Abstract
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death among non-contagious diseases in the world. PDE inhibitors are among current medicines prescribed for COPD treatment of which, PDE-4 family is the predominant PDE isoform involved in hydrolyzing cyclic adenosine monophosphate (cAMP) that regulates the inflammatory responses in neutrophils, lymphocytes, macrophages and epithelial cells The aim of this study is to investigate the cellular and molecular mechanisms of cAMP-PDE signaling, as an important pathway in the treatment management of patients with COPD. In this review, a comprehensive literature review was performed about the effect of PDEs in COPD. Generally, PDEs are overexpressed in COPD patients, resulting in cAMP inactivation and decreased cAMP hydrolysis from AMP. At normal amounts, cAMP is one of the essential agents in regulating metabolism and suppressing inflammatory responses. Low amount of cAMP lead to activation of downstream inflammatory signaling pathways. PDE4 and PDE7 mRNA transcript levels were not altered in polymorphonuclear leukocytes and CD8 lymphocytes originating from the peripheral venous blood of stable COPD subjects compared to healthy controls. Therefore, cAMP-PDE signaling pathway is one of the most important signaling pathways involved in COPD. By examining the effects of different drugs in this signaling pathway critical steps can be taken in the treatment of this disease.
Keywords: COPD Therapy, Phosphodiesterase inhibitors, cAMP signaling
Highlights
-
•
cAMP-PDE signaling pathway is one of the most important signaling pathways involved in COPD.
-
•
PDE4 increases in COPD patients.
-
•
PDE4 inhibitors such as Roflumilast can be effective in the treatment of COPD.
1. Introduction
As one of the top ten non-infectious diseases, chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease that causes obstructed airflow from the lungs [1]. 300 million individuals worldwide have COPD, with a prevalence of around 7.2% in 2021, the WHO proposed COPD as the third leading cause of death among non-contagious diseases by 2020. Alarmingly, COPD took third place in 2016, four years ahead of WHO estimation. The majority of COPD patients experience pulmonary inflammatory exacerbations resulting in greater mortality, increased hospitalization costs [[1], [2], [3], [4]], necessitating appropriate clinical management. Viral and bacterial infections trigger inflammation exacerbations in COPD patients. Patients with exacerbation are susceptible to repeated acute exacerbations (AECOPD) [5], which accelerates the decline of lung function and increase the rates morbidity and mortality [6]. COPD exacerbations also significantly boost inflammatory mediators such as c-reactive protein [7], IL-6 [8], and TNF-α levels [9], for which, a variety of anti-inflammatory medications targeting primary inflammatory pathways have been developed to date, though with a wide range of side effects and adverse reactions. Inhaled corticosteroids (ICS) are linked to an increased risk of oral candidiasis, hoarseness, skin bruising, and pneumonia [10], as well as increased incidence of hip and upper extremities fractures in high dosages [11]. According to the GOLD 2018 study, although bronchodilators are used to ameliorate symptoms, using short-acting bronchodilators on a regular basis is not advisable [12]. Accordingly, drugs that target cAMP-phosphodiesterase (PDE) signaling pathway have recently gained particular attention for the treatment of COPD [13]. There are at least 11 different families of PDE enzymes in mammals, encoding more than 50 isoforms [14], some of which show tissue-specific expression. PDE11A3, PDE7B2, and PDE11A4 are expressed specifically in the testis and prostate tissues [15,16], while PDE3, PDE4, and PDE7 are specific to lungs [17,18]. These gene families have been classified according to their functional characteristic such as affinities for cAMP and cGMP, inhibitor sensitivity, response to specific effectors, and mechanism of regulation [4]. cAMP and cGMP modulate several intracellular signaling pathways, thus playing a pivotal role in the regulation of different physiologic processes, including apoptosis, cell proliferation, inflammation, immune response, and bone remodeling [4]. Conversely, the inhibition of cAMP and cGMP biological effects by PDEs seems to be a pathogenic mechanism involved in the onset and maintenance of different pathological states, including COPD, depression, diabetes, erectile dysfunction, inflammatory bowel diseases (IBD), and psoriatic arthritis [19]. Therefore, as drugs targeting the cAMP-PDE signaling pathway may be effective in the treatment of COPD, a large number of studies have focused on targeting PDEs, particularly PDE4, as a novel treatment for COPD. These drugs have several effects on signaling pathways in various cells, including neutrophils, lymphocytes, macrophages, and epithelial cells [20,21]. On the other hand, cAMP plays a key role in balancing the microbiome content and mucosal immune system. The microbiome subpopulations ferment fiber rich nutrients and produce short-chain fatty acids such as butyrate and acetate and thus increase cAMP levels that in turn, regulate the mucosal immune system to reach a homeostasis in the body that can dysregulate through any infection or antibiotic administration. Therefore, co-administration of PDE inhibitors is recommended for the treatment and control of inflammatory diseases such as COPD [22], despite considerable side effects in case of PDE4 inhibitors [23,24]. This review aims to evaluate data on cAMP-PDE signaling pathway and PDE4 inhibitors mechanism of action to elucidate the efficacy of targeting cAMP-PDE signaling pathway as a promising therapeutic strategy in COPD patients.
2. Role of cAMP-PDE signaling pathway in COPD pathogenesis
Although a number of PDE inhibitors are used in clinical practice (Table 1), PDE-4 is the major therapeutic target in respiratory diseases, including COPD and asthma [[24], [25], [26], [27], [28]]. As previously mentioned, PDE-4 is the predominant isoform involved in the catabolism of cAMP that regulates the inflammatory responses in several cells.
Table 1.
List of clinical trials assessing the efficacy of different PDE inhibitors in COPD. (COPD: Chronic Obstructive Pulmonary Disease, PDE: Phosphodiesterases, BID: abbreviation for “bis in die” which in Latin means twice a day.)
| 1 | NCT Numbera | Drug used | last update | condition | Mechanism of action | Dosing | Phases | Enrollment | Publication |
|---|---|---|---|---|---|---|---|---|---|
| 2 | NCT02097992 | Roflumilast | November 2017 | Airway Blood Flow as an Expression of Airway Inflammation in COPD | inhibition of the PDE(4) isoenzyme with a consequent increase of cyclic adenosine monophosphate | 500ug daily, followed by a 4 week | Phase 1 Phase 2 | 11 participants | [29] |
| 3 | NCT01973998 | Roflumilast | March 2020 | COPD | inhibition of the PDE (4) | 500 μg tablet daily for 180 days | Phase 3 | 68 participants | – |
| 4 | NCT01313494 | Roflumilast | February 2017 | COPD | inhibition of the PDE (4) | 500 μg, tablet, oral, once daily for up to 24 weeks | Phase 3 | 626 participants | [30] |
| 5 | NCT01572948 | Roflumilast | October 2015 | Inflammation in COPD | inhibition of the PDE (4) | – | Not Applicable | 27 participants | [31] |
| 6 | NCT00242320 | Roflumilast | December 2016 | Patients Older Than 40 Years With COPD | inhibition of the PDE (4) | 500 μg, tablet, oral, once daily for 12 weeks | Phase 3 | 551 participants | – |
| 7 | NCT00062582 | Roflumilast | November 2016 | Pulmonary Function and Respiratory Symptoms in Patients With COPD | inhibition of the PDE (4) | 500 mcg Daily for 12 weeks | Phase 3 | 1000 participants | – |
| 8 | NCT01509677 | Roflumilast | November 2019 | COPD | inhibition of the PDE (4) | 500 μg tablets once daily | Phase 3 | 158 participants | [32] |
| 9 | NCT00297102 | Roflumilast | January 2017 | COPD | inhibition of the PDE (4) | 500 mcg, once daily, oral for 56 weeks | Phase 3 | 1523 participants | [33] |
| 10 | NCT01745848 | Roflumilast | August 2016 | Bone Metabolism and Endothelial Function in COPD | inhibition of the PDE (4) | 500 μg daily for 30 days | Phase 4 | 26 participants | – |
| 11 | NCT02165826 | Roflumilast | April 2017 | Evaluation of Tolerability and Pharmacokinetics of Roflumilast, 250 μg and 500 μg, as add-on to Standard COPD Treatment to Treat Severe COPD | inhibition of the PDE (4) | 250 μg and 500 μg OD for 12 weeks | Phase 3 | 1323 participants | [34] |
| 12 | NCT00103922 | Cilomilast | October 2016 | COPD | inhibition of the PDE (4) | ARIFLO® (15 mg BID) for 24 weeks | Phase 3 | 600 participants | – |
| 13 | NCT00671151 | Theophylline | May 2008 | COPD | It acts as a competitive nonselective phosphodiesterase inhibitor (inhibiting type III and type IV phosphodiesterase), which increases the concentration of intracellular cAMP | 100 mg bid for 3 months | Not Applicable | 35 participants | [35] |
| 14 | NCT00299858 | Theophylline | July 2017 | COPD | competitive nonselective phosphodiesterase inhibitor (inhibiting type III and type IV phosphodiesterase) | 10 mg/kg, titrated to blood levels between 55 and 110 μmol/L, for a period of 4 weeks. | Phase 2/Phase 3 | 24 participants | [36] |
| 15 | NCT02261727 | Theophylline | August 2021 | Theophylline and Steroids in COPD | competitive nonselective phosphodiesterase inhibitor (inhibiting type III and type IV phosphodiesterase) | Theophylline 100 mg 1 tab twice daily and Prednisone placebo 1 tab once daily | Phase 4 | 1670 participants | [37] |
| 16 | NCT01599871 | Theophylline | August 2017 | Low-dose Theophylline as Anti-inflammatory Enhancer in Severe COPD | competitive nonselective phosphodiesterase inhibitor (inhibiting type III and type IV phosphodiesterase) | 100 mg, twice a day for 52 Weeks | Phase 3 | 70 participants | [38] |
| 17 | NCT03984188 | Theophylline ER | May 2022 | Management of Biomass-Associated COPD | competitive nonselective phosphodiesterase inhibitor (inhibiting type III and type IV phosphodiesterase) | 200 mg extended release (ER) low-dose theophylline taken orally daily | Phase 3 | 110 participants | [39] |
| 18 | NCT02340520 | Theophylline and Roflumilast | June 2018 | Enhancement of Corticosteroid Efficacy in COPD | inhibition of the PDE (4) | Theophylline for one week, followed by the addition of Roflumilast for a further one week. | Phase 3 | 13 participants | – |
ClinicalTrials.gov Identifier.
As a second messenger molecule, cAMP is a critical component in metabolic regulation. The E class of G Protein-Coupled Receptors (GPCRs) is tightly regulated by cAMP [40]. Currently, it has been found that this ubiquitous second messenger molecule is stimulated in response to a multitude of extracellular and intracellular stimuli to trigger a mass of downstream biochemical and signaling pathways, including PKA, AMPK, and Epac signaling modules. These three major signaling pathways are involved in the regulation of inflammation and energy metabolism [41,42]. The final cellular concentration of cAMP depends on several parameters, including energy metabolism and activity of PDEs. A normal concentration of cAMP suppresses key inflammatory responses. However, augmented level of PDEs hydrolyzes the cAMP to its inactive form, AMP, which leads to the decline in cAMP. Finally, decreased amount of cAMP leads to the activation of downstream inflammatory signaling pathways [23,41]. Since an abnormal inflammatory response to noxious irritants is a critical hallmark of COPD, efforts to treat COPD patients have been focused on blocking inflammation enhancers. Inhaled corticosteroids (ICS) are anti-inflammatory drugs that decrease the transcription of pro-inflammatory genes such as NF-κB genes in COPD patients [43]. Furthermore, PDE inhibitors have recently introduced as anti-inflammatory regulators with demonstrated anti-exacerbation capabilities in COPD [44]. PDE inhibition raises cAMP concentrations in inflammatory cells, resulting in inflammation suppression, smooth muscle relaxation, bronchodilation, and sensory nerve modulation [14,45,46].
PDE4 is the most common type of PDE in COPD-related inflammatory cells, and PDE4A4 isoform has significantly different expression levels in COPD patients' macrophages [47]. As macrophages are the hallmark of inflammation in COPD, and PDE4 inhibitors are cost effective drugs, PDE signaling pathway is an appropriate target in the war against COPD [48,49]. Roflumilast (a PDE4 inhibitor) suppresses severe skeletal muscle wasting and sputum neutrophilia in COPD patients [50,51]. A clinical trial comprising more than 4000 COPD patients demonstrated that Roflumilast reduced both moderate and severe COPD exacerbations by 12% and 16%, respectively. The efficacy of Roflumilast is positively associated strongly with higher count of eosinophils [52]. Moreover, the numbers of neutrophils are also associated with severity and frequency of COPD exacerbations [[53], [54], [55], [56]]. But despite its efficacy, the side effects reported with Roflumilast have limited the use of this drug [57]. It seems that inhibition of PDEs, especially PDE4, to reduce inflammatory responses and exacerbation shows a promising treatment in COPD patients with acceptable side effects [58]. This necessitates detecting key regulators of cAMP concentration in PDE signaling pathways (Fig. 1).
Fig. 1.
A) The E class of G Protein-Coupled Receptors (GPCRs) is tightly regulated by cAMP. PDE causes the deactivation of AMP and the conversion of cAMP to AMP. In response to a wide range of stimuli, cAMP is universally produced and controlled, influencing a wide range of downstream biochemical and signaling pathways, including PKA, AMPK, and Epac signaling modules, which modulates inflammatory responses. B) Increased cAMP levels trigger PKA, which then inhibits the pro-inflammatory effector RhoA (NF-κB pathway activator). In addition, endothelial dysfunction in individuals with COPD is related to the GTP-RhoA family (ROCK). C) Epac is a cAMP-sensitive guanine exchange factor (GEF) that connects members of the Ras superfamily including Rho, Rac, and Ras at the molecular level. Pro-inflammatory interleukins are less activated when Epac1 is activated by cAMP by altering many signaling pathways, such as PI3k/Akt, ERK, phospholipase D, and NF-κB. D) AMPK (AMP-activated protein kinase) interacts with cAMP- stimulated PKA to restrict the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway and reduce inflammation. The oxidant-antioxidant balance is related to AMPK's anti-inflammatory properties, and AMPK activation increases Nrf2 expression. Furthermore, AMPK regulates protein synthesis by activating tuberous sclerosis complex 2 (TSC2), suppressing mTORC1, and regulating autophagy via ULK1 (UNC51-like kinase 1). STAT3 may be blocked by both cAMP and AMPK triggered by cAMP, which inhibits the downstream inflammatory pathways. E) The effect of symbiosis and dysbiosis in the downstream signaling pathways and their effect on cAMP turn-over.
2.1. cAMP-PKA-RhoA signaling axis
PKA (protein kinase A) is a cAMP effector. Despite unchanged expression level in the lung tissue of COPD patients, PKA activation by a selective cAMP-dependent PKA activator (i.e., 6-Bnz-cAMP) can completely reverse the IL-8 expression levels induced by cigarette smoke in the airway smooth muscle cell line [59]. Therefore, high levels of IL-8 in the serum of COPD patients can be a sign of attenuated PKA activity subsequent to reduced cAMP [[60], [61], [62]]. Normally, higher levels of cAMP activate PKA which, in turn, suppress the pro-inflammatory effector RhoA (a small G-protein). But, RhoA is significantly hyperactive in proximal pulmonary arteries of COPD patients compared to non-smoker healthy controls [63]. In addition, the GTP-RhoA family (ROCK-I or ROCKb and ROCK-II or ROCKa) is involved in endothelial dysfunction in both healthy smokers and COPD patients. The activity of RhoA can potentially activate NF-kB signaling pathway mediating COPD inflammation both at stable and exacerbation COPD phases [[64], [65], [66]]. Induction of the NF-kB pathway results in the secretion of several pro-inflammatory cytokines, chemokines, and proteases such as IL-1B, IL-18, IL-8, IL-6, TNF-α, MMP2, and MMP9 in COPD [62,66,67]. Besides, RELB, a subunit of NF-kB that is a suppressor of cigarette-smoke induced inflammation [68], is significantly downregulated in patients at acute exacerbation of AECOPD compared to stable patients (Fig. 2) [69].
Fig. 2.
PDE inhibitor drugs prevent the conversion of cAMP to AMP, thereby activating cAMP. Activation of PKA is one of the downstream pathways of cAMP, which can inactivate and reverse many inflammatory pathways involved in COPD. Suppressing the pro-inflammatory factor RhoA as a result of inhibiting the inflammatory pathways of p38 MAPK and finally the transcription factor NFKB, leads to inhibiting the release of many inflammatory factors such as IL-1B, IL-18, IL-8, IL-6, TNF-α, MMP2, and MMP9. PKA also leads to phosphorylation and inhibition of JAK-STAT3 and mTORC1 through AMPK, which can prevent the progress of inflammation by cytokines such as IL-22, IL-17 and IL-22. The activation of AMPK can also lead to the activation of FOXO family transcription factors, which will result in the inhibition of NFKB and pro-inflammatory factors. Finally, the blocking of the HIF1 pathway and the inactivation of IL17 leads to a decrease in the concentration of MMPs, preventing tissue damage due to the overactivation of the immune system.
Matrix Metalloproteinases (MMPs) play pivotal roles in lung development and repair processes [70]. This zinc-dependent protease family consists of 24 endopeptidases that are important in lung inflammatory diseases such as asthma and COPD. The imbalance of protease (MMPs) and antiproteases (TIMP-1) is the hallmark of COPD that involved in immune cell infiltration and migration and tissue destruction. For instance, serum level of MMP1 is significantly higher in COPD patients compared to healthy control [70]. Accordingly, blockade of MMP1 using Duloxetine, a selective serotonin reuptake inhibitor, resulted in decreased levels of TLR4 and IL8, and prevented alveolar tissue damage [71]. MMP-9, another member of MMP family that acts as gelatinase and degrades collagen IV, fibronectin, and plasminogen is believed to initiate angiogenesis [72]. Under hyperoxia conditions, the expression levels (both in mRNA and protein) and the activity of MMP-9 are decreased, and TIMP-1 protein level is elevated, both of which may lead to lung remodeling [73]. Nevertheless, under hypoxia conditions, MMP-9 and MMP-2 levels have been shown to be upregulated subsequent to tissue ischemia, which is involved in neoangiogenesis initialization through degrading nonfibrillar collagens [[74], [75], [76]]. MMP-9 level is also elevated in chronic bronchitis and emphysema, which can increase the risk of COPD development [76]. The MMP-9 expression level is significantly higher in COPD patients in comparison with controls [77]. The MMP-9 overexpression is also involved in AECOPD than healthy controls and asthmatic patients [78]. The MMP-9 expression level is simultaneously correlated negatively with lung function and positively with smoking index, suggesting a link between higher smoking index, higher MMP-9 expression level, and lower pulmonary function [67]. The mean total activity of MMP-9 and MMP-8 is significantly higher in the sputum of COPD patients compared to adjusted healthy controls [79]. Taking all these data together indicate that MMPs’ higher expression has been correctly considered as a key factor for development of COPD. According to the basic function of MMP-9 in normal alveolarizations [73,80], in addition to damaging lung parenchyma alveoli cell wall leading to emphysema, the MMP-9 expression and activity may increase for repairing damaged alveoli either. cAMP- activated PKA also interacts with AMPK (AMP-activated protein kinase) which normally suppresses Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway through JAK phosphorylation and inhibits inflammation [81].
2.2. cAMP-Epac signaling axis
The exchange protein directly activated by cAMP (Epac) was the answer to how several cAMP-mediated cellular functions happen in a PKA-independent manner. Scientists came up with Epac by looking for cAMP-dependent but PKA-insensitive activation of Rap1 small GTPase (RAP1A, a member of the RAS oncogene family) in 1998 [82,83]. Epac, a cAMP-sensitive guanine exchange factor (GEF) catalyzing GTP/GDP exchange, serves as a molecular link between Ras superfamily members such as Rho, Rac, and Ras [84]. Epac1 (cAMP-GEFI), Epac2 (cAMP-GEFII), and GEFs of Rap1 and Rap2 regulate calcium handling, cell migration, differentiation, proliferation, and inflammatory responses through targeting various downstream targets, including phospholipase D, Phospholipase C-ε, phosphoinositide 3-kinase dependent protein-kinase B (PKB)/Akt, Extracellular signal-regulated kinases (ERK1/2), and NF-κB [85]. Since Epac hurdles the induction of pro-inflammatory molecules (Fig. 1), decreased expression level of Epac1 in lung tissue of COPD patients can resulted in NF-κB activation that is crucial for inducing IL-8 production in response to cigarette smoke extract (CSE) [59,86,87]. Increased activation of PI3k/Akt signaling pathway has been shown in the airway epithelial of COPD patients [88]. The expression levels of phosphatase and tensin homolog deleted from chromosome 10 (PTEN) decrease significantly in COPD patients. PTEN is a negative regulator of PI3K, a convertor of phosphatidylinositol-3, 4, 5-phosphate (PIP3) to phosphatidylinositol-4,5-phosphate (PIP2).
Activated Akt phosphorylates the forkhead transcription factor FOXO3a and provokes its accumulation, ubiquitination, and degradation in the cytoplasm [89]. Accordingly, in COPD patients’ bronchial epithelial cells, reduced expression of nuclear FOXO3a results in a higher IL-8 expression [88]. Based on CSE-treated human bronchial cells, FOXO3a normally inhibits NF-кB binding to the IL-8 promoter through interacting with the RelA/p65 component of NF-κB in the nucleus [90]. Following the activation of the PI3K/Akt signaling pathway inflammatory cytokines are produced with lower levels of PTEN and FOXO3A expression levels that leads to greater levels of IL-6 and IL-8 cytokines, respectively (Fig. 3).
Fig. 3.
Epac1 is activated by the inhibition of PDEs and as a result the activation of cAMP and leads to the inhibition of the PI3K/Akt pathway, which as a result of this inhibition and dephosphorylation of FOXO3 (activation) blocks NF-KB and other inflammatory factors caused by it. PTEN is a negative regulator of PI3K, converting phosphatidylinositol-3,4,5-phosphate (PIP3) to phosphatidyl-4,5-phosphate (PIP2), which results in the production of many pro-inflammatory factors such as IL6, IL1, IL-8 and TNFA are inhibited and inflammation is prevented.
2.3. cAMP-AMPK signaling axis
In COPD pathogenesis, AMPK plays a critical role through modulating inflammatory signaling pathways, mitochondrial impairment, metabolic irregularities, and senescence. AMPK has three subunits, including the catalytic subunit α (α1 & α2), and two regulatory subunits β (β1, β2) and γ (γ1, γ2, γ3) [91]. AMPK α1 genetic deletion under cigarette smoke and poly conditions (I:C) lead to elevated airway inflammatory responses and emphysema in mice [92]. While AMPK activators such as 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and metformin reduce the release of IL-6 and IL-8 inflammatory mediators in human lung epithelial cell lines, AMPK inhibitors such as THE compound C increase their release [93]. In mouse model of COPD, prophylactic treatment with AICAR and compound C resulted in lower level of CXCL1 and higher level of CCL2 in BAL fluid [93]. In mice intratracheally injected with CSE, metformin decreased not only CXCL1 and CCL2 but also IL-6. This anti-inflammatory effect of AMPK is linked to oxidant-antioxidant balance where AMPK activation causes higher Nrf2 expression [94]. Nrf2 and NFE2L2 (found in rodents and human, respectively) are critical lung defensive mechanisms against oxidative stress [95,96]. Moreover, AMPK is involved in controlling protein synthesis through activation of tuberous sclerosis complex 2 (TSC2), inhibiting the mTORC1 (Fig. 1), and regulating autophagy by ULK1 (UNC51-like kinase 1) [97,98]. Therefore, AMPK pathways may offer novel prospective therapeutic possibilities for COPD. However, under stress conditions, epithelial cells and macrophages in patients with COPD produce CSE, which activates AMPK, leading to the production of inflammatory mediators [99,100]. AMPK activation in inflammatory conditions can vary depending on the type of activated tissue and the type of disease; therefore, more research is needed to understand this important pathway in a variety of diseases, including COPD.
2.4. Inhibition of AMPK through inactived PKA
The mRNA expression level of STAT3 is 4 times higher in lung tissue of smokers COPD patients compared to healthy controls. The remaining STAT3 downstream genes are also moderately more expressed in COPD than in healthy controls [101]. The activated JAK/STAT signaling pathway plays a pathological role in inflammatory diseases such as atherosclerosis [102], rheumatoid arthritis [103], and COPD [104]. The reason why PDE inhibitors, especially PDE-4 inhibitor Roflumilast, are highly effective in diminishing the frequency of COPD exacerbations may be due to HIF-1 signaling inhibition caused by the inactivation of STAT3 by cAMP second messenger, as declared in our network. mTORC1 (mammalian target of rapamycin complex 1), Transforming growth factor (TGF) β1, and IL-6 can also activate JAKs, all of which are significantly activated in COPD patients [61,[105], [106], [107]]. Activated JAKs can stimulate STAT3, as JAKs bound to IL-6 receptor signal transducing subunit (gp130) are activated following IL-6-receptor complex formation and then stimulate STAT3 [108,109]. HIF-1 (Hypoxia inducible factor 1) can be stimulated by significantly high expression levels of STAT3 in smokers and nonsmokers with COPD. Although HIF-1 expression has been shown to be lower in COPD patients than non-COPD patients, because more than half of non-COPD controls had lung cancer, it was unreliable [102]. Higher expression levels of HIF-1α correlate with poor prognosis in lung cancer patients [110]. However, following ex vivo hypoxia condition, induction levels of HIF-1α and its downstream VEGF (vascular endothelial growth factor), and subsequently, the activity of HIF-1α signaling pathway decrease dramatically in PBMCs of COPD patients [111]. However, both HIF-1α and VEGF expression increased in the muscles of COPD patients [112], which can be due to lower gas exchange in the lung of COPD patients. The following downstream molecule of HIF-1α is the retinoic-acid-related orphan receptor (ROR) gamma t (ROR gamma t), which is the most critical component for the development of IL-17 producing T cell, and is highly expressed in lung tissue of COPD patients [113,114]. Accordingly, expression levels of IL-17A and its correlating cytokines IL-22 and IL-23 are increased dramatically in the peripheral [115] and epithelium of lungs in end-stage COPD patients [116].
3. PDE inhibitors in treatment of COPD
3.1. Corticosteroid resistance in COPD
Inhaled corticosteroids (ICSs) act by binding to the cytoplasmic glucocorticoid receptor (GR) and produce a complex that penetrates the nucleus and prevents transcription factors that activate multiple inflammatory genes [43]. In addition to reducing the frequency of exacerbations, ICSs improve quality of life, lung function, and symptoms in COPD cases with FEV1<50% predicted [117,118]. However, long-term oral immunotherapy is not recommended in COPD patients due to several side effects, including hoarseness of voice, oral candidiasis, skin bruising, and a higher risk of pneumonia during long-term administration, especially in the case of fluticasone propionate [117,[119], [120], [121]]. Fluticasone also causes higher rates of morbidity in COPD patients compared to other ICSs as well as no ICS therapy [122]. Using corticosteroids also exacerbates muscle dysfunction in COPD [123]. Furthermore, not only corticosteroids are less effective in COPD patients than in asthmatics, but also COPD responders sometimes lose responsiveness to the anti-inflammatory action of corticosteroids [124,125]. Corticosteroid resistance in COPD is mediated by inflammatory components such as IL-17, p38 MAPK, PI3K/Akt, and JAK/STAT signaling pathways [[126], [127], [128], [129]]. Blocking p38 MAPK with the GW856553 inhibitor restores the suppressive effect of dexamethasone on IL-8 and IL-6 in PBMCs of COPD patients [128]. Unfortunately, corticosteroids have little or no effect on modulating chronic inflammation in COPD. ICSs are prescribed to 42–86% of COPD patients as mono- or combination therapy regardless of exacerbation risk and COPD severity [[130], [131], [132]]. Because of the predominance of eosinophilic bronchial inflammation in this group, ICS therapy may be more effective for asthma-COPD syndrome (ACOS) patients [133,134]. While ICS/LABA treatment has already been suggested by GOLD for patients with ≥2 exacerbations, the combination is currently proposed for patients with exacerbating ACOS. In addition, PDE-4 inhibitor Roflumilast should be prescribed for chronic bronchitis [134,135]. Therefore, comprehensive molecular and clinical studies on COPD inflammation signaling pathways are demanded to provide physicians with either novel drugs or strategies to improve ICS efficacy (Table 3). Much data is available demonstrating the effectiveness of long-acting bronchodilators, up to 20–30% with long-acting β2-agonist (LABA) and 35% with long-acting muscarinic antagonist (LAMA), for exacerbation reduction in COPD patients. Interestingly, combining LABA and LAMA resulted in a risk reduction compared with LAMA alone, and more importantly, a significant reduction compared with LABA/ICS [[136], [137], [138], [139]]. However, there is growing evidence indicating that not all COPD patients respond to ICS treatment. Given the potential for pneumonia and other important side effects of ICS, emerging data have revealed that it may be possible to withdraw the ICS component in certain COPD patient groups provided that an adequate bronchodilation is in place (Table 2) [[139], [140], [141], [142], [143]].
Table 3.
Pharmacokinetics difference between the PDE inhibitor drugs.
| Drug name | Absorption | Metabolism | Route of elimination | Half-life | Possible adverse reactions |
|---|---|---|---|---|---|
| Roflumilast | After 500mcg measurements, the bioavailability of roflumilast is approximately 80%. Greatest plasma concentrations are come to in 0.5–2 h [180]. | Roflumilast is metabolized to roflumilast N-oxide, the active metabolite of roflumilast in humans, by CYP3A4 and CYP1A2 [180,181]. | Roflumilast is excreted 70% in the urine as roflumilast N-oxide [181]. | Taking after oral organization, the plasma half-lives of roflumilast and roflumilast N-oxide are 17 h and 30 h, individually [182]. | Headache, gastrointestinal disorders, dizziness, palpitations, lightheadedness, clamminess, arterial hypotension and weight loss [183]. |
| Cilomilast | Totally absorbed taking after oral organization and has unimportant first-pass digestion system, bioavailability is reliably near to 100% [184]. | Plasma clearance (around 2 L/h) is nearly completely metabolic, through numerous parallel pathways. The most inexhaustible metabolite, shaped by the activity of cytochrome P450 2C8, has <10% of the movement of the parent molecule [185]. | Most of the drug is excreted in the urine (∼90%) and faeces (6–7%) with unchanged cilomilast accounting for less than 1% of the administered dose [185]. | Its terminal elimination half-life is around 6.5 h and relentless state is quickly accomplished with twice-daily administration [185]. | Gastrointestinal adverse events, nausea, vomiting, and headache [186]. |
| Theophyline | Theophylline is rapidly and completely absorbed after oral administration in solution or immediate-release solid oral dosage form [187]. | Biotransformation takes put through demethylation to 1-methylxanthine and 3-methylxanthine. Approximately 6% of a theophylline dosage is N-methylated to caffeine. Caffeine and 3-methylxanthine are the as it were theophylline metabolites with pharmacologic activity [188]. | Renal excretion of unaltered theophylline in neonates sums to almost 50% of the dosage, compared to approximately 10% in children more seasoned than three months and in grown-ups [187]. | Serum half-lives ranges from approximately 3–12.8 (normal 7–9) hours in something else sound, nonsmoking asthmatic grown-ups, from almost 1.5–9.5 h in children, and from around 15–58 h in untimely newborn children [187]. | Chest pain or discomfort, dizziness |
| fainting | |||||
| fast, slow, or irregular heartbeat | |||||
| increase in urine volume | |||||
| lightheadedness | |||||
| persistent vomiting | |||||
| pounding or rapid pulse | |||||
| seizures | |||||
| shakiness | |||||
| [187] | |||||
| ensifentrine (RPL554) | Not Available | metabolized by CYP2C9 [189] | Not Available | Not Available | According to the information obtained from the clinical trial studies, the side effects are very few compared to the previous PDA inhibitor drugs, except for rare cases of cough, shortness of breath, headache and liver problems [189]. |
Table 2.
Genes involved in PDE signaling in COPD in different biological samples.
| Biological Sample | Gene | Difference | Case | Control | P-Value | Ref(s) |
|---|---|---|---|---|---|---|
| ADIPOSE TISSUE | CD40 | Up | AECOPD | COPD (Stable) | 0.013 | [144] |
| IKBKAP | 0.014 | |||||
| MADD | 0.011 | |||||
| MAP2K4 | 0.017 | |||||
| MAP2K4 | 0.002 | |||||
| MAPK8 | 0.002 | |||||
| MAPK8 | 0.001 | |||||
| NFKBIA | 0.021 | |||||
| TRAF2 | 0.017 | |||||
| BLOOD | CCR2 | Up | COPD | Healthy control | 0.001 | [145] |
| DNTTIP2 | Down | COPD | 0.001 | |||
| GDAP1 | Down | COPD | 0.01 | |||
| IL6R | Up | COPD | 0.001 | |||
| LIPE (HSL) | Down | COPD | 0.01 | |||
| MMP2 | Up | AECOPD | 0.05 | [146] | ||
| MMP2 | Up | AECOPD | COPD (Stable) | 0.05 | ||
| MyD88 | Up | COPD | Healthy control | 0.01 | [147] | |
| PPP2CB | Down | COPD | 0.01 | [145] | ||
| RASSF2 | Up | COPD | 0.001 | |||
| WTAP (Wt1 Associated protein) | Up | COPD | 0.001 | |||
| LUNG BIOPSY | FOXP3 | Down | COPD | Smoker/Non-smoker | <0.05 | [113] |
| IL6 | Up | AECOPD (Phlegm-dampness syndrome) | AECOPD (Phlegm-heat syndrome) | 0.01 | [148] | |
| PPARG | Down | COPD | Non-Smoker | 0.001 | [149] | |
| RAPGEF3 (Epac1) | Down | COPD | Healthy control | 0.05 | [59] | |
| RHOA | activity Up | COPD | Healthy control | 0.001 | [63] | |
| RHOA | Expression Up | COPD | Healthy control | 0.5 | ||
| RORG | Up | COPD | Smoker/Non-smoker | <0.05 | [113] | |
| STAT3 | Up | COPD | Healthy control | 0.05 | [101] | |
| TGFB1 | Up | COPD | Healthy Non-smoker | 0.01 | [107] | |
| TNF | Up | AECOPD (Phlegm-heat syndrome) | AECOPD | 0.01 | [148] | |
| PLSAMA | CXCL8 | Up | AECOPD | Healthy | 0.05 | [9] |
| IL6 | Up | AECOPD | COPD (Stable) | 0.001 | [150] | |
| MMP9 | Down | AECOPD (Stage III&IV) | Healthy control | 0.077 | [151] | |
| MMP9 | Down | COPD (Stage III&IV) | Healthy control | 0.077 | ||
| SERUM | IFNG | Up | AECOPD | COPD (Stable) | 0.05 | [112] |
| Healthy control | 0.05 | |||||
| IL17A | Up | COPD | Healthy Smoker | 0.00302 | [60] | |
| IL1A | Up | COPD (Stable) | Healthy smoker | 0.00096 | ||
| IL2 | Down | AECOPD | Healthy control | 0.014 | [152] | |
| TNF | Up | COPD (Stable) | 0.001 | [62] | ||
| SPUTUM | CXCL8 | Up | Healthy | 0.05 | [9] | |
| IL18 | Up | COPD (Stable) | Healthy control | 0.01 | [153] | |
| IL18 | Up | AECOPD | COPD (Stable) | 0.5 | ||
| IL1B | Up | Healthy (Smoker&nonsmoker) | 0.05 | [154] | ||
| IL1B | Up | COPD (Stable) | 0.05 | |||
| IL6 | Up | Healthy control | 0.05 | [105] | ||
| TNF | Up | Healthy | 0.05 | [9] |
IKBKAP: inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase complex-associated protein, MADD: MAP Kinase Activating Death Domain, MAP2K4: Mitogen-Activated Protein Kinase 4, NFKBIA: NFKB Inhibitor Alpha, TRAF2: TNF receptor-associated factor 2, CCR2: C–C Motif Chemokine Receptor 2, DNTTIP2: Deoxynucleotidyltransferase Terminal Interacting Protein 2, MMP2: Matrix Metallopeptidase 2, MYD88: Innate Immune Signal Transduction Adaptor, PPP2CB: Protein Phosphatase 2 Catalytic Subunit Beta, RASSF2: Ras Association Domain Family Member 2, FOXP3: Forkhead Box P3, PPARG: Peroxisome proliferator-activated receptor gamma, Epac: Exchange protein directly activated by cAMP, RHOA: Ras Homolog Family Member A, RORG: RAR-related orphan receptor gamma, STAT3: Signal Transducer And Activator Of Transcription 3, TGFB1: Transforming Growth Factor Beta 1, IFNG: Interferon Gamma, CXCL8: C-X-C Motif Chemokine Ligand 8.
3.2. The impact of Roflumilast in COPD
A hallmark feature of COPD exacerbations is a marked increase in systemic inflammatory mediators [150,[155], [156], [157]] which are appropriate therapeutic targets. Roflumilast, the only FDA-approved oral PDE4 inhibitor, blocks cAMP hydrolysis to AMP in inflammatory cells, and thereby raises intracellular cAMP level. cAMP activates anti-inflammatory signaling pathways that reduce cytokines production, neutrophil inflammatory mediators, and cell surface indicators of numerous cell types, allergen, and lipopolysaccharide-induced inflammation, and finally result in fewer exacerbations [[158], [159], [160], [161], [162]]. The frequency of exacerbations requiring corticosteroids can be reduced by Roflumilast in moderate to severe COPD patients suffering from chronic bronchitis [33]. In addition, Roflumilast results in a decreased rate of exacerbation-induced hospitalization and adverse events in high risk COPD patients despite ICS and LABA therapy [163]. Furthermore, it improves forced expiratory volume (FEV1) and lowers hyperinflation [164]. Roflumilast can also be used with corticosteroids. In comparison to dexamethasone alone, coadministration of Roflumilast with dexamethasone causes significant anti-inflammatory effects on CD8+ T cells isolated from COPD patients through hindering releasing IL-2 and IFN-γ [165]. Nonetheless, Roflumilast caused adverse effects as well as an unexpected pharmacological response in these surveys [30,33]. Roflumilast therapy is now discontinued due to a variety of adverse effects, including diarrhea, nausea, pneumonia, headache, and carcinogenic risk [163,[166], [167], [168]]. Weight loss is another common systemic side effect of Roflumilast that is associated with higher primary BMI and is thought to be a consequence of elevated cAMP effects on regulating signaling pathways of lipolysis [44,169]. According to FDA report, Roflumilast therapy is also associated with more psychiatric symptoms than placebo [170]. In fact, in comparison with other inhaled medications, PDE4 inhibitors have more side effects in COPD, in a dose dependent manner [23]. Compared with 500 μg/day dose of Roflumilast, 250 μg/day regimens correlated with much less side effects, including nausea, vomiting, and diarrhea, lack of appetite, gastrointestinal difficulties, and cessation [[171], [172], [173]]. The severity of COPD also affects the efficacy and side effects of Roflumilast. The beneficial effects of Roflumilast are higher in patients with a prior history of an acute exacerbation induced hospitalization [174,175]. In terms of health status and quality of life scores, patients with moderate-to-severe frequent exacerbations also experience greater benefit of Roflumilast therapy than patients with non-frequent exacerbations [176]. Furthermore, as with ICS-responsiveness, Roflumilast efficacy is positively associated with eosinophil count in COPD patients [52], a criterion for the selection of appropriate cases. To avoid side effects, much lower dosages of a drug can be used if multiple drugs with distinct targets or drugs with different pharmaceutical actions are administered [177]. Ensifentrine, a bi-functional PDE3/PDE4 inhibitor, causes an apparent synergic effect. It was previously known as Ensifentrine [LS-193,855; 9,10-dimethoxy-2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2 H-pyrimido- [6,1-a] iso. While the exact mechanism of this effect is unclear, it is thought to be due to different pools of cAMP regulated by these two isozymes; PDE3 is predominantly located in a particulate cellular fraction and PDE4 is predominantly cytosolic (Table 3) [178,179]. Considering PDE4 inhibitors as rare, effective, and long-lasting therapies in COPD compared to early stage and short-term therapies (i.e., bronchodilators), further investigations are required to come up with an appropriate strategy to make PDE4 inhibitors, especially Roflumilast, side effects ignorable.
4. The effect of PDE inhibitors on different cells in COPD
4.1. Neutrophils
Neutrophil elevation is associated with chronic inflammation in COPD patients. A further enhancement in neutrophil frequency also occurs in the peripheral blood of COPD patients based on the disease severity [7,190]. Nonetheless, there is a discrepancy in the neutrophil count in COPD patients' BAL and sputum [191] that can be due to differences in the disease stage or applied experimental techniques. In response to higher levels of chemoattractants such as IL-8 and IL-6, neutrophils infiltrate lung tissue and secreting neutrophil elastase (NE), oxidative reaction species, and MMPs to contribute in local tissue damage [192], which results in emphysema and reduced lung function. In terms of COPD therapeutic interventions, it seems that neutrophils play a decisive role. While the inefficacy of glucocorticoids [193,194] is linked to neutrophil dominance in COPD [195], PDE4 inhibitors, ASP3258, and Roflumilast (but not prednisolone) are effective. They significantly inhibit human neutrophil chemotaxis and superoxide production [196], which finally lead to neutrophil migration suppression. Roflumilast reduces the rate of neutrophils (35%) and eosinophils (50%) in sputum of COPD patients [177]. Roflumilast's reversed inhibitory mechanism on CXCL1-induced neutrophil migration by the exchange factor directly activated by cAMP1 (Epac1) specific (CE3F4) and pan-Epac (ESI-09) inhibitors shows that Epac1 is a mediator of Roflumilast's anti-neutrophil migration impact [197]. PDE7 exists in human peripheral blood and airway neutrophils [198]. In contrast to Roflumilast that significantly inhibits neutrophil degranulation, PDE7 isoenzyme selective inhibitor PF 0332040 had no inhibitory effect on NE from human peripheral blood neutrophils stimulated with FMLP (N-Formyl-methionyl-leucyl-phenylalanine) [18]. Due to higher level of TNF-α in the serum and sputum of COPD patients, the responsiveness of FMLP-induced neutrophils to a given stimulus or the integrin-mediated ability of neutrophils to adhere to human umbilical vein endothelial cells (HUVECs) improves. PDE4 inhibitors hinder not only hypersensitivity and neutrophil degranulation, but also inhibit neutrophil HUVEC adhesion through lowered secretion of NE, Myeloproxidase (MPO), and MMP-9 under both presence and absence of TNF-α [9,60,199]. Therefore, further studies are required on the mechanism of action of PDE inhibitors on neutrophils, as a biomarker of COPD, to deepen our understanding of COPD pathogenesis and design novel therapeutic interventions.
4.2. Macrophages
According to the T-helper immune response, there are two main types of macrophages including macrophage 1 (M − 1) linked to Th1, and macrophage 2 (M − 2) linked to Th2. While the former plays role in the progression of inflammation, the latter is important in resolution and tissue remodeling [200,201]. Macrophages aggregate in the lung of frequent smokers and COPD patients. Due to the augmentation of circulating monocyte recruitment, they usually accumulate in the alveoli, bronchiole, and small airways. Elevated levels of chemokines such as CCL2 and CXCL1 in the sputum and BAL fluid of COPD patients attract circulating monocytes chemotactically [202]. In the lungs of young smokers, macrophages also accumulate in the bronchiolar region and cause bronchiolitis [203,204]. The higher numbers of airway macrophages correlate with COPD severity that can be explained partly by macrophage participation in the emphysema process [205]. Higher rates of inflammatory cells, including macrophages, lymphocytes, and neutrophils, have been significantly shown in the emphysematous lung tissues and airspaces of emphysema patients as well as heavy smokers. The highest number of inflammatory cells belongs to macrophages, indicating their possibly critical role in emphysema progression [206]. Since cytokine and chemokine production by BAL derived macrophages are lower, the alveolar macrophages of smokers do not regulate pulmonary inflammation through production of proinflammatory cytokines [[207], [208], [209]]. Given the role of alveolar macrophages in COPD progression as well as the presence of several PDE isoforms in alveolar macrophages, it seems that PDE inhibition is a logical approach to blocking COPD inflammation [210,211]. In a mouse model of COPD, treatment with the PDE-4 inhibitor Piclamilast prevented significantly the smoke-induced increase of alveolar macrophages [212]. Furthermore, another PDE4 inhibitor, ASP3258, also significantly decreased neutrophil and macrophage infiltration into alveoli in the LPS-instilled lung of rats [196]. While alveolar macrophages express PDE3, 4, and 7A [213], inhibitors of both PDE3 and PDE4 isoforms suppress macrophage activity. However, PDE4 inhibitors are less effective in macrophages than monocytes [214]. Therefore, it seems that the attenuation of macrophages (CD68+ cells) with Cilomilast, a PDE4 inhibitor, in the airway of COPD patients during a clinical trial is through the blockade of monocyte recruitment into the lungs [215].
4.3. Lymphocytes
Together with macrophages and neutrophils, lymphocytes are characterized factors of inflammatory infiltration in COPD [216]. Through enhancing the recruitment and reactivity of neutrophils, T lymphocytes enhance the endothelial cell dysfunction mediated by neutrophils, and contribute to the inflammation-induced vascular protein leakage [217,218]. The close positive correlation between neutrophils and lymphocytes implies the higher lymphocyte count in COPD patients. The level of CD8+ lymphocytes is associated with COPD severity, with a significant difference in the blood cell count between healthy and stable COPD blood samples, as well as stable and acute exacerbation COPD cases [62]. In addition, the rate of NK cells in BAL from COPD patients is higher than never-smokers and non-smokers. Ex-smokers with COPD had a much larger percentage of NK cells than ex-smokers with normal lung function, indicating persistency of this difference [219]. PDEs activity can affect lymphocyte proliferation, as reduced PDE7 expression levels resulting from the PDE7 antisense oligonucleotide could decrease the proliferation of CD4+ lymphocyte [220]. Accordingly, combination of PDE4 and PDE7 inhibitors amplified inhibition of lymphocyte proliferation [20]. Moreover, both PF 0332040 and Rolipram, isoenzyme specific inhibitors of PDE7 and PDE4, reduced PHA-stimulated proliferation of human peripheral blood mixed mononuclear cells (HPBMNC) in a concentration-dependent manner. Co-administration of Rolipram and PF 0332040 significantly increased the inhibition rate compared to single drug administration [18]. Therefore, the effectiveness of PDEs on neutrophils’ function may be partly through the blockade of lymphocytes. Besides, Treg cells have a key role to immune tolerance network. Recent studies showed that the cAMP- induced activation of PKA and EPAC are implicated in the Treg homeostasis. Some mechanisms and factors are involved by Treg to provide suppression potency. Treg cells apply cAMP in several mechanisms due to its inhibitory functions. Treg cells influx cAMP into gap junction and microenvironment to affect the effector T cells [221]. Second, additional to Treg contain a vast amount of adenosine which release into microenvironment, Treg can convert the adenosine triphosphate to adenosine by their surface CD39 and CD37 and liberate into gap junction to promote the intracellular adenylate cyclase in the effector T cells [222]. The downstream cAMP can activate PKA pathway. PKA can activate the cAMP responsive element modulator (CREM) proteins that affect epigenetic modifications and transcription of cytokines involved in T cell differentiation [223]. CREM can repress IL-2 and IL-17 gene expression resulting in effector T cell suppression (such as Th17 cells). In addition, it seems that cAMP elevating agents such as cholerae toxin can induce upregulation of inhibitory molecules such as CTLA-4 in Treg cells which acquired suppressive function [222].
4.4. Epithelial
Epithelial cells are the frontline cell population in tracheobronchial tree affecting by inhaled environmental irritants through the airways, such as cigarette smoke as the major risk factor associated with COPD. Epithelial cells play pivotal roles in airway innate immunity with tight junctions blocking penetration of microorganisms into the body, and anti-protease, anti-oxidant, and anti-bacterial compounds production as well as ciliary clearance [224]. Novel histopathological findings such as epithelial remodeling and fibrosis in tiny airway sub-epithelial cells are now recognized as a major structural change in COPD [225,226]. Hyperplasia (high levels of mucus exudation) and metaplastic changes in epithelial cells of small airway were demonstrated in COPD [227,228]. Moreover, the bronchial accumulation of myofibroblasts that causes small airway narrowing in COPD can emanate from epithelial to mesenchymal transition (EMT) as a part of airway remodeling [229]. Following EMT, epithelial cells lose their cellular polarity and adhesiveness, migration capacity, and develop a mesenchymal phenotype [230]. Cigarette smoke can cause EMT and subsequent bronchial narrowing [[231], [232], [233]]. EMT-related markers (Vimenting and S100A4) are strongly associated with airway obstruction and lower lung function [234]. Study of primary bronchial epithelial cells in the small bronchi demonstrated the presence of EMT in smokers as well as smoker COPD patients [235]. Therefore, studies have focused on EMT inhibition as an appropriate therapeutical strategy for COPD. As anti-inflammatory drugs, PDE4 inhibitors can curb lung structural remodeling and mucociliary malfunction [162]. In vitro administration of Roflumilast N-oxide to human bronchial epithelial cells (HBEC) attenuates EMT in cigarette smoke-induced EMT [235]. In a treatment procedure, Roflumilast reduced the development of bleomycin-induced lung damage and alleviated the bleomycin-induced lung fibrotic and vascular remodeling responses, the latter of which was resistant to glucocorticoids [236,237]. Other COPD drug classes, such as ICSs, are also capable of reducing EMT in COPD patients [238]. Next, the surface epithelium of the upper and lower respiratory tract is composed of goblet cells that exudate mucin [239]. In response to a variety of airway irritants like gasses, bacterial products, inflammatory mediators, and cigarette smoke, the frequency of airway goblet cells increases following non-granulated progenitor cells differentiation [240,241]. Goblet cell hyperplasia and subsequent higher mucus exudation is a remarkable characteristic of remodeled airway epithelium in COPD that negatively affects exacerbation rates, hospitalization, and mortality [[242], [243], [244]]. Roflumilast has also been used as an expectorant therapy in attenuating mucus hypersecretion, but this function restricts to a small group of patients [242,243,245,246]. In addition, it showed some central nervous system related side effects, including nausea and vomiting [33]. However, another PDE4 inhibitor TAS203 that poses lower emetogenicity suppresses goblet cell hyperplasia of the airway epithelium in a cigarette smoke induced guinea pig model [247]. The continuous inflammation caused by cigarette smoke is assumed to be the cause of structural alterations in lungs that contributes to COPD [194]. Inhaled irritants such as cigarette smoke stimulate epithelial cells of lung tissue to produce innate inflammatory mediators, including TNF-α, IL-1β, IL-6, GM-CSF, and CXCL8 (IL-8) [248] (Table 1 and Fig. 1). In response to airborne irritants, the epithelium of small airways expresses TGF-, which can enhance the induction of local fibrosis [249]. Bronchial epithelial cells cause accumulation of CD8+ cells through expressing higher levels of IP-10. Following the release of TNF-α, granzyme B, and perforins by CD8+ cells, apoptosis and cytolysis of alveolar epithelial cells and subsequently airway epithelial destruction occur [250,251]. Since ROS induces higher levels of proinflammatory cytokines through the activation of NF-kB signaling pathway in human epithelial cell lines [252], we postulate that cigarette smoke-produced ROS promotes airway epithelial inflammation by activating the NF-kB, mitochondria, and inflammasome signaling pathway [253]. In the bronchial epithelium and submucosa of COPD patients, the number of IL-22+ and IL-23+ cells is much higher than in non-smokers [116]. IL-23 can induce IL-17A production [254,255] with potential positive-feedback loops. IL-17A and IL-17F may recruit neutrophils indirectly and play an important role in chronic pulmonary inflammation [256,257]. Anti-IL-17 and IL-23 antibodies have been shown to be effective against neutrophilic inflammation in a variety of diseases as well as animal models. Therefore, understanding the underlying processes of COPD inflammation may aid in the development of new therapeutic targets against COPD [258]. As previously stated, high levels of cAMP can activate Treg cells to suppress effector T cells such as Th17, and high levels of cAMP can activate CREM to suppress IL-17 gene expression. Furthermore, by increasing cAMP levels, PDE4 inhibitors such as apremilast reduced pro-inflammatory TNF, IFN, and IL-17 production while increasing anti-inflammatory IL-10 production [259].
5. Infection and PDE signaling in COPD
5.1. Microbiome and cAMP- PDE
Microbiome is microorganisms residing in the human body together with their genomes, which lives on the skin and in mucosal surfaces [260]. Although the lower respiratory system was not included in the “Human Microbiome project”, it did not take long to find out that the lower respiratory system is not sterile [261] and contains 103 CFU/ml of microorganisms, possibly as a Transient “But Not Resident (TBNR)” model, mainly originating from mouth aspiration, gut, and environment [262]. Studies have shown that the diversity and frequency of the lung microbiome are in intricate homeostasis with the lung immune system being beneficial to organize the appropriate composition of residing microbiome, known as symbiosis. So, any variation in this balance resulting in the loss of beneficial micorbiome (dysbiosis) can result in effector T cells (e.g., Th17) immune response induction, inflammation, and, if the host is susceptible, infection. In this regard, the considerable theory “vicious cycle” explains that a defective immune system and dysbiosis ignite each other cyclically [263,264]. As a crucial second messenger, cAMP is pivotal in establishing homeostasis between the microbiome and the immune system. The healthy microbiome elevates cAMP levels by producing short-chain fatty acids, including butyrate, propionate, and acetate, through the fermentation of dietary fibers. Higher cAMP levels correspond to Treg-mediated responses and functional T cell suppression [265]. The association between cAMP level, dysbiosis and symbiosis is depicted in Fig. 4.
Fig. 4.
The association between cAMP, dysbiosis, and symbiosis. cAMP has a central role in the homeostasis between the immune system and the human microbiome in the body. In the physiological condition, the symbiotic microbiome produces short-chain fatty acids that lead to the inhibition of cAMP-CRP and thus increase the level of cAMP. cAMP activates T-reg inhibitory responses resulting in homeostasis. On the contrary, microbiome dysbiosis decreases cAMP levels and leads to local or systemic inflammation.
5.2. Infections and cAMP-PDE
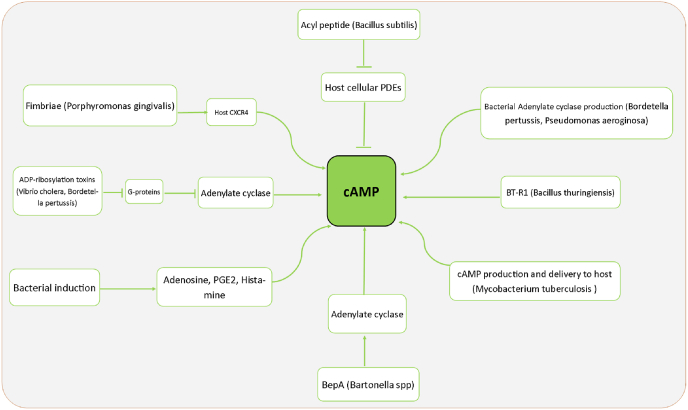
cAMP level is a very important factor in modulating the innate and specific immune systems of the host. Accordingly, many pathogenic bacteria target the cAMP level in several ways as a virulence strategy to suppress host defense immune to regulate the expression of inflammatory mediators and reduce the phagocytic activity of host cells [266]. Some pathogenic bacteria increase cellular cAMP levels through various pathogenic and host immune evasion mechanisms. For example, Bordetella pertussis and Vibrio cholerae produce ADP-ribosylating toxins that inhibit G-protein, and Bartonella Spp. produce BepA metabolite, which all increases adenylate cyclase levels in the cells to produce cAMP. Some bacteria can produce class III PDE enzymes as the significant and most frequent class of PDE that can degrade cyclic nucleotides, including cAMP and cGMP. For example, Rv0805 is a PDE enzyme of Mycobacterium tuberculosis [267]. In addition, CpdA is a top member of class III PDE found in E coli [268], Vibrio vulnificus, and Pseudomonas aeruginosa [269]. that hydrolyzes cAMP. Bacterial PDEs play roles in regulating peptidoglycan biosynthesis, cell wall permeability, and bacterial virulence. Using CpdA PDE, Pseudomonas aeruginosa can produce adenylate cyclase to increase cAMP levels in the cells [[270], [271], [272]]. Mycobacterium tuberculosis can produce and inject cAMP directly into the host cells [273]. Some important examples of such bacterial mechanisms are demonstrated in Fig. 3. Hence, along with antibiotic prescriptions that re-establish the symbiotic state and decrease cAMP levels, PDE inhibitors are sometimes prescribed for exacerbated chronic respiratory diseases such as COPD. By inhibiting PDEs, the reduction in the cAMP level is compensated. For example, Bacillus subtilis produces a metabolite Acyl peptide that directly inhibits host PDEs and increases cAMP levels of the host cells [271,274]. Another immune defect in exacerbated COPD patients is incomplete autophagy [275]. Although in normal autophagy, macrophages and neutrophils clear the lungs from invasive microorganisms, incomplete autophagy leads to bacterial survival and proliferation inside phagocytic cells and causes chronic infection [276]. As discussed earlier, dysbiosis and the loss of beneficial microbiome members, especially butyrate-producing bacteria, can induce decreased cAMP levels that, in turn, aggravate immune responses and autophagy. Therefore, in COPD patients with low levels of cAMP, higher levels of incomplete autophagy may develop more chronic infections [276,277]. As a result, it seems that simultaneous administration of a PDE inhibitor and antibiotic may be a therapeutic key in converting dysbiosis to symbiosis and managing COPD (Fig. 5).
Fig. 5.
Some important examples of bacterial mechanisms to increase host cell levels of cAMP. Pathogenic bacteria directly or indirectly (through producing some metabolites that impact cAMP) induce cAMP to increase in the host cells. For example, Bordetella pertussis and Vibrio cholerae produce ADP-ribosylating toxins, and Bartonella produces BepA metabolite to produce cAMP. Some bacteria produce class III PDE enzymes that degrade cAMP. For example, Mycobacterium tuberculosis produces Rv0805, and E coli, Vibrio vulnificus, and Pseudomonas aeruginosa produce CpdA as a major class III PDE that hydrolyzes cAMP. Using CpdA PDE, Pseudomonas aeruginosa can produce adenylate cyclase to increase cAMP levels in the cells, and Mycobacterium tuberculosis can produce and inject directly cAMP into the host cells.
6. Conclusion
COPD is now considered as a global challenge for which many efforts are being made to prevent, diagnose, and treat. The administration of corticosteroids is currently the main treatment in these patients, but drug-resistance is observed in significant number of patients. Recently, the cAMP- phosphodiesterase (PDE) signaling pathway has gained much attraction of researchers. Beside various in vitro and animal studies, several clinical trials have also been conducted to investigate the efficiency and efficacy of the PDE-inhibitors as diagnostic and therapeutic targets. Among the different isoforms of this molecule, PDE4 has been the focus of many studies. Considering the central role of cAMP in cell metabolism and body homeostasis, and at the same time, its key role in the suppression of inflammatory processes, the activity of PDE molecules can adjust the consumption/accumulation of cAMP. According to the studies, the accumulation of cAMP suppresses the cellular inflammation, and the expression level of PDE4 increases in COPD patients. Evidences indicate that PDE-inhibitors can reduce the rate of COPD recurrences as well as the duration of hospitalization. Furthermore, promising results have been obtained regarding to the combination therapy of this drug along with corticosteroids. The current challenge with these inhibitors is their side effects, as seen for Roflumilast and some others. The medicate ensifentrine (RPL554) is one of the PDE3/4 inhibitors that different clinical trial studies have been conducted for the adequacy of this medicate in COPD patients, and the comes about of these ponders appear that this medicate (in inhalation form) Alone and in combination with other drugs used in COPD, it has been exceptionally compelling and has appeared much less side effects than older drugs such as Roflumilast and Cilomilast, which can promise the development of effective drugs within the treatment of this chronic disease.
Ethical Approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
No funding was obtained for this study.
Authors' contributions
The supervisor of the project was S.A.J. S.A.J and M.GH conceived of the presented idea.Y.H.N and A.A writing an article. Y.H.N and J.S carried out data gathering. M.K and A.E Corrections and search. All authors discussed the results and contributed to the final manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
Authors have no conflict of interest to declare.
Acknowledgments
Authors would thank Baqiyatallah University of Medical Sciences for their support.
Data availability
Data will be made available on request.
References
- 1.Rennard S.I., et al. Effects of roflumilast in COPD patients receiving inhaled corticosteroid/long-acting beta2-agonist fixed-dose combination: RE(2)SPOND rationale and study design. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:1921–1928. doi: 10.2147/COPD.S109661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurst J.R., et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 3.Soler-Cataluna J.J., et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera P.N., et al. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9(2):131–141. doi: 10.3109/15412555.2011.650239. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., et al. Resolving viral-induced secondary bacterial infection in COPD: a concise review. Front. Immunol. 2018;9:2345. doi: 10.3389/fimmu.2018.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson G., et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandara L., et al. Systemic inflammation up-regulates serum hepcidin in exacerbations and stabile chronic obstructive pulmonary disease. Clin. Biochem. 2015;48(18):1252–1257. doi: 10.1016/j.clinbiochem.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmik A., et al. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daldegan M.B., Teixeira M.M., Talvani A. Concentration of CCL11, CXCL8 and TNF-alpha in sputum and plasma of patients undergoing asthma or chronic obstructive pulmonary disease exacerbation. Braz. J. Med. Biol. Res. 2005;38(9):1359–1365. doi: 10.1590/s0100-879x2005000900010. [DOI] [PubMed] [Google Scholar]
- 10.Yang I.A., et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012;(7) doi: 10.1002/14651858.CD002991.pub3. 2012. CD002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A.V., et al. Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest. 2018;153(2):321–328. doi: 10.1016/j.chest.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. American journal of respiratory and critical care medicine. 2017 Mar 1;195(5):557-82, doi:10.1164/rccm.201701-0218PP . [DOI] [PubMed]
- 13.Barber R., et al. vol. 287. 2004. pp. L332–L343. (Differential Expression of PDE4 cAMP Phosphodiesterase Isoforms in Inflammatory Cells of Smokers with COPD, Smokers without COPD, and Nonsmokers). 2. [DOI] [PubMed] [Google Scholar]
- 14.Brown W.M. Treating COPD with PDE 4 inhibitors. Int. J. Chronic Obstr. Pulm. Dis. 2007;2(4):517–533. [PMC free article] [PubMed] [Google Scholar]
- 15.Yuasa K., et al. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J. Biol. Chem. 2000;275(40):31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T., Kotera J., Omori K. Novel alternative splice variants of rat phosphodiesterase 7B showing unique tissue-specific expression and phosphorylation. Biochem. J. 2002;361(Pt 2):211–220. doi: 10.1042/0264-6021:3610211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Chung K. Phosphodiesterase inhibitors in airways disease. Eur. J. Pharmacol. 2006;533(1–3):110–117. doi: 10.1016/j.ejphar.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 18.Jones N.A., et al. Phosphodiesterase (PDE) 7 in inflammatory cells from patients with asthma and COPD. Pulm. Pharmacol. Ther. 2007;20(1):60–68. doi: 10.1016/j.pupt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Azevedo M.F., et al. Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr. Rev. 2014;35(2):195–233. doi: 10.1210/er.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips J.E. Inhaled phosphodiesterase 4 (PDE4) inhibitors for inflammatory respiratory diseases. Front. Pharmacol. 2020;11:259. doi: 10.3389/fphar.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamatawong T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J. Thorac. Dis. 2017;9(4):1144–1154. doi: 10.21037/jtd.2017.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAleer J.P., Kolls J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018;48(1):39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong J., Leung B., Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013;(11):CD002309. doi: 10.1002/14651858.CD002309.pub4. [DOI] [PubMed] [Google Scholar]
- 24.Boswell-Smith V., Cazzola M., Page C.P. Are phosphodiesterase 4 inhibitors just more theophylline? J. Allergy Clin. Immunol. 2006;117(6):1237–1243. doi: 10.1016/j.jaci.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 25.Torphy T.J., Undem B.J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giembycz M.A., Dent G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin. Exp. Allergy. 1992;22(3):337–344. doi: 10.1111/j.1365-2222.1992.tb03095.x. 10.1111/j.1365-2222.1992.tb03095.x. [DOI] [PubMed] [Google Scholar]
- 27.Torphy T.J., Livi G.P., Christensen S.B. Novel phosphodiesterase inhibitors for the therapy of asthma. Drug News Perspect. 1993;6:203. [Google Scholar]
- 28.Lowe J.A., Chang J. The PDE IV family of calcium-independent phosphodiesterase enzymes. Drugs Future. 1992;17:799. [Google Scholar]
- 29.Akram M.F., et al. Doxofylline and theophylline: a comparative clinical study. J. Clin. Diagn. Res. 2012;6(10):1681–1684. doi: 10.7860/JCDR/2012/4697.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng J., et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest. 2014;145(1):44–52. doi: 10.1378/chest.13-1252. 10.1378/chest.13-1252. [DOI] [PubMed] [Google Scholar]
- 31.Wells J.M., et al. A randomized, placebo-controlled trial of roflumilast. Effect on proline-glycine-proline and neutrophilic inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015;192(8):934–942. doi: 10.1164/rccm.201503-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabe K.F., et al. Anti-inflammatory effects of roflumilast in chronic obstructive pulmonary disease (ROBERT): a 16-week, randomised, placebo-controlled trial. Lancet Respir. Med. 2018;6(11):827–836. doi: 10.1016/S2213-2600(18)30331-X. [DOI] [PubMed] [Google Scholar]
- 33.Calverley P.M., et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 34.Facius A., et al. Pharmacokinetic and pharmacodynamic modelling to characterize the tolerability of alternative up-titration regimens of roflumilast in patients with chronic obstructive pulmonary disease. Clin. Pharmacokinet. 2018;57(8):1029–1038. doi: 10.1007/s40262-018-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosio B.G., et al. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004;200(5):689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voduc N., et al. Effect of theophylline on exercise capacity in COPD patients treated with combination long-acting bronchodilator therapy: a pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2012;7:245–252. doi: 10.2147/COPD.S29990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins C.R., et al. The effect of low-dose corticosteroids and theophylline on the risk of acute exacerbations of COPD: the TASCS randomised controlled trial. Eur. Respir. J. 2021;57(6) doi: 10.1183/13993003.03338-2020. [DOI] [PubMed] [Google Scholar]
- 38.Cosio B.G., et al. Oral low-dose theophylline on top of inhaled fluticasone-salmeterol does not reduce exacerbations in patients with severe COPD: a pilot clinical trial. Chest. 2016;150(1):123–130. doi: 10.1016/j.chest.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Bonjour S., et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environ. Health Perspect. 2013;121(7):784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng W.T., et al. Emerging roles of G protein-coupled receptors in hepatocellular carcinoma. Int. J. Mol. Sci. 2018;19(5) doi: 10.3390/ijms19051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng X., et al. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. 2008;40(7):651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke B., et al. vol. 64. 2015. pp. 3355–3362. (Inactivation of NF-Κb P65 (RelA) in Liver Improves Insulin Sensitivity and Inhibits cAMP/PKA Pathway). 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond.) 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 44.Fabbri L.M., et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 45.Grootendorst D.C., et al. Efficacy of the novel phosphodiesterase-4 inhibitor BAY 19-8004 on lung function and airway inflammation in asthma and chronic obstructive pulmonary disease (COPD) Pulm. Pharmacol. Ther. 2003;16(6):341–347. doi: 10.1016/S1094-5539(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y.J., et al. Zl-n-91, a selective phosphodiesterase 4 inhibitor, suppresses inflammatory response in a COPD-like rat model. Int. Immunopharm. 2010;10(2):252–258. doi: 10.1016/j.intimp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Barber R., et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287(2):L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- 48.van der Schans S., et al. Systematic review and quality appraisal of cost-effectiveness analyses of pharmacologic maintenance treatment for chronic obstructive pulmonary disease: methodological considerations and recommendations. Pharmacoeconomics. 2017;35(1):43–63. doi: 10.1007/s40273-016-0448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeffery P.K. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 2000;117 doi: 10.1378/chest.117.5_suppl_1.251s. 5 Suppl 1. 251S-60S. [DOI] [PubMed] [Google Scholar]
- 50.Barreiro E., et al. The phosphodiesterase-4 inhibitor roflumilast reverts proteolysis in skeletal muscle cells of patients with COPD cachexia. J. Appl. Physiol. 1985;125(2):287–303. doi: 10.1152/japplphysiol.00798.2017. 2018. [DOI] [PubMed] [Google Scholar]
- 51.Dunne A.E., et al. Direct inhibitory effect of the PDE4 inhibitor roflumilast on neutrophil migration in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2019;60(4):445–453. doi: 10.1165/rcmb.2018-0065OC. [DOI] [PubMed] [Google Scholar]
- 52.Martinez F.J., et al. Determinants of response to roflumilast in severe chronic obstructive pulmonary disease. Pooled analysis of two randomized trials. Am. J. Respir. Crit. Care Med. 2018;198(10):1268–1278. doi: 10.1164/rccm.201712-2493OC. [DOI] [PubMed] [Google Scholar]
- 53.Quint J.K., Wedzicha J.A. The neutrophil in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2007;119(5):1065–1071. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro S.D., et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am. J. Pathol. 2003;163(6):2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stănescu D., et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51(3):267–271. doi: 10.1136/thx.51.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurst J.R., et al. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;173(1):71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 57.Matera M.G., et al. Phosphodiesterase inhibitors for chronic obstructive pulmonary disease: what does the future hold? Drugs. 2014;74(17):1983–1992. doi: 10.1007/s40265-014-0303-8. [DOI] [PubMed] [Google Scholar]
- 58.Barnes P.J. Frontrunners in novel pharmacotherapy of COPD. Curr. Opin. Pharmacol. 2008;8(3):300–307. doi: 10.1016/j.coph.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Oldenburger A., et al. Anti-inflammatory role of the cAMP effectors Epac and PKA: implications in chronic obstructive pulmonary disease. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto-Plata V., et al. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax. 2007;62(7):595–601. doi: 10.1136/thx.2006.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piehl-Aulin K., et al. Increased serum inflammatory markers in the absence of clinical and skeletal muscle inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2009;78(2):191–196. doi: 10.1159/000207793. [DOI] [PubMed] [Google Scholar]
- 62.Lim S.C., et al. Apoptosis of T lymphocytes isolated from peripheral blood of patients with acute exacerbation of chronic obstructive pulmonary disease. Yonsei Med. J. 2011;52(4):581–587. doi: 10.3349/ymj.2011.52.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bei Y., et al. Activation of RhoA/Rho-kinase pathway accounts for pulmonary endothelial dysfunction in patients with chronic obstructive pulmonary disease. Phys. Rep. 2013;1(5) doi: 10.1002/phy2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes P.J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 65.Barnes P.J. Transcription factors in airway diseases. Lab. Invest. 2006;86(9):867–872. doi: 10.1038/labinvest.3700456. [DOI] [PubMed] [Google Scholar]
- 66.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia T.G., Zhao J.Q., Liu J.H. Serum inflammatory factor and cytokines in AECOPD. Asian Pac J Trop Med. 2014;7(12):1005–1008. doi: 10.1016/S1995-7645(14)60177-2. [DOI] [PubMed] [Google Scholar]
- 68.McMillan D.H., et al. Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation. Am. J. Pathol. 2011;179(1):125–133. doi: 10.1016/j.ajpath.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labonte L., et al. Alterations in the expression of the NF-kappaB family member RelB as a novel marker of cardiovascular outcomes during acute exacerbations of chronic obstructive pulmonary disease. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohil V., et al. A study on the correlation of matrix metalloproteinase MMP1 in COPD and smoking in the North Indian population. Asian J. Med. Sci. 2017;8(1):5–14. [Google Scholar]
- 71.Sonett J., et al. American Thoracic Society; 2018. Inhibition of cigarette smoke induced MMP1 Using a novel Drug to prevent alveolar tissue damage, in A28; p. A1202. (Advances in COPD and Asthma). [Google Scholar]
- 72.Hendrix A.Y., Kheradmand F. In: Progress in Molecular Biology and Translational Science. Khalil R.A., editor. Academic Press; 2017. Chapter one - the role of matrix metalloproteinases in development, repair, and destruction of the lungs; pp. 1–29. [DOI] [PubMed] [Google Scholar]
- 73.Hosford G.E., Fang X., Olson D.M. Hyperoxia decreases matrix metalloproteinase-9 and increases tissue inhibitor of matrix metalloproteinase-1 protein in the newborn rat lung: association with arrested alveolarization. Pediatr. Res. 2004;56(1):26–34. doi: 10.1203/01.PDR.0000130658.45564.1F. [DOI] [PubMed] [Google Scholar]
- 74.Itoh T., et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58(5):1048–1051. [PubMed] [Google Scholar]
- 75.Collen A., et al. Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood. 2003;101(5):1810–1817. doi: 10.1182/blood-2002-05-1593. [DOI] [PubMed] [Google Scholar]
- 76.Huang P.-H., et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow–derived endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2009;29(8):1179–1184. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 77.Tan L.H., et al. The cytoprotective role of DJ-1 and p45 NFE2 against human primary alveolar type II cell injury and emphysema. Sci. Rep. 2018;8(1):3555. doi: 10.1038/s41598-018-21790-3. 10.1038/s41598-018-21790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mercer P.F., et al. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir. Res. 2005;6(1):151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vernooy J.H., et al. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest. 2004;126(6):1802–1810. doi: 10.1378/chest.126.6.1802. [DOI] [PubMed] [Google Scholar]
- 80.Fukuda Y., et al. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-2 in fetal rabbit lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279(3):L555–L561. doi: 10.1152/ajplung.2000.279.3.L555. [DOI] [PubMed] [Google Scholar]
- 81.Rutherford C., et al. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci. Signal. 2016;9(453):ra109. doi: 10.1126/scisignal.aaf8566. [DOI] [PubMed] [Google Scholar]
- 82.De Rooij J., et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 83.Kawasaki H., et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282(5397):2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 84.Krugmann S., et al. ARAP3 is a PI3K-and rap-regulated GAP for RhoA. Curr. Biol. 2004;14(15):1380–1384. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 85.Dekkers B.G., Racke K., Schmidt M. Distinct PKA and Epac compartmentalization in airway function and plasticity. Pharmacol. Ther. 2013;137(2):248–265. doi: 10.1016/j.pharmthera.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Mortaz E., et al. Salmeterol with fluticasone enhances the suppression of IL-8 release and increases the translocation of glucocorticoid receptor by human neutrophils stimulated with cigarette smoke. J. Mol. Med. (Berl.) 2008;86(9):1045–1056. doi: 10.1007/s00109-008-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oenema T.A., et al. Pro-inflammatory mechanisms of muscarinic receptor stimulation in airway smooth muscle. Respir. Res. 2010;11(1):130. doi: 10.1186/1465-9921-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ganesan S., et al. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax. 2013;68(2):131–141. doi: 10.1136/thoraxjnl-2012-201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dejean A.S., Hedrick S.M., Kerdiles Y.M. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxidants Redox Signal. 2011;14(4):663–674. doi: 10.1089/ars.2010.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang J.W., et al. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 2011;187(2):987–998. doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Z., et al. Molecular pathogenesis in chronic obstructive pulmonary disease and therapeutic potential by targeting AMP-activated protein kinase. J. Cell. Physiol. 2018;233(3):1999–2006. doi: 10.1002/jcp.25844. [DOI] [PubMed] [Google Scholar]
- 92.Lee J.S., et al. Role of AMP-activated protein kinase (AMPK) in smoking-induced lung inflammation and emphysema. Tuberc. Respir. Dis. 2015;78(1):8–17. doi: 10.4046/trd.2015.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng X.Y., et al. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget. 2017;8(14):22513–22523. doi: 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui W., et al. Nrf2 attenuates inflammatory response in COPD/emphysema: crosstalk with Wnt3a/beta-catenin and AMPK pathways. J. Cell Mol. Med. 2018;22(7):3514–3525. doi: 10.1111/jcmm.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rangasamy T., et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J. Clin. Investig. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iizuka T., et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Gene Cell. 2005;10(12):1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 97.Kim J., et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeYoung M.P., et al. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang G.J., et al. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic. Biol. Med. 2011;50(11):1492–1502. doi: 10.1016/j.freeradbiomed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 100.Ko H.K., et al. Regulation of cigarette smoke induction of IL-8 in macrophages by AMP-activated protein kinase signaling. J. Cell. Physiol. 2015;230(8):1781–1793. doi: 10.1002/jcp.24881. [DOI] [PubMed] [Google Scholar]
- 101.Qu P., et al. Stat3 downstream genes serve as biomarkers in human lung carcinomas and chronic obstructive pulmonary disease. Lung Cancer. 2009;63(3):341–347. doi: 10.1016/j.lungcan.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ortiz-Munoz G., et al. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29(4):525–531. doi: 10.1161/ATVBAHA.108.173781. [DOI] [PubMed] [Google Scholar]
- 103.Isomaki P., et al. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology. 2015;54(6):1103–1113. doi: 10.1093/rheumatology/keu430. [DOI] [PubMed] [Google Scholar]
- 104.Yew-Booth L., et al. JAK-STAT pathway activation in COPD. Eur. Respir. J. 2015;46(3):843–845. doi: 10.1183/09031936.00228414. [DOI] [PubMed] [Google Scholar]
- 105.Liang R., Zhang W., Song Y.M. Levels of leptin and IL-6 in lungs and blood are associated with the severity of chronic obstructive pulmonary disease in patients and rat models. Mol. Med. Rep. 2013;7(5):1470–1476. doi: 10.3892/mmr.2013.1377. [DOI] [PubMed] [Google Scholar]
- 106.Houssaini A., et al. Senescence of pulmonary vascular cells is related to overactivation of the mTOR signaling pathway in chronic obstructive pulmonary disease (COPD) Rev. Mal. Respir. 2015;32(3):306. doi: 10.1016/j.rmr.2015.02.010. [DOI] [Google Scholar]
- 107.Takizawa H., et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am. J. Respir. Crit. Care Med. 2001;163(6):1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 108.O'Shea J.J., et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garbers C., et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23(3):85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Berezowska S., et al. Glycine decarboxylase and HIF-1alpha expression are negative prognostic factors in primary resected early-stage non-small cell lung cancer. Virchows Arch. 2017;470(3):323–330. doi: 10.1007/s00428-016-2057-z. [DOI] [PubMed] [Google Scholar]
- 111.To M., et al. Defect of adaptation to hypoxia in patients with COPD due to reduction of histone deacetylase 7. Chest. 2012;141(5):1233–1242. doi: 10.1378/chest.11-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Remels A.H., et al. TNF-alpha-induced NF-kappaB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1alpha. Endocrinology. 2015;156(5):1770–1781. doi: 10.1210/en.2014-1591. [DOI] [PubMed] [Google Scholar]
- 113.Chu S., et al. The expression of Foxp3 and ROR gamma t in lung tissues from normal smokers and chronic obstructive pulmonary disease patients. Int. Immunopharm. 2011;11(11):1780–1788. doi: 10.1016/j.intimp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 114.Miossec P., Korn T., Kuchroo V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 115.Roos A.B., et al. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke–induced lymphoid neogenesis. Am. J. Respir. Crit. Care Med. 2015;191(11):1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 116.Di Stefano A., et al. T helper type 17‐related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin. Exp. Immunol. 2009;157(2):316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calverley P.M., et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 118.Jones P.W., et al. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. Eur. Respir. J. 2003;21(1):68–73. doi: 10.1183/09031936.03.00013303. [DOI] [PubMed] [Google Scholar]
- 119.Singh S., Amin A.V., Loke Y.K. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch. Intern. Med. 2009;169(3):219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 120.Calverley P.M.A., et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139(3):505–512. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 121.Tashkin D.P., et al. Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: a matched-subgroup post hoc analysis of the UPLIFT(R) trial. Respir. Res. 2018;19(1):196. doi: 10.1186/s12931-018-0874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morjaria J.B., Rigby A., Morice A.H. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT study. Lung. 2017;195(3):281–288. doi: 10.1007/s00408-017-9990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Casaburi R. Skeletal muscle function in COPD. Chest. 2000;117 doi: 10.1378/chest.117.5_suppl_1.267s-a. 5 Suppl 1. 267S-71S. [DOI] [PubMed] [Google Scholar]
- 124.Milara J., et al. MUC1 deficiency mediates corticosteroid resistance in chronic obstructive pulmonary disease. Respir. Res. 2018;19(1):226. doi: 10.1186/s12931-018-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roche N., et al. Beyond corticosteroids: future prospects in the management of inflammation in COPD. Eur. Respir. Rev. 2011;20(121):175–182. doi: 10.1183/09059180.00004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flayer C.H., Larson E.D., Haczku A. Breaking steroid resistance: effect of vitamin D on IL-23. Am. J. Respir. Cell Mol. Biol. 2017;57(3):267–269. doi: 10.1165/rcmb.2017-0199ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun X.-J., et al. Combination of erythromycin and dexamethasone improves corticosteroid sensitivity induced by CSE through inhibiting PI3K-δ/Akt pathway and increasing GR expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309(2):L139–L146. doi: 10.1152/ajplung.00292.2014. [DOI] [PubMed] [Google Scholar]
- 128.Khorasani N., et al. Reversal of corticosteroid insensitivity by p38 MAPK inhibition in peripheral blood mononuclear cells from COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:283–291. doi: 10.2147/COPD.S72403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Southworth T., et al. IFN-gamma synergistically enhances LPS signalling in alveolar macrophages from COPD patients and controls by corticosteroid-resistant STAT1 activation. Br. J. Pharmacol. 2012;166(7):2070–2083. doi: 10.1111/j.1476-5381.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barrecheguren M., et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir. Med. 2016;111:47–53. doi: 10.1016/j.rmed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 131.Reddel H.K., et al. Assessment and management of asthma and chronic obstructive pulmonary disease in Australian general practice. Aust. Fam. Physician. 2017;46(6):413–419. [PubMed] [Google Scholar]
- 132.Rubio M.C., et al. Clinical audit of COPD in outpatient respiratory clinics in Spain: the EPOCONSUL study. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:417. doi: 10.2147/COPD.S124482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barrecheguren M., Esquinas C., Miravitlles M. The asthma–chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr. Opin. Pulm. Med. 2015;21(1):74–79. doi: 10.1097/MCP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 134.Miravitlles M., et al. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir. Res. 2016;17(1):112. doi: 10.1186/s12931-016-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vestbo J., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 136.Wedzicha J.A., et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir. Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 137.Zhong N., et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:1015–1026. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wedzicha J.A., et al. Indacaterol-Glycopyrronium versus salmeterol-fluticasone for COPD. N. Engl. J. Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 139.Miravitlles M., et al. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir. Res. 2016;17(1):112. doi: 10.1186/s12931-016-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magnussen H., et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 141.Nadeem N.J., Taylor S.J., Eldridge S.M. Withdrawal of inhaled corticosteroids in individuals with COPD--a systematic review and comment on trial methodology. Respir. Res. 2011;12(1):107. doi: 10.1186/1465-9921-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rossi A., et al. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO) Respir. Res. 2014;15(1):77. doi: 10.1186/1465-9921-15-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rossi A., et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur. Respir. J. 2014;44(6):1548–1556. doi: 10.1183/09031936.00126814. [DOI] [PubMed] [Google Scholar]
- 144.Tkacova R., et al. Increased adipose tissue expression of proinflammatory CD40, MKK4 and JNK in patients with very severe chronic obstructive pulmonary disease. Respiration. 2011;81(5):386–393. doi: 10.1159/000319957. [DOI] [PubMed] [Google Scholar]
- 145.Edmiston J.S., et al. Gene expression profiling of peripheral blood leukocytes identifies potential novel biomarkers of chronic obstructive pulmonary disease in current and former smokers. Biomarkers. 2010;15(8):715–730. doi: 10.3109/1354750X.2010.512091. [DOI] [PubMed] [Google Scholar]
- 146.Shi L., et al. Selection of AECOPD-specific immunomodulatory biomarkers by integrating genomics and proteomics with clinical informatics. Cell Biol. Toxicol. 2018;34(2):109–123. doi: 10.1007/s10565-017-9405-x. [DOI] [PubMed] [Google Scholar]
- 147.Zeng X., et al. [Effects of sulforaphane on Toll-like receptor 4/myeloid differentiation factor 88 pathway of monocyte-derived macrophages from patients with chronic obstructive pulmonary disease] Zhonghua Jiehe He Huxi Zazhi. 2014;37(4):250–254. [PubMed] [Google Scholar]
- 148.Mei X., et al. [A comparative study of inflammatory factor expression of phlegm-heat syndrome and phlegm-dampness syndrome model with acute exacerbation of chronic obstructive pulmonary disease] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25(6):343–346. doi: 10.3760/cma.j.issn.2095-4352.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 149.Lakshmi S.P., et al. Down-regulated peroxisome proliferator-activated receptor γ (PPARγ) in lung epithelial cells promotes a PPARγ agonist-reversible proinflammatory phenotype in chronic obstructive pulmonary disease (COPD) J. Biol. Chem. 2014;289(10):6383–6393. doi: 10.1074/jbc.M113.536805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.Hurst J.R., et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;174(8):867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y., et al. Decreased CD34+ cell number is correlated with cardiac dysfunction in patients with acute exacerbation of COPD. Heart Lung Circ. 2014;23(9):875–882. doi: 10.1016/j.hlc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 152.Almansa R., et al. Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J. Interferon Cytokine Res. 2011;31(5):409–413. doi: 10.1089/jir.2010.0131. [DOI] [PubMed] [Google Scholar]
- 153.Haaland I., et al. Interleukin-18 levels in induced sputum of stable and exacerbated COPD patients. Eur. Respir. J. 2014;44(Suppl 58):P848. [Google Scholar]
- 154.Kersul A.L., et al. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch. Bronconeumol. 2011;47(4):176–183. doi: 10.1016/j.arbres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 155.Bathoorn E., et al. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int. J. Chronic Obstr. Pulm. Dis. 2009;4:101–109. doi: 10.2147/copd.s4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mallia P., et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 2011;183(6):734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tkacova R., et al. Systemic inflammation and systemic oxidative stress in patients with acute exacerbations of COPD. Respir. Med. 2007;101(8):1670–1676. doi: 10.1016/j.rmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 158.Hatzelmann A., et al. The preclinical pharmacology of roflumilast–a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Therapeut. 2010;23(4):235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 159.Gauvreau G.M., et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir. Res. 2011;12(1):140. doi: 10.1186/1465-9921-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schick M.A., et al. Phosphodiesterase‐4 inhibition as a therapeutic approach to treat capillary leakage in systemic inflammation. J. Physiol. 2012;590(11):2693–2708. doi: 10.1113/jphysiol.2012.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wedzicha J.A., Calverley P.M., Rabe K.F. Roflumilast: a review of its use in the treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:81–90. doi: 10.2147/COPD.S89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hatzelmann A., et al. The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010;23(4):235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 163.Martinez F.J., et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 164.Vos W., et al. Functional respiratory imaging to assess the interaction between systemic roflumilast and inhaled ICS/LABA/LAMA. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:263. doi: 10.2147/COPD.S93830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grundy S., et al. Additive anti-inflammatory effects of corticosteroids and phosphodiesterase-4 inhibitors in COPD CD8 cells. Respir. Res. 2016;17:9. doi: 10.1186/s12931-016-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Giembycz M.A., Field S.K. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des. Dev. Ther. 2010;4:147–158. doi: 10.2147/dddt.s7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rabe K.F., et al. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 168.Kim K.H., et al. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:3449–3456. doi: 10.2147/COPD.S143967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rabe K.F. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br. J. Pharmacol. 2011;163(1):53–67. doi: 10.1111/j.1476-5381.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chong J., Leung B., Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017;9(9):CD002309. doi: 10.1002/14651858.CD002309.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Watz H., et al. Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:813–822. doi: 10.2147/COPD.S154012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lahu G., Facius A. Application of population pharmacokinetic modeling to explore the impact of alternative roflumilast dosing regimens on tolerability. Int. J. Clin. Pharm. Ther. 2013;51(11):832–836. doi: 10.5414/cp201906. [DOI] [PubMed] [Google Scholar]
- 173.Joo H., et al. Incidence of adverse effects and discontinuation rate between patients receiving 250 micrograms and 500 micrograms of roflumilast: a comparative study. Tuberc. Respir. Dis. 2018;81(4):299–304. doi: 10.4046/trd.2018.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han M.K., et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am. J. Respir. Crit. Care Med. 2014;189(12):1503–1508. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rabe K.F., et al. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur. Respir. J. 2017;50(1) doi: 10.1183/13993003.00158-2017. [DOI] [PubMed] [Google Scholar]
- 176.Kardos P., et al. Health status in patients with COPD treated with roflumilast: two large noninterventional real-life studies: DINO and DACOTA. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:1455–1468. doi: 10.2147/COPD.S159827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cazzola M., et al. Ensifentrine (RPL554): an inhaled 'bifunctional' dual PDE3/4 inhibitor for the treatment of asthma and chronic obstructive pulmonary disease. Pharm Pat Anal. 2018;7(6):249–257. doi: 10.4155/ppa-2018-0030. [DOI] [PubMed] [Google Scholar]
- 178.Matera M.G., Page C.P., Cazzola M. Novel bronchodilators for the treatment of chronic obstructive pulmonary disease. Trends Pharmacol. Sci. 2011;32(8):495–506. doi: 10.1016/j.tips.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 179.Denis D., Riendeau D. Phosphodiesterase 4-dependent regulation of cyclic AMP levels and leukotriene B4 biosynthesis in human polymorphonuclear leukocytes. Eur. J. Pharmacol. 1999;367(2–3):343–350. doi: 10.1016/s0014-2999(98)00987-x. [DOI] [PubMed] [Google Scholar]
- 180.Huang J., et al. vol. 12. 2018. p. 4047. (Pharmacokinetics of Single-And Multiple-Dose Roflumilast: an Open-Label, Three-Way Crossover Study in Healthy Chinese Volunteers). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li Q., et al. vol. 42. 2017. pp. 371–381. (Pharmacokinetics of Roflumilast and its Active Metabolite Roflumilast N-Oxide in Healthy Chinese Subjects after Single and Multiple Oral Doses). 3. [DOI] [PubMed] [Google Scholar]
- 182.Moussa B.A., et al. Roflumilast analogs with improved metabolic stability, plasma protein binding, and pharmacokinetic profile. 2019;11(6):886–897. doi: 10.1002/dta.2562. [DOI] [PubMed] [Google Scholar]
- 183.Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. International journal of chronic obstructive pulmonary disease. 2016 Jan;6:81–90. doi: 10.2147/COPD.S89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zussman B.D., et al. vol. 21. 2001. pp. 653–660. (Bioavailability of the Oral Selective Phosphodiesterase 4 Inhibitor Cilomilast). 6. [DOI] [PubMed] [Google Scholar]
- 185.Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert opinion on investigational drugs. 2001 Jul 1;10(7):1361-79, doi:10.1517/13543784.10.7.1361. [DOI] [PubMed]
- 186.Liu F.-C., et al. vol. 16. 2018. pp. 1–14. (Use of Cilomilast-Loaded Phosphatiosomes to Suppress Neutrophilic Inflammation for Attenuating Acute Lung Injury: the Effect of Nanovesicular Surface Charge). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jilani T.N., Preuss C.V., Sharma S. StatPearls Publishing; 2021. Theophylline. in StatPearls [Internet] [DOI] [PubMed] [Google Scholar]
- 188.Zhao X., et al. vol. 48. 2020. pp. 345–352. (Theophylline Acetaldehyde as the Initial Product in Doxophylline Metabolism in Human Liver). 5. [DOI] [PubMed] [Google Scholar]
- 189.Singh D., et al. vol. 21. 2020. pp. 1–11. (A Dose-Ranging Study of the Inhaled Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine in COPD). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Günay E., et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37(2):374–380. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 191.Eapen M.S., et al. Airway inflammation in chronic obstructive pulmonary disease (COPD): a true paradox. Expet Rev. Respir. Med. 2017;11(10):827–839. doi: 10.1080/17476348.2017.1360769. [DOI] [PubMed] [Google Scholar]
- 192.Shaykhiev R., Crystal R.G. Innate immunity and chronic obstructive pulmonary disease: a mini-review. Gerontology. 2013;59(6):481–489. doi: 10.1159/000354173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pauwels R.A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 194.Barnes P.J., Shapiro S., Pauwels R. Chronic obstructive pulmonary disease: molecular and cellularmechanisms. Eur. Respir. J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 195.Belvisi M.G. Regulation of inflammatory cell function by corticosteroids. Proc. Am. Thorac. Soc. 2004;1(3):207–214. doi: 10.1513/pats.200402-002MS. [DOI] [PubMed] [Google Scholar]
- 196.Kubo S., et al. Anti-neutrophilic inflammatory activity of ASP3258, a novel phosphodiesterase type 4 inhibitor. Int. Immunopharm. 2012;12(1):59–63. doi: 10.1016/j.intimp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 197.Dunne A.E., et al. Direct inhibitory effect of the phosphodiesterase-4 inhibitor, roflumilast, on neutrophil migration in COPD. Am. J. Respir. Cell Mol. Biol. 2018 doi: 10.1165/rcmb.2018-0065OC. (ja) [DOI] [PubMed] [Google Scholar]
- 198.Smith S.J., et al. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284(2):L279–L289. doi: 10.1152/ajplung.00170.2002. [DOI] [PubMed] [Google Scholar]
- 199.Jones N.A., et al. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm. Pharmacol. Ther. 2005;18(2):93–101. doi: 10.1016/j.pupt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 200.He S., et al. Characteristics and potential role of M2 macrophages in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:3029–3039. doi: 10.2147/COPD.S147144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 202.Traves S., et al. Increased levels of the chemokines GROα and MCP-1 in sputum samples from patients with COPD. Thorax. 2002;57(7):590–595. doi: 10.1136/thorax.57.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jeffery P.K. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53(2):129–136. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Finkelstein R., et al. Alveolar inflammation and its relation to emphysema in smokers. Am. J. Respir. Crit. Care Med. 1995;152(5 Pt 1):1666–1672. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- 205.Di Stefano A., et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am. J. Respir. Crit. Care Med. 1998;158(4):1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 206.Retamales I., et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am. J. Respir. Crit. Care Med. 2001;164(3):469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 207.Dandrea T., et al. Differential inhibition of inflammatory cytokine release from cultured alveolar macrophages from smokers and non-smokers by NO2. Hum. Exp. Toxicol. 1997;16(10):577–588. doi: 10.1177/096032719701601005. [DOI] [PubMed] [Google Scholar]
- 208.McCrea K.A., et al. Altered cytokine regulation in the lungs of cigarette smokers. Am. J. Respir. Crit. Care Med. 1994;150(3):696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 209.Ohta T., et al. Cigarette smoking decreases interleukin-8 secretion by human alveolar macrophages. Respir. Med. 1998;92(7):922–927. doi: 10.1016/s0954-6111(98)90191-3. [DOI] [PubMed] [Google Scholar]
- 210.Barnes P.J. New treatments for COPD. Nat. Rev. Drug Discov. 2002;1(6):437–446. doi: 10.1038/nrd820. [DOI] [PubMed] [Google Scholar]
- 211.Barnes P. Chronic obstructive pulmonary disease• 12: new treatments for COPD. Thorax. 2003;58(9):803–808. doi: 10.1136/thorax.58.9.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Seimetz M., et al. Cigarette smoke-induced emphysema and pulmonary hypertension can Be prevented by phosphodiesterase 4 and 5 inhibition in mice. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smith S.J., et al. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284(2):L279–L289. doi: 10.1152/ajplung.00170.2002. [DOI] [PubMed] [Google Scholar]
- 214.Dent G., et al. Theophylline suppresses human alveolar macrophage respiratory burst through phosphodiesterase inhibition. Am. J. Respir. Cell Mol. Biol. 1994;10(5):565–572. doi: 10.1165/ajrcmb.10.5.8179921. [DOI] [PubMed] [Google Scholar]
- 215.Gamble E., et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;168(8):976–982. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 216.Saetta M., et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163(6):1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 217.Tennenberg S.D., Weller J.J. Endotoxin-induced, neutrophil-mediated endothelial cytotoxicity is enhanced by T-lymphocytes. J. Surg. Res. 1997;69(1):11–13. doi: 10.1006/jsre.1996.4996. [DOI] [PubMed] [Google Scholar]
- 218.Rodrigues S.F., Granger D.N. Role of blood cells in ischaemia–reperfusion induced endothelial barrier failure. Cardiovasc. Res. 2010;87(2):291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Szabo M., et al. Deficiency of innate-like T lymphocytes in chronic obstructive pulmonary disease. Respir. Res. 2017;18(1):197. doi: 10.1186/s12931-017-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li L., Yee C., Beavo J.A. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283(5403):848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 221.Rueda C.M., Jackson C.M., Chougnet C.A. Regulatory T-cell-mediated suppression of conventional T-cells and dendritic cells by different cAMP intracellular pathways. Front. Immunol. 2016;7:216. doi: 10.3389/fimmu.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Riccomi A., et al. Modulation of phenotype and function of human CD4(+)CD25(+) T regulatory lymphocytes mediated by cAMP-elevating agents. Front. Immunol. 2016;7:358. doi: 10.3389/fimmu.2016.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Klein M., Bopp T. Cyclic AMP represents a crucial component of Treg cell-mediated immune regulation. Front. Immunol. 2016;7:315. doi: 10.3389/fimmu.2016.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Puchelle E., et al. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006;3(8):726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- 225.Hogg J.C., et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 226.McDonough J.E., et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Araya J., et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Invest. 2007;117(11):3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Auerbach O., et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung; a report of progress. N. Engl. J. Med. 1957;256(3):97–104. doi: 10.1056/NEJM195701172560301. [DOI] [PubMed] [Google Scholar]
- 229.Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 230.Nowrin K., et al. Epithelial-mesenchymal transition as a fundamental underlying pathogenic process in COPD airways: fibrosis, remodeling and cancer. Expet Rev. Respir. Med. 2014;8(5):547–559. doi: 10.1586/17476348.2014.948853. [DOI] [PubMed] [Google Scholar]
- 231.Milara J., et al. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 232.Zhang H., et al. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic. Biol. Med. 2012;52(8):1437–1442. doi: 10.1016/j.freeradbiomed.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zou W., et al. Nicotine-induced epithelial-mesenchymal transition via Wnt/beta-catenin signaling in human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;304(4):L199–L209. doi: 10.1152/ajplung.00094.2012. [DOI] [PubMed] [Google Scholar]
- 234.Mahmood M.Q., et al. Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:1515–1524. doi: 10.2147/COPD.S81032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Milara J., et al. Roflumilast N-oxide inhibits bronchial epithelial to mesenchymal transition induced by cigarette smoke in smokers with COPD. Pulm. Pharmacol. Ther. 2014;28(2):138–148. doi: 10.1016/j.pupt.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 236.Cortijo J., et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br. J. Pharmacol. 2009;156(3):534–544. doi: 10.1111/j.1476-5381.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Martorana P.A., et al. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am. J. Respir. Crit. Care Med. 2005;172(7):848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- 238.Sohal S.S., et al. A randomized controlled trial of inhaled corticosteroids (ICS) on markers of epithelial-mesenchymal transition (EMT) in large airway samples in COPD: an exploratory proof of concept study. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:533–542. doi: 10.2147/COPD.S63911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rogers D.F. The airway goblet cell. Int. J. Biochem. Cell Biol. 2003;35(1):1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 240.Rogers D.F. Airway goblet cells: responsive and adaptable front-line defenders. Eur. Respir. J. 1994;7(9):1690–1706. doi: 10.1183/09031936.94.07091690. [DOI] [PubMed] [Google Scholar]
- 241.Nadel J.A., Burgel P.R. The role of epidermal growth factor in mucus production. Curr. Opin. Pharmacol. 2001;1(3):254–258. doi: 10.1016/s1471-4892(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 242.Fahy J.V., Dickey B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tamaoki J. Targeting airway mucus hypersecretion in chronic obstructive pulmonary disease. Respir Investig. 2015;53(6):247–248. doi: 10.1016/j.resinv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 244.Ramos F.L., Krahnke J.S., Kim V. Clinical issues of mucus accumulation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:139–150. doi: 10.2147/COPD.S38938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rennard S.I., et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast--the importance of defining different subsets of patients with COPD. Respir. Res. 2011;12(1):18. doi: 10.1186/1465-9921-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang T., Zhou X. Clinical application of expectorant therapy in chronic inflammatory airway diseases (Review) Exp. Ther. Med. 2014;7(4):763–767. doi: 10.3892/etm.2014.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Demizu S., et al. TAS-203, an oral phosphodiesterase 4 inhibitor, exerts anti-inflammatory activities in a rat airway inflammation model. Eur. J. Pharmacol. 2019;849:22–29. doi: 10.1016/j.ejphar.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 248.Gao W., et al. Bronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015;20(5):722–729. doi: 10.1111/resp.12542. [DOI] [PubMed] [Google Scholar]
- 249.Pérez-Padilla R., Schilmann A., Riojas-Rodriguez H. Respiratory health effects of indoor air pollution. Int. J. Tubercul. Lung Dis. 2010;14(9):1079–1086. [PubMed] [Google Scholar]
- 250.Hashimoto S., et al. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2000;161(1):237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 251.Saetta M., et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;165(10):1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 252.Rusznak C., et al. Ozone-induced mediator release from human bronchial epithelial cells in vitro and the influence of nedocromil sodium. Eur. Respir. J. 1996;9(11):2298–2305. doi: 10.1183/09031936.96.09112298. [DOI] [PubMed] [Google Scholar]
- 253.Yoon C.M., et al. Mitochondrial regulation of inflammasome activation in chronic obstructive pulmonary disease. J Innate Immun. 2016;8(2):121–128. doi: 10.1159/000441299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Langrish C.L., et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202(1):96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 255.Oppmann B., et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 256.Pelletier M., et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 257.Alcorn J.F., Crowe C.R., Kolls J.K. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 258.Liang S.C., et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 259.I Sakkas L., Mavropoulos A., P Bogdanos D.J.C.M.C. vol. 24. 2017. P; pp. 3054–3067. (Hosphodiesterase 4 Inhibitors in Immune-Mediated Diseases: Mode of Action, Clinical Applications, Current and Future Perspectives). 28. [DOI] [PubMed] [Google Scholar]
- 260.Marchesi J.R., Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Turnbaugh P.J., et al. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Saeedi P., et al. The transient but not resident (TBNR) microbiome: a Yin Yang model for lung immune system. Inhal. Toxicol. 2015;27(10):451–461. doi: 10.3109/08958378.2015.1070220. [DOI] [PubMed] [Google Scholar]
- 263.Dickson R.P., Huffnagle G.B. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moffatt M.F., Cookson W.O. The lung microbiome in health and disease. Clin. Med. 2017;17(6):525–529. doi: 10.7861/clinmedicine.17-6-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.McDonough K.A., Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat. Rev. Microbiol. 2011;10(1):27–38. doi: 10.1038/nrmicro2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Serezani C.H., et al. vol. 39. 2008. pp. 127–132. (Cyclic AMP: Master Regulator of Innate Immune Cell Function). 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Thomson M., et al. vol. 298. 2022. (Expression of a Novel Mycobacterial Phosphodiesterase Successfully Lowers cAMP Levels Resulting in Reduced Tolerance to Cell Wall–Targeting Antimicrobials). 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Matange N.J.F.m.l. vol. 362. 2015. (Revisiting Bacterial Cyclic Nucleotide Phosphodiesterases: Cyclic AMP Hydrolysis and beyond). 22. fnv183. [DOI] [PubMed] [Google Scholar]
- 269.Fuchs E.L., et al. vol. 192. 2010. pp. 2779–2790. (In V Itro and in V Ivo Characterization of the Pseudomonas aeruginosa Cyclic AMP (cAMP) Phosphodiesterase CpdA, Required for cAMP Homeostasis and Virulence Factor Regulation). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Serezani C.H., et al. Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008;39(2):127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pulliainen A.T., et al. Bacterial effector binds host cell adenylyl cyclase to potentiate Galphas-dependent cAMP production. Proc. Natl. Acad. Sci. U. S. A. 2012;109(24):9581–9586. doi: 10.1073/pnas.1117651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hajishengallis G., et al. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U. S. A. 2008;105(36):13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ahidjo B.A., Bishai W.R. Phosphodiesterase inhibitors as adjunctive therapies for tuberculosis. EBioMedicine. 2016;4:7–8. doi: 10.1016/j.ebiom.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Matange N. Revisiting bacterial cyclic nucleotide phosphodiesterases: cyclic AMP hydrolysis and beyond. FEMS Microbiol. Lett. 2015;362(22) doi: 10.1093/femsle/fnv183. [DOI] [PubMed] [Google Scholar]
- 275.Vishnupriya S., et al. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mizumura K., et al. Autophagy: friend or foe in lung disease? Ann Am Thorac Soc. 2016;13(Suppl 1):S40–S47. doi: 10.1513/AnnalsATS.201507-450MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Barnes P.J., Baker J., Donnelly L.E. Autophagy in asthma and chronic obstructive pulmonary disease. Clin. Sci. (Lond.) 2022;136(10):733–746. doi: 10.1042/CS20210900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data will be made available on request.