Abstract
Higher-order cycloadditions are a powerful strategy for the construction of polycycles in one step. However, an efficient and concise version for the induction of asymmetry is lacking. N-heterocyclic carbenes are widely used organocatalysts for asymmetric synthesis and could be an ideal choice for enantioselective higher-order cycloadditions. Here, we report an enantioselective [10 + 2] annulation between catalytically formed aza-benzofulvene intermediates and trifluoromethyl ketone derivatives. This protocol exhibits a wide scope, high yields, and good ee values, reflecting a robust and efficient higher-order cycloaddition. Density functional theory calculations provide an accurate prediction of the reaction enantioselectivity, and in-depth insight to the origins of stereocontrol.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology
High-order cycloaddition reactions are useful for the construction of polycycles in a single step, but versions that induce asymmetry are limited. Here the authors report the construction of asymmetric polycycles via N-heterocyclic carbene-catalyzed hetero-[10 + 2] cycloaddition of indole-2-carbaldehydes with trifluoromethyl ketone derivatives.
Introduction
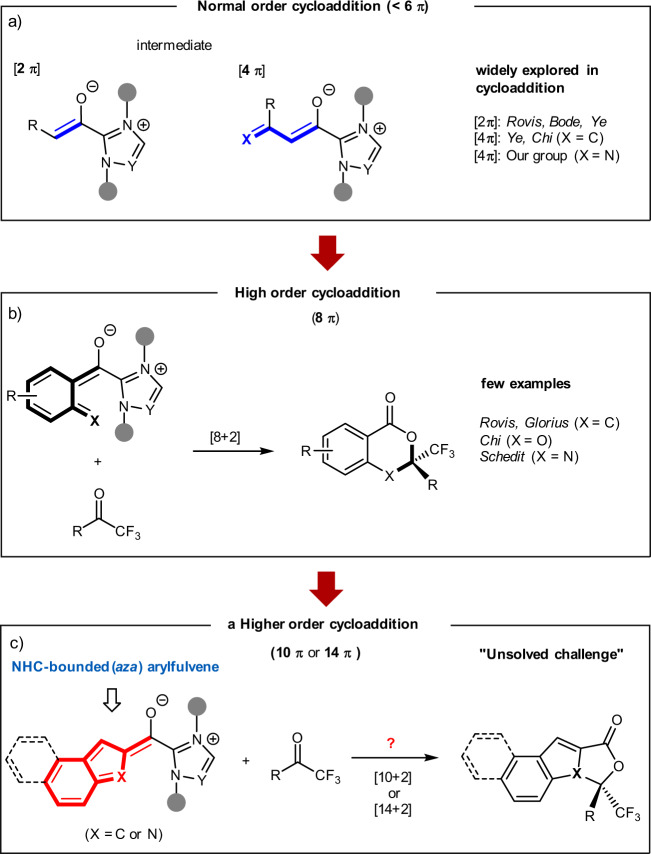
In the past few decades, the use of chiral N-heterocyclic carbenes (NHCs) as asymmetric organocatalysts1–10, with the associated advantages of their easy operation and of carrying out enantioselective transformations in a benign environment and under mild reaction conditions, has led to impressive and continuous growth in their use. Specifically, the NHC-catalyzed asymmetric cycloaddition for the assembly of chiral mono- or polycyclic molecules has received broad attention, driven by the predominance of these chiral cyclic structures in natural products and pharmaceuticals11,12. In this context, normal order cycloadditions (cycloaddition that involves <6π-electron components) have been investigated in NHC catalysis in terms of in situ generated active enolate13,14 or dienolate intermediates15 (Fig. 1a). These pioneer works include [2 + 2]16–20, [2 + 3]21–23, [2 + 4]24–29, [4 + 2]30–35, etc.36,37. In 2008, Zhang et al.18 and Duguet et al.16 simultaneously realized an NHC-catalyzed [2 + 2] cycloaddition of enolates with imines, yielding versatile chiral β-lactams. The enantioselective [2 + 3] cycloaddition of enolates with oxaziridines or nitrovinylindoles has been reported by Shao et al.21 and Ni et al.22 groups, using NHC organocatalysis, independently. Asymmetric carbene-catalyzed [2 + 4] reaction of enolates with azadienes was also disclosed by He et al.24 to furnish chiral dihydropyridinones. In addition to enolates, NHC-bounded dienolates have also been successfully studied in [4 + 2]30 or [4 + 3]38 cycloadditions to generate six- or seven-membered heterocycles, respectively.
Fig. 1. NHC-catalyzed normal and higher-order cycloaddition.
a NHC-catalyzed normal order cycloaddition. b NHC-catalyzed high order cycloaddition. c NHC-catalyzed higher-order cycloaddition (this work).
Although the above-mentioned normal order cycloaddition reactions are widely explored, the higher-order cycloaddition (≥6π-electrons) has received a high level of attention and is somehow used to concisely construct polycycles in one step fashion. Significant progress of highly stereoselective higher-order cycloadditions has been made in recent years39–41. Elegant relevant works in this direction involve Feng’s Ni-catalyzed [8 + 2] cycloaddition of azaheptafulvenes with alkylidene malonates42,43. The Jørgensen group enriched this field by developing a series of highly enantioselective cycloaddition reactions (e.g., [8 + 2]44,45, [6 + 4]46, and [10 + 4]47) promoted via enamine catalysis. However, to a certain extent48–51, this class of higher-order cycloadditions suffers from some drawbacks (i.e., difficult stereocontrol and lack of periselectivity), thus resulting in slower growth than normal order cycloaddition. Despite the difficulties ahead, some encouraging progress was still achieved in the direction of NHC catalysis52–55. Janssen-Muller56 and Chen and Rovis57 reported a [8 + 2] cycloaddition of NHC-bounded o-quinodimethane intermediates (8π-electron) with ketones, respectively (Fig. 1b)58,59. A recent Chen et al.60 work indicated that salicylaldehydes could be oxidized to generate NHC-bounded o-quinone methide intermediates (8π-electron), which participated in a [8 + 2] cycloaddition with electrophile trifluoromethyl ketones. Besides the above accomplishments, Lee et al.61 then successfully found that the NHC-bounded aza-o-quinone methide intermediates (8π-electron), first generated from decarboxylation of N-methylisatoic anhydrides, could react with trifluoromethyl ketones to deliver enantioenriched dihydrobenzoxazin-4-ones via a [8 + 2] cycloaddition strategy.
In brief, NHC-catalyzed cycloadditions ranged from [2 + 2] to [8 + 2] have been extensively investigated over the past few years, but there is a remarkable lack of higher-order cycloadditions (e.g., [10 + 2]62,63 and [14 + 2]). Although the intricately competitive pathways make the reaction-control difficult, these higher-order cycloadditions can provide a direct way to efficiently build polycyclic scaffolds.
Herein, we report a hetero-[10 + 2] higher-order cycloaddition of indole-2-carbaldehydes with trifluoromethyl ketone derivatives, proceeding via an NHC-bounded aza-benzofulvene intermediate (Fig. 1c). This discovery represents the initial use of NHC-bounded aza-arylfulvene intermediates in catalytic and enantioselective [10 + 2] or [14 + 2] reaction. In addition, in medicinal chemistry, the incorporation of “F”-containing fragments normally provides an effective route to enhance the metabolic stability, as well as other chemical or physical properties, of target molecules64–66. Based on the importance of polycyclic structures and incorporated “F”-containing fragments, the potential of these synthesized molecules in drug discovery is worth our expectation.
Results
Reaction optimization
We commenced our studies by investigating the reaction of indole-2-carbaldehyde 1a and 2,2,2-trifluoroacetophenone 2a as the model substrates, K2CO3 as the base, DQ as the oxidant, tetrahydrofuran as the solvent, and the results are briefly summarized in Table 1. When L-phenylalanine-derived triazolium NHC precatalyst A was exploited, the expected cycloadduct 3a was not observed. Replacing the mesitylene group with pentafluorophenyl group triazolium NHC precatalyst B gave desired product 3a in 40% yield and 0% ee, whereas the use of precatalyst C and D resulted in almost no reaction. To our delight, when indanol-derived triazolium catalyst E was tested, the [10 + 2] cycloadduct 3a was successfully formed in 61% yield with 35% ee and implies that this highly enantioselective [10 + 2] annulation can be achieved in the presence of ideal conditions. The catalytic performance could be further improved by changing the X group of precatalyst E from H to NO2 (entry 6). After evaluating bases and solvents, we found that a combination of PhCO2Na as the base and hexane as the solvent gave the product 3a in 80% yield and 88% ee (entry 10). Improvements in yield and enantioselectivity were found when thiourea was used as the additive to form 3a (entry 12, 85% yield, 91% ee).
Table 1.
Optimization of the reaction conditionsa.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | NHC cat. | Solvent | Base | Additive | Yield (%)b | ee (%)c |
| 1 | A | THF | K2CO3 | / | trace | – |
| 2 | B | THF | K2CO3 | / | 40 | 0 |
| 3 | C | THF | K2CO3 | / | trace | – |
| 4 | D | THF | K2CO3 | / | trace | – |
| 5 | E | THF | K2CO3 | / | 61 | 35 |
| 6 | F | THF | K2CO3 | / | 70 | 51 |
| 7 | F | DCM | K2CO3 | / | 53 | 59 |
| 8 | F | Toluene | K2CO3 | / | 40 | 42 |
| 9 | F | Hexane | Et3N | / | 42 | 68 |
| 10 | F | Hexane | PhCO2Na | / | 80 | 88 |
| 11 | F | Hexane | PhCO2Na | G | 93 | 88 |
| 12 | F | Hexane | PhCO2Na | H | 85 | 91 |
| 13d | F | Hexane | PhCO2Na | H | 74 | 91 |
aConditions: 1a (0.1 mmol), 2a (0.12 mmol), catalyst (15 mol%), base (0.10 mmol) and DQ (0.11 mmol), solvent (1.0 mL), room temperature, 4 Å MS (30 mg), Ar, 48 h.
bIsolated yield after flash column chromatography.
cEnantiomeric excess (ee) determined via chiral-phase HPLC analysis.
dcat. F (10 mol%) was used, 72 h.
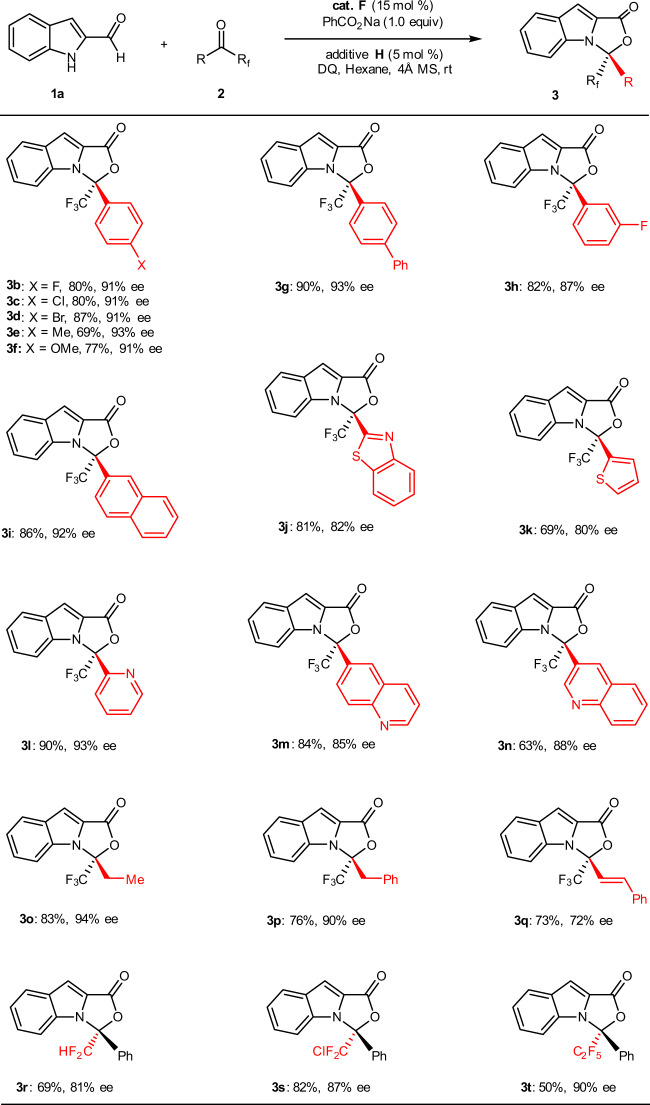
Substrate scope
With the optimal catalytic system in hand, we moved our attention to exploring the generality of this asymmetric higher order [10 + 2] annulation. As illustrated in Fig. 2, by reacting with indole-2-carbaldehyde 1a, an array of aryl trifluoromethyl ketones 2 was examined first. In the reactions to generate the [10 + 2] cycloadducts 3, yields and enantioselectivities were found to be independent of the electronic properties of the substituents on the aryl group in 2 (3b−i). When the heteroaryl trifluoromethyl ketones were reacted with indole-2-carbaldehyde 1a under optimal conditions, an [10 + 2] annulation was efficiently realized in all cases (3j−n). Reactions attempted using the alkyl trifluoromethyl ketones gave their corresponding [10 + 2] cycloadducts in good yields with high ee values (3o and 3p). Whereas the alkenyl trifluoromethyl ketone 2q was reacted with 1a, product 3q was also obtained in a good yield (73%) but with a slightly diminished enantioselectivity (72% ee). Switching the fluorinated substituent from CF3 to CF2H, ClCF2, or C2F5 in ketones, synthetic useful yields, and high to excellent enantioselectivities were still obtained under current conditions (3r−t).
Fig. 2. Scope of ketones.
Reaction conditions: 1a (0.2 mmol), 2 (0.24 mmol), cat. F (15 mol%), additive H (5 mol%), PhCO2Na (0.20 mmol) and DQ (0.22 mmol), hexane (2.0 mL), room temperature, 4 Å MS (60 mg), Ar, 36–96 h.
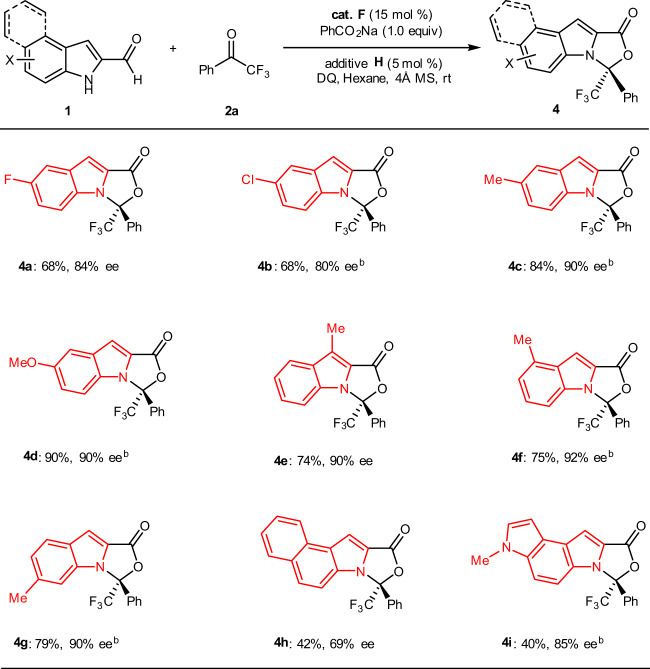
Next, we turned our focus to investigate the scope of substrate 1 (Fig. 3). Different substituents and substitution patterns on the indole skeleton were examined comprehensively. Electron-withdrawing substituents such as halo (4a and 4b) units on the phenyl ring of the aldehyde substrates were well tolerated. Electron-releasing groups such as methyl (4c, 4e, 4f, and 4g) and methoxyl unit (4d) could also be installed on the indole scaffold of the aldehyde substrates. It is worth to note that this [10 + 2] protocol could be extended to a higher-order [14 + 2] cycloaddition, affording their corresponding cycloadducts (4h and 4i) in good enentioselectivities albeit with acceptable but dropped yields under the current standard conditions. The absolute configuration of 3e (CCDC 1961662) was determined by single-crystal X-ray analysis and other products were assigned by analogy.
Fig. 3. Scope of indole-2-carbaldehydes 1.
Reaction conditions: 1 (0.2 mmol), 2a (0.24 mmol), cat. F (15 mol%), additive H (5 mol%), PhCO2Na (0.20 mmol) and DQ (0.22 mmol), hexane (2.0 mL), room temperature, 4 Å MS (60 mg), Ar, 36–96 h. bDCM-Hexane (1:5) was used.
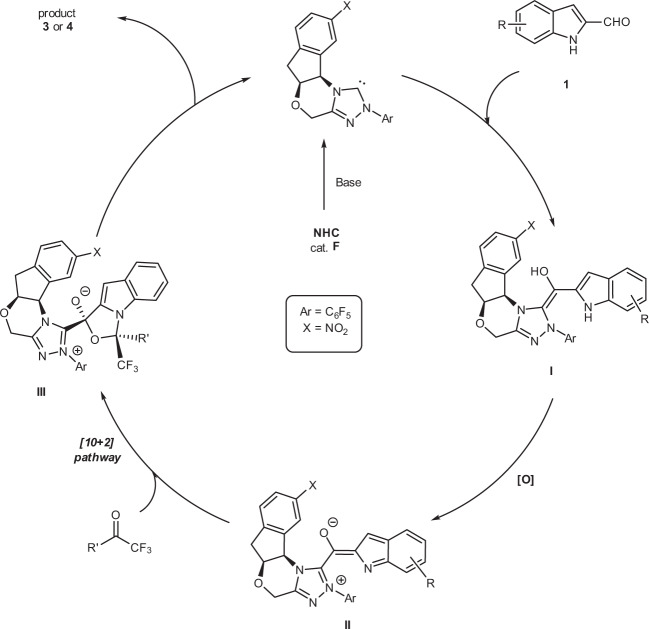
Postulated mechanism
A postulated catalytic mechanism of [10 + 2] annulation is summarized in Fig. 4. Deprotonation of NHC precatalyst F gives the corresponding NHC, which adds to aldehyde 1 to give the corresponding tetrahedral intermediate,67,68 with further deprotonation giving the Breslow intermediate I. Intermediate I is subsequently oxidized to the key NHC-bounded aza-benzofulvene intermediate II. A mass correlating to intermediate II was observed via high-resolution mass spectrometry (See Supplementary information (SI) Supplementary Table 1 for details). This critical intermediate II can promote a concerted [10 + 2] pathway or a stepwise Michael addition–acylation to form intermediate III, which undergoes N-acylation to release the NHC catalyst F for the next catalytic cycle. Kinetic experiments were conducted to gain a better insight into the mechanistic details. The initial rate constants of the reaction were determined in situ 1H-nuclear magnetic resonance (NMR) and 19F-NMR spectroscopy. The results show that the reaction appeared to have a nearly first-order dependence on NHC catalyst F (Fig. 5a), and zero-order dependence on substrates 1a (Figs. 5b), 2a (Fig. 5c), and DQ (Fig. 5d).
Fig. 4. Postulated mechanistic pathways.
Postulated catalytic mechanism of [10 + 2] annulation.
Fig. 5. Plot of initial rates vs catalyst and substrates.
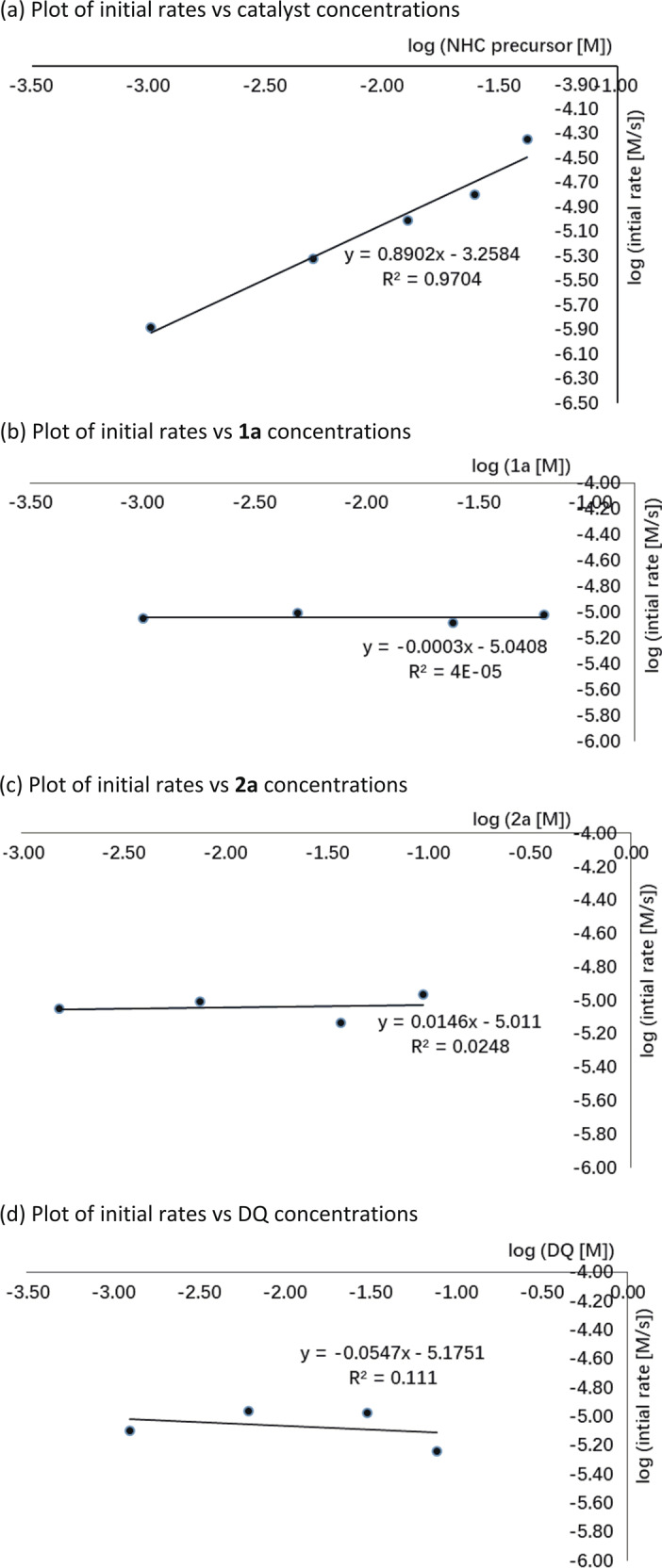
a Plot of initial rates vs. catalyst concentrations. b Plot of initial rates vs. 1a concentrations. c Plot of initial rates vs. 2a concentrations. d Plot of initial rates vs. DQ concentrations.
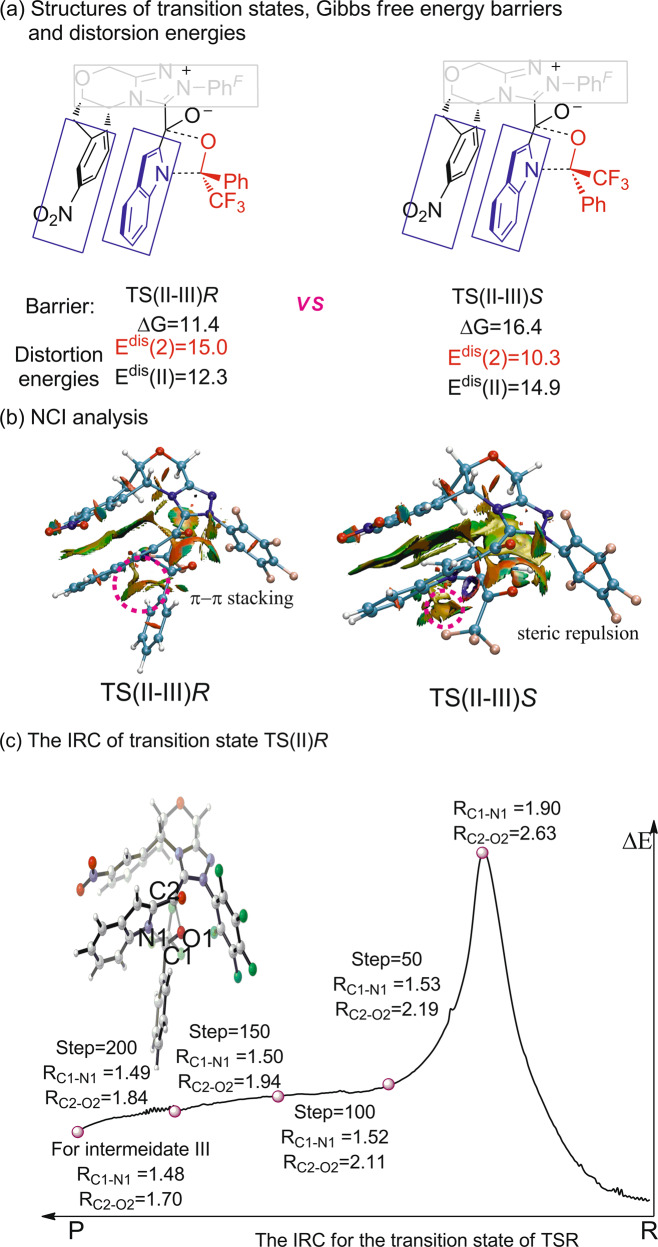
To further reveal the enantioselectivity of this [10 + 2] annulation, density functional theory (DFT) calculation was performed to study the key step of nucleophilic attack of intermediate II onto trifluoroacetophenone. As shown in Fig. 6a, two transition states named TS(II–III)R and TS(II–III)S was located, where the re- or si-face of trifluoroacetophenone was attacked, respectively. The calculated relative free energy of transition state TS(III–IV)R is 5.0 kcal/mol lower than that of TS(II–III)S, which predicts that the generation of R-configuration product 4a is favorable. The calculated results overestimate the level of enantioinduction in this reaction process but are consistent with predicting the observed experimental product configuration. The geometry of those two transition states is also given in Fig. 6b. After the absorption of indole reactant onto the NHC catalyst, a strong π–π stacking between indolyl moiety and the aryl in the NHC catalyst can significantly stabilize the deprotonated indolyl moiety. The π–π attraction is clearly shown in calculated noncovalent interaction (NCI) maps. In addition, kinetic experiments revealed that electron-rich indoles or electron-deficient aryl ketones reacted more quickly, which partially elucidated the potential π−π interaction. When the nucleophilic attack occurs, trifluoromethyl of trifluoroacetophenone appears at the more bulky inner side in transition state TS(II–III)R. It is more favorable than the case in transition state TS(II–III)S that the phenyl group is set to the inner side. The NCI map of transition state TS(II–III)R clearly reveals that the repulsion between phenyl group of trifluoroacetophenone and the NHC catalyst leads to instability of transition state TS(III–IV)S, while this repulsion is absent in transition state TS(II–III)R.
Fig. 6. The DFT investigation on the enantioselectivity of the [10 + 2] annulation.
a The two transition states of TS(II–III)R and TS(II–III)S Gibbs free energy barriers and distortion energies comparing. b NCI analysis of the TS(II–III)R and TS(II–III)S. c The IRC of transition state TS(II)R.
In order to figure out whether the process from II to III would be concerted or stepwise, the intrinsic reaction coordinate calculation (IRC) of transition state TS(II)R has been performed (Fig. 6c). The result clearly shows the C1 of trifluoroacetophenone and N1 of indole would form the covalent bond firstly. Along with the decreasing distance of C1–N1, the bond of oxygen atom O1–C2 gradually formed until the intermediate III generate. Hence, we speculate that the process tends to be a concerted asynchronous process59,69.
Synthetic transformations and applications
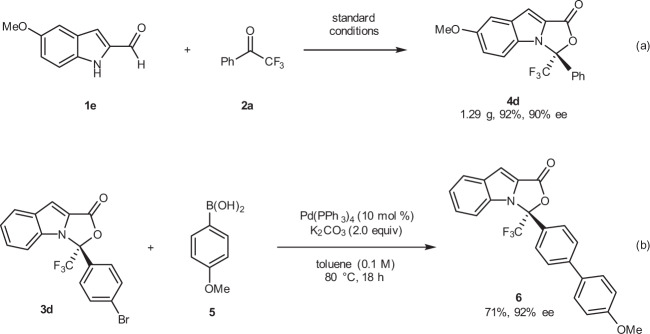
Our protocol is amenable to large-scale preparation. For example, the use of standard conditions was sufficient to produce 4d (1.29 g) in 92% yield and with 90% ee (Fig. 7a). A facile Pd-catalyzed Suzuki coupling of 3d with 4-methoxyphenylboronic acid 5 led to product 6 in a 72% yield and with a remained enantioselectivity (Fig. 7b).
Fig. 7. Gram scale synthesis and transformation.
a Reaction conditions: 1e (4.0 mmol), 2a (4.8 mmol), F (0.60 mmol), DQ (4.4 mmol), additive H (0.20 mmol), PhCO2Na (4.0 mmol), Hexane (33.4 mL) and DCM (6.6 mL), room temperature, 4 Å M.S. (600 mg), Ar, 40 h. b Reaction conditions: 3d (0.05 mmol), 5 (0.10 mmol), K2CO3 (0.10 mmol) and Pd(PPh3)4 (0.005 mmol), toluene (0.5 mL), 80 °C, 18 h.
In summary, a unique NHC-catalyzed enantioselective hetero-[10 + 2] annulation of indole-2-carbaldehydes with trifluoromethyl ketone derivatives has been developed. This process generates a new NHC-bounded aza-benzofulvene as a key intermediate. This new protocol allows the rapid assembly of enantioenriched polycycles from readily available starting materials under mild conditions. DFT calculations elucidated the origins of the [10 + 2] process. Further investigations on new NHC-bounded aza-arylfulvene as an active intermediate in asymmetric synthesis are currently ongoing in our laboratory.
Methods
Synthesis of 3/4
To a flame-dried Schlenk reaction tube equipped with a magnetic stir bar, was added the precatalyst F (15.4 mg, 0.03 mmol), DQ (90.0 mg, 0.22 mmol), additive H (5.0 mg, 0.01 mmol), PhCO2Na (28.8 mg, 0.20 mmol), 1 (0.20 mmol) and 4 Å MS (60 mg). The Schlenk tube was closed with a septum, evacuated, and refilled with an argon atmosphere. Hexane (2.0 mL) and 2 (0.24 mmol) was added. The mixture was then stirred at 25 °C and monitored by TLC until 1 was consumed. The mixture was concentrated under reduced pressure and purified by column chromatography on silica gel (hexane/EtOAc = 100:1) to afford the desired product 3 or 4. Full experimental details can be found in the Supplementary Methods.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Generous financial support for this work is provided by the National Natural Science Foundation of China (Nos. 21672121 and 21871160), the Tsinghua University, the Bayer Investigator fellow, the Fellowship of Tsinghua-Peking Centre for Life Sciences (CLS).
Author contributions
Q.P.P. conducted the main experiments. S.J.L. and Y.L. conducted computational studies. B.Z. and D.H.G. prepared several starting materials, including substrates. J.W. conceptualized and directed the project, and drafted the paper with the assistance from co-authors. All authors contributed to discussions.
Data availability
For 1H NMR, 13C NMR, and 19F NMR spectra see Supplementary Figs. 1–93 and high-performance liquid chromatography spectra see Supplementary Figs. 94–153. The supplementary crystallographic data (Supplementary Data 1) for this paper could be obtained free of charge from The Cambridge Crystallographic Data Centre (3e: CCDC 1961662) via www.ccdc.cam.ac.uk/data_request/cif. The coordinates for the corresponding structures and IRC of transition state TS(II)R in Supplementary Data 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Lan, Email: lanyu@cqu.edu.cn.
Jian Wang, Email: wangjian2012@tsinghua.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42004-020-00425-7.
References
- 1.Enders D, Niemeier O, Henseler A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]
- 2.Marion N, Díez-González S, Nolan SP. N-heterocyclic carbenes as organocatalysts. Angew. Chem. Int. Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- 3.Nair V, Vellalath S, Babu BP. Recent advances in carbon–carbon bond-forming reactions involving homoenolates generated by NHC catalysis. Chem. Soc. Rev. 2008;37:2691–2698. doi: 10.1039/b719083m. [DOI] [PubMed] [Google Scholar]
- 4.Biju AT, Kuhl N, Glorius F. Extending NHC-catalysis: coupling aldehydes with unconventional reaction partners. Acc. Chem. Res. 2011;44:1182–1195. doi: 10.1021/ar2000716. [DOI] [PubMed] [Google Scholar]
- 5.Ryan SJ, Candish L, Lupton DW. Acyl anion free N-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 2013;42:4906–4917. doi: 10.1039/c3cs35522e. [DOI] [PubMed] [Google Scholar]
- 6.Hopkinson MN, Richter C, Schedler M, Glorius F. An overview of N-heterocyclic carbenes. Nature. 2014;510:485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 7.Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015;115:9307–9387. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon RS, Biju AT, Nair V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon–carbon bond-forming reactions. Chem. Soc. Rev. 2015;44:5040–5052. doi: 10.1039/C5CS00162E. [DOI] [PubMed] [Google Scholar]
- 9.Wang MH, Scheidt KA. Cooperative catalysis and activation with N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2016;55:14912–14922. doi: 10.1002/anie.201605319. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Hooper JF, Lupton DW. N-heterocyclic carbene catalysis via the α, β-unsaturated acyl azolium. ACS Catal. 2017;7:2583–2596. doi: 10.1021/acscatal.6b03663. [DOI] [Google Scholar]
- 11.Grossmann A, Enders D. N-heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 2012;51:314–325. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]
- 12.Izquierdo J, Hutson GE, Cohen DT, Scheidt KA. A continuum of progress: applications of N-hetereocyclic carbene catalysis in total synthesis. Angew. Chem. Int. Ed. 2012;51:11686–11698. doi: 10.1002/anie.201203704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds NT, Rovis T. Enantioselective protonation of catalytically generated chiral enolates as an approach to the synthesis of α-chloroesters. J. Am. Chem. Soc. 2005;127:16406–16407. doi: 10.1021/ja055918a. [DOI] [PubMed] [Google Scholar]
- 14.Mahatthananchai J, Bode JW. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 2014;47:696–707. doi: 10.1021/ar400239v. [DOI] [PubMed] [Google Scholar]
- 15.Chen XY, Liu Q, Chauhan P, Enders D. N-heterocyclic carbene catalysis via azolium dienolates: an efficient strategy for remote enantioselective functionalizations. Angew. Chem. Int. Ed. 2018;57:3862–3873. doi: 10.1002/anie.201709684. [DOI] [PubMed] [Google Scholar]
- 16.Duguet N, Campbell CD, Slawin AM, Smith AD. N-heterocyclic carbene catalysed β-lactam synthesis. Org. Biomol. Chem. 2008;6:1108–1113. doi: 10.1039/b800857b. [DOI] [PubMed] [Google Scholar]
- 17.He L, Lv H, Zhang Y-R, Ye S. Formal cycloaddition of disubstituted ketenes with 2-oxoaldehydes catalyzed by chiral N-heterocyclic carbenes. J. Org. Chem. 2008;73:8101–8103. doi: 10.1021/jo801494f. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y-R, He L, Wu X, Shao P-L, Ye S. Chiral N-heterocyclic carbene catalyzed Staudinger reaction of ketenes with imines: highly enantioselective synthesis of N-Boc β-lactams. Org. Lett. 2008;10:277–280. doi: 10.1021/ol702759b. [DOI] [PubMed] [Google Scholar]
- 19.Wang XN, Zhang YY, Ye S. Enantioselective synthesis of spirocyclic oxindole-β-lactones via N-heterocyclic carbene-catalyzed cycloaddition of ketenes and isatins. Adv. Synth. Catal. 2010;352:1892–1895. doi: 10.1002/adsc.201000187. [DOI] [Google Scholar]
- 20.Jian TY, He L, Tang C, Ye S. N-heterocyclic carbene catalysis: enantioselective formal [2+ 2] cycloaddition of ketenes and N-sulfinylanilines. Angew. Chem. Int. Ed. 2011;50:9104–9107. doi: 10.1002/anie.201102488. [DOI] [PubMed] [Google Scholar]
- 21.Shao PL, Chen XY, Ye S. Formal [3+ 2] cycloaddition of ketenes and oxaziridines catalyzed by chiral Lewis bases: enantioselective synthesis of oxazolin-4-ones. Angew. Chem. Int. Ed. 2010;49:8412–8416. doi: 10.1002/anie.201003532. [DOI] [PubMed] [Google Scholar]
- 22.Ni Q, et al. Asymmetric synthesis of pyrroloindolones by N-heterocyclic carbene catalyzed [2+ 3] annulation of α-chloroaldehydes with nitrovinylindoles. Angew. Chem. Int. Ed. 2013;52:13562–13566. doi: 10.1002/anie.201305957. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Lv Y, Wang F, Zhong G. Chiral NHC-catalyzed 1, 3-dipolar [3+ 2] cycloaddition of azomethine imines with α-chloroaldehydes for the synthesis of bicyclic pyrazolidinones. Org. Biomol. Chem. 2018;16:4433–4438. doi: 10.1039/C8OB00925B. [DOI] [PubMed] [Google Scholar]
- 24.He M, Struble JR, Bode JW. Highly enantioselective azadiene Diels− Alder reactions catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2006;128:8418–8420. doi: 10.1021/ja062707c. [DOI] [PubMed] [Google Scholar]
- 25.He M, Uc GJ, Bode JW. Chiral N-heterocyclic carbene catalyzed, enantioselective oxodiene Diels− Alder reactions with low catalyst loadings. J. Am. Chem. Soc. 2006;128:15088–15089. doi: 10.1021/ja066380r. [DOI] [PubMed] [Google Scholar]
- 26.Kaeobamrung J, Kozlowski MC, Bode JW. Chiral N-heterocyclic carbene-catalyzed generation of ester enolate equivalents from α, β-unsaturated aldehydes for enantioselective Diels–Alder reactions. Proc. Natl Acad. Sci. USA. 2010;107:20661–20665. doi: 10.1073/pnas.1007469107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jian T-Y, Shao P-L, Ye S. Enantioselective [4+ 2] cycloaddition of ketenes and 1-azadienes catalyzed by N-heterocyclic carbenes. Chem. Commun. 2011;47:2381–2383. doi: 10.1039/C0CC04839A. [DOI] [PubMed] [Google Scholar]
- 28.Ryan SJ, Candish L, Lupton DW. N-heterocyclic carbene-catalyzed (4+ 2) cycloaddition/decarboxylation of silyl dienol ethers with α, β-unsaturated acid fluorides. J. Am. Chem. Soc. 2011;133:4694–4697. doi: 10.1021/ja111067j. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, et al. Enantioselective annulations for dihydroquinolones by in situ generation of azolium enolates. J. Am. Chem. Soc. 2014;136:10589–10592. doi: 10.1021/ja505880r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen LT, Shao PL, Ye S. N-heterocyclic carbene-catalyzed cyclization of unsaturated acyl chlorides and ketones. Adv. Synth. Catal. 2011;353:1943–1948. doi: 10.1002/adsc.201100178. [DOI] [Google Scholar]
- 31.Mo J, Chen X, Chi YR. Oxidative γ-addition of enals to trifluoromethyl ketones: enantioselectivity control via Lewis acid/N-heterocyclic carbene cooperative catalysis. J. Am. Chem. Soc. 2012;134:8810–8813. doi: 10.1021/ja303618z. [DOI] [PubMed] [Google Scholar]
- 32.Chen XY, Xia F, Cheng JT, Ye S. Highly enantioselective γ-amination by N-heterocyclic carbene catalyzed [4+ 2] annulation of oxidized enals and azodicarboxylates. Angew. Chem. Int. Ed. 2013;52:10644–10647. doi: 10.1002/anie.201305571. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Li F, Wang J. Intermolecular dynamic kinetic resolution cooperatively catalyzed by an N-heterocyclic carbene and a Lewis acid. Angew. Chem. Int. Ed. 2015;54:1629–1633. doi: 10.1002/anie.201410030. [DOI] [PubMed] [Google Scholar]
- 34.Chen X-Y, et al. N-Heterocyclic carbene catalyzed [4+2] annulation of enals via a double vinylogous michael addition: asymmetric synthesis of 3,5-diaryl cyclohexenones. Angew. Chem. Int. Ed. 2017;56:6241–6245. doi: 10.1002/anie.201702881. [DOI] [PubMed] [Google Scholar]
- 35.Peng Q, Zhang B, Xie Y, Wang J. Carbene-catalyzed [4+ 2] annulation of 2 H-azirine-2-carboxaldehydes with ketones via azolium aza-dienolate intermediate. Org. Lett. 2018;20:7641–7644. doi: 10.1021/acs.orglett.8b03378. [DOI] [PubMed] [Google Scholar]
- 36.Gillard RM, Fernando JE, Lupton DW. Enantioselective N-heterocyclic carbene catalysis via the dienyl acyl azolium. Angew. Chem. Int. Ed. 2018;57:4712–4716. doi: 10.1002/anie.201712604. [DOI] [PubMed] [Google Scholar]
- 37.Zhu T, et al. N-heterocyclic carbene-catalyzed δ-carbon LUMO activation of unsaturated aldehydes. J. Am. Chem. Soc. 2015;137:5658–5661. doi: 10.1021/jacs.5b02219. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Huang Z, Xu J, Chi YR. N-heterocyclic carbene-catalyzed [3+ 4] cycloaddition and kinetic resolution of azomethine imines. J. Am. Chem. Soc. 2014;136:1214–1217. doi: 10.1021/ja411110f. [DOI] [PubMed] [Google Scholar]
- 39.Rigby JH. Transition metal promoted higher-order cycloaddition reactions in organic synthesis. Acc. Chem. Res. 1993;26:579–585. doi: 10.1021/ar00035a003. [DOI] [Google Scholar]
- 40.Palazzo TA, Mose R, Jørgensen KA. Cycloaddition reactions: why is it so challenging to move from six to ten electrons? Angew. Chem. Int. Ed. 2017;56:10033–10038. doi: 10.1002/anie.201701085. [DOI] [PubMed] [Google Scholar]
- 41.Palazzo TA, Jørgensen KA. Higher-order cycloaddition reactions: a computational perspective. Tetrahedron. 2018;74:7381–7387. doi: 10.1016/j.tet.2018.11.008. [DOI] [Google Scholar]
- 42.Xie M, et al. Catalytic asymmetric [8+ 2] cycloaddition: synthesis of cycloheptatriene-fused pyrrole derivatives. Angew. Chem. Int. Ed. 2013;52:5604–5607. doi: 10.1002/anie.201209601. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, et al. Catalytic asymmetric [8+ 3] annulation reactions of tropones or azaheptafulvenes with meso-aziridines. Chem. Eur. J. 2018;24:13428–13431. doi: 10.1002/chem.201803507. [DOI] [PubMed] [Google Scholar]
- 44.Mose R, et al. Organocatalytic stereoselective [8+ 2] and [6+ 4] cycloadditions. Nat. Chem. 2017;9:487. doi: 10.1038/nchem.2682. [DOI] [PubMed] [Google Scholar]
- 45.Donslund BS, et al. Organocatalytic enantioselective higher-order cycloadditions of in situ generated amino isobenzofulvenes. Angew. Chem. Int. Ed. 2018;57:1246–1250. doi: 10.1002/anie.201710694. [DOI] [PubMed] [Google Scholar]
- 46.Bertuzzi G, et al. Catalytic enantioselective hetero-[6+ 4] and-[6+ 2] cycloadditions for the construction of condensed polycyclic pyrroles, imidazoles, and pyrazoles. J. Am. Chem. Soc. 2019;141:3288–3297. doi: 10.1021/jacs.8b13659. [DOI] [PubMed] [Google Scholar]
- 47.Donslund BS, et al. Catalytic enantioselective [10+4] cycloadditions. Angew. Chem. Int. Ed. 2018;57:13182–13186. doi: 10.1002/anie.201807830. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi Y, et al. Organocatalytic, enantioselective intramolecular [6+ 2] cycloaddition reaction for the formation of tricyclopentanoids and insight on its mechanism from a computational study. J. Am. Chem. Soc. 2011;133:20175–20185. doi: 10.1021/ja108516b. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Rodríguez-Escrich C, Pericàs MA. Catalytic asymmetric [8+2] annulation reactions promoted by a recyclable immobilized isothiourea. Angew. Chem. Int. Ed. 2017;56:15068–15072. doi: 10.1002/anie.201707341. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z, et al. Switchable regioselectivity in amine-catalysed asymmetric cycloadditions. Nat. Chem. 2017;9:590. doi: 10.1038/nchem.2698. [DOI] [PubMed] [Google Scholar]
- 51.Yu P, et al. Mechanisms and origins of periselectivity of the ambimodal [6+ 4] cycloadditions of tropone to dimethylfulvene. J. Am. Chem. Soc. 2017;139:8251–8258. doi: 10.1021/jacs.7b02966. [DOI] [PubMed] [Google Scholar]
- 52.He C, Li Z, Zhou H, Xu J. Stereoselective [8 + 2] cycloaddition reaction of azaheptafulvenes with α-chloro aldehydes via N-heterocyclic carbene catalysis. Org. Lett. 2019;21:8022–8026. doi: 10.1021/acs.orglett.9b03014. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Rodríguez-Escrich C, Fianchini M, Maseras F, Pericàs MA. Diastereodivergent enantioselective [8 + 2] annulation of tropones and enals catalyzed by N-heterocyclic carbenes. Org. Lett. 2019;21:3187–3192. doi: 10.1021/acs.orglett.9b00906. [DOI] [PubMed] [Google Scholar]
- 54.Nair V, Poonoth M, Vellalath S, Suresh E, Thirumalai R. An N-heterocyclic carbene-catalyzed [8+ 3] annulation of tropone and enals via homoenolate. J. Org. Chem. 2006;71:8964–8965. doi: 10.1021/jo0615706. [DOI] [PubMed] [Google Scholar]
- 55.Xia F, Gao ZH, Zhang CL, Ye S. Oxidative N-heterocyclic carbene-catalyzed [8+ 2] annulation of tropone and aldehydes: synthesis of cycloheptatriene-fused furanones. Adv. Synth. Catal. 2019;361:2291–2294. doi: 10.1002/adsc.201801679. [DOI] [Google Scholar]
- 56.Janssen-Müller D, Singha S, Olyschläger T, Daniliuc CG, Glorius F. Annulation of o-quinodimethanes through N-heterocyclic carbene catalysis for the synthesis of 1-isochromanones. Org. Lett. 2016;18:4444–4447. doi: 10.1021/acs.orglett.6b02335. [DOI] [PubMed] [Google Scholar]
- 57.Chen D-F, Rovis T. N-heterocyclic carbene and chiral brønsted acid cooperative catalysis for a highly enantioselective [4+ 2] annulation. Synthesis. 2017;49:293–298. doi: 10.1055/s-0036-1589015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, et al. Addition of N-heterocyclic carbene catalyst to aryl esters induces remote C–Si bond activation and benzylic carbon functionalization. Org. Lett. 2017;20:333–336. doi: 10.1021/acs.orglett.7b03544. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y, et al. NHC-catalyzed efficient syntheses of isoquinolinones or isochromanones through formal [4+ 2] cycloaddition of o-quinodimethanes with acylhydrazones or ketones. ChemistrySelect. 2018;3:1708–1712. doi: 10.1002/slct.201702925. [DOI] [Google Scholar]
- 60.Chen X, et al. A reaction mode of carbene-catalysed aryl aldehyde activation and induced phenol OH functionalization. Nat. Commun. 2017;8:15598. doi: 10.1038/ncomms15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee A, et al. Carbene-catalyzed enantioselective decarboxylative annulations to access dihydrobenzoxazinones and quinolones. Angew. Chem. Int. Ed. 2019;58:5941–5945. doi: 10.1002/anie.201900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, et al. Carbene-catalyzed enantioselective aromatic n-nucleophilic addition of heteroarenes to ketones. Angew. Chem. Int. Ed. 2020;59:442–448. doi: 10.1002/anie.201912160. [DOI] [PubMed] [Google Scholar]
- 63.Balanna K, et al. N-heterocyclic carbene-catalyzed formal [6+2] annulation reaction via cross-conjugated aza-trienolate intermediates. Chem. Eur. J. 2020;26:818–822. doi: 10.1002/chem.201905177. [DOI] [PubMed] [Google Scholar]
- 64.Hagmann WK. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 65.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y, et al. Modern approaches for asymmetric construction of carbon–fluorine quaternary stereogenic centers: synthetic challenges and pharmaceutical needs. Chem. Rev. 2018;118:3887–3964. doi: 10.1021/acs.chemrev.7b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collett CJ, et al. Mechanistic insights into the triazolylidene-catalysed Stetter and benzoin reactions: role of the N-aryl substituent. Chem. Sci. 2013;4:1514–1522. doi: 10.1039/c2sc22137c. [DOI] [Google Scholar]
- 68.Collett CJ, et al. Rate and equilibrium constants for the addition of N-heterocyclic carbenes into benzaldehydes: a remarkable 2-substituent effect. Angew. Chem. Int. Ed. 2015;54:6887–6892. doi: 10.1002/anie.201501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ess DH, et al. Bifurcations on potential energy surfaces of organic reactions. Angew. Chem. Int. Ed. 2008;47:7592–7601. doi: 10.1002/anie.200800918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
For 1H NMR, 13C NMR, and 19F NMR spectra see Supplementary Figs. 1–93 and high-performance liquid chromatography spectra see Supplementary Figs. 94–153. The supplementary crystallographic data (Supplementary Data 1) for this paper could be obtained free of charge from The Cambridge Crystallographic Data Centre (3e: CCDC 1961662) via www.ccdc.cam.ac.uk/data_request/cif. The coordinates for the corresponding structures and IRC of transition state TS(II)R in Supplementary Data 2.