Abstract
With the ever-increasing burden of obesity and Type 2 diabetes, it is generally acknowledged that there remains a need for developing new therapeutics. One potential mechanism to combat obesity is to raise energy expenditure via increasing the amount of uncoupled respiration from the mitochondria-rich brown and beige adipocytes. With the recent appreciation of thermogenic adipocytes in humans, much effort is being made to elucidate the signaling pathways that regulate the browning of adipose tissue. In this review, we focus on the ligand–receptor signaling pathways that influence the cyclic nucleotides, cAMP and cGMP, in adipocytes. We chose to focus on G-protein–coupled receptor (GPCR), guanylyl cyclase and phosphodiesterase regulation of adipocytes because they are the targets of a large proportion of all currently available therapeutics. Furthermore, there is a large overlap in their signaling pathways, as signaling events that raise cAMP or cGMP generally increase adipocyte lipolysis and cause changes that are commonly referred to as browning: increasing mitochondrial biogenesis, uncoupling protein 1 (UCP1) expression and respiration.
Introduction
Obesity is a disorder that leads to complications such as insulin resistance and diabetes. Changes in diet and exercise are the most commonly recommended methods to treat obesity, but rarely succeed over the long term [1]. More recently, invasive procedures such as bariatric surgery have also been prescribed [2]. In addition, pharmacological approaches to reduce obesity, including blockade of fat absorption and central nervous system stimulants, have been developed [2], but include unacceptable side effects or eventual lack of efficacy. The lack of effective therapeutics for obesity is a serious deficiency in the currently available pharmacological compendium, as recent studies have reported that approximately 30% of Americans are clinically obese [3] and the prevalence of obesity worldwide is rising [4]. Obesity is co-morbid with Type 2 diabetes and related diseases such as heart disease, nephropathy and sleep apnea. Thus, finding ways to treat or reduce obesity are important for decreasing the amounts of diabetes and related diseases.
A novel strategy for achieving weight loss that was attempted early in the 20th century targeted ‘uncoupled respiration’ by using the drug 2,4-dinitrophenol (DNP), which globally and indiscriminately uncouples oxidative phosphorylation from ATP production in mitochondria of all cells. This therapy was employed extensively between 1933 and 1938, but its use was largely discontinued after a number of deaths, and the US FDA labeled this drug as “extremely dangerous and not fit for human consumption” [5]. While DNP is not an acceptable anti-obesity agent, it did shed light on the fact that wasteful metabolic processes may be an attractive means of achieving weight loss. Moreover, recently a modified form of DNP that targets the liver has been shown in rodents as well as non-human primates to reverse dyslipidemia and hepatic steatosis by increasing fat oxidation in the liver [6,7].
One naturally occurring process that involves regulated energy-expenditure is non-shivering thermogenesis. This is the production of heat in warm-blooded organisms without skeletal muscle shivering. An important site of thermogenesis in mice and humans is brown adipose tissue (BAT), which is a type of adipose tissue that has large numbers of mitochondria, contributing to its brown color [8]. Activation of the BAT signature protein, uncoupling protein 1 (UCP1), allows protons to cross the inner mitochondrial membrane without ATP production. This uncoupled electron transport causes heat to be the main product of metabolism [9]. It has recently been appreciated that adult humans possess significant amounts of UCP1 and mitochondria-rich brown adipocytes [10–12], and the activation of UCP1 is now seen as a potential therapeutic target for obesity and metabolic disease as a mechanism to consume energy and reduce or prevent obesity. Even in the absence of appreciable weight loss, reducing plasma glucose and fatty acids could be very beneficial for risk of diabetes and cardiovascular disease. In addition to BAT, organisms possess white adipose tissue (WAT) that primarily functions to store caloric energy in the form of triglycerides. However, cells in the WAT depot can increase a population of cells, called “beige” or “brite” adipocytes, that have elevated levels of mitochondria, express UCP1, and are more thermogenic. This process is called “beiging” or “browning” [8].
The therapeutic implications of a pharmacological agent(s) which could safely increase a subject’s uncoupled-energy expenditure via their endogenous UCP1 proteins are attractive. Indeed, recent studies have identified a wide range of neural, circulating, and dietary factors that purportedly increase adipose browning. As there are too many agents that have alleged browning effects to be included in this review, we have decided to only focus on the pathways that enhance/inhibit adipocyte browning and BAT thermogenesis via their regulation of the cyclic nucleotides, cAMP and cGMP (cyclic Adenosine MonoPhosphate and cyclic Guanosine MonoPhosphate). As such, much of this review will focus on G-Protein Coupled Receptors (GPCRs) whose downstream signaling events regulate the production of cAMP. In the event that the reader is unfamiliar with this family of receptors and their downstream signaling, we have suggested a few recent reviews [13–15]. We will also review browning via the much smaller receptor–enzyme families of guanylyl cyclases (GC), which produce cGMP, because there is large overlap in the downstream signaling pathways of cAMP and cGMP. Finally, we also review the emerging concept of modulating adipocyte thermogenesis by targeting the phosphodiesterase (PDE) enzymes, which degrade cAMP and cGMP. We chose to focus on these areas because we estimate that they represent some of the most fertile ground for developing potential pharmaceutical agents that augment adipocyte browning as an estimated 35% of all approved drugs target GPCRs [16] and large portions of the much smaller GC and PDE families are the targets of many currently approved therapeutics.
In this review, we discuss in alphabetical order the GPCR, GC, and PDE families that have been implicated in the context of regulating adipocyte cyclic nucleotide concentrations, lipolysis, oxygen consumption, mitochondrial biogenesis, UCP1 expression, and uncoupled respiration starting with the adrenergic receptors. In Table 2 at the end of this chapter we have listed many of these receptor/ligand signaling pathways in order to provide a compendium of the currently available information about these signaling pathways and how they affect adipocyte lipolysis and thermogenesis. This is not meant to be a comprehensive list as not all receptor/ligand signaling pathways were able to be included. Hopefully, this will allow us to better understand the subtle differences in these signaling pathways that will help us choose the best pathway(s) to target for the development of therapeutics that augment adipocyte thermogenesis.
Table 2.
Summary of ligand–receptor signaling pathways that regulate adipocyte lipolysis and browning
| Ligand | Receptor | G-protein | Cyclic nucleotide | Lipolysis | Browning/Thermogenesis |
|---|---|---|---|---|---|
| Adenosine | A1R [466,467] | Gαi | Adenosine opposes the actions of other agents that raise cAMP [468–472]. A1 R knock-out blocks the 2-chloroadenosine-evoked reduction in cAMP [473]. | Adenosine opposes the actions of other agents which raise lipolysis both in vitro/ex vivo [468,469,471,472,474–480] and in vivo [481]. A1 R knock-out blocks the 2-chloroadenosine-evoked reduction in lipolysis [473,482]. | Adenosine inhibits adipocyte respiration [479,483,484]. |
| Adenosine | A2AR, A2BR [466] | Gαs | Adenosine increases cAMP in some cell lines [485]. A2AR and A2BR agonists increase cAMP [485]. | Adenosine increases lipolysis in some cell lines [485]. A2AR and A2BR agonists increase lipolysis [485]. | Activation of A2AR increases thermogenic gene expression in human and mouse brown and white adipocytes [485]. |
| Adrenergic: (norepinephrine, epinephrine) | β1AR, β2AR, β3AR | Gαs | Increase cAMP [486] | Increase lipolysis [486]. | Increase browning [486]. |
| Adrenergic: (norepinephrine, epinephrine) | α1AR | Gαq/11 | Augment the βAR response in brown adipocytes to increase thermogenesis [38,39]. | ||
| Adrenergic: (norepinephrine, epinephrine) | α2AR | Gαi | Decrease cAMP by inhibiting adenylyl cyclase [486]. | Decrease lipolysis [25,40]. | Central nervous system activation of α2AR can suppress peripheral SNS activation of BAT [487]. Peripheral actions variable in literature. |
| Bile acids (cholic acid, chenodeoxycholic acid) | GPBAR1 (aka TGR5) [466,488–493] | Gαs | Bile acids (including cholic acid) increase cAMP in BAT cells [488]. | Cholic acid and chenodeoxycholic acid both have been reported to increase adipocyte thermogenesis [488,490,491,494–496]. GPBAR1 selective agonists have similar browning effects [489,492,493,497,498]. | |
| Gastric inhibitory polypeptide (GIP) | GIPR [147,167–177,466] | Gαs | GIP raises cAMP concentrations and PKA-evoked signaling [95,167,171,174,177,188–191]. | The effects of GIP on lipolysis have observed it to be increased [168,181,185,189], unaffected [193] and decreased [116,192]. GIP increased both glycerol release and fatty acid re-esterification, which resulted in an overall decrease in free fatty acid release despite the increased lipolysis [185]. | Both GIP treatment and Gipr knockdown were reported to increase UCP1 in immortalized BAT cells along with an increase in their maximal oxygen consumption [204]. Similar findings were observed in Gipr−/− mice where Ucp1 mRNAwas increased in brown adipose tissue of mice fed a high fat diet [151]. |
| Glucagon | GCGR [75,85–96,466] | Gαs | Glucagon increases intracellular cAMP in adipocytes [85,104,107,110,111,113,115,117, 118,120,124–128,284,285,469,499,500] | Glucagon increases circulating glycerol and free fatty acids in vivo [60,65,66,77–84]. Glucagon increases adipocyte lipolysis in vitro and ex vivo [75,87,89,97–103,105–110,112–114,116,118–123,126–130,501–504]. | Glucagon increases body temperature, oxygen consumption, metabolic rate and thermogenic markers in vivo, but some studies indicate that this can be blocked by inhibiting β-adrenergic signaling [56–76,135]. Glucagon increases oxygen consumption and thermogenesis in vitro and ex vivo in adipocytes and adipose tissue [65,71,75,122,129–133]. |
| Glucagon-like peptide-1 (GLP-1) | GLP1R [85,86,94,95,142–147,466] | Gαs | GLP-1 has been reported to increase [85,113,126,128] or not alter [117] intracellular cAMP in adipocytes. | GLP-1 has been reported to increase [85,113,126,128] or not alter [148,149] lipolysis. | Central administration of synthetic GLP1R agonists increase adipose tissue browning [134,152,153]. Peripheral administration of synthetic GLP1R agonists increase UCP1 in adipocytes [155–159]. |
| Melanocyte stimulating hormones (MSH): α-MSH, β-MSH, γ-MSH etc. Adrenocorticotropic hormone (ACTH) | MC2R [228–233,236], MC5R [228,230–233,236,466] Human adipocytes lack MC2R but may express other receptors [229,239,240] | Gαs | ACTH, which binds to all melanocortin receptors, increases cAMP in adipocytes [104,107,110,115,120,219,222–224,228,230,233,234,236,284,285,292,469,499,500,505–507]. α-MSH, which does not bind to MC2R, similarly increases cAMP in adipocytes [228,230,233]. | ACTH stimulates lipolysis [97–99,101,103,106,107,109,110,120, 217,218,220–223,226,227,229,231,233,234,501,502] α-MSH and β-MSH, which do not bind MC2R, stimulate lipolysis [103,226,231,233,235,241]. Lipolysis is not affected by ACTH in primates or canines [229,237,238,240], though a-MSH may have a small lipolytic effect [240]. | ACTH increases oxygen consumption and thermogenic gene expression in adipocytes [59,130,133,225,232,234,236]. α-MSH, which does not bind to MC2R, increases thermogenic gene expression in adipocytes [235,236,242]. |
| Neuropeptide Y (NPY), peptide YY (PYY), pancreatic polypeptide (PP) | YR1, YR2, YR5 [147,466,508–513] | Gαi | Both NPY and PYY inhibit lipolysis in vitro [238,241,514–516]. NPY and PYY inhibit lipolysis in vivo in sympathectomized rats [517]. | NPY administration reduces [518] while global NPY knockout increases adipocyte thermogenic markers [519,520]. NPY suppress thermogenic gene expression and oxygen consumption in C3H10T1/2 adipocytes [521]. | |
| Parathyroid hormone (PTH), Parathyroid hormone-related protein (PTHrP) | PTH1R, PTH2R [236,266,268] | Gαs,Gαq/11 | PTH stimulates cAMP in adipocytes [260–264]. | PTH stimulates lipolysis in adipocytes [238,258–261,265,266]. | Both PTH and PTHrP increase respiration and promote the expression of thermogenic genes in adipocytes [236,267,268]. |
| Pituitary adenylate cyclase-activating peptide (PACAP), Vasoactive intestinal peptide (VIP) | PAC1, VPAC1, VPAC2 [236,466,511,522–528] | Gαs | VIP increases cAMP in adipocytes [85,111,115,264,529]. | PACAP- and VIP-stimulated adipocyte lipolysis [112,116,123,149,181,226,264,524]. This can only be inhibited by a VPAC2 antagonist [524]. | PACAP has been reported to stimulate Ucp1 gene expression and has a small stimulatory effect on respiration in brown adipocytes [236]. Central administration of either PACAP or VIP has been shown to promote brown adipose tissue thermogenesis [530,531]. |
| Prostaglandin E2 (PGE2) | EP2, EP4 [236,466,532–538] | Gαs | In the absence of elevated cAMP, high concentrations of PGE2 by itself have been reported to increase cAMP in adipocytes [288,293]. Under similar circumstances, PGE1, a similar PGE analog, has also been reported to increase cAMP in adipocytes [284,539]. | PGE2 has been shown to increase UCP1 [236,303,304,540]. 16,16-dimethyl-PGE2, an EP2/EP3/EP4 agonist, has been reported to also increase the expression of thermogenic gene expression in BAT in vivo [297]. | |
| Prostaglandin E2 (PGE2) | EP3 [296,466,532,533,535–538,541] | Gαi | PGE2 opposes the actions of other agents which raise cAMP [286–290,292,295,542–544]. PGE1 also lowers cAMP after it has been raised by other agents. [284,285,506,542,545–547]. In the absence of raising cAMP with other agents, PGE2 by itself lowers cAMP, but only at low concentrations [288,293]. Sulprostone, an EP1 and EP3 selective agonist, has also been shown to oppose actions of other agents that raise cAMP in adipocytes [293,544]. The EP3 antagonist, L-798,106, blocks the PGE2-evoked reduction in cAMP [295]. | PGE2 opposes actions of other agents that raise lipolysis both in vitro/ex vivo [276,286,289,290,292,294,296,303,482,502,505,541,543,544,548–552] and in vivo/in situ [553,554]. PGE1 also reduces lipolysis after it has been raised by other agents both in vitro/ex vivo [434,435,500–502,505,515,542,547, 555–560] and in vivo/in situ [103,502,553,561]. Sulprostone, an EP1 and EP3 selective agonist, has also been shown to oppose actions of other agents that raise lipolysis in adipocytes [294,544]. EP3 knock-out or the antagonist L-826,266 blocks the PGE2-evoked reduction in lipolysis [296,541]. | |
| Prostacyclin (PGI2) | IP [466,532,562] | Gαs | PGI2 raises cAMP in adipocytes [279–283,563]. | PGI2 increases lipolysis [276]. | PGI2 and its synthetic analog carbaprostacyclin have been observed to increase Ucp1 and other thermogenic gene expression in adipocytes [298–301]. |
| Secretin | SCTR [85,236,466,564–566] | Gαs | Secretin increases cAMP in adipocytes [85,107,111,124,125,545,564]. | Secretin stimulates lipolysis both in vivo [79] and in vitro [102,106,107,112,114,116,545,564, 565,567,568]. | Secretin increases oxygen consumption and thermogenic gene expression in brown adipocytes [71,236,566]. |
| Thyroid-stimulating hormone (TSH, aka thyrotropin) | TSHR [236,466,569–582] | Gαs | TSH increases intracellular cAMP levels in adipocytes [104,285,499,570,573,574,579,580]. | TSH induces lipolysis [103,501,502,577,583–589]. | TSH increases Ucp1 and oxygen consumption in adipocytes in vitro [124,579,582]. |
| Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) | NPRA (aka GC-A) [315,383,390–396,399,402,404,406,412,418,590] | – | ANP signaling increases intracellular cGMP concentrations in adipocytes [393,404,410,418]. BNP signaling also increases intracellular cGMP concentrations in adipocytes [404]. | ANP stimulates lipolysis in vitro [395,406,411,413,414,418,421,591] and in vivo [395,402,414,417,419,591]. BNP stimulates lipolysis in vitro [419] and in vivo [395,419,420]. | ANP increases oxygen consumption and UCP1 expression in adipocytes in vitro [315,[404,[406,421,[422]. BNP increases UCP1 expression in vitro [404] and in vivo [315,400,423]. |
| C-type natriuretic peptide (CNP) | NPRB (aka GC-B) [392,394,396,404] | – | CNP signaling increases intracellular cGMP concentrations in adipocytes [393,409,410]. | Mice overexpressing CNP in adipose tissue have increased oxygen consumption and UCP1 in inguinal WAT [409]. |
G-protein–coupled receptors
Adrenergic receptors
The adrenergic receptors are in many respects the prototypical GPCR. Owing to the ability to purify significant quantities of the β2AR protein and G-protein complexes in some laboratories, many of the fundamental characteristics of this family of receptors were described in studies of the β2AR [17]. The gene and cDNA for the β2AR was also the first of this large family of seven transmembrane domain-containing proteins to be cloned [18], followed soon thereafter by the β1AR [19]. There are nine adrenergic receptors (ARs): three alpha1- (α1ARs), three α2ARs and three beta-(β)ARs (Figure 1). Each of these receptors is the products of separate genes. The βARs are by far the most studied and discussed regarding adipocyte catabolism and increasing energy expenditure through non-shivering thermogenesis, and there are so many review articles on this topic that the reader can easily find one (Figure 2). Therefore, we will not spend a great deal of time on this topic here, except to make some general statements and points of interest to us. In general, β1AR and β2AR are broadly expressed throughout organs and cell types, and both can be found expressed together in some tissues such as the heart, adipose tissues and vasculature. The β3AR, which was the last of the ARs to be cloned and characterized [20–22], tends to be found primarily in adipocytes; although there are reports of functionally relevant β3AR in organs such as urinary bladder. In rodent adipose tissue, β3AR is the most highly expressed relative to β1AR and β2AR [22,23], while in human adipose tissues β3AR represents a much smaller proportion compared with β1AR and β2AR [22,24–26]. Further, β3AR is found in all rodent adipose depots – white and brown – but its lower level in primate adipose tissue at this point also appears to be more restricted to ‘brown’ or ‘beige’ adipocytes that are able to express UCP1 [27–29]. This difference in the level of expression of the β3AR between rodents and primates is important to remember. In humans and non-human primates, the β1AR is preferentially activated by norepinephrine, and β2AR can also be activated in human adipocytes through circulating epinephrine [26]. A recent report suggests that β1AR, and not β3AR, may be more important for activating human brown adipocytes [30]. There are also subtle and not-so-subtle differences in the signaling properties of the three βARs [31,32]. While β1AR and β2AR utilize β-arrestins in their signaling scenarios, β3AR is unique in this regard in that it does not recruit β-arrestin [33], although all three βARs are nevertheless able to activate ERK pathways by different means [31]. Activation of p38 MAPK downstream of PKA is also an important feature of βAR signaling in adipocytes and is required for orchestrating the signaling components to increase transcription of the Ucp1 and Pgc1α genes, among others [31,34]. Finally, a more recent discovery in the complexity of βAR signaling in the adipocyte for driving ‘browning’ and brown fat development is the unexpected activation of the rapamycin-sensitive mTOR complex 1 (mTORC1) by PKA [35]. These signaling pathways through the βARs in adipocytes also afford remarkable plasticity between the energy-storing ‘white’ adipocyte and the energy-consuming, thermogenic ‘brown’ or ‘beige’ adipocyte [36,37]. The α1AR and α2AR are also expressed in adipocytes, the former having been reported to also contribute to brown adipocyte function [38,39] and the latter largely involved in blocking the actions of the βARs through their coupling to the heterotrimeric Gαi protein and inhibition of adenylyl cyclase [25,40]. Moreover, increases in α2ARs in human obesity are reported to contribute to the refractoriness of the tissue for catabolism.
Figure 1. Catecholamine biosynthetic pathway and signaling.
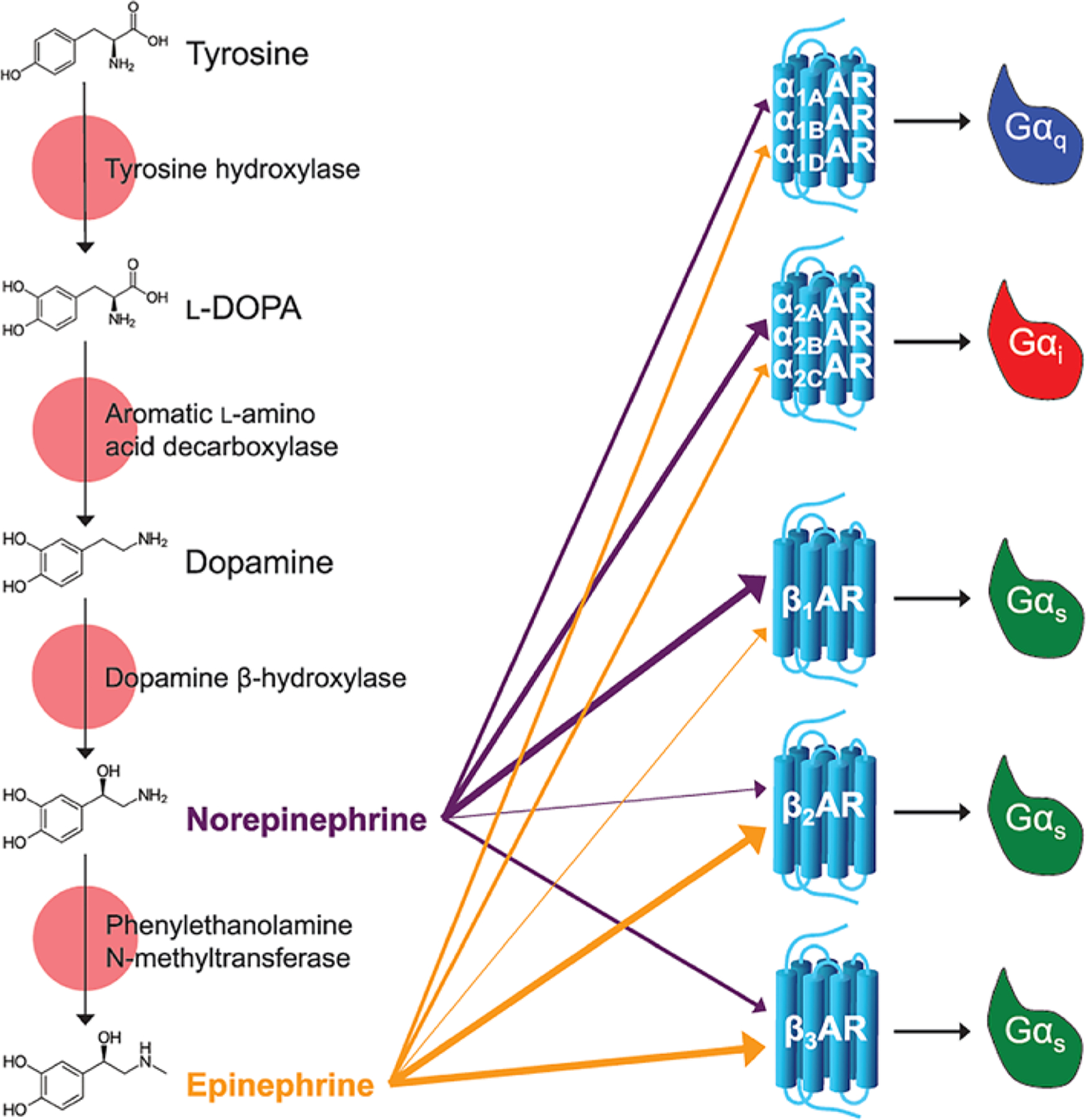
Epinephrine and norepinephrine (also called adrenaline and noradrenaline, respectively) are made from the amino acid tyrosine via the catecholamine biosynthetic pathway. Epinephrine and norepinephrine signal through nine GPCRs called the adrenoceptors. The α1 receptors are Gαq-coupled, α2 receptors are Gαi-coupled, and the β-receptors are Gαs-coupled. The α-receptors are activated by epinephrine and norepinephrine, with epinepherine having a lower potency than norepinephrine on the α2 receptors. For the β-receptors, in β1 norepinephrine is more potent than epinephrine, in β2 epinephrine is more potent than norepinephrine, and in β3 epinephrine and norepinephrine have similar potencies (adrenoceptors in the IUPHAR/BPS Guide to Pharmacology Database.
Available from: https://doi.org/10.2218/gtopdb/F4/2019.4).
Figure 2. Sympathetic activation of adipocyte lipolysis and thermogenesis.
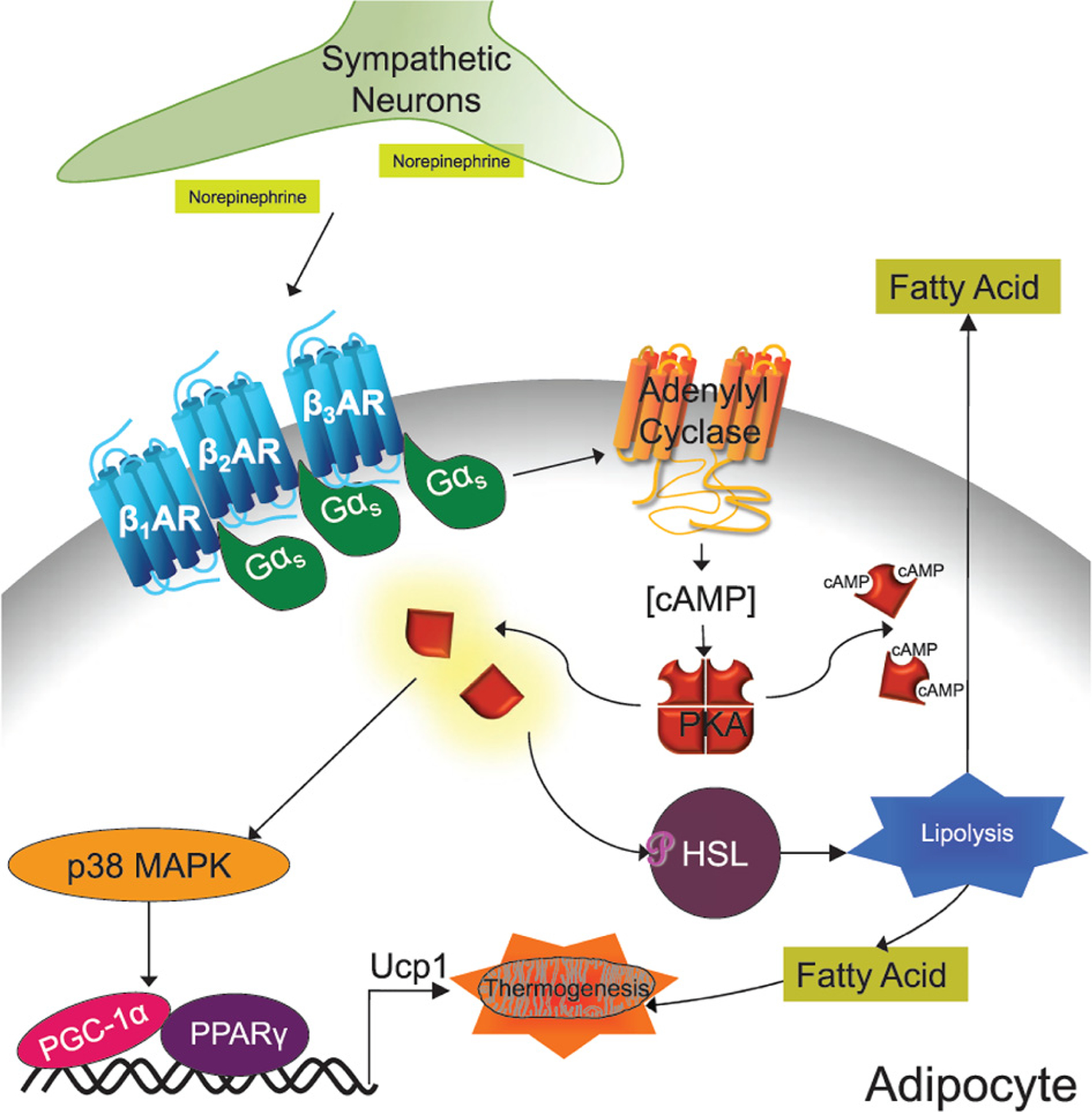
Canonical activation of adipocyte lipolysis and thermogenesis occurs via the sympathetic nervous system. Sympathetic neurons release norepinephrine which activate β-adrenergic receptors on the adipocytes. Activation of these receptors turn on their associated G-proteins by causing the Gβγ (not shown) and Gαs subunits to dissociate. The Gαs subunit in turn augments adenylyl cyclase activity. The cAMP produced by adenylyl cyclase causes the regulatory subunits of PKA to dissociate from the catalytic subunits thereby liberating its activity. PKA phosphorylates and activates Hormone Sensitive Lipase (HSL) and other components of the lipolytic pathway. PKA also phosphorylates and activates p38 MAPK that leads to increased Ucp1 transcription and the expression of other pro-thermogenic genes.
Glucagon/PACAP/secretin family
The B1 subfamily of GPCRs consists of 15 members, all of which bind polypeptide hormones and couple to Gαs, although some can also couple to other G-proteins [41]. Nine of the ligands for this family (Glucagon, Glucagon-Like Peptides [GLP-1, GLP-2], Gastric Inhibitory Polypeptide [GIP], Growth-Hormone Releasing Hormone [GHRH], Pituitary Adenylate Cyclase-Activating Peptide [PACAP], peptide histidine methionine [PHM], Secretin, Vasoactive Intestinal Peptide [VIP]) are structurally related, many of which can bind to and activate each other’s receptors at high concentrations [41,42]. Furthermore, glucagon, GLP-1 and GLP-2 are all derived from the same proglucagon gene [43,44]. This family of receptors is also referred to as the Secretin Family, because the first hormone discovered in this (or any) family was secretin [45]; the PACAP Family, because secretin is absent in fish and PACAP appears to be the most ancient gene in the family [46]; or the Glucagon Family, though this generally refers only to the receptors for glucagon, GLP-1, GLP-2 and GIP, while other closely related receptors such as GHRH and secretin are also sometimes included [42]. Another similarity that is shared between GLP-1, GLP-2, GIP, secretin and VIP is that they are secreted from the intestines in response to food. These hormones are collectively referred to as “Incretins” due to their ability to enhance glucose stimulated insulin secretion in response to food intake [47]. Several of these family members, including glucagon, GLP-1 and GIP, are degraded by dipeptidyl peptidase-4 (DPP-4) [48]. DPP-4 inhibitors, also called gliptins, are currently used as therapeutic treatments for Type 2 diabetes due to their ability to prolong the half-life of these incretins [49]. Here, we will review yet another commonality among several of these closely related ligands and receptors: their ability to induce adipocyte browning and thermogenesis. Readers should be advised that there is considerable variation in perspective among studies in that it is still not clear what effects are ‘direct’ effects of the peptides on adipose tissue per se versus ‘indirect’ effects that may stem from inter-organ cross-talk or central effects. Therefore, as they will become apparent in the following sections, the literature is still somewhat conflicted.
Glucagon
Glucagon is best known as the hormone that opposes insulin, acting as a counter-regulatory hormone produced by α-cells in the pancreatic islets of Langerhans [50]. Glucagon was discovered while studying methods of insulin purification; it was found that a certain fraction was able to cause a rise in blood glucose in pancreatectomized dogs [51]. After pancreatic glucagon was discovered, it was found that a similar substance was also produced in the intestinal mucosa [52]. The gene that encodes glucagon also encodes GLP-1 and GLP-2, which are produced by the L-cells in the intestines [43,44,53,54]. The canonical function of glucagon is to promote glycogenolysis and gluconeogenesis in the liver thereby raising blood glucose levels [55].
In addition to its gluconeogenic effects, glucagon has long been known to exhibit thermogenic properties with numerous reports of glucagon increasing body temperature, oxygen consumption, metabolic rate and thermogenic markers in vivo [56–76]. The concomitant lipolytic effects, such as increasing circulating glycerol and free fatty acids, are also observed in response to glucagon administration in vivo [60,65,66,77–84]. Glucagon has apparent direct effects on adipose tissue as the glucagon receptor (Gcgr) is expressed in adipocytes [75,85–96]. There are numerous reports of glucagon increasing intracellular cAMP and adipocyte lipolysis in vitro and ex vivo [75,85,87,97–128]. Furthermore, several studies have shown that glucagon increases oxygen consumption and thermogenesis in isolated adipose tissue or cells [65,71,75,129–133]. Nevertheless, in vivo studies present a much more complicated picture of how glucagon regulates body temperature and adipose thermogenesis. Glucagon appears to partially exert its pro-thermogenic effects through an indirect, centrally mediated mechanism, as central administration of glucagon increases brown adipose tissue thermogenesis [134]. Additionally, these thermogenic-effects of glucagon may be indirect, as they are oftentimes blocked by propranolol or adrenalectomy [56,57,71]. Somewhat contradicting these findings are studies showing that denervation is ineffective at altering glucagon-evoked changes in thermogenic mitochondrial markers or lipolysis [67,81]. In one instance the effects of glucagon on energy expenditure were partially blunted in mice lacking Fgf21, further suggesting that much of its effects are indirect [75]. Further complications regarding glucagon’s thermogenic role in vivo arise from the observations that the glucagon-evoked rise in body temperature is intact in mice that have a Ucp1 gene knockout [75] and in swine, which have an ancestral disruption of the Ucp1 gene [135,136], indicating that the effects of glucagon may not be entirely dependent upon uncoupled respiration.
Despite the uncertainty behind the mechanism, the glucagon family of peptides appear to be an important component of the thermogenic response, as mice with a global gene knockout of the pro-glucagon gene have impaired responses to cold or β3-agonist treatment [137]. Additionally, mice with a global gene knockout of Gcgr, though they remain cold tolerant and have unaltered sympathetic tone, exhibit impaired cold-induced browning of white adipose tissue [76]. From these studies, it appears that glucagon augments energy expenditure through both direct and indirect effects. While studies have demonstrated that glucagon directly binds its own receptor on adipocytes and promotes fatty acid liberation and thermogenesis, glucagon also acts through secondary pathways, such as catecholamines and FGF21, to promote adipocyte lipolysis and browning. Though the extent at which glucagon acts through direct versus indirect mechanisms to augment energy expenditure remains a bit unclear, this remains an important component of its activities and has been a therapeutic goal for agonists that target the glucagon receptor.
Glucagon like peptide-1
GLP-1 and GLP-2 are derived from the same gene as glucagon and are produced in the L-cells of the intestines and released in response to food intake [43,44]. As such, one of their main functions as incretins is to augment glucose stimulated insulin secretion from the islet β-cell [138]. While GLP-2 has been less well studied, the effects of GLP-1 on the β-cell have been exploited for therapeutic benefit, as several GLP-1 receptor agonists have been developed to treat Type 2 diabetes. These include exenatide (synthetic exendin-4, Byetta), liraglutide (Victoza), dulaglutide (Trulicity), albiglutide (Tanzeum), lixisenatide (Adlyxin) and semaglutide (Ozempic). In addition to their effect on the β cell, these GLP-1 agonists also have roles in other tissues through which they can regulate food intake and weight gain [139]. This ‘side effect’ of GLP-1 agonists first became clinically applicable in December 2014, when liraglutide was also approved for weight loss under the product label Saxenda.
Although GLP-1 has a well-established role in the β cell, its function in adipose tissue is a bit more controversial. Even the question of whether or not the GLP-1 receptor is expressed in adipose tissue has produced conflicting results. For example, a few studies utilizing Northern Blots failed to observe the GLP-1 receptor (Glp1r) mRNA [140,141], most studies using more sensitive methods, such as PCR or radioligand binding, did find the mRNA or protein in adipose tissue [85,86,94,95,142–147]. Similarly, GLP-1 has been observed to both increase [85,113,126,128] or not alter [117,148,149] intracellular cAMP and lipolysis in adipocytes. Globally, GLP-1 was suggested to play some role in thermogenesis in a study of mice with a whole body knockout of Glp1r: Glp1r−/− mice had relatively lower energy expenditure at room temperature, but this difference was ablated at thermoneutrality [150]. Furthermore, although chow fed Glp1r−/− mice showed a very modest increase in oxygen consumption in response to norepinephrine treatment, high fat diet fed Glp1r−/− mice had a significant reduction in response to norepinephrine [150]. By contrast, another group found increased oxygen consumption in Glp1r−/− mice on both regular chow and high fat diet, which was attributed to increased locomotor activity [151]. Despite these conflicting results for direct effects of GLP-1 on adipose tissue, central administration of GLP1R agonists: GLP-1, liraglutide and exendin-4; all found increased adipose tissue browning and energy expenditure [134,152,153]. Nevertheless, it has been reported that signaling through the GLP1R in vagal afferent neurons decreases energy expenditure and brown adipose tissue thermogenesis [154]. These findings make interpreting results from peripheral administration of these agonists difficult, as the contribution of the indirect stimulation of adipose tissue remains possible. In studies where humans were administered GLP-1, no effect on energy expenditure has been observed [72,73]. However, when exendin-4, liraglutide, GLP-1 [32–36] amide (LVKGR amide) and supaglutide (a tool compound that is not in the clinic) were administered to rodents, oxygen consumption was increased and adipose tissue browning was observed [155–159]. This effect was lost in mice housed at thermoneutrality, as 6 weeks of liraglutide treatment failed to affect thermogenic gene expression in the adipose tissue of mice and any changes in oxygen consumption in response to norepinephrine were comparable with that of untreated mice that were pair-fed with the liraglutide treated mice [150]. This indicates that these apparent changes in energy expenditure may be secondary to changes in body weight. While the central effects of GLP1R agonists on weight gain and energy expenditure are well established, their peripheral effects that directly impact the adipocyte are much less understood and it is questionable how much of an impact adipocyte GLP1R have on thermogenesis.
Gastric inhibitory polypeptide
Entero-gastrone was the name first given to what was then a putative hormone that was reported to be released in response to dietary fat and inhibits gastric secretion [160]. Later efforts to purify Entero-gastrone yielded the hormone that we now call Gastric Inhibitory Polypeptide or Glucose-dependent Insulinotropic Polypeptide (GIP) [161–164]. GIP is expressed in the K cells of the intestines [165] and is well known for its role augmenting glucose stimulated insulin secretion as an incretin [166]. The GIP receptor (GIPR) is also highly expressed in mature rodent and human adipocytes [147,167–177]. It appears that GIP–GIPR signaling on adipocytes has surprisingly similar effects to that of insulin: they both promote glucose and fatty acid uptake and lipid accretion in adipocytes [173,178–186]. In vivo, this may not be surprising given the close association between GIP and insulin as elevation of GIP levels will result in a concomitant rise in insulin in the fed state that may itself be mediating these effects [187]. However, in vitro, where there is no accompanying insulin release, this association is somewhat unexpected in adipocytes as GIPR is Gαs coupled and raises cAMP concentrations and PKA-evoked signaling [95,167,171,174,177,188–191]. Though most studies find that GIP raises cAMP in adipocytes, its effects on lipolysis are somewhat controversial with some studies finding an increase [168,181,185,189], and others a decrease [116,192] in lipolysis, and yet others with no effects [193]. One study somewhat reconciled the conflicting observation of GIP promoting both fatty acid uptake and lipolysis: they found that GIP increased both glycerol release and fatty acid re-esterification, which resulted in an overall decrease in free fatty acid release despite the increased lipolysis [185].
GIP–GIPR signaling also has an important role in weight gain in response to high fat diet. Several maneuvers including GIPR knockout mice, GIPR antagonists, Gip-expressing K cell ablation and neutralization of GIP or GIPR with an antibody all exhibited resistance to diet-induced obesity [151,177,184,194–203]. GIPR signaling in adipose tissue appears to contribute significantly to this effect as mice with adipose tissue specific GIPR knockout are resistant to high fat diet-mediated weight gain [201], and re-expression of GIPR in the adipose tissue of whole body GIPR knockout restores the weight gain phenotype in response to high fat diets [198]. Brown adipose tissue was not considered to be mediating the effect, since mice with a Gipr knockout driven by Myf5-Cre had similar body weights upon high fat diet feeding [204]. However, the specificity of Myf5-Cre is not confined to the brown adipocyte, but also includes skeletal muscle; and there have been unexpected phenotypes associated with this Cre driver [205]. The effect of GIPR on lipid metabolism may mediate much of its effects on weight gain as GIPR ablation reduces the respiratory quotient, indicative of greater lipid oxidation. However, that said, most, but not all, studies observe no effects on energy expenditure [177,184,199,204,206]. Paradoxically, administration of a DPP-4-resistant GIP analog or GIP overexpression also appears to reduce weight gain when fed a high fat diet [207,208]. Along with this, both GIP treatment and Gipr knockdown were reported to increase UCP1 in immortalized BAT cells along with an increase in their maximal oxygen consumption [204]. Similar findings were observed in Gipr−/− mice where Ucp1 mRNA was increased in brown adipose tissue of mice fed a high fat diet [151]. These studies indicate that GIP–GIPR signaling in adipocytes exists and may regulate metabolic phenotype, but its role is unclear at this point and more work is needed to clarify it.
The engineered polyagonists
An innovative concept has emerged in this area that takes advantage of the similarities between these incretin peptides and their receptors in order to marshal the potential therapeutic benefits of stimulating each of these receptors, by developing single molecules that can activate multiple receptors in this family, to unique benefit [209]. The first ‘designer’ dual-agonists contain features and activities at the glucagon and GLP-1 receptors [210,211]. The idea behind this was to combine the thermogenic and lipolytic properties of glucagon with the anti-hyperglycemic properties of GLP-1 in order to counteract the glucose raising glycogenolytic and gluconeogenic properties of glucagon. Moreover, being a single molecular entity has clear benefits for patenting purposes. These peptides reduce body weight in mice with diet induced obesity [210,211] as well as reducing steatohepatitis [212]. While there is an effect to decrease food intake, despite this, energy expenditure is also increased, respiratory quotient is decreased, and some versions of this dual agonist increase UCP1 in brown adipose tissue. Another dual-agonist that was developed is a peptide that activates the GLP-1 and GIP receptors [213]. This peptide also reduces food intake, body weight and blood glucose, but it had no effect on energy expenditure or respiratory quotient. A tri-agonist that combines the activities of glucagon, GLP-1, and GIP has also been made [191]. This tri-agonist was found to reduce body weight and fat mass, reduce food intake, increase energy expenditure, decrease respiratory quotient, and improve glucose homeostasis in mice with diet induced obesity. Some of the weight-lowering effects of the tri-agonist were clearly due to increased energy expenditure as the weight lost in tri-agonist-treated mice was greater than that of their pair-fed controls. The glucagon-like actions of the tri-agonist were largely responsible for the reduction in body weight and food intake as these effects were lost in Gcgr−/− mice. The GLP-1-like actions were important for glycemic control as the tri-agonist increased blood glucose in Glp1r−/− mice. Also, the loss of body weight and food intake were more modest in these Glp1r−/− mice. The GIP-like functions were also important for the glucose-lowering effect as this was lost in Gipr−/−mice. Apparently, the activation of Glp1r prevented the tri-agonist from raising blood glucose in these Gipr−/− mice. The body weight, food intake and fat mass lowering effects of the tri-agonist were unaltered in the Gipr−/− mice, indicating that the GIP-like effects were not responsible for these phenotypes. This tri-agonist probably has a role in adipose tissue as it stimulates cAMP in 3T3-L1 adipocytes [191], and increased circulating free fatty acid levels in vivo [214]. Although less work has been done to understand the adipose browning potential of these polyagonists, it remains possible that some of their beneficial metabolic effects stem from adipocyte thermogenesis. Some of these polyagonists are now in clinical trials, and it is an exciting new area of basic and translational research.
In addition to these peptide polyagonists, chemical hybrid molecules have also been designed that have potential browning effects. One such molecule is a glucagon and T3 (triiodothyronine) hybrid that consists of the glucagon peptide linked to a T3 molecule [96]. This glucagon/T3 was designed because it would deliver the T3 primarily to the liver and adipose tissue and thereby avoid the adverse effects of T3 in other organs. Glucagon/T3 potently lowered body weight due to increased energy expenditure as it did not alter food intake and was significantly blunted in Ucp1−/− mice. This increased the thermogenic gene profile of inguinal white adipose tissue but had minimal effect on the brown adipose tissue. It is unclear if the glucagon- or T3-activities are the primary drivers of this browning phenotype as the glucagon receptor is necessary for the targeting of glucagon/T3 to the adipose tissue, which is lost in Gcgr−/− mice.
Melanocortins
The endogeneous melanocortins are all products of a single gene, produced via cleavage of the proopiomelanocortin (POMC) polypeptide by prohormone convertases. These melanocortins are agonists for five different Gαs-coupled receptors that are termed MC1R–MC5R. MC1R is expressed in melanocytes and white blood cells, consequently being involved in pigmentation and immune responses. MC2R is expressed in adrenal glands and is an important regulator of glucocorticoid production. MC3R and MC4R are both expressed in the central nervous system and are important regulators of food intake and energy homeostasis. MC5R is expressed in skeletal muscle and adipocytes where it regulates fatty acid oxidation and lipolysis, in addition to exocrine glands where it is associated with sebum production. There are many biologically active peptides that are derived from POMC; these include α-melanocyte stimulating hormone (MSH), β-MSH, γ-MSH and adrenocorticotropic hormone (ACTH; also known as corticotropin), in addition to several others. In addition, there are two endogeneous antagonists/inverse-agonists of the melanocortin receptors that are products of their own genes: agouti and agouti-related protein (AGRP). α-MSH, β-MSH, γ-MSH, ACTH are all agonists for MC1R, MC3R, MC4R and MC5R, though their affinities for the receptors vary. MC2R is unique in that ACTH is its only endogenous ligand; hence, ACTH is the only melanocortin that can bind to and activate all five receptors. The expression patterns of melanocortins vary, with α-MSH being expressed in the central nervous system and hypothalamus, β-MSH in the hypothalamus (except in rodents where POMC has an amino acid substitution that disrupts a cleavage site necessary to produce β-MSH), γ-MSH in the pituitary and hypothalamic arcuate, and ACTH in the anterior pituitary corticotrophs. The ‘Agouti’ peptide serves as an antagonist for MC1R and MC4R and is expressed in skin, testis, ovary and adipose tissues. AGRP is an antagonist for MC3R and MC4R and is produced in the adrenal cortex and certain regions of the central nervous system (reviewed in [215,216]).
ACTH is well known for its ability to stimulate the release of glucocorticoids (cortisol in humans and corticosterone in rodents) from the adrenal cortex and thereby regulate one of the most important aspects of glucose metabolism. In addition to its effects on glucocorticoids, in many species ACTH acts directly upon MC2R in adipocytes increasing intracellular cAMP–PKA signaling and thereby stimulating lipolysis, oxygen consumption and uncoupled thermogenesis [59,97–99,101,103,104,106,107,109,110,115,120,130,133,217–236]. However, MC2R is not expressed in the adipocytes of some species, including primates; as a result, ACTH has no effect on intracellular cAMP, lipolysis or uncoupled thermogenesis in human or canine adipocytes [229,237–240]. In vivo administration of ACTH increases adipocyte browning in rodents; however, this effect is significantly blunted by the concomitant rise in corticosterone, which is a potent inhibitor of adipocyte browning [234]. In addition to MC2R, MC5R is also expressed on rodent adipocytes [228,230–233,236]. α-MSH, which does not bind to MC2R, similarly increases cAMP, lipolysis and uncoupled thermogenesis in these adipocytes; however, the relevance of this pathway in vivo is questionable as the endogenous levels of ACTH and α-MSH in adipose tissue are not likely to be high enough to fully activate MC5R [103,226,228,230,231,233,236,241,242]. Central administration of melanocortins also induces adipocyte thermogenesis, but this effect is dependent upon the central nervous system as effects of centrally administered melanocortins are lost in denervated mice [243–245]. Though agonists activating the melanocortin receptors are potent browning agents in many species, the clinical utility of this pathway in humans is negligible due to the low receptor expression in human adipose tissue.
Parathyroid hormones
Parathyroid hormone (PTH) is an 84-amino acid peptide that is produced by the ‘chief’ cells in the parathyroid glands for which it is named [246,247]. PTH is best known for its effects on the kidney and bone. In the kidney, PTH promotes calcium reabsorption, inhibits phosphate reabsorption, and promotes the synthesis of the vitamin D metabolite, 1,25 dihydroxy-vitamin D3, which itself also promotes calcium reabsorption [248]. In bone, intermittent PTH augments bone growth but continuous PTH signaling favors bone resorption [248]. Certain cancers had long been known to produce a factor that behaved like PTH, but when it was demonstrated that PTH was in fact not the factor, Parathyroid Hormone-related Protein (PTHrP) was identified [249–251]. Mature human PTHrP is a 141-, 139-, or 173-amino acid peptide, depending on the splicing pattern of the transcript [252,253]. PTHrP is produced in a wide variety of tissues in which it acts in a paracrine manner [254]. PTHrP, like PTH, is crucial for bone development, but unlike PTH−/− mice that have bone abnormalities, PTHrP−/− mice die immediately after birth via respiratory failure due to defective rib cage development [255,256]. Aside from its role in bone, PTHrP also plays a role in other diverse processes such as placental calcium transport, lactation and smooth muscle relaxation [254].
There are two receptors for PTH and PTHrP: Parathyroid Hormone 1 Receptor (PTH1R, also known as the PTH/PTHrP Receptor) and Parathyroid Hormone 2 Receptor (PTH2R). Both PTH and PTHrP are agonists for PTH1R, but PTH2R is not activated by PTHrP [257]. Both receptors are primarily coupled to Gαs, but can also be coupled with Gαq/11 [248].
The ability of PTH to stimulate cAMP production and, through the cAMP-PKA signaling is well established, and in adipocytes can stimulate lipolysis [238,258–266]. However, it has only been recently been observed that PTH and PTHrP can have ‘browning’ properties as tumor-derived PTHrP was found to promote cachexia via adipose browning [267]. Shortly thereafter, the same authors found that PTH also promotes browning and cachexia in a 5/6 nephrectomy model of kidney failure [268]. Both PTH and PTHrP promote browning and cachexia via PTH1R signaling through the Gαs–PKA pathway [267,268]. PTH and PTHrP both increase respiration and promote the expression of thermogenic genes in adipocytes [236,267,268].
Prostaglandins
The ‘E-series’ prostaglandins are known to raise the body temperature by inducing fever. In fact, during the first study examining the effect of prostaglandin E2 (PGE2) infusion in humans, a ‘feeling of warmth’ was among the first symptoms reported [269]. This pyretic response of PGE2 is mediated through the EP3 receptor that is located in hypothalamic neurons of the preoptic nucleus [270,271]. Downstream neural signaling events lead to classical SNS activation of BAT and thereby raise body temperature [272,273]. It appears that PGE2 primarily regulates fever-induced thermogenesis and has minimal effects on normal body temperature regulation (reviewed in [274]; the role of other prostaglandins in hyperthermia are reviewed in [275]). Despite this, prostaglandins have very important roles in adipocytes, including adipogenesis, lipolysis and the regulation of thermogenic adipocytes.
Prostaglandins are bioactive lipid-signaling molecules that are oxidative metabolites of arachidonic acid; they are produced by cyclooxygenase enzyme (COX1 and COX2) metabolism of arachidonic acid into an intermediary metabolite (PGH2) followed by its rapid conversion into one of the five bioactive prostanoids (PGE2, PGD2, PGF2α, PGI2, TXA2) by their respective synthases (Figure 3). Prostaglandins act locally in an autocrine or paracrine manner that is dependent on the activation of a family of specific GPCRs. The two predominant prostaglandins in adipose tissue appear to be PGE2 and PGI2. These generally have an opposing effect in adipose tissue [276] with prostacyclin (PGI2) signaling through its lone Gαs-coupled receptor, IP, and PGE2 signaling through its predominantly Gαi-coupled receptor, EP3 [277,278]. As such, many studies have shown that in adipocytes, signaling through IP raises cAMP [279–283] while PGE2 and its synthetic analogs lower cAMP [284–295]. It should be noted that three other receptors for PGE2 exist, with EP2 and EP4 being Gαs-coupled and EP1 being Gαq-coupled [277,278]; hence the actions of PGE2 may raise cAMP depending on the circumstances. EP3 antagonists or gene knockout blocks the cAMP- and lipolysis-lowering effects of PGE2 in adipocytes [295,296], indicating that these effects of PGE2 occur via EP3.
Figure 3. Prostaglandin biosynthetic pathway and signaling.
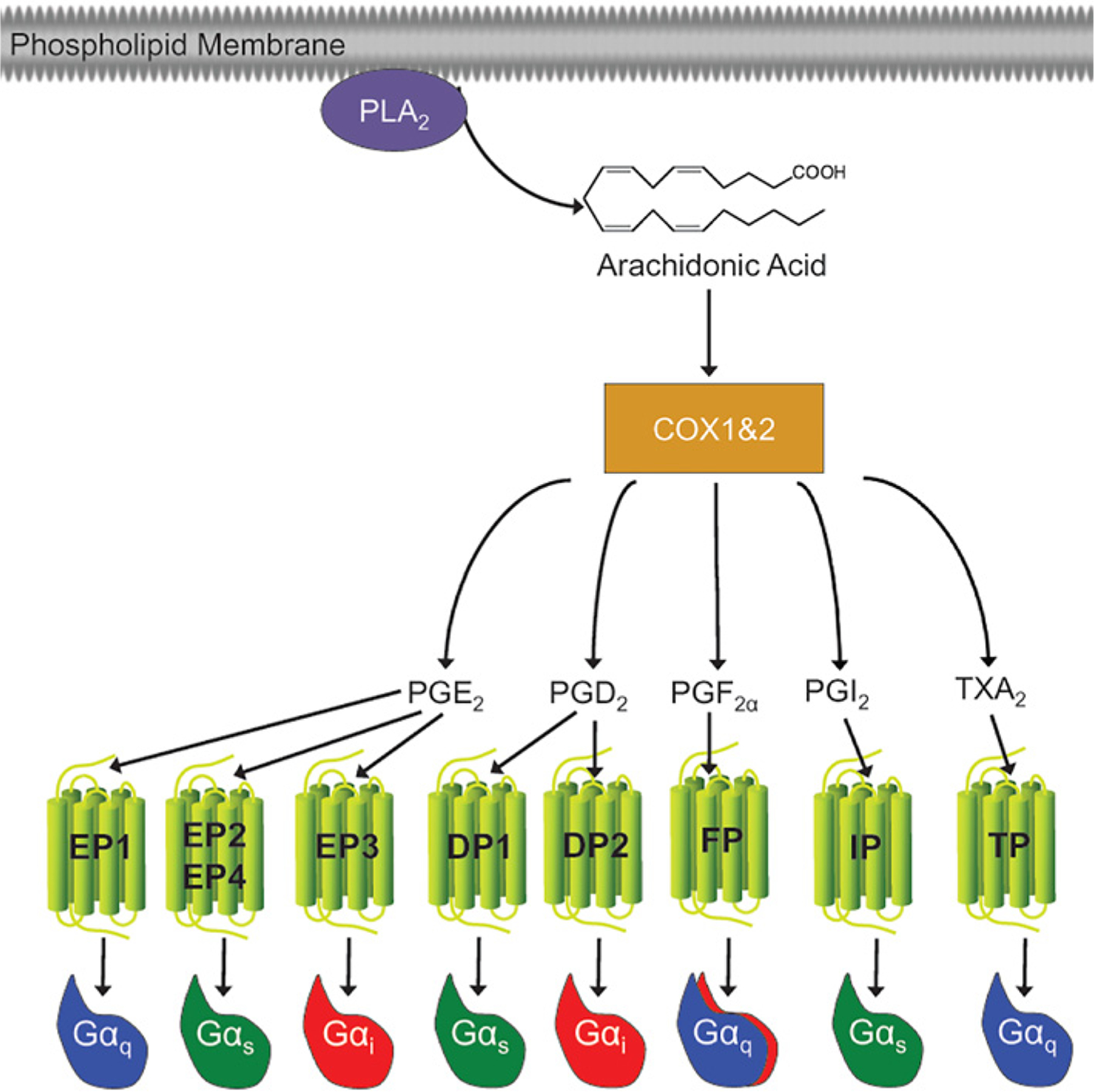
Prostaglandins are oxidative metabolites of arachidonic acid that are produced by cyclooxygenase (COX) enzymes. Arachidonic acid is liberated from the plasma membrane by phospholipase A2 (PLA2). COX converts arachidonic acid into the short lived PGH2 (not shown) which is converted into five primary bioactive prostanoids: PGE2, PGD2, PGF2α, PGI2 (prostacyclin) and TXA2 (thromboxane). These prostanoids act locally in an autocrine or paracrine manner. The local action of prostaglandins depends on activation of a family of GPCRs designated EP (for E prostanoid), DP, FP, IP and TP receptors, for the other prostaglandins, respectively.
COX inhibition with the nonselective inhibitor indomethacin, the COX2 selective inhibitor celecoxib, or COX2 gene deletion have been shown to inhibit beiging of inguinal WAT [297,298]. Consistent with its ability to stimulate Gαs-coupled receptors, PGI2 and its synthetic analog carbaprostacyclin have been observed to increase Ucp1 and other thermogenic gene expression in adipocytes [298–301]. Additionally, the PGI2 analog beraprost increases skin temperature in mice [302], though it is unclear if this is due to a direct effect on the adipose tissue or via neural pathways. The roles of PGE2 and other prostaglandins are less clear. PGE2 has been shown to increase UCP1 mRNA expression in human omental white adipose tissue explants [303], mouse primary brown adipocytes [236], and in mice inguinal white adipose tissue using an adipose-targeted nanoparticle delivery system [304]. 16,16-dimethyl-PGE2, an EP2/EP3/EP4 agonist, has been reported to also increase the expression of thermogenic gene expression in BAT in vivo [297]. However, 16,16-dimethyl-PGE2 was also shown to elicit a dose dependent decrease in UCP1 expression in rosiglitazone-treated hMADS [305]. The EP4 antagonist, AH-23848, and to a lesser extent the EP2 antagonist, AH-6809, attenuated isoproterenol-evoked Ucp1 mRNA and thermogenic gene expression in Retinoblastoma-null adipocytes [297]. These studies indicate that in adipocytes PGE2 and PGI2 may have a pro-thermogenic role (Figure 4). PGD2 may also have a role in BAT as the expression of one of its synthases, lipocalin prostaglandin D synthase, is induced in BAT by cold exposure and global knockout is associated with a change in fuel utilization during cold-exposure [306]. Additionally, the subcutaneous WAT of these knockout mice showed a 4-fold increase in Ucp1 mRNA expression over the wild-type [307].
Figure 4. Prostaglandin thermogenic signaling.
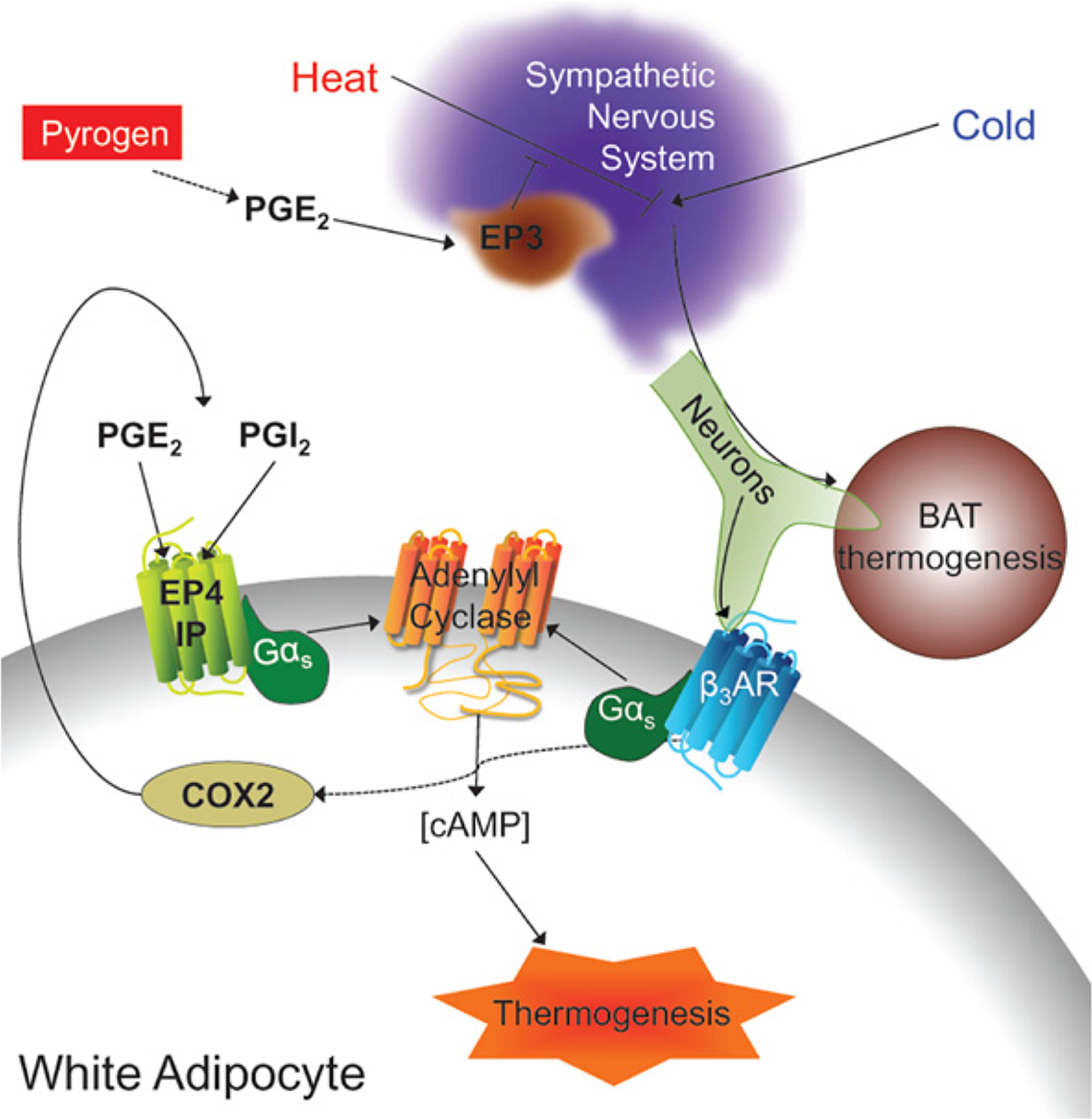
PGE2−EP3 signaling is well known to be an important regulator of the increased thermogenesis that occurs during fever. In addition, recent studies have shown that prostaglandins are important regulators of adipocyte browning.
Guanylyl cyclases
In addition to cAMP, another cyclic nucleoside, cGMP, is an important second messenger. Unlike the hundreds of GPCRs that influence cAMP levels, cGMP is regulated by a much more finite group of upstream signaling mediators. cGMP is made by guanylyl cyclases that convert GTP into cGMP in much the same way that adenylyl cyclase converts ATP into cAMP. Guanylyl cyclases can be divided into two categories: soluble and membrane bound (also called ‘particulate’). Soluble guanylyl cyclases are heterodimeric being made up of α and β subunits, both of which have two isoforms, and are activated by nitric oxide (NO) [308,309]. Nitric oxide synthases (NOS), of which there are three isoforms termed endothelial (eNOS), inducible (iNOS), and neuronal (nNOS) [310], mediate the endogenous production of NO by converting l-arginine into citrulline and NO [311]. In addition, nitrate (NO3−) and nitrite (NO2−), which had been considered inert end products of NO metabolism, have been recognized for their contribution to NO production and signaling via the nitrate–nitrite–NO pathway [312]. There are seven membrane bound guanylyl cyclases (though not all are found in humans) termed Guanylyl Cyclase A-G (GC-A and -B are also commonly referred to as Natriuretic Peptide Receptor A and B, respectively); they are homodimers consisting of an extracellular ligand binding domain, a transmembrane domain, followed by the intracellular kinase homology domain, hinge region, and guanylyl cyclase catalytic domains [308,313]. cGMP activates two serine/threonine kinase termed protein kinase G-I and -II (PKG), which are similar to PKA in that they phosphorylate many of the same targets and are both activated by cyclic nucleoside binding to their regulatory subunits. PKG differs from PKA in that its regulatory subunit is part of the same polypeptide as its catalytic subunit. Like activation of PKA, activation of PKG increases the browning of adipose tissue, increasing mitochondrial biogenesis and UCP1 expression, and these effects are ablated with PKG inhibitors or PKG-I gene knockout [314–316]. Though there have been numerous studies that have demonstrated increased adipocyte browning by targeting GPCRs and thereby cAMP, targeting of cGMP-regulated pathways remains less studied (Figure 5).
Figure 5. Guanylyl cyclase dependent activation of adipocyte lipolysis and thermogenesis.
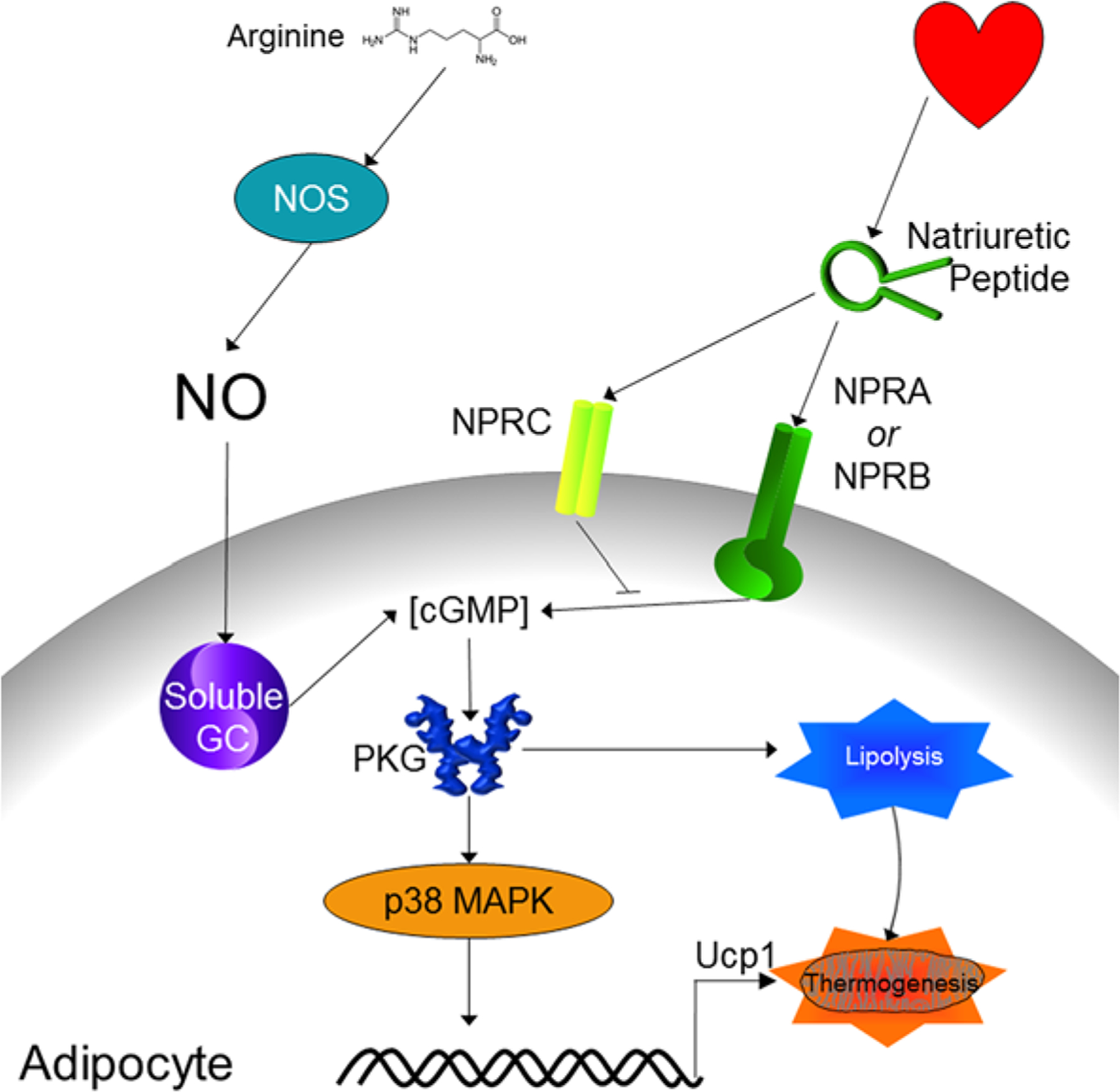
Activation of both soluble and membrane bound guanylyl cyclases in adipocytes increases intracellular cGMP concentrations. This leads to increased PKG activity, which phosphorylates and activates signaling pathways that cause increased lipolysis and thermogenic gene expression.
Soluble guanylyl cyclases
Drugs that activate soluble guanylyl cyclases by acting as NO donors, called nitrovasodilators, have long been utilized to relax the vascular endothelium and reduce blood pressure. Nitroglycerine is one of the earliest nitrovasodilators; it was suspected of being a vasodilator a few years after its initial synthesis [317,318]. Within three decades, it was already being utilized therapeutically to treat angina pectoris and reduce blood pressure [319]. A century later, the mechanism behind nitroglycerine’s vasodilation was elucidated when it was found to be a NO donor around the same time that NO produced by the endothelium was recognized as an important signaling molecule for the relaxation of smooth muscle [311,320–323]. Nitroglycerin and other traditional vasodilators aremain important pharmacological tools and are still used to treat acute heart failure [324].
In addition to its effects on vasodilation, NO has important effects on energy expenditure and adipose tissue. Several studies have shown that NO stimulates mitochondrial biogenesis in adipocytes [325–327]. In vitro, it appears that NO-cGMP signaling disrupts pre-adipocyte proliferation and pushes them into differentiating into a more brown-like adipocyte [314,316,328–332]. This mitochondria-generating effect is not limited only to adipose, as this phenomenon has been reported in several other tissues [333]. In addition to promoting mitochondrial biogenesis, NO itself directly affects mitochondrial respiration. NO can directly bind to the O2 binding site in cytochrome oxidase inhibiting its turnover and thereby mitochondrial respiration [334–337]. As with other tissues, this reaction also occurs in brown adipose tissue [338].
NO–cGMP evoked pathways appear to be an important component of the cold-induced browning phenotype as cold-norepinephrine-cAMP-evoked signaling induces iNOS and eNOS expression and activity in adipocytes [339–343]. This appears to be of functional importance as inhibition of NOS activity with Nω-nitro-l-arginine methyl ester (l-NAME) impairs this catecholamine-induced browning [339,344–347]. In vivo treatment with l-NAME actually decreases body weight and adipose tissue mass, but this appears to be primarily the result of decreased food intake [345,348–351]. Conflicting results were obtained for l-NAME’s effect on UCP1 in BAT [351,352], but l-NAME administration to rat pups reduced their oxygen consumption during cold stress [353].
Targeting of the individual NOS’s produces somewhat different results. Though Enos−/− mice maintain similar body weights and food consumption; oxygen consumption, Ucp1 expression, mitochondrial β-oxidation, and cold-induced mitochondrial biogenesis are all reduced [325,327,354,355]. Overexpression of eNOS is beneficial as it increases mitochondrial biogenesis in adipose tissue conferring a brown color, increases oxygen consumption, and reduces weight gain conferring resistance to diet induced obesity [356].
On the other hand, iNOS appears to be maladaptive as iNOS−/− mice are protected against proinflammatory macrophage infiltration of adipose tissue and the associated extracellular matrix deposition, fibrosis and insulin resistance [357–359]. On a normal standard chow diet, iNOS−/− mice maintain body weights similar to that of wild-type, thought they have been reported to be slightly heavier or to have reduced fat mass [360,361]. One study reported that these mice have reduced food intake, heat production, CO2 output and respiratory exchange ratio [361]. However, in Lepob/ob iNOS−/− mice, body weight was reduced, core body temperature was increased, and the brown adipose tissue had increased UCP1 and fluorodeoxyglucose uptake [362,363]. Similarly, the decreased expression of genes associated with mitochondrial biogenesis upon high fat diet feeding was rescued in iNOS−/− mice [357].
Though iNOS produced NO appears to be detrimental to a healthy metabolic phenotype, activation of the soluble guanylyl cyclases in adipose tissue still appears to be beneficial. Mice lacking the β1 subunit of soluble guanylyl cyclase have impaired thermogenesis due to reduced mitochondria and UCP1 in the brown adipose tissue [332]. In addition, activation of soluble guanylyl cyclases with the agonist, BAY41–8543, increases whole-body energy expenditure, induces browning of WAT, and protects against diet-induced weight gain [332]. Similarly, the addition of sodium nitrate to the diet, which leads to the production of biological NO independent of NOS via the nitrate–nitrite–NO pathway, has beneficial metabolic effects. When sodium nitrate is added to the drinking water, body weight is reduced in both wild-type and eNOS−/− mice [364,365], and significant morphological changes to the white adipose tissue, inducing the formation of multilocular adipocytes and the expression of Ucp1 and other thermogenic genes.
As with most other agents that induce adipocyte browning, NO–cGMP signaling also plays a role in adipocyte lipolysis. NO donors stimulate lipolysis, increasing glycerol release from adipocytes [366–369]. However, NO donors also reduce and NOS inhibitors augment isoproterenol-stimulated lipolysis [366,370–372]. This occurs because NO can directly interact with isoproterenol altering its chemical composition converting it into a molecule that is not a β-agonist [373]. NO-signaling may be an important component of PKA-mediated lipolysis, as NOS inhibitors have been reported to reduce the lipolytic response to β3- and cAMP-stimulated lipolysis [369,374]. It has been posited that the antioxidant properties of NO mediate this effect as antioxidants rescue the lipolysis lost to NOS inhibitors [374]. Additionally, cAMP-signaling increases nitrite levels and this may be an important source of NO for lipolysis [368,369]. Contradicting these observations of NO-stimulated lipolysis, the NOS inhibitor NG-monomethyl l-arginine (l-NMMA), but not its inactive isomer d-NMMA, increase lipolysis [370,375]. A similarly enhanced lipolytic response was observed it the NOS inhibitor l-NAME, but its inactive isomer d-NAME also enhanced lipolysis indicating that it was caused by an off-target effect [374].
Membrane bound/particulate guanylyl cyclases – natriuretic peptide receptors
Natriuretic peptides (NPs) are peptides that are well known for their abilities to lower blood pressure via diuresis and the eponymous natriuresis [376]. There are three types of NPs: Atrial Natriuretic Peptide (ANP), B-type Natriuretic Peptide (also called Brain Natriuretic Peptide, BNP), and C-Type Natriuretic Peptide (CNP). Mature ANP is 28-, CNP is 22-, and BNP can vary between 26- and 45-amino acids depending on species, with each containing a 17-amino acid loop bound by a disulfide bond [377]. ANP and BNP are secreted primarily from atrial cardiomyocytes in response to mechanical stretch, whereas CNP is produce by a variety of tissues including vasculature, brain and pituitary gland [378].
NPs act on three receptors termed Natriuretic Peptide Receptor A (NPRA, also called Guanylyl Cyclase A, GC-A), Natriuretic Peptide Receptor B (NPRB, also called Guanylyl Cyclase B, GC-B), and Natriuretic Peptide Receptor C (NPRC, also called the Natriuretic Peptide Clearance Receptor; note NPRC is not the same as GC-C which is the guanylyl cyclase-coupled receptor for guanylin). NPRA binds to both ANP and BNP with pM affinities but does not interact with CNP at physiological concentrations. Conversely, NPRB only interacts with CNP, which it binds in the pM range, and does not appreciably bind ANP and BNP whose affinities are in the nM range. All NPs interact with NPRC with pM affinities [377,379].
NPRA and NPRB have a similar structure, containing an extracellular ligand-binding domain, a single trans-membrane region, and the kinase homology domain (which represses the guanylyl cyclase activity in the inactive state) and guanylyl cyclase catalytic domain in the intracellular portion [313,377,380]. Due to their guanylyl cyclase activity, NPRA and NPRB are also referred to as Particulate Guanylyl Cyclases or Membrane-Bound Guanylyl Cyclases [313]. Circulating NPs bind to inactive homodimers of these NPRs, and this is thought to produce a conformational change that leads to the activation of their intrinsic guanylyl cyclase activity. NPRC also contains an extracellular ligand-binding domain and single trans-membrane region, but only has 37-amino acids in the intracellular portion and hence does not synthesize cGMP [313,377,380]. NPRC was first described as a ‘clearance’ receptor because early studies found that though it has no effect on cGMP levels, the NPRC selective ligand C-ANP4–23 increases circulating NPs in vivo (i.e. it is thought that NPRC clears NPs from the circulation because the NPRC selective ligand competes with the other NPs to be cleared) [381,382]. NPRC also reduces the efficacy of ANP in vitro, as we have shown that adding NPRC to cells causes a rightward shift in the ANP dose–response curve [383]. Despite its minimal intracellular domain, NPRC has been reported to promote clathrin-mediated endocytosis [384] and some studies have indicated that NRPC is capable of activating Gαi signaling [385–389].
Both white and brown adipose tissues are important sites of NP action, demonstrating high levels of NP binding and high expression of all three receptors [315,390–396]. Numerous studies have shown that in adipose tissue the expression levels of the NP receptors are dynamic and that the ratio of the guanylyl cyclase-coupled receptors, NPRA and NPRB, to the clearance receptor, NPRC, is an important component of metabolic function (i.e. with a higher NPRA/NPRC or NPRB/NPRC ratio, NPs will have a stronger signaling effect and will be more able to raise intracellular cGMP concentrations). In obese patients and animal models of obesity, the adipose tissue NPRA&B/NPRC ratio is lower indicating impaired NP signaling [396–405]. Additionally, in adipocyte NPRA/NPRC ratio can be lowered by insulin, which is elevated during obesity associated Type 2 diabetes [396,403,406]. Conversely, dieting/fasting and cold exposure raises the NPRA&B/NPRC ratio in adipose tissue indicating improved NP signaling [315,398,407,408]. Consistent with this, increased NP signaling has been demonstrated to improve metabolic dysfunction [383,398–400,409]. In adipose tissue, NP signaling increases intracellular cGMP concentrations [393,410], which have effects that are largely similar to increasing intracellular cAMP concentrations. For instance, NPs promote glucose uptake in adipocytes [383,411,412] and are potent lipolytic agents [383,395,402,406,409,411,413–421]. As expected, in obese subjects with a reduced NPRA/NPRC ratio the lipolysis response is reduced, but is increased in patients on a low-calorie diet [402,419].
Perhaps not surprisingly, given that PKA and PKG phosphorylate highly similar peptide motifs (RRXS*/T*), cardiac NPs have also been shown to stimulate brown fat thermogenesis as well as promote ‘browning’ in white adipose tissue [315,398,400,404,421–423]. Treatment of adipocytes with ANP increases mitochondrial gene expression and increases uncoupled respiration [315,421]. Like the βARs and PKA, the NPs and PKG similarly activate p38 MAPK [315,424] as well as the mTORC1 machinery [423]. It has also been shown that mice overexpressing BNP (driven by the serum amyloid P promoter which expresses BNP in the liver) also have increased oxygen consumption, although increased mitochondria in other tissues such as skeletal muscle may also be playing a role in that model [398]. The metabolically beneficial effects of the cardiac NPs can also be augmented by either eliminating NPRC or by compounds that selectively bind NPRC and effectively block the binding of ANP/BNP [315,383,425]. Increased adipose thermogenesis in mice fed a high fat diet was observed not only in Nprc−/− mice with a global gene knockout, but also in mice with an adipose tissue specific Nprc gene deletion [383]. In both mouse models, there was improved insulin sensitivity, and a generally improved metabolic phenotype [383]. Muscle-specific Nprc knock-out had a minimal impact on metabolic phenotype with regard to high-fat diet feeding, indicating that the beneficial metabolic effects of NPRC ablation are primarily due to changes in the adipose tissue [383]. However, we do not rule out a role for NPRC in skeletal muscle for affecting fatty acid oxidation and outcomes such as muscle performance or endurance.
Phosphodiesterases
While many studies have demonstrated that stimulating the production of cyclic nucleotides in adipose tissue leads to increased browning, thermogenesis and decreased obesity, the fact remains that levels of cAMP and cGMP can also be elevated by decreasing their degradation. The phosphodiesterase (PDE) enzymes inactivate cAMP and cGMP by breaking the phosphodiester bond in these cyclic nucleotides. There are 11 families of PDEs and each family has between one and four members for a total of 24 genes (Table 1) (there is a PDE12, but it breaks the phosphodiester bond in the poly(A) tails of mitochondrial RNA [426]). In addition, complexity increases as each gene product can have multiple splice variants, each of which can then play a role in their subcellular localization and action. Despite this class of enzymes being considerably smaller than GPCRs, they are nevertheless important and well-known therapeutic targets. Of these 11 families, currently 3 families are targets of FDA approved drugs. These PDE inhibitors serve as therapeutics for many diverse conditions such as chronic obstructive pulmonary disease (nonselective: theophylline [427]), acute heart failure (PDE3: milrinone, aka Primacor), intermittent claudication (PDE3: cilostazol, aka Pletaal [428]), chronic obstructive pulmonary disease (PDE4: roflumilast, aka Daliresp), psoriatic arthritis (PDE4: apremilast, aka Otezla [429]), atopic dermatitis (PDE4B: crisaborole, Eucrisa [430]), erectile dysfunction (PDE5: sildenafil, aka Viagra; vardenafil, aka Levitra; tadalafil, aka Cialis; avanafil, aka Stendra [431]), and pulmonary arterial hypertension (PDE5, sildenafil, aka Revatio; tadalafil, aka Adcirca [431]).
Table 1.
Phosphodiesterase family members
| Family | Gene(s) | Affinity (Km) (μM) | Miscellaneous |
|---|---|---|---|
| 1 | Pde1a Pde1b Pde1c | cAMP 0.3–120 cGMP 0.6–6.0 |
Ca2+/calmodulin stimulated |
| 2 | Pde2a | cAMP 30–50 cGMP 10–30 |
cGMP stimulated |
| 3 | Pde3a Pde3b | cAMP 0.02–0.4 cGMP 0.02–0.2 |
cGMP inhibited |
| 4 | Pde4a Pde4b Pde4c Pde4d | cAMP 1.5–10 cGMP NA |
cAMP specific |
| 5 | Pde5a | cAMP NA cGMP 1–6.2 |
cGMP specific |
| 6 | Pde6a Pde6b Pde6c | cAMP NA cGMP 10–17 |
cGMP specific |
| 7 | Pde7a Pde7b | cAMP 0.1–0.2 cGMP NA |
cAMP specific |
| 8 | Pde8a Pde8b | cAMP 0.06–0.6 cGMP NA |
cAMP specific IBMX-insensitive |
| 9 | Pde9a | cAMP NA cGMP 0.17–0.7 |
cGMP specific IBMX-insensitive |
| 10 | Pde10a | cAMP 0.17–1 cGMP 0.3–14 |
cAMP inhibited |
| 11 | Pde11a | cAMP 2.0–3.2 cGMP 0.4–2.1 |
In adipocytes, PDEs have been studied extensively and have a well-established role. Canonical insulin inhibition of lipolysis occurs via activation of PDE3B which in turn degrades cAMP thereby decreasing lipolysis. Non-selective PDE inhibitors, such as IBMX, theophylline and caffeine, have long been known to increase adipocyte lipolysis and thermogenesis [432–437]. Resveratrol, which is found in red wine and other foods [438], is also a non-selective PDE inhibitor, inhibiting PDEs 1, 3 and 4 [439], and has been shown to increase whole body oxygen consumption and Ucp1 expression in mouse WAT and BAT [440]. Despite the common knowledge that PDE inhibition increases adipocyte thermogenesis and the fact that highly selective PDE inhibitors are readily available, much less work has been done to elucidate the role of the individual PDEs.
PDE1 has the unique distinction of being the only family of PDEs that are regulated by calcium-calmodulin signaling [441]. PDE1 was suggested to influence cAMP accumulation in brown adipocytes by an early study that showed that the calcium/calmodulin-mediated decrease in cAMP could be blocked by a non- or PDE1-selective inhibitor [442]. PDE1 activity in brown adipocytes was independently verified in a subsequent study [443]. PDE1B was more conclusively identified as having a role in browning because it is a target of microRNA-378 (miR-378) [444]. miR-378 comes from an intron of PGC-1β, an important regulator of mitochondrial biogenesis and brown fat-specific gene expression; consequently, it has a similar expression pattern. miR-378 overexpression in mice induces brown adipocyte hyperplasia that improves the metabolic phenotype. This miR-378 overexpression also increased cAMP levels and lipolysis in BAT.
PDE3 is perhaps the PDE most commonly associated with adipocytes. This is due, in no small part, to its canonical role in the insulin-mediated inhibition of lipolysis [445]. As expected for an enzyme that degrades cAMP in adipocytes, inhibition or gene knock-out of PDE3 increases browning [446,447]. The PDE3 inhibitor, cilostamide, augments isoproterenol-evoked cAMP and Ucp1 mRNA accumulation in brown adipocytes in vitro [443]. In the absence of β-adrenergic stimulation, cilostamide only increased cAMP and Ucp1 mRNA in the presence of the PDE4 inhibitor, rolipram [443]. In vivo studies by Vincent Manganiello’s group found that Pde3b−/− mice are resistant to high fat diet feeding [447]. This appears to be due to increased mitochondrial activity and thermogenic gene expression, which was observed in epididymal WAT in response to Pde3b knockout and cilostamide [446,447]. Additionally, Pde3b knockout and cilostamide enhanced the effects of β3-adrenergic stimulation on mitochondrial activity, thermogenic gene expression, oxygen consumption, respiration and weight loss [446,447]. This was attributed to a PKA–AMPK mediated signaling pathway [447].
PDE4 is one of the three cAMP specific PDEs. PDE4 inhibitors have recently garnered some attention as a possible therapeutic target for obesity [448,449]. Potential weight loss benefits of PDE4 inhibition has already been observed in chronic obstructive pulmonary disease patients treated with roflumilast, whom display reductions in body weight, though the decline in body weight was greater in the patients that experienced the side effects of diarrhea, nausea, vomiting or headache [450]. Another PDE4 inhibitor, rolipram, also showed a beneficial metabolic response, but in mice [439]. In addition to reduced weight gain when fed a high fat diet, these mice had increased oxygen consumption, body temperature and Ucp1; although increased skeletal mitochondria may be, at least partially, mediating some of these effects [439]. Genetic evidence for a role of PDE4 comes from Pde4b knockout mice, which have a slightly reduced body weight and improved white adipose tissue when fed a high fat diet [451]. However, these mice also displayed increased locomotor activity which, again, may be mediating some of these effects [451]. The protein stability of PDE4D5 is positively regulated by Rheb; consequently, Rheb knockout mice have increased adipocyte cAMP and browning [452]. Though there is significant evidence implicating PDE4 in the augmentation of adipocyte thermogenesis, inhibition of both PDE3 and PDE4 in brown adipocytes results in greater UCP1 expression than inhibiting either alone [443].
PDE5, which is cGMP specific, is perhaps best known as the primary target of sildenafil, the active ingredient in ‘the little blue pill’ Viagra. There is some evidence that PDE5 inhibitors might improve the metabolic phenotype and increase adipocyte thermogenesis. In one study, sildenafil has been shown to improve insulin sensitivity during prediabetes in a randomized, controlled clinical trial [453]. Tadalafil, another PDE5 inhibitor, has been reported to improve forearm glucose uptake in people following a meal [454]. Additionally, mice treated with sildenafil had reduced body weight and fat mass due to increased energy expenditure [455]. This was accompanied by improved insulin sensitivity with increased glucose uptake into the skeletal muscle [455]. Similarly, Wistar rats made insulin resistant with an isocaloric fructose-enriched diet have reduced plasma triglycerides and improved oral glucose tolerance when treated with sildenafil [456]. Given that PDE5 inhibition improves capillary permeability and blood flow, and that blood flow to insulin sensitive tissues is a critical factor regulating insulin action [457], it is possible that some of these beneficial effects of PDE5 inhibition are through its vascular effects. However, direct effects of PDE5 inhibition on adipocytes have been observed. Sildenafil treatment of mice induced browning of inguinal WAT [316], but did not affect UCP1 expression in BAT [455]. Similar results were found in humans, where sildenafil increased UCP1 and mitochondrial respiration in subcutaneous adipose tissue, but not BAT [458] and vardenafil increased PPARGC1A and mitochondrial DNA in omental adipose tissue [326]. Additionally, the PDE5 inhibitor, udenafil, has been found to increase the oxygen consumption rate in 3T3-L1 adipocytes [459]. PDE6 is structurally similar to PDE5, and many of these inhibitors also block PDE6 activity, but PDE6 is primarily expressed in the rod and cone cells of the retina and is therefore unlikely to mediate these effects [460,461].
Pde8a, one of two genes in the IBMX-insensitive and cAMP specific PDE8 family, may also play a role in brown adipose tissue [443].
In addition to the aforementioned PDEs, PDE7B, PDE9A and PDE10A, have been detected in human adipose tissue via Western Blot [462]. Though PDE10A is highly expressed in the striatum with minimal expression in peripheral tissues, PDE10A expression in human supraclavicular BAT and mouse BAT can be seen using the PDE10A radioligand, [18F]-AQ28A [463]. Early studies noted that Pde10a−/− mice weighed less than their Pde10a+/+ counterparts, though no explanation was given [464]. It was only some years later that THPP-6, a PDE10 inhibitor, was found to increase energy expenditure and suppress food intake explaining the reduced body weight [465]. Another PDE10 inhibitor, MP-10, had no effect on food intake, but similarly increased energy expenditure, which was due to increased thermogenesis from brown and visceral white adipose tissue [463]. Similar to other means of elevating cyclic nucleotides, MP-10 also elevated lipolysis in cultured brown adipocytes [463]. These PDE10 inhibitors both caused a net improvement in the metabolic phenotype, improving glucose tolerance and insulin sensitivity [463,465].
Summary/Conclusion
In this review, we have covered studies of second messenger signaling pathways implicated in brown and beige adipocytes from studies performed in cell culture models, rodents and humans (Figure 6 and Table 2). Each of these approaches has its limitations. For example, one procedural – almost philosophical – aspect of studies in rodents is the environmental housing temperature that is used. It is currently a topic of discussion in the field as to whether rodent studies should be conducted at their thermoneutral temperature (~28–30°C; 82°F) instead of our ambient indoor temperature that is thermoneutral for humans. This is because at the latter temperature (~23°C), there is a slight thermal stress to rodents and could impact energy expenditure. We increasingly see publications that include experiments at both temperatures, but the jury is still out. Nevertheless, it is accepted in the field that measurements of energy expenditure should be corrected to body mass (lean mass) and not total body mass.
Figure 6. G-protein coupled receptors and cyclic nucleotide regulation of adipose tissue lipolysis and energy expenditure.
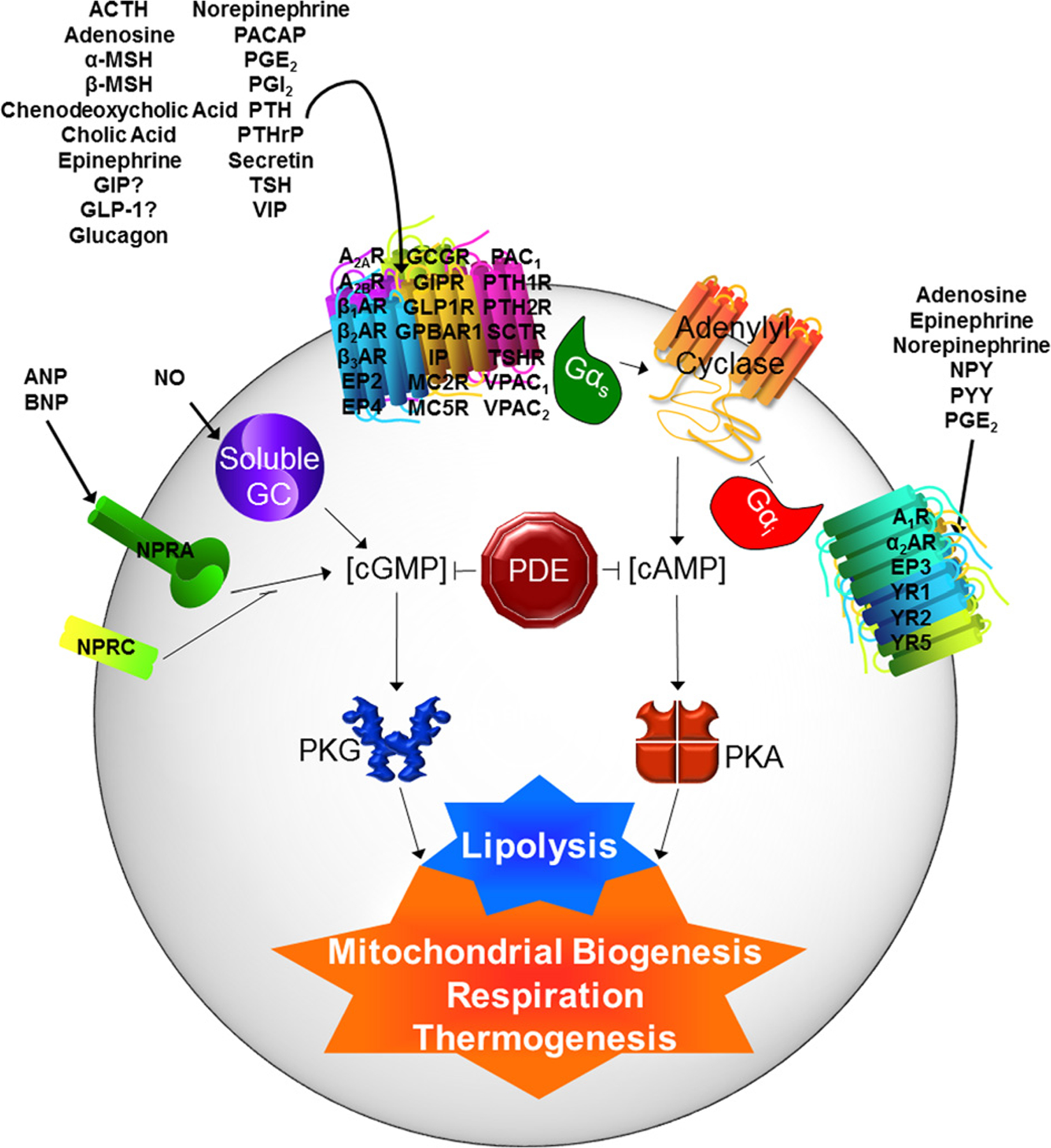
Many hormones utilize GPCR-cAMP- or GC-cGMP-mediated pathways to regulate adipocyte lipolysis and energy expenditure. These pathways are inherently intertwined as the liberation of fatty acids by lipolysis supplies the fuel for the thermogenesis. It is interesting to note that so many of these hormones utilize the same downstream signaling mechanism. For this reason, treatment of adipocytes with these hormones produces (at least superficially) similar results.
The vast majority of studies on this topic of brown and beige adipocytes, energy expenditure and fuel utilization, originally stem from the catecholamines, and particularly the βARs, because of the importance of the sympathetic innervation of adipose depots and to a lesser extent circulating catecholamines. These receptors and cAMP are by far and away the most heavily studied for understanding adipocyte metabolism, and especially the expansion and activation of energy-consuming brown/beige adipocytes. The current optimism in the field that these thermogenic cells may provide some therapeutic benefit for improving insulin sensitivity, reducing inflammation and promoting overall metabolic health has led to exploration of other potential pathways. In this review, we have drawn attention to these many other receptors and mediators of cyclic nucleotide signaling and summarize their effects on adipocytes and adipose tissue. While some of these receptors and ligands are already well studied, others might benefit from deeper investigation into their mechanisms and physiology. In our view, there is never a shortage of surprises to be uncovered when it comes to dissecting signal transduction mechanisms and the web of interactions that they mediate. Perhaps new insight and discoveries from these lesser studied receptors and pathways will yield important new research directions. It is important to remember that “You can’t make the puzzle if you don’t have all the pieces”.
Funding
R.P.C. was supported by NIH F32 DK116520.
Abbreviations
- GPCR
G-protein–coupled receptor
- DNP
2,4-dinitrophenol
- BAT
brown adipose tissue
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- GC
guanylyl cyclase
- PDE
phosphodiesterase
- AR
adrenergic receptor
- PKA
protein kinase A
- PPARγ
peroxisome proliferator-activated receptor gamma
- PGC-1α
PPARγ coactivator-1α
- mTORC1
mTOR complex 1
- GLP
glucagon-like peptide
- GIP
gastric inhibitory peptide
- GHRH
growth-hormone releasing hormone
- PACAP
pituitary adenylate cyclase-activating peptide
- PHM
peptide histidine methionine
- DPP-4
dipeptidyl peptidase-4
- HSL
hormone sensitive lipase
- GCGR
glucagon receptor
- T3
triiodothyronine
- POMC
proopiomelanocortin
- MSH
melanocyte stimulating hormone
- ACTH
adrenocorticotropic hormone
- AGRP
agouti-related peptide
- PTH
parathyroid hormone
- PTHrP
PTH-related protein
- PG
prostaglandin
- COX
cyclooxygenase
- PLA2
phospholipase A2
- NO
nitric oxide
- PKG
protein kinase G
- l-NAME
Nω-nitro-l-arginine methyl ester
- l-NMMA
NG-monomethyl l-arginine
- NP
natriuretic peptide
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R et al. (2016) Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 24, 1612–1619, 10.1002/oby.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laddu D, Dow C, Hingle M, Thomson C and Going S (2011) A review of evidence-based strategies to treat obesity in adults. Nutr. Clin. Pract.: Off. Pub. Am. Soc. Parent. Enteral. Nutr 26, 512–525, 10.1177/0884533611418335 [DOI] [PubMed] [Google Scholar]
- 3.Myers CA, Slack T, Martin CK, Broyles ST and Heymsfield SB (2015) Regional disparities in obesity prevalence in the United States: A spatial regime analysis. Obesity (Silver Spring) 23, 481–487, 10.1002/oby.20963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781, 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundlingh J, Dargan PI, El-Zanfaly M and Wood DM (2011) 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol.: Off. J. Am. College Med. Toxicol 7, 205–212, 10.1007/s13181-011-0162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry RJ, Zhang D, Zhang XM, Boyer JL and Shulman GI (2015) Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256, 10.1126/science.aaa0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedeke L, Peng L, Montalvo-Romeral V, Butrico GM, Dufour S, Zhang XM et al. (2019) Controlled-release mitochondrial protonophore (CRMP) reverses dyslipidemia and hepatic steatosis in dysmetabolic nonhuman primates. Sci. Transl. Med 11, 10.1126/scitranslmed.aay0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeifer A and Hoffmann LS (2015) Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu. Rev. Pharmacol. Toxicol 55, 207–227, 10.1146/annurev-pharmtox-010814-124346 [DOI] [PubMed] [Google Scholar]
- 9.Arechaga I, Ledesma A and Rial E (2001) The mitochondrial uncoupling protein UCP1: a gated pore. IUBMB Life 52, 165–173, 10.1080/15216540152845966 [DOI] [PubMed] [Google Scholar]
- 10.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517, 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND et al. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508, 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 12.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525, 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 13.Hill SJ (2006) G-protein-coupled receptors: past, present and future. Br. J. Pharmacol 147, S27–S37, 10.1038/sj.bjp.0706455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahouth SW and Nooh MM (2017) Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell. Signal 36, 42–55, 10.1016/j.cellsig.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilger D, Masureel M and Kobilka BK (2018) Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol 25, 4–12, 10.1038/s41594-017-0011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sriram K and Insel PA (2018) G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol 93, 251–258, 10.1124/mol.117.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ, Stadel JM and Caron MG (1983) Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu. Rev. Biochem 52, 159–186, 10.1146/annurev.bi.52.070183.001111 [DOI] [PubMed] [Google Scholar]
- 18.Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T et al. (1986) Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79, 10.1038/321075a0 [DOI] [PubMed] [Google Scholar]
- 19.Frielle T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ and Kobilka BK (1987) Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc. Natl. Acad. Sci. U. S. A 84, 7920–7924, 10.1073/pnas.84.22.7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emorine LJ, Marullo S, Briend-Sutren M-M, Patey G, Tate K, Delavier-Klutchko C et al. (1989) Molecular characterization of the human β3-adrenergic receptor. Science 245, 1118–1121, 10.1126/science.2570461 [DOI] [PubMed] [Google Scholar]
- 21.Nahmias C, Blin N, Elalouf J-M, Mattei MG, Strosberg AD and Emorine LJ (1991) Molecular characterization of the mouse β3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 10, 3721–3727, 10.1002/j.1460-2075.1991.tb04940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granneman JG, Lahners KN and Chaudhry A (1991) Molecular cloning and expression of the rat β3-adrenergic receptor. Mol. Pharmacol 40, 895–899 [PubMed] [Google Scholar]
- 23.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL and Gettys TW (1994) Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinol 8, 518–527 [DOI] [PubMed] [Google Scholar]
- 24.Lafontan M, Berlan M and Carpene C (1985) Fat cell adrenoceptors: inter- and intraspecific differences and hormone regulation. Int. J. Obes 9, 117–127 [PubMed] [Google Scholar]
- 25.Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpene C et al. (1997) Adrenergic regulation of adipocyte metabolism. Hum. Reprod 12, 6–20, 10.1093/humrep/12.suppl_1.6 [DOI] [PubMed] [Google Scholar]
- 26.Arner P (2005) Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract. Res. Clin. Endocrinol. Metab 19, 471–482, 10.1016/j.beem.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Garruti G and Ricquier D (1992) Analysis of uncoupling protein and its mRNA in adipose tissue deposits of adult humans. Int. J. Obesity Related Metabolic Disorders: J. Int. Assoc. Study Obesity 16, 383–390 [PubMed] [Google Scholar]
- 28.Krief S, Lönnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P et al. (1993) Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Invest 91, 344–349, 10.1172/JCI116191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lönnqvist F, Krief S, Strosberg AD, Nyberg S, Emorine LJ and Arner P (1993) Evidence for a functional β3-adrenoceptor in man. Br. J. Pharmacol 110, 929–936, 10.1111/j.1476-5381.1993.tb13902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riis-Vestergaard MJ, Richelsen B, Bruun JM, Li W, Hansen JB and Pedersen SB (2019) Beta-1 and not beta-3-adrenergic receptors may be the primary regulator of human brown adipocyte metabolism. J. Clin. Endocrinol. Metab, 10.1210/clinem/dgz298 [DOI] [PubMed] [Google Scholar]
- 31.Collins S (2012) beta-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front. Endocrinol. (Lausanne) 2, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cernecka H, Sand C and Michel MC (2014) The odd sibling: features of beta3-adrenoceptor pharmacology. Mol. Pharmacol 86, 479–484, 10.1124/mol.114.092817 [DOI] [PubMed] [Google Scholar]
- 33.Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM et al. (2000) Direct binding of activated c-Src to the β3-adrenergic receptor is required for MAP kinase activation. J. Biol. Chem 275, 38131–38134, 10.1074/jbc.C000592200 [DOI] [PubMed] [Google Scholar]
- 34.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X et al. (2004) p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol 24, 3057–3067, 10.1128/MCB.24.7.3057-3067.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL et al. (2016) Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J. Clin. Invest 126, 1704–1716, 10.1172/JCI83532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cinti S (1999) The Adipose Organ, Editrice Kurtis, Milano [Google Scholar]
- 37.Rosen ED and Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156, 20–44, 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster DO (1985) Participation of alpha-adrenoreceptors in brown adipose tissue thermogenesis in vivo. Int. J. Obes 9, 25–29 [PubMed] [Google Scholar]
- 39.Zhao J, Cannon B and Nedergaard J (1997) α1-Adrenergic stimulation potentiates the thermogenic action of β3-adrenoreceptor-generated cAMP in brown fat cells. J. Biol. Chem 272, 32847–32856, 10.1074/jbc.272.52.32847 [DOI] [PubMed] [Google Scholar]
- 40.Lafontan M and Berlan M (1995) Fat cell α-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr. Rev 16, 716–738 [DOI] [PubMed] [Google Scholar]
- 41.Harmar AJ (2001) Family-B G-protein-coupled receptors. Genome Biol. 2, REVIEWS3013, 10.1186/gb-2001-2-12-reviews3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B et al. (2003) International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev 55, 167–194, 10.1124/pr.55.1.6 [DOI] [PubMed] [Google Scholar]
- 43.Bell GI, Sanchez-Pescador R, Laybourn PJ and Najarian RC (1983) Exon duplication and divergence in the human preproglucagon gene. Nature 304, 368–371, 10.1038/304368a0 [DOI] [PubMed] [Google Scholar]
- 44.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L and Habener JF (1986) Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J. Biol. Chem 261, 11880–11889 [PubMed] [Google Scholar]
- 45.Bayliss WM and Starling EH (1902) The mechanism of pancreatic secretion. J. Physiol 28, 325–353, 10.1113/jphysiol.1902.sp000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takei Y (2016) Secretin (Pituitary Adenylate Cyclase-Activating Polypeptide) Family. In Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research (Takei Y, Ando H and Tsutsui K, eds), Academic Press, San Diego, 140-e18–2 [Google Scholar]
- 47.Rehfeld JF (2018) The Origin and Understanding of the Incretin Concept. Front. Endocrinol. (Lausanne) 9, 387, 10.3389/fendo.2018.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drucker DJ (2007) Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30, 1335–1343, 10.2337/dc07-0228 [DOI] [PubMed] [Google Scholar]
- 49.Ahrén B (2019) DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. (Lausanne) 10, 376, 10.3389/fendo.2019.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baum J, Simons BE, Unger RH and Madison LL (1962) Localization of glucagon in the alpha cells in the pancreatic islet by immunofluorescent technics. Diabetes 11, 371–374 [PubMed] [Google Scholar]
- 51.Kimball CP and Murlin JR (1923) Aqueous extracts of pancreas: III. Some precipitation reactions of insulin. J. Biol. Chem 58, 337–346 [Google Scholar]
- 52.Sutherland EW and de Duve C (1948) Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J. Biol. Chem 175, 663–674 [PubMed] [Google Scholar]
- 53.Orci L, Pictet R, Forssmann WG, Renold AE and Rouiller C (1968) Structural evidence for glucagon producing cells in the intestinal mucosa of the rat. Diabetologia 4, 56–67, 10.1007/BF01241034 [DOI] [PubMed] [Google Scholar]
- 54.Vaillant CR and Lund PK (1986) Distribution of glucagon-like peptide I in canine and feline pancreas and gastrointestinal tract. J. Histochem. Cytochem 34, 1117–1121, 10.1177/34.9.3755450 [DOI] [PubMed] [Google Scholar]
- 55.Sutherland EW and Cori CF (1948) Influence of insulin preparations on glycogenolysis in liver slices. J. Biol. Chem 172, 737–750 [PubMed] [Google Scholar]
- 56.Davidson IWF, Salter JM and Best CH (1957) Calorigenic action of glucagon. Nature 180, 1124, 10.1038/1801124a0 [DOI] [PubMed] [Google Scholar]
- 57.Davidson IWF, Salter JM and Best CH (1960) The effect of glucagon on the metabolic rate of rats. Am. J. Clin. Nutr 8, 540–546, 10.1093/ajcn/8.5.540 [DOI] [Google Scholar]
- 58.Miller WL and Krake JJ (1963) Comparative expiratory and oxidative effects of glucagon and epinephrine in mice. P. Soc. Exp. Biol. Med 113, 784–788 [Google Scholar]
- 59.Heim T and Hull D (1966) The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J. Physiol 187, 271–283, 10.1113/jphysiol.1966.sp008088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiser PC and Grande F (1974) Calorigenic effects of glucagon and epinephrine in anesthetized dogs. Proc. Soc. Exp. Biol. Med 145, 912–917, 10.3181/00379727-145-37923 [DOI] [PubMed] [Google Scholar]
- 61.Yahata T, Ohno T and Kuroshima A (1981) Improved cold tolerance in glucagon-treated rats. Life Sci. 28, 2603–2610, 10.1016/0024-3205(81)90717-7 [DOI] [PubMed] [Google Scholar]
- 62.Doi K and Kuroshima A (1982) Modified metabolic responsiveness to glucagon in cold-acclimated and heat-acclimated rats. Life Sci. 30, 785–791, 10.1016/0024-3205(82)90614-2 [DOI] [PubMed] [Google Scholar]
- 63.Doi K and Kuroshima A (1982) Thermogenic response to glucagon in cold-acclimated mice. Jpn. J. Physiol 32, 377–385, 10.2170/jjphysiol.32.377 [DOI] [PubMed] [Google Scholar]
- 64.Barre H and Rouanet JL (1983) Calorigenic effect of glucagon and catecholamines in king penguin chicks. Am. J. Physiol 244, R758–R763 [DOI] [PubMed] [Google Scholar]
- 65.Kuroshima A, Yahata T, Habara Y and Ohno T (1984) Hormonal regulation of brown adipose tissue—with special reference to the participation of endocrine pancreas. J. Therm. Biol 9, 81–85, 10.1016/0306-4565(84)90042-1 [DOI] [Google Scholar]
- 66.Barré H, Cohen-Adad F and Rouanet J-L (1987) Two daily glucagon injections induce nonshivering thermogenesis in Muscovy ducklings. Am. J. Physiol 252, E616–E620 [DOI] [PubMed] [Google Scholar]
- 67.Billington CJ, Bartness TJ, Briggs J, Levine AS and Morley JE (1987) Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am. J. Physiol 252, R160–R165 [DOI] [PubMed] [Google Scholar]
- 68.Nair KS (1987) Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J. Clin. Endocrinol. Metab 64, 896–901, 10.1210/jcem-64-5-896 [DOI] [PubMed] [Google Scholar]
- 69.Billington CJ, Briggs JE, Link JG and Levine AS (1991) Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am. J. Physiol 261, R501–R507 [DOI] [PubMed] [Google Scholar]
- 70.Calles-Escandón J (1994) Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism 43, 1000–1005, 10.1016/0026-0495(94)90180-5 [DOI] [PubMed] [Google Scholar]
- 71.Dicker A, Zhao J, Cannon B and Nedergaard J (1998) Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am. J. Physiol 275, R1674–R1682 [DOI] [PubMed] [Google Scholar]
- 72.Tan TM, Field BCT, McCullough KA, Troke RC, Chambers ES, Salem V et al. (2013) Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes 62, 1131–1138, 10.2337/db12-0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA et al. (2014) Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 63, 3711–3720, 10.2337/db14-0242 [DOI] [PubMed] [Google Scholar]
- 74.Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES, Comninos AN et al. (2016) Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes. Metab 18, 72–81, 10.1111/dom.12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beaudry JL, Kaur KD, Varin EM, Baggio LL, Cao X, Mulvihill EE et al. (2019) The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab 22, 37–48, 10.1016/j.molmet.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Townsend LK, Medak KD, Knuth CM, Peppler WT, Charron MJ and Wright DC (2019) Loss of glucagon signaling alters white adipose tissue browning. FASEB J. 33, 4824–4835, 10.1096/fj.201802048RR [DOI] [PubMed] [Google Scholar]
- 77.Lefebvre P (1966) The physiological effect of glucagon on fat-mobilisation. Diabetologia 2, 130–132, 10.1007/BF00423023 [DOI] [PubMed] [Google Scholar]
- 78.Grande F (1969) Lack of insulin effect on free fatty acid mobilization produced by glucagon in birds. Proc. Soc. Exp. Biol. Med 130, 711–713, 10.3181/00379727-130-33639 [DOI] [PubMed] [Google Scholar]
- 79.Rudman D, Del Rio AE, Garcia LA, Barnett J, Bixler T and Hollins B (1970) Lipolytic substances in bovine thyroid, parotid and pineal glands. Endocrinology 87, 27–37, 10.1210/endo-87-1-27 [DOI] [PubMed] [Google Scholar]
- 80.Pozza G, Pappalettera A, Melogli O, Viberti G and Ghidoni A (1971) Lipolytic effect of intra-arterial injection of glucagon in man. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 3, 291–292, 10.1055/s-0028-1096783 [DOI] [PubMed] [Google Scholar]
- 81.Lefebvre P, Luyckx A and Bacq ZM (1973) Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 5, 245–250, 10.1055/s-0028-1093959 [DOI] [PubMed] [Google Scholar]
- 82.Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW et al. (1974) Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J. Clin. Invest 53, 190–197, 10.1172/JCI107537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schade DS and Eaton RP (1975) Modulation of fatty acid metabolism by glucagon in man. I. Effects in normal subjects. Diabetes 24, 502–509, 10.2337/diabetes.24.5.502 [DOI] [PubMed] [Google Scholar]
- 84.Carlson MG, Snead WL and Campbell PJ (1993) Regulation of free fatty acid metabolism by glucagon. J. Clin. Endocrinol. Metab 77, 11–15 [DOI] [PubMed] [Google Scholar]
- 85.Bataille D, Freychet P and Rosselin G (1974) Interactions of glucagon, gut glucagon, vasoactive intestinal polypeptide and secretin with liver and fat cell plasma membranes: binding to specific sites and stimulation of adenylate cyclase. Endocrinology 95, 713–721, 10.1210/endo-95-3-713 [DOI] [PubMed] [Google Scholar]
- 86.Mérida E, Delgado E, Molina LM, Villanueva-Peñacarrillo ML and Valverde I (1993) Presence of glucagon and glucagon-like peptide-1-(7–36)amide receptors in solubilized membranes of human adipose tissue. J. Clin. Endocrinol. Metab 77, 1654–1657 [DOI] [PubMed] [Google Scholar]
- 87.Livingston JN, Cuatrecasas P and Lockwood DH (1974) Studies of glucagon resistance in large rat adipocytes: 125I-labeled glucagon binding and lipolytic capacity. J. Lipid Res 15, 26–32 [PubMed] [Google Scholar]
- 88.Iwanij V, Amos TM and Billington CJ (1994) Identification and characterization of the glucagon receptor from adipose tissue. Mol. Cell. Endocrinol 101, 257–261, 10.1016/0303-7207(94)90242-9 [DOI] [PubMed] [Google Scholar]
- 89.Slavin BG, Ong JM and Kern PA (1994) Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid Res 35, 1535–1541 [PubMed] [Google Scholar]
- 90.Svoboda M, Tastenoy M, Vertongen P and Robberecht P (1994) Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol. Cell. Endocrinol 105, 131–137, 10.1016/0303-7207(94)90162-7 [DOI] [PubMed] [Google Scholar]
- 91.Burcelin R, Li J and Charron MJ (1995) Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene 164, 305–310, 10.1016/0378-1119(95)00472-I [DOI] [PubMed] [Google Scholar]
- 92.Hansen LH, Abrahamsen N and Nishimura E (1995) Glucagon receptor mRNA distribution in rat tissues. Peptides 16, 1163–1166, 10.1016/0196-9781(95)00078-X [DOI] [PubMed] [Google Scholar]
- 93.Morales A, Lachuer J, Duchamp C, Vera N, Georges B, Cohen-Adad F et al. (1998) Tissue-specific modulation of rat glucagon receptor mRNA by thyroid status. Mol. Cell. Endocrinol 144, 71–81, 10.1016/S0303-7207(98)00150-6 [DOI] [PubMed] [Google Scholar]
- 94.Richards MP and McMurtry JP (2008) Expression of proglucagon and proglucagon-derived peptide hormone receptor genes in the chicken. Gen. Comp. Endocrinol 156, 323–338, 10.1016/j.ygcen.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 95.Vendrell J, El Bekay R, Peral B, García-Fuentes E, Megia A, Macias-Gonzalez M et al. (2011) Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 152, 4072–4079, 10.1210/en.2011-1070 [DOI] [PubMed] [Google Scholar]
- 96.Finan B, Clemmensen C, Zhu Z, Stemmer K, Gauthier K, Müller L et al. (2016) Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell 167, 843.e14–857.e14, 10.1016/j.cell.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 97.Vaughan M (1961) Effect of hormones on glucose metabolism in adipose tissue. J. Biol. Chem 236, 2196–2199 [PubMed] [Google Scholar]
- 98.Vaughan M and Steinberg D (1963) Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J. Lipid Res 4, 193–199 [PubMed] [Google Scholar]
- 99.Vaughan M, Berger JE and Steinberg D (1964) Hormone-sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J. Biol. Chem 239, 401–409 [PubMed] [Google Scholar]
- 100.Goodridge AG and Ball EG (1965) Studies on the metabolism of adipose tissue. 18. In vitro effects of insulin, epinephrine and glucagon on lipolysis and glycolysis in pigeon adipose tissue. Comp. Biochem. Physiol 16, 367–381, 10.1016/0010-406X(65)90303-8 [DOI] [PubMed] [Google Scholar]
- 101.Rodbell M and Jones AB (1966) Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens α toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J. Biol. Chem 241, 140–142 [PubMed] [Google Scholar]
- 102.Lazarus NR, Voyles NR, Devrim S, Tanese T and Recant L (1968) Extra-gastrointestinal effects of secretin, gastrin, and pancreozymin. Lancet 2, 248–250, 10.1016/S0140-6736(68)92353-2 [DOI] [PubMed] [Google Scholar]
- 103.Lewis GP and Matthews J (1968) The mobilization of free fatty acids from rabbit adipose tissue in situ. Br. J. Pharmacol 34, 564–578, 10.1111/j.1476-5381.1968.tb08485.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Birnbaumer L and Rodbell M (1969) Adenyl cyclase in fat cells. II. Hormone receptors. J. Biol. Chem 244, 3477–3482 [PubMed] [Google Scholar]
- 105.Lefebvre PJ and Luyckx AS (1969) Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, 195–197, 10.1007/BF01213680 [DOI] [PubMed] [Google Scholar]
- 106.Rudman D and Del Rio AE (1969) Lipolytic activity of synthetic porcine secretin. Endocrinology 85, 214–217, 10.1210/endo-85-2-214 [DOI] [PubMed] [Google Scholar]
- 107.Rodbell M, Birnbaumer L and Pohl SL (1970) Adenyl cyclase in fat cells. 3. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones. J. Biol. Chem 245, 718–722 [PubMed] [Google Scholar]
- 108.Prigge WF and Grande F (1971) Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. Comp. Biochem. Physiol. B 39, 69–82, 10.1016/0305-0491(71)90254-9 [DOI] [PubMed] [Google Scholar]
- 109.Manganiello V and Vaughan M (1972) Selective loss of adipose cell responsiveness to glucagon with growth in the rat. J. Lipid Res 13, 12–16 [PubMed] [Google Scholar]
- 110.Schwabe U and Ebert R (1972) Different effects of lipolytic hormones and phosphodiesterase inhibitors on cyclic 3’,5’-AMP levels in isolated fat cells. Naunyn Schmiedebergs Arch. Pharmacol 274, 287–298, 10.1007/BF00501938 [DOI] [PubMed] [Google Scholar]
- 111.Desbuguois B, Laudat MH and Laudat P (1973) Vasoactive intestinal polypeptide and glucagon: stimulation of adenylate cyclase activity via distinct receptors in liver and fat cell membranes. Biochem. Biophys. Res. Commun 53, 1187–1194, 10.1016/0006-291X(73)90590-1 [DOI] [PubMed] [Google Scholar]
- 112.Frandsen EK and Moody AJ (1973) Lipolytic action of a newly isolated vasoactive intestinal polypeptide. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 5, 196–199, 10.1055/s-0028-1093951 [DOI] [PubMed] [Google Scholar]
- 113.Gutman RA, Fink G, Voyles N, Selawry H, Penhos JC, Lepp A et al. (1973) Specific biologic effects of intestinal glucagon-like materials. J. Clin. Invest 52, 1165–1175, 10.1172/JCI107283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Langslow DR (1973) The action of gut glucagon-like immunoreactivity and other intestinal hormones on lipolysis in chicken adipocytes. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 5, 428–432, 10.1055/s-0028-1093917 [DOI] [PubMed] [Google Scholar]
- 115.Bennett V, Mong L and Cuatrecasas P (1975) Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. Relations to the mode of activation by hormones. J. Membr. Biol 24, 107–129, 10.1007/BF01868618 [DOI] [PubMed] [Google Scholar]
- 116.Dupre J, Greenidge N, McDonald TJ, Ross SA and Rubinstein D (1976) Inhibition of actions of glucagon in adipocytes by gastric inhibitory polypeptide. Metabolism 25, 1197–1199, 10.1016/S0026-0495(76)80002-9 [DOI] [PubMed] [Google Scholar]
- 117.Kitabgi P, Rosselin G and Bataille D (1976) Interactions of glucagon and related peptides with chicken adipose tissue. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 8, 266–270, 10.1055/s-0028-1093652 [DOI] [PubMed] [Google Scholar]
- 118.Jean-Baptiste E, Rizack MA and Epand RM (1982) Lipolytic and adenyl-cyclase-stimulating activity of Nα-trinitrophenyl glucagon: comparison with other glucagon derivatives modified at the amino terminus. Biosci. Rep 2, 163–167, 10.1007/BF01116379 [DOI] [PubMed] [Google Scholar]
- 119.Heckemeyer CM, Barker J, Duckworth WC and Solomon SS (1983) Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Endocrinology 113, 270–276, 10.1210/endo-113-1-270 [DOI] [PubMed] [Google Scholar]
- 120.Honnor RC, Dhillon GS and Londos C (1985) cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J. Biol. Chem 260, 15130–15138 [PubMed] [Google Scholar]
- 121.Howland RJ and Benning AD (1986) Differential effects of noradrenaline and glucagon on lipolysis and fatty-acid utilization in brown adipose tissue. FEBS Lett. 208, 128–132, 10.1016/0014-5793(86)81546-0 [DOI] [PubMed] [Google Scholar]
- 122.Howland RJ and Bond KD (1987) Modulation by insulin and glucagon of noradrenaline-induced activation of isolated brown adipocytes from the rat. Eur. J. Biochem 169, 155–166, 10.1111/j.1432-1033.1987.tb13593.x [DOI] [PubMed] [Google Scholar]
- 123.Richter WO, Robl H and Schwandt P (1989) Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, 333–335, 10.1016/0196-9781(89)90039-9 [DOI] [PubMed] [Google Scholar]
- 124.Strassheim D, Palmer T and Houslay MD (1991) Genetically acquired diabetes: adipocyte guanine nucleotide regulatory protein expression and adenylate cyclase regulation. Biochim. Biophys. Acta 1096, 121–126, 10.1016/0925-4439(91)90049-F [DOI] [PubMed] [Google Scholar]
- 125.Palmer TM, Taberner PV and Houslay MD (1992) Alterations in G-protein expression, Gi function and stimulatory receptor-mediated regulation of adipocyte adenylyl cyclase in a model of insulin-resistant diabetes with obesity. Cell. Signal 4, 365–377, 10.1016/0898-6568(92)90031-3 [DOI] [PubMed] [Google Scholar]
- 126.Ruiz-Grande C, Alarcón C, Mérida E and Valverde I (1992) Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides 13, 13–16, 10.1016/0196-9781(92)90134-O [DOI] [PubMed] [Google Scholar]
- 127.Perea A, Clemente F, Martinell J, Villanueva-Peñacarrillo ML and Valverde I (1995) Physiological effect of glucagon in human isolated adipocytes. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 27, 372–375, 10.1055/s-2007-979981 [DOI] [PubMed] [Google Scholar]
- 128.Villanueva-Peñacarrillo ML, Márquez L, González N, Díaz-Miguel M and Valverde I (2001) Effect of GLP-1 on lipid metabolism in human adipocytes. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 33, 73–77, 10.1055/s-2001-12428 [DOI] [PubMed] [Google Scholar]
- 129.Hagen JH (1961) Effect of glucagon on the metabolism of adipose tissue. J. Biol. Chem 236, 1023–1027 [PubMed] [Google Scholar]
- 130.Joel CD (1966) Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J. Biol. Chem 241, 814–821 [PubMed] [Google Scholar]
- 131.Kuroshima A, Kurahashi M and Yahata T (1979) Calorigenic effects of noradrenaline and glucagon on white adipocytes in cold- and heat-acclimated rats. Pflugers Arch. 381, 113–117, 10.1007/BF00582341 [DOI] [PubMed] [Google Scholar]
- 132.Kuroshima A and Yahata T (1979) Thermogenic responses of brown adipocytes to noradrenaline and glucagon in heat-acclimated and cold-acclimated rats. Jpn. J. Physiol 29, 683–690, 10.2170/jjphysiol.29.683 [DOI] [PubMed] [Google Scholar]
- 133.Marette A and Bukowiecki LJ (1990) Mechanism of norepinephrine stimulation of glucose transport in isolated rat brown adipocytes. Int. J. Obes 14, 857–867 [PubMed] [Google Scholar]
- 134.Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C et al. (2012) Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 61, 2753–2762, 10.2337/db11-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ingram DL and Kaciuba-Uscilko H (1980) Metabolic effects of glucagon in the young pig. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 12, 430–433, 10.1055/s-2007-999167 [DOI] [PubMed] [Google Scholar]
- 136.Berg F, Gustafson U and Andersson L (2006) The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2, e129, 10.1371/journal.pgen.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinoshita K, Ozaki N, Takagi Y, Murata Y, Oshida Y and Hayashi Y (2014) Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology 155, 3484–3492, 10.1210/en.2014-1175 [DOI] [PubMed] [Google Scholar]
- 138.Drucker DJ (2018) Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 27, 740–756, 10.1016/j.cmet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 139.Flint A, Raben A, Astrup A and Holst JJ (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest 101, 515–520, 10.1172/JCI990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei Y and Mojsov S (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 358, 219–224, 10.1016/0014-5793(94)01430-9 [DOI] [PubMed] [Google Scholar]
- 141.Bullock BP, Heller RS and Habener JF (1996) Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137, 2968–2978, 10.1210/endo.137.7.8770921 [DOI] [PubMed] [Google Scholar]
- 142.Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W and Wolfrum C (2012) Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem 287, 6421–6430, 10.1074/jbc.M111.310342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ et al. (2013) GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 154, 127–139, 10.1210/en.2012-1937 [DOI] [PubMed] [Google Scholar]
- 144.Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y et al. (2017) GLP-1/GLP-1R Signaling in Regulation of Adipocyte Differentiation and Lipogenesis. Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 42, 1165–1176, 10.1159/000478872 [DOI] [PubMed] [Google Scholar]
- 145.Iacobellis G, Camarena V, Sant DW and Wang G (2017) Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et Metabolisme 49, 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dozio E, Vianello E, Malavazos AE, Tacchini L, Schmitz G, Iacobellis G et al. (2019) Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int. J. Cardiol 292, 218–224, 10.1016/j.ijcard.2019.04.039 [DOI] [PubMed] [Google Scholar]
- 147.Zapata RC, McMillan C, Tong J and Chelikani PK (2019) Short communication: Expression of transcripts for proglucagon, glucose-dependent insulinotropic peptide, peptide YY, and their cognate receptors, in feline peripheral tissues. Res. Vet. Sci 124, 223–227, 10.1016/j.rvsc.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 148.Bertin E, Arner P, Bolinder J and Hagström-Toft E (2001) Action of glucagon and glucagon-like peptide-1-(7–36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J. Clin. Endocrinol. Metab 86, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 149.Åkesson L, Ahrén B, Manganiello VC, Holst LS, Edgren G and Degerman E (2003) Dual effects of pituitary adenylate cyclase-activating polypeptide and isoproterenol on lipid metabolism and signaling in primary rat adipocytes. Endocrinology 144, 5293–5299, 10.1210/en.2003-0364 [DOI] [PubMed] [Google Scholar]
- 150.Heppner KM, Marks S, Holland J, Ottaway N, Smiley D, Dimarchi R et al. (2015) Contribution of brown adipose tissue activity to the control of energy balance by GLP-1 receptor signalling in mice. Diabetologia 58, 2124–2132, 10.1007/s00125-015-3651-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y et al. (2007) Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J. Clin. Invest 117, 143–152, 10.1172/JCI25483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F et al. (2014) GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358, 10.2337/db14-0302 [DOI] [PubMed] [Google Scholar]
- 153.Kooijman S, Wang Y, Parlevliet ET, Boon MR, Edelschaap D, Snaterse G et al. (2015) Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58, 2637–2646, 10.1007/s00125-015-3727-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krieger JP, Santos da Conceição EP, Sanchez-Watts G, Arnold M, Pettersen KG, Mohammed M et al. (2018) Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R708–R720, 10.1152/ajpregu.00068.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tanaka K, Masaki Y, Tanaka M, Miyazaki M, Enjoji M, Nakamuta M et al. (2014) Exenatide improves hepatic steatosis by enhancing lipid use in adipose tissue in nondiabetic rats. World J. Gastroenterol 20, 2653–2663, 10.3748/wjg.v20.i10.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tomas E, Stanojevic V, McManus K, Khatri A, Everill P, Bachovchin WW et al. (2015) GLP-1(32–36)amide Pentapeptide Increases Basal Energy Expenditure and Inhibits Weight Gain in Obese Mice. Diabetes 64, 2409–2419, 10.2337/db14-1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei Q, Li L, Chen JA, Wang SH and Sun ZL (2015) Exendin-4 improves thermogenic capacity by regulating fat metabolism on brown adipose tissue in mice with diet-induced obesity. Ann. Clin. Lab. Sci 45, 158–165 [PubMed] [Google Scholar]
- 158.Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H et al. (2016) GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 59, 1059–1069, 10.1007/s00125-016-3896-5 [DOI] [PubMed] [Google Scholar]
- 159.Wan Y, Bao X, Huang J, Zhang X, Liu W, Cui Q et al. (2017) Novel GLP-1 Analog Supaglutide Reduces HFD-Induced Obesity Associated with Increased Ucp-1 in White Adipose Tissue in Mice. Front. Physiol 8, 294, 10.3389/fphys.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kosaka T and Lim RKS (1930) Demonstration of the Humoral Agent in Fat Inhibition of Gastric Secretion. P. Soc. Exp. Biol. Med 27, 890–891, 10.3181/00379727-27-5024 [DOI] [Google Scholar]
- 161.Brown JC, Pederson RA, Jorpes E and Mutt V (1969) Preparation of highly active enterogastrone. Can. J. Physiol. Pharmacol 47, 113–114, 10.1139/y69-020 [DOI] [PubMed] [Google Scholar]
- 162.Brown JC, Mutt V and Pederson RA (1970) Further purification of a polypeptide demonstrating enterogastrone activity. J. Physiol 209, 57–64, 10.1113/jphysiol.1970.sp009155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brown JC (1971) A gastric inhibitory polypeptide. I. The amino acid composition and the tryptic peptides. Can J. Biochem 49, 255–261, 10.1139/o71-037 [DOI] [PubMed] [Google Scholar]
- 164.Brown JC and Dryburgh JR (1971) A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J. Biochem 49, 867–872, 10.1139/o71-122 [DOI] [PubMed] [Google Scholar]
- 165.Buffa R, Polak JM, Pearse AG, Solcia E, Grimelius L and Capella C (1975) Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry 43, 249–255, 10.1007/BF00499706 [DOI] [PubMed] [Google Scholar]
- 166.Dupre J, Ross SA, Watson D and Brown JC (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab 37, 826–828, 10.1210/jcem-37-5-826 [DOI] [PubMed] [Google Scholar]
- 167.Yip RG-C, Boylan MO, Kieffer TJ and Wolfe MM (1998) Functional GIP receptors are present on adipocytes. Endocrinology 139, 4004–4007, 10.1210/endo.139.9.6288 [DOI] [PubMed] [Google Scholar]
- 168.McIntosh CHS, Bremsak I, Lynn FC, Gill R, Hinke SA, Gelling R et al. (1999) Glucose-dependent insulinotropic polypeptide stimulation of lipolysis in differentiated 3T3-L1 cells: wortmannin-sensitive inhibition by insulin. Endocrinology 140, 398–404, 10.1210/endo.140.1.6464 [DOI] [PubMed] [Google Scholar]
- 169.Rudovich N, Kaiser S, Engeli S, Osterhoff M, Gögebakan O, Bluher M et al. (2007) GIP receptor mRNA expression in different fat tissue depots in postmenopausal non-diabetic women. Regul. Pept 142, 138–145, 10.1016/j.regpep.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 170.Song DH, Getty-Kaushik L, Tseng E, Simon J, Corkey BE and Wolfe MM (2007) Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 133, 1796–1805, 10.1053/j.gastro.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weaver RE, Donnelly D, Wabitsch M, Grant PJ and Balmforth AJ (2008) Functional expression of glucose-dependent insulinotropic polypeptide receptors is coupled to differentiation in a human adipocyte model. Int. J. Obes. (Lond.) 32, 1705–1711, 10.1038/ijo.2008.148 [DOI] [PubMed] [Google Scholar]
- 172.Dekker MJ, Graham TE, Ooi TC and Robinson LE (2010) Exercise prior to fat ingestion lowers fasting and postprandial VLDL and decreases adipose tissue IL-6 and GIP receptor mRNA in hypertriacylglycerolemic men. J. Nutr. Biochem 21, 983–990, 10.1016/j.jnutbio.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 173.Kim S-J, Nian C and McIntosh CHS (2011) Adipocyte expression of the glucose-dependent insulinotropic polypeptide receptor involves gene regulation by PPARγ and histone acetylation. J. Lipid Res 52, 759–770, 10.1194/jlr.M012203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Timper K, Grisouard J, Radimerski T, Dembinski K, Peterli R, Häring A et al. (2011) Glucose-dependent insulinotropic polypeptide (GIP) induces calcitonin gene-related peptide (CGRP)-I and procalcitonin (Pro-CT) production in human adipocytes. J. Clin. Endocrinol. Metab 96, E297–E303, 10.1210/jc.2010-1324 [DOI] [PubMed] [Google Scholar]
- 175.Ahlqvist E, Osmark P, Kuulasmaa T, Pilgaard K, Omar B, Brøns C et al. (2013) Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes 62, 2088–2094, 10.2337/db12-0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ceperuelo-Mallafré V, Duran X, Pachón G, Roche K, Garrido-Sánchez L, Vilarrasa N et al. (2014) Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab 99, E908–E919, 10.1210/jc.2013-3350 [DOI] [PubMed] [Google Scholar]
- 177.Killion EA, Wang J, Yie J, Shi SD-H, Bates D, Min X et al. (2018) Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med 10, 10.1126/scitranslmed.aat3392 [DOI] [PubMed] [Google Scholar]
- 178.Eckel RH, Fujimoto WY and Brunzell JD (1979) Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes 28, 1141–1142, 10.2337/diab.28.12.1141 [DOI] [PubMed] [Google Scholar]
- 179.Beck B and Max JP (1983) Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regul. Pept 7, 3–8, 10.1016/0167-0115(83)90276-8 [DOI] [PubMed] [Google Scholar]
- 180.Beck B and Max JP (1987) Hypersensitivity of adipose tissue to gastric inhibitory polypeptide action in the obese Zucker rat. Cell. Mol. Biol 33, 555–562 [PubMed] [Google Scholar]
- 181.Hauner H, Glatting G, Kaminska D and Pfeiffer EF (1988) Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann. Nutr. Metab 32, 282–288, 10.1159/000177467 [DOI] [PubMed] [Google Scholar]
- 182.Oben J, Morgan L, Fletcher J and Marks V (1991) Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7–36) amide, on fatty acid synthesis in explants of rat adipose tissue. J. Endocrinol 130, 267–272, 10.1677/joe.0.1300267 [DOI] [PubMed] [Google Scholar]
- 183.Knapper JME, Puddicombe SM, Morgan LM and Fletcher JM (1995) Investigations into the actions of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1(7–36)amide on lipoprotein lipase activity in explants of rat adipose tissue. J. Nutr 125, 183–188 [DOI] [PubMed] [Google Scholar]
- 184.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H et al. (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med 8, 738–742, 10.1038/nm727 [DOI] [PubMed] [Google Scholar]
- 185.Getty-Kaushik L, Song DH, Boylan MO, Corkey BE and Wolfe MM (2006) Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring) 14, 1124–1131, 10.1038/oby.2006.129 [DOI] [PubMed] [Google Scholar]
- 186.Kim S-J, Nian C and McIntosh CHS (2007) Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem 282, 8557–8567, 10.1074/jbc.M609088200 [DOI] [PubMed] [Google Scholar]
- 187.Thondam SK, Daousi C, Wilding JP, Holst JJ, Ameen GI, Yang C et al. (2017) Glucose-dependent insulinotropic polypeptide promotes lipid deposition in subcutaneous adipocytes in obese type 2 diabetes patients: a maladaptive response. Am. J. Physiol. Endocrinol. Metab 312, E224–E233, 10.1152/ajpendo.00347.2016 [DOI] [PubMed] [Google Scholar]
- 188.Mohammad S, Ramos LS, Buck J, Levin LR, Rubino F and McGraw TE (2011) Gastric inhibitory peptide controls adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110β isoform of phosphatidylinositol 3-kinase. J. Biol. Chem 286, 43062–43070, 10.1074/jbc.M111.289009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Timper K, Grisouard J, Sauter NS, Herzog-Radimerski T, Dembinski K, Peterli R et al. (2013) Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am. J. Physiol. Endocrinol. Metab 304, E1–E13, 10.1152/ajpendo.00100.2012 [DOI] [PubMed] [Google Scholar]
- 190.Mohammad S, Patel RT, Bruno J, Panhwar MS, Wen J and McGraw TE (2014) A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol. Cell. Biol 34, 3618–3629, 10.1128/MCB.00256-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C et al. (2015) A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med 21, 27–36, 10.1038/nm.3761 [DOI] [PubMed] [Google Scholar]
- 192.Gögebakan Ö, Andres J, Biedasek K, Mai K, Kühnen P, Krude H et al. (2012) Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes 61, 292–300, 10.2337/db10-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Montrose-Rafizadeh C, Yang H, Wang Y, Roth J, Montrose MH and Adams LG (1997) Novel signal transduction and peptide specificity of glucagon-like peptide receptor in 3T3-L1 adipocytes. J. Cell. Physiol 172, 275–283, [DOI] [PubMed] [Google Scholar]
- 194.Gault VA, McClean PL, Cassidy RS, Irwin N and Flatt PR (2007) Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 50, 1752–1762, 10.1007/s00125-007-0710-4 [DOI] [PubMed] [Google Scholar]
- 195.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA and Flatt PR (2007) GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am. J. Physiol. Endocrinol. Metab 293, E1746–E1755, 10.1152/ajpendo.00460.2007 [DOI] [PubMed] [Google Scholar]
- 196.Althage MC, Ford EL, Wang S, Tso P, Polonsky KS and Wice BM (2008) Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J. Biol. Chem 283, 18365–18376, 10.1074/jbc.M710466200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fulurija A, Lutz TA, Sladko K, Osto M, Wielinga PY, Bachmann MF et al. (2008) Vaccination against GIP for the treatment of obesity. PLoS One 3, e3163, 10.1371/journal.pone.0003163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ugleholdt R, Pedersen J, Bassi MR, Füchtbauer E-M, Jørgensen SM, Kissow H-L et al. (2011) Transgenic rescue of adipocyte glucose-dependent insulinotropic polypeptide receptor expression restores high fat diet-induced body weight gain. J. Biol. Chem 286, 44632–44645, 10.1074/jbc.M111.311779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nasteska D, Harada N, Suzuki K, Yamane S, Hamasaki A, Joo E et al. (2014) Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 63, 2332–2343, 10.2337/db13-1563 [DOI] [PubMed] [Google Scholar]
- 200.Boylan MO, Glazebrook PA, Tatalovic M and Wolfe MM (2015) Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. Am. J. Physiol. Endocrinol. Metab 309, E1008–E1018, 10.1152/ajpendo.00345.2015 [DOI] [PubMed] [Google Scholar]
- 201.Joo E, Harada N, Yamane S, Fukushima T, Taura D, Iwasaki K et al. (2017) Inhibition of Gastric Inhibitory Polypeptide Receptor Signaling in Adipose Tissue Reduces Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice. Diabetes 66, 868–879, 10.2337/db16-0758 [DOI] [PubMed] [Google Scholar]
- 202.Maekawa R, Ogata H, Murase M, Harada N, Suzuki K, Joo E et al. (2018) Glucose-dependent insulinotropic polypeptide is required for moderate high-fat diet- but not high-carbohydrate diet-induced weight gain. Am. J. Physiol. Endocrinol. Metab 314, E572–E583, 10.1152/ajpendo.00352.2017 [DOI] [PubMed] [Google Scholar]
- 203.Nakamura T, Tanimoto H, Mizuno Y, Okamoto M, Takeuchi M, Tsubamoto Y et al. (2018) Gastric inhibitory polypeptide receptor antagonist, SKL-14959, suppressed body weight gain on diet-induced obesity mice. Obes. Sci. Pract 4, 194–203, 10.1002/osp4.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Beaudry JL, Kaur KD, Varin EM, Baggio LL, Cao X, Mulvihill EE et al. (2019) Physiological roles of the GIP receptor in murine brown adipose tissue. Mol. Metab 28, 14–25, 10.1016/j.molmet.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H and Guertin DA (2012) PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362, 10.1016/j.cmet.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Naitoh R, Miyawaki K, Harada N, Mizunoya W, Toyoda K, Fushiki T et al. (2008) Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochem. Biophys. Res. Commun 376, 21–25, 10.1016/j.bbrc.2008.08.052 [DOI] [PubMed] [Google Scholar]
- 207.Szalowska E, Meijer K, Kloosterhuis N, Razaee F, Priebe M and Vonk RJ (2011) Sub-chronic administration of stable GIP analog in mice decreases serum LPL activity and body weight. Peptides 32, 938–945, 10.1016/j.peptides.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 208.Kim S-J, Nian C, Karunakaran S, Clee SM, Isales CM and McIntosh CHS (2012) GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One 7, e40156, 10.1371/journal.pone.0040156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Knerr PJ, Finan B, Gelfanov V, Perez-Tilve D, Tschop MH and DiMarchi RD (2018) Optimization of peptide-based polyagonists for treatment of diabetes and obesity. Bioorg. Med. Chem 26, 2873–2881, 10.1016/j.bmc.2017.10.047 [DOI] [PubMed] [Google Scholar]
- 210.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J et al. (2009) A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol 5, 749–757, 10.1038/nchembio.209 [DOI] [PubMed] [Google Scholar]
- 211.Henderson SJ, Konkar A, Hornigold DC, Trevaskis JL, Jackson R, Fritsch Fredin M et al. (2016) Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab 18, 1176–1190, 10.1111/dom.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Valdecantos MP, Pardo V, Ruiz L, Castro-Sanchez L, Lanzon B, Fernandez-Millan E et al. (2017) A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology 65, 950–968, 10.1002/hep.28962 [DOI] [PubMed] [Google Scholar]
- 213.Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM et al. (2013) Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med 5, 209ra151, 10.1126/scitranslmed.3007218 [DOI] [PubMed] [Google Scholar]
- 214.Jall S, Sachs S, Clemmensen C, Finan B, Neff F, DiMarchi RD et al. (2017) Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol. Metab 6, 440–446, 10.1016/j.molmet.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ericson MD, Lensing CJ, Fleming KA, Schlasner KN, Doering SR and Haskell-Luevano C (2017) Bench-top to clinical therapies: A review of melanocortin ligands from 1954 to 2016. Biochim. Biophys. Acta. Mol. Basis Dis 1863, 2414–2435, 10.1016/j.bbadis.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang Y and Harmon CM (2017) Molecular signatures of human melanocortin receptors for ligand binding and signaling. Biochim. Biophys. Acta. Mol. Basis Dis 1863, 2436–2447, 10.1016/j.bbadis.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 217.White JE and Engel FL (1958) Lipolytic action of corticotropin on rat adipose tissue in vitro. J. Clin. Invest 37, 1556–1563, 10.1172/JCI103748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Herberg L, Gries FA and Hesse-Wortmann C (1970) Effect of weight and cell size on hormone-induced lipolysis in New Zealand obese mice and American obese hyperglycemic mice. Diabetologia 6, 300–305, 10.1007/BF01212242 [DOI] [PubMed] [Google Scholar]
- 219.Rodbell M (1975) On the mechanism of activation of fat cell adenylate cyclase by guanine nucleotides. An explanation for the biphasic inhibitory and stimulatory effects of the nucleotides and the role of hormones. J. Biol. Chem 250, 5826–5834 [PubMed] [Google Scholar]
- 220.Spirovski MZ, Kovacev VP, Spasovska M and Chernick SS (1975) Effect of ACTH on lipolysis in adipose tissue of normal and adrenalectomized rats in vivo. Am. J. Physiol 228, 382–385, 10.1152/ajplegacy.1975.228.2.382 [DOI] [PubMed] [Google Scholar]
- 221.Müller K and Maier R (1976) Influence of the assay conditions on the lipolytic potency in vitro of different adrenocorticotrophins. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 8, 271–273, 10.1055/s-0028-1093633 [DOI] [PubMed] [Google Scholar]
- 222.Ramachandran J and Lee V (1976) Divergent effects of adrenocorticotropin and melanotropin on isolated rat and rabbit adipocytes. Biochim. Biophys. Acta 428, 339–346, 10.1016/0304-4165(76)90041-6 [DOI] [PubMed] [Google Scholar]
- 223.Dehaye JP, Winand J and Christophe J (1977) Lipolysis and cyclic AMP levels in epididymal adipose tissue of obese-hyperglycaemic mice. Diabetologia 13, 553–561, 10.1007/BF01236307 [DOI] [PubMed] [Google Scholar]
- 224.Honnor RC, Dhillon GS and Londos C (1985) cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J. Biol. Chem 260, 15122–15129 [PubMed] [Google Scholar]
- 225.Rothwell NJ and Stock MJ (1985) Acute and chronic effects of ACTH on thermogenesis and brown adipose tissue in the rat. Comp. Biochem. Physiol. A Comp. Physiol 81, 99–102, 10.1016/0300-9629(85)90273-7 [DOI] [PubMed] [Google Scholar]
- 226.Ng TB (1990) Studies on hormonal regulation of lipolysis and lipogenesis in fat cells of various mammalian species. Comp. Biochem. Physiol. B 97, 441–446, 10.1016/0305-0491(90)90141-F [DOI] [PubMed] [Google Scholar]
- 227.Bousquet-Mélou A, Galitzky J, Moreno CM, Berlan M and Lafontan M (1995) Desensitization of beta-adrenergic responses in adipocytes involves receptor subtypes and cAMP phosphodiesterase. Eur. J. Pharmacol 289, 235–247, 10.1016/0922-4106(95)90100-0 [DOI] [PubMed] [Google Scholar]
- 228.Boston BA and Cone RD (1996) Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology 137, 2043–2050, 10.1210/endo.137.5.8612546 [DOI] [PubMed] [Google Scholar]
- 229.Kiwaki K and Levine JA (2003) Differential effects of adrenocorticotropic hormone on human and mouse adipose tissue. J. Comp. Physiol. B 173, 675–678, 10.1007/s00360-003-0377-1 [DOI] [PubMed] [Google Scholar]
- 230.Norman D, Isidori AM, Frajese V, Caprio M, Chew SL, Grossman AB et al. (2003) ACTH and α-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central-peripheral melanocortin-leptin pathway. Mol. Cell. Endocrinol 200, 99–109, 10.1016/S0303-7207(02)00410-0 [DOI] [PubMed] [Google Scholar]
- 231.Cho KJ, Shim JH, Cho MC, Choe YK, Hong JT, Moon DC et al. (2005) Signaling pathways implicated in α-melanocyte stimulating hormone-induced lipolysis in 3T3-L1 adipocytes. J. Cell. Biochem 96, 869–878, 10.1002/jcb.20561 [DOI] [PubMed] [Google Scholar]
- 232.Iwen KA, Senyaman O, Schwartz A, Drenckhan M, Meier B, Hadaschik D et al. (2008) Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. J. Endocrinol 196, 465–472, 10.1677/JOE-07-0299 [DOI] [PubMed] [Google Scholar]
- 233.Møller CL, Raun K, Jacobsen ML, Pedersen TÅ, Holst B, Conde-Frieboes KW et al. (2011) Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Mol. Cell. Endocrinol 341, 9–17, 10.1016/j.mce.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 234.van den Beukel JC, Grefhorst A, Quarta C, Steenbergen J, Mastroberardino PG, Lombés M et al. (2014) Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. FASEB J. 28, 4857–4867, 10.1096/fj.14-254839 [DOI] [PubMed] [Google Scholar]
- 235.Stefanidis A, Wiedmann NM, Tyagi S, Allen AM, Watt MJ and Oldfield BJ (2018) Insights into the neurochemical signature of the Innervation of Beige Fat. Mol. Metab 11, 47–58, 10.1016/j.molmet.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schnabl K, Westermeier J, Li Y and Klingenspor M (2019) Opposing Actions of Adrenocorticotropic Hormone and Glucocorticoids on UCP1-Mediated Respiration in Brown Adipocytes. Front. Physiol 9, 1931, 10.3389/fphys.2018.01931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carlson LA, Butcher RW and Micheli H (1970) Fat mobilizing lipolysis and levels of cyclic AMP in human and dog adipose tissue. Acta Med. Scand 187, 525–528, 10.1111/j.0954-6820.1970.tb02979.x [DOI] [PubMed] [Google Scholar]
- 238.Bousquet-Mélou A, Galitzky J, Lafontan M and Berlan M (1995) Control of lipolysis in intra-abdominal fat cells of nonhuman primates: comparison with humans. J. Lipid Res 36, 451–461 [PubMed] [Google Scholar]
- 239.Hoch M, Eberle AN, Wagner U, Bussmann C, Peters T and Peterli R (2007) Expression and localization of melanocortin-1 receptor in human adipose tissues of severely obese patients. Obesity (Silver Spring) 15, 40–49, 10.1038/oby.2007.525 [DOI] [PubMed] [Google Scholar]
- 240.Møller CL, Pedersen SB, Richelsen B, Conde-Frieboes KW, Raun K, Grove KL et al. (2015) Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res. Notes 8, 559, 10.1186/s13104-015-1539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bradley RL, Mansfield JP and Maratos-Flier E (2005) Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes. Obes. Res 13, 653–661, 10.1038/oby.2005.73 [DOI] [PubMed] [Google Scholar]
- 242.Rodrigues AR, Salazar MJ, Rocha-Rodrigues S, Gonçalves IO, Cruz C, Neves D et al. (2019) Peripherally administered melanocortins induce mice fat browning and prevent obesity. Int. J. Obes. (Lond.) 43, 1058–1069, 10.1038/s41366-018-0155-5 [DOI] [PubMed] [Google Scholar]
- 243.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS and Mantzoros CS (2002) Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51, 1337–1345, 10.2337/diabetes.51.5.1337 [DOI] [PubMed] [Google Scholar]
- 244.Williams DL, Bowers RR, Bartness TJ, Kaplan JM and Grill HJ (2003) Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144, 4692–4697, 10.1210/en.2003-0440 [DOI] [PubMed] [Google Scholar]
- 245.Li G, Zhang Y, Wilsey JT and Scarpace PJ (2004) Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J. Endocrinol 182, 123–132, 10.1677/joe.0.1820123 [DOI] [PubMed] [Google Scholar]
- 246.Habener JF, Rosenblatt M and Potts JT (1984) Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol. Rev 64, 985–1053, 10.1152/physrev.1984.64.3.985 [DOI] [PubMed] [Google Scholar]
- 247.Potts JT (2005) Parathyroid hormone: past and present. J. Endocrinol 187, 311–325, 10.1677/joe.1.06057 [DOI] [PubMed] [Google Scholar]
- 248.Bastepe M, Turan S and He Q (2017) Heterotrimeric G proteins in the control of parathyroid hormone actions. J. Mol. Endocrinol 58, R203–R224, 10.1530/JME-16-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC et al. (1987) Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J. Biol. Chem 262, 7151–7156 [PubMed] [Google Scholar]
- 250.Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ et al. (1987) Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc. Natl. Acad. Sci. U. S. A 84, 5048–5052, 10.1073/pnas.84.14.5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC et al. (1987) Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J. Clin. Invest 80, 1803–1807, 10.1172/JCI113275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mangin M, Ikeda K, Dreyer BE, Milstone L and Broadus AE (1988) Two distinct tumor-derived, parathyroid hormone-like peptides result from alternative ribonucleic acid splicing. Mol. Endocrinol 2, 1049–1055, 10.1210/mend-2-11-1049 [DOI] [PubMed] [Google Scholar]
- 253.Thiede MA, Strewler GJ, Nissenson RA, Rosenblatt M and Rodan GA (1988) Human renal carcinoma expresses two messages encoding a parathyroid hormone-like peptide: evidence for the alternative splicing of a single-copy gene. Proc. Natl. Acad. Sci. U. S. A 85, 4605–4609, 10.1073/pnas.85.13.4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McCauley LK and Martin TJ (2012) Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J. Bone Miner. Res 27, 1231–1239, 10.1002/jbmr.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM et al. (1994) Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289, 10.1101/gad.8.3.277 [DOI] [PubMed] [Google Scholar]
- 256.Miao D, He B, Karaplis AC and Goltzman D (2002) Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest 109, 1173–1182, 10.1172/JCI0214817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Usdin TB, Gruber C and Bonner TI (1995) Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J. Biol. Chem 270, 15455–15458, 10.1074/jbc.270.26.15455 [DOI] [PubMed] [Google Scholar]
- 258.Werner S and Löw H (1973) Stimulation of lipolysis and calcium accumulation by parathyroid hormone in rat adipose tissue in vitro after adrenalectomy and administration of high doses of cortisone acetate. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 5, 292–296, 10.1055/s-0028-1093931 [DOI] [PubMed] [Google Scholar]
- 259.Gozariu L, Forster K, Faulhaber JD, Minne H and Ziegler R (1974) Parathyroid hormone and calcitonin: influences upon lipolysis of human adipose tissue. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 6, 243–245, 10.1055/s-0028-1095713 [DOI] [PubMed] [Google Scholar]
- 260.Sinha TK, Thajchayapong P, Queener SF, Allen DO and Bell NH (1976) On the lipolytic action of parathyroid hormone in man. Metabolism 25, 251–260, 10.1016/0026-0495(76)90083-4 [DOI] [PubMed] [Google Scholar]
- 261.Thajchayapong P, Queener SF, McClintock R, Allen DO and Bell NH (1976) Demonstration that cyclic adenosine 3’,5’-monophosphate mediates the lipolytic action of parathyroid hormone. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 8, 190–195, 10.1055/s-0028-1093658 [DOI] [PubMed] [Google Scholar]
- 262.Kather H and Simon B (1977) Adenylate cyclase of human fat cell ghosts. Stimulation of enzyme activity by parathyroid hormone. J. Clin. Invest 59, 730–733, 10.1172/JCI108692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kather H, Tschöpe W and Simon B (1977) Human fat cell adenylate cyclase. Modulation of parathyroid hormone action by guanine nucleotides. Res. Exp. Med. (Berl) 171, 201–204, 10.1007/BF01851367 [DOI] [PubMed] [Google Scholar]
- 264.Kather H and Simon B (1979) Adenylate cyclase of human fat cell ghosts: effects of the vasoactive intestinal polypeptide (VIP) on the enzyme system of preadolescent and adult fat cells. FEBS Lett. 100, 145–147, 10.1016/0014-5793(79)81151-5 [DOI] [PubMed] [Google Scholar]
- 265.Taniguchi A, Kataoka K, Kono T, Oseko F, Okuda H, Nagata I et al. (1987) Parathyroid hormone-induced lipolysis in human adipose tissue. J. Lipid Res 28, 490–494 [PubMed] [Google Scholar]
- 266.Larsson S, Jones HA, Göransson O, Degerman E and Holm C (2016) Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cell. Signal 28, 204–213, 10.1016/j.cellsig.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 267.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE et al. (2014) Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104, 10.1038/nature13528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B et al. (2016) PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 23, 315–323, 10.1016/j.cmet.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bergström S, Duner H, von Euler US, Pernow B and Sjövall J (1959) Observations on the effects of infusion of prostaglandin E in man. Acta Physiol. Scand 45, 145–151, 10.1111/j.1748-1716.1959.tb01686.x [DOI] [PubMed] [Google Scholar]
- 270.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T et al. (1998) Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395, 281–284, 10.1038/26233 [DOI] [PubMed] [Google Scholar]
- 271.Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB et al. (2007) EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci 10, 1131–1133, 10.1038/nn1949 [DOI] [PubMed] [Google Scholar]
- 272.Monda M, Amaro S, Sullo A and De Luca B (1995) Nitric oxide reduces body temperature and sympathetic input to brown adipose tissue during PGE1-hyperthermia. Brain Res. Bull 38, 489–493, 10.1016/0361-9230(95)02020-R [DOI] [PubMed] [Google Scholar]
- 273.Morrison SF, Madden CJ and Tupone D (2014) Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756, 10.1016/j.cmet.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Aronoff DM and Romanovsky AA (2007) Eicosanoids in non-febrile thermoregulation. In Neurobiology of Hyperthermia. Progress in Brain Research (Sharma HS, ed.), vol. 162, pp. 15–25, Elsevier B.V., 2007/07/25 ed: [DOI] [PubMed] [Google Scholar]
- 275.Fraifeld V and Kaplanski J (1998) Brain eicosanoids and LPS fever: species and age differences. In Brain Function in Hot Environment. Progress in Brain Research (Sharma HS and Westman J, eds), vol. 115, pp. 141–157, Elsevier Science BV, 1998/06/20 ed: [DOI] [PubMed] [Google Scholar]
- 276.Chatzipanteli K, Rudolph S and Axelrod L (1992) Coordinate control of lipolysis by prostaglandin E2 and prostacyclin in rat adipose tissue. Diabetes 41, 927–935, 10.2337/diab.41.8.927 [DOI] [PubMed] [Google Scholar]
- 277.Breyer RM, Bagdassarian CK, Myers SA and Breyer MD (2001) Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol 41, 661–690, 10.1146/annurev.pharmtox.41.1.661 [DOI] [PubMed] [Google Scholar]
- 278.Hata AN and Breyer RM (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther 103, 147–166, 10.1016/j.pharmthera.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 279.Vassaux G, Gaillard D, Darimont C, Ailhaud G and Negrel R (1992) Differential response of preadipocytes and adipocytes to prostacyclin and prostaglandin E2: physiological implications. Endocrinology 131, 2393–2398, 10.1210/endo.131.5.1330499 [DOI] [PubMed] [Google Scholar]
- 280.Vassaux G, Gaillard D, Ailhaud G and Négrel R (1992) Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP-and Ca2+-elevating agent. J. Biol. Chem 267, 11092–11097 [PubMed] [Google Scholar]
- 281.Négrel R (1999) Prostacyclin as a critical prostanoid in adipogenesis. Prostaglandins Leukot. Essent. Fatty Acids 60, 383–386, 10.1016/S0952-3278(99)80017-9 [DOI] [PubMed] [Google Scholar]
- 282.Aubert J, Saint-Marc P, Belmonte N, Dani C, Négrel R and Ailhaud G (2000) Prostacyclin IP receptor up-regulates the early expression of C/EBPβ and C/EBPδ in preadipose cells. Mol. Cell. Endocrinol 160, 149–156, 10.1016/S0303-7207(99)00210-5 [DOI] [PubMed] [Google Scholar]
- 283.Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S et al. (2003) Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J. Lipid Res 44, 271–279, 10.1194/jlr.M200346-JLR200 [DOI] [PubMed] [Google Scholar]
- 284.Butcher RW and Sutherland EW (1967) The effects of the catecholamines, adrenergic blocking agents, prostaglandin E1, and insulin on cyclic AMP levels in the rat epididymal fat pad in vitro. Ann. N. Y. Acad. Sci 139, 849–859, 10.1111/j.1749-6632.1967.tb41255.x [DOI] [PubMed] [Google Scholar]
- 285.Butcher RW and Baird CE (1968) Effects of prostaglandins on adenosine 3’,5’-monophosphate levels in fat and other tissues. J. Biol. Chem 243, 1713–1717 [PubMed] [Google Scholar]
- 286.Dalton C, Hope H and Martikes L (1974) Prostaglandin inhibition of cyclic-AMP accumulation and rate of lipolysis in fat cells. Prostaglandins 7, 319–326, 10.1016/S0090-6980(74)80087-0 [DOI] [PubMed] [Google Scholar]
- 287.Fredholm BB and Hamberg M (1976) Metabolism and effect of prostaglandin H2 in adipose tissue. Prostaglandins 11, 507–518, 10.1016/0090-6980(76)90098-8 [DOI] [PubMed] [Google Scholar]
- 288.Kather H and Simon B (1979) Biphasic effects of prostaglandin E2 on the human fat cell adenylate cyclase. J. Clin. Invest 64, 609–612, 10.1172/JCI109500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kather H (1981) Effects of prostaglandin E2 on adenylate cyclase activity and lipolysis in human adipose tissue. Int. J. Obes 5, 659–663 [PubMed] [Google Scholar]
- 290.Kather H and Simon B (1981) Stimulatory and inhibitory effects of catecholamines and of prostaglandin E2 on human fat cell adenylate cyclase. Adv. Cyclic Nucleotide Res 14, 555–562 [PubMed] [Google Scholar]
- 291.Kather H (1982) Sodium ions amplify prostaglandin E2-induced inhibition of human fat cell adenylate cyclase. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 14, 556, 10.1055/s-2007-1019078 [DOI] [PubMed] [Google Scholar]
- 292.Steinfelder HJ, Schramm S and Joost H-G (1987) Prostaglandin E2 differentiates between two forms of glucose transport inhibition by lipolytic agents. Naunyn Schmiedebergs Arch. Pharmacol 336, 105–110, 10.1007/BF00177759 [DOI] [PubMed] [Google Scholar]
- 293.Cohen-Luria R and Rimon G (1992) Prostaglandin E2 can bimodally inhibit and stimulate the epididymal adipocyte adenylyl cyclase activity. Cell. Signal 4, 331–335, 10.1016/0898-6568(92)90073-H [DOI] [PubMed] [Google Scholar]
- 294.Strong P, Coleman RA and Humphrey PPA (1992) Prostanoid-induced inhibition of lipolysis in rat isolated adipocytes: probable involvement of EP3 receptors. Prostaglandins 43, 559–566, 10.1016/0090-6980(92)90115-A [DOI] [PubMed] [Google Scholar]
- 295.Iyer A, Lim J, Poudyal H, Reid RC, Suen JY, Webster J et al. (2012) An Inhibitor of Phospholipase A2 Group IIA Modulates Adipocyte Signaling and Protects Against Diet-Induced Metabolic Syndrome in Rats. Diabetes 61, 2320–2329, 10.2337/db11-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ceddia RP, Lee D, Maulis MF, Carboneau BA, Threadgill DW, Poffenberger G et al. (2016) The PGE2 EP3 Receptor Regulates Diet-Induced Adiposity in Male Mice. Endocrinology 157, 220–232, 10.1210/en.2015-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Madsen L, Pedersen LM, Lillefosse HH, Fjære E, Bronstad I, Hao Q et al. (2010) UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One 5, e11391, 10.1371/journal.pone.0011391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A et al. (2010) Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328, 1158–1161, 10.1126/science.1186034 [DOI] [PubMed] [Google Scholar]
- 299.Bayindir I, Babaeikelishomi R, Kocanova S, Sousa IS, Lerch S, Hardt O et al. (2015) Transcriptional Pathways in cPGI2-Induced Adipocyte Progenitor Activation for Browning. Front. Endocrinol. (Lausanne) 6, 129, 10.3389/fendo.2015.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ghandour RA, Giroud M, Vegiopoulos A, Herzig S, Ailhaud G, Amri EZ et al. (2016) IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim. Biophys. Acta 1861, 285–293, 10.1016/j.bbalip.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 301.Babaei R, Schuster M, Meln I, Lerch S, Ghandour RA, Pisani DF et al. (2018) Jak-TGFβ cross-talk links transient adipose tissue inflammation to beige adipogenesis. Sci. Signal 11, 10.1126/scisignal.aai7838 [DOI] [PubMed] [Google Scholar]
- 302.Sato N, Kaneko M, Tamura M and Kurumatani H (2010) The prostacyclin analog Beraprost sodium ameliorates characteristics of metabolic syndrome in obese Zucker (fatty) rats. Diabetes 59, 1092–1100, 10.2337/db09-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.García-Alonso V, Titos E, Alcaraz-Quiles J, Rius B, Lopategi A, López-Vicario C et al. (2016) Prostaglandin E2 Exerts Multiple Regulatory Actions on Human Obese Adipose Tissue Remodeling, Inflammation, Adaptive Thermogenesis and Lipolysis. PLoS One 11, e0153751, 10.1371/journal.pone.0153751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Xue Y, Xu X, Zhang XQ, Farokhzad OC and Langer R (2016) Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc. Natl. Acad. Sci. U. S. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pisani DF, Ghandour RA, Beranger GE, Le Faouder P, Chambard JC, Giroud M et al. (2014) The ω6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol. Metab 3, 834–847, 10.1016/j.molmet.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Virtue S, Feldmann H, Christian M, Tan CY, Masoodi M, Dale M et al. (2012) A new role for lipocalin prostaglandin D synthase in the regulation of brown adipose tissue substrate utilization. Diabetes 61, 3139–3147, 10.2337/db12-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Virtue S, Masoodi M, Velagapudi V, Tan CY, Dale M, Suorti T et al. (2012) Lipocalin prostaglandin D synthase and PPARγ2 coordinate to regulate carbohydrate and lipid metabolism in vivo. PLoS One 7, e39512, 10.1371/journal.pone.0039512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S et al. (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev 52, 375–414 [PubMed] [Google Scholar]
- 309.Derbyshire ER and Marletta MA (2012) Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem 81, 533–559, 10.1146/annurev-biochem-050410-100030 [DOI] [PubMed] [Google Scholar]
- 310.Griffith OW and Stuehr DJ (1995) Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol 57, 707–736, 10.1146/annurev.ph.57.030195.003423 [DOI] [PubMed] [Google Scholar]
- 311.Palmer RM, Ferrige AG and Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526, 10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- 312.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A et al. (2009) Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol 5, 865–869, 10.1038/nchembio.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kuhn M (2016) Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev 96, 751–804, 10.1152/physrev.00022.2015 [DOI] [PubMed] [Google Scholar]
- 314.Haas B, Mayer P, Jennissen K, Scholz D, Berriel Diaz M, Bloch W et al. (2009) Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci. Signal 2, ra78, 10.1126/scisignal.2000511 [DOI] [PubMed] [Google Scholar]
- 315.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C et al. (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest 122, 1022–1036, 10.1172/JCI59701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P et al. (2013) Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 27, 1621–1630, 10.1096/fj.12-221580 [DOI] [PubMed] [Google Scholar]
- 317.Sobrero A (1849) Sopra alcuni nuovi composti fulminanti ottenuti col mezzo dell’azione dell’acido nitrico sulle sostante organiche vegetali. Memorie della Reale Accademia delle Scienze di Torino, vol. 10, pp. 195–201, Torino [Google Scholar]
- 318.Sobrero A (1847) Sur plusieur composés détonants produits avec l’acide nitrique et le sucre, la dextrine, la lactine, la mannite et la glycérine. Comptes Rendus, vol. 24, Bachelier, Imprimeur-Libraire, Paris [Google Scholar]
- 319.Murrell W (1879) Nitro-glycerine as a remedy for angina pectoris. Lancet North Am. Ed 113, 80–81, 10.1016/S0140-6736(02)46032-1 [DOI] [Google Scholar]
- 320.Arnold WP, Mittal CK, Katsuki S and Murad F (1977) Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U. S. A 74, 3203–3207, 10.1073/pnas.74.8.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Katsuki S, Arnold W, Mittal C and Murad F (1977) Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cyclic Nucleotide Res 3, 23–35 [PubMed] [Google Scholar]
- 322.Furchgott RF and Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376, 10.1038/288373a0 [DOI] [PubMed] [Google Scholar]
- 323.Ignarro LJ, Buga GM, Wood KS, Byrns RE and Chaudhuri G (1987) Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A 84, 9265–9269, 10.1073/pnas.84.24.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Holt DB and Pang PS (2019) Vasodilator Therapies in the Treatment of Acute Heart Failure. Curr. Heart Fail Rep 16, 32–37, 10.1007/s11897-019-0421-4 [DOI] [PubMed] [Google Scholar]
- 325.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C et al. (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299, 896–899, 10.1126/science.1079368 [DOI] [PubMed] [Google Scholar]
- 326.De Toni L, Strapazzon G, Gianesello L, Caretta N, Pilon C, Bruttocao A et al. (2011) Effects of type 5-phosphodiesterase inhibition on energy metabolism and mitochondrial biogenesis in human adipose tissue ex vivo. J. Endocrinol. Invest 34, 738–741, 10.1007/BF03346724 [DOI] [PubMed] [Google Scholar]
- 327.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L et al. (2014) Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63, 2800–2811, 10.2337/db13-1234 [DOI] [PubMed] [Google Scholar]
- 328.Nisoli E, Clementi E, Tonello C, Sciorati C, Briscini L and Carruba MO (1998) Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. Br. J. Pharmacol 125, 888–894, 10.1038/sj.bjp.0702131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yan H, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A et al. (2002) Nitric oxide promotes differentiation of rat white preadipocytes in culture. J. Lipid Res 43, 2123–2129, 10.1194/jlr.M200305-JLR200 [DOI] [PubMed] [Google Scholar]
- 330.Hemmrich K, Gummersbach C, Paul NE, Goy D, Suschek CV, Kröncke K-D et al. (2010) Nitric oxide and downstream second messenger cGMP and cAMP enhance adipogenesis in primary human preadipocytes. Cytotherapy 12, 547–553, 10.3109/14653241003695042 [DOI] [PubMed] [Google Scholar]
- 331.Jennissen K, Siegel F, Liebig-Gonglach M, Hermann MR, Kipschull S, van Dooren S et al. (2012) A VASP-Rac-soluble guanylyl cyclase pathway controls cGMP production in adipocytes. Sci. Signal 5, ra62, 10.1126/scisignal.2002867 [DOI] [PubMed] [Google Scholar]
- 332.Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AWC et al. (2015) Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat. Commun 6, 7235, 10.1038/ncomms8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M et al. (2004) Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. U. S. A 101, 16507–16512, 10.1073/pnas.0405432101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wainio WW (1955) Reactions of cytochrome oxidase. J. Biol. Chem 212, 723–733 [PubMed] [Google Scholar]
- 335.Brown GC and Cooper CE (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 356, 295–298, 10.1016/0014-5793(94)01290-3 [DOI] [PubMed] [Google Scholar]
- 336.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S and Schapira AHV (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54, 10.1016/0014-5793(94)00424-2 [DOI] [PubMed] [Google Scholar]
- 337.Schweizer M and Richter C (1994) Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem. Biophys. Res. Commun 204, 169–175, 10.1006/bbrc.1994.2441 [DOI] [PubMed] [Google Scholar]
- 338.Koivisto A, Matthias A, Bronnikov G and Nedergaard J (1997) Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 417, 75–80, 10.1016/S0014-5793(97)01258-1 [DOI] [PubMed] [Google Scholar]
- 339.Nisoli E, Tonello C, Briscini L and Carruba MO (1997) Inducible nitric oxide synthase in rat brown adipocytes: implications for blood flow to brown adipose tissue. Endocrinology 138, 676–682, 10.1210/endo.138.2.4956 [DOI] [PubMed] [Google Scholar]
- 340.Saha SK and Kuroshima A (2000) Nitric oxide and thermogenic function of brown adipose tissue in rats. Jpn. J. Physiol 50, 337–342, 10.2170/jjphysiol.50.337 [DOI] [PubMed] [Google Scholar]
- 341.Giordano A, Tonello C, Bulbarelli A, Cozzi V, Cinti S, Carruba MO et al. (2002) Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 514, 135–140, 10.1016/S0014-5793(02)02245-7 [DOI] [PubMed] [Google Scholar]
- 342.Kikuchi-Utsumi K, Gao B, Ohinata H, Hashimoto M, Yamamoto N and Kuroshima A (2002) Enhanced gene expression of endothelial nitric oxide synthase in brown adipose tissue during cold exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol 282, R623–R626, 10.1152/ajpregu.00310.2001 [DOI] [PubMed] [Google Scholar]
- 343.Mehebik N, Jaubert AM, Sabourault D, Giudicelli Y and Ribière C (2005) Leptin-induced nitric oxide production in white adipocytes is mediated through PKA and MAP kinase activation. Am. J. Physiol. Cell Physiol 289, C379–C387, 10.1152/ajpcell.00320.2004 [DOI] [PubMed] [Google Scholar]
- 344.Nagashima T, Ohinata H and Kuroshima A (1994) Involvement of nitric oxide in noradrenaline-induced increase in blood flow through brown adipose tissue. Life Sci. 54, 17–25, 10.1016/0024-3205(94)00573-7 [DOI] [PubMed] [Google Scholar]
- 345.Saha SK, Ohinata H and Kuroshima A (1996) Effects of acute and chronic inhibition of nitric oxide synthase on brown adipose tissue thermogenesis. Jpn. J. Physiol 46, 375–382, 10.2170/jjphysiol.46.375 [DOI] [PubMed] [Google Scholar]
- 346.Petrović V, Korać A, Buzadžić B and Korać B (2005) The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J. Exp. Biol 208, 4263–4271, 10.1242/jeb.01895 [DOI] [PubMed] [Google Scholar]
- 347.Petrović V, Buzadžić B, Korać A, Vasilijević A, Janković A and Korać B (2010) NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp. Biochem. Physiol. C Toxicol. Pharmacol 152, 147–159, 10.1016/j.cbpc.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 348.Morley JE and Flood JF (1994) Effect of competitive antagonism of NO synthetase on weight and food intake in obese and diabetic mice. Am. J. Physiol 266, R164–R168 [DOI] [PubMed] [Google Scholar]
- 349.Stricker-Krongrad A, Beck B and Burlet C (1996) Nitric oxide mediates hyperphagia of obese Zucker rats: relation to specific changes in the microstructure of feeding behavior. Life Sci. 58, PL9–PL15 [DOI] [PubMed] [Google Scholar]
- 350.Saha SK, Ohno T, Ohinata H and Kuroshima A (1997) Effects of nitric oxide synthase inhibition on phospholipid fatty acid composition of brown adipose tissue. Jpn. J. Physiol 47, 477–480, 10.2170/jjphysiol.47.477 [DOI] [PubMed] [Google Scholar]
- 351.Tsuchiya K, Sakai H, Suzuki N, Iwashima F, Yoshimoto T, Shichiri M et al. (2007) Chronic blockade of nitric oxide synthesis reduces adiposity and improves insulin resistance in high fat-induced obese mice. Endocrinology 148, 4548–4556, 10.1210/en.2006-1371 [DOI] [PubMed] [Google Scholar]
- 352.Vasilijević A, Vojčić L, Dinulović I, Buzadžić B, Korać A, Petrović V et al. (2010) Expression pattern of thermogenesis-related factors in interscapular brown adipose tissue of alloxan-treated rats: beneficial effect of L-arginine. Nitric Oxide 23, 42–50, 10.1016/j.niox.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 353.Malik SS and Fewell JE (2003) Thermoregulation in rats during early postnatal maturation: importance of nitric oxide. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R1366–R1372, 10.1152/ajpregu.00280.2003 [DOI] [PubMed] [Google Scholar]
- 354.Le Gouill E, Jimenez M, Binnert C, Jayet P-Y, Thalmann S, Nicod P et al. (2007) Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes 56, 2690–2696, 10.2337/db06-1228 [DOI] [PubMed] [Google Scholar]
- 355.Jurrissen TJ, Sheldon RD, Gastecki ML, Woodford ML, Zidon TM, Rector RS et al. (2016) Ablation of eNOS does not promote adipose tissue inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol 310, R744–R751, 10.1152/ajpregu.00473.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA et al. (2012) Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ. Res 111, 1176–1189, 10.1161/CIRCRESAHA.112.266395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jang JE, Ko MS, Yun J-Y, Kim M-O, Kim JH, Park HS et al. (2016) Nitric Oxide Produced by Macrophages Inhibits Adipocyte Differentiation and Promotes Profibrogenic Responses in Preadipocytes to Induce Adipose Tissue Fibrosis. Diabetes 65, 2516–2528, 10.2337/db15-1624 [DOI] [PubMed] [Google Scholar]
- 358.Becerril S, Rodríguez A, Catalán V, Méndez-Giménez L, Ramírez B, Sáinz N et al. (2018) Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. Int. J. Obes. (Lond.) 42, 1458–1470, 10.1038/s41366-018-0005-5 [DOI] [PubMed] [Google Scholar]
- 359.Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S et al. (2018) Chronic hyperinsulinemia promotes meta-inflammation and extracellular matrix deposition in adipose tissue: Implications of nitric oxide. Mol. Cell. Endocrinol 477, 15–28, 10.1016/j.mce.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 360.Gómez-Ambrosi J, Becerril S, Oroz P, Zabalza S, Rodríguez A, Muruzábal FJ et al. (2004) Reduced adipose tissue mass and hypoleptinemia in iNOS deficient mice: effect of LPS on plasma leptin and adiponectin concentrations. FEBS Lett. 577, 351–356, 10.1016/j.febslet.2004.10.028 [DOI] [PubMed] [Google Scholar]
- 361.Kanuri BN, Kanshana JS, Rebello SC, Pathak P, Gupta AP, Gayen JR et al. (2017) Altered glucose and lipid homeostasis in liver and adipose tissue pre-dispose inducible NOS knockout mice to insulin resistance. Sci. Rep 7, 41009, 10.1038/srep41009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Becerril S, Rodríguez A, Catalán V, Sáinz N, Ramírez B, Collantes M et al. (2010) Deletion of inducible nitric-oxide synthase in leptin-deficient mice improves brown adipose tissue function. PLoS One 5, e10962, 10.1371/journal.pone.0010962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Becerril S, Rodríguez A, Catalán V, Sáinz N, Ramírez B, Gómez-Ambrosi J et al. (2012) Transcriptional analysis of brown adipose tissue in leptin-deficient mice lacking inducible nitric oxide synthase: evidence of the role of Med1 in energy balance. Physiol. Genomics 44, 678–688, 10.1152/physiolgenomics.00039.2012 [DOI] [PubMed] [Google Scholar]
- 364.Carlström M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E et al. (2010) Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. U. S. A 107, 17716–17720, 10.1073/pnas.1008872107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Roberts LD, Ashmore T, Kotwica AO, Murfitt SA, Fernandez BO, Feelisch M et al. (2015) Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes 64, 471–484, 10.2337/db14-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Gaudiot N, Jaubert AM, Charbonnier E, Sabourault D, Lacasa D, Giudicelli Y et al. (1998) Modulation of white adipose tissue lipolysis by nitric oxide. J. Biol. Chem 273, 13475–13481, 10.1074/jbc.273.22.13475 [DOI] [PubMed] [Google Scholar]
- 367.Frühbeck G and Gómez-Ambrosi J (2001) Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell. Signal 13, 827–833, 10.1016/S0898-6568(01)00211-X [DOI] [PubMed] [Google Scholar]
- 368.Canová N, Lincová D and Farghali H (2005) Inconsistent role of nitric oxide on lipolysis in isolated rat adipocytes. Physiol. Res 54, 387–393 [PubMed] [Google Scholar]
- 369.Canová NK, Lincová D, Kmoníčková E, Kameníková L and Farghali H (2006) Nitric oxide production from rat adipocytes is modulated by β3-adrenergic receptor agonists and is involved in a cyclic AMP-dependent lipolysis in adipocytes. Nitric Oxide 14, 200–211, 10.1016/j.niox.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 370.Andersson K, Gaudiot N, Ribiere C, Elizalde M, Giudicelli Y and Arner P (1999) A nitric oxide-mediated mechanism regulates lipolysis in human adipose tissue in vivo. Br. J. Pharmacol 126, 1639–1645, 10.1038/sj.bjp.0702430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lincová D, Mišeková D, Kmoníčková E, Canová N and Farghali H (2002) Effect of nitric oxide donors on isoprenaline-induced lipolysis in rat epididymal adipose tissue: studies in isolated adipose tissues and immobilized perfused adipocytes. Physiol. Res 51, 387–394 [PubMed] [Google Scholar]
- 372.Yamada Y, Eto M, Ito Y, Mochizuki S, Son B-K, Ogawa S et al. (2015) Suppressive Role of PPARγ-Regulated Endothelial Nitric Oxide Synthase in Adipocyte Lipolysis. PLoS One 10, e0136597, 10.1371/journal.pone.0136597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Klatt P, Cacho J, Crespo MD, Herrera E and Ramos P (2000) Nitric oxide inhibits isoproterenol-stimulated adipocyte lipolysis through oxidative inactivation of the β-agonist. Biochem. J 351, 485–493, 10.1042/bj3510485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gaudiot N, Ribiére C, Jaubert A-M and Giudicelli Y (2000) Endogenous nitric oxide is implicated in the regulation of lipolysis through antioxidant-related effect. Am. J. Physiol. Cell Physiol 279, C1603–C1610, 10.1152/ajpcell.2000.279.5.C1603 [DOI] [PubMed] [Google Scholar]
- 375.Hickner RC, Kemeny G, Clark PD, Galvin VB, McIver KL, Evans CA et al. (2012) In vivo nitric oxide suppression of lipolysis in subcutaneous abdominal adipose tissue is greater in obese than lean women. Obesity (Silver Spring) 20, 1174–1178, 10.1038/oby.2011.91 [DOI] [PubMed] [Google Scholar]
- 376.Vesely DL, Douglass MA, Dietz JR, Gower WR, McCormick MT, Rodriguez-Paz G et al. (1994) Three peptides from the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce diuresis, natriuresis, and/or kaliuresis in humans. Circulation 90, 1129–1140, 10.1161/01.CIR.90.3.1129 [DOI] [PubMed] [Google Scholar]
- 377.Koller KJ and Goeddel DV (1992) Molecular biology of the natriuretic peptides and their receptors. Circulation 86, 1081–1088, 10.1161/01.CIR.86.4.1081 [DOI] [PubMed] [Google Scholar]
- 378.Ogawa T and de Bold AJ (2014) The heart as an endocrine organ. Endocr. Connect 3, R31–R44, 10.1530/EC-14-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G et al. (1992) Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130, 229–239, 10.1210/endo.130.1.1309330 [DOI] [PubMed] [Google Scholar]
- 380.Potter LR, Abbey-Hosch S and Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev 27, 47–72, 10.1210/er.2005-0014 [DOI] [PubMed] [Google Scholar]
- 381.Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA et al. (1987) Physiological role of silent receptors of atrial natriuretic factor. Science 238, 675–678, 10.1126/science.2823385 [DOI] [PubMed] [Google Scholar]
- 382.Almeida FA, Suzuki M, Scarborough RM, Lewicki JA and Maack T (1989) Clearance function of type C receptors of atrial natriuretic factor in rats. Am. J. Physiol 256, R469–R475 [DOI] [PubMed] [Google Scholar]
- 383.Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W et al. (2017) Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci. Signal 10, 10.1126/scisignal.aam6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Cohen D, Koh GY, Nikonova LN, Porter JG and Maack T (1996) Molecular determinants of the clearance function of type C receptors of natriuretic peptides. J. Biol. Chem 271, 9863–9869, 10.1074/jbc.271.16.9863 [DOI] [PubMed] [Google Scholar]
- 385.Anand-Srivastava MB and Cantin M (1986) Atrial natriuretic factor receptors are negatively coupled to adenylate cyclase in cultured atrial and ventricular cardiocytes. Biochem. Biophys. Res. Commun 138, 427–436, 10.1016/0006-291X(86)90299-8 [DOI] [PubMed] [Google Scholar]
- 386.Anand-Srivastava MB, Srivastava AK and Cantin M (1987) Pertussis toxin attenuates atrial natriuretic factor-mediated inhibition of adenylate cyclase. Involvement of inhibitory guanine nucleotide regulatory protein. J. Biol. Chem 262, 4931–4934 [PubMed] [Google Scholar]
- 387.Anand-Srivastava MB, Sehl PD and Lowe DG (1996) Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J. Biol. Chem 271, 19324–19329, 10.1074/jbc.271.32.19324 [DOI] [PubMed] [Google Scholar]
- 388.Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR and Makhlouf GM (2000) G(i-1)/G(i-2)-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am. J. Physiol. Gastrointest. Liver Physiol 278, G974–G980, 10.1152/ajpgi.2000.278.6.G974 [DOI] [PubMed] [Google Scholar]
- 389.Pagano M and Anand-Srivastava MB (2001) Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J. Biol. Chem 276, 22064–22070, 10.1074/jbc.M101587200 [DOI] [PubMed] [Google Scholar]
- 390.Bacay AC, Mantyh CR, Vigna SR and Mantyh PW (1988) Receptor binding sites for atrial natriuretic factor are expressed by brown adipose tissue. Peptides 9, 1021–1026, 10.1016/0196-9781(88)90083-6 [DOI] [PubMed] [Google Scholar]
- 391.Okamura H, Kelly PA, Chabot JG, Morel G, Belles-Isles M and Heisler S (1988) Atrial natriuretic peptide receptors are present in brown adipose tissue. Biochem. Biophys. Res. Commun 156, 1000–1006, 10.1016/S0006-291X(88)80943-4 [DOI] [PubMed] [Google Scholar]
- 392.Sarzani R, Paci VM, Dessì-Fulgheri P, Espinosa E and Rappelli A (1993) Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J. Hypertens. Suppl 11, S214–S215, 10.1097/00004872-199312050-00086 [DOI] [PubMed] [Google Scholar]
- 393.De León H, Bonhomme MC, Thibault G and Garcia R (1995) Localization of atrial natriuretic factor receptors in the mesenteric arterial bed. Comparison with angiotensin II and endothelin receptors. Circulation Res. 77, 64–72, 10.1161/01.RES.77.1.64 [DOI] [PubMed] [Google Scholar]
- 394.Sarzani R, Dessì-Fulgheri P, Paci VM, Espinosa E and Rappelli A (1996) Expression of natriuretic peptide receptors in human adipose and other tissues. J. Endocrinol. Invest 19, 581–585, 10.1007/BF03349021 [DOI] [PubMed] [Google Scholar]
- 395.Sengenès C, Berlan M, De Glisezinski I, Lafontan M and Galitzky J (2000) Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 14, 1345–1351, 10.1096/fasebj.14.10.1345 [DOI] [PubMed] [Google Scholar]
- 396.Nakatsuji H, Maeda N, Hibuse T, Hiuge A, Hirata A, Kuroda Y et al. (2010) Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem. Biophys. Res. Commun 392, 100–105, 10.1016/j.bbrc.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 397.Dessì-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G et al. (1997) Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J. Hypertens 15, 1695–1699, 10.1097/00004872-199715120-00074 [DOI] [PubMed] [Google Scholar]
- 398.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M et al. (2009) Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 58, 2880–2892, 10.2337/db09-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Coué M, Badin PM, Vila IK, Laurens C, Louche K, Marquès MA et al. (2015) Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes 64, 4033–4045, 10.2337/db15-0305 [DOI] [PubMed] [Google Scholar]
- 400.Plante E, Menaouar A, Danalache BA, Broderick TL, Jankowski M and Gutkowska J (2014) Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia 57, 1257–1267, 10.1007/s00125-014-3201-4 [DOI] [PubMed] [Google Scholar]
- 401.Kovacova Z, Tharp WG, Liu D, Wei W, Xie H, Collins S et al. (2016) Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring) 24, 820–828, 10.1002/oby.21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Rydén M, Bäckdahl J, Petrus P, Thorell A, Gao H, Coue M et al. (2016) Impaired atrial natriuretic peptide-mediated lipolysis in obesity. Int. J. Obes. (Lond.) 40, 714–720, 10.1038/ijo.2015.222 [DOI] [PubMed] [Google Scholar]
- 403.Gentili A, Frangione MR, Albini E, Vacca C, Ricci MA, De Vuono S et al. (2017) Modulation of natriuretic peptide receptors in human adipose tissue: molecular mechanisms behind the “natriuretic handicap” in morbidly obese patients. Transl. Res 186, 52–61, 10.1016/j.trsl.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 404.Glöde A, Naumann J, Gnad T, Cannone V, Kilic A, Burnett JC et al. (2017) Divergent effects of a designer natriuretic peptide CD-NP in the regulation of adipose tissue and metabolism. Mol. Metab 6, 276–287, 10.1016/j.molmet.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Dinas PC, Nintou E, Psychou D, Granzotto M, Rossato M, Vettor R et al. (2018) Association of fat mass profile with natriuretic peptide receptor alpha in subcutaneous adipose tissue of medication-free healthy men: A cross-sectional study. F1000Res. 7, 327, 10.12688/f1000research.14198.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Bordicchia M, Ceresiani M, Pavani M, Minardi D, Polito M, Wabitsch M et al. (2016) Insulin/glucose induces natriuretic peptide clearance receptor in human adipocytes: a metabolic link with the cardiac natriuretic pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R104–R114, 10.1152/ajpregu.00499.2015 [DOI] [PubMed] [Google Scholar]
- 407.Sarzani R, Paci VM, Zingaretti CM, Pierleoni C, Cinti S, Cola G et al. (1995) Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. J. Hypertens 13, 1241–1246, 10.1097/00004872-199511000-00004 [DOI] [PubMed] [Google Scholar]
- 408.Haufe S, Kaminski J, Utz W, Haas V, Mähler A, Daniels MA et al. (2015) Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. J. Hypertens 33, 1458–1464, 10.1097/HJH.0000000000000573 [DOI] [PubMed] [Google Scholar]
- 409.Bae C-R, Hino J, Hosoda H, Son C, Makino H, Tokudome T et al. (2018) Adipocyte-specific expression of C-type natriuretic peptide suppresses lipid metabolism and adipocyte hypertrophy in adipose tissues in mice fed high-fat diet. Sci. Rep 8, 2093, 10.1038/s41598-018-20469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Thibault G, Lacasse A and Garcia R (1996) Specific potentiation by cyclic AMP of natriuretic peptide-mediated cyclic GMP production in adipose tissue. Life Sci. 58, 2345–2353, 10.1016/0024-3205(96)00235-4 [DOI] [PubMed] [Google Scholar]
- 411.Ruiz-Ojeda FJ, Aguilera CM, Rupérez AI, Gil Á and Gomez-Llorente C (2016) An analogue of atrial natriuretic peptide (C-ANP4–23) modulates glucose metabolism in human differentiated adipocytes. Mol. Cell. Endocrinol 431, 101–108, 10.1016/j.mce.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 412.Coué M, Barquissau V, Morigny P, Louche K, Lefort C, Mairal A et al. (2018) Natriuretic peptides promote glucose uptake in a cGMP-dependent manner in human adipocytes. Sci. Rep 8, 1097, 10.1038/s41598-018-19619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Galitzky J, Sengenès C, Thalamas C, Marques MA, Senard J-M, Lafontan M et al. (2001) The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J. Lipid Res 42, 536–544 [PubMed] [Google Scholar]
- 414.Moro C, Polak J, Richterova B, Sengenès C, Pelikanova T, Galitzky J et al. (2005) Differential regulation of atrial natriuretic peptide- and adrenergic receptor-dependent lipolytic pathways in human adipose tissue. Metabolism 54, 122–131, 10.1016/j.metabol.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 415.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Tank J et al. (2006) Beta-adrenergic and atrial natriuretic peptide interactions on human cardiovascular and metabolic regulation. J. Clin. Endocrinol. Metab 91, 5069–5075, 10.1210/jc.2006-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Moro C, Klimcakova E, Lafontan M, Berlan M and Galitzky J (2007) Phosphodiesterase-5A and neutral endopeptidase activities in human adipocytes do not control atrial natriuretic peptide-mediated lipolysis. Br. J. Pharmacol 152, 1102–1110, 10.1038/sj.bjp.0707485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Polak J, Moro C, Klimcakova E, Kovacikova M, Bajzova M, Vitkova M et al. (2007) The atrial natriuretic peptide- and catecholamine-induced lipolysis and expression of related genes in adipose tissue in hypothyroid and hyperthyroid patients. Am. J. Physiol. Endocrinol. Metab 293, E246–E251, 10.1152/ajpendo.00688.2006 [DOI] [PubMed] [Google Scholar]
- 418.Nishikimi T, Iemura-Inaba C, Akimoto K, Ishikawa K, Koshikawa S and Matsuoka H (2009) Stimulatory and Inhibitory regulation of lipolysis by the NPR-A/cGMP/PKG and NPR-C/Gi pathways in rat cultured adipocytes. Regul. Pept 153, 56–63, 10.1016/j.regpep.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 419.Sengenes C, Stich V, Berlan M, Hejnova J, Lafontan M, Pariskova Z et al. (2002) Increased lipolysis in adipose tissue and lipid mobilization to natriuretic peptides during low-calorie diet in obese women. Int. J. Obesity Related Metab. Disorders: J. Int. Assoc. Study Obesity 26, 24–32, 10.1038/sj.ijo.0801845 [DOI] [PubMed] [Google Scholar]
- 420.Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J et al. (2011) Lipolytic effects of B-type natriuretic peptide 1–32 in adipose tissue of heart failure patients compared with healthy controls. J. Am. Coll. Cardiol 58, 1119–1125, 10.1016/j.jacc.2011.05.042 [DOI] [PubMed] [Google Scholar]
- 421.Souza SC, Chau MD, Yang Q, Gauthier M-S, Clairmont KB, Wu Z et al. (2011) Atrial natriuretic peptide regulates lipid mobilization and oxygen consumption in human adipocytes by activating AMPK. Biochem. Biophys. Res. Commun 410, 398–403, 10.1016/j.bbrc.2011.05.143 [DOI] [PubMed] [Google Scholar]
- 422.Kimura H, Nagoshi T, Yoshii A, Kashiwagi Y, Tanaka Y, Ito K et al. (2017) The thermogenic actions of natriuretic peptide in brown adipocytes: The direct measurement of the intracellular temperature using a fluorescent thermoprobe. Sci. Rep 7, 12978, 10.1038/s41598-017-13563-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Liu D, Ceddia RP and Collins S (2018) Cardiac natriuretic peptides promote adipose ‘browning’ through mTOR complex-1. Mol. Metab 9, 192–198, 10.1016/j.molmet.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Collins S (2014) A heart-adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol 10, 157–163, 10.1038/nrendo.2013.234 [DOI] [PubMed] [Google Scholar]
- 425.Inuzuka M, Tamura N, Yamada N, Katsuura G, Oyamada N, Taura D et al. (2010) C-type natriuretic peptide as a new regulator of food intake and energy expenditure. Endocrinology 151, 3633–3642, 10.1210/en.2010-0141 [DOI] [PubMed] [Google Scholar]
- 426.Rorbach J, Nicholls TJ and Minczuk M (2011) PDE12 removes mitochondrial RNA poly(A) tails and controls translation in human mitochondria. Nucleic Acids Res. 39, 7750–7763, 10.1093/nar/gkr470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Somerville LL (2001) (NHLBI) NHLaBI. Theophylline revisited. Allergy Asthma Proc. 22, 347–351 [PubMed] [Google Scholar]
- 428.Kumar M and Bhattacharya V (2007) Cilostazol: a new drug in the treatment intermittent claudication. Recent Pat. Cardiovasc. Drug Discov 2, 181–185, 10.2174/157489007782418991 [DOI] [PubMed] [Google Scholar]
- 429.Fala L (2015) Otezla (Apremilast), an Oral PDE-4 Inhibitor, Receives FDA Approval for the Treatment of Patients with Active Psoriatic Arthritis and Plaque Psoriasis. Am. Health Drug Benefits 8, 105–110 [PMC free article] [PubMed] [Google Scholar]
- 430.Hoy S (2017) Crisaborole Ointment 2%: A Review in Mild to Moderate Atopic Dermatitis. Am. J. Clin. Dermatol 18, 837–843, 10.1007/s40257-017-0327-4 [DOI] [PubMed] [Google Scholar]
- 431.Ciulla MM and Vivona P (2017) PDE5 Inhibitors, Erectile Dysfunction and beyond: How, Sometimes, Indications are the Consequences of Marketing Strategies and/or Serendipity. JSM Sexual Med. 2, 1005 [Google Scholar]
- 432.Dole VP (1961) Effect of nucleic acid metabolites on lipolysis in adipose tissue. J. Biol. Chem 236, 3125–3130 [PubMed] [Google Scholar]
- 433.Cockburn F, Hull D and Walton I (1967) The effect of lipolytic hormones and theophylline on heat production in brown adipose tissue in vivo. Br. J. Pharmacol. Chemother 31, 568–577, 10.1111/j.1476-5381.1967.tb00421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Mühlbachova E, Sólyom A and Puglisi L (1967) Investigations on the mechanism of the prostaglandin E1 antagonism to norepinephrine and theophylline-induced lipolysis. Eur. J. Pharmacol 1, 321–325, 10.1016/0014-2999(67)90074-X [DOI] [Google Scholar]
- 435.Sólyom A, Puglisi L and Muhlbachova E (1967) Effect in vitro of theophylline and prostaglandin E1 on free fatty acid release and on triglyceride synthesis in rat adipose tissue. Biochem. Pharmacol 16, 521–525, 10.1016/0006-2952(67)90099-8 [DOI] [PubMed] [Google Scholar]
- 436.Davies JI (1968) In vitro regulation of the lipolysis of adipose tissue. Nature 218, 349–352, 10.1038/218349a0 [DOI] [PubMed] [Google Scholar]
- 437.Izawa T, Komabayashi T, Mochizuki T, Suda K and Tsuboi M (1991) Enhanced coupling of adenylate cyclase to lipolysis in permeabilized adipocytes from trained rats. J. Appl. Physiol 71, 23–29, 10.1152/jappl.1991.71.1.23 [DOI] [PubMed] [Google Scholar]
- 438.Baur JA and Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov 5, 493–506, 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 439.Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H et al. (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433, 10.1016/j.cell.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW et al. (2010) AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563, 10.2337/db09-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Goraya TA and Cooper DM (2005) Ca2+-calmodulin-dependent phosphodiesterase (PDE1): current perspectives. Cell. Signal 17, 789–797, 10.1016/j.cellsig.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 442.Bronnikov GE, Zhang SJ, Cannon B and Nedergaard J (1999) A dual component analysis explains the distinctive kinetics of cAMP accumulation in brown adipocytes. J. Biol. Chem 274, 37770–37780, 10.1074/jbc.274.53.37770 [DOI] [PubMed] [Google Scholar]
- 443.Kraynik SM, Miyaoka RS and Beavo JA (2013) PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol. Pharmacol 83, 1155–1165, 10.1124/mol.112.084145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY et al. (2014) MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun 5, 4725, 10.1038/ncomms5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Makino H, Manganiello VC and Kono T (1994) Roles of ATP in insulin actions. Annu. Rev. Physiol 56, 273–295, 10.1146/annurev.ph.56.030194.001421 [DOI] [PubMed] [Google Scholar]
- 446.Guirguis E, Hockman S, Chung YW, Ahmad F, Gavrilova O, Raghavachari N et al. (2013) A role for phosphodiesterase 3B in acquisition of brown fat characteristics by white adipose tissue in male mice. Endocrinology 154, 3152–3167, 10.1210/en.2012-2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Chung YW, Ahmad F, Tang Y, Hockman SC, Kee HJ, Berger K et al. (2017) White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci. Rep 7, 40445, 10.1038/srep40445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wu C and Rajagopalan S (2016) Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders. Obesity Rev.: An Off. J. Int. Assoc. Study Obesity, 10.1111/obr.12385 [DOI] [PubMed] [Google Scholar]
- 449.Clapcote SJ (2017) Phosphodiesterase-4B as a Therapeutic Target for Cognitive Impairment and Obesity-Related Metabolic Diseases. In Adv Neurobiol. Phosphodiesterases: CNS Functions and Diseases (Zhang H-T, Xu Y and O’Donnell JM, eds), vol. 17, pp. 103–131, Springer; [DOI] [PubMed] [Google Scholar]
- 450.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ et al. (2009) Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374, 685–694, 10.1016/S0140-6736(09)61255-1 [DOI] [PubMed] [Google Scholar]
- 451.Zhang R, Maratos-Flier E and Flier JS (2009) Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology 150, 3076–3082, 10.1210/en.2009-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Meng W, Liang X, Chen H, Luo H, Bai J, Li G et al. (2017) Rheb Inhibits Beiging of White Adipose Tissue via PDE4D5-Dependent Downregulation of the cAMP-PKA Signaling Pathway. Diabetes 66, 1198–1213, 10.2337/db16-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Ramirez CE, Hui N, Yu C, Gamboa JL, Luther JM, Brown NJ et al. Treatment with Sildenafil Improves Insulin Sensitivity in Prediabetes: A Randomized, Controlled Trial. J. Clin. Endocrinol. Metab 2015, jc20153415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sjögren L, Olausson J, Strindberg L, Mobini R, Fogelstrand P, Mattsson Hultén L et al. (2016) Postprandial effects of the phosphodiesterase-5 inhibitor tadalafil in people with well-controlled Type 2 diabetes mellitus: a randomized controlled trial. Diabet. Med 33, 1299–1301, 10.1111/dme.12999 [DOI] [PubMed] [Google Scholar]
- 455.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT and Wasserman DH (2007) Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56, 1025–1033, 10.2337/db06-0883 [DOI] [PubMed] [Google Scholar]
- 456.Behr-Roussel D, Oudot A, Caisey S, Coz OL, Gorny D, Bernabé J et al. (2008) Daily treatment with sildenafil reverses endothelial dysfunction and oxidative stress in an animal model of insulin resistance. Eur. Urol 53, 1272–1280, 10.1016/j.eururo.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 457.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP and Wasserman DH (2013) Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62, 572–580, 10.2337/db12-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Li S, Li Y, Xiang L, Dong J, Liu M and Xiang G (2018) Sildenafil induces browning of subcutaneous white adipose tissue in overweight adults. Metabolism 78, 106–117, 10.1016/j.metabol.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 459.Yu HM, Chung HK, Kim KS, Lee JM, Hong JH and Park KS (2017) PDE 5 inhibitor improves insulin sensitivity by enhancing mitochondrial function in adipocytes. Biochem. Biophys. Res. Commun 493, 631–636, 10.1016/j.bbrc.2017.08.140 [DOI] [PubMed] [Google Scholar]
- 460.Cote RH (2004) Characteristics of photoreceptor PDE (PDE6): similarities and differences to PDE5. Int. J. Impot. Res 16, S28–S33, 10.1038/sj.ijir.3901212 [DOI] [PubMed] [Google Scholar]
- 461.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J and Manganiello VC (2014) Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov 13, 290–314, 10.1038/nrd4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Omar B, Banke E, Ekelund M, Frederiksen S and Degerman E (2011) Alterations in cyclic nucleotide phosphodiesterase activities in omental and subcutaneous adipose tissues in human obesity. Nutr. Diab 1, e13, 10.1038/nutd.2011.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Hankir MK, Kranz M, Gnad T, Weiner J, Wagner S, Deuther-Conrad W et al. (2016) A novel thermoregulatory role for PDE10A in mouse and human adipocytes. EMBO Mol. Med 8, 796–812, 10.15252/emmm.201506085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD et al. (2006) Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology 51, 374–385, 10.1016/j.neuropharm.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 465.Nawrocki AR, Rodriguez CG, Toolan DM, Price O, Henry M, Forrest G et al. (2014) Genetic deletion and pharmacological inhibition of phosphodiesterase 10A protects mice from diet-induced obesity and insulin resistance. Diabetes 63, 300–311, 10.2337/db13-0247 [DOI] [PubMed] [Google Scholar]
- 466.Regard JB, Sato IT and Coughlin SR (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571, 10.1016/j.cell.2008.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Dhalla AK, Santikul M, Chisholm JW, Belardinelli L and Reaven GM (2009) Comparison of the antilipolytic effects of an A1 adenosine receptor partial agonist in normal and diabetic rats. Diabetes Obes. Metab 11, 95–101, 10.1111/j.1463-1326.2008.00902.x [DOI] [PubMed] [Google Scholar]
- 468.Hjemdahl P and Fredholm BB (1976) Cyclic AMP-dependent and independent inhibition of lipolysis by adenosine and decreased pH. Acta Physiol. Scand 96, 170–179, 10.1111/j.1748-1716.1976.tb10186.x [DOI] [PubMed] [Google Scholar]
- 469.Schwabe U, Ebert R and Erbler HC (1973) Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3’,5’-AMP levels and lipolysis. Naunyn Schmiedebergs Arch. Pharmacol 276, 133–148, 10.1007/BF00501186 [DOI] [PubMed] [Google Scholar]
- 470.Fain JN, Pointer RH and Ward WF (1972) Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J. Biol. Chem 247, 6866–6872 [PubMed] [Google Scholar]
- 471.Trost T and Stock K (1977) Effects of adenosine derivatives on cAMP accumulation and lipolysis in rat adipocytes and on adenylate cyclase in adipocyte plasma membranes. Naunyn Schmiedebergs Arch. Pharmacol 299, 33–40, 10.1007/BF00508634 [DOI] [PubMed] [Google Scholar]
- 472.Ohisalo JJ, Ranta S and Huhtaniemi IT (1984) Inhibition of adenosine 3’,5’-monophosphate accumulation and lipolysis by adenosine analogs in human subcutaneous adipocytes. J. Clin. Endocrinol. Metab 58, 32–35, 10.1210/jcem-58-1-32 [DOI] [PubMed] [Google Scholar]
- 473.Johansson SM, Lindgren E, Yang J-N, Herling AW and Fredholm BB (2008) Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur. J. Pharmacol 597, 92–101, 10.1016/j.ejphar.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 474.Turpin BP, Duckworth WC and Solomon SS (1977) Perifusion of isolated rat adipose cells. Modulation of lipolysis by adenosine. J. Clin. Invest 60, 442–448, 10.1172/JCI108794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Fredholm BB (1978) Effect of adenosine, adenosine analogues and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol. Scand 102, 191–198, 10.1111/j.1748-1716.1978.tb06062.x [DOI] [PubMed] [Google Scholar]
- 476.Hjemdahl P and Sollevi A (1978) Antilipolytic effect of adenosine in isolated perifused fat cells. Acta Physiol. Scand 103, 270–274, 10.1111/j.1748-1716.1978.tb06214.x [DOI] [PubMed] [Google Scholar]
- 477.Sollevi A (1980) The sensitivity of perifused fat cells to the antilipolytic action of adenosine. Acta Physiol. Scand 110, 211–213, 10.1111/j.1748-1716.1980.tb06654.x [DOI] [PubMed] [Google Scholar]
- 478.Ohisalo JJ (1981) Effects of adenosine on lipolysis in human subcutaneous fat cells. J. Clin. Endocrinol. Metab 52, 359–363, 10.1210/jcem-52-2-359 [DOI] [PubMed] [Google Scholar]
- 479.Szillat D and Bukowiecki LJ (1983) Control of brown adipose tissue lipolysis and respiration by adenosine. Am. J. Physiol 245, E555–E559 [DOI] [PubMed] [Google Scholar]
- 480.Knospe S and Köhler E (1984) Impaired hormonal regulation of lipolysis and the interference with adenosine in adipose tissue from hyperglycemic sand rats in vitro. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 16, 349–353, 10.1055/s-2007-1014788 [DOI] [PubMed] [Google Scholar]
- 481.Sollevi A, Hjemdahl P and Fredholm BB (1981) Endogenous adenosine inhibits lipolysis induced by nerve stimulation without inhibiting noradrenaline release in canine subcutaneous adipose tissue in vivo. Naunyn Schmiedebergs Arch. Pharmacol 316, 112–119, 10.1007/BF00505303 [DOI] [PubMed] [Google Scholar]
- 482.Johansson SM, Yang JN, Lindgren E and Fredholm BB (2007) Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiol. (Oxf.) 190, 87–96, 10.1111/j.1365-201X.2007.01692.x [DOI] [PubMed] [Google Scholar]
- 483.Schimmel RJ and McCarthy L (1984) Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am. J. Physiol 246, C301–C307, 10.1152/ajpcell.1984.246.3.C301 [DOI] [PubMed] [Google Scholar]
- 484.Schimmel RJ, Elliott ME and Dehmel VC (1987) Interactions between adenosine and α1-adrenergic agonists in regulation of respiration in hamster brown adipocytes. Mol. Pharmacol 32, 26–33 [PubMed] [Google Scholar]
- 485.Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A et al. (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399, 10.1038/nature13816 [DOI] [PubMed] [Google Scholar]
- 486.Lafontan M and Berlan M (1993) Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lipid Res 34 [PubMed] [Google Scholar]
- 487.Madden CJ, Tupone D, Cano G and Morrison SF (2013) α2 Adrenergic receptor-mediated inhibition of thermogenesis. J. Neurosci 33, 2017–2028, 10.1523/JNEUROSCI.4701-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489, 10.1038/nature04330 [DOI] [PubMed] [Google Scholar]
- 489.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G et al. (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177, 10.1016/j.cmet.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A et al. (2015) The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 22, 418–426, 10.1016/j.cmet.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 491.Zietak M and Kozak LP (2016) Bile acids induce uncoupling protein 1-dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am. J. Physiol. Endocrinol. Metab 310, E346–E354, 10.1152/ajpendo.00485.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Carino A, Cipriani S, Marchianò S, Biagioli M, Scarpelli P, Zampella A et al. (2017) Gpbar1 agonism promotes a Pgc-1α-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci. Rep 7, 13689, 10.1038/s41598-017-13102-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Velazquez-Villegas LA, Perino A, Lemos V, Zietak M, Nomura M, Pols TWH et al. (2018) TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun 9, 245, 10.1038/s41467-017-02068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Watanabe M, Morimoto K, Houten SM, Kaneko-Iwasaki N, Sugizaki T, Horai Y et al. (2012) Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 7, e38286, 10.1371/journal.pone.0038286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Teodoro JS, Zouhar P, Flachs P, Bardova K, Janovska P, Gomes AP et al. (2014) Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int. J. Obes. (Lond.) 38, 1027–1034, 10.1038/ijo.2013.230 [DOI] [PubMed] [Google Scholar]
- 496.Chen X, Yan L, Guo Z, Chen Y, Li M, Huang C et al. (2017) Chenodeoxycholic acid attenuates high-fat diet-induced obesity and hyperglycemia via the G protein-coupled bile acid receptor 1 and proliferator-activated receptor γ pathway. Exp. Ther. Med 14, 5305–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Carino A, Cipriani S, Marchianò S, Biagioli M, Santorelli C, Donini A et al. (2017) BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Sci. Rep 7, 42801, 10.1038/srep42801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Carino A, Marchianò S, Biagioli M, Bucci M, Vellecco V, Brancaleone V et al. (2018) Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis. FASEB J. fj201801373RR [DOI] [PubMed] [Google Scholar]
- 499.Butcher RW, Baird CE and Sutherland EW (1968) Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J. Biol. Chem 243, 1705–1712 [PubMed] [Google Scholar]
- 500.Blecher M, Merlino NS, Ro’Ane JT and Flynn PD (1969) Independence of the effects of epinephrine, glucagon, and adrenocorticotropin on glucose utilization from those on lipolysis in isolated rat adipose cells. J. Biol. Chem 244, 3423–3429 [PubMed] [Google Scholar]
- 501.Steinberg D, Vaughan M, Nestel PJ and Bergström S (1963) Effects of prostaglandin E opposing those of catecholamines on blood pressure and on triglyceride breakdown in adipose tissue. Biochem. Pharmacol 12, 764–766, 10.1016/0006-2952(63)90053-4 [DOI] [PubMed] [Google Scholar]
- 502.Steinberg D, Vaughan M, Nestel PJ, Strand O and Bergström S (1964) Effects of the Prostaglandins on Hormone-Induced Mobilization of Free Fatty Acids. J. Clin. Invest 43, 1533–1540, 10.1172/JCI105030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Honnor RC and Saggerson ED (1980) Altered lipolytic response to glucagon and adenosine deaminase in adipocytes from starved rats. Biochem. J 188, 757–761, 10.1042/bj1880757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Scanes CG, Peterla TA and Campbell RM (1994) Influence of adenosine or adrenergic agonists on growth hormone stimulated lipolysis by chicken adipose tissue in vitro. Comp. Biochem. Physiol. Pharmacol. Toxicol. Endocrinol 107, 243–248, 10.1016/1367-8280(94)90047-7 [DOI] [PubMed] [Google Scholar]
- 505.Illiano G and Cuatrecasas P (1971) Endogenous prostaglandins modulate lipolytic processes in adipose tissue. Nature: New Biol. 234, 72–74 [DOI] [PubMed] [Google Scholar]
- 506.Hittelman KJ and Butcher RW (1973) Effects of antilipolytic agents and α-adrenergic antagonists on cyclic AMP metabolism in hamster white adipocytes. Biochim. Biophys. Acta 316, 403–410, 10.1016/0005-2760(73)90079-9 [DOI] [PubMed] [Google Scholar]
- 507.Hittelman KJ, Wu CF and Butcher RW (1973) Control of cyclic AMP levels in isolated fat cells from hamsters. Biochim. Biophys. Acta 304, 188–196, 10.1016/0304-4165(73)90127-X [DOI] [PubMed] [Google Scholar]
- 508.Gong HX, Guo XR, Fei L, Guo M, Liu QQ and Chen RH (2003) Lipolysis and apoptosis of adipocytes induced by neuropeptide Y-Y5 receptor antisense oligodeoxynucleotides in obese rats. Acta Pharmacol. Sin 24, 569–575 [PubMed] [Google Scholar]
- 509.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD et al. (2007) Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med 13, 803–811, 10.1038/nm1611 [DOI] [PubMed] [Google Scholar]
- 510.Yang K, Guan H, Arany E, Hill DJ and Cao X (2008) Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 22, 2452–2464, 10.1096/fj.07-100735 [DOI] [PubMed] [Google Scholar]
- 511.Gericke MT, Kosacka J, Koch D, Nowicki M, Schroder T, Ricken AM et al. (2009) Receptors for NPY and PACAP differ in expression and activity during adipogenesis in the murine 3T3-L1 fibroblast cell line. Br. J. Pharmacol 157, 620–632, 10.1111/j.1476-5381.2009.00164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Singer K, Morris DL, Oatmen KE, Wang T, DelProposto J, Mergian T et al. (2013) Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One 8, e57929, 10.1371/journal.pone.0057929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Zhang W, Sumners LH, Siegel PB, Cline MA and Gilbert ER (2013) Quantity of glucose transporter and appetite-associated factor mRNA in various tissues after insulin injection in chickens selected for low or high body weight. Physiol. Genomics 45, 1084–1094, 10.1152/physiolgenomics.00102.2013 [DOI] [PubMed] [Google Scholar]
- 514.Valet P, Berlan M, Beauville M, Crampes F, Montastruc JL and Lafontan M (1990) Neuropeptide Y and peptide YY inhibit lipolysis in human and dog fat cells through a pertussis toxin-sensitive G protein. J. Clin. Invest 85, 291–295, 10.1172/JCI114425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Castan I, Valet P, Quideau N, Voisin T, Ambid L, Laburthe M et al. (1994) Antilipolytic effects of α2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am. J. Physiol 266, R1141–R1147 [DOI] [PubMed] [Google Scholar]
- 516.Turtzo LC, Marx R and Lane MD (2001) Cross-talk between sympathetic neurons and adipocytes in coculture. Proc. Natl. Acad. Sci. U. S. A 98, 12385–12390, 10.1073/pnas.231478898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Labelle M, Boulanger Y, Fournier A, St Pierre S and Savard R (1997) Tissue-specific regulation of fat cell lipolysis by NPY in 6-OHDA-treated rats. Peptides 18, 801–808, 10.1016/S0196-9781(97)00028-4 [DOI] [PubMed] [Google Scholar]
- 518.Billington CJ, Briggs JE, Grace M and Levine AS (1991) Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am. J. Physiol 260, R321–R327 [DOI] [PubMed] [Google Scholar]
- 519.Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y et al. (2006) Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 55, 3091–3098, 10.2337/db05-0624 [DOI] [PubMed] [Google Scholar]
- 520.Park S, Komatsu T, Kim SE, Tanaka K, Hayashi H, Mori R et al. (2017) Neuropeptide Y resists excess loss of fat by lipolysis in calorie-restricted mice: a trait potential for the life-extending effect of calorie restriction. Aging Cell 16, 339–348, 10.1111/acel.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Wan Y, Xue R, Wang Y, Zhang Q, Huang S, Wu W et al. (2015) The effect of neuropeptide Y on brown-like adipocyte’s differentiation and activation. Peptides 63, 126–133, 10.1016/j.peptides.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 522.Wei Y and Mojsov S (1996) Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J. Neuroendocrinol 8, 811–817, 10.1046/j.1365-2826.1996.05191.x [DOI] [PubMed] [Google Scholar]
- 523.Nakata M, Shioda S, Oka Y, Maruyama I and Yada T (1999) Insulinotropin PACAP potentiates insulin-stimulated glucose uptake in 3T3 L1 cells. Peptides 20, 943–948, 10.1016/S0196-9781(99)00085-6 [DOI] [PubMed] [Google Scholar]
- 524.Åkesson L, Ahrén B, Edgren G and Degerman E (2005) VPAC2-R mediates the lipolytic effects of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide in primary rat adipocytes. Endocrinology 146, 744–750, 10.1210/en.2004-0504 [DOI] [PubMed] [Google Scholar]
- 525.Yu RJ, Zhang L, Yi TH, Xie SS and Dai Y (2008) In vivo anti-obesity effect of the agonist for receptor VPAC1. Sheng Li Xue Bao 60, 751–758 [PubMed] [Google Scholar]
- 526.Arsenijevic T, Gregoire F, Chiadak J, Courtequisse E, Bolaky N, Perret J et al. (2013) Pituitary adenylate cyclase activating peptide (PACAP) participates in adipogenesis by activating ERK signaling pathway. PLoS One 8, e72607, 10.1371/journal.pone.0072607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Cline DL, Short LI, Forster MAM and Gray SL (2019) Adipose Tissue Expression of PACAP, VIP, and Their Receptors in Response to Cold Stress. J. Mol. Neurosci 68, 427–438, 10.1007/s12031-018-1099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Santangelo C, Filardi T, Perrone G, Mariani M, Mari E, Scazzocchio B et al. (2019) Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol. 56, 681–689, 10.1007/s00592-019-01304-x [DOI] [PubMed] [Google Scholar]
- 529.Saito Y, Matsuoka N, Shirai K, Yamamoto M, Kumagai A and Yanaihara N (1978) Effects of adrenergic blocking agents on lipolysis and adenyl cyclase activity induced by vasoactive intestinal polypeptide (VIP). Endocrinol. Jpn 25, 403–405, 10.1507/endocrj1954.25.403 [DOI] [PubMed] [Google Scholar]
- 530.Shido O, Yoneda Y and Nagasaka T (1989) Changes in brown adipose tissue metabolism following intraventricular vasoactive intestinal peptide and other gastrointestinal peptides in rats. Jpn. J. Physiol 39, 359–369, 10.2170/jjphysiol.39.359 [DOI] [PubMed] [Google Scholar]
- 531.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS and Choi S (2011) Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am. J. Physiol. Regul. Integr.omparative Physiol 301, R1625–R1634, 10.1152/ajpregu.00334.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Børglum JD, Pedersen SB, Ailhaud G, Négrel R and Richelsen B (1999) Differential expression of prostaglandin receptor mRNAs during adipose cell differentiation. Prostaglandins Other Lipid Mediat. 57, 305–317, 10.1016/S0090-6980(98)00082-3 [DOI] [PubMed] [Google Scholar]
- 533.Tran POT, Gleason CE and Robertson RP (2002) Inhibition of interleukin-1β-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet β-cell function. Diabetes 51, 1772–1778, 10.2337/diabetes.51.6.1772 [DOI] [PubMed] [Google Scholar]
- 534.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E and Sul HS (2007) Regulation of lipolysis in adipocytes. Annu. Rev. Nutr 27, 79–101, 10.1146/annurev.nutr.27.061406.093734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Inazumi T, Shirata N, Morimoto K, Takano H, Segi-Nishida E and Sugimoto Y (2011) Prostaglandin E2-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res 52, 1500–1508, 10.1194/jlr.M013615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Michaud A, Lacroix-Pépin N, Pelletier M, Daris M, Biertho L, Fortier MA et al. (2014) Expression of genes related to prostaglandin synthesis or signaling in human subcutaneous and omental adipose tissue: depot differences and modulation by adipogenesis. Mediators Inflamm. 2014, 451620, 10.1155/2014/451620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Tang EHC, Cai Y, Wong CK, Rocha VZ, Sukhova GK, Shimizu K et al. (2015) Activation of prostaglandin E2-EP4 signaling reduces chemokine production in adipose tissue. J. Lipid Res 56, 358–368, 10.1194/jlr.M054817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Vianello E, Dozio E, Bandera F, Froldi M, Micaglio E, Lamont J et al. (2020) Correlative Study on Impaired Prostaglandin E2 Regulation in Epicardial Adipose Tissue and its Role in Maladaptive Cardiac Remodeling via EPAC2 and ST2 Signaling in Overweight Cardiovascular Disease Subjects. Int. J. Mol. Sci 21, 10.3390/ijms21020520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kather H and Simon B (1980) Inhibition of the stimulatory effect of adrenaline and prostaglandin E1 on the human fat cell adenylate cyclase by adenosine. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 12, 169–172, 10.1055/s-2007-996232 [DOI] [PubMed] [Google Scholar]
- 540.Zhang X, Luo Y, Wang C, Ding X, Yang X, Wu D et al. (2018) Adipose mTORC1 Suppresses Prostaglandin Signaling and Beige Adipogenesis via the CRTC2-COX-2 Pathway. Cell Rep. 24, 3180–3193, 10.1016/j.celrep.2018.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK et al. (2009) AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med 15, 159–168, 10.1038/nm.1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Stock K and Prilop M (1974) Dissociation of catecholamine-induced formation of adenosine 3’5’-monophosphate and release of glycerol in fat cells by prostaglandin E1, E and N6 2 -phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 282, 15–31, 10.1007/BF00647400 [DOI] [PubMed] [Google Scholar]
- 543.García-Sáinz JA (1981) Decreased sensitivity to α adrenergic amines, adenosine and prostaglandins in white fat cells from hamsters treated with pertussis vaccine. FEBS Lett. 126, 306–308, 10.1016/0014-5793(81)80267-0 [DOI] [PubMed] [Google Scholar]
- 544.Kather H (1982) Inhibition of hormone-stimulated human fat cell-lipolysis by prostaglandin E2 and its synthetic analogue sulprostrone. Prostaglandins Leukot. Med 8, 525–529 [PubMed] [Google Scholar]
- 545.Butcher RW and Carlson LA (1970) Effects of secretin on fat mobilizing lipolysis and cyclic AMP levels in rat adipose tissue. Acta Physiol. Scand 79, 559–563, 10.1111/j.1748-1716.1970.tb04758.x [DOI] [PubMed] [Google Scholar]
- 546.Hoffman BB, Prokocimer P, Thomas JM, Vagelos R, Chang H and Reaven GM (1989) Cellular tolerance to adenosine receptor-mediated inhibition of lipolysis: altered adenosine 3’,5’-monophosphate metabolism and protein kinase activation. Endocrinology 124, 2434–2442, 10.1210/endo-124-5-2434 [DOI] [PubMed] [Google Scholar]
- 547.Lambert B and Jacquemin C (1982) Inhibition by cholera toxin of the antilipolytic action of prostanoids, N6-(phenylisopropyl) adenosine and insulin. FEBS Lett. 143, 188–192, 10.1016/0014-5793(82)80096-3 [DOI] [PubMed] [Google Scholar]
- 548.Richelsen B and Beck-Nielsen H (1985) Decrease of prostaglandin E2 receptor binding is accompanied by reduced antilipolytic effects of prostaglandin E2 in isolated rat adipocytes. J. Lipid Res 26, 127–134 [PubMed] [Google Scholar]
- 549.Richelsen B and Pedersen SB (1987) Antilipolytic effect of prostaglandin E2 in perifused rat adipocytes. Endocrinology 121, 1221–1226, 10.1210/endo-121-4-1221 [DOI] [PubMed] [Google Scholar]
- 550.Lönnqvist F, Wennlund A and Arner P (1989) Antilipolytic effects of insulin and adenylate cyclase inhibitors on isolated human fat cells. Int. J. Obes 13, 137–146 [PubMed] [Google Scholar]
- 551.Fain JN, Leffler CW, Bahouth SW, Rice AM and Rivkees SA (2000) Regulation of leptin release and lipolysis by PGE2 in rat adipose tissue. Prostaglandins Other Lipid Mediat. 62, 343–350, 10.1016/S0090-6980(00)00088-5 [DOI] [PubMed] [Google Scholar]
- 552.Wang H and Edens NK (2007) mRNA expression and antilipolytic role of phosphodiesterase 4 in rat adipocytes in vitro. J. Lipid Res 48, 1099–1107, 10.1194/jlr.M600519-JLR200 [DOI] [PubMed] [Google Scholar]
- 553.Bergström S, Carlson LA and Orö L (1964) Effect of Prostaglandins on Catecholamine Induced Changes in the Free Fatty Acids of Plasma and in Blood Pressure in the Dog. Prostaglandin and Related Factors 22. Acta Physiol. Scand 60, 170–180, 10.1111/j.1748-1716.1964.tb02880.x [DOI] [PubMed] [Google Scholar]
- 554.Fredholm BB and Hedqvist P (1973) Role of pre- and postjunctional inhibition by prostaglandin E2 of lipolysis induced by sympathetic nerve stimulation in dog subcutaneous adipose tissue in situ. Br. J. Pharmacol 47, 711–718, 10.1111/j.1476-5381.1973.tb08198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Bergström S and Carlson LA (1965) Influence of the nutritional state on the inhibition of lipolysis in adipose tissue by Prostaglandin E1 and nicotinic acid. Prostaglandin and related factors 46. Acta Physiol. Scand 65, 383–384, 10.1111/j.1748-1716.1965.tb04289.x [DOI] [PubMed] [Google Scholar]
- 556.Bergström S and Carlson LA (1965) Inhibitory Action of Prostaglandin E1 on the Mobilization of Free Fatty Acids and Glycerol from Human Adipose Tissue in Vitro Prostaglandin and related factors. Acta Physiol. Scand 63, 195–196, 10.1111/j.1748-1716.1965.tb04059.x [DOI] [PubMed] [Google Scholar]
- 557.Moskowitz J and Fain JN (1969) Hormonal regulation of lipolysis and phosphorylase activity in human fat cells. J. Clin. Invest 48, 1802–1808, 10.1172/JCI106146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Efendić S (1970) Influence of prostaglandin E1 on lipolysis induced by noradrenaline, isopropylnoradrenaline, theophylline, and dibutyryl cAMP in human omental adipose tissue in vitro. Acta Med. Scand 187, 503–507, 10.1111/j.0954-6820.1970.tb02976.x [DOI] [PubMed] [Google Scholar]
- 559.Lambert B and Jacquemin C (1980) Synergic effect of insulin and prostaglandin E1 on stimulated lipolysis. Prostaglandins Med. 5, 375–382, 10.1016/0161-4630(80)90109-3 [DOI] [PubMed] [Google Scholar]
- 560.Schimmel RJ, Mcmahon KK and Serio R (1981) Interactions between Alpha-Adrenergic Agents, Prostaglandin-E1, Nicotinic-Acid, and Adenosine in Regulation of Lipolysis in Hamster Epididymal Adipocytes. Mol. Pharmacol 19, 248–255 [PubMed] [Google Scholar]
- 561.Fredholm BB and Rosell S (1970) Effects of prostaglandin E1 in canine subcutaneous adipose tissue in situ. Acta Physiol. Scand 80, 450–458, 10.1111/j.1748-1716.1970.tb04813.x [DOI] [PubMed] [Google Scholar]
- 562.Rahman MS, Khan F, Syeda PK, Nishimura K, Jisaka M, Nagaya T et al. (2014) Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology 66, 635–646, 10.1007/s10616-013-9616-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Hopkins NK and Gorman RR (1981) Regulation of 3T3-L1 fibroblast differentiation by prostacyclin (prostaglandin I2). Biochim. Biophys. Acta 663, 457–466, 10.1016/0005-2760(81)90174-0 [DOI] [PubMed] [Google Scholar]
- 564.Miegueu P, Cianflone K, Richard D and St-Pierre DH (2013) Effect of secretin on preadipocyte, differentiating and mature adipocyte functions. Int. J. Obes. (Lond.) 37, 366–374, 10.1038/ijo.2012.73 [DOI] [PubMed] [Google Scholar]
- 565.Sekar R and Chow BKC (2014) Lipolytic actions of secretin in mouse adipocytes. J. Lipid Res 55, 190–200, 10.1194/jlr.M038042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A et al. (2018) Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 175, 1561.e12–1574.e12, 10.1016/j.cell.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 567.Raptis S, Faulhaber JD and Schröder KE (1969) The effect of intestinal hormones upon lipolysis of isolated human fat cells. Hormone Metab. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 1, 249–250, 10.1055/s-0028-1096843 [DOI] [PubMed] [Google Scholar]
- 568.Bourdeaux AM, Giudicelli Y, Rebourcet MC, Nordmann J and Nordmann R (1980) Influence of some gastrointestinal hormones on adipose tissue lipoprotein lipase activity in vitro: evidence of a stimulatory effect of pancreozymin and gastrin. Biochem. Biophys. Res. Commun 95, 212–219, 10.1016/0006-291X(80)90726-3 [DOI] [PubMed] [Google Scholar]
- 569.Gill DL, Marshall NJ and Ekins RP (1978) Characterization of thyrotropin binding to specific receptors in human fat tissue. Mol. Cell. Endocrinol 12, 41–51, 10.1016/0303-7207(78)90100-4 [DOI] [PubMed] [Google Scholar]
- 570.Holmes SD, Gitlin J, Titus G and Field JB (1980) Effect of increased circulating thyroid-stimulating hormone on in vitro thyroid-stimulating hormone stimulation of thyroid and adipose tissues. Endocrinology 106, 1892–1899, 10.1210/endo-106-6-1892 [DOI] [PubMed] [Google Scholar]
- 571.Gennick SE, Thomas CG and Nayfeh SN (1986) Characterization of the guinea pig adipocyte thyrotropin receptor. Biochem. Biophys. Res. Commun 135, 208–214, 10.1016/0006-291X(86)90964-2 [DOI] [PubMed] [Google Scholar]
- 572.Roselli-Rehfuss L, Robbins LS and Cone RD (1992) Thyrotropin receptor messenger ribonucleic acid is expressed in most brown and white adipose tissues in the guinea pig. Endocrinology 130, 1857–1861 [DOI] [PubMed] [Google Scholar]
- 573.Endo T, Ohta K, Haraguchi K and Onaya T (1995) Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J. Biol. Chem 270, 10833–10837, 10.1074/jbc.270.18.10833 [DOI] [PubMed] [Google Scholar]
- 574.Haraguchi K, Shimura H, Lin L, Endo T and Onaya T (1996) Differentiation of rat preadipocytes is accompanied by expression of thyrotropin receptors. Endocrinology 137, 3200–3205, 10.1210/endo.137.8.8754740 [DOI] [PubMed] [Google Scholar]
- 575.Crisp MS, Lane C, Halliwell M, Wynford-Thomas D and Ludgate M (1997) Thyrotropin receptor transcripts in human adipose tissue. J. Clin. Endocrinol. Metab 82, 2003–2005, 10.1210/jcem.82.6.2003 [DOI] [PubMed] [Google Scholar]
- 576.Shimura H, Haraguchi K, Endo T and Onaya T (1997) Regulation of thyrotropin receptor gene expression in 3T3-L1 adipose cells is distinct from its regulation in FRTL-5 thyroid cells. Endocrinology 138, 1483–1490, 10.1210/endo.138.4.5048 [DOI] [PubMed] [Google Scholar]
- 577.Janson A, Rawet H, Perbeck L and Marcus C (1998) Presence of thyrotropin receptor in infant adipocytes. Pediatr. Res 43, 555–558, 10.1203/00006450-199804000-00020 [DOI] [PubMed] [Google Scholar]
- 578.Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ and Sorisky A (2000) Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am. J. Physiol. Cell Physiol 279, C335–C340, 10.1152/ajpcell.2000.279.2.C335 [DOI] [PubMed] [Google Scholar]
- 579.Murakami M, Kamiya Y, Morimura T, Araki O, Imamura M, Ogiwara T et al. (2001) Thyrotropin receptors in brown adipose tissue: thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinology 142, 1195–1201, 10.1210/endo.142.3.8012 [DOI] [PubMed] [Google Scholar]
- 580.Bell A, Gagnon A, Dods P, Papineau D, Tiberi M and Sorisky A (2002) TSH signaling and cell survival in 3T3-L1 preadipocytes. Am. J. Physiol. Cell Physiol 283, C1056–C1064, 10.1152/ajpcell.00058.2002 [DOI] [PubMed] [Google Scholar]
- 581.Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X et al. (2012) Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis 11, 17, 10.1186/1476-511X-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Martinez-deMena R, Anedda A, Cadenas S and Obregon MJ (2015) TSH effects on thermogenesis in rat brown adipocytes. Mol. Cell. Endocrinol 404, 151–158, 10.1016/j.mce.2015.01.028 [DOI] [PubMed] [Google Scholar]
- 583.Swislocki NI (1970) Dissociation of lipolysis and protein anabolism by ACTH, bovine growth hormone, and thyroid stimulating hormone in adipose tissue with dibutyryl cyclic AMP and theophylline. Biochim. Biophys. Acta 201, 242–249, 10.1016/0304-4165(70)90298-9 [DOI] [PubMed] [Google Scholar]
- 584.Farmer SW, Ramachandran J and Papkoff H (1972) Lipolytic activity of gonadotropins and their subunits. Endocrinology 91, 543–548, 10.1210/endo-91-2-543 [DOI] [PubMed] [Google Scholar]
- 585.Hartree AS, Thomas M, Furnival BE, Burns TW and Langley P (1972) Thyroid-stimulating and lipolytic activities of purified preparations of human thyroid-stimulating hormone. J. Endocrinol 53, 95–100, 10.1677/joe.0.0530095 [DOI] [PubMed] [Google Scholar]
- 586.Gospodarowicz D (1973) A comparative study of the lipolytic activity of thyroid-stimulating hormone and luteinizing hormone. J. Biol. Chem 248, 1314–1317 [PubMed] [Google Scholar]
- 587.Elgadi A, Zemack H, Marcus C and Norgren S (2010) Tissue-specific knockout of TSHr in white adipose tissue increases adipocyte size and decreases TSH-induced lipolysis. Biochem. Biophys. Res. Commun 393, 526–530, 10.1016/j.bbrc.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 588.Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA et al. (2010) Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism 59, 547–553, 10.1016/j.metabol.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 589.Endo T and Kobayashi T (2012) Expression of functional TSH receptor in white adipose tissues of hyt/hyt mice induces lipolysis in vivo. Am. J. Physiol. Endocrinol. Metab 302, E1569–E1575, 10.1152/ajpendo.00572.2011 [DOI] [PubMed] [Google Scholar]
- 590.Shibasaki I, Nishikimi T, Mochizuki Y, Yamada Y, Yoshitatsu M, Inoue Y et al. (2010) Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul. Pept 165, 210–217, 10.1016/j.regpep.2010.07.169 [DOI] [PubMed] [Google Scholar]
- 591.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Tank J et al. (2006) β-adrenergic and atrial natriuretic peptide interactions on human cardiovascular and metabolic regulation. J. Clin. Endocrinol. Metab 91, 5069–5075, 10.1210/jc.2006-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]


