Abstract
It has been demonstrated that oxidative stress is related to periodontitis, and that pyrroloquinoline quinine (PQQ) acts as a powerful antioxidant. This study aimed to explore the effect of PQQ on ligature-induced alveolar bone loss in experimental periodontitis (EP) mice with/without PQQ in the diet. EP mice received a diet supplemented with PQQ for 2 weeks and were compared with sham (control) mice as well as untreated EP mice. Additionally, human periodontal ligament cells (hPDLCs) were treated with PQQ in the presence or absence of lipopolysaccharide (LPS). We found that the bone volume fraction, alkaline phosphatase activity, and the number of antioxidant cells were significantly decreased in EP mice compared with the sham mice, whereas PQQ administration rescued the above effects. In contrast, alveolar bone loss, osteoclast number, cell senescence-associated cells, and cytokines’ expression were significantly increased in EP mice compared with the sham mice but were significantly decreased with PQQ supplementation in periodontal tissues. Furthermore, we found that antioxidant enzymes and Bmi-1 protein expression levels were downregulated, whereas the protein expression levels of cell senescence-related proteins including γ-H2AX, IL-6, IL-1β, p16, and p21 were significantly up-regulated in LPS-induced hPDLCs compared with the control cells. However, PQQ administration partially prevented these changes. These findings suggest that PQQ may alleviate periodontal damage through regulation of the redox balance and cell senescence.
Keywords: Pyrroloquinoline quinone, periodontitis, alveolar bone resorption, oxidative stress, inflammation
Introduction
Over the past decade, periodontitis has become an alarming concern worldwide due to its high prevalence and the current long life expectancy, and thus is an increasing burden to the healthcare system [1,2]. Several studies have demonstrated the relationship of periodontal pathogens with systemic diseases, such as cardiovascular disease, diabetes, and kidney disease [3,4]. However, besides some surgical measures, there are few other therapeutic approaches that are clinically available for treatment of periodontitis [5]. Therefore, it is imperative to develop effective treatment methods to slow down the progression of periodontitis.
Periodontitis, a chronic destructive disease, is caused by the build-up of dental plaque. Bacterial pathogens cause both immune and inflammatory cells to the periodontal site [6]. These cells can resist the invasion of bacteria by producing reactive oxygen species (ROS), leading to oxidative stress (OS) in the periodontal area [7,8]. Local and systemic changes in the redox balance in the periodontium cause cytotoxic effects and periodontal destruction [9]. If the antioxidant defense system fails to clear out ROS in time, ROS accumulation in the periodontium may contribute to the degradation of the periodontal tissue [10]. Therefore, antioxidant therapy, a ROS antagonist, may help patients with periodontal diseases [11].
Pyrroloquinoline quinone (PQQ), which was originally discovered as a new coenzyme in methylotrophic bacteria in 1979, is ubiquitously found in foods [12,13]. Interestingly, PQQ has gained attention because of its role as an antioxidant and its function as a nutritional growth-promoting factor [14,15]. Several studies have shown the protective effects of PQQ on osteoporosis and neuroinjury, as well as on cardiac and immune events [16,17]. PQQ has been reported to be a promising compound that could improve human health [18].
Recently, PQQ has evoked much interest in terms of bone metabolism. Previous studies have demonstrated that PQQ can suppress osteoclast differentiation and bone resorptive activity, suggesting that it is a potential drug for bone resorption inhibition [19]. PQQ administration alleviated testosterone deficiency-induced osteoporosis through mitigation of ROS production and DNA damage, promotion of osteoblast proliferation and differentiation, as well as reduction in osteoclastic bone resorption [20]. Furthermore, previous studies have reported the benefits of the chondroprotective effect of PQQ during osteoarthritis development [21,22]. These findings indicated that PQQ can decrease bone resorption and promote bone formation. High doses of PQQ in a mouse model protected the mandible from developing osteoporosis [23]. Destructive erosion of bone is a major complication of periodontal diseases [24]. However, it is unclear whether application of PQQ can suppress alveolar bone loss and attenuate periodontitis.
To investigate the effect of PQQ on periodontitis, an experimental periodontitis (EP) mouse model was developed by silk thread ligature, followed by supplementation with/without PQQ in the diet. To explore whether PQQ inhibits periodontal destruction, the effects of PQQ on alveolar bone changes and the expression of inflammatory-related factors in the gingiva were examined. The present study offers preliminary evidence for using PQQ to attenuate periodontitis.
Materials and methods
Animals
Mice were bred in specific pathogen free (SPF) conditions in the laboratory animal center of Nanjing Medical University. The animal procedures were approved by the Institutional Animal Care and Use Committee (approval No.: IACUC-2104032) and were conducted according to the guide of the Nanjing medical University.
Experimental design for ligature-induced periodontitis
This was a randomized, controlled, single blind (the analyst was blinded to the treatments given to the experimental mice) animal study. Eight-week-old C57BL/6J female mice were divided into three groups: (1) sham group with normal diet (Sham, n=10); (2) ligature-induced EP group with normal diet (Ligature, n=10); and (3) ligature-induced EP group supplemented with PQQ in the diet (Ligature + PQQ, n=10). EP was induced in mice by 5-0 silk thread ligature as described previously [25]. All mice were anesthetized by intraperitoneal administration of 10 ml kg-1 of 4% chloral hydrate. Thread was gently tied around the cervical portion of the maxillary second molars (M2).
Sham mice received the same surgical procedure as that of EP mice, but the threads were not kept in M2 position. For EP mice, the threads were maintained for 2 weeks, and kept in a submarginal position of M2 to induce microbial accumulation and inflammation. The ligature status was checked daily, wherein the lost or loose ligatures were replaced. The EP mice were given PQQ supplement immediately after the surgery or normal diet. PQQ-treated diet (4 mg PQQ/kg in normal diet) [22] was given to the animals during the entire experiment. The mice were sacrificed at 2 weeks after surgery.
Micro-CT scanning
After removing the maxilla, the maxilla were scanned using micro-computed tomography (micro-CT) as described previously [26]. The two- and three-dimensional micro-CT images were then harvested. For maxilla reconstruction, the region of interest (ROI) in the maxilla was located in the region of M2, which was under the roof of furcation and above the root apex. Bone volume fraction (BV/TV) is defined as the ratio of bone volume (BV) to total volume (TV) of the maxillary ROI. Volumetric measurements were obtained following the procedure as previously described [27].
Histology
All animals were sacrificed after inhalation of ether. The maxilla were fixed with PLP fixative for 24 h [28], and then decalcified in EDTA glycerol solution for 4 weeks at 4°C. All samples were then embedded in paraffin, sectioned into 5 µm thickness, and used in the mesio-distal plane for histological analysis. All sections were histochemically stained with hematoxylin-eosin (HE) staining, or total collagen, or alkaline phosphatase activity (ALP), or tartrate-resistant acid phosphatase (TRAP) as previously described [26,29].
To elucidate the alveolar bone loss (ABL) in mice with PQQ supplement diet, the vertical distance from the point of root bifurcation to the top of alveolar bone were measured in the maxillary M2 root furcation sections of HE samples. Furthermore, total collagen, ALP and TRAP positive sections were assessed in the maxillary M2 root furcation area.
Immunohistochemistry
Immunohistochemical staining was conducted using avidin-biotin-peroxidase complex technique with affinity-purified rabbit anti-mouse interleukin-1 beta (IL-1β), interleukin-6 (IL-6), antioxidant enzymes superoxide dismutase 2 (SOD2), CD3 and nuclear factor-кB p65 (NF-кB p65) antibodies (Santa Cruz, CA, USA). According to the previous procedure [26,29], immunohistochemical staining was performed.
Real-time RT-PCR
The gene expression levels of matrix metalloproteinase-3 (MMP3), matrix metalloproteinase-8 (MMP8), IL-1β and tumor necrosis factor-α (TNF-α) were measured through real-time PCR. For measuring the relative gene expression of interest, total RNA was isolated from the mouse maxillary gingiva through Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse-transcription reactions were performed using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) as described previously [30]. Real-time PCR analyses were carried out in an Applied Biosystems Cycler with a SYBR Green PCR reagent kit as described previously [26].
Cell cultures
As directed by previous protocol [19], PDLCs were flushed out from human normal premolars for orthodontic treatment. Informed consents were gained from all participants. Cells between passages 4 and 7 were applied in the present experiments. When the degree of cell density reached 80%, cells were given the fresh complete culture medium containing 1 µg/ml lipopolysaccharides (LPS) with or without PQQ (10 µmol/L). After 48 hours, whole-cell lysates were used for western blotting. Primary antibodies against SOD1, SOD2, γ-H2AX, p16, and β-actin (Cell Signaling Technology), as well as IL-6, IL-1β, and p21 (Santa Cruz) were used for immunoblotting. Immunoreactive bands were then measured through enhanced chemiluminescence (ECL) (Bio-Rad) and Image J, respectively.
Statistical analysis
GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA) was used for data analysis. The data were presented as means ± SE, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. All P values were two-tailed, and P<0.05 was considered to be statistically significant.
Results
Effects of PQQ on alveolar bone loss in EP mice
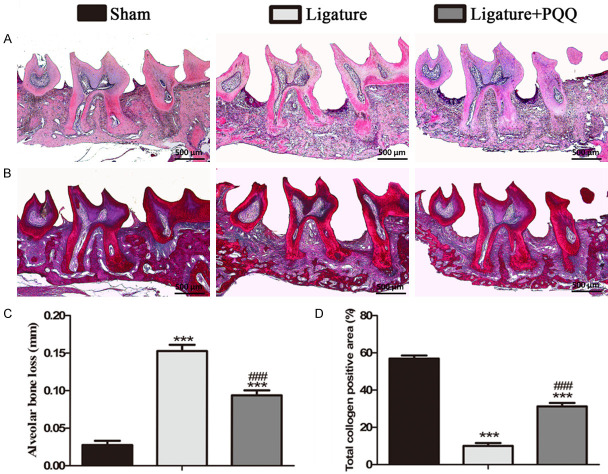
To examine the effects of PQQ on alveolar bone degradation, the maxillary phenotypes were examined by micro-CT and histomorphometry (Figures 1 and 2). The results showed that the second molar (M2) root was more exposed because of less alveolar bone around the M2 in the ligated EP group, compared with the sham group. These mice were then treated with PQQ supplementation, which rescued this phenotype (Figure 1A-C). The 3D reconstruction of the micro-CT scans revealed that the bone volume fraction was enhanced by PQQ treatment in the EP group (Figure 1D). Furthermore, the ABL was significantly greater in the EP mice than in the sham mice, whereas it was lower in the EP mice administered a PQQ diet than in the mice on a normal diet (Figure 2A and 2C). To further elucidate the ABL in mice on a PQQ diet, the total collagen-positive area in the M2 root furcation sections was assessed by histochemical techniques (Figure 2B and 2D). Compared with the sham group, the above parameter was significantly decreased in the periodontal tissues of the EP mice, while it was significantly increased after PQQ supplementation, though it did not return to the level of the sham-operated mice. These data suggest that PQQ treatment partially prevented ABL in EP mice.
Figure 1.
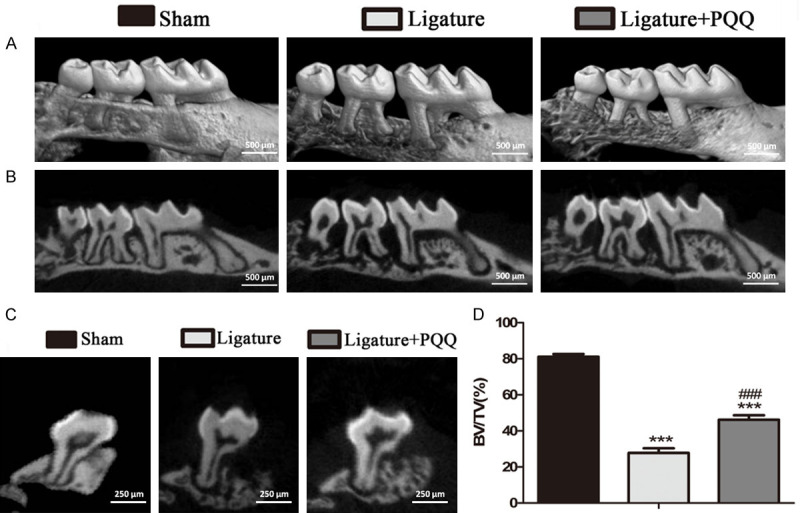
The effect of PQQ on alveolar bone destruction in EP mice. A. Representative Micro-CT scans and 3D reconstructed sections of mouse maxilla. B. Two-dimensional (2D) sagittal plane of micro-CT scanned maxillary sections. C. Two-dimensional (2D) transverse plane of micro-CT scanned maxillary second molar (M2). D. The alveolar bone volume fraction (bone volume/total volume, BV/TV) was determined by micro-CT images of 3D reconstructed sections of M2 root furcation. The vertical data point is presented as means ± SEM, and the data were the means of ten specimens, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. ***P<0.001, vs shame group; ###P<0.001, vs PQQ-untreated ligated mice.
Figure 2.
The effect of PQQ on alveolar bone loss (ABL) in EP mice. A, B. Representative photomicrographs of paraffin sections of maxilla of each group histochemically stained for HE and total collagen. C. ABL was determined by HE image analysis. D. The percentage of total collagen positive area was determined by image analysis. Data are presented as means ± SEM, and each data point was the means of ten specimens, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. ***P<0.001, vs shame group; ###P<0.001, vs PQQ-untreated ligated mice. A and B. Magnification, 50×.
Effects of PQQ on bone formation and resorption of alveolar bone in EP mice
To investigate whether PQQ suppresses ligature-induced ABL by regulating osteoblastic bone formation, the ALP area at the M2 furcation was assessed by histochemical staining (Figure 3A). Compared with the sham-operated mice, the ALP-positive areas were decreased in untreated EP mice, but PQQ supplementation in the diet rescued this effect (Figure 3C). These results indicated that PQQ may inhibit ABL by promoting bone formation in mice.
Figure 3.
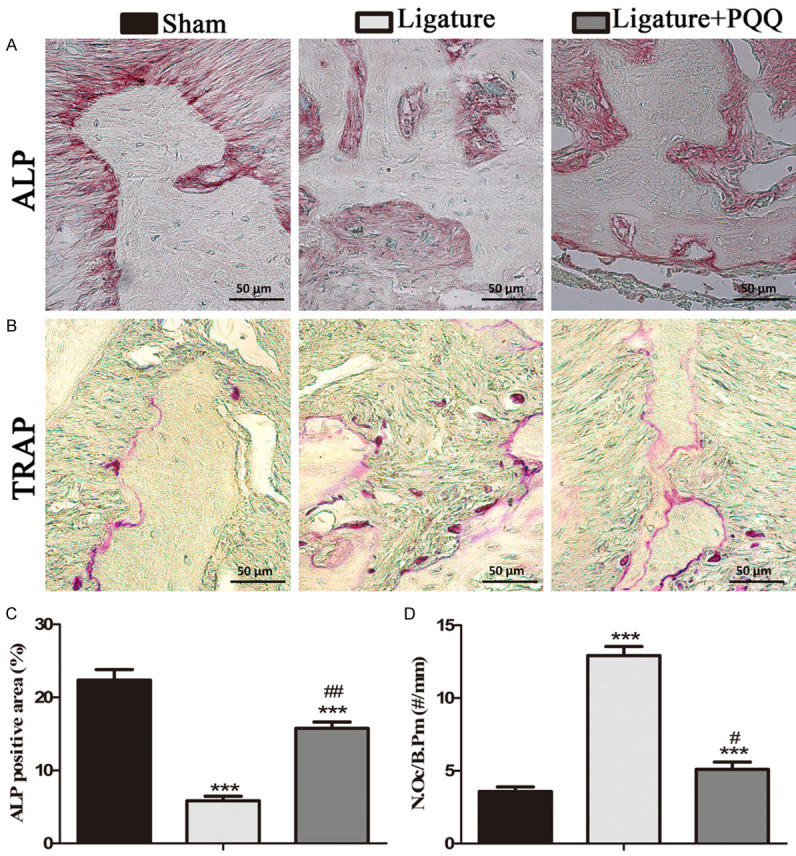
The effect of PQQ on osteoblastic and osteoclastic alveolar bone formation in EP mice. A. Representative photomicrographs of paraffin sections of maxilla of each group histochemically stained for ALP. B. Representative photomicrographs of paraffin sections of maxilla of each group histochemically stained for TRAP. C. The percentage of ALP positive area was determined by image analysis. D. The number of TRAP-positive osteoclasts (N.Oc) per mm bone perimeter (B.Pm) was measured in the alveolar bone. Data are presented as means ± SEM, and each data point was the means of ten specimens. Data are presented as means ± SEM, and each data point was the means of ten specimens, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. ***P<0.001, vs shame group; #P<0.05, ##P<0.01, vs PQQ-untreated ligated mice. A and B. Magnification, 400×.
To assess whether PQQ reduced ligature-induced ABL through inhibiting osteoclastic bone resorption, the number of TRAP-positive osteoclasts (N.Oc) per mm alveolar bone perimeter (B.Pm) in the M2 root furcation regions was assessed by histochemical staining (Figure 3B). The results showed that the osteoclast number was significantly greater in the EP mice compared with that in the sham mice, which was inhibited by PQQ supplementation in mice that underwent surgery (Figure 3D). These results suggest that PQQ not only increased osteoblastic alveolar bone formation, but also decreased osteoclastic bone resorption in the EP mice.
Effects of PQQ on senescence-associated cells and cytokines expression in gingival regions in EP mice
To explore the effects of PQQ on senescence-associated cells in vivo, alveolar sections were subjected to immunohistochemical staining using CD3-specific and NF-κB p65-specific antibodies (Figure 4A and 4B). The results revealed that the number of CD3 and NF-κB p65-positive cells was higher in the gingival regions of the EP group than that in the sham group, but it was lower in EP mice on a PQQ diet than in mice on a normal diet (Figure 4C and 4D).
Figure 4.
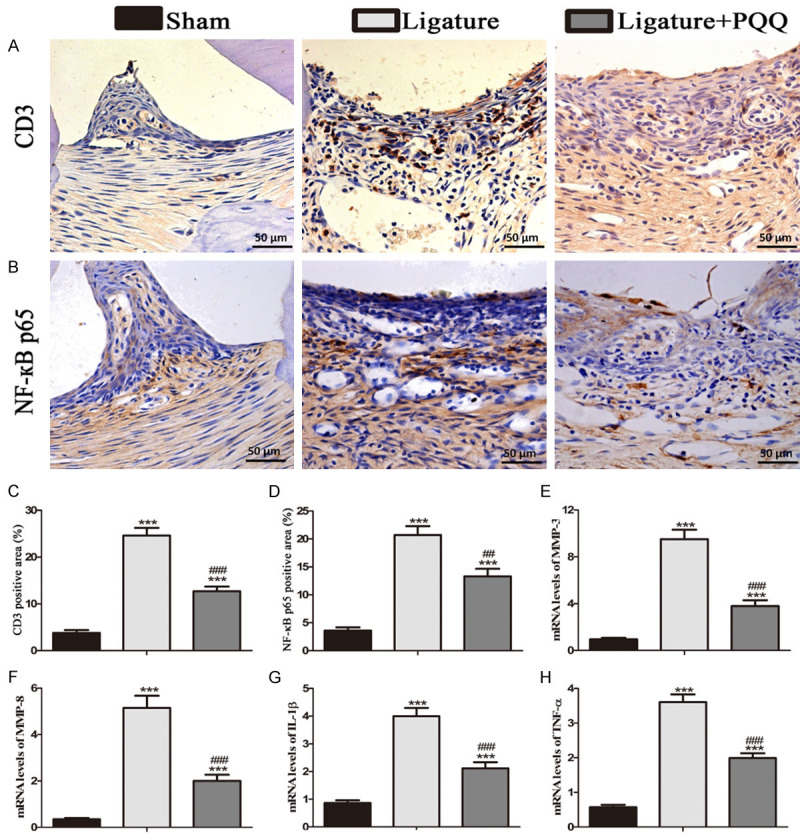
The effect of PQQ on senescence-related inflammatory cells and cytokines in the gingiva in EP mice. A. Representative micrographs of paraffin sections of maxillae immunohistochemically stained for CD3. B. Representative micrographs of paraffin sections of maxillae immunohistochemically stained for NF-кB p65. C. The percentages of CD3 positive cells. D. The percentages of NF-кB p65 positive cells. E-H. Real-time RT-PCR was performed on the extracts of gingiva for gene expression of MMP-3, MMP-8, IL-1β and TNF-α. Messenger RNA expression, assessed by real-time RT-PCR analysis, is calculated as a ratio to that of β-actin mRNA level. Each value is presented as means ± SEM, and the data were the means of ten specimens, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. ***P<0.001, vs shame group; ##P<0.01, ###P<0.001, vs PQQ-untreated ligated mice. A and B. Magnification, 400×.
To determine whether PQQ inhibits the release of cell senescence-associated cytokines in the gingiva, the mRNA expression levels of cytokines were examined by real-time RT-PCR (Figure 4E-H). The gene expression levels of MMP3, MMP8, IL-1β, and TNF-α were significantly upregulated in the untreated EP mice compared with the sham-operated group. However, the expression of these genes was significantly lower in the PQQ-treated EP group than that in the untreated EP group.
Effects of PQQ on senescence-associated cells and antioxidant enzyme expression in the alveolar bone in EP mice
Next, we determined whether the PQQ rescue of alveolar bone loss was associated with enhanced expression of antioxidant proteins and decreased cell senescence in alveolar bone (Figure 5). The results demonstrated that the expression of antioxidant proteins decreased in the EP mice compared with the sham mice, while it was partially rescued by PQQ supplementation in the EP mice (Figure 5A and 5D). Furthermore, inflammation markers, including IL-1β and IL-6, were significantly increased in the alveolar bone of the EP mice, while they were significantly reduced by PQQ supplementation in the EP mice, though they did not return to the level of the sham-operated mice (Figure 5B, 5C, 5E and 5F). These data suggest that PQQ played antioxidant roles that protected the EP mice from inflammation.
Figure 5.
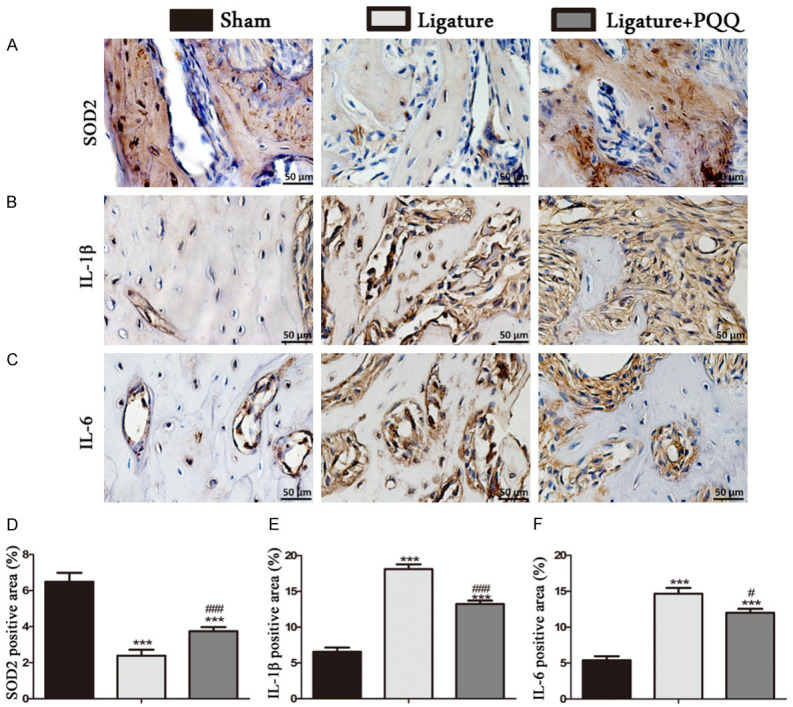
The effect of PQQ on antioxidant enzyme expression and senescence-related inflammatory cells in the alveolar bone area in EP mice. A-C. Representative micrographs of paraffin sections of maxillae immunohistochemically stained for SOD2, IL-1β and IL-6. D-F. The percentages of SOD2, IL-1β and IL-6 positive cells. Each value is presented as means ± SEM, and the data were the means of ten specimens, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. ***P<0.001, vs shame group; #P<0.05, ###P<0.001, vs PQQ-untreated ligated mice. A-C. Magnification, 400×.
Effects of PQQ on the redox balance and cell senescence in LPS-induced hPDLCs
To determine whether PQQ inhibits cell senescence through regulation of the redox balance, we examined the effects of PQQ on LPS-induced hPDLCs (Figure 6). Our data revealed that the antioxidant enzymes (SOD1, SOD2) and Bmi-1 protein expression levels were significantly reduced in LPS-induced hPDLCs compared with the control cells, but partially rescued by PQQ supplementation in LPS-induced hPDLCs. Furthermore, there was a significant increase in the protein expression levels of cell senescence-related molecules including γ-H2AX, IL-6, IL-1β, p16, and p21 in LPS-induced hPDLCs compared with the control cells, which was largely suppressed by PQQ treatment. These results demonstrated that PQQ treatment decreased cell senescence in LPS-induced hPDLCs and upregulated antioxidant reactions.
Figure 6.
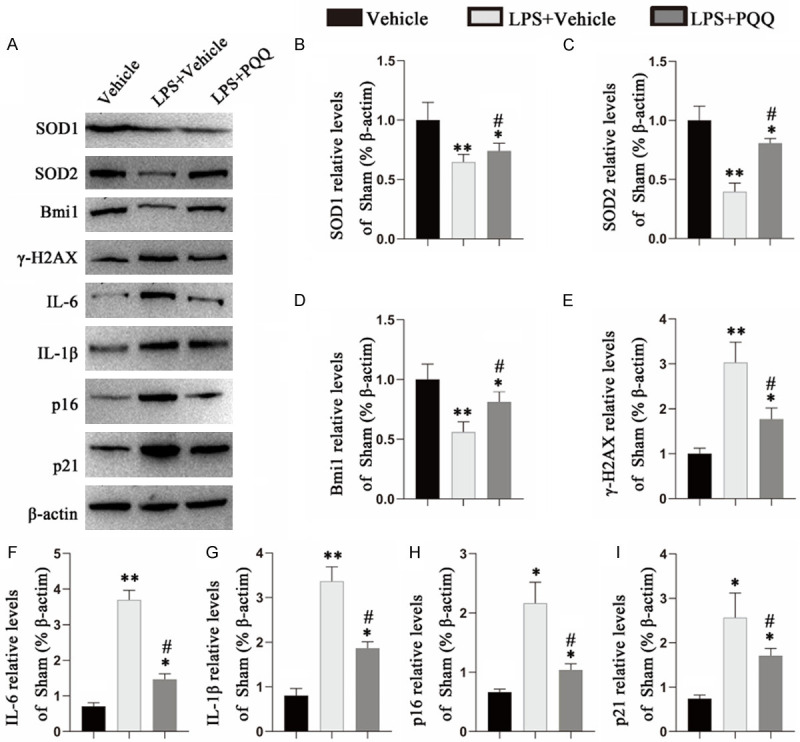
Effects of PQQ on redox balance and cell senescence-related markers in LPS-induced hPDLCs. A. Western blots of cell extracts for the expression of SOD1, SOD2, Bmi-1, γ-H2AX, IL-6, IL-1β, p21, p16. ß-actin was used as loading control for Western blots. B-I. Quantification of protein relative expression levels of SOD1, SOD2, Bmi-1, γ-H2AX, IL-1β, IL-6, p21 and p16 assessed by densitometric analysis. Values are means ± SEM of 6 determinations per group, and the differences between the groups were statistically analyzed using a one-way ANOVA followed by Bonferroni’s test. *P<0.05, **P<0.01, vs vehicle-induced group; #P<0.05, vs PQQ-untreated LPS-induced group.
Discussion
Periodontitis is characterized by pathologic loss of periodontal ligament and dental alveolar bone, hence it is necessary to find more effective and less expensive therapeutic options to mitigate it [31]. The current study investigated whether PQQ administration can prevent periodontal damage in EP mice.
The 3D micro-CT reconstruction and H&E images showed more bone loss in EP mice on a normal diet compared with those on a PQQ diet. In the PQQ-treated EP group, ABL was significantly lower than that in the EP group on a normal diet, though it was still more pronounced than that in the sham group. The above measurements were accompanied by loss of bone volume and total collagen in the alveolar bone in all three groups. These results revealed that PQQ inhibited alveolar bone destruction in EP mice. A previous study has also demonstrated that PQQ rescued mice with jaw osteoporosis by scavenging ROS [23].
In view of the suppressive action of PQQ on ABL, the status of bone remodeling was examined. PQQ treatment partially alleviated the alveolar bone destruction in the EP mice by enhancing osteoblastic bone formation and inhibiting osteoclastic bone resorption. Other studies have demonstrated promotion of osteoblasts and prevention of osteoclasts in long bones after PQQ intake [20,22]. It has been shown that PQQ influenced osteoclast precursors and suppressed osteoclast generation, and played a preventive role in NF-кB ligand (RANKL)-induced osteoclast differentiation [19,32]. As a putative redox vitamin, PQQ has been reported to participate in regulation of bone remodeling [33]. In periodontitis, PQQ may be involved in improving alveolar bone quality by influencing bone metabolism.
Considering T cells are the major source of RANKL in bone destruction in periodontal diseases and RANKL expression by activated lymphocytes acts as a prerequisite to promote osteoclast differentiation in the inflamed gingiva [34], the number of CD3-positive T cells was analyzed by immunohistochemistry. Our data clearly demonstrated that ligature-induced EP promoted a higher immune reaction in the gingival tissues with a greater number of CD3-positive cells compared with the sham group, but PQQ supplementation mitigated this immunoreaction. Furthermore, there is a growing recognition of the importance of the NF-κB signaling pathway in immune cell development, particularly the T cell lineage [35]. NF-кB is essential for modulating events for the function and survival of immune cells when gingivitis transforms into periodontitis [36]. To explore the effect of PQQ on inflammation in EP mice, the number of NF-кB p65-positive cells and its downstream proteins including IL-1β and IL-6 was examined by immunohistochemistry. Compared with the PQQ-treated EP group, the inflammatory reaction in the periodontal tissues was more pronounced in the EP mice on a normal diet and had a greater number of inflammatory cells. This is consistent with previous reports that PQQ exerted anti-inflammatory effects by regulating the biosynthesis of proinflammatory molecules [22]. Furthermore, PQQ treatment markedly suppressed the nuclear translocation of NF-κB in LPS-evoked neuroinflammation and in collagen-induced rheumatoid arthritis, and the inhibitory activity of PQQ on inflammation was partially modulated through downregulation of NF-κB activation [37,38]. Suppression of NF-кB is believed to prevent osteoclast bone resorption and promote osteoblast bone formation in inflammatory bone diseases [39]. PQQ treatment reduced the inflammatory cells in the gingival region, indicating a protective role against periodontitis.
Through inflammatory/immune factors, a plethora of cells in the periodontium secrete cytokines and chemokines, such as interleukins, TNF-α, and other proteases, such as matrix metalloproteinases (MMPs) [40]. These inflammatory mediators influence the occurrence and development of periodontitis, and may increase the formation of osteoclasts via upregulation of RANKL expression and promotion of osteoclast differentiation [41-43]. To further assess the effect of PQQ on inflammation in the current study, the levels of these factors in the gingiva were examined by RT-PCR. Following ligation, all mice exerted an inflammatory response, as evidenced by increased secretion of MMP3, MMP8, IL-1β and TNF-α. PQQ treatment significantly downregulated these cytokines, compared with EP mice on a normal diet. Thus, our results revealed that PQQ intake exerted anti-inflammatory effects on EP mice. The extensive release of the above inflammatory mediators may result in the breakdown of periodontal tissues [40].
In the present study, we further investigated whether PQQ treatment can affect oxidative stress by increasing the protein expression levels of antioxidants. Our data suggest that PQQ treatment efficiently increased the expression levels of antioxidant enzymes in the alveolar bone area in EP mice and LPS-induced hPDLCs. Next, we examined the downstream proteins of oxidative stress and found that PQQ suppressed cell senescence-related proteins including γ-H2AX, IL-6, IL-1β, p16, and p21 in LPS-induced hPDLCs, which implied that PQQ treatment regulated cell senescence by inhibiting oxidative stress. It has been reported that ROS were produced in osteoclasts under RANKL stimulation [44], and that they contributed to the activation of NF-κB signaling and osteoclast formation as secondary messengers [45]. A previous study has also revealed that PQQ suppressed osteoclast differentiation and bone loss [19].
In conclusion, our study demonstrated that PQQ may play a protective role against ligature-induced alveolar bone loss in EP through regulation of the redox balance and cell senescence. The present study indicated that PQQ is a potential therapeutic option for attenuation of periodontitis. However, additional research with longer experimental time will help further elucidate the effect of PQQ on periodontitis. Additionally, the role of PQQ in large animals and humans still needs to be elucidated.
Acknowledgements
This work was supported by grants from the Science and Technology Development Foundation of Nanjing Medical University (Nanjing, China; award no. 2017NJMUZD049 and NMUB2019211). We thank Michal Bell, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.CDC researchers find close to half of American adults have periodontitis. J Can Dent Assoc. 2012;78:c136. [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bui FQ, Almeida-Da-Silva C, Huynh B, Trinh A, Ojcius DM. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000. 2009;51:208–19. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 6.Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol 2000. 2018;76:43–50. doi: 10.1111/prd.12153. [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res. 2010;44:43–51. doi: 10.1111/j.1600-0765.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis G. New developments in neutrophil biology and periodontitis. Periodontol 2000. 2020;82:78–92. doi: 10.1111/prd.12313. [DOI] [PubMed] [Google Scholar]
- 9.Donos N, Nibali L. Periodontitis and redox status: a review. Curr Pharm Des. 2013;19:2687. doi: 10.2174/1381612811319150003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HY, Liu LC. Progress on the relationship betwten oxygen-derived free radical and periodontitis. Chin J Conserv Dent. 2009;19:46–49. [Google Scholar]
- 11.Tóthová L, Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. 2017;8:1055. doi: 10.3389/fphys.2017.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salisbury SA, Forrest HS, Cruse WB, Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979;280:843–844. doi: 10.1038/280843a0. [DOI] [PubMed] [Google Scholar]
- 13.Kumazawa T, Sato K, Seno H, Ishii A, Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem J. 1995;307:331–333. doi: 10.1042/bj3070331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000;130:719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- 15.Misra HS, Khairnar NP, Barik A, Priyadarsini KI, Apte SK. Pyrroloquinoline-quinone: a reactive oxygen species scavenger in bacteria. FEBS Lett. 2005;578:26–30. doi: 10.1016/j.febslet.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Wen H, He Y, Zhang K, Yang X, He B. Mini-review: functions and action mechanisms of PQQ in osteoporosis and neuro injury. Curr Stem Cell Res Ther. 2018;15:32–36. doi: 10.2174/1574888X14666181210165539. [DOI] [PubMed] [Google Scholar]
- 17.Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Altern Med Rev. 2009;14:268–277. [PubMed] [Google Scholar]
- 18.Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci U S A. 2018;115:10836–10844. doi: 10.1073/pnas.1809045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Yang C, Yu L, Smith W, Zhu S, Zhu J, Zhu Q. Pyrroloquinoline quinine inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos in mouse bone marrow cells and inhibits wear particle-induced osteolysis in mice. PLoS One. 2013;8:e61013. doi: 10.1371/journal.pone.0061013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan W, Jie L, Zhang H, Hui W, Miao D. Pyrroloquinoline quinone prevents testosterone deficiency-induced osteoporosis by stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption. Am J Transl Res. 2017;9:1230–1242. [PMC free article] [PubMed] [Google Scholar]
- 21.Tao R, Wang S, Xia X, Wang Y, Cao Y, Huang Y, Xu X, Liu Z, Liu P, Tang X, Liu C, Shen G, Zhang D. Pyrroloquinoline quinone slows down the progression of osteoarthritis by inhibiting nitric oxide production and metalloproteinase synthesis. Inflammation. 2015;38:1546–1555. doi: 10.1007/s10753-015-0129-x. [DOI] [PubMed] [Google Scholar]
- 22.Qin R, Sun J, Wu J, Chen L. Pyrroloquinoline quinone prevents knee osteoarthritis by inhibiting oxidative stress and chondrocyte senescence. Am J Transl Res. 2019;11:1460–1472. [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Chen N, Miao D. Effect and mechanism of pyrroloquinoline quinone on anti-osteoporosis in Bmi-1 knockout mice-anti-oxidant effect of pyrroloquinoline quinone. Am J Transl Res. 2017;9:4361–4374. [PMC free article] [PubMed] [Google Scholar]
- 24.Consensus Report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong AX, Chen J, Wu J, Li J, Wang L, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D deficiency accelerates alveolar bone loss independent of aging and extracellular calcium and phosphorus. J Periodontol. 2018;89:983–994. doi: 10.1002/JPER.17-0542. [DOI] [PubMed] [Google Scholar]
- 27.Liu YF, Wu LA, Wang J, Wen LY, Wang XJ. Micro-computerized tomography analysis of alveolar bone loss in ligature- and nicotine-induced experimental periodontitis in rats. J Periodontal Res. 2010;45:714–719. doi: 10.1111/j.1600-0765.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 28.Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–939. doi: 10.1210/endo.142.2.7976. [DOI] [PubMed] [Google Scholar]
- 29.Miao D, Scutt A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem. 2002;50:333–340. doi: 10.1177/002215540205000305. [DOI] [PubMed] [Google Scholar]
- 30.Jin J, Lv X, Chen L, Zhang W, Li J, Wang Q, Wang R, Lu X, Miao D. Bmi-1 plays a critical role in protection from renal tubulointerstitial injury by maintaining redox balance. Aging Cell. 2014;13:797–809. doi: 10.1111/acel.12236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 32.Odkhuu E, Koide N, Haque A, Tsolmongyn B, Naiki Y, Hashimoto S, Komatsu T, Yoshida T, Yokochi T. Inhibition of receptor activator of nuclear factor-kB ligand (RANKL)-induced osteoclast formation by pyrroloquinoline quinine (PQQ) Immunol Lett. 2012;142:34–40. doi: 10.1016/j.imlet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Gallop PM, Paz MA, Fluckiger R, Henson E. Is the antioxidant, anti-inflammatory utative new vitamin, PQQ, involved with nitric oxide in bone metabolism? Connect Tissue Res. 1993;29:153–161. doi: 10.3109/03008209309014242. [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF-κB control of T cell development. Nat Immunol. 2014;15:15–25. doi: 10.1038/ni.2785. [DOI] [PubMed] [Google Scholar]
- 36.Kurgan S, Kantarci A. Molecular basis for immunohistochemical and inflammatory changes during progression of gingivitis to periodontitis. Periodontol 2000. 2018;76:51–67. doi: 10.1111/prd.12146. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Sun C, Tao R, Xu X, Xu L, Cheng H, Wang Y, Zhang D. Pyrroloquinoline quinone decelerates rheumatoid arthritis progression by inhibiting inflammatory responses and joint destruction via modulating NF-κB and MAPK pathways. Inflammation. 2016;39:248–256. doi: 10.1007/s10753-015-0245-7. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Yu L, Kong L, Ma R, Zhang J, Zhu Q, Zhu J, Hao D. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-κB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS One. 2014;9:e109502. doi: 10.1371/journal.pone.0109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimi E, Takakura N, Hiura F, Nakamura I, Hirata-Tsuchiya S. The role of NF-κB in physiological bone development and inflammatory bone diseases: is NF-κB inhibition “killing two birds with one stone”? Cells. 2019;8:1636. doi: 10.3390/cells8121636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trindade F, Oppenheim FG, Helmerhorst EJ, Amado F, Gomes PS, Vitorino R. Uncovering the molecular networks in periodontitis. Proteomics Clin Appl. 2014;8:748–61. doi: 10.1002/prca.201400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stadler AF, Angst P, Arce RM, Gomes SC, Oppermann RV, Susin C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J Clin Periodontol. 2016;43:727–745. doi: 10.1111/jcpe.12557. [DOI] [PubMed] [Google Scholar]
- 42.Sukriti KC, Wang XZ, Gallagher JE. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: systematic review. J Clin Periodontol. 2020;47:289–308. doi: 10.1111/jcpe.13218. [DOI] [PubMed] [Google Scholar]
- 43.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 44.Steinbeck MJ, Appel WH Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol. 1994;126:765–72. doi: 10.1083/jcb.126.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SH, Zhou B, Kim JK, Ra JC, Kim YH, Jang HD. Scopoletin and scopolin isolated from artemisiaiwayomogi suppress differentiation of osteoclastic macrophage RAW264.7 cells via scavenging reactive oxygen species. J Nat Prod. 2013;76:615–620. doi: 10.1021/np300824h. [DOI] [PubMed] [Google Scholar]