Abstract
We reviewed the prevalence, the likely aetiopathogenesis, and the management of oro-facial mucocutaneous manifestations of Coronavirus Disease-2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus -2 (SARS-CoV-2). English language manuscripts searched using standard databases yielded 26 articles that met the inclusion criteria. In total, 169 cases (75 females; 94 males) from 15 countries with a spectrum of COVID-19 severities were reviewed. Gustatory perturbations were prevalent in over 70%. Mucocutaneous manifestations were reported predominantly on the tongue, palate, buccal mucosa, gingivae, and lips and included ulcers, blisters, erosions, papillary hyperplasia, macules, glossitis, and mucositis. Ulcerative lesions, present in over 50 percent, were the most common oral manifestation. Lesions resembling candidal infections, with burning mouth, were prevalent in 19%. Petechiae and angina bullosa were generally seen, subsequent to COVID-19 therapies, in 11%. Ulcerated, necrotic gingivae were documented in severely ill with poor oral hygiene. These manifestations, present across the COVID-19 disease spectrum, were commonly associated with the immunosuppressed state and/ or the concurrent antimicrobial/steroidal therapies. In summary, a wide variety of orofacial mucocutaneous lesions manifest in COVID-19. They are likely to be secondary to the disease-associated immune impairment and/or pharmaco-therapy rather than a direct result of SARS-CoV-2 infection per se.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters the human cells via the angiotensin-converting enzyme II (ACE2) receptors and trans-membrane serine protease (TMPRSS2 and TMPRSS4) that are ubiquitously expressed in numerous tissues and organs of the body [1–3]. The oral cavity is thought to be particularly susceptible to SARS-CoV-2 infection due to the high degree of expression of ACE2 receptors in its mucosa lining, the tonsillar crypts, and the salivary gland epithelia [4, 5]. Therefore, it is highly likely that such readily expressed oral targets for SARS-CoV-2 infection may result in now well recognized oral manifestations such as xerostomia and dysgeusia as well as other nondescript mucocutaneous lesions that are reported in COVID-19 patients [5–7].
The array of COVID-19 manifestations in the oral mucosa is expansive and includes ulcers, blisters, enanthems, hemorrhagic lesions, and cheilitis [4, 8]. Several hypotheses have been proposed for their pathogenesis [4]. Brandão and colleagues [9] theorized that interaction between SARS-CoV-2 and ACE2-expressing epithelial cells of the lining mucosa could lead to a surge in the permeability and disruption of oral keratinocyte integrity and the barrier function, leading to epithelial necrosis and ulceration [9]. Others posit that thrombocytopenia, disseminated intravascular coagulation, anticoagulant therapy, or systemic inflammation could cause oral manifestations seen in COVID-19 patients [10–12]. While still others speculate the possibility of primary or secondary vascular inflammation associated with COVID-19 may be the reason for the oral lesions [12, 13]. Apart from these hypothesized reasons, there could be other factors that contribute to the loss of integrity of the oral epithelium, and these include generalized or localized immunosuppression, oral microbiome dysbiosis, and, more importantly, drug therapy [14, 15].
Despite the reported broad spectrum of orofacial manifestations of COVID-19, there are no systematic reviews, to our knowledge, that specifically address their prevalence or aetiopathogenesis. Although very recently [7] has begun a series of live systematic reviews (LSR) on oral manifestations of COVID-19, they are general in nature and do not address in detail the oral mucocutaneous manifestations. Hence, here we review the oral-mucocutaneous lesions either directly linked to SAR-CoV-2 infection or secondary to COVID-19 treatment protocols. For the sake of completion, we also review the general oral manifestations, including taste dysfunction, xerostomia, and burning mouth sensation in SARS-CoV-2, as they too are indirectly linked to mucocutaneous disease.
Methods
Data sources
We (LPS, KSF, and HCN) executed an electronic data search of the English language manuscripts using PubMed via OVID, SCOPUS, and Web of Science databases. Published reports between March 01, 2020, and May 01, 2021, were accessed. We identified a total of twenty-five case reports and a case series.
The Systematic review protocol was registered with the PROSPERO database (CRD42020183714).
Study selection
Inclusion criteria.
Study design: Case reports, case series, observational studies
Population: Cases with suspected, asymptomatic, mild, moderate, or severe COVID-19 infection
Setting: any healthcare setting providing consultation or treatment for COVID-19 infection (hospitals, dental clinics)
Country or date enforced no limitations
Exclusion criteria.
Review articles
Comments, abstracts, and grey literature
Reports presenting incomplete outcome details
Studies evaluating gustatory dysfunction without data on oral mucocutaneous lesions
Studies that do not meet the set study objectives
Search terms
A specific search string was planned for each of the databases, which included the following search terms:
SARS-CoV-2 OR COVID-19 OR Coronavirus OR novel coronavirus disease OR nCoV-19 AND oral manifestations OR oral lesions OR oro-facial manifestations OR oro-facial findings AND gustatory dysfunction OR taste disorder OR taste alteration OR dysgeusia OR hypogeusia OR ageusia AND dry mouth OR xerostomia AND glossodynia OR burning mouth syndrome (BMS) OR glossalgia AND mucosal lesion OR mucocutaneous lesion OR oral mucocutaneous lesion OR oral lesion.
Summary measure
The primary outcome was to review the clinical presentation of oral mucocutaneous lesions expression during SARS-CoV-2 infection, its causality, and temporal association during infection. The secondary outcome included the prevalence of gustatory dysfunctions, xerostomia, and burning mouth sensations in those presenting with oral mucocutaneous lesions.
Electronic data search and analysis. We followed PRISMA (Preferred Reporting Items for Systematic Reviews) guidelines for a systematic and comprehensive approach.
We examined the titles and abstracts of all relevant published reports that met our set inclusion criteria during stage one of the three-staged, electronic data-search and analysis. A full-text review of all the related articles was performed during stage two to view the data comprehensively. A thorough analysis of the full text of the retrieved literature ensured that the eligibility criteria were met and the reported outcomes were according to the set outcome measures. In addition, references of the included reports were examined as a backward search. During stage three: the reviewers (LPS and KSF) extracted and evaluated the data.
After the full-text review, specific points related to the characteristics of each included study were charted. This facilitated in classifying the setting, study design, intervention, and reporting jurisdiction. Besides, the sample size, evaluation time, assessment methods, and study conclusions were systematically examined. Finally, the third reviewer (HCN) cross-checked the data to verify its accuracy. The identified manuscripts were compiled using a bibliographic software tool, Endnote version 9 (Clarivate Analytics, USA). A summary of the characteristics of included reports is provided in (Table 1).
Table 1. Characteristics of the included studies with risk of bias.
| Study (Study design) Country | Sample (n) Age Gender | Comorbid conditions | Oral Manifestation | Oral mucocutaneous lesions | Risk of Bias (acc. JBI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gustatory dysfunction (Dysgeusia/ hypogeusia) |
Xerostomia/ Others |
Ulcer & erosion/ Aphthous-like lesions (ALL) | Herpetiform /zosteriform lesion | White/red plaque | Erythema multiforme-(EM)-like lesions | Petechiae & macular lesions | Vesicles and Pustules/ Angina bullosa like lesions |
Necrotizing periodontal disease | Non-specific lesions (mucositis) | ||||
| Aghazadeh N et al. 2020 (CR) USA | N = 1 9 yrs. F |
Healthy | NM | NM | + | L | |||||||
| Al-Khanati et al. 2020. (CR) Syria | N = 1 24 yrs. M |
NM | NM | Burning sensation on the tongue | + | M | |||||||
| Ansari R et al. 2021 (CR) Iran | N = 2, 56 yrs. F 75 yrs. M |
Case 1: well-controlled HT, COPD Case 2: HT, Diabetes, Obesity, and RF Case 3: Obesity, Parkinson, HT, COPD Case 4: Diabetes, HT Case 5 to 8: NCC | NM | NM | + | L | |||||||
|
Brandão TB et al. 2021 (CS) Brazil |
N = 8, 28–81 yrs. F = 3; M = 5 |
NCC | Present in all cases except case 3 & 4 | NM | + | L | |||||||
| Cebeci K et al. 2020 (CR) Turkey | N = 1 51 yrs. M |
NM | Present | NM | + | + | + | L | |||||
| Chaux‑ Bodard A-G et al. 2020 (CR) France | N = 1, 45 yrs. F |
NCC | Present | NM | + | M | |||||||
| Ciccarese G et al. 2021 (CR) Italy | N = 1 19 yrs. F | Atopic person, mostly on analgesics and antibiotics prescriptions | NM | NM | + | L | |||||||
| Corchuelo J et al. 2020 (CR) Colombia | N = 1 40 yrs. F |
NM | No alteration in taste | Xerostomia/Post-inflammatory pigmentation | + | + | L | ||||||
| Cruz Tapia RO et al. 2020 (CR) Mexico | N = 4 41–55 yrs. F = 3, M = 1 |
Case 1: NM Case 2: NM Case 3: NM Case 4: NM |
Dysgeusia in the male patient | Burning mouth symptom in a male patient | + | + | M | ||||||
| Díaz Rodríguez M et al. 2020 (CR) Spain | N = 3 43–78 yrs. F = 2, M = 1 |
Case 1: NM; history of RAS Case 2: NM; No history of RAS Case 3: NM; No history of RAS |
Case 1&2: present | Case 1&2: burning sensation Case 3: intense xerostomia | + | M | |||||||
|
Dominguez-Santas M et al. 2020 (CR) Spain |
N = 4 19–43 yrs. F = 1, M = 3 |
HT; Renal transplant; On regular immunosuppressants and daily prophylactic cover with Enoxaparin sodium for PVT | NM | NM | + | L | |||||||
|
dos Santos JA et al. 2020 (CR) Brazil |
N = 1 67 yrs. M |
NCC | Hypogeusia | Extremely viscous saliva | + | + | L | ||||||
|
Favia G et al. 2021 Observational descriptive study Italy |
N = 123 Median age 72 yrs. F = 53 M = 70 |
NM | Present in over 80% [Dysgeusia 64% Hypogeusia 27% Ageusia 9%] |
Pain & burning symptoms. Moderate COVID cases (87%) Severe COVID cases (88%) Critical Cases (83%) |
+ | + | + | + | + | + | + | ||
|
Glavina A et al. 2020 (CR) Croatia |
N = 1 40 yrs. F |
NM | Present | Pain and burning sensation | + | + | + | L | |||||
|
Indu S et al. 2020 (CR) India |
N = 1 Age (NR) M |
NM | NM | NM | + | M | |||||||
|
Jimenez-Cauhe J et al. 2020 (CR) Spain |
N = 3 58–77 yrs. F |
Hypercholesterinemia and Coronary heart disease | NM | NM | + | M | |||||||
|
Kämmerer T et al. 2021 (CR) Germany |
N = 1 46 yrs. M |
NCC |
NM | NM | + | L | |||||||
|
Kitakawa et al. 2020 (CR) Brazil |
N = 1 20 yrs. F |
NCC | NM | Severe pruritis | + | L | |||||||
|
Labé P et al. 2020 (CR) France |
N = 2 6 yrs. (M) 3 yrs. (M) |
Healthy | NM | NM | + | + | + | M | |||||
|
Malih N et al. 2020 (CR) Iran |
N = 1 38 yrs. M |
Case 1: NCC Case 2: Diabetes and HT Case 3: Obesity and HT |
Dysgeusia | NM | + | L | |||||||
|
Martín Carreras- Presas C et al. 2020 (CR) Spain |
N = 3, 56–65 yrs. F = 1, M = 2 |
NCC |
Case 1: present Case 2 & 3: NR |
NM | + | + | L | ||||||
|
Patel J et al. 2021 (CR) United Kingdom |
N = 1 35 yrs. F |
NM | NM | NM | + | L | |||||||
|
Sakaida T et al. 2020 (CR) Japan |
N = 1 52 yrs. F |
Diabetes and HT | NM | NM | + | L | |||||||
|
Soares CD et al. 2020 (CR) Brazil |
N = 1 42 yrs. M |
History of MRS | Hypogeusia | Xerostomia | + | + | L | ||||||
|
Taşlıdere B et al. 2021 (CR) Turkey |
N = 1 51 yrs. F |
NCC | NM | NM | L | ||||||||
|
Tomo S et al. 2020 (CR) Brazil |
N = 1 37 yrs. F |
Dysgeusia | Xerostomia. Burning tongue sensation |
+ | L | ||||||||
NR = Not reported; CR = Case report; CS = Case series; NCC = No chronic condition; F = female; M = male; MRS = Melkersson–Rosenthal syndrome; HT = hypertension; COPD = chronic obstructive pulmonary disease; RF = renal failure; PVT = pulmonary venous thromboembolism JBI (Joanna Briggs Institute) critical appraisal tool for CR [low risk of bias (> 70% scores); moderate risk of bias, (scores between 50% and 69%); and high risk of bias (scores were below 49%)].
Quality and overall risk of bias assessment
During stage three, two investigators (LPS and KSF) independently performed the quality assessment of the eligible studies using the Joanna Briggs Institute Critical Appraisal Checklist for (i) case series, (ii) case reports, and (iii) analytical cross-sectional studies’ Critical appraisal checklists’ [16]. The third and fourth reviewers (HCN and BB) were referred to in case of any disagreement. The evaluated studies were documented as low-risk, moderate, or high-risk (Table 1). Case reports with a high risk of bias were excluded from the present review. All reviewers discussed decisions based on cumulative scores. The report was characterized as low risk when the ’yes’ score reached ≥70%, moderate when the score is between 50%-69%, and low when the score is ≤49%.
Results
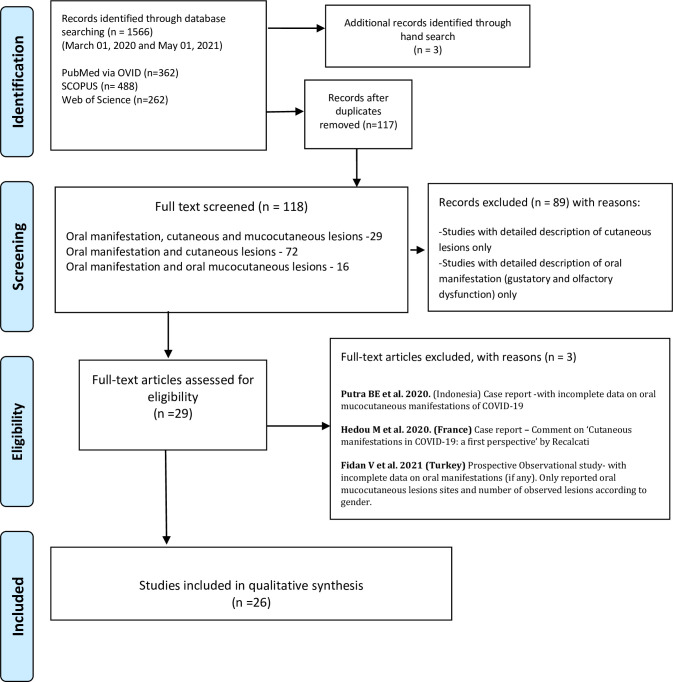
Of the 118 full texts reviewed, only 26 articles met the set inclusion criteria, Fig 1. The reviewed articles encompassed an observational study [12], a case series [9], and 24 case reports [10, 11, 13, 17–37], encompassing a total of 169 patients (75 females; 94 males) with various degrees of COVID-19 disease severities, ranging from suspected, to intensive care bound critical cases.
Fig 1. PRISMA flow chart of the literature search and study selection.
The largest number of reported cases were from an observational study, which included 123 COVID-19 patients with moderate, severe, and critically ill-disease severities [12]. Moreover, most of the reviewed case reports included one to four cases, while the only available case series described oral manifestations in eight COVID-19 patients [9]. Incidentally, the included case reports comprised case studies of three children aged between 3-to-9-years [17, 31] and 19-to-81-year-old adults.
Oral mucocutaneous lesions were almost equal among both females and males with 46% (n = 77) and 54% (92) representation, respectively. In several reports, single or comorbid-chronic systemic conditions such as hypertension, diabetes mellitus, obesity were prevalent (Table 1).
Related to SARS-CoV-2 infection, the spectrum of taste dysfunctions, including dysgeusia, hypogeusia, and ageusia, was prevalent in 74% (125) cases (Table 1). Symptoms of xerostomia were reported in few reviewed case reports with no gender predilection [10, 23, 24, 37]. A Brazilian case report [11] of a 67-year-old male COVID case reported extremely viscous saliva (Table 1). Additionally, we found quite a few reports with symptoms of burning mouth sensations in moderate to severe severity COVID-patients, some of which were associated with suspected candidal infection [12, 13, 18, 24, 26, 37].
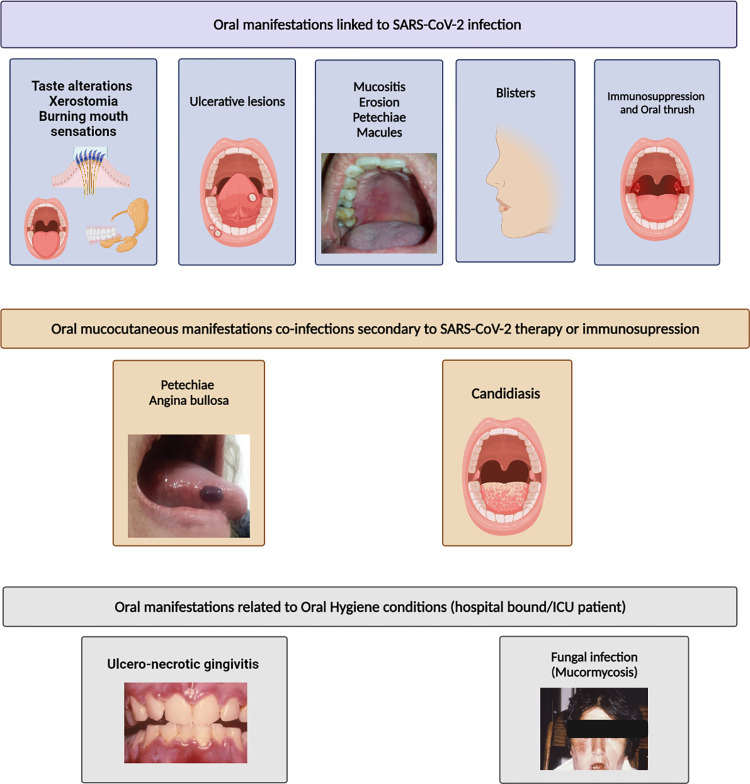
COVID-related oral mucocutaneous manifestations were varied and diverse. Painful, ulcerative lesions were the most predominant (Fig 2), present in over 50% of cases. Other manifestations included blisters, erosive lesions akin to erythema multiforme, macules, non-specific mucositis, and post-inflammatory pigmentation.
Fig 2.
The mucosal lesions differed in quantity, size, and appearance either at single or multiple sites of the oral mucosa. There was no specific intraoral anatomical predilection for the lesion presentation, and an equal proportion of lesions were present in both the keratinized as well as the non-keratinized mucosa. For instance, a 40-years-old female with an asymptomatic COVID-19 infection presented with a painful aphthous ulcer on the attached gingiva, with multiple painless petechiae on her lower lip and a whitish area at the back of the tongue [23]. In several cases, blisters preceded ulcerative lesions [12, 26, 30, 33]. Clusters of herpetiform-like blisters progressed into erythematous ulcerative lesions followed by mild bleeding and pain, in another case [12].
Lesions either similar to or reported as candidal infections were present in 19%, some of which presented with burning oral sensation. Petechiae alone or associated with angina bullosa were expressed either prior or together with other COVID-19 symptoms in 11% [12, 20, 22, 23, 28]. Papillary hyperplasia on the dorsum and lateral borders of the tongue were also common signs observed in about 28% of the cases [12]. In addition, non-specific mucositis, including erythematous-macules and papules, pustules, and plaques, was reported in various intra-oral sites.
Other oral manifestations included fissured or de-papillated tongue, pigmentation, white/red plaques, necrotizing periodontal infection, erosive cheilitis, and spontaneous bleeding (Table 2). Interestingly, a case with a history of Melkersson–Rosenthal syndrome (MRS) had a recurrence of MRS symptoms associated with severe COVID-19 infection [36].
Table 2. SARS-CoV-2 related oral manifestation latency time, duration, and therapy.
| Study | COVID Severity | Appearance of mucocutaneous lesions | Time to resolution of mucocutaneous lesions after treatment | Treatment |
|---|---|---|---|---|
| Aghazadeh et al. 2020 | COVID-Confirmed. Severity NM |
Eruption preceded to a typical COVID‐19 pneumonia. | In a weeks’ time | Treated conservatively with hydration, supplemental oxygen therapy at home |
| Al-Khanati et al. 2020 | COVID-Confirmed. Severity NM |
Aphthous ulcers appear at the same time as COVID-19 symptoms. | NM | NM |
| Ansari et al. 2021 | COVID-Confirmed. Severity NM |
Case 1: Fifth day after the onset of COVID-19 symptoms Case 2: One-week post-hospitalization |
In about one week without scarring in both cases | Topical medications, (mixture of diphenhydramine, dexamethasone, tetracycline, and lidocaine) |
| Brandão TB et al. 2021 |
Case 1&2: Severe Case 3,4,5,7 & 8: Mild Case 6: Moderate |
Case 1: 5th day. Case 2: 4th day. Case 3: 2nd day. Case 4: 5th day. Case 5: 10th day. Case 6: 6th day. Case 7: 8th day Case 8: 8th day of COVID symptoms. |
Case 1: 11 days. Case 2: >15 days. Case 3, 5 & 7: 5 days. Case 4: 7 days Case 6: 8 days. Case 8: 6 days |
Case 1 & 2: HSV-1 detected. Initially, IV Acyclovir for 10 days, with no clinical improvement. Alternatively, for painful oral ulcers PBMT for another 10 days. Case 2: Lip ulcerations did not respond after 15 days of PBMT. Case 3 & 4: Patients receiving PBMT improved. Case 5, 6, 7 & 8: No treatment required. |
| Cebeci K et al. 2020 | COVID-Confirmed. Severity NM |
Ten days after the onset of COVID symptoms | Lesions resolved after few days of antibiotic therapy | Antibiotic therapy |
| Chaux‑ Bodard A-G et al. 2020 | COVID-Confirmed. Severity NM |
Ulcer preceded the COVID-19 confirmatory test. | After 10 days. | NM |
| Ciccarese G et al. 2021 | Mild | About 5 days from the onset of COVID-19 symptoms | After 5 days | IV immune globulins (400 mg/kg) and methylprednisolone (1 mg/kg) |
| Corchuelo J et al. 2020 | Asymptomatic | Three weeks after COVID-19 confirmatory test | Lesion on the tongue resolved after two weeks of anti-fungal treatment. Time of resolution of ulcer NR |
Nystatin for two weeks. Use of CHX 0.12%. Advised washing toothbrush in a NaOCl (1:100 dilution of 5% sodium hypochlorite) for 30 min, rinsing and drying before use. |
| Cruz Tapia RO et al. 2020 |
Case 1&3: (Mild) Case 2: (Hospitalized) Case 4: (NM) |
Case 1: After some 8–9 days of COVID-19 symptoms Case 2: NM Case 3: 2nd day after COVID symptoms Case 4: Same time as COVID-19 symptoms |
Case 1: NM Case 2: NM Case 3: After 5th days Case 4: After 7th days |
Case 1, 2, &3: Nothing specific mentioned Case 4: Topical Cortisone solution and CHX 0.12% mouthwash |
| Díaz Rodríguez M et al. 2020 |
Case 1: Self isolated Case 2&3: Hospitalized |
Case 1: Last two weeks of 56 days COVID symptoms duration. Case 2: Oral symptoms seen after hospital discharge. Case 3: Since hospitalization for COVID-19 symptoms |
Case 1: Lingual de-papillation persisted but ulcers and burning sensation resolved. Case 2: Dysgeusia persists, oral lesion resolved. Case 3: Pseudomembranous lesions and commissural fissures resolved. |
Case 1: Triamcinolone acetonide 0.05%, 3 times a day for 10‐days Case 2: Ointment (neomycin, nystatin, and triamcinolone acetonide) 3 times/d Case 3: Nystatin solution rinses; Ointment (neomycin, nystatin, and triamcinolone acetonide) |
| Dominguez-Santas M et al. 2020 | COVID-Confirmed. Severity NM |
Case 1: 4th days. Case 2: 3rd days. Case 3: 5th days. Case 4: same time as COVID-19 symptoms |
NM | NM |
| dos Santos JA et al. 2020 | Severe | About twenty-fourth days of hospitalization | White lesion resolved after two -weeks. Geographic tongue condition improved from ‘severe’ to ‘moderate’ acc. to severity index after 54 days. |
IV Fluconazole and oral nystatin. CHX (0.12%) alcohol-free mouth rinses with 1% hydrogen peroxide daily |
| Favia G et al. 2021 | Confirmed Moderate, Severe and Critical COVID Cases |
• In (n = 82; 65.9%) had oral lesions together with general COVID symptoms or within a week prior to any COVID therapy. • In seven cases blisters preceded ulcerative lesions • In 92% cases, blisters and ulcerative lesions appear either with general COVID symptoms or within one-week of the onset of COVID symptoms. • Petechiae often in association with angina bullosa appeared mostly after the initiation of COVID therapies. • In seven cases ulcero-necrotic gingivitis was observed in critical cases with poor oral hygiene. |
Within 14 days | In patients with ulcero-erosive lesions Hyaluronic acid gel and 2% chlorhexidine mouthwash/gel (twice a day) for 14 days In patients with cytological diagnosis of candidiasis Miconazole Nitrate twice a day Tranexamic acid for local hemorrhages |
| Glavina A et al. 2020 | Confirmed | About seven days after COVID-19 confirmatory test | After 3 weeks | Systemic Acyclovir therapy for five days. Local therapy (antiseptic, nystatin, panthenol, local anesthetic) for two weeks. |
| Indu S et al. 2020 | Asymptomatic | Same time as the initial manifestation of COVID-symptom | After 10 days | NM |
| Jimenez-Cauhe J et al. 2020 | COVID-Confirmed. Severity NM |
Lesions appeared after Hospital discharge. | NM | Systemic corticosteroids |
| Kämmerer T et al. 2021 | Severe | Three days after extubation from the ICU (secondary herpetic gingivostomatitis in the context of COVID‐19 infection) | NM | Oral acyclovir therapy 400 mg five times daily |
| Kitakawa et al. 2020 | Confirmed Severity NM |
At the same time as the initial manifestation of COVID-symptom | After 14 days | Nebacetin (Neomycin sulfate) ointment for 2 days |
| Labé P et al. 2020 |
Case 1: Confirmed Case 2: Suspected |
Case 1: After 14 days of COVID-19 symptoms Case 2: NM |
Case 1: After 2 weeks Case 2: NM |
Case 1: NM Case 2: Intravenous gamma globulin |
| Malih N et al. 2020 | COVID-Confirmed. Severity NM |
Tonsillar aphthous lesion precedes COVID symptoms. | NM | Acetaminophen for pain control |
| Martín Carreras- Presas C et al. 2020 |
Case 1: Suspected Case 2: Suspected Case 3: Mild |
Case 1: About 2–3 days. Case 2: NM Case 3: After a month. |
Case 1: Resolved after 10 days. Case 2: 1 week. Case 3: After three days |
Case 1: Valacyclovir 500 mg 3 times/d for 10 d, with topical CHX and hyaluronic acid. Case 2: Antiseptic mouthwash. Case 3: Hyaluronic acid and CHX mouthwash. prednisolone 30 mg/d |
| Patel J et al. 2021 | Suspected | Oral symptoms appear same time as COVID-19 symptoms. | After 5-days | Metronidazole 400mg 3 times for 5 days and 0.12% chlorhexidine mouthwash twice daily for 10 days |
| Sakaida T et al. 2020 | Severe | Erythematous lesions and erosions on the lips preceded a week before COVID-19 symptoms. | After 5-days | Oral prednisolone (20 mg/day) |
| Soares CD et al. 2020 | COVID-Confirmed. Severity NM |
NM | In three-weeks’ time | Dexamethasone, dipyrone |
| Taşlıdere B et al. 2021 | Severe | COVID-19 infection can be considered among the causes of recurrence of the Melkersson–Rosenthal syndrome | NM | Hydroxychloroquine; Azithromycin, and steroid therapy |
| Tomo S et al. 2020 | Confirmed Asymptomatic |
About 9th day of COVID-19 symptom | After 2 weeks | Chlorhexidine (0.12%) mouthwash |
The majority of the oral lesions were painful. In addition, a case of a young female patient with symptoms of severe pruritus with painful, herpetic-like-lesion in the median lower lip area was also reported [30] (Table 2).
The collated cumulative data indicate, in general, oral lesions show no specific gender or age predilection. The common oral sites of presentation of the lesions, in descending order of prevalence, were the dorsum of the tongue, followed by the hard palate, soft palate, lips, buccal/labial mucosa, gingiva, tonsillar region, and the commissures (Table 2).
In terms of the temporality of presentation, oral mucocutaneous lesions preceded other COVID-19 symptoms in 2.4% (n = 4) of reported cases [17, 21, 32, 35], while in 51%, (n = 86) cases the lesions appeared simultaneously, or within a week of systemic SARS-CoV-2 symptoms [9, 12, 13, 18, 19, 22, 25–27, 30, 34]. Nonetheless, in some cases, this latency period stretched from two-to-three weeks after the onset of systemic COVID-19 symptoms [11, 24, 29, 31, 33, 37]. However, in two reported instances [24, 28], oral lesions emerged after the hospital discharge. Curiously, in a mild and asymptomatic case of COVID-19, the oral lesions manifested even after three weeks [23] and one month [33] after disease diagnosis (Table 3). Likewise, the time to resolution of the oral lesions also varied, from five days to two weeks. Few reports also documented persistent lingual de-papillation, dysgeusia, and geographic tongue even after the resolution of oral lesions following therapy for COVID-19 (Table 3).
Table 3. SARS-CoV-2 related oral mucocutaneous lesions clinical appearance, locations, and differential diagnosis.
| Study | Location of oral muco-cutaneous lesion | Sign & Symptoms | Differential Diagnosis |
|---|---|---|---|
| Aghazadeh N et al. 2020 | Lips, anterior tongue, and buccal mucosa | • Vesicular/herpetiform oral eruptions | Hand‐foot‐mouth disease; Atypical herpes simplex infection; Mycoplasma‐induced rash and mucositis; Erythema multiforme; Drug eruption |
| Al-Khanati et al. 2020. | Lower lip | • Aphthous‐like ulcers on the mucosa which became enlarged and painful over 3 days. • Burning sensation related to the tongue. • Halitosis. |
NM |
| Ansari R et al. 2021 |
Case 1: Almost the entire hard palate Case 2: Anterior part of the tongue |
Case 1: Painful ulcers of varying sizes; irregular margins, with red, non‐hemorrhagic background Case 2: Painful small ulcers; irregular margins with red, and non‐hemorrhagic background |
Herpes simplex virus type 1 and type 2 |
| Brandão TB et al. 2021 |
Case 1 & 2: Upper and lower lip mucosa, anterior dorsal tongue Case 3: Lateral border of tongue and anterior hard palate Case 4: Upper and lower lip Case 5: Apex and lateral borders of the tongue Case 6: Tonsillar pillar Case 7: Ventral portion of the tongue Case 8: Upper and lower labial mucosae and lateral border of the tongue |
Case 1, 3 & 4: Painful aphthous like ulcers with necrosis Case 2: Painful hemorrhagic ulcers Case 5–8: Painful aphthous like ulcers Case 6: Hemorrhagic ulcers with necrotic areas |
NM |
| Cebeci K et al. 2020 | Oropharynx, hard and soft palate | • Erythematous surface, few petechiae, numerous pustular enanthema | NM |
| Chaux‑Bodard A-G et al. 2020 | Dorsal surface of the tongue | • Initially, painful inflammation, followed by painful erythematous macula, then an asymptomatic irregular ulcer | NM |
| Ciccarese G et al. 2021 | Lips (inner surface), palate and gingiva | • Erosions, ulcerations, and blood crusts on the inner surface of the lips. • Palatal and gingival petechiae |
NM |
| Corchuelo J et al. 2020 | Lower lip, attached gingiva of the lower left first premolar, and tongue | • Multiple painless petechiae on the lower lip. • Whitish area at the back of tongue. • Painful aphthous ulcer on the attached gingiva of premolar |
Whitish area on the tongue suggestive of Candidiasis. |
| Cruz Tapia RO et al. 2020 | Hard palate, tongue |
Case 1&3: asymptomatic non-bleeding purple bulla (hard palate & tongue, respectively). Case 2: macule and papule‐plaque (hard palate). Case 4: Erythematous small macules (hard palate) |
Angina bullosa hemorrhagic‐like lesion |
| Díaz Rodríguez M et al. 2020 | Tongue, palate, and commissure |
Case 1: aphthous‐like lesions with tongue de-papillation Case 2: burning mouth sensation and unilateral commissural fissures. Case 3: lesions compatible with pseudomembranous candidiasis and angular cheilitis |
NM |
| Dominguez-Santas M et al. 2020 | Buccal and labial mucosa; mucogingival junction; ventral surface of tongue | Case 1 to 4: single to cluster of aphthous ulcers | Herpes simplex virus, Epstein‐Barr virus, and Cytomegalovirus |
| dos Santos JA et al. 2020 | Tongue | • White plaque, with multiple pinpoint yellowish ulcers on the dorsum of the tongue dorsum. | Oral candidiasis; Herpetic recurrent oral lesions |
| Favia G et al. 2021 | Tongue, hard palate, lip, buccal mucosa, soft palate, gingiva |
Moderate COVID: Geographic tongue (5); Fissured tongue (4); Painful ulcerative lesion (51); Blisters (14); Hyperplasia of papillae (33); Angina bullosa (8); Candidiasis (18); Ulcero-necrotic gingivitis (1); Petechiae (4) Severe COVID: Geographic tongue (2); Fissured tongue (1); Painful ulcerative lesion (11); Blisters (5); Hyperplasia of papillae (13); Angina bullosa (2); Candidiasis (4); Ulcero-necrotic gingivitis (2); Petechiae (6) Critical Cases: Painful ulcerative lesion (3); Hyperplasia of papillae (2) - Angina bullosa (1); Candidiasis (6); Ulcero-necrotic gingivitis (4); Petechiae (4); Spontaneous oral hemorrhage (1) |
NM |
| Glavina A et al. 2020 | Lip, hard palate, and tongue | • Herpetic vesicles on the lips and hard palate • White hairy tongue and non-specific white lesion on the ventral surface of the tongue • Pain and burning sensation in the oral cavity |
NM |
| Indu S et al. 2020 | Labial mucosa and ventral surface of the tongue | • Unilateral, painful, shallow, round to oval shape ulcers surrounded by an inflammatory halo | Herpes Zoster infection |
| Jimenez-Cauhe J et al. 2020 | Palate | Macules and petechiae | NM |
| Kämmerer T et al. 2021 | Oral mucosa | Multiple, sharply circumscribed, painful ulcerations covered by yellow–grey membranes | Herpes simplex virus (HSV)‐1/2 antibodies, Behçet disease (have no prior history of Herpes) |
| Kitakawa et al. 2020 | Lip | A herpetic like-lesion in the median lower lip semi-mucosa, with severe pruritis | Recurrent Herpes |
| Labé P et al. 2020 et al. 2020 | Lip, oral mucosa, tongue |
Case 1: Severe painful, erosive cheilitis, diffuse gingival erosions with thick hemorrhagic crusts Case 2: Painful cheilitis and glossitis |
Case 1: Herpes simplex virus (HSV) Case 2: COVID‐19‐associated Kawasaki disease |
| Malih N et al. 2020 | Tonsillar region | Erythema and painful aphthous lesion on the left tonsil | Herpes simplex lesions |
| Martín Carreras- Presas C et al. 2020 |
Case 1 & 2: Hard palate Case 3: Inner lip mucosa and gingiva |
Case 1: Painful, orange‐colored ulcers with an erythematous halo. Case 2: Multiple unilateral, painful, pinpoint yellowish ulcers with an erythematous halo. Case 3: Painful blisters on lip mucosa and desquamative gingivitis |
Case1 & 2: Herpes simplex lesions Case 3: Erythema multiforme |
| Patel J et al. 2021 | Gingiva in both the maxillary and mandibular labial sextants | • Severe halitosis with generalized erythematous and edematous gingivae. • Necrotic interdental papillae. • Gingival sulcus bleeding without any provocation |
Necrotizing gingivitis |
| Sakaida T et al. 2020 | Lower lip and buccal mucosa | Erythematous lesions and erosions | NM |
| Soares CD et al. 2020 | Buccal mucosa, hard palate, tongue, and lips | Painful, scattered, ulcerated lesion, reddish macules of varying sizes | NM |
| Taşlıdere B et al. 2021 | Face, lips, and tongue | • Edema in the right lower lip • Right facial paralysis • Fissured tongue |
Cytomegalovirus; Herpes simplex virus, Epstein–Barr virus; Coxsackie virus infection |
| Tomo S et al. 2020 | Tongue and soft palate | • Oral mucositis characterized by diffuse, bilateral erythema with de-papillation in the borders of the tongue. • Burning sensation in the borders of the tongue and soft palate |
NM |
Depending on the type of lesion, a wide range of therapeutic measures was employed for their management (Table 3). These included chlorhexidine mouthwashes, topical or systemic corticosteroids, antibiotics, antifungals, antiviral drugs alone or in combination with antibiotics, and drugs for pain relief. In one case, a young 9-year-old female patient’s vesicular herpetic eruptions resolved approximately a week after conservative treatment using only hydration therapy [17]. Interestingly, photo-biomodulation (PBMT) therapy was employed for pain management of oral ulcers in few other cases [9] (Table 3).
Discussion
The receptor-binding domain of SARS-CoV-2 has an intense affinity for ACE2 -functional receptors [38]. The ubiquitous presence of ACE2 in the lining mucosa of the nose, lung, pharynx, oral mucosa, and salivary glands, as well as multiple organ systems of humans, make them not only essay portals of viral access to the body but also vulnerable to viral damage [39]. The varied oral-facial mucocutaneous manifestations of COVID-19 reported in the literature and reviewed here could, therefore, be due either to the primary, focal damage caused by the virus at the point of tissue entry, and/or a reflection of secondary damage due to the generalized effect of systemic SARS-CoV-2 infection and the ensuing immunologic abnormalities [4, 8]. We discuss our findings under two major categories, generalized oral manifestations, and muco-cutaneous manifestations. The former, which addresses i) gustatory dysfunction ii) xerostomia, and iii) burning mouth, is included in the narrative as they are intimately linked to mucocutaneous disease.
Generalized oral manifestations
Gustatory dysfunction
From the beginning of the SARS-CoV-2 pandemic, multiple reports from several regions of the world indicated that aberrations of taste sensation either as dysgeusia or ageusia were particularly common in diseased individuals and presented mainly as a premonitory or an early symptom of the disease [40, 41] (Fig 2). The studies reviewed here corroborate these data from the early stage of the pandemic and indicate that either the total acute loss of taste–ageusia, or alterations in the taste sensation–dysgeusia or hypogeusia, are a frequent symptom of SARS-CoV-2 infection. However, contradicting the previous literature [6, 42, 43], we found no specific gender predilection in the alterations of taste sensation in the collated and reviewed data.
A number of postulates have been proposed for the pathogenesis of the gustatory disorders associated with COVID-19. Some contend that ACE2 receptors on lingual keratinocytes and supporting cells of taste buds are the first to be infected, leading to virus-induced cell dysfunction and death, and subsequent alterations of taste perception [44]. A postmortem report by Favia et al. (2021) supports this hypothesis. They provide histopathological data of lingual papillae of COVID-19 patients with enlarged, inflamed papillae of the lining mucosa and heavy monocytic/ lymphocytic infiltrates, as well as vascular hyperplasia in tissues subjacent to taste buds [12]. Furthermore, it is noteworthy that ACE2 expression is higher in the tongue than in other oral mucosal tissues [45].
Loss of smell or anosmia is simultaneously present in most COVID-19 patients with dysgeusia/ageusia [40, 41]. Hence some have surmised that, as gustatory and olfactory sensations are closely linked, the viral damage to the olfactory epithelium may either fully or partly account for the acute onset ageusia [46, 47]. Indeed, profuse expression of ACE2 receptors is seen in the olfactory neuroepithelium, that are infected during COVID-19.
Other explanations for dysgeusia have been offered, particularly when it persists after the initiation of COVID-19 therapy and after hospital discharge. These include afflictions of the peripheral nervous system innervating the taste buds [48] and the possible side effects of medications such as antibiotics, corticosteroids, and immunosuppressants, prescribed for managing COVID-19 [48, 49].
Xerostomia
In the reviewed reports, gustatory dysfunction (dysgeusia/hypogeusia) was present either as the sole oral manifestation or associated with xerostomia and/or burning mouth sensation in 69.8 percent of the patients (Fig 2) In a recent report, Huang et al., using single-cell RNA (scRNA) analyses, elegantly illustrated the rich expression of ACE2 and transmembrane serine protease (TMPRSS) receptors for SARS-CoV-2 in the acinar and duct epithelial cells of both the major and minor salivary glandular tissue derived from COVID-19 patients [5]. Such salivary gland invasion by SARS-CoV-2 may have two significant consequences: first, the infection per se may affect the functionality, and the quality and the quantity of the salivary secretions, and second, saliva could, coincidentally, serve as a rich source of infectious particles promoting the spread of infection.
Interestingly, one report described a COVID-19 patient who complained of extremely viscous saliva [11]. Therefore, it is tempting to speculate that the latter manifestation could be due to the primary viral infection affecting the glandular tissue of the major, serous salivary gland (parotid), leading to the predominantly mucus/viscous secretions [50]. Nevertheless, the pathobiology of xerostomia secondary to the SARS-CoV-2 infection remains to be determined through further studies.
As mentioned above, a few of the reviewed cases simultaneously presented with both dysgeusia and xerostomia [10, 11, 37]. Physiologically, the taste sensation is a critical central stimulant for initiating salivary secretions [51]. It could, therefore, be postulated that gustatory dysfunction noted in a vast proportion of COVID-19 patients could be bimodal in nature: primarily neuronal, affecting the physiology and functionality of the taste buds due to the well-known neuro-tropic and invasiveness of the virus [52], and a secondary effect due to the coincidental xerostomia. However, more data are needed to ascertain the relationship between xerostomia and salivary gland dysfunction in COVID-19.
Burning mouth
It is known that the burning mouth sensation usually presents in a majority of patients with either dysgeusia and/or xerostomia [53, 54]. Yet, the burning mouth sensation is a rather non-specific disease entity related to a multiplicity of other conditions, including psychiatric disorders, candidal infections, diabetes, various drugs, vitamin and/or mineral deficiencies [53]. Though not specifically recorded, one or more of these may have been etiologically involved in the reviewed cohort of patients who complained of burning mouth. For instance, it is noteworthy that a few with burning oral sensations showed signs of candidal infection [12, 24].
Oral mucocutaneous manifestations
Viral infections in the oro-facial regions are relatively common. They mostly manifest as maculopapular lesions and ulcers of various shapes and sizes, in addition to neurologic manifestations, including facial palsies [55]. Herpes group viruses, such as herpes simplex, herpes zoster, cytomegalovirus, Epstein-Barr virus, are the commonest viruses that infect the oro-facial region [55, 56]. Recrudescence of viral infections, mostly due to the herpes group of viruses, may also occur as secondary diseases, due to generalized immunosuppression consequential to radiotherapy, cytotoxic or steroid drugs, or in systemic infections such as the human immunodeficiency virus (HIV) disease that incapacitates the immune system in general [57]. Additionally, viruses such as the human papillomavirus (HPV), present as innocuous passengers in the salivary virome of a high proportion of individuals [58], may also induce dysplastic and neoplastic changes of the mucosal epithelium, as well [59].
Whilst the foregoing viruses are common agents affecting the oro-facial region, a string of novel viruses that cause respiratory tract infections in humans, including Severe Acute Respiratory Syndrome Coronavirus– 1(SARS-CoV-1), Middle East Respiratory Syndrome Coronavirus (MERS‐CoV), and H7N9 influenza A virus, a swine‐like influenza H3N2 variant virus, and a human adenovirus 14p1, are now known to affect the oral cavity with ill-defined, nondescript manifestations [60]. Furthermore, it now appears that the newest addition to this list is SARS-CoV-2 that may induce both primary and secondary oral infections, as noted in the reviewed publications.
Clearly, the oro-pharyngeal region appears to be a major reservoir of the SARS-CoV-2 in the infected cohorts [4]. In addition, molecular studies indicate the profuse expression of ACE2 receptors in the epithelia of the dorsal lingual surface, hard palate, soft palate, lips, buccal, and labial mucosa [4, 39]. This was reflected by the predominance of mucocutaneous lesions in these anatomical locales. The lesions, in general, had a nondescript expression profile, ranging from aphthous-like ulcerations, erosions, mucositis, pigmentations, hemorrhagic crusts, desquamative gingivitis, as well as cheilitis. It is, however, difficult to state with any degree of certainty whether the lesions were due primarily to SARS-CoV-2 infection or a secondary effect consequential to the general debility and/or drug therapy, or possible immune dysfunction seen in these patients.
A case in point is the enanthems observed by some that were categorized as petechial, erythematous, vesicular, macular, or macular with petechiae [4, 61]. This is in contradistinction to enanthems in other viral infections such as herpangina, measles, human herpesvirus (HHV) infections, and roseola infantum that present with specific, pathognomonic characteristics identifiable with the disease entity [61]. Thus, for example, enanthem in herpangina appears as small vesicular or ulcerative lesions confined to the posterior oropharyngeal region, or in the case of roseola infantum, ’Nagayama spots’ presenting as erythematous papules on the soft palate and at the base of the uvula [62].
Oral ulcers
Oral ulcers were the most frequent lesion reported in the reviewed COVID-19 cases. The pathogenesis of these lesions remains speculative at present. Disruption of the superficial epithelial barrier of the oral mucosa due either to viral invasion and/or the collateral damage due to the host immune responses against the viral antigenic components of the mucosal epithelium is yet to be resolved. For instance, increased level of tumor necrosis factor (TNF)-α noted in COVID-19 infection [63] may lead to resultant IL-1, and IL-6 production and chemotaxis of inflammatory cells into the viral-laden epithelium, which in turn may cause apoptosis, necrosis and consequent ulceration [64, 65]. The work of Favia et al. [12] lends some credence to this notion, as they observed complete disruption of the epithelial covering, vascular hyperplasia, perivascular hemorrhage, and lymphomonocytic infiltrations in the sub-epithelial tissues in the central necrotic area of the ulcerated regions, in addition to thrombosis of small and medium-sized vessels in subjacent tissues. Histopathological observations of oral mucosal lesions in COVID-19 by other workers also attest to the presence of inflammatory infiltrates in the affected epithelium [10, 19, 28].
Angina bullosa and petechial lesions
The exaggerated inflammatory response evoked by SARS-CoV-2 is now recognized as a harbinger of vascular endothelial dysfunction and subsequent thrombus formation [66, 67]. Hence, anticoagulants remain an essential management tool for the thromboembolic phenomena seen in COVID-19 patients [67]. Therefore, petechiae and angina bullosa in the oral mucosa reported in several studies [12] could conceivably be linked to such anticoagulant therapies. Furthermore, in several reports, petechial spots were observed on the hard and soft palate, lips, and the oropharynx [12, 22, 24, 25, 30]. However, as these reports did not indicate whether the patients were undergoing anticoagulant therapy prior to such presentation, further studies are required to confirm or refute the aforesaid contention.
Oral mycoses
The portrayal of an asymptomatic COVID-19 patient with oral candidiasis by Corchuelo and Ullioa (2020) [23] brings to light another facet of COVID-19 manifestation. The latter group opined that generalized immunosuppression might lead to opportunistic fungal infections even in the absence of overt signs of COVID-19. A subsequent report has described two COVID-19 patients with esophageal candidiasis but with no history of any antibiotic or immunosuppressant use [68]. Nevertheless, they too surmised that the profound and acute T-cell immunologic deficit due to SARS-CoV-2 infection might have led to the fungal infections [68].
Another multicenter study by Salehi and colleagues (2020) [69] reported the risk of opportunistic fungal infection in ventilation supported COVID-19 patients with respiratory distress syndrome, receiving broad-spectrum antibiotics and/or corticosteroids, both of which are well known to cause oral candidiasis [70]. Others have also published reports of hospitalized COVID-19 patients having oral candidiasis, which improved with antifungal medication [11, 12, 24]. Interestingly, a case series of COVID-19 patients with angular cheilitis, known to be due to mixed candidal-bacterial infection [71] is also noteworthy in this context [72].
Incidentally, mucormycosis, colloquially known as `black fungus disease,`is a relatively common presentation, mainly reported from the Indian sub-continent [73], and may present as oral ulcerations in post-COVID-19 states after recovery [74, 75]. However, our reviewed reports did not encompass any cases of oral mucormycosis, possibly due to its very recent recognition in COVID-19 patients, particularly in the South Asian region. Apart from candidiasis and mucormycosis, the third common fungal disease reported in COVID-19 patients is pulmonary aspergillosis [76]. Yet again, we did not note any oral mucocutaneous lesions caused by this fungus in the current review. The foregoing strongly suggests the not uncommon prevalence of opportunistic oral fungal coinfections superimposed on systemic viral disease, not unlike that seen in HIV infection, where oral candidiasis is a predominant early manifestation [77].
Taken together, these observations support the hypothesis that oral ulcerations in COVID-19 may be due to a triad of effects, first, as a direct result of the viral damage to the epithelium, second, an indirect effect of the immune dysregulation of the mucosal epithelium, and third due to the underlying therapeutic regimens such as corticosteroids used in the disease management. However, deciphering the role of each of these entities need to be performed when a more extensive database of COVID-19 patients is available, and when such cohorts are followed up for a significant period.
Periodontal health
Regarding the periodontal health of the reviewed cohort, we noted in some individuals the relatively uncommon condition of necrotizing ulcerative gingivitis [12, 34, 78] (Fig 2). This is a painful and precipitously progressive disease involving free/attached gingivae and the alveolar mucosa, with ulceration, pain, necrosis, and bleeding. The condition may occur as a complication of chronic gingivitis when oral hygiene is neglected, especially in debilitated individuals, and/or when the systemic immune system is compromised, as in the case of HIV disease [79]. In general, the condition was documented in several critically ill COVID-19 patients with poor oral hygiene, and further, follow-up studies are required to ascertain the pathogenesis of such lesions.
Treatment algorithms
A well-recognized, standard therapeutic algorithm for managing the oral manifestations of COVID-19 is unavailable, as yet, most likely due to the novelty of the disease entity. This, not surprisingly, has led to a variety of management regimens in different jurisdictions (Table 2). Most of the reviewed cases were managed by topical steroids alone or combined with antibacterial and antivirals, mainly as ingredients in ointments (Table 2). For instance, Glavina et al. (2020) [26] reports systemic Acyclovir combined with a topical antiseptic, a polyene antifungal nystatin, panthenol (a skin antiseptic), as well as a local anesthetic for treating painful herpetic vesicles, and non-specific white lesions in a 40-years old female. Likewise, Ansari et al. (2021) [19] and Diaz Rodríguez et al. (2020) [24] used topical ointment with an assortment of anti-fungal, antibacterial, and steroidal suspensions with painkillers to manage non-specific complaints resembling candidiasis on the lingual surface. On the contrary, in a major departure from the conventional treatment, Brandão et al. (2021) used photo biomodulation therapy (PBMT) for managing painful oral ulcers, with some degree of success [9]. In two reports, ulcero-erosive lesions were treated using hyaluronic acid gel with chlorhexidine [12, 33]. The former also used tranexamic acid to arrest bleeding in ulcers [12].
Stand-alone therapies used by clinicians include the administration of systemic antibiotics by Cebeci et al. (2020) for treating pustular oral manifestation [20] and the application of neomycin ointment to treat herpetiform and zosteriform lesions [30]. Jimenez-Cauhe (2020) used systemic corticosteroids for erythema multiform-like manifestations that appeared in a severe case of COVID following hospital discharge [28]. Others have used various conservative techniques for the management of oral manifestations, such as home-based hydration and supplemental oxygen therapy [17] or chlorhexidine mouthwash alone [37].
The foregoing, wide, and disparate variety of medications used to treat oral manifestations of COVID-19 testifies to the urgent need for a well-formulated, consensus therapeutic regimentation for managing oral manifestations of COVID-19.
Data quality and limitations
The critical appraisal of the reviewed data indicates that most reports could be categorized as having a low to moderate risk of biases (Table 1). We noted, for instance, deficiencies in the self-assessment reports of subjectively perceived taste disorder symptoms. The accuracy of such self-diagnosed symptomatology can introduce intrinsic response biases unless confirmed by an experienced clinician using standardized and validated instruments. Also, some authors did not explicitly state the degree of severity of COVID-19 of their patients. Furthermore, most case reports of mucosal lesions were devoid of histopathological information despite categorization as a specific disease entity.
Conclusions
Oral manifestations of COVID-19 patients encompassing the current review varied considerably for a number of reasons. Apart from those mentioned above, these included the non-specific reference point descriptors. For instance, in several reports, hospital admission, treatment commencements, hospital discharge, other respiratory and systemic manifestations are utilized as reference points to describe the onset of oral signs and symptoms, leading to discrepant temporal associations of COVID-19 onset and oral manifestations. This, notwithstanding, our review highlights that in over half of the COVID-19 cases, oral mucosal manifestations either precede or appear simultaneously with symptoms of SARS-CoV-2 infection, suggesting an association, and not necessarily causation, between the viral infection and oral disease. Furthermore, apart from the now well-known taste disorders in COVID-19 [80], the pathogenesis of mucocutaneous lesions, such as candidiasis and hemorrhagic lesions (petechiae/angina bullosa), is likely to be secondary to antimicrobial or steroidal or anticoagulant therapies, and/or the COVID-19 associated immune impairment, rather than a direct result of SARS-CoV-2 infection per se. Nevertheless, prompt recognition and management of oral mucocutaneous manifestations are essential to improve the quality of life of these patients. Last but not least, the proper care and the management of the varied oral manifestations, particularly in the severe and hospital-bound COVID-19 patients should be performed by a multispecialty team including dental practitioners versed in oral medicine and pathology.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
Professor Samaranayake gratefully acknowledges the Thammasat University of Thailand for the award of a Bualuang ASEAN Chair Professorship to support this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Wu J, Deng W, Li S, Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cellular and molecular life sciences: CMLS. 2021;78(2):531–44. Epub 2020/08/11. doi: 10.1007/s00018-020-03611-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I, Timens W, Bulthuis M, Lely A, Navis Gv, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203(2):631–7. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–92. Epub 2020/03/15. doi: 10.1007/s11684-020-0754-0 ; PubMed Central PMCID: PMC7088738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Rosa GRM, Libra M, De Pasquale R, Ferlito S, Pedullà E. Association of Viral Infections With Oral Cavity Lesions: Role of SARS-CoV-2 Infection. Frontiers in Medicine. 2021;7(1059). doi: 10.3389/fmed.2020.571214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nature Medicine. 2021. doi: 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaranayake LP, Fakhruddin KS, Mohammad OE, Panduwawala C, Bandara N, Ngo HC. Attributes of dysgeusia and anosmia of coronavirus disease 2019 (COVID-19) in hospitalized patients. Oral Dis. 2020. Epub 2020/11/12. doi: 10.1111/odi.13713 . [DOI] [PubMed] [Google Scholar]
- 7.Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral Manifestations in Patients with COVID-19: A 6-Month Update. J Dent Res. 2021:220345211029637. Epub 2021/07/30. doi: 10.1177/00220345211029637 . [DOI] [PubMed] [Google Scholar]
- 8.Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J Dent Res. 2021;100(2):141–54. Epub 2020/09/12. doi: 10.1177/0022034520957289 . [DOI] [PubMed] [Google Scholar]
- 9.Brandão TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah ACFA, Prado GVB, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2021;131(2):e45–e51. doi: 10.1016/j.oooo.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to Editor: Oral lesions in a patient with Covid-19. Medicina oral, patologia oral y cirugia bucal. 2020;25(4):e563–e4. doi: 10.4317/medoral.24044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, De Paula RM, Cembranel AC, Santos-Silva AR, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020;97:326–8. Epub 2020/06/09. doi: 10.1016/j.ijid.2020.06.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favia G, Tempesta A, Barile G, Brienza N, Capodiferro S, Vestito MC, et al. Covid-19 Symptomatic Patients with Oral Lesions: Clinical and Histopathological Study on 123 Cases of the University Hospital Policlinic of Bari with a Purpose of a New Classification. J Clin Med. 2021;10(4). Epub 2021/03/07. doi: 10.3390/jcm10040757 ; PubMed Central PMCID: PMC7918830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, Matos Valdez LH. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spec Care Dentist. 2020;40(6):555–60. Epub 2020/09/04. doi: 10.1111/scd.12520 . [DOI] [PubMed] [Google Scholar]
- 14.Din AU, Mazhar M, Waseem M, Ahmad W, Bibi A, Hassan A, et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomedicine & Pharmacotherapy. 2021;133:110947. doi: 10.1016/j.biopha.2020.110947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, De Nitto E, et al. The human respiratory system and its microbiome at a glimpse. Biology. 2020;9(10):318. doi: 10.3390/biology9100318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: Systematic Reviews of Etiology and Risk. JBI Manual for Evidence Synthesis2020. [Google Scholar]
- 17.Aghazadeh N, Homayouni M, Sartori-Valinotti JC. Oral vesicles and acral erythema: report of a cutaneous manifestation of COVID-19. International Journal of Dermatology. 2020;59(9):1153–4. doi: 10.1111/ijd.15047 [DOI] [PubMed] [Google Scholar]
- 18.Al-Khanati NM, Riad A, Sahloul ME, Klugar M. Aphthous-like stomatitis of COVID-19 patients. Brazilian Journal of Oral Sciences. 2020;19. doi: 10.20396/bjos.v19i0.8661354 [DOI] [Google Scholar]
- 19.Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. 2021;27 Suppl 3:771–2. Epub 2020/06/09. doi: 10.1111/odi.13465 . [DOI] [PubMed] [Google Scholar]
- 20.Cebeci Kahraman F, Çaşkurlu H. Mucosal involvement in a COVID-19-positive patient: A case report. Dermatol Ther. 2020;33(4):e13797. Epub 2020/06/11. doi: 10.1111/dth.13797 ; PubMed Central PMCID: PMC7300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaux-Bodard A-G, Deneuve S, Desoutter A. Oral manifestation of Covid-19 as an inaugural symptom? Journal of Oral Medicine and Oral Surgery. 2020;26(2):18. [Google Scholar]
- 22.Ciccarese G, Drago F, Boatti M, Porro A, Muzic SI, Parodi A. Oral erosions and petechiae during SARS-CoV-2 infection. J Med Virol. 2021;93(1):129–32. Epub 2020/06/25. doi: 10.1002/jmv.26221 ; PubMed Central PMCID: PMC7362051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int J Infect Dis. 2020;100:154–7. Epub 2020/09/01. doi: 10.1016/j.ijid.2020.08.071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. 2020. Epub 2020/07/23. doi: 10.1111/odi.13555 ; PubMed Central PMCID: PMC7404436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, Suarez-Valle A. Minor aphthae associated with SARS-CoV-2 infection. International journal of dermatology. 2020;59(8):1022–3. Epub 2020/06/18. doi: 10.1111/ijd.15004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glavina A, Biočina-Lukenda D, Mravak-Stipetić M, Markeljević J. Oral symptoms and lesions in SARS-CoV-2-positive patient. Oral Diseases. 2020;n/a(n/a). doi: 10.1111/odi.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indu S. Multiple oral ulcerations—An initial manifestation of COVID 19 infection: A personal experience!! J Oral Maxillofac Pathol. 2020;24(2):227–9. Epub 2020/09/09. doi: 10.4103/jomfp.JOMFP_324_20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, Suarez-Valle A, Saceda-Corralo D, Moreno-Garcia Del Real C, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. 2020;45(7):892–5. Epub 2020/05/10. doi: 10.1111/ced.14281 ; PubMed Central PMCID: PMC7272969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kämmerer T, Walch J, Flaig M, French LE. COVID-19-associated herpetic gingivostomatitis. Clin Exp Dermatol. 2021;46(1):174–6. Epub 2021/01/07. doi: 10.1111/ced.14402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho L. Short report—Herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. 2020;24(17):9151–3. Epub 2020/09/24. doi: 10.26355/eurrev_202009_22863 . [DOI] [PubMed] [Google Scholar]
- 31.Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Ben Saïd P, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. 2020;34(10):e539–e41. Epub 2020/05/27. doi: 10.1111/jdv.16666 ; PubMed Central PMCID: PMC7283825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malih N, Hajinasrollah G, Zare M, Taheri M. Unexpected Presentation of COVID-19 in a 38-Year-Old Male Patient: A Case Report. Case Reports in Dermatology. 2020;12(2):124–31. doi: 10.1159/000509994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021;27 Suppl 3:710–2. Epub 2020/05/06. doi: 10.1111/odi.13382 ; PubMed Central PMCID: PMC7267423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel J, Woolley J. Necrotizing periodontal disease: Oral manifestation of COVID-19. Oral diseases. 2021;27 Suppl 3:768–9. Epub 2020/06/22. doi: 10.1111/odi.13462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaida T, Tanimoto I, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID-19: Is drug eruption specific to COVID-19? Journal of dermatological science. 2020;99(1):62–4. Epub 2020/05/16. doi: 10.1016/j.jdermsci.2020.05.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taşlıdere B, Mehmetaj L, Özcan AB, Gülen B, Taşlıdere N. Melkersson-Rosenthal Syndrome Induced by COVID-19. Am J Emerg Med. 2021;41:262.e5–.e7. Epub 2020/08/15. doi: 10.1016/j.ajem.2020.08.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomo S, Miyahara GI, Simonato LE. Oral mucositis in a SARS-CoV-2-infected patient: Secondary or truly associated condition? Oral Dis. 2020. Epub 2020/07/30. doi: 10.1111/odi.13570 . [DOI] [PubMed] [Google Scholar]
- 38.Delgado JM, Duro N, Rogers DM, Tkatchenko A, Pandit SA, Varma S. Molecular basis for higher affinity of SARS-CoV-2 spike RBD for human ACE2 receptor. Proteins. 2021. Epub 2021/04/18. doi: 10.1002/prot.26086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamanna F, Maglio M, Landini MP, Fini M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Frontiers in Medicine. 2020;7(935). doi: 10.3389/fmed.2020.594495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samaranayake LP, Fakhruddin KS, Panduwawala C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontologica Scandinavica. 2020:1–7. doi: 10.1080/00016357.2020.1787505 [DOI] [PubMed] [Google Scholar]
- 41.Carrillo-Larco RM, Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020;5:94–. doi: 10.12688/wellcomeopenres.15917.1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harikrishnan P. Dysgeusia and dysosmia in asymptomatic COVID-19 patients for contact tracing and isolation. Infectious Diseases. 2021;53(3):212–3. doi: 10.1080/23744235.2020.1854849 [DOI] [PubMed] [Google Scholar]
- 43.Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020;277(12):3519–23. Epub 2020/06/04. doi: 10.1007/s00405-020-06102-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariz B, Brandão T, Ribeiro A, Lopes M, Santos-Silva A. New insights for the pathogenesis of COVID-19-related dysgeusia. Journal of Dental Research. 2020;99(10):1206–. doi: 10.1177/0022034520936638 [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrobel BB, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin North Am. 2004;12(4):459–vii. doi: 10.1016/j.fsc.2004.04.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, et al. Detection of 2019-nCoV in saliva and characterization of oral symptoms in COVID-19 patients. Available at SSRN 3556665. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heckmann JG, Heckmann SM, Lang CJG, Hummel T. Neurological Aspects of Taste Disorders. Archives of Neurology. 2003;60(5):667–71. doi: 10.1001/archneur.60.5.667 [DOI] [PubMed] [Google Scholar]
- 49.Schiffman SS. Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):84–91. doi: 10.1016/j.wjorl.2018.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2009;2(3):303–7. . [PMC free article] [PubMed] [Google Scholar]
- 51.Pushpass R-AG, Daly B, Kelly C, Proctor G, Carpenter GH. Altered Salivary Flow, Protein Composition, and Rheology Following Taste and TRP Stimulation in Older Adults. Front Physiol. 2019;10:652–. doi: 10.3389/fphys.2019.00652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saniasiaya J. Xerostomia and COVID-19: Unleashing Pandora’s Box. Ear, Nose & Throat Journal. 2020;100(2_suppl):139S–S. doi: 10.1177/0145561320960353 [DOI] [PubMed] [Google Scholar]
- 53.Gurvits GE, Tan A. Burning mouth syndrome. World J Gastroenterol. 2013;19(5):665–72. doi: 10.3748/wjg.v19.i5.665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamey PJ. Burning mouth syndrome. Dermatol Clin. 1996;14(2):339–54. Epub 1996/04/01. doi: 10.1016/s0733-8635(05)70361-2 . [DOI] [PubMed] [Google Scholar]
- 55.Nair R, Ariana A, Itthagarun A, Pakneshan S, Brennan M, Samaranayake L. Orofacial viral infections—An update for clinicians. Dental Update. 2014;41. doi: 10.12968/denu.2014.41.6.518 [DOI] [PubMed] [Google Scholar]
- 56.Asai D, Nakashima H. Pathogenic Viruses Commonly Present in the Oral Cavity and Relevant Antiviral Compounds Derived from Natural Products. Medicines (Basel). 2018;5(4):120. doi: 10.3390/medicines5040120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leao JC, Ribeiro CMB, Carvalho AAT, Frezzini C, Porter S. Oral complications of HIV disease. Clinics (Sao Paulo). 2009;64(5):459–70. doi: 10.1590/s1807-59322009000500014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun CX, Bennett N, Tran P, Tang KD, Lim Y, Frazer I, et al. A Pilot Study into the Association between Oral Health Status and Human Papillomavirus-16 Infection. Diagnostics (Basel). 2017;7(1). Epub 2017/03/04. doi: 10.3390/diagnostics7010011 ; PubMed Central PMCID: PMC5373020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gheit T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Frontiers in Oncology. 2019;9(355). doi: 10.3389/fonc.2019.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scully C, Samaranayake LP. Emerging and changing viral diseases in the new millennium. Oral Diseases. 2016;22(3):171–9. doi: 10.1111/odi.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drago F, Ciccarese G, Merlo G, Trave I, Javor S, Rebora A, et al. Oral and cutaneous manifestations of viral and bacterial infections: Not only COVID-19 disease. Clin Dermatol. 2021. doi: 10.1016/j.clindermatol.2021.01.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horstmann DM. VIRAL EXANTHEMS AND ENANTHEMS. Pediatrics. 1968;41(5):867. [PubMed] [Google Scholar]
- 63.Hajjar R, Chan G. Anti-tumor necrosis factor agents and COVID-19: A word of caution. J Clin Transl Res. 2020;6(3):94–6. . [PMC free article] [PubMed] [Google Scholar]
- 64.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. Epub 2020/06/02. doi: 10.1016/j.cytogfr.2020.06.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The Role of Interleukin 6 During Viral Infections. Front Microbiol. 2019;10(1057). doi: 10.3389/fmicb.2019.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang R, Mamun A, Dominic A, Le N-T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front Physiol. 2021;11:605908–. doi: 10.3389/fphys.2020.605908 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandra A, Chakraborty U, Ghosh S, Dasgupta S. Anticoagulation in COVID-19: current concepts and controversies. Postgraduate Medical Journal. 2021:postgradmedj-2021-139923. doi: 10.1136/postgradmedj-2021-139923 [DOI] [PubMed] [Google Scholar]
- 68.Baraboutis IG, Gargalianos P, Aggelonidou E, Adraktas A, Collaborators. Initial Real-Life Experience from a Designated COVID-19 Centre in Athens, Greece: a Proposed Therapeutic Algorithm. SN Compr Clin Med. 2020:1–5. doi: 10.1007/s42399-020-00324-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic Fungal Infections in the Epidemic Area of COVID-19: A Clinical and Diagnostic Perspective from Iran. Mycopathologia. 2020;185(4):607–11. Epub 2020/07/31. doi: 10.1007/s11046-020-00472-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellepola ANB, Samaranayake LP. Oral Candidal Infections and Antimycotics. Critical Reviews in Oral Biology & Medicine. 2000;11(2):172–98. doi: 10.1177/10454411000110020301 [DOI] [PubMed] [Google Scholar]
- 71.Warnakulasuriya KAAS, Samaranayake LP, Peiris JSM. Angular cheilitis in a group of Sri Lankan adults: a clinical and microbiologic study. Journal of Oral Pathology & Medicine. 1991;20(4):172–5. doi: 10.1111/j.1600-0714.1991.tb00915.x [DOI] [PubMed] [Google Scholar]
- 72.Riad A, Kassem I, Issa J, Badrah M, Klugar M. Angular cheilitis of COVID-19 patients: A case-series and literature review. Oral Dis. 2020. Epub 2020/10/13. doi: 10.1111/odi.13675 ; PubMed Central PMCID: PMC7675282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raut A, Huy NT. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? The Lancet Respiratory Medicine. 2021;9(8):e77. doi: 10.1016/S2213-2600(21)00265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta KK, Singh A, Kalia A, Kandhola R. Anaesthetic considerations for post-COVID-19 mucormycosis surgery- A case report and review of literature. Indian J Anaesth. 2021;65(7):545–7. Epub 2021/07/23. doi: 10.4103/ija.ija_470_21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pauli MA, Pereira LdM, Monteiro ML, de Camargo AR, Rabelo GD. Painful palatal lesion in a patient with COVID-19. Oral surgery, oral medicine, oral pathology and oral radiology. 2021;131(6):620–5. Epub 2021/03/28. doi: 10.1016/j.oooo.2021.03.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLOS ONE. 2021;16(3):e0238825. doi: 10.1371/journal.pone.0238825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samaranayake L. Oral mycoses in HIV infection. Oral Surgery, Oral Medicine, Oral Pathology. 1992;73(2):171–80. doi: 10.1016/0030-4220(92)90191-r [DOI] [PubMed] [Google Scholar]
- 78.Samaranayake L. Essential Microbiology for Dentistry. 5th Edition, Elsevier, 2018. 2018:1–400. [Google Scholar]
- 79.Aaron SL, DeBlois KW. Acute Necrotizing Ulcerative Gingivitis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021. [PubMed] [Google Scholar]
- 80.Ibekwe TS, Fasunla AJ, Orimadegun AE. Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19. OTO Open. 2020;4(3):2473974X20957975–2473974X. doi: 10.1177/2473974X20957975 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.