Abstract
Inflammatory bowel disease (IBD), a disorder characterized by chronic inflammation of the gastrointestinal (GI) tract and a range of adverse health effects including diarrhea, abdominal pain, vomiting, and bloody stools, affects nearly 3.1 million genetically susceptible adults in the United States today. Although the etiology of IBD remains unclear, genetics, stress, diet, and gut microbiota dysbiosis— especially in immunocompromised individuals— have been identified as possible causes of disease. Although previous research has largely focused on the role of bacteria in IBD pathogenesis, recently observed alterations of fungal load and biodiversity in the GI tract of afflicted individuals suggest interkingdom interactions amongst different gut microbial communities, particularly between bacteria and fungi. These discoveries point to the potential utilization of treatment approaches such as antibiotics, antifungals, probiotics, and postbiotics that target both bacteria and fungi in managing IBD. In this review, we discuss the impact of specific fungi on disease pathogenesis, with a focus on the highly virulent genus Candida and how the presence of certain co-enzymes impacts its virulence. In addition, we evaluate current gut microbiome-based therapeutic approaches with the intention of better understanding the mechanisms behind novel therapies.
Keywords: mycobiome, Crohn’s disease, probiotics
Key Messages.
What is already known?
Gut microbiome dysbiosis is a distinct feature of IBD. Compared with healthy individuals, the gut microbiome of IBD patients is often much more diverse, encompassing a wider variety of bacteria, fungi, and other microbes. In addition, there appears to be significantly less beneficial bacteria in the guts of IBD patients. These compositional distinctions are suggestive of interkingdom interactions amongst varying microbial communities, which in turn identifies dysbiosis as a potential cause of disease.
What is new here?
In this review, we discuss the impact of specific fungi on disease pathogenesis. In addition, we evaluate current gut microbiome-based therapeutic approaches with the intention of improving our understanding of the mechanisms behind novel, microbia-based therapies.
How can this study help patient care?
By exploiting what we currently know about the fungal influence on the microbial composition and biodiversity of afflicted individuals, we can better address disease symptoms through the development of novel nutritional-based therapies and by further clarifying the microbiome-mycobiome interactions and defining how they affect intestinal inflammation.
Introduction
Consisting of 2 clinical entities, namely Crohn’s disease (CD) and ulcerative colitis (UC), inflammatory bowel disease (IBD) is characterized by chronic relapsing-remitting inflammatory disorders of the gastrointestinal (GI) tract associated with a deregulation of the T cell–mediated immune responses toward intestinal microbes. Although both CD and UC are generally characterized by chronic inflammation of the gastrointestinal tract and symptoms of abdominal discomfort, bloody stool, diarrhea, urgent bowel movements, increased frequency of bowel movements, increased gas release, passage of mucus, and cramping with bowel movements, there are some differences between the 2 conditions. For instance, abdominal pain and fatigue are more common CD symptoms, whereas diarrhea and bloody bowel are more common UC symptoms.1 In terms of physical manifestation, CD presents as patches of inflammation throughout the entire GI tract,2 but UC presents continuously and exclusively in the large intestine, typically extending proximally from the rectum.3
Although the etiology of IBD remains unclear, some evidence suggests that a combination of genetic susceptibility, immune system dysregulation, host gut microbiome, and environmental conditions contribute to its pathogenesis. In addition, associated risk factors include diet, stress, smoking, drug use, and gut microbial interactions.4 A study based on systematic analyses for the global, national, and regional burden of IBD from 1990 to 2017 in 195 different countries reported that IBD incidence rates increased by nearly 85.1% globally, affecting a significantly higher proportion of females than males. Females 60 to 64 of age and males 70 to 74 of age5 saw the highest rates of IBD. From 2007 to 2016 in the United States, IBD incidence amongst children and adults increased by 133% and 123%, respectively.6 The upward trend of disease prevalence leads us to believe that incidence rates will continue to increase in the coming years and that the need for more effective therapies and medications will be of higher demand.
The gut microbiome comprises numerous microorganisms including bacteria, fungi, viruses, archaea, and protozoa, all of which function independently or commensally to maintain intestinal homeostasis or in some cases exacerbate disease. Bacterial dysbiosis of the gut results from the interactions between various fungi and bacteria and is related to gut mucosal damage and inflammation. Interkingdom interactions have also been observed in multiple diseases including colorectal cancer,7 gastric cancer, and leukemia.8 In 2007, National Institute of Health (NIH) funded a multicomponent community resource known as the Human Microbiome Project, which encompasses the trillions of microbes inhabiting the human body. This large database allows us to examine the bacterial, fungal, and viral patterns related to various health conditions affecting the skin, blood, oral cavity, genitourinary tract, nasopharynx, and GI tract.9 These microbial profiles are then compared with the “healthy” human microbiome. In September 2021, the NIH expanded their investigations beyond bacteria, incorporating information regarding the gut mycobiome. Data from the Human Microbiome Project has been used to further our understanding of CD and UC etiology. For example, CD patients show greater proportions of the fungus Candida tropicalis and bacteria Escherichia coli and Serratia marcescens compared with their non-Crohn’s disease healthy relatives (NCDR).10 Interactions amongst these fungi and bacteria are related to the formation of pathogenic biofilm, which can cause localized inflammation. The threatening effects of microbiome dysbiosis points to the potential utilization of treatments like antibiotics, antifungals, and probiotics, which can restore microbial gut composition to offset some IBD symptoms.11
Role of Fungi in IBD
Recent evidence of mycobiome dysbiosis suggests that gut fungi play a significant role in regulation of IBD, much like bacteria (Table 1).
Table 1.
Evidence of fungi in regulating IBD
| Damage of epithelial membranes by Candidalysin (a fungal peptide toxin) secretion12 |
| Increased biofilm production resulting from interaction of C. tropicalis with S. marcescens and E. coli10 |
| Damage of macrophages caused by fungal peptide leading to activation of NLP3 inflammasome-dependent IL-1β production 13 |
| Mycobiota-induced hyphal formation in hosts with genetic deficiencies 14 |
| Increased intestinal permeability through microbiota dysbiosis induction in mucin-degrading bacteria 15 |
| Induction of regulatory response of dendritic cells 16 |
In a study conducted by Li et al, internal transcribed spacer analysis revealed that several C. albicans strains were steadily overrepresented in the gut of IBD patients compared with individuals without IBD.17 Macrophages play a pivotal role in antifungal immunity, and it has been previously reported that C. albicans is capable of damaging these cells.13Candida albicans were, therefore, divided into high-damaging group (HD-Ca) intestinal macrophages and low-damaging group (LD-Ca) based on their ability to damage intestinal macrophages through hyphal formation and production of virulence factors. Additionally, genetic ablation of ECE1 cell elongation 1 (ECE1) gene-encoded protein, candidalysin, drastically decreased the capacity of HD-Ca strains of damaging macrophages. This gene-expression analysis revealed that ECE1 played a major role in inducing hyphal formation of HD-Ca strains and consequent macrophage damage.
Tampakakis et al observed impaired viability and decreased abundance of C. albicans in the presence of the bacteria Salmonella enterica, suggesting that S. enterica limit successful colonization of C. albicans.18 Evidence of disease-specific fungal dysbiosis was also observed in a cohort of pediatric IBD patients in which heightened anti-Saccharomyces cerevisiae antibody (ASCA) load—suggestive of elevated Candida— and a higher ratio of the phylum Basidiomycota to Ascomycota in IBD flare vs IBD remission were observed.19 Of the varying fungi studied, the particular species C. albicans has been established as one of the most common IBD-associated fungal species due to its virulence factors (eg, hyphal formation, adherence, secreted phospholipase and aspartic proteinases, and biofilm formation20) and mechanisms to elude antifungal treatments and host immune response.21 Moreover, host environments are more susceptible to Candida dissemination depending on their preexisting environmental conditions in which pathogens colonize. For example, the use of broad spectrum antibiotics that kills off beneficial bacteria such as Lactobacillus or epithelial damage from chemotherapy prime the gut lining for fungal hyphae penetration, potentially leading to oral and intestinal mucosa candidiasis and increased systemic inflammation.22 The pathogenicity of C. albicans is based on its ability to convert from commensal to pathogen and form resistant biofilm on the surface of a host cell, especially in immunosuppressed conditions.23 Successful C. albicans–induced candidiasis has been observed and characterized by elevated interleukin (IL)-1β expression, increased stomach lesion occurrence, mucosal neutrophilia, and eventual fatality within 28 days in immunocompromised untreated mice.24 Doron et al recently showed how the immune system maintains C. albicans in its commensal, nonpathogenic form. Specifically, the conversion of C. albicans from yeast to hyphal forms induces plasma cells to secret immunoglobin A (sIgA), which binds with higher affinity to the hyphae, stopping its spread.14 The apparent link between C. albicans and CD led to a pilot study in 2021 that evaluated the effect of a 6-month treatment with antifungal fluconazole (FCZ) on postoperative recurrence of CD and provided therapeutic evidence of the Candida-CD connection.25 The study showed a decrease in CD recurrence in patients receiving FCZ compared with placebo-dosed patients 6 months after surgery.
Differences in fungal load—particularly increases of Candida, Gibberella moniliformis, Alternaria brassiciciola, and Cryptococcus neoformans in CD— have also been identified based on the type of mucosa they inhabit (ie, inflamed vs noninflamed). As such, fungi that are more abundant in inflamed mucosa may be more “harmful” or disease exacerbating than those found in noninflamed mucosa.26 Huo et al found that C. metapsilosis M2006B was significantly more abundant in IBD patients and that transplantation with M2006B attenuated experimental colitis in mice by activation of farnesoid X receptor (FXR).27 In addition, 2 secondary metabolites of M2006B, F4 and F5, also activated FXR. Therefore, cultivated C. metapsilosis M2006B and its metabolites are promising fungal-based therapies that could protect against gut inflammation.
A recent study published by Di Martino et al demonstrated that C. tropicalis infection in Black 6 mice led to increased susceptibility to dextran sodium sulfate (DDS)-induced colitis compared with uninfected controls.28 The increased susceptibility was caused by dysbiosis related to mucin-degrading bacteria Akkermansia muciniphila and Ruminococcus gnavus, which in turn led to significant damage to the gut mucosal barrier of the infected Black 6 mice.
When grown together, C. tropicalis and bacteria E. coli and S. marcescens formed significantly thicker biofilm than when grown independently.29 The mechanism behind this successful tissue invasion involves highly specific and close interkingdom interactions in which S. marcescens formed a fimbriae bridge between E coli and C tropicalis, in addition to the direct fusion of E. coli with the cell wall of C. tropicalis.11
The non-albicans Candida species (NAC spp.) have also been found to possess virulence traits similar to that of their C. albicans relatives.30 Studies comparing the levels of NAC spp. to C. albicans from various clinical candidiasis samples showed that C. albicans, C. tropicalis, and C. glabrata produced the most phospholipase compared with the other fungal strains studied.31 As a biomarker for Candida presence, the hydrolytic enzyme phospholipase is produced by Candida and aids in its colonization by degrading the intestinal cell membrane.32 Comparatively, NAC spp. were also associated with heightened phospholipase production, highlighting their relatively high pathogenicity.33
Current IBD Treatments
Despite the variety of IBD therapies available today, positive response rates to treatment remain relatively low due to the complexity of IBD and its unknown causes. Common drug therapies such as monoclonal antibodies, anti-inflammatory drugs, immune system suppressors, antibiotics, and other therapies like surgery and nutritional intervention have been associated with several adverse health effects including impaired immune function, abdominal discomfort, headache, and nausea, posing great challenges for both health care professionals treating their patients, and patients seeking the necessary care.
Furthermore, immunomodulatory therapies have been associated with increased risk of bacterial, viral, and fungal opportunistic infection in IBD patients.34 Although no specific association has been observed between a particular drug and type of infection, Toruner et al found a positive correlation between corticosteroid use and fungal infection (specifically caused by Candida strains) and between antitumor necrosis factor (TNF) therapy with mycobacterial infections.35 The interactions between bacteria, fungi, and human gut’s microbial profiles are known to speed up or slow disease progression. Discussing the effect of current IBD treatments on diseased guts can help us identify their shortcomings and further investigate safer, more effective therapeutic approaches.
Biologics
Biologic medications have become one of the most common ways to treat IBD and have also has been shown to be both safe and effective. This class of immunosuppressants involve the preparation of antibodies that target proteins associated with inflammation in diseases such as multiple sclerosis, psoriasis, rheumatoid arthritis, and IBD.36 Inflammatory bowel disease biologics are classified as TNF-α blockers, integrin blockers, or interleukin blockers, each of which modulate the body’s immune response to pathogens in the GI tract.
Anti-TNF agents such as infliximab (IFX)37 and adalimumab (ADA)38 have been used for over 25 years to treat a number of inflammatory diseases. Their mechanism of action involves downregulating the overexpression of pro-inflammatory cytokine TNF.39 Since their approval for use, anti-TNF agents have been well tolerated by UC and CD patients and typically do not require drug discontinuation, albeit some adverse effects such headache, nausea, abdominal pain, and other minor health inconveniences have been reported. Based on the data collected from various randomized controlled trials, large observational studies, meta-analyses, and postmarketing registries, Papamichael et al confirmed that IFX has been associated with lowered CD disease activity, clinical remission, and similar rates of adverse effects between the IFX and placebo groups.40 Likewise, 2 out of 3 IBD patients with symptomatic small bowel stricture responded to treatment with ADA, and over 50% of them avoided surgery 4 years after the start of treatment.41
Another monoclonal antibody, ustekinumab (UST), inhibits IL-12 and IL-23 activation and proliferation of pro-inflammatory cytokines, downregulating the body’s immune response in CD and psoriatic conditions.42 Sands et al reported significantly higher rates of clinical remission in UC patients after exposure to UST and similar rates of adverse effects compared with placebo. Clinical remission was also observed in IFX-refractory pediatric UC patients after UST treatment.43
Vedolizumab (VDZ)44 is another monoclonal antibody that has been approved to treat moderate to severe CD and UC in adult patients and is generally prescribed after the failure of other anti-TNF-α therapies. Vedolizumab works against the immune system to inhibit leukocyte proliferation of the GI tract, thereby limiting the production of pro-inflammatory cytokines and recruitment of other inflammatory cells.45 A combined analysis of 6 VDX efficacy trials from 2009 to 2013 showed no significant risk of infection, progressive multifocal leukoencephalopathy, or malignancies after exposure to the drug, establishing its favorable safety profile.46
Aminosalicylates
Aminosalicylates—another commonly prescribed IBD medication—functions by limiting leukocyte accumulation in GI tract, transitively reducing localized inflammation.47 Several types of aminosalicylates including sulfasalazine (SASP) and 5-aminosalicylic acid (5-ASA) have effectively induced clinical remission in both UC and CD patients across a number of studies.48 Yoshino et al showed, for example, that of 36 UC refractory patients treated with SASP, 69.4% experienced clinical remission and that SASP contributed to continued clinical remission even after discontinuation of other medications like 5-ASA and concomitant tacrolimus.49 Takeshima et al showed a possible correlation between drug effectiveness and length of treatment in which short-term (≤105 days) treatment with 5-ASA showed a significantly higher percentage of UC relapse and a shorter time to relapse than their long-term (>105 days) counterparts. This data favors sustained use of 5-ASA over short term, although no such preference was established for dosage.50 Treatment with 1 round of 2-1600 mg of 5-ASA tablets and 2 rounds of 4-400 mg tablets induced disease remission in 22.4% and 24.6% of patients, respectively, showing no significant correlation between number of tablets taken per day and disease improvement.51 Despite its beneficial effects of treating IBD symptoms, SASP along with other aminosalicylates have resulted in several adverse effects such as nausea, vomiting, headache, dizziness, rash, and drug-drug interactions, as observed in rheumatoid arthritis patients.49
Immunosuppressants
Immunosuppressants—which are often used to treat autoimmune diseases like IBD— function by suppressing a host’s immune response to foreign invaders, particularly inflammation.52 Common IBD immunosuppressants include methotrexate (MTX), 6-mercaptopurine (6-MP), and azathioprine (AZA) and typically reduce localized inflammation by inhibiting lymphocyte production and pro-inflammatory cytokine proliferation. A wide range of negative health effects including as nausea, vomiting, hepatitis, arthritis, pneumonitis, pancreatitis and an increased risk of lymphoma have been observed, however, in patients treated with 6-MP and AZA. A study examining the effect of MTX in UC revealed that although MTX was not superior at maintaining or inducing steroid-free remission in UC patients compared with placebo, a greater portion of UC patients did experience clinical remission, leading to fewer withdrawals from treatment caused by active UC.53 On the other hand, effective induction therapy via administration of upadacitinib, a Janus kinase 1 inhibitor, has been demonstrated in both UC and CD patients in which a significant portion of diseased individuals underwent endoscopic remission after phase 2 of drug administration, despite frequently reported adverse effects such as headache, fatigue, vomiting, upper respiratory and urinary tract infections, and even worsening CD.54 A systematic review and meta-analysis of the safety of Janus kinase inhibitors concluded that patients with immune-mediated diseases treated with Janus kinase inhibitors were at an increased risk of herpes foster infection, whereas risk for all other adverse effects studied were unaffected.55
Gut Microbiome-based Therapeutic Approaches: Interventions for Promoting Human Health and Combating Disease
Will Microbiome Research Help Us to Become Healthier?
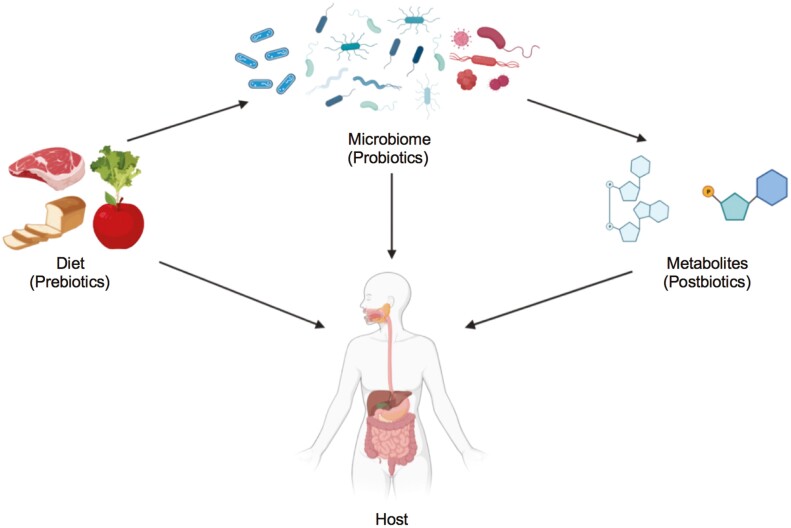
Advances in the treatment of Clostridium difficile provided proof that manipulation of the microbiome could treat disease: patients who received fecal microbiome transplant (FMT) from healthy donors were rapidly cured.56 Based on this, it is believed that microbiome research could lead to the development of new therapies (a targeted intervention of the microbiome for health). Different approaches to balance the gut microbiome include nutritional intervention, probiotic supplementation, or bacteria-derived metabolites (Figure 1).57
Figure 1.
Interventions for promoting human health, including nutritional intervention, probiotic supplementation, and postbiotics (microorganisms-derived metabolites).
Microbiome Modulation: With Nutritional Intervention (Prebiotics or by Individualized Diets)
A number of nutritional studies have examined how specialized diets can change the gut microflora and reduce disease severity. The Mediterranean diet (MD), for example, primarily consists of whole grains, legumes, nuts, olive oil, foods rich in omega-3, fruits and limits eggs and foods with added sugars.58 The MD is often recommended to elderly individuals at risk of frailty, which can be related to the upregulation of CD- and UC-specific pro-inflammatory cytokines and resulting cognitive decline.59 Those consistently following this dietary pattern are less likely to develop cardiovascular disease and cancer and can typically expect to live longer than non-MD consumers.60 As such, the health benefits of MD were highlighted in a study that showed that intervention with a plant-based diet—comprising berries, grapes, peanuts, and seeds—was associated with an anti-inflammatory GI microbiota profile, whereas adherence to a meat-based diet—composed of processed meat, cheese, dairy, and other cholesterol-rich foods—was associated with a pro-inflammatory GI microbiota profile.61 Despite these positive outcomes, stronger adherence to MD diet has been associated with elevated C. albicans and more apparent disease symptoms.62
Due to the disproportionately high levels of fat mass surrounding the GI tract of IBD patients, afflicted individuals commonly experience health complications similar to those resulting from obesity.63 Therefore, examining the effects of MD in obese and overweight individuals provides insight to its potential healing properties in IBD. Although daily consumption of 179 ± 50 g of whole grains vs daily consumption of 13 ± 10 g of refined grains had no distinguishable effects on microbiome composition in adults at risk of metabolic syndrome, a whole grain diet was associated with weight loss and reduced serum levels of pro-inflammatory cytokine IL-6.64 Reduced systemic inflammation, increased insulin sensitivity, lowered serum cholesterol levels, and microbiome alterations were observed amongst another cohort of overweight and obese individuals whose previous sedentary lifestyles and low intake of fruits and vegetables exacerbated their risk of systemic inflammation.65 Similarly, adoption of a low fat vegan diet in overweight adults resulted in a reduction of fat mass and increased insulin sensitivity— both of which were associated with weight loss and elevated Bacteroides fragilis.66 Despite the fact that Bacteroidetes phylum is typically less abundant in obese individuals compared with nonobese individuals,67 the results of this study are better explained by other findings that have shown that caloric intake and Bacteroidetes abundance are negatively correlated, which is why intervention with a relatively low calorie diet (ie, low fat vegan)68 is correlated to elevated Bacteroides fragilis.
Excessive caloric intake, which is linked to a number of negative health outcomes, is another factor to consider when evaluating IBD severity. Excessive availability of macronutrients to adipose tissue stimulates their upregulation of pro-inflammatory cytokines IL-6 and TNF-α, leading to persistent systemic inflammation in individuals with excessive visceral fat.69 Panizza et al showed that in combination with MD, intermittent energy restriction effectively reduced visceral adiposity in 60 East Asian Americans living in Hawaii.70 Therefore, a reduction in caloric intake, generally achievable through the adoption of MD, demonstrates effective modulation of inflammatory responses. Additionally, consumption of low fat, high fiber foods has also been associated with reduced systemic inflammation in IBD. Chicco et al found that short-term adherence to MD not only improved body mass index (UC, 0.42; CD, 0.48) and decreased waist circumference (UC, 1.25 cm; CD, 1.37 cm), but also significantly reduced disease activity and pro-inflammatory biomarkers of UC and CD, such as C-reactive protein and fecal calprotectin.18
A recent study revealed, however, that MD or vegetarian diet intervention lasting less than 3 months failed to induce significant gut microbiome alterations and subsequent reductions in inflammatory biomarkers, suggesting that significant health benefits are best achieved through sustained adherence to a diet.71 This study also confirmed the anti-inflammatory properties of the MD, as the production of gut-benefiting short chain fatty acids (SCFA)s were negatively correlated with the release of pro-inflammatory cytokines IL-17 and IL-12. With an emphasis on whole foods-based diets, nutritional studies have identified specific foods and dietary patterns whose induced microbiome alterations pose potential strategies for managing symptom severity of several metabolic and inflammatory diseases. Ghannoum et al highlighted how effective the mycobiome diet was in improving GI symptoms and overall health.72 The mycobiome diet includes a plant-based protein, a “good” fat source (such as fat-rich foods primarily containing monounsaturated or polyunsaturated fats) and a starch-rich food (such as whole grain, legumes or starchy vegetables) with each meal. Adherence to this diet for 4 weeks resulted in improved GI symptoms, weight loss, heightened energy levels, better sleep, and reduced hot flashes. Microbial analysis of participants’ fecal samples showed heightened levels of beneficial fungi Pichia kluyveri and Galactomyces geotrichum and lowered levels of Candida (specifically C. albicans and C. tropicalis) compared with the fecal microbial population before the mycobiome diet.
Several dietary studies have also discovered elevated levels of Candida species in the intestine of malnourished children and hypothesize that vitamin and iron deficiencies may be risk factors for candidiasis.73 Vitamin B6, for example, is a water-soluble vitamin that performs a variety of functions in the body mostly related to protein metabolism,74 hemoglobin formation, and glycogen breakdown.75 The triad of magnesium-related latent tetany, essential fatty acids deficiencies, and vitamin B6 dependency and deficiency was present with great consistency in the work with Candida-infected patients by Galland et al.76 Vitamin A, especially its most active metabolite all-trans retinoic acid, has been shown to have a great impact on the innate immune response against candidiasis by suppressing Dectin-1, a fungal pattern recognition receptor, and therefore leading to a significant downregulation of Candida-induced expression of pro-inflammatory cytokines such as Il-6, Il-12 and TNF-α.77 Vitamin D has been strongly associated with IBD and also has been demonstrated to have fungicidal properties. A meta-analysis study conducted in 2015 showed that patients with IBD were 64% more likely to develop vitamin D deficiency, whereas UC patients were 50% more likely to develop vitamin D deficiency compared with controls.78 One study showed that vitamin D3 (VD3), in particular, has been shown to limit C. albicans colonization in culture plates in vitro. The results indicated that treatment of C. albicans with VD3 resulted in a minimum fungistatic concentrations to minimum fungicidal concentrations quotient of 1, a value falling below the quotient 4 threshold, and therefore classifying vitamin D3 as a “good antifungal therapy.”79 Neumann et al explained such antifungal effects, with the tendency of fat soluble molecules to jeopardize eukaryotic cell membranes, may induce cell lysis and prevent further fungal invasion.80 Vitamin D3’s role in limiting successful fungal infection is consistent with the findings that vitamin D-deficient mice infected with Aspergillus fumigatus exhibited increased fungal spore formation and activity, worsened lung inflammation, and physical damage to lung tissue when compared with vitamin D-sufficient mice.81 Vitamin D deficiency has also shown positive associations with oral candidiasis in HIV seropositive women.82 Silymarin, a compound derived from milk thistle, is a dietary supplement which successfully suppressed yeast cell formation by inhibiting Candida secretion of proteinase and phospholipase, known Candida virulence factors.32 This compound was also safely administered in combination with amphotericin B, fluconazole, and caspofungin antifungals, supporting the use of silymarin in different combination therapies.83
The immunoregulatory effects of all-trans retinoic acid, an active vitamin A metabolite, on C. albicans infection is characterized by its suppression of fungi-associated inflammatory cytokine (TNF-α, IL-6 and IL-12) production.77 In addition, vitamin A suppressed Dectin-1 expression and subsequently production of Dectin-1 dependent cytokines, but its effects increased nearly 5-fold over a 16-hour period, shedding light on the prolonged impact of its fungal fighting properties. Thus, it is not surprising to see that, like VD3 deficiency, vitamin A deficiency is associated with a number of negative health implications including increased risk of fungal infection. Majewski et al showed that when compared with healthy individuals, psoriatic patients had lower systemic vitamin A levels, which was also associated with increased disease activity.84 Given that psoriatic patients show greater susceptibility to Candida infection compared with any other skin disease, vitamin A deficiency is a possible explanation for such successful fungal colonization.85 Specific nutrient supplementation has also been documented to exhibit health-benefitting effects. In particular, zinc supplementation has been shown to decrease the prevalence of candidemia and candiduria in pediatric intensive care units, where the incidence of yeast infections has greatly increased in recent years.86 In this particular trial designed to study the efficacy of zinc supplementation against candidiasis, zinc supplementation is shown to help reducing Candida infections in patients receiving broad-spectrum antibiotics.
Directly Impacting the Host: Probiotic Supplementation or FMT
New developments in our current understanding of the synergistic relationships amongst gut bacteria, fungi, and other gut microbes point to the potential effectiveness of probiotics in treating IBD. Probiotic usage has become increasingly common in managing a variety of health afflictions such as high blood cholesterol, lactose intolerance, traveler’s diarrhea, compromised immune systems, and various IBD symptoms, especially in the field of nutrition.87 Moreover, research has shown that probiotics E. coli, VSL#3, Saccharomyces boulardii, and Bifidobacterium longum, for example, induce and maintain UC remission, as well as prevent chronic pouchitis relapse.88 These beneficial bacteria which naturally occur in fermented foods and milk regulate the gut’s microbial compositional profile, as well as prevent GI abnormalities and diseases, often in combination with other diet modifications.63 Although little is known about the potential benefits of probiotics in disease management, recent research has shown increases in gut bacteria, including Bifidobacterium spp., in metastatic renal cell carcinoma and CD patients adhering to a gluten-free diet.89 In addition, CD patients reported reduced physical pain and severity of irritable bowel syndrome–like symptoms after supplementation with a probiotic mix.90
Investigations of the gut-brain axis also shed light on the interrelatedness of gut microbiome alterations and disease pathogenesis, as well as the beneficial effects of probiotics. The gut-brain axis, a phenomenon in which the central and enteric nervous systems communicate in a way that connects the emotional and cognitive parts of the brain to the GI tract, is the mechanistic link between leaky gut and a number of neuropsychiatric complications.91 Disruptions in the gut-brain axis as a result of gut microbiota disorder, such as that in Autism Spectrum Disorder (ASD), may account for the high frequency and increased severity of GI symptoms associated with this disorder.92 Therefore, treatment with probiotics in ASD may be a safer alternative to classical pharmacology which involves a number of side effects. Indeed, 2 months of twice a day supplementation with Lactobacillus acidophilus has been shown to directly affect gut metabolism and indirectly address behavioral and emotional effects such as concentration in ASD children via the gut-brain axis.92 Similarly, probiotic supplementation has also improved cognitive functions and reduced stress in older adults. The results of these studies demonstrate the ability of probiotics to effectively alter the microbiota and in turn activate other physiological and psychological responses in the body to alleviate symptoms of various mental disorders.93 Finally, probiotic usage has been shown to reduce depressive symptoms and improve sleep quality without significant risk of adverse side effects in moderately depressed individuals.94 Although these studies are heavily focused on the psychological impact of probiotic supplementation, they nonetheless reveal that changes to the gut microflora via probiotic supplementation offer a number of health benefits and therefore can be useful in IBD to restore intestinal homeostasis with minimal risk.
As previously mentioned, the high abundance of C. albicans in the gut of IBD patients is largely due to its ability to adhere to and colonize a host environment.95 Probiotic BIOHM, which consists of Bifidobacterium breve, S. boulardii, L. acidophilus, L. rhamnosus, and amylase has recently been used to restore gastrointestinal microbiome dysbiosis.96 Mycobiome analysis after once a day consumption of BIOHM for 4 weeks showed significant reductions of Candida. C. albicans tended to be lower in subjects who consumed the probiotic, albeit this was not statistically significant. Mycobiome analysis after probiotic treatment also revealed an increase in ascomycota levels and a reduction in zygomycota levels (P < .01) in enrolled individuals. We can therefore take advantage of the therapeutic properties of probiotics to elevate beneficial fungi or destroy harmful fungi and restore a more balanced gut microbiome in IBD patients. By doing so, we limit gut dysbiosis and symptoms of inflammation and mucosal damage.96
Bacteria-derived Metabolites: Administration, Reduction, or Activity Blocking of Metabolites Through Treatment With or Inhibition of Postbiotics
Although probiotics offer a number of health benefits to the gut, they function differently from postbiotics which indirectly impact the host via secretion of beneficial molecules known as metabolites. Postbiotics comprehend any substance produced through the metabolic activity of bacteria or fungi, which can positively affect the host by contributing to number of physiological functions such as food digestion, immunity and intercellular communication,97 and maintaining a healthy gut microbial balance.98 There are various types of postbiotics including SCFAs, lipopolysaccharides, enzymes, cell wall fragments, bacterial lysates, cell-free supernatants, vitamins, and amino acids. They result naturally from the existence of the gut microbiome and therefore offer therapeutic potential.99 Here we discuss how the administration, reduction, or functional blocking of metabolites through various treatment methods impacts disease progression.
Microbial derived SCFAs, mainly acetate, propionate, and butyrate, have been shown to resist pathogen colonization of the GI tract and maintain intestinal homeostasis by preventing colonization of certain fungal strains. Compared with health individuals, some SCFA-producing bacteria are less abundant in IBD patients, limiting the anti-inflammatory and mucosal healing properties of these metabolites 100-102.
Bhaskaran et al showed that treatment with antibiotics in mice caused a reduction in Foxp3 + regulatory cells and IL-17A-producing cells and exacerbated C. albicans-dependent inflammation in the gut and oral cavity. Bacteria-derived SCFAs were shown to increase levels of Foxp3+, IL-17A + and Foxp3 + IL-17A + double positive Treg17 cells in mice, playing a protective role against inflammation. Treatment with SCFAs, however, did not fully treat inflammation, suggesting that some resident microbes partially contribute to microbial homeostasis 103.
Short chain fatty acids are released by gut bacteria in the process of carbohydrate fermentation 104. Butyric acid (BA), a major SCFA, produced by strains belonging to genera Butyribacterium, Clostridium, Eubacterium and Butyvibrio, inhibits activation of Nuclear Factor kappa B (NF-κB) and degrades I kappa B alpha (IκBα) protein, ultimately decreasing pro-inflammatory cytokine levels and inducing an anti-inflammatory effect on intestinal epithelial cells 105. Likewise, the gut microbial profiles of IBD patients have shown reduced numbers of SCFAs-producing bacteria and consequently a reduced BA concentration, which is linked to heightened pro-inflammatory immune cells in the gut mucosa of these patients 106. The anti-inflammatory effect of BA is mediated by its capacity of regulating gene expression by inhibiting histone deacetylaces,107 leading to hyperacetylation of histones, open structure of chromatin and therefore to DNA accessible to initiate gene transcription. As a consequence, the histone deacetylases inhibition can tame the inflammatory response by inhibiting NF-κB activation and production of pro-inflammatory cytokines, such as IL-6, IL-1β, TNF-α, and IFN-γ.108 The discovery of the BA mechanism led to the development of a histone deacetylaces inhibitor for treatment of pro-inflammatory disorders such as idiopathic arthritis and IBD.109
The body’s fermentation of fiber produces SCFAs. Therefore, those with diets rich in dietary fiber are likely to have higher levels of SCFAs than those not consuming as much. The positive association between SCFA production and consumption of dietary fiber was evident in adolescents with celiac disease whose levels of acetic acid, BA, and other SCFAs were significantly higher than nonoat consuming controls after 1 year of diet treatment.110 In addition, increased intake of fiber-rich foods has been related to weight loss, structural alterations to the gut microbiota, and reduced inflammation of the GI tract.111 A recent study has shown that catered low-fat, high-fiber diet was well tolerated, increased quality of life (which was measured by an IBD questionnaire at baseline and week 4 of the diet), and led to a greater increase in fecal acetate compared with those consuming the improved standard American diet.111 The increase level of acetate was paired with lower inflammatory markers such as fecal calprotectin, C-reactive protein, and serum amyloid A. It was suggested, however, that dietary intervention should last at least 3 months in order to fully observe its therapeutic effects. Increased intake of other fermentable foods and drinks such as L. acidophilus and Bifidobacterium animalis-containing milk have also been associated with increased acetic acid levels and decreased inflammatory cytokines TNF-α and resistin in patients with type 2 diabetes.112 Healy et al showed that supplementation with an inulin-type fructan prebiotic only increased Bifidobacterium in the low-fiber diet group, whereas in the high fiber group, the same treatment increased Bifidobacterium and Faecalibacterium and decreased Coprococcus, Dorea, and Ruminococcus.113 Therefore, the presence of dietary fiber appears to prime the gut microbiota for a better response to prebiotics, which is particularly useful information when studying how dietary patterns can impact prebiotic effectiveness. Microbiome alterations and increased fecal acetate and propionate concentration have also been observed postinsulin supplementation, although no major increases in bacteria-derived metabolites were noted.114 Another study showed that supplementation with Lactobacillus plantarum based probiotic, Lp299v, in men with coronary artery disease resulted in reduced systemic inflammation, likely a result of increased circulating gut-derived metabolites such as propionate. Interestingly, the levels of acetate were lowered postconsumption.115
However, carbohydrates are not the only metabolite producing nutrients that ease the inflammatory response of disease. A study evaluating the effect of daily avocado consumption on the gut microflora showed positive correlations between avocado consumption and the alpha diversity of Faecalibacterium, Lachnospira, Alistipes (known beneficial bacteria) and fecal acetate and stearic acid levels.116 Therefore, consistent consumption of foods rich in monounsaturated fatty acids is another potential disease management strategy that has yet to be explored.
Conclusions
While previous studies have pointed to bacterial dysbiosis as a contributing factor to IBD pathogenesis, recent evidence suggest that fungal dysbiosis is equally contributing. Furthermore, although traditional IBD treatments such as monoclonal antibodies, aminosalicylates, and immunosuppressants have shown great success in inducing and maintaining disease remission, evolving therapies such as dietary intervention, vitamin administration, probiotic, and postbiotic supplementation are alternative modes of therapy that may be more promising. By targeting specific members of the gut microbiome, these novel therapeutic approaches can reestablish microbial order and potentially reduce disease severity.
Contributor Information
Caitlyn Hsu, Case Digestive Health Research Institute, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA.
Mahmoud Ghannoum, Center for Medical Mycology and Integrated Microbiome Core, Department of Dermatology, Case Western Reserve University, and University Hospitals Cleveland Medical Center, Cleveland, Ohio, 44106, USA.
Fabio Cominelli, Case Digestive Health Research Institute, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA; Department of Medicine, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA; Department of Pathology, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA.
Luca Di Martino, Case Digestive Health Research Institute, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA; Department of Medicine, Case Western University School of Medicine, Cleveland, Ohio, 44106, USA.
Funding
This work was supported by the National Institutes of Health Grant R01AI145289-01A1 to Mahmoud Ghannoum and by the Grant DK125526 to Luca Di Martino.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1. Perler BK, Ungaro R, Baird G, et al. Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019;19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, LoftusEV, Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139(4):1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF.. Ulcerative colitis. Lancet. 2017;389(10080):1756-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye Y, Pang Z, Chen W, Ju S, Zhou C.. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med. 2015;8(12):22529-22542. [PMC free article] [PubMed] [Google Scholar]
- 5. Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye Y, Manne S, Treem WR, Bennett D.. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007-2016. Inflamm Bowel Dis. 2020;26(4):619-625. [DOI] [PubMed] [Google Scholar]
- 7. Wang T, Fan C, Yao A, et al. The Adaptor Protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity. 2018;49(3):504-514.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson S, Peterson CB, Sahasrabhojane P, et al. Observational cohort study of oral mycobiome and interkingdom interactions over the course of induction therapy for leukemia. mSphere. 2020;5(2):e00048-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Group NHW, Peterson J, Garges S, et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoarau G, Mukherjee PK, Gower-Rousseau C, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. mBio. 2016;7(5):e01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hager CL, Ghannoum MA.. The mycobiome: role in health and disease, and as a potential probiotic target in gastrointestinal disease. Dig Liver Dis. 2017;49(11):1171-1176. [DOI] [PubMed] [Google Scholar]
- 12. Moyes DL, Wilson D, Richardson JP, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasper L, Konig A, Koenig PA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9(1):4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doron I, Mesko M, Li XV, et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat Microbiol. 2021;6(12):1493-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Martino L, De Salvo C, Buela KA, et al. Candida tropicalis infection modulates the gut microbiome and confers enhanced susceptibility to colitis in mice. Cell Mol Gastroenterol Hepatol. 2022;13(3):901-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li XV, Leonardi I, Putzel GG, et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature. 2022;603(7902):672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chicco F, Magri S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birimberg-Schwartz L, Wilson DC, Kolho KL, et al. pANCA and ASCA in Children with IBD-unclassified, Crohn’s colitis, and ulcerative colitis-a longitudinal report from the IBD porto group of ESPGHAN. Inflamm Bowel Dis. 2016;22(8):1908-1914. [DOI] [PubMed] [Google Scholar]
- 20. de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N.. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12(4):470-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirayama T, Miyazaki T, Ito Y, et al. Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci Rep. 2020;10(1):3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gulati M, Nobile CJ.. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18(5):310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsui C, Kong EF, Jabra-Rizk MA.. Pathogenesis of Candida albicans biofilm. Pathog Dis. 2016;74(4):ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan CH, Lo HJ, Yan JY, et al. Candida albicans colonizes and disseminates to the gastrointestinal tract in the presence of the microbiota in a severe combined immunodeficient mouse model. Front Microbiol. 2020;11:619878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sendid B, Salvetat N, Sarter H, et al. A pilot clinical study on post-operative recurrence provides biological clues for a role of Candida yeasts and fluconazole in Crohn’s disease. J Fungi (Basel). 2021;7(5):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Wang C, Tang C, He Q, Li N, Li J.. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J Clin Gastroenterol. 2014;48(6):513-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huo X, Li D, Wu F, et al. Cultivated human intestinal fungus Candida metapsilosis M2006B attenuates colitis by secreting acyclic sesquiterpenoids as FXR agonists. Gut 2022:gutjnl-2021-325413. [DOI] [PubMed] [Google Scholar]
- 28. Di Martino L, De Salvo C, Buela KA, et al. Candida tropicalis infection modulates the gut microbiome and confers enhanced susceptibility to colitis in mice. Cell Mol Gastroenterol Hepatol. 2022;13(3):901-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandra J, Mukherjee PK, Ghannoum MA.. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3(12):1909-1924. [DOI] [PubMed] [Google Scholar]
- 30. Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS.. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10-24. [DOI] [PubMed] [Google Scholar]
- 31. Deorukhkar SC, Saini S, Mathew S.. Non-albicans Candida infection: an emerging threat. Interdiscip Perspect Infect Dis. 2014;2014:615958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13(1):122-143, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cafarchia C, Romito D, Coccioli C, Camarda A, Otranto D.. Phospholipase activity of yeasts from wild birds and possible implications for human disease. Med Mycol. 2008;46(5):429-434. [DOI] [PubMed] [Google Scholar]
- 34. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-468. [DOI] [PubMed] [Google Scholar]
- 35. Toruner M, LoftusEV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. [DOI] [PubMed] [Google Scholar]
- 36. Monoclonal Antibodies. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [PubMed] [Google Scholar]
- 37. Melsheimer R, Geldhof A, Apaolaza I, Schaible T.. Remicade((R)) (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellis CR, Azmat CE.. Adalimumab. Treasure Island (FL): StatPearls, 2022. [Google Scholar]
- 39. Gerriets V, Goyal A, Khaddour K.. Tumor Necrosis Factor Inhibitors. Treasure Island (FL): StatPearls, 2022. [PubMed] [Google Scholar]
- 40. Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS.. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67(1):53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colquhoun M, Kemp AK.. Ustekinumab. Treasure Island (FL): StatPearls, 2022. [Google Scholar]
- 43. Dhaliwal J, McKay HE, Deslandres C, et al. One-year outcomes with ustekinumab therapy in infliximab-refractory paediatric ulcerative colitis: a multicentre prospective study. Aliment Pharmacol Ther. 2021;53(12):1300-1308. [DOI] [PubMed] [Google Scholar]
- 44. Scribano ML. Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence. World J Gastroenterol. 2018;24(23):2457-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crooks B, Barnes T, Limdi JK.. Vedolizumab in the treatment of inflammatory bowel disease: evolving paradigms. Drugs Context. 2020;9:2019-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Greenfield SM, Punchard NA, Teare JP, Thompson RP.. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7(4):369-383. [DOI] [PubMed] [Google Scholar]
- 48. Tamura S, Ishida N, Miyazu T, et al. Mesalazine granule formulation improves clinical data in Crohn’s disease compared with tablet formulation. Sci Rep. 2020;10(1):21353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoshino T, Sono M, Yazumi S.. Usefulness of sulfasalazine for patients with refractory-ulcerative colits. BMJ Open Gastroenterol. 2016;3(1):e000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takeshima F, Matsumura M, Makiyama K, et al. Efficacy of long-term 4.0 g/day mesalazine (Pentasa) for maintenance therapy in ulcerative colitis. Med Sci Monit. 2014;20:1314-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D’Haens GR, Sandborn WJ, Zou G, et al. Randomised non-inferiority trial: 1600 mg vs 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2017;46(3):292-302. [DOI] [PubMed] [Google Scholar]
- 52. Sahasranaman S, Howard D, Roy S.. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64(8):753-767. [DOI] [PubMed] [Google Scholar]
- 53. Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology. 2016;150(2):380-388 e384. [DOI] [PubMed] [Google Scholar]
- 54. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139-2149 e2114. [DOI] [PubMed] [Google Scholar]
- 55. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L.. Safety of janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554-1573 e1512. [DOI] [PubMed] [Google Scholar]
- 56. Silverman MS, Davis I, Pillai DR.. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471-473. [DOI] [PubMed] [Google Scholar]
- 57. Wong AC, Levy M.. New approaches to microbiome-based therapies. mSystems 2019;4(3):e00122-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Davis C, Bryan J, Hodgson J, Murphy K.. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139-9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsieh TJ, Chang HY, Wu IC, et al. Independent association between subjective cognitive decline and frailty in the elderly. PLoS One. 2018;13(8):e0201351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Widmer RJ, Flammer AJ, Lerman LO, Lerman A.. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Soest APM, Hermes GDA, Berendsen AAM, et al. Associations between pro- and anti-inflammatory gastro-intestinal microbiota, diet, and cognitive functioning in Dutch healthy older adults: the NU-AGE study. Nutrients. 2020;12(11):3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mitsou EK, Kakali A, Antonopoulou S, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117(12):1645-1655. [DOI] [PubMed] [Google Scholar]
- 63. Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS.. Beneficial properties of probiotics. Trop Life Sci Res. 2016;27(2):73-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roager HM, Vogt JK, Kristensen M, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meslier V, Laiola M, Roager HM, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69(7):1258-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kahleova H, Rembert E, Alwarith J, et al. Effects of a low-fat vegan diet on gut microbiota in overweight individuals and relationships with body weight, body composition, and insulin sensitivity. a randomized clinical trial. Nutrients 2020;12(10):2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y.. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Panizza CE, Lim U, Yonemori KM, et al. Effects of intermittent energy restriction combined with a Mediterranean diet on reducing visceral adiposity: a randomized active comparator pilot study. Nutrients. 2019;11(6):1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pagliai G, Russo E, Niccolai E, et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59(5):2011-2024. [DOI] [PubMed] [Google Scholar]
- 72. Ghannoum M, Smith C, Adamson E, Isham N, Salem I, Retuerto M.. Effect of mycobiome diet on gut fungal and bacterial communities of healthy adults. J Prob Health. 2019;7:215:1-6. [Google Scholar]
- 73. Gracey M, Stone DE, Suharjono, Sunoto. Isolation of Candida species from the gastrointestinal tract in malnourished children. Am J Clin Nutr. 1974;27(4):345-349. [DOI] [PubMed] [Google Scholar]
- 74. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. [PubMed] [Google Scholar]
- 75. Okada M, Ishikawa K, Watanabe K.. Effect of vitamin B6 deficiency on glycogen metabolism in the skeletal muscle, heart, and liver of rats. J Nutr Sci Vitaminol (Tokyo). 1991;37(4):349-357. [DOI] [PubMed] [Google Scholar]
- 76. Galland L. Nutrition and candidiasis. Journal of orthomolecular psychiatry. 1985;14(1):50-60. [Google Scholar]
- 77. Klassert TE, Hanisch A, Brauer J, et al. Modulatory role of vitamin A on the Candida albicans-induced immune response in human monocytes. Med Microbiol Immunol. 2014;203(6):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F.. Association between inflammatory bowel disease and vitamin d deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(11):2708-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouzid D, Merzouki S, Bachiri M, Ailane SE, Zerroug MM.. Vitamin D3 a new drug against Candida albicans. J Mycol Med. 2017;27(1):79-82. [DOI] [PubMed] [Google Scholar]
- 80. Neumann A, Wieczor M, Zielinska J, Baginski M, Czub J.. Membrane sterols modulate the binding mode of amphotericin b without affecting its affinity for a lipid bilayer. Langmuir. 2016;32(14):3452-3461. [DOI] [PubMed] [Google Scholar]
- 81. Hu S, Dai J, Chen X.. Vitamin D reduces autophagy by regulating NF-kappaB resistance to Aspergillus fumigatus infection. Gene. 2020;753:144819. [DOI] [PubMed] [Google Scholar]
- 82. Sroussi HY, Burke-Miller J, French AL, et al. Association among vitamin D, oral candidiasis, and calprotectinemia in HIV. J Dent Res. 2012;91(7):666-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Janeczko M, Kochanowicz E.. Silymarin, a popular dietary supplement shows anti-candida activity. Antibiotics (Basel). 2019;8(4):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Majewski S, Janik P, Langner A, et al. Decreased levels of vitamin A in serum of patients with psoriasis. Arch Dermatol Res. 1989;280(8):499-501. [DOI] [PubMed] [Google Scholar]
- 85. Campione E, Cosio T, Lanna C, et al. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J Pharmacol Sci. 2020;144(1):52-56. [DOI] [PubMed] [Google Scholar]
- 86. Xie J, Zhu L, Zhu T, et al. Zinc supplementation reduces Candida infections in pediatric intensive care unit: a randomized placebo-controlled clinical trial. J Clin Biochem Nutr. 2019;64(2):170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nagpal R, Kumar A, Kumar M, et al. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334(1):1-15. [DOI] [PubMed] [Google Scholar]
- 88. Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105(10):2218-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dizman N, Hsu J, Bergerot PG, et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 2021;10(1):79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Francavilla R, Piccolo M, Francavilla A, et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol. 2019;53(3):e117-e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sekirov I, Russell SL, Antunes LC, Finlay BB.. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859-904. [DOI] [PubMed] [Google Scholar]
- 92. Santocchi E, Guiducci L, Fulceri F, et al. Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry. 2016;16:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kim CS, Cha L, Sim M, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. 2021;76(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wallace CJK, Milev RV.. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12:618279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vautier S, Drummond RA, Chen K, et al. Candida albicans colonization and dissemination from the murine gastrointestinal tract: the influence of morphology and Th17 immunity. Cell Microbiol. 2015;17(4):445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ghannoum MA, McCormick TS, Retuerto M, et al. Evaluation of microbiome alterations following consumption of BIOHM, a novel probiotic. Curr Issues Mol Biol. 2021;43(3):2135-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tsilingiri K, Rescigno M.. Postbiotics: what else? Benef Microbes 2013;4(1):101-107. [DOI] [PubMed] [Google Scholar]
- 98. Klemashevich C, Wu C, Howsmon D, et al. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr Opin Biotechnol. 2014;26:85-90. [DOI] [PubMed] [Google Scholar]
- 99. Zolkiewicz J, Marzec A, Ruszczynski M, Feleszko W.. Postbiotics-a step beyond pre- and probiotics. Nutrients 2020;12(8):2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang W, Chen L, Zhou R, et al. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lawley TD, Walker AW.. Intestinal colonization resistance. Immunology 2013;138(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Noverr MC, Huffnagle GB.. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun. 2004;72(11):6206-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bhaskaran N, Quigley C, Paw C, et al. Role of short chain fatty acids in controlling tregs and immunopathology during mucosal infection. Front Microbiol. 2018;9:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pascale A, Marchesi N, Marelli C, et al. Microbiota and metabolic diseases. Endocrine 2018;61(3):357-371. [DOI] [PubMed] [Google Scholar]
- 105. Segain JP, Raingeard de la Bletiere D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 2000;47(3):397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. De Preter V, Arijs I, Windey K, et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18(6):1127-1136. [DOI] [PubMed] [Google Scholar]
- 107. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7 Suppl):2485S-2493S. [DOI] [PubMed] [Google Scholar]
- 108. Han SB, Lee JK.. Anti-inflammatory effect of Trichostatin-A on murine bone marrow-derived macrophages. Arch Pharm Res. 2009;32(4):613-624. [DOI] [PubMed] [Google Scholar]
- 109. Vojinovic J, Damjanov N, D’Urzo C, et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(5):1452-1458. [DOI] [PubMed] [Google Scholar]
- 110. Tjellstrom B, Stenhammar L, Sundqvist T, et al. The effects of oats on the function of gut microflora in children with coeliac disease. Aliment Pharmacol Ther. 2014;39(10):1156-1160. [DOI] [PubMed] [Google Scholar]
- 111. Fritsch J, Garces L, Quintero MA, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(6):1189-1199 e1130. [DOI] [PubMed] [Google Scholar]
- 112. Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS.. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36(1):85-92. [DOI] [PubMed] [Google Scholar]
- 113. Healey G, Murphy R, Butts C, et al. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2018;119(2):176-189. [DOI] [PubMed] [Google Scholar]
- 114. Biruete A, Cross TL, Allen JM, et al. Effect of dietary inulin supplementation on the gut microbiota composition and derived metabolites of individuals undergoing hemodialysis: a pilot study. J Ren Nutr. 2021;31(5):512-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Malik M, Suboc TM, Tyagi S, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. 2018;123(9):1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Thompson SV, Bailey MA, Taylor AM, et al. Avocado consumption alters gastrointestinal bacteria abundance and microbial metabolite concentrations among adults with overweight or obesity: a randomized controlled trial. J Nutr. 2021;151(4):753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]