Background:
Bundled interventions usually reduce surgical site infection (SSI) when implemented at single hospitals, but the feasibility of their implementation at the nationwide level and their clinical results are not well established.
Materials and Methods:
Pragmatic interventional study to analyze the implementation and outcomes of a colorectal surgery care bundle within a nationwide quality improvement program. The bundle consisted of antibiotic prophylaxis, oral antibiotic prophylaxis (OAP), mechanical bowel preparation, laparoscopy, normothermia, and a wound retractor. Control group (CG) and Intervention group (IG) were compared. Overall SSI, superficial (S-SSI), deep (D-SSI), and organ/space (O/S-SSI) rates were analyzed. Secondary endpoints included microbiology, 30-day mortality, and length of hospital stay.
Results:
A total of 37 849 procedures were included, 19 655 in the CG and 18 194 in the IG. In all, 5462 SSIs (14.43%) were detected: 1767 S-SSI (4.67%), 847 D-SSI (2.24%), and 2838 O/S-SSI (7.5%). Overall SSI fell from 18.38% (CG) to 10.17% (IG), odds ratio (OR) of 0.503 [0.473–0.524]. O/S-SSI rates were 9.15% (CG) and 5.72% (IG), OR of 0.602 [0.556–0.652]. The overall SSI rate was 16.71% when no measure was applied and 6.23% when all six were used. Bundle implementation reduced the probability of overall SSI (OR: 0.331; CI95: 0.242–0.453), and also O/S-SSI rate (OR: 0.643; CI95: 0.416–0.919). In the univariate analysis, all measures except normothermia were associated with a reduction in overall SSI, while only laparoscopy, OAP, and mechanical bowel preparation were related to a decrease in O/S-SSI. Laparoscopy, wound retractor, and OAP decreased overall SSI and O/S-SSI in the multivariate analysis.
Conclusions:
In this cohort study, the application of a specific care bundle within a nationwide nosocomial infection surveillance system proved feasible and resulted in a significant reduction in overall and O/S-SSI rates in the elective colon and rectal surgery. The OR for SSI fell between 1.5 and 3 times after the implementation of the bundle.
Keywords: bundle, colorectal surgery, mechanical bowel preparation, normothermia, oral antibiotic prophylaxis, surgical site infection, surveillance program, systemic antibiotic prophylaxis, wound retractor

Introduction
Highlights
Colorectal surgery has the highest rates of surgical site infection (SSI).
Bundled interventions usually reduce SSI in colorectal surgery.
The feasibility of implementing bundles in a large group of hospitals has not been well established, nor has their clinical efficacy.
A six-measure bundle was successfully introduced in the context of a nationwide healthcare-related infection surveillance system.
This bundle, which included mechanical and oral antibiotic bowel preparation, lowered rates of SSI in all sites in elective surgery for both the colon and rectum.
Surgical site infections (SSIs) are among the most dreaded postoperative complications and also the most frequent, accounting for 20% of all healthcare-associated infections in Europe1. Surgical operations are associated with varying risks of SSI, depending on the underlying clinical diagnosis, the patient’s medical condition, and the type of procedure2. Despite the implementation of evidence-based prevention measures, the incidence of SSI after colorectal surgery is the highest among elective abdominal procedures, affecting 15–30% of patients3–9.
Along with additional surgical procedures, added morbidity, and often higher mortality, SSI places considerable financial strain on the healthcare system owing to the prolonged length of hospital stay (LOS), readmission10, and its significant negative impact on patients’ quality of life11. In colorectal surgery, organ/space-SSI (O/S-SSI) triples hospital stay and has a readmission rate of 23%, a reoperation rate of 60%, and a 29% rate of need for intensive care12.
Although SSIs are a direct consequence of surgery, it is estimated that 60% of them could be prevented with an increased and controlled use of the best evidence-based measures13,14. Preventive bundles or sets of evidence-based interventions are structured strategies for improving patient outcomes15. Some of these intensive quality improvement projects were first implemented for high-risk surgical procedures such as colorectal surgery16. However, the adoption of best practice measures within colorectal bundles did not consistently lead to overall SSI reductions17–23; most have been shown to reduce superficial-SSI, but their impact on deep and O/S-SSI is variable20,24–26. Furthermore, bundles may be easy to introduce in a single hospital, but the feasibility of implementing comprehensive SSI prevention bundles within a larger and more diverse population of hospitals is unclear, and their clinical efficacy has not been well established27. Regarding the choice of the components of a colorectal bundle, recent meta-analyses support the efficacy of bundles, including oral antibiotic prophylaxis (OAP), to reduce SSI but also note that certain questions remain unanswered and that well-designed pragmatic studies are needed28.
This pragmatic cohort study was designed with the following aims: to assess the feasibility of the implementation of a bundle for SSI reduction in colorectal surgery at the multicenter level in the setting of a nationwide quality improvement program; to evaluate the efficacy of the bundle in reducing SSI in any surgical space; and to examine the association between the degree of bundle adherence and clinical outcomes. Additionally, the study analyses the differences between colon and rectal surgery and the influence of hospital size on SSI outcomes in a large cohort of hospitals.
We hypothesized that a coordinated, guided implementation strategy would allow successful implementation of the bundle and would lower risk-adjusted SSI rates and complications associated with colorectal surgeries at the participating hospitals.
Material and methods
Design
This pragmatic, prospective, cohort, multicenter study compares two phases: a baseline period before bundle implementation (Control Group, CG), from January 2011 to June 2016; and the bundle implementation period (Intervention Group, IG), from July 2016 to December 2020.
Setting and patients
The study uses data collected prospectively within a nationwide infection surveillance system covering a network of public and private hospitals. Data from 55 hospitals participating in the network were included in the analysis. The program is described in detail on the institutional website29 and also in previous publications7,30.
Patients who underwent elective colorectal surgery between January 2011 and December 2020 were included. Cases of elective wound class 2 (clean-contaminated) and 3 (contaminated), according to the National Healthcare Safety Network classification31, were followed. Patients with previous ostomies or peritonitis at the time of intervention (wound class 4) were excluded. Table 1 shows in detail the inclusion criteria for colorectal surgery surveillance. Prospective surveillance was performed by training the infection control team (ICT) at each hospital to ensure appropriate data collection. A detailed operational definition document was generated and shared with all network hospitals. The definitions, criteria, and surveillance methodology used by the ICT staff were identical in the two study periods. The ICTs received prior training to ensure consistent and accurate data collection, and audits of the data provided were conducted at different times during the development of the program. Active mandatory postdischarge surveillance was performed up to day 30 postsurgery.
Table 1.
Inclusion and exclusion criteria for colorectal surgery surveillance.
| Inclusion criteria Colon or rectal elective resection surgery (all diseases that require surgical resection are included: malignant and benign neoplastic diseases, chronic inflammatory disease, and diverticulosis). Delayed surgery (patient admitted as an emergency, but surgery performed on a scheduled basis during the same hospital admission, for example colonic bowel obstruction treated with an endoscopic stent and operated days later) Elective wound class 2 (Clean-contaminated) and 3 (Contaminated) cases. Minimum of 100 consecutive procedures per year per hospital or continuous monitoring throughout the year for those centers that perform fewer than 100 procedures per year. |
| Exclusion criteria Emergency surgery. Peritonitis at the time of intervention (wound class 4 surgery). Patients who underwent multiple procedures during the surgery itself, for example resection of liver metastases (until 2015). From 2016, cases with other procedures that can accompany colon surgery, such as cholecystectomy, herniorrhaphy, appendicectomy, nephrectomy, liver segmentectomy, or partial bladder resection were included. Patients with previous ostomies. Centers that performed fewer than 10 surgical procedures annually. |
Intervention
A multidisciplinary team of nurses and medical and surgical specialists was recruited to formulate a bundle of preventive measures specific to colorectal surgery. The literature for optimal care during the preoperative, intraoperative, and postoperative phases was reviewed, including evidence on OAP and mechanical bowel preparation (MBP)32. Practices were chosen either for their high level of scientific evidence or for being considered reasonable, associated with minimal risk, and potentially beneficial. On this basis, the working group created a 6-measure bundle to be implemented voluntarily by the participating hospitals. The measures in the bundle were adequate antibiotic intravenous prophylaxis (antibiotic type, dose, timing within 60 min, intraoperative re-dosing, and duration <24 h), OAP, MBP, laparoscopic surgery, maintenance of normothermia (goal >36°C), and the use of a double-ring plastic wound retractor in open and laparoscopic surgery (Table 2).
Table 2.
Measures included in the colorectal bundle.
| ‘Adequate’ systemic iv antibiotic prophylaxis | ‘Adequate’=all the following items must be fulfilled. Start 30–60 min before incision. Intraoperative re-dosing when indicated. Do not prolong >24 h. Type of antibiotic according to hospital protocol. Recommended: • Metronidazole 15 mg/kg+gentamycin 5 mg/kg or • Cefuroxime 1.5 g+metronidazole 15 mg/kg or • Cefazolin 2 g+metronidazole 15 mg/kg or • Amoxicillin-clavulanate 2 g |
| Mechanical bowel preparation | Day before the procedure |
| Oral antibiotic prophylaxis | Day before the procedure. Recommended: • Metronidazole 750 mg+neomycin 1 g (three doses the day before surgery). or • Erythromycin 1 g+metronidazole 750 mg (three doses the day before surgery) |
| Laparoscopic surgery | |
| Maintenance of normothermia | Goal: >36° at the end of operation |
| Double-ring plastic wound edge retractor | In open or laparoscopic surgery |
The intervention began on 1 January 2016, with the dissemination of the bundle measures via e-mail to all participating hospitals, and a workshop addressed to the surgical and ICTs. Hospitals were given the option to implement either all or a set of individual bundle components. The bundle involved a systematic approach to improving the use of SSI preventive measures across the phases of perioperative care. It was a multidisciplinary project in which surgeons, anaesthesiologists, surgical nurses, operating room staff, unit nurses, house staff, and hospital mid-level providers were asked to enact the prescribed elements. Participating institutions created local improvement teams with the support of senior leaders from the hospital to facilitate the implementation of SSI prevention measures.
Study outcomes, variables, definitions, and data source
Basic demographic data were recorded, including age, gender, American Society of Anaesthesiologists (ASA) score, and information on surgical details, including surgical approach, wound contamination class, and duration of surgery. The National Nosocomial Infections Surveillance (NNIS) score was also calculated for each patient.
The primary outcome was the development of a SSI within 30 days after operation, according to the Centers for Disease Control and Prevention (CDC) definitions33. SSIs were defined as superficial incisional (S-SSI), deep incisional (D-SSI), and organ space (O/S-SSI). The term ‘overall SSI’ refers to the sum of the SSI at all three anatomical levels. When necessary, ‘incisional SSI’ (I-SSI) means the addition of S-SSI and D-SSI. The incidence of SSI was measured as events per 100 procedures included.
Secondary outcome variables included postdischarge SSI, readmission, postoperative 30-day mortality, LOS, time from surgical procedure to SSI, microbiological etiology of infections, and compliance with the bundle of six perioperative measures.
Ethical issues
The implementation of the bundle precluded randomization. The data were taken from a large nonpublicly available national database. Patients’ confidential information was protected in accordance with European regulations. Anonymity and data confidentiality (access to records, data encryption, and archiving of information) were maintained throughout the research process. Data extraction was approved by the Institutional Research Board, and the study was approved by the Clinical Research Ethics Committee. The need for informed consent and the provision of an information sheet were waived because data were routinely collected as part of hospital surveillance and quality improvement. The project has the Research Registry UIN: researchregistry8407 at https://www.researchregistry.com (https://www.researchregistry.com/browse-the-registry#home/registrationdetails/634d398305178e002191c978/) and was also registered with ClinicalTrials.gov Identifier: NCT04129177 (https://clinicaltrials.gov/ct2/show/NCT04129177). The study has been reported in accordance with the STROCSS (Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery) criteria34.
Statistical analysis
Descriptive statistical analyses were performed using frequencies and proportions for categorical variables, while medians and interquartile range (IQR) or means and SD were used for continuous variables. Infection rates were expressed as cumulative incidence, that is, the crude percentage of operations resulting in SSI/number of surgery procedures. Furthermore, some analyses were stratified by year, risk index category, hospital size, and SSI type. Spearman correlation coefficient (ρ) was used to describe the evolution of infection rates and mortality over the years. Any relationship between two qualitative variables was analyzed using contingency tables and performing the χ2 test or the likelihood ratio test as appropriate.
A univariate logistic regression model was performed to analyze the individual effects of the bundle measures, and a multinomial logistic regression model was performed to study the combined effect of all bundle measures over the years.
The results are presented in terms of OR (estimated infection rates), with the corresponding 95% confidence intervals (CI95). The significance level was set at 5% in all tests. The results are analyzed using the statistical package SAS v9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
The study included 37 849 patients, 19 655 in the CG (13 886 colon surgery and 5769 rectal surgery) and 18 194 in the IG (13 363 colon surgery and 4831 rectal surgery). The demographic and baseline characteristics of the two cohorts are displayed in Table 3.
Table 3.
Characteristics of patients in Control Group and Intervention Group.
| Overall | Control group | Intervention group | P | |
|---|---|---|---|---|
| Colorectal surgery | ||||
| Number of procedures | 37 849 | 19 655 | 18 194 | |
| Wound class | <0.0001 | |||
| 3 (clean/contaminated) | 36 883 (97.60%) | 18 930 (96.40%) | 17 953 (89.90%) | |
| 4 (contaminated) | 906 (2.40%) | 706 (3.60%) | 200 (1.10%) | |
| Age, years (mean, SD) | 68.67 (12.45) | 68.73 (12.41) | 68.61 (12.50) | 0.3231 |
| Sex, male (%) | 22 690 (59.95%) | 11 899 (60.54%) | 10 791 (59.31%) | 0.0148 |
| Median duration of intervention, minutes (Q1, Q3) | 165 (125, 220) | 165 (120, 216) | 170 (129, 225) | <0.0001 |
| ASA score | 0.0007 | |||
| ASA score 1 | 1979 (5.27%) | 1098 (5.59%) | 881 (4.91%) | |
| ASA score 2 | 20 827 (55.44%) | 10 826 (55.16%) | 10 001 (55.74%) | |
| ASA score 3 | 13 895 (36.99%) | 7207 (36.72%) | 6688 (37.28%) | |
| ASA score 4 | 858 (2.28%) | 492 (2.51%) | 366 (2.04%) | |
| Laparoscopy (%) | 25 069 (66.51%) | 11 493 (58.68%) | 13 576 (74.97%) | <0.0001 |
| NNISS ≥1 (%) | 11 507 (30.40%) | 6646 (33.81%) | 4861 (26.72%) | <0.0001 |
| Colon surgery | ||||
| Number of procedures | 27 249 | 13 886 | 13 363 | |
| Wound class | <0.0001 | |||
| 3 (clean/contaminated) | 26 671 (98.02%) | 13 434 (96.86%) | 13 237 (99.23%) | |
| 4 (contaminated) | 538 (1.98%) | 435 (3.14%) | 103 (0.77%) | |
| Age, years (mean, SD) | 69.10 (12.41) | 69.09 (12.38) | 69.10 (12.44) | 0.9851 |
| Sex, male (%) | 15 845 (58.15%) | 8174 (58.87%) | 7671 (57.40%) | 0.0146 |
| Median duration of intervention, minutes (Q1, Q3) | 154 (120, 200) | 150 (115, 195) | 157(120, 204) | <0.0001 |
| ASA score | 0.0027 | |||
| ASA score 1 | 1430 (5.29%) | 774 (5.58%) | 656 (4.97%) | |
| ASA score 2 | 14 899 (55.07%) | 7547 (54.44%) | 7353 (55.73%) | |
| ASA score 3 | 10 056 (37.17%) | 5158 (37.21%) | 4898 (37.13%) | |
| ASA score 4 | 665 (2.46%) | 380 (2.74%) | 284 (2.16%) | |
| Laparoscopy (%) | 18 082 (66.63%) | 8103 (58.57%) | 9979 (75.01%) | <0.0001 |
| NNISS ≥1 (%) | 8311 (30.50%) | 4756 (34.25%) | 3555 (26.60%) | <0.0001 |
| Rectal surgery | ||||
| Number of procedures | 10 600 | 5769 | 4831 | |
| Wound class | <0.0001 | |||
| 3 (clean/contaminated) | 10 212 (96.52%) | 5496 (95.30%) | 4716 (97.98%) | |
| 4 (contaminated) | 368 (3.48%) | 262 (4.70%) | 97 (2.02%) | |
| Age, years (mean, SD) | 67.59 (12.49) | 67.87 (12.43) | 67.26 (12.56) | 0.0120 |
| Sex, male (%) | 6845 (64.58%) | 3725 (64.57%) | 3120 (64.58%) | 0.9883 |
| Median duration of intervention, minutes (Q1, Q3) | 210 (155, 270) | 205 (150, 265) | 215 (160, 276) | <0.0001 |
| ASA score | 0.0161 | |||
| ASA score 1 | 549 (5.22%) | 324 (5.62%) | 225 (4.97%) | |
| ASA score 2 | 5928 (56.39%) | 3279 (56.89%) | 2649 (55.79%) | |
| ASA score 3 | 3839 (36.52%) | 2049 (35.55%) | 1790 (37.70%) | |
| ASA score 4 | 193 (1.84%) | 112 (1.94%) | 81 (1.71%) | |
| Laparoscopy (%) | 6987 (66.19%) | 3390 (58.95%) | 3597 (74.86%) | <0.0001 |
| NNISS ≥1 (%) | 3196 (30.15%) | 1890 (32.76%) | 1306 (27.03%) | <0.0001 |
Adequate surgical prophylaxis: type of antibiotic according to local guidelines, in addition to correct timing, dosage, and duration.
ASA, American Society of Anesthesiologists; NNISS, National Nosocomial Infections Surveillance System Index; Q1, first quartile; Q3, third quartile.
SSI rates
Overall colorectal surgery
Figure 1 shows the trends of SSI incidence over the course of the study period. There were 5462 SSIs, representing a cumulative incidence of 14.43%. This incidence fell significantly over the years (ρ=−0.98788). With regard to the surgical site affected, 1767 (4.67%) infections were S-SSI, 847 (2.24%) D-SSI, and 2838 (7.50%) O/S-SSI (Table 4 and Fig. 1).
Figure 1.

Aggregate colorectal SSI, and superficial, deep, and organ/space-SSI rates during the period of the study (2011–2020). SSI, surgical site infection.
Table 4.
Overall SSI rates and comparison of SSI rates in the pragmatic trial.
| Overall (%) | Control group (%) | Intervention group (%) | OR [CI95%] | P | |
|---|---|---|---|---|---|
| Colorectal surgery | 14.43 | 18.38 | 10.17 | 0.503 [0.473–0.534] | <0.0001 |
| Superficial-SSI | 4.67 | 6.09 | 3.13 | 0.499 [0.450–0.552] | <0.0001 |
| Deep-SSI | 2.24 | 3.12 | 1.29 | 0.405 [0.348–0.471] | <0.0001 |
| O/S-SSI | 7.50 | 9.15 | 5.72 | 0.602 [0.556–0.652] | <0.0001 |
| Colon surgery | 13.05 | 17.09 | 8.85 | 0.471 [0.437–0.507] | <0.0001 |
| Superficial-SSI | 4.64 | 6.30 | 2.92 | 0.447 [0.396–0.505] | <0.0001 |
| Deep-SSI | 1.73 | 2.44 | 0.99 | 0.399 [0.326–0.488] | <0.0001 |
| O/S-SSI | 6.65% | 8.32 | 4.90 | 0.568 [0.514–0.627] | <0.0001 |
| Rectal surgery | 17.99 | 21.48 | 13.83 | 0.587 [0.529–0.650] | <0.0001 |
| Superficial-SSI | 4.74 | 5.58 | 3.73 | 0.655 [0.543–0.789] | <0.0001 |
| Deep-SSI | 3.55 | 4.75 | 2.11 | 0.433 [0.343–0.545] | <0.0001 |
| O/S-SSI | 9.69 | 11.13 | 7.97 | 0.692 [0.606–0.790] | <0.0001 |
O/S-SSI, organ space-surgical site infection; SSI, surgical site infection.
Comparing the two study groups, the overall SSI rate for colorectal surgery was 18.38% in the CG and 10.17% in the IG (OR: 0.503 [CI95: 0.473–0.534]; P<0.0001). In all locations, SSI fell significantly, in O/S-SSI it was 9.15% in the CG and 5.72% in the IG (OR: 0.602; CI95: 0.556–0.652; P<0.0001). The decrease in overall and O/S-SSI rates was similar in high-volume, medium-volume, and low-volume hospitals, as shown in Figure 2.
Figure 2.

Aggregate colorectal surgical site infection rates according to hospital size.
Colon surgery
The overall SSI rate was 17.09% in the CG and 8.85% in the IG (OR: 0.471 [CI95: 0.437–0.507]; P<0.0001). The trend in SSI rates was also significant, with ρ −0.97576 (Table 4). O/S-SSI rates were 8.32% in the CG and 4.90% in the IG (OR: 0.568 [CI95: 0.514–0.627]); P<0.0001), with Spearman coefficient −0.95152.
Rectal surgery
The overall SSI rate was 21.48% in the CG and 13.83% in the IG, OR of 0.587 [CI95: 0.529–0.650]; P less than 0.0001. The overall SSI decrease in rectal surgery was also significant, with ρ −0.96364 (Table 4). O/S-SSI rates were 11.13% in the CG and 7.97% in the IG (OR: 0.692 [CI95: 0.606–0.790]; P<0.0001), with a significant, but less evident decrease (ρ –0.72121).
Bundle adherence and SSI rates
Table 5 shows the percentage of use of the measures. The rates of correct compliance with each measure were 82.37% for IV prophylaxis, 74.97% for laparoscopy, 92.23% for maintenance of normothermia, 73.78% for OAP, 78.87% for MBP, and 75.61% for wound protection. The level of adherence to each recommendation of the bundle did not differ according to the type of surgery. Comparing the two periods of the study, the use of laparoscopy increased in both colon (58.57% vs. 75.01%; P<0.0001) and rectal surgery (58.95% vs. 74.86%; P<0.0001).
Table 5.
Percentage of use of bundle measures in the study groups.
| Control group, N (%) | Intervention group, N (%) | P | |
|---|---|---|---|
| Colorectal surgery | |||
| Adequate antibiotic prophylaxis* | 16 701 (86.91) | 14 965 (82.37) | <0.001 |
| Oral antibiotic prophylaxis (OAP) | NA | 8868 (73.78) | |
| Mechanical bowel preparation (MBP) | NA | 9744 (78.87) | |
| Laparoscopy | 11 493 (58.68) | 13 576 (74.97) | <0.001 |
| Maintenance of normothermia | NA | 10 135 (92.23%) | |
| Double-ring wound retractor | NA | 8781 (75.61%) | |
| Colon surgery | |||
| Adequate antibiotic prophylaxis* | 11 850 (87.25%) | 11 048 (82.76%) | <0.001 |
| OAP | NA | 6326 (71.22%) | |
| MBP | NA | 6761 (73.89%) | |
| Laparoscopy | 8103 (58.57%) | 9979 (75.01%) | <0.001 |
| Maintenance of normothermia | NA | 7558 (92.32%) | |
| Double-ring wound retractor | NA | 6876 (78.97%) | |
| Rectal surgery | |||
| Adequate antibiotic prophylaxis* | 4851 (86.09%) | 3917 (81.28%) | <0.001 |
| OAP | NA | 2542 (81.03%) | |
| MBP | NA | 2983 (93.10) | |
| Laparoscopy | 3390 (58.95%) | 3597 (74.86%) | <0.001 |
| Maintenance of normothermia | NA | 2577 (91.97%) | |
| Double-ring wound retractor | NA | 1895 (65.50%) | |
Only information on the adequation of systemic antibiotic prophylaxis and the use of laparoscopy was available in the period before the implementation of the bundle.
Adequate surgical prophylaxis: type of antibiotic according to local guidelines, in addition to correct timing, dosage and duration.
NA, not available.
Overall SSI rates ranged from 16.71% when no bundle measures were used to 6.23% when all six measures were appropriately applied (Fig. 3). Bundle implementation reduced the probability of overall SSI (OR: 0.331; CI95: 0.242–0.453) and O/S-SSI (OR: 0.643; CI95: 0.416–0.919). Analyzing colon and rectal cases separately, the bundle effect was well maintained in colon surgery (overall SSI: OR, 0.273 [CI95: 0.188–0.395]; O/S-SSI, OR of 0.720 [CI95: 0.532–0.974]). In rectal surgery, however, its effect was less robust, OR of 0.545 [CI95: 0.301–0.985] for overall SSI and 0.626 [CI95: 0.425–0.923] for O/S-SSI. Figure 4 shows the relation between the increase in the implementation of the bundle elements over time and the decrease in overall SSI throughout the two periods of study. To show this relation more clearly, Figure 5 displays only the Intervention Group. In the first year of implementation of the bundle, a 19% drop in overall SSI rates was achieved, the largest annual fall recorded since surveillance began.
Figure 3.

Colorectal SSI rates according to compliance with the elements included in the bundle. SSI, surgical site infection.
Figure 4.
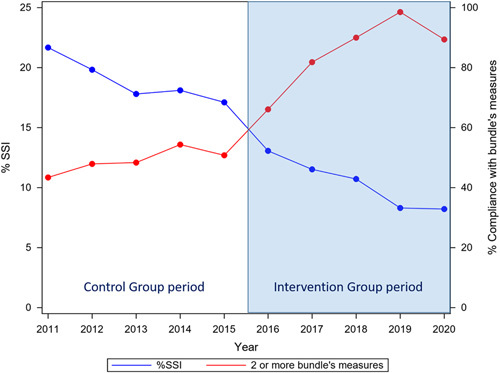
Relationship between the implementation of the elements of the bundle throughout the entire study period (covering the Control Group and the Intervention Group) and the evolution of the overall SSI rates. The most marked decrease in SSI occurred in 2016, the first year of the dissemination of the bundle. SSI, surgical site infection.
Figure 5.

Relationship between compliance with the elements of the bundle and the evolution of overall SSI rates in the Intervention Group period (2016–2020). The information collected on compliance in 2020 may have been affected by the coronavirus disease 2019 pandemic. SSI, surgical site infection.
Individual effect of bundle measures on SSI rates
In the univariate analysis of colon and rectal cases considered together (Table 6), all measures except for normothermia were associated with a decrease in overall SSI. For O/S-SSI, laparoscopy, OAP, and MBP were protective factors. Multivariate analysis confirmed that laparoscopy, OAP, and wound protectors decreased overall colorectal SSI (Fig. 6) and O/S-SSI (Fig. 7).
Table 6.
Univariate and multivariate analysis of the effect of the bundle measures on SSI rates.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | CI95 | P | OR | CI95 | P | |
| Colorectal overall SSI | ||||||
| Adequate antibiotic prophylaxis | 0.858 | 0.760–0.969 | 0.0139 | 0.953 | 0.771–1.180 | 0.6611 |
| Laparoscopic surgery | 0.561 | 0.507–0.621 | <0.0001 | 0.592 | 0.501–0.700 | <0.0001 |
| Maintenance of normothermia | 0.1597 | 1.315 | 0.973–1.777 | 0.0748 | ||
| Oral antibiotic prophylaxis (OAP) | 0.586 | 0.515–0.666 | <0.0001 | 0.623 | 0.516–0.751 | <0.0001 |
| Mechanical bowel preparation (MBP) | 0.720 | 0.627–0.827 | <0.0001 | 1.002 | 0.819–1.225 | 0.9871 |
| Double-ring wound retractor | 0.660 | 0.576–0.755 | <0.0001 | 0.592 | 0.500–0.701 | <0.0001 |
| Colorectal O/S-SSI | ||||||
| Adequate antibiotic prophylaxis | 0.6636 | 0.981 | 0.747–1.289 | 0.8903 | ||
| Laparoscopic surgery | 0.817 | 0.711–0.939 | 0.0045 | 0.795 | 0.637–0.993 | 0.0434 |
| Maintenance of normothermia | 0.6716 | 1.117 | 0.779–1.601 | 0.5482 | ||
| OAP | 0.664 | 0.563–0.784 | <0.0001 | 0.699 | 0.551–0.888 | 0.0033 |
| MBP | 0.819 | 0.682–0.983 | 0.0322 | 1.057 | 0.816–1.369 | 0.6761 |
| Double-ring wound retractor | 0.1248 | 0.772 | 0.618–0.964 | 0.0224 | ||
| Colon overall SSI | ||||||
| Adequate antibiotic prophylaxis | 0.804 | 0.692–0.933 | 0.0042 | 0.980 | 0.755–1.273 | 0.8822 |
| Laparoscopic surgery | 0.490 | 0.433–0.555 | <0.0001 | 0.506 | 0.414–0.619 | <0.0001 |
| Maintenance of normothermia | 0.0915 | 1.418 | 0.970–2.071 | 0.0713 | ||
| OAP | 0.473 | 0.405–0.553 | <0.0001 | 0.577 | 0.463–0.718 | <0.0001 |
| MBP | 0.582 | 0.497–0.683 | <0.0001 | 0.896 | 0.714–1.124 | 0.3416 |
| Double-ring wound retractor | 0.733 | 0.614–0.876 | <0.0006 | 0.683 | 0.548–0.851 | <0.0007 |
| Colon O/S-SSI | ||||||
| Adequate antibiotic prophylaxis | 0.9953 | 1.060 | 0.754–1.491 | 0.7381 | ||
| Laparoscopic surgery | 0.689 | 0.582–0.816 | <0.0001 | 0.602 | 0.465–0.780 | 0.0001 |
| Maintenance of normothermia | 0.2504 | 1.345 | 0.836–2.164 | 0.2212 | ||
| OAP | 0.608 | 0.496–0.744 | <0.0001 | 0.681 | 0.515–0.902 | 0.0072 |
| MBP | 0.697 | 0.565–0.859 | 0.0007 | 0.986 | 0.737–1.319 | 0.9238 |
| Double-ring wound retractor | 0.9257 | 0.937 | 0.696–1.261 | 0.6658 | ||
| Rectal overall SSI | ||||||
| Adequate antibiotic prophylaxis | 0.9923 | 0.936 | 0.646–1.358 | 0.7291 | ||
| Laparoscopic surgery | 0.728 | 0.608–0.872 | 0.0006 | 0.804 | 0.585–1.104 | 0.1769 |
| Maintenance of normothermia | 0.8599 | 1.147 | 0.694–1.896 | 0.5936 | ||
| OAP | 0.736 | 0.576–0.939 | 0.0138 | 0.682 | 0.472–0.985 | 0.0415 |
| MBP | 0.666 | 0.467–0.950 | 0.0251 | 0.976 | 0.584–1.632 | 0.9263 |
| Double-ring wound retractor | 0.687 | 0.554–0.853 | 0.0007 | 0.598 | 0.453–0.789 | 0.0003 |
| Rectal O/S-SSI | ||||||
| Adequate antibiotic prophylaxis | 0.6325 | 0.897 | 0.566–1.422 | 0.6443 | ||
| Laparoscopic surgery | 0.2933 | 1.585 | 0.992–2.532 | 0.0541 | ||
| Maintenance of normothermia | 0.4669 | 0.811 | 0.462–1.426 | 0.4676 | ||
| OAP | 0.651 | 0.482–0.880 | 0.0053 | 0.670 | 0.423–1.061 | 0.0875 |
| MBP | 0.1114 | 0.846 | 0.446–1.604 | 0.6088 | ||
| Double-ring wound retractor | 0.2866 | 0.733 | 0.514–1.045 | 0.0861 | ||
O/S-SSI, organ/space-surgical site infection; SSI, surgical site infection.
Figure 6.

Multivariate analysis of the effect of the measures of the bundle on overall surgical site infection rates.
Figure 7.

Multivariate analysis of the effect of the measures of the bundle on organ/space-surgical site infection rates.
The results were similar when only colon surgery was analyzed. In the univariate analysis of rectal cases, MBP showed a protective effect on O/S-SSI but not on overall SSI. In the multivariate analysis, only systemic IV prophylaxis and plastic wound retractor were protective factors for overall SSI for rectal surgery, while none of the measures showed a significant effect on O/S-SSI (Table 6).
Secondary variables
Median length of stay
The median postoperative LOS for the whole group was 7 days (IQR 5–11). A significant decrease was noted after the implementation of the bundle (ρ=−0.98414), with a fall from 8 days in the CG to 6 days in the IG (P<0.0001).
Time to SSI
No differences were detected between the groups in the median time elapsed between the intervention and the appearance of overall SSI, with medians of 7 days (IQR 5–12) vs. 8 days (IQR 5–13); P=0.2895. However, differences were found in O/S-SSI, with a median of 7 days (4–11) in the CG and 6 days (4–11.5) in the IG; P=0.0075.
Postdischarge SSI
Overall colorectal SSI was diagnosed during the first admission in 3856 (70.69%) cases and at postdischarge surveillance in 1596 patients (29.26%). In the latter group, 870 (54.5%) required readmission. Postdischarge SSI rates were 27.89% in the CG group and 32.09% in the IG group (P=0.0099). Readmission was also more frequent in the IG (15.21% vs. 17.40%; P=0.0099).
Mortality
Overall mortality was 1.12% and decreased significantly over the course of the study: from 1.49% in the CG to 0.80% in the IG for colorectal SSI (P<0.0001), from 1.67% to 0.86% for colon surgery (P<0.0001), and from 1.05% to 0.65% for rectal surgery (P=0.0203).
Pathogens detected in SSI
An etiological diagnosis was achieved in 3840 patients with SSI (70.30%) (Table 7). There were 3620 microorganisms isolated from the 3612 SSI in the CG and 1525 from 1850 in the IG. Comparison of the two groups demonstrated differences only in the etiology of O/S-SSI, in which more Gram-positive cocci (22.07% vs. 36.41%), fewer Gram-negative bacteria (72.20% vs. 53.15%), and more fungi (2.38% vs. 6.41%) were isolated in the IG. In this group, the isolation of Enterococcus faecalis, Enterococcus faecium, and Candida spp. doubled in O/S-SSI.
Table 7.
Comparison of etiology of incisional (I-SSI) and organ/space (O/S-SSI) colorectal surgical site infection according to the study group.
| Control group (%) | Intervention group (%) | P | |
|---|---|---|---|
| Organisms in incisional SSI | |||
| Gram-positive bacteria | 31.76 | 35.38 | 0.0736 |
| Enterococcus faecalis | 9.65 | 12.05 | 0.0678 |
| Enterococcus faecium | 3.50 | 4.36 | 0.2972 |
| Enterococcus spp. | 1.03 | 0.51 | 0.1935 |
| MRSA | 0.86 | 1.67 | 0.00746 |
| Others | 16.71 | 16.79 | 0.9602 |
| Gram-negative bacteria | 61.92 | 59.62 | 0.2727 |
| Escherichia coli | 36.24 | 29.87 | 0.0018 |
| Klebsiella spp. | 3.91 | 4.87 | 0.2639 |
| Pseudomonas spp. | 8.10 | 9.49 | 0.2486 |
| Enterobacter spp. | 5.11 | 4.62 | 0.5954 |
| Others | 8.56 | 10.77 | 0.0764 |
| Anaerobes | 5.34 | 3.97 | 0.1422 |
| Clostridium spp. | 0.17 | 0.51 | 0.1520 |
| Bacteroides spp. | 5.17 | 3.46 | 0.0595 |
| Yeasts | 0.98 | 1.03 | 0.9083 |
| Candida albicans | 0.98 | 1.03 | 0.9083 |
| Organisms in organ/space-SSI | |||
| Gram-positive bacteria | 22.07 | 36.41 | <0.0001 |
| Enterococcus faecalis | 7.68 | 15.00 | <0.0001 |
| Enterococcus faecium | 7.02 | 13.59 | <0.0001 |
| Enterococcus spp. | 1.32 | 1.63 | 0.5064 |
| MRSA | 0.40 | 0.54 | 0.5794 |
| Others | 5.65 | 5.65 | 0.9969 |
| Gram-negative bacteria | 72.20 | 53.15 | <0.0001 |
| Escherichia coli | 26.57 | 27.39 | 0.6339 |
| Klebsiella spp. | 4.24 | 7.50 | 0.0002 |
| Pseudomonas spp. | 5.83 | 6.63 | 0.3879 |
| Enterobacter spp. | 3.40 | 4.46 | 0.1517 |
| Others | 32.17 | 7.17 | <0.0001 |
| Anaerobes | 3.35 | 4.02 | 0.3557 |
| Clostridium spp. | 0.62 | 0.65 | 0.9114 |
| Bacteroides spp. | 2.74 | 3.37 | 0.3358 |
| Yeasts | 2.38 | 6.41 | <0.0001 |
| Candida albicans | 2.38 | 6.41 | <0.0001 |
Incisional surgical site infection includes superficial-SSI and deep-SSI.
MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
This prospective cohort study provided strong support for the implementation of a colorectal SSI reduction bundle in a broad cohort of hospitals and demonstrated its efficacy in reducing SSI.
Little is known about the implementation of SSI preventive bundles in large groups of hospitals. Most of the colorectal bundles described to date have been implemented in single hospitals35; very few are regional or national bundles designed to be introduced at multiple centers. Previous research has highlighted that prevention bundles may be more difficult to introduce at multicenter level and that their clinical efficacy in this setting has not been demonstrated27.
Although several international guidelines regarding SSI prevention have been published36,37, guidelines are not self-implementing, and suboptimal compliance rates have been reported38,39, even in colorectal surgery40. Internal barriers to implementation are mainly related to human factors, while external barriers are environmental factors such as lack of leadership or organizational culture41,42. To overcome these difficulties, bundles of evidence-based interventions have been proposed15.
At least three meta-analyses have shown that when correct adherence to specific evidence-based bundles is achieved, SSI risk in colorectal surgery is reduced by an average of 40–50%35,43,44. However, these bundles are not homogeneous in terms of the measures included, and they are not widely used21. In some cases, even high compliance with the measures was not directly associated with reducing SSI rates23,45, and the adequate selection of the components of a given bundle is probably the key to its success46.
A systematic review42 studied the effect of implementation strategies on the prevention of SSI in abdominal surgery, defined as techniques designed to increase the adoption of health promotion activities47. The review showed that the highest risk reduction was achieved by applying a set of ‘top five’ activities: audit and feedback, organizational culture, monitoring the performance of healthcare delivery, reminders, and educational meetings. This bundle was successfully introduced in less than 1 year, leveraging a nationwide infection surveillance system that was already implementing these five strategies. The application of bundles in similar multicenter collaborative settings has shown that quality improvement studies should consider not only surgeon behavior, but also institutional traits for their optimal implementation48.
Most studies have analyzed colon and rectal surgeries together; separate assessments of patients undergoing colon and rectal surgery are scarce49,50. Although the risk factors and SSI rates of colon and rectal surgery differ49,51,52, it should be highlighted that the present bundle had an effect on both types of procedures.
More importantly, the bundle was effective at all three surgical sites, including the organ space site, where the consequences in terms of mortality and LOS are more severe than in I-SSI53,54. However, although most published colorectal bundles have demonstrated their beneficial effect on I-SSI, most of them did not improve rates of O/S-SSI24,55.
The observed reduction in SSI rates is likely due to the implementation of the bundle, in view of the strong association found between increasing bundle compliance and lower levels of SSI. The most efficient measures were OAP, laparoscopic surgery, and the use of a double-ring plastic wound retractor. The bundle’s efficacy in decreasing SSI rates was linearly correlated with the number of elements used. While some of the bundle measures appeared specifically designed to prevent either incisional or intra-abdominal infection, they worked together to reduce SSI at all levels. All of them showed individual efficacy for overall SSI prevention, except for maintenance of normothermia. For O/S-SSI, only laparoscopy, MBP, and OAP were effective. Multivariate analysis confirmed laparoscopy, OAP and wound retractor as protective factors against overall and O/S-SSI.
The relatively low impact of systemic antibiotic prophylaxis may be explained by the fact that only properly administered prophylaxis was considered for the analysis. The criteria used to consider it ‘adequate’ were very strict and comprised: the type of drug, dose, the timing of infusion, completion before surgical incision, and duration of therapy. Although prophylaxis was performed and recorded in all patients, a single deviation from the recommended guidelines was enough for the process to be considered inadequate.
The lack of effectiveness in maintaining body temperature may also seem surprising, but it should be noted that the difference in temperature between patients with and without infection was found to be 0.1°C. Seminal randomized clinical trials56,57 demonstrated the detrimental effect of severe hypothermia (around 34°C) on SSI rate after colorectal surgery and led to the current recommendation of keeping a core body temperature above 36°C in the perioperative period. However, subsequent cohort studies and a meta-analysis58 found no association between perioperative hypothermia and SSI risk. It should be noted that the differences between normothermic and hypothermic patients in the original studies56,57 were in the order of 1–2°C. In contrast, the differences in the cohort studies that reported negative results had an average of 0.1°C, as observed in this study. Since today the vast majority of patients are actively warmed, it is likely that these minor temperature differences between those with SSI and those without will no longer be statistically significant.
OAP and MBP are controversial SSI preventive measures that are exclusively used for colorectal surgery59–61. Although there is broad consensus that intravenous antibiotic prophylaxis is essential before colorectal surgery, it is still debated whether oral antibiotics should be added. In addition, the development of multimodal rehabilitation programs62 and the publication of several conflicting studies has fueled the controversy surrounding MBP and its potential combination with OAP, leading to a significant decrease in their prescription rates worldwide. In 2017, a European survey recorded an oral prophylaxis use of only 11% and routine use of MBP of 29.6%63.
When designing the bundle, a multidisciplinary team decided to include OAP combined with MBP (mechanical and oral antibiotic bowel preparation, MOABP). Subsequently, two randomized trials compared MOABP64 or OAP65 with no bowel preparation, the first of which found no differences in SSI rate and the second only reduction in S-SSI rates. While waiting for the confirmation of these results, some researchers think that the MOABP strategy should be continued, albeit with the adjustments made necessary by the new findings in the gut microbiome66. Recent guidelines of several scientific societies have recommended the inclusion of OAP in their bundles for colorectal surgery, even in the setting of Enhanced Recovery After Surgery programs67,68.
After the implementation of the bundle, increases in E. faecalis, E. faecium, Klebsiella spp., and Candida albicans were detected in O/S-SSI. It could be argued that this change in the infecting flora is due to the administration of OAP. In experiments with mice, oral administration of antibiotics, including neomycin, changed the composition of the gut microbiota and increased the abundance of potentially pathogenic genera such as Enterococcus 69. Other authors have documented a risk of selection of resistant Enterobacteriaceae after treatment with oral colistin and neomycin70. Similarly, another study found that intestinal preparation with erythromycin and neomycin may be an independent risk factor for the selection of nosocomial strains of enterococci71.
Strengths and limitations of the study
This study has several limitations. First, even though the sequential groups are, to some extent, homogeneous, certain changes in practices during the time frame of the study, such as the increased use of laparoscopy, may have interfered with the results. However, the pragmatic nature of the study and the fact that it was carried out within a consolidated infection surveillance program allowed prospective recording of the data from the two study groups and the use of the same methodology. Second, the improvement in the results may be due only to the surveillance program itself. Surveillance activities are known to reduce the tendency of healthcare-associated infections72, although the surveillance effect lasts only a few years73, and in most cases, it is difficult to disentangle it from the result of implementing specific interventions. In this case, the decline in SSI rates appears to be related to the introduction of the bundle, as reported elsewhere74. Third, as in similar nationwide databases, the number of variables collected was restricted, and some risk factors, such as BMI, smoking, and diabetes, or secondary outcomes, such as anastomotic leakage, were not evaluated. Finally, the level of compliance with some of the bundle measures was uneven at the participating hospitals. The strengths of the study include its large number of cases followed up prospectively as part of a consolidated program, which means that its results can probably be extrapolated to other settings.
Implications
The current study describes the successful prospective implementation of a comprehensive SSI prevention bundle in a large, diverse network of hospitals. The opportunity to leverage a bundle of this kind within a long-established surveillance program allowed its controlled implementation in a short period of time and the use of a large prospective database to analyze the clinical outcomes. The study provides a pragmatic insight into bundle implementation as well as clinical evidence to further the efforts to reduce SSI.
Conclusions
These results show that a common series of measures can be successfully introduced in the setting of a nationwide healthcare-related infection surveillance system. The proposed bundle, including OAP, decreased overall SSI, O/S-SSI, LOS, and mortality, both in the elective colon and rectal surgery in a wide population of patients undergoing elective procedures. The implementation of the bundle halved the OR for SSI. Preoperative OAP, the use of a double-ring plastic wound retractor, and the laparoscopic technique were the measures with the strongest impact on outcomes.
Ethical approval
Data extraction was approved by the Institutional Research Board with code 20166009, and the study was approved by the Clinical Research Ethics Committee of Hospital General de Granollers with code 2021006. The need for informed consent and the provision of an information sheet were waived because data were routinely collected as part of hospitals surveillance and quality improvement.
Sources of funding
This study received no external funding. The VINCat Surveillance Program, from which the data was obtained, is supported by public funding from the Catalan Health Service, Department of Health, Generalitat de Catalunya.
Author contribution
F.G., M.P., J.M.B., N.A.: conceptualization and design; A.V., A.A., and J.M.B.: methodology; E.L., A.V., M.P.: acquisition of data; A.V., A.A., E.L., J.M.B., N.A., and M.P.: formal analysis and interpretation of data; J.M.B. and N.A.: drafting of the manuscript – original draft preparation; J.M.B., M.P., N.A., A.A., D.P., M.P., A.V., M.P.-A., D.F., D.P., A.A.-T., M.P., M.P.-A., A.S.-P., E.L., and F.G.: critical revision of the manuscript.
Conflicts of interest disclosure
The authors declare no conflict of interest. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Research registration unique identifying number (UIN)
Name of the registry: Research Registry.
Unique identifying number or registration ID: researchregistry8407.
Hyperlink: www.researchregistry.com, https://www.researchregistry.com/browse-the-registry#home/registrationdetails/634d398305178e002191c978/
Name of the registry: ClinicalTrials.gov
Identifier: NCT04129177
Hyperlink: https://clinicaltrials.gov/ct2/show/NCT04129177.
Guarantor
Guarantor: J.M. Badia.
Data availability statement
The research data is prospectively registered and belongs to the Nosocomial Infection Surveillance System in Catalonia (VINCat), a program from the Catalan Health Service, Department of Health, Generalitat de Catalunya. Anonymous data extraction was approved by the Institutional Research Board of the VINCat. All data will be made available on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Appendix 1. Members of Infection Control Teams participating in the program
Dolors Castellana and Elisa Montiu González, Hospital Universitari Arnau de Vilanova de Lleida; Graciano García Pardo and Francesc Feliu Villaró, Hospital Universitari Joan XXIII de Tarragona; Josep Rebull Fatsini and M. France Domènech Spaneda, Hospital Verge de la Cinta de Tortosa; Marta Conde Galí and Anna Oller Pérez-Hita, Hospital Universitari Dr. Josep Trueta Girona; Lydia Martín and Ana Lerida, Hospital de Viladecans; Sebastiano Biondo and Emilio Jiménez Martínez, Hospital Universitari de Bellvitge; Nieves Sopena Galindo and Ignasi Camps Ausàs, Hospital Universitari Germans Tries i Pujol; Carmen Ferrer and Luis Salas, Hospital Universitari Vall d’Hebron; Rafael Pérez Vidal and Dolors Mas Rubio, Althaia Xarxa Assistencial de Manresa; Irene García de la Red, Hospital HM Delfos; Mª Angels Iruela Castillo and Eva Palau i Gil, Clínica Girona; José Antonio Martínez Martínez and Mª Blanca Torralbo Navarro, Hospital Clínic de Barcelona; Maria López and Carol Porta, Hospital Universitari Mútua de Terrassa; Alex Smithson Amat and Guillen Vidal Escudero, Fundació Hospital de l'Esperit Sant; José Carlos de la Fuente Redondo and Montse Rovira Espés, Hospital Comarcal Mora d'Ebre; Arantxa Mera Fidalgo and Luis Escudero Almazán, Hospital de Palamós; Monserrat Ortega Raya and Aina Gomila, Hospital Parc Taulí de Sabadell; Vicens Diaz-Brito and Mª Carmen Álvarez Moya, Parc Sanitari Sant Joan de Déu (Hospital de Sant Boi); Laura Grau Palafox and Yésika Angulo Gómez, Hospital de Terrassa; Anna Besolí Codina and Carme Autet Ricard, Consorci Hospitalari de Vic; Carlota Hidalgo López and Marta Pascual Damieta, Hospital del Mar; Jordi Cuquet Pedragosa and Demelsa Mª Maldonado López, Hospital General de Granollers; David Blancas and Esther Moreno Rubio, Consorci Sanitari del Garraf; Roser Ferrer i Aguilera, Hospital Sant Jaume de Calella; Simona Iftimie Iftimie and Antoni Castro-Salomó, Hospital Universitari Sant Joan de Reus; Rosa Laplace Enguídanos and Maria Carmen Sabidó Serra, Hospital de Sant Pau i Santa Tecla; Núria Bosch Ros, Hospital de Santa Caterina; Virginia Pomar Solchaga and Marta Piriz Marabaján, Hospital de la Santa Creu i Sant Pau; Laura Lázaro Garcia and Angeles Boleko Ribas, Hospital Universitari Quirón Dexeus; Jordi Palacín Luque and Alexandra Lucía Moise, Pius Hospital de Valls; Mª Carmen Fernández Palomares and Santiago Barba Sopeña, Hospital Universitari Sagrat Cor; Eduardo Sáez Huertas and Sara Burges Estada, Clínica NovAliança; Josep María Tricas Leris and Eva Redon Ruiz, Fundació privada Hospital de Mollet; Montse Brugués Brugués and Susana Otero Acedo, Consorci Sanitari de l’Anoia. Igualada; Maria Cuscó Esteve and Lourdes Gabarró, Hospital Comarcal de l’Alt Penedès; Fco. José Vargas-Machuca and Mª de Gracia García Ramírez, Centre MQ de Reus; Elena Vidal Díez and Ana Maria Ciscar Bellés, Consorci Hospitalari del Maresme. Hospital de Mataró; Mariló Marimón Morón and Marisol Martínez Sáez, Hospital Universitari General de Catalunya; Josep Farguell and Mireia Saballs, QUIRON Salud; Montserrat Vaqué Franco and Leonor Invernón Garcia, Hospital de Barcelona; Rosa Laplace Enguídanos and Meritxell Guillemat Marrugat, Hospital Comarcal del Vendrell; Ana Coloma Conde and Lucrecia López González, Hospital Moisès Broggi.
Acknowledgments
The authors thank the CERCA Program/Generalitat de Catalunya for institutional support and all colorectal surgical teams, surgical nurses, infection control teams, and quality improvement professionals for all participating hospitals.
The authors also thank Michael Maudsley for the review of the manuscript.
Footnotes
Josep M. Badia and Nares Arroyo-Garcia contributed equally to this article.
Members of the VINCat Colorectal Surveillance Team: Lucrecia López, Infection control team, Hospital de Sant Joan Despí Moisès Broggi, Spain; Marta Piriz, Infection control team, Hospital Universitari Sant Pau, Barcelona, Spain; Mercè Hernández, Department of Surgery, Hospital Universitari Parc Taulí, Sabadell, Spain; Cecilia Díez, Department of Anaesthesiology, Hospital Universitari Sant Pau, Barcelona, Spain.
On behalf of the VINCat Program, the members of Infection Control Teams participating in the program appear in Appendix 1.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 14 March 2023
Contributor Information
Josep M. Badia, Email: jmbadia@fphag.org.
Nares Arroyo-Garcia, Email: narroyo@fphag.org.
Ana Vázquez, Email: Ana.Vazquez@uab.cat.
Alexander Almendral, Email: alexanderalmendral@iconcologia.net.
Aina Gomila-Grange, Email: agomila@tauli.cat.
Domenico Fraccalvieri, Email: dofrac@yahoo.es.
David Parés, Email: dapares@gmail.com.
Ana Abad-Torrent, Email: ana.abad@vallhebron.cat.
Marta Pascual, Email: mpascual@parcdesalutmar.cat.
Alejandro Solís-Peña, Email: alejandro_solis85@hotmail.com.
Mireia Puig-Asensio, Email: mpuiga@bellvitgehospital.cat.
Miguel Pera, Email: mpera@parcdesalutmar.cat.
Francesc Gudiol, Email: fgudiol@ub.edu.
Enric Limón, Email: elimon@iconcologia.net.
Miquel Pujol, Email: mpujol@bellvitgehospital.cat.
References
- 1. ECDC, European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011.2012; 2013. Accessed 20 May 2022. https://ecdc.europa.eu/sites/portal/file
- 2. Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg 2001;234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keenan JE, Speicher PJ, Thacker JKM, et al. The preventive surgical site infection bundle in colorectal surgery an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg 2014;149:1045–52. [DOI] [PubMed] [Google Scholar]
- 4. Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg 2004;239:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pastor C, Baek JH, Varma MG, et al. Validation of the risk index category as a predictor of surgical site infection in elective colorectal surgery. Dis Colon Rectum 2010;53:721–727. [DOI] [PubMed] [Google Scholar]
- 6. Cannon JA, Altom LK, Deierhoi RJ, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum 2012;55:1160–1166. [DOI] [PubMed] [Google Scholar]
- 7. Pujol M, Limón E, López-Contreras J, et al. Surveillance of surgical site infections in elective colorectal surgery. Results of the VINCat Program (2007–2010). Enferm Infecc Microbiol Clin 2012;30:20–25. [DOI] [PubMed] [Google Scholar]
- 8. Paulson EC, Thompson E, Mahmoud N. Surgical site infection and colorectal surgical procedures: a prospective analysis of risk factors. Surg Infect (Larchmt) 2017;18:520–526. [DOI] [PubMed] [Google Scholar]
- 9. Gomila A, Badia JM, Carratal J, et al. , Current outcomes and predictors of treatment failure in patients with surgical site infection after elective colorectal surgery. A multicentre prospective cohort study. J Infect 2017;74:555–563. [DOI] [PubMed] [Google Scholar]
- 10. Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 11. Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw E, Badia JM, Piriz M, et al. O053: What surgical site infection rates in colorectal surgery should be considered for benchmarking standards? Antimicrob Resist Infect Control 2013;2:25–28.24088259 [Google Scholar]
- 13. Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. J Hosp Infect 2003;54:258–266. [DOI] [PubMed] [Google Scholar]
- 14. Meeks DW, Lally KP, Carrick MM, et al. Compliance with guidelines to prevent surgical site infections: as simple as 1-2-3? Am J Surg 2011;201:76–83. [DOI] [PubMed] [Google Scholar]
- 15. Resar R, Griffin FA, Haraden C, et al. Using Care Bundles to Improve Health Care Quality, IHI Innovation Series White Paper. Institute for Healthcare Improvement; 2012. [Google Scholar]
- 16. Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA 2015;313:483–495. [DOI] [PubMed] [Google Scholar]
- 17. Larochelle M, Hyman N, Gruppi L, et al. Diminishing surgical site infections after colorectal surgery with surgical care improvement project: is it time to move on? Dis Colon Rectum 2011;54:394–400. [DOI] [PubMed] [Google Scholar]
- 18. Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections,. JAMA 2010;303:2479–2485. [DOI] [PubMed] [Google Scholar]
- 19. Serra-Aracil X, García-Domingo MI, Parés D, et al. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg 2011;146:606–612. [DOI] [PubMed] [Google Scholar]
- 20. Hoang SC, Klipfel AA, Roth LA, et al. Colon and rectal surgery surgical site infection reduction bundle: to improve is to change. Am J Surg 2019;217:40–45. [DOI] [PubMed] [Google Scholar]
- 21. Guerrero MA, Anderson B, Carr G, et al. Adherence to a standardized infection reduction bundle decreases surgical site infections after colon surgery: a retrospective cohort study on 526 patients. Patient Saf Surg 2021;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hedrick TL, Heckman JA, Smith RL, et al. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg 2007;205:432–438. [DOI] [PubMed] [Google Scholar]
- 23. Anthony T, Murray BW, Sum-Ping JT, et al. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Arch Surg 2011;146:263–269. [DOI] [PubMed] [Google Scholar]
- 24. Weiser MR, Gonen M, Usiak S, et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br J Surg 2018;105:1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waits SA, Fritze D, Banerjee M, et al. Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surg (United States) 2014;155:602–606. [DOI] [PubMed] [Google Scholar]
- 26. Hewitt DB, Tannouri SS, Burkhart RA, et al. Reducing colorectal surgical site infections: a novel, resident-driven, quality initiative. Am J Surg 2017;213:36–42. [DOI] [PubMed] [Google Scholar]
- 27. Mcgee MF, Kreutzer L, Quinn CM, et al. Leveraging a comprehensive program to implement a colorectal surgical site infection reduction bundle in a statewide quality improvement collaborative. Ann Surg 2019;270:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodfield JC, Clifford K, Schmidt B, et al. , Has network meta-analysis resolved the controversies related to bowel preparation in elective colorectal surgery? Color Dis 2022;24:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nosocomial Infection Surveillance Programme at Catalan Hospitals (VINCat) 2015 Manual; 2015. Accessed 30 July 2020. https://catsalut.gencat.cat/ca/proveidors-professionals/vincat/prevencio-infeccio/metodologia-resultats/objectiu-7/metodologia
- 30. Arroyo-Garcia N, Badia JM, Vázquez A, et al. An interventional nationwide surveillance program lowers postoperative infection rates in elective colorectal surgery. A cohort study (2008–2019). Int J Surg 2022;102:106611. [DOI] [PubMed] [Google Scholar]
- 31. National Healthcare Safety Network, Surgical Site Infection (SSI) Event: National Healthcare Safety Network, 2023. Accessed 16 December 2022. https://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf?agree=yes&next=Accept
- 32. Badia JM, Arroyo-García N. Mechanical bowel preparation and oral antibiotic prophylaxis in colorectal surgery: analysis of evidence and narrative review. Cir Esp 2018;96:317–325. [DOI] [PubMed] [Google Scholar]
- 33. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 34. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int J Surg Open 2021;37:100430. [DOI] [PubMed] [Google Scholar]
- 35. Pop-Vicas AE, Abad C, Baubie K, et al. Colorectal bundles for surgical site infection prevention: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2020;41:805–812. [DOI] [PubMed] [Google Scholar]
- 36. Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276–e287. [DOI] [PubMed] [Google Scholar]
- 37. Berriós-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 38. Leaper DJ, Tanner J, Kiernan M, et al. Surgical site infection: poor compliance with guidelines and care bundles. Int Wound J 2015;12:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Badia JM, Casey AL, Rubio-Pérez I, et al. A survey to identify the breach between evidence and practice in the prevention of surgical infection: time to take action. Int J Surg 2018;54:290–297. [DOI] [PubMed] [Google Scholar]
- 40. Badia JM, Casey AL, Rubio-Pérez I, et al. Awareness of practice and comparison with best evidence in surgical site infection prevention in colorectal surgery. Surg Infect (Larchmt) 2019;21:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jun J, Kovner CT, Stimpfel AW. Barriers and facilitators of nurses’ use of clinical practice guidelines: an integrative review. Int J Nurs Stud 2016;60:54–68. [DOI] [PubMed] [Google Scholar]
- 42. Tomsic I, Heinze NR, Chaberny IF, et al. Implementation interventions in preventing surgical site infections in abdominal surgery: a systematic review. BMC Health Serv Res 2020;20:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanner J, Padley W, Assadian O, et al. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surg (United States) 2015;158:66–77. [DOI] [PubMed] [Google Scholar]
- 44. Zywot A, Lau CSM, Stephen Fletcher H, et al. Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg 2017;21:1915–1930. [DOI] [PubMed] [Google Scholar]
- 45. Tanner J, Kiernan M, Hilliam R, et al. Effectiveness of a care bundle to reduce surgical site infections in patients having open colorectal surgery. Ann R Coll Surg Engl 2016;98:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunt TK, Hopf HW. Selection of bundle components. Arch Surg 2011;146:1220–1221. [DOI] [PubMed] [Google Scholar]
- 47. Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brajcich BC, Schlick CJR, Halverson AL, et al. Association between patient and hospital characteristics and adherence to a surgical site infection reduction bundle in a statewide surgical quality improvement collaborative. J Am Coll Surg 2022;234:783–792. [DOI] [PubMed] [Google Scholar]
- 49. Konishi T, Watanabe T, Kishimoto J, et al. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg 2006;244:758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petrosillo N, Drapeau CMJ, Nicastri E, et al. Surgical site infections in Italian hospitals: a prospective multicenter study. BMC Infect Dis 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morikane K, Honda H, Yamagishi T, et al. Factors associated with surgical site infection in colorectal surgery: the Japan nosocomial infections surveillance. Infect Control Hosp Epidemiol 2014;35:660–666. [DOI] [PubMed] [Google Scholar]
- 52. Gomila A, Carratalà J, Camprubí D, et al. Risk factors and outcomes of organ-space surgical site infections after elective colon and rectal surgery. Antimicrob Resist Infect Control 2017;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–397. [DOI] [PubMed] [Google Scholar]
- 54. Eagye KJ, Nicolau DP. Deep and organ/space infections in patients undergoing elective colorectal surgery: incidence and impact on hospital length of stay and costs. Am J Surg 2009;198:359–367. [DOI] [PubMed] [Google Scholar]
- 55. Dixon LK, Biggs S, Messenger D, et al. Surgical site infection prevention bundle in elective colorectal surgery. J Hosp Infect 2022;122:162–167. [DOI] [PubMed] [Google Scholar]
- 56. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996;334:1209–1216. [DOI] [PubMed] [Google Scholar]
- 57. Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet, 358 2001:876–880. [DOI] [PubMed] [Google Scholar]
- 58. Bu N, Zhao E, Gao Y, et al. Association between perioperative hypothermia and surgical site infection: a meta-analysis. Medicine (United States) 2019;98:e14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nichols RL, Choe EU, Weldon CB. Mechanical and antibacterial bowel preparation in colon and rectal surgery. Chemotherapy 2005;51:115–121. [DOI] [PubMed] [Google Scholar]
- 60. Hayashi MS, Wilson SE. Is there a current role for preoperative non-absorbable oral antimicrobial agents for prophylaxis of infection after colorectal surgery? Surg Infect (Larchmt) 2009;10:285–288. [DOI] [PubMed] [Google Scholar]
- 61. Murray BW, Huerta S, Dineen S, et al. Surgical site infection in colorectal surgery: a review of the nonpharmacologic tools of prevention. J Am Coll Surg 2010;211:812–822. [DOI] [PubMed] [Google Scholar]
- 62. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–641. [DOI] [PubMed] [Google Scholar]
- 63. Devane LA, Proud D, O’Connell PR, et al. A European survey of bowel preparation in colorectal surgery. Color Dis 2017;19:O402–O406. [DOI] [PubMed] [Google Scholar]
- 64. Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840–848. [DOI] [PubMed] [Google Scholar]
- 65. Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–738. [DOI] [PubMed] [Google Scholar]
- 66. Alverdy JC, Shogan BD. Preparing the bowel for surgery: rethinking the strategy. Nat Rev Gastroenterol Hepatol 2019;16:708–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holubar SD, Hedrick T, Gupta R, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659–695. [DOI] [PubMed] [Google Scholar]
- 69. Huang C, Feng S, Huo F, et al. Effects of four antibiotics on the diversity of the intestinal microbiota. Microbiol Spectr 2022;10:e0190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Lastours V, Poirel L, Huttner B, et al. Emergence of colistin-resistant Gram-negative Enterobacterales in the gut of patients receiving oral colistin and neomycin decontamination. J Infect 2020;80:578–606. [DOI] [PubMed] [Google Scholar]
- 71. Rhinehart E, Smith NE, Wennersten C, et al. Rapid dissemination of β-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant–toddler surgical ward. N Engl J Med 1990;323:1814–1818. [DOI] [PubMed] [Google Scholar]
- 72. Li Y, Gong Z, Lu Y, et al. Impact of nosocomial infections surveillance on nosocomial infection rates: a systematic review. Int J Surg 2017;42:164–169. [DOI] [PubMed] [Google Scholar]
- 73. Abbas M, de Kraker MEA, Aghayev E, et al. Impact of participation in a surgical site infection surveillance network: results from a large international cohort study. J Hosp Infect 2019;102:267–276. [DOI] [PubMed] [Google Scholar]
- 74. Rudder NJ, Borgert AJ, Kallies KJ, et al. Reduction of surgical site infections in colorectal surgery: a 10-year experience from an independent academic medical center. Am J Surg 2019;217:1089–1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data is prospectively registered and belongs to the Nosocomial Infection Surveillance System in Catalonia (VINCat), a program from the Catalan Health Service, Department of Health, Generalitat de Catalunya. Anonymous data extraction was approved by the Institutional Research Board of the VINCat. All data will be made available on request.


