Abstract
The novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19 outbreak, spread rapidly and infected more than 140 million people with more than three million victims worldwide. The SARS-CoV-2 causes destructive changes in the immunological and hematological system of the host. These alterations appear to play a critical role in disease pathology and the emerging of clinical manifestations. In this review, we aimed to discuss the effect of COVID-19 on the count, function and morphology of immune and blood cells and the role of these changes in the pathophysiology of the disease. Knowledge of these changes may help with better management and treatment of COVID-19 patients.
Keywords: SARS-COV-2, Immunity, Granulocytes, Monocytes, Blood platelets, Erythrocytes
1. Background
In December 2019, the novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2) emerged from Wuhan, China and rapidly spread over more than 200 countries. Eventually, the World Health Organization (WHO) officially declared Coronavirus Disease-2019 (COVID-19) a pandemic on March 11, 2020 [1], [2]. SARS-CoV-2 is highly contagious and it has so far infected more than 140 million patients with more than three million victims across the world [3]. COVID-19 mostly involves the lower respiratory tract causing respiratory symptoms ranging from mild respiratory illness to acute respiratory distress syndrome (ARDS) [4]. The most common reported symptoms of COVID-19 patients are fever, cough, dyspnea, myalgia or fatigue, and sputum production [5].
Infection with SARS-CoV-2 causes crucial hematological and immunological changes in patients, which are involved in the pathophysiology of the disease [6], [7]. The immune system has an essential role in the control and eradication of viral infections. However, an insufficient or over-responsive immune system may cause serious adverse conditions [8], [9]. Inflammatory cells like macrophages are recruited in almost all viral infections and release cytotoxic cytokines, lipid mediators, metalloproteinases, and reactive oxygen species that induce tissue damage [9]. In severe cases of COVID-19, elevated levels of cytokines and chemokines (IL1α/β, IP-10, MCP-1) promote hyper inflammation in the lungs and underlies ARDS. Furthermore, postmortem analysis has shown infiltration of inflammatory cells such as macrophages and lymphocytes in the lung tissue of COVID-19 patients [5], [10], [11]. Several studies have found a decreased number of CD8 + and NK cells and functional exhaustion of these cells due to overexpression of inhibitory markers like NKG2A and PD-1 [12], [13], [14].
The most common hematological changes in covid-19 patients include lymphopenia [15], [16], neutrophilia [17], [18], eosinopenia [19], [20], mild thrombocytopenia [15], and thrombocytosis with a lower frequency. The leukocyte count varies between different cases and may be normal, reduced [15], [20], [21], or increased [3], [22]. In some studies, the lymphocyte count is proposed as an indicator for classification of disease severity besides pulmonary imaging as the main test for classification of disease type [23], [24]. The platelet count is another indicator of disease severity and prognosis and can reflect the pathophysiological alterations in COVID-19 patients in the early stages [25]. A combination of thrombocytopenia, prolonged prothrombin time (PT) and increased D-dimer is indicative of disseminated intravascular coagulation (DIC) in COVID-19 patients. In addition, deposition of fibrin and thrombin in the pulmonary microvasculature is a factor that contributes to ARDS and coagulopathy in patients dying from COVID-19 [26], [27].
Daily global reports warn that the number of patients is rising sharply. Moreover, a lack of information about different aspects of the disease is still a serious obstacle despite all efforts [28]. Hence, the study of the pathophysiology of disease and the effect of the virus on the immune cells and other blood cells, besides improving our knowledge about the disease, can also help to choose effective treatment strategies. In this comprehensive study, we reviewed the destructive effects of COVID-19 on the quality and quantity of immune and blood cells and discussed the role of these changes in the pathophysiology of the disease.
2. NK cells
2.1. Alteration in count
While many studies have reported findings related to the NK cell function in COVID-19, the exact role of these cells in defense against SARS-CoV-2 and the pathophysiology of the disease is not defined. In SARS-CoV-2 infected patients, the frequency of NK cells in the blood is decreased [13], [29], [30], [31], [32], or increased [33], or does not show any significant changes [34]. A similar NK cell reduction was reported in SARS-CoV-1 infection previously [35]. After recovery, the NK cell count returns to normal levels in severe COVID-19 patients [33], [36]. In addition, the NK cell frequency has the potential to predict COVID-19 severity. The NK cell frequency is lower in patients with ARDS and pneumonia compared to those with a mild phenotype of the disease, which suggests an indirect link between the NK cells count and disease severity in COVID-19 patients [12]. It is still unclear whether NK cell reduction is because of cell death resulting from SARS-CoV-2 or infiltration of inflammatory NK cells into infected organs (particularly the lung) [37]. However, it is unlikely that SARS-CoV-2 induces cell death in NK cells similar to what is observed in other viral infections like influenza [38] because NK cells lack the entry receptor, angiotensin-converting enzyme 2 (ACE-2) [39]. In a study, analysis of the bronchoalveolar lavage fluid (BLAF) of ARDS patients showed no CD16 +CD57 + mature NK cells in the lung tissue, suggesting that the reduction of circulatory NK cells is not a consequence of the migration of these cells into infected lungs [12]. On the contrary, the single cell landscape of bronchoalveolar immune cells in two recent studies reported an increased frequency of NK cells in BALF of COVID-19 patients [40], [41]. Therefore, more studies are needed to determine the etiology of NK cell reduction in COVID-19 patients.
2.2. Alteration in cell surface markers and function
While several studies have investigated the potential responses of NK cells during COVID-19, there are still many debates in this regard. In a cohort study by Demaria et al. [12], the frequency of NK group 2 member A (NKG2A) expressing NK cells was lower in ARDS patients compared to healthy controls while the NKG2A density on the surface of NK cells was up-regulated. Likewise, other regulatory molecules such as programmed cell death protein-1 (PD-1), CD244 (also known as 2B4), and CD39 were upregulated in COVID-19 patients. More importantly, the levels of cytotoxic effector molecules including granzyme A and perforin decreased in the patients [12], [32]. Besides, isolated NK cells from BALF of patients had higher expression of NKG2A, CD39, and PD-1 molecules, even higher than those observed in NK cells derived from blood samples. Interestingly, incubation of NK cells isolated from ARDS patients with monalizumab, an anti-NKG2A monoclonal antibody, unleashed their cytotoxic functions and killing ability [12]. In a study comparing mild and severe forms of COVID-19 patients, Zheng et al. [31] found a significant reduction in the frequency of NK and CD8 + T cells in severe COVID-19 patients. In addition, they reported higher expression levels of inhibitory receptor NKG2A in NK and CD8 + T cells in the severe form of the disease. The expression level of CD107a (a degranulation marker of NK cell) [29] and production of IFN-γ and TNF-α are lower in severe COVID-19 patients [31]. Accordingly, it is hypothesized that NK and CD8 + T cells have an exhausted phenotype in severe COVID-19 patients, which might be due to NKG2A overexpression ( Table 1) [31]. To evaluate the possible role of SARS-CoV-2 spike protein (SP) in altering the NK cell function, an in vitro study explored NK cell responses towards lung epithelial cells transfected with spike protein. The results showed that the spike protein might induce chemo-attraction and IFN-γ production of NK cells [42]. Furthermore, CD107a degranulation marker decreased in SP1 transfected cell culture. Further analysis exhibited that the HLA-E/NKG2A/CD94 interaction may be a possible mechanism for the reduction in NK cell cytotoxicity functions [42]. These studies emphasize the exhausted phenotype of NK cells with over-expression of inhibitory molecules and describe NKG2A as a potential cause for this exhaustion during COVID-19 infection. In addition, the data suggests that the exhausted phenotype of NK cells can be a prognostic factor for a more severe phenotype of COVID-19.
Table 1.
Alterations in NK cells and lymphocytes in COVID-19 patients.
| Demaria et al. [12] | 10 healthy 10 paucisymptomatic COVID-19 34 pneumonia 28 ARDS |
Blood BALF |
NK | Expression of CD39, PD-1, and NKG2A in Severe COVID-19 patients↑ KIR2DL1/S1 expression in ARDS↑ CD16 + CD57+ in BALF of ARDS↓ CD39, PD-1, and NKG2A in BALF of Severe COVID-19 patients↑ |
| Varchetta et al. [14] | 32 patients 25 healthy |
Blood | NK CD8+T |
Mature NK↑ Expression of Tim-3 and CD69 in NK and CD8+T↑ NK exhaustion (IFN-γ ↓), FcεRIγ- CD56+ CD57+ (mature NK)↑ |
| Osman et al. [29] | 78 controls and 10 COVID-19 |
Blood | NK | NK count↓ Cytotoxic activity↓ Serum NK activating cytokines↓ |
| Wan et al. [30] | 123 patients | Blood | NK, WBC Lymph, Plt |
NK, WBC, Lymph, Plt count in severe comparing to mild patients↓ |
| Zheng et al. [31] | 55 mild 13 severe |
Blood | NK CD8+ T |
NK and CD8+ T count↓ NK and CD8+ T exhausted |
| Li et al. [32] | 16 mild 16 severe |
Blood | NK, NKT CD4+ T CD8+ T |
NK, CD4+ T, CD8+ T, and NKT count and percentages in severe patients ↓ Regulatory molecules CD244 and PD-1 on T and NK in severe patients↑ Serum perforin and granzyme A ↓ |
| Yan et al. [33] | 11 convalescent 11 controls |
Blood | NK, CD8+T Tfh |
NK counts↑, Effector memory CD8+ T counts↑ Tim-3+ Tfh like cell counts ↑ CD226+ Tfh like cell counts ↓ ICOS+ Tfh like cell counts↓ |
| Gil-Etayo et al. [44] | 55 patient 21 control |
Blood | T cell | Percentage of Th1, Th2, Th17 ↓ Th2 activity ↑ (78% of died patient have over-reactive Th2 response) |
| Gutierrez-Bautista et al. [45] | 144 patient 42 control |
Blood | T cell | Significantly reduction in Th1 subset HLA-DR and CD38 expression on CD8+ T cell ↓ Active CD4+ T cell in severe case ↑ Exhausted profile in CD4+ and CD8+ of died case ↑ |
| Shi et al. [48] | 54 patient 16 control |
Blood | B cell T cell NK cell |
The count of B cell, T cell and NK cell ↓ in sever case The % of B cell and NK cell ↑ (in patient relative to control) The percentage of CD8+ T cell ↓ (associated with severity) IL-2R and JAK/STAT expression↓ (lead to T cell reduction) |
| Sosa-Hernández et al. [65] | 52 patient 7 control |
Blood | B cell | severe cases relative to mild cases: CD19+ B cells ↑ Transitional B cells ↓ in severe cases Memory B cell ↓ in severe and critical cases Ab-secreting cell ↑ (associated with severity) |
| Shuwa et al. [66] | 58 acute patients 83 convalescent patients |
Blood | B cell T cell |
IL-6 production by B cell during acute phase ↑ cytotoxic activity of CD8+ in convalescent phase ↑ CXCR5/CXCR3 expression in acute phase ↓ |
3. Lymphocytes
3.1. Alteration in count
Lymphopenia following SARS-CoV-2 infection has been reported in many studies [43], [44], [45], [46], [47], [48], [49]. About 85% of patients who need to be hospitalized have lymphopenia [45], [50] with a decrease in both T and B lymphocytes [44], [45], [51], [52], [53], [54]. However, a study noted that the lymphocyte count remained in the normal range in mild COVID-19 patients [55]. The B cell count is not affected by SARS-CoV-2 infection [47], [55], [56] even in the severe phase [55]. A decrease in lymphocyte populations can be used as a predictive parameter for determining the severity of the disease and the risk of death in patients with SARS-CoV-2 infection [43], [50], [52], [53], [54], [55], [57], [58]. However, a recent systematic review found that a low lymphocyte level was correlated with a poor prognosis in general but the association between lymphocyte subsets and the mortality rate of COVID-19 patients was not significant [59]. Possible mechanisms that may cause lymphopenia include bone marrow suppression [45], [52], [60], direct infection of lymphocytes with SARS-CoV-2 [44], [45], [59], [60], [61], [62], pulmonary entrapment [44], [59], [60], [62] high amounts of inflammatory cytokines [44], [52], [58], [60] that prevent T cell proliferation and survival [44], [45], [63], the destructive effects of SARS-CoV-2 on the lymphatic tissue such as the thymus and spleen [51], [52], epigenetic changes in lymphocytes [52] and dendritic cell dysfunction leading to T cell apoptosis [45].
In the disease course, a dynamic alteration is seen in the lymphocyte count. According to a recent study, during the disease progression, the lowest level of B cells and CD4+ T cells is seen in the second week after the onset of symptoms, whereas the CD8+ T cells show the lowest level in the third week after the onset of symptoms [58]. In addition, in the recovery phase, the T cell population is larger compared to the acute phase of infection [58] but a sustained lymphopenia with decreased CD3+, CD4+, and CD8+ T cells is found in more severe cases of COVID-19 [46]. After treatment and in the convalescent phase of the disease, the lymphocyte count gradually returns to the range of COVID-19 mild cases [46], [64].
3.1.1. B lymphocytes and SARS-CoV-2 infection
The data on B-lymphocytes is still conflicting and needs further investigation. A study on the total B cells and its different subsets indicated that the frequency and the absolute number of total B cells did not change considerably in COVID-19 patients relative to healthy controls [65]. The results of this study showed that transitional B cells and antibody secreting cells (plasmablast/plasma cell) increased in COVID-19 patients whereas the memory B cells reduced significantly indicating a positive correlation with disease severity (Table 1) [65]. Other studies also reported that the number or proportion of plasma cells increased in COVID-19 patients [52], [65], [66], [67].
3.1.2. T lymphocyte subset and SARS-CoV-2 infection
A decrease in both CD4+ and CD8+ T cells is reported in COVID-19 patients with different disease severities, which is more significant in severe and critical patients [43], [45], [51], [52], [54], [57], [58], [60]. Histological analysis of deceased COVID-19 patients showed that the CD4+ and CD8+ T cells decreased in the spleen and lymph nodes of these patients [68].
3.1.2.1. CD4+ T cells
The absolute count of Th1, Th2, Th17, Th22, and Treg is reduced in COVID-19 patients [45]. The Th subset differentiation and Th1/Th2 coordination can determine the COVID-19 severity and outcome [44]; for instance, it has been observed that a high percentage of exhausted Th2 cells is associated with a high rate of mortality in COVID-19 patients (Table 1) [44]. Furthermore, the memory T cells also decrease in COVID-19 patients, which can be one of the reasons why infected individuals are not immune to the virus after infection [52].
Th1 cells play a vital role in anti-viral response and induce proliferation and activation of cytotoxic T cell, which leads to removal virus-infected cells [45]. On the other hand, Th2 cytokines are reported to be high in COVID-19 patients [45], [51], [61]; these cytokines have a suppressive effect on inflammatory Th1 response and induce antibody response [44], [45].
Reduction of Treg cells [52], [54] and exhaustion of CD4+ and CD8+ T cells in COVID-19 [52], [54], [68] patients exacerbate the inflammatory status and cause more damage to the lung [46], [54]. On the other hand, intense inflammatory cytokines may promote lymphopenia and lymphocyte exhaustion [52] creating a damage cycle all together. A possible reason for Treg cell reduction is direct infection with SARS-CoV-2 or migration of these cells to the lungs [52]. In addition, a decrease in IL-2 level is seen in bronchoalveolar lavage of severe COVID-19 patients, which may lead to Treg apoptosis [52].
3.1.2.2. CD8+ T cells
One study found that only CD8+ T cells reduced in asymptomatic COVID-19 patients and the other subsets of lymphocytes in these patients were similar to healthy individuals [58]. It may indicate that CD8+ T cells are more susceptible to SARS-CoV-2 infection [48], [51], [52], [55], [63] and their reduction in the early phase of infection may predict the need for ICU admission [69]. Interestingly, a study found that the baseline levels of ACE2 and TMPRSS2 in the lung tissue were negatively related to the level of CD8+ T cells that reside in the lungs before infection [70]. This finding may indicate why some people are more susceptible to more severe infections with the SARS-CoV-2 [70] since the presence of cytotoxic cells in the lungs can play an important role in rapid containment of the infection in the early stages of the virus infection [70]. Moreover, this study revealed that the baseline level of chemokines attracting CD8+ T cells to the lung had a negative correlation with ACE2 and TMPRSS2 expression levels, which, in return, exacerbates the lack of cytotoxic cells [70]. However, it has been observed that the level of these chemokines increases following the SARS-CoV-2 infection resulting in the migration of CD8+ T cells and other cytotoxic cells to the lungs [70].
3.2. Alteration in cell surface markers and function
Although ACE2 is not expressed on lymphocytes, CD147 may act as an alternative receptor for SARS-CoV2 entry, which is expressed on B cells as well as CD4+ and CD8+ T cells [51]. In addition, CD26 (DPP4) is also suggested to be an entry receptor for SARS-CoV-2 on T cells (but does not exist on B cells) [51]. However, the exact role of these receptors is not yet proven warranting further research [51].
3.2.1. CD4+ T cells
The percentage of CD4+ T cells that express HLA-DR and CD38 is also elevated in severe disease, which indicates the activation of these cells [45], [56], [68]. Increased expression of HLA-DR may be due to a high level of inflammatory cytokines in these patients and therefore it does not indicate specific activation of these cells (Table 1) [45]. A recent study found that the expression of OX40 and 4–1BB increased on CD4+ and CD8+ T cells of COVID-19 patients, respectively. Both markers indicate the active phenotype of these cells and increase more in severe cases [56]. The expression of PD-1 and TIM-3 as exhaustion markers on T lymphocytes increases in severe COVID-19 patients, which is associated with a poor prognosis [51], [52], [56], [68], [71].
It has been reported that SARS-CoV-2 specific CD4+ T cells cannot efficiently generate am interferon response, specifically in ICU patients [71]. Additionally, it has been shown that in critical patients admitted to the ICU, the neutralizing antibody titer is not concordant with the level of CD4+ T lymphocytes indicating discordance between B cells, as major participants in the humoral response, and CD4+ T cells [71].
3.2.2. CD8+ T cells
Generally, most studies have reported a hyperactive status in CD4+ and CD8+ T cells [51], [63], [68], [69]. A recent study found that the expression of CD38 and HLA-DR on CD8+ T cells reduced following SARS-CoV-2 infection, which is possibly associated with Th1 dysfunction [45]. However, on the opposite side, it has been revealed that the cytotoxicity of these cells is increased in COVID-19 patients for compensating their count reduction [47], [52], [69]. In this regard, some studies reported increased expression of HLA-DR, CD38, GrA, GrB, and perforin on CD8+ T cell in SARS-CoV-2 infected patients [47], [66], [72].
4. Neutrophils
4.1. Alteration in count
Neutrophils, as the innate immune cells, are the first cells that are recruited in inflammations. They have important roles in defense against COVID-19 [73]. Neutrophils are recruited to the lungs of COVID-19 patients, and neutrophilia acts as a prognostic factor of ARDS in these patients [22], [74], [75], [76], [77].
The neutrophil to lymphocyte ratio (NLR) as the prognostic value is used to predict the severity of disease in the early stage of COVID-19 [25], [34], [78], [79], [80], [81], [82], [83], [84], [85]. Some studies reported that diabetic patients with COVID-19 had higher NLRs [6], [76], [86], [87]. A study found that the neutrophil to CD4 + lymphocyte ratio (NCD4LR) provided a better prediction of virus negative conversion time [49]. Another study showed that the neutrophil count to albumin ratio (NAR) could be a predictor of the mortality rate of COVID-19 patients [88]. Infiltration of neutrophils in pulmonary capillaries and alveolar space is reported in some studies; moreover, in infiltration of immature and/or dysfunctional neutrophils has been observed severe cases of COVID-19 [89], [90], [91], [92].
4.2. Alteration in cell surface markers and function
BALF analysis of COVID-19 patients has shown increased levels of some chemokines such as CXCL2 and CXCL8 leading to recruitment of PMNs to lungs. Although neutrophils are important in defense against viruses, neutrophilia can cause lung injury and ARDS [17], [93], [94], [95], [96], [97], [98], [99]. A study reported an increase in the expression of some genes such as Cathepsin G, IFNγR1, ATP6AP2, CD36 that induce neutrophil apoptosis and downregulation of the TGFB gene in macaques model of COVID-19 infection [78].
Degranulation of neutrophils and release of some proteolytic enzymes such as elastase can cause more injury to lung capillaries and result in edema formation [100]. The level of calprotectin, as a neutrophil activation marker, is correlated with the severity of COVID-19 disease. In addition, it has positive correlations with C-reactive protein, ferritin, lactate dehydrogenase, absolute neutrophil count, and platelet count. Therefore, calprotectin, as an inflammatory marker, can activate neutrophils and lead to their degranulation and phagocytosis. Furthermore, cross-talk between neutrophils and platelets and calprotectin can cause artery and vein thrombosis in COVID-19 [101] . A study revealed strong associations between markers of hyperactive neutrophils (calprotectin and cell-free DNA) and D-dimer [102]. Neutrophils and platelets interact closely [103] via P-selectin and its ligand (PSGL-1) [104], [105]. Platelets induce the release neutrophil extracellular traps (NET) from neutrophils in viral infections [106], [107]. Neutrophils and platelets are entrapped in the fibrin meshwork in alveolar capillaries of SARS-COV-2 patients. these neutrophils are destructed to some extent and form NETs [77], [89], [102], [108], [109].
4.2.1. NETosis during COVID-19 infection
NET is composed of chromatin fibers and citrullinated histones, some special enzymes such as neutrophil elastase, myeloperoxidase, and cathepsin G [110]. The NETosis process can act as a double-edged sword; although it is an essential mechanism for virus entrapment, excessive NET formation increases inflammation, autoimmunity, vascular disease, and thrombosis [102], [111], [112], [113], [114]. COVID-19 patients may develop thromboembolism and arterial thrombosis [95], [115]. NETs are associated with the severity of COVID-19 disease and can trigger macrophages to produce more IL-1β precursors [116]. An active form of IL-1β can help with NET formation in vivo and in vitro [117], [118]. The NET–IL1β loop is activated in severe COVID-19. An enhanced production of NETs and IL1β can accelerate respiratory decompensation, formation of micro thrombi, and aberrant immune responses. Importantly, IL1β induces IL6 [119], and IL6 is considered as a promising target for COVID-19 treatment [31], [120]. NETs can activate the coagulation cascade by acting as a scaffold for VWF and tissue factor (TF) and also by activating the contact pathway. NETs also has an inhibitory effect on anticoagulant pathway such as protein C and tissue factor pathway inhibitors (TFPI) Fig. 1 [111], [121]. Some studies found evidence for NETosis in COVID-19 patients, such as aggregation of neutrophils and platelets in the blood, presence of cell-free DNA and MPO-DNA complex [102], [109], [112], [121], [122], [123], [124], [125]. Elevated levels of soluble platelet derived factors that trigger NETosis, including PF4 and RANTES can be observed as the prognosis of the severity of the disease in these patients Table 2 [121]. One study found that the branch length of the NETs released by neutrophils was larger in COVID-19 patients compared to healthy controls [112]. NETs are positively correlated with ARDS [121], multi-organ failure and mortality [122] but NET formation normalizes during convalescence after COVID-19 [121]. NET levels are also significantly higher in the tracheal aspirate fluid than in plasma samples in COVID-19 patients [121]. NET is related to the complement system, and inhibition of C3 and C5 can reduce NET formation [126], [127]. Therefore, targeting some parts of the NET formation, thrombosis, or complement system can be a good therapeutic approach for reducing the mortality of COVID-19 patients [128].
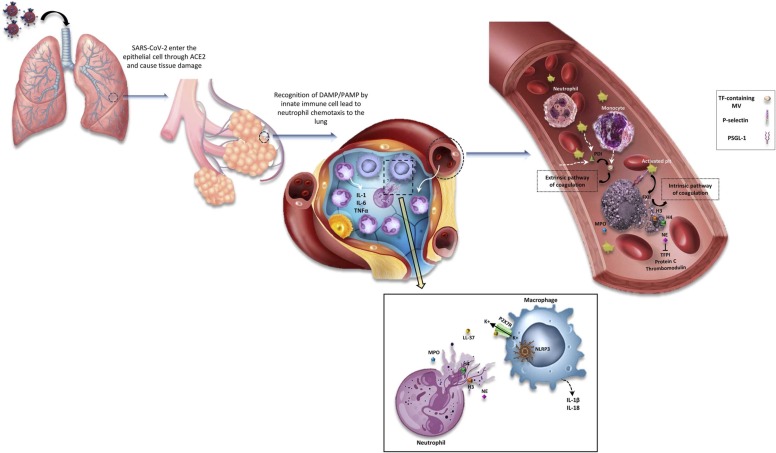
Fig. 1.
Role of neutrophils in hypercoagulable state of COVID-19 patients. After the entrance of the SARS-CoV2 virus into the respiratory system, it enters the epithelial cells through ACE2 and causes some damages. This virus travels to the alveoli of the lungs and innate immune cells are recruited. Monocytes activate by identifying PAMPs and DAMPs and neutrophils are recruited and cytokine storm occurs by producing some inflammatory cytokines such as IL-6, IL-1b, TNF-α. Therefore, infiltration of neutrophils occurs, which produce NETs. The NETosis process has some connections with macrophages in the tissue. NETs are composed of MPO, NE, H3, H4, LL37, etc. LL37 activates the p2x7r channel on macrophages and then the efflux of K+ ions occur. Hence, NLRP3 inflammasome activates and macrophages produce some cytokines such as IL-1b and IL-18 that can help neutrophils to produce more NETs. On the other hand, the neutrophils that produce NETs can link to platelets in the blood through PSGL1 and p-selectin on the platelets and activate them. These activated platelets release poly p and PDI. PDI, as an enzyme, causes the release of TF micro- vesicles from activated monocytes. Not only this TF activates the extrinsic pathway of coagulation but also adheres to the NETs. VWF adheres to these NETs too, and they can form a scaffold for thrombosis happening. Also, the intrinsic pathway can be activated by activating factor XII on this scaffold. NE extraction of neutrophils inhibits some inhibitory factors of the coagulation system such as protein C, thrombomodulin, and TFPI. Finally, all of the above cause thrombosis in the veins or arteries of COVID19 patients.
Table 2.
Quantitative and qualitative changes in granulocytes due to SARS-CoV-2 infection.
| Author (year) | Sample size (case/control) | Sample | Parameter studied | Study findings |
|---|---|---|---|---|
| Yufei et al. (2020) [79] | 191 COVID19 patients and 50 healthy individuals as control |
Blood (retrospective study) |
Neutrophil Lymphocyte |
NLR and CRP↑ lymphocyte percentage↓ Neutrophilia |
| Xie et al. (2021) [83] | 227 pneumonia patients and 97 hospitalized COVID19 patients | Blood | Eosinophil | Eosinopenia in COVID19 patients CRP level in pneumonia patients↑ |
| Shi et al. (2021) [101] | 172 hospitalized COVID19 patients | Serum Plasma |
Neutrophil | Serum and plasma calprotectin↑ Positive correlation of calprotectin and NET release |
| Middleton et al. (2020) [121] | 33 COVID19 patients,17 age and sex matched healthy adults | Whole blood Autopsy of lung specimen |
Neutrophil NETosis Platelet |
Plasma NET↑ MPO-DNA complexes↑ PMN granularity↓ Co-localization of Cit H3 with platelets and thrombosis Platelet-neutrophil aggregations |
| Kong et al. (2020) [122] | 210 COVID19 patients(retrospective cohort study) | Blood | Neutrophil Lymphocyte |
NLR↑ Neutrophilia Lymphopenia APPt and D-dimer↑ |
| Sun et al. (2020) [133] | 63 confirmed COVID-19 patients | Throat swab or sputum, Urine Blood Stool |
Eosinophil | Leukopenia Lymphopenia Eosinopenia in moderate and severe form of COVID19 |
5. Eosinophil alterations in COVID-19 patients
Eosinophils account for 1–3% of circulating leukocytes and have some roles in antiviral responses [129]. Some studies found that more than half the patients admitted with COVID-19 had eosinopenia (defined as an absolute eosinophil count<0.02 * 109 cells/L) [83], [95], [130], [131]. This eosinopenia is correlated with the severity of COVID-19 disease [129], [132], [133]. A low eosinophil count will normalize over time (Table 2) [83]. No eosinophil enrichment has been found in the pulmonary tissue of patients with COVID-19 in postmortem analyses [134]. Type 2 immune response, including type 2 cytokines (IL-4, IL-13, etc.) and accumulation of eosinophils, might provide potential protective effects against COVID-19 [135]. However, there is controversy over the protective or exacerbating role of eosinophils during SARS-CoV-2 infection [129].
6. Monocytes/macrophages
Monocytes and macrophages are the main cells of the mononuclear phagocyte system (MPS) that play an important role in both innate and adaptive immune systems [136]. The presence of these two cell populations in all phases of SARS-CoV-2 infection can act as a double-edged sword that can ameliorate infection or exacerbate it [136]. They are the main players in the innate anti-viral immunity. They can also trigger systemic inflammation, pro-coagulant syndrome and cytokine release syndrome (CRS) leading to ARDS and multi organ failure in COVID-19 patients [136], [137], [138], [139], [140]. Monocytes and tissue macrophages express a broad set of membrane receptors and secretory elements that mediate their role in both anti-viral and inflammatory responses [136]; the balance between these two responses and the contribution of other immunity mechanisms can determine the severity and outcome of SARS-CoV-2 infection [136].
6.1. Alteration in count and morphology
The absolute number of total monocytes and their frequencies are not affected by SARS-CoV-2 virus [141], [142], [143], [144], [145]; however, some studies found a decrease in the number of monocytes in critical patients [146], [147] and an increase in mild cases [147]. Based on previous studies, it can be concluded that monocytes increase their antiviral response in the early stages, which may indicate a good prognosis. However, in the severe stages of the disease, a decrease occurs in monocytes, suggesting the destructive effects of the virus on monocytes [146]. In patients with SARS-CoV-2 infection, the monocyte distribution width (MDW) shows a higher value compared to healthy individuals. Moreover, among infected individuals, those requiring ICU admission have significantly higher MDW values relative to patients with mild symptoms [148]. Therefore, MDW can be considered a prognostic parameter along with other laboratory parameters for predicting unflavored outcomes [148].
6.2. Alteration in morphology
Large monocytes with vacuolated cytoplasm are seen specifically in COVID-19 patients [145]. Meanwhile, the results of several studies have confirmed a change in the frequency of different subsets of monocytes in COVID-19 patients [143]; A study showed that the non-classical (NC) monocytes could determine the severity of the disease; NC monocytes below 4% indicate more severe disease and the recovery phase also occurs when the NC monocytes start to increase [144]. Two studies reported a mixed phenotype expressing both M1 and M2 macrophage markers in this specific population ( Table 3) [142], [145]. Nonetheless, there is a profound contradiction in the results of studies on each cell population, and more research is needed to draw a definitive conclusion about the changes in each monocyte subset.
Table 3.
Alterations in monocytes, macrophages and dendritic cells in COVID-19 patients.
| Author (year) | Sample size (case/control) | Sample | Parameter studied | Study findings |
|---|---|---|---|---|
| Sánchez-Cerrillo (2020) [138] | 64 COVID-19 cases 22 Non-COVID-19 |
Blood Bronchial sample | DCs Monocytes |
DCs and monocytes ↓ (blood samples). CD1+c DCs and monocyte ↑ (bronchial sample). CD40 on monocyte and pDCs in blood ↓ and on bronchial sample ↑ |
| Gatti (2020) [141] | 30 COVID-19 cases 20 healthy control |
Blood | Monocyte | Monocytes number patients control NC monocyte↓ in severe cases. INT monocyte and CD11b ↑ in moderate case HLA-DR ↓ in severe cases. |
| Matic (2020) [142] | 57 COVID-19 patients 5 healthy control |
Blood | DCs Monocyte Macrophage |
Monocyte/macrophage ↓. C monocyte ↑ / INT and NC monocyte ↓. Co-expressing of CD23 & CD38 on INT and NC monocytes. |
| Peruzzi (2020) [143] | 40 COVID-19 patients 8 healthy control |
Blood | DCs Monocytes |
Total monocytes patients controls. NC monocyte ↓ and T and C monocyte ↑. HLA-DR ↓ and CD11b / CD64 ↑ |
| Silvin (2020) [144] | 13 COVID-19 cases 12 flu-like cases |
Blood | Monocyte | Total monocytes severe cases controls. INT monocyte ↑ in mild cases. NC monocyte ↓ in severe cases CD11b+ C monocyte ↑/ HLA-DR expression ↓ (in severe cases) |
| Zhang (2021) [145] | 34 COVID-19 patients Unknown number of healthy control |
Blood | Monocyte | Atypical monocyte ↑ C monocytes ↓ / INT & NC monocyte ↑ ACE2 on monocyte of patients ↓ |
| Bedin (2021) [151] | 32 COVID-19 cases 30 healthy control |
Blood | Monocytes | Monocyte CD169 ↑ during active infection. Monocyte CD169 in mild cases severe cases Monocyte CD169 lower in cases with producing IgG against SARS-CoV-2 |
6.3. Alteration in cell surface markers and function
Comparison of COVID-19 patients with healthy individuals shows a significant reduction in HLA-DR expression on monocytes of COVID-19 patients, which has a direct relationship with the severity of symptoms [92], [143], [147], [149]. The high amounts of IL-6 and CRP in COVID-19 patients can downregulate the HLA-DR expression on classical monocytes [143], [144]. Impaired HLA-DR expression leads to less antigen presentation and lower anti-viral response by T cells [143], [149]. On the other hand, the expression of some functional markers such as CD64 and CD11b increases on monocytes, which is independent of COVID-19 severity [143]. Moreover, one study reported an increase in CD38 expression on monocytes in critical patients, which indicates the enhanced inflammatory status of monocytes [147]. A recent study found reduced CD4 expression on monocytes in severe COVID-19 patients [146]. CD40 expression as an activation marker in blood monocytes has a propensity to decrease in all monocyte subtypes in critically ill patients; however, this reduction is not associated with IL-6 and other inflammatory elements (Table 3) [138].
Monocytes, macrophages (particularly M1), and dendritic cells have a transporting role for SARS-CoV-2 as they bind to the virus by their lectin-binding receptors such as CD169 and carry it to local lymph nodes. Through this transportation, the virus gains access to the lower respiratory tract and even other tissues through the bloodstream [136], [149], [150]. When SARS-CoV-2 disseminates in the body and reaches other organs, resident tissue macrophages evoke an inflammatory response and cause some dysfunction in the related organ [136]. During the early phase of infection with SARS-CoV-2, overexpression of CD169 on monocytes has been observed; however, its expression decreases in the severe phase of the disease (Table 3) [151]. Under inflammatory conditions, it has been shown that mononuclear phagocytes can activate platelets and produce procoagulant factors such as tissue factors [136], [137], factor 5, and factor 13, which indicates their role in hyper-coagulation and generation of thrombo-embolism ( Fig. 2) [136]. Furthermore, a recent study reported an increase in monocyte-platelet aggregation (MPA) in COVID-19 patients compared to healthy individuals and a positive relationship between MPA and the severity of disease [152]. These MPAs can be one of the underlying mechanisms for increased risk of thrombotic complications in COVID-19 patients [152].
Fig. 2.
Interaction between Monocytes-Platelets-T cells in COVID-19 patients. In COVID-19 patients, the relationship between platelets, monocytes, and T cells exacerbates the cytokine storm, enhances coagulopathies, and diminished the antiviral response of T lymphocytes. As shown figure, the monocyte-platelet interaction through Mac-1/αIIbβ3 and PSGL-1/CD62P leads to the release of TF, FV, and FXIII from platelets. On the other hand, activated platelets can affect the T cell responses by altering some CD4+ T cell functions and Antigen presentation to CD8+ T cells. Moreover, excessive secretion of GM-CSF by T cells exacerbates cytokine release and TF production by monocytes and differentiates them toward M1 pro-inflammatory macrophages. A decrease in the HLA-DR expression on monocytes results in more attenuation of the T cell response due to a decrease in antigen presentation.
On the opposite side of the pathological processes that are driven by these cells, the recovery process also depends on macrophages in COVID-19 infection [136]. M2-macrophages can reduce inflammation by producing some lipid metabolites such as resolvins, protectins, and maresins [136]. Besides, secretion of elastase, collagenase, TGFβ, and IGF-1 and blotting out the necrotic cells is a cooperative mechanism done by macrophages resulting in recovery [136]. It has been shown that the number and function of monocytes are restored in the course of COVID-19 remission [147].
7. Platelets
Platelets, in addition to being the major cells in thrombosis and homeostasis, are also involved in inflammatory processes and immune responses [153], [154], [155], [156], [157]. Adverse changes in the platelet count and function are responsible for coagulation drawbacks and thrombotic complications in COVID-19 patients and increase the risk of lethal outcomes [156], [158], [159], [160], [161], [162].
7.1. Alteration in count
Thrombocytopenia is a common occurrence in COVID-19 patients [158], [161], [162], [163], [164]. The platelet count shows a decreasing trend throughout the disease progression proportional to the severity of the COVID-19 disease [25], [158], [161], [162], [163], [164], [165], [166]. However, it gradually begins to increase after admission in survivors [25], [166]. Several retrospective studies showed a more severe reduction in the platelet count in non-survivor COVID-19 patients compared to survivors [25], [167]. A low platelet count at the onset of infection can indicate a poor prognosis and a higher chance of mortality [25], [166]. A decrease in the platelet count in COVID-19 patients may be associated with several factors including hyper activation of platelets and platelet consumption [25], [155], [162], [168], [169]. However, some studies found that the platelet count increased or did not change in COVID-19 patients [153], [156], [157], [158], [166], [170]. Elevated levels of IL-6 and other inflammatory cytokines as characteristic inflammatory conditions in COVID-19 patients may promote platelet biogenesis through increasing thrombopoietin production by hepatocytes [153], [157], [158], [170].
An increase in the mean platelet volume (MPV) is directly related to the severity of COVID-19 disease and the rate of platelet activation [155], [162], [166], [168]. However, a prospective study of asymptomatic children that tested positive for SARS-CoV-2 infection found a high MPV even without any clinical signs ( Table 4) [154]. A possible underlying mechanism for a high MPV in COVID-19 patients may be an increase in the release of young platelet in response to platelet destruction after infection and cytokines such as IL-6 [154], [157]. A study reported that MPV/platelet count ratio (MPR) had a correlation with COVID-19 severity as the patients with elevated MPR were more prone to severe pneumonia and were at a high risk of death [157].
Table 4.
Morphological, functional, and count alterations in red blood cells and platelets of COVID-19 patients.
| Author (year) [Ref.] | Sample size (case/control) | Sample | Parameter studied | Study findings |
|---|---|---|---|---|
| Gumus et al. (2021) [154] | 55 children infected with COVID-19 and 60 healthy children | Blood | Platelet Lymphocyte |
MPV↑ Lymphopenia |
| Manne et al.(2020) [156] | 41 COVID19 patients | Blood | Platelet | platelet-neutrophil, -monocyte, and -T-cell aggregates↑ Platelet hyperactivity TPO levels↑ Expression of P-selection on plt↑ |
| Si Zhang et al. (2020) [162] | 422 COVID19 patients and 201 non COVID19 patients | Blood | Platelet | Thrombocytopenia PCT↓ MPV,PDW,PT,INR,APPT,D-dimer and FDPs↑ PTA↓ Plt hyperactivity Releasing factor V and XIII from platelets |
| Yang et al. (2020) [164] | 1476 consecutive patients with COVID-19 | Blood | Platelet | Thrombocytopenia |
| Paola Canzano et al. (2021) [169] | 46 consecutive COVID-19 patients | Blood | Platelet | Expression of p-selectin on plt↑ Granulocyte-plt and monocyte-plt aggregations, Plt activation |
| Venter et al. (2020) [177] | 37 COVID-19 patients | Blood | RBC Platelet |
Serum ferritin↑ CRP↑ P-selectin concentrations on plt↑ Plt hyperactivity Erythrocyte-platelet aggregations |
| Yuan et al. (2020) [180] | 117 COVID19 patients | Blood | RBC | RBC and Hb↓ |
| Henry et al. (2020) [181] |
49 COVID19 patients | Blood | RBC RDW |
progressive increase of RDW |
| Berzuini et al. (2021) [182] | RBC | Mild to severe anemia, morphology alterations such as polychromasia and basophilic stippling, Rouleaux formation spherocytes, schistocyte Retic count↑ |
7.2. Alteration in morphology and coagulation parameters
A short report showed the presence of macro thrombocytes and platelet aggregation in peripheral blood smears of ICU COVID-19 patients while such a manifestation was not observed in stable non-ICU patients [170]. Macro thrombocytes indicate the increased turnover of platelets [168], [170] leading to PDW elevation [168]. An autopsy examination showed an increased number of megakaryocytes in the lungs and bone marrow of dead COVID-19 patients [159]. The morphology of BM megakaryocyte indicates the active platelet biogenesis. Moreover, electron microscopy examination of these cells has shown the presence of small amounts of virions in megakaryocytes [159].
Severe thrombocytopenia causes some abnormalities in the results of PT, APTT, INR, D-dimer, and FDP measurement in COVID-19 patients [158], [161], [162], [163]. The levels of D-dimer [158], [161], [166], [171], [172], [173] and fibrinogen [166], [172], [174] and the fibrinogen-albumin ratio (FAR) [171] are higher in severe COVID-19 patients and the platelet count and albumin level were lower in these patients compared to non-severe patients [25], [169], [171]. It has been reported that a high level of fibrinogen in COVID-19 patients can make platelets more active and intensify coagulopathies [162], [174].
7.3. Alteration in cell surface markers and function
7.3.1. Platelet and map kinase pathway
An in vitro study showed direct infection of platelets by SARS-CoV-2 through ACE2 and TMPRSS2 expression on platelets [162] while other studies did not report such a finding [156], [169], [175]. The effect of SARS-CoV-2 on platelets is mediated by activation of the MAPK pathway and production of thromboxane A2, which has been shown to increase in COVID-19 patients [156], [162], [174]. Activation of the MAPK pathway rearranges the platelet cytoskeleton, which subsequently results in the reinforcement of platelet secretory profile, clot retraction, and platelet aggregation [156], [162]. Enhanced platelet-leukocyte aggregates are seen in COVID-19 patients [153], [156], [162], [176] and makes platelets respond to IL-6 by assembling the IL-6R subunits and subsequently activates them significantly [175].
7.3.2. Platelet and hyper activation
Hyper activation of platelets following the SARS-CoV-2 infection is reported by several studies [153], [156], [157], [169]. It can occur in several ways including TLR response to DAMP released from damaged tissues and stimulation of complement receptors on platelets leading to thrombosis and platelet depletion [163], [175]. Moreover, Fc receptor activation on platelets may be involved in antibody-mediated platelet count reduction in COVID-19 patients [163], [175]. There are reports of the increased expression of αIIbβ3 integrin [153], [162] and CD62P (P-selectin) on the platelets of COVID-19 patients, indicating the active state of these cells (Table 4) [156], [162], [174], [177]. TF release by damaged endothelial cells together with decreased levels of nitric oxide and prostacyclin are the possible causative mechanisms of this hyper activation [169]. CD62P (P-selectin) expression as a marker of platelet activation increases in COVID-19 patients, especially in severe ones [153], [156], [157], [162], [169], [174]. Enhancement of CD62P expression results in augmentation of its interaction with PSGL-1 on leukocytes, which causes platelet-leukocyte aggregation [153], [156], [162], [169]. Furthermore, SARS-CoV-2 and its spike protein can trigger the secretion of factor V, factor XIII, PF4, IL-1β, IL-8, and TNFα from platelets (Table 4) [162]. The PF4 level is significantly elevated in COVID-19 patients and is correlated with disease severity [162]. It has been found that spike protein (S1, not S2) can increase αIIbβ3 integrin activation and CD62P expression on platelets in the presence or absence of agonist stimulation [153], [162].
7.3.3. Platelets and other blood cells
The platelet-monocyte and platelet-neutrophil interactions play an auxiliary role in tissue factor secretion, micro-thrombosis formation, and ARDS in COVID-19 [175]. The interrelation between platelets and neutrophils can promote the formation of neutrophil extracellular traps (NETs) [153], [158], [174], resulting in platelet activation and thrombotic complications in COVID-19 patients [158]. In addition, the platelet-monocyte aggregation has been shown to increase in COVID-19 patients, particularly in severe patients, and is associated with the mortality rate [169], [174]. In vitro and in vivo observations indicate that adhesion of platelets to monocytes induces TF expression on monocytes [174], [178], which is mediated by CD62P adhesion molecule and αIIbβ3 integrin and subsequently contributes to hyper-coagulation in COVID-19 patients [174]. Aggregation of platelets and T cells is increased in COVID-19 patients, which may affect the T cell inflammatory and immune response [156]. In addition, the platelet-to-lymphocyte ratio is higher in patients with SARS-CoV-2 infection compared to non-infected individuals [179].
7.3.4. Platelets and endothelial cells
Activation of endothelial cells, monocytes, granulocytes, and platelets after viral infection may lead to the secretion of pro-coagulant macrovesicles (MVs) containing phosphatidyl serine (PS) and TF, which plays a role in triggering the coagulation cascade [169]. It has been shown that TF-containing MVs are more abundant in the plasma of COVID-19 patients compared to healthy individuals [169]. According to a prospective study of COVID-19 patients, platelets, monocytes, and granulocytes that express tissue factor are higher in COVID-19 patients compared to healthy subjects [169]. In COVID-19, ACE2 expression on endothelial cells causes some damage to these cells, which in turn leads to an increased expression of VonWillebrand factor and a hyper coagulation state in the patients [158], [163], [175]. Destructed endothelial cells increase platelet adhesion and activation through collagen-GPIV interaction [175] and cause platelet aggregation in the lungs [25], [167], [168]. On the other hand, due to lung damage, the function of lung megakaryocytes may be impaired and they may no longer be able to produce platelets efficiently [25], [155], [158], [164], [165], [166], [170]. Moreover, megakaryocyte secretions, which contain cytokines, may cause further injury to the lungs [170]. Adverse immune mechanisms such as autoantibodies against platelets and immune complexes may result in platelet loss [25], [158].
8. Red blood cells
8.1. Alteration in count and morphology
COVID-19 as a viral infection causes some changes in erythrocytes and their hematological markers. One study found that red blood cell counts were lower in COVID-19 patients compared to normal individuals. It is suggested that inhibition of hematopoiesis by this virus in the bone marrow of patients causes anemia [180]. Anisocytosis, some changes in the lipid and protein membrane of circulating RBCs, erythrophagocytosis, and hemophagocytosis by macrophages engulfed with both erythroblasts and mature erythrocytes are reported in COVID-19 patients [181]. Polychromasia and basophilic stippling can be seen in the blood film of patients, probably as a consequence of an increased reticulocyte count [182]. Huge rouleaux formations and auto agglutination are reported in the blood film too [182]. Spherocytes and schistocytes are also observed, probably as a result of membrane damage induced by fibrin strands occurring in vascular thrombosis with microangiopathy [183]. Another common observation is the presence of high percentages of stomatocytes, knizocytes, and cup-shaped erythrocytes in the blood smear of COVID-19 patients. These morphological changes are associated with COVID-19-related anemia [182], [184]. One study reported a few mushroom‐shaped cells in the blood film [185]. Red blood cell distribution width (RDW) is increased in severe COVID-19 patients so it can be used as a significant predictor and prognostic factor for the severity and kidney damage of COVID-19 patients [181]. The hemoglobin (Hb) concentration tends to decrease during COVID-19. Therefore, a mild to severe anemia may ensue [186]. There are reports of coated RBCs with some complement proteins such as C3b/iC3b/C3dg. C4d is also seen on the RBCs of COVID-19 patients [187].
9. Conclusions
In conclusion, the SARS-CoV-2 infection can change immunological and hematological cells in different aspects. These changes appear to play a critical role in disease pathology and the emerging of clinical manifestations. Infection with SARS-CoV-2 contributes to lymphopenia, neutrophilia, eosinopenia, and mild thrombocytopenia. Severe thrombocytopenia in COVID-19 patients causes abnormalities in the results of PT, APTT, INR, D-dimer, and FDP measurements. Hyperactivation of platelets and development of DIC are observed in COVID-19 patients. Furthermore, SARS-CoV-2 and its spike protein can trigger the secretion of factor V, factor XIII, PF4, IL-1β, IL-8, and TNFα from platelets. Accordingly, SARS-CoV-2 can induce coagulopathy and thrombosis in infected patients. COVID19 as a viral infection causes some changes in erythrocytes and its related hematological markers. Anisocytosis, echinocytosis, spherocytosis, schistocytes, stomatocytes, knizocytes, polychromasia, basophilic stippling, and huge rouleaux formation may appear in the blood film of patients. In addition, the hemoglobin (Hb) concentration tends to decrease during COVID19 disease, therefore, mild to severe anemia may occur in the patients. The virus can cause attenuated immune responses or uncontrolled inflammatory responses, which lead to improper defense against the virus and damage to the host respectively. Neutrophils, as first responders to inflammation, can trigger inflammation and lung injury by production of proteolytic enzymes, ROS, and NET. Furthermore, inflammatory cells like monocytes and macrophages are associated with systemic inflammation, pro-coagulant syndrome, and cytokine release syndrome, which can result in ARDS condition and multi-organ failure in COVID-19 patients. Large monocytes (high MDW) with vacuolated cytoplasm are another feature of SARS-CoV-2 infection. The presence of DC-SIGN, L-SIGN, and CD147 along with ACE2 expression on DCs makes these cells a vulnerable target for SARS-CoV-2 entry and infection. HLA-DR and co-stimulatory molecules such as CD80 and CD86 are downregulated in DCs following infection with SARS-CoV-2 virus and can subsequently affect the T cell responses and even cause their apoptosis. The absolute count of CD4+ and CD8+ T cells is lower in severe patients. The effect of SARS-CoV-2 on lymphocyte function is a controversial subject and further studies are needed. Lymphocyte morphology is also affected by SARS-CoV-2 and atypical lymphocytes with plasmacytoid features are common in the blood smear of COVID-19 patients. The SARS-CoV-2 can turn normal NK and CD8+ T cells, which are important to eliminate the virus, to exhausted phenotype, which makes the patients prone to other viral infections. Considering these changes may help to manage and treat COVID-19 patients better.
Author contributions
EA: Originated the study, Drafted the manuscript. AO: final approval of the version to be published. ZB: Made substantial contributions to the conception. EZ: revised article for important intellectual content. All authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Ehsan Ahmadi: Originated the study, acquired data, Drafted the manuscript. Azadeh Omidkhoda: Made substantial contributions to the conception and substantively revised manuscript. Zahra Bagherpour: Acquired data and Design some part of the work. Elmira Zarei: Design some part of the work. All authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Dr. Hossein Dehdari for her encouraging feed-back.
Data Availability
Not applicable.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad A., Prasad M. SARS-CoV-2: the emergence of a viral pathogen causing havoc on human existence. J. Genet. 2020;99:37. doi: 10.1007/s12041-020-01205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Zhang R., He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann. Hematol. 2020;99:1421–1428. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S.H., Zhao Y.S., Zhou D.X., Zhou F.C., Xu F. Coronavirus disease 2019 (COVID-19): cytokine storms, hyper-inflammatory phenotypes, and acute respiratory distress syndrome. Genes Dis. 2020;7:520–527. doi: 10.1016/j.gendis.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaria O., Carvelli J., Batista L., Thibult M.L., Morel A., Andre P., Morel Y., Vely F., Vivier E. Identification of druggable inhibitory immune checkpoints on natural killer cells in COVID-19. Cell Mol. Immunol. 2020;17:995–997. doi: 10.1038/s41423-020-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varchetta S., Mele D., Oliviero B., Mantovani S., Ludovisi S., Cerino A., Bruno R., Castelli A., Mosconi M., Vecchia M., Roda S., Sachs M., Klersy C., Mondelli M.U. Unique immunological profile in patients with COVID-19. Cell Mol. Immunol. 2021;18:604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., C. China Medical Treatment Expert Group for Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian G.Q., Yang N.B., Ding F., Ma A.H.Y., Wang Z.Y., Shen Y.F., Shi C.W., Lian X., Chu J.G., Chen L., Wang Z.Y., Ren D.W., Li G.X., Chen X.Q., Shen H.J., Chen X.M. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM Mon. J. Assoc. Physicians. 2020;113:474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., Yu W., Zhang J. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S., Cai X., Wang H., He G., Lin Y., Lu B., Chen C., Pan Y., Hu X. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin. Chim. Acta Int. J. Clin. Chem. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao K., Li R., Wu X., Zhao Y., Wang T., Zheng Z., Zeng S., Ding X., Nie H. Clinical features in 52 patients with COVID-19 who have increased leukocyte count: a retrospective analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:2279–2287. doi: 10.1007/s10096-020-03976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y., Weng Z., Yang L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X., Wang K., Zuo P., Liu Y., Zhang M., Xie S., Zhang H., Chen X., Liu C. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients-indications for predictive, preventive, and personalized medical approach. EPMA J. 2020;11:1–7. doi: 10.1007/s13167-020-00208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Mesa J.E., Galindo-Coral S., Montes M.C., Munoz Martin A.J. Thrombosis and coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021;46 doi: 10.1016/j.cpcardiol.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezalek Z.T., Khibri H., Ammouri W., Bouaouad M., Haidour S., Harmouche H., Maamar M., Adnaoui M. COVID-19 associated coagulopathy and thrombotic complications. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620948137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman M., Faridi R.M., Sligl W., Shabani-Rad M.T., Dharmani-Khan P., Parker A., Kalra A., Tripathi M.B., Storek J., Cohen Tervaert J.W., Khan F.M. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020;4:5035–5039. doi: 10.1182/bloodadvances.2020002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y., Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020;189:428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M., Guo W., Dong Y., Wang X., Dai D., Liu X., Wu Y., Li M., Zhang W., Zhou H., Zhang Z., Lin L., Kang Z., Yu T., Tian C., Qin R., Gui Y., Jiang F., Fan H., Heissmeyer V., Sarapultsev A., Wang L., Luo S., Hu D. Elevated exhaustion levels of NK and CD8(+) T cells as indicators for progression and prognosis of COVID-19 disease. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan L., Cai B., Li Y., Wang M.J., An Y.F., Deng R., Li D.D., Wang L.C., Xu H., Gao X.D., Wang L.L. Dynamics of NK, CD8 and Tfh cell mediated the production of cytokines and antiviral antibodies in Chinese patients with moderate COVID-19. J. Cell. Mol. Med. 2020;24:14270–14279. doi: 10.1111/jcmm.16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., Dwyer D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R., Mathew D., Baxter A.E., Vella L., Kuthuru O., Apostolidis S., Bershaw L., Dougherty J., Greenplate A.R., Pattekar A., Kim J., Han N., Gouma S., Weirick M.E., Arevalo C.P., Bolton M.J., Goodwin E.C., Anderson E.M., Hensley S.E., Jones T.K., Mangalmurti N.S., Luning Prak E.T., Wherry E.J., Meyer N.J., Betts M.R. Immunologic perturbations in severe COVID-19/SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.05.18.101717. [DOI] [Google Scholar]
- 37.Masselli E., Vaccarezza M., Carubbi C., Pozzi G., Presta V., Mirandola P., Vitale M. NK cells: a double edge sword against SARS-CoV-2. Adv. Biol. Regul. 2020;77 doi: 10.1016/j.jbior.2020.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao H., Tu W., Qin G., Law H.K., Sia S.F., Chan P.L., Liu Y., Lam K.T., Zheng J., Peiris M., Lau Y.L. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J. Virol. 2009;83:9215–9222. doi: 10.1128/JVI.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Travaglini K.J., Nabhan A.N., Penland L., Sinha R., Gillich A., Sit R.V., Chang S., Conley S.D., Mori Y., Seita J., Berry G.J., Shrager J.B., Metzger R.J., Kuo C.S., Neff N., Weissman I.L., Quake S.R., Krasnow M.A. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;587:619–625. doi: 10.1038/s41586-020-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thurmann L., Kurth F., Volker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Muller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L.E., Eils R. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 41.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 42.Bortolotti D., Gentili V., Rizzo S., Rotola A., Rizzo R. SARS-CoV-2 spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cells. 2020;9 doi: 10.3390/cells9091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantenys-Molina S., Fernandez-Cruz E., Francos P., Lopez Bernaldo de Quiros J.C., Munoz P., Gil-Herrera J. Lymphocyte subsets early predict mortality in a large series of hospitalized COVID-19 patients in Spain. Clin. Exp. Immunol. 2021;203:424–432. doi: 10.1111/cei.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Etayo F.J., Suarez-Fernandez P., Cabrera-Marante O., Arroyo D., Garcinuno S., Naranjo L., Pleguezuelo D.E., Allende L.M., Mancebo E., Lalueza A., Diaz-Simon R., Paz-Artal E., Serrano A. T-helper cell subset response is a determining factor in COVID-19 progression. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.624483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez-Bautista J.F., Rodriguez-Nicolas A., Rosales-Castillo A., Jimenez P., Garrido F., Anderson P., Ruiz-Cabello F., Lopez-Ruz M.A. Negative clinical evolution in COVID-19 patients is frequently accompanied with an increased proportion of undifferentiated Th cells and a strong underrepresentation of the Th1 subset. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.596553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han M., Xu M., Zhang Y., Liu Z., Li S., He T., Li J., Gao Y., Liu W., Li T., Chen Z., Huang X., Cheng G., Wang J., Dittmer U., Witzke O., Zou G., Li X., Lu M., Zhang Z. Assessing SARS-CoV-2 RNA levels and lymphocyte/T cell counts in COVID-19 patients revealed initial immune status as a major determinant of disease severity. Med. Microbiol. Immunol. 2020;209:657–668. doi: 10.1007/s00430-020-00693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Y., Wei X., Guan J., Qin S., Wang Z., Lu H., Qian J., Wu L., Chen Y., Chen Y., Lin X. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin. Immunol. 2020;218 doi: 10.1016/j.clim.2020.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi H., Wang W., Yin J., Ouyang Y., Pang L., Feng Y., Qiao L., Guo X., Shi H., Jin R., Chen D. The inhibition of IL-2/IL-2R gives rise to CD8(+) T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. 2020;11:429. doi: 10.1038/s41419-020-2636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Zhang Y., Mo P., Liu J., Wang H., Wang F., Zhao Q. Neutrophil to CD4+ lymphocyte ratio as a potential biomarker in predicting virus negative conversion time in COVID-19. Int. Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lafarge A., Martin J.E., Longval T., Dupont T., De Jong A., Azoulay E. Two different patterns of lymphocyte alterations in critically ill COVID-19 patients. Intern. Emerg. Med. 2021;16:1411–1414. doi: 10.1007/s11739-020-02575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berthelot J.M., Liote F., Maugars Y., Sibilia J. Lymphocyte changes in severe COVID-19: delayed over-activation of STING? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.607069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delshad M., Tavakolinia N., Pourbagheri-Sigaroodi A., Safaroghli-Azar A., Bagheri N., Bashash D. The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19. Int. Immunopharmacol. 2021;95 doi: 10.1016/j.intimp.2021.107586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang W., Berube J., McNamara M., Saksena S., Hartman M., Arshad T., Bornheimer S.J., O’Gorman M. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97:772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu D., Wang Y., Zhao B., Lan L., Liu Y., Bao L., Chen H., Yang M., Li Q., Zeng Y. Overall reduced lymphocyte especially T and B subsets closely related to the poor prognosis and the disease severity in severe patients with COVID-19 and diabetes mellitus. Diabetol. Metab. Syndr. 2021;13:5. doi: 10.1186/s13098-020-00622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. eBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S., Sheng Y., Tu J., Zhang L. Association between peripheral lymphocyte count and the mortality risk of COVID-19 inpatients. BMC Pulm. Med. 2021;21:55. doi: 10.1186/s12890-021-01422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu X., Yang R. Changes of peripheral lymphocyte subset in patients with SARS-CoV-2 infection during the whole course of disease. Expert Rev. Respir. Med. 2021;15:553–559. doi: 10.1080/17476348.2021.1866991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Q., Wang Z., Yin Y., Zhao Y., Tao P., Zhong P. Association of peripheral lymphocyte and the subset levels with the progression and mortality of COVID-19: a systematic review and meta-analysis. Front. Med. 2020;7 doi: 10.3389/fmed.2020.558545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalicinska E., Szymczak D., Andrasiak I., Bogucka-Fedorczuk A., Zinczuk A., Szymanski W., Biernat M., Rymko M., Semenczuk G., Jablonowska P., Rybka J., Simon K., Wrobel T. Lymphocyte subsets in haematological patients with COVID-19: Multicentre prospective study. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2020.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akbari H., Tabrizi R., Lankarani K.B., Aria H., Vakili S., Asadian F., Noroozi S., Keshavarz P., Faramarz S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng Z., Zhang M., Zhu T., Zhili N., Liu Z., Xiang R., Zhang W., Xu Y. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;98:353–358. doi: 10.1016/j.ijid.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni M., Tian F.B., Xiang D.D., Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J. Med. Virol. 2020;92:2600–2606. doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y., Tan W., Chen H., Zhu Y., Wan L., Jiang K., Guo Y., Tang K., Xie C., Yi H., Kuang Y., Luo Y. Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect. Dis. 2021;21:79. doi: 10.1186/s12879-021-05792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sosa-Hernandez V.A., Torres-Ruiz J., Cervantes-Diaz R., Romero-Ramirez S., Paez-Franco J.C., Meza-Sanchez D.E., Juarez-Vega G., Perez-Fragoso A., Ortiz-Navarrete V., Ponce-de-Leon A., Llorente L., Berron-Ruiz L., Mejia-Dominguez N.R., Gomez-Martin D., Maravillas-Montero J.L. B cell subsets as severity-associated signatures in COVID-19 patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shuwa H.A., Shaw T.N., Knight S.B., Wemyss K., McClure F.A., Pearmain L., Prise I., Jagger C., Morgan D.J., Khan S., Brand O., Mann E.R., Ustianowski A., Bakerly N.D., Dark P., Brightling C.E., Brij S., Circo, Felton T., Simpson A., Grainger J.R., Hussell T., Konkel J.E., Menon M. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med. 2021;2:720–735e724. doi: 10.1016/j.medj.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., Dong L., Cui X., Miao Y., Wang D., Dong J., Xiao C., Chen W., Wang H. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W., Li L., Liu J., Chen L., Zhou F., Jin T., Jiang L., Li X., Yang M., Wang H. The characteristics and predictive role of lymphocyte subsets in COVID-19 patients. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;99:92–99. doi: 10.1016/j.ijid.2020.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calvet J., Gratacos J., Amengual M.J., Llop M., Navarro M., Moreno A., Berenguer-Llergo A., Serrano A., Orellana C., Cervantes M. CD4 and CD8 lymphocyte counts as surrogate early markers for progression in SARS-CoV-2 pneumonia: a prospective study. Viruses. 2020;12 doi: 10.3390/v12111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duijf P.H.G. Low baseline pulmonary levels of cytotoxic lymphocytes as a predisposing risk factor for severe COVID-19. mSystems. 2020;5 doi: 10.1128/mSystems.00741-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oja A.E., Saris A., Ghandour C.A., Kragten N.A.M., Hogema B.M., Nossent E.J., Heunks L.M.A., Cuvalay S., Slot E., Linty F., Swaneveld F.H., Vrielink H., Vidarsson G., Rispens T., van der Schoot E., van Lier R.A.W., Ten Brinke A., Hombrink P. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur. J. Immunol. 2020;50:1998–2012. doi: 10.1002/eji.202048908. [DOI] [PubMed] [Google Scholar]
- 72.Agrati C., Sacchi A., Bordoni V., Cimini E., Notari S., Grassi G., Casetti R., Tartaglia E., Lalle E., D’Abramo A., Castilletti C., Marchioni L., Shi Y., Mariano A., Song J.W., Zhang J.Y., Wang F.S., Zhang C., Fimia G.M., Capobianchi M.R., Piacentini M., Antinori A., Nicastri E., Maeurer M., Zumla A., Ippolito G. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh K., Mittal S., Gollapudi S., Butzmann A., Kumar J., Ohgami R.S. A meta‐analysis of SARS‐CoV‐2 patients identifies the combinatorial significance of D‐dimer, C‐reactive protein, lymphocyte, and neutrophil values as a predictor of disease severity. Int. J. Lab. Hematol. 2020 doi: 10.1111/ijlh.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan J., Wei X., Qin S., Liu X., Jiang Y., Chen Y., Chen Y., Lu H., Qian J., Wang Z., Lin X. Continuous tracking of COVID-19 patients’ immune status. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosa B.A., Ahmed M., Singh D.K., Choreno-Parra J.A., Cole J., Jimenez-Alvarez L.A., Rodriguez-Reyna T.S., Singh B., Gonzalez O., Carrion R., Jr., Schlesinger L.S., Martin J., Zuniga J., Mitreva M., Kaushal D., Khader S.A. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. Commun. Biol. 2021;4:290. doi: 10.1038/s42003-021-01829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yufei Y., Mingli L., Xuejiao L., Xuemei D., Yiming J., Qin Q., Hui S., Jie G. Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19) Scand. J. Clin. Lab. Investig. 2020;80:536–540. doi: 10.1080/00365513.2020.1803587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gormez S., Ekicibasi E., Degirmencioglu A., Paudel A., Erdim R., Gumusel H.K., Eroglu E., Tanboga I.H., Dagdelen S., Sariguzel N. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil–lymphocyte ratio, D-Dimer and clinical severity of COVID-19 in hospitalized patients: a multicenter, observational study. J. Hum. Hypertens. 2020:1–10. doi: 10.1038/s41371-020-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song C.-Y., Xu J., He J.-Q., Lu Y.-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020 [Google Scholar]
- 82.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie G., Ding F., Han L., Yin D., Lu H., Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021;76:471–482. doi: 10.1111/all.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., Zhang M., Tan J., Xu Y., Song R., Song M., Wang L., Zhang W., Han B., Yang L., Wang X., Zhou G., Zhang T., Li B., Wang Y., Chen Z., Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020;92:1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu J., Kong J., Wang W., Wu M., Yao L., Wang Z., Jin J., Wu D., Yu X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb. Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Webb B.J., Peltan I.D., Jensen P., Hoda D., Hunter B., Silver A., Starr N., Buckel W., Grisel N., Hummel E., Snow G., Morris D., Stenehjem E., Srivastava R., Brown S.M. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varim C., Yaylaci S., Demirci T., Kaya T., Nalbant A., Dheir H., Senocak D., Kurt R., Cengiz H., Karacaer C. Neutrophil count to albumin ratio as a new predictor of mortality in patients with COVID-19 ınfection. Rev. Assoc. Méd. Bras. 2020;66:77–81. doi: 10.1590/1806-9282.66.S2.77. [DOI] [PubMed] [Google Scholar]
- 89.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Dassler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao X., Li T., He Z., Ping Y., Liu H., Yu S., Mou H., Wang L., Zhang H., Fu W. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing li xue za zhi Chin. J. Pathol. 2020;49 doi: 10.3760/cma.j.cn112151-20200312-00193. E009-E009. [DOI] [PubMed] [Google Scholar]
- 91.Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e1423. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parackova Z., Zentsova I., Bloomfield M., Vrabcova P., Smetanova J., Klocperk A., Meseznikov G., Casas Mendez L.F., Vymazal T., Sediva A. Disharmonic inflammatory signatures in COVID-19: augmented neutrophils’ but impaired monocytes’ and dendritic cells’ responsiveness. Cells. 2020;9:2206. doi: 10.3390/cells9102206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Didangelos A. COVID-19 hyperinflammation: what about neutrophils? mSphere. 2020;5 doi: 10.1128/mSphere.00367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890e882. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ronit A., Berg R.M.G., Bay J.T., Haugaard A.K., Ahlstrom M.G., Burgdorf K.S., Ullum H., Rorvig S.B., Tjelle K., Foss N.B., Benfield T., Marquart H.V., Plovsing R.R. Compartmental immunophenotyping in COVID-19 ARDS: a case series. J. Allergy Clin. Immunol. 2021;147:81–91. doi: 10.1016/j.jaci.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 98.Miyazawa M. Immunopathogenesis of SARS-CoV-2-induced pneumonia: lessons from influenza virus infection. Inflamm. Regen. 2020;40:39. doi: 10.1186/s41232-020-00148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aikawa N., Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther. Clin. Risk Manag. 2014;10:621–629. doi: 10.2147/TCRM.S65066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi H., Zuo Y., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Blair C., Woodward W., Lezak S.P., Lugogo N.L., Woods R.J., Lood C., Knight J.S., Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2021;109:67–72. doi: 10.1002/JLB.3COVCRA0720-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Woodard W., Lezak S.P., Lugogo N.L., Knight J.S., Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis. 2021;51:446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim S.-J., Jenne C.N. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 2016:546–554. doi: 10.1016/j.smim.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Sreeramkumar V., Adrover J.M., Ballesteros I., Cuartero M.I., Rossaint J., Bilbao I., Nacher M., Pitaval C., Radovanovic I., Fukui Y., McEver R.P., Filippi M.D., Lizasoain I., Ruiz-Cabello J., Zarbock A., Moro M.A., Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jenne C.N., Wong C.H., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A., McFadden G., Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 107.Tveit I.A., Nielsen H.Z. Morphologically abnormal neutrophil granulocytes in COVID-19 cases. Tidsskr. Nor. Laegeforen. 2020;140 doi: 10.4045/tidsskr.20.0484. [DOI] [PubMed] [Google Scholar]
- 108.Zarbock A., Polanowska-Grabowska R.K., Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Schonrich G., Raftery M.J. Neutrophil extracellular traps go viral. Front. Immunol. 2016;7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vorobjeva N.V., Chernyak B.V. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry. 2020;85:1178–1190. doi: 10.1134/S0006297920100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jayarangaiah A., Kariyanna P.T., Chen X., Jayarangaiah A., Kumar A. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620943293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., Dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decote-Ricardo D., Ferreira-Pereira A., Freire-de-Lima C.G., Barroso S.P.C., Takiya C., Conceicao-Silva F., Savino W., Morrot A. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci. Rep. 2020;10:19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sorvillo N., Cherpokova D., Martinod K., Wagner D.D. Extracellular DNA NET-Works With Dire Consequences for Health. Circ. Res. 2019;125:470–488. doi: 10.1161/CIRCRESAHA.119.314581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yaqinuddin A., Kashir J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1beta/neutrophil extracellular traps feedback loop. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nahrendorf M., Swirski F.K. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–238. doi: 10.1126/science.aac7801. [DOI] [PubMed] [Google Scholar]
- 117.Meher A.K., Spinosa M., Davis J.P., Pope N., Laubach V.E., Su G., Serbulea V., Leitinger N., Ailawadi G., Upchurch G.R., Jr. Novel role of IL (Interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018;38:843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., Barthwal M.K., Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dinarello C.A. Targeting the pathogenic role of interleukin 1β in the progression of smoldering/indolent myeloma to active disease. Mayo Clin. Proc. 2009:105–107. doi: 10.4065/84.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh U.K. Across speciality collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kong M., Zhang H., Cao X., Mao X., Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muraro S.P., De Souza G.F., Gallo S.W., Da Silva B.K., De Oliveira S.D., Vinolo M.A.R., Saraiva E.M., Porto B.N. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raftery M.J., Lalwani P., Krautkrmer E., Peters T., Scharffetter-Kochanek K., Kruger R., Hofmann J., Seeger K., Kruger D.H., Schonrich G. beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014;211:1485–1497. doi: 10.1084/jem.20131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., Yamamoto N., Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 126.Mastellos D.C., Pires da Silva B.G.P., Fonseca B.A.L., Fonseca N.P., Auxiliadora-Martins M., Mastaglio S., Ruggeri A., Sironi M., Radermacher P., Chrysanthopoulou A., Skendros P., Ritis K., Manfra I., Iacobelli S., Huber-Lang M., Nilsson B., Yancopoulou D., Connolly E.S., Garlanda C., Ciceri F., Risitano A.M., Calado R.T., Lambris J.D. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., Tektonidou M., Konstantinidis T., Papagoras C., Mitroulis I., Germanidis G., Lambris J.D., Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cavalcante-Silva L.H.A., Carvalho D.C.M., de Almeida Lima É., Galvão J.G., da Silva J.S.d.F., de Sales-Neto J.M., Rodrigues-Mascarenhas S. Neutphils and COVID-19: the road so far. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jimenez‐Cauhe J., Ortega‐Quijano D., Suarez‐Valle A., Dominguez‐Santas M., Diaz‐Guimaraens B., Fernandez‐Nieto D. Comment on “are erythema multiforme and urticaria related to a better outcome of COVID 19?” Eosinophil count in seven patients with COVID‐19 and urticarial rash. Dermatol. Ther. 2020 doi: 10.1111/dth.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lippi G., Henry B.M. Eosinophil count in severe coronavirus disease 2019. QJM Mon. J. Assoc. Physicians. 2020;113:511–512. doi: 10.1093/qjmed/hcaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C., Wang F.S. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J. Autoimmun. 2020;112 doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin. Rev. Allergy Immunol. 2020;59:78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez F.O., Combes T.W., Orsenigo F., Gordon S. Monocyte activation in systemic Covid-19 infection: assay and rationale. eBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanchez-Cerrillo I., Landete P., Aldave B., Sanchez-Alonso S., Sanchez-Azofra A., Marcos-Jimenez A., Avalos E., Alcaraz-Serna A., de Los Santos I., Mateu-Albero T., Esparcia L., Lopez-Sanz C., Martinez-Fleta P., Gabrie L., Del Campo Guerola L., de la Fuente H., Calzada M.J., Gonzalez-Alvaro I., Alfranca A., Sanchez-Madrid F., Munoz-Calleja C., Soriano J.B., Ancochea J., Martin-Gayo E., Reinmun C., E. groups COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020;130:6290–6300. doi: 10.1172/JCI140335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., Nie X., Zhou L., Liu Z., Ren Y., Yuan L., Zhang Y., Zhang J., Liang L., Chen X., Liu X., Wang P., Han X., Weng X., Chen Y., Yu T., Zhang X., Cai J., Chen R., Shi Z.L., Bian X.W. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. eBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T., Cai J.P., Zhou J., Yuan S., Zhang A.J., Chan J.F., Yuen K.Y. Attenuated interferon and proinflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222:734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gatti A., Radrizzani D., Vigano P., Mazzone A., Brando B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Cytometry A. 2020;97:887–890. doi: 10.1002/cyto.a.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matic S., Popovic S., Djurdjevic P., Todorovic D., Djordjevic N., Mijailovic Z., Sazdanovic P., Milovanovic D., Ruzic Zecevic D., Petrovic M., Sazdanovic M., Zornic N., Vukicevic V., Petrovic I., Matic S., Karic Vukicevic M., Baskic D. SARS-CoV-2 infection induces mixed M1/M2 phenotype in circulating monocytes and alterations in both dendritic cell and monocyte subsets. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peruzzi B., Bencini S., Capone M., Mazzoni A., Maggi L., Salvati L., Vanni A., Orazzini C., Nozzoli C., Morettini A., Poggesi L., Pieralli F., Peris A., Bartoloni A., Vannucchi A.M., Liotta F., Caporale R., Cosmi L., Annunziato F. Quantitative and qualitative alterations of circulating myeloid cells and plasmacytoid DC in SARS-CoV-2 infection. Immunology. 2020;161:345–353. doi: 10.1111/imm.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L., Almire C., Henon C., Kosmider O., Droin N., Rameau P., Catelain C., Alfaro A., Dussiau C., Friedrich C., Sourdeau E., Marin N., Szwebel T.A., Cantin D., Mouthon L., Borderie D., Deloger M., Bredel D., Mouraud S., Drubay D., Andrieu M., Lhonneur A.S., Saada V., Stoclin A., Willekens C., Pommeret F., Griscelli F., Ng L.G., Zhang Z., Bost P., Amit I., Barlesi F., Marabelle A., Pene F., Gachot B., Andre F., Zitvogel L., Ginhoux F., Fontenay M., Solary E. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418e1418. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., Qian H., Dai T., Zhang T., Lai Y., Wang J., Liu Z., Chen T., He A., O’Dwyer M., Hu J. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021;109:13–22. doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kazancioglu S., Yilmaz F.M., Bastug A., Sakalli A., Ozbay B.O., Buyuktarakci C., Bodur H., Yilmaz G. Lymphocyte subset alteration and monocyte CD4 expression reduction in patients with severe COVID-19. Viral Immunol. 2021;34:342–351. doi: 10.1089/vim.2020.0166. [DOI] [PubMed] [Google Scholar]
- 147.Qin S., Jiang Y., Wei X., Liu X., Guan J., Chen Y., Lu H., Qian J., Wang Z., Lin X. Dynamic changes in monocytes subsets in COVID-19 patients. Hum. Immunol. 2021;82:170–176. doi: 10.1016/j.humimm.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ognibene A., Lorubbio M., Magliocca P., Tripodo E., Vaggelli G., Iannelli G., Feri M., Scala R., Tartaglia A.P., Galano A., Pancrazzi A., Tacconi D. Elevated monocyte distribution width in COVID-19 patients: the contribution of the novel sepsis indicator. Clin. Chim. Acta Int. J. Clin. Chem. 2020;509:22–24. doi: 10.1016/j.cca.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alamri A., Fisk D., Upreti D., Kung S.K.P. A missing link: engagements of dendritic cells in the pathogenesis of SARS-CoV-2 infections. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22031118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abassi Z., Knaney Y., Karram T., Heyman S.N. The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Front. Immunol. 2020;11:1312. doi: 10.3389/fimmu.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bedin A.S., Makinson A., Picot M.C., Mennechet F., Malergue F., Pisoni A., Nyiramigisha E., Montagnier L., Bollore K., Debiesse S., Morquin D., Veyrenche N., Renault C., Foulongne V., Bret C., Bourdin A., Le Moing V., Van de Perre P., Tuaillon E. Monocyte CD169 expression as a biomarker in the early diagnosis of coronavirus disease 2019. J. Infect. Dis. 2021;223:562–567. doi: 10.1093/infdis/jiaa724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Le Joncour A., Biard L., Vautier M., Bugaut H., Mekinian A., Maalouf G., Vieira M., Marcelin A.G., Rosenzwajg M., Klatzmann D., Corvol J.C., Paccoud O., Carillion A., Salem J.E., Cacoub P., Boulaftali Y., Saadoun D. Neutrophil-platelet and monocyte-platelet aggregates in COVID-19 patients. Thromb. Haemost. 2020;120:1733–1735. doi: 10.1055/s-0040-1718732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bongiovanni D., Klug M., Lazareva O., Weidlich S., Biasi M., Ursu S., Warth S., Buske C., Lukas M., Spinner C.D., Scheidt M.V., Condorelli G., Baumbach J., Laugwitz K.L., List M., Bernlochner I. SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis. 2021;12:50. doi: 10.1038/s41419-020-03333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gumus H., Demir A., Yukkaldiran A. Is mean platelet volume a predictive marker for the diagnosis of COVID-19 in children? Int. J. Clin. Pract. 2021;75 doi: 10.1111/ijcp.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He J., Wei Y., Chen J., Chen F., Gao W., Lu X. Dynamic trajectory of platelet-related indicators and survival of severe COVID-19 patients. Crit. Care. 2020;24:607. doi: 10.1186/s13054-020-03339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhong Q., Peng J. Mean platelet volume/platelet count ratio predicts severe pneumonia of COVID-19. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Amgalan A., Othman M. Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Platelets. 2020;31:740–745. doi: 10.1080/09537104.2020.1768523. [DOI] [PubMed] [Google Scholar]
- 159.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S., Thomas S., Adler N.M., Charytan D.M., Gasmi B., Hochman J.S., Reynolds H.R. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. eClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ruberto F., Chistolini A., Curreli M., Frati G., Marullo A.G.M., Biondi-Zoccai G., Mancone M., Sciarretta S., Miraldi F., Alessandri F., Ceccarelli G., Barone F., Santoro C., Alvaro D., Pugliese F., Pulcinelli F.M., Policlinico Umberto I.C.-G. Von Willebrand factor with increased binding capacity is associated with reduced platelet aggregation but enhanced agglutination in COVID-19 patients: another COVID-19 paradox? J. Thromb. Thrombolysis. 2021;52:105–110. doi: 10.1007/s11239-020-02339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sahebnasagh A., Saghafi F., Safdari M., Khataminia M., Sadremomtaz A., Talaei Z., Rezai Ghaleno H., Bagheri M., Habtemariam S., Avan R. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J. Clin. Pharm. Ther. 2020;45:1515–1519. doi: 10.1111/jcpt.13251. [DOI] [PubMed] [Google Scholar]
- 162.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang S., Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S., Long D., Yu L. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31:490–496. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang S.Q., Huang Q.F., Xie W.M., Lv C., Quan X.Q. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br. J. Haematol. 2020;190:e29–e33. doi: 10.1111/bjh.16817. [DOI] [PubMed] [Google Scholar]
- 168.Bommenahalli Gowda S., Gosavi S., Ananda Rao A., Shastry S., Raj S.C., Menon S., Suresh A., Sharma A. Prognosis of COVID-19: red cell distribution width, platelet distribution width, and C-reactive protein. Cureus. 2021;13 doi: 10.7759/cureus.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., Poggio P., Cascella A., Pengo M.F., Veglia F., Fiorelli S., Bonomi A., Cavalca V., Trabattoni D., Andreini D., Omodeo Sale E., Parati G., Tremoli E., Camera M. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl. Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rampotas A., Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206933. [DOI] [PubMed] [Google Scholar]
- 171.Bi X., Su Z., Yan H., Du J., Wang J., Chen L., Peng M., Chen S., Shen B., Li J. Prediction of severe illness due to COVID-19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets. 2020;31:674–679. doi: 10.1080/09537104.2020.1760230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Salamanna F., Maglio M., Landini M.P., Fini M. Platelet functions and activities as potential hematologic parameters related to coronavirus disease 2019 (Covid-19) Platelets. 2020;31:627–632. doi: 10.1080/09537104.2020.1762852. [DOI] [PubMed] [Google Scholar]
- 174.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pao C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parra-Izquierdo I., Aslan J.E. Perspectives on platelet heterogeneity and host immune response in coronavirus disease 2019 (COVID-19) Semin. Thromb. Hemost. 2020;46:826–830. doi: 10.1055/s-0040-1715093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Denorme F., Manne B.K., Portier I., Petrey A.C., Middleton E.A., Kile B.T., Rondina M.T., Campbell R.A. COVID-19 patients exhibit reduced procoagulant platelet responses. J. Thromb. Haemost. 2020;18:3067–3073. doi: 10.1111/jth.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Venter C., Bezuidenhout J.A., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B., Pretorius E. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21218234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Campo G., Contoli M., Fogagnolo A., Vieceli Dalla Sega F., Zucchetti O., Ronzoni L., Verri M., Fortini F., Pavasini R., Morandi L., Biscaglia S., Di Ienno L., D’Aniello E., Manfrini M., Zoppellari R., Rizzo P., Ferrari R., Volta C.A., Papi A., Spadaro S. Over time relationship between platelet reactivity, myocardial injury and mortality in patients with SARS-CoV-2-associated respiratory failure. Platelets. 2021;32:560–567. doi: 10.1080/09537104.2020.1852543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seyit M., Avci E., Nar R., Senol H., Yilmaz A., Ozen M., Oskay A., Aybek H. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am. J. Emerg. Med. 2021;40:110–114. doi: 10.1016/j.ajem.2020.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yuan X., Huang W., Ye B., Chen C., Huang R., Wu F., Wei Q., Zhang W., Hu J. Changes of hematological and immunological parameters in COVID-19 patients. Int. J. Hematol. 2020;112:553–559. doi: 10.1007/s12185-020-02930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Henry B.M., Benoit J.L., Benoit S., Pulvino C., Berger B.A., Olivera M.H.S., Crutchfield C.A., Lippi G. Red blood cell distribution width (RDW) predicts COVID-19 severity: a prospective, observational study from the cincinnati SARS-CoV-2 emergency department cohort. Diagnostics. 2020;10:618. doi: 10.3390/diagnostics10090618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Berzuini A., Bianco C., Migliorini A.C., Maggioni M., Valenti L., Prati D. Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus. 2021;19:34–36. doi: 10.2450/2020.0242-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bessis M. Corpuscles. Springer; 1974. Spherocytes and knizocytes; pp. 65–70. [Google Scholar]
- 185.Gerard D., Ben Brahim S., Lesesve J.F., Perrin J. Are mushroom-shaped erythrocytes an indicator of COVID-19? Br. J. Haematol. 2021;192:230. doi: 10.1111/bjh.17127. [DOI] [PubMed] [Google Scholar]
- 186.Stanworth S.J., New H.V., Apelseth T.O., Brunskill S., Cardigan R., Doree C., Germain M., Goldman M., Massey E., Prati D. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lam L.M., Murphy S.J., Kuri-Cervantes L., Weisman A.R., Ittner C.A.G., Reilly J.P., Pampena M.B., Betts M.R., Wherry E.J., Song W.C., Lambris J.D., Cines D.B., Meyer N.J., Mangalmurti N.S. Erythrocytes reveal complement activation in patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.20.20104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.