Abstract
Introduction
Chronic pain is a common condition with high socioeconomic and public health burden. A wide range of psychiatric conditions are often comorbid with chronic pain and chronic pain conditions, negatively impacting successful treatment of either condition. The psychiatric condition receiving most attention in the past with regard to chronic pain comorbidity has been major depressive disorder, despite the fact that many other psychiatric conditions also demonstrate epidemiological and genetic overlap with chronic pain. Further understanding potential mechanisms involved in psychiatric and chronic pain comorbidity could lead to new treatment strategies both for each type of disorder in isolation and in scenarios of comorbidity.
Methods
This article provides an overview of relationships between DSM-5 psychiatric diagnoses and chronic pain, with particular focus on PTSD, ADHD, and BPD, disorders which are less commonly studied in conjunction with chronic pain. We also discuss potential mechanisms that may drive comorbidity, and present new findings on the genetic overlap of chronic pain and ADHD, and chronic pain and BPD using linkage disequilibrium score regression analyses.
Results
Almost all psychiatric conditions listed in the DSM-5 are associated with increased rates of chronic pain. ADHD and BPD are significantly genetically correlated with chronic pain. Psychiatric conditions aside from major depression are often under-researched with respect to their relationship with chronic pain.
Conclusion
Further understanding relationships between psychiatric conditions other than major depression (such as ADHD, BPD, and PTSD as exemplified here) and chronic pain can positively impact understanding of these disorders, and treatment of both psychiatric conditions and chronic pain.
Keywords: Chronic pain, Psychiatric conditions, Genetic correlation, Comorbidity
Introduction
Chronic pain, broadly defined as pain persisting beyond a period of 3 months, is a highly prevalent condition [1, 2] with significant negative socioeconomic and quality-of-life impact [3]. Chronic pain is a core feature of a range of conditions, as well as presenting as a primary disorder (as recently outlined for the ICD-11, [4, 5]). Mechanisms of development, and factors contributing to increased likelihood of development of chronic pain, are not fully understood, and treatment and management can be less effective as a result. Chronic pain is often comorbid with psychiatric conditions, with one of the most well-studied psychiatric conditions in relation to chronic pain being major depressive disorder (MDD). However, almost every psychiatric diagnosis in the DSM-5 is also associated with increased rates of chronic pain, and for many psychiatric disorders, this chronic pain relationship is understudied, particularly in personality disorders and neurodevelopmental disorders such as attention-deficit hyperactivity disorder (ADHD).
Importantly, comorbid psychiatric disorder and chronic pain can often contribute to worse outcomes for the individual, as compared to having chronic pain in the absence of psychiatric disorder and vice versa. For example, treatment of chronic pain with SNRI monotherapy [6] in a person with comorbid bipolar disorder can be dangerous and induce mania [7, 8].
Comorbidity between chronic pain and psychiatric disorder could indicate shared neurobiological mechanisms, and so understanding this overlap can inform effective, safe treatment and add to understanding of both chronic pain and psychiatric disorder etiology. In this review, we discuss the relationship between psychiatric disorders and chronic pain, presenting broad evidence for co-occurrence of chronic pain and a range of psychiatric disorders, and discussing potential underlying biological mechanisms of these comorbidities. We focus in particular on three psychiatric disorders with as yet understudied relationships with chronic pain: post-traumatic stress disorder (PTSD), borderline personality disorder (BPD), and ADHD. This knowledge gap may have particularly devastating consequences in cases of chronic pain comorbidity.
Section 1: Epidemiology of Psychiatric Disorders and Chronic Pain
Prevalence of chronic pain and chronic pain disorders is higher in populations with a range of psychiatric disorders compared to those without (Table 1; online suppl. information; for all online suppl. material, see www.karger.com/doi/10.1159/000527041). Some of these relationships are more well studied and understood than others − e.g., there is a large body of literature on the relationship between major depression and chronic pain [1, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. In contrast, chronic pain comorbid with personality disorders, such as BPD, and neurodevelopmental disorders, such as ADHD and autism spectrum disorder, is less well understood. Although neurological disorders and associated neurocognitive disease are referenced in the DSM-5, the relationship between neurological disorders and chronic pain has been reviewed elsewhere [19, 20, 21], and is beyond the scope of this article.
Table 1.
Summary of relationships between psychiatric conditions and chronic pain
| Psychiatric diagnosis | Specific sub-type (if any) | Comorbidity patterns | Evidence for dysregulation of Specific areas or types of chronic pain? pain perception? | |
|---|---|---|---|---|
| Schizophrenia spectrum disorders | Schizophrenia | ↑ [22] ⇓ [23, 24, 25] | Yes [23, 26, 27, 28, 29] | |
| Other schizophrenia spectrum disorders | ↑ [30] | |||
| Bipolar disorder | ↑ [10, 31, 32, 33, 34, 35] | Migraine [34] | ||
| General chronic pain [10, 33] | ||||
| Obsessive-compulsive and related disorders | OCD | ↑ [36, 37, 38] | Yes [39, 40] | Fibromyalgia [36, 41] General chronic pain [37] |
| Body dysmorphic disorder | ? | |||
| Excoriation disorder | ? | Yes [42, 43] | ||
| Hoarding disorder | ↑ [44, 45] | |||
| Trichotillomania | ? | Yes [42] | ||
| Neurodevelopmental disorders | Autism spectrum disorder | ↑ | Yes [46, 47, 48, 49, 50] | Gastrointestinal [51, 52, 53, 54] |
| Joint pain (hypermobility spectrum disorder) [55, 56] |
||||
| Intellectual disability | Mixed [57, 58, 59, 60, 61] | |||
| Other neurodevelopmental disorders* | ? | |||
| Feeding and eating disorders | Anorexia nervosa | ↑ [62, 63] | Yes [64, 65, 66, 67] | Endometriosis-low BMI relationship [68, 69, 70, 71] |
| Bulimia nervosa | ↑ [65, 72] | Yes [67, 73, 74] | Abdominal [74] | |
| Migraine, IBS, facial pain [65] | ||||
| Binge eating disorder | ↑ [75, 76, 77] | |||
| General disordered eating | ↑ [72, 78] | |||
| ARFID | ↑ [65, 79] | Abdominal [79] | ||
| Pica | ? [80, 81] | Sickle cell [82, 83] | ||
| Rumination disorder | ↑ [84] | |||
| Anxiety disorders | Generalized anxiety disorder | ↑ [85] | ||
| Any anxiety disorder | ↑ [1, 86, 87] | |||
| Panic disorder | ↑ [36] | |||
| Social anxiety disorder | ↑ [36] | |||
| Depressive disorders other than MDD | Premenstrual dysphoric disorder | ? [88, 89, 90] | ||
| Substance-related/addictive disorders | Opioid use disorder | ↑ [91, 92, 93, 94] | ||
| Alcohol use disorder | ↑ [95, 96] |
PTSD occurs following exposure to traumatic or stressful life events [97, 98], and presents with symptoms grouped (following DSM-5 diagnostic criteria) into 4 categories: intrusion, avoidance, negative changes to mood and cognition, and arousal/reactivity changes. Lifetime prevalence of PTSD in adult Americans has been estimated at ∼6–7% [97, 98] and at 15.3–26.53% in conflict-affected populations [99, 100]. Other studies have shown similar rates of PTSD between US civilian and veteran populations [101, 102]. PTSD in both veterans and civilians is associated with increased rates of chronic pain [103, 104]. Chronic pain may also represent a significant stressor and inciting event for an adjustment disorder [105] or development of PTSD [106], particularly if the pain is associated with (or caused by) an injury sustained at the time of the index trauma − direct exposure (e.g., injury on 9/11) has been found to be a significant risk factor for subsequent PTSD [107, 108, 109]. Higher pain levels in the early post-injury period are also associated with increased risk of later PTSD symptoms [110, 111].
BPD is characterized by affective dysregulation and an unstable sense of self, in addition to chronic self-destructive behaviors [112, 113, 114, 115]. BPD is common in both general population and clinical settings, with point prevalence and lifetime prevalence in US general population samples found to be 1.6% and 5.9%, respectively [116]. In populations meeting criteria for personality disorder diagnosis of all kinds, chronic pain is more prevalent compared to populations without personality disorder diagnoses [117], and having BPD comorbid with a chronic pain condition is associated with worsening chronic pain symptoms [118]. Those with BPD also report more pain and chronic pain compared to the general population [119, 120, 121]. BPD has also been associated with worse pain symptoms even in comparison with other personality disorders [122]. BPD also shares significant diagnostic cross-over with complex PTSD [123, 124], and both conditions often occur together [125].
Finally, ADHD is a common neurodevelopmental disorder, with symptoms of developmentally inappropriate inattention, impulsivity, and hyperactivity, with diagnosis according to DSM-5 requiring symptom onset before age 12 [114, 126]. Prevalence is estimated at 2.8% in adult populations [127]. As well as pain perception changes in adolescents with chronic pain, higher rates of neuropsychiatric conditions, including ADHD, are present in those with chronic pain [128]. Other studies also found a sex-related relationship, where adolescent girls were more likely to have a neurodevelopmental disorder go undiagnosed or misdiagnosed if they also had a chronic pain condition [129]. High rates of ADHD have also been observed in populations with fibromyalgia, a chronic pain condition [130, 131].
Arrows pointing upward indicate positive relationship between rates of chronic pain and/or chronic pain conditions and psychiatric disorder; arrows pointing downward indicate negative relationship between chronic pain/chronic pain conditions and this psychiatric disorder. Question marks indicate lack of available research on relationships between chronic pain/chronic pain conditions and this psychiatric disorder. *Other neurodevelopmental disorders were childhood onset fluency disorder, specific learning disorders, motor disorder, tic disorder, stereotypic movement disorder. More in-depth discussion is shown in online supplementary Table 1.
With most psychiatric disorders, comorbid chronic pain negatively impacts treatment and management of both psychiatric and chronic pain symptoms. These negative impacts can be due to more general factors shared across several psychiatric disorders such as differences in communication, or clinician biases, or may be related to factors that are more specific to each psychiatric disorder. Factors can also be loosely described as “above the skin” or “below the skin,” with above the skin including social epidemiologic factors such as interactions with others, individual behaviors, and higher level factors such as environment at the neighborhood or country level, and government policies and their implementation and below the skin including genetic and molecular factors [132, 133]. An example of an above-the-skin factor in the relationship between schizophrenia and chronic pain could be differences in access to healthcare for people with schizophrenia [134, 135, 136], which then influences chronic pain development. Here, chronic pain is subsequent to schizophrenia in a causal pathway and is related to individual behavior and social epidemiologic factors (healthcare access). In comparison, a potential below-the-skin factor in chronic pain and schizophrenia could be crosstalk between the dopaminergic and immune systems. In the following section, mostly below-the-skin factors are discussed, but impacts on successful treatment involving more above-the-skin type factors are also briefly covered.
Section 2: Potential Causes for and Mechanisms of Overlap with Chronic Pain
Post-Traumatic Stress Disorder
Psychological Factors
Several psychological models to explain chronic pain and PTSD comorbidity have been proposed. These include the shared vulnerability, mutual maintenance, fear avoidance, and the triple vulnerability models. The shared vulnerability model suggests that certain individual differences, such as increased anxiety sensitivity (fear of anxiety-related bodily sensations and the belief these sensations are physically harmful), increase risk of co-occurring PTSD and chronic pain [103, 137], specifically due to increased likelihood of intense emotional reaction to traumatic incidents involving physical injury [138]. Mutual maintenance theory outlines seven components as drivers of comorbid chronic pain and PTSD: anxiety sensitivity, pain acting as a reminder of the traumatic event, attentional bias (where attention is focused on pain and/or trauma cues), avoidance as a coping mechanism, fatigue/lethargy contributing to depression, general fear in both conditions, and finally overwhelming cognitive demands in both conditions, which limit energy and use of healthy coping mechanisms [139]. With fear avoidance, the individual seeks to avoid potentially beneficial movement in fear of pain, exacerbating both chronic pain and PTSD [140, 141]. The triple vulnerability model suggests that three vulnerabilities are required to be present in order to develop a disorder: biological, generalized psychological, and specific psychological vulnerabilities [137, 142, 143] − these could be shared between chronic pain and PTSD, or elements of either disorder could represent one or more vulnerabilities for the other.
Across models, catastrophizing represents a key component and important link between chronic pain and PTSD. Related to this, PTSD networks in the brain representing information about fear become “highly elaborated and accessible,” changing attentional bias and interpretation of neutral stimuli (reviewed by [144]) − this is similar to being in a chronic pain state, where networks involved in fear and distress also show increased activity [145, 146, 147], and previously neutral sensory stimuli may become noxious and painful (allodynia). Additional theories suggest comorbidity in pain and PTSD could be driven by “pain flashbacks” [148], though this moreso describes pain from injuries happening at the time of trauma.
Genetic Correlation
There is evidence of a shared genetic basis for PTSD and multisite chronic pain (0.41 [149]) and with having at least one chronic pain condition (0.61, men only [150]). In comparison, genetic correlation between schizophrenia and PTSD, and between MDD and PTSD, is ∼0.34 [151], and bipolar disorder and PTSD is 0.65 [152]. However, research into potential pathways driving comorbidity is generally scarce (reviewed by [153]).
Opioid and Endocannabinoid Systems
One potential area of mechanistic overlap between PTSD and chronic pain is the opioid and endocannabinoid systems (ECSs). Interactions between adrenergic alpha2 receptors and mu-opioid receptors have an effect on morphine efficacy [154, 155]: when agonists for both receptors are administered together, this can have synergistic effects for pain relief [156]. These adrenergic receptors have also been found to be reduced in those with PTSD [157, 158], and agonists can be used off-label to treat hyperarousal in PTSD [159] and painful conditions [160]. Activation of cannabinoid and mu-opioid receptors in tandem increases efficacy of morphine pain relief, and this activation is also essential for fear extinction [161, 162, 163]. Models of PTSD involving conditioning and fear extinction have been outlined, where neutral cues become associated with danger, contributing to hyperarousal [164]. The ECS has also been investigated as a potential therapeutic target in PTSD [165].
Immune Factors
Immune factors also play a key role in both PTSD and chronic pain. Higher levels of inflammatory cytokines have been found in those with PTSD versus those without [157, 166, 167, 168, 169, 170, 171], circulating both peripherally and centrally. Other studies show epigenetic changes sustained during trauma may have long-term effects on the immune system and inflammation [172, 173, 174]. The immune system and inflammation also play a key role in both acute and chronic pain [175, 176, 177, 178, 179, 180, 181].
GABAergic Neurosteroids and the HPA Axis
Another set of compounds that may represent a link between PTSD and chronic pain is GABAergic neurosteroids allopregnanolone (ALLO) and pregnanolone (reviewed by [182, 183, 184]). These steroids have an antinociceptive effect in studies of human and rodents [185, 186, 187, 188, 189, 190], have been shown to be effective in reducing neuropathic pain [191], and are also found to be reduced in CSF [192, 193] in brain tissue samples [194], and serum [195] of those with PTSD. Some studies also show women with PTSD may have a “block” in the pathway from progesterone to conversion to neurosteroids [196]. ALLO has also been linked to fear extinction in studies of women with PTSD [197].
ALLO neurosteroids exert their action in part by modulation of the HPA axis. Stress activates the HPA, leading to the eventual release of stress hormones (glucocorticoids, cortisol in humans), regulating gene transcription and stress response. In those with PTSD, studies have shown HPA axis dysfunction, with reduced cortisol levels found in those with PTSD versus controls [198, 199, 200, 201], which may be due to oversensitivity of the negative feedback loop [202, 203] following a period of chronic over-activation of the HPA axis [204]. Other studies found more mixed results for the relationship between cortisol and PTSD [205]. Evidence for HPA axis dysfunction is more mixed for chronic pain [206, 207, 208, 209, 210, 211, 212, 213, 214], and it is not clear whether HPA axis dysfunction in chronic pain is a cause or consequence.
Bone-Derived Neurotrophic Factor
Bone-derived neurotrophic factor (BDNF) expression is altered in a range of brain regions in response to stress (reviewed by [215]), including PTSD, as well as being expressed in many different tissues outside the CNS and playing a part in development [216], and with BDNF-mediated neuroplasticity linked to maintenance of the hippocampus [217, 218, 219]. Function and structure of the hippocampus have been shown to be negatively impacted by stress from trauma exposure, and PTSD symptoms have been associated with hippocampal activity (reviewed by [220]). BDNF is also involved in neuroglial proliferation in response to injury and as part of normal development [216, 219, 221].
Treatment and Management Impacts of Comorbidity
PTSD has been found to make painful symptoms worse in veterans [222] and in civilians [223], and those with PTSD and comorbid chronic pain tend to use more analgesic medication (including opioids) than those with chronic pain who do not have PTSD [224, 225] − since risk of substance misuse is higher in populations with PTSD [226, 227, 228, 229, 230], this can also have a detrimental impact on successfully treating PTSD and comorbid chronic pain. Also, if chronic stress without achieving hormone homeostasis is associated with PTSD, this can directly impact drug processing and so treatment efficacy [231, 232, 233], negatively impacting success of treatments for both conditions.
Comorbidity and potential shared underlying mechanisms can also be positive in terms of treatment: some studies show treatment for PTSD can be helpful in chronic pain, e.g., eye movement desensitization reprocessing [234, 235]. Cognitive behavioral therapy treatment for PTSD has also been found to improve chronic pain symptoms [227, 236], as have a range of other nonpharmaceutical treatments such as narrative exposure therapy, yoga programs, and emotional freedom techniques [227, 237].
Borderline Personality Disorder
Psychological Factors
There are theories that chronic pain is individual failure to self-regulate pain [238], or that a “pain personality” exists [119, 238], referring to specific personality traits that may increase risk of chronic pain development such as reduced self-directedness and increased harm avoidance [239]. Similarly, BPD has been characterized as a pathological inability to self-regulate emotion − these potentially stigmatizing conceptualizations of BPD and chronic pain and their impact on treatment are discussed further below. More recent work conceptualizes BPD as a consequence of stressful or traumatic life events − BPD can be “latent,” with severe symptoms only brought on by sufficient stress [240] − developing chronic pain could be this stressor.
Genetic Correlation
In contrast to PTSD and chronic pain, genetic correlations have not previously been calculated between chronic pain, chronic pain disorders, and BPD. This may in part be due to the fact that GWAS and other genetic studies of BPD are relatively small in sample size in comparison with GWAS of, e.g., MDD − recent GWAS sample sizes in BPD range from 2,750 (with 1075 BPD cases) [241] to 8,426 participants in GWAS analyses of borderline personality features (as opposed to diagnosed BPD) [242]). For the purposes of this review, genetic correlation between multisite chronic pain and BPD was calculated using summary statistics from the 2017 GWAS of BPD (see online suppl. Table 2) carried out by Witt et al. [241] and was found to be 0.39 (SE 0.095), comparable to genetic correlation values found between chronic pain and PTSD, and chronic pain and neuroticism [149].
HPA Axis Dysfunction
HPA axis dysfunction has also been implicated in BPD, as “acute” BPD symptoms tend to be stress-reactive (reviewed by [243]). HPA axis dysfunction has also been linked to BPD through its effects on memory (reviewed by [244]). A recent large meta-analysis also found HPA axis changes in individuals with BPD, measured through cortisol levels [245]. Early life stress and childhood trauma, experienced extremely commonly by those with BPD, also affect HPA axis function [246, 247], to the extent that BPD could be conceptualized as a “stress-related neurodevelopmental disorder” [247], with cortisol-related dysfunction worsening with age and BPD chronicity [248].
Opioid and ECS
Similar to PTSD, studies have also highlighted the endogenous opioid system in BPD pathophysiology. The brain opioid theory of social attachment refers to observations that those addicted to narcotics and those involved in “intense” social relationships show similar emotional and behavioral qualities [249], and suggests that feelings of pleasure from social interaction are due to activity of the endogenous opioid system [250]. Degree of opioid receptor availability has been linked to adult attachment styles [251], and administering naltrexone (an opioid antagonist) has been found to reduce feelings of social connection [252]. In BPD, the endogenous opioid system may be dysregulated, contributing to unstable relationships, chronic feelings of emptiness, and increased susceptibility to substance use disorders − an underactive endogenous opioid system, with reduced receptor density, could explain BPD-associated pathological behavior as a way of seeking exogenous opioid satiety [253]. Symptoms may also be explained by an underactive endogenous opioid system leading to an upregulation of opioid receptors [254], leading to lower baseline levels of pleasure being achievable and hyper-sensitized opioid receptor populations [255, 256].
Opioid drug treatments and the endogenous opioid system play a key role in pain, chronic pain, and treatment of pain (reviewed by [257]), emotional pain has been shown to be neurally similar to physical pain (reviewed by [258, 259]), and emotional euphoria associated with, e.g., romantic relationships has been shown to reduce pain [260]. In addition, opioid receptors can be thought of generally as representing the interface between nervous and immune systems in hyperalgesia (reviewed by [261]). Taken together, evidence suggests dysregulation in the endogenous opioid system could contribute to the relationship between BPD and chronic pain.
BDNF
Changes in neurotrophic factors such as BDNF, and associated changes in neuroplasticity, have also been linked to BPD (reviewed by [246]). Changes to methylation at specific promoters of the BDNF gene have been associated with a range of psychiatric disorders including BPD (reviewed by [262, 263]). These neuroplastic changes may alter the way the brain is able to process and react to various stimuli including stress. Neuroplasticity has also been implicated in the transition from acute to chronic pain [264, 265, 266, 267, 268, 269, 270, 271].
Treatment and Management Impacts of Comorbidity
Chronic pain has been found to worsen personality disorder symptoms [272], and in the case of BPD clinical severity of pain symptoms is worse for patients who have both chronic pain and BPD [118]. Another concern in the impact of chronic pain on BPD treatment (and vice versa) is increased risk of substance use issues in those with BPD [273, 274, 275], particularly if chronic pain treatment involves drug treatment(s) associated with addiction and dependency. This is of particular importance as the overlap between BPD and chronic pain is understudied, and so clinicians and healthcare professionals may be less likely to screen for PDs including BPD in those with chronic pain compared to screening, e.g., MDD or depressive symptoms. BPD is estimated to be 15 times more common in populations with chronic pain compared to those who are pain-free, and in comparison with MDD, studies examining this relationship are relatively scarce (e.g., there are 8 studies from ‘99–2011 [119]).
Patients with a BPD diagnosis are often stigmatized by healthcare professionals as “difficult” (reviewed by [276]; see also [277, 278, 279, 280]). In BPD, in comparison with other psychiatric disorders, symptoms may be seen as the responsibility or failing of the individual instead of part of the disorder: “perceived as purposefully misbehaving rather than experiencing an illness” [281]. There may be parallels in this stigmatization with chronic pain, particularly when chronic pain is not explained by injury, illness, or other physical or medical findings [282], with patients' symptoms (chronic pain) again reacted to as a personal failing − e.g., in studies of adolescents, pain believed to be more medically based (i.e., associated with clear physical signs or injury) was reacted to more positively by healthcare providers [283, 284]. In BPD, this stigmatization can directly negatively impact care, e.g., by affecting patients already heightened rejection sensitivity − clinicians may emotionally withdraw during psychotherapy, which can have devastating effects [277, 279]. The framing of BPD diagnosis and the manner of delivering diagnosis can also have significant effects on subsequent engagement by the patient with services and treatment [285]. Similarly, disbelief of chronic pain patients can cause significant distress and depression (reviewed by [286]; see also [287]). Types of psychotherapeutic interventions under the cognitive behavioral therapy umbrella, in particular dialectical behavioral therapy and acceptance and commitment therapy, aim to improve skills in coping with (physical) distress in the context of chronic pain, and being able to function or attempt to function regardless − this can be compared to the role these therapies play in BPD treatment with aim to increase coping with emotional distress and discomfort (reviewed by [288]).
Attention-Deficit Hyperactivity Disorder
Psychological Factors
Additionally, as implicated in a range of other psychiatric disorders including PTSD and BPD as mentioned above, emotional dysregulation has also been highlighted as a shared aspect in ADHD and fibromyalgia [289]. Executive function deficits are key features of ADHD [290, 291, 292] and may also affect attentional processes and so the extent to which positive coping strategies can be used for chronic pain, increasing risk of symptoms both feeling worse and having a greater negative impact on daily functioning [293].
Genetic Correlation
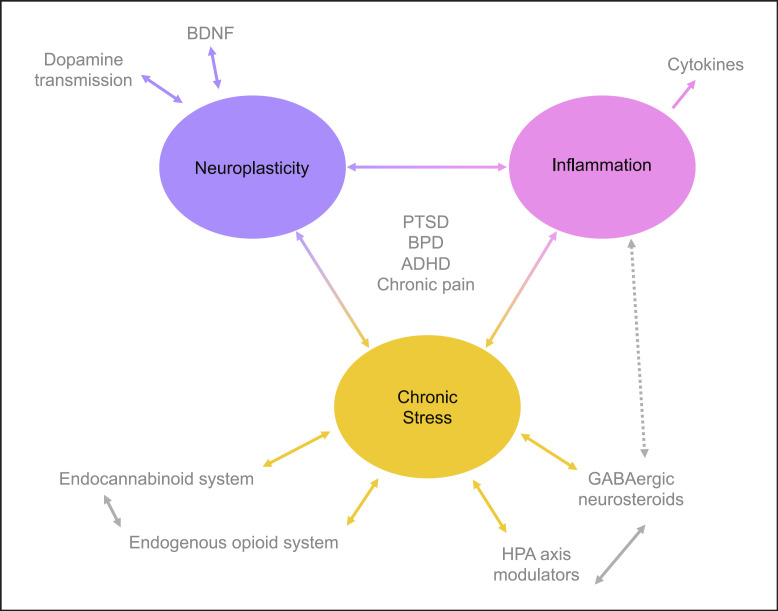
Recent large GWAS, in addition to past twin and family studies [294, 295, 296], highlights that ADHD is a complex trait with a genetic component [294]. ADHD is genetically correlated with multisite chronic pain at a value of 0.56 (see online suppl. Table 3) − this is comparable to genetic correlation values seen between chronic pain traits and MDD [149]. Genetic correlation of 0.26 between migraine and ADHD has also been described [297], and Lundberg et al. [298] also note a “positive” genetic correlation value between ADHD and chronic pain (Fig. 1).
Fig. 1.
Visual representation of possible systems and their interaction in relationships between chronic pain, ADHD, BPD, and PTSD.
Dopamine and ECS
Links between dopamine and ECSs could also be involved in comorbidity between chronic pain and ADHD [299]. The ECS consists of two receptor types, CB1 and CB2. ECS components act as neuromodulators of several neuron types (glutamatergic, GABAergic, serotonergic) aside from dopaminergic and have been linked to a range of psychiatric disorders beyond ADHD [300]. CB2 receptors of the ECS were originally thought to be found entirely peripherally (i.e., outside the CNS), interacting with the immune system to modulate the release of cytokines [301, 302, 303]. Later, they were also found in CNS in microglia, neurons, and astrocytes [304, 305, 306, 307], whereas CB1 receptors are found primarily in the CNS [308]. The ECS also plays a role in nociceptive signaling and chronic pain [309, 310, 311, 312], again involving dopamine transmission and reward circuitry in the brain [312, 313], in addition to being highlighted as a potential therapeutic target in ADHD [299, 314, 315, 316].
Immune Factors
A potential shared mechanism driving ADHD and chronic pain comorbidity could be neuroinflammation. There is an existing body of literature on the importance of neuroinflammation (inflammatory responses in the brain and spinal cord) in chronic pain and chronic pain conditions [178], the role of the complement system in synaptic pruning [317, 318, 319], and crosstalk between the nervous system and immune system in chronic pain development [320, 321]. Neuroinflammation has also been implicated in the pathophysiology of ADHD [128, 322, 323, 324], based on the high comorbidity seen between ADHD and autoimmune and inflammatory disorders in children and adolescents [325, 326, 327], and association between family history of autoimmune and inflammatory disease and individual risk of developing ADHD [327, 328, 329, 330]. Infection during the first few months of life, involving systemic inflammation, has also been associated with increased risk of later ADHD [322, 331, 332, 333].
Studies have also shown ADHD and increased levels of serum cytokines to be associated with one another [333, 334, 335, 336], with the activity of these cytokines implicated in dopaminergic transmission dysfunction. Lower levels of serum cytokines have also been found in studies comparing pharmacologically treated and treatment-naive groups of people with ADHD, again implicating inflammation in ADHD pathology [337], although other studies showed higher levels of inflammatory markers in medicated versus unmedicated children and adults with ADHD [338].
HPA Axis Dysfunction
In ADHD, under-reactivity of the HPA axis as shown through low salivary cortisol levels has been linked to ADHD in adolescents [200, 339, 340, 341], with other studies showing cortisol-level improvements after completing an ADHD treatment program [342], and a relationship between level of executive dysfunction and lower cortisol levels [339].
BDNF
BDNF is a neurotrophic factor in the CNS and nervous system, involved in neural structure and plasticity, and its downregulation and polymorphisms at the gene have been associated with schizophrenia, mood disorders, Alzheimer's disease, and stress (reviewed by [343, 344]; see also [221]). BDNF can also stimulate proliferation of glial cells, involved in pain and chronic pain (reviewed by [345, 346]; see also [347, 348]), and interact with other immune cells in the regulation of pain response [221].
BDNF may play a role in ADHD pathogenesis, through its effects on neurogenesis, particularly development of dopaminergic neurons (reviewed by [349, 350, 351, 352]). Studies of BDNF levels in ADHD show mixed results: BDNF has been found at lower levels in saliva [340] and serum [353] in cohorts with ADHD, while other studies measuring plasma BDNF found higher levels [354, 355], or no difference in serum BDNF between those with ADHD and those without [356]; see also [357] for review. With regard to ADHD and fibromyalgia specifically, dopamine neurotransmission dysfunction (potentially involving BDNF) may contribute to comorbidity between the two conditions. In fibromyalgia, attention and cognitive problems are a common and debilitating issue [358], with dopaminergic transmission dysfunction implicated as a mechanism for these parallel experiences in both ADHD and fibromyalgia [128, 358, 359].
Note that a lot of research is on pain perception and acute pain, in both neuropathic and inflammatory cases, as opposed to chronic pain (reviewed by [360]; see also [361, 362]), and that results are mixed in terms of anti- and pro-nociceptive effects of BDNF. Other studies in rodents look at BDNF and chronic pain more specifically, and show BDNF as a regulator of atypical protein kinase Cs in initiating the transition from acute to chronic pain, and maintaining central sensitization [363], and that BDNF generally mediates acute to chronic pain transition [364, 365]. BDNF may also be involved in both opioid dependence and opioid-induced hyperalgesia as found in studies on rodents [366, 367, 368, 369].
Treatment and Management Impacts of Comorbidity
Having neurodevelopmental disorder ASD and/or ADHD traits in addition to chronic pain makes both quality of life and pain interference worse, in comparison with cases of chronic pain only [293]. In addition, children with chronic pain, particularly girls, may be more at risk of having a comorbid neurodevelopmental disorder go undiagnosed [129] − this relationship between chronic pain and missed diagnoses was also seen across genders in adults [370]. Although ADHD is widely considered a disorder of childhood and adolescence, ADHD persists into adulthood in the majority of those diagnosed [371, 372, 373] − it may be the case that healthcare professionals, particularly those treating chronic pain, assume ADHD is not worth screening for in their adult patient populations. ADHD is also a significant risk factor for development of addiction and substance use issues [374, 375, 376], and this issue may be further compounded when comorbid chronic pain is present [358], again potentially impacting chronic pain treatment particularly in the case of opioid prescription. Similar to some treatments for co-occurring PTSD and chronic pain showing promise, successful treatment of ADHD with stimulant medications has been found to improve pain symptoms in general [130, 370, 377], in addition to improving cognitive symptoms associated with chronic pain disorders such as “fibrofog,” cognitive impairments seen in fibromyalgia (reviewed by [359]).
Discussion
Chronic pain is commonly comorbid with most psychiatric disorders. Having comorbid chronic pain means effective treatment for either disorder is less likely, relative quality of life is reduced, and the impact that pain and psychiatric symptoms have on daily life is increased. Many psychiatric disorders, including ADHD, BPD, and PTSD as disorders of focus here, are more likely to be missed or misdiagnosed when comorbid chronic pain is present − conversely, where psychiatric diagnoses tend to be associated with more profound differences in or difficulties with communication (e.g., autism spectrum disorder, schizophrenia), chronic pain may be going underdiagnosed and undertreated. In addition, the overlap between chronic pain and many psychiatric disorders aside from MDD is understudied, despite, as shown here with ADHD, genetic overlap being comparable to that of chronic pain and MDD, and comorbidity being extremely common. Disorders such as ADHD and BPD are less likely to be screened for in chronic pain patients compared to MDD, which may be due to assumptions that these disorders are disorders only of childhood or are generally uncommon.
One over-arching theme in the overlap between chronic pain and PTSD, and chronic pain and BPD, is trauma, as discussed in previous sections. Despite ADHD appearing to fit less well into a trauma-related narrative compared to BPD and PTSD, this condition is also associated with trauma, with some studies showing children with ADHD more likely to have experienced a traumatic event [378, 379], longstanding observation that ADHD and “maltreatment” are related (reviewed by [379, 380]), and findings that accidents and injuries (potentially traumatic events) are more common in those with ADHD [381, 382, 383, 384, 385].
ADHD may also seem out of place as a neurodevelopmental disorder − however, studies in BPD suggest benefits to viewing BPD through neurodevelopmental lens [386, 387], in part due to the kind of contributing factors, such as traumatic events during developmental periods (childhood), that are extremely common in BPD [388, 389, 390]. Additionally, as reviewed in previous sections and despite being classed as a neurodevelopmental disorder, ADHD often persists into adulthood and remains an important consideration in psychiatric condition − chronic pain overlap.
In addition to each disorder having a relationship with trauma and being an important factor to consider in psychiatric condition − chronic pain comorbidity, all three are also inter-related. ADHD has been noted as a risk factor in BPD diagnosis later in life, and 18–34% of adults with ADHD have been estimated to have comorbid BPD (reviewed by [391, 392]). BPD and PTSD are also commonly comorbid, with up to 70% of individuals with BPD having comorbid PTSD and up to 24% of individuals with PTSD also having BPD in some studies (reviewed by [393, 394]). Finally, higher rates of PTSD have also been observed in those with ADHD and vice versa in both child and adult populations [395].
Overall, and in addition to the points outlined above, we choose to highlight these three conditions as their relationship with chronic pain receives less attention that psychiatric disorders such as MDD, and reduced understanding and awareness of them in chronic pain overlap contribute to worse quality of life for individuals who have both conditions at once. A fuller understanding of the causes and consequences of comorbid psychiatric and chronic pain diagnoses could lead to more effective treatments for both disorders separately, and in cases where they are comorbid. Understanding what makes treatment of comorbid chronic pain and psychiatric disorder successful can lead to further understanding of the shared (and distinct) etiology of each disorder. Key areas that could explain mechanistic overlap in ADHD, BPD, PTSD, and chronic pain reviewed here include the endogenous opioid system, neurosteroids and the HPA axis, and neuro and systemic inflammation, and trauma is also highlighted as a key above-the-skin factor in the relationships between these psychiatric conditions and chronic pain. However, this list is nonexhaustive, and interaction between biological, psychological, and social/environmental factors remains important for chronic pain vulnerability and development, as well as for understanding the overlap between chronic pain and psychiatric conditions.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
Dr. Huckins was funded by the NIMH (R01MH118278, R01MH124839), NIEHS (R01ES033630), and the Klarman Family Foundation. Dr. Johnston was funded by the NIMH (R01MH118278, R01MH124839).
Author Contributions
Dr. Johnston and Dr. Huckins conceived and designed the study, and edited and revised the manuscript. Dr. Johnston performed literature searches and was responsible for the primary drafting of the manuscript.
Supplementary Material
Supplementary data
Supplementary data
Funding Statement
Dr. Huckins was funded by the NIMH (R01MH118278, R01MH124839), NIEHS (R01ES033630), and the Klarman Family Foundation. Dr. Johnston was funded by the NIMH (R01MH118278, R01MH124839).
References
- 1.Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, et al. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135((1–2)):82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392((10159)):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160((1)):28–37. doi: 10.1097/j.pain.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 5.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160((1)):19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira GE, McLachlan AJ, Lin C-WC, Zadro JR, Abdel-Shaheed C, O'Keeffe M, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ. 2021;372:m4825. doi: 10.1136/bmj.m4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyall LM, Penades N, Smith DJ. Changes in prescribing for bipolar disorder between 2009 and 2016: national-level data linkage study in Scotland. Br J Psychiatry. 2019;215((1)):415–421. doi: 10.1192/bjp.2019.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall T, Morriss R, Mayo-Wilson E, Marcus E, Guideline Development Group of the National Institute for Health and Care Excellence Assessment and management of bipolar disorder: summary of updated NICE guidance. BMJ. 2014;349:g5673. doi: 10.1136/bmj.g5673. [DOI] [PubMed] [Google Scholar]
- 9.Brown GK. A causal analysis of chronic pain and depression. J Abnorm Psychol. 1990;99((2)):127–137. doi: 10.1037//0021-843x.99.2.127. [DOI] [PubMed] [Google Scholar]
- 10.Nicholl BI, Mackay D, Cullen B, Martin DJ, Ul-Haq Z, Mair FS, et al. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149, 611 participants in UK Biobank. BMC Psychiatry. 2014;14:350. doi: 10.1186/s12888-014-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13((2)):116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163((20)):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 13.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68((2)):262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 14.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9((10)):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60((1)):39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. Pain. 2012;153((2)):293–304. doi: 10.1016/j.pain.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Jaracz J, Gattner K, Jaracz K, Górna K. Unexplained painful physical symptoms in patients with major depressive disorder: prevalence, pathophysiology and management. CNS Drugs. 2016;30((4)):293–304. doi: 10.1007/s40263-016-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, et al. Pain and depression: a systematic review. Harv Rev Psychiatry. 2018;26((6)):352–363. doi: 10.1097/HRP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 19.Nandi PR. Pain in neurological conditions. Curr Opin Support Palliat Care. 2012;6((2)):194–200. doi: 10.1097/SPC.0b013e328352edff. [DOI] [PubMed] [Google Scholar]
- 20.Borsook D. Neurological diseases and pain. Brain. 2012;135((Pt 2)):320–344. doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cragg JJ, Warner FM, Shupler MS, Jutzeler CR, Cashman N, Whitehurst DGT, et al. Prevalence of chronic pain among individuals with neurological conditions. Health Rep. 2018;29((3)):11–16. [PubMed] [Google Scholar]
- 22.Birgenheir DG, Ilgen MA, Bohnert ASB, Abraham KM, Bowersox NW, Austin K, et al. Pain conditions among veterans with schizophrenia or bipolar disorder. Gen Hosp Psychiatry. 2013;35:480–484. doi: 10.1016/j.genhosppsych.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Engels G, Francke AL, van Meijel B, Douma JG, de Kam H, Wesselink W, et al. Clinical Pain in Schizophrenia: A Systematic Review. J Pain. 2014;15:457–467. doi: 10.1016/j.jpain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Stubbs B, Mitchell AJ, De Hert M, Correll CU, Soundy A, Stroobants M, et al. The prevalence and moderators of clinical pain in people with schizophrenia: A systematic review and large scale meta-analysis. Schizophr Res. 2014;160:1–8. doi: 10.1016/j.schres.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Owen-Smith A, Stewart C, Sesay MM, Strasser SM, Yarborough BJ, Ahmedani B, et al. Chronic pain diagnoses and opioid dispensings among insured individuals with serious mental illness. BMC Psychiatry. 2020;20:40. doi: 10.1186/s12888-020-2456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumensohn R, Ringler D. Pain perception in patients with schizophrenia. J Nerv Ment Dis. 2002;190:481–483. doi: 10.1097/00005053-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S. Are Patients With Schizophrenia Insensitive to Pain? A Reconsideration of the Question. Clin J Pain. 2009;25:244–252. doi: 10.1097/AJP.0b013e318192be97. [DOI] [PubMed] [Google Scholar]
- 28.Singh MK, Giles LL, Nasrallah HA. Pain Insensitivity in Schizophrenia: Trait or State Marker? J Psychiatr Pract. 2006;12:90–102. doi: 10.1097/00131746-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fond G, Boyer L, Andrianarisoa M, Godin O, Bulzacka E, Berna F, et al. Self-reported pain in patients with schizophrenia. Results from the national first-step FACE-SZ cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:62–68. doi: 10.1016/j.pnpbp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Almeida JG de, Braga PE, Lotufo Neto F, Pimenta CA, de M. Chronic Pain and Quality of Life in Schizophrenic Patients. Rev Bras Psiquiatr. 2013;35:13–20. doi: 10.1016/j.rbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 31.De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho AF, Firth J, Vieta E. Bipolar Disorder. N Engl J Med. 2020;383:58–66. doi: 10.1056/NEJMra1906193. [DOI] [PubMed] [Google Scholar]
- 33.Failde I, Dueñas M, Agüera-Ortíz L, Cervilla JA, Gonzalez-Pinto A, Mico JA. Factors associated with chronic pain in patients with bipolar depression: a cross-sectional study. BMC Psychiatry. 2013;13:112. doi: 10.1186/1471-244X-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leo RJ, Singh J. Migraine headache and bipolar disorder comorbidity: A systematic review of the literature and clinical implications. Scand J Pain. 2016;11:136–145. doi: 10.1016/j.sjpain.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Stubbs B, Eggermont L, Mitchell AJ, De Hert M, Correll CU, Soundy A, et al. The prevalence of pain in bipolar disorder: a systematic review and large-scale meta-analysis. Acta Psychiatr Scand. 2015;131:75–88. doi: 10.1111/acps.12325. [DOI] [PubMed] [Google Scholar]
- 36.Asmundson GJG, Katz J. Understanding the co‐occurrence of anxiety disorders and chronic pain: state‐of‐the‐art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 37.Subramaniam M, Abdin E, Vaingankar JA, Chong SA. Obsessive–compulsive disorder: prevalence, correlates, help-seeking and quality of life in a multiracial Asian population. Soc Psychiatry Psychiatr Epidemiol. 2012;47:2035–2043. doi: 10.1007/s00127-012-0507-8. [DOI] [PubMed] [Google Scholar]
- 38.Walid MS. Pain in Nursing Home Residents andCorrelation with Neuropsychiatric Disorders. Pain Physician. 2009;5(12):877–880. [PubMed] [Google Scholar]
- 39.Hezel DM, Riemann BC, McNally RJ. Emotional distress and pain tolerance in obsessive-compulsive disorder. J Behav Ther Exp Psychiatry. 2012;43:981–987. doi: 10.1016/j.jbtep.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Eng GK, Collins KA, Brown C, Ludlow M, Tobe RH, Iosifescu DV, et al. Dimensions of interoception in obsessive-compulsive disorder. J Obsessive-Compuls Relat Disord. 2020;27:100584. doi: 10.1016/j.jocrd.2020.100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppens E, Van Wambeke P, Morlion B, Weltens N, Giao Ly H, Tack J, et al. Prevalence and impact of childhood adversities and post-traumatic stress disorder in women with fibromyalgia and chronic widespread pain. Eur J Pain. 2017;21:1582–1590. doi: 10.1002/ejp.1059. [DOI] [PubMed] [Google Scholar]
- 42.Grant JE, Chamberlain SR. Exploring the neurobiology of OCD: clinical implications. Psychiatr Times. 2020;2020 exploring-neurobiology-ocd-clinical-implications. [PMC free article] [PubMed] [Google Scholar]
- 43.Grant JE, Chamberlain SR. Trichotillomania and Skin-Picking Disorder: An Update. FOCUS. 2021;19:405–412. doi: 10.1176/appi.focus.20210013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates S, Chang WC, Hamilton CE, Chasson GS. Hoarding disorder and co-occurring medical conditions: A systematic review. J Obsessive-Compuls Relat Disord. 2021;30:100661. [Google Scholar]
- 45.Nutley SK, Camacho MR, Eichenbaum J, Nosheny RL, Weiner M, Delucchi KL, et al. Hoarding disorder is associated with self-reported cardiovascular / metabolic dysfunction, chronic pain, and sleep apnea. J Psychiatr Res. 2021;134:15–21. doi: 10.1016/j.jpsychires.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summers J, Shahrami A, Cali S, D'Mello C, Kako M, Palikucin-Reljin A, et al. Self-Injury in Autism Spectrum Disorder and Intellectual Disability: Exploring the Role of Reactivity to Pain and Sensory Input. Brain Sci. 2017;7:140. doi: 10.3390/brainsci7110140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuBois D, Ameis SH, Lai M, Casanova MF, Desarkar P. Interoception in Autism Spectrum Disorder: A review. Int J Dev Neurosci. 2016;52:104–111. doi: 10.1016/j.ijdevneu.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease: Neuroscience of interoception. Acad Sci. 2018;1428:112–128. doi: 10.1111/nyas.13915. [DOI] [PubMed] [Google Scholar]
- 49.Bonaz B, Lane RD, Oshinsky ML, Kenny PJ, Sinha R, Mayer EA, et al. Diseases, Disorders, and Comorbidities of Interoception. Trends Neurosci. 2021;44:39–51. doi: 10.1016/j.tins.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Chen LL, Shen S, Mao J, Lopes M, Liu S, et al. Challenges in the Diagnosis and Management of Pain in Individuals with Autism Spectrum Disorder. Rev J Autism Dev Disord. 2020;7:352–363. [Google Scholar]
- 51.Lanyi J, Flynn C, Mannion A, Maher L, Naughton K, Leader G. Abdominal Pain in Children and Adolescents with Autism Spectrum Disorder: a Systematic Review. Rev J Autism Dev Disord. 2022;9((2)):280–289. [Google Scholar]
- 52.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 53.Wasilewska J, Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: links and risks − a possible new overlap syndrome. Pediatr Health Med Ther. 2015;6:153–166. doi: 10.2147/PHMT.S85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penzol MJ, Salazar de Pablo G, Llorente C, Moreno C, Hernández P, Dorado ML, et al. Functional Gastrointestinal Disease in Autism Spectrum Disorder: A Retrospective Descriptive Study in a Clinical Sample. Front Psychiatry. 2019;10:179. doi: 10.3389/fpsyt.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baeza-Velasco C, Cohen D, Hamonet C, Vlamynck E, Diaz L, Cravero C, et al. Joint Hypermobility-Related Disorders and Pain. Front Psychiatry. 2018;9:656. doi: 10.3389/fpsyt.2018.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baeza-Velasco C, Sinibaldi L, Castori M. Attention-deficit/hyperactivity disorder, joint hypermobility-related disorders and pain: expanding body-mind connections to the developmental age. ADHD Atten Deficit Hyperact. Disord. 2018;10:163–175. doi: 10.1007/s12402-018-0252-2. [DOI] [PubMed] [Google Scholar]
- 57.McGuire BE, Kennedy S. Pain in people with an intellectual disability. Curr Opin Psychiatry. 2013;26:270–275. doi: 10.1097/YCO.0b013e32835fd74c. [DOI] [PubMed] [Google Scholar]
- 58.Doody O, Bailey ME. Interventions in pain management for persons with an intellectual disability. J Intellect Disabil. 2019;23:132–144. doi: 10.1177/1744629517708679. [DOI] [PubMed] [Google Scholar]
- 59.Doody O, Bailey ME. Understanding pain physiology and its application to person with intellectual disability. J Intellect Disabil. 2019;23:5–18. doi: 10.1177/1744629517708680. [DOI] [PubMed] [Google Scholar]
- 60.Doody O, Bailey EM. Pain and pain assessment in people with intellectual disability: Issues and challenges in practice. Br J Learn Disabil. 2017;45:157–165. [Google Scholar]
- 61.Raiter A, Merbler A, Burkitt CC, Symons FJ, Oberlander TF. Clin Pain Manag. John Wiley & Sons; 2022. Pain in individuals with intellectual disabilities; pp. p. 439–449. [Google Scholar]
- 62.Tegethoff M, Belardi A, Stalujanis E, Meinlschmidt G. Comorbidity of Mental Disorders and Chronic Pain: Chronology of Onset in Adolescents of a National Representative Cohort. J Pain. 2015;16:1054–1064. doi: 10.1016/j.jpain.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Gerhardt A, Hartmann M, Schuller-Roma B, Blumenstiel K, Bieber C, Eich W, et al. The Prevalence and Type of Axis-I and Axis-II Mental Disorders in Subjects with Non-Specific Chronic Back Pain: Results from a Population-Based Study. Pain Med. 2011;12:1231–1240. doi: 10.1111/j.1526-4637.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 64.Di Lernia D, Serino S, Cipresso P, Riva G. Ghosts in the Machine. Interoceptive Modeling for Chronic Pain Treatment. Front Neurosci. 2016;10:314. doi: 10.3389/fnins.2016.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sim L, Harbeck Weber C, Harrison T, Peterson C. Central Sensitization in Chronic Pain and Eating Disorders: A Potential Shared Pathogenesis. J Clin Psychol Med Settings. 2021;28:40–52. doi: 10.1007/s10880-019-09685-5. [DOI] [PubMed] [Google Scholar]
- 66.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 67.Clelia Malighetti, Santino Gaudio, Daniele Di Lernia, Marta Matamala-Gomez Altered inner body perception in anorexia and bulimia nervosa: a systematic review. PsyArXiv. 2020 doi: 10.3390/jcm11237134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holdsworth-Carson SJ, Dior UP, Colgrave EM, Healey M, Montgomery GW, Rogers PA, et al. The association of body mass index with endometriosis and disease severity in women with pain. J Endometr Pelvic Pain Disord. 2018;10:79–87. [Google Scholar]
- 69.Tang Y, Zhao M, Lin L, Gao Y, Chen GQ, Chen S, et al. Is body mass index associated with the incidence of endometriosis and the severity of dysmenorrhoea: a case–control study in China? BMJ Open. 2020;10:e037095. doi: 10.1136/bmjopen-2020-037095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lafay Pillet M-C, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, et al. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case-control study. Hum Reprod. 2012;27:265–272. doi: 10.1093/humrep/der346. [DOI] [PubMed] [Google Scholar]
- 71.Yong L, Weiyuan Z. Association between body mass index and endometriosis risk: a meta-analysis. Oncotarget. 2017;8:46928–36. doi: 10.18632/oncotarget.14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sim LA, Lebow J, Weiss K, Harrison T, Bruce B. Eating Disorders in Adolescents With Chronic Pain. J Pediatr Health Care. 2017;31:67–74. doi: 10.1016/j.pedhc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Stein D, Kaye WH, Matsunaga H, Myers D, Orbach I, Har-Even D, et al. Pain perception in recovered bulimia nervosa patients. Int J Eat Disord. 2003;34:331–336. doi: 10.1002/eat.10164. [DOI] [PubMed] [Google Scholar]
- 74.Klabunde M, Collado D, Bohon C. An interoceptive model of bulimia nervosa: A neurobiological systematic review. J Psychiatr Res. 2017;94:36–46. doi: 10.1016/j.jpsychires.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brownley KA, Berkman ND, Peat CM, Lohr KN, Cullen KE, Bann CM, et al. Binge-Eating Disorder in Adults. Ann Intern Med. 2016;165:409–420. doi: 10.7326/M15-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olguin P, Fuentes M, Gabler G, Guerdjikova AI, Keck PE, McElroy SL. Medical comorbidity of binge eating disorder. Eat Weight Disord Stud Anorex Bulim Obes. 2017;22:13–26. doi: 10.1007/s40519-016-0313-5. [DOI] [PubMed] [Google Scholar]
- 77.Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, et al. The Prevalence and Correlates of Binge Eating Disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73:904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pianucci L, Sonagra M, Greenberg BA, Priestley DR, Gmuca S. Disordered eating among adolescents with chronic pain: the experience of a pediatric rheumatology subspecialty pain clinic. Pediatr Rheumatol. 2021;19:16. doi: 10.1186/s12969-021-00506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourne L, Bryant-Waugh R, Cook J, Mandy W. Avoidant/restrictive food intake disorder: A systematic scoping review of the current literature. Psychiatry Res. 2020;288:112961. doi: 10.1016/j.psychres.2020.112961. [DOI] [PubMed] [Google Scholar]
- 80.Advani S, Kochhar G, Chachra S, Dhawan P. Eating everything except food (PICA): A rare case report and review. J Int Soc Prev Community Dent. 2014;4:1–4. doi: 10.4103/2231-0762.127851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nayak SV, Kini R, Shetty U, Rao PK, Kashyap RR, Bhandarkar G. Pica - an eating disorder: a report and review. Arch Med Health Sci. 2017;5:82. [Google Scholar]
- 82.O'Callaghan ET, Gold JI. Pica in Children With Sickle Cell Disease: Two Case Reports. J Pediatr Nurs. 2012;27:e65–e70. doi: 10.1016/j.pedn.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigues N, Shih S, Cohen LL. Pica in Pediatric Sickle Cell Disease. J Clin Psychol Med Settings. 2021;28:6–15. doi: 10.1007/s10880-019-09671-x. [DOI] [PubMed] [Google Scholar]
- 84.Khan S, Hyman PE, Cocjin J, Lorenzo CD. Rumination syndrome in adolescents. J Pediatr. 2000;136:528–531. doi: 10.1016/s0022-3476(00)90018-0. [DOI] [PubMed] [Google Scholar]
- 85.Csupak B, Sommer JL, Jacobsohn E, El-Gabalawy R. A population-based examination of the co-occurrence and functional correlates of chronic pain and generalized anxiety disorder. J Anxiety Disord. 2018;56:74–80. doi: 10.1016/j.janxdis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Kroenke K, Outcalt S, Krebs E, Bair MJ, Wu J, Chumbler N, et al. Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen Hosp Psychiatry. 2013;35:359–365. doi: 10.1016/j.genhosppsych.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 87.Carleton RN, Afifi TO, Taillieu T, Turner S, El-Gabalawy R, Sareen J, et al. Anxiety-related psychopathology and chronic pain comorbidity among public safety personnel. J Anxiety Disord. 2018;55:48–55. doi: 10.1016/j.janxdis.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Terzi R, Terzi H, Kale A, editors. Evaluating the relation of premenstrual syndrome and primary dysmenorrhea in women diagnosed with fibromyalgia. Rev Bras Reumatol Engl. 2015;55:334–339. doi: 10.1016/j.rbr.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 89.Maharaj S, Trevino K. A Comprehensive Review of Treatment Options for Premenstrual Syndrome and Premenstrual Dysphoric Disorder. J Psychiatr Pract. 2015;21:334–350. doi: 10.1097/PRA.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 90.Palit S, Bartley EJ, Kuhn BL, Kerr KL, DelVentura JL, Terry EL, et al. Endogenous inhibition of pain and spinal nociception in women with premenstrual dysphoric disorder. J Pain Res. 2016;9:57–66. doi: 10.2147/JPR.S97109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Speed TJ, Parekh V, Coe W, Antoine D. Comorbid chronic pain and opioid use disorder: literature review and potential treatment innovations. Int Rev Psychiatry. 2018;30:136–146. doi: 10.1080/09540261.2018.1514369. [DOI] [PubMed] [Google Scholar]
- 92.Lipari RN, Park-Lee E. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Subst Abuse Ment Health Serv Admin. 2018:82. [Google Scholar]
- 93.Hser Y-I, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D. Chronic pain among patients with opioid use disorder: results from electronic health records data. J Subst Abuse Treat. 2017;77:26–30. doi: 10.1016/j.jsat.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orhurhu V, Olusunmade M, Urits I, Viswanath O, Peck J, Orhurhu MS, et al. Trends of opioid use disorder among hospitalized patients with chronic pain. Pain Pract. 2019;19:656–663. doi: 10.1111/papr.12789. [DOI] [PubMed] [Google Scholar]
- 95.Maleki N, Tahaney K, Thompson BL, Oscar-Berman M. At the intersection of alcohol use disorder and chronic pain. Neuropsychology. 2019;33:795–807. doi: 10.1037/neu0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vadivelu N, Kai AM, Kodumudi G, Haddad D, Kodumudi V, Kuruvilla N, et al. Recommendations for Substance Abuse and Pain Control in Patients with Chronic Pain. Curr Pain Headache Rep. 2018;22:25. doi: 10.1007/s11916-018-0679-3. [DOI] [PubMed] [Google Scholar]
- 97.Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, et al. The Epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51((8)):1137–1148. doi: 10.1007/s00127-016-1208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62((6)):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 99.Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet. 2019;394((10194)):240–248. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoppen TH, Morina N. The prevalence of PTSD and major depression in the global population of adult war survivors: a meta-analytically informed estimate in absolute numbers. Eur J Psychotraumatology. 2019;10((1)):1578637. doi: 10.1080/20008198.2019.1578637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wisco BE, Marx BP, Wolf EJ, Miller MW, Southwick SM, Pietrzak RH. Posttraumatic stress disorder in the US veteran population: results from the National Health and Resilience in veterans study. J Clin Psychiatry. 2014;75((12)):1338–1346. doi: 10.4088/JCP.14m09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26((5)):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Asmundson GJG, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47((10)):930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- 104.Fishbain DA, Pulikal A, Lewis JE, Gao J. Chronic pain types differ in their reported prevalence of Post -Traumatic Stress Disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: an evidence-based structured systematic review. Pain Med. 2017;18((4)):711–735. doi: 10.1093/pm/pnw065. [DOI] [PubMed] [Google Scholar]
- 105.Katz J, Rosenbloom BN, Fashler S. Chronic pain, psychopathology, and DSM-5 somatic symptom disorder. Can J Psychiatry Rev Can Psychiatr. 2015;60((4)):160–167. doi: 10.1177/070674371506000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pengpid S, Peltzer K. Associations of serious physical injuries with posttraumatic stress and depressive symptoms: a cross-sectional survey among university students in 26 countries. BMC Psychol. 2020;8((1)):129. doi: 10.1186/s40359-020-00501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiGrande L, Neria Y, Brackbill RM, Pulliam P, Galea S. Long-term posttraumatic stress symptoms among 3, 271 civilian survivors of the September 11, 2001, terrorist attacks on the World Trade Center. Am J Epidemiol. 2011;173((3)):271–281. doi: 10.1093/aje/kwq372. [DOI] [PubMed] [Google Scholar]
- 108.Liu B, Tarigan LH, Bromet EJ, Kim H. World Trade Center disaster exposure-related probable posttraumatic stress disorder among responders and civilians: a meta-analysis. PLoS One. 2014;9((7)):e101491. doi: 10.1371/journal.pone.0101491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neria Y, DiGrande L, Adams BG. Posttraumatic stress disorder following the September 11, 2001, terrorist attacks: a review of the literature among highly exposed populations. Am Psychol. 2011;66((6)):429–446. doi: 10.1037/a0024791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zatzick DF, Rivara FP, Nathens AB, Jurkovich GJ, Wang J, Fan M-Y, et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med. 2007;37((10)):1469–1480. doi: 10.1017/S0033291707000943. [DOI] [PubMed] [Google Scholar]
- 111.Brennstuhl M-J, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51((4)):295–304. doi: 10.1111/ppc.12093. [DOI] [PubMed] [Google Scholar]
- 112.Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364((21)):2037–2042. doi: 10.1056/NEJMcp1007358. [DOI] [PubMed] [Google Scholar]
- 113.Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet. 2011;377((9759)):74–84. doi: 10.1016/S0140-6736(10)61422-5. [DOI] [PubMed] [Google Scholar]
- 114.Diagnostic and statistical manual of mental disorders: DSM-5TM. 5th ed. Arlington, VA US: American Psychiatric Publishing, Inc; 2013. [Google Scholar]
- 115.Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013;12((2)):92–98. doi: 10.1002/wps.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey replication. Biol Psychiatry. 2007;62((6)):553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carvalho LF, Primi R, Capitão CG. Personality assessment in chronic pain patients. Estud Psicol Camp. 2016;33((4)):645–653. [Google Scholar]
- 118.Johnson BN, Lumley MA, Cheavens JS, McKernan LC. Exploring the links among borderline personality disorder symptoms, trauma, and pain in patients with chronic pain disorders. J Psychosom Res. 2020;135:110164. doi: 10.1016/j.jpsychores.2020.110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sansone RA, Sansone LA. Chronic pain syndromes and borderline personality. Innov Clin Neurosci. 2012;9((1)):10–14. [PMC free article] [PubMed] [Google Scholar]
- 120.Dixon-Gordon KL, Conkey LC, Whalen DJ. Recent advances in understanding physical health problems in personality disorders. Curr Opin Psychol. 2018;21:1–5. doi: 10.1016/j.copsyc.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heath LM, Paris J, Laporte L, Gill KJ. High prevalence of physical pain among treatment-seeking individuals with borderline personality disorder. J Personal Disord. 2018;32((3)):414–420. doi: 10.1521/pedi_2017_31_302. [DOI] [PubMed] [Google Scholar]
- 122.Dixon-Gordon KL, Whalen DJ, Layden BK, Chapman AL. A systematic review of personality disorders and health outcomes. Can Psychol Can. 2015;56((2)):168–190. doi: 10.1037/cap0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bisson JI, Cosgrove S, Lewis C, Robert NP. Post-traumatic stress disorder. BMJ. 2015;351:h6161. doi: 10.1136/bmj.h6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aliev G, Beeraka NM, Nikolenko VN, Svistunov AA, Rozhnova T, Kostyuk S, et al. Neurophysiology and psychopathology underlying PTSD and recent insights into the PTSD therapies: a comprehensive review. J Clin Med. 2020;9:2951. doi: 10.3390/jcm9092951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ford JD, Courtois CA. Complex PTSD and borderline personality disorder. Borderline Personal Disord Emot Dysregulation. 2021;8((1)):16. doi: 10.1186/s40479-021-00155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tarver J, Daley D, Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. 2014;40((6)):762–774. doi: 10.1111/cch.12139. [DOI] [PubMed] [Google Scholar]
- 127.Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, Al-Hamzawi A, et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten Deficit Hyperact Disord. 2017;9((1)):47–65. doi: 10.1007/s12402-016-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kerekes N, Sanchéz-Pérez AM, Landry M. Neuroinflammation as a possible link between attention-deficit/hyperactivity disorder (ADHD) and pain. Med Hypotheses. 2021;157:110717. doi: 10.1016/j.mehy.2021.110717. [DOI] [PubMed] [Google Scholar]
- 129.Lipsker CW, Bölte S, Hirvikoski T, Lekander M, Holmström L, Wicksell RK. Prevalence of autism traits and attention-deficit hyperactivity disorder symptoms in a clinical sample of children and adolescents with chronic pain. J Pain Res. 2018;11:2827–2836. doi: 10.2147/JPR.S177534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Asztély K, Kopp S, Gillberg C, Waern M, Bergman S. Chronic pain and health-related quality of life in women with autism and/or ADHD: a prospective longitudinal study. J Pain Res. 2019;12:2925–2932. doi: 10.2147/JPR.S212422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Rensburg R, Meyer HP, Hitchcock SA, Schuler CE. Screening for adult ADHD in patients with fibromyalgia syndrome. Pain Med. 2018;19((9)):1825–1831. doi: 10.1093/pm/pnx275. [DOI] [PubMed] [Google Scholar]
- 132.Gillman MW, Hammond RA. Precision treatment and precision prevention: integrating “below and above the skin”. JAMA Pediatr. 2016;170((1)):9–10. doi: 10.1001/jamapediatrics.2015.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galea S, Hernán MA. Win-Win: reconciling social epidemiology and causal inference. Am J Epidemiol. 2020;189((3)):167–170. doi: 10.1093/aje/kwz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ewart SB, Happell B, Bocking J, Platania-Phung C, Stanton R, Scholz B. Social and material aspects of life and their impact on the physical health of people diagnosed with mental illness. Health Expect. 2017;20((5)):984–991. doi: 10.1111/hex.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bellamy CD, H Flanagan E, Costa M, O'Connell-Bonarrigo M, Tana Le T, Guy K, et al. Barriers and facilitators of healthcare for people with mental illness: why integrated patient centered healthcare is necessary. Issues Ment Health Nurs. 2016;37((6)):421–428. doi: 10.3109/01612840.2016.1162882. [DOI] [PubMed] [Google Scholar]
- 136.Lawrence D, Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol Oxf Engl. 2010;24((4 Suppl)):61–68. doi: 10.1177/1359786810382058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003;40((5)):397–405. doi: 10.1682/jrrd.2003.09.0397. [DOI] [PubMed] [Google Scholar]
- 138.Asmundson GJG, Hadjistavropolous HD. Addressing shared vulnerability for comorbid PTSD and chronic pain: a cognitive-behavioral perspective. Cogn Behav Pract. 2006;13((1)):8–16. [Google Scholar]
- 139.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21((6)):857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 140.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85((3)):317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 141.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17((9 Suppl)):T70–T92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kind S, Otis JD. The interaction between chronic pain and PTSD. Curr Pain Headache Rep. 2019;23((12)):91. doi: 10.1007/s11916-019-0828-3. [DOI] [PubMed] [Google Scholar]
- 143.Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. 2nd ed. New York, NY US: Guilford Press; 2002. [Google Scholar]
- 144.Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meier ML, Stämpfli P, Humphreys BK, Vrana A, Seifritz E, Schweinhardt P. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain. Pain Rep. 2017;2((3)):e601. doi: 10.1097/PR9.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, et al. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157((9)):1970–1978. doi: 10.1097/j.pain.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Elman I, Borsook D. Threat response system: parallel brain processes in pain vis-à-vis fear and anxiety. Front Psychiatry. 2018;9:29. doi: 10.3389/fpsyt.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Macdonald B, Salomons TV, Meteyard L, Whalley MG. Prevalence of pain flashbacks in posttraumatic stress disorder arising from exposure to multiple traumas or childhood traumatization. Can J Pain. 2018;2((1)):48–56. doi: 10.1080/24740527.2018.1435994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLOS Genet. 2019;15((6)):e1008164. doi: 10.1371/journal.pgen.1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gasperi M, Panizzon M, Goldberg J, Buchwald D, Afari N. Post-traumatic stress disorder and chronic pain conditions in men: a Twin Study. Psychosom Med. 2021;83((2)):109–117. doi: 10.1097/PSY.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2018;23((3)):666–673. doi: 10.1038/mp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smoller JW. The genetics of stress-related disorders: PTSD depression, and anxiety disorders. Neuropsychopharmacology. 2016;41((1)):297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morgan L, Aldington D. Comorbid chronic pain and post-traumatic stress disorder in UK veterans: a lot of theory but not enough evidence. Br J Pain. 2020;14((4)):256–262. doi: 10.1177/2049463719878753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003 Dec;64((6)):1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 155.Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The α2aAdrenergic receptor subtype mediates spinal analgesia evoked by α2Agonists and is necessary for spinal adrenergic: opioid synergy. J Neurosci. 1997;17((18)):7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chabot-Doré A-J, Schuster DJ, Stone LS, Wilcox GL. Analgesic synergy between opioid and α2-adrenoceptors. Br J Pharmacol. 2015;172((2)):388–402. doi: 10.1111/bph.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maes M, Lin AH, Verkerk R, Delmeire L, Van Gastel A, Van der Planken M, et al. Serotonergic and noradrenergic markers of post-traumatic stress disorder with and without major depression. Neuropsychopharmacology. 1999;20((2)):188–197. doi: 10.1016/S0893-133X(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 158.Krystal JH, Kosten T. Neurobiological aspects of PTSD: review of clinical and preclinical studies. Behav Ther. 1989;20((2)):177–198. [Google Scholar]
- 159.Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98((1–2)):87–92. doi: 10.1159/000446441. [DOI] [PubMed] [Google Scholar]
- 161.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418((6897)):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 162.Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30((3)):516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 163.Ney LJ, Akhurst J, Bruno R, Laing PAF, Matthews A, Felmingham KL. Dopamine, endocannabinoids and their interaction in fear extinction and negative affect in PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110118. doi: 10.1016/j.pnpbp.2020.110118. [DOI] [PubMed] [Google Scholar]
- 164.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trezza V, Campolongo P. The endocannabinoid system as a possible target to treat both the cognitive and emotional features of post-traumatic stress disorder (PTSD) Front Behav Neurosci. 2013;7:100. doi: 10.3389/fnbeh.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62((2)):663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 167.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45((4)):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 168.von Känel R, Begré S, Abbas CC, Saner H, Gander M-L, Schmid J-P. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17((1)):39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- 169.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41((9)):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 170.Gill JM, Szanton S. Inflammation and traumatic stress: the society to cells resiliency model to support integrative interventions. J Am Psychiatr Nurses Assoc. 2011;17((6)):404–416. doi: 10.1177/1078390311418652. [DOI] [PubMed] [Google Scholar]
- 171.Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol Psychiatry. 2020;87((10)):885–897. doi: 10.1016/j.biopsych.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de los Santos R, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107((20)):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Daskalakis NP, Rijal CM, King C, Huckins LM, Ressler KJ. Recent genetics and epigenetics approaches to PTSD. Curr Psychiatry Rep. 2018;20((5)):30. doi: 10.1007/s11920-018-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bam M, Yang X, Zhou J, Ginsberg JP, Leyden Q, Nagarkatti PS, et al. Evidence for epigenetic regulation of pro-inflammatory cytokines, interleukin-12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. J Neuroimmune Pharmacol. 2016;11((1)):168–181. doi: 10.1007/s11481-015-9643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6((7)):521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 176.McMahon SB, Cafferty WBJ, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192((2)):444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 177.Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195((1–2)):157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 178.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16((11)):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lacagnina MJ, Heijnen CJ, Watkins LR, Grace PM. Autoimmune regulation of chronic pain. PAIN Rep. 2021;6((1)):e905. doi: 10.1097/PR9.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A. T cells as an emerging target for chronic pain therapy. Front Mol Neurosci. 2019;12:216. doi: 10.3389/fnmol.2019.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grace PM, Tawfik VL, Svensson CI, Burton MD, Loggia ML, Hutchinson MR. The neuroimmunology of chronic pain: from rodents to humans. J Neurosci. 2021;41((5)):855–865. doi: 10.1523/JNEUROSCI.1650-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Scioli-Salter ER, Forman DE, Otis JD, Gregor K, Valovski I, Rasmusson AM. The shared neuroanatomy and neurobiology of comorbid chronic pain and PTSD: therapeutic implications. Clin J Pain. 2015;31((4)):363–374. doi: 10.1097/AJP.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 183.Almeida FB, Barros HMT, Pinna G. Neurosteroids and Neurotrophic factors: what is their promise as biomarkers for major depression and PTSD? Int J Mol Sci. 2021;22((4)):1758. doi: 10.3390/ijms22041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pinna G. Allopregnanolone, the neuromodulator turned therapeutic agent: thank you, next? Front Endocrinol. 2020;11:507. doi: 10.3389/fendo.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mechlin B, Morrow AL, Maixner W, Girdler SS. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: evidence for ethnic differences. Pain. 2007;131((1–2)):142–152. doi: 10.1016/j.pain.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008;139((3)):603–609. doi: 10.1016/j.pain.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 187.Coronel MF, Villar MJ, Brumovsky PR, González SL. Spinal neuropeptide expression and neuropathic behavior in the acute and chronic phases after spinal cord injury: effects of progesterone administration. Peptides. 2017;88:189–195. doi: 10.1016/j.peptides.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 188.Kawano T, Soga T, Chi H, Eguchi S, Yamazaki F, Kumagai N, et al. Role of the neurosteroid allopregnanolone in the hyperalgesic behavior induced by painful nerve injury in rats. J Anesth. 2011;25((6)):942–945. doi: 10.1007/s00540-011-1216-2. [DOI] [PubMed] [Google Scholar]
- 189.Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy-Pour A. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience. 2014;256:403–411. doi: 10.1016/j.neuroscience.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 190.Huang C-T, Chen S-H, Lue J-H, Chang C-F, Wen W-H, Tsai Y-J. Neurosteroid allopregnanolone suppresses median nerve injury: induced mechanical hypersensitivity and glial extracellular signal − regulated kinase activation through γ-aminobutyric acid type A receptor modulation in the rat cuneate nucleus. Anesthesiology. 2016;125((6)):1202–1218. doi: 10.1097/ALN.0000000000001360. [DOI] [PubMed] [Google Scholar]
- 191.Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog Neurobiol. 2014;113:70–78. doi: 10.1016/j.pneurobio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 192.Rasmusson AM, King MW, Valovski I, Gregor K, Scioli-Salter E, Pineles SL, et al. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104. doi: 10.1016/j.psyneuen.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60((7)):704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 194.Cruz DA, Glantz LA, McGaughey KD, Parke G, Shampine LJ, Kilts JD, et al. Neurosteroid levels in the orbital frontal cortex of subjects with PTSD and controls: a preliminary report. Chronic Stress. 2019;3:247054701983857. doi: 10.1177/2470547019838570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kinzel P, Marx CE, Sollmann N, Hartl E, Guenette JP, Kaufmann D, et al. Serum neurosteroid levels are associated with cortical thickness in individuals diagnosed with posttraumatic stress disorder and history of mild traumatic brain injury. Clin EEG Neurosci. 2020;51((4)):285–299. doi: 10.1177/1550059420909676. [DOI] [PubMed] [Google Scholar]
- 196.Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. doi: 10.1016/j.psyneuen.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 197.Pineles SL, Nillni YI, Pinna G, Webb A, Arditte Hall KA, Fonda JR, et al. Associations between PTSD-Related extinction retention deficits in women and plasma steroids that modulate brain GABAA and NMDA receptor activity. Neurobiol Stress. 2020;13:100225. doi: 10.1016/j.ynstr.2020.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Meewisse M-L, Reitsma JB, de Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 199.Schumacher S, Niemeyer H, Engel S, Cwik JC, Laufer S, Klusmann H, et al. HPA axis regulation in posttraumatic stress disorder: a meta-analysis focusing on potential moderators. Neurosci Biobehav Rev. 2019;100:35–57. doi: 10.1016/j.neubiorev.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 200.Pan X, Wang Z, Wu X, Wen SW, Liu A. Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis. BMC Psychiatry. 2018;18((1)):324. doi: 10.1186/s12888-018-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pan X, Kaminga AC, Wen SW, Wang Z, Wu X, Liu A. The 24-hour urinary cortisol in post-traumatic stress disorder: a meta-analysis. PLoS One. 2020;15((1)):e0227560. doi: 10.1371/journal.pone.0227560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38((9)):1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42((3)):503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 204.Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. 2016;71:127–135. doi: 10.1016/j.psyneuen.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 205.Speer KE, Semple S, Naumovski N, D'Cunha NM, McKune AJ. HPA axis function and diurnal cortisol in post-traumatic stress disorder: a systematic review. Neurobiol Stress. 2019;11:100180. doi: 10.1016/j.ynstr.2019.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136((Pt 3)):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 207.Muhtz C, Rodriguez-Raecke R, Hinkelmann K, Moeller-Bertram T, Kiefer F, Wiedemann K, et al. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013;14((4)):498–503. doi: 10.1111/j.1526-4637.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 208.Sveinsdottir V, Eriksen HR, Ursin H, Hansen ÅM, Harris A. Cortisol, health, and coping in patients with nonspecific low back pain. Appl Psychophysiol Biofeedback. 2016;41((1)):9–16. doi: 10.1007/s10484-015-9300-2. [DOI] [PubMed] [Google Scholar]
- 209.Schell E, Theorell T, Hasson D, Arnetz B, Saraste H. Stress biomarkers' associations to pain in the neck, shoulder and back in healthy media workers: 12-month prospective follow-up. Eur Spine J. 2008;17((3)):393–405. doi: 10.1007/s00586-007-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buryanov A, Kostrub A, Kotiuk V. Endocrine disorders in women with complex regional pain syndrome type. I. Eur J Pain. 2017;21((2)):302–308. doi: 10.1002/ejp.924. [DOI] [PubMed] [Google Scholar]
- 211.Generaal E, Milaneschi Y, Jansen R, Elzinga BM, Dekker J, Penninx BWJH. The brain-derived neurotrophic factor pathway, life stress, and chronic multi-site musculoskeletal pain. Mol Pain. 2016;12:1744806916646783. doi: 10.1177/1744806916646783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Villafañe JH, Pedersini P, Bertozzi L, Drago L, Fernandez-Carnero J, Bishop MD, et al. Exploring the relationship between chronic pain and cortisol levels in subjects with osteoarthritis: results from a systematic review of the literature. Osteoarthritis Cartilage. 2020;28((5)):572–580. doi: 10.1016/j.joca.2020.02.836. [DOI] [PubMed] [Google Scholar]
- 213.Lundh D, Hedelin H, Jonsson K, Gifford M, Larsson D. Assessing chronic pelvic pain syndrome patients: blood plasma factors and cortisol saliva. Scand J Urol. 2013;47((6)):521–528. doi: 10.3109/21681805.2013.769460. [DOI] [PubMed] [Google Scholar]
- 214.Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress. 2017;1:247054701770476. doi: 10.1177/2470547017704763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Miao Z, Wang Y, Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int J Mol Sci. 2020;21((4)):1375. doi: 10.3390/ijms21041375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38((3)):579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112((2)):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 219.Harley SBR, Willis EF, Shaikh SN, Blackmore DG, Sah P, Ruitenberg MJ, et al. Selective ablation of BDNF from microglia reveals novel roles in self-renewal and hippocampal neurogenesis. J Neurosci. 2021;41((19)):4172–4186. doi: 10.1523/JNEUROSCI.2539-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Miller JK, Wiener JM. PTSD recovery, spatial processing, and the val66met polymorphism. Front Hum Neurosci. 2014;8:100. doi: 10.3389/fnhum.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jin Y, Sun LH, Yang W, Cui RJ, Xu SB. The role of BDNF in the neuroimmune axis regulation of mood disorders. Front Neurol. 2019;10:515. doi: 10.3389/fneur.2019.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Benedict TM, Keenan PG, Nitz AJ, Moeller-Bertram T. Post-traumatic stress disorder symptoms contribute to worse pain and health outcomes in veterans with PTSD compared to those without: a systematic review with meta-analysis. Mil Med. 2020;185((9–10)):e1481–e91. doi: 10.1093/milmed/usaa052. [DOI] [PubMed] [Google Scholar]
- 223.Phifer J, Skelton K, Weiss T, Schwartz AC, Wingo A, Gillespie CF, et al. Pain symptomatology and pain medication use in civilian PTSD. Pain. 2011;152((10)):2233–2240. doi: 10.1016/j.pain.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, et al. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47((2)):136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.López-Martínez AE, Reyes-Pérez Á, Serrano-Ibáñez ER, Esteve R, Ramírez-Maestre C. Chronic pain, posttraumatic stress disorder, and opioid intake: a systematic review. World J Clin Cases. 2019;7((24)):4254–4269. doi: 10.12998/wjcc.v7.i24.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liebschutz JM, Saitz R, Weiss RD, Averbuch T, Schwartz S, Meltzer EC, et al. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11:1047–1055. doi: 10.1016/j.jpain.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rometsch-Ogioun El Sount C, Windthorst P, Denkinger J, Ziser K, Nikendei C, Kindermann D, et al. Chronic pain in refugees with posttraumatic stress disorder (PTSD): a systematic review on patients' characteristics and specific interventions. J Psychosom Res. 2019;118:83–97. doi: 10.1016/j.jpsychores.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 228.Brady KT, Back SE, Coffey SF. Substance abuse and posttraumatic stress disorder. Curr Dir Psychol Sci. 2004;13((5)):206–209. [Google Scholar]
- 229.Brady KT, McCauley JL, Back SE. The comorbidity of Post-traumatic Stress Disorder (PTSD) and substance use disorders. In: el-Guebaly N, Carrà G, Galanter M, Baldacchino AM, editors. Textbook of addiction treatment: international perspectives. Cham: Springer International Publishing; 2021. pp. p. 1327–1339. [Google Scholar]
- 230.Koven SG. Veteran treatments: PTSD interventions. Healthcare. 2018;6((3)):94. doi: 10.3390/healthcare6030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tennant F. The physiologic effects of pain on the endocrine system. Pain Ther. 2013;2:75–86. doi: 10.1007/s40122-013-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Konstandi M, Johnson EO, Lang MA. Consequences of psychophysiological stress on cytochrome P450-catalyzed drug metabolism. Neurosci Biobehav Rev. 2014;45:149–167. doi: 10.1016/j.neubiorev.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 233.Stanke-Labesque F, Gautier-Veyret E, Chhun S, Guilhaumou R, French Society of Pharmacology and Therapeutics Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627. doi: 10.1016/j.pharmthera.2020.107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tesarz J, Leisner S, Gerhardt A, Janke S, Seidler GH, Eich W, et al. Effects of eye movement desensitization and reprocessing (EMDR) treatment in chronic pain patients: a systematic review. Pain Med. 2014;15((2)):247–263. doi: 10.1111/pme.12303. [DOI] [PubMed] [Google Scholar]
- 235.Valiente-Gómez A, Moreno-Alcázar A, Treen D, Cedrón C, Colom F, Pérez V, et al. EMDR beyond PTSD: a systematic literature review. Front Psychol. 2017;8:1668. doi: 10.3389/fpsyg.2017.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bosco MA, Gallinati JL, Clark ME. Conceptualizing and treating comorbid chronic pain and PTSD. Pain Res Treat. 2013;2013:174728–10. doi: 10.1155/2013/174728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: an intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma Theory Res Pract Policy. 2020;12((8)):888–896. doi: 10.1037/tra0000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry Edgmont. 2007;4:40–46. [PMC free article] [PubMed] [Google Scholar]
- 239.Conrad R, Wegener I, Geiser F, Kleiman A. Temperament, character, and personality disorders in chronic pain. Curr Pain Headache Rep. 2013;17((3)):318. doi: 10.1007/s11916-012-0318-3. [DOI] [PubMed] [Google Scholar]
- 240.Shapiro H, Kulich RJ, Schatman ME. Manifestation of borderline personality symptomatology in chronic pain patients under stress: an understated and exacerbated consequence of the COVID-19 crisis. J Pain Res. 2020;13:1431–1439. doi: 10.2147/JPR.S264761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Witt SH, Frank J, Awasthi S, Streit F, Jungkunz M, Reinbold CS, et al. Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. Transl Psychiatry. 2017;7((6)):e1155. doi: 10.1038/tp.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lubke GH, Laurin C, Amin N, Hottenga JJ, Willemsen G, van Grootheest G, et al. Genome-wide analyses of borderline personality features. Mol Psychiatry. 2014;19((8)):923–929. doi: 10.1038/mp.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zimmerman DJ, Choi-Kain LW. The hypothalamic-pituitary-adrenal axis in borderline personality disorder: a review. Harv Rev Psychiatry. 2009;17((3)):167–183. doi: 10.1080/10673220902996734. [DOI] [PubMed] [Google Scholar]
- 244.Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther. 2011;17((6)):714–722. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Drews E, Fertuck EA, Koenig J, Kaess M, Arntz A. Hypothalamic-pituitary-adrenal axis functioning in borderline personality disorder: a meta-analysis. Neurosci Biobehav Rev. 2019;96:316–334. doi: 10.1016/j.neubiorev.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 246.Cattane N, Rossi R, Lanfredi M, Cattaneo A. Borderline personality disorder and childhood trauma: exploring the affected biological systems and mechanisms. BMC Psychiatry. 2017;17((1)):221. doi: 10.1186/s12888-017-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Thomas N, Gurvich C, Kulkarni J. Borderline personality disorder, trauma, and the hypothalamus-pituitary-adrenal axis. Neuropsychiatr Dis Treat. 2019;15:2601–2612. doi: 10.2147/NDT.S198804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rausch J, Flach E, Panizza A, Brunner R, Herpertz SC, Kaess M, et al. Associations between age and cortisol awakening response in patients with borderline personality disorder. J Neural Transm. 2021;128((9)):1425–1432. doi: 10.1007/s00702-021-02402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148((9–10)):985–1025. [Google Scholar]
- 250.Inagaki TK. Opioids and social connection. Curr Dir Psychol Sci. 2018;27((2)):85–90. [Google Scholar]
- 251.Turtonen O, Saarinen A, Nummenmaa L, Tuominen L, Tikka M, Armio R-L, et al. Adult attachment system links with brain Mu opioid receptor availability in vivo. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6((3)):360–369. doi: 10.1016/j.bpsc.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 252.Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI. Opioids and social bonding: naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci. 2016;11((5)):728–735. doi: 10.1093/scan/nsw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychol Rev. 2010;117((2)):623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- 254.Prossin AR, Love TM, Koeppe RA, Zubieta J-K, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167((8)):925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stanley B, Siever LJ. The interpersonal dimension of borderline personality disorder: toward a neuropeptide model. Am J Psychiatry. 2010;167((1)):24–39. doi: 10.1176/appi.ajp.2009.09050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.New AS, Stanley B. An opioid deficit in borderline personality disorder: self-cutting, substance abuse, and social dysfunction. Am J Psychiatry. 2010;167((8)):882–885. doi: 10.1176/appi.ajp.2010.10040634. [DOI] [PubMed] [Google Scholar]
- 257.Ballantyne JC, Sullivan MD. Discovery of endogenous opioid systems: what it has meant for the clinician's understanding of pain and its treatment. Pain. 2017;158((12)):2290–2300. doi: 10.1097/j.pain.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 258.Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8((7)):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 259.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13((6)):421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 260.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One. 2010;5((10)):e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Malafoglia V, Ilari S, Vitiello L, Tenti M, Balzani E, Muscoli C, et al. The interplay between chronic pain, opioids, and the immune system. Neuroscientist. 2021 doi: 10.1177/10738584211030493. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ikegame T, Bundo M, Murata Y, Kasai K, Kato T, Iwamoto K. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. J Hum Genet. 2013;58((7)):434–438. doi: 10.1038/jhg.2013.65. [DOI] [PubMed] [Google Scholar]
- 263.Mitchelmore C, Gede L. Brain derived neurotrophic factor: epigenetic regulation in psychiatric disorders. Brain Res. 2014;1586:162–172. doi: 10.1016/j.brainres.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 264.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Apkarian VA, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152((3 Suppl)):S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136((Pt 9)):2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mansour AR, Farmer MA, Baliki MN, Apkarian AV. Chronic pain: the role of learning and brain plasticity. Restor Neurol Neurosci. 2014;32((1)):129–139. doi: 10.3233/RNN-139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2017;18((2)):113–130. doi: 10.1038/nrn.2017.5. [DOI] [PubMed] [Google Scholar]
- 270.Khoutorsky A, Price TJ. Translational control mechanisms in persistent pain. Trends Neurosci. 2018;41((2)):100–114. doi: 10.1016/j.tins.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim W, Kim SK, Nabekura J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J Neurochem. 2017;141((4)):499–506. doi: 10.1111/jnc.14012. [DOI] [PubMed] [Google Scholar]
- 272.McWilliams LA, Higgins KS. Associations between pain conditions and borderline personality disorder symptoms: findings from the National Comorbidity Survey replication. Clin J Pain. 2013;29((6)):527–532. doi: 10.1097/AJP.0b013e31826ab5d0. [DOI] [PubMed] [Google Scholar]
- 273.Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Personal Disord. 2016;30((3)):336–350. doi: 10.1521/pedi_2015_29_197. [DOI] [PubMed] [Google Scholar]
- 274.Trull TJ, Freeman LK, Vebares TJ, Choate AM, Helle AC, Wycoff AM. Borderline personality disorder and substance use disorders: an updated review. Borderline Personal Disord Emot Dysregulation. 2018;5:15. doi: 10.1186/s40479-018-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bassir Nia A. Opioid addiction and borderline personality disorder. Am J Addict. 2018;27((1)):54–55. doi: 10.1111/ajad.12664. [DOI] [PubMed] [Google Scholar]
- 276.Sulzer SH. Does “difficult patient” status contribute to functional demedicalization? The case of borderline personality disorder. Soc Sci Med. 2015;142:82–89. doi: 10.1016/j.socscimed.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Aviram RB, Brodsky BS, Stanley B. Borderline personality disorder, stigma, and treatment implications. Harv Rev Psychiatry. 2006;14((5)):249–256. doi: 10.1080/10673220600975121. [DOI] [PubMed] [Google Scholar]
- 278.Veysey S. People with a borderline personality disorder diagnosis describe discriminatory experiences. Kōtuitui N Z J Soc Sci Online. 2014;9((1)):20–35. [Google Scholar]
- 279.Sansone RA, Sansone LA. Responses of mental health clinicians to patients with borderline personality disorder. Innov Clin Neurosci. 2013;10((5–6)):39–43. [PMC free article] [PubMed] [Google Scholar]
- 280.Bodner E, Cohen-Fridel S, Mashiah M, Segal M, Grinshpoon A, Fischel T, et al. The attitudes of psychiatric hospital staff toward hospitalization and treatment of patients with borderline personality disorder. BMC Psychiatry. 2015;15:2. doi: 10.1186/s12888-014-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sheehan L, Nieweglowski K, Corrigan P. The stigma of personality disorders. Curr Psychiatry Rep. 2016;18((1)):11. doi: 10.1007/s11920-015-0654-1. [DOI] [PubMed] [Google Scholar]
- 282.De Ruddere L, Craig KD. Understanding stigma and chronic pain: a-state-of-the-art review. Pain. 2016;157((8)):1607–1610. doi: 10.1097/j.pain.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 283.Wakefield EO, Zempsky WT, Puhl RM, Litt MD. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. Pain Rep. 2018;3((Suppl 1)):e679. doi: 10.1097/PR9.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Betsch TA, Gorodzinsky AY, Finley GA, Sangster M, Chorney J. What's in a name? Health care providers' perceptions of pediatric pain patients based on diagnostic labels. Clin J Pain. 2017;33:694–698. doi: 10.1097/AJP.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 285.Lester R, Prescott L, McCormack M, Sampson M, North West Boroughs Healthcare NHS Foundation Trust Service users' experiences of receiving a diagnosis of borderline personality disorder: a systematic review. Personal Ment Health. 2020;14((3)):263–283. doi: 10.1002/pmh.1478. [DOI] [PubMed] [Google Scholar]
- 286.Newton BJ, Southall JL, Raphael JH, Ashford RL, LeMarchand K. A narrative review of the impact of disbelief in chronic pain. Pain Manag Nurs. 2013;14((3)):161–171. doi: 10.1016/j.pmn.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 287.Wakefield EO, Puhl RM, Litt MD, Zempsky WT. If it ever really hurts, i try not to let them know”: the use of concealment as a coping strategy among adolescents with chronic pain. Front Psychol. 2021;12:666275. doi: 10.3389/fpsyg.2021.666275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kalira V, Treisman GJ, Clark MR. Borderline personality disorder and chronic pain: a practical approach to evaluation and treatment. Curr Pain Headache Rep. 2013;17((8)):350. doi: 10.1007/s11916-013-0350-y. [DOI] [PubMed] [Google Scholar]
- 289.Bou Khalil R, Khoury E, Richa S. The comorbidity of fibromyalgia syndrome and attention deficit and hyperactivity disorder from a pathogenic perspective. Pain Med. 2018;19:1705–1709. doi: 10.1093/pm/pny142. [DOI] [PubMed] [Google Scholar]
- 290.Brown TE. ADD/ADHD and impaired executive function in clinical practice. Curr Atten Disord Rep. 2009;1:37–41. [Google Scholar]
- 291.Chang S, Yang L, Wang Y, Faraone SV. Shared polygenic risk for ADHD executive dysfunction and other psychiatric disorders. Transl Psychiatry. 2020;10((1)):182. doi: 10.1038/s41398-020-00872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Silverstein MJ, Faraone SV, Leon TL, Biederman J, Spencer TJ, Adler LA. The relationship between executive function deficits and DSM-5-defined ADHD symptoms. J Atten Disord. 2020;24((1)):41–51. doi: 10.1177/1087054718804347. [DOI] [PubMed] [Google Scholar]
- 293.Wiwe Lipsker C, Hirvikoski T, Balter LJT, Bölte S, Lekander M, Holmström L, et al. Autistic traits and attention-deficit hyperactivity disorder symptoms associated with greater pain interference and depression, and reduced health-related quality of life in children with chronic pain. Front Neurosci. 2021;15:716887. doi: 10.3389/fnins.2021.716887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tistarelli N, Fagnani C, Troianiello M, Stazi MA, Adriani W. The nature and nurture of ADHD and its comorbidities: A narrative review on twin studies. Neurosci Biobehav Rev. 2020;109:63–77. doi: 10.1016/j.neubiorev.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 296.Thapar A. Discoveries on the genetics of ADHD in the 21st century: new findings and their implications. Am J Psychiatry. 2018;175((10)):943–950. doi: 10.1176/appi.ajp.2018.18040383. [DOI] [PubMed] [Google Scholar]
- 297.Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. Brainstorm Consortium Analysis of shared heritability in common disorders of the brain. Science. 2018;360((6395)):eaap8757. doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lundberg M, Campos AI, Farrell SF, Wang G, Sterling M, Ngo TT, et al. Genetic, lifestyle and environmental risk factors for chronic pain revealed through GWAS. 2020 Epub ahead of print. [Google Scholar]
- 299.Dawson DA. Targeting the endocannabinoid system in the treatment of ADHD. 2021;7 [Google Scholar]
- 300.Navarro D, Gasparyan A, Navarrete F, Torregrosa AB, Rubio G, Marín-Mayor M, et al. Molecular alterations of the endocannabinoid system in psychiatric disorders. Int J Mol Sci. 2022;23((9)):4764. doi: 10.3390/ijms23094764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365((6441)):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 302.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006;30((Suppl 1)):S13–S8. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 303.Kelly MEM, Lehmann C, Zhou J. The endocannabinoid system in local and systemic inflammation. Colloq Ser Integr Syst Physiol Mol Funct. 2017;9((2)):192. [Google Scholar]
- 304.Navarrete F, García-Gutiérrez MS, Aracil-Fernández A, Lanciego JL, Manzanares J. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson's disease. Neurotherapeutics. 2018;15((2)):459–469. doi: 10.1007/s13311-018-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.García-Gutiérrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, et al. Alterations in gene and protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics. 2018;15((3)):796–806. doi: 10.1007/s13311-018-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci. 2014;111((46)):E5007–E15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153((2)):240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Glass M, Faull RL, Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77((2)):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 309.Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos Trans R Soc B Biol Sci. 2012;367((1607)):3300–3311. doi: 10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Burston JJ, Woodhams SG. Endocannabinoid system and pain: an introduction. Proc Nutr Soc. 2014;73((1)):106–117. doi: 10.1017/S0029665113003650. [DOI] [PubMed] [Google Scholar]
- 311.Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice AS. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain. 2021 doi: 10.1097/j.pain.0000000000002268. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Brodermann HM. Pain, pleasure and placebo: the cannabinoids in reward processing and the perception of pain. Ment Health Addict Res. 2016;1 [Google Scholar]
- 313.Mlost J, Wąsik A, Starowicz K. Role of endocannabinoid system in dopamine signalling within the reward circuits affected by chronic pain. Pharmacol Res. 2019;143:40–47. doi: 10.1016/j.phrs.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 314.Spanagel R. Cannabinoids and the endocannabinoid system in reward processing and addiction: from mechanisms to interventions. Dialogues Clin Neurosci. 2020;22((3)):241–250. doi: 10.31887/DCNS.2020.22.3/rspanagel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Castelli M, Federici M, Rossi S, De Chiara V, Napolitano F, Studer V, et al. Loss of striatal cannabinoid CB1 receptor function in attention-deficit/hyperactivity disorder mice with point-mutation of the dopamine transporter. Eur J Neurosci. 2011;34((9)):1369–1377. doi: 10.1111/j.1460-9568.2011.07876.x. [DOI] [PubMed] [Google Scholar]
- 316.Hupli AMM. Medical cannabis for adult attention deficit hyperactivity disorder: sociological patient case report of cannabinoid therapeutics in Finland. Med Cannabis Cannabinoids. 2019;1((2)):112–118. doi: 10.1159/000495307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gomez-Arboledas A, Acharya MM, Tenner AJ. The role of complement in synaptic pruning and neurodegeneration. ImmunoTargets Ther. 2021;10:373–386. doi: 10.2147/ITT.S305420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in development and disease. Adv Immunol. 2017;135:53–79. doi: 10.1016/bs.ai.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 319.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 320.Kwiatkowski K, Mika J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol Rep. 2018;70((4)):821–830. doi: 10.1016/j.pharep.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 321.Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38((1)):5–19. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Dunn GA, Nigg JT, Sullivan EL. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav. 2019;182:22–34. doi: 10.1016/j.pbb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Song Y, Lu M, Yuan H, Chen T, Han X. Mast cell-mediated neuroinflammation may have a role in attention deficit hyperactivity disorder (Review) Exp Ther Med. 2020;20((2)):714–726. doi: 10.3892/etm.2020.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Corona JC. Role of oxidative stress and neuroinflammation in attention-deficit/hyperactivity disorder. Antioxidants. 2020;9((11)):1039. doi: 10.3390/antiox9111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Miyazaki C, Koyama M, Ota E, Swa T, Mlunde LB, Amiya RM, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17((1)):120. doi: 10.1186/s12888-017-1281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schans J, Çiçek R, de Vries TW, Hak E, Hoekstra PJ. Association of atopic diseases and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. Neurosci Biobehav Rev. 2017;74((Pt A)):139–148. doi: 10.1016/j.neubiorev.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 327.Nielsen PR, Benros ME, Dalsgaard S. Associations between autoimmune diseases and attention-deficit/hyperactivity disorder: a nationwide study. J Am Acad Child Adolesc Psychiatry. 2017;56((3)):234.e1–40.e1. doi: 10.1016/j.jaac.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 328.Ellul P, Acquaviva E, Peyre H, Rosenzwajg M, Gressens P, Klatzmann D, et al. Parental autoimmune and autoinflammatory disorders as multiple risk factors for common neurodevelopmental disorders in offspring: a systematic review and meta-analysis. Transl Psychiatry. 2022;12((1)):112. doi: 10.1038/s41398-022-01843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hegvik T-A, Chen Q, Kuja-Halkola R, Klungsøyr K, Butwicka A, Lichtenstein P, et al. Familial co-aggregation of attention-deficit/hyperactivity disorder and autoimmune diseases: a cohort study based on Swedish population-wide registers. Int J Epidemiol. 2022;51((3)):898–909. doi: 10.1093/ije/dyab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.He H, Yu Y, Liew Z, Gissler M, László KD, Valdimarsdóttir UA, et al. Association of maternal autoimmune diseases with risk of mental disorders in offspring in Denmark. JAMA Netw Open. 2022;5((4)):e227503. doi: 10.1001/jamanetworkopen.2022.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Fichorova RN, Dammann O, Hooper SR, Hunter SJ, Joseph RM, et al. The ELGAN Study ADHD symptoms writing group for the ELGAN Study Investigators Systemic inflammation during the first postnatal month and the risk of attention deficit hyperactivity disorder characteristics among 10 year-old children born extremely preterm. J Neuroimmune Pharmacol. 2017;12((3)):531–543. doi: 10.1007/s11481-017-9742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rand KM, Austin NC, Inder TE, Bora S, Woodward LJ. Neonatal infection and later neurodevelopmental risk in the very preterm infant. J Pediatr. 2016;170:97–104. doi: 10.1016/j.jpeds.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 333.O'Shea TM, Joseph RM, Kuban KCK, Allred EN, Ware J, Coster T, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr Res. 2014;75((6)):781–787. doi: 10.1038/pr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Anand D, Colpo GD, Zeni G, Zeni CP, Teixeira AL. Attention-deficit/hyperactivity disorder and inflammation: what does current knowledge tell us? A systematic review. Front Psychiatry. 2017;8:228. doi: 10.3389/fpsyt.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Donfrancesco R, Nativio P, Di Benedetto A, Villa MP, Andriola E, Melegari MG, et al. Anti-Yo antibodies in children with ADHD: first results about serum cytokines. J Atten Disord. 2020;24((11)):1497–1502. doi: 10.1177/1087054716643387. [DOI] [PubMed] [Google Scholar]
- 336.Darwish AH, Elgohary TM, Nosair NA. Serum interleukin-6 level in children with attention-deficit hyperactivity disorder (ADHD) J Child Neurol. 2019;34((2)):61–67. doi: 10.1177/0883073818809831. [DOI] [PubMed] [Google Scholar]
- 337.Oades RD, Dauvermann MR, Schimmelmann BG, Schwarz MJ, Myint A-M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism: effects of medication. Behav Brain Funct. 2010;6:29. doi: 10.1186/1744-9081-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yang LL, Stiernborg M, Skott E, Söderström Å, Giacobini M, Lavebratt C. Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur Neuropsychopharmacol. 2020;41:118–131. doi: 10.1016/j.euroneuro.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 339.Llorens M, Barba M, Torralbas J, Nadal R, Armario A, Gagliano H, et al. Stress-related biomarkers and cognitive functioning in adolescents with ADHD: effect of childhood maltreatment. J Psychiatr Res. 2022;149:217–225. doi: 10.1016/j.jpsychires.2022.02.041. [DOI] [PubMed] [Google Scholar]
- 340.Chang JP-C, Mondelli V, Satyanarayanan SK, Chiang Y-J, Chen H-T, Su K-P, et al. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain Behav Immun. 2020;88:105–113. doi: 10.1016/j.bbi.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 341.Buske-Kirschbaum A, Trikojat K, Tesch F, Schmitt J, Roessner V, Luksch H, et al. Altered hypothalamus-pituitary-adrenal axis function: a relevant factor in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder? Psychoneuroendocrinology. 2019;105:178–186. doi: 10.1016/j.psyneuen.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 342.Okabe R, Okamura H, Egami C, Tada Y, Anai C, Mukasa A, et al. Increased cortisol awakening response after completing the summer treatment program in children with ADHD. Brain Dev. 2017;39((7)):583–592. doi: 10.1016/j.braindev.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 343.Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18((10)):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- 344.Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20((8)):916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- 345.Gosselin R-D, Suter MR, Ji R-R, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16((5)):519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gazerani P. Satellite glial cells in pain research: a targeted viewpoint of potential and future directions. Front Pain Res. 2021;2:646068. doi: 10.3389/fpain.2021.646068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, et al. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics. 2020;17((3)):846–860. doi: 10.1007/s13311-020-00905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhou L-J, Peng J, Xu Y-N, Zeng W-J, Zhang J, Wei X, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27((13)):3844.e6–3859.e30. doi: 10.1016/j.celrep.2019.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Liu D-Y, Shen X-M, Yuan F-F, Guo O-Y, Zhong Y, Chen J-G, et al. The physiology of BDNF and its relationship with ADHD. Mol Neurobiol. 2015;52((3)):1467–1476. doi: 10.1007/s12035-014-8956-6. [DOI] [PubMed] [Google Scholar]
- 350.Tsai S-J. Role of neurotrophic factors in attention deficit hyperactivity disorder. Cytokine Growth Factor Rev. 2017;34:35–41. doi: 10.1016/j.cytogfr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 351.Mehta T, Mannem N, Yarasi NK, Bollu PC. Biomarkers for ADHD: the present and future directions. Curr Dev Disord Rep. 2020;7((3)):85–92. [Google Scholar]
- 352.Camuso S, La Rosa P, Fiorenza MT, Canterini S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol Dis. 2022;163:105606. doi: 10.1016/j.nbd.2021.105606. [DOI] [PubMed] [Google Scholar]
- 353.Cubero-Millán I, Ruiz-Ramos M-J, Molina-Carballo A, Martínez-Serrano S, Fernández-López L, Machado-Casas I, et al. BDNF concentrations and daily fluctuations differ among ADHD children and respond differently to methylphenidate with no relationship with depressive symptomatology. Psychopharmacology. 2017;234((2)):267–279. doi: 10.1007/s00213-016-4460-1. [DOI] [PubMed] [Google Scholar]
- 354.Shim S-H, Hwangbo Y, Kwon Y-J, Jeong H-Y, Lee B-H, Lee H-J, et al. Increased levels of plasma brain-derived neurotrophic factor (BDNF) in children with attention deficit-hyperactivity disorder (ADHD) Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1824–1828. doi: 10.1016/j.pnpbp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 355.El Ghamry R, El-Sheikh M, Abdel Meguid M, Nagib S, Aly El Gabry D. Plasma brain-derived neurotrophic factor (BDNF) in Egyptian children with attention deficit hyperactivity disorder. Middle East Curr Psychiatry. 2021;28((1)):22. [Google Scholar]
- 356.Scassellati C, Zanardini R, Tiberti A, Pezzani M, Valenti V, Effedri P, et al. Serum brain-derived neurotrophic factor (BDNF) levels in attention deficit-hyperactivity disorder (ADHD) Eur Child Adolesc Psychiatry. 2014;23((3)):173–177. doi: 10.1007/s00787-013-0447-1. [DOI] [PubMed] [Google Scholar]
- 357.Bonvicini C, Faraone SV, Scassellati C. Common and specific genes and peripheral biomarkers in children and adults with attention-deficit/hyperactivity disorder. World J Biol Psychiatry. 2018;19((2)):80–100. doi: 10.1080/15622975.2017.1282175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pallanti S, Porta F, Salerno L. Adult attention deficit hyperactivity disorder in patients with fibromyalgia syndrome: assessment and disabilities. J Psychiatr Res. 2021;136:537–542. doi: 10.1016/j.jpsychires.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 359.Kravitz HM, Katz RS. Fibrofog and fibromyalgia: a narrative review and implications for clinical practice. Rheumatol Int. 2015;35((7)):1115–1125. doi: 10.1007/s00296-014-3208-7. [DOI] [PubMed] [Google Scholar]
- 360.Cappoli N, Tabolacci E, Aceto P, Dello Russo C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J Neuroimmunol. 2020;349:577406. doi: 10.1016/j.jneuroim.2020.577406. [DOI] [PubMed] [Google Scholar]
- 361.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 362.Smith PA. BDNF: no gain without pain? Neuroscience. 2014;283:107–123. doi: 10.1016/j.neuroscience.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 363.Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, et al. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain. 2013;9:12. doi: 10.1186/1744-8069-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, et al. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141((4)):1028–1039. doi: 10.1093/brain/awy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28((44)):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Fu R, Li S, Li S, Gong X, Zhou G, Wang Y, et al. P2X4 receptor in the dorsal horn contributes to BDNF/TrkB and AMPA receptor activation in the pathogenesis of remifentanil-induced postoperative hyperalgesia in rats. Neurosci Lett. 2021;750:135773. doi: 10.1016/j.neulet.2021.135773. [DOI] [PubMed] [Google Scholar]
- 367.Liang D-Y, Sun Y, Shi X-Y, Sahbaie P, Clark JD. Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia. Mol Pain. 2014;10:59. doi: 10.1186/1744-8069-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ferrini F, Trang T, Mattioli T-AM, Laffray S, Del'Guidice T, Lorenzo L-E, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl homeostasis. Nat Neurosci. 2013;16((2)):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Vargas-Perez H, Ting-A-Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in Naïve rats. Science. 2009;324((5935)):1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kasahara S, Okamura Y, Matsudaira K, Oka H, Suzuki Y, Murakami Y, et al. Diagnosis and treatment of attention-deficit hyperactivity disorder in patients with chronic pain. Open J Psychiatry. 2017;07((04)):261–275. [Google Scholar]
- 371.Zalsman G, Shilton T. Adult ADHD: a new disease? Int J Psychiatry Clin Pract. 2016;20((2)):70–76. doi: 10.3109/13651501.2016.1149197. [DOI] [PubMed] [Google Scholar]
- 372.Turgay A, Goodman DW, Asherson P, Lasser RA, Babcock TF, Pucci ML, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73((2)):192–201. doi: 10.4088/JCP.10m06628. [DOI] [PubMed] [Google Scholar]
- 373.Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177((3)):299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50((6)):543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Crunelle CL, van den Brink W, Moggi F, Konstenius M, Franck J, Levin FR, et al. International Consensus Statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur Addict Res. 2018;24((1)):43–51. doi: 10.1159/000487767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kaye S, Ramos-Quiroga JA, van de Glind G, Levin FR, Faraone SV, Allsop S, et al. Persistence and subtype stability of ADHD among substance use disorder treatment seekers. J Atten Disord. 2019;23((12)):1438–1453. doi: 10.1177/1087054716629217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sinita E, Coghill D. The use of stimulant medications for non-core aspects of ADHD and in other disorders. Neuropharmacology. 2014;87:161–172. doi: 10.1016/j.neuropharm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 378.Schilpzand EJ, Sciberras E, Alisic E, Efron D, Hazell P, Jongeling B, et al. Trauma exposure in children with and without ADHD: prevalence and functional impairment in a community-based study of 6–8-year-old Australian children. Eur Child Adolesc Psychiatry. 2018;27((6)):811–819. doi: 10.1007/s00787-017-1067-y. [DOI] [PubMed] [Google Scholar]
- 379.Spalletta G, Janiri D, Piras F, Sani G, editors. Childhood trauma in mental disorders: a comprehensive approach. Cham: Springer International Publishing; 2020. [Google Scholar]
- 380.Craig SG, Bondi BC, O'Donnell KA, Pepler DJ, Weiss MD. ADHD and exposure to maltreatment in children and youth: a systematic review of the past 10 years. Curr Psychiatry Rep. 2020;22((12)):79. doi: 10.1007/s11920-020-01193-w. [DOI] [PubMed] [Google Scholar]
- 381.Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385((9983)):2190–2196. doi: 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- 382.Kittel-Schneider S, Wolff S, Queiser K, Wessendorf L, Meier AM, Verdenhalven M, et al. Prevalence of ADHD in accident victims: results of the PRADA Study. J Clin Med. 2019;8((10)):1643. doi: 10.3390/jcm8101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Adeyemo BO, Biederman J, Zafonte R, Kagan E, Spencer TJ, Uchida M, et al. Mild traumatic brain injury and ADHD: a systematic review of the literature and meta-analysis. J Atten Disord. 2014;18((7)):576–584. doi: 10.1177/1087054714543371. [DOI] [PubMed] [Google Scholar]
- 384.Brunkhorst-Kanaan N, Libutzki B, Reif A, Larsson H, McNeill RV, Kittel-Schneider S. ADHD and accidents over the life span: a systematic review. Neurosci Biobehav Rev. 2021;125:582–591. doi: 10.1016/j.neubiorev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 385.Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallón S, Alvarez Zallo N, Luis EO, et al. Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;84:63–71. doi: 10.1016/j.neubiorev.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 386.Chanen AM, Kaess M. Developmental pathways to borderline personality disorder. Curr Psychiatry Rep. 2012;14((1)):45–53. doi: 10.1007/s11920-011-0242-y. [DOI] [PubMed] [Google Scholar]
- 387.Winsper C, Marwaha S, Lereya ST, Thompson A, Eyden J, Singh SP. A systematic review of the neurobiological underpinnings of borderline personality disorder (BPD) in childhood and adolescence. Rev Neurosci. 2016;27((8)):827–847. doi: 10.1515/revneuro-2016-0026. [DOI] [PubMed] [Google Scholar]
- 388.Battle CL, Shea MT, Johnson DM, Yen S, Zlotnick C, Zanarini MC, et al. Childhood maltreatment associated with adult personality disorders: findings from the Collaborative Longitudinal Personality Disorders Study. J Pers Disord. 2004 Apr;18((2)):193–211. doi: 10.1521/pedi.18.2.193.32777. [DOI] [PubMed] [Google Scholar]
- 389.Yen S, Tracie Shea M, Cynthia L, Battle Dawn M, Johnson Caron Zlotnick Regina Dolan-Sewell. Traumatic exposure and posttraumatic stress disorder in borderline, schizotypal, avoidant, and obsessive-compulsive personality disorders: findings from the collaborative longitudinal personality disorders study. J Nerv Ment Dis. 2002 Aug;190((8)):510–518. doi: 10.1097/00005053-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 390.de Aquino Ferreira LF, Queiroz Pereira FH, Neri Benevides AML, Aguiar Melo MC. Borderline personality disorder and sexual abuse: a systematic review. Psychiatry Res. 2018;262:70–77. doi: 10.1016/j.psychres.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 391.Ditrich I, Philipsen A, Matthies S. Borderline personality disorder (BPD) and attention deficit hyperactivity disorder (ADHD) revisited: a review-update on common grounds and subtle distinctions. Borderline Personal Disord Emot Dysregulation. 2021;8((1)):22. doi: 10.1186/s40479-021-00162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Weiner L, Perroud N, Weibel S. Attention deficit hyperactivity disorder and borderline personality disorder in adults: a review of their links and risks. Neuropsychiatr Dis Treat. 2019;15:3115–3129. doi: 10.2147/NDT.S192871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Frías Á, Palma C. Comorbidity between post-traumatic stress disorder and borderline personality disorder: a review. Psychopathology. 2015;48:1–10. doi: 10.1159/000363145. [DOI] [PubMed] [Google Scholar]
- 394.Matthies SD, Philipsen A. Common ground in Attention Deficit Hyperactivity Disorder (ADHD) and Borderline Personality Disorder (BPD): review of recent findings. Borderline Personal Disord Emot Dysregulation. 2014;1:3. doi: 10.1186/2051-6673-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Spencer AE, Faraone SV, Bogucki OE, Pope AL, Uchida M, Milad MR, et al. Examining the association between posttraumatic stress disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77((1)):72–83. doi: 10.4088/JCP.14r09479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data