Trypanosoma cruzi is the etiological agent of Chagas disease, usually transmitted by triatomine vectors. An estimated 20 to 30% of infected individuals develop potentially lethal cardiac or gastrointestinal disease. Sylvatic transmission cycles exist in the southern United States, involving 11 triatomine vector species and infected mammals such as rodents, opossums, and dogs. Nevertheless, imported chronic T. cruzi infections in migrants from Latin America vastly outnumber locally acquired human cases.
KEYWORDS: Chagas disease, triatomine, Trypanosoma cruzi, United States
SUMMARY
Trypanosoma cruzi is the etiological agent of Chagas disease, usually transmitted by triatomine vectors. An estimated 20 to 30% of infected individuals develop potentially lethal cardiac or gastrointestinal disease. Sylvatic transmission cycles exist in the southern United States, involving 11 triatomine vector species and infected mammals such as rodents, opossums, and dogs. Nevertheless, imported chronic T. cruzi infections in migrants from Latin America vastly outnumber locally acquired human cases. Benznidazole is now FDA approved, and clinical and public health efforts are under way by researchers and health departments in a number of states. Making progress will require efforts to improve awareness among providers and patients, data on diagnostic test performance and expanded availability of confirmatory testing, and evidence-based strategies to improve access to appropriate management of Chagas disease in the United States.
INTRODUCTION
Trypanosoma cruzi is the causative agent of Chagas disease (1, 2). Infection is lifelong without treatment; thus, prevalence can be high despite low incidence. Current estimates of 6 million infections and 1.2 million cases of cardiomyopathy place Chagas disease first in disease burden among parasitic diseases in the Americas (3, 4). Trypanosoma cruzi organisms are transmitted when infected vector feces enter the bite site or mucous membranes of a mammalian host. Transmission can also occur through blood component transfusion, organ transplantation, consumption of food or beverages contaminated by the vector or vector feces, and in utero from mother to fetus (5).
The classic setting for Chagas disease is rural Latin America, where adobe houses and the presence of domestic animals favor domestic and peridomestic vector infestation (2). However, transmission in many rural areas has decreased due to vector control programs, and infected individuals have migrated to Latin American cities (6), the United States, and Europe (7, 8). Unlike Europe, the United States has well-described enzootic T. cruzi transmission, involving 11 triatomine species and a range of mammalian hosts (9). Nevertheless, the vast majority of T. cruzi-infected individuals in the United States are Latin American immigrants infected in their countries of origin. We will review clinical, epidemiological, and public health aspects of Chagas disease in the United States, with a focus on the most recent relevant publications.
BIOLOGY AND TRANSMISSION OF TRYPANOSOMA CRUZI
In 1909, Carlos Chagas, a young physician working in rural Brazil, demonstrated the etiological agent, its vector, several of its reservoir hosts, and the salient manifestations of the disease that now bears his name, a feat unrivaled in medical history (10). He named the parasite in honor of his mentor, Oswaldo Cruz (11). Chagas proceeded to isolate T. cruzi from the blood of a domestic cat and, finally, from a symptomatic toddler. This “first patient” remained infected for life but never developed chronic manifestations of Chagas disease and died at 73 from unrelated causes (12). Finally, Chagas fulfilled Koch’s postulates by reproducing the infection experimentally in laboratory animals (11).
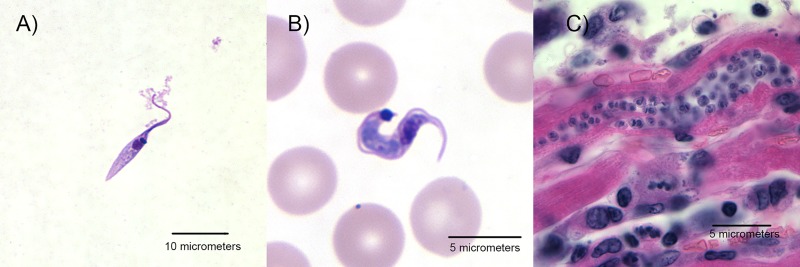
In the years since 1909, the life cycle has been more fully characterized. In order to successfully colonize the mammalian host and triatomine vector, T. cruzi assumes three distinct morphological forms at different developmental stages (Fig. 1) (13). Amastigote and epimastigote forms replicate by binary fission in mammalian cells and the hindgut of the triatomine vector, respectively. Trypomastigote forms are nonreplicative and are present at two distinct life cycle stages: (i) in the bloodstream of the mammalian host (bloodstream-form trypomastigotes) and (ii) in the rectum and feces of vectors (infective metacyclic trypomastigotes).
FIG 1.
Trypanosoma cruzi morphological forms. (A) The replicating epimastigote form in culture (Giemsa stain). (B) Trypomastigote in a peripheral blood smear from a patient with acute Chagas disease (Giemsa stain). (C) Nest of amastigotes within a cardiac myocyte in a patient with chronic Chagas disease (hematoxylin and eosin stain). Courtesy of the Division of Parasitic Diseases and Malaria, U.S. Centers for Disease Control and Prevention.
Infective metacyclic trypomastigotes are deposited on the skin of the mammalian host in fecal droplets extruded by a blood-feeding triatomine bug. Parasites enter through the bite site, skin abrasions, or mucosa, such as the conjunctiva. This mechanism, via the vector feces rather than mouthparts, is known as stercorarian transmission. Once internalized, motile trypomastigotes invade nucleated cells via both lysosome-dependent and -independent mechanisms (reviewed in references 14 and 15). The parasite is then taken up into a membrane-bound (parasitophorous) vacuole, which subsequently fuses with a lysosome; exposure to decreasing pH stimulates parasite differentiation to the intracellular amastigote form and its concomitant release into the cytosol over a period of 4 to 5 days. Here, amastigotes multiply asexually to form pseudocysts, which can arise in a variety of host tissues but predominantly do so in cardiac, smooth, and skeletal muscles and reticuloendothelial cells in the liver, spleen, and lymphatic system. Within pseudocysts, amastigotes differentiate into trypomastigotes that, upon cell lysis, can either infect adjacent tissues to initiate new replicative cycles or disseminate throughout the bloodstream and lymph. Without antitrypanosomal treatment, infection persists for the duration of the mammalian host’s life.
Triatomine bugs feeding on an infected host may ingest extracellular trypomastigotes, which pass to the midgut where transformation to an intermediate spheromastigote form occurs. Differentiation of spheromastigotes into epimastigotes occurs in response to decreasing environmental glucose levels as the blood meal is digested (13). Epimastigotes multiply by binary fission in the hindgut and migrate to the rectum, where they attach hydrophobically to the waxy gut cuticle by their flagella and transform into infective metacyclic trypomastigotes, thus completing the life cycle.
Routes of Transmission
Vector-borne transmission.
Vector-borne transmission remains the predominant route of new human infections in regions of endemicity. Historically, vector-borne transmission has occurred in ecologically determined areas throughout continental Latin America, from Mexico to the northern 50 to 60% of the territories of Argentina and Chile (16). Infected vectors and reservoir animals are not infrequent in the southern half of the continental United States, but vector-borne transmission to humans is rarely detected (reviewed in multiple sections that follow).
Congenital transmission.
Reported vertical transmission rates are variable, ranging from 0% in some studies to more than 15%; the pooled transmission risk in a recent meta-analysis was 4.7% (17). Factors associated with a higher risk include younger maternal age (reflecting more recent infection), maternal immunological responses, higher maternal parasitemia, twin births, and HIV coinfection (18–21). Infected infants are detected regularly in screening programs in Spain and sporadically in other countries with Latin American immigrant populations (22–24).
Blood-borne transmission.
In the early 1990s, T. cruzi infection was found in 1 to 60% of donated blood units in Latin American blood banks (25). Since then, blood donation screening has been established as a major component of Chagas disease control programs (26). With the addition of Mexico in 2012, screening of blood components for T. cruzi is now required in all countries of endemicity in Latin America, and reported donor prevalence has decreased markedly (26, 27).
Organ-derived transmission.
Transplantation of an organ from a T. cruzi-infected donor can transmit T. cruzi to the recipient, but the risk varies by organ type. In cohorts of kidney recipients from infected donors in the United States and Argentina, transmission occurred in 13% and 19%, respectively (28, 29). The transmission rate among 10 U.S. liver transplant recipients was 20% (28). The risk from heart transplant in the same U.S. series was 75% (3 of 4); use of the heart from an infected donor is contraindicated (28, 30).
Oral transmission.
Outbreaks of acute T. cruzi infection due to contaminated fruit or sugar cane juice have been reported in several countries of Latin America (31, 32). Most case clusters are small, affecting family groups in the Amazon and attributed to fruits like açaí (33). The largest reported outbreak was associated with a 10% attack rate among students and staff at a school in Caracas; home-pressed guava juice was implicated (34).
EPIDEMIOLOGY AND ECOLOGY
Global Burden of Chagas Disease
The Chagas disease control initiatives instituted throughout Latin America since 1991 constitute a major public health success story. Thanks to vector control programs, blood bank screening, and in some countries, congenital Chagas disease screening programs, global estimates have decreased from 18 million in 1991 to less than 6 million infected individuals in 2010 (Table 1) (3, 35). Incidence estimates have fallen from 500,000 in 1991 to 30,000 new T. cruzi infections per year in 2010 (3). As vectorial transmission has come under increasing control, the proportion attributable to other routes has grown: currently, 22.5% of incident infections are estimated to occur through congenital transmission, and in some areas, oral transmission may be more frequent than the traditional vector-borne route (3, 31).
TABLE 1.
Countries in which Chagas disease is endemic, estimates of national seroprevalence, and numbers of infected inhabitantsa
| Estimated T. cruzi infection prevalenceb
|
|||
|---|---|---|---|
| Region | Country | % prevalence | No. of infections |
| North America | United States | NDA | 240,000 to 350,000c |
| Mexico | 0.779 | 876,458 | |
| Central America | Belize | 0.330 | 1,040 |
| Costa Rica | 0.170 | 7,667 | |
| El Salvador | 1.298 | 90,222 | |
| Guatemala | 1.230 | 166,667 | |
| Honduras | 0.918 | 73,333 | |
| Nicaragua | 0.523 | 29,300 | |
| Panama | 0.515 | 18,337 | |
| South America | Argentina | 3.641 | 1,505,235 |
| Bolivia | 6.104 | 607,186 | |
| Brazil | 0.606 | 1,156,821 | |
| Chile | 0.700 | 119,660 | |
| Colombia | 0.956 | 437,960 | |
| Ecuador | 1.380 | 199,872 | |
| Guyana, French Guiana, & Suriname | 0.839 | 12,600 | |
| Paraguay | 2.130 | 184,669 | |
| Peru | 0.440 | 127,282 | |
| Uruguay | 0.238 | 7,852 | |
| Venezuela | 0.710 | 193,339 | |
| Total | 1.056 | 5,742,167d | |
Vector-borne T. cruzi transmission occurs or occurred until recently in parts of these countries.
Disease burden estimates are for the year 2010 and are based on references 3 and 7. NDA, no data available.
The figure for the United States reflects the estimated number of infected immigrants from countries of disease endemicity of Latin America. No estimate of locally acquired infections is currently available.
Excluding the United States.
Triatomine Vector Biology
More than 130 triatomine species have been reported in the Western Hemisphere, many known to carry T. cruzi (16, 36). However, a few species are disproportionately responsible for T. cruzi transmission to humans, due to their propensity to colonize human houses and/or the peridomestic environment. These include Triatoma infestans and Panstrongylus megistus in the Southern Cone (Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay), Triatoma dimidiata in southern Mexico and Central America, and Rhodnius prolixus in Central America and northern South America (16). These species have been the major targets of the regional control initiatives. The elimination of R. prolixus from Central America and the near elimination of domestic T. infestans from much of the Southern Cone are responsible for the steep decline in new infections and very low prevalence in children throughout most of the historic zone of endemicity (37, 38). However, in the Gran Chaco, an ecological zone that straddles southern Bolivia, northeastern Argentina, and parts of Paraguay, the prevalence of house infestation and transmission remains very high (39, 40).
The domestic environment is rich in blood meal sources, both human and animal. Crevices in adobe walls and dark spaces within animal corrals and poultry nests provide safe diurnal refuges for triatomines. Rhodnius species, which nest in palm crowns in the sylvatic environment, can infest thatch roofs. Triatomines of both sexes require at least one blood meal during each of the five nymphal stages, and females need a blood meal to lay eggs. Thus, both male and female nymphs and adults may carry T. cruzi, with infection rates increasing with age. Only adults have wings. Most domestic triatomine species feed nocturnally and complete their blood meals without waking the host (36). The major Latin American vectors defecate during or immediately after taking a blood meal (41). Many sylvatic triatomine species colonize the nests of their blood meal sources and are found in close association with specific rodent or marsupial species (16, 36). Sylvatic triatomine adults may be attracted by light to invade human dwellings, which can lead to sporadic human infections (42, 43). Some triatomine species, such as T. dimidiata, can infest both domestic and sylvatic sites (44).
Triatomine Distribution in the United States
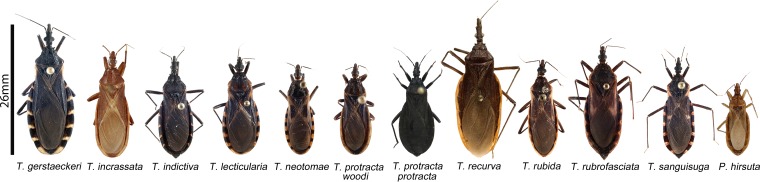
Eleven triatomine species have been reported in the United States: Triatoma gerstaeckeri, T. incrassata, T. indictiva, T. lecticularia, T. neotomae, T. protracta, T. recurva, T. rubida, T. rubrofasciata, T. sanguisuga, and Paratriatoma hirsuta (Fig. 2 and Table 2) (9, 36). Triatomines are present from coast to coast across the southern two-thirds of the continental United States (Fig. 3). In field collections, vectors are often found in specific microenvironments (woodpiles, rock piles, rodent nests, livestock pens, and dog kennels) (9). Natural T. cruzi infections have been documented in all species except the rarely collected T. incrassata and P. hirsuta (9, 45).
FIG 2.
Photographs of U.S. triatomine species of the genera Triatoma and Paratriatoma. Image size relative to the scale bar represents the average length of each species. The Triatoma incrassata photo is courtesy of E. Barrera Vargas, the T. recurva and Paratriatoma hirsuta photos are courtesy of R. Hoey-Chamberlain and C. Weirauch, and the T. protracta protracta photo is courtesy of G. Lawrence (DPDM/CDC). All other images are from reference 9 (photos by S. Kjos).
TABLE 2.
Triatomine vectors in the United Statesa
| Species | Frequency of collectionb | Rangeb | Ecological association(s) | T. cruzi prevalence |
|---|---|---|---|---|
| T. sanguisuga | Frequent | AL, AR, DE, FL, GA, IL, IN, KS, KY, LA, MD, MO, MS, NC, NJ, OH, OK, PA, SC, TN, TX, VA, WV | Highly diverse, including woodrats, other rodents, armadillos, opossums, dogs, chickens, horses; frequent in peridomestic settings; invades houses | Moderate prevalence, very widespread |
| T. gerstaeckeri | Very frequent | Eastern NM, central TX | Sylvatic and peridomestic settings, dog kennels, rodent burrows; frequently invades houses | High prevalence, especially in dog kennel collections |
| T. protracta | Frequent | AZ, CA, CO, NM, NV, west TX, UT | Close association with woodrats (Neotoma spp.); attracted by lights | Moderate prevalence, widespread |
| T. rubida | Frequent | AZ, southern CA, NM, southwest TX | Woodrat nests, disturbed environments in AZ and Mexico; reports of house colonization in Sonora, Mexico | Usually low, but focal collections with high prevalence |
| T. lecticularia | Infrequent | FL, GA, MO, NM, OK, SC, TN, TX, UTc | Houses, dog kennels, woodrat nests in TX; peridomestic settings | Can be high in collections from woodrat nests |
| T. indictiva | Infrequent | AZ, NM, TX | Found in woodrat nests and near lights | Moderate in sparse data |
| T. recurva | Infrequent | Southern half of AZ | Associated with rodents, especially rock squirrels | Low to moderate |
| T. neotomae | Rare | TX | Found in woodrat nests | Can be high in collections from woodrat nests |
| T. incrassata | Rare | Southern AZ | Unknown | No naturally infected specimen reported |
| P. hirsuta | Rare | CA, AZ, NV | Found in woodrat nests, near lights, and invading houses | No naturally infected specimen reported |
| T. rubrofasciata | Rare | Jacksonville, FL; Honolulu, HI | Roof rats (Rattus rattus); found in houses in FL and HI, chicken coops in HI | 2 infected bugs reported in HI |
Based on our review of literature from 1939 to 2011 (9) plus new data in references 46, 47, and 335.
Frequency and range are based on published reports; absence of reports from a given area often reflects lack of field research rather than true absence of vectors. Ranges of all species except T. rubrofasciata extend into Mexico.
Several other states are listed for T. lenticularia in reference 45 and reproduced by reference 278 but were not confirmed in our 2011 review (9). We follow the approach advocated by Ryckman (49), in which reports prior to Usinger (336) are treated with caution in the absence of later verification. Utah was added based on the recent report in reference 335.
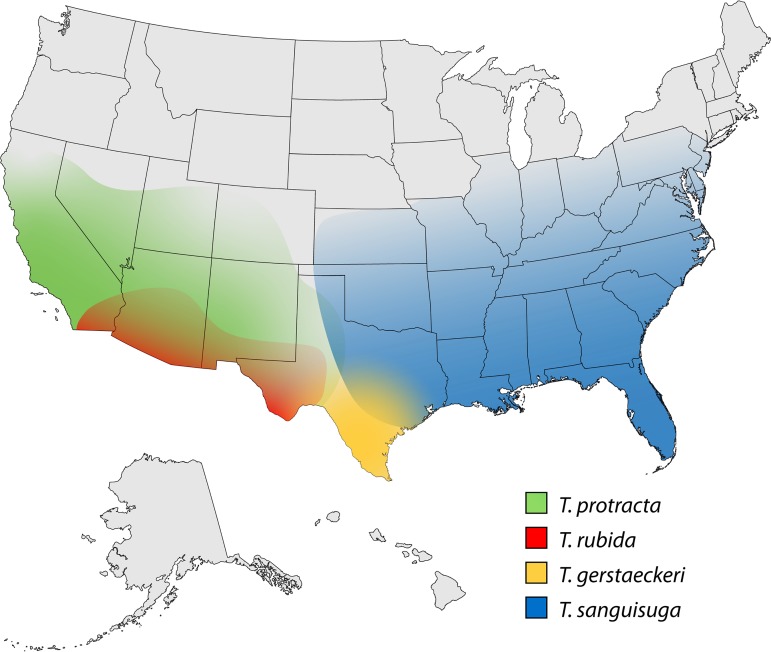
FIG 3.
Ranges of the four most frequently identified triatomine species in the continental United States. Based on references provided in Table 2.
The two species with the widest geographic distribution in the United States are T. sanguisuga and T. protracta. The former has been reported from Texas to the Atlantic coast and as far north as Illinois, the latter from Texas to California (9, 46). A recent review by the Wheeling-Ohio County West Virginia Health Department turned up 10 specimens of T. sanguisuga archived since 1969 and adds this state (long assumed to have the vector) to the confirmed list (46). T. sanguisuga was also recently reported in Delaware (47). T. protracta has been extensively collected in association with its favored blood meal hosts, the woodrats (Neotoma spp.); the prominent above-ground nests of these rodents make sylvatic collection relatively straightforward for this species (9, 48). T. protracta includes three morphologically distinct subspecies in the United States, T. protracta protracta in California, Nevada, Utah, Arizona, and New Mexico, T. protracta woodi in Texas, and T. protracta navajoensis in the Four Corners area, where Colorado, Utah, Arizona, and New Mexico meet (49). Thousands of specimens of T. sanguisuga and T. protracta have been reported in literature dating back to the 1930s, and these species were found in or near the residences of humans with locally acquired T. cruzi infection in Tennessee, Louisiana, Mississippi (T. sanguisuga), and California (T. protracta) (9, 50–53). In field collections, both species frequently have T. cruzi infection, with rates generally in the 15 to 30% range (9, 48).
T. gerstaeckeri has a more limited range, encompassing south-central Texas and southeastern New Mexico, but is one of the most frequently collected species, perhaps in part because of its propensity to infest dog kennels and other peridomestic structures (54). T. gerstaeckeri constituted more than 70% of several thousand vectors submitted through a citizen science project based at Texas A&M University (55). Collections of T. gerstaeckeri show high rates of T. cruzi infection, often >60% (9, 55). Infected T. gerstaeckeri insects were collected in the house of a child with acute T. cruzi infection in south Texas in 2006 (56).
Texas and the southwestern states have the highest triatomine species diversity, with at least seven species in Texas and six in Arizona (Table 3) (9, 54). A spatial analysis of bugs submitted through the Texas citizen science initiative showed geographic overlap among species but with T. gerstaeckeri predominantly in south-central Texas, T. sanguisuga in the eastern portion, T. rubida in west Texas, and T. indictiva in a small area of central Texas (54). T. gerstaeckeri reports showed earlier seasonality than T. sanguisuga, possibly because of the earlier arrival of high temperatures in the southern part of the state. Like all passive surveillance, there may be reporting biases in these data. The authors observe that they received few submissions from west Texas (and perhaps for this reason, few T. protracta specimens). They attribute this to lower human population density and/or less effective outreach (54), but lower rates of Internet access in rural counties could also play a role.
TABLE 3.
Triatomine vectors and Trypanosoma cruzi-infected mammals by state in published reports
| State | Vector(s) reported |
T. cruzi infection identified in: |
||
|---|---|---|---|---|
| Vector(s) | Wildlife | Dogs | ||
| AL | T. sanguisuga | Yes | Raccoon, opossum | |
| AR | T. sanguisuga | |||
| AZ | T. protracta, T. rubida, T. indictiva, T. recurva, T. incrassata, P. hirsuta | Yes | Raccoon, ringtail, skunk, woodrats, other rodents | |
| CA | T. protracta, T. rubida, P. hirsuta | Yes | Skunk, woodrats, other rodents | Yes |
| CO | T. protracta | |||
| DE | T. sanguisuga | |||
| FL | T. sanguisuga, T. lecticularia, T. rubrofasciata | Yes | Raccoon, opossum, skunk, gray fox | |
| GA | T. sanguisuga, T. lecticularia | Yes | Raccoon, opossum, skunk, gray fox, bobcat, coyote, feral swine | Yes |
| HI | T. rubrofasciata | Yes | ||
| IL | T. sanguisuga | |||
| IN | T. sanguisuga | Yes | ||
| KS | T. sanguisuga | Yes | ||
| KY | T. sanguisuga | Raccoon, opossum | ||
| LA | T. sanguisuga | Yes | Opossum, nine-banded armadillo | Yes |
| MD | T. sanguisuga | Raccoon, opossum | ||
| MO | T. sanguisuga, T. lecticularia | Yes | Raccoon | |
| MS | T. sanguisuga | Yes | ||
| NC | T. sanguisuga | Raccoon, opossum | ||
| NJ | T. sanguisuga | |||
| NM | T. lecticularia, T. protracta, T. gerstaeckeri, T. rubida, T. indictiva | Yes | Woodrats, other rodents | |
| NV | T. protracta, P. hirsuta | |||
| OH | T. sanguisuga | |||
| OK | T. sanguisuga, T. lecticularia | Yes | Raccoon, opossum | Yes |
| PA | T. sanguisuga | |||
| SC | T. sanguisuga, T. lecticularia | Gray fox | Yes | |
| TN | T. sanguisuga, T. lecticularia | Yes | Raccoon | Yes |
| TX | T. sanguisuga, T. lecticularia, T. protracta, T. gerstaeckeri, T. rubida, T. indictiva, T. neotomae | Yes | Raccoon, opossum, nine-banded armadillo, skunk, American badger, coyote, woodrats, other rodents, bat | Yes |
| UT | T. protracta, T. lecticularia | |||
| VA | T. sanguisuga | Yes | Raccoon, opossum, coyote | Yes |
| WV | T. sanguisuga | |||
The ranges of all United States species extend into Mexico, with the exception of T. rubrofasciata (49, 57). T. rubrofasciata is associated with rats and is thought to have been carried from North America globally on sailing ships in the 18th century (58). In the United States, this species has been reported in Jacksonville, Florida, and Honolulu, Hawaii, consistent with its predominant distribution in ports.
Allergic Reactions to Triatomine Antigens
T. gerstaeckeri, T. protracta, T. recurva, T. rubida, and T. sanguisuga have been implicated in allergic reactions in the United States (59). Such reactions are due to vector salivary antigens, not the infection status of the vector. Most reactions consist of a pruritic welt where the bite occurred. Severe reactions may involve angioedema, urticaria, dyspnea, gastrointestinal symptoms, and/or anaphylaxis (59). Severe reactions may necessitate treatment with epinephrine (60). Reports are most frequent in Arizona and California; the most commonly identified species are T. protracta and T. rubida, and the most frequent scenario is house invasion by an adult triatomine (59). In a study in southern California, allergic reactions consistent with those provoked by triatomine exposure were reported by 13% of residents of desert areas with frequent triatomine sightings, compared to 4% of those living in suburban Los Angeles County (61).
Wild and Domestic Animal Reservoirs
Wildlife reservoirs.
Trypanosoma cruzi infection has been reported in more than 150 species of mammals from eight orders, and it is widely believed that all mammals are susceptible (62). Birds and cold-blooded vertebrates are refractory to infection (63). The epidemiological importance of particular species is highly variable, depending on local ecology and parasite transmission dynamics. Maintenance reservoirs have persistent infection, while amplifier reservoirs are those that display characteristics that favor transmission, such as high parasitemia levels (64). As with humans, most infected animals are chronically infected, and therefore, detection may be reliant on a combination of examination of peripheral blood smears, culture isolation, serological testing, and PCR; both relative ease of trapping and variations in the performance of diagnostic assays contribute to bias in reported prevalence levels. Across the range of endemicity, Dasypus novemcinctus (nine-banded armadillo) and Didelphis species (opossums) are prominent sylvatic reservoirs and amplifiers of infection. Trypanosoma cruzi is able to infect almost all tissues in its mammalian hosts, including atypical sites like the cornea of Thrichomys apereoides (spiny rat) (65) and the anal scent glands of Didelphis species (66), enabling the latter to function as both host and vector. In addition to vector-borne transmission, many sylvatic mammals are prone to alternate transmission routes, including oral infection via ingestion of infected vectors, congenital infection, and exposure to contaminated bodily secretions (67). These biological features may predispose such hosts to infection with multiple strains, due to high transmission intensity and efficiency (68, 69).
In the United States, T. cruzi infection has been demonstrated in more than 24 wildlife species, including raccoons, opossums, armadillos, foxes, mice, squirrels, coyotes, skunks, and wood rats (9). Recent studies have expanded this list to include additional rodent (70, 71), bat (72), and deer species (73). Reported seroprevalence rates fluctuate quite widely within species, ranging from 15 to 90% in raccoons (70, 74, 75), 9 to 100% in skunks (70, 76), 8 to 33% in opossums (76, 77), and 20 to 76% in woodrats and other rodents (70, 78–80). The prevalence varies depending on ecology and local diversity and density of vector species and, in some cases, between sexes, with female denning activities being associated with increased triatomine contact (76, 81). High infection rates in some mammals, such as wood rats and raccoons, may result from frequent insectivory (82). Experimental infection studies and the high attack rates in human outbreaks of orally transmitted Chagas disease suggest that ingestion of infected vectors or vector fecal material is a very efficient transmission route (34, 82). In contrast, consumption of raw T. cruzi-infected meat did not result in experimental infection in one study (82).
Canine Chagas disease.
Dogs are important in peridomestic cycles in Latin America, both as vector blood meal sources and T. cruzi infection reservoirs (83, 84). In the Chaco region of Argentina where the disease is hyperendemic, dogs have been shown to be highly infective to vectors and are thought to be a key reservoir sustaining transmission to humans (85). In the United States, T. cruzi-infected dogs have been reported from Tennessee, South Carolina, Georgia, Virginia, Louisiana, California, Oklahoma, and Texas (reviewed in reference 9). Infected dogs may develop acute and chronic manifestations similar to those in humans, including acute myocarditis, arrhythmias, chronic dilated cardiomyopathy, congestive heart failure, and sudden death (86). Several recent surveys in Texas demonstrate widespread canine T. cruzi infection, especially in working dogs and those living in kennels (87–90). The prevalence in these surveys varied widely. Some studies demonstrated significant discordance between diagnostic tests and a substantial number of dogs whose infection status was unresolved with the testing performed (89). The highest infection rate, 71% by serology, was reported in the investigation of a Texas kennel where several dogs suffered sudden death suspected to be due to acute Chagas disease (87). Triatomines collected in dog kennels and near houses in Texas show high prevalences of canine blood meals and T. cruzi infection (87, 91), suggesting that dogs may be an important peridomestic host.
Transmission Potential in the United States
With the exception of the rarely collected species T. neotomae, T. incrassata, and P. hirsuta, all U.S. vector species have been reported to invade human dwellings (54, 60). A total of 2,883 specimens of 7 different vector species were submitted to the Texas citizen science project (54). Of these, 17% were collected inside human dwellings; the highest proportions were for T. rubida and T. protracta. Houses with refuges like woodpiles, rock piles, and brush and those with structural gaps through which vectors can pass are more vulnerable to vector invasion (50, 60, 92). Rarely, the presence of T. protracta or T. recurva nymphs has been reported inside houses, suggesting possible colonization (60). Window screens, air conditioning, and caulking of gaps in house construction may be protective against such invasion (60).
Detection of human blood meals is frequent in tested triatomines, especially those collected in and around human dwellings and in other spaces where humans congregate. In a recent study in Texas, human blood was detected in 59% (30/51) of T. gerstaeckeri bugs; the second most frequent blood meal was canine (17/51, 33%), followed by more than a dozen other vertebrate hosts (93). In this study, collection sites were largely domestic or peridomestic, and human blood meals were found in 77% (17/22) of T. gerstaeckeri bugs collected inside homes; mixed blood meals were frequent. In another study from Texas, vectors were collected in dog kennels and woodrat nests, as well as domestic settings (91). In this study, dogs (10/33, 30%) were the predominant blood meal source for T. gerstaeckeri, followed by woodrats (Neotoma micropus) (7/33, 21%); human blood was identified in a single bug. All 40 T. protracta bugs were collected in woodrat nests and had fed exclusively from Neotoma micropus (91). In a Louisiana study conducted near the house of the 2006 autochthonous human infection, 43 T. sanguisuga bugs were collected; 53% had fed from American green tree frogs, 49% from humans, and 30% from raccoons; detection of blood from multiple host species was frequent (94). In the Arizona-Sonora Desert Zoo, human blood was detected in all 7 T. rubida bugs tested; 5 of 7 had other blood meal sources detected, including pig, sheep or goat, dog, mouse, rat, or woodrat (95). Human blood was also detected in 2 of 3 T. recurva bugs collected elsewhere in southern Arizona (95).
The coincidence of human blood meals and T. cruzi infection in triatomines has been described as indicating the “potential for Chagas disease” in the United States (93–95). Clearly, transmission to humans occurs; more investigation is needed to quantify the risk, since most infections likely go undetected. However, the small number of locally acquired T. cruzi infections detected in humans stands in contrast to the moderate to high T. cruzi prevalence rates in dogs, raccoons, opossums, and woodrats. Compared to major South American vectors, such as R. prolixus and T. infestans, North American vectors appear to have somewhat longer time intervals from blood meal to defecation and may be less likely to defecate on the host (96–99). Vectors rarely colonize houses in the United States, and well-constructed houses with window screens provide effective barriers against domestic invasion. Perhaps most importantly, stercorarian transmission is inefficient; mathematical models based on data from the Gran Chaco, where transmission to humans is the highest in the world, estimate that a single human T. cruzi infection requires on average 900 to 4,000 contacts with infected vectors (69).
MOLECULAR EPIDEMIOLOGY
Trypanosoma cruzi Genotypes
Trypanosoma cruzi is a highly genetically diverse parasite, estimated to have diverged from its most recent common ancestor 3 to 4 million years ago (100). Scientific consensus currently defines a minimum of six genetic lineages, or discrete typing units (DTUs [TcI to TcVI]) (101), plus a potential seventh, bat-associated genotype (TcBat), most closely related to TcI (102–105). Multiple molecular markers confirm a largely clonal population structure, which maintains the identity of major DTUs, interspersed with recombination events (106). TcI through TcIV form monophyletic clades, while TcV and TcVI resulted from recent hybridization of TcII and TcIII (100, 107). Genomic data support this evolutionary model. TcI to TcIV display substantial allelic homozygosity resulting from long-term, recurrent, and dispersed gene conversion, whereas TcV and TcVI have natural heterozygosity and minimal distinction, with shared intact alleles from their parental DTUs (100, 107–111).
Each T. cruzi DTU is characterized by distinct but often overlapping transmission ecologies (112). TcI, TcII, TcV, and TcVI are commonly associated with domestic cycles and are the genotypes found in most human infections. Investigators have long observed that gastrointestinal Chagas disease is more frequent in the Southern Cone than further north in Latin America and hypothesized a connection to different circulating T. cruzi strains (113). However, there remains no clear, unequivocal evidence of influence of particular lineages on the progression or clinical outcome of human Chagas disease (reviewed in reference 114). Domestic TcI is distributed from the Amazon Basin northwards and is the principal DTU found in humans in Venezuela, Ecuador, and Colombia (115–117). TcI also circulates in arboreal ecotopes between Didelphis species and the triatomine tribe Rhodniini (118, 119), with secondary cycles among rodents and sylvatic Triatoma species in highland valleys in Bolivia, Peru, and Chile (120–123). Sylvatic TcI populations are characterized by high levels of genetic diversity (124–130), while human infections are associated with divergent, more genetically homogenous strains (128, 131–133). In contrast, TcII, TcV, and TcVI appear less variable overall (100) and are predominant in domestic cycles in the Southern Cone (112, 134, 135). However, recent whole-genome sequencing of clinical TcII isolates has revealed more extensive intra-DTU diversity than previously reported (110). Sylvatic reservoirs of TcII, TcV, and TcVI are less well delineated than for TcI, but TcII has been increasingly detected in Brazilian primates (130, 136, 137). In addition, TcV and TcVI have been demonstrated in domestic dogs from Argentina to as far north as Colombia (107, 138–141). TcIII is transmitted by Panstrongylus geniculatus to D. novemcinctus and other burrowing mammals in terrestrial transmission cycles from the Amazon Basin to Argentina (142–144). The known host range of this DTU has expanded to include dogs, grisons, and foxes in Brazil (145). TcIV, perhaps the most neglected DTU, circulates sympatrically with TcI in wild primates in the Amazon (146) and raccoons and dogs in the United States (147). TcIV can invade the domestic environment in Venezuela (113, 116) and has been isolated from patients during oral-infection outbreaks in the Brazilian Amazon (146, 148–151) and Colombia (152). Finally, TcBat has been isolated from Chiroptera species across Brazil (102), Panama (103), Colombia (105), and Ecuador (104) and is potentially infective to humans (153).
Trypanosoma cruzi Molecular Epidemiology in the United States
The majority of genotyping activities have concentrated on vectors and reservoir hosts. In an extensive analysis of U.S. vectors and mammalian hosts, all five autochthonous human cases were reported as TcI (online supplement in reference 119). In a more recent series of presumed-autochthonous chronic T. cruzi infections in Texas blood donors, the authors were limited by genetic marker resolution and therefore unable to distinguish among TcII, TcV, and TcVI (154).
To date, TcI and TcIV are the only DTUs detected among the six triatomine species examined, with no absolute associations between parasite genotype and vector (Table 4). Higher proportions of TcIV have usually been identified in T. sanguisuga, T. indictiva, and T. lenticularia, compared to a predominance of TcI in T. gerstaeckeri, T. protracta, and T. rubida (78, 87, 89, 119, 155–157). However, except for studies of vectors collected by the Texas citizen science initiative (156, 157), samples sizes were far too small to make any meaningful extrapolations. The observation of potential TcII/V/VI autochthonous human infections in Texas is noteworthy but challenging to interpret without clear evidence of these genotypes circulating in local vector species (154). Similarly, a study of T. protracta collected in California encountered issues distinguishing among TcII/V/VI and was unable to establish the presence of infections with these lineages (158). Further investigations are warranted to confirm the presence of these DTUs in the United States, using a larger panel of more highly resolutive markers, in conjunction with phylogenetic analyses incorporating all representative T. cruzi DTUs; neither of these studies examined parasite sequence homology to TcI (154, 158).
TABLE 4.
Trypanosoma cruzi genotypes reported in triatomine vectors in the United States
| Location | Vector | Total no. examined | No. (%) T. cruzi positive | No. typed | No. (%) that were: |
Genotyping methoda | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| TcI | TcIV | TcI/IV | |||||||
| TX | T. gerstaeckeri | 16 | 16 (100) | 16 | 10 (63) | 4 (25) | 2 (13) | SL-IR; TcSC5D gene and SNPs in subset | 87 |
| TX | T. gerstaeckeri | 897 | 574 (64) | 548 | 294 (54) | 189 (34) | 65 (12) | TcSC5D; SL-IR on subset | 156 |
| South TX | T. gerstaeckeri | 18 | 9 (50) | 9 | 6 (67) | 1 (11) | 2 (22) | SL-IR | 89 |
| TX | T. gerstaeckeri | 11 | 1 (9) | NR | 100% | NR | SL-IR | 157 | |
| TX | T. gerstaeckeri | NR | NR | 3 | 2 (67) | 0 | 1 (33) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 |
| TX | T. gerstaeckeri | 19 | 13 (100) | 13 | 13 (100) | 0 | 0 | 18S rRNA sequencing | 71 |
| TX | T. gerstaeckeri | 1 | 1 (100) | 0 | 0 | SL-IR | 337 | ||
| TX | T. indictiva | 67 | 32 (48) | 28 | 9 (32) | 17 (61) | 2 (7) | TcSC5D; SL-IR on subset | 156 |
| TX | T. lenticularia | 66 | 44 (67) | 42 | 9 (21) | 25 (60) | 8 (19) | TcSC5D; SL-IR on subset | 156 |
| TX | T. lenticularia | 2 | 2 (100) | 2 | 2 (100) | 18S rRNA sequencing | 71 | ||
| TX | T. protracta | 19 | 3 (16) | 2 | 2 (100) | 0 | 0 | TcSC5D; SL-IR on subset | 156 |
| Southwestb | T. protracta | 14 | 1 (7) | 1 | 1 (100) | 0 | 0 | TcSC5D; SL-IR on subset | 156 |
| Northern CA | T. protracta | 29 | 16 (55) | 13 | 13 (100) | 0 | 0 | RFLP (HPS60, GPI), SL-IR, 24S α-subunit rRNA, sequencing of Rb19, TR, and COII-ND1 | 48 |
| Southern CA | T. protracta | 68 | 21 (31) | 9 | 7 (78) | 2 (22) | 0 | RFLP (HPS60, GPI), SL-IR, 24S α-subunit rRNA, sequencing of Rb19, TR and COII-ND1 | 48 |
| Southern CA | T. protracta | 161 | 34 (21.1) | 2c | 0 | 0 | 0 | 24S α-subunit RNA | 158 |
| TX | T. protracta | 9 | 4 (44) | NR | 100% | NR | SL-IR | 157 | |
| TX | T. rubida | 64 | 11 (17) | 7 | 6 (86) | 1 (14) | 0 | TcSC5D; SL-IR on subset | 156 |
| Southwestd | T. rubida | 40 | 7 (18) | 5 | 5 (100) | 0 | 0 | TcSC5D; SL-IR on subset | 156 |
| South TX | T. rubida | 2 | 0 | 0 | 0 | 0 | 0 | SL-IR | 89 |
| TX | T. rubida | 299 | 69 (23) | NR | 100% | NR | SL-IR | 157 | |
| West TX | T. rubida | 39 | 24 (62) | 24 | 24 (100) | 0 | 0 | TcSC5D | 155 |
| TX | T. sanguisuga | 20 | 13 (65) | 13 | 2 (15) | 9 (69) | 2 (15) | SL-IR; TcSC5D and SNPs in subset | 87 |
| TX | T. sanguisuga | 315 | 158 (50) | 135 | 21 (16) | 107 (79) | 7 (5) | TcSC5D; SL-IR on subset | 156 |
| Southeaste | T. sanguisuga | 45 | 12 (27) | 12 | 2 (17) | 10 (83) | 0 | TcSC5D; SL-IR on subset | 156 |
| Midwestf | T. sanguisuga | 7 | 4 (57) | 3 | 0 | 3 (100) | 0 | TcSC5D; SL-IR on subset | 156 |
| FL, GA | T. sanguisuga | 4 | 4 (100) | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | ||
| LA | T. sanguisuga | 12 | 8 (67) | 6 | 6 (100) | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 78 |
SL-IR, spliced-leader intergenic region; SNPs, single-nucleotide polymorphisms; RFLP, restriction fragment length polymorphism.
AZ, CA, NM.
Typed as II/VI.
AZ, NM.
AL, FL, GA, KY, LA, NC, TN, VA.
IN, KS, MO, OH, OK.
Among reservoir hosts, TcI and TcIV are the principal DTUs identified in United States (Table 5). Similar to vector surveys, sample sizes are insufficient to reveal any strict correlations between host and parasite genotype; current data demonstrate both lineages circulating among mammalian hosts in variable proportions. Finally, a few studies in Louisiana reported rodents harboring TcII, alongside other mixed TcI, TcIV, and TcVI infections (78). Additional sampling efforts will be necessary to delineate the frequency and ecology of TcII/V/VI in the United States.
TABLE 5.
Trypanosoma cruzi genotypes reported in mammalian hosts in the United States
| Location(s) (no. of individuals) | Host | Total no. genotyped | No. (%) of samples typed as: |
Other DTUs reported (no. of samples) | Genotyping method | Reference | ||
|---|---|---|---|---|---|---|---|---|
| TcI | TcIV | TcI/IV | ||||||
| CA (2), LA (1), TX (2) | Humans | 5 | 5 (100) | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| TX | Humans | 6 | 0 | 0 | 0 | TcII-V-VI (4), TcI/TcII-V-VI (2)a | PCR-RFLP (SL-IR, 24S α-subunit rRNA, 18S rRNA), sequencing | 154 |
| CA (1), OK (1), SC (2), TN (1), Unknown (2) | Canis lupus familiaris (domestic dog) | 7 | 0 | 6 (86) | 1 (14) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| TX | C. lupus familiaris (domestic dog) | 2 | 1 (50) | 0 | 1 (50) | SL-IR | 89 | |
| TX | C. lupus familiaris (domestic dog) | 15 | 9 (60) | 5 (33) | 1 (7) | SL-IR, sequencing of TcSC5D | 87 | |
| TX | C. lupus familiaris (domestic dog) | 4 | 4 (100) | 0 | 0 | SL-IR | 337 | |
| TX | C. lupus familiaris (domestic dog) | 6 | 5 (83) | 1 (17) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 88 | |
| FL (16), GA (45), MD (1), TN (1), SC (1) | Procyon lotor (raccoon) | 64 | 2 (3) | 61 (95) | 1 (2) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| GA | P. lotor (raccoon) | 5 | 0 | 5 (100) | 0 | SL-IR, confirmatory sequencing | 70 | |
| TX | P. lotor (raccoon) | 11 | 10 (91) | 0 | 1 (9) | TcSC5D | 75 | |
| TX | P. lotor (raccoon) | 2 | 0 | 2 (100) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 338 | |
| IL | P. lotor (raccoon) | 5 | 0 | 5 (100) | 0 | SL-IR, 24S α-subunit rRNA, confirmatory sequencing | 339 | |
| KY | P. lotor (raccoon) | 2 | 0 | 2 (100) | 0 | SL-IR, 24S α-subunit rRNA, confirmatory sequencing | 339 | |
| MO | P. lotor (raccoon) | 1 | 0 | 1 (100) | 0 | SL-IR, 24S α-subunit rRNA, confirmatory sequencing | 339 | |
| GA | Lemur catta (ring-tailed lemur) | 3 | 0 | 3 (100) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| GA (1), unknown (1) | Macaca mulatta (rhesus macaque) | 2 | 1 (50) | 0 | 1 (50) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| Tx | M. mulatta (rhesus macaque) | 33 | 18 (55) | 13 (39) | 2 (6) | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 338 | |
| AL (1), FL (6), GA (6), LA (2) | Didelphis virginiana (opossum) | 15 | 15 (100) | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| TX | D. virginiana (opossum) | 4 | 4 | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 338 | |
| GA (1), LA (2) | Dasypus novemcinctus (nine-banded armadillo) | 3 | 2 (67) | 1 (33) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| GA | Mephitis mephitis (striped skunk) | 1 | 0 | 1 (100) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA | 119 | |
| GA | M. mephitis (striped skunk) | 4 | 1 (25) | 3 (75) | 0 | SL-IR, confirmatory sequencing | 70 | |
| TX | M. mephitis (striped skunk) | 2 | 1 (50) | 1 (50) | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 338 | |
| TX | Neotoma micropus (Southern plains woodrat) | 23 | 10 (43) | 13 (57) | 0 | SL-IR, confirmatory sequencing | 70 | |
| TX | N. micropus (Southern plains woodrat) | 1 | 1 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| GA | Sigmodon hispidus (hispid cotton rat) | 2 | 0 | 2 (100) | 0 | SL-IR, confirmatory sequencing | 70 | |
| GA | Otospermophilus variegatus (rock squirrel) | 1 | 0 | 1 (100) | 0 | SL-IR, confirmatory sequencing | 70 | |
| TX | Peromyscus leucopus (white-footed mouse) | 3 | 3 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| TX | Chaetodipus hispidus (hispid pocket mouse) | 1 | 1 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| TX | S. hispidus (hispid cotton rat) | 1 | 1 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| TX | Baiomys taylori (northern pygmy mouse) | 1 | 1 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| TX | Liomys irroratus (Mexican spiny pocket mouse) | 1 | 1 (100) | 0 | 0 | 18S rRNA sequencing | 71 | |
| LA | Peromyscus gossypinus and Mus musculus (mouse spp.) | 20 | 16 (80) | 0 | 0 | TcII (2), TcI/TcII (1), TcII/TcIV (1) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 78 |
| LA | Neotoma floridana | 3 | 2 (67) | 0 | 0 | TcII/TcIV (1) | SL-IR, 24S α-subunit rRNA, 18S rRNA | 78 |
| TX | Nycticeius humeralis (evening bat) | 1 | 1 (100) | 0 | 0 | SL-IR, 24S α-subunit rRNA, 18S rRNA, COII | 72 | |
The authors were unable to distinguish between non-TcI DTUs.
Issues Underlying T. cruzi Genotyping Data Collection
To accurately interpret T. cruzi genotypic data, biological and logistical limitations relating to both parasite infection dynamics and genotyping methodologies must be considered. Trypanosoma cruzi genotyping can be conducted on clinical samples (blood or tissue) or parasite axenic cultures, obtained through hemoculturing or xenodiagnosis. Due to low levels of peripheral parasitemia, especially in chronically infected patients, direct genotyping is insensitive but may be improved if multiple specimens are tested (159). The principal limitation of parasite isolation is selection bias for specific clones, due to higher growth rates or culture conditions to begin with (160–162) and, subsequently, by loss of diversity from long-term maintenance in axenic culture or animals (163–167). Hemoculture recovery rates are usually less than 30% among chronic patients (168) and are dependent upon parasite load and distribution in the initial inoculum. Xenodiagnosis can generally recover more parasite strains, but biases may result from variable vector permissibility to specific strains (169–171). Furthermore, circulating clones isolated by hemoculture or xenodiagnosis may be different from those sequestered in tissues due to differential strain tropisms (172–174) and can vary even between sequential blood samples (175). Similar biases affect sylvatic T. cruzi sampling, with certain reservoir species more heavily represented in survey data due to their relative ease of capture and the presence of detectable parasitemia.
The most commonly used genotyping techniques for clinical and field specimens involve analysis of size polymorphisms in multicopy genetic markers, particularly the nuclear spliced-leader intergenic region (SL-IR), 24α rRNA, and 18S ribosomal DNA (rDNA) (Tables 4 and 5) (176, 177). The major confounder associated with the use of any multicopy gene is the level of intraclone copy number and undefined chromosomal orthology, which can prevent direct comparability between strains. The SL-IR is present in many hundreds of copies per parasite genome; the copies are not necessarily identical and may instead comprise a predominant haplotype accompanied by a low abundance of minor paralogous sequence types more closely related in identity to other DTUs, likely resulting from their shared evolution (110, 178, 179). In this scenario, it is virtually impossible to distinguish between a monoclonal DTU infection containing multiple divergent SL-IR sequences and a polyclonal infection consisting of different major DTU parasites. This issue is minimized when using conventional PCR, as generally, the most common gene sequence is amplified in a reaction. However, recently, a number of deep sequencing studies reported results based on sequencing millions of copies of the SL-IR locus that seem to indicate the occurrence of almost all DTUs in infected rodents and primates in the United States (180, 181). Parallel deep sequencing of appropriate biologically cloned controls to exclude low-abundance haplotypes has thus far yielded equivocal evidence; further investigations are essential to define the applicability of this technique to characterize natural multiclonal infections (182, 183).
CLINICAL MANIFESTATIONS
Acute T. cruzi Infection
The acute phase begins 1 to 2 weeks after vector-borne transmission and lasts approximately 8 weeks. Patients are most commonly asymptomatic or experience mild, nonspecific symptoms such as fever. A T. cruzi abscess or chagoma may occur at the site of inoculation. Parasite entry via the conjunctiva may result in unilateral eyelid swelling (the Romaña sign) (184). However, eyelid swelling can be caused by an allergic reaction to triatomine salivary or fecal antigens; confirmed diagnosis of T. cruzi infection is obligatory, even in the setting of vector exposure and an apparent Romaña sign. Severe acute Chagas disease, including myocarditis, pericardial effusion, and/or meningoencephalitis, is rare, but when it occurs, the mortality risk is high (5, 185). In the absence of the Romaña sign or severe manifestations, individual infections are seldom diagnosed during the acute phase.
Orally transmitted T. cruzi infection has been reported to cause more severe acute morbidity and higher mortality than vector-borne infection (186, 187). Microepidemics appear to be fairly frequent in the Amazon basin, due to sylvatic vectors contaminating produce, such as açaì or sugarcane (31). In the Caracas outbreak, mentioned above, 103 people were infected, of whom 59% had electrocardiogram (ECG) abnormalities and 20% were admitted to hospital, and one person died from acute Chagas myocarditis (32, 34). Alterations in T. cruzi surface glycoproteins caused by exposure to gastric acid may increase parasite invasiveness, providing a possible explanation for the increased severity of orally acquired Chagas disease (188, 189).
Congenital Chagas disease is acute infection in the newborn. Most infected infants are asymptomatic or have mild findings, but a small percentage present with severe disease or die in utero (18, 190). Manifestations may include low birth weight, prematurity, low Apgar scores, hepatosplenomegaly, anemia, and thrombocytopenia (18, 190). Severely affected neonates may have meningoencephalitis, gastrointestinal megasyndromes, myocarditis, pneumonitis, and/or respiratory distress (18). Women who receive antitrypanosomal therapy prior to conception are significantly less likely to transmit T. cruzi to their infants (23, 191).
Chronic T. cruzi Infection
One to 2 months after infection, parasitemia falls below levels detectable by microscopy, and the patient passes into the chronic phase of T. cruzi infection (2, 5). Chronic T. cruzi infection without signs or symptoms of Chagas disease is designated the indeterminate form (2, 5, 192). Over a period of years to decades, an estimated 20 to 30% of infected individuals develop cardiomyopathy (2, 5). A retrospective cohort analysis of Brazilian blood donors estimated progression to cardiomyopathy to occur at a rate of 1.85% per year (193). Chagas cardiomyopathy features chronic inflammation in all chambers and damage to the conduction system and cardiac muscle (194). The most frequent early signs are right bundle branch block or left anterior fascicular block and segmental left ventricular wall motion abnormalities (185, 194, 195). Later manifestations appear decades after infection and include ventricular arrhythmias, sinus node dysfunction and bradycardia, persistent or intermittent complete heart block, an apical aneurysm usually in the left ventricle, thromboembolic phenomena, and progressive dilated cardiomyopathy. Patients may experience palpitations, syncope, and systemic and pulmonary emboli, with high risk of sudden death or death from progressive heart failure. (185, 194, 195).
Gastrointestinal involvement is much less frequent than cardiomyopathy. Esophageal manifestations range from asymptomatic motility disorders through mild achalasia to megaesophagus (196). Patients may experience dysphagia, odynophagia, esophageal reflux, weight loss, aspiration, and regurgitation. Patients with colonic involvement may have prolonged constipation, fecaloma, volvulus, bowel ischemia, or megacolon. Symptomatic gastrointestinal disease, like symptomatic cardiac disease, usually appears several decades after infection.
Chagas Disease in the Immunocompromised Host
Organ-derived infection.
Acute T. cruzi infection in organ transplantation recipients may lead to a relatively severe clinical spectrum, with manifestations that include acute myocarditis and congestive heart failure (197). In recent years, as screening of donors has become more frequent, most donor-derived infections have been detected by PCR monitoring prior to symptom onset, allowing prompt antitrypanosomal treatment and favorable outcomes (28). Current recommendations suggest monitoring the recipient of an organ from an infected donor for at least 6 months, at which point the frequency can be decreased (Table 6) (30).
TABLE 6.
Chagas disease diagnostic testing by clinical context
| Clinical scenario | Testing modality(ies), specimen type(s), and schedule (reference[s]) |
|---|---|
| Suspected chronic T. cruzi infection | |
| All persons (symptomatic or asymptomatic) with epidemiological risk factorsa ; high priority to screen children and women of child-bearing age, especially if pregnant or planning pregnancy | IgG serologyb by two distinct assays, preferably based on different antigens (16) |
| Persons at risk for acute T. cruzi infection | |
| Suspected contact with infected vector | PCR (microscopy)c in blood between 2 and 8 wks postexposure, IgG serology at 6–8 wks |
| Infant of T. cruzi-infected mother | PCR (microscopy)c in blood at birth and 1–3 mos; IgG serology at 9–12 mos (216) |
| Recipient of blood components, organ, or tissue from infected donor | Serial PCR in blood 1×/wk in mos 1–2, 1×/2 wks in mos 3–4, 1×/mo in mos 5–6, then based on clinical scenario (30) |
| Laboratory accident | Serial PCR (microscopy)c in blood 1×/wk for 6–8 wks, IgG serology at 6 to 8 wks (340) |
| Persons at risk for T. cruzi reactivation | |
| Prospective organ or tissue recipient with risk factors | IgG serology by two distinct assays (341, 342) |
| Transplant recipient with chronic T. cruzi infection | Serial quantitative PCR in bloodd 1×/wk in mos 1–2, 1×/2 wks in mos 3–4, 1×/mo in mos 5–6, then based on clinical scenario (203); for heart transplant patients, histology in endomyocardial biopsy specimen, especially in setting of suspected rejection |
| HIV-T. cruzi-coinfected patient with signs of reactivation | PCRd /microscopy in tissue, blood, CSF as clinically indicated |
| T. cruzi-infected patient with iatrogenic immunosuppression (chemotherapy, corticosteroids) and signs of reactivation | PCRd /microscopy in tissue, blood, CSF as clinically indicated |
Epidemiological risk factors for T. cruzi infection include birth or residence or maternal birth or residence in a country with endemic vectorial transmission (see Table 1); residence in areas of the United States with high rates of vector-human contact, especially if the patient reports triatomine bites and/or house invasion.
Plasma is not an approved biospecimen for some FDA-cleared tests; serum is acceptable for all FDA-cleared tests.
PCR is substantially more sensitive than microscopy in peripheral blood.
Positive PCR in blood does not diagnose reactivation; rising parasite load in blood is generally the first indication. Positive PCR in cerebrospinal fluid (CSF) indicates reactivation.
Reactivation in cardiac transplant recipients.
Cardiac transplantation is an accepted treatment for end-stage Chagas cardiomyopathy (198, 199). In a large Brazilian cohort, survival of patients transplanted for Chagas cardiomyopathy was better than among those with idiopathic or ischemic cardiomyopathy and T. cruzi reactivation was a rare cause of death (200, 201). Data from a smaller cohort of patients transplanted for end-stage Chagas cardiomyopathy in the United States also demonstrated survival similar to that among patients transplanted for other etiologies (202). The most common manifestations of reactivation are fever and acute myocarditis in the transplanted heart. Patients may also develop inflammatory panniculitis and cutaneous nodules (197). Central nervous system (CNS) involvement occurs infrequently. All patients with dilated cardiomyopathy and a history of significant residence in continental Latin America should be screened (203). For those found to be infected, posttransplant monitoring should include histopathology of the explanted heart and subsequent endomyocardial biopsy specimens and serial peripheral blood monitoring by quantitative PCR (Table 6) (203).
Reactivation of Chagas disease in HIV-coinfected patients.
The most common clinical manifestation of T. cruzi reactivation in HIV-coinfected patients is meningoencephalitis with or without a mass lesion (204). The case fatality rate for CNS reactivation is very high. The presentation is often confused with CNS toxoplasmosis (205, 206); T. cruzi should be considered in the differential diagnosis of CNS mass lesions in HIV-infected patients (207, 208). Acute reactivated myocarditis is another frequent manifestation and may be obscured by preexisting chronic cardiomyopathy (209). New arrhythmias or conduction system abnormalities, pericardial effusions, or cardiac decompensation should prompt testing for reactivation. Subcutaneous nodules resembling erythema nodosum and parasitic invasion of the peritoneum, stomach, or intestine can occur but are uncommon (210). Five cases of T. cruzi reactivation in HIV-infected Latin American immigrants have been reported in the United States since 1992; all presented as CNS syndromes and were treated initially as toxoplasmosis (205, 206, 211–213).
DIAGNOSTIC TECHNIQUES
The choice of modality to diagnose Chagas disease is determined by the clinical setting and suspected phase of infection. In general, techniques to directly detect the parasite are used in the acute phase and suspected reactivation, whereas IgG serology is the mainstay of diagnosis in the chronic phase (Table 6).
Microscopy
In acute, congenital, or reactivated infection, trypomastigotes may be detectable by light microscopy in thick and thin smears from whole blood or buffy coat with routine staining (e.g., Giemsa stain) (214). When acute or reactivation meningoencephalitis is suspected, cerebrospinal fluid samples should be concentrated by the thin-layer cell preparation technique, stained, and examined by light microscopy. Microscopy is useful due to fast turnaround time, wide availability, and high specificity, but its sensitivity is lower than that of molecular techniques (215, 216).
Molecular Techniques
The most sensitive primer sequences originate from satellite or kinetoplast minicircle DNA (217–219). A recent publication from the CDC outlines an algorithm that incorporates testing by multiple primer sets in a quantitative assay to optimize performance and reliability (218). Several recent initiatives have addressed standardization of qualitative and quantitative PCR for clinical use (217, 220). In acute or early congenital infection, PCR has substantially higher sensitivity than microscopy and is the diagnostic test of choice (190, 219). PCR results are variably positive in chronic T. cruzi infection, depending on specimen volume, primers, DNA extraction methods, and level of experience of the laboratory (220). Blood clot or buffy coat preparations may provide higher sensitivity than whole blood, but these preparations may not be widely available in routine clinical laboratories (218, 221). PCR has recently been utilized in several clinical trials as an early indicator of treatment failure; use in this setting requires rigorous standardization and criteria for patient inclusion (for example, positive results by PCR in at least one of 3 pretrial 10-ml specimens) (222, 223). In chronically infected patients at risk because of immunosuppression, a rise in parasite load by quantitative PCR in serial specimens is the earliest indicator of reactivation, enabling treatment before onset of symptoms (224, 225).
Diagnostic Serology
Diagnosis in the chronic phase of Chagas disease relies on detection of host IgG against T. cruzi antigens (16). Currently, the main methods in use are enzyme-linked immunosorbent assays (ELISAs), immunofluorescence assays (IFAs), and immunochromatographic strip or cassette tests. Confirmed diagnosis requires positive results by at least two assays, preferably based on different antigens (for example, parasite lysate and recombinant antigens) (16). The sensitivities and specificities of the available assays are not sufficient for a single assay to be used alone for diagnosis, especially in a setting where prevalence is low and pretest probability is not high. The IgG trypomastigote excreted-secreted antigen immunoblot (TESA-blot) is used as a confirmatory test in blood banks and clinical practice in Brazil (226, 227). Preparation of the test strips using antigen from trypomastigotes in cell culture requires specialized infrastructure and may be prone to interbatch and laboratory variability. The banding pattern may differ by T. cruzi DTU, suggesting different antigenic characteristics between strains (228). Conventional serology in cord and infant blood reflects transferred maternal IgG until around 9 months of age.
IgM-based assays have been evaluated for the diagnosis of acute T. cruzi infection, with a special focus on use for congenitally infected infants in settings where molecular assays are not available (226). IgM TESA-blot showed sensitivity of 58% compared to a consensus definition of infection and 80% compared to PCR in congenitally infected infants in Bolivia; in these two analyses, the specificities were 98% and 94%, respectively (190, 229). The specificity of IgM assays utilizing whole T. cruzi lysate was <30% in a similar population of congenitally infected infants (230). In the United States, PCR is the assay of choice for the diagnosis of acute and congenital T. cruzi infection (216).
HCT/P
Serological screening is recommended for donors of human cells, tissues, and tissue-based products (HCT/P) with epidemiological risk factors; for example, those who were born or lived in areas of endemicity or whose mothers were born in such areas. Two assays are approved by the U.S. Food and Drug Administration (FDA) for living and cadaveric donor screening, Ortho ELISA (Ortho-Clinical Diagnostics, Inc., Raritan, NJ) and Abbott PRISM (Abbott Diagnostics, Abbott Park, IL) (231). These tests are currently available only in blood donor testing laboratories. The Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) Disease Transmission Advisory Committee also recommends the use of an FDA-cleared diagnostic ELISA to test living donors (232, 233). For living donors, a positive screening test should prompt referral for appropriate diagnostic testing and clinical evaluation (192).
Histopathology
Trypanosoma cruzi causes tissue damage through cellular lysis, inflammatory response, and fibrotic replacement (234). The spectrum of histopathology related to T. cruzi infection has been the subject of several recent reviews (235–239). The most important target organ is the heart, where chronic pathology includes multichamber damage, most prominent in the ventricles and often severe enough to form an apical aneurysm (235, 236). The spectrum of microscopic pathology includes myofiber degeneration, interstitial fibrosis, and patchy inflammation predominantly comprised of lymphocytes, macrophages, plasma cells, and eosinophils; neutrophils are not commonly observed. The patterns of inflammation and fibrosis can be focal or diffuse throughout the layers of the myocardium. Fibrotic plaques may be observed on the epicardium (235, 236). Intracellular amastigote pseudocysts are rarely observed in chronic pathology, especially with limited tissue sampling, but demonstrable parasite persistence appears to be associated with higher-grade inflammation in the chronic phase (236, 240, 241). Histopathology can play an important diagnostic role in the setting of suspected reactivation. Careful examination of endomyocardial biopsy specimens for nests of intracellular parasites can help distinguish rejection from T. cruzi reactivation in cardiac transplant recipients (203, 242). In immunosuppressed patients with suspected skin manifestations of T. cruzi reactivation, histopathology may reveal the parasite and confirm the diagnosis (238). The diagnosis of T. cruzi reactivation was made on a brain biopsy specimen in a patient with HIV and cerebral lesions of unknown etiology (205).
ETIOLOGICAL TREATMENT AND CLINICAL MANAGEMENT
Antitrypanosomal Drugs
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease (2). Benznidazole is considered the first-line treatment, because of better tolerance and more comprehensive efficacy data (1, 243). Benznidazole is a prodrug that requires metabolism by parasite enzymes to become active; metabolites appear to act through multiple mechanisms to interrupt T. cruzi glutathione and trypanothione pathways (243). Dermatologic side effects are frequent and consist of rashes and photosensitization (244, 245). Dermatitis occurs with significantly higher frequency in females than males (244). Most rashes are mild and can be managed with antihistamines or topical steroids without interrupting treatment (246). Treatment should be suspended immediately for severe or exfoliative dermatitis or dermatitis associated with fever and lymphadenopathy. Peripheral neuropathy is dose dependent, usually occurs late in the course of therapy, and should prompt immediate treatment interruption; resolution may take months. Bone marrow suppression is rare and requires immediate interruption of treatment. Clinical and laboratory monitoring for side effects should occur regularly throughout the course of treatment. Benznidazole tolerance is substantially better in children than in adults and in children younger than 7 years than in older children (247). This better safety profile correlates directly with more rapid elimination of the drug in younger age groups (248).
Benznidazole was approved by the FDA in August 2017 (249) and became commercially available in the United States as of 14 May 2018. The drug is marketed in the United States by Exeltis, a U.S.-based division of Insud Pharma (previously called Chemo Group) (250). The approval covers treatment of T. cruzi infection in children 2 to 12 years of age (250); usage for other age groups is off-label. As of 2019, prescriptions require submission of a completed order form to Exeltis FastAccess (https://www.benznidazoletablets.com/en/Treatment). Urgent requests for benznidazole should be made by telephone (877-303-7181).
Nifurtimox, a nitrofuran, impedes T. cruzi carbohydrate metabolism through the inhibition of pyruvic acid synthesis. The most common side effects are anorexia and weight loss, experienced by up to 70% of patients. Other frequent adverse effects include nausea, vomiting, irritability, and insomnia (251, 252). Rarely, patients develop peripheral neuropathy, usually manifested as paresthesias. The peripheral neuropathy is dose dependent, appears late in the course of therapy, and requires cessation of the drug. Nifurtimox is better tolerated by children than adults. As of 2019, nifurtimox is not approved by the FDA but is provided by the CDC under investigational protocols (https://www.cdc.gov/parasites/chagas/health_professionals/tx.html; telephone no., 404-718-4745).
Several new drug candidates (posaconazole and the ravuconazole prodrug E1224) have been tested in recent trials, but so far, none has shown acceptable efficacy (222, 223). All of the participants in these trials were from the Southern Cone. Although the posaconazole trial was carried out in Spain, 75 of the 78 subjects acquired their infections in Bolivia (223). Recent reviews have called for the inclusion of patients from diverse locations within Latin America, representing all of the major T. cruzi strains that infect humans (253). A novel aspect of these trials was the use of carefully standardized PCR assays to document treatment failure (254). Of those treated with posaconazole, 80 to 90% had detectable parasitemia by 12 months posttreatment, compared to benznidazole failure rates of 6% (per protocol) to 38% (intention to treat) (223). Similar results were demonstrated in a Bolivian trial of E1224, a related drug (222). These trials demonstrated that, with rigorous standardization, PCR may be useful as an early indicator of treatment failure, at least in populations of patients with infection acquired in the Southern Cone.
Acute and Congenital T. cruzi Infection
Acute infection has been an absolute indication for treatment since the drugs first became available in the 1970s (255). In acute and early congenital T. cruzi infection, antitrypanosomal therapy reduces the severity of symptoms, shortens the clinical course, and decreases the duration of detectable parasitemia (255, 256). In severe acute disease, treatment can be lifesaving. Cure rates in acute and congenital infection are estimated at 80 to 99% (255–258).
Treatment of Chronic T. cruzi Infection
Evaluation of antitrypanosomal drug efficacy in chronic T. cruzi infection is challenging. PCR, while a potential indicator of treatment failure, is not a true test of cure, since many persons with chronic T. cruzi infection will have circulating parasite levels below the threshold of detection of the assay. Conventional IgG serology is considered the only sensitive indicator of infection, but it requires years to decades to revert to negative after successful treatment (259). The longer the duration of infection, the more durable the antibody response, with women treated after age 15 taking a median of 27 years to revert to negative serology (191). Age is often used as a proxy for infection duration, since in communities where the disease is endemic, most infections are acquired in childhood. Testing using experimental lytic antibody assays shows conversion to negative results more quickly than with conventional serology but still requires years, even in children (260). In the 1990s, two placebo-controlled trials of benznidazole treatment in children with chronic T. cruzi infection showed approximately 60% cure rates based on lytic antibody assays 3 to 4 years after treatment (261, 262). These studies made early diagnosis and antitrypanosomal drug therapy the standard of care for children and prompted the establishment of large-scale pediatric screening programs in high-prevalence locations (16, 263).
Treatment of chronic infection in adults remains a topic of debate (264, 265). The fundamental question is whether antitrypanosomal therapy decreases the risk that an infected person will develop cardiac morbidity from T. cruzi. Observational data published in 2006 suggested that benznidazole treatment significantly decreased the progression of Chagas cardiomyopathy in adults (266). Since progression only occurs in 20 to 30% of those with infection and takes decades to become clinically evident, the ideal trial would require large study populations followed for 20 years or more, a virtually impossible clinical trial design. The design of the BENEFIT trial (Benznidazole Evaluation for Interrupting Trypanosomiasis; ClinicalTrials.gov registration no. NCT00123916), a randomized, double-blinded, placebo-controlled trial, was based on the observation that patients who already have cardiac morbidity are more likely to have further progression than those with normal cardiac status (267). Eligible patients were required to have cardiac findings consistent with established Chagas cardiomyopathy, and the primary outcome consisted of any of the following: death, resuscitated cardiac arrest, sustained ventricular tachycardia, insertion of a pacemaker or implantable cardioverter-defibrillator, cardiac transplantation, new heart failure, or other thromboembolic event. To the disappointment of many in the Chagas disease community, the trial showed no significant difference for the primary composite outcome, despite significantly higher conversion to negative PCR results in the treatment group compared to the results for the placebo group (268).
The patient populations in the observational and trial populations differed substantially. The nonrandomized study subjects had a mean age of 39, and two-thirds had normal cardiac function at baseline (266). In contrast, the BENEFIT trial population had a mean age of 55, all had cardiac damage based on electrocardiographic abnormalities, and nearly half had decreased ejection fraction at baseline, indicating ventricular dysfunction (268). The question of whether treatment provides clinical benefit for those with no or very early cardiac signs therefore remains unanswered (269). The only clear take-away messages are that the younger the patient the higher the probability of benefit and that active screening is essential to identify infected individuals before they become symptomatic. As in earlier publications (192), treatment recommendations remain stratified by age and clinical status and require balancing the risk of adverse effects with the probability and uncertainty of benefit (Table 7).
TABLE 7.
Recommendations for antitrypanosomal drug treatment according to Chagas disease phase and form, patient age, and clinical status
| Recommendation on antitrypanosomal drug treatment by Chagas disease phase, form, and demographic group | Strength of recommendation; quality of evidencea |
|---|---|
| Should always be offered | |
| Acute T. cruzi infection (including congenital infection in 1st mo of life) | Strong; moderate |
| Children ≤12 yrs old with chronic T. cruzi infection | Strong; high |
| Children 13–18 yrs old with chronic T. cruzi infection | Strong; low |
| Reactivated T. cruzi infection in immunosuppressed patient | Strong; moderate |
| Reproductive-age women planning future pregnancies | Strong; moderate |
| May be offered with consideration of potential risks and benefits, uncertainties, and patient preferences | |
| Adults with normal ECG and cardiac function | Discretionary; weak |
| Adults with early signs of cardiomyopathy | Discretionary; weak |
| Recommendation against treatment | |
| During pregnancy | Strong; weak |
| During lactation | Weak; weak |
| Patients with advanced cardiomyopathy | Strong; moderate |
| Patients with gastrointestinal Chagas disease that impairs absorption | Weak; weak |
Based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (343). The GRADE system offers only two grades of recommendations: “strong” and “weak” or “discretionary.” Strong recommendations are provided when the balance of desirable versus undesirable effects is clear. Weak or discretionary recommendations require an assessment of the evidence and decision making based on a consideration of potential risks and benefits, uncertainties, and patient preferences (344).
Management of the Immunocompromised Host
In organ transplant recipients with reactivation, a standard course of benznidazole or nifurtimox is effective in ameliorating clinical symptoms and shortening the duration of microscopically detectable parasitemia. Prior treatment or posttransplant prophylaxis has not been shown to decrease the risk of reactivation; posttransplant prophylaxis is not generally administered in heart transplant centers in Latin America (270). As no reliable test of cure exists, treated patients are considered to remain at risk for reactivation (225). Organ recipients at risk of reactivation should have regular monitoring of blood by quantitative PCR, with treatment based on demonstration of rising parasite load in blood (Table 6) (225, 271). T. cruzi reactivation should be included in the differential diagnosis of febrile episodes and apparent rejection crises, and endomyocardial biopsy specimens should be examined for evidence of T. cruzi myocarditis in the transplanted heart. Reactivation in an HIV-coinfected patient is treated with standard courses of antitrypanosomal treatment and optimization of antiretroviral therapy (272). The utility of and optimal regimen for secondary prophylaxis are unknown.
HUMAN CHAGAS DISEASE IN THE UNITED STATES
Disease Burden among Latin American Immigrants
No population-representative data exist to make an unbiased estimate of T. cruzi infection prevalence in the United States. Several studies of T. cruzi seroprevalence in convenience samples of Latin American immigrants have been conducted. In Los Angeles, 59 (1.24%) of 4,755 Latin-American-born residents had confirmed T. cruzi infection (273). The prevalence was higher among participants older than 40 than in those 18 to 40 (1.42% versus 0.95%), and it was higher among immigrants from El Salvador than those from Mexico (3.45% versus 0.79%) (273). A community health clinic-based program to screen Latin American immigrants in East Boston reported an overall prevalence of 0.87% (19/2,183), with prevalence rising with age (0/101 [0%] for age <20, 10/1,562 [0.64%] for age 20 to 39, and 9/507 [1.78%] among those 40 years or older) (274).
Based on the reported number of immigrants from countries of Chagas disease endemicity of Latin America and estimated national T. cruzi seroprevalence in their countries of origin, there were an estimated 240,000 to 350,000 infected persons in the United States in 2010; the upper end of the range includes an estimate for undocumented immigrants, whereas the lower end does not (7). All estimates of Chagas disease burden in the United States have major uncertainties, and the method used for these estimates carries several potential biases. The demographics of Latin American immigrants in the United States may not reflect those of the general population in their countries of origin, and their significant exposure risk ended when they left their home countries years earlier (275). Chagas disease prevalence is highly heterogeneous in countries of endemicity; depending on geographic sources of immigrants, the prevalence in immigrants could be either higher or lower than the national average. For example, in Spain, Bolivian immigrants appear to have a higher prevalence of Chagas disease than the estimated national prevalence, possibly because they are more likely to come from departments with high prevalence, such as Cochabamba and Santa Cruz, than from departments with low prevalence, such as Oruro or Potosí (3, 8). Similar systematic information for Mexican and Central American immigrants in the United States is lacking, although data from Los Angeles support the notion that infection prevalence is higher among immigrants from some Mexican states than others (273). Finally, the composition of migrant populations entering the United States has changed in recent years, with a higher proportion of families and children from Central America than in earlier migrations, in which adult men from Mexico predominated (275). National T. cruzi prevalences are higher in El Salvador, Guatemala, and Honduras than in Mexico (3), but the younger age of migrants would have the effect of decreasing the likely prevalence in migrants compared to that in earlier waves of older migrants. The success of vector control programs has dramatically decreased the prevalence of T. cruzi infection among children in Latin America over the past 30 years, and the limited data from Boston suggest that pediatric Chagas disease is infrequent in Latin American immigrants in the United States (274).
Autochthonous Vector-Borne Transmission to Humans
Available data indicate that autochthonous vector-borne T. cruzi transmission to humans is rare in the United States (276, 277). A longitudinal study in repeat blood donors yielded a point estimate of zero incidence, with an upper 95% confidence limit of 0.61 per million (277). House colonization is rare, and vector-human contact occurs primarily in peridomestic areas, when vectors invade houses, or when humans spend time in sylvatic sites with enzootic T. cruzi transmission (9, 278).
Prior to initiation of blood bank screening in 2007, all reported vector-borne infections in the United States were detected because of acute symptoms and/or the presence of a vector in the vicinity of the case (Table 8). Four infections were reported in Texas and one each in California, Tennessee, and Louisiana. Five of seven cases occurred in infants or small children, and six were in the acute phase at the time of detection. In retrospect, one of the Texas infections, reported to be in a 2- to 3-week old infant with no other details provided, may actually have been congenital (279). In California, a contemporaneous survey demonstrated positive complement fixation results in 6 (2.5%) of 241 residents of the community associated with the 1982 case compared to 1 (0.2%) of 637 persons surveyed in a major urban area (53).
TABLE 8.
Case data for reported autochthonous vector-borne Trypanosoma cruzi infections in the United States
| State of residence | Age | Sex | Diagnostic modality | Phase | Yr detected | Yr infected | Evidence of autochthonous vector-borne origin; putative state of acquisition | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| TX | 10 mos | F | Microscopy of peripheral blood | Acute | 1955 | 1955 | Peridomestic infestation; TX | 345 |
| TX | 2–3 wks | M | Not reported | Acute | 1955 | 1955 | No details provided—perhaps congenital, given reported age; TX | 279 |
| CA | 56 yrs | F | Microscopy of peripheral blood | Acute | 1982 | 1982 | Adult uninfected T. protracta in house; CA | 53, 346 |
| TX | 7 mos | M | Histology of cardiac tissue | Acute | 1983 | 1983 | No vectors found but search made in winter, house in poor condition; TX | 347 |
| TN | 18 mos | M | T. cruzi PCR in peripheral blood | Acute | 1998 | 1998 | T. cruzi-infected T sanguisuga found in child’s crib; TN | 52 |
| TX | 12 mos | M | Microscopy of pericardial fluid | Acute | 2006 | 2006 | Mother uninfected, T. cruzi-infected T. gerstaeckeri near house; TX | 56 |
| LA | 74 yrs | F | Serology and hemoculture | Chronic | 2006 | Unknown | T. sanguisuga infestation, 10/18 positive by T. cruzi PCR; LA | 51 |
| MS | 44 yrs | M | Blood donor screening | Chronic | 2007 | Unknown | T. sanguisuga found on property, also extensive hunting of known T. cruzi reservoir species; MS | 50 |
| NRa | NR | NR | 14 cases detected on blood donor screening | Chronic | 2006–2010 | Unknown | Blood donors not from Latin America and not primarily Spanish speaking | 50 |
| TX | 59 yrs | M | Blood donor screening | Chronic | 2007 | Unknown | Rural TX, including deer hunting in a place with infected T. gerstaeckeri | 282 |
| TX | 69 yrs | M | Blood donor screening | Chronic | 2007 | Unknown | Rural TX, some travel to Mexico | 282 |
| TX | 47 yrs | F | Blood donor screening | Chronic | 2007 | Unknown | Residence in rural TX and LA | 282 |
| TX | 72 yrs | M | Blood donor screening | Chronic | 2010 | Unknown | Residence in rural TX | 282 |
| TX | 21 yrs | M | Blood donor screening | Chronic | 2011 | Unknown | Extensive camping in TX and MO | 282 |
| TX | 83 yrs | M | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 61 yrs | F | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 71 yrs | M | Blood donor screening | Chronic | NR | Unknown | Occupation considered high risk; TX | 283 |
| TX | NR | NR | Blood donor screening | Chronic | NR | Unknown | Camping considered likely risk; TX | 283 |
| TX | 19 yrs | M | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 60 yrs | M | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 56 yrs | F | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 52 yrs | M | Blood donor screening | Chronic | NR | Unknown | Current residence, occupation, hunting considered moderate risk; TX | 283 |
| TX | 25 yrs | F | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 51 yrs | F | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| TX | 52 yrs | F | Blood donor screening | Chronic | NR | Unknown | Former/current residence considered high risk; TX | 283 |
| CA | 19 yrs | M | Blood donor screening | Chronic | 2009 | Unknown | Lack of international travel, extensive camping history; TX | 285 |
| TX | 28 yrs | M | Blood donor screening | Chronic | 2014 | Unknown | Reported vectors near childhood home in AZ; TX resident at time of detection | 284 |
| AZ | 16 yrs | F | Blood donor screening | Chronic | NR | Unknown | T. cruzi-infected T. rubida found near home; AZ | 280 |
NR, not reported.
Since 2007, blood bank screening has resulted in the publication of an additional 35 putative autochthonous T. cruzi infections (50, 280–285). All were in the chronic phase and detected on serological screening by blood centers. States postulated to be sources of infection include Mississippi, Texas, Louisiana, Arizona, and California, but with the exception of the CDC investigation (50), all were either individual case reports or focused on a single state, raising the likelihood of sampling bias. The CDC investigation estimated that locally acquired T. cruzi infection was likely to account for between 5.5% and 7.5% of confirmed positive blood donations (50). Unlike earlier case reports, all of these putative autochthonous infections were in adults in the chronic phase; thus, the location and timing of transmission events are unknown. Some reports speculate on potential sources of triatomine contact, including hiking, camping, hunting, and peridomestic woodpiles and brush (50, 282, 283, 285). In other cases, infected individuals had exposure in multiple states with known sylvatic cycles and no hypotheses could be formed as to which was the most likely site of acquisition (280, 282).
Blood Donor Screening and Transfusion Transmission
Prior to the institution of blood donor screening, five transfusion-associated T. cruzi infections were documented in the United States, two in 1988, one in 1989, one in 1997, and one in 2002 (Table 9) (9, 286). Evaluation of the recipients of blood components from infected donors identified two additional transmission events in 2004 and 2006, from separate donations from the same donor (287). Most infected recipients had underlying malignancies and were immunosuppressed (9, 287). Donors from the Southern Cone were implicated in 5 of the 6 cases where the source was known. In two cases, the blood component was unknown; in all others, the implicated units were platelets. Based on tracing of 350 recipients of blood components from infected donors, the risk associated with a platelet unit was estimated to be 13.3% (95% confidence interval [CI], 5.6 to 25.7), compared to zero for packed red blood cells (RBCs) (95% CI, 0 to 0.15) and frozen plasma/cryoprecipitate (95% CI, 0 to 3.7) (286). Several of the acutely infected recipients had severe manifestations of Chagas disease, including acute myocarditis, acute atrioventricular block, severe congestive heart failure, pericarditis with T. cruzi in the pericardial fluid, and possible meningoencephalitis.
TABLE 9.
Published reports of transfusion-related Trypanosoma cruzi transmission in the United States
| Yr of transmission | State | Recipient characteristics | Implicated blood component, donor origin | Reference |
|---|---|---|---|---|
| 1988 | NY | 11-yr-old female with Hodgkin lymphoma, developed fever and pericarditis, trypomastigotes seen on blood smear; treated with nifurtimox and recovered | Platelets, Bolivia | 348 |
| 1988 | CA | 17-yr-old male post-bone marrow transplant with fulminant acute Chagas disease | Not specified, Mexico | 349 |
| 1989 | TX | 59-yr-old female with metastatic colon cancer on chemotherapy, granulocytopenic, disseminated intravascular coagulation; developed fever, pulmonary infiltrates, bradycardia and atrioventricular block; parasites seen on bone marrow aspirate; died within 36 h of diagnosis | Unknown; had received >500 units, including RBCs, platelets | 350 |
| 1997 | FL | 60-yr-old female with multiple myeloma; T. cruzi-infected donor unit detected during research study; recipient asymptomatic, treated with nifurtimox; died of underlying disease several yrs later | Platelets, Chile | 351 |
| 2002 | RI | 3-yr-old female with stage 4 neuroblastoma on chemotherapy, neutropenic, fever, trypomastigotes seen on blood smear; treated with nifurtimox but died of her underlying disease | Platelets, Bolivia | 352 |
| 2004 | NY | 64-yr-old male with non-Hodgkin lymphoma and chemotherapy-induced thrombocytopenia; T. cruzi found on serological testing during look-back study | Platelets, Argentina | 287 |
| 2006 | NY | 62-yr-old male; T. cruzi found on serological testing during look-back study | Platelets, Argentina | 287 |
Blood donation screening began in January 2007 using the Ortho ELISA, which had been licensed by FDA the previous month, with the radioimmunoprecipitation assay (RIPA) as the confirmatory test (288). The FDA licensed the Abbott PRISM chemiluminescent immunoassay (ChLIA) as a screening test in 2010 and the Abbott enzyme strip assay (ESA) as a supplemental test in 2011. Guidance from the FDA in 2010 recommended screening of all donations, regardless of previous screening results (277). Universal screening enabled an analysis of results from two or more serial specimens from 4.22 million repeat donors representing 6.06 million person-years of follow-up (277, 290). No incident T. cruzi infections were detected, corresponding to zero autochthonous transmission incidence, with an upper 95% confidence limit of 0.61 incident cases per million person-years (277). In 2017, final FDA guidance endorsed a one-time screening approach, recommended against the use of donor questions to assess risk based on low sensitivity, and required further testing of screen-positive donations with the Abbott ESA (291, 292). Screening was estimated to cover 75 to 90% of the U.S. blood supply as of 2008 (293).
Several ancillary studies were conducted during the early years of blood donor screening. In an analysis of approximately 14 million blood donations in 2008, the overall seroprevalence was 1/27,500, with the highest rates in Florida (1/3,800), followed by California (1/8,300) (293). Of 104 T. cruzi-infected donors with epidemiological data, 29 (28%) were born in Mexico, 27 (26%) in the United States, 17 (16%) in El Salvador, and 11 (11%) in Bolivia; the remaining 20 donors were born in nine other countries of Central and South America. In a subsequent study of 22 million donations collected between 2007 and 2011, 717 donations were confirmed seropositive by RIPA, corresponding to a seropositivity rate of 1/31,000 (215). Among 263 donors who provided 30-ml blood specimens, 18 (6.8%) had positive results by hemoculture and 17 had parasite genotyping results. Only 2 (1.3%) of 157 donors from areas where TcI predominates (Mexico, Central America, and northern South America) had positive hemocultures (both TcI). In contrast, T. cruzi grew in cultures from 13 (34.2%) of 38 donors from the Southern Cone (1 TcII, 10 TcV, 1 TcVI, and 1 not typed). Three donors born in the United States had positive results by hemoculture, two TcV and one TcVI; no data were available to determine the likely source of their infections. Together with the predominance of Southern Cone donors implicated in recognized transfusion transmissions, these data support the hypothesis that TcII/V/VI infections result in higher parasite loads and, therefore, higher blood-borne transmission risk than TcI infections (215, 286).
As of 26 October 2019, a total of 2,435 confirmed seropositive donors in 47 states have been detected in screening (AABB Chagas Biovigilance Network, Chagas data reports through 26 October 2019; www.aabb.org/programs/biovigilance/Pages/chagas.aspx). Over the period from 2007 to 2016, the mean prevalence in first-time donors was 64 per million donors overall and 3.64 per 10,000 donors in southern California and showed a nonsignificant decreasing trend (277). The highest number of positive donations by calendar year was 420 in 2008; since 2014, yearly detections have ranged from 84 to 98 (Chagas data reports mentioned above).
Organ Donor-Derived Transmission and Organ Donor Screening
A total of 14 investigations, involving organs from 14 T. cruzi-infected donors transplanted to 32 recipients between 2001 and 2011, were reported in a recent review (28). Transmission occurred to 9 recipients of organs from six donors; no transmission occurred from the remaining 8 donors (Table 10) (28). Transmission risk differs by organ type: 3 (75%) of 4 heart, 2 (20%) of 10 liver, and 2 (13%) of 15 kidney recipients became infected.
TABLE 10.
Published reports of organ transplant-derived cases of Chagas disease in the United States
| Yr | State of organ harvest | Donor origin | Implicated organ | Recipient characteristics and outcome | Reference |
|---|---|---|---|---|---|
| 2001 | GA | El Salvador | Kidney-pancreas | 37-yr-old female with fever 6 wks posttransplant and T. cruzi in blood smear, died of Chagas myocarditis 7 mo posttransplant despite prolonged course of nifurtimox | 294 |
| 2001 | GA | El Salvador | Kidney | 69-yr-old female, asymptomatic, T. cruzi hemoculture positive, diagnosis sought because of recipient 1 above, treated with nifurtimox, survived | 294 |
| 2001 | GA | El Salvador | Liver | 32-yr-old female, asymptomatic, T. cruzi hemoculture positive, diagnosis sought because of recipient 1 above, treated with nifurtimox but died of unrelated causes | 294 |
| 2005 | CA | U.S.-born (mother from Mexico) | Heart | 64-yr-old male with anorexia, fever, diarrhea, diagnosed with organ rejection and treated with steroids, T. cruzi found in blood smear 8 wks posttransplant, PCRs became negative on nifurtimox, died of rejection 20 wks posttransplant | 295 |
| 2006 | CA | El Salvador | Heart | 73-yr-old male with fever, fatigue, rash, T. cruzi in blood smear 7 wks posttransplant, parasitemia cleared with nifurtimox, switched to benznidazole because of tremors, died of heart failure 25 wks posttransplant | 295 |
| 2006 | PA | Bolivia | Liver | 56-yr-old male, T. cruzi detected in PCR monitoring, died from gastrointestinal bleed 244 wks posttransplant | 28 |
| 2006 | PA | Bolivia | Bilateral kidney | 73-yr-old female, T. cruzi detected in PCR monitoring, died from kidney failure 15 wks posttransplant | 28 |
| 2010 | NY | Mexico | Heart | 20-yr-old female, T. cruzi detected in PCR monitoring and successfully treated with benznidazole, survived at least to 24 mos posttransplant | 28 |
| 2011 | TN | El Salvador | Bilateral lung | 36-yr-old male with cystic fibrosis, T. cruzi detected in PCR monitoring, completed course of benznidazole but had intermittent positive PCRs posttreatment, Chagas disease possible contributing factor to death 2 yrs posttransplant | 297 |
The earliest reported instances of transmission in 2001 and 2006 were not suspected until at least one recipient presented with symptomatic acute Chagas disease (294, 295). More recently, some organ procurement organizations have begun selective or universal screening of donated organs (30, 296). Four subsequent published transmission events (in a liver recipient in 2006, two heart recipients in 2006 and 2010, and a bilateral lung recipient in 2011) were detected through systematic laboratory monitoring. Three of these patients were treated and survived their T. cruzi infection (28). The bilateral lung transplant recipient died 2 years posttransplantation from respiratory failure; his T. cruzi PCR was intermittently positive despite prolonged benznidazole therapy, and Chagas disease was considered a possible contributing factor in his death (28, 297).
In the United States, screening of donors has been based largely on risk assessment; donors born in or with significant periods of residence in Latin America, born of women from Latin America, and/or noted to have clinical findings such as cardiomegaly consistent with Chagas disease should be screened by IgG serology (30). Some organ procurement organizations contract with a blood bank for donor testing, as the Abbott PRISM and Ortho ELISA have approval for use in specimens from living and cadaveric organ donors (231).
The recipient of an organ from an infected donor should be monitored by microscopy and/or PCR in serial specimens (28, 30). Seroconversion may be delayed or never occur in immunocompromised individuals. Positive results by PCR occur days to weeks before parasites are detectable by microscopy (218). The incubation period of transplant-transmitted T. cruzi infection is typically 2 to 3 months, but detection may be delayed as long as 6 months (28). A frequently recommended monitoring schedule consists of weekly specimens for 2 months, every 2 weeks up to 4 months, and then monthly afterwards (Table 6) (28, 30). In the absence of other indications and assuming no evidence of infection has been detected, the monitoring interval can be lengthened after 6 months posttransplantation.
Chagas Cardiomyopathy and Heart Transplantation for Chagas Heart Disease in the United States
Chagas heart disease has been recognized in U.S. health care facilities for nearly 30 years (298). Ten years ago, we estimated that there were 30,000 to 45,000 patients with Chagas cardiomyopathy in the United States (299). Recent studies have confirmed that Chagas disease is frequent among Latin American-born patients with cardiac signs, including 13% (5/39) of patients with left ventricular ejection fraction (LVEF) of <45% without evidence of ischemic heart disease in New York City (300), 19% (25/135) of patients with LVEF of <40% without evidence of ischemic heart disease in Los Angeles (301), 5.3% (17/327) of patients with any bundle branch block in Los Angeles (302), and 7.5% (6/80) of patients with pacemaker implantation in Los Angeles (303). As in studies from Latin America (304, 305), the most common conduction system abnormalities were right bundle branch block, left anterior hemiblock, and the combination of the two (302, 306), and Chagas cardiomyopathy was associated with more rapid progression than other cardiac etiologies to severe disease requiring transplantation or resulting in death (301, 306, 307). Data from 17 Texas blood donors suggest that locally acquired Chagas disease can also result in cardiomyopathy, but data are insufficient to assess the relative risk for autochthonous versus imported infection (281).
In Brazil and Argentina, heart transplantation is an accepted modality to treat end-stage Chagas cardiomyopathy, and survival for those transplanted for Chagas heart disease is the same or better than that of recipients of cardiac transplants for other etiologies (200, 201, 308, 309). The incidence of T. cruzi reactivation in Latin American heart transplant cohorts varies widely, from 20 to 90% (225). In the United States, data are published for 40 patients who underwent heart transplantation for end-stage Chagas cardiomyopathy since 2006 (199, 225). In one review from a Los Angeles medical center, 31 of 405 patients who received heart transplants between 2006 and 2012 were born in Latin America; 20 of the 31 had serological testing for T. cruzi and 11 (2.7% of the total number of 405) had positive results (199). Only two of the T. cruzi-infected transplant recipients received their diagnosis prior to the transplant, both in their home countries. Two (18.2%) patients were diagnosed with T. cruzi reactivation when they experienced dysfunction of the transplanted heart; their infections had not previously been suspected, and one died of cardiogenic shock. One additional patient was asymptomatic but treated based on the finding of parasites in the explanted heart. One of the patients in the Los Angeles cohort is included in the CDC’s comprehensive review of 31 patients that underwent heart transplantation for Chagas cardiomyopathy from 2012 to 2016; 19 (61%) had reactivation (225). In the CDC review, reactivation was defined by rising parasite load by quantitative PCR in peripheral blood, a finding that precedes both microscopically detectable parasitemia and the development of symptoms (218). Only one instance of reactivation was symptomatic at the time of diagnosis, and all patients with reactivation were alive at the end of follow-up.
Congenital Chagas Disease in the United States
Based on births to Latin American-born women in the United States and T. cruzi infection prevalence in their home countries, it was estimated that between 60 and 315 congenitally infected infants are born in the United States each year (299). Nevertheless, only two congenital infections have been reported, both in infants of Bolivian women (310, 311). The first infant was delivered by caesarian section at 29 weeks gestation because of fetal hydrops (a classic presentation of severe congenital Chagas disease) (18). The diagnosis was not suspected until the mother reported a prior diagnosis of Chagas disease (311). The second infant was also delivered by caesarian section at 30 weeks due to placental abruption and had a pericardial effusion, ascites, and respiratory distress requiring intubation until the 10th day of life (310). The diagnosis was made on histopathological examination of the placenta. Both infants were successfully treated with antitrypanosomal therapy.
SPECIAL CONSIDERATIONS IN THE UNITED STATES
An adequate public health response to Chagas disease in the United States faces critical challenges, including limited patient and provider awareness of the disease, societal, economic, and health system barriers to patient access, and sparse diagnostic options with an inadequate evidence base to assess performance.
Physician Awareness
Surveys indicate that the majority of physicians practicing in the United States have limited knowledge of Chagas disease and seldom consider the diagnosis, even when caring for Latin American-born patients at high risk or with typical clinical syndromes (312, 313). In one survey, 23% of cardiologists, 47% of obstetrician/gynecologists, and 25% of transplantation specialists reported that they had never heard of Chagas disease (312). In a survey of more than 400 obstetrician/gynecologists, 78% reported that they never considered the diagnosis of Chagas disease in their Latin American patients and fewer than 10% were cognizant of the risk of vertical transmission to the infant (313).
Patient Awareness and Access
Awareness and knowledge of Chagas disease are also limited among those at risk. Of Latin American immigrants interviewed in community outreach settings in southern California, 86% had never heard of Chagas disease, even though 62% reported having seen triatomines in their countries of origin (314). Patients with Chagas disease encounter multiple barriers to health care access in general, not just for Chagas disease: these patients disproportionately live below the poverty line, have less than a high school education, lack health insurance, and have difficulty taking time off from work for medical appointments (315). Even legal immigrants have lower access than citizens to publicly funded health insurance in most states; concerns about revealing immigration status may make patients reluctant to seek care (315).
Diagnostic Issues
There are currently four commercial IgG serological tests cleared by the FDA for diagnostic use in the United States, including three ELISA kits (Hemagen [Hemagen Diagnostics, Waltham MA], Chagatest Recombinante 3.0 [Wiener Laboratories, Rosario, Argentina], and Ortho [Ortho-Clinical Diagnostics, Inc., Raritan, NJ]) and one point-of-care test (ChagasDetectPlus [InBios International, Seattle, WA]) (316). With the exception of the Ortho ELISA, which is also licensed for blood donor screening (215, 277, 317), there is a paucity of data on the performance of these assays in specimens from populations in the United States or in the likely predominant countries of origin of infected U.S. residents (Mexico, El Salvador, Guatemala, and Honduras) (273, 293, 299). Discordant serology has been reported as a particular problem in Mexico (318). Some recombinant tests with excellent performance in the Southern Cone show discordance or low sensitivity when applied in some areas where TcI is predominant (319, 320). In addition, no commercial laboratory in the United States currently offers more than one validated IgG serological assay. The diagnosis of chronic T. cruzi infection requires positive results by two distinct IgG assays and is therefore not possible with commercial testing alone (16). Currently, the only laboratory that conducts multiple IgG serological assays under Clinical Laboratory Improvement Amendments (CLIA) is the CDC Division of Parasitic Diseases and Malaria (DPDM) laboratory.
FDA Approval of Benznidazole and Resulting Changes
Until May 2018, when benznidazole became commercially available, prescribing antitrypanosomal treatment necessitated a consultation with CDC epidemiologists (321). Confirmation of the diagnosis, recommendations for treatment, and advice on monitoring and management of side effects were necessary components of these consultations, and reporting of adverse events was mandatory under the investigational protocol. Ensuring appropriate diagnostic confirmation, judicious treatment decisions, and adequate side effect monitoring going forward will require efforts to raise provider awareness and knowledge about Chagas disease and antitrypanosomal therapy.
From October 2011 to May 2018, the CDC released benznidazole for 365 patients with confirmed T. cruzi infection (321). Four (1.1%) patients had acute-phase infection, two organ derived, one congenital, and one presumed to be vector borne. Treatment was administered for T. cruzi reactivation in 35 (9.6%) patients, comprising 29 organ transplant recipients, 5 HIV-coinfected patients, and 1 on chemotherapy for malignancy. The vast majority of patients were adults, 236 (64.7%) aged 19 to 50 and 97 (26.6%) older than 50 years. Only 2 (0.5%) of 365 treated patients were aged 2 to 12 years, the age range for which the FDA approved benznidazole.
Insud Pharma’s approach to FDA approval and drug marketing represents a new paradigm in the United States, with patient access and affordability as central concerns (250). The FDA Priority Review Voucher (PRV) program was established in 2008 to provide an incentive for new drug development for neglected tropical diseases (NTD) but has had limited impact and unintended negative consequences (322). In one recent example, FDA approval of miltefosine, a drug used for leishmaniasis, was followed by an astronomical price increase, and the company ceased production after receiving and selling the PRV, leading to a global shortage (323). In contrast, the Insud Pharma/Exeltis Patient Assistance Program, funded in part by the benznidazole PRV, ensures that the cost to the patient will not exceed $60 per course (250).
Public Health Surveillance and Response
As of 2017, Chagas disease was a reportable condition in six states, Arizona, Arkansas, Louisiana, Mississippi, Tennessee, and Texas (324); Utah added Chagas disease to its notifiable disease list recently. All of these states except Arkansas and Utah have had published reports of locally acquired T. cruzi infection (50–52, 280, 282, 283). Most of the states focus on autochthonous transmission and incident cases, and several have provisions for submission of triatomines for identification (324). In Texas, the Department of State Health Services (DSHS) provides extensive on-line guidance and data to health care providers and the public, including a summary of reported Chagas disease case data; of 124 cases reported from 2013 to 2017, all were chronic infections and 22 were judged to have resulted from local transmission (325). These figures likely overlap substantially with the published locally acquired infections detected in blood donors in recent years (282, 283). The Texas Chagas Taskforce, which includes the DSHS and several universities, addresses many public health aspects of Chagas disease, including provider and patient resources and educational materials on local vectors (326).
PUBLIC HEALTH APPROACHES
Public health approaches to Chagas disease comprise primary prevention (prevention of transmission), secondary prevention (early treatment of infection to prevent sequelae), and tertiary prevention (medical and surgical management of morbidity to improve survival and quality of life) (Table 11) (309). In Latin America, primary prevention through vector control is responsible for the vast majority of the decrease in estimated annual incidence from 500,000 in 1991 to 30,000 today (35). Primary prevention through vector control is virtually impossible in the United States, since the vectors are sylvatic; however, barriers to house invasion, elimination of microhabitats close to houses, and improved awareness among those living in areas of risk could help decrease the likelihood of autochthonous transmission. Blood bank screening has also been highly successful in both Latin America and the United States. The last detected blood component-derived infection in the United States occurred in 2006, before screening was instituted (277, 287).
TABLE 11.
Public health screening options for Chagas disease in the United States
| Target population | Screening method(s) | Primary goal | Secondary goal | Intervention details and effectiveness | Published estimates of screening yield (reference[s]) |
|---|---|---|---|---|---|
| Blood donors | Serologic screening | Prevent transmission | Refer infected persons for management | Discard screen-positive donations; highly effective | ∼1/15,000 first-time donors, up to 1/2,700 in high-risk area (277) |
| Organ donors | Serologic or risk-based screening | Prevent transmission | Heart from infected donor not used, other organs used with appropriate monitoring; highly effective | 0.9% in combined risk-based and serologic donor screening (296) | |
| Pregnant women from Latin America; infants of infected women | Maternal serology, serial testing of infants, serology in siblings | Detect infected infants early in life | Refer infected women and their other children for treatment | Early treatment of infants, treatment of women after lactation ends, treatment of infected siblings; highly effective in infants and children, moderately effective in young women | ∼10 mothers, <1 infected child per 4,000 high-risk women (majority born in Latin America) (329) |
| Latin American immigrants | Serologic screening and confirmatory testing | Detect asymptomatic infected individuals | Treatment of infected individuals; effectiveness high in children, uncertain in adults | 0.5–1% in high-risk populations; many of those detected were >50 yrs old, for whom treatment is not generally recommended (273, 274) | |
| Patients from Latin America with nonischemic cardiac syndromes | Serologic screening and confirmatory testing | Detect symptomatic infected individuals | Standard cardiac management, prospective monitoring for reactivation in transplant recipients; effective in improving survival and quality of life | Latin American-born patients with bundle branch blocks, 5%; pacemakers, 7.5%; depressed left ventricular ejection fraction, 13–19% (300–303) |
In Latin America, secondary prevention efforts include congenital Chagas disease screening programs and mass testing and treatment of children in high-prevalence zones (263, 327). Prenatal and congenital screening programs are attractive for several reasons. Cure rates are >90% in infected infants and drug tolerance is excellent (257, 258); treatment of infected women, once lactation ends, significantly decreases the risk of transmission in future pregnancies (191); and detection of maternal infection provides a screening opportunity for her other children, who are also at risk for T. cruzi infection (192). However, congenital screening programs are also complicated. With current diagnostic modalities, effective congenital Chagas disease screening requires a multistep algorithm consisting of prenatal serology in women and testing of infants of seropositive mothers 2 or 3 times (parasitological or molecular testing in the first months of life, and for those who test negative, serological testing at 9 to 12 months) (216). Even in Bolivia, where infection prevalence in women is often 15% or higher and awareness is high, congenital Chagas disease detection is challenging, because of low sensitivity of microscopy and >80% loss to follow-up for the 9- to 12-month visits (328).
A pilot study in a hospital in Houston screened 4,000 women, 75% of whom were born in Latin America (329). Ten (0.25%) women had confirmed T. cruzi infection; no infected infants were detected. A recent analysis concluded that in the United States, universal congenital Chagas disease screening and treatment would be cost saving with congenital transmission rates of ≥0.001% and maternal prevalence of >0.06% (330). The results vary substantially depending on the cost and performance of the maternal screening test; thus, it will be essential to ascertain the currently unknown sensitivity of available serological assays in at-risk populations in the United States.
The effectiveness of secondary prevention strategies depends strongly on the effectiveness of antitrypanosomal treatment in preventing the development and progression of cardiac disease, which remains a controversial topic without a clear answer (269). Current practice in Latin America prioritizes the diagnosis and treatment of children 15 years old or younger, but the vast majority of infected individuals in the United States are adults. In the sparse available community screening data, the T. cruzi prevalence in Latin American adults 40 years old or younger was 0.64 to 0.95%, compared to 1.42 to 1.78% among those older than 40 years (273, 274). In limited community screening to date, no infections have been detected among children (274).
Tertiary prevention has had a major impact on the survival and quality of life of persons living with T. cruzi infection in Latin America (331), and the experience of a dedicated center of excellence based in the cardiology service of a large Los Angeles hospital confirms this model in the United States (301, 302, 332–334). Expanding this effort will require outreach efforts to cardiologists and primary care physicians and making accurate diagnostic testing more widely available.
CONCLUSIONS
Chagas disease causes disease of the heart and/or gastrointestinal tract in 20 to 30% of those infected by T. cruzi. The southern half of the United States contains enzootic cycles of T. cruzi, involving 4 major and 7 minor triatomine vector species. T. cruzi infection has been detected in multiple mammalian species, including raccoons, opossums, woodrats, and dogs. Locally acquired Chagas disease has been increasingly recognized in the United States over the past 10 years, largely due to screening of blood donations and investigations of infected blood donors without exposure in Latin America. Nevertheless, imported chronic T. cruzi infections in migrants from Latin America vastly outnumber autochthonous human cases, and locally acquired infection is rarely detected in the acute phase. Benznidazole is now FDA approved as a treatment, and clinical and public health efforts are under way by researchers and some state health departments to broaden access to diagnosis and treatment.
Making progress will require work on many fronts, including innovative ways to improve the knowledge base among providers, expand the availability of high-quality diagnostic and confirmatory testing, and pilot public health screening data to develop evidence-based targeting strategies. However, increased awareness of Chagas disease is crucial to all aspects of this effort. Providers with awareness of the disease can screen those at risk when they present for clinical care, with the highest priority for children and women of child-bearing age, since the benefit of antitrypanosomal therapy is clear for these groups. The appropriate index of suspicion saves lives when reactivation of chronic infection and donor-derived T. cruzi infection are recognized in a timely fashion. Diagnosis of T. cruzi infection and follow-up for the onset or progression of cardiomyopathy or gastrointestinal disease can mitigate morbidity and improve survival and quality of life.
ACKNOWLEDGMENTS
We thank Ernesto Barrera Vargas, Rochelle Hoey-Chamberlain, Christiane Weirauch, Gena Lawrence, and Sonia Kjos for images of the triatomine vectors and Henry Bishop for images of T. cruzi.
Caryn Bern received consulting fees from Exeltis in 2017 and 2018. James H. Maguire received consulting fees from Bayer in 2017. Louisa A. Messenger and Jeffrey D. Whitman report no financial interests.
Biographies

Caryn Bern is Professor of Epidemiology and Biostatistics in the School of Medicine, University of California, San Francisco. She holds an M.D. from Stanford University School of Medicine and an M.P.H. from the Johns Hopkins University School of Public Health and is board certified in internal medicine. She was a medical epidemiologist at the Centers for Disease Control and Prevention from 1990 to 2011 and worked for 15 years in the CDC Division of Parasitic Diseases and Malaria. Dr. Bern has worked on clinical and epidemiological aspects of Chagas disease since 2003, with projects in Peru, Bolivia, and the United States. Dr. Bern’s current research focuses on diagnosis, treatment, epidemiology, and control of Chagas disease and visceral leishmaniasis.

Louisa A. Messenger is an Assistant Professor in the Department of Disease Control of the London School of Hygiene and Tropical Medicine. She holds a B.A. from the University of Cambridge and an M.Sc. in Medical Parasitology and Ph.D. in Molecular Biology from the London School of Hygiene and Tropical Medicine. She has been a recipient of the L’Oréal-UNESCO For Women in Science UK and Ireland Fellowship and an American Society for Microbiology/Centers for Disease Control and Prevention Fellowship. Dr. Messenger has lived and worked extensively in South America and Africa to improve methods of Chagas disease and malaria control, respectively.

Jeffrey D. Whitman is currently a postdoctoral research fellow in the Department of Laboratory Medicine at the University of California, San Francisco, School of Medicine. He has an M.S. in biochemistry and molecular biology from the University of California, Riverside, and an M.D. from the David Geffen School of Medicine at UCLA. His research interests include improving diagnostic disparities in global health settings with a methodological focus on mass spectrometry-based metabolomics and proteomics. He is currently pursuing research related to improving Chagas disease diagnostics.

James H. Maguire is Professor of Medicine, Harvard Medical School, and Senior Physician, Brigham and Women’s Hospital, Boston, MA. He completed his M.D. from Harvard Medical School and M.P.H. from Harvard School of Public Health and is board certified in internal medicine and infectious diseases. He was the chief of CDC Parasitic Diseases Branch from 2001 to 2005. He was Professor and Head, Division of International Health, Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine from 2005 to 2008. Dr. Maguire performed seminal research in the epidemiology and cardiac manifestations of Chagas disease in rural Brazil in the 1980s and collaborated on studies of Chagas disease in Peru and Bolivia from 2003 to the present.
REFERENCES
- 1.Bern C. 2015. Chagas’ disease. N Engl J Med 373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Molina JA, Molina I. 2018. Chagas disease. Lancet 391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–44. [PubMed] [Google Scholar]
- 4.World Health Organization. 2018. Global health estimates 2016 summary tables: DALYs by cause, age and sex, by WHO region, 2000–2016. World Health Organization, Geneva, Switzerland: https://www.who.int/healthinfo/global_burden_disease/GHE2016_DALY_WHOReg_2000_2016_.xls?ua=1. [Google Scholar]
- 5.López-Vélez R, Norman FF, Bern C. 2019. American trypanosomiasis (Chagas disease), p 762–765. In Ryan ET, Hill DR, Solomon T, Endy TP, Aronson N (ed), Hunter’s tropical medicine and emerging infectious disease, 10th ed Elsevier, London, United Kingdom. [Google Scholar]
- 6.Vizzoni AG, Varela MC, Sangenis LHC, Hasslocher-Moreno AM, do Brasil P, Saraiva RM. 2018. Ageing with Chagas disease: an overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors 11:354. doi: 10.1186/s13071-018-2929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manne-Goehler J, Umeh CA, Montgomery SP, Wirtz VJ. 2016. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis 10:e0005033. doi: 10.1371/journal.pntd.0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Requena-Méndez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DAJ, Gascon J, Muñoz J. 2015. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis 9:e0003540. doi: 10.1371/journal.pntd.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bern C, Kjos S, Yabsley M, Montgomery SP. 2011. Trypanosoma cruzi and Chagas disease in the United States. Clin Microbiol Rev 24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steverding D. 2014. The history of Chagas disease. Parasit Vectors 7:317. doi: 10.1186/1756-3305-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chagas C. 1909. Nova tripanosomiaze humana. Estudos sobre morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n.g., n. sp., ajente etiolojico de nova entidade morbida do homen. Mem Inst Oswaldo Cruz 1:159–218. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 12.de Lana M, Chiari CA, Chiari E, Morel CM, Goncalves AM, Romanha AJ. 1996. Characterization of two isolates of Trypanosoma cruzi obtained from the patient Berenice, the first human case of Chagas’ disease described by Carlos Chagas in 1909. Parasitol Res 82:257–260. doi: 10.1007/s004360050106. [DOI] [PubMed] [Google Scholar]
- 13.Tyler KM, Engman DM. 2001. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol 31:472–481. doi: 10.1016/s0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 14.Epting CL, Coates BM, Engman DM. 2010. Molecular mechanisms of host cell invasion by Trypanosoma cruzi. Exp Parasitol 126:283–291. doi: 10.1016/j.exppara.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caradonna KL, Burleigh BA. 2011. Mechanisms of host cell invasion by Trypanosoma cruzi. Adv Parasitol 76:33–61. doi: 10.1016/B978-0-12-385895-5.00002-5. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Committee. 2002. Control of Chagas Disease: second report of the WHO expert committee. WHO technical report series number 905. World Health Organization, Brasilia, Brazil. [Google Scholar]
- 17.Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. 2014. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG 121:22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico MC, Dramaix M, Truyens C, Carlier Y. 2004. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 70:201–209. doi: 10.4269/ajtmh.2004.70.201. [DOI] [PubMed] [Google Scholar]
- 19.Kaplinski M, Jois M, Galdos-Cardenas G, Rendell VR, Shah V, Do RQ, Marcus R, Burroughs Pena MS, Del Carmen Abastoflor M, LaFuente C, Bozo R, Valencia E, Verastegui M, Colanzi R, Gilman RH, Bern C. 2015. Sustained domestic vector exposure is associated with increased Chagas cardiomyopathy risk but decreased parasitemia and congenital transmission risk among young women in Bolivia. Clin Infect Dis 61:918–926. doi: 10.1093/cid/civ446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann E, Truyens C, Alonso-Vega C, Rodriguez P, Berthe A, Torrico F, Carlier Y. 2004. Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon-gamma in response to parasite antigens. J Infect Dis 189:1274–1281. doi: 10.1086/382511. [DOI] [PubMed] [Google Scholar]
- 21.Scapellato PG, Bottaro EG, Rodriguez-Brieschke MT. 2009. Mother-child transmission of Chagas disease: could coinfection with human immunodeficiency virus increase the risk? Rev Soc Bras Med Trop 42:107–109. doi: 10.1590/s0037-86822009000200002. [DOI] [PubMed] [Google Scholar]
- 22.Jackson Y, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, Loutan L, Gervaix A. 2009. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis 15:601–603. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murcia L, Carrilero B, Munoz-Davila MJ, Thomas MC, López MC, Segovia M. 2013. Risk factors and primary prevention of congenital Chagas disease in a nonendemic country. Clin Infect Dis 56:496–502. doi: 10.1093/cid/cis910. [DOI] [PubMed] [Google Scholar]
- 24.Rodari P, Angheben A, Gennati G, Trezzi L, Bargiggia G, Maino M, Ruggeri M, Rampello S, Soavi L, Rizzi M. 2018. Congenital Chagas disease in a non-endemic area: results from a control programme in Bergamo province, Northern Italy. Travel Med Infect Dis 25:31–34. doi: 10.1016/j.tmaid.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Schmunis GA. 1991. Trypanosoma cruzi, the etiologic agent of Chagas’ disease: status in the blood supply in endemic and nonendemic countries. Transfusion 31:547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmunis GA, Cruz JR. 2005. Safety of the blood supply in Latin America. Clin Microbiol Rev 18:12–29. doi: 10.1128/CMR.18.1.12-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Gonzalez G, Figueroa-Lara A, Elizondo-Cano M, Wilson L, Novelo-Garza B, Valiente-Banuet L, Ramsey JM. 2016. Cost-effectiveness of blood donation screening for Trypanosoma cruzi in Mexico. PLoS Negl Trop Dis 10:e0004528. doi: 10.1371/journal.pntd.0004528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huprikar S, Bosserman E, Patel G, Moore A, Pinney S, Anyanwu A, Neofytos D, Ketterer D, Striker R, Silveira F, Qvarnstrom Y, Steurer F, Herwaldt B, Montgomery S. 2013. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001–2011. Am J Transplant 13:2418–2425. doi: 10.1111/ajt.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riarte A, Luna C, Sabatiello R, Sinagra A, Schiavelli R, De Rissio A, Maiolo E, Garcìa MM, Jacob N, Pattin M, Lauricella M, Segura EL, Vázquez M. 1999. Chagas’ disease in patients with kidney transplants: 7 years of experience 1989–1996. Clin Infect Dis 29:561–567. doi: 10.1086/598634. [DOI] [PubMed] [Google Scholar]
- 30.Chin-Hong PV, Schwartz BS, Bern C, Montgomery SP, Kontak S, Kubak B, Morris MI, Nowicki M, Wright C, Ison MG. 2011. Screening and treatment of Chagas disease in organ transplant recipients in the United States: recommendations from the Chagas in Transplant Working Group. Am J Transplant 11:672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 31.Shikanai-Yasuda MA, Carvalho NB. 2012. Oral transmission of Chagas disease. Clin Infect Dis 54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 32.Noya BA, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Muñoz-Calderón A, Noya O. 2015. Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz 110:377–386. doi: 10.1590/0074-02760140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santana RAG, Guerra M, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, Ferreira LS, Souza KR, Tavares IC, Morais RF, Silva GAV, Melo GC, Vergel GM, Albuquerque BC, Arcanjo ARL, Monteiro WM, Ferreira J, Lacerda MVG, Silveira H, Guerra J. 2019. Oral transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis 25:132–135. doi: 10.3201/eid2501.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcón de Noya B, Díaz‐Bello Z, Colmenares C, Ruiz‐Guevara R, Mauriello L, Zavala‐Jaspe R, Suarez JA, Abate T, Naranjo L, Paiva M, Rivas L, Castro J, Márques J, Mendoza I, Acquatella H, Torres J, Noya O. 2010. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis 201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 35.Dias JC, Silveira AC, Schofield CJ. 2002. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz 97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 36.Lent H, Wygodzinsky P. 1979. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus Nat Hist 63:123–520. [Google Scholar]
- 37.Hashimoto K, Schofield CJ. 2012. Elimination of Rhodnius prolixus in Central America. Parasit Vectors 5:45. doi: 10.1186/1756-3305-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dias JC. 2007. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusional Chagas disease. Historical aspects, present situation, and perspectives. Mem Inst Oswaldo Cruz 102(Suppl 1):11–18. doi: 10.1590/S0074-02762007005000092. [DOI] [PubMed] [Google Scholar]
- 39.Rumi MM, Perez Brandan C, Gil JF, D’Amato AM, Ragone PG, Lauthier JJ, Tomasini N, Cimino RO, Orellana V, Lacunza CD, Nasser JR, Basombrio MA, Diosque P. 2013. Benznidazole treatment in chronic children infected with Trypanosoma cruzi: serological and molecular follow-up of patients and identification of discrete typing units. Acta Trop 128:130–136. doi: 10.1016/j.actatropica.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Samuels AM, Clark EH, Galdos-Cardenas G, Wiegand RE, Ferrufino L, Menacho S, Gil J, Spicer J, Budde J, Levy MZ, Bozo RW, Gilman RH, Bern C, Working Group on Chagas Disease in Bolivia and Peru. 2013. Epidemiology of and impact of insecticide spraying on Chagas disease in communities in the Bolivian Chaco. PLoS Negl Trop Dis 7:e2358. doi: 10.1371/journal.pntd.0002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeledon R, Alvarado R, Jiron LF. 1977. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae). Acta Trop 34:65–77. [PubMed] [Google Scholar]
- 42.Dumonteil E, Nouvellet P, Rosecrans K, Ramirez-Sierra MJ, Gamboa-León R, Cruz-Chan V, Rosado-Vallado M, Gourbière S. 2013. Eco-bio-social determinants for house infestation by non-domiciliated Triatoma dimidiata in the Yucatan Peninsula, Mexico. PLoS Negl Trop Dis 7:e2466. doi: 10.1371/journal.pntd.0002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waleckx E, Gourbiere S, Dumonteil E. 2015. Intrusive versus domiciliated triatomines and the challenge of adapting vector control practices against Chagas disease. Mem Inst Oswaldo Cruz 110:324–338. doi: 10.1590/0074-02760140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miles MA, Feliciangeli MD, de Arias AR. 2003. American trypanosomiasis (Chagas’ disease) and the role of molecular epidemiology in guiding control strategies. BMJ 326:1444–1448. doi: 10.1136/bmj.326.7404.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeledón R, Beard CB, Pinto Dias JC, Leiby DA, Dorn PL, Coura JR. 2012. An appraisal of the status of Chagas disease in the United States, p 5–32. Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 46.Wheeling-Ohio County Health Department. 2015. First record of Triatoma sanguisuga (Hemiptera: Reduviidae), vector of Chagas disease, in West Virginia. http://www.ohiocountyhealth.com/wp-content/uploads/12-07-15_xt_WVKissingBug20151.pdf.
- 47.Centers for Disease Control and Prevention. 2019. Notes from the Field: Identification of a Triatoma sanguisuga “kissing bug” — Delaware, 2018. MMWR Morb Mortal Wkly Rep 68:359. doi: 10.15585/mmwr.mm6815a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shender LA, Lewis MD, Rejmanek D, Mazet JA. 2016. Molecular diversity of Trypanosoma cruzi detected in the vector Triatoma protracta from California, USA. PLoS Negl Trop Dis 10:e0004291. doi: 10.1371/journal.pntd.0004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryckman RE. 1986. The vertebrate hosts of the Triatominae of North and Central America and the West Indies (Hemiptera: Reduviidae: Triatominae). Bull Soc Vector Ecol 11:221–241. [Google Scholar]
- 50.Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, Todd CW, Currier M, Hand S, Varnado W, Dotson E, Hall C, Jett PL, Montgomery SP. 2012. The United States Trypanosoma cruzi Infection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion 52:1922–1930. doi: 10.1111/j.1537-2995.2012.03581.x. [DOI] [PubMed] [Google Scholar]
- 51.Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, Wesson D. 2007. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 13:605–607. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herwaldt BL, Grijalva MJ, Newsome AL, McGhee CR, Powell MR, Nemec DG, Steurer FJ, Eberhard ML. 2000. Use of polymerase chain reaction to diagnose the fifth reported US case of autochthonous transmission of Trypanosoma cruzi, in Tennessee, 1998. J Infect Dis 181:395–399. doi: 10.1086/315212. [DOI] [PubMed] [Google Scholar]
- 53.Navin TR, Roberto RR, Juranek DD, Limpakarnjanarat K, Mortenson EW, Clover JR, Yescott RE, Taclindo C, Steurer F, Allain D. 1985. Human and sylvatic Trypanosoma cruzi infection in California. Am J Public Health 75:366–369. doi: 10.2105/ajph.75.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. 2018. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states, USA. Am J Trop Med Hyg 98:113–121. doi: 10.4269/ajtmh.17-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. 2015. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl Trop Dis 9:e0004235. doi: 10.1371/journal.pntd.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kjos SA, Snowden KF, Olson JK. 2009. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis 9:41–50. doi: 10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- 57.Cruz-Reyes A, Pickering-López JM. 2006. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years—a review. Mem Inst Oswaldo Cruz 101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 58.Schofield CJ, Galvao C. 2009. Classification, evolution, and species groups within the Triatominae. Acta Trop 110:88–100. doi: 10.1016/j.actatropica.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Klotz JH, Dorn PL, Logan JL, Stevens L, Pinnas JL, Schmidt JO, Klotz SA. 2010. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 50:1629–1634. doi: 10.1086/652769. [DOI] [PubMed] [Google Scholar]
- 60.Klotz SA, Shirazi FM, Boesen K, Beatty NL, Dorn PL, Smith S, Schmidt JO. 2016. Kissing bug (Triatoma spp.) intrusion into homes: troublesome bites and domiciliation. Environ Health Insights 10:45–49. doi: 10.4137/EHI.S32834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter J, Fletcher E, Moussaoui R, Gandhi K, Weirauch C. 2012. Do bites of kissing bugs cause unexplained allergies? Results from a survey in triatomine-exposed and unexposed areas in Southern California. PLoS One 7:e44016. doi: 10.1371/journal.pone.0044016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noireau F, Diosque P, Jansen AM. 2009. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res 40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kierszenbaum F, Gottlieb CA, Budzko DB. 1981. Antibody-independent, natural resistance of birds to Trypanosoma cruzi infection. J Parasitol 67:656–660. doi: 10.2307/3280439. [DOI] [PubMed] [Google Scholar]
- 64.Jansen AM, Roque ALR. 2010. Domestic and wild mammalian reservoirs, p 249–276. In Telleria J, Tibayrenc M (ed), American trypanosomiasis (Chagas Disease): one hundred years of research. Elsevier, London, United Kingdom. [Google Scholar]
- 65.Herrera L, Martinez C, Carrasco H, Jansen AM, Urdaneta-Morales S. 2007. Cornea as a tissue reservoir of Trypanosoma cruzi. Parasitol Res 100:1395–1399. doi: 10.1007/s00436-006-0403-9. [DOI] [PubMed] [Google Scholar]
- 66.Deane MP, Lenzi HL, Jansen A. 1984. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz 79:513–515. doi: 10.1590/s0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- 67.Carreira JC, Jansen AM, de Nazareth Meirelles M, Costa e Silva F, Lenzi HL. 2001. Trypanosoma cruzi in the scent glands of Didelphis marsupialis: the kinetics of colonization. Exp Parasitol 97:129–140. doi: 10.1006/expr.2001.4603. [DOI] [PubMed] [Google Scholar]
- 68.Roellig DM, Yabsley MJ. 2010. Infectivity, pathogenicity, and virulence of Trypanosoma cruzi isolates from sylvatic animals and vectors, and domestic dogs from the United States in ICR strain mice and SD strain rats. Am J Trop Med Hyg 83:519–522. doi: 10.4269/ajtmh.2010.09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nouvellet P, Dumonteil E, Gourbiere S. 2013. The improbable transmission of Trypanosoma cruzi to human: the missing link in the dynamics and control of Chagas disease. PLoS Negl Trop Dis 7:e2505. doi: 10.1371/journal.pntd.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charles RA, Kjos S, Ellis AE, Barnes JC, Yabsley MJ. 2013. Southern plains woodrats (Neotoma micropus) from southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and host of a putative novel Trypanosoma species. Vector Borne Zoonotic Dis 13:22–30. doi: 10.1089/vbz.2011.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aleman A, Guerra T, Maikis TJ, Milholland MT, Castro-Arellano I, Forstner MR, Hahn D. 2017. The prevalence of Trypanosoma cruzi, the causal agent of Chagas disease, in Texas rodent populations. Ecohealth 14:130–143. doi: 10.1007/s10393-017-1205-5. [DOI] [PubMed] [Google Scholar]
- 72.Hodo CL, Goodwin CC, Mayes BC, Mariscal JA, Waldrup KA, Hamer SA. 2016. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop 164:259–266. doi: 10.1016/j.actatropica.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunter SM, Cordray C, Gorchakov R, Du I, Dittmar B, Brown EL, Murray KO, Nolan MS. 2018. Identification of white-tailed deer (Odocoileus virginianus) as a novel reservoir species for Trypanosoma cruzi in Texas, USA. J Wildl Dis 54:814–818. doi: 10.7589/2017-09-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maloney J, Newsome A, Huang J, Kirby J, Kranz M, Wateska A, Dunlap B, Yabsley MJ, Dunn JR, Jones TF, Moncayo AC. 2010. Seroprevalence of Trypanosoma cruzi in raccoons from Tennessee. J Parasitol 96:353–358. doi: 10.1645/GE-2312.1. [DOI] [PubMed] [Google Scholar]
- 75.Curtis-Robles R, Lewis BC, Hamer SA. 2016. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. Int J Parasitol Parasites Wildl 5:117–123. doi: 10.1016/j.ijppaw.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ. 2010. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis 10:757–763. doi: 10.1089/vbz.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pung OJ, Banks CW, Jones DN, Krissinger MW. 1995. Trypanosoma cruzi in wild raccoons, opossums, and triatomine bugs in southeast Georgia, U.S.A. J Parasitol 81:324–326. doi: 10.2307/3283947. [DOI] [PubMed] [Google Scholar]
- 78.Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. 2015. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors 8:123. doi: 10.1186/s13071-015-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikenga JO, Richerson JV. 1984. Trypanosoma cruzi (Chagas) (protozoa: Kinetoplastida: Trypanosomatidae) in invertebrate and vertebrate hosts from Brewster County in Trans-Pecos Texas. J Econ Entomol 77:126–129. doi: 10.1093/jee/77.1.126. [DOI] [PubMed] [Google Scholar]
- 80.Pinto CM, Baxter BD, Hanson JD, Mendez-Harclerode FM, Suchecki JR, Grijalva MJ, Fulhorst CF, Bradley RD. 2010. Using museum collections to detect pathogens. Emerg Infect Dis 16:356–357. doi: 10.3201/eid1602.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yabsley MJ, Noblet GP. 2002. Seroprevalence of Trypanosoma cruzi in raccoons from South Carolina and Georgia. J Wildl Dis 38:75–83. doi: 10.7589/0090-3558-38.1.75. [DOI] [PubMed] [Google Scholar]
- 82.Roellig DM, Ellis AE, Yabsley MJ. 2009. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J Parasitol 95:360–364. doi: 10.1645/GE-1740.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardinal MV, Sartor PA, Gaspe MS, Enriquez GF, Colaianni I, Gurtler RE. 2018. High levels of human infection with Trypanosoma cruzi associated with the domestic density of infected vectors and hosts in a rural area of northeastern Argentina. Parasit Vectors 11:492. doi: 10.1186/s13071-018-3069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castillo-Neyra R, Chou Chu L, Quispe-Machaca V, Ancca-Juarez J, Malaga Chavez FS, Bastos Mazuelos M, Naquira C, Bern C, Gilman RH, Levy MZ. 2015. The potential of canine sentinels for reemerging Trypanosoma cruzi transmission. Prev Vet Med 120:349–356. doi: 10.1016/j.prevetmed.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Enriquez GF, Bua J, Orozco MM, Wirth S, Schijman AG, Gurtler RE, Cardinal MV. 2014. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites for domestic transmission. Infect Genet Evol 25:36–43. doi: 10.1016/j.meegid.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Kjos SA, Snowden KF, Craig TM, Lewis B, Ronald N, Olson JK. 2008. Distribution and characterization of canine Chagas disease in Texas. Vet Parasitol 152:249–256. doi: 10.1016/j.vetpar.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 87.Curtis-Robles R, Snowden KF, Dominguez B, Dinges L, Rodgers S, Mays G, Hamer SA. 2017. Epidemiology and molecular typing of Trypanosoma cruzi in naturally-infected hound dogs and associated triatomine vectors in Texas, USA. PLoS Negl Trop Dis 11:e0005298. doi: 10.1371/journal.pntd.0005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hodo CL, Rodriguez JY, Curtis-Robles R, Zecca IB, Snowden KF, Cummings KJ, Hamer SA. 2019. Repeated cross-sectional study of Trypanosoma cruzi in shelter dogs in Texas, in the context of Dirofilaria immitis and tick-borne pathogen prevalence. J Vet Intern Med 33:158–166. doi: 10.1111/jvim.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyers AC, Meinders M, Hamer SA. 2017. Widespread Trypanosoma cruzi infection in government working dogs along the Texas-Mexico border: discordant serology, parasite genotyping and associated vectors. PLoS Negl Trop Dis 11:e0005819. doi: 10.1371/journal.pntd.0005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tenney TD, Curtis-Robles R, Snowden KF, Hamer SA. 2014. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas. Emerg Infect Dis 20:1323–1326. doi: 10.3201/eid2008.131843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kjos SA, Marcet PL, Yabsley MJ, Kitron U, Snowden KF, Logan KS, Barnes JC, Dotson EM. 2013. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J Med Entomol 50:1126–1139. doi: 10.1603/me12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moudy RM, Michaels S, Jameson SB, Londono B, Lopez V, Caillouet KA, Hallmark CJ, Davis JK, Foppa IM, Dorn PL, Wesson DM. 2014. Factors associated with peridomestic Triatoma sanguisuga (Hemiptera: Reduviidae) presence in southeastern Louisiana. J Med Entomol 51:1043–1050. doi: 10.1603/me13234. [DOI] [PubMed] [Google Scholar]
- 93.Gorchakov R, Trosclair LP, Wozniak EJ, Feria PT, Garcia MN, Gunter SM, Murray KO. 2016. Trypanosoma cruzi Infection prevalence and bloodmeal analysis in triatomine vectors of Chagas disease from rural peridomestic locations in Texas, 2013–2014. J Med Entomol 53:911–918. doi: 10.1093/jme/tjw040. [DOI] [PubMed] [Google Scholar]
- 94.Waleckx E, Suarez J, Richards B, Dorn PL. 2014. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg Infect Dis 20:2141–2143. doi: 10.3201/eid2012.131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klotz SA, Schmidt JO, Dorn PL, Ivanyi C, Sullivan KR, Stevens L. 2014. Free-roaming kissing bugs, vectors of Chagas disease, feed often on humans in the Southwest. Am J Med 127:421–426. doi: 10.1016/j.amjmed.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klotz SA, Dorn PL, Klotz JH, Pinnas JL, Weirauch C, Kurtz JR, Schmidt J. 2009. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop 111:114–118. doi: 10.1016/j.actatropica.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Martínez-Ibarra JA, Alejandre-Aguilar R, Paredes-González E, Martínez-Silva MA, Solorio-Cibrián M, Nogueda-Torres B, Trujillo-Contreras F, Novelo-López M. 2007. Biology of three species of North American Triatominae (Hemiptera: Reduviidae: Triatominae) fed on rabbits. Mem Inst Oswaldo Cruz 102:925–930. doi: 10.1590/s0074-02762007000800006. [DOI] [PubMed] [Google Scholar]
- 98.Pippin WF. 1970. The biology and vector capability of Triatoma sanguisuga texana Usinger and Triatoma gerstaeckeri (Stal) compared with Rhodnius prolixus (Stal) (Hemiptera: Triatominae). J Med Entomol 7:30–45. doi: 10.1093/jmedent/7.1.30. [DOI] [PubMed] [Google Scholar]
- 99.Reisenman CE, Gregory T, Guerenstein PG, Hildebrand JG. 2011. Feeding and defecation behavior of Triatoma rubida (Uhler, 1894) (Hemiptera: Reduviidae) under laboratory conditions, and its potential role as a vector of Chagas disease in Arizona, USA. Am J Trop Med Hyg 85:648–656. doi: 10.4269/ajtmh.2011.11-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis MD, Llewellyn MS, Yeo M, Acosta N, Gaunt MW, Miles MA. 2011. Recent, independent and anthropogenic origins of Trypanosoma cruzi hybrids. PLoS Negl Trop Dis 5:e1363. doi: 10.1371/journal.pntd.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 102.Marcili A, Lima L, Cavazzana M, Junqueira AC, Veludo HH, Maia Da Silva F, Campaner M, Paiva F, Nunes VL, Teixeira MM. 2009. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and histone H2B genes and genotyping based on ITS1 rDNA. Parasitology 136:641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- 103.Pinto CM, Kalko EK, Cottontail I, Wellinghausen N, Cottontail VM. 2012. TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal Zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infect Genet Evol 12:1328–1332. doi: 10.1016/j.meegid.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Pinto CM, Ocana-Mayorga S, Tapia EE, Lobos SE, Zurita AP, Aguirre-Villacis F, MacDonald A, Villacis AG, Lima L, Teixeira MM, Grijalva MJ, Perkins SL. 2015. Bats, trypanosomes, and triatomines in Ecuador: new insights into the diversity, transmission, and origins of Trypanosoma cruzi and Chagas disease. PLoS One 10:e0139999. doi: 10.1371/journal.pone.0139999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramírez JD, Tapia-Calle G, Muñoz-Cruz G, Poveda C, Rendón LM, Hincapié E, Guhl F. 2014. Trypanosome species in neo-tropical bats: biological, evolutionary and epidemiological implications. Infect Genet Evol 22:250–256. doi: 10.1016/j.meegid.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Messenger LA, Miles MA. 2015. Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi. Acta Trop 151:150–155. doi: 10.1016/j.actatropica.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Messenger LA, Ramirez JD, Llewellyn MS, Guhl F, Miles MA. 2016. Importation of hybrid human-associated Trypanosoma cruzi strains of southern South American origin, Colombia. Emerg Infect Dis 22:1452–1455. doi: 10.3201/eid2208.150786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeo M, Mauricio IL, Messenger LA, Lewis MD, Llewellyn MS, Acosta N, Bhattacharyya T, Diosque P, Carrasco HJ, Miles MA. 2011. Multilocus sequence typing (MLST) for lineage assignment and high resolution diversity studies in Trypanosoma cruzi. PLoS Negl Trop Dis 5:e1049. doi: 10.1371/journal.pntd.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diosque P, Tomasini N, Lauthier JJ, Messenger LA, Monje Rumi MM, Ragone PG, Alberti-D’Amato AM, Pérez Brandán C, Barnabé C, Tibayrenc M, Lewis MD, Llewellyn MS, Miles MA, Yeo M. 2014. Optimized multilocus sequence typing (MLST) scheme for Trypanosoma cruzi. PLoS Negl Trop Dis 8:e3117. doi: 10.1371/journal.pntd.0003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reis-Cunha JL, Baptista RP, Rodrigues-Luiz GF, Coqueiro-dos-Santos A, Valdivia HO, de Almeida LV, Cardoso MS, D’Ávila DA, Dias FHC, Fujiwara RT, Galvão LMC, Chiari E, Cerqueira GC, Bartholomeu DC. 2018. Whole genome sequencing of Trypanosoma cruzi field isolates reveals extensive genomic variability and complex aneuploidy patterns within TcII DTU. BMC Genomics 19:816. doi: 10.1186/s12864-018-5198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talavera-Lopez C, Messenger LA, Lewis MD, Yeo M, Reis-Cunha JL, Bartholomeu DC, Calzada JE, Saldana A, Ramirez JD, Guhl F, Ocana-Mayorga S, Costales JA, Gorchakov R, Jones K, Garcia MN, Carrasco H, Grisard EC, Teixeira SMR, Bottazzi ME, Hotez PJ, Murray KO, Grijalva MJ, Burleigh B, Miles MA. 2018. Repeat-driven generation of antigenic diversity in a major human pathogen, Trypanosoma cruzi. bioRxiv. doi: 10.1101/283531. [DOI] [PMC free article] [PubMed]
- 112.Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R, Fitzpatrick S, Gaunt MW, Mauricio IL. 2009. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology 136:1509–1528. doi: 10.1017/S0031182009990977. [DOI] [PubMed] [Google Scholar]
- 113.Miles MA, Cedillos RA, Povoa MM, de Souza AA, Prata A, Macedo V. 1981. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas’ disease? Lancet 1:1338–1340. doi: 10.1016/s0140-6736(81)92518-6. [DOI] [PubMed] [Google Scholar]
- 114.Messenger LA, Miles MA, Bern C. 2015. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev anti Infect Ther 13:995–1029. doi: 10.1586/14787210.2015.1056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramirez JD, Guhl F, Rendon LM, Rosas F, Marin-Neto JA, Morillo CA. 2010. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Negl Trop Dis 4:e899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carrasco HJ, Segovia M, Llewellyn MS, Morocoima A, Urdaneta-Morales S, Martinez C, Martinez CE, Garcia C, Rodriguez M, Espinosa R, de Noya BA, Diaz-Bello Z, Herrera L, Fitzpatrick S, Yeo M, Miles MA, Feliciangeli MD. 2012. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis 6:e1707. doi: 10.1371/journal.pntd.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Costales JA, Jara-Palacios MA, Llewellyn MS, Messenger LA, Ocaña-Mayorga S, Villacís AG, Tibayrenc M, Grijalva MJ. 2015. Trypanosoma cruzi population dynamics in the Central Ecuadorian Coast. Acta Trop 151:88–93. doi: 10.1016/j.actatropica.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaunt M, Miles M. 2000. The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz 95:557–565. doi: 10.1590/s0074-02762000000400019. [DOI] [PubMed] [Google Scholar]
- 119.Roellig DM, Brown EL, Barnabe C, Tibayrenc M, Steurer FJ, Yabsley MJ. 2008. Molecular typing of Trypanosoma cruzi isolates, United States. Emerg Infect Dis 14:1123–1125. doi: 10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cortez MR, Pinho AP, Cuervo P, Alfaro F, Solano M, Xavier SC, D’Andrea PS, Fernandes O, Torrico F, Noireau F, Jansen AM. 2006. Trypanosoma cruzi (Kinetoplastida Trypanosomatidae): ecology of the transmission cycle in the wild environment of the Andean valley of Cochabamba, Bolivia. Exp Parasitol 114:305–313. doi: 10.1016/j.exppara.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 121.Barnabé C, De Meeûs T, Noireau F, Bosseno M-F, Monje EM, Renaud F, Brenière SF. 2011. Trypanosoma cruzi discrete typing units (DTUs): microsatellite loci and population genetics of DTUs TcV and TcI in Bolivia and Peru. Infect Genet Evol 11:1752–1760. doi: 10.1016/j.meegid.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 122.Breniere SF, Aliaga C, Waleckx E, Buitrago R, Salas R, Barnabe C, Tibayrenc M, Noireau F. 2012. Genetic characterization of Trypanosoma cruzi DTUs in wild Triatoma infestans from Bolivia: predominance of TcI. PLoS Negl Trop Dis 6:e1650. doi: 10.1371/journal.pntd.0001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arenas M, Campos R, Coronado X, Ortiz S, Solari A. 2012. Trypanosoma cruzi genotypes of insect vectors and patients with Chagas of Chile studied by means of cytochrome b gene sequencing, minicircle hybridization, and nuclear gene polymorphisms. Vector Borne Zoonotic Dis 12:196–205. doi: 10.1089/vbz.2011.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, Vallejo GA, Guhl F. 2007. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol 7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 125.Herrera C, Guhl F, Falla A, Fajardo A, Montilla M, Adolfo Vallejo G, Bargues MD. 2009. Genetic variability and phylogenetic relationships within Trypanosoma cruzi I isolated in Colombia based on miniexon gene sequences. J Parasitol Res 2009:897364. doi: 10.1155/2009/897364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Connor O, Bosseno MF, Barnabe C, Douzery EJ, Breniere SF. 2007. Genetic clustering of Trypanosoma cruzi I lineage evidenced by intergenic miniexon gene sequencing. Infect Genet Evol 7:587–593. doi: 10.1016/j.meegid.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Falla A, Herrera C, Fajardo A, Montilla M, Vallejo GA, Guhl F. 2009. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop 110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. 2009. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog 5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ocana-Mayorga S, Llewellyn MS, Costales JA, Miles MA, Grijalva MJ. 2010. Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLoS Negl Trop Dis 4:e915. doi: 10.1371/journal.pntd.0000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lima VS, Jansen AM, Messenger LA, Miles MA, Llewellyn MS. 2014. Wild Trypanosoma cruzi I genetic diversity in Brazil suggests admixture and disturbance in parasite populations from the Atlantic Forest region. Parasit Vectors 7:263. doi: 10.1186/1756-3305-7-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV, Kjos S, Gurgel-Gonçalves R, Blanchet D, De Pablos LM, Tomasini N, da Silva A, Russomando G, Cuba CAC, Aznar C, Abate T, Levin MJ, Osuna A, Gürtler RE, Diosque P, Solari A, Triana-Chávez O, Schijman AG. 2010. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol 40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramirez JD, Guhl F, Messenger LA, Lewis MD, Montilla M, Cucunuba Z, Miles MA, Llewellyn MS. 2012. Contemporary cryptic sexuality in Trypanosoma cruzi. Mol Ecol 21:4216–4226. doi: 10.1111/j.1365-294X.2012.05699.x. [DOI] [PubMed] [Google Scholar]
- 133.Zumaya-Estrada FA, Messenger LA, Lopez-Ordonez T, Lewis MD, Flores-Lopez CA, Martinez-Ibarra AJ, Pennington PM, Cordon-Rosales C, Carrasco HV, Segovia M, Miles MA, Llewellyn MS. 2012. North American import? Charting the origins of an enigmatic Trypanosoma cruzi domestic genotype. Parasit Vectors 5:226. doi: 10.1186/1756-3305-5-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baptista RD, D’Ávila DA, Segatto M, do Valle ÍF, Franco GR, Valadares HMS, Gontijo ED, Galvão LMD, Pena SDJ, Chiari E, Machado CR, Macedo AM. 2014. Evidence of substantial recombination among Trypanosoma cruzi II strains from Minas Gerais. Infect Genet Evol 22:183–191. doi: 10.1016/j.meegid.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 135.de Oliveira MT, de Assis GF, Oliveira e Silva JC, Machado EM, da Silva GN, Veloso VM, Macedo AM, Martins HR, de Lana M. 2015. Trypanosoma cruzi discrete typing units (TcII and TcVI) in samples of patients from two municipalities of the Jequitinhonha Valley, MG, Brazil, using two molecular typing strategies. Parasit Vectors 8:568. doi: 10.1186/s13071-015-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kerr CL, Bhattacharyya T, Xavier SC, Barros JH, Lima VS, Jansen AM, Miles MA. 2016. Lineage-specific serology confirms Brazilian Atlantic forest lion tamarins, Leontopithecus chrysomelas and Leontopithecus rosalia, as reservoir hosts of Trypanosoma cruzi II (TcII). Parasit Vectors 9:584. doi: 10.1186/s13071-016-1873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Araujo CA, Waniek PJ, Xavier SC, Jansen AM. 2011. Genotype variation of Trypanosoma cruzi isolates from different Brazilian biomes. Exp Parasitol 127:308–312. doi: 10.1016/j.exppara.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 138.Maffey L, Cardinal MV, Ordóñez-Krasnowski PC, Lanati LA, Lauricella MA, Schijman AG, Gürtler RE. 2012. Direct molecular identification of Trypanosoma cruzi discrete typing units in domestic and peridomestic Triatoma infestans and Triatoma sordida from the Argentine Chaco. Parasitology 139:1570–1579. doi: 10.1017/S0031182012000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Enriquez GF, Cardinal MV, Orozco MM, Lanati L, Schijman AG, Gurtler RE. 2013. Discrete typing units of Trypanosoma cruzi identified in rural dogs and cats in the humid Argentinean Chaco. Parasitology 140:303–308. doi: 10.1017/S003118201200159X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fernández MDP, Cecere MC, Lanati LA, Lauricella MA, Schijman AG, Gürtler RE, Cardinal MV. 2014. Geographic variation of Trypanosoma cruzi discrete typing units from Triatoma infestans at different spatial scales. Acta Trop 140:10–18. doi: 10.1016/j.actatropica.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 141.Guhl F, Ramirez JD. 2013. Retrospective molecular integrated epidemiology of Chagas disease in Colombia. Infect Genet Evol 20:148–154. doi: 10.1016/j.meegid.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 142.Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, Miles GA, Lopez E, Gonzalez N, Patterson JS, Gaunt MW, de Arias AR, Miles MA. 2005. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol 35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 143.Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira AC, Souza AI, da Rosa JA, Campaner M, Lewis MD, Llewellyn MS, Miles MA, Teixeira MM. 2009. Comparative phylogeography of Trypanosoma cruzi TcIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol 9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 144.Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, Segovia M, Vargas J, Torrico F, Miles MA, Gaunt MW. 2009. Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl Trop Dis 3:e510. doi: 10.1371/journal.pntd.0000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barros JHS, Xavier SCC, Bilac D, Lima VS, Dario MA, Jansen AM. 2017. Identification of novel mammalian hosts and Brazilian biome geographic distribution of Trypanosoma cruzi TcIII and TcIV. Acta Trop 172:173–179. doi: 10.1016/j.actatropica.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 146.Marcili A, Valente VC, Valente SA, Junqueira AC, da Silva FM, Pinto AY, Naiff RD, Campaner M, Coura JR, Camargo EP, Miles MA, Teixeira MM. 2009. Trypanosoma cruzi in Brazilian Amazonia: lineages TcI and TcIIa in wild primates, Rhodnius spp. and in humans with Chagas disease associated with oral transmission. Int J Parasitol 39:615–623. doi: 10.1016/j.ijpara.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 147.Roellig DM, Savage MY, Fujita AW, Barnabe C, Tibayrenc M, Steurer FJ, Yabsley MJ. 2013. Genetic variation and exchange in Trypanosoma cruzi isolates from the United States. PLoS One 8:e56198. doi: 10.1371/journal.pone.0056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roque AL, Xavier SC, da Rocha MG, Duarte AC, D’Andrea PS, Jansen AM. 2008. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am J Trop Med Hyg 79:742–749. doi: 10.4269/ajtmh.2008.79.742. [DOI] [PubMed] [Google Scholar]
- 149.Valente SAD, da Costa Valente V, das Neves Pinto AY, de Jesus Barbosa César M, dos Santos MP, Miranda COS, Cuervo P, Fernandes O. 2009. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: human cases, triatomines, reservoir mammals and parasites. Trans R Soc Trop Med Hyg 103:291–297. doi: 10.1016/j.trstmh.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 150.Monteiro WM, Magalhaes LK, de Sa AR, Gomes ML, Toledo MJ, Borges L, Pires I, Guerra JA, Silveira H, Barbosa M. 2012. Trypanosoma cruzi IV causing outbreaks of acute Chagas disease and infections by different haplotypes in the Western Brazilian Amazonia. PLoS One 7:e41284. doi: 10.1371/journal.pone.0041284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Monteiro WM, Magalhaes LK, Santana Filho FS, Borborema M, Silveira H, Barbosa M. 2010. Trypanosoma cruzi TcIII/Z3 genotype as agent of an outbreak of Chagas disease in the Brazilian Western Amazonia. Trop Med Int Health 15:1049–1051. doi: 10.1111/j.1365-3156.2010.02577.x. [DOI] [PubMed] [Google Scholar]
- 152.Ramirez JD, Montilla M, Cucunuba ZM, Florez AC, Zambrano P, Guhl F. 2013. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis 7:e2041. doi: 10.1371/journal.pntd.0002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramirez JD, Hernandez C, Montilla M, Zambrano P, Florez AC, Parra E, Cucunuba ZM. 2014. First report of human Trypanosoma cruzi infection attributed to TcBat genotype. Zoonoses Public Health 61:477–479. doi: 10.1111/zph.12094. [DOI] [PubMed] [Google Scholar]
- 154.Garcia MN, Burroughs H, Gorchakov R, Gunter SM, Dumonteil E, Murray KO, Herrera CP. 2017. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol 49:151–156. doi: 10.1016/j.meegid.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 155.Buhaya MH, Galvan S, Maldonado RA. 2015. Incidence of Trypanosoma cruzi infection in triatomines collected at Indio Mountains Research Station. Acta Trop 150:97–99. doi: 10.1016/j.actatropica.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Curtis-Robles R, Auckland LD, Snowden KF, Hamer GL, Hamer SA. 2018. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect Genet Evol 58:171–180. doi: 10.1016/j.meegid.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 157.Curtis-Robles R, Meyers AC, Auckland LD, Zecca IB, Skiles R, Hamer SA. 2018. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop 188:225–233. doi: 10.1016/j.actatropica.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 158.Hwang WS, Zhang G, Maslov D, Weirauch C. 2010. Infection rates of Triatoma protracta (Uhler) with Trypanosoma cruzi in Southern California and molecular identification of trypanosomes. Am J Trop Med Hyg 83:1020–1022. doi: 10.4269/ajtmh.2010.10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parrado R, Ramirez JC, de la Barra A, Alonso-Vega C, Juiz N, Ortiz L, Illanes D, Torrico F, Gascon J, Alves F, Flevaud L, Garcia L, Schijman AG, Ribeiro I. 2019. Usefulness of serial blood sampling and PCR replicates for treatment monitoring of patients with chronic Chagas disease. Antimicrob Agents Chemother 63:e01191-18. doi: 10.1128/AAC.01191-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Devera R, Fernandes O, Coura JR. 2003. Should Trypanosoma cruzi be called “cruzi” complex? a review of the parasite diversity and the potential of selecting population after in vitro culturing and mice infection. Mem Inst Oswaldo Cruz 98:1–12. doi: 10.1590/s0074-02762003000100001. [DOI] [PubMed] [Google Scholar]
- 161.Alves AM, De Almeida DF, von Krüger WM. 1994. Changes in Trypanosoma cruzi kinetoplast DNA minicircles induced by environmental conditions and subcloning. J Eukaryot Microbiol 41:415–419. doi: 10.1111/j.1550-7408.1994.tb06099.x. [DOI] [PubMed] [Google Scholar]
- 162.Dvorak JA, Hartman DL, Miles MA. 1980. Trypanosoma cruzi: correlation of growth kinetics to zymodeme type in clones derived from various sources. J Protozool 27:472–474. doi: 10.1111/j.1550-7408.1980.tb05401.x. [DOI] [Google Scholar]
- 163.Alves AM, Tanuri A, de Almeida DF, von Krüger WM. 1993. Reversible changes in the isoenzyme electrophoretic mobility pattern and infectivity in clones of Trypanosoma cruzi. Exp Parasitol 77:246–253. doi: 10.1006/expr.1993.1081. [DOI] [PubMed] [Google Scholar]
- 164.Engel JC, Dvorak JA, Segura EL, Crane MS. 1982. Trypanosoma cruzi: biological characterization of 19 clones derived from two chronic chagasic patients. I. Growth kinetics in liquid medium. J Eukaryot Microbiol 29:555–560. doi: 10.1111/j.1550-7408.1982.tb05441.x. [DOI] [PubMed] [Google Scholar]
- 165.Deane MP, Mangia RH, Pereira NM, Momen H, Goncalves AM, Morel CM. 1984. Trypanosoma cruzi: strain selection by different schedules of mouse passage of an initially mixed infection. Mem Inst Oswaldo Cruz 79:495–497. doi: 10.1590/s0074-02761984000400016. [DOI] [PubMed] [Google Scholar]
- 166.Deane MP, Sousa MA, Pereira NM, Goncalves AM, Momen H, Morel CM. 1984. Trypanosoma cruzi: inoculation schedules and re-isolation methods select individual strains from doubly infected mice, as demonstrated by schizodeme and zymodeme analyses. J Protozool 31:276–280. doi: 10.1111/j.1550-7408.1984.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 167.Morel CM, Deane MP, Goncalves AM. 1986. The complexity of Trypanosoma cruzi populations revealed by schizodeme analysis. Parasitol Today 2:97–101. doi: 10.1016/0169-4758(86)90038-4. [DOI] [PubMed] [Google Scholar]
- 168.Siriano L, Luquetti AO, Avelar JB, Marra NL, de Castro AM. 2011. Chagas disease: increased parasitemia during pregnancy detected by hemoculture. Am J Trop Med Hyg 84:569–574. doi: 10.4269/ajtmh.2011.10-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miles MA, Apt BW, Widmer G, Povoa MM, Schofield CJ. 1984. Isozyme heterogeneity and numerical taxonomy of Trypanosoma cruzi stocks from Chile. Trans R Soc Trop Med Hyg 78:526–535. doi: 10.1016/0035-9203(84)90076-2. [DOI] [PubMed] [Google Scholar]
- 170.Luquetti AO, Miles MA, Rassi A, de Rezende JM, de Souza AA, Póvoa MM, Rodrigues I. 1986. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas’ disease in central Brazil. Trans R Soc Trop Med Hyg 80:462–470. doi: 10.1016/0035-9203(86)90347-0. [DOI] [PubMed] [Google Scholar]
- 171.Ortiz S, Zulantay I, Apt W, Saavedra M, Solari A. 2015. Transferability of Trypanosoma cruzi from mixed human host infection to Triatoma infestans and from insects to axenic culture. Parasitol Int 64:33–36. doi: 10.1016/j.parint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Burgos JM, Begher S, Silva HM, Bisio M, Duffy T, Levin MJ, Macedo AM, Schijman AG. 2008. Molecular identification of Trypanosoma cruzi I tropism for central nervous system in Chagas reactivation due to AIDS. Am J Trop Med Hyg 78:294–297. doi: 10.4269/ajtmh.2008.78.294. [DOI] [PubMed] [Google Scholar]
- 173.Burgos JM, Diez M, Vigliano C, Bisio M, Risso M, Duffy T, Cura C, Brusses B, Favaloro L, Leguizamon MS, Lucero RH, Laguens R, Levin MJ, Favaloro R, Schijman AG. 2010. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clin Infect Dis 51:485–495. doi: 10.1086/655680. [DOI] [PubMed] [Google Scholar]
- 174.Vago AR, Andrade LO, Leite AA, d’Ávila Reis D, Macedo AM, Adad SJ, Tostes S, Moreira MDV, Filho GB, Pena SDJ. 2000. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol 156:1805–1809. doi: 10.1016/S0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sanchez LV, Bautista DC, Corredor AF, Herrera VM, Martinez LX, Villar JC, Ramirez JD. 2013. Temporal variation of Trypanosoma cruzi discrete typing units in asymptomatic Chagas disease patients. Microbes Infect 15:745–748. doi: 10.1016/j.micinf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 176.Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. 1996. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol 83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- 177.Brisse S, Verhoef J, Tibayrenc M. 2001. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol 31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- 178.Wagner W, So M. 1990. Genomic variation of Trypanosoma cruzi: involvement of multicopy genes. Infect Immun 58:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Minning TA, Weatherly DB, Flibotte S, Tarleton RL. 2011. Widespread, focal copy number variations (CNV) and whole chromosome aneuploidies in Trypanosoma cruzi strains revealed by array comparative genomic hybridization. BMC Genomics 12:139. doi: 10.1186/1471-2164-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Herrera C, Majeau A, Didier P, Falkenstein KP, Dumonteil E. 2019. Trypanosoma cruzi diversity in naturally infected nonhuman primates in Louisiana assessed by deep sequencing of the mini-exon gene. Trans R Soc Trop Med Hyg 113:281–286. doi: 10.1093/trstmh/try119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pronovost H, Peterson AC, Chavez BG, Blum MJ, Dumonteil E, Herrera CP. 21 December 2018. Deep sequencing reveals multiclonality and new discrete typing units of Trypanosoma cruzi in rodents from the southern United States. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 182.Sánchez JM, Beffi Sueto SO, Schwabl P, Grijalva MJ, Llewellyn MS, Costales JA. 2019. Remarkable genetic diversity of Trypanosoma cruzi and Trypanosoma rangeli in two localities of southern Ecuador identified via deep sequencing of mini-exon gene amplicons. bioRxiv. doi: 10.1101/542993. [DOI] [PMC free article] [PubMed]
- 183.Villanueva-Lizama L, Teh-Poot C, Majeau A, Herrera C, Dumonteil E. 2019. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite discrete typing units in Chagasic patients From Yucatan, Mexico. J Infect Dis 219:1980–1988. doi: 10.1093/infdis/jiz047. [DOI] [PubMed] [Google Scholar]
- 184.Carter YL, Juliano JJ, Montgomery SP, Qvarnstrom Y. 2012. Acute Chagas disease in a returning traveler. Am J Trop Med Hyg 87:1038–1040. doi: 10.4269/ajtmh.2012.12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Acquatella H, Asch FM, Barbosa MM, Barros M, Bern C, Cavalcante JL, Echeverria Correa LE, Lima J, Marcus R, Marin-Neto JA, Migliore R, Milei J, Morillo CA, Nunes MCP, Campos Vieira ML, Viotti R. 2018. Recommendations for multimodality cardiac imaging in patients with Chagas disease: a report from the American Society of Echocardiography in Collaboration with the InterAmerican Association of Echocardiography (ECOSIAC) and the Cardiovascular Imaging Department of the Brazilian Society of Cardiology (DIC-SBC). J Am Soc Echocardiogr 31:3–25. doi: 10.1016/j.echo.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 186.Beltrao B, Cerroni P, Freitas DR, Pinto AY, Valente C, Valente SA, Costa Ede G, Sobel J. 2009. Investigation of two outbreaks of suspected oral transmission of acute Chagas disease in the Amazon region, Para State, Brazil, in 2007. Trop Doct 39:231–232. doi: 10.1258/td.2009.090035. [DOI] [PubMed] [Google Scholar]
- 187.Barreto-de-Albuquerque J, Silva-dos-Santos D, Perez AR, Berbert LR, de Santana-van-Vliet E, Farias-de-Oliveira DA, Moreira OC, Roggero E, de Carvalho-Pinto CE, Jurberg J, Cotta-de-Almeida V, Bottasso O, Savino W, de Meis J. 2015. Trypanosoma cruzi infection through the oral route promotes a severe infection in mice: new disease form from an old infection? PLoS Negl Trop Dis 9:e0003849. doi: 10.1371/journal.pntd.0003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Covarrubias C, Cortez M, Ferreira D, Yoshida N. 2007. Interaction with host factors exacerbates Trypanosoma cruzi cell invasion capacity upon oral infection. Int J Parasitol 37:1609–1616. doi: 10.1016/j.ijpara.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 189.Yoshida N. 2008. Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity. Parasitol Int 57:105–109. doi: 10.1016/j.parint.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 190.Messenger LA, Gilman RH, Verastegui M, Galdos-Cardenas G, Sanchez G, Valencia E, Sanchez L, Malaga E, Rendell VR, Jois M, Shah V, Santos N, Abastoflor MDC, LaFuente C, Colanzi R, Bozo R, Bern C, Working Group on Chagas Disease in Bolivia and Peru. 2017. Toward improving early diagnosis of congenital Chagas disease in an endemic setting. Clin Infect Dis 65:268–275. doi: 10.1093/cid/cix277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fabbro DL, Danesi E, Olivera V, Codebo MO, Denner S, Heredia C, Streiger M, Sosa-Estani S. 2014. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 8:e3312. doi: 10.1371/journal.pntd.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 193.Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, Ianni BM, Nastari L, Fernandes F, Patavino GM, Sachdev V, Capuani L, de Almeida-Neto C, Carrick DM, Wright D, Kavounis K, Goncalez TT, Carneiro-Proietti AB, Custer B, Busch MP, Murphy EL. 2013. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation 127:1105–1115. doi: 10.1161/CIRCULATIONAHA.112.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverria LE, Dutra WO, Gascon J, Morillo CA, Oliveira-Filho J, Ribeiro ALP, Marin-Neto JA, American Heart Association Rheumatic Fever Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Stroke Council. 2018. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation 138:e169–e209. doi: 10.1161/CIR.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 195.Benck L, Kransdorf E, Patel J. 2018. Diagnosis and management of Chagas cardiomyopathy in the United States. Curr Cardiol Rep 20:131. doi: 10.1007/s11886-018-1077-5. [DOI] [PubMed] [Google Scholar]
- 196.de Oliveira RB, Troncon LE, Dantas RO, Menghelli UG. 1998. Gastrointestinal manifestations of Chagas’ disease. Am J Gastroenterol 93:884–889. doi: 10.1111/j.1572-0241.1998.270_r.x. [DOI] [PubMed] [Google Scholar]
- 197.Bern C. 2012. Chagas disease in the immunosuppressed host. Curr Opin Infect Dis 25:450–457. doi: 10.1097/QCO.0b013e328354f179. [DOI] [PubMed] [Google Scholar]
- 198.Dias JC, Ramos AN Jr, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, Torres RM, Melo JR, Almeida EA, Oliveira W Jr, Silveira AC, Rezende JM, Pinto FS, Ferreira AW, Rassi A, Fragata AAF, Sousa AS, Correia D, Jansen AM, Andrade GM, Britto CF, Pinto AY, Rassi A Jr, Campos DE, Abad-Franch F, Santos SE, Chiari E, Hasslocher-Moreno AM, Moreira EF, Marques DS, Silva EL, Marin-Neto JA, Galvao LM, Xavier SS, Valente SA, Carvalho NB, Cardoso AV, Silva RA, Costa VM, Vivaldini SM, Oliveira SM, Valente VD, Lima MM, Alves RV. 2016. 2nd Brazilian consensus on Chagas Disease, 2015. Rev Soc Bras Med Trop 49(Suppl 1):3–60. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 199.Kransdorf EP, Czer LS, Luthringer DJ, Patel JK, Montgomery SP, Velleca A, Mirocha J, Zakowski PC, Zabner R, Gaultier CR, Qvarnstrom Y, Benedict T, Steurer F, Bosserman E, Paddock CD, Rafiei M, Kobashigawa JA. 2013. Heart transplantation for Chagas cardiomyopathy in the United States. Am J Transplant 13:3262–3268. doi: 10.1111/ajt.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bocchi EA, Fiorelli A. 2001. The paradox of survival results after heart transplantation for cardiomyopathy caused by Trypanosoma cruzi. First Guidelines Group for Heart Transplantation of the Brazilian Society of Cardiology. Ann Thorac Surg 71:1833–1838. doi: 10.1016/S0003-4975(01)02587-5. [DOI] [PubMed] [Google Scholar]
- 201.Bocchi EA, Fiorelli A. 2001. The Brazilian experience with heart transplantation: a multicenter report. J Heart Lung Transplant 20:637–645. doi: 10.1016/S1053-2498(00)00235-7. [DOI] [PubMed] [Google Scholar]
- 202.Benatti RD, Al-Kindi SG, Bacal F, Oliveira GH. 2018. Heart transplant outcomes in patients with Chagas cardiomyopathy in the United States. Clin Transplant 32:e13279. doi: 10.1111/ctr.13279. [DOI] [PubMed] [Google Scholar]
- 203.Kransdorf EP, Zakowski PC, Kobashigawa JA. 2014. Chagas disease in solid organ and heart transplantation. Curr Opin Infect Dis 27:418–424. doi: 10.1097/QCO.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 204.Perez-Molina JA, Rodriguez-Guardado A, Soriano A, Pinazo MJ, Carrilero B, Garcia-Rodriguez M, Salas J, Torrus D, Soler-Ferrer C, Puente S, Haro-Gonzalez JL, Martin-Rabadan P, Gascon J, the Chagas Study Group Of The SEMTSI (Sociedad Espanola de Medicina Tropical y Salud Internacional [Spanish Society of Tropical Medicine and International Health]). 2011. Guidelines on the treatment of chronic coinfection by Trypanosoma cruzi and HIV outside endemic areas. HIV Clin Trials 12:287–298. doi: 10.1310/hct1206-287. [DOI] [PubMed] [Google Scholar]
- 205.Campo M, Phung MK, Ahmed R, Cantey P, Bishop H, Bell TK, Gardiner C, Arias CA. 2010. A woman with HIV infection, brain abscesses, and eosinophilia. Clin Infect Dis 50:239–240. doi: 10.1086/649538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yasukawa K, Patel SM, Flash CA, Stager CE, Goodman JC, Woc-Colburn L. 2014. Trypanosoma cruzi meningoencephalitis in a patient with acquired immunodeficiency syndrome. Am J Trop Med Hyg 91:84–85. doi: 10.4269/ajtmh.14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cordova E, Boschi A, Ambrosioni J, Cudos C, Corti M. 2008. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int J Infect Dis 12:587–592. doi: 10.1016/j.ijid.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 208.Diazgranados CA, Saavedra-Trujillo CH, Mantilla M, Valderrama SL, Alquichire C, Franco-Paredes C. 2009. Chagasic encephalitis in HIV patients: common presentation of an evolving epidemiological and clinical association. Lancet Infect Dis 9:324–330. doi: 10.1016/S1473-3099(09)70088-X. [DOI] [PubMed] [Google Scholar]
- 209.Sartori AM, Ibrahim KY, Nunes Westphalen EV, Braz LM, Oliveira OC Jr, Gakiya E, Lopes MH, Shikanai-Yasuda MA. 2007. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann Trop Med Parasitol 101:31–50. doi: 10.1179/136485907X154629. [DOI] [PubMed] [Google Scholar]
- 210.Martinez-Perez A, Norman FF, Monge-Maillo B, Perez-Molina JA, Lopez-Velez R. 2014. An approach to the management of Trypanosoma cruzi infection (Chagas’ disease) in immunocompromised patients. Expert Rev anti Infect Ther 12:357–373. doi: 10.1586/14787210.2014.880652. [DOI] [PubMed] [Google Scholar]
- 211.Gluckstein D, Ciferri F, Ruskin J. 1992. Chagas’ disease: another cause of cerebral mass in the acquired immunodeficiency syndrome. Am J Med 92:429–432. doi: 10.1016/0002-9343(92)90275-g. [DOI] [PubMed] [Google Scholar]
- 212.Lambert N, Mehta B, Walters R, Eron JJ. 2006. Chagasic encephalitis as the initial manifestation of AIDS. Ann Intern Med 144:941–943. doi: 10.7326/0003-4819-144-12-200606200-00018. [DOI] [PubMed] [Google Scholar]
- 213.Yoo TW, Mlikotic A, Cornford ME, Beck CK. 2004. Concurrent cerebral American trypanosomiasis and toxoplasmosis in a patient with AIDS. Clin Infect Dis 39:e30–e34. doi: 10.1086/422456. [DOI] [PubMed] [Google Scholar]
- 214.Garcia LS. 2016. Diagnostic medical parasitology. ASM Press, Washington, DC. [Google Scholar]
- 215.Leiby DA, Nguyen ML, Proctor MC, Townsend RL, Stramer SL. 2017. Frequency of Trypanosoma cruzi parasitemia among infected blood donors with a potential association between parasite lineage and transfusion transmission. Transfusion 57:1426–1432. doi: 10.1111/trf.14082. [DOI] [PubMed] [Google Scholar]
- 216.Messenger LA, Bern C. 2018. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis 31:415–421. doi: 10.1097/QCO.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 217.Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, Favaloro RR, Freilij H, Schijman AG. 2009. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl Trop Dis 3:e419. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qvarnstrom Y, Schijman AG, Veron V, Aznar C, Steurer F, da Silva AJ. 2012. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Negl Trop Dis 6:e1689. doi: 10.1371/journal.pntd.0001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schijman AG. 2018. Molecular diagnosis of Trypanosoma cruzi. Acta Trop 184:59–66. doi: 10.1016/j.actatropica.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 220.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Sanchez Leon Z, Galvao L, Nolder D, Monje Rumi M, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Anez N, De Castro AM, Gonzalez CI, Acosta Viana K, Yachelini P, Torrico F, Robello C, Diosque P, Triana Chavez O, Aznar C, Russomando G, Buscher P, Assal A, Guhl F, Sosa Estani S, DaSilva A, Britto C, Luquetti A, Ladzins J. 2011. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis 5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mayta H, Romero YK, Pando A, Verastegui M, Tinajeros F, Bozo R, Henderson-Frost J, Colanzi R, Flores J, Lerner R, Bern C, Gilman RH, Chagas Working Group in Peru and Bolivia. 2019. Improved DNA extraction technique from clot for the diagnosis of Chagas disease. PLoS Negl Trop Dis 13:e0007024. doi: 10.1371/journal.pntd.0007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Torrico F, Gascon J, Ortiz L, Alonso-Vega C, Pinazo M-J, Schijman A, Almeida IC, Alves F, Strub-Wourgaft N, Ribeiro I, E1224 Study Group, Santina G, Blum B, Correia E, Garcia-Bournisen F, Vaillant M, Morales JR, Pinto Rocha JJ, Rojas Delgadillo G, Magne Anzoleaga HR, Mendoza N, Quechover RC, Caballero MYE, Lozano Beltran DF, Zalabar AM, Rojas Panozo L, Palacios Lopez A, Torrico Terceros D, Fernandez Galvez VA, Cardozo L, Cuellar G, Vasco Arenas RN, Gonzales I, Hoyos Delfin CF, Garcia L, Parrado R, de la Barra A, Montano N, Villarroel S, Duffy T, Bisio M, Ramirez JC, Duncanson F, Everson M, Daniels A, Asada M, Cox E, Wesche D, Diderichsen PM, Marques AF, Izquierdo L, Sender SS, Reverter JC, Morales M, Jimenez W. 2018. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis 18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Molina I, Gómez I Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 224.Schijman AG, Vigliano C, Burgos J, Favaloro R, Perrone S, Laguens R, Levin MJ. 2000. Early diagnosis of recurrence of Trypanosoma cruzi infection by polymerase chain reaction after heart transplantation of a chronic Chagas’ heart disease patient. J Heart Lung Transplant 19:1114–1117. doi: 10.1016/S1053-2498(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 225.Gray AEB, La Hoz RM, Green JS, Vikram HR, Benedict T, Rivera H, Montgomery SP. 2018. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012–2016. Transpl Infect Dis 20:e12996. doi: 10.1111/tid.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Umezawa ES, Nascimento MS, Kesper N Jr, Coura JR, Borges-Pereira J, Junqueira AC, Camargo ME. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol 34:2143–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Silveira-Lacerda EP, Silva AG, Junior SF, Souza MA, Kesper N, Botelho-Filho A, Umezawa ES. 2004. Chagas’ disease: application of TESA-blot in inconclusive sera from a Brazilian blood bank. Vox Sang 87:204–207. doi: 10.1111/j.1423-0410.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 228.Kesper N Jr, de Almeida KA, Stolf AM, Umezawa ES. 2000. Immunoblot analysis of trypomastigote excreted-secreted antigens as a tool for the characterization of Trypanosoma cruzi strains and isolates. J Parasitol 86:862–867. doi: 10.1645/0022-3395(2000)086[0862:IAOTES]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 229.Noazin S, Lee JA, Malaga ES, Valencia Ayala E, Condori BJ, Roca C, Lescano AG, Bern C, Castillo W, Mayta H, Menduiña MC, Verastegui MR, Tinajeros F, Gilman RH, Miranda-Schaeubinger M, Chakravarti I, Ferriss EL, Chavez C, Velarde JK, Urquizu F, Gorena M, Bowman N, Hinojosa E, Chagas Working Group in Bolivia and Peru. 2019. Trypomastigote excretory secretory antigen blot is associated with Trypanosoma cruzi load and detects congenital T. cruzi infection in neonates, using anti-shed acute phase antigen immunoglobulin M. J Infect Dis 219:609–618. doi: 10.1093/infdis/jiy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rodriguez P, Truyens C, Alonso-Vega C, Flores A, Cordova M, Suarez E, Torrico F, Carlier Y. 2005. Serum levels for IgM and IgA antibodies to anti-Trypanosoma cruzi in samples of blood from newborns from mothers with positive serology for Chagas disease. Rev Soc Bras Med Trop 38(Suppl 2):62–64. (In Spanish.) [PubMed] [Google Scholar]
- 231.US Food and Drug Administration. 2019. Testing HCT/P donors for relevant communicable disease agents and diseases. https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/TissueSafety/ucm095440.htm. Accessed 30 May 2019.
- 232.Levi ME, Kumar D, Green M, Ison MG, Kaul D, Michaels MG, Morris MI, Schwartz BS, Echenique IA, Blumberg EA, AST ID Community of Practice. 2014. Considerations for screening live kidney donors for endemic infections: a viewpoint on the UNOS policy. Am J Transplant 14:1003–1011. doi: 10.1111/ajt.12666. [DOI] [PubMed] [Google Scholar]
- 233.OPTN/UNOS Disease Transmission Advisory Committee. 2014. Recognizing seasonal and geographically endemic infections in organ donors: considerations during living donor evaluation. https://optn.transplant.hrsa.gov/media/1138/seasonal_disease_guidance.pdf.
- 234.Lewis MD, Kelly JM. 2016. Putting infection dynamics at the heart of Chagas disease. Trends Parasitol 32:899–911. doi: 10.1016/j.pt.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM. 2019. Pathology and pathogenesis of Chagas heart disease. Annu Rev Pathol 14:421–447. doi: 10.1146/annurev-pathol-020117-043711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kransdorf EP, Fishbein MC, Czer LS, Patel JK, Velleca A, Tazelaar HD, Roy RR, Steidley DE, Kobashigawa JA, Luthringer DJ. 2016. Pathology of chronic Chagas cardiomyopathy in the United States: a detailed review of 13 cardiectomy cases. Am J Clin Pathol 146:191–198. doi: 10.1093/ajcp/aqw098. [DOI] [PubMed] [Google Scholar]
- 237.Jabari S, de Oliveira EC, Brehmer A, da Silveira AB. 2014. Chagasic megacolon: enteric neurons and related structures. Histochem Cell Biol 142:235–244. doi: 10.1007/s00418-014-1250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hemmige V, Tanowitz H, Sethi A. 2012. Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. Int J Dermatol 51:501–508. doi: 10.1111/j.1365-4632.2011.05380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Duaso J, Yanez E, Castillo C, Galanti N, Cabrera G, Corral G, Maya JD, Zulantay I, Apt W, Kemmerling U. 2012. Reorganization of extracellular matrix in placentas from women with asymptomatic Chagas disease: mechanism of parasite invasion or local placental defense? J Trop Med 2012:758357. doi: 10.1155/2012/758357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Benvenuti LA, Roggerio A, Freitas HF, Mansur AJ, Fiorelli A, Higuchi ML. 2008. Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol 102:481–487. doi: 10.1179/136485908X311740. [DOI] [PubMed] [Google Scholar]
- 241.Benvenuti LA, Roggerio A, Cavalcanti MM, Nishiya AS, Levi JE. 2017. An autopsy-based study of Trypanosoma cruzi persistence in organs of chronic chagasic patients and its relevance for transplantation. Transpl Infect Dis 19:e12783. doi: 10.1111/tid.12783. [DOI] [PubMed] [Google Scholar]
- 242.Benvenuti LA, Aiello VD. 2017. The challenging diagnosis of Chagas disease reactivation in endomyocardial biopsies from heart-transplanted patients. J Heart Lung Transplant 36:1360–1361. doi: 10.1016/j.healun.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 243.Muller Kratz J, Garcia Bournissen F, Forsyth CJ, Sosa-Estani S. 2018. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev Clin Pharmacol 11:943–957. doi: 10.1080/17512433.2018.1509704. [DOI] [PubMed] [Google Scholar]
- 244.Crespillo-Andújar C, Venanzi-Rullo E, López-Vélez R, Monge-Maillo B, Norman F, López-Polín A, Pérez-Molina JA. 2018. Safety profile of benznidazole in the treatment of chronic Chagas disease: experience of a referral centre and systematic literature review with meta-analysis. Drug Saf 41:1035–1048. doi: 10.1007/s40264-018-0696-5. [DOI] [PubMed] [Google Scholar]
- 245.Miller DA, Hernandez S, Rodriguez De Armas L, Eells SJ, Traina MM, Miller LG, Meymandi SK. 2015. Tolerance of benznidazole in a United States Chagas disease clinic. Clin Infect Dis 60:1237–1240. doi: 10.1093/cid/civ005. [DOI] [PubMed] [Google Scholar]
- 246.Hasslocher-Moreno AM, do Brasil PE, de Sousa AS, Xavier SS, Chambela MC, Sperandio da Silva GM. 2012. Safety of benznidazole use in the treatment of chronic Chagas’ disease. J Antimicrob Chemother 67:1261–1266. doi: 10.1093/jac/dks027. [DOI] [PubMed] [Google Scholar]
- 247.Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. 2011. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127:e212–e218. doi: 10.1542/peds.2010-1172. [DOI] [PubMed] [Google Scholar]
- 248.Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME, Ballering G, Bisio M, Koren G, Garcia-Bournissen F. 2014. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl Trop Dis 8:e2907. doi: 10.1371/journal.pntd.0002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.US Food and Drug Administration. 2017. FDA approves first U.S. treatment for Chagas disease. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm573942.htm. Accessed 17 September 2018.
- 250.Drugs for Neglected Diseases Initiative. 2017. U.S. FDA approves Chemo Group’s benznidazole to treat children with Chagas disease. DNDi, Geneva, Switzerland.
- 251.Crespillo-Andujar C, Chamorro-Tojeiro S, Norman F, Monge-Maillo B, Lopez-Velez R, Perez-Molina JA. 2018. Toxicity of nifurtimox as second-line treatment after benznidazole intolerance in patients with chronic Chagas disease: when available options fail. Clin Microbiol Infect 24:1344.e1–1344.e4. doi: 10.1016/j.cmi.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 252.Forsyth CJ, Hernandez S, Olmedo W, Abuhamidah A, Traina MI, Sanchez DR, Soverow J, Meymandi SK. 2016. Safety profile of nifurtimox for treatment of Chagas disease in the United States. Clin Infect Dis 63:1056–1062. doi: 10.1093/cid/ciw477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zingales B, Miles MA, Moraes CB, Luquetti A, Guhl F, Schijman AG, Ribeiro I, Drugs for Neglected Disease Initiative, Chagas Clinical Research Platform Meeting. 2014. Drug discovery for Chagas disease should consider Trypanosoma cruzi strain diversity. Mem Inst Oswaldo Cruz 109:828–833. doi: 10.1590/0074-0276140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ramirez JC, Parrado R, Sulleiro E, de la Barra A, Rodriguez M, Villarroel S, Irazu L, Alonso-Vega C, Alves F, Curto MA, Garcia L, Ortiz L, Torrico F, Gascon J, Flevaud L, Molina I, Ribeiro I, Schijman AG. 2017. First external quality assurance program for bloodstream real-time PCR monitoring of treatment response in clinical trials of Chagas disease. PLoS One 12:e0188550. doi: 10.1371/journal.pone.0188550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cancado JR, Brener Z. 1979. Terapeutica, p 362–424. In Brener Z, Andrade Z (ed), Trypanosoma cruzi e doença de Chagas, 1st ed. Guanabara Koogan, Rio de Janeiro, Brazil. [Google Scholar]
- 256.Wegner DH, Rohwedder RW. 1972. The effect of nifurtimox in acute Chagas’ infection. Arzneimittelforschung 22:1624–1635. [PubMed] [Google Scholar]
- 257.Chippaux JP, Clavijo AN, Santalla JA, Postigo JR, Schneider D, Brutus L. 2010. Antibody drop in newborns congenitally infected by Trypanosoma cruzi treated with benznidazole. Trop Med Int Health 15:87–93. doi: 10.1111/j.1365-3156.2009.02431.x. [DOI] [PubMed] [Google Scholar]
- 258.Chippaux JP, Salas-Clavijo AN, Postigo JR, Schneider D, Santalla JA, Brutus L. 2013. Evaluation of compliance to congenital Chagas disease treatment: results of a randomised trial in Bolivia. Trans R Soc Trop Med Hyg 107:1–7. doi: 10.1093/trstmh/trs004. [DOI] [PubMed] [Google Scholar]
- 259.Sguassero Y, Roberts KN, Harvey GB, Comandé D, Ciapponi A, Cuesta CB, Aguiar C, Castro A. M d, Danesi E, de Andrade AL, de Lana M, Escribà JM, Fabbro DL, Fernandes CD, Flores-Chávez M, Hasslocher-Moreno AM, Jackson Y, Lacunza CD, Machado-de-Assis GF, Maldonado M, Meira WSF, Molina I, Monje-Rumi MM, Muñoz-San Martín C, Murcia L, Nery de Castro C, Sánchez Negrette O, Segovia M, Silveira CAN, Solari A, Steindel M, Streiger ML, Vera de Bilbao N, Zulantay I, Sosa-Estani S. 2018. Course of serological tests in treated subjects with chronic Trypanosoma cruzi infection: a systematic review and meta-analysis of individual participant data. Int J Infect Dis 73:93–101. doi: 10.1016/j.ijid.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Krettli AU. 2009. The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem Inst Oswaldo Cruz 104(Suppl 1):142–151. doi: 10.1590/S0074-02762009000900020. [DOI] [PubMed] [Google Scholar]
- 261.Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 262.Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am J Trop Med Hyg 59:526–529. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- 263.Yun O, Lima MA, Ellman T, Chambi W, Castillo S, Flevaud L, Roddy P, Parreno F, Albajar Vinas P, Palma P. 2009. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Medecins Sans Frontieres. PLoS Negl Trop Dis 3:e488. doi: 10.1371/journal.pntd.0000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Issa VS, Bocchi EA. 2010. Antitrypanosomal agents: treatment or threat? Lancet 376:768. (Letter.) doi: 10.1016/S0140-6736(10)61372-4 (Reply, 376:768–769, 10.1016/S0140-6736(10)61373-6.) [DOI] [PubMed] [Google Scholar]
- 265.Viotti R, Alarcon de Noya B, Araujo-Jorge T, Grijalva MJ, Guhl F, Lopez MC, Ramsey JM, Ribeiro I, Schijman AG, Sosa-Estani S, Torrico F, Gascon J. 2014. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother 58:635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 267.Marin-Neto JA, Rassi A Jr, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S. 2008. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas’ cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am Heart J 156:37–43. doi: 10.1016/j.ahj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 268.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S. 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 269.Maguire JH. 2015. Treatment of Chagas’ disease—time is running out. N Engl J Med 373:1369–1370. doi: 10.1056/NEJMe1510170. [DOI] [PubMed] [Google Scholar]
- 270.Campos SV, Strabelli TM, Amato Neto V, Silva CP, Bacal F, Bocchi EA, Stolf NA. 2008. Risk factors for Chagas’ disease reactivation after heart transplantation. J Heart Lung Transplant 27:597–602. doi: 10.1016/j.healun.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 271.Diez M, Favaloro L, Bertolotti A, Burgos JM, Vigliano C, Lastra MP, Levin MJ, Arnedo A, Nagel C, Schijman AG, Favaloro RR. 2007. Usefulness of PCR strategies for early diagnosis of Chagas’ disease reactivation and treatment follow-up in heart transplantation. Am J Transplant 7:1633–1640. doi: 10.1111/j.1600-6143.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- 272.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. 2018. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 273.Meymandi SK, Forsyth CJ, Soverow J, Hernandez S, Sanchez D, Montgomery SP, Traina M. 2017. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin Infect Dis 64:1182–1188. doi: 10.1093/cid/cix064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Manne-Goehler J, Davis J, Perez JH, Collins K, Harakawa H, Hochberg N, Hamer D, Barnett E, Köhler J. 2018. The results of a primary care-based screening program for Trypanosoma cruzi in East Boston, Massachusetts, abstr 442. IDWeek, San Francisco, CA, 3 to 7 October 2018.
- 275.Pew Research Center. 2017. Facts on U.S. Latinos, 2015: statistical portrait of Hispanics in the United States. http://www.pewhispanic.org/2017/09/18/facts-on-u-s-latinos/. Accessed 2 January 2019.
- 276.Webber BJ, Pawlak MT, Valtier S, Daniels CC, Tully CC, Wozniak EJ, Roachell WD, Sanchez FX, Blasi AA, Cropper TL. 2017. Prevalence and seroprevalence of Trypanosoma cruzi infection in a military population in Texas. Am J Trop Med Hyg 97:1477–1481. doi: 10.4269/ajtmh.17-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dodd RY, Groves JA, Townsend RL, Notari EP, Foster GA, Custer B, Busch MP, Stramer SL. 2019. Impact of one‐time testing for Trypanosoma cruzi antibodies among blood donors in the United States. Transfusion 59:1016–1023. doi: 10.1111/trf.15118. [DOI] [PubMed] [Google Scholar]
- 278.Klotz SA, Dorn PL, Mosbacher M, Schmidt JO. 2014. Kissing bugs in the United States: risk for vector-borne disease in humans. Environ Health Insights 8:49–59. doi: 10.4137/EHI.S16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Anonymous. 1956. Found: two cases of Chagas disease. Texas Health Bull 9:11–13. [Google Scholar]
- 280.Beatty NL, Perez-Velez CM, Yaglom HD, Carson S, Liu E, Khalpey ZI, Klotz SA, Elliott SP. 2018. Evidence of likely autochthonous transmission of Chagas disease in Arizona. Am J Trop Med Hyg 99:1534–1536. doi: 10.4269/ajtmh.18-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Garcia MN, Murray KO, Hotez PJ, Rossmann SN, Gorchakov R, Ontiveros A, Woc-Colburn L, Bottazzi ME, Rhodes CE, Ballantyne CM, Aguilar D. 2015. Development of Chagas cardiac manifestations among Texas blood donors. Am J Cardiol 115:113–117. doi: 10.1016/j.amjcard.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 282.Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, Woc-Colburn L, Hotez PJ, Murray KO. 2015. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg 92:325–330. doi: 10.4269/ajtmh.14-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gunter SM, Murray KO, Gorchakov R, Beddard R, Rossmann SN, Montgomery SP, Rivera H, Brown EL, Aguilar D, Widman LE, Garcia MN. 2017. Likely autochthonous transmission of Trypanosoma cruzi to humans, south central Texas, USA. Emerg Infect Dis 23:500–503. doi: 10.3201/eid2303.161157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Harris N, Woc-Colburn L, Gunter SM, Gorchakov R, Murray KO, Rossmann S, Garcia MN. 2017. Autochthonous Chagas disease in the southern United States: a case report of suspected residential and military exposures. Zoonoses Public Health 64:491–493. doi: 10.1111/zph.12360. [DOI] [PubMed] [Google Scholar]
- 285.Hernandez S, Flores CA, Viana GM, Sanchez DR, Traina MI, Meymandi SK. 2016. Autochthonous transmission of Trypanosoma Cruzi in southern California. Open Forum Infect Dis 3:ofw227. doi: 10.1093/ofid/ofw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Benjamin RJ, Stramer SL, Leiby DA, Dodd RY, Fearon M, Castro E. 2012. Trypanosoma cruzi infection in North America and Spain: evidence in support of transfusion transmission. Transfusion 52:1913–1921. doi: 10.1111/j.1537-2995.2011.03554.x. [DOI] [PubMed] [Google Scholar]
- 287.Kessler DA, Shi PA, Avecilla ST, Shaz BH. 2013. Results of lookback for Chagas disease since the inception of donor screening at New York Blood Center. Transfusion 53:1083–1087. doi: 10.1111/j.1537-2995.2012.03856.x. [DOI] [PubMed] [Google Scholar]
- 288.Centers for Disease Control and Prevention. 2007. Blood donor screening for Chagas disease—United States, 2006–2007. MMWR Morb Mortal Wkly Rep 56:141–143. [PubMed] [Google Scholar]
- 289.Reference deleted.
- 290.Stramer SL, Notari EP, Townsend RL, Custer B, Kamel H, Busch MP, Dodd RY. 2011. No evidence of Trypanosoma cruzi incidence in US blood donors: a 4-year study, abstr P1-020A. AABB Annual Meeting, San Diego, CA, 22 to 25 October 2011. [Google Scholar]
- 291.Food and Drug Administration. 2017. Use of serological tests to reduce the risk of transmission of Trypanosoma cruzi infection in blood and blood components: guidance for industry. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM528600.pdf. Accessed 3 January 2019.
- 292.Steele WR, Hewitt EH, Kaldun AM, Krysztof DE, Dodd RY, Stramer SL. 2014. Donors deferred for self-reported Chagas disease history: does it reduce risk? Transfusion 54:2092–2097. doi: 10.1111/trf.12590. [DOI] [PubMed] [Google Scholar]
- 293.Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. 2008. Chagas disease and the US blood supply. Curr Opin Infect Dis 21:476–482. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 294.Centers for Disease Control and Prevention. 2002. Chagas disease after organ transplantation—United States, 2001. MMWR Morb Mortal Wkly Rep 51:210–212. [PubMed] [Google Scholar]
- 295.Kun H, Moore A, Mascola L, Steurer F, Lawrence G, Kubak B, Radhakrishna S, Leiby D, Herron R, Mone T, Hunter R, Kuehnert M. 2009. Transmission of Trypanosoma cruzi by heart transplantation. Clin Infect Dis 48:1534–1540. doi: 10.1086/598931. [DOI] [PubMed] [Google Scholar]
- 296.Schwartz BS, Paster M, Ison MG, Chin-Hong PV. 2011. Organ donor screening practices for Trypanosoma cruzi infection among US Organ Procurement Organizations. Am J Transplant 11:848–851. doi: 10.1111/j.1600-6143.2011.03436.x. [DOI] [PubMed] [Google Scholar]
- 297.Corey AB, Sonetti D, Maloney JD, Montgomery SP, Rademacher BL, Taylor LJ, Smith JA, Striker R. 2017. Transmission of donor-derived Trypanosoma cruzi and subsequent development of Chagas disease in a lung transplant recipient. Case Rep Infect Dis 2017:5381072. doi: 10.1155/2017/5381072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hagar JM, Rahimtoola SH. 1991. Chagas’ heart disease in the United States. N Engl J Med 325:763–768. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- 299.Bern C, Montgomery SP. 2009. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 300.Kapelusznik L, Varela D, Montgomery SP, Shah AN, Steurer FJ, Rubinstein D, Caplivski D, Pinney SP, Turker D, Factor SH. 2013. Chagas disease in Latin American immigrants with dilated cardiomyopathy in New York City. Clin Infect Dis 57:e7. doi: 10.1093/cid/cit199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Traina MI, Sanchez DR, Hernandez S, Bradfield JS, Labedi MR, Ngab TA, Steurer F, Montgomery SP, Meymandi SK. 2015. Prevalence and impact of Chagas disease among Latin American immigrants with nonischemic cardiomyopathy in Los Angeles, California. Circ Heart Fail 8:938–943. doi: 10.1161/CIRCHEARTFAILURE.115.002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Traina MI, Hernandez S, Sanchez DR, Dufani J, Salih M, Abuhamidah AM, Olmedo W, Bradfield JS, Forsyth CJ, Meymandi SK. 2017. Prevalence of Chagas disease in a U.S. population of Latin American immigrants with conduction abnormalities on electrocardiogram. PLoS Negl Trop Dis 11:e0005244. doi: 10.1371/journal.pntd.0005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Park S, Sanchez DR, Traina MI, Bradfield JS, Hernandez S, Ufion AJA, Dufani J, Bergin P, Wachsner RY, Meymandi SK. 2017. The prevalence of Chagas disease among Latin American immigrants with pacemakers in Los Angeles, California. Am J Trop Med Hyg 96:1139–1142. doi: 10.4269/ajtmh.16-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ribeiro AL, Sabino EC, Marcolino MS, Salemi VM, Ianni BM, Fernandes F, Nastari L, Antunes A, Menezes M, Oliveira CD, Sachdev V, Carrick DM, Busch MP, Murphy EL, NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II), International Component. 2013. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis 7:e2078. doi: 10.1371/journal.pntd.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Vilas Boas LG, Bestetti RB, Otaviano AP, Cardinalli-Neto A, Nogueira PR. 2013. Outcome of Chagas cardiomyopathy in comparison to ischemic cardiomyopathy. Int J Cardiol 167:486–490. doi: 10.1016/j.ijcard.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 306.Rassi A Jr, Rassi A, Little WC. 2000. Chagas’ heart disease. Clin Cardiol 23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bertolino ND, Villafanha DF, Cardinalli-Neto A, Cordeiro JA, Arcanjo MJ, Theodoropoulos TA, Bestetti RB. 2010. Prognostic impact of Chagas’ disease in patients awaiting heart transplantation. J Heart Lung Transplant 29:449–453. doi: 10.1016/j.healun.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 308.Bestetti RB. 1997. Heart transplantation as a treatment for patients with end-stage Chagas’ heart disease. Circulation 96:2744–2745. [PubMed] [Google Scholar]
- 309.Rassi A Jr, Dias JC, Marin-Neto JA, Rassi A. 2008. Challenges and opportunities for primary, secondary, and tertiary prevention of Chagas’ disease. Heart 95:524–534. doi: 10.1136/hrt.2008.159624. [DOI] [PubMed] [Google Scholar]
- 310.Alarcon A, Morgan M, Montgomery SP, Scavo L, Wong EC, Hahn A, Jantausch B. 2016. Diagnosis and treatment of congenital Chagas disease in a premature infant. J Ped Infect Dis 5:e28–e31. doi: 10.1093/jpids/piw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Centers for Disease Control and Prevention. 2012. Congenital transmission of Chagas disease—Virginia, 2010. MMWR Morb Mortal Wkly Rep 61:477–479. [PubMed] [Google Scholar]
- 312.Stimpert KK, Montgomery SP. 2010. Physician awareness of Chagas disease, USA. Emerg Infect Dis 16:871–872. doi: 10.3201/eid1605.091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Verani JR, Montgomery SP, Schulkin J, Anderson B, Jones JL. 2010. Survey of obstetrician-gynecologists in the United States about Chagas disease. Am J Trop Med Hyg 83:891–895. doi: 10.4269/ajtmh.2010.09-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sanchez DR, Traina MI, Hernandez S, Smer AM, Khamag H, Meymandi SK. 2014. Chagas disease awareness among Latin American immigrants living in Los Angeles, California. Am J Trop Med Hyg 91:915–919. doi: 10.4269/ajtmh.14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Forsyth CJ, Hernandez S, Flores CA, Roman MF, Nieto JM, Marquez G, Sequeira J, Sequeira H, Meymandi SK. 2018. “It's like a phantom disease”: patient perspectives on access to treatment for Chagas disease in the United States. Am J Trop Med Hyg 98:735–741. doi: 10.4269/ajtmh.17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Whitman JD, Bulman CA, Gunderson EL, Irish AM, Townsend RL, Stramer SL, Sakanari JA, Bern C. 2019. Chagas disease serological test performance in U.S. blood donor specimens. J Clin Microbiol 57:e01217-19. doi: 10.1128/JCM.01217-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gorlin J, Rossmann S, Robertson G, Stallone F, Hirschler N, Nguyen KA, Gilcher R, Fernandes H, Alvey S, Ajongwen P, Contestable P, Warren H. 2008. Evaluation of a new Trypanosoma cruzi antibody assay for blood donor screening. Transfusion 48:531–540. doi: 10.1111/j.1537-2995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 318.Guzmán-Gómez D, López-Monteon A, de la Soledad Lagunes-Castro M, Álvarez-Martínez C, Hernández-Lutzon MJ, Dumonteil E, Ramos-Ligonio A. 2015. Highly discordant serology against Trypanosoma cruzi in central Veracruz, Mexico: role of the antigen used for diagnostic. Parasit Vectors 8:466. doi: 10.1186/s13071-015-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sosa-Estani S, Gamboa-León MR, Del Cid-Lemus J, Althabe F, Alger J, Almendares O, Cafferata ML, Chippaux J-P, Dumonteil E, Gibbons L, Padilla-Raygoza N, Schneider D, Belizán JM, Buekens P. 2008. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg 79:755–759. doi: 10.4269/ajtmh.2008.79.755. [DOI] [PubMed] [Google Scholar]
- 320.Verani J, Seitz A, Gilman R, LaFuente C, Galdos-Cardenas G, Kawai V, de LaFuente E, Ferrufino L, Bowman N, Pinedo-Cancino V, Levy M, Steurer F, Todd C, Kirchhoff L, Cabrera L, Verastegui M, Bern C. 2009. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg 80:410–415. doi: 10.4269/ajtmh.2009.80.410. [DOI] [PubMed] [Google Scholar]
- 321.Herwaldt BL, Dougherty CP, Allen CK, Jolly JP, Brown MN, Yu P, Yu Y. 2018. Characteristics of patients for whom benznidazole was released through the CDC-sponsored investigational New Drug Program for Treatment of Chagas Disease—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 67:803–805. doi: 10.15585/mmwr.mm6729a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Doshi P. 2014. US incentive scheme for neglected diseases: a good idea gone wrong? BMJ 349:g4665. doi: 10.1136/bmj.g4665. [DOI] [PubMed] [Google Scholar]
- 323.Sunyoto T, Potet J, Boelaert M. 2018. Why miltefosine—a life-saving drug for leishmaniasis—is unavailable to people who need it the most. BMJ Glob Health 3:e000709. doi: 10.1136/bmjgh-2018-000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bennett C, Straily A, Haselow D, Weinstein S, Taffner R, Yaglom H, Komatsu K, Venkat H, Brown C, Byers P, Dunn J, Moncayo A, Mayes BC, Montgomery SP. 2018. Chagas disease surveillance activities—seven states, 2017. MMWR Morb Mortal Wkly Rep 67:738–741. doi: 10.15585/mmwr.mm6726a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Texas Department of State Health Services. 2018. Chagas disease data, 12/17/2018 revision. https://www.dshs.texas.gov/DCU/disease/chagas/Chagas-Disease-Data.aspx. Accessed 15 February 2019.
- 326.Texas Chagas Taskforce. 2018. Kissing bugs & Chagas disease: what you need to know. Texas Department of Health Services, San Antonio, TX: https://www.dshs.texas.gov/IDCU/disease/chagas/UT_Health_Kissing_Bug_Chagas_Guide-061918.pdf. [Google Scholar]
- 327.Oliveira I, Torrico F, Munoz J, Gascon J. 2010. Congenital transmission of Chagas disease: a clinical approach. Expert Rev anti Infect Ther 8:945–956. doi: 10.1586/eri.10.74. [DOI] [PubMed] [Google Scholar]
- 328.Alonso-Vega C, Billot C, Torrico F. 2013. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: results 2004–2009. PLoS Negl Trop Dis 7:e2304. doi: 10.1371/journal.pntd.0002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Edwards MS, Rench MA, Todd CW, Czaicki N, Steurer FJ, Bern C, Montgomery SP. 2015. Perinatal screening for Chagas disease in southern Texas. J Pediatric Infect Dis Soc 4:67–70. doi: 10.1093/jpids/pit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Stillwaggon E, Perez-Zetune V, Bialek SR, Montgomery SP. 2018. Congenital Chagas disease in the United States: cost savings through maternal screening. Am J Trop Med Hyg 98:1733–1742. doi: 10.4269/ajtmh.17-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sousa GR, Costa HS, Souza AC, Nunes MC, Lima MM, Rocha MO. 2015. Health-related quality of life in patients with Chagas disease: a review of the evidence. Rev Soc Bras Med Trop 48:121–128. doi: 10.1590/0037-8682-0244-2014. [DOI] [PubMed] [Google Scholar]
- 332.Meymandi S, Hernandez S, Park S, Sanchez DR, Forsyth C. 2018. Treatment of Chagas disease in the United States. Curr Treat Options Infect Dis 10:373–388. doi: 10.1007/s40506-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Traina M, Meymandi S, Bradfield JS. 2016. Heart failure secondary to Chagas disease: an emerging problem in non-endemic areas. Curr Heart Fail Rep 13:295–301. doi: 10.1007/s11897-016-0305-9. [DOI] [PubMed] [Google Scholar]
- 334.Meymandi SK, Hernandez S, Forsyth CJ. 2017. A community-based screening program for Chagas disease in the USA. Trends Parasitol 33:828–831. doi: 10.1016/j.pt.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 335.Texas A&M University. 2019. Kissing bugs and Chagas disease in the United States: interactive map. https://kissingbug.tamu.edu/Map/. Accessed 6 March 2019.
- 336.Usinger RL. 1944. The Triatominae of North and Central America and the West Indies and their public health significance. Public health bulletin no. 288 Federal Security Agency, US Public Health Service, Washington, DC. [Google Scholar]
- 337.Curtis-Robles R, Zecca IB, Roman-Cruz V, Carbajal ES, Auckland LD, Flores I, Millard AV, Hamer SA. 2017. Trypanosoma cruzi (agent of Chagas disease) in sympatric human and dog populations in “Colonias” of the Lower Rio Grande Valley of Texas. Am J Trop Med Hyg 96:805–814. doi: 10.4269/ajtmh.16-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hodo CL, Wilkerson GK, Birkner EC, Gray SB, Hamer SA. 2018. Trypanosoma cruzi transmission among captive nonhuman primates, wildlife, and vectors. Ecohealth 15:426–436. doi: 10.1007/s10393-018-1318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vandermark C, Zieman E, Boyles E, Nielsen CK, Davis C, Jimenez FA. 2018. Trypanosoma cruzi strain TcIV infects raccoons from Illinois. Mem Inst Oswaldo Cruz 113:30–37. doi: 10.1590/0074-02760170230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Herwaldt BL. 2001. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 14:659–688. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.FACT-JACIE. 2018. International standards for hematopoietic cellular therapy product collection, processing, and administration, 7th ed Foundation for the Accreditation of Cellular Therapy (FACT) and Joint Accreditation Committee—ISCT and EBMT, Omaha, NE: https://www.ebmt.org/sites/default/files/2018-06/FACT-JACIE%207th%20Edition%20Standards.pdf. [Google Scholar]
- 342.Guiang KM, Cantey P, Montgomery SP, Ailawadhi S, Qvarnstrom Y, Price T, Blodget E. 2013. Reactivation of Chagas disease in a bone marrow transplant patient: case report and review of screening and management. Transpl Infect Dis 15:E264–E267. doi: 10.1111/tid.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, GRADE Working Group. 2008. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. 2016. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol 72:45–55. doi: 10.1016/j.jclinepi.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 345.Woody NC, Woody HB. 1955. American trypanosomiasis (Chagas’ disease); first indigenous case in the United States. JAMA 159:676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]
- 346.Schiffler RJ, Mansur GP, Navin TR, Limpakarnjanarat K. 1984. Indigenous Chagas’ disease (American trypanosomiasis) in California. JAMA 251:2983–2984. doi: 10.1001/jama.1984.03340460061025. [DOI] [PubMed] [Google Scholar]
- 347.Ochs DE, Hnilica VS, Moser DR, Smith JH, Kirchhoff LV. 1996. Postmortem diagnosis of autochthonous acute chagasic myocarditis by polymerase chain reaction amplification of a species-specific DNA sequence of Trypanosoma cruzi. Am J Trop Med Hyg 54:526–529. doi: 10.4269/ajtmh.1996.54.526. [DOI] [PubMed] [Google Scholar]
- 348.Grant IH, Gold JW, Wittner M, Tanowitz HB, Nathan C, Mayer K, Reich L, Wollner N, Steinherz L, Ghavimi F. 1989. Transfusion-associated acute Chagas disease acquired in the United States. Ann Intern Med 111:849–851. doi: 10.7326/0003-4819-111-10-849. [DOI] [PubMed] [Google Scholar]
- 349.Gerseler PJ, Ito JI, Tegtmeier BR, Kerndt PR, Krance R. 1988. Fulminant Chagas disease in bone marrow transplantation, abstr 418. Abstr 27th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 350.Cimo PL, Luper WE, Scouros MA. 1993. Transfusion-associated Chagas’ disease in Texas: report of a case. Tex Med 89:48–50. [PubMed] [Google Scholar]
- 351.Leiby DA, Lenes BA, Tibbals MA, Tames-Olmedo MT. 1999. Prospective evaluation of a patient with Trypanosoma cruzi infection transmitted by transfusion. N Engl J Med 341:1237–1239. doi: 10.1056/NEJM199910143411615. [DOI] [PubMed] [Google Scholar]
- 352.Young C, Losikoff P, Chawla A, Glasser L, Forman E. 2007. Transfusion-acquired Trypanosoma cruzi infection. Transfusion 47:540–544. doi: 10.1111/j.1537-2995.2006.01147.x. [DOI] [PubMed] [Google Scholar]