Abstract

Here, we exploit our mechanochemical synthesis for co-crystallization of an organic antiseptic, proflavine, with metal-based antimicrobials (silver, copper, zinc, and gallium). Our previous studies have looked for general antimicrobial activity for the co-crystals: proflavine·AgNO3, proflavine·CuCl, ZnCl3[Proflavinium], [Proflavinium]2[ZnCl4]·H2O, and [Proflavinium]3[Ga(oxalate)3]·4H2O. Here, we explore and compare more precisely the bacteriostatic (minimal inhibitory concentrations) and antibiofilm (prevention of cell attachment and propagation) activities of the co-crystals. For this, we choose three prominent “ESKAPE” bacterial pathogens of Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The antimicrobial behavior of the co-crystals was compared to that of the separate components of the polycrystalline samples to ascertain whether the proflavine–metal complex association in the solid state provided effective antimicrobial performance. We were particularly interested to see if the co-crystals were effective in preventing bacteria from initiating and propagating the biofilm mode of growth, as this growth form provides high antimicrobial resistance properties. We found that for the planktonic lifestyle of growth of the three bacterial strains, different co-crystal formulations gave selectivity for best performance. For the biofilm state of growth, we see that the silver proflavine co-crystal has the best overall antibiofilm activity against all three organisms. However, other proflavine–metal co-crystals also show practical antimicrobial efficacy against E. coli and S. aureus. While not all proflavine–metal co-crystals demonstrated enhanced antimicrobial efficacy over their constituents alone, all possessed acceptable antimicrobial properties while trapped in the co-crystal form. We also demonstrate that the metal–proflavine crystals retain antimicrobial activity in storage. This work defines that co-crystallization of metal compounds and organic antimicrobials has a potential role in the quest for antimicrobials/antiseptics in the defense against bacteria in our antimicrobial resistance era.
Keywords: antimicrobials, quaternary-ammonium compound, silver, copper, gallium, zinc, co-crystallization
Introduction
The current state of the antibiotic resistance crisis puts pressure for more research toward the development of new and novel antimicrobial agents, as we find ourselves in the antimicrobial resistance (AMR) era.1 The rates at which clinically and agriculturally relevant pathogens are gaining resistance toward treatment with available antimicrobials are outpacing the rates at which new antimicrobials arrive at the market.1 WHO now considers AMR the hidden pandemic, claiming that we will see 10 million human deaths per year by 2050, which will be greater than those due to cancer. The issues of the emerging crisis of antibiotic resistance and inadequate focus in bacterial susceptibility assessment call for re-evaluation of conventional approaches in antimicrobial development. We also have the issue of the paradigm of antibiotic research and development almost exclusively focusing on the planktonic (free-swimming) form of microbial growth. However, it is now well appreciated that in nature, bacteria predominantly appear in the form of aggregations or surface-attached communities—biofilms.2 Biofilms are far less susceptible to antibiotics, due to their extracellular matrix of exopolysaccharides, proteins, nucleic acids, and other biomolecules such as metabolites, which are thought to generate a barrier of penetration from the external environment.2 Additionally, there are remarkable changes in cellular biochemistry and physiology, compared to the planktonic form.2 Thus, biofilms provide a unique challenge in antimicrobial formulation development.
Among the promising directions to address AMR are metal- and metalloid-based antimicrobials (MBAs).3 Unlike conventional antibiotics, which tend to demonstrate target-specific biochemical activities, metal ions and their related chemical species are believed to have multiple biochemical targets within the bacterial cell, providing multifactorial modes of action. As recently reviewed in detail,4 MBAs may cause protein damage by direct redox chemistry of biomolecules and substitution of the essential metals both in active and structural sites of proteins. Some MBAs interfere with nutrient intake, damage the cell membrane, and may lead to electron transport chain (ETC) decoupling. MBAs may bind to nucleic acids leading to differential regulation and/or mutations. Another MBA toxicity factor is the generation of various reactive oxygen species (ROS) that may be catalyzed directly by the metal or indirectly (via ETC decoupling or [Fe–S] disruption), leading to further cellular damage.
Although many metals have antimicrobial activities, those that have shown strong potential and are in use include Ag(I),5 Cu(II),6 Zn(II),7 and Ga(III).8 Ag(I) and Cu(II) are examples of “soft” acids according to hard–soft acids and bases theory9 and a major part of their antibacterial activity depends on interactions with “soft” bases (thiols) within the bacterial cell. Cu(II) and Cu(I) ions were shown to interact with thiol groups, such as cysteine side chains and glutathione, interfering with natural thiol biochemistry and depleting their ability to act as antioxidants.10 Ag(I) and Cu(II) bind and/or oxidize “soft” sulfur atoms and thus cause functional changes in proteins, frequently inactivating them. [Fe–S] clusters are yet another target,11,12 and destruction of [Fe–S] clusters by replacing the Fe with the antimicrobial metal leads, first, to the respective protein malfunction and, second, to the release of highly Fenton-active Fe(II) ions leading to oxidative damage by means of ROS production. As such, exposure to excessive amounts of Ag(I) and Cu(II) leads directly to the impairment of [Fe–S] reactions in the ETC and indirectly to ROS generation due to increased Fe activity. Zn(II) has been shown to interfere with essential metal uptake, specifically Mn.13 Ag(I) is known to disrupt bacterial cell wall continuity in as yet undefined mechanism.14 Cu(II) can also interfere with bacterial cell wall biosynthesis by inhibiting ld-transpeptidase.15 Ag(I) has also been shown to inhibit urease through restricting key conformational changes.16 Antimicrobial activity of the Ga(III) ion is based on its chemical similarity to Fe(III). Ga(III) competes for Fe(III) transport systems, thus depleting the bacterial Fe(III) supply. Ga(III) may also be incorporated in metalloproteins instead of Fe(III); however, unlike Fe(III), Ga(III) is not redox-active under physiological conditions.17
Using a combination of antimicrobials that affect a cell very differently is an approach to combat AMR, as it reduces the rate of resistance. The probability that mutations will occur in a single cell within two different genes at the same time to provide resistance to both antimicrobials is astronomically low. An additional advantage of the combinatorial approach of any two different antimicrobials is that one may obtain a greater efficacy than would be expected from summing their effects up and demonstrate synergistic outcomes. Previous work has shown MBAs to have synergistic effects with some quaternary cation compounds (QCCs) used as antiseptics.18 QCCs are a class of molecules that possess a positively charged quaternary atom, typically nitrogen, and less frequently arsenic or phosphorus. Amphiphilic members of the group demonstrate antimicrobial activity due to their ability to be incorporated into lipid bilayers and thus impair bacterial membrane functions.19 Planar QCC molecules can also be intercalated between the nucleic acid bases and thus are genotoxic.
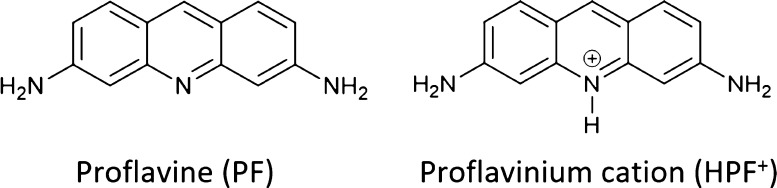
We have begun to use co-crystallization in our exploration to mix metal-based antimicrobials with QCCs. We have chosen proflavine (acridine-3,6-diamine), a notable example of planar QCCs,20 which has been used as a disinfectant since early in the 20th century and has continued to find use as a topical antimicrobial in 21st century. Its antimicrobial properties are based on three principles: (i) photosensitizing the intercalated region of DNA and causing frameshift mutations, (ii) inhibition of DNA and RNA polymerases, thus interfering with DNA replication and transcription, and (iii) affecting cell membrane fluidity. The molecular diagrams of proflavine (PF) and of its monoprotonated proflavinium cation (HPF+) are shown in Scheme 1. The character of this QCC provided a foundational compound for co-crystallization with metals for proof of principle work.
Scheme 1. Neutral PF and Its Monoprotonated Proflavinium Cation (HPF+).
There are several strategies that aim to mix organics with metals to provide novel superior antimicrobial, anti-cancer, and anti-fungal drugs and those for other purposes. Crystal engineering, that is, the design, preparation, and characterization of multi-component systems based on supramolecular interactions,21 is now being actively exploited also to tackle the problem of AMR. Crystal engineering approaches utilizing metal–organic complexes and coordination networks can enhance the delivery and pharmaceutical properties of both the counterparts.
Examples of metals containing multi-component materials are metal organic frameworks, metal–organic gels, incorporation of antimicrobial metals into nanopolymers, or metal-based coordination polymers. An alternative to these approaches is that of antimicrobial co-crystals, that is, multicomponent solid materials whereby the antimicrobial activity of metal ions could be used together with that of an active organic molecule to enhance the physicochemical and microbiological properties of the constituent compounds. The focus of this approach is not on the properties of the individual components, but rather on collective properties of the extended supramolecular aggregates formed in the solid state via non-covalent interactions such as hydrogen bonds and coordination interactions.22 There is increasing literature on applications of co-crystallization in the quest for new drugs or as a means to rejuvenate old drugs.23,24 However, in most cases, the focus of studies is on the changes in the physical properties of the crystalline phase of the active ingredient (solubility, dissolution rate, thermal stability, etc.) imparted by the association with an ancillary component and not on the mechanism of action. We and others have concentrated our efforts on exploring if the roles of the organic/inorganic constituents may be enabling, enhancing, or inhibiting the respective antimicrobial activities. This is the case of metal salts of Lauric acid25 or a metabolite such as urea,26 or that of enhancing the activity of an organic antiseptic.27−29
In this study, we aim to quantify and compare the antimicrobial properties of earlier reported co-crystals and supramolecular complex/salts of PF and MBAs: PF·AgNO3,27 PF·CuCl,27 ZnCl3(HPF),28 [HPF]2[ZnCl4]·H2O,28 and [HPF]3[Ga(ox)3]·4H2O.29 Characteristic antimicrobial concentrations of these crystals and their constituents were established for inhibition of planktonic growth (minimum inhibitory concentration, MIC) and biofilm growth (minimum biofilm inhibitory concentration, MBIC) for three WHO priority list pathogens.30 The Gram-negative bacteria Pseudomonas aeruginosa and Escherichia coli are listed as critical priority pathogens and the Gram-positive Staphylococcus aureus as high priority; all three are part of the concerning ESKAPE list of pathogens.
Methods
Crystal Preparation
The compounds investigated in this study, namely, PF·AgNO3,27 PF·CuCl,27 ZnCl3(HPF),28 [HPF]2[ZnCl4]·H2O,28 and [HPF]3[Ga(ox)3]·4H2O,29 have all been prepared and fully characterized by solid state methods, as summarized in Table 1. PF has been used in the co-crystallization processes either as neutral PF·H2O or as its hydrochloride salt [HPF]Cl2·H2O. All compounds discussed herein were prepared by mechanochemical (grinding) or slurry methods, that is, by direct mixing of solid PF·H2O or [HPF]Cl2·H2O with the inorganic salts, CuCl2, AgNO3, ZnCl2, as well as with K3[Ga(ox)3]. This approach yielded, in most cases, higher purity target products with respect to conventional crystallization from solution. Structural characterization for all materials was conducted via single crystal and/or powder X-ray diffraction.
Table 1. Crystalline Materials Investigated in This Work.
When single crystal structure information was obtained, correspondence between the structure of the single crystal and that of the bulk polycrystalline material was verified by comparing observed powder patterns to those calculated based on single crystal data. The preparation methods are briefly described below; however, the interested reader is addressed to the original crystal engineering papers (and their supplementary structural material) for more specific details.
Figure 1 shows the structure of the PF copper(I) chloride co-crystal, PF·CuCl. The crystal is formed by a 1-D polymer of CuCl monomers and by the neutral PF molecules arranged in a herring-bone fashion.
Figure 1.
Relevant packing features in crystalline PF·CuCl, showing the herring-bone arrangement of the PF molecules (a) and the 1D (CuCl···CuCl···)n chains (b); H atoms omitted for clarity.
The reaction of PF and AgNO3, both by slurry and grinding, yielded the anhydrous solid PF·AgNO3.27 The reaction with ZnCl2 yielded two different products, depending on the preparation method (grinding or slurry) and based on the Zn-PF stoichiometric ratio and on the experimental conditions. The two materials contain the (HPF+) cation and were characterized as the complex ZnCl3(HPF) and the monohydrate supramolecular salt [HPF]2[ZnCl4]·H2O.28 The structures of the two compounds are compared in Figures 2 and 3. In both materials, stacking of the proflavinium cations is observed. The same interaction is present in the crystal structures of neutral PF and of the hydrochloride salt [HPF]Cl·2H2O.
Figure 2.

(a) Projection along the a-axis of crystalline ZnCl3(HPF), showing the stackings of proflavinium ligands along the c-axis direction. (b) Space-filling representation. H atoms omitted for clarity.
Figure 3.

(a) Projection down the crystallographic b-axis of crystalline [HPF]2[ZnCl4]·H2O, showing the herring-bone pattern of HPF+ cationic pairs. (b) Space-filling representation. Water oxygens in blue; H atoms omitted for clarity.
Here, our study also comprises the co-crystals obtained by using as a preformed building unit a gallium oxalate complex [Ga(ox)3]3– in order to prepare the tetrahydrate [HPF]3[Ga(ox)3]·4H2O.29 The structure of [HPF]3[Ga(ox)3]·4H2O is shown in Figure 4. The proflavinium cations envelop the [Ga(ox)3]3– anions forming stackings of PF cations analogous to those observed in the neutral co-crystals discussed above.
Figure 4.

Packing in crystalline [HPF]3[Ga(ox)3]·4H2O viewed along the crystallographic b-axis (a). Proflavinium cations envelop around the [Ga(ox)3]3– anion (b). OW in blue; H atoms omitted for clarity.
Antimicrobial Testing; Strains and Growth Medium
P. aeruginosa ATCC27853, S. aureus ATCC25923, and E. coli ATCC25922 pathogen indicator strains were used in this study. Lysogeny broth (LB) was prepared in distilled water with 10 g/L NaCl (VWR International Co., Mississauga, Canada), 5 g/L yeast extract (EMD Chemicals Inc., Darmstadt, Germany), and 10 g/L tryptone (VWR Chemicals LLC, Solon, USA). The pH of the media was not altered by the addition of any of the compounds used in the antimicrobial testing.
Working Stocks
24 mg/mL of stock solutions of metal salts or suspensions/slurries of target co-crystals was prepared. Stocks were prepared freshly and used within 24 h. No experiments were performed to determine the speciation of released metals nor the decomposition of the crystals in the complex microbial media during the time course of the experiment. Crystal violet (CV) (VWR Chemicals LLC, Solon, USA) was prepared with distilled water to 0.1% (w/v) stock for biofilm biomass assessment.
Inoculum
Frozen cultures were revived on LB agar plates overnight at 37 °C, subsequently passaged on fresh LB agar, and incubated overnight again. From the second passage of overnight cultures, several colonies were picked using sterile cotton swab and transferred to saline until the suspension reached McFarland 1.0 turbidity that approximately corresponds to the concentration of colony forming units in the suspension of 3 × 108 CFU/mL. Bacterial suspension then was diluted 30-fold in LB and used as inoculum for minimum effective concentration assays.
Antimicrobial Assays
For all assays, the original metal salt and the PF were assayed as comparator controls. The original studies27−29 evaluated if the compounds worked additively or not, and as such, experiments of adding the individual components of each co-crystal into solution were not performed here, allowing us to focus on comparing the co-crystals to each other. These earlier studies provided the list of compounds for this study (Table 2). All antimicrobial assays were repeated with two to four biological replicates from a fresh bacterial culture inoculated from the ATCC stock. Each biological replicate also had three technical replicates within a given experiment. Variation between trials is observed in standard deviations. If no deviations are indicated, there was no variation between experiments or replicates. AMR data are presented (Figures 5 and 6) as log-2 scale as per the dilution series.
Table 2. Antimicrobials under Evaluation.
| compound | number |
|---|---|
| AgNO3 | 1 |
| CuCl2 | 2 |
| ZnCl2 | 3 |
| Ga(NO3)3 | 4 |
| K3[Ga(ox)3]·3H2O | 5 |
| K2[Ga2(ox)2(OH)2]·2H2O | 6 |
| PF | 7 |
| PF·AgNO3 | CC-1 |
| PF·AgNO3 (aged) | CC-2 |
| PF·CuCl | CC-3 |
| PF·CuCl (aged) | CC-4 |
| ZnCl3(HPF) | CC-5 |
| [HPF]2[ZnCl4]·H2O | CC-6 |
| [HPF]3[Ga(ox)3]·4H2O | CC-7 |
Figure 5.
Bacteriostatic MIC efficacy of the metal salt and PF starter compounds and their co-crystallization products. Bars with no visible errors indicate that there was no variation in the values obtained between trials. Note y-axis scale is log-2.
Figure 6.
MBIC efficacy of the metal salt and PF starter compounds and their co-crystallization products. These values represent the ability of the compounds to inhibit cell attachment and proliferation of biofilm biomass. Bars with no visible error bars indicate that there was no variation in the values obtained between trials. Note the y-axis scale is log-2.
Note that in Table 2, compounds CC-1 and CC-2 are the same co-crystal formulation, as well as CC-3 and CC-4. Samples CC-2 and CC-3 were synthesized 3 years earlier (March 2019) and used for the preliminary antimicrobial testing by disk diffusion.27 The samples were stored in the lab at room temperature in the dark in a stoppered vial.
Minimum Inhibitory Concentration
Classical broth dilution assay31 was used to quantitate the antimicrobial activity serial two-fold dilutions of compounds were prepared in LB broth in a standard microtiter plate, which thus had 8 sterility control wells (150 μL of medium), 8 growth control wells (135 μL of medium), and 2 replicates of dilution series for different compound (total of 80 wells, 135 μL of medium/compound mixture). Growth and test wells were inoculated with 15 μL of inoculum and incubated for 24 h (E. coli and P. aeruginosa) or 48 h (S. aureus) under 37 °C and 150 rpm shaking. Cultured plates were analyzed visually and by optical density at 600nm, and MICs were identified as the concentration of the compound in the first well that has no turbidity increase and/or coloration change, adjacent to the well with such changes present.
Minimal Biocidal Concentration
Ten microliters from each well from the MIC determination plate was either spot-plated to solid media or used to inoculate 150 μL of fresh media and incubated for 36 h at 37 °C. The lack of colony growth on solid media and/or no growth as determined by an OD600 measurement was defined as the end point defining the MBC values, that is, MBC gives an impression of bactericidal activity compared to MIC’s suggestion of bacteriostatic activity.
Minimum Biofilm Inhibition Concentration
Total biofilm biomaterial was evaluated using the popular method of biomass staining with CV detected with spectrophotometric analysis.32 The remaining liquid from the initial MIC plates was removed by inverting the plate upside-down and shaking three times. Plates were then rinsed twice by submerging into the distilled water. 170 μL of 0.1% (w/v) CV solution was added into each well and left at room temperature for 10 min. Then the CV solution was removed by inverting the plate with subsequent double rinsing in distilled water by submerging. After rinsing, plates were inverted and tapped on paper towel five times to remove the remaining free dye solution. Plates were then left at room temperature for 2 h for drying. The dye in each well, which would be bound to microbial biofilm biomass, was solubilized by adding 200 μL of acetic acid and mixed pipetting up and down for 5 times. 150 μL of solubilized dye from each well was then transferred to a new microtiter plate, and absorbance was evaluated by a microtiter plate reader. MBICs were identified as the concentration of the compound in the respective well of the initial MIC plate that has no observable coloration increase in the MBIC plate compared to sterility control, adjacent to a well in the MBIC plate that has the same coloration or higher than that of a growth control well. Although the rinsing step removed the co-crystal slurry, a control experiment performed showed no spectroscopic modification of the CV by trace co-crystals.
Results
The antimicrobial activity of all compounds is shown for P. aeruginosa, E. coli, and S. aureus together in Figures 5 and 6. The raw data numbers are available in Tables S1 (MIC), S2 (MBC), and S3 (MBIC). Evaluation of the antimicrobial activity saw good reproducibility as seen by the absence of standard deviation error bars for most of the compounds for both MIC and MBIC endpoints of the biological replicants. Note that to read these figures, the smaller bar height reflects superior antimicrobial activity. This is highlighted by compound 1 (AgNO3), which is known for its superior antimicrobial activity and thus also a good comparator to evaluate novel metal-based antimicrobials.
A cursory comparison of the antimicrobial data for the three test bacteria highlights the remarkable difference in efficacy of the same metal ions against these strains. Even the two Gram-negative strains (P. aeruginosa and E. coli) show striking differences in their susceptibility profiles.
Antimicrobial Efficacy
The MIC is an effective measure of how bacteriostatic an antimicrobial agent is against microbial growth in the free-swimming planktonic state. The MIC data are seen in Figure 5. For these planktonic MIC data, we see some compounds showing poor efficacy (4, 5, 6), yet have improved efficacy in their co-crystal forms. For a compound to be considered to have good antimicrobial activity, one generally looks for MIC concentrations below 0.125 mg/mL. Overall, we see that the co-crystals gave MIC values against all three organisms at or below this value, and in many cases, there is greater bactericidal activity than the PF alone (compound 7). Looking at the data of P. aeruginosa, we see the potential of the gallium compounds (5, 6) acting specifically to this strain and for our co-crystallization products CC-6 and CC-7 also showing superior performance. ZnCl3(HPF) (CC-5) MIC was at least 2-fold lower than that of PF (7) and more than 10-fold lower than that of Zn2+ alone (3). [HPF]2[ZnCl4]·H2O (CC-6) had stronger anti-planktonic properties against this strain—at least 4-fold, compared to PF, and more than 60-fold, compared to Zn2+. The PF·CuCl (CC-3) co-crystal had 2-fold and 10-fold decreased MIC, compared to PF and Cu2+ alone, respectively. [HPF]3[Ga(ox)3]·4H2O (CC-7), although presenting a range of MIC values (large error bar), was at least 2 times more effective than K3[Ga(ox)3]·3H2O (6) or PF (7) alone.
For E. coli and S. aureus, we see that all the co-crystallization products provided good bacteriostatic activities being below the 0.125 mg/mL cut off except for CC-3 against S. aureus. Certainly E. coli appeared the most susceptible in the planktonic state to the silver co-crystals, with CC-1 showing the lowest MIC values, and as such, the highest efficacy. Although not as impressive, both CC-1 and CC-7 would be quite effective to control growth of S. aureus.
Bactericidal activity as the minimal biocidal concentration (MBC) at 100% kill was determined (Table S2). Biocidal assay reflects the lack of ability of a microbe to recover and grow after the antimicrobial has been removed. All the co-crystal compounds demonstrated biocidal properties. The MBC values generally followed the same trend as the MIC values for the various antimicrobials tested but at higher concentrations than those of MIC, which is typical, and were more variable, particularly for the S. aureus strain.
Prevention of Biofilm
Here, we assayed the antimicrobial plate for the ability of cells to attach to the surface to initiate and proliferate a biofilm. Often the MBIC is expected to be equal to the MIC values as seen with most antibiotics. However, we have observed often for projects within the Calgary Biofilm Research group that for many non-“antibiotic” antimicrobials, antiseptics, and biocides, particularly those that are bacterial static versus bactericidal, the MBIC can be quite different from the MIC. Many bacteriostatic compounds still allow slow growth, and bacteria can adsorb to a surface still viable. This changes their physiology to biofilm state, and thus they begin a biofilm growth cycle. Thus, in our case, we expect and see MBIC > MIC or MBC.
The CV dye assay measures surface-adhered biomass (both live and dead cells as well as the variety of biomolecules of the biofilm matrix). Our comparator control of silver nitrate (1) showed strong biofilm inhibition as expected (Figure 6) and reflects why this simple metal salt is so popular as an antimicrobial.5 The other metals on their own did not have very good antibiofilm activity, but for each strain, we see interesting second choices (based on a 0.25 mg/mL target) such as ZnCl2 (3) for S. aureus and E. coli and CuCl2 (2) for E. coli. The gallium oxalate chelates K3[Ga(ox)3]·3H2O (5) and K2[Ga2(ox)2(OH)2]·2H2O (6) only showed antimicrobial activity against P. aeruginosa, supporting our previous observations.29
P. aeruginosa is a robust biofilm-forming bacterial species, and thus there is a need for novel antibiofilm compounds. Of our tested antimicrobials, the PF-silver co-crystals (CC-1) showed strong antibiofilm activity at useful concentrations and this compound showed remarkable antibiofilm activity toward S. aureus and E. coli. For E. coli, all the co-crystals were able to prevent this organism from forming and proliferating a biofilm below 0.065 mg/mL. S. aureus biofilm production was effectively inhibited with the Zn co-crystal, CC-6. Although the other PF-metal compounds showed efficacy within very effective MBIC values below the 0.25 mg/mL cut off for both S. aureus and E. coli.
Effect on Extended Storage Time
Due to COVID-19 isolation restrictions, we paused working and stored co-crystal samples of PF with silver (CC-2) and copper (CC-4), which were used in our original pilot study published in 2020.27 The compounds were at room temperature and ∼35% relative humidity (on average) in a covered container away from direct light exposure. This fortuitous event allowed us to revisit these older samples in our present study and evaluate them besides freshly prepared co-crystals of the same compounds (CC-1 and CC-3) Figure 7. The only observable difference was that the aged compounds had an overall larger grain size by 2–4 times, which may have originated by using a different grinding apparatus in the earlier preparations. We observed only minor changes in antimicrobial efficacy between the fresh and aged co-crystals, suggesting that they are very stable to room temperature storage conditions.
Figure 7.

Comparison of efficacy from storage of co-crystals CC-1, CC-2, CC-3, and CC-4.
Discussion
This study explores novel antimicrobial formulation efficacies against these pathogens by means of co-crystallization of potential metal atom-based antimicrobials (MBAs) with PF. Co-crystallization of pharmaceuticals is a growing research field that harvests the change of physicochemical properties of the co-crystal, compared to properties of single compound crystals as reviewed in detail elsewhere.33 Briefly, co-crystallization has been shown to increase solubility, photo and mechanical stability, as well as bioavailability.33 As a part of the whole combinatorial approach in dealing with antibiotic resistance development, which was proposed more than 30 years ago, co-crystallization of antimicrobials is viewed by us as a viable way of combining antimicrobials which have different modes of action into a single drug complex. Such a combination would deliver both antimicrobials with the possibility for increased antimicrobial efficacy, compared to the single antimicrobials used to produce it.
MBAs were used to treat microbial infections and preserve food well before discovery of conventional antibiotics.34 MBAs, including metals, metal salts, and metal complexes, are viewed as a viable alternative to antibiotics.3 QCCs are well known antimicrobial agents and are currently used as antiseptics, biocides, and preservatives.35 PF and proflavinium cation, as an example of a ridged planar QCC, is of our interest due to its previous use. We see co-crystallization of MBAs with QCCs as a unique area of combinatorial antimicrobials, and it provides an excellent test case for further antimicrobial co-crystallization development.
In our previous works,27−29 we described the co-crystalline materials of PF with the MBAs of Ag, Cu, Zn, and Ga, exploring their antimicrobial properties by means of disk diffusion assays. The disk diffusion assay, however, does not provide quantitative data regarding antimicrobial properties of the compound, which is required for adequate assessment and further development of any antimicrobial agent. Thus, in the present study, we provide quantification of anti-planktonic and anti-biofilm efficacy of those compounds in a direct comparison to each other. We evaluated the standard approach of determining the MIC required for inhibition of planktonic growth of bacteria (bacteriostatic effect). Growing awareness of the dramatic difference in physiology and biochemistry between planktonic and biofilm mode of bacterial growth and a realization of the prevalence of biofilm in biofouling, infection spread, and chronic infections36 led us to also evaluate the anti-biofilm activity (MIBC) of all crystalline materials. Both the spread of infection and infection itself are tightly associated with biofilms, as bacteria tend to form biofilms on inanimate surfaces that serve as hotspots of institutional and nosocomial infection transfer.37 Thus, our study here is a crucial initial step that compares the potential of co-crystals of antimicrobial efficacy of metal ions with a QCC antiseptic. Below, we briefly overview strategies against the three bacterial species studied here providing literature examples of other organo-metal complexes as comparators to the co-crystal results we present here.
Co-crystallization Products’ Antimicrobial Activity against P. aeruginosa
Studies have been performed using silver against either planktonic and biofilm growth of P. aeruginosa, demonstrating good antimicrobial and antifouling properties.38 However, besides economic burden caused by high price of silver, there are other factors that limit its practical use in free ionic form, such as low photostability and possible precipitation in the form of AgCl or Ag-thiol group aggregates under physiological conditions.39 To address the issue of silver’s bioavailability and stability, multiple approaches have been proposed, such as stabilized speciation and/or complexation of silver with organic ligands. Copper, similar to silver, is a coinage metal and was used since ancient times to preserve water and treat infectious diseases.40 Zinc ions were shown to interfere with P. aeruginosa biofilm formation and inhibit planktonic growth of the bacterium.41 Gallium is attracting attention as an antimicrobial agent,42 with forms of gallium-containing drugs currently being discussed with regard to P. aeruginosa treatment, such as gallium complexes with organic molecules, such as acetate43 or desferrioxamin.44
Co-crystallization of silver with PF in our work reflected MIC values between those of PF and AgNO3 individually. Biofilm prevention properties of these two co-crystals (CC-1 and CC-2) were at the level of AgNO3 (1). It is worth noting that the molar presence of silver in co-crystal is half that for the dosage provided by 1. This suggests that the physicochemical properties of the Ag-PF co-crystal enhance the Ag efficacy and thus provide an advantage in the use of the silver-PF co-crystals over AgNO3 alone. Our studies demonstrated decrease in MIC with PF-CuCl co-crystals (CC-3) compared to PF (7) or CuCl2 (2) alone. Antibiofilm properties of (CC-3) were on the same order as that of (7) or (2), suggesting no advantage of the crystal form. However, CC-4 did not lose any efficacy with storage over 3 years, whereas PF was unstable when stored in a similar fashion. This suggests that the co-crystal of PF-CuCl could be useful for applications against P. aeruginosa requiring longevity or shelf-life stability.
Our approach also demonstrated impressive results where the Zn-PF co-crystals had lower MICs of PF or ZnCl2 alone for P. aeruginosa. Promising data were also obtained regarding ZnCl3(HPF) anti-biofilm properties—2-fold decrease in MBIC, compared to PF alone. Gallium, as an effective bacteriostatic agent, is also recently receiving attention in various organometallic complexes. Here, we see that the Ga-oxalate ligand complexes 5 and 6 showed selective antimicrobial activity toward P. aeruginosa compared to the other two strains. The co-crystal CC-7 retained antibiofilm properties to the level of Ga(NO3)3 (4).
Co-crystallization Products’ Antimicrobial Activity against E. coli
Like P. aeruginosa, silver is the most studied and widely applied antimicrobial metal against E. coli. Dozens of patents were assigned over 21st century,5 yet silver resistance is now widespread including resistance to the nanoparticle form.45 Following the combinatorial narrative, silver–sulfodiazine combination is used in clinic settings for various microbes including E. coli.46 Copper has also been shown to increase efficacy of several organic compounds against E. coli, including lactic acid47 and chloro-catechols.48 Although on its own Zn is less antimicrobial to E. coli compared to silver or copper, Zn2+ is being explored in combinations with other therapeutics, in part due to its immunostimulant properties and its ability to reverse E. coli resistance to amikacin,49 for example.
PF-silver co-crystals (CC-1/2) demonstrated good bacteriostatic results against planktonic growth of E. coli, where they remained as effective as AgNO3 (1) or averaged between MICs of 1 and 7. Antibiofilm properties of CC1/2 provided equal or better efficacy level of the constituents. Here, we also see the same trend for the copper-PF co-crystals (CC3/4) in both bacteriostatic and antibiofilm properties toward E. coli. This trend continues with the two different zinc–PF complexes (CC-5 and CC-6) and the gallium co-crystal (CC-7), where higher bacteriostatic activity was observed over the metal salts (3 and 4) or Ga- oxalate chelates (5 and 6) alone. The Cu, Zn, and Ga PF co-crystals showed anti-biofilm properties with efficacy comparable to or better than PF (7) alone for E. coli. It is worth reiterating that the molar presence of metal and/or PF in the co-crystal is considerably smaller than that of these compounds on their own, which implies that the same or better antimicrobial activity is seen when using less of each antimicrobial. At this stage, we cannot define this observation to be synergistic or additive.
Co-crystallization Products’ Antimicrobial Activity against S. aureus
Generally, silver has been shown to demonstrate only moderate activity against S. aureus, although the silver–sulfodiazine combination is reasonably active against S. aureus biofilms.50 Multiple attempts have been made to increase its antimicrobial properties against Gram-positive bacteria, including complexation with carbene compounds51 or coordination with camphorimines.52 Considerable attention has also been dedicated to anti-Staphylococcal research of copper compounds. High-throughput screening of copper complexation with organic molecules yielded several active bis(thiosemicarbazone)–Cu complexes, including specific copper-dependent as glyoxal-bis(N4-methylthiosemicarbazone) (GTSM)-Cu as an anti-MRSA complex.53 Cu-Schiff base complexes were also studied for interactions with oxacillin and vancomycin and were shown to be additive/synergistic. [Cu(bitpy)2](ClO4)2 was demonstrated to possess S. aureus anti-biofilm properties.54 Zn is the MBA that is receiving the most attention regarding S. aureus treatment. Multiple complexes of Zn(II) with heterocyclic ligands were reported to have antimicrobial activity.55 Phthalocyanine56 and menthol–phthalocyanine57 complexes with Zn(II) are viewed as a viable photo-assisted solution to S. aureus infection. Like copper, zinc Schiff-bases complexes such as (N-allylsalicylideneiminato)-zinc are reported to be twice as effective as zinc acetate alone.58 The Ga(III) complex with maltolate59 was reported to show anti-planktonic properties against S. aureus, while recent success of galbofloxacin, a ciprofloxacin-containing gallium compound, demonstrates success of a combinational approach to gallium-bearing drugs development against S. aureus.60
In our studies, bacteriostatic properties against S. aureus were observed from all co-crystallization products. Beyond the silver co-crystals (CC-1/2) having good antibiofilm properties, the zinc co-crystal (CC-6) has excellent antibiofilm activity, even though its bacteriostatic activity is less effective. This gives another example of the co-crystalline structure showing selective antimicrobial efficacy, but in this case in antibiofilm properties.
Overall, at this time, it is not clear if the co-crystal activity mechanism is simply a delivery platform for the two different antimicrobials or it is that features of their structures mediate bacterial cell structure and physiology damage as well. Our previous work26−28 suggested that the co-crystals are more than simply the additive efficacy of the components and as a co-crystal are enhancing the interaction with the bacteria or perhaps and facilitating a localized “burst” of combined antimicrobials. It is well known that the toxicity of a metal is in its speciation. Given the chemical complexity of the microbial growth media and the bacterium’s biochemistry, one can imagine an overwhelming diversity and complexity of different metal species and chelates in the biological soup. With this in mind, we can postulate further that our crystalline materials are able to deliver, at least initially, a unique and/or uniform metal species to the bacterium cell.
Conclusions
It is well appreciated in toxicology that a metal’s chemical speciation influences its bioavailability, and as such, the metal organic co-crystal provides a unique speciation that warranted the antimicrobial investigation here. We find that all metal-PF co-crystals explored here have merit as antimicrobials. Although the best broad-spectrum antibiofilm compound is the silver-based CC-1, we identified other effective co-crystals for each species and growth state that could also move forward for specific bacterial problem situations. We find that select co-crystals are required for best results against each bacterium and growth state. We observe that no single metal-PF co-crystal works equally well against all three bacterial species. This is not too much of a surprise, given the dramatic difference in physiology between the strains and growth states. While not all metal-PF co-crystals demonstrated enhanced antimicrobial efficacy over one or both of their constituents, their compounds did not lose their antimicrobial properties while trapped in the crystal form. This is an excellent observation, and it clearly shows that co-crystallization of MBAs with an antiseptic QCC (PF) does not affect the activity of individual antimicrobials and potentially protects them within the co-crystal. How the antimicrobial activity might be related to changes in thermodynamic solubility or kinetic dissolution rate, at least for some of the best performing co-crystals, will require further studies, and how co-crystallization may be further used to fine-tune physicochemical properties of metal–organic antimicrobial combinations must be explored. This work gives a solid proof of principle to apply these co-crystals to specific applications such as in cosmetics, biofouling, agriculture, general antiseptic, disinfectant, coatings, and so forth.
Acknowledgments
A.L. received MSc scholarship from MITACs. The synthesis of the material is based on the work of O.S., C.F., F.G., and D.B. supported by the University of Bologna and by MUR, project “Nature Inspired Crystal Engineering” (PRIN2020). R.J.T. is supported by an NSERC Discovery Grant (RGPIN/04811-2015).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.2c00404.
AMR values (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ventola C. The antibiotic resistance crisis: part 1: causes and threats. Pharm. Ther. 2015, 40, 277–283. [PMC free article] [PubMed] [Google Scholar]
- Harrison J.; Turner R.; Marques L.; Ceri H. Biofilms. Am. Sci. 2005, 93, 508. 10.1511/2005.56.508. [DOI] [Google Scholar]
- Turner R. J. Metal-Based Antimicrobial Strategies. Microbiol. Biotechnol. 2017, 10, 1062–1065. 10.1111/1751-7915.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J. A.; Harrison J. J.; Turner R. J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- Sim W.; Barnard R. T.; Blaskovich M. A. T.; Ziora Z. M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. 10.3390/ANTIBIOTICS7040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendsen L. P.; Thakar R.; Sultan A. H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32, SEP19. 10.1128/CMR.00125-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R. Why Zinc Is Bad for Bacteria. Nat. Rev. Microbiol. 2011, 10, 4. 10.1038/nrmicro2726. [DOI] [Google Scholar]
- Hijazi S.; Visaggio D.; Pirolo M.; Frangipani E.; Bernstein L.; Visca P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. 10.3389/FCIMB.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 2002, 85, 3533–3539. 10.1021/JA00905A001. [DOI] [Google Scholar]
- Rigo A.; Corazza A.; Luisa di Paolo M.; Rossetto M.; Ugolini R.; Scarpa M. Interaction of Copper with Cysteine: Stability of Cuprous Complexes and Catalytic Role of Cupric Ions in Anaerobic Thiol Oxidation. J. Inorg. Biochem. 2004, 98, 1495–1501. 10.1016/j.jinorgbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Macomber L.; Imlay J. A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 8344–8349. 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. F.; Imlay J. A. Silver(I), Mercury(II), Cadmium(II), and Zinc(II) Target Exposed Enzymic Iron-Sulfur Clusters When They Toxify Escherichia Coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. 10.1128/AEM.07368-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp B. A.; Morey J. R.; Ween M. P.; Ong C. L. Y.; McEwan A. G.; Paton J. C.; McDevitt C. Extracellular Zinc Competitively Inhibits Manganese Uptake and Compromises Oxidative Stress Management in Streptococcus Pneumoniae. PLoS One 2014, 9, e89427 10.1371/JOURNAL.PONE.0089427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q. L.; Wu J.; Chen G. Q.; Cui F. Z.; Kim T. N.; Kim J. O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia Coli and Staphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. . [DOI] [PubMed] [Google Scholar]
- Peters K.; Pazos M.; Edoo Z.; Hugonnet J. E.; Martorana A. M.; Polissi A.; VanNieuwenhze M. S.; Arthur M.; Vollmer W. Copper Inhibits Peptidoglycan LD-Transpeptidases Suppressing β-Lactam Resistance Due to Bypass of Penicillin-Binding Proteins. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 10786–10791. 10.1073/PNAS.1809285115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei L.; Cianci M.; Gonzalez Vara A.; Ciurli S. The Structure of Urease Inactivated by Ag(I): A New Paradigm for Enzyme Inhibition by Heavy Metals. Dalton Trans. 2018, 47, 8240–8247. 10.1039/C8DT01190G. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [PubMed] [Google Scholar]
- Harrison J. J.; Turner R. J.; Joo D. A.; Stan M. A.; Chan C. S.; Allan N. D.; Vrionis H. A.; Olson M. E.; Ceri H. Copper and Quaternary Ammonium Cations Exert Synergistic Bactericidal and Antibiofilm Activity against Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. 10.1128/AAC.00203-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Cui F.; Zeng G. M.; Jiang M.; Yang Z.; Yu Z. G.; Zhu M. Y.; Shen L. Q. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in the Environment. Sci. Total Environ. 2015, 518–519, 352–362. 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Acridine--a neglected antibacterial chromophore. J. Antimicrob. Chemother. 2001, 47, 1–13. 10.1093/JAC/47.1.1. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R. Crystal Engineering: A Holistic View. Angew. Chem., Int. Ed. 2007, 46, 8342–8356. 10.1002/ANIE.200700534. [DOI] [PubMed] [Google Scholar]
- Grepioni F.; Casali L.; Fiore C.; Mazzei L.; Sun R.; Shemchuk O.; Braga D. Steps towards nature inspired inorganic crystal engineering. Dalton Trans. 2022, 51, 7390–7400. 10.1039/d2dt00834c. [DOI] [PubMed] [Google Scholar]
- Pharmaceutical Salts and Co-Crystals; Wouters J., Quéré L., Eds.; Drug Discovery; Royal Society of Chemistry: Cambridge, 2011. [Google Scholar]
- Steed J. W. The Role of Co-Crystals in Pharmaceutical Design. Trends Pharmacol. Sci. 2013, 34, 185–193. 10.1016/J.TIPS.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Morikawa T.; Kawai T.; Nonomura Y. Selective Bactericidal Activity of Divalent Metal Salts of Lauric Acid. ACS Omega 2017, 2, 113–121. 10.1021/acsomega.6b00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali L.; Mazzei L.; Shemchuk O.; Honer K.; Grepioni F.; Ciurli S.; Braga D.; Baltrusaitis J. Smart Urea Ionic Co-Crystals with Enhanced Urease Inhibition Activity for Improved Nitrogen Cycle Management. Chem. Commun. 2018, 54, 7637–7640. 10.1039/C8CC03777A. [DOI] [PubMed] [Google Scholar]
- Shemchuk O.; Braga D.; Grepioni F.; Turner R. J. Co-Crystallization of Antibacterials with Inorganic Salts: Paving the Way to Activity Enhancement. RSC Adv. 2020, 10, 2146–2149. 10.1039/C9RA10353H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C.; Shemchuk O.; Grepioni F.; Turner R. J.; Braga D. Proflavine and Zinc Chloride “Team Chemistry”: Combining Antibacterial Agents via Solid-State Interaction. CrystEngComm 2021, 23, 4494–4499. 10.1039/D1CE00612F. [DOI] [Google Scholar]
- Guerrini M.; d’Agostino S.; Grepioni F.; Braga D.; Lekhan A.; Turner R. J. Antimicrobial Activity of Supramolecular Salts of Gallium(III) and Proflavine and the Intriguing Case of Trioxalate Complex. Sci. Rep. 2022, 12, 3673. 10.1038/s41598-022-07813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . “Global Priority List of Antibiotic-Resistant Bacteria To Guide Research, Discovery, And Development Of New Antibiotics” [Online]. Available: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed April 5, 2022).
- Balouiri M.; Sadiki M.; Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allkja J.; Bjarnsholt T.; Coenye T.; Cos P.; Fallarero A.; Harrison J. J.; Lopes S. P.; Oliver A.; Pereira M. O.; Ramage G.; Shirtliff M. E.; Stoodley P.; Webb J. S.; Zaat S. A. J.; Goeres D. M.; Azevedo N. F. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm 2020, 2, 100010. 10.1016/j.bioflm.2019.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Jafari M.; Padrela L.; Walker G. M.; Croker D. M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. 10.1021/ACS.CGD.8B00933. [DOI] [Google Scholar]
- Landecker H. Antimicrobials before Antibiotics: War, Peace, and Disinfectants. Palgrave Commun. 2019, 5, 45. 10.1057/s41599-019-0251-8. [DOI] [Google Scholar]
- Gerba C. P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. 10.1128/AEM.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T. The Role of Bacterial Biofilms in Chronic Infections. APMIS 2013, 121, 1–58. 10.1111/APM.12099. [DOI] [PubMed] [Google Scholar]
- Kramer A.; Schwebke I.; Kampf G. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BMC Infect. Dis. 2006, 6, 130. 10.1186/1471-2334-6-130/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T.; Kirketerp-møller K.; Kristiansen S.; Phipps R.; Nielsen A. K.; Jensen P. Ø.; Høiby N.; Givskov M. Silver against Pseudomonas Aeruginosa Biofilms. APMIS 2007, 115, 921–928. 10.1111/J.1600-0463.2007.APM_646.X. [DOI] [PubMed] [Google Scholar]
- Betts H. D.; Neville S. L.; McDevitt C. A.; Sumby C. J.; Harris H. H. The Biochemical Fate of Ag+ Ions in Staphylococcus Aureus, Escherichia Coli, and Biological Media. J. Inorg. Biochem. 2021, 225, 111598. 10.1016/J.JINORGBIO.2021.111598. [DOI] [PubMed] [Google Scholar]
- Dollwet H. H. A.; Sorenson J. R. J.. Historic Uses of Copper Compounds in Medicine. In Trace Elements in Medicine; The Humana Press Inc.: Arkansas, 2001; pp 80–87. [Google Scholar]
- Wu C.; Labrie J.; Tremblay Y. D. N.; Haine D.; Mourez M.; Jacques M. Zinc as an Agent for the Prevention of Biofilm Formation by Pathogenic Bacteria. J. Appl. Microbiol. 2013, 115, 30–40. 10.1111/JAM.12197. [DOI] [PubMed] [Google Scholar]
- Kaneko Y.; Thoendel M.; Olakanmi O.; Britigan B. E.; Singh P. K. The Transition Metal Gallium Disrupts Pseudomonas Aeruginosa Iron Metabolism and Has Antimicrobial and Antibiofilm Activity. J. Clin. Invest. 2007, 117, 877–888. 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Han B.; Xie Y.; Wang H.; Wang R.; Xia W.; Li H.; Sun H. Combination of Gallium(III) with Acetate for Combating Antibiotic Resistant Pseudomonas Aeruginosa. Chem. Sci. 2019, 10, 6099–6106. 10.1039/C9SC01480B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E.; Lozinski A.; Brady K. M.; Berenshtein E.; Butterfield P. W.; Moshe M.; Chevion M.; Greenberg E. P.; Banin E. The Potential of Desferrioxamine-Gallium as an Anti-Pseudomonas Therapeutic Agent. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 16761–16766. 10.1073/pnas.0808608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. L.; Tajkarimi M.; Cunningham Q.; Campbell A.; Nonga H.; Harrison S. H.; Barrick J. E. Rapid Evolution of Silver Nanoparticle Resistance in Escherichia Coli. Front. Genet. 2015, 6, 42. 10.3389/FGENE.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.; Cooper R.. Silver Sulfadiazine: A Review of the Evidence; Wounds UK, 2005; Vol. 1. [Google Scholar]
- Gyawali R.; Ibrahim S. A.; Abu Hasfa S. H.; Smqadri S. Q.; Haik Y. Antimicrobial Activity of Copper Alone and in Combination with Lactic Acid against Escherichia Coli O157:H7 in Laboratory Medium and on the Surface of Lettuce and Tomatoes. J. Pathog. 2011, 2011, 650968. 10.4061/2011/650968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigert N.; Hunziker R. W.; Escher B. I.; Eggen R. I. L. Acute Toxicity of (Chloro-)Catechols and (Chloro-)Catechol-Copper Combinations in Escherichia Coli Corresponds to Their Membrane Toxicity in Vitro. Environ. Toxicol. Chem. 2001, 20, 239–247. . [DOI] [PubMed] [Google Scholar]
- Lin D. L.; Tran T.; Alam J. Y.; Herron S. R.; Ramirez M. S.; Tolmasky M. E. Inhibition of Aminoglycoside 6′-N-Acetyltransferase Type Ib by Zinc: Reversal of Amikacin Resistance in Acinetobacter Baumannii and Escherichia Coli by a Zinc Ionophore. Antimicrob. Agents Chemother. 2014, 58, 4238–4241. 10.1128/AAC.00129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y.; Miyazaki M.; Mashima K.; Takagi S.; Hara S.; Kamimura H.; Jimi S. The Effects of Silver Sulfadiazine on Methicillin-Resistant Staphylococcus Aureus Biofilms. Microorg 2020, 8, 1551. 10.3390/MICROORGANISMS8101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esarte Palomero O.; Cunningham A. L.; Davies B. W.; Jones R. A. Antibacterial Thiamine Inspired Silver (I) and Gold (I) N-Heterocyclic Carbene Compounds. Inorg. Chim. Acta 2021, 517, 120152. 10.1016/J.ICA.2020.120152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso J. M. S.; Galvão A. M.; Guerreiro S. I.; Leitão J. H.; Suarez A. C.; Carvalho M. F. N. N. Antibacterial Activity of Silver Camphorimine Coordination Polymers. Dalton Trans. 2016, 45, 7114–7123. 10.1039/C6DT00099A. [DOI] [PubMed] [Google Scholar]
- Haeili M.; Moore C.; Davis C. J. C.; Cochran J. B.; Shah S.; Shrestha T. B.; Zhang Y.; Bossmann S. H.; Benjamin W. H.; Kutsch O.; Wolschendorf F. Copper Complexation Screen Reveals Compounds with Potent Antibiotic Properties against Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 2014, 58, 3727–3736. 10.1128/AAC.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi S.; Fathima A.; Rao J. R.; Nair B. U. Antibacterial Activity of Copper(II) Complexes against Staphylococcus Aureus. RSC Adv. 2014, 4, 32004–32012. 10.1039/C4RA03241A. [DOI] [Google Scholar]
- Yamgar R. S.; Nivid Y.; Nalawade S.; Mandewale M.; Atram R. G.; Sawant S. S. Novel Zinc(II) Complexes of Heterocyclic Ligands as Antimicrobial Agents: Synthesis, Characterisation, and Antimicrobial Studies. Bioinorg. Chem. Appl. 2014, 2014, 276598. 10.1155/2014/276598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçel A.; Öztürk İ.; Ince M.; Ocakoglu K.; Hoşgör-Limoncu M.; Yurt F. Antimicrobial Photodynamic Therapy against Staphylococcus Aureus Using Zinc Phthalocyanine and Zinc Phthalocyanine-Integrated TiO2 Nanoparticles. J. Porphyrins Phthalocyanines 2019, 23, 206–212. 10.1142/S1088424619500238. [DOI] [Google Scholar]
- Szymczak J.; Sobotta L.; Dlugaszewska J.; Kryjewski M.; Mielcarek J. Menthol Modified Zinc(II) Phthalocyanine Regioisomers and Their Photoinduced Antimicrobial Activity against Staphylococcus Aureus. Dyes Pigm. 2021, 193, 109410. 10.1016/J.DYEPIG.2021.109410. [DOI] [Google Scholar]
- Poulter N.; Donaldson M.; Mulley G.; Duque L.; Waterfield N.; Shard A. G.; Spencer S.; Jenkins A. T. A.; Johnson A. L. Plasma Deposited Metal Schiff-Base Compounds as Antimicrobials. New J. Chem. 2011, 35, 1477–1484. 10.1039/C1NJ20091G. [DOI] [Google Scholar]
- Arnold C. E.; Bordin A.; Lawhon S. D.; Libal M. C.; Bernstein L. R.; Cohen N. D. Antimicrobial Activity of Gallium Maltolate against Staphylococcus Aureus and Methicillin-Resistant S. Aureus and Staphylococcus Pseudintermedius: An in Vitro Study. Vet. Microbiol. 2012, 155, 389–394. 10.1016/J.VETMIC.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Pandey A.; Śmiłowicz D.; Boros E. Galbofloxacin: A Xenometal-Antibiotic with Potent in Vitro and in Vivo Efficacy against S. Aureus. Chem. Sci. 2021, 12, 14546–14556. 10.1039/D1SC04283A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.