Circular RNAs (circRNAs) are a class of noncoding RNAs produced by a noncanonical form of alternative splicing called back-splicing. To investigate a potential role of circRNAs in the p53 pathway, we analyzed RNA sequencing (RNA-seq) data from colorectal cancer cell lines (HCT116, RKO, and SW48) that were untreated or treated with a DNA-damaging agent. Surprisingly, unlike the strong p53-dependent induction of hundreds of p53-induced mRNAs upon DNA damage, only a few circRNAs were upregulated from p53-induced genes.
KEYWORDS: circRNA, p53, MDM2, CRC, cell cycle, DNA damage, circular RNA
ABSTRACT
Circular RNAs (circRNAs) are a class of noncoding RNAs produced by a noncanonical form of alternative splicing called back-splicing. To investigate a potential role of circRNAs in the p53 pathway, we analyzed RNA sequencing (RNA-seq) data from colorectal cancer cell lines (HCT116, RKO, and SW48) that were untreated or treated with a DNA-damaging agent. Surprisingly, unlike the strong p53-dependent induction of hundreds of p53-induced mRNAs upon DNA damage, only a few circRNAs were upregulated from p53-induced genes. circ-MDM2, an annotated circRNA from the MDM2 locus, was one of the handful of circRNAs that originated from a p53-induced gene. Given the central role of MDM2 in suppressing p53 protein levels and p53 activity, we investigated the function of circ-MDM2. Knocking down circ-MDM2 with small interfering RNAs (siRNAs) that targeted circ-MDM2 did not alter MDM2 mRNA or MDM2 protein levels but resulted in increased basal p53 levels and growth defects in vitro and in vivo. Consistent with these results, transcriptome profiling showed increased expression of several direct p53 targets, reduced retinoblastoma protein (Rb) phosphorylation, and defects in G1-S progression upon silencing circ-MDM2. Our results on the initial characterization of circ-MDM2 identify a new player from the MDM2 locus that suppresses p53 levels and cell cycle progression.
INTRODUCTION
The transcription factor p53 is a major tumor suppressor, mutated in more than half of all human cancers (1, 2). p53-null mice develop spontaneous tumors within 6 months, and patients with Li-Fraumeni syndrome having germ line mutations in p53 are predisposed to cancers (3, 4). In response to various stress signals, including DNA damage, p53 induces cell cycle arrest, apoptosis, and senescence (5, 6). p53 mediates its effects by enhancing the transcription of hundreds of protein-coding genes and noncoding RNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) (7–17). Given the central role of p53 in the stress response, it is plausible that the outcome of p53 activation is also mediated through the recently discovered circular RNAs (circRNAs), since some are differentially expressed during the stress response (18–21).
circRNAs form a covalently closed continuous loop and contain scrambled exons. These scrambled exons are exons of an mRNA transcript that are not in the same order as their positions in the genomic DNA. The existence of scrambled exons was reported in the early 1990s; however, they were believed to be the by-products of aberrant splicing (22–24). Recent advancements in genomics approaches, including RNA sequencing (RNA-seq) of nonpolyadenylated transcripts or a prior treatment of total RNA with RNase R, an enzyme that degrades linear RNAs but not circRNAs, have uncovered the existence of thousands of circRNAs in the mammalian genome (25–28). circRNAs are often expressed at low levels but are more stable and occasionally more abundant than their associated linear mRNAs (25, 29–31).
Most circRNAs originate from an alternative form of splicing known as back-splicing in which a downstream 5′ splice site interacts with an upstream 3′ splice site and forms a covalently closed circle (24, 27, 29). The mechanisms underlying these events are an area of active research; it has been shown that exon circularization requires the spliceosome machinery and canonical splice sites (32). circRNAs are generated cotranscriptionally, and in some cases, their production depends on the flanking intronic sequences (33). RNA binding proteins (RBPs) also play an important role in circRNA biogenesis. For example, splicing factor musclebind (MBNL1), FUS, and quacking (QKI) facilitate circularization (33–35), whereas the RNA-editing enzyme ADAR1 inhibits circRNA formation (36). The molecular and cellular functions of only a handful of circRNAs have been established. For example, ciRS-7 (26, 37), circ-Sry (38), circ-Foxo3 (39, 40), circ-RasGEF1B (41), circ-ANRIL (20), and circ-PVT1 (42), have been shown to act by sponging or interacting with miRNAs or RBPs to regulate gene splicing and transcription.
In this study, we set out to identify circRNAs induced by DNA damage in multiple colorectal cancer (CRC) cell lines. We focused on a previously uncharacterized circRNA, circ-MDM2, that originates from MDM2, a direct transcriptional target of p53 and an established regulator of p53 (43–46). We showed that circ-MDM2 regulates basal p53 levels, cell proliferation in vitro, and tumor growth in vivo. Together, our results on the initial characterization of circ-MDM2 uncover a negative feedback loop between circ-MDM2 and p53.
RESULTS
Genome-wide identification of DNA damage-regulated circRNAs.
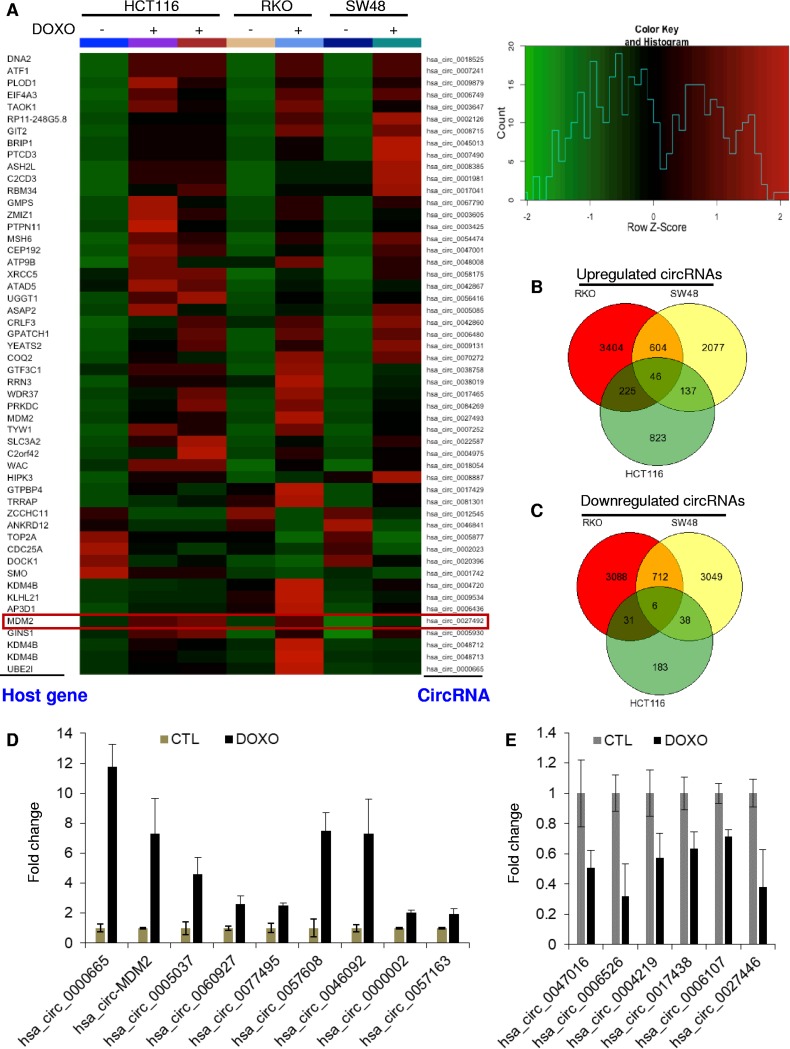
To identify circRNAs whose expression was altered upon DNA damage, we analyzed our recently published RNA-seq data from three p53 wild-type (WT) CRC lines (HCT116, RKO, and SW48 cells), untreated or treated with the DNA-damaging agent doxorubicin (DOXO; 300 nM) for 16 h (12). The TopHat-Fusion (version 2.1.0) program was used to find the fusion junctions, and following the identification of fused junctions, the CIRCexplorer program (version 1.10) was utilized to identify which of these fused junctions can circularize to form circular junctions. The identified circularizing junctions were annotated using circBase identifiers (IDs). Using a cutoff of a ≥2-fold change and minimum of 1 read, 52 annotated circRNAs (Fig. 1A; also see Tables S1 to S3 in the supplemental material) were differentially expressed upon DOXO treatment in all three lines; 46 were upregulated (Fig. 1B), and 6 were downregulated (Fig. 1C). When we used a cutoff minimum of 2 reads in all three cell lines, the levels of 27 circRNAs changed upon DOXO treatment; 22 were upregulated, and 5 were downregulated (Table S3, top part). We validated upregulation of 9 circRNAs (Fig. 1D) and downregulation of 6 circRNAs (Fig. 1E) from a subset of differentially expressed circRNAs by quantitative reverse transcription-PCR (qRT-PCR) from HCT116 cells, untreated or treated with DOXO for 16 h, using divergent primers to amplify the circularized junctions. It should be noted that regardless of the cutoff used, the changes in levels of specific circRNAs in all three cell lines upon DOXO treatment (Fig. 1A) should be used with caution because we did not have triplicate samples for each cell line.
FIG 1.
Genome-wide identification of DNA damage-inducible circRNAs from multiple CRC lines. (A) A heat map (left panel) is shown for the differentially expressed circRNAs identified by RNA-seq from HCT116, RKO, and SW48 cells untreated or treated with DOXO for 16 h. Upregulated genes are shown in red, and downregulated genes are shown in green. circ-MDM2 (hsa_circ_0027492) is shown in the red box. The scale for the heat map is shown in the upper right panel. (B and C) Venn diagram shows the number of all the circRNAs upregulated ≥2-fold and downregulated ≤2-fold after DOXO treatment of HCT116, RKO, and SW48 cells and the overlap between the three CRC cell lines. (D and E) qRT-PCR analysis from HCT116 cells untreated or treated with DOXO for 16 h. Error bars represent standard deviations from three independent experiments.
To determine if the induction of a subset of the upregulated circRNAs was p53 dependent, we analyzed the host genes of the 46 upregulated circRNAs. We found that the associated host genes of five circRNAs (hsa_circ_0027492, hsa_circ_0027493, hsa_circ_0048713, hsa_circ_0048712, and hsa_circ_0004720) are direct transcriptional targets of p53 by examining the intersection of the 46 upregulated circRNAs with p53 Global Run-on sequencing (GRO-seq) data (47). Of these, two circRNAs (hsa_circ_0027492 and hsa_circ_0027493) originated from the MDM2 locus, and three circRNAs (hsa_circ_0048713, hsa_circ_0048712, and hsa_circ_0004720) originated from the KDM4B locus. Interestingly, among the 46 circRNAs, we did not find a circRNA originating from other well-established p53 targets such as p21, GDF15, and TP53I3. Although there are annotated circRNAs from these p53 targets, either their expression was undetectable in our RNA-seq data or they were not induced after DOXO treatment (Tables S1 and S2).
MDM2 is a direct transcriptional target of p53 and also the primary negative regulator of p53 levels and activity (48, 49). Given the central role of MDM2 in regulating p53, we decided to investigate a potential role of circ-MDM2 (hsa_circ_0027492) in the p53 network. In addition, circ-MDM2 was more abundant than hsa_circ_0027493 (Table S3).
circ-MDM2 is resistant to RNase R and is induced in a p53-dependent manner.
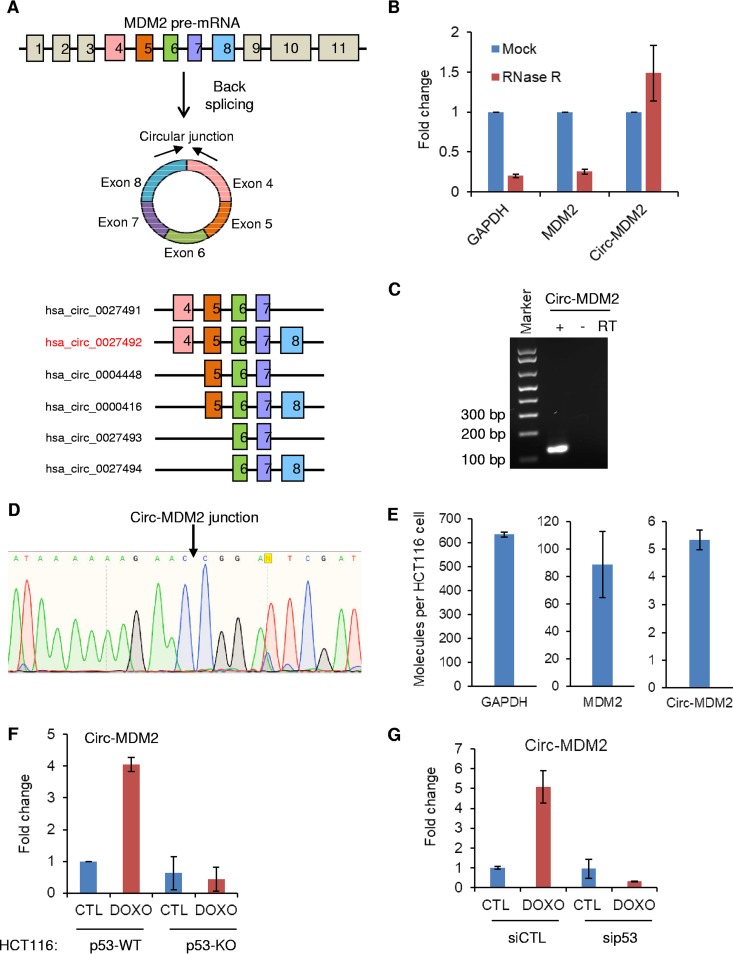
According to circBase (http://www.circbase.org/) (50), spliced circ-MDM2 RNA is 510 nucleotides long and consists of five exons. circ-MDM2 is formed by back-splicing of exon 8 to exon 4 of MDM2 transcript annotated as GenBank accession number NM_002392 that has 11 exons (Fig. 2A). In our screen, we identified multiple circRNAs which are derived from the same locus as circ-MDM2, several of which share the same back-spliced exon junction (Fig. 2A and Tables S1 to S3). To validate the expression of circ-MDM2 in HCT116 cells, total RNA was treated with RNase R, an enzyme that degrades linear RNAs but not the covalently closed circRNAs. The levels of circ-MDM2, MDM2 mRNA, and GAPDH mRNA were evaluated by qRT-PCR (Fig. 2B). In response to RNase R treatment, GAPDH and MDM2 mRNAs were strongly reduced. However, circ-MDM2 levels remained unaltered (Fig. 2B). When the circ-MDM2 qRT-PCR product (Fig. 2C) was subjected to Sanger sequencing, the sequence matched the sequence of the annotated circ-MDM2 junction (Fig. 2D), further demonstrating that circ-MDM2 is a bona fide circRNA. Moreover, we conducted a digital droplet PCR (ddPCR) assay and estimated that ∼5 circ-MDM2 molecules are expressed per HCT116 cell (Fig. 2E). As expected, in HCT116 cells, MDM2 mRNA was more abundant than circ-MDM2 and expressed at ∼90 molecules per cell whereas the housekeeping GAPDH mRNA was expressed at ∼ 635 molecules per cell (Fig. 2E).
FIG 2.
circ-MDM2 is upregulated in a p53-dependent manner in response to DNA damage. (A) The upper panel shows a pictorial representation of MDM2 pre-mRNA. Boxes represent annotated exons. circ-MDM2 as shown is formed by back-splicing of exon 8 to exon 4. In the lower panel, exons and introns in the MDM2 pre-mRNA corresponding to other circRNAs from the MDM2 locus are shown. (B) qRT-PCR analysis was performed for GAPDH mRNA, MDM2 mRNA, and circ-MDM2 from HCT116 cells, untreated or treated with RNase R. (C) The circ-MDM2 qRT-PCR product, with or without reverse transcriptase (RT), was visualized by electrophoresis by being run on an agarose gel. (D) Sequence of the circ-MDM2 junction amplified by qRT-PCR was determined by Sanger sequencing. The arrow indicates the junction. (E) Digital droplet PCR results showing the number of molecules per HCT116 cell for GAPDH, MDM2 mRNA, and circ-MDM2, as indicated. Graphs represent the average of three RNA samples from different passages. (F) qRT-PCR analysis from p53-WT and isogenic p53-KO HCT116 cells untreated or treated with DOXO for 16 h. (G) qRT-PCR analysis from HCT116 cells transfected with CTL siRNA or p53 siRNAs untreated or treated with DOXO for 16 h. Error bars represent standard deviations from three independent experiments.
Because circ-MDM2 originates from the p53-regulated MDM2 locus, we reasoned that the expression of circ-MDM2 is p53 dependent. Therefore, we performed qRT-PCR from isogenic WT p53 and p53 knockout (KO) HCT116 cells in the presence or absence of DOXO. In response to DNA damage, we observed significant upregulation of circ-MDM2 in WT p53 HCT116 cells but not in p53 KO HCT116 cells, suggesting that circ-MDM2 is induced in a p53-dependent manner (Fig. 2F). A similar p53-dependent upregulation of circ-MDM2 was observed upon transient knockdown of p53 with small interfering RNAs (siRNAs) upon DOXO treatment (Fig. 2G). circ-MDM2 upregulation in response to DNA damage was also observed in the osteosarcoma cell lines U2OS and SJSA (Fig. 3A and B) and in normal human fibroblast BJ cells (Fig. 3C), suggesting that the induction of circ-MDM2 is not restricted to CRC cells. SJSA cells have 25-fold amplification of MDM2 (51). We therefore measured the expression of MDM2 mRNA and circ-MDM2 in U2OS and SJSA cells. By comparing the expression to that of GAPDH mRNA, we found that MDM2 mRNA was more abundant in SJSA cells than in U2OS cells (Fig. 3D). Furthermore, we observed ∼24-fold-higher levels of circ-MDM2 in SJSA cells than in U2OS cells (Fig. 3E). The levels of the control (CTL) β-actin mRNA were similar among these two cell lines (Fig. 3F). In these experiments, we noticed that in SJSA cells, although MDM2 mRNA was ∼2-fold less abundant than GAPDH mRNA (Fig. 3D), circ-MDM2 was ∼900-fold less abundant than GAPDH mRNA (Fig. 3E). These data are consistent with the notion that circRNAs are generally expressed at much lower levels than the host gene mRNA (25, 27, 29, 50). Next, qRT-PCR from nuclear and cytoplasmic fractions of HCT116 cells (Fig. 3G) indicated that the majority (>80%) of circ-MDM2 is cytoplasmic. The nuclear enriched MALAT1 and cytoplasmic GAPDH mRNA were used as controls.
FIG 3.
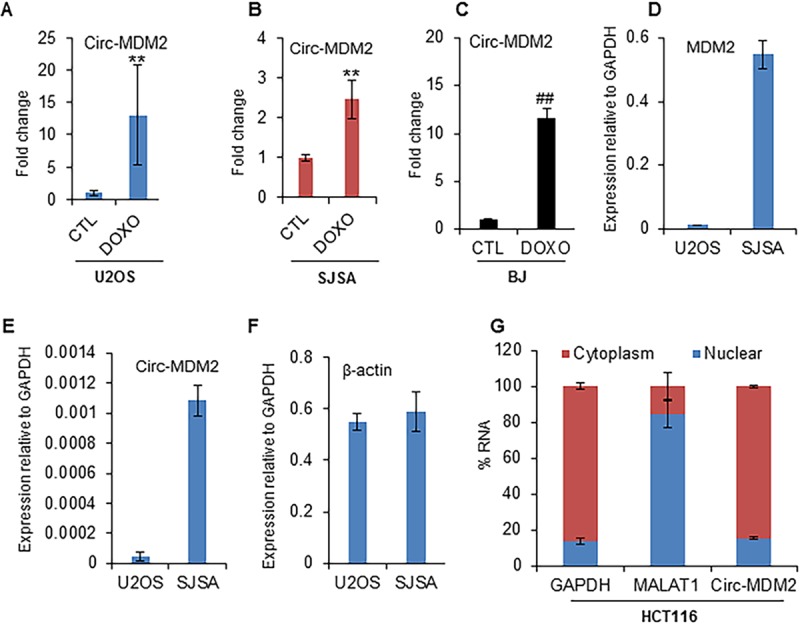
circ-MDM2 is cytoplasmic and induced in multiple cell types in response to DNA damage. (A to C) qRT-PCR analysis from osteosarcoma U2OS cells, SJSA cells, and normal human diploid fibroblast BJ cells, as indicated, untreated or treated with DOXO for 16 h. (D to F) The relative abundance of MDM2 mRNA, circ-MDM2 RNA, and β-actin mRNA, as indicated, relative to that of GAPDH mRNA, as measured by qRT-PCR. (G) qRT-PCR analysis from nuclear and cytoplasmic fractions of HCT116 cells; the cytoplasmic GAPDH mRNA and the nuclear lncRNA MALAT1 were used as controls. Error bars represent standard deviations from independent experiments. #, P < 0.01; **, P < 0.005; ##, P < 0.001.
Silencing circ-MDM2 increased p53 levels and reduced E2F targets.
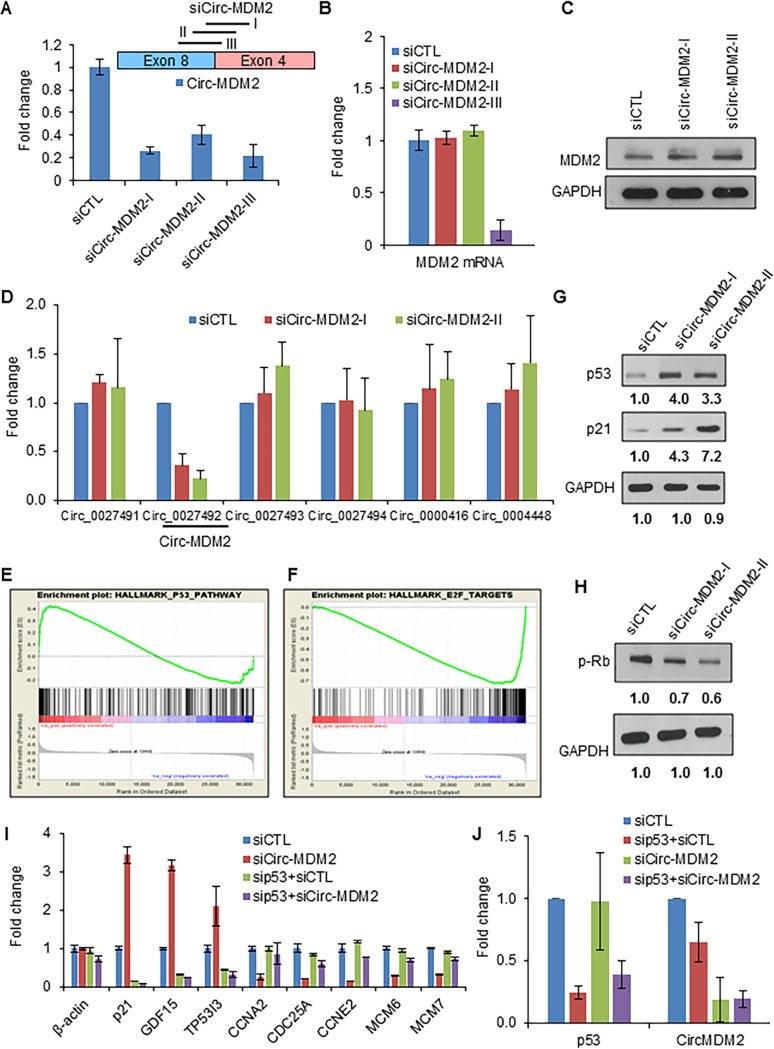
Due to the p53-dependent upregulation of circ-MDM2 in response to DNA damage, we hypothesized that circ-MDM2 functions as a downstream effector of p53. To begin to test this hypothesis, we knocked down circ-MDM2 using three independent siRNAs that target the circular junction. Although all three siRNAs significantly knocked down circ-MDM2 by ∼70 to 80% (Fig. 4A), only two siRNAs, siCirc-MDM2-I and siCirc-MDM2-II, had no effect on MDM2 mRNA (Fig. 4B) or MDM2 protein levels (Fig. 4C). Due to the nonspecific effect of siCirc-MDM2-III (Fig. 4B), this siRNA was excluded from further experiments.
FIG 4.
circ-MDM2 suppresses basal p53 levels. (A, top) Pictorial representation of three siRNAs targeting the circ-MDM2 junction. (Bottom) HCT116 cells were transfected with siCTL or circ-MDM2 siRNA for 48 h, and circ-MDM2 knockdown efficiency was evaluated by qRT-PCR. (B) HCT116 cells were transfected with siCTL or circ-MDM2 siRNA for 48 h, and the effect on MDM2 mRNA was determined by qRT-PCR. (C) Immunoblotting shows that siCirc-MDM2-I and -II do not alter MDM2 protein levels. GAPDH was used as a loading control. (D) qRT-PCR analysis from HCT116 cells transfected with CTL siRNA or circ-MDM2 siRNA to determine the effect on the levels of other circRNAs derived from the MDM2 locus. (E and F) Gene set enrichment analysis (GSEA) for the upregulated genes (E) and downregulated genes (F) in the microarrays from untreated HCT116 cells transfected with siCTL or circ-MDM2 siRNA. The normalized enrichment scores were 1.70 and −2.56 for the p53 pathway and E2F targets, respectively. The FDR q values were 0.010 and 0.000 for the p53 pathway and E2F targets, respectively. (G and H) Immunoblot analysis for p53, p21, phosphorylated Rb, and the loading control GAPDH, as indicated, was performed from HCT116 cells transfected with siCTL or circ-MDM2 siRNA. (I and J) qRT-PCR analysis from HCT116 cells transfected with siCTL, circ-MDM2 siRNA, p53 siRNA, or combinations of these siRNAs. Error bars represent standard deviations from three independent experiments.
Since there are several circRNAs derived from the MDM2 locus, it is not surprising that circ-MDM2 shares some of the back-spliced junction and exonic sequences with multiple circRNAs expressed in our tested cell lines and derived from the same locus (Fig. 2A). This raised the concern that the siRNAs used to knock down circ-MDM2 may be targeting other circ-MDM2 isoforms as well. To exclude the possibility of off-target effects of the siRNAs, we examined the levels of other circRNAs from the MDM2 locus upon transfection of HCT116 cells with the two circ-MDM2 siRNAs we had used (Fig. 4A) to specifically knock down circ-MDM2. The result from this experiment showed that the other five circ-MDM2 isoforms did not change significantly, suggesting that our siRNAs were specific to circ-MDM2 (Fig. 4D).
circRNAs regulate gene expression (26, 37, 38, 52). To determine if circ-MDM2 could play a role in regulating gene expression, we performed mRNA microarrays from HCT116 cells transfected with CTL siRNA or circ-MDM2 siRNA. Using a false-discovery rate (FDR) cutoff (adjusted P value of < 0.05), we found that 1,725 genes were upregulated and that 1,638 genes were downregulated upon knockdown of circ-MDM2 (Table S4); as expected, the levels of MDM2 mRNA did not change significantly. Interestingly, in our microarrays we also found that some E2F targets were significantly downregulated after circ-MDM2 depletion in untreated cells (Table S4). Gene set enrichment analysis (GSEA) of circ-MDM2-depleted cells versus CTL cells indicated significant enrichment of the p53 pathway in the upregulated genes and significant enrichment of the E2F pathway in the downregulated genes upon depletion of circ-MDM2 (Fig. 4E and F). Consistent with these data, we found that depletion of circ-MDM2 resulted in increased basal p53 levels (Fig. 4G) and upregulation of p21 (Fig. 4G), suggesting that circ-MDM2 negatively regulates p53. Because p21 is known to block retinoblastoma protein (Rb) phosphorylation, we next examined the effects on Rb phosphorylation by immunoblotting. We found that depletion of circ-MDM2 resulted in a decrease in Rb phosphorylation (Fig. 4H).
We further validated these findings by knocking down circ-MDM2 and performing qRT-PCR for several p53 and E2F targets in HCT116 cells. Transient knockdown of circ-MDM2 resulted in significant upregulation of p53 targets such as p21, GDF15, and TP53I3 and downregulation of E2F targets such as CCNA2, CCNE2, MCM6, and MCM7 (Fig. 4I). Concurrent knockdown of circ-MDM2 and p53 mitigated gene expression changes upon circ-MDM2 knockdown only, suggesting that the observed circ-MDM2-mediated effects are p53 dependent (Fig. 4I). Simultaneous knockdown of circ-MDM2 and p53 downregulated circ-MDM2 and p53 levels as efficiently as when these siRNAs were used independently (Fig. 4J).
circ-MDM2-mediated effects are p53 dependent.
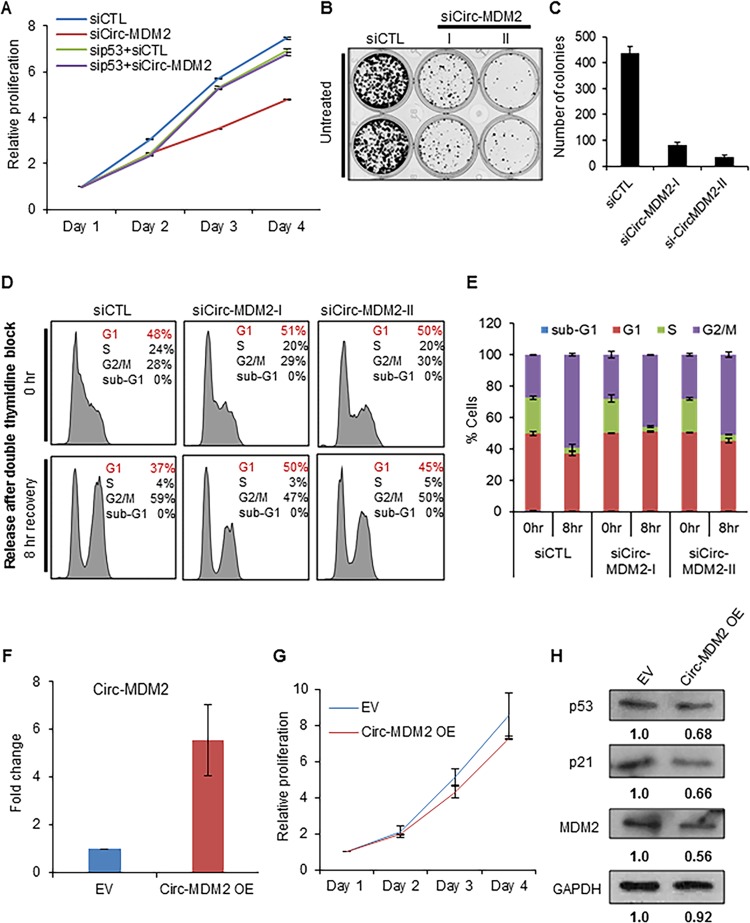
To determine the functional consequences of circ-MDM2 depletion, we first performed cell proliferation assays because we found increased p53 levels upon knockdown of circ-MDM2. We found that circ-MDM2-depleted cells showed significant growth defects compared to growth of CTL siRNA transfected cells (Fig. 5A). The reduced proliferation was p53 dependent because concurrent knockdown of p53 and circ-MDM2 completely rescued the proliferation defect (Fig. 5A). Moreover, we found that circ-MDM2-depleted cells showed significant growth defects in long-term colony formation assays compared to growth of the CTL siRNA transfected cells (Fig. 5B and C).
FIG 5.
circ-MDM2 knockdown alters cell proliferation and cell cycle progression. (A) Cell proliferation assays at the indicated time points were performed from untreated HCT116 cells transfected with siCTL, circ-MDM2 siRNA, or p53 siRNA 24 h after the cells were seeded in 96-well plates. (B and C) HCT116 cells were transfected with siCTL or circ-MDM2 siRNA for 48 h; cells were then trypsinized and seeded, and colony formation assays were performed after 10 days. (D and E) HCT116 cells were transfected with siCTL or circ-MDM2 siRNA, and after 48 h double-thymidine block assays were performed. After an 8-h recovery, the effect on cell cycle was assessed by performing propidium iodide (PI) staining followed by flow cytometry analysis (FACS). (F) qRT-PCR from HCT116 cells stably overexpressing circ-MDM2 (OE) compared to that of empty vector (EV) shows upregulation of circ-MDM2 levels. GAPDH was used as a loading control. (G) Cell proliferation assays at the indicated time points were performed from HCT116 cells stably overexpressing circ-MDM2 24 h after the cells were seeded in 96-well plates. (H) Immunoblotting was performed for p53, p21, and MDM2 from circ-MDM2 OE cells or empty vector cells. GAPDH was used as a loading control. Error bars represent standard deviations from three independent experiments.
Given the role of E2Fs in cell cycle progression (53–55) and the observed decrease in Rb phosphorylation upon circ-MDM2 depletion, we next determined if circ-MDM2 regulates G1-to-S progression. To test this, we transfected HCT116 cells with CTL or circ-MDM2 siRNAs and synchronized the cells in the G1/S boundary by double-thymidine block. Then, fresh medium was added to allow the cells to progress to S and G2/M phases of the cell cycle. When we performed propidium iodide (PI) staining followed by fluorescence-activated cell sorter (FACS) analysis, we found that in the CTL cells ∼60% of the cells reached G2/M phase after 8 h of recovery. However, in the circ-MDM2-depleted cells ∼47 to 50% of cells progressed into G2/M (Fig. 5D and E). In addition, after 8 h of recovery from the double-thymidine block, in the CTL cells the G1 population decreased from 48% to 37%, but in the circ-MDM2-depleted cells, there was less of a decrease in the G1 population (from 51% to 50% for siCirc-MDM2-I and from 50% to 45% for siCirc-MDM2-II). These data indicate that circ-MDM2 plays an important role in cell cycle progression from G1 to S and into G2/M.
Next, to determine the effects of overexpression (OE) of circ-MDM2, we conducted experiments in HCT116 cells that stably overexpress circ-MDM2 (see Materials and Methods for details of OE). Using qRT-PCR, we observed >5-fold OE of circ-MDM2 in HCT116 cells stably overexpressing circ-MDM2 (Fig. 5F). In contrast to results with our siRNA experiments, when we performed cell proliferation assays from empty vector and circ-MDM2 OE cells, we did not observe a significant change in cell proliferation upon circ-MDM2 OE (Fig. 5G). However, consistent with results of our circ-MDM2 siRNA experiments, we observed a modest decrease in p53, p21, and MDM2 protein levels upon circ-MDM2 OE (Fig. 5H). It may be that the modest decreases in p53 and p21 levels are not sufficient to provide growth advantage to the cells in this short time course experiment.
Loss of circ-MDM2 results in impaired tumor growth in vivo.
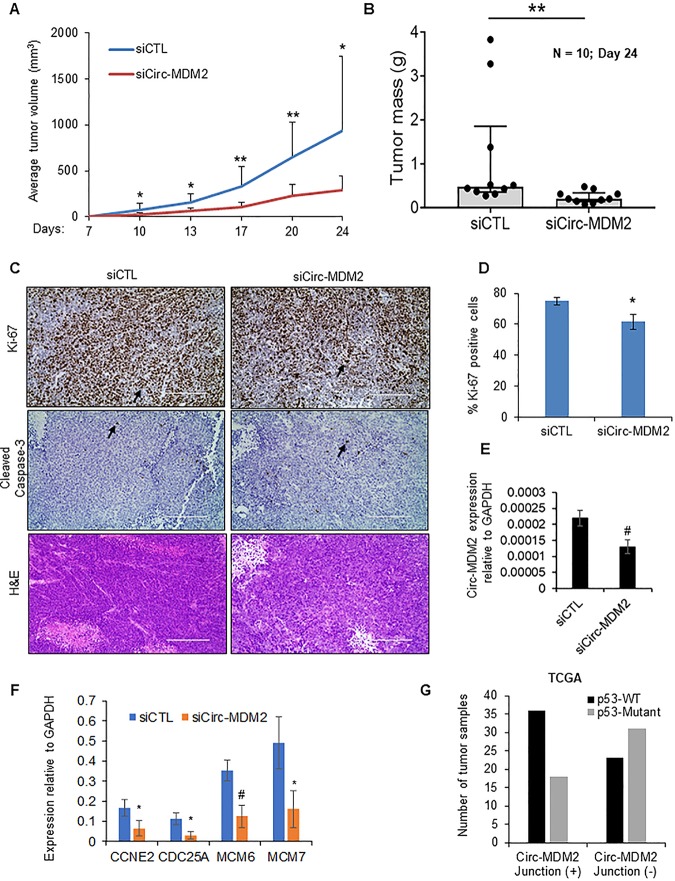
To investigate the effects of circ-MDM2 depletion in an in vivo setting, we subcutaneously injected nude mice with HCT116 cells transfected with CTL siRNA or circ-MDM2 siRNA for 48 h and examined the effect on tumor growth. We found that depletion of circ-MDM2 significantly reduced the rate of xenograft growth over a period of 4 weeks (Fig. 6A and B). The difference in tumor volumes between the siCTL and siCirc-MDM2 tumors was ∼2-fold (P < 0.05) at 13 days after injection and increased to ∼3-fold (P < 0.005) after 24 days (Fig. 6A). Immunohistochemical staining of the tumors for the proliferation marker Ki67 revealed that the siCirc-MDM2 tumors had ∼18% decreased Ki67-positive cells (Fig. 6C and D) compared to levels in siCTL tumors, suggesting that the observed reduced tumor volume is mediated, in part, by inhibition of cell proliferation. For the apoptosis marker, cleaved caspase-3, no significant staining (<1%) was observed in either CTL tumors or circ-MDM2-depleted tumors (Fig. 6C). We also performed qRT-PCR from total RNA extracted from three control and three circ-MDM2 knockdown tumors at the endpoint (24 days after injecting the cells into mice) and observed a significant downregulation of circ-MDM2 levels (Fig. 6E). This was a surprising result, but it may be that the circ-MDM2 siRNAs were not completely lost in this in vivo experiment due to the decreased proliferation of cells transfected with circ-MDM2 siRNA. Additionally, although due to limited material we were unable to compare p53 protein levels by immunoblotting between the tumors from CTL siRNA- and circ-MDM2 siRNA-injected cells, we observed significant downregulation of some E2F targets (CCNE2, CDC25A, MCM6, and MCM7) in the circ-MDM2 siRNA-injected cells by the qRT-PCR (Fig. 6F). Together, these results indicate that circ-MDM2 plays a role in facilitating tumor cell growth in vivo.
FIG 6.
circ-MDM2 depletion results in reduced tumor growth in vivo. (A and B) HCT116 cells were transfected with siCTL or siCirc-MDM2, and after 48 h, cells were injected subcutaneously into the flanks of athymic nude mice (five mice for each group, two tumors per mouse). Average tumor volume and tumor mass are shown. (C) Immunohistochemical staining of the siCTL and siCirc-MDM2 tumors for the proliferation marker Ki67 and apoptosis marker cleaved caspase-3 and hematoxylin and eosin staining. Arrows indicate cells positive for Ki67 or cleaved caspase-3. (D) Quantitation of Ki67-positive cells is shown. Error bars represent standard deviations from three different tumor samples. (E and F) qRT-PCR was performed from tumors (n = 3) isolated from mice 24 days after injecting HCT116 cells transfected with siCTL or siCirc-MDM2. RNA levels were normalized to the level of GAPDH. (G) Correlation of circ-MDM2 occurrence and the p53 mutation status among TCGA COAD samples. *, P < 0.05; #, P < 0.01, **, P < 0.005.
Next, we analyzed the expression of circ-MDM2 in patients with colon adenocarcinoma (COAD). We searched for the circ-MDM2 junction across 411 COAD samples from The Cancer Genome Atlas (TCGA) and identified 283 hits across 173 (159 tumor and 14 normal) COAD TCGA IDs (Table S5). To determine if circ-MDM2 occurrence correlates with p53 mutations, we compared circ-MDM2 occurrences between WT p53 and mutant p53 tumors. Of the 108 tumor samples, 59 had WT p53, and 49 had missense or nonsense p53 mutations. We found that the occurrence of the circ-MDM2 junction was higher in WT p53 tumors than in mutant p53 tumors (odds ratio [OR], 2.67; P = 0.019, Fisher’s exact test) (Fig. 6G and Table S6). These data are consistent with the regulation of circ-MDM2 by p53 in CRC cell lines. Taken together, our results suggest that the MDM2 locus suppresses basal p53 levels and maintains cellular homeostasis via circ-MDM2, a novel circRNA in the p53 network.
DISCUSSION
In this study, we report the initial characterization of circ-MDM2, a circRNA originating from MDM2 pre-mRNA and induced in response to DNA damage in a p53-dependent manner. Our loss-of-function data show that circ-MDM2 suppresses basal p53 levels, implying an autoregulatory feedback loop.
circ-MDM2 is not the only circRNA that regulates cell cycle progression and p53 levels. Recent studies have reported roles of circRNAs in cell cycle progression, apoptosis, senescence, and p53 regulation. O’Leary et al. reported circRNAs that are differentially expressed in response to ionizing radiation and identified two circRNAs, KIRKOS-71 and KIRKOS-73, that originated from the gene WWOX, a tumor suppressor and a p53 regulator (19). circ-Foxo3 is another example of a circRNA that regulates p53 levels. circ-Foxo3 forms a ternary complex with p21 and CDK2 and functions as a negative regulator of cell cycle progression (18). Interaction of circ-Foxo3 with antisenescence proteins ID-1 and E2F1 causes their retention into the cytoplasm and thus results in increased cellular senescence (40). Likewise, circ-PVT1, a circRNA from the lncRNA PVT1, has been shown to regulate p53 levels and senescence (42). Additionally, circ-PVT1 has recently been shown to play an oncogenic role in head and neck cancer in which the mutant p53/YAP/TEAD complex regulates circ-PVT1 transcription (56). Our study expands this growing list of circRNAs in the p53 pathway.
Our loss-of-function experiments show that circ-MDM2 suppresses basal p53 levels and results in decreased proliferation and tumor growth in mice. Conversely, we found that ectopic OE of circ-MDM2 resulted in a modest decrease in p53, p21, and MDM2 protein levels, but we did not observe an effect on cell proliferation. It would be interesting to determine if circ-MDM2 OE has an effect on these cells in long-term proliferation assays and in vivo in mice. That said, the molecular mechanisms that govern the regulation of p53 by circ-MDM2 remain to be thoroughly investigated. Very little is known about the mechanisms of action of circRNAs. Some circRNAs, such as the extensively characterized CDR1as, act as miRNA sponges. CDR1as has many binding sites to miRNA 7 (130 and 73 in mouse and humans, respectively) and dampens neuronal activity (26, 37, 52). Other circRNAs such as SRY (26, 37) and circ-HIPK3 (57) have also been shown to act as miRNA sponges in neuronal tissues and in human cancers, respectively. Because circ-MDM2 is predominantly cytoplasmic, it has the potential to act by sponging specific miRNAs. However, based on the number of reads of circ-MDM2 in our RNA-seq data and the relative expression level in our qRT-PCR and absolute number of molecules of circ-MDM2 per cell (∼5 molecules per HCT116 cell), circ-MDM2 is likely to be expressed at levels that are substantially lower than those of the miRNAs and/or RBPs that it could bind to in a population of cells. However, it may be the case that some cells in the cell population may express higher levels of circ-MDM2, making it a potential regulator of miRNAs and/or RBPs. This could be assessed in the future by single-cell RNA-seq. Nevertheless, the observed low expression of circ-MDM2 is consistent with the notion that circRNAs are generally expressed at low levels (25, 26, 29, 30). One might argue that, like other circRNAs (58), circ-MDM2 is resistant to exonucleases as it lacks free ends. Therefore, circ-MDM2 should presumably be a very stable molecule, and this feature could make it more potent. However, we believe that despite these features, identifying the factors (miRNAs or RBPs) that bind to endogenous circ-MDM2 in a cell lysate will be extremely challenging experimentally due to the low abundance of this circRNA. We also considered the possibility that circ-MDM2 could mediate its effects via an encoded polypeptide. However, unlike the recently discovered protein-coding circ-ZNF609 (59), it is likely that circ-MDM2 is truly noncoding based on our coding potential analysis of circ-MDM2 using CPAT (http://lilab.research.bcm.edu/cpat/index.php).
Finally, in addition to the yet unknown factors that interact with endogenous circ-MDM2 to suppress basal p53 levels, we noticed that in addition to MDM2 mRNA, the mRNA levels of other key regulators of p53, such as Wip1/PPM1D (60) and PTEN (61), did not change significantly in our gene expression microarray data obtained after knockdown of circ-MDM2. An important though technically challenging question that needs to be resolved in the future is the molecular mechanism(s) by which circ-MDM2 regulates p53 levels. Our insights on the regulation of basal p53 levels by circ-MDM2 help lay the foundation to address these important questions in future investigations to better understand how circRNAs, an enigmatic class of noncoding RNAs, function in normal physiology and human diseases.
MATERIALS AND METHODS
Cell culture, treatments, and siRNA transfections.
All cell lines used in this study were purchased from the ATCC; colorectal cancer cell lines HCT116 (ATCC CCL-247), SW48 (ATCC CCL-231), and RKO (ATCC CRL-2577) cells, osteosarcoma cell lines U2OS (ATCC HTB-96) and SJSA (ATCC CRL-209), and normal human fibroblasts BJ (ATCC CRL-2522) cells were purchased from the ATCC. Isogenic p53 WT and p53-KO HCT116 lines were previously generated by Bert Vogelstein’s lab (Johns Hopkins University). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin at 37°C in 5% CO2. All cell lines were routinely checked for mycoplasma using a Venor Gem Mycoplasma detection kit (MP0025-1KT; Sigma-Aldrich). For the experiments in Fig. 1 to 3, cells were treated with 300 nM doxorubicin (DOXO; catalog no. D1515) for the indicated time.
The Allstars Negative (CTL) siRNAs were purchased from Qiagen, and siRNAs for p53 (L-003329-00; SMARTpool siRNAs), for MDM2 (L-003279-00; SMARTpool siRNAs), for circ-MDM2 (custom siRNAs; siCirc-MDM2-I-sense, 5′-AUCCGGUUCUUUUUUAUCUUGdTdT-3′, and antisense, 5′-CAAGAUAAAAAAGAACCGGAUdTdT-3′; siCirc-MDM2-II-sense, 5′-GAAUCCGGUUCUUUUUUAUCUdTdT-3′, and antisense, 5′-AGAUAAAAAAGAACCGGAUUCdTdT-3′; and siCirc-MDM2-III-sense, 5′-GACGCCAUCGAAUCCGGUUCUdTdT-3′, and antisense, 5′-AGAACCGGAUUCGAUGGCGUCdTdT 3′), and p53 (L-003329-005; SMARTpool siRNAs) were purchased from Dharmacon. All siRNA transfections were performed by reverse transfection at a final concentration of 20 nM, unless mentioned otherwise, using Lipofectamine RNAiMAX (Life technologies) as directed by the manufacturer. All reverse transfections were performed for 48 h.
For circRNA overexpression, we cloned the genomic sequences including exons 4 to 8 of the MDM2 locus into pcDNA3.1(+) Laccase2 MCS Exon Vector for RNA circularization (10.1101/gad.270421.115). Stable HCT116 cells were generated with electroporation of the circ-MDM2-expressing plasmid or empty vector. A total of 1.5 × 106 cells were electroporated using a Nucleofector II system (Lonza) and plated on six-well plates. After 48 h, medium was changed and supplemented with 800 μg/ml Geneticin (Gibco). Fresh medium was changed every 2 days for 12 days until stable cells were generated.
Identification of circRNAs.
To identify DNA damage-responsive circRNAs, we utilized our published RNA-seq data from three p53 WT CRC lines (HCT116, RKO, and SW48 cells), untreated or treated with DOXO (12). For the identification of circRNAs, adapter contamination was removed from the raw fastq files, and TopHat2 (version 2.1.0) was used to align the sequences to the human genome (hg19). The reads which remain unaligned were used to find fusion junctions using TopHat-Fusion (version 2.1.0). The CIRCexplorer program (version 1.10) was used with the identified fusion junctions obtained using the Ensembl GRCh37, release 72, human transcripts to identify the circularizing junctions. The identified circularizing junctions were annotated using circBase IDs. The circRNA junction counts were normalized to reads per million, and quantile normalization was performed before the fold changes were calculated. Finally, the results were annotated using the Ensembl GRCh37, release 72, database.
RNA isolation and qRT-PCR.
Total RNA from cell lines was isolated using an RNeasy minikit (Qiagen). For qRT-PCR analysis, 500 ng of total RNA was reverse transcribed using an iScript reverse transcription kit (Bio-Rad), and quantitative PCR (qPCR) was performed using Fast SYBR green Master Mix (Life technologies) per the manufacturer’s instructions. Primer sequences are provided Table S7 in the supplemental material.
Droplet digital PCR analysis (ddPCR) was conducted to quantify the absolute RNA copy numbers of circ-MDM2 and the linear host gene MDM2. To do this, 1 μg of total RNA was reversely transcribed, and the PCR using the droplets was generated using EvaGreen Supermix (1864033; Bio-Rad) containing 5 μl of cDNA for quantifying the copy numbers of circ-MDM2 or 1 μl of 4% (vol/vol) cDNA for MDM2 and GAPDH. Primers were used at concentrations of 250 nM. For circ-MDM2, a different forward primer was used from the one used for qRT-PCR (TGAAAGCCTGGCTCTGTGTG). The droplets were generated using a QX200 AutoDG Droplet Digital PCR System. The absolute RNA copy numbers were assessed using aQX200 Droplet Digital PCR System.
For RNase R treatment, 5 μg of total RNA was treated with 10 units (0.5 μl) of RNase R (+RNase R) or with water (mock) in 10-μl reaction mixtures and incubated at 37°C for 20 min. Following incubation, we combined 8 μl (of 10 μl) of the samples with 1 μl of random primers (catalog no. 58875; Invitrogen) and 1 μl of deoxynucleoside triphosphates (dNTPs) (18427-088; Invitrogen) from a Superscript III kit (52118; Invitrogen) and performed reverse transcription.
Nuclear and cytoplasmic extract preparations.
Nuclear and cytoplasmic extracts were prepared from HCT116 cells using digitonin permeabilization as previously described (62). RNA was isolated from cytoplasmic and nuclear fractions using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol.
Microarray analysis.
To identify the circ-MDM2-regulated transcriptome, HCT116 cells were transfected with control (CTL) siRNAs or circ-MDM2 siRNAs for a period of 48 h. RNA samples were prepared in duplicates as described above and labeled using an Illumina TotalPrep RNA amplification kit (Ambion), and microarrays were performed with a HumanHT-12, version 4, Expression BeadChip kit (Illumina). After hybridization, raw data were extracted with Illumina GenomeStudio software. Raw probe intensities were converted to expression values using the lumi package in Bioconductor with background correction, variance stabilization, and quantile normalization. Differential expression was computed by an empirical Bayes analysis of a linear model using the limma package in Bioconductor. Adjusted P values were calculated with the Benjamini-Hochberg method.
Flow cytometry, colony formation, and cell proliferation assays.
To perform cell proliferation assays, HCT116 cells were reverse transfected with siCTL or circ-MDM2 or p53 siRNA, and cell proliferation was determined by Cell Counting Kit-8 (Dojindo).
For cell cycle analysis after double-thymidine block (Fig. 5), HCT116 cells were reverse transfected with siCTL or circ-MDM2 siRNA. After 48 h, 2 mM thymidine was added for a period of 24 h. After the first thymidine block, the cells were washed and reseeded in six-well plates, allowing a 16-h recovery. Following recovery, 2 mM thymidine was added for a period of 24 h to synchronize the cells at G1/S. After this second block, thymidine was removed, and fresh medium was added for cells to recover. Cells were collected at the indicated times and were fixed with ice-cold ethanol for 2 h and stained with propidium iodide (Sigma) in the presence of RNase A (Qiagen). Cell cycle profiles were captured using a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (FloJo, LLC).
For colony formation, HCT116 cells were reverse transfected with siCTL and two independent circ-MDM2 siRNAs. After 48 h, cells were trypsinized and seeded in a 12-well plate at a density of 500 cells per well. After 10 days, colonies were fixed with ice-cold 100% methanol for 5 min and visualized by staining with crystal violet (C3886; Sigma).
Immunoblotting.
To determine the protein levels of MDM2, p53, p21, phospho-Rb, or the loading control glyceraldehyde-3-phosphate dehydrogenase (GAPDH), HCT116 cells were reverse transfected with siCTL or circ-MDM2 siRNA. After 48 h, whole-cell lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail (Roche). Proteins were quantified using a bicinchoninic acid (BCA) protein quantitation kit (Thermo Scientific). For immunoblotting, 20 μg of whole-cell lysate per lane was loaded onto SDS-PAGE gels, transferred to polyvinylidene difluoride (PVDF) membrane, and immunoblotted with anti-MDM2 (catalog no. OP115; Calbiochem), anti-p53 (DO-1) (sc-126; Santa Cruz), anti-p21 (sc-397; Santa Cruz), anti-phospho-Rb (catalog no. 9307P, 9208P, and 9301P; Cell Signaling), and anti-GAPDH (14C10; Cell Signaling) antibodies.
Xenograft assays.
Animal protocols were approved by the National Cancer Institute Animal Care and Use Committee following AALAAC guidelines and policies, using the animal study protocol LC-070. HCT116 cells were reverse transfected with siCTL and siCirc-MDM2 siRNAs at a final siRNA concentration of 50 nM. Live cells were counted with trypan blue exclusion; 1 million of the transfected HCT116 cells were mixed with 30% Matrigel in phosphate-buffered saline (PBS) on ice, and the mixture was injected into the flanks of 6- to 8-week-old female athymic nude mice (Animal Production Program, Frederick, MD) (each group, n = 10). Tumor volume was measured twice a week starting at a week after injection. The following formula was used for the caliper measurements: 0.52 × l × s × s, where l is the longest dimension and s is the shorter orthogonal dimension.
To evaluate the effect of proliferation and/or apoptosis in the tumors, the xenograft tumors were collected from three siCTL and siCirc-MDM2 tumors and fixed in 10% neutral buffered formalin (Sigma, St. Louis, MO). Paraffin sectioning, Ki67 staining, and cleaved caspase-3 staining were performed by Histoserv, Inc. (Gaithersburg, MD). The following antibodies were used for immunohistochemistry staining: anti-Ki67 (Ab16667; Abcam) and anti-cleaved caspase-3 (9661; Cell Signaling). The images were acquired at ×20 magnification using an EVOS FL Auto Imaging System (Life technologies). RNA was extracted using TRIzol reagent from three control and three circ-MDM2 siRNA-treated tumors upon tumor removal to assay for circ-MDM2 levels with qRT-PCR.
Identification of circ-MDM2 in COAD.
To identify the presence of circ-MDM2 in COAD, we employed a de novo method and performed a BLAST search for the circ-MDM2 back-spliced exon junction sequence (30 + 30 nucleotides), across 411 COAD samples from TCGA. The BLAST search was performed by making a BLAST custom database from each sample’s unmapped reads, and the 60-nucleotide sequence was queried across these reads. Hits with greater than 20 nucleotides of the query sequence and with an E value of <0.001 were filtered and used further. After the filter was imposed, a total of 283 hits were recorded across 173 (159 tumor and 14 normal) COAD TCGA IDs.
To observe the influence of p53 mutation on occurrence of circ-MDM2 in COAD, we analyzed 130 mutation annotation format (MAF) files from the FireBrowse database and extracted p53 mutation information. We observed that across the 108 samples which had both mutation information and RNA-seq data, 49 TCGA samples had mutated p53 proteins and 59 had WT p53 proteins. We further analyzed these samples to observe the occurrence of circ-MDM2 isoforms (see Table S7 in the supplemental material) and performed Fisher’s exact test on this data set to compute statistical significance for enrichment.
Data availability.
Microarray data were deposited in the Gene Expression Omnibus under accession number GSE138016.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bert Vogelstein (Johns Hopkins University) for the isogenic cell lines. The circRNA overexpression plasmid was a generous gift from Jeremy Wilusz (University of Pennsylvania).
This research was supported by the Intramural Research Program (R.C., B.R.M., X.L.L., Y.Z., P.S.M., and A.L.) of the National Cancer Institute (NCI), Center for Cancer Research (CCR), NIH, and the Intramural Research Program (S.D., K.A., and M.G.) of the National Institute on Aging (NIA). S.C.J.’s lab is supported by an RO1 project grant from the National Institute of General Medical Sciences (NIGMS) under award number R01GM123314.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Olivier M, Hollstein M, Hainaut P. 2010. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature 408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 4.Malkin D, Li FP, Strong LC, Fraumeni JF Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA. 1990. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 5.Zilfou JT, Lowe SW. 2009. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol 1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vousden KH, Prives C. 2009. Blinded by the light: the growing complexity of p53. Cell 137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary R, Lal A. 2017. Long noncoding RNAs in the p53 network. Wiley Interdiscip Rev RNA 8:e1410. doi: 10.1002/wrna.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley T, Sontag E, Chen P, Levine A. 2008. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 9.Beckerman R, Prives C. 2010. Transcriptional regulation by p53. Cold Spring Harb Perspect Biol 2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermeking H. 2012. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 12:613–626. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 11.Freeman JA, Espinosa JM. 2013. The impact of post-transcriptional regulation in the p53 network. Brief Funct Genomics 12:46–57. doi: 10.1093/bfgp/els058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XL, Subramanian M, Jones MF, Chaudhary R, Singh DK, Zong X, Gryder B, Sindri S, Mo M, Schetter A, Wen X, Parvathaneni S, Kazandjian D, Jenkins LM, Tang W, Elloumi F, Martindale JL, Huarte M, Zhu Y, Robles AI, Frier SM, Rigo F, Cam M, Ambs S, Sharma S, Harris CC, Dasso M, Prasanth KV, Lal A. 2017. Long noncoding RNA PURPL suppresses basal p53 levels and promotes tumorigenicity in colorectal cancer. Cell Rep 20:2408–2423. doi: 10.1016/j.celrep.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mello SS, Sinow C, Raj N, Mazur PK, Bieging-Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J, Hirose T, Nakagawa S, Rinn J, Attardi LD. 2017. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes Dev 31:1095–1108. doi: 10.1101/gad.284661.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. 2010. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. 2007. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Kopp F, Chang TC, Sataluri A, Chen B, Sivakumar S, Yu H, Xie Y, Mendell JT. 2016. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO Proteins. Cell 164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary R, Gryder B, Woods WS, Subramanian M, Jones MF, Li XL, Jenkins LM, Shabalina SA, Mo M, Dasso M, Yang Y, Wakefield LM, Zhu Y, Frier SM, Moriarity BS, Prasanth KV, Perez-Pinera P, Lal A. 2017. Prosurvival long noncoding RNA PINCR regulates a subset of p53 targets in human colorectal cancer cells by binding to Matrin 3. Elife 6:e23244. doi: 10.7554/eLife.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z, Yang BB. 2017. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ 24:357–370. doi: 10.1038/cdd.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Leary VB, Smida J, Matjanovski M, Brockhaus C, Winkler K, Moertl S, Ovsepian SV, Atkinson MJ. 2017. The circRNA interactome-innovative hallmarks of the intra- and extracellular radiation response. Oncotarget 45:78397–78409. doi: 10.18632/oncotarget.19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gabel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D. 2016. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer JW, Leung AK. 2017. CircRNAs: a regulator of cellular stress. Crit Rev Biochem Mol Biol 52:220–233. doi: 10.1080/10409238.2016.1276882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. 1991. Scrambled exons. Cell 64:607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 23.Cocquerelle C, Daubersies P, Majerus MA, Kerckaert JP, Bailleul B. 1992. Splicing with inverted order of exons occurs proximal to large introns. EMBO J 11:1095–1098. doi: 10.1002/j.1460-2075.1992.tb05148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. 1993. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73:1019–1030. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 25.Guo JU, Agarwal V, Guo H, Bartel DP. 2014. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, Le Noble F, Rajewsky N. 2013. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 27.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. 2012. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Duff MO, Graveley BR, Carmichael GG, Chen LL. 2011. Genomewide characterization of non-polyadenylated RNAs. Genome Biol 12:R16. doi: 10.1186/gb-2011-12-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. 2013. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. 2013. Cell-type specific features of circular RNA expression. PLoS Genet 9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Yang L, Chen LL. 2018. The biogenesis, functions, and challenges of circular RNAs. Mol Cell 71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH, Bindereif A. 2015. Exon circularization requires canonical splice signals. Cell Rep 10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. 2014. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. 2015. The RNA binding protein quaking regulates formation of circRNAs. Cell 160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Errichelli L, Dini Modigliani S, Laneve P, Colantoni A, Legnini I, Capauto D, Rosa A, De Santis R, Scarfo R, Peruzzi G, Lu L, Caffarelli E, Shneider NA, Morlando M, Bozzoni I. 2017. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun 8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N. 2015. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. 2013. Natural RNA circles function as efficient microRNA sponges. Nature 495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. 2015. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 39.Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, Li X, Yang BB. 2017. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 38:1402–1412. doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 40.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. 2016. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng WL, Marinov GK, Liau ES, Lam YL, Lim YY, Ea CK. 2016. Inducible RasGEF1B circular RNA is a positive regulator of ICAM-1 in the TLR4/LPS pathway. RNA Biol 13:861–871. doi: 10.1080/15476286.2016.1207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panda AC, Grammatikakis I, Kim KM, De S, Martindale JL, Munk R, Yang X, Abdelmohsen K, Gorospe M. 2017. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res 45:4021–4035. doi: 10.1093/nar/gkw1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, Shohet JM. 2005. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A 102:731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manfredi JJ. 2010. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev 24:1580–1589. doi: 10.1101/gad.1941710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moll UM, Petrenko O. 2003. The MDM2-p53 interaction. Mol Cancer Res 1:1001–1008. [PubMed] [Google Scholar]
- 46.Haupt Y, Maya R, Kazaz A, Oren M. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 47.Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL, Dowell RD, Espinosa JM. 2014. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife 3:e02200. doi: 10.7554/eLife.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. 2000. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 49.Barak Y, Gottlieb E, Juven-Gershon T, Oren M. 1994. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev 8:1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- 50.Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, Robinson DR, Nesvizhskii AI, Chinnaiyan AM. 2019. The landscape of circular RNA in cancer. Cell 176:869–881.e13. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. 2006. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A 103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piwecka M, Glažar P, Hernandez-Miranda LR, Memczak S, Wolf SA, Rybak-Wolf A, Filipchyk A, Klironomos F, Cerda Jara CA, Fenske P, Trimbuch T, Zywitza V, Plass M, Schreyer L, Ayoub S, Kocks C, Kühn R, Rosenmund C, Birchmeier C, Rajewsky N. 2017. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357:eaam8526. doi: 10.1126/science.aam8526. [DOI] [PubMed] [Google Scholar]
- 53.Rowland BD, Bernards R. 2006. Re-evaluating cell-cycle regulation by E2Fs. Cell 127:871–874. doi: 10.1016/j.cell.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev 16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen HZ, Tsai SY, Leone G. 2009. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verduci L, Ferraiuolo M, Sacconi A, Ganci F, Vitale J, Colombo T, Paci P, Strano S, Macino G, Rajewsky N, Blandino G. 2017. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol 18:237. doi: 10.1186/s13059-017-1368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S. 2016. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebbesen KK, Hansen TB, Kjems J. 2017. Insights into circular RNA biology. RNA Biol 14:1035–1045. doi: 10.1080/15476286.2016.1271524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. 2017. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell 66:22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, Vande Woude GF, O'Connor PM, Appella E. 1997. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A 94:6048–6053. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. 2001. Regulation of PTEN transcription by p53. Mol Cell 8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 62.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. 2004. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microarray data were deposited in the Gene Expression Omnibus under accession number GSE138016.