Abstract
Keratomycosis or mycotic keratitis is recognized as one of the major causes of ophthalmic morbidity worldwide. The most common organisms linked to keratomycosis include Candida spp., Fusarium spp., and Aspergillus spp. However, varieties of saprobic fungi have been reported as causative agents of keratomycosis. Amongst these are members of the genus Colletotrichum. Herein we present the first reported case of C. chlorophyti infection in a post-corneal transplant patient, suggesting an increasing role for Colletotrichum species as emerging human pathogens, particularly in the transplant population.
Keywords: Colletotrichum chlorophyti, Fungal, Keratitis, Emerging, Pathogen
Highlights
-
•
Keratomycosis is a major cause of ophthalmic morbidity and blindness worldwide.
-
•
Colletotrichum spp are increasingly recognized as etiologic agents of keratomycosis.
-
•
This is the first report of human infection by Colletotrichum chlorophyti.
-
•
Differentiation among the Colletotrichum species is phenotypically difficult, requiring further characterization at a molecular level.
1. Introduction
Keratomycosis, or mycotic keratitis, is a major cause of ophthalmic morbidity, and can cause blindness [1,2]. Most keratomycoses are linked to Candida spp., Fusarium spp., and Aspergillus spp, although various saprobic fungi [3,4], including several Colletotrichum species, have been reported to cause non-traumatic [3] and traumatic [5] keratomycosis. Here, we describe the first reported human infection caused by the plant pathogen Colletotrichum chlorophyti, in a patient with keratomycosis and endophthalmitis following corneal transplant, highlighting its potential role in human disease.
2. Case
An 82-year-old man was referred for management of a failed corneal transplant with a disorganized anterior chamber presumed due to fungal infection. Eight months earlier, he underwent corneal transplantation in his left eye, which failed, requiring a repeat corneal transplant six weeks after the initial surgery. Histopathology of specimens from the second surgery demonstrated fungal hyphae in the previous graft, but cultures were without growth. Otherwise, the patient history was notable only for hypertension. He denied trauma, contact lens use, recent travel, or exposures to plants, pets, animals, or environmental debris.
Following removal of the intraocular lens implant, anterior chamber reconstruction, pars plana vitrectomy and a third corneal transplantation (Day 0), he appeared to improve, but during the third postoperative week developed white, fluffy pre/intraretinal lesions. Microscopic examination of the vitreous fluid was unrevealing, and cultures were without growth. Empiric treatment was started with weekly intravitreal voriconazole injections (50mcg/0.1ml) for a total six doses, plus topical and oral voriconazole (200 mg BID) for 8 days. After two weeks, examination of a repeat vitreous fluid sample (week 9) demonstrated hyphal elements, and intravitreal amphotericin B injection was added (5mcg/0.1ml) for a total four doses. Over the next ten days, the corneal and retinal infiltrates worsened, with development of a traction retinal detachment. A repeat corneal transplant with pars plana vitrectomy, membrane peel, endolaser and silicone oil injection was performed on week 11. Postoperatively, the patient did not tolerate intravenous amphotericin B. Treatment with weekly intravitreal amphotericin B was unsuccessful, requiring enucleation of his left eye 11 months after the initial corneal transplant.
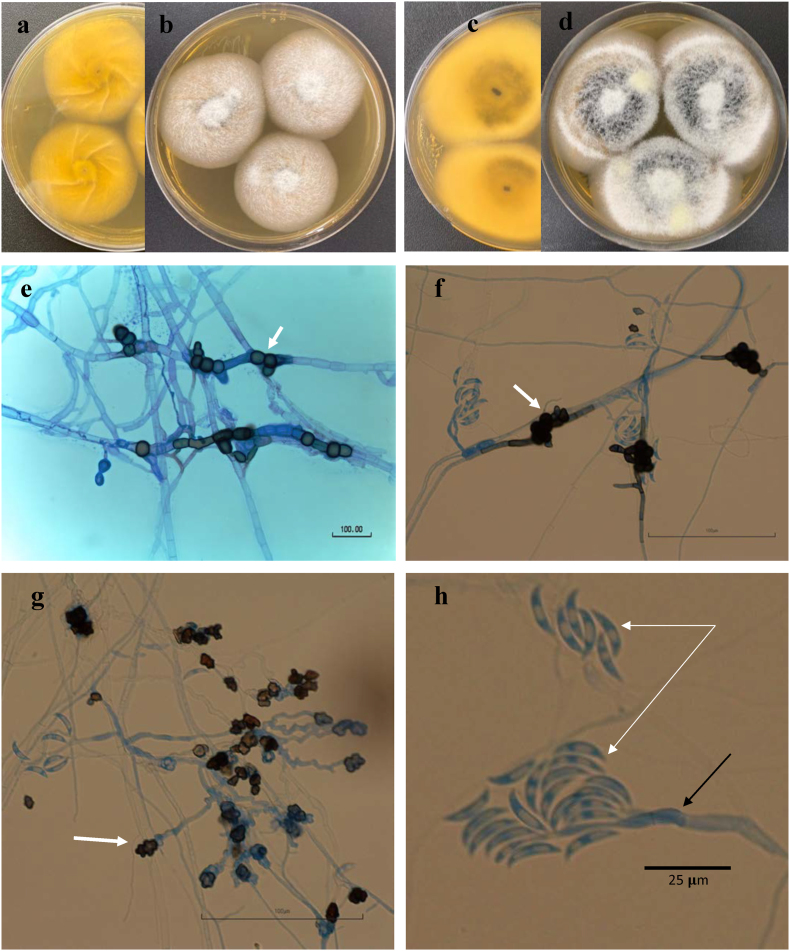
Histopathology of vitreous fluid cytospin and corneal and retinal tissues (Fig. 1), confirmed the diagnosis of acute fungal keratitis and endophthalmitis. Vitreous fluid was inoculated to Sabouraud-dextrose agar, and after one-week incubation at 25 °C yielded growth of a filamentous fungus. Colonies exhibited dark-brown pigment with a lighter brown reverse (Fig. 2). Microscopic examination of lactophenol blue-stained slide culture-mounts demonstrated many branching, septate hyaline and phaeoid filaments with brown-colored, crenated, lobed appresoria (Fig. 2).
Fig. 1.
Fungal keratitis (a–d). Periodic acid–Schiff (PAS)–depicting fungal elements (a). Higher magnification showing multiple hyphae (PAS, original magnification × 100) and numerous infiltrating eosinophils (b). Grocott-Gomori methenamine-silver (GMS) stain highlighting fungal elements (c). Branching hyphae throughout the corneal stroma (GMS, original magnification ×100).
Fig. 2.
Colletotrichum chlorophyti (from UTHSCSA DI19-239 isolate): colony on MEA at 25 °C: 5d, (a) reverse - (b) obverse; 7d, (c) reverse - (d) obverse; (e–f) chlamydospores (arrow); (g) appressoria (arrow); (h) conidia (white arrow), conidiophore (black arrow). e: from Sabouraud dextrose agar (SDA), f-h from PFA, 14d at 25 °C.
Preliminary identification of our isolate as Colletotrichum chlorophyti was based on colony growth, conidial morphology (Methods S1), and BLASTn searches in GenBank of the ITS and BenA sequences. Top matches in BLASTn searches were: 99–100% with C. chlorophyti followed by 98–99% C. phaseolorum for ITS, and 98–99% with C. chlorophyti followed by 96% with C. phaseolorum for BenA. Confirmatory phylogenetic analyses were done with GADPH as additional genE (Methods S2). Isolate UTHSCSA DI19-239 was confirmed as Colletotrichum chlorophyti as shown in 3 individual gene-datasets and combined. Phylograms for each individual loci and combined (Appendix A, Appendix A, Appendix A, Appendix A, Appendix A, Appendix A, Appendix A, Appendix A) were concordant and showed that our isolate clustered with C. chlorophyti strains with high support of 0.99–1.00 BPP and 87–100% BT. A close relationship of C. phaseolorum and C. chlorophyti is also shown in all three loci individually, and combined. The growth and morphology of the organism along with the sequencing data were consistent with the published description of C. chlorophyti.
Antifungal susceptibility testing was performed by broth microdilution following standard methods [6]. Minimum inhibitory concentrations (MIC) for amphotericin B, itraconazole, posaconazole, and voriconazole were read as the lowest concentration resulting in 100% inhibition of growth. For the echinocandins anidulafungin, caspofungin, and micafungin, the minimum effective concentrations (MEC) were read as the lowest concentration resulting in morphologic abnormalities. Good activity was found for amphotericin B (MIC 0.125 μg/ml), but activity was reduced for the azoles itraconazole (4 μg/ml), voriconazole (2 μg/ml), and posaconazole (1 μg/ml). The MEC values were consistent among the echinocandins (0.25 μg/ml for each).
3. Discussion
The genus Colletotrichum includes several hundred species within the coelomycetous Glomerellales, that are traditionally recognized as the anamorph (asexual) stages of the family Glomerellaceae, lacking stromatic tissue, and often appearing as hyphomycete-like in culture by producing sporodochium-like acervuli [7]. Although isolated more frequently in tropical and subtropical areas, Colletotrichum species are found worldwide as major pathogens of many herbaceous and woody plants [8]. Uncommonly, Colletotrichum species infections have been reported in insects [9], turtles [10], and humans [8]. Human cases include reports of keratitis, subcutaneous infections and arthritis [8,11], often linked to outdoor and agricultural activities [8]. Rosa et al. described the first association of Colletotrichum with human corneal infection in a 1994 retrospective study [12], followed by a 1997 report of its isolation from a postoperative patient with recurrent fungal keratitis by Ritterband et al [1]. Previously, five Colletotrichum species were reported to cause keratomycoses in humans: C.atramentum, C. graminicola, C. dematium, C. gloeosporioides, and C. truncatum; we now add C. chlorophyti.
C. chlorophyti is a pathogen of common herbaceous plants [8] including crops such as tomato, soybean [13] and legumes [14]. Prior to our report, C. chlorophyti had been isolated only from leaves and seeds; no other environmental sources or human infection had been reported. Because routine morphological methods do not allow species level identification, we combined phenotypic characterization, molecular methods supplemented by ITS sequencing, and confirmatory phylogenetic analysis, to identify our isolate.
Infections with Colletotrichum species typically follow fungal implantation associated with corneal trauma [5] but also can occur in patients without known trauma [3]. Pre-existing corneal abrasions [15] may predispose to spore colonization and infection; similar strategies are used by these pathogens to invade plant cells [4]. Consistently, the most important risks reported are exposure to potentially colonized tree branches, thorns or leaves [4], aerial debris (sand and dust) or insects [4], or other foreign body implantation [3]. Diabetes mellitus and corticosteroid (topical or systemic) therapy have been identified as predisposing factors for Colletotrichum keratitis [16]. In industrialized countries, widespread contact lens use and eye surgery may also predispose to infection [3]. As in our patient, invasion into deeper tissues may follow corneal involvement [17].
In the absence of other contributing factors in our patient, fungal contamination from a donor cornea must be considered. Although fungal infections after corneal transplantation have been rare, the sequelae can be devastating, and the potential for fungal contamination of donor corneas and post-keratoplasty fungal infections has become a trending issue. According to a 2013 report from the Eye Bank Association of America, post-transplant infections almost doubled between 2008 (0.009%) and 2010 (0.016%), with fungal outnumbering bacterial pathogens [18].
In conclusion, Colletotrichum species primarily cause opportunistic infections in patients with local trauma or underlying immune suppression. Previously, C. chlorophyti had been reported as a pathogen only in plants; this is the first report of human infection by C. chlorophyti. Our case serves as a reminder that common plant pathogens can cause human disease in select circumstances, and highlights potential benefits from optimized corneal donor screening and inclusion of antifungal supplementation in storage solutions in eye banks.
Declaration of competing interest
The authors declare not to have any conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mmcr.2021.04.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ritterband D.C., Shah M., Seedor J.A. Colletotrichum graminicola: a new corneal pathogen. Cornea. 1997;16(3):362–364. PMID: 9143813. [PubMed] [Google Scholar]
- 2.Tabatabaei S.A., Tabatabaei M., Soleimani M., Tafti Z.F. Fungal keratitis caused by rare organisms. J Curr Ophthalmol. 2017;30(1):91–96. doi: 10.1016/j.joco.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llamos R., Al-Hatmi A.M., Martínez G., Hagen F., Velar R., de la Caridad Castillo Pérez A. Non-traumatic keratitis due to Colletotrichum truncatum. JMM Case Rep. 2016;3(4) doi: 10.1099/jmmcr.0.005047. e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchta V., Nekolová J., Jirásková N., Bolehovská R., Wipler J., Hubka V. Fungal keratitis caused by Colletotrichum dematium: case study and review. Mycopathologia. 2019;184:441–453. doi: 10.1007/s11046-019-00335-w. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan S.V., Rekha N.S., Sharda R.D., Mahalingam N. Colletotrichum keratitis: a rare but definite clinical entity. J. Clin. Diagn. Res. 2013;7(7):1430–1433. doi: 10.7860/JCDR/2013/5513.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference C.L.S.I. third ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. [Google Scholar]
- 7.Seifert K.A., Gams W. The genera of Hyphomycetes - 2011 update. Persoonia. 2011;27:119–129. doi: 10.3767/003158511X617435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon P.F., Damm U., Johnston P.R., Weir B.S. Colletotrichum - current status and future directions. Stud. Mycol. 2012;73(1):181–213. doi: 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcelino J., Giordano R., Gouli S., Gouli V., Parker B.L., Skinner M. Colletotrichum acutatum var. fioriniae (teleomorph: glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia. 2008;100(3):353–374. doi: 10.3852/07-174r. [DOI] [PubMed] [Google Scholar]
- 10.Manire C.A., Rhinehart H.L., Sutton D.A., Thompson E.H., Rinaldi M.G., Buck J.D. Disseminated mycotic infection caused by Colletotrichum acutatum in a Kemp's ridley sea turtle (Lepidochelys kempi) J. Clin. Microbiol. 2002;40(11):4273–4280. doi: 10.1128/jcm.40.11.4273-4280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho J., Sharma R., Sutton D., Wiederhold N., Sanders C., Wickes B. Fungal arthritis secondary to Colletotrichum gloeosporioides. JMM Case Rep. 2015;2(1) doi: 10.1099/jmmcr.0.000012. [DOI] [Google Scholar]
- 12.Rosa R.H., Jr., Miller D., Alfonso E.C. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101(6):1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 13.Yang H.C., Stewart J.M., Hartman G.L. First report of Colletotrichum chlorophyti infecting soybean seed in Arkansas, United States. Plant Dis. 2013;97(11):1510. doi: 10.1094/PDIS-04-13-0441-PDN. [DOI] [PubMed] [Google Scholar]
- 14.Damm U., Woudenberg J.H.C., Cannon P.F., Crous P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009;39:45–87. [Google Scholar]
- 15.Pote S.T., Chakraborty A., Lahiri K.K., Patole M.S., Deshmukh R.A., Shah S.R. Keratitis by a rare pathogen Colletotrichum gloeosporioides: a case report. J. Mycol. Med. 2017;27(3):407–411. doi: 10.1016/j.mycmed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez V., Dursun D., Miller D., Alfonso E.C. Colletotrichum keratitis. Am. J. Ophthalmol. 2002;134(3):435–438. doi: 10.1016/s0002-9394(02)01576-3. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti A., Shivaprakash M.R., Singh R., Tarai B., George V.K., Fomda B.A. Fungal endophthalmitis: fourteen years' experience from a center in India. Retina. 2008;28(10):1400–1407. doi: 10.1097/iae.0b013e318185e943. [DOI] [PubMed] [Google Scholar]
- 18.Thareja T., Kowalski R., Kamyar R., Dhaliwal D., Jeng B.H., Tu E. Fungal infection after keratoplasty and the role of antifungal supplementation to storage solution: a review. Br. J. Ophthalmol. 2020;104(8):1036. doi: 10.1136/bjophthalmol-2019-314664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.