Abstract
Objective
To compare the outcomes of digital breast tomosynthesis (DBT) screening combined with ultrasound (US) with those of digital mammography (DM) combined with US in women with dense breasts.
Materials and Methods
A retrospective database search identified consecutive asymptomatic women with dense breasts who underwent breast cancer screening with DBT or DM and whole-breast US simultaneously between June 2016 and July 2019. Women who underwent DBT + US (DBT cohort) and DM + US (DM cohort) were matched using 1:2 ratio according to mammographic density, age, menopausal status, hormone replacement therapy, and a family history of breast cancer. The cancer detection rate (CDR) per 1000 screening examinations, abnormal interpretation rate (AIR), sensitivity, and specificity were compared.
Results
A total of 863 women in the DBT cohort were matched with 1726 women in the DM cohort (median age, 53 years; interquartile range, 40–78 years) and 26 breast cancers (9 in the DBT cohort and 17 in the DM cohort) were identified. The DBT and DM cohorts showed comparable CDR (10.4 [9 of 863; 95% confidence interval {CI}: 4.8–19.7] vs. 9.8 [17 of 1726; 95% CI: 5.7–15.7] per 1000 examinations, respectively; P = 0.889). DBT cohort showed a higher AIR than the DM cohort (31.6% [273 of 863; 95% CI: 28.5%–34.9%] vs. 22.4% [387 of 1726; 95% CI: 20.5%–24.5%]; P < 0.001). The sensitivity for both cohorts was 100%. In women with negative findings on DBT or DM, supplemental US yielded similar CDRs in both DBT and DM cohorts (4.0 vs. 3.3 per 1000 examinations, respectively; P = 0.803) and higher AIR in the DBT cohort (24.8% [188 of 758; 95% CI: 21.8%–28.0%] vs. 16.9% [257 of 1516; 95% CI: 15.1%–18.9%; P < 0.001).
Conclusion
DBT screening combined with US showed comparable CDR but lower specificity than DM screening combined with US in women with dense breasts.
Keywords: Breast cancer, Digital breast tomosynthesis, Mammography, Screening, Ultrasound
INTRODUCTION
Early detection using screening mammography has contributed to a reduction in breast cancer mortality [1]. However, dense breasts on mammography represent an important reason for failed early diagnosis of breast cancer with a sensitivity as low as 30%–48% [2,3] and an increased incidence of interval or advanced breast cancers [4,5,6]. To overcome the limitations of mammography, digital breast tomosynthesis (DBT) has been developed to improve cancer detection and reduce the rate of false positives by removing overlapping tissues [7,8,9], especially in women with dense breasts [10] and those under the age of 50 years [11]. In patients with negative findings on digital mammography (DM), supplemental DBT showed incremental cancer detection of 1.2–2.7 per 1000 screening examinations [7,12,13,14,15,16]. A recent meta-analysis reported a pooled incremental cancer detection rate (CDR) of 1.6 per 1000 screening examinations after a negative assessment on DM [17]. DBT combined with DM, or synthesized mammography (SM), is rapidly becoming a routine breast screening tool [18]. Although DBT showed improved diagnostic performance compared with DM, a few recent studies have investigated additional cancer yield by using supplemental screening ultrasound (US) after negative DBT [19,20]. Studies that directly compared the diagnostic performance of supplemental DBT and US in women with dense breasts showed a higher CDR with more false-positive findings with respect to US [21,22]. As DBT replaced DM as a baseline breast cancer screening modality, the options for breast cancer screening in women with dense breasts have broadened, including the sole use of DBT with DM or SM, DM with US, and combined DBT, DM or SM, and US. However, the comparative performances of these options remain unclear. Since higher cancer detection can be achieved by whole-breast US screening, baseline screening with DBT would not have any benefit compared with DM. Furthermore, the performance of DBT compared with DM and supplemental US in matched cohorts has not been well investigated. Therefore, we aimed to compare the outcomes of DBT screening combined with US with those of DM screening combined with US in a matched cohort of women with dense breasts.
MATERIALS AND METHODS
The Seoul National University Hospital Institutional Review Board (IRB No. 2004-177-1119) approved this retrospective study and waived the requirement for informed consent. A total of 573 of the 2589 women in the current study were previously reported [20]. A previous study [20] evaluated the additional value of US to DBT in combination with DM for screening in average-risk women with both dense and non-dense breasts. The current study focused on the outcomes of DBT screening combined with US versus DM combined with US in women with dense breasts using matched cohorts.
Study Cohort
Since 2016, Seoul National University Hospital Healthcare System Gangnam Center has offered simultaneous DM and DBT (DM/DBT, herein DBT) combined with whole-breast handheld US or DM combined with whole-breast handheld US as a part of routine screening program. Baseline screening for DM was performed at our institution. The reason behind selection of DBT for screening was not recorded, but the selection of DBT was likely dependent on machine availability at the time of imaging, individual risk of breast cancer, and choice of women wanting to be screened more thoroughly with this advanced technology.
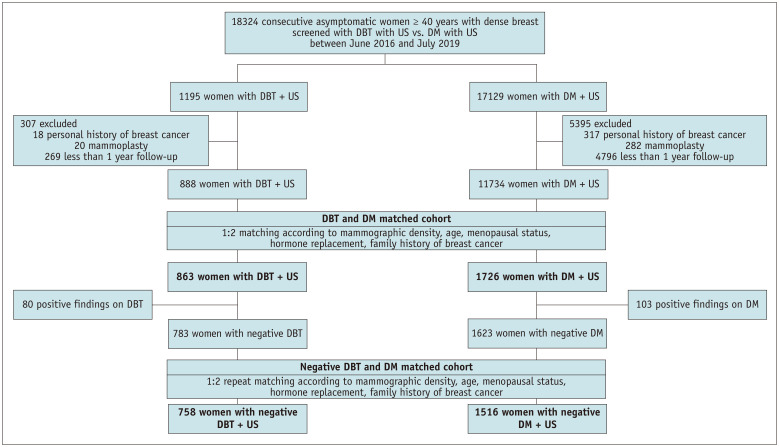
We performed a retrospective search of screening breast examinations performed between June 2016 and July 2019 at Seoul National University Hospital Healthcare System Gangnam Center for asymptomatic women (≥ 40 years old) with mammographic density classified as Breast Imaging Reporting and Data System (BI-RADS) categories c (heterogeneously dense) or d (extremely dense) [23]. A total of 1195 consecutive asymptomatic women screened with DBT and 17129 screened with DM were identified. Among 1195 asymptomatic women who had undergone DBT + US (DBT cohort), 307 women were excluded because of a personal history of breast cancer (n = 18), follow-up period of less than 1 year (n = 269), and augmentation mammoplasty (n = 20). Among the 17129 asymptomatic women who had undergone DM + US (DM cohort), we excluded 5395 women because of a personal history of breast cancer (n = 317), follow-up period of less than 1 year (n = 4796), and augmentation mammoplasty (n = 282). After 1:2 matching of women in the DBT and DM cohorts, 863 and 1726 women in the DBT and DM cohorts, respectively, were finally included in the analysis. Among them, 783 and 1623 women were assessed as negative for DBT and DM, respectively. Subsequently, 1:2 repeat matching was performed, resulting in inclusion of 758 women in the DBT cohort and 1516 women in the DM cohort (Fig. 1).
Fig. 1. Study flow chart. This flow chart show the study population selection process, including inclusion criteria, and exclusion criteria. 1:2 matching was performed for women in digital breast tomosynthesis (DBT) and digital mammography (DM) cohorts,and another 1:2 matching was performed for women with negative assessments on DBT and DM. US = ultrasound.
DM, DBT, and US Examinations
All imaging data were obtained prospectively as part of our routine clinical practice and stored in a picture archiving and communication system. All mammographic imaging data were acquired using a full-field mammography unit (Selenia Dimensions, Hologic Inc.; Senographe 2000D, GE Medical Systems). Standard mammography includes bilateral two-view (mediolateral oblique and craniocaudal) mammograms. DBT was performed using a full-field DM unit with integrated DBT acquisition (Selenia Dimensions, Hologic Inc.). SM images were acquired automatically after the DBT acquisition. The software created SM from raw DBT data [24]. Whole-breast handheld US examinations were performed by one of the three dedicated breast radiologists (B.R.K., S.U.S., and A.Y. with 6, 8, and 14 years of experience on breast US, respectively) who used a handheld US 14–16 MHz linear transducer with a EUB-8500 (Hitachi Medical) (Supplementary Materials and Methods).
Image Analysis
At our health care center, for women scheduled for DBT or DM and US on the same day, the radiologists reported the DBT or DM findings separately using the fifth edition of the BI-RADS score of 1–5 [23] before the US examination and then reported the combined final assessment after the US examination. Cases assigned a BI-RADS category of 3, 4, or 5 were considered positive screening results, while those assigned a BI-RADS category of 1 or 2 were considered negative. Recommendations for routine annual follow-up examinations were made for negative cases, whereas a short-interval follow-up of 6 months, additional mammographic views, or biopsy were recommended for positive cases (Supplementary Materials and Methods).
Data Collection
Among variables known to be related to breast cancer risk [25], we collected data that were identifiable from our medical records, including age, menopausal status, history of hormone replacement therapy, and family history of breast cancer. Histological examinations and 1-year follow-up data were used as reference standards. For cancers, data on pathologic tumor size, histologic type, TNM stage, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor type 2 were collected (Supplementary Materials and Methods).
Statistical Method
To balance women who underwent DBT with supplemental US (DBT + US, DBT cohort) with those who underwent DM with supplemental US (DM + US, DM cohort), 1:2 matching was performed on matching variables, including age (within 4 years), mammographic density, menopausal status, history of hormone replacement therapy, and family history of breast cancer. For matching, a 1:2 matching ratio was used considering the larger number of patients in the DM cohort than in the DBT cohort to improve precision without an increased bias [26]. Another 1:2 matching was performed for women with negative assessments on DBT and DM. CDR was defined as the number of positive examinations with cancers detected per 1000 screening examinations. The sensitivity, specificity, and abnormal interpretation rate (AIR) of the DBT and DM cohorts were calculated for the entire population and for women with negative findings on DBT or DM (Supplementary Materials and Methods). The 95% confidence intervals (CIs) were estimated using the Clopper–Pearson exact CI for proportions. P-values for comparison of outcomes and differences of proportions were obtained from a logistic model using the generalized estimating equation to account for the correlation among matched sets. We additionally provided P-values for the comparison of outcomes from the multivariable logistic model adjusted for the matching variables. The characteristics of the detected cancers were compared using the independent sample t-test for continuous variables and Fisher’s exact test for categorical variables. A two-sided P-value of less than 0.05 indicated a statistically significant difference (SAS software, version 9.4; SAS Institute).
RESULTS
Study Cohort
Table 1 summarizes the demographic characteristics of the matched DBT cohort, DM cohort, and 26 patients with breast cancer. In the matched cohort of 2589 women, the median age was 53 years (interquartile range [IQR]: 40–78 years). Breast density was classified as heterogeneously dense in 1695 (66%) and extremely dense in 894 patients (34%). Among the 2589 women, 1173 (45%) were premenopausal, 330 (13%) had undergone hormone replacement therapy, and 156 (6%) had a family history of breast cancer. The demographic characteristics of the women included in the subsequent analysis in the groups with negative findings on DBT and DM and eight patients with breast cancer are described in Supplementary Table 1.
Table 1. Patient Characteristics.
| Characteristic | Total (n = 2589) | DBT Cohort (n = 863) | DM Cohort (n = 1726) | Patients with Breast Cancer (n = 26) | |
|---|---|---|---|---|---|
| Median age (IQR) at initial breast cancer diagnosis, yr | 53 (40–78) | 53 (40–78) | 53 (40–77) | 53 (44–64) | |
| Age group at initial breast cancer diagnosis | |||||
| < 50 years | 899 (35) | 297 (34) | 602 (35) | 10 (39) | |
| ≥ 50 years | 1690 (65) | 566 (66) | 1124 (65) | 16 (61) | |
| First-degree family history of breast cancer | |||||
| Absent | 2433 (94) | 811 (94) | 1622 (94) | 24 (92) | |
| Present | 156 (6) | 52 (6) | 104 (6) | 2 (8) | |
| Breast density | |||||
| Heterogeneously dense | 1695 (66) | 565 (66) | 1130 (66) | 13 (50) | |
| Extremely dense | 894 (34) | 298 (34) | 596 (34) | 13 (50) | |
| Menopausal status | |||||
| Premenopausal | 1173 (45) | 391 (45) | 782 (45) | 15 (58) | |
| Peri- or postmenopausal | 1416 (55) | 472 (55) | 944 (55) | 11 (42) | |
| Hormone replacement | |||||
| No | 2259 (87) | 753 (87) | 1506 (87) | 23 (89) | |
| Yes | 330 (13) | 110 (13) | 220 (13) | 3 (11) | |
Data are numbers with percentages in parentheses, unless otherwise specified. DBT = digital breast tomosynthesis, DM = digital mammography, IQR = interquartile range
Outcome Measures in the DBT and DM Cohorts
Among the 2589 women, 26 (1%) were diagnosed with breast cancer, of which 9 (35%) were in the DBT cohort and 17 (65%) were in the DM cohort. We found no difference in the CDRs between DBT + US (10.4 [95% CI: 4.8–19.7] per 1000 examinations) and DM + US (9.8 [95% CI: 5.7–15.7] per 1000 examinations) (P = 0.889), despite the higher AIR of DBT + US (31.6% [95% CI: 28.5%–34.9%] vs. 22.4% [95% CI: 20.5%–24.5%]; P < 0.001) (Table 2). The sensitivities of both DBT + US and DM + US were 100% (95% CI: 66.4%–100%) and 100% (95% CI: 80.5%–100%), respectively, with no interval cancers. The specificity of DM + US was higher than that of DBT + US (78.4% [95% CI: 76.3%–80.3%] vs. 69.1% [95% CI: 65.9%–72.2%]; P < 0.001). The detailed outcomes of DM and DBT are provided in the Supplementary Table 2.
Table 2. Performance Outcomes of Screening DBT + US and DM + US in All Women with Dense Breasts.
| Parameters | DBT Cohort (n = 863) | DM Cohort (n = 1726) | P * | P † | ||
|---|---|---|---|---|---|---|
| DBT | DBT + US | DM | DM + US | |||
| Cancer detection rate per 1000 examinations | 7.0 (2.6, 15.1) [6/863] | 10.4 (4.8, 19.7) [9/863] | 7.0 (3.6, 12.1) [12/1726] | 9.8 (5.7, 15.7) [17/1726] | 0.889 | 0.889 |
| Sensitivity, % | 66.7 (29.9, 92.5) [6/9] | 100 (66.4, 100) [9/9] | 70.6 (44.0, 89.7) [12/17] | 100 (80.5, 100) [17/17] | - | - |
| Specificity, % | 91.2 (89.1, 93.0) [779/854] | 69.1 (65.9, 72.2) [590/854] | 94.2 (92.9, 95.2) [1609/1709] | 78.4 (76.3, 80.3) [1339/1709] | < 0.001 | < 0.001 |
| Abnormal interpretation rate, % | 9.4 (7.5, 11.5) [81/863] | 31.6 (28.5, 34.9) [273/863] | 6.5 (5.4,7.8) [112/1726] | 22.4 (20.5, 24.5) [387/1726] | < 0.001 | < 0.001 |
The data in parentheses are 95% Clopper-Pearson exact confidence intervals, and the data in brackets are the number of examinations.
*P-value of DBT + US vs. DM + US from a logistic model using generalized estimating equation to account for the correlation among matched sets, †P-value of DBT + US vs. DM + US from a multivariable logistic model adjusted for the matching variables. DBT = digital breast tomosynthesis, DM = digital mammography, US = ultrasound
Outcomes Measures in the DBT and DM Cohorts with Negative Findings
In women with negative findings on DBT and DM, CDRs were not statistically different between DBT + US (4.0 [95% CI: 0.8–11.5] per 1000 examinations) and DM + US (3.3 [95% CI: 1.1–7.7] per 1000 examinations) (P = 0.803), despite the higher AIR of DBT + US (24.8% [95% CI: 21.8%–28.0%] vs. 16.9% [95% CI: 15.1%–18.9%]; P < 0.001) (Table 3). The sensitivities of the DBT and DM cohorts were 100% (95% CI: 29.2%–100%) and 100% (95% CI: 47.8%–100%), respectively, with no interval cancers. The specificity of DM + US was higher than that of DBT + US (83.3% [95% CI: 81.4%–85.2%] vs. 75.5% [95% CI: 72.3%–78.5%]; P < 0.001).
Table 3. Performance Outcomes of Supplemental US in Women with Dense Breasts with Negative Results at DBT and DM.
| Parameters | DBT + US (n = 758) | DM + US (n = 1516) | P * | P† |
|---|---|---|---|---|
| Cancer detection rate per 1000 examinations | 4.0 (0.8, 11.5) [3/758] | 3.3 (1.1, 7.7) [5/1516] | 0.803 | 0.803 |
| Sensitivity, % | 100 (29.2, 100) [3/3] | 100 (47.8, 100) [5/5] | - | - |
| Specificity, % | 75.5 (72.3, 78.5) [570/755] | 83.3 (81.4, 85.2) [1259/1516] | < 0.001 | < 0.001 |
| Abnormal interpretation rate, % | 24.8 (21.8, 28.0) [188/758] | 16.9 (15.1, 18.9) [257/1516] | < 0.001 | < 0.001 |
The data in parentheses are 95% Clopper-Pearson exact confidence intervals, and the data in brackets are the number of examinations.
*P-value of DBT + US vs. DM + US from a logistic model using generalized estimating equation to account for the correlation among matched sets, †P-value of DBT + US vs. DM + US from a multivariable logistic model adjusted for the matching variables. DBT = digital breast tomosynthesis, DM = digital mammography, US = ultrasound
Characteristics of Detected Breast Cancers
The characteristics of the 26 cancers detected are listed in Table 4. Overall, 58% (15 of 26) were invasive cancers, and 42% (11 of 26) were ductal carcinoma in situ (DCIS). Of the nine cancers detected by DBT + US, five (56%) were invasive (mean invasive tumor size, 1.4 cm), four (44%) were DCIS, and 89% (8 of 9) were lymph node-negative. Of the 17 cancers detected by DM + US, 10 (59%) were invasive (mean invasive tumor size, 1.9 cm), 7 (41%) were DCIS, and 82% (14 of 17) were lymph node-negative. Among the nine cancers detected by DBT + US, there were two asymmetries (22%), two masses (22%), two calcifications (22%), and three occult (33%). Among the 17 cancers detected by DM + US, the most common mammographic feature of cancers detected by DM was six calcifications (35%), followed by three asymmetries (18%), three mass (18%), and five occult (29%).
Table 4. Comparison of Characteristics between Cancers Detected on DBT + US and DM + US.
| DBT + US | DM + US | P | |||
|---|---|---|---|---|---|
| All women | |||||
| Invasive or DCIS | 9 | 17 | > 0.999 | ||
| DCIS | 4 (44) | 7 (41) | |||
| Invasive | 5 (56) | 10 (59) | |||
| Mean size of invasive cancers, cm | 1.4 ± 0.7 (0.9–2.5) | 1.9 ± 1.4 (0.5–5.4) | 0.400 | ||
| Stage | 0.999 | ||||
| DCIS | 4 (44) | 7 (41) | |||
| Invasive, I | 3 (33) | 5 (29) | |||
| Invasive, II or III | 2 (22) | 5 (30) | |||
| T stage | 0.627 | ||||
| 0, I | 8 (89) | 13 (77) | |||
| II or higher | 1 (11) | 4 (23) | |||
| Lymph node status | > 0.999 | ||||
| Negative | 8 (89) | 14 (82) | |||
| Positive | 1 (11) | 3 (18) | |||
| Women with negative results on DBT and DM | |||||
| Invasive or DCIS | 3 | 5 | > 0.999 | ||
| DCIS | 1 (33) | 1 (20) | |||
| Invasive | 2 (67) | 4 (80) | |||
| Mean size of invasive cancers, cm | 1.8 ± 1.0 (1.0–2.5) | 2.6 ± 2.0 (0.9–5.4) | 0.606 | ||
| Stage | 0.785 | ||||
| 0 | 1 (33) | 1 (20) | |||
| I | 2 (66) | 2 (40) | |||
| II or III | 1 (33) | 1 (20) | |||
| T stage | > 0.999 | ||||
| 0, I | 2 (67) | 3 (60) | |||
| II or higher | 1 (33) | 2 (40) | |||
| Lymph node status | 0.196 | ||||
| Negative | 3 (100) | 2 (40) | |||
| Positive | 0 (0) | 3 (60) | |||
Data are mean ± standard deviation (range) or number of patients with percentages in parentheses. DBT = digital breast tomosynthesis, DCIS = ductal carcinoma in situ, DM = digital mammography, US = ultrasound
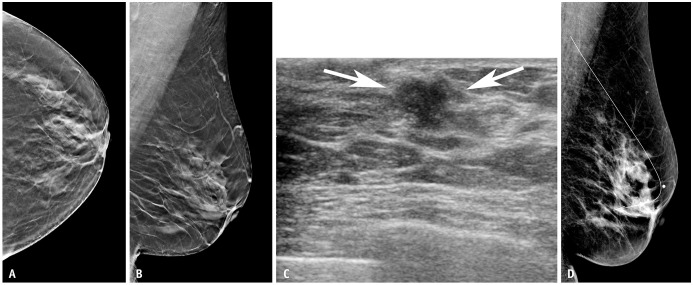
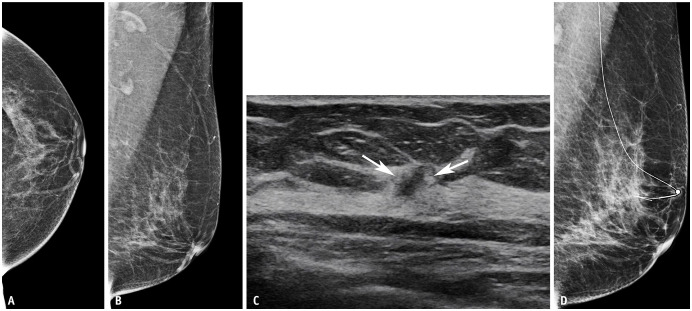
Supplemental US after negative findings on DBT or DM allowed the detection of eight cancers: 75% (6 of 8) invasive and 25% (2 of 8) DCIS. Of the three false-negative cancers that were not detected on DBT but detected on US, two (67%) were lymph node-negative invasive cancers (mean invasive tumor size, 1.8 cm) (Fig. 2) and one (33%) was DCIS. Of the five false-negative cancers that were not detected on DM but detected on US, four (80%) were invasive (mean invasive tumor size, 2.6 cm) (Fig. 3) and one (20%) was DCIS. Three of the five invasive cancers (60%) were lymph node-positive. Although not statistically significant, larger size and higher stage cancers were detected with supplemental US after negative findings on DM than in the DBT cohort. Detailed characteristics of the 26 detected cancers are presented in Supplementary Table 3.
Fig. 2. Imaging of a 56-year-old woman with a screening breast ultrasound (US) detected invasive ductal cancer (T1N0M0, estrogen receptor, progesterone receptor positive, and human epithelial growth factor receptor 2 negative). Images from the breast tomosynthesis in the left craniocaudal (A) and left mediolateral oblique (B) views show no definite suspicious lesions in the left breast. C: Breast US showing an irregular mass in the left breast (arrows). D: The patient underwent US-guided wire localization before breast-conserving surgery, and digital mammography in the left mediolateral oblique view obtained after US-guided wire localization did not show any suspicious findings at the site of the wire. A marker was attached on the skin of the nipple entry site.
Fig. 3. Imaging of a 61-year-old woman with a screening breast ultrasound (US) revealed invasive ductal cancer (T1N1M0, estrogen receptor, progesterone receptor positive, and human epithelial growth factor receptor 2 negative). Digital mammography images of the left craniocaudal (A) and left mediolateral oblique (B) views show no definite suspicious lesions in the left breast. C: Breast US showing an irregular mass in the left breast (arrows). D: The patient underwent US-guided wire localization before breast-conserving surgery. Digital mammography in the left mediolateral oblique view obtained after US-guided wire localization did not show any suspicious findings at the site of the wire. A marker was attached on the skin of the nipple entry site.
DISCUSSION
In our analysis of matched cohorts of 2589 women with dense breasts screened using DBT (n = 863) or DM (n = 1726) in combination with supplemental US, there was no significant improvement in screening performance with DBT + US compared with DM + US. The CDR of DBT + US was comparable to that of DM + US (10.4 vs. 9.8 per 1000 examinations). In women with negative findings on DBT and DM, the incremental CDRs by supplemental US were not statistically different (4.0 vs. 3.3 per 1000 examinations). Our findings suggest that, in women with dense breasts undergoing concurrent breast US screening, the contribution of DBT is not significantly different from that of DM. Regarding the characteristics of detected cancers, both DBT and DM with supplemental US allowed the detection of all cancers without interval cancers. After negative assessment of both DBT and DM, US allowed the detection of an additional 75% (6 of 8) of invasive cancers.
Since 2011, DBT has been approved and rapidly implemented as a robust mammographic technique, and many breast imaging practices have been adopted for DBT combined with DM or SM [18]. However, DBT is not the standard technique used for screening in many other countries [27]. Thus, it is important to evaluate the optimal screening methods for women with dense breasts to determine whether DBT would make any difference to DM as a replacement in combination with US. According to our results, the contribution of either mammographic technique is limited in women with dense breasts, especially in those planning to be screened with supplemental US. Our results were similar to those of Dibble et al. [19], who analyzed the screening performance of DBT + US and DM + US in women with dense breasts. They found comparable CDR of DM + US (3.5 per 1000 women) and DBT + US (3.0 per 1000 women) (P = 0.999). Our findings suggest that the addition of screening US to both DBT and DM increased cancer detection in women with dense breasts; however, the benefit of using DBT as baseline imaging compared with DM was not evident.
Over the years, supplemental imaging modalities, including US and DBT, have been increasingly utilized for screening women with dense breast tissue. According to the American College of Radiology appropriateness criteria [28], supplemental DBT is recommended for both screening and diagnostic purposes in women with dense breasts tissue due to improved CDR and reduced recall rate [29,30,31]. In contrast, supplemental screening with breast US is categorized as “may be appropriate” for average-risk women with dense breast due to disagreement between studies associated with improved sensitivity and decreased specificity and positive predictive value [32,33,34,35,36,37]. We expected better diagnostic performance with a combination of two different modalities with strengths in different aspects regarding the higher sensitivity of breast US and higher specificity of DBT, although this was not evident in our study result. In our study, compared with DBT alone, DM combined with US showed higher sensitivity with lower specificity but with comparable CDR (9.8 vs. 7.0 per 1000 examinations, P = 0.463). The sole use of DBT as the primary stand-alone modality showed lower recall rates and higher feasibility than DM with time- and labor-intensive US screening. However, regarding sensitivity, DM combined with US may still be a better choice for detecting cancers, especially in settings where DBT is unavailable, or US costs are lower than DBT.
Recently, Destounis et al. [38] evaluated the use of supplemental US in women with mammographically dense breasts who underwent DBT screening. Almost 96% (49 of 51) of cancers detected by US alone were invasive (49 invasive and 2 DCIS) [38], while most of the cancers detected on DBT alone were DCIS. Similarly, in a previous Adjunct Screening with Tomosynthesis or US in women with Mammography-Negative Dense Breasts (ASTOUND-2) trial [22], adjunct screening detected 27 additional invasive cancers: 12 (44%) detected on both DBT and US, 1 (4%) detected on DBT alone, and 14 (52%) detected on US alone. In our study, most were early-stage invasive cancers with a lymph node-negative status, and a significant number of non-invasive cancers were detected in both groups. Among the 18 cancers detected on DM or DBT, 50% (9 of 9) were DCIS, and 50% (9 of 9) were invasive, while supplemental US detected an additional 75% (6 of 8) invasive cancers. A few cancers in the DM cohort were larger and lymph node-positive (two-stage III cancers with breast density c and d on DM).
In our study, the recall rates for DM and DBT were 6.5% and 9.4%, respectively, and higher recall rates persisted with supplemental US (31.6% vs. 22.4%). The higher AIR of DBT overall and in the negative cohort may be attributed to intensive screening within the DBT cohort, our screening setting with a relatively lower recall rate with DM, or the availability of prior DM imaging for comparison in the DM cohort. Results from previous studies regarding the recall rate of DBT have been inconsistent; some studies [13,39] reported a reduction in false-positive recalls, while others have shown that the proportion of women recalled for further assessment has increased [12,15]. Regardless of the baseline screening modalities of either DM or DBT, AIR increased with the addition of US, similar to the results of other studies [36,40,41]. Interestingly, in women with a negative assessment on DBT, the US still led to higher recall rates than those with a negative assessment of DM. The reasons for the higher recall rates in the DBT cohort were uncertain in the current study. Selection bias, availability of prior examination, or relatively shorter experience in DBT interpretation might affect these different recall rates; thus, further studies are necessary.
Our study had several limitations. First, this was a retrospective study conducted at a single academic institution; thus, selection bias exists. Due to the retrospective design, we used mammographic density, menopausal status, history of hormone replacement therapy, and family history of breast cancer as matching variables; however, there may be other characteristics that should be considered. Second, the independent diagnostic performance of US could not be assessed because US was interpreted based on mammographic findings. Third, our results may not be generalizable to communities in which US is performed by a technologist. Fourth, we did not have detailed risk information for all patients. Fifth, our performance was calculated using data from a single-round screening and could not be extended. Our reference standard of 1-year follow-up may be insufficient; thus, larger studies with longer follow-up periods are needed to verify our results. In addition, we did not consider whether women underwent prevalence or incidence screening. Lastly, since our institution is a hybrid screening setting with both tests available, patients with specific characteristics may be preferentially referred to a particular imaging modality; however, we accounted for these differences by matching the two cohorts.
Our results suggest that supplemental US combined with DBT yielded comparable CDR but lower specificity than US combined with DM in women with dense breasts. In an environment where whole-breast handheld US is performed, the benefit of DBT does not exceed that of DM.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung Min Chang.
- Data curation: Su Min Ha, Ann Yi, Bo Ra Kwon, Sung Ui Shin, Jung Min Chang.
- Formal analysis: Su Min Ha, Dahae Yim, Myoung-jin Jang.
- Investigation: Su Min Ha, Jung Min Chang.
- Supervision: Dahae Yim, Myoung-jin Jang, Eun Jae Lee, Soo Hyun Lee, Woo Kyung Moon.
- Writing—original draft: Su Min Ha.
- Writing—review & editing: Jung Min Chang.
Funding Statement: This study was supported by JW Medical Corporation’s research grant, Seoul National University Hospital (grant no. 06-2020-2460).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2022.0649.
References
- 1.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 2.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 3.Buchberger W, Geiger-Gritsch S, Knapp R, Gautsch K, Oberaigner W. Combined screening with mammography and ultrasound in a population-based screening program. Eur J Radiol. 2018;101:24–29. doi: 10.1016/j.ejrad.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 5.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm J, Humphreys K, Li J, Ploner A, Cheddad A, Eriksson M, et al. Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol. 2015;33:1030–1037. doi: 10.1200/JCO.2014.58.9986. [DOI] [PubMed] [Google Scholar]
- 7.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 8.Chong A, Weinstein SP, McDonald ES, Conant EF. Digital breast tomosynthesis: concepts and clinical practice. Radiology. 2019;292:1–14. doi: 10.1148/radiol.2019180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard HR, Nicholson BE, Rochman CM, Merchant JK, Mayo RC, 3rd, Harvey JA. Digital breast tomosynthesis in the diagnostic setting: indications and clinical applications. Radiographics. 2015;35:975–990. doi: 10.1148/rg.2015140204. [DOI] [PubMed] [Google Scholar]
- 10.Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings at digital breast tomosynthesis occult to conventional digital mammography: imaging features and pathology findings. Breast J. 2015;21:538–542. doi: 10.1111/tbj.12446. [DOI] [PubMed] [Google Scholar]
- 11.Conant EF, Barlow WE, Herschorn SD, Weaver DL, Beaber EF, Tosteson ANA, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019;5:635–642. doi: 10.1001/jamaoncol.2018.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fantò C, Ostillio L, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016;17:1105–1113. doi: 10.1016/S1470-2045(16)30101-2. [DOI] [PubMed] [Google Scholar]
- 13.Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14:583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 14.Houssami N, Macaskill P, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading--evidence to guide future screening strategies. Eur J Cancer. 2014;50:1799–1807. doi: 10.1016/j.ejca.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol. 2013;23:2061–2071. doi: 10.1007/s00330-013-2820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zackrisson S, Lång K, Rosso A, Johnson K, Dustler M, Förnvik D, et al. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018;19:1493–1503. doi: 10.1016/S1470-2045(18)30521-7. [DOI] [PubMed] [Google Scholar]
- 17.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J Natl Cancer Inst. 2018;110:942–949. doi: 10.1093/jnci/djy121. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Radiation-emitting products. MQSA national statistics. U.S. Food and Drug Administration.com Web site. [Accessed July 14, 2022]. https://www.fda.gov/radiation-emitting-products/mqsa-insights/mqsa-national-statistics .
- 19.Dibble EH, Singer TM, Jimoh N, Baird GL, Lourenco AP. Dense breast ultrasound screening after digital mammography versus after digital breast tomosynthesis. AJR Am J Roentgenol. 2019;213:1397–1402. doi: 10.2214/AJR.18.20748. [DOI] [PubMed] [Google Scholar]
- 20.Yi A, Jang MJ, Yim D, Kwon BR, Shin SU, Chang JM. Addition of screening breast US to digital mammography and digital breast tomosynthesis for breast cancer screening in women at average risk. Radiology. 2021;298:568–575. doi: 10.1148/radiol.2021203134. [DOI] [PubMed] [Google Scholar]
- 21.Tagliafico AS, Calabrese M, Mariscotti G, Durando M, Tosto S, Monetti F, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016;34:1882–1888. doi: 10.1200/JCO.2015.63.4147. [DOI] [PubMed] [Google Scholar]
- 22.Tagliafico AS, Mariscotti G, Valdora F, Durando M, Nori J, La Forgia D, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2) Eur J Cancer. 2018;104:39–46. doi: 10.1016/j.ejca.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 23.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS atlas: breast imaging reporting and data system. 5th ed. Reston: American College of Radiology; 2013. [Google Scholar]
- 24.Hofvind S, Hovda T, Holen ÅS, Lee CI, Albertsen J, Bjørndal H, et al. Digital breast tomosynthesis and synthetic 2D mammography versus digital mammography: evaluation in a population-based screening program. Radiology. 2018;287:787–794. doi: 10.1148/radiol.2018171361. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy AM, Guan Z, Welch M, Griffin ME, Sippo DA, Deng Z, et al. Performance of breast cancer risk-assessment models in a large mammography cohort. J Natl Cancer Inst. 2020;112:489–497. doi: 10.1093/jnci/djz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172:1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon PB, Berg WA. Is it really time to close the chapter on screening breast US? Radiology. 2021;301:E414. doi: 10.1148/radiol.2021210104. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein SP, Slanetz PJ, Lewin AA, Battaglia T, Chagpar AB, Dayaratna S, et al. ACR Appropriateness Criteria(R) supplemental breast cancer screening based on breast density. J Am Coll Radiol. 2021;18(11S):S456–S473. doi: 10.1016/j.jacr.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Phi XA, Tagliafico A, Houssami N, Greuter MJW, de Bock GH. Digital breast tomosynthesis for breast cancer screening and diagnosis in women with dense breasts - a systematic review and meta-analysis. BMC Cancer. 2018;18:380. doi: 10.1186/s12885-018-4263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RE, Jr, Venkataraman S, Phillips J, Dialani V, Fein-Zachary VJ, Prakash S, et al. Increased cancer detection rate and variations in the recall rate resulting from implementation of 3D digital breast tomosynthesis into a population-based screening program. Radiology. 2016;278:698–706. doi: 10.1148/radiol.2015142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skaane P, Sebuødegård S, Bandos AI, Gur D, Østerås BH, Gullien R, et al. Performance of breast cancer screening using digital breast tomosynthesis: results from the prospective population-based Oslo Tomosynthesis Screening Trial. Breast Cancer Res Treat. 2018;169:489–496. doi: 10.1007/s10549-018-4705-2. [DOI] [PubMed] [Google Scholar]
- 32.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang JM, Koo HR, Moon WK. Radiologist-performed hand-held ultrasound screening at average risk of breast cancer: results from a single health screening center. Acta Radiol. 2015;56:652–658. doi: 10.1177/0284185114538252. [DOI] [PubMed] [Google Scholar]
- 34.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Arao RF, Sprague BL, Kerlikowske K, Lehman CD, Smith RA, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Intern Med. 2019;179:658–667. doi: 10.1001/jamainternmed.2018.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CI, Cevik M, Alagoz O, Sprague BL, Tosteson AN, Miglioretti DL, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology. 2015;274:772–780. doi: 10.1148/radiol.14141237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Destounis S, Arieno A, Santacroce A. Comparison of cancers detected by screening breast ultrasound and digital breast tomosynthesis. Acad Radiol. 2022;29:339–347. doi: 10.1016/j.acra.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Cohen EO, Tso HH, Phalak KA, Mayo RC, Leung JWT. Screening mammography findings from one standard projection only in the era of full-field digital mammography and digital breast tomosynthesis. AJR Am J Roentgenol. 2018;211:445–451. doi: 10.2214/AJR.17.19023. [DOI] [PubMed] [Google Scholar]
- 40.Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162:157–166. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.