Abstract
Background:
The spontaneously hypertensive rat (SHR) is extensively used to study hypertension. Gut microbiota dysbiosis is a notable feature in SHR for reasons unknown. Immunoglobulin A (IgA) is a major host factor required for gut microbiota homeostasis. We hypothesized that inadequate IgA contributes to gut microbiota dysbiosis in SHR.
Methods:
IgA was measured in feces, cecum, serum, liver, gut-associated lymphoid tissue and milk from SHR and Wistar Kyoto (WKY) rats. IgA regulatory factors like IgM, IgG and polymeric immunoglobulin receptor (pIgR) were analyzed. IgA and IgG antibodies and blood pressure (BP) were measured before and after adminstrating a bacterial antigen (i.e., flagellin).
Results:
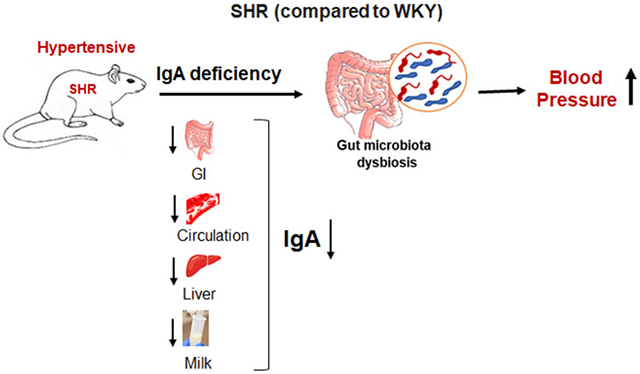
Compared to WKY rats, SHR displayed remarkably near-deficient IgA levels accompanied with compensatory increases in serum IgM and IgG and gut-liver pIgR expression. Inadequate milk IgA in SHR emphasized this immune defect stemmed from the neonatal stage. Reduced IgA+ B cells in circulation and Peyer’s patches indicated a possible reason for the lower IgA in SHR. Noteworthy, a genetic insufficiency was unlikely because administering flagellin to SHR induced anti-flagellin IgA antibodies. This immune response surprisingly accelerated hypertension development in SHR, suggesting IgA quiescence may help maintain lower BP.
Conclusions:
This study is the first to reveal IgA deficiency in SHR as one host factor associated with gut microbiota dysbiosis and invigorates future research to determine the pathophysiological role of IgA in hypertension.
Keywords: IgA, IgG, IgM, B cells, Gut Microbiota, Flagellin, Milk
Graphical Abstract
Introduction
Hypertension is the leading cause of cardiovascular disease and a prevalent reason of early mortality. The spontaneously hypertensive rat (SHR) is widely used to study essential hypertension and the Wistar Kyoto (WKY) rat is its normotensive counterpart. One of the first reported features in SHR was an immunological depression of lymphocytes observed throughout its lifespan.1 Additionally, recent evidence highlights gut microbiota dysbiosis, where the normal gut microorganism ecology is disturbed, as an attribute of SHR.2 Considering that the gut microbiota shapes and is shaped by host immune responses, it could be hypothesized that their bidirectional relationship, when altered, contributes to hypertension.
Among the interactions between commensal bacteria and mucosal immunity, one vital interface is the secretion of immunoglobulins into the gut. Microbial antigens can activate B cells that subsequently undergo receptor editing to generate mucosal antibodies of diverse reactivities.3 Immunoglobulin A (IgA) is the major antibody that provides gut humoral immunity to maintain, rather than eliminate, commensal microorganism biodiversity.4 Gut microbiota dysbiosis requires additional IgA-dependent mechanisms such as limiting microbial encroachment on the gut epithelia and promoting antigen sampling by innate immune cells.4 Underscoring the critical importance of IgA in the gastrointestinal tract, we hypothesized that dampened IgA responses may be associated with gut microbiota dysbiosis and hypertension in the SHR.
Results obtained in this study demonstrate that, irrespective of their source and sex, SHR have near-deficient IgA levels in their cecal content, feces, serum, liver and small intestine compared to WKY. Importantly, we observed this IgA deficiency in milk collected during lactation from SHR dams, suggesting that SHR pups experience developmental programming of IgA insufficiency via maternal malnourishment. One potential reason for the lack of IgA in SHR could be due to the significantly lower level of IgA+ B cells in circulation and gut-associated lymphoid tissue. Of note, SHR had compensatory immune responses with significant increases in circulating IgM and IgG and intestinal pIgR. Interestingly, IgA antibodies were induced in SHR and their blood pressure (BP) increased when immunized with bacterial flagellin, thus suggesting that IgA remains quiescent in SHR rather than overt deficiency. Collectively, these findings demonstrate that early onset of selective IgA deficiency might be a contributor to gut microbiota dysbiosis, but when hypertension is established, its IgA-quiescent state could be an adaptive response.
Methods
An expanded Materials and Methods section is available in the Supplemental Material. Further data and details on materials and protocols related to this study are also available upon reasonable request from the corresponding authors.
Animals
SHR and WKY rats were bred and have been maintained in the Department of Laboratory Animal Resources, University of Toledo College of Medicine and Life Sciences for the last 20 years.5 WKY, stroke-prone spontaneously hypertensive-A3 (SHR-SP) and stroke-resistant spontaneously hypertensive-B2 (SHR-SR) were maintained at the University of Texas.6 For some experiments, male WKY rats and SHR were purchased from a commercial vendor, Charles River Laboratories (www.crl.com). Animals were maintained in specific-pathogen-free (SPF) conditions, housed 2-3 rats per cage with Enrich-n’Pure paper bedding material, and provided ad libitum standard chow diet from Envigo Teklad (Cat #7034) and autoclaved water. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Toledo and University of Texas (protocol approval numbers 104573 and 108390, respectively).
In a separate study, male, seven-week-old germ-free (GF) Sprague Dawley (SD) rats and germ-free rats co-housed with conventional SD rats (1:1 ratio) for 10 days (conventionalized GF [GFC, aka ‘germ full’) were obtained from Taconic Biosciences (https://www.taconic.com/). This co-housing allowed GF rats to obtain a natural, continuous infusion of microbiota via coprophagy. Upon arrival at the University of Toledo, the animals were euthanized after blood pressure (BP) measurement was taken asceptically. Serum samples were collected and stored at −80°C until analysis. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the IACUC at University of Toledo, Toledo, OH.
Statistical Analysis
GraphPad Prism version 9.0 was used for statistical analysis. Nonparametric Mann-Whitney test was used to compare between 2 groups, whereas one-way ANOVA post hoc test was used for studies with >2 groups. Statistically significant values were represented as p≤0.05 (*), p≤0.01 (**), p≤0.001 (***) and p≤0.0001 (****). All data were expressed as mean ± SEM.
Results
Gut microbiota dysbiosis in SHR is marked by an altered bacterial load
A previous study by Yang et al.2 demonstrated a substantial decrease in fecal bacterial load, microbial richness, diversity, and evenness in the SHR. In accordance, we observed a significant reduction of fecal bacterial load in SHR compared to WKY rats (figure S1A). Representation of the obligate anaerobe Bifidobacteria spp. was nearly undetectable in the feces from SHR (figure S1B) while there were no differences in the taxa Lachnospiraceae and Ruminococcaceae and γ-Proteobacteria (figure S1C and D). As the cecum is a thin-walled ‘anaerobic bioreactor’ that houses a large part of the gut microbiome, we then analyzed the total bacterial load and select species in the cecal contents. In contrast to the fecal data, the total bacterial load in the cecal content from SHR was significantly elevated compared to WKY (figure S1E). Also, the elevated cecal bacterial load in SHR was accompanied by a high abundance of Bifidobacteria spp. (figure S1F). However, there were no differences in the taxa Lachnospiraceae, Ruminococcaceae and γ-Proteobacteria (figure S1G and H). Collectively, the skewed bacterial loads between the feces and cecum of SHR are suggestive not only of microbiota dysbiosis, but a reduction in fecal bacterial shedding compared with WKY rats.
SHR exhibit strikingly reduced levels of IgA in mucosal, systemic, and tissue compartments
High microbial density in the ceca, coupled with reduced fecal bacterial shedding, is indicative of an inability for bacterial clearance. Since immunoglobulin A (IgA) facilitates the clearance of gut microbes, we sought to determine whether mucosal (fecal and cecal contents) IgA differed in SHR compared to WKY. Fecal IgA was practically undetectable in young (8 week), adult (12 week), and old SHR (18 week) for both male and female rats (Figure 1A-C). This observation was irrespective of source (i.e., Charles River and U of Toledo). WKY rats had a lower trend of fecal IgA at 18 weeks of age compared to their earlier time points (Figure 1B and C). Neither IgG nor IgM were detectable in the fecal samples of SHR and WKY rats (data not shown), which is in line with evidence that IgA is the main immunoglobulin isotype actively secreted and transported into the gut lumen.7 In support, the cecal content from SHR, but not WKY rats, also had negligible IgA levels (figure S2A and B). Having noted the marked differences in mucosal IgA levels, we next asked whether these differences exist in systemic circulation. Indeed, compared to WKY rats, serum IgA levels were remarkably reduced in SHR from Charles River and the U of Toledo and this was irrespective of sex (Figure 1D-F). Taken together, these findings substantiate for reduced IgA as a potential contributor to gut microbiota dysbiosis in the SHR.
Figure 1. SHR display substantially reduced fecal and serum levels of IgA compared to WKY rats.
Feces were collected from Charles River Laboratory (18 weeks old, male, n=10) and U of Toledo (8, 12 and 18 weeks old, male and female, n=6) WKY and SHR and immediately snap frozen. Fecal samples were analyzed to measure (A-C) Fecal IgA. Hemolysis-free sera were obtained from the above rats for IgA quantification to quantify (D-F) Serum IgA. Results are expressed as means ± SEM. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
Circulating IgA can enter the liver by first binding to the transmembrane region (i.e., secretory component) of the polymeric immunoglobulin receptor (pIgR) located on hepatocytes. The complex is then transported across hepatocytes into the canalicular bile duct and enters into the small intestine.8 The lack of fecal and serum IgA in SHR implied a defect in its enterohepatic circulation. Impressively, SHR had near-deficient IgA levels in their liver and small intestine when compared to WKY rats (Figure 2A and B). Of note, the hepatic and small intestinal expression of pIgR were significantly increased in SHR (Figure 2C and D), suggesting a potential compensatory response to increase IgA importation.
Figure 2. Compensatory increase in gut-liver pIgR and systemic IgM in IgA deficient SHR.
Liver and small intestine (10 cm above the cecum) were collected from the U of Toledo (18 weeks old, male, n=7) WKY and SHR and were analyzed for (A) Liver IgA and (B) Small intestine IgA, by ELISA. (C) Hepatic pIgR and (D) Small intestinal pIgR expression by qRT-PCR, normalized by 18S. SHR and WKY rat hemolysis-free sera were obtained from U of Toledo (8, 12 and 18 weeks old, male and female, n=6). Serum IgM were quantified via commercially available rat ELISA kits from Invitrogen. (E, F) Serum IgM. Results are expressed as means ± SEM. *p<0.05, ***p<0.001 and ****p<0.0001.
SHR have a compensatory increase in systemic IgM and IgG
Selective IgA deficiency is a common primary immune deficiency in humans and is generally asymptomatic. IgM and to a lesser degree IgG are presumed to compensate for the lack of IgA.9-13 Therefore, we next asked if SHR displayed these compensatory immunoglobulin responses to rescue IgA insufficiency. As anticipated, serum IgM levels in SHR were considerably higher than WKY rats (Figure 2E and F, figure S3A) for both male and female rats and this was irrespective of source. Serum IgG levels were also slightly elevated in SHR, albeit only significant for male SHR at 18 weeks old (figure S3B-D). These results demonstrate an attemptive adaptive response to rescue IgA deficiency in SHR.
IgA deficiency in SHR is independent of genetic drift
To rule out differences in their genetics, we investigated the IgA status in two distinct SHR lines of genetic homozygosity i.e., SHR-SP line (stroke prone) and SHR-SR line (stroke resistant).6, 14 Of note, the gut microbiota dysbiosis in SHR-SP correlates with their high BP.15 Consistent with our findings, WKY rats had a ~40-60-fold greater amount of serum IgA than SHR-SP and SHR-SR (figure S4A). Interestingly, there was an age dependent increase of IgA in WKY rats and both SHR-SP and SHR-SR lines from 18 to 40 weeks of age (figure S4A). Most notable was the transition from undetectable to detectable levels at 18 and 40 weeks old, respectively, in the SHR-SP and SHR-SR lines, but both strains had comparable levels at the later time point (figure S4A). Interestingly, both SHR-SP and SHR-SR lines had a similar age-dependent increase in compensatory IgM and this was absent in the WKY rats (figure S4B). Serum IgG levels were also higher in SHR but the differences, when compared to WKY rats, were not as prominent as IgM (figure S4C). The SHR and WKY rats each had augmented IgG levels from 18 to 40 weeks of age, but this age-dependent increase was most pronounced in the SHR-SP and SHR-SR lines (figure S4C). Collectively, these results demonstrate that the SHR, regardless of their maintenance and associated possibilities for genetic drifts, exhibit selective IgA deficiency.
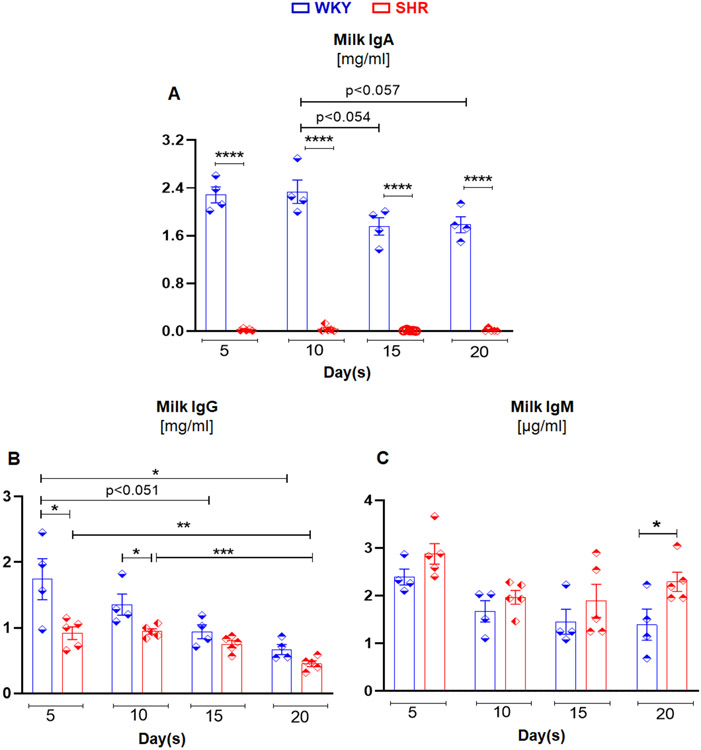
SHR milk has significantly reduced levels of IgA and IgG
Next, we investigated whether IgA deficiency also exists in other mucosal secretions such as breast milk, a complete edible immune system for newborns. Notably, the first source of IgA for a newborn is mother’s milk and provides the first-line of immune defense at mucosal surfaces. This includes regulating initial microbial colonization in the neonatal intestine.16 Accordingly, we collected breast milk from WKY and SHR dams on days 5, 10, 15 and 20 of lactation17 and measured IgA. Impressively, milk IgA levels were nearly undetectable in SHR at each of the lactation time points (Figure 3A). IgG levels were also considerably lower in milk from the SHR at days 5 and 10 of lactation, but at later time points (15 and 20 days) milk IgG levels were comparable between WKY and SHR dams (Figure 3B). Milk IgM levels were similar in both WKY and SHR dams, though there was a higher trend from SHR at days 5 and 15 of lactation and significant elevation on day 20 (Figure 3C). Despite the lack of IgA in milk, when incubated with E. coli K12 (MG1655), the anti-microbial property of milk from SHR was comparable to WKY rats (figure S5). These data support the view that exposure to a compromised, but quiescent, immune system (i.e., low IgA in milk) early in life could be contributing to the onset of gut microbiota dysbiosis in the SHR.
Figure 3. Milk from SHR contains reduced levels of IgA and IgG with some degree of compensatory IgM response in comparison to milk from WKY rats.
Milk samples were collected from 12 week old WKY (n=4) and SHR (n=5) dams at 5, 10, 15 and 20 days of postnatal stage. Milk (A) IgA, (B) IgG, and (C) IgM levels in WKY and SHR. Results are expressed as means ± SEM. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
SHR have diminished B cells in circulation and Peyer’s patches
B cells are essential for humoral responses via production of antigen-specific antibodies, including IgA. After observing the lack of IgA in SHR, we next measured the B cell population in peripheral blood and Peyer’s patches (representative gut-associated lymphoid tissue). We also investigated B cell levels in the spleen and bone marrow because circulating IgA is primarily secreted by mature plasma cells in the bone marrow.4, 18 The % CD45RA+ total B cell population in circulation and Peyer’s patches were significantly lower in SHR compared to WKY rats (Figure 4A and B). In addition, the % CD45RA+CD127+ immature B cell subset in SHR was substantially reduced in circulation, Peyer’s patches and spleen, but not in bone marrow (Figure 4C and D, figure S6A and S7A), implicating that SHR has no defect in B cell development in the bone marrow. We also observed that IgA+ B cells were trending lower in circulation and substantially reduced in Peyer’s patches and spleens of SHR (Figure 4E and F, figure S7B), but no changes were seen in bone marrow (figure S6B). These results indicated that less IgA+ B cells could be, in part, a potential factor for the IgA deficiency in SHR.
Figure 4. Reduced IgA+ B cells in circulation and Peyer's patches in SHR.
Blood from WKY rats and SHR (12 weeks old, males, n=6) were collected in EDTA tubes. Whole blood erythrocytes were lysed with red blood cell lysis buffer and stained with PE anti-rat CD45RA and Alexa fluor 647 anti-rat CD127. Cells were fixed and permeabilized followed by incubation with FITC anti-rat IgA for 1 h at RT, washed and analyzed on BD Accuri C6 Plus (BD Biosciences). Peyer’s patches immune cells were isolated from WKY rats and SHR (12 weeks old, males, n=6) and stained for PE anti-rat CD45RA and Alexa fluor 647 anti-rat CD127. Cells were washed, fixed and permeabilized, followed by incubation with FITC anti-rat IgA. Cells were analyzed by flow cytometry. (A) Peripheral and (B) Peyer’s Patches CD45RA+ B cells; (C) Peripheral and (D) Peyer’s patches CD45RA+CD127+ B cells. (E) Peripheral blood CD45RA+IgA+ B cells and (C) Peyer’s patches CD45RA+CD127+ B cells. Results are expressed as means ± SEM. *p<0.05, ***p<0.001.
Induction of IgA via microbial colonization or microbe-associated molecular patterns in germ-free rats and SHR, respectively, increases BP
The presence of microbes is well-acknowledged to be required for IgA induction in germ-free animals.19 Further, introducing microbes to germ-free rats was found to increase BP through restored vascular contractility.20 To assess the possible contribution of the gut microbiota induced-IgA on BP, germ-free rats were co-housed with conventional rats (1:1 ratio) for 10 days to introduce the gut microbiota via copraphagy. Interestingly, we observed an increasing trend in fecal IgA and a striking elevation in systemic IgA levels in the conventionalized germ-free rats (Figure 5A and B), which was positively associated with an increase in mean arterial pressure (MAP, Figure 5C and D). Moreover, these conventionalized germ-free rats expressed higher levels of pIgR in their liver and small intestine relative to the germ-free counterparts (Figure 5E and F). Collectively, these findings from a non-genetic hypertensive model suggest that microbiota status, rather than pre-existing elevated BP, is the determinant of IgA levels.
Figure 5. Conventionalization of germ-free rats robustly induced IgA and increased mean arterial pressure.
Seven-week-old male Sprague Dawley (SD, n=6) rats that were either germ-free (GF) or germ-free conventionalized (GFC) for 10 days. (A) Fecal IgA, (B) Serum IgA, (C) mean arterial pressure (MAP), and (D) Spearman correlation between MAP and serum IgA levels. pIgR expression in (E) Liver and (F) Small intestine by qRT-PCR, normalized by 18S. Results are expressed as means ± SEM. *p<0.05, **p<0.01, ***p<0.001.
As SHR already have a residing gut microbiota community, we sought to investigate whether microbe-associated molecular patterns (MAMPs) such as flagellin could restore systemic IgA levels and regulate BP. MAMPs have been well documented in its involvement of BP regulation.21-23 Of note, systemic IgA reactive to flagellin was positively associated with BP in humans.24 To determine if SHR could mount an IgA response, we administered purified bacterial flagellin (a Toll-Like Receptor 5 agonist)25-27 from Salmonella typhimurium on days 0 and 14 to SHR and WKY rats, and quantitated anti-flagellin IgA28 in serum one week post last injection (day 21) (Figure 6Ai). Impressively, SHR responded readily by generating similar anti-flagellin IgA reactivity as observed in WKY rats (Figure 6B). The anti-flagellin IgG was also similar in both SHR and WKY rats (Figure 6C). These data suggest that the observed IgA deficiency is likely not due to genetic defects since SHR can still generate IgA in respone to an antigen challenge.
Figure 6. SHR exhibited anti-flagellin IgA and increased BP following flagellin challenge.
(A). Experimental flow charts of flagellin treatments for measuring (i) anti-flagellin IgA and IgG and (ii) BP via radio-telemetry. Serum was collected from SHR and WKY rats (n=5, 6 weeks old, day 0 and day 21 of flagellin treatment). Anti-flagellin (B) IgA and (C) IgG were analyzed via ELISA. (D) Systolic and diastolic BP (mmHg) for SHR and WKY for flagellin intervention. Results are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001***and ****p<0.0001.
We next hypothesized that rescuing IgA deficiency in SHR could lower BP. Seven week old SHR and WKY rats were administered with 2 weekly doses (days 14 and 21) of flagellin and monitored for BP by radio-telemetry (Figure 6Aii). Disproving our hypothesis, both systolic and diastolic BP were increased 24 h after each flagellin treatment in SHR when compared to baseline (5 weeks old, day 0) BP values (Figure 6D). Though we can not rule out the possible effect of age-related BP elevation in SHR, the observed BP spike in SHR was associated with flagellin treatment. At the 360 h time point (day 15, 24 h after 1st injection), BP (systolic: 143 mmHg; diastolic: 95 mmHg) was within the standard deviation from baseline (systolic: 136 mmHg ± 2.05; diastolic 90 mmHg ± 1.00), and we observed a significant BP increase 28-40 h post flagellin treatment (systolic: 153 mmHg ± 2.64; diastolic: 102 mmHg ± 1.80). A similar pattern in BP elevation was noted with the second injection of flagellin and it further increased BP (systolic: 159 mmHg ± 2.72; diastolic 106 mmHg ± 1.84). Intriguingly, flagellin treatment also increased the BP of WKY rats albeit modestly and they remained normotensive (Figure 6D). Thus, contrary to our hypothesis, induction of immunoreactive IgA did not decrease, but increased, BP in SHR. Collectively, these data support our overall conclusion that the SHR display a quiesent IgA response and succumb to gut microbiota dysbiosis to protect against an elevation in BP beyond its natural propensity to develop hypertension.
Discussion
SHR and WKY rats are widely used animal models to study essential hypertension.29, 30 One notable feature of SHR is a depression of lymphocytes observed before the onset of hypertension and lasting through its lifespan.1, 31 Immunological research in SHR has focused on T cell depression, including the lack of anti-inflammatory regulatory T cells.32, 33 Accordingly, restoration of T cells was found to reduce BP in SHR.34-38 Contrary, a recent study highlights the pro-hypertensive effects of Th17 cells in SHR.39 This finding supports an earlier hypothesis by Takeichi et al. (1980)1 that, over generations, SHR inadvertently selected immunological depression to limit the possible pro-hypertensive effects from immune cells. This hypothesis, however, has been only partially investigated because there is little knowledge to whether B cell suppression in SHR results in altered humoral functions and changes in BP.
Immunoglobulins are major effector molecules from B cells that provide protection against bacterial pathogens. IgA is the most produced antibody isotype in mucosal secretions, and its role in gut microbiota homeostasis has been well-studied.40 Gut microbiota dysbiosis may participate in the etiology of hypertension in SHR.2, 41-43 However, it is unclear as to what factor(s) contribute to gut microbiota dysbiosis preceding hypertension. Integrating the well-recognized features of lymphocyte depression and gut microbiota dysbiosis in SHR, we discovered IgA deficiency as a plausible link between the two attributes.
Our work discovered that SHR have near-deficient IgA levels in enterohepatic circulation (cecal, serum, liver, and small intestine) and mucosal secretions (fecal and milk) compared to normotensive WKY rats. Our results also demonstrated that SHR had a certain degree of IgM and IgG compensatory responses, in addition to increased hepatic and small intestinal pIgR expression, but these were not sufficient to re-establish the gut microbiota eubiosis nor correct IgA levels. The low level of total and IgA-producing B cells in circulation and Peyer’s patches implicated a plausible reason to why SHR are inherently prone to IgA deficiency.
Our profound observation of IgA deficiency in SHR contradicts a previous study by Chen and Schachter (1993)44 wherein both SHR and WKY rats were shown to progressively elevate systemic IgA levels with age, but only the SHR developed hypertension. We postulate that the discrepancy could be due to the prior study’s (i) use of outbred WKY [Tac:N-(WKY)fflR] and SHR [Tac:N(SHR)fBR] from Taconic facility (no longer available) whereas the inbred SHR used in our study could have selectively retained IgA deficiency, and (ii) different methodology to quantify serum IgA levels. For our study, we have confirmed the sensitivity and specificity is stringent by using monoclonal capture and detection antibodies for our ELISA procedures.
Selective IgA deficiency (sIgAD) is the most common immunodeficiency in humans and this condition has been connected to autoimmunity and nephropathy,10, 45-49 but whether sIgAD is associated with hypertension is underexplored. Importantly, sIgAD patients have been reported to exhibit reduced gut microbiota diversity and enrichment of opportunistic pathogens like E. coli.50 One potential reason underlying sIgAD in SHR could be that the bacterial composition may poorly elicit an IgA response. Evidence that gut microbiota is responsible for eliciting an IgA response is strengthened by our observation that introduction of microbiota to germ-free rats elevated systemic IgA levels and BP. However, the prevailing dysbiotic microbiota in the SHR, for unknown reasons, does not basally evoke an IgA response, as noted by their IgA deficiency. Interestingly, the ability of SHR to mount an IgA response (despite insufficient IgA levels) after immunization with flagellin is evidence for intact IgA production and affinity maturation. This would implicate that SHR maintains IgA levels in a quiescent state.
It is tempting to speculate that, during inbreeding, a ‘natural selection’ process resulted in sIgAD to protect SHR against IgA-nephropathy and autoreactive antibodies that would exacerbate hypertension. This idea is supported with a report that SHR display autoreactive IgA antibodies to single and double stranded DNA or thyroglobulin44 and from our SHR data wherein BP further increased by rescuing the IgA via flagellin. Although it could not be ruled out if inflammatory responses from innate immune cells contribute to this observation, these data support the notion that increasing IgA could be detrimental to the already hypertensive state. As such, our study tracing IgA deficiency at the early life window during lactation, when the pups are acquiring microbiota, could be thought of as preparing SHR for a life-long adaptation to limit the development of exaggerated hypertension. Our findings serve as the basis for further studies to test the hypothesis that SHR have adapted the lesser of two adverse possibilities, which is to develop microbiota dysbiosis as a consequence rather than raise IgA levels that may further increase BP.
In conclusion, our study has shed light on a previously undiscovered IgA deficiency as a potential cause of microbiota dysbiosis in the SHR and raises interesting mechanistic possibilities for the risk of hypertension among humans with gut microbiota dysbiosis and sIgAD.
Perspectives
Our results provide a novel phenotype in SHR i.e., IgA deficiency and proposes two possibilities. First, the early onset of near IgA deficiency could be the precursor for gut microbiota dysbiosis that later contributes to hypertension. Second, the quiescent feature of IgA in the SHR is an ‘inherited adaptation’ to limit autoreactive IgA induced end-organ damage, such as IgA-nephropathy. Importantly, SHR appear to have a developmental programming of IgA deficiency via maternal malnourishment because its unavailable in breast milk for SHR pups during lactation. Another interesting observation was the IgA deficiency triggered compensatory immune responses in other immunoglobulins and transporters, but such compensations were ineffective to restore gut microbiota eubiosis. In conclusion, our finding of SHR being a natural model of IgA quiescence, microbiota dysbiosis and hypertension warrants similar investigations of IgA deficiency in humans as a contributor to microbiota dysbiosis and hypertension. IgA supplement holds tremendous potential for targeted microbiome manipulation that could also mitigate the negative effects of antibiotic use in primary antibody deficiency. Finally, IgA supplements have a potential application in clinical translation as they could be used to restore microbial homeostasis even if a patient's condition is not limited to antibody defects.
Supplementary Material
Novelty and Relevance.
1. What Is New?
Discovered a naturally-occuring selective IgA deficiency as a previously unrecognized feature in the spontaneously hypertensive rat (SHR).
Insufficient IgA is one of the potential factors contributing to gut microbiota dysbiosis in SHR.
Despite this inherent lack in IgA levels, the SHR readily generated immunoreactive IgA when challenged with a bacterial antigen, indicating that IgA is maintained at a low, quiescent state.
2. What Is Relevant?
Selective IgA deficiency is a very common primary immune deficiency in humans and is generally asymptomatic.
IgA is the major immune protein that regulates gut microbiota homeostasis and patients with hypertension present with gut dysbiosis, but their IgA status is unknown.
SHR is a model of gut microbiota dysbiosis, lymphocyte depression and hypertension, but knowledge on the status of IgA in this model is limiting. Considering the importance of IgA in gut microbiota homeostasis, its deficiency is certain to result in a disturbed microbial ecology, i.e., gut microbiota dysbiosis.
3. Clinical/Pathophysiological Implications
IgA supplements could be a potential target in clinical translation as they could be used to reinstate microbial homeostasis in the patient with IgA or primary antibody deficiency.
Acknowledgements:
P.S. and M.V-K for conception and design. P.S., B.M., R.M.G., and V.R.B. for acquisition of data. P.S., B.M., R.M.G., V.R.B., A.A.A., X.M., and B.S.Y. for analysis and interpretation of data. P.S. and R.M.G. for drafting the manuscript. P.S, B.M., R.M.G, B.S.Y., P.A.D., A.T.G., B.J., and M.V-K for revising it critically for important intellectual content. P.S., B.M., R.M.G, V.R.B., A.A.A., X.M., B.S.Y., P.A.D., A.T.G., B.J., and M.V-K for final approval of the version to be published.
Sources of Funding:
This work was supported by grants from the National Institutes of Health (NIH) to M.V-K, BJ and PAD (CA219144 to M.V.-K., HL143082 to BJ, HG011252 and R01DK114235 to PAD), grants from Crohn’s and Colitis Foundation (CCF) and American Heart Association (AHA) Career Development Awards (854385 and 855256 respectively) to PS, and grant from AHA (831112) to BSY.
Non-standard Abbreviations and Acronyms
- BP
blood pressure
- GF
germ-free
- IACUC
institutional animal care and use committee
- IgA
immunoglobulin A
- MAP
mean arterial pressure
- MAMP
microbe-associated molecular patterns
- pIgR
polymeric immunoglobulin receptor
- SD
sprague dawley
- SHR
spontaneously hypertensive rat
- SHR-SP
spontaneously hypertensive rat – stroke prone
- SHR-SR
spontaneously hypertensive rat – stroke resistant
- sIgAD
selective immunoglobulin A deficiency
- SPF
specific pathogen free
- WKY
wistar kyoto
Footnotes
Disclosures: None
References
- 1.Takeichi N, Suzuki K, Okayasu T, Kobayashi H. Immunological depression in spontaneously hypertensive rats. Clin Exp Immunol. 1980;40:120–126 [PMC free article] [PubMed] [Google Scholar]
- 2.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW. Microbial colonization influences early b-lineage development in the gut lamina propria. Nature. 2013;501:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abokor AA, McDaniel GH, Golonka RM, Campbell C, Brahmandam S, Yeoh BS, Joe B, Vijay-Kumar M, Saha P. Immunoglobulin a, an active liaison for host-microbiota homeostasis. Microorganisms. 2021;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie Y, Kumarasamy S, Waghulde H, Cheng X, Mell B, Czernik PJ, Lecka-Czernik B, Joe B. High-resolution mapping of a novel rat blood pressure locus on chromosome 9 to a region containing the spp2 gene and colocalization of a qtl for bone mass. Physiol Genomics. 2016;48:409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmitrieva RI, Hinojos CA, Boerwinkle E, Braun MC, Fornage M, Doris PA. Hepatocyte nuclear factor 1 and hypertensive nephropathy. Hypertension. 2008;51:1583–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haneberg B, Aarskog D. Human faecal immunoglobulins in healthy infants and children, and in some with diseases affecting the intestinal tract or the immune system. Clin Exp Immunol. 1975;22:210–222 [PMC free article] [PubMed] [Google Scholar]
- 8.Brown WR, Kloppel TM. The liver and iga: Immunological, cell biological and clinical implications. Hepatology. 1989;9:763–784 [DOI] [PubMed] [Google Scholar]
- 9.Yazdani R, Azizi G, Abolhassani H, Aghamohammadi A. Selective iga deficiency: Epidemiology, pathogenesis, clinical phenotype, diagnosis, prognosis and management. Scand J Immunol. 2017;85:3–12 [DOI] [PubMed] [Google Scholar]
- 10.Yel L Selective iga deficiency. J Clin Immunol. 2010;30:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikfarjam J, Pourpak Z, Shahrabi M, Nikfarjam L, Kouhkan A, Moazeni M, Aghamohammadi A. Oral manifestations in selective iga deficiency. Int J Dent Hyg. 2004;2:19–25 [DOI] [PubMed] [Google Scholar]
- 12.Cunningham-Rundles C Physiology of iga and iga deficiency. J Clin Immunol. 2001;21:303–309 [DOI] [PubMed] [Google Scholar]
- 13.Reich RH, Berten JL. [growth induction via condylar reconstruction in children]. Fortschr Kieferorthop. 1991;52:40–43 [DOI] [PubMed] [Google Scholar]
- 14.Gigante B, Rubattu S, Stanzione R, Lombardi A, Baldi A, Baldi F, Volpe M. Contribution of genetic factors to renal lesions in the stroke-prone spontaneously hypertensive rat. Hypertension. 2003;42:702–706 [DOI] [PubMed] [Google Scholar]
- 15.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr., Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandtzaeg P The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–15 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T An automated experimental milker for rat. J Vet Med Sci. 2003;65:557–562 [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of iga induction and function. Mucosal Immunol. 2008;1:11–22 [DOI] [PubMed] [Google Scholar]
- 19.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R 3rd, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of iga immune responses. Science. 2010;328:1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joe B, McCarthy CG, Edwards JM, Cheng X, Chakraborty S, Yang T, Golonka RM, Mell B, Yeo JY, Bearss NR, Furtado J, Saha P, Yeoh BS, Vijay-Kumar M, Wenceslau CF. Microbiota introduced to germ-free rats restores vascular contractility and blood pressure. Hypertension. 2020;76:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JM, Roy S, Galla SL, Tomcho JC, Bearss NR, Waigi EW, Mell B, Cheng X, Saha P, Vijay-Kumar M, McCarthy CG, Joe B, Wenceslau CF. Fpr-1 (formyl peptide receptor-1) activation promotes spontaneous, premature hypertension in dahl salt-sensitive rats. Hypertension. 2021;77:1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrentraut S, Frede S, Stapel H, Mengden T, Grohe C, Fandrey J, Meyer R, Baumgarten G. Antagonism of lipopolysaccharide-induced blood pressure attenuation and vascular contractility. Arterioscler Thromb Vasc Biol. 2007;27:2170–2176 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Xu H, Tu X, Gao Z. The role of short-chain fatty acids of gut microbiota origin in hypertension. Front Microbiol. 2021;12:730809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locks LM, Mwiru RS, Mtisi E, Manji KP, McDonald CM, Liu E, Kupka R, Kisenge R, Aboud S, Gosselin K, Gillman M, Gewirtz AT, Fawzi WW, Duggan CP. Infant nutritional status and markers of environmental enteric dysfunction are associated with midchildhood anthropometry and blood pressure in tanzania. J Pediatr. 2017;187:225–233 e221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijay-Kumar M, Gewirtz AT. Flagellin: Key target of mucosal innate immunity. Mucosal Immunol. 2009;2:197–205 [DOI] [PubMed] [Google Scholar]
- 26.Sanders CJ, Yu Y, Moore DA 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires t cells and activation of innate immunity. J Immunol. 2006;177:2810–2818 [DOI] [PubMed] [Google Scholar]
- 27.Gewirtz AT, Simon PO Jr., Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler TR, Luo M, Estivariz CF, Moore DA 3rd, Sitaraman SV, Hao L, Bazargan N, Klapproth JM, Tian J, Galloway JR, Leader LM, Jones DP, Gewirtz AT. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:R402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padmanabhan S, Joe B. Towards precision medicine for hypertension: A review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev. 2017;97:1469–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev. 2000;80:135–172 [DOI] [PubMed] [Google Scholar]
- 31.Takeichi N, Suzuki K, Kobayashi H. Characterization of immunological depression in spontaneously hypertensive rats. Eur J Immunol. 1981;11:483–487 [DOI] [PubMed] [Google Scholar]
- 32.Katsuki M, Hirooka Y, Kishi T, Sunagawa K. Decreased proportion of foxp3+ cd4+ regulatory t cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertens. 2015;33:773–783; discussion 783 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Ba D, Takeichi N, Hosokawa M, Kobayashi H. Screening of various immunopotentiators in spontaneously hypertensive rats with t-cell depression. Tohoku J Exp Med. 1983;139:187–193 [DOI] [PubMed] [Google Scholar]
- 34.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of t cell depression and suppression of blood pressure in spontaneously hypertensive rats (shr) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216 [PubMed] [Google Scholar]
- 35.Fernandes G, Rozek M, Troyer D. Reduction of blood pressure and restoration of t-cell immune function in spontaneously hypertensive rats by food restriction and/or by treadmill exercise. J Hypertens Suppl. 1986;4:S469–474 [PubMed] [Google Scholar]
- 36.Khraibi AA, Norman RA Jr., Dzielak DJ. Chronic immunosuppression attenuates hypertension in okamoto spontaneously hypertensive rats. Am J Physiol. 1984;247:H722–726 [DOI] [PubMed] [Google Scholar]
- 37.Norman RA Jr., Dzielak DJ. Immunological dysfunction and enhanced sympathetic activity contribute to the pathogenesis of spontaneous hypertension. J Hypertens Suppl. 1986;4:S437–439 [PubMed] [Google Scholar]
- 38.Norman RA Jr., Dzielak DJ, Bost KL, Khraibi AA, Galloway PG. Immune system dysfunction contributes to the aetiology of spontaneous hypertension. J Hypertens. 1985;3:261–268 [DOI] [PubMed] [Google Scholar]
- 39.Kim JY, Lee E, Koo S, Kim CW, Kim I. Transfer of th17 from adult spontaneous hypertensive rats accelerates development of hypertension in juvenile spontaneous hypertensive rats. Biomed Res Int. 2021;2021:6633825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson DA, McNulty NP, Guruge JL, Gordon JI. Iga response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339 [DOI] [PubMed] [Google Scholar]
- 41.Kumar D, Mukherjee SS, Chakraborty R, Roy RR, Pandey A, Patra S, Dey S. The emerging role of gut microbiota in cardiovascular diseases. Indian Heart J. 2021;73:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. 2015;47:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CM, Schachter D. Elevation of plasma immunoglobulin a in the spontaneously hypertensive rat. Hypertension. 1993;21:731–738 [DOI] [PubMed] [Google Scholar]
- 45.Saifi M, Wysocki CA. Autoimmune disease in primary immunodeficiency: At the crossroads of anti-infective immunity and self-tolerance. Immunol Allergy Clin North Am. 2015;35:731–752 [DOI] [PubMed] [Google Scholar]
- 46.Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the pd-1/pd-l1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12:1091–1100 [DOI] [PubMed] [Google Scholar]
- 47.Baldovino S, Montin D, Martino S, Sciascia S, Menegatti E, Roccatello D. Common variable immunodeficiency: Crossroads between infections, inflammation and autoimmunity. Autoimmun Rev. 2013;12:796–801 [DOI] [PubMed] [Google Scholar]
- 48.Gu Y, Jensen PE, Chen X. Immunodeficiency and autoimmunity in h2-o-deficient mice. J Immunol. 2013;190:126–137 [DOI] [PubMed] [Google Scholar]
- 49.Sciascia S, Ceberio L, Garcia-Fernandez C, Roccatello D, Karim Y, Cuadrado MJ. Systemic lupus erythematosus and infections: Clinical importance of conventional and upcoming biomarkers. Autoimmun Rev. 2012;12:157–163 [DOI] [PubMed] [Google Scholar]
- 50.Moll JM, Myers PN, Zhang C, Eriksen C, Wolf J, Appelberg KS, Lindberg G, Bahl MI, Zhao H, Pan-Hammarstrom Q, Cai K, Jia H, Borte S, Nielsen HB, Kristiansen K, Brix S, Hammarstrom L. Gut microbiota perturbation in iga deficiency is influenced by iga-autoantibody status. Gastroenterology. 2021;160:2423–2434 e2425 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.