Summary
Background
Intranasal esketamine has received regulatory approvals for the treatment of depression. Recently a large trial of repeated dose racemic ketamine also demonstrated efficacy in severe depression. However, uncertainties remain regarding comparative efficacy, dosage, and the time course of response.
Methods
In this systematic review and meta-analysis, we searched Embase, Medline, Pubmed, PsycINFO, and CENTRAL up to April 13, 2023, for randomised controlled trials (RCTs) investigating ketamine for depression. Two investigators independently assessed study eligibility and risk of bias and extracted the data on depression severity scores, response and remission rates, and all-cause dropouts. Multivariable mixed-effects meta-regressions incorporated drug formulation (racemic (Rac) or esketamine (Esket)) and dose (Low or High) as covariates. Treatment effects were assessed: immediately following the first dose, during further repeated dosing, and follow-up after the final dose of a treatment course. This study is registered with PROSPERO (CRD42021221157).
Findings
The systematic review identified 687 articles, of which 49 RCTs were eligible for analysis, comprising 3299 participants. Standardised mean differences (95% confidence intervals) immediately following the first/single treatment were moderate-high for all conditions (Rac-High: −0.73, −0.91 to −0.56; Esket-High: −0.48, −0.75 to −0.20; Rac-Low: −0.33, −0.54 to −0.12; Esket-Low: −0.55, −0.87 to −0.24). Ongoing effects during repeated dosing were significantly greater than the control for Rac-High (−0.61; −1.02 to −0.20) and Rac-Low (−0.55, −1.09 to −0.00), but not Esket-Low (−0.15, −0.49 to 0.19) or Esket-High (−0.22, −0.54 to 0.10). At follow-up effects remained significant for racemic ketamine (−0.65; −1.23 to −0.07) but not esketamine (−0.33; −0.96 to 0.31). All-cause dropout was similar between experiment and control conditions for both formulations combined (Odds Ratio = 1.18, 0.85–1.64). Overall heterogeneity varied from 5.7% to 87.6%
Interpretation
Our findings suggested that effect sizes for depression severity, as well as response and remission rates, were numerically greater for racemic ketamine than esketamine. Higher doses were more effective than low doses. Differences were evident in initial effects, ongoing treatment, and lasting effects after the final dose.
Funding
None.
Keywords: Ketamine, Depression, Systematic review, Meta-analysis
Research in context.
Evidence before this study
Prior meta-analyses examined the effects of drug formulation before phase 3 data on racemic ketamine were available, did not examine single versus repeated dosing or route of administration on the antidepressant effects of ketamine. The issue of dosing has been minimally investigated. We searched PubMed, Embase, PsycINFO, and CENTRAL databases, as well as clinical trial registries, up to April 13, 2023, for randomised controlled trials (RCT) investigating ketamine for the treatment of depression and included data from our recent phase 3 RCT of racemic ketamine. We used the search terms: (Depression [Mesh] OR depression OR “depressive disorder” [MeSH Major Topic] OR “bipolar disorder” [MeSH Major Topic]) AND (“ketamine” [MeSH Major Topic] OR “Receptors, N-Methyl-d-Aspartate/”) AND (“randomised controlled trial” [Publication Type] OR “controlled clinical trial” [Publication Type] OR “randomised controlled trial” [Publication Type] OR “randomised” [Title/Abstract] OR “randomly” [Title/Abstract] OR “clinical trials as topic” [MeSH Major Topic] OR “trial” [Title]). This search identified 687 articles.
Added value of this study
This updated systematic review and meta-analysis involves 49 RCTs consisting of 3299 participants. It adds new data from a recently completed phase 3 RCT of racemic ketamine, new data on the effects of dose and ketamine formulation, and new information on the time course of response. Multivariable mixed effects meta-regression analyses showed that racemic ketamine had numerically greater efficacy than esketamine. Higher doses reduced depressive symptoms and increased response rates at all stages in a treatment course (initial, ongoing, and lasting effects). Ketamine at all doses and formulations was acceptable, with comparable rates of all-cause dropouts to the placebo control.
Implications of all the available evidence
Ketamine formulation and adequate dosage are important factors contributing to the antidepressant efficacy of ketamine. Therefore, clinics and research studies should adopt protocols that ensure adequate dosing when giving ketamine for depression.
Introduction
Ketamine, an n-methyl-d-aspartate antagonist commonly used as an anaesthetic agent, has emerged as a new treatment for depression, with efficacy demonstrated in severe and treatment-resistant depression.1, 2, 3, 4, 5, 6 Meta-analyses have provided valuable information regarding potential effect modifiers, including improved response in unipolar compared to bipolar depression,7 sustained benefit following repeated sessions of treatment,2 routes of administration,8 and indirect comparisons of efficacy for racemic and esketamine enantiomer formulations.3 However, for these meta-analyses, data for repeated treatment sessions were only available from phase 3 randomised controlled trials (RCTs) of esketamine, the S-enantiomer of ketamine. This updated meta-analysis incorporates data from the first large phase 3 RCT of racemic ketamine,9 addressing a key gap in the literature.
Variable approaches to dosing have been used in studies to date, with some data suggesting this may be an important factor in treatment outcomes.1,10, 11, 12 Whereas earlier trials typically delivered a fixed dose relative to the patient's body weight,13, 14, 15 recent studies used a titrated dosing approach,9,11,12,16,17 which allows for dose increases in the event of non-response.18 An early meta-analysis of nine RCTs, consisting of only 201 participants, found greater reductions in depression severity scores in patients receiving a higher dose.1 The present study extends prior work by classifying studies according to the dose levels used.
Clinical considerations for a new treatment include the time onset of effects, efficacy attained at the end of treatment and the persistence of benefits after treatment cessation. Prior meta-analyses mainly focussed on effects at the primary study endpoint. The present meta-analysis modelled the acute onset of antidepressant effects, the maintenance of effects during an ongoing treatment course of repeated ketamine administrations, and any lasting benefits following treatment cessation.
Therefore, this systematic review and meta-analysis aimed to update findings on racemic ketamine and esketamine antidepressant efficacy and acceptability, incorporate new data from a substantive trial of racemic ketamine, evaluate outcomes over the treatment course and at follow-up, and examine the effect of the dosing approach.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis were conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19,20 SN and AS independently searched Embase, Medline Pubmed, PsycINFO and CENTRAL for randomised controlled trials (RCTs) investigating the use of ketamine primarily as a treatment for participants with a clinical diagnosis of major depressive disorder, published in any language. There was no limit for the earliest year of publication, and the search concluded on April 13, 2023. Search terms included “randomised controlled trial” AND “ketamine” AND “depression”, in addition to permutations and variations of these terms detailed in the Appendix (pp 5–7). The search strategy for each bibliographic database was peer reviewed by a UNSW librarian according to the PRESS Checklist.21 We also searched reference lists of studies meeting inclusion criteria and clinical trial registries (e.g., ClinicalTrials.gov). SN and AS successively screened identified studies according to their title, abstract, and full text and assessed for risk of bias.22 Any disagreement following full-text screening was resolved through discussion until consensus was achieved. The clinical trial registration number of all included studies was inspected to ensure no duplicates in the data.
Data analysis
We extracted data relating to trial design, including the number of treatments, treatment duration, route of ketamine administration, ketamine enantiomer, type of control condition, and dose converted to the intravenous racemic ketamine equivalent23 to facilitate comparisons between different routes and enantiomers. The dose was categorised as a binary factor of ‘High’ if delivered at the intravenous equivalent of ≥0.5 mg/kg (or option of titrating up to this level) or ‘Low’ if < 0.5 mg/kg. Studies using an intranasal route of administration were categorised as a high dose if the dosing was predominantly at ≥ 84 mg or low if <84 mg (see Appendix pp 7–8). We further extracted participant characteristics, including age, sex, and the presence of treatment-resistant depression. Primary and secondary outcome measures were obtained at all available time points up to four weeks following the final treatment dose.
The primary efficacy outcome was a change in depression severity relative to the control group obtained using a standardised psychometric scale such as the Hamilton Depression Rating Scale (HDRS) or Montgomery-Asperg Depression Rating Scale (MADRS). Secondary efficacy outcomes included response (≥50% reduction in depressive symptoms) and remission rates. Each of these efficacy outcomes was assessed at three time periods: 1) acute effects of the first treatment, obtained from 4 h after the first dose up to 3 days after or the time of the second treatment, whichever occurred first; 2) effects of further repeated treatments, i.e., second treatment to last treatment (for studies involving ≥2 ketamine sessions), obtained from day 4 onwards during the treatment course; 3) follow up effects after a course of repeated treatments obtained up to 28 days after the final session. The secondary safety outcome was acceptability using all-cause dropout rates at the primary study endpoint for studies using repeated administrations of ketamine. Effect sizes were calculated using standardised mean differences (SMD) for continuous outcomes and Odds Ratios (OR) for binary outcomes.
Risk of bias was assessed using the Cochrane risk-of-bias tool for randomised trials.22 Publication and small study biases were assessed using the Egger test24 and visual inspection of contoured funnel plots.25 To generate funnel plots, we performed aggregate meta-analyses for each combination of ketamine formulation (Racemic vs Esketamine) and dose (High vs Low) at the following prespecified times for the primary outcome of depression severity: i) 4 h, days 1 and 3 for initial effects after a single/first dose; ii) days 7, 14, 21, and 28 for effects of repeated ketamine administrations; and iii) day 28 for effects following the final dose of a course of treatments.
Statistical analyses were performed using the metafor package in R (version 4.1.1),26 using mixed-effects models with significance set at p < 0.05. Multivariable mixed-effect meta-regressions were initialised with 1st and 2nd order fixed effects of time (linear and quadratic, respectively). As the present study focused on ketamine formulation (racemic vs esketamine) and dosage (high vs low), these variables and their interaction were also incorporated into the model as fixed effects, and ‘study’ was included as a random effect. This approach was used to account for within-study interdependency of effect sizes arising due to multiple observations from the same study. Furthermore, we used a restricted maximum-likelihood estimation method due to the potential for non-independent sampling errors within studies.
We additionally report meta-regression and subgroup analysis outcomes for several effect modifiers of interest, including ketamine formulation, dose, control condition type (active or saline), treatment-resistant depression (TRD) or non-treatment-resistant depression, and route of administration. These were added individually to a base mixed effects meta-regression model incorporating a polynomial time factor for all investigated periods.
The Higgins’ I2 statistic was used to estimate heterogeneity. An I2 value greater than 50% was considered indicative of substantial heterogeneity. The quality of the body of evidence was assessed using the GRADE approach.27
This study was a systematic review and thus did not require ethics approval. This study was registered on PROSPERO (CRD42021221157).
Role of the funding source
There was no funding source for this study. All authors had access to all meta-analysed data used in this study and accept responsibility for the decision to submit the manuscript for publication.
Results
Our search of bibliographic databases, reference lists and clinical trial registers identified 687 articles (Fig. 1). Following the screening, we included data from 49 eligible studies in the systematic review and meta-analysis, consisting of 52 placebo arms and 72 ketamine arms, with 3299 participants. In addition to 45 peer-reviewed RCTs,11, 12, 13, 14, 15, 16, 17,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 we included data from clinical trials registries (NCT02106325, NCT03434041, and NCT03539887) and a recently completed study.9 Of the included studies, 13 (27%) used a crossover study design, for which we prioritised data obtained before the crossover event (Table 1). 27 (55%) studies investigated the effects of ketamine following a single dose, and 22 (45%) reported the effects of repeated (≥2) administrations of ketamine. 39 (80%) studies used racemic ketamine, and 10 (20%) reported esketamine use. Most studies (33, 67%) sourced participants from a treatment-resistant cohort. Five routes of administration were identified in the meta-analysis dataset experiment arms: 48 intravenous, 17 intranasal, 4 subcutaneous, 2 oral, and 1 intramuscular.
Fig. 1.
Study selection.
Table 1.
Trials of ketamine efficacy for depression meeting inclusion criteria.
| Author | Design | Diagnosis | Sample |
Treatment |
||
|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | |||
| Berman et al. (2000) | Single dose Crossover—washout ≥1 week |
DSM-IV | 9 | – | IV racemic 0.5 mg/kg | Saline placebo |
| Zarate et al. (2006) | Single dose Crossover—washout ≥1 week |
DSM-IV HAMD-21 ≥ 18 failed ≥2 antidepressants | 18 | – | IV racemic 0.5 mg/kg | Saline placebo |
| Diazgranados et al. (2010) | Single dose Crossover—washout ≥2 weeks |
DSM-IV MADRS ≥20 failed ≥1 antidepressant & ≥ 1 mood stabiliser | 18 | – | IV racemic 0.5 mg/kg | Saline placebo |
| Zarate et al. (2012) | Single dose Crossover—washout ≥2 weeks |
DSM-IV MADRS ≥20 failed ≥1 antidepressant & ≥ 1 mood stabiliser | 15 | – | IV racemic 0.5 mg/kg | Saline placebo |
| Murrough et al. (2013) | Single dose Parallel |
DSM-IV IDS-C ≥ 32 | 47 | 25 | IV racemic 0.5 mg/kg | Midazolam 0.045 mg/kg |
| Sos et al. (2013) | Single dose Crossover—washout ≥1 week |
DSM- IV MADRS ≥20 failed ≥3 antidepressants | 30 | – | IV racemic 0.27 mg/kg loading 0.27 mg/kg maintenance | Saline placebo |
| Lai et al. (2014) | Up to 5 treatmentsa Crossover—washout ≥1 week |
DSM-IV MADRS ≥20 failed ≥5 antidepressants | 4 | – | IV racemic ketamine 0.1, 0.2, 0.3 & 0.4 mg/kg | Saline placebo |
| Lapidus et al. (2014) | Single dose Crossover—washout ≥1 week |
DSM-IV IDS-C ≥30 failed ≥1 antidepressant | 20 | – | IN racemic ketamine 50 mg | Saline placebo |
| Downey et al. (2016) | Single dose Parallel |
DSM-IV | 21 ketamine 20 lanicemineb | 19 | IV racemic ketamine 0.5 mg/kg, lanicemine 100 mg | Saline placebo |
| Hu et al. (2016) | Single dose Parallel |
DSM-IV HDRS-17 ≥24 | 13 | 14 | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| Li et al. (2016) | Single dose Parallel |
DSM-IV failed ≥3 medications | 32 | 16 | IV racemic ketamine 0.2 & 0.4 mg/kg | Saline placebo |
| Loo et al. (2016) | Up to 5 treatmentsc Crossover—washout ≥1 week |
DSM IV MADRS ≥20 failed ≥1 antidepressant | 15 | – | IV, IM & SC racemic ketamine 0.5 mg/kg | Midazolam 0.01 mg/kg |
| Singh et al. (2016a) | 2 treatmentsd Parallel |
DSM-IV IDS-CR ≥ 34 failed ≥2 antidepressants | 20 | 10 | IV esketamine 0.2 & 0.4 mg/kg | Saline placebo |
| Singh et al. (2016b) | 4 treatments twice weekly & 6 treatments thrice weekly Parallel |
DSM-IV IDS-CR ≥34 failed ≥2 antidepressants | 18 × 2 weekly, 17 × 3 weekly |
17 × 2 weekly, 16 × 3 weekly | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| George et al. (2017) | Up to 5 treatmentse Crossover—washout ≥1 week |
DSM IV MADRS ≥20 failed ≥1 antidepressant | 16 | – | SC racemic ketamine 0.1, 0.2, 0.3, 0.4 & 0.5 mg/kg | Midazolam 0.01 mg/kg |
| Grunebaum et al. (2017) | Single dose Parallel |
DSM-IV HDRS-17 ≥16 | 7 | 9 | IV racemic ketamine 0.5 mg/kg | Midazolam 0.02 mg/kg |
| Su et al. (2017) | Single dose Parallel |
DSM-IV failed ≥2 antidepressantsss | 23, 24 | 24 | IV racemic ketamine 0.2 & 0.5 mg/kg | Saline placebo |
| Arabzadeh et al. (2018) | 84 treatments twice weekly Parallel |
DSM-V HDRS-17 ≥20 | 41 | 40 | O racemic ketamine 50 mg/day | Saline placebo |
| Canuso et al. (2018) | 8 treatments Parallel |
DSM-IV-TR MADRS ≥22 | 35 | 31 | IN esketamine 84 mg | Saline placebo |
| Cao et al. (2018) | Single dose Parallel |
DSM-IV-TR HDRS-17 ≥ 18 failed ≥2 antidepressants | 55 | 18 | IV racemic ketamine 0.2 & 0.5 mg/kgf | Saline placebo |
| Chen et al. (2018) | Single dose Parallel |
DSM-IV-TR failed ≥3 antidepressants | 8, 8 | 8 | IV racemic ketamine 0.2 & 0.5 mg/kg | Saline placebo |
| Daly et al. (2018) | Single dose Parallel |
DSM-IV-TR IDS-CR ≥ 34 failed ≥2 antidepressants | 11, 11, 12 | 33 | IN esketamine 28, 56 & 84 mg | Saline placebo |
| Fava et al. (2018) | Single dose Parallel |
DSM-IV-TR MADRS ≥20 failed ≥2 antidepressants | 18, 20, 22, 20 | 19 | IV racemic ketamine 0.1, 0.2, 0.5 & 1.0 mg/kg | Midazolam 0.045 mg/kg |
| Gálvez et al. (2018) | 8 treatments Parallel |
DSM-V-TR MADRS ≥22 failed ≥2 antidepressants | 3 | 2 | IN racemic ketamine 100 mg | Midazolam 4.5 mg |
| Grunebaum et al. (2018) | Single dose Parallel |
DSM-IV-TR HDRS-17 ≥ 16 | 40 | 40 | IV racemic ketamine 0.5 mg/kg | Midazolam 0.02 mg/kg |
| Nugent et al. (2018) | Single dose Crossover—washout ≥2 weeks |
DSM-IV MADRS ≥20 failed ≥1 antidepressant | 35 | – | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| Sinyor et al. (2018) | 6 treatments Parallel |
MINI SSI >20 | 5 | 4 | IV racemic ketamine 0.5 mg/kg | Midazolam 0.045 mg/kg |
| Domany et al. (2019) | 9 treatments Parallel |
MINI MADRS ≥19 failed ≥2 antidepressants | 22 | 19 | O racemic ketamine 1.0 mg/kg | Saline placebo |
| Fedgchin et al. (2019) | 8 treatments Parallel |
DSM-IV MADRS ≥28 failed ≥2 antidepressants | 117, 116 | 113 | IN esketamine 56 & 84 mg | Saline placebo with bittering agent |
| Ionescu et al. (2019) | 6 treatments Parallel |
DSM-IV HDRS-28 ≥20 failed ≥3 antidepressants | 13 | 13 | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| Phillips et al. (2019) | Single dose Crossover—washout ≥1 week |
DSM-IV-TR MADRS ≥25 failed ≥2 antidepressants | 43 | – | IV racemic ketamine 0.5 mg/kg | Midazolam 30 μg/kg |
| Popova et al. (2019) | 8 treatments Parallel |
DSM-V IDS-CR ≥34 failed ≥2 antidepressants | 114 | 109 | IN esketamine 56 & 84 mg | Saline placebo with bittering agent |
| Domany et al. (2020) | Single dose Parallel |
DSM-IV-TR C-SSRS ≥3 | 9 | 9 | IV racemic ketamine 0.2 mg/kg | Saline placebo |
| Fu et al. (2020) | 8 treatments Parallel |
DSM-V MADRS >28 | 111 | 112 | IN esketamine 84 mg | Saline placebo with bittering agent |
| Milak et al. (2020) | Single dose Parallel |
DSM-IV MADRS ≥22 | 5, 6, 8, 5, 9 | 5 | IV racemic ketamine 0.1, 0.2, 0.3, 0.4 & 0.5 mg/kg | Saline placebo |
| Ochs-Ross et al. (2020) | 8 treatments Parallel |
DSM-V IDS-CR ≥31 failed ≥2 antidepressants | 72 | 66 | IN esketamine 84 mg | Saline placebo with bittering agent |
| Shiroma et al. (2020) | 6 treatments Fg Parallel |
DSM-IV-TR IDS-CR ≥ 32 failed ≥2 antidepressants | 25 | 29 | IV racemic ketamine 0.5 mg/kg | Midazolam 0.045 mg/kg |
| Sumner et al. (2020) | Single dose Crossover—washout ≥3 weeks |
DSM-IV MADRS ≥20 failed ≥2 treatments—drug or psychological | 30 | – | IV racemic ketamine 0.5 mg/kg | Remifentanil 1.7 ng/ml |
| Tiger et al. (2020) | Single dose Parallel |
MINI MADRS ≥20 failed ≥1 selective serotonin reuptake inhibitor | 20 | 10 | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| Dwyer et al. (2021) | Single dose Crossover—washout ≥2 weeks |
DSM-V CDRS-R >40 failed ≥1 antidepressant | 17 | – | IV racemic ketamine 0.5 mg/kg | Midazolam 0.045 mg/kg |
| Ionescu et al. (2021) | 8 treatments Parallel |
DSM-V MADRS >28 | 114 | 113 | IN esketamine 84 mg | Saline placebo with bittering agent |
| Lijffijt et al. (2021) | Single dose Parallel |
DSM-V MADRS ≥27 | 20 | 13 | IV racemic ketamine 0.1, 0.25 & 0.5 mg/kg | Midazolam 0.03 mg/kg |
| Takahashi et al. (2021) | 8 treatments Parallel |
DSM-V MADRS ≥20 failed ≥1 & <5 antidepressants | 122 | 80 | IN esketamine 28, 56 & 84 mg | Saline placebo |
| Gallagher et al. (2022) | 4 treatments Parallel |
DSM-V HDRS-24 ≥27 | 13 | 12 | IV racemic ketamine 0.5 mg/kg | Midazolam 0.045 mg/kg |
| Ahmed et al. (2023) | 2 treatments Parallel |
“current diagnosis of treatment-resistant MDD” failed ≥2 antidepressants | 18 | 18 | IV racemic ketamine 0.5 mg/kg | Saline placebo |
| Loo et al. (under review) | 8 treatments Parallel |
DSM-V MADRS ≥20 failed ≥2 antidepressants | 86 | 88 | SC racemic ketamine 0.5 & 0.5–0.9 mg/kg | Midazolam 0.025 & 0.025–0.045 mg/kg |
| NCT02106325 | Single dose Parallel |
MINI MADRS ≥24 | 11 | 10 | IV racemic ketamine 0.25 mg/kg | Diphenhydramine 25 mg |
| NCT03434041 | 8 treatments Parallel |
DSM-V MADRS ≥28 failed ≥1 & ≤5 antidepressants | 124 | 126 | IN esketamine 56–84 mg | Intranasal spray |
| NCT03539887 | Single dose Parallel |
DSM-IV-TR MADRS ≥12 | 17 | 11 | IN racemic ketamine 50 mg | Saline placebo |
CDRS-R: Children's Depression Rating Scale–Revised, C-SSRS: Columbia Suicide Severity Rating Scale, DSM: Diagnostic and Statistical Manual of Mental Disorders, HDRS: Hamilton Depression Rating Scale, IDS-CR: Inventory of Depressive Symptomatology—Clinician Rating, MADRS: Montgomery-Asberg Depression Rating Scale, MDD: Major Depressive Disorder, MINI: Mini International Neuropsychiatric Interview, SSI: Scale of Suicidal Ideation, IV: Intravenous, IN: Intranasal, SC: subcutaneous, O: oral, IM: intramuscular.
Data from first dose analysed only.
Lanicemine group not included in analyses.
Data from first dose analysed only.
Data from before the second dose was analysed as responders/non-responders received dose-escalation.
Data from first dose analysed only.
Results were presented for both active conditions combined (0.2 and 0.5 mg/kg) so the weighted combined average was calculated for analysis.
Data was extracted from the first five treatments only as the final sixth dose was active in both ketamine and midazolam experiment arms.
Overall, the Cochrane risk-of-bias tool found most studies had a low or unclear bias risk—see Appendix pp 27–28. However, an inspection of contoured funnel plots showed potential signs of publication bias. Use of the trim and fill procedure suggested missing null findings from the acute phase 4 h, 1 day and 3 days following an initial dose. This suggests possible bias in the direction of greater effect sizes favouring ketamine over control during the acute phase (see Appendix pp 29–30 for further details).
Primary outcome—change in depression severity: initial effects after a single/first dose
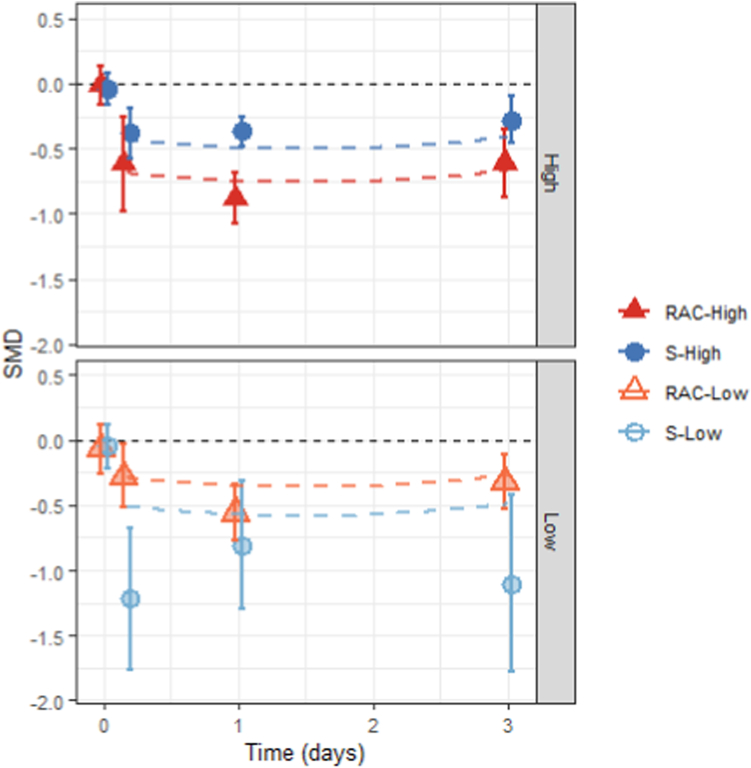
Acute improvements in mood were observed in all conditions, with a large effect size for racemic high dose treatments (Rac-High = −0.73; p < 0.0001; 95% CI −0.91 to −0.56), moderate effects for esketamine formulations at low and high doses (Esket-Low = −0.55; p = 0.0006; 95% CI −0.87 to −0.24; Esket-High = −0.48; p = 0.0007; 95% CI −0.75 to −0.20), and modest effects for low dose racemic administrations (Rac-Low = −0.33; p = 0.0019; 95% CI −0.54 to −0.12). The model showed substantial heterogeneity (k = 43, I2 = 60.5%). There was a significant quadratic effect of time during the acute period following an initial dose (Timequad = 0.81; p = 0.025; 95% CI 0.10–1.52; Timelinear = 0.20; p = 0.56; 95% CI −0.48 to 0.89). These findings suggest a rapid reduction in depression severity, reaching a peak approximately one day after treatment, followed by a return towards the baseline (Fig. 2). Note that the meta-regression fitted line for Esket-Low suggests weaker effects than the point estimates derived from simple univariable meta-analyses at each time point for this group, even exceeding the 95% confidence limits at 4 h. Point estimates for this period showed potential signs of publication bias (see Appendix pp 30–31), with exaggerated effect sizes likely due to small, underpowered trials. The larger, adequately powered Esket-Low studies show more moderate effect sizes in line with meta-regression estimates. Additionally, the meta-regression model uses data from studies reporting both Esket-Low and Esket-High results,45,50,62 to infer comparable effect sizes at both doses, whilst leveraging the improved precision available from larger studies to provide a more accurate estimate of effect sizes by ketamine formulation and dose.
Fig. 2.
Acute effects following the single/first dose of ketamine to a control condition. Data points represent random effects aggregate meta-analyses conducted separately for each combination of ketamine formulation (Racemic vs Esketamine) and dose (High vs Low) at specific time points; baseline, as well as 4 h, and days 1 and 3 following the initial dose. Error bars denote the 95% confidence interval. Dashed lines indicate findings from the mixed effects multivariable meta-regression incorporating 1st and 2nd order fixed effects of time (linear and quadratic, respectively) as well as factors of ketamine formulation and dosage as fixed effects, and ‘study’ as a random effect. Plots are separated to show results for High (top panel) and Low (bottom panel) dose categories. Negative standardised mean differences (SMDs) indicate reduced scores on standardised depression scales (e.g., MADRS or HDRS) for participants receiving ketamine compared to a control condition. Differences between groups at baseline (day 0) are included for visual reference and were not incorporated in the meta-regression analysis. RAC: racemic; S: esketamine.
Change in depression severity: effects of repeated doses
We examined the ongoing antidepressant effects of repeated ketamine administrations from day four following the first treatment until the final treatment (Fig. 3). After initial acute effects, a course of repeated ketamine treatments maintained antidepressant effects relative to the control condition, as evidenced by the lack of significant linear or quadratic effects of time (Timelinear = −0.16; p = 0.41; 95% CI −0.56 to 0.23; Timequad = −0.13; p = 0.53; 95% CI −0.52 to 0.27). Racemic ketamine at high and low doses was significantly better than control (Rac-High = −0.61; p = 0.0038; 95% CI −1.02 to −0.20; Rac-Low = −0.55; p = 0.049; 95% CI −1.09 to −0.00). Low-dose as well as high-dose esketamine produced numerically but not statistically greater antidepressant effects compared to control during treatment (Esket-Low = −0.15; p = 0.40; 95% CI −0.49 to 0.19; Esket-High = −0.22; p = 0.18; 95% CI −0.54 to 0.10). There was large heterogeneity between included studies (k = 16; I2 = 84.2%).
Fig. 3.
Repeated administrations as part of a treatment course. Data points represent random effects aggregate meta-analyses conducted separately for each combination of ketamine formulation (Racemic vs Esketamine) and dose (High vs Low) at specific time points; days 7, 14, 21, and 28 during a treatment course. Due to differences in data collection times between studies, findings were aggregated for results reported within ±3 days from these time points (e.g., findings at day 25 were aggregated into day 28). Error bars denote the 95% confidence interval. Dashed lines indicate findings from the mixed effects multivariable meta-regression incorporating 1st and 2nd order fixed effects of time (linear and quadratic, respectively) as well as factors of ketamine formulation and dosage as fixed effects, and ‘study’ as a random effect. Plots are separated to show results for High (top panel) and Low (bottom panel) dose categories. Negative standardised mean differences (SMDs) indicate reduced scores on standardised depression scales (e.g., MADRS or HDRS) for participants receiving ketamine compared to a control condition. RAC: racemic; S: esketamine.
Change in depression severity: effects following the final dose of a course of treatments
For follow-up effects after cessation of treatment with repeated sessions of ketamine, data were only available from seven studies and eight experimental arms. Only one experimental arm used a low dose, delivering fixed-dose subcutaneous racemic ketamine.9 Thus, dose was not included as a factor. We analysed effects up to four weeks following the final treatment session (Fig. 4). There was a trend-level linear reduction in antidepressant effects over time (Timelinear = 0.01; p = 0.051; 95% CI −0.00 to 0.01). Mood improvements were significantly maintained for racemic ketamine formulations (Rac = −0.65; p = 0.029; 95% CI −1.23 to −0.07) and only numerically maintained for esketamine (Esket = −0.33; p = 0.31; 95% CI −0.96 to 0.31), i.e., there was no longer a difference compared to control. The model had high heterogeneity (k = 7; I2 = 87.6%). A sensitivity analysis excluded the single experiment arm that used a low dose of racemic ketamine. The effect size for racemic ketamine remained significant and numerically larger than esketamine (Rac = −0.87; p = 0.0023; 95% CI −1.43 to −0.31; Esket = −0.32; p = 0.19; 95% CI −0.81 to 0.16; Appendix pp 57).
Fig. 4.
Effects following the final dose. Data points represent random effects aggregate meta-analyses conducted separately for each combination of ketamine formulation (Racemic vs Esketamine) and dose (High vs Low) at specific time points; baseline, as well as days 7, 14, 21, and 28 following the final dose in a treatment course. Error bars denote the 95% confidence interval. Dashed lines indicate findings from the mixed effects multivariable meta-regression incorporating 1st and 2nd order fixed effects of time (linear and quadratic, respectively) as well as the factor of ketamine formulation as a fixed effect, and ‘study’ as a random effect. Model findings are only provided for the High dose category because all but one study contributing to the meta-regression outcomes used a high dose. Plots are separated to show results for High (top panel) and Low (bottom panel) dose categories. Negative standardised mean differences (SMDs) indicate reduced scores on standardised depression scales (e.g., MADRS or HDRS) for participants receiving ketamine compared to a control condition. Time is represented as the number of days since the final dose of a treatment course. RAC: racemic; S: esketamine.
Response rates
Analyses of response rates across the three time periods reveal similar findings to depression severity outcomes. Response rates were significantly higher than control after the first treatment session, with the largest Odds Ratio observed using high racemic doses (Rac-High = 5.35; p < 0.0001; 95% CI 3.48–8.22), followed by low dose racemic and esketamine formulations (Rac-Low = 2.69; p = 0.0004; 95% CI 1.56–4.64; Esket-Low = 3.48; p = 0.0009; 95% CI 1.66–7.27), and modest effects for high dose esketamine (Esket-High = 2.27; p = 0.0075; 95% CI 1.24–4.14). Unlike mean depression severity scores, there were no significant linear or quadratic effects of time (Timelinear = 0.30; p = 0.15; 95% CI 0.06–1.57; Timequad = 0.75; p = 0.75; 95% CI 0.13–4.39). There was low-moderate heterogeneity (k = 37; I2 = 44.0%).
A course of repeated sessions maintained improved response rates for high-dose racemic and esketamine formulations (Rac-High = 2.83; p < 0.0001; 95% CI 1.68–4.76; Esket-High = 1.42; p = 0.016; 95% CI 1.07–1.90), but not low dose formulations (Rac-Low = 1.56; p = 0.38; 95% CI 0.57–4.26; Esket-Low = 1.37; p = 0.13; 95% CI 0.91–2.05). Time was not a significant factor (Timelinear = 1.67; p = 0.29; 95% CI 0.64–4.30; Timequad = 1.29; p = 0.58; 95% CI 0.52–3.18) and there was low-moderate heterogeneity (k = 16; I2 = 37.8%).
Following the final dose after a treatment course, response rates were significantly better than control for racemic ketamine but only numerically better for esketamine (Rac = 2.53; p = 0.049; 95% CI 1.00–6.37; Esket = 1.76; p = 0.10; 95% CI 0.89–3.49) and there was no effect of time (Timelinear = 0.99; p = 0.32; 95% CI 0.98–1.01). In addition, there was moderate heterogeneity in response rates (k = 7; I2 = 61.6%).
Remission rates
Remission rates were significantly greater than control after the first treatment session (Rac-Low = 3.31; p = 0.0017; 95% CI 1.57–7.01; Rac-High = 2.97; p < 0.0001; 95% CI 1.77–4.99; Esket-Low = 5.05; p < 0.0001; 95% CI 2.41–10.54; Esket-High = 2.17; p = 0.0002; 95% CI 1.43–3.29). Time was not a significant factor (Timelinear = 0.59; p = 0.60; 95% CI 0.09–4.11; Timequad = 1.72; p = 0.59; 95% CI 0.23–12.67). Heterogeneity between studies was low (k = 25; I2 = 9.9%).
Repeated ketamine administrations maintained remission rates for high-dose formulations (Rac-High = 2.44; p = 0.0004; 95% CI 1.49–3.99; Esket-High = 1.52; p < 0.0001; 95% CI 1.27–1.82) but not low dose formulations (Rac-Low = 1.05; p = 0.93; 95% CI 0.35–3.12; Esket-Low = 1.30; p = 0.15; 95% CI 0.91–1.85). Time was not a significant factor (Timelinear = 1.28; p = 0.66; 95% CI 0.42–3.88; Timequad = 1.36; p = 0.57; 95% CI 0.47–3.91) and there was low heterogeneity (k = 16; I2 = 5.7%).
Following the final dose, remission rates were significantly higher than control for esketamine (Esket = 1.80; p = 0.011; 95% CI 1.14–2.82) but not racemic ketamine (Rac = 1.31; p = 0.56; 95% CI 0.52–3.28). Time was not a significant factor (Timelinear = 0.99; p = 0.33; 95% CI 0.98–1.01). Heterogeneity between studies was low (k = 7; I2 = 37.7%).
Effect modifiers
Further multivariable analyses incorporating individual effect modifiers, including dose, ketamine formulation, control comparator type, treatment resistance, and route of administration, are reported in Appendix pp 49–58.
Acceptability
Acceptability, measured by all-cause dropouts at the study's primary endpoint for RCTs using repeated sessions, was not significantly different between ketamine and control conditions (k = 20; OR = 1.18; p = 0.31; 95% CI 0.85–1.64, I2 = 17.3%). Compared to control, there was no significant difference in dropouts for low and high doses using either racemic or esketamine formulations (Rac-Low = 0.94; p = 0.94; 95% CI 0.06–15.83; Rac-High = 1.23; p = 0.67; 95% CI 0.47–3.22; Esket-Low = 1.06; p = 0.88; 95% CI 0.48–2.34; Esket-High = 1.20; p = 0.33; 95% CI 0.83–1.74).
Discussion
This systematic review and meta-analysis builds on previous work, incorporating data from a recent large RCT of racemic ketamine provides the most comprehensive evaluation to date of the effects of ketamine formulations. Results from 49 RCTs with a total of 3299 participants show that the racemic formulation of ketamine is superior to esketamine in both acute onset and benefits during ongoing treatment, reflected in the magnitude of effects relative to control.
Previous meta-analyses have reported that racemic or arketamine may have larger effect sizes than esketamine.2,3 However, to our knowledge, only one small pilot RCT has directly compared esketamine to racemic ketamine, demonstrating non-inferiority following a single session.66 This updated meta-analysis suggests that active versus control differences were larger for high-dose racemic formulations compared to esketamine, although statistical comparison did not significantly differentiate these conditions. Interestingly, esketamine did not significantly improve antidepressant effects compared to the control condition during repeated sessions (Esket-Low = −0.15; Esket-High = −0.22). The pivotal study by Popova, Daly17 demonstrated the efficacy of intranasal esketamine in depression. These results were supported by Fu, Ionescu,54 a study of esketamine on suicidality. The present meta-analysis considers the totality of all available esketamine studies. The remaining RCTs included in this meta-analysis did not report statistically significant benefits of esketamine relative to control,16,42,50,60,62 particularly in the later stages of a treatment course. Reasons proposed to explain the lack of statistical significance in these studies have included: i) improved effects in the placebo control group due to heightened expectations upon receiving a novel drug,67 ii) poor effects in participants aged over 75 years old who comprise a sizeable minority in some studies,16 iii) relatively short treatment course durations (e.g., 3–4 weeks) which may not allow sufficient time to maximise antidepressant effects; and iv) frequent and lengthy participant clinical interactions in the control group which may further heighten placebo and non-specific treatment effects. However, several of these explanations are equally applicable to racemic ketamine, which nonetheless showed antidepressant effects relative to control under high doses (Rac-High = −0.61). Considering the lack of direct comparisons between racemic ketamine and esketamine formulations during and following an adequately dosed trial of repeated administrations, there is a need for large RCTs to conclusively establish the non-inferiority, or superiority, of racemic ketamine to esketamine. Likewise, there is a need for further trials to establish the suspected superiority of the arketamine enantiomer, which is included in the racemic mixture, to esketamine.
Higher doses were associated with improved antidepressant effects following a single session and during repeated administrations of ketamine, with insufficient data available to explore dose effects during the follow-up period. A high dose of racemic ketamine produced the largest reduction in depression severity symptoms after the initial dose (SMD = −0.73) and during repeated administrations (SMD = −0.61), supporting prior observations that adequate dosage is important for optimal efficacy. Early dose–response pilot studies found increased antidepressant response following a single session of racemic ketamine at higher doses, with greater proportions of participants reaching response and remission criteria as the dosage was increased according to a response-guided approach.11,31 Similar findings were later obtained for participants with treatment-resistant depression,40,44,46 including those over sixty years.12,61 More recently, Milak, Rashid55 showed a positive correlation between intravenous racemic ketamine doses, ranging from 0.1 to 0.5 mg/kg, and improvements in depression severity scores. A similar positive dose–response relationship has been observed following a single session of intranasal esketamine,45 although not consistently.37 Interestingly, repeated sessions of intranasal esketamine do not exhibit a clear association between dose and antidepressant response when dosing was assigned at a group level rather than based on individual response-guided titration.50,62 These findings suggest that adequate dosage, ideally response-guided on an individual basis, should be recommended in clinical settings to optimise efficacy outcomes.
An important question in the field is whether improvements in depressive symptoms following repeated administrations of ketamine can be maintained. Minimal data from only seven studies were available to evaluate effects following cessation of treatment. Due to the limited data available, meta-regression analysis could not incorporate dose as a factor, and the model was instead restricted to investigating the effects of ketamine formulation. This available data found a significant benefit of esketamine relative to the control condition and a similar benefit for racemic ketamine once the low-dose experiment arm was excluded from analyses. Racemic and esketamine formulations produced small effect sizes for mood reductions relative to the placebo control arms. However, there are some important caveats to this finding. Firstly, the number of studies providing data for racemic (k = 4) and esketamine (k = 3) effects was low. Secondly, there was a significant number of dropouts (0.0–35.5%) in the follow-up period for included studies, further reducing statistical power. Dropouts may introduce a bias to the findings either in favour of the experiment condition, participants not benefiting from treatment may leave the study prematurely, or in the opposite direction, those who remitted/responded may no longer be interested in engaging with study investigators and so may be lost to contact. Future studies are encouraged to collect and report comprehensive follow-up data to establish the ongoing benefits of ketamine and estimate the rate of relapse.
All ketamine formulations and doses showed similar acceptability to the control condition at the primary study endpoint measured using all-cause dropout rates. There was no evidence from the present analysis that increased doses resulted in reduced acceptability. However, evidence from the literature identified during the systematic review suggests that care should be taken to ensure that the use of higher doses to achieve greater efficacy is not accompanied by intolerable treatment-emergent adverse events, which also show dose-dependence.11,18,37,68 The response-guided individual dose titration approach described above may be useful for optimising safety and efficacy outcomes.9,11,12,31 As all-cause dropouts are a coarse measure of safety, more in-depth meta-analyses are needed to assess adverse events using a comprehensive range of psychomimetic, physical (e.g., liver and bladder functioning, blood pressure, etc.) and cognitive (e.g., executive functioning) assessments.
There are several limitations of the present analysis that must be acknowledged. First, there was some evidence of publication or small study bias observed following a visual inspection of funnel plots (see Appendix pp 28–34). However, this may reflect a gradual shift from early, small pilot studies using saline placebo controls and non-treatment resistant cohorts to large RCTs that use control conditions with improved blinding (i.e., midazolam or saline with a bittering agent) and investigate effects in individuals with treatment-resistant depression, leading to more modest effects. Secondly, the categorisation of studies as low or high dose involved estimates of the bioavailability of the parent compound, though emerging pre-clinical data suggest that ketamine metabolites may be more important than ketamine for antidepressant efficacy.69,70 Thirdly, studies utilising a crossover design were included in the meta-analysis. Crossover studies can be particularly problematic for ketamine interventions, due to the potential for compromised blinding from the unique, subjective drug effects.71 Additionally, there is a possibility of carryover effects (i.e., participants randomised to ketamine treatment in the first crossover may not entirely return to baseline by the second crossover), as well as withdrawal/rebound effects (i.e., relapse and subsequent worsening of mood for participants initially randomised to the ketamine treatment). To mitigate these concerns, we adopted a conservative approach to dealing with crossover studies by prioritising data from before the crossover event. Finally, an inspection of the study protocols of the 49 RCTs included in the present analyses reveals a large parameter space, which includes the use of single or repeated sessions, varying frequency of treatment delivery, routes of administration, ketamine types (racemic or esketamine), control conditions (saline or active control), treatment resistance of the sample, a wide range of doses, and whether an individualised titration approach was adopted, among others. In addition, these factors were often confounded, e.g., ketamine formulation was frequently associated with the route of administration. This limits the ability to conduct a comprehensive multivariable analysis to quantify the effect of each modifier within a single model, which has implications for future study trial designs (e.g., choice of saline or active control), and suggests the need for a platform trial to address the relative impact of each of these factors.
This meta-analysis confirmed prior findings that ketamine is effective for treating depression and racemic ketamine may be more effective than esketamine. Dosage was shown to be an important factor, with higher doses associated with superior effects relative to low doses. These findings were observed in acute efficacy after a single dose and ongoing treatment effects with repeated dosing. However, limited benefit of dose or formulation was detected regarding the durability of effects after the final dose, though few studies were available to evaluate these factors. Despite efficacy differences, no differences in acceptability were seen. Future research should investigate head-to-head randomised comparisons of esketamine and racemic ketamine and routes of administration.
Contributors
Conceptualisation: SN, AR, CL; Study design: SN, AR, CL; Literature search: SN, AS; Risk of bias assessment: SN, AS; Data extraction: SN, AS; Data verification: SN, AS, CL; Formal analysis: SN; Writing—original draft: SN; Writing—review and editing: SN, AR, CL, AS, AB, GV, CZ. All authors had access to all meta-analysed data used in this study and accept responsibility for the decision to submit the manuscript for publication.
Data sharing statement
Data collected for this systematic review and meta-analysis, including search strategy, the review protocol, and data extraction sheets, are available immediately after publication and are either published as supplementary material or can be accessed through the corresponding author.
Declaration of interests
AB has been awarded doctoral studies research funding from the Canadian Institutes of Health Research Fellowship and research funding through the Calgary Health Trust. AB receives a small honorarium for teaching undergraduate and postgraduate medical trainees in the Cumming School of Medicine at the University of Calgary. AB is an unpaid member of the Canadian Network for Mood and Anxiety Treatments editorial committee, the International Society of Addiction Journal Editors, the Canadian Society of Addiction Medicine policy committee, and the Addiction Psychiatry section of the Canadian Psychiatric Association. AB is also an unpaid associate editor of the Canadian Journal of Addiction and a mental health educator for TED-Ed, where he receives a small honorarium for supporting online educational content.
CZ is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R, 6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R, S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R, 6R)-hydroxynorketamine and (2S, 6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties the government may receive.
GV has received grants from the Canadian Institute of Health Research; Canadian Biomarker Integration Network in Depression; Canadian Ontario Ministry of Health and Long-Term Care; Queen's University Medical School (RIG and Dean's Doctoral Award); Research Innovation Fund (Providence Care Hospital); Women's Giving Circle UHKK. GV is a speaker or member of the advisory board for: Abbvie, Allergan, Janssen, Lundbeck/Otsuka, NeonMind Biosciences, Asofarma, Raffo, Gador, Eurofarma, Elea/Phoenix, Psicofarma, Tecnofarma, Sunovion, Janssen.
CL is supported by an NHMRC (Australian National Health and Medical Council) investigator Grant (1195651). CL has served on an advisory board for Janssen Cilag and a scientific advisory committee for Douglas Pharmaceuticals. She is the Medical Director of Neurostimulation/Interventional Psychiatry at the Ramsay Northside Clinic.
The remaining authors have no conflicts of interest to declare.
Acknowledgements
This study, carried out under YODA Project #2021-4658, used data obtained from the Yale University Open Data Access Project, which has an agreement with Janssen Research & Development, L.L.C. The interpretation and reporting of research using this data is solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or Janssen Research & Development, L.L.C.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102127.
Appendix A. Supplementary data
References
- 1.Xu Y., Hackett M., Carter G., et al. Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2016;19(4) doi: 10.1093/ijnp/pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kryst J., Kawalec P., Mitoraj A.M., Pilc A., Lasoń W., Brzostek T. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharmacol Rep. 2020;72(3):543–562. doi: 10.1007/s43440-020-00097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahji A., Vazquez G.H., Zarate C.A., Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2020 doi: 10.1016/j.jad.2020.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee E.E., Della Selva M.P., Liu A., Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37(2):178–184. doi: 10.1016/j.genhosppsych.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Conley A.A., Norwood A.E., Hatvany T.C., Griffith J.D., Barber K.E. Efficacy of ketamine for major depressive episodes at 2, 4, and 6-weeks post-treatment: a meta-analysis. Psychopharmacology. 2021;238(7):1737–1752. doi: 10.1007/s00213-021-05825-8. [DOI] [PubMed] [Google Scholar]
- 6.Han Y., Chen J., Zou D., et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859. doi: 10.2147/NDT.S117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romeo B., Choucha W., Fossati P., Rotge J.-Y. Meta-analysis of short-and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015;230(2):682–688. doi: 10.1016/j.psychres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre R.S., Carvalho I.P., Lui L.M., et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576. doi: 10.1016/j.jad.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 9.Loo C., Glozier N., Barton D., et al. Efficacy and safety of a 4-week course of repeated subcutaneous ketamine injections for treatment-resistant depression (KADS study): randomised double-blind active-controlled trial. B J Psych. 2023:1–9. doi: 10.1192/bjp.2023.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartberg J., Garrett-Walcott S., De Gioannis A. Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: a retrospective study. Psychopharmacology. 2018;235(2):393–398. doi: 10.1007/s00213-017-4786-3. [DOI] [PubMed] [Google Scholar]
- 11.Loo C., Gálvez V., O'keefe E., et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134(1):48–56. doi: 10.1111/acps.12572. [DOI] [PubMed] [Google Scholar]
- 12.George D., Gálvez V., Martin D., et al. Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry. 2017;25(11):1199–1209. doi: 10.1016/j.jagp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Zarate C.A., Singh J.B., Carlson P.J., et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 14.Diazgranados N., Ibrahim L., Brutsche N.E., et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman R.M., Cappiello A., Anand A., et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 16.Ochs-Ross R., Daly E.J., Zhang Y., et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Popova V., Daly E.J., Trivedi M., et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- 18.Short B., Fong J., Galvez V., Shelker W., Loo C.K. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 21.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glue P., Russell B., Medlicott N.J. Influence of formulation and route of administration on ketamine's safety and tolerability: systematic review. Eur J Clin Pharmacol. 2021;77(5):671–676. doi: 10.1007/s00228-020-03047-z. [DOI] [PubMed] [Google Scholar]
- 24.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R . Computing RFfS. 3.5.1 ed. 2020. A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- 27.Schunemann H., Brozek J., Guyatt G., Oxman A. 2013. GRADE handbook for grading quality of evidence and strength of recommendations (The GRADE Working Group)http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Google Scholar]
- 28.Zarate C.A., Brutsche N.E., Ibrahim L., et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murrough J.W., Iosifescu D.V., Chang L.C., et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sos P., Klirova M., Novak T., Kohutova B., Horacek J., Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. 2013;34(4):101–107. [PubMed] [Google Scholar]
- 31.Lai R., Katalinic N., Glue P., et al. Pilot dose–response trial of iv ketamine in treatment-resistant depression. World J Biol Psychiatry. 2014;15(7):579–584. doi: 10.3109/15622975.2014.922697. [DOI] [PubMed] [Google Scholar]
- 32.Lapidus K.A., Levitch C.F., Perez A.M., et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76(12):970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downey D., Dutta A., McKie S., et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. 2016;26(6):994–1003. doi: 10.1016/j.euroneuro.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y.-D., Xiang Y.-T., Fang J.-X., et al. Single iv ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016;46(3):623–635. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- 35.Li C.T., Chen M.H., Lin W.C., et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: a randomized controlled study. Hum Brain Mapp. 2016;37(3):1080–1090. doi: 10.1002/hbm.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh J.B., Fedgchin M., Daly E.J., et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 37.Singh J.B., Fedgchin M., Daly E., et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Grunebaum M.F., Ellis S.P., Keilp J.G., et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19(3):176–183. doi: 10.1111/bdi.12487. [DOI] [PubMed] [Google Scholar]
- 39.Grunebaum M.F., Galfalvy H.C., Choo T.-H., et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327–335. doi: 10.1176/appi.ajp.2017.17060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su T.-P., Chen M.-H., Li C.-T., et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482–2492. doi: 10.1038/npp.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabzadeh S., Hakkikazazi E., Shahmansouri N., et al. Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double-blind controlled trial. J Affect Disord. 2018;235:236–241. doi: 10.1016/j.jad.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 42.Canuso C.M., Singh J.B., Fedgchin M., et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. doi: 10.1176/appi.ajp.2018.17060720. [DOI] [PubMed] [Google Scholar]
- 43.Cao Z., Lin C.-T., Ding W., Chen M.-H., Li C.-T., Su T.-P. Identifying ketamine responses in treatment-resistant depression using a wearable forehead EEG. IEEE Trans Biomed Eng. 2018;66(6):1668–1679. doi: 10.1109/TBME.2018.2877651. [DOI] [PubMed] [Google Scholar]
- 44.Chen M.-H., Li C.-T., Lin W.-C., et al. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: a randomized control study. J Affect Disord. 2018;225:709–714. doi: 10.1016/j.jad.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Daly E.J., Singh J.B., Fedgchin M., et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fava M., Freeman M.P., Flynn M., et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol Psychiatry. 2020;25(7):1592–1603. doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gálvez V., Li A., Huggins C., et al. Repeated intranasal ketamine for treatment-resistant depression–the way to go? Results from a pilot randomised controlled trial. J Psychopharmacol. 2018;32(4):397–407. doi: 10.1177/0269881118760660. [DOI] [PubMed] [Google Scholar]
- 48.Nugent A.C., Ballard E.D., Gould T.D., et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. 2019;24(7):1040–1052. doi: 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domany Y., Bleich-Cohen M., Tarrasch R., et al. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. 2019;214(1):20–26. doi: 10.1192/bjp.2018.196. [DOI] [PubMed] [Google Scholar]
- 50.Fedgchin M., Trivedi M., Daly E.J., et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1) Int J Neuropsychopharmacol. 2019;22(10):616–630. doi: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ionescu D.F., Bentley K.H., Eikermann M., et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord. 2019;243:516–524. doi: 10.1016/j.jad.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 52.Phillips J.L., Norris S., Talbot J., et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176(5):401–409. doi: 10.1176/appi.ajp.2018.18070834. [DOI] [PubMed] [Google Scholar]
- 53.Domany Y., Shelton R.C., McCullumsmith C.B. Ketamine for acute suicidal ideation. An emergency department intervention: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Depress Anxiety. 2020;37(3):224–233. doi: 10.1002/da.22975. [DOI] [PubMed] [Google Scholar]
- 54.Fu D.-J., Ionescu D.F., Li X., et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I) J Clin Psychiatry. 2020;81(3):6605. doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- 55.Milak M.S., Rashid R., Dong Z., et al. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of Glx and GABA responses in adults with major depression: a randomized clinical trial. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiroma P.R., Thuras P., Wels J., et al. A randomized, double-blind, active placebo-controlled study of efficacy, safety, and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression. Transl Psychiatry. 2020;10(1):1–9. doi: 10.1038/s41398-020-00897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumner R.L., McMillan R., Spriggs M.J., et al. Ketamine enhances visual sensory evoked potential long-term potentiation in patients with major depressive disorder. Biol Psychiatry. 2020;5(1):45–55. doi: 10.1016/j.bpsc.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Tiger M., Veldman E.R., Ekman C.-J., Halldin C., Svenningsson P., Lundberg J. A randomized placebo-controlled PET study of ketamine' s effect on serotonin1B receptor binding in patients with SSRI-resistant depression. Transl Psychiatry. 2020;10(1):159. doi: 10.1038/s41398-020-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dwyer J.B., Landeros-Weisenberger A., Johnson J.A., et al. Efficacy of intravenous ketamine in adolescent treatment-resistant depression: a randomized midazolam-controlled trial. Am J Psychiatry. 2021;178(4):352–362. doi: 10.1176/appi.ajp.2020.20010018. [DOI] [PubMed] [Google Scholar]
- 60.Ionescu D.F., Fu D.-J., Qiu X., et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II) Int J Neuropsychopharmacol. 2021;24(1):22–31. doi: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lijffijt M., Murphy N., Iqbal S., et al. Identification of an optimal dose of intravenous ketamine for late-life treatment-resistant depression: a Bayesian adaptive randomization trial. Neuropsychopharmacology. 2022;47(5):1088–1095. doi: 10.1038/s41386-021-01242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi N., Yamada A., Shiraishi A., Shimizu H., Goto R., Tominaga Y. Efficacy and safety of fixed doses of intranasal Esketamine as an add-on therapy to Oral antidepressants in Japanese patients with treatment-resistant depression: a phase 2b randomized clinical study. BMC Psychiatry. 2021;21(1):1–13. doi: 10.1186/s12888-021-03538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinyor M., Williams M., Belo S., et al. Ketamine augmentation for major depressive disorder and suicidal ideation: preliminary experience in an inpatient psychiatry setting. J Affect Disord. 2018;241:103–109. doi: 10.1016/j.jad.2018.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallagher B., Foley M., Slattery C.M., Gusciute G., Shanahan E., McLoughlin D.M. Ketamine as an adjunctive therapy for major depression-a randomised controlled pragmatic pilot trial (Karma-Dep Trial) HRB Open Res. 2020;3:90. doi: 10.12688/hrbopenres.13182.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed G.K., Elserogy Y.M., Elfadl G.M.A., Abdelsalam K.G., Ali M.A. Antidepressant and anti-suicidal effects of ketamine in treatment-resistant depression associated with psychiatric and personality comorbidities: a double-blind randomized trial. J Affect Disord. 2023;325:127. doi: 10.1016/j.jad.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Correia-Melo F.S., Leal G.C., Vieira F., et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–534. doi: 10.1016/j.jad.2019.11.086. [DOI] [PubMed] [Google Scholar]
- 67.Singh J.B., Daly E.J., Mathews M., et al. Approval of esketamine for treatment-resistant depression. Lancet Psychiatry. 2020;7(3):232–235. doi: 10.1016/S2215-0366(19)30533-4. [DOI] [PubMed] [Google Scholar]
- 68.Zhu W., Ding Z., Zhang Y., Shi J., Hashimoto K., Lu L. Risks associated with misuse of ketamine as a rapid-acting antidepressant. Neurosci Bull. 2016;32(6):557–564. doi: 10.1007/s12264-016-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanos P., Moaddel R., Morris P.J., et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J.-C., Li S.-X., Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 71.Muthukumaraswamy S.D., Forsyth A., Lumley T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol. 2021;14(9):1133–1152. doi: 10.1080/17512433.2021.1933434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.