This randomized clinical trial assesses the efficacy and safety of zuranolone, a neuroactive steroid γ-aminobutyric acid receptor–positive allosteric modulator, in individuals with postpartum depression.
Key Points
Question
Does treatment with zuranolone reduce depressive symptoms in female individuals experiencing postpartum depression?
Findings
In this phase 3, double-blind, randomized, placebo-controlled trial of 151 adult women with postpartum depression, patients taking daily zuranolone for 2 weeks displayed greater statistically significant reductions in depressive symptoms compared with placebo at day 15, assessed by change from baseline in the 17-item Hamilton Rating Scale for Depression. Reductions in depressive symptoms were observed by day 3 and sustained at all measured time points through day 45.
Meaning
Zuranolone provided significant reductions in depressive symptoms and was generally well tolerated, supporting its further development in the treatment of postpartum depression.
Abstract
Importance
Postpartum depression (PPD) is one of the most common medical complications during and after pregnancy, negatively affecting both mother and child.
Objective
To demonstrate the efficacy and safety of zuranolone, a neuroactive steroid γ-aminobutyric acid receptor–positive allosteric modulator, in PPD.
Design, Setting, and Participants
This phase 3, double-blind, randomized, outpatient, placebo-controlled clinical trial was conducted between January 2017 and December 2018 in 27 enrolling US sites. Participant were women aged 18 to 45 years, 6 months or fewer post partum, with PPD (major depressive episode beginning third trimester or ≤4 weeks postdelivery), and baseline 17-item Hamilton Rating Scale for Depression (HAMD-17) score of 26 or higher. Analysis was intention to treat and began December 2018 and ended March 2019.
Interventions
Randomization 1:1 to placebo:zuranolone, 30 mg, administered orally each evening for 2 weeks.
Main Outcomes and Measures
Primary end point was change from baseline in HAMD-17 score for zuranolone vs placebo at day 15. Secondary end points included changes from baseline in HAMD-17 total score at other time points, HAMD-17 response (≥50% score reduction) and remission (score ≤7) rates, Montgomery-Åsberg Depression Rating Scale score, and Hamilton Rating Scale for Anxiety score. Safety was assessed by adverse events and clinical assessments.
Results
Of 153 randomized patients, the efficacy set comprised 150 patients (mean [SD] age, 28.3 [5.4] years), and 148 (98.7%) completed treatment. A total of 76 patients were randomized to placebo, and 77 were randomized to zuranolone, 30 mg. Zuranolone demonstrated significant day 15 HAMD-17 score improvements from baseline vs placebo (−17.8 vs −13.6; difference, −4.2; 95% CI, −6.9 to −1.5; P = .003). Sustained differences in HAMD-17 scores favoring zuranolone were observed from day 3 (difference, −2.7; 95% CI, −5.1 to −0.3; P = .03) through day 45 (difference, −4.1; 95% CI, −6.7 to −1.4; P = .003). Sustained differences at day 15 favoring zuranolone were observed in HAMD-17 response (odds ratio, 2.63; 95% CI, 1.34-5.16; P = .005), HAMD-17 score remission (odds ratio, 2.53; 95% CI, 1.24-5.17; P = .01), change from baseline for Montgomery-Åsberg Depression Rating Scale score (difference, −4.6; 95% CI, −8.3 to −0.8; P = .02), and Hamilton Rating Scale for Anxiety score (difference, −3.9; 95% CI, −6.7 to −1.1; P = .006). One patient per group experienced a serious adverse event (confusional state in the zuranolone group and pancreatitis in the placebo group). One patient in the zuranolone group discontinued because of an adverse event vs none for placebo.
Conclusions and Relevance
In this randomized clinical trial, zuranolone improved the core symptoms of depression as measured by HAMD-17 scores in women with PPD and was generally well tolerated, supporting further development of zuranolone in the treatment of PPD.
Trial Registration
ClinicalTrials.gov Identifier: NCT02978326
Introduction
Postpartum depression (PPD) is a perinatal major depressive episode that negatively affects new female parents and their families.1,2 Although symptoms of PPD affects around 13.2% of new female parents across the US3 and is among the most common medical complications during and after pregnancy,3,4,5,6,7,8,9 it is underdiagnosed and undertreated10,11 and can persist for years.12,13 Complications of untreated PPD include maternal suicide,14,15,16 lasting negative effects on infant and child development,12,17,18,19,20,21,22 and depression in partners.23,24,25
The pathophysiology of PPD is likely multifactorial,26,27 with evidence supporting a role for disruption of perinatal γ-aminobutyric acid (GABA) signaling, the major inhibitory signaling pathway of the central nervous system.28 One potential factor affecting GABAergic signaling and PPD development are dramatic perinatal changes in circulating levels of allopregnanolone, a neuroactive steroid (NAS) GABAA receptor (GABAAR)–positive allosteric modulator (PAM).29,30,31,32 In brain regions associated with emotion and self-perception, neural network connectivity, supported by GABAergic signaling, is positively correlated with plasma allopregnanolone concentrations in individuals with PPD vs healthy postpartum female individuals.33 The role of GABAergic signaling is further supported by animal model studies demonstrating PPD-like behaviors in mice lacking the extrasynaptic GABAAR δ subunit or potassium/chloride cotransporter KCC2, and these PPD models also functionally link GABA with the hypothalamic-pituitary axis, a stress pathway implicated in PPD.34,35 Administration of a preclinical NAS GABAAR PAM that targets both synaptic and extrasynaptic receptors (SAGE-516; Sage Therapeutics, Inc) in late pregnancy reduced PPD-like behaviors in these mouse models, supporting the development of NAS GABAAR PAMs in PPD treatment.36
Clinical research in the treatment of PPD supports a role for NAS GABAAR PAMs. Brexanolone injection, a NAS GABAAR PAM, demonstrated reductions in depressive symptoms in 3 double-blind, randomized, placebo-controlled trials and was approved by the US Food and Drug Administration for treatment of adults with PPD.37,38 Zuranolone (SAGE-217; Sage Therapeutics, Inc) is an investigational NAS GABAAR PAM with a similar mechanism of action and a pharmacokinetic profile suitable for once-daily oral dosing.39 NAS GABAAR PAMs have pharmacological profiles and binding sites distinct from benzodiazepines,40,41 with the ability to modulate the activity of both synaptic and extrasynaptic GABAARs.39,42 Therefore, we performed a phase 3, double-blind, randomized, placebo-controlled clinical trial comparing the efficacy and safety of zuranolone vs placebo in the outpatient treatment of adult women with PPD.
Methods
Study Design and Participants
Patients were screened at 33 centers in the US (first informed consent, January 4, 2017; final poststudy observation, December 11, 2018), with 27 sites randomizing and dosing at least 1 patient. The trial received institutional review board approval and was performed in accordance with ethical principles originating in the Declaration of Helsinki43 and are consistent with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice guidelines and all applicable regulatory requirements. All patients provided written informed consent prior to enrollment. The trial was completed on reaching predetermined target enrollment numbers.
Inclusion/Exclusion Criteria
Adult ambulatory female patients, aged 18 to 45 years old, 6 months or less post partum, with a major depressive episode without psychosis (diagnosed by the Structured Clinical Interview for DSM-5 Axis I Disorders for clinical trials)1 that began no earlier than the third trimester and no later than the first 4 weeks following delivery, were enrolled. This PPD definition was used in previous clinical trials.37,38 A baseline 17-item Hamilton Rating Scale for Depression (HAMD-17) score of 26 or higher was required.44 Patients taking psychotropic medications used to treat depressive symptoms were required to have been taking a stable dose for more than 30 days prior to day 1 and delay the start/alteration of psychotropic treatment regimens until after the treatment period and day 15 assessments were completed. Patients must have ceased lactating at screening or agreed to cease breastfeeding from just prior to receiving the study drug until 7 days after the last dose. Race/ethnicity was self-reported by patients, or the data were pulled from external medical records, if available. The principal investigator at each site was responsible for recruitment and enrollment of patients. The trial protocol, which contains additional inclusion and full exclusion criteria, is available in Supplement 1.
Randomization/Masking
Patients were randomized 1:1 to receive zuranolone, 30 mg, or matching placebo capsules for 2 weeks, based on previous studies.39,45 Randomization codes were generated with a block size of 4 by an independent statistical vendor not affiliated with Sage Therapeutics, Inc. The randomization scheme was initially performed manually using SAS statistical software version 9.3 (SAS Institute); a decision was then made to contract randomization to an interactive response technology system vendor using SAS statistical software version 9.4 (SAS Institute). Of 153 total randomized patients, 80 were randomized manually and 73 were randomized by interactive response technology implementation. Study site–designated pharmacy staff, responsible for dispensing the study drug, were the only study personnel unblinded to the randomization scheme. All other site personnel were blinded to treatment assignments during the study. In the event of a medical emergency, the clinic pharmacist could reveal the study drug assignment to the investigator, after which the patient could be unmasked and terminated from the study.
Procedures
The study drug (placebo or zuranolone, 30 mg) was administered once each evening with food. Four initial patients were administered the study drug by a health care professional in a clinic, and the rest of the patients self-administered the study drug at home. Patients unable to tolerate 30 mg of the study drug once daily could be administered 20 mg for the remainder of the treatment period. All assessments were conducted via outpatient clinic visits. Posttreatment follow-up was through day 45.
Outcome Measures
The primary efficacy end point was the change from baseline in HAMD-1744 total score at day 15. Secondary efficacy end points included the change from baseline in HAMD-17 total score at all other time points (days 3, 8, 21, and 45) with no modifications, Montgomery-Åsberg Depression Rating Scale (MADRS) score with no modifications,46 Hamilton Rating Scale for Anxiety (HAM-A) score,47 HAMD-17 response (≥50% reduction in score from baseline), HAMD-17 remission (score ≤7, which is widely adopted as criteria for remission using this clinician-rated scale of depressive symptoms48,49), and Clinical Global Impression Improvement (CGI-I) response (1 indicated very much improved or 2, much improved).50 Other end points included change from baseline in the Barkin Index of Maternal Functioning (BIMF), a validated measure of patient-reported maternal function within the first year of childbirth that may be used in clinical settings.51
Safety and tolerability were evaluated by adverse events (AEs), vital signs, clinical laboratory evaluations, electrocardiogram parameters, and the Columbia-Suicide Severity Rating Scale.52 Serious AEs were defined as any untoward medical occurrence that resulted in death, was life threatening (at the time of event), required inpatient hospitalization, resulted in persistent or significant disability, or was a congenital anomaly.
Statistical Analysis
By 2-sided t test (α level of .05), a sample size of approximately 65 evaluable patients per treatment group provided 90% power to detect a placebo-adjusted treatment difference of approximately 4 points in the primary end point assuming an SD of 7 points. Assuming a 10% dropout rate and 1:1 randomization ratio, approximately 72 randomized patients per treatment group were required to obtain 130 evaluable patients. The safety set included all patients who received at least 1 dose of the double-blind study drug. The efficacy set, based on intention to treat, included all patients in the safety set who had a valid baseline and at least 1 postbaseline efficacy assessment.
A mixed-effects model for repeated measures including treatment, baseline HAMD-17 total score, baseline antidepressant use, assessment time point, and time point by treatment as explanatory variables was used for analysis of the primary end point. An unstructured covariance structure was used to model the within-patient errors. Secondary (HAMD-17, MADRS, HAM-A scores change from baseline) and other end points (BIMF score) were assessed using the same mixed-effects model for repeated measures as the primary end point. Model-based point estimates (ie, least-squares means), 95% CIs, and P values were reported. Treatment comparisons were made between placebo and treatment groups. Categorical secondary end points (HAMD-17 response and remission and CGI-I response) were analyzed by a generalized estimating equations approach. Odds ratios (ORs), 95% CIs, and P values were reported. P values and 95% CIs for secondary end points, which are highly associated with the primary end point, were not adjusted for multiplicity, and therefore reported as point estimates. Statistical analyses used SAS statistical software version 9.4 (SAS Institute). No imputation was used to estimate missing data (Supplement 1). Analysis began December 2018 and ended March 2019.
Results
Disposition and Demographics
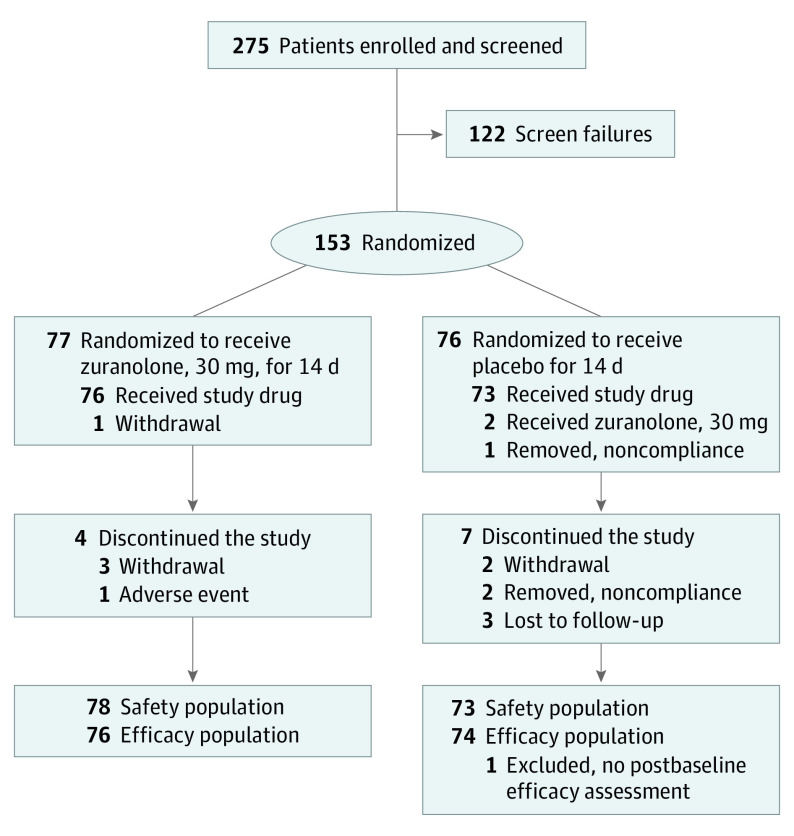
A total of 275 women were screened and enrolled; 153 were randomized (placebo, n = 76; zuranolone, n = 77; Figure 1). Two patients were randomized but did not receive dosing (zuranolone, n = 1 [withdrawal]; placebo, n = 1 [noncompliance]). The safety set included 78 patients treated with zuranolone and 73 patients treated with placebo, as 2 patients were randomized to placebo but received at least 1 dose of zuranolone. In the safety set, 76 of 78 patients in the zuranolone group (97%) completed treatment. Reasons for treatment discontinuation in the zuranolone group were AE (n = 1) and noncompliance (n = 1). Of patients in the zuranolone group who completed treatment, 3 patients’ study drug doses were reduced to 20 mg. Of 73 patients receiving placebo, 72 (99%) completed treatment, with no dose reductions and 1 patient was lost to follow-up. One patient in the placebo group did not complete a postbaseline efficacy assessment, resulting in 150 evaluable patients (placebo, n = 74; zuranolone, n = 76).
Figure 1. CONSORT Diagram.
The 122 patients who were considered screen failures were screened but deemed ineligible for the study. Two patients randomized to placebo each received in error 1 dose of zuranolone, 30 mg (1 patient on day 1 and the other on day 3). Both were logged as such for safety analysis, but the patients received placebo at all other time points. Therefore, the safety population included 78 patients treated with zuranolone and 73 patients treated with placebo. Two patients were randomized but did not receive dosing (zuranolone, n = 1 [withdrawal]; placebo, n = 1 [noncompliance]), and 1 patient in the placebo group did not complete a postbaseline efficacy assessment, resulting in an efficacy population of 76 patients in the zuranolone group and 74 patients in the placebo group.
Baseline characteristics and demographics were well balanced between treatment groups (efficacy set, Table 1; safety set, eTables 1-5 in Supplement 2). The mean (SD) age was 29.3 (5.4) years in the zuranolone group and 27.4 (5.3) years in the placebo group (range, 18-44 years). Most patients identified as White (placebo group, 54% [40 of 74]; zuranolone group, 58% [44 of 76]) or Black/African American (placebo group, 42% [31 of 74]; zuranolone group, 41% [31 of 76]). More than 20% of patients in the placebo (24% [18 of 74]) and zuranolone groups (21% [16 of 76]) identified as Hispanic or Latina. At baseline, 21% (16 of 76) and 18% (13 of 74) of patients in the zuranolone and placebo groups, respectively, were taking stable doses of antidepressant medications.
Table 1. Baseline Characteristics of the Efficacy Population.
| Characteristic | No. (%) | |
|---|---|---|
| Zuranolone, 30 mg (n = 76) | Placebo (n = 74) | |
| Age, mean (SD), y | 29.3 (5.4) | 27.4 (5.3) |
| Hispanic/Latina ethnicity | 16 (21) | 18 (24) |
| Race | ||
| African American | 31 (41) | 31 (42) |
| White | 44 (58) | 40 (54) |
| Othera | 1 (1) | 3 (4) |
| Weight, mean (SD), kg | 85.1 (19) | 80.2 (24) |
| BMI, mean (SD) | 31.1 (6) | 30.3 (8) |
| Baseline antidepressant use | 16 (21) | 13 (18) |
| Onset of PPD | ||
| Third trimester | 32 (42) | 31 (42) |
| ≤4 wk After delivery | 44 (58) | 43 (58) |
| Family history of PPD | 10 (13) | 10 (14) |
| Baseline HAMD-17 total score, mean (SD)b | 28.4 (2) | 28.8 (2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HAMD-17, 17-item Hamilton Rating Scale for Depression; PPD, postpartum depression.
Other includes Asian, Native Hawaiian or Other Pacific Islander, and more than 1 race.
The HAMD-17 scale ranges from 0 to 52, and a higher score indicates increased depression severity.
Efficacy Outcomes
Primary End Point
The primary end point was achieved. There was a significantly greater reduction from baseline in HAMD-17 total score with zuranolone compared with placebo at day 15 (least-squares mean, −17.8 points vs −13.6 points; 95% CI, −6.9 to −1.5; P = .003; effect size, 0.53; Figure 2).
Figure 2. Least-Squares Mean (LSM) Change From Baseline in the 17-Item Hamilton Rating Scale for Depression (HAMD-17) Score.
Treatment with zuranolone, 30 mg, achieved the primary end point of a significant change from baseline HAMD-17 total score at day 15 compared with placebo using mixed-effects model for repeated measures. HAMD-17 total score at time points other than day 15 were secondary end points, not adjusted for multiplicity and therefore reported as point estimates, which also showed sustained improvements for the zuranolone group compared with the placebo group.
aP = .03.
bP = .01.
cP = .003.
Secondary End Points
Greater reductions from baseline in HAMD-17 score favoring zuranolone compared with placebo were observed at all measured time points from day 3 through day 45, 4 weeks after treatment cessation (day 3: least-squares means difference, −2.7; 95% CI, −5.1 to −0.3; P = .03; day 45: least-squares means difference, −4.1; 95% CI, −6.7 to −1.4; P = .003) (Figure 2; eTable 6 in Supplement 2).
Day 15 secondary end points supported the primary end point, including a sustained greater proportion of patients achieving HAMD-17 response and remission with zuranolone compared with placebo (response: 72% [53 of 74] in the zuranolone group vs 48% [35 of 73] in the placebo group; OR, 2.6; 95% CI, 1.3-5.2; P = .005; remission: 45% [33 of 74] in the zuranolone group vs 23% [17 of 73] in the placebo group; OR, 2.5; 95% CI, 1.2-5.2; P = .01) (Figure 3A; eTables 7 and 8 in Supplement 2). HAMD-17 score change from baseline by predefined baseline subgroups at day 15 were similar to overall HAMD-17 score analyses (race, age, stable concomitant antidepressant use, body mass index, onset of PPD, and family history of PPD; eFigure 1 in Supplement 2). There was also a sustained larger reduction from baseline in MADRS score with zuranolone vs placebo at day 15 (least-squares means difference, −4.6; 95% CI, −8.3 to −0.8; P = .02) (eFigure 2 in Supplement 2). Improvements in anxiety (HAM-A) and global functioning (CGI-I response) were observed at day 15. A sustained larger reduction from baseline in HAM-A score favored zuranolone compared with placebo at day 15 (least-squares means difference, −3.9; 95% CI, −6.7 to −1.1; P = .006) (Figure 3B). Sustained greater increase in CGI-I response rate was observed for zuranolone compared with placebo at day 15 (72% [53 of 74] in the zuranolone group vs 52% [38 of 73] in the placebo group; OR, 2.2; 95% CI, 1.1-4.3; P = .03) (eFigure 3 in Supplement 2).
Figure 3. HAMD-17 Response, HAMD-17 Remission, and LSM Change From Baseline in HAM-A Scores.
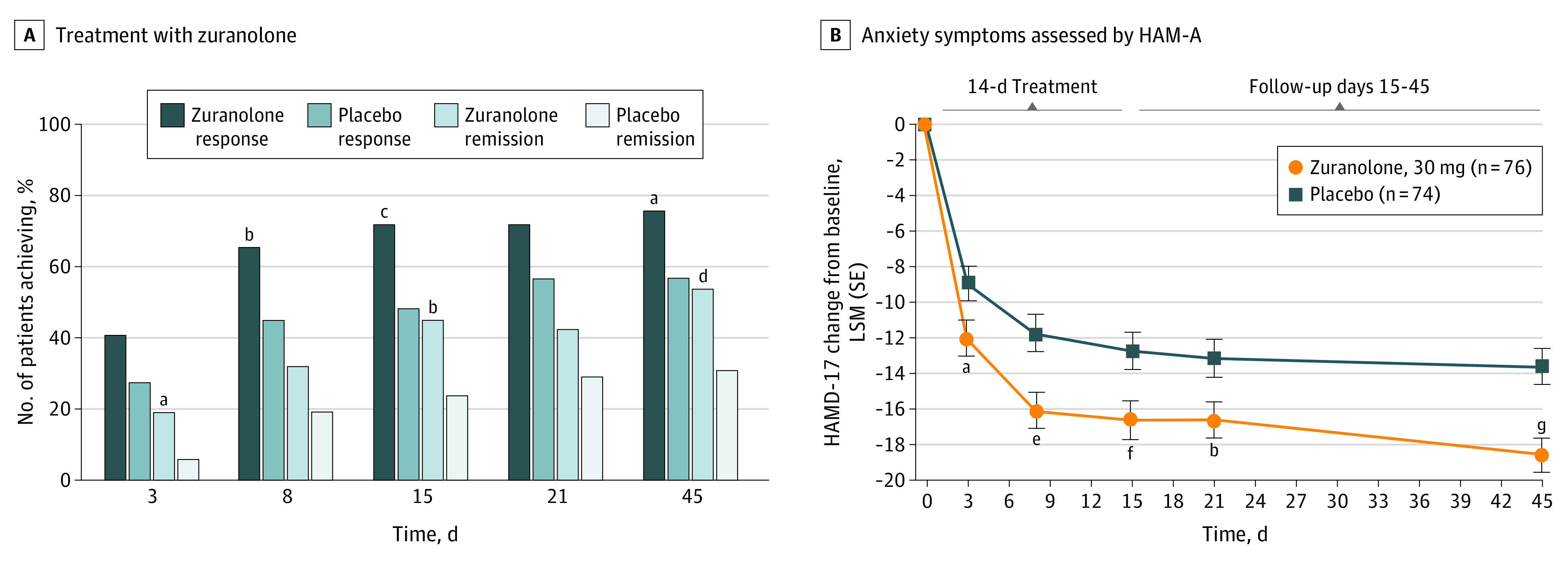
Key secondary end points were not adjusted for multiplicity, and P values are reported as point estimates. A, Treatment with zuranolone, 30 mg, resulted in a greater percentage of patients achieving 17-item Hamilton Rating Scale for Depression (HAMD-17) response (days 8, 15, and 45) and HAMD-17 remission (days 3, 15, and 45) compared with placebo. HAMD-17 response is defined as a 50% or greater reduction in total score from baseline, and HAMD-17 remission is defined as total score of 7 or less. B, Sustained improvements in anxiety symptoms as assessed by Hamilton Rating Scale for Anxiety (HAM-A) were observed across the treatment and follow-up periods in the zuranolone group compared with the placebo group. LSM indicates least-squares mean.
aP = .02.
bP = .01.
cP = .005.
dP = .009.
eP = .001.
fP = .006.
gP < .001.
At day 3, a higher percentage of patients in the zuranolone group achieved HAMD-17 remission vs the placebo group (19% [14 of 74] in the zuranolone group vs 5% [4 of 74] in the placebo group; OR, 3.9; 95% CI, 1.2-12.2; P = .02; eTable 8 in Supplement 2). The change from baseline in HAM-A score favoring zuranolone vs placebo was also apparent at day 3 (least-squares means difference, −3.1; 95% CI, −5.7 to −0.6; P = .02; Figure 3B).
At day 45, 4 weeks after study drug cessation, HAMD-17 response (75% [55 of 73] in the zuranolone group vs 57% [39 of 69] in the placebo group; OR, 2.3; 95% CI, 1.1-4.6; P = .02) and HAMD-17 remission (53% [39 of 73] in the zuranolone group vs 30% [21 of 69] in the placebo group; OR, 2.5; 95% CI, 1.3-5.0; P = .009) were sustained in the zuranolone vs placebo group (Figure 3; eTables 7 and 8 in Supplement 2). During the follow-up period, only a small proportion (4 patients [5.4%] and 6 patients [7.9%] in the zuranolone and placebo groups, respectively) had new antidepressant medication treatment initiated by investigators. There were sustained larger reductions from baseline in MADRS and HAM-A scores in the zuranolone group vs placebo at day 45 (MADRS: least-squares means difference, −5.8; 95% CI, −9.4 to −2.2; P = .002; HAM-A: least-squares means difference, −5.0; 95% CI, −7.5 to −2.4; P < .001) (eFigure 3 in Supplement 2; Figure 3).
In addition to assessing clinician-rated measures of depression, anxiety, and global function, the patient-reported BIMF scores were assessed for maternal function. Numerically greater increases in BIMF scores were observed for zuranolone vs placebo at all time points, with statistical significance at day 45 (least-squares means difference, 7.2; 95% CI, 1.3-13.0; P = .02) (eTable 9 in Supplement 2).
Safety and Tolerability
Zuranolone was generally well tolerated (Table 2). The most common treatment-emergent AEs in the zuranolone group (≥5%) were somnolence (15% [12 of 78]), headache (9% [7 of 78]), dizziness (8% [6 of 78]), upper respiratory tract infection (8% [6 of 78]), diarrhea (6% [5 of 78]), and sedation (5% [4 of 78]). The most common treatment-emergent AEs in the placebo group (≥5%) were headache (12% [9 of 73]), somnolence (11% [8 of 73]), nausea (8% [6 of 73]), dizziness (6% [4 of 73]), vomiting (6% [4 of 73]), abnormal dreams (6% [4 of 73]), and hyperhidrosis (6% [4 of 73]).
Table 2. Treatment-Emergent Adverse Events (TEAEs)a.
| Patients reporting TEAE | No. (%) | |
|---|---|---|
| Zuranolone, 30 mg (n = 78) | Placebo (n = 73) | |
| Any AE | 47 (60) | 38 (52) |
| Severe AE | 3 (4) | 3 (4) |
| Serious AE | 1 (1) | 1 (1) |
| AE drug discontinuation | 1 (1) | 0 |
| Death | 0 | 0 |
| Most common TEAEs, ≥5% patients occurring in either study arm | ||
| Somnolence | 12 (15) | 8 (11) |
| Headache | 7 (9) | 9 (12) |
| Dizziness | 6 (8) | 4 (6) |
| Upper respiratory tract infection | 6 (8) | 1 (1) |
| Diarrhea | 5 (6) | 2 (3) |
| Sedation | 4 (5) | 0 |
| Nausea | 3 (4) | 6 (8) |
| Vomiting | 1 (1) | 4 (6) |
| Abnormal dreams | 0 | 4 (6) |
| Hyperhidrosis | 0 | 4 (6) |
Abbreviation: AE, adverse event.
A TEAE is an AE with onset after the start of study drug or worsening of preexisting medical condition or AE with onset after the start of study drug. Severe AEs were defined as AEs that were incapacitating, with inability to perform normal activities. Serious AEs were defined as any untoward medical occurrence resulting in death, was life threatening (at time of event), required inpatient hospitalization, resulted in persistent or significant disability, or was a congenital anomaly. Data shown are from the safety population.
In both treatment groups, most treatment-emergent AEs were mild or moderate. Three patients in the zuranolone group experienced severe treatment-emergent AEs (sedation, n = 1; confusional state, n = 1; migraine, n = 1), and 3 patients in the placebo group experienced severe treatment-emergent AEs (back pain/muscle spasms, n = 1; headache/oropharyngeal pain, n = 1; menorrhagia, n = 1). Two patients experienced serious AEs. One patient in the zuranolone group, with no record of other medical or psychiatric conditions except for PPD, experienced a confusional state on day 3, involving inability to remember the exact sequence of the day’s events, along with sedation, which resolved within 7 hours. Following dose interruption on the day of the serious AE and reduction to 20 mg the following day (day 4), the patient continued treatment and completed the study without further incident. One patient in the placebo group experienced pancreatitis on day 32 of the follow-up period that was resolved on day 36 with cholecystectomy. In the zuranolone group, 1 patient discontinued because of an AE (intermittent sedation).
No notable or clinically significant changes in vital signals, electrocardiograms, or clinical laboratory parameters associated with any clinical findings were reported or resulted in study drug dose adjustment or discontinuation. No evidence for increased suicidal ideation or suicidal behavior was observed compared with baseline, measured by the Columbia-Suicide Severity Rating Scale. There were no medical emergencies requiring unblinding of any patient’s study drug and no evidence of withdrawal based on adverse events and vital signs.
Discussion
In this first phase 3, randomized, placebo-controlled outpatient trial in women with PPD, zuranolone demonstrated rapid (by day 3), clinically meaningful, and sustained (at all measured time points through day 45) antidepressant effects, as well as rapid and sustained improvements in anxiety and improved global and maternal functioning compared with placebo, despite the relatively high placebo response observed in this trial.
The rapid antidepressant effects, along with previous brexanolone injection clinical trials, support the development of NAS GABAAR PAMs as PPD therapies and potential fast-acting antidepressants.37,38,53 The mechanism of rapid antidepressant onset has yet to be completely elucidated.54 Currently, none of the monoaminergic antidepressants (eg, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or atypical antidepressants) have specific indications for PPD. A recent meta-analysis of controlled clinical trials of selective serotonin reuptake inhibitors in PPD reported that the strength of available evidence was low and inconclusive of whether selective serotonin reuptake inhibitors were more effective than psychosocial intervention, while demonstrating higher response and remission rates than placebo.55 Despite these limitations, current PPD standard-of-care pharmacotherapies are antidepressants approved for major depressive disorder treatment, which are often associated with delayed onset of response and/or failure to achieve remission.56,57,58 Even with dosing cessation after 2 weeks, zuranolone demonstrated sustained depressive symptom reduction over all measured time points during the 45-day study period, a high proportion of patients remained in remission over the study period, and a small proportion of patients initiated new antidepressant medications during the follow-up period. This sustained effect is clinically meaningful and similar to the effects observed in brexanolone injection studies.38 Improvements in anxiety, a common PPD symptom/comorbidity,59 and in clinician-measured global functioning (CGI-I) were also sustained through day 45. Notably, although PPD diagnosis requires that symptoms cause impaired functioning,1 standard clinician-rated trial instruments may only measure reductions in depressive symptoms, not functioning.44,46,60 Zuranolone was associated with clinically meaningful improvements in postpartum maternal functioning at day 45 using the patient-reported BIMF score, suggesting an overall treatment effect.51 Although the persistence of the zuranolone effect beyond 45 days is currently unknown, it suggests the potential for short-term, outpatient utility of zuranolone in PPD.
Zuranolone was generally well tolerated. No loss of consciousness events or AEs related to drug discontinuation after treatment/withdrawal syndrome or worsening depression were reported. The results of this double-blind, randomized, placebo-controlled clinical trial with zuranolone suggest the potential for an oral NAS GABAAR PAM in the treatment of PPD. The need for rapid and effective resolution of PPD symptoms cannot be overstated, given the prevalence of PPD and the negative effect untreated PPD can have on mothers, children, and partners.
Limitations
The study population represents a US population that may not be generalizable to broader global populations of women with PPD, although the study did enroll a higher proportion of African American and Hispanic individuals than generally observed in clinical trials. Since the follow-up period ended at day 45, the sustainability of any treatment response beyond this period is unknown. An ongoing open-label study with zuranolone will investigate the sustainability of effects and need for retreatment in major depressive disorder as a secondary end point (NCT03864614). Finally, patients were not permitted to breastfeed during the treatment period and for 1 week after treatment ended (21 days), so the safety of breastfeeding while being treated with zuranolone is currently unknown.
Conclusions
In this phase 3 randomized clinical trial of women with PPD, zuranolone achieved its primary end point of a statistically significant change from baseline in HAMD-17 total score compared with placebo at day 15. Zuranolone showed rapid (by day 3), sustained (all measured time points through day 45), and clinically meaningful improvements in depressive symptoms, anxiety, and global and maternal functioning and was generally well tolerated. Zuranolone has the potential to become a novel treatment for patients with PPD. Moreover, the findings of this study, along with prior PPD studies,37,38 support the potential for the development and therapeutic use of NAS GABAAR PAMs in the treatment of PPD.
Trial protocol
eMethods.
eTable 1. Baseline Patient Characteristics of the Safety Population
eTable 2. Psychiatric History of Depression or Anxiety Diagnosis
eTable 3. Psychiatric History of PPD Episodes and Hospitalizations due to Depression/Anxiety
eTable 4. Family Psychiatric History
eTable 5. Baseline HAMD-17 Stratified by Baseline Antidepressant Use
eTable 6. LS Mean Change from Baseline in HAMD-17
eTable 7. HAMD-17 Response
eTable 8. HAMD-17 Remission
eTable 9. LS Mean Change from Baseline in BIMF
eFigure 1. LS Mean Difference in HAMD-17 Change from Baseline at Day 15 by Baseline Subgroup
eFigure 2. LS Mean Change from Baseline in MADRS
eFigure 3. CGI-I Response
Data Sharing Statement
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2.National Institute of Mental Health. Perinatal depression. Accessed February 8, 2021. https://www.nimh.nih.gov/health/publications/postpartum-depression-facts/index.shtml
- 3.Bauman BL, Ko JY, Cox S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression: United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575-581. doi: 10.15585/mmwr.mm6919a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Osterman MJK, Driscoll AK, Rossen LM. Vital Statistics Rapid Release: Births: Provisional Data for 2018: Report No. 007. National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 5.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Diabetes during pregnancy. Updated June 12, 2018. Accessed February 8, 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy.htm
- 7.Centers for Disease Control and Prevention. Data on selected pregnancy complications in the United States. Updated February 28, 2019. Accessed February 8, 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm
- 8.Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(4):353. doi: 10.1016/j.ajog.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Cox EQ, Sowa NA, Meltzer-Brody SE, Gaynes BN. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77(9):1189-1200. doi: 10.4088/JCP.15r10174 [DOI] [PubMed] [Google Scholar]
- 11.Frieder A, Fersh M, Hainline R, Deligiannidis KM. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282. doi: 10.1007/s40263-019-00605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253. doi: 10.1001/jamapsychiatry.2017.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vliegen N, Casalin S, Luyten P. The course of postpartum depression: a review of longitudinal studies. Harv Rev Psychiatry. 2014;22(1):1-22. doi: 10.1097/HRP.0000000000000013 [DOI] [PubMed] [Google Scholar]
- 14.Johannsen BM, Larsen JT, Laursen TM, Bergink V, Meltzer-Brody S, Munk-Olsen T. All-cause mortality in women with severe postpartum psychiatric disorders. Am J Psychiatry. 2016;173(6):635-642. doi: 10.1176/appi.ajp.2015.14121510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar-Deren S, Klipstein K, Fersh M, Shemesh E, Howell EA. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224. doi: 10.1089/jwh.2015.5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153-160. doi: 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutra K, Chatzi L, Bagkeris M, Vassilaki M, Bitsios P, Kogevinas M. Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother-child cohort (Rhea Study) in Crete, Greece. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335-1345. doi: 10.1007/s00127-012-0636-0 [DOI] [PubMed] [Google Scholar]
- 18.Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70(12):1312-1319. doi: 10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surkan PJ, Ettinger AK, Hock RS, Ahmed S, Strobino DM, Minkovitz CS. Early maternal depressive symptoms and child growth trajectories: a longitudinal analysis of a nationally representative US birth cohort. BMC Pediatr. 2014;14:185. doi: 10.1186/1471-2431-14-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valla L, Wentzel-Larsen T, Smith L, Birkeland MS, Slinning K. Association between maternal postnatal depressive symptoms and infants' communication skills: a longitudinal study. Infant Behav Dev. 2016;45(pt A):83-90. doi: 10.1016/j.infbeh.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Verkuijl NE, Richter L, Norris SA, Stein A, Avan B, Ramchandani PG. Postnatal depressive symptoms and child psychological development at 10 years: a prospective study of longitudinal data from the South African Birth to Twenty cohort. Lancet Psychiatry. 2014;1(6):454-460. doi: 10.1016/S2215-0366(14)70361-X [DOI] [PubMed] [Google Scholar]
- 22.Woolhouse H, Gartland D, Mensah F, Giallo R, Brown S. Maternal depression from pregnancy to 4 years postpartum and emotional/behavioural difficulties in children: results from a prospective pregnancy cohort study. Arch Womens Ment Health. 2016;19(1):141-151. doi: 10.1007/s00737-015-0562-8 [DOI] [PubMed] [Google Scholar]
- 23.Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35. doi: 10.1046/j.1365-2648.2003.02857.x [DOI] [PubMed] [Google Scholar]
- 24.Matthey S, Barnett B, Ungerer J, Waters B. Paternal and maternal depressed mood during the transition to parenthood. J Affect Disord. 2000;60(2):75-85. doi: 10.1016/S0165-0327(99)00159-7 [DOI] [PubMed] [Google Scholar]
- 25.Vismara L, Rollè L, Agostini F, et al. Perinatal parenting stress, anxiety, and depression outcomes in first-time mothers and fathers: a 3- to 6-months postpartum follow-up Study. Front Psychol. 2016;7:938. doi: 10.3389/fpsyg.2016.00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165-180. doi: 10.1016/j.yfrne.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan C, Cosgrove J, Deligiannidis KM. Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep. 2017;19(10):70. doi: 10.1007/s11920-017-0824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front Cell Neurosci. 2019;13:83. doi: 10.3389/fncel.2019.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. Lower allopregnanolone during pregnancy predicts postpartum depression: an exploratory study. Psychoneuroendocrinology. 2017;79:116-121. doi: 10.1016/j.psyneuen.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433. doi: 10.1210/jcem.85.7.6675 [DOI] [PubMed] [Google Scholar]
- 31.Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97(1):77-80. [DOI] [PubMed] [Google Scholar]
- 32.Deligiannidis KM, Kroll-Desrosiers AR, Tan Y, Dubuke ML, Shaffer SA. Longitudinal proneuroactive and neuroactive steroid profiles in medication-free women with, without and at-risk for perinatal depression: a liquid chromatography-tandem mass spectrometry analysis. Psychoneuroendocrinology. 2020;121:104827. doi: 10.1016/j.psyneuen.2020.104827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554. doi: 10.1038/s41386-018-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207-213. doi: 10.1016/j.neuron.2008.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melón LC, Hooper A, Yang X, Moss SJ, Maguire J. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193. doi: 10.1016/j.psyneuen.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melón L, Hammond R, Lewis M, Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front Endocrinol (Lausanne). 2018;9:703. doi: 10.3389/fendo.2018.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489. doi: 10.1016/S0140-6736(17)31264-3 [DOI] [PubMed] [Google Scholar]
- 38.Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070. doi: 10.1016/S0140-6736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann E, Nomikos GG, Kaul I, et al. SAGE-217, a novel GABAA receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase i dose-finding studies. Clin Pharmacokinet. 2020;59(1):111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laverty D, Thomas P, Field M, et al. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat Struct Mol Biol. 2017;24(11):977-985. doi: 10.1038/nsmb.3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6(6):2311-2322. doi: 10.1096/fasebj.6.6.1347506 [DOI] [PubMed] [Google Scholar]
- 42.Martinez Botella G, Salituro FG, Harrison BL, et al. Neuroactive Steroids: 2: 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-Aminobutyric Acid)A receptor. J Med Chem. 2017;60(18):7810-7819. doi: 10.1021/acs.jmedchem.7b00846 [DOI] [PubMed] [Google Scholar]
- 43.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi: 10.1016/j.neuropharm.2020.108333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 48.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855. doi: 10.1001/archpsyc.1991.01810330075011 [DOI] [PubMed] [Google Scholar]
- 49.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5-9. [PubMed] [Google Scholar]
- 50.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 51.Barkin JL, Wisner KL, Bromberger JT, Beach SR, Terry MA, Wisniewski SR. Development of the Barkin Index of Maternal Functioning. J Womens Health (Larchmt). 2010;19(12):2239-2246. doi: 10.1089/jwh.2009.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanes SJ, Colquhoun H, Doherty J, et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol. 2017;32(2). doi: 10.1002/hup.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA Jr. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69(6):946-958. doi: 10.4088/JCP.v69n0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molyneaux E, Howard LM, McGeown HR, Karia AM, Trevillion K. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;(9):CD002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 57.Trivedi MH, Rush AJ, Wisniewski SR, et al. ; STAR*D Study Team . Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. doi: 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- 58.De Crescenzo F, Perelli F, Armando M, Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J Affect Disord. 2014;152-154:39-44. doi: 10.1016/j.jad.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 59.Nakić Radoš S, Tadinac M, Herman R. Anxiety during pregnancy and postpartum: course, predictors and comorbidity with postpartum depression. Acta Clin Croat. 2018;57(1):39-51. doi: 10.20471/acc.2018.57.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wisniewski SR, Rush AJ, Bryan C, et al. ; STAR*D Investigators . Comparison of quality of life measures in a depressed population. J Nerv Ment Dis. 2007;195(3):219-225. doi: 10.1097/01.nmd.0000258229.38212.6f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods.
eTable 1. Baseline Patient Characteristics of the Safety Population
eTable 2. Psychiatric History of Depression or Anxiety Diagnosis
eTable 3. Psychiatric History of PPD Episodes and Hospitalizations due to Depression/Anxiety
eTable 4. Family Psychiatric History
eTable 5. Baseline HAMD-17 Stratified by Baseline Antidepressant Use
eTable 6. LS Mean Change from Baseline in HAMD-17
eTable 7. HAMD-17 Response
eTable 8. HAMD-17 Remission
eTable 9. LS Mean Change from Baseline in BIMF
eFigure 1. LS Mean Difference in HAMD-17 Change from Baseline at Day 15 by Baseline Subgroup
eFigure 2. LS Mean Change from Baseline in MADRS
eFigure 3. CGI-I Response
Data Sharing Statement