Abstract
Intracerebral hemorrhage (ICH) is the deadliest form of stroke for which there is no effective treatment, despite an endless number of pre-clinical studies and clinical trials. The obvious therapeutic target is the neutralization of toxic products of red blood cell (RBC) lysis that lead to cytotoxicity, inflammation, and oxidative damage. We used rigorous approaches and translationally relevant experimental ICH models to show that lactoferrin-(LTF)-based monotherapy is uniquely robust in reducing brain damage after ICH. Specifically, we designed, produced, and pharmacokinetically/toxicologically characterized an optimized LTF, a fusion of human LTF and the Fc domain of human IgG (FcLTF) that has a 5.8-fold longer half-life in the circulation than native LTF. Following dose-optimization studies, we showed that FcLTF reduces neurological injury caused by ICH in aged male/female mice, and in young male Sprague Dawley (SD) and spontaneously hypertensive rats (SHR). FcLTF showed a remarkably long 24-h therapeutic window. In tissue culture systems, FcLTF protected neurons from the toxic effects of RBCs and promoted microglia toward phagocytosis of RBCs and dead neurons, documenting its pleotropic effect. Our findings indicate that FcLTF is safe and effective in reducing ICH-induced damage in animal models used in this study.
Keywords: Intracerebral hemorrhage, lactoferrin, neuroprotection, phagocytosis, cytoprotection
Introduction
Intracerebral hemorrhage (ICH) is the most devastating form of stroke for which there is no effective treatment. The mortality and morbidity of ICH are closely associated with the mass of extravasated blood that inflicts mechanical injury through brain tissue displacement caused by blood accumulation and then causes cytotoxic insults due to breakdown of the deposited blood.1–3 The biochemical pathway prominently involved in the cytotoxic process includes products of erythrolysis, such as hemoglobin, heme, and ultimately iron.2 These factors have unique abilities to promote damage through many mechanisms including oxidative stress,4 prolyl hydroxylase/hypoxia-inducible factor inhibition,5 and ferroptosis.6 In addition, to direct detrimental effects on surviving neurons, these factors induce pro-inflammatory responses that act as additional sources of secondary brain damage.7
A recently launched clinical trial investigated deferoxamine, an iron chelating agent that is approved to manage beta thalassemia, as an investigative treatment for ICH.8 The rationale for this trial was provided by extensive preclinical studies in animal ICH models, demonstrating beneficial effects of deferoxamine.9–11 While this trial showed potentially promising results,8 anticipated limitations included a narrow action mechanism focused on iron removal, and a risk of long-term treatment possible depleting the body’s iron. In exploring alternative therapeutic approaches to overcome these limitations, we searched for a pleiotropic and iron-sequestrating substance that would both bind iron with high affinity and able to relinquish the iron for recycling, to avoid global iron deficiency while supporting immune homeostasis.
One such substance is lactoferrin (LTF). This naturally occurring glycoprotein of the transferrin family is recognized for its antimicrobial effects and immunomodulatory functions, largely due to its ability to strongly (∼10−20 M Kd) bind and sequestrate free iron and prevent oxidative stress.12,13 LTF is an important first-line defense molecule involved in protection against microbial infections13 and subsequent development of systemic inflammatory response syndrome (SIRS) and sepsis.14,15 By modulating specific target cell responses, LTF bridges innate and adaptive immune functions.12 Importantly, LTF can access the brain by mechanisms involving transcytosis through brain endothelial cells at the capillary level.16,17
In our recent studies,18,19 we discovered that LTF can (1) reduce the toxicity of hemolytic products towards neurons in culture; (2) reduce lipid peroxidation in ICH-affected brain tissue; (3) improve iron removal from the ICH-affected brain; (4) reduce brain edema caused by ICH; and ultimately (5) reduce neurological deficits in randomized and blinded studies with clinically relevant models of ICH. Overall, these data suggest that LTF may have strong clinical relevance in ICH management. Since LTF is normally cleared rapidly from the circulation (half-life [t1/2] ∼15 min),20 a property that could limit clinical application, we designed and successfully generated a novel fusion protein based on human LTF and the Fc domain of immunoglobulin G (IgG), to increase the therapeutic window of such optimized LTF (FcLTF). The concept of using Fc domain of IgG to increase the serum t1/2 of specific peptides or proteins has been successfully tested in the development of many biotherapeutics.21–23 To date, more than 20 monoclonal antibody (mAb)/Fc products have received marketing authorization approval by the U.S. Food & Drug Administration (FDA).24
The goal of the present study was to develop and characterize a new human compatible LTF-based compound that could be delivered intravenously to achieve immediate activity in the central nervous system. The results provide a strong justification for use of FcLTF as an innovative monotherapy for ICH.
Materials and methods
Animals
All animal studies followed the guidelines outlined in Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Animal Welfare Committee of The University of Texas Health Science Center at Houston. All experiments used a randomization (coin toss; two coins were used if 4 animals were randomization) approach, and analyses were conducted by investigators blinded to treatment assignments (animals were coded for group allocation) following STAIR recommendations25 and ARRIVE guidelines.26 Animals were kept in a specific pathogen-free environment, fed a standard rodent diet, and housed in standard rodent cages on a 12-h inverted light–dark cycle. Behavioral analyses were carried out between 10:00 a.m. and 4:00 p.m. Young male Wistar rats were employed for pharmacokinetic evaluations (see below), and young male Sprague Dawley rats were used for toxicological testing. Different strains may have distinct metabolic profile, therefore having different rat strains for both toxicity and pharmacokinetic analysis may introduce limitations. However, these animal studies are only an approximation of the biological properties of FcLTF. Ultimately, these toxicological and pharmacokinetic parameters will have to be re-assessed in humans, pending FcLTF will be considered for clinical use. For ICH modeling, we used both rats and mice, as specified for each experiment below. Sprague Dawley rats were used as source of microglia and neurons culture.
ICH modeling
Mouse and rat ICH models were induced by intrastriatal infusion of autologous blood as described previously.27–29 Briefly, male Sprague Dawley (SD) rats (250–350 g) or male SHRs (300–350 g, 12–14 weeks old), or male 12 weeks old, and male or female 20–24-month-old C57BJ6 mice under chloral hydrate anesthesia (0.35 g/kg, i.p.) were immobilized onto a stereotaxic frame. A 1-mm-diameter burr hole was drilled in the skull, and a 26-gauge stainless steel cannula was inserted for blood infusion (collected from femoral artery, 15 µl/5 min for mice or 35 µl/5 min for rats) into the left corpus striatum (for rat, 0.5 mm anterior to bregma, 4.0 mm lateral to midline, and 5.6 mm deep to skull; for mouse, 0.0 mm anterior to bregma, 3.0 mm lateral to midline, and 3.5 mm deep to skull). Core body temperature was maintained at 37 ± 0.5°C during surgery and for 2 h afterward.
Neurological/functional deficits measurement
All behavioral assessments were performed in a quiet and low-lit room by an experimenter blinded to the treatment groups. Pretesting was performed to exclude animals with abnormal behaviors or deficits. Only animals with <20% foot-faults and animals that had no preference in forelimb placing were subjected to ICH. An individual test score and a combination test score from a battery of behavioral tests (foot-fault, forelimb placing, postural flexing, wire or cylinder) was used to calculate the NDS, as we reported earlier.19,28,30 The composite score (grand NDS) combined all the tests, which were equally weighted.
The tape remover test was performed as described by Schallert and Whishaw31 with minor modifications. Briefly, two pieces of 10 × 5-mm adhesive tape firmly attached to the animal’s front paws in the distal-radial region. The animal was then placed in a clean cage alone. We recorded the time that the animal made the first contact to the tape with its teeth, as well as the time required to remove the tape from its front paws. The tests were ended at 120 s, which was the maximum time. Each animal received three trials at indicated time points, and the best trial (shortest time to remove the tape) was recorded.
Blood pressure measurement
BP on SHR tails was performed with the noninvasive CODA® High Throughput System (Kent Scientific Corp, Torrington, CT).
Brain edema
Brain edema was measured using the wet-weight/dry-weight method.18,19 Briefly, the brains were removed without perfusion, and the ICH-affected hemispheres were dissected. A brain coronal section (4-mm-thick; 2-mm anterior and 2-mm posterior to the blood injection site) was excised. The tissue weight was determined before and after drying in a 95°C oven for 48 h. Brain edema is expressed as a percent of water content: (wet weight–dry weight)/wet weight × 100.
FcLTF t1/2
The principal objective for the pharmacokinetic study was to compare the t1/2 of FcLTF with rhLTF as a control. Both test articles were CHO-expressed products and administered as a single intravenous injection to 230–250 g Wistar rats (Harlan Lab, Indianapolis, IN). The test articles were formulated to account for differences in the molecular structures and weights of FcLTF and rhLTF; the concentration of FcLTF was normalized for LTF equivalency at 1 mg/ml. Rats were restrained in Broome-style restrainers for dosing, and an i.v. bolus (1 mg/kg) was delivered into the lateral tail vein of each rat. At the selected collection timepoints, blood (250 µl) was collected through a jugular vein catheter. The blood sample was placed in K2EDTA tube and inverted 10 times before being placed on ice, until centrifugation at 8000 rpm/min for 5 min at 4°C. Plasma samples were assayed for hLTF using an AssayPro human LTF ELISA Kit (catalog # EL2011-1) according to the manufacturer’s protocol. Rat LTF did not cross-react with the hLTF antibody.
Primary cortical neuron culture
Primary neuron cultures from embryonic day 18 (E-18) SD rats were prepared as we previously described.32 The cells were plated on poly-L-lysine-coated culture plates in Neurobasal medium with B27 and maintained in a 5% CO2 incubator for 12 days before modeling ICH.
ICH-like injury and neurotoxicity in vitro
Primary rat cortical neurons (12 days in culture) were subjected to lysed RBCs30 with 0–5 µg/ml FcLTF for 48 h. The degree of cell injury was assessed by lactate dehydrogenase (LDH) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays using the combination CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI). Briefly, to measure LDH in the culture medium, 50 µl of medium from cultured cells was collected and mixed with 50 µl of CytoTox 96 reagent in a 96-well, flat, clear-bottomed plate. The plate was incubated for 30 min at room temperature and then absorbance was recorded at 490 nm. The optical density (OD) of each sample was calculated by averaging the results from triplicate wells. For MTT measurement, the MTT assay reagent was added into each neuron culture well and incubated for 2 h in a CO2 incubator (5% CO2, 21% O2) at 37.0 ± 0.5°C. The cells were lysed in lysis buffer, and absorbance was recorded at 570 nm. The OD of each sample was calculated by averaging the results from triplicate wells.
Primary rat brain glial culture and microglia isolation
The cortices of one to two days-old postnatal SD rat pups were dissected and dissociated by triturating as we previously described.32 Briefly, the dissociated cells were suspended in Dulbecco’s minimum essential medium with 10% fetal bovine serum, plated in 75-cm2 tissue culture flasks, and maintained in a CO2 incubator (5% CO2) at 37.0 ± 0.5°C. Half of the culture medium was changed every three days. After 12–15 days in culture, the astrocytes formed a confluent layer and were ready for microglia isolation. The loosely adherent microglia were harvested by a shaking procedure (220 rpm for 15 min) and then placed in Petri dishes for 30 min. The unattached microglia were collected and replated onto poly-L-Lysine coated plates with or without German-glass inserts at a cell density of 1–4 × 105 cells/ml.
Phagocytosis assay for dead neurons
The phagocytosis assay was performed as we reported earlier,33 with minor modifications. The isolated cortical neuronal cells from E-18 prenatal SD rat embryos were seeded on 100-mm culture dishes and cultured in vitro for two days. Then, the neurons were subjected to γ-irradiation (32 Gy) to induce apoptosis and then incubated in a CO2 cell culture incubator for 48 h, followed by a short incubation with 1 µg/ml of propidium iodide (PI) for nuclear labeling to help visualize dead cells under the epifluorescence microscope, upon their engulfment by microglia. After washing in phosphate-buffered saline (PBS), the PI-labeled dead neurons were resuspended in Neurobasal medium at 1 − 5 × 108 cells/ml and used as an indicator/target of phagocytosis.
Two hours after exposing the microglia to the PI-labeled dead neurons, the cells were fixed in 2% paraformaldehyde for 2 min. After brief permeabilization with 0.1% Triton X-100, the cells were labeled with phalloidin-fluorescein isothiocyanate (Invitrogen, Carlsbad, CA) to define the shape of individual microglia, and Hoechst 33258 was used to label nuclei. The images from rat microglia were inspected under a Zeiss Axioskop 2 fluorescence microscope (Zeiss, Oberkochen, Germany), which was equipped with a CCD camera and operated by MetaMorph 7.4 software. Five images were collected under a 40× objective. The number of PI-labeled nuclei per microglial cell was determined manually on still images, counting 30 microglial cells per each experimental condition. The average number of phagocytized DNs within each microglial cell was calculated and used as the phagocytosis index.33
Microglia were incubated with 0–5 µg/ml FcLTF 30 min before adding the PI-labeled dead neurons. FcLTF was kept in the culture medium during the phagocytosis assay.
RBC phagocytosis assay
Assessment of RBC phagocytosis by rat microglia was performed as we previously reported,29 with minor modifications. Purified SD rat RBCs were suspended in PBS at 1 × 108 cells/ml and used as an indicator/target of phagocytosis. One hour after exposing rat microglia to purified RBCs at a ratio of 1:100, the cells were fixed in 2% formalin. The RBC and microglia were immunofluoresecently labeled with mouse anti-CD11b (MCA275; Serotec, Puchheim, Germany) and rabbit anti-rat RBC (20 R-RR012; Fitzgerald, Acton, MA). The RBC and microglia were visualized with goat anti-rabbit IgG-Alexa Fluor 546 and goat anti-mouse IgG-Alexa Fluor 488. The nuclei were counterstained with DAPI. Images were collected under a Zeiss Axioskop 2 fluorescence microscope, which was equipped with a CCD camera and operated by MetaMorph 7.4 software. Five images under a 40× objective were recorded, and the number of RBCs per microglial cell was determined manually on still images. We counted 50 microglial cells per each experimental condition. The average number of phagocytized RBC within each microglial cell was calculated as the phagocytosis index. Microglia were incubated with 0–5 g/ml FcLTF 30 min before adding the PI-labeled dead neurons. FcLTF were kept in the culture medium during the phagocytosis assay.
Statistical analyses
Data are expressed as mean ± SD. For in vitro experiments, we pooled samples from three culture wells and performed experiments in triplicate. We performed statistical analyses using the GraphPad and InStat programs (GraphPad Inc., San Diego, CA) and SAS 9.4 software (Cary, NC). One-way analysis of variance (ANOVA) followed by Newman–Keuls post-testing was used for multiple comparisons. Unpaired t-tests were used when two groups were compared. Two-way ANOVA followed by Bonferroni post-testing was performed to compare replicate means (e.g. NDS between two groups at different time points). Longitudinal data analysis was performed using a mixed-effects model.
Results
We reported recently that recombinant mouse19 or human LTF (rhLTF)18,19 can reduce neurological deficits caused by ICH in rodent models. While we discovered promising therapeutic effects of LTF in ICH,18,19 we also recognized that rhLTF has a considerably short plasma t1/2 that may limit the use of this substance as a therapeutic agent for ICH. Thus, we created a fusion of LTF with the Fc fragment of IgG, an approach that could extend the half-life and improve pharmacokinetic properties of the therapeutic proteins.21,23,34,35
The designed FcLTF fusion protein is based on the DNA sequence of human LTF (hLTF)36 and human IgG2-Fc (Fc) genes and was expressed under the CMV promoter in Chinese hamster ovary (CHO) cells (Figures S1 and S2). The generated fusion protein (FcLTF) has the mammalian glycosylation pattern,36 which is essential for optimal efficacy and biocompatibility. First, we tested its pharmacokinetic properties and compared it with the rhLTF. After injecting FcLTF and rhLTF to Wistar rats, we draw blood at different time points (1, 5, 10, 20, 40, 80, 160, and 320 min) from jugular vein and measured the blood LTF concentration using hLTF ELISA. The t1/2 and mean retention time for FcLTF were significantly (5.8 folds) increased when compared to hLTF. Detailed comparison of pharmacokinetic properties comparing hLTF and FcLTF is included in the Supplementary Material (Figure S3 and Table S1).
FcLTF is cytoprotective in an ICH-like injury in vitro model and it augments phagocytic properties of microglia
Since LTF has uniquely high binding affinity toward iron, we first tested if FcLTF could limit the toxicity of hemolyzed blood toward cortical neurons in culture. Using LDH and MTT assay, we found that FcLTF in a dose-dependent fashion indeed reduced the injury to neurons caused by lysed RBCs (Figure 1(a) and (b)).
Figure 1.
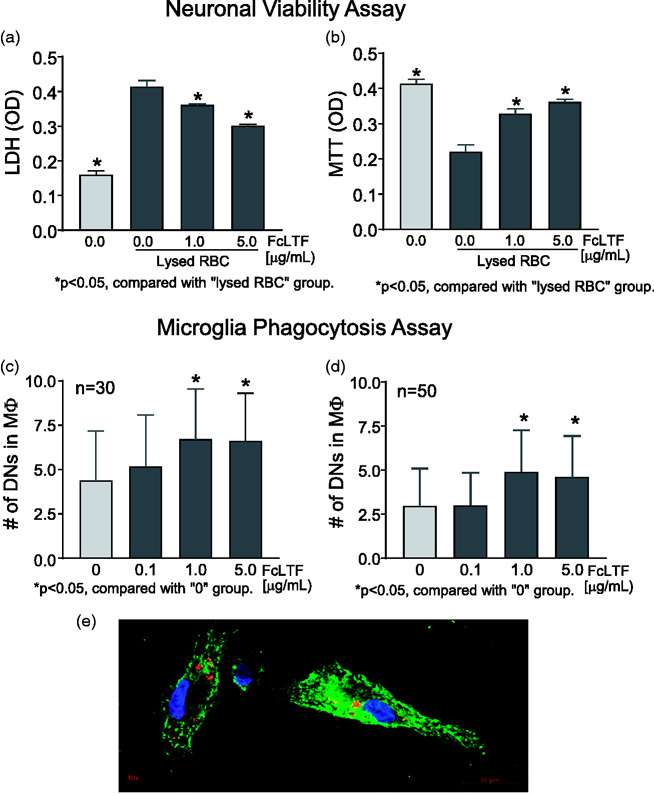
(a, b) FcLTF reduces the neurotoxicity of RBC lysates in vitro. Cultured SD rat cortical neurons at 12d of age were exposed to RBC lysates in the presence or absence of FcLTF, and viability was assessed using LDH release (a) and MTT assay (b). (c, d) FcLTF increased phagocytosis in vitro. Bar graphs quantifying the number of phagocytosed dead neurons (DNs) (c) or RBCs (d) per single rat microglial cell. FcLTF was given at 0–5 µg/ml. The n = 50 or 30 indicate the number of individual microglial cells that were inspected for internalized DN or RBC. (e) Representative confocal image of microglial cells (green) that engulfed DN (red). Nuclei were counterstained with DAPI (blue).
To further evaluate the mechanism of FcLTF, we used primary cultures to determine whether FcLTF could enhance the phagocytic functions of microglia. FcLTF effectively enhanced engulfment of both apoptotic neurons (Figure 1(c)) and RBCs (main component of hematoma) (Figure 1(d)), indicating that FcLTF could not only neutralize the toxicity of RBC lysis products, but also promote cleanup of RBCs and other dead brain cells (e.g., neurons).
FcLTF shows no-observed-adverse-effect-level (NOAEL) in rats
We evaluated FcLTF toxicology using male and female Sprague Dawley rats for either single-dose toxicity (5, 15, or 50 mg/kg) or 7-day repeated treatment (5, 15, or 50 mg/kg/day) effects on full standard panels for hematology, coagulation, serum chemistry, urinalysis, body weight, temperature, organ weight, necropsy, and histopathology (see Supplement for more details). There was no effect of a single treatment on any of the measured parameters. In repeated treatment, only the highest FcLTF dose (50 mg/kg/day) increased absolute monocyte counts in both sexes in parallel to increased spleen weight and cellularity of the red pulp of the spleen.
FcLTF is highly effective in reducing ICH-induced neurological deficits
We first compared the efficacy of rhLTF vs. FcLTF in their abilities to reduce neurological deficits in a mouse model of ICH. We employed a translationally relevant autologous blood injection model that we previously validated and characterized.1,27,29,37–40 To assure rigorous testing, all the treatments were delayed to 24 h after ICH onset. The dose for both compounds was 10 mg/kg and reflected an effective dose of rhLTF as established in our earlier studies.18,19 Using four distinct sensorimotor behavioral tests, we demonstrate that this delayed treatment with both rhLTF and Fc LTF significantly reduced neurological deficits. Interestingly, the therapeutic effect of FcLTF was more pronounced compared to rhLTF (Figure 2). These results indicate that FcLTF is at least as effective as rhLTF and warranted further studies on FcLTF as a potential new therapy for ICH.
Figure 2.
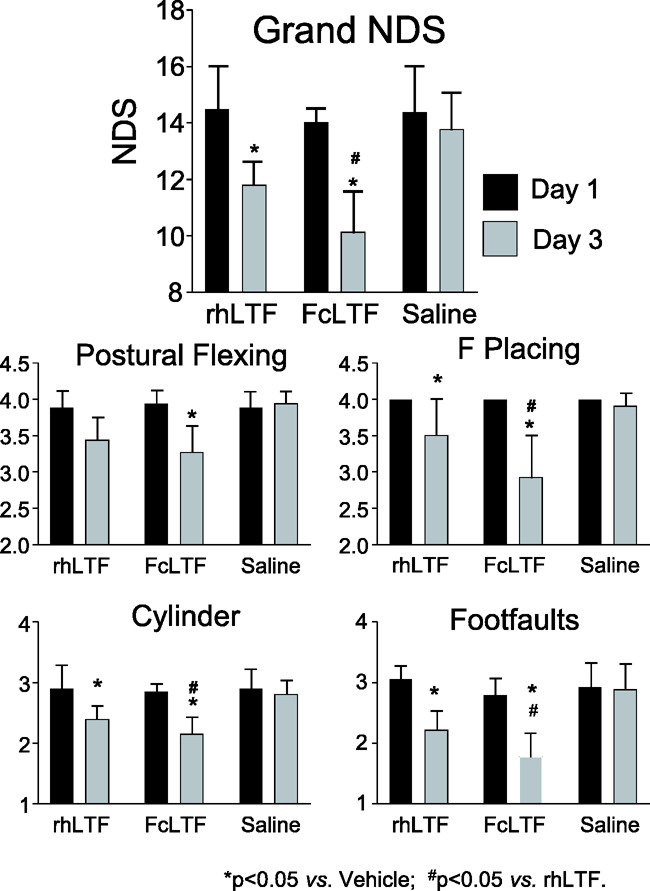
Comparison of efficacy of FcLTF vs. rhLTF in reducing neurological deficits (NDS) in three month male mice subjected to ICH. Treatment was initiated 24 h after ICH (10 mg/kg in 100 µL, i.v.) and then at 1 mg/kg in 100 µL by oral administration on d2 and d3. The NDS on d3 was measured after the last treatment. Bar graphs shows grand NDS (a combination score of NDS measured by using postural flexing, forward placing, foot faults and cylinder test) and performance of individual tests on d1 after ICH (immediately before treatment) and on d3, two days after treatment with saline, FcLTF, or rhLTF. *p ≤ 0.05, vs. saline group at the same time point (d3 after ICH). #p ≤ 0.05 between FcLTF and rhLTF groups. Data are presented as mean ± SD, n = 9 mice per group.
Determination of the optimal therapeutic dose of FcLTF
We again used the autologous blood injection model of ICH in three-month-old male mice. Since neurological outcome and brain edema are powerful clinically translational measures, we focused on assessment of these two injury indices in separate cohorts of mice. Neurological performance was gauged using four sensory-motor tests. A composite score (grand neurological deficit score [NDS], Figure 3(a)) and scores for individual tests (Figure 3(b)) were determined. All mice received three days of FcLTF treatment at 0–5 mg/kg/day. We found that the mice receiving 1 or 5 mg/kg FcLTF exhibited significant reductions in behavioral deficits as compared to saline-treated mice. Although there is no significant difference between 1 and 5 mg/kg groups, in two tests (forward placing and wire tests), 5 mg/kg appeared to be the most potent, especially when evaluating at 10 days after ICH (Figure 3(b)).
Figure 3.
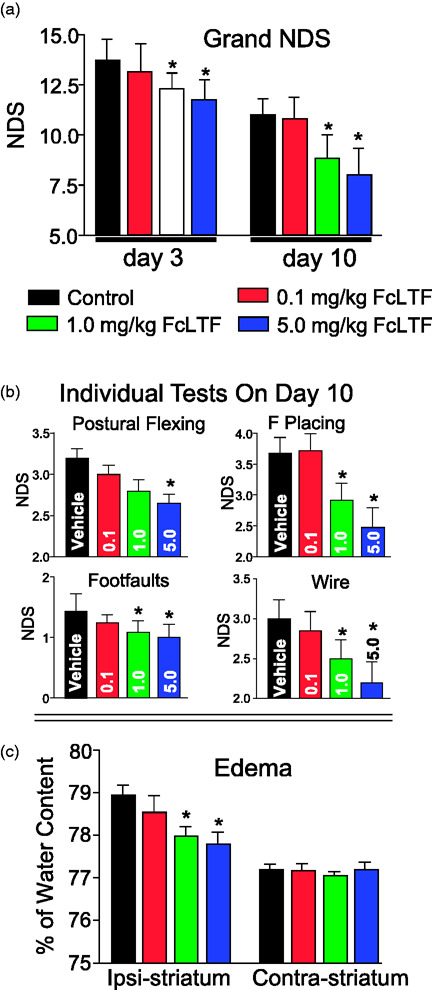
FcLTF dose response in NDS and brain edema in mice after ICH. (a) Grand NDS (A composite score of all 4 tests) on d3 and d10, and (b) individual test (postural flexing, forward placing, foot-faults, and wire test) scores on d10 after ICH. n = 10 animals per group. The FcLTF (0.1–5 mg/kg) was first administered 3 h after ICH i.v. and then i.p. on d1 and d2. *p ≤ 0.05, vs. control. (c) Brain edema = brain water content–([wet-dry]/wet weight) in the ICH-affected (Ipsi) and contralateral (Contra) striatum on day 3 after ICH. Data are presented as mean ± SD, n = 5/group. *p ≤ 0.05 vs. indicated group.
In agreement with the functional assessments (Figure 3(a) and (b)), we found that FcLTF at 1 and 5 mg/kg, but not at 0.1 mg/kg was effective in reducing brain edema after ICH (Figure 3(c)).
Therapeutic window for effective therapy with FcLTF
Based on the dose–response results described above, we further evaluated the FcLTF treatment regimen at a dose of 5 mg/kg.
While we know that FcLTF is effective in reducing neurological deficits when given 24 h after ICH (Figure 2), we did not know the optimal treatment timing or length that offer optimal effects. Thus, we measured the therapeutic efficacy of FcLTF using behavioral outcomes following treatment initiated between 3 and 48 h after ICH (Figure 4). Expecting that the FcLTF mechanism of action may involve immune cell re-programing and improved hematoma resolution (including iron sequestration and apoptotic cell cleanup), processes that normally continue over the course of five to seven days in our mouse model,18,19 in most of the experiments we continued FcLTF treatment for three to five days after ICH onset.
Figure 4.
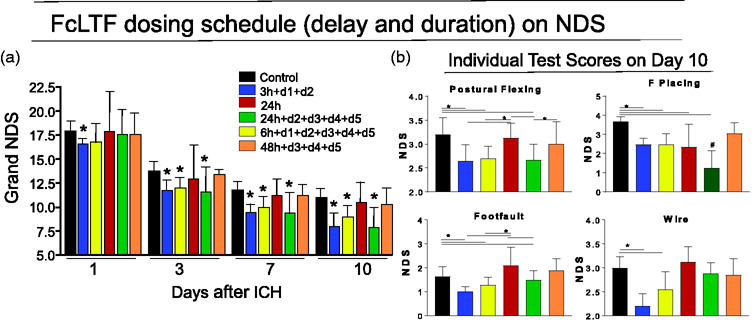
The effect of FcLTF treatment regimen on neurological deficit (NDS) in mice after ICH. (a) Mice received FcLTF (5 mg/kg/injection) starting at either 3, 6, 24, or 48 h after ICH onset. Some of the animals received FcLTF daily for either two or five days as indicated in the figure. The first treatment was given by i.v. infusion, and subsequent treatments were i.p. injection. Grand NDS (a composite score encompassing the Postural Flexing, Forward Placing, Footfaults and Wire tests) was determined on d1, d3, d7, and d10 after ICH; Values are mean ± SD, n = 10–12 mice per group. *p ≤ 0.05, vs. vehicle control. (b) Performance on each individual behavior test on d10 after ICH. *p ≤ 0.05, vs. indicated group; #p ≤ 0.05 vs. all remaining groups. A repeated two-way ANOVA was used to analyze the data.
As anticipated, both early initiated treatments (3-h delay blue bars and 6-h delay yellow bars) were highly effective in reducing NDS (Figure 4(a) and (b)) as compared to vehicle-treated mice (control). The most translationally exciting finding of this study was that the treatment delayed to 24 h was equally beneficial, assuming the follow-up treatment was continued for five additional days (Figure 4, green bars). A single FcLTF administration delayed to 24 h offered no therapeutic benefit (Figure 4, red bars). FcLTF treatment initiated 48 h after ICH that included the follow-up treatment (Figure 4, orange bars) was ineffective, as gauged based on control group performance.
FcLTF provides long-term benefits in aged female and male mice after ICH
Age and sex represent an important modifier for ICH outcome. In this experiment, we subjected 20–24-month-old male and female mice (51 males and 61 females) to ICH. FcLTF at 1 and 5 mg/kg was administered using the therapeutic window of 6 h. The first dose was delivered i.v., and the remaining treatments were delivered once a day from d1 to d7 as i.p. injections. Mortality, body weight, and neurological deficit (NDS, a combined score from 5 neurological tests) were measured over 21 days after ICH.
Despite the trend in reduced mortality in animals treated with FcLTF vs. control (11.1% vs. 17.5%), there was no overall difference in mortality among vehicle- and FcLTF-treated animals (p = 0.57). One unexpected finding was that independent of the FcLTF intervention, mice experienced much higher (p < 0.05) overall mortality in male (25.5%, 13 over 51) vs. females (4.9%, 3 over 61) as shown in Table S2 and Figure S4.
To better understand the nature of recovery in the aged animals, we measured behavioral performance over three weeks. We found that both male and females demonstrated a similar level of initial sensorimotor deficits and similar propensity to spontaneous recovery. Independent of sex and the dose, FcLTF did not influence animal performance at d3. Although we noted some trend toward better recovery at 1 mg/kg, only a dose of 5 mg/kg improved recovery with similar effects in both sexes (Figure 5). We established that besides when analyzing both sexes together, which showed significant improvement at 14 days after ICH, males and females analyzed separately only showed benefit 21 days after ICH. There was no significant difference regarding body weight among treatment groups or between males and females after ICH, as measured over three weeks (Figure 5).
Figure 5.
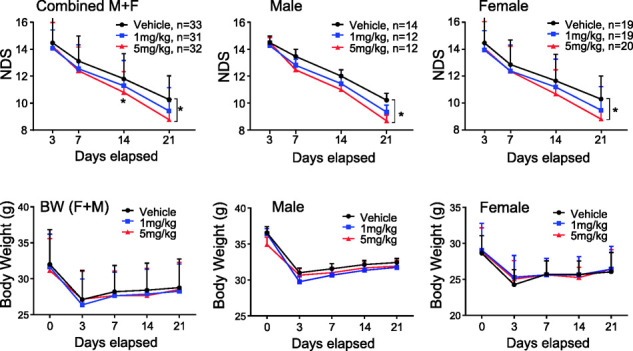
FcLTF reduces neurological deficit (NDS) in aged mice after ICH. Postural flexing, forward placing, corner, foot-faults, and wire tests were used to generate a composite grand neurological deficit (NDS) score for d3, d7, d14, and d21 after ICH. FcLTF at 1 or 5 mg/kg or saline (vehicle) was first given 6 h after ICH (i.v.) and then daily for an additional six days (i.p.). n indicates number of animals per group. *p ≤ 0.05, vs. vehicle control group at the same time point.
FcLTF improves functional outcomes after ICH in spontaneously hypertensive rats
Hypertension is a central risk factor for ICH, and chronic hypertension may contribute to a pro-inflammatory cerebrovascular phenotype that could affect the brain’s response to ICH and thus impact potential benefits of FcLTF. Thus, as a proof of concept, we evaluated the protective effect of FcLTF in ICH on animals with chronic hypertension. Twenty 12-week-old male SHRs were subjected to ICH. We measured blood pressure (BP) and NDS using a set of four neurobehavioral tests.
First, as measured between 30 min and seven days after ICH, SHR rats exhibited elevated blood pressure (170–190 mmHg) and found that FcLTF did not affect systolic or diastolic blood pressure in normotensive Sprague Dawley rats or SHRs (data not included).
To test the therapeutic effect of FcLTF, we subjected SHRs to ICH and treated them with 1 mg/kg FcLTF, first at 6 h, i.v. and then daily via i.p. injection for a total of six days. The NDS was measured with four tests on d3, d7, d14 and d21 after ICH. Despite using 1 mg/kg (a lower dose effective in mice; Figure 3), we observed a significant improvement in SHR performance on all tests (Figure 6). While we did not detect any improvement on d3 after ICH, we saw significant improvements on most of the tests on d7 and d21, indicating that FcLTF is important for the recovery process. In this experiment, four animals died after ICH: three from the vehicle group and one from the FcLTF-treated group.
Figure 6.
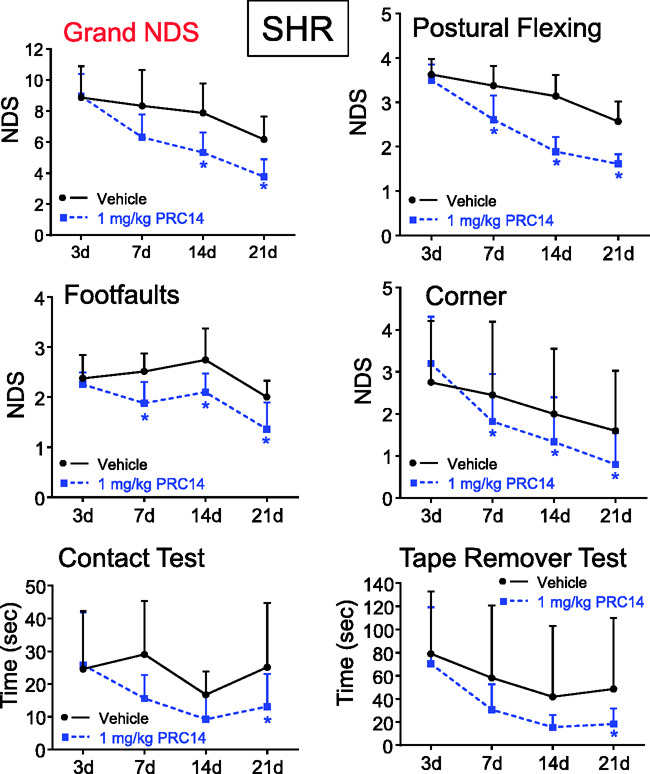
FcLTF reduces neurological deficits (NDS) in male SHR rats after ICH. Postural flexing, foot faults, corner, and tape removal test results were used to calculate a grand NDS on d3, d7, d14, and d21 after ICH. FcLTF (1 mg/kg in 100 µL, i.v.) or saline (vehicle) was given first at 6 h after ICH and then daily at 1 mg/kg i.p. for an additional six days. n = 10/group *p ≤ 0.05, vs. the vehicle control group at the same time point.
Discussion
This report identifies a novel potential treatment for ICH, which is essential since there is currently no effective treatment. ICH is a uniquely severe form of stroke with a 40% mortality rate that also leads to severe lifelong complications and disabilities in its survivors.41,42 One of the central component of ICH pathobiology is severely toxic blood products (especially RBC lysis products) that damage the ICH-affected brain parenchyma harboring the hematoma. The key molecules generated as a result of hemolysis are hemoglobin byproducts, heme, and iron. Hemoglobin, heme, and iron are cytotoxic virtually to all brain cells, resulting in pronounced brain damage accompanied by severe edema and long-lasting inflammation. Thus, neutralization of hemoglobin, heme, and/or iron represents an attractive target for reducing damage in response to ICH.
One recent search for the treatment for ICH identified LTF—a naturally occurring 80-kDa glycoprotein of the transferrin family—as one potential novel therapeutic agent.18,19 LTF has been known for decades as antimicrobial and immunomodulatory protein that is highly abundant in the colostrum, milk and other secreted fluids including saliva and tears and is involved in various homeostatic activities that could improve immune self-defense functions under various pathological conditions.12,13 One key property for LTF’s biological effect is its ability to sequestrate iron with extremely high affinity, effectively preventing free iron from engaging in free radical formation, and oxidative damage.12,13 By modulating specific target cell responses, LTF bridges innate and adaptive immune functions.12,19 Importantly, besides being able to enter the brain due to blood–brain barrier disruption after ICH, LTF can access the normal brain via transcytosis through brain endothelial cells at the capillary level.16,17,43 Because of these unique biological properties, we recently tested LTF as a potential treatment approach for ICH. We had demonstrated that human rLTF can (1) reduce the toxicity of hemolytic products toward neurons in culture, (2) reduce lipid peroxidation in the ICH-affected brain, (3) improve iron removal from the ICH-affected brain, (4) reduce brain edema caused by ICH, and (5) ameliorate neurological deficits in randomized and blinded studies with clinically relevant ICH models.18,19 Collectively, these results suggest that LTF may have strong clinical relevance in ICH management.
However, when considering the clinical utility of LTF for ICH, we recognized that due to its short t1/2 in circulation (∼15 min20), LTF may not be an optimal therapeutic candidate. Thus, we designed and successfully produced a fusion of the human Fc domain derived from IgG2 with human LTF. To date, more than 20 mAb/Fc products have received marketing authorization approval by the U.S. FDA.24 Most of these therapeutics have the Fc domain of human IgG1; however, the Fc of IgG2 offers potentially increased stability due to the higher number of disulfide bonds. Our fusion construct of the human Fc domain derived from IgG2 and human LTF has greater stability and a longer half-life, as well as fewer effector functions (antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity) compared to a fusion of the Fc domain derived from IgG1.21 Using this approach, we generated a product with improved pharmacokinetic properties (e.g. ∼4 h t1/2) while retaining potent cytoprotective properties within a dose range of 1–10 mg/kg with regard to neutralizing the hemolysates’ neurotoxicity and its robust therapeutic effects in rodent ICH models. Our toxicological study found FcLTF to be generally safe. However, we ought to point out that repeated doses of FcLTF at 50 mg/kg resulted in increased monocyte counts and spleen cellularity. These findings are in agreement with the well-established immunoregulatory effects of LTF.44 In fact, the induction of myelopoiesis, by LTF, resembles in some respect the phenomenon of sterile inflammation. While in our study this response was limited to a high 50 mg/kg dose in rats, one need to be cautious if similar dose–response exists in humans. The myelopoietic responses associated with the treatment need to be carefully recorded in the clinical settings, if this LTF-based therapy is to be tested in humans.
Pathogenic events triggered by ICH are sequential. Besides early physical damage that is caused by hematoma expansion and brain tissue compression during onset, ICH leads to delayed damage that is inflicted by the injurious components of blood plasma and RBC lysate-generated cytotoxins leading to brain tissue destruction, inflammation, and edema.1,2,4 RBCs lyse over time (one to five days from ICH onset4), releasing massive amounts of hemoglobin that are retained in the parenchymal space, generating hemoglobin, heme, and ultimately iron, all highly cytotoxic molecules virtually to all the cell types in the brain.2,4,45–47 Thus, our in vitro data showing LTF’s ability to sequester and neutralize toxic free iron to prevent cytotoxic effects of hemolysates in the hematoma provide a logical justification for FcLTF to be considered as a candidate molecule for ICH treatment. Also, since the onset of hemolysis is considerably late in ICH evolution,4 we predicted that the therapeutic window for interfering with hemolysate toxicity would be relatively wide. Indeed, the LTF-based therapeutic approach showed a long (at least 24 h) therapeutic window. Intriguingly, delayed treatment was only effective if FcLTF given at 24-h post injury was continued for additional five days after ICH; a single dose of FcLTF at 24 h after ICH was ineffective. This strongly suggests that the pathogenic processes targeted by FcLTF—likely including iron toxicity, inflammation, and phagocyte-assisted hematoma resolution—continue for several days after ICH. Accordingly, these pathogenic activities need to be mitigated during this time. This also raises an important caveat regarding translating FcLTF, or any other treatment targeting similar pathogenic processes, from rodents to human ICH: the need to consider differences in hematoma size (three orders of magnitude larger reservoir of toxic blood in humans; e.g. 0.05 ml vs. 50 ml). This also translates into months-long vs. days-long hematoma resolution duration,29 which may require adjustment of the treatment time necessary to achieve an equivalent therapeutic effect. This also unmasks the limitation of our study. We have not yet tried to use FcLTF for more than five days, which potentially could help to answer the question of how long after ICH treatment is needed. Furthermore, we have used FcLTF only as once a day approach. Assuming, considerably short FcLTF half-life, more frequent administration should be tested to better guide the translational relevance of this treatment.
While FcLTF-based therapy for ICH was beneficial in aged mice, its robustness was markedly reduced compared to younger mice. FcLTF at 1 mg/kg effectively reduced neurological deficits in young mice but was ineffective in aged mice; 5 mg/kg was necessary to offer significant benefit. In addition, the first signs of benefit with FcLTF were more delayed in aged mice and not significant until 14 days after ICH, as compared to younger mice with functional improvement just 3 days after ICH onset. The reasons behind these differences are unknown and could include variable pharmacokinetics and/or pharmacodynamics of FcLTF in aged vs. young mice. It is also likely that the severity of ICH-induced insult is greater in the aged brain and therefore less likely to benefit from any treatment as the aged system has less propensity for self-repair and recovery. In humans, advanced age represents one of the key unmodifiable risk factors for worse outcome after ICH, indicating that aging could contribute to more severe injury.48 In agreement with this notion, aged animals subjected to experimental ICH showed more severe inflammation and edema, greater neurological deficits, and more extensive white matter injury.49–51 Similarly to aged mice, when using SHR, we did not see a treatment effect or neurological improvement on day 3 after ICH. This could be due to a larger volume of blood deposited during the induction of ICH in a rat vs. mouse model (15 vs. 35 uL) and/or the fact that SHR’s brain is more penalized by the ICH-induced injury due to pre-existing comorbidities.
From the initial days of starting LTF testing, we struggled with the selecting i.v., i.p., or oral administration as the optimal approach for the treatment. While intravenous administration could be most practical, repetitive intravenous administration is highly stressful to animals and may represent a confounding factor regarding the outcome. Thus, we often combined the intravenous with intraperitoneal administration. Oral administration of LTF-based therapy is another part of the study that was not well defined, but could be highly practical. The oral route is especially relevant for continuation of the treatment after hospital discharge. Also, one potential limitation of our approach was that the sample size calculation for each experiment varied between the groups and it may not be optimal in each experiment. This could weaken interpretation of the effect size at the measured time-points and dosage groups.
We previously demonstrated that LTF after ICH improves neurological performance and accelerates iron removal in the ICH-affected hemisphere, suggesting that LTF-based therapy facilitates the removal of hematoma products. A recently completed multicenter, randomized, placebo-controlled, double-blind phase 2 clinical trial with the iron chelating agent deferoxamine mesylate (i-def) for ICH8 showed no sign of good outcome with deferoxamine at 90 days after ICH. Intriguingly, a similar analysis at 180 days after ICH onset showed non-futility of the treatment, suggesting benefit at this delayed time point. Here in our study, the beneficial effect of FcLTF in SHRs was noted as late as d7 and d21 after ICH onset, suggesting that FcLTF is also important for the recovery process. This could be in agreement with the i-def outcome, signifying that the post-ICH recovery process is uniquely long and could necessitate a long-term management. Besides affecting iron removal, our in vitro results suggest that FcLTF has significant effects on the phagocytic function of microglia. The post-ICH environment contains abundance of dislocated RBCs and dead cells compromised by the mechanical and biochemical insults associated with ICH. Effective removal of these components occurs through phagocytosis-mediated mechanisms and is essential for hematoma clearance. Removing the source of pro-inflammatory responses is critical for establishing new homeostatic neurovascular architecture and promoting recovery.29,39,52 We recently reported that microglia/macrophage-mediated phagocytosis is an important process for cleaning up dead and damaged brain tissue and is associated with improved post-stroke recovery, especially during subacute stage.29,32,39 Here we observed that FcLTF promoted engulfment of RBC and dead neurons by microglia/macrophages, which could be important mechanism underlying LTF in promoting hematoma resolution after ICH.19 While our tissue culture studies suggest that inhibition of iron-mediated toxicity and improved phagocyte-mediated cleanup process could explain the therapeutic function of FcLTF, these mechanisms of action and their relation to the outcome need to be confirmed in vivo (in animals after ICH). Based on the biological activity of LTF, it is likely that other mechanisms could also contribute to combating the pathology of ICH.
In conclusion, this study reports our novel, highly promising preclinical findings demonstrating a powerful clinical benefit of FcLTF in ICH. It was rigorously tested under translationally relevant settings including aging, hypertension, and sex, conditions that are of critical importance for ICH pathobiology. Remarkably, we showed that FcLTH has at least a 24-h therapeutic window that may predict a broad clinical utility of this approach. We are currently working on a large-scale manufacturing process to generate quantities of FcLTF needed to initiate human safety and feasibility for clinical studies.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20925667 for Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: Pre-clinical experience by Xiurong Zhao, Marian Kruzel, Shun-Ming Ting, Guanghua Sun, Sean I Savitz and Jaroslaw Aronowski in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Institute of Neurological Diseases and Stroke (NINDS), grants R41NS090650 and R01NS096308.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: JA, MK, and XZ designed and planned experiments. S-MT, GS, and XZ conducted experiments. JA, MK, SS, and XZ performed analyses and wrote the manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Keep RF, Hua Y, Xi G.Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 2012; 11: 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronowski J, Zhao X.Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011; 42: 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol 2015; 11: 111–122. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 2003; 23: 629–652. [DOI] [PubMed] [Google Scholar]
- 5.Karuppagounder SS, Ratan RR.Hypoxia-inducible factor prolyl hydroxylase inhibition: robust new target or another big bust for stroke therapeutics? J Cereb Blood Flow Metab 2012; 32: 1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zille M, Karuppagounder SS, Chen Y, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 2017; 48: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Gao C, Hua Y, et al. Role of iron in brain injury after intraventricular hemorrhage. Stroke 2011; 42: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selim M, Foster LD, Moy CS, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 2019; 18: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Hua Y, Keep RF, et al. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke 2009; 40: 2241–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okauchi M, Hua Y, Keep RF, et al. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke 2009; 40: 1858–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Q, Gu Y, Hua Y, et al. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke 2014; 45: 290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Actor JK, Hwang SA, Kruzel ML.Lactoferrin as a natural immune modulator. Curr Pharm Des 2009; 15: 1956–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez L, Calvo M, Brock JH.Biological role of lactoferrin. Arch Dis Childhood 1992; 67: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baynes RD, Bezwoda WR.Lactoferrin and the inflammatory response. Adv Exp Med Biol 1994; 357: 133–141. [DOI] [PubMed] [Google Scholar]
- 15.Kruzel ML, Harari Y, Mailman D, et al. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol 2002; 130: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RQ, Ke WL, Qu YH, et al. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J Biomed Sci 2007; 14: 121–128. [DOI] [PubMed] [Google Scholar]
- 17.Fillebeen C, Descamps L, Dehouck MP, et al. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem 1999; 274: 7011–7017. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Ting SM, Liu CH, et al. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat Commun 2017; 8: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Ting SM, Sun G, et al. Beneficial role of neutrophils through function of lactoferrin after intracerebral hemorrhage. Stroke 2018; 49: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiga Y, Oshima Y, Kojima Y, et al. Recombinant human lactoferrin-Fc fusion with an improved plasma half-life. Eur J Pharm Sci 2015; 67: 136–143. [DOI] [PubMed] [Google Scholar]
- 21.Czajkowsky DM, Hu J, Shao Z, et al. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 2012; 4: 1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck A, Reichert JM.Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. mAbs 2011; 3: 415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafari R, Zolbanin NM, Rafatpanah H, et al. Fc-fusion proteins in therapy: an updated view. Curr Med Chem 2017; 24: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 24.Liu XI, Dallmann A, Wang YM, et al. Monoclonal antibodies and fc-fusion proteins for pediatric use: dosing, immunogenicity, and modeling and simulation in data submitted to the US food and drug administration. J Clin Pharmacol 2019; 59: 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felberg RA, Grotta JC, Shirzadi AL, et al. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol 2002; 51: 517–524. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Sun G, Zhang J, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 2007; 38: 3280–3286. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007; 61: 352–362. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Song S, Sun G, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci 2009; 29: 15819–15827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schallert T, Whishaw IQ.Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci 1984; 98: 518–540. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Wang H, Sun G, et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci 2015; 35: 11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Zhang L, Ting SM, et al. Phagocytosis assay of microglia for dead neurons in primary rat brain cell cultures. Bio Protoc 2016; 6: e1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou QH, Boado RJ, Lu JZ, et al. Re-engineering erythropoietin as an IgG fusion protein that penetrates the blood-brain barrier in the mouse. Mol Pharm 2010; 7: 2148–2455. [DOI] [PubMed] [Google Scholar]
- 35.Bitonti AJ, Dumont JA, Low SC, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A 2004; 101: 9763–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi BK, Actor JK, Rios S, et al. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconjugate J 2008; 25: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belayev L, Saul I, Curbelo K, et al. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 2003; 34: 2221–2227. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Fields J, Dore S.The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res 2008; 1222: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Grotta J, Gonzales N, et al. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 2009; 40: S92–S94. [DOI] [PubMed] [Google Scholar]
- 40.Adeoye O, Clark JF, Khatri P, et al. Do current animal models of intracerebral hemorrhage mirror the human pathology? Transl Stroke Res 2011; 2: 17–25. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi AI, Mendelow AD, Hanley DF.Intracerebral haemorrhage. Lancet 2009; 373: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemorrhagic Stroke Academia Industry Roundtable P. Unmet needs and challenges in clinical research of intracerebral hemorrhage. Stroke 2018; 49: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji B, Maeda J, Higuchi M, et al. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci 2006; 78: 851–855. [DOI] [PubMed] [Google Scholar]
- 44.Zimecki M, Artym J, Kocieba M, et al. Homologous lactoferrin triggers mobilization of the myelocytic lineage of bone marrow in experimental mice. Stem Cells Develop 2013; 22: 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regan RF, Panter SS.Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma 1996; 13: 223–231. [DOI] [PubMed] [Google Scholar]
- 46.Imai T, Iwata S, Hirayama T, et al. Intracellular Fe(2+) accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci Rep 2019; 9: 6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan RF, Panter SS.Neurotoxicity of hemoglobin in cortical cell culture. Neurosci Lett 1993; 153: 219–222. [DOI] [PubMed] [Google Scholar]
- 48.Sacco S, Marini C, Toni D, et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009; 40: 394–399. [DOI] [PubMed] [Google Scholar]
- 49.Wasserman JK, Schlichter LC.White matter injury in young and aged rats after intracerebral hemorrhage. Exp Neurol 2008; 214: 266–275. [DOI] [PubMed] [Google Scholar]
- 50.Gong Y, Hua Y, Keep RF, et al. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke 2004; 35: 2571–2575. [DOI] [PubMed] [Google Scholar]
- 51.Gong Y, Xi GH, Keep RF, et al. Aging enhances intracerebral hemorrhage-induced brain injury in rats. Acta Neurochir Suppl 2005; 95: 425–427. [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson DA, Keep RF, Hua Y, et al. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: from macro to micro. J Cereb Blood Flow Metab 2018; 38: 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20925667 for Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: Pre-clinical experience by Xiurong Zhao, Marian Kruzel, Shun-Ming Ting, Guanghua Sun, Sean I Savitz and Jaroslaw Aronowski in Journal of Cerebral Blood Flow & Metabolism


