Abstract
Purpose
The purpose of this study was to evaluate computed tomography and magnetic resonance imaging of benign trichilemmal cysts and proliferating trichilemmal tumours.
Methods
Nineteen histologically confirmed cutaneous lesions with trichilemmal keratinisation (12 trichilemmal cysts and seven proliferating trichilemmal tumours) were enrolled. Among them, 10 lesions (six trichilemmal cysts and four proliferating trichilemmal tumours) were examined by computed tomography, while 13 lesions (eight trichilemmal cysts and five proliferating trichilemmal tumours) were examined by magnetic resonance imaging. Computed tomography and magnetic resonance imaging characteristics were retrospectively reviewed.
RESULTS
Sixteen lesions (84%, 10 trichilemmal cysts and six proliferating trichilemmal tumours) occurred on the scalp. Lobulated margins were observed in five lesions (26%, three trichilemmal cysts and two proliferating trichilemmal tumours). With respect to computed tomography attenuation, calcification (>200 Hounsfield units) was observed in seven lesions (70%, five trichilemmal cysts and two proliferating trichilemmal tumours), hyperdense areas (≥80 and ≤200 Hounsfield units) in six (60%, three trichilemmal cysts and three proliferating trichilemmal tumours), and soft tissue density areas (<80 Hounsfield units) in nine (90%, five trichilemmal cysts and four proliferating trichilemmal tumours). On T1-weighted images, intratumoral hyperintensity was only observed in eight trichilemmal cysts but no proliferating trichilemmal tumours (100% vs. 0%, P<0.01). On T2-weighted images, hypointense rim and intratumoral hypointensity was observed in all 13 lesions (100%, eight trichilemmal cysts and five proliferating trichilemmal tumours), and linear or reticular hypointensity was observed in 10 (77%, six trichilemmal cysts and four proliferating trichilemmal tumours).
Conclusion
Trichilemmal cysts and proliferating trichilemmal tumours predominantly occurred on the scalp with calcification, and usually exhibited linear or reticular T2 hypointensity. Intratumoral T1 hyperintensity may be a useful imaging feature for differentiating trichilemmal cysts from proliferating trichilemmal tumours.
Keywords: Trichilemmal cyst, proliferating trichilemmal tumour, scalp, CT, MRI
Introduction
Trichilemmal cyst (TC), also known as pilar cyst, is a benign common intradermal or subcutaneous cystic lesion. It occurs in 5–10% of the population with a predilection for middle-aged womens. It originates in the external root of the hair follicle and occurs preferentially in areas with dense hair follicle concentration. Approximately 90% of all cases develop on the scalp, accounting for 40% of all scalp benign lesions, but only occasionally on the face, trunk, groin and extremities.1–4 TCs are clinically indistinguishable from epidermoid cysts (ECs), except for their frequency and distribution. Histopathologically, TCs contain abrupt keratinisation and ample eosinophilic cytoplasm without a granular layer, whereas ECs include a granular layer and keratin lamellae within the lumen. Although ECs tend to rupture or become infected, 5 these complications are rare with TCs. 1
In 2% of TCs, proliferating cells in single or multiple foci grow into a proliferating trichilemmal tumour (PTT), which is categorised into three groups: benign, locally aggressive, and malignant in the 2018 World Health Organization classification of skin tumours. 2 Even though both TCs and PTTs have a trichilemmal type of keratinisation and can occur simultaneously, the former is a non-neoplastic cyst while the latter is a solid-cystic neoplasm. PTT develops either de novo or from an existing TC. 2 PTT usually appears as a solitary, slow-growing, exophytic lesion, ranging from 2 cm to 25 cm in size, but sometimes may grow rapidly. 1 Most of the case reports of PTT have shown benign biological behaviour. In rare instances, PTT occurs as local recurrence or distant metastasis and exhibits invasive growth extending beyond the cyst wall and may be indistinguishable from squamous cell carcinomas.2,6
Many case reports have described the computed tomography (CT) and magnetic resonance imaging (MRI) findings of TCs and PTTs.7–15 Although CT and MRI findings 16 and sonographic features 17 of TCs or PTTs have been reported in a few case series, to the best of our knowledge, no studies have published CT and MRI findings of TCs and PTTs. Thus, this study aimed to describe the CT and MRI findings of TCs and PTTs and assess the differences between the two pathologies.
Methods
Patients
This study was approved by the human research committee of the institutional review board, and complied with the guidelines of the Health Insurance Portability and Accountability Act. The requirement for informed consent was waived due to the retrospective nature of the study. The electronic medical record system of our university hospital was searched for patients with histologically confirmed TCs and PTTs using surgically resected specimens between January 2008 and June 2020. Among them, we selected patients who underwent preoperative CT and/or MRI. Nine patients with TCs (four men and five women; age range 32–77 years; mean age 54 years) and seven patients with PTTs (four men and three women; age range 57–82 years; mean age 72 years) were confirmed. One patient with TC had four lesions; therefore, 12 TCs and seven PTTs were included in this study. Ten lesions (six TCs and four PTTs) were examined by unenhanced CT, while 13 lesions (eight TCs and five PTTs) were examined by unenhanced MRI. Patient characteristics are summarised in Table 1. Pathological criteria for a TC were defined as a cyst lined by stratified squamous epithelium and containing abrupt keratinisation without a granular layer. Pathological criteria for a PTT were defined as prominent epithelial infoldings into the cystic lumen with trichilemmal keratinisation.1,2
Table 1.
Patient characteristics of TC and PTT.
| Characteristics | TC | PTT |
|---|---|---|
| Number of patients | n = 9 | n = 7 |
| Age (years) | ||
| Mean | 54.3 | 72 |
| Range | 32–77 | 57–82 |
| Gender | ||
| Male | 4 | 4 |
| Female | 5 | 3 |
| Number of lesions | n = 12 | n = 7 |
| Single | 8 | 7 |
| Multiple | 1 | 0 |
| CT | 6 | 4 |
| MRI | 8 | 5 |
TC: trichilemmal cyst; PTT: proliferating trichilemmal tumour.
CT imaging
CT imaging was performed for six TCs and four PTTs using an 8-slice CT scanner (LightSpeed Ultra; GE Healthcare, Milwaukee, WI, USA), 16-slice CT scanner (LightSpeed 16; GE Healthcare, Milwaukee, WI, USA), or a 64-slice CT scanner (Brilliance CT 64; Philips Medical Systems, Best, The Netherlands). Unenhanced transverse CT images were reconstructed using a soft tissue algorithm at 2.5–5.0 mm section thickness with no overlap.
Magnetic resonance imaging
MRI was performed using a 1.5-T magnetic resonance unit (Intera Achieva 1.5 T Pulsar; Philips Medical Systems, Amsterdam, The Netherlands) for three TCs and three PTTs or a 3-T magnetic resonance unit (Intera Achieva 3.0T Quasar Dual; Philips Medical Systems) for five TCs and two PTTs. All MRI scans were obtained using 20 × 20–24 × 24 cm field of view at 5 mm section thickness with 2 mm intersection gap. Axial and coronal/sagittal T2-weighted fast spin echo (TR/TE, 3170–4841/90–100 ms) and axial T1-weighted spin-echo (TR/TE, 500–782/10–15 ms) images were obtained for 13 lesions (eight TCs and five PTTs). Axial diffusion-weighted images (TR/TE, 4000–5000/67–75 ms) were obtained for nine lesions (five TCs and three PTTs) using a single-shot spin echo echoplanar imaging sequence with a b-value of 0 and 1000 s mm−2.
Imaging data analysis
Two radiologists with 21 and 7 years of post-training experience in musculoskeletal imaging, who were unaware of the clinical information and pathological diagnoses, individually reviewed all images. Any disagreements between the radiologists were resolved by consensus.
The reviewers initially recorded the location of the lesion and assessed its margin, shape and maximum diameter. The margin was classified into either well defined or ill defined, and the shape was classified as either oval/round or lobulated.
Next, the reviewers evaluated the presence and predominance of calcification, hyperdense areas and soft-tissue density areas on CT images. The CT attenuation values of calcification, hyperdense areas and soft tissue density areas were defined as greater than 200 Hounsfield units (HU), 80 HU or greater and 200 HU or less and less than 80 HU, respectively. The number and the maximum diameter of each calcification were also recorded.
Finally, the reviewers evaluated the predominant intensity of T1 and T2-weighted images, the homogeneity, the presence of intratumoral T1 hyperintensity, intratumoral T2 hypointensity and T2 reticular hypointensity on T1 or T2-weighted images. T1 and T2-weighted images were compared with signal intensity of gray matter or spinal cord. The distribution of intratumoral T1 hyperintensity and T2 hypointensity was classified as either focal or diffuse. Linear or reticular T2 hypointensity was defined as either hypointense, fine, septal, or folded linear structures that were visible within the tumour on T2-weighted images. Apparent diffusion coefficient (ADC) values (×10−3 mm2s−1) were measured on ADC maps by placing regions of interest (ROIs) over the lesions. ROIs were placed to encompass as much of the lesion as possible while avoiding cystic components by referring to T1 and T2-weighted images.
Statistical analysis
All statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). The Mann–Whitney U test was used to compare the quantitative results (the maximum diameters and ADC values) between TCs and PTTs. The chi-square test or Fisher’s exact test was performed to compare the frequencies of imaging findings (shape, calcification, hyperdense areas, soft-tissue density areas, homogeneity on T1 and T2-weighted images, intratumoral T1 hyperintensity, distribution of intratumoral T2 hypointensity and linear or reticular T2 hypointensity) between TCs and PTTs. Interobserver variability of qualitative assessments was assessed using kappa statistics. P values of less than 0.05 were considered to be statistically significant.
Results
Imaging findings of TCs and PTTs are summarised in Table 2. Seventeen lesions (84%, 10 TCs and six PTTs) occurred on the scalp. Other locations included the cheek (5%, one PTT), shoulder (5%, one TC) and foot (5%, one TC). All TCs and PTTs had well-defined margins. The shape was oval/round in 74% (nine TCs and five PTs) and lobulated in 26% (three TCs and two PTTs). The maximum diameter of the lesions ranged from 6 mm to 38 mm (mean 23 mm). There were no significant differences in the oval/round shape (75% vs. 71%, P = 0.634) and maximum diameter (21.3 ± 11.7 mm vs. 26.1 ± 9.7 mm, P = 0.340) between TCs and PTTs, respectively.
Table 2.
Imaging findings of TC and PTT.
| Characteristics | TC | PTT | P value |
|---|---|---|---|
| Location | |||
| Scalp | 10 (83%) | 6 (86%) | |
| Cheek | 0 | 1 (14%) | |
| Shoulder | 1 (8%) | 0 | |
| Foot | 1 (8%) | 0 | |
| Shape | |||
| Oval/round | 9 (75%) | 5 (71%) | 0.634 |
| Lobulated | 3 (25%) | 2 (29%) | |
| Maximum diameter (mm) | 21.3 ± 11.7 | 26.1 ± 9.7 | 0.340 |
| CT | n = 6 | n = 4 | |
| Calcification | 5 (83%) | 2 (50%) | 0.333 |
| Number (range) | 3 (1–4) | 6 (2–10) | |
| Maximum diameter (mm, range) | 6 (3–8) | 4 (3–5) | |
| Hyperdense area | 3 (50%) | 3 (75%) | 0.452 |
| Soft-tissue density area | 5 (83%) | 4 (100%) | 0.600 |
TC: trichilemmal cyst; PTT: proliferating trichilemmal tumour; CT: computed tomography
In qualitative assessment, data are numbers of patients, and numbers in parentheses are frequencies expressed as percentages. The maximum diameter of the lesion is expressed as the mean ± 1 standard deviation. In the number and maximum diameter of calcification, data are median values, and numbers in parentheses are ranges.
CT imaging findings of TCs and PTTs are summarised in Table 2. Calcification was observed in seven lesions (70%, five TCs and two PTTs) (Figures 1 and 2). The number of calcifications ranged from one to 10 (median two), and the maximum diameter of calcification ranged from 3 mm to 8 mm (median 5 mm). Hyperdense areas were observed in six lesions (60%, three TCs and three PTTs), and soft tissue density areas in nine (90%, five TCs and four PTTs) (Figures 1 and 2). The predominant components were calcification in one lesion (10%, one TC), hyperdense areas in two (20%, one TC and one PTT), and soft tissue density areas in seven (70%, four TCs and three PTTs). There were no significant differences in the frequency of calcification (83% vs. 50%, P = 0.333), hyperdense areas (50% vs. 75%, P = 0.452), and soft tissue density areas (83% vs. 100%, P = 0.600) between TCs and PTTs, respectively.
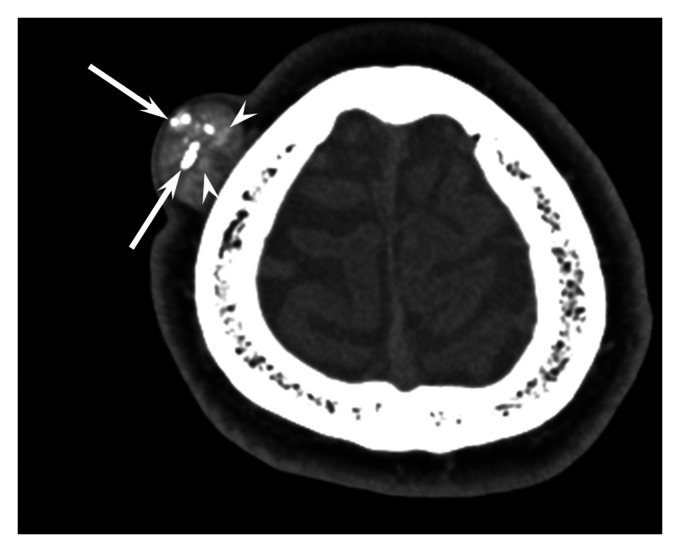
Figure 1.
36-Year-old man with trichilemmal cyst on the scalp.
Unenhanced computed tomography image shows a well-defined, oval, subcutaneous lesion with calcifications (arrows) and hyperdense areas (arrowheads).
Figure 2.
76-Year-old man with proliferating trichilemmal tumour on the scalp.
Unenhanced computed tomography shows a well-defined, lobulated, cutaneous lesion with calcifications (arrows) and hyperdense areas (arrowheads).
MRI findings of TCs and PTTs are summarised in Table 3. On T1-weighted images, predominant signal intensity was hyperintensity in six lesions (46%, six TCs), iso-intensity in three (23%, one TC and two PTTs), and hypointensity in four (31%, one TC and three PTTs). Six lesions (46%, four TCs and two2 PTTs) were homogeneous on T1-weighted images. Intratumoral T1 hyperintensity was observed in all eight TCs, but was not observed in PTTs. Intratumoral T1 hyperintensity was more frequent in TCs than in PTTs (100% vs. 0%, P < 0.01). The distribution of intratumoral T1 hyperintensity in TCs was focal in two and diffuse in six (Figures 3 and 4). On T2-weighted images, predominant signal intensity was hyperintensity in seven lesions (46%, four TCs and three PTTs) and hypointensity in seven (54%, four TCs and three PTTs). One lesion (8%, one TC) was homogeneous on T2-weighted images. T2 hypointense rim and intratumoral T2 hypointensity were observed in all 13 lesions (100%, eight TCs and five PTTs). The distribution of intratumoral T2 hypointensity was focal in nine lesions (69%, six TCs and three PTTs) and diffuse in four (31%, two TCs and two PTTs) (Figures 3 and 4). Linear or reticular T2 hypointensity was observed in 10 (77%, six TCs and four PTTs) (Figures 3 and 4). There were no significant differences in homogeneity on T1-weighted images (50% vs. 40%, P = 0.587), homogeneity on T2-weighted images (12% vs. 0%, P = 0.615), focal T2 hypointensity (75% vs. 60%, P = 0.510), linear or reticular T2 hypointensity (75% vs. 80%, P = 0.685) and ADC values (1.74 ± 0.41 × 10−3 mm2 s−1 vs. 1.49 ± 0.24 × 10−3 mm2 s−1, P = 0.327) between TCs and PTTs, respectively.
Table 3.
MRI findings of TC and PTT.
| TC | PTT | P value | |
|---|---|---|---|
| T1-weighted image | |||
| Predominant signal intensity | |||
| Hyperintensity | 6 (75%) | 0 (0%) | |
| Iso-intensity | 1 (13%) | 2 (40%) | |
| Hypointensity | 1 (13%) | 3 (60%) | |
| Homogeneity | |||
| Homogeneous | 4 (50%) | 2 (40%) | 0.587 |
| Heterogeneous | 4 (50%)3 (60%) | ||
| Intratumoral hyperintensity | 8 (100%) | 0 (0%) | 0.001* |
| Distribution | |||
| Focal | 2 (25%) | ||
| Diffuse | 6 (75%) | ||
| T2-weighted image | |||
| Predominant signal intensity | |||
| Hyperintensity | 4 (50%) | 2 (40%) | |
| Iso-intensity | 0 (0%) | 0 (0%) | |
| Hypointensity | 4 (50%) | 3 (60%) | |
| Hypointense rim | 8 (100%) | 5 (100%) | |
| Homogeneity | |||
| Homogeneous | 1 (12%) | 0 (0%) | 0.615 |
| Heterogeneous | 7 (88%) | 5 (100%) | |
| Intratumoral hypointensity | 8 (100%) | 5 (100%) | |
| Distribution | |||
| Focal | 6 (75%) | 3 (60%) | 0.510 |
| Diffuse | 2 (25%) | 2 (40%) | |
| Linear or reticular | 6 (75%) | 4 (80%) | 0.685 |
| ADC value (×10−3 mm2s−1) | 1.74 ± 0.41 (n = 5) | 1.49 ± 0.24 (n = 4) | 0.327 |
MRI: magnetic resonance imaging; TC: trichilemmal cyst; PTT: proliferating trichilemmal tumour; ADC: apparent diffusion coefficient.
*Significant differences in the values were observed between TCs and PTTs (P<0.01).
In qualitative assessment, data are numbers of patients, and numbers in parentheses are frequencies expressed as percentages. ADC value is expressed as the mean ± 1 standard deviation.
Figure 3.
56-Year-old man with trichilemmal cyst on the scalp. (a) T1-weighted image (TR/TE, 542/15 ms) shows a well-defined, oval, subcutaneous lesion (arrow) with diffuse hyperintensity. (b) T2-weighted image (TR/TE, 3,500/90 ms) shows a heterogeneous subcutaneous lesion (arrow) with linear or reticular hypointensity (arrowheads).
Figure 4.
57-Year-old woman with proliferating trichilemmal tumour on the scalp. (a) T1-weighted image (TR/TE, 778/15 ms) shows a well-defined, oval, subcutaneous lesion (arrow), which is hypointense to gray matter. (b) T2-weighted image (TR/TE, 3,170/90 ms) shows a heterogeneous subcutaneous lesion (arrow) with linear or reticular hypointensity (arrowheads).
Kappa values for the two observers with regard to assessing shape showed substantial agreement (0.69), and that with regard to assessing homogeneity on T1-weighted images, intratumoral T1 hyperintensity, T2 hypointense rim, homogeneity on T2-weighted images, distribution of intratumoral T2 hypointensity, and linear or reticular T2 hypointensity showed excellent agreement (0.85, 1.00, 0.90, 1.00, 0.85, and 0.92, respectively).
Discussion
In our series, TCs and PTTs most frequently occurred on the scalp (89%) as noted in earlier studies (68–83%).1,17 A literature review of 76 PTTs reported that PTTs also occurred on the scalp in 85.4% of cases. 6 Therefore, TCs and PTTs should be considered in the differential diagnosis of scalp lesions.
In this study, calcification (70%) and hyperdense areas (60%) were frequently observed in TCs and PTTs. Because dystrophic calcification and abundant cholesterol crystals can be observed histologically within the compact eosinophilic keratin of TCs, 1 these pathological features should be reflected as calcification and hyperdense areas on CT images. Prior studies evaluating imaging findings of TCs noted internal calcification in 65% of cases on ultrasound images 17 and in 75% on CT images. 16 No radiological studies have noted the frequency of calcification in PTTs, but a few case reports have described calcification of PTTs on CT images.7,9 Although no previous reports have mentioned hyperdense areas of TCs and PTTs on CT images, our results suggest that hyperdense areas on CT images would be one of the characteristic imaging features of TCs and PTTs.
Intratumoral T1 hyperintensity was observed only in TCs. Among the previous reports of histologically confirmed cases, T1-weighted images were presented in two TCs 12,13 and four PTTs.7,8,14,15 In these case reports, one TC showed hyperintensity while the other was isointense compared to gray matter on T1-weighted images. Meanwhile, all four PTTs showed iso to hypointensity on T1-weighted images. In the present study, as intratumoral T1 hyperintensity was observed in all TCs and not observed in any PTTs, this might be a useful imaging finding differentiating TCs from PTTs. As noted above, TCs histologically contain trichilemmal keratinisation with homogeneous compact eosinophilic material including calcification or viscous material. 1 Therefore, T1 relaxivity of TCs might be caused by the accumulation of surface relaxation effects of the calcium salt particles or high protein content. In contrast, PTTs are histologically characterised by prominent epithelial infoldings into the lumen and are composed of variably sized lobules of squamous epithelium exhibiting abrupt trichilemmal keratinisation. Low signal intensity of PTTs on T1-weighted images might be caused by a decreased deposition of calcification or viscous material.
Relatively high ADC values were observed in TCs (1.74 × 10−3mm2 s−1) and PTTs (1.49 × 10−3mm2 s−1) in this study. These ADC values were higher than those of ECs (0.81 × 10−3mm2 s−1). 18 Because trichilemmal keratinisation of TCs and PTTs did not show remarkable restricted diffusion, ADC values would be useful for differentiating trichilemmal keratinisation of TCs and PTTs from keratinisation of ECs.
TCs are usually surrounded by thick fibrous capsules containing small layers of cuboidal, dark-staining basal epithelial cells in a palisade arrangement without an obvious intercellular gap. Those cells coalesce with multiple layers of keratinocytes forming squamous epithelium; these cells showed more maturation with dense eosinophilic-staining keratin in the absence of a granular cell layer. Hypointense rim on T2-weighted images would correspond to the fibrous capsule.
Linear or reticular T2 hypointensity was observed in 77% of TCs and PTTs. Histologically, TCs include abundant trichilemmal keratinised debris. 2 In addition, because lobular proliferation of squamous cells with epithelial infoldings into the cystic lumen were found in PTTs, 1 these pathological findings look like the septa of multiloculated cystic lesions. Therefore, trichilemmal keratinised debris and epithelial infoldings might correspond to linear or reticular T2 hypointensity.
There are no established guidelines for the management of TCs and PTTs, and the usefulness of preoperative imaging is controversial. Benign TCs are suitable for tumour enucleation. In contrast, PTTs would need the resected surgical margin of the lesion because PTTs are classified into the malignant category by the World Health Organization.2,19 Therefore, preoperative imaging seemed to be essential for assessing tumour malignancy and determining the surgical margin.
The differential diagnosis of TCs and PTTs includes ECs, pilomatricoma, sebaceoma, cutaneous squamous cell carcinoma and cutaneous angiosarcoma. On T2-weighted images, linear dark debris in the non-dependent areas, reflecting layered and aggregated keratin debris, is characteristically seen in ECs, 20 similar to the T2 linear hypointensity of TCs and PTTs. ECs exhibit iso to mild hyperintensity on T1-weighted images, 20 whereas the ADC values of ECs in a prior study 18 are lower than those of TCs and PTTs in this study. Pilomatricoma exhibits homogeneous hypointensity (42%) on T2-weighted images, reticular or ring-like hyperintensity (50%) on fat-suppressed T2-weighted images is characteristic of pilomatricoma.21,22 In addition, pilomatricoma always shows homogeneous hypointensity on T1-weighted images, 21 unlike TCs in our series. Sebaceomas usually exhibit hyperintensity on T1 and T2-weighted images and decreased signal intensity on fat-suppressed sequences which reflects abundant oil content. 3 Cutaneous squamous cell carcinoma exhibits a flattened configuration, superficial ulceration, protrusion and ill-demarcated deep margins.23,24 Cutaneous angiosarcoma also occurs predominantly on the scalp and demonstrates hypointensity on T2-weighted images similar to TCs. Unlike TCs and PTTs, cutaneous angiosarcoma appears as a flat elevated lesion with invasion of subcutaneous fat tissue. 24
There are several limitations to this study. First, this was retrospective study with a small sample size from a single institution. Second, because contrast media was only administered to one patient on CT imaging and no patients underwent contrast-enhanced MRI, we could not evaluate contrast-enhanced imaging. According to earlier case reports which described contrast-enhanced MRI findings, TCs usually appear as rim-enhancing cystic lesions, 10 while PTTs usually show heterogeneous enhancement corresponding to a mixture of cellular and cystic/necrotic components.8,15 Contrast-enhanced MRI may contribute to differentiation between TCs and PTTs. Third, this study did not include atypical or malignant PTTs.
In conclusion, TCs and PTTs predominantly occurred on the scalp. Although calcification and hyperdense areas were common CT findings for both TCs and PTTs, the predominant components were frequently soft tissue density areas. On MRI scans, linear or reticular T2 hypointensity was characteristic of TCs and PTTs, whereas intratumoral T1 hyperintensity may be a useful MRI feature for differentiating these two lesions. As TCs and PTTs are common pathologies of the scalp, it is important that radiologists know their distinguishing characteristics.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Masaya Kawaguchi https://orcid.org/0000-0002-2807-0524
Hiroki Kato https://orcid.org/0000-0001-5926-1895
References
- 1.Ramaswamy A, Manjunatha H, Bylappa S, et al. Morphological Spectrum of Pilar Cysts. N Am J Med Sci 2013; 5: 124–128. DOI: 10.4103/1947-2714.107532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Requena L, Crowson AN, Kaddu S, et al. Proliferating trichilemmal tumor. In: WHO Classification of Skin Tumors 4. Lyon, France: IARC Press, 2018, pp. 196–197.
- 3.Kawaguchi M, Kato H, Matsuo M. CT and MRI features of scalp lesions. Radiol Med 2019; 124: 1049–1061. DOI: 10.1007/s11547-019-01060-6 [DOI] [PubMed] [Google Scholar]
- 4.Prodinger CM, Koller J, Laimer M. Scalp tumors. J Dtsch Dermatol Ges 2018; 16: 730–753. 2018/06/07. DOI: 10.1111/ddg.13546 [DOI] [PubMed] [Google Scholar]
- 5.Balasundaram P, Garg A, Prabhakar A, et al. Evolution of epidermoid cyst into dermoid cyst: embryological explanation and radiological-pathological correlation. Neuroradiol J 2019; 32: 92–97. 2019/01/04. DOI: 10.1177/1971400918821086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye J, Nappi O, Swanson P, et al. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol 2004; 122: 566–574. DOI: 10.1309/21DK-LY2R-94H1-92NK [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Kim TS, Lee KH, et al. Proliferating trichilemmal tumors: CT and MR imaging findings in two cases, one with malignant transformation. AJNR Am J Neuroradiol 2001; 22: 180–183. 2001/02/13. [PMC free article] [PubMed] [Google Scholar]
- 8.Kitajima K, Imanaka K, Hashimoto K, et al. Magnetic resonance imaging findings of proliferating trichilemmal tumor. Neuroradiology 2005; 47: 406–410. 2005/05/13. DOI: 10.1007/s00234-004-1328-6 [DOI] [PubMed] [Google Scholar]
- 9.Chang SJ, Sims J, Murtagh FR, et al. Proliferating trichilemmal cysts of the scalp on CT. AJNR Am J Neuroradiol 2006; 27: 712–714. 2006/03/23. [PMC free article] [PubMed] [Google Scholar]
- 10.Gossner J, Larsen J. Incidental subcutaneous nodules on the scalp in patients undergoing CT of the brain; frequency, appearance, and differential diagnosis. Clin Radiol 2010; 65: 427–428. 2010/04/13. DOI: 10.1016/j.crad.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 11.Fonseca TC, Bandeira CL, Sousa BA, et al. Proliferating trichilemmal tumor: case report. J Bras Patol Med Lab 2016; 52: 120–123. [Google Scholar]
- 12.Tan L, Harbhajanka A, Kasliwal M, et al. Giant trichilemmal cyst of the scalp. Neurology India 2016; 64: 357–358. Neuroimages. DOI: 10.4103/0028-3886.177589 [DOI] [PubMed] [Google Scholar]
- 13.Narra RKS, Putcha A. Trichilemmal cysts of scalp: imaging findings. Indian J Case Reports 2018; 4: 429–430. [Google Scholar]
- 14.Jang J, Kang B, Suh J, et al. MR findings of trichilemmal carcinoma: a case report. J Korean Soci Radiol 2009; 61: 87. DOI: 10.3348/jksr.2009.61.2.87 [Google Scholar]
- 15.Moon H, Kim M-W, Kim Y, et al. Recurrent proliferating trichilemmal tumor with malignant change on the F-18 fluorodeoxyglucose positron emission tomography/computed tomography. J Korean Soc Radiol 2016; 74: 389. DOI: 10.3348/jksr.2016.74.6.389 [Google Scholar]
- 16.Boruah D, Gogoi B, Prakash A, et al. Radiological and pathological evaluation of trichilemmal cysts of the scalp. J Evid Based Med Healthc 2017; 4: 4314–4320. DOI: 10.18410/jebmh/2017/859 [Google Scholar]
- 17.He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic features. J Ultrasound Med 2019; 38: 91–96. 2018/05/01. DOI: 10.1002/jum.14666 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki C, Maeda M, Matsumine A, et al. Apparent diffusion coefficient of subcutaneous epidermal cysts in the head and neck comparison with intracranial epidermoid cysts. Acad Radiol 2007; 14: 1020–1028. 2007/08/21. DOI: 10.1016/j.acra.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Verma P, Yadav P, et al. Proliferating trichilemmal tumor of scalp: benign or malignant, a dilemma. J Cutan Aesthet Surg 2012; 5: 213–215. DOI: 10.4103/0974-2077.101394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HK, Kim SM, Lee SH, et al. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol 2011; 40: 1415–1419. DOI: 10.1007/s00256-010-1072-4 [DOI] [PubMed] [Google Scholar]
- 21.Kato H, Kanematsu M, Watanabe H, et al. MR imaging findings of pilomatricomas: a radiological-pathological correlation. Acta Radiol 2016; 57: 726–732. 2015/08/09. DOI: 10.1177/0284185115597717 [DOI] [PubMed] [Google Scholar]
- 22.Kato H, Kawaguchi M, Ando T, et al. Hypointense head and neck lesions on T2-weighted images: correlation with histopathologic findings. Neuroradiology 2020; 62: 1207–1217. 2020/06/21. DOI: 10.1007/s00234-020-02483-z [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi M, Kato H, Tomita H, et al. Magnetic resonance imaging findings differentiating cutaneous basal cell carcinoma from squamous cell carcinoma in the head and neck region. Korean J Radiol 2020; 21: 325–331. 2020/02/25. DOI: 10.3348/kjr.2019.0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi M, Kato H, Suzui N, et al. Imaging findings of cutaneous angiosarcoma of the scalp: comparison with cutaneous squamous cell carcinoma. Neuroradiol J 2021; 1971400921998941. 2021/03/05. DOI: 10.1177/1971400921998941 [DOI] [PMC free article] [PubMed]