Abstract
Background
Low blood pressure (BP) in acute ischaemic stroke (AIS) is associated with poor functional outcome, death, or severe disability. Increasing BP might benefit patients with post-stroke hypotension including those with potentially salvageable ischaemic penumbra. This updated systematic review considers the present evidence regarding the use of vasopressors in AIS.
Methods
We searched the Cochrane Database of Systematic Reviews, MEDLINE, EMBASE and trial databases using a structured search strategy. We examined reference lists of relevant publications for additional studies examining BP elevation in AIS.
Results
We included 27 studies involving 1886 patients. Nine studies assessed increasing BP during acute reperfusion therapy (intravenous thrombolysis, mechanical thrombectomy, intra-arterial thrombolysis or combined). Eighteen studies tested BP elevation alone. Phenylephrine was the most commonly used agent to increase BP (n = 16 studies), followed by norepinephrine (n = 6), epinephrine (n = 3) and dopamine (n = 2). Because of small patient numbers and study heterogeneity, a meta-analysis was not possible. Overall, BP elevation was feasible in patients with fluctuating or worsening neurological symptoms, large vessel occlusion with labile BP, sustained post-stroke hypotension and ineligible for intravenous thrombolysis or after acute reperfusion therapy. The effects on functional outcomes were largely unknown and close monitoring is advised if such intervention is undertaken.
Conclusion
Although theoretical arguments support increasing BP to improve cerebral blood flow and sustain the ischaemic penumbra in selected AIS patients, the data are limited and results largely inconclusive. Large, randomised controlled trials are needed to identify the optimal BP target, agent, duration of treatment and effects on clinical outcomes.
Keywords: stroke, blood pressure, acute, elevation, induced hypertension, vasopressor, ischaemic
Introduction
Up to 25% of patients with acute ischaemic stroke (AIS) have a systolic blood pressure (BP) below 140 mm Hg within 48 hours of onset. 1,2 Low BP in acute stroke is associated with poor prognosis: observational data from a large randomised trial showed that early death (2-week) increased by ∼18% for every 10 mm Hg below 150 mm Hg. Another prospective study of patients with first-ever stroke reported that the relative risk of death increased at 1-month and 1-year by 28% and 17%, respectively, for every 10 mm Hg systolic BP below 130 mm Hg. 3 Recently, post-hoc analysis from the Multicentre Randomised Clinical Trial of Endovascular Treatment for Acute Ischaemic Stroke in the Netherlands (MR CLEAN) found that hypotension during mechanical thrombectomy (MT) was strongly associated with death and poor functional outcome at 3 months. 4 Similar results were reported in a prospective endovascular treatment registry. 5 The reasons for these observations are unclear but might be explained by concomitant factors including poor collateral circulation, prior cardiac disease, arrhythmias or sepsis; hypotension per se; or damage to the autonomic centres in the brain from the stroke itself. 6,7 Moreover, studies have reported that hypotension post-stroke is associated with reduced cerebral blood flow leading to infarct extension and neurological deterioration. 8,9 Thus, increasing BP in AIS patients with low BP, confirmed large vessel occlusion (LVO) and salvageable penumbra could potentially increase brain perfusion and improve functional outcomes.
Interventions to increase BP in acute stroke have been examined for >40 years. Volume expansion/haemodilution is one approach that has been assessed in a number of clinical trials. However, a Cochrane Review (21 studies, >4,000 patients) found no significant benefit. 10 Another approach is using pharmacological agents to raise BP, which is recognised for treating cerebral ischaemia in patients with vasospasm after subarachnoid haemorrhage. 11 However, little is known of the effects in AIS. A meta-analysis, led by the Blood Pressure in Acute Stroke Collaboration in 2014 found insufficient evidence to provide reliable guidance on deliberate pharmacological intervention in acute stroke. 12 An earlier review of pressor therapy in acute stroke concluded that there were few trials and the results were inconclusive. 13 Clinical guidelines on management of post-stroke hypotension provide no objective clarification, reflecting the paucity of evidence in this area. However, guidelines advise that patients with low BP may need inotropic support with close cardiac and neurological monitoring. 14,15 This suggests that potential causes of low BP in stroke should be addressed and vasopressor therapy may be considered in selected patients. However, it is unclear when to increase or maintain BP, duration, the ideal agent and which group of patients might benefit.
In the last decade, there are increasing reports of patients treated with intravenous thrombolysis (IVT) and or during mechanical thrombectomy (MT) receiving vasopressors to increase or maintain BP. This updated systematic review considers the present evidence regarding the use of vasopressors in patients with AIS.
Methods
Search strategy
We systematically searched several databases (August 2019–December 2020) to identify studies examining the effect of elevating BP in AIS. The databases included MEDLINE and EPub ahead of print, In-process, Ovid Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews, Web of Science and Scopus. No language restrictions were applied.
To further identify published, unpublished and ongoing trials we also searched:
reviews of high BP in acute stroke 16
the ongoing Trials Section of Stroke and the Internet Stroke Centre Stroke Trials Registry
In addition, we also searched Google Scholar and the reference lists from published reviews and relevant trials.
The search strategy was designed and developed by the study’s principal investigator (KK) with input from all collaborators. Keywords were used to search for studies testing BP elevation in AIS. An appendix detailing the search strategy is appended separately.
Inclusion criteria
We included any study which aimed to or reported on the effect of increasing BP in AIS. We included adults (aged 18 years or over) of either sex, eligible or not eligible for acute reperfusion therapy (ART) including IVT, MT, intra-arterial thrombolysis (IAT) or combined.
Exclusion criteria
We excluded studies where there was no classification or confirmation (neuroradiological or other) of AIS or where increasing BP was neither the aim nor the proposed mechanism of action.
Outcomes
The primary outcome was death or dependency assessed using the seven-level modified Rankin Scale (mRS). Secondary outcomes included stroke severity (National Institute of Health Stroke Scale-NIHSS), or any other clinical measures used to define neurological deficit, mortality and adverse events. Where available, we extracted information on systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), heart rate (before, during and after treatment) and radiological effects of treatment (e.g. infarct growth/extension, collateral circulation).
Study extraction
Using the selection criteria, three authors (TAS, MO, CC) independently assessed published and unpublished articles from the electronic searches and with input from authors (ECS, KK) selected studies for inclusion. We resolved any disagreements by discussion.
Data extraction
For this update, data were independently extracted by three authors (TAS, MO, TJKH) and one author (KK) checked each value.
Data management
We sought information on the agent, dose, route of administration, timing, inclusion criteria, method of randomisation, allocation concealment, blinding and method of analysis (intention-to-treat).
The protocol was registered with the international prospective register of systematic reviews (CRD42021227717).
Results
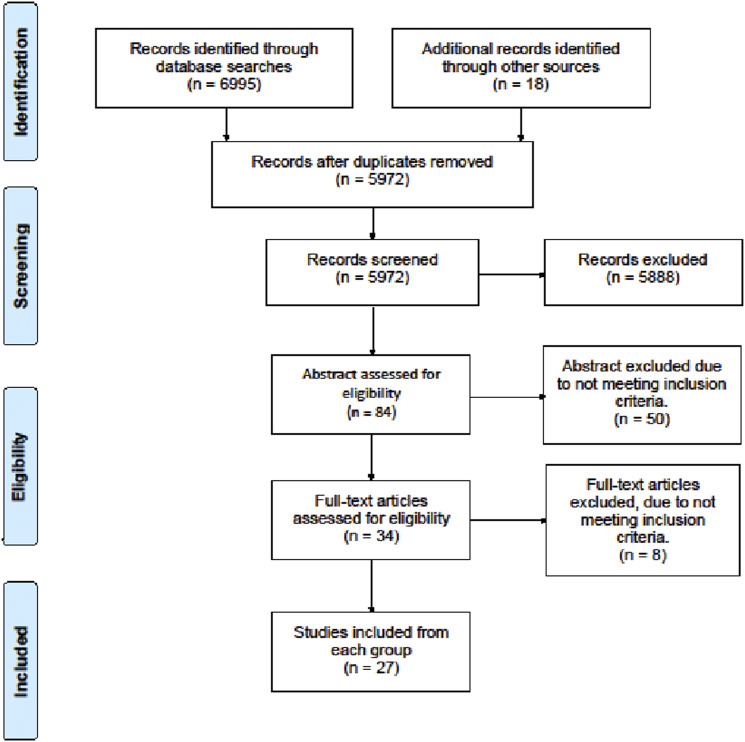
The searches yielded 6995 publications. After removal of duplicates, 5972 titles were screened. Of these, eighty-four articles were selected for initial assessment and thirty-four publications were extracted for full text review. After applying the predefined selection criteria, twenty-seven studies were finally included for this review. (Figure 1)
Figure 1.
Flow chart of searches
Eleven studies were prospective of which seven were Randomised Controlled Trials (RCTs). Sixteen studies were retrospective of which ten were observational studies, two case series and four case reports.
Nine studies deliberately increased BP in patients treated with acute reperfusion therapy (ART) including IVT, MT, intra-arterial thrombolysis (IAT) or combined. Among these, six studies were during MT only, one in IVT, one in IVT and MT and one in combination of IVT, MT and IAT.
The majority of studies (n = 18) either:
- were conducted before ART
- included patients who were ineligible for ART
- did not provide information on ART
Of these, only five studies were RCTs and the other thirteen comprised observational studies and case reports.
Patients
The total number of patients in the included studies were 1886 (range: one to 256). Of these, 1551 participants received vasopressors to increase BP and 335 participants did not (controls). (Table 1)
Table 1.
Characteristics of included studies
| Study | Design and main inclusion criteria | Intervention and duration of intervention | Outcomes | Results | Adverse effects | Modality of reperfusion |
|---|---|---|---|---|---|---|
| Studies of ART | ||||||
| Alcaraz 2019 | Retrospective N = 126 EVT anterior circulation | PhE., Ephedrine - | Haemodynamic intervention | 24 received vasopressor | - | EVT |
| Deng 2019 | RCT N = 51 A/C: 25/26 Anterior LVO mRS < 3 | Metaraminol - | 90 days mRS | No significant change in mRS | C: 1 Perforated vessel + 1 ICH | EVT |
| Rasmussen 2018 | RCT N = 128 A/C: 100/28 NIHSS ≥ 10 Infarct <70 ml, Independently living Groin puncture <6 h OTT Anterior circulation LVO | Ephedrine, PhE - | 90 days mRS TICI score Haemodynamic parameters | No significant change in mRS at 90 days | None | EVT |
| Schönenberger 2018 | Retrospective N = 150 (GA/CS: 73/77) AIS, ICA occlusion and/or MCA. NIHSS > 10. Planned EVT | NEp Median 105 min (IQR: 80–150) | 24h NIHSS 90 days mRS | No significant association between SBP, DBP and MAP and change in 24 h NIHSS. No change in 90-day mRS | - | 150 EVT 96 IVT + EVT |
| Treurniet 2018 | Subgroup analysis of MR CLEAN N = 60 BP data available. | NEp., Ep., PhE - | 90 days mRS | > change in MAP was associated with worse mRS (acOR) 0.95 per mmHg, (95% CI 0.92–0.99) decrease. MAP during GA 10 mmHg lower than baseline MAP constituted a 1.67 times lower odds of a shift towards good outcome on the mRS. | None | EVT |
| Whalin 2017 | Retrospective N = 256 EVT for AIS | PhE - | 90 days mRS ≤ 2 | No difference in BP between 2 groups. Low MAP before reperfusion was associated with mRS >2 (P = .04) | 19 ICH | EVT |
| Mundiyanapurath 2016 | Prospective study N = 64 EVT of ICA or MCA in AIS NIHSS>10 | NEp. - | Favourable outcome: mRS ≤ 2 Collateral status | 27 with mRS ≤ 2 37 with mRS > 2 mRS > 2 was associated with higher cumulative dose NEp (OR 0.142, CI: 0.038–0.525, p = .003) Median mRS at 90 days was 3 | None | EVT |
| Sivasankar 2016 | Retrospective N = 84 EVT of anterior circulation and/or treated with IA-tPA. | PhE., Ep., Ephedrine During EVT | mRS | Change in mRS was not associated with the use of vasopressor. | None | 77 EVT (47 MT alone, 6 IA-tPA alone, 24 MT + IA-tPA) – EVT + IV-tPA |
| Marzan 2004 | Retrospective N = 34 NIHSS ≥ 5, SBP < 140 mmHg, No evidence for hypovolemia | NEp. 14-96 h | mRS | mRS at discharge 3 ± 1 | N = 4 Died 1 ventricular tachycardia | 8 IVT 26 None |
| Studies of no ART | ||||||
| Bang 2019 | RCT N = 153 A:76/C: 77 Non-cardioembolicAIS NIHSS 4–18, ineligible EVT or IVT or with progressive stroke*. | PhE Mean 4.9 ±1.9 days | 7-day NIHSS 90 days mRS | A: NIHSS improvement in 44/76 (58%) favourable day 7 NIHSS OR 2.49 (95% CI 1.25–4.96, (p = .010) OR 90-day mRS (0–2): 2.97 (95% CI 1.32–6.68, (p = .009). C: NIHSS improvement in 24/77 (31.2%) | sICH: 1A/0C (p = .313) HTI: 5A/0C (p = .022) | * |
| Nasi 2019 | RCT N = 218 A1: 140–160 mmHg n = 77 A2: 161–180 mmHg n = 75 A3: 181–200 mmHg n = 66 AIS ≤ 12 OTT ineligible for IVT | NEp, Esmolol, Ntr.pru 24 h | 90 days mRS | No change in mRS at 90 days | sICH was more frequent in higher SBP range (p = .048). A1: 1, A2: 2, A3: 6 mRS 6: N = 30 (12 received vasopressor) | * |
| Kang 2017 | Retrospective N = 66 A/C: 25/41 Lacunar infarct diameter on MRI < 20 mm Motor progression | PhE Until 24 h stable NIHSS score + 24 h tapering dose. | NIHSS at discharge mRS ≤ 2 at discharge and 90 days | A vs C: NIHSS: 4.4 ± 2.5 vs 6.0 ± 3.7, (p = .036) mRS ≤ 2 at discharge: 21 vs 20 (p = .004) 90 days mRS ≤2: 18 vs 15 (p = .0011) | None | * |
| Lim 2011 | Retrospective N = 82 A/C: 52/30 Lacunar syndrome with motor weakness. DWI confirmed infarct <20 mm in diameter | PhE Until 24 h stable NIHSS score + 24 h tapering dose. | NIHSS mRS hospitalisation days | A vs C NIHSS motor score at discharge 1.01 ± 1.47 vs 1.86 ± 1.92 (p = .042) mRS ≤ 2: 32 vs 15 (p = .044) Hospitalisation days: 13.1 ± 7 vs 18.6 ± 17.3 (p = .047) | Chest tightness 4 (3A/ 1C) Dysuria (n = 2) | * |
| Koenig 2006 | Retrospective N = 100 Symptoms of AIS, MRI < 7 OTT MRI suggestive of AIS | PhE., Dpm., Mddr., Fl.cort. - | Safety NIHSS | No significant change in NIHSS | C:2 NSTEMI 2 PE. A:1 sICH, 1CE, 1PE | * |
| Chalela 2005 | Case report N = 1 A2 occlusion. perfusion/diffusion mismatch | PhE 5 min | MRI Perfusion | Perfusion deficit decreased 40% and hypoperfusion improved. CBV did not change. | None | * |
| Hillis 2004 | RCT N = 15 A/C: 10/5 Quantifiable, aphasia, hemi-spatial neglect and/or hemiparesis < 7 days OTT >20% + >30 ml MRI mismatch | PhE 24–72 hours | PWI volume at baseline vs follow-up mean NIHSS at baseline vs day 3 | PWI volume: A: 147ml to 58.3ml (p = <.001) C: 106 ml to 94 ml (p = .19) Mean NIHSS A: 9.3 to 4.8 (p < .001) C: 12 to 11.8 (p = .5) | HTI: C:1 | * |
| Hillis 2003 | RCT N = 15 A/C: 9/6 <7 days from AIS. Perfusion/diffusion mismatch neurological deficits | PhE <72 h | NIHSS and cognition | A: Mean NIHSS from day 1-3: 10.2-5.6 (p = .002) C: No significant difference in NIHSS between day 1 and 3 A vs C: Mean NIHSS: day 3: 5.6 vs 12.3 (p = .01) 6–8 weeks: mean 2.8 vs 9.7 (p = .04) A: cognitive battery improved 58.7 ± 36% to 27.9 ± 32% (p < .002) C: No significant change in cognitive battery (64.3 ± 31 vs. 67.3 +/- 34% errors) | None | * |
| Oliviera-Filho 2002 | Case report N = 1 | Dpm. Circa 48 h | NIHSS | Improved according to graph in the publication but no further details | - | * |
| Schwarz 2002 | Prospective N = 19 Acute MCA territory stroke | NEp. - | sICH, ICH GCS & Death | 15 severely disabled at discharge (GCS 3) 4 died | * | |
| Hillis 2001 | Case series N = 6 < 7 days from AIS. Aphasia PWI/DWI mismatch | PhE Until function improved and/or MAP 130 or adverse effects | Lexical semantics | 6 improvements in lexical semantics when MAP was elevated | - | * |
| Hillis 2001 | Case report N = 1 | PhE 12 h | Language comprehension | Improvement in parallel with MAP improved perfusion on MRI | - | * |
| Rordorf 2001 | Prospective N = 13 NIHSS > 4, No ICH, ≤ 12 hours from onset | PhE ≥ 30 min | NIHSS | 7 improved NIHSS | None | - |
| Saxena 1999 | RCT N = 85 A/C: 40/45 AIS in the anterior circulation≤ 18 hours from onset | DCLHb. Every 6 h for 72 h | Daily physical examination while admitted and at 90 days. mRS at 90 days. | Adverse events: A: Patient 1: scleral icterus, fever, hypertension, PE, CE, death. Patient 2: pancreatic insufficiency nausea, anaemia Outcome at 90 days was significantly worse in treatment group. (p = .002). Treatment with DCLHb (p = .015; OR, 3.9; CI, 1.4 to 12.0) were independent predictors of a worse outcome A: 85% 90 days mRS 3–6 (23% death) C: 51% 90 days mRS 3–6 (9% death) Reperfusion of the hemisphere through collateral flow with patients symptoms resolved | * | |
| Duke 1998 | Case report N = 1 Embolic occlusion of the middle cerebral artery | Dobutamine - | Clinical | Reperfusion of the hemisphere through collateral flow with patients symptoms resolved | - | |
| Rordorf 1997 | Retrospective N = 63 A/C: 30/33 Cerebral ischaemia | PhE Mean 110 h (range, 7–576 h). | Morbidity and mortality | No significant difference in outcome between groups. | CE A:2, C:6. Radiological abnormalities: A:8 C: 12, Arrhythmia A:1 | * |
| Meier 1991 | Prospective N = 81 A/C = 37/44 <6 h from stroke onset Acute blood flow defect in carotid system | Ep 5 min x 3 | Consciousness and severity of paresis Mortality | A: Improved Consciousness 22%, Improvement in Severity of paresis 24%, Improved paresis and consciousness 16%. No effect 38%, A: Mortality 21 days 14/37 C: Mortality 21 days 28/44 | None | * |
| Wise 1972 | Case series N = 13 Nonhypertensive with focal brain ischaemia without change BP ≤ 4 hours of onset | Vasopressor** - | Neurological function | N = 5/13 improvement of brain function immediately | - | * |
Patients under 18 years of age were not included.
Progressive stroke was defined as symptom worsening (i.e. a ≥2-point increase in NIHSS score, including an increase in the motor score for the affected upper and lower limbs) during hospitalisation and the presence of new lesions or infarct growth on diffusion-weight imaging magnetic resonance scan performed within 24 hours of treatment.
Not specified further in the publication
A = Active group; AIS = acute ischaemic stroke; ART: acute reperfusion therapy; BP = blood pressure; C = Control group; CBV = cerebral blood volume
CE = Cerebral oedema; CS = conscious sedation; DBP = diastolic blood pressure; DCLHb = Diaspirin cross-linked haemoglobin
Dpm = Dopamine; Ep = Epinephrine; EVT = endovascular treatment; Fl.cort = Fludrocortisone; GA = general anaesthesia
GCS = Glasgow coma scale; HTI = Haemorrhagic transformation of infarct; IA-tPA = Intra-arterial tissue plasminogen activator
ICA = Internal carotid artery; ICH: intracerebral haemorrhage; IVT = intravenous thrombolysis; MAP = mean arterial pressure; MCA = middle cerebral artery Mddr = Midodrine; mRS = modified Rankin scale; MT = Mechanical thrombectomy; NEp = norepinephrine
NIHSS = National Institutes of Health Stroke Scale; NSTEMI = non-ST elevation myocardial infarction; Ntr.pru. = Nitroprusside
OTT = onset to treatment; PE = Pulmonary oedema; PhE = Phenylepinephrine; SBP = systolic blood pressure; sICH = symptomatic intracranial haemorrhage; - = Not reported; *= indicates no ART/conducted before ART
Bias
We systematically assessed the risk of bias in the included seven RCTs using the Cochrane risk-of-bias tool. 17
Allocation
We assessed allocation concealment as ‘low risk’, ‘high risk’ or ‘unclear risk’ according to the Cochrane handbook for Systematic Reviews of Interventions (ref).
One study of ART used computer-generated randomisation and was therefore deemed Low-Risk and the other study did not report the method of randomisation (unclear risk) (Supplementary Table 1).
Two trials of no ART used a computer-generated randomisation method (low risk) and the remaining three trials did not describe randomisation (unclear risk).
Blinding
In the ART studies, participants and investigators were blinded to treatments as follows:
double-blind (n = 1)
open-label (n = 1)
Of the five RCTs of no ART, blinding was as follows:
single blind (n = 2)
open-label (n = 3)
Incomplete outcome data:
Both RCTs of ART were analysed by the intention-to-treat principle.
Of the five RCTs of no ART, two were analysed according to the intention-to-treat principle and the remaining three were on a per-protocol basis.
Selective reporting
We did not see evidence of selective reporting in any of the seven RCTs.
Other sources of bias:
We did not find any other risks to the validity of the included RCTs.
Outcomes
Eighteen studies reported on functional outcome after BP elevation: nine used mRS, five reported NIHSS and four studies assessed both. (Table 1)
Two studies reported on language comprehension using lexical semantics. 18,19 One study mainly assessed safety 20 and two others on morbidity, symptomatic intracerebral haemorrhage, Glasgow Coma scale and death. 21,22 Two studies reported neurological deficits using non-standard scales. 23,24 One study used day 90 mRS as primary outcome, but also examined revascularisation using the Thrombolysis in Cerebral Infarction (TICI) score and haemodynamics. 25
One small prospective study reported on the effect of BP elevation on collateral circulation. 26
Two studies reported on volume of cerebral infarct after BP elevation. 27,28
The primary outcome in one study was the need for haemodynamic intervention to maintain SBP target 140-180 mm Hg. 29
Intervention
Vasopressors were used as a single agent or in combination in the included twenty-seven studies. Overall, the most commonly used drug to increase BP was the selective-α 1 agonist phenylephrine.
In the ART studies, phenylephrine was used in four studies combined with other vasopressors 25,29–31 and in one study as a single agent. 32 Norepinephrine was used thrice as a single agent 26,33,34 and once in combination. 30 Ephedrine was used three times combined with other vasopressors. 25,29,31 Metaraminol was used as a single agent in one study. 35
Of the studies of no ART, phenylephrine was used in eleven studies as a single agent to elevate BP. Dopamine was used once as single agent 36 and once in combination with other vasopressors. 20 Norepinephrine was used once alone 21 and in one study combined with esmolol and sodium nitroprusside. 37 Dobutamine 38 and epinephrine 24 were as used single agents in one study each.
Saxena et al. tested Diaspirin Cross-Linked Haemoglobin (a haemoglobin based oxygen carrying solution) to increase BP for three days in 85 patients within 18 hours of stroke onset. 39 In one retrospective study, the sympathomimetic drug midodrine was tested in combination with phenylephrine, dopamine and fludrocortisone in 46 patients. 20 Another study reported using vasopressor therapy in patients but did not mention which agent was used. 23
The effects of vasopressors on receptors and pharmacodynamics are listed in Supplementary Table 2.
Haemodynamics
The baseline BP, definition of target achieved and duration of treatment varied between the studies (Table 2). The measures of BP were defined as: SBP (n = 15 studies), Diastolic BP (DBP) (n = 4), Mean Arterial Pressure (MAP) (n = 11) or combined (n = 5).
Table 2.
Haemodynamic characteristics of included studies.
| Study | Baseline BP (mmHg) | Target BP (mm Hg or % as reported) | BP during/end of treatment (mm Hg) | Change in BP (mm Hg or % as reported) | Attained target |
|---|---|---|---|---|---|
| Studies of ART | |||||
| Alcaraz 2019 | Mean SBP ± SD: 143.63 ± 24.8 Mean DBP ± SD: 79.34 ± 13.2 | SBP 140–180 | - | - | - |
| Deng 2019 | - | 160–180 | Mean SBP: A/C: 167 (IQR 150–175 [113–188]) / 139 (IQR 135–143 [115–154]) | diff in median SBP = 28 | 100% |
| Rasmussen 2018 | GA mean SBP ± SD 143 ± 15, MAP 95±8 CS mean SBP±SD 155±20, MAP 101±12 | SBP ≥ 140 and MAP ≥ 70 | - | - | - |
| Schönenberger 2018 | Mean SBP ± SD: 165.3 ± 26.3 Mean DBP ± SD: 93.9 ± 17.2 Mean MAP±SD: 117.7±17.8 | SBP : 140 to 160 | Mean SBP ± SD: 142.2 ± 20.5 No control group | - | - |
| Treurniet 2018 | Median (IQR): SBP: 140 (126–155) DBP: 80 (70–90) MAP: 100 (92–110) | No target | median (IQR) during GA: SBP: 119 (106–130) DBP: 64 (59–71) MAP: 81 (77–91) | - | - |
| Whalin 2017 | Mean SBP ± SD:157.6 ± 29.3 Mean MAP ± SD :108.5 ± 18.6 | SBP 140–180 | - | - | - |
| Mundiyanapurath 2016 | Median SBP: 150.0 (IQR 140.0–170.0) | SBP 140–160 | Median 146.0 (IQR 131.0–159.0) after extubation | - | - |
| Sivasankar 2016 | Baseline MAP ± SD: MAC 103.3 ± 28.1; TIVA 102.0 ± 25.5; Vol 109.5 ± 17.7; Comb 104.8 ± 13.7 | No target | Mean MAP ± SD during procedure: MAC 95.1 ± 16.2; TIVA 89.0 ± 10.8; Vol 95.8 ± 12.2; Comb 94.3 ± 11.0 | - | - |
| Marzan 2004 | Mean SBP ± SD 127 ± 14, Mean DBP ± SD 65 ± 10 | 10–20% > baseline SBP | Mean SBP ± SD: 151 ± 11, Mean DBP ± SD: 75 ± 7 | SBP: 17 ± 10% DBP: 18 ± 18% | |
| Studies of no ART | |||||
| Bang 2019 | Mean SBP ± SD: A/C: 144.2 ± 18.5/146.5 ± 16.8 | >20% from baseline | Mean SPB ± SD A/C: 178.6 ± 18.7/146.5 ± 16.8 | - | 86% |
| Nasi 2019 | Median SBP: A1:166 (144–185) A2:163 (140–189) A3:169 (151–203) | A1: 140–160 A2: 161–180 A3: 181–200 | Median SBP: A1: 153 (IQR 147–160) A2: 163 (IQR 151–170) A3: 178 (IQR 167–184) | - | A1: 70%, A2: 61%; A3: 60% |
| Kang 2017 | Mean SBP ± SD: A/C: 139.1 ± 18.5/150.4 ± 21.5 | >20% from baseline SBP | Mean SBP ± SD A/C: 175.4 ± 21.2/138.41 ± 17.9 (p= <0.0001) | - | 80% |
| Lim 2011 | Mean SBP±SD A/C:161.7 ± 24.8/168.0 ± 31.9 | >20% from baseline | Mean SBP ± SD A/C:168.7 ± 13.6/158.2 ± 17.9 | - | 42% |
| Koenig 2006 | Mean MAP*: A/C: 106/100 | MAP 10–20% > baseline | Mean MAPA/C: 103 ± 14/96 ± 13(p = 0.002) | - | 35% |
| Chalela 2005 | MAP 91 | - | MAP 142 | MAP increase 51 | - |
| Hillis 2004 | Mean MAP ± SD A/C: 97.9 ± 15 | 10–20% MAP increment, max 130 | - | - | 100% |
| Hillis 2003 | Mean MAP: A/C: 94.2 ± 15/103.5 ± 15 | MAP 90–130 | Mean MAP A/C: 115.8 ± 16/108.2 ± 14 | - | 100% |
| Olivia-Filho 2002 | SBP 120 | 160 SBP | - | - | - |
| Schwarz 2002 | Mean MAP ± SD: 83.6 ± 1.6 | > 10 MAP | Mean MAP ± SD:108.9 ± 2.0 | MAP increase 25.3 | - |
| Hillis 2001 | - | Increments of 10% MAP ≤ 130 | - | - | - |
| Hillis 2001 | MAP: 72–88 | MAP 90–100 | - | - | 100% |
| Rordorf 2001 | Mean SBP ± SD Responders: 140 ± 13 Not responders: 141 ± 23 | SBP 160 or > 20% from baseline | Mean SBP ± SD Responders: 174 ± 15 Not responders: - | - | 100% |
| Saxena 1999 | Mean MAP ± SDA/C: 113 ± 14/- | - | Mean MAP ± SDA/C: 134 ± 20/109 ± 16 | - | - |
| Duke 1998 | SBP 110–120 | - | SBP 110–120 | - | - |
| Rordorf 1997 | Mean SBP 152** | - | Mean SBP 156 | - | - |
| Meier 1991 | - | 210–220 | - | - | - |
| Wise 1972 | - | SBP: 150–170 DBP: 85–100 | - | - | 38% |
Heart rate was mentioned in Schönenberger, Marzena and Saxena. * Estimated from fig. 3 in the study publication. ** Calculated from Table 1 in original article.
Systolic blood pressure values varied within 5% of the target value in 16 (47%), within 10% of the target value in 14 (41%) and within 15% of the target value in the remaining 4 (12%) patients.
A: active; ART: acute reperfusion therapy; C: Control; Comb = Combined intravenous and volatile anaesthesia; CS = Conscious sedation; DBP = Diastolic blood pressure; GA = General anaesthesia; HR = Heart rate; MAC = Monitored anaesthesia care; MAP = Mean arterial pressure; PAWP = Pulmonary arterial wedge pressure; SBP = Systolic blood pressure; TIVA = Total intravenous anaesthesia; Vol = Volatile; - = Not reported
Target blood pressure
The target BP was predefined in twenty-one studies, either as a percentage increase from baseline (n = 8), or absolute SBP, DBP, MAP or as combined (n = 13).
Of the nine ART studies which assessed increasing BP, six studies aimed for a predefined SBP target which ranged between: 140 and 180 mm Hg (n = 2)29,32; 140 and 160 mm Hg (n = 2)26,34; 160 and 180 mm Hg (n = 1). 35 One study aimed for a target as SBP ≥ 140 mm Hg and MAP ≥70 mm Hg. 25 In one study, the target BP was set at 10–20% higher from baseline. 33
In the eighteen studies where participants did not receive ART or were not eligible, fifteen reported a predefined BP target. In eight studies, BP target was set as 10%, 20% or a 10–20% increase from baseline with seven assessing MAP and one using SBP. In the remaining seven studies, the BP target varied from SBP 140–220 mm Hg, MAP 90–130 and one study used DBP 85–100 mm Hg.
In one prospective RCT of no ART (n = 218), patients were randomised to three systolic BP (SBP) treatment groups: 140–160 mm Hg, 161–180 mm Hg and 181–200 mm Hg. 37
Duration of treatment
The duration of BP elevation was reported in only one of the ART studies and the median was one hundred and five minutes. 34 From the published data, we were unable to determine treatment duration in other ART studies.
Of the eighteen studies of no ART, thirteen reported on duration of BP. In these studies, the duration of BP elevation ranged from:
5 minutes to 1 hour (n = 3)
1 hour to 24 hours (n = 2)
24 hours to 72 hours (n = 3)
72 hours to 168 hours (n = 1)
> 168 hours (n = 1). In this study, the threshold SBP for neurological deterioration (defined as SBP below which a sustained neurological decline occurred at least twice, and above which the decline was rapidly reversed with increase in BP) was observed in 10 of 33 patients treated with phenylephrine. Patients in this study were treated for up to 24 days.
In one study, phenylephrine was used in 6 patients with ischaemic stroke (within 1 week of onset) with diffusion and perfusion-weighted imaging mismatch (DWI/PWI) mismatch on magnetic resonance imaging (MRI) until functional improvement or target MAP of 130 mm Hg was achieved. 19 In two other studies, patients had their infusion continued and maintained until the motor score on the National Institutes of Health Stroke Scale (NIHSS) remained stable for 24 hours. 40,41 (Table 2)
Blood pressure target achieved
In the ART studies, only one RCT achieved a predefined SBP target (160–180 mm Hg). 35 In this study, patients randomised to treatment with metaraminol had a mean SBP of 167 mm Hg throughout the duration of MT. There was insufficient information to make a judgement whether the predefined target was achieved in the other ART studies.
In the seventeen studies where participants did not receive ART, ten reported reaching the predefined BP target. The number of patients who reached the predefined target varied between studies and the timing within target also varied (Table 2).
In one retrospective case analysis of 100 patients, 46 received various agents to maintain 20% increase from baseline BP. Of these, only 16 patients reached the predefined target. 20 In a case series of acute lacunar ischaemic strokes, phenylephrine was successfully used to increase SBP to 20% from admission in 42% of patients. 40 In four small studies, the predefined BP target was attained in all patients. 18,19,27,42
Collateral circulation
Only one study of MT reported the effect of collateral status (assessed using Computed Tomography angiography) on functional outcome following treatment with norepinephrine to increase BP. 26 This study reported a favourable outcome (mRS ≤ 2) in 60% of patients with moderate or good collateral circulation compared to only 28% of patients with poor collaterals. 26
Amongst the studies where patients did not receive ART, none reported on collateral circulation before, during or after treatment.
Effect of blood pressure elevation on outcomes
Of the ART studies, three studies found no significant association between increasing BP during MT and mRS. 25,34,35 Similar results were observed in one study using a combination of MT, IAT and IVT. 31 In three studies of MT, worse outcomes (mRS > 2) were associated with haemodynamic fluctuations in MAP 30,32 or SBP. 26
One study of MT reported a non-significant improvement in NIHSS compared to baseline after vasopressor therapy. 34
In one study where the primary outcome was haemodynamic intervention during MT, intraprocedural hypotension (SBP < 140 mm Hg) was higher among patients who received conscious sedation compared to no sedation (80% vs 50%). 29 The majority were self-limiting, but 15% of all patients required treatment with phenylephrine and ephedrine to increase BP.
Of the studies of no ART, three studies reported significant improvement in mRS at discharge 40,41 or at day 9041,43 in non-cardioembolic stroke with vasopressor therapy.
Following treatment, changes in NIHSS were reported in eight studies. In each study, patients who had BP elevation had lower NIHSS scores compared to baseline, of which five were statistically significant (p ≤ .05) ( Table 2 ).
One study reported improvement in participant language assessed using the lexical semantics with increase in MAP. 19 The same group reported that in a single patient with large fronto-temporal stroke, language comprehension improved when perfusion of the Wernicke’s area increased with a parallel increase in MAP. 18
Three studies assessed safety as a key endpoint in patients (n = 95) assigned to vasopressors and each reported that BP elevation was safe and feasible. 20–22 The investigators concluded that there was an acceptable rate of complications, considering the severity of stroke and the relative aggressive nature of intervention.
In the study by Saxena et al., functional outcome was significantly worse in the group treated with Diaspirin cross-linked Haemoglobin (mRS 3 to 6 at 3 months: 85% in treated patients compared to 51% in the non-treated group). 39 The authors concluded that differences in baseline stroke severity and dose-dependent increase in endothelin levels could explain the results.
Using perfusion-weighted MRI, one study reported significant reduction in volume of hypoperfused brain after infusion of phenylephrine. 27 This corresponded to a significant improvement in mean NIHSS from 9.3 on admission to 4.8 at day 3. There were no adverse events in the group treated with phenylephrine.
Another group reported a case of a 76-year-old man with fluctuating right-hand clumsiness and mild right leg weakness and MRI confirmed a left anterior cerebral artery infarction with DWI/PWI mismatch. 28 After treatment with phenylephrine, a repeat MRI showed that the perfusion deficit had decreased by 40%.
The authors of three studies (2 case series and 1 prospective RCT) concluded that AIS patients with atherosclerosis were more likely to have post-stroke hypotension and therefore vasopressors were indicated. 20,41,43
Adverse effects
Of the studies with ART, only one study reported adverse events.
Marzan et al. reported a single episode of paroxysmal atrial fibrillation followed by ventricular tachycardia with norepinephrine in one patient, so treatment was discontinued. 33 In this study, 4 patients died; however, it is not clear if these patients received ART.
Eight studies of no ART reported adverse effects with vasopressor therapy. Of these, the most common was intracerebral haemorrhage (n = 44). Other complications included haemorrhagic transformation of infarct, brain herniation and worsening of cerebral oedema (n = 8). Pulmonary oedema and chest tightness were also reported, but less frequent (n = 3 each) with treatment.
Thirty-two patients who received vasopressor therapy and no ART died 21,23,24,37 but it is unclear whether this was directly related to treatment, duration or other complications. Other studies reported on mortality, but did not differentiate between the intervention group versus controls.
Discussion
This review found several retrospective observational studies evaluating BP elevation in AIS and seven small prospective RCTs. Although the studies in this review varied in inclusion/exclusion criteria and methodology, the results show that treatment was feasible. Although no formal meta-analysis was possible, this review found that patients who may be more likely to receive vasopressor therapy included:
stroke from atherosclerosis
large vessel occlusion in the anterior circulation including patients treated with MT, or IAT or combined
ischaemic stroke presenting outside time-windows for IVT
patients with fluctuating or worsening neurological symptoms including lacunar syndrome
A recent trial showed that increasing BP was significantly associated with early neurological improvement and functional independence day 90 (mRS 0–2) in patients with acute lacunar ischaemic stroke. 43 One explanation for the observed results is that increasing BP directly increases blood volume to an end-artery in the brain and improves microcirculation. 41
The relationship between increasing BP in acute stroke and collateral circulation was unclear but two studies reported that a modestly higher target (170–190 mm Hg) was significantly associated with improved collaterals including patients treated with IVT. 44,45 This suggests that there is no ‘ceiling effect’ for BP on collateral status, however, the effects when SBP exceeds 180 mm Hg need further exploration. The results are relevant in AIS, where cerebral autoregulation is impaired and blood flow is directly dependent on systemic BP. The development of collateral circulation is dynamic and failure is associated with increasing infarct growth and poor outcomes. 46 Nevertheless, these data suggest the prognostic importance of hypotension in AIS and a potential therapeutic role for vasopressors.
In this review, it was unclear whether the rates of mortality were in relation to treatment and adverse events were not consistently reported. The studies varied in the target BP for vasopressor therapy; earlier studies tested a SBP target of 150–175 mm Hg and one trial tested different BP thresholds. 37 In this trial, higher rates of symptomatic intracerebral haemorrhage (sICH) were seen in the patient group having a SBP target 180–200 mm Hg. This could be relevant as the association between BP and outcomes after MT indicate a J-shaped or U-shaped curve suggesting a narrow window of therapeutic target and response. 47
Amongst various vasopressors, this review found that phenylephrine was the most commonly used agent with few significant neurological adverse events. Phenylephrine has selective α1-agonist properties and likely to increase BP without substantial cerebral vasoconstriction; the effects are attributed to the low density of α1-receptors in the cerebral vessels. Additionally, phenylephrine has the advantage that it can be given as a bolus and infused though a peripheral line unlike other agents, which generally require a central venous catheter. In addition to phenylephrine, norepinephrine was commonly tested to augment cerebral blood flow and preserve the ischaemic penumbra. The study by Schwartz et al. and the results of a retrospective study 33 indicate that norepinephrine is effective in elevating BP but is more likely to cause adverse cardiac effects due to its β-1 receptor action. Atrial fibrillation was reported in one study with phenylephrine and norepinephrine but one case-note review reported no significant differences in mortality. 22 In one study, cumulative dose of norepinephrine to maintain SBP at 140–160 mm Hg during MT was associated with worse functional outcome (mRS > 2) at day 90. 26 This effect was independent of the duration of MT indicating that norepinephrine could have an adverse effect on collateral circulation. This finding is relevant as norepinephrine is widely used to stabilise BP during interventions. 26
Other vasoactive agents including epinephrine, dobutamine, dopamine and Diaspirin cross-linked Haemoglobin have been tested but the present results indicate less convincing evidence for increasing BP in AIS. Moreover, patients with history of recent congestive cardiac failure, myocardial infarction, unstable angina, bradycardia, uncontrolled hypertension or significant peripheral arterial disease, and treated with digoxin, mono-amine oxidase inhibitors or tricyclic antidepressants will not be eligible for treatment with these agents.
An important issue with the studies was that none clearly defined post-stroke hypotension. Moreover, there was very little information on whether patients had a history of high BP and whether they were on antihypertensives. Furthermore, it was unclear how BP was measured before, during and after treatment. As a result, it is difficult to assess the quality of the reported readings.
The studies in this review varied by examining SBP, MAP and/or DBP as the therapeutic physiological target during pressor therapy. This reflects the uncertainty on which measure best describes regional cerebral blood flow in AIS or may be more than one parameter is needed. MAP is dependent on cardiac output and peripheral vascular resistance and indirectly associated with Cerebral Perfusion Pressure, (i.e. Cerebral Perfusion Pressure = MAP-Intracranial Pressure). It is commonly used in critical care management of patients with subarachnoid haemorrhage or severe traumatic brain injury. 48 The role of MAP in AIS is unclear 49 and the interaction with pressor therapy and effects on outcome needs to be tested in well-designed prospective studies.
Considering the present results, it may be postulated that patients with AIS who might benefit from vasopressor therapy include those:
with sustained SBP < 130 to 150 mm Hg or those with significant drop in BP (>20 mm Hg). This could include those treated with IVT, LVO and low BP
ineligible for IVT to maintain cerebral perfusion until development of collateral circulation or recanalisation of the occluded vessel, but with levels of BP still below those where guidelines recommend antihypertensive treatment. 14,15
severe ipsilateral extracranial or intracranial large vessel occlusion or stenosis
eligible for MT for large vessel occlusion as ‘bridging therapy’ until collateral circulation is restored. This could include those presenting in later-time windows (12–24 hours) with a salvageable ischaemic penumbra.
patients with unstable collateral circulation including those fluctuant or progressive neurological symptoms
MT during GA when periprocedural BP is known to be labile 50
It is essential that future trials report on how BP is measured by including the following information:
equipment including manufacturer, model, method of measurement (invasive or non-invasive) and validity of the equipment used
who measured BP and were they trained, assessed or reassessed
measurements: the number of readings at each time point and site of reading
Future studies evaluating the efficacy of manipulating BP in acute stroke should also account for the volume of the ischaemic core at presentation (marker of salvageable brain and a risk factor for sICH) and the presence or absence of LVO (marker for the potential for BP manipulation to sustain collateral circulation or to maintain brain perfusion where normal autoregulatory mechanisms are impaired).
Conclusions
This updated review found that increasing BP is feasible in AIS (with close monitoring) and to date very few RCTs have been performed. The interpretation of the results of the published studies was complicated by differences in study methods, participants, measures of BP, duration of treatment and outcome measures. The small sample sizes of what were mostly retrospective studies, limit any reliable conclusion of the effects of BP elevation in AIS.
This review found that phenylephrine was the most common drug to increase BP in acute stroke. However, treatment should only be considered after possible causes of low BP are investigated and treated. This review suggests that vasopressor therapy should be ideally restricted to the acute phase of stroke when potential viable ischaemic penumbra exists. Investigations to demonstrate this viable penumbra should be performed before treating patients and beyond the acute phase of stroke.
This review indicates that the risk-benefit of BP elevation (SBP, DBP, MAP individually or combined) in patients with AIS needs to be tested in well-designed prospective RCTs. These studies should assess which measures of BP, (SBP, DBP, MAP) either alone, or combined), which targets best predict the effects of treatment and which clinical scenarios (such as confirmed LVO) are most likely to benefit from pressor therapy.
Supplemental Material
Supplemental meterial, sj-pdf-1-eso-10.1177_23969873221078136 for Pressor therapy in acute ischaemic stroke: an updated systematic review by Torbjørn Austveg Strømsnes, Truls Jørgen Kaugerud Hagen, Menglu Ouyang, Xia Wang, Chen Chen, Silje-Emilie Rygg, David Hewson FRCA, Rob Lenthall, Norman McConachie, Wazim Izzath, Philip M Bath, Permesh Singh Dhillon, Anna Podlasek, Timothy England, Nikola Sprigg, Thompson G Robinson, Rajiv Advani, Hege Ihle-Hansen, Else Charlotte Sandset and Kailash Krishnan in European Stroke Journal
Acknowledgements
We thank Finn Olav Levy, Ullevål University Hospital, Norway for the pharmacological expertise and input to Supplementary Table 2.
APPENDIX.
EMBASE search strategy
1. blood pressure.tw.
2. hypertension.tw
3. acute/
4. stroke.tw.
5. or/1–4
6. and/1–4
7. 1–4.kf.
8. 1–4.ti.
9. (trials/or randomisation/or controlled-study/or multicentre study/or phase-3 clinical trial/or phase-4-clinical trial/or double-blind-procedure/or single-blind procedure/) or ((random*or cross-over or factorial* or placebo* or volunteer*) or ((singl* or doubl* or treble*or tripl*) adj (blind* or mask*).ti.ab and human
10. 1–4 and 9
11. 1–4 and 9.ti.
12. ischaemic stroke.tw/ti or brain ischaemia.m
13. haemorrhagic stroke.tw/ti.
14. intracerebral haemorrhage.tw./ti.
15. blood pressure increase/
16. 1–4 or 12
17. 1–4 or 13
18. 1–4 or 14
19. 1–4 and 14
20. 1–4 and 15
21. cerebral
22. 1–4 or 22
23. vasoactive/
24. 12–15 or 24
25. dopamine.tw.
26. dobutamine.tw.
27. noradrenaline.tw.
28. phenylephrine.tw.
29. 26–29 and or 3, 4, 9, 12,13, 14, 15
30. cerebral blood flow
31. autoregulation
32. stroke outcome
33. 31–33 and or 1–4
34. 31–33 and or 12–14,15
35. cerebral occlusion.tw37. 36 and or 26–29 38. 36 and or 9,12–14,16
36. 36 and or 24
37. large vessel occlusion.tw41. 40 and or 26–29 42. 40 and or 3,4, 9,12,13,14,15
38. 40 and or 24
39. middle cerebral artery occlusion.tw45. 44 and or 26–29 46. 44 and or 9,12–14,16
40. 44 and or 24
41. basilar artery occlusion.tw
42. 48 and or 26–29
43. 48 and or 9,12–14,16
44. 48 and or 24
45. carotid artery occlusion.tw
46. 52 and or 26–29
47. 51 and or 9,12–14,16
48. 51 and or 24
49. adrenaline.tw
50. amphetamine.tw
51. caffeine.tw
52. theophylline.tw
53. diaspirin cross-linked haemoglobin.tw
54. 55–59 and or 3, 4, 9, 12,13, 14, 15
MEDLINE search strategy
1. stroke.mp.
2. infarction.mp.
3. exp brain infarction/
4. exp infarction, anterior cerebral artery/
5. exp infarction, middle cerebral artery/
6. exp infarction, posterior cerebral artery/
7. exp brain ischaemic/
8. cerebral ischaemia.mp
9. haemorrhage. mp.
10. exp. cerebral haemorrhage/
11. cerebral haemorrhage.mp.
12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
13. (vasodilators or haemodilution or haemodilution).mp
14. (dopamine or dobutamine or adrenaline or noradrenaline or phenylephrine or caffeine or amphetamine or caffeinol or theophylline or diaspirin cross-linked haemoglobin or DCLHb).mp
15. 14 or 15
16. 13 and 16
17. (randomised controlled trial.pt or randomised controlled trial.pt or controlled trial.pt or randomised.ab. or placebo.ab. or clinical trials as topic.sh or randomly.ab. or trial.ti.) and humans.sh
18. 17 and 18
19. (middle cerebral artery occlusion. or basilar artery occlusion. or carotid artery occlusion. or large vessel occlusion.).mp.
20. 13 and 20
21. 16 and 20
Science Citation Index search strategy
1. stroke.TS./TI
2. acute stroke.TS./TI.
3. cerebral infarction.TS./TI.
4. brain infarction.TS/TI.
5. brain ischaemia.TS./TI.
6. brain ischema. TS./TI.
7. cerebral ischaemia.TS./TI
8. cerebral haemorrhage.TS./TI.
9. cerebral haemorrhage. TS./TI.
10. cerebral bleed.TS./TI.
11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12. vasodilators or haemodilution or haemodilution.TS./TI.
13. dopamine or dobutamine or adrenaline or noradrenaline or phenylephrine or caffeine or amphetamine or caffeinol or theophylline or diaspirin cross-linked haemoglobin or DCLHb TS./TI.
14. 12 or 13
15. 11 and 14
16. (randomised controlled trial.TI. or randomised controlled trial.T1. or controlled trial.TI. or randomised.T1. or placebo.T1. or clinical trials TI. or randomly.TI. or trial.T1. or single blind.TI or double-blind.TI) and humans.TI.
17. 15 and 16
18. 15 and 17
19. (middle cerebral artery occlusion.TI or basilar artery occlusion.TI. or carotid artery occlusion.TI. or large vessel occlusion.TI.) and humans TS.TI
20. 11 and 19
21. 14 and 19
22. 16 and 19
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures: TAS, MO, TJKH, XW, CC, S-ER, DH, RL, NC, WI, PSD, AP, TE, NS, RA, H-IH, NS, ECS, KK have no disclosures. TGR is a National Institute of Health Research (UK) Senior Investigator. PMB is Stroke Association of Professor of Stroke Medicine and National Institute of Health Research (UK) Senior Investigator.
Guarantor: KK is the guarantor of this study.
Contributorship: This project was conceived by KK and ECS. KK designed the search strategy with input from all authors. TAS, MO, CC independently assessed published and unpublished articles from the electronic searches and with input from ECS, KK selected studies for inclusion. Data were independently extracted by TAS, MO, TJKH and KK checked each value. KK, TAS and TJKH analysed the results. KK wrote and prepared the first draft of the manuscript with input from the writing committee, all of whom approve for the final version of the manuscript to submitted and agree to be accountable for all aspects of the work undertaken in this research project.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Torbjørn Austveg Strømsnes https://orcid.org/0000-0002-7592-3622
Else Charlotte Sandset https://orcid.org/0000-0003-4353-4515
Kailash Krishnan https://orcid.org/0000-0001-7297-7169
References
- 1. CAST . Randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) collaborative group. Lancet 1997; 349: 1641–1649. 1997/06/07. [PubMed] [Google Scholar]
- 2. The International Stroke Trial (IST) . A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet 1997; 349: 1569–1581. 1997/05/31. [PubMed] [Google Scholar]
- 3. Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Int Med 2004; 255: 257–265. 2004/01/30. DOI: 10.1046/j.1365-2796.2003.01291.x. [DOI] [PubMed] [Google Scholar]
- 4. Mulder M, Ergezen S, Lingsma HF, et al. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in mr clean (multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in the Netherlands). Stroke 2017; 48: 1869–1876. 2017/04/23. DOI: 10.1161/strokeaha.116.016225. [DOI] [PubMed] [Google Scholar]
- 5. Maïer B, Gory B, Taylor G, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J American Heart Association 2017; 6 2017/10/12. DOI: 10.1161/jaha.117.006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colivicchi F, Bassi A, Santini M, et al. Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke, Stroke. 2005; 36: 1710–1715. DOI: 10.1161/01.STR.0000173400.19346.bd. [DOI] [PubMed] [Google Scholar]
- 7. Vemmos KN, Spengos K, Tsivgoulis G, et al. Factors influencing acute blood pressure values in stroke subtypes. J Hum Hypertens 2004; 18: 253–259. 2004/03/24. DOI: 10.1038/sj.jhh.1001662. [DOI] [PubMed] [Google Scholar]
- 8. Mullen MT, McKinney JS, Kasner SE. Blood pressure management in acute stroke. J Hum Hypertens 2009; 23: 559–569. DOI: 10.1038/jhh.2008.164. [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Kang DW. Induced hypertensive therapy in an acute ischemic stroke patient with early neurological deterioration. J Clin Neurol 2007; 3: 187–191. 2007/12/01. DOI: 10.3988/jcn.2007.3.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang TS, Jensen MB. Haemodilution for acute ischaemic stroke. Cochrane Database Syst Rev 2014; 2014, CD000103: Cd000103. 2014/08/28. DOI: 10.1002/14651858.CD000103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muzevich KM, Voils SA. Role of vasopressor administration in patients with acute neurologic injury. Neurocritical Care 2009; 11: 112–119. 2009/04/24. DOI: 10.1007/s12028-009-9214-z. [DOI] [PubMed] [Google Scholar]
- 12. Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. The Cochrane Database of Sys Rev 2014; 2014, CD000039–Cd000039. 2014/10/30. DOI: 10.1002/14651858.CD000039.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke: systematic review. Stroke 2006; 37: 1565–1571. 2006/05/06. DOI: 10.1161/01.Str.0000222002.57530.05. [DOI] [PubMed] [Google Scholar]
- 14. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage, Europ Stroke Jour 2021; 6: XLVIII–LXXXIX. DOI: 10.1177/23969873211012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aiyagari V, Gorelick PB. Management of blood pressure for acute and recurrent stroke, Stroke 2009; 40: 2251–2256. DOI: 10.1161/STROKEAHA.108.531574. [DOI] [PubMed] [Google Scholar]
- 16. Geeganage C, Bath PMW. Vasoactive drugs for acute stroke. Cochrane Database of Syst Rev 2010; 2010: CD002839. DOI: 10.1002/14651858.CD002839.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011; 343: d5928. 2011/10/20. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hillis AE, Barker PB, Beauchamp NJ, et al. Restoring blood pressure reperfused Wernicke’s area and improved languageRestoring blood pressure reperfused Wernicke’s area and improved language, J Neurol. 2001; 56: 670–672. DOI: 10.1212/WNL.56.5.670 % [DOI] [PubMed] [Google Scholar]
- 19. Hillis AE, Kane A, Tuffiash E, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain and Language 2001; 79: 495–510. 2002/01/10. DOI: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- 20. Koenig MA, Geocadin RG, de Grouchy M, et al. Safety of induced hypertension therapy in patients with acute ischemic stroke. Neurocri Care 2006; 4: 3–7. 2006/02/25. DOI: 10.1385/ncc:4:1:003. [DOI] [PubMed] [Google Scholar]
- 21. Schwarz S, Georgiadis D, Aschoff A, et al. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke 2002; 33: 998–1004. [DOI] [PubMed] [Google Scholar]
- 22. Rordorf G, Cramer SC, Efird JT, et al. Pharmacological elevation of blood pressure in acute stroke. Clinical effects and safety. Stroke 1997; 28: 2133–2138. 1997/11/22. DOI: 10.1161/01.str.28.11.2133. [DOI] [PubMed] [Google Scholar]
- 23. Wise G, Sutter R, Burkholder J. The treatment of brain ischemia with vasopressor drugs. Sroke 1972; 3: 135–140. DOI: 10.1161/01.STR.3.2.135. [DOI] [PubMed] [Google Scholar]
- 24. Meier F, Wessel G, Thiele R, et al. Induced hypertension as an approach to treating acute cerebrovascular ischaemia: possibilities and limitations. Experiment Pathol 1991; 42: 257–263. 1991/01/01. DOI: 10.1016/s0232-1513(11)80079-4. [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen M, Espelund US, Juul N, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Bri J Anaesth 2018; 120: 1287–1294. 2018/05/26. DOI: 10.1016/j.bja.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 26. Mundiyanapurath S, Stehr A, Wolf M, et al. Pulmonary and circulatory parameter guided anesthesia in patients with ischemic stroke undergoing endovascular recanalization. J NeuroInter Surg 2016; 8: 335–341. DOI: 10.1136/neurintsurg-2014-011523 %J [DOI] [PubMed] [Google Scholar]
- 27. Hillis AE, Wityk RJ, Beauchamp NJ, Perfusion-weighted MRI as a marker of response to treatment in acute and subacute stroke. Neuroradiol 2004; 46: 31–39. 2003/12/16. DOI: 10.1007/s00234-002-0918-4. [DOI] [PubMed] [Google Scholar]
- 28. Chalela JA, Dunn B, Todd JW, et al. Induced hypertension improves cerebral blood flow in acute ischemic stroke. Neurol 2005; 64: 1979. DOI: 10.1212/01.WNL.0000156360.70336.18 %J [DOI] [PubMed] [Google Scholar]
- 29. Alcaraz G, Chui J, Schaafsma J, et al. Hemodynamic management of patients during endovascular treatment of acute ischemic stroke under conscious sedation: a retrospective cohort study. J Neurosur Anesthe 2019; 31: 299–305. 2018/06/05. DOI: 10.1097/ana.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 30. Treurniet KM, Berkhemer OA, Immink RV, et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J NeuroInter Surg 2018; 10: 107–111. DOI: 10.1136/neurintsurg-2017-012988 % [DOI] [PubMed] [Google Scholar]
- 31. Sivasankar C, Stiefel M, Miano TA, et al. Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointervent Surg 2016; 8: 1101–1106. 2015/11/29. DOI: 10.1136/neurintsurg-2015-011998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whalin MK, Halenda KM, Haussen DC, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR 2017; 38: 294–298. 2016/11/05. DOI: 10.3174/ajnr.A4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marzan AS, Hungerbühler HJ, Studer A, et al. Feasibility and safety of norepinephrine-induced arterial hypertension in acute ischemic stroke. Neurol 2004; 62: 1193–1195. 2004/04/14. DOI: 10.1212/01.wnl.0000118303.45735.04. [DOI] [PubMed] [Google Scholar]
- 34. Schönenberger S, Uhlmann L, Ungerer M, et al. Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke 2018; 49: 1451–1456. 2018/05/04. DOI: 10.1161/strokeaha.117.019709. [DOI] [PubMed] [Google Scholar]
- 35. Deng C, Campbell D, Diprose W, et al. A pilot randomised controlled trial of the management of systolic blood pressure during endovascular thrombectomy for acute ischaemic stroke, Anaesthe. 2020; 75: 739–746. DOI: 10.1111/anae.14940. [DOI] [PubMed] [Google Scholar]
- 36. Oliveira-Filho J, Pedreira BB, Jesus PA, et al. [Pharmacologically-induced hypertension in a patient with vertebro-basilar territory ischemia associated with bilateral vertebral stenosis]. Arquivos de neuro-psiquiatria 2002; 60: 498–501. 2002/07/20. [PubMed] [Google Scholar]
- 37. Nasi LA, Martins SCO, Gus M, et al. Early manipulation of arterial blood pressure in acute ischemic stroke (mapas): results of a randomized controlled trial. Neurocri Care 2019; 30: 372–379. 2018/11/22. DOI: 10.1007/s12028-018-0642-5. [DOI] [PubMed] [Google Scholar]
- 38. Duke BJ, Breeze RE, Rubenstein D, et al. Induced hypervolemia and inotropic support for acute cerebral arterial insufficiency: an underused therapy. Surg Neurol 1998; 49: 51–54; discussion 54-57. [DOI] [PubMed] [Google Scholar]
- 39. Saxena R, Wijnhoud AD, Carton H, et al. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 1999; 30: 993–996. 1999/05/07. DOI: 10.1161/01.str.30.5.993. [DOI] [PubMed] [Google Scholar]
- 40. Lim TS, Hong JM, Lee JS, et al. Induced-hypertension in progressing lacunar infarction. J Neurol Sci 2011; 308: 72–76. 2011/06/28. DOI: 10.1016/j.jns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 41. Kang MJ, Yang JW, Lee YB, et al. The role of phenylephrine in patients with small deep subcortical infarct and progressive weakness. J Neurol Sci 2017; 377: 107–111. 2017/05/10. DOI: 10.1016/j.jns.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 42. Hillis AE, Ulatowski JA, Barker PB, et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis 2003; 16: 236–246. [DOI] [PubMed] [Google Scholar]
- 43. Bang OY, Chung JW, Kim SK, et al. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurol 2019; 93: e1955–e1963. 2019/10/28. DOI: 10.1212/wnl.0000000000008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang B, Churilov L, Kanesan L, et al. Blood pressure may be associated with arterial collateralization in anterior circulation ischemic stroke before acute reperfusion therapy. J Stroke 2017; 19: 222–228. 2017/05/04. DOI: 10.5853/jos.2016.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rusanen H, Saarinen JT, Sillanpää N. Collateral circulation predicts the size of the infarct core and the proportion of salvageable penumbra in hyperacute ischemic stroke patients treated with intravenous thrombolysis. Cerebrovas Dis 2015; 40: 182–190. DOI: 10.1159/000439064. [DOI] [PubMed] [Google Scholar]
- 46. Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. 2013/05/09. DOI: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasmussen M, Schönenberger S, Hendèn PL, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol 2020; 77: 622–631. DOI: 10.1001/jamaneurol.2019.4838 %J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012; 43: 1711–1737. 2012/05/05. DOI: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 49. Xiong L, Liu X, Shang T, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry 2017; 88: 520–531. 2017/05/26. DOI: 10.1136/jnnp-2016-314385. [DOI] [PubMed] [Google Scholar]
- 50. Schönenberger S, Hendén PL, Simonsen CZ, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA 2019; 322: 1283–1293. DOI: 10.1001/jama.2019.11455 %J JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental meterial, sj-pdf-1-eso-10.1177_23969873221078136 for Pressor therapy in acute ischaemic stroke: an updated systematic review by Torbjørn Austveg Strømsnes, Truls Jørgen Kaugerud Hagen, Menglu Ouyang, Xia Wang, Chen Chen, Silje-Emilie Rygg, David Hewson FRCA, Rob Lenthall, Norman McConachie, Wazim Izzath, Philip M Bath, Permesh Singh Dhillon, Anna Podlasek, Timothy England, Nikola Sprigg, Thompson G Robinson, Rajiv Advani, Hege Ihle-Hansen, Else Charlotte Sandset and Kailash Krishnan in European Stroke Journal