ABSTRACT
[Purpose] The purpose of this study was to compare the effectiveness of the Structural Diagnosis and Management (SDM) approach with Myofascial Release (MFR) in improving plantar heel pain, ankle range of motion, and disability. [Subjects] Sixty-four subjects, aged 30–60 years, with a diagnosis of plantar heel pain, plantar fasciitis, or calcaneal spur by a physician according to ICD-10, were equally allocated to the MFR (n = 32) and SDM (n = 32) groups by hospital randomization and concealed allocation. [Methods] In this assessor-blinded randomized clinical trial, the control group performed MFR to the plantar surface of the foot, triceps surae, and deep posterior compartment calf muscles, while the experimental group performed a multimodal approach utilizing the SDM concept for 12 sessions over 4 weeks. Both groups also received strengthening exercises, ice compression, and ultrasound therapy. Pain, activity limitations and disability were assessed as primary outcomes using the Foot Function Index (FFI) and Range of motion (ROM) assessment of the ankle dorsiflexors and plantar flexors using a universal goniometer. Secondary outcomes were measured using the Foot Ankle Disability Index (FADI) and a 10-point manual muscle testing process for the ankle dorsiflexors and plantar flexors. [Results] Both MFR and SDM groups exhibited significant improvements from baseline in all outcome variables, including pain, activity level, disability, range of motion, and function after the 12-week intervention period (p < .05). The SDM group showed more improvements than MFR for FFI pain (p < .01), FFI activity (p < .01), FFI (p < .01) and FADI (p = <.01). [Conclusion] Both MFR and SDM approaches are effective in reducing pain, improving function, ankle range of motion, and reducing disability in plantar heel pain, however, the SDM approach may be a preferred treatment option.
KEYWORDS: Plantar heel pain, MFR, SDM, FADI, FFI, Disability
Introduction
Plantar heel pain, which affects 7.9% of the population, is a disabling condition of the lower limbs characterized by pain in one or both feet [1]. Plantar heel pain clinically presents with heel pain, limited ankle range of motion (ROM), and reduced muscle strength of the foot and ankle. Plantar fasciitis (PF) is often associated with an increased body mass index and is prevalent in occupations that involve prolonged standing and multiple factors [2]. The pathophysiology involves repetitive abnormal stress, which leads to micro-tears in the plantar fascia at the insertion site, causing repetitive collagen degradation, inflammation, and thickening of the fascia [3]. PF also leads to localized pain in loading positions and tenderness and exaggeration of symptoms in the morning [4]. The biomechanical factors for plantar heel pain have been studied by many researchers and the most prominent theories are summarized in Table 1 [1–13]. These theories are based on increased stress to the plantar fascia due to biomechanical alteration of foot and lower limb position or decreased ankle dorsiflexion. These biomechanical abnormalities create an abnormal kinetic chain of forefoot varus, excessive pronation of the foot [5–7], hypermobility in plantar flexion [5,7], and increase or decrease in the arches of the foot [12,13]. The most accepted theory is described by Bolgla and Malone [14], who mention the biomechanical link between plantar fascia and the windlass mechanism. This is illustrated in Figure 1. The windlass mechanism is explained by an imaginary triangle connecting the calcaneus, midtarsal joint, and metatarsal, forming a ‘truss’ where the plantar fascia (along the horizontal line in Figure 1) acts like the ‘Spanish windlass.’ The body weight acts in an inferior direction on both the anterior and posterior parts of the tibia, and midtarsal joint [15]. The ground reaction force is directed upwards through the calcaneus and metatarsal joint. The ‘truss’ forms a stress tension that is necessary to maintain the medial longitudinal arch [16]. Biomechanical abnormalities in the anterior and posterior tibial line or the structures related to the ‘truss’ lead to increased stretch to the plantar fascia and perifascial structures, causing injury to the plantar fascia. The repetitive stress and injury to these soft tissues lead to the clinical manifestation of plantar heel pain and impairments [15,16].
Table 1.
The theories of Biomechanical abnormalities leading to increased stress to the plantar fascia and perifascial structures.
| Theory 1 | Theory 2 | Theory 3 |
|---|---|---|
| Forefoot varus causing excessive pronation during gait [5–7] ⇓ Excessive mobility of the foot [8] ⇓ increased stress level to soft tissues of foot [8–10] ⇓ Stress to plantar fascia and perifascial structures [8–10] |
Excessive mobility or over functioning of foot beyond normal range [5,6] ⇓ More stress to medial joint capsules and ligamentous structures of foot [5,6] ⇓ Adjustment of posterior tibialis by becoming lengthened and hypoactive [11] ⇓ Pain, discomfort and increasing stress to plantar fascia and perifascial structures [5,6] |
Increase or decrease arch of foot causing alteration of normal mobility [12,13] ⇓ Deviation from normal mobility required to absorb the ground reaction force through foot [12,13] ⇓ Inability to dissipate the forces from heel strike to midstance [13] ⇓ Increasing load to plantar fascia causing stress to plantar fascia and perifascial structures [12,13] |
Figure 1.

The windlass mechanism following Bolgla and Malone [14], plantar fascia supports the maintenance of arch and weight distribution of stress through the feet, forefoot varus contributes to excessive pronation and higher arch during ambulation, creating more stress to the plantar musculature and fascia, and creating biomechanical abnormality in the global mobilizer of the ankle. The horizontal line in the figure is the plantar fascia stress like “Spanish Windlass”.
In the UK, 41.0% of patients with plantar heel pain visit a physiotherapist regularly [17]. The treatment approaches prescribe the combined use of analgesics, orthotics, splints or taping, stretching exercises, self-directed exercise, ultrasound therapy, extracorporeal shock wave therapy, and corticosteroid therapy [18]. One study suggests [19] that the incorporation of a plantar fascia selective stretching program in addition to calf stretching exercise for eight weeks can reduce pain and improve ROM in chronic plantar fasciitis for up to two years. Some researchers suggest that stretching the plantar fascia in a non-weight-bearing position is the most effective treatment protocol [20]. Myofascial release (MFR) is applied using various techniques to reduce the tensile load of the plantar fascia at the attachment site. Common soft tissue management in MFR includes deep stripping on the plantar surface of the foot toward the calcaneus or may involve friction to the plantar fascia directed away from the calcaneus [21]. MFR over the pressure pain area of the fascia, over the calcaneus, and the gastrocnemii and soleus muscles was found effective in reducing pain and improving function within 12 sessions over 4 weeks [22]. A variety of studies suggest MFR is superior to friction massage, ultrasound therapy, and stretching to the fascia in the remission of pain and improvement of function [23]. The mechanism of the intervention describes stretching of the muscle components of the fascial layer, breaking cross-linkages, and changing the viscosity of substances in the fascia [24], although the mechanism for intervention effects on ankle range, strength, and function is not clear. SDM for plantar heel pain has been conceptualized based on three biomechanical abnormalities leading to increased strain on the plantar fascia and peri-fascial structures described in Table 1. The consequences of these abnormalities include forefoot varus causing excessive pronation of the foot during walking [5–7], hypermobility of ankle plantar flexion causing stretch to the joint capsule and soft tissues of the foot, and maladaptation of the posterior tibialis [5,6,11]. Another study [25] explains the understanding of the relationship among the ‘windlass mechanism,’ plantar fascia, and gastrocnemius, soleus muscle. As the plantar fascia supports the maintenance of arch and weight distribution of stress through the feet, the forefoot varus may contribute to excessive pronation and higher arch during ambulation, creating more stress to the plantar musculature and fascia, and creating biomechanical abnormality in the plantar flexors and dorsiflexors of the ankle [26]. Gastrocnemius and soleus are interconnected through fascial components connected to the plantar fascia [21]. The flexibility of this connected system of the gastrocnemius, soleus, and plantar fascia can direct the resultant force of the body weight downwards to both the anterior and posterior part of the tibia, and mid-tarsal joint [15]. Moreover, the inability to dissipate body weight through a lower limb in a normal pattern during the stance and swing phase causes abnormal absorption of ground reaction force through the foot [12,13], which may in turn cause alteration in the arch of the foot. The SDM approach includes dorsiflexion strain and mobilization, the release of myofascial trigger points of the gastrocnemius and soleus, improving the flexibility of the hamstrings, and the release of local myoneural adhesions of the posterior femoral cutaneous nerve, medial sural cutaneous nerve, superficial fibular nerve, and tibial nerve. These three maneuvers normalize the flexibility of the hamstrings and gastrocnemius, soleus muscle, reduce myofascial and myoneural adhesions, restore ankle dorsiflexion, improve mobility in plantar flexion, normalize weight distribution during gait, normalize the absorption of ground reaction force through the foot, reduce strain on the joint capsule and soft tissues of the foot, and accelerate the healing process in plantar fasciitis (Table 2).
Table 2.
The theoretical concept of SDM for plantar heel pain.
| Stretching to the gastrocnemii, and soleus muscles. | Release of local trigger points of calf muscles | Myoneural stretch to hamstring and nerves |
|---|---|---|
| Improves flexibility of Gastrocnemius and Soleus ⇓ Counterforce forefoot varus and prevents excessive pronation of the foot during gait [5–7] ⇓ Reduces and normalizes stress level to soft tissues of foot [8–10] ⇓ Normalizes the stress to plantar fascia and to the perifascial structures [21] |
Helps to improve flexibility of Gastrocnemius and Soleus ⇓ enhance normal mobility required [5,6] to absorb the ground reaction force through foot [12,13] ⇓ Promotes normal dissipation of the forces [13] from heel strike to midstance ⇓ Normalizes load to plantar fascia and perifascial structures [21,26] |
Prevents hypermobility or over functioning of foot causing excessive pronation [5–7,11] ⇓ Releasing myoneural adhesions of sciatic and tibial nerves impacts the pathophysiology of plantar fascia [27] ⇓ Normalize the body weight distribution to both the anterior and posterior part of the tibia, and mid-tarsal joint [15]. ⇓ Reduce pain, discomfort to plantar fascia and perifascial structures [15,27] |
SDM is a newly designed hypothetical concept and needs to be examined through a systematic process. Hence, this study aimed to compare the effectiveness of the SDM approach with the MFR approach to improve pain, ankle range of motion, and disability in subjects with plantar heel pain.
Subjects and method
The study was an assessor-blinded, randomized clinical trial that was carried out for 18 months at the Centre for the Rehabilitation of the Paralyzed in Savar, Bangladesh. The study was approved by the BHPI Institutional Review Board (IRB) and prospective trial registration was obtained from the WHO primary trial registry platform. A pilot RCT of 10 subjects was conducted to assess the feasibility of the study. The Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed for the study (Figure 2).
Figure 2.

CONSORT 2010 flow diagram for the study.
Patients
From May 2020 to November 2021, 69 patients aged 30–60 years with a diagnosis of plantar heel pain, plantar fasciitis, or calcaneal spur, as determined by a physician according to ICD-10 [28], were recruited to the study through hospital randomization. According to Chow, Shao, Wang, and Lokhnygina [29], a sample size of 377 patients in each group can provide a .05 superiority margin, with a 0.07 true difference in mean between treatment groups [30] according to the minimal clinically important differences (MCID) of the Foot Functional Index (FFI) with 10% standard deviation, and 80% power. In this time frame, 64 subjects met the eligibility criteria and were assigned, after giving written consent, to either the SDM group or MFR by computer-generated concealed allocation. The inclusion criteria were (i) subjects with a diagnosis of unilateral or bilateral plantar fasciitis, heel spur, or plantar heel pain, according to the ICD 10 criteria, (ii) limited ankle dorsiflexion range of motion (ROM) in any range, and (iii) pain lasting more than 4 weeks. The exclusion criteria were (i) any history of fracture of the foot or lower tibia in the last 6 weeks, (ii) co-morbidity associated with an infectious condition of the foot, for example, endocrine disease with visible cyanotic symptoms in the foot, severe osteopenia of foot in x-ray or carcinoma, (iii) preexisting phobia to physiotherapy or manipulative therapy. Both groups received interventions from two outpatient settings of the same hospital. Interventions were given by an expert physiotherapist with extensive in-service training to follow the specific treatment protocol. MFR was provided by a physiotherapist with post-graduation training in MFR techniques, and SDM was provided by another physiotherapist with post-graduation training in manual therapy. The single assessor was blinded to the assignment and performed all the assessments. Baseline data were collected before treatment and repeated after 12 sessions (3 sessions, 4 weeks) of treatment in the hospital setting.
Interventions
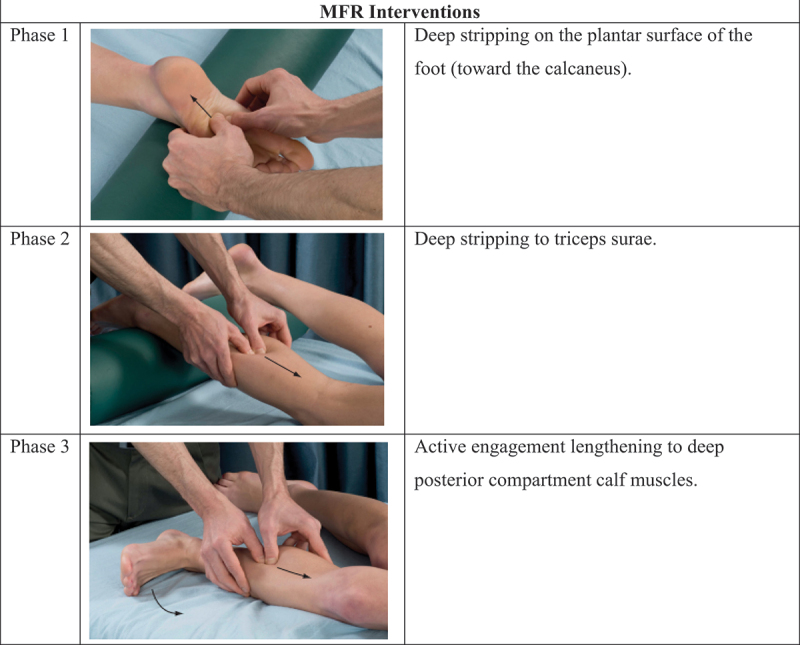
The MFR group received myofascial release of the plantar fascia and perifascial structures in a prone lying position. Patients received three maneuvers for 5–7 repetitions, with 15–30 seconds hold in a progressive manner applied by a specialist physiotherapist. The SDM group also received three interventions, a combination of myofascial release and stretching to the gastrocnemii, and soleus muscles. Additionally, stretching to the hamstring was added with myoneural stretch to the posterior femoral cutaneous nerve, medial sural cutaneous nerve, superficial fibular nerve, and tibial nerve, all in a progressive manner and performed by an experienced physiotherapist. In addition, both groups received strengthening exercises for the dorsiflexor and plantar flexor of the ankle, as well as other conventional treatments such as ultrasound therapy, shoe modification advice, and ice or hot compression as advised by the physiotherapist. Detailed interventions are supplied in (Figure 3(a,b)). From the day of baseline assessment, each participant received a total of 30 minutes of interventions, 3 times a week for 4 weeks.
Figure 3a.

MFR and SDM interventions.
Figure 3b.
(Continued).
Outcome measurement
Primary outcomes such as pain, activity limitations, and disability due to pain were assessed by the Foot Function Index (FFI) [30]. The FFI is a 17-item questionnaire, with five items on pain and 12 questions on painful activities causing disability. Each item has an individual score between 0 and 10, with 0 indicating no pain, difficulty, or disability, and 10 indicating the worst. The scale sum of scores was converted to 100% to generate the final interpretation, as higher scores indicate more pain and/or disability. The tool has good validity, reliability, and wider acceptance among clinicians [31,32]. In addition, the range of motion of ankle dorsiflexion and plantar flexion was measured using a universal goniometer [33]. The goniometer is also a globally accepted reliable tool for measuring joint range of motion. Secondary outcomes evaluated were overall disability by the Foot Ankle Disability Index (FADI) and ankle dorsiflexion and plantar flexion strength by a 10-point manual muscle testing process. FADI is a 26-item questionnaire containing 22 functional difficulty-related questions and four pain-related questions. Each item is scored between 0 and 4, with 0 indicating unable to do and 4 indicating no difficulties at all. The total score was converted to 100%, with the highest score indicating the best functional status. The scale has good validity, reliability, and responsiveness for chronic ankle-related impairments [34]. To determine the strength of dorsiflexion and plantar flexion, manual muscle testing (MMT) was used [35]. MMT has good construct validity and reliability in conditions of chronic musculoskeletal and neuromuscular disorders [35].
For the entire questionnaire, we used a translated Bengali questionnaire with a forward and backward translation by three bilingual researchers and small-scale piloting to maintain internal consistency. The Cronbach’s alpha was 0.7. To ensure reliability, an assessor blinded to the randomization assessed the baseline and posttest data with the aid of an independent data collector.
Statistical analysis
Statistical analyses were conducted on data that had been examined by a separate operator who was blinded to the data collection process. The data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 20.0. The ranges of motion (ROM) in degrees, converted scores of the FFI total score and all the sub-scales, and the FADI were considered as continuous data. The MMT was considered ordinal data. In the baseline compatibility test, differences were examined using an independent t-test for continuous data and a chi-square test for multivariate categorical data. The differences between groups in the posttest were analyzed using an independent t-test for continuous data and the Mann-Whitney U test for categorical data. The changes within the group from the pretest to the posttest were analyzed using a paired t-test or a Wilcoxon test, depending on the nature of the data. The statistical level of significance was set at 0.05 and the clinical level of significance was set as a change of 7 in the FFI score, as per the minimum clinically important difference (MCID) value.
Results
Statistically significant differences were observed in the baseline compatibility test (Table 3) between the MFR and SDM groups in age (p < .01), BMI (p < .01), duration of impairments in weeks (p < .05), FFI activity (p < .01), and FADI scores (p < .01). However, similar baseline criteria were noted in FFI pain (p = .885), FFI disability (p = .426), dorsiflexor (p = .122), and plantar flexor strength (p = .5). The respondents were mostly in their 4th decade of life (MFR 42.5 ± 8.7, SDM 48.2 ± 6.7), the majority of whom were female with overweight as calculated by BMI (MFR 25.7 ± 2.2, SDM 30.8 ± 4.8), who were housewives working nearly 8 hours a day and with chronic conditions (MFR 11.2 ± 11.2 weeks, SDM 19.0 ± 17.8). In both groups, the dorsiflexion range was limited to nearly half of the normal range (MFR 27.4 ± 2.8 degrees and SDM 28.5 ± 2.7 degrees). Both the MFR and SDM groups had statistically significant improvements from baseline in all variables (Table 4). In paired sample t-test, the MFR group had a mean, lower, and upper margin of 95% CI for FFI pain (p < .01), FFI disability (p < .01), FFI activity (p < .01), FADI (p < .05), Plantar flexion range (p < .05), and dorsiflexion range (p < .05); the SDM group had a mean, lower, and upper margin of 95% CI for FFI pain (p < .01), FFI disability (p < .01), FFI activity (p < .01), FADI (p < .01), Plantar flexion range (p < .05), and dorsiflexion range (p < .01). In the Wilcoxon test, the MFR group had statistically significant improvement in strength in plantar flexion (p < .01) and dorsiflexion strength (p < .01); similarly, the SDM group showed improvement in plantar flexion (p < .01) and dorsiflexion (p < .01). In between group analysis (Table 4), the SDM group was found to be better than the MFR group with statistical significance in FFI pain (p < .01), FFI activity (p < .01), FFI disability (p < .01), FADI (p < .01), and plantar flexion strength (p < .05). Detailed results are appended in Table 4.
Table 3.
Baseline compatibility tests.
| Variable | MFR (n=31) | SDM (n=29) | P |
|---|---|---|---|
| Age (years) | 42.5 ± 8.7 | 48.2 ± 6.7 | .0071 |
| Gender (M/F) | 9/22 | 7/22 | .6682* |
| BMI | 25.7 ± 2.2 | 30.8 ± 4.8 | .0011 |
| Occupation | |||
| Farmer | 1 | 3 | .0092 |
| Garments Worker | 6 | 2 | |
| Businessman | 4 | 0 | |
| Housewife | 16 | 20 | |
| Teacher | 4 | 0 | |
| Technical Professionals | 0 | 4 | |
| Walking habit daytime | |||
| Sandal | 9 | 7 | .0012 |
| High Heel | 12 | 2 | |
| Shoe | 8 | 4 | |
| Walk Barefoot | 2 | 16 | |
| Working hours | 7.1 ± 1.7 | 7.5 ± 1.1 | .3081* |
| Limb affected (1/2) | 28/3 | 26/3 | .9312* |
| Duration (Weeks) | 11.2 ± 11.2 | 19.0 ± 17.8 | .0461 |
| AROM PF | 38.2 ± 3.5 | 38.4 ± 3.5 | .8091* |
| AROM DF | 27.4 ± 2.8 | 28.5 ± 2.7 | .1231* |
| Strength PF (median, mode) | 7/7 | 6/6 | .1222* |
| Strength DF(median, mode) | 6/6 | 7/6 | .5092* |
| Pre FFI Pain | 60.4 ± 4.7 | 60.2 ± 7.9 | .8851* |
| Pre FFI disability | 60.8 ± 5.0 | 62.5 ± 10.1 | .4261* |
| Pre FFI activity | 30.2 ± 6.8 | 24.4 ± 8.7 | .0061 |
| Pre FADI total | 70.6 ± 8.3 | 78.7 ± 7.3 | .0011 |
1Independent sample t test; 2Chi square test; * both group are compatible in baseline; significance level (<.05); MFR, Myofascial Release; SDM, Structural Diagnosis and Management.
Table 4.
Between groups and within group outcome for all variables.
| Variables | Within group analysis |
Between group analysis |
|||||
|---|---|---|---|---|---|---|---|
| MFR alone |
SDM alone |
MFR VS SDM |
|||||
| change of mean | p | change of mean | p | mean difference | t | p | |
| FFI Pain | 30.381 | .001** | 29.731 | .001** | −.3492 | −.221 | .001** |
| FFI Disability | 31.291 | .002** | 29.121 | .001** | −3.0752 | −2.28 | .001** |
| FFI Activity | 14.771 | .001** | 10.351 | .001** | 1.2772 | .707 | .009** |
| FADI | −21.031 | .04* | −7.6771 | .001** | 5.2612 | 3.531 | .002** |
| ROM PF | −7.7421 | .014* | −5.8061 | .012** | 2.0022 | .719 | .176 |
| ROM DF |
−10.961 |
.02* |
−6.6771 |
.001** |
3.2152 |
3.890 |
.456 |
| |
Z value |
p |
Z value |
p |
U value |
p |
|
| Strength PF | −3.933 | .003** | −4.5443 | .003** | 3054 | .033* | |
| Strength DF | −4.893 | .002** | −4.7633 | .002** | 3484 | .150 | |
1Paired t test; 2Independent sample t test; 3Wilcoxon test; 4Mann–Whitney U test; * significant with p<.05; ** significant with p<.01, *** significant with p<.001; MFR, Myofascial Release; SDM, Structural Diagnosis and Management; FFI, Foot Function Index; FADI, Foot Ankle Disability Index; PF, Plantar flexion; DF, Dorsiflexion of foot; ROM, Range of motion.
Discussion
This study aimed to compare the effectiveness of the SDM approach to the MFR approach in improving pain, ankle range of motion, foot function, and disability in subjects with plantar heel pain. The randomized clinical study found that both SDM and MFR were effective in reducing pain, improving ankle range of motion, and improving foot function and disability in plantar heel pain within four weeks of intervention. At the baseline, there were discrepancies in age, BMI, symptom duration, and disability status in the FADI index, but similarities in pain, function, and disability in the FFI index. However, in post-treatment, the between-group analysis found that the SDM approach had statistically significant improvements in remission of pain, improving activity, and reducing disability in the FFI index for participants with plantar heel pain.
The majority of the respondents were between 30 and 40 years old, female, overweight, and worked more than 8 hours a day. Studies [2] have found that plantar heel pain is associated with higher BMI, decreased ankle dorsiflexion range of motion, and reductions in foot and ankle strength and flexibility. The MFR approach targeted interventions to release the plantar fascia and the triceps surae and deep parts of the gastrocnemius and soleus muscles. On the other hand, the SDM approach targeted multi-modal interventions to release local myofascial adhesions of the gastrocnemius and soleus muscles, improve the flexibility of the hamstring, and facilitate the neurodynamics of the posterior femoral cutaneous nerve, medial sural cutaneous nerve, superficial fibular nerve, and tibial nerve. Our study found that stretching the gastrocnemius and soleus muscles progressively, rather than targeting the plantar fascia only, is necessary for improvement. Stripping the plantar fascia is a common approach, but the study reveals the necessity of stretching the gastrocnemii and soleus muscles progressively, rather than interventions targeting plantar fascia only [2,5–8,11–13]. There are many approaches to stretching Gastrocnemius and soleus, DiGiovanni and colleagues [20] found stretching both muscles separately has a better result than stretching collectively. Garten [36] explained the isolated process of stretching the Gastrocnemius and soleus in the myofascial pain syndrome. He described the dorsiflexion stretch as keeping the knee extended only stretches the gastrocnemii, to stretch the soleus the knee should be flexed.
The SDM approach used specific and isolated stretching techniques for the hamstring, gastrocnemius, and soleus muscles and did not directly treat the plantar fascia, yet still had positive results. Studies find the flexibility of hamstrings, gastrocnemius, and soleus muscle reduces myofascial and myoneural adhesion [11,20,30], that restores ankle dorsiflexion, improves mobility in plantar flexion, normalizes weight distribution during gait, normalizes the absorption of ground reaction force through foot [12,13], reduce starch to the joint capsule and soft tissues of foot [8–10], and accelerate the healing process in plantar heel pain and associated impairments. Also, normalizing tension to the muscles of the deep posterior compartment muscles of the lower limb facilitates the normalization of tension in the plantar fascia, hence normalizing the biomechanics of the ‘windlass mechanism’ of the foot [26].
Our study found a significant reduction in pain and disability and improvements in inactivity and ankle range for both groups compared to the baseline. The SDM approach of releasing gastrocnemius and soleus muscles was found to be effective in reducing pain and disability and improving activity compared to the MFR approach of the gastrocnemius, soleus, and plantar fascia. These findings concur with previous findings and provide evidence that stretching to the plantar fascia, gastrocnemii, and soleus muscles [20] or stretching gastrocnemii and soleus muscles, alone, have a significant (p < .05) improvement effect, compared to baseline, in 4 weeks (12 sessions). Although the MFR process stated in the control was not supported by Garten [36], the reason for improvement might be the stated process of plantar fascia release. Between-group, the analysis found the SDM intervention for releasing gastrocnemii and soleus muscles was significantly superior in reducing pain and disability and improving activity (p = <.005) compared to the MFR process [20] of gastrocnemii, soleus, and plantar fascia. Thus, it can be concluded that the SDM intervention for gastrocnemii and soleus is superior to conventional MFR for gastrocnemii, soleus, and plantar fascia.
SDM approach used neural stretching techniques for different branches of the sciatic nerve and tibial nerves to improve plantar heel pain. One study [27] describes there is a deep relation with sciatic, tibial, and plantar nerves that can be stretched during modified straight leg raise stretch in the ankle dorsiflexion position. The study also suggests, releasing myoneural adhesions of sciatic and tibial nerves through neurodynamic approaches impacts the pathophysiology of plantar heel pain. In this study, both groups received common treatment as strengthening exercises for the ankle dorsiflexor or plantar flexor, ultrasound therapy, and ice as suggested by the physiotherapist. The added outcome was found as significant changes in dorsiflexion and plantar flexor strength in both groups. The treatment was provided to both groups, so the added effect was ruled out for both groups.
The study had some limitations. The sample size was less than the calculated sample size, and there was a statistically significant difference in age, BMI, symptom duration, and disability in the Foot and Ankle Disability Index (FADI) in both groups during baseline. Additionally, there were added treatments and the study outcome was not sufficient to have an external generalization of the treatment. Furthermore, the key difference in outcome in both groups was the Foot Function Index (FFI), The reason for the smaller sample size was that the study was conducted at a single center with high specificity in diagnosis, resulting in difficulty in finding eligible samples. The study did not conduct any intention-to-treat analysis because the drop-out rate was higher in the SDM group (9.37%) versus MFR (6.25%), mainly due to financial problems. No adverse events were reported in either group. Future studies should include a repeated measures evaluation to explore the long-term effects of the Self-Directed Management (SDM) intervention. Also, a larger sample size would allow the application of parametric statistical analysis, measuring strength with a standardized test, such as a dynamometer, and the inclusion of gait and posture analysis may provide a greater understanding of the multi-dimensional context and outcomes for the SDM approach.
Conclusion
MFR and SDM approaches are both effective interventions to reduce pain and disability, and improve activities and ankle ROM, in patients with plantar heel pain. However, the SDM approach can be a method of choice in the reduction of pain, improving activities related to foot function, and reducing disability in plantar heel pain.
Biographies
Sapia Akter is a consultant physiotherapist working in the field of musculoskeletal medicine and Women’s health. She completed her bachelor's and master’s degrees in Physiotherapy from the University of Dhaka. She is a Ph.D. fellow at Jashore University of Science and Technology. She has more than 16 year’s clinical experience. She is the co-founder of the Structural diagnosis and management (SDM) concept of Musculoskeletal Medicine.
Mohammad Shahadat Hossain is the chief consultant of the Department of musculoskeletal medicine at the Institute of Advanced mechanical correction therapy. He completed his bachelor's and master’s degree in Physiotherapy from the University of Dhaka. He is a Ph.D. fellow at Jahangirnagar University. He has more than 16 year’s clinical experience. He is the founder of the Structural diagnosis and management (SDM) concept of Musculoskeletal Medicine.
K M Amran Hossain is a full-time faculty of Jashore University of Science & Technology. Dr. Amran is the founder of 'Amran's School of Thoughts', which promotes healthcare research in lower-middle-income countries. He completed his graduation and post-graduation degree in Physiotherapy from the University of Dhaka. He is the organizing secretary of the national physiotherapy association accredited by the World Physiotherapy. Also, he is the equity, diversity, and belonging co-director of Long COVID Physio. He received 4 International awards for research and leadership. Dr. Amran is a member of a multi-specialty research team for Long COVID. He is also the Academic editor of PLOS One.
Dr. Zakir Uddin did his bachelor's degree from the University of Dhaka (Bangladesh); master's degree from Kobe University (Japan); doctoral degree from McMaster University and post-doctoral fellowship from McGill University. He has worked in 5 different countries (Bangladesh, Japan, Canada, South Korea, United Arab Emirates) and gained clinical, academic teaching, and research experiences in pain rehabilitation and interdisciplinary health approach. His notable academic works were a full-time teaching faculty at the University of Sharjah, UAE, and Woosong University, South Korea; part-time teaching faculty with McMaster University, Canada; Pain research work at McMaster & McGill Universities, Canada.
Mohammad Anwar Hossain is a Senior Consultant & Head of the Physiotherapy Department at the Centre for the Rehabilitation of the Paralysed (CRP), Dhaka, Bangladesh. He is also a faculty member and Associate Professor of Physiotherapy at the Bangladesh Health Professions Institute (BHPI). Dr. Hossain has expertise in Musculoskeletal Physiotherapy and nearly has 20 years of experience in the field. Professor Dr. Anwar completed his bachelor's and master’s degree in Physiotherapy from the University of Dhaka and his Ph.D. from Jashore University of Science and Technology.
Foisal Alom is a physiotherapist and academic working in the field of musculoskeletal medicine. He completed his bachelor's degree in Physiotherapy from the University of Dhaka, and a master’s degree in Public health from BSM Medical University. He has more than 6 year’s clinical experience. He is the faculty of the national specialized institute for orthopedics, and traumatology rehabilitation in Bangladesh.
Md. Feroz Kabir is an academic, clinician, and researcher. He is the founder of the Department of Physiotherapy and Rehabilitation at Jashore University of Science & Technology. He completed his graduation and post-graduation degree in Physiotherapy from the University of Dhaka. He is a Ph.D. fellow at the University of Malaysia Sabah. He is the awardee of the World Physiotherapy for promoting Physiotherapy education in lower-middle-income countries. He is also the Academic editor of PLOS One. As a clinician, Dr. Kabir is a consultant physiotherapist at BRB Hospitals, one of the largest corporate hospitals in Dhaka.
Lori Maria Walton is a professor of neuroscience in the Department of Physical Therapy at The University of Scranton in the USA. Dr. Walton completed a Ph.D. from Nova Southeastern University and a DPT from Creighton University. Dr. Walton was inducted into the International Research Scholars in 2011-present. Dr. Walton’s research is focused on community-based physical therapy programs for vulnerable populations, with a special emphasis on migrant & refugee health.
Veena Raigangar is a Senior Lecturer at the School of Sport and health sciences at the University of Brighton. She has a double Master’s in Physiotherapy and Education and is currently pursuing her Ph.D. in the UK. She is part of the Metabolic Syndrome and Related Disorders Research group at the University of Sharjah, with several high-impact peer-reviewed papers related to physical activity, biomarkers, and stress. She is also a part of funded ongoing research projects in the field of physical activity and pregnancy with the research group and is working on some publications in this area. Dr. Veena worked at the University of Sharjah for nearly 15 years.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Thomas MJ, Whittle R, Menz HB, et al. Plantar heel pain in middle-aged and older adults: population prevalence, associations with health status and lifestyle factors, and frequency of healthcare use. BMC Musculoskelet Disord. 2019. Dec;20(1):337. doi: 10.1186/s12891-019-2718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sullivan J, Burns J, Adams R, et al. Musculoskeletal and activity-related factors associated with plantar heel pain. Foot Ankle Int. 2015. Jan;36(1):37–45. doi: 10.1177/1071100714551021. [DOI] [PubMed] [Google Scholar]
- [3].Martin RL, Davenport TE, Reischl SF, et al. Heel pain—plantar fasciitis: revision 2014. J Orthop Sports Phys Ther. 2014. Nov;44(11):A1–33. doi: 10.2519/jospt.2014.0303. [DOI] [PubMed] [Google Scholar]
- [4].Beeson P. Plantar fasciopathy: revisiting the risk factors. Foot Ankle Surg. 2014 Sep 1;20(3):160–165. DOI: 10.1016/j.fas.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [5].Chandler TJ, Kibler WB. A biomechanical approach to the prevention, treatment and rehabilitation of plantar fasciitis. Sports Med. 1993. May;15(5):344–352. doi: 10.2165/00007256-199315050-00006. [DOI] [PubMed] [Google Scholar]
- [6].Kwong PK, Kay D, Voner RT, et al. Plantar fasciitis. Mechanics and pathomechanics of treatment. Clin Sports Med. 1988 Jan 1;7(1):119–126. [PubMed] [Google Scholar]
- [7].Whiting WC, Zernicke RF. Biomechanics of Musculoskeletal Injury. Champaign, IL: Human Kinetics; 1998. pp. 172–173. [Google Scholar]
- [8].Donatelli RA. Abnormal biomechanics. In Donatelli RA (editor) Biomechanics of the Foot and Ankle 2nd. Philadelphia, PA: FA Davis. 1996. pp. 34–72. [Google Scholar]
- [9].Backstrom KM, Moore A. Plantar fasciitis. Phys Ther Case Rep. 2000;3:154–162. [Google Scholar]
- [10].Cornwall MW, McPoil TG. Plantar fasciitis: etiology and treatment. J Orthop Sports Phys Ther. 1999;29(12):756–760. [DOI] [PubMed] [Google Scholar]
- [11].Cornwall MW. Common pathomechanics of the foot. Athl Ther Today. 2000;5(1):10–16. [Google Scholar]
- [12].Karr SD. Subcalcaneal heel pain. Orthop Clin North Am. 1994;25(1):161–175. [PubMed] [Google Scholar]
- [13].Hunter LJ, Fortune J. Foot and ankle biomechanics. S Afr J Physiother. 2000;56(1):17–20. [Google Scholar]
- [14].Bolgla LA, Malone TR. Plantar fasciitis and the windlass mechanism: a biomechanical link to clinical practice. J Athl Train. 2004. Jan;39(1):77. [PMC free article] [PubMed] [Google Scholar]
- [15].Fuller EA. The windlass mechanism of the foot: a mechanical model to explain pathology. J Am Podiatr Med Assoc. 2000;90(1):35–46. [DOI] [PubMed] [Google Scholar]
- [16].Sarrafian SK. Functional characteristics of the foot and plantar aponeurosis under tibiotalar loading. Foot Ankle. 1987;8(1):4–18. [DOI] [PubMed] [Google Scholar]
- [17].Fraser JJ, Glaviano NR, Hertel J. Utilization of physical therapy intervention among patients with plantar fasciitis in the United States. J Orthop Sports Phys Ther. 2017. Feb;47(2):49–55. [DOI] [PubMed] [Google Scholar]
- [18].Orchard J. Plantar fasciitis. BMJ. 2012;345(oct10 1):e6603. [DOI] [PubMed] [Google Scholar]
- [19].DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006 Aug 1;88(8):1775–1781. [DOI] [PubMed] [Google Scholar]
- [20].DiGiovanni BF, Nawoczenski DA, Lintal ME, et al. Tissue-specific plantar fascia-stretching exercise enhances outcomes in patients with chronic heel pain: a prospective, randomized study. J Bone Joint Surg Am. 2003 Jul 1;85(7):1270–1277. [DOI] [PubMed] [Google Scholar]
- [21].Lowe WW. Orthopedic Massage E-Book: theory and Technique. In: Elsevier Health Science. 2nd ed. 8 Apr, p. 83–86. [Google Scholar]
- [22].Ajimsha MS, Binsu D, Chithra S. Effectiveness of myofascial release in the management of plantar heel pain: a randomized controlled trial. Foot. 2014 Jun 1;24(2):66–71. [DOI] [PubMed] [Google Scholar]
- [23].Ajimsha MS, Al-Mudahka NR, Al-Madzhar JA. Effectiveness of myofascial release: systematic review of randomized controlled trials. J Bodyw Mov Ther. 2015 Jan 1;19(1):102–112. [DOI] [PubMed] [Google Scholar]
- [24].Shah S, Bhalara A. Myofascial release. Inter J Health Sci Res. 2012. May;2(2):69–77. [Google Scholar]
- [25].Cornwall MW. Common pathomechanics of the foot. Athletic Therapy Today. 2000. Jan;5(1):10–16. [Google Scholar]
- [26].Fuller EA. The windlass mechanism of the foot. A mechanical model to explain pathology. J Am Podiatr Med Assoc. 2000. Jan;90(1):35–46. [DOI] [PubMed] [Google Scholar]
- [27].Coppieters MW, Alshami AM, Babri AS, et al. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006;24(9):1883–1889. [DOI] [PubMed] [Google Scholar]
- [28].ICD 10 Data . 2020. Retrieved from https://www.icd10data.com/ICD10CM/Codes/M00-M99/M70-M79/M72-/M72.2 retrieved on 23/03/2023
- [29].Chow S-C, Shao J, Wang H, et al. Sample size calculations in clinical research. Third ed. 2017. DOI: 10.1201/9781315183084 [DOI] [Google Scholar]
- [30].Budiman-Mak E, Conrad KJ, Roach KE. The foot function index: a measure of Foot Pain and disability. J Clinical Epidemiol. 1991;44(6):561–570. [DOI] [PubMed] [Google Scholar]
- [31].Budiman-Mak E, Conrad KJ, Mazza J, et al. A review of the foot function index and the foot function index – revised. J Foot Ankle Res. 2013;6(1). DOI: 10.1186/1757-1146-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bennett PJ, Patterson C, Wearing S, et al. Development and validation of a questionnaire designed to measure foot-health status. J Am Podiatr Med Assoc. 1998;88(9):419–428. [DOI] [PubMed] [Google Scholar]
- [33].Martin RRL, McPoil TG. Reliability of ankle goniometric measurements. J Am Podiatr Med Assoc. 2005;95(6):564–572. [DOI] [PubMed] [Google Scholar]
- [34].Hale SA, Hertel J. Reliability and Sensitivity of the Foot and Ankle Disability Index in Subjects with Chronic Ankle Instability. J Athl Train. 2005;40(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- [35].Cuthbert SC, GJ G Jr.. On the reliability and validity of manual muscle testing: a literature review.Chiropr Osteopat. 2007. [Published 2007 Mar 6];15(1):4. DOI: 10.1186/1746-1340-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garten H. The Muscle Test Handbook. London, UK: Churchill Livingstone; 2012. p. 123. [Google Scholar]


