Abstract
Over the past 30 years, hypoglossal nerve stimulation has moved through a development pathway to become a viable treatment modality for patients with OSA. Initial pilot studies in animals and humans laid the conceptual foundation for this approach, leading to the development of fully implantable stimulating systems for therapeutic purposes. These devices were then shown to be both safe and efficacious in feasibility studies. One such closed-loop stimulating device was found to be effective in treating a limited spectrum of apneic patients and is currently approved by the US Food and Drug Administration for this purpose. Another open-loop stimulating system is currently being rigorously tested in a pivotal trial. Collectively, clinical trials of hypoglossal nerve stimulating systems have yielded important insights that can help optimize therapeutic responses to hypoglossal nerve stimulation. These insights include specific patient selection criteria and methods for delivering stimulation to specific portions of the hypoglossal nerve and/or genioglossus muscle. New approaches for activating efferent and afferent motor pathways are currently in early-stage laboratory development and hold some long-term promise as a novel therapy.
Key Words: hypoglossal nerve stimulation, OSA, upper airway stimulation
Abbreviations: AHI, apnea-hypopnea index; DISE, drug-induced endoscopy; IPG, implantable pulse generator; Pcrit, critical closing pressure
OSA is characterized by recurrent periods of upper airway obstruction (apneas and hypopneas) during sleep, leading to nocturnal hypercapnia, repeated oxyhemoglobin desaturations, and arousals from sleep.1 The prevalence of OSA is 24% to 27% in middle-aged men, 40% to 45% in older men, 9% in middle-aged women, and 25% to 30% in older women. Prevalence exceeds 50% in obesity, a potent risk factor for this disorder.2, 3, 4 OSA is a major cause of morbidity and mortality in Western society5, 6, 7, 8 and contributes significantly to the development and progression of neurocognitive, metabolic, cardiovascular, and oncologic diseases.9, 10, 11, 12, 13, 14
Nasal CPAP remains the mainstay for treating OSA and has proven efficacious in reducing the apnea-hypopnea index (AHI) and related oxyhemoglobin desaturations, as well as arousals from sleep.15 Unfortunately, suboptimal adherence severely limits its effectiveness,16 leading 5% to 50% of patients to discontinue treatment within the first week, and another 12% to 25% of patients by 3 years. Despite vigorous educational, behavioral, and technological advances, CPAP adherence remains challenging for a significant proportion of patients with OSA.17 Alternatives to CPAP include behavior modification (ie, weight loss, positional strategies, alcohol cessation), upper airway muscle exercises, intraoral appliances (eg, negative pressure devices, appliances designed to advance the jaw and tongue), and a variety of surgical procedures that remove soft tissue (eg, tonsillectomy, uvulopalatopharyngoplasty), widen the pharynx (eg, lateral pharyngoplasty, transpalatal advancement pharyngoplasty), and/or correct restriction from bony structures (hyoid suspension and maxillomandibular advancement).18 Over the past 25 years, electrical stimulation of the hypoglossal nerve, which innervates lingual muscles, has become a viable therapeutic approach for alleviating disturbances in neuromotor control during sleep. The present review examines the rationale, therapeutic approach, clinical outcomes, and future directions for this therapeutic modality.
Upper Airway Physiological Foundation
It is well recognized that upper airway neuromuscular activity plays a major role in the maintenance of pharyngeal patency during sleep. Several lines of evidence suggest that a fundamental defect in pharyngeal neuromuscular control is required for the pathogenesis of upper airway obstruction in OSA.19, 20 The lack of rigid bony support around the pharynx allows tissues to collapse when pharyngeal neuromuscular activity declines at sleep onset.21 In contrast, marked changes in neuromuscular activity during wakefulness modulate the collapsibility of the human upper airway from the choanae to the larynx,21, 22 and support highly specific tasks such as speech, swallowing, and breathing.21, 23
Disturbances in lingual and pharyngeal neuromuscular control play a critical role in the pathogenesis of OSA. Remmers et al24 first reported decrements in patients with OSA in the activity of the genioglossus, a major pharyngeal dilator muscle, just prior to upper airway closure, and marked increases in genioglossus electromyographic activity at apnea termination. Mezzanotte et al25 then showed that genioglossus activity was elevated in apneic individuals compared with normal individuals during wakefulness, leading the authors to postulate that apneic subjects are critically dependent on airway neuromuscular activity for the maintenance of airway patency during wakefulness. Patil et al22 further described defects in airway structural and neuromuscular control in apneic patients compared with well-matched normal individuals. Even after controlling for mechanical factors that compromise airway patency, these investigators found that a loss of compensatory neuromuscular responses played a pivotal role in the pathogenesis of airway obstruction during sleep.26 Disturbances in pharyngeal neuromuscular control can result from a loss of tonic and/or phasic neuromuscular activity,27, 28 reflecting decreased postural control and reflex responses to negative airway pressure.29 Reductions in neuromuscular tone have been closely linked to marked increases in the collapsibility of the pharynx in postmortem infants, heavily anesthetized animals, and sleeping humans.27 By contrast, marked reductions in collapsibility have been observed when neuromuscular activity is preserved in lightly anesthetized animals or awake healthy patients.30 These findings suggest that disturbances in lingual and pharyngeal neuromuscular control lead to increases in airway collapsibility during sleep.
Electrical Stimulation: Conceptual Development
Genioglossus Stimulation
Electrical stimulation of upper airway muscles was designed to augment dilator muscle tone and overcome defects in airway neuromuscular control. In early studies in anesthetized animals, investigators showed that electrical stimulation of the genioglossus can increase upper airway patency.31 Oliven et al32 suggested that improvements in airway patency during genioglossus stimulation in the anesthetized dog were related to its dilating and stiffening effects on pharyngeal structures. Further research found that reductions in pharyngeal collapsibility with electrical stimulation could be achieved by stimulating the proximal trunk and medial branch of the hypoglossal nerve, which primarily innervates the genioglossus muscle.31 Responses to genioglossus activation on airway collapsibility outpaced those observed from stimulating cervical strap muscles and other lingual muscles in the isolated feline airway.33 In contrast, severing the hypoglossal nerves in this model did not completely abolish responses in airway collapsibility to global stimulation of the airway musculature with CO2, suggesting that other pharyngeal and cervical muscles can also play a significant role in the maintenance of airway patency. Modest decreases in pharyngeal collapsibility were also elicited by stimulating the veli tensor palatini muscles, which stiffen the soft palate.34 Thus, evidence from animal studies highlights a major role for the genioglossus in maintaining pharyngeal patency, as well as an as yet undefined contribution from other lingual, pharyngeal, and cervical strap muscles.
Lingual Muscle Coactivation
The genioglossus can prevent the tongue from prolapsing into the pharynx and occluding the airway.25, 35, 36 Additional studies in rodents, however, suggested that other lingual muscles work in concert with the genioglossus to stabilize airway patency. Specifically, Fuller et al37 showed that marked increases in tongue protrudor (genioglossus) and retractor (styloglossus and hyoglossus) muscles during hypercapnic stimulation of the airway musculature, suggesting that both muscle groups play a role in stabilizing tongue structures when ventilatory drive is high. Mechanical effects of tongue protrudors, however, were different when they were activated with and without retractors. Although protrudors dilated the airway, they did not significantly decrease its collapsibility. In contrast, coactivating protrudors and retractors led to substantial decreases in pharyngeal collapsibility without significant airway dilation. These rodent studies suggest that tongue protrudors might be most effective in maintaining airway patency when they are coactivated with retractors, and that synergistic effects of these “antagonistic” muscles can stiffen and stabilize the tongue.
Further studies suggested that tongue protrudors and retractors both play a role in maintaining pharyngeal patency during sleep. Specifically, investigators documented markedly different activation patterns between sleep and wakefulness.38 Airway obstruction produced concomitant increases in both protrudor and retractor activity during wakefulness but only isolated protrudor activity with a loss of retractor activity during sleep.39 Moreover, combined electrical stimulation of protrudors and retractors during sleep led to greater reductions in pharyngeal collapsibility than did stimulating the protrudors alone.40 The findings suggest that costimulation of protrudors and retractors restores lingual muscle synergy and stabilizes airway patency during sleep.
Implantable Hypoglossal Stimulation Systems
Concurrent research in humans helped translate insights from anesthetized animals to sleeping humans. Early efforts using transcutaneous electrical stimulation of lingual muscles were confounded by concomitant arousals from sleep.37, 41 Shortly thereafter, investigators abandoned methods for stimulating tongue muscles transcutaneously in favor of intraoral superficial and fine wire electrodes.36, 42 In these studies, protrudors decreased and retractors markedly increased airflow obstruction during sleep.36 Moreover, combined stimulation of protrudors and retractors (via the proximal hypoglossal nerve) produced comparable increases in airway patency, suggesting a role for both tongue muscles in the maintenance of airway patency during sleep42, 43 (Fig 1). Thus, data accumulated from animal and human studies suggested therapeutic efficacy for stimulating the hypoglossal nerve to recruit lingual protrudors with and without retractors. These studies also spurred the development of suitable implantable technology for pacing the hypoglossal nerve during sleep.
Figure 1.
Putative effects of tongue protrudor and retractor stimulation. Upper left panel: resting tongue position and shape. Upper right panel: activation of lingual protrudors markedly dilates the oropharynx. Lower left panel: activation of retractors will lead to active pharyngeal occlusion. Lower right panel: coactivation of tongue protrudors and retractors is superior to protrudors alone in reduction of pharyngeal collapsibility without significant displacement of tongue body. (Illustration by Corinne Sandone, © 2016 Johns Hopkins University, used with permission.)
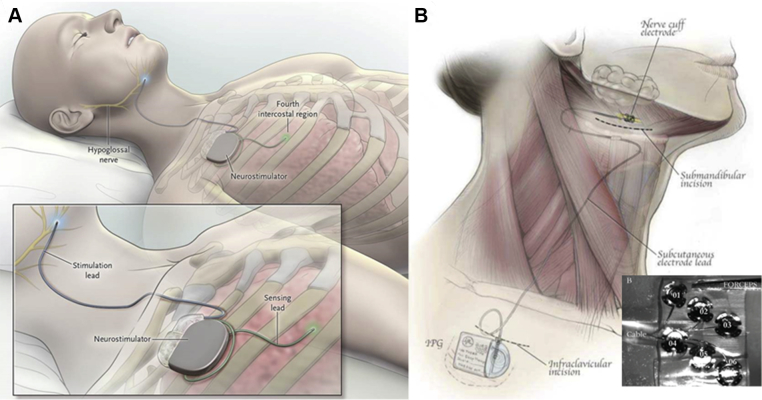
As investigators provided evidence for the potential efficacy of hypoglossal nerve stimulation, engineers worked in parallel to design fully implantable systems for treating OSA. In principle, these hypoglossal nerve stimulating systems incorporate several key components. An implantable pulse generator (IPG) is placed surgically in an infraclavicular subcutaneous pocket superficial to the pectoralis major. The IPG outputs electrical impulses to the hypoglossal nerve. Stimulation patterns are determined by the width, frequency, and amplitude of individual pulses, which sum over time to produce trains of a given burst duration. The bursts are transmitted through a lead to a cuff electrode that wraps around the hypoglossal nerve. Several closed-loop stimulating systems gate burst discharges with inspiration by means of an implantable chest sensor that monitors respiratory effort. The sensing and stimulation leads are tunneled subcutaneously from the IPG to the lower ribs and hypoglossal nerve, respectively. Open-loop stimulating systems deliver stimulation bursts independent of respiratory phase.
Stimulating systems have evolved significantly over the past 20 years. The first hypoglossal nerve stimulating system used a tripolar cuff electrode to stimulate the entire distal hypoglossal nerve.44 Newer technologies incorporated this design while refining methods for placing the cuff electrode on the distal nerve to target tongue protrudor muscles (Fig 2A).45 An alternative system was designed to target specific sectors of the proximal hypoglossal nerve with six unipolar stimulating contacts, which are furled circumferentially around the proximal nerve trunk (Fig 2B).
Figure 2.
Current systems for hypoglossal nerve stimulation. A, The stimulation lead is distally placed on the medial branch of the hypoglossal nerve to guarantee protrudor selection. The stimulating pulses are delivered via electrodes synchronized with ventilation detected by the sensing lead. B, The implantable six electrode cuff (detail) is furled around the hypoglossal nerve main trunk. Pulse generators are placed in an ipsilateral infraclavicular subcutaneous pocket in both systems.
(Adapted with permission from Eisele et al,43 Strollo et al,46 and Zaidi et al.47)
Each stimulating platform uses specific strategies to prevent overstimulating and fatiguing the hypoglossal nerve (Fig 3). In one embodiment (Medtronic, Apnex Medical, and Inspire Medical Systems), lingual protrudors are specifically targeted by placing the cuff electrode around the distal hypoglossal nerve. A respiratory sensor and related algorithms synchronize stimulation with inspiration while allowing for an unstimulated rest interval during expiration.28, 44, 46 Another system (ImThera Medical) places multiple stimulating electrodes around the proximal hypoglossal nerve, targeting specific fibers of the nerve. This system interposes rest intervals by sequentially stimulating sectors of the hypoglossal nerve trunk with discrete contacts.47, 48 Devices also differ in power supply with rechargeable or long-life precharged batteries, which are housed within the IPG. To date, nearly all implantable systems provide unilateral stimulation for the hypoglossal nerve. Another system (Nyxoah), however, is implanted around the genioglossus’ insertion at the mandibular symphysis (mental spine) bilaterally. It dissociates the stimulating interface from an external power source that is activated during sleep.49
Figure 3.
The anatomy and rationale for cuff placement. Left image shows the hypoglossal nerve and its branches to the retractors (styloglossus and hyoglossus) or protrudors (genioglossus and geniohyoid) muscles. Right image shows the two cuff placement sites, where the proximal site results in both group activations while the distal site selectively activates protrudors. Colors denote this difference (proximal results in all four arrows, distal only green arrows).
(Reproduced with permission from Schwab et al.57)
Hypoglossal Stimulation: Feasibility Studies
With the development of implantable hypoglossal nerve stimulating systems, early-stage feasibility trials were launched to examine the effects of stimulation on sleep apnea (Fig 4). The first such feasibility trial of distal nerve stimulation was conducted in eight patients with moderate to severe OSA, and it reported significant reductions in apnea-hypopnea indices.44 Subsequently, two single-arm interventional trials were conducted with similar technology on greater numbers of patients, and it reproduced comparable decreases in AHI with approximately 55% of patients receiving an implant fulfilling criteria for responses to surgical therapy.28 The latter trials resolved initial technical difficulties with hardware malfunction (IPG defects and lead fractures). In one such trial, however, favorable AHI responses were only achieved after significantly restricting patient inclusion criteria to those with a BMI < 32 kg/m2, AHI < 50 events per hour, and those with no evidence of concentric pharyngeal collapse on drug-induced sleep endoscopy (DISE). The latter suggested that hypoglossal nerve stimulation might not effectively treat patients with pronounced tongue and lateral pharyngeal collapse because they may have global rather than focal defects in pharyngeal neuromechanical control. The early success of the Inspire device may have also resulted from moving the electrode placement more distally on the hypoglossal nerve, effectively targeting tongue protrudor muscles rather than retractor muscles. Nonetheless, similar improvements in AHI have been shown with targeted stimulation of the proximal hypoglossal nerve over a somewhat wider range of apneic patients with a BMI < 35 kg/m2, an apnea index < 30 events per hour, and oxyhemoglobin desaturations < 10%.50 Taken together, these feasibility trials suggested therapeutic efficacy but that strict selection criteria with targeted stimulation of specific lingual muscles was required to optimize therapeutic responses.
Figure 4.
Results of pooled individual studies illustrating responses in mean apnea-hypopnea index outcome at 3, 6, and 12 months. Significant reductions were demonstrated with several stimulating platforms. HGNSS Hypoglossal nerve stimulation system.
(Reproduced with permission from Certal et al.53)
Hypoglossal nerve stimulation also yielded a range of therapeutic benefits with acceptable risks and tolerability. Objective improvements in sleep apnea were accompanied by clinically meaningful decreases in daytime sleepiness, mood, and daytime function. Although unblinded patient-reported outcomes are subjective and potentially biased, improvements in sleepiness and fatigue scales were comparable to those reported in CPAP-adherent patients with moderate to severe OSA.28, 45, 51 Procedure-related adverse events were limited to occasional instances of local infection28 and hardware dislodgements51 requiring either removal or replacement. Other minor adverse events included numbness and pain at the incision sites, as well as transient tongue paresis that resolved spontaneously on follow-up.48 Therapy-related adverse events included ventral tongue abrasion due to repetitive lingual movement over the lower incisors, which was managed with plastic dental guards.51 Nevertheless, pilot studies reported good overall tolerability and therapy usage, which seemed to exceed that seen with positive airway pressure therapy. Of note, tongue movement with stimulation did not produce significant discomfort or disrupt sleep. Thus, feasibility trials with different hypoglossal nerve stimulating systems offered strong evidence for therapeutic efficacy, with reasonable risks, tolerability, and acceptance, suggesting the need for definitive trials of these devices.
Hypoglossal Stimulation: Pivotal Trials
After reporting the overall efficacy of hypoglossal nerve stimulation, investigators launched prospective pivotal trials for two stimulating platforms. The Stimulation Therapy for Apnea Reduction (STAR) trial examined responses in sleep apnea, snoring, sleepiness, and quality of life to inspiratory stimulation of the distal hypoglossal nerve.46 This study consisted of a multicenter, single-arm intervention followed by a randomized controlled, therapy-withdrawal study in a subgroup of consecutive responders with participants serving as their own control subjects. In all, 126 patients underwent implantation following extensive screening of 929 apneic patients with polysomnography, clinical assessment, and DISE. Stringent criteria were applied to exclude patients with BMI > 32 kg/m2, AHI > 50 events per hour, central or positional sleep apnea, and any unfavorable anatomical feature or concentric palatal collapse. Intervention resulted in substantial decreases in sleep apnea severity (Fig 5) and resolution or significant improvement in sleep apnea in 66% of participants. Subjects also reported improvements in quality of life, snoring, and daytime sleepiness. Therapy withdrawal for 1 week in a subset of responders demonstrated recurrence of sleep apnea compared with treated control subjects. Long-term follow-up (at 36 and 60 months) showed that responses in sleep apnea and improvements in quality of life were sustained over time.45, 52 Comparable results have also been shown for a clinical registry of approximately 750 apneic patients, suggesting greater generalizability of the STAR trial results.53 Adverse events were limited to transient surgical complications and device dislodgement, with little deleterious impact on long-term therapeutic responses.51 The findings established the polysomnographic and clinical effectiveness of hypoglossal nerve stimulation for CPAP-intolerant patients with moderate to severe OSA.
Figure 5.
Primary outcomes at 12 months following implantation and during the randomized, therapy withdrawal. After 12 months of therapy, 46 consecutive participants who had a response to therapy were randomly assigned on equal ratio to the therapy-maintenance group or the therapy-withdrawal group (device turned off for at least 5 days). Significant difference was seen between the therapy-withdrawal group and the therapy-maintenance group. A, Apnea-hypopnea index; B, Oxygen desaturation index.
(Reproduced with permission from Strollo Jr et al.46)
Although the implant was effective in treating OSA, approximately one-third of patients receiving an implant in the STAR trial exhibited negligible or suboptimal responses to therapy.45, 46, 54 This finding challenges the long-held concept that the genioglossus is the major dilator muscle responsible for the maintenance of pharyngeal patency during sleep. An important long-term goal would be to identify predictors of therapeutic success with this modality that would enrich the population for responders by addressing specific fundamental physiologic and anatomic defects in airway control. In general, therapeutic responses to hypoglossal nerve stimulation can be enhanced by further refining patient selection and customizing the approach to stimulation delivery, as discussed in the next section.
Optimizing Therapeutic Responses to Hypoglossal Stimulation
Patient Selection
Upper Airway Collapsibility
Abundant evidence indicates the impact of upper airway collapsibility on sleep apnea pathogenesis and treatment responses. When airway collapsibility (as reflected by measurements of critical closing pressure [Pcrit]) rises during sleep toward or above atmospheric pressure, airflow obstruction results and sleep apnea ensues (Fig 6).55, 56 This outcome can be achieved most readily in patients with a baseline Pcrit in the minimally subatmospheric range, which predicts the presence of obstructive hypopneas rather than complete apneas. Pilot studies in apneic patients with a previous implant found that distal stimulation of the hypoglossal nerve decreased Pcrit by approximately 4.0 ± 2.3 cm H2O, suggesting that decreases in Pcrit below the –5 cm H2O threshold will be achieved mainly in patients with a baseline Pcrit near or below atmospheric pressure. In patients with baseline Pcrit above atmospheric pressure, however, electrical stimulation may not be sufficient to overcome anatomic loads and/or neuromuscular factors, which leave the pharynx more prone to obstruct. These findings advocate for assessing baseline Pcrit (or related surrogates) in selecting patients for hypoglossal nerve stimulation. Nonetheless, some variability in Pcrit responses to hypoglossal and genioglossal stimulation was observed, suggesting that other factors also play a role in determining the magnitude of the fall in Pcrit with therapy. Robust decreases in airway collapsibility may be achieved in those with retroglossal collapse, which enlarges more in responders than in nonresponders when the tongue moves forward and decompresses the oral cavity.57
Figure 6.
Effect of electrical stimulation of the genioglossus muscle with fine wire and of the distal hypoglossal nerve on Pcrit. Open circle stimulation (off), closed circle (on) diamond mean ± SD. Dashed red line indicates the target threshold for reductions in Pcrit below which sleep apnea remits. Pcrit = critical closing pressure.
(Reproduced with permission from Schwab et al.57)
Pharyngeal Shape
The shape of the pharyngeal lumen in the retropalatal and retroglossal segments and the overall size of the tongue may also predict stimulation responses. Leiter58 initially suggested that the tongue played a greater role in controlling pharyngeal patency in patients with an elliptical pharyngeal lumen oriented with its major axis in the lateral and small axis in the anterior-posterior dimension. Under these circumstances, a given degree of the tongue protrusion would produce greater increases in patency in patients with elliptical compared with concentric pharyngeal collapse (Fig 7). Support for this concept is provided by physiologic studies reporting much greater reductions in pharyngeal collapsibility in patients with predominantly anterior-posterior rather than lateral pharyngeal collapse under DISE.59 This concept can also account for apparent improvements in stimulation responses in this subgroup.54 Responses may be attenuated, however, in those with a bulky soft palate,57 suggesting that tongue and bony structures can also affect these responses.
Figure 7.
Relationship between the shape of the velopharynx and the response to electrical stimulation of the genioglossus. The shape is characterized by the ratio of the AP/L diameters. The response to stimulation is given by the change in the critical value of ΔPcrit during stimulation. The response to electrical stimulation was larger in patients with a narrow anteroposterior and large lateral diameter. Thick line, shadowed ellipse: baseline. Dashed line indicates shape during electrical stimulation. ΔPcrit = end-expiratory pressure; AP/L = anteroposterior to lateral. See Figure 6 legend for expansion of other abbreviation.
(Modified with permission from Dotan et al.59)
Site of Pharyngeal Collapse
Current evidence suggests that hypoglossal nerve stimulation can alleviate pharyngeal obstruction in both the retropalatal and retroglossal segments during natural and drug-induced sleep.57 Investigators previously found that hypoglossal nerve stimulation can relieve airflow obstruction in the retropalatal segment of experimental animals.31 The majority of apneic patients also exhibit retropalatal rather than retroglossal collapse as the primary site of airway obstruction during sleep,60 although secondary sites of obstruction can also be observed more distally.61 Mechanical coupling between the tongue and soft palate can account for improvements in retropalatal as well as retroglossal patency with genioglossus contraction, with concomitant decreases in the collapsibility and/or compliance of these pharyngeal segments.62 Increases in retropalatal patency can be further augmented by mandibular advancement, which allows for greater amounts of tongue protrusion within the oral cavity.63 Similarly, responders to hypoglossal nerve stimulation exhibited greater degrees of retropalatal enlargement than nonresponders, despite similar degrees of retroglossal widening.64 Responses to hypoglossal nerve stimulation may depend on the degree of palatoglossal coupling and/or spatial freedom within the maxillomandibular enclosure. Taken together, these findings suggest that anatomic assessment and prospectively assessing stimulation responses may predict stimulation responses.
Stimulation Delivery
Stimulation Titration
Pilot studies have also served to establish effects of stimulation intensity on upper airway patency during sleep. Investigators showed that responses in airway patency vary with stimulation amplitude, such that improvements in pharyngeal patency increase progressively once amplitude exceeds a capture threshold (Fig 8).65 Responses plateaued thereafter, and airway obstruction remitted completely in nearly one half of the patients but remained partially obstructed in the other half of the patients. The latter indicates that other pharyngeal muscles can play a significant role in the maintenance of airway patency during sleep and that methods are required to determine responses in selected patients.
Figure 8.
VImax response to increasing hypoglossal nerve stimulation current amplitude during non-rapid eye movement sleep for stimulated and unstimulated breaths. As stimulation current increased beyond the flow capture threshold, VImax increased linearly until the peak flow threshold was attained, at which point VImax plateaued as increasing stimulus current was applied. Note that inspiratory flow limitation persisted at intermediate current levels (closed circles). Further increases in current abolished inspiratory flow limitation (open circles). VImax = inspiratory airflow.
(Reproduced with permission from Schwartz et al.65)
Targeting Specific Genioglossus Fibers
The genioglossus comprises two major functional components with differing effects on pharyngeal patency. It is a fan-shaped muscle that originates from the mandibular symphysis with horizontal fibers that protrude and vertical fibers that depress the tongue.66, 67 Contracting the horizontal fibers leads to symmetric pharyngeal enlargement and reductions in pharyngeal collapsibility,59, 68 whereas isolated contraction of the vertical fibers can compress the tongue and compromise the posterior airspace59 without decreasing pharyngeal collapsibility. Reductions in airway collapsibility have been most closely associated with the dilating action of horizontal fibers, and are most pronounced in patients with a large tongue relative to the size of the bony enclosure. Physiological evidence suggests that therapeutic responses to hypoglossal nerve stimulation can be optimized by selectively targeting the horizontal fibers of the genioglossus, and they may be further enhanced by activating additional horizontal fibers in the geniohyoid through cervical spinal nerve 1 fibers in the hypoglossal nerve,69 particularly in patients with obstruction in the hypopharynx.64, 70
Targeting Other Lingual Muscles
Seminal findings in rodents and humans suggest that tongue protrudors and retractors act in synergy to stabilize pharyngeal patency during sleep.31, 37, 43, 71, 72, 73 This theory is supported by human studies showing that airway obstruction during sleep can elicit unopposed increases in genioglossus activity with a concomitant decrease in retractor activity that fails to stabilize airway patency in some cases40 (Fig 9).68, 72, 74 Rodent studies further show potential synergy between lingual protrudors and retractors.37, 40, 72, 74 These muscles appear to exert distinct dilating and stiffening effects on structures at specific sites of pharyngeal collapse, suggesting the need for personalized strategies for activating lingual muscles and stabilizing airway patency during sleep.
Figure 9.
EMG activity of protrudors and retractors during wakefulness and sleep in patients with OSA. During obstructed breathing, protrudor activity rose to levels that exceed those required to prevent pharyngeal collapse as seen in wakefulness. In contrast, in the absence of retractors coactivation, such robust activation of the genioglossus failed to prevent pharyngeal obstruction. EMG = electromyography.
(Reproduced with permission from Dotan et al.68)
Tonic vs Phasic Stimulation Patterns
Sleep is associated with a loss of phasic (inspiratory) and tonic activity to lingual and pharyngeal muscles that can predispose to pharyngeal obstruction.28, 56, 75 Despite OSA heterogeneity, inspiratory hypoglossal nerve stimulation has established therapeutic benefits.46 Nonetheless, during experimentally induced periods of airway obstruction, normal individuals mitigate the obstruction by recruiting tonic activity, whereas apneic patients do not,27 demonstrating an underlying pathogenic defect in tonic neuromuscular control. One stimulating platform currently in a pivotal clinical trial seeks to overcome the loss of neuromuscular activity with tonic rather than phasic stimulation of lingual muscles.47, 49 This strategy is supported by studies showing the therapeutic efficacy of isometric and isotonic oropharyngeal muscle training exercises,76, 77 and stands in contrast to supraphysiologic phasic hypoglossus stimulation, which more closely resembles quick phasic motions during speech and swallowing47, 78 rather than breathing. Previous studies have reported that asynchronous tonic hypoglossal nerve stimulation48, 50 for treating OSA is both efficacious and well tolerated.
The conceptual foundation for lingual muscle stimulation has been further developed by investigators showing that coordinated actions of multiple muscles determine the tongue’s position and shape. The tongue has been considered a muscular hydrostat, lacking skeletal support, capable of a broad range of complex movements and shapes. Its most important biomechanical characteristic is that it remains isovolemic, irrespective of muscle activation patterns. When muscles shorten the tongue in one dimension, a compensatory increase occurs in at least one more dimension. The interplay of multiple intrinsic and extrinsic tongue muscles are responsible for the wide dynamic range of tongue shape and movement.79 Current evidence suggests that balanced costimulation of opposing muscle groups can optimize the degree of tongue protrusion and stiffening required to maintain airway patency. Thus, different activation patterns can produce specific morphologic changes in posterior fatigue-resistant fibers47 that prevent retrograde prolapse by protruding the tongue and fixing its position (Fig 1).
Neural Stimulation: Future Directions
Upper Airway Efferent Stimulation
Current research in animals suggests that neuromodulating agents may ultimately provide a potent therapeutic alternative to electrical stimulation strategies. One approach involves amplifying adrenergic and serotoninergic input to respiratory motor neuron groups by administering direct agonists or antagonists to stimulatory or inhibitory receptors, respectively. In particular, yohimbine, an α2-adrenergic blocker, has been shown to recruit the genioglossus by disinhibiting specific pontine areas that project to upper airway motor neurons.80 Direct stimulation of these motor neurons can also be targeted by activating inward rectifying potassium 2.4 channels (Kir2.4), although selective agents have not yet been identified.81 Another approach aims to stimulate motor neurons by expressing specialized genetically modified transmembrane receptors on the cell surface. Designer receptors exclusively activated by designer drugs have been genetically engineered to recognize an inert ligand. After systemic administration of this ligand, genioglossus muscle activity and airway patency increase markedly in anesthetized82 and sleeping83 mice. Evidence also suggests that hormones such as leptin and oxytocin can improve upper airway patency in mice84, 85 and humans.86 Thus, neuropharmacologic, chemogenetic, and neuromodulatory agents may ultimately prove effective in stimulating upper airway motor neurons to treat sleep apnea.
Upper Airway Afferent Activation
Efferent motor pathways can also be stimulated by recruiting reflex afferent input to respiratory and upper airway motor control centers. Studies in humans and animals have suggested that compared with direct unilateral hypoglossal nerve stimulation, sciatic nerve stimulation,87 esophageal distention,88 electrical auricular stimulation,89 and pulsed nasal insufflation of heated and humidified air90 can activate and upregulate respiratory brainstem motor nuclei.91 Such reflexes seem to engage a coordinated brainstem response involving several cranial nerves and upper airway muscles, perhaps more broadly than targeted efferent stimulation approaches. Early findings suggest a role for sensory stimulation in ameliorating OSA.92, 93, 94
Conclusions
Investigators have charted a course for developing and applying hypoglossal nerve stimulation in the treatment of OSA. Initial pilot studies in animals and humans provided proof of concept for this approach, and impelled the development of fully implantable pacemakers for therapeutic purposes. The approach was then demonstrated in feasibility studies conducted with several stimulating platforms to be safe and efficacious. One such device is currently approved by the US Food and Drug Administration while another is in pivotal trials. Collectively, clinical trials have yielded important insights that can be used to optimize therapeutic responses. These include specific patient selection criteria and methods for delivering stimulation to various portions of the hypoglossal nerve and/or genioglossus muscle. Promising new neurohumoral and molecular approaches for activating efferent and afferent motor pathways are currently in early-stage laboratory development.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: Funding for the ImThera study described in this publication was provided by LivaNova. A. R. S. is also a paid consultant and advisory board member for LivaNova. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. None declared (T. F. C., A. O., L. U. S., V. Y. P., D. E.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Institutes of Health (NIH) under Grants NIH R01HL138932-01 and R01HL128970 to Dr Polotsky and R01 HL144859-01 to Dr Schwartz and the Coordination for the Improvement of Higher Education Personnel (CAPES) under grant PDSE 99999.010894/2014-04 and American Heart Association under 16POST31000017 to Dr Fleury Curado.
References
- 1.Gastaut H., Tassinari C.A., Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1(2):167–186. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Young T., Peppard P.E., Gottlieb D.J. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Strohl K.P., Brown D.B., Collop N. An official American Thoracic Society clinical practice guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. Am J Respir Crit Care Med. 2013;187(11):1259–1266. doi: 10.1164/rccm.201304-0726ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gami A.S., Howard D.E., Olson E.J., Somers V.K. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 7.Marshall N.S., Wong K.K., Liu P.Y., Cullen S.R., Knuiman M.W., Grunstein R.R. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 8.Punjabi N.M., Caffo B.S., Goodwin J.L. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drager L.F., Bortolotto L.A., Figueiredo A.C., Krieger E.M., Lorenzi-Filho G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 10.Drager L.F., Lopes H.F., Maki-Nunes C. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drager L.F., Togeiro S.M., Polotsky V.Y., Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto F.J., Peppard P.E., Young T., Finn L., Hla K.M., Farre R. Sleep disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peppard P.E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K., Laffan A.M., Harrison S.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay P., Weaver T., Loube D., Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer A.M., Gooneratne N.S., Marcus C.L., Ofer D., Richards K.C., Weaver T.E. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engleman H.M., Wild M.R. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7(1):81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 18.Spicuzza L., Caruso D., Di Maria G. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis. 2015;6(5):273–285. doi: 10.1177/2040622315590318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onal E., Lopata M., O’Connor T.D. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50(5):1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 20.Olson LG, Fouke JM, Hoekje PL, Strohl KP. A biomedical view of upper airway function. In: Mathew OP, Sant’Ambrogio G, eds. Respiratory Function of the Upper Airway. New York, NY: Marcel Dekker; 1988:35:359-389.
- 21.Eckert D.J., Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil S.P., Schneider H., Schwartz A.R., Smith P.L. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132(1):325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eikermann M., Jordan A.S., Chamberlin N.L. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131(6):1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmers J.E., deGroot W.J., Sauerland E.K., Anch A.M. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 25.Mezzanotte W.S., Tangel D.J., White D.P. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Investig. 1992;89(5):1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin C.H., Kirkness J.P., Patil S.P. Compensatory responses to upper airway obstruction in obese apneic men and women. J Appl Physiol. 2012;112(3):403–410. doi: 10.1152/japplphysiol.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinley B.M., Schwartz A.R., Schneider H., Kirkness J.P., Smith P.L., Patil S.P. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105(1):197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kezirian E.J., Goding G.S., Jr., Malhotra A. Hypoglossal nerve stimulation improves obstructive sleep apnoea: 12-month outcomes. J Sleep Res. 2014;23(1):77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suratt P.M., Wilhoit S.C., Atkinson R.L. Elevated pulse flow resistance in awake obese subjects with obstructive sleep apnea. Am Rev Respir Dis. 1983;127(2):162–165. doi: 10.1164/arrd.1983.127.2.162. [DOI] [PubMed] [Google Scholar]
- 30.Vranish J.R., Bailey E.F. A comprehensive assessment of genioglossus electromyographic activity in healthy adults. J Neurophysiol. 2015;113(7):2692–2699. doi: 10.1152/jn.00975.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz A.R., Thut D.C., Russ B. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis. 1993;147(5):1144–1150. doi: 10.1164/ajrccm/147.5.1144. [DOI] [PubMed] [Google Scholar]
- 32.Oliven A., Odeh M., Schnall R.P. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213–216. doi: 10.1159/000196547. [DOI] [PubMed] [Google Scholar]
- 33.Eisele D.W., Schwartz A.R., Hari A., Thut D.C., Smith P.L. The effects of selective nerve stimulation on upper airway airflow mechanics. Arch Otolaryngol Head Neck Surg. 1995;121(12):1361–1364. doi: 10.1001/archotol.1995.01890120021004. [DOI] [PubMed] [Google Scholar]
- 34.McWhorter A.J., Rowley J.A., Eisele D.W., Smith P.L., Schwartz A.R. The effect of tensor veli palatini stimulation on upper airway patency. Arch Otolaryngol Head Neck Surg. 1999;125(9):937–940. doi: 10.1001/archotol.125.9.937. [DOI] [PubMed] [Google Scholar]
- 35.Strohl M.D.K.P., Baskin M.D.J., Lance M.D.C. Origins of and implementation concepts for upper airway stimulation therapy for obstructive sleep apnea. Respir Investig. 2016;54(4):241–249. doi: 10.1016/j.resinv.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz A.R., Eisele D.W., Hari A., Testerman R., Erickson D., Smith P.L. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643–652. doi: 10.1152/jappl.1996.81.2.643. [DOI] [PubMed] [Google Scholar]
- 37.Fuller D., Mateika J.H., Fregosi R.F. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507(pt 1):265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dotan Y., Pillar G., Tov N. Dissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesia. Eur Respir J. 2013;41(1):74–84. doi: 10.1183/09031936.00159611. [DOI] [PubMed] [Google Scholar]
- 39.McNicholas W.T., Bowes G., Zamel N., Phillipson E.A. Impaired detection of added inspiratory resistance in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;129(1):45–48. doi: 10.1164/arrd.1984.129.1.45. [DOI] [PubMed] [Google Scholar]
- 40.Oliven A., Odeh M., Geitini L. Effect of co-activation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol. 2007;103(5):1662–1668. doi: 10.1152/japplphysiol.00620.2007. [DOI] [PubMed] [Google Scholar]
- 41.Miki H., Hida W., Chonan T., Kikuchi Y., Takishima T. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis. 1989;140(5):1285–1289. doi: 10.1164/ajrccm/140.5.1285. [DOI] [PubMed] [Google Scholar]
- 42.Oliven A., O’Hearn D.J., Boudewyns A. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95(5):2023–2029. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 43.Eisele D.W., Smith P.L., Alam D.S., Schwartz A.R. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1997;123(1):57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz A.R., Bennett M.L., Smith P.L. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127(10):1216–1223. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- 45.Woodson B.T., Strohl K.P., Soose R.J. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Neck Surg. 2018;159(1):194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 46.Strollo P.J., Jr., Soose R.J., Maurer J.T. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 47.Zaidi F.N., Meadows P., Jacobowitz O., Davidson T.M. Tongue anatomy and physiology, the scientific basis for a novel targeted neurostimulation system designed for the treatment of obstructive sleep apnea. Neuromodulation. 2013;16(4):376–386. doi: 10.1111/j.1525-1403.2012.00514.x. discussion 386. [DOI] [PubMed] [Google Scholar]
- 48.Mwenge G.B., Rombaux P., Dury M., Lengele B., Rodenstein D. Targeted hypoglossal neurostimulation for obstructive sleep apnoea: a 1-year pilot study. Eur Respir J. 2013;41(2):360–367. doi: 10.1183/09031936.00042412. [DOI] [PubMed] [Google Scholar]
- 49.Hormann K., Sommer J.U. innovative surgery for obstructive sleep apnea: nerve stimulator. Adv Otorhinolaryngol. 2017;80:116–124. doi: 10.1159/000470880. [DOI] [PubMed] [Google Scholar]
- 50.Friedman M., Jacobowitz O., Hwang M.S. Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: six-month results. Laryngoscope. 2016;126(11):2618–2623. doi: 10.1002/lary.25909. [DOI] [PubMed] [Google Scholar]
- 51.Eastwood P.R., Barnes M., Walsh J.H. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479–1486. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodson B.T., Soose R.J., Gillespie M.B. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg. 2016;154(1):181–188. doi: 10.1177/0194599815616618. [DOI] [PubMed] [Google Scholar]
- 53.Certal V.F., Zaghi S., Riaz M. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125(5):1254–1264. doi: 10.1002/lary.25032. [DOI] [PubMed] [Google Scholar]
- 54.Van de Heyning P.H., Badr M.S., Baskin J.Z. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122(7):1626–1633. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 55.Gold A.R., Schwartz A.R. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110(4):1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 56.Carberry J.C., Jordan A.S., White D.P., Wellman A., Eckert D.J. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep. 2015;39(3):511–521. doi: 10.5665/sleep.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwab R.J., Wang S.H., Verbraecken J. Anatomic predictors of response and mechanism of action of upper airway stimulation therapy in patients with obstructive sleep apnea. Sleep. 2018;41(4) doi: 10.1093/sleep/zsy021. [DOI] [PubMed] [Google Scholar]
- 58.Leiter J.C. Upper airway shape: Is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med. 1996;153(3):894–898. doi: 10.1164/ajrccm.153.3.8630569. [DOI] [PubMed] [Google Scholar]
- 59.Dotan Y., Golibroda T., Oliven R., Netzer A., Gaitini L., Toubi A. Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J. 2011;38(2):338–347. doi: 10.1183/09031936.00125810. [DOI] [PubMed] [Google Scholar]
- 60.Morrison D.L., Launois S.H., Isono S., Feroah T.R., Whitelaw W.A., Remmers J.E. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;148(3):606–611. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 61.Kotecha B.T., Hannan S.A., Khalil H.M.B., Georgalas C., Bailey P. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol. 2007;264(11):1361–1367. doi: 10.1007/s00405-007-0366-1. [DOI] [PubMed] [Google Scholar]
- 62.Isono S., Tanaka A., Nishino T. Effects of tongue electrical stimulation on pharyngeal mechanics in anaesthetized patients with obstructive sleep apnoea. Eur Respir J. 1999;14(6):1258–1265. doi: 10.1183/09031936.99.14612589. [DOI] [PubMed] [Google Scholar]
- 63.Oliven R., Tov N., Odeh M. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol. 2009;106(5):1668–1673. doi: 10.1152/japplphysiol.91501.2008. [DOI] [PubMed] [Google Scholar]
- 64.Safiruddin F., Vanderveken O.M., de Vries V.N., Maurer J.T., Lee K., Ni Q. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J. 2015;45(1):129–138. doi: 10.1183/09031936.00059414. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz A.R., Barnes M., Hillman D. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(4):420–426. doi: 10.1164/rccm.201109-1614OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mu L., Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat. 2010;23(7):777–791. doi: 10.1002/ca.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delaey P., Duisit J., Behets C., Duprez T., Gianello P., Lengelé B. Specific branches of hypoglossal nerve to genioglossus muscle as a potential target of selective neurostimulation in obstructive sleep apnea: anatomical and morphometric study. Surg Radiol Anat. 2017;39(5):507–515. doi: 10.1007/s00276-016-1778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dotan Y., Pillar G., Schwartz A.R., Oliven A. Asynchrony of lingual muscle recruitment during sleep in obstructive sleep apnea. J Appl Physiol. 2015;118(12):1516–1524. doi: 10.1152/japplphysiol.00937.2014. [DOI] [PubMed] [Google Scholar]
- 69.Heiser C. Advanced titration to treat a floppy epiglottis in selective upper airway stimulation. Laryngoscope. 2016;126(suppl 7):S22–S24. doi: 10.1002/lary.26118. [DOI] [PubMed] [Google Scholar]
- 70.Heiser C., Edenharter G., Bas M., Wirth M., Hofauer B. Palatoglossus coupling in selective upper airway stimulation. Laryngoscope. 2017;127(10):E378–E383. doi: 10.1002/lary.26487. [DOI] [PubMed] [Google Scholar]
- 71.Kezirian E.J., Boudewyns A., Eisele D.W. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14(5):299–305. doi: 10.1016/j.smrv.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Fuller D., Williams J.S., Janssen P.L., Fregosi R.F. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519(pt 2):601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowley J.A., Williams B.C., Smith P.L., Schwartz A.R. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am J Respir Crit Care Med. 1997;156(2 pt 1):515–521. doi: 10.1164/ajrccm.156.2.9607115. [DOI] [PubMed] [Google Scholar]
- 74.Fregosi R.F., Fuller D.D. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol. 1997;110(2-3):295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 75.Eckert D.J., White D.P., Jordan A.S., Malhotra A., Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guimaraes K.C., Drager L.F., Genta P.R., Marcondes B.F., Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179(10):962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 77.Puhan M.A., Suarez A., Lo C.C., Zahn A., Heitz M., Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. Br Med J. 2006;332(7536):266–270. doi: 10.1136/bmj.38705.470590.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meadows P.M., Whitehead M.C., Zaidi F.N. Effects of targeted activation of tongue muscles on oropharyngeal patency in the rat. J Neurol Sci. 2014;346(1-2):178–193. doi: 10.1016/j.jns.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 79.Sanders I., Mu L. A 3-dimensional atlas of human tongue muscles. Anat Rec (Hoboken) 2013;296(7):1102–1114. doi: 10.1002/ar.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song G., Poon C.S. α2-Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea. JCI Insight. 2017;2(4):e914556. doi: 10.1172/jci.insight.91456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grace K.P., Hughes S.W., Shahabi S., Horner R.L. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol. 2013;188(3):277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Curado T.F., Fishbein K., Pho H. Chemogenetic stimulation of the hypoglossal neurons improves upper airway patency. Sci Rep. 2017;7:44392. doi: 10.1038/srep44392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horton G.A., Fraigne J.J., Torontali Z.A. Activation of the hypoglossal to tongue musculature motor pathway by remote control. Sci Rep. 2017;7:45860. doi: 10.1038/srep45860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meister B., Håkansson M.L. Circumventricular organs: gateways to the brainleptin receptors in hypothalamus and circumventricular organs. Clin Exp Pharmacol Physiol. 2002;28(7):610–617. doi: 10.1046/j.1440-1681.2001.03493.x. [DOI] [PubMed] [Google Scholar]
- 85.Polotsky M., Elsayed-Ahmed A.S., Pichard L. Effects of leptin and obesity on the upper airway function. J Appl Physiol (1985) 2012;112(10):1637–1643. doi: 10.1152/japplphysiol.01222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain V., Marbach J., Kimbro S. Benefits of oxytocin administration in obstructive sleep apnea. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L825–L833. doi: 10.1152/ajplung.00206.2017. [DOI] [PubMed] [Google Scholar]
- 87.Haxhiu M.A., Van L.E., Mitra J., Cherniack N.S., Strohl K.P. Comparison of the responses of the diaphragm and upper airway muscles to central stimulation of the sciatic nerve. Respir Physiol. 1984;58(1):65–76. doi: 10.1016/0034-5687(84)90045-8. [DOI] [PubMed] [Google Scholar]
- 88.Daulatzai M.A. Role of sensory stimulation in amelioration of obstructive sleep apnea. Sleep Disord. 2011;2011:596879. doi: 10.1155/2011/596879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donic V., Tomori Z., Gresova S. Treatment of sleep apnea syndrome by electrical auricle stimulation using miniaturized system of second generation. Sleep Med. 2017;40:e80–e81. [Google Scholar]
- 90.Sowho M.O., Woods M.J., Biselli P., McGinley B.M., Buenaver L.F., Kirkness J.P. Nasal insufflation treatment adherence in obstructive sleep apnea. Sleep Breath. 2015;19(1):351–357. doi: 10.1007/s11325-014-1027-4. [DOI] [PubMed] [Google Scholar]
- 91.Hernández A.I., Pérez D., Feuerstein D. Kinesthetic stimulation for obstructive sleep apnea syndrome: an “on-off” proof of concept trial. Sci Rep. 2018;8:3092. doi: 10.1038/s41598-018-21430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deegan P.C., Mulloy E., McNicholas W.T. Topical oropharyngeal anesthesia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(4):1108–1112. doi: 10.1164/ajrccm/151.4.1108. [DOI] [PubMed] [Google Scholar]
- 93.Horner R.L. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19(10):827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 94.Wirth K.J., Steinmeyer K., Ruetten H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: investigations with AVE0118 in anesthetized pigs. Sleep. 2013;36(5):699–708. doi: 10.5665/sleep.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]