Abstract
Circadian (24-h) clocks are cell-autonomous biological oscillators that orchestrate many aspects of our physiology on a daily basis. Numerous circadian rhythms in mammalian and non-mammalian retinas have been observed and the presence of an endogenous circadian clock has been demonstrated. However, how the clock and associated rhythms assemble into pathways that support and control retina function remains largely unknown. Our goal here is to review the current status of our knowledge and evaluate recent advances. We describe many previously-observed retinal rhythms, including circadian rhythms of morphology, biochemistry, physiology, and gene expression. We evaluate evidence concerning the location and molecular machinery of the retinal circadian clock, as well as consider findings that suggest the presence of multiple clocks. Our primary focus though is to describe in depth circadian rhythms in the light responses of retinal neurons with an emphasis on clock control of rod and cone pathways. We examine evidence that specific biochemical mechanisms produce these daily light response changes. We also discuss evidence for the presence of multiple circadian retinal pathways involving rhythms in neurotransmitter activity, transmitter receptors, metabolism, and pH. We focus on distinct actions of two dopamine receptor systems in the outer retina, a dopamine D4 receptor system that mediates circadian control of rod/cone gap junction coupling and a dopamine D1 receptor system that mediates non-circadian, light/dark adaptive regulation of gap junction coupling between horizontal cells. Finally, we evaluate the role of circadian rhythmicity in retinal degeneration and suggest future directions for the field of retinal circadian biology.
Keywords: retina, circadian clock, circadian rhythmicity, energy metabolism, melatonin, dopamine, adenosine, pH, gap junctions, electrical synapses
1. Introduction
The earliest reported observation of a rhythmic event in a vertebrate retina that persisted in constant environmental conditions with a period of about 24 h was probably made by Welsh and Osborne (1937) in the brown bullhead catfish (Ictalurus nebulosus). Their work showed for the first time that when fish were kept in constant darkness, the distribution of retinal pigments changed between day and night, similarly to that in fish kept in a light/dark (L/D) cycle. Since then, many rhythmic processes have been documented in retinas from all vertebrate classes, and the presence of a circadian clock in the retina itself has been conclusively established. Retinal rhythms combine with light/dark adaptive processes to modulate the physiology of the retina, as evidenced by circadian rhythms of one or more components of the electroretinogram (ERG), light responses of cones, and psychophysical measurements. Extensive research in this field during the last 30 years has generated a substantial number of review articles (Cahill and Besharse, 1995; Barlow, 2001; Mangel, 2001; Tosini and Fukuhara, 2002; Green and Besharse, 2004; Iuvone et al., 2005; Tosini et al., 2008; Mangel and Ribelayga, 2010; McMahon et al., 2014; Besharse and McMahon, 2016; Felder-Schmittbuhl et al., 2018; Ko, 2020).
Major advances have been made in our understanding of the circadian clock organization of retinal function. Accumulative evidence now indicates that virtually every cell type in the retina contains a functional clock mechanism and that clocks are important for retinal cell function, survival, and maintenance. These discoveries have triggered intensive research to understand the local influence of individual clock cell types on retinal circuitry and how they interact with each other. In addition, during the last 25 years, electrophysiological recordings, mainly from single cells in intact retinal tissue, have yielded interesting insights into circadian clock control of functional pathways in the retina.
One of the most surprising findings in circadian research of the retina was the observation that dark-adapted cones (and their post-synaptic targets) in intact retinal tissue at night – in contrast to dark-adapted isolated cones – are almost as sensitive as rods to very dim (low scotopic) light stimuli (Wang and Mangel, 1996; Ribelayga et al., 2008). This finding challenged a long-standing idea about rods and cones. Based on recordings of isolated dark-adapted rods and cones, it had been accepted for decades that cones require light stimuli 100x-1000x more intense to produce a threshold response than do rods. In other words, it was thought that there is a range of very dim stimul (i.e., scotopic) to which rods, but not cones, respond. However, experiments performed under conditions of constant darkness in the day and night have demonstrated that cones in intact neural tissue respond to very dim scotopic stimuli because the retinal clock opens wide rod/cone gap junctions at night, which allows rod signals of very dim stimuli to reach cones. Therefore, both rods and cones respond to very dim scotopic light stimuli, but rods (and not cones) initiate the retinal response to scotopic illumination.
Here, we review the literature on circadian rhythms in the vertebrate retina with a focus on how the retinal circadian clock controls neuronal light responses and rod and cone pathways. In addition, we discuss evidence that suggests the presence of distinct clock pathways that control retinal processing during day and night. We will limit this review to the vertebrate retina. Circadian rhythmicity in invertebrate visual systems has been reviewed by others (Barlow et al., 2001; Battelle, 2002; Dunlap et al., 2003; Ashmore and Sehgal, 2003; Battelle, 2013).
2. General properties of circadian clock systems
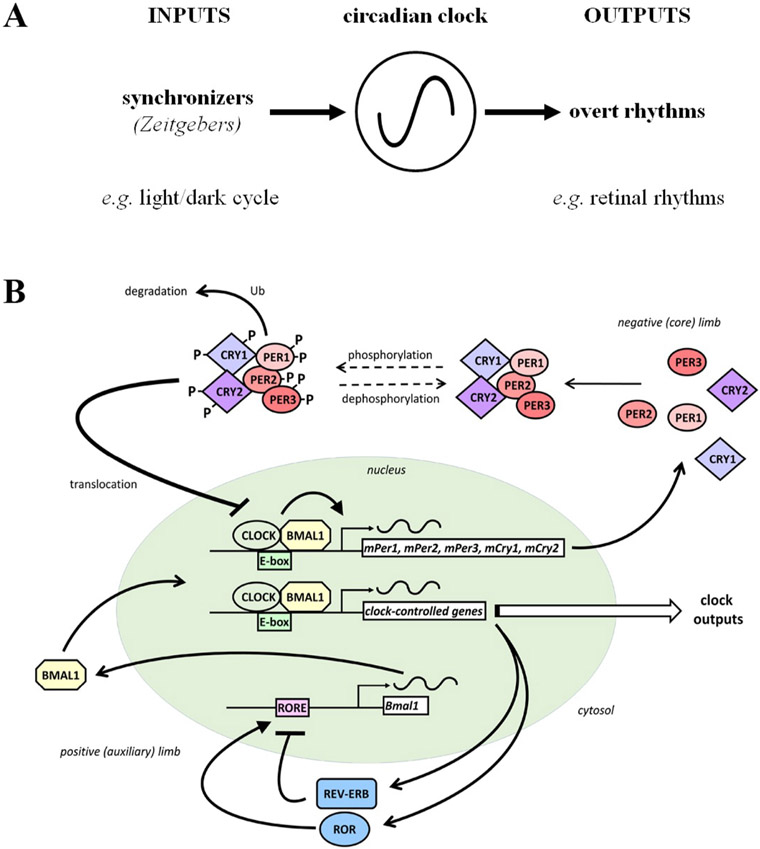
Daily and seasonal biological processes are governed by rhythmic geophysical phenomena: namely, the Earth’s rotation on its axis and its orbit around the Sun. As a result, life has evolved to adapt to periodically changing environmental demands by anticipating these demands given their predictability. This anticipatory activity has been shown to rely on endogenous biological clocks, which continue to run in constant environmental conditions (i.e. constant darkness). The most studied of these biological clock has a period of approximately 24 h, hence circadian clock, and can be synchronized to environmental rhythms through external cues called zeitgebers (German for “time-giver”). Of these, the light/dark (L/D) cycle is the most important, conveying information about the phases of both daily and annual geophysical cycles (Pittendrigh, 1981, 1993; Takahashi et al., 2001, 2017; Dunlap et al., 2003; Buhr and Takahashi, 2013). Figure 1A shows a characteristic representation of a circadian clock with its three levels of organization: inputs, core clock mechanism, and outputs.
Fig. 1. Schematic representation of a simplified circadian clock pathway.
(A) A simplified circadian clock pathway has three components. First, there is a molecular mechanism, which is referred to as a clock, clockwork, or a pacemaker, that has a period of approximately 24 hours and is able to maintain its daily rhythmicity in the absence of environmental cues (e.g., in constant darkness and temperature). Second, although circadian clocks can maintain rhythmicity in a constant environment, they are entrained or synchronized to the local environment by zeitgebers (German for time-giver). For example, the daily light/dark cycle acts as an input to clocks because the onset of morning light resets the “hands” of clocks so that they are set to local time, even though environments do not alter the period of clocks. Third, circadian clocks produce daily rhythms at the molecular, cellular, and systems levels. For example, the circadian clock in the retina produces a variety of daily rhythms within the retina, including increasing the hormone melatonin at night, decreasing the neurotransmitter dopamine in the day, and enabling cone photoreceptors to respond to very dim (scotopic) light stimuli at night (but not in the day). Because circadian clocks are affected by environmental stimuli, circadian experiments, which aim to determine whether time of day or night affects a function (e.g., level of extracellular dopamine), are conducted in the absence of environmental cues (e.g., under conditions of constant darkness and temperature). In a circadian experiment, the terms “subjective day” and “subjective night” refer to the day and night of the imposed light/dark cycle, respectively, when animals or isolated intact retinas were maintained in constant darkness.
(B) Simplified representation of the core components of the mammalian circadian clockwork. See text for details.
During the last 50 years, considerable work has laid down the fundamental properties of circadian clocks. In mammals, a circadian clock was localized for the first time in 1972 in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore and Eichler, 1972; Stephan and Zucker, 1972). The spectacular effect of SCN lesions on the circadian rhythms of drinking and locomotor activity in rodents helped forge the classical view of a circadian system organized around a principal clock in the brain which signals to tissues throughout the body. Through studying the SCN clock, a model of the molecular components that work together to produce 24-hour rhythms was proposed (Panda et al., 2002; Reppert and Weaver, 2002; Lowrey and Takahashi, 2004). This clock mechanism or clockworks within SCN neurons is responsible for the robust circadian rhythmic neuronal spiking observed both in vivo and in vitro (Lowrey and Takahashi, 2004).
Subsequent discoveries revealed that the core mechanism of the clock is contained within single SCN cells and relies on the self-sustained rhythmic expression of a specific set of genes (the clock genes) and their protein products (the clock proteins). The rhythmic expression is maintained through the interaction of transcriptional-translational feedback loops (reviewed in Takahashi, 2017). In mammals, the network relies on circadian locomotor output cycles protein kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) proteins which form a dimer (CLOCK:BMAL1) that binds to E-box sequences. CLOCK:BMAL1 serves as a transcription factor to activate the “clock” genes Period (Per) 1-3 and Cryptochrome (Cry) 1-2, whose protein products repress their own transcription through the repression of CLOCK:BMAL1, forming a negative feedback loop (Figure 1B). Additionally through E-box binding, CLOCK:BMAL1 proteins activate retinoic acid receptor like orphan receptor α/β/γ (RORα/β/γ) and Reverse Erythroblastosis α/β (nuclear receptor subfamily 1, group D, member1/2) α/β (REV-ERBα/β) gene expression, whose proteins initiate and inhibit Bmal1 transcription through ROR element (RORE) binding sites, respectively. These feedback loops take ~24 hours to complete. They interact to generate rhythmic expression not only of the clock genes but also of a large portion of the transcriptome (Burh and Takahashi, 2013; Zhang et al., 2014; Takahashi, 2017). Furthermore, the circadian clock has been shown to rely on a number of post-transcriptional mechanisms (Kojima et al., 2011; Takahashi, 2017; Green, 2018). Altogether, these translational and post-translational mechanisms within SCN cells subsequently translate into daily modulation of neuronal activity and eventually that of behavior (Koike et al., 2012; Cox and Takahashi, 2019).
Although the circadian clock molecular machinery has been extensively studied in mammals, it is surprisingly very similar in other vertebrates (Panda et al., 2002). In addition, in fungi, plants and bacteria, the clock mechanism still shares the same conceptual similarity to the mammalian clock in that it relies on interlocking transcriptional feedback loops (Dunlap et al., 2003; Refinetti, 2016). Thus, the transcriptional/translational molecular mechanism of circadian clocks is, with very few exceptions, universal.
A striking discovery following the identification of the clock genes in mammals was that their expression was not limited to the SCN cells but was widespread throughout the body (Lowrey and Takahashi, 2004; Panda and Hogenesch, 2004; Bell-Pedersen et al., 2005; Takahashi et al. 2008). These observations strongly challenged the view of a central principal clock and further suggested that the vertebrate timing system is in fact composed of numerous self-autonomous clocks dispersed in many organs and tissues. We now know that the SCN is not a “principal clock” but rather a “principal synchronizer” as it helps synchronize the entire circadian system, built on many clocks throughout the body, most of which harbor conserved core clock machinery (Mohawk et al., 2012; Takahashi, 2017). In mouse almost half of the transcriptome cycles in a circadian fashion (Zhang et al., 2014) and in primate up to 81.7% of protein-coding genes show daily rhythms of expression (Mure et al., 2018). Many of these transcriptional rhythms are tissue specific, in part as a result of varying contributions of the core clock genes (Panda et al., 2002; Storch et al., 2002; Panda and Hogenesch, 2004; Mohawk et al., 2012; Mure et al., 2018).
It is worth noting that retinal input is necessary to entrain circadian rhythms to daily L/D cycles in mammals (Yamazaki et al., 1999; Berson, 2003; Do, 2019). However, many non-mammalian vertebrates can use non-retinal photoreception in addition to retinal photoreception to entrain their clocks. For instance, reptiles, fish, birds, and amphibians have all been shown to use photoreceptors in the pineal gland to entrain to L/D cycles (Menaker, 1968; Mano and Fukada, 2006; Vatine et al., 2011; Cassone, 2014). Furthermore, in zebrafish, peripheral circadian clocks can be entrained by light without requiring direct input from the eyes, similar to Drosophila (Froland Steindal and Whitmore, 2019).
Deciphering the clock mechanism helped unravel a critical relationship between circadian rhythmicity and health. It has been known for a long time that perturbation of circadian rhythmicity by large shifts in the phase of the L/D cycle, such as during travel across time zones or shift working, accounts for many circadian-related malaises (e.g. diurnal sleepiness, depressed mood, decreased efficiency, premature awakening, alteration of reproduction) (Dunlap et al., 2003; Refinetti, 2016; Rijo-Ferreira and Takahashi, 2019). The importance of maintaining internal temporal homeostasis conferred by the circadian system has been clearly substantiated by animal models in which mutations in genes coding for core components of the clock result in serious diseases, including cancer (Fu and Lee, 2003; Lowrey and Takahashi, 2004; Lee, 2006; Takahashi et al., 2008; Masri and Sassone-Corsi, 2018; Sulli et al., 2019) and diabetes (Marcheva et al., 2010). Evidence also indicates that the circadian system is intimately linked to metabolism and the cell cycle (Green et al., 2008; Masri and Sassone-Corsi, 2018). Furthermore, a large portion of genes coding for druggable target proteins cycle in a rhythmic fashion (Zhang et al., 2014; Mure et al., 2018). Together, the data collected during the last two decades clearly support the view that circadian clock control expands down to very primary and vital functions of every cell in our body and that circadian rhythm dysregulation can contribute to disease.
Widespread clock gene expression has raised many interesting fundamental physiological and integrative issues that remain to be answered. How does a molecular clock regulate overt circadian rhythms? How are the individual activities of cellular clocks synchronized within a tissue? How are rhythms in various individual organs integrated into a coherent whole? How do clocks modulate neuronal activity and neural network function in the central nervous system (CNS) and eventually behavior? The retina has arguably emerged as the ideal model system to address circadian clock function in the CNS. The anatomy and functional organization of the retina are well known and its natural input (light) can be rigorously controlled experimentally, thereby constraining models of information processing. In addition, the retina is a great example of a self-optimizing network, which relies in part on circadian clocks intrinsic to the retina. Together, the interplay between ambient light and circadian clocks accounts for the changes in retinal computation that occur over the course of the day/night cycle. Below we review the current knowledge on retinal circadian clocks, associated clock pathways, and their impact on retinal physiology and function.
3. Circadian rhythmicity in the retina
The vertebrate retina is an integral part of the CNS (Rodieck, 1998; Dowling, 2012; see also http://webvision.med.utah.edu/) and functions as the first element of three different visual systems: (1) an image-forming visual system in which parallel signals from the retina reach sub-cortical regions of the brain such as the superior colliculus (optic tectum) enabling the non-conscious location of food and other objects, avoidance of predators, etc (Schneider, 1969; Fabbro et al., 2015); 2) a second image-forming visual system in which parallel channels from the retina reach the visual cortex allowing conscious perception of the visual environment (Rodieck, 1998; Dowling, 2012); and (3) the non-image-forming visual system, also called non-visual system, in which the retinal output signals the ambient light level in the day and controls non-conscious visual tasks such as the pupillary light reflex and circadian photoentrainment (Moore, 1996). In particular, the photic information that entrains the circadian system synchronizes many rhythmic physiological functions of organisms with the local environment. In mammals, the retina provides the only photic input to the circadian system (Miller et al., 1996; Moore, 1996; Yamazaki et al., 1999).
A key feature of the retina is its ability to adapt to a wide range of light intensities. Specifically, the retina is able to operate in starlight, in the midday sun, and at all times in between, during which the ambient or background illumination changes by 9 to 12 orders of magnitude (Rodieck, 1998; Mangel, 2001; Mangel and Ribelayga, 2010; Dowling, 2012). Light and dark adaptation in the retina rely on complex physiological, biochemical, and molecular mechanisms that are not yet fully understood. The switch between light- and dark-adapted states is particularly pronounced at dawn and dusk. Importantly, many of the L/D adaptive processes of the retina that normally arise at various times of the daily L/D cycle take place in the absence of light. That is, rhythmic reorganization of the morphology, biochemistry, and physiology of the retina persists to a great extent in constant darkness due to the activity of an endogenous circadian clock. Table 1 presents a survey of retinal circadian rhythms, including anatomical/morphological rhythms (Section 3.1) and physiological/behavioral rhythms (Section 3.2, i.e., rhythms in neuronal light responses). In addition, Table 2 presents a survey of retinal biochemical rhythms (Section 4). The different categories in which retinal rhythms fall are reviewed below.
Table 1.
Survey of circadian rhythms in the vertebrate retina (Anatomy/ morphology and Physiology/Behavior)
| Process | Peak time | Vertebrate class: Species (References) |
|---|---|---|
| Anatomy/morphology | ||
| Rod myoid contraction | night | Fish: Atlantic tarpon (Kopperud and Grace, 2017); blue acara (Kolbinger et al., 1996); zebrafish (Menger et al., 2005) |
| Rod disc shedding | dawn | Amphibians: Xenopus (Pierce and Besharse, 1986; Besharse et al., 1977); Mammals: rat (LaVail, 1976; Teirstein et al., 1980; Terman et al., 1993); Syrian hamster (Grace et al., 1996); mouse (Besharse and Hollyfield, 1979; Grace et al., 1999); Nile rat (Bobu and Hicks, 2009) |
| Cone myoid contraction | day | Fish: Atlantic tarpon (Kopperud and Grace, 2017); catfish (Welsh and Osborne, 1937); green sunfish (Burnside and Ackland, 1984); midas cichlid (Levinson and Burnside, 1981); zebrafish (Menger et al., 2005); Amphibians: Xenopus (Pierce and Besharse, 1985) |
| Cone disc shedding | dawn | Mammals: Nile rat (Bobu and Hicks, 2009) |
| dusk | Birds: chick (Young, 1978); Reptiles: lizard (Sceloporus occidentalis) (Bernstein et al., 1984); Mammals: squirrel (Young, 1967) | |
| RPE pigment dispersion | day | Fish: carp (Kohler et al., 1990); zebrafish (Menger et al., 2005); Amphibians: Xenopus (Pierce and Besharse, 1985) |
| Horizontal cell spinule formation | day | Fish: goldfish (Douglas and Wagner, 1983); blue acara (Wagner et al., 1992) |
| Synaptic ribbon length | day | Fish: blue acara (Wagner et al., 1992) |
| Mirochondria number | night | Fish: zebrafish (Giarmarco et al., 2020) |
| Physiology/Behavior | ||
| Cone CNGC affinity for cGMP | night | Birds: chick (Ko et al., 2001) |
| Photoreceptor gap-junctional coupling | night | Fish: goldfish (Ribelayga et al., 2008); Mammals: mouse (Ribelayga et al., 2008; Jin and Ribelayga, 2016; Jin et al., 2015, 2020); rabbit (Ribelayga and Mangel, 2010) |
| ERG sensitivity (1/threshold) | night | Birds: quail (Manglapus et al., 1998); Mammals: rabbit (Brandenburg et al., 1983); mouse (C3H, Baba et al., 2009) |
| dusk | Fish: zebrafish (Li and Dowling, 1998) | |
| ERG a-wave implicit time | day | Mammals: mouse (C3H, Baba et al., 2009) |
| night | Birds: chick (Schaeffel et al., 1991; McGoogan and Cassone, 1999; Peters and Cassone, 2005); pigeon (Wu et al., 2000) | |
| ERG a-wave amplitude | day | Birds: chick (Schaeffel et al., 1991; McGoogan and Cassone, 1999; Peters and Cassone, 2005); pigeon (Wu et al., 2000) |
| night | Birds: quail (Manglapus et al., 1998); Mammals: human (Rufiange et al., 2002); mouse (C3H, Baba et al., 2009) | |
| ERG b-wave implicit time | day | Mammals: mouse (C3H, Baba et al., 2009) |
| night | Amphibians: Xenopus (Solessio et al., 2004); Birds: chick (Schaeffel et al., 1991; McGoogan and Cassone, 1999; Peters and Cassone, 2005); pigeon (Wu et al., 2000); Mammals: human (Hankins et al., 1998); mouse (C57BL/6x129, Barnard et al., 2006; Storch et al., 2007; Cameron et al., 2008a) | |
| ERG b-wave amplitude | day | Amphibians: Xenopus (Barlow et al., 2000; Solessio et al., 2004); Reptiles: Anolis (Fowlkes et al., 1984); green iguana (Miranda-Anaya et al., 2002); Birds: chick (Schaeffel et al., 1991; McGoogan and Cassone, 1999; Peters and Cassone, 2005); pigeon (Barattini et al., 1981; Wu et al., 2000); domestic fowl (Lu et al., 1995); Mammals: mouse (C57BL/6x129, Barnard et al., 2006; Storch et al., 2007; Cameron et al., 2008a; Jackson et al., 2012; Zhang et al., 2020); human (Nozaki et al., 1983) |
| dusk | Fish: zebrafish (Li and Dowling, 1998; Ren and Li, 2004) | |
| night | Birds: quail (Manglapus et al., 1998); Mammals: rabbit (Brandenburg et al., 1983); human (Rufiange et al., 2002); mouse (C3H, Baba et al., 2009) | |
| Cone→Rod dominance switch of ERG | night | Birds: pigeon (Barattini et al., 1981); quail (Manglapus et al., 1998); chick (Schaeffel et al., 1991) |
| Cone→Rod dominance switch of cone light responses | night | Fish: goldfish (Ribelayga et al., 2008) |
| Cone→Rod dominance switch of horizontal cell light responses | night | Fish: goldfish (Wang and Mangel, 1996; Ribelayga et al., 2000, 2004); Mammals: rabbit (Ribelayga and Mangel, 2010) |
| Visual sensitivity | day | Mammals: human (Bassi and Powers, 1986; Barlow et al., 1997) |
| dusk | Fish: zebrafish (Li and Dowling, 1998) | |
| night | Fish: goldfish (Bassi and Powers, 1987); Mammals: rat (Rosenwasser et al., 1979; Remé et al., 1991) | |
| Photoreceptor light damage | night | Mammals: rat (Duncan and O’Steen, 1985; Organisciak et al., 2000; Vaughan et al., 2002) |
Table 2.
Survey of circadian rhythms in the vertebrate retina (Biochemical activity)
| Process | Peak time | Vertebrate class: Species (References) |
|---|---|---|
| Biochemical activity | ||
| Melatonin synthesis and release | night | Fish: goldfish (Iigo et al., 1997a,b); zebrafish (Cahill, 1996); wrasse (Iigo et al., 2003); flounder (Kulczykowska and Iuvone, 1998); Amphibians: Xenopus (Cahill and Besharse, 1990, 1991, 1992, 1993); greenfrog (Alonso-Gomez et al., 2000a,b); newt (Chiba et al., 2005); Reptiles: iguana (Tosini and Menaker, 1998a; Miranda-Anaya et al., 2002); Birds: chick (Hamm and Menaker, 1980; Reppert and Sagar, 1983; Thomas and Iuvone, 1991; Zawilska and Iuvone, 1992); quail (Underwood et al., 1990; Manglapus et al., 1999; Steele et al., 2006); pigeon (Adachi et al., 1998, 1999); duck (Zawilska et al., 2003a); goose (Zawilska et al., 2003b); Turkey (Zawilska et al., 2006); Mammals: rat (Pang et al., 1980; Yu et al., 1981; Sakamoto et al., 2004b; Tosini et al., 1998); mouse (Tosini and Menaker, 1998b); Syrian hamster (Faillace et al., 1995; Tosini and Menaker, 1996, 1998c) |
| day | Birds: chick (GC only, Garbarino-Pico et al., 2004b) | |
| Tpoh mRNA | night | Amphibians: Xenopus (Green and Besharse, 1994; Green et al., 1995a,b; Valenciano et al., 2000); Birds: chick (Chong et al., 1998; Bailey et al., 2004); Mammals: sheep (Privat et al., 1999); rat (Tosini and Fukuhara, 2002; Liang et al., 2004; Sakamoto et al., 2005) |
| TPOH activity | night | Amphibians: Xenopus (Valenciano et al., 1999); Birds: chick (Thomas and Iuvone, 1991; Thomas et al., 1993); quail (Manglapus et al., 1999) |
| Aaad mRNA | night | Mammals: rat (Tosini and Fukuhara, 2002; Fukuhara and Tosini, 2003) |
| Aa-nat mRNA | night | Fish: pike (aanat1, Coon et al., 1999); zebrafish (aanat2 is circadian, aanat1 is light driven, Appelbaum et al., 2006); Birds: chick (Bernard et al., 1997; Haque et al., 2002; Bailey et al., 2004; Toller et al., 2006); Mammals: rat (Niki et al., 1998; Sakamoto and Ishida, 1998a; Sakamoto et al., 2002, 2004b, 2006; Engel et al., 2004); mouse (Sakamoto and Ishida, 1998b); sheep (Privat et al., 1999); macaque (Coon et al., 2002) |
| AA-NAT activity | night | Amphibians: Xenopus (Besharse and Iuvone, 1983); greenfrog (Alonzo-Gomez et al., 2000a,b); Birds: chick (Hamm and Menaker, 1980; Bernard et al., 1997; Garbarino-Pico et al., 2004b); duck (Zawilska et al., 2003a); goose (Zawilska et al., 2003b); turkey (Zawilska et al., 2006); Mammals: rat (Niki et al., 1998); macaque (Coon et al., 2002) |
| Hiomt mRNA | night | Birds: chick (Guerlotte et al., 1996); Mammals: rat (Gauer and Craft, 1996; Tosini and Fukuhara, 2002; Fukuhara and Tosini, 2003); macaque (Coon et al., 2002) |
| Melatonin receptor mRNA | day | Birds: chick (MT1: Natesan and Cassone, 2002); Amphibians: Xenopus (MT2+ Mel1c, Wiechmann and Smith, 2001) |
| night | Birds: chick (Natesan and Cassone, 2002) | |
| Dopamine content | day | Fish: cichlid (Wulle et al., 1990); Birds: chick (Zawilska et al., 2003c); duck (Zawilska et al., 2003a); Reptiles: iguana (Miranda-Anaya et al., 2002); Mammals: rat (Wirz-Justice et al., 1984; Pozdeyev and Lavrikova, 2000; Doyle et al., 2002b); mouse (Doyle et al., 2002a) |
| Dopamine synthesis (as TH activity) | day | Fish: goldfish (Ribelayga and Mangel, unpublished observations); Birds: quail (Manglapus et al., 1999) |
| night | Fish: minas cichlid (McCormack and Burnside, 1993) | |
| Dopamine release | day | Fish: cichlid (Wulle et al., 1990); goldfish (Ribelayga et al., 2002b, 2004); Reptiles: iguana (Miranda-Anaya et al., 2002); Birds: pigeon (Adachi et al., 1998, 1999); duck (Zawilska et al., 2003a); chick (Zawilska et al., 2003c); Mammals: rat (Pozdeyev and Lavrikova, 2000; Doyle et al., 2000); mouse (Doyle et al., 2002a) |
| Dopamine D4 receptor mRNA | night | Mammals: rat (Bai et al., 2008; Klitten et al., 2008); mouse (Storch et al., 2007) |
| Adenosine (extr. and intrac. levels) | night | Fish: goldfish (Mangel and Ribelayga, 2001; Ribelayga et al., 2002a); Mammals: rabbit (Ribelayga et al., 2005) |
| GABA content/turnover | night | Mammals: Syrian hamster (Jaliffa et al., 2001) |
| Protein Kinase C (in rod BP) | night | Mammals: rat (Gabriel et al., 2001) |
| Parvalbumin (in AII Amacrine Cells) | night | Mammals: rat (Gabriel et al., 2004) |
| Phosducin phosphorylation (in PR) | night | Mammals: mouse (Pozdeyev et al., 2008) |
| Extracellular pH | day | Fish: goldfish (Dmitriev and Mangel, 2000); Mammals: rabbit (Dmitriev and Mangel, 2001) |
| Energy metabolism | night | Fish: goldfish (Dmitriev and Mangel, 2004) |
| Phospholipid metabolism (in INL, GCL) | day | Birds: chick (Guido et al., 2001; Garbarino-Pico et al., 2004a, 2005) |
| AC-cAMP-PKA-Ras-MAPK (in PR) | night | Birds: chick (Ko et al., 2001, 2004a,b; Ivanova and Iuvone, 2003a; Garbarino-Pico et al., 2004b); Mammals: rat (Fukuhara et al., 2004) |
| c-fos mRNA | night | Mammals: rat (ONL, Yoshida et al., 1993); mouse (Peirson et al., 2006) |
| midkine-a mRNA/Mdka protein | dawn | Fish: zebrafish (Calinescu et al., 2009) |
| Rhodopsin mRNA | day | Fish: african cyclid Haplochromis burtoni (Korenbrot and Fernald, 1989); Amphibians: toad (Korenbrot and Fernald, 1989) |
| dusk | Mammals: mouse (von Schantz, 1999); Fish: zebrafish (Yu et al., 2007) | |
| Cone opsin mRNA | day | Fish: African cyclid Haplochromis burtoni (Halstenberg et al., 2005) dawn Fish: zebrafish (Li et al., 2008b) |
| dusk | Birds: chick (Pierce et al., 1993; von Schantz et al., 1999; Zhang et al., 2003); quail (Pierce, 1999); Mammals: mouse (von Schantz, 1999) | |
| Melanopsin mRNA | day/night | Birds: chick (RPE, INL, GC: day, PR: night, Chaurasia et al., 2005) |
| dusk | Mammals: rat (Sakamoto et al., 2004a, 2005) | |
| Nocturnin mRNA | night | Amphibians: Xenopus (Green and Besharse, 1996a,b; Baggs and Green, 2003) |
| dusk | Mammals: mouse (Wang et al., 2001) | |
| Connexin36 mRNA/protein | night | Mammals: mouse (Katti et al., 2013) |
| Clock genes mRNA/protein | see Table 2 |
BP: bipolar cells; INL: inner nuclear layer; GCL: ganglion cell layer; Mdka: Midkine A; ONL: outer nuclear layer; PR: photoreceptors. All other abbreviations, see text.
3.1. Rhythms in morphology
3.1.1. Retinomotor movements
In fish, amphibians and birds, the pupil diameter is relatively fixed and does not change in response to ambient light intensity as much as it does in reptiles and mammals. Instead, photoreceptors and retinal pigmented epithelium (RPE) pigment granules undergo morphological rearrangement according to changes in ambient lighting and/or the circadian cycle. Collectively, these movements are known as retinomotor movements (Levinston and Burnside, 1981; Burnside and Nagle, 1983; Pierce and Besharse, 1985; Iuvone, 1995; Burnside, 2001; Menger et al., 2005; Burnside and King-Smith, 2010). Specifically, the inner segments of rod and cone photoreceptors contract or elongate in response to light and endogenous signals. Cone inner segments typically contract at or before dawn and elongate at or before dusk, whereas the inner segments of rod photoreceptors display the reverse pattern of movement (Table 1). Additionally, RPE cells possess apical processes that surround photoreceptor outer segments. During the day, RPE pigment granules characteristically disperse into these processes, catching unabsorbed photons and thereby enhancing visual acuity. At night, pigment granules aggregate in the RPE cell basal region, thereby minimizing RPE photon capture and maximizing the sensitivity under low light conditions. Therefore, at dawn, cone outer segments are optimally positioned for daylight perception while rod outer segments are buried in RPE cells, and, at night, rod outer segments are optimally positioned with RPE pigment granules basally aggregated increasing the light capturing capacity of the retina. In addition to optimally positioning photoreceptors for the given lighting conditions, it has been noted that photoreceptors are always elongated when they are not mediating vision. This suggests that additionally, retinomotor movements may be attenuating the signal from the outer segment by increasing the distance it must travel to the synapse (Burnside and King-Smith, 2010). Therefore, retinomotor movements, relying on coordinated action from both RPE cells and photoreceptors, contribute to prepare and adapt the retina to (incoming) changes in ambient light intensity (Levinston and Burnside, 1981; Menger et al., 2005; Burnside and King-Smith, 2010). Retinomotor movements are controlled by neuromodulators such as dopamine and adenosine, which act through their membrane receptors to control the intracellular messenger cyclic adenosine monophosphate (cAMP) and reorganize the cytosketon and change the shape of the photoreceptor cells and/or the concentration/dispersion of pigment granules in the RPE cells (Burnside and King-Smith, 2010; Beharse and McMahon, 2016; Lewis et al., 2018).
3.1.2. Disk shedding
The maintenance of the normal physiological function of photoreceptors (cones and rods) requires that their outer segment portion, which contains the visual pigment, be renewed regularly. Specifically, the stacks of membrane saccules (in the case of rods) and the continuous infolding of the membrane (in the case of cones) are renewed through a series of coordinated steps between the photoreceptor and the RPE (Eckmiller, 1997; Nguyen-Legros and Hicks, 2000; Baba et al., 2022). First, new membranous material is synthesized, transported, and incorporated into newly forming outer segment membranes. Second, a compensatory shedding of older membranous material occurs, thereby maintaining the segment at a constant length. This step consists in phagocytosis and degradation of the photoreceptor distal tip by the RPE. Both a circadian oscillator and the daily L/D cycle affect disk shedding. Rod disk shedding typically peaks at dusk or early morning while cone disk shedding shows some variability between species (Table 1). Disk shedding requires the coordination between the RPE and the photoreceptors (Besharse and Defoe, 1998).
Recent work has begun to unravel the molecular mechanism through which the circadian clocks in the RPE and neural retina interact and thereby control photoreceptor disk shedding. Both the RPE and the neural retina have circadian clocks which can function independently. However, they must work in tandem to establish rhythms of photoreceptor disk shedding (Felder-Schmittbuhl et al., 2018; Milićević et al., 2019, 2021). Two RPE expressed genes have been identified as major effectors for the early morning peak in mouse disk shedding rhythms. Knockouts of the phagocytic factor integrin beta-5 (Itbg5) or its ligand, milk fat globule EGF factor 8 (Mfge8), cause mice to undergo disk shedding constitutively (Nandrot et al., 2004; Nandrot et al., 2007; Nandrot and Finneman, 2008). This is likely because ITBG5 activates Rac1 which recruites phagocytic machinery (Mao and Finneman, 2012). Furthermore, in Itbg5−/− and Mfge8−/− mice, there is a decrease in the early morning peak of a phagocytic signal, phosphatidyl serine, on rod outer segment tips (Ruggiero et al., 2012). Recent work has found that plexin B1 and its ligand semaphorin 4D may act as a brake for the phagocytic burst prior to light onset (Bulloj et al., 2018). PlexinB1 is expressed in RPE whereas semaphorin 4D is expressed in the photoreceptors and together they inhibit Rac1 activity in the RPE, except during an early morning period (between one hour before and one hour after light onset) where there is a significant reduction in their protein levels and thereby an increase in phagocytic activity (Bulloj et al., 2018). As expected, in cultured RPE monolayers, there is rhythmic expression of phagocytic factors (Milićević et al., 2019). Interestingly, phagocytosis of photoreceptor outer segments may serve as an entrainment signal to the RPE clock, demonstrating the complex interplay between local circadian clocks (Milićević et al., 2019). Recent work analyzing the RPE transcriptome under constant darkness conditions prior to and immediately following the typical burst of phagocytic activity found that rhythmic expression of phagocytic factors was maintained under constant darkness and identified a number of novel genes potentially involved in disk shedding rhythms (Campbell et al., 2018; DeVera and Tosini, 2020). Finally, work exploring the contribution of L/D cycles has suggested that L/D cycles likely leverage their effect through the phototransduction machinery of the photoreceptors, because phosphodiesterase inhibitors mimic the effects of dark rearing (Campbell and Jensen, 2017).
Altogether, continued research is needed to fully elucidate the mechanism through which the RPE and retinal clocks interact to control photoreceptor disk shedding. This could reveal important therapeutic targets, as arrhythmic disk shedding leads to early onset of age-related photoreceptor degeneration (Nandrot et al., 2004). However, it is worth noting that a recent report has shown that dopamine regulates integrin signaling through D2/4 receptors (Gubin et al, 2020) and the loss of the morning peak of disk shedding and phagocytosis in dopamine D2/4 receptor knockout mice had no effect on photoreceptor degeneration at 3 or 12 months of age. Further investigation is required to understand the role of integrin signaling pathways in rhythmic disk shedding in the photoreceptors.
3.1.3. Horizontal cell spinule formation
Spinules are small finger-like evaginations of cone horizontal cell (cHC) dendrites that are observed in teleost fish (Raynauld et al., 1979). Spinules substantially increase the contact area between the cone membrane and cHC dendrites within the cone pedicle. Evidence suggests that spinules play a role in shaping the chromatic response properties of cHCs (Wagner and Djamgoz, 1993; De Juan and Garcia, 2001). It has also been proposed that spinules mediate feedback from cHCs to cones (Wagner and Djamgoz, 1993; De Juan and Garcia, 2001) but this idea has been questioned (Thoreson and Mangel, 2012). Spinules are clearly present in the light-adapted retina and absent in dark-adapted conditions, thus suggesting that spinule formation is exclusively triggered by light. However, a weak circadian rhythm in spinule formation has been found in goldfish (Douglas and Wagner, 1983) and a robust endogenous one in the tropical fish Blue acara (Aequidens pulcher). In Blue acara, spinule formation occurs during the subjective day in constant darkness conditions (Wagner et al., 1992). Recent evidence has suggested that horizontal cell spinules may also exist in mammalian retina (Morgans et al., 2016). However, it has yet to be investigated whether these spinules respond to light or undergo circadian modulation as in the fish retina.
3.1.4. Synaptic ribbons
Ribbons are dense elongated structures located in the photoreceptor and bipolar cell terminals (Heidelberger et al., 2005; Lagnado and Schmitz, 2015). These particular structures act as conveyer belts to help maintain the sustained and graded release of glutamate in photoreceptors and bipolar cells needed to faithfully transmit light information. In fish, photoreceptor synaptic ribbons are markedly longer during the day compared to the night and tend to disassemble at night (Wagner and Ali, 1977; Vollrath and Spiwoks-Becker, 1996; Spiwoks-Becker et al., 2004). However, in mouse retina these diurnal changes seem to be driven mostly by L/D adaptation, at least in rods (Spiwoks-Becker et al., 2004). Interestingly, there is evidence for mouse strain differences in rhythmic ribbon adaptations of rods, with only Balb/c mice exhibiting diurnal changes and not C57BL/6 (Fuchs et al., 2013). However, in the retina of the tropical fish Blue acara (Aequidens pulcher), the rhythm is truely circadian, at least in cones (Wagner et al., 1992). A study in goldfish found that ON-type bipolar cell terminals have fewer ribbons at night, and that this decrease is correlated to a decreased efficiency of exocytosis (Hull et al., 2006). Astonishingly, in larval zebrafish a circadian clock controls the nighttime degradation of almost all synaptic ribbons at photoreceptor terminals, leading to extreme dampening of ERG b-wave and loss of behavioral visual responses (Emran et al., 2010). Together these studies indicate that the diurnal/circadian cycle in number and shape of synaptic ribbons in the retina likely impacts retinal processing. It has been suggested that these diurnal changes are due to metabolic regulation of nicotinamide adenine dinucleotide hydride (NADH), which disrupts protein-protein interactions necessary to build the synaptic ribbon (Magupalli et al., 2008; Mercer and Thoreson, 2011).
While it remains unclear whether the diurnal changes in retinal ribbons are truly circadian, there is robust evidence that synaptic ribbons of many vertebrate species undergo circadian changes in size and/or number in pinealocytes, which are evolutionarily related to retinal photoreceptors (Kurumando and Mori, 1977; Vollrath and Spiwoks-Becker, 1996; Kikuchi et al., 2000; Mano and Fukada, 2006; Spiwoks-Becker et al., 2008). This suggests that the observed daily/circadian differences in ribbon size and number could be a common adaptive mechanism.
3.1.5. Cone mitochondria
Cone photoreceptors are the most energetically demanding cells in the retina (Ingram et al., 2020), and they have increased metabolic demands in the dark or at night (Okawa et al., 2008; DeVera et al., 2019; Gianmarco et al., 2020). Mitochondrial physiology is regulated by circadian clocks in many parts of the body (Manella and Asher, 2016). Recent work suggests that in order to account for these diurnal changes in energy demands, cones make changes in the number of mitochondria. Dynamin-related protein 1 (DRP1), a critical protein for mitochondrial fission or division, is truly circadian in the avian retina and in a mammalian cone cell line (Chang et al., 2018). In zebrafish retinas mitochondria in cones are more numerous at night than during the day, and the genes controlling mitochondria biogenesis are regulated by the circadian clock (Giarmarco et al., 2020). Furthermore, mitochondria autophagy appears to be upregulated during the subjective day (Giarmarco et al., 2020). These mitochondrial changes likely reflect the circadian regulation of metabolism in the retina (see 3.2.3). Recent work has also shown that in Bmal1 knockouts (Bmal1−/−), cone photoreceptor degeneration is accelerated, hypothesized to be caused at least partly by circadian dysregulation of mitochondrial function (Baba et al., 2018a, 2018b).
3.2. Rhythms in retinal light responses
Given the multitude of circadian rhythms, including those of morphology, it is not surprising that the retinal clock impacts the light responses and electrical properties of retinal neurons. Strategies to investigate these phenomena have generally utilized two different electrical recording approaches, each with its own set of advantages and limitations. The first one consists of recording the mass electrical response of the retina to photic stimulation using the global or full-field eletroretinogram (ERG). The second approach is to study the light responses and electrical properties of individual neurons in intact retinal tissue using electrophysiology techniques such as patch-clamp recording and intracellular and extracellular recording.
3.2.1. Electroretinogram (ERG) recordings
ERG recording of electrical activity is a noninvasive and relatively easy technique to investigate whether the retinal circadian clock affects retinal function (Barlow, 2001; Cameron et al., 2008b). Specifically, anesthetized animals are exposed to light stimuli at different times of the circadian cycle. The recorded responses are typically biphasic waveforms that include two main components: the a- and b-waves. The a-wave, which is the first component of the ERG, is a large negative wave that reflects photoreceptor activity. The a-wave is followed by the b-wave, which is corneal positive, usually larger in amplitude than the a-wave, and reflects the activity of the ON pathway (Dowling, 2012). There are two principal measures of the ERG waveform. The first measure is to determine the amplitudes of the a- and b-waves. The a-wave amplitude is the voltage change from the baseline to the negative trough of the a-wave, and the b-wave amplitude is the voltage change from the trough of the a-wave to the following peak of the b-wave. The second principal measure is the time from flash onset to the trough of the a-wave and the time from flash onset to the peak of the b-wave. These times, reflecting peak latencies of the a- and b-waves, are often referred to as implicit times. ERG measurements have been performed in most vertebrate classes, and the incidence of circadian rhythmicity of the ERG components has been investigated. Most of the different ERG measures vary on a circadian basis, although with some differences between species and even between mouse strains (Table 1). In one extreme case, the ERG of the larval zebrafish is typically light responsive during the day but becomes non-responsive at night (Emran et al., 2010).
ERG measurements are also used to determine two other properties of light-evoked responses: the response threshold and spectral sensitivity. In fact, studies that have focused on these response properties have shown that they vary during a circadian cycle in many species, oscillating between a cone-dominated configuration (high threshold and spectral sensitivity matching that of cones) and a rod-dominated one (low threshold and spectral sensitivity matching that of rods) (Table 1).
It is also of note that genetic ablation of key components of the circadian clock in the mouse retina leads to a loss of rhythmicity in ERG waveform components (Storch et al., 2007; Cameron et al., 2008b; Zhang et al., 2020). Furthermore, in clock knockout models (Bmal1−/− (Storch et al., 2007; Sawant et al., 2017; Zhang et al., 2020) and Cry1−/−/Cry2−/− (Cameron et al., 2008b)), the ERG a-wave is not affected whereas b-wave amplitude and implicit time are altered. However, this occurs only under photopic conditions when cones and second-order neurons are light-adapted. In contrast, as discussed below in Section 3.2.2, circadian studies of the light responses of individual goldfish cones and individual cone-connected horizontal cells in intact in vitro goldfish and rabbit retinas show day/night differences in light responses under dark-adapted, but not light-adapted conditions (Wang and Mangel, 1996; Ribelayga et al., 2008; Ribelayga and Mangel, 2010, 2019). Moreover, as also described in Section 3.2.2, cut-loading experiments in intact in vitro goldfish, rabbit, and mouse retinas show greater tracer coupling between photoreceptor cells under dark-adapted conditions at night than in the day (Ribelayga et al., 2008; Ribelayga and Mangel, 2010; Choi et al., 2012; Li et al., 2009; Li et al., 2013), a finding that supports the results of the single-cell studies.
Recent studies provide a possible esplanation of these apparently contradictory findings. Zhang et al. (2020) found that genetic elimination of photoreceptor coupling in mouse retina had two effects: 1) it abolished the circadian rhythm of the photopic ERG b-wave amplitude; and 2) it eliminated the progressive increase in the amplitude of the b-wave when dark-adapted retinas were placed under photopic conditions, a process called light adaptation that requires about 15-20 min to fully develop. Interestingly, in the absence of photoreceptor coupling, the photopic b-wave amplitude is locked in a light-adaptive state regardless of the time of day or time under photopic illumination (Zhang et al., 2020). These observations are consistent with increased rod/cone coupling reported in mouse at night in the dark and consequent control of the cone pedicle voltage by coupled rods (Jin et al., 2020). That is, when a rod-saturating background is presented to fully dark-adopted retinas, rods become saturated and unresponsive to light. Because rods are coupled to cones at night in the dark via rod/cone gap junctions, rods clamp cone pedicles under these experimental conditions in a hyperpolarized state, a state that constrains the cone synapse to transmit only small responses to flashes brighter than the background. It takes minutes for rod/cone gap junctions to close and for the cone pedicles to repolarize and regain high-amplitude signal transmission to second-order neurons. Thus, these results suggest that, under these specific experimental conditions, light adaptation of the previously dark-adapted ERG and the circadian rhythm in photopic b-wave amplitude and kinetics depend on the day/night difference in rod-cone coupling that occurs under dark-adapted conditions (Zhang et al., 2020).
3.2.2. Circadian clock modulation of the light responses of retinal neurons
While ERG measurements are useful tools for uncovering circadian changes in retinal processing (Barlow, 2001; Cameron et al., 2008b), they provide limited clues about the cellular types or neuronal networks modulated by the retinal clock, or the global impact on retinal output. A deeper look inside retinal circuitry requires the use of invasive cellular electrophysiological techniques performed in the subjective day and night using intact neural tissue. Although single-cell recording in constant darkness is difficult, accumulating evidence indicates that most classes of retinal neuron display circadian rhythmicity in their light responses and electrical properties. One of the best understood mechanisms of circadian clock control of retinal circuitry is the plasticity of gap junction coupling between photoreceptors. Below we review some of this work and discuss the potential implications of this circadian rhythm.
Gap junctions are made of tiny intercellular channels that form a continuum between the cytosols of two adjacent coupled cells through which electrical signals and small molecules can pass. Gap junction channels are made of connexins, of which there exist about 20 different genes in vertebrates (Harris, 2018). The presence of functional electrical coupling between photoreceptors has been established in fish, amphibians, and mammals (Bloomfield and Volgyi, 2009; Ribelayga and O’Brien, 2017). Connexin35 (Cx35) or its mammalian ortholog Cx36 is expressed in photoreceptors. A second connexin (Cx34.7) has been identified in fish cones (O’Brien et al., 1998, 2004). In mammals, both rods and cones express Cx36 exclusively (Jin et al., 2020). Gap junctions connecting rod and cone terminals allow rod input to enter the cone circuit; this forms the entry of an important rod pathway, called the secondary rod or rod/cone pathway in mammals (Bloomfield and Dacheux, 2001; Jin et al., 2022).
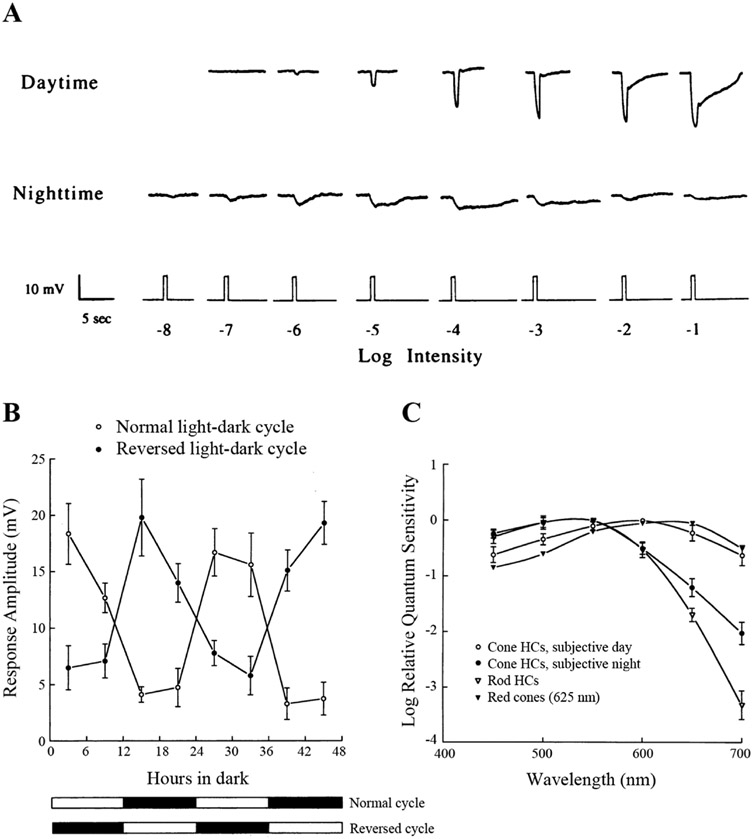
While early work had suggested that photoreceptor gap junctions are modulated by light (Yang and Wu, 1989; Krizaj et al., 1998), more recent work has shown that under natural illumination conditions photoreceptor gap junctions are under the control of the retinal circadian clock and not dependent on the response of the retina to the natural visual environment at night. First evidence of this was obtained when the Mangel lab performed circadian experiments on goldfish cHCs, which like those in other vertebrate retinas, receive glutamate input exclusively from cones (Ariel et al., 1984, 1986; Mangel et al., 1985; Zhang et al., 2002; Dmitriev and Mangel, 2006; Dowling, 2012). They studied a type of cHC, H1-type (H1), in the goldfish retina and found that it exhibits circadian rhythmicity in its light response properties (Wang and Mangel, 1996; Ribelayga et al., 2002; 2003; 2004; 2007). Specifically, they showed that the responses of dark-adapted goldfish cHCs to dim light stimuli resemble those of cones during the day or subjective day and those of rods or rod horizontal cells at night in the following ways: 1) absolute sensitivity increases 100-fold at night compared to the subjective day; 2) response waveforms are fast and transient in the subjective day, sluggish and slow to recover at light offset at night; and 3) spectral sensitivity peaks around 600 nm during the day and around 500 nm at night. Thus, during the day, H1-type cHC light responses are driven by long wavelength (red)-sensitive cones, whereas at night they are primarily driven by rods and respond to very dim (i.e., low scotopic) light stimuli (Figure 2).
Fig. 2. A circadian clock regulates the light responses of fish cone horizontal cells (cHCs).
(A) Cone input to L-type cHCs predominates during the subjective day and rod input predominates during the subjective night. Compared to the day, the responses at night are slower, smaller in size, longer in duration and the response threshold is approximately a hundred times lower. Retinas were dark adapted for at least 1 h after excision, following which L-type cHCs were impaled without the aid of any light flashes. Responses of the cells to dim full-field white light flashes (ranging from −8 log Io to −5 log Io) were then recorded. The responses of two different cells are shown in the subjective day and night.
(B) Average responses to a bright light stimulus (−3 log Io) as a function of time. Responses in the dark are greater during the subjective day than during the subjective night (open circles), even when the animals have been previously entrained to a reversed light/dark cycle (filled circles). The presence of a recording from a cHC was confirmed following cell impalement by flashing a series of dim (≤ −6 log Io) lights. Following this, a single bright (−3 log Io) light was flashed. Data were averaged only from responses to this single bright light stimulus (one bright light stimulus per retina). In each case, a response to a single bright light stimulus (−3 log Io) was obtained at the time indicated.
(C) Spectral sensitivity measurements demonstrate a circadian rhythm of rod and cone input to cHCs. Average L-type cHC spectral sensitivity during the subjective night (~ CT15) resembles that of goldfish rod horizontal cells (and rods) (Schwanzara, 1967), rather than red (625 nm) cones (Harosi and MacNichol, 1974), for wavelengths ≤ 600 nm. In contrast, average L-type cHC spectral sensitivity during the subjective day (~ CT03) was similar to that of red (625 nm) cones. The relative spectral sensitivity of rod horizontal cells closely resembled that of goldfish rods, regardless of time of day (Ribelayga et al., 2007). Narrow band interference filters were used to control stimulus wavelength. Relative quantum sensitivity was determined using a 1 mV criterion response in order to minimize light sensitization of the dark-adapted state. Surgical isolation of the retina occurred approximately 2 h before this bright light response was recorded. Each data point represents averages obtained from 5-8 cells (1 cell per retina). Intensity values are relative to the maximum, unattenuated intensity (Io, 2.0 mW.cm−2) of full-field white light stimuli generated by the photostimulator. The maximum, unattenuated light intensity of the stimulus at 550 nm was 7.2 x 1013 photons.cm−2.sec−1.
Adapted from Wang, Y., Mangel, S.C., 1996. Proc. Natl. Acad. Sci. USA 14, 4655-4660.
Because goldfish H1 cHCs make chemical synaptic contact exclusively with long wavelength-sensitive cones, and not with rods (Stell and Lightfoot, 1975), Wang and Mangel (1996) suggested that the increased rod input to H1 cells at night originated from rod signals that travelled to cones through open rod/cone gap junctions, and subsequently, reached horizontal cells through the cone synapse. Such a hypothesis implied that the rod/cone gap junction conductance would increase at night to allow rod signals to enter cones and decrease during the day to reduce rod input to cones, thereby causing the circadian change in the light responses of cHCs.
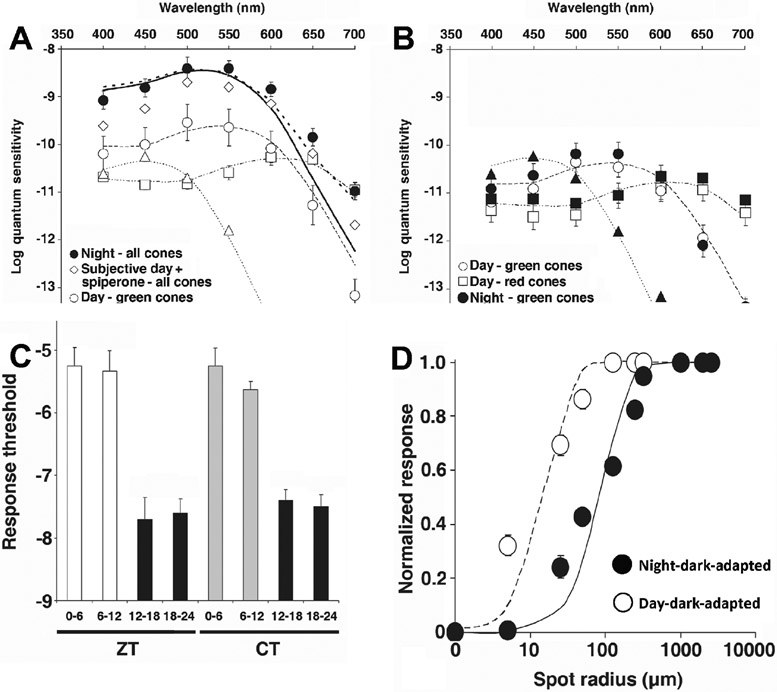
Patch-clamp recordings from cone inner segments in intact goldfish retinas, which were obtained subsequently by the Mangel lab during the subjective day and night, showed that cone light responses resemble those of typical cones during the day and those of rods at night, with respect to threshold, time course, and spectral sensitivity (Figure 3; Ribelayga et al., 2008). Moreover, the receptive field size of cones was significantly larger at night than in the day (Figure 3D), a finding that is consistent with increased photoreceptor gap junction coupling at night compared to the day. In addition, tracer injections into individually recorded cones revealed an increase in rod/cone tracer coupling at night compared to the day (Figures 4A, 4B, 4E; Ribelayga et al., 2008). Tracer coupling measurements in zebrafish (Li et al., 2009), rabbit (Ribelayga and Mangel, 2010), and mouse retinas (Ribelayga et al., 2008; Li et al., 2013; Jin et al., 2015) provided additional indirect evidence that electrical coupling between photoreceptors is stronger at night compared to the day or subjective day.
Fig. 3. Circadian Variations in Cone Spectral Sensitivity, Light Response Threshold, and Receptive Field Size.
(A) Average spectral sensitivity of cones recorded under dark-adapted conditions during the day or subjective day fit one of three nomograms (thin dotted curves) corresponding to the three major known types of goldfish cone pigments: L, M, and S. Data were obtained from recorded red cones (open squares; n = 9), green cones (open circles; n = 6) and blue cone (open triangle; n = 1). In contrast, the spectral sensitivity of all dark-adapted cones recorded at night peaked at ~ 535 nm (filled circles; n = 10). Although cone spectral sensitivity at night under dark-adapted conditions closely fits a rod nomogram (solid thick line) for 400 nm < λ < 600 nm, it does not fit the nomogram as well for λ > 600 nm. Rather, the data points closely fit a modified nomogram that combines goldfish rod and L-cone pigment nomograms (dotted thick curve; λmax = 537 ± 3 (s.d.) nm; r2 = 0.91). Following application of spiperone (10 μM) (open diamonds; n = 2), cone spectral sensitivity in the subjective day resembled that observed during the subjective night and data points fit well the modified nomogram (λmax = 537 ± 3 nm; r2 = 0.96).
(B) Following bright light adaptation at night or during the subjective night 3 groups of cones with different spectral sensitivities were observed: red cones (filled squares; n = 4), green cones (filled circles; n = 5) and blue cone (filled triangles; n = 1), whereas bright light adaptation during the day or subjective day did not affect the relative spectral sensitivity of the recorded cones (red cones: open squares; n = 2; green cones: open circles; n = 6) but slightly decreased the absolute sensitivity. Nomograms as in (A).
(A and B) Data points represent average sensitivity ± s.e.m.
(C) Average day/night and circadian rhythms of the cone light response threshold (i.e. intensity required to elicit a 0.5 mV response) under dark-adapted conditions. The average cone light response threshold (log intensity) was significantly higher during the day (p < 0.001) and subjective day (p < 0.001) than during the night and subjective night (Tukey post hoc analysis). Data points represent averages of 4 to 15 measurements.
(D) Average normalized response amplitudes of dark-adapted cones plotted against stimulus radius for a stimulus of intensity −5 log Io. These data indicate that the receptive field size of cones is larger at night than in the day. Measurements were performed during the day (open circles, n = 6) and night (filled circles, n = 6).
(C, D) Error bars indicate s.e.m.
Adapted from Ribelayga, C., Cao, Y., Mangel, S.C., 2008. Neuron 59, 790-801.
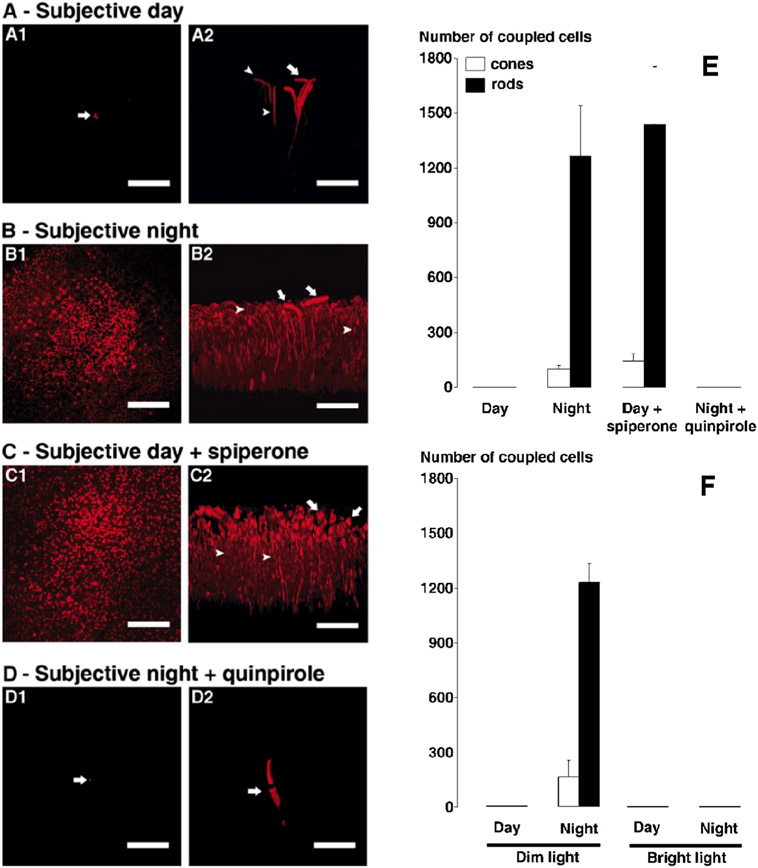
Fig. 4. A circadian clock in the goldfish retina controls rod/cone gap junction coupling.
(A-D) Following iontophoresis of biocytin into individual cones, the tracer remained in a few cells (indicated by arrows in A1, D1) near the injected cone during the subjective day (A) and during the subjective night in the presence of the D4 receptor agonist quinpirole (1 μM, D), but diffused into many rods and cones during the subjective night (B) and during the subjective day in the presence of the D4 receptor antagonist spiperone (10 μM, C). In each of A-D, confocal images of a whole-mount retina at the level of the rod inner segments are shown on the left and perpendicular views of the 3-D reconstruction of the photoreceptor cells from the same retina are shown on the right. Some cones (arrows) and rods (arrowheads) are indicated. Scale bars (A-D): 50 μm.
(E and F) Average numbers of stained cones (open bars) and rods (filled bars) following biocytin injections into individual cones (1 cone injected/retina) under dark-adapted conditions (E) during the day (n = 11) and subjective day (n = 5), night (n = 4) and subjective night (n = 5), subjective day in the presence of spiperone (n = 6), and subjective night in the presence of quinpirole (n = 6), and under dim light-adapted conditions (F-left) during the day (n = 6) and night (n = 3) and bright light-adapted conditions (F-right) during the day (n = 2) and night (n = 3). Under dark-adapted conditions, the number of tracer coupled rods and cones was significantly greater during the night (p < 0.001) and during the day following spiperone treatment (p < 0.001) than during the day under control conditions. Under dim light-adapted conditions, the number of tracer coupled rods and cones was significantly greater during the night (p < 0.001) compared to the day (Tukey post hoc analysis). Under bright light-adapted conditions, biocytin was restricted to the injected cone; no other cells were labeled. Error bars represent s.e.m.
Adapted from Ribelayga, C., Cao, Y., Mangel, S.C., 2008. Neuron 59, 790-801.
Interestingly, similar results were obtained when low-mesopic light stimuli, such as occur naturally right before dawn or just after dusk, were flashed onto the retinas for >60 min before tracer injections (Figure 4F). This result strongly suggests that the retinal clock, and not the retinal response to the natural visual environment at night, controls rod-cone coupling. Note that brighter light such as occurs typically during the day (but not naturally at night) closed rod-cone gap junctions in both day and night (Figure 4F). It is common for bright illumination to block circadian clock effects, a phenomenon known as “masking” (Ribelayga et al., 2008).
Direct measurements of the transjunctional conductance between adjacent mouse photoreceptors provided quantitative measures of the amplitude of the change. In the mouse retina, Cx36 is required and sufficient for photoreceptor electrical coupling (Asteriti et al., 2017; Jin et al., 2020). In dark-adapted wildtype (WT) B6 mice, which are melatonin-deficient, rod/cone conductance is about 300 pico Siemens (pS) (Jin et al., 2020). In melatonin-proficient congenic B6 mice (Zhang et al., 2018), the conductance changes between a minimum of about 100 pS during the subjective day to about 600 pS during the subjective night (Jin and Ribelayga, unpublished observations; see Section 4.1 for a discussion on the role of melatonin in the circadian modulation of photoreceptor coupling). Because the rod/cone gap junction is predominant in the mouse photoreceptor network and virtually every rod has electrical access to a cone (Jin et al., 2020; Ishibashi et al., 2022), the circadian changes in rod/cone coupling impact the strength of electrical coupling between rods through a rod-to-cone-to-rod route and results in weak rod coupling during the subjective day (98 pS) and stronger coupling during the subjective night (493 pS) (Jin and Ribelayga, 2016). The robust difference in gap junction conductance reflects the modulation of the phosphorylation state of Cx36, whose level is positively correlated to the extent of tracer coupling (Ribelayga and O’Brien, 2017). A nocturnal increase in Cx35/36 phosphorylation at photoreceptor terminals has been reported in zebrafish (Li et al., 2009), goldfish (Zhang and Ribelayga, unpublished observations), and mouse (Li et al., 2013; Zhang et al., 2015), suggesting that control of rod/cone Cx35/36-mediated coupling by the retinal clock is a general feature of the vertebrate retina.
A recent study of day/night differences in cone to cHC synaptic transmission in goldfish retina reported that although changes in rod/cone coupling can account for many day/night changes, such as changes in spectral tuning and response threshold of cones and cHCs, some day/night differences may result from distinct clock effects (Ribelayga and Mangel, 2019). For example, at night compared to the day, evidene suggests that cone to cHC synaptic transfer is highly non-linear and of lower gain. As a result, cHC light responses saturate at a lower intensity at night than in the day, and at a lower intensity than cones at night. These characteristics restrict cone to cHC signaling to very dim light stimuli, making the cone to cHC synapse more sensitive to small changes in dim light intensity at night (Ribelayga and Mangel, 2019). In mouse cone pedicles, complexin3, a SNARE regulator, is under the control of the cone clock (Bhoi et al., 2021). All together, these recent studies suggest that in addition to circadian modulation of rod/cone coupling, the synaptic transfer function from cones to second-order cells may also be regulated by the retinal clock. It is also worth noting that the results of Ribelayga and Mangel (2019) suggest that the retinal clock has little effect on the phototransduction process itself, although recent evidence suggests that the clock may modulate recovery of cone photoresponses in zebrafish (Zang et al., 2021).
In addition to controlling rod/cone gap junctions, other studies have revealed the presence of circadian clock regulation of ion channels and associated enzyme activities that mediate neuronal light responses. For example, the cyclic guanosine monophosphate (cGMP)-gated cation channel (CNGC), the final step in the phototransduction process, is under circadian control. Specifically, although the opening and closing of CNGCs depend on light stimulation, the affinity of CNGCs to cGMP is higher during the subjective night than the subjective day in avian cones (Ko et al., 2001; Ko, 2020), in part due to circadian regulation of adenylyl cyclase (AC), cAMP, Ras-MAP kinase (MAPK), and calcium/calmodulin-dependent kinase II (CaMKII) (Ko et al., 2001, 2003, 2004b). In addition to circadian regulation of cone CNGCs, Cav1.3, an L-type calcium channel (LTCC) located on cone synaptic terminals, is also under circadian control. The average maximal current amplitudes of cone LTCCs, which mediate tonic glutamate release from cone terminals, are larger at midnight than at midday, mainly due to increased expression of messenger ribonucleic acid (mRNA) and protein levels of Cav1.3 at night (Ko, 2020). However, in contrast to cone CNGCs, the activation and channel gating kinetics of cone LTCCs do not change over the course of day and night. The Ras-MAPK-CaMKII signaling pathway also plays a role in circadian regulation of Cav1.3, as do mechanistic/mammalian target of rapamycin complex 1 (mTORC) and AMP-activated protein kinase (AMPK) (Huang et al., 2015; Ko, 2020).
The circadian modulation of electrical coupling between photoreceptors impacts the light responses of photoreceptors themselves as well as those of downstream neurons, such as cHCs in goldfish and rabbits (Wang and Mangel, 1996; Ribelayga and Mangel, 2010). In addition, strong rod/cone coupling at night increases signal sharing in the rod network (Jin et al., 2015) and allows rod signals to enter cones (Ribelayga et al., 2008; Jin et al., 2020) and cone pathways, as evidenced by the presence of rod signals in cone-connected second order cells at night (Wang and Mangel, 1996; Ribelayga et al., 2002, 2004; Ribelayga and Mangel, 2003, 2010). The benefit of increased coupling between rods and cones and between rods under dim light conditions is expected to extend the range of scotopic vision by circumventing saturation at the rod to rod-bipolar cell synapse and increasing rod signal processing in cone pathways (Hornstein et al., 2005).
It has also been theorized that coupling between photoreceptors would render the rod synapse less effective at separating out single-photon signals from dark noise, an effect that would elevate the absolute threshold of dark-adapted observers (Smith et al., 1986; Hornstein et al., 2005; Okawa and Sampath, 2007). However, recent measurements in mouse indicate that this is not the case. Using a visually guided task, Koskela et al. (2020) elegantly demonstrated that under very dim light conditions close to the sensitivity limit of vision, the absolute visual threshold does not change between subjective day and subjective night. By directly recording from retinal ganglion cells (RGCs), the authors further show that the absolute threshold of the retinal output does not change either, thereby suggesting that the circadian modulation of rod/cone coupling does not impact the absolute threshold of the retinal output (Fahrenfort and Ribelayga, 2020). Conversely, others have reported evidence that suggests that the neuronal activity of RGCs in the mammalian retina is affected by a circadian clock. For instance, in the rabbit retina, the light response threshold of ON and OFF transient and sustained RGCs are approximately 2.5 orders of magnitude lower during the subjective night compared to the subjective day (Mangel, 2011). In addition, the spontaneous activity of RGCs is lower during the subjective night than in the day (Mangel, 2011). A recent study performed in the freely moving mouse suggests that the firing rate of RGCs changes in a circadian cycle (Hong et al., 2018).
In summary, it is now well established in mammalian and non-mammalian retinas that the circadian clock in the retina controls rod input to cones by modulating the conductance of rod-cone gap junctions, and that rod input reaches post-synaptic targets of cones (i.e., cHCs) in the night but not in the day. However, although some circadian changes have been reported for RGCs, it remains unclear how the retinal circadian clock modulates the light responses of RGCs, bipolar cells, and amacrine cells. Evidence suggests though that absolute visual threshold remains constrained by the statistics of the photon flux irrespective of the time of day.
3.2.3. Visual sensitivity
To determine whether circadian modulation of retinal function translates into a modulation of visual perception, investigators have used a variety of behavioral assays in various vertebrate pecies, including humans. These assays include optomotor responses generated by a rotating drum (Xenopus, zebrafish, mouse), conditioning paradigms including a visual stimulus followed by a mild tail shock (goldfish), and a forced-choice procedure to identify the presence or absence of very dim flashes presented against a dark background (human). It is important to note that these psychophysical tests assay various aspects of “behavioral visual sensitivity” including absolute visual sensitivity, contrast sensitivity, and movement sensitivity. Findings from these studies have consistently supported the view that visual sensitivity is affected by circadian clocks (Table 1). Moreover, specific characteristics of circadian rhythms in visual sensitivity such as peak time of a rhythm depend on the particular test used and/or on the species in the manner that is closely linked to the natural behavior and ecological niche of the species. In addition, it is worth noting that for each particular species the peak of behavioral visual sensitivity usually corresponds closely to that of retinal sensitivity, indicating that the former is likely a consequence of the latter. This view is strengthened by the finding that a robust circadian rhythm of visual sensitivity persists in mammals (rats) after the SCN have been lesioned (Terman and Terman, 1985).
It is possible that the circadian clock in the retina regulates the non-image-forming visual pathway that arrises from a subpopulation of RGCs, the melanopsin-expressing intrinsically-photosensitive RGCs (ipRGCs). Signals from ipRGCs do not reach the visual cortex but instead provide input to specific nuclei, including in mammals the SCN, the intergeniculate leaflet (IGL) of the thalamus, and the olivary pretectal nucleus (OPN) (Gooley et al., 2003; Paul et al., 2009). Thus, it is possibile that the circadian clock in the retina affects the activity of ipRGCs, and thereby, modulates the non-image forming visual pathway. Evidence includes the circadian rhythm of melanopsin mRNA expression in the retina (Bailey and Cassone, 2005; Chaurasia et al., 2005); the rhythms of kinase phosphorylation (Lee et al., 2003) and FOS expression (Chambile, 1998) in subpopulations of SCN neurons that are greatly altered in the absence of the eyes, and thus, presumably of the retinal clock; and a circadian rhythm of the pupil diameter (Liu et al., 1996), which is controlled by the OPN, to which ipRGCs project (Gooley et al., 2003). In fact, a circadian rhyhm of the intrinsic photosensitivity of ipRGCs has been described (Weng et al., 2009). These observations suggest that clocks within the retina (or elsewhere in the brain) may modulate the effects of light on the non-image-forming pathway, including the SCN, through their control of ipRGCs. Further investigation is needed in this new and exciting field of retinal physiology.
4. Mechanisms by which the retinal circadian clock exerts its effects on neuronal light responses
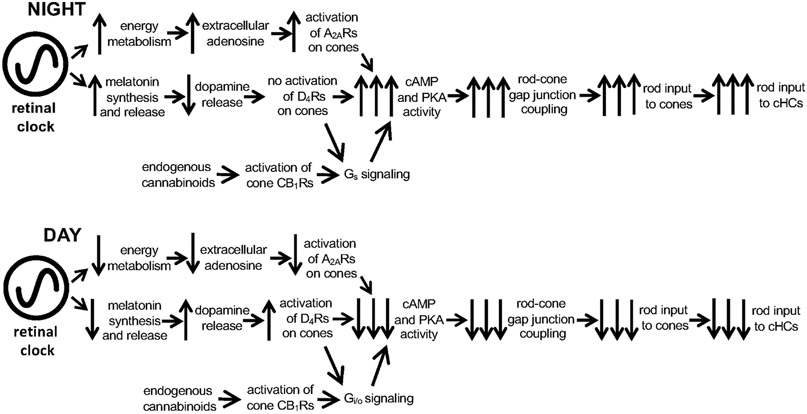
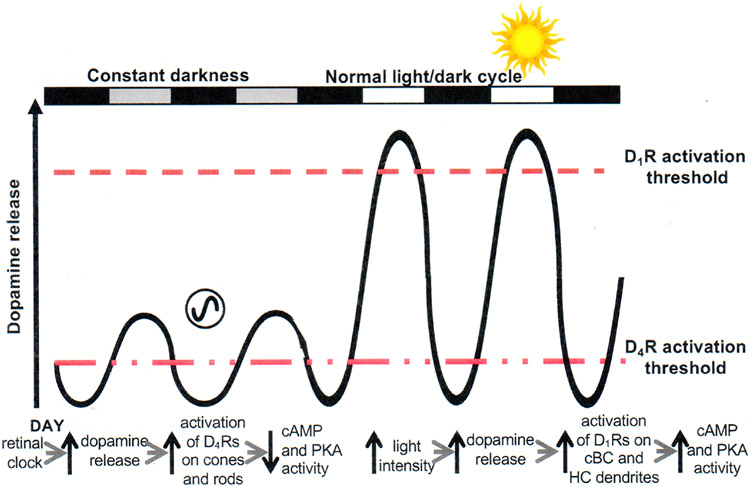
As illustrated in Figure 1, circadian clocks produce diverse daily rhythms. Because environmental stimuli can alter the phase of clocks and their rhythmic outputs, circadian experiments are performed under constant environmental conditions (e.g., constant darkness). Daily rhythms observed under these conditions can then be attributed as due to a circadian clock. Such circadian experiments have demonstrated that many retinal rhythms are produced by a circadian clock located in the retina itself (i.e., the circadian rhythms persist after retinal tissue is isolated from the body). Moreover, other circadian experiments have further shown how specific clock outputs (i.e., circadian rhythms in retinal melatonin, dopamine, and adenosine) produce daily rhythms in neuronal light responses. Section 4 will review these and other related findings.
4.1. Melatonin
4.1.1. Melatonin synthesis and melatonin receptors
The hormone melatonin (5-methoxy-N-acetyl-tryptamine) was first discovered in the pineal gland by Lerner et al. (1958), and its presence was later revealed in the retina of all classes of vertebrates. Similar to that which occurs in the pineal gland, melatonin synthesis in the retina is rhythmic and peaks during the nighttime, except in some rare fish species in which retinal melatonin is high during the day (Besseau et al., 2006). In all vertebrate retinas, with the possible exception of Salmonidae (Falcon, 1999 but see Zaunreiter et al., 1998a,b), the day/night rhythm of melatonin persists in constant darkness, thus revealing its control by a circadian clock (Cahill and Besharse, 1995; Tosini and Fukuhara, 2002; Iuvone et al., 2005; Table 2).
In most vertebrates, retinal melatonin is primarily synthesized in photoreceptors in which the enzymes of its synthetic pathway have been consistently observed (Cahill and Besharse, 1995; Iuvone et al., 2005; Tosini et al., 2012). Due to its lipophilic nature, melatonin release is regulated at the level of its synthesis. At the beginning of the night, the newly synthesized neurohormone diffuses out of cells and throughout the retina where it acts by interacting with its G-protein-coupled membrane receptors (see below). It is thought that retinal melatonin acts locally within the retina and does not contribute to circulating melatonin levels (Iuvone et al., 2005).
Melatonin is synthesized from tryptophan through a multiple step pathway involving numerous enzymes. These enzymes include, in sequential order, tryptophan hydroxylase (TPOH), aromatic amino acid decarboxylase (AAAD), arylalkylamine-N-acetyltransferase (AA-NAT), and hydroxyindole-O-methyltransferase [HIOMT (also known as acetylserotonin-O-methyltransferase (ASMT)) (Simonneaux and Ribelayga, 2003). Serotonin (5-hydroxytryptophan; 5-HT) and N-acetylserotonin (NAS) are important intermediates, and AA-NAT converts 5-HT into N-acetylserotonin. Much of the research on the regulation of melatonin synthesis has focused on the regulatory mechanism of AA-NAT because in the pineal gland large circadian variations of its activity generate an ~10-fold melatonin rhythm (King and Steinlechner, 1985; Klein et al., 1997; Simonneaux and Ribelayga, 2003; Iuvone et al., 2005; Klein, 2007; Tosini et al., 2012). The mechanisms controlling melatonin synthesis in the retina and pineal gland bear a high degree of similarity and have been reviewed (Simonneaux and Ribelayga, 2003; Iuvone et al., 2005). However, in the retina, TPOH activity and thus serotonin availability represents a critical, rate-limiting step prior to AA-NAT activity (Cahill and Besharse, 1990; Iuvone et al., 2005). Like AA-NAT, TPOH is under circadian control in a number of vertebrate species, including rat (Liang et al., 2004), chicken (Thomas et al., 1991), and Xenopus (Green and Besharse, 1994). Concomitantly with the nocturnal increase in melatonin synthesis, gene and/or protein expression of the enzymes of the melatonin synthesis pathway are upregulated at night (Table 2).
It is worth noting that human and primates might not synthesize melatonin endogenously within the retina, as hiomt mRNA transcripts are barely detected in their retinas (Rodriguez et al., 1994; Coon et al., 2002). This suggests that retinal melatonin in humans and primates could originate from circulating melatonin generated in the pineal gland.
Most mouse strains, including C57BL/6J (B6)--the most common inbred mouse strain used in biomedical research in the United States—are deficient in the biosynthesis of melatonin. Melatonin deficiency results from nonfunctional alleles of the Aanat and/or hiomt genes (Ebihara et al., 1986; Vivien-Roels et al., 1998; Kasahara et al., 2010; Zhang et al., 2018). Because melatonin is an important clock effector in the retina and elsewhere in the body, the full repertoire of circadian rhythms is incomplete in melatonin-deficient strains. By introducing functional alleles of the Aanat and hiomt genes from melatonin-proficient (e.g., CBA/CaJ; CBA) mouse strains to B6, the Ribelayga lab recently generated a B6 congenic line with the capacity of rhythmic melatonin synthesis (Zhang et al., 2018).
Melatonin relays its effects through high-affinity membrane G-protein coupled receptors. Three different melatonin receptor genes have been cloned, namely MT1 (formerly Mel1a), MT2 (formerly Mel1b), and Mel1c (Dubocovich et al., 1998; Vanecek, 1998; Dubocovich and Markowska, 2005; Jockers et al., 2016). The melatonin receptor is typically a high-affinity receptor (KD < 1 nM) negatively coupled to AC, yet other transduction cascades associated with them have been described. In addition, it has been shown that melatonin receptors can exist as monomers and can form dimers, and that this is essential for their activation. MT1 or MT2 monomeric receptor signaling is essentially negatively coupled to AC whereas MT1/MT2 heteromeric receptor signaling is positively coupled to the phospholipase C/inositol triphosphate pathway (Liu et al., 2016). Pharmacological actions of melatonin (at concentrations in the μM range and above) have been described and are thought to be mediated via interactions with intracellular proteins and to result in antioxidant effects and subsequent protection against neurodegeneration, apoptosis, and ischemia/reperfusion injury (Tosini et al., 2012; Liu et al., 2016, 2019). These actions are different from the physiological actions of melatonin, and in particular its “time-giver” (i.e., phase-shifting or Zeitgeber) actions, which rely on membrane receptors.
All 3 known melatonin receptor subtypes are expressed in the retina of all vertebrates, with one exception: the Mel1c subtype is not expressed in the mammalian retina (Reppert, 1997). In Xenopus and chicken retinas, melatonin receptors are widely expressed in all nuclear layers of the retina, including the RPE, as well as in many ocular structures (Xenopus: Wiechmann and Smith, 2001; Wiechmann, 2003; Wiechmann et al., 2004; chick: Natesan and Cassone, 2002; pigeon: Sheng et al., 2021; vertebrates: Wiechmann and Summers, 2008). In mammals, the use of radiolabeled melatonin and antibodies against melatonin receptor subtypes suggested widespread expression of the receptors among retinal layers and ocular tissues (Jockers et al., 2016; Felder-Schmittbuhl et al., 2018). However, in an attempt to identify retinal cell types that express MT1 and/or MT2, Klosen et al. (2019) used a specific reporter (LacZ) knockin strategy in C57/C3H mice, which are melatonin-proficient. They found much more limited expression of both MT1 and MT2 in the mouse retina than previously reported. MT1-LacZ expression was found in photoreceptor inner segments, as well as in a few specific subsets of amacrine cells and ganglion cells. MT2-LacZ expression was surprisingly not detected in photoreceptors but could be detected in subsets of cells in the inner nuclear layer (INL) and RGC layer, although these cells appeared different from those expressing MT1-LacZ. Double-immunochemical analysis with an antibody against tyrosine hydroxylase (TH), a marker of dopaminergic amacrine cells) found a single cell double labeled for both MT1-LacZ and TH. In summary, the results from the reporter gene expression assay of melatonin receptors in mouse retina indicate discrete expression of both MT1 and MT2 receptors in most nuclear layers, with little overlap, and noted their virtual absence from dopaminergic amacrine cells (Klosen et al., 2019).
4.1.2. Circadian clock-driven effects of melatonin
Melatonin affects retinal function, as illustrated by its effects on different components of the ERG (Lu et al., 1995; McGoogan and Cassone, 1999; Emser et al., 1993; Miranda-Anaya et al., 2002; Baba et al., 2009). Arguably, one of the most studied effects of melatonin in the vertebrate retina is on the release of dopamine. Melatonin acutely inhibits dopamine release in all vertebrate retinas (Dubocovich, 1983; Boatright et al., 1994; Adachi et al., 1998, 1999; Behrens et al., 2000; Ribelayga et al., 2004). It is still debated whether the suppressive effect of melatonin on dopaminergic cells is direct or indirect through an inhibitory interneuron. On the one hand, the pharmacological profile of dopamine suppression in the rabbit retina is consistent with the presence of MT2 receptors (Dubocovich et al., 1997). In addition, consistent with this idea, the circadian rhythm of dopamine release in mouse retina likely relies on MT2 receptors since it persists in MT1 knockout animals (Sengupta et al., 2011). On the other hand, transcript expression of both MT1 and MT2 has been found throughout the INL (Baba et al., 2013) but little evidence supports the presence of either receptor subtype in dopaminergic cells (Klosen et al., 2019). MT1 and Mel1c, but not MT2, are expressed in GABAergic and dopaminergic cells in the Xenopus retina (Wiechmann, 2003; Wiechmann et al., 2004; Klosen et al., 2019).
The melatonin/dopamine system has been one of the most studied clock pathways in the vertebrate retina. The observations that melatonin release is high during the night, that dopamine release is high during the subjective day, and that melatonin can inhibit dopamine release have led to the widespread view that retinal melatonin generates the circadian rhythm of dopamine release. In Xenopus and fish, clock genes are rhythmically expressed in photoreceptors where melatonin is produced, and perturbation of clock gene expression alters the melatonin rhythm (Hayasaka et al., 2002). The used direct measurements of dopamine overflow from explanted intact goldfish retinas and electrophysiological recordings to test whether the rhythm of dopamine release depended on the melatonin rhythm (Ribelayga et al., 2004). It was observed that endogenous dopamine release from isolated retinas, which were cultured in continuous darkness for 56 h, clearly exhibited a circadian rhythm with high values during the subjective day. These results demonstrated that a clock in the fish retina generates a circadian rhythm in dopamine release. In addition, the continuous presence of melatonin (1 nM) in the culture medium abolished the circadian rhythm of dopamine release by keeping values constantly low and equal to the nighttime values. More importantly, the selective melatonin receptor antagonist luzindole (1 μM), which blocks endogenous activation of melatonin receptors, also abolished the dopamine rhythm, but in this case, dopamine values remained high and equal to the daytime values. These findings thus demonstrated that endogenous activation of melatonin receptors at night produced the decrease in dopamine release. These observations support the view that the rhythm of melatonin generates the rhythm of dopamine release.
This conclusion was further strengthened by investigating the effects of pharmacological manipulation of melatonin receptors on the circadian rhythm of goldfish cHC light responsiveness. Melatonin application during the late subjective day introduced rod input and reduced cone input to fish cHCs, a state usually observed during the subjective night (Wang and Mangel, 1996; Ribelayga et al., 2004). In contrast, luzindole application during the subjective night decreased rod input and increased cone input, a state usually observed during the subjective day. Most significantly, prior application of dopamine or spiperone, a selective dopamine D2 receptor family antagonist, blocked the above effects of melatonin and luzindole, respectively (Ribelayga et al., 2004). These findings thus indicated that a circadian clock in the retina regulates dopamine release by endogenous activation of melatonin receptors and that melatonin increases rod input to cones at night by inhibiting dopamine release and D4 receptor activation. In summary, these results are consistent with the view that a circadian clock in the retina modulates endogenous dopamine release via the rhythmic production of melatonin, and that the clock-controlled increase in dopamine release is sufficient to activate D4 receptors on rods and cones.
Circadian rhythms of dopamine content and dopamine release are observed in the mammalian retina. However, attempts to investigate the functional relationship linking the melatonin and dopamine rhythms have reported conflicting results. For instance, a circadian rhythm of dopamine content and release is present in the retina of Royal College of Surgeons (RCS) rats (Doyle et al., 2002b). Because RCS rat retinas contain few photoreceptor cells (Dowling and Sidman, 1962; LaVail, 2001) and because melatonin is synthesized primarily, and possibly exclusively, in the photoreceptor cell layer (Iuvone et al., 2005), these observations suggest that a second circadian clock, in addition to the retinal clock that controls melatonin synthesis, controls dopamine content and release independently of melatonin. In contrast, a circadian rhythm of dopamine content and release is absent in mouse strains that are genetically incapable of rhythmically producing melatonin (C57Bl/6J, Doyle et al., 2002a; BALB/c, Nir et al., 2000), but a circadian rhythm is present in C3H/f mice that produce melatonin rhythmically (Doyle et al., 2002a), supporting the view that the retinal rhythm of melatonin is required to generate the dopamine rhythm in mice. Our preliminary data indicate that the melatonin rhythm is required and sufficient for the rhythm of dopamine release to exist in the mouse retina (Mangel and Ribelayga, 2009). Specifically, retinas from the melatonin-proficient CBA/CaJ mouse strain display a circadian rhythm of dopamine release when maintained in vitro for several days. In contrast, no circadian rhythm of dopamine release is found when retinas from the melatonin-deficient C57BL/6J strain are cultured under the same conditions. Finally, the Ribelayga lab recently rescued melatonin proficiency in a B6 congenic mouse line and showed that a rhythm of retinal dopamine release is present in the congenic animals but absent in the littermate controls C57Bl/6J deficient in melatonin (Zhang et al., 2018). Thus, these observations agree with the view that the melatonin rhythm generates the rhythm of dopamine release in the mammalian retina, as is the case in non-mammalian retinas. Whether retinal melatonin that originates from the pineal drives the rhythm of dopamine in RCS rat retinas or whether rats represent an exception to the rule remains to be determined.
Although the exact mechanism/circuit through which melatonin suppresses dopamine remains unclear, melatonin suppression of dopamine is believed to account for many of the clock-related effects of melatonin on retinal physiology. These effects include photoreceptor disk shedding and phagocytosis (Besharse and Dunis, 1983), cone retinomotor movements (Pierce and Besharse, 1985), rod/cone input to cHCs (Wang and Mangel, 1996; Ribelayga et al., 2004), electrical coupling between photoreceptors (Ribelayga et al., 2008; Li et al., 2013; Zhang et al., 2015; Jin et al., 2015; Jin and Ribelayga, 2016) in a way that clearly qualifies melatonin as a nighttime clock effector. It should be mentioned here that melatonin is often improperly referred to as a “darkness” effector. However, darkness by itself is not sufficient to trigger melatonin synthesis, while the action of the clock is required and sufficient to increase melatonin synthesis at night, as long as light is not present to repress it. Thus, darkness plays only a permissive role in the clock-controlled increase in melatonin synthesis at night. Under normal lighting conditions (i.e. darkness at night), melatonin is thus an exclusive output of the retinal circadian clock.
Direct effects of melatonin, which are not mediated by dopamine, have been proposed as well. For instance, melatonin may directly modulate rod photoreceptor responsiveness in the Xenopus retina in an autocrine/paracrine manner (Wiechmann et al., 2003) and horizontal cell sensitivity in the salamander retina (Wiechmann et al., 1988). Although direct effects of melatonin on neuronal activity are possible, it is not always clear whether these effects are direct or indirect through the control of dopamine release. In addition, it is possible that melatonin and dopamine interact at the level of their membrane receptors. A functional interaction between melatonin receptors and dopamine D1 receptors has been demonstrated in the chick retina (Iuvone and Gan, 1995). A molecular interaction between MT1/MT2 and the dopamine transporter (DAT) has also been suggested in mouse (Benleulmi-Chaachoua et al., 2018), consistent with a mechanism through which melatonin can modulate dopaminergic neurotransmission, in addition to inhibition of dopamine release. Studies performed on isolated cells suggest that melatonin indeed may act directly on specific targets (Cosci et al., 1997; Huang et al., 2005; Zhang et al., 2007; Ping et al., 2008; Baba et al., 2013). Thus, based on our current knowledge, it is likely that melatonin acts both dependently and independently of the dopaminergic system to modulate retinal physiology.
4.2. Dopamine
4.2.1. Dopamine synthesis, release, receptors, and pathways in the retina
Dopamine is the main catecholamine of the vertebrate retina, and its functional role has been extensively investigated. All vertebrate species have a single type of dopaminergic cell, which may contain several subtypes (Zhang et al., 2007). These cells, which in most species are called dopaminergic amacrine cells, and in other species dopaminergic interplexiform cells (Dowling and Ehinger, 1978) have cell bodies among amacrine cells in the inner nuclear layer. Dopaminergic interplexiform cells, which are found in fish and primates including humans, are so-called because one of their output processes travels to the outer plexiform layer where it makes synaptic contact with HCs and/or photoreceptors (Dowling and Ehinger, 1975). In contrast, these output processes in dopaminergic amacrine cells are shorter; in some species they barely leave the inner plexiform layer (e.g., rabbit) and in other species they travel a relatively short distance towards the outer retina (e.g., mouse). Both dopaminergic amacrine and interplexiform cells release dopamine onto other neurons from processes in the inner plexiform layer.
In addition to reaching its receptors via direct synaptic contact, evidence suggests that dopamine reaches retinal neurons via volume diffusion (Ribelayga et al., 2002; Witkovsky, 2004). Moreover, dopamine and TH, the rate limiting enzyme in dopamine synthesis, are observed throughout most, and possibly all, processes of dopaminergic cells, suggesting that dopamine may be synthesized and released all along the processes, and that dopamine release involves Na+-spiking (Ehinger and Floren, 1978; Puopolo et al., 2001; Witkovsky, 2004).
Dopamine plays a major role in light/dark adaptation by acting on most cell types in the retina and seting the gain of retinal networks in daylight (Mangel and Dowling, 1985, 1987; Witkovsky and Dearry, 1991; Witkovsky and Schutte, 1991; Djamgoz and Wagner, 1992; Weiler et al., 1997; Witkovsky, 2004). Both flickering and sustained light stimuli increase dopamine release in a variety of vertebrate species (Witkovsky, 2004). Measurements of goldfish cHC light responses suggest that the release of dopamine by flickering, but not sustained, light stimulation is dependent on NMDA receptor activation (Harsanyi et al., 1996). Dopamine synthesis, content and release are modulated both by light and a circadian clock, but to different extents. Light stimulates the activity of the rate-limiting enzyme in dopamine synthesis, namely TH. The effect of light likely involves phosphorylation at multiple sites of the enzyme (Witkovsky, 2004). Circadian variations of TH activity have been observed in many vertebrate species (Table 1). Conversely, dopamine content, which reflects the balance between dopamine synthesis and release, undergoes daily and/or circadian variations depending on the species. Light depletes dopamine retinal content in fish (Kolbinger et al., 1990; Kohler et al., 1990; Ribelayga et al., 2002) but increases it in rodents (Nowak and Zurawska, 1989; Nir et al., 2000; Zhang et al., 2018). Circadian variations of dopamine content are evident in Xenopus (Delgado et al., 2001), lizard (Miranda-Anaya et al., 2002) and rodents (Wirz-Justice et al., 1984; Doyle et al., 2002a, b; Zhang et al., 2018) but not in fish (Kolbinger et al., 1990; Ribelayga et al., 2002).
Direct measurements of dopamine overflow, or of the dopamine metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), have been used as indexes of dopamine release. Such experiments have clearly demonstrated light-stimulated dopamine release, which is consistent with the role of dopamine in light/dark-adaptive processes (Witkovsky, 2004). Dopamine release is under circadian clock control as well. High values of extracellular dopamine are observed during the subjective day, and low values are seen at night (Table 2). However, daylight and the circadian clock increase dopamine release to different extents. Daylight is a more powerful trigger of dopamine release than the circadian clock, and this dual regulation of dopamine release has pharmacological and physiological implications (see Section 4.2.3).
Dopaminergic amacrine and dopaminergic interplexiform cells receive inputs from rod and cone pathways as well as input from ipRGCs (Dumitrescu et al., 2009; Marshak, 2001; Qiao et al., 2016; Zhao et al., 2017). Dopaminergic amacrine cells exhibit a variety of response properties that make them functionally diverse. Dopaminergic cells receive both On and Off inhibitory responses from bipolar cells via GABAergic and glycinergic amacrine cells in the inner plexiform layer (Qiao et al., 2016). In the presence of light, dopaminergic amacrine cells exhibit two kinds of light responses- On sustained and On transient responses (Zhang et al., 2007). In dim light, dopaminergic amacrine cells also receive inhibitory input from the rod pathway via glycinergic amacrine cells (Newkirk et al., 2013; Perez-Fernandez et al., 2019). Some On bipolar cells make ectopic synapses onto dopaminergic amacrine cells and ipRGCs in the Off sub-lamina of inner plexiform layer (Dumitrescu et al., 2009; Hoshi et al., 2009). Somatostatin amacrine cells also form inhibitory synapses on dopaminergic amacrine cells as well as ipRGCs through somatostatin (sst) sst2 and sst4 receptors, respectively (Vuong et al., 2015). ipRGCs also provide retrograde signals to dopaminergic amacrine cells that can eventually alter the visual responses of RGCs (Prigge et al., 2016). Sustained light responses in some dopaminergic neurons may be mediated by inputs from ipRGCs in the inner plexiform layer (Zhang et al., 2008). Conversely, dopamine alters the visual responses of ipRGCs through D1 receptors (Van Hook et al., 2012).
Dopamine binds and acts through two dopamine receptor families, i.e., the D1 receptor family that includes D1 and D5 receptors and the D2 receptor family that includes D2, D3, and D4 receptors. All dopamine receptors are metabotropic, G protein coupled receptors, whose activation either enhances or reduces downstream signaling pathways. Activation of the D1 receptor family (Gs receptor type) increases AC activity, whereas activation of the D2 receptor family (Gi receptor) decreases AC activity. In addition, the D2 receptor family is 100-500x more sensitive to dopamine than the D1 receptor family (Kebabian and Calne, 1979; Missale et al., 1998). Dopamine D4 receptors are the most sensitive dopamine receptor type, binding endogenous dopamine in the low nM range. The difference in sensitivity of the various dopamine receptor subtypes may be an under-appreciated, but nonetheless, fundamental functional characteristic of these receptors, as has been proposed for dopamine receptors in the retina (Section 4.2.3; Ribelayga and Mangel, 2003, 2007, 2010; Ribelayga et al., 2008; Goel and Mangel, 2021).
In the outer plexiform layer, rods and cones express D4 receptors, but not dopamine D1 receptors (Cohen et al., 1992; Nguyen-Legros et al., 1999; Witkovsky, 2004; Iuvone et al., 2005). Conversely, the dendrites of horizontal cells and cone-bipolar cells (both ON- and OFF-cone-bipolar cells) express D1 receptors but not D4 receptors. In addition, the synaptic terminals/release sites of dopaminergic cells express D2 receptors, which function as dopamine autoreceptors, i.e., activation of D2 receptors decreases dopamine release (Harsanyi and Mangel, 1992; Wang et al., 1997). RGCs express both D1 and D2 receptors (Koulen, 1991; Ogata et al., 2012; Van Hook et al., 2012; Veruki, 1997).
The retinal circadian clock does not directly control dopamine levels in the retina. Rather, it affects dopamine through its action on the hormone melatonin (see Section 4.1.2). Because melatonin inhibits dopamine release in the retina (Dubocovich, 1983; Reppert et al., 1995; Ribelayga et al., 2004), the melatonin rhythm produces a dopamine rhythm that is opposite in phase, i.e., when melatonin is high at night, dopamine is lower, and when melatonin is lower in the day, dopamine is higher. Therefore, dopamine acts as a circadian clock signal for the day, while melatonin acts as a circadian signal for the night.
4.2.2. Circadian clock-driven effects of dopamine
As stated earlier, the circadian clock stimulates dopamine release in the retina during the subjective day because its release is not suppressed by melatonin (Ribelayga et al., 2004; see 4.1.2). The clock-induced increase in dopamine release affects many aspects of retinal physiology, morphology, gene expression, and biochemical activity. These effects include, but are not limited to, melatonin suppression, cone contraction, opsin mRNA expression, and neuronal light responses (Cahill and Besharse, 1995; Iuvone 1995; Green and Besharse, 2004; Iuvone et al., 2005; McMahon et al., 2014; see Table 2).
4.2.2.1. Circadian clock-driven effects of dopamine on horizontal cells
As described in Section 3.2.2, early studies had reported that cHCs could respond to dim light stimuli (low scotopic) following maintained dark adaptation (e.g., cat: Steinberg, 1969; goldfish: Mangel et al., 1994), but the mechanism underlying their sensitivity to scotopic stimuli remained unclear. “Scotopic” refers to the range of dim light intensities in which rods, but not cones, initiate the visual process. The first report at the single neuron level that the retinal clock affects the light responses of individual neurons in the vertebrate retina was the finding by the Mangel lab that the light responses of goldfish cHCs, which make synaptic contact with cones, but not with rods (Stell and Lightfoot, 1975), exhibit a day/night difference in constant darkness (Wang and Mangel, 1996). As shown in Figures 2A, 2B, goldfish cHCs respond to very dim light stimuli (low scotopic) at night in the dark but not in the day in the dark (Wang and Mangel, 1996). In this series of experiments, an important control was performed to demonstrate that this daily difference in sensitivity to dim light stimuli is controlled by a circadian clock. Specifically, even though the above experiments were performed in constant darkness, it is possible that the observed day/night difference was not due to a circadian clock but to a day/night difference in an environmental factor, such as a decrease in temperature at night. This possibility was ruled out by showing that prior reversal of the light/dark cycle for 10 days reversed the circadian rhythm in sensitivity (Figure 2B) (Wang and Mangel, 1996). Peak sensitivity occurred during what used to be the day (even though other factors such as daily temperature changes had not been reversed). Spectral sensitivity measurements also showed that a specific type of goldfish cHC, the L-type (H1) cHC, which receives synaptic contact primarily from long-wavelength or red cones (Stell and Lightfoot, 1975), had a spectral sensitivity similar to that of goldfish rods at night, but similar to that of red cones in the day (Figure 2C; Wang and Mangel, 1996). These findings thus indicated that rod input reaches cHCs at night but not in the day.
Because isolated cones require absorption of 100-1000x more photons compared to rods to produce small light responses (Dowling, 2012), the finding that cHCs in intact retina had sensitivity to very dim light stimuli similar to that of rods was striking and unexpected. Because goldfish cHCs do not make synaptic contact with rods (Stell and Lightfoot, 1975), Wang and Mangel (1996) hypothesized that rod input reaches cHCs at night via open rod/cone gap junctions and that rod input does not reach cHCs in the day because the gap junctions close. This idea was strengthened by subsequent studies on goldfish cHCs (Ribelayga et al., 2002; 2004) that showed that the circadian rhythms in light responses, spectral sensitivity, and dim light sensitivity depended on dopamine D2-like receptors (i.e., D4 receptors), which are on cones but not HCs (Witkovsky, 2004). If the hypothesis that rod input reaches cHCs at night via open gap junctions is correct, then cones should respond to very dim light at night like cHCs.
4.2.2.2. Circadian clock-driven effects of dopamine on rod/cone coupling and rod input to cones
Rod/cone gap junctions have been observed in diverse vertebrate species such as fish, amphibians, and mammals including primates (Raviola and Gilula, 1973; Bloomfield and Völgyi, 2009). The Mangel lab was able to confirm the hypothesis that the retinal circadian clock controls rod/cone coupling through activation of cone D4 receptors by injecting tracer into individual goldfish cones in day and night under dark- adapted conditions with or without spiperone (selective D2 receptor family antagonist) or quinpirole (selective D2 receptor family agonist) (Ribelayga et al., 2008). These studies showed that tracer diffused into an average of 1200 rods and 100 cones during the subjective night and during the subjective day in the presence of spiperone but was restricted to an average of 2 cones and 2 rods during the subjective day and during the subjective night in the presence of quinpirole (Figure 4). In addition, use of a technique called “cut loading” (Choi et al., 2012) in which a razor blade dipped in neurobiotin tracer cut through goldfish and mouse retinal tissue showed that tracer had diffused through photoreceptors further from the cut at night in the dark than in the day in the dark (Ribelayga et al., 2008; Li et al., 2013).
Interestingly, similar results were obtained when low-mesopic light stimuli, such as occur naturally right before dawn or just after dusk, were flashed onto the retinas for >60 min before tracer injections (Figure 4F). This result demonstrates that the retinal clock, and not the retinal response to the normal visual environment at night, controls rod/cone coupling. Note that brighter light (from mid-mesopic through photopic) such as occurs typically during the day (but not naturally at night) closed rod/cone gap junctions in both day and night (Figure 4F). It is common for bright illumination to block circadian clock effects, a phenomenon known as “masking” (Ribelayga et al., 2008).
Patch-clamp recordings from goldfish cones yielded results similar to those obtained from goldfish cHCs. Specifically, cone spectral sensitivity was similar to that of rods during the subjective night and during the subjective day in the presence of spiperone, but during the day in the dark or following bright light adaptation green-sensitive, red-sensitive, and blue-sensitive cone spectra were recorded (Figure 3A, B) (Ribelayga et al., 2008). These spectral sensitivity data demonstrate that rod/cone gap junctions are functionally open at night in the dark. In addition, patch-clamp recordings showed that cones were able to respond to very dim (low scotopic) light stimuli at night in the dark but not in the day (Figure 3C). It is worth noting that application of spiperone had no effect on cones at night in the dark (Ribelayga et al., 2008), as reported previously for cHCs (Ribelayga et al., 2002), indicating that the retinal clock decreases extracellular dopamine at night below the threshold of D4 receptor activation (see Sections 4.2.3 and 4.3 for discussion of functional consequences). In addition, measurements indicated that the receptive field size of cones was larger at night in the dark compared to the day in the dark (Figure 3D), consistent with the finding that rod/cone electrical synapses are open at night.
Thus, cones (and cHCs) are able to respond to very dim scotopic light stimuli at night in the dark due to the action of the retinal clock. In other words, cones and post-synaptic neurons in cone pathways respond to very dim (low scotopic) light stimuli at night because the conductance of the rod/cone electrical synapse is high. This phenomenon was observed by maintaining retinas under dark-adapted conditions with no illumination present brighter than low mesopic (i.e., not even a single brief flash) (Wang and Mangel, 1996; Ribelayga et al., 2002, 2004, 2008; Ribelayga and Mangel, 2003, 2010).
Before the demonstration that the retinal circadian clock opens rod/cone gap junctions at night in the dark, it had been assumed for years that rod/cone gap junctions open when background illumination reaches the mesopic range in the morning. “Mesopic” refers to the range of light intensities in which both rods and cones initiate the visual process. This idea was based in part on the observation by several labs that bright light stimulation of dark-adapted retinas introduces a “rod plateau potential” or “depolarizing afterpotential” into cone and cHC light responses by slightly increasing rod/cone coupling (e.g., Yang and Wu, 1989; Krizaj et al., 1998; Witkovsky, 2004). However, as has been shown (see Figure 2A here for cHCs; Figure 1 in Wang and Mangel, 1996; Figure 5A, B for cones in Ribelayga et al., 2008), rod plateau potentials in response to bright light stimulation occur when retinas are previously dark-adapted but not when retinas are previously light-adapted. Moreover, rod plateau potentials that occurred following dark adaptation were eliminated following 5 min of bright light stimulation (see Figure 2A here; Figure 1 in Wang and Mangel, 1996; Figure 5B in Ribelayga et al., 2008). In addition, rod plateau potentials occur when retinas are dark-adapted in the late afternoon or evening (when the retinal clock has begun to slowly open rod/cone gap junctions), but not when retinas are previously dark-adapted in the morning or at midday (Mangel, unpublished observations). Considered together, these results show that the presence of rod plateau potentials in cone (and cHC) responses to bright light stimulation following dark adaptation depends on the time of day and does not indicate that rod/cone coupling has been increased by bright illumination. Rather, the results suggest that the presence of rod plateau potentials in response to bright light stimulation following dark adaptation in the afternoon or evening only reveals that rod/cone gap junctions are slightly open due to action of the retinal clock. In fact, bright light stimulation over the course of ~5 minutes eliminates rod plateau potentials of cones and cHCs by closing rod/cone gap junctions (Wang and Mangel, 1996 Ribelayga et al., 2008; Goel and Mangel, 2021).
Fig. 5. Activation of dopamine D4, adenosine A2A, and cannabinoid CB1 receptors on rods and cones in the day and night work together to increase the day/night difference in rod/cone gap junction coupling.
The contribution of each receptor type (D4, A2A, CB1) increases coupling at night by increasing intracellular PKA/cAMP, but decreases it in the day by reducing PKA/cAMP.
Dopamine: Schematic shows that a retinal circadian clock increases melatonin synthesis and release during the night, which inhibits the release of dopamine from dopaminergic amacrine cells (not shown) sufficiently so that D4 receptors on photoreceptor cells are not activated. In contrast, the retinal clock decreases melatonin in the day, which enhances dopamine release, resulting in volume diffusion of dopamine throughout the retina and activation of D4 receptors on rods and cones. This decreases intracellular cAMP and PKA activity levels in photoreceptors, which lowers the conductance of rod/cone gap junctions so that rod input to cones and cHCs is reduced. Note that separate circadian clocks may influence adenosine vs. melatonin/dopamine although one clock is depicted here controlling both pathways.
Cannabinoids: Schematic also shows that endogenous activation of cone CB1 receptors increases cAMP/PKA and rod/cone coupling via a Gs signal at night when cone D4 receptors are not activated, but decreases cAMP/PKA and rod/cone coupling via a Gi/o protein signal in the day due to activation of cone D4 receptors.
Adenosine: Evidence shows that a circadian clock in the retina itself increases extracellular adenosine at night. The retinal clock is proposed to increase energy metabolism at night so that the extracellular level of adenosine increases. This in turn enhances activation of A2A receptors on rods and cones. As a result, intracellular cAMP and PKA activity levels in photoreceptors increase, thus enhancing the conductance of rod/cone gap junctions so that rod input to cones and then to cHCs is enhanced. Conversely, a clock-induced decrease in energy metabolism in the day lowers extracellular adenosine and A2AR activation. This lowers intracellular cAMP and PKA which closes rod/cone gap junctions so that rod input to cones and cHCs is decreased. This clock-controlled adenosine pathway is parallel to the clock-controlled melatonin/dopamine system.
See text for further details.
Recent studies from the Ribelayga and O’Brien labs have provided measures of the rod/cone transjunctional conductance in mouse retina (Jin et al., 2020) and the formal demonstration that dopamine modulates Cx35/Cx36 phosphorylation in fish (Li et al., 2009) and mouse photoreceptors (Li et al., 2013), and thereby rod/cone coupling. Together the data indicate that the dopamine-dependent signaling pathway that controls rod/cone coupling in mouse and fish appears to be surprisingly similar.
Considered together, these results demonstrate that the retinal clock increases dopamine release in the day by decreasing melatonin production in the day. Dopamine released from dopaminergic interplexiform cells (goldfish) or dopaminergic amacrine cells (mouse, rabbit) activates cone D4 receptors, which decreases intracellular cAMP/PKA and closes rod/cone gap junctions (Figure 5). In contrast, during the subjective night, the retinal clock increases melatonin release, which reduces dopamine release. As a result, cone D4 receptors are not activated. This results in an increase in cAMP/PKA, which increases phosphorylation of Cx35/36, and as a direct consequence, opens rod/cone gap junctions so that rod input reaches cones and then cHCs (Figure 5).
Generally speaking, dopamine, by activating cone D4 receptors in the day, regulates rod/cone coupling (Ribelayga et al., 2008; Ribelayga and Mangel, 2010; Jin et al., 2015, 2020), the rod/cone dominance of cones and cHCs in fish (Ribelayga et al., 2000, 2004; Ribelayga and Mangel, 2003), A-type horizontal cells in rabbit (Ribelayga and Mangel, 2010), and ERG (Manglapus et al., 1999; Jackson et al., 2012), as well as phosphorylation of phosducin (Pozdeyev et al., 2008) and of Cx35/36 (Li et al., 2010; Li et al., 2013; Zhang et al., 2015) in photoreceptors. In fact, the dopamine rhythm appears required and sufficient to generate these rhythms. For instance, the cone dominance of fish cones and fish and rabbit cHCs and quail ERG during daytime relies on the endogenous daytime peak of dopamine release. In support of this view, blockade of dopamine D4 receptors and/or dopamine release during the day abolishes these rhythms (Manglapus et al., 1999; Ribelayga et al., 2002, 2008; Ribelayga and Mangel, 2010). It is noteworthy that in all cases the clock, via dopamine, decreases visual sensitivity during the day, rather than increasing it at night. Thus, dopamine mediates some of the effects of the clock during the subjective day on retinal physiology and therefore qualifies as a daytime effector of the circadian clock. Moreover, as the above experimental evidence shows, the circadian clock-mediated melatonin/dopamine system is conserved among both mammalian and non-mammalian species.
4.2.3. Two dopamine systems in the retina
Evidence suggests that there are two dopamine systems in the retina (Figure 6) that function in a complementary fashion. One of these systems involves the retinal circadian clock acting through dopamine D4 receptors and the other system involves D1 receptors which mediate the retinal response to the ambient (background) light level. This idea arose from consideration of the extant literature concerning the circadian- and non-circadian-mediated effects of dopamine in the retina and from measurements of the modulation of gap junction coupling between rods and cones, on the one hand, and between horizontal cells on the other hand (Ribelayga and Mangel, 2003, 2007, 2010; Ribelayga et al., 2008; Jin et al., 2015, 2016, 2020; Cao and Mangel, 2021; Goel and Mangel, 2021). These studies indicated that the retinal circadian clock increases dopamine release in the day by ~3x compared to night (Ribelayga et al., 2004; Witkovsky, 2004; Iuvone et al., 2005) and acts through high-affinity D4 receptors on rods and cones. However, the increase in clock-mediated dopamine release in the day is not sufficient to affect coupling between cHCs or between rod HCs (Ribelayga and Mangel, 2003, 2007, 2010). cHC-cHC coupling in goldfish and rabbits and rod HC-rod HC coupling in goldfish are high in the dark in both day and night (i.e., coupling is not controlled by a circadian clock). Rather, bright illumination increases dopamine release to a greater extent than the retinal clock, and this higher level of dopamine activates low-affinity dopamine D1 receptors on the dendrites of HCs and bipolar cells.
Fig. 6. Two dopamine receptor systems in the retina.
Schematic representation of the dual control of dopamine release by the retinal circadian clock and light in the fish retina, which activate D4 receptors and D1 receptors, respectively. Although the retinal clock releases less dopamine in the day than bright illumination, circadian clock-induced dopamine release in the day is sufficient to activate cone and rod D4 receptors (but not D1 receptors on the dendrites of cone bipolar cells (cBCs) and horizontal cells (HCs)), because D4 receptors are ~500x more sensitive to dopamine than D1 receptors. As a result, cAMP/PKA in photoreceptors is low in the day. In constant darkness at night, dopamine levels are lower than in the day and not sufficient to activate cone D4 receptors. As a result, cAMP/PKA in cones increases at night. The circadian rhythm in dopamine release is due to the inhibitory action of melatonin on dopamine release. The retinal clock increases melatonin synthesis and release to a greater extent at night than in the day, which results in a circadian rhythm in dopamine release that is opposite in phase (i.e., higher in the day than at night). During the subjective day, melatonin levels are low, and as a result, so is its inhibitory action on dopamine release. Consequently, during the subjective day, extracellular dopamine levels increase sufficiently to activate D4 receptors, but not D1 receptors. During the regular light/dark cycle, daylight increases dopamine release sufficiently to activate D1 receptors.
Modified from Ribelayga and Mangel (2003).
The Mangel lab studied whether the retinal circadian clock modulates coupling between HCs, in addition to modulating gap junction coupling between rods and cones (see Section 4.2.2), based on the following evidence: 1) both light stimulation and the retinal circadian clock increase dopamine release in the retina (Witkovsky, 2004; Iuvone et al., 2005; Mangel and Ribelayga, 2010; Besharse and McMahon, 2016; Ko, 2020); and 2) gap junctions between HCs and between rods and cones are modulated by activation of dopamine receptors (Witkovsky, 2004; Mangel and Ribelayga, 2010). Both D1 and D4 receptors are expressed in the outer retina; D1 receptors are located on the dendrites of cHCs, rod HCs, and cone bipolar cells, whereas D4 receptors are expressed by rods and cones (Witkovsky, 2004; Iuvone t al., 2005; Besharse and McMahon, 2016). D4 receptors display sensitivity to dopamine two to three orders of magnitude higher than D1 receptors (Hillman et al., 1995; Wang et al., 1997; Missale et al., 1998; Ribelayga et al., 2002). It has been known since the 1980s that light stimulation uncouples HCs by activating their D1 receptors (Murakami et al., 1995; McMahon et al., 2001).
It was therefore hypothesized that if the circadian clock-controlled increase in dopamine release were sufficient to activate D1 receptors during the subjective day, then one would observe a clock-mediated decrease in HC coupling during the subjective day, i.e., in the dark in the day. The extent of gap junction coupling between cone- or rod-connected horizontal cells was determined by injecting a tracer molecule, which can diffuse through open (but not closed) gap junctions, into individual cHCs or rod HCs in the day or night under dark-adapted conditions. Use of this technique, which does not require the use of light (Bloomfield et al., 1995; Bloomfield and Xin, 1997), yielded unexpected results. The extent of HC tracer coupling, although extensive, remained unchanged under conditions of constant darkness in the day and night, both in goldfish (Ribelayga and Mangel, 2003, 2007) and rabbit (Ribelayga et al., 2009). Moreover, under these dark-adapted conditions, HC coupling was dramatically reduced by a D1 receptor agonist but was not affected by a D1 antagonist (or a D4 antagonist), suggesting that coupling under dark-adapted conditions is not under the sustained control of endogenous D1 receptor activation. These findings thus show that HC coupling is modulated by bright illumination acting through D1 receptors, but not by the retinal circadian clock acting via D4 receptors.
Moreover, the absence of activity of D1 receptors present on HCs following dark adaptation is consistent with other observations. Dopamine acts through D1 receptors to alter the gating kinetics of horizontal cell glutamate channels, an effect which results in a change of the resting membrane potential of the cells (Knapp and Dowling, 1987). There is, however, no circadian variation of horizontal cell membrane potential in dark-adapted fish (Wang and Mangel, 1996; Ribelayga et al., 2002) and rabbit (Ribelayga and Mangel, 2010) retinas, suggesting that the clock does not control D1 receptor activity. In addition, in fish, spinules, whose formation is controlled by light and requires the activation of D1 receptors, rapidly disappear during dark adaptation (Section 3.1.3; De Juan and Garcia, 2001).
In addition, evidence suggests that endogenous activation of D1 receptors on other cell types in the retina occurs in response to bright illumination. For example, Vaquero et al. (2001) reported that cAMP immunoreactivity in goldfish RGCs is controlled by D1 receptors and can be detected only after dopamine application in the μM range or after bright light adaptation of the retina. In mammals as well several observations suggest that D1 receptor activation requires the presence of light (He et al., 2000; Pérez-Fernández et al., 2019). Interestingly, bright light or D1, but not D4, receptor activation is able to entrain the clock in the inner retina of the mouse (Ruan et al., 2008).
Another D1 receptor-mediated example of neuromodulation in the retina that is not controlled by the retinal clock, but is regulated by the level of background illumination and D1 receptor activation is the receptive field surrounds of ganglion and bipolar cells. The surrounds of both cell types, which are provided by horizontal cells in mammalian and non-mammalian retinas (Mangel, Miller, 1987; Mangel, 1991; Mangel, Brucken, 1992; Thoreson and Mangel, 2012; Chaffiol et al., 2017), are strongest following maintained (>30 min) bright background illumination and minimal following maintained darkness (Barlow, Levick, 1969; Chaffiol et al., 2017). Evidence suggests that surround responses of light-adapted mammalian RGCs are blocked by D1 receptor antagonists (Jensen and Daw, 1989). The Mangel lab has recently shown that dopamine D1 receptors on the dendrites of ON-cone bipolar cells mediate L/D modulation of the strength of ON-cone bipolar cell receptive field surrounds by regulating GABAA receptors on the dendrites of the cells (Chaffiol et al., 2017). Specifically, as the ambient light level increases during the morning, dopamine release and activation of D1 receptors on ON-cone bipolar cell dendrites are enhanced. This in turn increases GABAA receptor expression and function, leading to increased surround strength. Conversely, as the ambient light level decreases during the afternoon, D1 receptor activation is reduced, leading to decreased GABAA receptor expression and function and a decrease in surround strength (Chaffiol et al., 2017).
In contrast to evidence that suggests that bright illumination activates D1 receptors, experimental evidence has shown that the retinal circadian clock uses D4 receptors to modulate other retinal processes in addition to controlling rod/cone gap junction coupling. For example, dopamine regulation of photoreceptor metabolism (Dearry and Burnside, 1986; Besharse et al., 1988; Iuvone, 1995; Burnside, 2001) and rod/cone dominance (Manglapus et al., 1999; Ribelayga et al., 2002, 2004, 2008; Ribelayga and Mangel, 2010) has been reported to be mediated by D4 receptors. In the inner retina, although dopamine D4 receptors are expressed and D2 receptor family agonists and antagonists modulate homologous and heterologous coupling between amacrine cells and RGCs (Mills et al., 2007), it is still unknown whether retinal clocks and endogenous dopamine modulate gap junction coupling in the inner retina on a daily basis.
Experimental evidence from diverse species also indicates that the D4 receptor subtype, and not the D2 autoreceptor, of the D2 receptor family (Section 4.2.1) is under circadian clock control. The D4 receptor subtype, whose sensitivity to dopamine is 2-3 orders of magnitude higher than that of D1 receptors and an order of magnitude higher than that of D2 autoreceptors, is a postsynaptic receptor expressed on photoreceptors that mediates the many effects of the clock on photoreceptor function (Cohen et al., 1992; Zawilska and Nowak, 1993; Hillman et al., 1995; Tosini and Dirden, 2000; Nir et al., 2002; Iuvone et al., 2005; Besharse and McMahon, 2016; Ko, 2020). To investigate whether the circadian clock affects the activity of the presynaptic D2 autoreceptor, we tested the effects of a general D2 receptor family agonist and an antagonist on cHC tracer coupling under dark-adapted conditions in the day and night (Ribelayga and Mangel, 2003). Neither the agonist nor the antagonist had an effect, indicating that any decrease or increase in dopamine release they would have produced were insufficient to affect D1 receptor activity. Although these data do not completely rule out the possibility that the clock modulates D2 autoreceptor activity, other studies have shown that D2 autoreceptor activity is linked to the level of extracellular dopamine and may be physiologically relevant only when extracellular dopamine levels remain low (in the nM range), in darkness or when a dim light background is present (Harsanyi and Mangel, 1992; Rashid et al., 1993; Wang et al., 1997). Thus, in dark-adapted conditions, D2 autoreceptors tend to keep dopamine levels low but are probably ineffective when dopamine release is high (in the μM range) due to bright illumination. According to this view, and assuming that the kinetic properties of D2 autoreceptors are constant throughout the L/D cycle, it is conceivable that the clock-induced increase in dopamine release at dawn may lower the threshold of saturation of D2 autoreceptors before the incoming day, thereby potentiating the daylight-induced increase in dopamine release.
In summary, evidence suggests that two dopamine receptor systems in the retina (Figure 6) work in complementary fashion as the ambient (background) light level gradually changes throughout day and night. In one of these systems, low levels of extracellular dopamine (low nM range), by selectively activating high-affinity D4 receptors, functions as an effector of an intrinsic circadian clock that serves as the primary adaptive mechanism in the retina at night in the dark and under dim illumination conditions at dawn and dusk (Section 4.2.2). In the second system, a higher extracellular concentration of dopamine, by activating low-affinity D1 receptors, constitutes a response to extrinsic changes in ambient illumination over the course of morning, midday, and afternoon, i.e., during mid-mesopic through photopic daylight conditions.
4.3. Interactions between cannabinoid and dopamine receptors in day and night
In addition to expressing dopamine D4 receptors, rods and cones express cannabinoid CB1 receptors, a type of G protein coupled receptor known to signal through Gi, a pathway that decreases intracellular cAMP (Straiker et al., 1999; Yazulla et al., 2000; Witkovsky, 2004; Yazulla, 2008; Bouchard et al., 2016). However, some studies have observed that CB1 receptors can also signal via Gs under certain conditions (Glass and Felder, 1997; Struik et al. 2006, Zheng et al. 2013, Bagher et al. 2016, Garcia et al. 2016), and that the mode of G-protein signaling may depend on dopamine receptor activation.
Although the circadian clock in the goldfish retina increases dopamine release by 3-fold in the day compared to the night (Ribelayga et al., 2004; Iuvone et al., 2005; Mangel and Ribelayga, 2010; Besharse and McMahon, 2016; Ko, 2020; Goel and Mangel, 2021), the day/night difference in rod-cone tracer coupling is remarkably large (~ 600-fold greater at night than in the day). Injecting Neurobiotin, a membrane impermeable tracer molecule that can diffuse through open, but not closed gap junctions, into a single cone in the dark-adapted intact goldfish retina results in its diffusion into an average of two nearby rods in the day, but into an average of 1,200 rods at night (Ribelayga et al., 2008; Goel and Mangel, 2021).
Cao and Mangel (2021) therefore tested the hypothesis that an interaction between CB1 receptors and D4 receptors receptors expressed by cones increases the day/night difference in rod/cone coupling compared to the effect of D4 receptors acting alone (Figure 5). Tracer injections into individual cones in the intact goldfish retina, electrical recordings of cones, and selective antagonists of CB1 receptors and D4 receptors (which reveal whether endogenous activation of CB1 receptors and D4 receptors is present) were used to determine whether endogenous activation of D4 receptors modulates the effects of endogenous activation of CB1 receptors on rod/cone coupling. The results that were obtained are consistent with the idea that endogenous activation of cone CB1 receptors increases rod/cone gap junction coupling at night when cone D4 receptors are not activated, but decreases rod/cone coupling in the day when D4 receptors are activated (Figure 5; Cao and Mangel, 2021).
Because these day/night changes are driven by the retinal circadian clock, they occur relatively slowly, i.e., over the course of hours. Thus, the interactions described by Cao and Mangel (2021) involve tonic endogenous activation of both CB1 receptors and D4 receptors. They observed a reversal in the effect of endogenous CB1 receptor activation after blocking D4 receptors for 30 minutes, suggesting that the D4 receptor-dependent switch in the effects of cone CB1 receptors may occur over the course of tens of minutes. This suggests that the reversal of D4 receptor-dependent CB1 receptor signaling occurs primarily at dawn and dusk as the retinal clock gradually increases and decreases dopamine release (and D4 receptor activation), respectively. Indeed, because 1) the change in dopamine extracellular concentration affects both CB1 receptors and D4 receptors and 2) endogenous activation of CB1 and D4 receptors on cones and rods have similar effects on rod/cone coupling in the day and night, the synergistic interaction between CB1 receptors and D4 receptors – compared to D4 receptors acting alone – may increase the day/night difference in rod/cone coupling and function as a means of speeding up changes in the strength of rod/cone coupling at dawn when rod vision changes to cone vision and at dusk when cone vision changes to rod vision. These daily synergistic interactions between CB1 receptors and D4 receptors may increase detection of very dim large objects at night and fine spatial details in the day and speed up transitions between day and night operating modes at dawn and dusk.
4.4. Metabolism and pH
Although all living tissue produces acid due to metabolic activity, it has been widely accepted that the acid/base ratio, as represented by pH, is strictly regulated to maintain normal function. In the nervous system, however, evidence has shown that neuronal activity can result in shifts in pH that are large enough to influence enzyme and channel functions (Chesler and Kaila, 1992; more recent refs). In the retina, the pH of the extracellular space varies with light stimulation and light/dark adaptation. Light stimulation produces an alkalinization of the extracellular space that surrounds photoreceptor cells (Borgula et al., 1989; Oakley and Wen, 1989; Yamamoto et al., 1992). Conversely, dark adaptation produces an acidification.
Using pH-sensitive microelectrodes, it has been shown that extracellular pH in the in vitro vertebrate retina exhibits circadian variations. These measurements demonstrated that a circadian clock regulates the pH of fish (Figure 7) and rabbit (Figure 8) retinas so that the pH is lower at night (by ~0.1 – 0.15 pH units) compared to the day (Dmitriev and Mangel, 2000, 2001). This day-night difference, which is produced by a circadian clock in the retina itself (Figure 8B, Dmitriev and Mangel, 2001), is several times greater than light-induced pH changes. Interestingly, a decrease in the superfusate pH from 7.6 to 7.4, which changed the extracellular pH of the fish retina by 0.1, reduced the size of cHC responses to full-field stimuli by ~50% (Figure 7C, Dmitriev and Mangel, 2000; see also Harsanyi and Mangel, 1993), suggesting that the clock-induced decrease in extracellular pH at night (= 0.1) reduces the size of light responses. In addition, the lowest extracellular pH value recorded in the retina in both the day and night was in the vicinity of the inner segments of photoreceptor cells, supporting the idea that photoreceptors serve as the primary source of protons (H+) (Figure 7A, Dmitriev and Mangel, 2000). These findings indicate that a circadian clock in the retina regulates the pH of the vertebrate retina and suggest that the shift is large enough to influence synaptic transmission between retinal neurons. In other words, extracellular protons may function as a nighttime effector of the retinal circadian clock.
Fig. 7. A circadian clock regulates extracellular pH in the fish retina.
(A) Extracellular pH (pHo) is shown as a function of distance (μm) from a superfused goldfish retina in the subjective day and night. pH-sensitive microelectrodes were advanced through the Ringer solution to the retina, then through the retina, and finally withdrawn. Retinal pHo was always lower than Ringer solution pH, and the difference between retinal and Ringer solution pH was greater in the subjective night than in the subjective day. The electrodes were moved in 100 μm steps every 30 s. Fast “spikes” on the records are movement artifacts.
(B) The mean difference between retinal and Ringer solution pH exhibits a circadian rhythm. Before the fish were maintained in constant darkness (24 to 48 h), they were entrained to a 12 h light-12 h dark cycle (12 L/12 D) for at least 14 days. The light-dark cycle is indicated at the top of the figure. Retinas were prepared in either the subjective day or the subjective night. The time of subjective day or night indicated corresponds to the time that pH measurements were taken. Each data point represents the mean value ± SEM for 7-12 measurements.
(C) The clock-induced pH changes may modulate synaptic transmission in the retina. A decrease in Ringer solution pH from 7.6 to 7.4 (indicated by the bar above the record) during the day, which reduces retinal pHo by about 0.1 pH units, reduced the size of cHC light responses by about 50 %. Full-field white light stimuli were repetitively flashed at −3 log Io, except when an intensity- response series (−5 log Io to −1 log Io) was obtained (indicated by asterisks). Intensity values are relative to the maximum, unattenuated intensity (Io, 2.0 mW.cm−2) of full-field white light stimuli generated by the photostimulator.
Adapted from Dmitriev, A.V., Mangel, S.C., 2000. J. Physiol. 522, 77-82.
Fig. 8. A circadian clock in the rabbit retina regulates retinal pH.
(A) The average difference between retinal and superfusate pH exhibits a circadian rhythm. Before an experiment, the rabbits were entrained for at least 10 days to a 12 L/12 D cycle or a 12 L/12 D cycle that had been phase-delayed by 9 h (shifted cycle). At the start of an experiment, the rabbits were placed in constant darkness for at least 24 h, after which the retinas were prepared in either the subjective day or night. The time of subjective day (ZT07-10) or night (ZT15-18) that is indicated corresponds to the time that pH measurements were taken. Retinal extracellular pH was defined as the lowest measured pH in the retina. Each data point represents mean value ± SEM for 7-12 measurements.
(B) Retinal pH shift occurs rapidly at the day/night transition. Extracellular pH was monitored continuously from 1 h before subjective dusk (ZT12) until 1 h after by placing microelectrodes into the in vitro retina at the level of the outer limiting membrane. Retinal pH decreased from a maximum (7.29) at ZT 12 to a minimum (7.16) at ZT13.
Adapted from Dmitriev, A.V., Mangel, S.C., 2001. J. Neurosci. 21, 2897-2902.
In addition to regulating synaptic transmission at the cone synapse, circadian modulation of extracellular pH may also have other roles. In fact, extracellular H+ have been reported to affect multiple aspects of retinal physiology, such as LTCCss in cones (Hosoi et al., 2005; Vessey et al., 2005), Na+ current in RGCs (Lilley et al., 2004), center-surround receptive field organization (Barnes, 2003; Thoreson and Mangel, 2012), and negative feedback from cones (DeVries, 2001) or horizontal cells (Kamermans and Fahrenfort, 2004; Barnes et al., 2020) to cones. However, because the circadian-controlled change in exptracellular pH is significantly larger than light-induced changes in exracellular pH in both goldfish and rabbits (Dmitriev and Mangel, 2000, 2001), future work that investigates the role of extracellular pH in synaptic function and retinal physiology should determine whether there is a day/night difference or a L/D adaptive dependency. For example, because the receptive field surround is strongest in the day under light-adapted conditions (Barlow and Levick, 1969; Chaffiol et al., 2017), it Is important to determine whether the effects of changing extracellular pH in the retina (and not just superfusate pH which is different from extracellular pH in the retina) on the surround depend on the time of day and/or the illumination conditions.
The retina requires both glycolysis and oxidative phosphorylation to produce enough energy for normal function (Winkler 1981; Dmitriev and Mangel, 2004). Although a circadian rhythm in energy metabolism was described in the SCN clock decades ago (Schwartz and Gainer, 1977), only recently has such a rhythm been investigated in the retina. Based on observations that retinal extracellular pH decreases at night (Dmitriev and Mangel, 2000, 2001), it has been speculated that this event may reflect an increase in energy metabolism. Another possibility, however, is that the nocturnal decrease in pH may result from a shift in the proportion between glycolysis and oxidative phosphorylation, because glycolysis is a much more acidic way to produce energy than oxidative phosphorylation. However, the selective suppression of either oxidative phosphorylation or glycolysis has almost identical effects on the dynamics and extent of H+ production during the subjective day and night (Dmitriev and Mangel, 2004). Thus, the proportion of glycolysis and oxidative phosphorylation is identical regardless of circadian time, and the circadian rhythm in pH likely reflects total energy production.
This finding is consistent with a subsequent study that calculated that in the dark mammalian rod photoreceptors consume approximately four times as much adenosine triphosphate (ATP) as in the light (Okawa et al., 2008). Interestingly, circulating blood glucose levels are high during the day in mammals (Owino et al., 2016), presenting a potential problem for the retina. However, it is hypothesized that retinal cells store glucose as glycogen during the day, as glycogen synthase 1, a critical enzyme for glycogen storage, is upregulated when glucose is high (Vancura et al., 2016). Furthermore, circadian regulation of retinal fatty acid oxidation also appears to play a role in the metabolic rhythm of the retina (Vancura et al., 2016). These experiments lead to the conclusion that the retinal circadian clock regulates the rate of energy metabolism in the retina so that it is higher at night than during the day. Thus, a circadian clock modulates metabolic activity and pH in the vertebrate retina as part of normal daily function.
Increased energy metabolism during the subjective night (Dmitriev and Mangel, 2004) indicates that a greater amount of work (e.g., ionic transport, biosynthesis), which requires more energy, is performed by retinal cells. Specifically, the nocturnal increase in energy metabolism is the result of an increase in ATP synthesis to compensate for the amount of ATP used by retinal cells and therefore keep the intracellular level of ATP constant. This theory holds only if oxygen and glucose supplies in the retina are sufficient, and in fact, they may not be. It has been known for years that the vertebrate retina utilizes oxygen at a very high rate, especially in the photoreceptor layer (Linsenmeier, 1986). Dark adaptation during the day has been reported to decrease the oxygen tension of the mammalian retina, especially in the synaptic layers and outer nuclear layer where it reaches ~ 0 mm Hg (Linsenmeier, 1986; Ahmed et al., 1993). It is conceivable that the nighttime increase in energy metabolism may increase oxygen consumption to a higher level so that a slightly hypoxic condition is generated. This situation in turn would decrease the steady-state level of ATP, and as a result, AMP would accumulate. The experimental proof to support this hypothesis may be hard to obtain, but the fact that the synthesis of adenosine, which is formed from adenosine diphosphate (ADP), is increased at night or under hypoxic conditions in retinal cells (Ribelayga and Mangel, 2005; Cao et al., 2021; see Section 4.5) supports this view. Additional experiments are needed to investigate in detail the impact of the circadian rhythm in energy metabolism on retinal function, but the prospect that endogenous mechanisms may generate periods of hypoxia of the retinal tissue is particularly intriguing.
4.5. Adenosine
In the CNS, the purine adenosine plays numerous modulatory roles in both normal and pathological conditions, including the modulation of neurotransmission, sleeping-waking, blood flow, inflammation, and pain, and the response to hypoxia and ischemia (Dunwiddie and Masino, 2001; Latini and Pedata, 2001; Sebastião and Ribeiro, 2009). The extracellular level of adenosine increases in response to various stimuli, such as increased tissue activity, hypoxia, and stress. The accumulation of extracellular adenosine arises from two different sources: either from the conversion of extracellular ATP into adenosine via the sequential actions of an ectoATPase and an ectonucleotidase or from the intracellular conversion of AMP via the action of an endonucleotidase. In the latter case, if the intracellular concentration of adenosine exceeds that on the outside, as occurs during ischemia-hypoxia, it is transported out. Clearance from the extracellular space requires reuptake and intracellular breakdown of adenosine by adenosine deaminase or adenosine kinase (Dunwiddie and Masino, 2001; Latini and Pedata, 2001).
Evidence has indicated that adenosine is also an important neuromodulator in the vertebrate retina. Adenosine typically acts as a neuromodulator and mediates its effects through membrane G-protein coupled adenosine receptors (Fredholm et al., 2001). Of the four known subtypes of adenosine receptor, two subtypes have been consistently found in the retina: A1 and A2A receptors (Blazynski and Perez, 1991; Sebastião and Ribeiro, 2009). Both of them display high affinity for adenosine in the nM range but opposing effects on AC activity, i.e., A1 receptors inhibit while A2A receptors activate AC (Ribeiro et al., 2002). Strikingly, the two subtypes are heterogenously distributed; A1 receptors are mainly expressed in the inner retina while A2A receptors are mainly expressed in the outer retina, especially by photoreceptors and RPE cells (Blazynski and Perez, 1991; Li et al., 2013). In addition, evidence suggests that the A3 receptor subtype is expressed by some RGC types (Zhang et al., 2006).
The presence of extracellular adenosine and A2A receptors in the outer retina is consistent with the effects of exogenous adenosine on the physiology of photoreceptor cells. These effects include the stimulation of cone myoid elongation in fish retina (Rey and Burnside, 1999) and a stimulatory effect on melatonin synthesis in Xenopus (Iuvone et al., 2000) and chicken (Haque et al., 2003; Ivanova and Iuvone, 2003a, b), processes known to occur at night under the control of a circadian clock. A variety of inhibitory effects have been reported as well. In the tiger salamander, adenosine inhibits rhodopsin mRNA expression (Alfinito et al., 2002), calcium influx through LTCCs in rods (Stella et al., 2002) and cones (Barnes and Hille, 1989), and, therefore, glutamate release (Stella et al., 2003), and suppresses exocytosis from cone terminals (Stella et al., 2009). Adenosine is also a competitive inhibitor of rhodopsin kinase and thereby inhibits rhodopsin phosphorylation (Donner and Hemila, 1985; Palczewski et al., 1988). Finally, adenosine increases phosphorylation of Cx35/36 proteins at the rod/cone gap junction at night (Li et al., 2009, 2013, 2014). Moreover, endogenous levels of adenosine in the mammalian retina are controlled both by light/dark adaptation and a circadian clock, so that extracellular adenosine is highest at night in the dark (Ribelayga and Mangel, 2005; Cao et al., 2021).
The effects of adenosine have been less characterized in the inner retina, but the purine has been shown to modulate the release of a number of neurotransmitters and neurohormones elsewhere in the brain, such as GABA (Hollins and Stone, 1980), dopamine (Michaelis et al., 1988), norepinephrine (Ebstein and Daly, 1982), and serotonin (Harms et al., 1979). In the inner retina, adenosine inhibits acetylcholine release (Perez et al. 1989) and the glutamate-induced calcium influx in rat RGCs (Hartwick et al., 2004). Consistent with its effects on several components of retinal circuitry, adenosine inhibits the light-evoked optic nerve responses and affects several components of the ERG (Blazynski et al., 1989).
Adenosine levels are intrinsically linked with metabolism, which is highly rhythmic in the retina (see Section 4.4), suggesting that adenosine could also be under the control of a circadian clock. In fact, it has been shown that levels of extracellular adenosine are modulated by a circadian clock and L/D adaptation in both goldfish and rabbit (Ribelayga and Mangel, 2005; Cao et al., 2021). Moreover, in isolated goldfish retinas, extracellular adenosine rhythms have been shown to persist over several days in constant darkness peaking during the subjective night (Cao et al., 2021). Interestingly, the clock and L/D adaptation appear to alter extracellular adenosine through different mechanisms (Ribelayga and Mangel, 2005; Cao et al., 2021). The clock-induced increase is primarily from an intracellular conversion of AMP into adenosine and subsequent transport out of the cells. In contrast, the dark-evoked increase arises primarily from the extracellular converson of ATP. Therefore, a circadian clock in the vertebrate retina regulates adenosine so that both extracellular and intracellular levels of the purine are increased at night.
Recent evidence has shown that circadian clock-controlled alterations in extracellular adenosine modulate neuronal light responses and information processing in the retina. Specifically, we studied whether adenosine functions as an endogenous effector of the retinal clock by activating cone A2A receptors at night. We used a variety of techniques in the intact goldfish retina including measurements of adenosine overflow and content, tracer injections into individual cones to measure the extent of photoreceptor gap junction coupling, and electrical recording of the light responses of cone photoreceptor cells and cHCs. The results show that a circadian clock in the retina itself controls extracellular and intracellular adenosine levels so that they are highest during the subjective night. Moreover, application of melatonin or a combination of spiperone (D4 receptor antagonist) and SCH23390 (D1 receptor antagonist) during the subjective day, or application of luzindole (a general melatonin receptor antagonist) or a combination of quinpirole (D4 receptor agonist) and SKF38393 (D1 receptor agonist) during the subjective night had no effect on extracellular or intracellular adenosine in the goldfish retina. These results suggest that the retinal circadian clock regulates adenosine independently of melatonin and dopamine receptors. In addition, we showed that circadian clock control of endogenous A2A receptor activation increases rod/cone gap junction coupling and rod input to cones and cHCs at night. These results, together with previous findings concerning the melatonin/dopamine system, suggest that the adenosine system is controlled by a retinal clock(s) independently of the melatonin/dopamine pathway. In addition, the findings also suggest that endogenous activation of cone A2A receptors at night increases rod/cone gap junction coupling and rod input to cones and cHCs, whereas endogenous activation of cone D4 receptors in the day decreases rod/cone gap junction coupling and rod input to cones and cHCs (Figure 5). Thus, adenosine serves as a nighttime effector of the retinal clock and dopamine acts as a daytime effector.
5. Circadian clock pathways in the retina
Historically, retinal rhythmicity was initially described as a cascade of interrelated processes that directly or indirectly originate from a unique retinal clock located in photoreceptors and whose primary output is melatonin (Cahill and Besharse, 1995; Mangel, 2001; Green Besharse, 2004; Iuvone et al., 2005). Based on the evidence reviewed here, there may be more than one functional clock and more than one clock pathway in the retina. However, much remains to be done to establish the exact cellular location of these clocks and their associated signaling pathways. Below, we focus on two clock pathways, namely, the melatonin/dopamine and the metabolism/pH/adenosine pathways, and on the contribution of endocannabinoids.
As illustrated in Figure 5 and described in Sections 4.1 and 4.2, a clock located in the retina, specifically in the photoreceptor cells, increases melatonin synthesis and release during the night so that the release of dopamine decreases (Ribelayga et al., 2004). This then lowers the extracellular levels of dopamine and activation of D4 receptors on photoreceptor cells (Ribelayga et al. 2002, 2004). Conversely, in the day when melatoin levels are low, endogenous activation of cone D4 receptors by dopamine increases. This then lowers cAMP and PKA activity in cones, resulting in the closing of rod/cone gap junctions and a decrease in rod input to cones and cone horizotal cells (Wang and Mangel, 1996; Ribelayga et al., 2002, 2004, 2008; Ribelayga and Mangel, 2010).
As also illustrated in Figure 5 and described in Sections 4.4 and 4.5, a circadian clock in the retina itself increases the extracellular levels of adenosine at night, leading to activation of A2A receptors on photoreceptors (Ribelayga and Mangel, 2005; Cao et al., 2021). As a result, because A2A receptors are positively coupled to AC, intracellular cAMP and PKA activity levels increase in photoreceptors during the night (Nir et al., 2002). This then results in the opening of rod/cone gap junctions and an increase in rod input to cones and cone horizotal cells (Ribelayga and Mangel, 2005; Cao et al., 2021).
Direct measurements of cAMP have confirmed that its intracellular levels indeed increase at night in photoreceptors due to the action of a circadian clock (Ivanova and Iuvone, 2003a; Fukuhara et al., 2004). Consistent with this view, superfusion of activators of the cAMP cascade during the subjective day increases Cx35/36 phosphorylation, photoreceptor coupling, and rod input to cones and cHCs (Ribelayga et al., 2002; Li et al., 2013; Choi and Mangel, in preparation). Conversely, blocking the cAMP cascade at night decreases Cx35/36 phosphorylation, photreceptor coupling, and rod input to cones and cHCs (Ribelayga et al., 2002; Li et al., 2013; Choi and Mangel, in preparation). Finally, application of octanol, an alcohol that closes gap junctions, during the subjective night also increases cone input and decreases rod input to cHCs (Ribelayga et al., 2002).
Considered together, these findings suggest that endogenous activation of cone A2A receptors and the lack of activation of cone D4 receptors at night increases intracellular cAMP/PKA, which results in increased rod/cone gap junction coupling and rod input to cones and cHCs, whereas endogenous activation of cone D4 receptors in the day decreases cAMP/PKA, which results in decreased rod/cone gap junction coupling and rod input to cones and cHCs (Figure 5). Thus, adenosine serves as a nighttime effector of the retinal clock and dopamine acts as a daytime effector.
As also illustrated in Figure 5 and described in Sections 4.4 and 4.5, a retinal circadian clock regulates energy metabolism, so that it is high at night and low during the day (Dmitriev and Mangel, 2004). The circadian rhythm in metabolism is directly responsible for the rhythm in extracellular pH (Dmitriev and Mangel, 2000, 2001) and likely for that of adenosine as well (Ribelayga and Mangel, 2005; Cao et al., 2021). Specifically, the high metabolic activity in the dark at night is likely responsible for the decrease in pH and the increase in adenosine. Moreover, evidence suggests that this pathway is independent of the melatonin/dopamine pathway. Adenosine has been reported to stimulate melatonin production (Iuvone et al., 2000; Ivanova and Iuvone, 2003a,b), an effect consistent with its role as a nocturnal clock effector (Ribelayga and Mangel, 2005). In contrast, we found that activation of dopamine and melatonin receptors has no effect on endogenous levels of adenosine in the retina in the day or night (Cao et al., 2021). Based on these observations, the metabolism/pH/adenosine pathway is likely independent of the melatonin/dopamine pathway, yet they may interact at some point, as suggested by the stimulatory effects of adenosine on melatonin synthesis. Additional experiments are needed to determine whether the two pathways are under the control of a single clock or if different clocks control each of them separately. Future experiments will also clarify the link between the molecular clockwork and metabolism. Observations from other brain regions and peripheral organs indicate that the clock molecular mechanism and metabolic homeostasis are tightly linked (Rutter et al., 2002; Green et al., 2008; Besharse and McMahon, 2016).
As described in Section 4.3, in addition to expressing D4 receptors and A2A receptors, photoreceptors express cannabinoid CB1 receptors. The Mangel lab has recently shown that endogenous activation of cone CB1 receptors in the day and night significantly increases the day/night difference in rod/cone gap junction coupling. As shown in Figure 5, they found that endogenous activation of cone CB1 receptors increases rod/cone gap junction coupling at night when cone D4 receptors are not activated, but decreases rod/cone coupling in the day when D4 receptors are activated. Because these opposite effects of endogenous CB1R activation augment the effects of D4R activation in both day and night, CB1R activation greatly increases the day/night difference in rod/cone coupling and speeds up transitions between day and night operating modes at dawn and dusk (see Section 4.3).
The findings that CB1 receptors, D4 receptors, and A2A receptors work together so that rod/cone coupling is minimal in the day and robust at night strongly suggest that photoreceptor coupling is functionally important. Moreover, the presence of these three transmitter receptor types in rods and cones in most vertebrate species and their synergistic effects on rod/cone coupling in the day and night likely represent the outcome of an evolutionary process that enhanced visual performance in day and night, and therefore, animal survival (Cangiano, Asteriti, 2021; Goel, Mangel, 2021). In rod-dominated retinas such as in fish and most mammals, each cone has gap junction connections with many rods and with other cones (Stell, 1967; Tsukamoto et al., 2001; Ribelayga and O’Brien, 2017; Jin et al., 2020; Munenori et al., 2022). Recordings from intact goldfish retinas showed that cones and cHCs, second order cells that receive synaptic input only from cones (Stell, 1967), respond to very dim light stimuli, i.e., sensitivity to dim illumination is increased by ~2 log units, when rod/cone gap junctions are wide open at night, but not in the day when coupling is minimal (Wang and Mangel, 1996; Ribelayga et al., 2008). A similar increase in sensitivity at night compared to the day has also been reported in rabbit cone-connected horizontal cells (Ribelayga and Mangel, 2010). In addition, other evidence suggestes that rod input reaches RGCs and other cells in the inner retina at night via open rod/cone gap junctions (Ribelayga and O’Brien, 2017; Jin et al., 2022).
In addition to providing a means by which very dim light signals from rods reach cones and cells in cone pathways, increased rod/cone coupling at night results in at least three other significant functional outcomes. First, as previously described (Lamb and Simon, 1976; Ribelayga et al., 2008; Ribelayga and Mangel, 2010; Ribelayga and O’Brien, 2017; Goel and Mangel, 2021), the robust increase in rod/cone coupling at night increases the signal-to-noise ratio of rods because photoreceptor coupling reduces noise more than synchronous light signals, especially in response to large, very dim objects. Specifically, photoreceptor coupling greatly decreases noise because noise in a photoreceptor is independent of the noise in its coupled neighbors, and coupling averages and thereby decreases this uncorrelated noise. In contrast, dim light stimuli that are absorbed simultaneously by nearby coupled rods produce synchronous light responses, lessening the reduction in rod signal strength due to coupling. This improves the reliability of the rod response to very dim light stimuli at night, especially to large, very dim objects, a visual ability that enhances animal survival. Conversely, detection of fine spatial details in the day is enhanced when rod/cone coupling is minimal and noise is no longer an issue, allowing individual cones to signal independently of each other (Goel and Mangel, 2021). Thus, synergistic interactions between CB1 receptors and D4 receptors and the action of A2A receptors enhance visual performance on moonless nights and in the day when it is bright.
In addition, the presence of three transmitter receptor types on rods and cones that mediate circadian clock control of rod/cone coupling in the day and night suggests the possibility that the regulation of rod/cone coupling is precisely tuned to visual performance needs throughout day and night. For example, circadian clock-mediated differential modulation of a CB1-D4-A2A receptor complex might open wide rod/cone gap junctions at night in the dark to maximize detection of large, very dim objects, might close rod/cone gap junctions but not cone-cone gap junctions at dawn when rod-initiated vision is switching to cone-initiated vision, and then close cone-cone gap junctions later in the morning to facilitate detection of fine spatial details. Moreover, although current evidence suggests that rods and cones express CB1 receptors, D4 receptors, and A2A receptors, it seems possible that cone types differentially express these transmitter receptor types, and/or that the expression of these three neurotransmitter receptors depends on retinal eccentricity/location. If so, the daily modulation of photoreceptor coupling would differentially affect post-synaptic cells, implying that RGC types and downstream neurons throughout the visual system and responsive to input from different retinal eccentricities (Caldwell and Daw, 1976; Lehmkuhle et al., 1978; Schneider et al., 1979; Mangel et al., 1983; Troy and Shou, 2002, Wienbar and Schwartz, 2017) may be differentially modulated as well.
6. Location and molecular machinery of the circadian clock in the retina
Numerous circadian rhythms have been described in the retina and the core clock proteins are locally expressed within the retina, suggesting that the circadian-generating system is present within the retina. Recent work has begun to unravel the complex relationships between the molecular circadian clocks in the retinal cells, the local circadian clock of the retina, and the principal circadian clock. Proper demonstration that a retinal clock directly regulates a rhythmic retinal process requires showing that the process in question exhibits a circadian rhythm over the course of several circadian cycles when the retina is maintained in vitro under constant environmental conditions and in isolation from the rest of the CNS (Takahashi et al., 2001). This proved to be a challenging endeavor the retina is highly sensitive to changing extracellular conditions, but such demonstrations have now been achieved in many vertebrate species. This provides strong evidence that many, maybe even most, retinal rhythms persist in conditions of isolation, consistent with their control by a local clock.
The emergence of genetic tools to study the retinal circadian clock has begun to strengthen this hypothesis. Storch et al. (2007) generated a conditional knockout of the Bmal1 gene, which is indispensable for clock activity. The specific ablation of Bmal1 in the retina suppressed the circadian rhythms of the ERG and gene expression. Furthermore, expression of reporter genes such as luciferase under the promotor of core clock proteins has demonstrated that many of the core clock genes are rhythmically expressed within the retina (see 5.2). Thus, these experiments demonstrate that at least in mammals, retinal rhythms reflect the activity of local clocks, and that the influence of extraretinal clocks is dispensable for retinal rhythmicity.
6.1. Early evidence for local clock within the retina: melatonin
The rhythmic expression of the melatonin synthesis enzyme, AA-NAT, in the retina was the first circadian rhythm that was found in isolated retinal tissue and persisted for several days under constant conditions (Besharse and Iuvone, 1983). This provided a direct demonstration that there is a circadian clock located locally within the retina which has significant physiological importance. Since this discovery, a local circadian clock has been shown to control many aspects of rhythmic retinal melatonin synthesis across a number of vertebrate species (see 3.2.1). However, the observations made in the Xenopus retina contributed to the assumption that the unique retinal clock in the vertebrate retina was located in the photoreceptors and controlled the rhythm of melatonin, through which the retinal clock affected retinal functions on a circadian basis (Cahill and Besharse, 1995). Recent observations, in particular the widespread expression of clock genes in the chick and the mammalian retinas, have challenged the view of a unique clock and suggest that the retinal timing system is rather dispersed throughout the different retinal layers and cell types (Ruan et al., 2006, 2008; Tosini et al., 2007a; Liu et al., 2012; Jaeger et al., 2015).
6.2. Location of the retinal circadian clock
Over the past 25 years, intensive research in the field of biological timing has provided valuable insights into the nature of the circadian clock mechanism (see 2). The core mechanism of circadian clocks is contained within single cells and relies on a specific set of core clock genes and their protein products (see Section 2). The fundamental organization of the clockwork is shared by virtually all living organisms ranging from the very simplest unicellular species to humans, and in vertebrates has been particularly well studied in mammals (Dunlap et al., 2003; Lowrey and Takahashi, 2004). In the retina, consistent with the presence of a circadian clock, the clock genes are expressed. However, whether there is a single circadian clock or an entire timing system dispersed throughout the retinal layers has been an exciting question.
In Xenopus, homologs of the clock genes have been cloned: xPer1 and xPer2 (Steenhard and Besharse, 2000; Zhuang et al., 2000); xCry1, xCry2a and xCry2b (Zhu and Green, 2001); xClock (Zhu et al., 2000); and xBmal (Anderson et al., 2001). Interestingly, in the retina, all these clock genes are primarily expressed in the outer nuclear layer (Table 2; Anderson and Green, 2000), a finding that is consistent with the presence in Xenopus photoreceptors of a circadian clock controlling the rhythm of melatonin synthesis (Cahill and Besharse, 1992, 1993). Additionally, weak clock gene expression has been found in the inner retina but does not appear to cycle (Steenhard and Besharse, 2000; Besharse et al., 2004).
In birds, rhythmic expression of the clock genes extends beyond the photoreceptor cells (Table 2). Although evidence indicates that a clock is present in the photoreceptors and regulates melatonin synthesis (Thomas et al., 1993; Ivanova and Iuvone, 2003b), as in the Xenopus retina. Melatonin and phospholipid measurements in enriched cell cultures have suggested that other functional clocks are located in the inner nuclear and RGC layers (Guido et al., 2001; Garbarino-Pico 2004a,b, 2005). Functional circadian clocks are thus likely present in different layers in the bird retina.
In mammals, numerous studies have investigated the presence and rhythmicity of clock gene expression among the retinal layers (Table 2). However, the retina contains a number of cell types (probably >130; Yan et al., 2020), each of which could drive cell-specific gene expression and oscillate with different phases and/or peak times. Therefore, a careful examination of individual cell types is necessary to uncover the complex interplay between individual circadian oscillators and their impact on retinal functions.
Cell-specific clock gene expression is readily observed in inner retinal neurons but observed less commonly in the photoreceptors. In the rat retina, rPer1 (Namihira et al., 2001) and rBmal1 and rClock (Namihira et al., 1999) mRNA levels are low in photoreceptors and high in inner retinal cells. In the mouse retina, single cell reverse transcription polymerase chain reaction (RT-PCR) detected co-expression of six core clock genes (mPer1, mPer2, mCry1, mCry2, mClock, and mBmal1) in inner retinal neurons, but was unable to detect all six clock genes together in photoreceptors (Ruan et al., 2006). Dorenbos et al. (2007) found expression of six clock core genes in dopaminergic amacrine whereas they failed to detect any of the six in the rods (Dorenbos et al., 2007). These data together raised the question of whether mammalian photoreceptors contain clocks, despite compelling evidence that a clock controls melatonin synthesis in the photoreceptor layer (Iuvone et al., 2005; Tosini et al., 2007a). In the rat retina, all of the core clock components were identified in isolated photoreceptors (Sandu et al., 2011). In addition, using a similar approach in rat, gene expression of many of the core clock genes was detected in isolated photoreceptors, but importantly, mPer2 and neuronal Per-Arnt-Sim domain-containing protein 2 mRNA (mNpas2) were not (Tosini et al., 2007a). Another study found that all of the core clock components are rhythmically expressed in photoreceptors, but not in the inner retinal layers (Dkhissi-Benyahya et al., 2013). However, it is possible that the lack of rhythmic expression observed in the inner retinal layers was due to individual circadian clocks in the various cell types oscillating at different times (i.e. not in phase).
At the protein level, the core clock components are expressed in every layer of the retina, and in most retinal neurons of the mouse (Liu et al., 2012). Notably, almost all cone photoreceptors but no rod photoreceptors displayed expression of clock proteins. Other cell types which were tested were horizontal cells, bipolar cells, amacrine cells, and RGCs. Surprisingly, while protein expression of all the core clock proteins was observed in cones under both L/D and constant darkness, only CRY2 showed rhythmic protein expression in dopaminergic amacrine cells. Altogether, this suggests that cone photoreceptors contain a functional circadian clock but questions the presence of a functional clock in the rods. However, a recent study provided evidence that rods indeed contain a functional clock (Gegnaw et al., 2021).
Despite the inconclusive results regarding the presence of photoreceptor expression of clock genes, bioluminescence recordings of retinal explants using the reporter luciferase (LUC) under the regulatory mechanisms of the clock genes Per1 and Per2 has provided strong evidence for circadian clocks dispersed in every layer of the retina (Tosini et al., 2007a; Ruan et al., 2008; Jaeger et al., 2015; Xu et al., 2016). In mouse and rat retinal explants of each isolated retinal layer, every layer could generate self-sustained oscillations, although with a period greater than 24 h (Jaeger et al., 2015), demonstrating the presence of an oscillatory clock within each layer of the retina, including the photoreceptor layer, and the importance of intercellular coupling to create rhythms of 24 h at the tissue level. Finally, the presence of functional circadian clocks is not restricted to the retinal neurons. Human and mouse Müller cells, a glial cell that spans through the entire retina, have sustained and robust circadian rhythms when cultured in isolation from other cell types (Xu et al., 2016). Altogether, evidence suggests that most retinal cells in mammals, including glial cells, contain a circadian clock.
6.3. Differences in the functional architecture of retinal circadian clock
While much of the work on the molecular clock in the retina has focused on its location within the retina, another interesting area of research is how the molecular architecture of the retinal clock differs within the retina and among other body clocks. Analysis of molecular clocks throughout the body has revealed that there are tissue specific variations in clock architecture (Takahashi, 2017). Namely, the contributions of each clock component and their interactions vary, allowing the clock to evoke temporally relevant responses in various tissues and control tissue-specific aspects of the transcriptome and function (Panda et al., 2002; Storch et al., 2002; Lowrey and Takahashi, 2004; Panda and Hogenesch, 2004; Mohawk et al., 2012; Mure et al., 2018). This holds true for the retina, where the circadian architecture appears to differ from that of the SCN (Ruan et al., 2012). Furthermore, within the retina there is evidence that each cell type leverages various clock components such that the clock functions in a cell-specific manner (Hwang et al., 2013; Liu et al., 2012; Xu et al., 2016; Wong et al., 2018).
One analysis of the mouse retinal clock and SCN clock used Per1::luc or PER2::LUC recording to analyze the effect of knocking out various components of the clock mechanism (Ruan et al., 2012). One key finding was that overall the clock mechanism in the retina was highly similar to the SCN, but the SCN clock was much more resilient to individual gene knockouts, likely due to strong intercellular coupling. In retinal explants, removal of mPer1, mCry1, and mClock individually abolished bioluminescent rhythms while only dampening their amplitude in the SCN. Interestingly, knocking out mPer2 had a more severe effect in the SCN than in the retina. Furthermore, the number of alleles of mPer1 and mPer3 had different effects on the period in the retina and SCN, with a reduction in mPer1 alleles shortening the retinal period but lengthening the SCN period and a reduction in mPer3 alleles shortening the retinal period but having no effect on the SCN period.
Furthermore, within the retina there appears to be cell-type specific differences in clock function. For example, in mouse Müller glia, mPer1 is necessary for circadian clock rhythmicity, but it does not appear to cycle and is instead constitutively expressed (Xu et al., 2016). Surprisingly, in human Müller glia, hPer1 does not appear to be required for rhythmicity because molecular rhythms were maintained after hPer1 knockdown (Xu et al., 2016). When comparing rhythms of mRNA expression of the core clock genes in the photoreceptors with those of the inner retina there appeared to be differences in the peak expression time, with mPer1 and mPer2 peaking at almost completely opposite phases in the inner and outer retina (Dkhissi-Benyahya et al., 2013). However, it is worth noting that while the rhythm in the outer retina likely reflected solely the cone circadian clock, the rhythm in the inner retina was a global reflection of gene expression from all of the different clocks in the inner retina.
A recent study showed that CRY1 is distributed throughout the retina and functionally important for rhythmic clock function as well as rhythmicity of retinal function, including contrast sensitivity, ERG b-wave, and pupillary light response (Wong et al., 2018). Interestingly, CRY2 appears only necessary for the outer retinal rhythm in the photoreceptors. CRY2 knockouts were still able to generate rhythms based on PERIOD2::LUCIFERASE (PER2::LUC) rhythms and still had rhythms of contrast sensitivity and pupillary light response, demonstrating the continued function of the inner retina. However, CRY2 knockouts exhibited an attenuation of the rhythm of photopic ERG b-wave, demonstrating the importance of CRY2 for cone circadian function.
Another particularly interesting divergence from the classical circadian clock mechanism is that a specific subpopulation of mouse RGCs appears to use NPAS2, a CLOCK homologue, as the partner of BMAL1 to drive circadian rhythms in gene expression (Hwang et al., 2013). Like CLOCK, NPAS2 can form a heterodimer with BMAL1 and serve as the positive limb of the circadian transcriptional feedback loop (Reick et al., 2001). NPAS2 is specifically expressed in Brn3a positive RGCs, and Npas2 mRNA was found to be rhythmically expressed in the RGC layer using a reporter gene (Hwang et al., 2013). Additionally, NPAS2:BMAL1 heterodimers were capable of binding and activating circadian E-boxes, demonstrating its regulatory capability (Hwang et al., 2013). Interestingly, the researchers propose a cell-type specific mechanism that confers a transcriptional preference for NPAS2:BMAL1 opposed to CLOCK:BMAL1, as observed in various cell lines. Thus, even though mouse RGCs express CLOCK (Storch et al., 2007; Liu et al., 2012), CLOCK is unable to bind to certain E-boxes. This transcriptional activation specificity has been observed previously in the cell line NG108-15, a murine neuroblastoma-rat glioma hybrid line (Hampp et al., 2008), and in Aa-nat expression, a clock-controlled gene necessary for melatonin synthesis that appears to involve both CLOCK and NPAS2 in the chick retina (Haque et al., 2010).
This reveals another interesting way in which the circadian clock can differentially exert control depending on the components of the core clock genes it uses. Specifically, Brn3a is a transcription factor that regulates Npas2 expression in RGCs (Sajgo et al., 2017). 94% of NPAS2 protein expressing RGCs are also Brn3a positive. There is a circadian rhythm in contrast sensitivity detection by Brn3a positive RGCs, i.e., peak contrast senstivity detection occurs during the day (Hwang et al., 2013). This rhythm is regulated by D4 receptors that regulate the signaling pathways involving NPAS2 which subsequently regulates AC 1 gene expression (Hwang et al., 2013).
6.4. Melatonin is a major output of a clock in the photoreceptors
At the molecular level, a direct link has been established between a retinal clock in the photoreceptors and melatonin synthesis (Iuvone et al., 2005). The clock controls melatonin synthesis at least through the rhythmic activity of the E-box in the promotor region of the Aa-nat gene. In vitro experiments have demonstrated that this E-box is functional and its activation able to induce the expression of a reporter gene in rat and chick retinas (Chen and Baler, 2000; Chong et al., 2000). In addition, transgenic experiments have demonstrated that a dominant-negative CLOCK protein alters the circadian rhythm of melatonin synthesis in the Xenopus retina (Hayasaka et al., 2002). These data are in agreement with a direct control of Aa-nat gene expression by a retinal clock. However, the nocturnal increase in melatonin synthesis also relies on second messengers, such as cAMP and calcium in the photoreceptors, which are required for the increase in AA-NAT activity (Iuvone, 1990; Iuvone et al., 2005). Whereas the intracellular increase in calcium results from dark adaptation, that in cAMP is likely triggered both by calcium increase and by the clock at night through the transcriptional activation of calcium-dependent type-1 AC (Ivanova and Iuvone, 2003a; Fukuhara et al., 2004). In turn, cAMP contributes to both the transcriptional and/or posttranslational regulation of AA-NAT (Iuvone et al., 2005). Thus, information about L/D information and time of day converge onto the second and third messenger systems to frame the daily profile of melatonin synthesis. Altogether, these observations agree with the presence within the same cells (photoreceptors) of a functional clock and melatonin synthetic enzymes.
7. Rhythms in gene expression
Many genes display daily and circadian variations of their expression in the vertebrate retina, and the circadian clock exerts a great amount of control over gene expression through the role of clock proteins as transcriptional regulators. Thought for a while to be restricted to the photoreceptors, rhythmic gene expression is widespread among the different retinal layers. Yet, studying rhythmic gene expression in the retina poses two major challenges. First, it is difficult to distinguish clock-controlled genes from genes controlled by the daily L/D cycle. Secondly, clock-controlled gene expression is heterogeneous across the different retinal cell types. Recently, advances in RNA sequencing and genetic engineering have allowed researchers to begin to unravel these varying contributions. The gene expression of the core clock components throughout the retina is reviewed in section 5.2.
In the mouse eye, a whole-genome microarray study found that 277 genes show circadian rhythmic expression and 2,670 show daily rhythmic expression in normal L/D conditions (out of 45,101 probe sets; Storch et al., 2007). The genes showing circadian rhythmicity represent a wide range of functions, including synaptic transmission, photoreceptor signaling, intercellular communication, and regulation of the cytoskeleton and chromatin, suggesting a control by the clock at multiple organizational levels of the retina and eye. Interestingly, gene expression is arrhythmic in clock-deficient mice even when kept in L/D conditions, thus unraveling an unsuspected role of the clock in light induction of retinal gene expression (Storch et al., 2007). Recently using chromatin immunoprecipitation sequencing (ChIPseq), BMAL1:CLOCK transcription factor binding sites in the mouse retina have been identified (Sawant et al., 2018). In the primate retina, 733 genes show daily rhythmic expression under normal L/D conditions (Mure et al., 2018). These studies provide crucial data to unravel how the circadian clock and L/D cycles interact to control retinal gene expression and thereby retinal functioning. However, they are limited in that they do not discriminate between retinal cell types. In a recent study, the circadian clock was specifically knocked out in cones, and its effects on the cone transcriptome was analyzed (Bhoi et al., 2021). During the day, 88 genes showed differential expression between cone-specific clock knockouts and their WT littermates, uncovering genes involved in a wide range of functions, including many genes not previously detected to be under circadian control in retina-wide transcriptomes. This demonstrates that within the retina the cell-specific circadian clocks in various cell types differentially control gene expression.
7.1. Visual pigments and phototransduction machinery
Photoreception for the image-forming visual pathway begins in rods and cones through light sensitive opsin proteins. A circadian rhythm in the expression of rhodopsin mRNA, the rod opsin, is under circadian control in many vertebrate species including toad, fish, and mouse (Korenbrot and Fernald, 1989; von Schantz et al., 1999; Yu et al., 2007). Surprisingly, rhodopsin mRNA levels do not display circadian variations in Xenopus (Green et al., 1995a) or chicken (Pierce et al., 1993). Across vertebrate species with rhodopsin rhythms, there is significant variance in when rhodopsin mRNA peaks (Table 1). Interestingly, in zebrafish maintained under constant darkness rhodopsin mRNA throughout the outer nuclear layer peaks at the beginning of the subjective night. However, in individual rods there is significant variance in the phase of rhodopsin rhythms, with different cells peaking at different times; light serves to synchronize individual rod clocks through dopamine D4 receptors (Yu et al., 2007). Cone opsin expression varies on a circadian basis as well (Table 1). Notably, the circadian clock in mouse cones is involved in determining the spectral identity of cones (Sawant et al., 2017).
In addition to photoreceptor visual pigments, other components of the transduction cascade display daily variations of their transcript content, but to date, the persistence of such rhythms in total darkness has not always been tested. This scenario is the case for α, β, and γ subunits of the G-protein transducin (Brann and Cohen, 1987; Bowes et al., 1988; Farber et al., 1991; Sokolov et al., 2002). Arrestin mRNA levels are increased by light but are not modulated by a circadian clock (Farber et al., 1991; McGinnis et al., 1992, 1994 but see Vancura et al., 2018). Notably, interphotoreceptor retinoid binding protein mRNA displays daily and circadian variations of its levels in the zebrafish retina (Rajendran et al., 1996).
Melanopsin (Opn4) is the photopigment of the non-image-forming visual pathway. In mammals, melanopsin expression is limited to ipRGCs and RPE (Berson, 2007; Hankins et al., 2008), while in birds and fish, the pigment expression is widespread throughout the retinal layers, including RPE (Bailey and Cassone, 2005; Cheng et al., 2009). An extensive survey of the functional role of melanopsin is beyond the scope of this review, but many excellent review articles on that topic are available (Berson, 2003; Van Gelder, 2003; Foster and Bellingham, 2004; Berson, 2007; Hankins et al., 2008; Schmidt et al., 2011; Do, 2019; Aranda and Schmidt, 2021). Nevertheless, it is important to note here that melanopsin mRNA expression displays circadian rhythmicity (Table 1), which in turn leads to a modest change in sensitivity of melanopsin cells (Weng et al., 2009). It is also of note that in the chick retina the phase angle of the rhythm depends on the cellular layer (Chaurasia et al., 2005). Together these data demonstrate that melanopsin mRNA expression is under the control of a circadian clock. Whether these rhythmic variations play a role in retinal rhythmicity and/or the light entrainment of the SCN clock is still unclear.
7.2. c-fos expression
The expression of immediate early gene c-fos mRNA and its protein Fos are considered in many cellular systems markers of cellular activation since they are rapidly and transiently induced upon stimulation (Dragunow and Faull, 1989). The expression of c-fos mRNA in the mammalian retina is under the control of both light and a circadian clock. Light transiently induces c-fos mRNA/Fos expression in the inner retina (Yoshida et al., 1993; Koistinaho and Sagar, 1995; Chambille, 1998; Hannibal et al., 2001), including ipRGCs (Semo et al., 2003) and dopaminergic amacrine cells (Zhang et al., 2008; Cameron et al., 2009). In contrast, in the outer nuclear layer, c-fos mRNA expression is primarily under the control of a circadian clock (Yoshida et al., 1993). Specifically, c-fos mRNA levels are low during the day and increase during the night, even under constant dark conditions. In addition, light applied during the night represses the clock-induced high levels of c-fos mRNA expression in the outer nuclear layer (Yoshida et al., 1993).
7.3. Nocturnin
Nocturnin was originally identified by differential display as a circadian-clock-regulated gene with high mRNA expression at nighttime in the photoreceptors of Xenopus (Green and Besharse, 1996a, b). The nocturnal rise in nocturnin mRNA (in the early night) is followed by a peak in the nocturnin protein levels around dawn (Baggs and Green, 2003). Nocturnin displays deadenylase activity, which is a key mechanism of posttranslational control of circadian-related mRNAs (Baggs and Green, 2003). Since the discovery of nocturnin in the retina, nocturnin has been found in many tissues throughout the body where it plays similar roles (Green et al., 2007; Kojima et al., 2011, 2015; Green, 2018; Hughes et al., 2018). For example, nocturnin has recently been found to be a nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) phosphatase that regulates oxidative stress in a circadian manner in the liver (Laothamatas et al. 2020).
7.4. Connexin36
Connexin36 (Cx36) is a gap junction protein expressed in photoreceptors, bipolar cells, amacrine cells, and RGCs, and contributes to cell-cell electrical coupling (Bloomfield and Völgyi, 2009). Notably, electrical coupling between photoreceptors and AII amacrine cells is controlled by light and/or the circadian clock (Bloomfield and Volgyi, 2009; Ribelayga and O’Brien, 2017). Electrical coupling between photoreceptors is increased at night (Ribelayga and O’Brien, 2017). Although the increase in coupling mostly results from post-translational modifications of Cx36 (Ribelayga and O’Brien, 2017), it has been shown that concomittantly, Cx36 gene expression in photoreceptors also peaks at night or subjective night (Katti et al., 2013). Interestingly, Cx36 protein levels show circadian rhythms of expression in melatonin proficient retinas (C3H), but not in melatonin deficient retinas (C57BL6/FVB), yet both strains exhibit rhythms of protein expression under L/D conditions (Katti et al., 2013). This suggests a post-transcriptional control mechanism which regulates the expression of Cx36 in photoreceptors and that is dependent on light and melatonin. It is currently unknown whether Cx36 is the only connexin in the retina whose expression varies with the L/D and/or circadian conditions.
8. Entrainment of retinal rhythmicity
An important hallmark of biological clocks, including the clocks in the retina, is their ability to entrain to environmental stimuli (also called Zeitgebers). The dominant and therefore physiologically most relevant entraining stimulus comes from environmental light cues (Pittendrigh, 1981, 1993). The effect of light on the phase of the retinal clock that controls the melatonin rhythm has been extensively studied in the Xenopus retina. Cahill and Besharse (1995) took advantage of their flow-through superfusion system to monitor the melatonin rhythm for several days and to manipulate it. Under these conditions, the effects of light and exogenously applied dopamine were studied. Both light and dopamine have two distinct effects on melatonin synthesis. First, they acutely suppress melatonin synthesis during the nighttime, an effect also known as negative masking, mainly through inhibition of the cAMP pathway and AA-NAT activity. Second, they phase shift the melatonin rhythm in a direction depending on the phase of the circadian rhythm when the light pulse or dopamine is applied (Cahill and Besharse, 1990; Iuvone et al., 2005). Surprisingly, although the effects of light and dopamine are similar and do not require an intact inner retina to display their effects, they use different intracellular pathways to reset the clock (Cahill and Besharse, 1992, 1993). Pharmacological experiments have demonstrated that dopamine acts through D4 receptors (Cahill and Besharse, 1990, 1993) that are known to decrease intracellular cAMP (Missale et al., 1998; Vallone et al., 2000; Martel, McArthur, 2020). But while artificially increasing cAMP inhibits the phase shifting effects of dopamine, it does not block the effect of light (Hasegawa and Cahill, 1998, 1999a,b). The effects of light are likely mediated by calcium, whose intracellular concentration drops when the cGMP-gated channels close as a result of the activation of the phototransduction cascade by light. Thus, dopamine and light act through separate pathways, parallel yet convergent at some point to control melatonin synthesis in photoreceptor cells.
Observations from isolated zebrafish, lizard, pigeon and rodent retinas have generalized the effect of light on retinal melatonin synthesis to all classes of vertebrates (Cahill, 1996; Tosini and Menaker, 1996, 1998a, 1998b; Adachi et al., 1998, 1999). However, although dopamine clearly acutely inhibits melatonin synthesis in rodents (Nguyen-Legros et al., 1996; Tosini and Dirden, 2000) and chicken (Zawilska, 1994), it is not clear whether dopamine is able to phase shift the clock in these species. Long-term flow-through experiments that test the phase shifting effects of dopamine in chicken and mammalian retinas are still needed.
The phase shifting effects of light and dopamine on the melatonin rhythm strongly suggest that the circadian clockwork itself may be affected, in addition to the melatonin rhythm. Observations in the Xenopus retina have strengthened this hypothesis (Steenhard and Besharse, 2000; Zhuang et al., 2000). However, the two isoforms of the Xenopus Per gene, namely xPer1 and xPer2, display radically different patterns and regulations. Although xPer1 is expressed with circadian rhythmicity and is relatively insensitive to acute exposure of light or dopamine, xPer2 expression does not display any rhythmicity in constant darkness; but, xPer2 is acutely upregulated by light or dopamine through D4 receptors (Steenhard and Besharse, 2000; Zhuang et al., 2000). These data suggest that xPer2 functions as the clock element involved in circadian entrainment and as the common target on which both light and dopamine converge to exert their effects on the clock.
In mammals, light entrains the rhythm of melatonin (Tosini and Menaker, 1996, 1998a). The mechanism of entrainment is unknown but thought to be based on the induction of clock gene expression, as in the SCN and the Xenopus retina. In support of this hypothesis, acute light exposure increases the expression of Bmal1 and Clock in the rat retina (Namihira et al., 1999). The behavior of the Per genes in the rat retina seems similar to their behavior in Xenopus, in that the gene expression of one isoform (rPer1) is higher than that of the other (rPer2) in the presence of light (Namihira et al., 2001; Yujnovsky et al., 2006). A recent in vitro study in the mPer2Luc mouse has focused on the entrainment of the PER2:LUC rhythm in the inner retina (Ruan et al., 2008). Interestingly, the rhythm in bioluminescence can be entrained by light or dopamine through D1, and not D4, receptors (Ruan et al., 2008). Recent work has identified a population of ipRGCs which extend axon collaterals that project distally towards the outer retina, and specifically appear target dopaminergic amacrine cells (Prigge et al., 2016). Through this retrograde pathway, ipRGCs modulate retinal light adaptation (Zhang et al., 2012; Prigge et al., 2016), and therefore possibly modulate retinal photoentrainment through dopaminergic activity. This view is strengthened by evidence in OPN4 knockout mice which have impaired photoreceptor layer rhythmicity (Dkhissi-Benyahya et al., 2013).
However, Buhr et al. (2014) made the surprising finding that mouse retinas lacking rod, cone, and melanopsin (OPN4) photoreception are still able to entrain intrinsic retinal circadian clocks as assayed in vitro. This observation soon led to the discovery of a role for an orphan opsin, neuropsin (OPN5), in local photoentrainment of the retina (Buhr et al., 2015). In mice with photoreceptor layer degeneration and OPN4 knockout, retinal explants were capable of photoentraining, whereas in genetic knockouts of solely OPN5, retinal explants were unable to entrain to L/D signals (Buhr et al., 2015). This suggests that OPN5 is necessary and sufficient for ex vivo photoentrainment. Interestingly, OPN5 knockout mice are still capable of behavioral photoentrainment and have typical visual responses (Buhr et al., 2015), suggesting that OPN5 plays little role, if any, in the photoentrainment of the SCN clock (Ota et al., 2018). These studies suggest that the photopigment responsible for entraining the local retinal clock (OPN5) is different from that responsible for photoentraining the SCN clock (OPN4) (Ribelayga, 2016; Guido et al., 2022). This functional distinction could serve as a safety mechanism preventing retinal arrhythmicity from severely impacting the function of the principal clock in the SCN.
Together the data collected in the different vertebrate classes are consistent with observations made in the SCN in that light entrainment requires clock gene induction and that specific clock components may be involved in the oscillatory mechanism of the clock, while others may be more specialized in the entrainment mechanism itself (Lowrey and Takahashi, 2004; Takahashi et al., 2008). Yet, it appears that the pathways of entrainment may be different according to the location of the clock in the retina. Specifically, dopamine D4 receptors may be involved in entrainment of the clock in the photoreceptor cells, while D1 receptors are likely involved in the entrainment of the clock in the inner retina. OPN5 is expressed in different types of RGC (Buhr et al., 2015; D’Souza et al., 2021; Guido et al., 2022) and appears to be required and sufficient for photoentrainment of all retinal clocks. However, a recent study indicates that rods may play a role as well in this process (Calligaro et al., 2019). We currently know little of the intraretinal pathway(s) through which OPN5+ RGCs set the clock in other retinal cells and whether rods (and cones) play a redundant role in this process.
9. Nonphotic inputs to the retinal clock
Although the major input to the retina is light, experimental data indicate that multiple nervous and humoral afferent inputs may influence retinal function and its rhythmic display.
Afferent innervation of the retina has been studied for over a century and reported in all classes of vertebrates (Repérant et al., 1989, 2006, 2007). The site of origin of retinal centrifugal fibers varies considerably among vertebrate species: mostly from the preoptic retinopetal nucleus and olfactory bulb in fish; anterior hypothalamus, lamina terminalis and pretectal region in amphibian; ventral thalamus, caudal mesencephalic tegmentum and telencephal in reptile; and the isthmo-optic nucleus and caudal mesencephalon in birds. In mammals, a retinal projection from the raphe nuclei has been proposed as the main source of 5-HT for the retina (Villar et al., 1987). Centrifugal fibers mostly project onto the outer and inner plexiform layers and the inner nuclear layer. Several neurotransmitters (5-HT) and peptides (i.e. FMRFamide, gonadotropin releasing hormone, substance P, met-enkephalin, histamine) have been detected in their synaptic endings (Repérant et al., 1989, 2006, 2007, Gastinger et al., 2006).
The role of centrifugal innervation on retinal physiology has been particularly well studied in teleosts. The teleost retina is innervated by a dense efferent projection from the olfactory bulb to the dopaminergic interplexiform cells (Zucker and Dowling, 1987; Umino and Dowling, 1991). This projection modulates the dopaminergic system, sufficiently enough to have an impact on visual sensitivity (Li and Dowling, 2000a, b), and may transmit olfactory signals directly to the retina (Maaswinkel and Li, 2003).
The general role played by centrifugal innervation on retinal circadian rhythmicity remains elusive however, and may be species-dependent (Remé et al., 1991). On the one hand, the fact that the melatonin rhythm persists in explanted retinas demonstrates that retinal centrifugal innervation is not required to generate this retinal rhythm (see Section 5.1). On the other hand, sectioning of the optic nerve abolishes the circadian rhythms of b-wave amplitude and of dopamine content in the lizard Iguana iguana (Miranda-Anaya et al., 2002), supporting an important role for centrifugal fibers in retinal rhythmicity. Other studies have reported that if optic nerve sectioning does not abolish the rhythm of rod disk shedding in the rat retina, the procedure abolishes the capacity of the rhythm to entrain to light (Goldman et al., 1980; Teirstein et al., 1980). Thus, at least in higher vertebrates, it seems that retinal centrifugal fibers are not required to generate most retinal rhythms, though they clearly affect some other aspects of retinal rhythmicity. Additional experiments are needed to determine whether this is a general rule and if there are exeptions to this rule. Based on current knowledge, a parsimonous suggestion is that centrifugal fibers modulate retinal function and that these effects likely interfere with clock pathways.
In addition to the retinal centrifugal inputs, several circulating substances may modulate retinal rhythms, including blood glucose and melatonin. A circadian rhythm in circulating glucose has been reported in mammals (La Fleur et al., 1999), including humans (Bolli et al., 1984), with peak values observed before the onset of activity, and this rhythm is under the control of the SCN clock (Kalsbeek et al., 2004). The variation in the glucose supply of the retina is correlated with a change in contrast sensitivity in humans (Barlow et al., 1997). Indeed, contrast sensitivity decreases four times at night, when the blood glucose level is the lowest. Ingestion of glucose at night reverses the decline in sensitivity whereas an insulin-induced decrease in circulating glucose during the day reduces contrast sensitivity ~ 10-fold. These experiments indicate that retinal function is tightly coupled to glucose supply and that even a small circadian-controlled drop (~ 15 %) in blood glucose may be sufficient enough to affect visual sensitivity profoundly (see Barlow, 2001 for details).
The effects of circulating melatonin on retinal rhythmicity have been extensively investigated as well. Pineal melatonin production is under the control of an extraretinal oscillator and contributes to circulating levels of melatonin that increase during the night (Simonneaux and Ribelayga, 2003). Circulating melatonin may penetrate into the retina and influence retinal rhythmicity through melatonin receptors (Grace et al., 1999; Doyle et al., 2002a). An easy way to abolish circulating melatonin levels is to surgically remove the pineal gland (pinealectomy). Such a procedure clearly increases the amplitude of the retinal rhythm of melatonin in goldfish (Iigo et al., 1997a), chicken (Cogburn et al., 1987), hamster (Faillace et al., 1995) and rat (Yu et al., 1981). These observations suggest that normal pineal melatonin production suppresses retinal melatonin synthesis in intact animals. In addition, pinealectomy strongly alters circadian regulation of ERG components in lizard (Shaw et al., 1993) and chick (McGoogan and Cassone, 1999). The clear impact of pineal melatonin on retinal rhythms may represent a way by which extraretinal clocks (located in the SCN or in the pineal gland) influence retinal rhythmicity. An attractive hypothesis is that pineal melatonin may consolidate the phase of the retinal clocks as it does in the SCN (McArthur et al., 1991). Future studies will aim to determine whether specific identified substances (e.g., glucocorticoids, retinoic acid) that are able to entrain peripheral clocks are also effective in the retina (Balsalobre et al., 2000; McNamara et al., 2001).
In conclusion, evidence indicates that nervous and humoral inputs of the retina impact the temporal organization of retinal functions. Their influence appears more pronounced in non-mammals than in mammals. Although it is likely that these inputs do not generate most retinal rhythms in mammals, such as the melatonin rhythm, they undoubtedly affect some aspects of retinal rhythmicity. The earlier observation that rhythmic expression of the clock gene rPer2 is disrupted in the rat retina following lesion of the SCN clearly reinforces this view (Sakamoto et al., 2000).
10. Development of the circadian clock system in the retina
Developmental studies on retinal rhythmicity have been undertaken in most classes of vertebrates. Most of these studies have focused on the developmental appearance of the melatonin rhythm. In zebrafish, a functional circadian clock regulates melatonin synthesis from 20-26 h after fertilization, although the study did not determine whether melatonin originated from the retina or the pineal gland (Kazimi and Cahill, 1999). Interestingly, larvae zebrafish have normal vision during the day but are totally blind at night (Emran et al., 2010). In the isolated Xenopus retina maintained in L/D conditions, day/night differences in melatonin release are observed as soon as melatonin is detectable (stage 26/29; Green et al., 1999). However, in constant darkness conditions, the circadian rhythm appears later (stage 41) and is fully established at stage 47 (Green et al., 1999). At this stage, photoreceptors have reached full development, and the retina exhibits light-evoked responses (Witkovsky et al., 1976; Hollyfield and Rayborn, 1979). It is noteworthy that the rhythmic synthesis of melatonin in the Xenopus retina appears after the rhythm of melatonin in the pineal gland (Green et al., 1999). In the chick retina, although AA-NAT activity is detectable as soon as embryonic day (ED) 6 (Iuvone, 1990), subjective day/night differences appear just before birth at ED 19-20, concomitantly with the first occurrence of a daily rhythm of melatonin (Reppert and Sagar, 1983; Iuvone, 1990) and the developmental stage when photoreceptor cells are first observed (Bruhn and Cepko, 1996). The day/night rhythm of AA-NAT activity and melatonin reach their adult amplitude two to three days after birth (Reppert and Sagar, 1983; Iuvone, 1990). As in Xenopus, the rhythmicity of melatonin synthesis appears later in the chick retina compared to the pineal gland (ED 16-18; Akasaka et al., 1995; Lamosova et al., 1995). In the rat retina, no data are available on the ontogeny of melatonin synthesis, but a postnatal study reported that Aa-nat mRNA is present one day after birth (P2) and displays day/night variations by P8-14 under the L/D cycle. However, the circadian nature of these daily variations is evident later, by P14-20, when eyelids open (Sakamoto et al., 2002). Thus, rhythmic melatonin synthesis occurs in the rat retina far after the SCN clock becomes functional (by P1) and the pineal gland is able to rhythmically synthesize melatonin (Klein et al., 1981; Ribelayga et al., 1998). It is interesting to note, however, that even if mammals are blind at birth, the newborn retina is nevertheless sensitive to light. Indeed, ipRGCs are functional at birth and already make functional connections with the SCN (Munoz Llamosas et al., 2000; Sernagor, 2005).
Interestingly, the circadian clock appears to have an important role in retinal neurogenesis, suggesting that prior to retina-wide circadian modulation cell-autonomous circadian clocks are functional and physiologically important. In mice lacking the circadian clock mechanism in the retina through retina-specific deletion of Bmal1, there are increased numbers of bipolar and Müller cells and decreases in specific populations of RGCs and amacrine cells (Sawant et al., 2019). The authors suggest that the perturbations in the timing of cell cycle exit in retinal progenitor cells leads to atypical cell fate which ultimately impacts visual function. Furthermore, cone-specific deletion of Bmal1 leads to an upregulation of short-wavelength cone opsin in the dorsal mouse retina whereas Per2 deletion has the opposite effect (Sawant et al., 2017). Additionally, comparing ocular transcriptomes between WT and mice with a loss of function mutation in Per1 and Per2 at various developmental timepoints revealed a number of genes and pathways in which the circadian clock is involved in the eye (Bagchi et al., 2020). These pathways include cell cycle and phototransduction as well as other novel pathways, with the number of differentially expressed genes increasing throughout development. These studies demonsrate the relevance of the circadian clock during retinal development and could inform future studies of the circadian clock during retinal development.
BMAL1 has also been implicated in the development of the retinal circuitry. Surprisingly, in retina-specific Bmal1 knockouts (ret-Bmal1−/−) retinas, in addition to perturbations of photopic ERG b-wave amplitude, there was a decrease in scotopic ERG b-wave amplitude (Baba et al., 2018a). This result is surprising because scotopic ERG b-wave amplitude is not rhythmic. Interestingly, the rod pathway deficits appear to be caused by stunted development of rod bipolar dendrites (Baba et al., 2018a). This was observed as early as 1 month and as late as 26 months in ret-Bmal1−/− retinas, suggesting it is a developmental rather than a degenerative result of Bmal1 deletion.
In conclusion, the daily rhythm of melatonin synthesis in the retina is clearly evident during the last stages of retinal development when photoreceptors become fully mature. Additionally, core clock proteins have a critical role in the developing retina, suggesting a role for the circadian clock at an ealier developmental stage. However, it remains unclear whether these developmental effects in Bmal1−/− retinas are due to the lack of a functional retinal clock or the inability for BMAL1 to serve as a transcription factor. Recently, Bagchi et al. (2020) demonstrated that all of the core clock genes are expressed by embryonic day 13 in the mouse retina using RT-PCR. While they did not analyze the rhythmicity, this provides evidence that in the mammalian retina rhythmicity could be functional very early in development. Future studies will aim to determine whether the development of melatonin rhythmicity parallels that of functional clocks in the retina or whether retinal clocks are functional at an earlier developmental stage.
11. Circadian rhythmicity and retinal degeneration
As has been suggested (Besharse and McMahon, 2016), evidence has indicated that circadian timing may be an important physiological process in the vertebrate retina and that disruption or even perturbation of the temporal organization of retinal physiology may contribute to retinal degeneration. Photoreceptors degenerate in response to many genetic and epigenetic malfunctions of the retina, leading to their permanent loss and possible blindness (Sahel et al., 2001). Abolishment of circadian rhythmicity in photoreceptors may be involved in the mediation of the degenerative effects of certain mutations affecting rhodopsin, rhodopsin kinase or arrestin (Fain and Lisman, 1999). Malfunction of the transduction cascade could have effects similar to chronic light exposure or deficiency in vitamin A, that is, abolishment of circadian rhythmicity in photoreceptors. In addition, many mutations have been linked to the death of either rods or cones but it is still unclear why the death of one photoreceptor type subsequently triggers the death of the other type (Sahel et al., 2001; Delyfer et al., 2004; Ma et al., 2018). Because rods and cones are coupled though gap junctions, which are large enough to allow the diffusion of intracellular signaling molecules (Bennett and Zukin, 2004; Connors and Long, 2004), it has been proposed that photoreceptor metabolic coupling may play a role in photoreceptor survival and/or death. Specifically, it has been suggested that cone survival might depend on the diffusion of nutrients and protective factors from coupled healthy rods (Striedinger et al., 2005; Ma et al., 2018; Xu et al., 2022) and/or cones might die due to the diffusion of pro-apoptotic factors from coupled dying rods (Ripps, 2002). Thus, by modulating the rod/cone gap junction conductance on a daily basis, the retinal clock may play a key role in the balance between life and death of photoreceptor cells.
As a direct consequence, abolition of the rhythms of clock effectors (such as dopamine, adenosine and melatonin) may affect photoreceptor survival. In fact, melatonin increases and dopamine decreases the susceptibility of the photoreceptors to light damage (Bubenik and Purtill, 1980; Wiechmann and O’Steen, 1992), and adenosine increases photoreceptor survival (Ivanova and Iuvone, 2003a,b; Paesde-Carvalho et al., 2003). The suppression of the rhythms of these clock effectors may also directly impact the complex regulation of fundamental functions, thereby leading to photoreceptor degeneration. Maintaining an appropriate pattern of gene expression in phase with the rhythm of photoreceptor tip shedding is obviously essential for photoreceptor outer segment renewal. Indeed, the size of rod outer segments increases in constant light when the normal rhythm of disk shedding is abolished (Besharse et al., 1977), and the blockade of disc turnover results in photoreceptor degeneration (LaVail, 2001).
Circadian factors could also influence retinal degeneration when inappropriately present. For instance, following a phase shift of 12 h, such as one may experience when traveling abroad, the out-of-phase melatonin rhythm may have detrimental effects and increase photoreceptor sensitivity to light damage during the daytime (Wiechmann and Summers, 2008). Additionally, it is well established that melatonin production normally decreases with age, and low levels of melatonin have been proposed to play a role in aging and/or age-related diseases, including in the eye and the retina (Pandi-Perumal, 2008). Thus, with frequent large shifts in the phase of the rhythms of clock effectors, genetic defects of the clock components and age may all lower melatonin levels, a condition that may be potentially harmful in the long-term for retinal cells.
Based on the indirect evidence stated above, one would logically presume that knocking down the clock in the retina would induce retinal degeneration. Surprisingly, the genetic ablation of one of the key clock components, BMAL1, specifically in the retina did not affect the gross morphology of the retina at 3 months of age, although it did dramatically affect light-induced gene expression and retinal processing (Storch et al., 2007). The state of the retina is more similar to “night” in Bmal1 knockout mice, as evidenced by the low amplitude and slow kinetics of the photopic ERG b-wave observed in WT mice only at night but both day and night in mutant mice (Storch et al., 2007). Although these results confirmed the importance of the retinal clock in visual processing, they argue against a role for the clock in retinal cell viability. However, a subsequent study demonstrated that proper melatonin signaling in the retina is indeed required for cell viability, especially in regard to aging (Baba et al., 2009). The effects of a lack of melatonin signaling were not seen in young animals but became obvious in mice that were at least 12 months old. Specifically, the authors found that 12-month-old mice lacking the melatonin MT1 receptor had 25% less photoreceptor nuclei compared to WT controls at the same age, a trend that was also observed at 18 months (−20%). At this age (12 months), a significant lower number of RGCs (−25%) was also observed in mice lacking the MT1 receptor.
This work motivated research into the impact of retinal clocks in aging. Interestingly, recent work has shown that in retina-specific clock knockouts there is 20-30% decrease in the number of nuclei in the outer nuclear layer at 8-9 months of age (Baba et al., 2018a). This result was found in both ret-Bmal1−/− and Clock/Npas2 knockout retinas, suggesting that the deficits in photoreceptor viability are the result of clock disruption rather than the loss in BMAL1 transcriptional activity. In line with this work, the number of cones in ret-Bmal1−/− compared to WT retinas was the same at 3 months of age, but significantly reduced in ret-Bmal1−/− retinas compared to WT at 26 months of age (Baba et al., 2018a). During aging there was a marked decrease in the number of cones in both genotypes, but the loss was severely accelerated in the knockouts. In addition to decreases in the number of cones in ret-Bmal1−/− retinas, surviving cones had shorter outer segments. These deficits in photoreceptor viability were associated with deficits in visual acuity and contrast sensitivity (Baba et al., 2018a), demonstrating the functional importance of retinal clock in normal aging process of the retina. The results imply that normal circadian rhythmicity in the retina is required for cell viability, and its impairment has deleterious consequences on the healthiness of the retinal tissue.
Circadian clock perturbations are associated with diabetic retinopathy, one of the most common complication of diabetes that leads to progressive vision loss and eventual blindness (Ola et al., 2012). Clock gene disruption in mice leads to diabetes and hypertension (Marcheva et al., 2010), and in the retina there is indirect evidence that circadian clock dysfunction is associated with and could contribute to diabetic retinopathy. Specifically, Per2 knockout mice exhibit the same symptoms as diabetic damage to the retina (Bhatwadekar et al., 2013), and diabetic retinopathy is associated with a reduction in clock gene expression in the retina of diabetic mice and rats (Busik et al., 2009). Furthermore, removal of Per2 causes disfunction of the retinal vasculature, a hallmark of diabetic retinopathy (Jadhav et al., 2016). Eliminating Bmal1 specifically in endothelial cells, a key cell type of blood vessels, results in diabetic retinopathy-like symptoms (Bhatwadekar et al., 2017). Altogether these studies provide strong evidence that circadian rhythmicity in retinal vasculature may play a critical role in maintaining the health of the neural retina.
12. Future directions
Despite recent advances in our understanding of how circadian clocks have evolved to regulate the functional organization of the vertebrate retina in the day compared to night, there is more work to be done. Here are some suggestions for further research:
(1). Study retinal clock pathway function under physiological conditions using WT animal models.
Animal (and plant) life evolved on a rotating planet on which the ambient light level gradually changes over the course of day and night. Circadian clock- and light/dark-adaptive processes modulate neuronal light responses in the vertebrate retina so that their operating ranges fit visual performance needs throughout day and night (e.g., high visual sensitivity on moonless nights; high acuity at midday), including at dawn and dusk (Wang and Mangel, 1996; Ribelayga et al., 2008; Ribelayga and Mangel, 2010; Chaffiol et al., 2017; Goel and Mangel, 2021). Moreover, evidence indicates that maintenance of the dark-adapted state during the night (or evening) requires continuous darkness, and that even a single light stimulus above the low mesopic range will light adapt a retina at night or in the evening (see Section 4.2.2.2; Wang and Mangel, 1996; Ribelayga et al., 2008; Ribelayga and Mangel, 2010; Goel and Mangel, 2021). In addition, local contrast (i.e., the difference in intensity between specific light and dark regions of a visual scene) in natural images tends to be less than 10% above and below the intensity of the ambient illumination (Srinivasan et al., 1982; Sakai and Naka, 1988; Sterling and Demb, 2004; Burkhardt et al., 2006), suggesting that illumination conditions in the laboratory should be restricted in this way (e.g., see Tikidji-Hamburyan et al., 2015). It is possible that experimental use of larger changes in contrast or sudden large increases or decreases in the intensity of background illumination may produce atypical light responses that do not usually occur under more natural illumination conditions (Thoreson and Mangel, 2012; Goel and Mangel, 2021).
Moreover, in addition to considering the importance of the illumination conditions and time of day or night, retinal studies need to recognize the advantages and limitations of each model system. For example, although investigations of dissociated rods and cones have uncovered highly significant findings, the dissociation process greatly alters many aspects of retinal function. Studies of dissociated cones have revealed many of the processes that underlie their light responses, but the study of individual cones in intact retinal tissue was essential to observe the following phenomena at night in the dark: 1) the robust increase in rod input to cones (and cHCs); 2) the ability of cones (and cHCs) to respond to very dim scotopic light stimuli; and 3) the robust increase in rod-cone gap junction coupling.
In addition, because many day/night differences in retinal function depend on melatonin (Iuvone et al., 2005; Besharse and McMahon, 2016), it is important to investigate the light responses of individual neurons in intact retinal tissue in melatonin-proficient animals, especially when experiments are conducted in the dark in the afternoon, evening, or night. Most common laboratory mouse strains are melatonin-deficient (e.g., C57BL/6J, BALB/c; Ebihara et al., 1986; Vivien-Roels et al., 1998; Kasahara et al., 2010; Zhang et al., 2018), which enables them to breed throughout the year. They may also lack other circadian pathway components. Thus, it is important to study specific circadian processes in WT animal models to identify and assess natural day/night differences in retina function. Genetic manipulation of circadian clock pathway components in melatonin-deficient animals may not reveal melatonin-dependent day/night differences in function.
(2). Determine the functional role of individual retinal clocks.
Compelling evidence indicates that clock gene expression is present in virtually all retinal cell types. A recent estimate reported that there are at least 128 neuronal cell types in the mouse retina (Yan et al., 2020). We still know little of the function of each of these clock cell types and their associated clock pathways with the exception of the photoreceptor clock that utilizes melatonin, dopamine, endocannabinoids, and adenosine to control photoreceptor electrical coupling (see Section 4 here; Ribelayga et al., 2008; Cao et al., 2021; Cao and Mangel, 2021; Goel and Mangel, 2021). Retina-specific elimination of Bmal1 provided the important demonstration that retinal clocks control retinal development, function, and maintenance (Storch et al., 2007; Baba et al., 2018a, b; Sawant et al., 2019). A floxed allele of Bmal1 is now available and has been used to selectively silence clock function in specific cell types (see for instance Sawant et al., 2017; Bhoi et al., 2021). Coupled to techniques of molecular biology and electrophysiology, these represent valuable tools to establish entire clock signaling pathways. In addition, clock activity in retinal cells is not limited to neurons. Muller cells--the predominant glia in the retina-- possess circadian clock function (Xu et al., 2016) that plays a key role in their development and lamination (Sawant et al., 2019). Further work is needed to fully understand how individual neuronal and glial clock cell types participate in the normal development of retinal tissue and maintain the integrity of retinal circuitry and function in adult mice.
(3). Identify entrainment pathways.
Entrainment is a critical aspect of circadian rhythmicity and much remains to be done to characterize entrainment pathways in the retina. The recent discovery of OPN5/neuropsin and its role in photoentrainment of retinal rhythms has transformed our view of the retinal circadian system. A greater understanding of the identity and physiology of the RGC type(s) that express OPN5, the means through which they entrain retinal rhythms (i.e., neurohormonal or synaptic), and the role of rod and cone pathways in photoentrainment is much needed.
(4). Clarify the link between circadian clock malfunction and retinal pathologies.
The first study that reported retina-specific genetic deletion of Bmal1 noted defects of retinal visual physiology but an overall normal structure using light and electron microscopy (Storch et al., 2007). The absence of morphological defects in this model has since been challenged. It is now clear that Bmal1 is required for normal development and maintenance of retinal tissue and cell survival (Sawant et al., 2017, 2019; Baba et al., 2018a, b). Cone photoreceptor viability during aging is particularly reduced (Baba et al., 2018a, b). Altogether, this provides strong support that circadian clock dysfunction contributes to aberrant visual function during development and aging. A greater understanding of clock function in retinal tissue may lead to advances in the prevention and/or treatment of some retinal diseases such as age-related macular degeneration. Toward this goal, clarifying the functional importance of melatonin should be a priority. The widespread effects of melatonin on retinal function, and also its protective effects on RPE cells, photoreceptors, and RGCs have suggested that administration of melatonin may be an important means of treating various ocular disorders, such as age-related macular degeneration, glaucoma, and myopia (Wiechmann and Summers, 2008; Tosini et al., 2012; Chakraborty et al., 2021; Gubin et al., 2021). It remains unclear though which of these effects are mediated by membrane receptors and the circadian control of retinal rhythms and which reflect the antioxidant properties of melatonin. These possibilities need to be clarified. It is also important to note that even though experiments in laboratory animals are promising, further translational and clinical investigations are much needed to establish the therapeutic potential of melatonin in the prevention and/or treatment of ocular diseases. Lastly, recent evidence suggests a link between disruption of melanopsin signaling and the development of refractive error and myopia (Chakraborty et al., 2022), an interesting finding worthy of further investigation.
In conclusion, despite 30 years of intense research, many aspects of how circadian clock systems in the retina impact retinal function remain unclear. Recent developments in the field have challenged many concepts and paved the way for future exciting studies. These new developments support the following possibilies: 1) distinct circadian clocks are present in different retinal cell types; 2) most, if not all, retinal rhythms are generated locally by clocks within the retina; 3) clocks use a variety of effectors and clock pathways to control the functional organization of the retina so that the retina optimally processes cone-initiated signals during the day and rod-initiated signals at night, and switches between rod and cone vision at dusk and dawn; and 4) clock-based rhythmicity is essential for proper development and viability of retinal cells, and the maintenance of normal retinal function. Future research will address the anatomical, molecular, cellular, and physiological bases of circadian rhythmicity in the retina as a hierarchical network of clocks and clock pathways that modulate signal processing. Experimental investigations have demonstrated that vision at night is not simply rod-mediated but rather is achieved by circadian clock-controlled re-organization of rod and cone pathways. A detailed understanding of the daily temporal organization of retinal function may be key to unraveling causes of retinal pathologies.
Table 3.
Current knowledge on the localization of the clock components in the vertebrate retina
GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. All other abbreviations, see text. Comparative quantitative analysis was based on measurements of mRNA expression lev-els using Northern blot analysis, Real time PCR or microarray analysis of retinal tissue, biolumines-cence (e.g. from PER2::LUC), or in situ hybridization (ISH). 0: no expression detected, +: weak ex-pression (usually detected after RNA amplification); ++: relative medium level and relative high of expression; +++: relative high level of expression (usually detected by ISH or bioluminescence). Blank indicates that gene expression was detected in retinal tissue (usually in retinal homogenate) but its geographical expression was not investigated.
Acknowledgements
We thank Dr. Kimberly A. Mankiewicz for critical reading and editing of the manuscript. Research in the Ribelayga lab is supported by National Institutes of Health Grants EY028647, EY029408, and MH127343, National Institutes of Health Vision Core Grant P30EY028102, and The Hermann Eye Fund. Research in the Mangel lab is supported by grants from the National Institutes of Health (R01-EY029777) and The Plum Foundation (Studio City, CA).
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- AAAD
Aromatic amino acid decarboxylase
- AA-NAT
Arylalkylamine-N-acetyltransferase
- AC
Adenylyl cyclase
- ADP
Adenosine diphosphate
- AMK
AMP-activated protein kinase
- AMP
Adenosine monophosphate
- ASMT
Acetylserotonin-O-methyltransferase
- ATP
Adenosine triphosphate
- BMAL1
Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1
- cAMP
Cyclic adenosine monophosphate
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- Cav1.3
Calcium channel, voltage-dependent, L type, alpha 1D subunit
- cGMP
Cyclic guanosine monophosphate
- cHC
Cone-connected horizontal cell
- ChiPseq
Chromatin immunoprecipitation sequencing
- CLOCK
Circadian locomotor output cycles protein kaput
- CNGC
Cyclic nucleotide gated channels
- CNS
Central nervous system
- Cry
Cryptochrome
- Cx
Connexin
- DAT
Dopamine transporter
- DOPAC
Dihydroxyphenylacetic acid
- DRP1
Dynamin related protein 1
- ERG
Electroretinogram
- GABA
Gamma aminobutyric acid
- GABAA
GABA receptor type-A
- GC
Ganglion cell
- HIOMT
Hydroxyindole-O-methyltransferase
- HVA
Homovanillic acid
- IGL
Intergeniculate leaflet
- INL
Inner nuclear layer
- Itgb5
Integrin beta-5
- ipRGC
intrinsically photosensitive retinal ganglion cell
- L/D
Light/dark
- LTCC
L-type voltage-gated calcium channel
- LUC
Luciferase
- MAPK
Mitogen-activated protein kinase
- Mdka
Midkine A
- Mel1c
Melatonin receptor type 1c
- Mfge8
Milk fat globule epidermal growth factor 8
- mRNA
Messenger ribonucleic acid
- MT
melatonin receptor type
- mTOR
Mammalian target of rapamycin
- NADH
Nicotinamide adenine dinucleotide hydride
- NADPH
Nicotinamide adenine dinucleotide phosphate hydrogen
- NAS
N-acetylserotonin
- Npas2
Neuronal Per-Arnt-Sim domain-containing protein 2
- OPN
Olivary pretectal nucleus
- Per1-3
Period 1-3
- pS
pico Siemens
- Rac1
Rac family small GTPase 1
- REV-ERBα/β
Reverse Erythroblastosis α/β (nuclear receptor subfamily 1, group D, member 1/2)
- RGC
Retinal ganglion cell
- ROR
Retinoic acid receptor like orphan receptor
- RORα/β
Retinoic acid receptor like orphan receptor α/β
- RORE
Retinoic acid receptor-like orphan receptor response element
- RPE
Retinal pigment epithelium
- RT-PCR
Reverse transcription polymerase chain reaction
- SCN
Suprachiasmatic nucleus
- sst
Somatostatin
- TH
Tyrosine hydroxylase
- TPOH
Tryptophan hydroxylase
- WT
Wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Nogi T, Ebihara S, 1998. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 792, 361–369. 10.1016/S0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- Adachi A, Suzuki Y, Nogi T, Ebihara S, 1999. The relationship between ocular melatonin and dopamine rhythms in the pigeon: effects of melatonin inhibition on dopamine release. Brain Res. 815, 435–440. 10.1016/S0006-8993(98)01077-4. [DOI] [PubMed] [Google Scholar]
- Ahmed J, Braun RD, Dunn R Jr., Linsenmeier RA, 1993. Oxygen distribution in the macaque retina. Invest. Ophthalmol. Vis. Sci 34, 516–521. [PubMed] [Google Scholar]
- Akasaka K, Nasu T, Katayama T, Murakami N, 1995. Development of regulation of melatonin release in pineal cells in chick embryo. Brain Res. 692, 283–286. 10.1016/0006-8993(95)00643-5. [DOI] [PubMed] [Google Scholar]
- Alfinito PD, Alli R, Townes-Anderson E, 2002. Adenosine A(2a) receptor-mediated inhibition of rod opsin mRNA expression in tiger salamander. J. Neurochem 83, 665–672. 10.1046/j.1471-4159.2002.01162.x. [DOI] [PubMed] [Google Scholar]
- Alonso-Gomez AL, Valenciano AI, Alonso-Bedate M, Delgado MJ, 2000a. Melatonin synthesis in the greenfrog retina in culture: II. Dopaminergic and adrenergic control. Life Sci. 66, 687–695. 10.1016/S0024-3205(99)00640-2. [DOI] [PubMed] [Google Scholar]
- Alonso-Gomez AL, Valenciano AI, Alonso-Bedate M, Delgado MJ, 2000b. Melatonin synthesis in the greenfrog retina in culture: I. Modulation by the light/dark cycle, forskolin and inhibitors of protein synthesis. Life Sci. 66, 675–685. 10.1016/S0024-3205(99)00639-6. [DOI] [PubMed] [Google Scholar]
- Anderson FE, Green CB, 2000. Symphony of rhythms in Xenopus laevis retina. Microsc. Res. Tech 50, 360–372. . [DOI] [PubMed] [Google Scholar]
- Anderson FE, Hayasaka N, Green CB, 2001. Rhythmic expression of BMAL in the retina of Xenopus laevis and non rhythmic expression in other tissues. Invest. Ophthalmol. Vis. Sci 42, S776. [Google Scholar]
- Appelbaum L, Vallone D, Anzulovich A, Ziv L, Tom M, Foulkes NS, Gothilf Y, 2006. Zebrafish arylalkylamine-N-acetyltransferase genes - targets for regulation of the circadian clock. J. Mol. Endocrinol 36, 337–347. 10.1677/jme.1.01893. [DOI] [PubMed] [Google Scholar]
- Aranda ML, Schmidt TM, 2021. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cell Mol. Life Sci 78, 889–907. 10.1007/s00018-020-03641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel M, Lasater EM, Mangel SC, Dowling JE, 1984. On the sensitivity of H1 horizontal cells of the carp retina to glutamate, aspartate and their agonists. Brain Res. 295, 179–183. doi: 10.1016/0006-8993(84)90828-x. [DOI] [PubMed] [Google Scholar]
- Ariel M, Mangel SC, Dowling JE, 1986. N-methyl-D-aspartate acts as an antagonist of the photoreceptor transmitter in the carp retina. Brain Res. 372, 143–148. doi: 10.1016/0006-8993(86)91467-8. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, Sehgal A, 2003. A Fly’s Eye View of Circadian Entrainment. J. Biol. Rhythms 18, 206–216. 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- Asteriti S, Gargini C, Cangiano L, 2017. Connexin 36 expression is required for electrical coupling between mouse rods and cones. Vis. Neurosci 34, E006. 10.1017/S0952523817000037. [DOI] [PubMed] [Google Scholar]
- Baba K, Benleulmi-Chaachoua A, Journe A-S, Kamal M, Guillaume J-L, Dussaud S, Gbahou F, Yettou K, Liu C, Contreras-Alcantara S, Jockers R, Tosini G, 2013. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Science Signaling 6, ra89–ra89. 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Goyal V, Tosini G 2022. Circadian regulation of retinal pigment epithelium function. Internatl J. Molec. Sci, 23, 2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G, 2009. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc. Natl. Acad. Sci. USA 106, 15043–15048. 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Piano I, Lyuboslavsky P, Chrenek MA, Sellers JT, Zhang S, Gargini C, He L, Tosini G, Iuvone PM, 2018a. Removal of clock gene Bmal1 from the retina affects retinal development and accelerates cone photoreceptor degeneration during aging. Proc. Natl. Acad. Sci. USA 115, 13099–13104. 10.1073/pnas.1808137115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Ribelayga CP, Michael Iuvone P, Tosini G, 2018b. The Retinal Circadian Clock and Photoreceptor Viability, in: Ash JD, Anderson RE, LaVail MM, Bowes Rickman C, Hollyfield JG, Grimm C (Eds.), Retinal Degenerative Diseases. Springer International Publishing, Cham, pp. 345–350. 10.1007/978-3-319-75402-4_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi U, Gegnaw ST, Milićević N, Sandu C, ten Brink JB, Jongejan A, Hicks D, Moerland PD, Felder-Schmittbuhl M-P, Bergen AA, 2020. Core-clock genes Period 1 and 2 regulate visual cascade and cell cycle components during mouse eye development. BBA - Gene Regul. Mech 1863, 194623. 10.1016/j.bbagrm.2020.194623. [DOI] [PubMed] [Google Scholar]
- Baggs JE, Green CB, 2003. Nocturnin, a deadenylase in Xenopus laevis retina. A mechanism for posttranscriptional control of circadian-related mRNA. Curr. Biol 13, 189–198. 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Bagher AM, Laprairie RB, Kelly ME, Denovan-Wright EM, 2016. Antagonism of dopamine receptor 2 long affects cannabinoid receptor 1 signaling in a cell culture model of striatal medium spiny projection neurons. Mol Pharmacol 89, 652–666. [DOI] [PubMed] [Google Scholar]
- Bai L, Zimmer S Rickes O, Rohleder N, Holthues H, Engel L, Leube R, Spessert R, 2008. Daily oscillation of gene expression in the retina is phase-advanced with respect to the pineal gland. Brain Res. 1203, 89–96. 10.1016/j.brainres.2008.01.073. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Beremand PD, Hammer R, Reidel E, Thomas TL, Cassone VM, 2004. Transcriptional profiling of circadian patterns of mRNA expression in the chick retina. J. Biol. Chem 279, 52247–52254. 10.1074/jbc.m405679200. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Cassone VM, 2005. Melanopsin expression in the chick retina and pineal gland. Brain Res. Mol. Brain Res 134, 345–348. 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Chong NW, Xiong J, Cassone VM, 2002. Chickens’ Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Lett. 513, 169–174. 10.1016/s0014-5793(02)02276-7. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U, 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347. 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Barattini S, Battisti B, Cervetto L, Marroni P, 1981. Diurnal changes in the pigeon electroretinogram. Rev. Can. Biol 40, 133–137. [PubMed] [Google Scholar]
- Barlow HB, Levick WR, 1969. Changes in the maintained discharge with adaptation level in the cat retina. J. Physiol 202, 699–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R, 2001. Circadian and efferent modulation of visual sensitivity. Prog. Brain Res 131, 487–503. 10.1016/s0079-6123(01)31039-7. [DOI] [PubMed] [Google Scholar]
- Barlow RB, Boudreau EA, Moore DC, Huckins SC, Lindstrom AM, Farell B, 1997. Glucose and time of day modulate human contrast sensitivity and fMRI signals from visual cortex. Invest. Ophthalmol. Vis. Sci 38, S735. [Google Scholar]
- Barlow R, Parshley MR, Kelly MJ, Knox BE, 2000. Visual sensitivity of Xenopus. Invest. Ophthalmol. Vis. Sci 41, S715. [Google Scholar]
- Barlow RB, Hitt JM, Dodge FA, 2001. Limulus vision in the marine environment. Biol. Bull 200, 169–176. 10.2307/1543311. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ, 2006. Melanopsin regulates visual processing in the mouse retina. Curr. Biol 16, 389–395. 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Barnes S, 2003. Center-surround antagonism mediated by proton signaling at the cone photoreceptor synapse. J. Gen. Physiol 122, 653–656. 10.1085/jgp.200308947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Grove JCR, McHugh CF, Hirano AA, Brecha NC (2020) Horizontal cell feedback to cone photoreceptors in mammalian retina: novel insights from the GABA-pH hybrid model. Front. Cell. Neurosci 14:595064. doi: 10.3389/fncel.2020.595064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Hille B, 1989. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J. Gen. Physiol 94, 719–743. 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK, 1986. Daily fluctuations in the detectability of dim lights by humans. Physiol. Behav 38, 871–877. 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK, 1987. Circadian rhythm in goldfish visual sensitivity. Invest. Ophthalmol. Vis. Sci 28, 1811–1815. [PubMed] [Google Scholar]
- Battelle BA, 2002. Circadian efferent input to the Limulus eyes: anatomy, circuitry, and impact. Microsc. Res. Tech 15, 345–355. 10.1002/jemt.10142. [DOI] [PubMed] [Google Scholar]
- Behrens UD, Douglas RH, Sugden D, Davies DJ, Wagner HJ, 2000. Effect of melatonin agonists and antagonists on horizontal cell spinule formation and dopamine release in a fish retina. Cell Tissue Res. 299, 299–306. 10.1007/s004419900161. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ, 2005. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet 6, 544–556. 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benleulmi-Chaachoua A, Hegron A, Le Boulch M, Karamitri A, Wierzbicka M, Wong V, Stagljar I, Delagrange P, Ahmad R, Jockers R, 2018. Melatonin receptors limit dopamine reuptake by regulating dopamine transporter cell-surface exposure. Cell. Mol. Life Sci 75, 4357–4370. 10.1007/s00018-018-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS, 2004. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41, 495–511. 10.1016/S0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC, 1997. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J. Neurochem 68, 213–224. 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- Bernstein SA, Breding DJ, Fisher SK, 1984. The influence of light on cone disk shedding in the lizard, Sceloporus occidentalis. J. Cell. Biol 99, 379–3893. 10.1083/jcb.99.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, 2003. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 26, 314–320. 10.1016/s0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Berson DM, 2007. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 454, 849–855. 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Defoe DM, 1998. Role of the retinal pigment epithelium in photoreceptor membrane turnover, in: Marmor MF, Wolfensberger TJ (Eds.) The retinal pigment epithelium. Oxford University Press, Oxford, pp. 152–172. [Google Scholar]
- Besharse JC, Dunis DA, 1983. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science 219, 1341–1343. 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, 1979. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest. Ophthalmol. Vis. Sci 18, 1019–1024. [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME, 1977. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J. Cell Biol 75, 507–527. 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, 1983. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature 305, 133–135. 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, 1992. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochem. Int 20, 193–199. 10.1016/0197-0186(92)90167-P. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, Pierce ME, 1988. Regulation of rhythmic photoreceptor metabolism: a role for post-receptoral neurons. Prog. Retin. Res 7, 21–61. 10.1016/0278-4327(88)90004-1. [DOI] [Google Scholar]
- Besharse JC, McMahon DG, 2016. The Retina and Other Light-sensitive Ocular Clocks. J. Biol. Rhythms 31, 223–243. 10.1177/0748730416642657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Zhuang M, Freeman K, Fogerty J, 2004. Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur. J. Neurosci 20, 167–174. 10.1111/j.1460-9568.2004.03479.x. [DOI] [PubMed] [Google Scholar]
- Besseau L, Benyassi A, Møller M, Coon SL, Weller JL, Boeuf G, Klein DC, Falcón J, 2006. Melatonin pathway: breaking the ‘high-at-night’ rule in trout retina. Exp. Eye Res 82, 620–627. 10.1016/j.exer.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Bhatwadekar AD, Beli E, Diao Y, Chen J, Luo Q, Alex A, Caballero S, Dominguez JM, Salazar TE, Busik JV, Segal MS, Grant MB, 2017. Conditional deletion of Bmal1 accentuates microvascular and macrovascular injury. Am. J. Pathol 187, 1426–1435. 10.1016/j.ajpath.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatwadekar AD, Yan Y, Qi X, Thinschmidt JS, Neu MB, Li Calzi S, Shaw LC, Dominiguez JM, Busik JV, Lee C, Boulton ME, Grant MB, 2013. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes 62, 273–282. 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoi JD, Zhang Z, Janz R, You Y, Wei H, Wu J and Ribelayga CP, 2021. The SNARE Regulator Complexin3 is a Target of the Cone Circadian Clock. J. Comp. Neurol 529, 1066–1080. 10.1002/cne.25004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazynski C, Cohen AI, Fruh B, Niemeyer G, 1989. Adenosine: autoradiographic localization and electrophysiologic effects in the cat retina. Invest. Ophthalmol. Vis. Sci 30, 2533–2536. [PubMed] [Google Scholar]
- Blazynski C, Perez MT, 1991. Adenosine in vertebrate retina: localization, receptor characterization, and function. Cell. Mol. Neurobiol 11, 463–484. 10.1007/bf00734810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF, 2001. Rod Vision: Pathways and Processing in the Mammalian Retina. Prog. Retin. Eye Res 20, 351–384. 10.1016/S1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Völgyi B, 2009. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci 10, 495–506. 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, 1997. A comparison of receptive-field and tracer-coupling size of amacrine and ganglion cells in the rabbit retina. Vis. Neurosci 14, 1153–1165. 10.1017/s0952523800011846. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Persky SE, 1995. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Vis. Neurosci 12, 985–999. 10.1017/S0952523800009524. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM, 1994. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis. Neurosci 11, 1013–1018. 10.1017/s095252380000393x. [DOI] [PubMed] [Google Scholar]
- Bobu C, Hicks D, 2009. Regulation of retinal photoreceptor phagocytosis in a diurnal mammal by circadian clocks and ambient lighting. Invest. Ophthalmol. Vis. Sci 50, 3495–3502. 10.1167/iovs.08-3145. [DOI] [PubMed] [Google Scholar]
- Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE, 1984. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes 33, 1150–1153. 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- Borgula GA, Karwoski CJ, Steinberg RH, 1989. Light-evoked changes in extracellular pH in frog retina. Vision Res. 29, 1069–1077. 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Casanova C, Cecyre B, Redmond WJ, 2016. Expression and function of the endocannabinoid system in the retina and the visual brain. Neural Plasticity Article ID 9247057, 14 pages. 10.1155/2016/9247057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C, van Veen T, Farber DB, 1988. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp. Eye Res 47, 369–390. 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Brandenburg J, Bobbert AC, Eggelmeyer F, 1983. Circadian changes in the response of the rabbit’s retina to flashes. Behav. Brain Res 7, 113–123. 10.1016/0166-4328(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Brann MR, Cohen LV, 1987. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science 235, 585–587. 10.1126/science.3101175. [DOI] [PubMed] [Google Scholar]
- Bruhn SL, Cepko CL, 1996. Development of the pattern of photoreceptors in the chick retina. J. Neurosci 16, 1430–1439. 10.1523/jneurosci.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik GA, Purtill RA, 1980. The role of melatonin and dopamine in retinal physiology. Can. J. Physiol. Pharmacol 58, 1457–1462. 10.1139/y80-220. [DOI] [PubMed] [Google Scholar]
- Buhr ED, Takahashi JS, 2013. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol 22, 3–27. 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Van Gelder RN, 2014. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc. Natl. Acad. Sci. USA 111, 8625–8630. 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yue WWS, Ren X, Jiang Z, Liao H-WR, Mei X, Vemaraju S, Nguyen M-T, Reed RR, Lang RA, Yau K-W, Van Gelder RN, 2015. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA 112, 13093–13098. 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloj A, Maminishkis A, Mizui M, Finnemann SC, 2018. Semaphorin4D-PlexinB1 signaling attenuates photoreceptor outer segment phagocytosis by reducing Rac1 activity of RPE cells. Mol. Neurobiol 55, 4320–4332. 10.1007/s12035-017-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA, Fahey PK, Sikora MA, 2006. Natural images and contrast encoding in bipolar cells in the retina of the land- and aquatic-phase tiger salamander. Vis. Neurosci 23, 35–47. [DOI] [PubMed] [Google Scholar]
- Burnside B, 2001. Light and circadian regulation of retinomotor movement. Prog. Brain Res 131, 477–485. 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- Burnside B, Ackland N, 1984. Effects of circadian rhythm and cAMP on retinomotor movements in the green sunfish, Lepomis cyanellus. Invest. Ophthalmol. Vis. Sci 25, 539–545. [PubMed] [Google Scholar]
- Burnside B, King-Smith C, 2010. Fish Retinomotor Movements, in: Encyclopedia of the Eye. Elsevier, pp. 142–150. 10.1016/B978-0-12-374203-2.00159-7 [DOI] [Google Scholar]
- Burnside B, Nagle B, 1983. Retinomotor movements of photoreceptors and pigment epithelium: mechanisms and regulation. In: Osborne N, Chader G (Eds), Progress in Retinal Research., Vol. 2. Pergamon Press, Oxford, pp. 67–109. [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Calzi SL, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB, 2009. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J. Exp. Med 206, 2897–2906. 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, 1996. Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 708, 177–181. 10.1016/0006-8993(95)01365-2. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC, 1990. Circadian regulation of melatonin in the retina of Xenopus laevis: limitation by serotonin availability. J. Neurochem 54, 716–719. 10.1016/0006-8993(95)01365-2. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC, 1991. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J. Neurosci 11, 2959–2971. 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC, 1992. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis. Neurosci 8, 487–490. 10.1017/s0952523800005009. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC, 1993. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron 10, 573–577. 10.1016/0896-6273(93)90160-S. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC, 1995. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog. Retin. Eye Res 14, 267–291. 10.1016/1350-9462(94)00001-Y. [DOI] [Google Scholar]
- Calinescu A-A, Raymond PA, Hitchcock PF, 2009. Midkine expression is regulated by the circadian clock in the retina of the zebrafish. Vis. Neurosci 26, 495–501. 10.1017/S0952523809990204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calligaro H, Coutanson C, Najjar RP, Mazzaro N, Cooper HM, Haddjeri N, Felder- Schmittbuhl M-P, Dkhissi-Benyahya O, 2019. Rods contribute to the light-induced phase shift of the retinal clock in mammals. PLoS Biol 17, e2006211. 10.1371/journal.pbio.2006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Barnard AR, Hut RA, Bonnefont X, van der Horst GT, Hankins MW, Lucas RJ, 2008a. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J. Biol. Rhythms 23, 489–4501. 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Barnard AR, Lucas RJ, 2008b. The electroretinogram as a method for studying circadian rhythms in the mammalian retina. J. Genet 87, 459–466. 10.1007/s12041-008-0068-5. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, Lucas RJ, 2009. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur. J. Neurosci 29, 761–767. 10.1111/j.1460-9568.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LJ, Jensen AM, 2017. Phosphodiesterase inhibitors Sildenafil and Vardenafil reduce zebrafish rod photoreceptor outer segment shedding. Invest. Ophthalmol. Vis. Sci 58, 5604. 10.1167/iovs.17-21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LJ, West MC, Jensen AM, 2018. A high content, small molecule screen identifies candidate molecular pathways that regulate rod photoreceptor outer segment renewal. Sci. Rep 8, 14017. 10.1038/s41598-018-32336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano L, Asteriti S, 2021. Interphotoreceptor coupling: an evolutionary perspective. Pfluger Archiv. Europ. J. Physiol, 473, 1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Mangel SC 2021. Interactions of cone cannabinoid CB1 and dopamine D4 receptors increase day/night difference in rod-cone gap junction coupling in goldfish retina. Journal of Physiology (Lond.), 599, 4085–4100. DOI: 10.1113/JP281308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Ribelayga CP, Mangel SC, 2021. The circadian clock in the retina regulates rod/cone gap junction coupling and neuronal light responses via activation of adenosine A2a receptors, Front. Cell. Neurosci 14, 605067. 10.3389/fncel.2020.605067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone VM, 2014. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol 35, 76–88. 10.1016/j.yfrne.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffiol AJ, Ishii M, Cao Y, Mangel SC, 2017. Dopamine regulation of GABAA receptors contributes to light/dark adaptive modulation of the ON-cone bipolar cell receptive field surround in the retina. Current Biology, 27, 1–10 (DOI: 10.1016/j.cub.2017.07.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, et al. 2021. Myopia, or near-sightedness, is associated with delayed melatonin circadian timing and lower melatonin output in young adult humans. Sleep 44, 1–12. DOI: 10.1093/sleep/zsaa208 [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Landis EG, Mazade R, Yang V, Strickland R, Hattar S, Stone RA, Iuvone PM, Pardue MT, 2022. Melanopsin modulates refractive development and myopia. Exp. Eye Res 214, 108866 DOI: 10.1016/j.exer.2021.108866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambille I, 1998. Temporospatial characteristics of light-induced Fos immunoreactivity in suprachiasmatic nuclei are not modified in Syrian hamsters treated neonatally with monosodium glutamate. Brain Res. 808, 250–261. 10.1016/S0006-8993(98)00831-2. [DOI] [PubMed] [Google Scholar]
- Chang JY-A, Shi L, Ko ML, Ko GY-P, 2018. Circadian Regulation of Mitochondrial Dynamics in Retinal Photoreceptors. J. Biol. Rhythms 33, 151–165. 10.1177/0748730418762152. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Pozdeyev N, Haque R, Visser A, Ivanova TN, Iuvone PM, 2006. Circadian clockwork machinery in neural retina: evidence for the presence of functional clock components in photoreceptor-enriched chick retinal cell cultures. Mol. Vis 12, 215–223. [PubMed] [Google Scholar]
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I, 2005. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem 92, 158–170. 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Baler R, 2000. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res. Mol. Brain Res 81, 43–50. 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- Cheng N, Tsunenari T, Yau KW, 2009. Intrinsic light response of retinal horizontal cells of teleosts. Nature 460, 899–903. 10.1038/nature08175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kaila K, 1992. Modulation of pH by neuronal activity. Trends Neurosci. 15, 396–402. 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chiba A, Hattori A, Iigo M, 2005. Daily and circadian variations of the pineal and ocular melatonin contents and their contributions to the circulating melatonin in the Japanese newt, Cynops pyrrhogaster. Zoolog. Sci 22, 65–70. 10.2108/zsj.22.65. [DOI] [PubMed] [Google Scholar]
- Choi H, Ribelayga C, Mangel SC, 2012. Cut-loading: a useful tool for examining the extent of gap junctional tracer coupling between retinal neurons. Journal of Visualized Experiments (JoVE), Online: http://www.jove.com/video/3180. doi: 10.3791/3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NW, Bernard M, Klein DC, 2000. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J. Biol. Chem 275, 32991–32998. 10.1074/jbc.m005671200. [DOI] [PubMed] [Google Scholar]
- Chong NW, Cassone VM, Bernard M, Klein DC, Iuvone PM, 1998. Circadian expression of tryptophan hydroxylase mRNA in chicken retina. Mol. Brain Res 61, 243–250. 10.1016/s0169-328x(98)00219-8. [DOI] [PubMed] [Google Scholar]
- Chong NW, Chaurasia SS, Haque R, Klein DC, Iuvone PM, 2003. Temporal-spatial characterization of chicken clock genes: circadian expression in retina, pineal gland, and peripheral tissues. J. Neurochem 85, 851–860. 10.1046/j.1471-4159.2003.01723.x. [DOI] [PubMed] [Google Scholar]
- Cogburn LA, Wilson-Placentra S, Letcher LR, 1987. Influence of pinealectomy on plasma and extrapineal melatonin rhythms in young chickens (Gallus domesticus). Gen. Comp. Endocrinol 68, 343–356. 10.1016/0016-6480(87)90073-6. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O'Malley KL, 1992. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc. Natl. Acad. Sci. USA 89, 12093–12097. 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Long MA, 2004. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci 27, 393–418. 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Constance CM, Fan JY, Preuss F, Green CB, Price JL, 2005. The circadian clock-containing photoreceptor cells in Xenopus laevis express several isoforms of casein kinase I. Brain Res. Mol. Brain Res 136, 199–211. 10.1016/j.molbrainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Coon SL, Begay V, Deurloo D, Falcon J, Klein DC, 1999. Two arylalkylamine N-acetyltransferase genes mediate melatonin synthesis in fish. J. Biol. Chem 274, 9076–82. 10.1074/jbc.274.13.9076. [DOI] [PubMed] [Google Scholar]
- Coon SL, Del Olmo E, Young III WS, Klein DC, 2002. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87). J. Clin. Endocrinol. Metab 87, 4699–4706. 10.1210/jc.2002-020683. [DOI] [PubMed] [Google Scholar]
- Cosci B, Longoni B, Marchiafava PL, 1997. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sci. 60, 1885–1889. 10.1016/S0024-3205(97)00150-1. [DOI] [PubMed] [Google Scholar]
- Cox KH, Takahashi JS, 2019. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol 63, R93–R102. 10.1530/JME-19-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Juan J, Garcia M, 2001. Spinules and nematosomes in retinal horizontal cells: a “thorny” issue. Prog. Brain Res 131, 519–537. [DOI] [PubMed] [Google Scholar]
- Dearry A, Burnside B, 1986. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinae. I. Induction of cone contraction is mediated by D2 receptors. J. Neurochem 46, 1006–1031. 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Delgado MJ, Cespedes MV, De Pedro N, Alonso-Bedate M, Alonso-Gomez AL, 2001. Day/night variations of dopamine ocular content during Xenopus laevis ontogeny. Neurosci. Lett 300, 129–132. 10.1016/S0304-3940(01)01560-9. [DOI] [PubMed] [Google Scholar]
- Delyfer MN, Léveillard T, Mohand-Saïd S, Hicks D, Picaud S, Sahel JA, 2004. Inherited retinal degenerations: therapeutic prospects. Biol. Cell 96, 261–269. 10.1016/j.biolcel.2004.01.006. [DOI] [PubMed] [Google Scholar]
- DeVera C, Baba K, Tosini G, 2019. Retinal circadian clocks are major players in the modulation of retinal functions and photoreceptor viability. Yale J. Biol. Med 92, 233–240. [PMC free article] [PubMed] [Google Scholar]
- DeVera C, Tosini G, 2020. Circadian analysis of the mouse retinal pigment epithelium transcriptome. Exp. Eye Res 193, 107988. 10.1016/j.exer.2020.107988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, 2001. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32, 1107–1117. 10.1016/S0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dinet V, Ansari N, Torres-Farfan C, Korf HW, 2006. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res 42, 83–91. 10.1111/j.1600-079x.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Wagner HJ, 1992. Localization and function of dopamine in the adult vertebrate retina. Neurochem. Int 20, 139–191. 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M, Cooper HM, 2013. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell. Mol. Life Sci 70, 3435–3447. 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC, 2000. A circadian clock regulates the pH of the fish retina. J. Physiol 522, 77–82. 10.1111/j.1469-7793.2000.0077m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC, 2001. Circadian clock regulation of pH in the rabbit retina. J. Neurosci 21, 2897–2902. 10.1523/JNEUROSCI.21-08-02897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC, 2004. Retinal pH reflects retinal energy metabolism in the day and night. J. Neurophysiol 91, 2404–2412. 10.1152/jn.00881.2003. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC, 2006. Electrical feedback in the cone pedicle: a computational analysis. J. Neurophysiol, 95, 1419–1427. doi: 10.1152/jn.00098.2005. [DOI] [PubMed] [Google Scholar]
- Do MTH, 2019. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 104, 205–226. 10.1016/j.neuron.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K, Hemila S, 1985. Rhodopsin phosphorylation inhibited by adenosine in frog rods: lack of effects on excitation. Comp. Biochem. Physiol. A 81, 431–439. 10.1016/0300-9629(85)90160-4. [DOI] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E, 2007. Expression of circadian clock genes in retinal dopaminergic cells. Vis. Neurosci 24, 573–580. 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Douglas RH, Wagner H-J, 1983. Endogenous control of spinule formation in horizontal cells of the teleost retina. Cell Tissue Res. 229, 443–449. 10.1007/bf00214985. [DOI] [PubMed] [Google Scholar]
- Dowling JE, 2012. The Retina, an Approachable Part of the Brain, rev. ed. Harvard University Press, Cambridge, CT. [Google Scholar]
- Dowling JE, Sidman RL, 1962. Inherited retinal dystrophy in the rat. J. Cell Biol 14, 73–109. 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing JE, Djamgoz MB, 1989. Quantitative analysis of cone photoreceptor-horizontal cell connectivity patterns in the retina of a cyprinid fish: electron microscopy of functionally identified and HRP-labelled horizontal cells. J. Comp. Neurol 289, 537–553. 10.1002/cne.902890402. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M, 2002a. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis. Neurosci 19, 593–601. 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M, 2002b. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J. Neurochem 83, 211–219. 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R, 1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29, 261–265. 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- D'Souza SP, Swygart DI, Wienbar SR, Upton BA, Zhang KX, Mackin RD, Casasent AK, Samuel MA, Schwartz GW, Lang RA, 2021. Retinal patterns and the cellular repertoire of neuropsin (Opn5) retinal ganglion cells. J. Comp. Neurol, online ahead of print. 10.1002/cne.25272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, 1983. Melatonin is a potent modulator of dopamine release in the retina. Nature 306, 782–784. 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Cardinali DP, Guardiola-Lemaitre B, Hagan RM, Krause DN, Sugden D, Vanhoutte PM, Yocca FD, 1998. Melatonin receptors. The IUPHAR Compendium of Receptor Characterization and Classification, IUPHAR Media, London, pp. 187–193. [Google Scholar]
- Dubocovich ML, Markowska M, 2005. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27, 101–110. 10.1385/endo:27:2:101. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Masana MI, Iacob S, Sauri DM, 1997. Melatonin receptor antagonists that differentiate between the human Mel1a, and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch. Pharmacol 355, 365–375. 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- Duncan TE, O’Steen WK, 1985. The diurnal susceptibility of rat retinal photoreceptors to light-induced damage. Exp. Eye Res 41, 497–507. 10.1016/s0014-4835(85)80007-5. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ, DeCoursey PJ, 2003. Chronobiology. Biological Timekeeping. Sinauer Associates, Inc. Sunderland, MA, USA. [Google Scholar]
- Dunwiddie TV, Masino SA, 2001. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci 24, 31–55. 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Marks T, Hudson DJ, Menaker M, 1986. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231, 491–493. 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Daly JW, 1982. Release of norepinephrine and dopamine from brain vesicular preparations: effects of adenosine analogues. Cell Mol. Neurobiol 2, 193–204. 10.1007/bf00711147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmiller MS, 1997. Morphogenesis and renewal of cone outer segments. Prog. Retin. Eye Res 16, 401–441. 10.1016/S1350-9462(96)00026-2. [DOI] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Dowling JE, 2010. Zebrafish larvae lose vision at night. Proc. Natl. Acad. Sci. U.S.A 107, 6034–6039. 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emser W, Dechoux R, Weiland M, Wirz-Justice A, 1993. Melatonin decreases the amplitude of the b-wave of the human electroretinogram. Experientia 49, 686–687. 10.1007/BF01923951. [DOI] [PubMed] [Google Scholar]
- Engel L, Vollrath L, Spessert R, 2004. Arylalkylamine N-acetyltransferase gene expression in retina and pineal gland of rats under various photoperiods. Biochem. Biophys. Res. Commun 318, 983–986. 10.1016/j.bbrc.2004.04.133. [DOI] [PubMed] [Google Scholar]
- Fabbro F, Aglioti SM, Bergamasco M, Clarici A, Panksepp J 2015. Evolutionary aspects of self- and world consciousness in vertebrates. Front. Hum. Neurosci 9:157. doi: 10.3389/fnhum.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort I, Ribelayga CP, 2020. Sensory processing: Visual sensitivity gets high at night. Curr. Biol 30, R18–R20. 10.1016/j.cub.2019.11.016. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Cutrera R, Sarmiento MI, Rosenstein RE, 1995. Evidence for local synthesis of melatonin in golden hamster retina. Neuroreport 6, 2093–2095. 10.1097/00001756-199510010-00033. [DOI] [PubMed] [Google Scholar]
- Fain GL, Lisman JE, 1999. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice. Invest. Ophthalmol. Vis. Sci 40, 2770–2772. [PubMed] [Google Scholar]
- Falcon J, 1999. Cellular circadian clocks in the pineal. Prog. Neurobiol 58, 121–162. 10.1016/s0301-0082(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Farber DB, Danciger JS, Organisciak DT, 1991. Levels of mRNA encoding proteins of the cGMP cascade as a function of light environment. Exp. Eye Res 53, 781–786. 10.1016/0014-4835(91)90114-T. [DOI] [PubMed] [Google Scholar]
- Felder-Schmittbuhl M-P, Buhr ED, Dkhissi-Benyahya O, Hicks D, Peirson SN, Ribelayga CP, Sandu C, Spessert R, Tosini G, 2018. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Invest. Ophthalmol. Vis. Sci 59, 4856. 10.1167/iovs.18-24957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Bellingham J, 2004. Inner retinal photoreceptors (IRPs) in mammals and teleost fish. Photochem. Photobiol. Sci 3, 617–627. 10.1039/B400092G. [DOI] [PubMed] [Google Scholar]
- Fowlkes DH, Karwoski CJ, Proenza LM, 1984. Endogenous circadian rhythm in electroretinogram of free-moving lizards. Invest. Ophthalmol. Vis. Sci 25, 121–124. [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J, 2001. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev 53, 527–552. [PMC free article] [PubMed] [Google Scholar]
- Frøland Steindal I, Whitmore D, 2019. Circadian Clocks in Fish—What Have We Learned so far? Biology 8, 17. 10.3390/biology8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Lee CC, 2003. The circadian clock: pacemaker and tumour suppressor. Nat. Rev. Cancer 3, 350–361. 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Sendelbeck A, Atorf J, Kremers J, Brandstätter JH, 2013. Strain differences in illumination-dependent structural changes at mouse photoreceptor ribbon synapses. J. Comp. Neurol 521, 69–78. 10.1002/cne.23161. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GCK, Storm DR, Iuvone PM, Tosini G, 2004. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J. Neurosci 24, 1803–1811. 10.1523/jneurosci.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Banvolgyi T, Petrovics G, Silver R, Witkovsky P, 2004. AII amacrine neurons of the rat retina show diurnal and circadian rhythms of parvalbumin immunoreactivity. Cell Tissue Res. 315, 181–186. 10.1007/s00441-003-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Silver R, Garcia-Espana A, Witkovsky P, 2001. Diurnal and circadian variation of protein kinase C immunoreactivity in the rat retina. J. Comp. Neurol 439, 140–150. 10.1002/cne.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino-Pico E, Carpentieri AR, Castagnet PI, Pasquare SJ, Giusto NM, Caputto BL, Guido ME, 2004a. Synthesis of retinal ganglion cell phospholipids is under control of an endogenous circadian clock: daily variations in phospholipid-synthesizing enzyme activities. J. Neurosci. Res 76, 642–652. 10.1002/jnr.20126. [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MI, Brocco MA, Panzetta P, Rosenstein RE, Caputto BL, Guido ME, 2004b. Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day. J. Biol. Chem 279, 51172–51181. 10.1074/jbc.m309248200. [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Valdez DJ, Contin MA, Pasquare SJ, Castagnet PI, Giusto NM, Caputto BL, Guido ME, 2005. Rhythms of glycerophospholipid synthesis in retinal inner nuclear layer cells. Neurochem. Int 47, 260–270. 10.1016/j.neuint.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Garcia CC, Palomo-Garo C, Gomez-Galvez Y, Fernandez-Ruiz J, 2016. Cannabinoid-dopamine interactions in the physiology and physiopathology of the basal ganglia. Br. J. Pharmacol 173, 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Tian N, Horvath T, Marshak DW, 2006. Retinopetal axons in mammals: emphasis on histamine and serotonin. Curr. Eye Res 31, 655–667. 10.1080/02713680600776119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauer F, Craft CM, 1996. Circadian regulation of hydroxyindole-O-methyltransferase mRNA levels in rat pineal and retina. Brain Res. 737, 99–109. 10.1016/0006-8993(96)00632-4. [DOI] [PubMed] [Google Scholar]
- Gegnaw ST, Sandu C, Mendoza J, Bergen AA, Felder-Schmittbuhl MP, 2021. Dark-adapted light response in mice is regulated by a circadian clock located in rod photoreceptors. Exp. Eye Res 213, 108807. 10.1016/j.exer.2021.108807. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ, 1998. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569. 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Giarmarco MM, Brock DC, Robbings BM, Cleghorn WM, Tsantilas KA, Kuch KC, Ge W, Rutter KM, Parker ED, Brockerhoff SE, 2020. Daily mitochondrial dynamics in cone photoreceptors (preprint). bioRxiv. 10.1101/2020.04.10.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Felder CC, 1997. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci 17, 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Mangel SC, 2021. Dopamine-mediated circadian and light/dark-adaptive modulation of chemical and electrical synapses in retina. Frontiers in Cellular Neuroscience, 15, 647541. doi: 10.3389/fncel.2021.647541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AI, Teirstein PS, O’Brien JO, 1980. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Invest. Ophthalmol 19, 1257–1267. [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fisher D, Saper CB, 2003. A broad role for melanopsin in nonvisual photoreception. J. Neurosci 23, 7093–7106. 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal V, DeVera C, Laurent V, Sellers J, Chrenek MA, Hicks D, Baba K, Iuvone PM, Tosini G, 2020. Dopamine 2 receptor signaling controls the daily burst in phagocytic activity in the mouse retinal pigment epithelium. Invest Ophthalmol Vis Sci. 61, 10. doi: 10.1167/iovs.61.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MS, Chiba A, Menaker M, 1999. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis. Neurosci 16, 909–918. 10.1017/s0952523899165106. [DOI] [PubMed] [Google Scholar]
- Green CB, 2018. Circadian Posttranscriptional Regulatory Mechanisms in Mammals. Cold Spring Harb. Perspect. Biol 10, a030692. 10.1101/cshperspect.a030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J, 2008. The meter of metabolism. Cell 134, 728–742. 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Besharse JC, 1994. Tryptophan hydroxylase expression is regulated by a circadian clock in Xenopus laevis retina. J. Neurochem 62, 2420–2428. 10.1046/j.1471-4159.1994.62062420.x. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC, 1996a. Identification of a novel vertebrate circadian clock-regulated gene encoding the protein nocturnin. Proc. Natl. Acad. Sci. USA 93, 14884–14888. 10.1073/pnas.93.25.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Besharse JC, 1996b. Use of a high stringency differential display screen for identification of retinal mRNAs that are regulated by a circadian clock. Mol. Brain Res 37, 157–165. 10.1016/0169-328X(95)00307-E. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC, 2004. Retinal circadian clocks and control of retinal physiology. J. Biol. Rhythms 19, 91–102. 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC, 1995a. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 677, 283–290. 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC, 1995b. Tryptophan hydroxylase is expressed by photoreceptors in Xenopus laevis retina. Vis. Neurosci 12, 663–670. 10.1017/s0952523800008956. [DOI] [PubMed] [Google Scholar]
- Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC, 2007. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc. Natl. Acad. Sci. USA 104, 9888–9893. 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Liang MY, Steenhard BM, Besharse JC, 1999. Ontogeny of circadian and light regulation of melatonin release in Xenopus laevis embryos. Brain Res. Dev. Brain Res 117, 109–116. 10.1016/s0165-3806(99)00109-1. [DOI] [PubMed] [Google Scholar]
- Gubin D, Neroev V, Malishevskaya T, Cornelissen G, Astakhov SY, Kolomeichuk S, Yuzhakova N, Kabitskaya Y, Weinert D, 2021. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cell function in glaucoma. J Pineal Res. 70(4):e12730. doi: 10.1111/jpi.12730. [DOI] [PubMed] [Google Scholar]
- Guerlotte J, Greve P, Bernard M, Grechez-Cassiau A, Morin F, Collin JP, Voisin P, 1996. Hydroxyindole-O-methyltransferase in the chicken retina: immunocytochemical localization and daily rhythm of mRNA. Eur. J. Neurosci 8, 710–715. 10.1111/j.1460-9568.1996.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Guido ME, Marchese NA, Rios MN, Morera LP, Diaz NM, Garbarino-Pico E, Contin MA., 2022. Non-visual opsins and novel photo-detectors in the vertebrate inner retina mediate light responses within the blue spectrum region. Cell Mol Neurobiol. 42(1):59–83. doi: 10.1007/s10571-020-00997-x. Epub 2020 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido ME, Pico EG, Caputto BL, 2001. Circadian regulation of phospholipid metabolism in retinal photoreceptors and ganglion cells. J. Neurochem 76, 835–845. 10.1046/j.1471-4159.2001.00081.x. [DOI] [PubMed] [Google Scholar]
- Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, Bono H, LeMieux J, Walsh P, Carninci P, Hayashizaki Y, Okazaki Y, Raviola E, 2004. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc. Natl. Acad. Sci. USA 101, 5069–5074. 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstenberg S, Lindgren KM, Samagh SP, Nadal-Vicens M, Balt S, Fernald RD, 2005. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni. Vis. Neurosci 22, 135–141. 10.1017/s0952523805222022. [DOI] [PubMed] [Google Scholar]
- Hamm HE, Menaker M, 1980. Retinal rhythms in chicks-circadian variation in melatonin and serotonin N-acetyltransferase. Proc. Natl. Acad. Sci. USA 77, 4998–5002. 10.1073/pnas.77.8.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U, 2008. Regulation of monoamine Oxidase A by circadian-clock components implies clock influence on mood. Cur.Biol. 18, 678–683. 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Jones RJM, Ruddock KH, 1998. Diurnal variation in the b-wave implicit time of the human electroretinogram. Vis. Neurosci 15, 55–67. 10.1017/s0952523898151118. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Peirson SN, Foster RG, 2008. Melanopsin: an exciting photopigment. Trends Neurosci. 31, 27–36. 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Vrang N, Card JP, Fahrenkrug J, 2001. Light-dependent induction of cFos during subjective day and night in PACAP-containing ganglion cells of the retinohypothalamic tract. J. Biol. Rhythms 16, 457–470. 10.1177/074873001129002132. [DOI] [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D, Iuvone PM, 2010. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N -acetyltransferase mRNA in chicken cone photoreceptors. J. Neurochem 113(5), 1296–1306. 10.1111/j.1471-4159.2010.06698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Alonso-Gomez AL, Chaurasia SS, Iuvone PM, 2003. Diurnal regulation of arylalkylamine N-acetyltransferase activity in chicken retinal cells in vitro: analysis of culture conditions. Mol. Vis 9, 52–59. [PubMed] [Google Scholar]
- Haque R, Chaurasia SS, Wessel III JH, Iuvone PM, 2002. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport 13, 2247–2251. 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- Harms HH, Wardeh G, Mulder AH, 1979. Effect of adenosine on depolarization-induced release of various radiolabelled neurotransmitters from slices of rat corpus striatum. Neuropharmacology 18, 577–580. 10.1016/0028-3908(79)90107-2. [DOI] [PubMed] [Google Scholar]
- Harosi FI, MacNichol EF Jr., 1974. Visual pigments of goldfish cones. Spectral properties and dichroism. J. Gen. Physiol 63, 279–304. 10.1085/jgp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L. Harris A, 2001. Emerging issues of connexin channels: biophysics fills the gap. Quart. Rev. Biophys 34, 325–472. 10.1017/S0033583501003705. [DOI] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC, 1992. Activation of a D2 receptor increases electrical coupling between retinal horizontal cells by inhibiting dopamine release. Proc. Natl. Acad. Sci. USA 89, 9220–9224. 10.1073/pnas.89.19.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC, 1993. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Visual Neuroscience. 10, 81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- Harsanyi K, Wang Y, Mangel SC 1996. Activation of NMDA receptors produces dopamine-mediated changes in fish retinal horizontal cell light responses. J. Neurophysiol 75, 629–639. doi: 10.1152/jn.1996.75.2.629. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Lalonde MR, Barnes S, Baldridge WH, 2004. Adenosine A1-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci 45, 3740–3748. 10.1167/iovs.04-0214. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM, 1998. Cyclic AMP resets the circadian clock in cultured Xenopus retinal photoreceptor layers. J. Neurochem 70, 1523–1531. 10.1046/j.1471-4159.1998.70041523.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM, 1999a. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J. Neurochem 72, 1812–1820. 10.1046/j.1471-4159.1999.0721812.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM, 1999b. Modulation of rhythmic melatonin synthesis in Xenopus retinal photoreceptors by cyclic AMP. Brain Res. 824, 161–167. 10.1016/s0006-8993(99)01162-2. [DOI] [PubMed] [Google Scholar]
- Hayasaka N, LaRue SI, Green CB, 2002. In vivo disruption of Xenopus CLOCK in the retinal photoreceptor cells abolishes circadian melatonin rhythmicity without affecting its production levels. J. Neurosci 22, 1600–1607. 10.1523/JNEUROSCI.22-05-01600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI, 2000. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J. Comp. Neurol 418, 33–40. . [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Thoreson WB, Witkowsky P, 2005. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res 24, 682–720. 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DW, Lin D, Burnside B, 1995. Evidence for D4 receptor regulation of retinomotor movement in isolated teleost cone inner-outer segments. J. Neurochem 64, 1326–1335. 10.1046/j.1471-4159.1995.64031326.x. [DOI] [PubMed] [Google Scholar]
- Hollins C, Stone T, 1980. Characteristics of the release of adenosine from slices of cerebral cortex. J. Physiol 303, 73–82. 10.1113/jphysiol.1980.sp013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Rayborn ME, 1979. Photoreceptor outer segment development: light and dark regulate the rate of membrane addition and loss. Invest. Ophthalmol. Vis. Sci 18, 117–132. [PubMed] [Google Scholar]
- Hong G, Fu T-M, Qiao M, Viveros RD, Yang X, Zhou T, Lee JM, Park H-G, Sanes JR, Lieber CM, 2018. A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451. 10.1126/science.aas9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH, Schnapf JL, 2005. Gap-junctional coupling and absolute sensitivity of photoreceptors in Macaque retina. J. Neurosci 25, 11201–11209. 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Arai I, Tachibana M, 2005. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J. Neurosci 25, 4062–4072. 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Lee SC, Yang XL, 2005. Modulation by melatonin of glutamatergic synaptic transmission in the carp retina. J. Physiol 569, 857–871. 10.1113/jphysiol.2005.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KL, Abshire ET, Goldstrohm AC, 2018. Regulatory roles of vertebrate Nocturnin: insights and remaining mysteries. RNA Biol. 15, 1255–1267. 10.1080/15476286.2018.1526541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H (2006) Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J. Neurophysiol 96, 2025–2033. 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Chaurasia SS, Jackson CR, Chan GC-K, Storm DR, Iuvone PM, 2013. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-Adenylyl Cyclase 1 signaling pathway in retinal ganglion cells. J. Neurosci 33, 14989–14997. 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Furukawa K, Hattori A, Ohtani-Kaneko R, Hara M, Suzuki T, Tabata M, Aida K, 1997a. Ocular melatonin rhythms in the goldfish, Carassius auratus. J. Biol. Rhythms 12, 182–192. 10.1177/074873049701200209. [DOI] [PubMed] [Google Scholar]
- Iigo M, Hara M, Ohtani-Kaneko R, Hirata K, Tabata M, Aida K, 1997b. Photic and circadian regulations of melatonin rhythms in fishes. Biol. Signals 6, 225–232. 10.1159/000109132. [DOI] [PubMed] [Google Scholar]
- Iigo M, Sato M, Ikeda E, Kawasaki S, Noguchi F, Nishi G, 2003. Effects of photic environment on ocular melatonin contents in a labrid teleost, the wrasse Halichoeres tenuispinnis. Gen. Comp. Endocrinol 133, 252–259. 10.1016/s0016-6480(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Ingram NT, Fain GL, Sampath AP, 2020. Elevated energy requirement of cone photoreceptors. Proc. Natl. Acad. Sci. USA 117, 19599–19603. 10.1073/pnas.2001776117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Morgans C, Aicher S, Carroll J, Singer J, Li W, Fahrenfort I, Ribelayga CP, Massey SC, 2022. Analysis of rod/cone gap junctions from the reconstruction of mouse photoreceptor terminals. Elife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, 1990. Development of melatonin biosynthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillators, and cyclic AMP. J. Neurochem 54, 1562–1568. 10.1111/j.1471-4159.1990.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, 1995. Cell biology and metabolic activity of photoreceptor cells: light-evoked and circadian regulation. In: Djamgoz MBA, Archer SN, Vallerga S (Eds.), Neurobiology and Clinical Aspects of the Outer Retina. Chapman and Hall, London, pp. 25–55. [Google Scholar]
- Iuvone PM, Gan J, 1995. Functional interaction of melatonin receptors and D1 dopamine receptors in cultured chick retinal neurons. J. Neurosci 15, 2179–2185. 10.1523/jneurosci.15-03-02179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS, 2005. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res 24, 433–456. 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Valenciano AI, Alonso-Gomez AL, 2000. Adenosine: a circadian modulator of melatonin biosynthesis in Xenopus photoreceptor cells. Invest. Ophthalmol. Vis. Sci 41, S112. [Google Scholar]
- Ivanova TN, Iuvone PM, 2003a. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 991, 96–103. 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM, 2003b. Melatonin synthesis in retina: circadian regulation of arylalkylamine N-acetyltransferase activity in cultured photoreceptor cells of embryonic chicken retina. Brain Res. 973, 56–63. 10.1016/s0006-8993(03)02540-x. [DOI] [PubMed] [Google Scholar]
- Jackson CR, Ruan G-X, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG, 2012. Retinal dopamine mediates multiple dimensions of light-adapted vision. J. Neurosci 32, 9359–9368. 10.1523/JNEUROSCI.0711-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Luo Q, M. Dominguez J, Al-Sabah J, Chaqour B, Grant MB, Bhatwadekar AD, 2016. Per2-mediated vascular dysfunction is caused by the upregulation of the connective tissue growth factor (CTGF). PLoS ONE 11, e0163367. 10.1371/journal.pone.0163367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Sandu C, Malan A, Mellac K, Hicks D, Felder-Schmittbuhl M, 2015. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 29, 1493–1504. 10.1096/fj.14-261214. [DOI] [PubMed] [Google Scholar]
- Jaliffa CO, Saenz D, Resnik E, Keller Sarmiento MI, Rosenstein RE, 2001. Circadian activity of the GABAergic system in the golden hamster retina. Brain Res. 912, 195–202. 10.1016/s0006-8993(01)02736-6. [DOI] [PubMed] [Google Scholar]
- Jin NG, Chuang AZ, Masson PJ, Ribelayga CP, 2015. Rod electrical coupling is controlled by a circadian clock and dopamine in mouse retina: Circadian clock control of rod electrical coupling. J. Physiol 593, 1597–1631. 10.1113/jphysiol.2014.284919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin NG, Ribelayga CP, 2016. Direct Evidence for Daily Plasticity of Electrical Coupling between Rod Photoreceptors in the Mammalian Retina. Journal of Neuroscience 36, 178–184. 10.1523/JNEUROSCI.3301-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Zhang Z, Keung J, Youn SB, Ishibashi M, Tian L-M, Marshak DW, Solessio E, Umino Y, Fahrenfort I, Kiyama T, Mao C-A, You Y, Wei H, Wu J, Postma F, Paul DL, Massey SC, Ribelayga CP, 2020. Molecular and functional architecture of the mouse photoreceptor network. Sci. Adv 6, eaba7232. 10.1126/sciadv.aba7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Tian L-M, Zhang Z, Fahrenfort I, Postma F, Paul DL, Massey SC, Ribelayga CP, 2022. Genetic elimination of rod/cone coupling reveals the contribution of the secondary rod pathway to the retinal output. Sci. Adv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, Cecon E, Zlotos DP, 2016. Update on melatonin receptors: IUPHAR Review 20: Melatonin receptors. Br. J. Pharmacol 173, 2702–2725. 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM, 2004. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci 24, 7604–7613. 10.1523/jneurosci.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, 2004. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr. Opin. Neurobiol 14, 531–541. 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM, 2005. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem. Biophys. Res. Commun 330, 18–26. 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hernandez-Borsetti N, Cahill GM, 2006. Diversity of zebrafish peripheral oscillators revealed by luciferase reporting. Proc. Natl. Acad. Sci. U.S.A 103, 14614–14619. 10.1073/pnas.0606563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T, 2010. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 107, 6412–6417. 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimi N, Cahill GM, 1999. Development of a circadian melatonin rhythm in embryonic Zebrafish. Brain Res. Dev. Brain Res 117, 47–52. 10.1016/s0165-3806(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Katti C, Butler R, Sekaran S, 2013. Diurnal and circadian regulation of connexin 36 transcript and protein in the mammalian retina. Invest. Ophthalmol. Vis. Sci 54, 821. 10.1167/iovs.12-10375. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Chiba A, Aoki K, 2000. Daily melatonin injections entrain the circadian change of synaptic ribbon number in the pineal organ of the Japanese newt. Neurosci. Lett 285, 181–184. 10.1016/S0304-3940(00)01058-2. [DOI] [PubMed] [Google Scholar]
- King TS, Steinlechner S, 1985. Pineal indolalkylamine synthesis and metabolism: kinetic considerations. Pineal Res. Rev 3, 69–113. [Google Scholar]
- Klein DC, 2007. Arylalkylamine N-acetyltransferase: "the Timezyme". J. Biol. Chem 282, 4233–4237. 10.1074/jbc.r600036200. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcon J, Cahill GM, Cassone VM, Baler R, 1997. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Rec. Prog. Horm. Res 52, 307–358. [PubMed] [Google Scholar]
- Klein DC, Namboodiri MA, Auerbach DA, 1981. The melatonin rhythm generating system: developmental aspects. Life Sci. 28, 1975–1986. 10.1016/0024-3205(81)90644-5. [DOI] [PubMed] [Google Scholar]
- Klitten LL, Rath MF, Coon SL, Kim JS, Klein DC, Møller M, 2008. Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Exp. Eye Res 87, 471–477. 10.1016/j.exer.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosen P, Lapmanee S, Schuster C, Guardiola B, Hicks D, Pevet P, Felder-Schmittbuhl MP, 2019. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J. Pineal Res 67, e12575. 10.1111/jpi.12575. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE, 1987. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature 325, 437–439. 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Ko GY, 2020. Circadian regulation in the retina: From molecules to network. Eur. J. Neurosci 51(1):194–216. DOI: 10.1111/ejn.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko M, Dryer SE, 2004a. Circadian and cAMP dependent modulation of retinal cone cGMP-gated channels does not require protein synthesis or calcium influx through L-type channels. Brain Res. 1021, 277–280. 10.1016/j.brainres.2004.05.072. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE, 2001. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron 29, 255–266. 10.1016/S0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE, 2003. Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J. Neurosci 23, 3145–3153. 10.1523/JNEUROSCI.23-08-03145.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GYP, Ko ML, Dryer SE, 2004b. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J. Neurosci 24, 1296–1304. 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Ishikawa T, Hirayama J, Daiyasu H, Kanai S, Toh H, Fukuda I, Tsujimura T, Terada N, Kamei Y, Yuba S, Iwai S, Todo T, 2000. Molecular analysis of zebrafish photolyase/cryptochrome family: two types of cryptochromes present in zebrafish. Genes Cells 5, 725–738. 10.1046/j.1365-2443.2000.00364.x. [DOI] [PubMed] [Google Scholar]
- Kohler K, Kolbinger W, Kurz-Isler G, Weiler R, 1990. Endogenous dopamine and cyclic events in the fish retina, II: Correlation of retinomotor movement, spinule formation, and connexon density of gap junctions with dopamine activity during light/dark cycles. Vis. Neurosci 5, 417–428. 10.1017/s0952523800000547. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo S-H, Huang H-C, Kumar V, Lee C, Kim T-K, Takahashi JS, 2012. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354. 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho J, Sagar SM, 1995. Light-induced c-fos expression in amacrine cells in the rabbit retina. Brain Res. Mol. Brain Res 29, 53–63. 10.1016/0169-328x(94)00218-4. [DOI] [PubMed] [Google Scholar]
- Kojima S, Gatfield D, Esau CC, Green CB, 2010. MicroRNA-122 Modulates the rhythmic expression profile of the circadian deadenylase Nocturnin in mouse liver. PLoS ONE 5, e11264. 10.1371/journal.pone.0011264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Gendreau KL, Sher-Chen EL, Gao P, Green CB, 2015. Changes in poly(A) tail length dynamics from the loss of the circadian deadenylase Nocturnin. Sci. Rep 5, 17059. 10.1038/srep17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Shingle DL, Green CB, 2011. Post-transcriptional control of circadian rhythms. J. Cell Sci 124, 311–320. 10.1242/jcs.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbinger W, Kohler K, Oetting H, Weiler R, 1990. Endogenous dopamine and cyclic events in the fish retina, I: HPLC assay of total content, release, and metabolic turnover during different light/dark cycles. Vis. Neurosci 5, 143–149. 10.1017/s0952523800000183. [DOI] [PubMed] [Google Scholar]
- Kolbinger W, Wagner D, Wagner HJ, 1996. Control of rod retinomotor movements in teleost retinae: the role of dopamine in mediating light-dependent and circadian signals. Cell Tissue Res. 285, 445–451. 10.1007/s004410050661. [DOI] [PubMed] [Google Scholar]
- Kopperud K, Grace M, 2017. Circadian rhythms of retinomotor movement in a marine megapredator, the Atlantic Tarpon, Megalops atlanticus. Int. J. Mol. Sci 18, 2068. 10.3390/ijms18102068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD, 1989. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature 337, 454–457. 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Koskela S, Turunen T, Ala-Laurila P, 2020. Mice reach higher visual sensitivity at night by using a more efficient behavioral strategy. Curr. Biol 30, 42–53.e4. 10.1016/j.cub.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Gábriel R, Owen WG, Witkovsky PJ, 1998. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J. ., Comp Neurol 398: 529–538. [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Akiyama M, Fukada Y, Okano T, 2006. Molecular cloning, mRNA expression, and immunocytochemical localization of a putative blue-light photoreceptor CRY4 in the chicken pineal gland. J. Neurochem 97, 1155–1165. 10.1111/j.1471-4159.2006.03826.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG, 2000. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport 11, 1479–1482. 10.1097/00001756-200005150-00023. [DOI] [PubMed] [Google Scholar]
- Kulczykowska E, Iuvone PM, 1998. Highly sensitive and specific assay of plasma melatonin using high-performance liquid chromatography with fluorescence detection preceded by solid-phase extraction. J. Chromatogr. Sci 36, 175–178. 10.1093/chromsci/36.4.175. [DOI] [PubMed] [Google Scholar]
- Kurumado K, Mori W, 1977. A morphological study of the circadian cycle of the pineal gland of the rat. Cell Tissue Res. 182. 10.1007/BF00219839. [DOI] [PubMed] [Google Scholar]
- La Fleur SE, Kalsbeek A, Wortel J, Buijs RM, 1999. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J. Neuroendocrinol 11, 643–652. 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Schmitz F, 2015. Ribbon synapses and visual processing in the retina. Annu. Rev. Vis. Sci 1, 235–262. 10.1146/annurev-vision-082114-035709. [DOI] [PubMed] [Google Scholar]
- Lamosova D, Zeman M, Mackova M, Gwinner E, 1995. Development of rhythmic melatonin synthesis in cultured pineal glands and pineal cells isolated from chick embryo. Experientia 51, 970–975. 10.1007/BF01921750. [DOI] [PubMed] [Google Scholar]
- Laothamatas I, Gao P, Wickramaratne A, Quintanilla CG, Dino A, Crystal A Khan CA, Liou J, Green CB, 2020. Spatiotemporal regulation of NADP(H) phosphatase Nocturnin and its role in oxidative stress response. Proc. Natl. Acad. Sci. USA 117, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Pedata F, 2001. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem 79, 463–484. 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- LaVail MM, 1976. Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science 194, 1071–1074. 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail MM, 2001. Legacy of the RCS rat: impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog. Brain Res 131, 617–627. 10.1016/S0079-6123(01)31048-8. [DOI] [PubMed] [Google Scholar]
- Lee CC, 2006. Tumor suppression by the mammalian Period genes. Cancer Causes Control 17, 525–530. 10.1007/s10552-005-9003-8. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN, 2003. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat. Neurosci 6, 111–112. 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S, Kratz KE, Mangel SC, Sherman SM, 1978. An effect of early monocular lid suture upon the development of X-cells in the cat's lateral geniculate nucleus. Brain Res. 157 (2), 346–350. doi: 10.1016/0006-8993(78)90039-2. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Takahashi Y, Lee TH, Mori W, 1958. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc 80, 2587. 10.1021/ja01543a060. [DOI] [Google Scholar]
- Levinson G, Burnside B, 1981. Circadian rhythms in teleost retinomotor movement. A comparison of the effects of circadian rhythm and light condition on cone length. Invest. Ophthalmol. Vis. Sci 20, 294–303. [PubMed] [Google Scholar]
- Lewis TR, Zareba M, Link BA, Besharse JC, 2018. Cone myoid elongation involves unidirectional microtubule movement mediated by dynein-1. Mol. Biol. Cell 29, 180–190. 10.1091/mbc.E17-08-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chuang AZ, O’Brien J, 2009. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J. Neurosci 29, 15178–15186. 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J, 2013. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J. Neuro 33, 3135–3150. 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE, 1998. Zebrafish visual sensitivity is regulated by a circadian clock. Vis. Neurosci 15, 851–857. 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE, 2000a. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J. Neurosci 20, 1883–1892. 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE, 2000b. Effects of dopamine depletion on visual sensitivity of zebrafish. J. Neurosci 20, 1893–1903. 10.1523/JNEUROSCI.20-05-01893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Chaurasia SS, Gao Y, Carr AL, Iuvone PM, Li L, 2008. CLOCK is required for maintaining the circadian rhythms of opsin mRNA expression in photoreceptor cells. J. Biol. Chem 283, 31673–31678. 10.1074/jbc.m803875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML, 2016. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol 56, 361–383. 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Labani N, Cecon E, Jockers R, 2019. Melatonin Target Proteins: Too Many or Not Enough? Front. Endocrinol 10, 791. 10.3389/fendo.2019.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wessel JH 3rd, Iuvone PM, Tosini G, Fukuhara C, 2004. Diurnal rhythms of tryptophan hydroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport 15, 1497–1500. 10.1097/01.wnr.0000131007.59315.66. [DOI] [PubMed] [Google Scholar]
- Lilley S, LeTissier P, Robbins J, 2004. The discovery and characterization of a proton-gated sodium current in rat retinal ganglion cells. J. Neurosci 24, 1013–1022. 10.1523/JNEUROSCI.3191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, 1986. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol 88, 521–542. 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP, 2012. Heterogeneous Expression of the Core Circadian Clock Proteins among Neuronal Cell Types in Mouse Retina. PLoS ONE 7, e50602. 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS, 2000. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–492. 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS, 2004. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet 5, 407–441. 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zoran MJ, Cassone VM, 1995. Daily and circadian variation in the electroretinogram of domestic fowl: effects of melatonin. J. Comp. Physiol. A 177, 299–306. 10.1007/bf00192419. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han X, de Castro RB, Zhang P, Zhang K, Hu Z, Qin L, 2018. Analysis of the bystander effect in cone photoreceptors via a guided neural network platform. Sci. Adv 4(5), eaas9274. 10.1126/sciadv.aas9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Li L, 2003. Olfactory input increases visual sensitivity in Zebrafish: a possible function for the terminal nerve and dopaminergic interplexiform cells. J. Exp. Biol 206, 2201–2209. 10.1242/jeb.00397. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F, 2008. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J. Neurosci 28, 7954–7967. 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella G, Asher G, 2016. The circadian nature of mitochondrial biology. Front. Endocrinol 7. 10.3389/fendo.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel SC, 1991. Analysis of the horizontal cell contribution to the receptive field surround of ganglion cells in the rabbit retina. Journal of Physiology (Lond). 442, 211–234. doi: 10.1113/jphysiol.1991.sp018790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel SC, 2001. Circadian clock regulation of neuronal light responses in the vertebrate retina. Prog. Brain Res 131, 505–518. 10.1016/S0079-6123(01)31040-3. [DOI] [PubMed] [Google Scholar]
- Mangel SC, 2011. The circadian clock in the mammalian retina uses dopamine and melatonin receptor activation to control rod and cone input to ganglion cells. Invest. Ophthal. Vis. Sci, ARVO abstract. [Google Scholar]
- Mangel SC, Ariel M, Dowling JE, 1985. Effects of acidic amino acid antagonists on the spectral properties of fish horizontal cells: circuitry of the outer retina. J. Neurosci 5, 2839–2850. doi: 10.1523/JNEUROSCI.05-11-02839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel SC, Baldridge WH,R, Weiler R, Dowling JE, 1994.Threshold and chromatic sensitivity changes in fish cone horizontal cells following prolonged darkness. Brain Res. 659, 55–61. doi: 10.1016/0006-8993(94)90862-1. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Brunken WJ, 1992. Effects of serotonin drugs on horizontal and ganglion cells in the rabbit retina. Visual Neurosci. 8, 213–218. doi: 10.1017/s0952523800002868. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE, 1985. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science 229, 1107–1109. 10.1126/science.4035351. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE, 1987. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proc. R. Soc. Lond. B. Biol. Sci 231, 91–121. 10.1098/rspb.1987.0037. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Miller RF, 1987. Horizontal cells contribute to the receptive field surround of ganglion cells in the rabbit retina. Brain Res. 414, 182–186. doi: 10.1016/0006-8993(87)91344-8. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Ribelayga C, 2010. Comparative eye: The circadian clock in the retina regulates rod and cone pathways. In: Dartt DA, Besharse JC, Dana R (Eds.), Encyclopedia of the Eye. Elsevier, Oxford, Vol. 1, pp. 283–289. doi: 10.1016/s0079-6123(01)31040-3. [DOI] [Google Scholar]
- Mangel SC, Wilson JR, Sherman SM 1983. Development of neuronal response properties in the cat lateral geniculate nucleus during monocular lid suture. J. Neurophysiol 50, 240–264. doi: 10.1152/jn.1983.50.1.240 [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB, 1999. Dopamine mediates circadian rhythms of rod–cone dominance in the Japanese quail retina. J. Neurosci 19, 4132–4141. 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB, 1998. Circadian rhythms of rod–cone dominance in the Japanese quail retina. J. Neurosci 18, 4775–4784. 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Fukada Y, 2006. A Median Third Eye: Pineal Gland Retraces Evolution of Vertebrate Photoreceptive Organs. Photochem. Photobiol 2006-02-24-IR-813. 10.1562/2006-02-24-IR-813. [DOI] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC, 2012. Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol. Biol. Cell 23, 1104–1114. 10.1091/mbc.e11-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J, 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631. 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel JC, McArthur SG, 2020. Dopamine receptor subtypes, physiology and pharmacology: New ligands and concepts in schizophrenia. Front. Pharmacol 11, 1003. 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Sassone-Corsi P, 2018. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med 24, 1795–1803. 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AJ, Gillette MU, Prosser RA, 1991. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 565, 158–161. 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- McCormack CA, Burnside B, 1993. Light and circadian modulation of teleost retinal tyrosine hydroxylase activity. Invest. Ophthalmol. Vis. Sci 34, 1853–1860. [PubMed] [Google Scholar]
- McGinnis JF, Austin BJ, Stepanik PL, Lerious V, 1994. Light-dependent regulation of the transcriptional activity of the mammalian gene for arrestin. J. Neurosci. Res 38, 479–482. 10.1002/jnr.490380414. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Whelan JP, Donoso LA, 1992. Transient, cyclic changes in mouse visual cell gene products during the light-dark cycle. J. Neurosci. Res 31, 584–590. 10.1002/jnr.490310325. [DOI] [PubMed] [Google Scholar]
- McGoogan JM, Cassone VM, 1999. Circadian regulation of chick electroretinogram: effects of pinealectomy and exogenous melatonin. Am. J. Physiol 277, R1418–R1427. 10.1152/ajpregu.1999.277.5.R1418. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, Tosini G, 2014. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog. Retin. Eye Res 39, 58–76. 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Zhang DQ, Ponomareva L, Wagner T, 2001. Synaptic mechanisms of network adaptation in horizontal cells. Prog. Brain Res 131, 419–436. 10.1016/S0079-6123(01)31034-8. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA, 2001. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105, 877–889. 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Menaker M, 1968. Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc. Nat.Acad. Sci. USA 59, 414–421. 10.1073/pnas.59.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger GJ, Koke JR, Cahill GM, 2005. Diurnal and circadian retinomotor movements in zebrafish. Vis. Neurosci 22, 203–209. 10.1017/s0952523805222083. [DOI] [PubMed] [Google Scholar]
- Mercer AJ, Thoreson WB, 2011. The dynamic architecture of photoreceptor ribbon synapses: Cytoskeletal, extracellular matrix, and intramembrane proteins. Vis. Neurosci 28, 453–471. 10.1017/S0952523811000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis ML, Johe KK, Moghadam B, Adams RN, 1988. Studies on the ionic mechanism for the neuromodulatory actions of adenosine in the brain. Brain Res. 473, 249–260. 10.1016/0006-8993(88)90854-2. [DOI] [PubMed] [Google Scholar]
- Milićević N, Mazzaro N, de Bruin I, Wils E, ten Brink J, Asbroek A, ten, Mendoza J, Bergen A, Felder-Schmittbuhl M-P, 2019. Rev-Erbα and photoreceptor outer segments modulate the circadian clock in retinal pigment epithelial cells. Sci. Rep 9, 11790. 10.1038/s41598-019-48203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milićević N, Ait-Hmyed Hakkari O, Bagchi U, Sandu C, Jongejan A, Moerland PD, Ten Brink JB, Hicks D, Bergen AA, Felder-Schmittbuhl MP., 2021. Core circadian clock genes Per1 and Per2 regulate the rhythm in photoreceptor outer segment phagocytosis. FASEB J.;35(7):e21722. doi: 10.1096/fj.202100293RR. [DOI] [PubMed] [Google Scholar]
- Miller JD, Morin LP, Schwartz WJ, Moore RY, 1996. New insights into the mammalian circadian clock. Sleep 19, 641–667. https://psycnet.apa.org/doi/10.1093/sleep/19.8.641. [DOI] [PubMed] [Google Scholar]
- Mills SL, Xia XB, Hoshi H, Firth SI, Rice ME, Frishman LJ, Marshak DW, 2007. Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Vis. Neurosci 24, 593–608. 10.1017/s0952523807070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Anaya M, Bartell PA, Menaker M, 2002. Circadian rhythm of iguana electroretinogram: the role of dopamine and melatonin. J. Biol. Rhythms 17, 526–538. 10.1177/0748730402238235. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG, 1998. Dopamine receptors: from structure to function. Physiol. Rev 78, 189–225. 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Sancar A, 1998. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl. Acad. Sci. USA 95, 6097–6102. 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS, 2012. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci 35, 445–462. 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, 1996. Entrainment pathways and the functional organization of the circadian system. Prog. Brain Res 111, 103–119. 10.1016/S0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB, 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206. 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Boelen MK, 1996. A retinal dark-light switch: a review of the evidence. Vis. Neurosci 13, 399–409. 10.1017/s0952523800008087. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Haley TL, Ren G, Duvoisin RM, 2016. Changes in the outer plexiform layer of the retina between light- and dark-adapted states. ARVO Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz Llamosas M, Huerta JJ, Cernuda-Cernuda R, Garcia-Fernandez JM, 2000. Ontogeny of a photic response in the retina and suprachiasmatic nucleus in the mouse. Dev. Brain Res 120, 1–6. 10.1016/s0165-3806(99)00175-3. [DOI] [PubMed] [Google Scholar]
- Murakami M, Miyachi EI, Takahashi KI, 1995. Modulation of gap junctions between horizontal cells by second messengers. Prog. Retin. Eye Res 14, 197–221. 10.1016/1350-9462(95)90003-4. [DOI] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S, 2018. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318. 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namihira M, Honma S, Abe H, Masubuchi S, Ikeda M, Honmaca K, 2001. Circadian pattern, light responsiveness and localization of rPer1 and rPer2 gene expression in the rat retina. Neuroreport 12, 471–475. 10.1097/00001756-200103050-00010. [DOI] [PubMed] [Google Scholar]
- Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, Honma K, 1999. Circadian rhythms and light responsiveness of mammalian clock gene, Clock and BMAL1, transcripts in the rat retina. Neurosci. Lett 271, 1–4. 10.1016/s0304-3940(99)00407-3. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC, 2007. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 104, 12005–12010. 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Finnemann SC, 2008. Lack of αvβ5 integrin receptor or its ligand MFG-E8: Distinct effects on retinal function. Ophthalmic Res. 40, 120–123. 10.1159/000119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC, 2004. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J. Exp. Med 200, 1539–1545. 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan AK, Cassone VM, 2002. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis. Neurosci 19, 265–274. 10.1017/s0952523802192042. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH, 1996. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J. Neurochem 67, 2514–2520. 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Hicks D, 2000.Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int. Rev. Cytol 196, 245–313. 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P, 1999. Dopamine receptor localization in the mammalian retina. Mol. Neurobiol 19, 181–204. 10.1007/bf02821713. [DOI] [PubMed] [Google Scholar]
- Niki T, Hamada T, Ohtomi M, Sakamoto K, Suzuki S, Kako K, Hosoya Y, Horikawa K, Ishida N, 1998. The localization of the site of arylalkylamine N-acetyltransferase circadian expression in the photoreceptor cells of mammalian retina. Biochem. Biophys. Res. Commun 248, 115–120. 10.1006/bbrc.1998.8916. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM, 2000. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 870, 118–125. 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM, 2002. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J. Neurosci 22, 2063–2073. 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ, Zurawska E, 1989. Dopamine in the rabbit retina and striatum: diurnal rhythm and effect of light stimulation. J. Neural Transm 75, 201–212. 10.1007/BF01258631. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Wakakura M, Ishikawa S, 1983. Circadian rhythm of human electroretinogram. Jpn J. Ophthalmol 27, 346–352. [PubMed] [Google Scholar]
- Oakley B 2nd, Wen R, 1989. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J. Physiol 419, 353–378. 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Bruzzone R, White TW, Al-Ubaidi MR, Ripps H, 1998. Cloning and expression of two related connexins from the perch retina define a distinct subgroup of the connexin family. J. Neurosci 18, 7625–7637. 10.1523/JNEUROSCI.18-19-07625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Nguyen HB, Mills SL, 2004. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. J. Neurosci 24, 5632–5642. 10.1523/JNEUROSCI.1248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N, 1998. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem. Biophys. Res. Commun 253, 199–203. 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, 2007. Optimization of Single-Photon Response Transmission at the Rod-to-Rod Bipolar Synapse. Physiology 22, 279–286. 10.1152/physiol.00007.2007. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL, 2008. ATP consumption by mammalian rod photoreceptors in darkness and in light. Cur. Biol 18, 1917–1921. 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM, 2012. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complicat 26, 56–64. 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B, 2000. Circadian-dependent retinal light damage in rats. Invest. Ophthalmol. Vis. Sci 41, 3694–3701. [PubMed] [Google Scholar]
- Ota W, Nakane Y, Hattar S, Yoshimura T, 2018. Impaired circadian photoentrainment in Opn5-null mice. iScience 6, 299–305. 10.1016/j.isci.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino S, Contreras-Alcantara S, Baba K, Tosini G, 2016. Melatonin signaling ccontrols the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS ONE 11, e0148214. 10.1371/journal.pone.0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes-de-Carvalho R, Maia GA, Ferreira JM, 2003. Adenosine regulates the survival of avian retinal neurons and photoreceptors in culture. Neurochem. Res 28, 1583–1590. 10.1023/A:1025686812298. [DOI] [PubMed] [Google Scholar]
- Palczewski K, McDowell JH, Hargrave PA, 1988. Rhodopsin kinase: substrate specificity and factors that influence activity. Biochemistry 27, 2306–2313. 10.1021/bi00407a010. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, 2004. It’s all in the timing: many clocks, many outputs. J. Biol. Rhythms 19, 374–387. 10.1177/0748730404269008. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA, 2002. Circadian rhythms from flies to human. Nature 417, 329–335. 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP, 2008. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol 85, 335–353. 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Pang SF, Yu HS, Suen HC, Brown GM, 1980. Melatonin in the retina of rats: a diurnal rhythm. J. Endocrinol 87, 89–93. 10.1677/joe.0.0870089. [DOI] [PubMed] [Google Scholar]
- Park K, Kang HM, 2004. Cloning and circadian expression of rat Cry1. Mol. Cells 18, 256–260. [PubMed] [Google Scholar]
- Park K, Kang HM, 2006. Circadian expression of clock genes in the rat eye and brain. Mol. Cells 22, 285–290. [PubMed] [Google Scholar]
- Paul KN, Saafir TB, Tosini G, 2009. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev. Endocr. Metab. Disord 10, 271–278. 10.1007/s11154-009-9120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG, 2006. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem. Biophys. Res. Commun 351, 800–807. 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]
- Perez MTR, Ehinger B, 1989. Adenosine inhibits evoked acetylcholine release from the rabbit retina. J. Neurochem 52, S157. [Google Scholar]
- Pérez-Fernández V, Milosavljevic N, Allen AE, Vessey KA, Jobling AI, Fletcher EL, Breen PP, Morley JW, Cameron MA, 2019. Rod photoreceptor activation alone defines the release of dopamine in the retina. Curr. Biol 29, 763–774.e5. 10.1016/j.cub.2019.01.042. [DOI] [PubMed] [Google Scholar]
- Peters JL, Cassone VM, 2005. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J. Pineal Res 38, 209–215. 10.1111/j.1600-079X.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Pierce ME, 1999. Circadian organization in quail retina: differential regulation of melatonin synthesis and iodopsin gene expression in vitro. Visual Neurosci. 16, 843–848. 10.1017/s0952523899165040. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC, 1986. Melatonin and dopamine interactions in the regulation of rhythmic photoreceptor metabolism. In: O’Brien PJ, Klein DC (Eds.), Pineal and Retinal relationships. Academic press, New-York, pp. 219–237. [Google Scholar]
- Pierce ME, Zhang Z, Fox LE, Takahashi JS, 1993. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Cell 10, 579–584. 10.1016/0896-6273(93)90161-J. [DOI] [PubMed] [Google Scholar]
- Ping Y, Huang H, Zhang XJ, Yang XL, 2008. Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J. Physiol 586, 2683–2694. 10.1113/jphysiol.2008.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, 1981. Circadian systems: General perspective. In: Aschoff J (Ed.), Handbook of Behavioral Neurobiology. Biological Rhythms, Vol. 4. Plenum Press, New York, pp. 57–80. [Google Scholar]
- Pittendrigh CS, 1993. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol 55, 16–54. 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Pozdeyev NV, Lavrikova EV, 2000. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J. Mol. Neurosci 15, 1–9. 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM, 2008. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur. J. Neurosci 27, 2691–2700. 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge CL, Yeh P-T, Liou N-F, Lee C-C, You S-F, Liu L-L, McNeill DS, Chew KS, Hattar S, Chen S-K, Zhang D-Q, 2016. M1 ipRGCs influence visual function through retrograde signaling in the retina. J. Neurosci 36, 7184–7197. 10.1523/JNEUROSCI.3500-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privat K, Ravault JP, Chesneau D, Fevre-Montange M, 1999. Day/night variation of tryptophan hydroxylase and serotonin N-acetyltransferase mRNA levels in the ovine pineal gland and retina. J. Pineal Res 26, 193–203. 10.1111/j.1600-079x.1999.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Rajendran RR, Van Niel EE, Stenkamp DL, Cunningham LL, Raymond PA, Gonzalez-Fernandez F, 1996. Zebrafish interphotoreceptor retinoid-binding protein: differential circadian expression among cone subtypes. J. Exp. Biol 199, 2775–2787. [DOI] [PubMed] [Google Scholar]
- Rashid K, Baldridge WH, Ball AK, 1993. Evidence for D2 receptor regulation of dopamine release in the goldfish retina. J. Neurochem 61, 2025–2033. 10.1111/j.1471-4159.1993.tb07438.x. [DOI] [PubMed] [Google Scholar]
- Raviola RF, Dacheux RF, 1990. Axonless horizontal cells of the rabbit retina: synaptic connections and origin of the rod aftereffect. J. Neurocytol 19, 731–736. 10.1007/BF01188041. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB, 1973. Gap junctions between photoreceptor cells in the vertebrate retina. Proc. Natl. Acad. Sci. USA 70, 1677–1681. 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld JP, Laviolette JR, Wagner HJ, 1979. Goldfish retina: a correlate between cone activity and morphology of the horizontal cell in cone pedicule. Science 204, 1436–1438. 10.1126/science.451577. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL, 2001. NPAS2: an analog of clock operative in the mammalian forebrain. Science 293, 506–509. 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Remé CE, Wirz-Justice A, Terman M, 1991. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? J. Biol. Rhythms 6, 5–29. 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Li L, 2004. A circadian clock regulates the process of ERG b- and d-wave dominance transition in dark-adapted zebrafish. Vision Res. 44, 2147–2152. 10.1016/j.visres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Refinetti R 2016. Circadian Physiology, third ed. CRC Press, Boca Raton, FL. [Google Scholar]
- Repérant J, Médina M, Ward R, Miceli D, Kenigfest NB, Rio JP, Vesselkin NP, 2007. The evolution of the centrifugal visual system of vertebrates. A cladistic analysis and new hypotheses. Brain Res. Rev 53, 161–97. 10.1016/j.brainresrev.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Repérant J, Miceli D, Vesselkin NP, Molotchnikoff S, 1989. The centrifugal visual system of vertebrates: a century-old search reviewed. Int. Rev. Cytol 118, 115–71. 10.1016/s0074-7696(08)60874-8. [DOI] [PubMed] [Google Scholar]
- Repérant J, Ward R, Miceli D, Rio JP, Médina M, Kenigfest NB, Vesselkin NP, 2006. The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain Res. Rev 52, 1–57. 10.1016/j.brainresrev.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Reppert SM, 1997. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J. Biol. Rhythms 12, 528–531. 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Sagar SM, 1983. Characterization of the day-night variation of retinal melatonin content in the chick. Invest. Ophthalmol. Vis. Sci 24, 294–300. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, 2002. Coordination of circadian timing in mammals. Nature 418, 935–941. 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rey HL, Burnside B (1999) Adenosine stimulates cone photoreceptor myoid elongation via an adenosine A2-like receptor. J. Neurochem 72, 2345–2355. 10.1046/j.1471-4159.1999.0722345.x [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A, 2002. Adenosine receptors in the nervous system: pathophysiological implications. Prog. Neurobiol 68, 377–392. 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC, 2008. The circadian clock in the retina controls rod/cone coupling. Neuron 59, 790–801. 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Gauer F, Pévet P, Simonneaux V, 1998. Ontogenesis of hydroxyindole-O-methyltransferase gene expression and activity in the rat pineal gland. Brain Res. Dev. Brain Res 110, 235–239. 10.1016/s0165-3806(98)00114-x. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2003. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J. Comp. Neurol 467, 243–253. 10.1002/cne.10927. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2005. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J. Neurosci 25, 215–222. 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2007. Tracer coupling between fish rod horizontal cells: Modulation by light and dopamine but not the retinal circadian clock. Vis Neurosci 24, 333–344. 10.1017/S0952523807070319. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2010. Identification of a Circadian Clock-Controlled Neural Pathway in the Rabbit Retina. PLoS ONE 5, e11020. 10.1371/journal.pone.0011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2019). Circadian clock regulation of cone to horizontal cell synaptic transfer in the goldfish retina. PLoS ONE, 14(8): e0218818. 10.1371/journal.pone.0218818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga CP, O’Brien J, 2017. Circadian and light-adaptive control of electrical synaptic plasticity in the vertebrate retina. In Jing J (Ed.), Network Functions and Plasticity: Perspectives from Studying Electrical Coupling in Microcircuits, Elsevier, Cambridge, UK, pp. 209–241. 10.1016/B978-0-12-803471-2.00010-2. [DOI] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC, 2002. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J. Physiol 544, 801–816. 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC, 2004. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J. Physiol 554, 467–482. 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijo-Ferreira F, Takahashi JS, 2019. Genomics of circadian rhythms in health and disease. Genome Med. 11, 82. 10.1186/s13073-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, 2002. Cell death in retinitis pigmentosa: gap junctions and the “bystander” effect. Exp. Eye Res 74, 327–336. 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, 1998. The First Step in Seeing. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Rodriguez IR, Mazuruk K, Schoen TJ, Chader GJ, 1995. Structural analysis of the human hydroxyindole-O-methyltransferase gene. Presense of two distinct promotors. J. Biol. Rhythms 50, 31969–31977. [PubMed] [Google Scholar]
- Rohleder N, Langer C, Maus C, Spiwoks-Becker I, Emser A, Engel L, Spessert R, 2006. Influence of photoperiodic history on clock genes and the circadian pacemaker in the rat retina. Eur. J. Neurosci 23, 105–111. 10.1111/j.1460-9568.2005.04528.x. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Raibert M, Terman JS, Terman M, 1979. Circadian rhythm of luminance detectability in the rat. Physiol. Behav 23, 17–21. 10.1016/0031-9384(79)90115-X. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, McMahon DG, 2008. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 6, e249. 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan G-X, Gamble KL, Risner ML, Young LA, McMahon DG, 2012. Divergent Roles of Clock Genes in Retinal and Suprachiasmatic Nucleus Circadian Oscillators. PLoS ONE 7, e38985. 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG, 2006. Circadian organization of the mammalian retina. Proc. Natl. Acad. Sci. USA 103, 9703–9708. 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufiange M, Dumont M, Lachapelle P, 2002. Correlating retinal function with melatonin secretion in subjects with an early or late circadian phase. Invest. Ophthalmol. Vis. Sci 43, 2491–2499. [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC, 2012. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc. Natl. Acad. Sci USA 109, 8145–8148. 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J, Reick M, McKnight SL, 2002. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem 71, 307–331. 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- Sahel JA, Mohand-Said S, Leveillard T, Hicks D, Picaud S, Dreyfus H, 2001. Rod/cone interdependence: implications for therapy of photoreceptor cell diseases. Prog. Brain Res 131, 649–661. 10.1016/S0079-6123(01)31051-8. [DOI] [PubMed] [Google Scholar]
- Sakai HM, Naka K-I, 1988. Neuron network in catfish retina: 1968–1987. Prog. Retin. Res 7, 149–208. [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G, 2005. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur. J. Neurosci 22, 3129–3136. 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G, 2006. Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol. Vis 12, 117–124. [PubMed] [Google Scholar]
- Sakamoto K, Ishida N, 1998a. Circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci. Lett 245, 113–116. 10.1016/S0304-3940(98)00189-X. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Ishida N, 1998b. Molecular cloning of serotonin N-acetyltransferase gene from the mouse and its daily expression in the retina. Neurosci Lett. 250, 181–184. 10.1016/s0304-3940(98)00462-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G, 2004a. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J. Neurosci 24, 9693–9697. 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G, 2004b. Circadian rhythms in the retina of rats with photoreceptor degeneration. J. Neurochem 90, 1019–1024. 10.1111/j.1471-4159.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Oishi K, Ishida N, 2002. Ontogeny of circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci. Lett 317, 53–55. 10.1016/S0304-3940(01)02407-7. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Oishi K, Shiraishi M, Hamano S, Otsuka H, Miyake Y, Ishida N, 2000. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport 11, 3995–3997. 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- Sandu C, Hicks D, Felder-Schmittbuhl M-P, 2011. Rat photoreceptor circadian oscillator strongly relies on lighting conditions: Molecular clock in rat photoreceptors. Eur. J. Neurosci 34, 507–516. 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- Sawant OB, Horton AM, Zucaro OF, Chan R, Bonilha VL, Samuels IS, Rao S, 2017. The circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep. 21, 692–706. 10.1016/j.celrep.2017.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant OB, Jidigam VK, Fuller RD, Zucaro OF, Kpegba C, Yu M, Peachey NS, Rao S, 2019. The circadian clock gene Bmal1 is required to control the timing of retinal neurogenesis and lamination of Müller glia in the mouse retina. FASEB J. 33, 8745–8758. 10.1096/fj.201801832RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Rohrer B, Lemmer T, Zrenner E, 1991. Diurnal control of rod function in the chicken. Vis. Neurosci 6, 641–653. 10.1017/s0952523800002637. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Do MTH, Dacey D, Lucas R, Hattar S, Matynia A, 2011. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: From form to function. J. Neurosci 31, 16094–16101. 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B, Ehrlich DJ, Stein R, Flaum M, Mangel S (1978) Changes in the apparent lengths of lines as a function of degree of retinal eccentricity. Perception 7: 215–223. doi: 10.1068/p070215. [DOI] [PubMed] [Google Scholar]
- Schneider GE, 1969. Two visual systems. Science 163, 895–902. [DOI] [PubMed] [Google Scholar]
- Schneider K, Tippmann S, Spiwoks-Becker I, Holthues H, Wolloscheck T, Spatkowski G, Engel L, Frederiksen U, Spessert R, 2010. Unique clockwork in photoreceptor of rat: Clock of mammalian photoreceptor. J. Neurochem 115, 585–594. 10.1111/j.1471-4159.2010.06953.x. [DOI] [PubMed] [Google Scholar]
- Schorderet M, Nowak JZ, 1990. Retinal dopamine D1 and D2 receptors: characterisation by binding or pharmacological studies and physiological functions. Cell Mol. Neurobiol 10, 303–325. 10.1007/bf00711177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzara SA, 1967. The visual pigments of fresh water fishes. Vis. Res 7, 121–148. 10.1016/0042-6989(67)90079-X. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Gainer H, 1977. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science 197, 1089–1091. 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA, 2009. Adenosine receptors and the central nervous system. Handb. Exp. Pharmacol 193, 471–534. 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Semo M, Lupi D, Peirson SN, Butler JN, Foster RG, 2003. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur. J. Neurosci 18, 3007–3017. 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM, Tosini G, 2011. Localization of Melatonin Receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS ONE 6, e24483. 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, 2005. Retinal development: second sight comes first. Curr. Biol 15, R556–R559. 10.1016/j.cub.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Shaw AP, Collazo CR, Easterling K, Young CD, Karwoski CJ, 1993. Circadian rhythm in the visual system of the lizard Anolis carolinensis. J. Biol. Rhythms 8, 107–124. 10.1177/074873049300800202. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr., Reppert SM, 1997. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269. 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Sheng W, Weng S, Li F, Zhang Y, He Q, Sheng W, Fu Y, Yan H, Liu K, 2021. Immunohistological localization of Mel1a melatonin receptor in pigeon retina Nat Sci Sleep. 5;13:113–121. doi: 10.2147/NSS.S290757. eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Freed M, Sterling P, 1986. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod/cone network. J. Neurosci 6, 3505–3517. 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C, 2003. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev 55, 325–395. 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN Jr., Arshavsky VY, 2002. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron 34, 95–106. 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Solessio E, Scheraga D, Engbretson GA, Knox BE, Barlow RB, 2004. Circadian modulation of temporal properties of the rod pathway in larval Xenopus. J. Neurophysiol 92, 2672–2684. 10.1152/jn.00344.2004. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L, 2004. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur. J. Neurosci 19, 1559–1571. 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Maus C, tom Dieck S, Fejtová A, Engel L, Wolloscheck T, Wolfrum U, Vollrath L, Spessert R, 2008. Active zone proteins are dynamically associated with synaptic ribbons in rat pinealocytes. Cell Tissue Res. 333, 185–195. 10.1007/s00441-008-0627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan MV, Laughlin SB, Dubs A, 1982. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond. B Biol. Sci 216, 427–459. [DOI] [PubMed] [Google Scholar]
- Steele CT, Tosini G, Siopes T, Underwood H, 2006. Time keeping by the quail's eye: circadian regulation of melatonin production. Gen. Comp. Endocrinol 145, 232–236. 10.1016/j.ygcen.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Steenhard BM, Besharse JC, 2000. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J. Neurosci 20, 8572–8577. 10.1523/JNEUROSCI.20-23-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO, 1975. Colour-specific interconnections of cones and horizontal cells in the retina of the goldfish. J. Comp. Neurol 159, 473–502. 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Stella SL Jr., Bryson EJ, Cadetti L, Thoreson WB, 2003. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J. Neurophysiol 90, 165–174. 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- Stella SL Jr., Bryson EJ, Thoreson WB, 2002. A2 adenosine receptors inhibit calcium influx through L-type calcium channels in rod photoreceptors of the salamander retina. J. Neurophysiol 87, 351–360. 10.1152/jn.00010.2001. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl.Acad. Sci. USA 69, 1583–1586. 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Demb JB, 2004. Retina. In: Shepherd GM (Ed.), The Synaptic Organization of the Brain, 5th Edition. Oxford University Press, New York, pp. 217–269. [Google Scholar]
- Storch K-F, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ, 2002. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83. 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ, 2007. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130, 730–741. 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker AJ, Stella N, Piomelli D, Mackie K, Karten HJ, Maguire. G., 1999. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and function of an endogenous signaling system. Proc. Natl. Acad. Sci. U.S.A 96, 14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, Petrasch-Parwez E, Zoidl G, Napirei M, Meier C, Eysel UT, Dermietzel R, 2005. Loss of connexin36 increases retinal cell vulnerability to secondary cell loss. Eur. J. Neurosci 22, 605–616. 10.1111/j.1460-9568.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- Struik ML, Yazulla S, Kamermans M, 2006. Cannabinoid agonist WIN 55212-2 speeds up the cone response to light offset in goldfish retina. Vis. Neurosci 23, 285–293. [DOI] [PubMed] [Google Scholar]
- Sulli G, Lam MTY, Panda S, 2019. Interplay between circadian clock and cancer: New frontiers for cancer treatment. Trends Cancer 5, 475–494. 10.1016/j.trecan.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZC, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC, 1997. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90, 1003–1011. 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, 2017. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18, 164–179. 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Turek FW, Moore RY, 2001. Handbook of Behavioural Neurobiology. Vol. 12. Circadian Clocks. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL, 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet 9, 764–775. 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Nagamine Y, Miyake S, Matsubara C, Taguchi K, Takekida S, Sakakida Y, Nishikawa K, Kishimoto T, Niwa S, Okumura K, Okamura H, 1999. A mammalian ortholog of Drosophila timeless, highly expressed in SCN and retina, forms a complex with mPER1. Genes Cells 4, 67–75. 10.1046/j.1365-2443.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- Teirstein PS, Goldman AI, O’Brien PJ, 1980. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest. Ophthalmol. Vis. Sci 19, 1268–1273. [PubMed] [Google Scholar]
- Terman JS, Remé CE, Terman M, 1993. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 605, 256.264. 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman J, 1985. A circadian pacemaker for visual sensitivity? Ann. N. Y. Acad. Sci 453, 147–161. 10.1111/j.1749-6632.1985.tb11807.x [DOI] [PubMed] [Google Scholar]
- Thomas KB, Iuvone PM, 1991. Circadian rhythm of tryptophan hydroxylase activity in chicken retina. Cell. Mol. Neurobiol 11, 511–527. 10.1007/bf00734813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM, 1993. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 601, 303–307. 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Rickman CB, Shaw SJ, Ebright JN, Kelly U, Sancar A, 2003. Rickman DW.Expression of the blue-light receptor cryptochrome in the human retina. Invest. Ophthalmol. Vis. Sci 44, 4515–4521. 10.1167/iovs.03-0303. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC, 2012. Lateral interactions in the outer retina. Progress in Retinal and Eye Research, 31, 407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikidji-Hamburyan A, Reinhard K, Seitter H, Hovhannisyan A, Procyk CA, Allen AE, Schenk M, Lucas RJ, Münch TA., 2015. Retinal output changes qualitatively with every change in ambient illuminance. Nat Neurosci. 18, 66–74. doi: 10.1038/nn.3891. Epub 2014 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller GL, Nagy E, Horvath RA, Klausz B, Rekasi Z, 2006. Circadian expression of Bmal1 and serotonin-N-acetyltransferase mRNAs in chicken retina cells and pinealocytes in vivo and in vitro. J. Mol. Neurosci 28, 143–150. 10.1385/JMN:28:2:143. [DOI] [PubMed] [Google Scholar]
- Tosini G, Baba K, Hwang CK, Iuvone PM, 2012. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res 103, 82–89. 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O, 2007a. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 21, 3866–3871. 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Dirden JC, 2000. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci. Lett 286, 119–122. 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Tosini G, Doyle S, Menaker M, 1998. Melatonin synthesis in the rat retina: cellular localization and circadian regulation. Invest. Ophthalmol. Vis. Sci 39, S236. [Google Scholar]
- Tosini G, Kasamatsu M, Sakamoto K, 2007b. Clock gene expression in the rat retina: effects of lighting conditions and photoreceptor degeneration. Brain Res. 1159, 134–140. 10.1016/j.brainres.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M, 1996. Circadian rhythms in cultured mammalian retina. Science 272, 419–421. 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M, 1998a. Multioscillatory circadian organization in a vertebrate, iguana iguana. J. Neurosci 18, 1105–1114. 10.1523/JNEUROSCI.18-03-01105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M, 1998b. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 789, 221–228. 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M, 1998c. Temperature compensation of retinal circadian oscillators in wild-type and tau mutant hamsters. Neuroreport 9, 1001–1005. 10.1097/00001756-199804200-00009. [DOI] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM, 2008. The circadian clock system in the mammalian retina. Bioassays. 30, 624–633. 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy JB, Shou T, 2002. The receptive fields of cat retinal ganglion cells in physiological and pathological states: where we are after half a century of research. Prog. Retin. Eye Res 21, 263–302. [DOI] [PubMed] [Google Scholar]
- Umino O, Dowling JE, 1991. Dopamine release from interplexiform cells in the retina: effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J. Neurosci 11, 3034–3046. 10.1523/JNEUROSCI.11-10-03034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H, Barrett RK, Siopes T, 1990. The quail’s eye: a biological clock. J. Biol. Rhythms 5, 257–265. 10.1177/074873049000500307. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Alonso-Gomez AL, Iuvone PM, 1999. Diurnal rhythms of tryptophan hydroxylase activity in Xenopus laevis retina: opposing phases in photoreceptors and inner retinal neurons. Neuroreport 10, 2131–2135. 10.1097/00001756-199907130-00025. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Alonso-Gomez AL, Iuvone PM, 2000. Regulation of tryptophan hydroxylase activity in Xenopus laevis photoreceptor cells by cyclic AMP. J. Neurochem 74, 1961–1967. 10.1046/j.1471-4159.2000.0741961.x. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E, 2000. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev 24, 125–132. 10.1016/s0149-7634(99)00063-9 [DOI] [PubMed] [Google Scholar]
- Vancura P, Csicsely E, Leiser A, Iuvone PM, Spessert R, 2018. Rhythmic regulation of photoreceptor and RPE genes important for vision and genetically associated with severe retinal diseases. Invest. Ophthalmol. Vis. Sci 59, 3789. 10.1167/iovs.18-24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancura P, Wolloscheck T, Baba K, Tosini G, Iuvone PM, Spessert R, 2016. Circadian and dopaminergic regulation of fatty acid oxidation pathway genes in retina and photoreceptor cells. PLoS ONE 11, e0164665. 10.1371/journal.pone.0164665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder RN, 2003. Making (a) sense of non-visual ocular photoreception. Trends Neurosci. 26, 458–461. 10.1016/s0166-2236(03)00211-x. [DOI] [PubMed] [Google Scholar]
- Vanecek J, 1998. Cellular mechanisms of melatonin action. Physiol. Rev 78, 687–721. 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, Ishida AT, 2001. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J. Neurosci 21, 8624–8635. 10.1523/jneurosci.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine G, Vallone D, Gothilf Y, Foulkes NS, 2011. It’s time to swim! Zebrafish and the circadian clock. FEBS Letters 585, 1485–1494. 10.1016/j.febslet.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT, 2002. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem. Photobiol 75, 547–553. 10.1562/0031-8655(2002)0750547EFACRO2.0.CO2. [DOI] [PubMed] [Google Scholar]
- Velarde E, Haque R, Iuvone PM, Azpeleta C, Alonso-Gómez AL, Delgado MJ, 2009. Circadian clock genes of goldfish, Carassius auratus: cDNA cloning and rhythmic expression of period and cryptochrome transcripts in retina, liver, and gut. J. Biol. Rhythms 24, 104–113. 10.1177/0748730408329901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S, 2005. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J. Neurosci 25, 4108–4117. 10.1523/jneurosci.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar MJ, Vitale ML, Parisi MN, 1987. Dorsal raphe serotoninergic projection to the retina. A combined peroxidase tracing neurochemical / high performance liquid chromatography study in the rat. Neuroscience 22, 681–686. 10.1016/0306-4522(87)90364-2. [DOI] [PubMed] [Google Scholar]
- Vivien-Roels B, Malan A, Rettori M-C, Delagrange P, Jeanniot J-P, Pévet P, 1998. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J. Biol. Rhythms 13, 403–409. 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Spiwoks-Becker I, 1996. Plasticity of ribbon synapses. Microsc. Res. Tech 35, 472–487. . [DOI] [PubMed] [Google Scholar]
- von Schantz M, Lucas RJ, Foster RG, 1999. Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res. Mol. Brain Res 72, 108–114. 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- Wagner H-J, Ali MA, 1977. Cone synaptic ribbons and retinomotor changes in the brook trout, Salvelinus fontinalis (Salmonidae, Teleostei), under various experimental conditions. Can. J. Zool 55, 1684–1691. 10.1139/z77-217. [DOI] [PubMed] [Google Scholar]
- Wagner H-J, Behrens UD, Zaunreiter M, Douglas RH, 1992. The circadian component of spinule dynamics in teleost horizontal cells is dependent on the dopaminergic system. Vis. Neurosci 9, 345–351. 10.1017/S0952523800010750. [DOI] [PubMed] [Google Scholar]
- Wagner H-J, Djamgoz MB, 1993. Spinules: a case for retinal synaptic plasticity. Trends Neurosci. 16, 201–206. 10.1016/0166-2236(93)90155-F. [DOI] [PubMed] [Google Scholar]
- Wang Y, Harsanyi K, Mangel SC, 1997. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. J. Neurophysiol 78, 439–449. 10.1152/jn.1997.78.1.439.. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC, 1996. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc. Natl. Acad. Sci. USA 93, 4655–4660. 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Osterbur DL, Megaw PL, Tosini G, Fukuhara C, Green CB, Besharse JC, 2001. Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev. Biol 1, 9. 10.1186/1471-213x-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Baldridge WH, Mangel SC, Dowling JE, 1997. Modulation of endogenous dopamine release in the fish retina by light and prolonged darkness. Visual Neurosci. 14, 351–356. [DOI] [PubMed] [Google Scholar]
- Weiler R, Kolbinger W, Kohler K, 1989. Reduced light responsiveness of the cone pathway during prolonged darkness does not result from an increase of dopaminergic activity in the fish retina. Neurosci. Lett 99, 214–218. 10.1016/0304-3940(89)90292-9. [DOI] [PubMed] [Google Scholar]
- Welsh JH, Osborne CM, 1937. Diurnal changes in the retina of catfish. J. Comp. Neurol 66, 349–359. 10.1002/cne.900660206. [DOI] [Google Scholar]
- Weng S, Wong KY, Berson DM, 2009. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. J. Biol. Rhythms 24, 391–402. 10.1177/0748730409343767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechmann AF, 2003. Differential distribution of Mel(1a) and Mel(1c) melatonin receptors in Xenopus laevis retina. Exp. Eye Res 76, 99–106. 10.1016/s0014-4835(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O’Steen WK, 1992. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest. Ophthalmol. Vis. Sci 33, 1894–1902. [PubMed] [Google Scholar]
- Wiechmann AF, Smith AR, 2001. Melatonin receptor RNA is expressed in photoreceptors and displays a diurnal rhythm in Xenopus retina. Brain Res. Mol. Brain Res 91, 104–111. 10.1016/s0169-328x(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA, 2008. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res 27, 137–160. 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Udin SB, Summers Rada JA, 2004. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp. Eye Res 79, 585–594. 10.1016/j.exer.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe R, Hu Y, 2003. Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest. Ophthalmol. Vis. Sci 44, 4522–4531. 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Yang X-L, Wu SM, Hollyfield JG, 1988. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 453, 377–380. 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Wienbar S, Schwartz GW. 2017. The dynamic receptive fields of retinal ganglion cells. Prog Retin Eye Res. 67, 102–117. doi: 10.1016/j.preteyeres.2018.06.003. Epub 2018 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, 1981. Glycolytic and oxidative metabolism in relation to retinal function. J. Gen. Physiol 77, 667–692. 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, DaPrada M, Reme C, 1984. Circadian rhythm in rat retinal dopamine. Neurosci. Lett 45, 21–25. 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, 2004. Dopamine and retinal function. Doc. Ophthalmol 108, 17–40. 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dearry A, 1991. Functional roles of dopamine in the vertebrate retina. Prog. Retin. Res 11, 247–292. 10.1016/0278-4327(91)90031-V. [DOI] [Google Scholar]
- Witkovsky P, Gallin E, Hollyfield JG, Ripps H, Bridges CD, 1976. Photoreceptor thresholds and visual pigment levels in normal and vitamin A-deprived Xenopus tadpoles. J. Neurophysiol 39, 1272–1287. 10.1152/jn.1976.39.6.1272. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Nicholson C, Rice ME, Bohmaker K, Meller E, 1993. Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc. Natl. Acad. Sci. USA 90, 5667–5671. 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Schutte M, 1991. The organization of dopaminergic neurons in vertebrate retinas. Vis. Neurosci. 7, 113–124. 10.1017/S0952523800010981. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Shakib M, Ripps H, 1974. Interreceptoral junctions in the teleost retina. Invest. Ophthalmol 13, 996–1009. [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R, 2003. Cellular location and circadian rhythm of expression of the biological clock gene period 1 in the mouse retina. J. Neurosci 23, 7670–7676. 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JCY, Smyllie NJ, Banks GT, Pothecary CA, Barnard AR, Maywood ES, Jagannath A, Hughes S, Horst GTJ, MacLaren RE, Hankins MW, Hastings MH, Nolan PM, Foster RG, Peirson SN, 2018. Differential roles for cryptochromes in the mammalian retinal clock. FASEB J. 32, 4302–4314. 10.1096/fj.201701165RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QW, McGoogan JM, Cassone VM, 2000. Circadian regulation of visually evoked potentials in the domestic pigeon, Columba livia. J. Biol. Rhythms 15, 317–328. 10.1177/074873000129001422. [DOI] [PubMed] [Google Scholar]
- Wulle I, Kirsch M, Wagner HJ, 1990. Cyclic changes in dopamine and DOPAC content, and tyrosine hydroxylase activity in the retina of a cichlid fish. Brain Res. 515, 163–167. 10.1016/0006-8993(90)90591-x. [DOI] [PubMed] [Google Scholar]
- Xu H, Jin N, Chuang JZ, Zhang Z, Zhong X, Zhang Z, Sung CH, Ribelayga CP, Fu Y, 2022. Visual pigment-deficient cones survive and mediate visual signaling despite the lack of outer segments. Proc. Natl. Acad. Sci. U.S.A 119, e2115138119. 10.1073/pnas.2115138119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ruan G, Dai H, Liu AC, Penn J, McMahon DG, 2016. Mammalian retinal Müller cells have circadian clock function. Mol. Vis 22, 275–283. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Okano T, Fukada Y, 2001. Chicken pineal Cry genes: light-dependent up-regulation of cCry1 and cCry2 transcripts. Neurosci. Lett 313, 13–16. 10.1016/S0304-3940(01)02227-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH, 1992. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp. Eye Res 54, 685–697. 10.1016/0014-4835(92)90023-L. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Goto M, Menaker M, 1999. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J. Biol. Rhythms 14, 197–201. 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- Yan W, Laboulaye MA, Tran NM, Whitney IE, Benhar I, Sanes JR, 2020. Molecular identification of sixty-three amacrine cell types completes a mouse retinal cell atlas (preprint). bioRxiv. 10.1101/2020.03.10.985770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wu SM, 1989. Modulation of rod/cone coupling by light. Science 244, 352–354. 10.1126/science.2711185. [DOI] [PubMed] [Google Scholar]
- Yazulla S, 2008).Endocannabinoids in the retina: from marijuana to neuroprotection. Prog. Retin. Eye Res 27, 501–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, McIntosh HH, Fan SF, 2000. Cannabinoid receptors on goldfish bipolar cells: Electron-microscopic immunocytochemistry and whole-cell recording. Visual Neurosci. 17, 391–401. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J, 1993. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron 10, 1049–1054. 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S, 2000. Molecular analysis of avian circadian clock genes. Brain Res. Mol. Brain Res 78, 207–215. 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Young RW, 1967. The renewal of photoreceptor cell outer segments. J. Cell. Biol 33, 61–72. 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, 1978. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest.Ophthalmol. Vis. Sci 17, 105–116. [PubMed] [Google Scholar]
- Yu C-J, Gao Y, Li P, Li L, 2007. Synchronizing multiphasic circadian rhythms of rhodopsin promoter expression in rod photoreceptor cells. J. Exp. Biol 210, 676–684. 10.1242/jeb.02694. [DOI] [PubMed] [Google Scholar]
- Yu HS, Pang SF, Tang PL, 1981. Increase in the level of retinal melatonin and persistence of its diurnal rhythm in rats after pinealectomy. J. Endocrinol 91, 477–481. 10.1677/joe.0.0910477. [DOI] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P, 2006. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl. Acad. Sci. USA 103, 6386–6391. 10.1073/pnas.0510691103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang J, Gesemann M, Keim J, Samardzija M, Grimm C, Neuhauss SC., 2021. Circadian regulation of vertebrate cone photoreceptor function. Elife. 22;10:e68903. doi: 10.7554/eLife.68903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaunreiter M, Brandstatter R, Goldschmid A, 1998a. Evidence for an endogenous clock in the retina of the rainbow trout: I. Retinomotor movements, dopamine and melatonin. Neuroreport 9, 1205–1209. 10.1097/00001756-199804200-00045. [DOI] [PubMed] [Google Scholar]
- Zaunreiter M, Brandstatter R, Goldschmid A, 1998b. Evidence for an endogenous clock in the retina of the rainbow trout: II. Circadian rhythmicity of serotonin metabolism. Neuroreport 9, 1475–1479. 10.1097/00001756-199805110-00042. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, 1994. Stimulation of D4-like dopamine receptor suppresses serotonin N-acetyltransferase activity but does not phase-shift the circadian oscillator in chick retina. Neurosci. Lett 179, 107–110. 10.1016/0304-3940(94)90946-6. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Bednarek A, Berezinska M, Nowak JZ, 2003c. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J. Neurochem 84, 717–724. 10.1046/j.1471-4159.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Berezinska M, Rosiak J, Vivien-Roels B, Nowak JZ, 2003a. The relationship between melatonin and dopamine rhythms in the duck retina. Neurosci. Lett 347, 37–40. 10.1016/s0304-3940(03)00643-8. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Berezinska M, Rosiak J, Vivien-Roels B, Skene D, Pevet P, Nowak JZ, 2003b. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the goose pineal gland, retina, and plasma. Gen. Comp. Endocrinol 134, 296–302. 10.1016/s0016-6480(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Iuvone PM, 1992. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci. Lett 135, 71–74. 10.1016/0304-3940(92)90138-w. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Lorenc A, Berezinska M, Vivien-Roels B, Pevet P, Skene DJ, 2006. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen. Comp. Endocrinol 145, 162–168. 10.1016/j.ygcen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Nowak JZ, 1993. Evidence for a D4 dopamine receptor decreasing serotonin N-acetyltransferase activity in chick retina. Acta Neurobiol. Exp. (Warsz) 53, 473. [PubMed] [Google Scholar]
- Zhang D-Q, Belenky MA, Sollars PJ, Pickard GE, McMahon DG, 2012. Melanopsin mediates retrograde visual signaling in the retina. PLoS ONE 7, e42647. 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-Q, Ribelayga C, Mangel SC, McMahon DG, 2002. Suppression by zinc of AMPA receptor mediated synaptic transmission in the retina. J. Neurophysiol, 88, 1245–1251. doi: 10.1152/jn.2002.88.3.1245. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG, 2008. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci. U.S.A 105, 14181–14186. 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou T, Ruan GX, McMahon DG, 2005. Circadian rhythm of Period1 clock gene expression in NOS amacrine cells of the mouse retina. Brain Res. 1050, 101–109. 10.1016/j.brainres.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH, 2006. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol. Vis 12, 937–948. [PubMed] [Google Scholar]
- Zhang M, Cao LH, Yang XL, 2007. Melatonin modulates glycine currents of retinal ganglion cells in rat. Neuroreport 18, 1675–1678. 10.1097/WNR.0b013e3282f0b5a2. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB, 2014. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 111, 16219–16224. 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lyuboslavsky P, Dixon JA, Chrenek MA, Sellers JT, Hamm JM, Ribelayga CP, Zhang Z, Le YZ, Iuvone PM, 2020. Effects of cone connexin-36 disruption on light adaptation and circadian regulation of the photopic ERG. Invest. Ophthalmol. Vis. Sci 61, 24. 10.1167/iovs.61.6.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Coleman JE, Fuchs GE, Semple-Rowland SL, 2003. Circadian oscillator function in embryonic retina and retinal explant cultures. Brain Res. Mol. Brain Res 114, 9–19. 10.1016/s0169-328x(03)00122-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li H, Liu X, O’Brien J, Ribelayga CP, 2015. Circadian clock control of connexin36 phosphorylation in retinal photoreceptors of the CBA/CaJ mouse strain. Vis. Neurosci 32, E009. 10.1017/S0952523815000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Silveyra E, Jin N, Ribelayga CP, 2018. A congenic line of the C57BL/6J mouse strain that is proficient in melatonin synthesis. J. Pineal Res 65, e12509. 10.1111/jpi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Chen L, Chen X, He X, Yang J, Shi Y, Zhou N, 2013. The second intracellular loop of the human cannabinoid CB2 receptor governs G protein coupling in coordination with the carboxyl terminal domain. PLoS One 8, e63262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Green CB, 2001. Three cryptochromes are rhythmically expressed in Xenopus laevis retinal photoreceptors. Mol. Vis 7, 210–215. [PubMed] [Google Scholar]
- Zhu H, LaRue S, Whiteley A, Steeves TD, Takahashi JS, Green CB, 2000. The Xenopus clock gene is constitutively expressed in retinal photoreceptors. Brain Res. Mol. Brain Res 75, 303–338. 10.1016/s0169-328x(99)00309-5. [DOI] [PubMed] [Google Scholar]
- Zhuang M, Wang Y, Steenhard BM, Besharse JC, 2000. Differential regulation of two period genes in the Xenopus eye. Brain Res. Mol. Brain Res 82, 52–64. 10.1016/s0169-328x(00)00177-7. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE, 1987. Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature 330, 166–168. 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Levine JD, Jin X, Weaver DR, Reppert SM, 1998. Molecular analysis of mammalian timeless. Neuron 21, 1115–1122. 10.1016/s0896-6273(00)80628-5. [DOI] [PubMed] [Google Scholar]