Abstract
Background and Purpose
We aimed to determine 1) the frequency of mammillary body (MB) atrophy in patients with temporal lobe epilepsy (TLE) and hippocampal sclerosis (HS), 2) the clinical significance of MB atrophy, and 3) the association between MB atrophy and volume changes in other subcortical limbic structures.
Methods
We enrolled 69 patients with pathologically confirmed TLE with HS, who underwent a standard anterior temporal lobectomy, as well as 40 healthy controls. We used the FreeSurfer deep-learning tool of U-Net to obtain the volumes of the subcortical limbic structures, including the MB, hypothalamus, basal forebrain, septal nuclei, fornix, and nucleus accumbens. MB atrophy was considered to be present when the MB volume was decreased relative to the healthy controls.
Results
MB atrophy was present in 18 (26.1%) of the 69 patients with TLE and HS. Among the clinical characteristics, the mean age at seizure onset was higher (25.5 vs. 15.9 years, p=0.027) and the median duration of epilepsy was shorter (149 vs. 295 months, p=0.003) in patients with than without MB atrophy. The basal forebrain (0.0185% vs. 0.0221%, p=0.004) and septal nuclei (0.0062% vs. 0.0075%, p=0.003) in the ipsilateral hemisphere of HS were smaller in the patients with MB atrophy.
Conclusions
We observed ipsilateral MB atrophy in about one-quarter of patients with TLE and HS. The severity of subcortical limbic structure abnormalities was greater in patients without MB atrophy. These findings suggest that MB atrophy in TLE with HS is not rare, but it has little clinical significance.
Keywords: mammillary body, epilepsy, magnetic resonance imaging
INTRODUCTION
Hippocampal sclerosis (HS), also referred to as mesial temporal sclerosis, is the most common pathological feature of refractory focal epilepsy.1 There is accumulating evidence from advanced neuroimaging studies that neurological abnormalities in patients with HS extend beyond the hippocampus and may involve both gray- and white-matter structures on both the ipsilateral and contralateral sides, as well as the temporal lobe.2,3,4,5
The hippocampus, fornix, and mammillary body (MB) are all components of a limbic circuit, with the fornix connecting the hippocampus to the MB. MB has been implicated in several human functions such as memory and emotion. It is also thought to contribute to generating epileptic discharges in mesial temporal lobe epilepsy (TLE) due to existing connections with the hippocampus.6 Furthermore, neuronal degeneration in the hippocampus, including the subiculum, has been suggested to result in ipsilateral MB atrophy.7,8,9 The hippocampus has a well-established role in TLE. However, to fully understand the epilepsy mechanism in TLE, it is necessary to look beyond the hippocampus and focus on other inter-connected structures that are unfortunately often overlooked.
The volume of the region of interest is directly proportional to the neuron intensity observed in magnetic resonance imaging (MRI), and hence volume loss involves gliosis and neuronal loss.10 Numerous studies have assessed MB atrophy by visually comparing their sizes on brain MRI scans between the sides with and without HS in TLE.11,12 Several studies have also carried out semiautomatic volume analyses of MB in patients with TLE and HS.13,14 However, all of these studies involved visual analyses or semiautomatic methods by drawing the region of interest, which results in suboptimal inter- or intraobserver reliability. Also, anatomical expertise is required to correctly locate anatomical structures, which is tedious, error-prone, and time-consuming. The studies found a wide variation in MB atrophy in patients with TLE and HS, ranging from 3% to 35%.11,12,13,14
Greve et al.15 recently developed a tool (available with the FreeSurfer program) to automatically segment several subcortical limbic structures, including the MB, hypothalamus, basal forebrain, septal nuclei, fornix, and nucleus accumbens. This is one of the deep-learning tools of U-Net that uses spatial, intensity, contrast, and noise augmentation, and it demonstrates good test–retest reliability.15 Although several tools for automatically labeling the brain have been developed using parametric methods, machine-learning techniques, and nonlinear registration, this is the first tool for performing automatic MB segmentation.
This study aimed to determine 1) the frequency of MB atrophy in patients with TLE and HS, 2) the clinical significance of MB atrophy, and 3) the association between MB atrophy and volume changes in other subcortical limbic structures.
We only enrolled pathologically confirmed patients with TLE and HS, and carried out an MB volume analysis using the automatic segmentation toolbox. We initially hypothesized that patients with TLE and HS often have MB atrophy, which would be a predictor for poor surgical outcomes following anterior temporal lobectomy. We also hypothesized that MB atrophy was associated with volume changes in other subcortical limbic structures.
METHODS
Participants
The study involving human participants was reviewed and approved by Yonsei University College of Medicine, Seoul, Korea (IRB No. 2020-03-018-001). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The investigation was conducted retrospectively at a tertiary hospital. The following criteria were used to enroll patients with TLE and HS: 1) typical seizure semiology consistent with mesial TLE, 2) ictal electroencephalography originating from the anterior temporal lobe, 3) typical MRI findings consistent with HS, including increased signal intensity and decreased volume in the fluid attenuated inversion recovery (FLAIR) sequence, 4) standard anterior temporal lobectomy between January 2010 and December 2019, 5) pathologically confirmed HS, and 6) at least 12 months elapsed since the surgery. We collected the following clinical data of the patients: age, sex, age at seizure onset, epilepsy duration (time from seizure onset to MRI scan), febrile seizure history, generalized tonic–clonic seizure occurrence, and seizure frequency (number of seizures during 1 year before surgery). We excluded patients who had any other abnormalities on brain MRI scans other than HS identified using visual inspection, as well as patients who had bilateral HS. We divided the patients into two groups based on their surgical outcome: good (International League Against Epilepsy [ILAE] classes I and II) and poor (ILAE classes III and VI).16
We also enrolled age- and sex-matched control subjects from our database of healthy controls that had no medical or neurological disease history. They had been recruited from our previous study17 that had enrolled 150 healthy subjects. In the present study, we only included 40 healthy subjects as a control group in order to match ages and sexes with patients with TLE and HS. The brain MRI scans were normal in all of the healthy controls.
MB atrophy was considered when there was ipsilateral MB atrophy at a cutoff volume -1.5 standard deviations below the mean value for the healthy subjects. The cutoff volumes defining ipsilateral MB atrophy for the right and left sides were different.
Brain MRI data acquisition
Preoperative three-dimensional T1-weighted MRI scans were performed in all patients using an Achieva 3.0-T scanner (Phillips Healthcare, Best, The Netherlands) with the following acquisition parameters: 224×256 matrix, 256×256 reconstructed matrix with 182 slices, 220-mm field of view, 0.98×0.98×1.2 mm3 voxels, echo time 4.6 ms, repetition time 9.6 ms, 8-degree flip angle, and 0-mm slice gap.
Obtaining volumes in subcortical limbic structures, including the MB
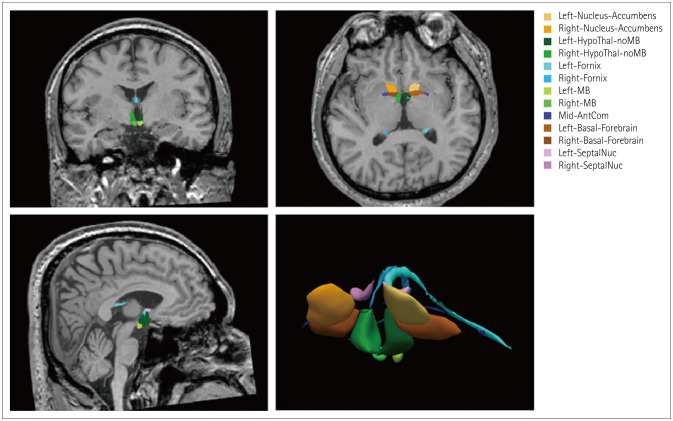
The structural volumes were obtained using the FreeSurfer program (development version; https://surfer.nmr.mgh.harvard.edu/fswiki/ScLimbic). We used the main FreeSurfer stream with the “recon-all” and “mri_sclimbic_seg” commands.15 This tool automatically segmented and obtained absolute structural volumes in subcortical limbic structures from T1-weighted images based on the U-Net deep-learning algorithm, including the MB, hypothalamus, basal forebrain, septal nuclei, fornix, and nucleus accumbens (Fig. 1).18 We also obtained hippocampus volumes. These absolute structural volumes were adjusted using the estimated total intracranial volume for each individual.
Fig. 1. Example of segmentation of subcortical limbic structures, including the mammillary body (MB). The segmentations are overlaid onto T1-weighted images in coronal, axial, and sagittal orientations, with a volume rendering also shown. AntCom, anterior commissure; HypoThal-noMB, hypothalamus; SeptalNuc, septal nucleus. Adapted from Kang et al. J Neurol 2022 Jul 9 (https://doi.org/10.1007/s00415-022-11263-z), with permission from Springer Nature.18.
Statistical analysis
The clinical characteristics of the groups were compared using chi-square tests for categorical variables and Student’s t-tests or Mann-Whitney tests for numerical variables that confirmed to the normal distribution. We investigated the differences in subcortical limbic structure volumes according to MB atrophy, including the ipsilateral and contralateral hypothalamus, basal forebrain, septal nuclei, fornix, and nucleus accumbens, according to MB atrophy using an analysis of covariance with hippocampal volumes as the covariates. In all statistical analyses, p<0.05 was considered statistically significant. When assessing the differences in subcortical limbic structure volumes, we also applied the Bonferroni correction (p=0.005, 0.05/10 structures). MedCalc statistical software (version 20.022, MedCalc Software, Ostend, Belgium; https://www.medcalc.org) was used for all statistical tests.
RESULTS
Clinical characteristics of patients with TLE and HS and of healthy controls
Table 1 lists the clinical characteristics of the 69 enrolled patients with TLE and HS: 32 (46.4%) had right HS and 37 (53.6%) had left HS. Surgical outcomes were poor and good in 29 (42.1%) and 40 patients (57.9%), respectively (30, 10, 19, 3, 5, and 2 patients with ILAE classes I, II, III, IV, V, and VI, respectively).
Table 1. Differences in clinical characteristics according to MB atrophy in temporal lobe epilepsy patients with HS.
| Characteristic | Overall (n=69) | Patients with MB atrophy (n=18) | Patients without MB atrophy (n=51) | p |
|---|---|---|---|---|
| Age (yr) | 36.8±10.5 | 33.7±10.4 | 37.9±10.4 | 0.151 |
| Sex, male | 26 (37.6) | 6 (33.3) | 20 (39.2) | 0.660 |
| Age at seizure onset (yr) | 18.4±16.0 | 25.5±21.4 | 15.9±13.0 | 0.027* |
| Epilepsy duration (month) | 242 [146–405] | 149 [81–236] | 295 [182–423] | 0.003* |
| History of febrile seizure | 20 (28.9) | 8 (44.4) | 12 (23.5) | 0.095 |
| Presence of GTC seizures | 48 (69.5) | 10 (55.5) | 38 (74.5) | 0.135 |
| Seizure frequency (/yr) | 24 [15–48] | 27 [12–80] | 24 [15–36] | 0.691 |
| Right HS | 32 (46.4) | 8 (44.4) | 24 (47.0) | 0.849 |
| Good surgical outcome | 40 (57.9) | 11 (61.1) | 29 (56.8) | 0.755 |
| Follow-up period after surgery (month) | 96 [72–132] | 84 [72–132] | 96 [69–132] | 0.937 |
Data are mean±SD, median [IQR], or n (%) values.
*p<0.05.
GTC, generalized tonic–clonic; HS, hippocampal sclerosis; IQR, interquartile range; MB, mammillary body; SD, standard deviation.
Age and sex did not differ between healthy control and patients with TLE and HS (age, 35.5±9.6 vs. 36.8±10.5 years [mean±standard deviation], p=0.533; males, 17/40 [42.5%] vs. 26/69 [37.3%], p=0.621).
Differences in clinical characteristics according to MB atrophy in patients with TLE and HS
MB atrophy was present in 18 (26.1%) of the 69 patients with TLE and HS. Table 1 lists the differences in clinical characteristics between patients with and without MB atrophy. The mean age at seizure onset was higher (25.5 vs. 15.9 years, p=0.027) and the median epilepsy duration was shorter (149 vs. 295 months, p=0.003) in patients with than without MB atrophy. However, the other clinical characteristics did not differ between the two groups.
Differences in subcortical limbic structure volumes according to MB atrophy in patients with TLE and HS
Table 2 lists the differences in subcortical limbic structure volumes according to MB atrophy in patients with TLE and HS. The basal forebrain (0.0185% vs. 0.0221%, p=0.004) and septal nuclei (0.0062% vs. 0.0075%, p=0.003) in the ipsilateral hemisphere of HS were significantly smaller in patients with than without MB atrophy.
Table 2. Volume differences of the subcortical limbic structures according to MB atrophy in temporal lobe epilepsy patients with HS.
| Structural volume (%) | p | |||
|---|---|---|---|---|
| Patients with MB atrophy (n=18) | Patients without MB atrophy (n=51) | |||
| Ipsilateral hemisphere of HS | ||||
| Hippocampus | 0.1774±0.0443 | 0.1986±0.0568 | 0.156 | |
| Hypothalamus | 0.0298±0.0028 | 0.0328±0.0058 | 0.141 | |
| Basal forebrain | 0.0185±0.0034 | 0.0221±0.0041 | 0.004* | |
| Septal nuclei | 0.0062±0.0015 | 0.0075±0.0012 | 0.003* | |
| Fornix | 0.0268±0.0068 | 0.0321±0.0060 | 0.006 | |
| Nucleus accumbens | 0.0257±0.0085 | 0.0295±0.0070 | 0.172 | |
| Contralateral hemisphere of HS | ||||
| Hippocampus | 0.2189±0.0291 | 0.2433±0.0376 | 0.014 | |
| Hypothalamus | 0.0305±0.0025 | 0.0334±0.0055 | 0.271 | |
| Basal forebrain | 0.0208±0.0019 | 0.0229±0.0034 | 0.196 | |
| Septal nuclei | 0.0066±0.0009 | 0.0075±0.0012 | 0.084 | |
| Fornix | 0.0292±0.0053 | 0.0339±0.0050 | 0.014 | |
| Nucleus accumbens | 0.0281±0.0038 | 0.0300±0.0061 | 0.626 | |
Data are mean±SD values.
*p<0.005.
HS, hippocampal sclerosis; MB, mammillary body; SD, standard deviation.
DISCUSSION
The main findings of this study were 1) 26% of patients with TLE and HS had MB atrophy, 2) patients with MB atrophy were older at seizure onset and had shorter epilepsy durations, 3) MB atrophy was not related to surgical outcomes following anterior temporal lobectomy, and 4) patients with MB atrophy had severe volume reductions in other subcortical limbic structures, such as the basal forebrain and septal nuclei in the ipsilateral hemisphere of HS.
The alveus is the primary efferent pathway of the hippocampus. The white-matter fibers of the alveus congregate to form the fimbria, which continues as the fornix. The fornix is bipolar in nature, with anterior and posterior fibers. The posterior bundle continues into the hypothalamus, where anterior fibers terminate in the MB.19 This anatomical relationship between hippocampus and MB means that MB atrophy in patients with TLE and HS is an expected consequence.
To the best of our knowledge, this was the first report of MB volume changes identified using automatic MB segmentation based on machine-learning techniques in pathologically confirmed patients with TLE and HS. When the neural pathway is disrupted by pathological abnormalities, two types of antegrade neuronal degeneration can occur: Wallerian degeneration20 and transneuronal degeneration.21 When a proximal axon or its cell body is damaged, Wallerian degeneration occurs in the axon and myelin sheath. When synaptic input is disrupted, transneuronal degeneration occurs in the postsynaptic neuron. In TLE with HS, neuronal damage to the hippocampus may result in MB atrophy due to this neuronal degeneration. Another possibility is that the MB plays a primary role in TLE.22 Shih et al.23 investigated aberrant intrinsic functional connectivity in TLE with HS, which revealed a significant relationship between seizure frequency and changes in functional connectivity between the hippocampus and MB, and between the MB and anterior thalamic nuclei. This implies that the correlation between neurons and seizure occurrence is not restricted to the seizure onset zones of the hippocampus in TLE with HS; instead, these might involve more neural pathways beyond the HS, including the MB.24 An animal study suggested that the main efferent mammillary tract projects into the anterior thalamic nucleus, named the mammillothalamic tract, which seems to play a role in mediating seizure activity.25 This is further supported by deep brain stimulation targeting the MB or anterior thalamic nucleus being effective in patients with refractory epilepsy, of which the most common form is TLE.26
A few studies have investigated the MB volume in patients with TLE and HS. One study identified an asymmetrically small MB in the ipsilateral side in 3% of those with TLE and HS.11 However, since the authors analyzed the difference in MB volume between the right and left sides, it was considered that there was no asymmetry if both sides had atrophy, and MB was only visually evaluated.11 Another assessment MB asymmetry found that 38% of patients had a small MB on the ipsilateral side; however, that study only evaluated MB volumes in 13 patients with TLE and HS.22 Ferreira et al.27 similarly found MB volume loss in 11.6% cases of patients with TLE and HS. We believe that the wide discrepancies in the results reported in the literature are due to the use of different techniques, pulse sequences, section thicknesses, and protocols for selecting patient groups.
Despite the advancement of surgical techniques, approximately one-third of patients with TLE and HS who undergo temporal lobectomy continue to experience medically refractory seizures.28 Some studies have assessed the association between surgical outcomes and MRI markers in TLE with HS.29,30,31 One of the purposes of our study was to determine the potential clinical significance of MB atrophy in predicting surgical outcomes. Surgical outcomes following anterior temporal lobectomy were good in 58% of patients in the present study, which was similar to previous reports. The present observations indicate that MB atrophy is not associated with surgical outcomes following anterior temporal lobectomy. A previous study investigated whether limbic system abnormalities (including in the MB) altered seizure outcomes following selective amygdalohippocampectomy. No MB volume differences were found according to surgical outcomes, which was consistent with the results of the present study.13 Although histological changes have been described in other limbic structures beyond the hippocampus, such as the amygdala, entorhinal cortex, subiculum, and MB, these abnormalities are usually less severe, suggesting that the hippocampus is the site of the main epileptogenic lesion and that MB atrophy has no effect on surgical outcomes.13 TLE with HS is also a dynamic process, since atrophy can be progressive and associated with seizure frequency.32 A similar study compared seizure outcomes after amygdalohippocampectomy in patients with HS with or without associated fornix atrophy, and found no significant differences between the groups.33
Only a few studies have investigated differences in clinical characteristics according to MB atrophy in patients with TLE and HS. One of these studies found no significant relationship between epilepsy duration and MB volume in patients with TLE and HS.14 It is particular interesting that the seizure onset age and epilepsy duration differed significantly between the groups in the present study. Patients with MB atrophy were older at seizure onset and had shorter epilepsy durations than those without. The short epilepsy duration in patients with MB atrophy is thought to be attributable to the higher mean seizure onset age. However, the reason why patients with MB atrophy were older at seizure onset remains unclear. A plausible explanation is that patients with MB atrophy experience more seizures over a short period of time, although the number of seizures did not differ significantly during 1 year before surgery. In fact, the MRI scans of our patients were taken as part of the presurgical workup for anterior temporal lobectomy. Despite the higher onset age in patients with MB atrophy, there was no difference in the age at which the operation was performed, which could explain the larger number of seizures within a shorter period of time in patients with MB atrophy.
This study also found that patients with MB atrophy had volume reductions in other subcortical limbic structures, such as the basal forebrain and septal nuclei. Our findings were consistent with data in the literature. One study of patients with TLE and HS found that 62% had unilateral abnormalities in the fornix and that 13% had bilateral atrophy.34 Lindboe et al.8 autopsied patients with TLE and HS and discovered that the fornix volumes on the same side of the affected hippocampus also decreased. This might be attributable to the fornix being the primary efferent pathway of hippocampal formation, and that forniceal fibers are primarily directed toward the MB. Another plausible hypothesis is that the recurrent propagation of abnormal electrical activity from the hippocampus on the affected side results in excitotoxic neuronal death of the subcortical limbic structures, such as the basal forebrain, septal nuclei, and fornix.
This study had several limitations. First, MB atrophy in patients with TLE and HS was considered to only reflect volume reduction in comparison to healthy controls; however, we did not consider the signal changes on FLAIR images. Second, factors other than age and sex could have influenced the MB volumes of the healthy subjects. We did not evaluate the cognitive function (including memory) of the patients with TLE and HS and of the healthy controls. However, all patients could perform the normal activities of daily living, and none of them showed definite intellectual disability. Third, this study was conducted at a single center. Multicenter studies with larger samples are warranted to confirm our findings. Fourth, this study had a cross-sectional design, and so could not reveal any causal relationship between seizures and MB atrophy in TLE with HS. Longitudinal follow-up studies are required to provide this sort of information.
In conclusion, we observed that ipsilateral MB atrophy was present in about one-quarter of patients with TLE and HS. However, it was not associated with surgical outcomes following anterior temporal lobectomy. Limbic structure abnormalities were more severe in patients with MB atrophy. These findings suggest that MB atrophy in TLE with HS is not rare, but it has little clinical significance.
Footnotes
- Conceptulation: Kyoo Ho Cho, Ho-Joon Lee, Kang Min Park.
- Data curation: Ho-Joon Lee, Dong Ah Lee.
- Formal analysis: Kyoo Ho Cho, Kang Min Park.
- Methodology: Kyoo Ho Cho, Kang Min Park.
- Supervision: Kang Min Park.
- Validation: Kyoo Ho Cho, Ho-Joon Lee.
- Visualization: Ho-Joon Lee.
- Writing—original draft: Kyoo Ho Cho, Ho-Joon Lee, Kang Min Park.
- Writing—review & editing: Kyoo Ho Cho, Ho-Joon Lee, Kang Min Park.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This work was supported by the Ministry of Science and ICT of the Republic of Korea (NRF-2021R1F1A1049605).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Blümcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15:34–39. doi: 10.1016/j.yebeh.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Voxel-based morphometry of sporadic epileptic patients with mesiotemporal sclerosis. Epilepsia. 2010;51:506–510. doi: 10.1111/j.1528-1167.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 3.Pail M, Brázdil M, Marecek R, Mikl M. An optimized voxel-based morphometric study of gray matter changes in patients with left-sided and right-sided mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE/HS) Epilepsia. 2010;51:511–518. doi: 10.1111/j.1528-1167.2009.02324.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Seo SA, Park KM. Quantification of thalamic nuclei in patients diagnosed with temporal lobe epilepsy and hippocampal sclerosis. Neuroradiology. 2020;62:185–195. doi: 10.1007/s00234-019-02299-6. [DOI] [PubMed] [Google Scholar]
- 5.Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol. 2018;14:129–140. doi: 10.3988/jcn.2018.14.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balak N, Balkuv E, Karadag A, Basaran R, Biceroglu H, Erkan B, et al. Mammillothalamic and mammillotegmental tracts as new targets for dementia and epilepsy treatment. World Neurosurg. 2018;110:133–144. doi: 10.1016/j.wneu.2017.10.168. [DOI] [PubMed] [Google Scholar]
- 7.Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci. 1990;10:3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindboe CF, Erichsen AA, Strøm EH. Atrophy and sponginess of the mammillary bodies with neuronal sparing: not only inactive Wernicke’s encephalopathy. APMIS. 1989;97:667–670. doi: 10.1111/j.1699-0463.1989.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 9.Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- 10.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N. Clinical significance of asymmetry of the fornix and mamillary body on MR in hippocampal sclerosis. AJNR Am J Neuroradiol. 1995;16:509–515. [PMC free article] [PubMed] [Google Scholar]
- 12.Ozturk A, Yousem DM, Mahmood A, El Sayed S. Prevalence of asymmetry of mamillary body and fornix size on MR imaging. AJNR Am J Neuroradiol. 2008;29:384–387. doi: 10.3174/ajnr.A0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbach H, Siebenhaar G, Koenig R, von Oertzen J, Scorzin J, Kurthen M, et al. Limbic system abnormalities associated with Ammon’s horn sclerosis do not alter seizure outcome after amygdalohippocampectomy. Epilepsia. 2005;46:549–555. doi: 10.1111/j.0013-9580.2005.29104.x. [DOI] [PubMed] [Google Scholar]
- 14.Morishita Y, Mugikura S, Mori N, Tamura H, Sato S, Akashi T, et al. Atrophy of the ipsilateral mammillary body in unilateral hippocampal sclerosis shown by thin-slice-reconstructed volumetric analysis. Neuroradiology. 2019;61:515–523. doi: 10.1007/s00234-019-02158-4. [DOI] [PubMed] [Google Scholar]
- 15.Greve DN, Billot B, Cordero D, Hoopes A, Hoffmann M, Dalca AV, et al. A deep learning toolbox for automatic segmentation of subcortical limbic structures from MRI images. Neuroimage. 2021;244:118610. doi: 10.1016/j.neuroimage.2021.118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE commission report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 17.Jang H, Lee JY, Lee KI, Park KM. Are there differences in brain morphology according to handedness? Brain Behav. 2017;7:e00730. doi: 10.1002/brb3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang J, Lee DA, Lee HJ, Park KM. Limbic covariance network alterations in patients with transient global amnesia. J Neurol. 2022 Jul 09; doi: 10.1007/s00415-022-11263-z. [Epub] [DOI] [PubMed] [Google Scholar]
- 19.Grewal SS, Gupta V, Vibhute P, Shih JJ, Tatum WO, Wharen RE. Mammillary body changes and seizure outcome after laser interstitial thermal therapy of the mesial temporal lobe. Epilepsy Res. 2018;141:19–22. doi: 10.1016/j.eplepsyres.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Khong PL, Zhou LJ, Ooi GC, Chung BH, Cheung RT, Wong VC. The evaluation of Wallerian degeneration in chronic paediatric middle cerebral artery infarction using diffusion tensor MR imaging. Cerebrovasc Dis. 2004;18:240–247. doi: 10.1159/000079961. [DOI] [PubMed] [Google Scholar]
- 21.Matthews MR. Further observations on transneuronal degeneration in the lateral geniculate nucleus of the macaque monkey. J Anat. 1964;98(Pt 2):255–263. [PMC free article] [PubMed] [Google Scholar]
- 22.Mamourian AC, Rodichok L, Towfighi J. The asymmetric mamillary body: association with medial temporal lobe disease demonstrated with MR. AJNR Am J Neuroradiol. 1995;16:517–522. [PMC free article] [PubMed] [Google Scholar]
- 23.Shih YC, Lin FH, Liou HH, Tseng WI. Seizure frequency is associated with effective connectivity of the hippocampal–diencephalic–cingulate in epilepsy with unilateral mesial temporal sclerosis. Brain Connect. 2021;11:457–470. doi: 10.1089/brain.2020.0835. [DOI] [PubMed] [Google Scholar]
- 24.Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013;74:223–231. doi: 10.1002/ana.23915. [DOI] [PubMed] [Google Scholar]
- 25.Mirski MA, Ferrendelli JA. Selective metabolic activation of the mammillary bodies and their connections during ethosuximide-induced suppression of pentylenetetrazol seizures. Epilepsia. 1986;27:194–203. doi: 10.1111/j.1528-1157.1986.tb03528.x. [DOI] [PubMed] [Google Scholar]
- 26.Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11:508–526. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira NF, de Oliveira V, Amaral L, Mendonça R, Lima SS. Analysis of parahippocampal gyrus in 115 patients with hippocampal sclerosis. Arq Neuropsiquiatr. 2003;61:707–711. doi: 10.1590/s0004-282x2003000500001. [DOI] [PubMed] [Google Scholar]
- 28.Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110:1135–1146. doi: 10.3171/2008.6.JNS17613. [DOI] [PubMed] [Google Scholar]
- 29.Cho KH, Lee HJ, Heo K, Kim SE, Lee DA, Park KM. Intrinsic thalamic network in temporal lobe epilepsy with hippocampal sclerosis according to surgical outcomes. Front Neurol. 2021;12:721610. doi: 10.3389/fneur.2021.721610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho KH, Park KM, Lee HJ, Cho H, Lee DA, Heo K, et al. Metabolic network is related to surgical outcome in temporal lobe epilepsy with hippocampal sclerosis: a brain FDG-PET study. J Neuroimaging. 2022;32:300–313. [Google Scholar]
- 31.Garcia MTFC, Gaça LB, Sandim GB, Assunção Leme IB, Carrete H, Júnior, Centeno RS, et al. Morphometric MRI features are associated with surgical outcome in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2017;132:78–83. doi: 10.1016/j.eplepsyres.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–416. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- 33.Burneo JG, Bilir E, Faught E, Morawetz R, Knowlton RC, Martin R, et al. Significance of fornix atrophy in temporal lobe epilepsy surgery outcome. Arch Neurol. 2003;60:1238–1242. doi: 10.1001/archneur.60.9.1238. [DOI] [PubMed] [Google Scholar]
- 34.Hakyemez B, Yucel K, Yildirim N, Erdogan C, Bora I, Parlak M. Morphologic and volumetric analysis of amygdala, hippocampus, fornix and mamillary body with MRI in patients with temporal lobe epilepsy. Neuroradiol J. 2006;19:289–296. doi: 10.1177/197140090601900303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.