Abstract
Biofilm-based infections pose a serious threat to public health. Biofilms are surface-attached communities of microorganisms, most commonly bacteria and yeast, residing in an extracellular polymeric substance (EPS). The EPS is composed of several secreted biomolecules that shield the microorganisms from harsh environmental stressors and promote antibiotic resistance. Due to the increasing prominence of multidrug-resistant microorganisms and a decreased development of bactericidal agents in clinical production, there is an increasing need to discover alternative targets and treatment regimens for biofilm-based infections. One promising strategy to combat antibiotic resistance in biofilm-forming bacteria is to trigger biofilm dispersal, which is a natural part of the bacterial biofilm life cycle. One signal for biofilm dispersal is the diatomic gas nitric oxide (NO). Low intracellular levels of NO have been well documented to rapidly disperse biofilm macrostructures and are sensed by a widely conserved NO-sensory protein, NosP, in many pathogenic bacteria. When bound to heme and ligated to NO, NosP inhibits the autophosphorylation of NosP’s associated histidine kinase, NahK, reducing overall biofilm formation. This reduction in biofilm formation is regulated by the decrease in secondary metabolite bis-(3’–5’)-cyclic dimeric guanosine monophosphate (c-di-GMP). The NosP/NahK signaling pathway is also associated with other major regulatory systems in the maturation of bacterial biofilms, including virulence and quorum sensing. In this review, we will focus on recent discoveries investigating NosP, NahK and NO-mediated biofilm dispersal in pathogenic bacteria.
1. Introduction
Biofilms are surface-bound, multicellular communities of microorganisms that reside and reproduce in an extracellular polymeric substance (EPS)1. Biofilm formation and secretion of the EPS, which is composed of secreted glycoproteins, polysaccharides, eDNA and glycolipids, limit the amount of antibiotic and chemical stresses that can enter the bacteria. This allows the bacteria to survive in harsh environmental conditions including extreme pH, UV exposure, temperature changes and low oxygen, promoting antibiotic tolerance2,3,4. In addition to gaining a protective layer, the EPS also enhances both cellular cohesion and surface adhesion; this increase allows for the bacteria to form biofilms on inorganic matter, most notably ventilators, catheters, and other medical equipment. As a result, hospitals are common sites of multidrug-resistant organisms5. Mature biofilms have multiple microenvironments. Bacteria on the outer layers of the biofilm will rapidly proliferate, whereas the bacteria in the deeper layers are dormant, allowing the biofilm to persist over time. The EPS and metabolic dormancy of the deeper layers allows for both adaptive and non-adaptive mechanisms for antibiotic resistance. Biofilm-forming pathogens have increasing resistance to bactericidal agents each year, and in response, institutions are examining alternative methods to combat biofilm formation. One promising strategy to circumvent antibiotic-resistant biofilms is to trigger biofilm dispersal, a native biofilm life-cycle event that allows the bacteria to leave the protection of the biofilm, therefore becoming more susceptible to current antibiotics6.
Nitric oxide (NO) is a diatomic gas that can penetrate the biofilm and serves as an important signaling molecule at pico-to-nanomolar intracellular concentrations. NO triggers biofilm dispersal through modulation of quorum sensing (QS) and biofilm-related processes, including flagella biosynthesis, modulation of secretion systems and chemotaxis factors, and EPS degradation 7,8,9,10. However, at higher intracellular concentrations, NO can become toxic by directly reacting with DNA, lipids, and proteins, resulting in oxidative damage and cleavage of macromolecules11. Accumulation of intracellular NO interferes with bacterial stress responses and cell morphology11. Bacteria thus have evolved multiple mechanisms to maintain intracellular NO concentrations12. Although the effect of NO on biofilm development has been documented, the proteins and molecular mechanisms that drive these NO-driven processes remain largely uncharacterized13,14.
Previous investigations into NO-mediated biofilm dispersal identified a class of bacterial heme-binding NO/O2 sensing proteins (H-NOXs)15,16,17. Although H-NOX proteins have since been well studied, many pathogenic bacteria that respond to low levels of NO either do not encode an hnoX gene or have more than one NO sensor18. Further investigation led our lab to discover a new family of nitric oxide sensing proteins (NosPs). Similar to H-NOXs, NosPs are hemoproteins. NosP is commonly found in the operons of two-component signaling systems (TCSSs), which enable bacteria to adapt to different environmental conditions by sensing and responding to external signals. TCSSs generally involve a phosphorelay from a conserved histidine residue of a histidine kinase (HK) to a conserved aspartate residue on its cognate response regulator (RR), thereby initiating a cellular response. In certain cases, the HK might also possess a receiver domain (hybrid histidine kinase; HHK) with a conserved aspartate residue allowing for an internal histidine-to-aspartate phosphorelay.
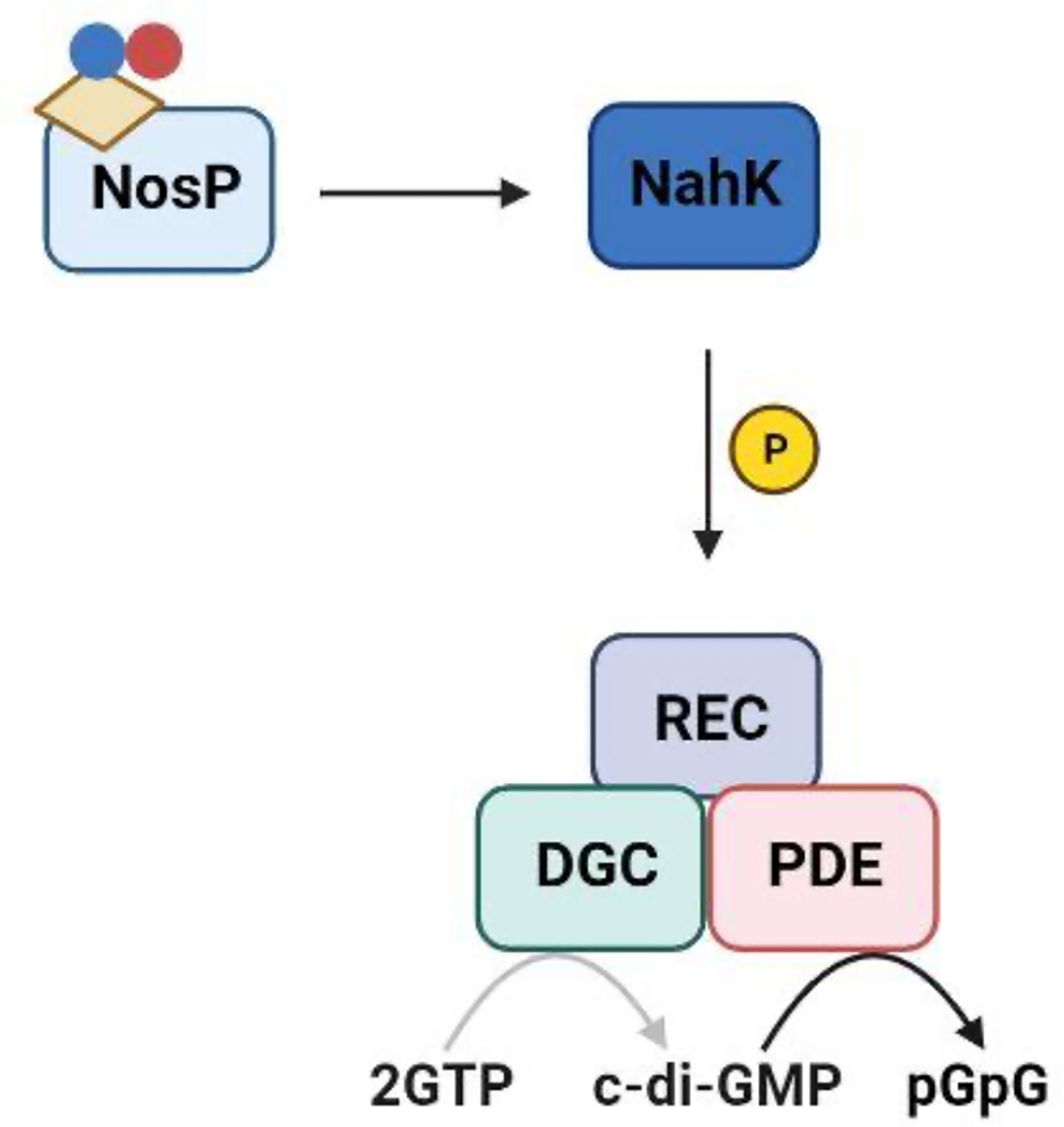
nosP is typically encoded next to a HK (or HHK) which we have named nahK (NosP-associated histidine kinase). Although the arrangement of the nosP/nahK TCSS operon varies from species to species, our lab has shown that NosP regulates the kinase activity of NahK as a function of iron oxidation and ligation state, eventually modulating chemotaxis proteins, flagellar biosynthesis and intracellular c-di-GMP (bis-(3’–5’)-cyclic dimeric guanosine monophosphate) (Figure 1). Change in c-di-GMP concentrations is especially relevant to NosP signaling, as c-di-GMP is a bacterial secondary messenger that regulates cellular processes including attachment, virulence and most importantly, biofilm formation19. Several studies have shown that an increase in intracellular c-di-GMP promotes biofilm formation, while a decrease leads to biofilm dispersal. These fluctuations in concentration are dynamically regulated by two enzyme classes: diguanylate cyclases (DGCs) that cyclize two GTP molecules to form c-di-GMP, and phosphodiesterases (PDEs), which breakdown c-di-GMP to form the linear molecule 5’-phosphoguanylyl-(3’→5’)-guanosine (pGpG) or two GMPs19. Interestingly, these enzymes are often encoded in the same operon as nosP or hnoX, indicating the direct involvement of c-di-GMP metabolism in the NO-triggered biofilm dispersal mediated by these hemoproteins.
Figure 1. NosP/NahK TCSSs regulate various processes amongst bacterial species.
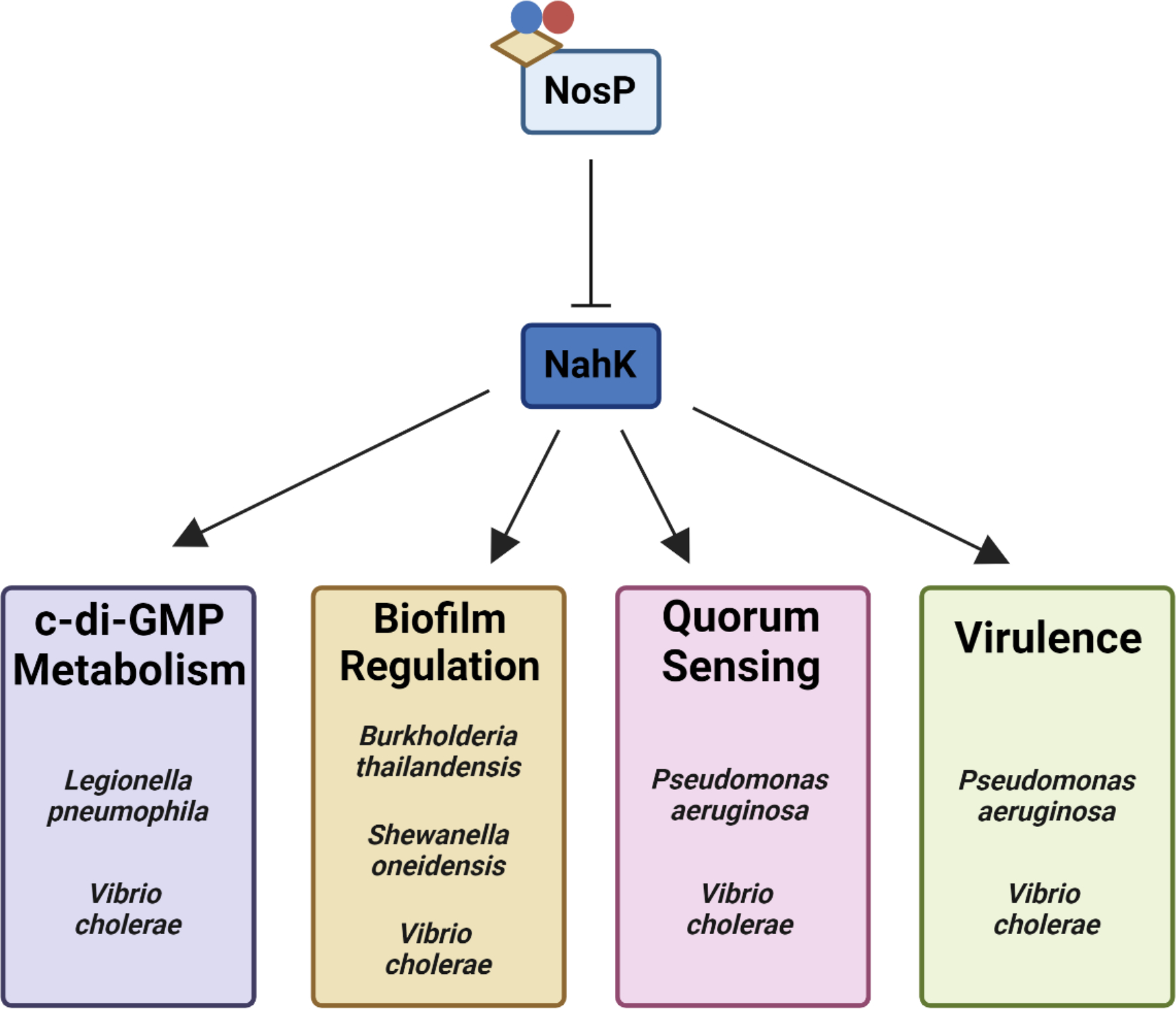
NosP is a heme-binding, NO-sensing protein that functions to inhibit the kinase activity of NosP’s associated histidine kinase, NahK. It has been reported that disruption of NahK activity disrupts various bacterial processes amongst Gram-negative biofilm-forming pathogens, including c-di-GMP metabolism, biofilm formation and dispersal, QS and virulence.62 In all figures, the beige parallelogram represents heme and the blue and red circles represent NO.
Currently, most studies on NO sensing in bacteria do not investigate the role of having multiple sensors. It is unknown why some bacteria may encode for a combination of H-NOXs and NosPs while other species have only one or neither. Below, where relevant, the contributions of H-NOX signaling to the biology of NosP signaling or general NO sensing will be discussed, however, the primary focus of this review is on NosP signaling. Here, we focus on recent reports of NosP/NahK signaling and the effect these proteins have on biofilm development and intracellular mechanisms16,20,21,22,23,25,25,26,27.
Computational Insight into the NosP Protein
Although a high-resolution structure of NosP has not been experimentally determined, multiple sequence alignments, protein structure prediction, and sequence similarity networking have provided an insight into the molecular mechanisms and homology of NosP that was previously unknown (Figure 2). NosP proteins commonly annotate to have both an N-terminal and a C-terminal FIST (F-Box Intracellular Signal Transduction) domain, a protein domain originally believed to be involved in small-molecule ligand detection and signal transduction28. AlphaFold 2.0 Protein Structure Database29,30 was used to generate a computational model of NosP to understand how the two annotated FIST domains are predicted to assemble after protein folding (Figure 2A). NosP is predicted to fold into two globular subunits, hereafter referred to as the tri-symmetrical base and the disordered cap. The N-terminal FIST domain (Figure 2A; red) is predicted to form two of the symmetrical units of the tri-symmetrical base, each unit consisting of two alpha helices and five antiparallel beta-strands, while the C-terminal FIST domain (Figure 2A; blue) is predicted to form the third symmetrical unit of the base and a majority of the disordered cap. Overlaying the predicted structures of several NosPs reveals that, although the amino acid sequences of the NosP proteins vary (~30% sequence similarity), the predicted NosP structures have a high structural similarity, predicting an RMSD ranging from 3.789 Å to 5.842 Å across all 366 amino acids (Figure 2B).
Figure 2: NosP is a structurally conserved protein more present in hnoX deficient bacteria.
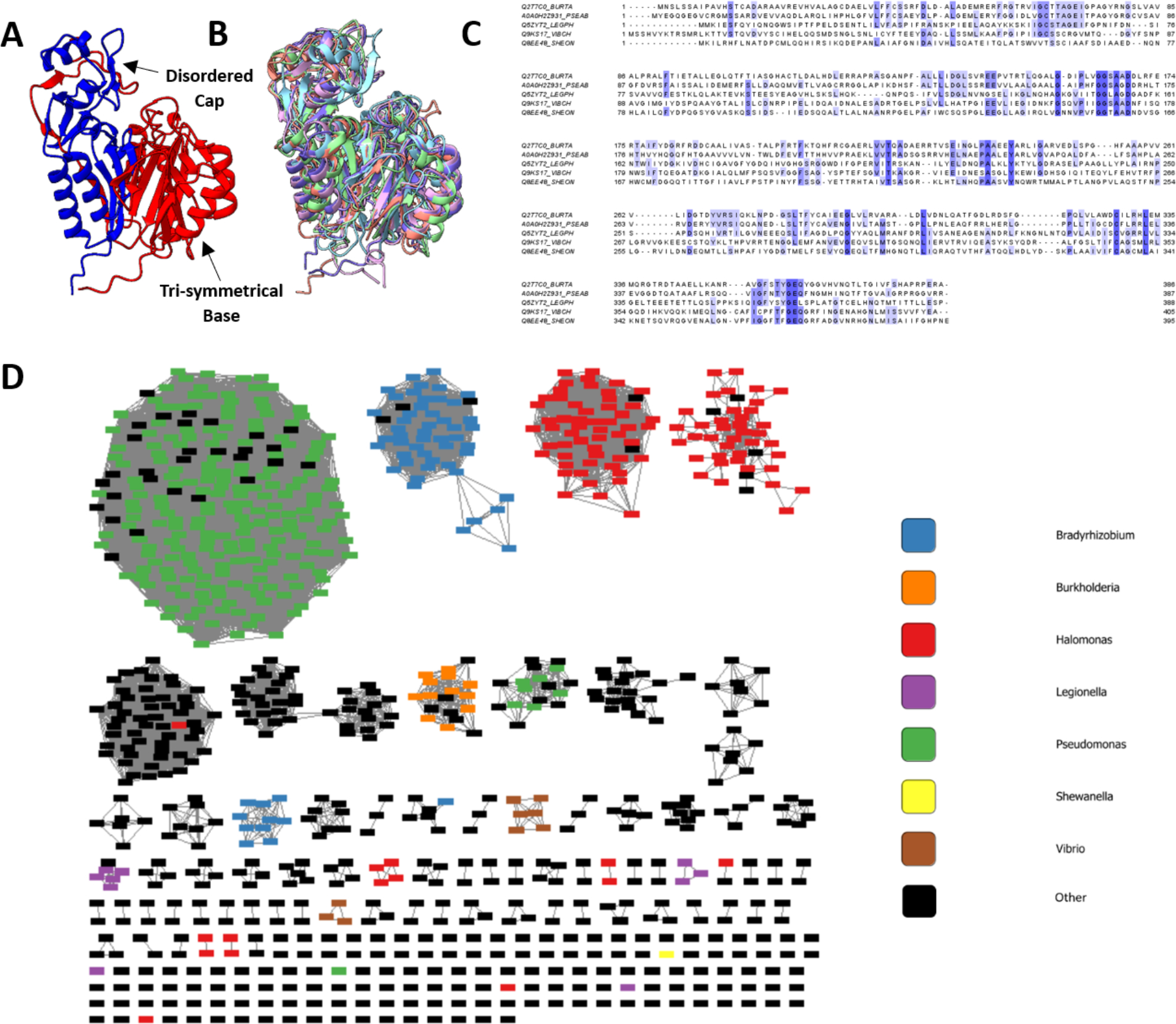
A. AlphaFold 2.0 Protein Structure Prediction Database predicts the Burkholderia thailandensis NosP to form a globular protein consisting of two distinct subunits, a tri-symmetrical base and a disordered cap. FIST_N (red) composes two of the symmetrical units of the base, while FIST_C composes the third symmetrical unit as well as the disordered cap. B. Using the matchmaker function on ChimeraX to visualize the protein overlay, the calculated structures of NosP from Burkholderia thailandesis, Legionella pneumophila, Pseudomonas aeruginosa, Shewanella oneidensis, and Vibrio cholerae have high structural similarity with calculated RMSDs from 3.789 Å to 5.842 Å across all 366 amino acids. C. Multiple sequence alignment of the amino acid NosP sequences from B. thailandesis, L. pneumophila, P. aeruginosa, S. oneidensis, and V. cholerae highlight several conserved amino acids across the N- and C-terminal FIST domains. D. Sequence Similarity Network (SSN) of Burkholderia thailandensis NosP generated using the EFI - Enzyme Similarity Tool. The SSN was visualized using Cytoscape with a %id edge filter set to 75%.
Multiple sequence alignment of the NosP protein across bacterial species discussed here reveals several conserved amino acids that have been identified previously as structural motifs of the FIST domain, such as several conserved glycine residues located across the protein and the highly conserved cysteine residue located on the 19th beta-strand of the protein (Figure 2C)28. Although these conserved amino acids have been previously noted, the role of each conserved residue in the overall function of NosP remains undetermined. The EFI - Enzyme Similarity Tool31,32 was used to generate a sequence similarity network (SSN) of Burkholderia thailandensis NosP, which highlights NosP as an alternative NO-sensing protein to H-NOX. Filtering the NosP amino acid sequences by a percent identity edge filter of 75%, reveals that as a majority of the clustered NosP sequences are present in hnoX-deficient bacteria, such as Pseudomonas, Bradyrhizobium, Halomonas, and Marinobacter (Figure 2D); combining this information with the computationally derived high structural similarity of the NosP proteins allows us to conclude that NosP is a structurally conserved protein, more prevalent in hnoX-deficient bacteria.
Heme-responsive NosP signaling in Burkholderia thailandensis
While NosP has been predominantly characterized as a NO-sensing hemoprotein, we have recently discovered a novel heme-sensing function for NosP in Burkholderia thailandensis (B. thailandensis), which is a model organism for its BSL3 homologue Burkholderia pseudomallei, the causative agent of Melioidosis20. In this bacterium, NosP (Bth_ii0731) is co-cistronic with an HK, BtNahK (Bth_ii0733), that can transfer a phosphoryl group to its cognate RR, NarR (NosP associated RR) (Bth_ii0731), a degenerate c-di-GMP phosphodiesterase containing an HD-GYP motif. BtNosP has flexible heme-binding properties reminiscent of heme-responsive sensor proteins, which are a class of proteins that affect transcription, translation or heme degradation based on reversible binding of labile heme. In vitro, BtNosP differentially regulates BtNahK activity, based on the presence or absence of heme; FeIII holo-BtNosP interacts strongly with BtNahK, repressing autophosphorylation in a concentration-dependent manner, while apo-BtNosP has weak interaction and minimal effect on BtNahK autophosphorylation (Figure 3). Therefore, the presence of heme acts as a signal for regulating BtNahK activity, suggesting a heme-responsive function for BtNosP. This function was also reflected in vivo in a static biofilm assay, where the wildtype strain formed less biofilm with increasing heme concentration, resembling a BtnahK deletion mutant. This was due to BtNosP mostly existing in its holo form, which represses BtNahK autophosphorylation. This recent evidence suggests that labile heme sensing by BtNosP might be just as significant as NO sensing in influencing bacterial biofilms. It also suggests that BtNosP may not exist only as a holo-protein inside the bacteria and may bind heme only under specific circumstances, depending on the availability of heme. Since BtNosP also displays NO sensing function in vitro, it would be interesting to investigate whether NO exerts its effect by altering BtNosP heme binding properties, as recently demonstrated in eukaryotic hemoproteins such as soluble guanylate cyclases and NO synthases, where NO either drives or blocks heme incorporation, respectively33,34,35.
Figure 3. NosP signaling pathway in B. thailandensis.
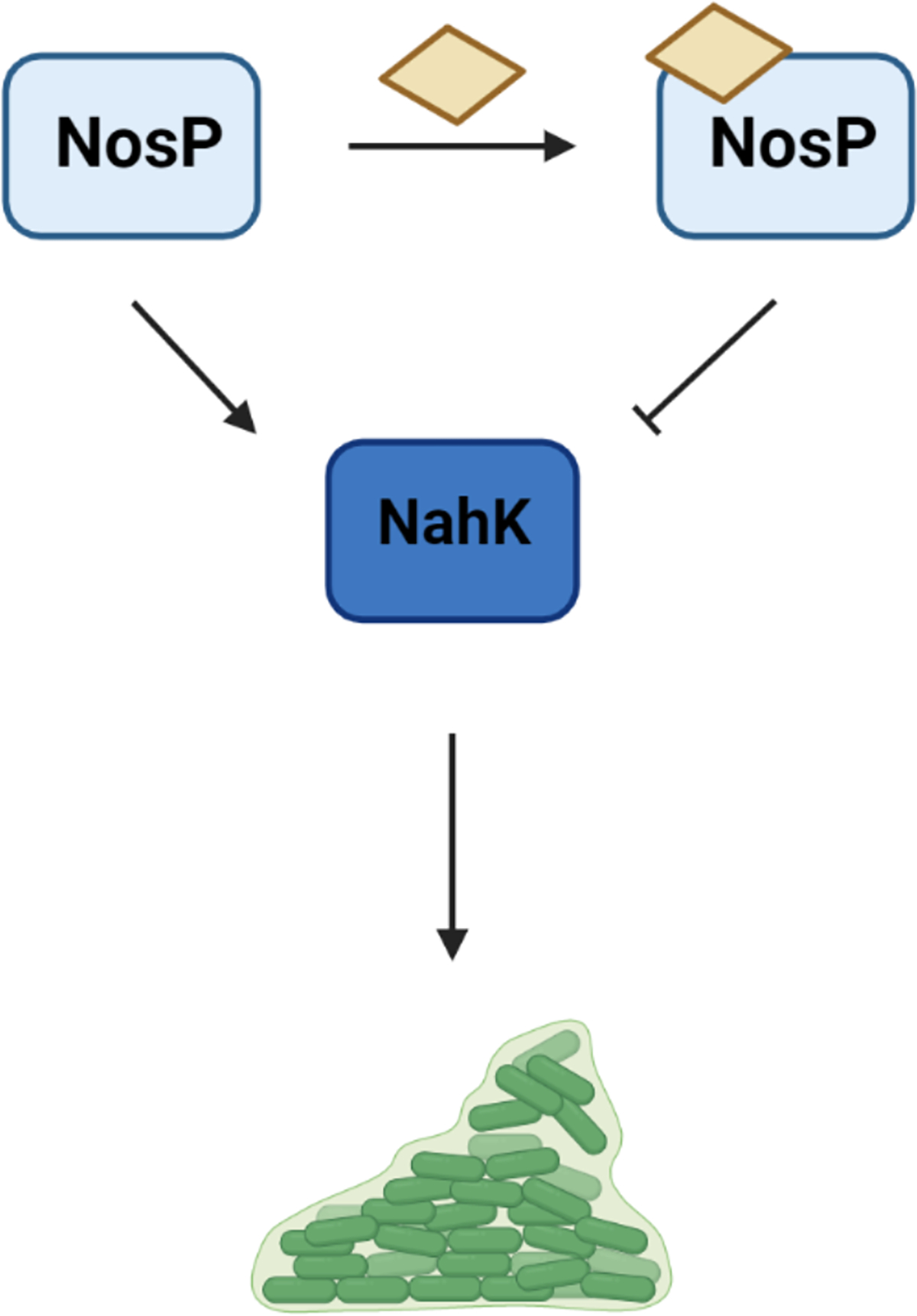
In B. thailandensis, BtNosP bound to heme (yellow parallelogram) inhibits the autophosphorylation activity of its co-cistronic BtNahK, leading to a decreased biofilm formation phenotype, similar to a ΔBtnahK mutant strain.62
NO signaling in Vibrio cholerae
The Vibrio cholerae (V. cholerae) genome encodes three putative NO sensors - one H-NOX and two NosPs. Out of the two NosPs, one exists as part of a multifunctional protein, CdpA (Vc0130), and the other as a stand-alone protein, VcNosP (Vc1444 or VspV)36,21,22. Both VcH-NOX (VCA0720) and CdpA have been demonstrated to affect c-di-GMP levels upon ligating NO. VcH-NOX is part of a complex multi-component network, which comprises an H-NOX associated HK HnoK (VCA0719) and two response regulators, one with an active EAL domain (HnoB, VC1086) and the other with a degenerate HD-GYP domain (HnoD, VC1087). Upon ligating NO, VcH-NOX inhibits the autophosphorylation of HnoK and thereafter the phosphotransfer to HnoB, inhibiting its PDE activity20,37.
CdpA affects c-di-GMP concentration by modulating the activity of its own C-terminal PDE domain, as a response to heme or NO. CdpA has been previously shown to be involved in biofilm regulation; inactivation of CdpA increased c-di-GMP levels 4-fold and biofilm formation 3-fold. Recent studies have shown that the PDE activity of CdpA can be directly affected by heme binding to the NosP domain39,23. Apo-CdpA can completely turnover c-di-GMP in 1 hour, and this PDE activity is greatly inhibited upon incubation with heme, indicating that the NosP domain in CdpA acts as a heme-responsive sensor, similar to what was recently shown in B. thailandensis.
CdpA also exhibits an NO-sensing function, as NO ligation by holo-CdpA resulted in rapid cell detachment due to decreased intracellular concentrations of c-di-GMP (Figure 4A–B). This was attributed to retraction of mannose sensitive hemagglutinin (MSHA) pili, which is used by V. cholerae to attach to surfaces39. In the presence of c-di-GMP, MSHA pili extends and facilitates attachment when c-di-GMP binds to the extension motor MshE (Figure 4A). When NO ligation activates CdpA, c-di-GMP is broken down and no longer binds to MshE, thereby causing pili retraction and subsequent cell detachment (Figure 4B)22. This dual functionality of CdpA in response to both heme and NO also suggests a possible effect of NO on incorporating heme into CdpA worth exploring in future studies.
Figure 4. NosP signaling in V. cholerae.
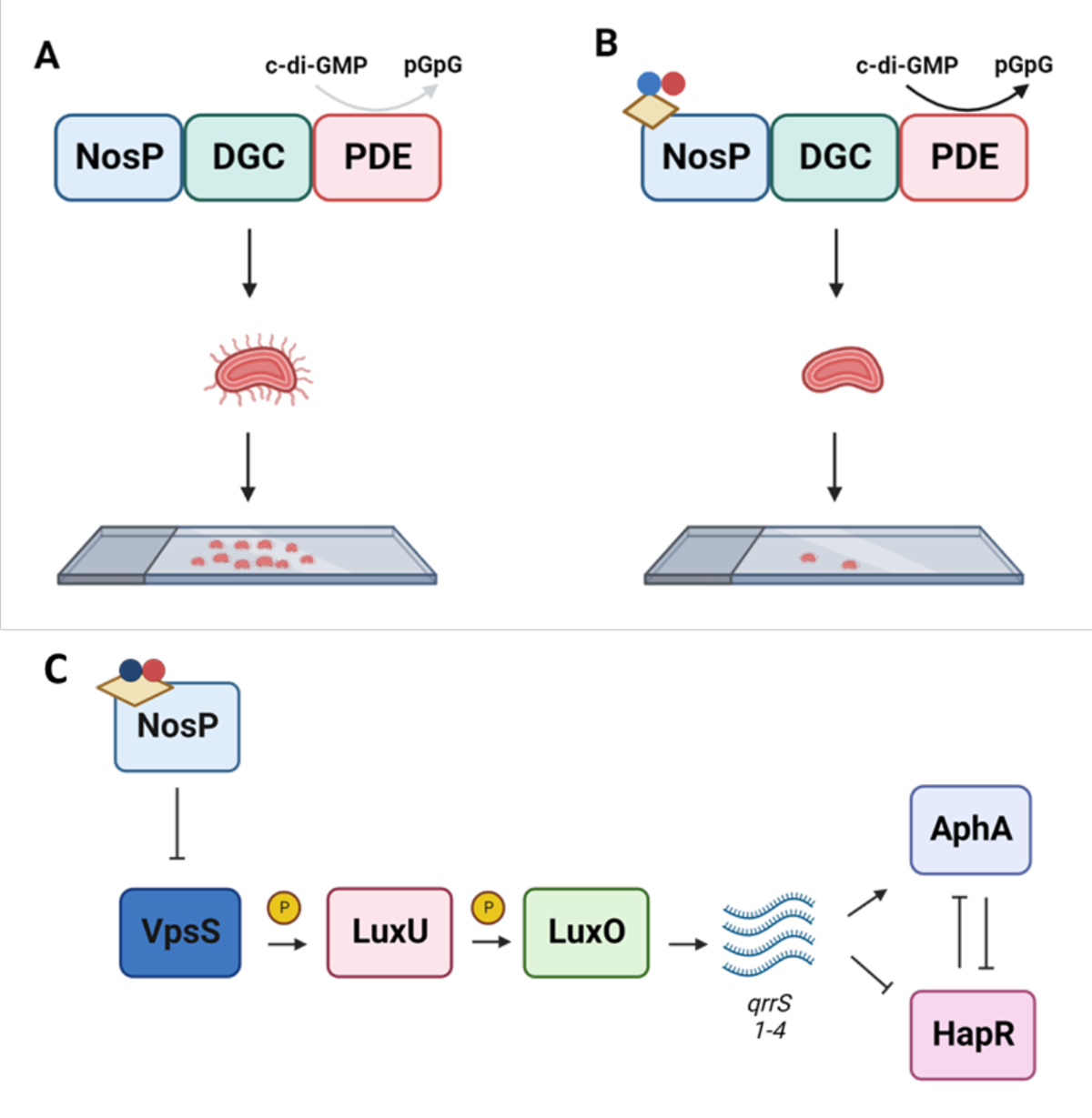
A. The NosP domain of CdpA is required for bacterial attachment in V. cholerae. Inhibition of the PDE activity of CdpA contributes to an accumulation of c-di-GMP which binds to MSHA pili. This allows for the pili to extend and promotes bacterial attachment. B. In the presence of NO, the NosP domain will promote PDE activity of CdpA contributing to pili retraction and bacterial detachment. C. The NosP (VpsV) and VpsS signaling cascade in the LuxU QS circuit. When NosP ligates ferrous NO, it inhibits VpsS, preventing VpsS from phosphorylating LuxU. This prevents QS signaling that leads to biofilm formation; overall, inactivation of VpsS promotes biofilm dispersal. Yellow parallelogram - heme, nitrogen - blue, oxygen - red.62
The second NosP protein in V. cholerae, VcNosP exists as a standalone protein in the same operon as a HHK VpsS (Vc1445) which is a part of a larger QS circuit21. QS controls community-based behaviors in bacterial cultures, including biofilm formation, virulence factor and secreted product production and swarming motility40. NO signaling is known to influence QS and biofilm formation in many bacteria and both H-NOX and NosP NO-responsive pathways have been shown to directly affect QS16,21,41,25. VpsS can phosphotransfer with LuxU, a QS transcription factor that downstream activates transcription of small regulatory RNAs qrrs 1–4. Activation of qrrs 1–4 leads to the stabilization of AphA, which leads to an increase in biofilm formation (Figure 4C)42. Here, NO signaling can act as a switch to activate QS signaling. In the presence of NO, VcNosP inhibits VpsS autophosphorylation, leading to decreased phosphorylation of LuxU, overall promoting biofilm dispersal21.
However, NO may not be the only signal that regulates the VpsVS signaling cascade. VpsS is one of several kinases that make up the LuxU QS circuit21. Currently, it is unclear what the individual role of each kinase is in this network, however, each kinase including VpsS is a HHK with a signaling domain. VpsS is predicted to have a PAS-like domain that responds to an unknown signal. It has been predicted that the sensor domain of VpsS may bind a QS autoinducer because all other kinases in the LuxU QS circuit bind QS autoinducers21. Overexpression of vpsS alone is sufficient for colonization of the mouse intestine, yet it is currently unknown how NO signaling affects QS and the VpsS signaling pathway in vivo43,44.
NosP signaling in Pseudomonas aeruginosa
In P. aeruginosa, PaNosP (PA1975/PA14_38990) is co-cistronic with a HHK, PaNahK (PA1976/ PA14_38970). There is no conserved hnoX in P. aeruginosa45. PaNahK has been previously identified as one of four kinases in a TCSS with phospho-transfer protein HptB. HptB is implicated as a “hub” in the GacS Multi-Kinase Network (MKN), which is the network that regulates the planktonic-to-sessile switch in P. aeruginosa (Figure 5)46,47. These kinases (GacS, PA1611, RetS, SagS and PaNahK) indirectly regulate activity of post-transcriptional global regulator protein, RsmA47,48. When active, RsmA inhibits translation of mRNAs related to biofilm formation and decreases c-di-GMP production, pushing towards the planktonic state47. Phosphorylation of HptB by the GacS MKN kinases drives inactivation of RsmA through the transcriptional upregulation of small regulatory RNA rsmY. This leads to de-repression of biofilm formation mRNAs, activating biofilm formation and increasing c-di-GMP production (Figure 5)48. All kinases in this network are HHKs that respond to extracellular signals, such as calcium ions and mucin glycans49. Through ligation to PaNosP, NO acts as one signal transduced through the GacS MKN to regulate the planktonic-to-sessile switch. In addition, PaNahK is a HHK containing several PAS domains of unknown function; it is currently unclear how these sensory domains modulate kinase activity.
Figure 5. NosP signals in the GacS MKN in P. aeruginosa PA14.
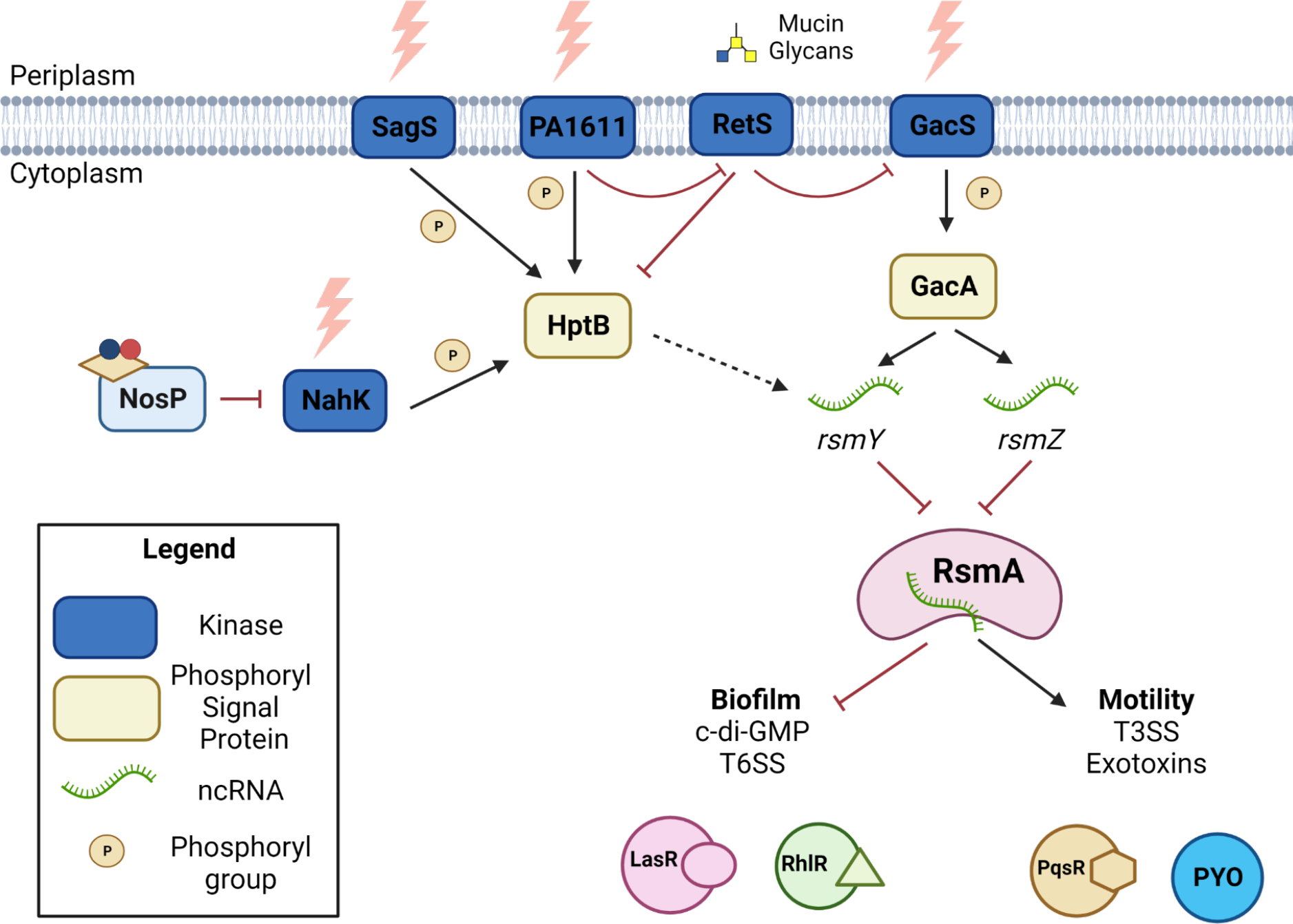
When PaNosP is not ligated to ferrous NO, PaNahK auto-phosphorylates and transfers a phosphoryl group to HptB. Downstream, this phosphorylation of HptB promotes transcriptional activation of sRNA rsmY. rsmY is an inhibitor of post-transcriptional regulator RsmA. rsmZ is another sRNA that inhibits RsmA, but is only known to be transcriptionally regulated by GacA. RsmA activity promotes PQS and PYO production while inhibiting las and rhl production. Yellow parallelogram - heme, nitrogen - blue, oxygen - red.62
In PA14, deletion of PanahK leads to an upregulation of pyocyanin (PYO), the main phenazine exotoxin that is a terminal output for QS systems. It was found that this upregulation of PYO is due to overproduction of the Pseudomonas quinolone signal (PQS) QS system. The PQS system produces 2-heptyl-3-hydroxy-4(1H)-quinolone as the signaling molecule that drives changes in gene expression. PQS has mostly been studied in its regulatory role for controlling transcription of phz2, the phenazine operon that produces most of the phenazines in infection and biofilm models51. The phenazines, including PYO, have a dual role in promoting P. aeruginosa health during infection by promoting electron shuttling in microaerobic layers of the biofilm while also generating reactive oxygen species against host mitochondrial respiratory chain machinery52,53.
PQS has also been shown to activate the P. aeruginosa denitrification system, contributing to intracellular NO levels. Excess PQS directly increases NIR reductase activity (and only NIR), which converts nitrite to NO. This suggests that there is a role for PQS in accumulation of intracellular NO, which may promote further NO signaling in P. aeruginosa54. NO detection and QS may also work together to regulate denitrification activity10. The NO sensor DNR (dissimilative nitrate respiration regulator), which modulates denitrification activity in the presence of NO, is transcriptionally upregulated in QS-disrupted strains, suggesting that QS is required to prevent the generation of excess NO53.
The relationship between NO and QS also may play an important role during an infection. Mammalian hosts typically encode for NOS (NO synthase) proteins that are activated in response to infection to curb microbial growth55. It is advantageous to the host immune system to disperse biofilms, as planktonic bacteria are more susceptible to host effectors and phagocytosis56. However, bacteria have also developed ways to counteract this nitrosative stress. By linking NO to QS, NO can act as a trigger to activate expression of QS-mediated virulence factors16,21,24. This potential feedback loop between NO and QS could be advantageous to the bacteria to promote survival and acquisition of nutrients. As a result, understanding how NO sensing and QS work together in mature biofilms must be further explored in bacterial infections.
NO signaling in Legionella pneumophila
In Legionella pneumophila, 2 H-NOX, H-NOX1 (lpg1056) and H-NOX2 (lpg2459), and 1 NosP (Lpg0279) exist, out of which both H-NOX1 and NosP can modulate c-di-GMP levels in response to NO57,58,37, while hnox2 is encoded in an operon with a HK and CheY-like RR that has not shown any effect on biofilm regulation. While H-NOX1 directly represses the DGC activity of the GGDEF-EAL enzyme lpg1057, NosP does so by activating its co-cistronic kinase (Lpg0278) which then phosphorylates the c-di-GMP metabolizing bifunctional enzyme NarR (Lpg0277), to generate an overall decrease in c-di-GMP through a decrease in DGC activity and increase in PDE activity (Figure 6). The direct involvement of both LpgNarR and Lpg1057 in biofilm regulation has been shown in the L. pneumophila Lens strain; the deletion of lpl0329, the Lens homologue of LpgNarR, increased biofilm formation while deletion of lpl1054, the Lens homologue of Lpg1057, promotes biofilm dispersal in the presence of NO compared to the wildtype strain59. The latter is presumably due to NO signaling by NosP as H-NOX2 does not affect biofilms. This also hints at the possibility that in the wildtype Lens strain, H-NOX1 might hinder the NO-NosP signaling pathway leading to biofilm dispersal, which could be explored in future studies. Besides biofilm regulation, the lpgnosP-nahK-narR operon is also involved in facilitating the persistence of L. pneumophila under low nutrient conditions, such as in the mature infectious forms found within protozoan hosts under prolonged starvation60.
Figure 6. NosP signaling pathway in L. pneumophila.
In L. pneumophila, NO (nitrogen – blue, oxygen – red) ligation by the heme (yellow parallelogram) bound NosP activates NahK autophosphorylation, causing an overall decrease in c-di-GMP concentration, due to an increase in PDE activity and decrease in DGC activity of the bifunctional response regulator NarR.62
HNOX and NosP Crosstalk in Shewanella oneidensis
Similar to Legionella pneumophila, in Shewanella oneidensis, NO-mediated biofilm dispersal occurs through changes in c-di-GMP concentrations. S. oneidensis encodes both a NosP (So_2542) and an H-NOX (So_2144), as well as associated histidine kinases (SoNahK/So_2543, SoHnoK/So_2145) for each NO sensor respectively24. Each of these kinases phosphorylate the response regulators HnoB, HnoC and HnoD which all directly or indirectly regulate intracellular c-di-GMP61. In S. oneidensis, SoNosP/SoNahK complex serves as a master regulator for biofilm formation through modulation of both SoHnoK and SoHnoB activity. In the absence of NO, SoNosP/SoNahK will promote biofilm formation and accumulation of c-di-GMP through inhibition of HnoK autophosphorylation. This prevents HnoK from activating HnoB, which has PDE activity, because HnoK cannot phosphorylate HnoD. De-phosphorylated HnoD directly inhibits the PDE activity of HnoB, overall promoting biofilm formation (Figure 7A). In the presence of NO, in vivo, SoNosP overall no longer inhibits HnoK activity. HnoK can then phosphorylate HnoD and HnoB, which relieves the repression of HnoB by HnoD (Figure 7B). Phosphorylated SoNahK prefers to phosphorylate HnoC, however, it is unknown what phenotypes HnoC contributes to. The combination of the decrease in HnoD activity and the increase in HnoB’s PDE activity contributes to the biofilm dispersal phenotype, however, it is unknown how the phosphorylation state of HnoB may contribute to its PDE activity27. Disruption of the SoNosP/SoNahK TCSS results in a decrease of c-di-GMP-mediated processes27. Genomic deletion of SonosP and SonahK resulted in a significant decrease of intracellular c-di-GMP when compared to planktonically grown wildtype. 24-hour flow cell biofilms of ΔSonosP and ΔSonahK show an increase in attached biomass and monolayer thickness compared to wildtype. After 48 hours mutant strains fail to develop and mature biofilm macrostructures, most likely due to the decreased concentration of intracellular c-di-GMP27. Future studies could investigate the contribution of H-NOX to HnoK activation in vivo, as it may fine-tune the HnoK response depending on ligation state. Similarly, the consequence of SoNahK phosphorylation to each response regulator could be investigated.
Figure 7. NosP signaling modulates biofilm formation through regulation of c-di-GMP in Shewanella oneidensis.
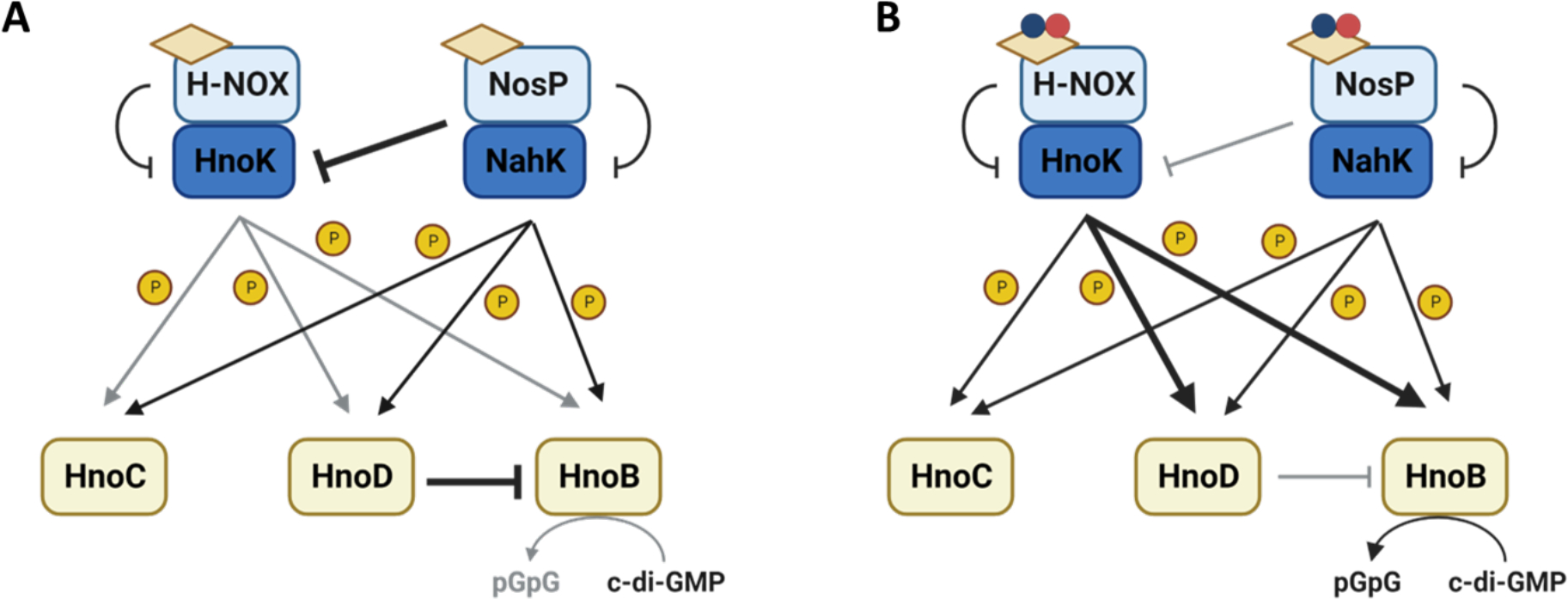
A. Both NosP and NahK promote biofilm formation in the absence of NO by inhibiting HnoK. This inhibition prevents the phosphotransfer from HnoK to HnoB, HnoC and HnoD, reducing PDE activity. The accumulation of c-di-GMP will allow for the development of mature biofilm macrostructures. B. NO-bound NosP/NahK complex promotes NO-mediated biofilm dispersal through HnoK. In the presence of NO, NosP will inhibit NahK and HnoK is now in the active state. This allows for the phosphorylation of HnoD and HnoB. This promotes the degradation of c-di-GMP into pGpG and a reduction in biofilm formation.62
Perspectives:
As the list of effective bactericidal agents against biofilm-forming pathogens is becoming limited, alternative approaches for the treatment of biofilm-associated infections need to be examined. Triggering biofilm dispersal is a promising area of research, that if characterized and targeted correctly, could be used to release the bacteria from the biofilm into the more antibiotic susceptible, planktonic state.
NosP is a widely conserved heme-binding, NO-sensing protein not found in bacteria native to the gut microbiome, making it an excellent target for therapies that will not harm our natural microbiota. Our lab has shown that NosP-mediated disruption of NahK activity reduces overall biofilm formation across several strains of biofilm-forming pathogens.
Further characterization into the NosP-NahK TCSS and its downstream effect need to be completed before this system can be targeted to induce biofilm dispersal in a clinical setting.
References
- 1.Maunders E, Welch M. Matrix exopolysaccharides; the sticky side of Biofilm Formation. FEMS Microbiol Lett. 2017;364(13). doi: 10.1093/femsle/fnx120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salgar-Chaparro SJ, Lepkova K, Pojtanabuntoeng T, Darwin A, Machuca LL. Nutrient level determines biofilm characteristics and subsequent impact on microbial corrosion and biocide effectiveness. Appl Environ Microb. 2020;86(7). doi: 10.1128/aem.02885-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narla AV, Borenstein DB, Wingreen NS. A biophysical limit for quorum sensing in biofilms. P Nat Acad Sci. 2021;118(21). doi: 10.1073/pnas.2022818118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Martino P Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4(2):274–88. doi: 10.3934/microbiol.2018.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rello J, Ramirez Estrada S, Borgatta B. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect Drug Resist. 2016;7. doi: 10.2147/idr.s50669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan JB. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2010;89(3):205–18. doi: 10.1177/0022034509359403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, et al. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther. 2022;7(1). doi: 10.1038/s41392-022-01056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barraud N, Hassett DJ, Hwang S-H, Rice SA, Kjelleberg S, Webb JS. Involvement of nitric oxide in biofilm dispersal of pseudomonas aeruginosa. J Bacteriol. 2006;188(21):7344–53. doi: 10.1128/jb.00779-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, et al. Nitric oxide signaling in pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and Enhanced Dispersal. J Bacteriol. 2009;191(23):7333–42. doi: 10.1128/jb.00975-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckler I, Boon EM. Insights into nitric oxide modulated quorum sensing pathways. Front Microbiol. 2019;10. doi: 10.3389/fmicb.2019.02174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich K, Leslie DJ, Jonas K. Modulation of bacterial proliferation as a survival strategy. Adv Appl Microbiol. 2015;127–71. doi: 10.1016/bs.aambs.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 12.Ciemniecki JA, Newman DK. The Potential for Redox-Active Metabolites To Enhance or Unlock Anaerobic Survival Metabolisms in Aerobes. J Bacteriol. 2020. May 11;202(11):e00797–19. doi: 10.1128/JB.00797-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microb Biotechnol. 2009;2(3):370–8. doi: 10.1111/j.1751-7915.2009.00098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barraud N, Kelso M, Rice S, Kjelleberg S. Nitric oxide: A key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des. 2014;21(1):31–42. doi: 10.2174/1381612820666140905112822 [DOI] [PubMed] [Google Scholar]
- 15.Plate L, Marletta MA. Nitric oxide-sensing H-NOX proteins govern bacterial communal behavior. Trends Biochem Sci. 2013;38(11):566–75. doi: 10.1016/j.tibs.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henares B, Xu Y, Boon E. A nitric oxide-responsive quorum sensing circuit in Vibrio harveyi regulates flagella production and biofilm formation. Int J Mol Sci. 2013;14(8):16473–84. doi: 10.3390/ijms140816473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nisbett L-M, Boon EM. Nitric oxide regulation of H-NOX signaling pathways in bacteria. Biochem. 2016;55(35):4873–84. doi: 10.1021/acs.biochem.6b00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain S, Nisbett L-M, Boon EM. Discovery of two bacterial nitric oxide-responsive proteins and their roles in bacterial biofilm regulation. Accounts Chem Res. 2017;50(7):1633–9. doi: 10.1021/acs.accounts.7b00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentini M, Filloux A. Multiple roles of c-di-GMP signaling in bacterial pathogenesis. Annu Rev Microbiol. 2019;73(1):387–406. doi: 10.1146/annurev-micro-020518-115555 [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Nisbett L-M, Gou Y, Boon EM. NosP detection of heme modulates Burkholderia thailandensis biofilm formation. Biochemistry 2023, in press. doi: 10.1021/acs.biochem.3c00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hossain S, Heckler I, Boon EM. Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio cholerae. ACS Chem Biol. 2018;13(8):1964–9. doi: 10.1021/acschembio.8b00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes HQ, Floyd KA, Hossain S, Anantharaman S, Kysela DT, Zöldi M, et al. Nitric oxide stimulates type IV msha pilus retraction in Vibrio cholerae via activation of the phosphodiesterase CdpA. P Nat Acad Sci USA. 2022;119(7). doi: 10.1073/pnas.2108349119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckler I, Hossain S, Boon EM. Heme inhibits the activity of a c-di-GMP phosphodiesterase in Vibrio cholerae. Biochem Bioph Res Co. 2020;529(4):1112–6. doi: 10.1016/j.bbrc.2020.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henares BM, Higgins KE, Boon EM. Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio harveyi. ACS Chem Biol. 2012;7(8):1331–6. doi: 10.1021/cb300215t [DOI] [PubMed] [Google Scholar]
- 25.Mendoza AG, Guercio D, Smiley MK, Sharma GK, Withorn JM, Hudson-Smith NV, Ndukwe C, Dietrich LEP, Boon EM. Histidine Kinase, NahK, Regulates Pyocyanin Production through the PQS system. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain S, Boon EM. Discovery of a novel nitric oxide binding protein and nitric-oxide-responsive signaling pathway in Pseudomonas aeruginosa. ACS Infect Dis. 2017;3(6):454–61. doi: 10.1021/acsinfecdis.7b00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nisbett L-M, Binnenkade L, Bacon B, Hossain S, Kotloski NJ, Brutinel ED. NosP signaling modulates the NO/H-NOX-mediated multicomponent d-di-GMP network and biofilm formation in Shewanella oneidensis. Biochem. 2019;58(48):4827–41. doi: 10.1021/acs.biochem.9b00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borziak K, Zhulin IB. Fist: A sensory domain for diverse signal transduction pathways in prokaryotes and ubiquitin signaling in eukaryotes. J Bioinform. 2007;23(19):2518–21. doi: 10.1093/bioinformatics/btm384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with alphafold. Nature. 2021;596(7873):583–9. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, et al. Alphafold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021;50(D1). doi: 10.1093/nar/gkab1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zallot R, Oberg N, Gerlt JA. The EFI web resource for genomic enzymology tools: Leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochem. 2019;58(41):4169–82. doi: 10.1021/acs.biochem.9b00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberg N, Zallot R, Gerlt JA. Efi-Est, Efi-GNT, and Efi-CGFP: Enzyme Function Initiative (EFI) web resource for genomic enzymology tools. J Mol Biol. 2023;435(14):168018. doi: 10.1016/j.jmb.2023.168018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai Y, Faul EM, Ghosh A, Stuehr DJ. No rapidly mobilizes cellular heme to trigger assembly of its own receptor. P Nat Acad Sci USA. 2022;119(4). doi: 10.1073/pnas.2115774119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waheed SM, Ghosh A, Chakravarti R, Biswas A, Haque MM, Panda K, et al. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic Biol Med. 2010;48(11):1548–58. doi: 10.1016/j.freeradbiomed.2010.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuehr DJ, Biswas P, Dai Y, Ghosh A, Islam S, Jayaram DT. A natural heme deficiency exists in biology that allows nitric oxide to control heme protein functions by regulating cellular heme distribution. BioEssays. 2023; doi: 10.1002/bies.202300055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plate L, Marletta MA. Nitric oxide modulates bacterial biofilm formation through a multicomponent cyclic-di-GMP signaling network. Molecular Cell. 2012;46(4):449–60. doi: 10.1016/j.molcel.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee-Lopez C, Yukl E. H-NOX proteins in the virulence of pathogenic bacteria. Biosci Rep. 2022;42(1). doi: 10.1042/bsr20212014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamayo R, Schild S, Pratt JT, Camilli A. Role of cyclic di-GMP during el tor biotype Vibrio cholerae infection: Characterization of the in vivo-induced cyclic di-GMP phosphodiesterase CdpA. Infect Immun. 2008;76(4):1617–27. doi: 10.1128/iai.01337-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae el tor biofilm. Mol Microbiol. 1999;34(3):586–95. doi: 10.1046/j.1365-2958.1999.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2014;6(1):26–41. doi: 10.1007/s13238-014-0100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henares BM, Higgins KE, Boon EM. Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio harveyi. ACS Chem Biol. 2012;7(8):1331–6. doi: 10.1021/cb300215t [DOI] [PubMed] [Google Scholar]
- 42.Shao Y, Bassler BL. Quorum-Sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets. Mol Microbiol. 2012;83(3):599–611. doi: 10.1111/j.1365-2958.2011.07959.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung SA, Chapman CA, Ng W-L. Quadruple quorum-sensing inputs control Vibrio cholerae virulence and maintain system robustness. PLOS Pathog. 2015;11(4). doi: 10.1371/journal.ppat.1004837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watve S, Barrasso K, Jung SA, Davis KJ, Hawver LA, Khataokar A, et al. Parallel quorum-sensing system in Vibrio cholerae prevents signal interference inside the host. PLOS Pathog. 2020;16(2). doi: 10.1371/journal.ppat.1008313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hossain S, Boon EM. Discovery of a novel nitric oxide binding protein and nitric-oxide-responsive signaling pathway in Pseudomonas aeruginosa. ACS Infect Dis. 2017;3(6):454–61. doi: 10.1021/acsinfecdis.7b00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu J-L, Chen H-C, Peng H-L, Chang H-Y. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J Biol Chem. 2008;283(15):9933–44. doi: 10.1074/jbc.m708836200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis VI, Porter SL. Multikinase networks: Two-component signaling networks integrating multiple stimuli. Annu Rev Microbiol. 2019;73(1):199–223. doi: 10.1146/annurev-micro-020518-115846 [DOI] [PubMed] [Google Scholar]
- 48.Valentini M, Laventie B-J, Moscoso J, Jenal U, Filloux A. The diguanylate cyclase HsbD intersects with the HptB regulatory cascade to control Pseudomonas aeruginosa biofilm and motility. PLOS Genet. 2016;12(10). doi: 10.1371/journal.pgen.1006354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broder UN, Jaeger T, Jenal U. Lads is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat Microbiol. 2016;2(1). doi: 10.1038/nmicrobiol.2016.184 [DOI] [PubMed] [Google Scholar]
- 50.Wang BX, Wheeler KM, Cady KC, Lehoux S, Cummings RD, Laub MT, et al. Mucin glycans signal through the sensor kinase Rets to inhibit virulence-associated traits in Pseudomonas aeruginosa. Curr Biol. 2021;31(1). doi: 10.1016/j.cub.2020.09.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recinos DA, Sekedat MD, Hernandez A, Cohen TS, Sakhtah H, Prince AS, et al. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. P Nat Acad Sci. 2012;109(47):19420–5. doi: 10.1073/pnas.1213901109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiessl KT, Hu F, Jo J, Nazia SZ, Wang B, Price-Whelan A, et al. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat Commun. 2019;10(1). doi: 10.1038/s41467-019-08733-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bm Jo J, Price-Whelan A, Dietrich LEP. Gradients and consequences of heterogeneity in biofilms. Nat Rev Microbiol. 2022. Oct;20(10):593–607. doi: 10.1038/s41579-022-00692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyofuku M, Nomura N, Kuno E, Tashiro Y, Nakajima T, Uchiyama H. Influence of the Pseudomonas quinolone signal on denitrification in Pseudomonas aeruginosa. J Bacteriol. 2008;190(24):7947–56. doi: 10.1128/jb.00968-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3(3):271–9. doi: 10.4161/viru.20328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alhede M, Bjarnsholt T, Givskov M, Alhede M. Pseudomonas aeruginosa biofilms. Adv Appl Microbiol. 2014;1–40. doi: 10.1016/b978-0-12-800262-9.00001-9 [DOI] [PubMed] [Google Scholar]
- 57.Carlson HK, Vance RE, Marletta MA. H-NOX Regulation of c-di-GMP metabolism and biofilm formation in Legionella pneumophila. Mol Microbiol. 2010; doi: 10.1111/j.1365-2958.2010.07259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer JT, Hossain S, Boon EM. NosP modulates cyclic-di-GMP signaling in Legionella pneumophila. Biochem. 2019;58(42):4325–34. doi: 10.1021/acs.biochem.9b00618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pécastaings S, Allombert J, Lajoie B, Doublet P, Roques C, Vianney A. New insights into Legionella pneumophila biofilm regulation by c-di-GMP signaling. Biofouling. 2016. Aug 5;32(8):935–48. doi: 10.1080/08927014.2016.1212988 [DOI] [PubMed] [Google Scholar]
- 60.Hughes ED, Byrne BG, Swanson MS. A two-component system that modulates cyclic-di-GMP metabolism promotes Legionella pneumophila differentiation and viability in low-nutrient conditions. J Bacteriol. 2019;201(17). doi: 10.1128/jb.00253-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plate L, Marletta MA. Phosphorylation-dependent derepression by the response regulator Hnoc in the Shewanella oneidensis nitric oxide signaling network. P Natl Acad Sci. 2013;110(48). doi: 10.1073/pnas.1318128110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Created with Biorender.com.


