Abstract
Orchestration of protein production and degradation and the regulation of protein lifetimes play a central role in many basic biological processes. Nearly all mammalian proteins are replenished by protein turnover in waves of synthesis and degradation. Protein lifetimes in vivo are typically measured in days, but a small number of extremely long-lived proteins (ELLPs) persist for months or even years. ELLPs are rare in all tissues but are enriched in tissues containing terminally differentiated post-mitotic cells and extracellular matrix. Consistently, emerging evidence suggests that the cochlea may be particularly enriched in ELLPs. Damage to ELLPs in specialized cell types, such as crystallin in the lens cells of the eye, causes organ failure such as cataracts. Similarly, damage to cochlear ELLPs is likely to occur with many insults, including acoustic overstimulation, drugs, anoxia, and antibiotics, and may play an underappreciated role in hearing loss. Furthermore, hampered protein degradation may contribute to acquired hearing loss. In this review, I highlight our knowledge of the lifetimes of cochlear proteins with an emphasis on ELLPs and the potential contribution that impaired cochlear protein degradation has on acquired hearing loss and the emerging relevance of ELLPs.
Keywords: long-lived proteins, proteostasis, protein degradation, proteasome, ubiquitin, autophagy, lysosome, noise induced hearing loss, post-mitotic cells
INTRODUCTION
The cochlea is a three-partition spiral-shaped cavity within the bony labyrinth. The middle partition (i.e., cochlear duct) is filled with endolymph while the outer two chambers (i.e., vestibular and tympanic ducts) contain perilymph. The cochlear duct is bordered by Reissner’s membrane (RM), the basilar membrane (BM) containing the sensory organ of Corti (OC), and the spiral ligament/stria vascularis. RM separates scala media from scala vestibuli and the BM separates scala media from scala tympani. The OC which sits on the BM is bordered by two zones of non-sensory cells, namely the inner sulcus (IS) and outer sulcus (OS). Formation of the cochlea during development requires a complex series of signaling events, outgrowth, cellular differentiation, and terminal mitosis across its three major axes. The human cochlea is fully established by 30 weeks of gestation and within 16–20 postnatal days in rodents (Pujol & Hilding 1973).
The complex and intricate structural arrangement of membranes and specialized cells within the cochlea makes it highly susceptible to dysfunction caused by physical damage from intense noise, drugs, anoxia, and antibiotics (Liberman 1990, Pujol et al 1990, Slepecky 1986, Wagner & Shin 2019). As a consequence of leaving the cell-cycle, many cochlear cells cannot dilute misfolded or damaged proteins through cell division as is the case in other organs and tissues (Toyama & Hetzer 2013). Thus, hampered protein turnover may contribute to cellular dysfunction and death of cochlear cells which could contribute to acquired hearing loss. Consistent with this line of reasoning, noise exposures that cause hearing loss in mice were shown to unbalance the proteome by driving the accumulation of hundreds of proteins in the cochlea. Notably, further analysis of the same cochlear extracts revealed that nearly the entire protein chaperone network and a large portion of the cellular protein degradation machinery had elevated gene expression (Jongkamonwiwat et al 2020). Our understanding of cochlear ELLPs and the emerging importance of robust regulation of cochlear protein lifetime by degradation is the focus of this review and may represent an underappreciated aspect of acquired hearing loss.
1. Long-lived proteins are enriched in post mitotic cells and extracellular structures.
Most cellular proteins are turned over by alternating cycles of gene-expression, protein synthesis, and degradation (Figure 1A). Until recently, ELLPs were primarily identified in small-scale studies using radio-isotope pulse-chase labeling (Toyama & Hetzer 2013). Additionally, amino acid racemization has also been used to track the lifetime of a few specific proteins. This process is based on the fact that in biological systems, amino acids are synthesized as L-isomers and spontaneous racemization very slowly converts L-form into a racemic mixture of L- and D-forms (Bada 1984, Sivan et al 2006), While somewhat limited in scope, these candidate-based small-scale studies provide the historical foundation for our understanding of ELLPs. These exceptionally stable proteins include collagen, elastin, and crystallins that persist for years or even the entire lifetime of the individual (Helfman & Bada 1975, Liu et al 2019, Shapiro et al 1991). Notably, ELLPs are frequently expressed in extracellular spaces and specialized long-lived cells such as those in the eye lens, brain, and heart (Hejtmancik & Shiels 2015, Yerbury et al 2005). These ELLPs accumulate physical damage and demonstrate age-related functional decline as they persist without replacement for months or years (Braverman & Fonferko 1982, Cantrell et al 2023, D’Angelo et al 2009).
Figure 1.
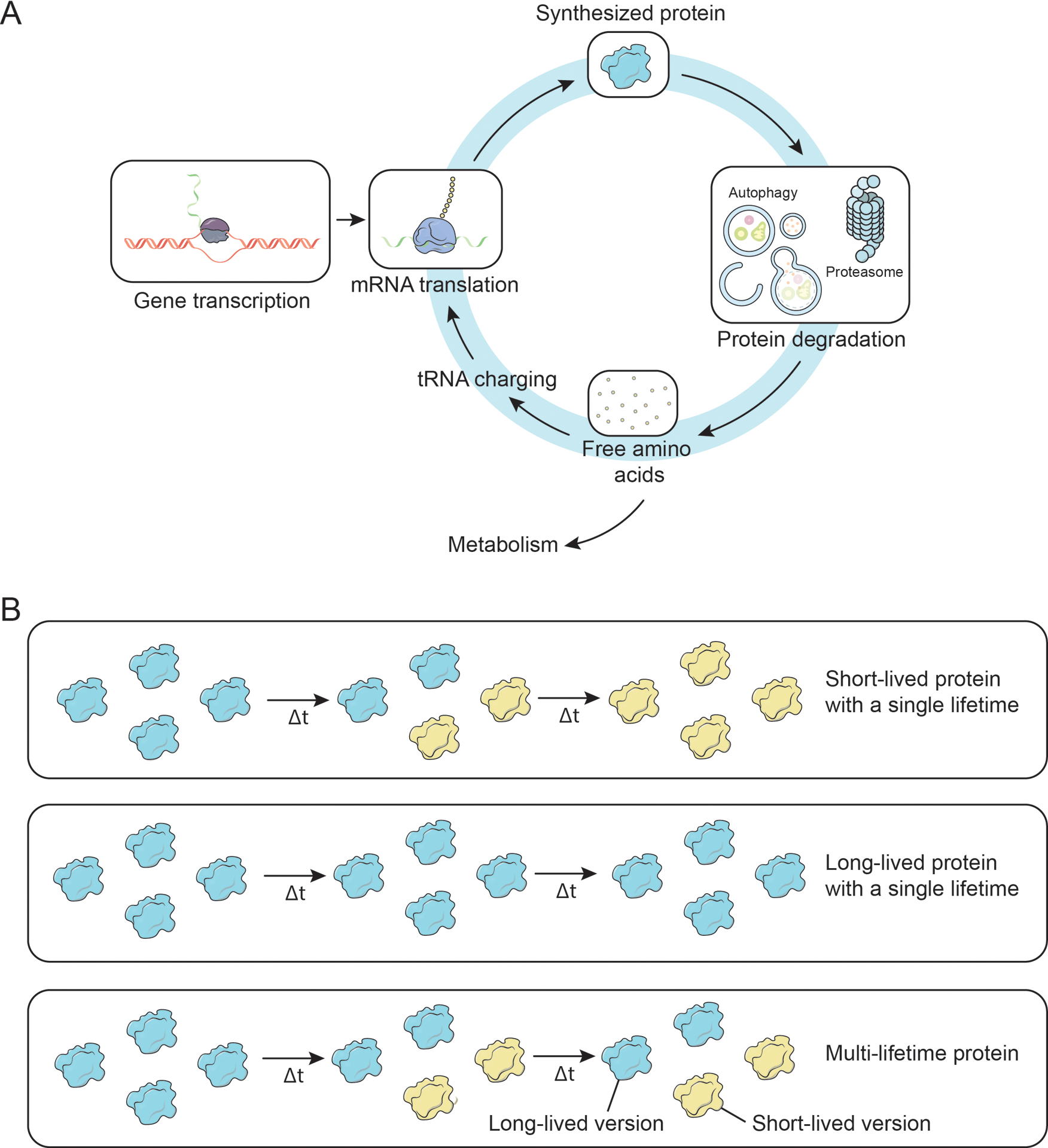
Cartoon depicting canonical gene expression, protein synthesis, protein degradation, and protein lifetimes patterns. (A) Summary of gene expression and protein life cycle. (B) Schematic illustrating short- and long-lived proteins. Cartoons depicting: (Top) a short-lived single lifetime protein, note that all protein molecules in the pool are replaced; (Middle) a long-lived single lifetime protein; (Bottom) a multi-lifetime protein.
Recently, we performed several large-scale global analyses aimed to identify ELLPs using stable-isotope pulse-chase metabolic labeling with liquid chromatography tandem mass spectrometry (LC-MS/MS) based proteomic analysis (Bomba-Warczak et al 2021, Fornasiero & Savas 2023, Hark & Savas 2021). In these studies, we confirmed the presence of extracellular ELLPs and identified a panel of additional intracellular ELLPs that localize to large structures including the core of the nuclear pore, mitochondrial cristae, and chromatin. Notably, almost all of these ELLPs, were enriched in organs containing long-lived and terminally differentiated cells (e.g. brain and heart). This is in contrast to results from organs that are continuously renewed by cell division (e.g. liver, blood, spleen, pancreas or lung), which do not harbor LLPs (Bomba-Warczak et al 2021, Savas et al 2012, Toyama et al 2013). However, long-lived histones are the exception and have been detected in almost all tissues. It is important to point out that some ELLPs exist as a single pool where 100% of the molecules are maintained together (Figure 1B). However, in many other cases only a subset of an individual protein’s pool persists for months. Recently it has been proposed that compromised physical interaction and functional harmony between new and old proteins may contribute to age-dependent functional decline (Arrojo et al 2019).
2. Perturbation of long-lived cochlear structures is associated with acquired hearing loss.
The cochlea contains three large membranes (i.e., RM, BM, and the tectorial membrane (TM)) that are formed during development and persist for the entire lifetime of the organism (Figure 2). RM is built from two layers of squamous epithelial cells separated by the basal lamina. It primarily functions to help transmit vibrations in the fluid and acts as a diffusion barrier that facilitates selective delivery of nutrients from the perilymph to the endolymph. Rupture of RM has been associated with sudden hearing loss, exposure to loud sound, and Ménière’s disease (Gussen 1981, Lawrence 1983). At least partial regeneration of all these membranes can occur; however, severe physical damage to the TM can be permanent, at least in birds, and represents the physical deterioration of a cochlear long-lived structure (Adler 1996, Cotanche 1987).
Figure 2.
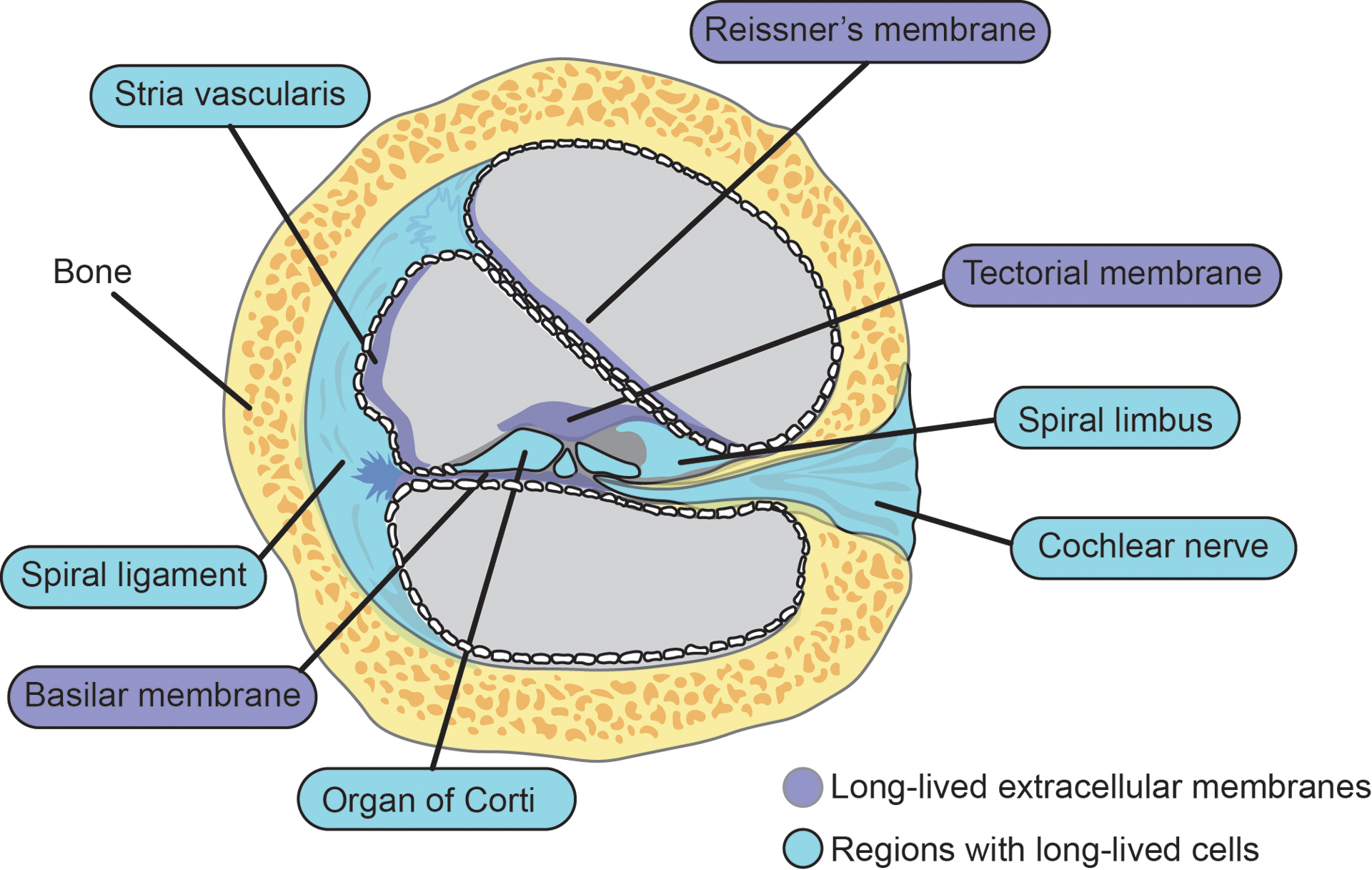
Cartoon depiction of cochlear long-lived structures and regions with long-lived cells.
The TM is composed of extracellular matrix attached to the spiral limbus, which extends across and above the internal spiral sulcus and lies just above the apical surface of the sensory epithelial (Goodyear & Richardson 2002, Goodyear & Richardson 2018). At least in guinea pigs, inner (IHC) and outer hair cell (OHC) stereocilia are embedded into the TM through filamentous Ca2+ ducts (Hakizimana & Fridberger 2021). The mammalian TM develops as two components: the major TM initially forms over the greater epithelial ridge starting at about E14.5 and the minor TM which is produced over the OHCs starts forming around P3 in mice (Goodyear & Richardson 2018). The TM is primarily composed of a small panel of proteins, namely four collagens (II, V, IX, and XI), alpha- and beta-tectorin (i.e., TECTA and TECTB), and carcinoma and embryonic antigen-related cell adhesion molecule 16 (CEACAM16), among others (Andrade et al 2016, Cohen-Salmon et al 1997, Yariz et al 2012, Zheng et al 2011). Recent unpublished results from our lab have revealed that alpha- and beta-tectorin are selectively expressed during development and are maintained as a homogenous pool of ELLPs which persist for the entire lifetime of a mouse. Interestingly, several other TM proteins, including numerous collagen proteins and CEACAM16, were present in both new and old versions, suggesting that they are either turned over slowly or expressed throughout life.
The BM, a long-lived pseudo-resonant structure that separates the scala media and scala tympani, is the base for both the sensory and supporting cells and facilitates sound frequency dispersion (Bor & Wu 1996, Robles & Ruggero 2001). The human BM consists of four independent layers: (1) epithelial basement membrane composed of laminin and collagen IV, (2) BM “proper” made of collagen II and XI, (3) a collagen IV layer, and (4) tympanic cover of integrins, fibronectin, and collagen IV (Liu et al 2015). Given that the protein degradation machinery is lowly expressed or even absent in extracellular spaces, ECM proteins generally have long lifetimes in the brain (Fornasiero et al 2018, Fornasiero & Savas 2022). It is likely that these structural proteins along with other yet to be identified proteins within the cochlea are also ELLPs. While the BM is a robust structure, exposure to intense sound of 130 dB SPL or more can cause it to tear (Rauchegger & Spoendlin 1981). It is also important to emphasize that misfolded and aggregated proteins are poor substrates for degradation in the brain (Hark et al 2021). Due to this, damage to extracellular cochlear proteins is likely to cause them to aggregate and extend their lifetimes.
3. Long-lived post mitotic cells in the OC.
The mammalian cochlea contains a diverse set of cell types, many of which are terminally differentiated and persist for the lifetime of the organism (Figure 2). These long-lived cochlear cells play a critical role in hearing and must maintain cellular homeostasis in the face of frequent insults throughout the life of the organism. Because these cells cannot dilute damaged proteins through cell division, long-lived cochlear cells are particularly vulnerable to proteotoxic stress. Most importantly, in the mammalian OC, the two classes of sensory cells (i.e., IHCs and OHCs), exit the cell cycle during development, persist for the entire lifetime of mammals, and do not regenerate (Groves 2010). Many insults (e.g., drugs, viral infection, acoustic over-stimulation) can cause HC death resulting in permanent hearing loss. Similarly, spiral ganglion neurons (SGNs) are also post mitotic long-lived cells that are essential for hearing. While HCs carry out the mechano-electrical transduction and auditory sensing, SGNs must receive and integrate those signals and propagate them long distances towards the brain.
The relatively large size of SGNs poses an additional challenge for regulating protein turnover, since protein transport is required for the delivery of newly synthesized proteins from the soma to dendritic and axonal outposts. Consistently, protein trafficking is required for delivering old proteins from these distal locations back to the soma for degradation. HCs and SGNs are also increasingly likely to accumulate misfolded or otherwise unwanted damaged proteins due to their high metabolic demands, which produce protein-damaging free radicals and reactive oxygen species (ROS) (Fetoni et al 2013, Wu et al 2020a).
The cochlear supporting cells include Hensen’s, Dieters, pillars, and Claudius cells that are also generally long-lived. These cells are less metabolically active than HCs and SGNs but provide ionic support and structural reinforcement across the OC through tight junctions (Monzack & Cunningham 2013). These junctions are linked to a complex cytoskeletal network composed of microtubules and microfilaments, but these cells have relatively few organelles (Li–dong et al 2008). For example, available data indicates that Deiters cells only display prominent rough endoplasmic reticulum (ER), mitochondria, and lysosomes. Additionally, Hensen’s cells have a thin cytoplasm, underdeveloped ER, phagosomes, and only a few mitochondria (Merchan et al 1980). Emerging findings from our laboratory have revealed that many cell junction protein complexes and their cytoskeletal elements are built for long term stability and persist for long periods (Bomba-Warczak et al 2021). Interestingly, a striking feature of eye lens cells is their abrupt degradation of cytoplasmic organelles, including the protein clearance machinery, which likely explains why crystallin is so long-lived (Bassnett 2002). Perhaps, cochlear supporting cells may also harbor ELLPs due to low level expression of the protein degradation machinery.
4. Long-lived cochlear cells beyond the OC.
The cochlea harbors additional long-lived cells outside the OC that play important roles in providing structural support and regulating the concentration of ions across several chambers. One example is the type I spiral ligament fibrocytes (SLFs), which are the major cell type comprising the lateral wall. These cells produce high levels of extracellular matrix proteins such as Cochlin during development but also throughout life. Type I SLFs do not appear to have a high degree of regeneration after noise exposure at 94 dB SPL; however, type II SLFs have robust regenerative potential (Hirose & Liberman 2003, Peeleman et al 2020). The stria vascularis forms the outer wall of the cochlear duct, maintains the ion composition of the endolymph, and establishes the endocochlear potential for the scala media. Several distinct cell types compose the stria including basal cells, intermediate cells, and marginal cells, in addition to endothelial cells, melanocytes, and pericytes (Liu et al 2016). Basal cells in the stria are interconnected and physically linked to fibrocytes and intermediate cells through gap junctions that may also be long-lived (Forge et al 2013, Kikuchi et al 1995). Moreover, while individuals suffering from presbycusis frequently suffer from IHC damage, they also have structurally abnormal stria (Wu et al 2020b). It’s also worth noting that at least some strial cells can regenerate ex vivo (Wang et al 2019). The spiral limbus plays an important structural role as well and harbors a pool of long-lived cells including the interdental cells that anchor the tectorial membrane.
In our previous studies in the brain, we identified a panel of ELLPs expressed by oligodendrocytes, including myelin basic protein, proteolipid protein, and myelin oligodendrocyte protein (Toyama et al 2013). This finding suggests that at least some oligodendrocytes, or at least their proteins, persist in the CNS for months. Similarly, some proteins produced by Schwann cells, such as myelin basic protein (MBP), proteolipid protein (PLP or lipophilin), and myelin oligodendrocyte glycoprotein (MOG), are also likely to be long-lived in the cochlea. Additional classes of post-proliferative cell types in the cochlea are associated with bone and include osteocytes and the osteocytic lining cells present on some bone matrix (Chole & Tinling 1994). Osteocytes produce important signaling proteins such as insulin-like growth factor 1, which plays an important role in neuroprotection and sensorineural hearing loss (Qin et al 2020, Rodriguez-de la Rosa et al 2017).
5. The role of impaired protein lifespans due to altered protein degradation in acquired hearing loss.
The importance of precisely regulating cochlear protein lifetimes through degradation mechanisms is supported by numerous previous studies of acquired hearing loss. Below I summarize the available evidence supporting this fact and discuss common and emerging themes in the context of the major protein degradation pathways.
5.1. Ubiquitin proteasome system
The ubiquitin proteasome system (UPS) is the main proteolytic protein degradation system within cells and plays a key role in regulating a wide range of cellular pathways and processes. The UPS contains a panel of specialized enzymes (i.e. E1, E2, and E3 ligases) that post-translationally modify protein substrates with a small protein called ubiquitin, which generally targets them to the 26S proteasome where they are proteolytically cleaved into amino acids. Multiple arms of the UPS are involved with acquired hearing loss, and the currently available data has suggested a particularly important role for E3 ubiquitin ligases. The Altschuler lab made some of the initial pioneering discoveries linking impaired protein degradation to NIHL. More than 20 years ago, they discovered that expression of the E3 ubiquitin ligase UBE3B gene is selectively elevated in basilar papilla from chicks exposed to 118 dB SPL for 6 h both acutely and during the recovery period (Lomax et al 2001). This provides important evidence that exposure to loud noise selectively activates the protein degradation machinery, presumably to remove damaged or unnecessary cochlear proteins. UBE3B is a regulated by calmodulin and may be involved with maintaining the mitochondrial reserve (i.e., mitostasis) by marking damaged proteins for degradation through the 26S proteasome (Braganza et al 2017). Furthermore, UBE3B is selectively expressed by SGNs in human cochlea thus linking this E3 to one cell class of long-lived cochlear cells. UBE3B was also identified in a genome-wide association study (GWAS) for hearing difficulty (Liu et al 2021). In flies, a forward genetic screen identified Ubr3, another E3 ligase, as being essential for proper auditory organ development (Li et al 2016). Ubr3 negatively regulates the mono-ubiquitination of non-muscle Myosin IIa, by ubiquitinating the E3 ubiquitin ligase Cul1 (SCF) and targeting it for degradation. Interestingly, many patients with dominant mutations in MYH9, which encodes the Myosin IIa protein, have sensorineural deafness (Lalwani et al 2000). Additionally, Fbx2 and Skp1 subunits of the multi-protein ubiquitin protein ligase complex SCF are highly expressed in the cochlea and Fbx2 knock out (KO) mice develop presbycusis beginning as young as 2 months of age (Nelson et al 2007). Finally, mice lacking RNF8 expression, a E3 ligase that plays an important role in DNA damage signaling, have accelerated cochlear aging (Li et al 2019). Taken all together these studies highlight the importance of E3 ligases in the cochlea.
In several other studies, a bulk increase in ubiquitinated cochlear proteins after exposure to noise causing hearing loss or during aging has been observed (Jongkamonwiwat et al 2020, Wang et al 2015). While the precise E3 ubiquitin ligase was not identified in these studies, they do however provide important evidence that the cochlear UPS is activated in acquired hearing loss. Several discoveries have been made that also support an important role for cochlear deubiquitinating enzymes (DUBs). These findings highlight that extending protein lifetimes by suppressing UPS mediated degradation is also important for cochlear function. For example, USP31 gene variants in Border Collies are associated with adult-onset deafness (Yokoyama et al 2012). Furthermore, support comes from another DUB, Usp53, which localizes to adherens junctions surrounding OHCs. Usp53 mutant mice are highly susceptible to high frequency noise injury that is likely to due to weakened adherens junctions (Kazmierczak et al 2015).
Recent observations in patients treated with bortezomib provide additional support for an association between UPS and hearing loss. Bortezomib is a small molecule that selectively inhibits the 26S proteasome, is thought to extend the lifespan of a variety of cochlear proteins. Bortezomib is a common chemotherapy drug used to treat multiple myeloma and mantle cell lymphoma, but often causes painful peripheral neuropathy as a side effect. In a few patients, it also caused severe irreversible bilateral deafness (Anoop et al 2016, Engelhardt et al 2005). Consistent with this, in vivo and ex vivo mouse experiments have shown that proteasome inhibitors disrupt hair cell stereocilia bundles, cause lipid droplet accumulation, and can induce hair cell death by impairing peroxisomes (Lee et al 2015). On the other hand, the proteasome inhibitor Z-LLF-CHO can protect zebrafish hair cells from acute but not from chronic gentamicin or neomycin treatment (Coffin et al 2013), suggesting that rodents and fish have different tolerance to proteasome inhibition.
5.2. Autophagy
Autophagy is an evolutionarily conserved cellular process responsible for the degradation and recycling of many long-lived proteins and damaged organelles to maintain energy homeostasis (Esselens et al 2004, Goldstein et al 2019, Rubinsztein et al 2005). Double-membraned autophagosomes expressing LC3-II form around these substrates and mature into autolysosomes. A growing body of evidence suggests that autophagic protein degradation plays an important but complex role in basic cochlear function and acquired hearing loss. For example, the autophagy related genes Becn1, Atg4g, Atg5, and Atg9, are expressed in the inner ear during development and throughout life (Magarinos et al 2017). Second, Atg5 is essential for HC survival and required for hearing in mice (Fujimoto et al 2017). Third, several studies have also found an association but not causative link between reduced autophagy during aging and protein turnover that may render long-lived cells more prone to cell death. For instance, reduced mitophagy has been observed in old aged C57BL/6J mice (Oh et al 2020), and LC3 expression in SGNs declines after twelve months of age (de Iriarte Rodriguez et al 2015). In terms of a mechanism, one group even found that expression of the microRNA, miR-34a, is elevated during aging and may reduce autophagy by down regulating Atg9A expression (Pang et al 2017).
Several additional associations between NIHL, drug-induced-toxicity, and cochlear autophagy have recently emerged. For example, auditory overstimulation can drive robust autophagic degradation of peroxisomes in HCs through LC3B in a pejvakin (Pjvk) dependent manner (Defourny et al 2019). Interesting, noise exposures causing a temporary threshold shift (TTS) induces autophagy and promote HC viability. However, damaging noise exposures causing a permanent threshold shift (PTS) overwhelm cochlear autophagic flux and contribute to NIHL through HC death (Yuan et al 2015). Taken all together, these findings suggest that noise exposures causing hearing loss activate autophagy in a complex manner. Several research groups have also reported that increased autophagy correlates with aminoglycoside-induced cell death, and it may require the ubiquitin carboxyl-terminal hydrolase isozyme L1 (Uchl1) (He et al 2017, Kim et al 2019, Kim et al 2017). However, conflicting results from another study suggest that mitophagic-independent mechanisms drive aminoglycoside ototoxicity (Setz et al 2018). Furthermore, kanamycin and furosemide have also been found to selectively impair autophagy in SGNs and play a key role in triggering SGN degeneration (Ye et al 2019). Somewhat paradoxically, the chemotherapeutic drug cisplatin has been found to promote autophagy in HCs (Xu et al 2021). Finally, several groups have found that enhancing autophagy with rapamycin or reducing expression of GSK-3β can minimize cisplatin-induced HC and SGN ototoxicity (Guo et al 2019, Liu et al 2016, Pang et al 2018). Taken all together, the available evidence suggests an association between autophagy and healthy cochlear function, however a causal role in acquired hearing loss remains to be shown and will require additional future research.
6.0. Conclusion
Protein turnover is essential for cellular homeostasis and is achieved through waves of synthesis and degradation. However, we and others have recently used discovery-based proteomics to identify a panel of proteins that are not degraded and persist for months or even the entire lifetime of the organism (Bomba-Warczak & Savas 2022, Savas et al 2012, Toyama et al 2013). While precisely how these proteins contribute to age-associated disease is only just now emerging, ELLPs are highly likely to represent points of vulnerability (Coyne & Rothstein 2022, D’Angelo et al 2009, Hark et al 2021). Seeing that the cochlea is fully constructed within 16–20 postnatal days in rodents, must persist for long time-periods, and contain numerous long-lived cells, membranes, and structures - it represents an organ that is likely to be particularly vulnerable to age-dependent proteotoxic stress in the form of impaired protein degradation (Jongkamonwiwat et al 2020). While yet to be proven experimentally, we anticipate that future studies will soon provide new insight into the importance of cochlear protein turnover and ELLPs in hearing function and acquired hearing loss.
AKNOWLEDGEMENTS:
I would like to thank Miguel Ramirez and Anika Wilen for their comments and suggestions on this review article. This work was supported by grants from the National Institutes of Health (R21AG072343) and Department of Defense (W81XWH-19-1-0627 and W81XWH-22-1-0773) to J.N.S.
ABBREVIATIONS:
- ATG
autophagy-related genes
- BM
basilar membrane
- ELLP
extremely long-lived protein
- IHC
inner hair cell
- IS
inner sulcus
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LC3
microtubule-associated protein 1A/1B-light chain 3
- LL
long-lived
- GWAS
genome-wide association studies
- HSP
heat shock response
- MS
mass spectrometry
- MBP
myelin basic protein
- MOG
myelin-oligodendrocyte glycoprotein
- OC
organ of Corti
- OHC
outer hair cell
- OS
outer sulcus
- PLP
Proteolipid protein (i.e., lipophilin)
- RM
Reissner’s membrane
- PTS
permanent threshold shift
- ROS
reactive oxygen species
- SGN
spiral ganglion neuron
- SLF
spiral ligament fibrocytes
- TM
tectorial membrane
- TTS
temporary threshold shift
- UB
Ubiquitin
- UPS
Ubiquitin proteasome system
Footnotes
DECLARATION OF COMPETING INTEREST:
None.
CrediT AUTHORSHIP CONTRIBUTION STATEMENT:
Jeffrey N. Savas: Conceptualization, writing, review, and editing.
REFERENCES:
- Adler HJ. 1996. Tectorial membrane repair in the quail following multiple exposures to intense sound. Audiol Neurootol 1: 65–79 [DOI] [PubMed] [Google Scholar]
- Andrade LR, Salles FT, Grati M, Manor U, Kachar B. 2016. Tectorins crosslink type II collagen fibrils and connect the tectorial membrane to the spiral limbus. J Struct Biol 194: 139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anoop P, Patil CN, Joshi VS, Maurya P, Hosamani P. 2016. Sensorineural deafness: An uncommon irreversible adverse effect of bortezomib. Indian J Cancer 53: 459. [DOI] [PubMed] [Google Scholar]
- Arrojo EDR, Lev-Ram V, Tyagi S, Ramachandra R, Deerinck T, et al. 2019. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metab 30: 343–51 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada JL. 1984. In vivo racemization in mammalian proteins. Methods Enzymol 106: 98–115 [DOI] [PubMed] [Google Scholar]
- Bassnett S 2002. Lens organelle degradation. Exp Eye Res 74: 1–6 [DOI] [PubMed] [Google Scholar]
- Bomba-Warczak E, Edassery SL, Hark TJ, Savas JN. 2021. Long-lived mitochondrial cristae proteins in mouse heart and brain. J Cell Biol 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomba-Warczak E, Savas JN. 2022. Long-lived mitochondrial proteins and why they exist. Trends Cell Biol 32: 646–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor JC, Wu CY. 1996. Analog electronic cochlea design using multiplexing switched-capacitor circuits. IEEE Trans Neural Netw 7: 155–66 [DOI] [PubMed] [Google Scholar]
- Braganza A, Li J, Zeng X, Yates NA, Dey NB, et al. 2017. UBE3B Is a Calmodulin-regulated, Mitochondrion-associated E3 Ubiquitin Ligase. J Biol Chem 292: 2470–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman IM, Fonferko E. 1982. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol 78: 434–43 [DOI] [PubMed] [Google Scholar]
- Cantrell LS, Gletten RB, Schey KL. 2023. Proteome Remodeling of the Eye Lens at 50 Years Identified With Data-Independent Acquisition. Mol Cell Proteomics 22: 100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chole RA, Tinling SP. 1994. Bone lining cells of the mammalian cochlea. Hear Res 75: 233–43 [DOI] [PubMed] [Google Scholar]
- Coffin AB, Williamson KL, Mamiya A, Raible DW, Rubel EW. 2013. Profiling drug-induced cell death pathways in the zebrafish lateral line. Apoptosis 18: 393–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, El-Amraoui A, Leibovici M, Petit C. 1997. Otogelin: a glycoprotein specific to the acellular membranes of the inner ear. Proc Natl Acad Sci U S A 94: 14450–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA. 1987. Regeneration of the tectorial membrane in the chick cochlea following severe acoustic trauma. Hear Res 30: 197–206 [DOI] [PubMed] [Google Scholar]
- Coyne AN, Rothstein JD. 2022. Nuclear pore complexes - a doorway to neural injury in neurodegeneration. Nat Rev Neurol 18: 348–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, Hetzer MW. 2009. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136: 284–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iriarte Rodriguez R, Pulido S, Rodriguez-de la Rosa L, Magarinos M, Varela-Nieto I. 2015. Age-regulated function of autophagy in the mouse inner ear. Hear Res 330: 39–50 [DOI] [PubMed] [Google Scholar]
- Defourny J, Aghaie A, Perfettini I, Avan P, Delmaghani S, Petit C. 2019. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc Natl Acad Sci U S A 116: 8010–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt M, Muller AM, Maier W, Wasch R. 2005. Severe irreversible bilateral hearing loss after bortezomib (VELCADE) therapy in a multiple myeloma (MM) patient. Leukemia 19: 869–70 [DOI] [PubMed] [Google Scholar]
- Esselens C, Oorschot V, Baert V, Raemaekers T, Spittaels K, et al. 2004. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J Cell Biol 166: 1041–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, et al. 2013. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci 33: 4011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Jagger DJ, Kelly JJ, Taylor RR. 2013. Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J Cell Sci 126: 1703–12 [DOI] [PubMed] [Google Scholar]
- Fornasiero EF, Mandad S, Wildhagen H, Alevra M, Rammner B, et al. 2018. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nature Communications 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Savas JN. 2022. Determining and interpreting protein lifetimes in mammalian tissues. Trends Biochem Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Savas JN. 2023. Determining and interpreting protein lifetimes in mammalian tissues. Trends Biochem Sci 48: 106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto C, Iwasaki S, Urata S, Morishita H, Sakamaki Y, et al. 2017. Autophagy is essential for hearing in mice. Cell Death Dis 8: e2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N, Haim Y, Mattar P, Hadadi-Bechor S, Maixner N, et al. 2019. Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte 8: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. 2002. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J Neurobiol 53: 212–27 [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. 2018. Structure, Function, and Development of the Tectorial Membrane: An Extracellular Matrix Essential for Hearing. Curr Top Dev Biol 130: 217–44 [DOI] [PubMed] [Google Scholar]
- Groves AK. 2010. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 235: 434–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu N, Chen P, Liu Y, Qi X, et al. 2019. Rapamycin Protects Spiral Ganglion Neurons from Gentamicin-Induced Degeneration In Vitro. J Assoc Res Otolaryngol 20: 475–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussen R 1981. Sudden hearing loss associated with cochlear membrane rupture. Two human temporal bone reports. Arch Otolaryngol 107: 598–600 [DOI] [PubMed] [Google Scholar]
- Hakizimana P, Fridberger A. 2021. Inner hair cell stereocilia are embedded in the tectorial membrane. Nat Commun 12: 2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark TJ, Rao NR, Castillon C, Basta T, Smukowski S, et al. 2021. Pulse-Chase Proteomics of the App Knockin Mouse Models of Alzheimer’s Disease Reveals that Synaptic Dysfunction Originates in Presynaptic Terminals. Cell Syst 12: 141–58 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark TJ, Savas JN. 2021. Using stable isotope labeling to advance our understanding of Alzheimer’s disease etiology and pathology. J Neurochem 159: 318–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Guo L, Shu Y, Fang Q, Zhou H, et al. 2017. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 13: 1884–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik JF, Shiels A. 2015. Overview of the Lens. Prog Mol Biol Transl Sci 134: 119–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman PM, Bada JL. 1975. Aspartic acid racemization in tooth enamel from living humans. Proc Natl Acad Sci U S A 72: 2891–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. 2003. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol 4: 339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongkamonwiwat N, Ramirez MA, Edassery S, Wong ACY, Yu J, et al. 2020. Noise Exposures Causing Hearing Loss Generate Proteotoxic Stress and Activate the Proteostasis Network. Cell Rep 33: 108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak M, Harris SL, Kazmierczak P, Shah P, Starovoytov V, et al. 2015. Progressive Hearing Loss in Mice Carrying a Mutation in Usp53. J Neurosci 35: 15582–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC. 1995. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 191: 101–18 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim K, Lee YY, Choo OS, Jang JH, Choung YH. 2019. Downregulated UCHL1 Accelerates Gentamicin-Induced Auditory Cell Death via Autophagy. Mol Neurobiol 56: 7433–47 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Tian C, Kim J, Shin B, Choo OS, et al. 2017. Autophagic flux, a possible mechanism for delayed gentamicin-induced ototoxicity. Sci Rep 7: 41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani AK, Goldstein JA, Kelley MJ, Luxford W, Castelein CM, Mhatre AN. 2000. Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am J Hum Genet 67: 1121–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M 1983. Regeneration of Reissner’s membrane. Acta Otolaryngol 95: 480–5 [DOI] [PubMed] [Google Scholar]
- Lee JN, Kim SG, Lim JY, Kim SJ, Choe SK, Park R. 2015. Proteasome inhibitors induce auditory hair cell death through peroxisome dysfunction. Biochem Biophys Res Commun 456: 269–74 [DOI] [PubMed] [Google Scholar]
- Li–dong Z, Jun L, Yin–yan H, Jian–he S, Shi–ming Y. 2008. Supporting cells–a new area in cochlear physiology study. Journal of Otology 3: 9–17 [Google Scholar]
- Li T, Giagtzoglou N, Eberl DF, Jaiswal SN, Cai T, et al. 2016. The E3 ligase Ubr3 regulates Usher syndrome and MYH9 disorder proteins in the auditory organs of Drosophila and mammals. Elife 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TT, Xu P, Bai ZG, Cui ZT, Cai XG, Zhou B. 2019. Expression of RNF8 on cochlear apoptosis and aging in mice of different ages. J Biol Regul Homeost Agents 33: 543–50 [PubMed] [Google Scholar]
- Liberman MC. 1990. Quantitative assessment of inner ear pathology following ototoxic drugs or acoustic trauma. Toxicol Pathol 18: 138–48 [DOI] [PubMed] [Google Scholar]
- Liu H, Li Y, Chen L, Zhang Q, Pan N, et al. 2016. Organ of Corti and Stria Vascularis: Is there an Interdependence for Survival? PLoS One 11: e0168953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Edassery SL, Ali L, Thomson BR, Savas JN, Jin J. 2019. Long-lived metabolic enzymes in the crystalline lens identified by pulse-labeling of mice and mass spectrometry. Elife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Atturo F, Aldaya R, Santi P, Cureoglu S, et al. 2015. Macromolecular organization and fine structure of the human basilar membrane - RELEVANCE for cochlear implantation. Cell Tissue Res 360: 245–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Johansson A, Rask-Andersen H, Rask-Andersen M. 2021. A combined genome-wide association and molecular study of age-related hearing loss in H. sapiens. BMC Med 19: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax MI, Gong TW, Cho Y, Huang L, Oh SH, et al. 2001. Differential Gene Expression Following Noise Trauma in Birds and Mammals. Noise Health 3: 19–35 [PubMed] [Google Scholar]
- Magarinos M, Pulido S, Aburto MR, de Iriarte Rodriguez R, Varela-Nieto I. 2017. Autophagy in the Vertebrate Inner Ear. Front Cell Dev Biol 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan MA, Merchan JA, Ludeña MD. 1980. Morphology of Hensen’s cells. Journal of anatomy 131: 519. [PMC free article] [PubMed] [Google Scholar]
- Monzack EL, Cunningham LL. 2013. Lead roles for supporting actors: critical functions of inner ear supporting cells. Hear Res 303: 20–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RF, Glenn KA, Zhang Y, Wen H, Knutson T, et al. 2007. Selective cochlear degeneration in mice lacking the F-box protein, Fbx2, a glycoprotein-specific ubiquitin ligase subunit. J Neurosci 27: 5163–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Youn CK, Jun Y, Jo ER, Cho SI. 2020. Reduced mitophagy in the cochlea of aged C57BL/6J mice. Exp Gerontol 137: 110946. [DOI] [PubMed] [Google Scholar]
- Pang J, Xiong H, Lin P, Lai L, Yang H, et al. 2017. Activation of miR-34a impairs autophagic flux and promotes cochlear cell death via repressing ATG9A: implications for age-related hearing loss. Cell Death Dis 8: e3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Xiong H, Zhan T, Cheng G, Jia H, et al. 2018. Sirtuin 1 and Autophagy Attenuate Cisplatin-Induced Hair Cell Death in the Mouse Cochlea and Zebrafish Lateral Line. Front Cell Neurosci 12: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeleman N, Verdoodt D, Ponsaerts P, Van Rompaey V. 2020. On the Role of Fibrocytes and the Extracellular Matrix in the Physiology and Pathophysiology of the Spiral Ligament. Front Neurol 11: 580639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Hilding D. 1973. Anatomy and physiology of the onset of auditory function. Acta Otolaryngol 76: 1–10 [DOI] [PubMed] [Google Scholar]
- Pujol R, Rebillard G, Puel JL, Lenoir M, Eybalin M, Recasens M. 1990. Glutamate neurotoxicity in the cochlea: a possible consequence of ischaemic or anoxic conditions occurring in ageing. Acta Otolaryngol Suppl 476: 32–6 [DOI] [PubMed] [Google Scholar]
- Qin L, Liu W, Cao H, Xiao G. 2020. Molecular mechanosensors in osteocytes. Bone Res 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchegger H, Spoendlin H. 1981. Damage of the basilar membrane by acoustic stimulation. Arch Otorhinolaryngol 232: 117–22 [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. 2001. Mechanics of the mammalian cochlea. Physiol Rev 81: 1305–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-de la Rosa L, Lassaletta L, Calvino M, Murillo-Cuesta S, Varela-Nieto I. 2017. The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front Aging Neurosci 9: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, et al. 2005. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 1: 11–22 [DOI] [PubMed] [Google Scholar]
- Savas JN, Toyama BH, Xu T, Yates JR 3rd, Hetzer MW. 2012. Extremely long-lived nuclear pore proteins in the rat brain. Science 335: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setz C, Benischke AS, Pinho Ferreira Bento AC, Brand Y, Levano S, et al. 2018. Induction of mitophagy in the HEI-OC1 auditory cell line and activation of the Atg12/LC3 pathway in the organ of Corti. Hear Res 361: 52–65 [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. 1991. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87: 1828–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan SS, Tsitron E, Wachtel E, Roughley PJ, Sakkee N, et al. 2006. Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J Biol Chem 281: 13009–14 [DOI] [PubMed] [Google Scholar]
- Slepecky N 1986. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res 22: 307–21 [DOI] [PubMed] [Google Scholar]
- Toyama BH, Hetzer MW. 2013. Protein homeostasis: live long, won’t prosper. Nat Rev Mol Cell Biol 14: 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, et al. 2013. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154: 971–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EL, Shin JB. 2019. Mechanisms of Hair Cell Damage and Repair. Trends Neurosci 42: 414–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sun Y, Chen S, Zhou X, Wu X, et al. 2015. Impaired unfolded protein response in the degeneration of cochlea cells in a mouse model of age-related hearing loss. Exp Gerontol 70: 61–70 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Li G, Sai N, Han J, et al. 2019. Vascular regeneration in adult mouse cochlea stimulated by VEGF-A165 and driven by NG2-derived cells ex vivo. Hear Res 377: 179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Xiong H, Sha S. 2020a. Noise-induced loss of sensory hair cells is mediated by ROS/AMPKalpha pathway. Redox Biol 29: 101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, O’Malley JT, de Gruttola V, Liberman MC. 2020b. Age-Related Hearing Loss Is Dominated by Damage to Inner Ear Sensory Cells, Not the Cellular Battery That Powers Them. J Neurosci 40: 6357–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu FL, Cheng Y, Yan W. 2021. Up-regulation of autophagy and apoptosis of cochlear hair cells in mouse models for deafness. Arch Med Sci 17: 535–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariz KO, Duman D, Zazo Seco C, Dallman J, Huang M, et al. 2012. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am J Hum Genet 91: 872–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Wang Q, Hu H, Shen Y, Fan C, et al. 2019. Restoring autophagic flux attenuates cochlear spiral ganglion neuron degeneration by promoting TFEB nuclear translocation via inhibiting MTOR. Autophagy 15: 998–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerbury JJ, Stewart EM, Wyatt AR, Wilson MR. 2005. Quality control of protein folding in extracellular space. EMBO Rep 6: 1131–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, Lam ET, Ruhe AL, Erdman CA, Robertson KR, et al. 2012. Variation in genes related to cochlear biology is strongly associated with adult-onset deafness in border collies. PLoS Genet 8: e1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Wang X, Hill K, Chen J, Lemasters J, et al. 2015. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal 22: 1308–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Miller KK, Yang T, Hildebrand MS, Shearer AE, et al. 2011. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4). Proc Natl Acad Sci U S A 108: 4218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]


