Abstract
Plants are subjected to a wide range of abiotic stresses, such as heat, cold, drought, salinity, flooding, and heavy metals. Generally, abiotic stresses have adverse impacts on plant growth and development which affects agricultural productivity, causing food security problems, and resulting in economic losses. To reduce the negative effects of environmental stress on crop plants, novel technologies, such as nanotechnology, have emerged. Implementing nanotechnology in modern agriculture can also help improve the efficiency of water usage, prevent plant diseases, ensure food security, reduce environmental pollution, and enhance sustainability. In this regard, nanoparticles (NPs) can help combat nutrient deficiencies, promote stress tolerance, and improve the yield and quality of crops. This can be achieved by stimulating the activity of certain enzymes, increasing the contents (e.g., chlorophyll) and efficiency of photosynthesis, and controlling plant pathogens. The use of nanoscale agrochemicals, including nanopesticides, nanoherbicides, and nanofertilizers, has recently acquired increasing interest as potential plant-enhancing technologies. This review acknowledges the positive impacts of NPs in sustainable agriculture, and highlights their adverse effects on the environment, health, and food chain. Here, the role and scope of NPs as a practical tool to enhance yield and mitigate the detrimental effects of abiotic stresses in crops are described. The future perspective of nanoparticles in agriculture has also been discussed.
Keywords: abiotic stress, crop yield, modern agriculture, nanoparticles, nanotechnology, plant performance
Introduction
Abiotic stress factors, that can affect modern agricultural productivity worldwide include high or low temperature, waterlogging, drought, salinity, heavy metals (HMs), and ultraviolet (UV) radiation (Seleiman et al., 2020a; Badawy et al., 2021). Plant response to abiotic stress involves alterations in various morphological, physiological, and biochemical processes depending on the crop type, stress type, and time of exposure (Semida et al., 2014; Desoky et al., 2020a; Rady et al., 2021; Abd El-Mageed et al., 2022; Shaaban et al., 2022). As such, sustainable agriculture and yield productivity can improve the quality of soil, water, and other resources required by plants (Badal et al., 2013; Saxena et al., 2016; Desoky et al., 2020b). To meet the increasing global food demand, researchers are striving to ameliorate the detrimental effects of abiotic stresses, enhance crop yield and food production, and achieve sustainability and food security. Indeed, for addressing these urgent global concerns, researchers must continue developing innovative technologies or solutions.
Nanotechnology is a fascinating and rapidly developing branch of research that has led to various innovations (El-Saadony et al., 2020, 2021a; Abd El-Ghany et al., 2021). In particular, nanotechnology can help provide effective solutions to agriculture-related problems and achieve a sustainable and secure future for agriculture (Seleiman et al., 2021b). Nanotechnology has gained tremendous attention in recent years owing to its wide range of applications in medicine, drug delivery, energy, poultry production, and the agrifood sector (Seleiman et al., 2020a; Yousry et al., 2020; Salem et al., 2021). In agriculture, nanotechnology is mainly utilized in the application of nanofertilizers and nanopesticides to track products and nutrient levels for enhancing growth and productivity and increasing plant resistance to insect pests and microbial diseases (Shang et al., 2019; Bhatt et al., 2020).
Nanoparticles (NPs) are tiny materials 1–100 nm in size (Khan and Upadhyaya, 2019). In contrast to their larger sized equivalents, NPs possess certain unique and diverse physicochemical properties. For instance, NPs have a large surface area-to-volume ratio, high adsorption efficacy, and increased connecting and working efficiencies owing to their extremely small size (Nel et al., 2006; Dubchak et al., 2010). Thus, NPs have been integrated into disease management strategies as bactericides/fungicides/pesticides to enhance plant health. NPs can also serve as macro and micro-nanofertilizers in plants to alleviate nutrient deficiency symptoms and supplement essential elements. Various biological, physical, and chemical techniques can be used for NPs synthesis (Singh et al., 2016).
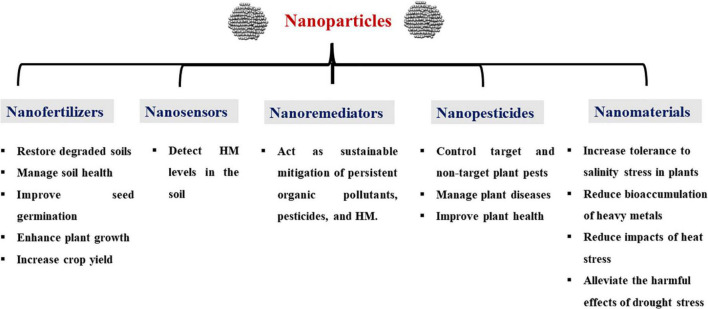
In agriculture and agrifood business, NPs can be applied in the form of nanosensors, nanofertilizers, nanoherbicides/nanopesticides, and nanoremediators (Figure 1; Elsakhawy et al., 2018; El-Saadony et al., 2021b). However, the mechanisms of underlying how NPs interact with plants have not been completely elucidated (Saxena et al., 2016; Khan et al., 2019). Therefore, this review highlights the current knowledge and potential uses of NPs that are widely used in agriculture, along with their effects on plants for better crop improvement and sustainable agriculture.
FIGURE 1.
Potential applications of nanotechnology in agriculture. HM, heavy metals.
Abiotic Stress
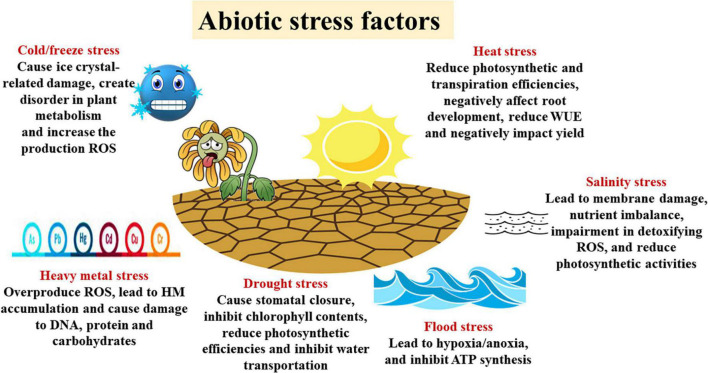
With the increasing world population, abiotic stress conditions are increasingly affecting crop production (Figure 2). During stress, physiological and biochemical changes occur in plant cells that can adversely affect plant growth, development, and productivity (Al-Ashkar et al., 2019; Seleiman et al., 2020b,2021a; Taha et al., 2021). Species and varieties bred to tolerate these challenges along with nanotechnology and other climate-sensitive agricultural technologies could be the most efficient adaptation strategy to cope with climate and abiotic stress factors, thereby achieving sustainable production (Kumari et al., 2022).
FIGURE 2.
Abiotic stress factors and their negative effects on plants. ROS, reactive oxygen species; WUE, water use efficiency; HM, heavy metals.
Major abiotic stressors that limit crop returns globally include heat stress and drought (Abd El-Mageed et al., 2019; Batool et al., 2020; Semida et al., 2020; Rasheed et al., 2021). Drought induces many morphological, physiological, molecular, and metabolic changes in plants which are relatively significant. Plants regulate their stomatal conductance to control the amount of water lost, and optimize CO2 assimilation to avoid photosynthetic inhibition, allowing them to resist water stress (Faraji and Sepehri, 2020). Under arid conditions, phenolics, flavonoids and antioxidant enzymes are affected to a large extent. Root-sourced signals are transported via the xylem to leaves, thus, affecting the cellular status in drought-stressed plants (Afshari et al., 2021). Turgor loss can also be observed in plants under drought stress. If dehydration is severe, the protoplasm may become rigid, consequently altering the cellular metabolism and inhibiting plant growth. Drought can severely disrupt cellular metabolism, ion accumulation, membrane structure integrity, and protein structures in plants. Therefore, leaf growth, photosynthetic rates and enzymatic activities are reduced. Drought can also induce the excessive generation of reactive oxygen species (ROS) in plants; and thereby results in oxidative stress (Cruz de Carvalho, 2008). In addition, salinity and HMs stress are also considered among the environmental factors that limit crop yield in many countries (Abd El-Mageed et al., 2018; Ye et al., 2019; Sofy et al., 2020; Taha et al., 2020; Dustgeer et al., 2021; Khan et al., 2021; Seleiman et al., 2022).
Salinity is a type of abiotic stress that is widespread and responsible for considerably decreasing plant growth. Soil salinity inhibits seed germination owing to the low osmotic potential generated around the seeds, which prevents water uptake (Tavakkoli et al., 2010; Seleiman et al., 2020c; Alkharabsheh et al., 2021; Taha et al., 2021). Sodium chloride (NaCl)-induced oxidative stress in legumes, considerably inhibits growth, decreases seed nutrient quality and lowers nodulation (Hernandez et al., 2000; Ahmad et al., 2008). Plants can employ various antioxidant defense mechanisms, both enzymatic and non-enzymatic, to reduce the effect of oxidative stress associated with salinity. Ascorbate and carotenoids are critical non-enzymatic defense mechanisms against salinity, whereas proline (Pro) is a known osmoregulatory stress-related compound (Anoop and Gupta, 2003).
Plant growth and development benefit from essential elements, such as cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), zinc (Zn), and non-essential elements such as cadmium (Cd), chromium (Cr), lead (Pb), and mercury However, all HMs are highly toxic to plants at high concentrations (White and Pongrac, 2017). The toxic levels of HMs adversely affect various metabolic processes. This may include, but not be limited to, degradation or displacement of protein structures resulting from the development of bonds between the HMs and sulfhydryl groups (Hall, 2002); disruption of cytoplasmic membrane integrity (Farid et al., 2013), and suppression of photosynthesis, respiration, and enzymatic actions (Hossain et al., 2012).
NPs
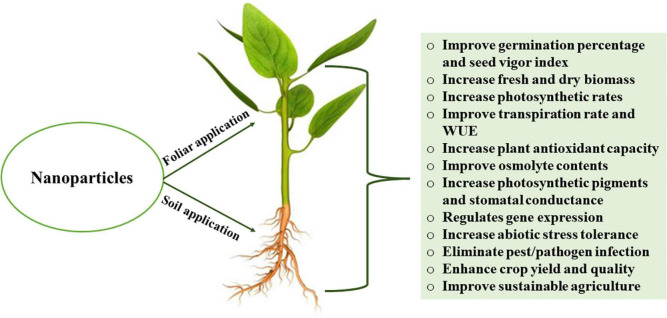
NPs are microscopic particles that can enter the cell through aboveground plant organs (cuticle, epidermis, stomata, hydathodes, or other openings) or underground organs (root tips, cortex, lateral root, wounds, or other openings). The physiological and morphological effects of NPs vary according to plant species, development period, development agents, application method, dose, and exposure time (Dietz and Herth, 2011; Rizwan et al., 2017). According to the mass flow/pressure flow hypothesis, NPs that enter through the stomata are carried within the plant by the phloem and are transported via pressure differences between the leaves and roots (Turgeon, 2010). The route through which NPs enter the plants alters many plant processes, including germination, antioxidant activity, macro and micronutrients, chlorophyll content, chloroplast number, and photosynthesis (Cinisli et al., 2019). In Arabidopsis, NPs application altered intraroot signals by affecting ethylene production (Syu et al., 2014). NPs can penetrate the cell membrane and the cell wall in order to be transported to the epidermis, xylem, central cylinder, and leaves (Tripathi et al., 2017a). Before reaching the central cylinder, NPs are passively transported in the endodermis (Judy et al., 2012). NPs are transported via the active route through osmotic pressure, capillary forces, cell wall pores, and plasmodesmata in plant roots or via the symplastic route (Usman et al., 2020). In general, NPs can bind to carrier proteins via ion channels, aquaporin, and endocytosis, as well as disrupt the plasma membrane to induce the formation of pores for crossing into the cells. The passage of NPs through the cell wall relies on their pore size. Small-sized NPs simply pass through the cell wall (Fleischer et al., 1999), while larger NPs pass through the hydathodes, stigma, and stomata (Hossain et al., 2016). NPs are transported via the stomata when their dimensions are < 15–40 nm (Eichert et al., 2008). Such NPs can act as a substitute for the vascular cambium, in the stomata and be transferred to various plant compartments through the phloem (Tripathi et al., 2017a). The NPs that are widely used in agriculture and their role in enhancing crop tolerance to abiotic stress (Figure 3) are summarized in Table 1. In seed coating, NPs enter through parenchymatous intercellular spaces in the seed coat in which aquaporins play an important role in controlling NPs entry (Abu-Hamdah et al., 2004; Lee et al., 2010).
FIGURE 3.
Effects of nanoparticles on plant health, growth performance and physiological parameters. WUE, water use efficiency.
TABLE 1.
Most commonly used nanoparticles in agriculture and their impacts in enhancing crop tolerance to abiotic stress.
| Nanoparticles | Administration | Plants species | Abiotic stresses | Impact | References |
| Zn | Nano-zinc solution was added to petri dishes containing soybean seeds | Soybean | Drought | Increased the germination rate and reduced seed residual weight | Sedghi et al., 2013 |
| Added to pot soil and the soil was mixed to uniform nanoparticles | Barley | Drought | Stimulated growth, improved production yield, and fortified edible grains with crucial nutrients and increased N acquisition | Dimkpa et al., 2019 | |
| Nano-ZnO suspension were added into half of these pots at 7 days after emergence | Zea mays L. cv. Jidan 27 | Drought | Enhanced melatonin synthesis. Promoted the functioning of the antioxidant system | Sun et al., 2020 | |
| Foliar application to leaf area | Sunflower and soybean | Salinity | Reduced Na levels in leaves and increased Zn levels, substomatal CO2 concentration, the CO2 acclimatization ratio, chlorophyll content, and Fv/Fm | Torabian et al., 2016 | |
| Nano-ZnO suspension were added into half of these pots | Lupinus termis | Salinity | Initiated plant growth; restored the levels of photosynthetic pigments, organic solutes, total phenols, ascorbic acid, and antioxidant enzymes; and decreased MDA level | Abdel Latef et al., 2017 | |
| Foliar application to leaf area | Wheat | Salinity | Improved plant development | Fathi et al., 2017 | |
| Foliar application to leaf area | Tomato | Salinity | Enhanced antioxidant enzyme levels. Promoted root and shoot growth. Increased biomass and photosynthetic pigment contents | Faizan et al., 2021 | |
| Foliar application to leaf area | Trigonella foenum-graecum | Salinity | Enhanced protein and Pro contents, promoted antioxidant activity, and reduced H2O2 and MDA levels | Noohpisheh et al., 2021 | |
| Foliar application to leaf area | Mangifera indica L. | Salinity | Increased the contents of antioxidant enzymes, total sugars, and Pro | Elsheery et al., 2020 | |
| Foliar application to leaf area | Pearl millet | Mineral nutrient | Increased shoot and root length, root area, chlorophyll content, total soluble leaf protein, plant dry biomass, enzymatic activity, growth, and net photosynthesis | Tarafdar et al., 2014 | |
| Foliar application | Coffea arabica | Mineral nutrient | Increased growth, biomass, and net photosynthesis | Rossi et al., 2019 | |
| Nano-ZnO suspension were added into pots | Wheat | Mineral nutrient | Improved grain production and biomass | Du et al., 2019 | |
| ZnO particles were sprayed by foliar (25-mL per pot) after 2 weeks of germination | Cyamopsis Tetragonoloba | Mineral nutrient | Stimulated plant development; increased biomass, nutrient and chlorophyll contents, levels of soluble protein, phytase, phosphatase, and alkaline phosphatase; and enhanced enzymatic activity | Raliya and Tarafdar, 2013 | |
| Added to seedlings’ nutrient solution | Nicotiana tabacum | Mineral nutrient | Positively impacted growth physiology and increased metabolite levels, enzymatic activities, and the anatomical features of plants | Tirani et al., 2019 | |
| The soil was amended with the NPs suspensions (well mixed) and kept 24 h for stabilization. | Pea | Mineral nutrient | Increased root length | Mukherjee et al., 2014 | |
| The seeds were soaked in different Zn NPs suspension | Rice | Mineral nutrient | Enhanced germination and antioxidant activity in plants | Panda, 2017 | |
| Added to seedling nutrient solution | Arabidopsis thaliana | Mineral nutrient | Modulated the transcription of different genes involved in Zn uptake, macronutrient and micronutrient homeostasis, and hormone control | Nair and Chung, 2017 | |
| Foliar application | Brassica juncea | Mineral nutrient | Increased plant development and improved antioxidant levels | Nayan et al., 2016 | |
| The seeds were soaked in different Zn NPs suspension | Arachis hypogaea | Mineral nutrient | Increased seed germination and seedling vigor | Prasad et al., 2012 | |
| The seeds were primed in different Zn NPs suspension | Lupinus termis | Mineral nutrient | Modulated growth, photosynthesis, and antioxidant responses | Abdel Latef et al., 2017 | |
| Foliar application | Eggplant | Mineral nutrient | Increased RWC and photosynthetic pigments. Enhanced fruit yield and growth parameters | Semida et al., 2021 | |
| Added to seedling nutrient solution | Oryza sativa L. | HMs | Increased phytochelatin content and promoted growth | Yan et al., 2021 | |
| Sprayed to plant foliage every alternate day for 2 weeks | Soy bean | Increased photosynthetic pigments, and induced antioxidant enzymes. Enhanced growth | Ahmad et al., 2020 | ||
| Foliar application | Maize | Increased biomass, photosynthetic pigments, and antioxidant enzymes | Rizwan et al., 2019a | ||
| Foliar application | Wheat | Increased Cd uptake and induced antioxidant enzymes. Enhanced growth | Rizwan et al., 2019b | ||
| Added to seedling nutrient solution | Lycopersicon leucocephala | Stimulated the antioxidant system. Mitigated lipid peroxidation. Enhanced protein and pigment contents. Enhanced growth | Venkatachalam et al., 2017 | ||
| Foliar application | Common bean | Salinity | Enhanced antioxidant enzyme activity and mitigated salinity-induced adverse effects | El-Saadony et al., 2021c | |
| Si | Plants were irrigated with silica nanoparticles for 2 weeks | Ocimum basilicum | Enhanced pigment and Pro levels. Improved growth parameters | Kalteh et al., 2014 | |
| Added to nutrient solution | Lens culinaris Medik. | Inhibited seed activity and germination and reduced FW | Sabaghnia and Janmohammadi, 2014 | ||
| Added to petri dishes containing seeds | Cucurbita pepo L. | Increased seed germination. Reduced H2O2 and MDA levels. Improved electrolyte levels. Increased photosynthetic pigments and antioxidant enzymes. Enhanced growth | Siddiqui et al., 2014 | ||
| Soaking seed in silicon nanoparticles suspension | Solanum lycopersicum L. | Increased the seed germination rate. Increased photosynthetic pigments. Regulated salt toxicity-associated genes. Improved root growth and root weight. Upregulated the expression of four salt stress genes (AREB, TAS14, NCED3, and CRK1) and downregulated that of six salt stress genes (RBOH1, APX2, MAPK2, ERF5, MAPK3, and DDF2). Inhibited salinity-induced adverse effects on the seed germination rate and seed development | Haghighi and Pessarakli, 2013; Almutairi, 2016a | ||
| Added to petri dishes containing seeds | Lycopersicum esculentum | Increased seed germination. Improved root growth and root weight | Haghighi et al., 2012 | ||
| Foliar application | Vicia faba L. | Stimulated the antioxidant system. Increased crop yield and RWC | Qados, 2015 | ||
| In irrigation water and foliar application | Fragaria sp. | Increased chlorophyll and Pro contents. Increased RWC | Avestan et al., 2019 | ||
| Added to nutrient solution of seedling | Hawthorn | Drought | Improved plant tolerance by retaining critical physiological and biochemical functions. Exhibited non-significant effects on chlorophyll and carotenoid contents | Ashkavand et al., 2015 | |
| Foliar application | Higher plants | Salinity, drought | Enhanced antioxidant enzyme activity and promoted plant stress resistance | Liang et al., 2007 | |
| Added to nutrient solution of seedling | Crataegus sp. | Increased biomass and photosynthetic pigment levels. Enhanced net photosynthesis and stomatal conductance by upregulating photosynthesis | Ashkavand et al., 2015 | ||
| Added to nutrient solution of shoots | Musa acuminata | Maintained Na+–K+ balance. Promoted photosynthesis. Increased chlorophyll levels and leaf growth | Mahmoud et al., 2020 | ||
| Foliar application | Tomato | Salinity | Increased germination, root length, DW, chlorophyll content, Pro precipitation, the photosynthetic rate, and leaf water content. Regulated antioxidant enzyme activity | Haghighi et al., 2012; Haghighi and Pessarakli, 2013; Haghighi and Pourkhaloee, 2013 | |
| Foliar application | Basil | Salinity | Increased FW and DW and chlorophyll and Pro contents. Increased antioxidant enzyme activity | Kalteh et al., 2014; Siddiqui et al., 2014 | |
| Added to nutrient solution | Lentil | Salinity | Increased seed germination and seedling development. Improved various defense mechanisms | Sabaghnia and Janmohammadi, 2014 | |
| Added to petri dishes containing seeds | Squash | Salinity | Increased germination and growth indices and enhanced the overall defense mechanism and antioxidant enzyme activity. Enhanced photosynthetic parameters | Siddiqui et al., 2014 | |
| Foliar application | Broad bean | Salinity | Increased seed germination and development, antioxidant enzyme activity, RWC and total production yield, total soluble sugars, and membrane strength | Qados, 2015; Qados and Moftah, 2015 | |
| Added to the nutrient solution immediately after the plants were transplanted. | Maize | Salinity | Increased FW | Gao et al., 2006 | |
| Added in irrigation water | Prunus mahaleb | Salinity | Increased seed germination and development, photosynthetic parameters, and antioxidant enzyme activity. Reduced MDA and H2O2 levels, chlorophyll destruction, and oxidative destruction | Ashkavand et al., 2018 | |
| Added in irrigation water | Sweet pepper | Salinity | Markedly regulated plant tolerance | Tantawy et al., 2015 | |
| Foliar application | Peregrina | Salinity | Increased growth and chemical constituents. Reduced Na and Cl precipitation and total phenolic and flavonoid content in leaves | Ashour and Abdel Wahab, 2017 | |
| Plants were irrigated with silica nanoparticles for 2 weeks | Basil | Salinity | Increased biomass and chlorophyll and Pro contents | Kalteh et al., 2014 | |
| Applied In irrigation water | Cucumber | Salinity | Elevated plant germination and growth parameters. Improved nutrient absorption and fruit production | Alsaeedi et al., 2018, 2019 | |
| Applied In irrigation water | Cucumber | Water deficit, salinity | Improved growth and productivity by alerting the plant of the nutrient uptake, such as increased N and K levels | Alsaeedi et al., 2019 | |
| Foliar application | Soybean | Salinity | Decreased oxidative damage through the expression of antioxidative enzymes | Farhangi-Abriz and Torabian, 2018 | |
| Applied In irrigation water | Soybean | Hg toxicity | Immobilized and inactivated Hg | Li et al., 2020 | |
| Added to petri dishes containing seeds | Common bean | Na+ stress | Improved the germination percentage, vigor index, seed germination rate, and the length and dry mass of shoots and roots. | Alsaeedi et al., 2017 | |
| Foliar application | Rice | HMs | Reduced HMs toxicity and promoted development by reducing bioaccumulation and translocation of HMs in plants | Wang et al., 2016 | |
| Applied In irrigation water | Pea | Cr (VI) | Decreased Cr (VI) absorption, promoted antioxidant defense mechanisms, and increased nutrient precipitation | Delfani et al., 2014 | |
| Added to nutrient solution of seedling | Pea | Cr (VI) | Alleviated Cr-induced phytotoxicity and improved overall growth potential | Tripathi et al., 2015b | |
| Seed soaking | Maize | As | Mitigated As toxicity and exhibited increased As resistance in maize cultivar compared to maize hybrid | Tripathi et al., 2016 | |
| Foliar application | Safflower | Mineral nutrient | Enhanced production yield | Janmohammadi et al., 2016 | |
| Added to petri dishes containing seeds | Wheatgrass | Cold | Averted seed dormancy and improved seed germination and seedling weight | Azimi et al., 2014 | |
| Foliar application | Wheat | UV-B radiation | Alleviated UV-B radiation stress in seedlings and resulted in improved protection of wheat seedlings via NO-mediated antioxidant defense, consequently counterbalancing toxic ROS generation | Tripathi et al., 2017b | |
| Foliar application | Wheat | Cd toxicity | Increased SOD and POD activity | Ali et al., 2019 | |
| Seed priming | Wheat | Cd toxicity | Mitigated oxidative stress, positively affected antioxidant enzyme activity, decreased Cd concentration mainly in grains, and increased Si concentration in plants | Hussain et al., 2019a,b | |
| Added to soil | Maize | UV-B radiation | Enhanced the growth and physiological responses | Suriyaprabha et al., 2012 | |
| Ag | Silver nanoparticles (15 ml) per every test plantlets was carried out for 14 days | Lentil | Drought | Decreased the germination ratio, root length, FW, and DW | Hojjat and Ganjali, 2016 |
| Seed soaking | Tomato | Salinity | Increased the germination percentage and germination ratio, root length, and seedling FW and DW. Upregulated four genes (AREB, MAPK2, P5CS, and CRK1) and downregulated three genes (TAS14, DDF2, and ZFHD1), thereby alleviating salt stress. | Almutairi, 2016b | |
| Seed soaking | Satureja hortensis L. | Increased germination percentage and enhanced growth parameters, such as shoot length. Improved salt stress tolerance | Nejatzadeh, 2021 | ||
| Seed priming | Wheat | Salinity | Mitigated the damaging impacts of salinity stress | Mohamed et al., 2017; Abou-Zeid and Ismail, 2018 | |
| Foliar application | Enhanced seed germination efficiency. Mitigated oxidative stress. Induced antioxidant enzymes | Wahid et al., 2020 | |||
| Seed priming | Increased plant growth hormones, including NAA, IBA and ABA. Promoted growth | Abou-Zeid and Ismail, 2018 | |||
| Silver nanoparticles (15 ml) per every test plantlets was carried out for 14 days | Promoted seed germination efficiency. Increased FW and DW | Hojjat and Kamyab, 2017 | |||
| Foliar application | Mitigated salt-stress–induced oxidative damage by inducing antioxidant enzymes. Regulated salt tolerance | Wahid et al., 2020 | |||
| Seed priming | Increased total sugar and Pro contents | Mohamed et al., 2017 | |||
| In irrigation water | Heat | Increased the leaf number and promoted growth | Iqbal et al., 2019 | ||
| Applied In irrigation water | Bok choy | Cd | Improved biomass, chlorophyll content, and vitamin C levels as well as SOD, CAT, and POD activity and reduced MDA levels | Li and Huang, 2014 | |
| Added in nutrient solution of seedling | Arabidopsis thaliana | Cold | Activated and increased the expression of antioxidant genes (MeCu/ZnSOD and MeAPX2) | Kohan-Baghkheirati and Geisler-Lee, 2015 | |
| Added in nutrient solution of seedling | Phaseolus vulgaris L. | Enhanced seedling quality and increased net photosynthesis. Regulated cold stress tolerance | Prazak et al., 2020 | ||
| In irrigation water | Wheat | Heat | Improved plant development and heat resistance | Iqbal et al., 2019 | |
| Spray | Horse-shoe pelargonium | Dark | Increased antioxidant enzyme activities, photosynthetic pigment content, and petal longevity. Reduced lipid peroxidation and petal abscission | Hatami and Ghorbanpour, 2013, 2014 | |
| Added in nutrient solution of seedling | Soybean | Flooding | Decreased the formation of cytotoxic byproducts of glycolysis as well as increased the abundance of stress-related proteins and seedling growth | Mustafa et al., 2015 | |
| Seed soaking | Saffron | Flooding | Blocked ethylene signaling, promoted root growth, and increased leaf DW and root length | Rezvani et al., 2012 | |
| Spraying | Chrysanthemum | Post-harvest | Enhanced the vitality and succulence of cut flowers and reduced FW loss and stem bacterial count | Kazemipour et al., 2013 | |
| In nutrient media | Brassica juncea | – | Improved growth and antioxidant potential in vitro | Sharma et al., 2012a | |
| In nutrient solution | Rice | – | Improved photosynthetic pigment content and enhanced CAT, APX, and GR activity | Gupta et al., 2018 | |
| In nutrient media | Arabidopsis thaliana | – | Improved anthocyanin precipitation in seedlings and stimulated protein precipitation | Syu et al., 2014 | |
| Spraying | Fenugreek | – | Increased shoot length, leaf number, plant number, and the production of photosynthetic pigments, phenolics, flavonoids, and tannins | Sadak, 2019 | |
| Foliar application | Cucumber | – | Activated antioxidant processes, enhanced phenolic features, and altered membrane characteristics | Zhang et al., 2018 | |
| Foliar application | Vigna sinensis | – | Promoted growth and increased biomass by increasing root nodulation and soil bacterial diversity | Pallavi et al., 2016 | |
| TiO2 | Foliar application | Wheat | Drought | Improved development, productivity, seed gluten, and starch content. Increased growth and starch content. Increased seedling DW. Increased chlorophyll and carotenoid contents, RWC, the transpiration rate, and stomatal conductance | Jaberzadeh et al., 2013; Faraji and Sepehri, 2020 |
| In irrigation water | Linum usitatissimum | Reduced H accumulation and increased chlorophyll and carotenoid contents and 2O2 and MDA levels | Aghdam et al., 2016 | ||
| In irrigation water | Ocimum basilicum L. | Mitigated drought-induced adverse effects and increased biomass and RWC | Kiapour et al., 2015 | ||
| Exogenous application | Vigna radiata L. | HMs | Induced antioxidant activity. Reduced MDA levels. Improved growth and increased biomass | Katiyar et al., 2020 | |
| Added in the soil | Glycine max L. | Mitigated Cd toxicity. Reduced lipid peroxidation. Increased chlorophyll content and reduce Pro content. Increased RWC, growth parameters, and net photosynthesis | Singh et al., 2016 | ||
| In irrigation water | Linseed or flax | Drought | Increased photosynthetic pigment content, plant development, production yield and reduced H2O2 and MDA levels | Aghdam et al., 2016 | |
| In irrigation water | Basil | Drought | Decreased the negative effects of drought stress | Kiapour et al., 2015 | |
| Spraying on shoot | Moldavian dragonhead | Drought (oxidative stress) | Alleviated oxidative stress. Increased Pro precipitation, and reduced H2O2 and MDA levels. | Mohammadi et al., 2016 | |
| Foliar application | Broad bean | Salinity | Increased plant development by improving antioxidant enzyme activities and increasing the levels of soluble sugars, amino acids, and Pro and other metabolites, thereby contributing to osmoprotection. | Abdel Latef et al., 2018 | |
| Seed priming | Zea mays L. | Enhanced seed germination efficiency Decreased Na+, Pro, and MDA levels and increased K+. Increased phenolic and antioxidant contents and RWC. Increased FW and DW. | Shah et al., 2021 | ||
| Added three times (three continuous days) to Hoagland solution 2 weeks after salinity stress application. | Dracocephalum moldavica | Positively impacted physiochemical properties by inducing antioxidant activities. | Gohari et al., 2020 | ||
| In nutrient solution | Chickpea | Cold | Improved antioxidative enzyme activities and reduced H2O2 levels and electrolyte leakage TiO2 precipitation was increased in the cold-sensitive genotype compared to the cold-tolerant genotype. | Mohammadi et al., 2013, 2014 | |
| TiO2 suspension was added to Petri dish containing seeds | Cold | Increased the expression of genes encoding Rubisco- and chlorophyll-binding proteins, decreased H2O2 levels, and increased the activity of phosphoenolpyruvate carboxylase | Hasanpour et al., 2015 | ||
| Soil and irrigation | Cold | Altered metabolic pathways as observed through transcription profiling | Amini et al., 2017 | ||
| Spraying | Tomato | Heat | Increased photosynthesis by regulating energy dissipation and caused leaf cooling by increasing stomatal opening | Qi et al., 2013 | |
| In irrigation water | Flax | Drought | Improved chlorophyll and carotenoid content, improved flax growth and yield attributes, and reduced H2O2 and MDA levels | Aghdam et al., 2016 | |
| Foliar application | Spinach | UV-B radiation | Reduced ROS and MDA levels and improved antioxidative enzyme activity and the oxygen evolution rate | Lei et al., 2007, 2008 | |
| Seed soaking | Spinach | Excessive light | Increased antioxidative enzyme activity, reduced ROS and MDA levels, and improved membrane stability and maintained an intact chloroplast structure | Hong et al., 2005 | |
| In nutrient media | Lemna minor | – | Increased the activities of different enzymes and eliminated accumulated ROS in plant cells | Song et al., 2012 | |
| In irrigation water | Wheat | – | Promoted leaf health and growth kinetic traits | Dawood et al., 2019 | |
| Spraying with hand automizer | Salvia ocinalis | – | Improved antioxidant action and increased phenol and flavonoid contents | Ghorbanpour, 2015 | |
| Spraying with hand automizer | Hyoscyamus niger | – | Increased SOD activity and exhibited the highest alkaloid (hyoscyamine and scopolamine) content | Ghorbanpour et al., 2015 | |
| Spraying | Wheat | – | Upregulated monosaccharides and azelaic acid, triggering tyrosine metabolism in roots. Upregulated reserve sugars and tocopherol, phenylalanine, and tryptophan pathways. | Silva et al., 2020 | |
| Foliar application | Cotton | Drought | Increased total phenolics, total soluble proteins, total free amino acids, Pro content, total antioxidant capacity, CAT, POD, and SOD activity | Shallan et al., 2016 | |
| In nutrient solution | Licorice | Cold | Decreased lipid peroxidation and H2O2 levels. Increased phenolics, total protein, and osmolyte contents | Kardavan Ghabel and Karamian, 2020 | |
| Foliar application | Rice | – | Increased biomass, decreased the photosynthetic ratio, and reduced energy consumption in metabolism | Zhang et al., 2020 | |
| Foliar application | Radish | – | Improved photosynthesis and total phenol levels | Tighe-Neira et al., 2020 | |
| CeO2 | Spraying | Mouse-ear Cress | Salinity | Improved leaf mesophyll K+ retention, chlorophyll content, biomass, and photosynthesis | Wu et al., 2018 |
| In the soil | Canola | Salinity | Shortened root apoplastic barriers, thereby allowing increased Na+ transport to shoots and reduced Na+ accumulation. Increased plant biomass and photosynthetic apparatus efficiency | Rossi et al., 2016 | |
| Chitosan | In soil and foliar application | Wheat | Drought | Increased leaf area, RWC, chlorophyll content, photosynthetic rate, CAT and SOD activities, crop yield, and biomass | Behboudi et al., 2019 |
| In soil and foliar application | Barley | Drought | Increased RWC, grain weight, grain protein, Pro content, and CAT and SOD activities | Behboudi et al., 2018 | |
| Al2O3 | Seed soaking | Soybean | Flooding | Regulated the ascorbate–glutathione pathway, membrane permeability, and tricarboxylic acid cycle activity | Mustafa and Komatsu, 2016 |
| Mn | Foliar application | Bell peppers | Salinity | Improved seed germination and root growth. Altered gene expression | Ye et al., 2020 |
| Mn3O4 | Foliar application | Cucumber | Salinity | Increased photosynthetic pigment content, net photosynthesis, in biomass which resulted in alterations in metabolomes. | Lu et al., 2020 |
| Fe | In nutrient media | Grape | Salinity | Increased total protein content and decreased Pro content, antioxidant enzymatic activity, and H2O2 levels. Decreased Na+ and increased K+ | Mozafari et al., 2018a |
| Foliar application | Moldavian balm | Salinity | Affected amino acid concentration and PPO, PAL, and SOD activities. Increased the gene expression of TAT, RAS, and RA | Moradbeygi et al., 2020a | |
| Foliar application | Moldavian balm | Salinity | Increased shoot and root leaf area, leaf length, FW, and DW | Moradbeygi et al., 2020b | |
| Seed soaking | Sorghum | Salinity | Improved the photosynthetic rate, chlorophyll index, PSII efficiency, RWC, and lipid peroxidation | Maswada et al., 2018 | |
| In nutrient media | Dracocephalum moldavica L. | Increased phenolic compound contents and enhanced APX, GR, CAT, and GPX activities | |||
| In nutrient media | Fragaria × ananassa Duch. | Increased photosynthetic pigments and total sugars, Fe levels, transpiration rate, and RWC. Enhanced membrane stability. Enhanced plant growth and weight. Decreased Na+ levels | Mozafari et al., 2018b | ||
| Foliar application | Mentha piperita L. | Decreased MDA and Pro contents. Decreased antioxidant enzymes | Askary et al., 2017 | ||
| Into soil | Wheat | Salinity and Cd | Increased photosynthetic pigments, NPK, and antioxidant enzymes activity. Increased growth, plant weight, and biomass. | Manzoor et al., 2021 | |
| Into soil and foliar application | Triticum aestivum L. | Drought and Cd | Enhanced Fe uptake, and improved growth parameters and photosynthetic activities | Adrees et al., 2020 | |
| Into soil and foliar application | Oryza sativa L. | Drought and Cd | Enhanced nutrient uptake and photosynthetic parameters. Enhanced growth and increased biomass | Ahmed et al., 2021 | |
| Seed soaking | Brassica juncea | Cr | Controlled the conversion and accumulation of Cr (VI) | Madhavi et al., 2013 | |
| In nutrient media | Arabidopsis thaliana | Drought | Promoted H+-ATPase activity, maintained stomatal opening and closure; increased biomass, photosynthetic pigments, and internal CO2 | Kim et al., 2015 | |
| Seed soaking | Brassica napus | Enhanced growth and increased chlorophyll levels and reduced H2O2 and MDA levels | Palmqvist et al., 2017 | ||
| Into soil and foliar application | Triticum aestivum L. | HMs | Reduced HMs-induced toxic effects and enhanced SOD and POX activities | Konate et al., 2017 | |
| Se | In soil and Foliar application | Wheat | Drought Heat Fungal infection | Maintained leaf water status and chlorophyll and carotenoid contents, which enhanced plant growth and increased biomass | El-Saadony et al., 2021d |
ABA, abscisic acid; Ag, silver; Al2O3, aluminum oxide; APX, ascorbate peroxidase; As, arsenic; CAT, catalase; Cd, cadmium; CeO2, cerium oxide; Cl, chlorine; CO2, carbon dioxide; Cr, chromium; DW, dry weight; Fe, iron; FW, fresh weight; GPX, glutathione peroxidase; GR, glutathione reductase; H, hydrogen; H2O2; hydrogen peroxide; Hg, mercury; HMs, heavy metals; IBA, indole-3-butyric acid; K, potassium; MDA, malondialdehyde; Mn, manganese; Mn3O4, manganese oxide; N, nitrogen; Na, sodium; NAA, 1-naphthaleneacetic acid; NO, nitric oxide; NPK, nitrogen:phosphorus:potassium; NPs, nanoparticles; PAL, phenylalanine ammonia-lyase; POD, peroxidase; POX, guaiacol peroxidase; PPO, polyphenol oxidase; PSII, photosystem II; RA, rosmarinic acid; RAS, rosmarinic acid synthase; ROS, reactive oxygen species; Pro, proline; RWC, relative water content; Se, selenium; Si, silicon; SOD, superoxide dismutase; TAT, tyrosine aminotransferase; TiO2, titanium dioxide; UV, ultraviolet; Zn, zinc.
With the emergence of new nanotechnological applications, the use of nanomaterials with a high surface area-to-volume rate has increased. The functions and usage of nanomaterials differ according to the size and structure of NPs (Tunca, 2015). When nanomaterials are used as a biofertilizer, plants are provided with nutrients slowly, small amounts are sufficient (in contrast to chemical fertilizers), and the environmental risks caused by chemical fertilizers are minimized (Cinisli et al., 2019; Usman et al., 2020). The chemical pesticides and fertilizers adversely affect ecosystems and human health, particularly when large doses are used to increase plant yield. Therefore, it has become desirable to replace conventional pesticides and fertilizers with nanopesticides and nanofertilizers, to reduce the use of chemical fertilizers, increase plant yield, and support agricultural development (Bratovcic et al., 2021). Thus, these nanopesticides and nanofertilizers are now receiving increasing research attention (Kah, 2015). Nanofertilizers vary in size (30–40 nm), pass through the stomata, bind to different ions, and release nutrients (Bal, 2019; Cinisli et al., 2019). In general, nanofertilizers affect plant growth and metabolism by improving soil quality and plant growth performance, increasing growth hormone production and enhancing resistance to biotic and abiotic stresses (Cinisli et al., 2019; Saad et al., 2021; El-Ashry et al., 2022; Khairy et al., 2022). Nanopesticides can be produced using physical, chemical, or biological methods. Nanopesticides and nanoformulations, including those incorporating silver (Ag), Cu, silica (SiO2), and zinc oxide (ZnO), exhibit an improved range of pesticide efficacy compared with conventional pesticides; thus, nanopesticides positively influence the control of plant pests and diseases (Chhipa, 2017). Chitosan-metal oxide NPs have been used to ensure that the fertilizers applied to plants are taken up more effectively. The application of chitosan increases the enzyme activity of nitrate reductase, glutamine synthetase, and protease during N metabolism, thereby affecting plant growth and development (Bal, 2019). In peanut and corn plants, ZnO NPs increase the germination percentage and improve seedling development (Prasad et al., 2012; Singh et al., 2017). In addition, treatment with 2000 mg L–1 of 60-nm aluminum (Al) NPs for 5 days reduced the root length of corn seedlings and did not exert any adverse effects on Lolium perenne, Raphanus sativus, Cucumis sativus, Brassica napus, and Lactuca sativa (Yang and Watts, 2005).
Application of 2000 mg L–1 of Zn NPs considerably hindered root development in maize and stopped the root growth of Brassica oleracea, C. sativus, Daucus carota, and Glycine max (Lin and Zhing, 2007). Few experiments have addressed the influence of NPs on seed germination and seedling growth. However, the application of NPs on seeds generally increases seed germination, seedling development, seedling viability, and emergence rate (Abbasi Khalaki et al., 2021). Seed germination, root and shoot length, and fresh weight (FW) and dry weight (DW) values of Agropyron elongatum were positively affected by SiO2 NPs application (Azimi et al., 2014). Ag NPs have been shown to increase the germination level, length of the roots and shoots, FW and DW, average germination time, and vitality indices in Thymus kotschyanus (Abbasi Khalaki et al., 2016). Similarly, Ag NPs have been found to increase the germination rate in Pennisetum glaucum (Parveen and Rao, 2015) and Festuca ovina (Abbasi Khalaki et al., 2019a). However, Ag NPs can reportedly adversely affect the germination of Brassica nigra (Amooaghaie et al., 2015) as well as the shoot length of Medicago sativa, the root length and shoot DW of Ocimum basilicum, and the shoot and root length of Hordeum vulgare, Linum usitatissimum, and L. perenne (El-Temsah and Joner, 2010; Ramezani et al., 2014; Yosefzaei et al., 2016).
In Onobrychis sativa, SiO2 NPs increased shoot length, whereas titanium dioxide (TiO2) NPs increased germination time and percentage (Moameri et al., 2018a). Iron oxide (Fe2O3) NPs increased the germination of L. perenne (Wang H. et al., 2011). In addition, FeO NPs reduced the mycorrhizal biomass and the shoot and root length of Trifolium repens (Feng et al., 2013), Satureja hortensis (Peyvandi et al., 2011a), H. vulgare, and L. perenne (El-Temsah and Joner, 2010). Studies have shown that TiO2 NPs can positively affect the germination of Foeniculum vulgare, and Petroselinum crispum (Dehkourdi and Mosavi, 2013; Feizi et al., 2013). Ag NPs increased the shoot length and chlorophyll content of Brassica juncea and Sorghum bicolor (Namasivayam and Chitrakala, 2011; Sharma et al., 2012b). The root development of T. kotschyanus and Alopecurus textilis was positively affected by SiO2 NPs application (Abbasi Khalaki et al., 2019a,b). Similarly, SiO2 application to M. sativa increased plant height, tiller count, yield, FW, and DW, chlorophyll content, and carotenoid levels (Ma and Yamaji, 2006; Zmeeva et al., 2017). Govorov and Carmeli (2007) reported that SiO2 NPs increase leaf FW and DW as well as chlorophyll content in O. basilicum, and also negatively affect shoot and root growth in S. bicolor, Stipa hohenackeriana, and Secale montanum (Lee et al., 2012; Moameri et al., 2018b; Moameri and Abbasi Khalaki, 2019). ZnO NPs increase biomass, root and shoot length, and chlorophyll content in many plant species (Peyvandi et al., 2011b; Raliya and Tarafdar, 2013; Najafi Disfani et al., 2017; García-López et al., 2018; Yuan et al., 2018). In addition, TiO2 NPs increased the essential oil content and yield of medicinal plants (Ahmad et al., 2018; Fazeli-Nasab et al., 2018). The application of copper oxide (CuO) NPs adversely affects the morphology, physiology, and biochemistry of H. vulgare, L. perenne, M. sativa, and Triticum aestivum (Lee et al., 2008; Atha et al., 2012; Ramezani et al., 2014; Shaw et al., 2014; Hong et al., 2016).
Application of NPs Under Salinity Conditions
NPs application is important to mitigate the abiotic stress effects of salinity on plants. At the germination stage, the use of Ag NPs in Lathyrus sativus under salt stress improves germination percentage, shoot and root length, and seedling FW and DW; thus, this enhanced osmotic regulation and reduced the negative effects associated with salinity (Hojjat, 2019). Noman et al. (2020) found that applying Cu NPs to the soil reduced oxidative stress in wheat and significantly increased plant development and yield. The use of NPs in wheat not only enhances plant development but also improves germination performance under salt-stress conditions (Shi et al., 2016). Preapplication of Ag NPs to wheat seeds alters antioxidant enzyme activities, reduces oxidative damage, and elevates salt-stress tolerance in such plants (Kashyap et al., 2015). In addition, ZnO NPs are known to increase the DW of sunflowers under salt stress (Torabian et al., 2016). CeO NPs (100 and 200 mg kg–1) was found to enhance the physiological responses of B. napus under salt stress (100 mM NaCl). CeO NPs are also known to boost plant biomass in salt-stressed canola (Rossi et al., 2016). The application of Ag NPs to basil seeds under salt-stress conditions increases seed germination (Darvishzadeh et al., 2015; Hojjat and Kamyab, 2017). Ag NPs applied to S. hortensis increase plant resistance to salt stress while reducing salt-stress–induced effects on germination percentage and plant shoot length (Nejatzadeh, 2021). Furthermore, the use of Ag NPs in salt-stressed cumin plants substantially improves plant salt resistance (Ekhtiyari and Moraghebi, 2012). Finally, Askary et al. (2017) reported that Fe3O4 NPs protects mint plants from oxidative stress caused by increased NaCl content.
Application of NPs Under Drought Conditions
Drought is considered a major abiotic stress that can drastically limit crop production (Al-Ashkar et al., 2021; Roy et al., 2021). NPs application is an efficient method for alleviating the impact of drought on plants by increasing antioxidant enzyme activity, improving phytohormone levels, and affecting physiological properties. The use of analcite NPs in soil under hot, dry conditions has been shown to promote germination and plant growth in wheat (Hossain et al., 2021). In addition, the use of ZnO NPs in soybean seeds under arid conditions increases the germination percentage of the seeds (Sedghi et al., 2013). Under drought stress, the use of Cu and Zn NPs in wheat plants increases their antioxidant enzyme activity and relative moisture content, decreases thiobarbituric acid levels, affects reagent precipitation, stabilizes photosynthetic pigment levels in leaves, and reduces the effects of stress (Taran et al., 2017; Semida et al., 2021). In response to drought stress, SiO2 NPs application can increase shoot length and relative water content (RWC) in barley, while reducing superoxide radical formation and membrane damage (Turgeon, 2010).
Jaberzadeh et al. (2013) have reported that foliar usage of TiO2 NPs in wheat is effective to overcome the yield reduction caused by drought stress. Furthermore, the application of Cu NPs to maize increased leaf water content, plant biomass, and anthocyanin, chlorophyll, and carotenoid contents under arid conditions (van Nguyen et al., 2022). Ashkavand et al. (2015) reported that SiO2 NPs applied to hawthorn grown under drought stress reduced photosynthesis and stomatal conductivity. However, silicon (Si) NPs have been reported to ameliorate the effects of drought stress in bananas (Mahmoud et al., 2020). Under moderate drought conditions, foliar application of Si NPs to coriander resulted in optimum antioxidant capacity and essential oil yield (Afshari et al., 2021). Shallan et al. (2016) have reported that foliar application of SiO2 and TiO2 NPs can reduce the negative effects of drought stress on cotton plants under arid conditions. In chickpea plants, the application of Si NPs to the soil reduces the negative effects of drought by increasing the relative moisture content in the plants (Gunes et al., 2007). Si- and selenium (Se)-NPs can reportedly help in enhancing growth, improving ion selectivity in roots, and increasing the yield of rice under saline conditions (Badawy et al., 2021). Although drought stress increases the adverse effects of Cd in wheat, the application of ZnO NPs can reduce both Cd and drought stress (Khan et al., 2019).
Application of NPs Under Heavy Metal Stress Conditions
Under HMs stress conditions, soil or foliar applications of NPs can eliminate the adverse effects of stress, improve plant development and photosynthesis, and reduce oxidative stress-induced toxicity. Therefore, the application of NPs contributes to in the remediation of HMs-contaminated environments. Under HMs stress conditions, the application of NPs to plants reduces the concentration of HMs in the soil, regulates the expression of HMs transfer genes in plants, increases the activity of plant antioxidant systems, improves physiological functions, and stimulates the production of protective substances such as root secretions, phytochelatin, and organic acids (Rui, 2021). The application of Si NPs on maize plants under arsenic (As) stress reduced the total chlorophyll, carotenoid content, and total protein content; in addition to mitigating the adverse effects of As stress on maximum quantum efficiency, photochemical quenching, and non-photochemical quenching of FS II (Tripathi et al., 2016). Soil application of TiO2 NPs can effectively limit Cd toxicity by enhancing the physiological parameters and photosynthetic rate in soybean plants; therefore, TiO2 NPs are vital to mitigate the effects of HMs-induced oxidative stress (Singh and Lee, 2016). When treated with SiO2 NPs, the activities of enzymes, such as ascorbate peroxidase (APX) and superoxide dismutase (SOD), increased; whereas the effects of oxidative stress were reduced in pea seedlings under Cr stress (Tripathi et al., 2015b). Furthermore, de Sousa et al. (2019) revealed that Si NPs can reduce Al toxicity by activating the antioxidant defense mechanism in maize plants. Konate et al. (2017) found that Fe3O4 NPs protected wheat against Cd-induced oxidative stress. Foliar applications of Se NPs to Chinese cabbage under Cd stress increased the biomass, plant height, leaf chlorophyll content, SOD levels, and plasma glutathione peroxidase (GPX) content, whereas the Cd and malondialdehyde (MDA) contents of the leaves were reduced (Zhang, 2019). Similarly, Si NPs alleviate the effect of Cd stress in rice (Wang et al., 2015). The combined use of foliar ZnO NPs and soil biochar in plants was found to be more effective against Cd stress (Rizwan et al., 2019a). Similarly, the coapplication of Fe NPs and biochar reduced the effects of Cd stress in rice (Hussain et al., 2019c). The use of FeO NPs in Cd-stressed wheat reduced the leaf electrolyte leakage ratio and Cd content in grains, while improving the antioxidant enzyme action and DW of the plants. Foliar application of Fe NPs is preferable over soil usage. Rahmatizadeh et al. (2019) also found that 20 mg L–1 of Fe3O4 NPs reduced Cd accumulation and improved Cd toxicity by increasing nutrient uptake in tomato plants.
Nanofertilizers Versus Commercial Fertilizers
Agrochemicals can be released in a controlled manner, and macromolecules can be delivered selectively. By incorporating nanoscale transporters and chemicals, the efficient use of fertilizers and pesticides can be improved, resulting in a reduction in the amount used without compromising the yield of crops. In contrast, commercial fertilizers, provide fewer benefits to plants because of their larger particle size and reduced solubility. In addition, repeated chemical fertilizer application result in a toxic build-up of HMs that disrupts the ecological balance in the soil. In addition, excessive application of chemical fertilizer can contribute to soil pollution due to leaching or being not fully utilized by plants; thus, the remaining is converted into insoluble salts in the soil.
Nanoagrochemicals play an important role in enhancing nutrient use efficiency and water quality management for sustainable agriculture. However, bioaccumulation and long-term exposure of NPs to plants may have a negative impact on edible plants and food chains (Rajput et al., 2020). According to Staroń et al. (2020), NPs can be taken up and deposited in the edible tissues of crop plants. The accumulation of NPs or metal ions in their natural state can disrupt plant physiological activities; affect the integrity of cellular and sub-cellular organelle organizations; and modify the content of proteins, lipids, and nucleic acids by creating hydroxyl radicals (Cota-Ruiz et al., 2018; Rajput et al., 2020). Overall, the wide-ranging applications of NPs may generate a slew of difficulties from an ecological, ethical, health, and safety standpoint (Rajput et al., 2018).
Until now, the potential negative effects of NPs on human health have been speculative and unsubstantiated (Staroń et al., 2020). By developing various NPs as new tools for the agriculture industry, nanotechnology has grown in popularity. There is an urgent need to increase our knowledge and understanding of the specific benefits and drawbacks associated with the use of NPs. The advancement of nanotechnology has resulted in significant amounts of manufactured NPs in the agroenvironment. Although this technology has numerous advantages, researchers and experts are concerned about the unsafe disposal of NPs in large quantities (several hundred tons) each year (Rajput et al., 2020).
The existence of NPs in a various controlled objects (atmospheric air, water objects, soils, hydrobionts, algae, fungi, tissues of land plants/animals) is recommended (Rajput et al., 2020). In comparison with other sources, the fate and movement of NPs in soil have undergone very little research. Simultaneously, the soil offers fundamental nutrients to food crops, which can also operate as NPs collector sink (Rajput et al., 2020). The current review sheds light on the potential impact of NPs on the environment, health, and food security.
Examples of NPs and the Roles They Play in Relieving Stress in Plants
Si NPs
Si-based materials and their oxides are found abundantly in the soil. Plants naturally contain high levels of Si (1–10%) as well. Si in plants is found in the form of amorphous silica (SiO2⋅nH2O) in the cell wall, providing it with strength and solidity, in addition to contributing to polyphenols and pectins as a reactant (Bhatt and Sharma, 2018). These substances are also active during plant defense and development. Because of their application in multiple agricultural fields, it has been reported that Si-based NPs can ameliorate abiotic stresses (Jeelani et al., 2020). However, little is known about the mechanisms by which Si alleviates stresses in plants (Ma, 2004; Liang et al., 2007; Datnoff et al., 2009). Si particles and Si NPs can increase tolerance to abiotic stress, nutrient element homeostasis, stimulation of antioxidant enzymes, and improved absorption, immobilization, and partition of metal ions (Liang et al., 2007; Monica and Cremonini, 2009; Qados, 2015). SiO2 NPs considerably enhance germination, development, and yield in plants under stress. This can be attributed to the uptake of these NPs via roots leading to the development of a thin layer in the cell wall helping plants to tolerate various stresses (DeRosa et al., 2010; Siddiqui et al., 2014). SiO2 NPs also increase the efficiency of water translocation, increase turgor pressure, and enhance relative water inclusion in leaves and water usage effectiveness in plants (Rawson et al., 1988; Wang and Naser, 1994). Sharifi-Rad et al. (2014) found that various concentrations of SiO2 NPs significantly promoted maize growth and affected different developmental stages. SiO2 NPs can also be involved in the regulation of protein and phenolics, which are important for the growth and development of Zea mays (Suriyaprabha et al., 2012). In addition, they found that a relatively high level of Si accumulated in roots would boost drought tolerance in maize.
Precipitated Si NPs within plant tissues are capable of increasing the expression of essential biochemical elements, improving development, and enhancing yield factors in maize (Suriyaprabha et al., 2012). Furthermore, the improved action of the enzymatic system, the build-up of nutrients, free Pro, amino acids, and water absorption are positive effects of NPs that improve stress tolerance in crops (Wang et al., 2015; Shalaby et al., 2016; Shojaei et al., 2019). Importantly, Si NPs can also increase plant tolerance to drought stress. Ashkavand et al. (2015) observed enhanced drought tolerance as well as retention of critical biochemical and physiological attributes in hawthorn seedlings following the application of SiO2 NPs under different levels of drought stress. Pretreatment with SiO2 NPs positively influences the photosynthetic rates, stomatal conductance, and augmented xylem water potential in hawthorn seedlings under drought stress. Large dosages of SiO2 NPs supplied with irrigation water before drought treatments mitigate drought stress effects on growth, and biochemical and physiological parameters of Prunus mahleb (Tripathi et al., 2015b). Improved drought tolerance, evident by an improvement in root development and retention of the photosynthetic ratio, was also reported in two cultivars of S. bicolor with varying drought sensitivities after the application of Si NPs. Thus, increase in drought resistance occurred regardless of the cultivar sensitivity to drought stress (Hattori et al., 2005).
Pei et al. (2010) noted that the use of an appropriate concentration of sodium silicate (i.e., 1.0 mM) could moderately diminish the negative effects of drought stress in wheat. In the same study, there was partial promotion of shoot development and chlorophyll content when Si was supplied. This also helped retain leaf water potential and reduced membrane lipid peroxidation in stressed plants (Pei et al., 2010). Under drought stress, Si deposition in plant cells could help reduce the transpiration ratio, and enhance the photosynthesis mechanism (Ali et al., 2012; Siddiqui et al., 2014). Thus, the effects of drought stress can be mitigated by various Si/SiO2 NPs applications in various plant species (Zargar et al., 2010). The improved performance of such NPs can be attributed to the increased absorption and/or penetration into plant tissues; however, the exact mechanisms are not yet understood (Ashkavand et al., 2015). Shallan et al. (2016) have reported that foliar sprays of TiO2 NPs (50 mg L–1) or SiO2 NPs (3200 mg L–1) increase the drought tolerance of cotton plants. In addition, Si can help plants acclimatize to various ecological stresses (Rastogi et al., 2019). Salinity stress restrains crop yield because of Na+ ion toxicity in approximately 23% of planted areas worldwide (Onaga and Wydra, 2016). However, the application of Si NPs and Si fertilizer under salinity stress has positive impacts on the physiological and morphological indices of vegetative characteristics in O. basilicum. This is evident from the remarkable enhancement in the developmental index, chlorophyll content, and Pro concentration. This, may be because of the involvement of NPs and Si fertilizers with increasing tolerance to salinity stress in plants (Kalteh et al., 2014). The use of SiO2 NPs has also been shown to enhance developmental parameters, chlorophyll content, Pro accumulation, and upregulation of antioxidant enzyme activity in tomato and squash plants under salinity stress (Haghighi et al., 2012; Siddiqui et al., 2014).
The application of SiO2 NPs improves not only seed germination and early seedling development but also other related characteristics in lentil genotypes under salinity stress. Thus, SiO2 NPs boost salt toxicity protection in plants (Sabaghnia and Janmohammadi, 2014). SiO2 NPs can also mitigate stress by reducing Na+ ion concentration, resulting in improved crop development, production, and survival under salinity stress (Savvas et al., 2009). The application of SiO2 NPs also increases FW in maize in response to salinity stress (Gao et al., 2006). Si NPs can improve plant development by reducing osmotic potential and Na+ toxicity associated with high salt stress (Raven, 1983). It has been reported that SiO2 NPs form a layer in the root cell wall that enables plants to tolerate several stresses (e.g., salinity) (DeRosa et al., 2010; Abdel Latef et al., 2018).
Wang et al. (2010) and Wang X. et al. (2011) and others have documented the capability of Si and SiO2 NPs in reducing the harmful effects of salt on plant development. Because of their small size, uptaking SiO2 NPs can be performed more efficiently than uptaking micro-SiO2, -Na2SiO3, or -H4SiO4 when added to maize roots and seeds (Suriyaprabha et al., 2012). The particles were subsequently used by plants to improve growth by affecting xylem humidity, water translocation, and increasing turgor pressure; which in turn, improves the RWC and water use efficiency (WUE). The enhancement of plant germination and developmental traits by SiO2 NPs may be associated with an enhanced K/Na ratio, which reduces Na+ uptake (Alsaeedi et al., 2018), and increases the expression of antioxidant enzymes (Torabian et al., 2016; Farhangi-Abriz and Torabian, 2018). According to Almutairi (2016a), it has been found strong interactions between the enhancement of seed germination and growth in tomato-stressed plants with high salt and the increased expression of salt tolerance genes when Si NPs are applied. In contrast to no treatment of Si NPs, Capsicum annuum plants showed increased growth when irrigated with saline water upon the application of Si treatments (Tantawy et al., 2015).
Several studies have demonstrated that nano-Si is effective in detoxifying HMs or reducing their toxic effects while promoting plant development under HMs stress (Shen et al., 2014; Keller et al., 2015). For instance, the toxicity of Cr in pea seedlings was alleviated by supplementing the growth media with Si NPs, which reduced oxidative stress by decreasing the precipitation of Cr and increasing antioxidant mechanisms (Tripathi et al., 2015a,b). In addition, Cui et al. (2020) have reported that SiO2 NPs application can reduce oxidative stress in As-exposed rice cell lines. Similarly, the foliar application of 2.5 mM nano-Si can markedly increase tolerance to Cd stress in rice through the regulation of Cd precipitation (Wang et al., 2015). Si NPs have also been shown to alleviate toxicity caused by Pb, Cu, Zn, and Cd HMs, and their use may be more effective at reducing HMs accumulation compared with traditional strategies (Wang et al., 2016). Liu and Lal (2015) demonstrated that Si NPs are more effective than bulk Si in reducing the detrimental effects of Pb on rice development. Si NPs prevent Pb movement from the rice roots to the shoots and reduce Pb precipitation in grains, especially in high-Pb-precipitating cultivars and in soils with high levels of Pb contamination. Si NPs can also reduce and chelate active HMs ions, stimulate antioxidant systems, enhance the complexation and coprecipitation of toxic metals with Si, and produce fundamental changes in plants by controlling the expression of metal transport genes. However, these processes are dependent on plant genotypes, plant species, metal elements, developmental requirements, and the duration of stress enforced. Therefore, Si-mediated reductions in metal toxicity might be generalized with caution (Adrees et al., 2015). According to studies conducted by Tripathi et al. (2015b), Si NPs are linked with mitigating the toxicity effects of Cr in Pisum sativum seedlings. Cr stress induces toxicity; however, Si NPs can protect pea seedlings from Cr (VI)-induced phytotoxicity by reducing Cr precipitation, enhancing the antioxidant defense system, and alleviating oxidative stress. Tripathi et al. (2016) have also evaluated the effects of Si NPs on alleviating As toxicity in a maize cultivars and hybrids. Hydroponic trials have shown that these NPs can considerably reduce As toxicity by increasing the levels of metabolites of the ascorbate–glutathione cycle, and decreasing the levels of oxidative stress indicators, resulting in reduced As precipitation in the Si NP-treated cultivars and hybrids. It has been hypothesized that Si NPs can be more effective than bulk Si particles for balancing ROS production and ameliorating ROS-mediated damage in treated plants. It has also been reported that Si NPs are more effective than Si in protecting plants against UV-B stress. In general, Si NPs may protect plants by activating their antioxidant defense mechanism and regulating ROS-induced oxidative stress (Tripathi et al., 2017b).
Ag NPs
Ag NPs are widely used in the agricultural sector, particularly in crop enhancement, food packaging, coating of domestic products, and pesticides. Their use in electronics, drug delivery, and biological-tagging medicine is also relatively common (Bechert et al., 1999; Davies, 2008; Korkin and Rosei, 2008; Jo et al., 2009; Ahamed et al., 2010; Kim et al., 2012). Ag is toxic when used in high concentrations; however, when reduced to a nanosize of 25–50 nm, it has unique properties compared with bulk Ag (Bhatt et al., 2020). Owing to these unique features, Ag NPs can be applied to enhance the vigor of plants and boost their overall development, productivity, and photosynthetic rate (Sharma et al., 2012a; Hatami and Ghorbanpour, 2013; Vannini et al., 2013; Shelar and Chavan, 2015). Ag NPs can also be used as antimicrobial substances to manage diseases on plants (Lamsal et al., 2011). The effect of different concentrations of chemically produced Ag NPs was investigated in B. juncea seedlings, specifically on the development and antioxidant status of the plants. Ag NPs were capable of improving growth and inducing the activity of specific antioxidant enzymes, which reduced ROS levels, improved overall antioxidant status, and reduced Pro and MDA levels. The growth-improving effect of Ag NPs in plants under stress is concentration-dependent; where a 50-ppm dose was ideal to improve growth (Sharma et al., 2012b). In another study on tomatoes, Ag NPs-treated seeds germinated earlier than those treated with deionized water; however, seed germination was inhibited when higher concentrations of Ag NPs were applied (Karami Mehrian et al., 2016).
Ag NPs may also play a role in the expression of stress genes. For instance, the up- and down-regulation of certain genes by Ag NPs was observed in microarray analysis: upregulated genes were mostly associated with responses to metal toxicity and oxidative stress, whereas downregulated genes were associated with responses to microbes and hormonal stimuli (Banerjee and Kole, 2016). Such responses may be associated plant defense mechanisms under adverse conditions; however, additional studies are required to elucidate the signaling cascades and genes controlled by Ag NPs and other NPs in various plant species.
The effects of Ag NPs on the hydraulic conductivity of the plant stem during drought stress have been studied; however, such NPs might also be capable of entering plant cells and tissues and impairing regular cellular activities (Tripathi et al., 2017a). Hojjat and Ganjali (2016) found that Ag NPs can alleviate the effect of drought stress effects in lentil (Lens culinaris). MahdiNezhad et al. (2018) reported that Ag NPs can reduce the levels of antioxidant enzymes in plants under drought stress; thus, this reduction can be attributed to the reduced antioxidant metabolism. NPs may be directly involved in the elimination of ROS, which reduces the levels of antioxidant enzymes. Seghatoleslami et al. (2015) reported the effects of Ag NPs on the yields and WUE of drought-stressed Carum copticum using a magnetic field.
Ag NPs application is useful to reduce the effect of salinity stress–induced toxicity. This has been demonstrated in studies on the germination of tomato, fennel, and cumin plants treated with Ag NPs; thus, enhancing germination, improving developmental performance, and mitigating the negative effects of salt stress (Ekhtiyari and Moraghebi, 2011; Ekhtiyari et al., 2011; Almutairi, 2016b). Positive effects of different concentrations of Ag NPs suspension have been reported on the germination and development of Solanum lycopersicum under salinity stress (Delfani et al., 2014). In the same study, AREB, P5CS, MAPK2, and CRK1 were induced and TAS14, ZFHD1, and DDF2 were repressed when salt-stressed plants were exposed to Ag NPs. A comparative study of the toxicity revealed that Ag NPs or AgNO3 had negative effects on C. sativus seedlings grown at higher concentrations; however, Ag NPs were less toxic than AgNO3 and had the potential to improve C. sativus yield (Cañas et al., 2008). The role of Ag NPs in relieving salt stress in wheat and B. juncea has been assessed, and Ag NPs were found to efficiently alleviate the effects of salinity stress (Sharma et al., 2012b; Mohamed et al., 2017; Abou-Zeid and Ismail, 2018). Ag NPs at 50 and 75 mg L–1 concentrations can protect plants from heat stress and improve their development (Iqbal et al., 2019).
TiO2 NPs
TiO2 is a typical oxide of titanium. As a metal, titanium is abundant in the Earth’s crust as well as found in plant and animal tissues. TiO2 and nano-TiO2 serve as UV blockers in sunscreens because they diminish the adverse effects of UV radiation. In addition, TiO2 NPs have photocatalytic sterilizing properties and can undergo redox reactions when subjected to light, resulting in the formation of superoxide anion radicals and hydroxide (Hong et al., 2005). Photosterilization by TiO2 NPs can promote photosynthesis and improve plant growth. The potential effects of TiO2 NPs on the photochemical responses of chloroplasts in spinach (Spinacia oleracea) were evaluated (Hong et al., 2005). TiO2 NPs treatment was found to improve the activities of SOD, catalase (CAT), and peroxidase (POD), decrease the accumulation of reactive oxygen free radicals and MDA levels, and maintain the stability of the membrane structure of chloroplast under the light. TiO2 NPs also play a role in plant biochemical processes, morphophysiological characteristics, and reactions to various stresses (Mishra et al., 2014). In S. oleracea, TiO2 NPs can increase antioxidant stress tolerance through decreasing superoxide radical precipitation, reducing stress indicator (H2O2 and MDA) levels, and stimulating antioxidant enzyme activities within the plants during the photochemical interactions in chloroplasts (Lei et al., 2008).
In spinach plants, nano-anatase TiO2 treatment markedly increased photosynthesis, electron transmission, photoreduction activity of photosystem II, oxygen evolution, and photophosphorylation of chloroplasts under visible and UV light illumination (Lei et al., 2007, 2008). In addition, the effects of TiO2 NPs on plant growth have been associated with enhanced photosynthetic rate and nitrogen metabolism (Yang et al., 2006). The photocatalytic degradation of pesticides by TiO2 has been demonstrated as a possible water remediation process (Lee et al., 2003). Moreover, TiO2 NPs increase plant water uptake and nitrogen use and stimulate antioxidant activity in canola (Mahmoodzadeh et al., 2013) and wheat (Jaberzadeh et al., 2013).
Several studies have confirmed the TiO2 NPs-mediated improvement of plant development. For instance, Changmei et al. (2002) found that TiO2 and SiO2 NPs positively affect seed germination and growth of G. max (Changmei et al., 2002). In addition, onion seedlings treated with TiO2 NPs increased the enzymatic activity of SOD, amylase, CAT, and POD (Laware and Raskar, 2014). Mohammadi et al. (2016) explored the potential effects of different concentrations of TiO2 NPs against drought stress in Dracocephalum moldavica. Foliar application of these NPs at higher concentrations (40 ppm) can reportedly alleviate the detrimental effects of drought stress by adjusting the level of antioxidant enzymes and oxidative stress indicators. TiO2 NPs have been reported to increase Rubisco activase activity, chlorophyll formation, and the photosynthetic ratio and plant dry mass (Gao et al., 2008). In Vigna unguiculata, seed yield increases with foliar application of NPs and TiO2. Thus, this could be attributed to the increase in photosynthetic rates (Owolade and Ogunleti, 2008). The activity of the antioxidant enzymes (POD and CAT) increases in response to TiO2 NPs; therefore, MDA precipitation also decreases (Ahmad et al., 2019). The ability of TiO2 NPs to alleviate the adverse effects of drought stress has been investigated in several studies. For instance, the foliar application of TiO2 NPs can promote growth and increase the yield of wheat under drought stress when TiO2 NPs (0.02%) has been used (Jaberzadeh et al., 2013).
TiO2 NPs also improved the ability of plants to capture sunlight in maize plants. Under drought stress, TiO2 NPs can affect the pigment formation, the transformation of light energy to the active electron, and chemical activity, thus, enhancing photosynthetic effectiveness in maize (Akbari et al., 2014). In a similar study, the effects of nano-TiO2 and -SiO2 on the biochemical components and productivity yield of drought-stressed cotton plants have also been tested (Shallan et al., 2016). In their findings, the pretreatment with nano-TiO2 or -SiO2 can improve the pigment content, antioxidant enzyme activity, and antioxidant capacity, and increase the yield of these plants. The optimum concentrations required to reduce the destructive effects of drought stress in cotton plants were 50 and 3200 ppm for nano-TiO2 and -SiO2, respectively. Foliar application of these NPs have also increased drought tolerance in cotton plants. Similar results have been obtained in drought-stressed L. usitatissimum treated with TiO2 NPs (Aghdam et al., 2016). The drought-stressed D. moldavica treated with TiO2 exhibited increased levels of Pro and considerably reduced levels of H2O2 and MDA compared with nontreated plants (Mohammadi et al., 2014). Thus, suggesting that TiO2 NPs can ameliorate stress-induced damage. TiO2 NPs significantly induced the antioxidant enzyme activity, and Pro and soluble sugar content, which in turn promoted osmotic balance in plant cells and growth recovery in plants (Abdel Latef et al., 2018). O. basilicum can tolerate drought stress owing to the combined effects of gibberellin and TiO2 (Hatami, 2017). Overall, the application of nano-TiO2 can alleviate stresses of HMs, light, cold, and heat.
Singh and Lee (2016) have shown that the application of TiO2 NPs can reduce Cd toxicity and enhance the photosynthetic rate in soybean (Singh and Lee, 2016). TiO2 NPs also play an important role in alleviating light stress. When subjected to light, these NPs catalyzed the redox reaction, thereby generating superoxide anion radicals and hydroxide (Khan and Siddiqui, 2018). The addition of TiO2 NPs reduced the impact of heat stress by controlling stomatal opening (Qi et al., 2013). TiO2 NPs also positively affect plant growth and development. The positive effects of NPs, including TiO2-NPs, include enhancement of the carboxylation of Rubisco (Gao et al., 2006), light absorption capabilities of chloroplasts (Ze et al., 2011), electron transport rates, and prevention of ROS formation (Giraldo et al., 2014). The use of nano-TiO2 increases the expression level of genes encoding Rubisco- and chlorophyll-binding proteins (Hasanpour et al., 2015) as well as the activity of antioxidant enzymes (Mohammadi et al., 2014); thus maintaining stable contents of chlorophyll and carotenoids, and improving tolerance to cold conditions. Furthermore, TiO2 NPs positively affect susceptible (ILC 533) and resistant (Sel 11439) genotypes of chickpea under cold stress. Under such stressful conditions, TiO2 dramatically reduced membrane damage indices, such as ion leakage index and MDA levels, resulting in reduced damage to the membrane (Mohammadi et al., 2013). TiO2 treatments can also hinder oxidative damage in chickpea and reduce membrane damage under cold stress; suggesting that TiO2 NPs can ameliorate the redox status under heat exposure (Mohammadi et al., 2014). It has been proposed that TiO2 NPs improves tolerance to cold stress by enhancing the mechanisms of protection and reducing the levels of injuries in chickpea plants. Future studies may confirm the effectiveness and mechanisms of TiO2 NPs in improving the tolerance of crops to cold stress.
Zn NPs
In plants, Zn is a critical micronutrient that regulates metabolic processes and facilitates development (Adhikary et al., 2010; Vitti et al., 2014). Zn also plays an important role in the survival of plants under adverse conditions. Plants use Zn in small amounts; therefore, accessibility of Zn at the nano level ensures that suitable amounts are transported to the plant while avoiding Zn toxicity in the plants and soil. ZnO is an ecofriendly compound that can be used as a “green” element (Pandey et al., 2010). Given its functions in maintaining membrane integrity, retaining the potassium content of cells, and the plant–water relationship, ZnO plays a major role in stomatal regulation (Khan et al., 2004). In a study on chickpeas, Zn deficiency decreased their ability to modulate osmotic pressure under drought stress (Khan et al., 2004). Auxin production can also be affected by Zn via the induction of tryptophan synthesis as a precursor for the production of indole acetic acid, which helps in root development and drought tolerance in plants (Waraich et al., 2011). The uptake of Zn can be improved when it is nano-sized; thus, the functions of Zn can be achieved more efficiently when using Zn NPs. The application of Zn NPs enhances radicle development in germinated seeds, and higher Zn content in grains; thereby improving seed survival and tolerance to environmental stresses, especially in Zn-deficient regions (Cakmak et al., 1996; Degenhardt and Gimmler, 2000; Cakmak, 2008).
Several studies have described the effects of Zn-based NPs on plant development and yield. The use of ZnO NPs, at appropriate concentrations, enhanced biomass production, seed germination, and seedling development in chickpeas, in contrast to the use of bulk ZnSO4. ZnO NPs can elevate auxin levels, and thus, promote plant development (Pandey et al., 2010; Burman et al., 2013). In another study, the stimulating effect of zinc sulfide (ZnS) NPs on the growth of B. juncea has been assessed (Nayan et al., 2016). They showed that chlorophyll content, sugar content, and plant biomass, increased significantly after the application of these NPs, and that the growth-stimulating effects were probably associated with improvements in the plant antioxidant system after ZnS NPs treatment. Furthermore, lower concentrations of ZnS NPs were more effective than higher concentrations in improving plant growth. Similar results have been reported in wheat plants treated with ZnO NPs at 66 mg L–1 (Awasthi et al., 2017). ZnO NPs can mediate the increases in photosynthetic pigments and a concomitant reduction in lipid peroxidation in soil-grown Coriandrum sativum plants (Bhatt et al., 2020). Thus, the ZnO-mediated NPs increases the photosynthetic pigments which may help plants cope with stressful conditions by stabilizing ROS generation. Sedghi et al. (2013) have reported that the germination ratio in soybean was potentially augmented by ZnO NPs under water-deficient conditions. Under drought stress, the applied ZnO NPs facilitate the rapid use of seed reservoirs for seedling development and reduced the effects of such stress (Sedghi et al., 2013).
Drought tolerance was also improved by the enhancement of antioxidant enzyme activity in wheat plants via ZnO NPs application (Yang et al., 2018). Both Cu NPs and Zn NPs can improve wheat plant tolerance to drought stress by enhancing the action of antioxidant enzymes and stabilizing the content of photosynthetic pigments (Taran et al., 2017). Seghatoleslami and Forutani (2015) have shown that biomass and WUE have been improved by ZnO NPs in plants under water stress, whereas plants provided with full irrigation achieved strong growth and yield with bulk ZnO treatment. Dimkpa et al. (2017, 2019) have noticed that a composite of ZnO, boric oxide, and CuO NPs can alleviate drought stress in G. max. Under drought stress, shoot development and grain production were enhanced by 33 and 36%, respectively, using these NPs; thus, crop productivity and uptake of P and N can be enhanced by the addition of micronutrient NPs. These findings are in agreement with those reported in another study in which ZnO NPs were demonstrated to mitigate drought-induced damage to sorghum productivity, grain fortification, and nutrient acquisition (Dimkpa et al., 2019).
Yang et al. (2018) found that remodeling of root shape by ZnO and CuO NPs could alter drought tolerance in T. aestivum plants colonized by Pseudomonas chlororaphis O6 (PcO6), a beneficial bacterial species. Zn NPs enhanced the formation of lateral roots, whereas Cu NPs stimulated the propagation of elongated root hairs close to the root tip in wheat seedlings. In the same study, the use of these NPs generally increased the expression of genes related to drought tolerance. In shoots, the expression of other genes, such as those associated with metal stress, increased, and this was consistent with the increases in Cu and Zn concentrations. Thus, plants that were subjected to CuO or ZnO NPs showed cross-protection to multiple challenges, including metal, and drought stress. Despite improvements in root hair formation and production of lateral roots caused by Cu NPs and Zn NPs, respectively, the decreased root length may be the reason for the reduction in water accessibility. In Arabidopsis and mustard, the increased lignification because of CuO may alter the water flow and restrict cell wall expansion. Lignification in the cell wall is a plant response that is associated with drought stress and water flow impairment; thus, this may also occur by the binding of Cu ions with cell wall pectins (Nair and Chung, 2014).
When exposed to CuO NPs, anthocyanin and Pro levels increased under water deficient stress. On the addition of CuO NPs, the precipitation of ROS improved in the roots of wheat. The increased levels of ROS and abscisic acid (ABA) due to drought stress may cause transcriptional changes, resulting in subsequent stress tolerance (Dimkpa et al., 2012). Alharby et al. (2016) have investigated the metabolic response of S. lycopersicum to ZnO NPs under salinity stress; and they found that the NPs can reverse the adverse effects of salinity stress by regulating tolerance-related proteins/enzymes, mainly through the upregulation of SOD and GPX gene expression. These results are consistent with those of Haripriya et al. (2018), who found that a foliar spray of ZnO NPs mitigates salinity stress effects in finger millet. ZnO NPs treatment in soil-grown sorghum can also improve drought-stress tolerance through the translocation of grain N and the restoration of total N content (Dimkpa et al., 2019). In contrast, ZnO NPs at concentrations ≥ 10 mg L–1 resulted in oxidative stress in tomato plants cultivated in 1/2 Murashige and Skoog media (Rédei, 2008). The differences in results could be attributed to the variation in ZnO NPs, levels of NPs used, plant development media used, and possible variation in plant liability to ZnO NPs.
ZnO NPs also reduced HMs stress by decreasing the uptake of HMs by plants; thus protecting plants from HMs toxicity (Baybordi, 2005; Venkatachalam et al., 2017). The symptoms of oxidative stress caused by Cd and Pb toxicity can be improved by ZnO NPs treatment. In addition, ZnO NPs can improve the total soluble protein and photosynthetic pigment levels, while reducing lipid peroxidation in developing seedlings of Leucaena leucocephala (Venkatachalam et al., 2017). When a foliar spray of ZnO NPs was applied to maize leaves, Cd absorption and Cd-induced oxidative stress were reduced (Rizwan et al., 2019c). Torabian et al. (2016) reported an increase in plant growth, photosynthetic index, and chlorophyll content and a decrease in the Na content in sunflower leaves supplied with ZnO NPs. Similarly, wheat plants treated with CuO/ZnO NPs showed improved growth, which could possibly due to the lower solubility of CuO NPs (Fathi et al., 2017). Taken together, these findings indicated that the application of Zn-based NPs enhanced plant stress tolerance.
Nanotechnology-Based Advances in Agriculture
Nanotechnology has several other possible applications and can play an important role in agriculture, forestry, energy production, food processing, environmental management as well as in ensuring water quality and utilizing waste resources (Prasad et al., 2017; Kim et al., 2018). The extensive scope of nanotechnology and its wide range of applications has led to advancements in the agricultural sector (Kim et al., 2018; Shang et al., 2019). Over the last two decades, the use of nanotechnology in agriculture has been supported by research and practical applications at the academic and industrial levels (Shang et al., 2019).
In particular, nanotechnology has been applied to increase crop production. In addition, nanotechnology has been used to produce nanofertilizers and nanoencapsulated nutrients, which are considered promising methods for achieving site-targeted and regulated delivery of nutrients to plants, thereby improving crop production and yield via “precision agriculture.” Nanoformulations of agrochemicals, such as nanopesticides and nanofertilizers, substantially reduce micronutrient losses of fertilizers through volatilization and leaching, enhance effective phytoavailability, feed plants gradually in a controlled manner, and eventually reduce environmental hazards caused by the excessive use of traditional fertilizers (Solanki et al., 2015; Shang et al., 2019; Zulfiqar et al., 2019). Nanofertilizers can be produced using Cu, SiO2, Zn, TiO2, and polymeric NPs as dendrimers acting as nanocarriers (Paramo et al., 2020).
Studies have shown that nanofertilizers can help crop productivity by improving stress tolerance as well as promoting plant germination, growth, and physiological processes. However, nanofertilizers have some drawbacks that have restricted their extensive application (Zulfiqar et al., 2019). Nanosensors have been reported as another application of nanotechnology that can improve crop quality and yield, while ensuring an output of high-quality and healthy food products. Novel nanosensors are primarily applied in crop safety for the detection and management of phytopathogens and for measuring and monitoring the uses, penetration, and residues of agrochemicals as well as environmental pollution (Ion et al., 2010; Chen et al., 2016; Prasad et al., 2017; Paramo et al., 2020). Their use has advanced the human management of soil and plant health, improved food quality, and protection, optimized packaging methods, and enhanced soil monitoring and crop conditions (Kim et al., 2018; Shang et al., 2019). Other agronomic uses of nanotechnology include the use of nanodevices in plant genetic engineering, postharvest management, and plant disease diagnostics (Ion et al., 2010; Prasad et al., 2017). Nanobiotechnology includes the use of novel methods to genetically modify and engineer crop programs that boost agricultural productivity, food safety, and processing capacity while promoting agricultural sustainability.
Different methods for the application of NPs in agriculture are shown in Figure 4. The application of nanomaterials enable efficient plant transformation for crop improvement (Anderson et al., 2016; Shafiee-Jood and Cai, 2016). Given their unique properties of small size, multiple binding sites and large surface area, nanomaterials are excellent nanocarriers of bioactive molecules (e.g., plasmid DNA and double-stranded RNA) (de Oliveira et al., 2014; Anderson et al., 2016; Shafiee-Jood and Cai, 2016; Kim et al., 2018; Zhao et al., 2020). Engineered NPs can also be used to increase crop safety and detect pesticide residues (Kim et al., 2018). Moreover, nanotechnology has become a common method used by engineers and designers to enhance and improve soil properties. Nano clay–polymer composites and nano-zeolites can be used in the soil to improve its moisture content, increase water-retention capacity, and slow water release during the cultivation season, and nanomagnets have been used to expel soil contaminants (Vundavalli et al., 2015; Prasad et al., 2017; Kim et al., 2018).
FIGURE 4.
Methods used for the application of nanoparticles in the field of agriculture.
The application of nanofertilizers can also help reduce soil toxicity caused by an accumulation of chemical substances applied to the soil, while also acting as an alternative means of enhancing resource-use efficiency (DeRosa et al., 2010; Nair et al., 2010; Jalil and Ansari, 2019). In addition, nanosensors are now widely used in agriculture for soil analysis, water management and transmission, environmental monitoring of pollution in soils and water, and pesticide and nutrient drop (Ion et al., 2010). Various sensors based on nanodetection technology, including biosensors, optical sensors, electrochemical sensors, and other instruments, are important tools for identifying HMs at trace levels (Ion et al., 2010; Prasad et al., 2017; Singhal et al., 2022).
Mechanisms of Action of NPs in Plants
Although NPs have a wide range of applications in agriculture, the majority of NPs are hazardous to plants when present in high concentrations. The uptake, accumulation and interference of NPs with key metabolic processes in different plant tissues may have positive or negative effects on plants, depending on their dosage, movement, characteristics, and reactivity.
Uptake of NPs
High concentrations of NPs can penetrate plant cells and cross the plasma membrane; thus, this may interfere with key cellular activities (Mazumdar and Ahmed, 2011; Mirzajani et al., 2013). NPs can reach plant tissues through the root system or above-ground parts such as root junctions and wounds. As a carrier, NPs must pass through several physiological barriers until they are taken up by the plant and translocated. Plant cell walls, which are made up of cellulose, allow small NPs, ranging between 5 and 20 nm in size, to pass through into the plant cells (Dietz and Herth, 2011).
Some NPs have been shown to develop larger pores in the cell wall to enter the cell (Navarro et al., 2008; Kurepa et al., 2010). NPs can be transferred to other plant tissues via the apoplastic and symplastic pathways (Etxeberria et al., 2006; Ma et al., 2010). Wong et al. (2016) suggested a lipid exchange mechanism for NPs transport into plant cells. The size, magnitude, and zeta potentials of NPs are important to determine their delivery in plant cells.
NPs-Plant Interaction Pathways
NPs may affect plant metabolism by delivering micronutrients (Liu and Lal, 2015), gene regulation (Nair and Chung, 2014), and interfering with several oxidative processes in plants (Hossain et al., 2015). Excessive contents of NPs can generate ROS; thus, interfering with the oxidative mechanism; while other pathways have yet to be deciphered. The NPs can disrupt the electron transport chain in mitochondria and chloroplast, causing an oxidative burst and an increase in ROS levels (Pakrashi et al., 2014; Cvjetko et al., 2017). The rate of carbon fixation is reduced in response to stressful conditions; thus, this increases photoinhibition, potentially leading to the overproduction of superoxide anion radicals and H2O2 in the photosystem (Foyer and Noctor, 2005). When ROS is generated as a result of NPs, all biological components are affected causing protein changes, lipid peroxidation, and DNA damage (Van Breusegem and Dat, 2006).
Several studies have found an increase in lipid peroxidation and DNA damage in plants while interacting with NPs (Atha et al., 2012; Saha and Dutta Gupta, 2017). The increase in ROS levels can cause apoptosis or necrosis, resulting in plant cell death (Faisal et al., 2013). Despite its destructive nature, ROS play a role in biological activities, including stress tolerance (Sharma et al., 2012a). The balance between ROS generation and scavenging determines whether ROS has a destructive or signaling function. The cells have developed a robust antioxidant mechanism to precisely control the quantity of ROS. Enzymatic (SOD, CAT, and guaiacol peroxidase) and non-enzymatic (ascorbate, glutathione, carotenoids, tocopherols, and phenolics) antioxidants are attributed to defense mechanisms in plants (Sharma et al., 2012b). Several studies have demonstrated that plants exposed to NPs produce more antioxidant molecules (Jiang et al., 2014; Costa and Sharma, 2016). Plant stress response signaling can also be influenced by phytohormones (Mengiste et al., 2010; O’Brien and Benková, 2013; Sham et al., 2019).
Plant hormones are endogenous molecules involved in the regulation of plant development and stress tolerance (Sham et al., 2017). In response to abiotic stresses, different hormonal pathways can be activated or suppressed (Kwak et al., 2006; O’Brien and Benková, 2013). In red pepper (Capsicum annuum), cytokinin levels increased in response to AgNPs stress; while in cotton (Gossypium sp.), a decrease in the levels of auxins and ABA in response to CuO NPs was detected. This suggests that NPs affect plant hormonal balance and plant metabolism.
Several studies have demonstrated that NPs can also affect the content and activity of photosynthetic pigments in plants (Perreault et al., 2014; Tripathi et al., 2017c). High concentrations of NPs have a negative impact on photosynthesis, resulting in growth retardation or death in plants (Tripathi et al., 2017c).
Future Prospects on NPs for Enhancing Crop Tolerance to Abiotic Stress
Nanobiotechnology has the potential to improve stress tolerance, stress sensing/detection, targeted delivery and controlled release of agrochemicals, transgenic events, and seed nanopriming in plants (Wu and Li, 2022). Such nanomaterials free of HMs and high dispersibility can be developed for agricultural use. Future research on evaluating the biological effects of nanozymes i.e., Mn3O4 NPs in plants under stress conditions should be on top of our priorities. Mechanisms underlying nanopriming-induced seed germination, breaking seed dormancy, and their interactions with seeds have to be investigated. Understanding how NPs improve plant stress tolerance will enable researchers to design tailor-made nanomaterials targeting agricultural challenges. In addition, nanomaterials have no doubt a bright future ahead, especially when it comes to their functionality in plants. For example, Santana et al. (2020) have developed a targeted delivery approach using nanomaterials to convert chloroplasts into “chloroplast factory” for better plant photosynthesis under low light conditions. The use of nanomaterials for CRISPR-Cas genome editing in cargo delivery (Demirer et al., 2021) will increase the efficiency of genetic engineering to enhance plant stress tolerance. Developing policies and regulations could help manage biosafety hazards associated with the use of nanomaterials in agriculture. We believe that nanomaterials will play a crucial role in the future of agriculture.
Conclusion
To achieve sustainable agriculture, the research community must identify appropriate ecofriendly solutions that address abiotic-stress–induced loss of crop yield (Figure 3). Nanotechnology is an innovative and effective means of promoting crop yield and quality, enhancing the farming sector, and managing global food demand. The potential role played by several NPs in alleviating abiotic stress-induced damage and improving plant development and crop yield is under intense investigation. NPs, such as TiO2, SiO2, and Ag NPs, can reduce the negative effects of abiotic stress by activating plant defense mechanisms via the induction of ROS production and phytotoxicity. NPs, given their small size, can also easily penetrate plant tissues, after which they positively influence plant morphological, physiological, and biochemical processes, promote plant development, and improve crop productivity in plants under various abiotic stresses. Moreover, NPs have a large surface area that improves the absorption and delivery of various targeted nutrients. Nevertheless, the applications of NPs in crop improvement and sustainable agriculture are still at an early stage of development, and the current research in the field is insufficient and, to some extent, inconsistent (Rajput et al., 2021). Therefore, additional investigations must explore the following issues, which will help limit the undesirable effects of NPs on ecosystems and crops: (a) the reaction of NPs with plants and metabolic process at the molecular and cellular levels, and optimization of NPs size and level before practical application in the field; (b) the effects of NPs and their possible toxicities in different plant species; (c) the impact of NPs on gene regulation and expression in plants under various abiotic stresses; (d) the behavior and fate of NPs in plants and the environment; (e) the effects of soil properties and different plant species on the efficiency of NPs; (f) the classification of NPs as stress initiators or stress in activators; and (g) the combined effects of NPs with other active ingredients and biotic stresses in plants.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank their respective institutions for their support. KE-T would also like to thank the library at Murdoch University, Australia, for providing the valuable online resources and comprehensive databases.
Funding
This project was funded by Abu Dhabi Education and Knowledge (Grant No. 21S105) to KE-T and Khalifa Center for Genetic Engineering and Biotechnology-UAEU (Grant No. 31R286) to SA.
References
- Abbasi Khalaki M., Ghorbani A., Dadjou F. (2019a). Influence of nanopriming on Festuca ovina seed germination and early seedling traits under drought stress in laboratory condition. Ecopersia 7 133–139. [Google Scholar]
- Abbasi Khalaki M., Ghorbani A., Esmali Ouri A., Shokouhian A. A., Jafari A. A. (2019b). Varying the vegetative and morphological traits of Thymus kotschyanus L. submitted to potassium silicate nanoparticles, superabsorbent hydrogel, effective microorganisms and animal manure. J. Biosci. 35 115–125. 10.14393/BJ-v35n1a2019-41832 33406856 [DOI] [Google Scholar]
- Abbasi Khalaki M., Ghorbani A., Moameri M. (2016). Effects of silica and silver nanoparticles on seed germination traits of Thymus kotschyanus in laboratory conditions. J. Rangel. Sci. 6 221–231. [Google Scholar]
- Abbasi Khalaki M., Moameri M., Asgari Lajayer B., Astatkie T. (2021). Influence of nanopriming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 93 13–28. 10.1007/s10725-020-00670-9 [DOI] [Google Scholar]
- Abd El-Ghany W. A., Shaalan M., Salem H. M. (2021). Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 77 1001–1025. 10.1080/00439339.2021.1960235 [DOI] [Google Scholar]
- Abd El-Mageed T. A., El-Sherif A. M. A., Abd El-Mageed S. A., Abdou N. M. (2019). A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric. Water Manag. 226:105831. 10.1016/j.agwat.2019.105831 [DOI] [Google Scholar]
- Abd El-Mageed T. A., Rady M. O. A., Abd El-Wahed M. H., Abd El-Mageed S. A., Omran W. M., Aljuaid B. S., et al. (2022). Consecutive seasonal effect on yield and water productivity of drip deficit irrigated sorghum in saline soils. Saudi J. Biol. Sci. 29 2683–2690. 10.1016/j.sjbs.2021.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Mageed T. A., Semida W. M., Taha R. S., Rady M. M. (2018). Effect of summer-fall deficit irrigation on morpho physiological, anatomical responses, fruit yield and water use efficiency of cucumber under salt affected soil. Sci. Hortic 237 148–155. 10.1016/j.scienta.2018.04.014 [DOI] [Google Scholar]
- Abdel Latef A. A. H., Alhmad M. F. A., Abdelfattah K. E. (2017). The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 36 60–70. 10.1007/s00344-016-9618-x [DOI] [Google Scholar]
- Abdel Latef A. A. H., Srivastava A. K., El Sadek M. S. A., Kordrostami M., Tran L. S. P. (2018). Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 29 1065–1073. 10.1002/ldr.2780 [DOI] [Google Scholar]
- Abou-Zeid H., Ismail G. (2018). The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L to salt stress. Egypt. J. Bot. 58 73–85. 10.21608/ejbo.2017.1873.1128 [DOI] [Google Scholar]
- Abu-Hamdah R., Cho W. J., Cho S. J., Jeremic A., Kelly M., Ilie A. E., et al. (2004). Regulation of the water channel aquaporin-1: isolation and reconstitution of the regulatory complex. Cell Biol. Int. 28 7–17. 10.1016/j.cellbi.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Adhikary B. H., Shrestha J., Baral B. R. (2010). Effects of micronutrients on growth and productivity of maize in acidic soil. Int. Res. J. Basic Appl. Sci. 1 8–15. [Google Scholar]
- Adrees M., Ali S., Rizwan M., Zia-Ur-Rehman M., Ibrahim M., Abbas F., et al. (2015). Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol. Environ. Saf. 119 186–197. 10.1016/j.ecoenv.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Adrees M., Khan Z. S., Ali S., Hafeez M., Khalid S., Rehman M. Z. U., et al. (2020). Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 238:124681. 10.1016/j.chemosphere.2019.124681 [DOI] [PubMed] [Google Scholar]
- Afshari M., Pazoki A., Sadeghipour O. (2021). Foliar-applied silicon and its nanoparticles stimulates physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum sativum L.) essential oil under different irrigation regimes. Silicon 13 4177–4188. 10.1007/s12633-021-01101-8 [DOI] [Google Scholar]
- Aghdam M. T. B., Mohammadi H., Ghorbanpour M. (2016). Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 39 139–146. 10.1007/s40415-015-0227-x [DOI] [Google Scholar]
- Ahamed M., AlSalhi M. S., Siddiqui M. K. J. (2010). Silver nanoparticle applications and human health. Clin. Chim. Acta 411 1841–1848. 10.1016/j.cca.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Ahmad B., Shabbir A., Jaleel H., Khan M. M., Sadiq Y. (2018). Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr Plant Biol. 13 6–15. 10.1016/j.cpb.2018.04.002 [DOI] [Google Scholar]
- Ahmad J., Ali A. A., Baig M. A., Iqbal M., Haq I., Qureshi M. I. (2019). “Role of phytochelatins in cadmium stress tolerance in plants,” in Cadmium Toxicity and Tolerance in Plants, eds Hasanuzzaman M., Prasad M. N. V., Fujita M. (Cambridge, MA: Academic Press; ), 185–212. [Google Scholar]
- Ahmad P., Alyemeni M. N., Al-Huqail A. A., Alqahtani M. A., Wijaya L., Ashraf M., et al. (2020). Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 9:825. 10.3390/plants90708252020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P., John R., Sarwat M., Umar S. (2008). Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant Prod. 2 353–366. 10.22069/ijpp.2012.626 [DOI] [Google Scholar]
- Ahmed T., Noman M., Manzoor N., Shahid M., Abdullah M., Ali L., et al. (2021). Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 209:111829. 10.1016/j.ecoenv.2020.111829 [DOI] [PubMed] [Google Scholar]
- Akbari G. A., Morteza E., Moaveni P., Alahdadi I., Bihamta M. R., Hasanloo T. (2014). Pigments apparatus and anthocyanins reactions of borage to irrigation, Methylalcohol and titanium dioxide. Int. J. Biosci. 4 192–208. 10.12692/ijb/4.7.192-208 [DOI] [Google Scholar]
- Al-Ashkar I., Alderfasi A., El-Hendawy S., Al-Suhaibani N., El-Kafafi S., Seleiman M. F. (2019). Detecting salt tolerance in doubled haploid wheat lines. Agronomy 9:211. 10.3390/agronomy9040211 [DOI] [Google Scholar]
- Al-Ashkar I., Al-Suhaibani N., Abdella K., Sallam M., Alotaibi M., Seleiman M. F. (2021). Combining genetic and multidimensional analyses to identify interpretive traits related to water shortage tolerance as an indirect selection tool for detecting genotypes of drought tolerance in wheat breeding. Plants 10:931. 10.3390/plants10050931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharby H. F., Metwali E. M., Fuller M. P., Aldhebiani A. Y. (2016). The alteration of mRNA expression of SOD and GPX genes, and proteins in tomato (Lycopersicon esculentum Mill) under stress of NaCl and/or ZnO nanoparticles. Saudi J. Biol. Sci. 23 773–781. 10.1016/j.sjbs.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Basra S. M. A., Hussain S., Iqbal J., Haji M. A. A., Bukhsh A., et al. (2012). Salt stress alleviation in field crops through nutritional supplementation of silicon. Pak. J. Nutr. 11 735–753. 10.3923/pjn.2012.735.753 [DOI] [Google Scholar]
- Ali S., Rizwan M., Hussain A., Ur Rehman M. Z., Ali B., Yousaf B., et al. (2019). Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 140 1–8. 10.1016/j.plaphy.2019.04.041 [DOI] [PubMed] [Google Scholar]
- Alkharabsheh H. M., Seleiman M. F., Hewedy O. A., Battaglia M. L., Jalal R. S., Alhammad B. A., et al. (2021). Field crop responses and management strategies to mitigate soil salinity in modern agriculture: a review. Agronomy 11:2299. 10.3390/agronomy11112299 [DOI] [Google Scholar]
- Almutairi Z. M. (2016a). Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics 9 106–114. [Google Scholar]
- Almutairi Z. M. (2016b). Influence of silver nano-particles on the salt resistance of tomato (Solanum lycopersicum) during germination. Int. J. Agric. Biol. 18 449–457. 10.17957/ijab/15.0114 [DOI] [Google Scholar]
- Alsaeedi A., El-Ramady H., Alshaal T., El-Garawani M., Elhawat N., Al-Otaibi A. (2018). Exogenous nanosilica improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress. Plant Physiol. Biochem. 125 164–171. 10.1016/j.plaphy.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Alsaeedi A., El-Ramady H., Alshaal T., El-Garawany M., Elhawat N., Al-Otaibi A. (2019). Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 139 1–10. 10.1016/j.plaphy.2019.03.008 [DOI] [PubMed] [Google Scholar]
- Alsaeedi A. H., El-Ramady H., Alshaal T., El-Garawani M., Elhawat N., Almohsen M. (2017). Engineered silica nanoparticles alleviate the detrimental effects of Na+ stress on germination and growth of common bean (Phaseolus vulgaris). Environ. Sci. Pollut. Res. 24 21917–21928. 10.1007/s11356-017-9847-y [DOI] [PubMed] [Google Scholar]
- Amini S., Maali-Amiri R., Mohammadi R., Kazemi-Shahandashti S. S. (2017). cDNAAFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol. Biochem. 111 39–49. 10.1016/j.plaphy.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Amooaghaie R., Tabatabaei F., Ahadi A. M. (2015). Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol. Environ. Saf. 113 259–270. 10.1016/j.ecoenv.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Gipmans M., Hurst S., Layton R., Nehra N., Pickett J., et al. (2016). Emerging agricultural biotechnologies for sustainable agriculture and food security. J. Agric. Food Chem. 64 383–393. 10.1021/acs.jafc.5b04543 [DOI] [PubMed] [Google Scholar]
- Anoop N., Gupta A. K. (2003). Transgenic indica rice cv IR-50 overexpressing Vigna aconitifolia Δ1-pyrroline -5- carboxylate synthetase cDNA shows tolerance to high salt. J. Plant Biochem. Biotechnol. 12 109–116. 10.1007/bf03263170 [DOI] [Google Scholar]
- Ashkavand P., Tabari M., Zarafshar M., Tomášková I., Struve D. (2015). Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap. 76 350–359. [Google Scholar]
- Ashkavand P., Zarafshar M., Tabari M., Mirzaie J., Nikpour A., Bordbar S. K., et al. (2018). Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Bol. Soc. Argent. Bot. 53 207–219. 10.31055/1851.2372.v53.n2.20578 [DOI] [Google Scholar]
- Ashour H. A., Abdel Wahab M. (2017). Response of Jatropha integerrima plants irrigated with different levels of saline water to nano silicon and gypsum. J. Agric. Sci. 5 136–160. 10.5296/jas.v5i4.12170 [DOI] [Google Scholar]
- Askary M., Talebi S. M., Amini F., Bangan A. D. B. (2017). Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 63 65–67. 10.6001/biologija.v63i1.3476 [DOI] [Google Scholar]
- Atha D. H., Wang H., Petersen E. J., Cleveland D., Holbrook R. D., Jaruga P., et al. (2012). Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 46 1819–1827. 10.1021/es202660k [DOI] [PubMed] [Google Scholar]
- Avestan S., Ghasemnezhad M., Esfahani M., Byrt C. S. (2019). Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 9:246. 10.3390/agronomy9050246 [DOI] [Google Scholar]
- Awasthi A., Bansal S., Jangir L. K., Awasthi G., Awasthi K. K., Awasthi K. (2017). Effect of ZnO nanoparticles on germination of Triticum aestivum seeds. Macromol. Symp. 376:1700043. 10.1002/masy.201700043 [DOI] [Google Scholar]
- Azimi R., Borzelabad M. J., Feizi H., Azimi A. (2014). Interaction of SiO2 nanoparticles with seed prechilling on germination and early seedling growth of tall wheatgrass (Agropyron elongatum L.). Pol. J. Chem. Technol. 16 25–29. 10.2478/pjct-2014-0045 [DOI] [Google Scholar]
- Badal E., Abd El-Mageed T. A., Buesa I., Guerra D., Bonet L., Intrigliolo D. S. (2013). Moderate plant water stress reduces fruit drop of “Rojo Brillante” persimmon (Diospyros kaki) in a Mediterranean climate. Agric. Water Manage. 119 154–160. 10.1016/j.agwat.2012.12.020 [DOI] [Google Scholar]
- Badawy S. A., Zayed B. A., Bassiouni S. M. A., Mahdi A. H. A., Majrashi A., Ali E. F., et al. (2021). Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 10:1657. 10.3390/plants10081657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A. (2019). Effect of Foliar Application of Nanoparticles of Chitosan, Iron Oxide and Chitosan-Iron Oxide Complex on Secondary Metabolites of Hypericum Triquetrifolium Turra. [Master’s Thesis]. Diyarbakir: Dicle University. [Google Scholar]
- Banerjee J., Kole C. (2016). “Plant nanotechnology: an overview on concepts, strategies, and tools,” in Plant Nanotechnology, eds Kole C., Kumar D. S., Khodakovskaya M. V. (Switzerland: Springer; ), 1–14. [Google Scholar]
- Batool T., Ali S., Seleiman M. F., Naveed N. H., Ali A., Ahmed K., et al. (2020). Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 10:16975. 10.1038/s41598-020-73489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybordi A. (2005). Effect of zinc, iron, manganese and copper on wheat quality under salt stress conditions. J. Water Soil 140 150–170. [Google Scholar]
- Bechert T., Böswald M., Lugauer S., Regenfus A., Greil J., Guggenbichler J. P. (1999). The Erlanger silver catheter: in vitro results for antimicrobial activity. Infection 27 S24–S29. 10.1007/bf02561613 [DOI] [PubMed] [Google Scholar]
- Behboudi F., Tahmasebi Sarvestani Z., Kassaee M. Z., Modares Sanavi S. A. M., Sorooshzadeh A., Ahmadi S. B. (2018). Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 3 22–39. 10.22090/jwent.2018.01.003 [DOI] [Google Scholar]
- Behboudi F., Tahmasebi-Sarvestani Z., Kassaee M. Z., Modarres-Sanavy S. A. M., Sorooshzadeh A., Mokhtassi-Bidgoli A. (2019). Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 42 1439–1451. 10.1080/01904167.2019.1617308 [DOI] [Google Scholar]
- Bhatt D., Bhatt M. D., Nath M., Dudhat R., Sharma M., Bisht D. S. (2020). “Application of nanoparticles in overcoming different environmental stresses,” in Protective Chemical Agents in The Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives, eds Roychoudhury A., Tripathi D. K. (Hoboken: Wiley-Blackwell; ), 635–654. [Google Scholar]
- Bhatt D., Sharma G. (2018). Role of silicon in counteracting abiotic and biotic plant stresses. IJCS 6 1434–1442. [Google Scholar]
- Bratovcic A., Hikal W. M., Said-Al Ahl H. A., Tkachenko K. G., Baeshen R. S., Sabra A. S., et al. (2021). Nanopesticides and nanofertilizers and agricultural development: scopes, advances and applications. Open Ecol. J. 11 301–316. 10.4236/oje.2021.114022 [DOI] [Google Scholar]
- Burman U., Saini M., Kumar P. (2013). Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 95 605–612. 10.1080/02772248.2013.803796 [DOI] [Google Scholar]
- Cakmak I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302 1–17. 10.1007/s11104-007-9466-3 [DOI] [Google Scholar]
- Cakmak I., Yilmaz A., Kalayci M., Ekiz H., Torun B., Ereno B., et al. (1996). Zinc deficiency as a critical problem in wheat production in Central Anatolia. Plant Soil 180 165–172. 10.1007/bf00015299 [DOI] [Google Scholar]
- Cañas J. E., Long M., Nations S., Vadan R., Dai L., Luo M., et al. (2008). Effects of functionalized and nonfunctionalized single walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 27 1922–1931. 10.1897/08-117.1 [DOI] [PubMed] [Google Scholar]
- Changmei L., Chaoying Z., Junqiang W., Guorong W., Mingxuan T. (2002). Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21 168–171. [Google Scholar]
- Chen Y. W., Lee H. V., Juan J. C., Phang S. M. (2016). Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 151 1210–1219. 10.1016/j.carbpol.2016.06.083 [DOI] [PubMed] [Google Scholar]
- Chhipa H. (2017). Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15 15–22. 10.1007/s10311-016-0600-4 [DOI] [Google Scholar]
- Cinisli K. T., Uçar S. M., Dikbaş N. (2019). Use of nanomaterials in agriculture. Yuzuncu Yil Univ. J. Agric. Sci. 29 817–831. 10.29133/yyutbd.595658 [DOI] [Google Scholar]
- Costa M. V. J. D., Sharma P. K. (2016). Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54 110–119. 10.1007/s11099-015-0167-5 [DOI] [Google Scholar]
- Cota-Ruiz K., Delgado-Rios M., Martínez-Martínez A., Núñez-Gastelum J. A., Peralta-Videa J. R., Gardea-Torresdey J. L. (2018). Current findings on terrestrial plants–Engineered nanomaterial interactions: are plants capable of phytoremediating nanomaterials from soil? Curr. Opin. Environ. Sci. Health 6 9–15. [Google Scholar]
- Cruz de Carvalho M. H. (2008). Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal. Behav. 3 156–165. 10.4161/psb.3.3.5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li Y., Jin Q., Li F. (2020). Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 7 162–171. 10.1039/c9en01035a [DOI] [Google Scholar]
- Cvjetko P., Milošic A., Domijan A. M., Vinkovic-Vrcek I., Tolic S., Peharec-Štefanic P., et al. (2017). Toxicity of silver ions and differently coate ’ d silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 137 18–28. 10.1016/j.ecoenv.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Darvishzadeh F., Najatzadeh F., Iranbakhsh A. R. (2015). Effect of silver nanoparticles on salinity tolerance of basil plant in germination stages under laboratory conditions. J. Cell. Biotechnol. Mol. 20 63–70. [Google Scholar]
- Datnoff L. E., Rodrigues F. A., Seebold K. W. (2009). “Silicon and plant disease,” in Mineral Nutrition and Plant Disease, eds Datnoff L. E., Elmer W. H., Huber D. M. (Saint Paul: American Phytopathological Society; ), 233–246. [Google Scholar]
- Davies J. C. (2008). Nanotechnology Oversight: An Agenda for the New Administration. The Project on Emerging Nanotechnologies. Washington, DC: Project on Emerging Nanotechnologies. [Google Scholar]
- Dawood M. F., Abeed A. H., Aldaby E. E. (2019). Titanium dioxide nanoparticles model growth kinetic traits of some wheat cultivars under different water regimes. Plant Physiol. Rep. 24 129–140. 10.1007/s40502-019-0437-5 [DOI] [Google Scholar]
- de Oliveira J. L., Campos E. V. R., Bakshi M., Abhilash P. C., Fraceto L. F. (2014). Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotechnol. Adv. 32 1550–1561. 10.1016/j.biotechadv.2014.10.010 [DOI] [PubMed] [Google Scholar]
- de Sousa A., Saleh A. M., Habeeb T. H., Hassan Y. M., Zrieq R., Wadaan M. A. M., et al. (2019). Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 693:133636. 10.1016/j.scitotenv.2019.133636 [DOI] [PubMed] [Google Scholar]
- Degenhardt B., Gimmler H. (2000). Cell wall adaptations to multiple environmental stresses in maize roots. J. Exp. Bot. 51 595–603. 10.1093/jexbot/51.344.595 [DOI] [PubMed] [Google Scholar]
- Dehkourdi E. H., Mosavi M. (2013). Effect of anatase nanoparticles (TiO2) on parsley seed germination (Petroselinum crispum) in vitro. Biol. Trace Elem. Res. 155 283–286. 10.1007/s12011-013-9788-3 [DOI] [PubMed] [Google Scholar]
- Delfani M., Baradarn Firouzabadi M., Farrokhi N., Makarian H. (2014). Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 45 530–540. 10.1080/00103624.2013.863911 [DOI] [Google Scholar]
- Demirer G. S., Silva T. N., Jackson C. T., Thomas J. B., Ehrhardt W., Rhee S. Y., et al. (2021). Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 16 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosa M. C., Monreal C., Schnitzer M., Walsh R., Sultan Y. (2010). Nanotechnology in fertilizers. Nat. Nanotechnol. 5:91. [DOI] [PubMed] [Google Scholar]
- Desoky E. S. M., Merwad A. R. M., Semida W. M., Ibrahim S. A., El-Saadony M. T., Rady M. M. (2020a). Heavy metals-resistant bacteria (HM-RB): potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 198:110685. [DOI] [PubMed] [Google Scholar]
- Desoky E. S. M., Saad A. M., El-Saadony M. T., Merwad A. R. M., Rady M. M. (2020b). Plant growth-promoting rhizobacteria: potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 30:101878. [Google Scholar]
- Dietz K. J., Herth S. (2011). Plant nanotoxicology. Trends Plant Sci. 16 582–589. [DOI] [PubMed] [Google Scholar]
- Dimkpa C. O., McLean J. E., Latta D. E., Manangón E., Britt D. W., Johnson W. P., et al. (2012). CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 14:1125. [Google Scholar]
- Dimkpa C. O., Singh U., Bindraban P. S., Elmer W. H., Gardea-Torresdey J. L., White J. C. (2019). Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 688 926–934. [DOI] [PubMed] [Google Scholar]
- Dimkpa C. O., White J. C., Elmer W. H., Gardea-Torresdey J. (2017). Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 65 8552–8559. 10.1021/acs.jafc.7b02961 [DOI] [PubMed] [Google Scholar]
- Du W., Yang J., Peng Q., Liang X., Mao H. (2019). Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: from toxicity and zinc biofortification. Chemosphere 227 109–116. 10.1016/j.chemosphere.2019.03.168 [DOI] [PubMed] [Google Scholar]
- Dubchak S., Ogar A., Mietelski J. W., Turnau K. (2010). Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res. 8 103–108. 10.5424/sjar/201008S1-1228 [DOI] [Google Scholar]
- Dustgeer Z., Seleiman M. F., Khan I., Chattha M. U., Ali E., Alhammad B., et al. (2021). Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti. Agrobot. Cluj Napoca 49:12248. [Google Scholar]
- Eichert T., Kurtz A., Steiner U., Goldbach H. E. (2008). Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and watersuspended nanoparticles. Physiol. Plant. 134 151–160. 10.1111/j.1399-3054.2008.01135.x [DOI] [PubMed] [Google Scholar]
- Ekhtiyari R., Mohebbi H., Mansouri M. (2011). Effect of nanosilver particles on salinity tolerance of fennel plants in early growth under laboratory conditions. J. Plant Biotechnol. 7 55–62. [Google Scholar]
- Ekhtiyari R., Moraghebi F. (2011). The study of the effects of nano silver technology on salinity tolerance of cumin seed (Cuminum cyminum L.). Plant Ecosyst. 25 99–107. [Google Scholar]
- Ekhtiyari R., Moraghebi F. (2012). Effect of nanosilver particles on salinity tolerance of cumin (Cuminum cyminum L.). J. Plant Biotechnol. 25 99–107. [Google Scholar]
- El-Ashry R. M., El-Saadony M. T., El-Sobki A. E., El-Tahan A. M., Al-Otaibi S., El-Shehawi A. M., et al. (2022). Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 29 920–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M. T., Abd El-Hack M. E., Taha A. E., Fouda M., Ajarem J. S., N Maodaa S., et al. (2020). Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 10:587. 10.3390/nano10030587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M. T., Sitohy M. Z., Ramadan M. F., Saad A. M. (2021a). Green nanotechnology for preserving and enriching yogurt with biologically available iron (II). Innov. Food Sci. Emerg. Technol. 69:102645. 10.1016/j.ifset.2021.102645 [DOI] [Google Scholar]
- El-Saadony M. T., ALmoshadak A. S., Shafi M. E., Albaqami N. M., Saad A. M., El-Tahan A. M., et al. (2021b). Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review Saudi J. Biol. Sci. 28 7349–7359. 10.1016/j.sjbs.2021.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M. T., Desoky E. S. M., Saad A. M., Eid R. S., Selem E., Elrys A. S. (2021c). Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 106 1–14. 10.1016/j.jes.2021.01.012 [DOI] [PubMed] [Google Scholar]
- El-Saadony M. T., Saad A. M., Najjar A. A., Alzahrani S. O., Alkhatib F. M., Shafi M. E., et al. (2021d). The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 28 4461–4471. 10.1016/j.sjbs.2021.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsakhawy T., Omara A. E. D., Alshaal T., El-Ramady H. (2018). Nanomaterials and plant abiotic stress in agroecosystems. EBSS 2 73–94. 10.21608/jenvbs.2018.3897.1030 [DOI] [Google Scholar]
- Elsheery N. I., Helaly M. N., El-Hoseiny H. M., Alam-Eldein S. M. (2020). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10:558. 10.3390/agronomy10040558 [DOI] [Google Scholar]
- El-Temsah Y. S., Joner E. J. (2010). Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 27 42–49. 10.1002/tox.20610 [DOI] [PubMed] [Google Scholar]
- Etxeberria E., Gonzalez P., Pozueta-Romero J., Romero J. P. (2006). Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells: evidence for the distribution of solutes to different intracellular compartments. Plant Signal. Behav. 1 196–200. 10.4161/psb.1.4.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal M., Saquib Q., Alatar A. A., Al-Khedhairy A. A., Hegazy A. K., Musarrat J. (2013). Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J. Hazard. Mater. 250–251 318–332. 10.1016/j.jhazmat.2013.01.063 [DOI] [PubMed] [Google Scholar]
- Faizan M., Bhat J. A., Chen C., Alyemeni M. N., Wijaya L., Ahmad P., et al. (2021). Zinc oxide nanoparticles (Zno-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 161 122–130. 10.1016/j.plaphy.2021.02.002 [DOI] [PubMed] [Google Scholar]
- Faraji J., Sepehri A. (2020). Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil. Sci. Plant Nutr. 20 703–714. 10.1007/s42729-019-00158-0 [DOI] [Google Scholar]
- Farhangi-Abriz S., Torabian S. (2018). Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 255 953–962. 10.1007/s00709-017-1202-0 [DOI] [PubMed] [Google Scholar]
- Farid M., Shakoor M. B., Ehsan S., Ali S., Zubair M., Hanif M. A. (2013). Morphological, physiological and biochemical responses of different plant species to Cd stress. Int. J. Chem. Biochem. Sci. 3 53–60. [Google Scholar]
- Fathi A., Zahedi M., Torabian S., Khoshgoftar A. (2017). Response of wheat genotypes to foliar spray of ZnO and Fe2O3 nanoparticles under salt stress. J. Plant Nutr. 40 1376–1385. 10.1080/01904167.2016.1262418 [DOI] [Google Scholar]
- Fazeli-Nasab B., Sirousmehr A. R., Azad H. (2018). Effect of titanium dioxide nanoparticles on essential oil quantity and quality in Thymus vulgaris under water deficit. J. Med. Plants Products 2 125–133. 10.22092/JMPB.2018.118140 [DOI] [Google Scholar]
- Feizi H., Kamali M., Jafari L., Rezvani Moghaddam P. (2013). Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 91 506–511. 10.1016/j.chemosphere.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Feng Y., Cui X., He S., Dong G., Chen M., Wang J., et al. (2013). The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 47 9496–9504. 10.1021/es402109n [DOI] [PubMed] [Google Scholar]
- Fleischer A., O’Neill M. A., Ehwald R. (1999). The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester crosslinking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 12 829–838. 10.1104/pp.121.3.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C. H., Noctor G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17 1866–1875. 10.1105/tpc.105.033589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Liu C., Qu C., Zheng L., Yang F., Su M., et al. (2008). Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? Biometals 21 211–217. 10.1007/s10534-007-9110-y [DOI] [PubMed] [Google Scholar]
- Gao X., Zou C., Wang L., Zhang F. (2006). Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 29 1637–1647. 10.1080/01904160600851494 [DOI] [Google Scholar]
- García-López J. I., Zavala-García F., Olivares-Sáenz E., Lira-Saldívar R. H., Díaz Barriga-Castro E., Ruiz-Torres N. A., et al. (2018). Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 8:215. 10.3390/agronomy8100215 [DOI] [Google Scholar]
- Ghorbanpour M. (2015). Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Ind. J. Plant Physiol. 20 249–256. 10.1007/s40502-015-0170-7 [DOI] [Google Scholar]
- Ghorbanpour M., Hatami M., Hatami M. (2015). Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nanosized titanium dioxide and bulk application. Acta Agric. Slov. 105 23–32. 10.14720/aas.2015.105.1.03 [DOI] [Google Scholar]
- Giraldo J. P., Landry M. P., Faltermeier S. M., McNicholas T. P., Iverson N. M., Boghossian A. A., et al. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 13 400–408. 10.1038/nmat3890 [DOI] [PubMed] [Google Scholar]
- Gohari G., Mohammadi A., Akbari A., Panahirad S., Dadpour M. R., Fotopoulos V., et al. (2020). Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 10:912. 10.1038/s41598-020-57794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorov A. O., Carmeli I. (2007). Hybrid structures composed of photosynthetic system and metal nanoparticles: plasmon enhancement effect. Nano Lett. 7 620–625. 10.1021/nl062528t [DOI] [PubMed] [Google Scholar]
- Gunes A., Pilbeam D. J., Inal A., Bagci E. G., Coban S. (2007). Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact. 2 105–113. 10.1080/17429140701529399 [DOI] [Google Scholar]
- Gupta S. D., Agarwal A., Pradhan S. (2018). Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 161 624–633. 10.1016/j.ecoenv.2018.06.023 [DOI] [PubMed] [Google Scholar]
- Haghighi M., Afifipour Z., Mozafarian M. (2012). The effect of N-Si on tomato seed germination under salinity levels. J. Biol. Environ. Sci. 6 87–90. [Google Scholar]
- Haghighi M., Pessarakli M. (2013). Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 161 111–117. 10.1016/j.scienta.2013.06.034 [DOI] [Google Scholar]
- Haghighi M., Pourkhaloee A. (2013). Nanoparticles in agricultural soils: their risks and benefits for seed germination. Minerva Biotecnol. 25 123–132. [Google Scholar]
- Hall J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53 1–11. 10.1093/jexbot/53.366.1 [DOI] [PubMed] [Google Scholar]
- Haripriya P., Stella P. M., Anusuya S. (2018). Foliar spray of zinc oxide nanoparticles improves salt tolerance in finger millet crops under glasshouse condition. Sciol. Biotechnol. 1 20–29. [Google Scholar]
- Hasanpour H., Maali-Amir R., Zeinali H. (2015). Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 62 779–787. 10.1134/S1021443715060096 [DOI] [Google Scholar]
- Hatami M. (2017). Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol. Environ. Saf. 142 274–283. [DOI] [PubMed] [Google Scholar]
- Hatami M., Ghorbanpour M. (2013). Effect of nanosilver on physiological performance of Pelargonium plants exposed to dark storage. J. Hortic. Res. 21 15–20. 10.2478/johr-2013-0003 [DOI] [Google Scholar]
- Hatami M., Ghorbanpour M. (2014). Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turk. J. Biol. 38 130–139. 10.3906/biy-1304-64 31411186 [DOI] [Google Scholar]
- Hattori T., Inanaga S., Araki H., An P., Morita S., Luxová M., et al. (2005). Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 123 459–466. 10.1111/j.1399-3054.2005.00481.x [DOI] [Google Scholar]
- Hernandez J. A., Jimenez A., Mullineaux P., Seviela F. (2000). Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated to induction of antioxidant defences. Plant Cell Environ. 23 853–862. 10.1046/j.1365-3040.2000.00602.x [DOI] [Google Scholar]
- Hojjat S. S. (2019). Effect of interaction between Ag nanoparticles and salinity on germination stages of Lathyrus sativus L. J. Environ. Soil Sci. 2 186–191. 10.32474/oajess.2019.02.000132 [DOI] [Google Scholar]
- Hojjat S. S., Ganjali A. (2016). The effect of silver nanoparticle on lentil seed germination under drought stress. Intl. J. Farm. Alli. Sci. 5 208–212. [Google Scholar]
- Hojjat S. S., Kamyab M. (2017). The effect of silver nanoparticle on fenugreek seed germination under salinity levels. Russ. Agric. Sci. 43 61–65. 10.3103/S1068367417010189 [DOI] [Google Scholar]
- Hong F., Yang F., Liu C., Gao Q., Wan Z., Gu F., et al. (2005). Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 104 249–260. 10.1385/BTER:104:3:249 [DOI] [PubMed] [Google Scholar]
- Hong J., Rico C. M., Zhao L., Adeleye A. S., Keller A. A., Peralta-Videa J. R., et al. (2016). Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ. Sci. Process. Impacts 17 177–185. 10.1039/c4em00551a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A., Skalicky M., Brestic M., Maitra S., Ashraful Alam M., Syed M. S., et al. (2021). Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 11:241. 10.3390/agronomy11020241 [DOI] [Google Scholar]
- Hossain M. A., Piyatida P., da Silva J. A. T., Fujita M. (2012). Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012:872875. 10.1155/2012/872875 [DOI] [Google Scholar]
- Hossain Z., Mustafa G., Komatsu S. (2015). Plant responses to nanoparticle stress. Int. J. Mol. Sci. 16 26644–26653. 10.3390/ijms161125980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z., Mustafa G., Sakata K., Komatsu S. (2016). Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J. Hazard Mater. 304 291–305. 10.1016/j.jhazmat.2015.10.071 [DOI] [PubMed] [Google Scholar]
- Hussain A., Oves M., Alajmi M. F., Hussain I., Amir S., Ahmed J., et al. (2019a). Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv. 9 15357–15369. 10.1039/c9ra01659g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Rizwan M., Ali Q., Ali S. (2019b). Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 26 7579–7588. 10.1007/s11356-019-04210-5 [DOI] [PubMed] [Google Scholar]
- Hussain A., Ali S., Rizwan M., Rehman M., Qayyum M. F., Wang H. (2019c). Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 173 156–164. 10.1016/j.ecoenv.2019.01.118 [DOI] [PubMed] [Google Scholar]
- Ion A. C., Ion I., Culetu A., Gherase D. (2010). Carbon-based nanomaterials. Environmental applications. Romania. Univ. Politehn. Bucharest 38 129–132. [Google Scholar]
- Iqbal M., Raja N. I., Mashwani Z., Hussain M., Muhammad E., Yasmeen F. (2019). Effect of silver nanoparticles on growth of wheat under heat stress. Iran. J. Sci. Technol. Trans. A Sci. 43 387–395. 10.1007/s40995-017-0417-4 [DOI] [Google Scholar]
- Jaberzadeh A., Moaveni P., Tohidi Moghadam H. R., Zahedi H. (2013). Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti. Agrobot. 41 201–207. 10.15835/nbha4119093 [DOI] [Google Scholar]
- Jalil S. U., Ansari M. I. (2019). “Nanoparticles and abiotic stress tolerance in plants: synthesis, action, and signaling mechanisms,” in Plant Signaling Molecules Role and Regulation Under Stressful Environments, eds Khan I. S., Reddy S. P., Ferrante A., Khan A. N. (Duxford: UK-Woodhead Publishing; ), 549–561. [Google Scholar]
- Janmohammadi M., Amanzadeh T., Sabaghnia N., Ion V. (2016). Effect of nano-silicon foliar application on safflower growth under organic and inorganic fertilizer regimes. Bot. Lith. 22 53–64. 10.1515/botlit-2016-0005 [DOI] [Google Scholar]
- Jeelani P. G., Mulay P., Venkat R., Ramalingam C. (2020). Multifaceted application of silica nanoparticles. A review. Silicon 12 1337–1354. 10.1007/s12633-019-00229-y [DOI] [Google Scholar]
- Jiang H. S., Qiu X. N., Li G. B., Li W., Yin L. Y. (2014). Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 33 1398–1405. 10.1002/etc.2577 [DOI] [PubMed] [Google Scholar]
- Jo Y.-K., Kim B. H., Jung G. (2009). Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 93 1037–1043. 10.1094/PDIS-93-10-1037 [DOI] [PubMed] [Google Scholar]
- Judy J. D., Unrine J. M., Rao W., Wirick S., Bertsch P. M. (2012). Bioavailability of gold nanomaterials to plants: importance of particle size and surface coating. Environ. Sci. Technol. 46 8467–8474. 10.1021/es3019397 [DOI] [PubMed] [Google Scholar]
- Kah M. (2015). Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation? Front. Chem. 3:64. 10.3389/fchem.2015.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalteh M., Taj A. Z., Shahram A., Maryam M. A., Alireza F. N. (2014). Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks. 4 49–55. [Google Scholar]
- Karami Mehrian S., Heidari R., Rahmani F., Najafi S. (2016). Effect of chemical synthesis silver nanoparticles on germination indices and seedlings growth in seven varieties of Lycopersicon esculentum Mill (tomato) plants. J. Clust. Sci. 27 327–340. 10.1007/s10876-015-0932-4 [DOI] [Google Scholar]
- Kardavan Ghabel V., Karamian R. (2020). Effects of TiO2 nanoparticles and spermine on antioxidant responses of Glycyrrhiza glabra L. to cold stress. Acta Bot. Croat. 79 137–147. 10.37427/botcro-2020-025 [DOI] [Google Scholar]
- Kashyap P. L., Xiang X., Heiden P. (2015). Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 77 36–51. 10.1016/j.ijbiomac.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Katiyar P., Yadu B., Korram J., Satnami M. L., Kumar M., Keshavkant S. (2020). Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 92 18–27. 10.1016/j.jes.2020.02.013 [DOI] [PubMed] [Google Scholar]
- Kazemipour S., Hashemabadi D., Kaviani B. (2013). Effect of silver nanoparticles on the vase life and quality of cut chrysanthemum (Chrysanthemum morifolium L.) flower. Eur. J. Exp. Biol. 3 298–302. [Google Scholar]
- Keller C., Rizwan M., Davidian J.-C., Pokrovsky O. S., Bovet N., Chaurand P., et al. (2015). Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241 847–860. 10.1007/s00425-014-2220-1 [DOI] [PubMed] [Google Scholar]
- Khairy A. M., Tohamy M. R., Zayed M. A., Mahmoud S. F., El-Tahan A. M., El-Saadony M. T., et al. (2022). Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi J. Biol. Sci. 29 2199–2209. 10.1016/j.sjbs.2021.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H. R., McDonald G. K., Rengel Z. (2004). Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arientinum L.). Plant Soil 267 271–284. 10.1007/s11104-005-0120-7 [DOI] [Google Scholar]
- Khan I., Seleiman M. F., Chattha M. U., Jalal R. S., Mahmood F., Hassan F. A., et al. (2021). Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti. Agrobot. Cluj Napoca 49:12303. [Google Scholar]
- Khan M., Siddiqui Z. A. (2018). Zinc oxide nanoparticles for the management of Ralstonia solanacearum, Phomopsis vexans and Meloidogyne incognita incited disease complex of eggplant. Indian Phytopathol. 71 355–364. 10.1007/s42360-018-0064-5 [DOI] [Google Scholar]
- Khan Z., Upadhyaya H. (2019). “Impact of nanoparticles on abiotic stress responses in plants: an overview,” in Nanomaterials in Plants, Algae and Microorganisms, eds Tripathi D. K., Ahmad P., Sharma S., Chauhan D. K., Dubey N. K. (Cambridge, MA: Academic Press; ), 305–322. [Google Scholar]
- Khan Z. S., Rizwan M., Hafeez M., Ali S., Javed M. R., Adrees M. (2019). The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 26 19859–19870. 10.1007/s11356-019-05333-5 [DOI] [PubMed] [Google Scholar]
- Kiapour H., Moaveni P., Habibi D., Sani B. (2015). Evaluation of the application of gibbrellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.). Int. J. Agron. Agric. Res. 6 138–150. [Google Scholar]
- Kim D.-Y., Kadam A., Shinde S., Saratale R. G., Patra J., Ghodake G. (2018). Recent developments in nanotechnology transforming the agricultural sector: a transition replete with opportunities. J. Sci. Food Agric. 98 849–864. 10.1002/jsfa.8749 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Oh Y., Yoon H., Hwang I., Chang Y.-S. (2015). Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 49 1113–1119. 10.1021/es504375t [DOI] [PubMed] [Google Scholar]
- Kim S. W., Jung J. H., Lamsal K., Kim Y. S., Min J. S., Lee Y. S. (2012). Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40 53–58. 10.5941/myco.2012.40.1.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan-Baghkheirati E., Geisler-Lee J. (2015). Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 5 436–467. 10.3390/nano5020436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konate A., He X., Zhang Z., Ma Y., Zhang P., Alugongo G. M., et al. (2017). Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 9:790. 10.3390/su9050790 [DOI] [Google Scholar]
- Korkin A., Rosei F. (2008). Nanoelectronics and Photonics: From Atoms to Materials, Devices, and Architectures. New York, NY: Springer. 453 p. [Google Scholar]
- Kumari S., Khanna R. R., Nazir F., Albaqami M., Chhillar H., Wahid I., et al. (2022). Bio-synthesized nanoparticles in developing plant abiotic stress resilience: a new boon for sustainable approach. Int. J. Mol. Sci. 23:4452. 10.3390/ijms23084452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J., Paunesku T., Vogt S., Arora H., Rabatic B. M., Lu J., et al. (2010). Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 10 2296–2302. 10.1021/nl903518f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J. M., Nguyen V., Schroeder J. I. (2006). The role of reactive oxygen species in hormonal responses. Plant Physiol. 141 323–329. 10.1104/pp.106.079004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsal K., Kim S. W., Jung J. H., Kim Y. S., Kim K. S., Lee Y. S. (2011). Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 39 194–199. 10.5941/myco.2011.39.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laware S. L., Raskar S. (2014). Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 3 749–760. [Google Scholar]
- Lee C. W., Mahendra S., Zodrow K., Li D., Tsai Y. C., Braam J., et al. (2010). Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 29 669–675. 10.1002/etc.58 [DOI] [PubMed] [Google Scholar]
- Lee D. J., Senseman S. A., Sciumbato A. S., Jung S.-C., Krutz L. J. (2003). The effect of titanium dioxide alumina beads on the photocatalytic degradation of picloram in water. J. Agric. Food Chem. 51 2659–2664. 10.1021/jf026232u [DOI] [PubMed] [Google Scholar]
- Lee W. M., An Y. J., Yoon H., Kweon H. S. (2008). Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 27 1915–1921. 10.1897/07-481.1 [DOI] [PubMed] [Google Scholar]
- Lee W. M., Kwak J. I., An Y. J. (2012). Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere 86 491–499. 10.1016/j.chemosphere.2011.10.013 [DOI] [PubMed] [Google Scholar]
- Lei Z., Mingyu S., Chao L., Liang C., Hao H., Xiao W., et al. (2007). Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol. Trace Elem. Res. 119 68–76. 10.1007/s12011-007-0047-3 [DOI] [PubMed] [Google Scholar]
- Lei Z., Mingyu S., Xiao W., Chao L., Chunxiang Q., Liang C., et al. (2008). Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 121 69–79. 10.1007/s12011-007-8028-0 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhu N., Liang X., Bai X., Zheng L., Zhao J., et al. (2020). Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg(ii) in soybean (Glycine max). Environ. Sci. Nano 7 1807–1817. 10.1039/d0en00091d [DOI] [Google Scholar]
- Li Z., Huang J. (2014). Effects of nanoparticle hydroxyapatite on growth and antioxidant system in pakchoi (Brassica chinensis L.) from cadmium-contaminated soil. J. Nanomater. 2014:470962. 10.1155/2014/470962 [DOI] [Google Scholar]
- Liang Y., Sun W., Zhu Y. G., Christie P. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut. 147 422–428. 10.1016/j.envpol.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Lin D., Zhing B. (2007). Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 150 243–250. 10.1016/j.envpol.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Liu R., Lal R. (2015). Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 514 131–139. 10.1016/j.scitotenv.2015.01.104 [DOI] [PubMed] [Google Scholar]
- Lu L., Huang M., Huang Y., Corvini P. F.-X., Ji R., Zhao L. (2020). Mn3O4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environ. Sci. Nano 7 1692–1703. 10.1039/d0en00214c [DOI] [Google Scholar]
- Ma J. F. (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50 11–18. 10.1080/00380768.2004.10408447 [DOI] [Google Scholar]
- Ma J. F., Yamaji N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11 392–397. 10.1016/j.tplants.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Ma X., Geisler-Lee J., Deng Y., Kolmakov A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ. 408 3053–3061. 10.1016/j.scitotenv.2010.03.031 [DOI] [PubMed] [Google Scholar]
- Madhavi V., Prasad T., Reddy A. V. B., Madhavi G. (2013). Plant growth promoting potential of nano-bioremediation under Cr (VI) stress. Int. J. Nanotechnol. Appl. 3 1–10. [Google Scholar]
- Mahmoodzadeh H., Nabavi M., Kashefi H. (2013). Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornament. Plants 3 25–32. [Google Scholar]
- Mahmoud L. M., Dutt M., Shalan A. M., El-Kady M. E., El-Boray M. S., Shabana Y. M., et al. (2020). Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 132 155–163. 10.1016/j.sajb.2020.04.027 [DOI] [Google Scholar]
- Manzoor N., Ahmed T., Noman M., Shahid M., Nazir M. M., Ali L., et al. (2021). Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 769:145221. 10.1016/j.scitotenv.2021.145221 [DOI] [PubMed] [Google Scholar]
- Maswada H. F., Djanaguiraman M., Prasad P. V. V. (2018). Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agro. Crop Sci. 204 577–587. 10.1111/jac.12280 [DOI] [Google Scholar]
- Mazumdar H., Ahmed G. U. (2011). Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int. J. ChemTech. Res. 3 1494–1500. [Google Scholar]
- Mahdi Nezhad N., Mousavi H., Fakheri B., Heidari F. (2018). The effect of some nanoparticles on the activity of antioxidant enzymes and parthenolide yield of feverfew plant (Tanacetum parthenium L.) under water deficit stress. Env. Stresses Crop Sci. 11 917–929. 10.22077/ESCS.2018.1051.1210 [DOI] [Google Scholar]
- Mengiste T., Laluk K., AbuQamar S. (2010). “Mechanisms of induced resistance against B. cinerea,” in Post-harvest Pathology, Plant Pathology in the 21st Century, eds Prusky D., Gullino M. L. (Dordrecht: Springer; ), 13–30. [Google Scholar]
- Mirzajani F., Askari H., Hamzelou S., Farzaneh M., Ghassempour A. (2013). Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 88 48–54. 10.1016/j.ecoenv.2012.10.018 [DOI] [PubMed] [Google Scholar]
- Mishra V., Mishra R. K., Dikshit A., Pandey A. C. (2014). “Interactions of nanoparticles with plants: an emerging prospective in the agriculture industry,” in Emerging Technologies and Management of Crop Stress Tolerance, Vol. 1 eds Ahmad P., Rasool S. (San Diego, CA: Academic press; ), 159–180. [Google Scholar]
- Moameri M., Abbasi Khalaki M. (2019). Capability of Secale montanum trusted for phytoremediation of lead and cadmium in soils amended with nano-silica and municipal solid waste compost. Environ. Sci. Pollut. Res. 26 24315–24322. 10.1007/s11356-017-0544-7 [DOI] [PubMed] [Google Scholar]
- Moameri M., Abbasi Khalaki M., Ghorbani A. (2018a). Effects of nanopriming and biopriming on growth characteristics of Onobrychis sativa Lam. under laboratory conditions. Rangelands 12 101–111. [Google Scholar]
- Moameri M., Jafari M., Tavili A., Motasharezadeh B., Zare Chahouki M. A., Madrid Diaz F. (2018b). Investigating lead and zinc uptake and accumulation by Stipa hohenackeriana trin and rupr in field and pot experiments. Biosci. J. 34 138–150. 10.14393/BJ-v34n1a2018-37238 33406856 [DOI] [Google Scholar]
- Mohamed A., Qayyum M. F., Abdel-Hadi A., Rehman R. A., Ali S., Rizwan M. (2017). Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 63 1736–1747. 10.1080/03650340.2017.1300256 [DOI] [Google Scholar]
- Mohammadi H., Esmailpour M., Gheranpaye A. (2016). Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 107 385–396. 10.14720/aas.2016.107.2.11 [DOI] [Google Scholar]
- Mohammadi R., Maali-Amiri R., Abbasi A. (2013). Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 152 403–410. 10.1007/s12011-013-9631-x [DOI] [PubMed] [Google Scholar]
- Mohammadi R., Maali-Amiri R., Mantri N. L. (2014). Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ. J. Plant Physiol. 61 768–775. 10.1134/S1021443714050124 [DOI] [Google Scholar]
- Monica R. C., Cremonini R. (2009). Nanoparticles and higher plants. Caryologia 62 161–165. 10.1080/00087114.2004.10589681 [DOI] [Google Scholar]
- Moradbeygi H., Jamei R., Heidari R., Darvishzadeh R. (2020a). Fe2O3 nanoparticles induced biochemical responses and expression of genes involved in rosmarinic acid biosynthesis pathway in Moldavian balm under salinity stress. Physiol. Plantarum. 169 555–570. 10.1111/ppl.13077 [DOI] [PubMed] [Google Scholar]
- Moradbeygi H., Jamei R., Heidari R., Darvishzadeh R. (2020b). Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 272:109537. 10.1016/j.scienta.2020.109537 [DOI] [Google Scholar]
- Mozafari A., Ghadakchi Asl A., Ghaderi N. (2018a). Grape response to salinity stress and role of iron nanoparticle and potassium silicate to mitigate salt induced damage under in vitro conditions. Physiol. Mol. Biol. Plants. 24 25–35. 10.1007/s12298-017-0488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari A., Havas F., Ghaderi N. (2018b). Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria× ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ. Cult. 132 511–523. 10.1007/s11240-017-1347-8 [DOI] [Google Scholar]
- Mukherjee A., Peralta-Videa J. R., Bandyopadhyay S., Rico C. M., Zhao L., Gardea-Torresdey J. L. (2014). Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6 132–138. 10.1039/c3mt00064h [DOI] [PubMed] [Google Scholar]
- Mustafa G., Komatsu S. (2016). Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteome Res. 15 4464–4475. 10.1021/acs.jproteome.6b00572 [DOI] [PubMed] [Google Scholar]
- Mustafa G., Sakata K., Hossain Z., Komatsu S. (2015). Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J. Proteomics 122 100–118. 10.1016/j.jprot.2015.03.030 [DOI] [PubMed] [Google Scholar]
- Nair P. M. G., Chung I. M. (2014). Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 21 12709–12722. 10.1007/s11356-014-3210-3 [DOI] [PubMed] [Google Scholar]
- Nair P. M. G., Chung I. M. (2017). Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 575 187–198. 10.1016/j.scitotenv.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Nair R., Varghese S. H., Nair B. G., Maekawa T., Yoshida Y., Kumar D. S. (2010). Nanoparticulate material delivery to plants. Plant Sci. 179 154–163. 10.1016/j.plantsci.2010.04.012 [DOI] [Google Scholar]
- Najafi Disfani M., Mikhak A., Kassaeec M. Z., Magharid A. H. (2017). Effects of nano Fe/SiO2 fertilizers on germination and growth of barley and maize. Arch. Agron. Soil Sci. 63 817–826. 10.1080/03650340.2016.1239016 [DOI] [Google Scholar]
- Namasivayam S., Chitrakala K. (2011). Ecotoxicological effect of Lecanicillium lecanii (Ascomycota: Hypocreales) based silver nanoparticles on growth parameters of economically important plants. J. Biopestic. 4 97–101. [Google Scholar]
- Navarro E., Piccapietra F., Wagner B., Marconi F., Kaegi R., Odzak N., et al. (2008). Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 42 8959–8964. 10.1021/es801785m [DOI] [PubMed] [Google Scholar]
- Nayan R., Rawat M., Negi B., Pande A., Arora S. (2016). Zinc sulfide nanoparticle mediated alterations in growth and anti-oxidant status of Brassica juncea. Biologia 71 896–902. 10.1515/biolog-2016-0107 [DOI] [Google Scholar]
- Nejatzadeh F. (2021). Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during in vitro and in vivo germination tests. Heliyon 7:e05981. 10.1016/j.heliyon.2021.e05981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A., Xia T., Mädler L., Li N. (2006). Toxic potential of materials at the nanolevel. Science 311 622–627. 10.1126/science.1114397 [DOI] [PubMed] [Google Scholar]
- Noman M., Shahid M., Ahmed T., Tahir M., Naqqash T., Muhammad S., et al. (2020). Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 192:110303. 10.1016/j.ecoenv.2020.110303 [DOI] [PubMed] [Google Scholar]
- Noohpisheh Z., Amiri H., Mohammadi A., Farhadi S. (2021). Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst. 155 267–280. 10.1080/11263504.2020.1739160 [DOI] [Google Scholar]
- O’Brien J. A., Benková E. (2013). Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 4:451. 10.3389/fpls.2013.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaga G., Wydra K. (2016). “Advances in plant tolerance to abiotic stresses,” in Plant Genomics, ed. Abdurakhmonov I. Y. (London: IntechOpen; ), 229–272. [Google Scholar]
- Owolade O., Ogunleti D. (2008). Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J. Plant Prot. Res. 48 329–335. 10.2478/v10045-008-0042-5 [DOI] [Google Scholar]
- Pakrashi S., Jain N., Dalai S., Jayakumar J., Chandrasekaran P. T., Raichur A. M., et al. (2014). In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS One 9:e87789. 10.1371/journal.pone.0087789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavi, Mehta C. M., Srivastava R., Arora S., Sharma A. K. (2016). Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 6:254. 10.1007/s13205-016-0567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist N. G. M., Seisenbaeva G. A., Svedlindh P., Kessler V. G. (2017). Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 12:631. 10.1186/s11671-017-2404-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S. (2017). Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed. J. Plant Sci Phytopathol. 1 62–70. [Google Scholar]
- Pandey A. C., Sanjay S. S., Yadav R. S. (2010). Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 5 488–497. 10.1080/17458081003649648 [DOI] [Google Scholar]
- Paramo L. A., Feregrino-Pérez A. A., Guevara R., Mendoza S., Esquivel K. (2020). Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials 10:1654. 10.3390/nano10091654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen A., Rao S. (2015). Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J. Clust. Sci. 26 693–701. 10.1007/s10876-014-0728-y [DOI] [Google Scholar]
- Pei Z. F., Ming D. F., Liu D., Wan G. L., Geng X. X., Gong H. J., et al. (2010). Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 29 106–115. 10.1007/s00344-009-9120-9 [DOI] [Google Scholar]
- Perreault F., Samadani M., Dewez D. (2014). Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology 8 374–382. 10.3109/17435390.2013.789936 [DOI] [PubMed] [Google Scholar]
- Peyvandi M., Kamali Jamakani Z., Mirza M. (2011a). Comparison of nano Fe chelate with Fe chelate effect on growth parameters and antioxidant enzymes activity of Satureja hortensis. New Cell Mol. Biotech. 2 2–32. [Google Scholar]
- Peyvandi M., Parandeh H., Mirza M. (2011b). Comparison of nano Fe chelate with Fe chelate effect on growth parameters and antioxidant enzymes activity of Ocimum basilicum. New Cell Mol. Biotech. J. 1 89–98. [Google Scholar]
- Prasad R., Bhattacharyya A., Nguyen Q. D. (2017). Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 8:1014. 10.3389/fmicb.2017.01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T. N. V. K. V., Sudhakar P., Sreenivasulu Y., Latha P., Munaswamy V., Reddy K. R., et al. (2012). Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 35 905–927. 10.1080/01904167.2012.663443 [DOI] [Google Scholar]
- Prazak R., Swieciło A., Krzepiłko A., Michałek S., Arczewska M. (2020). Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 10:312. 10.3390/agriculture10080312 [DOI] [Google Scholar]
- Qados A. M. A. (2015). Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean (Vicia faba L.) plants. Am. J. Exp. Agric. 7 78–95. 10.9734/ajea/2015/15110 [DOI] [Google Scholar]
- Qados A. M. A., Moftah A. E. (2015). Influence of silicon and nano-silicon on germination, growth and yield of faba bean (Vicia faba L.) under salt stress conditions. J. Exp. Agric. Int. 5 509–524. 10.9734/ajea/2015/14109 [DOI] [Google Scholar]
- Qi M., Liu Y., Li T. (2013). Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 156 323–328. 10.1007/s12011-013-9833-2 [DOI] [PubMed] [Google Scholar]
- Rady M. M., Boriek S. H. K., Abd El-Mageed T. A., Seif El-Yazal M. A., Ali E. F., Hassan F. A. S., et al. (2021). Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10:748. 10.3390/plants10040748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatizadeh R., Javad Arvin S. M., Jamei R., Mozaffari H., Nejhad F. R. (2019). Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact. 14 474–481. 10.1080/17429145.2019.1626922 [DOI] [Google Scholar]
- Rajput V., Minkina T., Ahmed B., Sushkova S., Singh R., Soldatov M., et al. (2020). Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: critical review of the state of the science and future perspectives. Rev. Environ. Contamin. Toxicol. 252 51–96. [DOI] [PubMed] [Google Scholar]
- Rajput V., Minkina T., Fedorenko A., Sushkova S., Mandzhieva S., Lysenko V., et al. (2018). Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 645 1103–1113. [DOI] [PubMed] [Google Scholar]
- Rajput V. D., Minkina T., Kumari A., Singh V. K., Verma K. K., Mandzhieva S., et al. (2021). Coping with the challenges of abiotic stress in plants: new dimensions in the field application of nanoparticles. Plants 10:1221. 10.3390/plants10061221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raliya R., Tarafdar J. C. (2013). ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2 48–57. 10.1007/s40003-012-0049-z [DOI] [Google Scholar]
- Ramezani F., Shayanfar A., Tavakkol Afshari R., Rezaee K. (2014). Effects of silver, nickel, zinc and zinc-copper nanoparticles on germination, seedling establishment and enzyme activity of alfalfa (Medicago sativa) seed. Iran J. Field Crop Sci. 45 107–118. 10.22059/ijfcs.2014.51038 [DOI] [Google Scholar]
- Rasheed A., Seleiman M. F., Nawaz M., Mahmood A., Anwar M. R., Ayub M. A., et al. (2021). Agronomic and genetic approaches for enhancing tolerance to heat stress in rice: a review. Not. Bot. Horti. Agrobot. Cluj Napoca 49:12501. [Google Scholar]
- Rastogi A., Tripathi D. K., Yadav S., Chauhan D. K., Živèák M., Ghorbanpour M., et al. (2019). Application of silicon nanoparticles in agriculture. 3 Biotech 9:90. 10.1007/s13205-019-1626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. A. (1983). The transport and function of silicon in plants. Biol. Rev. 58 179–207. 10.1111/j.1469-185X.1983.tb00385.x [DOI] [Google Scholar]
- Rawson H. M., Long M. J., Munns R. (1988). Growth and development in NaCl-treated plants. I. leaf Na+ and Cl– concentrations do not determine gas exchange of leaf blades in barley. Funct. Plant Biol. 15 519–527. 10.1071/PP9880519 [DOI] [Google Scholar]
- Rédei G. P. (2008). Encyclopedia of Genetics, Genomics, Proteomics, and Informatics, 3rd Edn. Dordrecht: Springer. 2201 p. [Google Scholar]
- Rezvani N., Sorooshzadeh A., Farhadi N. (2012). Effect of nano-silver on growth of saffron in flooding stress. World Acad. Sci. Eng. Technol. 6 517–522. [Google Scholar]
- Rizwan M., Ali S., Qayyum M. F., Ok Y. S., Adrees M., Ibrahim M., et al. (2017). Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J. Hazard. Mater. 322 2–16. 10.1016/j.jhazmat.2016.05.061 [DOI] [PubMed] [Google Scholar]
- Rizwan M., Ali S., Zia Ur Rehman M., Adrees M., Arshad M., Qayyum M. F., et al. (2019a). Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 248 358–367. 10.1016/j.envpol.2019.02.031 [DOI] [PubMed] [Google Scholar]
- Rizwan M., Noureen S., Ali S., Anwar S., Rehman M. Z. U., Qayyum M. F., et al. (2019b). Influence of biochar amendment and foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. J. Soils Sediments 19 3749–3759. 10.1007/s11368-019-02327-1 [DOI] [Google Scholar]
- Rizwan M., Ali S., Ali B., Adrees M., Arshad M., Hussain A., et al. (2019c). Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214 269–277. 10.1016/j.chemosphere.2018.09.120 [DOI] [PubMed] [Google Scholar]
- Rossi L., Fedenia L. N., Sharifan H., Ma X., Lombardini L. (2019). Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 135 160–166. 10.1016/j.plaphy.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Rossi L., Zhang W., Lombardini L., Ma X. (2016). The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 219 28–36. 10.1016/j.envpol.2016.09.060 [DOI] [PubMed] [Google Scholar]
- Roy R., Núñez-Delgado A., Sultana S., Wang J., Munir A., Battaglia M. L., et al. (2021). Additions of optimum water spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J. Environ. Manag. 295:113076. 10.1016/j.jenvman.2021.113076 [DOI] [PubMed] [Google Scholar]
- Rui Y. (2021). Nanoparticles Alleviate Heavy Metals Stress. Available online at: https://encyclopedia.pub/7093, (accessed June 2, 2021). [Google Scholar]
- Saad A. M., El-Saadony M. T., El-Tahan A. M., Sayed S., Moustafa M. A., Taha A. E., et al. (2021). Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 28 5674–5683. 10.1016/j.sjbs.2021.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaghnia N., Janmohammadi M. (2014). Effect of nano-silicon particles application on salinity tolerance in early growth of some lentil genotypes. Ann. UMCS Biol. 69 39–55. 10.1515/umcsbio-2015-0004 [DOI] [Google Scholar]
- Sadak M. S. (2019). Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 43 1–6. 10.1186/s42269-019-0077-y [DOI] [Google Scholar]
- Saha N., Dutta Gupta S. (2017). Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 330 18–28. 10.1016/j.jhazmat.2017.01.021 [DOI] [PubMed] [Google Scholar]
- Salem H. M., Ismael E., Shaalan M. (2021). Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomedicine 16 6783–6796. 10.2147/IJN.S319708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana I., Wu H., Hu P., Giraldo J. P. (2020). Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 11:2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvas D., Giotis D., Chatzieustratiou E., Bakea M., Patakioutas G. (2009). Silicon supply in soilless cultivations of zucchini alleviates stress induced by salinity and powdery mildew infections. Environ. Exp. Bot. 65 11–17. 10.1016/j.envexpbot.2008.07.004 [DOI] [Google Scholar]
- Saxena R., Tomar R. S., Kumar M. (2016). Exploring nanobiotechnology to mitigate abiotic stress in crop plants. J. Pharm Sci. Res. 8 974–980. [Google Scholar]
- Sedghi M., Mitra H., Sahar T. (2013). Effect of nano zinc oxide on the germination of soybean seeds under drought stress. Ann. West Univ. Timiş. Ser. Biol. 16 73–78. [Google Scholar]
- Seghatoleslami M., Feizi H., Mousavi G., Berahmand A. (2015). Effect of magnetic field and silver nanoparticles on yield and water use efficiency of Carum copticum under water stress conditions. Pol. J. Chem. Technol. 17 110–114. 10.1515/pjct-2015-0016 [DOI] [Google Scholar]
- Seghatoleslami M. J., Forutani R. (2015). Yield and water use efficiency of sunflower as affected by nano ZnO and water stress. J. Adv. Agric. Technol. 2 34–37. [Google Scholar]
- Seleiman M. F., Alotaibi M. A., Alhammad B. A., Alharbi B. M., Refay Y., Badawy S. A. (2020a). Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 10:790. 10.3390/agronomy10060790 [DOI] [Google Scholar]
- Seleiman M. F., Santanen A., Mäkelä P. (2020b). Recycling sludge on cropland as fertilizer-Advantages and risks. Resour. Conserv. Recycle 155:104647. 10.1016/j.resconrec.2019.104647 [DOI] [Google Scholar]
- Seleiman M. F., Semida W. M., Rady M. M., Mohamed G. F., Hemida K. A., Alhammad B. A., et al. (2020c). Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants 9:1783. 10.3390/plants9121783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleiman M. F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., et al. (2021a). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. 10.3390/plants10020259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleiman M. F., Almutairi K. F., Alotaibi M., Shami A., Alhammad B. A., Battaglia M. L. (2021b). Nano-fertilization as an emerging fertilization technique: why can modern agriculture benefit from its use? Plants 10:2. 10.3390/plants10010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleiman M. F., Aslam M. T., Alhammad B. A., Hassan M. U., Maqbool R., Chattha M. U., et al. (2022). Salinity stress in wheat: effects, mechanisms and management strategies. Phyton 91:667. 10.32604/phyton.2022.017365 [DOI] [Google Scholar]
- Semida W. M., Abd El-Mageed T. A., Howladar S. M. (2014). A novel organo-mineral fertilizer can alleviate negative effects of salinity stress for eggplant production on reclaimed saline calcareous soil. Acta Hortic. 1034 493–499. [Google Scholar]
- Semida W. M., Abdelkhalik A., Mohamed G. F., Abd El-Mageed T. A., Abd El-Mageed S. A., Rady M. M., et al. (2021). Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10:421. 10.3390/plants10020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semida W. M., Abdelkhalik A. A., Rady M. O. A., Marey R. A., Abd El-Mageed T. A. (2020). Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 272:109580. 10.1016/j.scienta.2020.109580 [DOI] [Google Scholar]
- Shaaban A., Al-Elwany O. A., Abdou N. M., Hemida K. A., El-Sherif A., Abdel-Razek M. A., et al. (2022). Filter mud enhanced yield and soil properties of water-stressed Lupinus termis L. in saline calcareous soil. J. Soil Sci. Plant Nutr. 22 1572–1588. 10.1007/s42729-021-00755-y [DOI] [Google Scholar]
- Shafiee-Jood M., Cai X. (2016). Reducing food loss and waste to enhance food security and environmental sustainability. Environ. Sci. Technol. 50 8432–8443. 10.1021/acs.est.6b01993 [DOI] [PubMed] [Google Scholar]
- Shah T., Latif S., Saeed F., Ali I., Ullah S., Abdullah Alsahli A., et al. (2021). Priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud. Univ. Sci. 33:101207. 10.1016/j.jksus.2020.10.004 [DOI] [Google Scholar]
- Shalaby T. A., Bayoumi Y., Abdalla N., Taha H., Alshaal T., Shehata S., et al. (2016). “Nanoparticles, soils, plants and sustainable agriculture,” in Nanoscience in Food and Agriculture 1, eds Ranjan S., Dasgupta N., Lichtfouse E. (Switzerland: Springer; ), 283–312. [Google Scholar]
- Shallan M. A., Hassan H. M., Namich A. A., Ibrahim A. A. (2016). Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharm. Biol. Chem. Sci. 7 1540–1551. [Google Scholar]
- Sham A., Al-Ashram H., Whitely K., El-Tarabily K. A., Iratni R., AbuQamar S. F. (2019). Metatranscriptomic analysis of multiple environmental stresses identifies RAP2.4 gene associated with Arabidopsis immunity to Botrytis cinerea. Sci. Rep. 9:17010. 10.1038/s41598-019-53694-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham A., Moustafa K., Al-Shamisi S., Alyan S., Iratni R., AbuQamar S. F. (2017). Microarray analysis of Arabidopsis WRKY33 mutants in response to the necrotrophic fungus Botrytis cinerea. PLoS One 12:e0172343. 10.1371/journal.pone.0172343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Hasan M., Ahammed G. J., Li M., Yin H., Zhou J. (2019). Applications of nanotechnology in plant growth and crop protection: a review. Molecules 24:2558. 10.3390/molecules24142558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Karimi J., Mohsenzadeh S., Sharifi-Rad M., Moradgholi J. (2014). Evaluating SiO2 nanoparticles effects on developmental characteristic and photosynthetic pigment contents of Zea mays L. Bull. Environ. Pharm. Life Sci. 3 194–201. [Google Scholar]
- Sharma P., Bhatt D., Zaidi M. G. H., Saradhi P. P., Khanna P. K., Arora S. (2012a). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167 2225–2233. 10.1007/s12010-012-9759-8 [DOI] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M. (2012b). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:217037. 10.1155/2012/217037 [DOI] [Google Scholar]
- Shaw A. K., Ghosh S., Kalaji H. M., Bosa K., Brestic M., Zivcak M., et al. (2014). Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ. Exp. Bot. 102 37–47. 10.1016/j.envexpbot.2014.02.016 [DOI] [Google Scholar]
- Shelar G. B., Chavan A. M. (2015). Myco-synthesis of silver nanoparticles from Trichoderma harzianum and its impact on germination status of oil seed. Biolife 3 109–113. 10.13140/RG.2.2.17609.16484 [DOI] [Google Scholar]
- Shen X., Xiao X., Dong Z., Chen Y. (2014). Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant 36 3063–3069. 10.1007/s11738-014-1676-8 [DOI] [Google Scholar]
- Shi Y., Zhang Y., Han W., Feng R., Hu Y., Guo J., et al. (2016). Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 7:196. 10.3389/fpls.2016.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei T. R., Salleh M. A. M., Tabatabaei M., Mobli H., Aghbashlo M., Rashid S. A., et al. (2019). “Applications of nanotechnology and carbon nanoparticles in agriculture,” in Synthesis, Technology and Applications of Carbon Nanomaterials, eds Abdul Rashid S., Othman R. N. I. R., Hussein M. Z. (Amsterdam: Elsevier; ), 247–277. [Google Scholar]
- Siddiqui M. H., Al Whaibi M. H., Faisal M., Al Sahli A. A. (2014). Nano silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 33 2429–2437. 10.1002/etc.2697 [DOI] [PubMed] [Google Scholar]
- Silva S., Ribeiro T. P., Santos C., Pinto D. C., Silva A. M. (2020). TiO2 nanoparticles induced sugar impairments and metabolic pathway shift towards amino acid metabolism in wheat. J. Hazard. Mater. 399:122982. 10.1016/j.jhazmat.2020.122982 [DOI] [PubMed] [Google Scholar]
- Singh J., Lee B. K. (2016). Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manage. 170 88–96. 10.1016/j.jenvman.2016.01.015 [DOI] [PubMed] [Google Scholar]
- Singh M. D., Jayadeva H. M., Chirag Gautam Mohan M. H. (2017). Effects of nano zinc oxide particles on seedling growth of maize (Zea mays L.) in germinating paper test. Int. J. Microbiol. Res. 9 897–898. [Google Scholar]
- Singh S., Tripathi D. K., Dubey N. K., Chauhan D. K. (2016). Effects of nano-materials on seed germination and seedling growth: striking the slight balance between the concepts and controversies. Mater. Focus 5 195–201. 10.1166/mat.2016.1329 [DOI] [Google Scholar]
- Singhal J., Verma S., Kumar S. (2022). The physio-chemical properties and applications of 2D nanomaterials in agricultural and environmental sustainability. Sci. Total Environ. 837:155669. 10.1016/j.scitotenv.2022.155669 [DOI] [PubMed] [Google Scholar]
- Sofy M. R., Seleiman M. F., Alhammad B. A., Alharbi B. M., Mohamed H. I. (2020). Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 10:699. 10.3390/agronomy10050699 [DOI] [Google Scholar]
- Solanki P., Bhargava A., Chhipa H., Jain N., Panwar J. (2015). “Nano-fertilizers and their smart delivery system,” in Nanotechnologies in Food and Agriculture, eds Rai M., Ribeiro C., Mattoso L., Duran N. (Switzerland: Springer; ), 81–101. [Google Scholar]
- Song G., Gao Y., Wu H., Hou W., Zhang C., Ma H. (2012). Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ. Toxicol. Chem. 31 2147–2152. 10.1002/etc.1933 [DOI] [PubMed] [Google Scholar]
- Staroń A., Długosz O., Pulit-Prociak J., Banach M. (2020). Analysis of the exposure of organisms to the action of nanomaterials. Materials 13:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Song F., Guo J., Zhu X., Liu S., Liu F., et al. (2020). Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int. J. Mol. Sci. 21:782. 10.3390/ijms21030782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyaprabha R., Karunakaran G., Yuvakkumar R., Prabu P., Rajendran V., Kannan N. (2012). Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 14:1294. 10.1007/s11051-012-1294-6 [DOI] [Google Scholar]
- Syu Y. Y., Hung J. H., Chen J. C., Chuang H. W. (2014). Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 83 57–64. 10.1016/j.plaphy.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Taha R., Seleiman M. F., Alotaibi M., Alhammad B. A., Rady M. M., Mahdi A. H. A. (2020). Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy 10:1741. 10.3390/agronomy10111741 [DOI] [Google Scholar]
- Taha R. S., Seleiman M. F., Shami A., Alhammad B. A., Mahdi A. H. A. (2021). Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants 10:1040. 10.3390/plants10061040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy A. S., Salama Y. A. M., El-Nemr M. A., Abdel-Mawgoud A. M. R. (2015). Nano silicon application improves salinity tolerance of sweet pepper plants. Int. J. ChemTech Res. 8 11–17. [Google Scholar]
- Tarafdar J. C., Raliya R., Mahawar H., Rathore I. (2014). Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric. Res. 3 257–262. 10.1007/s40003-014-0113-y [DOI] [Google Scholar]
- Taran N., Storozhenko V., Svietlova N., Batsmanova L., Shvartau V., Kovalenko M. (2017). Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 12:60. 10.1186/s11671-017-1839-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli E., Rengasamy P., McDonald G. (2010). High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 61 4449–4459. 10.1093/jxb/erq251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe-Neira R., Reyes-Díaz M., Nunes-Nesi A., Recio G., Carmona E., Corgne A., et al. (2020). Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci. Hortic. 269:109418. 10.1016/j.scienta.2020.109418 [DOI] [Google Scholar]
- Tirani M. M., Haghjou M. M., Ismaili A. (2019). Hydroponic grown tobacco plants respond to zinc oxide nanoparticles and bulk exposures by morphological, physiological and anatomical adjustments. Funct. Plant Biol. 46 360–375. 10.1071/fp18076 [DOI] [PubMed] [Google Scholar]
- Torabian S., Zahedi M., Khoshgoftar A. H. (2016). Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 39 172–180. 10.1080/01904167.2015.1009107 [DOI] [Google Scholar]
- Tripathi D. K., Singh S., Singh S., Mishra S., Chauhan D. K., Dubey N. K. (2015a). Micronutrients and their diverse role in agricultural crops: advances and future prospective. Acta Physiol. Plant. 37:139. 10.1007/s11738-015-1870-3 [DOI] [Google Scholar]
- Tripathi D. K., Singh V. P., Prasad S. M., Chauhan D. K., Dubey N. K. (2015b). Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 96 189–198. 10.1016/j.plaphy.2015.07.026 [DOI] [PubMed] [Google Scholar]
- Tripathi D. K., Singh S., Singh S., Pandey R., Singh V. P., Sharma N. C., et al. (2017a). An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 110 2–12. 10.1016/j.plaphy.2016.07.030 [DOI] [PubMed] [Google Scholar]
- Tripathi D. K., Singh S., Singh V. P., Prasad S. M., Dubey N. K., Chauhan D. K. (2017b). Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 110 70–81. 10.1016/j.plaphy.2016.06.026 [DOI] [PubMed] [Google Scholar]
- Tripathi D. K., Singh S., Singh S., Srivastava P. K., Singh V. P., Singh S., et al. (2017c). Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 110 167–177. 10.1016/j.plaphy.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Tripathi D. K., Singh S., Singh V. P., Prasad S. M., Chauhan D. K., Dubey N. K. (2016). Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 4:46. 10.3389/fenvs.2016.00046 [DOI] [Google Scholar]
- Tunca E. (2015). Nanoparticles as the base of nanotechnology and phytoremediation of nanoparticles. Ordu Univ. J. Sci. Technol. 5 23–34. [Google Scholar]
- Turgeon R. (2010). The puzzle of phloem pressure. Plant Physiol. 154 578–581. 10.1104/pp.110.161679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M., Farooq M., Wakeel A., Nawaz A., Cheema S. A., Rehman H., et al. (2020). Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ. 721:137778. 10.1016/j.scitotenv.2020.137778 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F., Dat J. F. (2006). Reactive oxygen species in plant cell death. Plant Physiol. 141 384–390. 10.1104/pp.106.078295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nguyen D., Nguyen H. M., Le N. T., Nguyen K. H., Nguyen H. T., Le H. M., et al. (2022). Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 41 364–375. 10.1007/s00344-021-10301-w [DOI] [Google Scholar]
- Vannini C., Domingo G., Onelli E., Prinsi B., Marsoni M., Espen L., et al. (2013). Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS One 8:e68752. 10.1371/journal.pone.0068752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam P., Jayaraj M., Manikandan R., Geetha N., Rene E. R., Sharma N. C., et al. (2017). Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol. Biochem. 110 59–69. 10.1016/j.plaphy.2016.08.022 [DOI] [PubMed] [Google Scholar]
- Vitti A., Nuzzaci M., Scopa A., Tataranni G., Tamburrino I., Sofo A. (2014). Hormonal response and root architecture in Arabidopsis thaliana subjected to heavy metals. Int. J. Plant Biol. 5:5226. 10.4081/pb.2014.5226 [DOI] [Google Scholar]
- Vundavalli R., Vundavalli S., Nakka M., Rao D. S. (2015). Biodegradable nano-hydrogels in agricultural farming-alternative source for water resources. Proc. Mater. Sci. 10 548–554. 10.1016/j.mspro.2015.06.005 [DOI] [Google Scholar]
- Wahid I., Kumari S., Ahmad R., Hussain S. J., Alamri S., Siddiqui M. H., et al. (2020). Silver nanoparticle regulates salt tolerance in wheat through changes in ABA concentration, ion homeostasis, and defense systems. Biomolecules 10:1506. 10.3390/biom10111506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kou X., Pei Z., Xiao J. Q., Shani X., Xing B. (2011). Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 5 30–42. 10.3109/17435390.2010.489206 [DOI] [PubMed] [Google Scholar]
- Wang J., Naser N. (1994). Improved performance of carbon paste amperometric biosensors through the incorporation of fumed silica. Electroanalysis 6 571–575. 10.1002/elan.1140060707 [DOI] [Google Scholar]
- Wang S., Wang F., Gao S. (2015). Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 22 2837–2845. 10.1007/s11356-014-3525-0 [DOI] [PubMed] [Google Scholar]
- Wang S., Wang F., Gao S., Wang X. (2016). Heavy metal accumulation in different rice cultivars as influenced by foliar application of nano-silicon. Water Air Soil Pollut. 227:228. 10.1007/s11270-016-2928-6 [DOI] [Google Scholar]
- Wang X., Ou-yang C., Fan Z. R., Gao S., Chen F., Tang L. (2010). Effects of exogenous silicon on seed germination and antioxidant enzyme activities of Momordica charantia under salt stress. J. Anim. Plant Sci. 6 700–708. [Google Scholar]
- Wang X., Wei Z., Liu D., Zhao G. (2011). Effects of NaCl and silicon on activities of antioxidative enzymes in roots, shoots and leaves of alfalfa. Afr. J. Biotechnol. 10 545–549. 10.5897/AJB10.1353 [DOI] [Google Scholar]
- Waraich E. A., Ahmad R., Ashraf M. Y. (2011). Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 5:764. [Google Scholar]
- White P. J., Pongrac P. (2017). “Heavy-metal toxicity in plants,” in Plant Stress Physiology, ed. Shabala S. (Wallingford: CABI; ), 300–331. [Google Scholar]
- Wong M. H., Misra R. P., Giraldo J. P., Kwak S. Y., Son Y., Landry M. P., et al. (2016). Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett. 16 1161–1172. 10.1021/acs.nanolett.5b04467 [DOI] [PubMed] [Google Scholar]
- Wu H., Li Z. (2022). Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 10 1–12. [Google Scholar]
- Wu H., Shabala L., Shabala S., Shabala S., Giraldo J. P. (2018). Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 5 1567–1583. 10.1039/c8en00323h [DOI] [Google Scholar]
- Yan S., Wu F., Zhou S., Yang J., Tang X., Ye W. (2021). Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease them accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 21:150. 10.1186/s12870-021-02929-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Hong F., You W., Liu C., Gao F., Wu C., et al. (2006). Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 110 179–190. [DOI] [PubMed] [Google Scholar]
- Yang K. Y., Doxey S., McLean J. E., Britt D., Watson A., Al Qassy D., et al. (2018). Remodeling of root morphology by CuO and ZnO nanoparticles: effects on drought tolerance for plants colonized by a beneficial pseudomonad. Botany 96 175–186. [Google Scholar]
- Yang L., Watts D. J. (2005). Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 158 122–132. 10.1016/j.toxlet.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Ye Y., Cota-Ruiz K., Hernandez-Viezcas J. A., Valdes C., Medina-Velo I. A., Turley R. S., et al. (2020). Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS Sustain. Chem. Eng. 8 1427–1436. 10.1021/acssuschemeng.9b05615 [DOI] [Google Scholar]
- Ye Y., Medina-Velo I. A., Cota-Ruiz K., MorenoOlivas F., Gardea-Torresdey J. (2019). Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 184:109671. 10.1016/j.ecoenv.2019.109671 [DOI] [PubMed] [Google Scholar]
- Yosefzaei F., Poorakbar L., Farhadi K. (2016). The effect of silver nanoparticles on morphological and physiological indexes of Ocimum basilicum L. Iranian J. Plant Physiol. Biochem. 1 63–73. [Google Scholar]
- Yousry C., Zikry P. M., Salem H. M., Basalious E. B., El-Gazayerly O. N. (2020). Integrated nanovesicular/self-nanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 10 801–814. 10.1007/s13346-020-00716-5 [DOI] [PubMed] [Google Scholar]
- Yuan J., Chen Y., Li H., Lu J., Zhao H., Liu M. M., et al. (2018). New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 8 1–9. 10.1038/s41598-017-18055-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar S. M., Nazir M., Kumar Agrawal G., Kim D. W., Rakwal R. (2010). Silicon in plant tolerance against environmental stressors: towards crop improvement using omics approaches. Curr. Proteomics 7 135–143. 10.2174/157016410791330507 [DOI] [Google Scholar]
- Ze Y., Liu C., Wang L., Hong M., Hong F. (2011). The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace Elem. Res. 143 1131–1141. 10.1007/s12011-010-8901-0 [DOI] [PubMed] [Google Scholar]
- Zhang H., Du W., Peralta-Videa J. R., Gardea-Torresdey J. L., White J. C., Keller A., et al. (2018). Metabolomics reveals how cucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress. Environ. Sci. Technol. 52 8016–8026. 10.1021/acs.est.8b02440 [DOI] [PubMed] [Google Scholar]
- Zhang S. (2019). Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. [master’s thesis]. Jinan: Shandong University. [Google Scholar]
- Zhang Y., Liu N., Wang W., Sun J., Zhu L. (2020). Photosynthesis and related metabolic mechanism of promoted rice (Oryza sativa L.) growth by TiO2 nanoparticles. Front. Environ. Sci. Eng. 14:103. 10.1007/s11783-020-1282-5 [DOI] [Google Scholar]
- Zhao L., Lu L., Wang A., Zhang H., Huang M., Wu H., et al. (2020). Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 68 1935–1947. 10.1021/acs.jafc.9b06615 [DOI] [PubMed] [Google Scholar]
- Zmeeva O. N., Daibova E. B., Proskurina L. D., Petrova L. V., Kolomiets N. E., Svetlichnyi V. A., et al. (2017). Effects of silicon dioxide nanoparticles on biological and physiological characteristics of Medicago sativa L. nothosubsp. varia (Martyn) in natural agroclimatic conditions of the subtaiga zone in Western Siberia. BioNanoSci 7 672–679. 10.1007/s12668-017-0395-1 [DOI] [Google Scholar]
- Zulfiqar F., Navarro M., Ashraf M., Akram N. A., Munné-Bosch S. (2019). Nanofertilizer use for sustainable agriculture: advantages and limitations. Plant Sci. 289 110270. 10.1016/j.plantsci.2019.110270 [DOI] [PubMed] [Google Scholar]