Abstract
Cartilage tissue engineering strategies seek to repair damaged tissue using approaches that include scaffolds containing components of the native extracellular matrix (ECM). Articular cartilage consists of glycosaminoglycans (GAGs) which are known to sequester growth factors. In order to more closely mimic the native ECM, this study evaluated the chondrogenic differentiation of mesenchymal stem cells (MSCs), a promising cell source for cartilage regeneration, on fibrous scaffolds that contained the GAG-mimetic cellulose sulfate. The degree of sulfation was evaluated, examining partially sulfated cellulose (pSC) and fully sulfated cellulose (NaCS). Comparisons were made with scaffolds containing native GAGs (chondroitin sulfate A, chondroitin sulfate C and heparin). Transforming growth factor-beta3 (TGF-β3) sequestration, as measured by rate of association, was higher for sulfated cellulose-containing scaffolds as compared to native GAGs. In addition, TGF-β3 sequestration and retention over time was highest for NaCS-containing scaffolds. Sulfated cellulose-containing scaffolds loaded with TGF-β3 showed enhanced chondrogenesis as indicated by a higher Collagen Type II:I ratio over native GAGs. NaCS-containing scaffolds loaded with TGF-β3 had the highest expression of chondrogenic markers and a reduction of hypertrophic markers in dynamic loading conditions, which more closely mimic in vivo conditions. Studies also demonstrated that TGF-β3 mediated its effect through the Smad2/3 signaling pathway where the specificity of TGF-β receptor (TGF- βRI)-phosphorylated SMAD2/3 was verified with a receptor inhibitor. Therefore, studies demonstrate that scaffolds containing cellulose sulfate enhance TGF-β3-induced MSC chondrogenic differentiation and show promise for promoting cartilage tissue regeneration.
Keywords: cellulose, chondrogenesis, chondroitin sulfate, glycosaminoglycans, mesenchymal stem cells
1 ∣. INTRODUCTION
Cartilage tissue engineering strategies aim to repair damaged tissue using constructs containing biological, natural and/or synthetic components that promote cellular infiltration, host tissue integration, regeneration and maintenance of tissue functionality.1 Articular or hyaline cartilage has limited intrinsic healing capability due to factors that include the lack of vasculature, chondrocyte replication and repair potential, and recruitment of extrinsic stem cells capable of repair.2,3 Cell-based tissue engineering techniques have been widely used for cartilage tissue repair, especially the use of autologous chondrocyte implantation (ACI), with or without scaffolds.4-6 However, the use of mature chondrocytes in cartilage tissue repair has several disadvantages including: donor site morbidity with autologous sources, limited availability of cells and lack of functional tissue repair due to fibrocartilage production.7,8 Thus, multipotent adult mesenchymal stem cells (MSCs) have gained considerable interest. MSCs can be sourced from adipose, bone marrow and synovial fluid and membrane tissues. MSCs, in comparison to mature chondrocytes, have higher proliferation and chondro-differentiation capacity and are resistant to senescence.7,9 Studies have shown that in middle-aged and older patients, MSC implantation can be effective, specifically in arthroscopic and histological scoring, depending on the size of the lesions in cartilage.10 Furthermore, in a 11-year follow-up, these patients did not develop tumors or infections following stem cell treatment.10 The challenge in the use of MSCs is to differentiate them into mature chondrocytes with reduced collagen type I production and expression of hypertrophic markers such as collagen type X (ColX), vascular endothelial growth factor (VEGF), matrix metalloproteinase 13 (MMP13) and thus, preventing hypertrophic/terminal differentiation.11 Approaches have focused on providing appropriate chemical and physical signals such as growth factors and cytokines, components of the extracellular matrix (ECM), and topographical and mechanical cues of the scaffold.12
One of the main factors important in promoting the chondrogenic differentiation of MSCs is the presentation of growth factors.13 Most in vitro studies use exogenous supplementation of growth factors such as transforming growth factor – beta (TGF-β), bone morphogenetic protein (BMP), growth differentiation factor-5 (GDF-5), insulin-like growth factor (IGF-1) and fibroblast growth factor (FGF).14 However, in vivo, during the development of cartilage tissue, these soluble factors are found bound to sulfated glycosaminoglycans (GAGs), such as chondroitin sulfates and heparan sulfate, in the ECM.15 This suggests the need for spatial and temporal control over the availability of these growth factors to the cells.
Sulfated polysaccharide-containing materials loaded with TGF-β have been shown to enhance chondrogenic activity both in vivo and in vitro.13,16-18 TGF-β3 activates the expression of chondrogenic genes through the TGF-β3/Smad2-3 signaling pathway that can reduce chondrocyte hypertrophy.19,20 In a previous study, gelatin scaffolds containing the GAG-mimetic, sulfated cellulose, have been shown to sequester TGF-β3 to a higher extent than gelatin scaffolds alone and is dependent upon the degree of sulfation of the sulfated cellulose.21,22 Therefore, in this study, MSC chondrogenesis was investigated on scaffolds containing the GAG-mimetic, sulfated cellulose, in comparison to scaffolds containing native GAGs, loaded with or without TGF-β3. Sequestration kinetics, association rate and release of TGF-β3 from the scaffolds, as well as TGF-β3/Smad2-3 signaling were investigated to gain an understanding of the effect of TGF-β3 and these scaffolds on chondrogenesis. In addition, MSC chondrogenesis on scaffolds loaded with TGF- β3 was evaluated in a bioreactor that applied physiological mechanical loading in order to more closely mimic in vivo conditions. Findings demonstrated that scaffolds containing sulfated cellulose had higher TGF-β3 sequestration and promoted the greatest chondrogenesis over native GAGs in static conditions. The fully sulfated cellulose (NaCS), having the highest sequestration and the lowest release of TGF-β3, promoted the greatest MSC chondrogenesis with a reduction in hypertrophic markers in dynamic conditions. The potential mechanism was also elucidated by the expression of TGF-β3 associated receptors and phosphorylated Smad2/3.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Materials
Gelatin type B, from bovine skin (Sigma, St Louis, MO); chondroitin sulfate A sodium salt from bovine trachea (CS-A, Sigma, degree of sulfation was 0.32 sulfates/monosaccharide,23) chondroitin sulfate sodium salt from shark cartilage (CS-C, Sigma, degree of sulfation was 0.54 sulfates/monosaccharide23), heparin sodium salt from porcine intestinal mucosa (Heparin, Sigma, degree of sulfation was 1.2 sulfates/monosaccharide (value obtained from elemental analysis)), partially sulfated sodium cellulose (pSC, prepared as previously published, degree of sulfation was 0.5 sulfates/monosaccharide24) and fully sulfated sodium cellulose (NaCS, degree of sulfation was 2.3 sulfates/monosaccharide,23 Dextran products limited, Ontario, Canada); Ethanol (200 proof, Sigma), N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide (EDC) (Sigma), N-hydroxysulfosuccinimide (NHS) (Sigma); human bone marrow (Lonza, Walkersville, MD); tissue culture polystyrene flasks (NunC, Thermo Fisher Scientific, Waltham, MA); Dulbecco's Modified Eagle's Medium (DMEM, Thermo Fisher Scientific), fetal bovine serum (Hyclone, Thermo Fisher Scientific) and antibiotic-antimycotic (Thermo Fisher Scientific); trypsin (Thermo Fisher Scientific); polypropylene 96-well plates (Thermo Fisher Scientific); DMEM high glucose containing 4 mM L-Glutamine (Thermo Fisher Scientific), Dexamethasone (Sigma), Ascorbic acid-2-phosphate (Wako Chemicals, Richmond, VA), ITS+ Premix Culture Supplement (Corning, Teterboro, NJ), Sodium Pyruvate (Sigma), Proline (Sigma), Transforming Growth Factor – beta 3 (TGF-β3, ProSpec, Israel); Human TGF-beta 3 Duoset ELISA (R&D Systems, Minneapolis, MN); Quant-iT dsDNA Picogreen Assay Kit (Thermo Fisher Scientific); Papain (Sigma); Triton X-100 (Sigma); TE buffer (Thermo Fisher Scientific); paraformaldehyde (Sigma); donkey serum (Sigma); monoclonal rabbit anti-human collagen type II antibody (EMD Millipore, Burlington, MA), polyclonal rabbit anti-human aggrecan (Abcam, Cambridge, MA), polyclonal rabbit anti-Smad2 + anti-Smad3 antibody (anti-Smad2/3) (Abcam), polyclonal rabbit anti-Smad2 + anti-Smad3 (phospho T8) (anti-pSmad2/3) antibody (Abcam), donkey anti-rabbit Immunoglobin G conjugated to Alexa Fluor 488 (EMD Millipore); rhodamine phalloidin (Thermo Fisher Scientific); 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific); Dimethylmethylene blue (DMMB); QuantiTect SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany); RNeasy Micro Kit (Qiagen); Collagen type II ELISA kit (Chondrex, Redmond, WA); Collagen type I ELISA kit (Chondrex).
2.2 ∣. Fabrication and evaluation of TGF-β3 loaded scaffolds
Gelatin scaffolds containing 5% (w/w) GAGs and GAG mimetics were fabricated using the electrospinning technique, as previously described.24 Briefly, solutions containing 24 w/w% gelatin and different GAGs and GAG mimetics in deionized water (DI water) were prepared in a 60°C water bath. Also, depending on the ambient humidity and the solubility of the GAGs and GAG mimetics, the solvent may have included either 30% or 50% 200 proof ethanol. Modified electrospinning technique was used where the syringe containing gelatin solution was kept at 60°C using a heating chamber. 40 kV was applied to a 14-gauge needle attached to the syringe containing the solution—20 kV was applied to the flat collector, approximately 30 cm distance from the needle. The flow rate of the gelatin solution was maintained at 6.5 ml/h. Humidity of 25%–30% and temperature of 24°C were maintained in the electrospinning chamber throughout the process. Similar conditions were used to prepare all of the scaffolds. Scaffolds were cross-linked using N-(3-dimethyl aminopropyl)-N′-ethyl carbodiimide (EDC) with N-hydroxysucciamide (NHS). Scaffold groups consisted of Gelatin only (Gel), Gel-CS-A (Chondroitin-4-sulfate), Gel-CS-C (Chondroitin-6-sulfate), Gel-Heparin, Gel-pSC (partially sulfated cellulose) and Gel-NaCS (fully sulfated cellulose) groups. All materials characterization was performed, as previously described.24 Average fiber diameter and inter-fiber spacing for all scaffolds were approximately 3 and 30 μm, respectively. All scaffolds were sterilized in 200 proof ethanol prior to studies, as previously described.21,24
2.3 ∣. Sequestering lysozyme as a model protein
To visualize the sequestering of protein, lysozyme was initially investigated as a model protein, following a previously reported protocol.25 Briefly, scaffolds were incubated in 200 units/ml of lysozyme (Chicken Lysozyme, Sigma) in 0.1% BSA (Millipore, IL) for 24 h. After incubation, the scaffolds were washed twice with sterile 1X DPBS and then fixed using 4% PFA for 20 min followed by blocking with 5% goat serum for 1 h. The samples were incubated with a primary antibody polyclonal rabbit antilysozyme (Thermo Fisher Scientific) overnight at 4°C, followed by a secondary antibody Alexa 594 goat anti-rabbit (Thermo Fisher Scientific) for 1 h. Scaffolds were viewed using a confocal microscope (Nikon, C1Si, Melville, NY) at 60X magnification.
2.4 ∣. TGF-β3 sequestration kinetics
The sequestration kinetics of TGF-β3 on the GAG containing scaffolds was evaluated by the pull down sequestration assay26 where scaffolds were incubated in a known concentration of TGF-β3 and the amount of TGF-β3 remaining in the solution was measured as an indirect measure of the amount loaded on the scaffold over time. Gelatin scaffold without GAG was used as a control. The scaffolds were incubated in 100 ng/ml TGF-β3 solution in 0.1% BSA (10 ng TGF-β3/scaffold or 1.76 ng/mm3) for 1 min, 10 min, 30 min, 1 h, 2 h, 6 h, and 8 h (n = 5 per group per time point). At each time point, the amount of TGF- β3 remaining in the solution was measured using Duoset Development ELISA kit for human TGF-β3 according to the manufacturer's protocol. The amount obtained at each time point was subtracted from the initial TGF- β3 amount in the solution to get an indirect measure of TGF- β3 on the scaffolds.
Exponential one-phase association equation was used to solve for the association rate constant (Ka). The equation used was:
Where Y is the amount of TGF- β3 on the scaffold (ng). Ymax is the amount of TGF- β3 on the scaffold at equilibrium (ng). K is the association rate constant or Ka (min−1) and x is time (min). Ka was calculated using GraphPad Prism software (GraphPad Software, San Diego, CA).
2.5 ∣. TGF-β3 release
In order to ascertain the amount of growth factor retained in the scaffold, the release was measured over time. The scaffolds were loaded with TGF-β3 by incubating in the 100 ng/ml TGF-β3 solution (10 ng TGF-β3/scaffold or 1.76 ng/mm3) for 6 h, which was the time required to reach equilibrium/Ymax as mentioned in section 2.4. Scaffolds were then incubated in chondrogenic induction media without TGF-β3 (CCM−, which consisted of high-glucose DMEM supplemented with 1 mM sodium pyruvate, 0.35 mM L-proline, 4 mM L-glutamine, 1% ITS-Premix, and 1% antibiotic-antimycotic) for 4 weeks. The media was collected at days 1, 2, 4, 10, 14, 21, and 28 days (n = 5 per group per time point). After day 28, any remaining TGF-β3 was extracted from the scaffolds by homogenizing them in Tris/CHAPs (50 mM Tris-HCl, 0.5% CHAPS, 150 mM NaCl at pH 7.2) buffer, as previously described.21 The cumulative percentage of TGF-β3 was determined as the sum of the total TGF-β3 released over time divided by the total amount of TGF-β3 loaded on the scaffold. The total amount of TGF-β3 loaded was calculated as the sum of the total TGF-β3 released in the media plus the remaining TGF-β3 on the scaffold. The amount of TGF-β3 was determined using Duoset Development ELISA for human-TGF-β3.
2.6 ∣. Evaluation of in vitro chondrogenesis
2.6.1 ∣. Isolation and culture of MSCs
Mesenchymal stem cells were isolated from commercially available human bone marrow aspirated from the iliac crest of male and female donors ranging from ages 18–30 (Lonza Biosciences, MD), according to previous published protocols and cryopreserved before use.27 Briefly, marrow samples were fractionated by centrifugation over a density cushion and plated on tissue culture flasks in general media (GM) comprised of Dubelcco's Modified Eagle's Medium (DMEM), 10% fetal bovine serum (FBS) and 1% 1× antibiotic-antimycotic (Gibco, Carlsbad, CA). Colony formation was monitored for a 14–17 day period and then, cells were subcultured. MSCs were characterized for morphology and for the expression of cell surface markers CD44, CD29, and CD90 and lack of expression for CD14 and CD45. Multipotency was characterized by differentiation assays, osteogenesis, chondrogenesis and adipogenesis, using established protocols. Cryopreserved MSCs were thawed and expanded in GM until 70%–80% confluent. MSCs were used at passage 3 for all studies.
2.6.2 ∣. TGF-β3 loading onto scaffolds
All scaffolds were incubated with 100 ng/ml TGF-β3 in 0.1% BSA (10 ng TGF-β3/scaffold or 1.76 ng/mm3) for 6 h to achieve the maximum amount of TGF-β3 on the scaffold (Ymax). Scaffolds were then washed with 1× DPBS three times before cell seeding. Control scaffolds without TGF-β3 were incubated with 0.1% BSA.
2.6.3 ∣. Chondrogenic differentiation
Scaffolds were inserted into low attachment polypropylene plates (96 well-plates, Non-treated, Corning, UT) for 6 mm disks or ultra-low attachment 24 well-plates (Corning, UT) for 12 mm disks. 6 mm disks were used for all assays except for the DMMB assay and the ELISAs for collagens type I and II, which used 12 mm disks. MSCs were seeded on scaffolds at 500,000 cells/ml and cultured in CCM− media. The samples were collected at days 1, 4, 7, 14, and 28 for assays. The scaffolds without loaded TGF-β3 and cultured in CCM− was considered as a control group.
Cell growth and chondrogenic differentiation were performed using the following techniques as previously described.24 Briefly, cell growth was evaluated using the picogreen assay. Chondrogenic gene expression were studied on TGF-β3 loaded scaffolds using qRT-PCR for expression of matrix metalloproteinase 2 (MMP2), SRY-Box transcription factor 9 (Sox9), collagen type I alpha 1 (Col I), collagen type II alpha 1 (Col II), aggrecan (Agg), chondroadherin (CHAD), collagen type X (Col X) and VEGF. The values obtained were normalized to housekeeping gene ribosomal protein, large, PO (RPLPO). Production of sulfated GAGs was evaluated using the DMMB assay. The amount of sulfated GAGs produced was determined by subtracting the amount of sulfated GAGs in the scaffolds cultured without cells from the amount of sulfated GAGs in the samples cultured with cells. Immunofluorescence staining was used to detect collagen type II and aggrecan. ELISA was used to determine production of collagen types I and II in culture at day 28. Unless otherwise noted, an n of 3 or 4 per group per time point was used for each quantitative assay.
2.6.4 ∣. Evaluation of TGF-β/Smad signaling
Scaffolds made up of gelatin and gelatin containing pSC and NaCS with and without loaded TGF-β3 were evaluated for TGF-β/Smad signaling. MSCs were cultured on these scaffolds in CCM− media with or without 5 μM SB 431542 (Insolution TGF- β RI kinase inhibitor VI, EMD Millipore, Burlington, MA) for 7 days. The cultures were immunostained with polyclonal rabbit anti- Smad2/3 antibody and polyclonal rabbit anti- pSmad2/3 antibody. The nucleus was counter-stained blue using DAPI.
The western blot technique was also used to analyze the TGF-β signaling on the cellulose sulfate containing scaffolds. After 7 days of culture, cells were lysed using RIPA buffer (Pierce, Rockford, IL), 0.1% protease inhibitor cocktail (Thermo Scientific), 0.1% phosphatase inhibitor cocktail 2 (Sigma). A series of freeze thaw cycles were used to facilitate cell lysis in order to obtain intracellular proteins. The samples were centrifuged, and lysate was analyzed for total protein extraction using micro BCA™ protein assay kit (Thermo Fisher Scientific). Approximately 15 μg/ml protein content from each lysate was loaded in 12% (w/v) sodium dodecyl sulfate polyacrylamide gels followed by electrophoresis for separation of proteins. The proteins were then transferred on polyvinylidene difluoride (PVDF) membranes (Perkin Elmer Life Sciences, Boston, MA). The membranes were blocked using 5% Fraction V BSA (Hyclone, Thermo Fisher Scientific) in 0.1% Tween 20 and 1X DPBS. This was followed by overnight incubation of membranes with primary antibodies at dilutions of 1:1000 at 4°C. After incubation, the membranes were washed with 0.1% Tween 20 and 1X DPBS followed by incubation with 1:2000 dilution of HRP conjugated secondary antibody. Enhanced chemiluminescence (ECL) reagent (Pierce) was used to develop the membrane. The membrane was imaged using Chemidoc XRS+ (Bio Rad, Hercules, CA). The primary antibodies used were polyclonal rabbit anti-pSmad2/3 and polyclonal rabbit anti-Smad2/3 antibody. The membranes were stripped with Restore stripping buffer (Pierce) between each primary antibody. The secondary antibody used was anti- rabbit HRP conjugated IgG (Thermo Fisher Scientific). The phosphorylation levels were analyzed by measuring the intensity of pSmad2/3 to total intensity of Smad2/3.
2.7 ∣. Evaluation of in vitro chondrogenesis under dynamic loading conditions
To further evaluate MSC chondrogenesis on gelatin scaffolds containing pSC and NaCS loaded with TGF-β3, studies were conducted in bioreactors where constructs were subjected to dynamic loading to more closely represent physiological conditions. Gelatin only scaffolds with loaded TGF-β3 were used as control. TGF-β3 was loaded onto each 6 mm diameter × 3 mm height scaffold by incubating each scaffold at room temperature for 6 h in 500 μl 0.1% BSA solution containing 150 ng TGF-β3 (or 1.76 ng TGF-β3/mm3 for each scaffold). After incubation, scaffolds were rinsed with sterile PBS to remove any unbound TGF-β3. The cells were seeded at density of 1 × 106 cells per scaffold (or approx. 12,000 cells/mm3) and were seeded using a vacuum loading seeding technique, as previously described.28 This cell density is similar to native cartilage.29 The cells were cultured on the scaffolds in static conditions for 14 days at 37°C, 5% CO2 and 85% humidity with 500 μl CCM− media changed every 3–4 days prior to transferring in the bioreactor.
The C9x-Cartigen bioreactor (Tissue Growth Technology, TGT, Minnetonka, MN) was used for this study. This bioreactor can apply cyclic unconfined compression along with perfusion.28,30,31 The bioreactor set up included computer controlled (GrowthWorks software) force sensor-load cell (FUTEK, CA), nine-well chamber, peristaltic pump (Masterflex L/S, Cole palmer) for perfusion of media through the porous sample at a constant flow rate. After 14 days of culture in static conditions, cell-containing scaffolds were transferred to the bioreactor and cultured in CCM−. The samples were subjected to 10% dynamic compressive strain using a sinusoidal waveform at 1 Hz for total of 3 h (one-hour break between each loading hour) for 5 days per week along with continuous perfusion at a flow rate of 0.5 ml/min. The samples were maintained in these conditions for a total of 14 days in the bioreactor. All studies were performed in the incubator at 37°C, 5% CO2. The samples were collected at Days 14, 21 (7 days after dynamic loading culture condition) and Day 28 (14 days after dynamic loading culture condition) for tissue analysis. n = 3 per group per time point for tissue analysis.
Cell number and gene expression for chondrogenic and hypertrophic markers of the cells on the scaffolds were evaluated at days 0 and 14 (in static culture) and days 21 and 28 (7 and 14 days of dynamic culture) using the picogreen assay and qRT-PCR, respectively, using methods as previously described.24,28 Briefly, mRNA was extracted using RNAeasy kit. mRNA expression for Col I, Col II, Sox9, Agg, CHAD, Col X, VEGF and MMP13 were determined and normalized to the housekeeping gene ribosomal protein, large, PO (RPLPO). Immunostaining was performed for Col I, and Col II and Agg at days 14, 21, and 28, as previously described24 and imaged using confocal microscopy. Protein production for Col I, Col II and Agg was evaluated using ELISA techniques at days 14, 21, and 28. Samples were processed according to the manufacturer's protocols. Briefly, the samples were treated with 3 M guanidine chloride, which is known to solubilize aggrecan, followed by treatment with acetic acid, pepsin and elastase to solubilize collagen from the ECM of cultured cells.
2.8 ∣. Statistical analysis
Statistical analysis was performed using multi-factorial two-way analysis of variance (ANOVA) to determine significant differences between groups and time (p < .05). Shapiro Wilk test and Levene's equal variance test was used to determine the normality. The multiple comparisons within groups were made using Tukey's posthoc test (p < .05). All the statistical analyses were performed using GraphPad Prism software (La Jolla, CA). Graphs were plotted using either Microsoft excel, Origin or GraphPad Prism software. All the values are reported as Mean ± SD.
3 ∣. RESULTS
3.1 ∣. Sequestering lysozyme
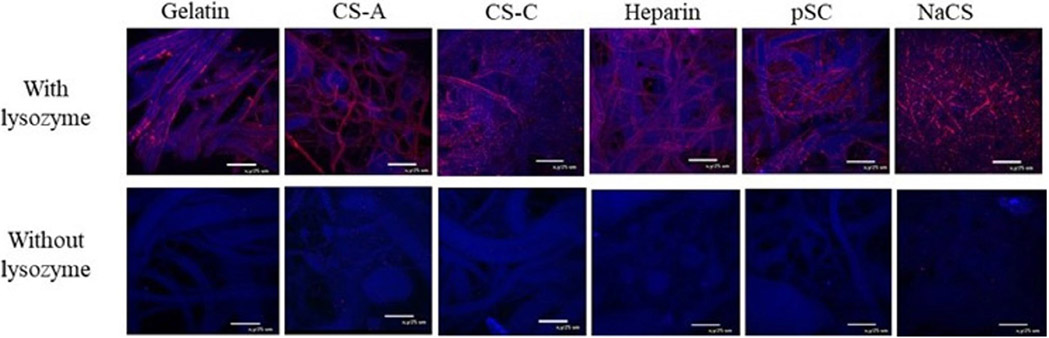
To visualize the sequestering of protein, lysozyme was initially investigated as a model protein. Figure 1 shows confocal images of scaffolds loaded with or without lysozyme. Gelatin only scaffolds appeared to have the least amount of lysozyme staining in comparison to native GAGs and sulfated cellulose containing scaffolds. Gelatin containing NaCS showed the most pronounced staining for lysozyme. Samples without lysozyme incubation had no staining for lysozyme.
FIGURE 1.
Confocal images of gelatin and gelatin scaffolds containing native GAGs and cellulose sulfate that were incubated with or without lysozyme-containing solution. Lysozyme (red) and scaffold fibers (blue), 60× magnification, 25 μm scale bar
3.2 ∣. TGF-β3 sequestration kinetics
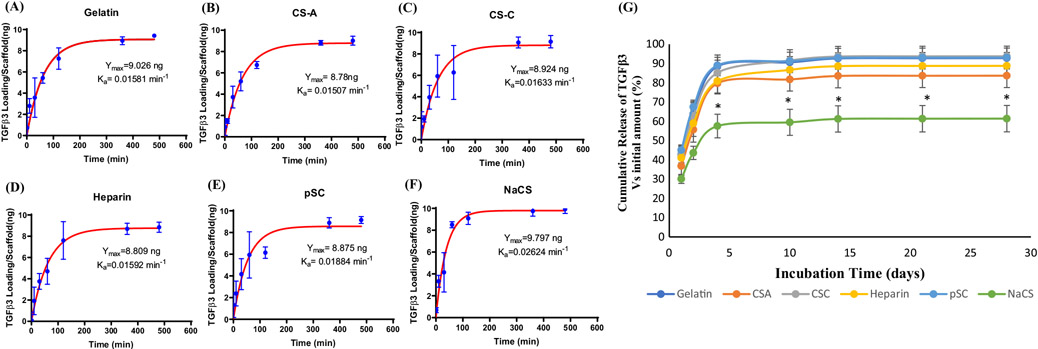
The sequestration/association rate, Ka, of the growth factor TGF-β3 was determined for gelatin scaffolds and gelatin scaffolds containing CS-A, CS-C, heparin, pSC, NaCS (Figure 2A-F). All scaffolds reached the maximum amount of TGF-β3 or equilibrium (Ymax) at around 6 h of incubation in TGF-β3 solution with a Ymax of approximately 9–10 ng of TGF-β3. The highest Ka was detected on scaffolds containing NaCS (0.0262 min1) followed by pSC (0.0188 min−1). Gelatin was also able to sequester TGF-β3 at Ka of 0.0158 min−1. Among the native GAGs, CS-C containing scaffolds had the highest Ka (0.0163 min−1) compared to gelatin. Scaffolds containing CS-A and heparin had Ka (0.0151 min−1 and 0.0159 min−1 respectively) similar to gelatin.
FIGURE 2.
Sequestration of TGF-β3 as a function of incubation time for (A) gelatin scaffolds and gelatin scaffolds containing (B) CS-A, (C) CS-C, (D) Heparin, (E) pSC and (F) NaCS (Values are Mean ± SD, n = 5 per group per time point). Ymax and Ka represent TGF-β3 at equilibria and association rate, respectively. (G) Cumulative release of TGF-β3 over 28 days in culture conditions from gelatin and gelatin containing CS-A, CS-C, Heparin, pSC and NaCS. (Values are Mean ± SD). *Significantly different from all other groups for each time point (p < .05, n = 5 per group per time point)
3.3 ∣. TGF-β3 release
A burst release of TGF-β3 was detected for all scaffolds within 4 days in media (Figure 2G). Gelatin and gelatin scaffolds containing CS-A, CS-C, heparin, and pSC showed similar trends in release profile with approximately 85% of TGF-β3 released by day 4. The lowest release of TGF-β3 was detected on scaffolds containing NaCS with approximately 55% cumulative release of TGF-β3 by day 4 with approximately 40% of TGF-β3 still remaining on the scaffold by day 28 (Figure S1).
3.4 ∣. Evaluation of in vitro chondrogenesis
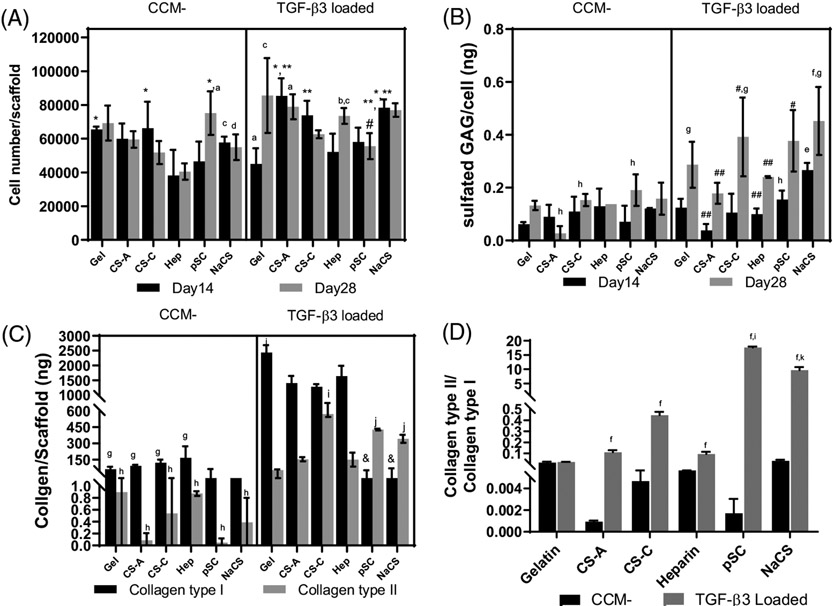
Higher cell numbers were detected on gelatin scaffolds and gelatin scaffolds containing CS-A, heparin and NaCS in TGF-β3 loaded groups in comparison to groups without TGF-β3 (Figure 3A). NaCS containing gelatin scaffolds loaded with TGF-β3 had higher cell number at both time points in comparison to NaCS containing gelatin scaffold without TGF-β3. The lowest cell number was determined for heparin containing gelatin scaffolds without TGF-β3 at both 14 and 28 days in comparison to all groups with or without TGF-β3. The sulfated GAGs produced per cell increased significantly by day 28 for all scaffolds loaded with TGF-β3 in comparison to all scaffolds without growth factor (Figure 3B). The highest GAG production per cell was determined for NaCS containing gelatin scaffolds loaded with TGF-β3 on day 14 in comparison to all other groups with or without TGF-β3 and at day 28 in comparison to all groups without TGF-β3.
FIGURE 3.
Cells cultured on gelatin (Gel) and gelatin scaffolds containing CS-A, CS-C, Heparin (Hep), pSC and NaCS with TGF-β3 loaded onto scaffolds cultured in CCM− media (TGF-β3 loaded) or without TGF-β3 (CCM−). (A) Cell number. (B) Sulfated GAGs produced per cell. (C) Production of collagens type I and II. (D) Ratio of collagen types II to I. *Significantly different from heparin at same time point and loading condition, **Significantly different from gelatin at same time point and loading condition, #Significantly different from CS-A at same time point and loading condition, ##Significantly different than NaCS group at same time point and loading condition, &Significantly lower than all scaffolds loaded with TGF-β3, aSignificantly different from CCM− for corresponding scaffold groups at day 14, bSignificantly different from CCM− for corresponding scaffold groups at day 28, cSignificantly different from corresponding scaffold groups loaded with TGF-β3 at day 14, dSignificantly different from corresponding scaffold groups loaded with TGF-β3 at day 28. (p < .05), eSignificantly different from CCM− for all scaffold groups at day 14, fSignificantly different from CCM− for all scaffold groups at day 28, gSignificantly different from all scaffold groups loaded with TGF-β3 at day 14, hSignificantly different from all scaffold groups loaded with TGF-β3 at day 28, iSignificantly higher than all groups with and without TGF-β3 loading, jSignificantly higher than Gel and CS-A and Hep groups with and without TGF-β3 loading, kSignificantly higher than gel and native GAG-containing scaffolds in both conditions (p < .05). (All values are Mean ± SD, n = 4 per group per time point for cell number and GAG production, n = 3 per group per time point for collagen production)
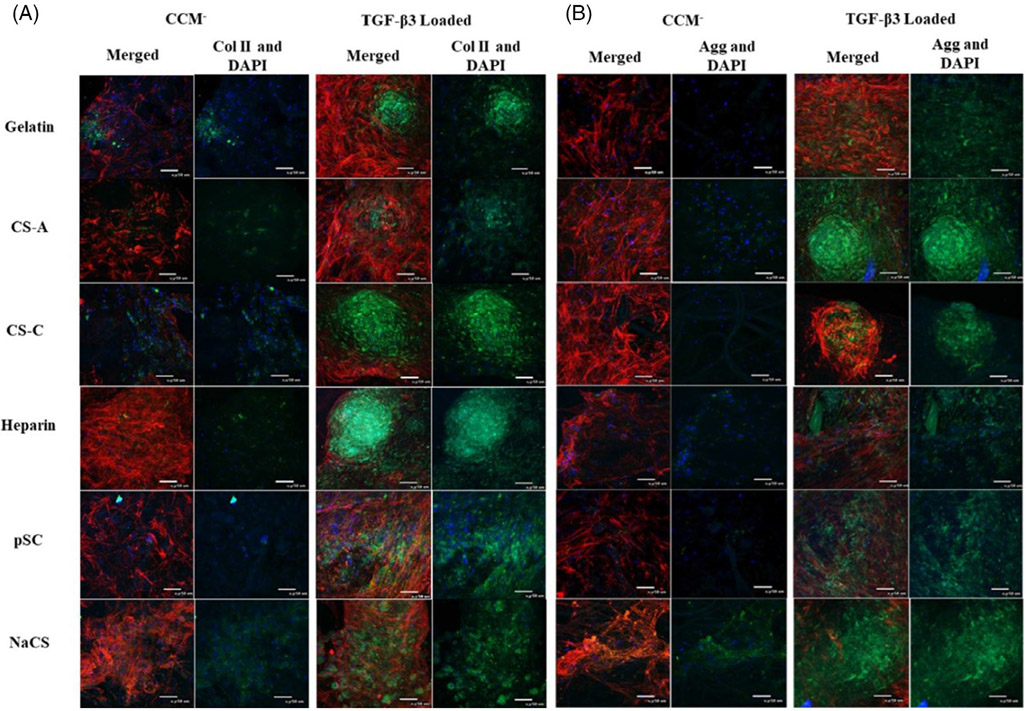
Immunofluorescent staining for collagen type II and aggrecan (Figure 4) showed enhanced staining for both chondrogenic proteins in all scaffolds loaded with TGF-β3 in comparison to scaffolds without TGF-β3. NaCS and pSC-containing scaffolds loaded with TGF-β3 appeared to have a more uniform distribution of immunostaining for collagen type II and aggrecan. Immunostaining for collagen type II and aggrecan for the scaffolds containing native GAGs loaded with TGF-β3 appeared more localized within the aggregates formed on the scaffolds. Collagen types I and II production as measured by ELISA by cells on all scaffolds without TGF-β3 was lower in comparison to scaffolds loaded with TGF-β3 (Figure 3C). Ratio of collagen type II to I was highest for pSC followed by NaCS-containing scaffolds loaded with TGF-β3 in comparison to all other groups (Figure 3D). Highest collagen I was produced by cells cultured on gelatin scaffolds loaded with TGF-β3 followed by scaffolds containing native GAGs. Cells cultured on pSC and NaCS containing scaffolds loaded with TGF-β3 showed the least production of collagen I, whereas production of collagen type II was higher for gelatin, CS-A and heparin containing gelatin scaffold groups.
FIGURE 4.
Confocal images of cells cultured on gelatin and gelatin scaffolds containing native GAGs and cellulose sulfate without TGF-β3 (CCM−) or with TGF-β3 loading (TGF-β3 loaded) for 28 days in CCM−. Immunostaining for (A) collagen type II (green) and (B) aggrecan (green). Actin (red) and nucleus (blue) for both A and B panels (40× magnification, scale bar is 50 μm)
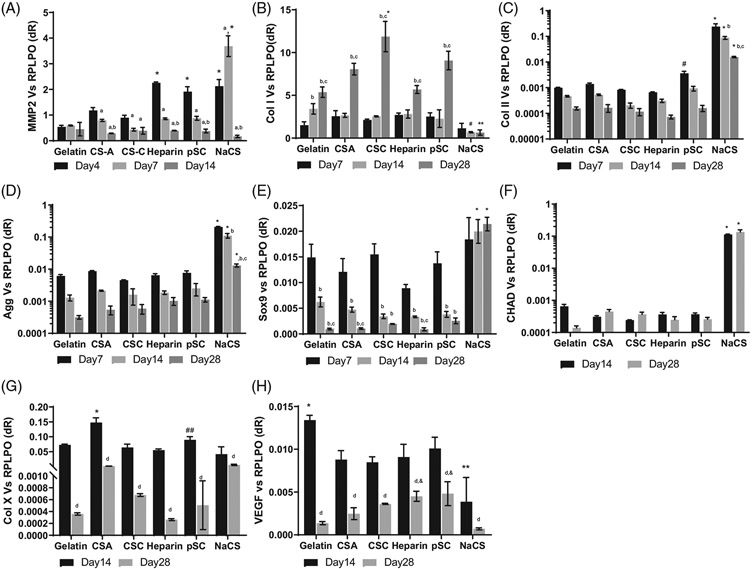
Gene expression for cells cultured on TGF- β3 loaded scaffolds was evaluated for mesenchymal markers MMP2, Col I, chondrogenic markers Sox 9, Col II, Agg, CHAD and hypertrophic markers Col X and VEGF (Figure 5). Expression of MMP2 was evaluated as an early chondrogenic marker. MSCs secrete MMP2 to allow for the remodeling of the initial matrix.32 Expression of MMP2 was highest for gelatin scaffold containing NaCS group. Expression of MMP2 reduced by day 14 for all scaffold groups. Col I expression was higher by day 28 for all scaffolds except for the NaCS scaffold group. Highest Col I expression was seen for cells on gelatin containing CS-C scaffolds. Col I expression was the least for cells on NaCS scaffolds at all time points. Sox9 expression for cells on all scaffolds except for NaCS containing scaffolds showed a reduction with time. Cells cultured on NaCS containing scaffolds showed a high expression of Sox 9 at all time points. Expression of the Col II gene was the highest for cells cultured on NaCS containing scaffolds and decreased from days 7 to 28 for all scaffold groups. Same trend was detected for aggrecan expression. The highest expression of CHAD was detected for cells cultured on NaCS containing scaffolds. Expression of hypertrophic markers Col X and VEGF was lower at day 28 in comparison to day 14 for all scaffold groups. Higher expression of Col X was detected for cells cultured on CS-A containing scaffolds as compared to all groups at day 14. Highest expression of VEGF was detected for cells cultured on gelatin only scaffolds and the least, or negligible levels of VEGF expression was detected for cells on NaCS containing scaffolds at day 14.
FIGURE 5.
Gene expression for cells on gelatin and gelatin containing native GAGs and cellulose sulfate scaffolds loaded with TGF-β3 in CCM− media for 28 days. (A) MMP2, (B) Collagen I (Col I), (C) Collagen type II (Col II), (D) Aggrecan (Agg), (E) Sox 9, (F) Chondroadherin (CHAD), (G) Collagen type X (Col X), and (H) VEGF. *Significantly higher than all other groups at the time point, **Significantly lower than all other scaffolds at same time point, #Significantly different than gelatin and native GAGs groups, ##Significantly higher than CS-C, heparin and NaCS scaffold groups, &Significantly higher than NaCS scaffold group aSignificantly different than day 4, bSignificantly different than day 7, cSignificantly different than day 14, dSignificantly different from day 14 in same scaffold group (p < .05). All values are normalized to RPLPO represent Mean ± SD (n = 3 per group per time point)
3.5 ∣. Evaluation of TGF-β/Smad signaling
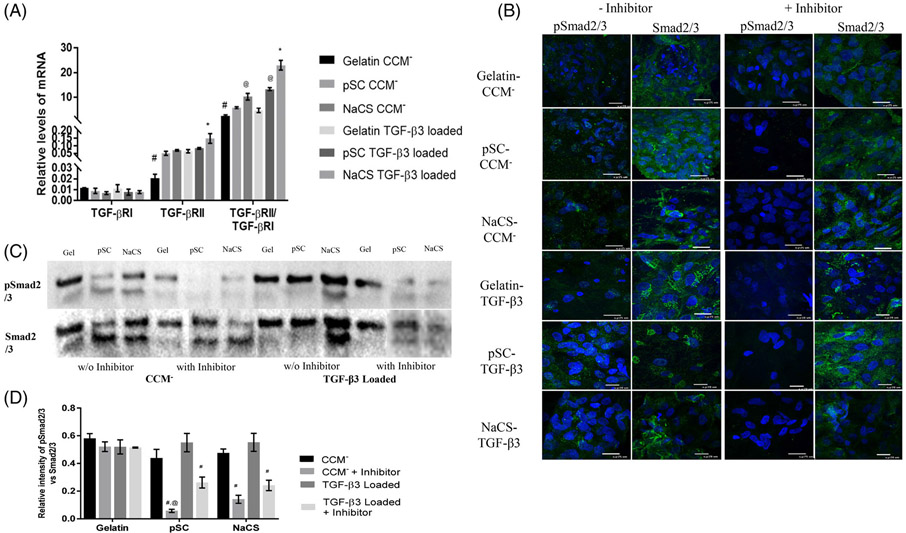
The TGF- β3/Smad signaling pathway was evaluated for cells cultured on gelatin and pSC and NaCS containing gelatin scaffolds loaded with and without TGF- β3 in CCM− media condition for 7 days. Gene expression for TGF-β receptors, TGF- βRI and TGF- βRII, were evaluated. Later, the cultures were subjected to TGF- βRI kinase inhibitor which blocks the downstream process of TGF- β/Smad2/3 signaling to assess the role of TGF- β/Smad2/3 signaling for cells on cellulose sulfate scaffolds.
Gene expression for TGF- βRI was similar for cells cultured on all scaffolds regardless of the presence of TGF-β3 (Figure 6A). Significant difference in TGF-βRII expression was determined where the least was detected for cells cultured on gelatin only scaffolds without TGF- β3. Cells cultured on NaCS containing gelatin scaffolds loaded with TGF- β3 showed enhanced expression of TGF- βRII. The ratio of TGF- βRII to TGF- βRI increased with the addition of TGF- β3 for all scaffold groups. The highest ratio was determined on scaffolds containing NaCS loaded with TGF- β3 followed by pSC containing scaffold loaded with TGF- β3. The least ratio was observed on cells cultured on gelatin only scaffolds without TGF-β3. Cells cultured on NaCS scaffolds without TGF- β3 also showed a higher ratio of TGF-βRII to TGF-βRI in comparison to gelatin and pSC scaffolds without loaded TGF-β3. Immunostaining for Smad2/3 revealed the presence of Smad2/3 for cells cultured on all the scaffolds with or without the presence of TGF- βRI kinase inhibitor (Figure 6B). A stronger immunostaining appeared for pSmad2/3 for cells cultured on scaffolds containing pSC and NaCS as compared to gelatin only scaffolds loaded with TGF-β3. However, pSmad2/3 staining was weak or not present for cells cultured on all scaffolds in the presence of the inhibitor. By western blot (Figure 6C,D), the phosphorylation of Smad2/3 was reduced in the case of pSC and NaCS containing scaffold groups in the presence of inhibitor with or without TGF- β3 loading. For cells on gelatin scaffolds, pSmad2/3 levels did not reduce in the presence of the TGF- βRI kinase inhibitor, indicating smad2/3 signaling is not dependent on TGF-β receptors.
FIGURE 6.
(A) Gene expression of TGF- βRI and TGF- βRII normalized to RPLPO and ratio of TGF-βRII to TGF-βRI for cells cultured on gelatin and gelatin containing cellulose sulfate scaffolds with and without TGF- β3 loading. (Values are Mean ± SD, n = 3 per group) *Significantly higher than all other scaffolds in both conditions. #Significantly lower than all other scaffolds with and without TGF- β3 loading. @Significantly higher than pSC containing scaffolds without TGF- β3 loading. (p < .05) (B) Immunofluorescent staining of Smad2/3 (green) and pSmad2/3 (green) for cells cultured on gelatin and gelatin containing cellulose sulfate scaffolds loaded with and without TGF- β3 in presence or absence of TGF- βRI kinase inhibitor. Nuclei (DAPI, Blue). 100× Magnification (C) Western blot for protein expression of pSmad2/3 and total Smad2/3 (D) Relative intensity of pSmad2/3 normalized to Smad2/3 for cells cultured on gelatin and gelatin containing cellulose sulfate scaffolds with and without loaded TGF-β3 in presence or absence of TGF- βRI kinase inhibitor. (Values are Mean ± SD, n = 3 per group) #Significantly lower than cells cultured on the same scaffold w/o inhibitor. @Significantly lower than cells cultured on TGF- β3 loaded scaffold with inhibitor. (p < .05)
3.6 ∣. Evaluation of in vitro chondrogenesis under dynamic loading conditions
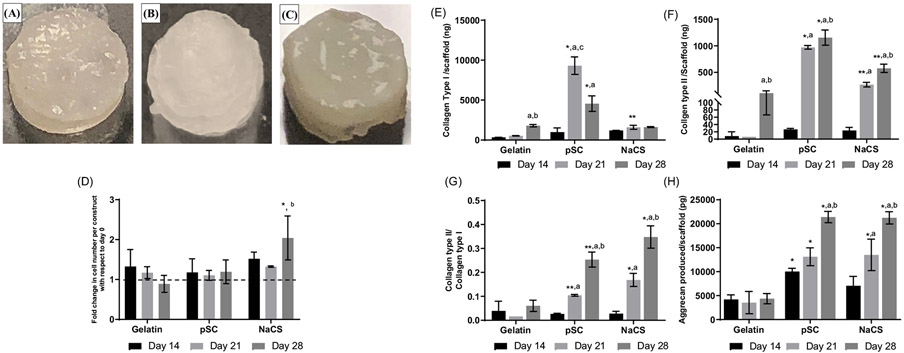
The cells were cultured on the gelatin and gelatin containing pSC and NaCS scaffolds with loaded TGF-β3 in static conditions for 14 days, and then were placed in the bioreactor for 14 days where they were subjected to dynamic compression with perfusion (for a total of 28 days in culture). A shiny tissue was present on all scaffolds (Figure 7A-C). No statistical differences in cell numbers were detected over time for cells on gelatin scaffolds and pSC containing scaffolds by day 28 (Figure 7D). However, a significant increase in cell number was determined for cells on scaffolds containing NaCS by day 28 in comparison to day 0 (Figure 7D).
FIGURE 7.
Cells cultured on scaffolds loaded with TGF-β3 and subjected to dynamic loading conditions in the bioreactor (a total of 28 days in culture - 14 days of static culture followed by 14 days of dynamic loading with perfusion) on (A) gelatin, (B) gelatin containing pSC scaffolds, and (C) gelatin containing NaCS scaffolds. Cells cultured on gelatin and gelatin scaffolds containing pSC and NaCS loaded with TGF-β3 in bioreactor. (D) Fold change in cell number with respect to day 0. (E) Collagen type I (Col I). (F) Collagen type II (Col II). (G) Ratio of collagen types II to I. (H) Aggrecan (Agg). *Significantly different than all other scaffolds at same time point, **Significantly higher than gelatin scaffold groups at same time point, aSignificantly different than day 14 in same scaffold group, bSignificantly different than day 21 in same scaffold group, cSignificantly different than day 28 in same scaffold group. (p < .05) (Values are Mean ± SD, n = 3 per group per time point)
Production of Col I was significantly higher on tissue constructs containing pSC after 21 days of culture, whereas a significant decrease could be observed by day 28 (Figure 7E). In the case of gelatin and NaCS containing tissue constructs, they showed lower collagen type I production throughout the culture. The production of Col II was highest for cells on pSC containing scaffolds at days 21 and 28 (Figure 7F). Tissue constructs containing NaCS scaffolds also had high production of Col II in comparison to gelatin only scaffolds at days 21 and 28. The ratio of Col II to I was determined where the highest ratio was for tissue constructs consisting of the NaCS scaffolds at day 28 (Figure 7G). Cells cultured on pSC scaffolds also had a higher ratio of Col II to I than gelatin only scaffolds. An increase in the ratio of Col II to Col I could be detected for the cellulose sulfated groups from days 14 to 28, when dynamic compressive loading was applied. Agg production was highest at days 21 and 28 for pSC and NaCS containing scaffold groups as compared to the gelatin only group (Figure 7H).
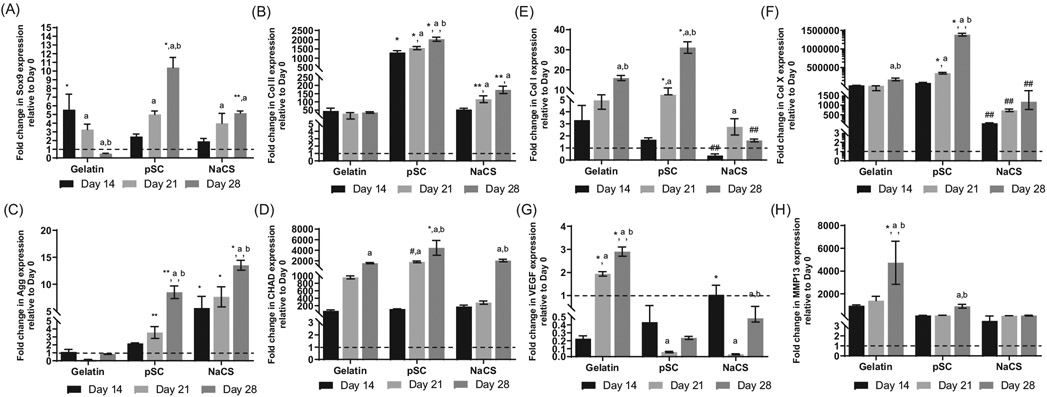
Gene expression for chondrogenic and hypertrophic markers were examined for up to 28 days (14 days in static culture followed by 14 days in the bioreactor). Sox9 was significantly higher for cells cultured on pSC and NaCS as compared to gelatin only scaffold groups at day 28 (Figure 8A). Sox9 expression decreased for gelatin containing scaffolds by day 28. High expression of Col II was determined for cells cultured on pSC and NaCS containing scaffold groups, with the highest for pSC group as compared to the gelatin only group (Figure 8B). Expression of aggrecan (Agg) was also enhanced for pSC and NaCS as compared to gelatin only groups for both days 21 and with the highest expression for the NaCS group at day 28 (Figure 8C). The expression of Agg was low for the gelatin group throughout the culture. CHAD expression was expressed for all groups by day 28 with significantly higher levels for cells cultured on the pSC containing scaffold as compared to other groups (Figure 8D). Col I was significantly higher for cells cultured on pSC containing scaffolds for both days 21 and 28, with the highest level at day 28 as compared to all other groups (Figure 8E). The least expression of Col I was for the NaCS group. The highest expression of Col X was for cells cultured on pSC containing gelatin scaffolds by day 28 (Figure 8F). Lowest expression of Col X was detected for cells cultured on NaCS containing scaffolds. High expression for VEGF was detected for cells cultured on gelatin only scaffolds at days 21 and 28 (Figure 8G). An additional hypertrophy marker MMP13 was evaluated Expression of MMP13, which degrades cartilage matrix collagens and proteoglycans, was low for cells cultured on pSC and NaCS containing scaffolds in comparison to cells cultured on gelatin only scaffolds at day 28 (Figure 8H).
FIGURE 8.
Gene expression for cells cultured on scaffolds loaded with TGF-β3 and subjected to dynamic loading conditions in the bioreactor (a total of 28 days in culture - 14 days of static culture followed by 14 days of dynamic loading with perfusion) normalized to day 0 (after 14 days in static culture). (A) Sox9. (B) Collagen II (Col II). (C) Aggrecan (Agg). (D) Chondroadherin (CHAD). (E) Collagen I (Col I). (F) Collagen X (Col X). (G) VEGF. (H) MMP13. *Significantly higher than all other scaffold groups at same time point, **Significantly higher than gelatin group at the same time point, #Significantly higher than NaCS group at the same time point, ##Significantly lower than all other scaffolds at the same time point, aSignificantly different than day 14 in same scaffold group, bSignificantly different than day 21 in same scaffold group (p < .05). (Values are Mean ± SD, n = 3 per group per time point). Gene expression at each time point was normalized to gene expression at day 0
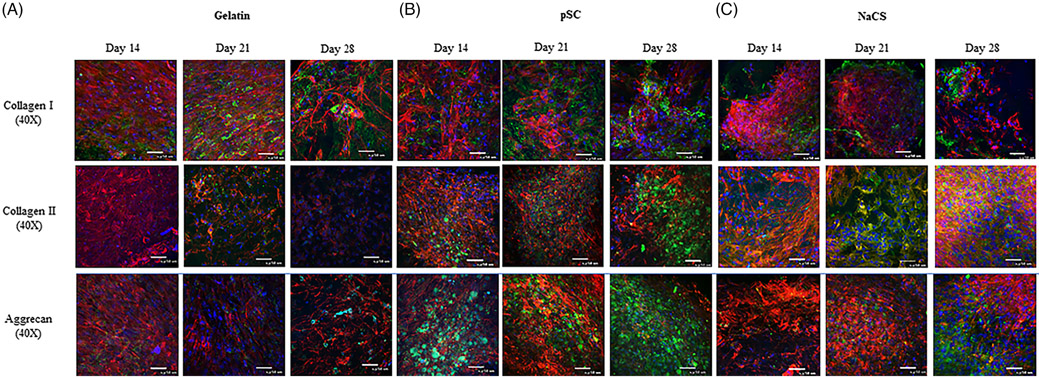
Immunofluorescent staining (Figure 9) for chondrogenic matrix proteins Col II and Agg could be observed, with greater intensity after day 14. Whereas, Col I could be detected throughout the culture. The more intense immunostaining for Col I, Col II and Agg could be observed at all time points for the pSC group. A stronger staining for Col II and Agg appeared to be for cells on NaCS scaffolds by day 28 as compared to gelatin only scaffold. Col I appeared less detectable for cells on NaCS scaffolds in comparison to gelatin and gelatin containing pSC scaffolds.
FIGURE 9.
Immunofluoroscent confocal images of cells grown on (A) gelatin, (B) gelatin containing pSC, and (C) gelatin containing NaCS scaffolds loaded with TGF-β3 and cultured in the bioreactor for 28 days. Collagen type I, collagen type II and aggrecan (Green). F-actin (Red) and nucleus (Blue) (scale bar = 50 μm)
4 ∣. DISCUSSION
The aim of this study was to evaluate the chondroinductive properties of fibrous gelatin scaffolds containing GAG mimetics–cellulose sulfate pSC and NaCS–in comparison to native GAGs CS-A, CS-C, and heparin. All scaffolds were loaded with TGF- β3 and evaluated for MSC chondrogenesis. Scaffolds were designed in order to mimic the native ECM of articular cartilage tissue where the bioactivity of growth factors is regulated by sequestering via GAGs, proteoglycans and glycoproteins found in the ECM.15,33,34 This study revealed that cellulose sulfate containing scaffolds were able to sequester TGF- β3 and enhance MSC chondrogenic differentiation over native GAGs. Moreover, in dynamic conditions, NaCS, having a higher degree of sulfation (DOS) than pSC, promoted the greatest chondrogenic differentiation as determined by a higher ratio of collagen type II to I ratio and lower gene expression for hypertrophic markers. The ratio of collagen type II to I is used as a measure of the differentiation of precursor cells into mature chondrocytes.35,36 MSCs secrete collagen type I and therefore, a higher production of collagen type II over collagen type I indicates a later stage of chondrogenic differentiation.21 The ratio of collagen type II to I is also used to describe the differences in the quality of matrix in normal human articular cartilage to osteoarthritic joints, where normal articular cartilage consists of collagen type II.37 Future studies are needed to determine the spatial distribution of collagen types I and II in the matrix.
The degree of sulfation, sulfation position/pattern, disaccharide unit sequence, and the conformation of the sulfated GAGs have been known to affect the biological activity and binding affinity of proteins.38-40 Electrostatic interaction where the positively charged protein binds to the negatively charged sulfate and carboxyl groups of the sulfated GAG molecules can also play a role in the sequestration of proteins.41 A stronger stain for positively charged protein lysozyme, which was used as a model protein, was observed for the fully sulfated NaCS scaffolds, which also complements earlier studies where the amount of NaCS in the gelatin scaffolds resulted in increased sequestration of lysozyme.22 Lysozyme was also observed on gelatin scaffolds, which may have been due to the electrostatic interaction with negatively charged gelatin type B. Gelatin was prepared using acid hydrolysis of collagen resulting in a negative charge at neutral pH.42 The sequestration rate of TGF- β3 on the scaffolds was dependent upon the DOS for the GAG mimetics but sulfation pattern and other structural differences may have played a role for the native GAGs. Highest Ka of TGF- β3 was determined on NaCS containing scaffolds followed by pSC and CS-C containing scaffolds and the retention of TGF- β3 was also highest for NaCS containing scaffolds. Taken together, these measurements indicate NaCS containing scaffolds have a greater affinity for TGF- β3. Additional studies measuring dissociation rate constant, Kd, would be useful to determine unbound and bound/strongly associated TGF- β3 in the scaffolds. In previous work examining GAGs alone, not incorporated into scaffolds, similar findings were reported where highly sulfated hyaluronic acid (DOS ~ 3) had a higher binding affinity for TGF- β1 in comparison to lower sulfated CS (mixture of 70% CS-A and 30% CS-C).43 Also, the same study reported that sulfated hyaluronic acid had higher binding affinity toward TGF- β1 in comparison to CS at similar DOS suggesting that the binding affinity could also be due to the sulfate position or due to the chain conformation of HA polymer being more beneficial for interaction with TGF- β1 protein.43 In this study, differences in the rate of sequestration for CS-C and pSC, although similar in DOS, may be attributed to the presence of some amount of CS-A since the native GAGs, CSC-C and CS-A, are not homogeneous.23,43 The sulfate position for CS-A differs from CS-C. CS-A containing scaffolds also showed the lowest Ka for TGF- β3 along with gelatin only and heparin containing scaffolds. The lower rate of sequestration of heparin-containing scaffolds, although heparin has a higher DOS than pSC, could be due to heparin and heparan sulfate having a lower binding to TGF- β3 and higher binding to TGF- β1 and TGF- β2.44 Thus, the rate of sequestration or binding affinity to these scaffolds may be dependent upon the isoform of TGF- β.
TGF- β3 loading on scaffolds promoted MSC chondrogenesis with the highest TGF- β3 sequestration for cellulose sulfate – containing scaffolds as compared to native GAGs. In addition, in dynamic conditions, the collagen type II/I ratio was highest for NaCS-containing scaffolds. NaCS-containing scaffolds had the highest TGF- β3 sequestration and retention of TGF- β3 over time. The TGF- β3 release profile for the scaffolds was a burst release in the first 4 days. However, the amount of release was dependent upon the DOS for GAG mimetics where the least was determined for NaCS containing scaffolds. Whereas the rest of the scaffold groups released most of the TGF- β3 by day 4. The majority of the GAGs and GAG mimetics incorporated into the scaffolds are retained over time, as previously reported.24 Therefore, the initial burst release may be due to diffusion of unbound TGF- β3. Similar release profile of growth factors was seen in published studies using gelatin as well as sulfated GAGs.16,45 Studies have indicated that continuous exposure of TGF- β3 in the first week is necessary to induce chondrogenesis in MSCs.46,47 Also, another study has indicated that transient exposure to high dosages of TGF-β3 (100 ng/ml) in the first week of MSC culture followed by culture in growth factor-free media for 9 weeks enhanced cartilage matrix deposition in comparison to MSCs cultured in standard induction media containing continuous supplementation of 10 ng/ml of TGF- β3.48 The scaffolds in this study had approximately 9–10 ng of TGF- β3 per scaffold. If the TGF- β3 was released, this would result in concentrations of 90–100 ng/ml in the culture media where the volume is 100 μl. This study, however, demonstrates that TGF- β3 sequestration to the scaffold with limited release, as indicated by the fully sulfated group, may be more favorable for enhanced chondrogenesis.
Various sulfated GAGs and polysaccharides have known to modulate TGF- β signaling response by modulating its interaction with their receptors.49-51 To study the molecular mechanism by which cellulose sulfate enhances chondrogenesis via TGF- β3 present on these scaffolds and to determine if cellulose sulfate enhances TGF- β signaling, the expression of TGF- βRI and TGF- βII mRNA and phosphorylation of pSmad2/3 were evaluated. The increased ratio of TGF- βRII to TGF- βRI was reported as an indicator of augmented TGF- β cellular response in heparan sulfate induced TGF- β signaling studies.49,52 Increased ratio of TGF- βRII to TGF- βRI was determined on the NaCS and pSC containing gelatin scaffolds loaded with TGF- β3. Smad2/3 phosphorylation also appeared to increase with the presence of TGF- β3 with a significant reduction when a TGF- βRI kinase inhibitor was used, suggesting that the TGF- β signaling plays a role in modulating the chondrogenesis of MSCs on cellulose sulfate scaffolds. Interestingly, in media without TGF- β3, Smad2/3 phosphorylation also reduced when TGF- βRI kinase inhibitor was used, indicating that other factors in the media, such as insulin and high glucose, may be contributing to receptor signaling.53 MSCs cultured on gelatin only scaffolds showed reduced expression of ratio of TGF- βRII to TGF- βRI in the absence or presence of TGF- β3 in comparison to pSC and NaCS scaffolds in similar culture conditions. In addition, TGF- βRI kinase inhibitor did not affect the phosphorylation of Smad2 in cells cultured on gelatin only scaffolds. Thus, suggesting that smad2 signaling is not dependent on TGF- β3 and TGF-β receptors in cells cultured on gelatin only scaffolds. Smads have also been known to be phosphorylated by other kinases apart from TGF- βRI. Erk-family MAP kinase54 and Ca2+/Calmodulin dependent protein kinase II,55 protein kinase C56 and cyclin dependent kinase CDK2 and CDK457 have also been known to phosphorylate smad 2/3 resulting in reduced transcriptional activity of smads. MSCs are also known to attach to gelatin via αvβ3, α5β1 integrins.58 Integrin linked kinase (ILK) associated with β3 integrin have been shown to induce smad2/3 phosphorylation and downstream signaling in fibroblasts.59 Thus, the presence of pSmad2/3 in the presence of TGF- βRI kinase inhibitor could be due to other pathways resulting in smad2/3 phosphorylation. Smad-independent pathways, such as p38 and ERK1/2,60,61 can be evaluated for cells cultured on cellulose sulfate-containing scaffolds. The high gene expression for Sox9 for cells on NaCS containing scaffolds could be due to the availability of TGF- β3 in the media as well as the prolonged availability on the scaffolds. TGF- β3 signaling through smad2/3 is known to be responsible for sox9 expression.62,63 Also, a low expression of hypertrophic markers VEGF and Col X was seen for cells on scaffolds containing NaCS in static and dynamic culture conditions, respectively. Similar findings were also seen for highly sulfated hyaluronic acid scaffolds loaded with TGF- β164 and Collagen type I/III and fibrin based matrices with localized TGF- β1.65 This suggests that the presentation of TGF- β is important for cartilage tissue regeneration and maintenance.
A greater expression of chondrogenic markers with reduced hypertrophic markers was demonstrated in dynamic loading conditions for cells on NaCS-containing scaffolds. The greater retention of TGF-β3 on NaCS-containing scaffolds in combination with static culture conditions may influence the chondrogenic differentiation of MSCs when later subjected to dynamic loading conditions.66 In native cartilage tissue, the pericellular matrix of chondrocytes plays an important role in regulating the mechanotransduction on the cell.67 It is speculated that chondrogenic pre-culture prior to mechanical loading allows the MSCs to produce pericellular matrix which helps the MSCs when undergoing mechanical loading to form mature chondrocytes.68 Dynamic load parameters in the bioreactor, loading frequency, compressive loading amplitude and loading duration, mimic physiological loading conditions.69 Frequency of 1 Hz is known to be the optimal frequency since it is similar to the frequency of walking cadence.70 Studies using lower or higher frequency loading have resulted in either no effect or detrimental effect on chondrogenesis of MSCs.71,72 Also, loading amplitude higher than 15 to 20% has resulted in higher expression of hypertrophic markers than when 5 to 10% strain is used.47,71,73,74 Loading duration for 1–4 h/day is adequate to induce chondrogenesis of MSCs whereas, longer loading durations 12 to 24 h/day have resulted in cell apoptosis. Dynamic loading parameters used in this study were frequency of 1 Hz, compressive loading amplitude of 10% stain and loading duration of 3 h (1 h on and 1 h off) per day for 5 days a week, which have been previously reported.28,75
Dynamic loading led to a decreased expression of chondrogenic hypertrophic genes on NaCS containing scaffolds and increased production of collagen type II with the highest collagen type II to I ratio. pSC containing gelatin scaffold group also showed a decrease in expression of hypertrophic markers VEGF and MMP13 however, Col X marker was still expressed. Col I production was also higher for this group. Longer duration of dynamic culture may result in lower hypertrophic marker expression.76 The mechanical properties of the constructs in the bioreactor were also determined and showed an increase after 2 weeks of dynamic loading (data not shown). However, the scaffolds were not able to form mechanically robust constructs equivalent to that of native cartilage tissue.77 Longer culture in dynamic loading conditions may improve the mechanical properties of the construct with increased production of a cartilage-like ECM.47,75,76
5 ∣. CONCLUSIONS
This study demonstrated cellulose-derived GAG mimetics incorporated into fibrous scaffolds and loaded with TGF-β3 enhanced chondrogenic differentiation of human MSCs. These GAG mimetics may be a suitable replacement for native GAGs for cartilage tissue regeneration. The sequestering of TGF-β3 to these scaffolds containing GAG-mimetics increased with the degree of sulfation where the fully sulfated cellulose sulfate (NaCS) containing scaffold had the highest sequestration rate and retention. NaCS-containing scaffolds loaded with TGF-β3 also promoted the greatest chondrogenesis where findings also reveal that TGF-β receptor and SMAD2/3 signaling play a role. This study demonstrates the potential of cellulose sulfate-containing constructs for cartilage tissue engineering applications.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) R01AR077056 and the National Science Foundation - Center for Engineering MechanoBiology STC (CMMI: 15-48571).
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- 1.O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95. [Google Scholar]
- 2.McAdams TR, Mithoefer K, Scopp JM, Mandelbaum BR. Articular cartilage injury in athletes. Cartilage. 2010;1(3):165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell KL, Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthr Cartil. 2015;23(3):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1–2):1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Jt Surg Br Ed. 2005;87(5):640–645. [DOI] [PubMed] [Google Scholar]
- 7.Hardingham T, Tew S, Murdoch A. Tissue engineering: chondrocytes and cartilage. Arthritis Res. 2002;4(Suppl 3):S63–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruano-Ravina A, Jato DM. Autologous chondrocyte implantation: a systematic review. Osteoarthr Cartil. 2006;14(1):47–51. [DOI] [PubMed] [Google Scholar]
- 9.Qi Y, Feng G, Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39(5):5683–5689. [DOI] [PubMed] [Google Scholar]
- 10.Brittberg M, Gomoll AH, Canseco JA, Far J, Lind M, Hui J. Cartilage repair in the degenerative ageing knee. Acta Orthop. 2016;87(sup363):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Fu P, Cong R, Wu H, Pei M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015;2(1):76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed TAE, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16(3):305–329. [DOI] [PubMed] [Google Scholar]
- 13.Re'em T, Kaminer-Israeli Y, Ruvinov E, Cohen S. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials. 2012;33(3):751–761. [DOI] [PubMed] [Google Scholar]
- 14.Danišovič L, Varga I, Polák S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell. 2011;44(2):69–73. [DOI] [PubMed] [Google Scholar]
- 15.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. [DOI] [PubMed] [Google Scholar]
- 16.Sun PJ, Jung YH, Gyun WD, Na YH, Kun N, Keun-Hong P. Chondrogenic differentiation of mesenchymal stem cells embedded in a scaffold by long-term release of TGF-β3 complexed with chondroitin sulfate. J Biomed Mater Res A. 2010;92A(2):806–816. [DOI] [PubMed] [Google Scholar]
- 17.Hempel U, Hintze V, Möller S, Schnabelrauch M, Scharnweber D, Dieter P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor β1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012;8(2):659–666. [DOI] [PubMed] [Google Scholar]
- 18.Waghmare NA, Arora A, Bhattacharjee A, Katti DS. Sulfated polysaccharide mediated TGF-β1 presentation in pre-formed injectable scaffolds for cartilage tissue engineering. Carbohydr Polym. 2018;193: 62–72. [DOI] [PubMed] [Google Scholar]
- 19.Ng F, Boucher S, Koh S, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Chen L, Xu X, Li C, Huang C, Deng C-X. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang GP, Menezes R, Vincent R, et al. Gelatin scaffolds containing partially sulfated cellulose promote mesenchymal stem cell Chondrogenesis. Tissue Eng Part A. 2017;23(17–18):1011–1021. [DOI] [PubMed] [Google Scholar]
- 22.Huang GP, Molina A, Tran N, Collins G, Arinzeh TL. Investigating cellulose derived glycosaminoglycan mimetic scaffolds for cartilage tissue engineering applications. J Tissue Eng Regen Med. 2018;12(1):e592–e603. [DOI] [PubMed] [Google Scholar]
- 23.Menezes R, Hashemi S, Vincent R, et al. Investigation of glycosaminoglycan mimetic scaffolds for neurite growth. Acta Biomater. 2019;90:169–178. [DOI] [PubMed] [Google Scholar]
- 24.Menezes R, Arinzeh TL. Comparative study of electrospun scaffolds containing native GAGs and a GAG mimetic for human mesenchymal stem cell Chondrogenesis. Ann Biomed Eng. 2020;48(7):2040–2052. [DOI] [PubMed] [Google Scholar]
- 25.Briggs T, Matos J, Collins G, Livingston AT. Evaluating protein incorporation and release in electrospun composite scaffolds for bone tissue engineering applications. J Biomed Mater Res A. 2015;103(10): 3117–3127. [DOI] [PubMed] [Google Scholar]
- 26.Hortensius RA, Harley BAC. The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials. 2013;34(31):7645–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–88. [DOI] [PubMed] [Google Scholar]
- 28.Damaraju SM, Shen Y, Elele E, et al. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51–62. [DOI] [PubMed] [Google Scholar]
- 29.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr Cartil. 2002;10:564–572. [DOI] [PubMed] [Google Scholar]
- 30.Tran SC, Cooley AJ, Elder SH. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnol Bioeng. 2011;108(6):1421–1429. [DOI] [PubMed] [Google Scholar]
- 31.Remya NS, Nair PD. Mechanoresponsiveness of human umbilical cord mesenchymal stem cells in in vitro chondrogenesis-a comparative study with growth factor induction. J Biomed Mater Res A. 2016;104(10):2554–2566. [DOI] [PubMed] [Google Scholar]
- 32.Chameetachal S, Midha S, Ghosh S. Regulation of chondrogenesis and hypertrophy in silk fibroin-gelatin based 3D bioprinted constructs. ACS Biomater Sci Eng. 2016;2(9):1450–1463. [DOI] [PubMed] [Google Scholar]
- 33.Chuang CY, Lord MS, Melrose J, et al. Heparan sulfate-dependent signaling of fibroblast growth factor 18 by chondrocyte-derived perlecan. Biochemistry. 2010;49(26):5524–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrand A, Romarís M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht C, Tichy B, Nürnberger S, et al. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthr Cartil. 2011;19(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 36.Marlovits S, Hombauer M, Truppe M, Vecsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Jt Surg Br Vol. 2004;86-B(2):286–295. [DOI] [PubMed] [Google Scholar]
- 37.Hamada T, Sakai T, Hiraiwa H, et al. Surface markers and gene expression to characterize the differentiation of monolayer expanded human articular chondrocytes. Nagoya J Med Sci. 2013;75:101–111. [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug des. 2008;72(6):455–482. [DOI] [PubMed] [Google Scholar]
- 39.Scott JE. Structure and function in extracellular matrices depend on interactions between anionic glycosaminoglycans. Pathol Biol. 2001;49(4):284–289. [DOI] [PubMed] [Google Scholar]
- 40.Shipp EL, Hsieh-Wilson LC. Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem Biol. 2007;14(2):195–208. [DOI] [PubMed] [Google Scholar]
- 41.Miller T, Goude MC, McDevitt TC, Temenoff JS. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10(4):1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aramwit P, Jaichawa N, Ratanavaraporn J, Srichana T. A comparative study of type A and type B gelatin nanoparticles as the controlled release carriers for different model compounds. Mater Express. 2015;5(3):241–248. [Google Scholar]
- 43.Hintze V, Miron A, Moeller S, et al. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1). Acta Biomater. 2012;8(6):2144–2152. [DOI] [PubMed] [Google Scholar]
- 44.Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-βs with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997;272(29):18000–18006. [DOI] [PubMed] [Google Scholar]
- 45.Fan H, Zhang C, Li J, et al. Gelatin microspheres containing TGF-β3 enhance the chondrogenesis of mesenchymal stem cells in modified pellet culture. Biomacromolecules. 2008;9(3):927–934. [DOI] [PubMed] [Google Scholar]
- 46.Mehlhorn A, Schmal H, Kaiser S, et al. Mesenchymal stem cells maintain TGF- β -mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12(6):1393–1403. [DOI] [PubMed] [Google Scholar]
- 47.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Wang Y, Chen C, et al. Exogenous heparan sulfate enhances the TGF-β3-induced chondrogenesis in human mesenchymal stem cells by activating TGF-β/Smad signaling. Stem Cells Int. 2016;2016: 1520136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehler L, Samsonov S, Rother S, et al. Sulfated hyaluronan derivatives modulate TGF-β1:receptor complex formation: possible consequences for TGF-β1 signaling. Sci Rep. 2017;7(1):1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TH, Lee EK, Lee MJ, Kim JH, Yang WS. Fucoidan inhibits activation and receptor binding of transforming growth factor-β1. Biochem Biophys Res Commun. 2013;432(1):163–168. [DOI] [PubMed] [Google Scholar]
- 52.Chen C-L, Huang SS, Huang JS. Cellular heparan sulfate negatively modulates transforming growth factor-β1 (TGF-β1) responsiveness in epithelial cells. J Biol Chem. 2006;281(17):11506–11514. [DOI] [PubMed] [Google Scholar]
- 53.Budi EH, Muthusamy BP, Derynck R. The insulin response integrates increases TGF-b signaling through Akt-induced enhancement of cell surface delivery of TGF-b receptors. Sci Signal. 2015;8(396):ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFβ/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wicks SJ, Lui S, Abdel-Wahab N, Mason RM, Chantry A. Inactivation of smad-transforming growth factor β signaling by Ca2+-Calmodulin-dependent protein kinase II. Mol Cell Biol. 2000;20(21):8103–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yakymovych I, Ten Dijke P, Heldin CH, Souchelnytskyi S. Regulation of Smad signaling by protein kinase C. FASEB J. 2001;15(3):553–555. [DOI] [PubMed] [Google Scholar]
- 57.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. [DOI] [PubMed] [Google Scholar]
- 58.Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci. 2011;124(8):1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boo S, Dagnino L. Integrins as modulators of transforming growth factor Beta signaling in dermal fibroblasts during skin regeneration after injury. Adv Wound Care. 2013;2(5):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-[beta] family signalling. Nature. 2003;425(6958):577–584. [DOI] [PubMed] [Google Scholar]
- 61.Oshimori N, Fuchs E. The harmonies played by TGF-beta in stem cell biology. Cell Stem Cell. 2012;11(6):751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furumatsu T, Ozaki T, Asahara H. Smad3 activates the Sox9-dependent transcription on chromatin. Int J Biochem Cell Biol. 2009;41(5):1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coricor G, Serra R. TGF-β regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci Rep. 2016;6:38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng Q, Lin S, Zhang K, et al. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017;53:329–342. [DOI] [PubMed] [Google Scholar]
- 65.Diederichs S, Baral K, Tanner M, Richter W. Interplay between local versus soluble transforming growth factor-beta and fibrin scaffolds: role of cells and impact on human mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2012;18(11–12):1140–1150. [DOI] [PubMed] [Google Scholar]
- 66.O'Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther. 2013;4(4):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guliak F, Alexopoulus LG, Upton ML, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068(1):498–512. [DOI] [PubMed] [Google Scholar]
- 68.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43(1):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim E, Guilak F, Haider MA. The dynamic mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions under cyclic compressive loading. J Biomech Eng. 2008;130(6):061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JR, Yong KW, Choi JY. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J Cell Physiol. 2018;233(3):1913–1928. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Yao S-J, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin–polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16(2):575–584. [DOI] [PubMed] [Google Scholar]
- 72.Natoli RM, Athanasiou KA. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J Biomech Eng. 2008;130(4):041012. [DOI] [PubMed] [Google Scholar]
- 73.Michalopoulos E, Knight RL, Korossis S, Kearney JN, Fisher J, Ingham E. Development of methods for studying the differentiation of human mesenchymal stem cells under cyclic compressive strain. Tissue Eng Part C Methods. 2012;18(4):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang T, Wen F, Wu Y, et al. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials. 2015;38:72–85. [DOI] [PubMed] [Google Scholar]
- 75.Mauck RL, Wang CCB, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr Cartil. 2003;11(12):879–890. [DOI] [PubMed] [Google Scholar]
- 76.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18(7–8):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jurvelin JS, Buschmann MD, Hunziker EB. Mechanical anisotropy of the human knee articular cartilage in compression. Proc Inst Mech Eng. 2003;217(3):215–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors