Highlights
-
•
A numerical model is presented for acoustic cavitation in water with non-condensable bubble nuclei.
-
•
The phase change has a significant effect on bubble growth and collapse dynamics during acoustic cavitation.
-
•
As the bubble nucleus size increases and the acoustic frequency increases, the cavitation threshold increases beyond the Blake threshold.
-
•
The threshold predictions fitted as a function of bubble nucleus size and acoustic frequency can be applied to acoustic cavitation in water with a wide range of threshold data in the previous works.
Keywords: Cavitation, Heat and mass transfer, Nanobubble, Threshold, Ultrasound
Abstract
Numerical modelling of acoustic cavitation threshold in water is presented taking into account non-condensable bubble nuclei, which are composed of water vapor and non-condensable air. The cavitation bubble growth and collapse dynamics are modeled by solving the Rayleigh-Plesset or Keller-Miksis equation, which is combined with the energy equations for both the bubble and liquid domains, and directly evaluating the phase-change rate from the liquid and bubble side temperature gradients. The present work focuses on elucidating acoustic cavitation in water with a wide range of cavitation thresholds (0.02–30 MPa) reported in the literature. Computations for different nucleus sizes and acoustic frequencies are performed to investigate their effects on bubble growth and cavitation threshold. The numerical predictions are observed to be comparable to the experimental data in the previous works and show that the cavitation threshold in water has a wide range depending on the bubble nucleus size.
Nomenclature
constants in the Van der Waals equation ()
speed of sound ()
specific heat ()
diffusion coefficient ()
specific energy ()
acoustic frequency ()
phase-change mass flux ()
latent heat of vaporization ()
molar mass ()
pressure ()
acoustic amplitude ()
Blake threshold ()
cavitation threshold ()
air mass fraction
bubble radius ()
gas constant ()
spherical coordinate ()
temperature ()
Greek symbols
accommodation coefficient
viscosity ()
thermal conductivity ()
surface tension ()
density ()
Subscripts
air, vapor
average
bubble, liquid
critical
initial
bubble-liquid interface
saturation
ambient
1. Introduction
Cavitation is a vapor generation process in a liquid under the local saturation pressure [1]. When cavitation occurs due to intense ultrasound pulses, the generated bubbles expand and experience rapid collapse, which is called acoustic cavitation. When the bubble collapses, it is greatly compressed and heated to a high temperature, releasing enormous energy into the surrounding fluid [2]. The acoustic cavitation has been extensively studied for many engineering applications including water treatment [3], medical therapy [4] and surface cleaning [5], as reviewed in Ref. [2]. However, a general predictive model for the threshold of acoustic cavitation in water, which has been measured over a wide range of 0.02–30 MPa [6], [7], [8], [9], [10], is lacking in the literature.
Cavitation is divided into two categories depending on its inception: homogeneous cavitation of vapor bubbles in a metastable pure liquid [11] and heterogeneous cavitation due to impurities such as solid particles and non-condensable gas nuclei [12]. Recent experimental studies have shown that bulk nanobubbles with a radius of less than exist in ambient water and survive for more than a few weeks [13]. The nanobubbles have great potential in acoustic cavitation because they act as heterogeneous cavitation nuclei and significantly reduce the cavitation threshold than the homogeneous bubble cavitation case. However, their quantitative effect on the cavitation threshold has been rarely reported in the literature.
The cavitation threshold has been estimated theoretically by two models based on vapor bubble nucleation or large growth of pre-existing bubbles. Classical nucleation theory (CNT) [11] is a popular model that describes homogeneous cavitation of bubble nuclei in a pure liquid. The prediction of CNT for cavitation threshold is higher than 100 MPa in standard water. However, this value is very different from the cavitation threshold below 30 MPa measured in typical experiments [6], [7], [8], [9], [10]. In heterogeneous cavitation from pre-existing bubble nuclei, the Blake threshold [14] is a useful concept for predicting the minimum liquid pressure that causes explosive growth of bubble nuclei. The minimum pressure can be evaluated by combining the bubble nucleus size, surface tension and vapor pressure. However, the Blake threshold concept does not take into account the temporal influence of acoustic frequency, which causes a deviation from the acoustic cavitation threshold at ultrasonic frequencies of a few MHz [15].
Analytical and numerical models for predicting the acoustic cavitation threshold have been developed in several studies. Holland and Apfel [15] presented an analytical approach for the cavitation threshold of air bubbles in water over various acoustic frequencies of 0.5–10 MHz and initial bubble radii of 0.1–3 . Assuming that the bubble follows an adiabatic process and using the cavitation criterion that the maximum collapse temperature exceeds 5000 K, causing free radical production. they observed that when the initial bubble radius was , the cavitation threshold increased linearly with acoustic frequency and minimized to at a frequency of . Maxwell et al. [9] experimentally observed that the cavitation threshold in water is about at an acoustic frequency of . In their numerical model, cavitation was assumed to originate from a spherical gas nucleus with a radius of 2.5 nm. When the cavitation threshold was defined as the condition that the maximum bubble radius is times larger than the initial radius, the predicted threshold of 28.1 MPa was comparable to the experimental observation. Suo et al. [16] also conducted a similar analysis to explore the influence of multi-frequency ultrasound on the microbubble cavitation threshold using two criteria based on the maximum bubble radius larger than twice the initial radius and the collapse rate larger than the speed of sound.
Although cavitation represents a phase-change phenomenon with bubble generation, the previous analytical and numerical models for acoustic cavitation have often neglected the phase-change effect [9], [15], [16], [17], [18], [19]. The growth and collapse of gas bubbles without phase change, which is called gaseous cavitation (or pseudo cavitation) [20], differs from actual cavitation [21]. The influences of heat transfer and phase change on cavitation bubble growth and collapse were investigated earlier by Fujikawa and Akamatsu [22] considering the collapse of a bubble with initial radii of 0.1–1 mm. The bubble collapse rate was observed to be slightly lower in the case with heat transfer and vapor condensation than in the adiabatic case. Yasui [23] studies the effects of thermal conduction and phase change on acoustic cavitation of air-vapor mixture bubbles with initial radii of 4.5–10.5 at an acoustic frequency of and acoustic amplitudes of 1–1.275 atm. The numerical results were observed to match with the experimental data including thermal conduction than the case without thermal conduction. Using similar acoustic and bubble conditions, Storey and Szeri [24] performed a systematic analysis to demonstrate the influences of phase change and chemical reaction on collapsing bubble dynamics. They showed that excess water vapor is trapped in the collapsing bubble and significantly reduces the bubble peak temperature. Recently, Peng et al. [25] conducted a similar analysis for acoustic cavitation of vapor and argon mixture bubbles with initial radii of 1.5 and 4.5 and obtained the optimum liquid temperature that maximizes the bubble collapse intensity, depending on the acoustic frequency and amplitude. Dehane et al. [26], [27], [28], [29] also performed a numerical analysis for acoustic cavitation of ambient (or initial) bubbles with radii of 0.5–14 , including the effects of heat and mass transfer and chemical reaction. They investigated the bubble collapse temperature and pressure in various acoustic amplitude and frequency conditions. The effects of thermal conduction and mass transfer were observed to be dominant mechanisms depending on bubble size and acoustic amplitude. The chemical reaction had an insignificant influence on the maximum bubble radius, but had a tendency to lower the maximum bubble temperature due to the endothermal chemical reactions occurring within the bubble.
Although the previous works [23], [24], [25], [26], [27], [28], [29] have advanced the analysis of acoustic cavitation including the phase-change effect, their analysis was limited in that the heat and mass transfer rates were calculated from the boundary layer approximations with adjusting factors instead of solving the conservation equations. Their applications were mainly acoustic cavitation of microbubbles, which are relatively larger than the nanobubble nuclei [13] expected in real tap or degassed water. Few studies have applied such a model to predict the wide range of acoustic cavitation thresholds (0.02–30 MPa) measured in typical experiments [6], [7], [8], [9], [10].
In this work, a general numerical modelling of acoustic cavitation in water is developed by combining the Rayleigh-Plesset (RP) or Keller-Miksis (KM) equation with the energy equations for both the bubble and liquid domains and directly evaluating the phase-change rate from the liquid and bubble side temperature gradients. The numerical model is applied to acoustic cavitation in water with non-condensable bubble nuclei to clarify a broad range of cavitation thresholds reported in the literature. We consider nanobubbles with a radius of less than , which are known to exist in degassed or tap water and survive for a few weeks [13], and acoustic frequencies of 0.1–5 MHz, which are used in many engineering applications [2] including sonochemistry, water treatment and surface cleaning. Various acoustic amplitudes are tested to find the cavitation threshold depending on the bubble nucleus size and acoustic frequency.
2. Numerical analysis
The current analysis focuses on acoustic cavitation in water with a non-condensable bubble nucleus, which is assumed to be a sphere with a radius of and a mixture of non-condensable air and water vapor. It is initially in mechanical and thermal equilibrium with the ambient water at 1 atm and 293 K and then grows or collapses as an acoustic pulse is applied. Water is considered an incompressible fluid, whereas the bubble is treated a Van der Waals (VDW) gas to describe the high-pressure state during collapse. The equation of state (EOS) is written as
| (1) |
where and b are the gas constant and VDW constants. For a mixture bubble, the constants are determined using the air mass fraction Y of the bubble as [23]
| (2) |
| (3) |
Here, the subscripts and b denote air, vapor and bubble, respectively. The mixture molar mass is calculated as .
2.1. Governing equations
The conservation equations of mass, air mass fraction Y, momentum and energy in the spherical bubble region are written as
| (4) |
| (5) |
| (6) |
| (7) |
Here, D is the diffusion coefficient, is the viscosity and is the thermal conductivity. The temperature and pressure are calculated from and the VDW Eq. (1), where is the specific heat evaluated as .
Assuming that the liquid is incompressible and contains no dissolved gases, the conservation equations in the liquid region are written as
| (8) |
| (9) |
| (10) |
The conservation equations in the bubble and liquid regions are coupled through the matching conditions at ,
| (11) |
| (12) |
| (13) |
| (14) |
Here, and G is the phase-change mass flux. Eq. (11) represents that the phase-change mass flux across the bubble surface is the same on the liquid and gas sides. In Eq. (12), non-condensable gas is assumed to have no mass flux across the the bubble surface. Eq. (13) indicates the force balance at the bubble surface including the effects of pressure difference, surface tension, phase change and viscous stresses. In Eq. (14), the temperature discontinuity at the bubble surface is neglected. Considering the energy balance at , G is related to the liquid and bubble side heat fluxes as
| (15) |
Using the kinetic theory and assuming that the phase-change mass flux is low, G can be expressed as [22], [23], [24], [25], [26], [27], [28], [29], [30]
| (16) |
where the accommodation coefficient varies in the range of 0–0.35 depending on [25], [28], [31], the saturated vapor pressure at is determined by the Antoine Eq. (17), and the vapor pressure at the bubble surface, is calculated from the VDW Eq. (1) of water vapor using .
| (17) |
The bubble surface temperature can be iteratively determined by combining Eqs. (15), (16), (17).
The boundary conditions at are described as
| (18) |
| (19) |
| (20) |
where is an acoustic pressure.
The liquid velocity profile is solved from the mass Eq. (8) with the boundary conditions as
| (21) |
Integrating the momentum Eq. (9) over the liquid region and using the boundary conditions, we obtain the RP equation as
| (22) |
2.2. Numerical methods
To efficiently treat the moving bubble surface, we introduce the moving coordinates for the bubble region and for the liquid region. The conservation Eqs. (4), (5), (6), (7) are rewritten as
| (23) |
| (24) |
| (25) |
| (26) |
| (27) |
The conservation equations are spatially discretized using a 2nd-order essentially non-oscillatory scheme [32], [33] for convection terms, and a central difference scheme for diffusion terms. The bubble region is discretized using 17–50 grid points, and the water region of is chosen to be large enough to exclude the influence of domain size, e.g. . A grid spacing of is used for , and non-uniform grids are used for the outer region. While introducing the moving coordinate for the liquid region, the outer range, , changes with time. A 3rd-order total variation diminishing Runge–Kutta method [34] is employed to solve the transient differential equations in combination with an adaptive time-step algorithm that keeps the numerical errors estimated with two different time steps constant [35].
Eq. (22) and Eqs. (23), (24), (25), (26), (27) are solved for and with the matching and boundary conditions, given by Eqs. (11), (12), (14), (20), where and G is calculated by Eqs. (11), (15).
We consider a bubble nucleus composed of non-condensable air and water vapor at and and use the following water and air properties: , , , , , , , , , . The mixture properties in the bubble are interpolated using the air mass fraction Y of the bubble as , and .
When the bubble temperature exceeds a critical value at bubble collapse and the thermodynamic difference between liquid water and vapor disappears [36], we assume no phase change (), as done by Refs. [36], [37]. The bubble surface temperature is determined from the heat balance, . When the bubble is at the supercritical state where the bubble surface velocity is above the speed of sound, the following Keller-Miksis equation [38] is solved instead of the RP equation to include the effect of liquid compressibility:
| (28) |
The following acoustic pressure pulse is imposed on ambient water:
| (29) |
where and f represent the acoustic amplitude and frequency, respectively. The acoustic pulse is applied for two cycles () in the current computations.
3. Results and discussion
We choose a spherical bubble nucleus of as a base case. The bubble is a mixture of air and water vapor, and is in mechanical and thermal equilibrium with the surrounding water at and . The initial bubble pressure and temperature are evaluated as and , respectively. The partial vapor pressure of the bubble can be determined from the Antoine Eq. (17). Using and the VDW equations for air-vapor mixture and pure vapor, the initial air mass fraction of the bubble is iteratively obtained as .
3.1. Model validation
To validate the present numerical model, computations are first carried out for the case of and , for which numerical results and experimental data are available in the literature [38], [39]. The results are plotted in Fig. 1. The initial bubble expands during the negative pressure cycle of acoustic pulse, reaching a maximum radius of . As the acoustic pressure turns into a positive pulse, the bubble shrinks and collapses rapidly. The predicted bubble radius matches well with the previous numerical result [38] and experimental data [39]. The bubble temperature averaged over the bubble region () reaches upon bubble collapse, which is similar to the previous numerical result of [38].
Fig. 1.
Validation of the present numerical model: (a) the acoustic pressure used in the calculation and (b) comparison of the predicted bubble radius with the previous numerical result [38] and experimental data [39].
3.2. Acoustic cavitation of an air-vapor mixture bubble nucleus
Fig. 2 shows the results of the phase-change cavitation at and . The initial air mass fraction of the mixture bubble is as previously described. During the first negative pulsing, the bubble grows to and the bubble mass increases to with evaporation. During the bubble expansion, the bubble average pressure decreases, as seen in Fig. 2c. However, does not drop below the saturated vapor pressure at because of the phase-change vapor in the bubble. During the subsequent positive pulsing, the bubble rapidly shrinks and the bubble mass decreases. At the main bubble collapse near , water vapor accounts for 5.5% of the mass in the bubble, which is consistent with the observation in Ref. [24]. Thereafter, the bubble mass increases and decreases with subsequent bubble rebounds and recollapses. The water vapor continues to condense immediately after the first rebound due to the still high bubble pressure [22]. As significantly increases with bubble collapse, the bubble temperature reaches . The cavitation threshold for bubble collapse temperature above 5000 K is obtained by increasing by , resulting in .
Fig. 2.
Acoustic cavitation of an air-vapor mixture bubble nucleus at and : (a) bubble radius, (b) bubble mass, (c) bubble average pressure, (d) bubble average temperature. In the figure d, the dash line represents .
The phase-change mass flux G at the bubble surface is determined from , where and are the liquid and bubble side heat fluxes directly evaluated by temperature gradients. The heat fluxes and are determined by solving the full energy equations in the bubble and liquid domains, unlike most previous works [22], [23], [24], [25], [26], [27], [28], [29], [31] using the boundary layer approximations with adjusting factors.
The computed local temperature distribution inside and outside the bubble is plotted in Fig. 3. During the early period of bubble expansion, the temperature inside the bubble decreases faster than the bubble surface temperature, while the liquid temperature drops slightly in the region of . During the bubble expansion period of , the temperature in the bubble and liquid regions increases due to heat transfer from the surrounding liquid at . As the bubble shrinks and collapses, the bubble temperature rises up rapidly, as seen in Fig. 3c. The temperature fields inside and outside the bubble are observed to vary more complex than predicted from the boundary layer approximation.
Fig. 3.
Temperature distributions inside the mixture bubble (left) and adjacent liquid (right) at and during: (a) the early expansion (), (b) expansion () and (c) shrinkage ().
Fig. 4 presents the bubble surface temperature , the bubble average temperature and the heat fluxes and obtained from the local temperature distributions. During the negative pulsing period, the bubble surface temperature is observed to remain near except for the bubble collapse periods, whereas the bubble average temperature is slightly lower than and increases with the bubble shrinkage. During the bubble expansion, is higher than , as the bubble temperature drops below , which causes evaporation. Thereafter, as the bubble temperature rises rapidly with the positive pressure pulsing, is lower than and the vapor in the mixture bubble is condensed.
Fig. 4.
Bubble temperature and heat flux at and : (a) bubble surface temperature, (b) bubble average temperature and (c) heat flux at the bubble surface.
The effect of phase change on the acoustic cavitation at a lower frequency of and is plotted in Fig. 5. As the first negative pulsing period increases at the lower frequency, and significantly increase to and , respectively. While the bubble expands, the mixture bubble pressure remains near the water saturation pressure at , as seen in Fig. 5c. This is coincident with the fact that cavitation is a phenomenon that occurs to maintain equilibrium with the saturation pressure when the pressure is lower than the saturation pressure [11], [33]. During the first bubble collapse, the bubble average temperature increases to a peak of with a considerable water vapor accounting for 9.4% of the total bubble mass. This indicates that the phase change has a significant effect on both bubble growth and collapse dynamics during acoustic cavitation.
Fig. 5.
Acoustic cavitation of an air-vapor mixture bubble nucleus at and : (a) bubble radius, (b) bubble mass, (c) bubble average pressure, (d) bubble average temperature. In the figure d, the dash line represents .
3.3. Blake threshold and acoustic cavitation threshold
The Blake threshold concept is useful to estimate the pressure required for explosive growth of a bubble, taking into account the quasi-static pressure and surface tension [14]. We briefly review the Blake threshold formulas for ideal and VDW gases. Assuming that the air-vapor mixture bubble is an ideal gas, bubble growth is an isothermal process and the vapor pressure is constant, the ambient liquid pressure is approximated as
| (30) |
where the initial air density is determined by . The critical radius for unstable or explosive bubble growth is obtained by differentiating Eq. (30) with respect to [14], and the corresponding Blake threshold pressure is expressed as
| (31) |
Using the EOS of VDW gas, Eq. (30) is rewritten as
| (32) |
and and are iteratively obtained with Eqs. (2), (3) for and .
The predictions of and using the ideal and VDW gas equations for various initial radii keeping and are compared in Fig. 6. It is noted that the applied pressure is quasi-static with no frequency. The predictions for the VDW gas are almost identical to those for the ideal gas over a wide range of . However, when decreases below , as in distilled water [9], [10], and the effect of surface tension becomes pronounced, the predictions for the VDW gas differ significantly from those for the ideal gas. Considering that the bubble is initially at and , its initial density is evaluated directly from the ideal gas equation and iteratively from the VDW gas equation. As decreases below and increases, the initial density becomes different between the ideal and VDW gases, as seen in Fig. 6c. When the initial bubble radius is reduced to , the pressure required for bubble growth, evaluated as in the Blake threshold model, is significantly different between the ideal and VDW gases, as depicted in Fig. 6d. It is noted that the maximum value of corresponds to the Blake threshold .
Fig. 6.
Blake threshold pressure and critical radius for the ideal and VDW gases: (a) in a wide range of and (b) in an narrow range of ; (c) versus and (d) versus for .
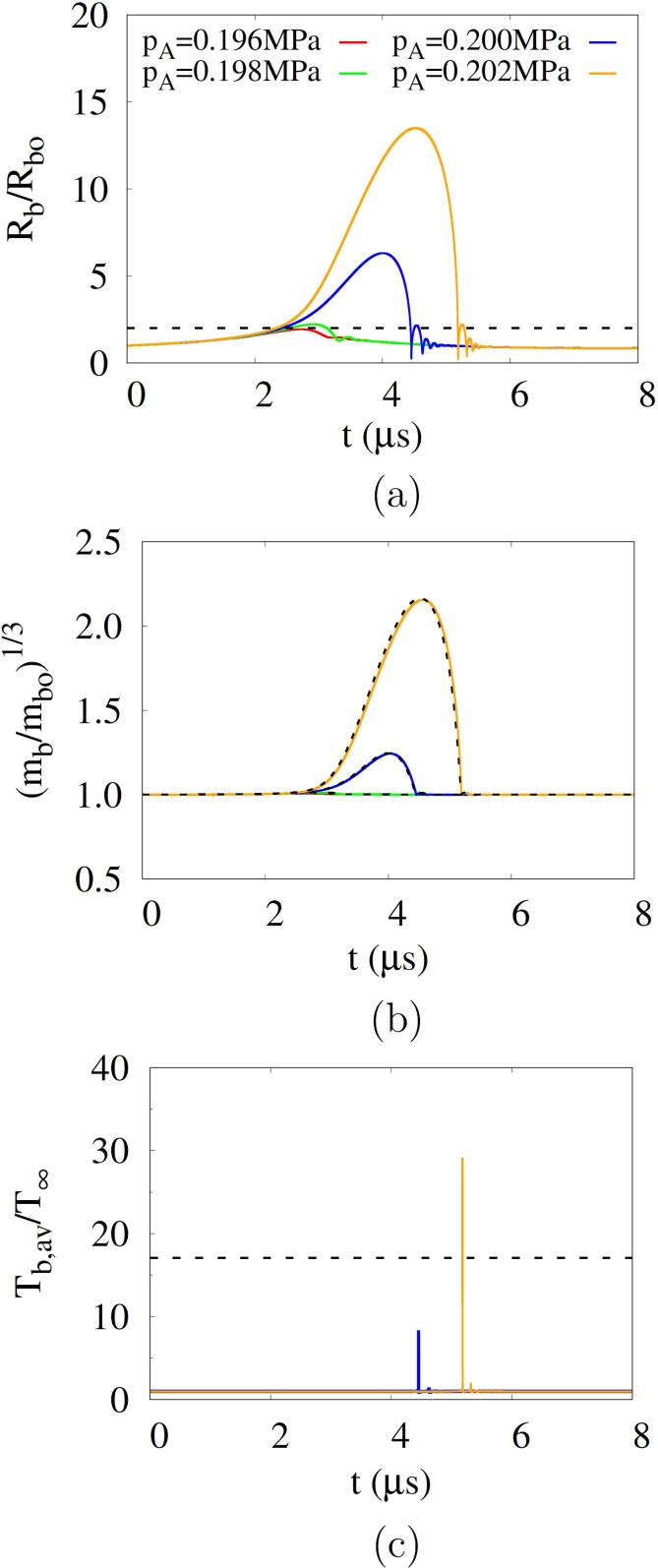
Fig. 7 shows the influence of on the acoustic cavitation at and . For , the predicted Blake threshold is and the corresponding critical radius is , which is coincident with the cavitation threshold criterion of [16], [17]. The curves of appears to be sensitive to the acoustic amplitude when is close to . For , the bubble grows to , whereas for , the bubble becomes larger than at and then shrinks during the positive pulsing. As increases above 0.200 MPa, the bubble grows significantly and then collapses rapidly. The temporal variation of bubble mass also depends on . The relation between and is expressed as
| (33) |
Fig. 7.
Influence of acoustic amplitude on acoustic cavitation at and : (a) bubble radius, (b) bubble mass and (c) bubble average temperature. In the figures a, b and c, the dash lines represent , the approximated bubble mass and , respectively.
Using and , Eq. (33) can be approximated as
| (34) |
The approximations match well with the numerical predictions (Fig. 7b). When the bubble collapses strongly for , sharp peaks appear in the bubble temperature (Fig. 7c). The peak values increase with increasing and exceed the cavitation criterion of for . At the low acoustic frequency of , the cavitation threshold is observed to be close to the Blake threshold. However, the Blake threshold concept, which does not take into account the temporal influence of acoustic frequency, can causes a deviation from the acoustic cavitation threshold at higher frequencies. The influence of acoustic frequency will be investigated in the next section.
3.4. Effect of acoustic frequency
Fig. 8 presents the effects of f and on the maximum bubble radius and temperature. The vertical black solid line denotes the Blake threshold whereas the red solid and black dash lines denote the minimum acoustic amplitudes and for and , respectively. The results are obtained only during the period prior to the first bubble rebound to remove the uncontrolled influences of subsequent bubble collapses and rebounds. For , is less than regardless of the acoustic frequency. For a low frequency of (Fig. 8a), the bubble begins to grow abruptly at and the thresholds and are close to the Blake threshold . However as the frequency increases to 1 MHz and 5 MHz, the increases of and with are reduced because the negative pulsing period decreases. The threshold based on increases to for and for as depicted in Fig. 8b and c. The threshold based on increases to and for and , respectively. This indicates that as f increases, the cavitation thresholds and become larger than the Blake threshold , which was derived without taking into account the temporal influence of acoustic frequency. The threshold difference also increases with the acoustic frequency.
Fig. 8.
Effect of acoustic amplitude on the maximum bubble radius and temperature during acoustic cavitation at and different frequencies: (a) , (b) and (d) . The vertical black solid line represents , and the red solid and black dash lines represent and , respectively.
3.5. Effect of bubble nucleus size
Fig. 9 shows the combined effects of bubble nucleus size and acoustic amplitude and frequency on the bubble growth in acoustic cavitation. For , the thresholds and are observed to be close to the Blake threshold regardless of the bubble nucleus size. For , as seen in Fig. 9a, at increases abruptly near , and as f increases and the first negative pulsing period decreases, the variation of slows and the thresholds increase. As the nucleus size is reduced to and (Fig. 9b and c), the thresholds and as well as increase significantly. The change of for becomes steep over a wide range of acoustic frequencies. The thresholds and are closer to as decreases.
Fig. 9.
Combined effects of acoustic frequency and amplitude on the bubble maximum radius during acoustic cavitation at different bubble nuclei: (a) , (b) and (c) . In the figures, the horizontal and vertical dash lines represent and , respectively, and the circle symbols represent the result of .
In Fig. 10, the present predictions of cavitation threshold (or ) are compared with experimental data reported in the literature [6], [7], [8], [9], [10]. The experimental data were obtained using air-saturated or degassed water. The acoustic cavitation thresholds measured in air-saturated water are for [6]. The experimental data are comparable to the numerical prediction for a relatively large bubble nucleus with , as seen in Fig. 10a. The bubble nuclei in gas-saturated water are expected to easily grow to larger sizes due to coalescence during acoustic pulsing periods [40], and the cavitation threshold is observed to decrease as the air saturation in water increases [41]. For degassed water [7], [8], [9], [10], the cavitation threshold increases as depicted in Fig. 10b and c. The present predictions for can be compared with the experimental data of Atchley et al. [7] for and using less than two acoustic pulses. The present predictions for are also comparable to the experimental data of Refs. [9], [10], in a range of for . The effect of acoustic frequency on the cavitation threshold weakens as decreases. This can be explained by considering the scales of inertia, surface tension and viscous stress that affect the bubble pressure, as expressed in Eq. (22). Selecting and as length and time scales, which is based on the observation in Fig. 9 that the cavitation threshold is close to the minimum acoustic amplitude for , the scales of inertia, surface tension and viscous stress can be estimated as and , respectively. Therefore, as decreases, the surface tension effect is dominant and the frequency effect becomes relatively weak.
Fig. 10.
Predicted cavitation thresholds versus acoustic frequencies at different bubble nucleus radii: (a) , (b) and (c) . The dash lines are the fitted curves from the numerical results of (circle symbols), and the black symbols are the experimental data in the previous works.
The predicted thresholds are fitted within the root-mean-square error of 0.03 MPa as
| (35) |
where and are in and nm, respectively. This fitted equation is applicable to acoustic cavitation in water with a wide range of cavitation thresholds (0.2–30 MPa) reported in the literature, as seen in Fig. 10. Eq. (35) is not very useful unless the bubble nucleus size is known. However, the equation is effective in quantifying the combined effect of bubble nucleus size and acoustic frequency on the difference between the cavitation threshold and the Blake threshold.
4. Conclusion
A numerical model for acoustic cavitation threshold in water was developed by coupling the Rayleigh-Plesset or Keller-Miksis equation with the energy equation for the bubble and liquid regions and directly evaluating the phase-change rate from the liquid and bubble side temperature gradients. The numerical model was applied to elucidate acoustic cavitation in water with a wide range of cavitation thresholds (0.02–30 MPa) reported in the literature. The numerical results showed that the temperature distribution inside and outside the bubble varies more complex than predicted from the boundary layer approximation. The phase-change vapor was observed to have a significant effect on bubble growth and collapse dynamics during acoustic cavitation. As the bubble nucleus size increases and the acoustic frequency increases, the cavitation threshold increases beyond the Blake threshold, which was developed without taking into account the temporal influence of acoustic frequency. The predicted thresholds were fitted as a function of bubble nucleus size and acoustic frequency and could be applied to acoustic cavitation in water with a wide range of threshold data reported in the literature.
CRediT authorship contribution statement
Seongjin Hong: Writing - original draft, Conceptualization, Software. Gihun Son: Writing - review & editing, Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (MSIP) (Grant No. 2019R1A2C2004109) and Korea Environment Industry & Technology Institute (KEITI), funded by the Korea Ministry of Environment (MOE) (Grant No. 2019002790006).
References
- 1.Brennen C.E. Cambridge University Press; 2014. Cavitation and bubble dynamics. [Google Scholar]
- 2.Gevari M.T., Abbasiasl T., Niazi S., Ghorbani M., Koşar A. Direct and indirect thermal applications of hydrodynamic and acoustic cavitation: A review. Appl. Therm. Eng. 2020;171 doi: 10.1016/j.applthermaleng.2020.115065. [DOI] [Google Scholar]
- 3.Yi C., Lu Q., Wang Y., Wang Y., Yang B. Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ultrason. Sonochem. 2018;43:156–165. doi: 10.1016/j.ultsonch.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Zilonova E.M., Solovchuk M., Sheu T.W. Simulation of cavitation enhanced temperature elevation in a soft tissue during high-intensity focused ultrasound thermal therapy. Ultrason. Sonochem. 2019;53:11–24. doi: 10.1016/j.ultsonch.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Vyas N., Wang Q.X., Manmi K.A., Sammons R.L., Kuehne S.A., Walmsley A.D. How does ultrasonic cavitation remove dental bacterial biofilm? Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105112. [DOI] [PubMed] [Google Scholar]
- 6.Thanh Nguyen T., Asakura Y., Koda S., Yasuda K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem. 2017;39:301–306. doi: 10.1016/j.ultsonch.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Atchley A.A., Frizzell L.A., Apfel R.E., Holland C.K., Madanshetty S., Roy R.A. Thresholds for cavitation produced in water by pulsed ultrasound. Ultrasonics. 1988;26:280–285. doi: 10.1016/0041-624X(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 8.Briggs L.J. Limiting negative pressure of water. J. Appl. Phys. 1950;21:721–722. doi: 10.1063/1.1699741. [DOI] [Google Scholar]
- 9.Maxwell A.D., Cain C.A., Hall T.L., Fowlkes J.B., Xu Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 2013;39:449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlaisavljevich E., Lin K.W., Maxwell A., Warnez M.T., Mancia L., Singh R., Putnam A.J., Fowlkes B., Johnsen E., Cain C., Xu Z. Effects of ultrasound frequency and tissue stiffness on the histotripsy intrinsic threshold for cavitation. Ultrasound Med. Biol. 2015;41:1651–1667. doi: 10.1016/j.ultrasmedbio.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caupin F., Herbert E. Cavitation in water: a review. Comptes Rendus Phys. 2006;7:1000–1017. doi: 10.1016/j.crhy.2006.10.015. [DOI] [Google Scholar]
- 12.Li B., Gu Y., Chen M. Cavitation inception of water with solid nanoparticles: A molecular dynamics study. Ultrason. Sonochem. 2019;51:120–128. doi: 10.1016/j.ultsonch.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Yasui K., Tuziuti T., Kanematsu W. Mysteries of bulk nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason. Sonochem. 2018;48:259–266. doi: 10.1016/j.ultsonch.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Leighton T. Academic press; 2012. The acoustic bubble. [Google Scholar]
- 15.Holland C.K., Apfel R.E. An Improved Theory for the Prediction of Microcavitation Thresholds. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1989;36:204–208. doi: 10.1109/58.19152. [DOI] [PubMed] [Google Scholar]
- 16.Suo D., Govind B., Zhang S., Jing Y. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrason. Sonochem. 2018;41:419–426. doi: 10.1016/j.ultsonch.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Church C.C., Labuda C., Nightingale K. A Theoretical Study of Inertial Cavitation from Acoustic Radiation Force Impulse Imaging and Implications for the Mechanical Index. Ultrasound Med. Biol. 2015;41:472–485. doi: 10.1016/j.ultrasmedbio.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerboua K., Hamdaoui O. Computational study of state equation effect on single acoustic cavitation bubble’s phenomenon. Ultrason. Sonochem. 2017;38:174–188. doi: 10.1016/j.ultsonch.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Qin D., Zou Q., Lei S., Wang W., Li Z. Nonlinear dynamics and acoustic emissions of interacting cavitation bubbles in viscoelastic tissues. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari A. Modelling approaches to acoustic cavitation in transmission pipelines. Int. J. Heat Mass Transf. 2010;53:4193–4203. doi: 10.1016/j.ijheatmasstransfer.2010.05.042. [DOI] [Google Scholar]
- 21.Watanabe S., Hidaka T., Horiguchi H., Furukawa A., Tsujimoto Y. Steady analysis of the thermodynamic effect of partial cavitation using the singularity method. J. Fluids Eng. Trans. ASME. 2007;129:121–127. doi: 10.1115/1.2409333. [DOI] [Google Scholar]
- 22.Fujikawa S., Akamatsu T. Effects of the non-equilibrium condensation of vapour on the pressure wave produced by the collapse of a bubble in a liquid. J. Fluid Mech. 1980;97:481–512. doi: 10.1017/S0022112080002662. [DOI] [Google Scholar]
- 23.Yasui K. Effects of thermal conduction on bubble dynamics near the sonoluminescence threshold. J. Acoust. Soc. Am. 1995;98:2772–2782. doi: 10.1121/1.413242. [DOI] [Google Scholar]
- 24.B.D. Storey, A.J. Szeri, Water vapour, sonoluminescence and sonochemistry, Proc. R. Soc A Math. Phys. Eng. Sci. 2000;456:1685–1709. doi: 10.1098/rspa.2000.0582. [DOI] [Google Scholar]
- 25.Peng K., Qin F.G., Jiang R., Kang S. Interpreting the influence of liquid temperature on cavitation collapse intensity through bubble dynamic analysis. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105253. [DOI] [PubMed] [Google Scholar]
- 26.Dehane A., Merouani S., Hamdaoui O., Alghyamah A. A comprehensive numerical analysis of heat and mass transfer phenomenons during cavitation sono-process. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehane A., Merouani S., Hamdaoui O., Alghyamah A. Insight into the impact of excluding mass transport, heat exchange and chemical reactions heat on the sonochemical bubble yield: Bubble size-dependency. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehane A., Merouani S., Hamdaoui O. Methanol sono-pyrolysis for hydrogen recovery: Effect of methanol concentration under an argon atmosphere. Chem. Eng. J. 2021 doi: 10.1016/j.cej.2021.133272. [DOI] [Google Scholar]
- 29.Dehane A., Merouani S., Hamdaoui O., Alghyamah A. A complete analysis of the effects of transfer phenomenons and reaction heats on sono-hydrogen production from reacting bubbles: Impact of ambient bubble size. Int. J. Hydrogen Energy. 2021;46:18767–18779. doi: 10.1016/j.ijhydene.2021.03.069. [DOI] [Google Scholar]
- 30.Carey V.P. Taylor & Francis; 2008. Liquid-vapor phase-change phenomena. [Google Scholar]
- 31.Yasui K. Alternative model of single-bubble sonoluminescence. Phys. Rev. E. 1997;56:6750. doi: 10.1103/PhysRevE.56.6750. [DOI] [Google Scholar]
- 32.Sussman M., Smereka P., Osher S. A level set approach for computing solution to incompressible two-phase flow. J. Comput. Phys. 1994;114:146–159. doi: 10.1006/jcph.1994.1155. [DOI] [Google Scholar]
- 33.Hong S., Son G. Numerical simulation of cavitating flows around an oscillating circular cylinder. Ocean Eng. 2021;226 doi: 10.1016/j.oceaneng.2021.108739. [DOI] [Google Scholar]
- 34.Osher S., Fedkiw R. Springer; 2003. Level Set Methods and Dynamic Implicit Surfaces. [Google Scholar]
- 35.Press W.H., Teukolsky S.A. Adaptive Stepsize Runge-Kutta Integration. Comput. Phys. 1992;6:188–191. doi: 10.1063/1.4823060. [DOI] [Google Scholar]
- 36.Akhatov I., Lindau O., Topolnikov A., Mettin R., Vakhitova N., Lauterborn W. Collapse and rebound of a laser-induced cavitation bubble. Phys. Fluids. 2001;13:2805–2819. doi: 10.1063/1.1401810. [DOI] [Google Scholar]
- 37.Park S., Son G. Numerical investigation of acoustic vaporization threshold of microdroplets. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadi Man Y.A., Trujillo F.J. A new pressure formulation for gas-compressibility dampening in bubble dynamics models. Ultrason. Sonochem. 2016;32:247–257. doi: 10.1016/j.ultsonch.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Löfstedt R., Barber B.P., Putterman S.J. Toward a hydrodynamic theory of sonoluminescence. Phys. Fluids A. 1992;5:2911–2928. doi: 10.1063/1.858700. [DOI] [Google Scholar]
- 40.Pflieger R., Bertolo J., Gravier L., Nikitenko S.I., Ashokkumar M. Impact of bubble coalescence in the determination of bubble sizes using a pulsed US technique: Part 1 – Argon bubbles in water. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galloway W.J. An experimental study of acoustically induced cavitation in liquids. J. Acoust. Soc. Am. 1954;26:849–857. doi: 10.1121/1.1907428. [DOI] [Google Scholar]