Abstract
Temporal sensitivity to multisensory stimuli has been shown to be reduced in obesity. We sought to investigate the possible role of the pro-inflammatory state on such alteration, considering the effect of the expression of markers, such as leptin and IL6, which are notably high in obesity. The performance of 15 male individuals affected by obesity and 15 normal-weight males was compared using two audiovisual temporal tasks, namely simultaneity judgment and temporal order judgment. Analyses of serum levels of inflammatory markers of leptin and IL6, and of neurotrophic factors of BDNF and S100SB were quantified. At the behavioral level we confirmed previous evidence showing poorer temporal sensitivity in obesity compared to normal-weight participants. Furthermore, leptin, that is a cytokine overexpressed in obesity, represented the best predictor of behavioral differences between groups in both tasks. The hypothesis we put forward is that the immune system, rather than overall cerebral dysfunction, might contribute to explain the altered temporal sensitivity in obesity. The present finding is discussed within the context of the role of cytokines on the brain mechanisms supporting temporal sensitivity.
Subject terms: Perception, Human behaviour
Introduction
The brain mechanism through which two or more different sensory input, when occur at the same time and place, are coordinate together to create an unified and coherent internal representation of the world, is known as multisensory integration1. It has a substantial survival value for humans: the construction of a coherent multimodal representation of the external world allows humans to take advantage of the redundancies and complementarities provided by multiple sensory modalities2. Thus, considering the large impact of this process on our perception, it is not surprising to observe a growing interest in studying multisensory integration, and under what circumstances this process takes place or defects. Indeed, multisensory integration difficulties has been recognized in several clinical conditions3–5; for example, our group recently provided evidence about aberrant temporal multisensory process in obesity6. Specifically, the behavioral measure of the audiovisual sensory integration, that it the temporal binding window, (i.e. “the epoch of time within which stimuli from different modalities is likely to be integrated and perceptually bound” according to the definition provided by Wallace and Stevenson (2014)7, resulted to be markedly different in the participants affected by obesity as compared to healthy-weight controls; our result mirrored what found previously by Wan and colleagues (2014)8 about the integration of audio-vibrotactile sensory input in overweight individuals.
A growing body of evidence suggests that the correct functioning of cerebral mechanisms, such as the synchronized network oscillations9 (i.e. the rhythmic or repetitive electrical activity generated spontaneously and in response to stimuli by neural tissue in the central nervous system; it is one of the neural mechanisms implicated in multisensory integration process10–13), is related to the immune systems14, which act on the central nervous system through chemical messengers15. In fact, as neural cells have receptors for cytokines, immune cells have receptors for various neurotransmitters16 involved in brain activity14. For example, the well-known γ-aminobutyric acid (GABA), that is the principal inhibitory neurotransmitter in the central nervous system17, is essential for the efficient brain functioning, mediating neuronal activity, information processing and plasticity, and the synchronized network oscillations14. Glutamate18 and GABA19 were found to predict individual differences in multisensory processing in healthy individuals: these components have inhibitory and exhibitory neuromodulatory effects, regulating the brain’s response to sensory perception20. Interestingly, as suggested by multiple research in animals and humans, as well as in pathological and clinical conditions, both glutamate21 and GABA22 are strictly related to inflammation: they can be considered as immunomodulators.
As in our knowledge, there is very few recent experimental evidence on the relationship between temporal sensitivity and inflammation. Focusing on obesity, as previously stated, our recent study6 and Wan and colleagues (2014)8’s work provided evidence about aberrant temporal multisensory process in this clinical condition. On the other hand, few research showed a correlation between resting state network dynamics and obesity23, and specifically an increased resting-state functional connectivity in severe obese adolescents24. How might these two different experimental evidences - the first one about an alteration of multisensory integration, the secondabout an alteration of cerebral activity in obesity be jointed? What might be the link between the functional cognitive process (i.e. multisensory integration) and cerebral activity? The turning point might be to look at obesity as not just a clinical condition caused by the accumulation of fat, but as a more complex condition in which metabolic dysfunction affects central nervous system. Specifically, it is now, even though not fully25 established, that obesity is associated with chronic low-grade systemic inflammation26,27; indeed the adipose tissue secretes a range of bioactive peptides and proteins28, included pro-inflammatory cytokines, with knock-on effects on other complex metabolic processes. Cytokines are a type of regulators of host responses to infection, immune responses, inflammation, and trauma29. When the cytokines actions result in a worsening of the disease, they are defined as pro-inflammatory; when they are overexpressed, as in in obesity28, they define a state of chronic systemic inflammation, meaning a prolonged condition in which the entire body is active to react to an illness. One the most studied product of the adipose tissue is leptin: it mediates the relationship between environment (intended as availability of nutrients), metabolism, and immune responses30. Another known product of the fat is the interleukin 6 (IL-6); the expression of this cytokine increases with adiposity31 with an important role in the expression of the other cytokines32. Interestingly, even though cytokines are produced at the level of adipose tissue (i.e. a peripheral inflammation), their negative consequences in obesity can be observed far away from the production site (i.e. systemic inflammation), influencing behavior as well as brain development and functions33,34. Brain is, indeed, a key target of the chronic low-grade inflammation, which is a risk factor for neuroinflammation and anatomical alterations25,35–38. The mechanisms according to inflammatory state might alter anatomically and functionally brain activity is not clear yet (see Miller and Spencer (2014)25, for a review). However, the existing relationship between and brain functioning, and the evidence showing altered brain functioning in obesity, pose the question whether inflammation might represent a link between obesity and altered brain mechanisms25, such as temporal sensitivity to multisensory stimuli.
Moreover, factors that are generally considered as indices of cerebral plasticity are found to be altered in obesity, in absence of neurological disease: this is the case for brain-derived neurotrophic factor (BDNF)39,40 and S100 calcium binding protein B (S100B)41,42. In details, BDNF plays a prominent role in the survival, growth, and maintenance of neurons during development43,44 in the adult age45. S100B, that is a member of the S100 protein family, is commonly used as a parameter of glial activation and/or death in different disorders of the central nervous system; since its role in normal cerebral development and recovery after injury, it was suggest to be a potential neurobiochemical marker of neurological disorders46.
Thus, given the tight link between the brain and the immune system15, we tested the hypothesis that inflammatory state in obesity might be related to poorer temporal sensitivity6. In particular, we address the question as to whether circulating levels of pro-inflammatory markers, such as leptin and IL-6, or markers of cerebral plasticity, such as BDND and S100B, are related to the temporal sensitivity in obesity, through a cross-sectional study. To this aim, the neurocognitive behavior of a sample of otherwise healthy obese men was tested by two experimental tasks assessing temporal sensitivity to multisensory stimuli6 compared to that obtained in a sample of control individuals with normal weight.
Blood levels of leptin, IL-6, BDNF and S100B were measured at the time of the testing in order to investigate the potential association with the pro-inflammatory pattern and its relation with neurotrophins. Pro-inflammatory cytokines production increases in response to acute psychological stress in humans47,48, suggesting a relationship between stress and immune functions49,50. Since this relationship appears to be critical in obesity51–53, we also measured the level of cortisol in our population sample.
Results
Demographic and clinical data
The participants with obesity and the healthy-weight participants were comparable in terms of Age (t(28) = 1.26; p = 0.21; d = 0.61; 95% CI [2; 8.4]) and years of Education (t(28) = 1.94; p = 0.062; d = 0.63; 95% CI [0.14; 5.34]). Data are reported in Table 1. As expected, participants affected by obesity reported a significantly higher BMI than normal-weight participants (t(28) = 18.68; p < 0.001; d = 6.83; 95% CI [18.21; 22.69]); specifically for participants with obesity the mean value was higher than 44, suggesting that this condition can be classified as “morbidly obese stage III”54. No differences were observed when comparing Beck Depression Inventory55,56 scores between the two groups (t(28) = 1.88; p = 0.07; d = 0.69; 95% CI [0.21; 5.28]). Moreover, both groups reported the same level of daytime sleepiness, as suggested by the score at the Epworth Sleepiness Scale57 (t(28) = 1.48; p = 0.14; d = 0.54; 95% CI [0.83; 5.23]).
Table 1.
Demographic and clinical data for the participants with obesity and the normal-weight participants.
| Participants with obesity | Healthy-weight participants | p-value | |
|---|---|---|---|
| Age | 34 (6) | 30 (7) | 0.21 |
| Education | 13 (4) | 15 (2) | 0.06 |
| BMI | 44.24 (3.7) | 23.7 (2) | <0.001* |
| BDI score | 6.4 (4.2) | 3.86 (3.06) | 0.07 |
| ESS score | 6.6 (4.98) | 4.4 (2.84) | 0.14 |
| Leptin | 42.77 (21.84) | 2.92 (2.04) | <0.001* |
| IL6 | 1.64 (0.79) | 0.49 (0.29) | <0.001* |
| BDNF | 17.82 (3.56) | 13.83 (3.02) | 0.003* |
| S100B | 23.24 (12.39) | 20.05 (17.53) | 0.57 |
| Cortisol | 12.84 (4.33) | 14.39 (3.63) | 0.3 |
Means and standard deviations (in brackets) are reported. Age in years is reported; Education is reported as years of school attended; BMI = body mass index express in units of kg/m2. BDI stands for the Beck Depression Inventory; ESS for the Epworth Sleepiness Scale. *Identifies a significant difference between the two groups.
Serum markers
Means and standard deviations of the serum marker levels are reported in Table 1. A significant difference emerged between groups in the serum levels of leptin (Levene’s test F = 27.82; p < 0.001; t (14.24) = 7.036; p < 0.001: d = 2.56; 95% CI [27.72; 51.98]), as well as IL6 (Levene’s test F = 12.17; p = 0.002; t (17.84) = 5.28; p < 0.001; d = 1.93; 95% CI [0.69; 1.61]) and BDNF (t(28) = 7.036; p = 0.003; d = 1.2; 95% CI [1.5; 6.45]). Instead, no differences were found for S100B (Levene’s test F = 4.59; p = 0.041; t(25.19) = 0.57; p = 0.57; 95% CI [8.17; 14.54]) and cortisol (t(27) = 1.03; p = 0.3; 95% CI [1.51; 4.6]).
Experimental tasks
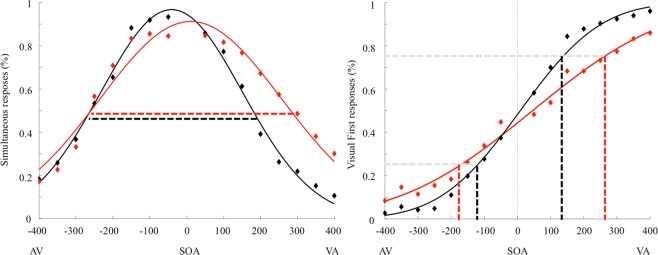
Regarding the SJ, we evaluated the goodness of fit of our mathematical model: considering the data relative to the participants with obesity, the mean R2 was 0.86, while for the normal-weight group it was 0.93. The Temporal Binding Windows (TBW) values violated normality (Shapiro-Wilk test, p = 0.046), hence data were log-transformed to obtain a normal distribution. According to the independent sample t-test, participants with obesity (Mean = 343 ms; SD = 84 ms) showed a wider TBW respect to the normal-weight group (Mean = 277 ms; SD = 80 ms) (t(28) = 2.16; p = 0.039; d = 0.8; 95% CI [− 126.67; −3.58]) (Fig. 1, left plot), confirming our previous results6.
Figure 1.
Left plot: Group mean TBW defined using the Simultaneity Judgment (SJ) task. Right plot: Group mean JND values defined using the Temporal Order Judgment (TOJ) task. Red curves represent obese participants, black curves represent healthy-weight controls. Symbols represent the raw, unfitted data.
According to the linear regression analysis, a significant regression was found [F(5, 23) = 3.23; p = 0.023] with an R2 of 0.41. A low level of multicollinearity was present (VIF = 2.5 for leptin, 2.17 for IL6, 1.1 for BDNF, 1.15 for S100B and 1.36 for cortisol). Leptin was the only significant predictor of the TBW [B = 2.097; p = 0.023] (Fig. 2); no other predictors were significant [IL6 B = −13.69; p = 0.58; BDNF B = −4.39; p = 0.25; S100B B = 1.12; p = 0.27; Cortisol B = −3; p = 0.46].
Figure 2.

The relationship between the TBW (x-axys) defined using the Simultaneity Judgment (SJ) Task and the level of leptin (y axys) split for the two groups (filled circle: participants with obesity; empty circle: normal-weight participants).
Regarding TOJ, we evaluated the goodness of fit: for the data relative to the participants with obesity, we observed a mean R2 of 0.84, while in the normal-weight group it was 0.96. The Just Noticeable Difference (JND) values violated normality (Shapiro-Wilk test, p < 0.001), hence again data were log-transformed to obtain a normal distribution. According to the independent sample t-test, the results showed larger JND in participants with obesity (Mean = 210 ms; SD = 99) compared to healthy-weight controls (Mean = 115 ms; SD = 42), t(28) = 3.76; p = 0.001; d = 1.24; 95% CI [−0.37; −0.11] (Fig. 1, right plot). This result is in line with our previous report23. According to the linear regression analysis, a significant regression equation was found [F(5, 23) = 8.34 p < 0.001] with an R2 of 0.64. Leptin [B = 3.29; p < 0.001] was the only significant predictor also for the JND (Fig. 3); no other predictors were significant [IL6 B = −32.77; p = 0.1; BDNF B = 0.15; p = 0.96; S100B B = 1.16; p = 0.15; Cortisol B = 0.14; p = 0.96].
Figure 3.
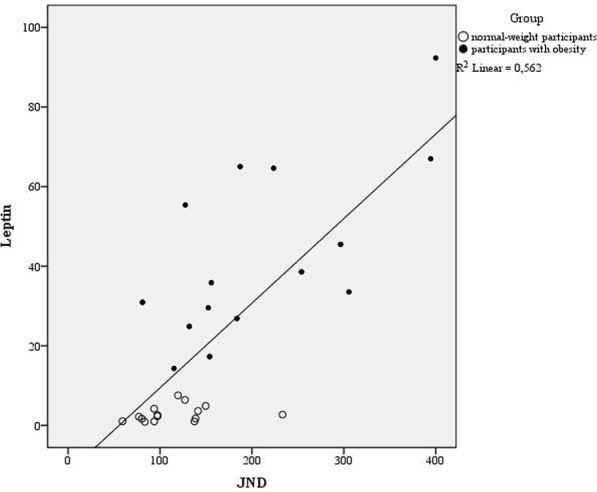
The relationship between the JND (x-axys) using the Temporal Order Judgment (TOJ) task. and the level of leptin (y axys) split for the two groups (filled circle: participants with obesity; empty circle: normal-weight participants).
Overall, we confirmed that individuals with obesity showed a wider TBW in SJ and a higher JND in TOJ respect to the healthy-weight controls, suggesting an alteration in the process of integration of multiple sensory stimuli. The behavior was predicted by the level of leptin measured in the serum in both tasks, as indicated by results in the linear regression analyses.
Discussion
In the present work, we searched for an alteration of temporal sensitivity to multisensory stimuli in individuals affected by obesity. Moreover, we extend our investigation to test a potential relationship linking neurocognitive behavior to blood-based markers for pro-inflammatory state (leptin and IL6) and to those neurotrophic factors considered sensitive biochemical markers of cerebral injury (BDNF and S100B).
Focusing on the behavioral task, we found that temporal sensitivity to multisensory stimuli was markedly wider in participants affected by obesity compared to healthy-weight participants. This result is in line with our previous results6 and also it mirrors what reported by Wan and colleagues (2000)8. Furthermore, in the present study, we showed that the difficulties of individuals affected by obesity seem to be related to higher levels of pro-inflammatory markers, instead of indexes of cerebral lesion, when measured in the blood. More specifically, according to the regression analyses, leptin, but not IL-6, was the best predictor for individuals’ performance in both tasks. However, it can be observed that both peripheral leptin and IL-6 are known as pro-inflammatory cytokines and they are able to cross the blood brain barrier and affect brain function58. Our results appeared to be in line with previous works suggesting the role of leptin in brain activity48,59–61. Moreover, GABA, that is involved in the long-lasting brain changes (as we have underlined in the Introduction), is also implicated not only in the regulation of food intake62, appetite63 and feeding behavior64, that are really crucial in obesity, but also in leptin expression65,66. This link might be a key research for the future in which the involvement of GABA in the long-lasting brain changes that occur in obesity. If leptin was found to be a significant predictor for individuals’ performance in both experimental tasks, a negative result emerged about IL-6, that represented the other index of inflammatory state adopted in the present study. Research has shown that also IL-6 plays a role in brain functioning67–71 and in this sense it might be expected a relationship with the participants’ behavior in experimental tasks. However, it should be considered that even though IL-6 is a critical factor in mediating obesity-related consequences, the question related to which of the several mechanisms through which the adipose tissue-derived IL-6 may affect metabolism is still debated72.
The two markers of cerebral plasticity (BDNF and S100B) were not significant predictors of the behavior in our participants, corroborating our hypothesis about the relationship between inflammatory state and poorer temporal sensitivity in obesity. However, while the two groups shown different level of serum concentration of BDNF, such a difference did not emerge about S100B. The prominent role of the neurotrophin BDNF in survival, growth, and maintenance of neurons during development43,44 also in pathological conditions (see Habtemariam, 201873 for a recent review) is well established, while it still need to be completely clarified its role in obesity; indeed we registered higher level of BNDF in our sample of individuals affected by obesity, respect to healthy-weight controls, and this result is in line with previous studies in the field74,75; however, it should be noted that reduced level of circulating BDNF levels in individuals affected by obesity respect to controls76,77 as well as no differences between the two groups78 were also reported. Nevertheless, there is an overall consensus in suggesting the possible critical role of BDNF in obesity; altered BDNF production was found to be associated with weight loss and food behavior in experimental animal models79–81 and in humans82. Interestingly, BDNF is also associated with energy homeostasis83; thus changes in circulating BDNF in obesity are likely secondary to the altered energy balance occurring in this condition84.
As mentioned earlier, the two groups did not differ in the serum level of S100B. This is as unexpected result, and possibly due to the magnitude of interindividual variation in S100B values observed especially among normal-weight participants. S100B it is produced primarily by brain astrocytes and its elevated levels are generally linked to glial damage or dysfunction in blood-brain barrier85,86, as usually observed in different cerebral diseases85–87. Also high concentration of S100B is observed in the serum of obese patients and it is significantly correlated with higher level of body mass index88, even though Pham and colleagues (2010)89 reported opposite results, since they did not observed statistically significant relationship between BMI and S100B levels in their large samples of studied individuals. Thus, they concluded that “an increase in fat mass might not in isolation be a major contributor to elevated S100B levels”89, suggesting the possible contribution of other obesity-related diseases in the expression of the protein. According to Aleksovska and colleagues (2014)87, S100B is more than a marker of brain damage; rather, its higher level in the brain would make the individuals more exposed to higher risk of developing psychiatric disorders in a stressful environment. Further research about S100B and its meaning in terms of cerebral wellbeing are necessary.
Finally, the two groups did not differ in the serum level of cortisol, that is a marker of acute psychological stress47,48. Individual variations in endocrine response to stress is reported to impact the level of expression of pro-inflammatory cytokines, as well as both high and low cortisol stress responsiveness has potentially adverse effects in maintain mental wellbeing90. Thus the absence of statistically higher level of cortisol in the participants affected by obesity respect to the normal-weight group might related to the pro-inflammatory condition mediated by the immune system, instead of a spread, unspecific response to a stressful condition.
In conclusion, we suggest that aberrant performance of individuals affected by obesity in temporal tasks might be, at least in part, explained by the serum level of pro-inflammatory marker of leptin. Future studies should investigate what might be the physiological mechanism maintaining the observed effects: other possible immunological components91, measured not only at the peripheral level (i.e. in the blood) but also at a central level (i.e. in the brain), should be considered. Another interesting investigation might be to consider the temporal time-course of immune products in relation to behavior, and to adopt neurophysiological techniques allowing to measure directly the cerebral activity associated to the behavior. Moreover, considering the magnitude of interindividual variation in markers concentrations, in future the numbers of participants should be enlarged. Finally, in this work only male individuals were assessed. Sex hormones appear to influence the immune response in humans (see Bouman et al.92 for a review); in other words, females and males show different hormones with different effect on immune systems. Thus, in future, it would be interesting to replicate this study focusing on the females’ behaviour, taken carefully in account the specificity of the female immune system. Even though this study is not exhaustive, it might shed the light of on a possible link between immune system, temporal sensitivity and brain activity93.
Methods
Participants
Fifteen male individuals affected by obesity and fifteen male normal-weight individuals took part in the study. All participants were right-handed. Subjects volunteer to participate; they gave informed written consent, were free to withdraw at will and were naïve to the rationale of the experiment. The study was approved by the IRCCS Istituto Auxologico Italiano Ethics Committee. The study protocol conformed to the guidelines of the European Convention on Human Rights and Biomedicine concerning biomedical research. All participants with obesity were consecutively recruited at admission to our institution (IRCCS Istituto Auxologico Italiano, Ospedale San Giuseppe). All subjects were nonsmokers and free from gastrointestinal, cardiovascular, psychiatric, or metabolic disorders or any concurrent medical condition not related to obesity. Moreover, no participant with diagnosis of Obstructive Obstructive Sleep Apnea syndrome (according to routinely clinical assessment) was included in the present study. We measured the level of daytime sleepiness through the Italian version94 of the Epworth Sleepiness Scale57; according to this scale, a score under the cut-off of 6 indicates no difficulty in daily level of alertness. All subjects underwent body measurements wearing light underwear, in fasting conditions after voiding. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using standard methods. BMI was expressed as body mass (kg)/height (m)2. Obesity was defined for any BMI over 30 kg/m2. We measured also the level of depressive symptoms through the Italian version56 of the Beck Depression Inventory55; a score under the cut-off of 9 indicates no pathological level of depressive symptoms. Demographic and clinical data for the participants with obesity and the normal-weight participants were reported in Table 1.
Serum markers
Testing was performed at 08.00 a.m. in fasting conditions and after voiding. About participants with obesity, the testing was performed within one week after admission while patients were fed a balanced diet (30% lipids, 50% carbohydrates, and 20% proteins).
Blood samples were drawn under fasting conditions and were separated by centrifugation after clotting, processed for routine measurement or aliquoted and stored at −80 °C until required.
Serum leptin concentrations were quantified using a commercially available ELISA kit (Mediagnost GmbH, Reutlingen, Germany) with sensitivity of 0.2134 ng/mL and overall inter- and intra-assay coefficients of variation (CV) of 6.8–8.3% and 5.5–6.9% respectively.
Serum IL-6 levels were measured using a Human IL-6 Quantikine HS ELISA (R&D systems, Minneapolis, MN, USA). Assay sensitivities for IL-6 is estimated at 0.039 pg/ml. Intra- and inter-assay precision CV for IL-6 were estimated at less than 7.4%, and less than 9.6%, respectively.
Serum BDNF were measured using a Human BDNF Quantikine ELISA (R&D systems, Minneapolis, MN, USA). Assay sensitivities, or minimum detectable concentrations for BDNF is estimated at 20 pg/ml. Respective intra- and inter-assay precision CV for BDNF are estimated at less than 6.2% and less than 11.3%,respectively, as reported by the manufacturer.
Serum S-100B level were measured using a human S100B ELISA (Diametra, Spello, Italy) having a sensitivity of 35.27 pg/ml, inter- and intra-assay CV of less than 11.7% and 10.2% respectively.
Levels of serum cortisol was measured using Cobas 6000 (Roche Diagnostics, Indianapolis, IN, USA).
Experimental tasks
All stimuli were presented using OpenSesame 2.9.695. Visual stimuli consisted of a white ring circumscribing a visual fixation cross on a black background and were 1.8 cm in diameter or 1.7° of visual angle. They were presented at a distance of approximately 60 cm from the participants and lasted 30 ms. Auditory stimuli consisted of a 3.500 Hz pure tone. They were presented binaurally via noise-cancelling headphones and lasted 30 ms.
Participants performed the Simultaneity Judgment Task (SJ) and the Temporal Order Judgment Task (TOJ) in separate sessions. In both tasks, visual and auditory stimuli were delivered sequentially with one of the following Stimulus Onset Asynchronies (SOAs): 0; ±50, ±100, ±150, ±200, ±250, ±300, ±350, ±400 for SJ and SOAs: ±50, ±100, ±150, ±200, ±250, ±300, ±350, ±400 for TOJ. Negative SOAs indicate that the auditory stimulus is presented first (auditory leading trials), whereas positive SOAs indicate that the visual stimulus is presented first (visual leading trials). In the SJ task, participants reported whether the auditory and visual stimuli were presented at the same or different times. In the TOJ task, participants reported which stimulus came first. The intertrial interval (ITI) ranged between 1500 and 2500 ms. The presentation of the stimuli was pseudo-randomized. Participants performed two blocks for each task. In each block, each SOA was presented 16 times, for a total of 288 trials per block in SJ and 256 trials per block in TOJ. Overall participants completed 576 trials for the SJ task and 512 trials for TOJ task. Tasks order was counterbalanced across participants.
During the experiment, participants were seated in a dimly lit room with their corporeal midline aligned with a fixation point located 60 cm from the plane of their eyes. They rested their right and left index fingers on two response buttons located on a table. Each hand was in its homonymous hemispace. Participants were instructed to fixate toward a fixation cross at all times. They provided their answers by pressing a response button with the right or the left index finger, with the button representation (synchronous/asynchronous or auditory-first/visual-first) being balanced across blocks. In the Fig. 4, a graphical representation of the experiment is reported.
Figure 4.
Graphical representation of stimuli presentation.
Statistical analyses
The differences between groups in serum marker levels were assessed using independent sample t-tests. The individual’s Temporal Binding Windows (TBW) in the SJ task, and the Just Noticeable Difference (JND) in the TOJ task (i.e. measures of temporal sensitivity) for each group were calculated using standard procedures96,97. Specifically, to calculate the individual’s TBWs in the SJ task, we first computed the percentage of simultaneous responses across all SOAs for each participant. The observed distribution of responses was fitted to a Gaussian function using the fit function implemented in MATLAB (fit type: gauss1). The peak of this curve is referred to as the point of subjective simultaneity (PSS). It is assumed that, at this particular SOA, the information from the different modalities is perceived as being maximally simultaneous. Another measure that can be derived from this curve is its standard deviation. The standard deviation is reflected in the width of the curve and is taken as the TBW, because it represents the range of SOAs at which the brain treats the two sensory information as occurring simultaneously. For the TOJ task, data analysis was as follows: first we calculated a rate of visual-first responses with each SOA. Then, a single psychometric function was fitted to the response rates across all SOAs, using the glmfit function in MATLAB, so as to determine the just noticeable difference (JND) for each group. The JND was defined as half of the difference between the two x values for which the psychometric function had a y value of 25% and 75%. For both tasks, we report the goodness of fit through the adjusted R-square. Possible differences between the TBW and the JND between groups were tested using independent sample t-tests. A post hoc power analysis was conducted using the software package, GPower 3.0.198. For both groups, a sample size of 15 was used; moreover the alpha level used for this analysis was p < 0.05. The post hoc analyses revealed the statistical power for this study was 0.37 for detecting a medium effect size (d = 0.5), whereas it was of 0.68 for a large effect size d = 0.8. Finally, a linear regression was calculated to predict TWB and JND based on the all serum marker levels; the variance inflation factor (VIF) is reported as measure of multicollinearity.
Author Contributions
F.S. and M.C conceived the study, performed the analyses and wrote the main manuscript. F.S. collected data. P.M. and S.M. collected and performed the serum markers analyses. A.M. and M.S. supervised the experiment. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing financial and non-financial interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Federica Scarpina, Email: f.scarpina@auxologico.it.
Marcello Costantini, Email: marcello.costantini@unich.it.
References
- 1.Stein BE, Meredith MA. Multisensory integration. Neural and behavioral solutions for dealing with stimuli from different sensory modalities. Ann. N. Y. Acad. Sci. 1990;608:51–65. doi: 10.1111/j.1749-6632.1990.tb48891.x. [DOI] [PubMed] [Google Scholar]
- 2.Alais D, Newell FN, Mamassian P. Multisensory processing in review: from physiology to behaviour. Seeing Perceiving. 2010;23:3–38. doi: 10.1163/187847510X488603. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson RA, Segers M, Ferber S, Barense MD, Wallace MT. The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Front. Psychol. 2014;5:379. doi: 10.3389/fpsyg.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams LE, Light GA, Braff DL, Ramachandran VS. Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia. 2010;48:3128–3136. doi: 10.1016/j.neuropsychologia.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrar V, et al. Multisensory integration and attention in developmental dyslexia. Curr. Biol. 2014;24:531–535. doi: 10.1016/j.cub.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Scarpina F, et al. Altered multisensory temporal integration in obesity. Sci. Rep. 2016;6:28382. doi: 10.1038/srep28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace MT, Stevenson RA. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. 2014;64:105–123. doi: 10.1016/j.neuropsychologia.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan X, Spence C, Mu B, Zhou X, Ho C. Assessing the benefits of multisensory audiotactile stimulation for overweight individuals. Exp. Brain Res. 2014;232:1085–1093. doi: 10.1007/s00221-013-3792-x. [DOI] [PubMed] [Google Scholar]
- 9.Senkowski D, Schneider TR, Foxe JJ, Engel AK. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci. 2008;31:401–409. doi: 10.1016/j.tins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser J, Hertrich I, Ackermann H, Lutzenberger W. Gamma-band activity over early sensory areas predicts detection of changes in audiovisual speech stimuli. NeuroImage. 2006;30:1376–1382. doi: 10.1016/j.neuroimage.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel, A. K., Senkowski, D., & Schneider, T. R. Multisensory Integration through Neural Coherence. In: Murray, M. M., & Wallace, M. T., editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): CRC Press/Taylor & Francis (2012). [PubMed]
- 13.Ghazanfar AA, Chandrasekaran CF. Paving the way forward: integrating the senses through phase-resetting of cortical oscillations. Neuron. 2007;53:162–164. doi: 10.1016/j.neuron.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 16.Bhat R, et al. Inhibitory role for GABA in autoimmune inflammation. PNAS. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res. Rev. 1998;26:113–135. doi: 10.1016/S0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 18.Ferri F, et al. A Neural “Tuning Curve” for Multisensory Experience and Cognitive-Perceptual Schizotypy. Schizophr. Bull. 2017;43:801–813. doi: 10.1093/schbul/sbw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balz J, et al. GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. Neuroimage. 2016;125:724–730. doi: 10.1016/j.neuroimage.2015.10.087. [DOI] [PubMed] [Google Scholar]
- 20.Wyss C, et al. GABA metabolism and its role in gamma‐band oscillatory activity during auditory processing: An MRS and EEG study. Hum. Brain Mapp. 2017;38:3975–3987. doi: 10.1002/hbm.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/S1092852900016734. [DOI] [PubMed] [Google Scholar]
- 22.Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids. 2013;45:87–94. doi: 10.1007/s00726-011-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochner CN, Green D, van Steenburgh JJ, Kounios J, Lowe MR. Asymmetric prefrontal cortex activation in relation to markers of overeating in obese humans. Appetite. 2009;53:44–49. doi: 10.1016/j.appet.2009.04.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olde Dubbelink KT, et al. Increased resting-state functional connectivity in obese adolescents; a magnetoencephalographic pilot study. PLoS One. 2008;3:e2827. doi: 10.1371/journal.pone.0002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 27.Spencer SJ. Perinatal nutrition programs neuroimmune function long-term: mechanisms and implications. Front. Neurosci. 2013;7:144. doi: 10.3389/fnins.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 30.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed-Ali V, et al. Human subcutaneous adipose tissue secretes interleukin-6 but not TNF-a in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 32.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nature Rev. Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 34.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2014;96:124–134. doi: 10.1016/j.neuropharm.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am. J. Geriatr. Psychiatry. 2012;20:753–763. doi: 10.1097/JGP.0b013e31825d08d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Banks, W. A., Farr, S. A. & Morley, J. E. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation10, 319–327, 10.1159/000071472 (2002–2003). [DOI] [PubMed]
- 39.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002;328:261–264. doi: 10.1016/S0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neurosci. 2003;6:736–742. doi: 10.1038/nn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steiner, J., et al. S100B serum levels in schizophrenia are presumably related to visceral obesity and insulin resistance. Cardiovasc. Psychiatry Neurol. 480707, 10.1155/2010/480707 (2010). [DOI] [PMC free article] [PubMed]
- 42.Steiner J, et al. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology. 2010;35:321–324. doi: 10.1016/j.psyneuen.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Leibrock J, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 44.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 45.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yardan T, et al. Usefulness of S100B protein in neurological disorders. J. Pak. Med. Assoc. 2011;61:276–281. [PubMed] [Google Scholar]
- 47.Maes M, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 48.Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin. Sci. 2001;101:185–92. doi: 10.1042/cs1010185. [DOI] [PubMed] [Google Scholar]
- 49.Abraham E. Effects of stress on cytokine production. Methods Achiev. Exp. Pathol. 1991;14:45–62. [PubMed] [Google Scholar]
- 50.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998;105:83–107. doi: 10.1037/0033-295X.105.1.83. [DOI] [PubMed] [Google Scholar]
- 51.Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1983;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 52.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/S0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization Obesity: preventing and managing the global epidemic: report of a WHO consultation. TRS. 2000;894:1–253. [PubMed] [Google Scholar]
- 55.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 56.Sica, C., & Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In: Leading-Edge Psychological Tests and Testing Research, Editors: M.A. Lange NOVA Science Publishers (2007).
- 57.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 58.Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014;24:1982–1999. doi: 10.1016/j.euroneuro.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunstad J, et al. Serum leptin levels are associated with cognitive function in older adults. Brain Res. 2008;1230:233–236. doi: 10.1016/j.brainres.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 60.Tsai CL, Huang TH, Tsai MC. Neurocognitive performances of visuospatial attention and the correlations with metabolic and inflammatory biomarkers in adults with obesity. Exp Physiol. 2017;102:1683–1699. doi: 10.1113/EP086624. [DOI] [PubMed] [Google Scholar]
- 61.Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int. J. Clin. Pract. 2010;264:1808–12. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 63.Delgado TC. Glutamate and GABA in Appetite Regulation. Front. Endocrin. 2013;4:103. doi: 10.3389/fendo.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, et al. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology. 2012;153:2223–2233. doi: 10.1210/en.2011-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanley BG. GABA: a cotransmitter linking leptin to obesity prevention. Endocrinology. 2012;153:2057–2058. doi: 10.1210/en.2012-1246. [DOI] [PubMed] [Google Scholar]
- 67.Spooreen A, et al. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Trapero I, Cauli O. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 2014;29:593–608. doi: 10.1007/s11011-014-9551-2. [DOI] [PubMed] [Google Scholar]
- 69.Schram MT, et al. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 70.Marioni ME, et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:710–703. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trollor JM, et al. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr.) 2012;34:1295–1308. doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- 73.Habtemariam S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs. Neural Regen. Res. 2018;13:983–984. doi: 10.4103/1673-5374.233438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golden E, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the baltimore longitudinal study of aging. PLoS One. 2010;5:e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slusher AL, et al. Brain-derived neurotrophic factor and substrate utilization following acute aerobic exercise in obese individuals. J. Neuroendocrinol. 2015;27:370–376. doi: 10.1111/jne.12275. [DOI] [PubMed] [Google Scholar]
- 76.Roth CL, Elfers C, Gebhardt U, Müller HL, Reinehr T. Brain-derived neurotrophic factor and its relation to leptin in obese children before and after weight loss. Metabolism. 2013;62:226–234. doi: 10.1016/j.metabol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Celik Guzel E, et al. Can low brain-derived neurotrophic factor levels be a marker of the presence of depression in obese women? Neuropsychiatr. Dis. Treat. 2014;10:2079–2086. doi: 10.2147/NDT.S72087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hinderberger P, et al. The effect of serum BDNF levels on central serotonin transporter availability in obese versus non-obese adults: A [(11)c]dasb positron emission tomography study. Neuropharmacology. 2016;110:530–536. doi: 10.1016/j.neuropharm.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 79.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 80.Yan Q, et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 1997;378:135–157. doi: 10.1002/(SICI)1096-9861(19970203)378:1<135::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 81.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee IT, Wang JS, Fu CP, Lin SY, Sheu WH. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome: A prospective study. Medicine. 2016;95:e5260. doi: 10.1097/MD.0000000000005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakagawa T, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.1097/MD.0000000000005260. [DOI] [PubMed] [Google Scholar]
- 84.Monteleone P, DiLieto A, Castaldo E, Maj M. Leptin functioning in eating disorders. CNS Spectr. 2004;9:523–529. doi: 10.1017/S1092852900009615. [DOI] [PubMed] [Google Scholar]
- 85.Rothermundt M, Ponath G, Arolt V. S100B in schizophrenic psychosis. Int. Rev. Neurobiol. 2004;59:445–470. doi: 10.1016/S0074-7742(04)59017-7. [DOI] [PubMed] [Google Scholar]
- 86.Schroeter ML, Steiner J. Elevated serum levels of the glial marker protein S100B are not specific for schizophrenia or mood disorders. Mol. Psychiatry. 2009;14:235–237. doi: 10.1038/mp.2008.85. [DOI] [PubMed] [Google Scholar]
- 87.Aleksovska K, et al. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS One. 2014;9:e106342. doi: 10.1371/journal.pone.0106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr. Opin. Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pham N, et al. Extracranial sources of S100B do not affect serum levels. PLoS One. 2010;5:e12691. doi: 10.1371/journal.pone.0012691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Imm. 2003;17:373–383. doi: 10.1016/S0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 91.Ranabir S, Reetu K. Stress and hormones. Indian J. Endocr. Metab. 2011;15:18–22. doi: 10.4103/2230-8210.77573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 93.Costantini M. Bodily self and immune self: is there a link? Front. Hum. Neurosci. 2014;8:138. doi: 10.3389/fnhum.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vignatelli L, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol. Sci. 2003;23:295–300. doi: 10.1007/s100720300004. [DOI] [PubMed] [Google Scholar]
- 95.Mathot S, Schreij D, Theeuwes J. OpenSesame: an open-source, graphical experiment builder for the social sciences. Behav. Res. Meth. 2012;44:314–324. doi: 10.3758/s13428-011-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vroomen J, Keetels M. Perception of intersensory synchrony: a tutorial review. Atten. Percept. Psychophys. 2010;72:871–884. doi: 10.3758/APP.72.4.871. [DOI] [PubMed] [Google Scholar]
- 97.Keetels, M., & Vroomen, J. Perception of Synchrony between the Senses. In: Murray MM, & Wallace, M.T., editors. The Neural Bases of Multisensory Processes, Taylor & Francis (2012).
- 98.Faul F, Erdfelder E, Lang AG, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Meth. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]