Abstract
Background:
Heightened motor activity is a hallmark of attention-deficit/hyperactivity disorder (ADHD), yet high activity levels are also often reported in young children with autism spectrum disorder (ASD). It is currently unclear whether increased motor activity represents a distinct versus shared early predictor of ASD and ADHD; no prior studies have directly examined this prospectively. We investigated differences in longitudinal patterns of objectively-measured motor activity during early development.
Methods:
Participants included 113 infants at high and low risk for ASD or ADHD. Continuous motion-based activity was recorded using tri-axial accelerometers at 12, 18, 24, and 36 months of age. At 36-months, participants were categorized into one of three outcome groups: ASD (n=19), ADHD Concerns (n=17), and Typically Developing (TD; n=77). Group differences in trajectories of motor activity were examined in structured and semi-structured contexts. Associations with behaviors relevant to ASD, ADHD, and general development were also examined.
Results:
In both structured and semi-structured contexts, both the ASD and ADHD Concerns groups exhibited heightened activity relative to the TD group by 18 months; the ASD group exhibited higher activity than the ADHD Concerns group at 24–36 months in the structured context only. Attention/behavior regulation, nonverbal and verbal development—but not social engagement—were differentially associated with objectively-measured activity by outcome group across contexts.
Conclusions:
Overactivity may be a shared, rather than distinct, precursor of atypical development in infants/toddlers developing ASD and concerns for ADHD, emerging as early as 18 months. Group differences in overactivity may be context-specific and associated with different underlying mechanisms.
Keywords: Activity level, Attention-deficit/hyperactivity disorder, Autism spectrum disorder, Infancy
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are two prevalent neurodevelopmental conditions (Baio et al., 2018; Sayal et al., 2018). Both have an early-onset, are highly heritable, and are associated with significant long-term impairment (Dalsgaard et al., 2015; Rommelse et al., 2010). Although ADHD and ASD are distinct conditions (DSM-5, American Psychiatric Association, 2013), children with ASD often present with elevated ADHD symptoms (Simonoff et al., 2008) and vice versa (Grzadzinski et al., 2016), which can lead to challenges in differential diagnosis, especially at early ages (Kentrou et al., 2019).
Increased motor activity is a core feature of ADHD, associated with the combined and predominantly hyperactivity-impulsivity presentations. Longitudinal investigations have found associations between high levels of parent-reported activity in infancy/toddlerhood and later ADHD symptoms (Einziger et al., 2018; Shephard et al., 2019). Emerging evidence utilizing accelerometers—which objectively measure motion-based activity (Hurd et al., 2013)—has further demonstrated that increased activity distinguishes children and adults diagnosed with ADHD from controls (Murillo et al., 2015), highlighting the potential diagnostic utility of accelerometry (Hall et al., 2016). However, the age at which objectively-measured overactivity first becomes evident in ADHD is underexplored.
Although not a core symptom of ASD, several studies have examined parent-reported measures of temperament-based motor activity during early development in infant siblings of children with ASD. Findings indicate that infants developing ASD exhibit reduced activity levels at 12 months of age (Del Rosario et al., 2014), followed by heightened activity into the second and third years of life compared to controls (Bolton et al., 2012; Del Rosario et al., 2014; Garon et al., 2009). However, it is unclear whether heightened motor activity represents a distinct versus shared early predictor of ASD and ADHD (Johnson et al., 2015), as no prior studies have directly examined this in a prospectively-evaluated sample enriched for outcomes of both elevated ADHD symptoms (i.e., ADHD Concerns) and ASD.
Studies examining activity level as a potential early indicator of ASD and/or ADHD in infants at-risk for ASD have revealed mixed findings. Utilizing a prospective longitudinal sibling risk design, one study found that increased parent-reported levels of locomotor activity in infants and toddlers aged 7, 14, and 24 months were correlated with ADHD (but not ASD) symptoms at 7 years (Shephard et al., 2019). In contrast, a population-based sample found that hyperactivity, as observed and recorded by community health nurses from birth to 10 months, predicted symptoms of both ASD and ADHD in early-to-mid childhood (Elberling et al., 2014). Consistent with these findings, a different population-based, case-control investigation found higher Aberrant Behavior Checklist Hyperactivity scores in 2- to 5-year-old children with ASD compared to those with developmental delay and typical development (Lyall et al., 2017). The different methods of measurement implemented to assay activity level across studies, paired with the subjective nature of measurement, may account for some of the observed differences across studies. In general, however, these studies suggest that heightened levels of activity early in life may be a hallmark of not only ADHD, but also evident in ASD. Based on a recently-described inherited polygenic risk framework (Constantino et al., 2021), overactivity early in development may be a possible overlapping inherited phenotype present in children with ASD and ADHD. Studying possible shared developmental phenotypes in at-risk infants might aid in elucidating the effects of inherited influences on children with ASD and ADHD, and potentially point to targets for transdiagnostic preventive and early intervention efforts (Talbott & Miller, 2020).
Determining whether activity levels deviate from typical development in early life is a challenge since it is not atypical for young children to exhibit high levels of motor activity (Fewell & Deutscher, 2002). This highlights the need to implement objective-measurement to aid in the quantification and detection of concerning activity levels exhibited during early development. No study to our knowledge has objectively-quantified activity level during the prodromal period of development for both disorders. The sensitivity of accelerometer-measured activity–which has been shown to distinguish preschool-aged children with ADHD from those with typical development (De Crescenzo et al., 2016; Murillo et al., 2015)–has the potential to determine the extent to which overactivity during the prodromal period is a specific early marker of ADHD versus a more general indicator of atypical development.
The current study examined differences in longitudinal patterns of objectively-measured activity from 12–36 months of age in a sample enriched for outcomes of ASD and ADHD Concerns. Since environmental task demands may influence the presentation of both ADHD (Kofler et al., 2016) and ASD (Kanne et al., 2009) symptoms, we examined activity level group differences across two assessment contexts (structured and semi-structured). We hypothesized that: (1) in line with findings based on parent-report measures of activity, infants developing ASD or ADHD Concerns would exhibit higher activity levels compared to typically developing infants across assessment contexts beginning in the second year of life; and (2) differences between participants with ADHD Concerns versus Typically Developing (TD) outcomes would be more pronounced during structured assessments, since higher activity levels in children with ADHD are often associated with increased task demands (Kofler et al., 2016). Exploratory analyses examined whether ADHD- and ASD-relevant behaviors and verbal/nonverbal development were differentially associated with objectively-measured activity by outcome group.
Methods
Ethical Considerations
The study protocol was approved by the University of California, Davis Institutional Review Board; written informed consent was obtained from parents before participation.
Participants
One-hundred and eighty-five infants with an older sibling diagnosed with ASD (ASD-risk group; n=100), an older sibling or parent diagnosed with ADHD (ADHD-risk group; n=46), or no family history of either diagnosis (low-risk group; n=39) participated in developmental assessments at 12, 18, 24, and 36 months of age as part of an ongoing longitudinal study. Additional inclusion/exclusion criteria details are provided in Appendix S1 of the Supporting Information.
At 36-months, participants were classified into one of three outcome groups by a licensed psychologist. Consistent with previous work (Hatch et al., 2020; Hill et al., 2020), participants were classified into the “ADHD Concerns” outcome group if they (a) received an examiner-rated clinical best estimate outcome of “ADHD Concerns,” and (b) exhibited ≥4 DSM-5 ADHD symptoms within any one symptom domain (i.e., inattentive or hyperactive-impulsive) or ≥5 DSM-5 symptoms across both symptom domains (i.e., inattentive and hyperactive-impulsive combined) across reporters (i.e., parent and teacher report on the ADHD Rating Scale Preschool version, examiner observation); at least one of the endorsed symptoms must have been reported on the ADHD Rating Scale by the parent or teacher as evidence of some degree of cross-situational presence of symptoms. That is, no child was classified into the ADHD Concerns outcome group based on examiner report alone. Participants with ASD met DSM-5 criteria for ASD and received an ADOS-2 calibrated severity score ≥4 (Lord et al., 2012). Participants who met criteria for ASD were also considered for inclusion in the ADHD Concerns group; n=2 participants met criteria for both outcomes but were excluded from analyses due to having too few accelerometer datapoints (see below). The TD group included all participants who did not meet criteria for ASD or ADHD Concerns, had an ADOS-2 comparison score <3 and had no more than one Mullen Scales of Early Learning (MSEL) subscale T-scores <35 with no subscales <30.
Participants included in the current study completed the 36-month assessment and received an outcome classification of ASD, ADHD Concerns, or TD and had ≥20-min of accelerometer data during ≥2 visits (Cliff et al., 2009). A total of 160 participants (86%) completed a 36-month assessment and received an outcome classification, 15 of whom (9%) were excluded due to outcome classification of non-TD that was inconsistent with either ASD or ADHD Concerns (of whom 12 had usable accelerometer data). An additional 32 participants (20%) were excluded due to having only one visit with useable accelerometer data (ADHD Concerns=3; ASD=5; ASD+ADHD Concerns=2; TD=22). The final analyzed sample consisted of 113 participants: ADHD Concerns (n=17), ASD (n=19), and TD (n=77). Table 1 displays sample characteristics by outcome group. Thirty-five percent had accelerometer data for all 4 visits, 32% for 3 visits, and 34% for 2 visits. Table S1 shows available and missing accelerometer data by group and visit age.
Table 1.
Participant characteristics.
| ADHD Concerns | ASD | TD | p-value | |
|---|---|---|---|---|
| (n=17) | (n=19) | (n=77) | ||
| Mean Age, Mean (SD) | 36.7 (0.8) | 36.2 (0.7) | 36.5 (1.0) | 0.29 |
| Male Sex, n (%) | 11 (64.7) | 11 (57.9) | 36 (53.2) | 0.68 |
| Risk Group, n (%) | <0.001 | |||
| ADHD Concerns | 11 (64.7) | 0 (0) | 22 (28.6) | |
| ASD | 6 (35.3) | 19 (100) | 29 (37.7) | |
| Low Risk | 0 (0.0) | 0 (0.0) | 26 (33.8) | |
| Racea, n (%) | 0.79 | |||
| Non-White | 7 (41.2) | 10 (52.6) | 35 (45.5) | |
| White | 10 (58.8) | 9 (47.4) | 40 (51.9) | |
| Hispanic Ethnicityb, n (%) | 2 (11.8) | 6 (31.6) | 14 (18.2) | 0.67 |
| Maternal Education, n (%) | 0.09 | |||
| No college degree | 7 (41.2) | 4 (21.1) | 13 (16.9) | |
| College degree or higher | 10 (58.8) | 15 (78.9) | 64 (83.1) | |
| Household Incomec, n (%) | 0.14 | |||
| Under $20,000 | 2 (11.8) | 0 (0) | 1 (1.3) | |
| $20,001–$60,000 | 6 (35.3) | 5 (26.3) | 14 (18.2) | |
| $60,001–$100,000 | 3 (17.6) | 7 (36.8) | 17 (22.1) | |
| $100,001 or higher | 5 (29.4) | 7 (36.8) | 36 (46.8) | |
| Autism Diagnostic Observation Schedule, 36 months d | <0.001 | |||
| Comparison Score, Mean (SD) | 1.6 (1.0) | 6.8 (2.3) | 1.2 (0.4) | |
| Attention-Deficit/Hyperactivity Disorder Rating Scale, 36 months e | <0.001 | |||
| Total, Mean (SD) | 18.6 (8.8) | 20.2 (9.9) | 9.3 (5.7) | |
Note: ADHD=attention-deficit/hyperactivity disorder; ASD=autism spectrum disorder; TD=typical development, SD=standard deviation. Overall group differences examined using chi-square tests for sex, risk group, race/ethnicity, maternal education, and Fisher’s Exact test for household income, and one-way analysis of variance for continuous measures. Missing or parent preferred not to answer:
n=2 TD;
n=3 ADHD Concerns, n=2 TD;
n=1 ADHD Concerns, n=9 TD;
n=1 ADHD Concerns;
n=3 ADHD Concerns, n=2 ASD, n=2 TD;
n=1 ASD, n=3 TD.
Procedure
During assessments at 12, 18, 24, and 36 months of age, continuous motion-based activity was recorded (range ±2g) from participants’ ankles using Empatica E4 multi-sensor devices (McCarthy et al., 2016) at a sampling rate of 32-Hz across the duration of each visit (Mduration=74.3 min, SDduration=46.9 min). Accelerometer-derived dependent variables were examined within two assessment contexts: structured and semi-structured testing. Participants first completed structured table testing (MSEL, several brief experimental tasks), followed by semi-structured, play-based testing (Autism Diagnostic Observation Schedule, 2nd Ed. [ADOS-2]). Testing order was consistent across participants and visits, except for the 12-month visit which consisted only of structured testing. Due to potential variation in our sample related to ADOS-2 guidelines (i.e., nonverbal mental age ≥12 months, ability to walk at least a few steps independently; Lord et al., 2012), the ADOS-2 was not administered at the 12-month visit.
Measures
Sample Characterization
ADOS-2 (Lord et al., 2012).
This semi-structured standardized observation of social communication and repetitive behaviors was used to verify inclusion criteria in probands with ASD and determine 36-month outcomes. The ADOS-2 was administered at the 18-, 24-, and 36-month visits, representing the “semi-structured” context.
Child & Adolescent Symptom Inventory, 5th Edition (CASI-5; Gadow & Sprafkin, 2013).
Caregivers completed the ADHD section of this checklist for DSM-5-defined symptoms of common childhood disorders on sibling probands to confirm familial risk classification.
Attention-Deficit/Hyperactivity Disorder Rating Scale, Preschool Version (ADHD-RS; McGoey et al., 2007).
Caregivers completed the preschool version of this ADHD symptom measure at the 36-month visit to inform outcome classification. An additional observer familiar with the child (e.g., preschool teacher, daycare provider) also completed this rating scale whenever possible.
Mullen Scales of Early Learning (MSEL; Mullen, 1995).
Four subscales from this standardized developmental test were administered during structured tabletop testing at each study visit: Fine Motor, Visual Reception, Expressive Language, and Receptive Language. MSEL subscales have excellent internal consistency (median 0.91) and test-retest reliability (median 0.84). Subscale T-scores (M=50; SD=10) and age equivalents can be obtained. The MSEL was utilized to inform participant 36-month outcome classification.
Accelerometer-derived Measures of Activity Level
Accelerometer preprocessing details are included in Appendix S2 of the Supporting Information. Filtered accelerometer activity was segmented into units of acceleration (mm/s2) over 15-s time intervals (epochs) within each assessment context for each visit and participant. Two dependent variables of activity level were derived (Hurd et al., 2013; Wood et al., 2009): Mean activity, by calculating the average of the epoch activity, and Mean intensity of activity, by calculating the mean of the maximum magnitude of epoch activity within each assessment context for each visit and participant. Duration of accelerometer recording at each visit, across assessment contexts, was not correlated with either mean activity or mean intensity of activity (range of Spearman’s rank correlation coefficients, −0.11 to −0.02, p≥0.38).
ASD, ADHD, and Verbal/Nonverbal Behaviors
Social engagement.
Frequencies of three social engagement behaviors were rated by examiners at each visit on a five-point scale ranging from 1=None to 5=Very Frequent: eye-contact, shared affect, and overall social responsiveness. A social engagement composite (range=3–15) was derived by summing these items, with higher scores reflecting a higher frequency of social engagement behaviors. This measure has been used in prior studies and was correlated with behaviorally-coded social behaviors (Ozonoff et al., 2010).
Behavior Rating Inventory for Children (BRIC).
Three ADHD-relevant behaviors (attention, activity, impulsivity; Gopin et al., 2010) were rated by examiners at each visit using a five-point Likert scale, with lower scores reflecting fewer problematic behaviors. An attention/behavior regulation composite was derived by summing and rescaling the three items so that higher scores reflect better attention/behavior regulation (range=3–15). The BRIC was modified for use with infants and toddlers and has been shown to correlate with behaviorally-coded attention/behavior regulation (Miller et al., 2020).
Verbal and Nonverbal Developmental Quotients (DQs).
DQs were calculated by dividing each MSEL subscale age-equivalent score by the child’s chronological age and multiplying by 100 (Messinger et al., 2013). Verbal (i.e., the average of the Receptive and Expressive Language DQs) and nonverbal (i.e., the average of the Fine Motor and Visual Reception DQs) DQs were derived.
Statistical Analyses
Linear mixed-effects models were implemented using the nlme package in R version 3.6.2 (R Core Team, 2020) to examine longitudinal trajectories of activity level and associations with outcome group, stratified by assessment context (structured, semi-structured). The models that were used to represent the observed accelerometer variable Yij for individual i at the measurement visit j for each context had the form:
Cubic age effects were fitted only for the structured context models. Age (in months) was centered at 12 months in the structured context, and 18 months in the semi-structured context. Therefore, the coefficients α0, β0, β3, and β6 describe the mean trajectory for participants with TD outcomes. The coefficients α1 and α2 provide tests of the differences at baseline between participants with ASD and TD outcomes and between ADHD Concerns and TD outcomes. The coefficients β1-β2, β4-β5, and β7-β8 (for structured context only) provide tests of whether the linear, quadratic, or cubic slopes differ between participants with ASD and TD outcomes and between ADHD Concerns and TD outcomes. Normally distributed random effects for participants intercepts (τi) were included to allow for deviations from the mean scores predicted by the model and to account for within-child correlation. Finally, the errors εij were assumed independent and normally distributed and independent of τi.
After fitting these initial models, we tested and sequentially removed higher-level terms that did not add significantly to the model. Heterogeneous variances (by group) were considered for the error terms. Final fixed effect structures were identified through comparisons between models with the same random effects structure using maximum likelihood estimation. All final models predicting mean activity included a heterogeneous residual variance structure within outcome groups since this improved model fit for mean activity models, but not mean intensity.
To evaluate whether time-varying characteristics relevant to ADHD (attention/behavior regulation), ASD (social engagement), or verbal/nonverbal development—measured at each assessment time point (12, 18, 24, and 36 months of age)—were differentially associated with accelerometer metrics by group, we tested each behavior individually by adding terms for the behavior (grand mean centered) and its interactions with group to the final models. Table S2 shows descriptive statistics by group and visit for all behaviors considered as covariates. We additionally conducted Spearman’s rank correlations to examine the relationship between accelerometer variables at each age and 36-month examiner-rated social engagement and 36-month attention/behavior regulation total scores (Table S3).
Following significant interaction effects, linear contrasts were used to evaluate pairwise group differences at each visit age. All tests were 2-sided with p-values of <0.05 considered statistically significant.
Results
Longitudinal Trajectories of Activity Level
Tables S4–S7 report tested models and model fit indices.
Structured Context.
Linear mixed-effects models indicated that the three groups did not differ in either accelerometer metric at 12 months of age. However, the interactions between outcome group and age effects were significant for both dependent variables in the structured assessment context, indicating different group developmental trajectories of mean activity (MA) and mean intensity of activity (MI) from baseline (12 months). These were driven by the ASD group having significantly different trajectories than the TD group (difference in both linear and quadratic slopes p<0.05). See Table 2 and Figure 1.
Table 2.
Parameter Estimates (SE) for the final linear mixed-effects models.
| Structured | Semi-Structured | |||
|---|---|---|---|---|
| Mean Activity | Mean Intensity | Mean Activity | Mean Intensity | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| (Intercept) | 50.07 (4.18)c | 715.33 (54.04)b | 117.91 (4.33)c | 1584.01 (43.12)c |
| ADHD Concerns | 7.25 (8.73) | 96.00 (106.54) | 21.57 (5.73)c | 233.70 (57.00)c |
| ASD | −3.11 (12.22) | −39.74 (105.42) | 19.97 (6.79)b | 161.71 (57.17)b |
| Age | 5.43 (1.43)c | 54.45 (17.78)b | −0.93 (0.22)c | −12.50 (2.28)c |
| Age2 | −0.55 (0.14)c | −5.37 (1.78)b | _ | _ |
| Age3 | 0.01 (0.004)c | 0.13 (0.05)b | _ | _ |
| ADHD Concerns×Age | 0.58 (1.48) | 8.18 (17.71) | _ | _ |
| ASD×Age | 5.93 (2.22)b | 49.40 (18.22)b | _ | _ |
| ADHD Concerns×Age2 | −0.01 (0.05) | −0.15 (0.62) | _ | _ |
| ASD×Age2 | −0.18 (0.08)a | −1.29 (0.65)a | _ | _ |
Note:
p<0.05,
p<0.01,
p<0.001.
Age measured in months was centered at 12 months in structured models and 18 months in semi-structured models. Mean activity and mean intensity measured in mm/s2. Estimates are from linear mixed-effects models with fixed effects reflected above and random effects for child-specific intercept.
SE=standard error; ADHD=attention-deficit/hyperactivity disorder; ASD=autism spectrum disorder; Age2=quadratic age effect; Age3=cubic age effect.
Figure 1. Longitudinal trajectories of motor activity by outcome group.
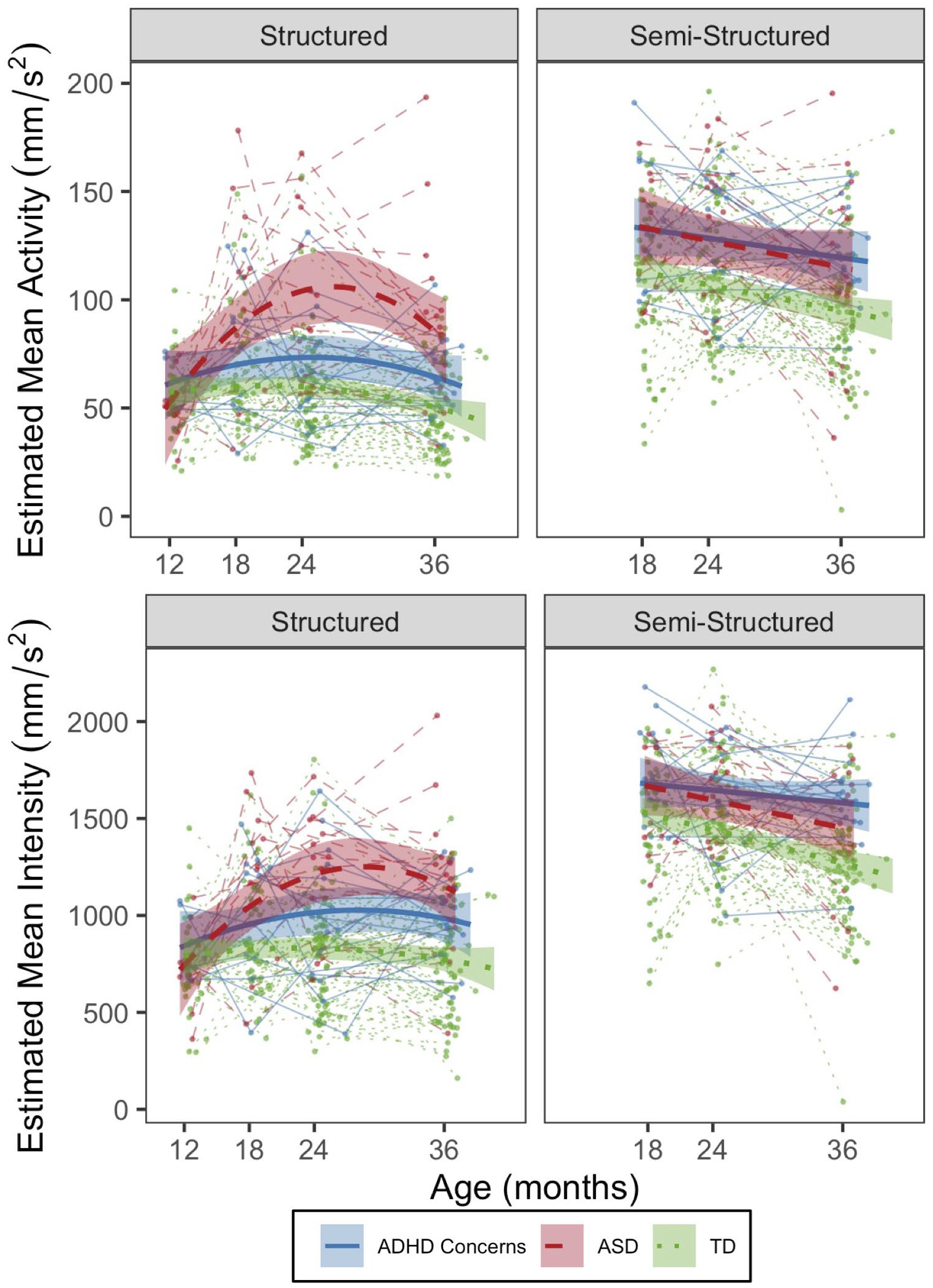
Mean activity (top panel) and mean intensity of activity (bottom panel) by structured (left panel) and semi-structured (right panel) assessment context. Error bands denote 95% Confidence Intervals.
Pairwise comparisons derived from the final models (Table S8) indicated that, over time, both the ASD and ADHD Concerns groups had significantly higher MA and MI compared to the TD group, with differences first observed at 18 months (ASD vs TD: MA: estimated difference [est.]=26.1, p<0.001; MI: est.=210.1, p=0.001; ADHD Concerns vs TD: MA: est.=10.3, p=.047; MI: est.=139.8, p=0.03), and becoming more pronounced through 36 months of age (ASD vs. TD: all ps<0.001; ADHD Concerns vs TD: all ps≤0.03). At 24 and 36 months, the ASD group had significantly higher MA and MI relative to the ADHD Concerns group (24-month MA: est.= −30.2, p=0.002; MI: est.= −194.1, p=0.04; 36-month MA: est.=−23.4, p=0.01; MI: est.= −194.7, p=0.04).
Semi-structured Context.
Table S9 shows descriptive statistics of accelerometer measures by group across visit ages. The quadratic age effect and interaction terms for ADHD Concerns and ASD outcome groups with age effects were not significant and therefore removed from the final models (Table 2), suggesting that the groups exhibited parallel patterns of MA and MI over time. The fixed effects for ASD and ADHD Concerns were significant, indicating greater MA and MI than the TD group across the age span examined (18–36 months). No significant differences in either accelerometer metric were observed between the ADHD Concerns and ASD groups.
Finally, the results of a secondary analysis conducted in the TD group only and reported in Table S10 suggest that risk group may not be differentially associated with activity level across the ages examined, as risk group effects and interactions between risk group and age effects were not significant.
Analysis of Time-varying Covariates
Structured Context.
Interaction terms with age were non-significant (Table S11), indicating significant effects described below did not differ by age. In terms of behaviors relevant to ADHD and ASD, for both accelerometer dependent variables, there was a significant effect of attention/behavior regulation for TD participants (MA: β=−1.91, p<0.001; MI: β=−27.27, p<0.001), indicating that activity level was lower in children who had better attention/behavior regulation. Both models yielded a significant ASD outcome group by attention/behavior regulation interactions (MA: β=−7.89, p<0.001; MI: β=−58.79, p=0.002), suggesting reduced attention/behavior regulation was more strongly associated with increased MA in the ASD group compared to the TD group. The strength of this relationship did not differ between the ADHD Concerns and TD groups, nor did it differ between the ADHD Concerns and ASD groups. Effects of social engagement were non-significant.
Regarding general developmental functioning, for TD participants, there was a significant association between nonverbal DQ and MA (β=−0.26, p=0.02), but not MI, suggesting that MA was higher in children with lower nonverbal functioning. Significant ASD outcome group by nonverbal DQ interactions indicated that lower nonverbal DQ was more strongly associated with increased activity levels in the ASD group compared to the TD group (MA: β=−1.02, p<0.001; MI: β=−9.03, p<0.001). This relationship did not differ between the ADHD Concerns and TD groups, nor between the ADHD Concerns and ASD groups. In terms of verbal DQ, there was a significant fixed effect, but not interaction, on both dependent variables, suggesting that increased activity was associated with lower verbal DQs (MA: β=−0.28, p<0.001; MI: β=−2.80, p=0.004).
Semi-structured Context.
There was a significant fixed effect of attention/behavior regulation, but no interaction with group, indicating that both accelerometer metrics were higher in children who had lower examiner-rated attention/behavior regulation skills (MA: β=−2.16, p=0.001; MI: β=−18.99, p=0.005). Effects of social engagement were non-significant.
Effects of nonverbal DQ were non-significant. However, for both accelerometer metrics, the effect of verbal DQ was significant (MA: β=0.39, p=0.01; MI: β=2.99, p=0.009) in the TD group, indicating that higher verbal functioning was associated with increased activity levels. The interaction between ADHD Concerns outcome and verbal DQ on MA (but not MI) was significant (β=−0.74, p=0.02), indicating a stronger negative association between verbal functioning and MA in the ADHD Concerns group compared to the TD group. The strength of this association did not differ between the ASD and TD groups, nor between the ASD and ADHD Concerns groups.
Discussion
To our knowledge, this is the first study to examine differences in longitudinal patterns of objectively-measured activity in a sample of infants at familial risk for ADHD or ASD. Although no differences in activity levels were observed among groups at baseline (12-months) in the structured assessment context, both the ASD and ADHD Concerns groups exhibited heightened activity levels compared to the TD group by 18 months, persisting through 36 months of age. In the semi-structured assessment context both the ASD and ADHD Concerns groups exhibited heightened activity levels compared to the TD group across all ages examined (18–36 months).
Although lower parent-reported activity levels have been documented in infants with later ASD at 12 months (Del Rosario et al., 2014), we did not find significant differences in objectively-measured motor activity among groups at this age. Instead, our findings suggest heightened activity levels emerge in the second year of life among infants developing ASD, consistent with our hypothesis and previous studies utilizing parent-report measures of activity level (Bolton et al., 2012; Del Rosario et al., 2014; Garon et al., 2009). Our study extends these previous findings by being the first to characterize activity level trajectories in infants with later ASD using objective accelerometer measures.
Contrary to our hypothesis, we did not find context-dependent effects on activity level in the ADHD Concerns group. The ADHD Concerns group exhibited increased MA and MI in both the structured and the semi-structured assessment contexts compared to the TD group, emerging at 18 months in the structured context, and evident at all ages examined (18–36 months) in the semi-structured context. Findings related to contextual influences on activity level in ADHD are mixed. Although several studies suggest that higher activity levels in participants with ADHD are associated with increased task demands (Kofler et al., 2016), others have found minimal differences in activity levels between ADHD participants and controls (Kam et al., 2011). Our results suggest that toddlers who develop ADHD Concerns by age 3 exhibit heightened activity levels across both structured and less structured contexts. These results may differ among children who later meet full diagnostic criteria for ADHD, which we hope to address as these children are followed over time.
By 18 months of age, the ASD and ADHD Concerns groups both exhibited higher activity levels relative to the TD group, but mean activity levels were significantly higher in the ASD group relative to the ADHD Concerns group in the structured (but not semi-structured) assessment context at 24–36 months of age. Given the limited sample size in the ASD and ADHD group, and in consideration of the medium effect sizes observed (Tables S8 and S9), it is possible that group differences would have emerged (e.g., been statistically significant) at an earlier time point in the structured context, with the ASD group showing greater MA than the ADHD Concerns group at 18 months of age. In the semi-structured context, it is possible that with a larger sample the ADHD Concerns group may have shown greater MI at 36 months of age relative to the ASD group.
Although ADHD symptoms like elevated activity level are often observed in young children with ASD (Antshel & Russo, 2019; Johnson et al., 2015), it is currently unclear whether heightened motor activity in ASD reflects the same underlying mechanisms that are associated with overactivity in ADHD. To begin to address this question, we examined whether ADHD- and ASD-relevant behaviors (i.e., attention/behavior regulation, social engagement) were differentially associated with the group effects described above. For all groups, lower attention/behavior regulation was associated with higher mean activity and intensity across both assessment contexts. This makes sense, as activity level was one of the three behaviors rated by examiners comprising the attention/behavior regulation composite (along with ratings of attention and impulsivity). In the structured context, the associations between attention/behavior regulation and both accelerometer metrics were stronger in the ASD group compared to the TD group, with no difference in the strength of the associations between the ASD and ADHD Concerns groups. In contrast, associations between social engagement and accelerometer metrics were non-significant. These findings are consistent with research describing associations between increased parent-rated activity level in infancy and later ADHD symptoms, but not ASD (Shephard et al., 2019). Although attention/behavior regulatory problems are implicated in ADHD, such problems also frequently co-occur in children with ASD (for a review see, Johnson et al., 2015), suggesting the importance of transdiagnostic approaches to understanding mechanisms underlying these behaviors that may cut across diagnostic boundaries. Given the strong association between the examiner rated measure of attention/behavior regulation and objective-measures of motor activity, our findings suggest that accelerometry may be a useful metric for not only objectively measuring heightened levels of activity early in development, but also for potentially indexing early manifestations of more generalized attentional and/or behavioral dysregulation. Future work should aim to build upon these initial analyses by applying causal modeling to describe the direction of the associations between objectively-measured activity level and specific behavioral symptoms of ADHD, as well as ASD.
We also examined the effect of general developmental functioning on group effects on activity level, finding some context-specific group differences. Specifically, the relationship between nonverbal development and activity level was especially strong in the ASD group in the structured context, consistent with evidence suggesting that children with ASD plus high ADHD symptoms tend to present with lower developmental functioning (Antshel & Russo, 2019; Karalunas et al., 2018). In contrast, the association between verbal development and mean activity level was particularly strong in the ADHD Concerns group in the semi-structured context. Among children with ADHD Concerns outcomes, those with lower language skills appeared to be more active in an environment in which there were fewer physical constraints and fewer “instructions” to follow. Indeed, inattentive-hyperactive behaviors have previously been associated with lower language abilities (Petersen et al., 2013), and language skills are suggested to serve as a self-regulatory mechanism in children with or at-risk for developing ADHD (Petersen et al., 2015). It is possible that heightened levels of activity in young children with ADHD concerns may partially arise from deficits in using language for planning and monitoring behavior in environments with limited structure/constraints.
Limitations
Strengths of this study include incorporating infants at familial risk for ADHD, a rarely-studied population (but see Gui et al., 2020); the use of an objective-measure of motor activity; and rigorous, longitudinal behavioral phenotyping of the sample. Several limitations are also apparent, including our relatively small sample size. In addition, our sample had a lower rate of co-occurrence between ASD and ADHD Concerns than has been found in other studies (Antshel & Russo, 2019). There are several potential explanations for this finding. First, our ADHD Concerns algorithm integrated ratings from examiners, parents, and (whenever possible) teachers. Some of the DSM ADHD symptoms cannot be observed by examiners in a structured test setting, which may have led to lower reporting rates for certain behaviors. In addition, we cannot rule out the possibility that parents and teachers may report on ADHD-related behaviors differently in children with ASD than those without ASD especially at such young ages. Finally, given the average age of ADHD diagnosis of age 7 (Visser et al., 2014), many children who develop ADHD may not show overt symptoms as early as 36 months of age. Therefore, it is likely that some of the children with ASD in the current study will go on to develop ADHD after 36 months of age. We hope to further explore potential co-occurrence between children with ASD and later ADHD in follow-up investigations.
We also obtained accelerometer measures from a single location (ankle). While this is a common location (De Crescenzo et al., 2016), additional recordings from other locations (e.g., waist) could provide more robust information about the nature of overactivity during early development. We also did not collect data on the proportion of time participants were seated in their caregiver’s laps during structured testing. To account for potential movement artifacts from caregivers, future work could aim to record accelerometer metrics from caregivers to allow for subtraction of caregiver movement from child movement. Finally, due to the testing guidelines of the ADOS-2, the semi-structured context excluded 12-month-old participants and, as a result, accelerometer data were only available for the structured assessment context at 12 months. Since group differences in the semi-structured context were not associated with age-related changes, it is possible that we did not capture the developmental transition point at which activity levels in children with ADHD Concerns and ASD begin to diverge from typically developing children.
Conclusion
Overall, our findings suggest that overactivity observed as early as 18 months of age may be a shared, rather than distinct, characteristic of infants/toddlers developing ADHD concerns and ASD. However, subtle group differences in overactivity may be context-specific and associated with different underlying mechanisms. From a clinical perspective, it is critical to be aware of such overlap in symptom presentation between infants/toddlers developing ADHD Concerns and ASD, as previous evidence suggests that ADHD symptoms in young children with ASD may delay formal diagnosis (Kentrou et al., 2019) and impact treatment outcomes (Antshel & Russo, 2019).
Our findings suggest that accelerometry may be a useful supplementary metric to complement behavioral observation for objectively detecting heightened activity levels early in development among young children with ASD and concerns for ADHD. However, future work is needed to identify whether overactivity is a transitory versus stable early marker in infants/toddlers who later meet full diagnostic criteria for ADHD and ASD before prognostic validity and transdiagnostic guidelines can be established. It is further necessary to understand what combination of metrics is most predictive of outcome and the extent to which child age may impact the predictability of certain metric combinations. Finally, to establish the clinical utility of accelerometry, future work is needed to examine the feasibility and acceptability of clinician use of this metric for monitoring heightened activity levels early in development.
Supplementary Material
Key points.
Although it is currently unclear whether increased activity level represents a distinct versus shared early predictor of ASD and ADHD, no study to date has directly examined this prospectively.
The current study examined differences in longitudinal patterns of objectively-measured activity from 12–36 months of age in a sample enriched for outcomes of ASD and ADHD Concerns across two assessment contexts.
Heightened motor activity emerged by 18 months in infants with later ADHD Concerns and ASD; group differences were observed across assessment contexts.
From a clinical standpoint, it is important to be aware of potential symptom overlap, as ADHD symptoms present in young children with ASD have been found to delay formal diagnosis and impact treatment outcomes.
Acknowledgements
This study was supported by grants from the National Institute of Mental Health R00 MH106642 (Miller), R01 MH068398 (Ozonoff), P50 MH106438-7776, the UC Davis Conte Center Biostatistics Core (Iosif), and the National Institute of Child Health and Human Development Intellectual and Developmental Disabilities Research Center P50 HD103526 (Abbeduto). R.R.’s time was supported by the Human Resources Services Agency grant HRSA-16-031 through the Northern California Leadership Education in Neurodevelopmental and Related Disorders Program. The authors gratefully acknowledge the families who have participated in their ongoing longitudinal investigation. The authors also thank Dr. Costin Tanase for contributing initial coding syntax for the accelerometer preprocessing pipeline.
A-M.I. has received honoraria for reviewing activities from Elsevier. S.O. has received research grant funding from the National Institutes of Health and Autism Speaks, travel reimbursement and honoraria for editorial activities from Autism Speaks, Autism Science Foundation, and Wiley, and book royalties from Guilford Press and American Psychiatric Press, Inc. M.M. has received research grant funding from the National Institutes of Health and travel reimbursement and/or honoraria from the Society for Clinical Child and Adolescent Psychology and the Help Group. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Conflict of interest statement: See Acknowledgements for full disclosures.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Appendix S1. Participants
Appendix S2. Accelerometer Recording and Preprocessing.
Table S1–S11.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. BMC Med, 17, 133–137. [Google Scholar]
- Antshel KM, & Russo N (2019). Autism spectrum disorders and ADHD: Overlapping phenomenology, diagnostic issues, and treatment considerations. Current Psychiatry Reports, 21(5), 34. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg CR, & White T (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton PF, Golding J, Emond A, & Steer CD (2012). Autism spectrum disorder and autistic traits in the Avon Longitudinal Study of Parents and Children: Precursors and early signs. Journal of the American Academy of Child & Adolescent Psychiatry, 51(3), 249–260. [DOI] [PubMed] [Google Scholar]
- Cliff DP, Reilly JJ, & Okely AD (2009). Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0–5 years. Journal of Science and Medicine in Sport, 12(5), 557–567. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Charman T, & Jones EJ (2021). Clinical and translational implications of an emerging developmental substructure for autism. Annual Review of Clinical Psychology, 17, 365–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, & Pedersen MG (2015). Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. The Lancet, 385(9983), 2190–2196. [DOI] [PubMed] [Google Scholar]
- De Crescenzo F, Licchelli S, Ciabattini M, Menghini D, Armando M, Alfieri P, Mazzone L, Pontrelli G, Livadiotti S, & Foti F (2016). The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Medicine Reviews, 26, 9–20. [DOI] [PubMed] [Google Scholar]
- Del Rosario M, Gillespie-Lynch K, Johnson S, Sigman M, & Hutman T (2014). Parent-reported temperament trajectories among infant siblings of children with autism. Journal of Autism and Developmental Disorders, 44(2), 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einziger T, Levi L, Zilberman-Hayun Y, Auerbach JG, Atzaba-Poria N, Arbelle S, & Berger A (2018). Predicting ADHD symptoms in adolescence from early childhood temperament traits. Journal of Abnormal Child Psychology, 46(2), 265–276. [DOI] [PubMed] [Google Scholar]
- Elberling H, Linneberg A, Olsen EM, Houmann T, Rask CU, Goodman R, & Skovgaard AM (2014). Infancy predictors of hyperkinetic and pervasive developmental disorders at ages 5–7 years: Results from the Copenhagen Child Cohort CCC 2000. Journal of Child Psychology and Psychiatry, 55(12), 1328–1335. [DOI] [PubMed] [Google Scholar]
- Fewell RR, & Deutscher B (2002). Attention deficit hyperactivity disorder in very young children: Early signs and interventions. Infants & Young Children, 14(3), 24–32. [Google Scholar]
- Gadow K, & Sprafkin J (2013). Child & Adolescent Symptom Inventory-5 manual. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, & Szatmari P (2009). Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology, 37(1), 59–78. [DOI] [PubMed] [Google Scholar]
- Gopin C, Healey D, Castelli K, Marks D, & Halperin JM (2010). Usefulness of a clinician rating scale in identifying preschool children with ADHD. Journal of Attention Disorders, 13(5), 479–488. [DOI] [PubMed] [Google Scholar]
- Grzadzinski R, Dick C, Lord C, & Bishop S (2016). Parent-reported and clinician-observed autism spectrum disorder (ASD) symptoms in children with attention deficit/hyperactivity disorder (ADHD): Implications for practice under DSM-5. Molecular Autism, 7(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui A, Mason L, Gliga T, Hendry A, Ali JB, Pasco G, Shephard E, Curtis C, Charman T, & Johnson MH (2020). Look duration at the face as a developmental endophenotype: Elucidating pathways to autism and ADHD. Development and Psychopathology, 32(4), 1303–1322. [DOI] [PubMed] [Google Scholar]
- Hall CL, Valentine AZ, Groom MJ, Walker GM, Sayal K, Daley D, & Hollis C (2016). The clinical utility of the continuous performance test and objective measures of activity for diagnosing and monitoring ADHD in children: A systematic review. European Child & Adolescent Psychiatry, 25(7), 677–699. [DOI] [PubMed] [Google Scholar]
- Hatch B, Iosif A-M, Chuang A, de la Paz L, Ozonoff S, & Miller M (2020). Longitudinal Differences in Response to Name Among Infants Developing ASD and Risk for ADHD. Journal of Autism and Developmental Disorders, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Gangi D, Miller M, Rafi SM, & Ozonoff S (2020). Screen time in 36-month-olds at increased likelihood for ASD and ADHD. Infant Behavior and Development, 61, 101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd WJ, Morrow MM, & Kaufman KR (2013). Tri-axial accelerometer analysis techniques for evaluating functional use of the extremities. Journal of Electromyography and Kinesiology, 23(4), 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Gliga T, Jones E, & Charman T (2015). Annual Research Review: Infant development, autism, and ADHD–early pathways to emerging disorders. Journal of Child Psychology and Psychiatry, 56(3), 228–247. [DOI] [PubMed] [Google Scholar]
- Kam HJ, Lee K, Cho S-M, Shin Y-M, & Park RW (2011). High-resolution Actigraphic analysis of ADHD: a wide range of movement variability observation in three school courses-a pilot study. Healthcare Informatics Research, 17(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Abbacchi AM, & Constantino JN (2009). Multi-informant ratings of psychiatric symptom severity in children with autism spectrum disorders: The importance of environmental context. Journal of Autism and Developmental Disorders, 39(6), 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Hawkey E, Gustafsson H, Miller M, Langhorst M, Cordova M, Fair D, & Nigg JT (2018). Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. Journal of Abnormal Child Psychology, 46(8), 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentrou V, de Veld DM, Mataw KJ, & Begeer S (2019). Delayed autism spectrum disorder recognition in children and adolescents previously diagnosed with attention-deficit/hyperactivity disorder. Autism, 23(4), 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Raiker JS, Sarver DE, Wells EL, & Soto EF (2016). Is hyperactivity ubiquitous in ADHD or dependent on environmental demands? Evidence from meta-analysis. Clinical Psychology Review, 46, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Lyall K, Schweitzer JB, Schmidt RJ, Hertz-Picciotto I, & Solomon M (2017). Inattention and hyperactivity in association with autism spectrum disorders in the CHARGE study. Research in Autism Spectrum Disorders, 35, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C, Pradhan N, Redpath C, & Adler A (2016). Validation of the Empatica E4 wristband. 1–4. [Google Scholar]
- McGoey KE, DuPaul GJ, Haley E, & Shelton TL (2007). Parent and teacher ratings of attention-deficit/hyperactivity disorder in preschool: The ADHD Rating Scale-IV Preschool Version. Journal of Psychopathology and Behavioral Assessment, 29(4), 269–276. [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Landa RJ, Charman T, Stone WL, & Constantino JN (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry, 52(3), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif A-M, Bell LJ, Farquhar-Leicester A, Hatch B, Hill A, Hill MM, Solis E, Young GS, & Ozonoff S (2020). Can Familial Risk for ADHD Be Detected in the First Two Years of Life? Journal of Clinical Child & Adolescent Psychology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. AGS Circle Pines, MN. [Google Scholar]
- Murillo LG, Cortese S, Anderson D, Di Martino A, & Castellanos FX (2015). Locomotor activity measures in the diagnosis of attention deficit hyperactivity disorder: Meta-analyses and new findings. Journal of Neuroscience Methods, 252, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, & Sigman M (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 256–266. [PMC free article] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, D’Onofrio BM, Coyne CA, Lansford JE, Dodge KA, Pettit GS, & Van Hulle CA (2013). Language ability predicts the development of behavior problems in children. Journal of Abnormal Psychology, 122(2), 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, & Staples AD (2015). The role of language ability and self-regulation in the development of inattentive-hyperactive behavior problems. Development and Psychopathology, 27(1), 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, & Buitelaar JK (2010). Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European Child & Adolescent Psychiatry, 19(3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Prasad V, Daley D, Ford T, & Coghill D (2018). ADHD in children and young people: Prevalence, care pathways, and service provision. The Lancet Psychiatry, 5(2), 175–186. [DOI] [PubMed] [Google Scholar]
- Shephard E, Bedford R, Milosavljevic B, Gliga T, Jones EJ, Pickles A, Johnson MH, Charman T, BASIS Team, & Baron‐Cohen S (2019). Early developmental pathways to childhood symptoms of attention‐deficit hyperactivity disorder, anxiety and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 60(9), 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Talbott MR, & Miller MR (2020). Future directions for infant identification and intervention for autism spectrum disorder from a transdiagnostic perspective. Journal of Clinical Child & Adolescent Psychology, 49(5), 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC (2020). R: A language and environment for statistical computing. R foundation for statistical computing. Austria; 2019. [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, & Blumberg SJ (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Asherson P, Rijsdijk F, & Kuntsi J (2009). Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. Journal of the American Academy of Child & Adolescent Psychiatry, 48(10), 1023–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


