Abstract
There is limited research evaluating the diagnosis and treatment of patients with autoimmune gastritis (AIG) and pernicious anemia (PA). We used a 2-phase data collection process to examine the literature and individual patient accounts. Phase one comprised a systematically conducted literature review focusing on diagnosis and treatment, relationships with healthcare practitioners and health-related quality of life (HRQOL). Phase two involved analysis of individual accounts via posts in online patient forums. We identified 6 main themes: the diagnosis journey, seeking treatment, patient-provider relationships, HRQOL, patient disempowerment, and the “expert patient.” Our findings confirm significant knowledge gaps concerning AIG/PA across the healthcare community. These have a cascading effect starting with delays in diagnosis and poor treatment protocols and often lead to complete withdrawal from care seeking. The establishment of standard consensus guidelines and improved clinical awareness should be urgently addressed. Interventions that better help patients understand their illness are also needed to improve psychological health. Without these changes disengagement from health systems, and poor health outcomes, will continue for this population group.
Keywords: pernicious anemia, autoimmune gastritis, autoimmune atrophic gastritis, patient journeys
Introduction
Autoimmune gastritis (AIG) is a chronic inflammatory disease with gastric parietal cell destruction. Its prevalence varies from 0.5% (1,2). to 19.5% depending on the setting (1,3–9). AIG is slowly progressive and usually asymptomatic for years. However, it raises the risk of gastric cancers, reduces acid output and triggers malabsorption of several micronutrients including iron and vitamin B12 (1). Chronic B12 malabsorption results in pernicious anemia (PA) (1,4,10). PA is associated with neurological, hematological, and gastrointestinal manifestations and a broad clinical presentation. Diagnosis is often delayed; over half of AIG/PA patients have another autoimmune disease including thyroiditis and type 1 diabetes (1,11,12).
Research into such delays is limited but a recent study found an average delay of 14 months (13). AIG is frequently overlooked by endoscopists; in Japan only 31.7% of study participants received a confirmatory endoscopic diagnosis compared to 68.3% receiving their diagnosis pathologically (14). Hooper et al's survey of nearly 1200 UK patients found that one-third experienced a 12 month delay and 14% waited over 10 years for a diagnosis (15). They also found inconsistency concerning types of tests conducted and treatment prescribed (15). Less than one-third of respondents were satisfied with their treatment; half stated they received poor medical care (15). Patients with AIG have a decreased health-related quality of life (HRQOL) compared to healthy controls, largely attributable to impaired physical functioning (16).
In response to diagnostic delays and suboptimal treatment many turn to online discussion forums where patients devise their own protocols. Anecdotal evidence of successful self-management suggests a gap between “lived experience” and medical advice. We aimed to identify these gaps by exploring peer-reviewed literature alongside individual accounts of AIG/PA.
Methods
The study comprised 2 phases. Phase one was a systematic review of peer-reviewed literature exploring lived experience of AIG/PA patients focusing on diagnosis and treatment, practitioner relationships and HRQOL. Phase two involved analyzing individual accounts via posts in online patient forums.
Phase One
Search Strategy
Phase one involved systematically searching academic literature using 5 medical databases: PUBMED, EMBASE, MEDLINE, WEB OF SCIENCE, CINAHL. Search terms derived from the literature included PA, megaloblastic anemia, B12 deficiency, autoimmune atrophic gastritis, parietal cell antibodies, guideline, symptom, protocol, treatment, criteria, unmet needs, lived experience, patient journey, case study, prevalence, incidence, and a large array of related terms. Co-citation and bibliographic coupling listings were also identified using connectedpapers.com to identify relevant prior and derivative works.
Inclusion/Exclusion Criteria
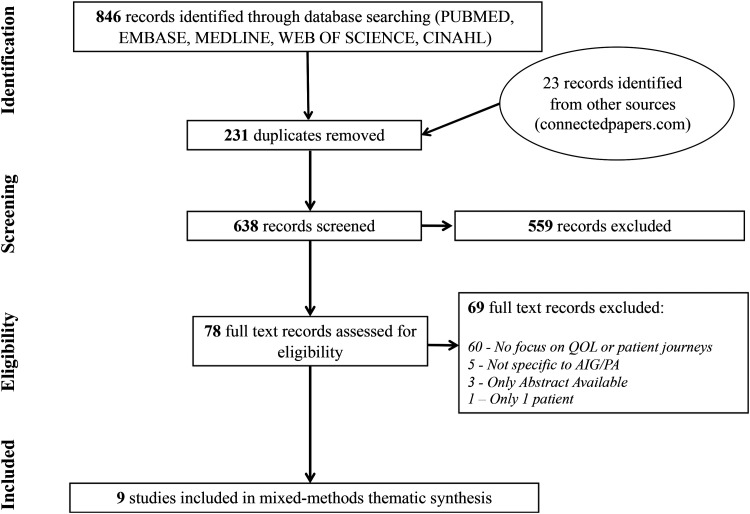
Inclusion criteria were English language studies to August 2021 focusing on the patient journey. All designs including qualitative, quantitative, cross-sectional, and mixed methods research were included. Exclusion criteria were a lack of focus on HRQOL or patient journeys and a lack of focus on AIG/PA. About 869 papers were imported into Covidence, a software package supporting systematic screening and extraction of literature. After removing duplicates, 638 titles and abstracts were screened and 559 excluded (Figure 1). Seventy-eight full text articles were screened, with 69 excluded due to a lack of focus on patient experiences, or availability only in abstract or non-English form. Nine publications remained for inclusion and data extraction. One comprised 4 separate studies (3 quantitative and one qualitative) bringing the number of unique studies for analysis to 12 (Appendix).
Figure 1.
Phase one literature search.
Analysis
The literature heterogeneity suggested a narrative synthesis analytic approach to identify common themes (17). This facilitates the identification of common stories and themes from multiple sources. Data from each study were extracted into a spreadsheet. Key themes included diagnostic delays, suboptimal treatment, and poor doctor-patient relationships. These were coded in line with areas of focus for this study: diagnosis experiences, treatment experiences, relationships with healthcare practitioners, and HRQOL.
Phase Two
This involved systematically searching online AIG/PA patient forums. A search identified active forums using search terms including support OR patient groups (AND) pernicious anaemia, autoimmune gastritis, and associated synonyms. Social platforms with AIG/PA support groups were found on Reddit, Healthunlocked, and Facebook (Table 1). Reddit was excluded; the only thread was comparatively small and relatively inactive. Healthunlocked, the social platform used by the Pernicious Anaemia Society (PAS), contained nearly 27 000 members and almost 20 000 posts. Nineteen English-language groups were identified on Facebook, comprising over 50 000 global members. Groups focusing solely on B12 deficiency were excluded. Three online groups—PAS (HealthUnlocked), Pernicious Anaemia Support (Facebook), and Pernicious Anaemia/B12 Deficiency-Support Group (Facebook) represented the largest number of members. The Autoimmune Atrophic Gastritis & Pernicious Anaemia group on Facebook were also included because of their specific focus on AIG.
Table 1.
Online Patient Forums.
| Group name | Membership | Demographics | Notes |
|---|---|---|---|
| * Pernicious Anaemia Society (private group) | 26 666 | n/a | * Included based on size and engagement |
| * Pernicious Anaemia Support (private group) | 7800 | F:94.2% M5.6%, primary age groups 45-54, 25-44, 25-34 primary countries: United Kingdom, United States, Australia | * Included based on size and engagement |
| * Pernicious Anaemia/B12 Deficiency-Support Group (private group) | 29 300 | F:93% M:7% primary age groups 25-44, 45-54, 25-34 primary countries: United Kingdom, United States, Australia | * Included based on size and engagement |
| * AutoImmune Atrophic Gastritis & Pernicious Anaemia (private group) | 1700 | F:89.5% M:10.1% Other 0.5% | * Included based on engagement and being only group dedicated to AIG |
| primary age groups 45-54, 25-44, 25-34 | |||
| primary countries: United Kingdom, United States, Australia | |||
| 14 other private English language groups, often region specific | 10 889 in total | Demographic data n/a; Region specific groups included United Kingdom, Australia, United States, Missouri and Midwest, Canada, Leicestershire & Midlands | Excluded based on size and/or low engagement |
| r/PerniciousAnaemia | 356 | n/a | Excluded based on size and/or low engagement |
| 76 711 |
*Groups included for data extraction.
Data Extraction and Analysis
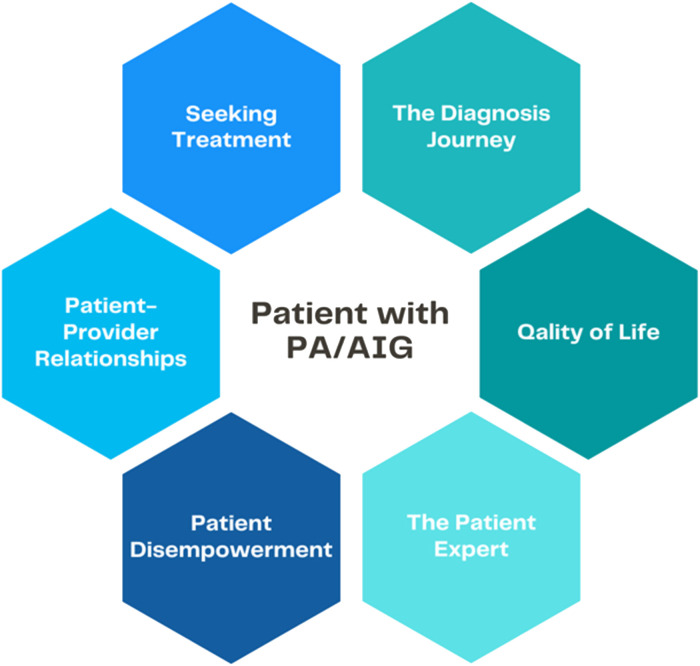
Over 3 dates in September 2021 (6th, 11th, and 20th) the most recent 50 forum posts were extracted to a spreadsheet. Each was treated as a unit of data. Using Braun and Clarke's (18) thematic analysis framework, common codes and themes were identified and cross-referenced with literature. Analysis began with reading and coding each post independently. Taking a deductive approach, we looked specifically at data related to focus areas for this study—diagnosis, treatment, healthcare professional relationships, and HRQOL. Subsequent readings used an inductive approach to identify additional codes not previously considered. All codes were then summarized into 6 over-arching themes: the diagnosis journey, seeking treatment, patient-provider relationships, quality of life, patient disempowerment, and the expert patient (Figure 2).
Figure 2.
Themes identified across the literature and patient forums.
Results
Phase One
Of the 12 studies, 9 originated in the United Kingdom, 2 in Italy and 1 in Germany. Seven were cross-sectional and 2 were single-center evaluations. Median sample size was 198 (range: 102-889). The 3 qualitative studies used semi-structured interviews and patient workshops involving 7 to 12 participants.
The cross-sectional studies were online surveys, with recruitment via advocacy groups like the PAS in the United Kingdom, B12 deficiency/PA information websites and online patient forums. The single-center evaluations were from a UK doctor's surgery and an Italian gastrointestinal clinic. The qualitative studies all recruited participants through the PAS member databases, university volunteer newsletters, and social media.
Phase Two
The 4 online forums represented a diverse collection of members, ranging from 1700 to nearly 29 300 worldwide participants (Table 1). The Facebook groups shared similar demographics: predominantly female (on average 92%), aged between 25 and 54 and mostly living in the United Kingdom, United States, and Australia. Demographics were unavailable for HealthUnlocked. Engagement was high in all groups; new posts averaged between 4 and 14 daily within individual groups, with up to 200 nested responses.
The 6 themes identified (see above) are considered consecutively, with illustrative forum quotes highlighting key themes (Table 2).
Table 2.
Illustrative Quotes From Online Forums.
| Themes | Forum comments |
|---|---|
| Diagnosis journey | I haven't been right for 3 years, the extreme fatigue started after I had pleurisy and gradually got worse every time I was unwell, usually throat infections etc. Doctor was happy to diagnose me with ME/CFS and Fibro without any further investigation. Very lucky to have found this group, or I would still be lying in bed thinking 'This is it, this is my life now'. I'm really, really angry. I feel sorry for my children more than anything! But bitterness won't help. Just relieved that I am not suffering like I was. |
| I wish I had been tested decades ago. I’m frustrated it went undetected for so long. I had multiple symptoms. Glad to have a proper dx now. It also gives me more self-awareness about my generalized anxiety and knowing now what the root cause is. Having that knowledge is so empowering! | |
| How did you get diagnosed? Still wondering this 3 years down the line! Low B12, high MCV and MCH wasn't enough. Being in bed for weeks on end wasn't either. Neurological symptoms responding to B12 injections was 'just one of those unexplainable things’ according to my GP. The system is broken. Haematologists are not following protocol. | |
| Seeking treatment | When my former GI advised me I had AIG he still NEVER thought to take me off the PPI. When I went to a 2nd GI he never thought to take me off the PPI either. Again, if AIG attacks your Parietal Cells and PPI inhibits something in the parietal cells, which are involved in the final steps of gastric acid secretion, why continue to prescribe a PPI? I no longer take a PPI. |
| Guys I’m so angry!! Went to the GP's today to get my injection (I have them every 2 months) when I got there the nurse said you need a blood test to see if you need it. I’ve been having them for over 20 years. I said going back I had a Schillings test to determine if I had PA and was told I had it and would need injections for life. Today she said she's look at my papers and I didn’t have it [PA]. So why when I’m due do I feel so rough? I’m so confused at the moment I could cry. | |
| As is the problem with most of medicine, there seems to be a big lag between what the latest data/research shows and what is practiced by the physicians. They are stuck in outdated models and follow old guidelines that lack nuance, preventative actions and don't take into account patient experience. For example, while my doctor told me that proton pump inhibitors (PPIs) are contra-indicated for AIG, he never talked to me about vitamin deficiencies or inhibited acid production. My primary doctor missed the PA completely and told me I need to "exercise more" as I was experiencing muscle aches. It is all very frustrating and sometimes downright dangerous. | |
| Patient-provider relationships | In my experience finding a new GI is really pointless. I’ve seen multiple GI spec for symptoms for years, and it's the consensus I’ve experienced none of them want or care about PA. Or truly want to give individualized care and that would include individual person specific tx. I also have multiple other medical problems and no one works together, same ‘ole song and dance! |
| Never has one doctor shown any signs any concerns over anti-parietal and my internal medicine doctor didn’t know what it was when I said well isn’t that why I have scarring from low stomach acid he said he had never heard of it …. as he booked me for my 2nd scope! Hard to work out if it's actual autoimmune Atrophic Gastritis if doctors don’t know about it. | |
| Quality of life | Being so ill off and on for most of my life had me believing I was crazy and lazy and stupid. Then when my body started failing, I gave up. Treatment was slow. But I have improved a lot. But I still have damage physically and mentally from it. |
| Feeling a little defeated! I’m recently separated and needing to find work. I haven’t worked in 20 years – and have had PA for 3 years. I self-inject every 3 days and feel pretty good, but any sort of stress wipes me out (neurological issues, brain and muscles). Well, I had an interview yesterday and it went well, but I broke down afterwards. There is no way I can commit to being “that up” (energy speaking) for several hours a day! Now, I don’t think I will be able to commit to a job at all! I say that because after I put in all that work into preparing for the interview, mentally, etc…. today I’m basically in bed because of what the stress has done. HOW DO WE EVER WORK? Is there medical compensation for PA? Do we end up on the street because of it?? I’m serious. How do we swing this disease with work? | |
| Feel dreadful and fearful! I’m due to have my 6th loading dose of B12 tomorrow. Yesterday I really thought things were improving and I had a good active and energised day. Today I feel dreadful! Fatigued, lightheaded, unbalanced, nauseous. I can’t believe the difference from yesterday. My quality of life is effected as I’m often in bed. I’ve read things get worse before getting better but when will I get better? What are other people's experiences please? I’m craving positive stories as currently I’m really depressed and anxious. | |
| Patient disempowerment | I'd recently been with my adult son (bedbound and blatantly obviously B12d) to a neurologist, after waiting for more than a year. This neurologist told us there's no such thing as active and inactive B12, it's all active, obviously, because it's all in the bloodstream and reaches the tissues - how could it not be active? I was open-mouthed with disbelief at what he was saying (because I had of course done my homework, with the group's help). But there was no discussion to be had, he had pronounced, and he was the expert. A year's wait and my son was dismissed with no information, no diagnosis, no treatment plan. My son left the building and cried. I can't remember seeing him ever before reduced to tears. |
| I saw [doctor] today and he asked what meds I'm currently on. I told him I have been SI [self-injecting] for past 4 weeks eod [every other day]. He shouted at me and said I should not be doing this. Didn’t matter what I had to say he kept on talking above me. I explained, treating the symptoms, and reducing over time. He just kept saying my levels will be ridiculously high. We ended the appointment with him telling me he doesn't approve of what I'm doing. Now I’m confused if I should continue with this or reduce to once a month. I feel better on the days that I take it, but nowhere near 'normal' whatever that might feel like, as I have forgotten. | |
| Looking for positives. I’m new to AIG and need something positive to focus on. I’ve read enough on cancer to scare me stiff! I have PA, which only affects 0.1% of the population and unfortunately, I managed to have it. I have such a phobia of the “c” word. I know that AIG and PA both raise cancer risk so I’m freaking out. I’m looking for positive reminders to focus on please. Some say that even though there's a cancer risk, it's still a low risk. Is this true? | |
| The patient expert | People with a diagnosis of PA need treatment with B12 injections FOR LIFE! Testing the blood for B12 levels is not required (You will find this mentioned in U.K. guidance.) You will always have a high B12 level with injections, but this doesn’t mean the B12 is getting into the cells. You need injections often enough so that you keep all your symptoms at bay. This can mean anything from daily to once every 3 months. We are all very different - with symptoms and how often we need injections. PA is unfortunately not understood by the medical profession. On this forum we have made our discoveries by trial and error! When first treating PA, patients often feel worse before feeling better. And yes, some get acne. But this will all pass. With the medical ignorance that is involved I had to take my treatment into my own hands and self-inject B12 (weekly in my case.) |
| I know I haven’t posted on here in a while, but I want to express my gratitude. If it weren’t for you all and others in another group, I wouldn’t have learned about AIAG, been brushed off, and not gotten any answers. I can now officially say that I have AIAG. Not sure if any of you remember, but I had gotten a upper endoscopy in 2017, and failed to speak up before I had it completed. The Dr. failed to release my results until a year later and was open to writing a letter stating he thought it might be AIAG. My primary took it into account but didn’t think I would need anything further as I was giving myself B12 shots. This year, I finally got a referral and had another endoscopy (last Wednesday) and spoke at my appointment to both my Dr. and to the assistant Dr. who performed my procedure. They biopsied the right places and confirmed the diagnosis. She wrote a letter saying it might be better to try iv Iron and or B12 injections. I can’t tell you how at peace I am and thankful. So thank you! |
Diagnosis Journey
The diagnosis journey was consistently described as “difficult” in the literature, with significant delays. Hooper et al found that 80% of patients described diagnostic delays of between 1 and 10 years (15). Lenti et al reported a median overall delay of 14 months; 65% of their study participants experienced delays of between 2 and 3 years and 8% more than 10 years (13). Many authors suggested this was due to a lack of awareness, lack of routine screening and inconsistencies across testing protocols and diagnostic criteria (13,15,19,20). Lenti et al found delays were longest for women, suggesting a confounding of menstruation and iron deficiency (13). Other confounding issues included autoimmune comorbidities in many patients (13). Paralleling the literature, forum participants also recounted misdiagnosis and diagnostic delays:
PA for me took almost 15 years to diagnose. I only found out by doing my own research after years of misdiagnosis and being sent to multiple specialists on a wild goose chase, with so many weird symptoms that adversely affected my confidence and career, including my own sanity. I finally requested doctor to run an IF antibodies test. I was thrilled to be positive as B12 shots began and they immediately improved my overall symptoms.
Seeking Treatment
Suboptimal treatment was commonly described. Hooper et al's survey found that 64% of patients reported suboptimal care; 51% describing their care as poor or very poor (15). Across the literature inconsistent treatment was reported, including poor understanding of symptom severity and neuropsychological distress (13,15,19–23). Little is offered in terms of treatment for gastrointestinal issues; practitioners commonly prescribe proton pump inhibitors (PPIs) which are contra-indicated for AIG (13).
Forum participants often reported clinicians’ reliance on B12 biomarkers, leading to treatment cancellation despite symptom persistence. Current research indicates basic B12 biomarkers should not be considered without other essential markers, including holotranscobalamin, MMA, homocysteine, and patient symptoms (1,24). Frustration concerning the lack of patient-centered care and adherence to outdated guidelines was common (Table 2).
Patient-Provider Relationships
Across the literature and forums, participants expressed dissatisfaction with medical care, poor doctor relationships, and disengagement from healthcare (15,19,20,22,23,25). Many described symptoms that were frequently trivialized, leaving them feeling misunderstood and unheard (19,22). Seage et al reported high levels of stigma and a delegitimization of patient experiences (19). They found a perceived gender bias in patient-provider relationships with females commonly told their symptoms were stress-based, psychosomatic, or hormonal (19). Complaints concerning fragmented care and lack of patient-centered care were also commonly expressed (Table 2). Though “trust” and fragmented services were often identified, not all patients felt unheard:
I have had brilliant treatment from my GP & get annoyed when they are all tarred with the same brush!
Quality of Life
Literature survey responses indicated that most people with AIG/PA experienced low HRQOL and psychological well-being compared with other gastro-intestinal diseases such as celiac disease (13,16). HRQOL results were poor across domains of physical functioning, bodily pain, general health, vitality, social functioning, emotional and mental health (16). Stigmatization was commonly described in the literature and individual forum stories:
Being so ill off and on for most of my life had me believing I was crazy and lazy and stupid. Then when my body started failing, I gave up. Treatment was slow. I have improved a lot, but I still have damage physically and mentally from it.
Semedo found that a better understanding of AIG/PA positively enhances people's HRQOL (20). Cognitive deficits and psychological disturbances due to B12 deficiency were a common source of distress across the literature and forums (20,22,23). Semedo also found concerns around managing work due to physical and mental fatigue from stress and ill-health (20). Mirroring this, one forum participant explained that just preparing for and completing an employment interview left her feeling depleted and concerned for her future (Table 2).
Stigma was perceived across the main quality of life domains of health, work, and family (22). High anxiety levels were also common as people tried to deal with their condition and expressed concerns about their prognosis (20).
Patient Disempowerment
Patient forums provided additional data not identified in the literature. Lack of awareness in healthcare about AIG/PA had a cascading effect for patients who, like their doctors, did not fully understand their condition, its associated complications and its management. Across the forums, misinformation was commonly discussed. Patients often failed to realize how broad disease symptoms could be, or alternatively assumed that all ill health was caused by AIG/PA. Poor patient-provider relationships and HRQOL compounded fear, uncertainty, and disengagement from healthcare. One forum participant described feeling dismissed by a clinician who disregarded their understanding of B12.
Forum participants described benefits from self-administering B12 in response to symptom recurrence, but also reported a lack of approval and support from healthcare providers, creating self-doubt and concern.
Poor insight into their condition resulted in many forum members expressing concern for their long-term prognosis, particularly around the association between AIG and cancer (20). A poor understanding of micronutrient deficiency was also widespread. People living with AIG/PA commonly perceived stigma around the practitioner-patient relationship with the perception that practitioners may lose patience when patients constantly present with new symptoms (19,22).
The Expert Patient
Another novel theme identified via patient forums was the emergence of the “expert patient.” Widespread suboptimal AIG/PA treatment leads to the emergence of individuals who achieve high levels of self-efficacy and who invest significant time and energy into researching their condition. These forum participants frequently become community leaders, providing advice and encouragement to other members.
Some engage in long research discussions, offering theories and advising others of treatment based on anecdotes. Given the widespread lack of knowledge in general, these patient experts often become key forum players, with patients consulting them for treatment advice rather than arranging medical appointments (Table 2).
Patient experts commonly encourage others to self-educate and instruct their healthcare practitioners. Others offer informative commentary to fill information gaps. Newer members often expressed gratitude to patient experts for empowering them:
…. after several attempts I just go to the experts now – you!
Discussion
Our study identified 6 themes concerning diagnosing and treating AIG/PA. Significant knowledge gaps exist. These have a cascading effect starting with delayed diagnosis and poor treatment which impact quality of life, lead to stigmatization, poor patient/doctor relationships, and often to complete withdrawal from care seeking (19,20,22,23,25).
Routine screening is commonly overlooked (13,15,20). Inconsistent testing protocols and guidelines contribute to delayed diagnosis (1,26–28). For example, there are currently more than 5 sets of UK guidelines on PA, all proffering different advice (29). Chronic B12 deficiency results in significant harm; diagnostic delays can lead to irreversible complications (13,16).
Lenti et al developed an AIG “red-flags” questionnaire incorporating 7 diagnostic features (neurological, gastrointestinal, hematological, cardiological, autoimmune, fertility, and family history) to help avoid such delays (30). They also identified affordable laboratory test scores to confirm AIG including parietal cell antibody, plasma MMA, and homocysteine (1,31). These collective scores, alongside gastric biopsies, are considered essential for diagnosis (30). Importantly, “normal” serum B12 and MMA alone do not exclude B12 malabsorption (27,32). Adopting a red-flags questionnaire would clearly promote early diagnosis.
Suboptimal treatment is common. Issues range from clinicians’ lack of awareness and inconsistent management to frankly inappropriate treatment. For example, the use of PPI's to inhibit acid production in AIG patients with reflux symptoms is common. Another issue concerns treatment based on B12 biomarkers despite evidence that, once PA is diagnosed, these become irrelevant (21,27,33). Continued testing is especially controversial when doctors discontinue treatment based on B12 laboratory ranges. This is a key source of negative practitioner-patient relationships (15,29).
Significant debate surrounds B12 dosage. Kornic found that symptoms were significantly improved with daily/weekly parenteral treatment rather than every 2 to 3 months (21). Hooper suggests there is no firm evidence for 3-monthly injections to treat PA (29). It is unclear why clinicians refuse additional supplementation or discourage self-treatment since B12 has no upper toxicity and few side-effects (15,21,27). The reliance of biomarkers over symptoms to guide treatment indicates a significant unmet need, likely resolvable using a more “patient-centered” approach. Moreover, research is urgently needed to explore individual variance in B12 treatment requirements.
Equally controversial is the debate over oral versus parenteral supplementation. A Cochrane Systematic Review found there is some evidence for oral B12 efficacy, although based on 2 small studies (34). While oral supplementation restores blood abnormalities, other research and forum anecdotal reports suggest it is ineffective for many; parenteral supplementation is recommended for those with neurological symptoms (1,21,27,28). Combined oral and parenteral supplementation often provides optimum symptom relief (21).
Wolffenbuttel et al identified and debunked 7 common myths associated with B12 treatment (27): (1) oral B12 equals or is better than parenteral to alleviate neurological symptoms; (2) once serum B12 is normalized treatment can be stopped; (3) elevated serum B12 requires treatment cessation; (4) more than 5 injections is harmful; (5) serum B12 should be tested after 3 injections to assess whether supplementation is working; (6) treatment should be stopped if symptoms worsen, and (7) treatment must stop during pregnancy. Correcting these myths would address many of the complaints in patient forums, indicating a need for improved education and standard consensus guidelines.
Patient-provider relationships are impacted by inconsistent and inappropriate treatment, gender-bias, perceived stigma, and delegitimization of patient experiences (19,20,22,23,25). Shared decision-making can result in happier patients, with improved health outcomes and quality of life (35,36). Across the literature there were also few mentions of the implications of broader nutrient malabsorption. Deficiencies in folate, iron, vitamin C, calcium, and vitamin D are commonly associated with AIG/PA, leading to various neurological, blood, and skeletal issues that directly impact HRQOL (15,37,38).
At the far end of the AIG/PA disempowerment spectrum sit the patient experts and community leaders. The patient expert is usually a veteran of the AIG/PA journey, who has studied the literature extensively and shares anecdotes and learnings for others’ benefit. Given the widespread lack of awareness of AIG/PA they become important leaders; people consult them for advice rather than seek medical help. Patient experts help newer members with improved health literacy and self-efficacy by pushing them to educate themselves and their doctors about AIG/PA. Using such experts as “consumer engagement consultants” (39) in the development of patient-centered guidelines might provide experiential input often missed by researchers and clinicians.
Limitations
Our literature search was conducted systematically to ensure all relevant publications were included; however, while ad-hoc citation and reference list searches were carried out, a more systematic process of searching may have identified additional studies. Online forums provide a unique insight into the “lived experience” of an international audience of AIG/PA patients; however, the countries represented were all high income, clearly introducing bias. Importantly, there is a tendency for forums to attract participants with biased views (eg, those dissatisfied with their diagnosis and treatment). Despite these limitations, our study provides unique insight into a diverse group of people experiencing a condition that frequently goes undiagnosed and under-treated.
Conclusion
This study is the first to examine empirical evidence on the lived experience of people with AIG/PA and the accounts of those engaging in online AIG/PA patient forums. It provides insight into the challenging journey such patients experience. Interventions to prevent diagnostic delays and improve clinical awareness should be urgently addressed through the establishment of standard consensus guidelines and education. The implementation of a “red-flags” questionnaire by primary health practitioners might assist early diagnosis and improve health outcomes. The reliance of biomarkers rather than symptoms to manage treatment is a significant unmet need, possibly resolvable by a “patient-centered” approach to AIG/PA. Interventions that provide patients with an improved understanding of their illness may also significantly improve psychological health outcomes. Using the experiences of AIG/PA expert patients as consumer engagement consultants might also prove beneficial. Without better awareness and understanding of this condition, improved patient HRQOL support and a patient-centered approach, disengagement from health systems will likely continue.
Supplemental Material
Supplemental material, sj-docx-1-jpx-10.1177_23743735231151767 for Examining the Diagnosis and Treatment Experiences of People Living With Autoimmune Gastritis and Pernicious Anemia by Martine Cotton and Andrew McCaddon in Journal of Patient Experience
Acknowledgements
The authors thank the indefatigable duo Dr Allyson Mutch and Dr Lisa Fitzgerald from the UQ School of Public Health for their invaluable guidance and support throughout this research.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AM is a Scientific Advisor and shareholder of COBALZ Limited.
Ethical Statement: Written informed consent was obtained from legally authorized representatives for anonymized patient information to be published in this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by COBALZ Limited, Pernicious Anaemia Society.
ORCID iDs: Martine Cotton https://orcid.org/0000-0003-2040-0936
Andrew McCaddon https://orcid.org/0000-0001-8588-9061
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lenti MV, Rugge M, Lahner Eet al. Autoimmune gastritis. Nature Rev Dis Primers. 2020;6(1):56. [DOI] [PubMed] [Google Scholar]
- 2.Notsu T, Adachi K, Mishiro T, et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern Med. 2019;58(13):1817‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afsar I, Sener A, Kaya S. Prevalence of high-level positivity of anti-parietal cell antibodies in Turkish population. Scand J Gastroenterol. 2015;50(10):1304‐5. [DOI] [PubMed] [Google Scholar]
- 4.Lahner E, Zagari R, Zullo Aet al. Chronic atrophic gastritis: natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis. 2019;51(12):1621‐32. [DOI] [PubMed] [Google Scholar]
- 5.Miceli E, Vanoli A, Lenti MVet al. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther. 2019;50(11–12):1172‐80. [DOI] [PubMed] [Google Scholar]
- 6.Telaranta-Keerie A, Kara R, Paloheimo L, Härkönen M, Sipponen P. Prevalence of undiagnosed advanced atrophic corpus gastritis in Finland: an observational study among 4,256 volunteers without specific complaints. Scand J Gastroenterol. 2010;45(9):1036‐41. [DOI] [PubMed] [Google Scholar]
- 7.Terao S, Suzuki S, Yaita Het al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig Endosc. 2020;32(3):364‐72. [DOI] [PubMed] [Google Scholar]
- 8.Weck MN, Stegmaier C, Rothenbacher D, Brenner H. Epidemiology of chronic atrophic gastritis: population–based study among 9444 older adults from Germany. Aliment Pharmacol Ther. 2007;26(6):879‐87. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Weck M, Scḧottker B, Rothenbacher D, Brenner H. Gastric parietal cell antibodies, helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in Germany. Cancer Epidemiol Biomarkers Prev. 2013;22(5):821‐6. [DOI] [PubMed] [Google Scholar]
- 10.Toh BH, Chan J, Kyaw T, Alderuccio F. Cutting edge issues in autoimmune gastritis. Clin Rev Allergy Immunol. 2012;42(3):269‐78. [DOI] [PubMed] [Google Scholar]
- 11.Zulfiqar AA, Andres E. Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study. J Med Life. 2017;10(4):250‐3. [PMC free article] [PubMed] [Google Scholar]
- 12.Weyermann D, Spinas G, Roth S, Guglielmetti M, Viollier E, Staub JJ. Polyglandular autoimmune syndrome: incidence, forms and clinical significance. Schweiz Med Wochenschr. 1994;124(44):1971‐5. [PubMed] [Google Scholar]
- 13.Lenti MV, Miceli E, Cococcia Set al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment Pharmacol Ther. 2019;50(2):167‐75. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Yoshida S, Watanabe H, et al. Clue of diagnosis for autoimmune gastritis. Digestion. 2021;102(6):903-10. [DOI] [PubMed] [Google Scholar]
- 15.Hooper M, Hudson P, Porter F, McCaddon A. Patient journeys: diagnosis and treatment of pernicious anaemia. Br J Nurs. 2014;23(7):376‐81. [DOI] [PubMed] [Google Scholar]
- 16.Miceli E, Brondino N, Lenti MVet al. Impaired quality of life in patients with autoimmune atrophic gastritis. Dig Dis Sci. 2020;66(10):3322-9. [DOI] [PubMed] [Google Scholar]
- 17.Popay J, Roberts H, Sowden Aet al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. UK; 2006.
- 18.Clarke V, Braun V. Thematic analysis. J Posit Psychol. 2017;12(3):297‐8. [Google Scholar]
- 19.Seage CH, Glover E, Mercer J. Receiving a diagnosis of pernicious Anemia: exploring experiences of relationships with health professionals. J Patient Exp. 2020;7(5):766‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semedo L. Developing a patient-centred outcome measure for patients suffering with pernicious anaemia. ProQuest Dissertations Publishing; 2018. [Google Scholar]
- 21.Kornic P, Harty M, Grant J. Influence of treatment parameters on symptom relief in individuals with vitamin B12 deficiency. Annu Res Rev Biol. 2017;11(5):1‐8. [Google Scholar]
- 22.Seage CH. Living with pernicious anaemia: exploring the link between anticipated stigma and wellbeing. J Psychosom Res. 2018;113(1):72‐3. [DOI] [PubMed] [Google Scholar]
- 23.Seage CH, Semedo L. How do patients receiving prescribed B-12 injections for the treatment of PA perceive changes in treatment during the COVID-19 pandemic? A UK-based survey study. J Patient Exp. 2021;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Htut TW, Thein KZ, Oo TH. Pernicious anemia: pathophysiology and diagnostic difficulties. J Evid Based Med. 2021;14(2):161‐9. [DOI] [PubMed] [Google Scholar]
- 25.Tyler N, Giles S, Daker-White G, McManus BC, Panagioti M. A patient and public involvement workshop using visual art and priority setting to provide patients with a voice to describe quality and safety concerns: vitamin B12 deficiency and pernicious anaemia. Health Expect. 2021;24(1):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahner E, Conti L, Annibale B, Corleto VD. Current perspectives in atrophic gastritis. Curr Gastroenterol Rep. 2020;22(8):38. [DOI] [PubMed] [Google Scholar]
- 27.Wolffenbuttel BHR, Wouters H, Heiner-Fokkema M, van der Klauw MM. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clin Proc Innov Qual Outcomes. 2019;3(2):200‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 2017;129(19):2603‐11. [DOI] [PubMed] [Google Scholar]
- 29.Hooper M. Martyn Hooper MBE – Chair of the PAS ∼ The Chair’s Blog ∼ [Internet]. martynhooper.com, editor 2020. Available from: https://www.martynhooper.com/2020/06/03/reflections-on-treating-pernicious-anaemia/.
- 30.Lenti MV, Cococcia S, Miceli Eet al. et al. Red flags for the diagnosis of autoimmune gastritis. Clin Res Hepatol Gastroenterol. 2021;46(1):101780. [DOI] [PubMed] [Google Scholar]
- 31.Miceli E, Padula D, Lenti MV, et al. A laboratory score in the diagnosis of autoimmune atrophic gastritis A prospective study. J Clin Gastroenterol. 2015;49(1):E1‐5. [DOI] [PubMed] [Google Scholar]
- 32.Mazokopakis EE. Normal serum cobalamin levels do not exclude the diagnosis of pernicious anaemia: a case report. Fam Pract. 2020;37(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 33.Dobson R, Alvares D. The difficulties with vitamin B12. Pract Neurol. 2016;16(4):308‐11. [DOI] [PubMed] [Google Scholar]
- 34.Wang HY, Li LY, Qin LL, Song YN, Vidal-Alaball J, Liu TH. Oral vitamin B-12 versus intramuscular vitamin B-12, for vitamin B-12 deficiency. Cochrane Database Syst Rev. 2018;3(1):1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: a review. World J Gastroenterol. 2017;23(4):563‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zilli A, Cavalcoli F, Ciafardini C, Massironi S. Deficiency of micronutrients in patients affected by chronic atrophic autoimmune gastritis: a single-institution observational study. Dig Liver Dis. 2019;51(4):505‐9. [DOI] [PubMed] [Google Scholar]
- 39.Young CE, Boyle FM, Brooker KS, Mutch AJ. Incorporating patient preferences in the management of multiple long-term conditions: is this a role for clinical practice guidelines? J Comorb. 2015;5(1):122‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jpx-10.1177_23743735231151767 for Examining the Diagnosis and Treatment Experiences of People Living With Autoimmune Gastritis and Pernicious Anemia by Martine Cotton and Andrew McCaddon in Journal of Patient Experience