T., Taconnat L., Renou J.P., Hofte H., Verbelen J.P., Vissenberg K. Identification of genes involved in ACC-mediated control of root cell elongation in Arabidopsis thaliana. BMC Plant Biol. 2014;14:214. doi: 10.1186/1471-2229-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shan X., Yan J., Xie D. Comparison of phytohormones signalling mechanisms. Curr. Opin. Plant Biol. 2012;15:84–91. doi: 10.1016/j.pbi.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Keunen E., Schellingen K., Vangronsveld J., Cuypers A. Ethylene and metal stress: Small molecules, big molecules. Front. Plant Sci. 2016;7:23. doi: 10.3389/fpls.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arteca R.N., Arteca J.M. Heavy-metal-induced ethylene production in Arabidopsis thaliana. J. Plant Physiol. 2007;164:1480–1488. doi: 10.1016/j.jplph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 86.Masood A., Iqbal N., Khan N.A. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012;35:524–533. doi: 10.1111/j.1365-3040.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 87.Asgher M., Khan N.A., Khan M.I., Fatma M., Masood A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol. Environ. Saf. 2014;106:54–61. doi: 10.1016/j.ecoenv.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Vassilev A., Lidon F., Scotti P., Da Graca M., Yordanov I. Cadmium-induced changes in chloroplast lipids and photosystem activities in barley plants. Biol. Plant. 2004;48:153–156. [Google Scholar]
- 89.Khan M.I., Khan N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma. 2014;251:1007–1019. doi: 10.1007/s00709-014-0610-7. [DOI] [PubMed] [Google Scholar]
- 90.Khan M.I., Nazir F., Asgher M., Per T.S., Khan N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015;173:9–18. doi: 10.1016/j.jplph.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Ruduś I., Sasiak M., Kêpczyñski J. Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol. Plant. 2012;35:295–307. [Google Scholar]
- 92.Yoon G.M., Kieber J.J. 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants. 2013;5:plt017. [Google Scholar]
- 93.Schellingen K., Van Der Straeten D., Vandenbussche F., Prinsen E., Remans T., Vangronsveld J., Cuypers A. Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 2014;14:214. doi: 10.1186/s12870-014-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srivastava A.K., Venkatachalam P., Raghothama K.G., Sahi S.V. Identification of lead-regulated genes by suppression subtractive hybridization in the heavy metal accumulator Sesbania drummondii. Planta. 2007;225:1353–1365. doi: 10.1007/s00425-006-0445-3. [DOI] [PubMed] [Google Scholar]
- 95.Cao F., Chen F., Sun H., Zhang G., Chen Z-H., Wu F. Genome-wide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genomics. 2014;15:611. doi: 10.1186/1471-2164-15-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wasternack C., Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kombrink E. Chemical and genetic exploration of jasmonate biosynthesis and signalling paths. Planta. 2012;236:1351–1366. doi: 10.1007/s00425-012-1705-z. [DOI] [PubMed] [Google Scholar]
- 98.Yan Y., Stolz S., Chetelat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. A downstream mediator in the growth repression limb of the jasmonates pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pauwels L., Barbero G.F., Geerinck J., Tilleman S., Grunewald W., Perez A.C., Chico J.M., Bossche R.V., Sewell J., Gil E., García-Casado G., Witters E., Inzé D., Long J.A., De Jaeger G., Solano R., Goossens A. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464:788–791. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dar T.A., Moin U., Khan M.M., Hakeem K.R., Jaleel H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015;115:49–57. [Google Scholar]
- 101.Maksymiec W., Wójcik M., Krupa Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere. 2007;66:421–427. doi: 10.1016/j.chemosphere.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Serrano M., Romero-Puertas M.C., Pazmino D.M., Testillano P.S., Risueno M.C., del Rio L.A., Sandalio L.M. Cellular responses of pea plants to cadmium toxicity: Cross talk between ROS, Nitric oxide and calcium. Plant Physiol. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanaka A., Wojcik M., Dreslar S., Mroczek-Zdyrska M., Maksymiec W. Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with copper? Ecotoxicol. Environ. Saf. 2016;124:480–488. doi: 10.1016/j.ecoenv.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 104.Sirhindi G., Mir M.A., Sharma P., Gill S. Singh, Kaur Harpreet, Mushtaq, R. Modulatory role of jasmonic acid on photosynthesis pigments, antioxidants and stress makers of Glycine max L. under nickel stress. Physiol. Mol. Biol. Plants. 2015;21:559–565. doi: 10.1007/s12298-015-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poonam S., Kaur H., Geetika S. Effect of jasmonic acid on photosynthetic pigments and stress makers in Cajanus cajan (L.) Milsp. seedlings under copper stress. Am. J. Plant Sci. 2013;4:817–823. [Google Scholar]
- 106.Yu L., Luo Y.F., Liao B., Xie L.J., Chen L., Xiao S., Li J., Hu S., Shu W. Comparative transcriptomics analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol. 2012;195:97–112. doi: 10.1111/j.1469-8137.2012.04154.x. [DOI] [PubMed] [Google Scholar]
- 107.Maksymiec W. Effects of jasmonate and some other signalling factors on bean and onion growth during the initial phase of cadmium action. Biol. Plant. 2011;55:112–118. [Google Scholar]
- 108.Clouse S.D. Brassinosteroids. Arabidopsis Book. 2002;1:e0009. doi: 10.1199/tab.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim T.W., Wang Z.Y. Brassinosteroids signal transduction from receptor kinase to transcription factors. Annu. Rev. Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- 110.Sharma P., Kumar A., Bhardwaj R. Plant steroidal Hormone epibrassinolide regulate-Heavy metal stress tolerance in Oryza sativa L.by modulating antioxidant defense expression. Environ. Exp. Bot. 2016;122:1–9. [Google Scholar]
- 111.Li M., Ahammed G.J., Li C. Xiao, Bao.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroids ameliorates ZnO nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016;7:615. doi: 10.3389/fpls.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma P., Bhardwaj R. Efeects of 24-epibrassinolide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol. Plant. 2007;29:259–263. [Google Scholar]
- 113.Villiers F., Jourdain A., Bastein O., Leonhardt N., Fujioka S., Tichtincky G., Parcy F., Bouurguinon J., Hugouvieux V. Evidences for functional interaction between brassinosteroids and cadmium response in Arabidopsis thaliana. J. Exp. Bot. 2012;63:1185–1200. doi: 10.1093/jxb/err335. [DOI] [PubMed] [Google Scholar]
- 114.Ramakrishna B., Rao S.S. Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma. 2015;252:665–677. doi: 10.1007/s00709-014-0714-0. [DOI] [PubMed] [Google Scholar]
- 115.Yusuf M., Khan T.A., Fariduddin Q. Interaction of epibrassinolide and selenium ameliorates the excess copper in Brassica juncea through altered proline metabolism and antioxidants. Ecotoxicol. Environ. Saf. 2016;129:25–34. doi: 10.1016/j.ecoenv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Wu X.X., Chen J.L., Xu S., Zhu Z.W., Zha D.S. Exogenous 24-epibrasinosteroids alleviates zinc-induced toxicity in eggplant (Solanum melongena L.) seedlings by regulating the glutathione-ascorbate-dependent detoxification pathway. J. Hortic. Sci. Biotechnol. 2016;91:412–420. [Google Scholar]
- 117.Kanwar M.K., Bhardwaj R., Arora P., Chowdhary S.P., Sharma P., Kumar S. Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere. 2012;86:41–49. doi: 10.1016/j.chemosphere.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 118.Kanwar M.K. Poonam, Bhardwaj, R. Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol. Environ. Saf. 2015;115:119–125. doi: 10.1016/j.ecoenv.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 119.Rajewska I., Talarek M., Bajguz A. Brassinosteroids and responses of plants to heavy metal action. Front. Plant Sci. 2016;7:629. doi: 10.3389/fpls.2016.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumar D. Salicylic acid signalling in disease resistance. Plant Sci. 2014;228:127–134. doi: 10.1016/j.plantsci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 121.Buscail P., Rivas S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014;20:35–46. doi: 10.1016/j.pbi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Janda M., Ruelland E. Magical mystery tour: Salicylic acid signalling. Environ. Exp. Bot. 2014;114:117–128. [Google Scholar]
- 123.Boatwright J.L., Pajerowska-Mukhtar K. Salicylic acid: An old hormone up to new tricks. Mol. Plant Pathol. 2013;14:623–634. doi: 10.1111/mpp.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Belkadhi A., Djebali W., Hediji H., Chaibi W. Cellular and signalling mechanisms supporting Cd-tolerance in salicylic acid treated seedlings. Plant Sci. Today. 2016;3:41–47. [Google Scholar]
- 125.Singh V.P., Kumar J., Singh M., Singh S., Prasad S.M., Dwivedi R., Singh M.P. Role of salicylic acid-seed priming in the regulation of Cr(VI) and UV-B toxicity in maize seedlings. Plant Growth Regul. 2016;78:79–91. [Google Scholar]
- 126.Zhang F., Zhang H., Xia Y., Wang G., Xu L., Shen Z. Exogenous application of salicylic acid alleviates Cd-toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 2011;30:1475–1483. doi: 10.1007/s00299-011-1056-4. [DOI] [PubMed] [Google Scholar]
- 127.Shi G.R., Cai Q.S., Liu Q.Q., Wu L. Salicylic acid-mediated alleviation of cadmium-toxicity in hemp plants in relation to cadmium uptake, photosynthesis and antioxidant enzymes. Acta Physiol. Plant. 2009;31:969–977. [Google Scholar]
- 128.Singh A.P., Dixit G., Mishra S., Dwivedi S., Tiwari M., Mallick S., Pandey V., Trivedi P.K., Chakrabarty D., Tripathi R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015;6:340. doi: 10.3389/fpls.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kong J., Dong Y., Xu L., Liu S., Bai X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot. Stud. (Taipei, Taiwan) 2014;55:9. doi: 10.1186/1999-3110-55-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DalCorso G., Farinati S., Furini A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010;5:663–667. doi: 10.4161/psb.5.6.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gupta D.K., Inouhe M., Rodriguez-Serrano M., Romero-Puertas M.C., Sandalio L.M. Oxidative stress and arsenic toxicity: Role of NADPH oxidase. Chemosphere. 2013;90:1987–1996. doi: 10.1016/j.chemosphere.2012.10.066. [DOI] [PubMed] [Google Scholar]
- 132.Leymarie J., Vitkauskaite G., Hoang H.H., Gendreau E., Chazoule V., Meimoun P., Corbineau F., El-Maarouf-Bouteau H., Bailly C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012;53:96–106. doi: 10.1093/pcp/pcr129. [DOI] [PubMed] [Google Scholar]
- 133.Schopfer P. Hydroxyl radical-induced cell wall loosening in vitro and in vivo: Implications for the control of elongation growth. Plant J. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- 134.Iglesias M.J., Terrile M.C., Bartoli C.G., D’Ippolito S., Casalongue C.A. Auxin signalling participates in the adaptive response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol. Biol. 2010;74:215–222. doi: 10.1007/s11103-010-9667-7. [DOI] [PubMed] [Google Scholar]
- 135.Xia X.J., Wang Y.J., Zhou Y.H., Tao Y., Mao W.H., Shi K., Asami T., Chen Z., Yu J.Q. Reactive oxygen species are involved in brassinosteroids induced stress tolerance in cucumber. Plant Physiol. 2009;150:801–814. doi: 10.1104/pp.109.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiao Y., Sun L., Song Y., Wang L., Liu L., Zhang L., Liu B., Li N., Miao C., Hao F. AtrbohD and AtrbohF positively regulates primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J. Exp. Bot. 2013;64:4183–4192. doi: 10.1093/jxb/ert228. [DOI] [PubMed] [Google Scholar]
- 137.Desikan R., Last K., Harrett-Williams R., Tagliavia C., Harter K., Hooley R., Hancock J.T., Neil S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006;47:907–916. doi: 10.1111/j.1365-313X.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 138.Mou Z., Fan W., Dong X. Inducers of plant systemic acquired resistance regulates NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 139.Tada Y., Spoel S.H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., Zuo J., Dong X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miura K., Okamoto H., Okuma E., Shiba H., Kamada H., Hasegawa P.M., Murata Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 2013;73:91–104. doi: 10.1111/tpj.12014. [DOI] [PubMed] [Google Scholar]
- 141.Martinez M.C., Andriantsitohaina R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009;11:669–672. doi: 10.1089/ars.2007.1993. [DOI] [PubMed] [Google Scholar]
- 142.Gill S.S., Hasanuzzaman M., Nahar K., Macovei A., Tuteja N. Importance of nitric oxide in cadmium stress in crop plants. Plant Physiol. Biochem. 2013;63:254–261. doi: 10.1016/j.plaphy.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 143.Grun S., Lindermayr C., Sell S., Durner J. Nitric oxide and gene regulation in plants. J. Exp. Bot. 2016;57:507–516. doi: 10.1093/jxb/erj053. [DOI] [PubMed] [Google Scholar]
- 144.Hao G.P., Zhang J.H. The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In: Hayat S., Mori M., Pichtel J., Ahmad A., editors. Nitric Oxide In: Plant Physiology. Weinheim: Wiley-VCH Verlag; 2010. pp. 115–138. [Google Scholar]
- 145.Mahmood T., Gupta K.J., Kaiser W.M. Cadmium stress stimulates nitric oxide production by wheat roots. Pak. J. Bot. 2009;41:1285–1290. [Google Scholar]
- 146.Feigl G., Lehotai N., Molnar A., Ordog A., Rodriguez-Ruiz M., Palma J.M., Corpas F.J., Erdei L., Kolbert Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015;116:613–625. doi: 10.1093/aob/mcu246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leterrier M., Airaki M., Palma J.M., Chaki M., Barroso J.B., Corpas F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012;166:136–143. doi: 10.1016/j.envpol.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 148.Corpas F.J., Barroso J.B. Peroxynitrite (ONOO-) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Ann. Bot. 2014;113:87–96. doi: 10.1093/aob/mct260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arasimowicz-Jelonek M., Floryszak-Wieczorek J., Gwóźdź E.A. The message of nitric oxide in cadmium challenged plants. Plant Sci. 2011;181:612–620. doi: 10.1016/j.plantsci.2011.03.019. [DOI] [PubMed] [Google Scholar]