Abstract
The current prevalence of obesity has been linked to the consumption of highly palatable foods and may be mediated by a dysregulated or hyposensitive orosensory perception of dietary fat, thereby contributing to the susceptibility to develop obesity. The goal of the current study was to investigate the role of lingual taste input in obesity-prone (OP, Osborne-Mendel) and obesity-resistant (OR, S5B/Pl) rats on the consumption of a high-fat diet (HFD). Density of fungiform papillae was assessed as a marker of general orosensory input. To determine if orosensory afferent input mediates dietary fat intake, surgical transection of the chorda tympani and glossopharyngeal nerves (GLX/CTX) was performed in OP and OR rats and HFD caloric intake and body weight were measured. Fungiform papillae density was lower in OP rats, compared with OR rats. GLX/CTX decreased orosensory input in both OP and OR rats, as measured by an increase in the intake of a bitter, quinine solution. Consumption of low-fat diet was not altered by GLX/CTX in OP and OR rats; however, GLX/CTX decreased HFD intake in OR, without altering HFD intake in OP rats. Overall, these data suggest that inhibition of orosensory input in OP rats do not decrease fat intake, thereby supporting that idea that hyposensitive and/or dysregulated orosensory perception of highly palatable foods contribute to the susceptibility to develop obesity.
Keywords: chorda tympani, glossopharyngeal nerve, high-fat diet, Osborne-Mendel, S5B/Pl
Introduction
The prevalence of obesity has been linked to increased intake of highly palatable, high-fat foods (Cvijanovic et al. 2015). Recently, “fat” taste has been studied for its role in the susceptibility to develop obesity. Detection of fatty acids in foods is similar to detection of other tastants by receptors within tongue papillae and the processing of fatty acids by lingual taste receptors follows similar pathways to the central nervous system as other tastants (Khan et al. 2020). The relationship between taste and obesity is complex. The ability to detect a tastant is hypothesized to be weakened by obesity and obese individuals report taste hyposensitivity (Bartoshuk et al. 2006; Pepino et al. 2010; Sartor et al. 2011; Mameli et al. 2019). It is unclear whether this hyposensitivity is preexisting and increases the susceptibility to develop obesity or if the development of obesity contributes to taste hyposensitivity. Regardless, there is an association between the orosensory perception of fat and increased fat intake. People hypersensitive to the orosensory perception of fat consume less fat and hyposensitive people consume more fat-rich meals (Keast et al. 2014; Bolhuis et al. 2016).
One potential mechanism for decreased orosensory perception in obesity is the number and/or function of lingual taste receptor cells. The most studied papillae are the fungiform papillae, located on the anterior portion of the tongue and the circumvallate papillae, located on the posterior portion of the tongue. In humans, the number of fungiform papillae is associated with body mass index (BMI) such that people with obesity have fewer number of fungiform papillae (Mameli et al. 2019) and increasing adiposity is associated with a decrease in the number of fungiform papillae (Kaufman et al. 2020). Similarly, mice fed a high-fat diet (HFD) have fewer fungiform papillae and markers of taste bud proliferation compared with controls (Kaufman et al. 2020). The circumvallate papillae are similarly affected by a HFD and mice fed a HFD have fewer taste buds in the circumvallate papillae compared with mice fed a normal diet (Kaufman et al. 2018). Recent work from our laboratory suggests that serotonin signaling between Type I and Type II taste cells in the circumvallate papillae is altered with continuous access to HFD potentially interfering with the ability of taste receptor cells to inhibit overstimulation (Gaudet et al. 2019).
Another potential candidate for decreased orosensory fat perception is the innervation of lingual fat receptors. Two nerves carry information from papillae to the central nervous system for further processing—the chorda tympani (CT) and the glossopharyngeal (GL). The CT primarily innervates the fungiform papillae and the anterior foliate papillae (Cheal 1977; Kopka et al. 2000; Hummel et al. 2011). The GL innervates the posterior foliate and the circumvallate papillae (Dinger et al. 1985; King et al. 2000; Hummel et al. 2011). In the clinical population, damage to either the CT, from repeated episodes of otitis media, or GL damage from head and neck pathology, lead to decreased taste sensation and these individuals were more likely to have obesity (Bartoshuk et al. 2012). In rodent models, transection of both CT and GL leads to loss of preference for sweet taste and loss of aversion to bitter tastes such as quinine (St John and Spector 1998; Geran et al. 2004; Gaillard et al. 2008). Though few studies have investigated the effects of GL and CT transection on dietary fat intake, Pittman and colleagues reported that transection of CT impairs gustatory detection of long-chain fatty acids (Pittman et al. 2007). In a separate study, transection of the GL decreases preference for lipid-enriched solutions while transection of both the CT and the GL in mice led to abolishment of the preference for lipid-enriched solutions (Gaillard et al. 2008). These studies suggest that both the CT and GL are important for detection of fat.
Several lingual fatty acid receptors have been studied, including delayed rectifying K+ channels, GPR120 and CD36. Fatty acids inhibit delayed rectifying K+ channels in the fungiform papillae (Liu et al. 2005; Gilbertson and Khan 2014) and long-chain fatty acids are detected by CD36 and GP120 (Ozdener et al. 2014; Khan et al. 2020). CD36, which mediates the response to dietary fat, is located on the circumvallate papillae on the back of the tongue in rats and is also expressed in the fungiform papillae and foliate in humans (Laugerette et al. 2005; Liu et al. 2005; Sclafani et al. 2007; Gaillard et al. 2008; Simons et al. 2011; Degrace-Passilly and Besnard 2012; Chen et al. 2013; Douglas Braymer et al. 2017). Dysregulation of the CD36 receptor has been studied as a potential mechanism for susceptibility to develop obesity. In obese people, a common variant in the CD36 gene (SNP rs1761667-A allele) has been associated with hyposensitive orosensory fat perception and obesity (Pepino et al. 2012; Mrizak et al. 2015; Sayed et al. 2015). In animal models of obesity, continuous intake of HFD in rats alters CD36 mRNA expression (Zhang et al. 2011; Gaudet et al. 2019) and CD36 knockout mice demonstrate decreased fat preference and intake compared with wild-type mice (Sclafani et al. 2007). Alterations in taste perception may contribute to overconsumption of high-fat foods and studies have shown that differences in susceptibility to develop obesity after consuming a HFD exist (Cvijanovic et al. 2015; Chamoun et al. 2018). Therefore, animal models that differ in obesity susceptibility provide a valuable tool to study potential mediators of obesity and discover factors that contribute to obesity-resistance. The obesity-prone (OP) Osborne-Mendel and obesity-resistant (OR) S5B/PI rats differ in physiologic, behavioral, and neurochemical responses to HFD (Gilbertson et al. 1997, 2005; Schaffhauser et al. 2002; White et al. 2005; Primeaux et al. 2007, 2008, 2010, 2013; Petrescu et al. 2008; Pittman et al. 2008; Thanos et al. 2010; Chen et al. 2013; Poret et al. 2018; Gaudet et al. 2019). The consumption of HFD significantly increased lingual CD36 mRNA expression in the OP, but not the OR rats (Primeaux et al. 2013), which led to multiple studies investigating the role of lingual CD36 in OP and OR rats. The effects of lingual CD36 on taste preference thresholds, intake and preference for HFD in OP and OR rats were assessed by experimentally decreasing the expression using RNA silencing (Chen et al. 2013; Douglas Braymer et al. 2017). Experimentally decreasing lingual CD36 did not alter fat intake or preference in OP rats. However, lingual CD36 siRNA application in OP rats was able to decrease the preference for a single concentration of linoleic acid, suggesting that lingual CD36 is functional in this strain (Chen et al. 2013). The OR rats, which are resistant to developing obesity, are responsive to lingual application of CD36 siRNA, which produced a robust decrease in HFD intake and preference. These findings led us to hypothesize that there is a dysregulation in the orosensory perception of fat in OP rats, which leads to increased fat intake, fat preference, weight gain and contributes to the susceptibility to develop obesity.
The goal of the current study was to expand our previous findings with lingual CD36 and determine if lingual taste input is a mediator of HFD intake in OP rats. Density of fungiform papillae in experimentally naive OP and OR rats provided an indication of general orosensory input. Surgical transection of the GL and CT was used to inhibit afferent taste input from the tongue and taste receptor cells to the brain. Quinine is commonly used to assess bitter taste and is known to be aversive to rodents, therefore quinine intake was measured to verify the surgical transection of the GL (GLX) and CT (CTX). We hypothesized that GLX/CTX in OR rats would decrease the intake of HFD and decrease weight gain. In OP rats, if lingual taste input is dysregulated and does not mediate dietary fat intake, then GLX/CTX will not alter HFD intake or weight gain in the OP strain.
Materials and methods
Animals
The male OP Osborne-Mendel and OR S5B/Pl rats (8–9 weeks old) used in these studies were bred in the AAALAC approved Pennington Biomedical Research Center vivarium. All rats were individually housed on a 12 h/12 h light/dark cycle (lights on at 0700) with standard laboratory chow and water available ad libitum, unless otherwise described. All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Assessment of fungiform papillae
To assess number of fungiform papillae, tongues from experimentally naive OP (n = 6) and OR (n = 5) rats were extracted following sacrifice and stored in 10% formalin (Fisher Scientific) for 3 days. The tongues were sectioned anterior to the intermolar eminence with a surgical blade. Each tongue was stained with a 0.5% methylene blue solution (Sigma-Aldrich). Following staining, tongues were viewed under a dissecting microscope and images were recorded for each tongue. In order to count a consistent area between rats, ImageJ (NIH) was used to measure the length of the median sulcus. Using ImageJ, fungiform papillae were counted in the right and left anterior 2/3 regions near the median sulcus.
GL and CT nerve transection surgery
Rats were anesthetized with a ketamine cocktail (ketamine, 80 mg/mL; acepromazine, 1.6 mg/mL; xylazine, 5 mg/mL, i.p.) and the ventral neck region was shaved, cleaned, and injected with a local anesthetic (bupivicaine/lidocaine, 1 mg/kg, s.c.). For bilateral transection of the GL (GLX), a single midline incision was made in the neck and the sublingual and submaxillary glands, the sternohyoid, omohyoid, and digastric muscles were retracted to expose the GL. The left and right GL were visualized using an illuminated stereo microscope and a large section of the nerve (5–10 mm) was removed. The wound was closed with sterile wound clips. Following GLX, the CT was transected (CTX). For bilateral CTX, the auditory meatus was widened using forceps and the tympanic membrane and ossicles were removed and the CT was avulsed using microforceps. Sham-operated controls (SHAM) were treated like GLX/CTX, however the GL and CT were not transected. Carprofen (1 mg/kg, s.c.) was given for postoperative analgesia. Body weight and food intake of standard laboratory chow were measured daily and overall health was closely monitored. Subsequent assessments of quinine intake or HFD/low-fat diet (LFD) intake in separate groups of animals began 8–9 days following surgery and once rats had achieved at least 85% of their presurgery body weight (King et al. 1999; Geran et al. 2004). At the conclusion of the experiment, following sacrifice, tongues were extracted and stored in 10% formalin. The lingual epithelium was dissected from the tongue and stained with 0.5% methylene blue and rinsed with distilled water. The presence of taste pores was examined using a dissecting microscope (Geran et al. 2004; Pittman et al. 2007; Gaillard et al. 2008). These nerve transections effectively removed input from ~90% of the taste receptor cells, which degenerate after transection. Based on these results, 2 OP-GLX/CTX-LF, 1 OP-GLX-CTX-HF, 1 OR-GLX/CTX-LF, and 2 OR-GLX/CTX-HF were removed from analyses due to taste pores in the majority of papillae. It is important to note that regeneration of damaged nerves can occur when CT and GL nerves are damaged (State 1977). Furthermore, taste buds may begin to reappear around 28-day and by 70-day postsurgery, ~80% of taste buds have returned (Iwayama and Nada 1969; Kopka et al. 2000).
Effect of GLX/CTX on quinine intake
As verification of the GLX/CTX surgical technique, quinine intake was assessed in a separate group of GLX/CTX and SHAM OP and OR rats 8–9 days following surgery (OR-Sham [n = 5], OR-GLX/CTX [n = 5], OP-Sham [n = 4], and OP-GLX/CTX [n = 4]). Following an overnight (16 h) water restriction, rats were given a single bottle containing 1 mM quinine (Sigma-Aldrich) solution in water for 60 min. Total intake was measured (mL).
Effect of GLX/CTX on HFD and LFD intake
Eight-nine days following GLX/CTX, experimentally naive OP and OR rats were randomly assigned to receive either a HFD (56% kilocalories from fat, 24% kilocalories from protein, 20% kilocalories from carbohydrates, 4.79 kcal/g, Research Diets #D01080902) or a LFD (10% kilocalories from fat, 24% kilocalories from protein, 66% kilocalories from carbohydrates, 3.67 kcal/g, Research Diets #D01080901) (Primeaux et al. 2010; Gaudet et al. 2019) These diets did not contain sucrose (OR-SHAM-LFD [n = 10], OR-GLX/CTX-LFD [n = 8], OR-SHAM-HFD [n = 11], OR-GLX/CTX-HFD [n = 10], OP-SHAM-LFD [n = 11], OP-GLX/CTX-LFD [n = 8], OP-SHAM-HFD [n = 10], and OP-GLX/CTX-HFD [n = 9]). Food intake was measured daily (every 24 h) for 20 days and reported in kilocalories, due to differences in caloric density between the diets. Body weight was measured on alternating days and reported as weight change from prediet weight.
Statistical analyses
The number of fungiform papillae in naive OP and OR rats was assessed by a 2-tailed, independent t-test. Quinine intake was assessed by a 2-way ANOVA with surgery and strain as factors. Caloric intake and body weight change over 20 days were assessed using a mixed ANOVA for each strain, with diet and surgical condition as the between-subject factors and days as the repeated measure. Bonferroni post hoc analyses were used to assess the difference between surgical treatment for each diet. A significance level of P < 0.05 was used for all tests.
Results
Assessment of fungiform papillae
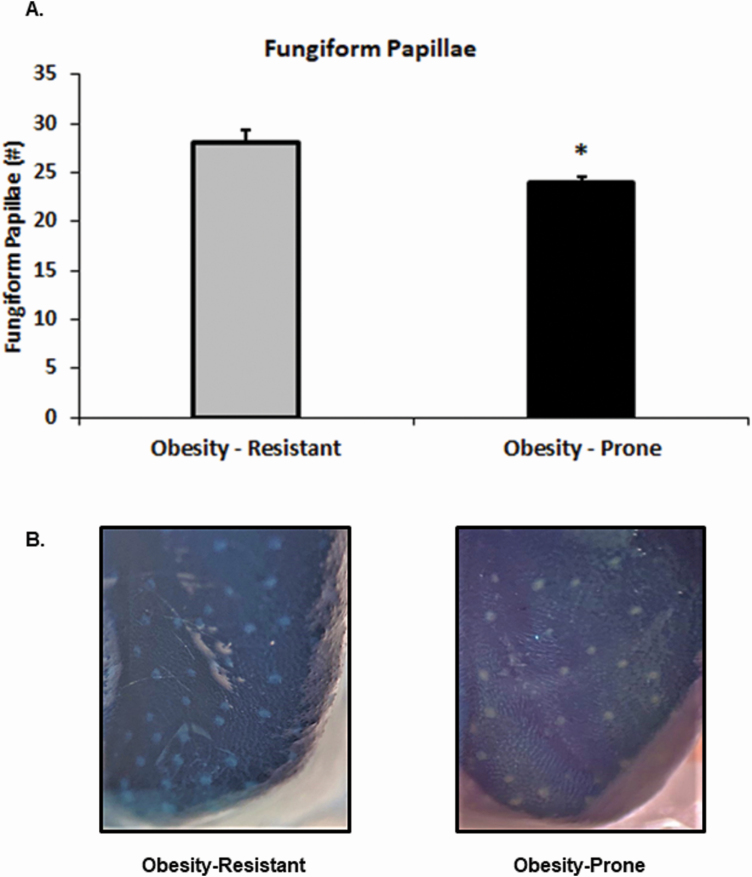
The density of fungiform papillae was assessed as a general marker of orosensory input in OP and OR rats. OP rats had significantly fewer fungiform papillae than OR rats (t(9) = 2.70, P < 0.05; Figure 1).
Figure 1.
Fungiform papillae in OP (n = 6) and OR (n = 5) rats were visualized and counted. (A) OP rats had significantly fewer fungiform papillae compared with OR rats. (B) Image of fungiform papillae following 0.5% methylene blue staining. Data shown as mean ± SEM; *P < 0.05.
Effect of GLX/CTX on quinine intake
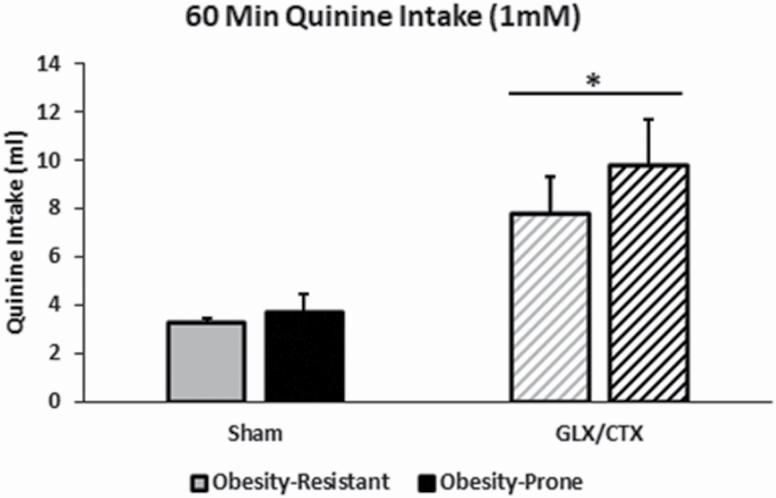
Quinine intake was used to verify the surgical transection of the GL and CT. As expected, there was a main effect of GLX/CTX to increase quinine intake (F(1,14) = 42.94, P < 0.01; Figure 2). There was no effect of strain on quinine intake (F(1,14) = 2.41, P > 0.05), nor was there an interaction between surgical condition and strain (F(1,14) = 0.91, P > 0.05).
Figure 2.
Quinine intake was measured in OP and OR rats with and without GLX/CTX. Following GLX/CTX, rats consumed more quinine solution than sham-operated rats. OR-SHAM (n = 5), OR-GLX/CTX (n = 5), OP-SHAM (n = 5), and OP-GLX/CTX (n = 4). Data shown as mean ± SEM; *P < 0.05.
Effect of GLX/CTX on HFD and LFD intake
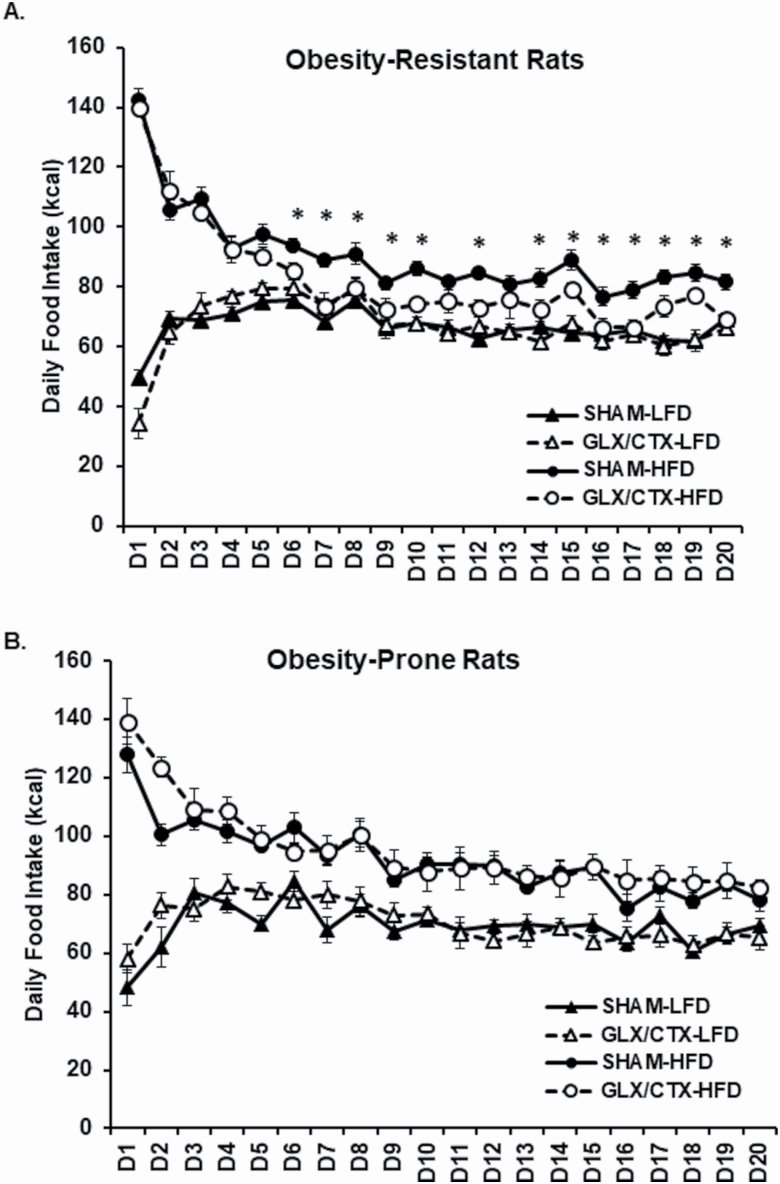
Following GLX/CTX, OR and OP rats were given continuous access to either a HFD or LFD for 20 days. Statistical analyses between surgical and diet conditions for each strain are reported in Table 1. A main effect of days and significant interactions between days and diet and days, diet, and surgical condition on caloric intake were detected in OR rats (Figure 3A). Additionally, there was a significant main effect of surgical condition and diet and a significant interaction between diet and surgical condition on caloric intake. In OR rats, post hoc analyses revealed that GLX/CTX decreased caloric intake of the HFD for 13 of 20 days, beginning on Day 6. A main effect of days and significant interactions between days and surgical condition and days and diet were detected in OP rats (Figure 3B). Post hoc analyses suggest that GLX/CTX did not alter caloric intake in OP rats (P > 0.05).
Table 1.
Statistical analyses between surgical and diet conditions in OR and OP rats fed HFD or LFD for 20 days
| OR rats | OP rats | |
|---|---|---|
| Caloric intake (kcal) | ||
| Repeated measures | ||
| Days | F(19,627) = 32.83*** | F(19,627) = 17.94*** |
| Days × surgery | F(19,627) = 1.28 | F(19,627) = 2.76** |
| Days × diet | F(19,627) = 60.53*** | F(19,627) = 18.55*** |
| Days × surgery × diet | F(19,627) = 2.31** | F(19,627) = 0.57 |
| Between-subjects | ||
| Surgery | F(1,33) = 5.78* | F(1,34) = 0.54 |
| Diet | F(1,33) = 160.47*** | F(1,34) = 81.51*** |
| Surgery × diet | F(1,33) = 6.07* | F(1,34) = 0.26 |
| Body weight change (g) | ||
| Repeated measures | ||
| Days | F(10,350) = 716.18*** | F(10,340) = 605.50*** |
| Days × surgery | F(10,350) = 0.35 | F(10,340) = 1.58 |
| Days × diet | F(10,350) = 8.51*** | F(10,340) = 33.76*** |
| Days × surgery × diet | F(10,350) = 2.25** | F(10,340) = 0.41 |
| Between-subjects | ||
| Surgery | F(1,35) = 0.004 | F(1,34) = 3.24 |
| Diet | F(1,35) = 26.89*** | F(1,34) = 95.39*** |
| Surgery × diet | F(1,35) = 0.79 | F(1,34) = 0.22 |
*P < 0.05.
**P < 0.01.
***P < 0.001.
Figure 3.
Caloric intake was measured daily for 20 days. (A) OR consumed more HFD than LFD. GLX/CTX significantly decreased caloric intake of HFD in OR rats in 13 out of 20 days beginning on Day 6. OR-SHAM-LFD (n = 10), OR-GLX/CTX-LFD (n = 8), OR-SHAM-HFD (n = 11), and OR-GLX/CTX-HFD (n = 10). (B) OP rats consumed more HFD than LFD. GLX/CTX did not affect HFD or LFD caloric intake in OP rats. OP-SHAM-LFD (n = 11), OP-GLX/CTX-LFD (n = 8), OP-SHAM-HFD (n = 10), and OP-GLX/CTX-HFD (n = 10). Data shown as mean ± SEM; *P < 0.05 between GLX/CTX and SHAM rats fed HFD.
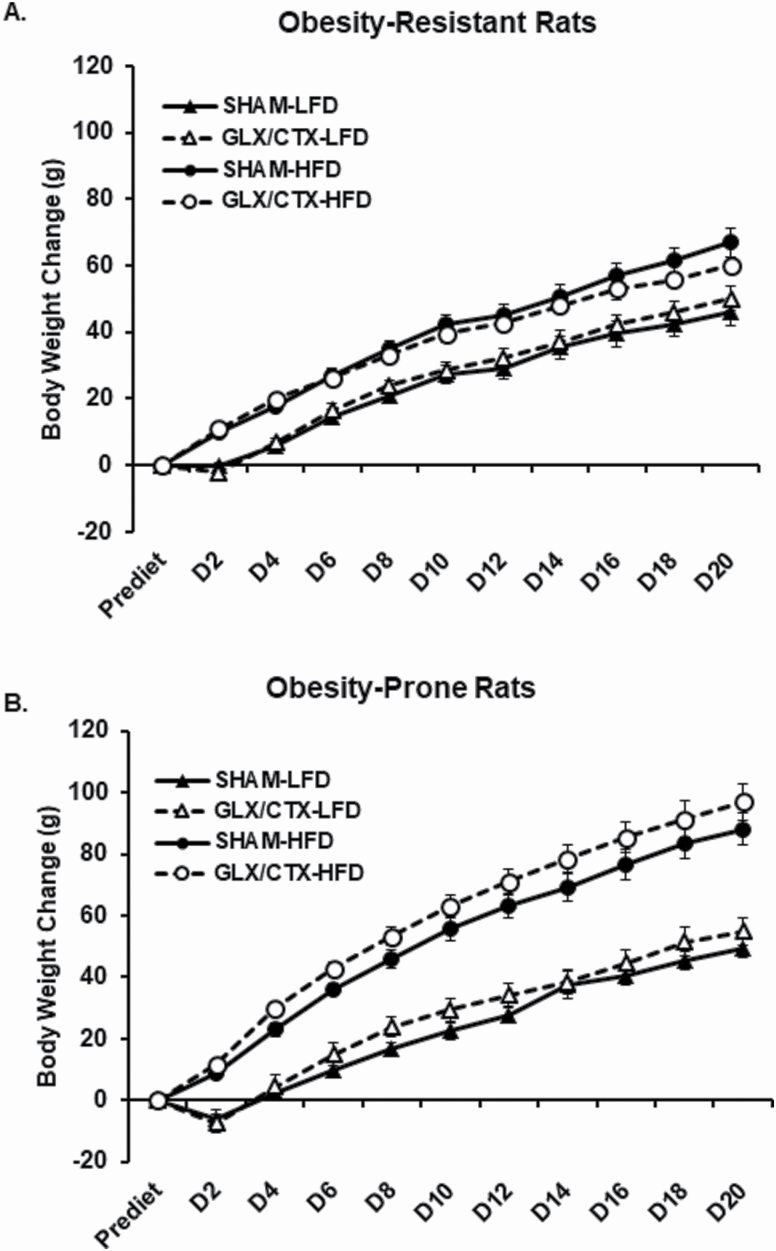
A main effect of days and a significant interaction between days and diet and days, surgical condition, and diet were detected on body weight change in OR rats (Figure 4A). In OP rats, a significant main effect of days, a main effect of diet and a significant days by diet interaction were detected (Figure 4B). Overall, body weight increased during the 20 days of HFD or LFD feeding in OR and OP rats. Rats consuming the HFD gained more weight than rats consuming the LFD diet. The significant interaction for weight gain between days, surgical condition, and diet that was detected in OR rats may be reflected by lower cumulative weight gain following GLX/CTX in rats consuming HFD.
Figure 4.
Change in body weight was assessed in OP and OR rats consuming HFD or LFD. (A) OR rats consuming HFD gained more weight than OR rats consuming LFD. OR-SHAM-LFD (n = 10), OR-GLX/CTX-LFD (n = 8), OR-SHAM-HFD (n = 11), and OR-GLX/CTX-HFD (n = 10). (B) OP rats consuming HFD gained more weight than OP rats consuming LFD. OP-SHAM-LFD (n = 11), OP-GLX/CTX-LFD (n = 8), OP-SHAM-HFD (n = 10), and OP-GLX/CTX-HFD (n = 10). Data shown as mean ± SEM.
Conclusion
The current prevalence of obesity in the United States has been linked to the intake of energy dense, highly palatable foods. These foods typically contain high levels of dietary fat and recent studies have shown that orosensory perception is an important factor in mediating fat intake (Abumrad 2005; Mattes 2005; Calder and Deckelbaum 2006; Chalé-Rush et al. 2007; Cartoni et al. 2010; Simons et al. 2011; Degrace-Passilly and Besnard 2012; Tucker and Mattes 2012; Chen et al. 2013; Primeaux et al. 2015; Besnard et al. 2016; Gaudet et al. 2019). Differences in orosensory fat perception and gustatory signaling may also influence the susceptibility to develop diet-induced obesity, with a decreased or dysregulated orosensory perception of fat mediating dietary fat intake (Gilbertson et al. 1998, 2005; Little and Feinle-Bisset 2010; Pepino et al. 2012; Chen et al. 2013; Primeaux et al. 2013; Pittman et al. 2015; Douglas Braymer et al. 2017). Previous research from our laboratory suggests that orosensory input differentially regulates fat intake and preference in OP, Osborne-Mendel and OR, S5B/Pl rats. OP rats, fed a standard chow diet, exhibited a higher fat preference threshold than OR rats when given increasing concentrations of linoleic acid (Chen et al. 2013). Furthermore, in OR rats, an overnight fast increases lingual CD36 expression and the preference threshold for fatty acids, while experimentally reducing lingual CD36 expression decreases the intake and preference for HFD (Douglas Braymer et al. 2017). In OP rats, changes in nutritional status (e.g., fasting or consuming HFD) do not alter the preference thresholds for fatty acids and experimentally reducing lingual CD36 does not decrease HFD intake or preference (Douglas Braymer et al. 2017). Gilbertson et al. (2005) examined the effects of fatty acid on delayed rectifying K+ in these animal models using patch clamp and reported a decrease in the responsiveness to linoleic acid in OP rats. These findings led us to hypothesize that a hyposensitive or dysregulated orosensory perception occurs in OP rats, which inhibits their ability to use information from taste receptor cells to mediate fat intake. Our previous studies focused on changes in lingual CD36 expression as a modulator of fat intake, while our current study investigated HFD caloric intake following a more comprehensive approach to limiting taste input from the tongue to the brain, the transection of the GL and CT.
Taste information is regulated by ascending projections from taste receptor cells on taste buds in the fungiform papillae, which are located on the anterior portion of the tongue, and in the circumvallate papillae, which are located on the posterior portion of the tongue, to homeostatic and hedonic regions of the brain (Rolls 2006; Kaye et al. 2009; Yarmolinsky et al. 2009; Gaudet et al. 2019). The density of fungiform papillae has been associated with altered taste sensitivity. Early studies suggested that supertasters, which had a heightened perception of bitter taste, expressed a greater density of fungiform papillae than nontasters, who had a reduced perception of bitter taste and much fewer fungiform papillae (Bartoshuk et al. 1994). More recent studies have linked fungiform papillae density to BMI, suggesting that individuals with obesity have fewer fungiform papillae (Mameli et al. 2019) and those with higher adiposity levels have fewer fungiform papillae (Kaufman et al. 2020). Additionally, mice consuming HFD also showed a reduction in fungiform papillae density. In the current study, we measured fungiform papillae density in experimentally naive OP and OR rats. OP rats had significantly fewer fungiform papillae than OR rats (Figure 1). These data support previous findings linking BMI and adiposity to lower fungiform papillae density and our previous findings that OP rats are hyposensitive to the “taste” of fat (Chen et al. 2013; Douglas Braymer et al. 2017).
Bilateral transection of the GL and CT has previously been shown to alter sweet taste perception by affecting sucrose responsiveness, bitter taste perception by decreasing quinine detection and fat taste perception by inhibiting the development of a conditioned taste aversion to linoleic acid, decreasing the preference for a lipid-enriched solution, decreasing the intake of corn oil, and altering the pattern of intake of oil–chow mix (Spector et al. 1996; St John and Spector 1996, 1998; King et al. 2000; Pittman et al. 2007; Gaillard et al. 2008; Dotson et al. 2012; Foo and Norgren 2014). In the current study, quinine solution consumption was assessed in a separate group of OP and OR rats to verify a successful bilateral GLX/CTX procedure. As expected, in both OP and OR rats, quinine intake was higher in rats that received GLX/CTX compared with sham-operated controls (Figure 2).
To our knowledge, the effects of bilateral transection of the GL and CT on HFD intake have not been measured. Previous studies have investigated the effects of CTX on a conditioned taste aversion to linoleic acid, GLX/CTX on preference for a lipid-enriched solution, GLX on corn oil intake and GL/CT/greater superficial petrosal nerve transections on oil–chow eating patterns (Pittman et al. 2007; Gaillard et al. 2008; Dotson et al. 2012; Foo and Norgren 2014). These studies reported reduced fat perception as measured by a suppression of a conditional aversion to linoleic acid, reduced short-term preference for linoleic acid, and reduced intake of corn oil. Interestingly, transection of GL/CT/greater superficial petrosal nerve significantly altered the feeding pattern of oil–chow mash and sweetened milk and importantly decreased body mass gain (Dotson et al. 2012). The goal of the current study was to expand our previous findings and determine if gustatory input, via GL and CT, is a mediator of HFD intake in OP rats. We hypothesized that GLX/CTX would decrease HFD intake in OR rats. Based on our previous findings, we predicted that if OP rats do not rely on lingual input to mediate dietary fat intake, then GLX/CTX would not alter HFD intake.
Following recovery from the GLX/CTX procedures, OP and OR rats were provided ad libitum access to either HFD or LFD for 20 days. The total duration of the current study was 28–29 days. This timeline was selected because previous reports suggested that taste buds will begin to reappear around 28-day and by 70-day postsurgery, ~80% of taste buds have returned (Iwayama and Nada 1969; Kopka et al. 2000). Initial caloric intake of HFD was high in both OP and OR rats, regardless of surgery, and after approximately 3 days, daily caloric intake levels stabilized. In OR rats, GLX/CTX significantly decreased HFD intake beginning on Day 6 (Figure 3A). This decrease in HFD intake continued for the duration of the study. Interestingly, GLX/CTX-induced decreases in HFD intake did not result in overt decreases in weight gain in OR rats (Figure 4A). However, a significant interaction was detected between surgery, diet, and experimental days, which may reflect a decrease in cumulative weight gain in the OR-GLX/CTX-HFD group beginning around Day 18. These results differ from Dotson et al. (2012), which reported significant and prolonged decreases in body weight following nerve transection. These data support our previous findings and suggest that orosensory fat perception mediates fat intake in OR rats and likely mediates their resistance to developing obesity. Unlike OR rats, bilateral transection of GL and CT did not affect caloric intake from the HFD in the OP rats (Figure 3B). Cumulative weight gain in OP rats was significantly higher in rats consuming the HFD but was not affected by GLX/CTX. These data support our previous findings indicating that a targeted approach to the disruption of lingual fat sensing, via CD36, did not affect HFD intake or preference in OP rats (Douglas Braymer et al. 2017).
The overall goal of the current study was to examine the disruption of afferent input from taste receptor cells on HFD intake in rats that differed in their susceptibility to develop obesity, the OP, Osborne-Mendel rat and the OR, S5B/Pl rat. Individuals that are susceptible to developing obesity may be particularly vulnerable to the effects of a dysregulated or hyposensitive orosensory response to dietary fat. This hyposensitivity or dysregulated orosensory perception may be an important mediator of obesity by leading to the overconsumption of highly palatable foods. Previous studies in these 2 strains of rats investigated specific regulators of lingual taste input (e.g., CD36, delayed rectifying K+ channels) on fatty acid preference thresholds, HFD intake and preference, and fatty acid-induced potassium current suppression via patch clamp (Gilbertson et al. 2005; Chen et al. 2013; Primeaux et al. 2013; Douglas Braymer et al. 2017). These studies indicate that though lingual expression of CD36 and delayed rectifying K+ channels is higher in OP rats, the responsiveness of OP rats to dietary fat is decreased. The current study applied a more comprehensive technique, transection of afferent nerves from the tongue, to elucidate the importance of orosensory input on the susceptibility to develop obesity. It should be noted that the current study did not investigate differences in taste signaling mechanisms between OR and OP rats and that though histology was performed to verify taste bud degeneration, previous studies have reported regeneration of taste buds at 28 days. Our findings support the hypothesis that dysregulated or hyposensitive orosensory perception mediates fat intake, however, more studies should be conducted to confirm the role of fat taste signaling in obese and/or OP individuals.
Acknowledgments
The authors would like to thank Christine Blackmon for her technical assistance with these studies.
Funding
This research was supported by LSU Health Sciences Center to S.D.P. This work used core facilities that are supported by Nutrition Obesity Research Center (NORC) Center grant #P30DK072476 and Centers of Biomedical Research Excellence (COBRE) Center grant #P30GM118430-01 from the National Institutes of Health.
Conflict of interest
The authors have no conflicts of interest to report.
References
- Abumrad NA. 2005. CD36 may determine our desire for dietary fats. J Clin Invest. 115(11):2965–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Catalanotto F, Hoffman H, Logan H, Snyder DJ. 2012. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol Behav. 107(4):516–526. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. 2006. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 361(1471):1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. 1994. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 56(6):1165–1171. [DOI] [PubMed] [Google Scholar]
- Besnard P, Passilly-Degrace P, Khan NA. 2016. Taste of fat: a sixth taste modality? Physiol Rev. 96(1):151–176. [DOI] [PubMed] [Google Scholar]
- Bolhuis DP, Costanzo A, Newman LP, Keast RS. 2016. Salt promotes passive overconsumption of dietary fat in humans. J Nutr. 146(4):838–845. [DOI] [PubMed] [Google Scholar]
- Calder PC, Deckelbaum RJ. 2006. CD36: taste the difference? Curr Opin Clin Nutr Metab Care. 9(2):77–78. [DOI] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 30(25):8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalé-Rush A, Burgess JR, Mattes RD. 2007. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 32(5):423–431. [DOI] [PubMed] [Google Scholar]
- Chamoun E, Mutch DM, Allen-Vercoe E, Buchholz AC, Duncan AM, Spriet LL, Haines J, Ma DWL; Guelph Family Health Study 2018. A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit Rev Food Sci Nutr. 58(2):194–207. [DOI] [PubMed] [Google Scholar]
- Cheal M. 1977. Taste responses of the chorda tympani nerve in the mouse. Physiol Behav. 19(1):175–177. [DOI] [PubMed] [Google Scholar]
- Chen CS, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP 3rd, Primeaux SD. 2013. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol. 305(11):R1346–R1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvijanovic N, Feinle-Bisset C, Young RL, Little TJ. 2015. Oral and intestinal sweet and fat tasting: impact of receptor polymorphisms and dietary modulation for metabolic disease. Nutr Rev. 73(5):318–334. [DOI] [PubMed] [Google Scholar]
- Degrace-Passilly P, Besnard P. 2012. CD36 and taste of fat. Curr Opin Clin Nutr Metab Care. 15(2):107–111. [DOI] [PubMed] [Google Scholar]
- Dinger B, Fidone SJ, Stensaas LJ. 1985. Regeneration of taste buds by nongustatory nerve fibers. Exp Neurol. 89(1):189–203. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Colbert CL, Garcea M, Smith JC, Spector AC. 2012. The consequences of gustatory deafferentation on body mass and feeding patterns in the rat. Am J Physiol Regul Integr Comp Physiol. 303(6):R611–R623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas Braymer H, Zachary H, Schreiber AL, Primeaux SD. 2017. Lingual CD36 and nutritional status differentially regulate fat preference in obesity-prone and obesity-resistant rats. Physiol Behav. 174:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo H, Norgren R. 2014. Concentration and state dependent reductions in corn oil intakes after glossopharyngeal nerve transections in rats. Physiol Behav. 128:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. 2008. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 22(5):1458–1468. [DOI] [PubMed] [Google Scholar]
- Gaudet DA, El-Desoky D, Poret JM, Braymer HD, Primeaux SD. 2019. Expression of neural markers of gustatory signaling are differentially altered by continuous and intermittent feeding patterns. Physiol Behav. 212:112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran LC, Garcea M, Spector AC. 2004. Nerve regeneration-induced recovery of quinine avoidance after complete gustatory deafferentation of the tongue. Am J Physiol Regul Integr Comp Physiol. 287(5):R1235–R1243. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. 1997. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 272(4 Pt 1):C1203–C1210. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Khan NA. 2014. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res. 53:82–92. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. 2005. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 86(5):681–690. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Liu L, York DA, Bray GA. 1998. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 855:165–168. [DOI] [PubMed] [Google Scholar]
- Hummel T, Landis BN, Hüttenbrink KB. 2011. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 10:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayama T, Nada O. 1969. Histochemical observation on phosphatase activities of degenerating and regenerating taste buds. Anat Rec. 163(1):31–38. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Choo E, Koh A, Dando R. 2018. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 16(3):e2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kim J, Noel C, Dando R. 2020. Taste loss with obesity in mice and men. Int J Obes (Lond). 44(3):739–743. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. 2009. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 10(8):573–584. [DOI] [PubMed] [Google Scholar]
- Keast RS, Azzopardi KM, Newman LP, Haryono RY. 2014. Impaired oral fatty acid chemoreception is associated with acute excess energy consumption. Appetite. 80:1–6. [DOI] [PubMed] [Google Scholar]
- Khan AS, Keast R, Khan NA. 2020. Preference for dietary fat: from detection to disease. Prog Lipid Res. 78:101032. [DOI] [PubMed] [Google Scholar]
- King CT, Garcea M, Spector AC. 2000. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci. 20(22):8426–8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Travers SP, Rowland NE, Garcea M, Spector AC. 1999. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 19(8):3107–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka SL, Geran LC, Spector AC. 2000. Functional status of the regenerated chorda tympani nerve as assessed in a salt taste discrimination task. Am J Physiol Regul Integr Comp Physiol. 278(3):R720–R731. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 115(11):3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. 2010. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: modification by diet and obesity. Front Neurosci. 4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hansen DR, Kim I, Gilbertson TA. 2005. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol. 289(4):C868–C880. [DOI] [PubMed] [Google Scholar]
- Mameli C, Cattaneo C, Panelli S, Comandatore F, Sangiorgio A, Bedogni G, Bandi C, Zuccotti G, Pagliarini E. 2019. Taste perception and oral microbiota are associated with obesity in children and adolescents. PLoS One. 14(9):e0221656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. 2005. Fat taste and lipid metabolism in humans. Physiol Behav. 86(5):691–697. [DOI] [PubMed] [Google Scholar]
- Mrizak I, Šerý O, Plesnik J, Arfa A, Fekih M, Bouslema A, Zaouali M, Tabka Z, Khan NA. 2015. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br J Nutr. 113(8):1330–1337. [DOI] [PubMed] [Google Scholar]
- Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. 2014. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 146(4):995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. 2010. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring). 18(5):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Love-Gregory L, Klein S, Abumrad NA. 2012. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 53(3):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. 2008. Differences in adipocyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes (Lond). 32(5):853–862. [DOI] [PubMed] [Google Scholar]
- Pittman D, Crawley ME, Corbin CH, Smith KR. 2007. Chorda tympani nerve transection impairs the gustatory detection of free fatty acids in male and female rats. Brain Res. 1151:74–83. [DOI] [PubMed] [Google Scholar]
- Pittman DW, Hansen DR, Gilbertson TA. 2015. High-fat diet alters the orosensory sensitivity to fatty acids in obesity-resistant but not obesity-prone rats. J Mol Genet Med. 9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman DW, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, Gilbertson TA. 2008. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Senses. 33(5):449–460. [DOI] [PubMed] [Google Scholar]
- Poret JM, Souza-Smith F, Marcell SJ, Gaudet DA, Tzeng TH, Braymer HD, Harrison-Bernard LM, Primeaux SD. 2018. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int J Obes (Lond). 42(3):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Barnes MJ, Bray GA. 2007. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone but not obesity-resistant rats. Behav Brain Res. 180(2):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Barnes MJ, Braymer HD, Bray GA. 2010. Sensitivity to the satiating effects of Exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes (Lond). 34(9):1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Blackmon C, Barnes MJ, Braymer HD, Bray GA. 2008. Central administration of the RFamide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides. 29(11):1994–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Braymer HD, Bray GA. 2013. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci. 58:369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Tzeng TH, Allerton TD, Chiang MC, Cosentino G, Dubin RL, Varughese A, Moore R, Geiselman PJ, Greenway FL, et al. 2015. Differences in short-term food preferences following vertical sleeve gastrectomy and Roux-en-Y gastric bypass surgery. Obes Res Clin Pract. 9(6):628–632. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2006. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 361(1471):1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. 2011. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite. 57(1):237–246. [DOI] [PubMed] [Google Scholar]
- Sayed A, Šerý O, Plesnik J, Daoudi H, Rouabah A, Rouabah L, Khan NA. 2015. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int J Obes (Lond). 39(6):920–924. [DOI] [PubMed] [Google Scholar]
- Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. 2002. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 10(11):1188–1196. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, Abumrad NA. 2007. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 293(5):R1823–R1832. [DOI] [PubMed] [Google Scholar]
- Simons PJ, Kummer JA, Luiken JJ, Boon L. 2011. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113(8):839–843. [DOI] [PubMed] [Google Scholar]
- Spector AC, Redman R, Garcea M. 1996. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci. 110(5):1096–1109. [PubMed] [Google Scholar]
- St John SJ, Spector AC. 1996. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus). Behav Neurosci. 110(6):1456–1468. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. 1998. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 18(11):4353–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State FA. 1977. Histological changes following unilateral re-innervation of the circumvallate papilla of rat. Acta Anat (Basel). 98(3):343–352. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, Bray GA, Wang GJ, Robinson JK, Volkow ND. 2010. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rat. Physiol Behav. 10:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RM, Mattes RD. 2012. Are free fatty acids effective taste stimuli in humans? J Food Sci. 77:S148–S150. [DOI] [PubMed] [Google Scholar]
- White CL, Braymer HD, York DA, Bray GA. 2005. Effect of a high or low ambient perinatal temperature on adult obesity in Osborne-Mendel and S5B/Pl rats. Am J Physiol Regul Integr Comp Physiol. 288(5):R1376–R1384. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. 2009. Common sense about taste: from mammals to insects. Cell. 139(2):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM. 2011. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 113(6):663–667. [DOI] [PubMed] [Google Scholar]