Abstract
Insect odorant-binding proteins (OBPs) are members of a rapidly evolving multigene family traditionally thought to facilitate chemosensation. However, studies on Drosophila have shown that members of this family have evolved functions beyond chemosensation, as evident from their expression in reproductive tissues and the brain. Previous studies implicated diverse functions of Obp56h, a member of the largest gene cluster of the D. melanogaster Obp repertoire. Here, we examined the effect of CRISPR/Cas9-mediated deletion of Obp56h on 2 fitness phenotypes, on resistance to starvation stress and heat stress, and on locomotion and sleep phenotypes. Obp56h−/− mutants show a strong sexually dimorphic effect on starvation stress survival, with females being more resistant to starvation stress than the control. In contrast, Obp56h−/− females, but not males, are highly sensitive to heat stress. Both sexes show changes in locomotion and sleep patterns. Transcriptional profiling of RNA from heads of Obp56h−/− flies and the wildtype control reveals differentially expressed genes, including gene products associated with antimicrobial immune responses and members of the Turandot family of stress-induced secreted peptides. In addition, differentially expressed genes of unknown function were identified in both sexes. Genes encoding components of the mitochondrial electron transport chain, cuticular proteins, gene products associated with regulation of feeding behavior (Lst and CCHa2), ribosomal proteins, lncRNAs, snoRNAs, tRNAs, and snRNAs show changes in transcript abundances in Obp56h−/− females. These differentially expressed genes are likely to contribute to Obp56h-mediated effects on the diverse phenotypes that arise upon deletion of this OBP.
Keywords: Obp56h, CRISPR-Cas9, pleiotropy, fitness, starvation resistance, heat stress, RNA sequencing, chemosensation, sleep, sexual dimorphism
Introduction
Multigene families that arise through repeated gene duplication events are one of the key drivers of adaptive evolution (Long et al., 2013; Magadum et al., 2013; Vaschetto and Ortiz, 2019). Functional redundancy within such gene families confers stability in the face of environmental fluctuations (Zhou et al., 2012), and relaxed selection pressures on daughter genes allow neofunctionalization and functional diversification. It is generally accepted that the diverse family of insect odorant-binding proteins (OBPs) evolved rapidly to facilitate chemosensation by promoting solubilization of airborne odorants in the perilymph surrounding olfactory sensory neurons (Hekmat-Scafe et al., 2002; Vieira et al., 2007; Pelosi et al., 2014; Sun, Xiao, et al., 2018). Although CRISPR-mediated deletion of a subset of Drosophila melanogaster Obp genes did not affect electrophysiological responses to odorants (Xiao et al., 2019), behavioral measurements using RNAi knockdown of Obps implicated combinatorial interactions between odorants and OBPs (Swarup et al., 2011), underscoring functional redundancy among members of this multigene family.
In addition to their role in olfaction, studies in D. melanogaster have shown that members of the Obp gene family have been co-opted for functions other than chemosensation. Obp59a has been implicated in humidity sensing (Sun, Larter, et al., 2018). CRISPR-mediated deletion of the Obp50a-d gene cluster followed by reinsertion of different combinations of paralogs provided evidence for functional diversification, redundancy, and epistasis among paralogs of this cluster and implicated Obp50a in development and Obp50d in stress resistance (Johnstun et al., 2021). Association analyses with wild-derived inbred D. melanogaster lines of the D. melanogaster genetic reference panel identified two polymorphic markers in Obp19d that were associated with variation in lifespan (Arya et al., 2010). A paralog of Obp19d, Obp19c, is expressed in ovaries and has been implicated in oviposition and postmating behavior (Arya et al., 2010). In addition, Obp8a is abundant in the male accessory gland. These observations suggest that OBP8a and OBP19c, and potentially other OBPs found in seminal fluid (McGraw et al., 2004; Findlay et al., 2008), may bind thus far unidentified hydrophobic molecules associated with the transfer of sperm during mating and stimulation of oviposition.
The Obp56 cluster is the largest gene cluster of the multigene Obp family in D. melanogaster. Within this cluster, Obp56h provides an ideal target for CRISPR/Cas9 gene editing, since it is a small gene (651 bp) without nested genes, and the nearest genes are 12,891 bp upstream and 10,374 bp downstream. RNA interference of Obp56h affects olfactory behavior (Swarup et al., 2011), avoidance of bitter tastants (Swarup et al., 2014), mating behavior (Shorter et al., 2016), and expression of co-regulated genes associated with lipid metabolism, immune/defense response, and heat stress (Shorter et al., 2016). Obp56h is expressed in the antenna (Galindo and Smith, 2001) and labellum (Galindo and Smith, 2001) and in cells of the central brain (Baker et al., 2021; Mokashi et al., 2021), which suggests functional pleiotropy at the Obp56h locus, i.e. diverse physiological roles of its gene product. Here, we show that CRISPR/Cas9-mediated deletion of Obp56h reveals sexually dimorphic effects on starvation stress resistance, resistance to heat stress, locomotion, and sleep patterns. In addition to effects on organismal phenotypes, transcriptional profiling shows differentially expressed genes between Obp56h−/− flies and the Canton S-B (CSB) control, associated with the diverse Obp56h-related phenotypes that are affected upon deletion of this odorant binding protein.
Materials and methods
Drosophila lines
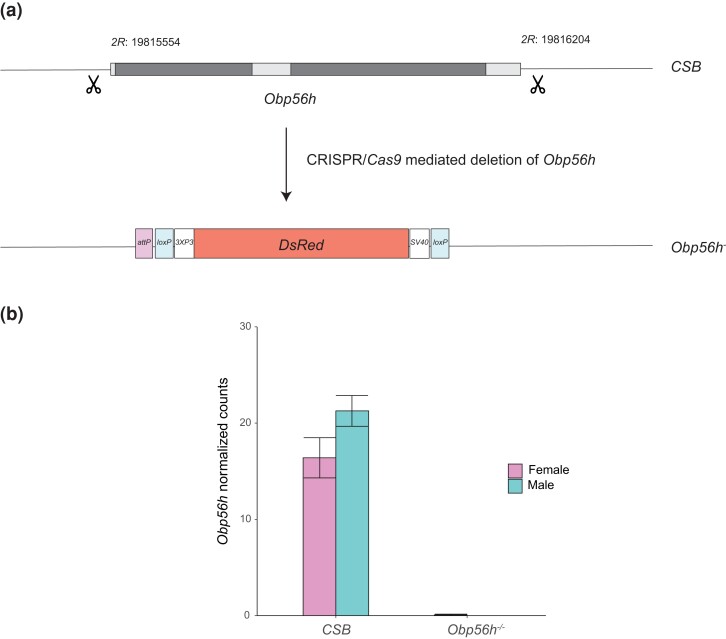
To generate a CRISPR/Cas9-mediated null allele of Obp56h in a CSB genetic background, we designed two guide RNAs flanking the gene using the Optimal Target Finder online tool (Gratz et al., 2014; Supplementary Table 1; Fig. 1a) and cloned them into the pU6-Bbs1-chiRNA plasmid. We used the pBS-Hsp70-Cas9 plasmid as a source for Cas9 and generated a donor plasmid containing 3XP3-driven DsRed flanked by 1kb sequences homologous to the regions flanking the Obp56h gene. This vector also has loxP sites flanking the DsRed cassette for subsequent removal of the cassette. We reared all flies at 25°C, 60–75% relative humidity, and a 12-hour light-dark cycle on standard cornmeal–molasses–agar medium. Before experimentation, we reared the flies for 2 generations at controlled densities (5 males and 5 females per vial allowed to lay eggs for 2 days). We used 3–5-day-old flies for all experiments.
Fig. 1.
CRISPR/Cas9-mediated deletion of Obp56h. a) Construction of the Obp56h null allele. Dark boxes represent exons of the Obp56h gene, and light boxes indicate the intron and 5′ and 3′ untranslated sequences. We designed guide RNAs flanking the Obp56h gene for CRISPR/Cas9-mediated deletion at the cut sites, indicated by the scissor symbols, in the CSB genetic background (Supplementary Table 1). We replaced the gene with a cassette that contains a DsRed fluorescent marker under the control of an eyespecific 3XP3 promoter and with SV40 polyadenylation sequences, loxP sites for Cre-mediated removal of the insert, and an attP site. b) Average normalized Obp56h cpm expression counts from whole genome RNA sequencing for males and females for Obp56h deletion flies and the CSB control (see also Supplementary Tables 3 and 4).
Quantification of organismal phenotypes
Starvation stress resistance
We used Drosophila activity monitors to measure starvation stress resistance. We placed one fly per tube containing starvation medium (1.5% agar in distilled water) and ran the assay for 4 days in accordance with previous work (Chiu et al., 2010), with a total of 64 flies per sex per genotype. We obtained activity bout data using Shiny-R DAM (Cichewicz and Hirsch, 2018) and used the time of last activity bout as the time of death.
Response to heat shock
The day before measuring the response to heat shock, flies of each genotype were lightly anesthetized with CO2 and sorted into single-sex groups of 20 individuals in standard vials containing 5 ml food. On the day of the heat stress exposure, flies from each replicate vial were transferred without anesthesia into vials without food and placed in an incubator at 37°C (±0.5°C) for 180 minutes. After heat stress exposure, flies were immediately transferred to vials containing 5 ml of standard cornmeal–agar–molasses medium and returned to the 25°C incubator for 24 hours. The percentage of surviving flies per vial was recorded 24 hours after the 3 hours heat shock. A fly was considered alive if it could move when the vial was gently tapped. We performed 5 replicates per genotype and sex.
Activity and sleep
We assessed total activity and proportion of sleep during the day and night (Shaw et al., 2000; Hendricks et al., 2000) using Drosophila Activity Monitors (TriKinetics). We ran the assay in accordance with previously published work (Chiu et al., 2010) and recorded data for 5 days on at least 64 flies per sex per genotype. We processed and analyzed the data using Shiny-R DAM (Cichewicz and Hirsh, 2018).
Statistical analyses of organismal quantitative traits
We assessed mean differences of phenotypic values using factorial, fixed effects ANOVA models: Y = μ + Genotype + Sex + Genotype × Sex + ɛ, where Y is the phenotype, μ is the overall mean, and ɛ is the residual (error) variance. All analyses were performed using SAS Studio release 3.71 (SAS Institute, Cary, NC, USA).
RNA sequencing
To prepare libraries for RNA sequencing, we collected 2 replicates/sex/genotype of 50 flies each, between 1 and 3 PM, and flash froze them on dry ice in 15 ml Falcon tubes (Thermo Fisher Scientific, Waltham, MA, USA). The flies were decapitated using a strainer (Carolina Biological Supply Company, Burlington, NC, USA) for head collections. The heads were collected on a dry ice-cooled fly pad and placed in 2 ml pre-filled tough microfuge tubes with glass beads. Total RNA was extracted using the Direct-Zol microprep kit RNA extraction protocol (Zymo Research, Irvine, CA, USA). The heads were homogenized in a bead mill (Thermofisher) for 1 minute at 4 m/s, after which the RNA was eluted with 15 μl water. We depleted ribosomal RNA using the NuQuant + UDI, Drosophila AnyDeplete kit (Tecan, Männedorf, Switzerland) and prepared bar-coded cDNA libraries for paired end sequencing on an S1 flow cell on the NovaSeq 6000 platform (Illumina, San Diego, CA, USA) using a 2 × 150 bp chemistry with an average of 36 million raw reads resulting in 20 million aligned reads and an average read length of 250–300 bp, as described previously (Johnstun et al., 2021).
Analysis of RNA sequences
We performed the initial steps of raw read processing and normalization of expression as previously described (Johnstun et al., 2021). Briefly, we used the AfterQC pipeline (Chen et al., 2017) to trim adapters, detect abnormal polynucleotide sequences, filter for low quality (Q < 20) and short (<35 nt) sequence reads, and generate basic sequence quality metrics. We used the bbduk command from the BBTools package (Bushnell, 2018) to detect rRNA contamination. We aligned high-quality sequence reads to the D. melanogaster reference genome release 6 (version 6.13) using GSNAP aligner (Wu and Nacu, 2010) and mapped unique alignments to genes using the Subread package (Liao et al., 2013). We used GeTMM (Smid et al., 2018) to normalize filtered expression counts. Differential expression between null and CSB within each sex was assessed using separate contrast statements after fitting a GLM model to the normalized counts in edgeR (Robinson et al., 2010). Prior to fitting, genes were filtered for expression using default parameters part of the filterbyexp function. Raw P-values derived from GLM likelihood ratio Test were adjusted for multiple hypothesis testing using Benjamini–Hochberg false discovery rate (BH-FDR) method and genes with BH–FDR < 0.05 were considered statistically significantly differentially expressed. We performed functional enrichment analysis on the differentially expressed genes for biological processes and Reactome pathways using Panther (Mi et al., 2017).
Results
Effects of Obp56h alleles on organismal phenotypes
We constructed an Obp56h deletion line using CRISPR/Cas9 technology (Fig. 1a) and confirmed the deletion of Obp56h with appropriate primers for Sanger sequencing (Supplementary Table 1) and the absence of its transcript from whole genome RNA sequences (Fig. 1b). Removal of Obp56h did not affect sex ratio or viability.
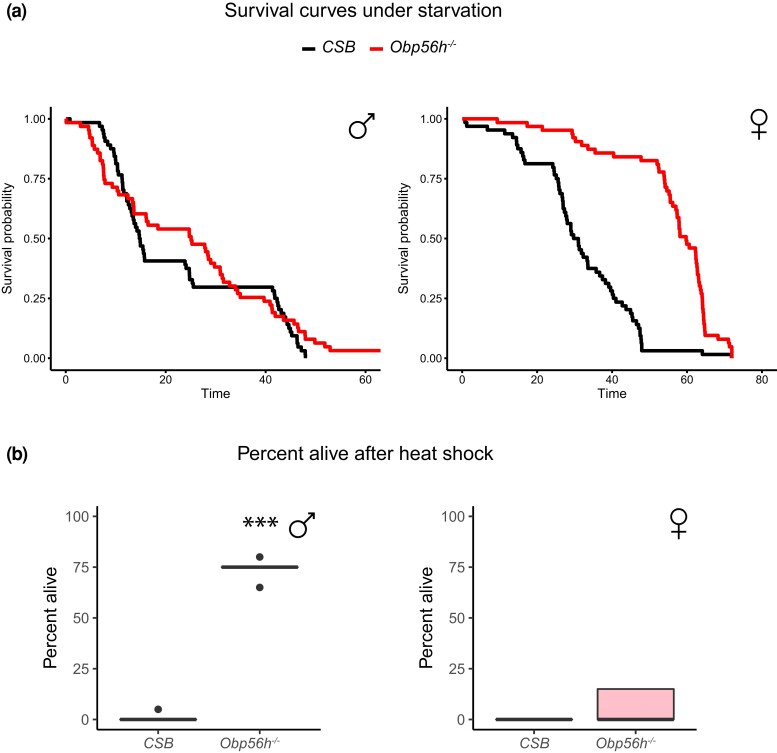
We assessed the effects of Obp56h deletion on starvation stress resistance, heat stress resistance, locomotor activity, and sleep traits (Supplementary Table 2). CSB females were more resistant to starvation stress than males. Deletion of Obp56h did not affect starvation resistance of males but greatly increased starvation stress resistance of Obp56h−/− females doubling their mean survival time (P < 0.0001; Fig. 2a). Obp56h−/− females were as sensitive to heat stress as the CSB control with few survivors. Males, however, were resistant to heat shock with about 75% survival compared to CSB [(P < 0.0001); Fig. 2b]. Thus, deletion of Obp56h has sexually dimorphic effects on stress resistance, with protective effects for females under starvation stress and for males under heat shock conditions.
Fig. 2.
Pleiotropic effects of Obp56h alleles on fitness-related quantitative traits. a) Survival curves under starvation stress. The black survival curve represents the CSB control, and the red survival curve represents the Obp56h−/− flies. b) Heat shock survival. Males are indicated in blue and females in pink. ***P < 0.0001 (Supplementary Table 2).
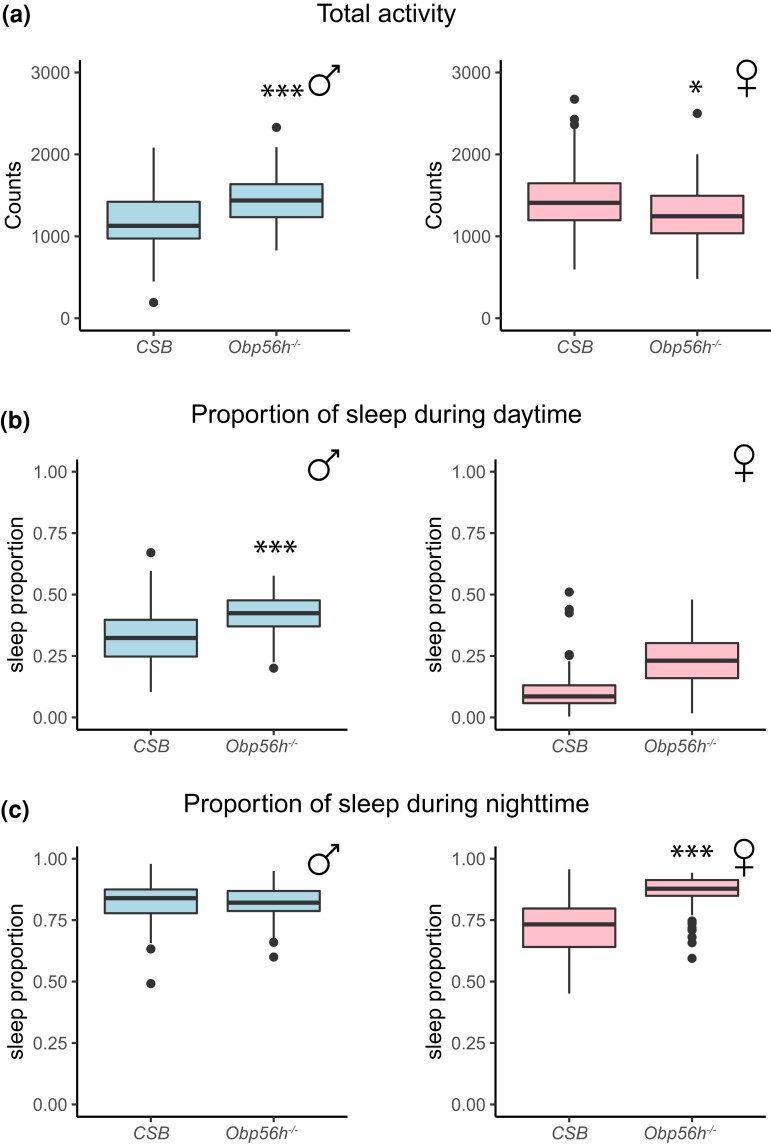
Deletion of Obp56h also affects locomotion and sleep. Males show an increase in total locomotion, and this increased activity is accompanied by an extended sleep proportion during the day (Fig. 3a; Supplementary Table 2). In contrast, females showed reduced locomotor activity and increased sleep proportions during the day and night (Fig. 3b and c; Supplementary Table 2).
Fig. 3.
Effects of Obp56h alleles on activity and sleep phenotypes. Box plots showing total activity (a), sleep proportion during the day (b), and sleep proportion during the night (c) for sexes separately. *P < 0.05; **P < 0.001; ***P < 0.0001 (Supplementary Table 2).
Effects of Obp56h alleles on genome-wide gene expression
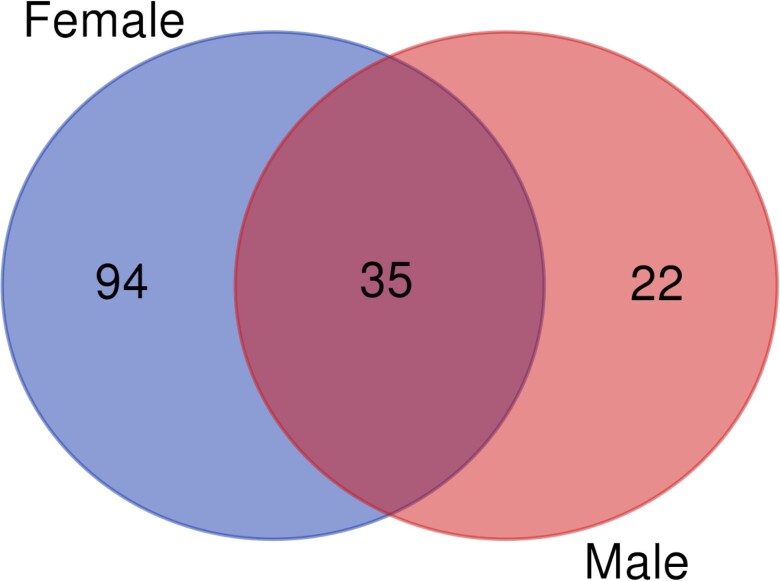
To gain insights into the cellular processes that might underlie the observed sexually dimorphic pleiotropic effects of Obp56h, we identified differentially expressed genes in the Obp56h−/− flies compared to the CSB control. The D. melanogaster transcriptome is highly modular (Ayroles et al., 2009). Mutations that disrupt a gene associated with complex organismal phenotypes generate trans-regulatory effects resulting in changes in co-regulated ensembles of genes (Anholt et al., 2003; Arya et al., 2010; Boyle et al., 2017; Anholt and Mackay, 2018). We obtained whole transcriptome profiles on heads of males and females separately (Supplementary Table 3). At a false discovery rate (FDR) < 0.05, we identified 130 differentially expressed genes in females and 57 in males (Supplementary Table 4; Fig. 4). Thirty-five genes were common among both sexes, including CG17224, predicted to encode a uridine phosphorylase (Gaudet et al., 2011), DNAJ-H, which encodes a chaperone heat shock protein (Gaudet et al., 2011; Whitaker et al., 2013), genes associated with antimicrobial immune responses, including def, Attb, DptA, CecA1 and pirk, and predicted genes with unknown functions. Long noncoding RNAs, snoRNAs, and tRNAs also show differential expression in both males and females. Most of the male-specific genes are genes of unknown function. As expected, expression of Obp56h was absent in both Obp56h−/− males and females compared to the CSB control.
Fig. 4.
Venn diagram of differentially expressed genes across both sexes in Obp56h−/− flies compared to the control.
Along with lncRNAs and snoRNAs, several snRNAs are differentially expressed in females, which are associated with control of mRNA splicing (Wilkinson et al., 2020). Genes that encode cuticular proteins (Cpr49Ae, Cpr100A, Acp1, and Edg91) are differentially expressed in females, along with CG12895, which is associated with the assembly of succinate dehydrogenase, ribosomal proteins (RpS2, RpS3, RpS15Ab, RpL3, RpL7 and RpL28), and components of the mitochondrial electron transfer chain (CG40472, Mt:ND2 and Mt:ND6) (Supplementary Table 4).
Several transcripts that are responsive to stress are also differentially expressed. In females, these include TotA, TotC, and TotM, and in males TotX, members of the Turandot family of peptides that are secreted in response to stress (Ekengren and Hultmark, 2001; Brun et al., 2006), in addition to the heat stress-related DNAJ-H chaperone (Supplementary Table 4). Obp56h−/− females show differential expression of Lst, which encodes the peptide hormone limostatin that is released by the corpora allata during starvation and suppresses insulin secretion (Alfa et al., 2016). CCHa2, which encodes a ligand for the CCHamide-2 receptor that stimulates food intake (Ida et al., 2012; Ren et al., 2015), is also differentially expressed in Obp56h−/− females (Supplementary Table 4).
Discussion
We used CRISPR/Cas9-mediated gene deletion to assess pleiotropic effects on fitness-related quantitative traits and changes in genome-wide gene expression upon deletion of Obp56h. We chose Obp56h for its favorable properties for CRISPR/Cas9 gene editing and because previous studies suggested that this gene might have pleiotropic effects on the transcriptome and organismal phenotypes (Swarup et al., 2011, 2014; Shorter et al., 2016). We found considerable sexual dimorphism in the effects of Obp56h deletion on survival under starvation and heat stress, locomotor activity, and day and night sleep. Obp56h−/− mutants show a strong sexually dimorphic effect on starvation stress survival, with females being more resistant to starvation stress than the control. In contrast, Obp56h−/− females, but not males, are highly sensitive to heat stress. The molecular mechanisms and cellular processes that give rise to these sexually dimorphic effects that result from deletion of Obp56h remain to be determined. We cannot exclude additional organismal phenotypes that may be affected by deletion of Obp56h. Whole-genome transcript analyses showed common genes affected by Obp56h deletion in both sexes as well as sexually dimorphic differences in differential gene expression (Supplementary Table 4). The mechanisms by which mutations in a focal gene cause correlated changes in coregulated genes are not known. Such changes may change the function of neurons or glia in the brain and could possibly also result in trans-cellular effects on gene expression.
It is not surprising that there are differences in differential gene expression in Obp56h knock-out flies between males and females. There is a vast body of evidence in the literature that the genetic architectures that underlie complex traits are distinct between males and females. What gives rise to these differences is a big question in quantitative genetics that has not yet been resolved.
Differential expression of Antennal dehydrogenase (Antdh) in both sexes and Obp56a and Obp19d in females (Supplementary Table 4) is consistent with a previously reported role for Obp56h in chemosensation (Swarup et al., 2011, 2014). A previous study, in which expression of Obp56h was reduced through RNA interference, also found up-regulation of antimicrobial response peptides and Turandot peptides in heads of males and females (Shorter et al., 2016). This study also reported changes in cuticular pheromones in whole flies upon RNAi-targeted inhibition of Obp56h under a Dll-GAL4 driver (Shorter et al., 2016).
It is intriguing to note that the increased starvation stress resistance of Obp56h−/− females is consistent with a reduction in transcript abundance for limostatin, which suppresses insulin production, thereby allowing increased levels of insulin during starvation stress (Alfa et al., 2016), while up-regulation of the orexigenic peptide CCHa2 enhances appetite (Ren et al., 2015). We can speculate that increased mitochondrial metabolism, as reflected by up-regulation of electron transfer components, might offer protection during starvation stress by promoting energy generation to maintain cellular integrity. It is possible that changes in gene expression that promote resistance to starvation stress are not protective against heat stress.
The ligand(s) for Obp56h in the brain is unknown but could be hydrophobic metabolites, which play a role in fundamental cellular processes. In addition, the link between Obp56h and gene regulation remains unknown. Altered expression of lncRNAs that may control gene expression, snRNAs that regulate splicing (Wilkinson et al., 2020), and snoRNAs associated with ribosomal function (Reichow et al., 2007) may be part of the mechanisms that regulate gene expression upon deletion of Obp56h.
The pleiotropic fitness effects of this member of the Obp family are likely not unique to Obp56h but may also pertain to other members of this rapidly evolving gene family. The results from this study, together with previous observations by us (Arya et al., 2010; Johnstun et al., 2021) and others (McGraw et al., 2004; Findlay et al., 2008; Sun, Larter, et al., 2018), contribute to the mounting evidence that the functions of OBPs extend well beyond chemosensation and affect a variety of fundamental cellular processes.
Supplementary Material
Acknowledgments
We thank Dr. Lakshmi Sunkara for help with RNA sequencing and Rachel C. Hannah for technical assistance with behavioral assays.
Contributor Information
Sneha S Mokashi, Department of Genetics and Biochemistry and Center for Human Genetics, Clemson University, 114 Gregor Mendel Circle, Greenwood, SC 29646, USA.
Vijay Shankar, Department of Genetics and Biochemistry and Center for Human Genetics, Clemson University, 114 Gregor Mendel Circle, Greenwood, SC 29646, USA.
Joel A Johnstun, Department of Biological Sciences, Program in Genetics, North Carolina State University, Raleigh, NC 27695, USA.
Trudy F C Mackay, Department of Genetics and Biochemistry and Center for Human Genetics, Clemson University, 114 Gregor Mendel Circle, Greenwood, SC 29646, USA.
Robert R H Anholt, Department of Genetics and Biochemistry and Center for Human Genetics, Clemson University, 114 Gregor Mendel Circle, Greenwood, SC 29646, USA.
Data availability
RNA sequence data have been deposited in the GEO repository under accession number GSE215148. All raw data and code are available at: https://github.com/snehamokashi/Obp56h_KO_vs_CSB.git.
Supplemental material available at G3 online.
Communicating editor: S. Lott
Literature cited
- Alfa RW, Park S, Skelly K-R, Poffenberger G, Jain N, Gu X, Kockel L, Wang J, Liu Y, Powers AC, et al. Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 2018;27(2):479. doi: 10.1016/j.cmet.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt RRH, Dilda CL, Chang S, Fanara J-J, Kulkarni NH, Ganguly I, Rollmann SM, Kamdar KP, Mackay TFC. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat Genet. 2003;35(2):180–184. doi: 10.1038/ng1240. [DOI] [PubMed] [Google Scholar]
- Anholt RRH, Mackay TFC. The road less traveled: from genotype to phenotype in flies and humans. Mamm Genome. 2018;29(1–2):5–23. doi: 10.1007/s00335-017-9722-7. [DOI] [PubMed] [Google Scholar]
- Arya GH, Weber AL, Wang P, Magwire MM, Negron YL, Mackay TFC, Anholt RRH. Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics. 2010;186(4):1475–1485. doi: 10.1534/genetics.110.123166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, Rollmann SM, Duncan LH, Lawrence F, Anholt RRH, et al. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet. 2009;41(3):299–307. doi: 10.1038/ng.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Mokashi SS, Shankar V, Hatfield JS, Hannah RC, Mackay TFC, Anholt RRH. The Drosophila brain on cocaine at single-cell resolution. Genome Res. 2021;31(10):1927–1937. doi: 10.1101/gr.268037.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017;169(7):1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun S, Vidal S, Spellman P, Takahashi K, Tricoire H, Lemaitre B. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cells. 2006;11(4):397–407. doi: 10.1111/j.1365-2443.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- Bushnell B. BBTools: a suite of fast, multithreaded bioinformatics tools designed for analysis of DNA and RNA sequence data. Joint Genome Institute; 2018. Available from: https://jgi.doe.gov/data-and-tools/bbtools/. [Google Scholar]
- Chen S, Huang T, Zhou Y, Han Y, Xu M, Gu J. AfterQC: automatic filtering, trimming, error removing and quality control for fastq data. BMC Bioinformatics. 2017;18(1):91–100. doi: 10.1186/s12859-017-1498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J Vis Exp. 2010;43:2157. doi: 10.3791/2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz K, Hirsh J. ShinyR-DAM: a program analyzing Drosophila activity, sleep and circadian rhythms. Commun Biol. 2018;1(1):25. doi: 10.1038/s42003-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekengren S, Hultmark D. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem Biophys Res Commun. 2001;284(4):998–1003. doi: 10.1006/bbrc.2001.5067. [DOI] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6(7):e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo K, Smith DP. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 2001;159(3):1059–1072. doi: 10.1093/genetics/159.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet P, Livstone MS, Lewis SE, Thomas PD. Phylogenetic-based propagation of functional annotations within the gene ontology consortium. Brief Bioinform. 2011;12(5):449–462. doi: 10.1093/bib/bbr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O'Connor-Giles KM. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014;196(4):961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 2002;12(9):1357–1369. doi: 10.1101/gr.239402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. doi: 10.1016/S0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Ida T, Takahashi T, Tominaga H, Sato T, Sano H, Kume K, Ozaki M, Hiraguchi T, Shiotani H, Terajima S, et al. Isolation of the bioactive peptides CCHamide-1 and CCHamide-2 from Drosophila and their putative role in appetite regulation as ligands for G protein-coupled receptors. Front Endocrinol (Lausanne). 2012;3:177. doi: 10.3389/fendo.2012.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstun JA, Shankar V, Mokashi SS, Sunkara LT, Ihearahu UE, Lyman RL, Mackay TFC, Anholt RRH. Functional diversification, redundancy, and epistasis among paralogs of the Drosophila melanogaster Obp50a-d gene cluster. Mol Biol Evol. 2021;38(5):2030–2044. doi: 10.1093/molbev/msab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. The subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41(10):e108–e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, VanKuren NW, Chen S, Vibranovski MD. New gene evolution: little did we know. Annu Rev Genet. 2013;47(1):307–333. doi: 10.1146/annurev-genet-111212-133301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadum S, Banerjee U, Murugan P, Gangapur D, Ravikesavan R. Gene duplication as a major force in evolution. J Genet. 2013;92(1):155–161. doi: 10.1007/s12041-013-0212-8. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14(16):1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER Version 11: expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45(D1):D183–D189. doi: 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokashi SS, Shankar V, MacPherson RA, Hannah RC, Mackay TFC, Anholt RRH. Developmental alcohol exposure in Drosophila: effects on adult phenotypes and gene expression in the brain. Front Psychiatry. 2021;12:699033. doi: 10.3389/fpsyt.2021.699033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014;5:320. doi: 10.3389/fphys.2014.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, Didriksen AK, Schjøtt SR, Sembach FE, Li S, Søgaard KC, et al. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLoS One. 2015;10(7):e0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichow SL, Hamma T, Ferré-D'Amaré AR, Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35(5):1452–1464. doi: 10.1093/nar/gkl1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. Edger: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shorter JR, Dembeck LM, Everett LJ, Morozova TV, Arya GH, Turlapati L, St Armour GE, Schal C, Mackay TFC, Anholt RRH. Obp56h modulates mating behavior in Drosophila melanogaster. G3 (Bethesda). 2016;6(10):3335–3342. doi: 10.1534/g3.116.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M, Coebergh van den Braak R, van de Werken H, van Riet J, van Galen A, de Weerd V, van der Vlugt-Daane M, Bril SI, Lalmahomed ZS, Kloosterman WP, et al. Gene length corrected trimmed mean of M-values (GeTMM) processing of RNA-seq data performs similarly in intersample analyses while improving intrasample comparisons. BMC Bioinformatics. 2018;19(1):236. doi: 10.1186/s12859-018-2246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JS, Larter NK, Chahda JS, Rioux D, Gumaste A, Carlson JR. Humidity response depends on the small soluble protein Obp59a in Drosophila. eLife. 2018;7:e39249. doi: 10.7554/eLife.39249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JS, Xiao S, Carlson JR. The diverse small proteins called odorant-binding proteins. Open Biol. 2018;8(12):180208. doi: 10.1098/rsob.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S, Williams TI, Anholt RRH. Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 2011;10(6):648–657. doi: 10.1111/j.1601-183X.2011.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S, Morozova TV, Sridhar S, Nokes M, Anholt RR. Modulation of feeding behavior by odorant-binding proteins in Drosophila melanogaster. Chem Senses. 2014;39(2):125–132. doi: 10.1093/chemse/bjt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschetto LM, Ortiz N. The role of sequence duplication in transcriptional regulation and genome evolution. Curr Genomics. 2019;20(6):405–408. doi: 10.2174/1389202920666190320140721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira FG, Sánchez-Gracia A, Rozas J. Comparative genomic analysis of the odorant-binding protein family in 12 Drosophila genomes: purifying selection and birth-and-death evolution. Genome Biol. 2007;8(11):R235. doi: 10.1186/gb-2007-8-11-r235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R, Faulkner S, Miyokawa R, Burhenn L, Henriksen M, Wood JG, Helfand SL. Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner. Aging. 2013;5(9):682–691. doi: 10.18632/aging.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ME, Charenton C, Nagai K. RNA Splicing by the spliceosome. Annu Rev Biochem. 2020;89(1):359–388. doi: 10.1146/annurev-biochem-091719-064225. [DOI] [PubMed] [Google Scholar]
- Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26(7):873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Sun JS, Carlson JR. Robust olfactory responses in the absence of odorant binding proteins. Elife. 2019;8:e51040. doi: 10.7554/eLife.51040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Campbell TG, Stone EA, Mackay TFC, Anholt RRH. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet. 2012;8(3):e1002593. doi: 10.1371/journal.pgen.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequence data have been deposited in the GEO repository under accession number GSE215148. All raw data and code are available at: https://github.com/snehamokashi/Obp56h_KO_vs_CSB.git.
Supplemental material available at G3 online.