Abstract
Growing work suggests that interoception, i.e., representations of one’s internal bodily changes, plays a role in shaping emotional experiences. Past studies primarily examine how behavioral accuracy in detecting interoceptive signals (interoceptive ability) relates to emotional states, with less work examining self-reported interoceptive facets such as the characterizations of one’s interoceptive abilities (interoceptive sensibility) or evaluative beliefs about the value vs. danger of interoceptive signals (interoceptive beliefs). However, existing studies rarely examine physiological reactivity, behavioral, and self-reported dimensions of interoception together in the same sample. As such, it remains unclear whether and how much individual differences in interoceptive facets uniquely and in interaction with physiological reactivity may matter for emotional experience. Herein, 250 healthy young adults completed a heartbeat detection task assessing interoceptive ability and questionnaire measures of interoceptive sensibility and beliefs during an initial laboratory visit. At a follow-up session, 227 participants returned to undergo an acute psychosocial stressor. Measures of physiological arousal such as pre-ejection period and heart rate variability were acquired throughout the stressor with self-reported emotions acquired immediately after. Linear regressions revealed that greater sympathetic nervous system reactivity (i.e., pre-ejection period), poorer interoceptive ability (i.e., accuracy), and less positive interoceptive beliefs were related to more intense high arousal emotions during the stressor. Importantly, across models, interoceptive beliefs was the only interoceptive facet to moderate the concordance between physiological and emotional arousal. Implications for psychological theories of emotion, stress, and interoception are discussed.
Keywords: beliefs, emotion, interoception, psychophysiology, acute stress
Recall how your body felt the last time you were stressed. Perhaps your heart was racing, your stomach clenched, or you had sweaty palms. Laypeople commonly report bodily sensations as concomitant with their emotional states (Nummenmaa et al., 2014), while peripheral psychophysiology confirms that visceral changes (e.g., in heart rate, respiration, or blood pressure) often accompany emotions (Siegel et al., 2018). One perennial debate is whether bodily changes can contribute to vs. are merely the product of emotional experiences (Cannon, 1927; James, 1884; MacCormack & Lindquist, 2017; Oosterwijk & Barrett, 2014; Reisenzein, 1983; Schachter & Singer, 1962). Interoception—how the brain and mind represent internal bodily states and signals—is increasingly recognized as a key avenue by which bodily changes can translate to emotion and other aspects of social affective functioning (Arnold et al., 2019; Barrett, 2018; Garfinkel & Critchley, 2013; Khalsa et al., 2018; Quigley et al., 2021; Shah et al., 2017).
Yet relations between interoception and emotion are surprisingly unclear. Some studies measure interoceptive ability, or performance-based behavioral accuracy at accessing interoceptive signals such as the heartbeat (Barrett et al., 2004; Critchley et al., 2004; Herbert et al., 2010; Kindermann & Werner, 2014b). Still others measure interoceptive sensibility—self-reported beliefs, self-characterizations, or confidence in one’s perceived ability to access interoceptive signals (e.g., Forkmann et al., 2016; Garfinkel et al., 2015; Palser et al., 2018). Finally, little studied are evaluative beliefs about the meaning of bodily sensations, such as whether sensations are valuable or dangerous, herein called interoceptive beliefs. Existing evidence suggests that these facets tap different underlying constructs, but few studies have compared their roles side-by-side in relation to emotion.
Studies examining physiology and emotion also rarely account for individual differences in interoception, while inversely, studies on interoception and emotion rarely account for individual differences in physiology, despite good reasons to do so. For instance, the effect sizes linking physiological reactivity and self-reported emotions or stress are often small-to-medium (Campbell & Ehlert, 2012; Feldman et al., 1999; Mauss et al., 2005; Siegel et al., 2018), which could be due in part to unmodeled associations with interoception. Similarly, when measuring interoception, it is critical to consider state or trait differences in physiological intensity, which may alter the threshold for accessing internal sensations (Blascovich et al., 1992; Fairclough & Goodwin, 2007; Herbert et al., 2012; Murphy, Brewer, et al., 2018). Thus, there remains no demonstration comparing whether and which interoceptive constructs moderate the physiology-emotion relationship.
In line with calls for more comprehensive interoceptive assessments (Suksasilp & Garfinkel, 2022), we aimed to provide an integrative resolution to these outstanding questions. A sample of healthy young adults (N=250) completed measures of interoceptive ability, sensibility, and beliefs. At a follow-up session, participants returned (N= 227) to complete an acute psychosocial stressor and assessments of stressor-induced changes in physiological arousal (pre-ejection period, heart rate variability), with emotions self-reported immediately following the stressor. This study allowed us to first clarify the nature of interoceptive constructs in relation to each other, while testing the additive importance and validity of the construct of evaluative interoceptive beliefs, which has largely been ignored by previous literature. Second, we aimed to compare the unique relations of interoceptive constructs with emotional experience, above and beyond shared interrelations between interoceptive constructs and physiology, while testing their relative roles of in moderating the concordance between physiological reactivity (i.e., in autonomic nervous system arousal) and emotional experience (i.e., emotional arousal) during an acute stressor.
Theoretical Frameworks Bridging Physiology to Emotion
Longstanding research characterizes the extent to which peripheral physiological changes are associated with emotion. Psychophysiological measures are diverse, capturing signals across multiple domains, such as the cardiovascular system (e.g., heart rate, heart rate variability, blood pressure), skin and sweat (i.e., skin conductance), the gut (e.g., electrogastrography), and hormonal changes (e.g., cortisol, testosterone, adrenaline). Physiological reactivity refers to functional changes within a physiological measure in response to stimuli (e.g., a difficult interpersonal interaction) relative to a baseline or control period. On average, there are changes in physiological reactivity during emotional states (e.g., heart rate, respiration, blood pressure), and the intensity of physiological reactivity tends to correlate with the intensity of felt emotion (Golland et al., 2014; Mauss & Robinson, 2009). Similarly, experimental manipulation of a given physiological system or state (e.g., hunger, inflammation, sympathetic nervous system-related signaling) impacts the intensity or quality of concurrent emotions (Harrison et al., 2009; MacCormack, Armstrong-Carter, et al., 2021; MacCormack & Lindquist, 2019; Muscatell et al., 2016). Physiological changes are thus associated with and can even contribute to emotional states.
However, the association between physiological reactivity and self-reports (emotion, stress) is small or weak (Campbell & Ehlert, 2012; Feldman et al., 1999; Siegel et al., 2018), suggesting that certain moderators may alter the extent to which physiological dynamics contribute to emotional experiences. For instance, some individuals exhibit greater concordance between their physiology and emotion reports relative to others (Brown et al., 2019; Mauss et al., 2005; Sommerfeldt et al., 2019; Sze et al., 2010; Van Doren et al., 2021). Concordance differences could stem in part from individual differences in the intensity of physiological reactivity to evocative stimuli (Del Giudice et al., 2011; Kupper et al., 2021; Manuck et al., 1989). At the anatomical or physiological level, such variation may stem from structural or functional differences in the central or peripheral nervous systems and their target organs whether due to epigenetics, aging, or health (Ginty et al., 2014; Lovallo et al., 2012; Uchino et al., 2010).
However, individual differences in interoception may also moderate the association between physiological reactivity and emotion reports. Interoception refers to the processes wherein the brain and mind come to represent and predict afferent physiological states and signals (Cameron, 2001; Craig, 2003; Khalsa et al., 2018; Quigley et al., 2021; Tsakiris & De Preester, 2018; Vaitl, 1996). Interoception is an important construct in constructionist models of emotion, such as the theory of constructed emotion (Barrett, 2006, 2017a, 2017b, 2018; Lindquist, 2013), which argue that instances of emotion states emerge from domain-general neural processes that iteratively fuse together ongoing sensory inputs from the body’s internal state, external sensations from the world, and an individual’s accumulated working models (e.g., concepts, beliefs, learning) (Atzil et al., 2018; Barrett & Simmons, 2015; Gendron et al., 2020; Hoemann & Barrett, 2019; Kleckner et al., 2017; Lindquist et al., 2015; Lindquist & Barrett, 2012; MacCormack & Lindquist, 2017, 2019). As such, when individuals exhibit greater concordance between physiological and emotional arousal, a constructionist interpretation is that this is due to a heavier weighting of afferent bodily and interoceptive signals and predictions in emotion construction.
With this constructionist hypothesis in mind, greater concordance between physiological and emotional arousal could arise in part due to within-person state differences, such as times when bodily signals are more robust and/or situationally salient (e.g., an acute stressor, when hungry). For example, Brown et al. (2019) and Van Doren et al. (2021) have argued and found that coherence between physiology and emotion may be stronger under state conditions of more robust physiological reactivity. Thus, interoception may be particularly relevant for helping couple together physiological and emotional states (MacCormack, Henry, et al., 2021; Sze et al., 2010; Van Doren et al., 2021). It follows that variation in the coherence between physiological and emotional arousal could arise in part due to between-person trait differences in interoception.
Feeling the Body: The Role of Individual Differences in Interoception in Emotion
Just as individuals differ in their ability to taste bitterness or sweetness, so too do individuals differ in interoception. To date, many studies have investigated the physiological, neural, and psychological pathways underlying interoception and its implications for emotion, sociality, wellbeing, and health (reviews in Arnold et al., 2019; Dunn et al., 2010; Farb et al., 2015; Fotopoulou & Tsakiris, 2017; Owens et al., 2018; Schulz & Vögele, 2015; Simmons & DeVille, 2017). Interoception is often examined as interoceptive ability or sensibility, but much less commonly, as interoceptive beliefs.
Interoceptive Ability.
Interoceptive ability describes the behavioral ability to accurately discriminate between and track physiological changes, such as one’s heartbeat. Early studies revealed that individuals vary in their ability to perceive such physiological changes (Katkin et al., 1982; Schandry, 1981; Whitehead et al., 1977). Heartbeat tracking or counting tasks (Schandry, 1981) ask individuals to mentally track and count the number of heartbeats they perceive throughout a randomized set of presented time intervals, which are then compared against the actual number of heartbeats measured therein. Heartbeat detection or discrimination tasks (Brener et al., 1993; Brener & Kluvitse, 1988; Kleckner et al., 2015; Whitehead et al., 1977) present individuals with auditory tones that are either synchronous or asynchronous with their actual heartbeats. Signal detection theory is then used to calculate whether individuals are accurately identifying true heartbeats.
Consistent with the notion that interoception may moderate the concordance between physiology and emotion, interoceptive ability often correlates with self-reported emotional intensity across measures and contexts. For instance, some studies reveal that interoceptive ability is associated with more intense momentary emotional states induced by evocative images or film clips (e.g., Dunn, Galton, et al., 2010; Eichler et al., 1987; Ferguson & Katkin, 1996; Hantas et al., 1982; Herbert et al., 2007; Pollatos et al., 2005, 2007; Schandry, 1981; Wiens et al., 2000) or as induced by acute stressors (e.g., Blascovich et al., 1992; Durlik et al., 2014; Fairclough & Goodwin, 2007; Kindermann & Werner, 2014a, 2014b; Schandry, 1983; Werner et al., 2009). But there is also evidence that interoceptive ability is specifically linked to variation in the arousing features of emotion. Arousal describes the degree to which a state is experienced as highly activating vs. quiescent (Barrett & Bliss-Moreau, 2009; Satpute et al., 2019). Importantly, arousal is somewhat independent of valence (how pleasant vs. unpleasant a given state is), and both arousal and valence are considered descriptive features of all conscious states (Barrett & Russell, 1999). Although emotional intensity is not equivalent to emotional arousal (e.g., one can have intensely positive or negative states that are also low or neutral arousal; Kuppens et al., 2013), high arousal emotional states such as feeling angry, stressed, or anxious tend to involve more overt, robust physiological changes (e.g., Lavoie et al., 2001). As a result, interoceptive ability may especially moderate the link between physiology and emotion in the case of high arousal emotional experiences. Barrett et al. (2004) found that performance on a heartbeat detection task predicted more frequent endorsements of emotion categories that varied in arousal (relative to valence) across two months of experience sampling. Studies with the heartbeat counting task provide convergent evidence, such that higher ability was associated with more intense arousal ratings of affective images (Herbert et al., 2010) and higher arousal experiences of anxiety (Dunn, Stefanovitch, et al., 2010). Thus, one hypothesis is that interoception—and perhaps especially interoceptive ability—may be particularly relevant in creating concordance between physiological and emotional arousal (MacCormack, Henry, et al., 2021; Sze et al., 2010; Van Doren et al., 2021).
Interoceptive Sensibility.
Interoceptive sensibility reflects individuals’ beliefs about or self-characterizations of how interoceptively attuned they are (Garfinkel & Critchley, 2013). Interoceptive sensibility is often measured with questionnaires such as the Multidimensional Assessment of Interoceptive Awareness (MAIA; Mehling et al., 2012) or the Body Awareness Questionnaire (BAQ; Shields et al., 1989). The MAIA includes items assessing how much people think they notice and pay attention to their bodily sensations, as well as how much they think these bodily sensations contribute to emotions. The BAQ similarly assesses how much people think they notice and can accurately track different types of physiological changes such as being hungry or fatigued. Both older and recent studies find that sensibility is unrelated to interoceptive ability (Calì et al., 2015; Garfinkel et al., 2016; Horváth et al., 2021; Murphy et al., 2020; Whitehead et al., 1976), perhaps reflecting broader disconnects between performance-based and self-assessment measures in other domains (Dang et al., 2020; Guassi Moreira et al., 2020). On the other hand, sensibility measures people’s self-characterizations of their tendency to notice and direct attention towards interoceptive sensations (Garfinkel & Critchley, 2013; Khalsa et al., 2018; Murphy et al., 2020; Zamariola, Frost, et al., 2019) rather than objective detection of those signals. Thus, sensibility may reflect beliefs about interoceptive attention and accuracy regardless of participants actual engagement of attention or accuracy when trying to detect those signals.1
Greater interoceptive sensibility as measured by the MAIA or BAQ is associated with trait-level measures of anxiety and depression (Ehlers & Breuer, 1992; Hanley et al., 2017; Mehling et al., 2013; Palser et al., 2018). Yet whether sensibility relates to state measures of emotion, such as during an acute stressor, remains underexamined. Recent studies show no relation between sensibility (e.g., the BAQ) and emotion states (Horváth et al., 2021; Lustyk et al., 2012; Zamariola, Luminet, et al., 2019). Despite these null effects, it is possible that the subjective tendency to monitor bodily sensations is important for emotion states because greater attention to interoceptive cues during an emotional event could heighten emotional intensity and/or arousal. Clearly, more evidence in larger samples, while adjusting for shared relations with physiology and other interoceptive measures, is needed.
Interoceptive Beliefs.
Although the BAQ and MAIA may capture beliefs about one’s level of attention and accuracy for interoceptive signals, individuals also likely hold evaluative beliefs about the valenced meaning of interoceptive sensations. For simplicity, we refer to these as interoceptive beliefs. Just as individuals differ in their beliefs about whether emotions are valuable or dangerous (Garrett-Peters et al., 2017), so too may individuals differ in the extent to which they believe interoceptive sensations are valuable or dangerous. Such beliefs should have critical implications for the experience and management of emotion and stress. Although to our knowledge, no studies have explicitly addressed the role of evaluative interoceptive beliefs in emotional experience, findings with related constructs are suggestive.
For example, while the MAIA is largely used as a measure of interoceptive sensibility, its body trusting and not worrying subscales may help capture people’s positive and negative stances towards interoceptive sensations as experiences that one can trust or not worry about. Similarly, some mindfulness practices teach the belief that sensations should be treated with acceptance or can serve as valuable sources of self-insight (Farb et al., 2015; Hanley et al., 2017). On the other hand, people with eating disorders tend to believe that bodily sensations like hunger are dangerous (Lattimore et al., 2017; Merwin et al., 2010) and people high in anxiety sensitivity or distress intolerance tend to catastrophize their bodily sensations (De Cort et al., 2013; Deacon & Abramowitz, 2006; McHugh et al., 2019; Olthuis et al., 2012; Richards & Bertram, 2000). Work on illness and pain beliefs similarly shows that major health events or ongoing health conditions (e.g., cancer, chronic pain) can sometimes lead individuals to develop more negative, threatened, or vigilant mindsets towards their somatic symptoms (e.g., Caneiro et al., 2021; Cunningham et al., 2021; Fisher et al., 2018; Heathcote et al., 2021). In the context of acute stress, experimentally manipulating arousal reappraisals about the adaptive vs. maladaptive value of arousal impacts cortisol reactivity, behavioral performance (e.g., during an exam, interview, game), as well as emotional self-reports (Jacquart et al., 2020; Jamieson et al., 2010, 2012, 2016; Sammy et al., 2017). Similarly, stress mindset theory argues that individual differences in viewing stress as enhancing vs. debilitating helps buffer against vs. exacerbate the physiological, emotional, and cognitive effects of acutely stressful experiences (Crum et al., 2013, 2017; Park et al., 2018).
Herein, we use the term “interoceptive beliefs” to help unify these diverse constructs siloed across contexts and disciplines while also providing a place for interoceptive beliefs in the emerging framework of interoceptive constructs. As such, one secondary goal of the present study is to begin characterizing evaluative interoceptive beliefs (i.e., construct validity) while examining their potential implications (i.e., predictive validity) for research on emotion and related constructs.2
The Present Study
In the present study, we examined the role of interoception in emotional experience and as a moderator between physiology and emotion. We recruited 250 healthy young adults who, at an initial session, completed a heartbeat detection task to assess their interoceptive ability counterbalanced with questionnaires measuring interoceptive sensibility and beliefs. At a second session, 227 participants (90.8%) returned and completed a variant of the Trier Social Stress Test (TSST; Kirschbaum et al., 1993), wherein participants prepared and then gave a speech and performed math in front of neutral evaluators.
To assess physiological arousal, we examined pre-ejection period as an index of cardiac sympathetic nervous system (SNS) reactivity and heart rate variability as an index of cardiac parasympathetic nervous system (PNS) reactivity. The SNS and related nor/adrenergic systems play critical roles in supporting arousal, wakefulness, attention, and the marshalling of visceromotor activity for adaptive responding, including during situations involving threat, stress, and motivated performance (Berridge, 2008; Cacioppo et al., 1994). In contrast to the SNS, which accelerates the cardiac cycle and subsequent cardiac output, the PNS exerts a slowing “regulatory” effect on cardiac function via modulation of vagal nerve outflow to the sinoatrial cells in the heart (Berntson et al., 1993). However, during intensely demanding, challenging, or threatening events, SNS activation is often accompanied by PNS withdrawal via vagal withdrawal. Such PNS withdrawal allows SNS cardiac influences to predominate (Weissman & Mendes, 2021), producing a state of physiological arousal.
Finally, to assess emotional arousal, we focused on self-reported highly aroused emotions (e.g., frustration, stress, anxiety) in response to the TSST. We focused on emotional arousal given prior work linking heartbeat detection performance with the arousal component of emotion (e.g., Barrett et al., 2004). However, in support of future replications and meta-analyses, we report exploratory analyses examining negative, low arousal, and positive emotions in the Supplemental Materials (SMs).
Evaluating the Nature of Interoceptive Constructs After Including Interoceptive Beliefs
This study had two primary goals. First, we aimed to evaluate the interrelations between interoceptive constructs (ability, sensibility, beliefs). We were especially interested in understanding the predictive and discriminant validity of interoceptive beliefs as a new, underexamined construct. As described above, most prior interoceptive literature has focused on measures of interoceptive ability or sensibility, with less explicit focus on the role of value-based beliefs about one’s interoceptive sensations, despite good reasons for why such beliefs may be important for the experience and management of emotions and stress. Thus, to better characterize interrelations between interoceptive constructs, we first conducted exploratory and confirmatory factor analyses and bivariate correlations on our measures of interoceptive ability, sensibility, and beliefs. We predicted that these measures would be distinct, reflecting different interoceptive constructs, in line with prior work (e.g., interoceptive ability and sensibility are distinct; Ferentzi et al., 2018; Forkmann et al., 2016; Garfinkel et al., 2015, 2016).
Testing Interoceptive Constructs as Main Effects and Moderators of Emotion
Our second goal was to establish, through stepwise regressions, the relative main effect sizes for interoceptive constructs in relation to self-reported emotions, while also testing their roles as moderators between physiological reactivity and self-reported emotions during the acute stressor. In so doing, we aimed to establish the additive value (i.e., predictive validity and utility) of evaluative interoceptive beliefs as a new construct, clarifying whether such a construct would matter after accounting for shared variance with interoceptive ability and sensibility. Indeed, past studies tend to only examine one interoceptive construct at a time (e.g., ability) in relation to emotion, despite potential confounds with other interoceptive constructs (e.g., sensibility, beliefs) so the predictive validity of these constructs in the context of emotion remains unclear. Recent methodological work highlights that performance on the heartbeat tracking or counting task may be biased by prior knowledge and beliefs about one’s heartbeat and cannot be a pure behavioral measure of interoceptive accuracy (Desmedt et al., 2018; Murphy, Millgate, et al., 2018; Ring et al., 2015; Zamariola et al., 2018). Thus, measuring and modeling the underlying shared relations between interoceptive constructs alongside physiological reactivity could provide more accurate effect size estimates relating interoceptive constructs with emotion.
Methods
Participants
We recruited a total of 250 healthy young adults from the University of North Carolina at Chapel Hill’s Department of Psychology and Neuroscience psychology participant pool to participate in a first study session (57.6% female, 42.4% male; 57.6% White, 13.6% African American, 13.6% Asian American, 6.4% Latinx, 6.0% biracial, and 2.8% that were other ethnic identities; Mage= 19.20 years, SDage= 1.29 years, 17–29 years old; Table 1). Recruitment goals were a product of both power analyses and expectations that some attrition would occur between Session 1 and Session 2. Effect size estimates were determined based on prior literature. Prior studies that found a significant main effect between heartbeat detection performance and emotional arousal observed small-to-moderate effects (e.g., r= .23 in Barrett et al., 2004; rs= −.30, −.58 in Blascovich et al., 1992). Meta-analyses also reveal small-to-moderate associations (rs~.10−.50) between measures of cardiovascular reactivity to acute stressors and self-reported emotion (Campbell & Ehlert, 2012; Feldman et al., 1999). Power analyses in G*power (Faul et al., 2007) suggested that a sample of N=84 would have 80% power to detect small-to-moderate main effect size (d= .30) and a sample of N=193 would have 80% power to detect a small interaction (d=.20). However, given that we aimed to conduct factor analyses and stepwise linear regressions, we aimed for a higher sample size of N=250. Of the 250 participants who completed the first session, 227 participants (90.8%) returned. All data were collected in accordance with APA ethical standards for human participants, as reviewed and monitored by the university’s Internal Review Board (IRB# 14–3243).
Table 1.
Sample characteristics.
| Demographics | Session 1 (N=250) | Session 2 (N=227) c |
|---|---|---|
| Self-identified sex | ||
| N Female | 144 (57.6%) | 128 (56.4%) |
| N Male | 106 (42.4%) | 99 (43.6%) |
| Self-identified ethnicity | ||
| N African descent | 34 (13.6%) | 33 (14.6%) |
| N Asian descent | 34 (13.6%) | 31 (13.7%) |
| N European descent | 144 (57.6%) | 132 (58.4%) |
| N Latinx descent | 16 (6.4%) | 13 (5.8%) |
| N Bi- or multi-racial | 15 (6.0%) | 14 (6.2%) |
| N Other | 7 (2.8%) | 4 (1.3%) |
| Other demographics | ||
| Mean Age | 19.20 ± 1.29 yrs | 19.24 ± 1.34 yrs |
| Mean BMI (self-report) | 22.76 ± 2.86 kg/m2 | 22.80 ± 2.89 kg/m2 |
| Mean Subjective SES a | 4.62 ± 1.32 | 4.65 ± 1.30 |
| Somatization b | ||
| Mean Symptom reports | 1.49 ± .39 | 1.49 ± .37 |
| Mean Hypochondriasis | 1.53 ± .67 | 1.52 ± .66 |
Rather than asking students to report their family income, which they might not know, we instead assessed subjective SES with a 6-item scale about relative wealth in childhood and at college (Likert ratings ranged 1–7). See SMs for items.
Somatization tendencies were assessed using the Common Mental Disorders somatization and hypochondriasis subscales with Likert ratings ranged 1–5 (Søgaard & Bech, 2009). Note that we did not assess depressive or anxious tendencies or eating disorder status, given that these were conditions screened against during intake.
Twenty-three individuals did not return for Session 2, but sample characteristics did not significantly differ in demographic or psychological features as determined by t-tests (age, BMI, SES, somatization) and chi-square tests (sex, ethnicity), all ps> .250.
Procedure
Potential participants were prescreened for conditions that could affect autonomic physiological or emotional intensity (e.g., diagnosis of mental disorder, heart conditions or cardiac pacemakers, body mass index (BMI) > than 33, current illness, etc.). Full criteria are included in SMs. Participants completed two laboratory sessions, each at least one week but no more than two months apart. The goal of the first session was to assess interoceptive ability, sensibility, and beliefs alongside other participant characteristics. The goal of the second session was to induce robust changes in physiological arousal and emotional experience via the TSST. During recruitment and throughout the study, participants were told that the study assessed “individual differences in physiology and cognition.” Participants received participation credits upon completion.
Session 1: Interoceptive Assessments.
Participants completed Session 1 in a private testing room that included only the testing computer and psychophysiological equipment. Participants removed all jewelry and belts (as these can interfere with psychophysiological measures) and any wearable technology (e.g., Fitbits, Apple Watches that could distract or provide biofeedback), leaving their phones on silent outside the testing room. Participants first reported their current health and completed written informed consent, after which we collected five minutes of resting baseline electrocardiography (ECG). This baseline was only collected to help establish physiological parameters for the heartbeat detection task. After baseline, participants completed tasks in counterbalanced order: (1) a heartbeat detection task to assess interoceptive ability, (2) a Qualtrics survey that included measures of interoceptive sensibility and beliefs, and (3) an exploratory reaction time task not reported herein. Tasks were counterbalanced given that completing the heartbeat detection task might make interoceptive processes more accessible, temporarily altering ratings on interoceptive questionnaires. Questionnaires and their items as well as heartbeat detection trials were randomized to prevent order effects. At the end, the experimenter thanked the participant and provided reminders about the second laboratory visit.
Session 2: Trier Social Stress Test.
Participants completed Session 2 in a large private testing room that included the testing computer, a video camera, psychophysiological equipment, and a table with two chairs opposite the participant. Participants removed all jewelry, belts, and wearable technology and left their phones on silent outside the testing room. As in Session 1, participants first completed a “health check” to assess protocol adherence. Next, the experimenter prepared individuals for psychophysiological data collection. Specifically, we collected ECG and impedance cardiography (ICG) to assess autonomic nervous system indices of pre-ejection period and heart rate variability. After a 5-min resting ECG/ICG baseline, the experimenter re-entered the testing room and provided a new consent form. As required by the IRB, this consent form told participants that they were about to undertake a cognitive behavioral test that included public speaking so that we could assess their “cognitive performance under pressure.”
After consent, the TSST began. The TSST is a widely used motivated performance task that reliably increases sympathetic nervous system activity and experiences of negative, high arousal emotion and psychological stress. First, the experimenter introduced two judges dressed in white lab coats whom the participant learned were trained experts in nonverbal communication, public performance, and cognitive ability. The participant was told they should “imagine that this is a preliminary interview for a desirable job in your specific area of interest” and that they would provide a 10-min imaginary job interview speech. After these instructions, the participant had 2-min alone to mentally prepare their speech. After the judges re-entered, participants were expected to talk for the full 10-min. If they paused for more than 10 seconds, judges prompted them to continue. After the 10-min speech was over, judges introduced a surprise task that the participant was not expecting: counting backwards from the number 996 in steps of 7 as fast as possible with as few errors as possible. Participants did not know the duration of this task. Anytime the participant made an error, they were asked to start over from the beginning. After this 5-min math task, the TSST ended. See SMs for full TSST script. Immediately after, the experimenter re-entered the testing room and administered a Qualtrics survey in which participants rated their emotional experiences during the speech and math tasks.
Session 1 Measures
Interoceptive Ability.
We administered the heartbeat detection task with participants sitting in a low-light testing room with the door closed to increase privacy and reduce visual or auditory distractions. On each trial, participants heard 10 tones in headphones that were either coincident with their actual heartbeat (200 ms after the R-spike) or noncoincident with their heartbeat (500 ms after the R-spike). After each trial, participants indicated “yes” or “no” as to whether tones did or did not coincide with their heartbeats. Individuals also rated how confident they were using a slider scale ranging from 0–100% confident. The task was administered with a Matlab program (developed by Kleckner et al., 2015) that interfaced with a heartbeat detection module (v. 3.0.13) in MindWare’s BioLab. The Matlab program generated 60 trials whose order was randomized uniquely to the participant. We chose 60 trials based on simulations from Kleckner et al. (2015) who found that with a sample size of N=200 participants, 60 trials on the heartbeat detection task should be sufficient to detect a true effect size of .15 in the relation between interoceptive ability and a second variable. MindWare’s module collected trigger-based ECG data with the detection threshold set to 70% of individuals’ highest baseline R-peak (not including R-peaks that were due to overt movement), allowing us to tailor trials to participants’ cardiac physiology.
Using signal detection theory, we computed hits (trials wherein auditory tones were coincident with actual heartbeat and the individual correctly responded that their heartbeat was present), false alarms (trials wherein auditory tones were noncoincident with actual heartbeat but the individual incorrectly responded that their heartbeat was present)), correct rejections (trials wherein auditory tones were noncoincident with actual heartbeat and the individual correctly responded that their heartbeat was not present)), and misses (wherein auditory tones were coincident with actual heartbeat but the individual incorrectly responded that their heartbeat was not present). Most studies including heartbeat detection tasks operationalize interoceptive ability as either the fraction correct [hits + correct rejections / total number of trials] as an index of accuracy or compute d’ as an index of sensitivity by subtracting [z-scored hits – z-scored false alarms]. In addition to these performance metrics, the task allows for computation of within-person trial-by-trial confidence ratings and reaction times. Based on common reaction time data practices (Whelan, 2008), we excluded trials wherein reactions times were <200 milliseconds (ms) and also examined reaction time distributions across participants to identify and exclude trials where individuals were off task (>7000 ms). Ultimately, for our index of interoceptive ability, we used the fraction of correct trials or interoceptive accuracy. We did so for two reasons. First, we used the Kleckner et al. (2015) validated version of the HBD task. Give that they used the above accuracy metric in their assessment of trial number, sample size, and power, we used the same index to support interpretation of our observed effects in line with validated benchmarks. Second, this index of accuracy is commonly used in other studies with heartbeat detection (e.g., Critchley et al., 2004; Wiens et al., 2000).
Interoceptive Sensibility and Beliefs.
We collected two established measures of interoceptive sensibility and a novel measure of interoceptive beliefs, with the aim of clarifying the potential importance of evaluative interoceptive beliefs as a separate construct from interoceptive sensibility.
Body Awareness Questionnaire (BAQ; Shields et al., 1989).
The BAQ is an 18-item self-report measure of perceived awareness of bodily sensations (∝ = .82). In previous validations, this scale was not correlated with hypochondriasis, trait anxiety, trait neuroticism, or self-esteem (Shields et al., 1989), suggesting that it may reflect individuals’ self-reported attention or noticing of bodily sensations rather than whether individuals catastrophize or feel distress at those sensations. Example items include, “I can distinguish between tiredness that’s caused by hunger and tiredness that’s caused by a lack of sleep” and “I know in advance when I’m getting the flu.” Participants responded to items on a 7-point Likert-type scale from 1 (not at all true of me) to 7 (very true of me).
Multidimensional Assessment of Interoceptive Awareness (MAIA; Mehling et al., 2012).
The MAIA is a 32-item self-report measure of interoceptive sensibility. It was originally developed in focus groups with mind-body instructors (e.g., yoga, meditation) and has since become one of the best-validated and most widely used assessment of interoceptive sensibility. The MAIA measures individuals’ self-reported attentiveness and comfort with bodily sensations. Participants responded on 6-point Likert-type scale from 0 (never) to 5 (always). As the study was planned in Fall 2013 and began in 2014, we used the first version of the MAIA. The noticing subscale had 4 items (∝ = .56; e.g., “When I am tense, I notice where the tension is located in my body”). The not distracting subscale had 3 items (∝ = .53; e.g., reverse item example: “I distract myself from sensations of discomfort.”). The attention regulation subscale had 7 items (∝ = .81; e.g., “I can maintain awareness of my inner bodily sensations even when there is a lot going on around me.”). The emotional awareness subscale had 5 items (∝ = .69; “I notice how my body changes when I am angry.”). The body listening subscale had 3 items (∝ = .74; “I listen to my body to inform me about what to do”). The self-regulation subscale had 4 items (∝ = .71; “When I bring awareness to my body, I feel a sense of calm.”).
At face value, the above subscales capture noticing, attending to, or regulating attention to one’s interoceptive sensations. The MAIA also includes two subscales that may instead reflect interoceptive beliefs about the value or nature of interoceptive sensations rather than interoceptive sensibility. These two subscales were not worrying and body trusting, each with 3-items (not worrying: ∝= .50; “When I feel physical pain, I become upset”; body trusting: ∝= .70; “I trust my body sensations”). We included these with the goal of conducting factor analyses to best determine which subscales reflected sensibility vs. evaluative beliefs.
Bodily Signal Beliefs Questionnaire (BSBQ).
This was a novel 12-item questionnaire (∝ = .69) that we created to capture additional beliefs about the intensity, danger, and difficulties posed by interoceptive sensations. Prior factor analyses with this scale suggested a single factor structure of 7 items (∝ = .77) which represent negative beliefs. These final 7 items were: “My body is unpredictable,” “I have a hard time handling my bodily sensations,” “I believe that my body’s feelings can be misleading,” “Listening to my body’s sensations can be problematic,” “My bodily urges are difficult to control,” “Sometimes I’m afraid of my bodily feelings,” and “My body is an intense place.” All items were rated on a 7-point Likert-type scale ranging from 1 (not at all true of me) to 7 (extremely true of me). We reverse coded items so that higher endorsements suggest more positive beliefs about the body. One study aim was to explore the validity of this measure and its relation to other interoceptive facets; see (Bonar et al., 2021) for a revised version that examines interoceptive intensity, distress, and efficacy beliefs.
Session 2 Measures
Psychophysiology.
To assess stress-related changes in autonomic psychophysiology during the TSST, we collected ECG and ICG using MindWare Technologies (Gahanna, OH, USA) BioLab acquisition software at 500 Hz. For ECG, three non-invasive spot electrodes were placed on participants’ torso, with one electrode on the right collarbone (−) and two electrodes on the lowermost ribs (+ and ground). For ICG, two spot electrodes were placed at the top (+) and bottom (−) of the sternum and two more electrodes on the spine, with the lower back electrode being placed two fingers’ width below where the front bottom electrode was placed (4 electrodes total). Continuous ECG and ICG were measured in the 5-min Baseline, 2-min TSST Prep, 10-min TSST Speech, 5-min TSST Math, and 5-min Recovery.
ECG data were processed with MindWare’s Heart Rate Variability (HRV) Analysis software (v. 3.0.25). Respiration was imputed from ICG. High frequency HRV was set as the frequency band between 0.12–0.40 Hz. ICG data were similarly processed with MindWare’s Impedance (IMP) Analysis software (v. 3.2.4). We used Biolab’s default filter for the first derivative of basal thoracic impedance (dZ/dt), set at 0.5–45 Hz; we also selected the percent of dZ/dT Time + C as the initial B-point detection method. Trained research assistants visually inspected and independently scored each segment (60 seconds) of ECG and ICG data, to verify that R-spikes, B-point, and X-point were correctly detected and to identify segments with excessive artifact or arrhythmia. Initial agreement between scorers was 93.7% for ECG (based on the number of R-spikes identified per segment) and 85.3% for ICG (based on pre-ejection period values per segment). A third expert (JKM) resolved all segment-by-segment discrepancies.
From the ECG and ICG data, we extracted measures of pre-ejection period (PEP), heart rate variability (HRV), and heart rate (HR), described below in more details. As part of data preparation, we also examined outliers +/− 3SDs for PEP, HRV, and HR within each study timepoint (Session 2 Baseline, TSST Prep, TSST Speech, TSST Math, TSST Recovery). No outliers with undue influence were identified. Reactivity scores were calculated for each index by averaging the first segment (minute) from TSST Prep, Speech, and Math together to represent physiology during the stressor, and then second, subtracting [stressor-baseline] to derive average change or reactivity from the Session 2 Baseline.
Sympathetic Nervous System Reactivity.
Pre-ejection period or PEP is an index of SNS activity (Newlin & Levenson, 1979), reflecting the time (in milliseconds) between the onset of cardiac depolarization and the start of left ventricular contraction to expel blood from the heart. Practically, this is calculated as the time in ms between the Q-point and B-point in the corresponding ECG and dZ/dt ensembles. Smaller PEP values suggest faster periods of cardiac contractility driven by the SNS, and larger PEP values suggest slower periods, such as when individuals are more relaxed or at rest. However, to improve the interpretability of PEP in analyses throughout this paper, we multiplied PEP values by −1 such that greater PEP reactivity is equivalent to an increase in cardiac SNS activity from resting baseline and lower PEP reactivity is equivalent to a decrease in cardiac SNS activity from resting baseline. Greater SNS reactivity is our primary index of physiological arousal herein.
Parasympathetic Nervous System Reactivity.
Sinoatrial cells in the heart can self-excite, resulting in an intrinsic heart rate in the absence of autonomic, respiratory, or hormonal influences. Upon inhalation, heart rate speeds up and upon exhalation, heart rate slows. Furthermore, the SNS stimulates heart rate which is then gated or slowed by PNS influence via the vagus nerve. As a result, the time between heartbeats is irregular, reflected in measures of heart rate variability or HRV (Berntson et al., 1993; de Geus et al., 2019). For our measure of HRV, we extracted the root mean square of successive differences between heartbeats (RMSSD), reflecting the beat-to-beat (R-to-R) variance in heart rate. Importantly, RMSSD can be ascribed to vagus nerve outflow to sinoatrial cells, serving as an index of cardiac parasympathetic control, especially once RMSSD is adjusted for confounds with heart rate. To adjust RMSSD, we applied the formula found in de Geus et al. (2019), wherein a coefficient of variation or cvRMSSD = 100 * (RMSSD/IBI) with IBI being the interbeat interval in ms between consecutive heartbeats. We report statistical models with adjusted cvRMSSD in the main text (called “HRV” for simplicity) but provide findings with unadjusted RMSSD in the SMs for transparency and future meta-analyses. Note that results are consistent with either version of RMSSD. Importantly, we did not use respiratory sinus arrhythmia (RSA) as our index of HRV, given that this measure is highly influenced by respiration, which would be problematic given that participants speak throughout the TSST Speech and Math tasks. RMSSD, while correlated with high frequency HRV (e.g., RSA), appears to be less influenced by respiration and may reflect greater contributions of the PNS (Shaffer & Ginsberg, 2017).
Heart rate (HR) in beats per minute (bpm) was also extracted as a supplementary measure of physiological reactivity. Herein, we focus on reporting PEP reactivity (given that SNS changes are likely most relevant in the context of a stressor) and HRV reactivity with the goal of clarifying the specificity of effects between SNS reactivity vs. PNS reactivity. We did not examine HR reactivity as a primary model of interest given that HR reflects both SNS and PNS contributions and thus is less informative relative to PEP or HRV. However, heart rate may still be of interest to readers, given that heartbeat detection assesses the ability to detect shifts in heart rate. We present models with HR reactivity in the SMs.
Self-Reported Emotions.
Emotions during the TSST were measured using an expanded 30-item version of the Positive & Negative Affect Schedule or PANAS (Watson & Clark, 1994), The PANAS is a standard measure assessing emotions across a variety of affective states ranging in arousal (high vs. low) and valence (negative vs. positive). Participants rated how intensely they experienced each emotion on a Likert-type scale from 0 (not at all) to 6 (extremely). Items ranged across the four quadrants of valence and arousal: 17 high arousal emotions (e.g., excited, stressed; ∝= .86) vs. 9 low arousal emotions (e.g.,bored, serene; ∝= .51) and 16 negative emotions (e.g., embarrassed, stressed, bored, sad; ∝= .91) vs. 9 positive emotions (e.g., excited, proud, serene; ∝ = .86). For analyses, we focused on high arousal emotions, given that these are the emotions most likely to be experienced during an acute stressor and given that work with the heartbeat detection task most typically finds an effect on arousal as opposed to valence. See SMs for exploratory findings with negative, low arousal, and positive emotions.
Analytic Strategy
Preregistration, Transparency, and Openness.
This study was designed in 2013 and began data collection in 2014, before preregistration sites and resources were widely available. As such, this study is not preregistered. However, we publicly provide all de-identified data, code, and additional supplementary information on the Open Science Framework (https://osf.io/z7c2a/) and report supporting analyses in SMs to increase data transparency and reporting completeness. All questionnaires and tasks used in this study are widely available from their original sources, except for the novel measure assessing interoceptive beliefs, which we describe fully in the Methods.
Aim 1: Evaluating the Interrelationships of Interoceptive Constructs.
Given that interoceptive beliefs remain underexamined as a separate construct from interoceptive sensibility, we wanted to verify whether it was justified to examine interoceptive sensibility vs. interoceptive beliefs as separate constructs. Thus, we first conducted exploratory factor analyses (EFAs) to first identify how interoceptive measures might interrelate with one another. Specifically, we conducted common factor analyses in R using the psych package (Revelle, 2019) with the maximum likelihood approach (Fuller & Hemmerle, 1966). We used the promax rotation (Hendrickson & White, 1964) given that we expected interoceptive factors could be correlated (e.g., interoceptive sensibility and beliefs might be different but related). To test goodness-of-fit, we used a χ2 to evaluate whether the proposed number of factors sufficiently fit the data, indicated by a nonsignificant χ2. In our primary EFAs, we included participants’ interoceptive accuracy score from the heartbeat detection task as well as mean scores for the BAQ, all MAIA subscales, and the BSBQ. In supplementary EFAs reported in the SMs, we also ran the same EFAs but with mean confidence from the heartbeat detection task, given that some studies consider confidence a measure of interoceptive sensibility (Forkmann et al., 2016; Mai-Lippold et al., 2020). As a further confirmatory and data reduction step, we conducted confirmatory factor analyses (CFAs) using latent variable structural equation modeling (Bollen, 1989, 2002) to evaluate how well our EFA-clarified manifest indicators represented the latent constructs of interoceptive sensibility vs. beliefs. For concision, CFAs are reported in SMs, and we only share their final conclusions in the main text. Finally, we also conducted bivariate Pearson correlations between the interoceptive measures to further clarify potential inter- and intra-relations between measures of interoceptive ability, sensibility, and beliefs.
Aim 2: Testing Interoceptive Constructs as Main Effects and Moderators of Emotion.
Guided by the EFA/CFA measurement models and our hypotheses about the role of interoceptive facets in moderating the link between physiological reactivity and emotion, we conducted stepwise linear regressions in SPSS with regression predictors of either SNS reactivity (change in PEP reversed * −1 in line with greater SNS changes) or HRV reactivity (change in cvRMSSD), interoceptive ability (accuracy), interoceptive sensibility (mean of BAQ and MAIA notice, non-distract, attention regulation, self-regulation, emotional awareness, and body listening subscales), and interoceptive beliefs (mean BSBQ reverse-scored to represent more positive beliefs). Although there were no missing data for questionnaire measures, there were technical failures with the heartbeat detection task (usable n= 234 out of 250, 94% complete) and some individuals with unusable PEP or HRV data (clean PEP n= 200 out of 227, 88% complete; clean HRV n= 219 out of 227, 97% complete). Thus, in regression models, of n=227 who completed Session 2, there were n=191 individuals (84%) with complete PEP and heartbeat detection data plus n=208 (92%) with complete HRV and heartbeat detection data.
In all models, Step 1 included physiological reactivity (PEP or HRV) on its own, Step 2 added in interoceptive ability, Step 3 added in interoceptive sensibility, Step 4 added in interoceptive beliefs, Step 5 added in interaction terms between physiological reactivity x interoceptive ability, sensibility, and beliefs, and Step 6 (the final model) added the covariates of self-identified sex and log-transformed BMI, given prior work showing both may confound effects of psychophysiology, interoception, and emotion reports. Indeed, we found both were related to our measures of interest (Table S6 in SMs). Notably, sex was associated with interoceptive ability (r= .14, p= .037), such that males were more accurate on the HBD task than females. Females also tended to report experiencing more intense negative and high arousal emotions during the TSST (ps<.05). There was a marginal association of sex with the BSBQ, such that females tended to report more positive interoceptive beliefs relative to males (r= −.12, p= .060). Finally, although BMI was not significantly associated with our physiological, interoceptive, or emotion measures (likely because we prescreened against BMIs > 33), BMI was still marginally associated with interoceptive ability, r= −.13, p=.054, underlining the importance of including it alongside sex as a covariate. Note that BMI was skewed, thus was natural log transformed.
Below, we report findings for our primary emotion outcome (high arousal emotion) and only summarize findings for secondary measures (negative, low arousal, positive emotion) described in SMs. For simplicity, we also only report the final model step in the main text but again refer readers to SMs for tables with full model steps. Interactions in the final model step were probed using simple slopes tests in Preacher, Curran, & Bauer’s online simple slopes for MLR 2-way interactions online utility (Preacher et al., 2006). Standardized betas (β) served as effect size estimates, with model predictors z-score mean-centered to improve main effect and interaction estimation and comparison.
Results
Aim 1: Evaluating the Interrelationships of Interoceptive Constructs
Exploratory Factor Analyses (EFAs).
We first tested the possible fit of three factors to the data structure underlying interoceptive ability, sensibility, and belief measures (Table 2). This factor structure explained about 43% of the variance in the data, but the goodness-of-fit test was significant, χ2= 40.14 (df=25), p=.028, suggesting that three factors are likely an insufficient fit to the underlying data structure of these measures. We then tested the possibility of four factors (Table 2). The goodness-of-fit test was no longer significant, χ2= 11.73 (df=17), p=.816, suggesting that four factors may be sufficient. This set of factors explained about 52% of the variance in the data. In this final model, the overall accuracy score from the heartbeat detection task did not load onto any single factor, as would be expected (e.g., Ferentzi et al., 2018; Forkmann et al., 2016; Garfinkel et al., 2015, 2016), suggesting it represents a separate dimension of interoception that is not represented by the other measures included the EFA.
Table 2.
Exploratory factor models with interoceptive ability, sensibility, and belief measures.
| EFA with 3 factors | EFA with 4 factors | ||||||
|---|---|---|---|---|---|---|---|
| Factor loadings | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
| Interoceptive ability | |||||||
| HBD accuracy | .23 | −.18 | −.11 | .16 | |||
| Interoceptive sensibility | |||||||
| BAQ | .63 | −.20 | .64 | −.17 | |||
| MAIA Notice | .75 | .73 | −.10 | ||||
| MAIA Non-distract | −.37 | .10 | .99 | ||||
| MAIA Not worrying | −.19 | −.47 | −.13 | .16 | .34 | ||
| MAIA Attention regulation | .60 | .44 | .86 | .44 | |||
| MAIA Emotion awareness | .82 | −.27 | .63 | −.45 | |||
| MAIA Self-regulation | .64 | .26 | .70 | .15 | .11 | ||
| MAIA Body listening | .76 | .71 | .13 | .11 | −.15 | ||
| MAIA Body trust | .37 | .21 | .40 | .30 | −.13 | .59 | |
| Interoceptive beliefs | |||||||
| BSBQ mean | .70 | −.12 | .10 | .62 | |||
| Total | |||||||
| SS loadings | 3.17 | 0.80 | 0.74 | 3.18 | 1.07 | 0.84 | 0.58 |
| Proportion of variance | 0.29 | 0.07 | 0.07 | 0.29 | 0.10 | 0.08 | 0.05 |
| Cumulative variance | 0.29 | 0.36 | 0.43 | 0.29 | 0.39 | 0.46 | 0.52 |
Similarly, for our a priori measures of interoceptive sensibility, the BAQ and MAIA subscales largely fit together in one factor (Factor 1) that explained around 29% (out of a total 52%) of the model variance. In particular, the BAQ and subscales of the MAIA including notice, attention regulation, self-regulation, emotional awareness, and body listening loaded onto this factor, confirming the face validity of using these measures together to represent how much individuals self-characterize their ability to notice and attend to interoceptive sensations. Interestingly, the MAIA non-distract subscale formed its own separate unique factor (Factor 2), explaining an additional 10% of the variance, but MAIA non-distract did not fit well with any other measures or factors in the model.
Finally, mean BSBQ responses—which included items such as “my body is unpredictable,” “sometimes I’m afraid of my body sensations,” and “listening to my body sensations can be problematic”—were separate from the BAQ and MAIA sensibility measures, suggesting that, as intended, the BSBQ may more uniquely assess evaluative beliefs about the difficulties, safety, or predictability surrounding interoceptive sensations. Of note, we were curious if the MAIA not worrying and body trusting subscales might reflect evaluative interoceptive beliefs and thus fit clearly with the BSBQ rather than with sensibility measures. However, the MAIA not worrying subscale loosely loaded across three out of the four factors and did not strongly fit with any single factor. Similarly, the MAIA body trusting subscale weakly loaded with the other MAIA subscales and partially loaded with the BSBQ interoceptive beliefs measure in the 4-factor EFA model, but not in the 3-factor model.
Confirmatory Factor Analyses (CFAs).
To verify the factor structure obtained from the EFAs, we conducted CFAs using latent variable SEM. Full results are reported in SMs (Table S2), but herein we summarize final results. Overall, models indicated that the MAIA not worrying and body trusting subscales alongside the BSBQ did not fit with the primary latent factor comprised of the BAQ mean and MAIA notice, non-distract, attention regulation, emotional awareness, self-regulation, and body listening subscales. This factor structure suggested that the BAQ and remaining MAIA subscales may reflect a latent factor representing interoceptive sensibility as beliefs about noticing, attention, and awareness of one’s interoceptive signals, states, and sensations.
Bivariate Correlations.
Finally, we examined bivariate correlations between interoception measures (Table 3). Our measure of behavioral interoceptive ability—interoceptive accuracy—from the heartbeat detection task was unrelated to the BAQ, MAIA subscales, and BSBQ (all ps> .10), consistent with prior findings discussed in the Introduction. For interoceptive sensibility, the BAQ and MAIA subscales of notice, attention regulation, emotional awareness, self-regulation, and body listening were all moderately to strongly associated with each other (rs~.30−.70). The MAIA non-distract subscale also was associated with the BAQ and these MAIA subscales but it was a weaker relationship (rs~.15−.20), and it was non-significant in relation to the MAIA emotional awareness and body listening subscales (ps= .259, .813). The MAIA not worrying subscale was only sometimes related to other MAIA subscales, with small but significant rs suggesting weaker interrelations. The MAIA body trusting subscale, on the other hand, was consistently associated with the BAQ and all MAIA subscales. Finally, the BSBQ was significantly but weakly associated with the MAIA not worrying, attention regulation, and body trusting subscales and no other measures. See SMs (Table S5) for full intercorrelations across all variables.
Table 3.
Bivariate correlations between interoceptive ability, sensibility, and belief measures.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ability | ||||||||||
| 1. Accuracy | .00 | −.40 | −.06 | .09 | .03 | −.04 | −.01 | −.10 | .01 | −.08 |
| Sensibility | ||||||||||
| 2. BAQ | 1.00 | .50 *** | −.15* | −.04 | .39 *** | .46 *** | .40 *** | .44 *** | .17 ** | −.12 |
| 3. MAIA Notice | 1.00 | −.18** | −.10 | .54 *** | .57 *** | .50 *** | .54 *** | .31 *** | −.05 | |
| 4. MAIA Nondistract | 1.00 | −.18** | −.21*** | −.07 | .20 ** | −.02 | −.25*** | .03 | ||
| 5. MAIA Nonworry | 1.00 | .14 * | −.20** | .03 | −.05 | .14 * | .18 ** | |||
| 6. MAIA Attention | 1.00 | .40 *** | .67 *** | .54 *** | 51 *** | .16 * | ||||
| 7. MAIA Emotion | 1.00 | .46 *** | .58 *** | .32 *** | −.06 | |||||
| 8. MAIA SelfReg | 1.00 | .53 *** | .44 *** | .09 | ||||||
| 9. MAIA Listening | 1.00 | .39 ** | .02 | |||||||
| 10. MAIA Trust | 1.00 | .34 *** | ||||||||
| Beliefs | ||||||||||
| 11. BSBQ | 1.00 |
p<.05,
p<.01,
p<.001
In sum, across EFAs, CFAs, and correlations, we sought to provide converging insights about the measurement structure and construct validity underlying interoceptive ability, sensibility, and beliefs. With regards to interoceptive ability, the EFAs and bivariate correlations revealed that interoceptive ability as measured by accuracy on the heartbeat detection task reflects a separate construct from interoceptive sensibility and beliefs. With regards to interoceptive sensibility, using EFAs and CFAs, we found a clear, unique factor that consisted of the BAQ mean and MAIA notice, non-distract, attention regulation, emotional awareness, self-regulation, and body listening subscales, reflecting self-reports of how much individuals think they notice and attend to their interoceptive sensations. These intra-construct relations were further supported in bivariate correlations. Based on these findings, we created a mean score reflecting interoceptive sensibility using these measures.
Finally, the BSBQ seemed to reflect its own separate construct from the interoceptive sensibility factor. We considered the possibility that the MAIA not worrying and body trusting subscales could also reflect evaluative interoceptive beliefs, but we did not see convergent, convincing evidence across the EFAs, CFAs, and bivariate correlations that these two MAIA subscales consistently and strongly fit with the BSBQ. The MAIA not worrying and body trusting subscales also did not consistently and clearly fit with the factor of interoceptive sensibility. Thus, for this study, we dropped these two subscales from subsequent analyses and used the BSBQ mean score as our measure of interoceptive beliefs in subsequent regressions. However, future studies may wish to examine the MAIA more closely in other samples to assess possible divergence in beliefs about interoceptive attention and awareness (i.e., sensibility) vs. evaluative beliefs about the nature and meaning of interoceptive sensations.
Aim 2: Testing Interoceptive Constructs as Main Effects and Moderators for Emotion
In regressions, our goal was to (1) test the main effects of interoceptive constructs in relation to emotional arousal (above and beyond their shared variance with each other and physiology) and (2) compare whether interoceptive constructs moderate the concordance between physiological arousal (greater SNS reactivity, decreases in HRV reactivity) and emotional arousal during the acute stressor.
Physiological Arousal and Interoception Effects on High Arousal Emotion (Table 4, Fig. 1)
Table 4.
Effects of physiological reactivity and interoception on high arousal emotions.
| Predictors | R 2 | b | SE | β | p | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|
| Mean High Arousal Emotion as Outcome | |||||||
| SNS Reactivity Model | |||||||
| F(9,189)= 3.16, p= .001 | .085 | ||||||
| Intercept | 1.33 | 1.578 | .402 | −1.787 | 4.440 | ||
| SNS reactivity | 0.13 | 0.066 | 0.15 | .045 | 0.003 | 0.263 | |
| Interoceptive ability | −0.13 | 0.066 | −0.15 | .043 | −0.263 | −0.004 | |
| Interoceptive sensibility | −0.04 | 0.064 | −0.04 | .540 | −0.165 | 0.087 | |
| Interoceptive beliefs | −0.16 | 0.064 | −0.17 | .017 | −0.282 | −0.028 | |
| SNS x Interoceptive ability | 0.03 | 0.076 | 0.03 | .715 | −0.122 | 0.177 | |
| SNS x Interoceptive sensibility | 0.10 | 0.074 | 0.10 | .174 | −0.045 | 0.249 | |
| SNS x Interoceptive beliefs | 0.14 | 0.066 | 0.15 | .038 | 0.008 | 0.268 | |
| Sex | −0.33 | 0.134 | −0.18 | .016 | −0.589 | −0.061 | |
| logBMI | 0.20 | 0.508 | 0.03 | .689 | −0.798 | 1.205 | |
| HRV Reactivity Model | |||||||
| F(9,206)= 2.69, p= .001 | .086 | ||||||
| Intercept | 1.86 | 1.557 | .233 | −1.208 | 4.934 | ||
| HRV reactivity | −0.06 | 0.064 | −0.06 | .371 | −0.185 | 0.069 | |
| Interoceptive ability | −0.08 | 0.067 | −0.09 | .222 | −0.213 | 0.050 | |
| Interoceptive sensibility | −0.04 | 0.063 | −0.04 | .517 | −0.165 | 0.050 | |
| Interoceptive beliefs | −0.16 | 0.062 | −0.18 | .011 | −0.283 | −0.038 | |
| HRV x Interoceptive ability | −0.09 | 0.069 | −0.09 | .212 | −0.222 | 0.050 | |
| HRV x Interoceptive sensibility | −0.06 | 0.071 | −0.05 | .430 | −0.195 | 0.083 | |
| HRV x Interoceptive beliefs | 0.19 | 0.061 | 0.21 | .003 | 0.064 | 0.306 | |
| Sex | −0.29 | 0.130 | −0.15 | .026 | −0.548 | −0.036 | |
| logBMI | 0.52 | 0.500 | 0.01 | .918 | −0.935 | 1.038 | |
Note: SNS reactivity was indexed by PEP, with scores reversed such that positive SNS reactivity values reflect an acceleration of PEP (shorter intervals) during the TSST relative to baseline. HRV reactivity was indexed by RMSSD, reflecting the beat-to-beat (R-to-R) variance in heart rate. Bolded rows highlight significant effects at p<.05. Standard errors and confidence intervals are with respect to unstandardized coefficients. Adjusted R2 is reported. Standardized betas (β) serve as effect sizes. Sex is self-identified and coded 0=Female, 1=Male.
Fig. 1.
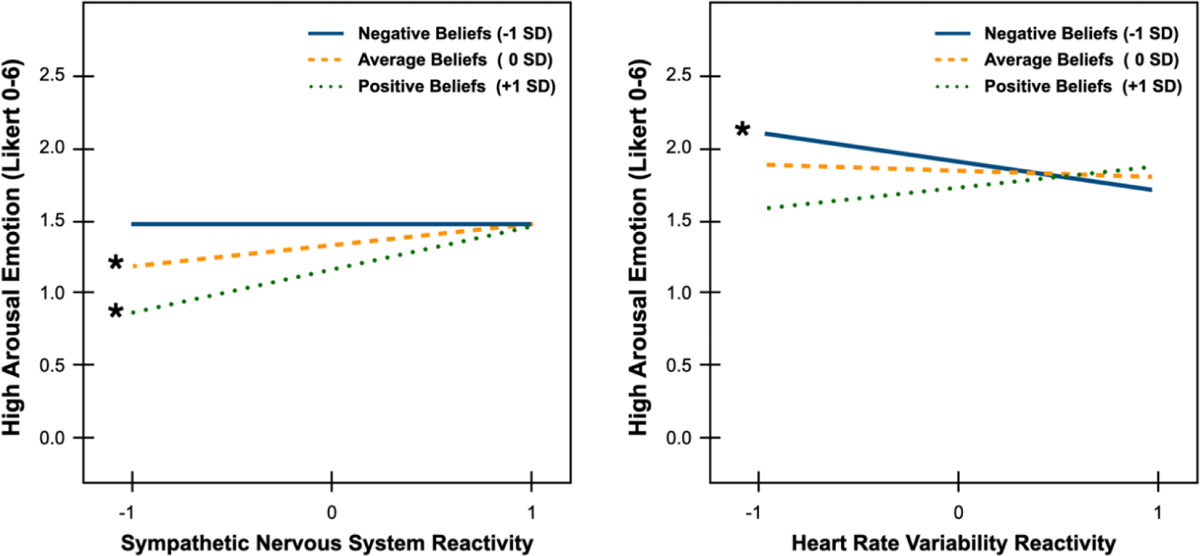
Probed interactions testing moderation by interoceptive beliefs on the relation between (a) sympathetic nervous system reactivity and (b) heart rate variability reactivity on high arousal emotion reports in response to the acute stressor. Significant slopes are indicated with an asterisk.
SNS Reactivity Model.
Consistent with the hypothesis that greater SNS reactivity is related to more intense high arousal emotions, there was a significant main effect of SNS reactivity, b= 0.13, p=.045, 95% CIs [0.00, 0.26], even after adjusting for shared variance with interoceptive constructs in the final model step. There was also a significant main effect of interoceptive ability, b= −0.13, p=.043, 95% CIs [−0.26, −0.00], such that greater interoceptive ability (i.e., accuracy) was related to less intense high arousal emotions. There was no effect of interoceptive sensibility (p=.540) but a significant inverse main effect of interoceptive beliefs, b= −0.16, p=.017, 95% CIs [−0.28, −0.03], wherein more positive beliefs about the value of interoceptive sensations was related to less intense high arousal emotions. Sex was also significant, b= −0.33, p=.016, 95% CIs [−0.59, −0.06], indicating that participants who self-identified as female reported more intense high arousal emotions from the stressor than did self-identified males.
Critically, there was a significant interaction of SNS reactivity x interoceptive beliefs (b= 0.14, p=.038, 95% CIs [0.01, 0.27]). Simple slopes analyses revealed that the slopes for more negative beliefs (−1SD) was not significant [t(180)= −0.05, p=.959], but the slopes for average/neutral beliefs (0SD) and more positive beliefs (+1SD) were significant [t(180)= 2.02, p=.045 and t(180)= 3.05, p=.003 respectively]. As depicted in Fig. 1, these slopes for interoceptive beliefs only diverged in individuals with less SNS reactivity (below 0SD). Lower SNS reactivity encompasses individuals who had very little change from baseline (i.e., low reactivity to the TSST) as well as individuals who had decreases in SNS activity from baseline (i.e., inhibition of SNS activity). These findings suggest that individuals who had positive or neutral interoceptive beliefs and who experienced less than average SNS reactivity tended to report lower levels of high arousal emotions. When individuals who had positive or neutral beliefs experienced greater than average SNS reactivity, they reported high levels of high arousal emotions. These findings suggest that individuals with positive or neutral beliefs tend to experience emotional arousal that tracks with their ongoing physiological arousal (i.e., greater concordance between emotional and physiological arousal), perhaps because they are more likely to value and thus incorporate physiology into ongoing emotional experiences. In contrast, individuals with negative interoceptive beliefs reported high levels of high arousal emotions regardless of whether they experienced relatively high or low SNS reactivity. These findings suggest that for at least some individuals with negative interoceptive beliefs, emotional experiences may be unmoored from ongoing physiological changes, with greater concordance between physiological and emotional arousal only emerging when SNS reactivity is robust and intense. Individuals who do not value their physiological sensations may have learned to ignore or suppress physiological experiences over time and may rely relatively more on external sensations (e.g., the features of the context) to inform ongoing emotional experiences.
HRV Reactivity Model.
Unlike the above model with SNS reactivity, PNS reactivity as indexed by HRV reactivity was unrelated to high arousal emotions (p=.371). The lack of significant effects for HRV reactivity may suggest that high arousal emotions are particularly connected to SNS-mediated physiological arousal and interoception thereof during an acute stressor. Neither interoceptive ability (p= .222) nor sensibility (p= .517) predicted high arousal emotion. However, there was a significant main effect of interoceptive beliefs, b= −0.16, p=.011, 95% CIs [−0.28, −0.04], such that individuals with more positive interoceptive beliefs reported less intense high arousal emotions during the TSST.
Replicating the SNS reactivity model, there was a significant interaction of HRV reactivity x interoceptive beliefs (b= 0.19, p=.003, 95% CIs [0.06, 0.31]). However, in contrast to the SNS reactivity model, simple slopes analyses revealed that it was the slope for negative interoceptive beliefs (−1SD) that was significant [t(197)= −2.84, p=.005] and not the slopes for average/neutral (0SD) or positive beliefs (+1SD), [t(197)= 0.90, p=.371 and t(197)= 1.39, p=.168 respectively]. As depicted in Fig. 1, these slopes for interoceptive beliefs only diverged in individuals with low HRV reactivity to the TSST (below 0SD). Lower HRV reactivity here reflects individuals who had decreasing HRV in response to the TSST. Decreases in HRV mean that the periods between heartbeats were becoming less variable or more regular, such as in the presence of greater cardiac SNS activation and PNS withdrawal (i.e., consistent with greater physiological arousal). On the other hand, increases in HRV mean that the periods between heartbeats were becoming more variable or irregular, consistent with greater parasympathetic control over the cardiac cycle. These findings suggest that individuals who have both negative interoceptive beliefs and greater decreases in HRV in response to the stressor (i.e., greater PNS withdrawal) tended to report more intense high arousal emotions than individuals with positive and neutral beliefs or individuals. On the other hand, greater HRV increases during the TSST (i.e., greater PNS control) was related to less intense high arousal emotions, regardless of interoceptive beliefs, suggesting coherence between PNS activity and lower levels of high arousal emotion. Altogether, more negative beliefs appeared to exacerbate high arousal emotions in the context of stressor-induced PNS withdrawal.
Discussion
Emotional experiences are often accompanied by objective physiological concomitants, but it has long been debated whether and to what extent these concomitants contribute to emotion (Cannon, 1927; James, 1884). Recent research breathes new life into these questions, showing that experimentally or pharmacologically manipulating physiological systems can alter subsequent emotional or stress experiences (e.g., Harrison et al., 2009; MacCormack, Armstrong-Carter, et al., 2021; MacCormack & Lindquist, 2019). The present work adds to this growing evidence that there are important within- and between-person factors determining when and how much the body matters for emotions. We found that both interoceptive ability and especially positive interoceptive beliefs were associated with lower emotional arousal during an acute stressor. Interestingly, interoceptive beliefs was the only interoceptive construct to moderate the concordance between physiological and emotional arousal. Collectively, these findings offer insights for the role of interoception in emotion, stress, and beyond.
The Role of Interoception in Emotion: Findings and Measurement Implications
Interoceptive science has exploded over the past decade, with hundreds of studies investigating relations between interoception and social affective processes, ranging from emotion (i.e., experience, awareness, perception, regulation), to social behavior (e.g., empathy, moral judgments), to intuitive decision-making, risk-taking, health behaviors, mood disorders, addiction, autism, and more (Tsakiris & De Preester, 2018). As such, the broader constructs underpinning interoception remain inconsistently defined and measured, making it difficult to interpret and integrate findings. Several scholars have begun building a taxonomy of constructs (Garfinkel & Critchley, 2013; Khalsa et al., 2018; Quigley et al., 2021), but few studies compare the relative predictive validity of multiple interoceptive constructs together for emotional experience. We aimed to clarify the nature of key interoceptive constructs (i.e., ability, sensibility, beliefs) and their unique relations to emotion, above and beyond shared interrelations between interoceptive constructs with each other and physiological reactivity.
Distinct Interoceptive Constructs.
Across bivariate correlations and factor analyses, we saw evidence for distinctions between interoceptive ability, sensibility, and beliefs (Tables 2–3 herein, also Table S5 in SMs for fuller correlations). For example, we found that the BAQ and most subscales of the MAIA formed a loose single factor distinct from the BSBQ, suggesting that the BAQ and MAIA may be tapping a similar construct or set of constructs related to people’s interoceptive sensibility, i.e., beliefs about noticing and regulating attention towards sensations. We also replicated past work showing that interoceptive ability vs. sensibility are unrelated (e.g., Garfinkel et al., 2014, 2015), affirming that self-characterizations of one’s interoceptive ability do not on average correlate with the behavioral ability to detect heartbeats. As such, researchers cannot use self-reported interoceptive sensibility as a more convenient substitute for behavioral measures of interoceptive ability.
In particular, our findings highlight differences between interoceptive sensibility and what we term interoceptive beliefs. Interoceptive beliefs include people’s evaluative beliefs about the nature and perceived utility of interoceptive sensations. To measure these beliefs, we designed the BSBQ to tap individuals’ beliefs about the value or danger, intensity, and utility of bodily sensations. Factor analyses suggest that these evaluative beliefs are distinct from interoceptive sensibility as measured by the BAQ and most MAIA subscales. Although subscales of the MAIA such as not worrying and body trusting may appear at face value to be similar to evaluative interoceptive beliefs, neither subscale fit consistently with the BSBQ in exploratory and confirmatory factor analyses (although these relations should be further examined in additional samples). Interoceptive sensibility, as measured herein, may therefore reflect individuals’ beliefs and perceptions about their access to and ability to regulate attention towards interoceptive sensations. In contrast, evaluative beliefs may instead capture individuals’ beliefs and accumulated knowledge about the meaning and utility of interoceptive sensations. Future research is needed to clarify the relations between these constructs and relevant outcomes (i.e., to identify the nomological networks of each construct; Cronbach & Meehl, 1955) but see Bonar et al., (2021) for initial construct validation.
Interoceptive Ability is Associated with Less Intense High Arousal Emotions.
When using stepwise regressions that adjusted for physiological reactivity, sex, and BMI, we found that greater interoceptive ability (accuracy on the heartbeat detection task) was inversely related to self-reported emotional arousal during the stressor. This was a small effect observed only in the presence of SNS reactivity. This inverse association runs counter to some theoretical assumptions (e.g., somatosensory amplification) arguing that interoception may “amplify” or exacerbate emotional arousal. The present data suggest more complicated inter-relations between physiology, interoception, and emotion.
First, the literature linking interoceptive ability as measured by the heartbeat detection task to emotional experience is small. Most studies reporting a positive relation between interoceptive ability and emotion use the heartbeat tracking or counting task, which critics suggest is biased by knowledge and beliefs about one’s heartbeat and is thus not a pure measure of behavioral accuracy (e.g., Desmedt et al., 2018; Murphy, Millgate, et al., 2018). Indeed, interoceptive ability metrics from the heartbeat counting vs. detection tasks are either weakly interrelated or unrelated (Hickman et al., 2020; Pennebaker & Hoover, 1984; Ring & Brener, 2018). In contrast, heartbeat detection studies—which better capture the behavioral ability to access heartbeat signals—report a mix of positive associations (Barrett et al., 2004 for arousal-focused emotions; Wiens et al., 2000), inverse associations (Barrett et al., 2004 for emotional intensity; Blascovich et al., 1992; Eichler et al., 1987), or null effects (Fairclough & Goodwin, 2007; Ferguson & Katkin, 1996) when linking interoceptive ability and emotion reports. These prior mixed effects, alongside our current findings with a larger sample size, call into question whether interoceptive ability necessarily corresponds to greater emotional arousal or whether this relation is more complex.
Indeed, divergent findings could be due to the degree of physiological arousal elicited by different kinds of affect inductions. The idea that interoceptive ability likely depends on one’s current physiological state is not new (Blascovich et al., 1992; Fairclough & Goodwin, 2007; Herbert et al., 2012), but this hypothesis remains underexamined. For example, positive associations between interoceptive ability and emotion are largely found in studies with affect inductions using images or film clips that may not robustly alter peripheral physiology relative to acute stress inductions (Eichler et al., 1987; Hantas et al., 1982; Pollatos et al., 2005, 2007). In such contexts where physiological changes are smaller, individuals with high interoceptive ability may be detecting more subtle, ambiguous changes to their physiology relative to individuals with poor interoceptive ability, affording more opportunity for highly interoceptive individuals to incorporate sensations into emotional experience. But during situations with robust physiological changes that are above the threshold of perception for most individuals (e.g., large changes in heart rate as occur during the TSST or a real-life intense stressor), the relationship between interoceptive ability and emotion may shift. Under robust physiological conditions, individuals with poorer interoceptive ability (i.e., who are less able to accurately perceive their heartbeat at rest) may now be able to detect physiological changes with greater accuracy and ease. On the other hand, higher interoceptive ability at rest may offer a different utility under robust physiological conditions: it may help some individuals better identify and regulate physiological responses earlier in the emotional time course, before physiological and emotional responses become too intense and difficult to manage. In this way, differences in physiological state (small vs. robust perturbations) could help explain prior mixed effects, such that interoceptive ability is positively related to emotional arousal under conditions of minimal physiological change but inversely related to emotional arousal under conditions of heightened physiological arousal. There may also be other third variable factors (e.g., adiposity, cardiovascular fitness, age, neuroticism, etc.) which could further complicate the extent to which resting vs. robust physiological activity moderate the interoceptive ability-emotion link.
More generally, inconsistencies across the interoception and emotion literature may be the product of low statistical power in past studies. We found that the effect of interoceptive ability with emotional experience was small, β = −.15. This suggests that many prior small sample studies using heartbeat counting or detection tasks in relation to emotion states are likely underpowered. Furthermore, both older and modern studies sometimes dichotomize samples into good vs. poor heartbeat perceivers, but dichotomization does not disentangle response tendencies and can lead to biases in parameter estimates, obscuring true effects (MacCallum et al., 2002; Preacher et al., 2005). We recruited a larger-than-typical sample and did not dichotomize in hopes of providing more reliable, robust effect estimates of the link between interoceptive ability and state emotion. We strongly recommend future studies using interoceptive behavioral tasks do the same in support of a more reliable interoceptive science.
Interoceptive Sensibility is Unrelated to Emotional States.
Interestingly, we did not observe a main effect of interoceptive sensibility on high arousal emotional experience (or negative, positive, or low arousal emotion for that matter; see SMs). These findings replicate other work showing no relation between interoceptive sensibility (as measured by the BAQ) and state emotion (Horváth et al., 2021; Lustyk et al., 2012; Zamariola, Luminet, et al., 2019). Previous work has mostly examined sensibility as a trait in the context of psychopathology, wellbeing, and health behaviors (Forkmann et al., 2019; Palser et al., 2018), suggesting that sensibility may be more relevant for trait emotion.
As with interoceptive ability, it is possible that interoceptive sensibility is less relevant for emotion under robust physiological conditions such as an acute stressor. This could be because interoceptive sensibility reflects beliefs about noticing or paying attention to interoceptive signals, which may be more useful in guiding individuals’ attention towards the body under conditions of calmer, less robust physiological arousal (e.g., low arousal or neutral states). Interoceptive sensibility may also be less relevant for emotion states because such tendencies say nothing about why individuals might be more vs. less motivated to notice their sensations. People could believe it is important to pay attention to their bodies for many reasons which then dictate how they interact with sensations. For example, some individuals may be hyper-vigilant and see interoceptive signals as dangerous or problematic. Other individuals may relish positively focusing on bodily sensations (e.g., exercising, taking a hot bath or shower, savoring certain kinds of comfort foods, etc.). Thus, sensibility—especially once clearly distinguished from more evaluative interoceptive beliefs—does not distinguish between these different attentional motivations (e.g., threatened vigilance vs. body/self-care) which may be especially relevant in the context of stressors. In contrast, evaluative interoceptive beliefs as measured herein help parse apart the underlying reasons why individuals might want to notice their sensations in the first place. Future studies should continue to distinguish interoceptive sensibility from other types of interoceptive beliefs and move beyond trait outcomes (e.g., trait anxiety) into extending observed effects in momentary experiences that involve varying degrees of physiological arousal (e.g., stressor vs. relaxation).
Positive Interoceptive Beliefs are Associated with Less Intense High Arousal Emotions.
Finally, the most robust interoceptive predictor of emotional arousal (and emotions more generally; see SMs) was interoceptive beliefs about the nature and meaning of internal bodily sensations. Specifically, more positive beliefs were related to less intense emotional arousal during the stressor, perhaps because participants who held more positive beliefs about their bodily states found sensations during the stressor to be less aversive on average than those who held more negative beliefs. Although evaluative beliefs about the body have been explored in research on mindfulness, anxiety sensitivity, panic disorders, eating disorders, and illness appraisals, this construct remains underexamined in the broader interoception literature and within non-clinical samples. Some work has examined the affective evaluations that people make of their heartbeats while completing interoceptive ability tasks (see discussions in Herbert & Pollatos, 2018; Pollatos & Herbert, 2018)—but it is presumably individuals’ a priori interoceptive beliefs that in part guide such interoceptive evaluations. We created the BSBQ to help address this gap.
Comparing Interoceptive Constructs as Moderators of Physiological and Emotional Arousal
Inspired by the hypothesis that interoception should help bridge physiological and emotional arousal (MacCormack, Henry, et al., 2021; Sze et al., 2010; Van Doren et al., 2021) and given broader constructionist hypotheses about interoceptive contributions to emotion construction (e.g., Barrett, 2017b, 2018; Lindquist, 2013; MacCormack & Lindquist, 2017), we compared the extent to which interoceptive ability, sensibility, and beliefs moderated concordance between physiological arousal (i.e., increases in SNS and decreases in PNS activity) and self-reported emotional arousal during the TSST. Interestingly, neither interoceptive ability (i.e., accuracy) nor sensibility moderated the link between physiological and emotional arousal. Only individuals’ interoceptive beliefs about the value and meaning of internal bodily signals was a significant moderator across our measures of physiological arousal.
First, the interaction of SNS reactivity and interoceptive beliefs suggests that individuals with more positive interoceptive beliefs experienced emotions that were more concordant with their ongoing physiological arousal. For instance, in the presence of low SNS reactivity, individuals with more positive and neutral interoceptive beliefs reported significantly less emotional arousal than individuals with negative beliefs. Yet in the presence of high SNS reactivity, individuals with positive and neutral interoceptive beliefs reported greater emotional arousal during the stressor. In contrast, individuals with negative interoceptive beliefs reported greater emotional arousal, regardless of their level of SNS activation. Insofar as individuals find bodily states to be dangerous and misleading, they may be more likely to ignore or direct attention away from bodily changes, weakening the concordance between physiological and emotional arousal when physiology is less robust. However, once SNS reactivity is more robust and difficult to ignore, individuals with negative beliefs again show concordance between physiological and emotional arousal comparable to individuals with positive and neutral beliefs. It is also possible that individuals who find their interoceptive states dangerous or misleading instead focus more on their external sensations to inform their emotions. Since the TSST manipulation was identical for all participants, this may explain why individuals with negative interoceptive beliefs reported comparable levels of high arousal emotions regardless of their SNS reactivity.
We observed complementary yet distinct findings in the context of PNS (HRV) reactivity. HRV, when appropriately adjusted and measured, can index PNS influence on the heart (Berntson et al., 1997; de Geus et al., 2019). When PNS-mediated cardiac control is high, such as during rest, the length between heartbeats becomes more variable. In contrast, when PNS influence is low (i.e., parasympathetic withdrawal), the length between heartbeats becomes more consistent. We found that when individuals had decreases in HRV (i.e., more consistent compared to baseline, indicating parasympathetic withdrawal during the stressor), individuals with negative interoceptive beliefs reported significantly more emotional arousal than their counterparts with average and positive beliefs. This may suggest that negative interoceptive beliefs may promote greater concordance between physiological and emotional arousal in the presence of greater PNS withdrawal. However, in the context of higher HRV (i.e., indicative of greater parasympathetic control over the heart), individuals reported similar levels of emotional arousal regardless of the nature of their interoceptive beliefs. These findings again suggest that positive beliefs may be more adaptive than negative beliefs insofar as negative beliefs may exacerbate the effect of parasympathetic withdrawal on high arousal emotion, whereas more positive and neutral beliefs may buffer against parasympathetic withdrawal effects. The fact that we found somewhat different patterns for SNS vs. PNS reactivity are consistent with evidence that individuals differ in the independence of SNS and PNS activity (Berntson et al., 2008). Future work could more deeply examine how the SNS-PNS relation (e.g., whether they are independent, inverse, or coactive) may interact with interoceptive beliefs.
Strengths, Limitations, Constraints on Generality, and Future Directions
The present study offers several strengths. For example, our sample used strict physical and mental health prescreening with a balanced sex distribution that together help rule out some alternate interpretations. However, by focusing on a healthy, young adult sample, it remains unclear whether observed findings will generalize to other populations (e.g., middle aged or older adults, individuals with health conditions or mood disorders) or other geographies and cultures. Similarly, this study best speaks to the role of interoception during times of higher physiological arousal—such as during acute psychosocial pressure and performance. Findings may replicate across other similarly evocative situations—such as an aggressive confrontation—but could differ in the context of positive events (e.g., an exciting win, enjoyable social activities) or low arousal, more neutral situations with less overt physiological changes (e.g., relaxing on the couch, a calm day at the office).
One study strength was sample size. Most interoception studies—especially those examining interoceptive ability—have historically relied on small samples which are likely underpowered to detect the likely small effects (as observed herein) for interoceptive ability and emotion. Samples with heartbeat detection and emotion typically range between Ns=10–60 (Blascovich et al., 1992; Critchley et al., 2004; Eichler et al., 1987; Fairclough & Goodwin, 2007; Ferguson & Katkin, 1996; Lyyra & Parviainen, 2018; Montgomery & Jones, 1984; Wiens et al., 2000). Thus, effect size estimates herein may provide an updated index of the magnitude in the relations between heartbeat detection and emotion.
Although we found that interoceptive beliefs was the most robust interoceptive predictor of emotions from the acute stressor, this effect size could be artificially inflated due to shared measurement variance between self-reported beliefs and self-reported emotions. It is a longstanding observation in behavioral science that behavior and self-report are often poorly correlated (Dang et al., 2020; Guassi Moreira et al., 2020), which may explain the relatively small (albeit still significant) effect size linking interoceptive ability and self-reported emotion. The strongest comparisons however can be drawn between interoceptive sensibility vs. beliefs, given that both constructs were measured using self-reports. The fact that interoceptive beliefs was a significant, more robust cross-sectional predictor of emotion self-reports relative to sensibility—regardless of whether sensibility was measured as a factor score or as the BAQ vs. MAIA on their own (see SMs)—suggests that evaluative interoceptive beliefs may capture important aspects of interoception relevant for the experience of acute stress and emotion.
Similarly, while we assessed physiological activity with SNS and PNS markers, these measures do not index broader cardiovascular changes reflecting challenge vs. threat dimensions of acute stress (Seery, 2013; Tomaka et al., 1993). Future work should examine how interoceptive beliefs alongside ability and sensibility map onto the physiological and subjective dimensions of challenge vs. threat. For example, we would hypothesize that positive interoceptive beliefs might support challenge responses (e.g., lower total peripheral resistance, higher cardiac output as physiological resources easily move to peripheral organs) while negative interoceptive beliefs might relate to threat responses (e.g., higher total peripheral resistance, lower cardiac output as physiological resources are conserved in the body’s core).
Another potential limitation is that emotion reports were measured immediately after rather than throughout the TSST, opening the possibility that the emotion reports reflect summative post-hoc recollections of the prior 15-minutes of stressful tasks. Yet asking participants to report their emotions throughout a task can alter associated physiological reactions (Kassam & Mendes, 2013), while other evidence suggests that labeling one’s state with emotion words serve a regulatory function (Torre & Lieberman, 2018). To avoid such effects, we decided to administer the PANAS immediately after the TSST ended (< 1 minute), in hopes of balancing concerns about induction interference or implicit emotion regulation effects with concerns about retrospective memory bias about emotional experiences. Importantly, work on retrospective memory bias suggests that emotion reports tend to reflect either the peak or end of an experience (Fredrickson & Kahneman, 1993). Given that we measured physiological reactivity as the difference in magnitude between baseline and changes averaged across TSST Prep, Speech, and Math, our physiological and emotion measurements may capture conceptually similar variance. Nonetheless, other person-level moderators (e.g., emotion beliefs, reporting style) could have influenced the intensity and types of emotions reported at post-TSST as compared to if we had measured momentary fluctuations in emotion reports throughout the TSST.
Finally, our study was quasi-experimental: we measured interoceptive ability, sensibility, and beliefs as trait-like measures in a first session, then manipulated physiological and emotion arousal within-subjects in a second session. As such, these findings cannot speak to the claim that physiological systems and interoception are causally necessary or sufficient for an emotional experience to occur. However, we point to other experimental evidence implicating both physiological changes and beliefs in shaping instances of emotion which could be combined in future experiments to assess causality. For example, as reviewed above, manipulating neurophysiological states and pathways, such as inducing hunger or a robust inflammatory state can alter subsequent emotional states (Harrison et al., 2009; MacCormack & Lindquist, 2019). Even healthy aging may impact emotion by altering interoception and related peripheral and central nervous system functioning (Levenson et al., 1991; MacCormack, Stein, et al., 2020; MacCormack, Henry, et al., 2021; Mendes, 2010). In parallel, experimentally manipulating beliefs (via appraisals) can alter emotions. Temporarily manipulating interoceptive beliefs is consistent with existing literature on arousal reappraisal during a stressor (Jamieson et al., 2013, 2018). Individuals who are taught to reappraise their stress-related arousal as adaptive during times of stress (e.g., an interview, a standardized exam, a sports game, etc.) tend to exhibit more challenge-oriented (rather than threat-oriented) physiological changes, report less negative emotions and more adaptive appraisals, and sometimes even perform better during stressors (Jacquart et al., 2020; Jamieson et al., 2010, 2012, 2016; Sammy et al., 2017). These effects contrast with effects for individuals asked to reappraise their arousal as a hindrance or in contrast to those completing the stressor normally (i.e., control group).
Although we did not examine whether interoceptive beliefs interact with interoceptive ability or sensibility, testing such interactions may prove fruitful for considering the temporal dynamics via which different interoceptive constructs can impact emotional awareness, experience, and regulation. For example, accuracy in interoceptive ability may set an important perceptual threshold by which afferent physiological signals become accessible and conscious to individuals, allowing them to act on and regulate physiology, feelings, and behavior accordingly. In contexts with low or neutral physiological activity and/or poor interoceptive ability, there is however greater potential ambiguity or uncertainty in how individuals make meaning of neurophysiological signals. In these instances, interoceptive beliefs may help “fill in the gaps,” imputing valenced meaning onto more ambiguous physiological states. Similarly, while interoceptive ability may help individuals become more aware of physiological changes related to their emotional or stress states, this may only be a first step in how people ultimately construct and navigate such states. After becoming aware of emotion- or tress-relevant physiological changes, interoceptive beliefs may add a layer of valenced meaning (i.e., “Are these bodily changes I’m feeling good vs. bad for me?”) which can in turn facilitate or hinder regulatory efforts in emotion and stress optimization. Altogether, it is likely that interoceptive accuracy and beliefs interact to predict emotion and stress experiences and regulation, a possibility that should be examined in future.
The role of interoceptive beliefs in linking physiological and emotional arousal could also have important consequences for wellbeing and health. For example, although with our sample, we could not examine the implications of interoceptive beliefs for mental health, past literature finds that individuals with anxiety sensitivity tend to have negative interoceptive beliefs, i.e., they catastrophize their physiological sensations and find them distressing (Eley et al., 2007; Olthuis et al., 2012). In contrast, positive interoceptive beliefs may help promote wellbeing. Indeed, we found that positive interoceptive beliefs was associated with greater concordance between physiology and self-reported emotion—and this concordance has been associated with improved wellbeing and health in other studies (Brown et al., 2019; Sommerfeldt et al., 2019). Positive interoceptive beliefs may promote this concordance by helping individuals more adaptively respond to and manage their physiological and emotional arousal.
Conclusions
In sum, we found that interoceptive beliefs moderated the relation between physiological and emotional arousal during an acute stressor, consistent with constructionist approaches to emotion which argue that predictions about the meaning of interoceptive signals should contribute to the construction of emotional experiences and perceptions (Barrett, 2017b, 2018; Barrett & Simmons, 2015; MacCormack & Lindquist, 2017, 2019). Individuals with positive beliefs had greater coherence between SNS-related physiological arousal and emotional arousal—consistent with evidence that greater coherence may support wellbeing and health (Brown et al., 2019; Sommerfeldt et al., 2019). Collectively, this work suggests that interoceptive beliefs may serve as priors that shape how people experience ongoing physiological arousal and translate that physiology to emotional arousal. Although past work has emphasized the importance of interoceptive ability for emotion states, there is growing recognition that interoceptive “predictions”—i.e., beliefs, schemas, mindsets, and other meta-cognitive inferences about interoceptive sensations, symptoms, arousal, and stress—may be powerful determinants of emotion, wellbeing, and health (Barrett, 2017b, 2018; Barrett & Simmons, 2015; Crum et al., 2013; Heathcote et al., 2021; Legrand et al., 2022; Paulus et al., 2019; Rouault et al., 2018; Yoris et al., 2015).
In constructionist models, interoceptive predictions derive from both idiographic experience and social transmission (e.g., garnered from upbringing, folk wisdom, cultural norms). This hypothesis is in line with models of knowledge and belief acquisition from cognitive and developmental science (Fotopoulou & Tsakiris, 2017; Hoemann & Barrett, 2019; Lindquist et al., 2015; Xu & Griffiths, 2011). One area of future research could be to understand how interoceptive beliefs develop in the first place and what implications these socialized beliefs have for downstream health and wellbeing. For example, mothers’ tendency to link interoceptive sensations to emotion predicts their children’s improved social skills and emotion regulation (MacCormack, Castro, et al., 2020), suggesting that caregivers’ understanding of and beliefs about interoceptive sensations are important yet underexamined socialization pathways. Beyond early life, concepts and beliefs about the relevance of interoceptive sensations for emotions, especially high arousal emotions, appear to differ across adulthood into later life (MacCormack, Henry, et al., 2021)—whether this be due to cohort differences in emotion knowledge or potentially even maturational changes in peripheral and central nervous system structure and function.
Ultimately, we hope that other researchers will see this paper as a call to action in two areas. First, future research must continue carefully disentangling the complicated interrelations between physiology, interoception, and emotion, be that through experimental or pharmacological manipulations, ambulatory and experience sampling approaches, or lifespan development approaches. Second, this paper highlights the potential promise and deep need for work documenting and delineating the variety of interoceptive beliefs that individuals may develop and hold—whether this be beliefs about one’s accuracy in perceiving interoceptive changes, beliefs about one’s tendency to be interoceptively attuned, or more evaluative beliefs about the value/danger, intensity, and management of interoceptive sensations as initially examined herein. The time has come for interoceptive scientists across fields to build a richer understanding of interoceptive beliefs and the extent of their importance for emotion, stress, and health across the lifespan.
Supplementary Material
Acknowledgements
During the completion of this project and manuscript, JKM received support from a Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship from the National Institute on Aging (1F31AG055265-01A1) at the University of North Carolina at Chapel Hill as well as a postdoctoral fellowship from the National Heart, Lung, and Blood Institute (5T32HL007560-37; PIs: Drs. Peter Gianaros and Rebecca Thurston) via the University of Pittsburgh Department of Psychiatry Cardiovascular Behavioral Medicine T32 Program. We would also like to acknowledge the many outstanding Carolina Affective Science Laboratory research assistants who helped collect participant data and served as TSST interviewers throughout this project’s duration.
Footnotes
OSF Repository Link: https://osf.io/z7c2a/
Occasionally, sensibility is used to refer to individuals’ confidence or self-reported belief about how accurate their interoceptive ability is, as rated in heartbeat detection or tracking tasks (e.g., Forkmann et al., 2016; Mai-Lippold et al., 2020). See the Supplementary Materials (SMs) for supplementary analyses and discussion that show behavioral confidence and MAIA/BAQ self-reports are unrelated in the present dataset.
Others have recently used the term “interoceptive beliefs” to refer to people’s perceptions about how fast their hearts are beating during interoceptive tasks (Legrand et al., 2022). Such beliefs likely draw on knowledge about one’s typical heart rate, confidence in one’s interoceptive ability, etc.
References
- Arnold AJ, Winkielman P, & Dobkins K (2019). Interoception and social connection. Frontiers in Psychology, 10, 2589. 10.3389/fpsyg.2019.02589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Gao W, Fradkin I, & Barrett LF (2018). Growing a social brain. Nature Human Behaviour, 2, 624–636. 10.1038/s41562-018-0384-6 [DOI] [PubMed] [Google Scholar]
- Barrett LF (2006). Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review : An Official Journal of the Society for Personality and Social Psychology, Inc, 10(1), 20–46. 10.1207/s15327957pspr1001_2 [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017a). How emotions are made: The secret life of the brain. Houghton Mifflin Harcourt. [Google Scholar]
- Barrett LF (2017b). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12, 1–23. 10.1093/scan/nsw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2018). Emotions are constructed with interoception and concepts within a predicting brain. In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion: Fundamental questions (pp. 33–38). Oxford University Press. [Google Scholar]
- Barrett LF, & Bliss-Moreau E (2009). Affect as a psychological primitive. In Zanna MP (Ed.), Advances in Experimental Social Psychology (pp. 167–218). Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Bliss-Moreau E, & Aronson KR (2004). Interoceptive sensitivity and self-reports of emotional experience. Journal of Personality and Social Psychology, 87, 684–697. 10.1037/0022-3514.87.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Russell JA (1999). The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science, 8, 10–14. 10.1111/1467-8721.00003 [DOI] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16, 419–429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, & Van der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30, 183–196. 10.1111/j.1469-8986.1993.tb01731.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45, 643–652. 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW (2008). Noradrenergic modulation of arousal. Brain Research Reviews, 58, 1–17. 10.1016/j.brainresrev.2007.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J, Brennan K, Tomaka J, Kelsey RM, Hughes P, Coad ML, & Adlin R (1992). Affect intensity and cardiac arousal. Journal of Personality and Social Psychology, 63, 164–174. 10.1037/0022-3514.63.1.164 [DOI] [PubMed] [Google Scholar]
- Bollen KA (1989). Structural equations with latent variables. Wiley. [Google Scholar]
- Bollen KA (2002). Latent variables in psychology and the social sciences. Annual Review of Psychology, 53, 605–634. 10.1146/annurev.psych.53.100901.135239 [DOI] [PubMed] [Google Scholar]
- Bonar AS, MacCormack JK, Feldman MJ, Inagaki TK, & Lindquist KA (2021). Assessing the role of interoceptive beliefs in emotion: Development of the Body Signal Beliefs Questionnaire (BSBQ). Affective Science. https://osf.io/z75gn/ [Google Scholar]
- Brener J, & Kluvitse C (1988). Heartbeat detection: Judgments of the simultaneity of external stimuli and heartbeats. Psychophysiology, 25, 554–561. 10.1111/j.1469-8986.1988.tb01891.x [DOI] [PubMed] [Google Scholar]
- Brener J, Liu X, & Ring C (1993). A method of constant stimuli for examining heartbeat detection: Comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology, 30, 657–665. 10.1111/j.1469-8986.1993.tb02091.x [DOI] [PubMed] [Google Scholar]
- Brown CL, Van Doren N, Ford BQ, Mauss IB, Sze JW, & Levenson RW (2019). Coherence between subjective experience and physiology in emotion: Individual differences and implications for well-being. Emotion, epub ahead of print. 10.1037/emo0000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, & Fieldstone A (1994). Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology, 31, 586–598. 10.1111/j.1469-8986.1994.tb02351.x [DOI] [PubMed] [Google Scholar]
- Calì G, Ambrosini E, Picconi L, Mehling WE, & Committeri G (2015). Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Frontiers in Psychology, 6, 1202. 10.3389/fpsyg.2015.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG (2001). Visceral sensory neuroscience: Interoception. Oxford University Press. [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37, 1111–1134. 10.1016/j.psyneuen.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Caneiro JP, Bunzli S, & O’Sullivan P (2021). Beliefs about the body and pain: The critical role in musculoskeletal pain management. Brazilian Journal of Physical Therapy, 25(1), 17–29. 10.1016/j.bjpt.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB (1927). The James-Lange theory of emotions: A critical examination and an alternative theory. The American Journal of Psychology, 39, 106. 10.2307/1415404 [DOI] [PubMed] [Google Scholar]
- Craig AD (2003). Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology, 13, 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, & Meehl PE (1955). Construct validity in psychological tests. Psychological Bulletin, 52, 281–302. 10.1037/h0040957 [DOI] [PubMed] [Google Scholar]
- Crum AJ, Akinola M, Martin A, & Fath S (2017). The role of stress mindset in shaping cognitive, emotional, and physiological responses to challenging and threatening stress. Anxiety, Stress, & Coping, 30, 379–395. 10.1080/10615806.2016.1275585 [DOI] [PubMed] [Google Scholar]
- Crum AJ, Salovey P, & Achor S (2013). Rethinking stress: The role of mindsets in determining the stress response. Journal of Personality and Social Psychology, 104, 716–733. 10.1037/a0031201 [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Patton M, Schulte F, Richardson PA, & Heathcote LC (2021). Worry about somatic symptoms as a sign of cancer recurrence: Prevalence and associations with fear of recurrence and quality of life in survivors of childhood cancer. Psycho-Oncology, 30, 1077–1085. 10.1002/pon.5647 [DOI] [PubMed] [Google Scholar]
- Dang J, King KM, & Inzlicht M (2020). Why are self-report and behavioral measures weakly correlated? Trends in Cognitive Sciences, 24, 267–269. 10.1016/j.tics.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cort K, Hermans D, Noortman D, Arends W, Griez EJL, & Schruers KRJ (2013). The weight of cognitions in panic: The link between misinterpretations and panic attacks. PloS One, 8(8), e70315. 10.1371/journal.pone.0070315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, & Berntson GG (2019). Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology, 56, e13287. 10.1111/psyp.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon B, & Abramowitz J (2006). Anxiety sensitivity and its dimensions across the anxiety disorders. Journal of Anxiety Disorders, 20, 837–857. 10.1016/j.janxdis.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35, 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt O, Luminet O, & Corneille O (2018). The heartbeat counting task largely involves non-interoceptive processes: Evidence from both the original and an adapted counting task. Biological Psychology, 138, 185–188. 10.1016/j.biopsycho.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, & Dalgleish T (2010). Listening to your heart: How interoception shapes emotion experience and intuitive decision making. Psychological Science, 21, 1835–1844. 10.1177/0956797610389191 [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, & Dalgleish T (2010). Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy, 48(11), 1133–1138. 10.1016/j.brat.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlik C, Brown G, & Tsakiris M (2014). Enhanced interoceptive awareness during anticipation of public speaking is associated with fear of negative evaluation. Cognition & Emotion, 28(3), 530–540. 10.1080/02699931.2013.832654 [DOI] [PubMed] [Google Scholar]
- Ehlers A, & Breuer P (1992). Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology, 101, 371–382. 10.1037/0021-843X.101.3.371 [DOI] [PubMed] [Google Scholar]
- Eichler S, Katkin ES, Blascovich J, & Kelsey RM (1987). Cardiodynamic factors in heartbeat detection and the experience of emotion. Psychophysiology, 24, 587. [Google Scholar]
- Eley TC, Gregory AM, Clark DM, & Ehlers A (2007). Feeling anxious: A twin study of panic/somatic ratings, anxiety sensitivity and heartbeat perception in children. Journal of Child Psychology and Psychiatry, 48, 1184–1191. 10.1111/j.1469-7610.2007.01838.x [DOI] [PubMed] [Google Scholar]
- Fairclough SH, & Goodwin L (2007). The effect of psychological stress and relaxation on interoceptive accuracy: Implications for symptom perception. Journal of Psychosomatic Research, 62(3), 289–295. 10.1016/j.jpsychores.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, Klein AC, Paulus MP, & Mehling WE (2015). Interoception, contemplative practice, and health. Frontiers in Psychology, 6, 763. 10.3389/fpsyg.2015.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Feldman PJ, Cohen S, Lepore SJ, Matthews KA, Kamarck TW, & Marsland AL (1999). Negative emotions and acute physiological responses to stress. Annals of Behavioral Medicine, 21, 216–222. 10.1007/BF02884836 [DOI] [PubMed] [Google Scholar]
- Ferentzi E, Drew R, Tihanyi BT, & Köteles F (2018). Interoceptive accuracy and body awareness – Temporal and longitudinal associations in a non-clinical sample. Physiology & Behavior, 184, 100–107. 10.1016/j.physbeh.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Ferguson ML, & Katkin ES (1996). Visceral perception, anhedonia, and emotion. Biological Psychology, 42(1–2), 131–145. 10.1016/0301-0511(95)05151-1 [DOI] [PubMed] [Google Scholar]
- Fisher E, Heathcote LC, Eccleston C, Simons LE, & Palermo TM (2018). Assessment of pain anxiety, pain catastrophizing, and fear of pain in children and adolescents with chronic pain: A systematic review and meta-analysis. Journal of Pediatric Psychology, 43, 314–325. 10.1093/jpepsy/jsx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkmann T, Scherer A, Meessen J, Michal M, Schächinger H, Vögele C, & Schulz A (2016). Making sense of what you sense: Disentangling interoceptive awareness, sensibility and accuracy. International Journal of Psychophysiology, 109, 71–80. 10.1016/j.ijpsycho.2016.09.019 [DOI] [PubMed] [Google Scholar]
- Forkmann T, Volz-Sidiropoulou E, Helbing T, Drüke B, Mainz V, Rath D, Gauggel S, & Teismann T (2019). Sense it and use it: Interoceptive accuracy and sensibility in suicide ideators. BMC Psychiatry, 19, 334. 10.1186/s12888-019-2322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulou A, & Tsakiris M (2017). Mentalizing homeostasis: The social origins of interoceptive inference. Neuropsychoanalysis, 19, 3–28. 10.1080/15294145.2017.1294031 [DOI] [Google Scholar]
- Fredrickson BL, & Kahneman D (1993). Duration neglect in retrospective evaluations of affective episodes. Journal of Personality and Social Psychology, 65(1), 45–55. 10.1037/0022-3514.65.1.45 [DOI] [PubMed] [Google Scholar]
- Fuller EL, & Hemmerle WJ (1966). Robustness of the maximum-likelihood estimation procedure in factor analysis. Psychometrika, 31, 255–266. 10.1007/BF02289512 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, & Critchley HD (2013). Interoception, emotion and brain: New insights link internal physiology to social behaviour. Social Cognitive and Affective Neuroscience, 8, 231–234. 10.1093/scan/nss140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, & Critchley HD (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, & Critchley HD (2016). Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biological Psychology, 114, 117–126. 10.1016/j.biopsycho.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Garrett-Peters PT, Castro VL, & Halberstadt AG (2017). Parents’ beliefs about children’s emotions, children’s emotion understanding, and classroom adjustment in middle childhood. Social Development, 26, 575–590. 10.1111/sode.12222 [DOI] [Google Scholar]
- Gendron M, Mesquita B, & Barrett LF (2020). The brain as a cultural artifact. In Kirmayer LJ, Worthman CM, Kitayama S, Lemelson R, & Cummings CA (Eds.), Culture, Mind, and Brain: Emerging Concepts, Models, and Applications (pp. 188–222). Cambridge University Press. [Google Scholar]
- Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, & de Rooij SR (2014). Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology, 48, 87–97. 10.1016/j.psyneuen.2014.05.023 [DOI] [PubMed] [Google Scholar]
- Golland Y, Keissar K, & Levit-Binnun N (2014). Studying the dynamics of autonomic activity during emotional experience. Psychophysiology, 51, 1101–1111. 10.1111/psyp.12261 [DOI] [PubMed] [Google Scholar]
- Guassi Moreira JF, Sahi R, Ninova E, Parkinson C, & Silvers JA (2020). Performance and belief-based emotion regulation capacity and tendency: Mapping links with cognitive flexibility and perceived stress. Emotion, epub ahead. 10.1037/emo0000768 [DOI] [PubMed] [Google Scholar]
- Hanley AW, Mehling WE, & Garland EL (2017). Holding the body in mind: Interoceptive awareness, dispositional mindfulness and psychological well-being. Journal of Psychosomatic Research, 99, 13–20. 10.1016/j.jpsychores.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantas M, Katkin ES, & Blascovich J (1982). Relationship between heartbeat discrimination and subjective experience of affective state [Abstract]. Psychophysiology, 19, 563. [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, & Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66, 407–414. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote LC, Loecher N, Simon P, Spunt SL, Jordan A, Tutelman PR, Cunningham S, Schapira L, & Simons LE (2021). Symptom appraisal in uncertainty: A theory-driven thematic analysis with survivors of childhood cancer. Psychology & Health, 36, 1182–1199. 10.1080/08870446.2020.1836180 [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, & White PO (1964). Promax: A quick method for rotation to oblique simple structure. British Journal of Statistical Psychology, 17, 65–70. 10.1111/j.2044-8317.1964.tb00244.x [DOI] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O, Weimer K, Enck P, Sauer H, & Zipfel S (2012). Effects of short-term food deprivation on interoceptive awareness, feelings and autonomic cardiac activity. Biological Psychology, 89(1), 71–79. 10.1016/j.biopsycho.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Herbert BM, & Pollatos O (2018). The relevance of interoception for eating behavior and eating disorders. In Tsakiris M & De Preester H (Eds.), The interoceptive mind: From homeostasis to awareness (pp. 165–186). Oxford University Press. 10.1093/oso/9780198811930.003.0009 [DOI] [Google Scholar]
- Herbert BM, Pollatos O, Flor H, Enck P, & Schandry R (2010). Cardiac awareness and autonomic cardiac reactivity during emotional picture viewing and mental stress. Psychophysiology, 47(2), 342–354. 10.1111/j.1469-8986.2009.00931.x [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, & Schandry R (2007). Interoceptive sensitivity and emotion processing: An EEG study. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 65(3), 214–227. 10.1016/j.ijpsycho.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Hickman L, Seyedsalehi A, Cook JL, Bird G, & Murphy J (2020). The relationship between heartbeat counting and heartbeat discrimination: A meta-analysis. Biological Psychology, 156, 107949. 10.1016/j.biopsycho.2020.107949 [DOI] [PubMed] [Google Scholar]
- Hoemann K, & Barrett LF (2019). Concepts dissolve artificial boundaries in the study of emotion and cognition, uniting body, brain, and mind. Cognition and Emotion, 33, 67–76. 10.1080/02699931.2018.1535428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth Á, Vig L, Ferentzi E, & Köteles F (2021). Cardiac and proprioceptive accuracy are not related to body awareness, perceived body competence, and affect. Frontiers in Psychology, 11, 575574. 10.3389/fpsyg.2020.575574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquart J, Papini S, Freeman Z, Bartholomew JB, & Smits JAJ (2020). Using exercise to facilitate arousal reappraisal and reduce stress reactivity: A randomized controlled trial. Mental Health and Physical Activity, 18, 100324. 10.1016/j.mhpa.2020.100324 [DOI] [Google Scholar]
- James W (1884). What is an emotion? Mind, 34, 188–205. [Google Scholar]
- Jamieson JP, Hangen EJ, Lee HY, & Yeager DS (2018). Capitalizing on appraisal processes to improve affective responses to social stress. Emotion Review, 10, 30–39. 10.1177/1754073917693085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, Blackstock E, & Schmader T (2010). Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology, 46(1), 208–212. 10.1016/j.jesp.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, & Nock MK (2013). Improving acute stress responses: The power of reappraisal. Current Directions in Psychological Science, 22(1), 51–56. 10.1177/0963721412461500 [DOI] [Google Scholar]
- Jamieson JP, Nock MK, & Mendes WB (2012). Mind over matter: Reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology. General, 141(3), 417–422. 10.1037/a0025719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Peters BJ, Greenwood EJ, & Altose AJ (2016). Reappraising stress arousal improves performance and reduces evaluation anxiety in classroom exam situations. Social Psychological and Personality Science, 7, 579–587. 10.1177/1948550616644656 [DOI] [Google Scholar]
- Kassam KS, & Mendes WB (2013). The effects of measuring emotion: Physiological reactions to emotional situations depend on whether someone is asking. PloS One, 8(7), e64959. 10.1371/journal.pone.0064959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katkin ES, Morell MA, Goldband S, Bernstein GL, & Wise JA (1982). Individual differences in heartbeat discrimination. Psychophysiology, 19, 160–166. 10.1111/j.1469-8986.1982.tb02538.x [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, Meuret AE, Nemeroff CB, Oppenheimer S, Petzschner FH, Pollatos O, Rhudy JL, Schramm LP, Simmons WK, Stein MB, … Zucker N (2018). Interoception and mental health: A roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann NK, & Werner NS (2014a). Cardiac perception enhances stress experience. Journal of Psychophysiology, 28, 225–232. 10.1027/0269-8803/a000114 [DOI] [Google Scholar]
- Kindermann NK, & Werner NS (2014b). The impact of cardiac perception on emotion experience and cognitive performance under mental stress. Journal of Behavioral Medicine, 37, 1145–1154. 10.1007/s10865-014-9564-7 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Wormwood JB, Simmons WK, Barrett LF, & Quigley KS (2015). Methodological recommendations for a heartbeat detection-based measure of interoceptive sensitivity. Psychophysiology, 52, 1432–1440. 10.1111/psyp.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, & Barrett LF (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P, Tuerlinckx F, Russell JA, & Barrett LF (2013). The relation between valence and arousal in subjective experience. Psychological Bulletin, 139, 917–940. 10.1037/a0030811 [DOI] [PubMed] [Google Scholar]
- Kupper N, Jankovic M, & Kop WJ (2021). Individual differences in cross-system physiological activity at rest and in response to acute social stress. Psychosomatic Medicine, 83, 138–148. 10.1097/PSY.0000000000000901 [DOI] [PubMed] [Google Scholar]
- Lattimore P, Mead BR, Irwin L, Grice L, Carson R, & Malinowski P (2017). ‘I can’t accept that feeling’: Relationships between interoceptive awareness, mindfulness and eating disorder symptoms in females with, and at-risk of an eating disorder. Psychiatry Research, 247, 163–171. 10.1016/j.psychres.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Lavoie KL, Miller SB, Conway M, & Fleet RP (2001). Anger, negative emotions, and cardiovascular reactivity during interpersonal conflict in women. Journal of Psychosomatic Research, 51(3), 503–512. 10.1016/S0022-3999(01)00217-3 [DOI] [PubMed] [Google Scholar]
- Legrand N, Nikolova N, Correa C, Brændholt M, Stuckert A, Kildahl N, Vejlø M, Fardo F, & Allen M (2022). The heart rate discrimination task: A psychophysical method to estimate the accuracy and precision of interoceptive beliefs. Biological Psychology, 168, 108239. 10.1016/j.biopsycho.2021.108239 [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, & Ekman P (1991). Emotion, physiology, and expression in old age. Psychology and Aging, 6, 28–35. [DOI] [PubMed] [Google Scholar]
- Lindquist KA (2013). Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emotion Review, 5(4), 356–368. 10.1177/1754073913489750 [DOI] [Google Scholar]
- Lindquist KA, & Barrett LF (2012). A functional architecture of the human brain: Emerging insights from the science of emotion. Trends in Cognitive Sciences, 16, 533–540. 10.1016/j.tics.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, MacCormack JK, & Shablack H (2015). The role of language in emotion: Predictions from psychological constructionism. Frontiers in Psychology, 6, 1–15. 10.3389/fpsyg.2015.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, & Vincent AS (2012). Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry, 71, 344–349. 10.1016/j.biopsych.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustyk MKB, Douglas HAC, Bentley JA, & Gerrish WG (2012). Cardiovascular responses to a laboratory stressor in women: Assessing the role of body awareness. Body, Movement and Dance in Psychotherapy, 7, 55–70. 10.1080/17432979.2011.617522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyyra P, & Parviainen T (2018). Behavioral inhibition underlies the link between interoceptive sensitivity and anxiety-related temperamental traits. Frontiers in Psychology, 9, 1026. 10.3389/fpsyg.2018.01026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, & Rucker DD (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7, 19–40. 10.1037/1082-989X.7.1.19 [DOI] [PubMed] [Google Scholar]
- MacCormack JK, Armstrong-Carter EL, Gaudier-Diaz MM, Meltzer-Brody S, Sloan EK, Lindquist KA, & Muscatell KA (2021). Beta-adrenergic contributions to emotion and physiology during acute psychosocial stress. Psychosomatic Medicine, 83, 959–968. 10.1097/PSY.0000000000001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormack J, Bonar AS, & Lindquist KA (2022, April 11). Interoceptive beliefs in acute stress. Retrieved from osf.io/z7c2a
- MacCormack JK, Castro VL, Halberstadt AG, & Rogers ML (2020). Mothers’ interoceptive knowledge predicts children’s emotion regulation and social skills in middle childhood. Social Development, 29, 578–599. 10.1111/sode.12418 [DOI] [Google Scholar]
- MacCormack JK, Henry TR, Davis BM, Oosterwijk S, & Lindquist KA (2021). Aging bodies, aging emotions: Interoceptive differences in emotion representations and self-reports across adulthood. Emotion, 21, 227–246. 10.1037/emo0000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormack JK, & Lindquist KA (2017). Bodily contributions to emotion: Schachter’s legacy for a psychological constructionist view on emotion. Emotion Review, 9, 36–45. 10.1177/1754073916639664 [DOI] [Google Scholar]
- MacCormack JK, & Lindquist KA (2019). Feeling hangry? When hunger is conceptualized as emotion. Emotion, 19, 301–319. 10.1037/emo0000422 [DOI] [PubMed] [Google Scholar]
- MacCormack JK, Stein AG, Giovanello KS, Kang J, Satpute AB, & Lindquist KA (2020). Affect in the aging brain: A neuroimaging meta-analysis of functional activation and connectivity differences in older vs. Younger adult affective experience and perception. Affective Science, 1, 128–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai-Lippold SA, Dettlinger CM, Khalsa SS, & Pollatos O (2020). A pilot study on the effect of an energy drink on interoception in high vs. Low anxiety sensitivity individuals. European Journal of Health Psychology, 27, 171–187. 10.1027/2512-8442/a000061 [DOI] [Google Scholar]
- Manuck SB, Kasprowicz AL, Monroe SM, Larkin KT, & Kaplan JR (1989). Psychophysiologic reactivity as a dimension of individual differences. In Handbook of Research Methods in Cardiovascular Behavioral Medicine (pp. 365–382). Springer; US. 10.1007/978-1-4899-0906-0_23 [DOI] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion (Washington, D.C.), 5(2), 175–190. 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- Mauss IB, & Robinson MD (2009). Measures of emotion: A review. Cognition & Emotion, 23(2), 209–237. 10.1080/02699930802204677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Kneeland ET, Edwards RR, Jamison R, & Weiss RD (2019). Pain catastrophizing and distress intolerance: Prediction of pain and emotional stress reactivity. Journal of Behavioral Medicine, epub ahead. 10.1007/s10865-019-00086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling, Daubenmier J, Price, Acree, Bartmess, & Stewart. (2013). Self-reported interoceptive awareness in primary care patients with past or current low back pain. Journal of Pain Research, 6, 403. 10.2147/JPR.S42418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, & Stewart A (2012). The Multidimensional Assessment of Interoceptive Awareness (MAIA). PloS One, 7(11), e48230. 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB (2010). Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review, 2, 240–244. 10.1177/1754073910364149 [DOI] [Google Scholar]
- Merwin RM, Zucker NL, Lacy JL, & Elliott CA (2010). Interoceptive awareness in eating disorders: Distinguishing lack of clarity from non-acceptance of internal experience. Cognition & Emotion, 24, 892–902. 10.1080/02699930902985845 [DOI] [Google Scholar]
- Montgomery WA, & Jones GE (1984). Laterality, emotionality, and heartbeat perception. Psychophysiology, 21, 459–465. 10.1111/j.1469-8986.1984.tb00227.x [DOI] [PubMed] [Google Scholar]
- Murphy J, Brewer R, Hobson H, Catmur C, & Bird G (2018). Is alexithymia characterised by impaired interoception? Further evidence, the importance of control variables, and the problems with the Heartbeat Counting Task. Biological Psychology, 136, 189–197. 10.1016/j.biopsycho.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Murphy J, Brewer R, Plans D, Khalsa SS, Catmur C, & Bird G (2020). Testing the independence of self-reported interoceptive accuracy and attention. Quarterly Journal of Experimental Psychology, 73, 115–133. 10.1177/1747021819879826 [DOI] [PubMed] [Google Scholar]
- Murphy J, Millgate E, Geary H, Ichijo E, Coll M-P, Brewer R, Catmur C, & Bird G (2018). Knowledge of resting heart rate mediates the relationship between intelligence and the heartbeat counting task. Biological Psychology, 133, 1–3. 10.1016/j.biopsycho.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR, & Eisenberger NI (2016). Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain, Behavior, and Immunity, 57, 21–29. 10.1016/j.bbi.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, & Levenson RW (1979). Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology, 16, 546–553. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Glerean E, Hari R, & Hietanen JK (2014). Bodily maps of emotions. Proceedings of the National Academy of Sciences of the United States of America, 111(2), 646–651. 10.1073/pnas.1321664111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthuis JV, Stewart SH, Watt MC, Sabourin BC, & Keogh E (2012). Anxiety sensitivity and negative interpretation biases: Their shared and unique associations with anxiety symptoms. Journal of Psychopathology and Behavioral Assessment, 34, 332–342. 10.1007/s10862-012-9286-5 [DOI] [Google Scholar]
- Oosterwijk S, & Barrett LF (2014). Embodiment in the construction of emotion experience and emotion understanding. In Shapiro L (Ed.), Routledge Handbook of Embodied Cognition (pp. 250–260). Routledge. [Google Scholar]
- Owens AP, Allen M, Ondobaka S, & Friston KJ (2018). Interoceptive inference: From computational neuroscience to clinic. Neuroscience & Biobehavioral Reviews, 90, 174–183. 10.1016/j.neubiorev.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Palser ER, Palmer CE, Galvez-Pol A, Hannah R, Fotopoulou A, & Kilner JM (2018). Alexithymia mediates the relationship between interoceptive sensibility and anxiety. PLOS ONE, 13, e0203212. 10.1371/journal.pone.0203212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Yu A, Metz SE, Tsukayama E, Crum AJ, & Duckworth AL (2018). Beliefs about stress attenuate the relation among adverse life events, perceived distress, and self-control. Child Development, 89, 2059–2069. 10.1111/cdev.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, & Khalsa SS (2019). An active inference approach to interoceptive psychopathology. Annual Review of Clinical Psychology, 15, 97–122. 10.1146/annurev-clinpsy-050718-095617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker JW, & Hoover CW (1984). Visceral perception versus visceral detection: Disentangling methods and assumptions. Biofeedback and Self-Regulation, 9, 339–352. 10.1007/BF00998977 [DOI] [PubMed] [Google Scholar]
- Pollatos O, & Herbert BM (2018). Interoception: Definitions, dimensions, neural substrates. In Hauke G & Kritikos A (Eds.), Embodiment in Psychotherapy (pp. 15–27). Springer. 10.1007/978-3-319-92889-0_2 [DOI] [Google Scholar]
- Pollatos O, Herbert BM, Matthias E, & Schandry R (2007). Heart rate response after emotional picture presentation is modulated by interoceptive awareness. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 63(1), 117–124. 10.1016/j.ijpsycho.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, & Schandry R (2005). On the relationship between interoceptive awareness, emotional experience, and brain processes. Brain Research. Cognitive Brain Research, 25(3), 948–962. 10.1016/j.cogbrainres.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, & Nicewander WA (2005). Use of the extreme groups approach: A critical reexamination and new recommendations. Psychological Methods, 10, 178–192. 10.1037/1082-989X.10.2.178 [DOI] [PubMed] [Google Scholar]
- Quigley KS, Kanoski S, Barrett LF, & Tsakiris M (2021). Functions of interoception: From energy regulation to experience of self. Trends in Neurosciences, 44, 29–38. 10.1016/j.tins.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenzein R (1983). The Schachter theory of emotion: Two decades later. Psychological Bulletin. 10.1037/0033-2909.94.2.239 [DOI] [PubMed] [Google Scholar]
- Revelle W (2019). psych: Procedures for psychological, psychometric, and personality research. Northwestern University. https://cran.r-project.org/package=psych [Google Scholar]
- Richards JC, & Bertram S (2000). Anxiety sensitivity, state and trait anxiety, and perception of change in sympathetic nervous system arousal. Journal of Anxiety Disorders, 14, 413–427. 10.1016/S0887-6185(00)00031-1 [DOI] [PubMed] [Google Scholar]
- Ring C, & Brener J (2018). Heartbeat counting is unrelated to heartbeat detection: A comparison of methods to quantify interoception. Psychophysiology, 55, e13084. 10.1111/psyp.13084 [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J, Knapp K, & Mailloux J (2015). Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: A cautionary tale about interoceptive awareness. Biological Psychology, 104, 193–198. 10.1016/j.biopsycho.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Rouault M, Seow T, Gillan CM, & Fleming SM (2018). Psychiatric symptom dimensions are associated with dissociable shifts in metacognition but not task performance. Biological Psychiatry, 84, 443–451. 10.1016/j.biopsych.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammy N, Anstiss PA, Moore LJ, Freeman P, Wilson MR, & Vine SJ (2017). The effects of arousal reappraisal on stress responses, performance and attention. Anxiety, Stress, & Coping, 30, 619–629. 10.1080/10615806.2017.1330952 [DOI] [PubMed] [Google Scholar]
- Satpute AB, Kragel PA, Barrett LF, Wager TD, & Bianciardi M (2019). Deconstructing arousal into wakeful, autonomic and affective varieties. Neuroscience Letters, 693, 19–28. 10.1016/j.neulet.2018.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, & Singer JE (1962). Cognitive, social, and physiological determinants of an emotional state. Psychological Review, 69, 379–399. [DOI] [PubMed] [Google Scholar]
- Schandry R (1981). Heart beat perception and emotional experience. Psychophysiology, 18, 483–488. 10.1111/j.1469-8986.1981.tb02486.x [DOI] [PubMed] [Google Scholar]
- Schandry R (1983). On the relationship between improvement of cardiac perception and the increase of emotional experience. Psychophysiology, 20, 419–479. 10.1111/j.1469-8986.1983.tb00923.x [DOI] [Google Scholar]
- Schulz A, & Vögele C (2015). Interoception and stress. Frontiers in Psychology, 6, 993. 10.3389/fpsyg.2015.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery MD (2013). The biopsychosocial model of challenge and threat: Using the heart to measure the mind. Social and Personality Psychology Compass, 7, 637–653. 10.1111/spc3.12052 [DOI] [Google Scholar]
- Shaffer F, & Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Catmur C, & Bird G (2017). From heart to mind: Linking interoception, emotion, and theory of mind. Cortex, 93, 220–223. 10.1016/j.cortex.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SA, Mallory ME, & Simon A (1989). The body awareness questionnaire: Reliability and validity. Journal of Personality Assessment, 53, 802–815. 10.1207/s15327752jpa5304_16 [DOI] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, Dy J, Quigley KS, & Barrett LF (2018). Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychological Bulletin, 144, 343–393. 10.1037/bul0000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, & DeVille DC (2017). Interoceptive contributions to healthy eating and obesity. Current Opinion in Psychology, 17, 106–112. 10.1016/j.copsyc.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard HJ, & Bech P (2009). Psychometric analysis of Common Mental Disorders—Screening Questionnaire (CMD-SQ) in long-term sickness absence. Scandinavian Journal of Public Health, 37, 855–863. 10.1177/1403494809344653 [DOI] [PubMed] [Google Scholar]
- Sommerfeldt SL, Schaefer SM, Brauer M, Ryff CD, & Davidson RJ (2019). Individual differences in the association between subjective stress and heart rate are related to psychological and physical well-being. Psychological Science, 30, 1016–1029. 10.1177/0956797619849555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksasilp C, & Garfinkel SN (2022). Towards a comprehensive assessment of interoception in a multi-dimensional framework. Biological Psychology, 168, 108262. 10.1016/j.biopsycho.2022.108262 [DOI] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, & Levenson RW (2010). Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion (Washington, D.C.), 10(6), 803–814. 10.1037/a0020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, & Leitten CL (1993). Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology, 65, 248–260. 10.1037/0022-3514.65.2.248 [DOI] [Google Scholar]
- Torre JB, & Lieberman MD (2018). Putting feelings into words: Affect labeling as implicit emotion regulation. Emotion Review, 10, 116–124. 10.1177/1754073917742706 [DOI] [Google Scholar]
- Tsakiris M, & De Preester H (Eds.). (2018). The interoceptive mind: From homeostasis to awareness. Oxford University Press. [Google Scholar]
- Uchino BN, Birmingham W, & Berg CA (2010). Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. The Journals of Gerontology: Series B, 65B, 154–162. 10.1093/geronb/gbp127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitl D (1996). Interoception. Biological Psychology, 42(1–2), 1–27. [DOI] [PubMed] [Google Scholar]
- Van Doren N, Dickens CN, Benson L, Brick TR, Gatzke-Kopp L, & Oravecz Z (2021). Capturing emotion coherence in daily life: Using ambulatory physiology measures and ecological momentary assessments to examine within-person associations and individual differences. Biological Psychology, 162, 108074. 10.1016/j.biopsycho.2021.108074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1994). The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. University of Iowa. [Google Scholar]
- Weissman DG, & Mendes WB (2021). Correlation of sympathetic and parasympathetic nervous system activity during rest and acute stress tasks. International Journal of Psychophysiology, 162, 60–68. 10.1016/j.ijpsycho.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner NS, Duschek S, Mattern M, & Schandry R (2009). Interoceptive sensitivity modulates anxiety during public speaking. Journal of Psychophysiology, 23, 85–94. 10.1027/0269-8803.23.2.85 [DOI] [Google Scholar]
- Whelan R (2008). Effective analysis of reaction time data. The Psychological Record, 58, 475–482. 10.1007/BF03395630 [DOI] [Google Scholar]
- Whitehead WE, Drescher VM, & Blackwell B (1976). Lack of a relationship between Autonomic Perception Questionnaire scores and actual sensitivity for perceiving one’s heart beat [Abstract]. Psychophysiology, 13, 177. [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P, & Blackwell B (1977). Relation of heart rate control to heartbeat perception. Biofeedback and Self-Regulation, 2, 317–392. [PubMed] [Google Scholar]
- Wiens S, Mezzacappa ES, & Katkin ES (2000). Heartbeat detection and the experience of emotions. Cognition & Emotion, 14, 417–427. 10.1080/026999300378905 [DOI] [Google Scholar]
- Xu F, & Griffiths TL (2011). Probabilistic models of cognitive development: Towards a rational constructivist approach to the study of learning and development. Cognition, 120(3), 299–301. 10.1016/j.cognition.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Yoris A, Esteves S, Couto B, Melloni M, Kichic R, Cetkovich M, Favaloro R, Moser J, Manes F, Ibanez A, & Sedeño L (2015). The roles of interoceptive sensitivity and metacognitive interoception in panic. Behavioral and Brain Functions, 11, 14. 10.1186/s12993-015-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamariola G, Frost N, Van Oost A, Corneille O, & Luminet O (2019). Relationship between interoception and emotion regulation: New evidence from mixed methods. Journal of Affective Disorders, 246, 480–485. 10.1016/j.jad.2018.12.101 [DOI] [PubMed] [Google Scholar]
- Zamariola G, Luminet O, Mierop A, & Corneille O (2019). Does it help to feel your body? Evidence is inconclusive that interoceptive accuracy and sensibility help cope with negative experiences. Cognition and Emotion, 33, 1627–1638. 10.1080/02699931.2019.1591345 [DOI] [PubMed] [Google Scholar]
- Zamariola G, Maurage P, Luminet O, & Corneille O (2018). Interoceptive accuracy scores from the heartbeat counting task are problematic: Evidence from simple bivariate correlations. Biological Psychology, 137, 12–17. 10.1016/j.biopsycho.2018.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


