Abstract
There is credible evidence that environmental factors influence individual risk and/or severity of autism spectrum disorders (hereafter referred to as autism). While it is likely that environmental chemicals contribute to the etiology of autism via multiple mechanisms, identifying specific environmental factors that confer risk for autism and understanding how they contribute to the etiology of autism has been challenging, in part because the influence of environmental chemicals likely varies depending on the genetic substrate of the exposed individual. Current research efforts are focused on elucidating the mechanisms by which environmental chemicals interact with autism genetic susceptibilities to adversely impact neurodevelopment. The goal is to not only generate insights regarding the pathophysiology of autism, but also inform the development of screening platforms to identify specific environmental factors and gene × environment (G × E) interactions that modify autism risk. Data from such studies are needed to support development of intervention strategies for mitigating the burden of this neurodevelopmental condition on individuals, their families and society. In this review, we discuss environmental chemicals identified as putative autism risk factors and proposed mechanisms by which G × E interactions influence autism risk and/or severity using polychlorinated biphenyls (PCBs) as an example.
1. Introduction
Autism spectrum disorder (ASD), hereafter referred to as autism, is a complex, multifactorial neurodevelopmental condition that is defined clinically by core deficits in social reciprocity and communication, restrictive interests and repetitive behaviors. The severity of core symptoms, the expression of co-morbidities, which include intellectual disability, anxiety, seizures, gastrointestinal disturbances, and immunological abnormalities, and the response to treatment vary considerably between individuals diagnosed with autism (Lord, Elsabbagh, Baird, & Veenstra-Vanderweele, 2018). The United States (U.S.) Center for Disease Control (CDC) estimated the prevalence of autism in U.S. children in 2021 to be 1 in 44, which is more than three times the CDC 2002 estimate of 1 in 150 U.S. children (https://www.autism-society.org). This trend of rising rates of autism, which dates back to the early 1990s, is a global occurrence not confined to the U.S. (Zeidan et al., 2022). Large-scale surveys estimate the current median worldwide prevalence of autism to be 1 in 100 children (Zeidan et al., 2022). When considered in the context of the tremendous costs that autism exacts on affected individuals, their families, and society (Hong et al., 2020; Leibson et al., 2020; Schofield et al., 2019), these statistics underscore the need to identify factors that confer autism risk and/or modify symptom severity.
Research on the etiology of autism has identified a strong hereditary component (Buxbaum & Hof, 2011; El-Fishawy & State, 2010; Geschwind, 2011). The candidate genes most strongly associated with autism encode proteins that regulate the patterning of synaptic connections in the developing brain (Delorme et al., 2013; Guang et al., 2018; Landrigan, Lambertini, & Birnbaum, 2012; Masini et al., 2020; Qiu, Aldinger, & Levitt, 2012; Stamou, Streifel, Goines, & Lein, 2013). However, genetic research has also shown that genes linked to autism rarely segregate in a simple Mendelian fashion (El-Fishawy & State, 2010) and that solely genetic causes account for approximately 10–30% of all autism cases (Ansel, Rosenzweig, Zisman, Melamed, & Gesundheit, 2016; Lai, Lombardo, & Baron, 2014; Masini et al., 2020). Moreover, there is incomplete concordance of autism in monozygotic twins (Herbert, 2010), and even in genetic syndromes strongly associated with autism, a significant percentage of carriers do not express autism phenotypes (Herbert, 2010; Levitt & Campbell, 2009; Masini et al., 2020). These observations are consistent with a model in which environmental factors act as modifiers of autism risk genes.
While the dramatic increase in autism rates over the past several decades is often presented as evidence that environmental factors are involved in autism, others have argued that the progressive rise in autism is the result of diagnostic substitution, or the labeling of individuals as autistic who previously would not have been so labeled, due to broadening of diagnostic criteria, coupled with increased awareness and improved detection of these disorders (Bishop, Whitehouse, Watt, & Line, 2008; Coo et al., 2008; Parner, Schendel, & Thorsen, 2008). However, studies that have tested this hypothesis found that diagnostic substitution accounted for less than half of new cases, indicating there has been a true increase in the number of individuals diagnosed with autism (Grether, Rosen, Smith, & Croen, 2009; Hertz-Picciotto & Delwiche, 2009; Hertz-Picciotto, Schmidt, & Krakowiak, 2018; King & Bearman, 2009). Consistent with these conclusions, two well-powered independent twin studies (Frazier et al., 2014; Hallmayer et al., 2011) and a third study of more than 14,000 Swedish children with autism (Sandin et al., 2014) concluded that environmental factors account for approximately 50–60% of autism cases. Collectively, these studies suggest that environmental factors can significantly influence autism susceptibility, thereby providing a plausible explanation for both the marked increase in autism rates and the significant clinical heterogeneity of autism.
In contrast to genetic risks, which are currently irreversible, environmental factors are modifiable risk factors. The identification of specific environmental factors that increase autism risk would enable the primary prevention of autism via public health policies aimed at reducing relevant environmental exposures in susceptible populations. Progress has been made in identifying environmental risk factors for autism, which include advanced paternal age at conception, complications during pregnancy, maternal diet, and prenatal exposure to psychotropic drugs (Bolte, Girdler, & Marschik, 2019; Carlsson, Molander, Taylor, Jonsson, & Bolte, 2020). Environmental chemical contaminants have also been proposed as autism risk factors (Landrigan, 2010; Landrigan et al., 2012). While experimental animal and predictive toxicology studies have identified environmental chemicals associated with autism-relevant phenotypes, it has been challenging to demonstrate this association in human studies (Carter & Blizard, 2016; Kalkbrenner, Schmidt, & Penlesky, 2014; Pelch, Bolden, & Kwiatkowski, 2019; Rock & Patisaul, 2018; Rossignol, Genuis, & Frye, 2014). A recent analysis of the relevant epidemiological literature concluded that overall, these studies were moderate in quality and of limited strength (Mari-Bauset et al., 2022). The authors identified numerous methodological challenges associated with epidemiological studies of environmental influences in autism that decreased quality and strength. These included difficulties in obtaining accurate measures of exposure, particularly for chemicals with short half-lives, such as some of the pesticides, phthalates and BPA; controlling for confounding factors, especially socioeconomic stressors that tend to co-vary with environmental exposures; and dealing with co-exposures, a not insignificant issue given the number of chemicals in the human chemosphere that vary in both composition and concentration across time and space. Epidemiologic studies of complex neurodevelopmental conditions face additional challenges in that these conditions are phenotypically and genetically heterogeneous. The former presents diagnostic challenges, while the latter likely creates a range of sensitivities to environmental risk factors (Pessah & Lein, 2008) that masks clear associations between exposure and diagnosis.
As a result of the difficulties associated with using epidemiological approaches to identify environmental chemicals that confer risk for autism, animal and cell-based models that demonstrate effects of environmental chemicals on outcomes with face validity to autism phenotypes are critically important for corroborating and informing human studies. Since autism is diagnosed based on behavioral criteria that are not readily translated to animal models, a key challenge has been identifying autism-relevant molecular and cellular phenotypes that can be quantified in experimental models, including cell-based and predictive toxicology models. An exciting advance in this regard is the convergence of genetic, histologic, in vivo imaging and functional data on altered patterns of synaptic connectivity as the biological basis underlying the behavioral phenotypes associated with autism (Alamdari, Sadeghi Damavandi, Zarei, & Khosrowabadi, 2022; Bourgeron, 2009; Geschwind & Levitt, 2007; Guang et al., 2018; Persichetti, Shao, Gotts, & Martin, 2022; Rubenstein & Merzenich, 2003; Zoghbi & Bear, 2012). As indicated earlier, candidate genes most strongly implicated in the causation of autism encode proteins that regulate the patterning of neuronal networks during development and influence the balance of excitatory to inhibitory signaling in the brain or E/I balance (Ansel et al., 2016; Bourgeron, 2009; Rubenstein & Merzenich, 2003; Stamou et al., 2013; Zoghbi & Bear, 2012). Histological analyses have identified abnormalities of dendritic and synaptic morphology (Penzes, Cahill, Jones, VanLeeuwen, & Woolfrey, 2011). For example, spine density was increased in Golgi-impregnated pyramidal neurons of Layer II in the frontal, temporal and parietal lobes and Layer V of the temporal lobe in autism (Hutsler & Zhang, 2010), suggesting skewed cortical synaptic activity towards excitation. Abnormal spine morphology was observed in the temporal and visual cortices of patients with Fragile-X, a genetic syndrome with a high incidence of autism (Irwin et al., 2001). Neuroimaging studies of children with autism have revealed both hypo- and hyper-connectivity in the brain of affected individuals (Baribeau & Anagnostou, 2013; Therien, Degre-Pelletier, Barbeau, Samson, & Soulieres, 2022). The available functional MRI data support a pattern of early brain overgrowth in the first few years of life followed by aberrant maturation in adolescence with impaired long-range connectivity but increased local and/or subcortical connectivity (reviewed in Baribeau & Anagnostou, 2013).
The differences in synaptic connectivity observed in individuals with autism may reflect influences of sex (Alaerts, Swinnen, & Wenderoth, 2016) and/or genotype (Dennis et al., 2011; Rudie et al., 2012; Scott-Van Zeeland et al., 2010). As an example of genotype, functional MRI studies of individuals expressing the autism risk variant of the Met Receptor Tyrosine Kinase (MET) gene revealed unique brain activity in response to social stimuli and altered connectivity in temporo-parietal brain regions, phenotypes that are exacerbated in individuals with autism (Eagleson, Xie, & Levitt, 2017; Rudie et al., 2012). These functional changes associated with MET genotype are not surprising considering that MET signaling is involved in regulating dendritic growth, spine formation and excitatory (glutamatergic) synaptogenesis during early development (Peng et al., 2016).
It has been proposed that the inherent imbalances in synaptic connectivity observed in autism present a genetic substrate that increases susceptibility to environmental insults that impact neurodevelopmental processes that determine synaptic connectivity, such as neuronal migration, apoptosis, elaboration of axons and dendrites, and activity-dependent refinements of synaptic connections (Belmonte & Bourgeron, 2006; Stamou et al., 2013). Perturbations of the spatiotemporal patterns and/or magnitude or extent of any of these neurodevelopmental events can modify the patterning of neural networks in the developing brain to give rise to behavioral phenotypes associated with autism (Belmonte & Bourgeron, 2006; Lein, Silbergeld, Locke, & Goldberg, 2005). Research findings from studies of transgenic animal models that express autism risk genes further support the hypothesis that perturbations of dendritic growth, synapse formation and/or synapse stabilization result in altered patterns of synaptic connectivity that coincide with autism-relevant behavioral phenotypes (Bourgeron, 2009; Zoghbi & Bear, 2012). How environmental and genetic risk factors interact to influence autism risk and/or severity remains an outstanding question.
In this review, we discuss current hypotheses regarding mechanisms by which environmental chemicals interact with genetic susceptibility factors to modify autism risk. These mechanisms are illustrated in the context of polychlorinated biphenyls (PCBs), an extensively studied class of environmental contaminants for which there is scientific consensus that they alter normal neurodevelopmental trajectories in the human brain (Pessah, Lein, Seegal, & Sagiv, 2019).
2. Environmental chemicals identified as putative autism risk factors
Early reports that in utero exposure to valproic acid or thalidomide during critical periods of development was associated with increased expression of autism-related traits in children (Rodier, Ingram, Tisdale, & Croog, 1997) raised significant interest regarding the role of chemical exposures in autism. Subsequent epidemiological studies reported an increased risk for autism associated with maternal use of various medications and drugs of abuse, including alcohol (Arndt, Stodgell, & Rodier, 2005; Christensen et al., 2013; Croen et al., 2011; Malm et al., 2012; Miller et al., 2005; Rodier et al., 1997; Rodier, Miller, & Brent, 2011), as well as prenatal or early postnatal exposure to diverse environmental chemicals. These epidemiological studies, as well as preclinical studies, have identified structurally and mechanistically diverse environmental chemicals as putative autism risk factors. These include legacy contaminants well-established to interfere with normal neurodevelopment, such as lead, mercury, and polychlorinated biphenyls (PCBs), as well as more contemporary contaminants, such as pesticides (organophosphorus and organochlorine pesticides, neonicotinoids and pyrethroids), flame retardants (polybrominated diphenyl ethers or PBDEs), plasticizers (phthalates and bisphenol A or BPA), and complex environmental mixtures, notably air pollution (Carter & Blizard, 2016; Kalkbrenner et al., 2014; Landrigan et al., 2012; Masini et al., 2020; Pelch et al., 2019).
The epidemiologic evidence linking environmental chemical exposures and autism was reviewed in Kalkbrenner et al. (2014). These authors identified 58 relevant articles in the peer-reviewed literature published prior to March 1, 2014, of which 32 met their inclusion criteria (individual-level data on autism, exposure measures pertaining to pregnancy or the 1st year of life, valid comparison groups, control for confounders and adequate sample sizes). These 32 articles included studies of autism and estimates of exposure to tobacco, air pollutants, volatile organic compounds and solvents, metals, pesticides, PCBs, PBDEs, PFCs, BPA and phthalates. Many of these chemicals have been shown to cause neurodevelopmental effects in experimental animal or cell-based models that have face validity to behavioral outcomes or cellular phenotypes associated with autism (reviewed in Kalkbrenner et al., 2014; Stamou et al., 2013). Some of these environmental exposures also showed associations with autism in the analysis of the epidemiologic literature by Kalkbrenner and colleagues. The most strongly and consistently associated were traffic-related air pollutants, some metals and the organophosphorus and organochlorine pesticides, with suggestive trends for some volatile organic compounds and phthalates (Kalkbrenner et al., 2014). Interestingly, neither tobacco smoke, which has been strongly associated with increased ADHD risk (Polanska, Jurewicz, & Hanke, 2012), nor alcohol showed a consistent association with increased risk for autism. The authors of this analysis reached the broad conclusion that with the possible exception of studies of tobacco and alcohol, the relevant publications available at the time were too limited in scope to either infer causality or to rule out the possibility that these or additional environmental chemicals confer risk for autism.
A later scoping review of the human and animal research was published in 2018, which aimed to identify and categorize primary research and reviews on the association between prenatal and early postnatal exposure to environmental chemicals and autism risk that were published through February 2018 (Pelch et al., 2019). Search criteria included studies that assessed exposure to environmental chemicals prior to 2 months of age in humans or 14 d in rodents and rodent studies that included at least one measurement of reciprocal social communicative behavior or repetitive and stereotyped behavior. The search identified 21,603 unique studies from which the authors selected 54 epidemiological studies, 46 experimental rodent studies, and 50 reviews as relevant. These studies covered 152 chemical exposures. The most frequently studied environmental chemicals in humans were particulate matter (n = 14), mercury (n = 14), nonspecific air pollution (n = 10) and lead (n = 10). Similarly, in rodents, mercury and lead were among the most studied environmental chemicals (n = 6 and 4, respectively) along with the organophosphorus pesticide, chlorpyrifos (n = 9). Similar to the conclusions reached by Kalkbrenner et al. (2014), Pelch et al. (2019) concluded that while research on this topic was rapidly expanding, the wide variability in study design and conduct, the exposures investigated and the outcomes assessed made it difficult to identify chemicals of concern, but based on the available literature, they recommended that chlorpyrifos, lead, and PCBs be systematically reviewed to assess their association with autism risk and severity.
A more recent narrative review of this literature published in 2020 (Cheroni, Caporale, & Testa, 2020) identified the following environmental chemicals as the most relevant in the context of autism risk: lead, methylmercury, air pollution, endocrine disruptors in general, phthalates, BPA, perfluoralkyl substances, and PCBs. In this review, we focus on the literature related to the association of PCBs with autism, but the reader is referred to recent reviews and meta-analysis of the literature regarding the association between autism phenotypes and either pesticides (Bertoletti et al., 2022; Maleki et al., 2022; Vester & Caudle, 2016) or air pollution (Cory-Slechta, Merrill, & Sobolewski, 2022; Fu, Guo, Cheung, & Yung, 2019; Yu et al., 2022).
3. Mechanisms by which gene × environment interactions influence autism risk
While it is now widely accepted that complex G × E interactions influence the etiology of autism, the mechanisms by which genetic and environmental factors interact to influence individual autism risk and/or severity remain speculative. Current hypotheses include (i) heritable deficits in xenobiotic metabolism, (ii) disruption of the gut microbiome, (iii) endocrine disruption, (iv) epigenetic alterations that alter gene expression, (v) inflammation and/or immune dysregulation, and (vi) convergence on signaling pathways that regulate brain development (Table 1). The scientific premise for each of these proposed mechanisms is briefly discussed in this section, with specific examples presented in subsequent sections focused on PCBs as putative autism risk factors. It is important to note that while there is evidence to support each of these G × E mechanisms in the determination of autism risk, at this time there is little evidence causally linking these mechanisms to autism. Establishing causality remains a significant goal of ongoing research.
Table 1.
Proposed mechanisms for how genetic and environmental factors interact to influence autism risk and severity.
| Mechanism | Rationale | References |
|---|---|---|
| Heritable deficits in xenobiotic metabolism | Genetic mutations in enzymes that metabolize environmental chemicals have been linked to increased autism risk. A decreased ability to detoxify environmental chemicals or an increased effectiveness in converting benign parent compounds to neurotoxic metabolites might increase the neurotoxicity of an environmental chemical contaminant. |
Bjorklund et al. (2021)
Herbert (2010) Rock and Patisaul (2018) |
| Disruption of the gut microbiome | Clinical and experimental data indicate that the gut microbiome regulates neurodevelopment; the gut microbiota in children with autism differs from that of neurotypical children. Toxicological studies show that the gut microbiome influences xenobiotic metabolism and disposition, and, conversely, environmental chemicals can alter the gut microbiome. |
Balaguer-Trias, Deepika, Schuhmacher, and Kumar (2022)
Bertotto, Catron, and Tal (2020) Needham et al. (2022) Rosenfeld (2017) Vuong et al. (2020) Yousefi et al. (2022) |
| Endocrine disruption | Endocrine signaling, including signaling by thyroid hormones, sex steroids and glucocorticoids, critically modulates neurodevelopment. The incidence of autism exhibits a pronounced sex bias, with a male:female ratio of 4:1. Diverse environmental chemicals have been shown to alter endocrine signaling via various mechanisms. |
Demeneix (2019)
Moosa, Shu, Sarachana, and Hu (2018) Mari-Bauset et al. (2022) Ramirez et al. (2022) Weiss (2012) |
| Epigenetic mechanisms | Epigenetic mechanisms are critically important in regulating brain development. Environmental chemicals can alter DNA methylation, histone acetylation and miRNA expression profiles, and these parameters are altered in at least some children with autism. |
Keil and Lein (2016)
Kong, Zhou, and Sun (2020) Park, Lee, Kim, and Yi (2022) Parra and Johnston (2022) |
| Inflammation and/or immune dysregulation | Crosstalk between nervous and immune systems is essential for normal neurodevelopment, and environmental chemicals can trigger systemic and neuroinflammation as well as alter immune function. Clinical and experimental data confirm neuroinflammation and immune dysregulation in autism. |
Arambula and McCarthy (2020)
McLellan, Kim, Bruce, Ramirez-Celis, and Van de Water (2022) Meltzer and Van de Water (2017) Sotgiu et al. (2020) |
| Convergence of G × E on signaling pathways that regulate brain development | Many genetic risk factors for autism converge on several major signaling pathways that critically regulate synaptic connectivity in the developing brain. Environmental chemicals have been identified that target these same signaling pathways. |
Ansel et al. (2016)
Ebert and Greenberg (2013) Pourtavakoli and Ghafouri-Fard (2022) Purushotham et al. (2022) Stamou et al. (2013) |
3.1. Genetic polymorphisms in xenobiotic metabolism
Genetic mutations in enzymes that metabolize xenobiotics, including environmental chemicals, have been linked to increased autism risk (Mordaunt et al., 2019; Rossignol et al., 2014). In a genome-wide transcriptomic study of cord blood from autistic vs. neurotypical children, 172 genes were found to be differentially expressed between the two groups with several genes grouped into the “response to toxic substances” category (Mordaunt et al., 2019). The genes included those that encode members of the cytochrome P450 (CYP) family of enzymes, such as CYP1A1, that play critical roles in metabolizing diverse environmental chemicals (Nagai, Fukuno, Suzuki, & Konishi, 2016; Uwimana, Ruiz, Li, & Lehmler, 2019). Polymorphisms in CYP genes that influence CYP expression levels and/or activity have been shown to decrease detoxification of environmental chemicals or increase metabolic conversion of a non-toxic parent compound to a neurotoxic metabolite. Either functional consequence can effectively increase the neurotoxic potential of an environmental chemical.
Glutathione-dependent enzymes also play a key role in the detoxification of environmental chemicals, but this is not their only critical function: glutathione-mediated metabolism is critically important in maintaining intracellular redox homeostasis (Bjorklund et al., 2021; Liu, Tan, Song, & Song, 2020). Mothers who express genetic polymorphisms that decrease glutathione-mediated processes are at higher risk for having a child with autism, and these genetic polymorphisms occur with higher frequency in children with autism vs. neurotypical children (Rossignol et al., 2014). Consistent with these observations, glutathione S-transferase (GST) detoxification activity was found to be lower in children with autism vs. neurotypical controls (Serajee, Nabi, Zhong, & Huq, 2004). It was also recently shown that imbalances in the glutathione redox system contribute to the pathogenesis of autism via not only increased oxidative stress, but also altered E/I balance in the brain (Bjorklund et al., 2021). Glutathione is involved in regulating the N-methyl-D-aspartate (NMDA) receptor, which mediates signaling by glutamate, the primary excitatory neurotransmitter in the brain. Autism-linked deficits in glutathione-related regulation of glutamate have been linked to E/I imbalance in the brain, and in extreme cases, to excitotoxic cell death of neurons (Bjorklund et al., 2021).
3.2. Disruption of the gut microbiome
The metabolism of environmental chemicals is also influenced by the gut microbiome (Li, Tian, et al., 2022; Lim et al., 2020; Zimmermann, Zimmermann-Kogadeeva, Wegmann, & Goodman, 2019), and changes in the composition of the gut microbiome have been shown to alter the disposition and concentration of environmental chemicals throughout the body, including the brain (Li, Tian, et al., 2022). Conversely, environmental chemicals can alter the gut microbiome (Gama, Neves, & Pereira, 2022; Lai, Chung, Li, Wan, & Wong, 2016; Li, Hefti, Marek, et al., 2022; Rude, Keogh, & Gareau, 2019). Given clinical and experimental data indicating that the gut microbiome regulates neurodevelopment and central nervous system function (Alam, Abdolmaleky, & Zhou, 2017; Muller et al., 2020; Ristori et al., 2019), it is widely posited that interactions between environmental chemicals and the gut microbiome during critical stages of neurodevelopment may alter neurodevelopmental trajectories to confer risk for autism. This is supported by reports that the gut microbiota in children with autism differs from that of neurotypical children (Kang et al., 2017; Liu et al., 2019; Mayer, Padua, & Tillisch, 2014; Sharon et al., 2019). However, data demonstrating that environmental chemical interactions with the gut microbiome are causally related to increased expression of autism phenotypes is largely lacking. Carefully designed experimental animal studies and prospective clinical studies are needed to better understand whether certain gut microbiome compositions are more likely to lead to adverse effects from environmental chemical exposures and whether therapeutic interventions targeting the gut microbiome could be employed to mitigate autism risk associated with G × E interactions.
3.3. Endocrine disruption
Diverse environmental chemicals have been classified as endocrine disruptors because they interfere with endogenous endocrine signaling either by mimicking endogenous hormones or by decreasing the production, transport or signaling of endogenous hormones (Mari-Bauset et al., 2022; Salazar, Villaseca, Cisternas, & Inestrosa, 2021; Sechman, Batoryna, Antos, & Hrabia, 2016; Takeuchi et al., 2011; Weiss, 2012). Since endocrine signaling influences neural circuitry in the developing brain via modulation of neurodevelopmental processes that regulate neuronal connectivity (Demeneix, 2019; Moosa et al., 2018; Ramirez et al., 2022), it is widely posited that endocrine disrupting chemicals interact with genetic factors that regulate endocrine signaling to alter neurodevelopmental trajectories.
One endocrine signaling system thought to be targeted by diverse environmental chemicals to cause developmental neurotoxicity is the thyroid hormone (TH) system. TH signaling is critical for normal neurodevelopment (Demeneix, 2019), and congenital hypothyroidism, if untreated, causes severe adverse neurodevelopmental outcomes (Oppenheimer & Schwartz, 1997; Rovet, 2014; Williams, 2008). While conflicting, there is some evidence that TH insufficiency increases autism risk (Salazar et al., 2021), and genetic mutations in TH receptors have been observed in children with autism (Kalikiri, Mamidala, Rao, & Rajesh, 2017). A large number of environmental chemicals have been shown to decrease circulating levels of TH and/or to interfere with TH signaling at the level of the receptor (Salazar et al., 2021). While these observations collectively support the hypothesis that environmental chemicals confer autism risk via modulation of TH signaling, evidence of a causal link is lacking, as are data demonstrating that expression of genetic mutations in TH receptors increase susceptibility to the neurotoxicity of environmental thyroid hormone disruptors.
Another observation that has promoted the hypothesis of endocrine signaling as a mechanisms by which G × E interactions confer autism risk is that autism disproportionately impacts males relative to females with a rate of diagnosis 3–4.5 times higher in males (Christensen et al., 2016; Zablotsky, Black, & Blumberg, 2017). This observation has led to the suggestion that sex steroids may be important in autism etiology. Sex steroid signaling determines the sexually dimorphic trajectories of brain development that produce sex differences in brain structure, synaptic connectivity and behavior (Collaer & Hines, 1995; McCarthy, 2016a, 2016b; McCarthy, Herold, & Stockman, 2018; Napolitano et al., 2022). Developmental surges of fetal testicular testosterone, which is converted to 17B-estradiol in the brain, are responsible for masculinizing the brain, and environmental chemicals that interfere with this developmental event can alter neural circuitry (McCarthy et al., 2018; McCarthy, Arnold, Ball, Blaustein, & De Vries, 2012; Napolitano et al., 2022). There is evidence of differential brain expression of genetic variants of the androgen receptor in autism compared to age- and sex-matched controls (Henningsson et al., 2009). Postmortem analysis has revealed decreased abundance of estrogen receptors in the brain of individuals with autism (Crider, Thakkar, Ahmed, & Pillai, 2014). Thus, environmental chemicals could contribute to autism risk by altering the spatiotemporal profiles and/or levels of sex hormones or their receptors to disrupt the normal sex specific patterning and development of the brain.
3.4. Epigenetic mechanisms
Epigenetic mechanisms are critically important in regulating brain development. DNA methylation patterns, chromatin remodeling, and miRNA expression profiles can be altered by environmental chemicals, and changes in these epigenetic modifiers have been documented in individuals with autism (Keil & Lein, 2016; Tremblay & Jiang, 2019). Perhaps the strongest evidence supporting epigenetic mechanisms as a means by which environmental chemicals might influence autism risk comes from studies of the DNA methylation binding protein, methyl-CpG binding protein 2 (MeCP2). Mutations in MeCP2 cause Rett syndrome, which has a high comorbidity of autism diagnosis, and mutations in MeCP2 have been identified in autism independent of Rett syndrome (Cukier et al., 2012; LaSalle & Yasui, 2009; Percy, 2011; Wen et al., 2017). MeCP2 is a transcriptional repressor regulated by miR132 that regulates dendritic and synaptic morphogenesis and function (Klein et al., 2007). Mice that lack MeCP2 have reduced expression of GABRB3, a gamma-aminobutyric acid (GABA) receptor important in determining the balance between excitatory and inhibitory neurotransmission (Samaco, Hogart, & LaSalle, 2005). Altered methylation of MeCP2 in the cord blood of mothers has been correlated with environmental chemical exposures (Eguchi et al., 2019; Pilsner et al., 2009).
The microRNA, miR132, plays an important role in synaptogenesis and connectivity (Xu, Li, Ji et al., 2019). In mice, genetic deletion of miR132 impaired performance in learning and memory tasks, and either deletion or overexpression of miR132 altered hippocampal transcription of genes related to synapse transmission, neuronal proliferation and learning and memory (Hansen et al., 2016). Brain levels of microRNAs have been reported to be elevated in autism (Abu-Elneel et al., 2008; Sarachana, Zhou, Chen, Manji, & Hu, 2010; Talebizadeh, Butler, & Theodoro, 2008), and environmental chemicals have been shown to alter brain expression of miR132 (Lesiak et al., 2014; Sima, Rossnerova, Simova, & Rossner Jr., 2021; Yu et al., 2021). Thus, microRNAs may represent a convergent target for genetic and environmental risk factors that alter synaptic connectivity.
There is evidence that epigenetic mechanisms influence xenobiotic metabolism. For example, comparative analyses of placental tissue from mothers who gave birth to a child later diagnosed with autism vs. placenta tissues from mothers whose children were neurotypical identified differentially methylated regions in a xenobiotic metabolism gene, CYP2E1, and the extent of differential methylation was further influenced by the mother’s CYP2E1 genotype (Zhu et al., 2019). Epigenetic mechanisms may also influence known genetic causes of autism. 15q11-q13 duplications are associated with a syndromic form of autism, and individuals with this duplication have lower levels of brain LINE-1 repetitive DNA methylation (Mitchell et al., 2012). The potential to alter gene expression across a wide array of genes important to neurodevelopment and neurotoxicity make epigenetic pathways a critical target of G × E interactions that modify autism risk and/or severity.
3.5. Inflammation and/or immune dysregulation
The immune system is inextricably linked with the central nervous system and plays a critical role in brain development and function (Ransohoff & Brown, 2012). For example, cytokines and microglia, the innate immune cells in brain, are necessary for establishing functional neural circuits in the developing brain, including those involved in social behavior (Deverman & Patterson, 2009; Garay & McAllister, 2010; Guedes, Ferreira, Costa, Cardoso, & Peca, 2022; Sullivan & Ciernia, 2022). While numerous cytokines have been implicated in modulating neurodevelopmental processes critical to determining synaptic connectivity, the proinflammatory cytokines interleukin (IL)-6 (Goines & Ashwood, 2013; Kummer, Zeidler, Kalpachidou, & Kress, 2021) and type II interferon, gamma (IFNγ) (Goines & Van de Water, 2010) are two key players. IL-6 is necessary for maintaining neural progenitor cells in mice (Bowen, Dempsey, & Vemuganti, 2011) and has been shown to promote neural differentiation in human induced pluripotent stem cells (Kummer et al., 2021; Xu et al., 2018). However, developmental timing is likely critical for these effects since IL-6 has also been reported to inhibit neural differentiation in hippocampal neurons from adult mice (Kong et al., 2019). IL-6 has also been reported to decrease dendrite number in cultured rat hippocampal neurons (Gadient, Lein, Higgins, & Patterson, 1998) and modulate dendrite growth in primary rat neurons in culture, promoting both dendrite retraction (Guo, Chandrasekaran, Lein, Kaplan, & Higgins, 1999) and increased dendrite number (Guo, Metzler-Northrup, Lein, Rueger, & Higgins, 1997) depending on cell type and culturing conditions. IL-6 also impacts synaptogenesis and connectivity, altering network activity in mouse hippocampal neurons, in part by reducing expression of glutamate receptors and calcium channel signaling (Vereyken, Bajova, Chow, de Graan, & Gruol, 2007). IFNγ also regulates dendritic growth and synaptogenesis, causing dendritic retraction and impairing synapse formation in rodent sympathetic and hippocampal neurons (Kim, Beck, Lein, & Higgins, 2002; Vikman, Owe-Larsson, Brask, Kristensson, & Hill, 2001). IFNγ can modulate synaptic activity in rat hippocampal neurons in vitro, causing marked increases in excitatory postsynaptic currents 48 h post exposure (Vikman et al., 2001). Similarly, microglia have been reported to play a critical role in regulating neural differentiation via engulfment of neural precursor cells and in pruning superfluous synapses in the developing brain (Guedes et al., 2022; Lebovitz, Ringel-Scaia, Allen, & Theus, 2018; Sullivan & Ciernia, 2022). Sex differences in microglial gene expression and function have been identified that play a key role in shaping sex-specific brain function and behavior. The levels of sex hormones, such as androgens, estrogens, and progesterone, vary in an age-dependent and sex-dependent manner, and microglia respond both directly and indirectly to changes in hormone levels by altering transcriptional gene expression, morphology, and function (Sullivan & Ciernia, 2022).
Human data support an association between the immune system and autism with a large body of evidence linking maternal or childhood infection with increased rates of autism (Krakowiak et al., 2017; Majerczyk, Ayad, Brewton, Saing, & Hart, 2022; Nielsen et al., 2022). A population-based study of children in Australia found that autoimmune disease in mothers or infection in children prior to age 2 were associated with increased odds of autism (Nielsen et al., 2022). Genetic studies have identified immune system genes differentially expressed in individuals with autism vs. neurotypical controls, and transcriptomic studies reported that pathways involved in immune function were significantly associated with autism diagnosis (Ansel et al., 2016). In a population-based case control study of children in California, levels of IL-1β and IL-4 from neonatal blood spots were associated with increased odds of autism (Krakowiak et al., 2017). Further IL-6 and IFNγ levels in mothers have been associated with elevated autism diagnosis in children (Majerczyk et al., 2022). Increased microglia cell activation has also been noted in postmortem analyses of brains from individuals with autism vs. neurotypical controls (Blagburn-Blanco, Chappell, De Biase, & DeNardo, 2022; Zhang, Wang, Li, Deng, & Shen, 2022). The role of the immune system in promoting masculinization of the brain likely provides a biological substrate for the sex bias in autism diagnosis among boys vs. girls (McCarthy, Nugent, & Lenz, 2017; McCarthy & Wright, 2017).
Given the importance of the immune system in establishing synaptic connectivity, and ample evidence that many environmental chemicals can disrupt immune function (Dietert, 2014; Eisner et al., 2022; Kreitinger, Beamer, & Shepherd, 2016; Winans, Humble, & Lawrence, 2011), it seems plausible that the immune system is a point of convergence for environmental and genetic factors to interact to confer risk for autism. However, as noted for other mechanisms of G × E interactions, to date, there is no evidence causally linking environmental chemical and genetic effects on immune function to increased risk of autism.
3.6. Convergence of genes and environment on neurodevelopmental signaling pathways
Many genetic risk factors for autism converge on signaling pathways that critically regulate synaptic connectivity in the developing brain (Delorme et al., 2013; Guang et al., 2018; Landrigan et al., 2012; Masini et al., 2020; Qiu et al., 2012). Therefore, these signaling pathways and the neurodevelopmental processes regulated by these signaling pathways represent molecular and cellular points of convergence for environmental and genetic risk factors for autism (Table 2) (Stamou et al., 2013).
Table 2.
Autism-associated genes important for neuronal connectivity, dendritogenesis and synaptogenesis that may interact with environmental chemicals to increase autism risk.
| Signaling pathway | Genes | References |
|---|---|---|
| Signaling pathways that regulate dendrite and/or synapse formation |
|
|
| Convergence on calcium signaling pathways |
|
A number of genes identified as autism risk genes regulate dendritogenesis or synapse formation, elimination or stabilization. These include genes for neuroligins (NLG3, NLG4) (Sudhof, 2008; Zoghbi & Bear, 2012), neurexins (NRXN1, NRXN3) (Sciacca et al., 2022), and contactin-associated protein-2 (CNTNAP2) (Dennis et al., 2011; Scott-Van Zeeland et al., 2010), all which have important roles in connecting and stabilizing presynaptic and postsynaptic components of the synapse. CNTNAP2 mutations have been linked to increased autism risk, and expression of CNTNAP2 gene variants are sufficient to alter connectivity in experimental models (Dennis et al., 2011; Scott-Van Zeeland et al., 2010). The SH3 and multiple ankyrin repeat domains 3 (SHANK3) gene has been identified as an autism risk gene (Huang et al., 2019; Lutz et al., 2021; Wan et al., 2022). SHANK3 functions to interact with neuroligin and neurexin complexes to regulate activity-dependent synaptogenesis (Craig & Kang, 2007; Stamou et al., 2013). ERK, PI3K and mTOR signaling pathways are also strongly implicated in autism (Chaudry & Vasudevan, 2022; Lutz et al., 2021) and are important in regulating dendritic growth and synaptogenesis (Levitt & Campbell, 2009).
Neuronal activity plays a significant role in sculpting neural circuits in the developing brain, and calcium signaling is the major mechanism linking neuronal activity to dendritogenesis and synaptogenesis in the developing brain (Pessah, Cherednichenko, & Lein, 2010; Wayman et al., 2006). Thus, it is not surprising that genes that either control calcium signaling or are regulated by calcium signaling, are predominant in the list of autism risk genes (Ansel et al., 2016; Nanou & Catterall, 2018; Nguyen, Medvedeva, Ayyagari, Schmunk, & Gargus, 2018; Pessah et al., 2010). Diverse environmental chemicals have also been shown to disrupt these same calcium signaling pathways, suggesting a “two hit” mechanism in which genetic and environmental factors converge to alter the fidelity of calcium signaling resulting in altered synaptic connectivity (Keil Stietz et al., 2021; Klocke & Lein, 2020; Panesar et al., 2020; Stamou et al., 2013).
Evidence that neuronal calcium signaling is disrupted in autism also derives from phenotypes observed in syndromic disorders that have a high incidence of autism. For example, Fragile × syndrome, which is caused by expansion of a CGG repeat (>200) in the 5’ non-coding portion of the fragile × mental retardation 1 gene (FMR1), is the most prevalent single gene disorder associated with increased risk for autism (Krueger & Bear, 2011; Leehey & Hagerman, 2012). FMR1 premutation (55–200 CGG repeats), which causes fragile X-associated tremor/ataxia syndrome marked by deficits in cognitive function, tremors and motor abilities in adults, is also associated with elevated risk of autism (Chonchaiya et al., 2012; Hagerman, Au, & Hagerman, 2011). Hippocampal neurons from FMR1 premutation mice displayed elevated cytoplasmic calcium concentrations and abnormal glutamatergic responses in vitro (Berman, Murray, Arque, Hunsaker, & Wenzel, 2012; Chen et al., 2010; Robin et al., 2017). FMR1 premutation mouse neurons also exhibited increased frequency and duration of clustered burst firing compared to wild type controls (Cao, Hulsizer, et al., 2012; Cao et al., 2013). FMR1 premutation neurons derived from human iPSC cells similarly displayed altered calcium signaling under baseline conditions and in response to glutamatergic stimulation (Liu et al., 2012). Since calcium signaling is a major driver of dendritic morphogenesis, it is not surprising that mice with FMR1 premutations also exhibited deficits in neuronal cytoarchitecture and learning and memory behavior (Berman et al., 2012; Chen et al., 2010; Diep et al., 2012; Keil et al., 2019; Sethi et al., 2021).
Dendrites can structurally remodel in response to environmental factors and experience. This structural plasticity is mediated by calcium signaling triggered by NMDA receptor activation that drives the sequential activation of calmodulin-dependent protein kinase kinase (CaMKK), CaMKI, and MAPK ERK/MEK ERK kinases to enhance cAMP response element-binding protein (CREB) resulting in Wnt2 transcription and dendritic growth (Wayman et al., 2006). CREB also regulates expression of miR132 during neuronal maturation and synaptogenesis (Hansen et al., 2013). CREB dysfunction is implicated in autism (Barnby et al., 2005; Ngounou Wetie et al., 2015; Todd & Mack, 2001; Zheng et al., 2016), and downstream genes such as miR132 and Wnt pathway members have also been linked to autism or autism-related phenotypes (Bocchi et al., 2017; Kalkman, 2012; Li et al., 2016). Environmental chemicals are also known to alter neurodevelopment via modulation of CREB dependent mechanisms (Connolly & Kingsbury, 2010; Jang et al., 2012; Sethi, Keil, & Lein, 2018).
Ryanodine receptors (RyRs) are important for regulating calcium release from the endoplasmic reticulum (Pessah et al., 2010). Point mutations in RYR are prevalent in the human population with ~35% of individuals carrying one or more RYR variants (Kim et al., 2013) that increase sensitivity to some anesthetics to trigger malignant hyperthermia (Pessah et al., 2010). Mice engineered to express human RYR mutations displayed enhanced sensitivity to inhaled anesthetics, and their neurons exhibited elevated intracellular calcium concentrations (Barrientos et al., 2012; Yuen et al., 2012). Neurons from RYR transgenic mice also exhibited altered dendrite arborization and deficits in behavioral tasks (Keil et al., 2019; Sethi et al., 2021). RyR2 mutations have been linked to autism (Soueid et al., 2016) and environmental chemicals have been shown to interact with genetic mutations in RyRs to influence dendritic arborization and behavior in mouse models (Keil Stietz et al., 2021; Sethi et al., 2021).
Genes that encode calcium ion channels other than RyRs, as well as channels that conduct ions other than calcium, have also been implicated in autism (Ansel et al., 2016; Nanou & Catterall, 2018; Schmunk & Gargus, 2013). These include the voltage-gated calcium channels (CACNA family), voltage-gated sodium channels (SCN family), ligand-gated chloride channels (GABRB family) and, more recently, calcium activated potassium channels (KCNM family) (Schmunk & Gargus, 2013). Mutations in the L-type calcium channel Ca(v)1.2 cause Timothy syndrome, which is associated with high rates of autism (Splawski et al., 2004). Mutations in Cav1.2 and Cav1.3 are also implicated as autism risk factors independent of Timothy syndrome (Marcantoni et al., 2020; Nanou & Catterall, 2018). Timothy syndrome is thought to cause autistic phenotypes via altered neuronal connectivity downstream of dysregulated calcium signaling (Nanou & Catterall, 2018). In neurons, this mutation elevated intracellular calcium levels and altered expression of genes regulated by calcium signaling, including CAMKII and CREB (Pasca et al., 2011). In a mouse model of Timothy syndrome, brains had reduced numbers of cortical projecting neurons, deficits in neuronal differentiation and elevated production of catecholamines, all of which are autism-relevant phenotypes (Pasca et al., 2011). Similarly, de novo mutations in voltage-gated sodium channels (SCN2A) are implicated as autism risk factors (Sanders et al., 2012). SCN2A are important in regulating action potentials and play an important role in synaptic plasticity (Spratt et al., 2019).
E/I imbalance is implicated in the pathogenesis of autism (Rubenstein & Merzenich, 2003), and this can result from structural changes in neural circuits and/or altered expression of receptors for excitatory vs. inhibitory neurotransmitters. For example, GABA receptor mutations are associated with increased autism risk (Deidda et al., 2014), and levels of GABRB3 were found to be lower in brains from children with autism compared to neurotypical controls (Samaco et al., 2005). GABRB3 is located in the chromosome 15q11–13 locus, which is implicated in syndromic forms of autism and is thought to increase susceptibility to environmental insults (Mitchell et al., 2012; Shelton, Hertz-Picciotto, & Pessah, 2012).
KCNM channels are also important for regulating neuronal excitability, and KCNMA1 mutations that reduce activity of the channel have been observed in individuals with autism (Du et al., 2005; Laumonnier et al., 2006; Schmunk & Gargus, 2013). KCNM channels have also been found to be regulated by Fragile × mental retardation protein (FMRP) via a KCNMB4 regulatory subunit and loss of FMRP reduced channel activity, which dysregulated neurotransmission (Deng et al., 2013). Mutations in the regulatory KCNMB4 subunit have been linked to autism, and KCNMB4 has been identified as one of the genes to be used in predictive panels of autism (Schmunk & Gargus, 2013; Skafidas et al., 2014).
4. PCBs as putative autism risk factors
PCBs belong to a larger grouping of environmental contaminants referred to as persistent organic pollutants (POPs) that have in common environmental persistence and resistance to metabolic degradation. PCBs are a family of 209 structurally similar congeners that vary according to the number and position of chlorine substitutions on the biphenyl backbone (Klocke, Sethi, & Lein, 2019). PCBs are classically divided into two groups based on their structure and binding affinity for the aryl hydrocarbon receptor (AhR), which is the canonical receptor for dioxin (2,3,7,8,-tetrachlorodibenzo-p-dioxin, TCDD): coplanar, dioxin-like PCBs bind the AhR with moderate to high affinity, whereas the non-coplanar, nondioxin-like (NDL) PCBs have little to no affinity for the AhR (Klocke et al., 2019). A subset of NDL PCBs have RyR activity (Holland et al., 2017; Pessah et al., 2019; Sethi et al., 2019). Some PCB congeners are reported to interact with steroid hormone receptors (Bell, Dryden, Will, & Gore, 2018; Bell, Hart, & Gore, 2016; Dickerson, Cunningham, Patisaul, Woller, & Gore, 2011; Sechman et al., 2016; Takeuchi et al., 2011; Takeuchi, Anezaki, & Kojima, 2017) and TH receptors (Itoh et al., 2018; Pessah et al., 2019; Salazar et al., 2021; Zoeller, 2007) and, thus, are postulated to cause developmental neurotoxicity via endocrine disruption (Bell et al., 2018; Walker, Goetz, & Gore, 2014; Winneke et al., 2014). The NDL PCBs are thought to have the most significant impact on neurodevelopmental outcomes (Klocke & Lein, 2020) and, therefore, are highlighted in this review.
Despite a ban on production of PCBs in the U.S. in the 1970s and globally in the early 2000s (Klocke et al., 2019; Melymuk et al., 2022), legacy PCBs produced prior to the ban persist in the environment to this day. Sources of legacy PCBs include leachate from waste sites, degradation of old building materials and release from PCB-containing fluorescent light ballasts, hydraulic equipment and capacitors still in use. In addition, there are contemporary sources of PCBs, including the unintentional production of PCBs as byproducts of the modern manufacturing processes used to produce pigments found in paints, varnishes and caulking (Hannah, Megson, & Sandau, 2022; Herkert, Jahnke, & Hornbuckle, 2018; Hu & Hornbuckle, 2010; Liu et al., 2004), and the breakdown of higher chlorinated PCBs into lower chlorinated PCBs (Xiang, Xing, Liu, Qin, & Huang, 2020). Some of these contemporary PCBs were not present in the commercial mixtures produced prior to the ban, making contemporary PCB exposures unique compared to those that occurred prior to 1979. Understanding the neurotoxicological implications of PCBs not found in the legacy mixtures is an expanding area of research.
PCBs are ubiquitous contaminants readily detected in human tissues, livestock, fish, dairy products, water, soil, sediments and both indoor and outdoor air worldwide (Barmpas et al., 2019; Chen, Streifel, et al., 2017; Kania-Korwel & Lehmler, 2016; Li, Lim, et al., 2022; Sethi, Chen, Kass, & Puschner, 2017; Thompson & Boekelheide, 2013; Yu, Liu, Shu, Ma, & Pan, 2020). Because they are generally lipophilic, PCBs bioaccumulate up the food chain. Thus, humans are primarily exposed to PCBs via diet, although inhalation is becoming an increasingly important route of exposure, particularly to the lower chlorinated congeners (Ampleman et al., 2015; Carpenter, 2006; Chan-Hon-Tong, Charles, Forhan, Heude, & Sirot, 2013; Durand et al., 2008). In addition, PCBs cross the placenta and are present in breastmilk (Foerster et al., 2021; Kim et al., 2018). The National Health and Nutrition Examination Survey (NHANES) has documented widespread PCB exposure in women of childbearing age in the U.S. (Thompson & Boekelheide, 2013). PCBs have been detected not only in serum of pregnant women but also in cord blood and brain tissue from young children (Baba et al., 2018; Eskenazi et al., 2017; Granillo et al., 2019; Hisada et al., 2014; Li, Lim, et al., 2022; Rosenquist et al., 2017). In one study, tissue levels of the NDL PCB congener, PCB 95, were found to be significantly higher in postmortem brain tissue from children with a genetic form of autism, 15q11-q13 duplication compared to neurotypical controls (Mitchell et al., 2012).
Reviews of the epidemiological evidence have almost universally concluded that the weight of evidence indicates that PCBs cause developmental neurotoxicity in humans; however, it is less clear whether PCBs are associated with increased risk for autism (Alampi et al., 2021; Bernardo et al., 2019; Lyall, Croen, Sjodin, et al., 2017; Mehri, Bashirian, Khazaei, & Jenabi, 2021; Pessah et al., 2019). For example, a recent narrative summary of the epidemiological literature published between 1990–2018 identified 29 peer-reviewed publications that fit inclusion criteria for sample size (>100), measurement of prenatal PCB levels in biological samples collected during pregnancy or delivery, and behavioral assessment of children at 3 years old or older (Pessah et al., 2019). The studies included in the analysis examined diverse cohorts from across North America, Asia, and Europe. The review identified 12 publications from 9 different cohorts that examined impacts of PCBs on cognitive function among children aged 3–11 years. Of these, most (8 of 12) found positive associations between PCB exposure and cognitive or language deficits in children (Pessah et al., 2019). A total of 17 studies from 11 different cohorts examined associations of prenatal PCBs with attention, behavioral regulation and social behavior among 3–12-year-old children (Pessah et al., 2019). The majority (10 of 17) reported PCB-related associations with impulse control, hyperactivity, and attention. Only two of the 17 studies examined PCBs in relation to social behavior and autistic traits, with one study reporting that total PCB levels were associated with fewer autistic traits (Nowack, Wittsiepe, Kasper-Sonnenberg, Wilhelm, & Scholmerich, 2015) and the other reporting congener-specific associations with autistic traits (Braun et al., 2014).
As acknowledged by the authors (Pessah et al., 2019), a limitation of the studies included in their review was that associations with specific PCB congeners or mechanism-based classes of congeners were not evaluated largely because most epidemiologic studies report total sum PCB levels or levels of “indicator” PCBs in biological samples. The significance of this limitation is underscored by a recent study suggesting that NDL PCBs with activity at the RyR, but not dioxin-like PCBs or total PCBs, are positively associated with autism (Granillo et al., 2019). The genetic substrate likely also influences the impact of PCBs on autism-relevant outcomes, as illustrated by a recent pilot study not included in this review that identified a trend towards a positive association between PCB 153 and autism in individuals with a deletion mutation in the gene encoding glutathione transferase but not in individuals who did not have this mutation (Bach et al., 2020). Also not included in the summary, because the age of the child at the time of autism diagnosis was not specified, was a 2017 study that found a positive association between PCB exposure and autism (Lyall, Croen, Sjodin, et al., 2017). Furthermore, a 2020 study reported an association between plasma PCB concentrations measured during pregnancy and increased incidence of autistic behaviors in children aged 3–4 years old when the data were analyzed using Bayesian predictive odds ratios (Bernardo et al., 2019). When considering these additional studies that have examined the impact of prenatal PCB on autism phenotypes together with the studies included in the review, most (five of six) report that PCBs increase the expression of autistic traits.
More recently, a meta-analysis was conducted of studies published through 2019 that reported odds ratios for PCB exposure and autism from case-control or cross-sectional studies (Mehri et al., 2021). The authors used random-effects models to examine the association among five different studies representing five independent cohorts from across North America and Finland using pooled odds ratios (ORs) and their 95% confidence intervals (CI). The pooled ORs indicated a significant association between PCB exposure during pregnancy and autism risk (OR, 1.80; 95% CI, 1.26–2.34) (Mehri et al., 2021). One of the studies included in the meta-analysis was a study from 546 mothers and infants in Canada that found children age 3–4 had increased social responsiveness scale (SRS) scores and increased odds of more autistic behavior in the highest plasma PCB concentration (sum of 24 PCB congeners) during pregnancy groups compared to the lowest PCB groups (Bernardo et al., 2019). A new study has since been conducted using the same cohort of 478 Canadian mothers and children but applying different regression models and found the sum of 4 PCBs (PCB 118, PCB 138, PCB 153 and PCB 180) was also associated with higher SRS scores (Alampi et al., 2021). These four PCBs were also identified in another study (Lyall, Croen, Sjodin, et al., 2017) included in the Mehri et al., 2021 meta-analysis that linked prenatal PCB exposure to autism risk. In a California cohort of 545 children with autism, mean levels of prenatal PCBs were higher in this group compared to general population controls, and autism risk was most elevated in the highest vs. lowest quartiles of prenatal PCB levels. Associated risk was especially prevalent for several individual congeners, including PCB 138/158, PCB 153, PCB 170 and PCB 180 that all had odds ratios of ~1.5 (Lyall, Croen, Sjodin, et al., 2017). Observations that specific congeners can differentially influence neurodevelopmental outcomes is an area of future study. This is particularly important given mechanistic data indicating that different subgroups of PCBs have different molecular targets (Klocke et al., 2019; Klocke & Lein, 2020). The implications are illustrated by the findings of a previously mentioned study of 104 mother-child pairs that found while total sum PCB levels were not associated with an increased risk of autism, prenatal levels of RyR-active congeners were linked to increased autism risk (Granillo et al., 2019). Thus, it is important to examine PCB links to autism phenotypes based on PCB structure and toxicological activity.
Clearly, these studies suggest that PCBs per se are not causing autism, but rather that PCBs increase risk for autism, likely by interacting with genetic susceptibilities. The questions then are which genes are PCBs interacting with to confer autism risk, and what are the mechanism(s) underlying G × PCB interactions? In a study of 206 autism susceptibility genes to determine which were targeted by chemicals included in the Comparative Toxicogenomics Database (CTD), over 1 million interactions were found with PCBs representing one of the major chemical classes of concern found to interact with autism risk genes (Carter & Blizard, 2016). In this study, the top three PCBs, PCB 153, PCB 138 and PCB 126, were found to affect a total 419, 569, and 1243 genes, respectively, and within those affected genes, 22, 20 and 24 were grouped as autism risk genes, respectively (Carter & Blizard, 2016). Below, we summarize the current literature related to how PCBs may interact with genetic risk factors to confer autism risk.
4.1. PCB interactions with xenobiotic metabolism
PCBs are metabolized by CYP enzymes, including Cyp1a1 (Grimm et al., 2015; Liu et al., 2020). Cyp1a1 is a transcriptional target of the AhR that is upregulated in the liver by PCB exposure in rodent models (Chubb et al., 2004), and the extent of Cyp1a1 induction following PCB exposure is influenced by the gut microbiome (Lim et al., 2020). A transcriptomic study found that CYP1A1 was downregulated in cord blood from individuals with autism vs. neurotypical controls, which would be predicted to decrease the rate and/or extent of PCB metabolism (Mordaunt et al., 2019; Thony, Auerbach, & Blau, 2000). This suggests that the population with autism, or at least a subgroup with decreased CYP1A1 expression, may be more susceptible to PCB effects on neurodevelopment compared to neurotypical controls.
Glutathione-mediated transformation is another major route of PCB detoxification (Mandic-Maravic et al., 2019; Matelski & Van de Water, 2016). Glutathione S-transferase (GST) activity was found to be lower in children with autism vs. neurotypical controls (Serajee et al., 2004). In addition, genetic polymorphisms in the glutathione pathway were detected at a higher frequency in autism vs. typically developing children, and mothers who expressed these genetic polymorphisms were at higher risk for having a child diagnosed with autism (Rossignol et al., 2014). Importantly, GST genotypes have been shown to influence PCB concentrations in children with autism (Amen et al., 2022). In a study of 125 children from Pakistan with autism, blood concentrations of PCB 77, PCB 118, PCB 128 and PCB 153 were significantly higher in autism cases compared to neurotypical controls (Amen et al., 2022). Conversely, PCB 187 was higher in control blood than autism cases, while other PCB congeners analyzed were not different between populations (Amen et al., 2022). This study also examined the impact on PCB levels of expression of genetic polymorphisms in the glutathione transferase gene, GSTT1. PCB 66 and PCB 156 concentrations were higher, while PCB 81 and PCB 118 were lower in the GSTT1 null genotype compared to the “wildtype” GSTT1 genotype. Among the autism cases, PCB 44 and PCB 128 concentrations were higher, while PCB 167 was lower in null vs. wildtype GSTT1 genotypes. Similar differences in PCB accumulation were also noted for another glutathione genotype, GSTM1 (Amen et al., 2022). These findings highlight the role that xenobiotic metabolism may play in determining the PCB profile present in the brain of exposed individuals. Moreover, this study clearly demonstrates that genotypic variations in glutathione genes influence autism risk, underscoring the challenge of conducting epidemiological studies with sufficient power to study causal relationships between PCB exposures and autism risk.
PCB metabolism is also important to consider in light of emerging data that PCB metabolites can have detrimental effects on the central nervous system (Berghuis, Soechitram, Hitzert, Sauer, & Bos, 2013; Dreiem, Rykken, Lehmler, Robertson, & Fonnum, 2009; Hisada et al., 2014; Nomiyama et al., 2019; Sethi, Keil, et al., 2017; Takeuchi et al., 2011; Tehrani & Van Aken, 2014; Uwimana et al., 2019) and that hydroxylated PCB metabolites are detected in human brain samples (Li, Lim, et al., 2022). Hydroxylated metabolites of some PCB congeners inhibited TH-dependent dendritic growth in cultured rodent Purkinje neurons (Kimura-Kuroda, Nagata, & Kuroda, 2005, 2007), while other hydroxylated PCB metabolites induced TH-independent dendritic growth in Purkinje neurons (Kimura-Kuroda et al., 2007). PCB 11 and its hydroxylated and sulfated metabolites enhanced rat hippocampal and cortical dendritic and axonal complexity in vitro, although the sulfated metabolites were more potent than the parent compound (Sethi, Keil, et al., 2017). PCBs have also been shown to trigger apoptosis of hippocampal neurons in vitro and in vivo via oxidative stress (Howard, Fitzpatrick, Pessah, Kostyniak, & Lein, 2003; Yang & Lein, 2010), and hydroxylated PCB metabolites similarly induced apoptosis of cultured rat cerebellar granule cells via oxidative stress, in part through a mechanism involving ERK1/2 signaling (Dreiem et al., 2009). Thus, while the ultimate purpose of PCB metabolism is to facilitate elimination of PCBs from the body, the metabolites appear to have deleterious effects on critical neurodevelopmental processes implicated in the pathogenesis of autism. Moreover, these observations support the hypothesis that heritable deficits in metabolic pathways can exacerbate the developmental neurotoxicity of PCBs.
4.2. PCB interactions with the gut microbiome
Exposure to PCBs has recently been demonstrated to alter the gut microbiome in mice exposed throughout gestation and lactation to PCBs in the maternal diet. Specifically, in offspring exposed developmentally to the MARBLES (Markers of Autism Risk in Babies—Learning Early Signs) PCB mixture, which simulates the relative proportions of the twelve most abundant PCB congeners found in the serum of pregnant women living in Northern California who are at increased risk for having a child with autism (Hertz-Picciotto, Schmidt, et al., 2018), the beta diversity of the gut microbiome was significantly altered compared to vehicle controls (Rude et al., 2019). This change was further exacerbated by expression of heritable human mutations in genes that alter the fidelity of calcium signaling (CGG premutation plus gain-of-function RyR mutation) (Rude, Keogh, & Gareau, 2019). Adult mice exposed to PCBs were found to have altered gut microbiota characterized by changed alpha diversity and reduced beneficial bacteria, and these effects were exacerbated when combined with a high fat diet (Chi et al., 2018; Petriello, Hoffman, Vsevolozhskaya, Morris, & Hennig, 2018; Wahlang et al., 2021). Recently, it was demonstrated that not only does PCB exposure alter the gut microbiome, but also that the gut microbiome influences PCB metabolism and disposition. Thus, tissue levels of PCBs were higher in conventional mice with a normal microbiome than in germ-free mice that lack a microbiome following comparable PCB exposures (Li, Tian, et al., 2022). The gut microbiome also altered the gut–liver axis as evidenced by differential patterns of gene expression in the liver of conventional vs. germ-free mice following exposure to a human-relevant PCB mixture (Lim et al., 2020). Genes that were differentially regulated included Cyp genes and other genes involved in PCB metabolism. These findings revealed significant interplay between PCB exposures and the microbiome with the microbiome influencing neurotoxic responses and PCBs influencing microbial diversity in the gut. Whether and how these interactions influence autism risk and/or severity represent an intriguing area of future study.
4.3. PCBs as endocrine disruptors
PCBs have been shown to disrupt endocrine signaling via diverse mechanisms. In humans, PCB levels were reported to be inversely correlated with testosterone levels in adults (Goncharov et al., 2009), and maternal serum PCB levels were linked to altered testosterone and estradiol levels in cord blood of female and male newborns (Cao et al., 2008). PCB levels in maternal serum were also associated with changes in sex-typical behaviors in children age 6–8, generally increasing feminine scores in boys and decreasing feminine scores in girls (Winneke et al., 2014). In rats, developmental exposure to an estrogenic PCB mixture (the commercial mixture Aroclor 1221) changed the sexual differentiation of the adult brain, most notably causing masculinization or defeminization of the female brain (Dickerson et al., 2011; Walker et al., 2014). Thus, PCBs appear capable of influencing steroid hormone levels or signaling pathways, but whether these endocrine effects are causally linked to PCB effects on autism-relevant phenotypes is unknown.
Multiple animal studies have demonstrated that PCB exposures can decrease serum TH levels in pregnant dams and disrupt TH homeostasis in dams and offspring via effects on TH synthesis, transport, and metabolism (Zoeller, 2007). While similar relationships between PCBs and serum TH levels have been reported in humans, recent epidemiologic studies (Itoh et al., 2018) suggest that this may not be a consistent relationship in humans. Early experimental studies demonstrated a causal link between PCB-mediated decreases in serum TH levels and neurotoxic effects. Specifically, TH supplementation prevented motor and auditory deficits induced by developmental exposure to Aroclor 1254 in rats (Goldey & Crofton, 1998) and reversed PCB effects on oligodendrocyte maturation and myelination in vitro (Nave & Werner, 2014), both phenotypes associated with autism (Lord et al., 2018). However, experimental animal studies suggest that the cognitive deficits associated with developmental PCB exposure occur independent of decreased serum TH levels. For example, developmental exposures of rats to Aroclor 1254 at levels that significantly reduced maternal serum TH levels were not associated with learning and memory deficits or changes in neuronal progenitor cell proliferation and survival in exposed offspring (Zahalka, Ellis, Goldey, Stanton, & Lau, 2001). Conversely, developmental exposure to Aroclor 1254 at lower levels that did not significantly decrease serum TH levels caused significant performance deficits in the Morris water maze (Yang et al., 2009). Studies with individual PCB congeners also provide little support for the hypothesis that TH deficits mediate the cognitive effects of PCBs. For example, the NDL PCBs 28, 118 and 153 produced similar deficits in spatial learning and memory, but their effects on serum T4 levels varied from a marked reduction to no effect. In contrast, DL PCBs 77 and 126 significantly reduced serum TH, but had few, if any, adverse effects on cognitive behavior (reviewed in Pessah et al., 2019). The inconsistencies observed across both human and rodent studies raise significant questions regarding a causal association between PCB effects on serum TH levels and cognitive behaviors. Fewer studies have focused on the effects of PCBs on sociability and repetitive behavior in rodent models (reviewed in Klocke & Lein, 2020). However, one study reported that developmental exposure to the MARBLES PCB mixture on behavioral phenotypes in mice were observed independent of altered serum TH levels (Sethi et al., 2021).
Alternatively, it has been proposed that the developmental neurotoxicity of PCBs is mediated by PCB interactions with TH receptors and/or the transcriptional machinery that regulates expression of TH-responsive genes. Early evidence in support of this hypothesis derived from a study showing that gestational exposure of rats to Aroclor 1254 in the maternal diet increased expression of TH-responsive genes in the fetal cortex despite significantly reducing maternal levels of serum TH, suggesting direct effects of PCBs on TH receptors in the fetal brain (reviewed in Zoeller, 2007). However, recent in vitro studies of PCBs abundant in the serum of pregnant women at increased risk for having a child diagnosed with autism found no significant agonistic or antagonistic interactions with canonical TH receptors expressed in a TH reporter cell line when exposed to these PCBs singly or in combination over a wide range of concentrations (Sethi et al., 2019). This same study also saw no effect of human-relevant hydroxylated or sulfated metabolites of PCB 11 and PCB 52 on TH receptor activity. These observations are consistent with an earlier study that failed to detect a direct interaction between Aroclor 1254 and TH receptors (Zoeller, 2007). The lack of convincing evidence demonstrating direct molecular interactions of PCBs with TH receptors has led to suggestions that PCBs may affect TH signaling via modulation of crosstalk between TH and other endocrine hormones and nuclear receptors (Kouidhi & Clerget-Froidevaux, 2018). However, to date, there are no experimental data directly linking PCB effects on TH signaling to effects of developmental PCB exposure on cognitive, social or repetitive behaviors. In contrast, evidence of causal relationships between PCB effects on serum TH levels and auditory and motor deficits and myelination suggest the possibility that interactions between PCBs and TH signaling may contribute to a subset of autism phenotypes.
4.4. Epigenetic effects of PCBs
In vitro and in vivo models have demonstrated that PCBs can alter epigenetic endpoints relevant to autism. One major target includes the family of genes responsible for maintaining or adding DNA methylation marks, the DNA methyltransferases (Dnmts) (Feng et al., 2010; Keil & Lein, 2016; Okano, Bell, Haber, & Li, 1999). PCB 153 decreased DNA methyltransferase activity in cultured embryos (Wu, Zhou, & Ohsako, 2006), a mixture of 15 PCBs reduced expression of Dnmts and methyl donor S-adenosylmethionine in the liver of developmentally exposed rats (Desaulniers et al., 2009), and developmental exposure to Aroclor 1221 increased Dnmt abundance in sexually dimorphic regions of the rat brain (Walker et al., 2014). These observations suggest that PCB effects on Dnmt expression and activity vary depending upon the timing of exposure, the PCB congeners and doses to which the system is exposed, and the target tissue. Evidence also suggests that PCBs alter global DNA methylation levels in the brain following developmental exposure. In a mouse model, developmental exposure to the MARBLES PCB mix caused significant brain hypermethylation compared to vehicle control, an effect unique to male offspring (Laufer et al., 2022). In addition to global changes, developmental exposure to the MARBLES PCB mix also resulted in differentially methylated genes within the brain and placenta of mouse offspring (Laufer et al., 2022). Several of the differentially methylated genes have been linked to PCB-induced developmental neurotoxicity, such as Wnt and calcium signaling molecules, as well as MeCP2 mutations associated with Rett syndrome and autism (Chen et al., 2015; Laufer et al., 2022).
These studies illustrate that PCBs can change DNA methylation in genes relevant to autism and are consistent with epidemiological evidence that PCB exposures are linked to alterations in the epigenome. For example, in a study of 399 Japanese women, serum levels of NDL PCBs were inversely associated with global DNA methylation levels in peripheral leukocytes (Itoh et al., 2014), which is similar to findings in another study of 89 Koreans (Kim et al., 2010) and 70 Greenlandic Inuit Indians (Rusiecki et al., 2008). Others have found levels of dioxin-like PCBs are associated with global DNA hypermethylation (Lind et al., 2013); thus, differences in PCB exposures with respect to congener profiles and levels and timing of exposure likely alter the influence of PCBs on the epigenetic landscape and make epidemiological studies extremely challenging. In addition to global changes, gene specific alterations have also been observed in humans exposed to PCBs. For example, altered methylation of MeCP2 in the cord blood of Japanese infants is correlated with environmental exposure to PCBs (Eguchi et al., 2019). Epigenetic mechanisms may also influence other known genetic causes of autism. For example, 15q11-q13 duplications are associated with a syndromic form of autism, and individuals with this duplication have lower levels of brain LINE-1 repetitive DNA methylation (Mitchell et al., 2012). Interestingly, these same individuals had higher levels of PCB 95 than typically developing controls, and this genetic mutation was the strongest predictor of PCB 95 levels (Mitchell et al., 2012). Considering the important role for epigenetic mechanisms in normal neurodevelopment and the epigenetic disruptions noted in autism, epigenetic modifications provide a convergent target by which PCBs may influence autism risk.
4.5. PCB effects on the immune system
Numerous studies in humans and both in vivo and in vitro experimental models have demonstrated that PCBs can modulate immune function (Kramer, Hikel, Adams, Hinds, & Moon, 2012; Liu, Lu, Zhong, Wang, & Xu, 2022). For example, it has been reported that developmental PCB exposures suppressed immune responses to diverse vaccines in children in Norway (Hochstenbach et al., 2012), the Netherlands (Stolevik et al., 2013; Weisglas-Kuperus et al., 2000), the Faroe Islands (Heilmann et al., 2010), Sweden (Glynn et al., 2008), and in Inuit infants from Nunavik, Quebec (Dallaire et al., 2004). In the Norwegian cohort, the immunosuppressive effects of PCBs coincided with transcriptional changes of several genes in cord blood related to immune function (Hochstenbach et al., 2012), and with increased infections (Stolevik et al., 2011). These associations are likely influenced by specific PCB congeners since increased rates of childhood infection in Swedish children were observed for PCB 28, PCB 52 and PCB 101, but not for other mono-and di-ortho substituted PCBs (Glynn et al., 2008). In mononuclear cells cultured from cord blood collected from children in Canada with relatively elevated PCB exposures via high dietary intake of PCB-contaminated fish, release of the proinflammatory cytokine tumor necrosis factor alpha (TNFα) was inversely associated with umbilical cord blood concentrations of PCBs (Bilrha et al., 2003). Gene expression analysis of human peripheral blood mononuclear cells incubated with various PCBs revealed differentially regulated gene expression in the presence of dioxin-like and NDL PCBs, with dioxin-like PCBs eliciting a greater number of changes (Leijs et al., 2019). Genes that were downregulated were predominately those involved in immune response (Leijs et al., 2019). Rodent studies further strengthen the association of PCBs with altered immune/inflammation responses. In pregnant rats, exposure to Aroclor 1254 increased expression of IL-6, IFNγ and IL-2 in liver and serum (Xu, Guo, Li, Pan, & Ma, 2019). In another rat study, developmental exposure to PCB 126 via the dam increased ILl-1B and IFNγ in the serum of offspring (Ahmed, El-Gareib, & Shaker, 2018). Similarly, gestational exposure to Aroclor mixtures via the maternal diet resulted in differential cytokine expression within the brain of rat offspring and further exacerbated serum cytokine IL-1β and IL-6 increases upon challenge with the inflammatory agent, lipopolysaccharide (LPS) (Bell et al., 2018). The findings in rodent models are consistent with the epidemiological observations (Dietert, 2014).
G × E interaction studies in rodent models have been conducted to better understand the convergence between PCBs and autism risk genes on the immune response. The impact of developmental exposure to the MARBLES PCB mixture on brain and serum cytokine levels was quantified in mice engineered to express genetic mutations that alter the fidelity of calcium signaling (Keil et al., 2019), e.g., a RyR gain-of-function mutation and/or CGG premutation repeat expansions in the FMR1 gene (Matelski et al., 2020). Mutations in RYR1 and FMR1 are both linked to dysregulated calcium signaling and confer heightened sensitivity to environmental stressors in multiple cell types from mice (Barrientos et al., 2012; Cao et al., 2012, 2013) and affect immune function in humans and mice (Careaga et al., 2014; Marek et al., 2012; Vukcevic et al., 2013). Dose-dependent effects of PCB exposure on cytokine concentrations were observed in the serum, but not hippocampus, with 13 of 23 serum cytokines increased by PCB exposure. Differential effects of genotype were observed in the serum and hippocampus. Hippocampal cytokines were consistently elevated in RyR mutant mice and also in wildtype animals for some cytokines compared to CGG premutation and double mutant mice, while serum cytokines were mostly elevated in the mutant genotypes compared to the wildtype group. Males had elevated levels of 19 cytokines in the serum and 4 in the hippocampus compared to females, but there were also interactions between sex and genotype for 7 hippocampal cytokines. Only serum levels of the chemokine CCL5 showed an interaction between PCB dose, genotype, and sex. Serum levels of CCL5 generally increased with increasing PCB dose, and this effect was exacerbated in the mutant mouse models (Matelski et al., 2020).
These observations support the hypothesis that the genetic substrate influences PCB effects on cytokine expression. However, it remains unknown whether these interactions are causally related to autism risk. Another notable data gap is the effects of PCBs on microglia. Given the critical role that microglia play in sculpting neural circuitry in the developing brain (Sullivan & Ciernia, 2022), this would appear to be an important question that warrants investigation.
4.6. PCB interactions with genes that regulate calcium-dependent signaling
PCBs have been shown to modulate signaling pathways important for regulating dendritic arborization, synaptogenesis and synaptic connectivity (Klocke et al., 2019; Klocke & Lein, 2020). Dendritic morphology is a critical determinant of synaptic connectivity and changes in dendritic morphology (increased or decreased dendrite number, branching and/or spine density) contribute to the altered patterns of neuronal connectivity observed in many neurodevelopmental disorders, including autism (Alaerts et al., 2016; Cooper et al., 2017; Coskun, Loveland, Pearson, Papanicolaou, & Sheth, 2013; Keown et al., 2013; Khan et al., 2015). Various NDL PCBs have been shown to promote dendritic arborization and spine formation in hippocampal and cortical neurons both in vitro and in vivo (Keil, Sethi, & Lein, 2018; Keil Stietz et al., 2021; Lesiak et al., 2014; Sethi et al., 2018; Wayman, Yang, et al., 2012; Yang et al., 2009, 2014). These morphometric effects of NDL PCBs are largely mediated by calcium-dependent signaling pathways (reviewed in Panesar et al., 2020). Several NDL PCBs, exemplified by PCB 95 and PCB 136, trigger calcium signaling pathways via interactions with RyRs that enhance the open probability of these calcium ion channels embedded in the membrane of the endoplasmic reticulum, which effectively increases intracellular calcium concentrations (Pessah et al., 2010; Wayman, Bose, et al., 2012). These effects of PCB 95 and PCB 136 on RyR activity (Wayman, Yang, et al., 2012; Yang et al., 2014) trigger calcium-dependent signaling pathways, which in turn activate CREB-dependent transcriptional mechanisms (Lesiak et al., 2014; Wayman, Bose, et al., 2012) and mTOR-dependent translational mechanisms (Keil, Miller, Chen, et al., 2018) to stimulate dendritic growth and spine formation. The dendrite promoting effects of PCB 95 and 136 were blocked by pharmacological antagonism or siRNA knockdown of RyRs. PCB 66, a congener with physicochemical properties comparable to that of PCB 95 that lacks RyR activity, did not promote dendritic growth or spine formation (Lesiak et al., 2014; Wayman, Bose, et al., 2012; Yang et al., 2014). Interestingly, point mutations in the RyR that increase the sensitivity of these ion channels to PCB 95 in animal models (Kim et al., 2013; Ta & Pessah, 2007) have been identified in autism candidate gene studies (Lu & Cantor, 2012). Collectively, these observations suggest that RyRs represent a convergent target for G × PCB interactions that confer autism risk.
While PCB 95 and PCB 136 were found to enhance dendritic arborization via RyR-dependent mechanisms that converged on CREB-mediated transcription (Wayman, Bose, et al., 2012), a lower chlorinated contemporary PCB of concern, PCB 11, also has been found to promote dendritic growth in cultured hippocampal and cortical neurons via CREB-dependent mechanisms (Sethi et al., 2018) despite having negligible RyR activity (Sethi et al., 2019). The upstream calcium signaling molecules that link PCB 11 to CREB activation are as yet unknown. CREB dysfunction has been observed in autism (Barnby et al., 2005; Ngounou Wetie et al., 2015; Russo, Albert, & Bowman, 2017; Todd & Mack, 2001; Zheng et al., 2016), suggesting that heritable deficits in CREB signaling may be a more universal convergent target than RyRs in G × PCB interactions that increase autism risk.
PCB effects on dendritic growth have been demonstrated to coincide with autism-relevant behavioral phenotypes. Gestational and lactational exposure to Aroclor 1254 at 1 or 6mg/kg/d (Yang et al., 2009) or to PCB 95 at 0.1, 1, but not 6, mg/kg/d (Wayman, Yang, et al., 2012) in the diet of rat dams increased dendritic complexity of pyramidal neurons in the hippocampus, cortex and cerebellum of offspring not subjected to training in the Morris Water maze. Experience-dependent dendritic growth associated with Morris water maze training was significantly diminished or even blocked in animals developmentally exposed to PCBs (Yang et al., 2009). PCB restriction of experience-dependent dendritic growth was associated with deficits in spatial learning and memory (Yang et al., 2009). Developmental exposure to PCB 95 also interfered with the synaptic plasticity and organization of the auditory cortex in rats, causing significant E/I imbalance (Kenet, Froemke, Schreiner, Pessah, & Merzenich, 2007). Establishing that RyR-dependent effects of PCBs on dendritic growth and synaptic plasticity are causally related to PCB effects on behavior is difficult since pharmacological or genetic deletion of RyR is embryolethal and in postnatal animals, cardiotoxic. However, a recent study using a larval zebrafish model to compare behavioral phenotypes following developmental exposures to PCBs with varying RyR activity ranging from little to very potent activity demonstrated that the structure-activity relationship (SAR) for RyR activity in zebrafish tissues predicted the SAR for PCB effects on behavior (Yaghoobi et al., 2022). This finding, coupled with observations that PCB causes comparable dose-response relationships for dendritic arborization and cognitive behavior in rodent models (Yang et al., 2009), provide strong support for a causal relationship between RyR-dependent effects of PCBs on dendritic growth and PCB-induced deficits in learning and memory.
Connectivity is influenced by not only the structural complexity of dendrites, but also by spine density (Chaudry & Vasudevan, 2022; Nanou & Catterall, 2018). In cultured primary rat hippocampal neurons and brain slices, PCB 95 significantly increased dendritic spine formation via RyR signaling and CREB-dependent upregulation of miR132 (Lesiak et al., 2014). The necessity of miR132 in this pathway is of particular interest since miR132 is also upregulated in the brain in response to valproic acid—a widely used model to induce autism phenotypes in mice (Hara et al., 2017). miR132 may be a convergent target for diverse environmental chemicals that confer autism risk. Interestingly, miR132 represses expression of MeCP2 (Klein et al., 2007), and dysfunction of MeCP2 is implicated in significant cognitive impairment, while, as mentioned earlier, both mutations and duplications of the MECP2 gene are associated with Rett syndrome and autism (Cukier et al., 2012; LaSalle & Yasui, 2009; Percy, 2011). MiR132 has also been shown to interact with FMRP to regulate synapse formation (Edbauer et al., 2010).
Genetic mouse models engineered to express heritable human mutations that alter the fidelity of calcium signaling have been leveraged to test the hypothesis that genetic mutations converging on the same signaling pathways as PCBs increase susceptibility to the neurotoxic effects of PCBs. Mice that expressed a human gain-of-function mutation in the RyR, a CGG repeat expansion in the premutation range in the Fmr1 gene, or both genetic mutations were exposed throughout gestation and lactation to varying doses of the MARBLES PCB mixture in the maternal diet. Morphometric and behavioral analyses of these mice revealed that dendritic morphogenesis and behavior were modulated by complex G × E interactions (Keil Stietz et al., 2021; Sethi et al., 2021). For example, in mouse cortical and hippocampal neurons, genotype influenced the dendritic response to PCBs in a dose, sex and brain region dependent manner. Expression of the RyR mutation or both the RyR and CGG expansion mutations increased dendritic complexity at lower doses of PCBs compared to the dose required to elicit the same dendritic response in wildtype neurons (Keil Stietz et al., 2021). Behavioral studies indicated that developmental exposure of wildtype males to the MARBLES PCB mixture phenocopied social behavior phenotypes observed in mice expressing heritable human mutations in calcium signaling, while expression of these mutations alleviated PCB effects on ultrasonic vocalizations and repetitive behavior, and modified the dose-response relationships and sex-dependent effects of PCBs on social behavior (Sethi et al., 2021). Additional studies using these same mouse models and PCB exposures indicated that G × E interactions influence the microbiome and intestinal physiology (Rude, Pusceddu, et al., 2019), as well as serum cytokine and chemokine levels (Matelski et al., 2020). Collectively, these observations support the hypothesis that heritable deficits in signaling pathways also impacted by environmental risk factors modify the neurotoxic response, which in turn influence autism risk.
4.7. PBDEs as autism risk factors
Polybrominated diphenyl ethers (PBDEs) were produced for use as flame retardants in diverse consumer products, including furniture and children’s clothing. These persistent organic pollutants (POPs) are of growing concern as putative autism risk factors because of widespread human exposure and their structural and toxicological similarity to NDL PCBs (Dingemans, Kock, & van den Berg, 2016; Pessah et al., 2019). Developmental exposure to BDE-47, one of the more prevalent of the 209 possible PBDE congeners detected in human tissues, impaired the performance of MeCP2 mutant mice in the Morris Water Maze task (Woods et al., 2012). In vitro studies revealed that PBDEs altered calcium signaling in primary rodent neurons via RyR-dependent mechanisms (Kim et al., 2011), modulated glutamate receptor signaling, disrupted TH signaling and increased production of reactive oxygen species via effects on mitochondrial function (Costa, Pellacani, Dao, Kavanagh, & Roque, 2015; Costa, Tagliaferri, Roque, & Pellacani, 2016; Dingemans et al., 2016). PBDEs have also been shown to alter hepatic biotransformation and CYP expression, an effect that varied depending on the enterotype (conventional vs. germ-free) of the exposed animal (Li et al., 2017).
Curiously, in contrast to NDL PCBs, neither BDE 47 nor BDE 49, two congeners detected in the human gestational environment, promoted dendritic growth in primary rat hippocampal neurons (Chen, Lin, Dang, & Puschner, 2017). Rather both congeners delayed neuronal polarization resulting in significant inhibition of axonal outgrowth during the first few days in vitro. The axon inhibitory effects of these PBDE congeners occurred independent of cytotoxicity, and were blocked by pharmacological antagonism of RyR or siRNA knockdown of RyR2. These results demonstrate that the molecular and cellular mechanisms by which PBDEs interfere with neurodevelopment overlap are distinct from those of NDL PCBs. In addition, altered patterns of neuronal connectivity may contribute to the developmental neurotoxicity of PBDEs and increase autism risk in individuals with heritable autism risk factors that converge on calcium signaling and/or pathways that regulate neuronal connectivity (Table 2).
Epidemiological observations suggest that PBDE exposures are associated with reduced IQ and behavioral abnormalities (Pessah et al., 2019; Zhang et al., 2017). Associations between developmental PBDE exposures and abnormal executive function and attention control in young children, as well as impairments in externalizing behaviors (Vuong et al., 2018), have been reported. However, the literature regarding PBDE links to autism is conflicting. In one study of pregnant women, PBDE 28 levels were positively correlated with SRS (autistic behavior) scores in children aged 4–5, while levels of PBDE 85 were linked to fewer autistic behaviors (Braun et al., 2014). In another study of 100 children, PBDE levels were similar in children with autism vs. typically developing controls; however; there were no data regarding levels of PBDEs during prenatal development (Hertz-Picciotto et al., 2011). In a study of mother and child pairs in Northern California, maternal serum levels of PBDEs were lower in mothers with a child with autism; yet, sex may play a role in these associations as these effects were prominent in boys for whom there was an indication of increased autism risk, especially with PBDE 28 and PBDE 47, but not in girls (Lyall, Croen, Weiss, et al., 2017).
5. Evolving areas of research
5.1. Emerging chemicals of concern
Increased awareness of the potential influence of environmental exposures on autism risk and/or severity has driven interest in determining which of the 80,000+ chemicals in the human chemosphere pose the greatest autism risk. This in turn has stimulated the development of experimental models for testing the impacts of environmental chemicals on phenotypes of relevance to autism. Data from these studies combined with exposure monitoring data have identified several chemical classes of emerging concern, notably per- and polyfluoroalkyl substances (PFAS), including perfluorooctane sulfonate (PFOS).
In preclinical rodent models, lactational exposure to PFOS decreased learning and memory function and elevated glutamate and GABA levels in the hippocampus of adult mice (Mshaty et al., 2020). Developmental PFOS exposure also caused deficits in motor function, changed short-term and long-term plasticity in Purkinje cells, and altered levels of proteins involved in neurotransmission, including glutamate receptors (Ninomiya et al., 2022). Postnatal exposure to perfluorohexane sulfonate (PFHxS), perfluorooctanoic acid (PFOA) and PFOS altered adult behavior and cognitive function in mice, in part by modulating cholinergic signaling in the brain (Johansson, Fredriksson, & Eriksson, 2008; Viberg, Lee, & Eriksson, 2013). While PFOS are less well studied than PCBs and other legacy pollutants, there is increasing evidence that PFOS are worth investigating as putative autism risk factors.
Epidemiological data suggest that certain types of PFAS, such as PFOA, increase autism risk in a non-monotonic (U-shaped) dose-related manner, while mixtures of PFAS show an inverse dose relationship with autism (Skogheim et al., 2021). PFAS were found in serum of women in California with a child age 2–5, and longer breastfeeding was linked to lower serum levels in the mothers, indicating that lactation may be a source of PFAS in children (Kim, Bennett, Calafat, Hertz-Picciotto, & Shin, 2020). In a study of mother-child pairs in the CHARGE study (CHildhood Autism Risk from Genetics and Environment), PFHxS and PFOS were higher in the serum of women who had a child with autism vs. typically developing controls (Shin, Bennett, Calafat, Tancredi, & Hertz-Picciotto, 2020). In 173 mother-child pairs from the MARBLES study, prenatal PFOA and perfluorononanoate (PFNA) levels were positively associated with autism risk, while PFHxS showed a negative association with autism risk (Oh, Bennett, Calafat, et al., 2021). In other studies, serum PFAS levels in children aged 5 and 7 were associated with behavioral problems at age 7 (Oulhote, Steuerwald, Debes, Weihe, & Grandjean, 2016). In 140 mother-child pairs from the MARBLES study, prenatal PFOA was inversely associated with scores for the Early Learning Composite, which is a measure of motor, visual and language function in children aged 2–3 (Oh, Schmidt, Tancredi, et al., 2021). However, two other studies that sampled maternal sera during early to mid-pregnancy found no or inverse associations between PFAS and autism diagnosis (Liew et al., 2015; Lyall et al., 2018).
The different outcomes observed between these studies likely result from differences in the study populations, including, location, year and stage of pregnancy at time of collection; differences in methodologies for measuring exposures; as well as well differences in clinical criteria used to diagnose autism. Despite the inconsistent findings, the available data suggest that PFAS warrant further investigation to better understand how much of a risk they pose to genetically susceptible individuals and to elucidate their mechanism(s) of developmental neurotoxicity to identify which genetic substrates are most susceptible.
5.2. Influence of environmental risk factors on autism co-morbidities
Children diagnosed with autism often suffer from several comorbid conditions, including GI complications as well as bladder dysfunction (von Gontard, Baeyens, Van Hoecke, Warzak, & Bachmann, 2011). The role of G × E interactions in inducing these comorbid conditions is currently understudied and discussed in a recent review (Panesar et al., 2020). As mentioned earlier, experimental evidence suggests that PCB exposures alter the gut microbiome (Li et al., 2017; Lim et al., 2020), which would be expected to alter GI function. PCBs may also contribute to GI dysfunction via mechanisms independent of effects on the gut microbiome. For example, developmental exposure to the MARBLES PCB mixture altered intestinal permeability and expression of inflammatory mediators in wild type mice, and these effects were exacerbated in mice with heritable mutations in calcium signaling (Li et al., 2017; Rude, Keogh, & Gareau, 2019). Interestingly, developmental exposure to the MARBLES PCB mixture influenced the ability of adult mice to urinate (Kennedy et al., 2022). Mechanistic studies of these observations are ongoing but suggest two testable possibilities: (i) PCBs directly affect peripheral target organs such as the intestine and bladder; and (ii) PCBs alter intestinal and bladder function indirectly via effects on the autonomic nervous system that innervates these organs. The latter hypothesis is derived from data linking disturbances in the autonomic nervous system to GI, bladder, and immune dysfunction (Matteoli & Boeckxstaens, 2013; Muller et al., 2020; Roy & Green, 2019). The peripheral nervous system may be a convergence point through which environmental factors such as PCBs influence the manifestation of autism comorbidities. How the genetic substrate ties into these phenotypes is currently not understood, but the mechanisms detailed above for the central nervous system likely play a role in the peripheral nervous system.
6. Concluding remarks
A significant contribution from environmental factors in determining autism risk and severity is consistent with both the rapid increase in autism rates and the clinical heterogeneity that is the hallmark of this neurodevelopmental condition. However, this phenotypic heterogeneity combined with the complex genetic substrate of autism significantly increases the challenge of identifying specific environmental factors that confer increased risk for autism as well as the genetic susceptibilities that are particularly vulnerable to these environmental factors. To close this significant data gap, we endorse the suggestion that research efforts focus on leveraging experimental models to identify mechanisms by which environmental factors interact with genetic factors to increase the risk of adverse neurodevelopmental outcomes of relevance to autism. Such mechanistic insights can then be used to develop rapid throughput screens for potential environmental risk factors, which in turn will inform and focus epidemiological studies.
The success of this strategy is predicated on choosing appropriate model systems. While a number of transgenic animals (mice, and more recently, rats) expressing autism-linked genetic mutations have been or are being developed, the costs associated with using these models for developmental neurotoxicity exposure studies limits their utility for early mechanistic and screening approaches (Lein et al., 2005). Such models do, however, serve an important role in confirming putative G × E interactions identified in simpler systems. But for initial mechanistic and screening studies, we propose the use of in vitro models (such as primary neuronal cell cultures or human iPSC-derived neural systems) or simple systems-based models (such as C. elegans or D. rerio) that recapitulate the neurodevelopmental processes disrupted in autism. Ongoing research demonstrates that such models can be adapted for rapid throughput screening of multiple culture wells simultaneously using, for example, high content imaging systems to analyze dendritic morphology and synaptic density (Harrill, Robinette, & Mundy, 2011), microelectrode arrays to measure network activity as a measure of functional connectivity (Johnstone et al., 2010; Robinette, Harrill, Mundy, & Shafer, 2011), the FLIPR® to assess spontaneous calcium oscillations (Cao, Hammock, et al., 2012) and “omic” technologies to identify changes in molecular and biochemical targets (Modafferi et al., 2021). In silico approaches that leverage databases, such as the Comparative Toxicogenomics Database, to interrogate chemical influences on the hundreds of autism susceptibility genes identified to date (Carter & Blizard, 2016) would also be an effective high-throughput approach for identifying potential autism-relevant G × E interactions.
Another consideration in studies of G × E interactions in autism is cross-talk between signaling systems and G × E mechanisms that influence neuronal connectivity, as well as between chemicals in mixtures (Fig. 1). For example, endocrine signaling regulates gene transcription of proinflammatory cytokines, and soluble mediators of the innate immune system, such as cytokines, can feed back on the brain to regulate endocrine signaling (Irwin & Cole, 2011). The immune system is also strongly influenced by the gut microbiome, and emerging evidence suggests that the microbiome influences not only peripheral immune calls, but also microglia in the brain (Lebovitz et al., 2018). There is also evidence that microbes within the human gut may play a significant role in the regulation of the brain via their influence on the production of inflammatory cytokines, antimicrobial peptides and neurotransmitters that affect the epigenome (Alam et al., 2017; Yousefi et al., 2022).
Fig. 1.
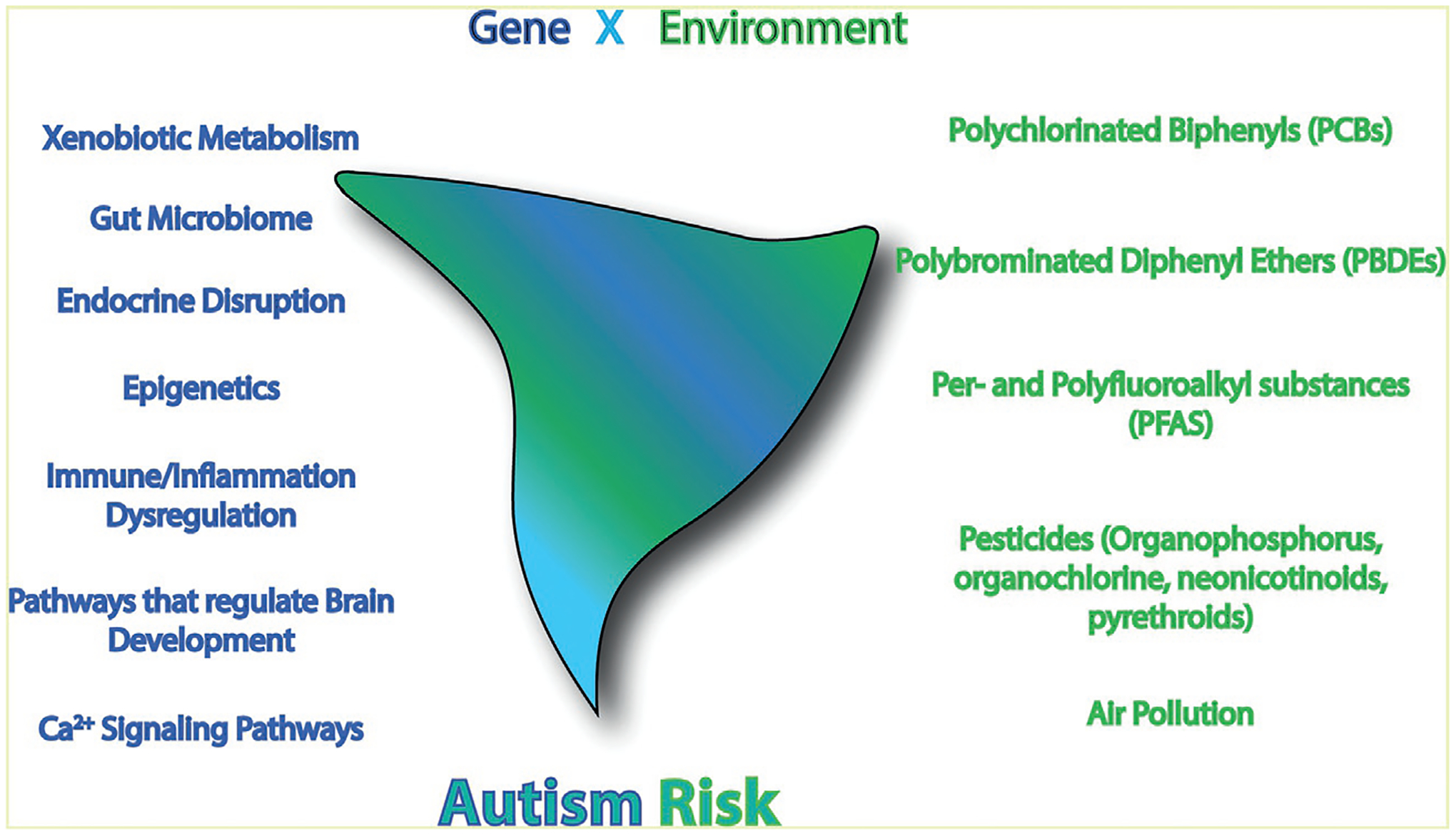
Autism risk is likely determined by cross talk between multiple signaling pathways, mechanisms of G × E interactions and interactions between structurally and mechanistically diverse chemicals in mixtures.
Clearly, work is urgently needed to better predict which combination of autism genes and environmental exposures pose the greatest autism risk. The fact that chemical exposures are more readily controlled than genetic factors to prevent or mitigate the expression of autism-related traits coupled with the significant toll that autism places on autistic children, their families and society, provides a compelling reason to engage in this endeavor.
Acknowledgments
This work was supported by funding from the National Institute of Environmental Health Sciences (grants R01 ES014901 to PJL, R00 ES029537 to KPKS, and P30 ES023513 to UC Davis) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant P50 HD103526 to the UC Davis MIND Institute).
References
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, et al. (2008). Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics, 9, 153–161. [DOI] [PubMed] [Google Scholar]
- Ahmed RG, El-Gareib AW, & Shaker HM (2018). Gestational 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction. Life Sciences, 192, 213–220. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, & Wenderoth N (2016). Sex differences in autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Social Cognitive and Affective Neuroscience, 11, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam R, Abdolmaleky HM, & Zhou JR (2017). Microbiome, inflammation, epigenetic alterations, and mental diseases. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 174, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamdari SB, Sadeghi Damavandi M, Zarei M, & Khosrowabadi R (2022). Cognitive theories of autism based on the interactions between brain functional networks. Frontiers in Human Neuroscience, 16, 828985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alampi JD, Lanphear BP, Braun JM, Chen A, Takaro TK, Muckle G, et al. (2021). Association between gestational exposure to toxicants and autistic behaviors using Bayesian quantile regression. American Journal of Epidemiology, 190, 1803–1813. [DOI] [PubMed] [Google Scholar]
- Amen NE, Eqani S, Bilal K, Ali N, Rajeh N, Adelman D, et al. (2022). Molecularly tracing of children exposure pathways to environmental organic pollutants and the Autism Spectrum Disorder Risk. Environmental Pollution, 315, 120381. [DOI] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, & Thorne PS (2015). Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environmental Science & Technology, 49, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel A, Rosenzweig JP, Zisman PD, Melamed M, & Gesundheit B (2016). Variation in gene expression in autism spectrum disorders: an extensive review of transcriptomic studies. Frontiers in Neuroscience, 10, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambula SE, & McCarthy MM (2020). Neuroendocrine-immune crosstalk shapes sex-specific brain development. Endocrinology, 161(6), bqaa055. 10.1210/endocr/bqaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, & Rodier PM (2005). The teratology of autism. International Journal of Developmental Neuroscience, 23, 189–199. [DOI] [PubMed] [Google Scholar]
- Baba T, Ito S, Yuasa M, Yoshioka E, Miyashita C, Araki A, et al. (2018). Association of prenatal exposure to PCDD/Fs and PCBs with maternal and infant thyroid hormones: The Hokkaido Study on Environment and Children’s Health. Sci Total Environ, 615, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Bach MA, Samms-Vaughan M, Hessabi M, Bressler J, Lee M, Zhang J, et al. (2020). Association of polychlorinated biphenyls and organochlorine pesticides with autism spectrum disorder in Jamaican children. Research in Autism Spectrum Disorder, 76, 101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer-Trias J, Deepika D, Schuhmacher M, & Kumar V (2022). Impact of contaminants on microbiota: linking the gut-brain axis with neurotoxicity. International Journal of Environmental Research and Public Health, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, & Anagnostou E (2013). A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: A review of the literature. Frontiers in Psychiatry, 4, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmpas M, Vakonaki E, Tzatzarakis M, Sifakis S, Alegakis A, Grigoriadis T, et al. (2019). Organochlorine pollutants’ levels in hair, amniotic fluid and serum samples of pregnant women in Greece. A cohort study. Environmental Toxicology and Pharmacology, 73, 103279. [DOI] [PubMed] [Google Scholar]
- Barnby G, Abbott A, Sykes N, Morris A, Weeks DE, Mott R, et al. (2005). Candidate-gene screening and association analysis at the autism-susceptibility locus on chromosome 16p: Evidence of association at GRIN2A and ABAT. American Journal of Human Genetics, 76, 950–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos GC, Feng W, Truong K, Matthaei KI, Yang T, Allen PD, et al. (2012). Gene dose influences cellular and calcium channel dysregulation in heterozygous and homozygous T4826I-RYR1 malignant hyperthermia-susceptible muscle. The Journal of Biological Chemistry, 287, 2863–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Dryden A, Will R, & Gore AC (2018). Sex differences in effects of gestational polychlorinated biphenyl exposure on hypothalamic neuroimmune and neuromodulator systems in neonatal rats. Toxicology and Applied Pharmacology, 353, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Hart BG, & Gore AC (2016). Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Molecular and Cellular Endocrinology, 420, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, & Bourgeron T (2006). Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience, 9, 1221–1225. [DOI] [PubMed] [Google Scholar]
- Berghuis SA, Soechitram SD, Hitzert MM, Sauer PJ, & Bos AF (2013). Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology, 38, 124–130. [DOI] [PubMed] [Google Scholar]
- Berman RF, Murray KD, Arque G, Hunsaker MR, & Wenzel HJ (2012). Abnormal dendrite and spine morphology in primary visual cortex in the CGG knock-in mouse model of the fragile X premutation. Epilepsia, 53(Suppl. 1), 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BA, Lanphear BP, Venners SA, Arbuckle TE, Braun JM, Muckle G, et al. (2019). Assessing the relation between plasma PCB concentrations and elevated autistic behaviours using bayesian predictive odds ratios. International Journal of Environmental Research and Public Health, 16(3), 457. 10.3390/ijerph16030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti ACC, Peres KK, Faccioli LS, Vacci MC, Mata IRD, Kuyven CJ, et al. (2022). Early exposure to agricultural pesticides and the occurrence of autism spectrum disorder: A systematic review. Rev Paul Pediatr, 41, e2021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotto LB, Catron TR, & Tal T (2020). Exploring interactions between xenobiotics, microbiota, and neurotoxicity in zebrafish. Neurotoxicology, 76, 235–244. [DOI] [PubMed] [Google Scholar]
- Bilrha H, Roy R, Moreau B, Belles-Isles M, Dewailly E, & Ayotte P (2003). In vitro activation of cord blood mononuclear cells and cytokine production in a remote coastal population exposed to organochlorines and methyl mercury. Environmental Health Perspectives, 111, 1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Whitehouse AJ, Watt HJ, & Line EA (2008). Autism and diagnostic substitution: evidence from a study of adults with a history of developmental language disorder. Developmental Medicine and Child Neurology, 50, 341–345. [DOI] [PubMed] [Google Scholar]
- Bjorklund G, Dosa MD, Maes M, Dadar M, Frye RE, Peana M, et al. (2021). The impact of glutathione metabolism in autism spectrum disorder. Pharmacological Research, 166, 105437. [DOI] [PubMed] [Google Scholar]
- Blagburn-Blanco SV, Chappell MS, De Biase LM, & DeNardo LA (2022). Synapse-specific roles for microglia in development: New horizons in the prefrontal cortex. Frontiers in Molecular Neuroscience, 15, 965756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi R, Egervari K, Carol-Perdiguer L, Viale B, Quairiaux C, De Roo M, et al. (2017). Perturbed Wnt signaling leads to neuronal migration delay, altered inter-hemispheric connections and impaired social behavior. Nature Communications, 8, 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Girdler S, & Marschik PB (2019). The contribution of environmental exposure to the etiology of autism spectrum disorder. Cellular and Molecular Life Sciences, 76, 1275–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T (2009). A synaptic trek to autism. Current Opinion in Neurobiology, 19, 231–234. [DOI] [PubMed] [Google Scholar]
- Bowen KK, Dempsey RJ, & Vemuganti R (2011). Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport, 22, 126–130. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. (2014). Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environmental Health Perspectives, 122, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, & Hof PR (2011). The emerging neuroscience of autism spectrum disorders. Brain Research, 1380, 1–2. [DOI] [PubMed] [Google Scholar]
- Cao Z, Hammock BD, McCoy M, Rogawski M, Lein P, & Pessah I (2012). Tetramethylenedisulfotetramine alters Ca2+ dynamics in cultured hippocampal neurons: Mitigation by NMDA blockade and GABAA receptor positive modulation. Toxicological Sciences, 130(2), 362–372. 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Hulsizer S, Cui Y, Pretto DL, Kim KH, Hagerman PJ, et al. (2013). Enhanced asynchronous Ca(2+) oscillations associated with impaired glutamate transport in cortical astrocytes expressing Fmr1 gene premutation expansion. The Journal of Biological Chemistry, 288, 13831–13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, et al. (2012). Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Human Molecular Genetics, 21, 2923–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Winneke G, Wilhelm M, Wittsiepe J, Lemm F, Furst P, et al. (2008). Environmental exposure to dioxins and polychlorinated biphenyls reduce levels of gonadal hormones in newborns: results from the Duisburg cohort study. International Journal of Hygiene and Environmental Health, 211, 30–39. [DOI] [PubMed] [Google Scholar]
- Careaga M, Rose D, Tassone F, Berman RF, Hagerman R, & Ashwood P (2014). Immune dysregulation as a cause of autoinflammation in fragile X premutation carriers: Link between FMR1 CGG repeat number and decreased cytokine responses. PLoS One, 9, 1–8. 10.1371/journal.pone.0094475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson T, Molander F, Taylor MJ, Jonsson U, & Bolte S (2020). Early environmental risk factors for neurodevelopmental disorders—A systematic review of twin and sibling studies. Development and Psychopathology, 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO (2006). Polychlorinated biphenyls (PCBs): Routes of exposure and effects on human health. Reviews on Environmental Health, 21, 1–23. [DOI] [PubMed] [Google Scholar]
- Carter CJ, & Blizard RA (2016). Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochemistry International. [DOI] [PubMed] [Google Scholar]
- Chan-Hon-Tong A, Charles MA, Forhan A, Heude B, & Sirot V (2013). Exposure to food contaminants during pregnancy. Sci Total Environ, 458–460, 27–35. [DOI] [PubMed] [Google Scholar]
- Chaudry S, & Vasudevan N (2022). mTOR-dependent spine dynamics in autism. Frontiers in Molecular Neuroscience, 15, 877609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen K, Lavery LA, Baker SA, Shaw CA, Li W, et al. (2015). MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America, 112, 5509–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin Y, Dang K, & Puschner B (2017). Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from California by gas chromatography-triple quadruple mass spectrometry. PLoS One, 12, e0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Streifel KM, Singh V, Yang D, Mangini L, Wulff H, et al. (2017). From the cover: BDE-47 and BDE-49 inhibit axonal growth in primary rat hippocampal neuron-glia co-cultures via ryanodine receptor-dependent mechanisms. Toxicological Sciences, 156, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, et al. (2010). Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Human Molecular Genetics, 19, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroni C, Caporale N, & Testa G (2020). Autism spectrum disorder at the crossroad between genes and environment: contributions, convergences, and interactions in ASD developmental pathophysiology. Molecular Autism, 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Lin Y, Zhu H, Huang Q, Ye G, & Dong S (2018). PCBs-high-fat diet interactions as mediators of gut microbiota dysbiosis and abdominal fat accumulation in female mice. Environmental Pollution, 239, 332–341. [DOI] [PubMed] [Google Scholar]
- Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, et al. (2012). Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Human Genetics, 131, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries, 65, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, et al. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA, 309, 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb LS, Andersen ME, Broccardo CJ, Legare ME, Billings RE, Dean CE, et al. (2004). Regional induction of CYP1A1 in rat liver following treatment with mixtures of PCB 126 and PCB 153. Toxicologic Pathology, 32, 467–473. [DOI] [PubMed] [Google Scholar]
- Collaer ML, & Hines M (1995). Human behavioral sex differences: a role for gonadal hormones during early development? Psychological Bulletin, 118, 55–107. [DOI] [PubMed] [Google Scholar]
- Connolly S, & Kingsbury TJ (2010). Caffeine modulates CREB-dependent gene expression in developing cortical neurons. Biochemical and Biophysical Research Communications, 397, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lloyd JE, Kasmara L, Holden JJ, & Lewis ME (2008). Trends in autism prevalence: diagnostic substitution revisited. Journal of Autism and Developmental Disorders, 38, 1036–1046. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Richter FR, Bays PM, Plaisted-Grant KC, Baron-Cohen S, & Simons JS (2017). Reduced hippocampal functional connectivity during episodic memory retrieval in autism. Cerebral Cortex, 27, 888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Merrill A, & Sobolewski M (2022). Air pollution-related neurotoxicity across the life span. Annual Review of Pharmacology and Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun MA, Loveland KA, Pearson DA, Papanicolaou AC, & Sheth BR (2013). Functional assays of local connectivity in the somatosensory cortex of individuals with autism. Autism Research, 6, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Pellacani C, Dao K, Kavanagh TJ, & Roque PJ (2015). The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology, 48, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Tagliaferri S, Roque PJ, & Pellacani C (2016). Role of glutamate receptors in tetrabrominated diphenyl ether (BDE-47) neurotoxicity in mouse cerebellar granule neurons. Toxicology Letters, 241, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, & Kang Y (2007). Neurexin-neuroligin signaling in synapse development. Current Opinion in Neurobiology, 17, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider A, Thakkar R, Ahmed AO, & Pillai A (2014). Dysregulation of estrogen receptor beta (ERbeta), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Molecular Autism, 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, & Zimmerman AW (2011). Prenatal exposure to beta2-adrenergic receptor agonists and risk of autism spectrum disorders. Journal of Neurodevelopmental Disorders, 3, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier HN, Lee JM, Ma D, Young JI, Mayo V, Butler BL, et al. (2012). The expanding role of MBD genes in autism: identification of a MECP2 duplication and novel alterations in MBD5, MBD6, and SETDB1. Autism Research, 5, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Dewailly E, Muckle G, Vezina C, Jacobson SW, Jacobson JL, et al. (2004). Acute infections and environmental exposure to organochlorines in Inuit infants from Nunavik. Environmental Health Perspectives, 112, 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deidda G, Bozarth IF, & Cancedda L (2014). Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives. Frontiers in Cellular Neuroscience, 8, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme R, Ey E, Toro R, Leboyer M, Gillberg C, & Bourgeron T (2013). Progress toward treatments for synaptic defects in autism. Nature Medicine, 19, 685–694. [DOI] [PubMed] [Google Scholar]
- Demeneix BA (2019). Evidence for prenatal exposure to thyroid disruptors and adverse effects on brain development. Eur Thyroid J, 8, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, et al. (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron, 77, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, Rudie JD, Brown JA, Johnson K, McMahon KL, et al. (2011). Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Connectivity, 1, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaulniers D, Xiao GH, Lian H, Feng YL, Zhu J, Nakai J, et al. (2009). Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. International Journal of Toxicology, 28, 294–307. [DOI] [PubMed] [Google Scholar]
- Deverman BE, & Patterson PH (2009). Cytokines and CNS development. Neuron, 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, & Gore AC (2011). Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology, 152, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep AA, Hunsaker MR, Kwock R, Kim K, Willemsen R, & Berman RF (2012). Female CGG knock-in mice modeling the fragile X premutation are impaired on a skilled forelimb reaching task. Neurobiology of Learning and Memory, 97, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR (2014). Developmental Immunotoxicity, Perinatal Programming, and Noncommunicable Diseases: Focus on Human Studies. Adv Med, 2014, 867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, Kock M, & van den Berg M (2016). Mechanisms of action point towards combined PBDE/NDL-PCB risk assessment. Toxicological Sciences, 153, 215–224. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Rykken S, Lehmler HJ, Robertson LW, & Fonnum F (2009). Hydroxylated polychlorinated biphenyls increase reactive oxygen species formation and induce cell death in cultured cerebellar granule cells. Toxicology and Applied Pharmacology, 240, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, et al. (2005). Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nature Genetics, 37, 733–738. [DOI] [PubMed] [Google Scholar]
- Durand B, Dufour B, Fraisse D, Defour S, Duhem K, & Le-Barillec K (2008). Levels of PCDDs, PCDFs and dioxin-like PCBs in raw cow’s milk collected in France in 2006. Chemosphere, 70, 689–693. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Xie Z, & Levitt P (2017). The pleiotropic MET receptor network: circuit development and the neural-medical interface of autism. Biological Psychiatry, 81, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, & Greenberg ME (2013). Activity-dependent neuronal signalling and autism spectrum disorder. Nature, 493, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. (2010). Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron, 65, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi A, Nishizawa-Jotaki S, Tanabe H, Rahmutulla B, Watanabe M, Miyaso H, et al. (2019). An altered DNA methylation status in the human umbilical cord is correlated with maternal exposure to polychlorinated biphenyls. International Journal of Environmental Research and Public Health, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner A, Gao Y, Collier F, Drummond K, Thomson S, Burgner D, et al. (2022). Cord blood immune profile: Associations with higher prenatal plastic chemical levels. Environmental Pollution, 120332. [DOI] [PubMed] [Google Scholar]
- El-Fishawy P, & State MW (2010). The genetics of autism: Key issues, recent findings, and clinical implications. The Psychiatric Clinics of North America, 33, 83–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, et al. (2017). In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: The CHAMACOS study. International Journal of Hygiene and Environmental Health, 220, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. (2010). Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature Neuroscience, 13, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster C, Zuniga-Venegas L, Enriquez P, Rojas J, Zamora C, Munoz X, et al. (2021). Levels of polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in human breast milk in chile: A pilot study. International Journal of Environmental Research and Public Health, 18(9), 4825. 10.3390/ijerph18094825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, et al. (2014). A twin study of heritable and shared environmental contributions to autism. Journal of Autism and Developmental Disorders, 44, 2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Guo X, Cheung FMH, & Yung KKL (2019). The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci Total Environ, 655, 1240–1248. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Lein P, Higgins D, & Patterson PH (1998). Effect of leukemia inhibitory factor (LIF) on the morphology and survival of cultured hippocampal neurons and glial cells. Brain Research, 798, 140–146. [DOI] [PubMed] [Google Scholar]
- Gama J, Neves B, & Pereira A (2022). Chronic effects of dietary pesticides on the gut microbiome and neurodevelopment. Frontiers in Microbiology, 13, 931440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay PA, & McAllister AK (2010). Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci, 2, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH (2011). Genetics of autism spectrum disorders. Trends in Cognitive Sciences, 15, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, & Levitt P (2007). Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology, 17, 103–111. [DOI] [PubMed] [Google Scholar]
- Glynn A, Thuvander A, Aune M, Johannisson A, Darnerud PO, Ronquist G, et al. (2008). Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: A prospective study. Environmental Health, 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, & Ashwood P (2013). Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicology and Teratology, 36, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, & Van de Water J (2010). The immune system’s role in the biology of autism. Current Opinion in Neurology, 23, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldey ES, & Crofton KM (1998). Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicological Sciences, 45, 94–105. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Rej R, Negoita S, Schymura M, Santiago-Rivera A, Morse G, et al. (2009). Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environmental Health Perspectives, 117, 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granillo L, Sethi S, Keil KP, Lin Y, Ozonoff S, Iosif AM, et al. (2019). Polychlorinated biphenyls influence on autism spectrum disorder risk in the MARBLES cohort. Environmental Research, 171, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether JK, Rosen NJ, Smith KS, & Croen LA (2009). Investigation of shifts in autism reporting in the California Department of Developmental Services. Journal of Autism and Developmental Disorders, 39, 1412–1419. [DOI] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, et al. (2015). Metabolism and metabolites of polychlorinated biphenyls. Critical Reviews in Toxicology, 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S, Pang N, Deng X, Yang L, He F, Wu L, et al. (2018). Synaptopathology involved in autism spectrum disorder. Frontiers in Cellular Neuroscience, 12, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes JR, Ferreira PA, Costa JM, Cardoso AL, & Peca J (2022). Microglia-dependent remodeling of neuronal circuits. Journal of Neurochemistry, 163, 74–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chandrasekaran V, Lein P, Kaplan PL, & Higgins D (1999). Leukemia inhibitory factor and ciliary neurotrophic factor cause dendritic retraction in cultured rat sympathetic neurons. The Journal of Neuroscience, 19, 2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Metzler-Northrup J, Lein P, Rueger D, & Higgins D (1997). Leukemia inhibitory factor and ciliary neurotrophic factor regulate dendritic growth in cultures of rat sympathetic neurons. Brain Research. Developmental Brain Research, 104, 101–110. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Au J, & Hagerman P (2011). FMR1 premutation and full mutation molecular mechanisms related to autism. Journal of Neurodevelopmental Disorders, 3, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah TJ, Megson D, & Sandau CD (2022). A review of the mechanisms of by-product PCB formation in pigments, dyes and paints. Sci Total Environ, 852, 158529. [DOI] [PubMed] [Google Scholar]
- Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, & Obrietan K (2013). miRNA-132: A dynamic regulator of cognitive capacity. Brain Structure & Function, 218, 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Aten S, Price KH, Loeser J, Hesse AM, et al. (2016). Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learning & Memory, 23, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, et al. (2017). Prenatal exposure to valproic acid increases miR-132 levels in the mouse embryonic brain. Molecular Autism, 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL, & Mundy WR (2011). Use of high content image analysis to detect chemical-induced changes in synaptogenesis in vitro. Toxicology In Vitro: an International Journal Published in Association with BIBRA, 25, 368–387. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jorgensen E, Nielsen F, Heinzow B, Weihe P, & Grandjean P (2010). Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environmental Health Perspectives, 118, 1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson S, Jonsson L, Ljunggren E, Westberg L, Gillberg C, Rastam M, et al. (2009). Possible association between the androgen receptor gene and autism spectrum disorder. Psychoneuroendocrinology, 34, 752–761. [DOI] [PubMed] [Google Scholar]
- Herbert MR (2010). Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Current Opinion in Neurology, 23, 103–110. [DOI] [PubMed] [Google Scholar]
- Herkert NJ, Jahnke JC, & Hornbuckle KC (2018). Emissions of tetrachlorobiphenyls (PCBs 47, 51, and 68) from polymer resin on kitchen cabinets as a non-aroclor source to residential air. Environmental Science & Technology, 52, 5154–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Bergman A, Fangstrom B, Rose M, Krakowiak P, Pessah I, et al. (2011). Polybrominated diphenyl ethers in relation to autism and developmental delay: A case-control study. Environmental Health, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, & Delwiche L (2009). The rise in autism and the role of age at diagnosis. Epidemiology, 20, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, & Krakowiak P (2018). Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Research, 11, 554–586. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, Walker CK, Bennett DH, Oliver M, Shedd-Wise KM, et al. (2018). A prospective study of environmental exposures and early bio-markers in autism spectrum disorder: Design, protocols, and preliminary data from the MARBLES Study. Environmental Health Perspectives, 126, 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada A, Shimodaira K, Okai T, Watanabe K, Takemori H, Takasuga T, et al. (2014). Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates. International Journal of Hygiene and Environmental Health, 217, 546–553. [DOI] [PubMed] [Google Scholar]
- Hochstenbach K, van Leeuwen DM, Gmuender H, Gottschalk RW, Stolevik SB, Nygaard UC, et al. (2012). Toxicogenomic profiles in relation to maternal immunotoxic exposure and immune functionality in newborns. Toxicological Sciences, 129, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, et al. (2017). An extended structure-activity relationship of nondioxin-like PCBs evaluates and supports modeling predictions and identifies picomolar potency of PCB 202 towards ryanodine receptors. Toxicological Sciences, 155, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Lee SM, Park S, Yoon SJ, Kim YE, & Oh IH (2020). Prevalence and economic burden of autism spectrum disorder in South Korea using national health insurance data from 2008 to 2015. Journal of Autism and Developmental Disorders, 50, 333–339. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, & Lein PJ (2003). Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicology and Applied Pharmacology, 190, 72–86. [DOI] [PubMed] [Google Scholar]
- Hu D, & Hornbuckle KC (2010). Inadvertent polychlorinated biphenyls in commercial paint pigments. Environmental Science & Technology, 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Chen S, Chen X, Zheng J, Xu Z, Doostparast Torshizi A, et al. (2019). Uncovering the functional link between SHANK3 deletions and deficiency in neurodevelopment using iPSC-derived human neurons. Frontiers in Neuroanatomy, 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, & Zhang H (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research, 1309, 83–94. [DOI] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews. Immunology, 11, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. (2001). Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. American Journal of Medical Genetics, 98, 161–167. [DOI] [PubMed] [Google Scholar]
- Itoh S, Baba T, Yuasa M, Miyashita C, Kobayashi S, Araki A, et al. (2018). Association of maternal serum concentration of hydroxylated polychlorinated biphenyls with maternal and neonatal thyroid hormones: The Hokkaido birth cohort study. Environmental Research, 167, 583–590. [DOI] [PubMed] [Google Scholar]
- Itoh H, Iwasaki M, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, et al. (2014). Association between serum organochlorines and global methylation level of leukocyte DNA among Japanese women: A cross-sectional study. Sci Total Environ, 490, 603–609. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Park HR, Kim TH, Yang WJ, Lee JJ, Choi SY, et al. (2012). High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology, 296, 73–82. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, & Eriksson P (2008). Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology, 29, 160–169. [DOI] [PubMed] [Google Scholar]
- Johnstone AF, Gross GW, Weiss DG, Schroeder OH, Gramowski A, & Shafer TJ (2010). Microelectrode arrays: A physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology, 31, 331–350. [DOI] [PubMed] [Google Scholar]
- Kalikiri MK, Mamidala MP, Rao AN, & Rajesh V (2017). Analysis and functional characterization of sequence variations in ligand binding domain of thyroid hormone receptors in autism spectrum disorder (ASD) patients. Autism Research, 10, 1919–1928. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, & Penlesky AC (2014). Environmental chemical exposures and autism spectrum disorders: A review of the epidemiological evidence. Current Problems in Pediatric and Adolescent Health Care, 44(10), 277–318. 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman HO (2012). A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Molecular Autism, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, & Lehmler HJ (2016). Toxicokinetics of chiral polychlorinated biphenyls across different species—A review. Environmental Science and Pollution Research International, 23, 2058–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil KP, & Lein PJ (2016). DNA methylation: A mechanism linking environmental chemical exposures to risk of autism spectrum disorders? Environ Epigenet, 2(1), dvv012. 10.1093/eep/dvv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil KP, Miller GW, Chen H, Sethi S, Schmuck MR, Dhakal K, et al. (2018). PCB 95 promotes dendritic growth in primary rat hippocampal neurons via mTOR-dependent mechanisms. Archives of Toxicology, 92, 3163–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil KP, Sethi S, & Lein PJ (2018). Sex-dependent effects of 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95) on dendritic arborization of primary mouse neurons. Toxicological Sciences, 168, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil KP, Sethi S, Wilson MD, Silverman JL, Pessah IN, & Lein PJ (2019). Genetic mutations in Ca(2+) signaling alter dendrite morphology and social approach in juvenile mice. Genes, Brain, and Behavior, 18(1), e12526. 10.1111/gbb.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil Stietz KP, Sethi S, Klocke CR, de Ruyter TE, Wilson MD, Pessah IN, et al. (2021). Sex and genotype modulate the dendritic effects of developmental exposure to a human-relevant polychlorinated biphenyls mixture in the juvenile mouse. Frontiers in Neuroscience, 15, 766802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, & Merzenich MM (2007). Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America, 104, 7646–7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CL, Spiegelhoff A, Lavery T, Wang K, Manuel RS, Wang Z, et al. (2022). Developmental polychlorinated biphenyl (PCB) exposure alters voiding physiology in young adult male and female mice. Am J Clin Exp Urol, 10, 82–97. [PMC free article] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, & Muller RA (2013). Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Reports, 5, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Michmizos K, Tommerdahl M, Ganesan S, Kitzbichler MG, Zetino M, et al. (2015). Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain, 138, 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Beck HN, Lein PJ, & Higgins D (2002). Interferon gamma induces retro-grade dendritic retraction and inhibits synapse formation. The Journal of Neuroscience, 22, 4530–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Bennett DH, Calafat AM, Hertz-Picciotto I, & Shin HM (2020). Temporal trends and determinants of serum concentrations of per- and polyfluoroalkyl substances among Northern California mothers with a young child, 2009–2016. Environmental Research, 186, 109491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, et al. (2011). Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environmental Health Perspectives, 119, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, et al. (2018). Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci Total Environ, 624, 377–384. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jarvik GP, Browning BL, Rajagopalan R, Gordon AS, Rieder MJ, et al. (2013). Exome sequencing reveals novel rare variants in the ryanodine receptor and calcium channel genes in malignant hyperthermia families. Anesthesiology, 119, 1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, et al. (2010). Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environmental Health Perspectives, 118, 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, & Kuroda Y (2005). Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Brain Research. Developmental Brain Research, 154, 259–263. [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, & Kuroda Y (2007). Disrupting effects of hydroxyl-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: A possible causal factor for developmental brain disorders? Chemosphere, 67, S412–S420. [DOI] [PubMed] [Google Scholar]
- King M, & Bearman P (2009). Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology, 38, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, & Goodman RH (2007). Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nature Neuroscience, 10, 1513–1514. [DOI] [PubMed] [Google Scholar]
- Klocke C, & Lein PJ (2020). Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (PCB) developmental neurotoxicity. International Journal of Molecular Sciences, 21(3), 1013. 10.3390/ijms21031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C, Sethi S, & Lein PJ (2019). The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): Similarities and differences. Environmental Science and Pollution Research International. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Gong Z, Zhang L, Sun X, Ou Z, Xu B, et al. (2019). JAK2/STAT3 signaling mediates IL-6-inhibited neurogenesis of neural stem cells through DNA demethylation/methylation. Brain, Behavior, and Immunity, 79, 159–173. [DOI] [PubMed] [Google Scholar]
- Kong Y, Zhou W, & Sun Z (2020). Nuclear receptor corepressors in intellectual disability and autism. Molecular Psychiatry, 25, 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouidhi S, & Clerget-Froidevaux MS (2018). Integrating thyroid hormone signaling in hypothalamic control of metabolism: crosstalk between nuclear receptors. International Journal of Molecular Sciences, 19(7), 2017. 10.3390/ijms19072017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, et al. (2017). Neonatal cytokine profiles associated with autism spectrum disorder. Biological Psychiatry, 81, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Hikel SM, Adams K, Hinds D, & Moon K (2012). Current status of the epidemiologic evidence linking polychlorinated biphenyls and non-hodgkin lymphoma, and the role of immune dysregulation. Environmental Health Perspectives, 120, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitinger JM, Beamer CA, & Shepherd DM (2016). Environmental immunology: Lessons learned from exposure to a select panel of immunotoxicants. Journal of Immunology, 196, 3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, & Bear MF (2011). Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annual Review of Medicine, 62, 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer KK, Zeidler M, Kalpachidou T, & Kress M (2021). Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine, 144, 155582. [DOI] [PubMed] [Google Scholar]
- Lai KP, Chung YT, Li R, Wan HT, & Wong CK (2016). Bisphenol A alters gut microbiome: Comparative metagenomics analysis. Environmental Pollution, 218, 923–930. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, & Baron-Cohen S (2014). Autism. Lancet, 383, 896–910. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ (2010). What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics, 22, 219–225. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Lambertini L, & Birnbaum LS (2012). A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environmental Health Perspectives, 120, a258–a260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM, & Yasui DH (2009). Evolving role of MeCP2 in Rett syndrome and autism. Epigenomics, 1, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer BI, Neier K, Valenzuela AE, Yasui DH, Schmidt RJ, Lein PJ, et al. (2022). Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal PCB exposure. Cell Reports, 38, 110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Roger S, Guerin P, Molinari F, M’Rad R, Cahard D, et al. (2006). Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. The American Journal of Psychiatry, 163, 1622–1629. [DOI] [PubMed] [Google Scholar]
- Lebovitz Y, Ringel-Scaia VM, Allen IC, & Theus MH (2018). Emerging developments in microbiome and microglia research: Implications for neurodevelopmental disorders. Frontiers in Immunology, 9, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey MA, & Hagerman PJ (2012). Fragile X-associated tremor/ataxia syndrome. Handbook of Clinical Neurology, 103, 373–386. [DOI] [PubMed] [Google Scholar]
- Leibson C, Weaver A, Myers S, Long K, Ransom J, Voigt R, et al. (2020). Objective estimates of direct-medical costs among persons aged 3 to 38 years with and without research-defined autism spectrum disorder ascertained during childhood: A population-based birth-cohort study. Value in Health, 23, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Gan L, De Boever P, Esser A, Amann PM, Ziegler P, et al. (2019). Altered gene expression in dioxin-like and non-dioxin-like PCB exposed peripheral blood mononuclear cells. International Journal of Environmental Research and Public Health, 16(12), 2090. 10.3390/ijerph16122090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P, Silbergeld E, Locke P, & Goldberg AM (2005). In vitro and other alternative approaches to developmental neurotoxicity testing (DNT). Environmental Toxicology and Pharmacology, 19, 735–744. [DOI] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, et al. (2014). The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. The Journal of Neuroscience, 34, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, & Campbell DB (2009). The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. The Journal of Clinical Investigation, 119, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hefti MM, Marek RF, Hornbuckle KC, Wang K, & Lehmler HJ (2022). Assessment of polychlorinated biphenyls and their hydroxylated metabolites in postmortem human brain samples: Age and brain region differences. Environmental Science & Technology, 56, 9515–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee S, Cade S, Kuo LJ, Schultz IR, Bhatt DK, et al. (2017). Novel interactions between gut microbiome and host drug-processing genes modify the hepatic metabolism of the environmental chemicals polybrominated diphenyl ethers. Drug Metabolism and Disposition, 45, 1197–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lim JJ, Wang K, Prasad B, Bhatt DK, Cui JY, et al. (2022). The disposition of polychlorinated biphenyls (PCBs) differs between germ-free and conventional mice. Environmental Toxicology and Pharmacology, 92, 103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Tian D, Lu M, Wang B, Li J, Xu B, et al. (2022). Gut microbiota dysbiosis induced by polychlorinated biphenyl 126 contributes to increased brain proinflammatory cytokines: Landscapes from the gut-brain axis and fecal microbiota transplantation. Ecotoxicology and Environmental Safety, 241, 113726. [DOI] [PubMed] [Google Scholar]
- Li RJ, Xu J, Fu C, Zhang J, Zheng YG, Jia H, et al. (2016). Regulation of mTORC1 by lysosomal calcium and calmodulin. eLife, 5, e19360. 10.7554/eLife.19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. (2015). Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: A nested case-control study in the Danish National Birth Cohort. Environmental Health Perspectives, 123, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Li X, Lehmler HJ, Wang D, Gu H, & Cui JY (2020). Gut microbiome critically impacts PCB-induced changes in metabolic fingerprints and the hepatic transcriptome in mice. Toxicological Sciences, 177, 168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Penell J, Luttropp K, Nordfors L, Syvanen AC, Axelsson T, et al. (2013). Global DNA hypermethylation is associated with high serum levels of persistent organic pollutants in an elderly population. Environment International, 59, 456–461. [DOI] [PubMed] [Google Scholar]
- Liu J, Koscielska KA, Cao Z, Hulsizer S, Grace N, Mitchell G, et al. (2012). Signaling defects in iPSC-derived fragile X premutation neurons. Human Molecular Genetics, 21, 3795–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, et al. (2019). Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Scientific Reports, 9, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lu Y, Zhong K, Wang C, & Xu X (2022). The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: A systematic review and meta-analysis. Ecotoxicology and Environmental Safety, 234, 113382. [DOI] [PubMed] [Google Scholar]
- Liu J, Tan Y, Song E, & Song Y (2020). A critical review of polychlorinated biphenyls metabolism, metabolites, and their correlation with oxidative stress. Chemical Research in Toxicology, 33, 2022–2042. [DOI] [PubMed] [Google Scholar]
- Liu W, Zheng M, Wang D, Xing Y, Zhao X, Ma X, et al. (2004). Formation of PCDD/Fs and PCBs in the process of production of 1,4-dichlorobenzene. Chemosphere, 57, 1317–1323. [DOI] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, & Veenstra-Vanderweele J (2018). Autism spectrum disorder. Lancet, 392, 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, & Cantor RM (2012). Allowing for sex differences increases power in a GWAS of multiplex Autism families. Molecular Psychiatry, 17, 215–222. [DOI] [PubMed] [Google Scholar]
- Lutz AK, Perez Arevalo A, Ioannidis V, Stirmlinger N, Demestre M, Delorme R, et al. (2021). SHANK2 mutations result in dysregulation of the ERK1/2 pathway in human induced pluripotent stem cells-derived neurons and Shank2(−/−) mice. Frontiers in Molecular Neuroscience, 14, 773571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Sjodin A, Yoshida CK, Zerbo O, Kharrazi M, et al. (2017). Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: Association with autism spectrum disorder and intellectual disability. Environmental Health Perspectives, 125, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Weiss LA, Kharrazi M, Traglia M, Delorenze GN, et al. (2017). Prenatal serum concentrations of brominated flame retardants and autism spectrum disorder and intellectual disability in the early markers of autism study: A population-based case-control study in California. Environmental Health Perspectives, 125, 087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. (2018). Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environmental Health Perspectives, 126, 017001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk D, Ayad EG, Brewton KL, Saing P, & Hart PC (2022). Systemic maternal inflammation promotes ASD via IL-6 and IFN-gamma. Bioscience Reports, 42(11), BSR20220713. 10.1042/BSR20220713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki M, Noorimotlagh Z, Mirzaee SA, Jaafarzadeh N, Martinez SS, Rahim F, et al. (2022). An updated systematic review on the maternal exposure to environmental pesticides and involved mechanisms of autism spectrum disorder (ASD) progression risk in children. Reviews on Environmental Health. [DOI] [PubMed] [Google Scholar]
- Malm H, Artama M, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomaki S, et al. (2012). Infant and childhood neurodevelopmental outcomes following prenatal exposure to selective serotonin reuptake inhibitors: Overview and design of a Finnish Register-Based Study (FinESSI). BMC Psychiatry, 12, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandic-Maravic V, Coric V, Mitkovic-Voncina M, Djordjevic M, Savic-Radojevic A, Ercegovac M, et al. (2019). Interaction of glutathione S-transferase polymorphisms and tobacco smoking during pregnancy in susceptibility to autism spectrum disorders. Scientific Reports, 9, 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantoni A, Calorio C, Hidisoglu E, Chiantia G, & Carbone E (2020). Cav1.2 channelopathies causing autism: New hallmarks on Timothy syndrome. Pflügers Archiv, 472, 775–789. [DOI] [PubMed] [Google Scholar]
- Marek D, Papin S, Eellefsen K, Niederhauser J, Isidor N, Ransijn A, et al. (2012). Carriers of the fragile X mental retardataion 1 (FMR1) premutation allele present with increased levels of cytokine IL-10. Journal of Neuroinflammation, 9, 1–8. 10.1186/1742-2094-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari-Bauset S, Peraita-Costa I, Donat-Vargas C, Llopis-Gonzalez A, Mari-Sanchis A, Llopis-Morales J, et al. (2022). Systematic review of prenatal exposure to endocrine disrupting chemicals and autism spectrum disorder in offspring. Autism, 26, 6–32. [DOI] [PubMed] [Google Scholar]
- Masini E, Loi E, Vega-Benedetti AF, Carta M, Doneddu G, Fadda R, et al. (2020). An overview of the main genetic, epigenetic and environmental factors involved in autism spectrum disorder focusing on synaptic activity. International Journal of Molecular Sciences, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelski L, Keil Stietz KP, Sethi S, Taylor ST, Van de Water J, & Lein PJ (2020). The influence of sex, genotype, and dose on serum and hippocampal cytokine levels in juvenile mice developmentally exposed to a human-relevant mixture of polychlorinated biphenyls. Current Research in Toxicology, 1, 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelski L, & Van de Water J (2016). Risk factors in autism: Thinking outside the brain. Journal of Autoimmunity, 67, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, & Boeckxstaens GE (2013). The vagal innervation of the gut and immune homeostasis. Gut, 62, 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Padua D, & Tillisch K (2014). Altered brain-gut axis in autism: Comorbidity or causative mechanisms? Bioessays, 36, 933–939. [DOI] [PubMed] [Google Scholar]
- McCarthy MM (2016a). Multifaceted origins of sex differences in the brain. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371, 20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2016b). Sex differences in the developing brain as a source of inherent risk. Dialogues in Clinical Neuroscience, 18, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, & De Vries GJ (2012). Sex differences in the brain: The not so inconvenient truth. The Journal of Neuroscience, 32, 2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Herold K, & Stockman SL (2018). Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiology & Behavior, 187, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, & Lenz KM (2017). Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nature Reviews. Neuroscience, 18, 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, & Wright CL (2017). Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biological Psychiatry, 81, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J, Kim DHJ, Bruce M, Ramirez-Celis A, & Van de Water J (2022). Maternal immune dysregulation and autism-understanding the role of cytokines, chemokines and autoantibodies. Frontiers in Psychiatry, 13, 834910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehri F, Bashirian S, Khazaei S, & Jenabi E (2021). Association between pesticide and polychlorinated biphenyl exposure during pregnancy and autism spectrum disorder among children: A meta-analysis. Clin Exp Pediatr, 64, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer A, & Van de Water J (2017). The role of the immune system in autism spectrum disorder. Neuropsychopharmacology, 42, 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melymuk L, Blumenthal J, Sanka O, Shu-Yin A, Singla V, Sebkova K, et al. (2022). Persistent problem: Global challenges to managing PCBs. Environmental Science & Technology, 56, 9029–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, & Gillberg C (2005). Autism associated with conditions characterized by developmental errors in early embryogenesis: A mini review. International Journal of Developmental Neuroscience, 23, 201–219. [DOI] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, et al. (2012). Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environmental and Molecular Mutagenesis, 53, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modafferi S, Zhong X, Kleensang A, Murata Y, Fagiani F, Pamies D, et al. (2021). Gene-environment interactions in developmental neurotoxicity: A case study of synergy between chlorpyrifos and CHD8 knockout in human brain spheres. Environmental Health Perspectives, 129, 77001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa A, Shu H, Sarachana T, & Hu VW (2018). Are endocrine disrupting compounds environmental risk factors for autism spectrum disorder? Hormones and Behavior, 101, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordaunt CE, Park BY, Bakulski KM, Feinberg JI, Croen LA, Ladd-Acosta C, et al. (2019). A meta-analysis of two high-risk prospective cohort studies reveals autism-specific transcriptional changes to chromatin, autoimmune, and environmental response genes in umbilical cord blood. Molecular Autism, 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshaty A, Haijima A, Takatsuru Y, Ninomiya A, Yajima H, Kokubo M, et al. (2020). Neurotoxic effects of lactational exposure to perfluorooctane sulfonate on learning and memory in adult male mouse. Food and Chemical Toxicology, 145, 111710. [DOI] [PubMed] [Google Scholar]
- Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, et al. (2020). Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature, 583(7816), 441–446. 10.1038/s41586-020-2474-7. Epub 2020 Jul 8. Erratum in: Nature. 2020 Sep;585(7823):E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Fukuno S, Suzuki H, & Konishi H (2016). Higher gene expression of CYP1A2, 2B1 and 2D2 in the brain of female compared with male rats. Pharmazie, 71, 334–336. [PubMed] [Google Scholar]
- Nanou E, & Catterall WA (2018). Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron, 98, 466–481. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Schiavi S, La Rosa P, Rossi-Espagnet MC, Petrillo S, Bottino F, et al. (2022). Sex differences in autism spectrum disorder: Diagnostic, neurobiological, and behavioral features. Frontiers in Psychiatry, 13, 889636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, & Werner HB (2014). Myelination of the nervous system: Mechanisms and functions. Annual Review of Cell and Developmental Biology, 30, 503–533. [DOI] [PubMed] [Google Scholar]
- Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, et al. (2022). A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature, 602, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngounou Wetie AG, Wormwood KL, Charette L, Ryan JP, Woods AG, & Darie CC (2015). Comparative two-dimensional polyacrylamide gel electrophoresis of the salivary proteome of children with autism spectrum disorder. Journal of Cellular and Molecular Medicine, 19, 2664–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen RL, Medvedeva YV, Ayyagari TE, Schmunk G, & Gargus JJ (2018). Intracellular calcium dysregulation in autism spectrum disorder: An analysis of converging organelle signaling pathways. Biochimica et Biophysica Acta, Molecular Cell Research, 1865, 1718–1732. [DOI] [PubMed] [Google Scholar]
- Nielsen TC, Nassar N, Shand AW, Jones HF, Han VX, Patel S, et al. (2022). Association of maternal autoimmune disease and early childhood infections with offspring autism spectrum disorder: A population-based cohort study. Autism Research. 10.1002/aur.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya A, Mshaty A, Haijima A, Yajima H, Kokubo M, Khairinisa MA, et al. (2022). The neurotoxic effect of lactational PFOS exposure on cerebellar functional development in male mice. Food and Chemical Toxicology, 159, 112751. [DOI] [PubMed] [Google Scholar]
- Nomiyama K, Eguchi A, Takaguchi K, Yoo J, Mizukawa H, Oshihoi T, et al. (2019) Targeted metabolome analysis of the dog brain exposed to PCBs suggests inhibition of oxidative phosphorylation by hydroxylated PCBs. Toxicology and Applied Pharmacology, 377, 114620. [DOI] [PubMed] [Google Scholar]
- Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, & Scholmerich A (2015). Influence of low-level prenatal exposure to PCDD/Fs and PCBs on empathizing, systemizing and autistic traits: Results from the Duisburg Birth Cohort Study. PLoS One, 10, e0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, et al. (2021). Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environment International, 147, 106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Schmidt RJ, Tancredi D, Calafat AM, Roa DL, Hertz-Picciotto I, et al. (2021). Prenatal exposure to per- and polyfluoroalkyl substances and cognitive development in infancy and toddlerhood. Environmental Research, 196, 110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, & Li E (1999). DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, & Schwartz HL (1997). Molecular basis of thyroid hormone-dependent brain development. Endocrine Reviews, 18, 462–475. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P, & Grandjean P (2016). Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environment International, 97, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panesar HK, Kennedy CL, Keil Stietz KP, & Lein PJ (2020). Polychlorinated biphenyls (PCBs): Risk factors for autism spectrum disorder? Toxics, 8(3), 70. 10.3390/toxics8030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee K, Kim K, & Yi SJ (2022). The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduction and Targeted Therapy, 7, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parner ET, Schendel DE, & Thorsen P (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics & Adolescent Medicine, 162, 1150–1156. [DOI] [PubMed] [Google Scholar]
- Parra AS, & Johnston CA (2022). Emerging roles of RNA-binding proteins in neurodevelopment. J Dev Biol, 10(2), 23. 10.3390/jdb10020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, et al. (2011). Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nature Medicine, 17, 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelch KE, Bolden AL, & Kwiatkowski CF (2019). Environmental chemicals and autism: A scoping review of the human and animal research. Environmental Health Perspectives, 127, 46001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Lu Z, Li G, Piechowicz M, Anderson M, Uddin Y, et al. (2016). The autism-associated MET receptor tyrosine kinase engages early neuronal growth mechanism and controls glutamatergic circuits development in the forebrain. Molecular Psychiatry, 21, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, & Woolfrey KM (2011). Dendritic spine pathology in neuropsychiatric disorders. Nature Neuroscience, 14, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy AK (2011). Rett syndrome: exploring the autism link. Archives of Neurology, 68, 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persichetti AS, Shao J, Gotts SJ, & Martin A (2022). Maladaptive laterality in cortical networks related to social communication in autism spectrum disorder. The Journal of Neuroscience. 10.1523/JNEUROSCI.1229-22.2022. JN-RM-1229–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, & Lein PJ (2010). Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacology & Therapeutics, 125, 260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, & Lein PJ (2008). Evidence for environmental susceptibility in autism: What we need to know about gene x environment interactions. In Zimmerman A (Ed.), Autism: Current theories and evidence (pp. 409–428). Totowa, NJ: Humana Press. [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, & Sagiv SK (2019). Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathologica, 138, 363–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, & Hennig B (2018). Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environmental Pollution, 242, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hu H, Ettinger A, Sanchez BN, Wright RO, Cantonwine D, et al. (2009). Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environmental Health Perspectives, 117, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J, & Hanke W (2012). Exposure to environmental and lifestyle factors and attention-deficit/hyperactivity disorder in children—A review of epidemiological studies. International Journal of Occupational Medicine and Environmental Health, 25, 330–355. [DOI] [PubMed] [Google Scholar]
- Pourtavakoli A, & Ghafouri-Fard S (2022). Calcium signaling in neurodevelopment and pathophysiology of autism spectrum disorders. Molecular Biology Reports, 49(11), 10811–10823. 10.1007/s11033-022-07775-6. [DOI] [PubMed] [Google Scholar]
- Purushotham SS, Reddy NMN, D’Souza MN, Choudhury NR, Ganguly A, Gopalakrishna N, et al. (2022). A perspective on molecular signalling dysfunction, its clinical relevance and therapeutics in autism spectrum disorder. Experimental Brain Research, 240, 2525–2567. [DOI] [PubMed] [Google Scholar]
- Qiu S, Aldinger KA, & Levitt P (2012). Modeling of autism genetic variations in mice: focusing on synaptic and microcircuit dysfunctions. Developmental Neuroscience, 34, 88–100. [DOI] [PubMed] [Google Scholar]
- Ramirez V, Gonzalez-Palacios P, Baca MA, Gonzalez-Domenech PJ, Fernandez-Cabezas M, Alvarez-Cubero MJ, et al. (2022). Effect of exposure to endocrine disrupting chemicals in obesity and neurodevelopment: The genetic and microbiota link. Sci Total Environ, 852, 158219. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, & Brown MA (2012). Innate immunity in the central nervous system. The Journal of Clinical Investigation, 122, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristori MV, Quagliariello A, Reddel S, Ianiro G, Vicari S, Gasbarrini A, et al. (2019). Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional interventions. Nutrients, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin G, Lopez JR, Espinal GM, Hulsizer S, Hagerman PJ, & Pessah IN (2017). Calcium dysregulation and Cdk5-ATM pathway involved in a mouse model of fragile X-associated tremor/ataxia syndrome. Human Molecular Genetics, 26, 2649–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette BL, Harrill JA, Mundy WR, & Shafer TJ (2011). In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Frontiers in Neuroengineering, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KD, & Patisaul HB (2018). Environmental mechanisms of neurodevelopmental toxicity. Curr Environ Health Rep, 5, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, & Croog VJ (1997). Linking etiologies in humans and animal models: studies of autism. Reproductive Toxicology, 11, 417–422. [DOI] [PubMed] [Google Scholar]
- Rodier P, Miller RK, & Brent RL (2011). Does treatment of premature labor with terbutaline increase the risk of autism spectrum disorders? American Journal of Obstetrics and Gynecology, 204, 91–94. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS (2017). Gut dysbiosis in animals due to environmental chemical exposures. Frontiers in Cellular and Infection Microbiology, 7, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist AH, Hoyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V, et al. (2017). Prenatal and postnatal PCB-153 and p,p’-DDE exposures and behavior scores at 5–9 years of age among children in Greenland and Ukraine. Environmental Health Perspectives, 125, 107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, & Frye RE (2014). Environmental toxicants and autism spectrum disorders: A systematic review. Translational Psychiatry, 4, e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet JF (2014). The role of thyroid hormones for brain development and cognitive function. Endocrine Development, 26, 26–43. [DOI] [PubMed] [Google Scholar]
- Roy HA, & Green AL (2019). The central autonomic network and regulation of bladder function. Frontiers in Neuroscience, 13, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior, 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude KM, Keogh CE, & Gareau MG (2019). The role of the gut microbiome in mediating neurotoxic outcomes to PCB exposure. Neurotoxicology, 75, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude KM, Pusceddu MM, Keogh CE, Sladek JA, Rabasa G, Miller EN, et al. (2019). Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environmental Pollution, 253, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, et al. (2012). Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron, 75, 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, & Bonefeld-Jorgensen EC (2008). Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environmental Health Perspectives, 116, 1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AJ, Albert A, & Bowman J (2017). Decreased phosphorylated CREB and AKT in individuals with autism normalized after zinc therapy. Acad J Ped Neonatol, 5, 57–60. [Google Scholar]
- Salazar P, Villaseca P, Cisternas P, & Inestrosa NC (2021). Neurodevelopmental impact of the offspring by thyroid hormone system-disrupting environmental chemicals during pregnancy. Environmental Research, 200, 111345. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, & LaSalle JM (2005). Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics, 14, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature, 485, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, & Reichenberg A (2014). The familial risk of autism. JAMA, 311, 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachana T, Zhou R, Chen G, Manji HK, & Hu VW (2010). Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Medicine, 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmunk G, & Gargus JJ (2013). Channelopathy pathogenesis in autism spectrum disorders. Frontiers in Genetics, 4, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield D, Zeppel MJB, Tanton R, Veerman JL, Kelly SJ, Passey ME, et al. (2019). Intellectual disability and autism: socioeconomic impacts of informal caring, projected to 2030. The British Journal of Psychiatry, 215(5), 654–660. 10.1192/bjp.2019.204. [DOI] [PubMed] [Google Scholar]
- Sciacca M, Marino L, Vitaliti G, Falsaperla R, & Marino S (2022). NRXN1 deletion in two twins’ genotype and phenotype: A clinical case and literature review. Children (Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, et al. (2010). Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Science Translational Medicine, 2, 56ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechman A, Batoryna M, Antos PA, & Hrabia A (2016). Effects of PCB 126 and PCB 153 on secretion of steroid hormones and mRNA expression of steroidogenic genes (STAR, HSD3B, CYP19A1) and estrogen receptors (ERalpha, ERbeta) in prehierarchical chicken ovarian follicles. Toxicology Letters, 264, 29–37. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Nabi R, Zhong H, & Huq M (2004). Polymorphisms in xenobiotic metabolism genes and autism. Journal of Child Neurology, 19, 413–417. [DOI] [PubMed] [Google Scholar]
- Sethi S, Chen X, Kass PH, & Puschner B (2017). Polychlorinated biphenyl and polybrominated diphenyl ether profiles in serum from cattle, sheep, and goats across California. Chemosphere, 181, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Chen H, Hayakawa K, Li X, Lin Y, et al. (2017). Detection of 3,3’-dichlorobiphenyl in human maternal plasma and its effects on axonal and dendritic growth in primary rat neurons. Toxicological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil KP, & Lein PJ (2018). 3,3’-Dichlorobiphenyl (PCB 11) promotes dendritic arborization in primary rat cortical neurons via a CREB-dependent mechanism. Archives of Toxicology, 92, 3337–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil Stietz KP, Valenzuela AE, Klocke CR, Silverman JL, Puschner B, et al. (2021). Developmental exposure to a human-relevant polychlorinated biphenyl mixture causes behavioral phenotypes that vary by sex and genotype in juvenile mice expressing human mutations that modulate neuronal calcium. Frontiers in Neuroscience, 15, 766826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Morgan RK, Feng W, Lin Y, Li X, Luna C, et al. (2019). Comparative analyses of the 12 most abundant PCB congeners detected in human maternal serum for activity at the thyroid hormone receptor and ryanodine receptor. Environmental Science & Technology, 53, 3948–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. (2019). Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell, 177, 1600–1618. e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Hertz-Picciotto I, & Pessah IN (2012). Tipping the balance of autism risk: Potential mechanisms linking pesticides and autism. Environmental Health Perspectives, 120, 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Calafat AM, Tancredi D, & Hertz-Picciotto I (2020). Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: A case-control study. Environmental Research, 186, 109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima M, Rossnerova A, Simova Z, & Rossner P Jr. (2021). The impact of air pollution exposure on the microRNA machinery and lung cancer development. J Pers Med, 11(1), 60. 10.3390/jpm11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skafidas E, Testa R, Zantomio D, Chana G, Everall IP, & Pantelis C (2014). Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Molecular Psychiatry, 19, 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogheim TS, Weyde KVF, Aase H, Engel SM, Suren P, Oie MG, et al. (2021). Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environmental Research, 202, 111692. [DOI] [PubMed] [Google Scholar]
- Sotgiu S, Manca S, Gagliano A, Minutolo A, Melis MC, Pisuttu G, et al. (2020). Immune regulation of neurodevelopment at the mother-foetus interface: the case of autism. Clin Transl Immunology, 9, e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soueid J, Kourtian S, Makhoul NJ, Makoukji J, Haddad S, Ghanem SS, et al. (2016). RYR2, PTDSS1 and AREG genes are implicated in a Lebanese population-based study of copy number variation in autism. Scientific Reports, 6, 19088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. (2004). Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell, 119, 19–31. [DOI] [PubMed] [Google Scholar]
- Spratt PWE, Ben-Shalom R, Keeshen CM, Burke KJ Jr., Clarkson RL, Sanders SJ, et al. (2019). The autism-associated gene Scn2a contributes to dendritic excitability and synaptic function in the prefrontal cortex. Neuron, 103, 673–685. e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M, Streifel KM, Goines PE, & Lein PJ (2013). Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicology and Teratology, 36, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolevik SB, Nygaard UC, Namork E, Haugen M, Kvalem HE, Meltzer HM, et al. (2011). Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants. Food and Chemical Toxicology, 49, 1843–1848. [DOI] [PubMed] [Google Scholar]
- Stolevik SB, Nygaard UC, Namork E, Haugen M, Meltzer HM, Alexander J, et al. (2013). Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food and Chemical Toxicology, 51, 165–172. [DOI] [PubMed] [Google Scholar]
- Sudhof TC (2008). Neuroligins and neurexins link synaptic function to cognitive disease. Nature, 455, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan O, & Ciernia AV (2022). Work hard, play hard: how sexually differentiated microglia work to shape social play and reproductive behavior. Frontiers in Behavioral Neuroscience, 16, 989011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TA, & Pessah IN (2007). Ryanodine receptor type 1 (RyR1) possessing malignant hyperthermia mutation R615C exhibits heightened sensitivity to dysregulation by non-coplanar 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95). Neurotoxicology, 28, 770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Anezaki K, & Kojima H (2017). Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environmental Pollution, 227, 306–313. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Shiraishi F, Kitamura S, Kuroki H, Jin K, & Kojima H (2011). Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology, 289, 112–121. [DOI] [PubMed] [Google Scholar]
- Talebizadeh Z, Butler MG, & Theodoro MF (2008). Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Research, 1, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani R, & Van Aken B (2014). Hydroxylated polychlorinated biphenyls in the environment: sources, fate, and toxicities. Environmental Science and Pollution Research International, 21, 6334–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien VD, Degre-Pelletier J, Barbeau EB, Samson F, & Soulieres I (2022). Differential neural correlates underlying mental rotation processes in two distinct cognitive profiles in autism. Neuroimage Clin, 36, 103221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, & Boekelheide K (2013). Multiple environmental chemical exposures to lead, mercury and polychlorinated biphenyls among childbearing-aged women (NHANES 1999–2004): Body burden and risk factors. Environmental Research, 121, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thony B, Auerbach G, & Blau N (2000). Tetrahydrobiopterin biosynthesis, regeneration and functions. The Biochemical Journal, 347(Pt 1), 1–16. [PMC free article] [PubMed] [Google Scholar]
- Todd PK, & Mack KJ (2001). Phosphorylation, CREB, and mental retardation. Pediatric Research, 50, 672. [DOI] [PubMed] [Google Scholar]
- Tremblay MW, & Jiang YH (2019). DNA methylation and susceptibility to autism spectrum disorder. Annual Review of Medicine, 70, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana E, Ruiz P, Li X, & Lehmler HJ (2019). Human CYP2A6, CYP2B6, AND CYP2E1 atropselectively metabolize polychlorinated biphenyls to hydroxylated metabolites. Environmental Science & Technology, 53, 2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereyken EJ, Bajova H, Chow S, de Graan PN, & Gruol DL (2007). Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. The European Journal of Neuroscience, 25, 3605–3616. [DOI] [PubMed] [Google Scholar]
- Vester A, & Caudle WM (2016). The synapse as a central target for neurodevelopmental susceptibility to pesticides. Toxics, 4(3), 18. 10.3390/toxics4030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Lee I, & Eriksson P (2013). Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology, 304, 185–191. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Owe-Larsson B, Brask J, Kristensson KS, & Hill RH (2001). Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Research, 896, 18–29. [DOI] [PubMed] [Google Scholar]
- von Gontard A, Baeyens D, Van Hoecke E, Warzak WJ, & Bachmann C (2011). Psychological and psychiatric issues in urinary and fecal incontinence. The Journal of Urology, 185, 1432–1436. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, et al. (2020). The maternal microbiome modulates fetal neurodevelopment in mice. Nature, 586, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukcevic M, Zorzato F, Keck S, Tsakiris DA, Keiser J, Maizels RM, et al. (2013). Gain of function in the immune system caused by a ryanodine receptor 1 mutation. Journal of Cell Science, 126, 3485–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, & Chen A (2018). Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Hormones and Behavior, 101, 94–104. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Alexander NC 2nd, Li X, Rouchka EC, Kirpich IA, & Cave MC (2021). Polychlorinated biphenyls altered gut microbiome in CAR and PXR knockout mice exhibiting toxicant-associated steatohepatitis. Toxicology Reports, 8, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, & Gore AC (2014). Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Molecular Endocrinology, 28, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Liu D, Xiao WB, Zhang BX, Yan XX, Luo ZH, et al. (2022). Association of SHANK family with neuropsychiatric disorders: An update on genetic and animal model discoveries. Cellular and Molecular Neurobiology, 42, 1623–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, et al. (2012). PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environmental Health Perspectives, 120, 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, et al. (2006). Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron, 50, 897–909. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, et al. (2012). PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environmental Health Perspectives, 120, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Patandin S, Berbers GA, Sas TC, Mulder PG, Sauer PJ, et al. (2000). Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environmental Health Perspectives, 108, 1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B (2012). The intersection of neurotoxicology and endocrine disruption. Neurotoxicology, 33, 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Cheng TL, Li GZ, Sun SB, Yu SY, Zhang Y, et al. (2017). Identification of autism-related MECP2 mutations by whole-exome sequencing and functional validation. Molecular Autism, 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of Neuroendocrinology, 20, 784–794. [DOI] [PubMed] [Google Scholar]
- Winans B, Humble MC, & Lawrence BP (2011). Environmental toxicants and the developing immune system: a missing link in the global battle against infectious disease? Reproductive Toxicology, 31, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Ranft U, Wittsiepe J, Kasper-Sonnenberg M, Furst P, Kramer U, et al. (2014). Behavioral sexual dimorphism in school-age children and early developmental exposure to dioxins and PCBs: A follow-up study of the Duisburg Cohort. Environmental Health Perspectives, 122, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, et al. (2012). Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Human Molecular Genetics, 21, 2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhou ZJ, & Ohsako S (2006). Effect of environmental contaminants on DNA methyltransferase activity of mouse preimplantation embryos. Wei sheng yan jiu = Journal of Hygiene Research, 35, 30–32. [PubMed] [Google Scholar]
- Xiang Y, Xing Z, Liu J, Qin W, & Huang X (2020). Recent advances in the biodegradation of polychlorinated biphenyls. World Journal of Microbiology and Biotechnology, 36, 145. [DOI] [PubMed] [Google Scholar]
- Xu L, Guo X, Li N, Pan Q, & Ma YZ (2019). Effects of quercetin on Aroclor 1254-induced expression of CYP450 and cytokines in pregnant rats. Journal of Immunotoxicology, 16, 140–148. [DOI] [PubMed] [Google Scholar]
- Xu N, Li AD, Ji LL, Ye Y, Wang ZY, & Tong L (2019). miR-132 regulates the expression of synaptic proteins in APP/PS1 transgenic mice through C1q. European Journal of Histochemistry, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Long J, Shi C, Zhang N, Lv Y, Feng J, et al. (2018). Effect of leukocyte inhibitory factor on neuron differentiation from human induced pluripotent stem cell-derived neural precursor cells. International Journal of Molecular Medicine, 41, 2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi B, Miller GW, Holland EB, Li X, Harvey D, Li S, et al. (2022). Ryanodine receptor-active non-dioxin-like polychlorinated biphenyls cause neurobehavioral deficits in larval zebrafish. Front Toxicol, 4, 947795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A, Chen H, Stamou M, Bose DD, et al. (2014). PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicological Sciences, 138, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, et al. (2009). Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environmental Health Perspectives, 117, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, & Lein PJ (2010). Polychlorinated biphenyls increase apoptosis in the developing rat brain. Curr Neurobiol, 1, 70–76. [PMC free article] [PubMed] [Google Scholar]
- Yousefi B, Kokhaei P, Mehranfar F, Bahar A, Abdolshahi A, Emadi A, et al. (2022). The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neuroscience and Biobehavioral Reviews, 132, 998–1009. [DOI] [PubMed] [Google Scholar]
- Yu H, Liu Y, Shu X, Ma L, & Pan Y (2020). Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere, 243, 125392. [DOI] [PubMed] [Google Scholar]
- Yu X, Rahman MM, Wang Z, Carter SA, Schwartz J, Chen Z, et al. (2022). Evidence of susceptibility to autism risks associated with early life ambient air pollution: A systematic review. Environmental Research, 208, 112590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Su Q, Chen Y, Wu L, Wu S, & Li H (2021). Epigenetics in neurodegenerative disorders induced by pesticides. Genes Environ, 43, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen B, Boncompagni S, Feng W, Yang T, Lopez JR, Matthaei KI, et al. (2012). Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. The FASEB Journal, 26, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, & Blumberg SJ (2017). Estimated prevalence of children with diagnosed developmental disabilities in the United States, 2014–2016. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Zahalka EA, Ellis DH, Goldey ES, Stanton ME, & Lau C (2001). Perinatal exposure to polychlorinated biphenyls Aroclor 1016 or 1254 did not alter brain catecholamines nor delayed alternation performance in Long-Evans rats. Brain Research Bulletin, 55, 487–500. [DOI] [PubMed] [Google Scholar]
- Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. (2022). Global prevalence of autism: A systematic review update. Autism Research, 15, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Wang T, Li YF, Deng YN, & Shen FG (2022). Roles of the Notch signaling pathway and microglia in autism. Behavioural Brain Research, 437, 114131. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN, et al. (2017). Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environmental Health Perspectives, 125, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Kasper LH, Bedford DC, Lerach S, Teubner BJ, & Brindle PK (2016). Mutation of the CH1 domain in the histone acetyltransferase CREBBP results in autism-relevant behaviors in mice. PLoS One, 11, e0146366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Mordaunt CE, Yasui DH, Marathe R, Coulson RL, Dunaway KW, et al. (2019). Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Human Molecular Genetics, 28, 2659–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, & Goodman AL (2019). Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature, 570, 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT (2007). Environmental chemicals impacting the thyroid: targets and consequences. Thyroid, 17, 811–817. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, & Bear MF (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor Perspectives in Biology, 4(3), a009886. 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]


