Abstract
Objective
The lockdown due to COVID-19 pandemic had a strong impact on daily habits, emotional experience, mental health and sleep. A large body of evidence suggests that dreams are affected by both waking experiences and sleep pattern. In this view, the lockdown should have induced intense modifications in dreaming activity. The aim of the study was to assess dream features during the lockdown in Italy.
Methods
We used an online survey to collect self-reported demographic, clinical, sleep and dream data. Our sample included 1091 participants.
Results
Results point to an increased dream frequency, emotional load, vividness, bizarreness and length during the lockdown, compared to a pre-lockdown period. Higher dream frequency and specific qualitative features were found in females and subjects with poor sleep quality, nocturnal disruptive behaviours and depressive symptoms. Most of the dream features assessed during the lockdown were predicted by age, gender, depressive symptoms, presence/absence of other people at home, and territorial area. A specific focus on sleep features revealed that sleep duration and several sleep quality indexes were the best predictors of dream variables. During the lockdown, dreams were also characterized by increased negative emotions, which were particularly frequent in females, younger adults, and participants with poor sleep quality, nocturnal disruptive behaviours, anxiety and depressive symptoms.
Conclusions
Our results confirm the hypothesis of a strong influence of the pandemic on dreaming, supporting both the hypothesis of continuity between wake and sleep mental processes and the view of a crucial influence of sleep quality and duration on dreaming activity.
Keywords: Dreams, COVID-19, Lockdown, Continuity hypothesis, Sleep pattern, Emotions
1. Introduction
The Coronavirus Virus disease 2019 (COVID-19), a respiratory illness due to the coronavirus SARS-Cov-2, has been declared a pandemic by the World Health Organization in March 2020. The high number of contagions and deaths led many countries worldwide to strong countermeasures (ie, lockdown/quarantine). Albeit heterogeneous, current findings on the general population point to a COVID-19 related reduction of the psychological well-being, particularly with increased symptoms of stress, depression and anxiety (for a review, see Ref. [1]).
At the beginning of April 2020, a task force of the European Academy of Cognitive-Behavioural Treatment of Insomnia warned about the possible effects of the forced confinement on sleep, suggesting that such peculiar situation may have both negative and positive consequences on sleep quality and daytime functioning [2]. Consistently with this hypothesis, on the one hand the present evidence highlights poor sleep quality and increased sleep disturbances associated with the consequence of the quarantine [[3], [4], [5], [6], [7], [8]]. On the other hand, several findings point to an increase of time in bed [5,[7], [8], [9]] and sleep duration [3,7,9] during the lockdown, associated with the higher flexibility of social schedules home-working [3]. Overall, the quarantine mainly led to a sleep pattern characterized by poorer quality and longer duration.
In this context, an interesting phenomenon has been observed during the COVID-19 outbreak: the enormous spread on the web of subjective reports of strong changes in the oneiric activity, with increased dream recall frequency, as well as higher vividness, bizarreness and emotional intensity of their dreams. Such experience led to the rise of many websites that collected dream reports in the COVID-19 period and to a broad worldwide press coverage. This phenomenon is consistent with findings suggesting a strong impact of potentially traumatic collective experiences on dreaming [[10], [11], [12], [13]].
At present, evidence about the phenomenology of dreaming activity during the COVID-19 lockdown is limited. A study focused on psychological distress and coping styles during the early period of the COVID-19 pandemic conducted on 1599 Chinese participants showed pandemic-related dreams in 38% of the sample, particularly in more distressed subjects [14]. Another study found that posttraumatic patients exhibited increased symptomatology (with disturbing dreams and nightmares) during the COVID-19 outbreak [15]. Only a few studies had dream activity during the pandemic as the main focus. Schredl and Bulkeley [16] assessed the effect of the pandemic in a large U.S. sample (N = 3031), confirming its strong impact on dreaming, particularly in subjects more affected by the pandemic, which showed higher dream recall, negative emotions in dreams and pandemic-related dream content. A higher effect of the pandemic on dreaming was also observed in women and participants with a high education level [16]. An Italian study on 796 participants focused on dream content, assessed with a dream questionnaire and the collection of the most recent dream [17]. The authors found that women, compared to men, had higher dream recall frequency, emotional intensity, and predominant negative emotions in dreams. Of the reported dreams, 20% were explicitly COVID-19 related, and participants having an acquaintance infected or deceased for the virus exhibited high emotional intensity and sensory impressions in the reported dreams. Finally, dreams were often set in an external location and were characterized by negative emotions [17]. The existence of a trend by gender was specifically assessed by Barrett [18] in a sample of COVID-19 related dream reports compared with normative dreams, showing that women exhibited higher rates of negative emotions, anxiety, sadness, anger, body content, references to biological processes, health and death in their dreams. MacKay and DeCicco [19] focused on dream imagery in university students at the beginning of the Canadian COVID-19 experience compared with a control group, showing an increase of COVID-19 related dream imagery, location changes and animal dream imagery, which several authors consider associated with daytime anxiety [20]. Finally, Pesonen and coworkers [21] assessed dream content during the COVID-19 pandemic with a network analysis approach, showing several pandemic-related contents associated with distressing events. Taken together, these findings highlight a strong effect of the pandemic on dream quality and quantity, and results have been mainly interpreted in line with the “continuity hypothesis” [22]. However, none of these studies assessed the relation between dreaming activity and sleep pattern, so it is not possible to conclude if daily experiences directly and exclusively affect the oneiric activity, or if also the sleep features impact on dreaming during the lockdown and in which way [23]. Moreover, starting from the observation of increased stress, anxiety and depression during the outbreak [1] and considering the hypothetical role of dreams in emotional processes [24], it is crucial to assess these clinical symptoms and their relations with dreaming.
One way to interpret such perceived intensification of oneiric activity is the “continuity hypothesis”, which claims that dreams reflects waking experience and in particular the emotional features of daily mental activity [22,25] and, from another standpoint, the existence of a continuum between waking and sleeping mental and neurobiological functioning [24,26]. Moreover, several findings support the notions that dreams may have a role in emotional memory and emotional regulation processes [24,27,28]. According to this view, it could be suggested that an intense and emotionally relevant experience like the pandemic-related lockdown should have a direct impact on dreaming activity, in particular concerning its emotional aspects.
On the other hand, another explanation for the intense changes in dreaming activity experienced during the lockdown concerns sleep patterns changes. Indeed, longer sleep duration is associated with higher dream recall [29,30]. Moreover, also sleep patterns characterized by high fragmentation [31] or high arousal [32,33] are associated with increased dream recall frequency, while recovery sleep after sleep deprivation (which usually exhibits a reduced number of awakenings) is associated with a near-complete abolition of dream recall after morning awakening [34]. Starting from the observation that the sleep pattern during COVID-19 quarantine seems characterized by poor quality and increased duration, we propose that a combination of these factors can explain the increased dream recall frequency during the lockdown. It is worth noting that the two proposed explanations (ie, continuity hypothesis and sleep pattern changes) are not mutually exclusive.
The aims of the present study were: a) to assess how the dreaming experience has changed during the COVID-19 lockdown compared to the pre-lockdown period in an Italian sample, considering the relation with demographic, clinical and sleep variables; b) to assess what variables predict dreams features during the pandemic; c) to assess the emotional tone of dreams during the pandemic and its relation with daily experience, clinical variables and sleep features. Our hypothesis states that changes in dream activity will be explained by both (A) waking experience and, in particular, the emotional features of daily mental activity and (B) parallel changes of sleep pattern.
2. Methods
2.1. Design and participants
To collect data in an Italian sample, we used an anonymous online survey, implemented with Google Forms and shared on several social-media. The survey was enabled from April 23, 2020. We considered only data collected until the end of the Italian lockdown (May 4, 2020). Each participant completed the survey after reading the informed consent form and declaring a) the explicit agreement to participate in the research and b) age ≥18 y. At any moment, the participant could withdraw from the procedure without data saving. No monetary compensation was provided for the participation in the survey. The study was approved by the Institutional Review Board of the Department of Psychology of the Sapienza University of Rome (#0000646/2020) and conducted in accordance with the Declaration of Helsinki.
2.2. Materials
Demographic data and COVID-19 related information: a questionnaire was administered to assess demographic data (ie, age, gender, education, occupation). Moreover, several COVID-19 and lockdown-related information were collected.
Anxiety symptoms: the State-Trait Anxiety Inventory (STAI-Y, I-II; [35]) was administered to assess anxiety symptoms. It is a self-reported anxiety assessment questionnaire, consisting of 40 items: 20 for the STAI-Y I version and 20 for the STAI-Y II version. The two versions evaluate state-like and trait-like anxiety, respectively. The participant is asked to indicate, choosing on a 4-point Likert scale (from nothing to very much), how much each item reflects his psycho-physical state at the administration time. Scores ≥40 indicate significant anxiety levels.
Depressive symptoms: the Beck Depression Inventory-II (BDI-II [36]; was administered to assess depressive symptoms. It is a self-reported questionnaire consisting of 21 multiple-choice questions. Each answer provides scores from 0 to 3, which positively correlate with the severity of depressive symptoms. Total scores >13 are indicative of the presence of depressive disorder.
Sleep Quality: the Pittsburgh Sleep Quality Index (PSQI; [37]) was administered to investigate the sleep quality during the last month preceding the assessment (ie, during the lockdown period). It is a self-reported questionnaire consisting of 19 items. The results are about partial scores in seven sub-scales and a sleep quality global score. The subscales measure subjective sleep quality (C1), sleep latency (C2), sleep duration (C3), habitual sleep efficiency (C4), sleep disturbances (C5), use of sleep medications (C6), daytime dysfunction (C7). A PSQI global score >5 indicates a subjectively perceived poor sleep quality. With the aim to assess the presence of trauma-related subjective sleep disturbances, we also administered the PSQI-Addendum (PSQI-A; [38]). The PSQI-A represents a specific self-report measure for the assessment of 7 disruptive nocturnal behaviours common among subjects with Post-Traumatic Stress Disorder (PTSD): flashes, general nervousness, memories or nightmares of traumatic experience, severe anxiety or panic not related to traumatic memories; bad dreams not related to traumatic memories, episodes of terror or screaming during sleep without fully awakening, episodes of acting out dreams, such as kicking, punching, running, or screaming. A PSQI score ≥4 is highly predictive for discriminating between subjects with and without PTSD [38].
Dream features: the participant was asked to self-report several features of his/her dreaming activity for two time periods: a) during the last month (Lockdown) and b) during the month that preceded the beginning of the Italian lockdown (Pre-Lockdown). Specifically, we asked the participant to score dream frequency on a 7-points Likert scale (0–6) and several qualitative dream features (emotional load, vividness, bizarreness, length) on a 6-points Likert scale (1–6) [34,[39], [40], [41], [42]]. Moreover, for each time period, the participants were asked to report the most frequent emotion in their dream, choosing from the following list: Happiness, Sadness, Fear, Anger, Disgust, Pleasure, Guilt, Shame, Surprise. Other qualitative dream measures have been collected as part of a wider project with different objectives regarding the pandemic's influence on dreaming activity in Italian population. These qualitative data will not be analysed and reported here.
2.3. Statistics
The statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 25.0 and Matlab R2011b.
Paired Student's t-tests were performed on each dream variable between Lockdown and Pre-Lockdown to assess pandemic-related changes in dream phenomenology. Subsequently, the Lockdown vs. Pre-Lockdown difference in each dream feature score was calculated, and the sample was divided into subgroups according to the following demographic, clinical and sleep variables: Gender (Males vs. Females), Age (18–24 y vs. 25–29 y vs. 30–39 y vs. ≥40 y); Exposure to COVID-19 (Not employed vs. Not exposed vs. Exposed), geographical area (North vs. Centre vs. South), daily time spent with digital media (<Median Time vs. ≥ Median Time), cohabitation (With others vs. Alone), PSQI global score (PSQI ≤5 vs. PSQI >5), PSQI-A global score (PSQI-A ≤ 3 vs. PSQI-A >3), STAI-I (STAI-I ≤ 39 vs. STAI-I > 39), STAI-II (STAI-II ≤ 39 vs. STAI-II > 39), BDI (BDI ≤ 13 vs. STAI-II > 13). Age ranges were defined with the aim to have a number of subjects as similar as possible between each subgroup. For each variable of interest, unpaired Student's t-test or one-way between-subjects ANOVAs (according to the number of subgroups) were performed to compare the different subgroups, with the Lockdown vs. Pre-Lockdown difference in each dream feature as the dependent variable.
Multiple linear regressions were performed to assess the best predictors for each dream feature reported during the lockdown period. In the first set of analysis, each multiple regression model had a dream feature as the dependent variable and the following as independent variables: age, gender, COVID-19 exposure, mean daily time passed with digital media, geographical area, cohabitation during the lockdown, PSQI global score, STAI-I score, STAI-II score, BDI score. The second set of multiple regressions was specifically focused on investigating the best predictors for each dream feature among different variables of the sleep pattern. In this case, each multiple regression model had a dream feature as the dependent variable and the different subscale of the PSQI as independent variables. It is worth noting that the PSQI-A global score has been not used as a possible predictor in our regression models. This choice has been made considering that several items of the PSQI-A assess nightmares and bad dreams. Consistently, we observed a strong positive correlation between dream variables and PSQI-A scores (Frequency: r = 0.387, p < 0.0001; Emotional Load: r = 0.476, p < 0.0001; Vividness: r = 0.328, p < 0.0001; Bizarreness: r = 0.295, p < 0.0001; Length: r = 0.317, p < 0.0001) but it is actually tautological, and we consider it as a possible confounding factor in a multiple regression model. For each multiple regression, collinearity was controlled using a normal linear regression collinearity diagnostic test. No variance inflation factor ≥5 was observed.
To assess the emotional tone of dreams during the pandemic, the percentage of each emotion reported in dreams during Lockdown and Pre-Lockdown was calculated, providing a first descriptive view on the distribution of the emotions in the considered time period. Then, emotions were divided into positive (Happiness, Pleasure, Surprise) and negative ones (Sadness, Fear, Anger, Disgust, Guilt, Shame). An exact McNemar test was performed to assess changes in the proportion of dream positive and negative emotions between Pre-Lockdown and Lockdown. Then, the proportion of positive and negative emotions during the Lockdown period was compared (χ 2) between the subgroups formed according to demographic, clinical, and sleep features, previously used for the t-tests.
For each analysis, uncorrected p-values < 0.05 were considered statistically significant.
3. Results
3.1. Demographic characteristics
We received 1.236 completed questionnaires during the period 23/04/2020–01/06/2020. Since the Italian lockdown ended the 04/05/2020, we considered only questionnaires received before this date (58 questionnaires completed after 04/05/2020 were excluded). Only 3 subjects were infected by COVID-19, so we decided to exclude them from the analyses. We also excluded: 45 subjects that completed the questionnaire more than one time; 15 subjects that clearly reported significant events not related with the pandemic during the last month (specifically: death, pregnancy, and abortion); 23 subjects that were located outside of Italy. Our final sample included 1.091 participants.
Table 1 reports information about the demographic characteristics of our sample. Considering gender, 785 were females (71.95%). The mean age ± SE was 31.3 ± 0.33 y (range 18–88 y). The most represented age was 18–24 range (30.16%), followed by 25–29 (29.51%), 30–39 (22.73%) and ≥40 (17.60%). The larger part of the sample achieved a high school degree (38.22%), followed by undergraduates (24.01%), graduates (23.65%) and post-graduates (12.83%); only 13 participants had middle school (1.19%), and 1 participant had primary school (0.09%) as the maximum educational degree. Considering the occupational status, 582 (53.3%) subjects were employed/self-employed, 371 (34%) were students, 102 (9.3%) were unemployed, 25 (2.3%) were house husband/wife, and 11 (1%) were retired. Of the considered participants, 244 (22.4%) referred themselves as potentially exposed to COVID-19 cause their job. Only 98 subjects (9%) were in a condition of forced quarantine for suspected infection, and 117 (10.7%) had a relative/friend affected by COVID-19. From a geographical standpoint, 131 (12%) subjects were located in the North of Italy, 549 (50.3%) in the Centre and 411 (37.7%) in the South. Subjects from Sardinia and Sicily were considered in the South group. The larger part of our sample (991 subjects; 90.8%) experienced the lockdown living with other people (family, friends), while 95 (8.7%) participants were alone at home, and 5 (0.4%) did not clearly report this information. The daily mean time of digital media use during the lockdown was 7.56 ± 0.099 h.
Table 1.
Demographic features of the sample.
| Overall sample |
||
|---|---|---|
| N | % | |
| Gender | ||
| Male | 306 | 28.05 |
| Female | 785 | 71.95 |
| Age | (Mean ± SE: 31.3 ± 0.33 y) | |
| 18–24 | 329 | 30.16 |
| 25–29 | 322 | 29.51 |
| 30–39 | 248 | 22.73 |
| ≥40 | 192 | 17.60 |
| Education | ||
| Primary school | 1 | 0.09 |
| Middle school | 13 | 1.19 |
| High school | 417 | 38.22 |
| Undergraduate | 262 | 24.01 |
| Graduate | 258 | 23.65 |
| Post-graduate | 140 | 12.83 |
| Occupation | ||
| Student | 371 | 34 |
| Employed/Self-Employed | 582 | 53.3 |
| Unemployed | 102 | 9.3 |
| House husband/wife | 25 | 2.3 |
| Retired | 11 | 1 |
| Geographical Area | ||
| North | 131 | 12 |
| Centre | 549 | 50.3 |
| South | 411 | 37.7 |
| COVID-19 exposure | ||
| Not employed during lockdown | 256 | 23.46 |
| Not exposed to COVID-19 | 591 | 54.17 |
| Exposed to COVID-19 | 244 | 22.36 |
| Cohabitation during lockdown | ||
| Alone | 95 | 8.7% |
| With others | 991 | 90.8% |
| Information not available | 5 | 0.4% |
| Forced quarantine for suspected COVID-19 infection | ||
| Yes | 98 | 9% |
| No | 993 | 91% |
| Knowing a relative/friend infected by COVID-19 | ||
| Yes | 117 | 10.7% |
| No | 974 | 89.3% |
| Daily time spent with digital media | (Mean ± SE: 7.56 ± 0.099 h) | |
3.2. Sleep quality, anxiety and depression
Table 2 reports the mean values concerning sleep quality and clinical features of our sample, and the proportion of subjects below and above the cut-off for each test. Concerning PSQI scores, they were available for 1084 subjects, of which 638 (58.9%) exhibited poor sleep quality. Data on the PSQI-A were available for 1083 subjects, of which 691 (63.8%) showed a PSQI-A score above the predictive cut-off for discriminating between subjects with and without PTSD. Considering results to the STAI, 783 subjects (71.8%) exhibited state anxiety symptoms, while 737 (67.6%) reported trait anxiety symptoms. Finally, 387 subjects (35.5%) showed the presence of depressive symptoms at the BDI.
Table 2.
Self-reported sleep and clinical characteristics of the sample.
| Overall sample (Mean ± SE) | Subgroups (N; %) | ||
|---|---|---|---|
| PSQIa | |||
| 1 - Self-reported Sleep Quality | 1.4 ± 0.022 | ||
| 2 - Sleep Latency | 1.5 ± 0.031 | ||
| 3 - Sleep Duration | 0.8 ± 0.027 | ||
| 4 - Habitual Sleep Efficiency | 0.7 ± 0.03 | ||
| 5 - Sleep Disturbance | 1.3 ± 0.017 | ||
| 6 - Use of Sleeping Medication | 0.2 ± 0.022 | ||
| 7 - Daytime Dysfunctions | 0.9 ± 0.021 | ||
| PSQI total score | PSQI ≤ 5 | PSQI >5 | |
| 6.8 ± 0.10 | 446; 41.1% | 638; 58.9% | |
| PSQI-Ab | PSQI-A ≤ 3 | PSQI-A >3 | |
| 5.5 ± 0.12 | 392; 36.2% | 691; 63.8% | |
| STAI-I | STAI-I ≤ 39 | STAI-I >39 | |
| 47.5 ± 0.35 | 308; 28.2% | 783; 71.8% | |
| STAI-II | STAI-II ≤ 39 | STAI-II >39 | |
| 45.3 ± 0.35 | 354; 32,4% | 737; 67.6% | |
| BDI-II | BDI ≤ 13 | BDI >13 | |
| 11.9 ± 0.26 | 704; 64.5% | 387; 35.5% | |
Abbreviations: BDI-II, Beck Depression Inventory II; PSQI, Pittsburgh Sleep Quality Index; PSQI-A, PSQI Addendum; STAI, State-Trait Anxiety Inventory.
Calculated on 1084 subjects due to missing values.
calculated on 1083 subjects due to missing values.
3.3. Lockdown-related changes in quantitative and qualitative features of dreams
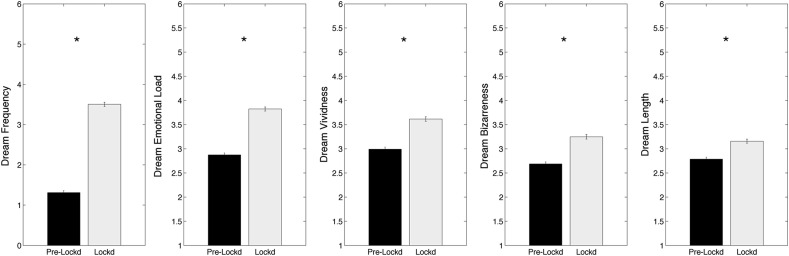
The comparisons (paired t-tests) between lockdown and pre-lockdown dream features (Fig. 1 ), showing a significant lockdown-related increase of dream frequency (mean ± SE: pre-lockdown: 1.31 ± 0.052; lockdown: 3.50 ± 0.052; t 1090 = 34.4; p < 0.000001), emotional load (pre-lockdown: 2.87 ± 0.043; lockdown: 3.82 ± 0.044; t 1090 = 20.7; p < 0.000001), vividness (pre-lockdown: 2.99 ± 0.045; lockdown: 3.61 ± 0.047; t 1090 = 13.8; p < 0.000001), bizarreness (pre-lockdown: 2.69 ± 0.043; lockdown: 3.25 ± 0.049; t 1090 = 12.9; p < 0.000001) and length (pre-lockdown: 2.79 ± 0.039; lockdown: 3.16 ± 0.043; t 1090 = 9.3; p < 0.000001).
Fig. 1.
Results of the comparisons (paired t-tests) between pre-lockdown (black bars) and lockdown (white bars), performed on the dream variables. Each box represents a dream feature. Error bars represent the standard errors. Asterisks index significant differences (p < 0.05).
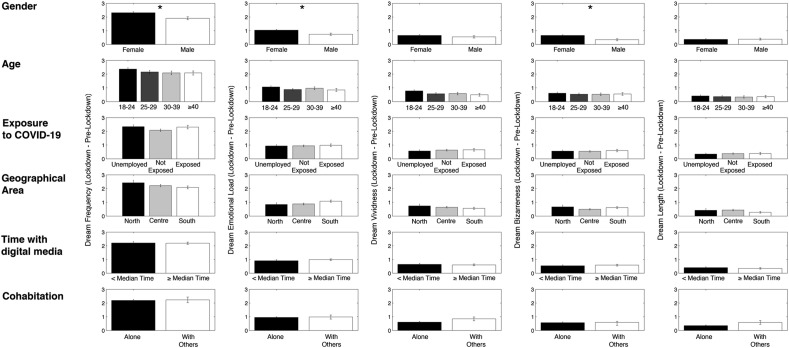
Fig. 2 depicts the results of the comparisons (t-tests and ANOVAs) performed on the lockdown vs. pre-lockdown differences in dream features between groups divided according to demographic variables. The only significant differences were observed considering gender: compared to males, females experienced a significantly higher increase during the lockdown in dream frequency (Males: 1.90 ± 0.11; Females: 2.30 ± 0.077; t 1089 = 2.85; p = 0.004), emotional load (Males: 0.74 ± 0.078; Females: 1.03 ± 0.056; t 1089 = 2.88; p = 0.004) and bizarreness (Males: 0.34 ± 0.073; Females: 0.65 ± 0.053; t 1089 = 3.23; p = 0.001).
Fig. 2.
Results of the comparisons between subgroups form on the basis of several demographic and COVID-19 related variables, performed on the lockdown vs. pre-lockdown differences in dream features. Each column represents a dream feature, each line represents a specific demographic or COVID-19 related variable. When only two subgroups were present, an unpaired t-test was performed on each dream features to compare them. When more than two groups were present, an ANOVA one way was performed to compare them. Error bars represent the standard errors. Asterisks index significant differences (p < 0.05).
Starting from the absence of differences in the ANOVAs, and guided by the fact that North Italy was the territorial area most involved in the sanitary emergency, we also split the sample in “North vs. Other areas”, transforming the variable “geographical area” in a dichotomous one. We compared the two subgroups with unpaired t-test, again showing the absence of significant differences. Similarly, we split the variable “Exposure to COVID” in “Exposed vs. Others”, and the variable “Age” in “<40 y vs. ≥40 y”, for both showing absence of significant differences (unpaired t-tests).
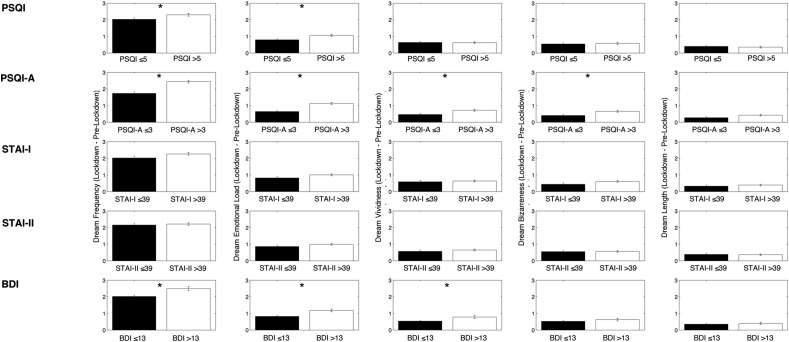
Fig. 3 shows the results of the comparisons (unpaired t-tests) performed on the lockdown vs. pre-lockdown differences in dream features between groups divided according to sleep and clinical variables. Concerning the PSQI, subjects with poor sleep quality (compared with participants with good sleep quality) exhibited a significantly higher lockdown-related increase of dream frequency (PSQI >5: 2.30 ± 0.083; PSQI ≤5: 2.03 ± 0.098; t 1082 = 2.09; p = 0.037) and emotional load (PSQI >5: 1.06 ± 0.061; PSQI ≤5: 0.80 ± 0.07; t 1082 = 2.83; p = 0.005). The analyses on PSQI-A scores shows that subjects with PTSD-related symptoms were characterized by a significantly higher enhancement during the lockdown of dream frequency (PSQI-A >3: 2.45 ± 0.083; PSQI-A ≤3: 1.73 ± 0.095; t 1081 = 5.4; p < 0.000001), emotional load (PSQI-A >3: 1.13 ± 0.061; PSQI-A ≤3: 0.6 ± 0.068; t 1081 = 5.1; p < 0.000001), vividness (PSQI-A >3: 0.72 ± 0.059; PSQI-A ≤3: 0.47 ± 0.069; t 1081 = 2.68; p = 0.007) and bizarreness (PSQI-A >3: 0.66 ± 0.058; PSQI-A ≤3: 0.40 ± 0.065; t 1081 = 2.78; p = 0.006). Participants with depressive symptoms, compared to subjects without depressive symptoms, exhibited an higher increase of dream frequency (BDI >13: 2.50 ± 0.11; BDI ≤13: 2.02 ± 0.076; t 1089 = 3.6; p = 0.0004), emotional load (BDI >13: 1.18 ± 0.082; BDI ≤13: 0.82 ± 0.055; t 1089 = 3.7; p = 0.0002) and vividness (BDI >13: 0.78 ± 0.081; BDI ≤13: 0.54 ± 0.053; t 1089 = 2.6; p = 0.009).
Fig. 3.
Results of the comparisons (unpaired t-tests) between subgroups form on the basis of self-reported sleep and clinical variables, performed on the lockdown vs. pre-lockdown differences in dream features. Specifically, then Pittsburgh Sleep Quality Index (PSQI) and the PSQI-Addendum (PSQI-A) global scores were used as sleep variables, while the global score at the State-Trait Anxiety Inventory I and II (STAI-I and STAI-II) and the Beck Depression Inventory (BDI) were used as clinical variables. Each column represents a dream feature, each line represents a specific sleep or clinical variable. Error bars represent the standard errors. Asterisks index significant differences (p < 0.05).
3.4. Predictor of quantitative and qualitative dream features during the lockdown
Table 3 reports the results of the multiple regressions performed on each dream feature and considering sleep, clinical and demographic variables as predictors. The multiple regression coefficients were statistically significant for each dream feature.
Table 3.
Results of multiple regressions (p < 0.05), considering dream features (frequency, emotional load, vividness, bizarreness, length) as criterion variables and age, gender, PSQI global score, STAI-I and STAI-II score, BDI score, COVID exposure, daily digital media use, geographical area, and cohabitation as predictors. Significant results are indicated in bold.
| Dependent Variables |
Predictors | Beta | Coefficients of Partial Correlation | t | p |
|---|---|---|---|---|---|
|
Dream frequency R = 0.313; adjusted R2 = 0.089; F10,1068 = 11.57; p < 0.0001 |
Age | −0.147 | −0.144 | −4.765 | <0.0001 |
| Gender | −0.170 | −0.172 | −5.710 | <0.0001 | |
| PSQI Global score | −0.018 | −0.016 | −0.516 | 0.606 | |
| STAI-I | 0.040 | 0.024 | 0.776 | 0.438 | |
| STAI-II | −0.017 | −0.010 | −0.313 | 0.755 | |
| BDI | 0.136 | 0.082 | 2.688 | 0.007 | |
| COVID Exposure | −0.017 | −0.018 | −0.576 | 0.565 | |
| Daily digital media use | −0.050 | −0.051 | −1.674 | 0.094 | |
| Area | 0.085 | 0.088 | 2.895 | 0.004 | |
| Cohabitation | 0.075 | 0.077 | 2.516 | 0.012 | |
|
Dream emotional load R = 0.417; adjusted R2 = 0.166; F10,1068 = 22.525; p < 0.0001 |
Age | −0.172 | −0.175 | −5.825 | <0.0001 |
| Gender | −0.151 | −0.160 | −5.281 | <0.0001 | |
| PSQI Global score | 0.067 | 0.062 | 2.044 | 0.041 | |
| STAI-I | 0.081 | 0.051 | 1.662 | 0.097 | |
| STAI-II | −0.023 | −0.014 | −0.452 | 0.651 | |
| BDI | 0.196 | 0.123 | 4.044 | <0.0001 | |
| COVID Exposure | −0.006 | −0.007 | −0.217 | 0.828 | |
| Daily digital media use | 0.044 | 0.047 | 1.531 | 0.126 | |
| Area | 0.067 | 0.073 | 2.395 | 0.017 | |
| Cohabitation | 0.082 | 0.088 | 2.900 | 0.004 | |
|
Dream vividness R = 0.320; adjusted R2 = 0.094; F10,1068 = 12.208; p < 0.0001 |
Age | −0.177 | −0.173 | −5.744 | <0.0001 |
| Gender | −0.149 | −0.151 | −4.997 | <0.0001 | |
| PSQI Global score | −0.008 | −0.007 | −0.242 | 0.809 | |
| STAI-I | −0.022 | −0.013 | −0.424 | 0.672 | |
| STAI-II | −0.039 | −0.022 | −0.728 | 0.467 | |
| BDI | 0.186 | 0.112 | 3.691 | <0.0001 | |
| COVID Exposure | −0.026 | −0.026 | −0.861 | 0.389 | |
| Daily digital media use | 0.005 | 0.005 | 0.158 | 0.875 | |
| Area | 0.093 | 0.097 | 3.188 | 0.001 | |
| Cohabitation | 0.085 | 0.087 | 2.858 | 0.004 | |
|
Dream bizarreness R = 0.287; adjusted R2 = 0.074; F10,1068 = 9.584; p < 0.0001 |
Age | −0.180 | −0.174 | −5.784 | <0.0001 |
| Gender | −0.045 | −0.046 | −1.490 | 0.137 | |
| PSQI Global score | −0.017 | −0.016 | −0.507 | 0.612 | |
| STAI-I | 0.098 | 0.058 | 1.905 | 0.057 | |
| STAI-II | −0.009 | −0.005 | −0.158 | 0.875 | |
| BDI | 0.081 | 0.048 | 1.580 | 0.114 | |
| COVID Exposure | 0.002 | 0.002 | 0.056 | 0.956 | |
| Daily digital media use | 0.032 | 0.032 | 1.061 | 0.289 | |
| Area | 0.110 | 0.113 | 3.728 | <0.0001 | |
| Cohabitation | 0.006 | 0.006 | 0.194 | 0.846 | |
|
Dream length R = 0.298; adjusted R2 = 0.080; F10,1068 = 10.421; p < 0.0001 |
Age | −0.187 | −0.181 | −6.021 | <0.0001 |
| Gender | −0.135 | −0.137 | −4.518 | <0.0001 | |
| PSQI Global score | −0.005 | −0.002 | 0.140 | 0.888 | |
| STAI-I | −0.00008 | −0.00005 | −0.002 | 0.999 | |
| STAI-II | 0.033 | 0.018 | 0.604 | 0.546 | |
| BDI | 0.095 | 0.057 | 1.863 | 0.063 | |
| COVID Exposure | −0.019 | −0.019 | −0.618 | 0.537 | |
| Daily digital media use | −0.019 | −0.020 | −0.640 | 0.522 | |
| Area | 0.067 | 0.070 | 2.286 | 0.022 | |
| Cohabitation | 0.070 | 0.072 | 2.358 | 0.019 |
Abbreviations: BDI-II, Beck Depression Inventory II; PSQI, Pittsburgh Sleep Quality Index; PSQI-A, PSQI Addendum; STAI, State-Trait Anxiety Inventory.
Gender: (1) Female; (2) Male.
COVID Exposure: (1) Others; (2) Exposed; Daily digital media use: (1) Below median time; (2) Above median time.
Area: (1) Others; (2) North.
Cohabitation: (1) With others; (2) Alone.
The partial correlations indicate that:
-
a)
Age, gender, BDI scores, geographical area and cohabitation were predictors of dream frequency and vividness;
-
b)
Age, gender, BDI scores, geographical area, cohabitation, and PSQI scores, were predictors of dream emotional load;
-
d)
Age and geographical area were predictors of dream bizarreness;
-
e)
Age, gender, geographical area and cohabitation were predictors of dream length.
Concerning the multiple regression performed to assess the contribution of specific sleep features (ie, PSQI scales) to oneiric activity during the lockdown, the multiple regression coefficients were statistically significant for each dream feature (Table 4 ). The partial correlations indicate that:
-
a)
Sleep duration and sleep disturbance were predictors of dream frequency;
-
b)
Sleep duration, sleep disturbance, self-reported sleep quality and daytime dysfunctions were predictors of dream emotional load;
-
c)
Sleep duration, sleep disturbance, and daytime dysfunctions were predictors of dream vividness;
-
d)
Sleep disturbance was the only predictor of dream bizarreness;
-
e)
Sleep duration and sleep disturbance were predictors of dream length.
Table 4.
Results of multiple regressions (p < 0.05), considering dream features (frequency, emotional load, vividness, bizarreness, length) as criterion variables and Pittsburgh Sleep Quality Index (PSQI) scales as predictors. Significant results are indicated in bold.
| Dependent Variables |
Predictors | Beta | Coefficients of Partial Correlation | t | p |
|---|---|---|---|---|---|
|
Dream frequency R = 0.240; adjusted R2 = 0.052; F7,1076 = 9.413; p < 0.0001 |
Self-reported sleep quality | 0.056 | 0.045 | 1.468 | 0.142 |
| Sleep Latency | −0.014 | −0.012 | −0.408 | 0.683 | |
| Sleep duration | −0.166 | −0.139 | −4.617 | <0.0001 | |
| Habitual Sleep Efficiency | 0.021 | 0.017 | 0.568 | 0.570 | |
| Sleep Disturbance | 0.175 | 0.156 | 5.164 | <0.0001 | |
| Use of Sleeping Medication | 0.054 | 0.054 | 1.766 | 0.078 | |
| Daytime Dysfunctions | 0.041 | 0.039 | 1.294 | 0.196 | |
|
Dream emotional load R = 0.339; adjusted R2 = 0.109; F7,1076 = 20.024; p < 0.0001 |
Self-reported sleep quality | 0.144 | 0.118 | 3.911 | <0.0001 |
| Sleep Latency | 0.035 | 0.032 | 1.035 | 0.301 | |
| Sleep duration | −0.126 | −0.110 | −3.624 | <0.0001 | |
| Habitual Sleep Efficiency | 0.001 | 0.001 | 0.021 | 0.983 | |
| Sleep Disturbance | 0.177 | 0.162 | 5.374 | <0.0001 | |
| Use of Sleeping Medication | 0.019 | 0.020 | 0.642 | 0.521 | |
| Daytime Dysfunctions | 0.132 | 0.130 | 4.311 | <0.0001 | |
|
Dream vividness R = 0.186; adjusted R2 = 0.028; F7,1076 = 5.531; p < 0.0001 |
Self-reported sleep quality | 0.021 | 0.017 | 0.548 | 0.584 |
| Sleep Latency | 0.045 | 0.038 | 1.261 | 0.208 | |
| Sleep duration | −0.094 | −0.079 | −2.596 | 0.010 | |
| Habitual Sleep Efficiency | −0.021 | −0.018 | −0.578 | 0.564 | |
| Sleep Disturbance | 0.077 | 0.068 | 2.237 | 0.025 | |
| Use of Sleeping Medication | 0.049 | 0.048 | 1.585 | 0.113 | |
| Daytime Dysfunctions | 0.103 | 0.098 | 3.218 | 0.001 | |
|
Dream bizarreness R = 0.166; adjusted R2 = 0.021; F7,1076 = 4.364; p < 0.0001 |
Self-reported sleep quality | 0.038 | 0.030 | 0.985 | 0.325 |
| Sleep Latency | 0.030 | 0.025 | 0.831 | 0.406 | |
| Sleep duration | −0.040 | −0.034 | −1.100 | 0.272 | |
| Habitual Sleep Efficiency | −0.073 | −0.059 | −1.949 | 0.052 | |
| Sleep Disturbance | 0.100 | 0.088 | 2.901 | 0.004 | |
| Use of Sleeping Medication | 0.047 | 0.046 | 1.519 | 0.129 | |
| Daytime Dysfunctions | 0.056 | 0.053 | 1.748 | 0.081 | |
|
Dream length R = 0.179; adjusted R2 = 0.026; F7,1076 = 5.066; p < 0.0001 |
Self-reported sleep quality | 0.008 | 0.007 | 0.218 | 0.827 |
| Sleep Latency | 0.027 | 0.023 | 0.754 | 0.451 | |
| Sleep duration | −0.099 | −0.082 | −2.713 | 0.007 | |
| Habitual Sleep Efficiency | −0.008 | −0.007 | −0.214 | 0.830 | |
| Sleep Disturbance | 0.120 | 0.106 | 3.505 | <0.0001 | |
| Use of Sleeping Medication | 0.054 | 0.053 | 1.744 | 0.81 | |
| Daytime Dysfunctions | 0.058 | 0.056 | 1.827 | 0.068 |
3.5. Dream emotions during the lockdown
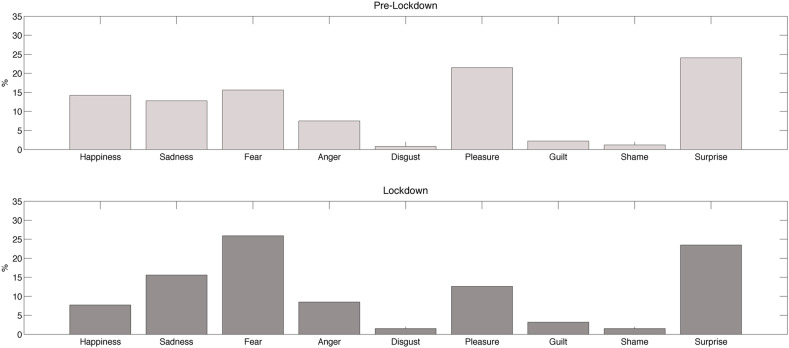
While in the pre-lockdown period the most frequent emotion was surprise (24.1%), followed by pleasure (21.5%) and fear (15.6%), during the lockdown fear was the most common emotion (25.9%), followed by surprise (23.5%) and sadness (15.6%) (Fig. 4 ).
Fig. 4.
Percentage of each assessed emotion reported in dreams during the pre-lockdown (upper box) and the lockdown period (lower box).
After dividing the emotions into positive and negative ones, we found a statistically significant difference (McNemar's test) in the proportion of positive emotions pre-lockdown and during the lockdown (p < 0.000001). Indeed, 272 participants passed from a pre-lockdown most frequent positive emotion to a most frequent negative emotion during the lockdown, while only 97 participants report the opposite.
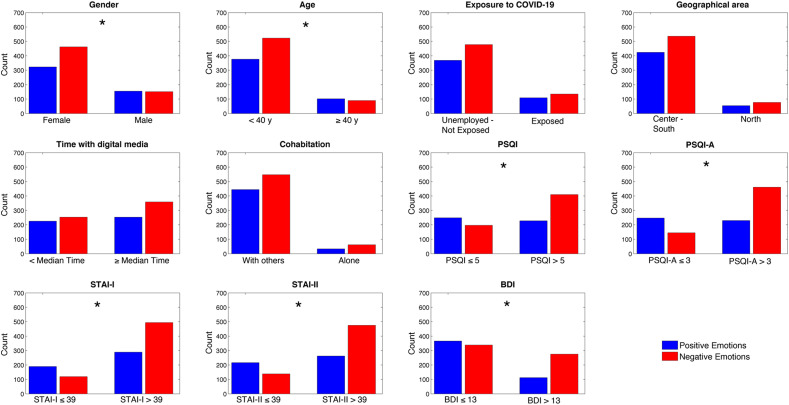
The proportion of dream negative emotions during the lockdown (Fig. 5 ) was significantly higher in females (females: 58%; males: 49.3%; χ 2 = 8.08; p = 0.004) and in subjects with poor sleep quality (PSQI >5: 64.3%; PSQI ≤5: 44.2%; χ 2 = 43.01; p < 0.00001), presence of PTSD-related symptoms (PSQI-A >3: 66.7%; PSQI-A ≤3: 37%; χ 2 = 89.67; p < 0.00001), depressive symptoms (BDI >13: 71.10%; BDI ≤13: 48%; χ 2 = 53.89; p < 0.00001), state (STAI-I >39: 63.10%; STAI-I ≤39: 38.6%; χ 2 = 53.70; p < 0.00001) and trait anxiety (STAI-II >39: 64.5%; STAI-II ≤39: 39%; χ 2 = 63.01; p < 0.00001), and younger age (<40 y: 58.2%; ≥40 y: 46.9%; χ 2 = 8.2; p = 0.004).
Fig. 5.
Number of subjects reporting a positive (blue bars) or a negative (red bars) emotion in dreams during the lockdown in subgroups formed according to demographic, clinical and sleep variables of interest. Asterisks index significant Chi-squares (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
To the best of our knowledge, this is the first study focused on dreaming during the COVID-19 pandemic that also assessed sleep and clinical self-reported data. Our findings show that: a) compared to the pre-lockdown period, the lockdown was characterized by a strong increase of dream frequency, as well as of dream qualitative features, larger in women, in participants with poor sleep quality, nocturnal disruptive behaviour and depressive symptoms; b) age, gender, depressive symptoms, geographical area, presence/absence of other people at home during the lockdown, sleep duration and several indexes of sleep quality were the best predictors of dream frequency and most of the qualitative dream features during the lockdown; c) negative emotions in dreams increased during the lockdown compared to the previous time period, and their proportion was higher in women, younger participants, and in subjects reporting poor sleep quality, nocturnal disruptive behaviour, depressive symptoms, state and trait anxiety symptoms.
The present findings depict a complex scenario, in which several factors contribute and interact to influence different aspects of the dreaming experience during the lockdown, provoking the intense changes reported: demographic characteristics (gender, age), features of the daily experience (geographical area; cohabitation) and particularly of the emotional experience (depressive and anxiety symptoms), as well as characteristics of the sleep pattern (sleep duration and quality).
4.1. Sleep pattern and clinical features during the lockdown
Our findings show that 59.1% of the participants reported poor sleep quality, 63.8% experienced nocturnal PTSD-like symptoms, 71.8% reported state anxiety symptoms, 67.6% indexed trait anxiety symptoms, and 35.5% showed depressive symptoms. It is worth noting that previous studies on the Italian population pointed to the presence of sleep difficulties in the 30% of subjects [43] and anxiety symptoms in 10.3% [44]. Moreover, the latest data provided by the Italian National Statistical Institute showed a 5.4% prevalence of depressive symptoms in the last two weeks and a 4.2% prevalence of severe anxiety during the year [45]. In light of these data, our findings suggest an increased level of psychological distress and sleep disruption during the lockdown period, consistently with other studies conducted in Italy during the COVID-19 lockdown [[4], [5], [6],[46], [47], [48]]. Compared to some of these works, the levels of sleep disruption and psychological difficulties reported in our study appear even higher, probably due to the different timing (ie, our survey was conducted during the final days of the lockdown period, compared to many other surveys conducted in its initial phase).
4.2. Changes in dream activity during the lockdown
As expected, we found a lockdown-related increase in dream frequency, emotional load, vividness, bizarreness and length. These findings confirm and extend the few previous COVID-19 related data [14,16], showing not only an increase of dream recall during the pandemic but also a strong enhancement of their qualitative features. Furthermore, these findings are consistent with the observation of an intense influence of potentially traumatic collective experiences on the phenomenology of the oneiric activity [11,12]. It is worth noting that the simultaneous increase in the perception of both frequency and intensity of dreams may be due, at least in part, to the strong association existing between quantitative and qualitative aspects of dreams. Indeed, the salience hypothesis [49] suggests that the subjective impact (ie, salience) of dreams represents a strong determinant of dream recall frequency. In this view, the increased intensity of dreams during the lockdown may have determined the higher dream recall frequency. On the other hand, the opposite view should also be considered: an increased number of dreams associated with a longer and disrupted sleep (see below) may lead to a subjective perception of increased dream qualitative properties. This issue needs further investigation.
Compared to men, women showed a higher lockdown-related increase of dream frequency, emotional load and bizarreness. Consistently, findings with different instruments indicated more frequent and intense dreams in women during the COVID-19 pandemic [[16], [17], [18]], confirming the well-known observation of an influence of gender on dreams [[50], [51], [52], [53]]. Albeit such phenomenon has not yet a clear explanation, Barrett [18], suggests that it’s observation during the pandemic can be interpreted in line with the “continuity hypothesis”, being women more disadvantaged [54] and more at risk of depression and anxiety [55] then men during the lockdown. Another possible explanation comes from several findings showing that women may present a stronger emotional reaction to negative stimuli [[56], [57], [58]]. In this view, dreams may have changed in a similar way between men and women during the lockdown, but they may have been perceived as more negative by women.
A higher lockdown-related increase in dream frequency and emotional load characterized also participants with poor sleep. This finding represents the first evidence supporting our hypothesis that lockdown-related changes in dreaming activity are at least in part influenced by the sleep pattern. The arousal-retrieval model suggests that a certain level of arousal during sleep and intra-sleep waking periods are necessary to encode the oneiric experience, leading to a more probable dream recall in the morning [34,59]. Consistently, more fragmented sleep is associated with increased dream recall frequency in healthy and clinical samples [[31], [32], [33]], and several electrophysiological findings showed that a desynchronized sleep electroencephalogram (EEG) can promote dream recall in both normal and pathological conditions [41,42,[60], [61], [62], [63], [64]]. In this view, it could be hypothesized that sleep in subjects with poor self-reported sleep quality during the lockdown should be lighter and more fragmented, promoting an increased dream recall. Moreover, participants with nocturnal PTSD symptoms showed a stronger increase of dream frequency, emotional load, vividness, bizarreness and length. Since nightmares represent a hallmark of PTSD [65], and PTSD patients experience vivid and emotionally negative dreams [65], it is possible that the increased frequency and intensity of dreams in participants with nocturnal PTSD symptoms mainly mirrors an increased number of nightmares (which are one of the disruptive nocturnal behaviour assessed by the PSQI-A). The increase of negative emotions during the lockdown, and the higher proportion of reported negative emotions in subjects with nocturnal PTSD symptoms converge in supporting this hypothesis. Clearly, the cross-sectional nature of this analysis and the absence of data on pre-lockdown sleep quality make it impossible to determine the direction of a possible causal relation between sleep difficulties and dream recall (see the “Limitations” section). For instance, we don't know if sleep has become worse during the lockdown compared to the previous period, inducing changes in dream recall, or subjects with pre-lockdown sleep difficulties more likely developed modifications in dream features under the stressful condition represented by the pandemic. We can only state that subjects with poorer sleep quality perceived a greater modification in their oneiric experience during the lockdown. Every conclusion about a causal relationship remains speculative.
Also, subjects with depressive symptoms showed an increased dream frequency during the lockdown, together with higher emotional load and vividness. Several studies found a reduced and less detailed dream recall in depressed subjects [66]. However, depressed patients showed an increased number of nightmares [67,68], and depressed patients with melancholic features exhibited a higher rate of nightmares compared to non-melancholic patients [69]. Moreover, depression is often associated with insomnia and disrupted sleep quality [70]. The higher increase of dream quantity and quality in subject with depressive symptoms, then, may be explained in three ways (not mutually exclusive): a) subjects with depressive symptoms experienced a larger number of nightmares (consistent with the observation of an increase of dream negative emotions during the lockdown), which determines the increased and more intense dream recall; b) subjects with depressive symptoms were characterized by higher/more frequent sleep disruption, which in turn provoked an increased dream recall; c) subjects have become depressed following a reduction of sleep quality due to nightmares and/or other factors. Consistently, in the present sample, the BDI score was positively associated with both PSQI (r = 0.497, p < 0.0001) and PSQI-A (r = 0.575, p < 0.0001) global scores.
4.3. Predictors of dream activity during the lockdown
The first set of multiple regressions conducted in the present study revealed age, gender, BDI scores, geographical area and cohabitation as predictors of dream frequency and almost all of the qualitative features of dreams during the lockdown. Specifically, reduced age, female gender, higher depressive symptoms, being in the north of Italy and living alone during the lockdown were associated with higher dream frequency, emotional load and vividness. Moreover, age and geographical area were also predictors of bizarreness, and age, gender, geographical area and cohabitation were also predictors of dream length. Finally, PSQI scores predicted dream emotional load, with poorer sleep quality associated with high emotional intensity.
The specific set of multiple regressions with self-reported sleep features as independent variables showed that sleep duration and sleep disturbance were the strongest predictors of the dream variables investigated, with longer sleep duration and higher sleep disturbance associated with increased scores in dream features. Moreover, daytime dysfunctions represented a predictor of dream emotional load and vividness: in this case, higher daytime dysfunction was associated with increased dream frequency and intensity. Finally, self-reported sleep quality was a further predictor for dream emotional load, with reduced sleep quality associated with higher emotional load.
Taken together, these findings suggest that the intervention of several factors explains the peculiarity of the oneiric activity during the lockdown. First, as predictable, demographic features classically associated with dream recall: gender [[50], [51], [52], [53]] and age [26,71]. Second, features characterizing the daily experience during the lockdown: living in the North of Italy, considered the core of the Italian COVID-19 emergency with a higher number of deaths and infected people during the lockdown, living alone, and daily mood (ie, depressive symptoms). Finally, the sleep patterns' characteristics, in terms of duration, disturbance, daytime dysfunctions and self-reported sleep quality. Along this line of reasoning, our results are consistent with both the “continuity hypothesis” [22,25] and with the view of an influence of the sleep pattern on dreaming activity [23]. In other words, lockdown-related changes in daily life and emotional experience may have had a strong impact on the quality and amount of dreams, but an increased sleep duration associated with more flexible schedule [3,7,9] and a reduced sleep quality [[3], [4], [5],7,8] may have represented a fertile ground for a boosted dream recall.
4.4. The emotional tone of dreams during the lockdown
The observed enhancement of negative emotions in dreams, with a predominance of fear, is consistent with the few existing data pointing to a spread of negative emotions [16], anxiety-related [19] and pandemic-related content [21] in dreams during the 2020 lockdown. Moreover, we found a higher proportion of dream negative emotions in females, younger subjects, and participants with lower sleep quality, disruptive nocturnal behaviours, depressive and state/trait anxiety symptoms. Beyond confirming the hypothesized impact of both daily emotional experience and sleep pattern on the oneiric activity during the lockdown also considering its emotional features, such finding can also be considered in light of the hypothesis that dreams may have a role in emotional processing and memory consolidation [24]. Dreaming activity and emotional regulation share similar neurobiological processes, suggesting the existence of a continuum between waking and REM sleep activity in several areas like amygdala, hippocampus and medial prefrontal cortex [39,[72], [73], [74], [75]]. Consistently, it has been proposed that dreams may represent an offline simulation of threatening events, working as problem-solving based emotional coping strategies for the rehearsal of threat-avoidance skills, sustained by the activation of amygdalocortical networks associated with fear [27,28]. Other authors propose a role for dreams in fear extinction [74,76], emotional conflict resolution, and reduction of negative mood [77]. Beyond the specific focus of these theoretical models, they all suggest that fear in dreams should be related to more adaptive behaviours in response to daily threatening stimuli [24]. Interestingly, a recent paper showed higher activation of insula and midcingulate cortex in dreams containing fear, and in a second study that subjects reporting a higher incidence of fear in their oneiric activity exhibited during wakefulness a decreased emotional arousal and fMRI response to adverse stimuli in the insula, amygdala and midcingulate cortex [74]. Consistently, the spread of negative emotions in lockdown-dreams may be the expression of a preparatory process to address the threats represented by the pandemic, aimed at the promotion of more adaptive behaviours during daily life. However, since we don't have the possibility to directly assess any causal relation between wake and sleep emotional activations during the lockdown, such hypothesis remains speculative.
5. Limitations
The cross-sectional design of the present research makes difficult to draw conclusions about the causality of the observed phenomena. Moreover, the online strategy used to recruit participants may introduce a significant bias in the final sample, and the online survey may have attracted a large number of subjects with sleep or psychopathological problems, or simply higher interest in dreams (ie, an issue of partial self-selection). Indeed, we had an unbalanced sample (eg, concerning age, gender, and geographical area). These observations lead to a relative difficulty in generalizing the present findings. However, it should be considered that the online survey actually remains the best strategy to reach a large sample, particularly during a period characterized by forced isolation.
An intrinsic limitation of our experimental design is represented by the lower reliability of retrospective questionnaires compared to experimental approaches in which dream reports are collected immediately upon awakening. Indeed, while the retrospective assessment of dreams allows a quick data collection in large samples, this method is affected by a strong memory bias, due to the possible influence of daytime activity on quantity and quality of recalled dreams [78], often leading to the underestimation of dream recall frequency [79]. In this view, the increased dream frequency and intensity during the lockdown compared to the pre-lockdown could be interpreted merely as higher accessibility to the memory of more recent dreams (ie, lockdown dreams), compared to dreaming activity referred to a previous period (ie, pre-lockdown dreams). Dreams can be re-processed with elapsed time, and the observed changes in dream features may represent a different subjective evaluation, elaboration and/or interpretation of dreams during the pandemic. Along this line, what we detected in our study is not the actual number of dreams recalled, but the subjective perception of dream frequency, resulting in an increased perception of dream recall during the lockdown. Albeit our results are in accordance with other retrospective studies using different analyses [14,16], studies through dream logs will be crucial to confirm our finding.
Another limitation of our study concerns the absence of sleep quality, depression and anxiety measures collected in the pre-lockdown period. Without this information, we can't clarify how pre-existent sleep difficulties and clinical symptoms affected the subjective evaluation of pandemic-related changes in dream features. We can't directly determine if sleep quality and depressive/anxiety symptoms changed or remained stable with the pandemic compared to the previous period, and how this presence/absence of modifications can affect changes in dream recall. It is possible that subjects with depression/anxiety symptoms or characterized by low sleep quality before the pandemic have been more likely to observe lockdown-related changes in their dreams without modification of clinical and sleep features. In this view, every conclusion about the relation between dream, sleep and clinical measures should be confirmed by longitudinal studies.
Finally, considering the between-groups comparisons conducted on the lockdown vs. pre-lockdown differences in dream features, it is worth noting that they should be considered with caution since in some cases we compared groups with very different sizes.
6. Conclusions
The lockdown associated with the COVID-19 pandemic had a significant impact on our life, strongly influencing daily habits, socio-economical conditions, relations, emotions, physical and mental health, wake–sleep cycles, and sleep patterns. Here, we highlighted that oneiric activity has been strongly influenced by the lockdown. We described a dramatic increase of dream frequency and intensity in an Italian sample, even larger in females and in the presence of poor sleep quality, disruptive nocturnal behaviours and depressive symptoms. Moreover, we found that demographic features, characteristics of the daily experience, emotional status and specific sleep pattern features can predict the phenomenology of dream during the lockdown. Finally, dreams during the lockdown were characterized by increased negative emotions, which were particularly frequent in females, younger participants, and those presenting poor sleep quality, disruptive nocturnal behaviours and higher depression and anxiety levels. These findings support both the hypothesis of continuity between wake and sleep mental processes and emotional experiences [22,24], and the view of a strong influence of the sleep pattern on dreaming activity [23]. Clearly, it is hard to disentangle the specific contribution of waking and sleeping variables and their possible interactions in determining changes in dreaming activity during the lockdown, starting from the complexity of the context and the online strategy's intrinsic limitations. Follow-up studies, more detailed analyses on dream content and daily sleep and dream log assessment would strongly help to clarify this issue.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Maurizio Gorgoni: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Supervision, Writing - original draft. Serena Scarpelli: Conceptualization, Methodology, Validation, Data curation, Supervision, Writing - original draft. Valentina Alfonsi: Investigation, Data curation. Ludovica Annarumma: Investigation, Data curation. Susanna Cordone: Investigation, Data curation. Serena Stravolo: Investigation, Data curation. Luigi De Gennaro: Conceptualization, Methodology, Validation, Supervision, Writing - original draft.
Acknowledgments
The authors thank Marianna Lanza, Chiara Pecorari, and Chiara Trivellone for their help in data collection.
Footnotes
None of the authors have potential conflicts of interest to be disclosed.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.02.006.
Conflict of interest
The following is the supplementary data related to this article:
Multimedia component 1
References
- 1.Xiong J., Lipsitz O., Nasri F., et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altena E., Baglioni C., Espie C.A., et al. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy. J Sleep Res. 2020;29(4) doi: 10.1111/jsr.13052. [DOI] [PubMed] [Google Scholar]
- 3.Blume C., Schmidt M.H., Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30:R795–R797. doi: 10.1016/j.cub.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casagrande M., Favieri F., Tambelli R., et al. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. doi: 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellini N., Canale N., Mioni G., et al. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020;29(4) doi: 10.1111/jsr.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschini C., Musetti A., Zenesini C., et al. Poor sleep quality and its consequences on mental health during COVID-19. Front Psychol. 2020 doi: 10.3389/fpsyg.2020.574475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Qin Q., Sun Q., et al. Insomnia and psychological reactions during the COVID-19 outbreak in China. J Clin Sleep Med. 2020;16(8):1417–1418. doi: 10.5664/jcsm.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marelli S., Castelnuovo A., Somma A., et al. Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J Neurol. 2020 doi: 10.1007/s00415-020-10056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright K.P., Linton S.K., Withrow D., et al. Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr Biol. 2020;30:R797–R798. doi: 10.1016/j.cub.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett D. Harvard University Press; London, UK: 2001. Trauma and dreams. [Google Scholar]
- 11.David D., Mellman T.A. Dreams following the hurricane Andrew. Dreaming. 1997;7:209–214. doi: 10.1037/h0094475. [DOI] [Google Scholar]
- 12.Hartmann E., Basile R. Dream imagery becomes more intense after 9/11/01. Dreaming. 2003;13:61–66. doi: 10.1023/A:1023398924124. [DOI] [Google Scholar]
- 13.Wood J.M., Bootzin R.R., Rosenhan D., et al. Effects of the 1989 San Francisco earthquake on frequency and content of nightmares. J Abnorm Psychol. 1992;101:219–224. doi: 10.1037/0021-843X.101.2.219. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Xia Q., Xiong Z., et al. The psychological distress and coping styles in the early stages of the 2019 coronavirus disease (COVID-19) epidemic in the general mainland Chinese population: a web-based survey. PloS One. 2020;15 doi: 10.1371/journal.pone.0233410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta Madhulika A. Spontaneous reporting of onset of disturbing dreams and nightmares related to early life traumatic experiences during the COVID-19 pandemic by patients with posttraumatic stress disorder in remission. J Clin Sleep Med. 2020;16:1419–1420. doi: 10.5664/jcsm.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schredl M., Bulkeley K. Dreaming and the COVID-19 pandemic: a survey in a U.S. sample. Dreaming. 2020;30(3):189–198. doi: 10.1037/drm0000146. [DOI] [Google Scholar]
- 17.Iorio I., Sommatico M., Parrello S. Dreaming in the time of COVID-19: a quali-quantitative Italian study. Dreaming. 2020;30(3):199–215. doi: 10.1037/drm0000142. [DOI] [Google Scholar]
- 18.Barrett D. Dreams about COVID-19 versus normative dreams: trends by gender. Dreaming. 2020;30(3):216–221. doi: 10.1037/drm0000149. [DOI] [Google Scholar]
- 19.MacKay C., DeCicco T.L. Pandemic dreaming: the effect of COVID-19 on dream imagery, a pilot study. Dreaming. 2020;30(3):222–234. doi: 10.1037/drm0000148. [DOI] [Google Scholar]
- 20.Miller N.J., DeCicco T.L., Dale A.L., et al. Assessing the effects of meditation on dream imagery, depression, and anxiety. Int J Dream Res. 2015;8:99–104. [Google Scholar]
- 21.Pesonen A.K., Lipsanen J., Halonen R., et al. Pandemic dreams: network analysis of dream content during the COVID-19 lockdown. Front Psychol. 2020;11:2569. doi: 10.3389/fpsyg.2020.573961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schredl M. Factors affecting the continuity between waking and dreaming: emotional intensity and emotional tone of the waking-life event. Sleep Hypn. 2006;8:1–5. [Google Scholar]
- 23.Bottary R., Simonelli G., Cunningham T.J., et al. Sleep extension: an explanation for increased pandemic dream recall? Sleep. 2020 doi: 10.1093/sleep/zsaa131. [DOI] [PubMed] [Google Scholar]
- 24.Scarpelli S., Bartolacci C., D'Atri A., et al. The functional role of dreaming in emotional processes. Front Psychol. 2019;10:459. doi: 10.3389/fpsyg.2019.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domhoff G.W. Springer; New York, NY: 1996. Finding meanings in dreams. A quantitative approach. [DOI] [Google Scholar]
- 26.Mangiaruga A., Scarpelli S., Bartolacci C., et al. Spotlight on dream recall: the ages of dreams. Nat Sci Sleep. 2018;10:1–12. doi: 10.2147/NSS.S135762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav Brain Sci. 2000;23:877–901. doi: 10.1017/S0140525X00004015. [DOI] [PubMed] [Google Scholar]
- 28.Revonsuo A., Tuominen J., Valli K. In: Open MIND. Metzinger T., Windt J.M., editors. MIND Group; Frankfurt am Main: 2015. The Avatars in the machine - dreaming as a simulation of social reality. [Google Scholar]
- 29.Schredl M., Fulda S. Dream recall and sleep duration: state or trait factor. Percept Mot Skills. 2005;101:613–616. doi: 10.2466/PMS.101.6.613-616. [DOI] [PubMed] [Google Scholar]
- 30.Schredl M., Reinhard I. Dream recall, dream length, and sleep duration: state or trait factor. Percept Mot Skills. 2008;106:633–636. doi: 10.2466/pms.106.2.633-636. [DOI] [PubMed] [Google Scholar]
- 31.van Wyk M., Solms M., Lipinska G. Increased awakenings from non-rapid eye movement sleep explains differences in dream recall frequency in healthy high and low recallers. Front Hum Neurosci. 2019;13:370. doi: 10.3389/fnhum.2019.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polini F., Principe R., Scarpelli S., et al. Use of varenicline in smokeless tobacco cessation influences sleep quality and dream recall frequency but not dream affect. Sleep Med. 2017;30:1–6. doi: 10.1016/j.sleep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Schredl M. Dreams in patients with sleep disorders. Sleep Med Rev. 2009;13(3):215–221. doi: 10.2190/IC.28.4.e. [DOI] [PubMed] [Google Scholar]
- 34.De Gennaro L., Marzano C., Moroni F., et al. Recovery sleep after sleep deprivation almost completely abolishes dream recall. Behav Brain Res. 2010;206:293–298. doi: 10.1016/j.bbr.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger C.D. 2nd ed. Consulting Psychologists Press; 1989. State-trait anxiety inventory: bibliography. [Google Scholar]
- 36.Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck depression inventory II. [Google Scholar]
- 37.Curcio G., Tempesta D., Scarlata S., et al. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI) Neurol Sci. 2013;34:511–519. doi: 10.1007/s10072-012-1085-y. 2013. [DOI] [PubMed] [Google Scholar]
- 38.Germain A., Hall M., Krakow B., et al. A brief sleep scale for posttraumatic stress disorder: Pittsburgh sleep quality index Addendum for PTSD. J Anxiety Disord. 2005;19:233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 39.De Gennaro L., Cipolli C., Cherubini A., et al. Amygdala and hippocampus volumetry and diffusivity in relation to dreaming. Hum Brain Mapp. 2011;32:1458–1470. doi: 10.1002/hbm.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Gennaro L., Ferrara M., Cristiani R., et al. Alexithymia and dream recall upon spontaneous morning awakening. Psychosom Med. 2003;65:301–306. doi: 10.1097/01.psy.0000058373.50240.71. [DOI] [PubMed] [Google Scholar]
- 41.Scarpelli S., D'Atri A., Bartolacci C., et al. Dream recall upon awakening from non-rapid eye movement sleep in older adults: electrophysiological pattern and qualitative features. Brain Sci. 2020;10(6):343. doi: 10.3390/brainsci10060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarpelli S., D'Atri A., Mangiaruga A., et al. Predicting dream recall: EEG activation during NREM sleep or shared mechanisms with wakefulness? Brain Topogr. 2017;30:629–638. doi: 10.1007/s10548-017-0563-1. [DOI] [PubMed] [Google Scholar]
- 43.Leger D., Poursain B., Neubauer D., et al. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24(1):307–317. doi: 10.1185/030079907x253771. [DOI] [PubMed] [Google Scholar]
- 44.de Girolamo G., Polidori G., Morosini P., et al. Prevalence of common mental disorders in Italy. Soc Psychiatr Psychiatr Epidemiol. 2006;41(11):853–861. doi: 10.1007/s00127-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 45.ISTAT Istituto Nazionale di Statistica . AT Istituto Nazionale di Statistica; Rome, Italy: 2018. La salute mentale nelle varie fasi della vita. Anni 2015-2017. [Google Scholar]
- 46.Barrea L., Pugliese G., Framondi L., et al. Does Sars-Cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Transl Med. 2020;18(1):318. doi: 10.1186/s12967-020-02465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gualano M.R., Lo Moro G., Voglino G., et al. Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Publ Health. 2020;17(13):4779. doi: 10.3390/ijerph17134779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazza C., Ricci E., Biondi S., et al. A nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: immediate psychological responses and associated factors. Int J Environ Res Publ Health. 2020;17:3165. doi: 10.3390/ijerph17093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen D.B., MacNeilage P.F. A test of the salience hypothesis of dream recall. J Consult Clin Psychol. 1974;42:699–703. doi: 10.1037/h0036948. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen T.A., Laberge L., Paquet J., et al. Development of disturbing dreams during adolescence and their relation to anxiety symptoms. Sleep. 2000;23:727–736. doi: 10.1093/sleep/23.6.1. [DOI] [PubMed] [Google Scholar]
- 51.Schredl M. Explaining the gender difference in dream recall frequency. Dreaming. 2010;20:96–106. doi: 10.1037/a0019392. [DOI] [Google Scholar]
- 52.Schredl M., Reinhard I. Gender differences in dream recall: a meta-analysis. J Sleep Res. 2008;17:125–131. doi: 10.1111/j.1365-2869.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 53.Settineri S., Frisone F., Alibrandi A., et al. Italian adaptation of the Mannheim Dream Questionnaire (MADRE): age, gender and dream recall effects. Int J Dream Res. 2019;12:119–129. doi: 10.11588/ijodr.2019.1.59328. [DOI] [Google Scholar]
- 54.Wenham C., Smith J., Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Özdin S., Bayrak Özdin S. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: the importance of gender. Int J Soc Psychiatr. 2020;66:504–511. doi: 10.1177/0020764020927051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley M.M., Codispoti M., Sabatinelli D., et al. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- 57.Lithari C., Frantzidis C.A., Papadelis C., et al. Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr. 2010;23(1):27–40. doi: 10.1007/s10548-009-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens J.S., Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Koulack D., Goodenough D.R. Dream recall and dream recall failure: an arousal-retrieval model. Psychol Bull. 1976;83:975–984. doi: 10.1037/0033-2909.83.5.975. [DOI] [Google Scholar]
- 60.Chellappa S.L., Frey S., Knoblauch V., et al. Cortical activation patterns herald successful dream recall after NREM and REM sleep. Biol Psychol. 2011;87:251–256. doi: 10.1016/j.biopsycho.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 61.D'Atri A., Scarpelli S., Schiappa C., et al. Cortical activation during sleep predicts dream experience in narcolepsy. Ann Clin Transl Neurol. 2019;6(3):445–455. doi: 10.1002/acn3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siclari F., Baird B., Perogamvros L., et al. The neural correlate s of dreaming. Nat Neurosci. 2017;20:872–878. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siclari F., Bernardi G., Cataldi J., et al. Dreaming in NREM sleep: a high-density study of slow waves and spindles. J Neurosci. 2018;38(43):9175–9185. doi: 10.1523/JNEUROSCI.0855-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J., Wamsley E.J. EEG predictors of dreaming outside of REM sleep. Psychophysiology. 2019;56(7):e13368. doi: 10.1111/psyp.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatr. 2013;170:372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palagini L., Rosenlicht N. Sleep, dreaming, and mental leatlh: a review of historical and neurobiological perspectives. Sleep Med Rev. 2011;15:179–186. doi: 10.1016/j.smrv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Li S.X., Zhang B., Li A.M., et al. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010;33:774–780. doi: 10.1093/sleep/33.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marinova P., Koychev I., Laleva L., et al. Nightmares and suicide: predicting risk in depression. Psychiatr Danub. 2014;26:159–164. [PubMed] [Google Scholar]
- 69.Agargun M.Y., Besiroglu L., Cilli A.S., et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98:267–270. doi: 10.1016/j.jad.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Steiger A., Pawlowski M. Depression and sleep. Int J Mol Sci. 2019;20:607. doi: 10.3390/ijms20030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scarpelli S., Bartolacci C., D'Atri A., et al. Mental sleep activity and disturbing dreams in the lifespan. Int J Environ Res Publ Health. 2019;16(19):3658. doi: 10.3390/ijerph16193658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Gennaro L., Lanteri O., Piras F., et al. Dopaminergic system and dream recall: an MRI study in Parkinson's disease patients. Hum Brain Mapp. 2016;37:1136–1147. doi: 10.1002/hbm.23095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eichenlaub J.B., Nicolas A., Daltrozzo J., et al. Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology. 2014;39:1594–1602. doi: 10.1038/npp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sterpenich V., Perogamvros L., Tononi G., et al. Fear in dreams and in wakefulness: evidence for day/night affective homeostasis. Hum Brain Mapp. 2020;41(3):840–850. doi: 10.1002/hbm.24843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallat R., Eichenlaub J.B., Nicolas A., et al. Dream recall frequency is associated with medial prefrontal cortex white-matter density. Front Psychol. 2018;9:1856. doi: 10.3389/fpsyg.2018.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nielsen T., Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11:295–310. doi: 10.1016/j.smrv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Cartwright R., Agargun M.Y., Kirkby J., et al. Relation of dreams to waking concerns. Psychiatr Res. 2006;141:261–270. doi: 10.1016/j.psychres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Schredl M. Continuity between waking and dreaming: a proposal for a mathematical model. Sleep Hypn. 2003;5:26–40. [Google Scholar]
- 79.Robert G., Zadra A. Measuring nightmare and bad dream frequency: impact of retrospective and prospective instruments. J Sleep Res. 2008;17:132–139. doi: 10.1111/j.1365-2869.2008.00649.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.