Abstract
Objective:To describe the impact of subjects’ family income, which was used as a proxy for socioeconomic status, with health characteristics and healthcare utilization of a large representative sample of population in Northern Greece, taking into account several socio-demographic characteristics and health behaviors of the participants.
Material and method:Eight hundred and twelve participants (43.7% males) with a mean age of 49.±14.8 years (range 19-83 years), from the area of Thrace, Greece, were enrolled in this cross-sectional populational study. A two-stage stratified sampling scheme was used and subjects were classified, according to the net mean monthly household income, into three financial levels: low .1000 Euro; medium 1001-2000 Euro; and high >2000 Euro. Self-reported questionnaires for socio-demographic, lifestyle and health related characteristics were collected. Sleep characteristics, utilizing Epworth Sleepiness Scale, Athens Insomnia Scale, Pittsburgh Sleep Quality Index and Berlin Questionnaire, and mental health, using Zung Self-rating Anxiety Scale and Beck Depression Inventory have been also assessed.
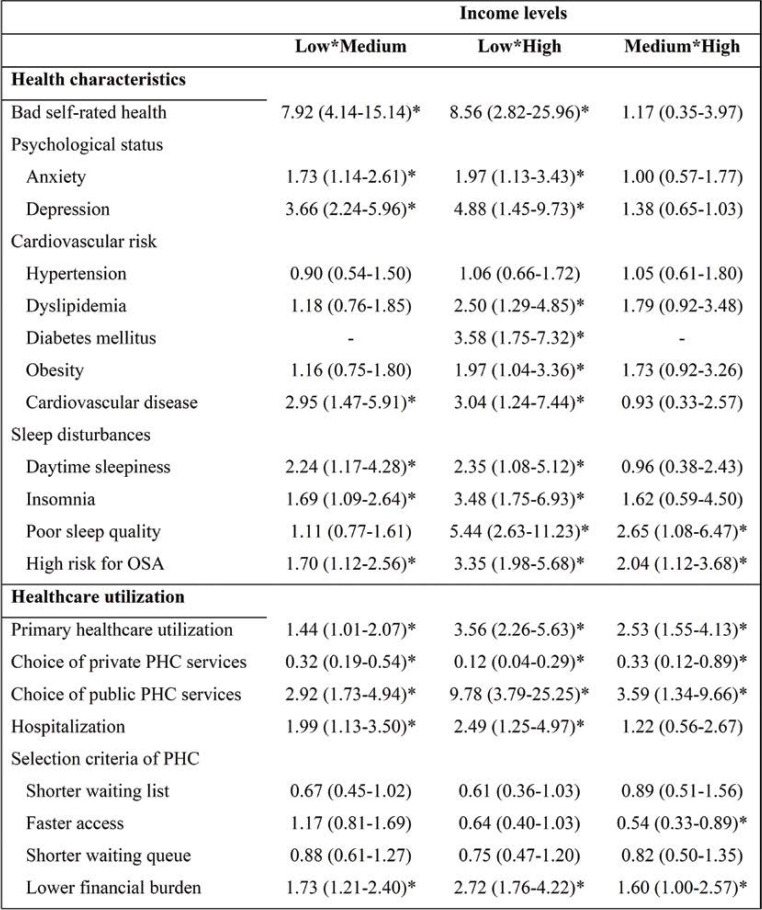
Results:The majority of participants belonged to the lower income level (476 subjects, 58.6%). Lower income level was associated with a higher prevalence of high alcohol consumption (p=0.030), low adherence to Mediterranean diet (p=0.016), low physical activity (p<0.001) and either short or long nocturnal sleep duration (p<0.001). After adjusting for all socio-demographic and lifestyle characteristics, subjects with low income had a higher risk for anxiety (aOR=1.97, p=0.017), depression (aOR=4.88, p<0.001), dyslipidemia (aOR=2.50, p=0.007), diabetes (aOR=3.58, p<0.001), obesity (aOR=1.97, p=0.038), cardiovascular disease (aOR=3.04, p=0.015) and sleep disorders, as well as for primary (aOR=3.56, p=0.017) and secondary (aOR=2.49, p=0.010) healthcare utilization compared to subjects with high income.
Conclusion:Low income is an important factor, which adversely affects the health of individuals via different pathways such as adaptation of harmful everyday habits. Large-scale prospective cohort studies are necessary to verify these associations in a methodologically more robust way.
Keywords:income, health, healthcare utilization, Greece.
INTRODUCTION
Nowadays, Greece is recovering from its abysmal financial crisis that commenced in mid-2009, the deepest and longest ever recorded in an Organization for Economic Co-operation and Development (OECD) country in the postwar period (1, 2). More than a decade of profound economic downturn, combined with relentless austerity measures, resulted in not only a significant reduction in family income, but also a dramatic increase in unemployment (3), thus leading to a remarkable decline in economic welfare and a sharp augmentation of poverty (4). More specifically, before the COVID-19 crisis, the in-work poverty rate was 14.1% (5), the second highest in the European Union. Furthermore, 34.8% of the whole population was at risk of poverty or social exclusion (6). Income inequality was the fifth highest in the EU, as the top 20% of earners made more than six times the amount of money compared to the bottom 20% (7).
There is strong evidence connecting income inequality to health imbalance. For example, Chetty et al. found a gap in life expectancy of about 15 years for men and 10 years for women, when comparing the richest 1% of individuals with the poorest 1% (8). Income, together with educational background and occupation, are indicators of socioeconomic status, which describe the position that a person holds within the structure of society (9). Socioeconomic status has been found to be adversely associated with a wide range of health problems (10-12). In particular, a recent meta-analysis revealed an approximately 1.8- to 3.7-fold increase in odds for cardiovascular disease, hypertension, diabetes and dyslipidemia, when comparing the bottom with the top of the subjective social status ladder (13). Self-rated health, a well established indicator of future mortality and morbidity (14), is frequently used in epidemiological research as a measure of health status (15). According to existing literature, subjects with a lower socioeconomic status are more likely to report poor self-rated health (16-18). There is also evidence for socioeconomic inequalities in common mental disorders, such as anxiety and depression; both disorders have been observed to occur more frequently among individuals of lower socioeconomic level (19, 20). Moreover, socioeconomic status has also been recognized as an important determinant of the prevalence of sleep disturbances, which are more frequent in subjects of lower income or education or those that are unemployed (21). In addition, income is strongly associated with healthcare utilization and has been identified as one of its most significant determinants (22).
The aim of this study was to describe the impact of subjects’ family income, which was used as a proxy for socioeconomic status, with health characteristics (self-rated health, anxiety and depression symptoms, hypertension, dyslipidemia, diabetes mellitus, cardiovascular disease, obesity, sleep disturbances) and healthcare utilization (primary healthcare utilization, hospitalization, selection criteria of healthcare utilization) of a large representative sample of population in Northern Greece, taking into account several socio-demographic characteristics and health behaviors (smoking, alcohol consumption, coffee consumption, adherence to Mediterranean diet, physical activity, sleep duration) of the participants.
MATERIAL AND METHOD
Study sample and research design
The study population in this cross-sectional study comprised individuals aged >18 years old. The sample selection was based on a two-stage stratified sampling scheme on all adults living in the region of Thrace, in Northeastern Greece, and it was conducted between September 2016 and May 2019. In the first stage of the sampling procedure, the area of Thrace was divided in two strata by the degree of urbanization. The urbanization levels were urban (.10,000 inhabitants) and semi-urban or rural (<10,000 inhabitants) areas. According to the 2011 census, which constituted the sampling frame in our study, the urban population of Thrace accounted for approximately 40% of the total population of this area. In the second stage, subjects were recruited proportionally to each stratum size, through a method of random generation of telephone numbers on the basis of the area code. After the aim of the study was explained to them, participants agreed to have field researchers visit their home and to complete the questionnaires of the study in a 30-min-long interview. The sampling scheme ensured that the sample was randomly selected and representative of the general population of Thrace.
Ethics
Informed consent was obtained from all participants of the study. All study procedures were in accordance with both the ethical standards of Democritus University Ethics Committee and standards of the Helsinki Declaration (1964) and its later amendments. The study protocol was approved by the Institutional Ethics Committee (Protocol Number 42570/294).
Socio-demographic characteristics
Socio-demographic characteristics were assessed, including gender, age (as continuous variables, categorized as .40, 40-60, >60 years old), marital status (married, single/divorced/widowed), place of residence (urban, rural), education level, employment status (employment, unemployment) and financial status. Education of the participants was categorized into three levels: low . basic education (.nine years); medium . up to high school or technical colleges; high . university.
Financial status
Financial status of the participants was categorized into three levels according to the net mean monthly household income during the past three years (low: ≤1000 Euro; medium: 1001-2000 Euro; and high: >2000 Euro), in line with previous studies conducted in Greece, which had examined the association between income and different health indices (23-25).
Health and lifestyle indicators
In order to accurately describe health behaviors, the following lifestyle and dietary habits were assessed. Regarding smoking, current smokers were defined those individuals who smoked at least one cigarette per day. The daily intake of alcohol was based on the number of drinks consumed per day, and an average intake of more than one drink per day for women and more than two drinks per day for men was considered as high. The daily intake of caffeine was based on the number of cups of coffee consumed per day, and an intake of more than two cups per day was considered as high. Adherence to the Mediterranean diet was considered as an indicator of healthy diet and it was assessed by the Mediterranean Diet Score (ranging from 0 to 55), with scores >35 being considered as high adherence (26). Physical activity was assessed according to self-reported weekly frequency, duration and intensity of regular exercise (running, cycling, swimming, football, basketball, tennis, volleyball) and walking. Moderate exercise for at least three days per week or walking for at least five days per week for at least 30 minutes per day was classified as high physical activity.
Participants provided information on their nighttime sleep by answering the following questions: “On the average, how many hours do you sleep per day?” The responses were obtained for an average weekday and weekend day over the previous month. As a proxy of the overall sleep duration on a weekly basis, weighted mean measures were calculated using the following formula: weighted sleep duration = 5/7* (sleep duration on a weekday) + 2/7* (sleep duration on a weekend day). Participants were classified into the following three sleep categories according to their sleep duration: short sleep duration (<six hours), normal sleep duration (6-8 hours), and long sleep duration (>eight hours), classification which is consistent with the majority of previous studies.
Health related characteristics
Self-reported general health, as an indicator of perceived health status, was initially characterized as very bad, bad, fair, good and very good, but in the sequence, categories very bad, bad and fair were considered as bad health status and categories good and very good were considered as good health status. Based on the self-reported height and weight of the subjects, body mass index (BMI; weight/height² in kg/m²) was calculated and obesity was defined if BMI ≥30 kg/m² according to the World Health Organization (WHO) criteria (27).
Participants were asked to report any preexisting health problems, based on diagnosis previously established by a health professional, such as hypertension, dyslipidemia diabetes mellitus and cardiovascular disease (CVD).
Anxiety symptoms were assessed using the Greek version (28) of the Zung Self-rating Anxiety Scale (SAS), a 20-item self-reporting Likert scale, in which items are rated by respondents according to how each item applied to them within the past week, using a four-point scale ranging from 1 (none, or a little of the time) to 4 (most, or all of the time). SAS items concern both affective and somatic symptoms; 15 expresses a negative experience such as “I feel afraid for no reason at all” and 5 expresses a positive experience and are reverse scored, such as “I can breathe in and out easily”. The scale has a raw score range of 20 to 80 points. Higher scores indicate greater severity of anxiety symptoms, while raw score of 36 was considered as a cut-off point for significant anxiety (29).
Depression symptoms were assessed using the Greek version (30) of the Beck Depression Inventory (BDI) (31), a widely used questionnaire that measures characteristic attitudes and symptoms of depression. It consists of 21 self-reporting Likert scale items, which are rated by respondents according to how each item applied to them during the past two weeks, using a four-point scale ranging from 0 (i.e., I do not feel sad) to 3 (i.e., “I am so sad and unhappy that I can’t stand it”). Items are summed to create a total score, with higher scores indicating higher levels of depression. A total score of 13 was considered as a screening cut-off point for significant depression due to the high sensitivity (32).
Sleep disturbances
Assessment of daytime sleepiness was performed using the Greek version of Epworth Sleepiness Scale (ESS) (33, 34), which includes eight questions pertaining to the chance of dozing off or falling asleep in eight day-to-day activities. The score of each question ranges from 0 to 3, and their sum is the final score (range, 0 to 24) of the questionnaire; excessive daytime sleepiness was considered for final scores >10 (35).
Assessment of insomnia symptoms was performed using the Greek version of Athens Insomnia Scale (AIS) (36), which was designed for quantifying sleep difficulty based on the ICD-10 criteria. This consists of eight items: the first five pertain to sleep induction, awakenings during the night, final awakening, total sleep duration, and sleep quality; while the last three refer to wellbeing, functioning capacity, and sleepiness during the day. A score of >5 was used to establish the diagnosis of insomnia (37).
Assessment of sleep quality was performed using the Greek version of Pittsburgh Sleep Quality Index (PSQI) (38, 39). It consists of 19 questions grouped into seven subcategories. Specifically, the seven distinct clinical subclasses of sleep difficulties are as follows: (1) Subjective sleep quality (one question), (2) Sleep latency (two questions), (3) Sleep duration (one question), (4) Sleep efficiency (three questions), (5) Sleep disorders (nine questions), (6) Use of sleep medication (one question), (7) Daytime dysfunction (two questions). These distinct subcategories are summed and produce an overall score; poor sleep quality was considered for final scores >5.
Risk assessment of OSA was performed using the Greek version of Berlin Questionnaire (BQ), one of the most common tools for identifying patients with an increased risk of OSA (40, 41). It consists of 10 questions grouped into three categories: (1) Snoring severity (questions 1-5), (2) Daytime sleepiness or fatigue (questions 6-9), (3) History of arterial hypertension or obesity (question 10). The questions also include information about sex, age, height, and weight. High risk for OSA was considered if at least two categories were positively rated.
Utilization of primary and secondary healthcare
Based on the survey questionnaire participants’ self-reported primary and secondary healthcare utilization were assessed. Primary healthcare utilization refers to visits to medical doctors, general practitioners and specialists, in private or public primary healthcare establishments during the last month, and secondary healthcare utilization refers to admissions to either private and public hospitals, during a one-year reference period preceding the survey. Participants were also asked to answer about the importance of the following criteria in selecting primary healthcare services: shorter waiting list, faster access, shorter waiting queue and lower financial burden.
Statistical analysis
Statistical analysis of the data was performed using IBM Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM Corp., Armonk, NY, USA). All variables were qualitative and they were expressed as absolute and relative (%) frequencies. In univariate analysis, the association of participants’ income with their socio-demographic characteristics such as habit factors, health characteristics and healthcare utilization was assessed using the chi-square test. Odds ratios (OR) with their 95% confidence intervals (CI) were estimated as the measure of the above associations. Multivariate logistic regression models were constructed to explore the independent effect of income on subjects’ characteristics. Explanatory variables included: gender, age, marital status, place of residence, education level, working status, smoking, alcohol and coffee consumption, adherence to Mediterranean diet, physical activity and sleep duration. Cronbach’s á coefficient was used to assess the internal consistency of all questionnaires. All tests were two tailed and statistical significance was considered for p values <0.05.
RESULTS
A total of 812 subjects, of which 355 (43.7%) males and 457 (56.3%) females, with a mean age of 49.3±14.8 years (range 19-83 years, median age 50 years), were included in the study out of the 1 144 people who have been initially approached (71% participation rate). Participants’ socio-demographic characteristics are summarized in Table 1.
Association of income with socio-demographic characteristics
More than half of the households were placed in rural settings (53.7%). The majority of the participants were married (66.5%), only 8.5% were unemployed, while their educational level was almost equally distributed into three levels (low 30.5%, medium 35% and high 34.5%). The majority of participants belonged to the lower income level (476 subjects, 58.6%), 200 (24.6%) to the medium and 136 (16.7%) to the higher income level. Low income level was more frequent among females (p=0.003), younger or older participants (p<0.001), unmarried family status (p<0.001), inhabitants of rural areas (p<0.001) and unemployed (p<0.001); income level has been also negatively associated with education (p<0.001) (Table 1).
Association of income with lifestyle indicators
Regarding lifestyle and other habits, 37.1% of the sample was current smokers, 21.8% had high alcohol consumption and 32.1% had high coffee consumption. The majority of participants reported low adherence to Mediterranean diet (77.6%) and low physical activity (83.3%). Based on participants’ self-reported sleep duration, the prevalence of short and long sleep duration was 22.3% and 13.3%, respectively. The association of income with these characteristics is displayed in Table 2. Low and medium income level was associated with a higher prevalence of high alcohol consumption (p=0.030), low adherence to Mediterranean diet (p=0.016), low physical activity (p<0.001) and short and long nocturnal sleep duration (p<0.001). The prevalence of high coffee consumption was greater in low and high income levels compared to medium level (p=0.014), while there was a trend towards higher prevalence of smoking in low and medium income levels compared to high level (p=0.085).
Association of income with health related characteristics
Regarding to the self-reported health indices, 180 participants (22.2%) reported bad health. In the univariate statistical analysis, income was strongly associated with self-reported health (p<0.001); 34.5% of the participants in the low income level reported bad health compared to only 6% and 2.9% in the medium and high income level, respectively. This negative association between income and self-reported health remained in the multivariate analysis even after adjusting for all possible confounders; in particular, in participants of the low income group a significantly higher likelihood for reporting bad health compared to those of medium (aOR=7.92, 95% CI=4.14-15.14, p<0.001) and high (aOR=8.56, 95% CI=2.82-25.96, p<0.001) income group.
Prevalence of hypertension, dyslipidemia, diabetes, obesity and CVD was 34.2%, 33.4%, 10.7%, 35.0% and 9.5%, respectively. The association between income and cardiovascular risk factor profile is shown in Tables 2-4. Participants’ income was inversely associated with the prevalence of hypertension (p=0.028), dyslipidemia (p<0.001), diabetes (p<0.001) and obesity (p<0.001). Low income level was associated with a 62%-increase in the risk of hypertension compared to high income level (OR=1.62, 95% CI=1.06-2.47, p=0.027), although this association did not remain significant after adjusting for other potential confounders (aOR=1.06, 95% CI=0.66-1.72, p=0.804), in particular when subjects’ age was included in the multivariate logistic model.
The risk of dyslipidemia was 3.6-times higher in the low income level (OR=3.62, 95% CI=2.18-6.03, p<0.001) and three-times higher in the medium income level (OR=2.99, 95% CI=1.71-5.22, p<0.001) compared to high income level; only the association between low and high income level remained significant after adjusting for other potential confounders (aOR=2.50, 95% CI=1.29-4.85, p=0.007).
The 5-fold increased risk for diabetes (OR=5.05, 95% CI=2.70-9.45, p<0.001), which was observed in the low income level compared to medium or high level, persisted even after adjusting for all other variables, but with smaller odds ratio (aOR=3.58, 95% CI=1.75-7.32, p<0.001).
Obesity was almost three-times more frequent in the low (OR=2.94, 95% CI=1.82-4.74, p<0.001) and in the medium income level (OR=2.86, 95% CI=1.69-4.84, p<0.001) compared to high income level. After adjustment for all other variables, the inverse association between income and obesity persisted, but it was attenuated in particular when subjects’ adherence to Mediterranean diet and physical activity were included in the multivariate logistic model; a twofold risk of obesity was found in low income level compared to high level (aOR=1.97, 95% CI=1.04-3.36, p=0.038), while there was a tendency towards higher risk of obesity in medium income level compared to high income level (aOR=1.73, 95% CI=0.92-3.26, p=0.087).
An overall 3-fold increased risk for CVD was observed in low income level compared to medium (aOR=2.95, 95% CI=1.47-5.91, p=0.002) and high (aOR=3.04, 95% CI=1.24-7.44, p=0.015) income level, even after adjusting for all demographic and behavioral risk factors.
Association of income with mental health
The internal consistency of Zung self-rating Anxiety Scale and Beck Depression Inventory (BDI) was very high (Cronbach’s á coefficient was 0.84 and 0.88, respectively). The prevalence of anxiety was 32.3% and the prevalence of depression was 26.6%. The association between income and psychological profile is also shown in Tables 2-4. The prevalence of anxiety was higher in the low income level compared to medium and high income levels (36.6% vs 26.0% and 26.5%, p<0.001), while participants’ income was inversely associated with the prevalence of depression (36.1%, 15.5% and 9.6% in low, medium and high income level, p<0.001). Multivariate logistic regression analyses revealed that lower income level was associated with significantly increased odds of: (i) anxiety compared to medium (aOR=1.73, 95% CI=1.14-2.61, p=0.010) and high (aOR=1.97, 95% CI=1.13-3.43, p=0.017) income levels; and (ii) depression compared to medium (aOR=3.66, 95% CI=2.24-5.96, p<0.001) and high (aOR=4.88, 95% CI=1.45-9.73, p<0.001) income levels.
Association of income with sleep disturbances
Regarding to the sleep disturbances, symptoms of daytime sleepiness and insomnia were found in the 9.9% and 20.7% of the entire sample, respectively. Poor sleep quality was found in 40.9%, while high risk for obstructive sleep apnea in 38.9% of the sample. The internal consistency of all four questionnaires was very high (Cronbach’s á coefficient was 0.75 for ESS, 0.92 for AIS, 0.85 for PSQI, and 0.87 for BQ. Participants’ income was inversely associated with the prevalence of daytime sleepiness (p=0.028), insomnia (p<0.001), bad sleep quality (p<0.001) and high risk for OSA (p<0.001) (Tables 2 and 3).
Multivariate logistic regression analysis, adjusting for all variables, revealed that (i) participants in the low income level had a higher likelihood for having daytime sleepiness (aOR=2.24, p=0.014), insomnia (aOR=1.69, p=0.019) and high risk for OSA (aOR=1.70, p=0.012) compared to those of medium income level, (ii) participants in the low income level had a higher likelihood for having daytime sleepiness (aOR=2.35, p=0.032), insomnia (aOR=3.48, p<0.001), poor sleep quality (aOR=5.44, p<0.001) and high risk for OSA (aOR=3.35, p<0.001) compared to those of high income level and (iii) participants in the medium income level had a higher likelihood for having poor sleep quality (aOR=2.65, p=0.033) and high risk for OSA (aOR=2.04, p=0.018) compared to those of high income level (Table 4).
Association of income with healthcare utilization
Regarding to the utilization of primary and secondary healthcare, 49.4% of the sample population utilized primary healthcare during the past month, and 13.1% reported having been admitted to hospital in the past year. The association between income and healthcare utilization is shown in Tables 2-4. Participants’ income was negatively associated with the use of PHC services (56.5%, 47.0% and 27.9% in low, medium and high income level, p<0.001) and hospitalization (16.0%, 9.0% and 8.8%, p=0.014). Both associations remained significant after adjusting for all other variables; in particular, compared to subjects of medium and high income level, those of the low income level had a higher likelihood for primary healthcare utilization (aOR=1.44, p=0.046 and aOR=3.56, p=0.017) and hospitalization (aOR=1.99, p<0.001 and aOR=2.49, p=0.010). Moreover, subjects of medium level had a higher likelihood for primary healthcare utilization (aOR=2.53, p<0.001) compared to those of high income level.
Lower income was associated with lower likelihood for choosing private primary healthcare (PHC) services (33.5%, 55.3% and 84.2% in low, medium and high income level, p<0.001) and with higher likelihood for choosing public PHC services (71.0%, 48.9% and 15.8%, p<0.001). Both gradient effects of income relationships persisted even after adjusting for all other variables (Table 4). In particular, subjects of low income level were almost 10 times more likely to use a public service (aOR=9.78, p<0.001) and 0.12 times less likely to use a private service (aOR=0.12, p<0.001) compared to those of high income level.
Regarding the selection criteria for health services, univariate statistical analysis showed that subjects of lower income levels were less likely to select health services with shorter waiting list (p<0.001), faster access (p=0.002) and shorter waiting queue (p=0.001) and more likely to select health services with lower financial burden (p<0.001) (Tables 2-3). In multivariate statistical analysis, although a trend for subjects of low income level to select services with shorter waiting list (aOR=0.61, p=0.064) and faster access (aOR=0.64, p=0.063), compared to those of high income level persisted even after adjustment for all other variables, the primary criterion for the selection of PHC services was financial burden. In this regards, subjects of the low income level had a higher likelihood for selecting PHC services with lower financial burden compared to subjects of medium (aOR=1.73, p=0.003) and high income level (aOR=2.72, p<0.001), as well as subjects of medium compared to those of high income level (aOR=1.60, p=0.050) (Table 4).
DISCUSSION
This study highlighted the impact of socio-economic status, as expressed by monthly income, in a large number of self-reported health indices of residents in Northern Greece. In line with previous reports, low income was found to predispose to worse health outcomes (13). Previous studies have attributed this association to the higher prevalence of harmful habits, such as tobacco smoking, alcohol drinking, unhealthy diet and lack of physical activity, among these populations (42-44). Indeed, unhealthy behaviors have previously explained the majority of the association between low socioeconomic status and high mortality in a civil service population in London, UK (45). Two nationally representative longitudinal studies in US adults revealed that, even after controlling for the effect of health behaviors, the mortality disadvantage of both lower income group and least disadvantaged socioeconomic status remained significant (46, 47).
Income, together with education and occupation, are indicators of socioeconomic status, outlining the position that a person holds within the structure of society (9). Socioeconomic status has been previously reported to be adversely associated with a wide range of health problems, such as all-cause mortality (45, 46), cardiovascular disease (45), hypertension (48), diabetes (49), dyslipidemia (50) and obesity (50). In our population, a significant association between income and selfreported health was also noted. Actually, 34.5% of participants in the low income group reported bad health, versus only 6% and 2.9% in the medium and high income level, respectively, a fact verified by multivariate analysis, after adjusting for all possible confounders. As mentioned previously, individuals from the low income group had a significantly higher likelihood to rate their health as bad, compared to those of the medium or high income group. In line with this, participants’ income was inversely associated with the prevalence of hypertension. However, in adjusted multivariate models, the association between hypertension and income did not remain significant. Regarding dyslipidemia only the association between low and high income level remained significant after adjusting for other potential confounders, while for diabetes, a significantly increased risk in the low income group compared to medium or high income was observed, even after adjusting for all other variables. Obesity was initially 3-times more frequent in the low and in the medium income group, compared to high income level. After adjustment for all other variables, this association was still observed, but it was attenuated in particular when subjects’ adherence to Mediterranean diet and physical activity were included in the multivariate logistic model; a two-fold risk of obesity was found in the low income group compared to high level. Finally, an overall 3-fold increased risk for CVD was observed in the low income group compared to mediumand high income group, even after adjusting for all demographic and behavioral risk factors. This provides evidence, which supports the strong association between low income and adverse health outcomes, independently of several confounding factors.
It should be also added that in our sample, lower income was associated with significantly increased odds for anxiety and depression, when compared with scores of individuals with both medium and high income, in line with previous studies that have reported the association between socio-economic inequalities and the development of anxiety and depression (19, 51). Regarding sleep characteristics of our sample, individuals with low income had higher odds for having disorders, such as daytime sleepiness, insomnia, poor sleep quality and high risk for OSA, in comparison with those with high income, a finding confirming previous reports in multiethnic populations, characterized by social diversity (21, 52).
Another notable finding in this study concerns the association between income and healthcare utilization. In this regards, low-income individuals were more likely to use primary health care services. Our findings are in agreement with the results of four previous studies from Greece, which all revealed that low-income groups appear to have increased healthcare utilization (22, 53-55). Moreover, there are reports from numerous European (56-60) and American (61, 62) studies, stating that subjects with a lower socioeconomic status are more likely to visit a general practitioner, despite the important differences in the type of health system between these countries. Our results indicate that the higher utilization rate among lower socio-economic groups is probably due to the greater health needs – in this study expressed in terms of self-rated health status and health problems. Moreover, our finding that hospital admissions occur more frequent in lower income groups is consistent with results from previous European (63, 64) and American (USA/Canada) (65) studies, and, in relation to greater health needs in the lower income group, may reflect the fact that there are no socio-economic barriers in the provision of hospital care. Indeed, access to hospital care in Greece is free of charge, providing also patients with free laboratory and diagnostic tests during their stay.
Certainly, this study has limitations. First, its cross-sectional design, which does not allow to establish clear causal relationships. Indeed, a longitudinal follow-up would add to the current knowledge, regarding the incidence of various health conditions in the three different income groups. Additionally, the self-report of health disorders instead of objective measurement decreases the value of the reported results. On the other hand, among the strengths of the study lie the following: its relatively large sample size and its representativeness, since it includes individuals of both sexes and all age groups, living in rural or urban settings, and the use of a large number of diagnostic tools and questionnaires.
CONCLUSION
In conclusion, this study highlights the fact that low income is an important factor, which adversely affects the health of individuals, via different pathways, such as the adaptation of harmful everyday habits. Large-scale prospective cohort studies are needed to establish these associations in a methodologically robust way.
Conflict of interests: none declared
Financial support: none declared.
TABLE 1.
Association of participants’ income with their sociodemographic characteristics
TABLE 2.
Association of subjects’ income with lifestyle factors, health characteristics and healthcare utilization
TABLE 3.
Association of subjects’ income with their health characteristics and healthcare utilization expressed as crude odds ratios (OR) with 95% confidence intervals (95% CIs) obtained by means logistic regression analysis.
TABLE 4.
Association of subjects’ income with their health characteristics and healthcare utilization expressed as adjusted odds ratios (aOR) with 95% confidence intervals (95% CI) obtained by means multivariate logistic regression analysis.
Contributor Information
Orestia ZISSIMOPOULOU, Laboratory of Medical Statistics, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Eleni LEONTIDOU, Laboratory of Medical Statistics, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Dimitrios TSIPTSIOS, Neurophysiology Department, South Tyneside & Sunderland NHS Foundation Trust, Sunderland, United Kingdom.
Apostolos MANOLIS, Laboratory of Medical Statistics, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Dimitrios IOANNIDES, Department of Economics, University of Macedonia, Thessaloniki, Greece.
Ioanna TRYPSIANI, Laboratory of Medical Statistics, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Paschalis STEIROPOULOS, Sleep Unit and Pulmonology Clinic, Medical School, Democritus University of Thrace.
Theodoros C. CONSTANTINIDIS, Laboratory of Hygiene and Environmental Protection, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
Gregory TRIPSIANIS, Laboratory of Medical Statistics, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Evangelia NENA, Laboratory of Social Medicine, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
References
- 1.Organization for Economic Co-operation and Development (OECD) Society at a Glance 2014. Available from https://read.oecd-ilibrary.org/social-issues-migration-health/society-at-a-glance-2014_soc_glance-2014-en#page1. Accessed on November 3rd, 2020. 2020.
- 2.Andriopoulou E, Karakitsios A, Tsakloglou P. Inequality and Poverty in Greece: Changes in Times of Crisis. In: Katsikas D, Sotiropoulos D, Zafiropoulou M, eds. Socioeconomic Fragmentation and Exclusion in Greece under the Crisis. New Perspectives on South-East Europe. Palgrave Macmillan/Cham. 2018. pp. 23–54.
- 3.Organization for Economic Co-operation and Development (OECD) Youth unemployment rate. 2018 figures. Available from: https://data.oecd.org/unemp/youthunemployment- rate.htm#indicator-chart. 2020.
- 4.Mitrakos T. Inequality, poverty and living conditions in Greece: Recent developments and prospects. Social Cohesion and Development. 2013;1:37–58. [Google Scholar]
- 5.Eurostat. In-work poverty in the EU. 2017 figures. Available from: https://ec.europa.eu/eurostat/web/ products-eurostat-news/-/DDN-20180316-1. Accessed on November 3rd, 2020. 2020.
- 6.Eurostat. People at risk of poverty or social exclusion (people with an income below 60% of the national median income). 2017 figures. Available from: https://ec.europa.eu/eurostat/statisticsexplained/ index.php/People_at_risk_of_ poverty_or_social_exclusion. Accessed on November 3rd, 2020. 2020.
- 7.Eurostat. In-work poverty in the EU. 2017 figures. Available from: https://ec.europa.eu/eurostat/web/ products-eurostat-news/-/EDN-20180426-1. Accessed on November 3rd, 2020. 2020.
- 8.Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;16:1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch J, Kaplan G. Socioeconomic position: Social epidemiology. New York: Oxford University Press. 2000.
- 10.Rethemiotaki I. Diabetes Mellitus and Stroke-A cross Sectional Study of 2.5 Million Adults in the United States. Maedica. 2020;1:24–31. doi: 10.26574/maedica.2020.15.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Lee B, Park M, et al. Prevalence of chronic disease and its controlled status according to income level. Medicine. 2016;95:44. doi: 10.1097/MD.0000000000005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanagh A, Bentley RJ, Turrell G. et al. Socioeconomic position, gender, health behaviours and biomarkers of cardiovascular disease and diabetes. Soc Sci Med. 2010;71:1150–1160. doi: 10.1016/j.socscimed.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Tang KL, Rashid R, Godley J, A Ghali WA. Association between subjective social status and cardiovascular disease and cardiovascular risk factors: a systematic review and meta-analysis. BMJ Open. 2016;6:e010137. doi: 10.1136/bmjopen-2015-010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstrom B, Fredlund P. Self rated health: Is it a good predictor of subsequent mortality among adults in lower as well as in higher social classes? J Epidemiol Community Health. 2001;55:836–840. doi: 10.1136/jech.55.11.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au N, Johnston DW. Self-assessed health: what does it mean and what does it hide? Soc Sci Med. 2014;121:21–8. doi: 10.1016/j.socscimed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Charonis A, Kyriopoulos I, Spanakis M, et al. Subjective social status, social network and health disparities: empirical evidence from Greece. Int J Equity Health. 2017;1:40. doi: 10.1186/s12939-017-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molarius A, Berglund K, Eriksson C, et al. Socioeconomic conditions, lifestyle factors, and self-rated health among men and women in Sweden. Eur J Public Health. 2006;17:125–133. doi: 10.1093/eurpub/ckl070. [DOI] [PubMed] [Google Scholar]
- 18.Lantz PM, Lynch JW, House JS, et al. Socioeconomic disparities in health change in a longitudinal study of US adults: the role of health-risk behaviors. Soc Sci Med. 2001;53:29–40. doi: 10.1016/s0277-9536(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 19.Green MJ, Benzeval M. The development of socioeconomic inequalities in anxiety and depression symptoms over the life course. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1951–1961. doi: 10.1007/s00127-013-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorant V, Deliège D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;2:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 21.Grandner M, Patel N, Gherman P, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;5:470–478. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geitona M, Zavras D, Kyriopoulos J. Determinants of healthcare utilization in Greece: Implications for decision-making, Eur J Gen Pract. 2007;3:144–150. doi: 10.1080/13814780701541340. [DOI] [PubMed] [Google Scholar]
- 23.Zavras D, Tsiantou V, Pavi E, et al. Impact of economic crisis and other demographic and socio-economic factors on self-rated health in Greece. Eur J Public Health. 2012;2:206–210. doi: 10.1093/eurpub/cks143. [DOI] [PubMed] [Google Scholar]
- 24.Liaropoulos L, Siskou O, Kaitelidou D, et al. Informal payments in public hospitals in Greece. Health Policy. 2008;87:72–81. doi: 10.1016/j.healthpol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Panagiotakos D, Georgousopoulou E, Notara V, et al. Education status determines 10-year (2002–2012) survival from cardiovascular disease in Athens metropolitan area: the ATTICA study, Greece. Health Soc Care Community. 2016;3:334–344. doi: 10.1111/hsc.12216. [DOI] [PubMed] [Google Scholar]
- 26.Panagiotakos D, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;4:335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation presented at: the WHO, June 3–5, 1997, Geneva, Switzerland, Publication WHO/NUT/NCD/ 98.1. 1997. [PubMed]
- 28.Samakouri M, Bouhos G, Kadoglou M, et al. Standardization of the Greek version of Zung’s Self-rating Anxiety Scale (SAS). Psychiatriki. 2012;3:212–220. [PubMed] [Google Scholar]
- 29.Zung WWK. How normal is anxiety? Upjohn: Durham. 1980.
- 30.Jemos J. Beck Depression Inventory, validation in a Greek sample. Athens University Medical School. 1984.
- 31.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 32.Lasa L, Ayuso-Mateos JL, Vazquez-Barquero JL, et al. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57:261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 33.Tsara V, Serasli E, Amfilochiou A, et al. Greek version of the Epworth Sleepiness Scale. Sleep Breath. 2004;8:91–95. doi: 10.1007/s11325-004-0091-6. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Johns MW. Sleep propensity varies with behaviour and the situation in which it is measured: the concept of somnificity. J Sleep Res. 2002;11:61–67. doi: 10.1046/j.1365-2869.2002.00274.x. [DOI] [PubMed] [Google Scholar]
- 36.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–60. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 37.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55:263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 38.Kotronoulas GC, Papadopoulou CN, Papapetrou A, Patiraki E. Psychometric evaluation and feasibility of the Greek Pittsburgh sleep quality index (GR-PSQI) in patients with cancer receiving chemotherapy. Support Care Cancer. 2011;19:1831–1840. doi: 10.1007/s00520-010-1025-4. [DOI] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Bouloukaki I, Komninos ID, Mermigkis C, et al. Translation and validation of Berlin questionnaire in primary health care in Greece. BMC Pulm Med. 2013;13:1. doi: 10.1186/1471-2466-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 42.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Affairs. 2002;2:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 43.Phelan JC, Link BG, Diez-Roux A, et al. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45(3):265–285. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- 44.Syme SL. Reducing racial and social-class inequalities in health: the need for a new approach. Health Affairs. 2008;2:456–459. doi: 10.1377/hlthaff.27.2.456. [DOI] [PubMed] [Google Scholar]
- 45.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandi A, Glymour MM, Subramanian SV. Association among socioeconomic status, health behaviors, and all-cause mortality in the United States. Epidemiology. 2014;25:170–177. doi: 10.1097/EDE.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 47.Lantz PM, Golberstein E, House JS, Morenoff J. Socioeconomic and behavioral risk factors for mortality in a national 19-year prospective study of U.S. adults. Soc Sci Med. 2010;70:1558–1566. doi: 10.1016/j.socscimed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng B, JinY, Li G, et al. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–229. doi: 10.1097/HJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 49.Bird Y, Lemstra M, Rogers M, Moraros J. The relationship between socioeconomic status/income and prevalence of diabetes and associated conditions: A cross-sectional population-based study in Saskatchewan, Canada. Int J Equity Health. 2015;14:93. doi: 10.1186/s12939-015-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manuck SB, Phillips JE, Gianaros PJ, et al. Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosom Med. 2010;1:35–45. doi: 10.1097/PSY.0b013e3181c484dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almeida-Filhoa N, Lessa I, Magalhães L, et al. Social inequality and depressive disorders in Bahia, Brazil: interactions of gender, ethnicity, and social class. Soc Sci Med. 2004;7:1339–1353. doi: 10.1016/j.socscimed.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Patel NP, Grandner MA, Xie D, et al. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. doi: 10.1186/1471-2458-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsaganis M, Mitrakos T. [Determinants of household expenditure in Greece. ] Econ Policy Stud. 2000;5:119–148. [Google Scholar]
- 54.Mergoupis T. Access to health care in Greece: a micro-level analysis. Proceedings of the First Conference of the Hellenic Social Policy Association. 2001.
- 55.Kyriopoulos J, Gregory S, Economou Ch. Health and health services in Greece. Athens: National School of Public Health; 2002.
- 56.Fernandez de la Hoz K, Leon DA. Self-perceived health status and inequalities in use of health services in Spain. Int J Epidemiol. 1996;25:593–603. doi: 10.1093/ije/25.3.593. [DOI] [PubMed] [Google Scholar]
- 57.Piperno A, Di Orio F. Social differences in health and utilization of health services in Italy. Soc Sci Med. 1990;31:305–312. doi: 10.1016/0277-9536(90)90277-y. [DOI] [PubMed] [Google Scholar]
- 58.Carr-Hill RA, Rice N, Roland M. Socioeconomic determinants of rates of consultation in general practice based on fourth national morbidity survey of general practices (see comments). BMJ. 1996;312:1008–12. doi: 10.1136/bmj.312.7037.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nolan B. General practitioner utilization in Ireland: the role of socio-economic factors. Soc Sci Med. 1994;5:711–716. doi: 10.1016/0277-9536(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 60.Van der Heyden JHA, Demarest S, Tafforeau J, Van Oyen H. Socio-economic differences in the utilisation of health services in Belgium. Health Policy. 2003;65:153–165. doi: 10.1016/s0168-8510(02)00213-0. [DOI] [PubMed] [Google Scholar]
- 61.Travassos C, Viacava F, Pinheiro R, Brito A. Utilization of health care services in Brazil: gender, family characteristics and social status. Rev Panam Salud Publica. 2000;11:365–373. doi: 10.1590/s1020-49892002000500011. [DOI] [PubMed] [Google Scholar]
- 62.Newbold KB, Eyles J, Birch S. Equity in health care: methodological contributions to the analysis of hospital utilization within Canada. Soc Sci Med. 1995;40:1181–1192. doi: 10.1016/0277-9536(94)00229-m. [DOI] [PubMed] [Google Scholar]
- 63.La Vecchia C, Negri E, Pagano R, Decarli A. Education, prevalence of disease, and frequency of health care utilisation. The 1983 Italian national health survey. J Epidemiol Community Health. 1987;2:161–165. doi: 10.1136/jech.41.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keskimaki I, Salinto M, Aro S. Socioeconomic equity in Finnish hospital care in relation to need. Soc Sci Med. 1995;41:425–431. doi: 10.1016/0277-9536(94)00339-u. [DOI] [PubMed] [Google Scholar]
- 65.Katz SJ, Hofer TP, Manning WG. Hospital utilization in Ontario and the United States: The impact of socioeconomic status and health status. Can J Public Health. 1996;4:253–256. [PubMed] [Google Scholar]