Abstract
Bcl-xL is a member of the Bcl-2 family of apoptotic regulators, responsible for inhibiting the permeabilization of the mitochondrial outer membrane, and a promising anti-cancer target. Bcl-xL exists in the following conformations, each believed to play a role in the inhibition of apoptosis: (a) a soluble folded conformation, (b) a membrane-anchored (by its C-terminal α8 helix) form, which retains the same fold as in solution and (c) refolded membrane-inserted conformations, for which no structural data are available. Previous studies established that in the cell Bcl-xL exists in a dynamic equilibrium between soluble and membranous states, however, no direct evidence exists in support of either anchored or inserted conformation of the membranous state in vivo. In this in vitro study, we employed a combination of fluorescence and EPR spectroscopy to characterize structural features of the bilayer-inserted conformation of Bcl-xL and the lipid modulation of its membrane insertion transition. Our results indicate that the core hydrophobic helix α6 inserts into the bilayer without adopting a transmembrane orientation. This insertion disrupts the packing of Bcl-xL and releases the regulatory N-terminal BH4 domain (α1) from the rest of the protein structure. Our data demonstrate that both insetion and refolding of Bcl-xL are modulated by lipid composition. We hypothesize that conformational rearrangements associated with the bilayer insertion of Bcl-xL result in its switching to a so- called non-canonical mode of apoptotic inhibition. Presented results suggest that the alteration in lipid composition before and during apoptosis can serve as an additional factor regulating the permeabilization of the mitochondrial outer membrane.
Keywords: Apoptosis, Bcl-2 proteins, conformational change, FRET, EPR, Depth-dependent fluorescence quenching
INTRODUCTION
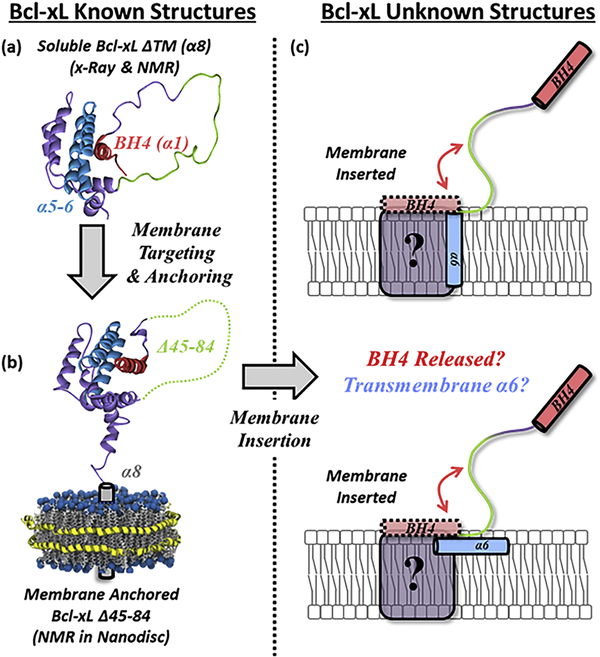
The critical step in triggering apoptosis is the permeabilization of the mitochondrial outer membrane (MOM), which releases apoptotic factors into the cytosol ultimately leading to cell death 1,2. MOM permeabilization (MOMP) is controlled and executed by the numerous proteins of the Bcl-2 family 3–5, which include three types: pro-apoptotic pore formers (e.g., Bax, Bak), anti-apoptotic pore inhibitors (e.g., Bcl-xL, BCL2), and BH3-only regulators (e.g., Bid, Bim) 6,7. According with the prevailing “Embedded Together” model, many functional interactions between Bcl-2 family proteins occur in membranes 4,7,8, however, the mechanisms by which the membrane induces conformational changes and modulates protein–protein interactions remain largely unknown 5,9. The main role of Bcl-xL in the cell is to prevent MOMP by interacting with and blocking pro-apoptotic pore forming proteins like Bax 10–15. The molecular details of this process, are not known, and multiple modes of inhibition have been suggested 4,5,12,13,16. Consistent with the postulated multiple modes of action, Bcl-xL exists in several conformations, both soluble and membranous (Fig. 1).
FIGURE 1. Bcl-xL membrane targeting/insertion and topology of membrane inserted form.
(a) The inactive form of the anti-apoptotic protein Bcl-xL exists in a soluble state that must interact with membranes to transition to its active conformation. (b) The targeting of Bcl-xL to the membrane leads to its anchoring through its C-terminal α8 helix. (c) The conformation of membrane inserted Bcl-xL, however, has not yet been determined. Here we characterize the lipid determinants that regulate the insertion of Bcl-xL into membranes. Additionally, we report the release of its N-terminal BH4 domain and its link to Bcl-xL insertion and the topology of its hydrophobic α6 helix.
The structure of Bcl-xL in solution is derived from high-resolution structures of the soluble construct Bcl-xL ΔTM, lacking its hydrophobic C-terminal α8 helix (Fig. 1a). The structure consists of a globular arrangement of α-helices with helices α5-α6 forming the hydrophobic core (Fig. 1, cyan) 17–19. The structural and functional features of Bcl-xL bound to the membrane, however, are less clear. The only available structure of a lipid-associated form is the NMR structure of the bilayer anchored form of Bcl-xL Δ45–82 (Fig. 1b), a construct with a deleted long loop between helices α1 and α2 20. The anchoring was achieved not by insertion into a membrane mimetic, but by forming nanodiscs around the hydrophobic α8 helix, and the resulting fold was found to be the same as in the soluble form of Bcl-xL ΔTM 20. Previous studies established that in the cell Bcl-xL exists in a dynamic equilibrium between soluble and membranous states 12–16, and the anchored conformation is often assumed for the latter. Nevertheless, no direct evidence exists in support of either anchored or inserted conformation of the membranous state in vivo.
Very little is known about the structure of the membrane-inserted conformation of Bcl-xL (Fig. 1c), other than it does not require the presence of its C-terminal α8 helix, and is likely to involve substantial refolding and membrane insertion of the α5-α6 helical hairpin 21–25. The proposed model of membrane-inserted Bcl-xL, in which α5-α6 adopts a transmembrane (TM) conformation 26–29, is based on the topology of the bilayer-inserted translocation domain in diphtheria toxin, which shares the solution fold with many Bcl-2 proteins, including Bcl-xL 17,30. This model has not been verified experimentally.
Previously, we compared the protonation-triggered membrane insertion pathway of Bcl-xL to that of diphtheria toxin and found that lipids can play a far more prominent role in the insertion of Bcl-xL 25. Here we follow up with direct testing of the putative insertion model of Bcl-xL using EPR and fluorescence spectroscopy. We also use FRET to examine the insertion-related refolding of Bcl-xL, specifically the release of the N-terminal BH4 (α1) regulatory domain from the folded structure. The latter is relevant to the so-called non-canonical mode of apoptotic inhibition, suggested for the close functional homologue of Bcl-xL, BCL2 31. Our results demonstrate that alteration in lipid composition can serve as a regulatory factor for preparing Bcl-xL for the switching between the canonical and non-canonical forms of inhibition.
MATERIALS AND METHODS
Materials:
All lipids used in this study: Palmitoyl-oleoyl-phosphatidylcholine (POPC), palmitoyl-oleoyl-phosphatidylglycerol (POPG), palmitoyl-oleoyl-phosphatidylserine (POPS), palmitoyl-oleoyl-phosphatidic acid (POPA), 1,1,2,2-tetraoleoyl-cardiolipin (TOCL), 1- palmitoyl-2-stearoyl-(5-doxyl)-sn-glycero-3-phosphocholine (5-Doxyl PC), 1-palmitoyl-2- stearoyl-(10-doxyl)-sn-glycero-3-phosphocholine (10-Doxyl PC), 1-palmitoyl-2-stearoyl-(12- doxyl)-sn-glycero-3-phosphocholine (12-Doxyl PC), 1-palmitoyl-2-stearoyl-(14-doxyl)-sn- glycero-3-phosphocholine (14-Doxyl PC), 1,2-dipalmitoyl-sn-glycero-3-phospho(tempo)choline (TEMPO-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Fluorescent dyes: IANBD-ester and AlexaFluor488-maleimide were obtained from Invitrogen (Carlsbad, CA)
Cloning, expression and labeling: mCherry2-C1 plasmid containing the mCherry encoding gene was purchased from Adgene (Cambridge, MA). The mCherry insert was PCR amplified using the following forward and reverse primers: GCGTATGCGGCCGGTGAGCAAGGGCGA-GGAGGATAAC and CGCATACCGGCCGGGTCCGCCGTTGTACAGCTCGTCCAT, both containing EagI sites. Both the insert and pET28b plasmid containing the Bcl-xL gene 25 were cleaved by EagI and ligated overnight at 4°C. The presence of the insert was determined by the red coloring from expressed mCherry in colonies grown on 1.0 mM IPTG containing plates and confirmed by sequencing. The resulting construct contained a N-terminal 6His-tag followed by mCherry at the N-terminus of Bcl-xL connected through a flexible 6 amino acid linker (GGPGRH) in-between mCherry and Bcl-xL. The desired mutations were introduced using QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). BL23DE3pLysS cells were transformed with the prepared plasmid for the recombinant expression of the construct. Single colonies were grown overnight in 5.0 mL of LB media supplied with 50 mg/mL of kanamycin. Overnight cultures were inoculated in 500 mL flasks with LB + 50 mg/mL kanamycin and grown to an OD600 = 0.6 at 37°C. At this point the temperature was decreased to 24°C and the synthesis of recombinant protein was induced by the addition of 0.8 mM IPTG. After 18 hours the cells were centrifuged at 4000 rpm for 15 min and frozen overnight at −80°C. Cells were thawed and resuspended in binding buffer containing 50 mM Tris-HCl, pH 8.0, 300 mM NaCl, and 5.0 mM imidazole and lysed by sonication. Cell debris was removed by centrifugation (10000 rpm, 30 min) and the clarified lysate was bound overnight to Ni-agarose. After several washes the bound protein was eluted with 0.5 M imidazole in binding buffer and further purified by FPLC on a Superose6 1×30 cm column in 50 mM Na-phosphate buffer, pH 8.0. The purity of mCherry-Bcl-xL containing fractions was checked by SDS-PAGE and the concentration determined using the following molar extinction coefficients: 41000 M−1cm−1 for Bcl-xL only, 72200 M−1cm−1 for mCherry-Bcl-xL at 280 nm and 72000 M−1cm−1 at 587 nm for mCherry. Bcl-xL ΔTM was treated with 4x molar excess MTSL ((1-oxy-2,2,5,5-tetramethyl-d-pyrroline-3-methyl)-methanethiosulfonate) for EPR measurements. Excess MTSL was removed with PD10 columns. The final concentration of MTSL Bcl-xL was assessed using ε = 36440 M−1 cm-1. Fluorescent labeling with IANBD and Alexa 488 was performed using a standard labeling protocol for thiol-reactive dyes 32. Excess dye was removed by gel-filtration in a Superose 61×30 cm column.
LUV preparation: Large unilamellar vesicles (LUV) were prepared by drying the required volume of chloroform lipid stocks under a nitrogen stream before overnight drying using high vacuum. Dried lipid films were re-suspended in 50 mM phosphate buffer (pH 8.0 for fluorescent measurements or 7.4 for EPR experiments) to a final concentration of 20 mM and vortexed. Rehydrated lipid solutions for EPR measurements were subjected to 7–10 freeze-thaw cycles. LUV were formed by extrusion using a Mini-Extruder (Avanti Polar Lipids, Alabaster, AL) through nucleopore polycarbonate membranes of 0.1 µm pore size (Whatman, Philadelphia, PA).
LUV stocks were stored at −4°C in 50 mM phosphate buffer, pH 8.0 33,34. LUV used in depth-dependent quenching experiments were prepared by including 30% molar content of the appropriate spin-lipid into the lipid mixture prior to nitrogen drying.
Fluorescence measurements and analysis: Steady-state fluorescence emission experiments were performed using a SPEX Fluorolog FL3–22 steady-state fluorescence spectrometer (Jobin Yvon, Edison, NJ) equipped with double-grating excitation and emission monochromators. Measurements were made using a 2×10 mm cuvette oriented perpendicular to the excitation beam and maintained at 25ºC using a Peltier device from Quantum Northwest (Spokane, WA.). All measurements were collected after the sample was equilibrated for at least 20 minutes. All spectral measurements were collected with 1.0 nm steps. FRET measurements between mCherry and A488 were collected between 480–720 nm using an excitation wavelength of 465 nm. Membrane insertion measurements using NBD labeled Bcl-xL were collected from 490 to 700 nm using an excitation wavelength of 470 nm. Depth-dependent measurements used an excitation wavelength of 465 nm. All emission measurements were collected using 5.0 nm slits on both monochromators and were averaged over 3–5 scans with at least 20 min incubation for equilibration. The samples normally contained 0.3 μM protein in 50 mM phosphate buffer at pH 8.0, unless otherwise indicated, and 1.0 mM of lipid. Sample acidification was achieved by the addition of small aliquots of acetic/acetate buffer. Fluorescence intensity of NBD measured at 510 nm was used to quantitate insertion, and fluorescence intensity of donor (Alexa 488) measured at 520 nm was used to quantitate FRET. The pH-dependencies of both insertion and refolding were fitted to the following equation 35:
| (Eq. 1) |
where I is the fluorescence intensity measured as a function of pH, IN and IL represent the limiting intensities at high and low pH respectively, pKa denotes the negative logarithm of the dissociation constant, and m is the transition slope.
The protonation-dependent free energies of both membrane insertion (ΔGTMH+) and BH4 domain release (ΔGBH4H+) were calculated using the determined pKa values using Eq. 1 in a rearranged Gibbs free energy equation:
| (Eq. 2) |
where R and T are the gas constant and absolute temperature, respectively, and the pKa is obtained with Eq. 1.
Membrane surface potential calculations: Membrane surface potential calculations were performed using Gouy-Chapman theory 36. This model describes the electric potential along the axial plane of the membrane, where the potential at the membrane surface Ψ0 depends on the surface charge density σ and the concentration of counterions according to:
| (Eq. 3) |
Where κB is the Boltzmann constant (1.38 × 10−23 J/K), T is the absolute temperature, z is the valence of the counterion (+1 in our case), e is the elementary electric charge (−1.602 × 10−19 C), N is the Avogadro number (6.022 × 1023 mol−1), ε0 is the permittivity in vacuum (8.854 × 10−12 C2m−1/J), εr is the dielectric constant of water (78.3). c is the concentration of counterions expressed in mol/m3, while Σ is the surface charge density expressed in C/m2.
The surface charge density for the different anionic LUV used was calculated according the following equation:
| (Eq. 4) |
where zAL is the net charge of the anionic lipid, χAL and χNL are the mole fractions of the anionic and neutral lipid, respectively, and AAL and ANL are the area per lipid of the anionic and neutral lipid, respectively, in units of m2. For the net charges, we used values of 0 for POPC, −1 for POPG, POPS and POPA, and −2 for TOCL. For the areas per lipid, we assumed a cylindrical shape and used the cross-sectional areas described in the literature for the crystalline phase: 64 Å2 for POPC 37, 66 Å2 for POPG 38, 63 Å2 for POPS 39, 68 Å2 for POPA 40, and 130 Å2 for TOCL 41. We further assumed that the area per lipid was not affected by the mixing of different lipids, the presence of the protein, or the concentration of ions.
Depth-Dependent Quenching:
Depth-dependent quenching profiles (QP’s) were generated using measurements of NBD fluorescence quenching with a series of lipids labeled with spin probes at different depths, h, defined as the distance from the bilayer center. Both steady-state and time-resolved and measurements were used to determine the intensities, F(h), and lifetimes, τ(h), as a function of quencher depth and the corresponding values in the absence of quenchers, F0 and τ0. Because of the heterogeneity of the fluorescence decay and scattering contributions, the following procedure was employed to calculate the average time of the decay 42: (i) time-resolved data were subjected to a standard deconvolution procedure that assumes three exponential components, with the shortest lifetime fixed at 0.1 ns; (ii) the amplitude-weighted average lifetime of the two longest components was used as the average τ. The two QP’s were generated as follows: steady-state (or total) QP(h)= (F0/F(h))-1, and differential QP(h)=(F0 /F(h))-(τ0 /τ(h))43 . The latter reduces the dynamic contribution from the transverse diffusion of a probe occurring during the excited-state lifetime, and has been shown to provide a more accurate position of the probe than conventional total QP, based only on steady-stat measurements 43–45. Both total and differential QP’s were subjected to Distribution Analysis (DA) methodology 46,47, which approximates the transverse quenching with a sum of two mirror-imaged Gaussian functions, G(h):
| (Eq. 5) |
where, hm is the most probable depth of the probe measured from bilayer center, σ, is the dispersion of the transverse profile, and S, corresponds to the overall quenching efficiency, related to lipid exposure of a probe. A symmetrical second Gaussian distribution, G(-h), added to account for trans-leaflet quenching, is important for analyzing deeply penetrating fluorophores 48. Both G(h) and G(-h) share the same three fitting parameters. The average positions of the spin quenchers, h, calculated from the center of the lipid bilayer have been previously determined by MD simulations of a series of spin-labeled lipids in a model membrane 49.
Support Plane Analysis:
The robustness of the determined hm parameters in the total quenching and differential quenching profiles obtained by DA analysis were determined by subjecting the fits to support plane analysis 50, as described previously 43. Briefly, a series of least-square fits were generated, in which the hm parameter was fixed at different values with 0.1–0.3 Å steps around the most optimal solution. The ratio between every Χ2 value and the optimal Χ2 solution (Χ2Min) were plotted and a cut-off corresponding to a single standard deviation (Χ2/ Χ2Min = 1.33) was used to identify the range of hm values 50
EPR measurements:
Power saturation experiments were performed in the range of 1.3 – 63.5 mW incident microwave power, on a Bruker EMX spectrometer equipped with a dielectric resonator. The O2 and NiEDDA accessibilities were estimated using a previously established method 51,52. The O2 accessibility (ΠO2) was measured with samples in the presence of air and the accessibility to NiEDDA (ΠNiEDDA) was obtained by the addition of 10.0 mM NiEDDA to a sample equilibrated under a constant stream of N2 gas. The depth parameter, Φ, was determined using the following equation:
| (Eq. 6) |
Measurements were performed on LUV containing either 3TOCL:2POPC or 75POPG:25POPC keeping the protein:lipid ratio >1:500. Bcl-xL V161C, I166C, and A168C were measured in 3TOCL:2POPC LUV, while V161C, V163C, I166C, A167C, A168C, W169C, M170C, N175C, and H177C were measured in 75POPG:25POPC LUV.
RESULTS
Bcl-xL membrane insertion correlates with the release of its N-terminal BH4 domain
The insertion of Bcl-xL ΔTM into membranes from its soluble form was determined by fluorescence emission spectroscopy using the environmentally sensitive fluorophore NBD. The emission spectrum of this probe increases in intensity and the position of its maximum shifts to lower wavelengths when the probe relocates to a more hydrophobic environment (i.e., lipid bilayers). These spectroscopic changes allow us to track the transition of NBD labeled Bcl-xL ΔTM from solution to membranes. NBD was introduced using the single-Cys mutant N175C located in the middle of the hydrophobic α6 helix (Fig. 1, cyan), expected to insert into bilayers as a function of pH 21–25. Acidification is a common way of efficiently inducing the membrane-inserted form of Bcl-xL in model systems 21–25. This is not meant to be a mimic of physiological conditions, but rather a way of extracting structural and thermodynamic information on the protonation-driven insertion transition. This methodology is similar to the use of high temperature in thermal stability studies, in which the conditions used are a tool and do not suggest that high temperatures are physiologically relevant. We have used large unilamellar vesicles (LUV) with a 1:2 ratio of cardiolipin and POPC, which approximates the cardiolipin content present at hot spots of apoptotic regulation in mitochondrial contact sites 53,54.
Compared to Bcl-xL in solution (Fig. 2a, grey), the addition of 1TOCL:2POPC LUV at pH 8.0 does not result in significant spectroscopic changes (Fig. 2a, black). Acidification of the solution, however, leads to large pH-dependent increases in NBD intensity that saturate at pH 4.5. The intensity increase is accompanied by a blue shift of the NBD emission maxima relative to the 535.1 nm at pH 8.0 to 525.9 nm at pH 4.5. Both spectroscopic changes are associated with the transition of NBD to hydrophobic environments and indicate the pH-dependent membrane insertion of the hydrophobic α6 helix. The transition of Bcl-xL into its inserted conformation is likely to destabilize the packing of its helices and result in its refolding in the membrane. The N- terminal BH4 helix (α1), in particular, is packed against the hydrophobic α6 helix and is connected through a long loop to the rest of the protein fold in its soluble conformation (Fig. 1a). Destabilization of the overall fold of Bcl-xL is likely to result in its release from the rest of the protein structure. This helix has been previously linked to apoptotic progression through a potential non-canonical anti-apoptotic mechanism 55–57, characterizing its topology on the inserted form of Bcl-xL is therefore crucial to our understanding of apoptotic repression.
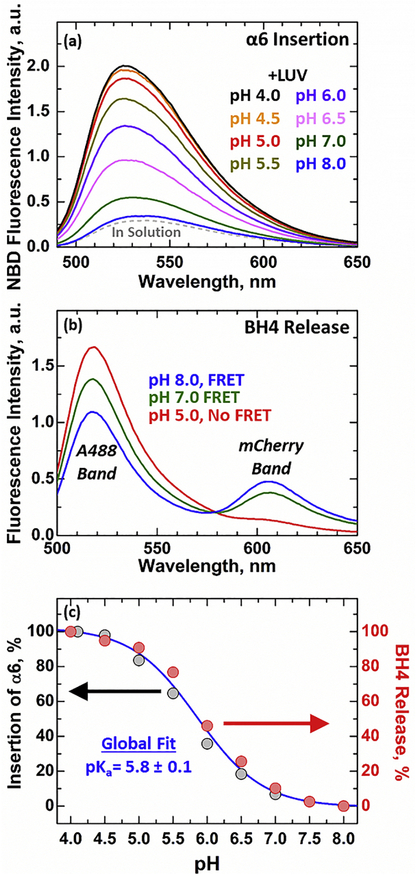
FIGURE 2. Protonation-dependent membrane insertion and refolding of Bcl-xL.
(a) Representative spectra of Bcl-xL ΔTM N175C-NBD inserting into cardiolipin containing LUV (1TOCL:2POPC). Increase in fluorescence intensity and blue shift in spectral position of maxima observed upon acidification in the presence of membranes are indicative of the membrane insertion of the NBD-labeled site. (b) Changes in fluorescence emission spectra of donor- and acceptor-labeled Bcl-xL ΔTM upon acidification in the presence of 1TOCL:2POPC LUV. The release of BH4 domain was determined by the loss of FRET between Alexa 488 (donor), attached to single-Cys mutant D189C, and mCherry (acceptor) fused to the N-terminus next to the BH4 domain. (c) Comparison of protonation-dependent insertion and refolding of Bcl-xL (see text for details). Both datasets can be accurately described by a single global fitting curve (blue).
The release of the N-terminal BH4 domain was characterized by conjugating the fluorescent protein mCherry to the N-terminus of Bcl-xL ΔTM. This construct was then used to perform FRET measurements between N-terminal mCherry and Alexa488 (A488) labeled Bcl-xL ΔTM. The fluorescent probe A488 was introduced using the single Cys D189C mutant, positioning the donor fluorophore in the folded structure <20 Å of the N-terminus to which the mCherry acceptor is attached. Both fluorophores form an efficient FRET pair (R0 ~61 Å) when Bcl-xL is in solution and its BH4 domain is tucked into the main body of the protein (Fig. 1a). This is evident by the two bands observed in their emission spectra when only the donor A488 is excited in the presence of 1TOCL:2POPC LUV, pH 8.0 (Fig. 2b, blue). Acidification in the presence of membranes, however, leads to complete loss of FRET, indicated by the large increase in the intensity of the donor A488 band and the elimination of the acceptor mCherry peak at low pH (Fig. 2b, red). These spectroscopic changes are related to the increase in spatial distance between the donor A488 at the main body of Bcl-xL ΔTM and the N-terminal acceptor mCherry and indicate the release of the N-terminal BH4 domain. Together with the observed protonation- dependent insertion of Bcl-xL ΔTM (Fig. 2a) these results suggest that the insertion of Bcl-xL into membranes leads to the release of its N-terminal BH4 domain.
To further explore the connection between the membrane insertion of Bcl-xL ΔTM and the release of its N-terminal BH4 domain we compared the relative signal changes in both events as a function of pH (Fig. 2c). The results are depicted as the percent change observed in the intensity signals for each respective event (NBD signal change: relative membrane insertion, % FRET loss: relative BH4 domain release). 100% release corresponds to the complete ablation of the acceptor mCherry band in FRET measurements and 100% insertion refers to the NBD intensity signal saturation observed at pH 4.5. This analysis showed that the relative changes in the insertion of Bcl-xL ΔTM into membranes completely overlapped with the release of its N- terminal BH4 domain. This overlap yielded a pKa = 5.8 ± 0.1 for both protonation-dependent transitions, indicating that both events are linked. Further kinetic studies will be required to investigate whether or not the two folding steps occur simultaneously.
Lipid modulation of Bcl-xL membrane insertion and BH4 domain release
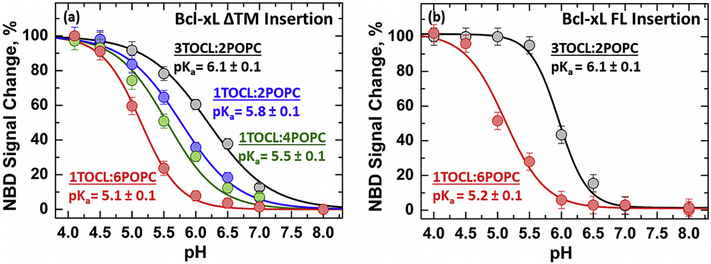
The activation of the apoptotic pathway leads to changes in the molar content of several mitochondrial lipids, including cardiolipin, that have been linked to apoptotic regulation 53,54,58–62, summarized in Fig. S1. To gain insight into the regulatory role of cardiolipin we measured the protonation-dependent insertion of Bcl-xL ΔTM into membranes containing increasing concentrations of cardiolipin (Fig. 3a). Our measurements show that the membrane insertion of Bcl-xL ΔTM, defined as the relative change in NBD intensity at a constant wavelength, becomes more favorable in membranes with higher cardiolipin content. This results in a 1.0 pH unit difference between the insertion pKa of Bcl-xL ΔTM in high cardiolipin membranes containing 3TOCL:2POPC (pKa = 6.1 ± 0.1) compared to the low cardiolipin membranes composed of 1TOCL:6POPC (pKa = 5.1 ± 0.1). Similar measurements were performed on the full-length variant of Bcl-xL (Bcl-xL FL N175C-NBD) to determine whether the cardiolipin-dependent effect observed was affected by the absence of the α8 helix in the ΔTM construct (Fig. 3b). The insertion of full-length Bcl-xL into membranes containing high (3TOCL:2POPC) or low (1TOCL:6POPC) cardiolipin content did not affect the calculated insertion pKa’s compared to the ΔTM variant. The only effect of the presence of the C-terminal helix α8 is a slight increase in the apparent cooperativity of the insertion transition. As pointed out in our previous publication, thermodynamic studies with a full-length protein are extremely difficult to carry out 25, therefore we do not attribute much significance to these changes. Nevertheless, it is clear that full-length Bcl-xL undergoes a very similar insertion process.
FIGURE 3. Membrane insertion of Bcl-xL is modulated by cardiolipin content.
Insertion of Bcl-xL was measured as a function of pH into membranes with increasing cardiolipin molar contents. Measurements were performed as in Fig. 2 using either Bcl-xL ΔTM (a) or full-length Bcl-xL (b). Increase in content of anionic cardiolipin shifts membrane insertion towards more neutral pH. This leads to a shift in the insertion pKa by 1.0 pH unit between low cardiolipin (1TOCL:6POPC) and high cardiolipin (3TOCL:2POPC) membranes. The presence of the C-terminal α8 helix in the full-length protein does not affect this lipid-dependent modulation of Bcl-xL insertion.
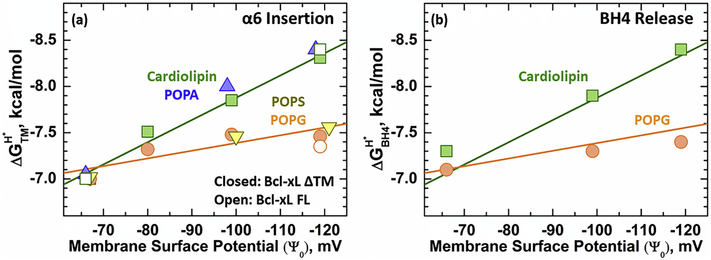
We further explored the modulation of Bcl-xL protonation-dependent membrane insertion by anionic membrane content using four different anionic lipids present in the mitochondria: cardiolipin (TOCL), phosphatidic acid (POPA), phosphatidylserine (POPS), and phosphatidylglycerol (POPG). The structure and relative abundance of studied lipids in the mitochondria before and during apoptosis are indicated in Fig. S1. Measurements were performed by inducing the insertion of Bcl-xL ΔTM N175C-NBD as a function of pH and measuring the changes in NBD intensity at a constant wavelength. The determined pKa’s were converted into protonation-dependent membrane insertion free energies (ΔGTMH+) using Eq. 2 in the methods section. The data is depicted in Fig. 4 as the calculated ΔGTMH+ plotted against membrane surface potential, which provides a measure of the electrostatic effect at the surface of anioinic membranes (calculated using the Gouy-Chapman model as described in the methods section). Under all membrane compositions tested, the protonation-dependent free energy of transmembrane insertion, ΔGTMH+ , showed a linear dependence with membrane surface potential, Ψ0 (Fig. 4a, solid symbols). Two different slopes arose to describe the linear releationship between Bcl-xL ΔTM membrane insertion and Ψ0, which were dependent on the type of anionic lipid present. The mitochondrial specific lipid cardiolipin, and POPA presented the most favorable slope; meanwhile, POPG and POPS had a shallower slope. Measurements performed at the endpoints of both slopes with Bcl-xL FL show that the lipid-specific effects on Bcl-xL membrane insertion are not affected by the presence or absence of the C-terminal α8 (Fig. 4a, clear symbols) anchor helix. The two different slopes observed in this analysis indicate that in addition to anionic lipid content, the insertion of Bcl-xL into membranes is modulated by other factors. In particular, it correlates with differences in lipid geometry between the tested anionic lipids. In the case of cardiolipin and POPA, the lack of a moiety in their headgroup is expected to result in a shallower interface compared to POPG and POPS (Fig. S1). Additional changes to non-electrostatic membrane properties such as differences in spontaneous curvature and lateral pressure, however, cannot be disregarded.
FIGURE 4. Effect of membrane surface potential (Ψo) on the insertion of α6 and release of Bcl-xL BH4 Domain.
Bcl-xL membrane insertion and the release of its N-terminal BH4 domain were characterized in several anionic lipid compositions. (a) Protonation-dependent free energy of transmembrane insertion (ΔGTMH+) was calculated from the measured transition pKa and plotted as a function of membrane surface potential (Ψ0). The membrane insertion of α6 is modulated by the bilayer anionic content and lipid geometry, giving rise to two different slopes. Closed symbols represent measurements performed with the Bcl-xL ΔTM variant, while open symbols denote the results using full-length Bcl-xL. (b) Release of the N-terminal BH4 domain was modulated in the same fashion as membrane insertion. The calculated protonation-dependent free energy for the release of the BH4 domain (ΔGBH4H+) overlapped with the previously observed membrane insertion slopes in Fig. 4a. These results confirm that both, the insertion of α6 and the release of the N-terminal BH4 domain correlate in all lipid compositions.
The protonation-dependent release of Bcl-xL ΔTM N-terminal BH4 domain was also measured in membranes with different anionic lipid species to confirm its correlation to Bcl-xL ΔTM membrane insertion. BH4 domain release was measured as previously described for Fig. 2b by measuring the loss of FRET in the mCherry-Bcl-xL ΔTM D189C-A488 construct in cardiolipin and POPG containing bilayers. The protonation-dependent free energy of BH4 domain release (ΔGBH4H+) was calculated from determined pKa’s as for Fig. 4a and plotted against membrane surface potential, Ψ0 (Fig. 4b). The calculated ΔGBH4H+ were compared to Bcl-xL ΔGTMH+ membrane insertion slopes determined in Fig. 4a. Our analysis shows that the calculated ΔGBH4H+ values overlap with ΔGTMH+ membrane insertion slopes regardless of lipid composition, confirming the link between BH4 domain release and Bcl-xL membrane insertion.
Topology of hydrophobic α6 helix in membrane inserted Bcl-xL
We confirmed the membrane insertion of Bcl-xL by characterizing the topology of α6 in membrane inserted Bcl-xL. This was achieved using a combination of electron paramagnetic resonance (EPR) and fluorescence depth-dependent quenching measurements. To ensure uniformity of our membrane inserted Bcl-xL population we used 3TOCL:2POPC or 75POPG:25POPC membranes, which showed the most favorable insertion ΔGBH4H+ Measurements were performed using the Bcl-xL ΔTM variant to prevent any anchored intermediate and insertion induced by decrease in pH.
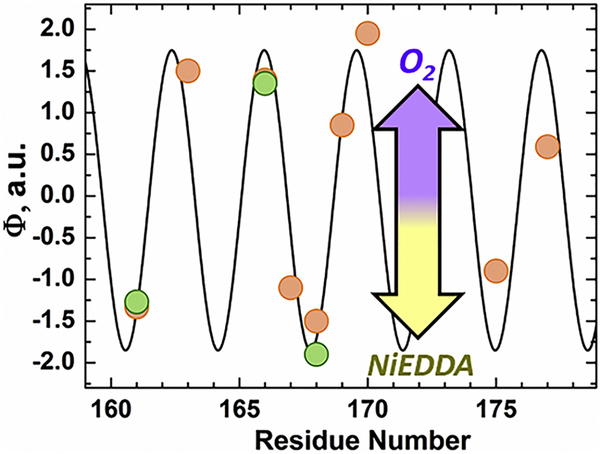
EPR experiments were first carried out to determine the membrane topology of selected residues along helix α6 in Bcl-xL ΔTM, which were replaced by spin-label R1, one amino acid at a time. (Fig. 5). The membrane immersion depths of spin-labeled side chains were then determined from their respective accessibilities to O2 and NiEDDA. These measurements are based on the fact that the more hydrophobic O2 preferentially partitions into the membrane while the more hydrophilic NiEDDA mainly remains in the aqueous environment 51,52. As a consequence, more deeply membrane inserted residues become progressively more accessible to O2 and less accessible to NiEDDA. The log of the ratio of the O2 and NiEDDA accessibilities is directly proportional to membrane immersion depth and it is typically summarized by the depth parameter Φ. As shown in Fig. 5, the Φ-values strongly depend on the labeling position. High values are observed for residues on the hydrophobic surface, while low (negative) values are obtained for residues on the hydrophilic helix surface. Such behavior is commonly observed for amphipathic helices that are asymmetrically solvated by the membrane on their hydrophobic side and by an aqueous environment on their hydrophilic side. The notion of an asymmetrically solvated amphipathic helix is further supported by the sinusoidal curve shown in Fig. 5. This curve fits the data well and has a periodicity of 3.6 amino acids, the number of amino acids required for one turn of an α-helix.
FIGURE 5. EPR O2/NiEDDA accessibility of MTSL labeled helix α6 in membrane inserted Bcl-xL.
Accessibility of spin labeled Bcl-xL ΔTM single-Cys mutants inserted into 75POPG:25POPC (orange) LUV at pH 4.5. Residues that were also measured in 3TOCL:2POPC LUV at pH 4.5 are indicated in green. Results are plotted as the ratio in quenching between membrane accessible O2 and water-soluble Ni-EDDA for each residue tested. Accessibility was determined as described in the methods section. A Φ > 0 represents membrane-protected spin probes and a Φ < 0 denotes a more solvent exposed probe. The black trace represents a cosine fit of the quenching data, which yielded a periodicity of 3.6 residues, consistent with an α-helix. The asymmetric protection of spin-labeled residues along α6 from O2/NiEDDA indicates an asymmetrically solvated α-helix.
Two distinct topologies can result in asymmetric solvation of membrane helices. The first scenario is for α6 to exist in a transmembrane orientation, where it is aligned with neighboring inserted helices to form a “pore”. The second possibility is an interfacial orientation for the hydrophobic α6 helix, perpendicular to the bilayer normal. The finding that the maxima for Φ have comparable values suggests that the immersion depth of membrane exposed residues is relatively constant. Such a behavior is typically seen for interfacial, but not transmembrane helices.
In either orientation half of the probes placed along α6 would be partially exposed to soluble quenchers, while the other half remain protected by the membrane. To distinguish between these possibilities, we performed depth-dependent fluorescent quenching using the distribution analysis (DA) methodology, which we have described recently for diphtheria toxin T-domain 43. This technique relies on measuring the steady-state and lifetime fluorescence of NBD labeled Bcl-xL, with fluorescent probes placed along α6. Four residues were selected along the α6 helix, two in the middle: W169C and A168C and one on each end: W177C and V161C (Fig. 6i). Measurements were conducted on membranes with high cardiolipin 3TOCL:2POPC at pH 4.5 to ensure insertion in the presence or absence of 30 mol% spin-lipid quenchers introduced by co- extrusion (as described in the methods section). Five different spin-lipid NBD quenchers were selected with either TEMPO or Doxyl moieties attached at progressively deeper positions along the lipids 63. Their spacing in the membrane therefore results in different quenching levels as a function of their distance to the fluorophore.
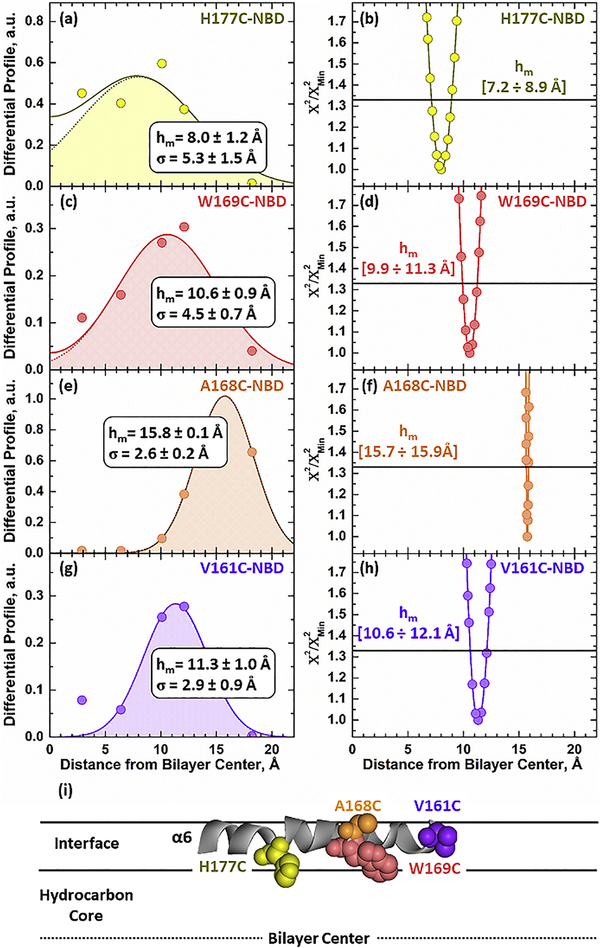
FIGURE 6. Depth-dependent fluorescence quenching of NBD selectively attached along the α6 helix in membrane inserted Bcl-xL.
The following positions along α6 were selectively labeled (top to bottom): 177, 169, 168 and 161. Insertion of Bcl-xL ΔTM NBD-labeled mutants was initiated by mixing the samples incubated with 3TOCL:2POPC LUV (no quencher composition) at pH 4.5. The vesicles that contained quenchers had 30% of one of the five spin-labeled lipids used substituting an equimolar concentration of POPC. Left panels: Differential Quenching Profiles for each tested mutant were obtained by subtracting the dynamic quenching component from steady-state quenching measurements (original data are presented in Fig. S2). The most probable depth (hm) of each labeled residue and the width of the transverse distribution (σ) were obtained by fitting the data to Eq. 5. Panels on the right: Support-plane analysis of the robustness of the fits used in the determination of the bilayer depth (see text for details). (i) All labeling sites along α6 were 8–16 Å away from the center of the bilayer, eliminating the possibility of a transmembrane orientation. The interfacial orientation for α6 is also consistent with EPR O2/NiEDDA accessibility measurements (Fig. 5).
The differential quenching profiles for the depth-dependent measurements in membranes containing 3TOCL:2POPC are shown in Fig. 6. They are obtained by subtracting the lifetime profile from the steady-state profile and depict the quenching distribution of the NBD fluorophore within the membrane. In the case of transmembrane helices, it is expected that residues near the center of the helix be located near the center of the bilayer at 0 Å, while the ends remain near the phosphates. All the quenching profiles of the α6 helix in membrane inserted Bcl-xL ΔTM, however, indicate that the labeled residues are far from the bilayer center. This includes residues W169C and A168C, located in the middle of the helix and indicate that the helix does not insert in a transmembrane orientation. Quantitative analysis of the depth- dependent measurements was performed using the distribution analysis (DA) methodology. This involves fitting the quenching data to a sum of two gaussian functions (Eq. 5) to account for quenching from the trans-leaflet. Determined parameters include the most probable depth (hm) and dispersion of the transverse position (σ), the colored profile depicts a data fit using a single gaussian function. Using this analysis, we determined that all tested residues were located far from the center of the bilayer at the following positions: H177C-NBD at 8.0 ± 1.2 Å, W169C- NBD at 10.6 ± 0.9 Å, A168C-NBD at 15.8 ± 0.1 Å, and V161C-NBD at 11.3 ± 1.0 Å away from the center of the bilayer (Fig. 6a, c, e, and g).
The robustness of the calculated depth, hm, parameter associated with the fit was determined by subjecting both the total quenching and differential profiles to support plane analysis 43,50 (Fig. 6b, d, f, and h). For this analysis the Χ2 goodness of the least-square fit was determined for a series of solutions using a fixed hm parameter around the optimal fit using 0.1–0.3 Å steps. A cutoff of 1 standard deviation (horizontal line at Χ2/Χ2Min = 1.33) was used to estimate the ranges in hm that yield an indistinguishable fit, indicated below the line. For every residue the support plane analysis shows a narrow error distribution around the optimal hm value calculated in the differential profile using the DA analysis. The optimization of the DA analysis compared to using raw data to analyze depth dependence can be clearly shown in the case of A168C-NBD (Fig. 6f). The support plane analysis shows that in the case of this residue the error associated with the fit of the raw data was greater than the thickness of a monolayer (hm= [15.0 ÷ >20 Å]). In contrast, a more defined fit is obtained by subtracting the dynamic quenching component, resulting in a more accurate error distribution. Together with our EPR measurements, these results confirm an asymmetrically solvated α6 helix in a deep interfacial orientation (Fig. 6i). The variance in depth between these residues was attributed to differences in the orientation of the tested labeled residues along the interfacial helix. Primary steady-state (blue triangles) and lifetime (green squares) data collected for all tested mutants is presented in Fig. S2. The lower quenching levels observed with the lifetime data (green) compared to the total quenching (steady-state, blue) are common 43–45 and reflects the “dynamic” component of the quenching process occurring on timescales longer than 0.1 ns.
Depth-dependent quenching measurements were also carried out in membranes containing 75POPG:25POPC (equivalent Ψ0 as 3TOCL:2POPC). Two mutants in the middle of α6 were selected for these measurements: W169C-NBD and A168C-NBD. Both residues yielded depths far from the center of the bilayer, with DA analysis placing them at hm = 9.9 ± 0.5 Å for W169C-NBD and hm = 11.3 ± 0.5 Å for A168C-NBD (Fig. S3). These results are consistent with our experiments in cardiolipin containing membranes (Fig. 6) and point to the interfacial orientation of the helix regardless of lipid composition.
DISCUSSION
The Bcl-2 family of proteins regulates the permeabilization of the mitochondrial outer membrane (MOMP), a key cellular process that results in cell death 64. The well-accepted “Embedded Together” model states that critical interactions between pro- and anti-apoptotic members of the Bcl-2 family leading to MOMP occur on the mitochondrial membrane 3,7.Generally, the transition between soluble and membraneous conformations can follow two scenarios (both implemented by the apoptotic inhibitor Bcl-xL, studied here): anchoring of a relatively unperturbed protein to the lipid bilayer (e.g., in tail-anchored proteins 65–67) or a complete refolding and bilayer insertion of the protein (e.g., in bacterial toxins). In the latter case, the properties of the lipid bilayer are expected to play an important role in modulating the structural and thermodynamic characteristics of the insertion. First, the membrane-inserted conformation will abide to general physicochemical rules that govern the organization of membrane proteins in the lipid bilayer 68. Second, the variation in lipid composition will modulate the insertion transition via changes in parameters such as charge density, spontaneous curvature and physical dimensions of the hydrocarbon core and interfacial regions. Below we discuss how structural and thermodynamic properties of the bilayer insertion of Bcl-xL are modulated by lipid composition.
Refolding of Membrane Inserted Bcl-xL
The anti-apoptotic regulation of the pore former Bax by Bcl-xL has been proposed to occur by two different non-exclusive mechanisms. 1) The canonical Bcl-xL anti-apoptotic mechanism involves its binding to the BH3 domain of pro-apoptotic Bax 69. 2) More recently Barclay et al. proposed an alternative non-canonical anti-apoptotic mechanism involving the N-terminal BH4 domain (α1 helix) of Bcl-2, an anti-apoptotic Bcl-xL homolog, binding BAX 31. This N-terminal domain is closely linked to apoptotic progression and regulation and its deletion is lethal when expressed in cells 55–57. The BH4 domain of Bcl-xL is connected through a very long loop consisting of 62 a.a. (≥ 100 Å in an extended conformation) to the rest of the protein. The release of BH4 from the inserted form of the protein would greatly enhance its ability to scan the surrounding area and increase its probability of interacting with Bax (Fig. 1c). We have studied the release of BH4 using FRET between Alexa 488 dye selectively attached at position 189 of Bcl-xL and mCherry fluorescent protein fused at the N-terminus of the BH4 domain via a short flixible linker. As expected, in the folded state the FRET is readily observed by the presence of the sensitized emission peak of the acceptor, when the donor is excited (Fig. 2b). Our results show that the intensity of sensitized emission decreases upon insertion, and the intensity of the donor increases, indicating the loss of FRET. The complete loss of FRET observed in our measurements indicates that the separation of donor and acceptor is greater than the R0 in the FRET pair used (~60 Å). Furthermore, the reduction in FRET appears to correlate with the insertion-associated increase in intensity of the environment-sensitive probe NBD attached at residue 175 of α6 (Fig. 2c). This indicates that the release of BH4 is closely associated with the refolding accompanying the membrane insertion of Bcl-xL. In addition, both BH4 release and insertion are modulated by membrane lipid composition in a similar fashion (Fig. 4).
The high-resolution structure of the membrane-inserted form of Bcl-xL remains unavailable, while the suggested low-resolution topology model is traditionally based on that for the diphtheria toxin translocation (T) domain 30. The latter undergoes a dramatic refolding on the membrane interface which results in a transmembrane positioning of the hydrophobic hairpin TH8–9 29,43,70–72. Because the soluble conformations of many Bcl-2 proteins (including pro-apoptotic Bax and anti-apoptotic Bcl-xL) are similar to that of the soluble T domain, it has been assumed that upon bilayer insertion their central helical hairpin (i.e., α5–6 in Bcl-xL and Bax) will be positioned in a transmembrane orientation. Here we have tested this assumption using EPR and fluorescence spectroscopy using selective labeling of a series of single cysteine mutants along the α6 helix.
The transition of Bcl-xL into its inserted conformation was confirmed by characterizing the topology of the hydrophobic α6 helix by EPR (Fig. 5) and depth-dependent quenching (Fig. 6 and S2-3). Both techniques are in agreement and indicate that the α6 helix inserts into the bilayer as a partially solvated helix in an interfacial orientation. Our depth-dependent quenching measurements place the deepest residue tested in the helix H177C-NBD at 8 Å and the shallowest A168C-NBD at 16 Å away from the center of the bilayer in cardiolipin membranes. Essentially the same results were observed in membranes containing POPG instead of cardiolipin, with both residues tested in the middle of the helix (A168C and W169C) being located at least 10 Å away from the center of the bilayer (Fig. S3). This is quite different from our published results on the corresponding helix in the diphtheria toxin T domain, for which the depth of several centrally located residues was <5 Å 43,73. Based on these EPR and fluorescence data we conclude that the central hairpin of Bcl-xL (unlike that of the T domain) does not form a transmembrane structure. Thus, the long-standing analogy of the structural aspects of the insertion of Bcl-xL (and perhaps other Bcl-2 proteins) to the insertion of the T domain can be put to rest. Previously, we have demonstrated that such analogy does not work from a thermodynamic perspective either and suggested that lipids may play a critical role in the protonation-triggered insertion of Bcl-xL 25, which has been demonstrated here.
Lipid-dependent Modulation of Bcl-xL Membrane Insertion
Several studies have suggested that mitochondrial membrane lipids play a role in the action of the Bcl-2 proteins and therefore in the regulation of apoptosis 2,23,25,58–62,74–80. The most prominent example is that of cardiolipin, an anionic phospholipid specific to mitochondria 81,82, which is strongly linked to apoptotic regulation 58–62. The exact mechanism behind the role of lipids on the modulation of apoptosis as a whole, however, remains unclear. Here we conducted a systematic study of Bcl-xL insertion and refolding into membranes containing different amounts of various anionic lipids (Figs. 3–4).
The free energy associated with the protonation-dependent membrane insertion of Bcl-xL has a complex dependence on lipid composition (Fig. 3 and 4a). It depends linearly on the surface membrane potential (Ψ0), but the slope is different for different lipids, and could be subdivided into two families: (1) Strong dependence between ΔGTMH+ on Ψ0 seen for cardiolipin (TOCL) and phosphatidic acid (POPA) and (2) a weaker dependence seen for membranes containing phosphatidylglycerol (POPG) and phosphatidylserine (POPS). One possibility for this differential regulation is the size of the phospholipid headgroup, as illustrated in Fig. S1. Cardiolipin and phosphatidic acid have no functional group attached to the phosphate group, and therefore the thickness of the membrane interface is expected to be relatively small. Phosphatidylglycerol and phosphatidylserine, on the other hand, have functional groups attached to the phosphate group, making the membrane interface thicker. The difference in the size of the interfacial region is also connected to the variation in the spontaneous curvature of the bilayer, which is expected to affect the insertion efficiency. Regardless of the exact interpretation, our results suggest that a combination of the surface membrane potential produced by anionic lipids and non-electrostatic membrane properties modulate the membrane insertion of Bcl-xL.
On the possible physiological role of the membrane-inserted conformation of Bcl-xL
The modulation of protonation states in Bcl-xL titratable residues will affect its interaction and refolding into the membrane, resulting in the release of the regulatory BH4 domain. We suggest that this release observed upon acidification in model systems can be relevant to the switching between canonical and non-canonical forms of inhibition of MOMP in the cells. Our results indicate that changes in lipid composition can bring the transition to the threshold of cellular pH, which is arguably the best position for the regulation. For example, any additional favorable interaction that brings as little as 1.0 kcal/mole in terms of free energy (equivalent of 0.8 pH unit shift in pKa), will result in an estimated 10-fold increase in the population of the inserted form according to the titration results in Fig. 2.
Multiple factors can influence protonation in the cellular environment 83, including variation in pH and specific interactions that can affect the pKa’s of residues (i.e., membrane binding) 84 critical for conformational switching. Our studies with other membrane-inserting proteins and peptides, such as diphtheria toxin 27,29,43, annexin B12 85, gp41-derived peptide 86, pHLIP 87,88, and Bcl-xL 25 itself, show that the membrane interface can modulate membrane insertion and refolding through membrane-driven changes in protonation propensity. The results presented here indicate that the membrane interactions of Bcl-xL depend heavily on the chemical properties of the membrane interface. Specifically, we show that negatively charged membranes shift the pKa of both its membrane insertion and refolding towards more neutral pH values. This type of changes on the mitochondria can lead to substantial fractions of Bcl-xL refolding into the lipid bilayer, provided that the membrane surface has a sufficient anionic charge. Thus, the physiological action of Bcl-xL may not require a dramatic change in pH in vivo, but instead, a change in lipid composition. We hypothesize that changes in the mitochondrial lipid composition, which are known to occur during apoptosis 2,59,74,76, modulate the membrane insertion and refolding of Bcl-xL into the membrane. This association would likely occur on regions of the membrane rich in anionic lipids, such as the contact sites between the inner and outer mitochondrial membranes, where the mole percent of the anionic lipid cardiolipin is increased 53,54. Indeed, several studies have found other Bcl-2 proteins localizing to these contact sites 89–91. It is possible that such hot spots will require non-canonical inhibition modes to prevent sporadic MOMP, something that should be tested in subsequent cellular studies.
Supplementary Material
Membrane interactions of Bcl-xL are characterized by fluorescence and EPR
Membrane insertion corelates with the release of BH4 regulatory domain
Lipids modulate insertion and folding transitions of the Bcl-xL
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (R01 GM126778).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang C & Youle RJ The role of mitochondria in apoptosis*. Annual review of genetics 43, 95–118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosentino K & García-Sáez AJ Mitochondrial alterations in apoptosis. Chemistry and physics of lipids (2014). [DOI] [PubMed] [Google Scholar]
- 3.Leber B, Lin J & Andrews DW Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene 29, 5221–5230, doi: 10.1038/onc.2010.283 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi X, Kale J, Leber B & Andrews DW Regulating cell death at, on, and in membranes. Biochimica et biophysica acta 1843, 2100–2113, doi: 10.1016/j.bbamcr.2014.06.002 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Moldoveanu T, Follis AV, Kriwacki RW & Green DR Many players in BCL-2 family affairs. Trends in biochemical sciences 39, 101–111, doi: 10.1016/j.tibs.2013.12.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardwick JM & Youle RJ SnapShot: BCL-2 proteins. Cell 138, 404, 404 e401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogner C, Leber B & Andrews DW Apoptosis: embedded in membranes. Current opinion in cell biology 22, 845–851, doi: 10.1016/j.ceb.2010.08.002 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Leber B, Lin J & Andrews DW Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis : an international journal on programmed cell death 12, 897–911, doi: 10.1007/s10495-007-0746-4 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna-Vargas MP & Chipuk JE The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. The FEBS journal, doi: 10.1111/febs.13624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei MC et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730, doi: 10.1126/science.1059108 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walensky LD & Gavathiotis E BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends in biochemical sciences 36, 642–652, doi: 10.1016/j.tibs.2011.08.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todt F, Cakir Z, Reichenbach F, Youle RJ & Edlich F The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ 20, 333–342, doi: 10.1038/cdd.2012.131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edlich F et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116, doi: 10.1016/j.cell.2011.02.034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolter KG et al. Movement of Bax from the cytosol to mitochondria during apoptosis. The Journal of cell biology 139, 1281–1292 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu YT, Wolter KG & Youle RJ Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proceedings of the National Academy of Sciences of the United States of America 94, 3668–3672 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billen LP, Kokoski CL, Lovell JF, Leber B & Andrews DW Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS biology 6, e147, doi: 10.1371/journal.pbio.0060147 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muchmore SW et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335–341 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Lessene G et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nature chemical biology 9, 390–397, doi: 10.1038/nchembio.1246 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Lee EF et al. The functional differences between pro-survival and pro-apoptotic B cell lymphoma 2 (Bcl-2) proteins depend on structural differences in their Bcl-2 homology 3 (BH3) domains. The Journal of biological chemistry 289, 36001–36017, doi: 10.1074/jbc.M114.610758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y et al. Conformation of BCL-XL upon membrane-integration. Journal of Molecular Biology, doi: 10.1016/j.jmb.2015.02.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuduppathy GR, Craig JW, Kholodenko V, Schon A & Hill RB Evidence that membrane insertion of the cytosolic domain of Bcl-xL is governed by an electrostatic mechanism. J Mol Biol 359, 1045–1058, doi: 10.1016/j.jmb.2006.03.052 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thuduppathy GR, Terrones O, Craig JW, Basanez G & Hill RB The N-terminal domain of Bcl-xL reversibly binds membranes in a pH-dependent manner. Biochemistry 45, 14533–14542, doi: 10.1021/bi0616652 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Saez AJ, Ries J, Orzaez M, Perez-Paya E & Schwille P Membrane promotes tBID interaction with BCL(XL). Nat Struct Mol Biol 16, 1178–1185 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Minn AJ et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 385, 353–357 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Vargas-Uribe M, Rodnin MV & Ladokhin AS Comparison of membrane insertion pathways of the apoptotic regulator Bcl-xL and the diphtheria toxin translocation domain. Biochemistry 52, 7901–7909, doi: 10.1021/bi400926k (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy JR Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process. Toxins 3, 294–308, doi: 10.3390/toxins3030294 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladokhin AS pH-triggered conformational switching along the membrane insertion pathway of the diphtheria toxin T-domain. Toxins 5, 1362–1380, doi: 10.3390/toxins5081362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodnin MV et al. Conformational switching of the diphtheria toxin T domain. J Mol Biol 402, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyrychenko A, Posokhov YO, Rodnin MV & Ladokhin AS Kinetic intermediate reveals staggered pH-dependent transitions along the membrane insertion pathway of the diphtheria toxin T-domain. Biochemistry 48, 7584–7594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antignani A & Youle RJ How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Current opinion in cell biology 18, 685–689 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Barclay LA et al. Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism. Mol Cell 57, 873–886, doi: 10.1016/j.molcel.2015.01.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haugland RP Handbook of Fluorescent Probes and Research Chemicals. 6th edn, (Molecular Probes, Inc., 1996). [Google Scholar]
- 33.Hope MJ, Bally MB, Mayer LD, Janoff AS & Cullis PR Generation of multilamellar and unilamellar phospholipid vesicles. Chem.Phys.Lipids 40, 89–107 (1986). [Google Scholar]
- 34.Mayer LD, Hope MJ & Cullis PR Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim.Biophys.Acta 858, 161–168 (1986). [DOI] [PubMed] [Google Scholar]
- 35.Ladokhin AS Fluorescence spectroscopy in thermodynamic and kinetic analysis of pH-dependent membrane protein insertion. Methods Enzymol. 466, 19–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin S The electrostatic properties of membranes. Annual review of biophysics and biophysical chemistry 18, 113–136 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Kučerka N, Nieh M-P & Katsaras J Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochimica et Biophysica Acta (BBA)-Biomembranes 1808, 2761–2771 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Pan J et al. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small angle neutron and X-ray scattering. Biochimica et Biophysica Acta (BBA)-Biomembranes 1818, 2135–2148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan J et al. The molecular structure of a phosphatidylserine bilayer determined by scattering and molecular dynamics simulations. Soft matter 10, 3716–3725 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Cheng MH, Liu LT, Saladino AC, Xu Y & Tang P Molecular dynamics simulations of ternary membrane mixture: phosphatidylcholine, phosphatidic acid, and cholesterol. The Journal of Physical Chemistry B 111, 14186–14192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan J et al. Structural and mechanical properties of cardiolipin lipid bilayers determined using neutron spin echo, small angle neutron and X-ray scattering, and molecular dynamics simulations. Soft matter 11, 130–138 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Posokhov YO & Ladokhin AS Lifetime fluorescence method for determining membrane topology of proteins. Analytical biochemistry 348, 87–93 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Kyrychenko A et al. Refining Protein Penetration into the Lipid Bilayer Using Fluorescence Quenching and Molecular Dynamics Simulations: The Case of Diphtheria Toxin Translocation Domain. The Journal of membrane biology, doi: 10.1007/s00232-018-0030-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyrychenko A & Ladokhin AS Refining membrane penetration by a combination of steady-state and time-resolved depth-dependent fluorescence quenching. Analytical biochemistry 446, 19–21, doi: 10.1016/j.ab.2013.10.015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyrychenko A, Rodnin MV & Ladokhin AS Calibration of Distribution Analysis of the Depth of Membrane Penetration Using Simulations and Depth-Dependent Fluorescence Quenching. The Journal of membrane biology, doi: 10.1007/s00232-014-9709-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ladokhin AS Distribution analysis of depth-dependent fluorescence quenching in membranes: A practical guide. Methods Enzymol. 278, 462–473 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Ladokhin AS Measuring membrane penetration with depth-dependent fluorescence quenching: distribution analysis is coming of age. Biochimica et biophysica acta 1838, 2289–2295, doi: 10.1016/j.bbamem.2014.02.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladokhin AS Analysis of protein and peptide penetration into membranes by depth-dependent fluorescence quenching: Theoretical considerations. Biophys.J. 76, 946–955 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyrychenko A & Ladokhin AS Molecular Dynamics Simulations of Depth Distribution of Spin-Labeled Phospholipids within Lipid Bilayer. The journal of physical chemistry. B 117, 5875–5885, doi: 10.1021/jp4026706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery DC & Peck EA Introduction to linear regression analysis. (Wiley, 1982). [Google Scholar]
- 51.Ambroso MR, Hegde BG & Langen R Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation. Proceedings of the National Acadey of Sciences of the United States of America 111, 6982–6987, doi: 10.1073/pnas.1402233111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altenbach C, Greenhalgh DA, Khorana HG & Hubbell WL A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: Application to spin-labeled mutants of bacteriorhodopsin. Proc.Natl.Acad.Sci.USA 91, 1667–1671 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ardail D et al. Mitochondrial contact sites: Lipid composition and dynamics. J.Biol.Chem. 265, 18797–18802 (1990). [PubMed] [Google Scholar]
- 54.Daum G Lipids of mitochondria. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes 822, 1–42 (1985). [DOI] [PubMed] [Google Scholar]
- 55.Ofengeim D et al. N-terminally cleaved Bcl-xL mediates ischemia-induced neuronal death. Nature neuroscience 15, 574–580, doi: 10.1038/nn.3054 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang DC, Adams JM & Cory S The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. The EMBO journal 17, 1029–1039, doi: 10.1093/emboj/17.4.1029 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clem RJ et al. Modulation of cell death by Bcl-XL through caspase interaction. Proceedings of the National Academy of Sciences of the United States of America 95, 554–559 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schug ZT & Gottlieb E Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochimica et Biophysica Acta (BBA)-Biomembranes 1788, 2022–2031 (2009). [DOI] [PubMed] [Google Scholar]
- 59.McMillin JB & Dowhan W Cardiolipin and apoptosis. Biochimica et biophysica acta 1585, 97–107 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Aisha S-D et al. Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. Biochemical Journal (2015). [DOI] [PubMed] [Google Scholar]
- 61.Lucken-Ardjomande S, Montessuit S & Martinou J-C Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death & Differentiation 15, 929–937 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Esposti MD Lipids, cardiolipin and apoptosis: a greasy licence to kill. Cell death and differentiation 9, 234 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Kyrychenko A, Tobias DJ & Ladokhin AS Validation of Depth-Dependent Fluorescence Quenching in Membranes by Molecular Dynamics Simulation of Tryptophan Octyl Ester in POPC Bilayer. The journal of physical chemistry. B 117, 4770–4778, doi: 10.1021/jp310638f (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Youle RJ & Strasser A The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular cell biology 9, 47–59 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Borgese N, Brambillasca S & Colombo S How tails guide tail-anchored proteins to their destinations. Current opinion in cell biology 19, 368–375 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Borgese N, Colombo S & Pedrazzini E The tale of tail-anchored proteins: coming from the cytosol and looking for a membrane. The Journal of cell biology 161, 1013–1019 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brambillasca S et al. Transmembrane topogenesis of a tail-anchored protein is modulated by membrane lipid composition. The EMBO journal 24, 2533–2542 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White SH, Ladokhin AS, Jayasinghe S & Hristova K How membranes shape protein structure. J.Biol.Chem. 276, 32395–32398 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Czabotar PE et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531, doi: 10.1016/j.cell.2012.12.031 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Oh KJ et al. Organization of diphtheria toxin T domain in bilayers: A site-directed spin labeling study. Science 273, 810–812 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Oh KJ et al. Conformation of the diphtheria toxin T domain in membranes: A site-directed spin-labeling study of the TH8 helix and TL5 loop. Biochemistry 38, 10336–10343 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Rosconi MP, Zhao G & London E Analyzing topography of membrane-inserted diphtheria toxin T domain using BODIPY-streptavidin: At low pH, helices 8 and 9 form a transmembrane hairpin but helices 5–7 form stable nonclassical inserted segments on the cis side of the bilayer. Biochemistry 43, 9127–9139 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Kurnikov IV et al. pH-Triggered Conformational Switching of the Diphtheria Toxin T-Domain: The Roles of N-Terminal Histidines. J Mol Biol 425, 2752–2764, doi: 10.1016/j.jmb.2013.04.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cristea IM & Degli Esposti M Membrane lipids and cell death: an overview. Chem Phys Lipids 129, 133–160, doi: 10.1016/j.chemphyslip.2004.02.002 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Martínez-Abundis E, Garcia N, Correa F, Franco M & Zazueta C Changes in specific lipids regulate BAX-induced mitochondrial permeability transition. Febs Journal 274, 6500–6510 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Crimi M & Esposti MD Apoptosis-induced changes in mitochondrial lipids. Biochimica et biophysica acta 1813, 551–557, doi: 10.1016/j.bbamcr.2010.09.014 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Zhao K et al. Phosphatidic acid mediates the targeting of tBid to induce lysosomal membrane permeabilization and apoptosis. Journal of lipid research 53, 2102–2114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho EY, Yun C-H & Ahn T Effects of phospholipids on the functional regulation of tBID in membranes. Molecular and cellular biochemistry 363, 395–408 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Mignard V, Lalier L, Paris F & Vallette F Bioactive lipids and the control of Bax pro-apoptotic activity. Cell death & disease 5, e1266–e1266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang T & Saghatelian A Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1831, 1542–1554 (2013). [DOI] [PubMed] [Google Scholar]
- 81.van Meer G, Voelker DR & Feigenson GW Membrane lipids: where they are and how they behave. Nature reviews. Molecular cell biology 9, 112–124, doi: 10.1038/nrm2330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olofsson G & Sparr E Ionization constants pKa of cardiolipin. PloS one 8, e73040, doi: 10.1371/journal.pone.0073040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casey JR, Grinstein S & Orlowski J Sensors and regulators of intracellular pH. Nature reviews Molecular cell biology 11, 50–61 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Teixeira VH, Vila-Vicosa D, Reis PB & Machuqueiro M pK(a) Values of Titrable Amino Acids at the Water/Membrane Interface. J Chem Theory Comput 12, 930–934, doi: 10.1021/acs.jctc.5b01114 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Posokhov YO, Rodnin MV, Lu L & Ladokhin AS Membrane insertion pathway of annexin B12: thermodynamic and kinetic characterization by fluorescence correlation spectroscopy and fluorescence quenching. Biochemistry 47, 5078–5087 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Kyrychenko A et al. Structural plasticity in the topology of the membrane-interacting domain of HIV-1 gp41. Biophysical journal 106, 610–620, doi: 10.1016/j.bpj.2013.12.032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasquez-Montes V, Gerhart J, King KE, Thevenin D & Ladokhin AS Comparison of lipid-dependent bilayer insertion of pHLIP and its P20G variant. Biochimica et biophysica acta 1860, 534–543, doi: 10.1016/j.bbamem.2017.11.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kyrychenko A, Vasquez-Montes V, Ulmschneider MB & Ladokhin AS Lipid Headgroups Modulate Membrane Insertion of pHLIP Peptide. Biophysical journal 108, 791–794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim T-H et al. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Molecular biology of the cell 15, 3061–3072 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lutter M, Perkins GA & Wang X The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC cell biology 2, 22 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinou J-C & Youle RJ Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Developmental cell 21, 92–101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.