Abstract
Background
The antibacterial effects of psychotropics may be part of their pharmacological effects when treating depression. However, limited studies have focused on gut microbiota in relation to prescribed medication.
Method
We longitudinally investigated the relationship between patients’ prescribed medications and intestinal bacterial diversity in a naturalistic treatment course for patients with major depressive disorders and anxiety disorders. Patients were recruited and their stool was collected at 3 time points during their usual psychiatric treatments. Gut microbiota were analyzed using 16S rRNA gene sequencing. We examined the impact of psychotropics (i.e., antidepressants, anxiolytics, antipsychotics) on their gut microbial diversity and functions.
Results
We collected 246 stool samples from 40 patients. Despite no differences in microbial diversity between medication groups at the baseline, over the course of treatment, phylogenic diversity whole-tree diversity decreased in patients on antipsychotics compared with patients without (P = .027), and beta diversity followed this trend. Based on a fixed-effect model, antipsychotics predicted microbial diversity; the higher doses correlated with less diversity based on the Shannon index and phylogenic diversity whole tree (estimate = −0.00254, SE = 0.000595, P < .0001; estimate = −0.02644, SE = 0.00833, P = .002, respectively).
Conclusion
Antipsychotics may play a role in decreasing the alpha diversity of the gut microbiome among patients with depression and anxiety, and our results indicate a relationship with medication dosage. Future studies are warranted and should consider patients’ types and doses of antipsychotics in order to further elucidate the mechanisms of gut-brain interactions in psychiatric disorders.
Keywords: anxiety, depression, microbial diversity, microbiome, psychotropics
Significance Statement.
Previous studies have shown a relationship between psychotropic medication and gut microbiome in animal studies and in vitro experiments. However, little is known about the evidence in humans. We prospectively followed-up 40 patients with depression and/or anxiety disorders and collected 246 stool samples. We found that antipsychotics are likely to decrease microbial diversity. Moreover, there was a negative correlation between doses of antipsychotics and gut diversity when adjusted for BMI and depression and anxiety severity scores.
To the best of our knowledge, this is the first study to investigate whether various class of psychotropics affect the gut microbiome among patients with depression and anxiety longitudinally, suggesting medication may play an important role to influence microbial diversity.
Introduction
The number of microorganisms in the microbiome of the human gastrointestinal tract has been reported to be almost equal to the number of cells in the human body elsewhere (Sender et al., 2016). These microorganisms assist in balancing our homeostasis by protecting us from pathogenic microbes, producing essential vitamins, strengthening gut integrity, and shaping the intestinal epithelium (Natividad and Verdu, 2013). Factors such as drugs (Maier et al., 2018), food habits (Senghor et al., 2018), nationality (Kovatcheva-Datchary et al., 2015), and age (Odamaki et al., 2016) are known to influence the gut microbiota composition. Moreover, recent studies have shown relationships between the gut microbiome and the brain: the so-called “microbiome-gut-brain axis” (Vuong et al., 2017). The dysregulation of the gut microbiota, known as dysbiosis, can affect the body’s immune response by activating the immune system or mediators that are able to penetrate the blood-brain barrier or other chemical-related substances such as tryptophan, which can freely enter the brain (Maes et al., 2012). The relationship between psychiatric disorders and gut microbiota is being actively investigated, with altered microbial compositions reported in disorders such as depression, schizophrenia, bipolar disorder, autism spectrum disorder (ASD), and attention-deficit hyperactivity disorder (ADHD) (Evans et al., 2017; Kang et al., 2018; Yuan et al., 2018; Huang et al., 2019).
Regarding major depressive disorder (MDD), Sanada et al. (2020) meta-analyzed the microbial features in patients with MDD compared with nondepressive controls based on 10 observational studies. The abundances of Coprococcus, Faecalibacterium, Ruminococcus, Bifidobacterium, and Escherichia were decreased in patients with MDD compared with nondepressed controls, while Paraprevotella was increased in patients with MDD compared with controls. Regarding schizophrenia, Xu et al. (2019) reported that 19 gut microbiota taxonomies were associated, with dysbiosis positively correlated with the diversity of microbiota-associated epitopes and gut IgA levels. Shen et al. (2018) reported that the abundances of Succinivibrio, Megasphaera, Collinsella, Clostridium, Klebsiella, and Methanobrevibacter were significantly higher, whereas the abundances of Blautia, Coprococcus, and Roseburia were decreased in patients with schizophrenia compared with health controls. They also conducted Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis and found that several metabolic pathways differed significantly between healthy controls and schizophrenia patients, including vitamin B6 and fatty acid.
In children with ASD, lower gut microbial diversity and reduced relative abundances of phylotypes most closely related to Prevotella copri were reported (Kang et al., 2017). A study in young patients with ADHD showed decreased alpha diversity compared with controls and that the beta diversity differed significantly between patients and controls (Prehn-Kristensen et al., 2018). In detail, the bacterial family Prevotellacae was associated with controls, while patients with ADHD showed elevated levels of Bacteroidaceae, and both Neisseriaceae and Neisseria species were found as possible biomarkers for juvenile ADHD.
Gut microbial diversity is described in terms of “richness” and “evenness”; that is, by the number of species in relation to the species’ abundance within a sample (alpha diversity). Decreased alpha diversity is generally observed in chronic disorders such as type 2 diabetes mellitus, obesity, and some psychiatric disorders such as ASD and ADHD (Cotillard et al., 2013; Karlsson et al., 2013; Kang et al., 2018; Prehn-Kristensen et al., 2018).
However, for patients with depression, the results from several studies (Jiang et al., 2015; Kelly et al., 2016; Liu et al., 2016; Huang et al., 2019; Sanada et al., 2020) reporting the alpha diversity of patients’ gut microbiota compared with that of healthy controls have been controversial. Results based on basic research by Macedo et al. (2017) and Maier et al. (2018) have shown that psychotropic drugs have antibacterial effects in vitro and have the potential to alter microbial compositions. Additionally, the side effects of antidepressants and antipsychotics, such as weight gain and extrapyramidal symptoms, are related to microbial composition in mice (Morgan et al., 2014; Munhoz et al., 2017). Therefore, it is important to consider medication usage when looking at the gut microbiome of patients with psychiatric disorders. However, studies focused on the association between medication and the gut microbiome in humans are limited. In addition, longitudinal studies in clinical settings are scarce in this area.
One previous study by Liskiewicz et al. (2019) focused longitudinally on the relationship between depression severity and gut microbial diversity among 17 patients in a hospital setting. A significant increase in alpha diversity in the Shannon index was observed after 6 weeks of pharmacotherapy, but not in the Chao1 index. However, medication usage was strictly limited to only escitalopram and other types of psychotropics such as antipsychotics and anxiolytics, which are widely used in patients with depression and anxiety, have not been investigated.
To further investigate the influence of psychotropics on the gut microbiome, we longitudinally investigated the relationship between patients’ prescribed medications and intestinal bacterial diversity in a naturalistic treatment course for Japanese patients with depression and anxiety. Our aim in this paper is to investigate the impact of antidepressants, anxiolytics, and antipsychotics on the intestinal microbiome.
Methods
Patients
Patients were recruited from inpatients and outpatients at Keio University Hospital (Tokyo, Japan), Komagino Hospital (Tokyo, Japan), and Showa University Karasuyama Hospital (Tokyo, Japan), and all participants gave written informed consent before enrollment.
The inclusion criteria were adult patients clinically diagnosed with depression and/or anxiety as described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Exclusion criteria were: those with any organic gastrointestinal disorders, those taking antibiotic medication at the time of recruitment, and those whose psychiatric symptoms could potentially worsen through participation in the study.
Psychiatric Assessment and Fecal Collection
The study flow chart is presented in Figure 1. For inpatients, all the stool sampling and psychiatric assessments were performed during hospitalization. Baseline data were obtained within 10 days of hospitalization (including the day of hospitalization), midterm data were obtained from days 14 to 20, and endpoint data were obtained after day 21 until the day of discharge. A minimum interval of 1 week was required between each time point. As a side note, the typical duration of hospitalization at the participating hospitals is 1 to 3 months for depression and anxiety disorders. For outpatients, the baseline was defined as the time when consent was obtained; the midterm point was the patient’s next visit to the outpatient ward (2 weeks to 2 months after baseline); and the endpoint was the patient’s third visit to the outpatient ward. A minimum interval of 1 week was required between each time point. For both settings, fecal samples were collected up to 3 times at each time point: baseline, midterm, and endpoint. When more than 1 sample was collected, the mean value of the data was used.
Figure 1.
Study flowchart. HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; Med., medication; d/c, discharge.
The patients’ psychiatric symptoms were assessed by trained psychiatrists or psychologists using the Hamilton Depression Rating Scale (HAM-D) and Hamilton Anxiety Rating Scale (HAM-A) at each time point.
This study was carried out in accordance with the latest version of the Declaration of Helsinki. The study protocol was approved by the ethics committee of Keio University School of Medicine (#20150368). The study is registered at the University Hospital Medical Information Network Center (no. 000021833).
DNA Extraction and 16S rRNA Gene Sequence
Samples were immediately frozen after collection and transported within 48 hours. They were kept in a −80°C freezer until further analysis could be conducted.
Using universal primers described in previous studies (Furusawa et al., 2013; Kim et al., 2013), we performed DNA extraction and sequenced the V1-V2 hypervariable region of the 16S rRNA genes in the fecal samples. The 16S rRNA gene was analyzed using some modifications previously indicated (Murakami et al., 2015). In short, one-half to 1 pellet of feces was washed with buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0). Next, fecal samples were lyophilized for approximately 18 hours using a VD-800R lyophilizer (TAITEC, Nagoya, Aichi, Japan). Each freeze-dried fecal sample was combined with four 3.0-mm zirconia beads, approximately 100 mg of 0.1-mm zirconia/silica beads, 400 µL DNA extraction buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0, containing 1% [w/v] sodium dodecyl sulfate), and 400 µL of phenol/chloroform/isoamyl alcohol (25:24:1) and subjected to vigorous shaking (1500 rpm for 15 minutes) using a Shake Master (Biomedical Science, Shinjuku, Tokyo, Japan). The resulting emulsion was subjected to centrifugation at 17 800 × g for 10 minutes at room temperature, and bacterial genomic DNA was purified from the aqueous phase by a standard phenol/chloroform/isoamyl alcohol protocol. RNA was removed from the sample by RNase A treatment; the resulting DNA sample then was purified again by another round of phenol/chloroform/isoamyl alcohol treatment. Filter-passed reads were randomly selected from each sample and used for further analysis. Reads were then processed using the Quantitative Insights into Microbial Ecology pipeline (ver. 1.9.1) (Caporaso et al., 2010). Sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity, and OTUs were assigned to the SILVA 132 Database (Quast et al., 2013). Beta diversity measures, such as weighted and unweighted UniFrac distance metrics analysis and principal coordinate analysis, were performed in the samples between antidepressants +/−, antipsychotics +/−, and anxiolytics +/− pairs respectively. In the UniFrac analysis, phylogenetic distance is used to evaluate the comparative relationship of individuals in a group. The quantitative version of UniFrac that considers bacterial numbers is called weighted UniFrac, and the qualitative version that considers only the existence of the microbiota is called unweighted UniFrac. PICRUSt analysis was performed to predict the group difference in the contributions of various OTUs to known biological pathways based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology groups using the KEGG databases. 16S rRNA gene sequences were clustered into OTUs based on a 97% similarity threshold, and OTUs were assigned to taxonomies based on the Greengenes Database (ver. 13.5) (DeSantis et al., 2006). The resultant OTU table was normalized with inferred 16S rRNA gene copy numbers and predicted microbial metagenomes using a script provided by PICRUSt (ver. 1.0.0) (Langille et al., 2013). Further information about the analysis is reported in our previous study (Ishii et al., 2018). As a post-hoc analysis, we conducted a Neuroactive Gut-Brain Modules (GBM) analysis, a metabolic reconstruction framework specific for translating metagenomic data into microbial neuroactive metabolic potential based on extensive literature (>300 peer-reviewed papers) and database (MetaCyc73) review. From a set of 56 GBMs, each corresponding to a process of synthesis or degradation of a neuroactive compound by members of the gut microbiota, we chose 6 modules related to GABA and tryptophan synthesis and degradation. We followed the method regarding GBM analysis previously reported (Valles-Colomer et al., 2019).
Patient Grouping
We categorized patients in the following 3 ways: patients who were taking antidepressants or not (antidepressants +/−), patients who were taking antipsychotics or not (antipsychotics +/−), and patients who were taking anxiolytics or not (anxiolytics +/−). We investigated and compared the gut microbial diversity between the groups defined above in terms of alpha diversity including Chao1 index, Shannon index, and phylogenic diversity (PD) whole tree and the beta diversity.
Dosage Assessment for Antidepressants, Antipsychotics, and Anxiolytics
Information on prescribed medications was collected from patients’ medical records. Information regarding the duration of use for the current medication was collected separately for each psychotropic class. To calculate the dosage equivalence of the prescribed medication, imipramine equivalent (Imipramine-eq) doses, chlorpromazine equivalent (CP-eq) doses, and diazepam equivalent (Diazepam-eq) doses were used for antidepressants, antipsychotics, and anxiolytics, respectively (Hayasaka et al., 2015; Inada and Inagaki, 2015).
Statistical Analysis
Means and SDs were calculated for normally distributed continuous variables, and numbers and percentages were calculated for categorical variables. All variables were inspected using histograms, q–q plots, and Kolmogorov-Smirnov tests before conducting statistical analyses to detect normal distribution. Mann-Whitney U-test was performed to assess the difference in the baseline microbial diversity between antidepressants +/−, antipsychotics +/−, and anxiolytics +/− pairs respectively, and multiple testing corrections were done by controlling the false discovery rate through the Benjamini-Hochberg procedure (Benjamini and Hochberg, 2000). For alpha, .05 was chosen for significance in the false discovery rate. Analysis of Similarities was conducted to determine if the beta diversity differences between groups were more significant than the intra-group differences in each psychotropic class. The relationship between drug dosage and alpha diversity was determined using a fixed-effect model with a diagonal covariance matrix. The effect on each alpha diversity index was analyzed from repeated measures of CP-eq, Imipramine-eq, and Diazepam-eq doses along with body mass index, HAM-D, and HAM-A scores across baseline, midterm, and endpoint. All analyses were 2-sided with alpha set at .05. Statistical analyses were conducted using SPSS 25.0 software (SPSS Inc. Chicago, IL), and R 3.5.3 and R Studio (version 1.2.1335).
Results
Demographic Characteristics
A total of 45 patients with depression and/or anxiety participated in the study. Among them, 5 patients did not provide any stool and/or did not receive clinical assessment. Thus, 40 patients (17 males and 23 females) who provided at least 1 clinical severity assessment were included in our analyses, and a total of 246 fecal samples were collected.
Patients’ clinical characteristics are shown in Table 1. Twenty-four patients (60.0%) were diagnosed with MDD, 8 (20.0%) with persistent depressive disorder, 6 (15.0%) with general anxiety disorder, 1 (3.0%) with social anxiety disorder, and 1 (3.0%) with panic disorder; of these, 12 patients (30.0%) were diagnosed with both depression and anxiety. Twenty-one patients (52.5%) had HAM-D scores above the threshold of moderate depression (HAM-D ≥ 14). Nineteen (47.5%) had HAM-A scores above the threshold of moderate anxiety (HAM-A ≥ 15).
Table 1.
Sociodemographic Data
| Sex (male, %) | 17 (42.5) |
| Age (y, mean ± SD) | 54.4 ± 19.0 |
| BMI (kg/m2, mean ± SD) | 22.06 ± 4.34 |
| Duration since first episode (y, mean ± SD) | 11.95 ± 10.70 |
| HAM-D (mean ± SD) | 14.53 ± 7.90 |
| HAM-A (mean ± SD) | 14.30 ± 8.10 |
| Chao1 index (median ± IQR) | 15 519.53 ± 4060.31 |
| Shannon index (median ± IQR) | 5.89 ± 1.36 |
| PD whole-tree index (median ± IQR) | 60.67 ± 9.28 |
| Duration on current antidepressant (median ± IQR) | 142 ± 364.50 |
| Duration on current antipsychotic (median ± IQR) | 63.5 ± 682.75 |
| Duration on current anxiolytics (median ± IQR) | 242 ± 618.75 |
Abbreviations: BMI, body mass index; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; IQR, interquartile range; PD, phylogenetic diversity.
Total n = 40.
Regarding antidepressants, 3 patients were taking tricyclic antidepressants (amitriptyline; amoxapine); 6 were taking selective serotonin reuptake inhibitors (sertraline; paroxetine; escitalopram); 6 were taking serotonin noradrenaline reuptake inhibitors (duloxetine; milnacipran; venlafaxine); 3 were taking noradrenergic and specific serotonergic antidepressants (mirtazapine); 16 were taking 2 or more kinds of antidepressants; and 6 were taking no antidepressants. Regarding antipsychotics, 1 patient was taking olanzapine, 4 patients were taking quetiapine, 3 were taking aripiprazole, 1 was taking perospirone, and 29 were taking no antipsychotics. All patients who were taking antipsychotics also took antidepressants. Regarding anxiolytics, 3 patients were taking etizolam, 1 was taking loflazepate, 2 were taking clonazepam, 1 was taking diazepam, 2 were taking alprazolam, 3 were taking lorazepam, 1 was taking etizolam and loflazepate, and 27 were taking no anxiolytics. Some patients were taking the medication for a very long time (Table 1). The median of the observation period from baseline sample collection to endpoint sample collection was 40 days (min 15, max 146). Seven patients in total (4 patients were taking antidepressants and 3 were not; 1 patient was taking antipsychotics and 6 were not; 7 patients were not taking anxiolytics) provided no stool sample at endpoint. Thus, a total 33 patients were analyzed at endpoint.
Baseline Alpha Diversity Between Different Drug Treatments
There were no significant differences in the baseline alpha diversity between patients with and without each type of psychotropics (Table 2).
Table 2.
Baseline Alpha Diversity at Baseline in Each Psychotropic +/− Group
| Antidepressants | Antipsychotics | Anxiolytics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + (n = 33) |
− (n = 7) |
P value | FDR | + (n = 9) |
− (n = 31) |
P value | FDR | + (n = 11) |
− (n = 29) |
P value | FDR | |
| Chao1 | 15 574.82 ± 3442.53 |
14 487.45 ± 5391.03 |
.383 | 1.000 | 15 275.10 ± 2082.85 |
15 577.55 ± 4408.22 |
.466 | 0.699 | 15 712.80 ± 2515.10 | 15 336.04 ± 3886.97 | .296 | 0.888 |
| Shannon | 5.90 ± 1.48 | 5.88 ± 1.00 |
.709 | 0.709 | 6.39 ± 1.29 | 5.87 ± 1.07 | .356 | 1.000 | 5.88 ± 0.50 | 5.93 ± 1.58 | .988 | 0.988 |
| PD whole tree | 59.73 ± 15.96 | 64.88 ± 12.90 |
.581 | 0.871 | 62.38 ± 17.96 |
59.79 ± 13.17 |
.758 | 0.758 | 58.61 ± 12.22 |
62.38 ± 17.30 |
.797 | 1.000 |
Abbreviations: BMI, Body Mass Index; FDR, false discovery rate; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; IQR, interquartile range; PD, phylogenetic diversity.
Median ± IQR, n = 40. P values were corrected by FDR for each medication category.
Alpha Diversity Change Between Baseline and Endpoint
There was a significant difference in PD whole tree between patients who were taking antipsychotics and patients who were not taking antipsychotics (P = .009). It remained significant after correction for multiple comparisons (P = .027) (Figure 2; supplementary Table 1).
Figure 2.
Box plot of alpha diversity (PD whole tree) change from baseline to endpoint in patients with or without antipsychotics (antipsychotic +/−); * P < .05.
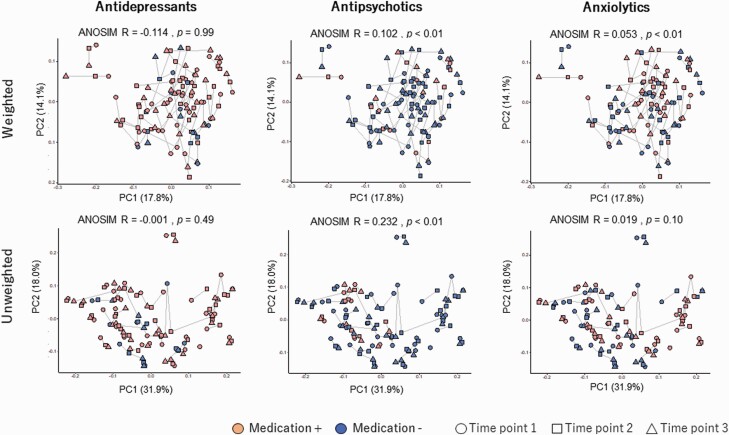
Beta Diversity and Functional Analysis Between Different Drug Treatments
Beta diversity using all samples grouped in each medication class are plotted in Figure 3. The PCoA plots based on weighted and unweighted UniFrac distances revealed significant compositional changes in the structure of the bacterial community between antipsychotics +/− (both weighted and unweighted Unifrac distances) and anxiolytics +/− (only weighted Unifrac distance) pairs by calculating Analysis of Similarities. The PICRUSt analysis using all samples showed that there are no significant predicted KEGG pathways that are enriched or decreased in each psychotropic class after multiple testing corrections (Figure 4). GBM analysis regarding each type of psychotropic is shown in supplementary Table 2 along with the correspondence table of individual enzymes in each function, which are shown in supplementary Table 3. Patients who were taking antidepressants presented increased GABA III synthesis and GABA degradation. Patients who were taking antipsychotics presented increased and decreased tryptophan synthesis and degradation, respectively, along with increased GABA II synthesis. Patients who were taking anxiolytics presented increased GABA II and III synthesis and decreased tryptophan synthesis, though significance disappeared after correction for multiple comparisons.
Figure 3.
Beta diversity shown in Weighted and Unweighted Unifrac Analysis and Analysis of Similarities (ANOSIM) for each psychotropic (+/−) group. Red dots indicate medication (+); blue dots indicate medication (−). Samples are assigned shapes (circle, square, triangle) according to timepoints. Lines connect projections of samples from the same patients. An R value close to 1.0 indicates the dissimilarity between groups, whereas an R value close to 0 indicates an even distribution. Abbreviations: PC, principal components. *P < .05.
Figure 4.
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis in each psychotropics (+/−) group. The average estimated function of the microbiome in each group is shown. Each function is presented in different colors and listed below. There was no significant difference between each pair.
Relationship Between Psychotropic Dosage and Microbial Diversity
The relationships between doses of psychotropics and microbial diversity are shown in Table 3 using a fixed-effect model. There was a negative correlation between antipsychotic doses and gut diversity based on the Shannon index and PD whole tree when adjusted for body mass index and depression and anxiety severities (estimate = −0.00254, SE = 0.000595, P < .0001; estimate = −0.02644, SE = 0.00833, P = 0.002, respectively). Other medication dosage parameters such as Imipramine-eq and Diazepam-eq did not show a significant relationship with microbial diversity.
Table 3.
The Dose Relationships of Psychotropics to Microbial Alpha Diversity Using a Fixed-Effect Model
| Chao1 Index | Shannon Index | PD Whole Tree | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P value | Estimate | SE | P value | Estimate | SE | P value | |
| Intercept | 16 795.45 | 1150.777 | <.001 | 5.705573 | 0.358251 | <.001 | 58.10886 | 4.979865 | <.001 |
| Imipramine-eq | −2.43114 | 2.29714 | .293 | −0.00052 | 0.000711 | .464 | −0.0015 | 0.00989 | .880 |
| CP-eq | −1.88646 | 1.931047 | .331 | −0.00254 | 0.000595 | <.001* | −0.02644 | 0.00833 | .002* |
| Diazepam-eq | −45.0931 | 60.14956 | .455 | −0.00178 | 0.018621 | .924 | 0.067608 | 0.258189 | .794 |
| HAM-D | 25.90793 | 72.32276 | .721 | −0.01281 | 0.020656 | .537 | −0.01169 | 0.279335 | .967 |
| HAM-A | 60.10727 | 72.99435 | .413 | 0.023183 | 0.020865 | .270 | 0.222334 | 0.281826 | .433 |
| BMI | −93.0798 | 54.45308 | .091 | 0.014021 | 0.016965 | .411 | 0.18641 | 0.235552 | .431 |
Abbreviations: BMI, body mass index; CP, chlorpromazine; -eq, equivalency scale; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; PD, phylogenetic diversity.
Repeated covariance type: diagonal; n = 40.
*P < .05.
Based on a fixed-effect model, only antipsychotics predicted microbial diversity, as higher doses correlated with less diversity. The sequence data in this study is available from the DNA DataBank of Japan (DDBJ) Sequence Read Archive (DRA) under the accession number DRA010810.
Discussion
In this study, the influence of psychotropics such as antidepressants, antipsychotics, and anxiolytics on the diversity of microbiota was examined during a naturalistic treatment course for 40 patients with depression and anxiety. Our study produced 3 main findings: (1) there was a significant difference in alpha diversity change in patients who were taking antipsychotics between the baseline and endpoint time points compared with those not taking antipsychotics; (2) there was a significant difference in beta diversity between patients who were taking antipsychotics and those not taking antipsychotics; and (3) the dose of antipsychotics was negatively correlated to the alpha diversity indices of the gut microbiome when adjusted for the severity of depression and anxiety. As far as we know, this is the first study to investigate longitudinal microbial diversity change as well as the relationship between psychotropics and gut microbial diversity in patients with depression and anxiety, with a focus on their prescribed medication types and dosage.
First, we investigated the baseline difference in gut microbial diversity in 3 different pairs: antidepressants +/−, antipsychotics +/−, and anxiolytics +/−. At baseline, there were no significant differences between any pairs. However, during the period from baseline to endpoint, PD whole-tree indices exhibited significant changes in diversity among patients taking antipsychotics compared with those who were not.
It is important to note that most of the previous studies on microbial diversity in patients with depression did not consider the patients’ prescribed psychotropic medications despite increasing evidence in animal models that psychotropics could influence gut microbiota composition (Kanji et al., 2018). Previous research by Macedo et al. (2017) indicates that antidepressants and antipsychotics have an antimicrobial effect. For instance, aripiprazole may induce changes in the gut microbiota composition in vivo when taken at approximately 20 mg/kg/d (Cussotto et al., 2019). Another study by Kanji et al. (2018) indicated that female rats receiving 2 mg of olanzapine had a decrease in the total diversity of their microbiota as measured by the Shannon index.
In humans, there is 1 study by Yuan et al. (2018) that reported that the diversity of specific species such as Bifidobacterium spp. and Escherichia coli changed after patients with first-episode schizophrenia took risperidone for 24 weeks, though the study investigated only a limited number of microbiota species with no reported alpha diversity indices. Another study by Flowers et al. (2019) investigating patients with bipolar disorder or schizophrenia showed that patients who were taking antipsychotics for at least 6 months had lower microbial diversity cross-sectionally compared with those not taking antipsychotics.
The results from our study, which suggest that patients who took antipsychotics may have decreased microbial diversity, could partly explain the controversial results of previous reports regarding alpha diversity among patients with depression and healthy controls (Jiang et al., 2015; Kelly et al., 2016; Liu et al., 2016; Zheng et al., 2016; Chen et al., 2018). Moreover, Xu et al. (2019) have shown that the diversity of gut microbiota in patients with schizophrenia was significantly lower compared with healthy controls; again, this may be influenced by the oral antipsychotics taken by these patients, a factor that was not considered in the report. In general, the diversity of gut microbiota is known to be decreased in various physical and mental pathologies compared with healthy controls, and this decrease is thought to have a negative influence on the host. However, a recent meta-analysis (Ma et al., 2019) has shown that higher diversity is seen in some disease states compared with healthy controls, such as Parkinson’s disease and HIV. We can only note that our findings in this human study were in line with similar results from previous studies showing that psychotropics changed the gut microbiome in animals, and further research will be needed to determine whether our results correlate with a negative or positive influence on patients with depression/anxiety.
Regarding beta diversity, our findings indicated that the composition of the microbiome in the antipsychotics group differed from each other, considering P value and R value in weighted and unweighted Unifrac distances, although the R value was relatively small. A previous animal study reported a significant difference in specific species composition between rats treated with escitalopram and those receiving vehicle, while the beta diversity in principal coordinate analysis did not differ significantly (Cussotto et al., 2018). Moreover, a recent human study did not show a significant change regarding beta diversity after 6 weeks’ intake of escitalopram in a controlled hospital setting (Liskiewicz et al., 2019). Our results were in line with these previous studies. Larger controlled studies are needed to determine whether each psychotropic type affects beta diversity.
The PICRUSt analysis showed no significant difference between the psychotropic groups regarding the biological pathways predicted from the metagenomic data. Although evidence is scarce regarding the relationship between psychotropics and altered predicted function of the microbiome in MDD patients, there is some evidence showing altered function in patients with MDD compared with healthy controls in cross-sectional studies. Three reports (Zheng et al., 2016; Chen et al., 2018; Chung et al., 2019) have indicated the pentose phosphate pathway, and starch and sucrose metabolism pathways were enriched in patients with MDD. However, a previous report (Human Microbiome Project Consortium, 2012) indicates that it is possible to have similar predicted functions even if the microbial composition is different in the group. Our results from the GBM analysis have shown that different types of psychotropic intake may influence the microbiome that is related to GABA and tryptophan metabolism in the host. A previous study (Valles-Colomer et al., 2019) has shown that GABA III synthesis was increased in patients with MDD compared with healthy controls. Since our patient groupings were based on pharmacotherapy and those by Valles-Colomer et al. (2019) were based on patients vs healthy controls, we cannot directly compare the 2 cases, but our results suggest that the microbiome metabolism related to GABA and tryptophan may be influenced by antidepressant and antipsychotic intake, though there is no indication of causal relationship at this point.
Finally, we found that there was a negative correlation between antipsychotic dosage and alpha diversity indices. Previous research indicates that the concentration of antipsychotics is proportional to their antimicrobial effects (Morgan et al., 2014). The result indicating the relationship between the antipsychotic dose and alpha diversity is independent after controlling for antidepressant dose, because all patients taking antipsychotics were on antidepressants as well. However, we cannot untangle the relative contribution of each drug class or draw any conclusions about antipsychotics themselves. The results from our findings suggest a correlation between antipsychotics and dysbiosis, although a causational relationship requires further study. For instance, antipsychotics generally induce side effects such as obesity, dyslipidemia, and gastrointestinal symptoms (Correll et al., 2015), which may secondarily induce dysbiosis in the gut (Dieterich et al., 2018).
Regarding the influence of antipsychotics on each microbial diversity index, our results show that longitudinal alpha diversity change was seen in PD whole-tree indices, while a relationship with dosage was seen in the Shannon and PD whole-tree indices. A possible reason for these differences is due to how the Chao1, Shannon, and PD whole-tree indices are calculated differently in terms of microbial composition. The Chao1 is an abundance-based estimator of species richness, while the Shannon index is an estimator of not only species richness but also species evenness (Kim et al., 2017). PD whole tree is defined as the minimum total length of all the phylogenetic branches required to span a given set of taxa on the phylogenetic tree (Faith and Baker, 2007).
The results of our study suggest that microbial diversity is altered not only by differences in study participants’ cultural and dietary patterns (Kovatcheva-Datchary et al., 2015) but also by differences in prescription backgrounds between patients.
The potential mechanism of the relationships between psychiatric disorders and microbiota is still under investigation; therefore, it is difficult to interpret how the resulting antipsychotic microbial alterations could be counteracted to the host. In a recent animal study where transplantation of gut microbiota was done from patients with depression to germ-free mice, the mice showed depressive-like and/or anxiety-like behavior that was accompanied with a downregulation of Stat5, a gene that regulates the hypothalamus-pituitary-adrenal (HPA) axis reaction (Luo et al., 2018).
Moreover, 1 study (Moya-Pérez et al., 2017) showed that after the level of cortisol increased in the depression model mice, it was then reduced after the administration of B. pseudocatenulatum CECT 7765, and the upregulated stress response of the HPA axis was suppressed. The above-mentioned effects on the HPA axis and inflammation are thought to be 1 of the mechanisms in the relationship between psychiatric disorders and microbiota, but the direct link is not fully understood in humans. Regarding psychotropics, a meta-analysis by McKay et al. (2010) evaluated antidepressant effects on cortisol in patients with unipolar depression, focusing on studies that measured pre- and post-treatment cortisol. The effect sizes for pre-/post-depression severity reductions were positively correlated with cortisol effect sizes, and similar results are reported for antipsychotics (Subramaniam et al., 2019).
Limitations
The results of this study must be interpreted in the context of the following limitations. First, this study’s sample size was small, which introduces the possibility that our results may demonstrate a false relationship. Second, we did not take into consideration the effect of prescribed medication taken before participating in this study, as we did not set a washout period using antibiotics. Third, to collect medication data, we referred only to the medical prescription records for each patient, which means there could possibly be effects from non-adherence that we did not take into account. Fourth, we investigated the patients with various psychotropics, which means our cohort should be interpreted as multi-psychotropics-treated patients’ cohort, and we cannot tease apart the relative contribution of each drug class. Fifth, there is a possibility for selection bias; for example, patients with gastrointestinal symptoms may have been more interested in participating in this research. Sixth, many other factors beyond medication are known to influence the diversity of the gut microbiome; 1 example of such a factor is dietary habits (Kovatcheva-Datchary et al., 2015), which will be investigated in our next paper.
This is the first study, to our knowledge, to demonstrate that antipsychotics may decrease alpha diversity during the course of treatment and that antipsychotic dosage has a negative relationship with alpha diversity in the gut microbiome among patients with depression and anxiety.
A failure to consider patients’ psychotropic medications may be one of the factors that made previous reports controversial. In future studies, it will be important to give consideration to the types and doses of psychotropic medications used by patients to further elucidate the mechanisms of gut-brain interactions in humans.
Supplementary Material
Acknowledgments
We thank Ms Kelley Cortright for proofreading this manuscript.
Contributor Information
Yoshihiro Tomizawa, Division of Pharmacotherapeutics, Faculty of Pharmacy, Keio University, Tokyo, Japan.
Shunya Kurokawa, Department of Neuropsychiatry, School of Medicine, Keio University, Tokyo, Japan.
Daiki Ishii, Division of Pharmacotherapeutics, Faculty of Pharmacy, Keio University, Tokyo, Japan; Institute for Advanced Biosciences, Keio University, Yamagata, Japan.
Katsuma Miyaho, Department of Psychiatry, Showa University School of Medicine, Tokyo, Japan.
Chiharu Ishii, Division of Pharmacotherapeutics, Faculty of Pharmacy, Keio University, Tokyo, Japan.
Kenji Sanada, Department of Psychiatry, Showa University School of Medicine, Tokyo, Japan.
Shinji Fukuda, Institute for Advanced Biosciences, Keio University, Yamagata, Japan; Intestinal Microbiota Project, Kanagawa Institute of Industrial Science and Technology, Kanagawa, Japan; Transborder Medical Research Center, University of Tsukuba, Ibaraki, Japan.
Masaru Mimura, Department of Neuropsychiatry, School of Medicine, Keio University, Tokyo, Japan.
Taishiro Kishimoto, Department of Neuropsychiatry, School of Medicine, Keio University, Tokyo, Japan.
This work was supported in part by Japan Society for the promotion of science KAKENHI (18H04805 to S.F.); Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology (JPMJPR1537 to S.F.); Japan Agency for Medical Research and Development Core Research for Evolutional Science and Technology (JP19gm1010009 to S.F.); Japan Science and Technology Agency Exploratory Research for Advanced Technology (JPMJER1902 to S.F.); the Takeda Science Foundation (to S.F.); the Food Science Institute Foundation (to S.F.); the Program for the Advancement of Research in Core Projects under Keio University’s Longevity Initiative (to S.F.); and Japan Dairy Association J-milk (to K.S.).
Statement of Interest
S.K. has received grants and/or speaker’s honoraria from Dainippon-Sumitomo Pharma, Meiji-Seika Pharma, and Mochida Pharmaceutical within the past 3 years. K.S. has received speaker’s honoraria from Eli Lilly, Dainippon Sumitomo Pharma, and Meiji Seika Pharma. T..K has received consultant fees from Dainippon Sumitomo, Novartis, and Otsuka; speaker’s honoraria from Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, MSD, Novartis, Otsuka, and Pfizer; and grant support from Takeda, Dainippon-Sumitomo, and Otsuka. M.M. has received grants and/or speaker’s honoraria from Asahi Kasei Pharma, Astellas Pharmaceutical, Daiichi Sankyo, Dainippon-Sumitomo Pharma, Eisai, Eli Lilly, Fuji Film RI Pharma, Janssen Pharmaceutical, Kracie, Meiji-Seika Pharma, Mochida Pharmaceutical, MSD, Novartis Pharma, Ono Yakuhin, Otsuka Pharmaceutical, Pfizer, Shionogi, Takeda Yakuhin, Tanabe Mitsubishi Pharma, and Yoshitomi Yakuhin; and research funding from Nishikawa Sangyo, Otsuka Pharmaceutical, MSD, Meiji-Seika Pharma, and Shionogi & Cc., Ltd within the past 3 years. For the remaining authors, no conflicts of interest were declared.
References
- Benjamini Y, Hochberg Y (2000) On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 25:60–83. [Google Scholar]
- Caporaso JG, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, Hu Z, Wang H, Zhong X, Zeng L, Chen K, Li P, Xie P (2018) Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 29:417–425. [DOI] [PubMed] [Google Scholar]
- Chung YE, Chen HC, Chou HL, Chen IM, Lee MS, Chuang LC, Liu YW, Lu ML, Chen CH, Wu CS, Huang MC, Liao SC, Ni YH, Lai MS, Shih WL, Kuo PH (2019) Exploration of microbiota targets for major depressive disorder and mood related traits. J Psychiatr Res 111:74–82. [DOI] [PubMed] [Google Scholar]
- Consortium - Human Microbiome Project (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Detraux J, De Lepeleire J, De Hert M (2015) Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 14:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Doré J, Zucker JD, Clément K, Ehrlich SD; ANR MicroObes consortium (2013) Dietary intervention impact on gut microbial gene richness. Nature 500:585–588. [DOI] [PubMed] [Google Scholar]
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF(2018) The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol 51:80–101. [DOI] [PubMed] [Google Scholar]
- Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, Stanton C, Dinan TG, Cryan JF (2019) Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl) 236:1671–1685. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich W, Schink M, Zopf Y (2018) Microbiota in the gastrointestinal tract. Med Sci (Basel) 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, McInnis MG (2017) The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res 87:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith DP, Baker AM (2007) Phylogenetic diversity (PD) and biodiversity conservation: some bioinformatics challenges. Evol Bioinform Online 2:121–128. [PMC free article] [PubMed] [Google Scholar]
- Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM, Ellingrod VL (2019) Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacotherapy 39:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, et al. (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. [DOI] [PubMed] [Google Scholar]
- Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA (2015) Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179–184. [DOI] [PubMed] [Google Scholar]
- Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH (2019) Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet 10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447. [DOI] [PubMed] [Google Scholar]
- Ishii C, Nakanishi Y, Murakami S, Nozu R, Ueno M, Hioki K, Aw W, Hirayama A, Soga T, Ito M, Tomita M, Fukuda S (2018) A metabologenomic approach reveals changes in the intestinal environment of mice fed on American diet. Int J Mol Sci 19:4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194. [DOI] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R (2017) Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, Lozupone CA, Hahn J, Adams JB, Krajmalnik-Brown R (2018) Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 49:121–131. [DOI] [PubMed] [Google Scholar]
- Kanji S, Fonseka TM, Marshe VS, Sriretnakumar V, Hahn MK, Müller DJ (2018) The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur Arch Psychiatry Clin Neurosci 268:3–15. [DOI] [PubMed] [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498:99–103. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Clarke G, Cryan JF, Dinan TG (2016) Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol 26:366–372. [DOI] [PubMed] [Google Scholar]
- Kim BR, Shin J, Guevarra R, Lee JH, Kim DW, Seol KH, Lee JH, Kim HB, Isaacson R (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27:2089–2093. [DOI] [PubMed] [Google Scholar]
- Kim SW, Suda W, Kim S, Oshima K, Fukuda S, Ohno H, Morita H, Hattori M (2013) Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res 20:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 22:971–982. [DOI] [PubMed] [Google Scholar]
- Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskiewicz P, Pelka-Wysiecka J, Kaczmarczyk M, Loniewski I, Wronski M, Baba-Kubis A, Skonieczna-Zydecka K, Marlicz W, Misiak B, Samochowiec J (2019) Fecal microbiota analysis in patients going through a depressive episode during treatment in a psychiatric hospital setting. J Clin Med 8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, Wang X, Wang K, Liu Z, Xia Z, Xu Z, Nie Y, Lv X, Wu X, Zhu H, Duan L (2016) Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol 14:1602–1611, e1605. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, Liu L, Wang H, Dong M, Pan J, Zheng P, Zhou C, Wei H, Xie P (2018) Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry 8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZS, Li L, Gotelli NJ (2019) Diversity-disease relationships and shared species analyses for human microbiome-associated diseases. Isme J 13:1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Júnior HVN, Freitas de Lucena D (2017) Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord 208:22–32. [DOI] [PubMed] [Google Scholar]
- Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M (2012) Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord 136:909–917. [DOI] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A (2018) Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay MS, Zakzanis KK (2010) The impact of treatment on HPA axis activity in unipolar major depression. J Psychiatr Res 44:183–192. [DOI] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF (2014) The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. Plos One 9:e115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Pérez A, Perez-Villalba A, Benítez-Páez A, Campillo I, Sanz Y (2017) Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun 65:43–56. [DOI] [PubMed] [Google Scholar]
- Munhoz RP, Bertucci Filho D, Teive HA (2017) Not all drug-induced parkinsonism are the same: the effect of drug class on motor phenotype. Neurol Sci 38:319–324. [DOI] [PubMed] [Google Scholar]
- Murakami S, Goto Y, Ito K, Hayasaka S, Kurihara S, Soga T, Tomita M, Fukuda S (2015) The consumption of bicarbonate-rich mineral water improves glycemic control. Evid Based Complement Alternat Med 2015:824395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad JM, Verdu EF (2013) Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 69:42–51. [DOI] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R (2016) Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A (2018) Reduced microbiome alpha diversity in young patients with ADHD. Plos One 13:e0200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO(2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools Nucleic Acids Res 41:D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, Mimura M, Iwanami A, Kishimoto T (2020) Gut microbiota and majore depressive disorder: a systematic review and meta-analysis. J Affect Disord 266:1–13. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. Plos Biol 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghor B, Sokhna C, Ruimy R, Lagier JC (2018) Gut microbiota diversity according to dietary habits and geographical provenance. Human Microbiome Journal 8:1–9. [Google Scholar]
- Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, Zhang M, Hu S, Liang Y (2018) Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res 197:470–477. [DOI] [PubMed] [Google Scholar]
- Subramaniam A, LoPilato A, Walker EF (2019) Psychotropic medication effects on cortisol: implications for research and mechanisms of drug action. Schizophr Res 213:6–14. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J (2019) The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4:623–632. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, Hsiao EY (2017) The microbiome and host behavior. Annu Rev Neurosci 40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Wu B, Liang J, He F, Gu W, Li K, Luo Y, Chen J, Gao Y, Wu Z, Wang Y, Zhou W, Wang M (2019) Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun 85:120–127. [DOI] [PubMed] [Google Scholar]
- Yuan X, Zhang P, Wang Y, Liu Y, Li X, Kumar BU, Hei G, Lv L, Huang XF, Fan X, Song X (2018) Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res 201:299–306. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.