Double-strand breaks (DSBs) are purposefully made during meiosis and must be repaired by homologous recombination (HR) to achieve a crossover. In mitotic cells, alternative repair pathways compete with HR for repair; however, little...
Keywords: DNA repair, meiosis, double-strand break, C. elegans, pathway choice, WormBase
Abstract
Double-strand breaks (DSBs) are among the most deleterious lesions DNA can endure. Yet, DSBs are programmed at the onset of meiosis, and are required to facilitate appropriate reduction of ploidy in daughter cells. Repair of these breaks is tightly controlled to favor homologous recombination (HR)—the only repair pathway that can form crossovers. However, little is known about how the activities of alternative repair pathways are regulated at these stages. We discovered an unexpected synthetic interaction between the DSB machinery and strand-exchange proteins. Depleting the Caenorhabditis elegans DSB-promoting factors HIM-5 and DSB-2 suppresses the formation of chromosome fusions that arise in the absence of RAD-51 or other strand-exchange mediators. Our investigations reveal that nonhomologous and theta-mediated end joining (c-NHEJ and TMEJ, respectively) and single strand annealing (SSA) function redundantly to repair DSBs when HR is compromised, and that HIM-5 influences the utilization of TMEJ and SSA.
DNA is under constant stress from endogenous and exogenous sources. The resulting lesions must be repaired to ensure genome integrity. In the germ line, improperly repaired damage can lead to inherited mutations or chromosomal rearrangements, which are often associated with heritable predispositions to cancer, recurrent miscarriage, and genetic disorders. Double-strand breaks (DSBs) are considered the most toxic type of lesion to the genome, and several pathways exist to repair them. The mechanism of repair depends on DSB structure and cell cycle phase (Shrivastav et al. 2008; Chang et al. 2017).
Cells in S and G2 phases primarily repair DSBs by homologous recombination (HR), which relies on the presence of a homologous template (the attached sister chromatid or the homologous chromosome) to ensure the most accurate repair. Alternatively, canonical nonhomologous end-joining (c-NHEJ) occurs by direct ligating the blunt ends of the DSB without using DNA sequence homology (Branzei and Foiani 2008). Cells leverage c-NHEJ throughout the whole cell cycle (G1, S, G2). Repair by c-NHEJ may generate mutations or chromosome rearrangements, but it can also be conservative if no base is lost after DSB formation (Eid et al. 2010). Although it seems that the c-NHEJ machinery may be involved in the repair of more intricate DSB structures (Löbrich and Jeggo 2017), other DSB repair pathways are error-prone by nature because they function by annealing sequence repeats. Alternative end-joining (alt-EJ, also called microhomology-mediated end-joining) relies on microhomologies, generally <10 nucleotides, to ligate cohesive DSB ends, and results in DNA insertions and deletions (Ma et al. 2003; Martin et al. 2005; Corneo et al. 2007; Yan et al. 2007; Sakuma et al. 2016). Theta-mediated end-joining (TMEJ) is a subtype of alt-EJ that depends on the polymerase theta (polɵ). TMEJ can anneal sequences sharing as few as one nucleotide of homology at the junction (Schendel et al. 2016). Similarly, but mechanistically independent, single-strand annealing (SSA) can anneal longer sequence repeats (>20 nt) (Maryon and Carroll 1991; Fishman-Lobell et al. 1992). Thus, SSA and TMEJ can cause mutations, and may be involved in the appearance of more complex chromosome rearrangements (reviewed in Bhargava et al. 2016).
Despite their potential toxicity, programmed DSBs occur at the onset of meiotic prophase I to induce the formation of crossovers (COs). COs are reciprocal exchanges of genetic material that must occur between each pair of homologs. They form a physical link—the chiasma—that is essential to prevent chromosome nondisjunction. Meiotic DSBs are catalyzed by the conserved and meiosis-specific topoisomerase-like protein SPO11 (Keeney et al. 1997). They are repaired preferentially by HR because it is the only repair pathway that forms COs to guarantee genomic integrity in the resulting gametes. Accordingly, mechanisms must exist in the germ line to ensure that HR predominates over other repair pathways. While many factors promoting HR have been described, it remains poorly understood how alternative repair pathways are suppressed in the germ line (Ranjha et al. 2018).
When HR is impaired, c-NHEJ has a well-demonstrated role in meiotic DSB repair (Clejan et al. 2006). More recently, TMEJ was shown to participate in DNA repair in C. elegans germ cells, although the timing of this repair is unknown (Schendel et al. 2016). Notably, SSA has been invoked in repair of DSBs on the X chromosome of hemizigous male nematodes (Checchi et al. 2014). Further investigation is needed to decipher how meiotic cells restrict TMEJ and SSA activities. Additionally, although it is known that c-NHEJ predominates in HR-deficient germ lines, it remains unclear whether c-NHEJ competes with HR, coordinates with HR machinery, or simply operates when HR is compromised (Clejan et al. 2006).
In Caenorhabditis elegans, like in most sexually reproducing eukaryotes, the formation of meiotic DSBs and their repair through HR is an essential event for proper chromosome disjunction. Recent studies of meiosis in HR-impaired animals indicate that c-NHEJ is restricted early during DSB processing by the formation of single-stranded DNA (ssDNA) to which the c-NHEJ machinery cannot bind. The formation of ssDNA around the DSB site is achieved by 5′-3′ resection of the double-stranded DNA. The MRN complex (MRE-11, RAD-50, NBS-1), together with COM-1 (the worm homolog of the tumor suppressor CtIP/Ctp1/Sae2) nick the DNA surrounding the catalyzed lesion, thereby removing SPO-11 with its attached small DNA oligo (Garcia et al. 2011). Further end resection generates a ssDNA template suitable for binding the RecA-like recombinases, RAD-51 or its meiotic-specific paralog DMC1 (Ma et al. 2015). In the absence of com-1, the Ku-complex (encoded by cku-70 and cku-80 in worms) can associate with the DSB and impede extended resection mediated by the exonuclease EXO-1, thereby facilitating end-to-end ligation by c-NHEJ (Lemmens et al. 2013). It has been proposed that meiotic inhibition of c-NHEJ is a result of competition between MRE-11/COM-1 and the Ku-complex for access to the DSB site. The extended resection by EXO-1 further prevents Ku binding (Lee et al. 1998; Zhou et al. 2014). It thus appears that early DSB-processing events are coordinated to promote HR over c-NHEJ.
The long single-stranded overhangs are bound by Rad51 and Dmc1 proteins, which drive strand invasion and the search for extended sequence homology. In worms, this strand-exchange activity relies on RAD-51, the only RecA homolog found in this species (Takanami et al. 1998). Subsequent repair using the homolog as a template eventually leads to formation of COs. Interestingly, the process of resection also produces DNA intermediates that are compatible with alternative repair pathways, including TMEJ and SSA. However, the role and inhibition of these repair pathways in meiosis remain poorly understood and mostly unexplored.
We report here that HIM-5 and DSB-2—two factors whose primary function is facilitating meiotic DSB induction—also promote HR-mediated repair. Depleting either of these genes suppresses chromosomal fusions that occur in rad-51 mutant germ cells without abrogating the formation of DSBs. This suppression leaves chromosomes strikingly intact, suggesting that repair occurs robustly through alternative pathways. Through analysis of the rad-51 and rad-51;him-5 double mutants, we provide evidence that, in addition to c-NHEJ, TMEJ, and SSA act as back-up repair mechanisms within the meiotic germ line of HR mutants. Further, we found that HIM-5 appears to restrict utilization of SSA and TMEJ, suggesting that meiotic repair pathway choice may be dictated by the DSB machinery itself in order to couple DSB formation to downstream repair via HR.
Materials and Methods
Strains and growth conditions
C. elegans strains were maintained at 20° under standard growth conditions (Brenner 1974). The N2 Bristol strain was used as the wild-type background (Sulston and Brenner 1974). The strains and genotyping conditions are provided in Supplemental Material, Table S1 and Table S2, respectively. All strains will be provided upon request and/or made available through the Caenorhabditis Genome Center.
CRISPR/Cas9 mutagenesis
We produced a complete knock-out of the him-5 gene using CRISPR/Cas9 mutagenesis. Removal of the endogenous him-5 locus was performed by injecting two gRNAs directed toward NGG motifs located on each end of the him-5 isoform A coding sequence (NM-HIM5sgRNA5 5′-GTCGTTATTAGAACGAATTC-3′ targeting a cut site in exon 1, and NM-HIM5sgRNA6 5′-ATGCGGAATGACCACCAGGC-3′ targeting a cut site in exon 7) with a repair template that bridged the gap with homology arms [JY-NM-086 5′-CCGAAAAAGCATACTTATCATCTGGTTTTTTTTGCTGAAAATGTC(A)AGAA_CATT(A)CAAAAAAACGCGCTCAATAAttcgtggtttaataatagtagtttt-3′, the gap is represented as an underscore. C to A mutations, shown between brackets were designed in the repair template to avoid Cas9 activity upon successful mutagenesis]. The injection was performed according to Paix et al. (2015). We obtained, and have analyzed, one allele, him-5(ea42), that lacks the complete coding sequence of him-5 (Table S1).
Sample preparation and imaging
L4 larvae were aged for 20 hr (day 1 adults) or 47–50 hr (Day 2 adults) before fixation and staining. For irradiated samples, day 1 adults were exposed to 10 Gy of ionizing radiation using a 137Cs source (Gammacell 1000 Elite; Nordion International). Irradiated animals were further aged for 27 hr (day 2 adults) prior to fixation and staining to assess diakinesis phenotypes.
Worm were fixed in Carnoy’s fixative solution (three parts absolute ethanol; two parts chloroform; one part glacial acetic acid), stained with DAPI (4’,6-diamidino-2-phenylindole) for at least 15 min, mounted in Prolong Gold Antifade Mountant with DAPI (P36931; ThermoFisher Scientific), and cured overnight prior to imaging. All images were acquired with a Nikon A1R inverted confocal microscope system, driven by the NIS-elements Software as 0.2 µm Z-stacks. Three dimensional (3D)-reconstructed acquisitions were analyzed and captured for publication with the Volocity software (PerkinElmer). Captured images were cropped and assembled using Adobe Photoshop and Illustrator.
Analysis of DNA structures in diakinesis oocytes and related statistics
DAPI-body content of the −1 nuclei were analyzed individually on 3D reconstructed images in the Volocity software, which allows for the free rotation of nuclei. A DAPI-body was defined as a continuous DAPI-stained chromatin structure, independent of its size. A chromatin fragment was defined as a DAPI-body with an estimated volume <25% of a univalent observed in spo-11 mutant −1 nuclei.
The number of DAPI-stained bodies per nucleus was plotted and analyzed with the Graphpad Prism software. We used nonparametric tests as we could not assume our samples would follow a normal distribution, especially samples with fusions and fragments. We compared the distribution of different DAPI-stained bodies counts (ranks). Pairwise comparisons were done with two-tailed Mann-Whitney tests, with the null hypothesis (H0) that it is equally likely that a randomly selected value from one sample will be less than or greater than a randomly selected value from the second sample, with an error risk of 5%. Multiple comparisons were processed as well with Kruskal-Wallis tests corrected to control the false discovery rate (FDR) using the two-stage step-up method of Benjamini, Krieger and Yekutieli (Benjamini et al. 2006). H0 is that the independent samples tested are indistinguishable from the combined population of samples. P-values and q-values (FDR-adjusted P-value) are given in Table S3. H0 was rejected when P <0.05 with a FDR <5%. We graphed the median and the interquartile range, which are more representative for how each population is distributed. However, we state the average number of DAPI-bodies per nucleus in the text as we thought it is more meaningful to the reader. Summary of statistical tests performed for each figure is provided in Table S3.
Data availability
Strains and plasmids are available upon request. Figure S1 confirms the univalent phenotype of dsb-2. Figure S2 presents suppression of rad-51 mutation-induced fusions by multiple alleles of him-5, including him-5(ea42) a full deletion of the locus created for this analysis. Figure S3 shows that him-5 suppresses the fusions formed by a number of mutations in early HR processing events. Figure S4 shows the phenotypes induced by single mutations in NHEJ, MMEJ, and SSA pathways. Figure S5 shows the redundancy between different repair pathways in rad-51; him-5 without the addition of exogenous DNA damage (low break conditions). Figure S6 presents analysis of loss of exo-1 function on diakinesis DNA morphology. Table S1 contains a list of all strains used in the paper; Table S2 shows PCR conditions used for genotyping; Table S3 provides statistical comparisons. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7087046.
Results
him-5 mutations suppress chromosome fusions formed by loss of rad-51
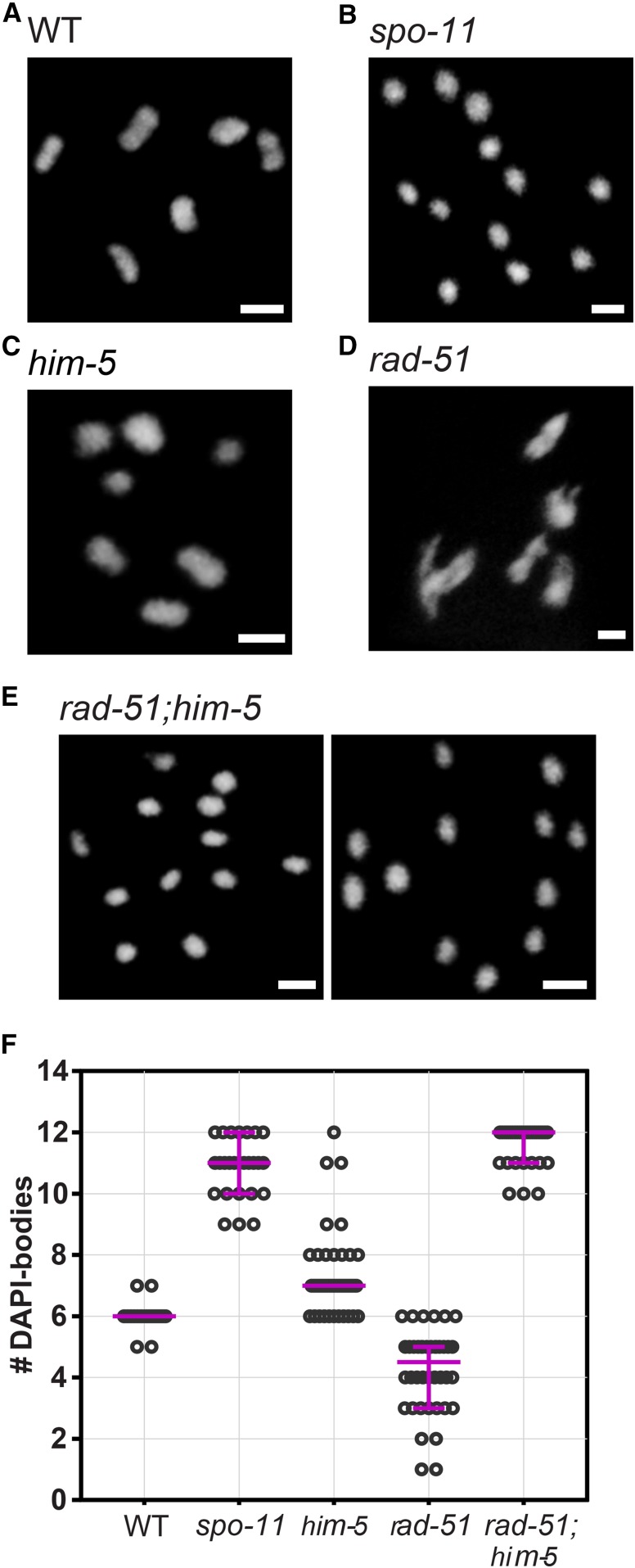
In C. elegans, defects in meiotic DNA repair can be seen in DAPI-stained nuclei of the diakinesis oocyte positioned just prior to fertilization (−1 nucleus). The morphology and number of DAPI-stained bodies is a readout of crossover formation, with wild-type worms exhibiting six ovoid, DAPI-stained bodies corresponding to the six pairs of homologs attached by chiasmata (also referred to as six bivalents) (Figure 1, A and F; Villeneuve 1994). When DSB formation is abrogated, the homologous chromosomes dissociate from one another and appear as smaller, round univalents (Dernburg et al. 1998). We observe an average of 10.8 DAPI bodies in spo-11 mutant nuclei, corresponding to a majority of univalent chromosomes, and an occasional bivalent that forms possibly as a consequence of ectopic breaks (Figure 1, B and F; see also Machovina et al. 2016). Combinations of univalents and bivalents are seen in mutants with impaired break capability, such as him-5 and dsb-2 (Figure 1, C and F and Figure S1) (Meneely et al. 2012; Rosu et al. 2013). In him-5 mutant animals, five bivalents and two univalents are seen in almost all nuclei, reflecting a defect in catalyzing DSBs on the X chromosomes (Meneely et al. 2012). In contrast, in mutants defective in strand-exchange activity, the DAPI-stained bodies appear irregularly shaped, often with aggregates (chromosomal fusions) and/or small fragments. For example, the disruption of rad-51 causes chromatin to form massive clumps (Takanami et al. 1998), with an average of 4.23 irregularly shaped and sized DAPI-stained bodies (Figure 1, D and F). This phenotype is reminiscent of chromosome end-to-end fusions, and is thought to result from random religation of SPO-11 cut chromosomes. Accordingly, these structures are completely dependent on the formation of meiotic DSBs, as seen by their suppression when spo-11 is codepleted (Rinaldo et al. 2002; Alpi et al. 2003; Takanami et al. 2003).
Figure 1.
The rad-51;him-5 double mutant phenocopies spo-11. (A–E) The rad-51;him-5 double mutant shows a phenotype similar to spo-11 worms with 12 univalent-like structures in diakinesis nuclei. Pictures show representative images of DAPI-stained diakinesis nuclei of wild type and mutants at day 2 of adulthood. Bar, 2 µm. (A) Wild type (n = 45); (B) spo-11(me44) (n = 26); (C) him-5(e1490) (n = 66); (D) rad-51(lg8701) (n = 40), (E) rad-51(lg8701);him-5(e1490) (n = 33). (F) Quantification of DAPI-bodies in diakinesis nuclei. Magenta bars demarcate the median and interquartile range.
We produced the rad-51;him-5 double mutants for which we expected an intermediate phenotype, with a mixture of chromosome clumps containing the autosomes due to the abnormal repair in absence of rad-51, and intact univalents that would not have received a DSB due to loss of HIM-5 function (Meneely et al. 2012). Surprisingly, we instead observed that rad-51;him-5 diakinesis nuclei contained ∼12 DAPI-positive bodies. Moreover, chromatin structures were indistinguishable from intact univalent chromosomes such as those seen in spo-11 mutants (Figure 1, E and F). This is surprising because the him-5 mutation alone does not prevent DSB formation (Meneely et al. 2012). Suppression of rad-51 chromosomal fusions was observed in rad-51;him-5(ok1896), rad-51;him-5(e1490), and rad-51;him-5(ea42) (Figure S2), arguing that the suppression is not allele-specific. We thus conclude that loss of HIM-5 function suppresses chromosome fusions formed in rad-51 mutant animals.
DSBs are formed and repaired in rad-51;him-5 mutants
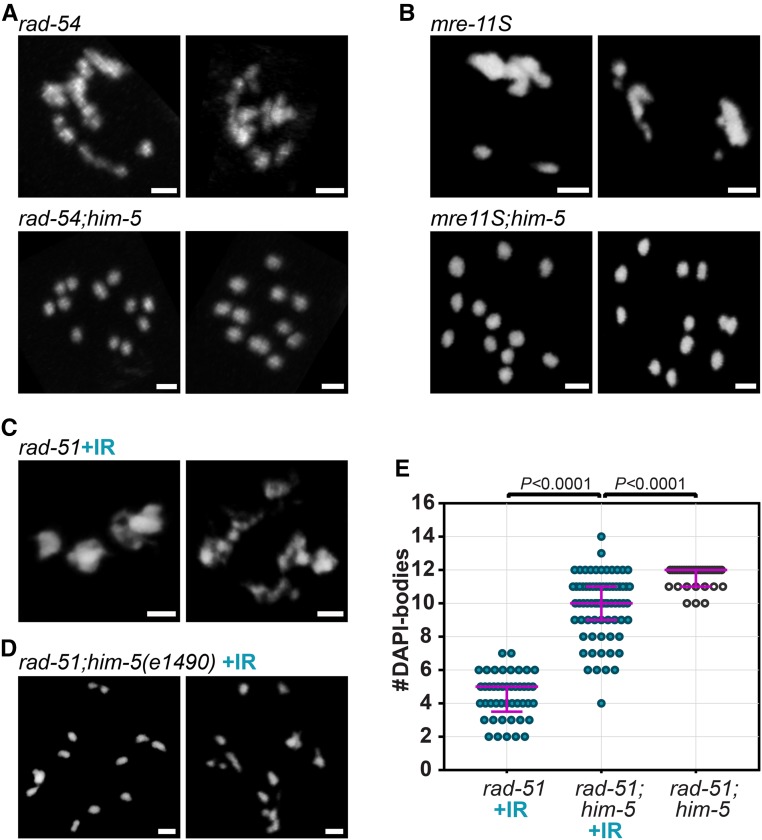
We reasoned that the presence of intact univalents in rad-51;him-5 could be explained in two ways: either RAD-51 contributes to DSB formation (either directly or via positive feedback); or, HIM-5 prevents the use of alternative DSB repair pathway(s) that can preserve the structure of (univalent) chromosomes. To address whether RAD-51 has a role in DSB induction, we first asked whether the univalents observed in rad-51;him-5 were specific to loss of rad-51, or whether depletion of other HR-promoting factors were suppressed by him-5. These factors include the RAD-51 cofactor RAD-54 (Ward et al. 2010) and the MRX/N complex subunit MRE-11 (Chin and Villeneuve 2001). Although MRE-11 has been shown to promote both DSB formation and DSB end resection, resulting in 12 univalents in null mutant diakinesis nuclei (Chin and Villeneuve 2001), we used separation-of-function allele mre-11(iow1) (referred to as mre-11S hereafter) which is deficient for end resection, but (mostly) proficient for DSB formation (Yin and Smolikove 2013). In the mre-11S,him-5 and rad-54;him-5 double mutants, we observed that, similar to rad-51;him-5, diakinesis nuclei contained ∼12 DAPI-stained, univalent-like structures, contrasting with the clumps observed in diakinesis nuclei of rad-54 and mre-11S single mutants (Figure 2, A and B). Suppression of fusions by him-5 was also observed in the double mutant rfs-1;helq-1 and in brc-2 (Figure S3, A and B). The fact that him-5 can suppress chromosome fusion formation induced by multiple early HR factors makes it unlikely that each of these factors contributes to DSB formation and supports the alternative hypothesis that him-5 functions to influence DNA repair.
Figure 2.
Depletion of him-5 suppresses chromosome fusions that arise in multiple early HR-defective mutants. (A) rad-54 and (B) mre-11S mutant animals exhibit chromosomal clumping that is suppressed by the depletion of him-5. (C and D) Irradiation of rad-51;him-5 does not restore a rad-51-like phenotype when him-5 is absent (C) rad-51 + IR control; (D) rad-51; him-5(e1490). DAPI-stained diakinesis nuclei at day 1 (A and B) or day 2 (C and D) of adulthood. Bar, 2 µm. (E) Quantification of DAPI-bodies in diakinesis nuclei of irradiated (+IR; blue dots) rad-51(lg8701) (n = 45), rad-51(lg8701);him-5(e1490) (n = 71), and nonirradiated (clear dots) rad-51(lg8701);him-5(e1490) (n = 33). Magenta bars indicate the median and the interquartile range. P-values calculated with two-tailed Mann-Whitney tests.
To further test whether meiotic DSBs are made in the rad-51;him-5 mutants, we relied on the fact that chiasma formation can be restored in spo-11 mutants upon exposure to gamma irradiation (IR) (Dernburg et al. 1998). A dose of 10 Gy is expected to induce ∼20 DSBs and is sufficient to recover at least one chiasma per chromosome pair (Yokoo et al. 2012; Machovina et al. 2016). If rad-51;him-5 were lacking DSBs entirely, one would expect that 10 Gy IR would lead to chromosome fusions, as seen in rad-51 single mutants. Contrary to this expectation, IR of rad-51;him-5 failed to induce the rad-51 phenotype (Figure 2, C and D). Instead, irradiated diakinesis nuclei looked remarkably like the unirradiated rad-51;him-5 with the addition of a few chromosome fusions and occasional chromatin fragments (Figure 2D and Figure S3C). The number of DAPI-stained bodies remained strikingly higher than in irradiated rad-51 mutants (9.87 vs. 4.42 DAPI-bodies/nucleus on average), and closer to the nonirradiated rad-51;him-5 double mutant (11.61 DAPI-bodies/nucleus). We also evaluated the distribution of DAPI-body counts since, in the presence of abnormal chromosome structures, the average can be misleading because outliers can dramatically affect the outcome. In this case, irradiated rad-51;him-5 nuclei were clearly distinguishable from irradiated rad-51 nuclei (Figure 2E and Figure S3D; P < 0.0001), as well as from unirradiated rad-51;him-5 nuclei (Figure 2E; P < 0.0001, two-tailed Mann-Whitney tests). Similar results were observed with mre-11S,him-5: irradiation did not recapitulate the fusions observed in mre-11S single mutants (Figure S3, E–G). The failure of IR-induced damage to lead to rad-51-like chromosome fusions is consistent with the hypothesis that loss of HIM-5 function alters repair outcomes even in the presence of exogenous DSBs.
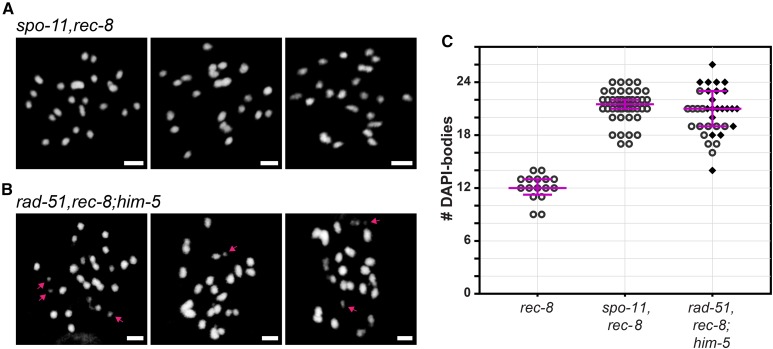
While the failure of IR to restore fusions in rad-51;him-5 suggests DSBs can be repaired by alternative mechanisms, this result did not preclude the other possibility that SPO-11-induced breaks were not formed. We thus wanted an alternative approach to verify that SPO-11-mediated DSBs are made in rad-51;him-5 mutant germ cells. Since there are no direct methods to monitor DSBs (worms do not have H2AX; nor do worms have meiotic crossover hotspots that can be physically monitored for DNA cleavage), we instead took advantage of a chromosome fragmentation assay that requires meiotic DSBs. REC-8 is a meiosis-specific component of a cohesin complex that promotes sister chromatids cohesion. In rec-8 mutants, DSBs are made, but sister chromatids separate from one another prior to repair, leading to chromosome fragmentation (Pasierbek et al. 2001). This fragmentation is dependent on meiotic DSBs as it can be suppressed by codepletion of spo-11 (Figure 3A) (Pasierbek et al. 2001). Diakinesis nuclei of spo-11,rec-8 double mutants contained an average of 21.27 DAPI-bodies, close to the expected 24 DAPI-bodies that correspond to the 12 pairs of unattached sister chromatids (the difference between the observed and expected DAPI-body counts might be explained by the inability to unambiguously distinguish closely apposed chromatids). These DAPI-bodies were well formed and uniform in appearance, and no chromosome masses or chromatin fragments were observed (n = 44; Figure 3, A and C), consistent with the lack of DSBs in the absence of spo-11 function. By contrast to spo-11;rec-8, diakinesis nuclei of rad-51,rec-8;him-5 triple mutants contained an average of 20.54 DAPI-bodies of irregular sizes and shapes (Figure 3B); >60% of cells contained at least one DNA fragment (n = 35; Figure 3, B and C), revealing that DSBs are formed in rad-51;him-5 mutant germ cells.
Figure 3.
SPO-11-mediated DSBs are catalyzed in rad-51;him-5. (A) DNA fragments are not observed in the control spo-11,rec-8 strain. (B) The presence of DSBs catalyzed by SPO-11 in rad-51;him-5 is revealed by the presence of chromatin fragments in rad-51,rec-8;him-5. Representative images of DAPI-stained diakinesis nuclei in 1-day-old adults. Arrows point to fragments of chromatin. Bar, 2 µm. (C) Quantification of DAPI-bodies in diakinesis nuclei: rec-8(ok978) (n = 16), spo-11(me44),rec-8(ok978) (A; n = 44), and rad-51(lg8701),rec-8(ok978);him-5(e1490) (B; n = 35). Diamonds indicate nuclei containing at least one fragment of chromatin. Magenta bars show the median and the interquartile range.
Overall, the presence of meiotic DSBs in rad-51;him-5 argues that alternative repair pathway(s) must be active in the rad-51;him-5 germ lines to allow for preservation of chromosome morphology without the formation of chiasmata or fragmentation. We thus hypothesized that HIM-5 could influence one or several alternative repair pathways such as c-NHEJ, TMEJ and SSA. However, the involvement of all of these pathways during meiosis, even as backup mechanisms when HR is not available, remains obscure or unexplored.
Multiple backup DSB repair pathways are active in the germline
It is well documented that c-NHEJ functions in meiosis when strand-exchange activity is depleted (Martin et al. 2005; Smolikov et al. 2007a; Yin and Smolikove 2013; Mateo et al. 2016). In mutant backgrounds where HR is impaired by the loss of rad-51 or strand exchange activity, chromatin clumps are observed in diakinesis nuclei. These clumps/fusions rely, at least partially, on c-NHEJ, as evidenced by the increase in number of DAPI bodies in diakinesis nuclei of cku-70;rad-51 vs. rad-51 (Martin et al. 2005). We confirmed the role of c-NHEJ activity in meiotic repair in wild type and HR-impaired animals by again quantifying DAPI bodies in −1 diakinesis oocytes. Consistent with prior studies (Clejan et al. 2006), we observed that depletion of c-NHEJ did not have a visible effect on meiotic crossover formation: diakinesis nuclei in cku-80 or cku-70 mutant animals contained predominantly six bivalent-shaped DAPI-bodies, which were indistinguishable from wild-type worms (Figure S4, A and B; two-tailed Mann-Whitney tests, P > 0.34). When HR function was impaired, chromosome fusions ensued with an average of 4.49 DAPI-stained bodies in rad-51. Removal of c-NHEJ functions in this background led to a partial suppression of these fusions with cku-70;rad-51 and cku-80;rad-51 exhibiting an average of 5.38 and 5.31 DAPI bodies, respectively, a significant increase in DAPI-bodies compared to rad-51 (Figure 4, A and B and Figure S4C; Kruskal-Wallis, P < 0.0050). Despite the increased number of chromatin masses, the overall chromatin morphology of these bodies was not qualitatively different (Figure 4, A, B and E). Of note, chromatin fusions were prevalent and chromosome fragments were rare in cku-70;rad-51 and cku-80;rad-51, leading us to posit that two, or more than two, pathways contribute to repair.
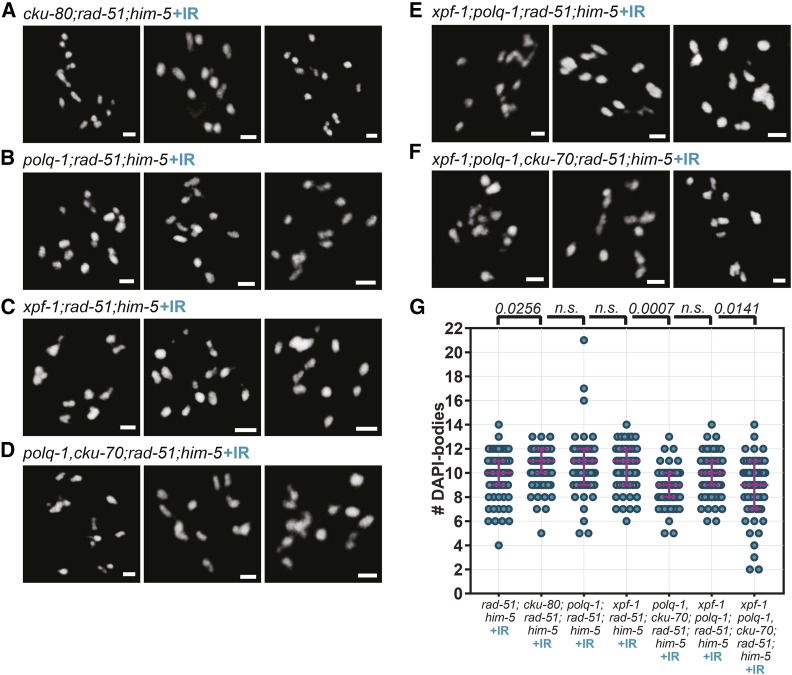
Figure 4.
c-NHEJ, TMEJ and SSA contribute to DSB repair in the HR defective rad-51 mutant. (A–D, F, and G) Representative images and (E) Quantification of DAPI-stained diakinesis nuclei from 1-day-old adults of designated genotypes. Bar, 2 µm. Magenta bars indicate the median and the interquartile range. P-values are calculated with Kruskal-Wallis multicomparison tests (n.s.: not significant, P > 0.19). rad-51(lg8701) (n = 106); cku-70(tm1524);rad-51(lg8701) (n = 53); polq-1(tm2026);rad-51(lg8701) (n = 43); xpf-1(e1487);rad-51(lg8701) (n = 40); polq-1(tm2026),cku-70(tm1524);rad-51(lg8701) (n = 60); xpf-1(e1487);polq-1(tm2026),cku-70(tm1524);rad-51(lg8701) (n = 108).
Although the specifics of meiotic DSB formation and processing have not been fully characterized in worms, short- and long-range resection by MRN/COM-1 and EXO-1, respectively, can generate strand-intermediates that are compatible with error-prone repair pathways known to be active in somatic cells (Truong et al. 2013). TMEJ-mediated repair necessitates the annealing of short homology tracks (1–15 bases) to repair DNA, and SSA needs longer tracks of homology (40–300 bases) to function (Sugawara et al. 2000; McVey and Lee 2008; reviewed in Lemmens and Tijsterman 2011). We set out to determine if TMEJ and SSA are contributing to repair when HR is impaired. To test the involvement of TMEJ, we analyzed mutants in polq-1, the worm homolog of the gene encoding polymerase theta (Schendel et al. 2016). During meiosis, depleting polq-1 function did not overtly impair CO formation as revealed by the presence of six bivalents in diakinesis nuclei (Figure S4, D and F; Mann-Whitney test, P > 0.86). In contrast, polq-1 mutations significantly increased the average number of DAPI-bodies per cell in the rad-51 mutant background (5.51 in polq-1;rad-51 vs. 4.49 in rad-51; Kruskal-Wallis, P = 0.0013) (Figure 4, C and E). This tendency to form fewer end-to-end fusions suggests that TMEJ, like c-NHEJ, contributes to repair, seen as chromosomal clumps when HR is defective.
To test the involvement of SSA in the absence of rad-51, we analyzed the effects of mutations in xpf-1, which encodes the worm homolog of the XPF/Rad1 nuclease. In C. elegans, XPF-1 has two described functions: first, as a key contributor to SSA in mitotic cells (Pontier and Tijsterman 2009); second as a component of a meiotic CO resolvase complex (Agostinho et al. 2013; O’Neil et al. 2013; Saito et al. 2013) The latter function leads to a defect in chiasmata formation, which we indeed observed in xpf-1 single mutants (Figure S4, E and F). Since RAD-51 filament formation precedes CO resolution, we hypothesized that any observed effect of xpf-1 depletion in rad-51 mutants should be attributed to its role in SSA. As in cku-70;rad-51 and polq-1;rad-51, chromosome morphology was abnormal in xpf-1;rad-51 (Figure 4D), and significantly less clumping occurred compared to rad-51 mutants (6.93 vs. 4.49 DAPI-bodies per nucleus on average, in xpf-1;rad-51 and rad-51, respectively, Kruskal-Wallis test, P < 0.0001) (Figure 4E). The marked increase in DAPI-body numbers (i.e., decrease in fusions), even compared to cku-70;rad-51 and polq-1;rad-51 (Figure 4E; Kruskal-Wallis, P < 0.002), suggests that SSA may play a more prominent role than c-NHEJ or TMEJ in DSB repair when HR is defective. This was further confirmed by the codepletion of alternative repair pathways: the depletion of xpf-1 further decreased the fusion phenotype in polq-1,cku-70;rad-51 (Figure 4, E–G; P = 0.0266).
Regulation of repair pathways in rad-51;him-5
Having ascertained that c-NHEJ, TMEJ, and SSA can be active in the meiotic germ line, at least when HR is impaired, we next wanted to ask whether these pathways contribute to repair in the rad-51;him-5 mutant background. None of the mutations that impair c-NHEJ, TMEJ or SSA by themselves or in pairwise combinations altered the number and morphology of DAPI bodies in rad-51;him-5 (Figure S5, A–E and G; Kruskal-Wallis, P > 0.43). However, a rad-51;him-5 strain with mutations in all three pathways had statistically significantly fewer DAPI-bodies per diakinesis oocyte (Figure S5, F and G; Kruskal-Wallis test, P = 0.0055). While this observation was confirmed using another statistical approach (Mann-Whitney test, P = 0.0072), we note that polq-1;cku-70;rad-51;him-5 mutants did not appear to be further affected by the depletion of xpf-1 (Mann-Whitney, P = 0.1168), suggesting that the repair pathways act redundantly to repair DSBs in the absence of both RAD-51 and HIM-5. These results also confirm that DSBs are induced in the rad-51;him-5 mutant germ cells (see Discussion).
The intact appearance of diakinesis-stage chromosomes in rad-51;him-5, in particular the lack of chromosome fragments, could also be attributed to the low level of DSBs that result from loss of him-5 function. We therefore wanted to reexamine the triple, quadruple, and quintuple mutant lines after irradiation, thereby compensating for the lack of DSBs. As discussed above, exposure of rad-51;him-5 mutant animals to 10 Gy IR led to a statistically significant decrease in the number of DAPI bodies in diakinesis oocytes, with the frequent appearance of one or more chromosome fusions and occasional small DNA fragments (Figure 2D). We note that this dose of IR did not dramatically alter the appearance of rad-51 diakinesis oocytes (two-tailed Mann-Whitney, P > 0.49), suggesting differences in the behavior of chromosomes in rad-51 vs. rad-51;him-5.
While the depletion of c-NHEJ eliminated some of the fusions that were formed in irradiated rad-51;him-5 (10.56 DAPI-bodies on average in cku-80;rad-51;him-5 vs. 9.87 in rad-51;him-5) (Figure 5, A and G; Kruskal-Wallis, P = 0.0256), the depletion of TMEJ or SSA, alone or in combination, did not have a significant effect on nuclear DAPI-body content (Figure 5, B, C, and G; Kruskal-Wallis, P > 0.05), suggesting that these two pathways were not essential to the repair of IR-generated breaks in the rad-51;him-5 background. In contrast, the presence of functional c-NHEJ was key; any mutant combination that lacked a component of the Ku-complex showed a significant difference in DAPI-body counts compared to rad-51;him-5 (Figure 5, D–G; Kruskal Wallis, P < 0.03). We note that in cku-80;rad-51;him-5, loss of c-NHEJ function prevented the formation of chromosomal fusions upon IR exposure, as observed by an increase in the average number in DAPI-bodies compared to rad-51;him-5. By contrast, codepletion of c-NHEJ and TMEJ and/or SSA led to a decrease in DAPI-body numbers (reflecting an increase in chromosome fusions; Figure 5, D, F, and G). This suggests that, while c-NHEJ, TMEJ and SSA pathways all act as backups to repair DSBs in rad-51 mutants, their use is disturbed by the additional loss of him-5.
Figure 5.
c-NHEJ is the major repair pathway for IR-induced breaks in rad-51;him-5 mutants. (A–F). Representative images and (G) Quantification of DAPI-stained diakinesis nuclei of 2-day-old, irradiated (+IR) adults. Bar, 2 µm. (G) Magenta bars indicate the median and the interquartile range. Kruskal-Wallis multiple comparisons to rad-51;him-5 (n.s.: not significant, P > 0.05). rad-51(lg8701);him-5(e1490) (n = 71), cku-80(ok851);rad-51(lg8701);him-5(e1490) (n = 80), polq-1(tm2027);rad-51(lg8701);him-5(e1490) (n = 67), xpf-1(e1487);rad-51(lg8701);him-5(e1490) (n = 61), polq-1(tm2026),cku-70(tm1524);rad-51(lg8701);him-5(e1490) (n = 63), xpf-1(e1487); polq-1(tm2026);rad-51(lg8701);him-5(e1490) (n = 64), xpf-1(e1487); polq-1(tm2026),cku-70(tm1524);rad-51(lg8701);him-5(e1490) (n = 59).
Exploring the accessibility of alternative repair machineries to DSB ends
The dependence of TMEJ and SSA, but not c-NHEJ, on the presence of him-5 led us to explore the differences in the requirements for these pathways. Specifically, we honed in on the role of resection in repair pathway choice. TMEJ and SSA rely on the annealing of ssDNA, the latter necessitates stretches of DNA sequence homology which are typically found between repetitive elements in the genome (Sugawara et al. 2000). The availability of ssDNA to the repair machinery requires 5′ to 3′ resection of DSB ends. Upon DSB formation, the activity of MRN/COM-1 generates short resected ends through the clipping of SPO-11 oligos. In mitotic cells, extensive resection requires the action of the exonuclease EXO- 1 (Lemmens and Tijsterman 2011), which has also been implicated in meiotic repair in nematodes and other species (Mimitou and Symington 2008; Zhu et al. 2008; Nimonkar et al. 2011; Lemmens et al. 2013; Yin and Smolikove 2013). c-NHEJ, by contrast, preferentially uses unresected ends. We thus hypothesized that the modulation of TMEJ and SSA by him-5 could be a consequence of a role for HIM-5 in EXO-1-mediated resection. To test this hypothesis, we compared the impact of depleting exo-1 or mre-11 in rad-51 and rad-51;him-5 mutant animals.
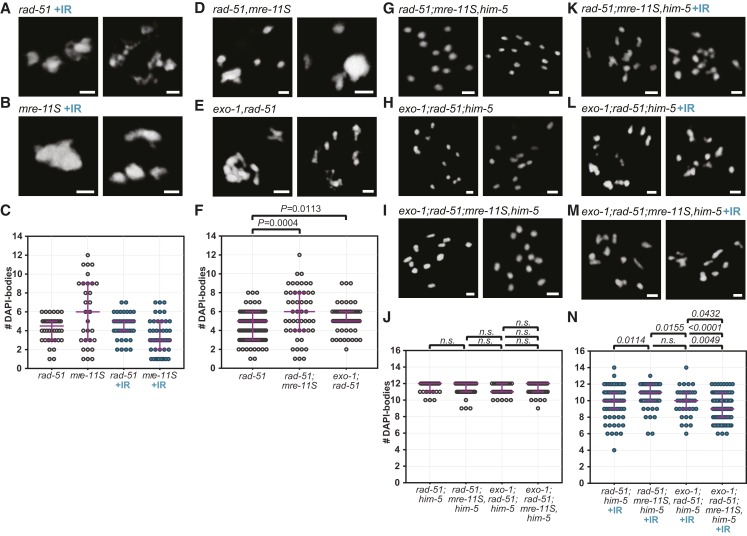
To our knowledge, the effect of depleting short and long resection specifically in a rad-51 mutant background has not been assayed. Consequently, we first examined the impact of loss of exo-1 or mre-11 in rad-51 mutant animals. The mre-11S mutant animals exhibit chromosome fusion phenotypes similar to rad-51 mutants, yet were more likely to result in more than six DAPI bodies (Figure 6C and Figure S6, D and F). With the addition of 10 Gy IR, mre-11S gave a more severe fusion phenotype than rad-51 (Figure 6, A–C). The spectrum of fusion phenotypes conferred by these mutations likely reflects differences in repair pathway usage in each mutant. As previously reported, loss of exo-1 function did not affect meiotic crossover formation (Figure S6, A–C), nor did it alter the phenotype of mre-11 (Figure S6, D–F; Yin and Smolikove 2013). The nuclear content in DAPI-stained bodies in rad-51;mre-11S (5.90 on average) and in exo-1;rad-51 (5.21) were both significantly higher than in rad-51 single mutants (4.49; Kruskal-Wallis, P < 0.012). The partial suppression of the rad-51 fusions suggests that both short and long resection contribute to fusions that form in rad-51 mutant animals (Figure 6, D–F).
Figure 6.
DSB repair is regulated differently in rad-51 and mre-11S mutants. (A–C) IR-induced breaks do not change the phenotype of rad-51 mutants, while they do affect mre-11S mutants. Shown are DAPI-bodies in representative diakinesis nuclei of unirradiated and irradiated (+IR) animals of indicated genotypes. (D–F) Depleting mre-11S or exo-1 in rad-51 mutants affects chromatin morphology, suggesting alterations in how DSBs are repaired. (A, B, D, and E) Images of diakinesis nuclei of 2-day-old adults of indicated genotypes. (C and F) Quantification of DAPI-stained bodies in diakinesis nuclei. (G–N) him-5 does not influence resection, but MRE-11 and EXO-1 act in concert for the repair of IR-induced breaks. (G–J) mre-11S or exo-1 mutations have no effect on diakinesis chromosome morphology in the rad-51;him-5 background. (K–N) By contrast, mre-11S and exo-1 affect repair of IR-induced damage, independently of him-5. (G–I and K–M) Pictures show DAPI-stained diakinesis nuclei of adults on day 2 of adulthood. Bar, 2 µm. (J and K) Quantification of DAPI-bodies in diakinesis nuclei of indicated genotypes. Magenta bars indicate median and interquartile range. P-values calculated from Kruskal Wallis tests (n.s.: not significant, P > 0.15). rad-51(lg8701) (n = 40), mre-11S(iow1) (n = 30), rad-51(lg8701)+IR (n = 39), mre-11S(iow1)+IR (n = 47). (F) rad-51(lg8701) (n = 106), rad-51(lg8701);mre-11S(iow1) (n = 50), exo-1(tm1842);rad-51(lg8701) (n = 63). (J) rad-51(lg8701);him-5(e1490) (n = 33), rad-51(lg8701);mre-11S(iow1),him-5(e1490) (n = 57), exo-1(tm1842);rad-51(lg8701);him-5(e1490) (n = 28), exo-1(tm1842);rad-51(lg8701);mre-11S(iow1),him-5(e1490) (n = 50). (N) rad-51(lg8701);him-5(e1490)+IR (n = 71), rad-51(lg8701);mre-11S(iow1),him-5(e1490)+IR (n = 60), exo-1(tm1842);rad-51(lg8701);him-5(e1490)+IR (n = 39), exo-1(tm1842);rad-51(lg8701);mre-11S(iow1),him-5(e1490)+IR (n = 101).
In the rad-51;him-5 double mutant context, the depletion or codepletion of mre-11S and exo-1 did not affect the univalent chromosomes (Figure 6, G–J). Upon IR, however, the depletion of mre-11S appears to cause a significant decrease in the formation of fusions (10.63 DAPI-bodies on average in rad-51;mre-11S,him-5 vs. 9.87 in rad-51;him-5, Kruskal-Wallis test, P = 0.0114), while the depletion of exo-1 alone did not have a significant effect (9.90 bodies per nucleus, P > 0.78) (Figure 6, K–N). Interestingly however, the codepletion of mre-11S and exo-1 caused significantly more fusions to occur (9.24 bodies per nucleus on average) compared to rad-51;him-5 double mutants, but also compared to rad-51;mre-11S,him-5 and exo-1;rad-51:him-5 triples (P < 0.05, Figure 6, G and H). While this data does not allow us to draw any conclusion about a role of HIM-5 on resection, we note a concerted action of MRE-11 and EXO-1 on dirty breaks. This is confirmed by the observation that, as discussed above, while the depletion of exo-1 did not alter the phenotype of mre-11S mutants in non-IR conditions, it did have an effect upon IR (Figure S6F). Indeed, fewer fusions were formed in irradiated exo-1;mre-11S compared to irradiated mre-11S (4.91 vs. 3.32 DAPI-bodies per nucleus on average, Mann-Whitney test, P = 0.0050). This emphasizes the fact that the nature of the DSB influences the way it will be repaired.
The DSB-promoting machinery regulates downstream repair events
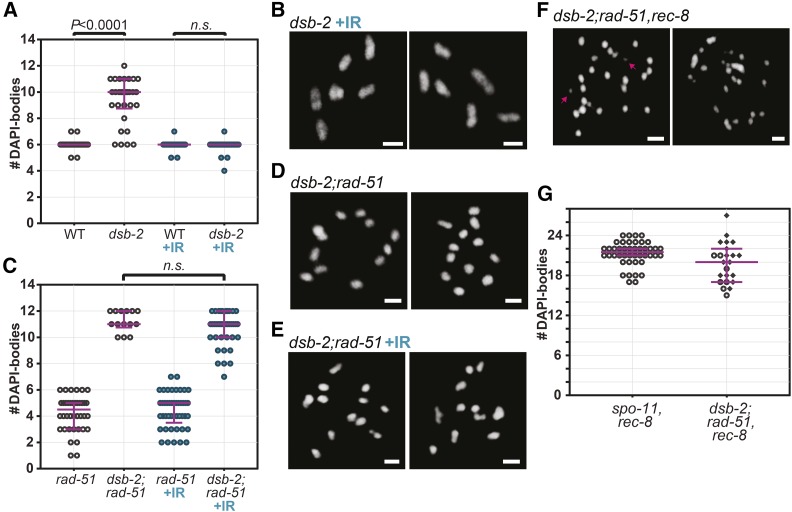
Having established a role for HIM-5 in influencing repair pathway choice, we next wanted to determine if this function was specific to HIM-5 or whether this is a more general feature of DSB-promoting factors. Mutations in dsb-2 confer a partial defect in DSB formation that worsens with maternal age, causing a shortage in chiasmata and increased nondisjunction (Rosu et al. 2013). In accordance with previous reports, we observed an average of 9.33 DAPI-bodies per nucleus in dsb-2 mutant (day 2 post-L4; Figure 7A). Upon treatment of dsb-2 mutant worms with 10 Gy IR, the average number of DAPI-bodies was restored to 5.93 intact ovoid structures—a level that is indistinguishable from wild-type worms (Figure 7, A and B; Mann-Whitney test, P = 0.5567). We discovered that diakinesis nuclei in dsb-2;rad-51 double mutants contained ∼12 DAPI-bodies (Figure 7, C and D). This phenotype is similar to what is observed in rad-51;him-5. Further, IR of dsb-2;rad-51 failed to recapitulate the rad-51 mutant phenotype and was instead not significantly different from unirradiated dsb-2;rad-51 (Figure 7, C and E; P = 0.1674). Moreover, we observed that SPO-11-mediated DSBs are still formed in dsb-2;rad-51. Indeed, 70% of dsb-2;rad-51,rec-8 triple mutant diakinesis nuclei contained fragments of chromatin (n = 23), contrasting with the absence of fragments in spo-11,rec-8 nuclei (n = 44), where DSBs are abolished (Figure 7, F and G). We were thus able to observe the same suppression of chromosome fusions that occur in rad-51 mutants as in rad-51;him-5 by depleting dsb-2. Again, we show that this is not due to an abrogation of DSBs.
Figure 7.
Loss of dsb-2 function impacts repair in rad-51 mutants, similar to him-5. (A and B) As described (Rosu et al. 2013), IR suppresses the univalent phenotype induced by loss of dsb-2, suggesting the gene is required for DSB induction. (A) Quantification of diakinesis oocytes of indicated genotypes. Wild type (WT, n = 45), dsb-2(me96) (n = 30), WT+IR (n = 52), dsb-2(me96) +IR (n = 44). Two-tailed Mann-Whitney tests are indicated on top of the graph as exact P-values (n.s.: not significant, P = 0.5567). (B) Representative images of diakinesis oocytes in irradiated 2-day-old, dsb-2 mutant animals. (C–E) Depletion of dsb-2 suppresses fusions in rad-51 mutants. (C–E) Diakinesis nuclei in dsb-2;rad-51 double mutants contain 12 intact univalents that are largely unaffected by IR-induced breaks. (C) Quantification and (D and E) Representative images of DAPI-stained diakinesis nuclei. rad-51(lg8701) (n = 40), dsb-2(me96);rad-51(lg8701) (n = 14), rad-51 +IR (n = 45), dsb-2;rad-51(lg8701) +IR (n = 39). two-tailed Mann-Whitney test (n.s.: not significant, P > 0.16). (F and G) SPO-11-mediated breaks are formed in dsb-2;rad-51 as seen by (F) chromatin fragments in the nuclei of dsb-2;rad-51,rec-8 (compare spo-11,rec8 in Figure 3A). (G) Quantification of DAPI bodies in diakinesis oocytes of spo-11(me44),rec-8(ok978) (n = 44), dsb-2(me96);rad-51(lg8701),rec-8(ok978) (n = 23). Diamonds correspond to nuclei containing at least one fragment of chromatin. Scale bars in images = 2 µm. Magenta bars in dot plots indicate median and interquartile range.
Discussion
Multiple error-prone DSB repair pathways are active in the meiotic germ line
Throughout the cell cycle, different DSB repair pathways are utilized to ensure the accurate repair of genetic information. In mitotically cycling cells, c-NHEJ is the dominant repair pathway in G1; whereas HR is preferentially utilized in S and G2 when a sister chromatid template is available. In meiotic cells, HR appears to be the major repair pathway since it is the only repair pathway that is considered “error-free” and can ensure the integrity of the genome across generations. It is well documented that c-NHEJ can step in to repair DSBs when HR is impaired during meioisis (Nairz and Klein 1997; Stark et al. 2004; Smolikov et al. 2007b; Lemmens et al. 2013; Yin and Smolikove 2013). The contributions of other DSB repair pathways are less well defined. Our data reveal multiple redundancies between c-NHEJ, TMEJ, and SSA during DSB repair in the meiotic germ line. To our knowledge, this is the first evidence that all of these pathways are active in meiotic germ cells and contribute as a failsafe mechanism to protect genomic integrity.
Checchi et al. (2014) revealed that, in the absence of HR, SSA can repair DSBs on the male X chromosome in C. elegans. We provide additional data to support a role for SSA as an alternative repair pathway, showing that SSA contributes to repair in the hermaphrodite oocyte germ lineage. Depletion of xpf-1 had the greatest impact on diakinesis DNA morphology in the rad-51 mutant background, suggesting that SSA is the major backup pathway of repair in the absence of RAD-51 and strand invasion functions. This may simply be a consequence of having long-resected ends which would normally promote strand invasion. c-NHEJ and TMEJ play important, but more minor, roles in repair when rad-51 function is impaired, consistent with their preference for shorter or blunt DNA ends. However, while we focus on SSA, we must point out that XPF-1 may have a role in other backup pathways (other than SSA), which could amplify the extent of our observations.
The redundancy between TMEJ, SSA, and c-NHEJ in meiotic DSB repair in the absence of HR function suggests that the simple dichotomy between c-NHEJ and HR repair is more complex. Our data suggest that a key decision point is between use of the ssDNA filament in SSA or HR. How is the bias for HR accomplished? Since SSA requires homologous direct repeats, it may be disfavored during meiosis, in which programmed DSBs are directed away from repetitive genomic sequences (Sasaki et al. 2010). Alternatively, specific components of the meiotic break and processing machinery may directly inhibit SSA. One good candidate for this is XPF-1, which also functions as part of a resolvase complex, potentially placing it configurations or contexts where it cannot promote SSA. Another possibility is that protein(s) required for SSA are not expressed or active during the leptotene to early pachytene stages of meiosis when crossovers are established.
HIM-5 and pathways choice functions
In the absence of HIM-5 proteins, repair pathway bias in rad-51 mutant animals shifted. c-NHEJ now becomes important for repair; whereas TMEJ and SSA adopt more auxiliary functions. Thus, HIM-5 function appears to promote use of TMEJ and SSA, at least when strand exchange is impaired. Several recent studies have pointed out that coupling DSB formation to downstream resection and repair facilitates repair through HR (Lemmens et al. 2013; Mateo et al. 2016; Girard et al. 2018). We previously showed that HIM-5, redundantly with the p53 homolog CEP-1, prevents access to c-NHEJ, a function also accomplished by COM-1/CtIP and MRN. We now show that HIM-5 also promotes homology-directed repair, either through HR or TMEJ/SSA.
We consider several different models for how HIM-5 can influence repair pathway choice. First, since HIM-5 is a chromatin-associated protein, it could regulate transcription or chromatin recruitment of key factors that promote HR and TMEJ/SSA. Decreased expression of these factors in him-5 mutant animals would impair HDR pathways and shift repair capacity to the more error-prone c-NHEJ. Second, HIM-5 could directly inhibit components of the c-NHEJ pathway, redundantly with CEP-1, under wild-type conditions. Third, based on our analysis of exo-1 and mre-11, we envision a role for HIM-5 in modulating the resection machinery. MRE-11, like HIM-5, functions in both DSB formation and resection. By contrast, EXO-1 is dispensable for both under wild-type situations. In rad-51;him-5, however, EXO-1 function became important for DNA repair in an MRE-11-dependent fashion. The shift in importance for EXO-1 could reflect a function for HIM-5 in coordinating assembly of the DSB machinery, including MRE-11. Consistent with this model, our data suggest that DSB-2, like HIM-5, may be involved in the promotion of HR-mediated DSB repair. Although deeper analysis of multi-mutants will be necessary to know if DSB-2 influences the same events as HIM-5. In fact, given the poorly conserved nature of him-5, the analysis of the role of dsb-2 and other DSB factors has the potential to reveal if the influence of the DSB formation machinery on repair pathway choice is a peculiarity or a more general feature of meiotic DNA repair regulation. Indeed, our results raise the possibility that the DSB-promoting machinery directly couples DSB induction with resection and also with the downstream events that promote HR during meiosis.
Timing of DSB break repair in meiosis shifts during early to late pachytene
A possibility that is not incompatible with other models is that HIM-5 acts as a timer for meiotic events, ensuring the appropriate formation of meiotic DSB formation in leptotene/zygotene and subsequent HR repair and crossover commitment in early pachytene. In this scenario, the loss of HIM-5 functions would delay DSB induction and/or repair until mid-to-late pachytene, leading to both a decrease in CO number (due a shorter window of opportunity for DSB formation) and the potential of aberrant repair by alternative DSB pathways.
This model is consistent with our prior data that loss of him-5 function delayed the appearance, accumulation, and total number of RAD-51 foci (Meneely et al. 2012; Mateo et al. 2016) and delayed the maturation of COs (Machovina et al. 2016). The timing model could also explain the shift in the dependence for exo-1 function. Smolikove and colleagues (Yin and Smolikove 2013) showed that exo-1 can contribute to CO repair when both mre-11 and c-NHEJ are mutated, but that accumulation of RAD-51 in these animals is delayed until late pachytene (LP), suggesting EXO-1 resection is either slower than MRE-11-induced resection or that EXO-1 is not active until LP. The contribution of exo-1 to repair in rad-51;him-5 might therefore be explained by either delayed timing of DSB formation or delayed end processing in him-5. Early pachytene may be conducive to DSB formation and repair via HR, whereas later events may be channeled to alternative repair pathways.
Further support for differences in DNA repair pathway accessibility throughout prophase comes from the observation that RAD-51 foci persist in rad-54 mutants in LP nuclei (Checchi et al. 2014), well after RAD-51 foci have disappeared in wild type (Koury et al. 2018; W. Li and J. L.Y., unpublished data). If the alternative repair pathways were active early in pachytene, RAD-51 should have disappeared with near normal kinetics. The late removal of RAD-51 suggests that these pathways are not active until late pachytene. Coincidently, the mid-to-late pachytene transition marks a change in HR repair from rad-50-dependent to rad-50 independent, which is thought to correspond to a shift from the interhomolog, CO pathway to an intersister, error-free HR repair pathway (Hayashi et al. 2007). The mid-to-late pachytene transition is also associated with the loss of meiotic DSB competency, assuring that additional SPO-11 mediated meiotic breaks are not made. Given that both HIM-5 and DSB-2 are components of the DSB machinery, and their accumulation in the nucleus is lost in LP, we suggest that barriers to alternative repair are also lifted in LP, perhaps as a consequence of the loss of the DSB proteins. The exact coordination of these events in the germ line will be an area of future investigation.
Evidence for additional back-up repair pathways
Surprisingly, xpf-1;polq-1;cku-70;rad-51 and xpf-1;polq-1;cku-70;rad-51;him-5 animals that are impaired for SSA, TMEJ, c-NHEJ, and HR functions exhibit genomes that are surprisingly intact. One might have anticipated seeing 12 large chromosome fragments corresponding to each of the unattached homologs and a large fraction of small-to-medium sized fragments corresponding to the pieces of chromosomes end that are cut by SPO-11. However, even the addition of IR in the him-5 background leaves a fairly intact genome. One possible explanation for these results is that we have only partially impaired the function of one or more of these DSB repair pathways, despite the use of null alleles. Alternatively, the mild phenotype may result from the activity of additional pathways that ligate chromosomes, such as other microhomology-mediated mechanisms. We note that, even in the presence of additional DSBs, xpf-1;polq-1;cku-70;rad-51 and xpf-1;polq-1;cku-70;rad-51;him-5, mutant germ cells are not identical, suggesting that HIM-5 function still biases repair outcomes even when SSA, TMEJ, NHEJ, and HR are not functional.
Dirty vs. clean DSBs
Examination of our data with and without IR, clearly points to distinct methods of repair for SPO-11-induced vs. IR-induced lesions. A clear example of this is seen in the comparison of rad-51 and mre-11S in the presence or absence of IR. Whereas IR has little impact on rad-51 (compare Figure 1D vs. Figure 2C), IR both reduced the variability of diakinesis phenotypes in mre-11S and led to more massive chromosome clumps compared to rad-51. The differences in the mre-11S background can be explained if the covalent attachment of SPO-11 to the DSBs occludes binding to Ku proteins until endonucleolytic cleavage. This would assist to bias repair toward HDR pathways. The damage created by IR tends to be staggered DSBs breaks that are ideal substrates for TMEJ or that would be rapidly processed into blunt ends for Ku complex recruitment for c-NHEJ.
Another example of the difference in SPO-11 vs. IR breaks comes from examination of the xpf-1 mutation in rad-51 and rad-51;him-5 mutant backgrounds. In rad-51 mutant animals, xpf-1 mutation confers the greatest suppression of fusion phenotypes. In rad-51;him-5, loss of xpf-1 has no effects with or without IR and, perhaps more significantly, loss of xpf-1 does not enhance the suppression conferred by polq-1;cku-70 double mutants both with and without IR. The differences in the processing of SPO-11 and IR-induced DSBs serves as a cautionary tale for a field that relies on IR as a surrogate for DSBs since they can be converted to COs.
Acknowledgments
The authors wish to thank Logan Russell for careful reading of the manuscript and members past and present of the Yanowitz laboratory for intellectual insights into the project. The work was funded by National Institutes of Health (NIH) grant: R01GM104007 to J.L.Y.; N.M. was also funded in part by the Magee-Womens Auxiliary Bright Star Award. Some strains were provided by the Caenorhabditis Genome Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7087046.
Communicating editor: J. Engebrecht
Literature Cited
- Agostinho A., Meier B., Sonneville R., Jagut M., Woglar A., et al. , 2013. Combinatorial regulation of meiotic Holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 9: e1003591 10.1371/journal.pgen.1003591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpi A., Pasierbek P., Gartner A., Loidl J., 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16. 10.1007/s00412-003-0237-5 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Krieger A. M., Yekutieli D., 2006. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507. 10.1093/biomet/93.3.491 [DOI] [Google Scholar]
- Bhargava R., Onyango D. O., Stark J. M., 2016. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet. 32: 566–575. 10.1016/j.tig.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2008. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 9: 297–308. 10.1038/nrm2351 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. H. Y., Pannunzio N. R., Adachi N., Lieber M. R., 2017. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 18: 495–506. 10.1038/nrm.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P. M., Lawrence K. S., Van M. V., Larson B. J., Engebrecht J., 2014. Pseudosynapsis and decreased stringency of meiotic repair pathway choice on the hemizygous sex chromosome of Caenorhabditis elegans males. Genetics 197: 543–560. 10.1534/genetics.114.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin G. M., Villeneuve A. M., 2001. C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev. 15: 522–534. 10.1101/gad.864101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clejan I., Boerckel J., Ahmed S., 2006. Developmental modulation of nonhomologous end joining in Caenorhabditis elegans. Genetics 173: 1301–1317. 10.1534/genetics.106.058628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneo B., Wendland R. L., Deriano L., Cui X., Klein I. A., et al. , 2007. Rag mutations reveal robust alternative end joining. Nature 449: 483–486. 10.1038/nature06168 [DOI] [PubMed] [Google Scholar]
- Dernburg A. F., McDonald K., Moulder G., Barstead R., Dresser M., et al. , 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398. 10.1016/S0092-8674(00)81481-6 [DOI] [PubMed] [Google Scholar]
- Eid W., Steger M., El-Shemerly M., Ferretti L. P., Pena-Diaz J., et al. , 2010. DNA end resection by CtIP and exonuclease 1 prevents genomic instability. EMBO Rep. 11: 962–968. 10.1038/embor.2010.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman-Lobell J., Rudin N., Haber J. E., 1992. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol. Cell. Biol. 12: 1292–1303. 10.1128/MCB.12.3.1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V., Phelps S. E., Gray S., Neale M. J., 2011. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479: 241–244. 10.1038/nature10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C., Roelens B., Zawadzki K. A., Villeneuve A. M., 2018. Interdependent and separable functions of Caenorhabditis elegans MRN-C complex members couple formation and repair of meiotic DSBs. Proc. Natl. Acad. Sci. USA 115: E4443–E4452. 10.1073/pnas.1719029115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Chin G. M., Villeneuve A. M., 2007. C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet. 3: e191 10.1371/journal.pgen.0030191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S., Giroux C. N., Kleckner N., 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. 10.1016/S0092-8674(00)81876-0 [DOI] [PubMed] [Google Scholar]
- Koury E., Harrell K., Smolikove S., 2018. Differential RPA-1 and RAD-51 recruitment in vivo throughout the C. elegans germline, as revealed by laser microirradiation. Nucleic Acids Res. 46: 748–764. 10.1093/nar/gkx1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. E., Moore J. K., Holmes A., Umezu K., Kolodner R. D., et al. , 1998. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94: 399–409. 10.1016/S0092-8674(00)81482-8 [DOI] [PubMed] [Google Scholar]
- Lemmens B. B., Tijsterman M., 2011. DNA double-strand break repair in Caenorhabditis elegans. Chromosoma 120: 1–21. 10.1007/s00412-010-0296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens B. B., Johnson N. M., Tijsterman M., 2013. COM-1 promotes homologous recombination during Caenorhabditis elegans meiosis by antagonizing Ku-mediated non-homologous end joining. PLoS Genet. 9: e1003276 10.1371/journal.pgen.1003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbrich M., Jeggo P., 2017. A process of resection-dependent nonhomologous end joining involving the goddess Artemis. Trends Biochem. Sci. 42: 690–701. 10.1016/j.tibs.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. L., Kim E. M., Haber J. E., Lee S. E., 2003. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol. Cell. Biol. 23: 8820–8828. 10.1128/MCB.23.23.8820-8828.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Milman N., Nambiar M., Smith G. R., 2015. Two separable functions of Ctp1 in the early steps of meiotic DNA double-strand break repair. Nucleic Acids Res. 43: 7349–7359. 10.1093/nar/gkv644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovina T. S., Mainpal R., Daryabeigi A., McGovern O., Paouneskou D., et al. , 2016. A surveillance system ensures crossover formation in C. elegans. Curr. Biol. 26: 2873–2884. 10.1016/j.cub.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. S., Winkelmann N., Petalcorin M. I., McIlwraith M. J., Boulton S. J., 2005. RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol. Cell. Biol. 25: 3127–3139. 10.1128/MCB.25.8.3127-3139.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon E., Carroll D., 1991. Characterization of recombination intermediates from DNA injected into Xenopus laevis oocytes: evidence for a nonconservative mechanism of homologous recombination. Mol. Cell. Biol. 11: 3278–3287. 10.1128/MCB.11.6.3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo A. R., Kessler Z., Jolliffe A. K., McGovern O., Yu B., et al. , 2016. The p53-like protein CEP-1 is required for meiotic fidelity in C. elegans. Curr. Biol. 26: 1148–1158. 10.1016/j.cub.2016.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M., Lee S. E., 2008. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet. 24: 529–538. 10.1016/j.tig.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely P. M., McGovern O. L., Heinis F. I., Yanowitz J. L., 2012. Crossover distribution and frequency are regulated by him-5 in Caenorhabditis elegans. Genetics 190: 1251–1266. 10.1534/genetics.111.137463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou E. P., Symington L. S., 2008. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455: 770–774. 10.1038/nature07312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz K., Klein F., 1997. mre11S–a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev. 11: 2272–2290. 10.1101/gad.11.17.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar A. V., Genschel J., Kinoshita E., Polaczek P., Campbell J. L., et al. , 2011. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 25: 350–362. 10.1101/gad.2003811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil N. J., Martin J. S., Youds J. L., Ward J. D., Petalcorin M. I., et al. , 2013. Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet. 9: e1003582 10.1371/journal.pgen.1003582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Folkmann A., Rasoloson D., Seydoux G., 2015. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics 201: 47–54. 10.1534/genetics.115.179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasierbek P., Jantsch M., Melcher M., Schleiffer A., Schweizer D., et al. , 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15: 1349–1360. 10.1101/gad.192701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D. B., Tijsterman M., 2009. A robust network of double-strand break repair pathways governs genome integrity during C. elegans development. Curr. Biol. 19: 1384–1388. 10.1016/j.cub.2009.06.045 [DOI] [PubMed] [Google Scholar]
- Ranjha L., Howard S. M., Cejka P., 2018. Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma 127: 187–214. 10.1007/s00412-017-0658-1 [DOI] [PubMed] [Google Scholar]
- Rinaldo C., Bazzicalupo P., Ederle S., Hilliard M., La Volpe A., 2002. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu S., Zawadzki K. A., Stamper E. L., Libuda D. E., Reese A. L., et al. , 2013. The C. elegans DSB-2 protein reveals a regulatory network that controls competence for meiotic DSB formation and promotes crossover assurance. PLoS Genet. 9: e1003674 10.1371/journal.pgen.1003674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. T., Lui D. Y., Kim H. M., Meyer K., Colaiacovo M. P., 2013. Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet. 9: e1003586 10.1371/journal.pgen.1003586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T., Nakade S., Sakane Y., Suzuki K. T., Yamamoto T., 2016. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat. Protoc. 11: 118–133. 10.1038/nprot.2015.140 [DOI] [PubMed] [Google Scholar]
- Sasaki M., Lange J., Keeney S., 2010. Genome destabilization by homologous recombination in the germ line. Nat. Rev. Mol. Cell Biol. 11: 182–195. 10.1038/nrm2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel V., Borca B., Pentegov I., Michnowicz T., Kraft U., et al. , 2016. Remotely controlled isomer selective molecular switching. Nano Lett. 16: 93–97. 10.1021/acs.nanolett.5b02974 [DOI] [PubMed] [Google Scholar]
- Shrivastav M., De Haro L. P., Nickoloff J. A., 2008. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18: 134–147. 10.1038/cr.2007.111 [DOI] [PubMed] [Google Scholar]
- Smolikov S., Eizinger A., Hurlburt A., Rogers E., Villeneuve A. M., et al. , 2007a Synapsis-defective mutants reveal a correlation between chromosome conformation and the mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics 176: 2027–2033. 10.1534/genetics.107.076968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov S., Eizinger A., Schild-Prufert K., Hurlburt A., McDonald K., et al. , 2007b SYP-3 restricts synaptonemal complex assembly to bridge paired chromosome axes during meiosis in Caenorhabditis elegans. Genetics 176: 2015–2025. 10.1534/genetics.107.072413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. M., Pierce A. J., Oh J., Pastink A., Jasin M., 2004. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 24: 9305–9316. 10.1128/MCB.24.21.9305-9316.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N., Ira G., Haber J. E., 2000. DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol. Cell. Biol. 20: 5300–5309. 10.1128/MCB.20.14.5300-5309.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S., 1974. The DNA of Caenorhabditis elegans. Genetics 77: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami T., Sato S., Ishihara T., Katsura I., Takahashi H., et al. , 1998. Characterization of a Caenorhabditis elegans recA-like gene Ce-rdh-1 involved in meiotic recombination. DNA Res. 5: 373–377. 10.1093/dnares/5.6.373 [DOI] [PubMed] [Google Scholar]
- Takanami T., Mori A., Takahashi H., Horiuchi S., Higashitani A., 2003. Caenorhabditis elegans Ce-rdh-1/rad-51 functions after double-strand break formation of meiotic recombination. Chromosome Res. 11: 125–135. 10.1023/A:1022863814686 [DOI] [PubMed] [Google Scholar]
- Truong L. N., Li Y., Shi L. Z., Hwang P. Y., He J., et al. , 2013. Microhomology-mediated end joining and homologous recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA 110: 7720–7725. 10.1073/pnas.1213431110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve A. M., 1994. A cis-acting locus that promotes crossing over between X chromosomes in Caenorhabditis elegans. Genetics 136: 887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. D., Muzzini D. M., Petalcorin M. I., Martinez-Perez E., Martin J. S., et al. , 2010. Overlapping mechanisms promote postsynaptic RAD-51 filament disassembly during meiotic double-strand break repair. Mol. Cell 37: 259–272. 10.1016/j.molcel.2009.12.026 [DOI] [PubMed] [Google Scholar]
- Yan C. T., Boboila C., Souza E. K., Franco S., Hickernell T. R., et al. , 2007. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 449: 478–482. 10.1038/nature06020 [DOI] [PubMed] [Google Scholar]
- Yin Y., Smolikove S., 2013. Impaired resection of meiotic double-strand breaks channels repair to nonhomologous end joining in Caenorhabditis elegans. Mol. Cell. Biol. 33: 2732–2747. 10.1128/MCB.00055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo R., Zawadzki K. A., Nabeshima K., Drake M., Arur S., et al. , 2012. COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers. Cell 149: 75–87. 10.1016/j.cell.2012.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Caron P., Legube G., Paull T. T., 2014. Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Res. 42: e19 10.1093/nar/gkt1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chung W. H., Shim E. Y., Lee S. E., Ira G., 2008. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134: 981–994. 10.1016/j.cell.2008.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Figure S1 confirms the univalent phenotype of dsb-2. Figure S2 presents suppression of rad-51 mutation-induced fusions by multiple alleles of him-5, including him-5(ea42) a full deletion of the locus created for this analysis. Figure S3 shows that him-5 suppresses the fusions formed by a number of mutations in early HR processing events. Figure S4 shows the phenotypes induced by single mutations in NHEJ, MMEJ, and SSA pathways. Figure S5 shows the redundancy between different repair pathways in rad-51; him-5 without the addition of exogenous DNA damage (low break conditions). Figure S6 presents analysis of loss of exo-1 function on diakinesis DNA morphology. Table S1 contains a list of all strains used in the paper; Table S2 shows PCR conditions used for genotyping; Table S3 provides statistical comparisons. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7087046.