Abstract
Hypoxia is a persistent physiological feature of many different solid tumors and a key driver of malignancy, and in recent years, it has been recognized as an important target for cancer therapy. Hypoxia occurs in the majority of solid tumors due to a poor vascular oxygen supply that is not sufficient to meet the needs of rapidly proliferating cancer cells. A hypoxic tumor microenvironment (TME) can reduce the effectiveness of other tumor therapies, such as radiotherapy, chemotherapy, and immunotherapy. In this review, we discuss the critical role of hypoxia in tumor development, including tumor metabolism, tumor immunity, and tumor angiogenesis. The treatment methods for hypoxic TME are summarized, including hypoxia‐targeted therapy and improving oxygenation by alleviating tumor hypoxia itself. Hyperoxia therapy can be used to improve tissue oxygen partial pressure and relieve tumor hypoxia. We focus on the underlying mechanisms of hyperoxia and their impact on current cancer therapies and discuss the prospects of hyperoxia therapy in cancer treatment.
Keywords: hyperoxia, hypoxia‐inducible factor (HIF), immunotherapy, targeted theraphy, tumor hypoxia
Solid tumors gradually form a hypoxic malignant microenvironment during growth, which promotes tumor proliferation and metastasis by affecting metabolism, angiogenesis, and immune escape, and finally leads to therapeutic resistance. In recent years, hypoxia‐inducible factor inhibitors, direct targeting of hypoxic cells, and respiratory hyperoxia have been widely used to improve the hypoxic microenvironment of tumors and enhance the sensitivity of tumor therapy.

1. INTRODUCTION
Oxygen is an indispensable element for aerobic metabolism in human cells. 1 Therefore, hypoxia often occurs in acute and chronic vascular diseases, pulmonary diseases, and cancer. 2 At the periphery of a tumor, 70–150 μm away from the tumor blood vessels, the available oxygen is consumed by rapidly proliferating cells, which limits the diffusion of oxygen into the deep layers of the tumor, resulting in oxygen tension below 10 mmHg, known as tumor hypoxia. 3 This harsh microenvironment is usually found in the core of most solid tumors, and the cause is related to the malformation of the vasculature. 4 Compared with normal tissues, one of the most prominent hallmarks of solid tumors is the structure of their vascular network, and the vasculature cannot provide essential oxygen and nutrients for the growing tumor. 5 Solid tumor hypoxia is typically classified into two subtypes, acute or chronic hypoxia. These two subtypes can lead to completely different hypoxia‐related responses, which may directly affect tumor development and response to treatment. 6 Studies have found that tumor cells induce hypoxia through multiple mechanisms, such as a high metabolic rate 7 , 8 and high‐rate oxygen consumption, 9 , 10 thereby causing endothelial dysfunction or destroying oxygen delivery due to various effects on blood vessels. 11 The formation of a chronic hypoxic environment activates the signaling pathway of hypoxia‐inducible factors (HIFs), accelerates tumor growth, improves tumor invasiveness, and promotes tumor metastasis. 12 Researchers have cultured cancer cells in normal and hypoxic environments and found that the proliferation, invasion, and metastasis activities of cells are significantly higher in hypoxic environments than in normal oxygen‐containing environments. 13 , 14
For more than a century, extensive efforts have been made to address tumor hypoxia in both experimental and clinical settings. 15 The key is to develop therapies that aim to target the hypoxic environment and improve intratumoral oxygenation to inhibit tumor progression and treatment resistance. To target hypoxic cells, inhibitors of hypoxia‐related pathways, such as HIFs and mammalian target of rapamycin (mTOR), have been developed. 16 Researchers have also found ways to improve tumor oxygenation and relieve the hypoxic tumor environment, such as supplemental hyperoxia therapy. 17 Oxygen therapy is one of the most widely used therapies in modern medicine and is also an intervention to relieve tissue hypoxia in critically ill patients. 18 Here, we will summarize the role of hypoxia in tumor development, ways to target tumor hypoxia, ways to improve tumor oxygenation, the application of hyperoxia in tumor therapy and the impact of hyperoxia on currently available therapies.
2. THE ROLE OF HYPOXIA IN TUMOR PROGRESSION
Many tumors can survive under hypoxic conditions, mainly due to the expression of HIF in tumor cells. 19 HIF was discovered serendipitously while studying the mechanism of erythropoietin (EPO) gene regulation. 20 HIF‐1 is an isoform consisting of one of three alpha subunits (HIF‐1α, HIF‐2α, or HIF‐3α) and a beta subunit (HIF‐1β, also known as aryl hydrocarbon nuclear transporter, or ARNT). 21 HIF‐1α is considered to be a master transcriptional regulator of the tumor cell response to hypoxia. 22 Jiang et al. exposed human cervical cancer HeLa cells to O2 concentrations ranging from 0.125% to 20% and then analyzed HIF‐1 expression as a function of intracellular O2 concentration. The HIF‐1 DNA‐binding activity of cells and the concentrations of HIF‐1α and HIF‐1β proteins increased exponentially with decreasing O2 concentration. 23
Von Hippel‒Lindau (VHL) syndrome is a rare autosomal dominant genetic disorder caused by pathogenic variants of the VHL gene. Approximately 70% of patients with VHL develop renal cell cancer (RCC) during their lifetime. The VHL protein acts as an E3 ubiquitin ligase and can ubiquitinate the HIF‐α subunit in an oxygen‐dependent manner, resulting in the proteolysis of HIF. VHL gene mutations lead to the stability of HIF subunits and the constitutive activation of HIF‐mediated transcriptional pathways in patients with VHL, causing the progression of renal cell carcinoma. 24 , 25 , 26 In VHL‐deficient renal clear cell carcinoma, aberrant activation of Akt (also known as protein kinase B, PKB) in a HIF‐independent and prolyl hydroxylation‐dependent manner is one of the underlying drivers of renal carcinogenesis and metastasis. 27 Ubiquitin‐specific protease 22 (USP22) can promote hypoxia‐induced hepatocellular carcinoma (HCC) stemness and glycolysis by deubiquitinating and stabilizing HIF1α. 28 As oxygen levels decline, HIF‐α escapes degradation by VHL and accumulates. The activation capacity of HIF‐α is controlled by an asparagine hydroxylase (factors that inhibit HIF, FIH) that acts on the C‐terminal domain (C‐TAD) of the HIF‐1α protein. Inhibiting FIH activity under hypoxia is necessary and sufficient for activating HIF‐1α c‐TAD activity. 29 In hypoxic brain tumor cells, an inhibitor of DNA binding protein 2 (ID2) binds to and disrupts the VHL complex, thereby preventing ubiquitin‐mediated proteasomal degradation of HIF‐2α. 30 Hypoxia‐induced upregulation of HIF‐1 also leads to the activation of vascular endothelial growth factor (VEGF). Chiarotto used different oxygen concentrations to verify the upregulation of VEGF mRNA in three cervical cancer cell lines (O2 concentrations of 21%, 6.25%, 4.85%, 3.46%, 2.11%, 1.57%, 1.00%, and 0%), demonstrating that the presence of VEGF mRNA may be a marker of hypoxia. 31 Takahiro and co‐workers also demonstrated that HIF‐1α was first produced in metastatic lymph nodes under hypoxia, leading to high expression of VEGF‐A and then increased expression of VEGF‐C. VEGF is highly expressed in most human cancer cell types, and hypoxia has been shown to be a major inducer of VEGF gene transcription (Figure 1). 32 , 33
FIGURE 1.
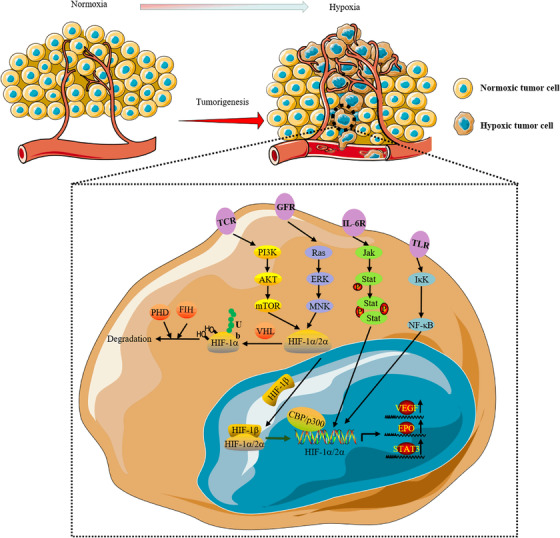
In the hypoxic tumor microenvironment, hypoxia‐inducible factor (HIF)‐1α is activated by a variety of receptors (such as TCR, GFR, IL‐6R, and TLR) on the plasma membrane through the mammalian target of rapamycin (mTOR), NF‐κB and JAK‐STAT signaling pathways. The accumulated HIF‐1α combines with HIF‐1β to form a dimer in the nucleus. CBP/P300 is a transcription cofactor, thus forming the HIF complex that initiates the transcriptional activity. The promotion of the transcription of multiple genes, such as vascular endothelial growth factor (VEGF), erythropoietin (EPO), and STAT3, is involved in cell adaptation to hypoxic stress. At the same time, HIF is degraded by proline hydroxylase (PHD), which is recognized by the ubiquitin ligase Von Hippel‒Lindau (VHL) for ubiquitination. Factors that inhibit HIF (FIH) can also suppress HIF‐1 expression by inhibiting its transcriptional activity
Rapid uncontrolled growth of tumor cells, inadequate blood supply, and hypoxia are typical microenvironmental features of most solid tumors. 34 Cancer cells rewire their metabolic programs, initiate angiogenesis and metastasis, influence immune responses, and launch rapid DNA damage repair responses to sustain growth and survival. 16 Next, we outline the effects of hypoxia on tumor behavior and the intrinsic mechanisms, underscoring the role of HIF‐mediated cell metabolism and immune responses.
2.1. Tumor hypoxia induces abnormal cell metabolism
The role of oxygen in the metabolic pathway of cells is paramount because cancer cells reside in nutrient‐ and oxygen‐deficient environments due to the rapid growth of tumors. 35 To maintain proliferation, cancer cells reconnect their metabolic pathways and find another way to maintain their own growth. Metabolic adaptation is a new feature of cancer. 36 , 37 This metabolic reprogramming is known as the Warburg effect in cancer cells and is mediated by HIF‐1. 38 , 39 , 40 Alterations in gene regulatory networks can drive the heterogeneous behavior of cancer metabolism. 41 Although some mutations have been identified, such as isocitrate dehydrogenase‐1 (IDH1) and IDH2, 42 , 43 mutations are only one of the reasons why hypoxia alters tumor metabolic pathways. Oxygen, glucose, and glutamine in the tumor microenvironment (TME) are considered to be the main nutrients that promote cell growth. 44 , 45 Hypoxia can provide energy to tumor cells by activating transcriptional programs that induce glucose uptake and glycolysis. 46 , 47 Tumor cells adaptively turn to androgen/androgen receptor‐independent pathways to survive and grow, resulting in therapeutic resistance. 48 This is mediated by glucose‐6‐phosphate isomerase (GPI), which is transcriptionally repressed by androgen receptors in response to hypoxia but recovers when androgen receptors are inhibited. GPI maintains glucose metabolism and energy homeostasis during hypoxia by redirecting glucose to hypoxia‐induced glycolytic pathways. 49 In response to hypoxia, pancreatic ductal cancer cells expressing activated Kirsten rat sarcoma viral oncogene homolog (KRAS) increase their expression of carbonic anhydrase 9 (CA9), which regulates cellular pH and is directly induced by hypoxia, by stabilizing HIF‐1α and HIF‐2α, thereby regulating pH and glycolysis. 50 The All1‐fused gene from chromosome 9 (AF9) is upregulated in HCC to recognize acetylated cMyc and promote the expression of hypoxia tolerance and glycolytic genes by forming a complex with HIF1α. 51 Macropinocytosis is an endocytic process caused by membrane folds, leading to phagocytosis of extracellular fluid (proteins), digestion of proteins, and subsequent incorporation into the cell biomass. Hypoxia supports macropinocytosis‐mediated cancer metabolic reprogramming through the HIF/EHD2 (EH dome‐containing protein 2) pathway and uses extracellular proteins as a nutrient for survival. 52
Glutamine is the most abundant amino acid in the blood and is critical for the synthesis of tricarboxylic acid cycle metabolites, nonessential amino acids, and adenosine triphosphate (ATP) energy, on which cancer cells and rapidly proliferating cells depend. 53 , 54 , 55 HIF‐2α‐induced expression of the glutamine transporter solute carrier family 1, member 5 (SLC1A5) mediates glutamine‐induced ATP production and glutathione synthesis and renders pancreatic cancer resistant to gemcitabine. 56 Glucose delivery is impeded in hypoxic regions of the body, which increases the cell's dependence on other carbon sources, such as L‐glutamine. Autophagy‐related 12 (ATG12) deficiency leads to decreased intracellular L‐glutamine levels during hypoxia, while cancer cell killing increases after L‐glutamine depletion. Tumor microenvironment analysis showed that ATG12 targeting resulted in a decreased tolerance to hypoxia, increased necrosis, and sensitivity to treatment. This suggests that ATG12 plays an essential role in maintaining L‐glutamine homeostasis. 57
Cell metabolism mainly refers to the metabolism of energy and substances. It is widely known that mitochondria are the energy reservoir in organisms. Many studies have focused on whether HIF regulates metabolic pathways that influence mitochondrial viability. 58 , 59 Mitochondrial serine hydroxymethyltransferase (SHMT2) is required for cancer cells to adapt to the tumor environment. 60 Jiangbin Ye proposed that HIF‐1 induces SHMT2 under hypoxia and that this induction is most pronounced in cells overexpressing the oncogenic transcription factor Myc. When these cells are hypoxic, they require SHMT2 expression to sustain the cellular NADPH/NADP+ ratio. Loss of SHMT2 in hypoxic cells increases reactive oxygen species (ROS) levels, resulting in cell death. 61 Inhibition of phospholipase C under hypoxia promotes the metabolic process of cancer cells, reduces the formation of mitochondrial ROS, and converts tumor bioenergy into glycolysis by inhibiting Ca2+ entry into mitochondria. 62 Collectively, the metabolic reprogramming associated with cancer has received much attention, and how cancer cells meet these needs in vivo is increasingly being studied. However, considering that the hypoxic TME and its interaction with metabolic processes are highly complex, tumor metabolism needs to be comprehensively evaluated. Hypoxia and tumor metabolism still need to be explored.
2.2. Tumor hypoxia promotes angiogenesis
In 1971, Professor Folkman proposed the theory that “tumor growth and metastasis depend on neovascularization”, which provided a new research direction and theoretical rationale for antiangiogenic antitumor drugs. 63 , 64 The mechanism of angiogenesis is complex, and there are many factors involved in promoting angiogenesis. More than 40 molecules have been identified to play a key role in vascular recruitment. 65 These include VEGF, transforming growth factor‐β (TGF‐β), platelet‐derived endothelial cell growth factor (PD‐ECGF), angiopoietin, HIF‐1, survivin, and EPO. 66 , 67 , 68 , 69 , 70 , 71 However, to date, most studies have focused on HIF and VEGF and their receptors. In the hypoxic environment of the internal tumor mass, the HIF‐1 dimeric complex remains stable and activates the expression of numerous genes responsible for the angiogenesis process. 71 HIF‐1‐induced proteins include VEGF, which promotes vascular permeability, and basic fibroblast growth factor (bFGF), which promotes endothelial cell growth. 72 , 73 Because of the essential function of angiogenesis in tumor development, the development of antiangiogenic drugs has been put on the agenda. The commonly used antiangiogenic drugs are bevacizumab, ramucirumab, and olaratumab, among others. 74 , 75 , 76 , 77 Traditional angiogenesis inhibitors mainly block VEGF signaling and prune rather than eradicate tumor vessels. 78 The expected antiangiogenic therapy has not been achieved thus far because these therapies are less effective against tumors with alternate blood supply sources. Angiogenesis inhibitors may be more beneficial in combination with other conventional chemotherapy agents. 79 , 80 , 81
2.3. Tumor hypoxia promotes metastasis
Hypoxia is associated with metastasis; however, how it affects metastasis progression is controversial. Currently, epithelial‐mesenchymal transition (EMT) has mostly been studied in relation to hypoxia and metastasis. EMT is a key process in the metastasis and colonization of cancer cells from the primary tumor to distant organs. Hypoxia plays an important role in triggering EMT by regulating HIFs. 82 , 83 , 84 Studies have demonstrated that HIF has a direct regulatory effect on EMT‐related proteins, such as zinc finger E‐box binding homeobox 1, Snail and Twist. 85 , 86 , 87 At the same time, HIF can also modulate microRNA (miRNA) to promote the cellular EMT process. 88 , 89 , 90 , 91 Many studies have reported the downregulation of drosha and dicer, two key enzymes involved in miRNA biogenesis. 92 , 93 Dicer is a key enzyme in miRNA maturation. HIF‐1α downregulates the expression of dicer by promoting ubiquitination of the E3 ligase parkin, which further inhibits known tumor suppressors, such as the microRNAs let‐7 and microRNA‐200B. Downregulation of dicer expression can significantly enhance cancer metastasis through microRNA‐200. 94 , 95 Hypoxia can promote EMT in tumor‐bearing mice by downregulating miRNA expression. 96 Recent studies have shown that CSN8, the smallest and most conserved subunit of the COP9 signalosome (CSN), is a key regulator of hypoxia‐induced EMT and dormancy, which endows colorectal cancer (CRC) cells with stronger invasion and metastasis abilities. 97
2.4. Tumor hypoxia accelerates DNA damage repair
Some cancer therapies kill tumor cells by causing DNA damage; however, cancer cells have been shown to activate a variety of repair mechanisms and signaling pathways as a response to overcome DNA damage. 98 The repaired cancer cells then become more resistant to standard treatment. The success of cancer treatment is limited by two factors: insufficient DNA damage during treatment and rapid DNA repair after treatment. 99 The ATM and RAD3‐related (ATR)/Chk1 and ataxia‐telangiectasia mutation (ATM)/Chk2 signaling pathways play key roles in the DNA damage response. 100 ATR/Chk1 signaling is usually activated by single‐stranded DNA or large‐volume DNA lesions, whereas the ATM/Chk2 pathway is activated by DNA double‐strand breaks. 101 After DNA damage caused by standard treatment, tumor hypoxia can drive DNA repair to allow tumor cells to continue to grow, giving rise to resistance to antitumor therapy. 102 The antiangiogenic agent cediranib inhibits the expression of the homology‐directed DNA repair (HDR) factor BRCA1/2 and RAD51 recombinase and promotes the sensitivity of the poly (ADP‐ribose) polymerase (PARP) inhibitor olaparib. The combination of cediranib and olaparib induces lethality by suppressing DNA repair. The activity of sildiranib as a DNA repair inhibitor has expanded its clinical utility. 103 There is a growing body of evidence that combination treatment with drugs with the ability to inhibit HDR (e.g., androgen receptor inhibitors) can modulate DNA repair, 104 , 105 with potential implications for the development of combination therapies aimed at exploiting DNA repair defects induced in cancer cells.
2.5. Tumor hypoxia promotes immune escape
Aside from obtaining an adequate energy supply, tumor occurrence and development must evade various immune surveillance mechanisms of the body. 106 The hypoxic TME can directly affect the biology of infiltrating immune cells and the response to therapy. (1) Hypoxia can directly upregulate the expression of various cellular immune checkpoints (such as programmed cell death‐ligand 1, PD‐L1) in the TME. 107 , 108 , 109 HIF‐1α interacts with histone deacetylase 1 (HDAC1) and simultaneously relies on polycomb repressive complex 2 protein with histone methyltransferase activity to induce chromatin remodeling, thereby leading to immune dysfunction. Hypoxic effects on immune cells also lead to insufficient PD‐L1 and PD‐L2 responses. 110 Targeting HIF‐1α with drugs or genes can inhibit PD‐L1 expression in the TME and enhance interferon production by T cells. 111 , 112 , 113 Targeting HIF‐1α reverses immune dysfunction and overcomes resistance to PD‐L1 blockade. 114 (2) Hypoxia maintains the function of myeloid‐derived suppressor cells (MDSCs), which have immunosuppressive activity, allowing cancer cells to escape immune surveillance and resist immune checkpoint blockade. 115 , 116 HIF‐1 induces extracellular nucleoside triphosphate diphosphate hydrolase 2 (ENTPD2/CD39L1) overexpression in hepatocellular carcinoma clinical specimens. ENTPD2 converts extracellular ATP to 5‐AMP, which prevents the differentiation of MDSCs and therefore promotes the maintenance of MDSCs. 117 HIFs can also activate the transcription of chemokine (C‐C motif) ligand 26 in cancer cells and recruit MDSCs expressing chemokine (C‐X3‐C motif) receptor 1 to primary tumors. 118 This suggests that targeting MDSC recruitment is an attractive approach for the treatment of solid tumors. (3) HIFs can also switch tumor‐associated macrophages (TAMs) from an antitumorigenic (M1‐like) phenotype to a protumorigenic (M2‐like) phenotype. 119 , 120 , 121 Mechanistically, HIF‐1α‐stabilizing long noncoding RNA (HISLA) can block the hydroxylation of HIF‐1α and inhibit the degradation of HIF‐1α by proline hydroxylase 2 (PHD 2). Simultaneously, lactate released from tumor cells upregulates HISLA in macrophages, and blocking the transmission of HISLA in vivo can inhibit drug resistance in breast cancer. 122 HIF‐1α can also induce TAMs to secrete IL‐23, which activates the proliferation of regulatory T cells (Tregs), promotes the expression of IL‐10 and TGF‐β, and thus inhibits the capacity of cytotoxic lymphocytes to kill tumor cells. 123 Specific deletion of HIF‐2, but not HIF‐1, in cancer‐associated fibroblasts, improve pancreatic cancer patient survival by reducing the recruitment of immunosuppressive macrophages. Treatment with the clinical HIF‐2 inhibitor PT2399 significantly reduced macrophage chemotaxis and M2 polarization in vitro. 124 (4) Does hypoxia inhibit T‐cell function? The effects of hypoxia on T cells are controversial. Some findings have indicated that O2 is required for T‐cell activation and the development of effector functions and that HIF‐1α acts as a negative regulator of T‐cell responses. 125 For example, tumor hypoxia activates the γδT‐cell protein kinase A pathway at the transcriptional level, resulting in the inhibition of the activating receptor natural killer cell group 2D (NKG2D). Alleviating tumor hypoxia enhanced the expression of NKG2D and enhanced the antitumor function of γδT cells. 126 The combination of the hypoxia‐activated prodrug TH‐302 and checkpoint blockade of PD‐L1 or cytotoxic T lymphocyte‐associated antigen‐4 can restore the killing function of T cells, and the tumor cure rate was more than 80%. 127 Hypoxia downregulates nuclear receptor coactivator 3, an epigenetic factor that maintains the basic level of cGMP‐AMP synthase (cGAS) expression, which leads to the inhibition of cGAS, thereby inhibiting the function of T cells. 128 Other studies using CD8+ T cells during resting periods of hypoxia or VHL‐deficient cells have reported that HIF‐1α signaling increases T‐cell function. 129 , 130 , 131 For example, 2‐hydroxyglutarate (2‐HG) is produced by CD8+ T lymphocytes in response to T‐cell receptor triggering and environmental hypoxia, and 2‐HG significantly boosts the proliferation, persistence, and antitumor capacity of CD8+ T lymphocytes in vivo. 132 VEGF‐A production by tumor cells is associated with a poor prognosis; however, deletion of the HIF target gene VEGF‐A in CD8+ T cells accelerated tumorigenesis and altered angiogenesis. The deletion of T‐cell‐specific genes demonstrated that HIF‐1α is an important regulator of T‐cell effector responses in the TME. Therefore, VEGF‐A in effector CD8+ T cells contributes to T‐cell infiltration. 133
3. HYPOXIA‐TARGETED THERAPEUTICS IN CANCER
Since the discovery of the hypoxic tumor environment, sensitization of hypoxic cells in tumors and improvement of the hypoxic tumor environment have been the focus of tumor research. 134 , 135 , 136 Hypoxia is arguably one of the most intriguing therapeutic targets for cancer. 137 , 138 The primary strategies for treating hypoxic tumors are to release hypoxia‐targeted drugs and improve the hypoxic environment. In this subsection, we review hypoxia‐targeted tumor therapy methods. Multiple targeting pathways have been proposed for hypoxic tumor cells, including specific targeting of HIFs, 139 , 140 , 141 gene therapy, 142 , 143 , 144 targeting hypoxic tumors, and targeting pathways that have important effects on hypoxic cells, such as the mTOR pathway. 145 , 146 In addition, because tumor tissue has a lower pH than healthy tissues, this special characteristic can be used to target acids to induce tumor death. 147 , 148 , 149
3.1. HIF inhibitors
HIFs are significantly upregulated in many cancer types, and the application of HIF inhibitors as anticancer therapies has captured great interest. 150 The oxygen‐dependent activity of HIFs is mainly transmitted by HIF‐α subunits. In human cells, there are three HIF‐α subtypes, among which HIF‐1α and HIF‐2α are the most widely studied. The first inhibitor of HIF‐1 discovered was camptothecin, which inhibits HIF‐1 through a mechanism that blocks protein accumulation. 151 Subsequently, a novel tricyclic carboxamide inhibitor of HIF‐1α, NSC 644221, was developed. NSC 644221 inhibits HIF‐1α protein expression in a time‐ and dose‐dependent manner. However, it does not inhibit HIF‐1β protein expression. 152 Px‐478, which has entered clinical stage I, plays an antitumor role by inhibiting HIF‐1α deubiquitination, leading to polyubiquitinated HIF‐1α degradation. 153 , 154 HIF‐2α contains the Per‐Arnt‐Sim B (PAS‐B) domain, and this particular PAS domain contains a relatively large cavity that can be occupied by water or small molecules, including PT2385. 155 , 156 PT2385 is a HIF‐2α antagonist that inhibits the expression of HIF‐2α by disrupting HIF‐2α/HIF‐1β (ARNT) heterodimerization. PT2385 has passed phase I clinical trials. 140 , 157 Belzutifan (MK‐6482) is a second‐generation small‐molecule HIF‐2α inhibitor that outperforms the pharmacological properties of the first‐generation compound PT2385 and has shown efficacy and safety in phase III trials in patients with advanced clear cell renal cell carcinoma. 24 , 72 , 158 James Brugarolas designed a selective HIF‐2α antagonist, PT2399, based on the structure of HIF‐2α PAS‐B; PT2399 selectively disrupts the heterodimerization of HIF‐2α and HIF‐1β. 159 Many compounds have been shown to antagonize HIF activity, including echinomycin, 160 17‐allylaminogeldanamycin, 161 acriflavine, 162 and the thioredoxin redox inhibitors 1‐methylpropyl 2‐imidazolyl disulfide 163 and 3‐(5′‐hydroxymethyl‐2′‐furyl)‐1‐benzylindazole (YC‐1). 164 The anticancer effect of these drugs is likely due to HIF inhibition; however, these drugs do not specifically target HIFs. Meanwhile, many of these compounds cannot be used as HIF inhibitors due to side effects or failure in clinical trials. Because HIFs are highly expressed not only in cancer cells but also in some normal tissues in some cases, it is unclear whether drugs targeting HIFs could have serious side effects in humans. Hopefully, clinical studies will provide an answer.
3.2. Gene therapy
Gene therapy is a promising strategy for the treatment of various genetic and acquired diseases. However, to minimize the side effects of gene therapy, gene expression must be targeted to specific tissues. 165 HIFs accumulate in anoxic tissues and have three domains: the DNA binding domain, transcription activation domain, and oxygen‐dependent degradation (ODD) domain. 166 An attractive strategy to inhibit HIF activity is to disrupt the HIF‐1α/P300 complex, as P300 is a key coactivator of hypoxia‐induced transcription. Blocking the interaction between HIF‐1 and the P300 region attenuates the expression of HIF‐1. 69 The ODD domain contains proline residues that are recognized and hydroxylated by proline hydroxylase (PHD 1–3). Prolyl hydroxylation promotes HIF‐α binding to the VHL complex, resulting in chromosomal degradation of HIF proteins. 167 IDH1 catalyzes the oxidative decarboxylation of isocitrate to α‐ketoglutaric acid (α‐KG). The stability of HIF‐1α is regulated by α‐KG, and increasing α‐KG reduces the expression of HIF‐1α. 168 Gene therapy may be one of the most promising cancer treatment strategies. Tumorigenesis is the result of genetic alterations and accumulation. Therefore, intervention with gene therapy for these genetic alterations may alleviate tumor growth.
3.3. Targeting hypoxic tumors
Researchers have harnessed the properties of hypoxic cells, targeting them with hypoxia‐activated prodrugs (HAPs) or bioreductive agents. 169 Appropriate prodrugs must diffuse efficiently to distant and hypoxic tumor regions and then specifically convert to the cytotoxic active form and diffuse over limited distances to kill adjacent hypoxic tumor cells. 170 For example, Tirapazamine (TPZ) is a bioreduction‐activated drug among a hypoxia‐selective benzotriazine series of antitumor drugs. 171 In a phase II trial of TPZ in combination with cisplatin and radiotherapy for advanced squamous cell carcinoma of the head and neck, TPZ enhanced the cytotoxicity of radiotherapy and cisplatin in the presence of hypoxic tumors. 172 In addition, Vlahovic demonstrated that the phosphorylated platelet‐derived growth factor receptor β (PDGFR‐β) inhibitor imatinib, in addition to inhibiting p‐PDGFR‐β, can also downregulate VEGF and reduce intratumoral interstitial fluid pressure in lung adenocarcinoma. 173 HAPs are important drugs for the treatment of hypoxic tumors because they have cytotoxic effects only in hypoxic environments and show reduced side effects in normal tissues under normoxic conditions. Although HAPs can kill hypoxic cells, insufficient tumor hypoxia limits their efficacy.
Multifunctional nanoparticles can integrate various key components, such as drugs, genes, and targeting ligands, using unique delivery platforms. Due to the enhanced permeability and retention effect (EPR effect), 174 selective accumulation in solid tumors can be used to target hypoxic tumors more effectively. 175 Bu et al. proposed a strategy of X‐ray‐induced nitrosation stress based on zeolitic imidazole framework‐82 with the assistance of polyvinyl pyrrolidone for the treatment of hypoxic prostate cancer. The proliferation of hypoxic prostate cancer cells was effectively inhibited by the continuous release of the hypoxic response ligand and Zn2+. 176 Live photosynthetic bacteria (PSB) have been used as hypoxia‐targeting vectors for the treatment of hypoxic tumors due to their near‐infrared chemotaxis and facultative aerobic physiological properties. PSB “integrates” the characteristics of hypoxia‐targeting agents and photothermal therapeutic agents and can achieve hypoxia‐targeted cancer therapy without later modification. 177 Lu integrated losartan (LOS), doxorubicin (DOX), and Fe ions into a system. LOS can reduce intratumoral collagen and alleviate tumor hypoxia. The Fenton reaction triggered by Fe ions promotes ROS production in tumors, and DOX enters tumors and inhibits tumor growth. 178 E6 chloride loaded with anoxic amphipathic dendritic nanoparticles can target hypoxic tumors and activate dendritic cells, induce strong antigen‐specific immune memory effects, and prevent tumor metastasis and recurrence in vivo. 179 Recently, a nanoparticle‐assisted immunometabolic therapy strategy targeting the ATP‐adenosine axis has been proposed to increase extracellular ATP levels while preventing the accumulation of immunosuppressive adenosine and alleviating hypoxia. 180 We propose that HAPs can be used in combination with nanomedicine to obtain a mutually beneficial nanosystem. For example, HAPs have been used in combination with vascular‐disrupting agent (VDA) nanomaterials for the effective treatment of solid tumors. This approach selectively enhanced tumor hypoxia and TPZ in the treatment of metastatic 4T1 breast tumors. 171 HAPs plus nanomedicine can be used for the synergistic treatment of tumors.
3.4. Others
Treatments targeting key components of tumor hypoxia signaling pathways, including mTOR, are one of the current approaches to cancer therapy. 181 , 182 mTOR activity promotes angiogenesis by regulating HIF‐1α transcription and translation. 183 The mTOR inhibitor everolimus, which has been tested in phase III clinical trials, can prolong the progression‐free survival of patients with advanced renal cell carcinoma by inhibiting the promotion of HIF‐1 by mTOR. 184 The dual inhibition of PI3K/mTOR by apitolisib did not have more effective antitumor activity than mTOR inhibition alone, possibly because the complete blockade of PI3K/mTOR signaling led to multiple adverse events. The beneficial effect of the mTOR inhibitor alone may be due to the inhibition of HIF‐1α. 185 Of course, more experiments are needed to verify this hypothesis. Hypoxic tumor cells show reduced intracellular pH compared with normal cells because tumors exhibit elevated metabolism, an acidic pH, and nutrient and oxygen deficiencies. 2 , 186 Nanoparticles responsive to the acidic TME can target hypoxia and be used in cancer therapy. Layer‐by‐layer (LbL) nanoparticles exhibit a pH‐sensitive outer layer that has been shown to target and be retained in hypoxic tumor areas. The neutral layer is shed in acidic environments, exposing a surface of charged nanoparticles, which can be easily absorbed by tumor cells. 187 Low oxygen and pH levels induce hyaluronic acid LbL nanoparticles to effectively target and penetrate hypoxic cells in vitro and in vivo. 188
Hypoxic TME is still one of the major causes of resistance to radiotherapy, chemotherapy, and immunotherapy, 189 and targeting hypoxic cells and improving tumor oxygenation have been a major focus of tumor therapy. In the above section, we introduced therapies targeting hypoxia. Next, we will focus on tumor therapies that can improve tumor oxygenation.
4. WAYS TO IMPROVE INTRATUMORAL OXYGENATION
To date, several methods have been proposed to increase the oxygen content of tumor tissue (Figure 2), including normobaric hyperoxia respiration, 190 hyperbaric hyperoxic respiration, 191 and carbogen inhalation 192 to promote tumor oxygen partial pressure (pO2); nanomaterials to deliver/produce oxygen 193 , 194 ; hyperthermia to increase tumor oxygen by increasing blood flow within the tumor 195 ; antihypoxic drugs to improve tumor oxygenation by killing hypoxic cells 196 ; antiangiogenic drugs 197 ; and promotion of oxygen accumulation by inhibiting tumor cell respiration. 198
FIGURE 2.
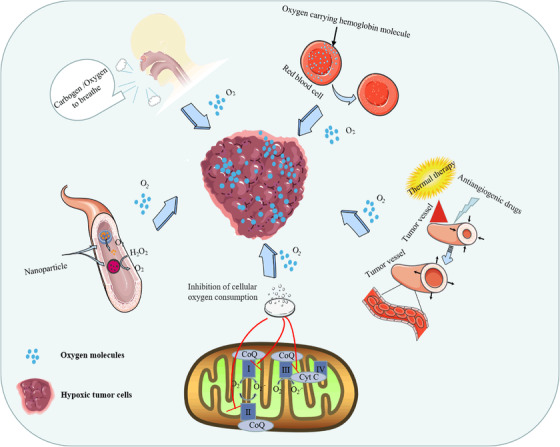
Schematic diagram of methods to improve tumor oxygenation. Tumor oxygenation improvement methods include the uptake of high‐concentration oxygen (normobaric/hyperbaric) or carbogen to increase vascular oxygen concentration, thereby increasing tumor oxygen partial pressure to reverse tumor hypoxia. Hemoglobin is an oxygen carrier that releases oxygen in tumor hypoxic tissue, and hyperthermia is used to increase blood flow by expanding blood vessels and leads to increases in oxygenation of the tumor. Antiangiogenic drugs normalize the overall function of tumor vessels to improve tumor blood supply and increase the tissue oxygen content. Drugs inhibit cellular respiration by inhibiting the oxidative respiratory chain, reducing the rate of oxygen consumption, and indirectly improving tumor oxygenation. Nanoparticles carry oxygen or catalyze the decomposition of H2O2 to obtain oxygen to relieve hypoxia in tumor tissue
4.1. Respiratory hyperoxia at normobaric pressure enhances the pO2
For normobaric hyperoxic respiration, in a standard atmosphere with an oxygen content between 20% and 100%, mice were treated with 60% O2 indefinitely without signs of toxicity. 199 , 200 However, long‐term respiratory hyperoxia may have adverse effects on multiple organ systems exposed to O2 > 60%. Sustained hyperoxia (>60% oxygen) normally results in pulmonary and ocular toxicity due to oxidative damage to endothelial and epithelial cells. 201 The initial use of hyperoxia therapy in cancer was to alleviate postoperative hypoxia in rectal and sigmoid cancers. 202 Respiration with 100% O2 (1 h) alleviates hepatic hypoxia by inhibiting HIF‐2α expression. 203 Multiple studies have been conducted showing that respiratory hyperoxia improves tumor oxygenation, which can have multiple beneficial effects as a cancer treatment modality. 204 , 205 Hyperoxia (60% O2) can also reverse the immunosuppressive TME and promote antitumor immunity. 206
4.2. Hyperbaric hyperoxic respiration enhances the tumor pO2
Hyperbaric hyperoxic respiration treatment involves exposure to a high level of oxygen at a higher atmospheric pressure The oxygen pressure is much higher than at sea level. The pressure at sea level is defined as standard atmospheric (ATM) pressure, a value of 101.325 kPa, which can increase the oxygen tension of tissue or venous blood vessels, likely resulting in increased oxygen concentration. The specified criteria in hyperbaric oxygen treatment (HBOT) stipulate that patients are offered pure oxygen (100%) for 2–3 h under 1.5–2.5 absolute atmospheres (ATA). 207 At present, respiratory hyperoxia is widely used as an adjuvant therapy for various pathological conditions, principally those related to hypoxia and/or ischemia. 208 In triple‐negative breast cancer, HBO treatment can alleviate tumor hypoxia, thereby inhibiting tumor growth and metastasis. 209 HBO can inhibit the hypoxia‐activated signal transducer and activator of transcription 3 (STAT3), slowing tumor growth and drug resistance. 210 As early as 1968, Churchill‐Davidson used HBO to relieve hypoxia and promote radiotherapy. 211 Based on clinical results, HBO has the effect of improving tumor oxygenation, and the use of HBO during radiotherapy has been proven to improve the radiation response of solid tumors. 212 In addition, HBO can promote the efficacy of cytotoxic drugs. 213 , 214
4.3. Delivery of nanoparticle oxygen‐generating/oxygen‐carrying systems to alleviate tumor hypoxia
Various researchers have made substantial efforts devoted to the development of different nanocarriers in the hope of using nanotechnology to deliver oxygen to reverse tumor hypoxia and improve the efficacy of existing treatments. 194 , 215 , 216 These methods can be simply divided into the following groups:
Oxygen‐loaded nanocarriers: Oxygen‐containing nanomaterials can directly deliver oxygen to hypoxic tissues and alleviate hypoxia. Researchers have developed a variety of nanomaterials with excellent oxygen‐carrying capacity, as shown by the following examples.
Perfluorinated compounds to deliver oxygen: Fluorine has low polarizability; thus, the low polarizability is converted to low intermolecular van der Waals forces, and van der Waals forces are the intermolecular forces that hold nonpolar molecules together. Accordingly, liquid perfluorocarbons behave close to ideal, gas‐like liquids. 217 , 218 Perfluorinated compounds are synthetic biologically inert compounds, and aqueous emulsions of fluorinated hydrocarbons are capable of dissolving large amounts of oxygen. 219 The only perfluorochemical O2 carrier approved for clinical use by the Food and Drug Administration (FDA) is fluorocarbon‐DA (Fluosol‐DA), but it has been withdrawn from the market due to its cumbersome implementation and limited efficacy. 220 Other perfluorochemical emulsions are being studied as oxygen transporters to alleviate tumor hypoxia. 221 , 222 The oxygen‐generating potential of several nanocomposite fluorine materials provides an extra strategy to enhance tumor oxygenation, supplemented by oxygen‐carrying nanocarriers to enhance tumor tissue oxygen levels and currently available therapies to overcome hypoxia‐related resistance and metastasis. 223 , 224
Hemoglobin‐based oxygen carriers to deliver oxygen: Hemoglobin‐based oxygen carriers (HBOCs) were originally developed to provide an alternative to blood transfusions. As the realization that hemoglobin solution is not only a substitute for erythrocytes but also has additional properties, including hemodynamic effects related to its osmotic pressure, nitric oxide scavenging effects, and effects on oxygen delivery and microcirculation, the broader concept of “hemoglobin therapy” was born. 225 Because HBOCs can regulate oxygen delivery in the circulatory system, hemoglobin therapy is a promising oxygen therapy that can enhance the efficacy of other tumor therapies, such as radiotherapy and chemotherapy. 226 , 227 , 228 , 229 , 230 Although HBOCs have been studied for decades and have undergone several generations, no HBOCs have yet been clinically approved. This is mainly due to side effects associated with HBOCs that have not been addressed, such as nephrotoxicity and elevated hypertension. 231
Oxygen‐producing nanocarriers: Excess hydrogen peroxide (H2O2) exists in the hypoxic microenvironment of tumors, which greatly limits the efficacy of tumor therapy. Materials such as catalase (CAT) 232 , 233 , 234 and manganese dioxide (MnO2) 235 , 236 can use H2O2 as a raw material to generate abundant oxygen, acting as oxygen generators to improve tumor oxygenation and existing therapeutic efficacy. 237 Song designed a novel bionanoreactor to decompose tumor endogenous H2O2 and relieve tumor hypoxia by encapsulating CAT in tantalum oxide (TaOx) nanoshells, resulting in TaOx@Cat nanoparticles. 238 Lalit Chudal encapsulated protoporphyrin IX in a liposome bilayer and then coated the lipid bilayer with MnO2 nanoparticles to improve the efficacy of photodynamic therapy under tumor hypoxia. 239 Therefore, integrating CAT and MnO2 into bionanoreactors provides an attractive nanotechnological route to effectively enhance tumor therapy.
Over the years, nanomaterials have been used as delivery platforms and in vivo diagnostic carriers due to their unique nano properties and physical and chemical properties, and many achievements have been made. Blood vessels in tumor tissues are very different from those in normal tissues and have a higher density. There is a large gap between vascular endothelial cells, and the blood vessels of tumor tissue have an EPR effect; therefore, nanomaterials can target tumor tissue and improve the accumulation of drugs in tumor tissue. Compared with atmospheric hyperoxia and hyperbaric hyperoxia, which improve tumor pO2, the delivery of nanomaterials can more specifically target tumor tissues and improve tumor oxygenation.
4.4. Hyperthermia increases tumor oxygenation by increasing the intratumoral blood flow supply
Hyperthermia (HT) is defined as an increase in the body tissue temperature above the normal physiological range, approximately 39–45°C. 240 In solid tumors, hyperthermia increases tumor blood flow and increases the tumor perfusion fraction in a temperature‐ and time‐dependent manner. Variations in blood circulation in tumors can create dramatic physiological changes, including enhanced vascular permeability, increased oxygenation, decreased interstitial fluid pressure, and restoration of normal physiological pH. 241 In the 1970s, a study of the effect of 45°C isotonic lavages of the bladder on proliferative transitional cell carcinoma showed a clear anticancer effect of hyperthermia. 242 HT therapy for cancer treatment has primarily focused on localized hyperthermia (i.e., heating the area surrounding the tumor), and existing clinical data suggest that the use of localized hyperthermia in combination with conventional radiotherapy may be valuable. 243 , 244 Other research groups have also investigated the application of systemic hyperthermia in antitumor therapy. 245 , 246 , 247 From these studies, two main whole‐body hyperthermia regimens have emerged: (1) the traditional short‐duration hyperthermia regimen, that is, core body temperature rises to 41.88°C for 2 h; (2) a mild hypothermic long‐duration regimen, that is, core body temperature rises to 39.5‐40.8°C for 6 h or more. Sun demonstrated that the effect of HT (41°C, 30/45 min) on tumor oxygenation was different in different regions in a xenograft model. Overall, tumor oxygenation improved during and after heating, but the effect was transient. 248 Thrall et al. treated 37 cases of spontaneous canine tumors with fractions of an equivalent total thermal dose of 30 CEM43T90 (a thermodosimetric measure, taking into account the 90th percentile temperature, not the median temperature of CEM43) divided weekly, and the animals received 1 or 3–4 divided treatments. Compared with baseline, the tumor PO₂ was significantly increased in both treatment groups. 249
HT increases the local temperature of the tumor, accelerates the circulation, and increases oxygen saturation, thus increasing the oxygen content of the tumor, increasing the number of cells in the sensitive phase of the tumor, and reducing the number of hypoxic cells in the center of the tumor. No damage to the surrounding normal tissue has been observed. As a cancer treatment method, studies have shown that HT can improve the efficacy of conventional cancer treatments, especially radiotherapy. 250 These studies suggest that further research on the antitumor effect of HT on tumor oxygenation enhancement is merited on a larger scale.
4.5. Carbogen respiration induces tumor hyperoxia
In the 1960s, Dusault explored the use of carbogen respiration in C3H breast tumor xenografts and found improvements. Studies have shown that carbogen improves blood flow and oxygenation more than pure oxygen because respiratory stimulation and vasodilation from carbon dioxide deliver more oxygen to the tumor. 251 Other studies have also supported this conclusion. 252 , 253 , 254 Carbogen respiration is defined as the inhalation of a 95% O2 + 5% CO2 mixture at standard atmospheric pressure. 255 Inhalation of different CO2 concentrations showed different effects on the time course of tumor oxygenation changes. The perfusion recovered to its starting value immediately after the end of hyperoxia (pure oxygen), whereas with high concentrations of CO2, the perfusion remained elevated for at least 30 min. Higher inhaled CO2 concentrations (2.5% or 5%) prolonged the time to improvement in tumor perfusion compared with pure oxygen respiration. 256 Carbogen respiration appears to have a greater effect on tumor oxygenation than respiration hyperoxia because carbogen induces vasodilation and increases blood flow to facilitate oxygen delivery. 191 Nevertheless, the results of carbogen respiration were inconsistent, which may be due to the inconsistent timing of patients receiving carbogen respiration.
4.6. Tumor vascular normalization to improve tumor oxygenation
The concept that growing tumors have a rich vascular network first emerged through the observations of the well‐known scientist Rudolf Virchow. 257 In 1939, Gordon and colleagues studied the relationship between tumor growth and blood supply in rabbit grafts, and they observed that tumor growth was accompanied by the rapid and extensive formation of new blood vessels. 258 Therefore, antiangiogenic drugs have a synergistic antitumor effect in combination with radiotherapy and chemotherapy in clinical practice. 259 , 260 , 261 , 262 , 263 However, hypoxia caused by the abnormal vascular state of tumors is the main cause of reduced radiotherapy and chemotherapy effectiveness, which contradicts the clinical facts. With the proposal of the “vascular normalization” theory, accumulating evidence has shown that antiangiogenic drugs normalize the overall function of tumor vessels before disrupting tumor vessels to improve tumor blood supply and increase the tissue oxygen content. 259 , 264 With the discovery of VEGF as a major driver of tumor angiogenesis, new therapies to inhibit VEGF activity have been studied. 265 In 1993, it was reported that treatment with anti‐VEGF monoclonal antibodies resulted in a significant reduction in vessel density and a significant delay in tumor growth in nude mice carrying rhabdomyosarcoma, glioblastoma multiforme (GBM), and leiomyosarcoma xenografts. 266 The efficacy of anti‐VEGF monotherapy in human solid tumors is generally less than satisfactory, 267 , 268 with only moderate objective response rates and meaningless survival differences in phase 3 clinical trials. 269 In metastatic CRC, the overall response rate of patients receiving bevacizumab (a monoclonal antibody to VEGF) was 3.3%. 270 Similarly, the response rate among patients with metastatic breast cancer treated with the drug alone was 6.7%. 271 In contrast, the addition of bevacizumab to standard first‐line chemotherapy regimens significantly improved progression‐free survival. 272 , 273 , 274 In conclusion, antiangiogenic drugs improve tumor blood supply and oxygen content, and this idea of vascular normalization provides a new direction for tumor treatment. Reasonable combinations of antiangiogenic drugs with radiotherapy and chemotherapy provide a feasible solution for tumor chemoradiotherapy resistance.
4.7. Promotion of oxygen accumulation via inhibition of tumor cell respiration
Inhibition of cellular respiration oxygen consumption provides a different type of intratumoral oxygen supply protocol to conserve endogenous oxygen and overcome hypoxia. 135 Cellular respiration is the main process by which living cells consume oxygen. The antimalarial atovaquone is a ubiquitin analog that reduces the oxygen consumption rate (OCR) by inhibiting mitochondrial complex III and reducing intratumoral hypoxia. 275 In addition, studies have shown that metformin (Met), a typical antidiabetic drug, also alleviates tumor hypoxia by reducing oxygen consumption by inhibiting complex I in the mitochondrial electron transport chain. 276 , 277 , 278 Hypoxic tumor cells mainly use glucose to produce glycolytic energy and release lactic acid, which can fuel the oxidative metabolism of tumor cells. Therefore, targeting lactate can selectively kill hypoxic tumor cells. 279 3‐Bromopyruvate inhibits hexokinase‐II activity, thereby inhibiting mitochondrial respiration and glycolysis and reducing mitochondrial oxygen consumption and ATP production. 280 Yu constructed a vesicular photodynamic therapy (PDT)‐specific PV‐TS nanoeconomizer, and an oxygen‐saver concept was proposed. PV‐TS can respond to tumor reduction conditions by releasing NO to inhibit cellular respiration, thereby reducing oxygen consumption and enabling biochemical redistribution of cellular oxygen resources to conserve oxygen. 281 Therefore, inhibition of mitochondrial respiration to reduce tumor oxygen consumption has become an alternative strategy to alleviate the hypoxic microenvironment.
5. ANTITUMOR EFFECTS OF HYPEROXIA THERAPY
Lung ventilation is the beginning of the respiratory function and the primary factor determining the efficiency of respiratory function. 282 , 283 Respiratory hyperoxia increases oxygen saturation in the lungs, increasing oxygen supply to the body. 284 Oxygen therapy is one of the most widely used therapies in modern medicine and is a frequently performed intervention for critically ill patients. 18 , 285 In 1951, hyperoxia was used as adjuvant therapy in the postoperative treatment of rectal cancer and sigmoid colon cancer and was able to slow the occurrence of postoperative shock. 202 In this section, the antitumor effects of hyperoxia therapy in the treatment of pulmonary tumors and non‐pulmonary tumors are the focus, and the landmark events in the application of hyperoxia for tumor treatment are outlined in Figure 3.
FIGURE 3.
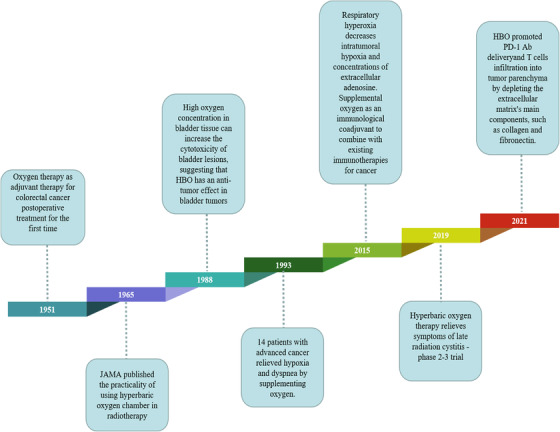
A timeline of respiratory hyperoxia discoveries and research history, highlighting key findings
5.1. Hyperoxia therapy in pulmonary cancer
In 1956, Heston and Pratt began to study the role of oxygen in lung tumors, and the results showed that elevated oxygen uptake increased lung tumor initiation in mice. 286 The application of early hyperoxia in tumor therapy is controversial, and Lindenschmidt et al. demonstrated that sustained exposure to 70% oxygen in mice inhibited the development of lung tumors. 287 , 288 Conversely, different groups have observed that respiration hyperoxia can act as a promoting stimulus to induce lung tumorigenesis of neuroendocrine tumors. 289 , 290 , 291 , 292 , 293 , 294 Initially, the effect of hyperoxia on cancer cells was poorly characterized. With the deepening of hyperoxia research and the understanding of the hypoxic TME, respiration hyperoxia was not thought to promote tumor development. Margaretten demonstrated that the effect of hyperoxia on lung tumors depends on the oxygen tolerance of lung tumors, and hyperoxia has a more pronounced inhibitory effect on oxygen‐sensitive cells. 295 Witschi also provided an explanation for the role of hyperoxia in tumor development. Exposure to hyperoxia before or after drug administration usually promotes tumor development, and further exposure to hyperoxia for a period of time begins to suppress tumor development, but the specific mechanism has not been deeply explored. 296 Liu demonstrated that intracellular metabolic reprogramming driven by the MYC/SLC1A5 axis is the main mechanism by which hyperoxia (60% O2, 6 h/day) inhibits lung cancer metastasis. 297 The antitumor effect of atmospheric hyperoxia may be due to oxidative stress and increased apoptosis (BCL2‐associated X [Bax]/Bcl‐2 mRNA expression rate), which is associated with the mitogen‐activated protein kinase (MAPK) pathway. 298 , 299 In the early stage, the antitumor effect of hyperoxia therapy was mainly studied in lung tumors. With in‐depth studies, scientists have gradually explored the expanded antitumor effect of hyperoxia therapy by increasing the investigated tumor types.
5.2. Hyperoxia therapy in non‐pulmonary cancer
Improved tumor oxygenation also has a suppressive effect on non‐pulmonary tumors. Studies have shown that atmospheric and hyperbaric hyperoxia can reduce the hypoxic area of tumors to varying degrees. 300 , 301 , 302 , 303 The EPO levels in hepatoblastoma HepG2 cells treated with 5% O2 and 1% O2 were 2.5 and 4 times higher than those in cells treated with 20% O2, respectively. In contrast, hyperoxia (40% O2) significantly inhibited EPO production. 304 Hypoxia also overexpresses/activates STAT3, leading to tumor progression and drug resistance. Human ovarian cancer xenograft mice were exposed to HBO (100% oxygen, 2 ATM, 1.5 h) for 21 days. Mice exposed to HBO had significant reductions in tumor volume, but no effect on body weight was observed. After HBO exposure, STAT3 (Tyr 705) activation and cyclin‐D1 protein/mRNA levels were significantly reduced. 210 Tumor cells and stromal cells were isolated, and the isolated cells had differential gene expression. HBO (2.5 bar, 100% O2, 90 min) had a significant inhibitory effect on tumor growth, and the MAPK pathway was significantly downregulated. An antiangiogenic effect was observed after intermittent HBO treatment. 305 To date, several reviews have been published on the use of respiratory hyperoxia in cancer therapy. 306 , 307 , 308 , 309 , 310
The different responses of cancer cells and normal cells to hyperoxia also provide a principle of action for hyperoxia in cancer therapy. Little attention has been given to the differences in the way normal cells and cancer cells respond to changes in oxidative stress. The basic antioxidant defense level is usually abnormal in tumor cells. Glutathione (GSH), an important antioxidant in the body, can scavenge free radicals and prevent the induction of ROS damage. 311 In normal cells, the GSH concentration is proportional to ambient oxygen tension. Compared with normal cells, tumor cells exhibited higher GSH concentrations under low oxygen tension but were unable to increase GSH with elevated oxygen tension. Tumor cells showed higher sensitivity to hyperoxia‐induced increases in ROS activity, while normal cells were not damaged by ROS due to elevated GSH. 312 Similarly, Kim and co‐workers demonstrated that the reduced GSH/oxidized GSH (GSSG) ratio was significantly reduced after hyperoxia in both normal and cancer mouse groups. After 24 h of hyperoxia, ROS levels in cancer cells were significantly increased compared with those in normal cells. This result suggests that oxidative stress is more pronounced in cancer tissues exposed to hyperoxia than in normal tissues exposed to hyperoxia and that an increase in oxidative stress above the toxicity threshold leads to cancer cell death (Figure 4A). 313
FIGURE 4.
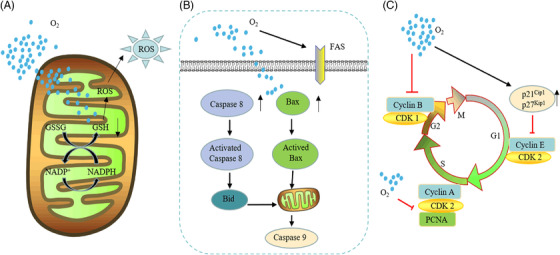
Antitumor effect of hyperoxia. Respiratory hyperoxia can through the following mechanisms. (A) Hyperoxia downregulates the concentration of glutathione in tumor cells, leading to intracellular oxidation‐reduction imbalance, excessive reactive oxygen species (ROS) production, and cell damage. (B) Hyperoxia promotes the increase of apoptosis‐related proteins Bax and Caspase 8, breaking mitochondria, and cell death. (C) The strong decrease in tumor cell proliferation under hyperoxia is associated with a marked inhibition of cell cycle progression, which is mainly characterized by the arrest in the G1, G2, and S phases. This G1/S phase arrest is associated with severe inhibition of cyclin‐dependent kinase 2 (CDK2) activity and DNA synthesis, and cell cycle progression from G1 to S is also inhibited. At the same time, hyperoxia induces cell arrest in the G2 phase by preventing the dimerization of cyclin B and the CDK1 complex and promotes cell apoptosis
Apoptotic factors are also involved in the necrosis of tumor cells under the hyperoxia response, and the activation of hyperoxia‐induced apoptosis factors (Bax) can lead to cell death with or without loss of mitochondrial function (Figure 4B). 314 , 315 Hyperoxia also has inhibitory effects on the cell cycle. Hyperoxia exposure (95% O2, 40–64 h) induces cyclin‐dependent kinase 2 activity and acute inhibition of DNA synthesis. The S‐phase block also has different degrees of blockade in the G1 and G2 phases (Figure 4C). 316 , 317 , 318 , 319 Multiple reports have suggested that reduced oxygen tension can induce cancer growth by adapting malignant cells to hypoxia, producing antitumor immunity and maintaining and promoting cancer progression. 46 , 52 , 126 , 320 Hyperoxia can overcome tumor tissue proliferation 321 and the issue of tumor development 322 , 323 , 324 by alleviating the hypoxic environment, inducing glandular loss, causing DNA damage, and promoting mesenchymal‐to‐epithelial transition (MET). 325
According to current studies, respiratory hyperoxia has inhibitory effects on lung tumors and other tumor types, with no significant difference in effect. Respiratory hyperoxia can alleviate tumor hypoxia and inhibit tumor progression through various mechanisms (Table 1). Although respiratory hyperoxia has been shown to be an effective way to eliminate tumor hypoxia, there are obvious limitations to oxygen transport and delivery far from blood vessels in the chaotic TME. Therefore, we recommend combining oxygen with other clinical treatment options. We believe that oxygen is an effective tool for improving radiotherapy and chemotherapy to maximize tumor rejection of current cancer therapies.
TABLE 1.
Summary of antitumor effects of hyperoxia in cancer therapy
| Year | Tumor type | Type of hyperoxia therapy | Molecular basis | Reference |
|---|---|---|---|---|
| 1989 | Hepatoblastoma | Atmospheric hyperoxia (40% O2) | Erythropoietin (EPO) | Ueno et al. 304 |
| 1998 | Breast cancer | Atmospheric hyperoxia (100% O2, 5 and 24 h) | Spermidine/spermine N1‐acetyltransferase (SSAT) | Chopra et al. 313 |
| 2000 | Breast cancer | Atmospheric hyperoxia (95% O2, 40 h) | Cdk2 | Bilodeau et al. 316 |
| 2002 | Fibrosarcoma | Atmospheric Hyperoxia (95% O2) | Bax/Bak | Budinger et al. 315 |
| 2009 | Breast cancer | Hyperbaric oxygen (2 bar, 100% O2, 90 min) | Mesenchymal‐to‐epithelial transition (MET) | Ingrid Moen et al. 325 |
| 2010 | Ovarian cancer | Hyperbaric oxygen (2 bar, 100% O2, 90 min) | STAT3 and cyclin‐D1 protein | Selvendiran et al. 210 |
| 2012 | Breast cancer | Hyperbaric oxygen (2 bar, 100% O2, 90 min) | MAPK pathway | Moen et al. 305 |
| 2017 | Breast cancer | Hyperbaric oxygen (O2 > 97%, 2.5 ATM) | N‐cadherin, Axl, and type I collagen | Sletta et al. 209 |
| 2017 | Lung adenocarcinoma, Colorectal cancer | Atmospheric hyperoxia (95% O2) | Suppressor of morphogenesis of genitalia (SMG‐1) and p53 | Resseguie et al. 326 |
| 2018 | Lung cancer | Atmospheric hyperoxia (95% O2 with normoxia cycle for 24 h) | MAPK pathway | Kim et al. 298 |
| 2018 | Breast cancer | Atmospheric hyperoxia (95% O2, 44 h) | Sirtuin 3 | Pinterić et al. 327 |
| 2020 | Lymphoblastoma | Atmospheric hyperoxia (O2 > 60%, 24 h) | Caspase‐3 | De Bels et al. 328 |
| 2020 | Triple negative breast cancer | Atmospheric hyperoxia (95% O2, 16 h) | Sirtuin 3 | Podgorski et al. 329 |
| 2022 | Lung cancer | Atmospheric hyperoxia (60% O2) | MYC/SLC1A5 | Liu et al. 297 |
6. ADJUNCTIVE ROLE OF HYPEROXIA IN CANCER THERAPY
6.1. Application of hyperoxia in radiotherapy
Radiation therapy has been used since the late 19th century as a means of controlling tumor burden, both for curative and palliative purposes. Following the discovery of the German physicist Wilhelm Konrad Roentgen in 1895, X‐rays were used for diagnosis, and less than 3 years later, radiotherapy was used to treat cancer. 330 , 331 In 1909, Schwarz demonstrated in a simple experiment that if the skin was clamped to reduce blood flow to the arm, the skin's response to radiation was significantly reduced. 332 However, it was not until 1953 that Gray and colleagues had an open‐label study showing that radiation sensitivity was dependent on oxygen concentration and that the effectiveness of X‐ray therapy might be enhanced if cancer patients were breathing oxygen while being irradiated. 333 Just 2 years later, Thomlinson and Gray proposed that hypoxia may be central to human tumors and is a major culprit of radiotherapy resistance. 334 These findings suggest that tumor cells near the oxygen diffusion limit exhibit improved survival under subnormal oxygen stress conditions, making them resistant to radiation therapy. There is increasing evidence that tumors with more hypoxic cells are poorly responsive to radiation therapy. 335 , 336 , 337 , 338 Results from earlier clinical studies confirmed the importance of oxygen content in the biological effects of radiation therapy. 339 , 340 , 341 When radiation is absorbed by biological matter, highly reactive free radicals are produced, and these free radicals ultimately cause tissue damage. The free radicals produced in the target body are unstable, and the damage caused by free radicals is permanent and fixed until further reactions produce stable changes. However, if this process occurs under hypoxia or in the presence of reducing substances, the free radicals chemically return to their original form, and the damage is repaired (Figure 5). Therefore, under hypoxic conditions, the radiation dose would need to be increased two‐ or threefold to achieve the same level of damage. 342
FIGURE 5.
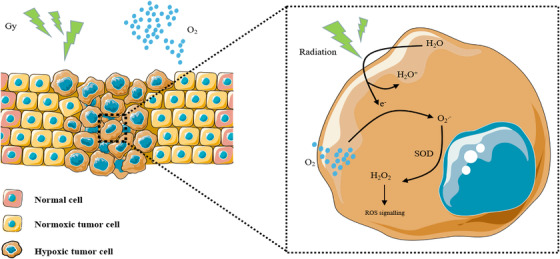
Schematic diagram of the antitumor effect of hyperoxia‐assisted radiation therapy. Radiation ionizes the tumor tissue water to generate electrons (e–), which need to be combined with oxygen (O2) to generate oxygen free radicals (O2.−). These radicals are oxidized by superoxide dismutase (SOD) to produce reactive oxygen species (H2O2). Hyperoxia promotes the antitumor effect of chemotherapy
Radiobiology researchers have improved the efficacy of radiotherapy by reversing tumor hypoxia through various means, including the use of HBO therapy, 343 nanoparticle oxygen platforms, 344 , 345 hypoxic cell sensitizers 346 , 347 and biological induction agents. 253 , 348 Molecular oxygen has long been recognized as a powerful regulator of cellular sensitivity to radiation. 211 It has been reported that the biological effects of ionizing radiation are increased approximately 3‐fold when irradiation occurs under well‐oxygenated conditions compared to hypoxic conditions. 333 Nordsmark applied hyperoxia (100% O2) and carbogen respiration (95% O2 + 5% CO2) to improve tumor oxygenation, and the results showed that both carbogen and oxygen inhalation increased tumor PO2 and radiation therapy effectiveness. 349 , 350 In mouse lung cancer, the combination of hyperoxia and radiation increased cell death and DNA damage compared to hyperoxia or radiation alone. 351 HBOT, which uses oxygen as a therapeutic, dissolves oxygen in plasma and has been found to improve oxygen supply to hypoxic tumor cells, a treatment that has been used in conjunction with radiation therapy to treat malignancies. 352 After HBO treatment, the elevated intratumoral oxygen tension is retained for a period of time, making it possible to enhance the tumor/normal tissue oxygenation ratio during radiotherapy. 353 , 354 , 355 In the past decade, nanoradiosensitization has been a research hotspot. Nanoparticles generate oxygen in hypoxic regions of tumors through oxygen‐carrying/oxygenation without any oxygen toxicity to other tissues and organs. 356 Recently, developed erythrocyte membrane‐encapsulated perfluorocarbon particles were shown to have fairly efficient oxygen loading and to efficiently deliver oxygen into solid tumors, facilitating radiotherapy‐based cancer therapy. 344 Membrane vesicle‐encapsulated CAT alleviates tumor hypoxia‐enhanced radiotherapy. 357
Fractionated radiotherapy is the irradiation mode adopted by most radiotherapy centers. 358 Fractionated radiotherapy can transform cancer cells in quiescent and hypoxic states into proliferating cancer cells with high oxygen content during the rest period of radiotherapy, thereby increasing the sensitivity to radiation. 359 In the phase I/II study, the hypoxic cell sensitizer misonidazole (MISO) was used as an adjunct to high‐fractional dose radiotherapy for unresectable stage III and IV oral, oropharyngeal, and hypopharyngeal squamous cell carcinoma. 360 However, the study showed that MISO does not improve the efficacy of high fractional dose radiotherapy. However, this was a useful finding for future clinical trials using HBO or new hypoxic cell sensitizers to facilitate high‐fractional dose radiotherapy. Recently, it has been found that an ultrahigh dose rate of ionizing radiation has the advantage of protecting normal tissue and killing tumor cells, and this approach was named “FLASH radiotherapy”. FLASH radiotherapy was first proposed by Vozenin and co‐workers in 2014. 361 FLASH radiotherapy applies radiation at an ultrahigh dose rate several orders of magnitude higher than the current clinical routine radiotherapy, which can make normal tissues resistant due to abnormal hypoxia while still maintaining local tumor control. 362 The underlying mechanisms leading to the FLASH effect have not been fully elucidated, but a significant effect on oxygen tension and ROS production is currently the most valid hypothesis. 363 , 364 It is well known that oxygen plays a key role in the DNA damage caused by ionizing radiation. 365 The fact that O2 is a powerful radiosensitizer has practical implications for cancer treatment with energetic heavy ions, such as carbon ions. 366 Additional carbon ions have a synergistic advantage when using flash radiotherapy because heavy ions can generate an oxygen‐containing microenvironment around their orbits due to water ionization; that is, near the end of the carbon ion path (Bragg peak region), they can produce abundant oxygen. 367 This suggests that flash radiotherapy using carbon ions, which are more toxic to the tumor, can significantly improve treatment.
Regarding clinical outcomes, several reports have demonstrated that irradiation occurring immediately after routine fractionated HBO has antitumor activity. 368 , 369 , 370 Early research showed that HBO promoted the curative effect of radiotherapy. In recent years, the effect of HBO seems to focus on the prognosis of radiotherapy. 371 , 372 The prognostic efficacy and safety of HBOT for overt osteoradionecrosis of the mandible were validated in 12 university hospitals. Patients received 30 preoperative HBOTs for 90 min at 2.4 ATA or placebo treatments, followed by 10 postoperative HBO sessions or placebo. The primary outcome measure was the 1‐year recovery rate from osteoradionecrosis, but the results showed that patients with pronounced osteoradionecrosis of the mandible did not benefit from HBO. 373 In 2012, a randomized controlled trial showed that HBOT was associated with improved outcomes in patients with post‐radiation tissue contusion following radiotherapy for the head, neck, anal, and rectal tissue cancers. 374 A randomized phase III trial to examine the clinical benefit of HBO in patients with chronic bowel dysfunction after radiotherapy for pelvic malignancies found no patients with radiation‐induced chronic gastrointestinal symptoms, including rectal bleeding, who benefited from HBO therapy. 375 Thus, the combination of HBO with radiotherapy appears to be an attractive strategy for cancer treatment, but as a method to improve the side effects of radiotherapy, the effect of HBO is not satisfactory.
In the past, hyperoxia therapy was considered to promote tumor proliferation and metastasis; thus, hyperoxia therapy was contraindicated. However, a large number of basic and clinical studies have found that hyperoxia therapy not only does not promote tumorigenesis but enhances the effect of radiotherapy. Hypoxia is an important reason for the poor effect of tumor radiotherapy. Under hyperoxia, the partial pressure of oxygen in tissues is more than 10 times that under normoxia, which can greatly improve the hypoxic state of tumors and enhance the effect of radiotherapy. On the other hand, when radiotherapy is used to treat malignant tumors, normal tissues will also be killed. Hyperoxia therapy can improve the blood supply to normal tissues to protect and promote the repair of normal tissues. In conclusion, hyperoxia is feasible and effective in the treatment of tumors, especially in adjuvant radiotherapy.
6.2. Application of hyperoxia in chemotherapy
Drug delivery and cellular uptake of drugs in hypoxic regions are known to be affected by hypoxia or associated acidity, resulting in the relative resistance of hypoxic tumor cells to chemotherapy. 376 , 377 Hypoxia is considered to be a major driver of multiple drug resistance (MDR) to chemotherapeutic drugs. 378 , 379 Glioma stem cells (GSCs) may be induced by dedifferentiation under hypoxic conditions to maintain the stemness of GSCs, which are maintained in chemoresistance. Hyperoxia inhibits the dedifferentiation process, promotes the differentiation of GSCs, and increases the sensitivity of glioma cells to chemotherapy. 14 In a phase III trial conducted before 1977, HBO was shown to act synergistically with radiotherapy to improve locoregional control of head and neck tumors. 380 In noncancerous hypoxic tissues, such as normal tissues after irradiation, HBO significantly increases oxygenation and promotes angiogenesis, ultimately leading to increased blood flow. 381 If HBO also induces angiogenesis in hypoxic tumors, the resulting improvements in long‐term oxygen and nutrient delivery and chemotherapeutic drug delivery may lead to enhanced antitumor effects of chemotherapeutic drugs. Alternatively, this improved method of administration may promote tumor growth that is too fast to control with traditional chemotherapy. Alagoz proposed complete HBO treatment before the first chemotherapy treatment to maximize angiogenesis and chemosensitivity. Once angiogenesis is established, HBO treatment can be stopped, and chemotherapy can be initiated. The experimental results demonstrated that HBO increases blood vessel density in large tumors, such as in epithelial ovarian cancer, and mice treated with cisplatin and HBO showed significantly delayed tumor growth compared with mice treated with cisplatin alone. HBO alters the tumor vascular architecture by inducing angiogenesis, which may resolve the problem of cisplatin entry into tumor cells and lead to the successful treatment of well‐vascularized tumors. 382 Additionally, intermittent normobaric hyperoxia (95% O2) can promote the toxic effects of carboplatin on lung tumors. 383 It is generally believed that some chemotherapeutic drugs require oxygen to generate oxygen free radicals, which in turn induce cytotoxicity and promote the effect of antitumor drugs. 384 An alginic acid solution containing calcium peroxide and CAT was dropped into a calcium chloride bath to form Ca2+‐crosslinked microcapsules. The cytotoxicity of DOX is promoted by increased ROS production. 385 However, due to the pathologically elevated interstitial fluid pressure and dense extracellular matrix (ECM) in solid tumors, the transport of antitumor drugs in tumor tissues becomes difficult. 386 , 387 , 388 HBO further enhances antitumor efficacy by increasing oxygen tension, reducing collagen deposition in the tumor ECM, promoting the penetration and accumulation of DOX liposomes into tumors, and increasing the sensitivity of tumor cells to DOX liposomes. 389 , 390 The hypoxic cell‐targeting drug tirapazamine promotes the toxicity of the PARP inhibitor olaparib/pazopanib by killing hypoxic cells. 344
Several studies on the remission of hypoxic tumors in combination with various chemotherapeutic agents have shown increased mean survival time and/or decreased tumor growth/metastasis. 391 , 392 In a mouse model of bladder cancer treated with a combination of HBO and nimustine, there was a therapeutic effect on a subset of bladder tumors. 393 GBM is a highly malignant primary brain tumor originating from the glial tissue of the central nervous system,394 and resistance to the chemotherapeutic drug temozolomide is commonly responsible for most disease recurrences. 395 , 396 , 397 Experimental results showed that hyperoxia leads to dysregulation of protein folding, which in turn induces apoptosis mediated by the unfolded protein response. Hyperoxia may induce resensitization of chemoresistant GBM cells. 398 The cytotoxicity of first‐line chemotherapeutics, such as cisplatin, carboplatin, DOX, paclitaxel, and 5‐fluorouracil, is enhanced in the presence of hyperoxia (Table 2). 383 , 389 , 399 , 400 , 401 , 402
TABLE 2.
Summary of hyperoxia combined with chemotherapy in antitumor treatment
| Year | Tumor type | Type of hyperoxia therapy | Combination therapy | Reference |
|---|---|---|---|---|
| 1989 | Breast cancer | Atmospheric hyperoxia (95% O2) | Doxorubicin | Mimnaugh et al. 407 |
| 2002 | GH3 prolactinoma | Carbogen inhalation (5% CO2+95% O2) | Ifosfamide | Rodrigues 401 |
| 2005 | Breast cancer | Hyperbaric hyperoxia (97.9% O2, 2.1% CO2, 2.4 ATA bar, 90 min) | Mefaram, Gemcitabine, and Paclitaxel | Granowitz 321 |
| 2005 | Transitional cell carcinoma | Atmospheric hyperoxia (95% O2) | Gemcitabine | Fechner et al. 377 |
| 2007 | Breast cancer | Hyperbaric hyperoxia (1.5 bar, 100% O2, 90 min) | 5‐Fluorouracil | Raa 322 |
| 2009 | Breast cancer | Hyperbaric hyperoxia (100% O2, 2 bar, 90 min) | 5‐Fluorouracil | Moen et al. 389 |
| 2011 | Ovarian cancer | Hyperbaric hyperoxia (100% O2, 2 ATM, 90 min) | Cisplatinum | Karuppaiyah et al. 210 |
| 2012 | Glioblastoma multiforme | Atmospheric hyperoxia (40% O2, 80% O2) | Temozolomide | Sun et al. 397 |
| 2014 | Glioblastoma multiforme | Atmospheric hyperoxia (40% O2) | Temozolomide | Lee et al. 398 |
| 2017 | Glioblastoma multiforme | Atmospheric hyperoxia (95% O2) | Temozolomide | Wang et al. 14 |
| 2018 | Lung cancer | Atmospheric hyperoxia (95% O2, 3 h/day) | Carboplatin | Lee et al. 383 |
| 2022 | Hepatocellular carcinoma | Hyperbaric hyperoxia (100% O2, 2.8 ATA, 120 min) | Teniposide | Li et al. 302 |
Hypoxia is a broadly studied phenotype at the clinical and molecular levels and a definite focus in biomarker studies; thus, exploring oxygen‐sensitive molecules to identify the degree of tumor hypoxia is a feasible approach. In addition to HIF, PHD, VHL, VEGF, CA9, and the other proteins mentioned above that can sense oxygen concentration, lysine demethylase 3A (KDM3A) and lysine demethylase 5C (KDM5C) should be included. Hypoxia inhibited the activities of KDM3A and KDM5C and reduced ROS production and tumor cell apoptosis. 403 , 404 In addition to inducing the transcriptional activation of multiple genes, hypoxia is also involved in the regulation of miRNAs. Studies have reported that miR‐210 is a highly upregulated miRNA in hypoxic cells and may serve as an in vivo marker of tumor hypoxia. 405 , 406 In the face of expanding drug combinations and measurable phenotypes associated with chemotherapy, it is necessary to explore the interrelationships between different oxygen‐related markers and tumor interventions. Clinical research is increasingly focusing on personalized treatment and the identification of biomarkers to guide treatment decisions for individual patients.
6.3. Application of hyperoxia in immunotherapy
Another antitumor mechanism of hyperoxia may be to promote the revitalization of immunity. High concentrations of oxygen activate the anticancer effects of T cells and NK cells by deactivating the adenosine immunosuppressive microenvironment. 127 , 206 , 408 In solid tumors, tumor hypoxia and HIF‐1α promote the upregulation of adenosine‐producing enzymes, such as CD39/CD73, which can dephosphorylate ATP to adenosine. High concentrations of adenosine impair the activation and function of T cells and NK cells and enhance the function of Tregs and the differentiation of M2 macrophages, resulting in strong immunosuppression. Increased tumor oxygenation may directly disrupt adenosine‐mediated immunosuppression by inhibiting the enzymatic activity of CD39 and CD73 to block adenosine production (Figure 6A). 206 Before the discovery of immunonegative regulators and hypoxia/A2‐adenosine immunosuppression, it was unclear what factors in the TME prevented hundreds of millions of adoptively transferred T cells from destroying tumors in vivo. Stephen demonstrated that combined immunotherapy with respiratory hyperoxia can reduce tumor hypoxia and attenuate hypoxic/HIF‐1α/A2 adenosine immunosuppression, thereby exerting a powerful antitumor effect. 306
FIGURE 6.
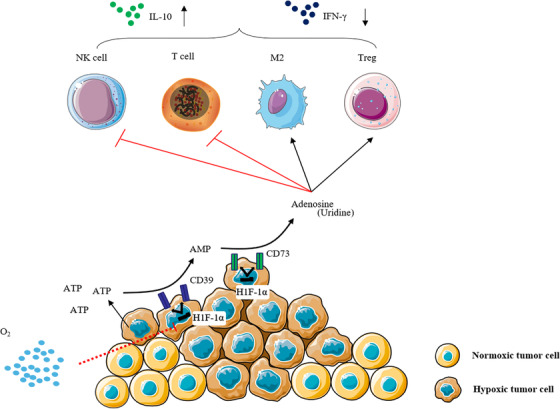
Hyperoxia reverses the hypoxic tumor microenvironment and promotes antitumor immunity. (A) Supplemental oxygen reduces the strength of downstream adenosine 2a (A2a)‐mediated tumor microenvironment (TME) immunosuppression by attenuating upstream tumor hypoxia. This in turn unleashes the antitumor activity of the otherwise suppressed T cells and natural killer (NK) cells, causing the tumor to regress
Polymorphonuclear neutrophils (PMNs) are thought to play an important role in cancer, but their role in tumors is unclear. 409 HBO exposure may promote neutrophil clearance by macrophages. 410 Mahiddine reported that hypoxia is a potent determinant of the tumor‐associated PMN phenotype and directly affects PMN‐tumor cell interactions. Hypoxia promotes the accumulation of PMNs inside tumors but then alters their phenotype in situ, limiting their ability to directly inhibit tumor growth. After the remission of hypoxia in respiratory hyperoxia, the tumor suppressor effect of PMNs was enhanced, resulting in a reduction in tumor burden despite reduced PMN infiltration. 411 Myeloid‐derived suppressor cells (MDSCs) promote CRC tumor progression through multiple mechanisms. MDSC‐derived exosomes are considered to be intercellular messengers. Hypoxia induces granulocytic MDSCs (G‐MDSCs) to secrete more exosomes in a HIF‐1α‐dependent manner. Respiratory hyperoxia can inhibit the generation of G‐MDSC exosomes, reduce the stemness of CRC cells and inhibit the development of CRC (Figure 7). 412
FIGURE 7.
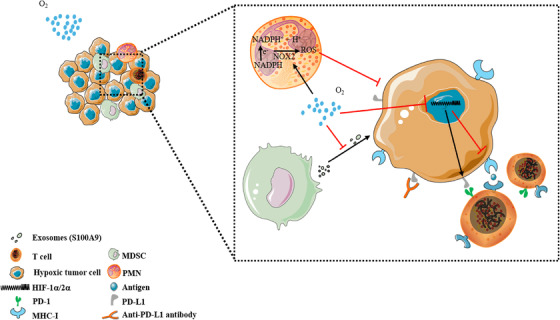
Hyperoxia promotes the expression of MCH‐1 and inhibits the expression of PDL‐1. PD‐1 binds to PD‐L1, which can transmit inhibitory signals and reduce the production of T lymphocytes. Hypoxia‐inducible factor (HIF) inhibits the in vivo immune effect by reducing MHC‐1 and increasing PD‐L1 expression in tumor cells. Hyperoxia has an antitumor effect by reversing tumor hypoxia and reducing PD‐L1 expression and can be applied in combination with an anti‐PD‐L1 antibody. Hyperoxia also promoted the upregulation of NOX2 in neutrophils, resulting in the increase of reactive oxygen species (ROS) and increased killing of tumor cells. Myeloid‐derived suppressor cells (MDSCs) can produce exosomes (mainly including S100A9) that promote the proliferation of tumor cells, and hyperoxia can reduce the production of exosomes by MDSCs and inhibit the proliferation of tumors
Enhancing tumor oxygenation as monotherapy can improve the tumor immune microenvironment; therefore, could combining hyperoxia and cancer immunotherapy improve the limitations of existing immunotherapies? Programmed death‐1 (PD‐1) blockade of the immune checkpoint has shown promising antitumor effects in a variety of cancer types. 413 However, clinical studies have shown that only 30% and 40% of melanoma patients benefit from PD‐1 inhibitors, and PD‐1 blockade therapy is ineffective in most patients. 414 , 415 , 416 Hypoxia, one of the most striking features of the TME, activates multiple signaling pathways that hinder the antitumor effects of various treatments, including immunotherapy. 417 For example, HIF reduces the expression of major histocompatibility complex I (MHC I) while increasing the expression of PD‐L1, thereby reducing the tumor cell killing ability of cytotoxic T lymphocytes (CTLs), 418 , 419 while hyperoxia (60% O2) upregulates MHC expression, confirming that MHC antigen presentation regulation is oxygen‐dependent. 420 The above findings suggest that alleviating the hypoxic TME may be an effective strategy to improve tumor immunotherapy represented by PD‐1 blockade (Figure 7). 421 In triple‐negative breast cancer, 60% O2 can not only reduce the secretion of exosomes from MDSCs caused by hypoxia but can also inhibit the upregulation of PD‐L1 protein expression. 422 HBO also promoted PD‐1 Ab delivery and T‐cell infiltration into the tumor parenchyma by depleting major components of the ECM, such as collagen and fibronectin. Furthermore, HBO disrupts hypoxia‐mediated immunosuppression and helps PD‐1 Ab trigger robust cytotoxic T lymphocyte responses and durable immune memory to suppress tumor recurrence. 423 , 424
Research has shown that an important component of hyperoxia therapy has been overlooked: the immune response. Normally, tumors grow faster than their blood supply, creating a hypoxic environment that stimulates cancer cells to produce a molecule called adenosine, which encourages antitumor T and NK cells to go into a dormant state. Given that respiratory hyperoxia inhibits the hypoxia‐adenosine pathway and promotes T cell infiltration into the tumor by depleting the dense ECM, we also expect hyperoxia to contribute to adoptive cell therapy, such as therapies that employ endogenous T cells and NK cells or genetically engineered T cells, NK cells and macrophages, by improving cell infiltration and functional maintenance in the malignant TME. Although the current studies have only been carried out in mouse models and this approach has not been tested in humans, they open up the possibility of using oxygen‐assisted immunotherapy to fight tumors.
6.4. Application of hyperoxia in PDT
PDT is a new therapeutic modality for the treatment of malignant tumors. It has been approved by the FDA for the palliative treatment of advanced lung and esophageal cancer. 425 , 426 , 427 PDT utilizes certain dyes absorbed by the target tissue, such as photosensitizers. Photochemical activation of photosensitizers at tumors generates free radicals and/or ROS. This process requires oxygen molecules, and ROS can induce apoptosis or necrosis, microvascular damage, and immune responses. 428 , 429 , 430 Therefore, the use of PDT for cancer treatment is based on the simultaneous participation of three factors, namely, retention of specific photosensitizers by tumor tissue, local illumination of lesions by visible light sources, and oxygen atoms. 431 The efficiency of PDT is limited due to hypoxic regions and vascular aberrations in tumor tissue. Therefore, improving tumor tissue oxygenation can increase the oxygen content in tumor tissue and the content of singlet oxygen (the excited state of ordinary oxygen, which is a type of ROS), which can improve the efficiency of PDT. 432 Studies have shown that the cytotoxicity of porphyrin and its related substances (photosensitizers) is mainly mediated by singlet oxygen, while hypoxic cells are less affected by porphyrin and light. 223 The availability of molecular oxygen during PDT has been shown to have a profound effect on treatment outcomes. 433 , 434 Hypoxic cells are resistant to PDT treatment, and without oxygen, PDT has no cell‐killing effect.
Hjelde and colleagues investigated the effect of oxygen tension on photodynamic therapy toxicity in an established cell line. 435 The authors demonstrated that hyperoxia did not affect the degree of toxicity of photodynamic therapy relative to normoxic conditions in vitro, in contrast to other experimental and clinical studies. 436 , 437 , 438 They attributed the lack of an enhanced phototoxicity effect of hyperoxia to the fact that cell cultures may not have preexisting hypoxic cells or sufficient oxygen during photochemical reactions to compensate for oxygen depletion. The main reasons for the significant oxygen effects observed in other studies are the presence of hypoxic cells in solid tumors and the simultaneous occurrence of light and oxidation. 439 Therefore, to take advantage of the oxygen effect, hyperoxic states must be ubiquitous. This is a very important detail because phototoxicity from PDT is dependent on both oxygen tension and photosensitizer concentration. 440 , 441 Nicola verified the effect of PDT under HBO on tumor tissue in the dorsal subcutaneous tissue of rats. Hematoporphyrin ester was used as a photosensitizer. Rats were pressurized to 3 ATM under 100% continuous oxygen ventilation in a specially designed hyperbaric chamber. The skin area above the tumor was photosensitized with a helium‐neon laser for 45 min. After 24 h, the tumor was excised for study. All PDT/HBO‐treated animals showed a marked reduction in the number of tumor cells and a large number of apoptotic and necrotic cells at the edges of the irradiated area compared to PDT control rats exposed to normal atmospheric conditions. 442 Recently, several studies have shown that nanoparticles can also generate superoxide radicals while splitting water to produce oxygen, which can promote photodynamic therapy against tumors. 443 , 444 , 445 , 446 The tumor growth inhibition effect of PDT was promoted by loading photosensitizer and O2 into perfluorocarbon nanodroplets. 447 In addition to generating ROS, oxygen can also assist PDT in decomposing collagen in the tumor ECM, thereby promoting the diffusion of oxygen and the penetration of photosensitizers deep into the tumor. This synergistic effect ultimately results in significantly improved therapeutic efficacy compared to photosensitizers alone at low laser power densities. 448 , 449 HBO combined with PDT can also significantly induce apoptosis and autophagy in human squamous cell carcinoma; therefore, HBO combined with PDT may be a promising approach for the treatment of human squamous cell carcinoma in the future. 450
Maier evaluated the use of PDT under HBO versus normobaric conditions in patients with advanced esophageal cancer. All patients underwent photosensitization with hematoporphyrin derivatives, and of these patients, 14 received PDT alone, and 17 received PDT under HBO (2 ATA). Tumor length was reduced in both groups, and the tumor reduction was more pronounced in the PDT/HBO group. The 12‐month survival rate was 28.6% in the PDT group and 41.2% in the PDT/HBO group. 451 Two clinical pilot studies demonstrated that combined PDT/HBO is a new and feasible therapy despite the small number of patients in the study. Combined PDT/HBO effectively and rapidly reduces intraluminal tumor burden and helps regulate further treatment procedures in patients. 452 , 453 Additionally, in the clinical treatment of advanced pleural malignant mesothelioma, investigators demonstrated that additional PDT/HBO is a safe and technically feasible approach (Table 3). 454 , 455 Currently, most PDT research focuses on nanomaterials and traditional PDT‐based nanotechnology to facilitate photosensitizer delivery and absorption while improving oxygenation in tumor tissue. 456 , 457 , 458
TABLE 3.
Clinical studies of hyperoxia combined with photodynamic therapy
| Year | Tumor type | Type of hyperoxia therapy | Progression | Result | Reference |
|---|---|---|---|---|---|
| 2000 | Esophageal and cardia Cancer | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | There were significant differences in dysphagia score (p = 0.0064) and tumor length (p = 0.0002) in the PDT/HBO group. The median overall survival time was 7 months in the PDT group and 12 months in the PDT/HBO group (p = 0.0098). | Maier et al. 437 |
| 2000 | Esophageal cancer | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | Tumor length was reduced in both groups, with a significant difference in the PDT/HBO group (P = 0.002). Kaplan‒Meier analysis showed that the median overall survival in the PDT group and PDT/HBO group was 7.0 months and 12 months, respectively. The 12‐month survival rate was 28.6% in the PDT group and 41.2% in the PDT/HBO group (p = 0.059). | Maier et al. 451 |
| 2001 | Lung cancer | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | Tumor stenosis was significantly reduced (p < 0.05), and Karnofsky's performance was improved (p < 0.05) at 1 and 4 weeks after PDT/HBO treatment. No treatment‐related complications were found. | Tomaselli et al. 452 |
| 2001 | Malignant bronchostenosis | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | At 1 week and 4 weeks after PDT/HBO treatment, tumor stenosis was significantly reduced (p < 0.05), and Karnofsky's performance status was significantly improved (p < 0.05). There were no treatment‐related complications. | Tomaselli et al. 453 |
| 2004 | Malignant pleural stromal tumor | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | CT scans showed focal tumor regeneration after 6 months in 10/12 patients in the non‐PDT/HBO group. However, in the PDT/HBO group, only 9/22 tumors regenerated at the 6‐month follow‐up. Survival analysis showed a significant advantage in the PDT/HBO group (p = 0.0179). | Matzi et al. 454 |
| 2004 | Malignant pleural stromal tumor | Hyperbaric hyperoxia (100% O2, 2 ATA) | Clinical pilot study | During the 6‐month follow‐up, 8/11 patients in the non‐PDT/HBO group had focal tumor regrowth. In the PDT/HB group, 4/14 tumors regenerated. In the non‐PDT/HBO group, 9/11 patients died due to local tumor progression and distant metastasis, and 10/14 patients in the PDT group died due to local tumor progression and/or distant metastasis. | Matzi et al. 455 |
Although hyperoxia can improve the efficacy of PDT treatment, HBO seems to be more effective for PDT. Study results indicate that HBO can promote the efficacy of PDT in addition to increasing blood pO2, but the specific mechanism remains to be explored. On the other hand, due to the variety of photosensitizers, the combination of hyperoxia therapy and photosensitizers that can better promote PDT requires more basic and clinical research. Oxygen supplementation can solve the problem of PDT resistance caused by tumor hypoxia, but the clinical application of hyperoxia combined with photodynamic therapy still needs further research.
7. FUTURE DIRECTIONS AND DISCUSSIONS
Cancer cells have a complex relationship with oxygen, and for decades, research has been conducted to investigate whether oxygen helps or harms cancer. Tumors that grow in hypoxic environments are known to be more metastatic and more lethal to patients. Tumors are initially susceptible to chemotherapy and radiotherapy, and advanced cancers maintain growth as cells adapt to a hypoxic microenvironment. 459 Hypoxia severely hinders various anticancer strategies and is also a major driver of the development of tumor resistance, invasion, and metastasis. 460 Tumor hypoxia has prompted the design of new approaches to fight cancer.
Theoretically, the application of hyperoxia therapy in tumor therapy is justified for the following reasons: improving oxygenation can remove hypoxia‐stimulated vascular malformations and degrade the dense ECM of tumor cells, improving drug delivery to hypoxic areas of tumors. The improvement in oxygenation causes cells to enter a proliferative phase, which makes them sensitive to radiation therapy and certain chemotherapeutic agents. Improved oxygenation also recruits immune cells to the tumor or enhances the antitumor ability of immune cells. With these facts in mind, although the antitumor effect of hyperoxia therapy is weak, it may improve the effectiveness of some therapies and be beneficial as an adjuvant in cancer treatment.
There are still many difficulties in the clinical application of hyperoxia therapy. Oxygen is breathed into the blood to provide nutrients for cells throughout the body. Cancer cells also need oxygen to survive. As earlier studies have shown, respiratory hyperoxia in the early stages of tumorigenesis accelerates tumor development by promoting angiogenesis. 289 , 461 This is the underlying theory of hyperoxia therapy as an adjunct therapy. Tumors thrive in the absence of oxygen and resist treatment; therefore, increasing oxygen supply to the tumor can return tumor cells to their normal state and thus resensitize them to existing therapies. Currently, hyperoxia is often used as adjunctive therapy for patients with advanced cancer, which reduces the antitumor efficacy of hyperoxia. The timing of hyperoxia intervention is the first problem to be solved. Another obstacle to the clinical application of hyperoxia therapy is the oxygen concentration and use time. Oxygen poisoning occurs with excess oxygen concentrations and oxygen inhalation times. Intermittent oxygen inhalation is recommended, and the patient treatment time is relatively flexible; thus, it is relatively easy to achieve clinical application. Several important questions remain unanswered about the mechanism of hyperoxia in tumor therapy and how to target hypoxia pathways in specific disease states. Every tissue, disease state, and individual has an optimal oxygen level. Mismatches in oxygen supply and demand can lead to pathological changes, and much remains unknown about the cellular response to hyperoxia. We should scientifically examine the hyperoxia therapy strategy and recognize the synergistic effect of hyperoxia therapy and existing tumor treatment methods. Isolated use of hyperoxia therapy to some extent restricts the further promotion of hyperoxia therapy in clinical practice. Therefore, hyperoxia therapy as a tumor treatment needs to be further validated.
At present, researchers seem to be highly interested in the effect of hyperoxia on immunity. Hyperoxia can recruit immune cells, such as T and NK cells, but its specific mechanism has not been fully studied. Why can hyperoxia recruit immune cells? One of the most important and basic effects of hyperoxia is to activate ROS, which kills tumor cells by damaging mitochondria. What is the connection with immunity? Although a large number of studies have verified the immune activation effect of hyperoxia, research on the antitumor effect of hyperoxia is limited. Therefore, the combination of hyperoxia therapy with existing immunotherapy can open up new treatment pathways and will need further exploration.
AUTHOR CONTRIBUTIONS
Y.Z. and J.H.W. conceptualized this review and drafted the paper. Y.Z., K.L. and Q.Y.H. produced the figures and tables. J.H.W., X.S.G. and C.P.J. revised the paper. All authors have read and approved the article.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Some of the images in the article are from ScienceSlides and BioRender. We are grateful for the support of the above websites.
Zhuang Y, Liu K, He Q, Gu X, Jiang C, Wu J. Hypoxia signaling in cancer: Implications for therapeutic interventions. MedComm. 2023;4:e203. 10.1002/mco2.203
Contributor Information
Chunping Jiang, Email: chunpingjiang@nju.edu.cn.
Junhua Wu, Email: wujunhua@nju.edu.cn.
DATA AVAILABILITY STATEMENT
Not appliable.
REFERENCES
- 1. Mills DB, Boyle RA, Daines SJ, et al. Eukaryogenesis and oxygen in Earth history. Nat Ecol Evol. 2022;6(5):520‐532. [DOI] [PubMed] [Google Scholar]
- 2. Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 3. Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer. 2011;11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17(12):738‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang M, Ye JJ, Xia Y, et al. Platelet‐Mimicking biotaxis targeting vasculature‐disrupted tumors for cascade amplification of hypoxia‐sensitive therapy. ACS Nano. 2019;13(12):14230‐14240. [DOI] [PubMed] [Google Scholar]
- 6. Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol (1985). 2001;90(5):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 7. Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26(9):877‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo W, Qiu Z, Wang Z, et al. MiR‐199a‐5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62(4):1132‐1144. [DOI] [PubMed] [Google Scholar]
- 9. Yang J, Staples O, Thomas LW, et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. J Clin Invest. 2012;122(2):600‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernansanz‐Agustín P, Choya‐Foces C, Carregal‐Romero S, et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020;586(7828):287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skuli N, Liu L, Runge A, et al. Endothelial deletion of hypoxia‐inducible factor‐2alpha (HIF‐2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114(2):469‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaGory EL, Giaccia AJ. The ever‐expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18(4):356‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer. 2001;84(9):1280‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang P, Wan W, Xiong S, et al. HIF1α regulates glioma chemosensitivity through the transformation between differentiation and dedifferentiation in various oxygen levels. Sci Rep. 2017;7(1):7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vilaplana‐Lopera N, Besh M, Moon EJ. Targeting hypoxia: revival of old remedies. Biomolecules. 2021;11(11):1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daruwalla J, Christophi C. Hyperbaric oxygen therapy for malignancy: a review. World J Surg. 2006;30(12):2112‐2131. [DOI] [PubMed] [Google Scholar]
- 18. Hochberg CH, Semler MW, Brower RG. Oxygen toxicity in critically ill adults. Am J Respir Crit Care Med. 2021;204(6):632‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrysik Z, Bender H, Galbraith MD, Espinosa JM. Multi‐omics analysis reveals contextual tumor suppressive and oncogenic gene modules within the acute hypoxic response. Nat Commun. 2021;12(1):1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149(1):63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510‐5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blouw B, Song H, Tihan T, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4(2):133‐146. [DOI] [PubMed] [Google Scholar]
- 23. Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia‐inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271(4):C1172‐80. Pt 1. [DOI] [PubMed] [Google Scholar]
- 24. Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for renal cell carcinoma in von Hippel‐Lindau disease. N Engl J Med. 2021;385(22):2036‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callahan CL, Moore L, Lenz P, et al. Differences in tumor VHL Mutation and Hypoxia‐inducible Factor 2α expression between African American and white patients with clear cell renal cell carcinoma. Eur Urol. 2019;75(5):882‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnan B, Rose TL, Kardos J, Milowsky MI, Kim WY. Intrinsic genomic differences between African American and White patients with clear cell renal cell carcinoma. JAMA Oncol. 2016;2(5):664‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo J, Chakraborty AA, Liu P, et al. pVHL suppresses kinase activity of Akt in a proline‐hydroxylation‐dependent manner. Science. 2016;353(6302):929‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling S, Shan Q, Zhan Q, et al. USP22 promotes hypoxia‐induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation. Gut. 2020;69(7):1322‐1334. [DOI] [PubMed] [Google Scholar]
- 29. Sim J, Cowburn AS, Palazon A, et al. The factor inhibiting HIF asparaginyl hydroxylase regulates oxidative metabolism and accelerates metabolic adaptation to hypoxia. Cell Metab. 2018;27(4):898‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SB, Frattini V, Bansal M, et al. An ID2‐dependent mechanism for VHL inactivation in cancer. Nature. 2016;529(7585):172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiarotto JA, Hill RP. A quantitative analysis of the reduction in oxygen levels required to induce up‐regulation of vascular endothelial growth factor (VEGF) mRNA in cervical cancer cell lines. Br J Cancer. 1999;80(10):1518‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakajima T, Anayama T, Koike T, et al. Endobronchial ultrasound doppler image features correlate with mRNA expression of HIF1‐α and VEGF‐C in patients with non‐small‐cell lung cancer. J Thorac Oncol. 2012;7(11):1661‐1667. [DOI] [PubMed] [Google Scholar]
- 33. Kato I, Nishinaka Y, Nakamura M, et al. Hypoxic adaptation of leukemic cells infiltrating the CNS affords a therapeutic strategy targeting VEGFA. Blood. 2017;129(23):3126‐3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Meng Q, Shi C, et al. Hypoxia‐inducible exosomes facilitate liver‐tropic premetastatic niche in colorectal cancer. Hepatology. 2021;74(5):2633‐2651. [DOI] [PubMed] [Google Scholar]
- 35. Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 2014;24(8):472‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 37. Li L, Yang L, Fan Z, et al. Hypoxia‐induced GBE1 expression promotes tumor progression through metabolic reprogramming in lung adenocarcinoma. Signal Transduct Target Ther. 2020;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309‐314. [DOI] [PubMed] [Google Scholar]
- 39. Jiang BH, Agani F, Passaniti A, Semenza GL. V‐SRC induces expression of hypoxia‐inducible factor 1 (HIF‐1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF‐1 in tumor progression. Cancer Res. 1997;57(23):5328‐5335. [PubMed] [Google Scholar]
- 40. Semenza GL. Regulation of metabolism by hypoxia‐inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347‐353. [DOI] [PubMed] [Google Scholar]
- 41. Hu J, Locasale JW, Bielas JH, et al. Heterogeneity of tumor‐induced gene expression changes in the human metabolic network. Nat Biotechnol. 2013;31(6):522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen C, Liu Y, Lu C, et al. Cancer‐associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev. 2013;27(18):1974‐1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dang L, White DW, Gross S, et al. Cancer‐associated IDH1 mutations produce 2‐hydroxyglutarate. Nature. 2009;462(7274):739‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Bai C, Ruan Y, et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat Commun. 2019;10(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Zhao H, Li Y, et al. The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer. 2018;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niu Y, Lin Z, Wan A, et al. Loss‐of‐function genetic screening identifies Aldolase A as an essential driver for liver cancer cell growth under hypoxia. Hepatology. 2021;74(3):1461‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li B, Qiu B, Lee DS, et al. Fructose‐1,6‐bisphosphatase opposes renal carcinoma progression. Nature. 2014;513(7517):251‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo H, Ci X, Ahmed M, et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Commun. 2019;10(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geng H, Xue C, Mendonca J, et al. Interplay between hypoxia and androgen controls a metabolic switch conferring resistance to androgen/AR‐targeted therapy. Nat Commun. 2018;9(1):4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDonald PC, Chafe SC, Brown WS, et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology. 2019;157(3):823‐837. [DOI] [PubMed] [Google Scholar]
- 51. Yu H, He J, Liu W, et al. The Transcriptional Coactivator, ALL1‐fused gene from chromosome 9, simultaneously sustains hypoxia tolerance and metabolic advantages in liver cancer. Hepatology. 2021;74(4):1952‐1970. [DOI] [PubMed] [Google Scholar]
- 52. Zhang MS, Cui JD, Lee D, et al. Hypoxia‐induced macropinocytosis represents a metabolic route for liver cancer. Nat Commun. 2022;13(1):954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reinfeld BI, Madden MZ, Wolf MM, et al. Cell‐programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593(7858):282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan M, Zorbas C, Sugaya M, et al. Glutamine deficiency in solid tumor cells confers resistance to ribosomal RNA synthesis inhibitors. Nat Commun. 2022;13(1):3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481(7381):380‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoo HC, Park SJ, Nam M, et al. A variant of SLC1A5 Is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 2020;31(2):267‐283. e12. [DOI] [PubMed] [Google Scholar]
- 57. Keulers TG, Koch A, van Gisbergen MW, et al. ATG12 deficiency results in intracellular glutamine depletion, abrogation of tumor hypoxia and a favorable prognosis in cancer. Autophagy. 2022;18(8):1898‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ikeda K, Horie‐Inoue K, Suzuki T, et al. Mitochondrial supercomplex assembly promotes breast and endometrial tumorigenesis by metabolic alterations and enhanced hypoxia tolerance. Nat Commun. 2019;10(1):4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma B, Cheng H, Mu C, et al. The SIAH2‐NRF1 axis spatially regulates tumor microenvironment remodeling for tumor progression. Nat Commun. 2019;10(1):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4(12):1406‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saliakoura M, Rossi Sebastiano M, Pozzato C, et al. PLCγ1 suppression promotes the adaptation of KRAS‐mutant lung adenocarcinomas to hypoxia. Nat Cell Biol. 2020;22(11):1382‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133(2):275‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182‐1186. [DOI] [PubMed] [Google Scholar]
- 65. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Batlle R, Andrés E, Gonzalez L, et al. Regulation of tumor angiogenesis and mesenchymal‐endothelial transition by p38α through TGF‐β and JNK signaling. Nat Commun. 2019;10(1):3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59(7):953‐962. [DOI] [PubMed] [Google Scholar]
- 68. Eppenberger U, Kueng W, Schlaeppi JM, et al. Markers of tumor angiogenesis and proteolysis independently define high‐ and low‐risk subsets of node‐negative breast cancer patients. J Clin Oncol. 1998;16(9):3129‐3136. [DOI] [PubMed] [Google Scholar]
- 69. Reece KM, Richardson ED, Cook KM, et al. Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF‐1α/p300 complex in a preclinical model of prostate cancer. Mol Cancer. 2014;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fernández JG, Rodríguez DA, Valenzuela M, et al. Survivin expression promotes VEGF‐induced tumor angiogenesis via PI3K/Akt enhanced β‐catenin/Tcf‐Lef dependent transcription. Mol Cancer. 2014;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xue Y, Lim S, Yang Y, et al. PDGF‐BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18(1):100‐110. [DOI] [PubMed] [Google Scholar]
- 72. Choueiri TK. Targeting the HIF2‐VEGF axis in renal cell carcinoma. Nat Med. 2020;26(10):1519‐1530. [DOI] [PubMed] [Google Scholar]
- 73. Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF‐1alpha‐dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107(7):2705‐2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou Q, Perakis SO, Ulz P, et al. Cell‐free DNA analysis reveals POLR1D‐mediated resistance to bevacizumab in colorectal cancer. Genome Med. 2020;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Akamatsu H, Toi Y, Hayashi H, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M‐mutated non‐small cell lung cancer previously treated with epidermal growth factor receptor‐tyrosine kinase inhibitor: West Japan Oncology Group 8715L phase 2 randomized clinical trial. JAMA Oncol. 2021;7(3):386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu AX, Baron AD, Malfertheiner P, et al. Ramucirumab as second‐line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH trial results by Child‐Pugh Score. JAMA Oncol. 2017;3(2):235‐243. [DOI] [PubMed] [Google Scholar]
- 77. Olaratumab approved for soft‐tissue sarcoma. Cancer Discov. 2016;6(12):1297. [DOI] [PubMed] [Google Scholar]
- 78. Jain RK. Normalizing tumor vasculature with anti‐angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987‐989. [DOI] [PubMed] [Google Scholar]
- 79. Xu RH, Zhang Y, Pan H, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second‐line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW‐Asia): a randomised, multicentre, double‐blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1015‐1024. [DOI] [PubMed] [Google Scholar]
- 80. Tap WD, Wagner AJ, Schöffski P, et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA. 2020;323(13):1266‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang T, Tang J, Yang H, et al. Effect of apatinib plus pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone on platinum‐resistant recurrent ovarian cancer: the APPROVE randomized clinical trial. JAMA Oncol. 2022;8(8):1169‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu HT, Kuo YC, Hung JJ, et al. K63‐polyubiquitinated HAUSP deubiquitinates HIF‐1α and dictates H3K56 acetylation promoting hypoxia‐induced tumour progression. Nat Commun. 2016;7:13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin CW, Wang LK, Wang SP, et al. Daxx inhibits hypoxia‐induced lung cancer cell metastasis by suppressing the HIF‐1α/HDAC1/Slug axis. Nat Commun. 2016;7:13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF‐1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295‐305. [DOI] [PubMed] [Google Scholar]
- 86. Luo D, Wang J, Li J, Post M. Mouse snail is a target gene for HIF. Mol Cancer Res. 2011;9(2):234‐245. [DOI] [PubMed] [Google Scholar]
- 87. Zhang W, Shi X, Peng Y, et al. HIF‐1α promotes epithelial‐mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS One. 2015;10(6):e0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xi Y, Shen Y, Wu D, et al. CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR‐27a‐3p. Mol Cancer. 2022;21(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA‐34a regulation of PPP1R11 and STAT3 and hypoxia‐induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153(2):505‐520. [DOI] [PubMed] [Google Scholar]
- 90. Xu Q, Liu X, Liu Z, et al. MicroRNA‐1296 inhibits metastasis and epithelial‐mesenchymal transition of hepatocellular carcinoma by targeting SRPK1‐mediated PI3K/AKT pathway. Mol Cancer. 2017;16(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xing S, Tian Z, Zheng W, et al. Hypoxia downregulated miR‐4521 suppresses gastric carcinoma progression through regulation of IGF2 and FOXM1. Mol Cancer. 2021;20(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Karube Y, Tanaka H, Osada H, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96(2):111‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Merritt WM, Lin YG, Han LY, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359(25):2641‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Martello G, Rosato A, Ferrari F, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141(7):1195‐1207. [DOI] [PubMed] [Google Scholar]
- 95. Villanueva T. Metastasis: it's nicer with DICER. Nat Rev Cancer. 2010;10(8):530‐531. [DOI] [PubMed] [Google Scholar]
- 96. Lai HH, Li JN, Wang MY, et al. HIF‐1α promotes autophagic proteolysis of Dicer and enhances tumor metastasis. J Clin Invest. 2018;128(2):625‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ju S, Wang F, Wang Y, Ju S. CSN8 is a key regulator in hypoxia‐induced epithelial‐mesenchymal transition and dormancy of colorectal cancer cells. Mol Cancer. 2020;19(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Olcina M, Lecane PS, Hammond EM. Targeting hypoxic cells through the DNA damage response. Clin Cancer Res. 2010;16(23):5624‐5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jiang W, Li Q, Xiao L, et al. Hierarchical multiplexing nanodroplets for imaging‐guided cancer radiotherapy via dna damage enhancement and concomitant DNA repair prevention. ACS Nano. 2018;12(6):5684‐5698. [DOI] [PubMed] [Google Scholar]
- 100. Wang M, Guo L, Wu Q, et al. ATR/Chk1/Smurf1 pathway determines cell fate after DNA damage by controlling RhoB abundance. Nat Commun. 2014;5:4901. [DOI] [PubMed] [Google Scholar]
- 101. Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ma X, Yao M, Gao Y, et al. Functional immune cell‐derived exosomes engineered for the trilogy of radiotherapy sensitization. Adv Sci. 2022;9(23):e2106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kaplan AR, Gueble SE, Liu Y, et al. Cediranib suppresses homology‐directed DNA repair through down‐regulation of BRCA1/2 and RAD51. Sci Transl Med. 2019;11(492):eaav4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration‐resistant prostate cancer: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975‐986. [DOI] [PubMed] [Google Scholar]
- 105. Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor‐induced “BRCAness” and PARP inhibition are synthetically lethal for castration‐resistant prostate cancer. Sci Signal. 2017;10(480):eaam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu Q, Zhou W, Yin S, et al. Blocking triggering receptor expressed on myeloid cells‐1‐positive tumor‐associated macrophages induced by hypoxia reverses immunosuppression and anti‐programmed cell death ligand 1 resistance in liver cancer. Hepatology. 2019;70(1):198‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shurin MR, Umansky V. Cross‐talk between HIF and PD‐1/PD‐L1 pathways in carcinogenesis and therapy. J Clin Invest. 2022;132(9):e159473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen X, Zhao J, Herjan T, et al. IL‐17‐induced HIF1α drives resistance to anti‐PD‐L1 via fibroblast‐mediated immune exclusion. J Exp Med. 2022;219(6):e20210693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ma S, Zhao Y, Lee WC, et al. Hypoxia induces HIF1α‐dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti‐PD‐1 immunotherapy. Nat Commun. 2022;13(1):4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bailey CM, Liu Y, Liu M, et al. Targeting HIF‐1α abrogates PD‐L1‐mediated immune evasion in tumor microenvironment but promotes tolerance in normal tissues. J Clin Invest. 2022;132(9):e150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD‐1/PD‐L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liang Y, Tang H, Guo J, et al. Targeting IFNα to tumor by anti‐PD‐L1 creates feedforward antitumor responses to overcome checkpoint blockade resistance. Nat Commun. 2018;9(1):4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salman S, Meyers DJ, Wicks EE, et al. HIF inhibitor 32–134D eradicates murine hepatocellular carcinoma in combination with anti‐PD1 therapy. J Clin Invest. 2022;132(9):e156774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Noman MZ, Desantis G, Janji B, et al. PD‐L1 is a novel direct target of HIF‐1α, and its blockade under hypoxia enhanced MDSC‐mediated T cell activation. J Exp Med. 2014;211(5):781‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. He Q, Liu M, Huang W, et al. IL‐1β‐induced elevation of solute carrier family 7 member 11 promotes hepatocellular carcinoma metastasis through up‐regulating programmed death ligand 1 and colony‐stimulating factor 1. Hepatology. 2021;74(6):3174‐3193. [DOI] [PubMed] [Google Scholar]
- 117. Chiu DK, Tse AP, Xu IM, et al. Hypoxia inducible factor HIF‐1 promotes myeloid‐derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8(1):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chiu DK, Xu IM, Lai RK, et al. Hypoxia induces myeloid‐derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C‐C motif) ligand 26. Hepatology. 2016;64(3):797‐813. [DOI] [PubMed] [Google Scholar]
- 119. Kumar V, Cheng P, Condamine T, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor‐associated macrophage differentiation. Immunity. 2016;44(2):303‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia‐induced changes in tumor‐associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189(5):593‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang J, Zhang Q, Lou Y, et al. Hypoxia‐inducible factor‐1α/interleukin‐1β signaling enhances hepatoma epithelial‐mesenchymal transition through macrophages in a hypoxic‐inflammatory microenvironment. Hepatology. 2018;67(5):1872‐1889. [DOI] [PubMed] [Google Scholar]
- 122. Chen F, Chen J, Yang L, et al. Extracellular vesicle‐packaged HIF‐1α‐stabilizing lncRNA from tumour‐associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21(4):498‐510. [DOI] [PubMed] [Google Scholar]
- 123. Fu Q, Xu L, Wang Y, et al. Tumor‐associated macrophage‐derived interleukin‐23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol. 2019;75(5):752‐763. [DOI] [PubMed] [Google Scholar]
- 124. Garcia Garcia CJ, Huang Y, Fuentes NR, et al. Stromal HIF2 regulates immune suppression in the pancreatic cancer microenvironment. Gastroenterology. 2022;162(7):2018‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia‐inducible factors as regulators of T cell development, differentiation, and function. Immunol Res. 2013;55(1‐3):58‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Park JH, Kim HJ, Kim CW, et al. Tumor hypoxia represses γδ T cell‐mediated antitumor immunity against brain tumors. Nat Immunol. 2021;22(3):336‐346. [DOI] [PubMed] [Google Scholar]
- 127. Jayaprakash P, Ai M, Liu A, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. 2018;128(11):5137‐5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wu MZ, Cheng WC, Chen SF, et al. miR‐25/93 mediates hypoxia‐induced immunosuppression by repressing cGAS. Nat Cell Biol. 2017;19(10):1286‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Doedens AL, Phan AT, Stradner MH, et al. Hypoxia‐inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14(11):1173‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li Q, Li D, Zhang X, et al. E3 ligase VHL promotes group 2 innate lymphoid cell maturation and function via glycolysis inhibition and induction of interleukin‐33 receptor. Immunity. 2018;48(2):258‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xu Y, Chaudhury A, Zhang M, et al. Glycolysis determines dichotomous regulation of T cell subsets in hypoxia. J Clin Invest. 2016;126(7):2678‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tyrakis PA, Palazon A, Macias D, et al. S‐2‐hydroxyglutarate regulates CD8(+) T‐lymphocyte fate. Nature. 2016;540(7632):236‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Palazon A, Tyrakis PA, Macias D, et al. An HIF‐1α/VEGF‐A axis in cytotoxic t cells regulates tumor progression. Cancer Cell. 2017;32(5):669‐683. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hua Y, Huang JH, Shao ZH, et al. Composition‐Dependent enzyme mimicking activity and radiosensitizing effect of bimetallic clusters to modulate tumor hypoxia for enhanced cancer therapy. Adv Mater. 2022;34(31):e2203734. [DOI] [PubMed] [Google Scholar]
- 135. Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33(48):e2103978. [DOI] [PubMed] [Google Scholar]
- 136. Bhandari V, Li CH, Bristow RG, Boutros PC. Divergent mutational processes distinguish hypoxic and normoxic tumours. Nat Commun. 2020;11(1):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li X, Jiang C, Wang Q, et al. A “valve‐closing” starvation strategy for amplification of tumor‐specific chemotherapy. Adv Sci. 2022;9(8):e2104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ma L, Craig AJ, Heinrich S. Hypoxia is a key regulator in liver cancer progression. J Hepatol. 2021;75(3):736‐737. [DOI] [PubMed] [Google Scholar]
- 139. Semenza GL. Pharmacologic targeting of hypoxia‐inducible factors. Ann Rev Pharmacol Toxicol. 2019;59:379‐403. [DOI] [PubMed] [Google Scholar]
- 140. Courtney KD, Infante JR, Lam ET, et al. Phase I dose‐escalation trial of PT2385, a first‐in‐class hypoxia‐inducible factor‐2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol. 2018;36(9):867‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hou L, Gong X, Yang J, Zhang H, Yang W, Chen X. Hybrid‐membrane‐decorated prussian blue for effective cancer immunotherapy via tumor‐associated macrophages polarization and hypoxia relief. Adv Mater. 2022;34(14):e2200389. [DOI] [PubMed] [Google Scholar]
- 142. Lee M. Hypoxia targeting gene expression for breast cancer gene therapy. Adv Drug Deliv Rev. 2009;61(10):842‐849. [DOI] [PubMed] [Google Scholar]
- 143. Li X, Pan Y, Chen C, et al. Hypoxia‐responsive gene editing to reduce tumor thermal tolerance for mild‐photothermal therapy. Angew Chem Int Ed Engl. 2021;60(39):21200‐21204. [DOI] [PubMed] [Google Scholar]
- 144. Koizume S, Ito S, Nakamura Y, et al. Lipid starvation and hypoxia synergistically activate ICAM1 and multiple genes in an Sp1‐dependent manner to promote the growth of ovarian cancer. Mol Cancer. 2015;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Saraswati S, Kumar S, Alhaider AA. α‐santalol inhibits the angiogenesis and growth of human prostate tumor growth by targeting vascular endothelial growth factor receptor 2‐mediated AKT/mTOR/P70S6K signaling pathway. Mol Cancer. 2013;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146. Semela D, Piguet AC, Kolev M, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46(5):840‐848. [DOI] [PubMed] [Google Scholar]
- 147. Walton ZE, Patel CH, Brooks RC, et al. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell. 2018;174(1):72‐87. e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yao J, Chakhoyan A, Nathanson DA, et al. Metabolic characterization of human IDH mutant and wild type gliomas using simultaneous pH‐ and oxygen‐sensitive molecular MRI. Neuro Oncol. 2019;21(9):1184‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang T, Wang D, Yu H, et al. A cancer vaccine‐mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018;9(1):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. 2019;15(10):641‐659. [DOI] [PubMed] [Google Scholar]
- 151. Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia‐inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62(15):4316‐4324. [PubMed] [Google Scholar]
- 152. Creighton‐Gutteridge M, Cardellina JH 2nd, Stephen AG, et al. Cell type‐specific, topoisomerase II‐dependent inhibition of hypoxia‐inducible factor‐1alpha protein accumulation by NSC 644221. Clin Cancer Res. 2007;13(3):1010‐1018. [DOI] [PubMed] [Google Scholar]
- 153. Welsh S, Williams R, Kirkpatrick L, Paine‐Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX‐478, an inhibitor of hypoxia‐inducible factor‐1alpha. Mol Cancer Ther. 2004;3(3):233‐244. [PubMed] [Google Scholar]
- 154. Ilegems E, Bryzgalova G, Correia J, et al. HIF‐1α inhibitor PX‐478 preserves pancreatic β cell function in diabetes. Sci Transl Med. 2022;14(638):eaba9112. [DOI] [PubMed] [Google Scholar]
- 155. Scheuermann TH, Li Q, Ma HW, et al. Allosteric inhibition of hypoxia inducible factor‐2 with small molecules. Nat Chem Biol. 2013;9(4):271‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wallace EM, Rizzi JP, Han G, et al. A small‐molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76(18):5491‐5500. [DOI] [PubMed] [Google Scholar]
- 157. Wu D, Su X, Lu J, et al. Bidirectional modulation of HIF‐2 activity through chemical ligands. Nat Chem Biol. 2019;15(4):367‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia‐inducible factor‐2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med. 2021;27(5):802‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF‐2 antagonist. Nature. 2016;539(7627):112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kong D, Park EJ, Stephen AG, et al. Echinomycin, a small‐molecule inhibitor of hypoxia‐inducible factor‐1 DNA‐binding activity. Cancer Res. 2005;65(19):9047‐9055. [DOI] [PubMed] [Google Scholar]
- 161. Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF‐1alpha and is required for O(2)‐independent and HSP90 inhibitor‐induced degradation of HIF‐1alpha. Mol Cell. 2007;25(2):207‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF‐1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A. 2009;106(42):17910‐17915. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163. Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1‐methylpropyl 2‐imidazolyl disulfide and pleurotin inhibit hypoxia‐induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2(3):235‐243. [PubMed] [Google Scholar]
- 164. Yeo EJ, Chun YS, Cho YS, et al. YC‐1: a potential anticancer drug targeting hypoxia‐inducible factor 1. J Natl Cancer Inst. 2003;95(7):516‐525. [DOI] [PubMed] [Google Scholar]
- 165. Dachs GU, Patterson AV, Firth JD, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3(5):515‐520. [DOI] [PubMed] [Google Scholar]
- 166. Hon WC, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF‐1 alpha by pVHL. Nature. 2002;417(6892):975‐978. [DOI] [PubMed] [Google Scholar]
- 167. Chowdhury R, Leung IK, Tian YM, et al. Structural basis for oxygen degradation domain selectivity of the HIF prolyl hydroxylases. Nat Commun. 2016;7:12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhao S, Lin Y, Xu W, et al. Glioma‐derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF‐1alpha. Science. 2009;324(5924):261‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sharma A, Arambula JF, Koo S, et al. Hypoxia‐targeted drug delivery. Chem Soc Rev. 2019;48(3):771‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lindner LH. Hypoxia‐activated prodrug: an appealing preclinical concept yet lost in clinical translation. Lancet Oncol. 2017;18(8):991‐993. [DOI] [PubMed] [Google Scholar]
- 171. Yang S, Tang Z, Hu C, et al. Selectively potentiating hypoxia levels by combretastatin A4 nanomedicine: toward highly enhanced hypoxia‐activated prodrug tirapazamine therapy for metastatic tumors. Adv Mater. 2019;31(11):e1805955. [DOI] [PubMed] [Google Scholar]
- 172. Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans‐Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol. 2005;23(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 173. Vlahovic G, Rabbani ZN, Herndon JE 2nd, Dewhirst MW, Vujaskovic Z. Treatment with Imatinib in NSCLC is associated with decrease of phosphorylated PDGFR‐beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer. 2006;95(8):1013‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17‐18):812‐818. [DOI] [PubMed] [Google Scholar]
- 175. Iyer AK, Singh A, Ganta S, Amiji MM. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv Drug Deliv Rev. 2013;65(13‐14):1784‐1802. [DOI] [PubMed] [Google Scholar]
- 176. Li Y, Gong T, Gao H, et al. ZIF‐based nanoparticles combine X‐ray‐induced nitrosative stress with autophagy management for hypoxic prostate cancer therapy. Angew Chem Int Ed Engl. 2021;60(28):15472‐15481. [DOI] [PubMed] [Google Scholar]
- 177. Zheng P, Fan M, Liu H, et al. Self‐Propelled and near‐infrared‐phototaxic photosynthetic bacteria as photothermal agents for hypoxia‐targeted cancer therapy. ACS Nano. 2021;15(1):1100‐1110. [DOI] [PubMed] [Google Scholar]
- 178. Hou L, Chen D, Wang R, et al. Transformable Honeycomb‐like nanoassemblies of carbon dots for regulated multisite delivery and enhanced antitumor chemoimmunotherapy. Angew Chem Int Ed Engl. 2021;60(12):6581‐6592. [DOI] [PubMed] [Google Scholar]
- 179. Wang Y, Gong N, Ma C, et al. An amphiphilic dendrimer as a light‐activable immunological adjuvant for in situ cancer vaccination. Nat Commun. 2021;12(1):4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wu L, Xie W, Li Y, et al. Biomimetic nanocarriers guide extracellular ATP homeostasis to remodel energy metabolism for activating innate and adaptive immunity system. Adv Sci. 2022;9(17):e2105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Weng ML, Chen WK, Chen XY, et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the FDFT1‐mediated AKT/mTOR/HIF1α pathway suppression. Nat Commun. 2020;11(1):1869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 182. Wander SA, Hennessy BT, Slingerland JM. Next‐generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121(4):1231‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Xiang T, Lin YX, Ma W, et al. Vasculogenic mimicry formation in EBV‐associated epithelial malignancies. Nat Commun. 2018;9(1):5009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double‐blind, randomised, placebo‐controlled phase III trial. Lancet. 2008;372(9637):449‐456. [DOI] [PubMed] [Google Scholar]
- 185. Powles T, Lackner MR, Oudard S, et al. Randomized open‐label phase II trial of Apitolisib (GDC‐0980), a novel inhibitor of the PI3K/Mammalian target of Rapamycin pathway, versus Everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2016;34(14):1660‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ovais M, Mukherjee S, Pramanik A, et al. Designing stimuli‐responsive upconversion nanoparticles that exploit the tumor microenvironment. Adv Mater. 2020;32(22):e2000055. [DOI] [PubMed] [Google Scholar]
- 187. Poon Z, Chang D, Zhao X, Hammond PT. Layer‐by‐layer nanoparticles with a pH‐sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5(6):4284‐4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Dreaden EC, Morton SW, Shopsowitz KE, et al. Bimodal tumor‐targeting from microenvironment responsive hyaluronan layer‐by‐layer (LbL) nanoparticles. ACS Nano. 2014;8(8):8374‐8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ma S, Zhao Y, Lee WC, et al. Hypoxia induces HIF1α‐dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti‐PD‐1 immunotherapy. Nat Commun. 2022;13(1):4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Vorum H, Østergaard M, Hensechke P, Enghild JJ, Riazati M, Rice GE. Proteomic analysis of hyperoxia‐induced responses in the human choriocarcinoma cell line JEG‐3. Proteomics. 2004;4(3):861‐867. [DOI] [PubMed] [Google Scholar]
- 191. Thews O, Vaupel P. Temporal changes in tumor oxygenation and perfusion upon normo‐ and hyperbaric inspiratory hyperoxia. Strahlenther Onkol. 2016;192(3):174‐181. [DOI] [PubMed] [Google Scholar]
- 192. Hou H, Dong R, Li H, et al. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson. 2012;214(1):22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhang S, Li Z, Wang Q, et al. NIR‐II photothermally triggered “oxygen bomb” for hypoxic tumor programmed cascade therapy. Adv Mater. 2022:e2201978. [DOI] [PubMed] [Google Scholar]
- 194. Wang D, Wu H, Lim WQ, et al. A mesoporous nanoenzyme derived from metal‐organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv Mater. 2019;31(27):e1901893. [DOI] [PubMed] [Google Scholar]
- 195. Kim W, Kim MS, Kim HJ, et al. Role of HIF‐1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int J Hyperthermia. 2018;34(3):276‐283. [DOI] [PubMed] [Google Scholar]
- 196. Zagvazdin Y, Bodo M, Sarkadi A, Szporny L. Cerebroprotective drugs shorten the hypoxia‐induced onset of electrical silence in unanesthetized rats. Pharmacol Res. 2000;42(3):261‐268. [DOI] [PubMed] [Google Scholar]
- 197. Guiard E, Baldini C, Pobel C, et al. Radiological patterns of tumour progression in patients treated with a combination of immune checkpoint blockers and antiangiogenic drugs. Eur J Cancer. 2022;167:42‐53. [DOI] [PubMed] [Google Scholar]
- 198. Meng X, Zhang X, Lei Y, Cao D, Wang Z. Biodegradable copper‐metformin nanoscale coordination polymers for enhanced chemo/chemodynamic synergistic therapy by reducing oxygen consumption to promote H(2)O(2) accumulation. J Mater Chem B. 2021;9(8):1988‐2000. [DOI] [PubMed] [Google Scholar]
- 199. Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58(1):123‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kaplan HP, Robinson FR, Kapanci Y, Weibel ER. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. 1969;20(1):94‐100. [PubMed] [Google Scholar]
- 201. Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol (1985). 1986;61(3):906‐911. [DOI] [PubMed] [Google Scholar]
- 202. Sherman LF, Bacon HE. An evaluation of oxygen therapy as an adjunct in the postoperative management of carcinoma of the rectum and sigmoid colon. Am J Surg. 1951;81(1):105‐110. [DOI] [PubMed] [Google Scholar]
- 203. Yu L, Wang H, Han X, et al. Oxygen therapy alleviates hepatic steatosis by inhibiting hypoxia‐inducible factor‐2α. J Endocrinol. 2020;246(1):57‐67. [DOI] [PubMed] [Google Scholar]
- 204. Hatfield SM, Kjaergaard J, Lukashev D, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α‐dependent and extracellular adenosine‐mediated tumor protection. J Mol Med. 2014;92(12):1283‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tiep B, Carter R, Zachariah F, et al. Oxygen for end‐of‐life lung cancer care: managing dyspnea and hypoxemia. Expert Rev Respir Med. 2013;7(5):479‐490. [DOI] [PubMed] [Google Scholar]
- 206. Hatfield SM, Kjaergaard J, Lukashev D, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7(277):277ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70(1):229‐236. Suppl. [DOI] [PubMed] [Google Scholar]
- 208. Caillard A, Collet M, Hong A, Chousterman BG. Periintubation ventilation and oxygenation of acutely ill patients. Am J Respir Crit Care Med. 2020;201(7):856‐858. [DOI] [PubMed] [Google Scholar]
- 209. Yttersian Sletta K, Tveitarås MK, Lu N, et al. Oxygen‐dependent regulation of tumor growth and metastasis in human breast cancer xenografts. PLoS One. 2017;12(8):e0183254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Selvendiran K, Kuppusamy ML, Ahmed S, et al. Oxygenation inhibits ovarian tumor growth by downregulating STAT3 and cyclin‐D1 expressions. Cancer Biol Ther. 2010;10(4):386‐390. [DOI] [PubMed] [Google Scholar]
- 211. Bennett MH, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst Rev. 2018;4(4):Cd005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Overgaard J. Sensitization of hypoxic tumour cells–clinical experience. Int J Radiat Biol. 1989;56(5):801‐811. [DOI] [PubMed] [Google Scholar]
- 213. Zembrzuska K, Ostrowski RP, Matyja E. Hyperbaric oxygen increases glioma cell sensitivity to antitumor treatment with a novel isothiourea derivative in vitro. Oncol Rep. 2019;41(5):2703‐2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lu Z, Ma J, Liu B, et al. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med. 2016;5(11):3147‐3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Liu Y, Jiang Y, Zhang M, Tang Z, He M, Bu W. Modulating hypoxia via nanomaterials chemistry for efficient treatment of solid tumors. Acc Chem Res. 2018;51(10):2502‐2511. [DOI] [PubMed] [Google Scholar]
- 216. Wang J, Sun J, Hu W, et al. A porous Au@Rh bimetallic core‐shell nanostructure as an H(2)O(2)‐driven oxygenerator to alleviate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater. 2020;32(22):e2001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):47‐63. [DOI] [PubMed] [Google Scholar]
- 218. Wang W, Cheng Y, Yu P, et al. Perfluorocarbon regulates the intratumoural environment to enhance hypoxia‐based agent efficacy. Nat Commun. 2019;10(1):1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tao Z, Ghoroghchian PP. Microparticle, nanoparticle, and stem cell‐based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014;32(9):466‐473. [DOI] [PubMed] [Google Scholar]
- 220. Winslow RM. Current status of oxygen carriers (‘blood substitutes’): 2006. Vox Sang. 2006;91(2):102‐110. [DOI] [PubMed] [Google Scholar]
- 221. Teicher BA, Rose CM. Perfluorochemical emulsions can increase tumor radiosensitivity. Science. 1984;223(4639):934‐936. [DOI] [PubMed] [Google Scholar]
- 222. Song G, Ji C, Liang C, et al. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials. 2017;112:257‐263. [DOI] [PubMed] [Google Scholar]
- 223. Liang X, Chen M, Bhattarai P, Hameed S, Dai Z. Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020;14(10):13569‐13583. [DOI] [PubMed] [Google Scholar]
- 224. Song G, Liang C, Yi X, et al. Perfluorocarbon‐loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near‐infrared light to enhance the radiotherapy of cancer. Adv Mater. 2016;28(14):2716‐2723. [DOI] [PubMed] [Google Scholar]
- 225. Creteur J, Vincent JL. Potential uses of hemoglobin‐based oxygen carriers in critical care medicine. Crit Care Clin. 2009;25(2):311‐324. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 226. Gundersen SI, Palmer AF. Hemoglobin‐based oxygen carrier enhanced tumor oxygenation: a novel strategy for cancer therapy. Biotechnol Prog. 2008;24(6):1353‐1364. [DOI] [PubMed] [Google Scholar]
- 227. Chen Z, Liu L, Liang R, et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti‐tumor immunity and abscopal effect. ACS Nano. 2018;12(8):8633‐8645. [DOI] [PubMed] [Google Scholar]
- 228. Xu T, Ma Y, Yuan Q, et al. Enhanced ferroptosis by oxygen‐boosted phototherapy based on a 2‐in‐1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14(3):3414‐3425. [DOI] [PubMed] [Google Scholar]
- 229. Wang Y, Luo S, Wu Y, et al. Highly penetrable and on‐demand oxygen release with tumor activity composite nanosystem for photothermal/photodynamic synergetic therapy. ACS Nano. 2020; [DOI] [PubMed] [Google Scholar]
- 230. Jiang L, Bai H, Liu L, Lv F, Ren X, Wang S. Luminescent, oxygen‐supplying, hemoglobin‐linked conjugated polymer nanoparticles for photodynamic therapy. Angew Chem Int Ed Engl. 2019;58(31):10660‐10665. [DOI] [PubMed] [Google Scholar]
- 231. Palmer AF, Intaglietta M. Blood substitutes. Annu Rev Biomed Eng. 2014;16:77‐101. [DOI] [PubMed] [Google Scholar]
- 232. Chen J, Liang C, Song X, et al. Hybrid protein nano‐reactors enable simultaneous increments of tumor oxygenation and iodine‐131 delivery for enhanced radionuclide therapy. Small. 2019;15(46):e1903628. [DOI] [PubMed] [Google Scholar]
- 233. Song X, Xu J, Liang C, et al. Self‐supplied tumor oxygenation through separated liposomal delivery of H(2)O(2) and catalase for enhanced radio‐immunotherapy of cancer. Nano Lett. 2018;18(10):6360‐6368. [DOI] [PubMed] [Google Scholar]
- 234. Fu LH, Wan YL, Li CY, et al. Biodegradable calcium phosphate nanotheranostics with tumor‐specific activatable cascade catalytic reactions‐augmented photodynamic therapy. Adv Funct Mater. 2021;31(14):2009848. [Google Scholar]
- 235. Meng L, Cheng Y, Gan S, et al. Facile deposition of manganese dioxide to albumin‐bound paclitaxel nanoparticles for modulation of hypoxic tumor microenvironment to improve chemoradiation therapy. Mol Pharm. 2018;15(2):447‐457. [DOI] [PubMed] [Google Scholar]
- 236. Meng L, Cheng Y, Tong X, et al. Tumor oxygenation and hypoxia inducible factor‐1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano. 2018;12(8):8308‐8322. [DOI] [PubMed] [Google Scholar]
- 237. He J, Fu LH, Qi C, Lin J, Huang P. Metal peroxides for cancer treatment. Bioact Mater. 2021;6(9):2698‐2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Song G, Chen Y, Liang C, et al. Catalase‐loaded TaOx nanoshells as bio‐nanoreactors combining high‐Z element and enzyme delivery for enhancing radiotherapy. Adv Mater. 2016;28(33):7143‐7148. [DOI] [PubMed] [Google Scholar]
- 239. Chudal L, Pandey NK, Phan J, Johnson O, Li X, Chen W. Investigation of PPIX‐Lipo‐MnO(2) to enhance photodynamic therapy by improving tumor hypoxia. Mater Sci Eng C Mater Biol Appl. 2019;104:109979. [DOI] [PubMed] [Google Scholar]
- 240. Kraybill WG, Olenki T, Evans SS, et al. A phase I study of fever‐range whole body hyperthermia (FR‐WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperthermia. 2002;18(3):253‐266. [DOI] [PubMed] [Google Scholar]
- 241. Dunne M, Regenold M, Allen C. Hyperthermia can alter tumor physiology and improve chemo‐ and radio‐therapy efficacy. Adv Drug Deliv Rev. 2020;163‐164:98‐124. [DOI] [PubMed] [Google Scholar]
- 242. Hall RR, Schade RO, Swinney J. Effects of hyperthermia on bladder cancer. Br Med J. 1974;2(5919):593‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Lindholm CE, Kjellén E, Nilsson P, Weber L, Hill S. Prognostic factors for tumour response and skin damage to combined radiotherapy and hyperthermia in superficial recurrent breast carcinomas. Int J Hyperthermia. 1995;11(3):337‐355. [DOI] [PubMed] [Google Scholar]
- 244. Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia. 1996;12(1):3‐20. [DOI] [PubMed] [Google Scholar]
- 245. Sakaguchi Y, Makino M, Kaneko T, et al. Therapeutic efficacy of long duration‐low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res. 1994;54(8):2223‐2227. [PubMed] [Google Scholar]
- 246. Hauck ML, Price GS, Ogilvie GK, et al. Phase I evaluation of mitoxantrone alone and combined with whole body hyperthermia in dogs with lymphoma. Int J Hyperthermia. 1996;12(3):309‐320. [DOI] [PubMed] [Google Scholar]
- 247. Robins HI, D'Oleire F, Grosen E, Spriggs D. Rationale and clinical status of 41.8 degrees C systemic hyperthermia tumor necrosis factor, and melphalan for neoplastic disease. Anticancer Res. 1997;17(4b):2891‐2894. [PubMed] [Google Scholar]
- 248. Sun X, Li XF, Russell J, et al. Changes in tumor hypoxia induced by mild temperature hyperthermia as assessed by dual‐tracer immunohistochemistry. Radiother Oncol. 2008;88(2):269‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Thrall DE, Maccarini P, Stauffer P, et al. Thermal dose fractionation affects tumour physiological response. Int J Hyperthermia. 2012;28(5):431‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Shoji S. Editorial comment to combination therapy with radiation and hyperthermia‐induced clinical complete response of small cell carcinoma of prostate. IJU Case Rep. 2022;5(2):116‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Dusault LA. The effect of oxygen on the response of spontaneous tumours in mice to radiotherapy. Br J Radiol. 1963;36:749‐754. [DOI] [PubMed] [Google Scholar]
- 252. Inch WR, McCredie JA, Kruuv J. Effect of breathing 5 per cent carbon dioxide and 95 per cent oxygen at atmospheric pressure on tumour radiocurability. Acta Radiol Ther Phys Biol. 1966;4(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 253. Robinson SP, Collingridge DR, Howe FA, Rodrigues LM, Chaplin DJ, Griffiths JR. Tumour response to hypercapnia and hyperoxia monitored by FLOOD magnetic resonance imaging. NMR Biomed. 1999;12(2):98‐106. [DOI] [PubMed] [Google Scholar]
- 254. Schuuring J, Rijpkema M, Bernsen H, et al. Effect of breathing a hyperoxic hypercapnic gas mixture on the oxygenation of meningiomas; preliminary results. J Neurooncol. 2002;57(2):127‐132. [DOI] [PubMed] [Google Scholar]
- 255. Oikawa H, al‐Hallaq HA, Lewis MZ, River JN, Kovar DA, Karczmar GS. Spectroscopic imaging of the water resonance with short repetition time to study tumor response to hyperoxia. Magn Reson Med. 1997;38(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 256. Thews O, Kelleher DK, Vaupel P. Dynamics of tumor oxygenation and red blood cell flux in response to inspiratory hyperoxia combined with different levels of inspiratory hypercapnia. Radiother Oncol. 2002;62(1):77‐85. [DOI] [PubMed] [Google Scholar]
- 257. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795‐803. [DOI] [PubMed] [Google Scholar]
- 258. Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5(12):1359‐1364. [DOI] [PubMed] [Google Scholar]
- 259. Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin‐1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553‐563. [DOI] [PubMed] [Google Scholar]
- 260. Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Ciric E, Sersa G. Radiotherapy in combination with vascular‐targeted therapies. Radiol Oncol. 2010;44(2):67‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Hoang T, Huang S, Armstrong E, Eickhoff JC, Harari PM. Enhancement of radiation response with bevacizumab. J Exp Clin Cancer Res. 2012;31(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Park JS, Kim IK, Han S, et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30(6):953‐967. [DOI] [PubMed] [Google Scholar]
- 264. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58‐62. [DOI] [PubMed] [Google Scholar]
- 265. Norden AD, Drappatz J, Wen PY. Novel anti‐angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152‐1160. [DOI] [PubMed] [Google Scholar]
- 266. Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor‐induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841‐844. [DOI] [PubMed] [Google Scholar]
- 267. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan‐VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Duda DG, Batchelor TT, Willett CG, Jain RK. VEGF‐targeted cancer therapy strategies: current progress, hurdles and future prospects. Trends Mol Med. 2007;13(6):223‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti‐VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24‐40. [DOI] [PubMed] [Google Scholar]
- 270. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539‐1544. [DOI] [PubMed] [Google Scholar]
- 271. Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose‐escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30(5):117‐124. Suppl 16. [DOI] [PubMed] [Google Scholar]
- 272. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335‐2342. [DOI] [PubMed] [Google Scholar]
- 273. Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first‐line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group randomized phase III MAX study. J Clin Oncol. 2010;28(19):3191‐3198. [DOI] [PubMed] [Google Scholar]
- 274. Konno H, Yamamoto M, Ohta M. Recent concepts of antiangiogenic therapy. Surg Today. 2010;40(6):494‐500. [DOI] [PubMed] [Google Scholar]
- 275. Ashton TM, Fokas E, Kunz‐Schughart LA, et al. The anti‐malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat Commun. 2016;7:12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Zuo H, Tao J, Shi H, He J, Zhou Z, Zhang C. Platelet‐mimicking nanoparticles co‐loaded with W(18)O(49) and metformin alleviate tumor hypoxia for enhanced photodynamic therapy and photothermal therapy. Acta Biomater. 2018;80:296‐307. [DOI] [PubMed] [Google Scholar]
- 277. Yang Z, Wang J, Liu S, et al. Defeating relapsed and refractory malignancies through a nano‐enabled mitochondria‐mediated respiratory inhibition and damage pathway. Biomaterials. 2020;229:119580. [DOI] [PubMed] [Google Scholar]
- 278. Wheaton WW, Weinberg SE, Hamanaka RB, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Sonveaux P, Végran F, Schroeder T, et al. Targeting lactate‐fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930‐3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Zuo L, Nie W, Yu S, et al. Smart tumor‐cell‐derived microparticles provide on‐demand photosensitizer synthesis and hypoxia relief for photodynamic therapy. Angew Chem Int Ed Engl. 2021;60(48):25365‐25371. [DOI] [PubMed] [Google Scholar]
- 281. Yu W, Liu T, Zhang M, et al. O(2) economizer for inhibiting cell respiration to combat the hypoxia obstacle in tumor treatments. ACS Nano. 2019;13(2):1784‐1794. [DOI] [PubMed] [Google Scholar]
- 282. Mannino DM. Insights into the spectrum of chronic lower respiratory disease: low lung function is still bad. Am J Respir Crit Care Med. 2021;204(8):873‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Heyman E, Daussin F, Wieczorek V, et al. Muscle oxygen supply and use in type 1 diabetes, from ambient air to the mitochondrial respiratory chain: is there a limiting step? Diabetes Care. 2020;43(1):209‐218. [DOI] [PubMed] [Google Scholar]
- 284. Ulrich S, Hasler ED, Saxer S, et al. Effect of breathing oxygen‐enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham‐controlled cross‐over trial. Eur Heart J. 2017;38(15):1159‐1168. [DOI] [PubMed] [Google Scholar]
- 285. Bruera E, de Stoutz N, Velasco‐Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal‐cancer patients. Lancet. 1993;342(8862):13‐14. [DOI] [PubMed] [Google Scholar]
- 286. Heston WE, Pratt AW. Increase in induced pulmonary tumors in mice associated with exposure to high concentration of oxygen. Proc Soc Exp Biol Med. 1956;92(2):451‐454. [DOI] [PubMed] [Google Scholar]
- 287. Lindenschmidt RC, Tryka AF, Witschi HP. Inhibition of mouse lung tumor development by hyperoxia. Cancer Res. 1986;46(4):1994‐2000. Pt 2. [PubMed] [Google Scholar]
- 288. Lindenschmidt RC, Margaretten N, Griesemer RA, Witschi HP. Modification of lung tumor growth by hyperoxia. Carcinogenesis. 1986;7(9):1581‐1586. [DOI] [PubMed] [Google Scholar]
- 289. Schüller HM, Nylen E, Park P, Becker KL. Nicotine, acetylcholine and bombesin are trophic growth factors in neuroendocrine cell lines derived from experimental hamster lung tumors. Life Sci. 1990;47(6):571‐578. [DOI] [PubMed] [Google Scholar]
- 290. Nylen ES, Becker KL, Joshi PA, Snider RH, Schuller HM. Pulmonary bombesin and calcitonin in hamsters during exposure to hyperoxia and diethylnitrosamine. Am J Respir Cell Mol Biol. 1990;2(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 291. Schuller HM, Correa E, Orloff M, Reznik GK. Successful chemotherapy of experimental neuroendocrine lung tumors in hamsters with an antagonist of Ca2+/calmodulin. Cancer Res. 1990;50(5):1645‐1649. [PubMed] [Google Scholar]
- 292. Schuller HM, Witschi HP, Nylen E, Joshi PA, Correa E, Becker KL. Pathobiology of lung tumors induced in hamsters by 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone and the modulating effect of hyperoxia. Cancer Res. 1990;50(6):1960‐1965. [PubMed] [Google Scholar]
- 293. Ning H, Oreffo VI, Gumerlock PH, Witschi H. Increased c‐Ki‐ras expression in hamster lung exposed to N‐nitrosodiethylamine and hyperoxia as detected by the polymerase chain reaction. Cancer Lett. 1991;59(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 294. Schuller HM, McGavin MD, Orloff M, Riechert A, Porter B. Simultaneous exposure to nicotine and hyperoxia causes tumors in hamsters. Lab Invest. 1995;73(3):448‐456. [PubMed] [Google Scholar]
- 295. Margaretten NC, Witschi HR. Effects of hyperoxia on growth of experimental lung metastasis. Carcinogenesis. 1988;9(3):433‐439. [DOI] [PubMed] [Google Scholar]
- 296. Witschi H. Effects of oxygen and ozone on mouse lung tumorigenesis. Exp Lung Res. 1991;17(2):473‐483. [DOI] [PubMed] [Google Scholar]
- 297. Liu X, Qin H, Li Z, et al. Inspiratory hyperoxia suppresses lung cancer metastasis through a MYC/SLC1A5‐dependent metabolic pathway. Eur Respir J. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Kim SW, Kim IK, Ha JH, et al. Normobaric hyperoxia inhibits the progression of lung cancer by inducing apoptosis. Exp Biol Med. 2018;243(9):739‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Wang X, Ryter SW, Dai C, et al. Necrotic cell death in response to oxidant stress involves the activation of the apoptogenic caspase‐8/bid pathway. J Biol Chem. 2003;278(31):29184‐29191. [DOI] [PubMed] [Google Scholar]
- 300. Thews O, Vaupel P. Spatial oxygenation profiles in tumors during normo‐ and hyperbaric hyperoxia. Strahlenther Onkol. 2015;191(11):875‐882. [DOI] [PubMed] [Google Scholar]
- 301. Mueller‐Klieser W, Vaupel P, Manz R. Effectiveness of respiratory hyperoxia, of normobaric and of hyperbaric oxygen atmospheres in improving tumor oxygenation. Adv Exp Med Biol. 1984;169:613‐619. [DOI] [PubMed] [Google Scholar]
- 302. Li K, Gong Y, Qiu D, et al. Hyperbaric oxygen facilitates teniposide‐induced cGAS‐STING activation to enhance the antitumor efficacy of PD‐1 antibody in HCC. J Immunother Cancer. 2022;10(8):e004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Nakada T. Hyperbaric oxygenation for experimental bladder tumor. I. Tissue oxygen tension of the rabbit bladder during hyperbaric oxygenation. Eur Urol. 1988;14(2):145‐149. [DOI] [PubMed] [Google Scholar]
- 304. Ueno M, Seferynska I, Beckman B, Brookins J, Nakashima J, Fisher JW. Enhanced erythropoietin secretion in hepatoblastoma cells in response to hypoxia. Am J Physiol. 1989;257(4):C743‐9. Pt 1. [DOI] [PubMed] [Google Scholar]
- 305. Moen I, Jevne C, Wang J, et al. Gene expression in tumor cells and stroma in dsRed 4T1 tumors in eGFP‐expressing mice with and without enhanced oxygenation. BMC Cancer. 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Hatfield SM, Sitkovsky MV. Antihypoxic oxygenation agents with respiratory hyperoxia to improve cancer immunotherapy. J Clin Invest. 2020;130(11):5629‐5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomed. 2018;13:6049‐6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Kim SW, Kim IK, Lee SH. Role of hyperoxic treatment in cancer. Exp Biol Med. 2020;245(10):851‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Churchill‐Davidson I, Sanger C, Thomlinson RH. High‐pressure oxygen and radiotherapy. Lancet. 1955;268(6874):1091‐1095. [DOI] [PubMed] [Google Scholar]
- 310. Ristescu AI, Tiron CE, Tiron A, Grigoras I. Exploring hyperoxia effects in cancer‐from perioperative clinical data to potential molecular mechanisms. Biomedicines. 2021;9(9):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21(6):363‐381. [DOI] [PubMed] [Google Scholar]
- 312. Allen RG, Balin AK. Effects of oxygen on the antioxidant responses of normal and transformed cells. Exp Cell Res. 2003;289(2):307‐316. [DOI] [PubMed] [Google Scholar]
- 313. Chopra S, Wallace HM. Induction of spermidine/spermine N1‐acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem Pharmacol. 1998;55(7):1119‐1123. [DOI] [PubMed] [Google Scholar]
- 314. Stuhr LE, Raa A, Oyan AM, et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J Neurooncol. 2007;85(2):191‐202. [DOI] [PubMed] [Google Scholar]
- 315. Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia‐induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl‐2 proteins. J Biol Chem. 2002;277(18):15654‐15660. [DOI] [PubMed] [Google Scholar]
- 316. Bilodeau JF, Faure R, Piedboeuf B, Mirault ME. Hyperoxia induces S‐phase cell‐cycle arrest and p21(Cip1/Waf1)‐independent Cdk2 inhibition in human carcinoma T47D‐H3 cells. Exp Cell Res. 2000;256(2):347‐357. [DOI] [PubMed] [Google Scholar]
- 317. Helt CE, Rancourt RC, Staversky RJ, O'Reilly MA. p53‐dependent induction of p21(Cip1/WAF1/Sdi1) protects against oxygen‐induced toxicity. Toxicol Sci. 2001;63(2):214‐222. [DOI] [PubMed] [Google Scholar]
- 318. Gehen SC, Vitiello PF, Bambara RA, Keng PC, O'Reilly MA. Downregulation of PCNA potentiates p21‐mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L716‐24. [DOI] [PubMed] [Google Scholar]
- 319. Cannizzaro A, Verga Falzacappa CV, Martinelli M, Misiti S, Brunetti E, Bucci B. O(2/3) exposure inhibits cell progression affecting cyclin B1/cdk1 activity in SK‐N‐SH while induces apoptosis in SK‐N‐DZ neuroblastoma cells. J Cell Physiol. 2007;213(1):115‐125. [DOI] [PubMed] [Google Scholar]
- 320. Kopecka J, Salaroglio IC, Perez‐Ruiz E, et al. Hypoxia as a driver of resistance to immunotherapy. Drug Resist Updat. 2021;59:100787. [DOI] [PubMed] [Google Scholar]
- 321. Granowitz EV, Tonomura N, Benson RM, et al. Hyperbaric oxygen inhibits benign and malignant human mammary epithelial cell proliferation. Anticancer Res. 2005;25(6b):3833‐3842. [PubMed] [Google Scholar]
- 322. Raa A, Stansberg C, Steen VM, Bjerkvig R, Reed RK, Stuhr LE. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA‐induced rat mammary tumors. BMC Cancer. 2007;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Kalns JE, Piepmeier EH. Exposure to hyperbaric oxygen induces cell cycle perturbation in prostate cancer cells. In Vitro Cell Dev Biol Anim. 1999;35(2):98‐101. [DOI] [PubMed] [Google Scholar]
- 324. Panayiotidis MI, Rancourt RC, Allen CB, et al. Hyperoxia‐induced DNA damage causes decreased DNA methylation in human lung epithelial‐like A549 cells. Antioxid Redox Signal. 2004;6(1):129‐136. [DOI] [PubMed] [Google Scholar]
- 325. Moen I, Øyan AM, Kalland KH, et al. Hyperoxic treatment induces mesenchymal‐to‐epithelial transition in a rat adenocarcinoma model. PLoS One. 2009;4(7):e6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Resseguie EA, Brookes PS, O'Reilly MA. SMG‐1 kinase attenuates mitochondrial ROS production but not cell respiration deficits during hyperoxia. Exp Lung Res. 2017;43(6‐7):229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Pinterić M, Podgorski II, Sobočanec S, et al. De novo expression of transfected sirtuin 3 enhances susceptibility of human MCF‐7 breast cancer cells to hyperoxia treatment. Free Radic Res. 2018;52(6):672‐684. [DOI] [PubMed] [Google Scholar]
- 328. De Bels D, Tillmans F, Corazza F, et al. Hyperoxia alters ultrastructure and induces apoptosis in leukemia cell lines. Biomolecules. 2020;10(2):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Podgorski II, Pinterić M, Marčinko D, et al. Combination of sirtuin 3 and hyperoxia diminishes tumorigenic properties of MDA‐MB‐231 cells. Life Sci. 2020;254:117812. [DOI] [PubMed] [Google Scholar]
- 330. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Vandenbrenk HA, Elliott K, Hutchings H. Further observations on radiocurability of a solid ehrlich and tissue reactions in the mouse with fractionated radiation doses and the effects of oxygen. Br J Cancer. 1963;17(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066‐4074. [DOI] [PubMed] [Google Scholar]
- 333. Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26(312):638‐648. [DOI] [PubMed] [Google Scholar]
- 334. Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9(4):539‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 336. Terris DJ, Dunphy EP. Oxygen tension measurements of head and neck cancers. Arch Otolaryngol Head Neck Surg. 1994;120(3):283‐287. [DOI] [PubMed] [Google Scholar]
- 337. Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24(5):727‐735. [DOI] [PubMed] [Google Scholar]
- 338. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF‐1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5(5):429‐441. [DOI] [PubMed] [Google Scholar]
- 339. Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet. 1977;2(8029):101‐103. [DOI] [PubMed] [Google Scholar]
- 340. Henk JM, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet. 1977;2(8029):104‐105. [DOI] [PubMed] [Google Scholar]
- 341. Wildermuth O. The case for hyperbaric oxygen radiotherapy. JAMA. 1965;191:986‐990. [DOI] [PubMed] [Google Scholar]
- 342. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674‐687. [DOI] [PubMed] [Google Scholar]
- 343. Carl UM, Ewert G, Vaupel P. Hyperbaric oxygen in radiation therapy: the role of respiratory hyperoxia. Strahlenther Onkol. 1996;172(2):2‐3. Suppl. [PubMed] [Google Scholar]
- 344. Gao M, Liang C, Song X, et al. Erythrocyte‐membrane‐enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater. 2017;29(35). [DOI] [PubMed] [Google Scholar]
- 345. Yang Y, Chen M, Wang B, et al. NIR‐II driven plasmon‐enhanced catalysis for a timely supply of oxygen to overcome hypoxia‐induced radiotherapy tolerance. Angew Chem Int Ed Engl. 2019;58(42):15069‐15075. [DOI] [PubMed] [Google Scholar]
- 346. Coleman CN, Bump EA, Kramer RA. Chemical modifiers of cancer treatment. J Clin Oncol. 1988;6(4):709‐733. [DOI] [PubMed] [Google Scholar]
- 347. Brown JM. Clinical perspectives for the use of new hypoxic cell sensitizers. Int J Radiat Oncol Biol Phys. 1982;8(9):1491‐1497. [DOI] [PubMed] [Google Scholar]
- 348. Hay MP, Denny WA, Wilson WR. Nitroimidazole‐based “extruded mustards” designed as reductively activated hypoxia‐selective cytotoxins. Anticancer Drug Des. 1996;11(5):383‐402. [PubMed] [Google Scholar]
- 349. Nordsmark M, Maxwell RJ, Horsman MR, Bentzen SM, Overgaard J. The effect of hypoxia and hyperoxia on nucleoside triphosphate/inorganic phosphate, pO2 and radiation response in an experimental tumour model. Br J Cancer. 1997;76(11):1432‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Al‐Hallaq HA, River JN, Zamora M, Oikawa H, Karczmar GS. Correlation of magnetic resonance and oxygen microelectrode measurements of carbogen‐induced changes in tumor oxygenation. Int J Radiat Oncol Biol Phys. 1998;41(1):151‐159. [DOI] [PubMed] [Google Scholar]
- 351. Pietrofesa RA, Velalopoulou A, Lehman SL, et al. Novel double‐hit model of radiation and hyperoxia‐induced oxidative cell damage relevant to space travel. Int J Mol Sci. 2016;17(6):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Hartmann KA, van der Kleij AJ, Carl UM, Hulshof MC, Willers R, Sminia P. Effects of hyperbaric oxygen and normobaric carbogen on the radiation response of the rat rhabdomyosarcoma R1H. Int J Radiat Oncol Biol Phys. 2001;51(4):1037‐1044. [DOI] [PubMed] [Google Scholar]
- 353. Kinoshita Y, Kohshi K, Kunugita N, Tosaki T, Yokota A. Preservation of tumour oxygen after hyperbaric oxygenation monitored by magnetic resonance imaging. Br J Cancer. 2000;82(1):88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Kunugita N, Kohshi K, Kinoshita Y, et al. Radiotherapy after hyperbaric oxygenation improves radioresponse in experimental tumor models. Cancer Lett. 2001;164(2):149‐154. [DOI] [PubMed] [Google Scholar]
- 355. Beppu T, Kamada K, Yoshida Y, Arai H, Ogasawara K, Ogawa A. Change of oxygen pressure in glioblastoma tissue under various conditions. J Neurooncol. 2002;58(1):47‐52. [DOI] [PubMed] [Google Scholar]
- 356. Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y. Emerging strategies of nanomaterial‐mediated tumor radiosensitization. Adv Mater. 2019;31(3):e1802244. [DOI] [PubMed] [Google Scholar]
- 357. Zai W, Kang L, Dong T, et al. E. coli membrane vesicles as a catalase carrier for long‐term tumor hypoxia relief to enhance radiotherapy. ACS Nano. 2021;15(9):15381‐15394. [DOI] [PubMed] [Google Scholar]
- 358. Arenas M, Algara M, De Febrer G, et al. Could pulmonary low‐dose radiation therapy be an alternative treatment for patients with COVID‐19 pneumonia? Preliminary results of a multicenter SEOR‐GICOR nonrandomized prospective trial (IPACOVID trial). Strahlenther Onkol. 2021;197(11):1010‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Chvetsov AV, Rajendran JG, Zeng J, Patel SA, Bowen SR, Kim EY. Theoretical effectiveness of cell survival in fractionated radiotherapy with hypoxia‐targeted dose escalation. Med Phys. 2017;44(5):1975‐1982. [DOI] [PubMed] [Google Scholar]
- 360. Lee DJ, Pajak TF, Stetz J, Order SE, Weissberg JB, Fischer JJ. A phase I/II study of the hypoxic cell sensitizer misonidazole as an adjunct to high fractional dose radiotherapy in patients with unresectable squamous cell carcinoma of the head and neck: a RTOG randomized study (#79‐04). Int J Radiat Oncol Biol Phys. 1989;16(2):465‐470. [DOI] [PubMed] [Google Scholar]
- 361. Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose‐rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93. [DOI] [PubMed] [Google Scholar]
- 362. Hughes JR, Parsons JL. FLASH radiotherapy: current knowledge and future insights using proton‐beam therapy. Int J Mol Sci. 2020;21(18):6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. El Khatib M, Van Slyke AL, Velalopoulou A, et al. Ultrafast tracking of oxygen dynamics during proton FLASH. Int J Radiat Oncol Biol Phys. 2022;113(3):624‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Guo Z, Buonanno M, Harken A, Zhou G, Hei TK. Mitochondrial damage response and fate of normal cells exposed to FLASH irradiation with protons. Radiat Res. 2022;197(6):569‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Lai Y, Jia X, Chi Y. Modeling the effect of oxygen on the chemical stage of water radiolysis using GPU‐based microscopic Monte Carlo simulations, with an application in FLASH radiotherapy. Phys Med Biol. 2021;66(2):025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Colangelo NW, Azzam EI. The importance and clinical implications of FLASH Ultra‐High Dose‐Rate Studies for Proton and Heavy Ion Radiotherapy. Radiat Res. 2020;193(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Zakaria AM, Colangelo NW, Meesungnoen J, Azzam EI, Plourde M, Jay‐Gerin JP. Ultra‐High dose‐rate, pulsed (FLASH) radiotherapy with carbon ions: generation of early, transient, highly oxygenated conditions in the tumor environment. Radiat Res. 2020;194(6):587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Kohshi K, Kinoshita Y, Imada H, et al. Effects of radiotherapy after hyperbaric oxygenation on malignant gliomas. Br J Cancer. 1999;80(1‐2):236‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Ogawa K, Ishiuchi S, Inoue O, et al. Phase II trial of radiotherapy after hyperbaric oxygenation with multiagent chemotherapy (procarbazine, nimustine, and vincristine) for high‐grade gliomas: long‐term results. Int J Radiat Oncol Biol Phys. 2012;82(2):732‐738. [DOI] [PubMed] [Google Scholar]
- 370. Chen JR, Xu HZ, Ding JB, Qin ZY. Radiotherapy after hyperbaric oxygenation in malignant gliomas. Curr Med Res Opin. 2015;31(11):1977‐1984. [DOI] [PubMed] [Google Scholar]
- 371. Creutzberg CL. Hyperbaric oxygen therapy for radiation‐induced injury: evidence is needed. Lancet Oncol. 2016;17(2):132‐134. [DOI] [PubMed] [Google Scholar]
- 372. Oscarsson N, Müller B, Rosén A, et al. Radiation‐induced cystitis treated with hyperbaric oxygen therapy (RICH‐ART): a randomised, controlled, phase 2–3 trial. Lancet Oncol. 2019;20(11):1602‐1614. [DOI] [PubMed] [Google Scholar]
- 373. Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo‐controlled, double‐blind trial from the ORN96 study group. J Clin Oncol. 2004;22(24):4893‐4900. [DOI] [PubMed] [Google Scholar]
- 374. Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012(5):Cd005005. [DOI] [PubMed] [Google Scholar]
- 375. Glover M, Smerdon GR, Andreyev HJ, et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double‐blind, sham‐controlled phase 3 trial. Lancet Oncol. 2016;17(2):224‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8(7):790‐797. [DOI] [PubMed] [Google Scholar]
- 377. Fechner G, Dederichs F, Schmidt D, Müller S, Vaupel P, Albers P. Hyperoxia‐induced improvement of the in vitro response to gemcitabine in transitional cell carcinoma. Anticancer Res. 2005;25(5):3413‐3418. [PubMed] [Google Scholar]
- 378. Shannon AM, Bouchier‐Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia‐related therapies. Cancer Treat Rev. 2003;29(4):297‐307. [DOI] [PubMed] [Google Scholar]
- 379. Cohen YC, Zada M, Wang SY, et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single‐cell sequencing. Nat Med. 2021;27(3):491‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Dische S. Hyperbaric oxygen: the Medical Research Council trials and their clinical significance. Br J Radiol. 1978;51(611):888‐894. [DOI] [PubMed] [Google Scholar]
- 381. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160(5):519‐524. [DOI] [PubMed] [Google Scholar]
- 382. Alagoz T, Buller RE, Anderson B, et al. Evaluation of hyperbaric oxygen as a chemosensitizer in the treatment of epithelial ovarian cancer in xenografts in mice. Cancer. 1995;75(9):2313‐2322. [DOI] [PubMed] [Google Scholar]
- 383. Lee HY, Kim IK, Lee HI, et al. Combination of carboplatin and intermittent normobaric hyperoxia synergistically suppresses benzo[a]pyrene‐induced lung cancer. Korean J Intern Med. 2018;33(3):541‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175‐176. [DOI] [PubMed] [Google Scholar]
- 385. Huang CC, Chia WT, Chung MF, et al. An implantable depot that can generate oxygen in situ for overcoming hypoxia‐induced resistance to anticancer drugs in chemotherapy. J Am Chem Soc. 2016;138(16):5222‐5225. [DOI] [PubMed] [Google Scholar]
- 386. Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039‐3051. [PubMed] [Google Scholar]
- 387. Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52(22):6371‐6374. [PubMed] [Google Scholar]
- 388. Nathanson SD, Nelson L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol. 1994;1(4):333‐338. [DOI] [PubMed] [Google Scholar]
- 389. Moen I, Tronstad KJ, Kolmannskog O, Salvesen GS, Reed RK, Stuhr LE. Hyperoxia increases the uptake of 5‐fluorouracil in mammary tumors independently of changes in interstitial fluid pressure and tumor stroma. BMC Cancer. 2009;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Wu X, Zhu Y, Huang W, et al. Hyperbaric oxygen potentiates doxil antitumor efficacy by promoting tumor penetration and sensitizing cancer cells. Adv Sci. 2018;5(8):1700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Wheeler RH, Dirks JW, Lunardi I, Nemiroff MJ. Effect of hyperbaric oxygen on the cytotoxicity of adriamycin and nitrogen mustard in cultured Burkitt's lymphoma cells. Cancer Res. 1979;39(2):370‐375. Pt 1. [PubMed] [Google Scholar]
- 392. Ma X, Yao M, Shi J, et al. High intensity focused ultrasound‐responsive and ultrastable cerasomal perfluorocarbon nanodroplets for alleviating tumor multidrug resistance and epithelial‐mesenchymal transition. ACS Nano. 2020;14(11):15904‐15918. [DOI] [PubMed] [Google Scholar]
- 393. Akiya T, Nakada T, Katayama T, et al. Hyperbaric oxygenation for experimental bladder tumor. II. Hyperbaric oxygenation in combination with chemotherapy in N‐butyl‐N‐(4‐hydroxybutyl)nitrosamine‐induced bladder tumors. Eur Urol. 1988;14(2):150‐155. [PubMed] [Google Scholar]
- 394. Argyriou AA, Antonacopoulou A, Iconomou G, Kalofonos HP. Treatment options for malignant gliomas, emphasizing towards new molecularly targeted therapies. Crit Rev Oncol Hematol. 2009;69(3):199‐210. [DOI] [PubMed] [Google Scholar]
- 395. Sun S, Lee D, Ho AS, et al. Inhibition of prolyl 4‐hydroxylase, beta polypeptide (P4HB) attenuates temozolomide resistance in malignant glioma via the endoplasmic reticulum stress response (ERSR) pathways. Neuro Oncol. 2013;15(5):562‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Lu XY, Cao K, Li QY, Yuan ZC, Lu PS. The synergistic therapeutic effect of temozolomide and hyperbaric oxygen on glioma U251 cell lines is accompanied by alterations in vascular endothelial growth factor and multidrug resistance‐associated protein‐1 levels. J Int Med Res. 2012;40(3):995‐1004. [DOI] [PubMed] [Google Scholar]
- 397. Sun S, Lee D, Lee NP, et al. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J Neurooncol. 2012;109(3):467‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Lee D, Sun S, Ho AS, et al. Hyperoxia resensitizes chemoresistant glioblastoma cells to temozolomide through unfolded protein response. Anticancer Res. 2014;34(6):2957‐2966. [PubMed] [Google Scholar]
- 399. Teicher BA, Holden SA, al‐Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50(11):3339‐3344. [PubMed] [Google Scholar]
- 400. Rasoulian B, Kaeidi A, Rezaei M, Hajializadeh Z. Cellular preoxygenation partially attenuates the antitumoral effect of cisplatin despite highly protective effects on renal epithelial cells. Oxid Med Cell Longev. 2017;2017:7203758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Rodrigues LM, Robinson SP, McSheehy PM, Stubbs M, Griffiths JR. Enhanced uptake of ifosfamide into GH3 prolactinomas with hypercapnic hyperoxic gases monitored in vivo by (31)P MRS. Neoplasia. 2002;4(6):539‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Im J, Kwon HY, Kim IK, et al. Normobaric hyperoxia re‐sensitizes paclitaxel‐resistant lung cancer cells. Mol Cell Toxicol. 2022;18(4):539‐548. [Google Scholar]
- 403. Qian X, Li X, Shi Z, et al. KDM3A senses oxygen availability to regulate PGC‐1α‐mediated mitochondrial biogenesis. Mol Cell. 2019;76(6):885‐895.e7. [DOI] [PubMed] [Google Scholar]
- 404. Li J, Zhang T, Ren T, et al. Oxygen‐sensitive methylation of ULK1 is required for hypoxia‐induced autophagy. Nat Commun. 2022;13(1):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Huang X, Ding L, Bennewith KL, et al. Hypoxia‐inducible mir‐210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35(6):856‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Fasanaro P, D'Alessandra Y, Di Stefano V, et al. MicroRNA‐210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin‐A3. J Biol Chem. 2008;283(23):15878‐15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Mimnaugh EG, Dusre L, Atwell J, Myers CE. Differential oxygen radical susceptibility of adriamycin‐sensitive and ‐resistant MCF‐7 human breast tumor cells. Cancer Res. 1989;49(1):8‐15. [PubMed] [Google Scholar]
- 408. Leone RD, Horton MR, Powell JD. Something in the air: hyperoxic conditioning of the tumor microenvironment for enhanced immunotherapy. Cancer Cell. 2015;27(4):435‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Vols S, Sionov RV, Granot Z. Always look on the bright side: anti‐tumor functions of neutrophils. Curr Pharm Des. 2017;23(32):4862‐4892. [DOI] [PubMed] [Google Scholar]
- 410. Almzaiel AJ, Billington R, Smerdon G, Moody AJ. Hyperbaric oxygen enhances neutrophil apoptosis and their clearance by monocyte‐derived macrophages. Biochem Cell Biol. 2015;93(4):405‐416. [DOI] [PubMed] [Google Scholar]
- 411. Mahiddine K, Blaisdell A, Ma S, Créquer‐Grandhomme A, Lowell CA, Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest. 2020;130(1):389‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Wang Y, Yin K, Tian J, et al. Granulocytic myeloid‐derived suppressor cells promote the stemness of colorectal cancer cells through exosomal S100A9. Adv Sci. 2019;6(18):1901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Noman MZ, Hasmim M, Lequeux A, et al. Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: new opportunities and challenges. Cells. 2019;8(9):1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Hussain SA, Birtle A, Crabb S, et al. From clinical trials to real‐life clinical practice: the role of immunotherapy with PD‐1/PD‐L1 inhibitors in advanced urothelial carcinoma. Eur Urol Oncol. 2018;1(6):486‐500. [DOI] [PubMed] [Google Scholar]
- 415. Najjar YG, Menk AV, Sander C, et al. Tumor cell oxidative metabolism as a barrier to PD‐1 blockade immunotherapy in melanoma. JCI Insight. 2019;4(5):e124989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Riera‐Domingo C, Audigé A, Granja S, et al. Immunity, hypoxia, and metabolism‐the Ménage à Trois of cancer: implications for immunotherapy. Physiol Rev. 2020;100(1):1‐102. [DOI] [PubMed] [Google Scholar]
- 418. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36(4):439‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Noman MZ, Buart S, Romero P, et al. Hypoxia‐inducible miR‐210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72(18):4629‐4641. [DOI] [PubMed] [Google Scholar]
- 420. Sethumadhavan S, Silva M, Philbrook P, et al. Hypoxia and hypoxia‐inducible factor (HIF) downregulate antigen‐presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One. 2017;12(11):e0187314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Jiang M, Qin B, Luo L, et al. A clinically acceptable strategy for sensitizing anti‐PD‐1 treatment by hypoxia relief. J Control Release. 2021;335:408‐419. [DOI] [PubMed] [Google Scholar]
- 422. Qian X, Zhang Q, Shao N, et al. Respiratory hyperoxia reverses immunosuppression by regulating myeloid‐derived suppressor cells and PD‐L1 expression in a triple‐negative breast cancer mouse model. Am J Cancer Res. 2019;9(3):529‐545. [PMC free article] [PubMed] [Google Scholar]
- 423. Liu X, Ye N, Liu S, et al. Hyperbaric oxygen boosts PD‐1 antibody delivery and T cell infiltration for augmented immune responses against solid tumors. Adv Sci. 2021;8(15):e2100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Herrera‐Campos AB, Zamudio‐Martinez E, Delgado‐Bellido D, et al. Implications of hyperoxia over the tumor microenvironment: an overview highlighting the importance of the immune system. Cancers. 2022;14(11):2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Blum NT, Zhang Y, Qu J, Lin J, Huang P. Recent advances in self‐exciting photodynamic therapy. Front Bioeng Biotechnol. 2020;8:594491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol. 2006;1(5):489‐493. [PubMed] [Google Scholar]
- 427. Sibille A, Lambert R, Souquet JC, Sabben G, Descos F. Long‐term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108(2):337‐344. [DOI] [PubMed] [Google Scholar]
- 428. Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Dalla Via L, Marciani Magno S. Photochemotherapy in the treatment of cancer. Curr Med Chem. 2001;8(12):1405‐1418. [DOI] [PubMed] [Google Scholar]
- 430. Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev. 2016;45(23):6488‐6519. [DOI] [PubMed] [Google Scholar]
- 431. Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative strategies for hypoxic‐tumor photodynamic therapy. Angew Chem Int Ed Engl. 2018;57(36):11522‐11531. [DOI] [PubMed] [Google Scholar]
- 432. Jiang W, Zhang Z, Wang Q, et al. Tumor reoxygenation and blood perfusion enhanced photodynamic therapy using ultrathin graphdiyne oxide nanosheets. Nano Lett. 2019;19(6):4060‐4067. [DOI] [PubMed] [Google Scholar]
- 433. Chen Q, Huang Z, Chen H, Shapiro H, Beckers J, Hetzel FW. Improvement of tumor response by manipulation of tumor oxygenation during photodynamic therapy. Photochem Photobiol. 2002;76(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 434. Dong GC, Hu SX, Zhao GY, Gao SZ, Wu LR. Experimental study on cytotoxic effects of hyperbaric oxygen and photodynamic therapy on mouse transplanted tumor. Chin Med J. 1987;100(9):697‐702. [PubMed] [Google Scholar]
- 435. Hjelde A, Gederaas OA, Krokan HE, Brubakk AO. Lack of effect of hyperoxia on photodynamic therapy and lipid peroxidation in three different cancer cell lines. Med Sci Monit. 2005;11(10):Br351‐6. [PubMed] [Google Scholar]
- 436. Maier A, Anegg U, Fell B, et al. Hyperbaric oxygen and photodynamic therapy in the treatment of advanced carcinoma of the cardia and the esophagus. Lasers Surg Med. 2000;26(3):308‐315. [DOI] [PubMed] [Google Scholar]
- 437. Maier A, Tomaselli F, Anegg U, et al. Combined photodynamic therapy and hyperbaric oxygenation in carcinoma of the esophagus and the esophago‐gastric junction. Eur J Cardiothorac Surg. 2000;18(6):649‐654. discussion 654–5. [DOI] [PubMed] [Google Scholar]
- 438. Jirsa M Jr, Síp M, Jirsa M. Influence of hyperbaric oxygenation on bilirubin and ditaurobilirubin auto‐oxidation and porphyrin‐sensitized photo‐oxidation. J Photochem Photobiol B. 1990;5(3‐4):295‐302. [DOI] [PubMed] [Google Scholar]
- 439. Henderson BW, Fingar VH. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem Photobiol. 1989;49(3):299‐304. [DOI] [PubMed] [Google Scholar]
- 440. Pham TC, Nguyen VN, Choi Y, Lee S, Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem Rev. 2021;121(21):13454‐13619. [DOI] [PubMed] [Google Scholar]
- 441. Roberts D, Cairnduff F, Dixon B, Brown S. Modulation of the response of a rodent fibrosarcoma to photodynamic therapy by hyperbaric‐oxygen treatment. Oncol Rep. 1995;2(3):387‐390. [DOI] [PubMed] [Google Scholar]
- 442. Nicola JH, Ester VCC, Nicola MD, Konradin Metze JHN, Institute of Physics, VCC, EMDN, KM. Medical School ‐Univ. Estadual de Campinas ‐ UNICAMP, Campinas, SP, Brazil . Enhancement of photodynamic therapy due to hyperbaric hyperoxia: an experimental study of walker 256 tumors in rats.
- 443. Huang X, Zhang W, Peng Y, et al. A multifunctional layered nickel silicate nanogenerator of synchronous oxygen self‐supply and superoxide radical generation for hypoxic tumor therapy. ACS Nano. 2022;16(1):974‐983. [DOI] [PubMed] [Google Scholar]
- 444. Wang C, Cao F, Ruan Y, Jia X, Zhen W, Jiang X. Specific generation of singlet oxygen through the Russell mechanism in hypoxic tumors and GSH depletion by Cu‐TCPP nanosheets for cancer therapy. Angew Chem Int Ed Engl. 2019;58(29):9846‐9850. [DOI] [PubMed] [Google Scholar]
- 445. Phua SZF, Yang G, Lim WQ, et al. Catalase‐integrated hyaluronic acid as nanocarriers for enhanced photodynamic therapy in solid tumor. ACS Nano. 2019;13(4):4742‐4751. [DOI] [PubMed] [Google Scholar]
- 446. Yang Y, Zhu W, Feng L, et al. G‐Quadruplex‐based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear‐targeted drug delivery for enhanced photodynamic therapy. Nano Lett. 2018;18(11):6867‐6875. [DOI] [PubMed] [Google Scholar]
- 447. Cheng Y, Cheng H, Jiang C, et al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun. 2015;6:8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Li J, Huang J, Ao Y, et al. Synergizing upconversion nanophotosensitizers with hyperbaric oxygen to remodel the extracellular matrix for enhanced photodynamic cancer therapy. ACS Appl Mater Interfaces. 2018;10(27):22985‐22996. [DOI] [PubMed] [Google Scholar]
- 449. Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle‐based photodynamic tumor therapy. Nano Lett. 2016;16(4):2512‐2521. [DOI] [PubMed] [Google Scholar]
- 450. Mei LH, Yang G, Fang F. Hyperbaric oxygen combined with 5‐aminolevulinic acid photodynamic therapy inhibited human squamous cell proliferation. Biol Pharm Bull. 2019;42(3):394‐400. [DOI] [PubMed] [Google Scholar]
- 451. Maier A, Anegg U, Tomaselli F, et al. Does hyperbaric oxygen enhance the effect of photodynamic therapy in patients with advanced esophageal carcinoma? A clinical pilot study. Endoscopy. 2000;32(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 452. Tomaselli F, Maier A, Sankin O, et al. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer–a clinical pilot study. Lasers Surg Med. 2001;28(5):399‐403. [DOI] [PubMed] [Google Scholar]
- 453. Tomaselli F, Maier A, Pinter H, Stranzl H, Smolle‐Jüttner FM. Photodynamic therapy enhanced by hyperbaric oxygen in acute endoluminal palliation of malignant bronchial stenosis (clinical pilot study in 40 patients). Eur J Cardiothorac Surg. 2001;19(5):549‐554. [DOI] [PubMed] [Google Scholar]
- 454. Matzi V, Maier A, Sankin O, et al. Photodynamic therapy enhanced by hyperbaric oxygenation in palliation of malignant pleural mesothelioma: clinical experience. Photodiagnosis Photodyn Ther. 2004;1(1):57‐64. [DOI] [PubMed] [Google Scholar]
- 455. Matzi V, Maier A, Woltsche M, Smolle‐Jüttner FM. Polyhematoporphyrin‐mediated photodynamic therapy and decortication in palliation of malignant pleural mesothelioma: a clinical pilot study. Interact Cardiovasc Thorac Surg. 2004;3(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 456. Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi‐Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26(11):612‐621. [DOI] [PubMed] [Google Scholar]
- 457. Jin CS, Lovell JF, Chen J, Zheng G. Ablation of hypoxic tumors with dose‐equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano. 2013;7(3):2541‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Zhang Y, Liao Y, Tang Q, Lin J, Huang P. Biomimetic nanoemulsion for synergistic photodynamic‐immunotherapy against hypoxic breast tumor. Angew Chem Int Ed Engl. 2021;60(19):10647‐10653. [DOI] [PubMed] [Google Scholar]
- 459. Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;18(12):751‐772. [DOI] [PubMed] [Google Scholar]
- 460. He G, Peng X, Wei S, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Crowley PD, Stuttgen V, O'Carroll E, Ash SA, Buggy DJ, Gallagher HC. Exposure to 60% oxygen promotes migration and upregulates angiogenesis factor secretion in breast cancer cells. Med Gas Res. 2017;7(4):226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not appliable.


