Abstract
Objectives:
Few studies have evaluated determinants of multidrug-resistant (MDR) Vibrio cholerae O1 in older children and adults. This study aimed to characterize the prevalence of MDR V. cholerae O1 and associated risk factors among patients over five years of age in Bangladesh.
Methods:
Stool culture and antimicrobial susceptibility testing were performed as a part of a larger study at Dhaka Hospital in Bangladesh from March 2019–March 2020. Univariate statistics and multiple logistic regression were used to assess the association between a range of variables and MDR V. cholerae O1.
Results:
MDR was found in 175 of 623 (28.1%) V. cholerae O1 isolates. High levels of resistance were found to erythromycin (99.2%), trimethoprim-sulfamethoxazole (99.7%), and ampicillin (88.9%), while susceptibility was high to tetracyclines (99.7%), azithromycin (99.2%), ciprofloxacin (99.8%), and cephalosporins (98.6%). MDR was associated with prior antibiotic use, longer transport time to hospital, higher income, non-flush toilet use, greater stool frequency, lower blood pressure, lower mid-upper arm circumference, and lower percent dehydration.
Conclusions:
MDR V. cholerae O1 was common among patients over five in an urban hospital in Bangladesh. Significant factors associated with MDR may be actionable in identifying patients with a high likelihood of MDR.
Keywords: Antimicrobial resistance, Multidrug resistance, Global health, Vibrio cholera, Diarrhea, Bangladesh
Introduction
Cholera, caused by toxin-producing strains (O1 and O139) of the bacterium Vibrio cholerae (V. cholerae), is characterized by acute watery diarrhea, vomiting, and muscle cramps and can lead to massive fluid losses and death within hours if untreated (Leibovici-Weissman et al., 2014; Mandal et al., 2011). While rehydration is the mainstay of cholera treatment, antibiotics are recommended by the World Health Organization (WHO) to reduce the volume and duration of diarrhea as well as bacterial shedding and transmission (Global Task Force on Cholera Control, 2018; Leibovici-Weissman et al., 2014). Despite global efforts for cholera control, cholera remains a significant cause of diarrhea-related morbidity and mortality in low- and middle-income countries (LMICs) due to unsafe water and poor sanitation (Mandal et al., 2011). Alarmingly, antimicrobial resistance (AMR) is rapidly increasing among V. cholerae as well as other enteric pathogens in large part due to unregulated antimicrobial overuse among humans and animals (Ventola, 2015). In response to these global trends, the WHO has identified AMR as a serious global public health concern (Ventola, 2015).
V. cholerae serogroup O1 is responsible for the majority of cholera cases globally and has been treated with multiple antibiotic classes over the years (e.g., tetracyclines, fluoroquinolones, macrolides) (Leibovici-Weissman et al., 2014; Sjölund-Karlsson et al., 2011). However resistance to all commonly used drugs has been documented, due to chromosomal mutations or acquisition of mobile genetic elements such as plasmids, transposons, and integrating conjugative elements (Ceccarelli et al., 2016; Global Task Force on Cholera Control, 2018). The WHO recommends first-line treatment of adults with doxycycline, followed by azithromycin or ciprofloxacin, while acknowledging that local resistance patterns must be considered (Global Task Force on Cholera Control, 2018). Currently, a single-dose of the macrolide azithromycin is the antimicrobial of choice for treating moderate-to-severe cholera in children and adults in Bangladesh (Parvin et al., 2020; Saha et al., 2006). Multidrug-resistant (MDR) V. cholerae has become an urgent issue, challenging clinical management, increasing healthcare costs, and straining fragile healthcare systems (Sjölund-Karlsson et al., 2011). Antibiotics such as third-generation cephalosporins, often unavailable or prohibitively expensive, are increasingly used, although reduced susceptibility to extended-spectrum β-lactamase (ESBL) producing strains (which can hydrolyze most β-lactams such as ampicillin, amoxicillin as well as third-generation cephalosporins) has been reported (Ceccarelli et al., 2016; Mandal et al., 2011).
AMR patterns of V. cholerae O1 strains vary significantly between regions and over time (Mandal et al., 2011). While the true burden of cholera remains severely under-reported, Bangladesh has one of the world’s highest cholera incidence rates, with a recent serosurvey estimating that up to 28 million cases of cholera occur annually in the country (Azman et al., 2020). Additionally, although cholera’s mortality risk is highest among children under five, a substantial burden of morbidity and mortality exists among older children, adults, and the elderly in whom MDR remains poorly characterized (Troeger et al., 2018). This study aimed to characterize the susceptibility pattern and prevalence of MDR among V. cholerae O1 in patients with acute diarrhea over five years of age in Bangladesh and assess individual factors associated with MDR. This knowledge may assist clinical and public health decision-making regarding appropriate antibiotic use and lead to more effective management of outbreaks in comparable LMIC settings.
Materials and methods
Study design
This was a secondary analysis of data collected from the “Novel, Innovative Research for Understanding Dehydration in Adults and Kids” (NIRUDAK) study, a prospective cohort study of patients over five years of age presenting with acute diarrhea to the rehydration unit at Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). The primary aim of the NIRUDAK study is to derive and validate age-specific clinical diagnostic models for the assessment of dehydration severity in older children, adolescents, adults and the elderly, and later incorporate these models in a mobile health tool as previously described (Gainey et al., 2019). Ethical approval for the NIRUDAK Study was obtained from the icddr,b Ethical Review Committee and Rhode Island Hospital Institutional Review Board.
Study setting and population
The study was conducted between March 2019 - March 2020 at icddr,b Dhaka Hospital, an urban referral hospital that provides free clinical services for over 100,000 patients annually. The inclusion and exclusion criteria used for the present study were the same as the parent (NIRUDAK) study. All patients over five years of age with acute diarrhea (defined as three or more loose stools in the past 24 h lasting less than seven days) were eligible for enrollment (Levine et al., 2015). Patients were screened for enrollment on arrival 24 h per day, seven days a week. Exclusion criteria included: having less than three loose stools in the past 24 h, diarrhea lasting more than seven days, a clear alternative diagnosis to gastroenteritis, and previous enrollment in the study (Gainey et al., 2019). Research staff provided patients and/or their guardians with information about the study’s risks and benefits and obtained verbal and written consent in the local language, Bangla. In cases where the patient or legal guardian could not read or write, research staff obtained verbal consent and asked the parent or guardian to mark the consent form with a thumbprint. For children over the age of eight and under 18, verbal or written assent was also obtained.
Study procedures
Enrolled subjects were assessed by a study nurse for historical, demographic, and socio-environmental data as well as clinical signs. Mid-upper area circumference (MUAC) was measured using standard measuring tapes. Study procedures were not allowed to delay emergent care. After an initial assessment, patients were treated according to standard icddr,b protocols for managing acute diarrhea and per physician discretion, including oral or intravenous rehydration and antibiotics. Percent weight change with rehydration was used as the criterion standard for percent dehydration (Hooper et al., 2015; Shirreffs, 2003; Steiner et al., 2004). Patients were then categorized as having severe (>9%) dehydration, some (3–9%) dehydration, or no (<3%) dehydration (Duggan et al., 1992; Levine et al., 2015).
All microbiological tests were performed under the supervision of a senior clinical microbiologist (co-author DA; Head of Clinical Microbiology and Immunology at icddr,b). Two stool specimens (at least 2 ml/vial) were collected from each subject–one for analysis to the clinical microbiology laboratory and one for storage in 70% ethanol. Each specimen was screened for common enteric pathogens using stool culture. Isolation, identification, serogrouping, and biotyping of stool samples were performed using standard procedures (Murray et al., 1995). V. cholerae was isolated by growth on tellurite taurocholate gelatin agar (TTGA) media with enrichment in bile peptone broth. Antimicrobial susceptibility testing (AST) was determined by the Kirby-Bauer standard disc diffusion method on Muller–Hinton agar. The selection of antibiotics used for AST followed the Clinical and Laboratory Standards Institute (CLSI) guidelines and the icddr,b Clinical Microbiology, and Immunology laboratory committee, which are regularly updated based on local susceptibility patterns (Clinical Laboratory Standards Institute, 2018). There were no issues relating to the availability of discs for disc diffusion testing that influenced AST. The results were reported as sensitive, intermediate, or resistant by a method based on the cutoff of the zone size for different antibiotics according to CLSI guidelines (Clinical Laboratory Standards Institute, 2018). Antibiotics tested included: amoxicillin/clavulanate, ampicillin, azithromycin, cefepime, cefotaxime, cefoperazone-sulbactam, cefuroxime, ciprofloxacin, trimethroprim-sulfamethoxazole (TMP-SMX), doxycycline, erythromycin, tetracycline, and tigecycline. Pathogens resistant to at least one agent in ≥3 antimicrobial categories were defined as MDR based on consensus definitions from the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC). (Magiorakos et al., 2012) Isolates with a result of “intermediate” and “resistant” were grouped together for the purpose of this analysis.
Statistical analysis
Categorical variables were described using frequencies with percentages. Continuous variables with normal distribution were presented as means with standard deviations (SD) and compared using t-tests. Univariate analysis was performed to evaluate differences between those with presence of MDR V. cholerae O1, with magnitudes of effect given as odds ratios (OR) and their respective 95% confidence intervals (CI). Multiple logistic regression analysis was performed to identify variables independently associated with the dependent variable of MDR and expressed in adjusted ORs with 95% CIs. All clinically relevant candidate variables were retained in the multiple logistic regression analysis. Continuous variables were recoded as binary variables by using the median value of the distribution or clinically relevant cutoff points. Nagelkerke’s pseudo-R2, a measure analogous to the R2 used in logistic regression, was calculated to provide a global measure of the estimated explained variance of the final model on a new data set; the pseudo-R2 ranges between 0 and 1 where 1 is a fully explained model (Nagelkerke, 1991). The multiple regression model discrimination was assessed using the area under the receiver-operator characteristic (ROC) curve (AUC) to predict MDR. For all analyses, a two-tailed p-value of 0.05 was considered statistically significant. STATA Version 14 (Stata Corp; College Station, USA) was used for all analyses.
Results
During the study period, 2172 patients ≥5 years of age with acute diarrhea were enrolled. Stool culture was completed for 2135 subjects, and 1198 had a growth on stool culture. V. cholerae was the most common pathogen isolated, representing 641 (30.0%) of those with positive cultures (Figure 1). Nearly all (623 of 641 or 97.2%) of the V. cholerae isolates were found to be O1 and were included for further analysis. The median age of subjects was 26 years (IQR, 15–60; range 5–90), and 346 (55.5%) were male. Antibiotic use prior to hospital presentation was reported by 199 (31.9%) subjects overall. Among included subjects, 76 (12.2%) were classified as no/mild dehydration, 226 (36.3%) as some, and 318 (51.0%) as severe. Mean percent dehydration was lower in those with MDR (5.9%; 95% CI 5.5–6.3) compared to those without MDR (6.4%; 95% CI 6.1–6.7).
Figure 1.
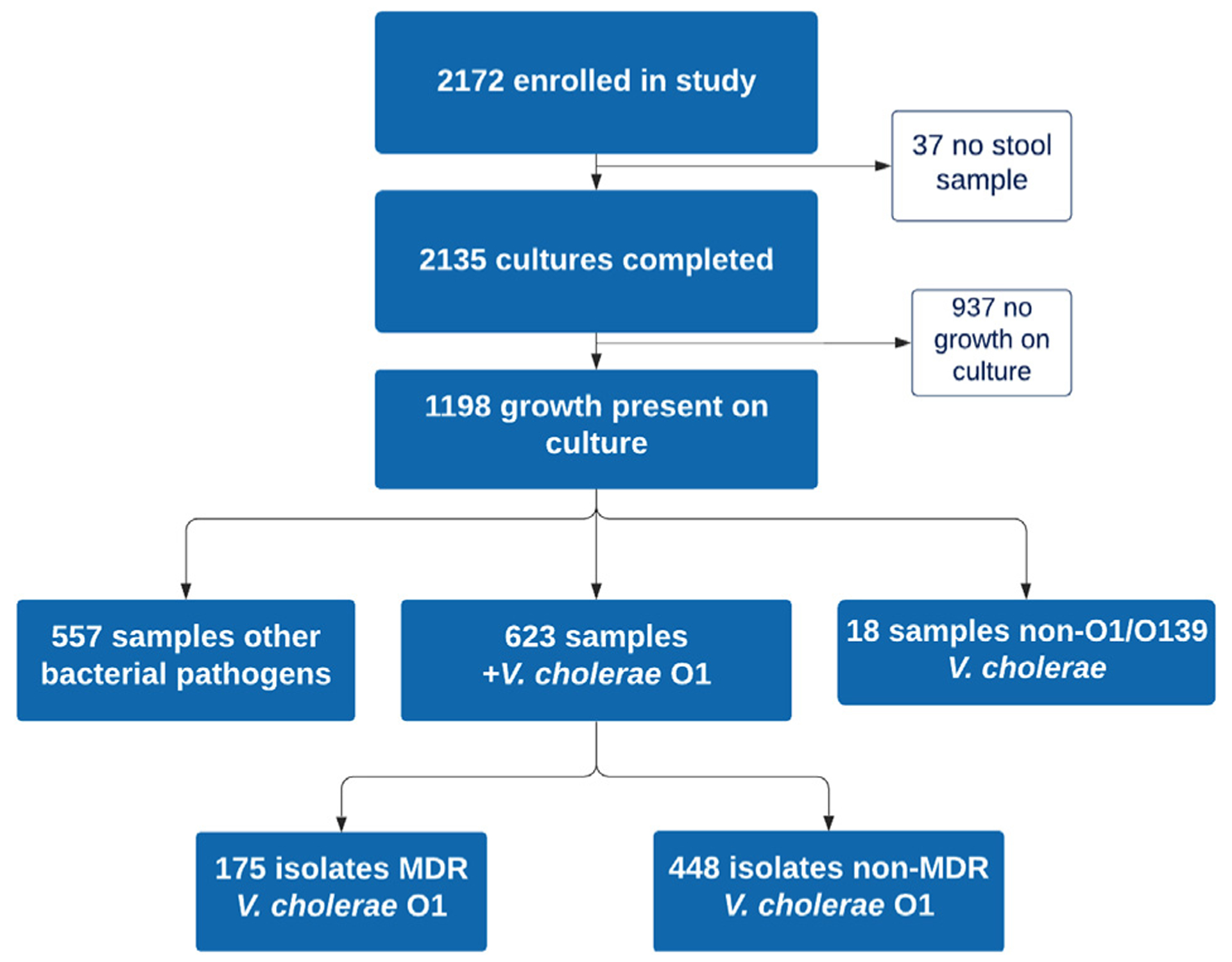
Study flow chart.
The AST profile of V. cholerae O1 isolates is shown in Figure 2. Isolates demonstrated the highest resistance to TMP-SMX (99.7%), erythromycin (99.2%), ampicillin (88.9%), and amoxicillin-clavulanate (61.6%). In contrast, there was high susceptibility to tetracycline and doxycycline (both 99.7%), ciprofloxacin (99.8%), and azithromycin (99.2%). Susceptibility was high to all cephalosporins tested. Isolates had 100% sensitivity to cefoperazone-sulbactam and tigecycline. A total of 175 (28.1%) isolates were found to have MDR with TMP-SMX + erythromycin + ampicillin or amoxicillin-clavulanate as the most common MDR patterns.
Figure 2.
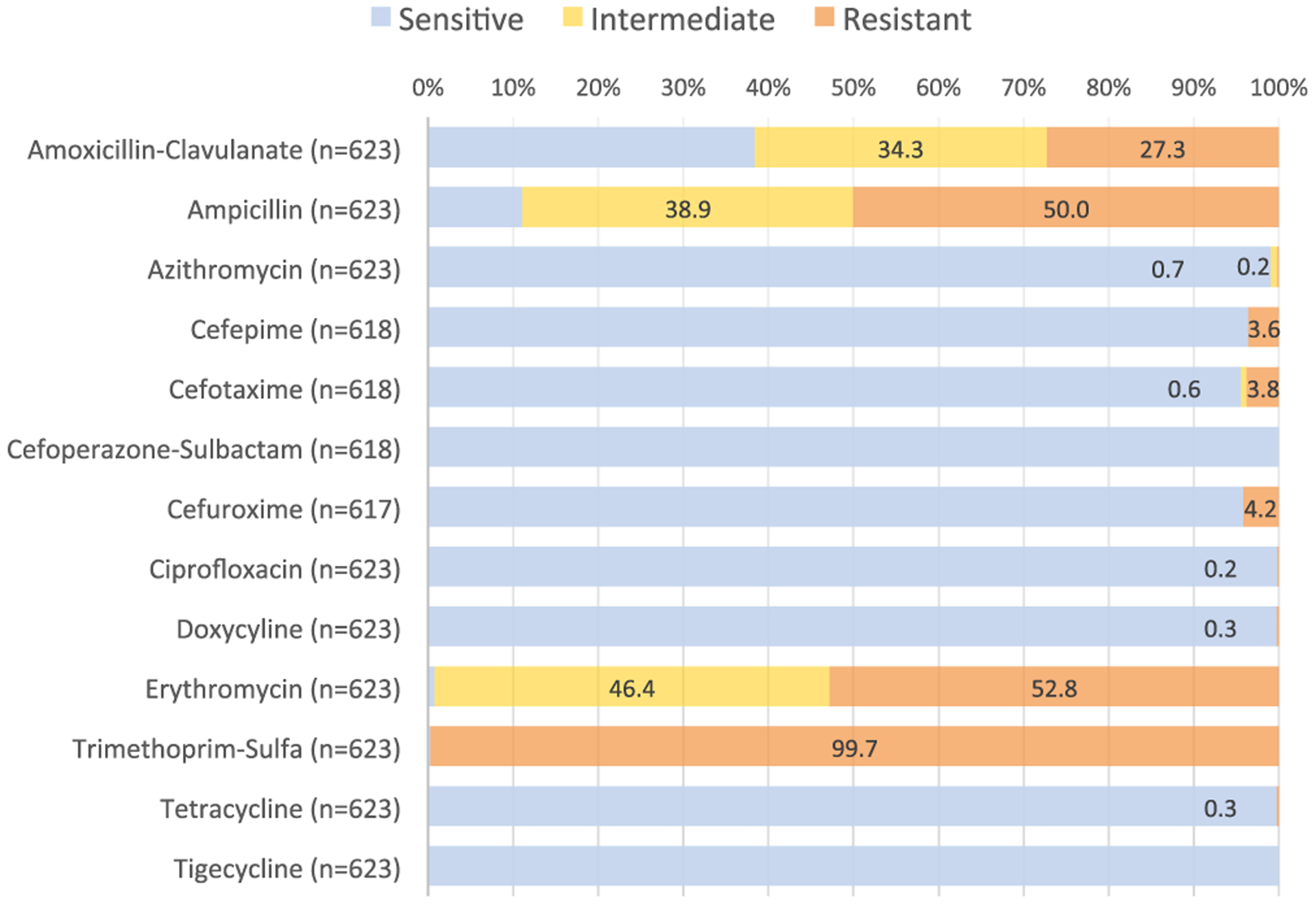
Antibiotic susceptibility profile of Vibrio cholerae O1 isolates.
In univariate analysis, prior antibiotic use (OR 2.17; 95% CI 1.51–3.13) and greater stool frequency (>10 episodes OR 1.80; 95% CI 1.20–2.70; >20 episodes OR 2.61; 95% CI 1.48–4.59) were positively associated with the presence of MDR (Table 1). Flush toilet use (versus other waste facility such as pit latrine or open defecation) was negatively associated (OR 0.33; 95% CI 0.23–0.48; Table 1). Mean heart rate, temperature, and arterial blood pressure (MAP) were lower in those with MDR compared to those without (Table 2).
Table 1.
Univariate and multiple logistic regression analysis for categorical variables associated with multidrug-resistant Vibrio cholerae O1.
| Variable | MDR (n = 175) | Non-MDR (n = 448) | Univariate | Multiple Logistic Regression | ||
|---|---|---|---|---|---|---|
| Categorical | N(%) | N (%) | OR (95% CI) | p | aOR (95% CI) | p |
| Male Sex | 88 (50.29) | 258 (57.59) | 0.74 (0.52–1.06) | 0.100 | 0.72 (0.47–1.10) | 0.132 |
| Altered Mental Status | 6 (3.43) | 37 (8.26) | 0.39 (0.16–0.95) | 0.038 | 0.42 (0.15–1.19) | 0.103 |
| Bloody Stool Reported | 1 (0.57) | 1 (0.22) | 2.57 (0.16–41.30) | 0.506 | 2.14 (0.03–140.18) | 0.721 |
| Mucoid Stool Reported | 32 (18.29) | 79 (17.63) | 1.04 (0.66–1.65) | 0.848 | 1.08 (0.67–1.85) | 0.769 |
| Abdominal pain | 71 (40.57) | 176 (39.29) | 1.06 (0.74–1.51) | 0.768 | 0.95 (0.63–1.45) | 0.827 |
| Vomiting (>3 episodes/24 h) | 132 (75.4) | 327 (73.0) | 1.14 (0.76–1.70) | 0.535 | 1.08 (0.67–1.75) | 0.755 |
| Diarrhea Frequency | 0.001* | 0.046* | ||||
| ≤10 episodes/24 h | 45 (25.71) | 180 (40.18) | - | - | ||
| >10 episodes/24 h | 100 (57.14) | 222 (49.55) | 1.80 (1.20–2.70) | 1.52 (0.95–2.42) | ||
| >20 episodes/24 h | 30 (17.14) | 46 (10.27) | 2.61 (1.48–4.59) | 2.21 (1.14–4.27) | ||
| Prior Antibiotic Useb | 78 (44.57) | 121 (27.01) | 2.17 (1.51–3.13) | <0.001* | 2.18 (1.42–3.36) | <0.001* |
| Sick Contacts in Household | 27 (21.14) | 109 (24.33) | 0.83 (0.55–1.27) | 0.399 | 0.93 (0.57–1.52) | 0.767 |
| Water Source - Indoor Piped | 130 (74.29) | 321 (71.65) | 1.14 (0.77–1.70) | 0.509 | 1.08 (0.65–1.79) | 0.768 |
| Use of Treated Watera | 192 (42.86) | 256 (57.14) | 0.89 (0.62–1.27) | 0.516 | 0.95 (0.60–1.50) | 0.830 |
| Flush Toilet Use | 65 (37.14) | 286 (63.84) | 0.33 (0.23–0.48) | <0.001* | 0.41 (0.27–0.63) | <0.001* |
| Highest Education Level | 0.989 | 0.469 | ||||
| None | 44 (25.14) | 116 (25.89) | - | - | ||
| Primary School | 65 (37.14) | 161 (35.94) | 1.06 (0.68–1.67) | 1.65 (0.88–3.06) | ||
| Junior Secondary School | 31 (17.71) | 78 (17.41) | 1.05 (0.61–1.80) | 1.55 (0.73–3.29) | ||
| Secondary School or Higher | 35 (20.00) | 93 (20.76) | 0.99 (0.59–1.67) | 1.50 (0.71–3.19) | ||
| >5 ppl in Household | 52 (29.71) | 156 (34.82) | 0.79 (0.54–1.15) | 0.225 | 0.86 (0.55–1.34) | 0.510 |
| >90-min Transport to Hospital | 40 (22.9) | 74 (16.52) | 1.50 (0.97–2.31) | 0.067 | 1.71 (1.01–2.92) | 0.048* |
Abbreviations: ORodds ratio; aORadjusted odds ratio; CIconfidence interval; pplpeople.
-:Reference level.
p < 0.05.
Any water treatment including chlorine treatment, boiling, filtering water.
Prior antibiotic use defined as any antibiotic use reported for the current illness before hospital arrival.
Table 2.
Univariate and multiple logistic regression analysis for continuous variables associated with multidrug-resistant Vibrio cholerae O1.
| Variable | MDR (n = 175) | Non-MDR (n = 448) | Univariate | Multiple Logistic Regression | ||
|---|---|---|---|---|---|---|
| Continuous | (mean ± SD) | (mean ± SD) | Mean Difference | p | aOR (95% CI) | p |
| Age (years) | 35.83 ± 1.78 | 33.03 ± 21.07 | 2.80 ± 1.94 | 0.151 | 1.00 (0.99–1.02) | 0.900 |
| Temperature (F) | 97.12 ± 0.80 | 97.68 ± 0.94 | 0.56 ± 0.08 | <0.001* | 0.44 (0.32–0.59) | <0.001* |
| Respiratory Rate (rpm) | 28.50 ± 5.17 | 29.00 ± 5.87 | 0.493 ± 0.51 | 0.331 | 0.97 (0.93–1.01) | 0.093 |
| Heart Rate (bpm) | 100.43 ± 24.02 | 107.52 ± 22.78 | 7.10 ± 2.06 | 0.001* | 0.99 (0.98–1.00) | 0.113 |
| MAP (mmHg) | 65.74 ± 14.10 | 71.85 ± 15.36 | 6.11 ± 1.33 | <0.001* | 0.98 (0.97–0.99) | 0.008* |
| MUAC (cm) | 23.06 ± 3.17 | 23.09 ± 4.09 | 0.04 ± 0.34 | 0.915 | 0.89 (0.83–0.96) | 0.002* |
| Percent Dehydration (%) | 5.92 ± 2.58 | 6.40 ± 3.11 | 0.48 ± 0.27 | 0.070 | 0.88 (0.82–0.96) | 0.003* |
| Monthly Household Income ($100USD) | 1.95 ± 1.52 | 1.83 ± 1.24 | 0.13 ± 0.12 | 0.280 | 1.22 (1.04–1.43) | 0.014* |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; MUAC, mid-upper arm circumference; MAP, mean arterial pressure; mmHg, millimeters of mercury; rpm, respirations per minute, bpm, beats per minute.
-: Reference level.
p < 0.05.
In multiple logistic regression analysis, prior antibiotic use (OR 2.25, 95% CI 1.42–3.36), >90-minute transport time to hospital (OR 1.71; 95% CI 1.01–2.92), greater stool frequency (>10 episodes OR 1.52; 95% CI 0.95–2.42; >20 episodes OR 2.21; 95% CI 1.14–4.27), higher monthly household income (OR 1.22; 95% CI 1.04–1.43), lower MAP (OR 0.98; 95% CI 0.97–0.99), lower MUAC (0.89; 95% CI 0.83–0.96) and lower percent dehydration (0.89; 95% CI 0.82–0.96) were associated with MDR (Tables 1 and 2). Flush toilet use was negatively associated with MDR (OR 0.41; 95% CI 0.27–0.63). The AUC of the multiple regression model for the outcome of MDR was 0.794. Nagelkerke’s pseudo-R2 was 0.298, indicating the modest explanatory power of the model.
Discussion
Multidrug-resistant V. cholerae O1 presents a major challenge for treating patients in areas with endemic cholera such as Bangladesh. While antimicrobial use and selection decisions should be guided by microbiological testing, these tests are unavailable in the vast majority of LMIC clinical settings. As a result, clinicians often decide on antimicrobial treatment empirically, based on syndromic guidelines and clinician suspicion, although a few studies have evaluated patient risk factors of MDR among enteric pathogens. This study found that MDR V. cholerae O1 was common in older children and adults with diarrhea in urban Bangladesh, with nearly one-third of V. cholerae O1 isolates demonstrating MDR. This rate is higher than the 18% MDR rate found in V. cholerae from a study in Dhaka in 2013, suggesting a potential increase of MDR in recent years (Das et al., 2013). This is a concerning finding given that 100% MDR V. cholera rates have been reported in two studies (2015 and 2019) in Nepal, as well as in a five-country study in sub-Saharan Africa in 2015 (Rijal et al., 2019; Smith et al., 2015; Thapa Shrestha et al., 2015).
Multiple individual-level factors were found to be significantly associated with MDR V. cholerae O1, which may enable more informed use of antimicrobial testing and selection in patients at high-risk for MDR. Reported antibiotic use before hospital presentation was strongly associated with MDR, emphasizing the critical role of assessing an individual’s antibiotic exposure history in determining risk for MDR infections. This finding is consistent with multiple other studies and known mechanisms of acquired resistance, demonstrating that patient antibiotic use places selective pressure towards MDR (Das et al., 2013). Antibiotic use was common, with nearly one-third of subjects reporting antibiotic use prior to hospital presentation, consistent with a study that found 39% home (non-prescription) antibiotic use among young children in rural Bangladesh (Mirzapur) (Ahmed et al., 2018).
Several physiologic characteristics were also found to be associated with MDR, including lower MAPs (6 mm Hg) and temperature (0.6 degrees F) than non-MDR subjects. As fever is rare in cholera, lower body temperatures and MAPs found in patients with MDR may reflect progression towards shock states, although, given the fairly small temperature difference, this finding’s clinical significance is unclear. Greater stool frequency (>20 episodes/24 h) was also associated with MDR and consistent with prior studies’ findings that MDR enteric infections are associated with greater severity of illness (Das et al., 2013). The mean percent dehydration was slightly lower (5.9%) in patients with MDR versus those with non-MDR (6.4%). However, this may be confounded by patients with access to antibiotics prior to presentation also having greater access to oral or intravenous rehydration fluids prior to arrival.
Several socio-environmental factors, including non-flush toilet use, longer transport time to hospital, and higher household income, were also associated with MDR. Patients living a greater distance from the hospital are more likely to come from suburban/rural areas with less access to trained health professionals, and more unprescribed antibiotic use may account for this finding. Higher household income has been associated with AMR in prior studies, possibly related to more antibiotic overuse in the private sector or higher AMR in more wealthy urban centers (Gruninger et al., 2017). Additionally, patients with more severe illness (such as from difficult-to-treat MDR pathogens) may prompt individuals to travel longer distances to the hospital versus seeking care at closer primary care facilities or may reflect differences between rural and urban locations (Das et al., 2013). While flush toilet use may be considered a proxy for higher income levels, flush toilet use (versus other waste facility use) was associated with lower odds of having MDR after adjusting for household income in the multivariate analysis. This finding suggests the vital role of improved sanitation systems in disrupting cycles of oral-fecal transmission of enteric pathogens.
Patients with lower MUAC (a marker of malnutrition) were also found to have greater odds of MDR. Malnutrition is strongly associated with an increased likelihood and greater severity of enteric infections, although there is a lack of research on the association specifically with MDR (Mata, 1992). Malnutrition is both a cause and consequence of acute infections, with increased frequency and severity of enteric infections due to impaired intake, digestion and absorption, nutrient losses, and altered immune responses (Bourke et al., 2016; Mata, 1992; Mondal et al., 2012). Given the small difference in mean MUAC between MDR and non-MDR groups, this finding’s clinical relevance may be spurious, although the link between malnutrition and enteric infections in older individuals is poorly described and warrants further study. A 2017 study of MDR among children under 15 years with diarrhea in Kenya showed that younger children (particularly those <24 months), acute malnutrition, and poor sanitation contributed to an increased risk of MDR enteric pathogens (Brander et al., 2017). However, age was not found to be associated with MDR in this population. Reasons for this may be due to a consistently high overall use of antibiotics among older children and adults, whereas more significant discrepancies in antibiotic use may exist in early childhood (Brander et al., 2017). These findings suggest that age-specific antimicrobial recommendations for cholera may not be warranted in older individuals.
V. cholerae O1 isolates in this study showed high susceptibility to azithromycin, ciprofloxacin, and cephalosporins and high levels of resistance to TMP-SMX, penicillins, and erythromycin, similar to findings from recent years in South Asia (Parvin et al., 2020). Resistance to these drugs has been hypothesized to be driven by overuse of these antibiotics, for both diarrheal illness as well as a multitude of other acute infectious illnesses. Notably, TMP-SMX and erythromycin have been previously used as first-line treatment for cholera in the region, which is a likely reason for the notably high resistance of the V. cholerae isolates to these antibiotics (Ceccarelli et al., 2016). However, susceptibility to tetracycline and doxycycline was notably higher (>99% susceptibility) than previously reported at icddr,b, which found a reversal in susceptibility to 76% in 2018 after <6% susceptibility between 2012–2017 (Parvin et al., 2020). A reversal to complete susceptibility has also been shown in Nepal’s recent national surveillance study (Rijal et al., 2019). AMR patterns have been shown to fluctuate rapidly in V. cholerae because it cannot stably carry resistance plasmids, and the organism naturally resides in aquatic environments without selective pressure from antibiotics (Rijal et al., 2019). Recent calls to change antibiotic guidelines to return to use of highly cost-effective tetracyclines for V. cholerae O1 have been suggested (Parvin et al., 2020). The study findings support this and highlight the vital role of antibiotic stewardship efforts in influencing AMR rates in LMICs.
Limitations & future directions
This data was obtained from a single study site and year and may not be generalizable to other populations or time periods. Selection bias may have occurred as icddr,b is a non-profit, urban referral hospital and may not reflect patients with more mild illness, those in rural areas, or wealthier patients more likely to attend private clinics. However, our findings are likely generalizable to other urban healthcare facilities in Bangladesh, a country with an extremely high burden of diarrheal disease. Additionally, while a wide variety of variables was included, unmeasured variables, including those shown to be associated with MDR (e.g., frequency of antibiotic use, prior hospitalizations, and chronic comorbidities), may confound the present results (Bischoff et al., 2018; Kristinsson, 1997). However, additional variables could not be assessed given the study’s retrospective nature. The modest pseudo-R2 suggests that other variables should be considered, to build a more robust predictive model of MDR V. cholerae O1. However, the model’s AUC for MDR was good (0.794), indicating that the included variables may accurately discriminate MDR in this population. Lastly, while the disk diffusion method of AST is common in LMICs due to its simplicity and low cost, these tests provide only a qualitative (sensitive, intermediate, or resistant) result, rather than quantitative result such as provided by dilutional methods, which generate a minimum inhibitory concentration (MIC) (Reller et al., 2003).
This study’s strengths include the large sample size, the broad age range included, and the robust clinical data collected to contextualize the AMR data. Further research is needed to confirm if MDR V. cholerae O1 is associated with more severe infections and the best strategy for clinical management of MDR cholera. Given the variability in AMR patterns, there is a great need to monitor resistance patterns locally and to continuously adapt treatment recommendations. Research developing similar models into clinical tools may help clinicians better identify patients at the highest MDR risk. More informed decision-making regarding antibiotic use and selection may subsequently help curb the development of MDR among V. cholerae and other enteric pathogens in LMICs.
Acknowledgments
The authors thank all study participants and the icddr,b Dhaka Hospital study staff for their help and support.
Funding
Funding for data collection was provided through grants from the NIH National Institute for Diabetes and Digestive and Kidney Diseases (DK116163). The funders had no role in the study design, data collection, data analysis, interpretation of data, or the writing or decision to submit the manuscript for publication.
Footnotes
Conflict of interest
None declared.
Ethical approval
Ethical approval was not required for this secondary analysis of de-identified data. Ethical approval for the NIRUDAK Study was obtained from the icddr,b Ethical Review Committee and Rhode Island Hospital Institutional Review Board.
References
- Ahmed S, Korpe P, Ahmed T, Chisti MJ, Faruque ASG. Burden and risk factors of antimicrobial use in children less than 5 years of age with diarrheal illness in rural Bangladesh. Am J Trop Med Hyg 2018;98:1571–6, doi: 10.4269/ajtmh.17-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azman AS, Lauer SA, Bhuiyan TR, Luquero FJ, Leung DT, Hegde ST, et al. Vibrio cholerae O1 transmission in Bangladesh: insights from a nationally representative serosurvey. Lancet Microbe 2020;1:e336–43, doi: 10.1016/S2666-5247(20)30141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis 2018;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 2016;37:386–98, doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander RL, Walson JL, John-Stewart GC, Naulikha JM, Ndonye J, Kipkemoi N, et al. Correlates of multidrug non-susceptibility in enteric bacteria isolated from Kenyan children with acute diarrhea. PLoS Negl Trop Dis 2017;11:1–18, doi: 10.1371/journal.pntd.0005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli D, Alam M, Huq A, Colwell RR. Reduced susceptibility to extended-spectrum βlactams in Vibrio Cholerae isolated in Bangladesh. Front Public Heal 2016;4:, doi: 10.3389/FPUBH.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests, approved standard-CLSI document M02-A11. 13th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- Das SK, Klontz EH, Azmi IJ, Ud-Din AIMS, Chisti MJ, Afrad MH, et al. Characteristics of multidrug-resistant Shigella and Vibrio cholerae O1 Infections in patients treated at an urban and a rural hospital in Bangladesh. Int Schol Res Not 2013;2013:, doi: 10.1155/2013/213915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan C, Santosham M, Glass R. The management of acute diarrhea in children: oral rehydration, maintenance and nutritional therapy. MMWR Recomm Rep 1992;41:1–20. [PubMed] [Google Scholar]
- Gainey M, Barry M, Levine AC. Developing a novel mobile health (mHealth) tool to improve dehydration assessment and management in patients with acute diarrhea in resource-limited settings. R I Med J 2019;. [PMC free article] [PubMed] [Google Scholar]
- Global Task Force on Cholera Control. Use of antibiotics for the treatment and control of cholera May 2018. 2018.
- Gruninger RJ, Johnson RA, Das SK, Nelson EJ, Spivak ES, Contreras JR, et al. Socioeconomic determinants of ciprofloxacin-resistant Shigella infections in Bangladeshi children. Pathog Immun 2017;2:89, doi: 10.20411/pai.v2i1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L, Abdelhamid A, Attreed NJ, Campbell WW, Channell AM, Chassagne P, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev 2015;, doi: 10.1002/14651858.CD009647.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson K Effect of antimicrobial use and other risk factors on antimicrobial resistance in pneumococci. Microb Drug Resist 1997;3:117–23. [DOI] [PubMed] [Google Scholar]
- Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, Paul M. Antimicrobial drugs for treating cholera. Cochrane Database Syst Rev 2014;2014:, doi: 10.1002/14651858.CD008625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Glavis-Bloom J, Modi P, Al E. Empirically derived dehydration scoring and decision tree models for children with diarrhea: assessment and internal validation in a prospective cohort study in Dhaka, Bangladesh. Glob Heal Sci Pr 2015;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81, doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Mandal S, Mandal MD, Pal NK. Cholera: a great global concern. Asian Pac J Trop Med 2011;4:573–80, doi: 10.1016/S1995-7645(11)60149-1. [DOI] [PubMed] [Google Scholar]
- Mata L Diarrheal disease as a cause of malnutrition. Am J Trop Med Hyg 1992;47:16–27. [DOI] [PubMed] [Google Scholar]
- Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 2012;54:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, Morgan DR. Manual of clinical microbiology (6th ed). Trends Microbiol 1995;3:449. [Google Scholar]
- Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika 1991;78:691–2. [Google Scholar]
- Parvin I, Shahunja KM, Khan SH, Alam T, Shahrin L, Ackhter MM, et al. Changing susceptibility pattern of Vibrio cholerae O1 isolates to commonly used antibiotics in the largest diarrheal disease hospital in Bangladesh during 2000–2018. Am J Trop Med Hyg 2020; tpmd200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, et al. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am J Trop Med Hyg 2003;69:411–9. [PubMed] [Google Scholar]
- Rijal N, Acharya J, Adhikari S, Upadhaya BP, Shakya G, Kansakar P, et al. Changing epidemiology and antimicrobial resistance in Vibrio cholerae: AMR surveillance findings (2006–2016) from Nepal. BMC Infect Dis 2019;19:1–8, doi: 10.1186/s12879-019-4432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med 2006;354:2452–62. [DOI] [PubMed] [Google Scholar]
- Shirreffs S Markers of hydration status. Eur J Clin Nutr 2003;57:9. [DOI] [PubMed] [Google Scholar]
- Sjölund-Karlsson M, Reimer A, Folster JP, Walker M, Dahourou GA, Batra DG, et al. Drug-resistance mechanisms in Vibrio Cholerae O1outbreak strain, Haiti, 2010. Emerg Infect Dis 2011;17:2151–4, doi: 10.3201/eid1711.110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Njanpop-Lafourcade B-M, Mengel MA, Gessner BD, Sauvageot D, Bidjada B, et al. Comparative characterization of Vibrio cholerae O1 from five Sub-Saharan African countries using various phenotypic and genotypic techniques. PLoS One 2015;10:e0142989, doi: 10.1371/journal.pone.0142989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, DeWalt D, Byerley J. Is this child dehydrated?. JAMA 2004;291:2746–54. [DOI] [PubMed] [Google Scholar]
- Thapa Shrestha U, Adhikari N, Maharjan R, Banjara MR, Rijal KR, Basnyat SR, et al. Multidrug-resistant Vibrio cholerae O1 from clinical and environmental samples in Kathmandu city. BMC Infect Dis 2015;15:104, doi: 10.1186/s12879-015-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018;18:1211–28, doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola CL. The antibiotic resistance crisis: causes and threats. PTJ 2015;40:277–83 https://doi.org/Article. [PMC free article] [PubMed] [Google Scholar]


