Abstract
The study was carried out to measure the glycemic index (GI) of an oral food supplement for people with CKD as well as on patients on maintenance dialysis. The study was conducted as per international protocols for testing GI, was approved by the local institutional ethics committee, and was registered with the Clinical Trial Registry of India (CTRI). This was a crossover randomized controlled study which enrolled 15 participants between the ages of 18 and 45 years. The participants were randomly allotted to one group that consumed either the reference food (27.5 g of glucose monohydrate) or 118 g of the nutritional supplement which contained 25 g of available carbohydrates. Fasting capillary blood samples as well as blood samples at different time intervals as per the GI protocol, after consumption of either the supplement or the reference food were taken from the participants. Each testing day was separated by a 3‐day washout period. GI was calculated from the incremental area under the blood glucose response elicited by the nutritional supplement as a percentage of the response after the consumption of 25 g of glucose (27.5 g of glucose monohydrate) by the same participant using a standard formula. The GI of the nutritional supplement was calculated to be 10.3 ± 2.0 which is considered to be low as per international GI testing standards. The product was created to supplement the diet of people with CKD at different stages and to help prevent the progression from CKD to ESRD as well as the risk for CVD. This product was found to have a low GI which is desirable for people with CKD as well as diabetics in general who are at risk for developing CKD.
Keywords: chronic kidney disease, glycemic index, nephropathy, nutritional supplement, renal nutrition
A study was carried out to measure the glycemic index (GI) of an oral food supplement for people with chronic kidney disease (CKD) as well as on patients on maintenance dialysis. The GI of the nutritional supplement was calculated to be 10.3 ± 2.0 which is considered to be low as per international GI testing standards.
1. INTRODUCTION
Chronic kidney disease (CKD) is a condition where there is decreased kidney function which is usually caused by diabetes and hypertension (Kramer, 2019; Webster et al., 2017) (United States Renal Data System, 2022). The prevalence of CKD across the world was 9.1% in 2017, i.e., around 700 million people with the disease (Bikbov et al., 2020).
With the rise in the prevalence of diabetes in pandemic proportions across the world, there has been a parallel rise in the prevalence of diabetic nephropathy. Globally diabetic nephropathy is a leading cause of end‐stage renal disease (ESRD) (Benjamin & Lappin, 2021) and approximately 40% of people with type 2 diabetes mellitus (DM2) end up developing diabetic nephropathy (Reutens, 2013) (Hussain et al., 2019).
High blood glucose level is not only an independent risk factor for the development of diabetic nephropathy but the most important one (Caramori et al., 2013). There are well designed randomized controlled studies that have shown the effect of blood glucose control on delayed onset of markers of CKD like albuminuria in DM2 (Ismail‐Beigi et al., 2010) (Duckworth et al., 2009).
CKD is a silent disease and studies have shown that more than 90% of people suffering from stage 2 or 3 CKD were not aware of their condition till they were late into the disease (Szczech et al., 2014) (Bakris et al., 2019). Another survey done on people with diabetes, who were detected to have stage 3 of 4 of CKD found that only 15% of them were actually aware that they have any kidney disease (Dharmarajan et al., 2017). Studies have shown that increased consumption of drinks high in sugar was associated with an increased risk for DM2 and obesity which have been linked with the progression toward ESRD in patients with CKD (Schulze et al., 2004), two major risk factors for progression toward chronic kidney disease (Fox et al., 2004). Consumption of sugary drinks has been shown to be associated with chronic kidney disease in well‐conducted studies (Saldana et al., 2007) (Shoham et al., 2008). With these established facts that DM2 has a direct correlation with CKD as well as its progression to ESRD, any intervention that helps in reducing the risk of DM2 and its complication can be logically thought to help in the incidence of ESRD too. A large randomized controlled trial looked at the impact of a multifactorial as well as a multidisciplinary team approach in patients with stages 3 and 4 CKD (Fogelfeld et al., 2017). An integrative approach comprising intensive diabetes and renal care in combination with behavioral as well as dietary interventions was used to manage the patients. The study showed that the patients with stages 3 and 4 CKD who were part of the intervention group and received the integrated intensive management had increased chances of achieving an HbA1C below 7% with a greater decrease in the albumin–creatinine ratio. The study further showed that only 13% of the patients in the intervention group progressed to ESRD in comparison with 28% in the control group, which roughly accounted for more than a 50% reduction in the chances of progressing toward ESRD in the intervention group. The study demonstrated that besides pharmacological interventions, there is a big role that dietary interventions could play especially in reducing hyperglycemia, albumin–creatinine ratio, and thus progression of CKD to ESRD.
A systematic review estimated that more than 24% of cases with CKD in industrialized countries could be attributed to nutritional factors which are a part of modifiable risk factors for the development of both CKD and its progression to ESRD (Wang et al., 2008). A 3‐fold increase in the incidence of CKD over 5 years was seen in a group of people consuming energy‐dense sources of carbohydrates which underlies the need for assessing the quality of carbohydrates, especially in people who are at risk of developing CKD or progressing toward ESRD (Gopinath et al., 2011). Although ESRD is a logical progression from CKD in people with uncontrolled diabetes mellitus, it has been seen that CKD is also associated with cardiovascular risk factors and majority of patients with CKD will not reach ESRD as they may die of cardiovascular risk factors that may precede it (USRDS, 2018). Therefore, interventions to prevent cardiovascular risk factors or to manage the same should be given a major impetus while designing the management for people with CKD. Foods containing different types of carbohydrates can have variable glycemic responses, where some foods can cause sudden surge as well as fall in blood glucose levels postconsumption and some foods can cause slow rise as well as fall in blood glucose levels. The quality of carbohydrates can be assessed by measuring the dietary glycemic index (GI) which represents the rate of rise in blood glucose levels after consumption of carbohydrates (Gopinath et al., 2011; Jenkins et al., 1987).
People with CKD whose diets are deficient in protein and energy requirements are often recommended oral nutritional supplements (Jensen, 2013). It was imperative that any oral nutritional supplement have a low GI besides fulfilling the protein as well as micronutrient requirements for this particular group of people. The present study, thus, aimed at analyzing the quality of carbohydrates in a healthy food drink tailored for people with CKD. This study measured the GI of the product for it to be a suitable nutritional supplement for people with CKD as well as for people with DM2, who are at a higher risk for developing CKD.
2. MATERIALS AND METHODS
2.1. GI testing
This study was conducted as per protocols recognized by Food and Agricultural Organization/World Health Organization (FAO/WHO, 1998), as per guidelines by international dietary carbohydrate task force for the measurement of GI in carbohydrate‐rich foods for GI methods (Brouns et al., 2005), as well as methods recognized by International Organization for Standardization (ISO) (ISO, 2010) (Henry et al., 2008). This was a crossover randomized controlled study as per international guidelines for GI studies and the participants from the study were recruited from a roster of the GI testing center of Madras Diabetes Research Foundation (MDRF) which was the site where the study was conducted. Participants were free‐living individuals from the community between 18 and 45 years of age. The inclusion criteria were males or females with a BMI of less than or equal to 22.9 kg/ m2. People with any known food allergy or intolerance, with a history of any medication known to have an effect on glucose metabolism, with any specific dietary restrictions, with a known history of diabetes mellitus, with a history of any disease or drug intake that influenced digestions as well as absorption of nutrients, with a history of any medical or surgical event in the last 3 months, and mothers who were pregnant or lactating, were excluded from the study. The study was approved by the institutional ethics committee of MDRF, Chennai, India and was conducted as per the good clinical practices and as per the declaration of Helsinki. Informed consent was taken from each participant before enrolling them in the study. The study was registered in the Clinical Trial Registry of India (CTRI); CTRI/2021/08/035929 (CTRI, n.d.), which is linked to the WHO clinical trial registry platform (WHO, n.d.).
2.2. Sample size
As per recommendations for the estimation of GI, a sample size of 10 participants was good enough (Brouns et al., 2005). This study enrolled 15 participants anticipating dropouts if any.
2.3. Intervention
As routine procedure participants were familiarized with capillary blood sampling prior to the start of the study. The study included a total of 15 participants who were randomly allocated to either group A or B. The reference food containing 25 g of available carbohydrates (27.5 g of glucose monohydrate) was given on three occasions (beginning‐R1, middle‐R2, and at the end of the study‐R3). The test food feeding (containing 25 g of available carbohydrates containing nutrition supplement powder) to both groups was randomized in such a way that group A consumed test food between R1 and R2, while group B participants consumed between R2 and R3. All the participants were hence had four visit days. Each visit day was separated by three washout days to prevent the carry‐over effect of the visit day's reference or test food intake. A 24‐h dietary recall was done, and the dietary intakes were assessed by trained research assistants with the help of visual food atlas with real food images, portion tools, and sizes to assist in the accuracy of the dietary intake assessment (Sudha et al., 2006). A history of physical activity was taken for the day prior to the testing using a standardized physical activity questionnaire (Anjana et al., 2015). A brief history of, smoking and alcohol were obtained for the pretest dates, and it was ensured that the participants refrained from smoking and alcohol during the study period. Participants were asked to report to the GI testing center each test day in the morning after a 10‐ to 12‐h overnight fast, to take the scheduled reference food or test food.
2.4. Estimation of GI
The operational definition for GI for this study is the incremental area under the blood glucose response elicited by 25 or 50 g of available carbohydrate‐containing test food portion expressed as a percentage of the response after the consumption of 25 or 50 g of anhydrous glucose by the same participant (Wolever, 2006) and is the comparison of mass to mass of carbohydrates in single foods.
The test product was a protein‐rich, proprietary formulation which is targeted for people with CKD and manufactured by Dr. Reddy's laboratories limited, India, the nutrient composition of which is given in Table 1. Proximate composition, dietary fiber, and available carbohydrate content (direct measurement) of the test product were estimated at the Food Quality Analysis Lab in MDRF. The test food quantity of 118 g was needed to obtain 25 g of available carbohydrates based on the analytical values. A 27.5 g of glucose dissolved in 125 mL of water was given as the reference test food to the participants and 118 g of the test food mixed with 531 mL of water was given as the test food. Participants were given an additional 125‐mL water with the test portion.
TABLE 1.
Nutritional composition of the nutritional supplement.
| Nutrients | Unit | 100 g |
|---|---|---|
| Energy | kcal | 440 |
| Protein a | g | 48 |
| Carbohydrate | g | 20.3 |
| Total sugars | g | 0 |
| Added sugar (sucrose) | g | 0 |
| Total fat | g | 17.2 |
| Monounsaturated fatty acids | g | 4 |
| Polyunsaturated fatty acids | g | 0.9 |
| Saturated fatty acids | g | 9.7 |
| Cholesterol | mg | <1.0 |
| Trans fatty acids | g | <0.1 |
| Dietary fiber | g | 6 |
| Sodium | mg | 251 |
| Vitamins | ||
| Vitamin C | mg | 78 |
| Vitamin B5 | mg | 5.5 |
| Vitamin E | mg | 11.2 |
| Vitamin B6 | mg | 1.5 |
| Vitamin B2 | mg | 1.55 |
| Vitamin B1 | mg | 1.55 |
| Vitamin B3 | mg | 11.5 |
| Vitamin A | mcg | 253 |
| Folic acid | mcg | 174 |
| Vitamin K | mcg | 23 |
| Biotin | mcg | 26 |
| Vitamin D | mcg | 8 |
| Vitamin B12 | mcg | 2.2 |
| Minerals | ||
| Chloride | mg | 160 |
| Magnesium | mg | 56 |
| Can describe Iron | mg | 5.05 |
| Zinc | mg | 2.5 |
| Manganese | mg | 1.8 |
| Selenium | mcg | 38 |
| Calcium | mg | 282 |
| Chromium | mcg | 57 |
| Phosphorous | mg | 213 |
| Copper | mcg | 562 |
| Potassium | mg | 445.5 |
| Other nutrients | ||
| Choline | mg | 169 |
| L‐Carnitine | mg | 70 |
| L‐Taurine | mg | 40 |
Whey protein isolate contributed to 80% and soy protein isolate to 20% of total proteins.
Fasting blood samples were taken at −5 min and 0 min by finger‐prick, using an automatic lancet device before consumption of the food and the baseline value taken was a mean of these two values. The participants then consumed 25 g available carbohydrate portion of the reference or the test food portion as per the schedule and randomization. The first bite/sip in the mouth was set as time 0 and the capillary blood samples were taken at 15, 30, 45, 60, 90, and 120 min.
GI was calculated using the following formula (Brouns et al., 2005) (ISO, 2010):
The Incremental Area under the Curve (IAUC) of blood glucose for the reference and test food were calculated geometrically using the trapezoid rule, ignoring the area below the fasting (FAO/WHO, 1998) (Brouns et al., 2005) (ISO, 2010) (Henry et al., 2008) (Augustin et al., 2015). The mean and standard errors (SEM) of the IAUC for the reference and test food were calculated. GI values were calculated by expressing each subject's IAUC after the test food as a percentage of the same subject's mean reference IAUC. The mean of the resulting value was the GI of the test food (Table 3).
TABLE 3.
Individual Mean IAUC of reference (Glucose) and GI of the Test food.
| S. No | Age in years | BMI kg/m2 | Mean IAUC – reference (mg/dL × min) | Glycemic index – test food (n = 10) |
|---|---|---|---|---|
| 1 | 30 | 20.8 | 5119.1 | 16.9 |
| 2 | 38 | 20.9 | 3620.9 | 14.4 |
| 3 | 29 | 18.7 | 5227.4 | 15.3 |
| 4 | 30 | 21.6 | 3983.0 | 7.5 |
| 5 | 24 | 21.6 | 1719.0 | 7.9 |
| 6 | 28 | 22.0 | 2352.5 | b |
| 7 | 31 | 21.3 | 2367.0 | 20.8 |
| 8 | 26 | 22.5 | 3072.1 | 0.1 |
| 9 | 28 | 21.6 | 3015.2 | 6.1 |
| 10 | 32 | 20.1 | 3471.5 | 7.3 |
| 11 | 21 | 19.4 | 3719.4 | c |
| 12 | 25 | 18.6 | 3653.2 | a |
| 13 | 26 | 21.3 | 2623.7 | b |
| 14 | 22 | 22.1 | 3127.6 | 7.1 |
| 15 | 22 | 20.5 | 1274.3 | b |
| Mean | 27.5 | 20.9 | 3223.1 | 10.3 |
| SEM | 1.2 | 0.3 | 283.1 | 2.0 |
Abbreviations: SEM, Standard Error of Mean.
Did not consume completely.
Dropout.
Outliers >30% CV.
3. RESULTS
A total of 15 participants (eight females and seven males) were enrolled for this and out of which data from 10 were used for the final analysis (Figure 1). The mean age of the study participants was 27.5 years, and their mean BMI was 20.9 kg/m2. The baseline characteristics of the participants in the study are given in Table 2.
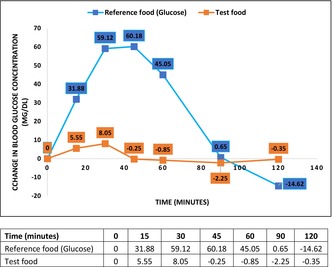
FIGURE 1.

Change in blood glucose between reference food (glucose) and nutritional supplement (test food) over 2 h.
TABLE 2.
Baseline characteristics of study participants.
| Characteristics | Mean ± SD |
|---|---|
| Age (Years) | 27.5 ± 4.5 |
| BMI (kg/m2) | 20.9 ± 1.2 |
| Male (n%) | 7 (46.7%) |
| Female (n%) | 8 (53.3%) |
| Fasting Blood Sugar (mg/dL) | 89 |
Note: n = No. of healthy volunteers who participated in the study.
Abbreviations: BMI, Body mass index; SD, Standard deviation.
Out of the 15 participants initially enrolled for the study, three participants dropped out from it and the data of one participant who could not consume the full test food was removed from the final analysis. As per standard methodology individual data of all participants, one participant with a coefficient of variation (CV) >30% (for reference glucose to reduce intraindividual variability) was removed as an outlier and did not find any individual values of GI > mean GI ± 2SD as outliers (Altman, 1991). The GI of the test product was calculated to be 10.3 ± 2.0 (Table 3) which is classified under the category of low GI foods (Vega‐López et al., 2018).
4. DISCUSSION
Glycemic index is the most reliable predictor of glycemic variations. Foods having GI ≥70 on the glucose scale are considered high‐GI foods, whereas those that have GI ≤55 on the glucose scale are considered low‐GI foods. It is important to highlight here that low‐GI foods provoke lesser blood glucose fluctuations than high‐GI foods over the day (Augustin et al., 2015).
Glycemic Index is considered to be a tool to help people with diabetes choose healthier carbohydrate food choices, was established more than 40 years back in 1981 (Jenkins et al., 1981), and has been used ever since by clinicians as well as nutritionists for the value it provides while prescribing a diet. The GI of the nutritional supplement (test food) used in the study was found to be low as per the GI classification (Augustin et al., 2015) and the GI value was 10.3 ± 2.0 (Table 3). The ingredients used to design the nutritional supplement, such as medium‐chain triglycerides (MCTs) and the combination of protein used could be the reason for low GI. This supplement is specially designed to meet the high protein requirement of CKD patients undergoing dialysis as per international guidelines (Cano et al., 2006) (Ikizler et al., 2020). One of the major challenges faced by physicians is glycemic control in people with CKD which comprises prescribing both medications and proper dietary advice. Dietary interventions should consist of not only correct combinations of protein and energy requirements for every stage of CKD but should also take into consideration the quality of both carbohydrates and proteins. A healthy diet may help in reducing the progression of CKD toward ESRD and combined with proper medications may go a long way in helping people with CKD (Stevens & Levin, 2013). A systematic review of cohort studies showed that diets with high GI and glycemic load (GL) were independently associated with increased risk of DM2 in healthy populations (Livesey et al., 2019). A seminal paper that examined evidence based on Bradford Hill criteria for causality (Hill, 1965) demonstrated GI and GL as causal factors for the risk of developing DM2 (Stevens & Levin, 2013). Alternatively, a cohort study showed that consumption of carbohydrates with low GI reduced the risk of developing CKD by 50% in the population studied (Gopinath et al., 2011).
Dietary interventions for people with CKD must be tailored to meet their protein and energy requirements while slowing the progression from CKD to ESRD. Interventions to prevent cardiovascular risk factors or to manage the same should have a major impetus while designing the management of people with CKD as most of the people with CKD die of cardiovascular risk factors rather than of ESRD. Genes play a big role in the incidence of ESRD in people under 50 years of age but it has also been seen that majority of cases with ESRD are associated with modifiable nutritional risk factors (Kramer, 2019). Management of cardiovascular disease risk factors such as hypertension, obesity, diabetes, and dyslipidemia should be an essential part for the management of people with CKD. A systematic review of randomized controlled trials showed that a low GI diet helped improved glycemic control in people with diabetes (Thomas & Elliott, 2009). A large multicontinental cohort study which analyzed data from 137,851 participants between 35 and 70 years of age from five continents showed that participants who consumed diets low in GI and GL had a significantly lower risk of cardiovascular disease and all‐cause mortality in comparison with participants who consumed diets high in GI and GL (Jenkins et al., 2021). A recently conducted systematic review also suggested that low GI foods can help improve risk factors for CVD like dyslipidemia, adiposity, blood pressure, and inflammation, even in people treated with medications for diabetes (Chiavaroli et al., 2021).
Indian diets are primarily low in both quality as well as quantity of protein and more than 70% of Indians have a protein‐deficient diet (Manish M., 2015). People with CKD and ESRD also suffer from protein malnutrition which is associated with increased morbidity and mortality. It is recommended that people with CKD not on dialysis have 0.6–0.8 g/kg body weight of protein per day and people on dialysis have 1–1.2 g/kg body weight of proteins per day, along with essential micronutrients (Zha & Qian, 2017). The International Society of Renal Nutrition and Metabolism (ISRNM) has coined “protein‐energy wasting” (PEW) for the loss of muscle as well as stored energy and “kidney wasting disease” for PEW in acute kidney disease (AKD) or CKD irrespective of the cause(s) (Fouque, Kalantar‐Zadeh et al., 2008). People suffering from CKD especially those who require dialysis tend to have malnutrition more so because this condition is usually accompanied by anorexia and that is why oral supplementation with macro‐ as well as micronutrients is recommended for this group of people. A cohort study done on patients with CKD requiring hemodialysis showed that more than 50% of the patients who were undernourished and were recommended medical nutritional therapy had better clinical outcomes (Tan et al., 2016). Among other biochemical and anthropometric measurements to determine PEW in AKD or CKD, dietary intake of proteins and carbohydrates form a part of the major criteria. Unintentional low dietary intake of protein lower than 0.8 g/kg body weight of proteins per day for at least 2 months for patients requiring dialysis, lower than 0.6 g/kg body weight of proteins per day for patients with stages of 2–5 CKD stages or unintentional low consumption of daily energy intake of less than 25 kcal/kg body weight per day for at least 2 months are important criteria to diagnose PEW in AKD or CKD (Fouque, McKenzie et al., 2008). Patients suffering from CKD, on either hemodialysis or peritoneal dialysis, have poor appetite mainly due to the presence of uremic toxins, increased catabolism, oxidative stress as well as the presence of other comorbidities like diabetes (Burrowes et al., 2003) (Malgorzewicz et al., 2016) and therefore may require specialized supplementation. Around 11% of patients who were started on specialized predialysis care were seen to suffer from moderate protein energy malnutrition (Westland et al., 2015). A systematic review of randomized controlled trials that analyzed the effect of oral protein‐based supplements in people with CKD requiring dialysis found that participants who were on protein‐rich food supplements improved nutritional markers as well as an increase in midarm muscle circumference which is one of the clinical measurements for PEW (Mah et al., 2020). Nutritional supplements specially designed for patients undergoing maintenance hemodialysis have been shown to prevent malnutrition, reduce hospitalization days, as well as improve quality of life (QOL) in these patients (Fouque, McKenzie et al., 2008) (Kalantar‐Zadeh et al., 2011) (Wilson et al., 2001). Whey or soy protein‐based supplements have helped improve health outcomes like, increased muscle mass to improve physical function and have reduced inflammatory markers like C‐reactive protein (CRP) and interleukin 6 (Il‐6) in patients with CKD on maintenance hemodialysis (Tomayko et al., 2015). The nutritional product in this study was a whey and soy protein‐based supplement, designed to support patients with CKD or undergoing dialysis, with other essential nutrients required on a daily basis, (Table 2). The product was created to supplement the diet of people with CKD at different stages and to help prevent the progression from CKD to ESRD as well as risk for CVD. A carbohydrate‐rich food is considered to have a low GI if the GI is <55 (Eleazu, 2016), the nutritional product studied in this study had a GI of 10.3 and therefore can be considered as a low GI food.
5. CONCLUSION
Consuming proper proportion of macronutrients (carbohydrates, proteins, and fats) is vital for sustaining caloric sufficiency, protein sparing, and achieving balance in nutrition. The test product is designed specifically to meet the nutritional needs of patients with CKD at different stages and can be used adjuvant to their daily diet to help fill the nutritional gap, especially their higher protein needs and to help prevent the progression from CKD to ESRD as well as risk for CVD. This product was found to have a low GI (10.3 ± 2.0) which is desirable for people with CKD as well as diabetics in general who are at risk for developing CKD.
6. PRACTICAL APPLICATION
The oral food supplement used in this study was a whey and soy‐based product with other essential nutrients, which was specially designed for people at different stages of CKD. The GI for the food supplement was assessed as per international norms for the testing of GI in carbohydrate‐rich foods. The study demonstrated that the oral nutritional supplement had a low GI as per international GI testing norms, which is desirable for people with CKD as well as diabetics in general who are at risk for developing CKD.
AUTHOR CONTRIBUTIONS
Bhoite Rachana: Conceptualization (equal); supervision (equal); validation (equal); writing – review and editing (equal). Shanmugam Shobana: Conceptualization (equal); supervision (equal); validation (equal); visualization (equal). Pratti Varalakshmi Lalithya: Supervision (equal); validation (equal); writing – review and editing (equal). Vasudevan Sudha: Conceptualization (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (equal). Satyvarat Vinita: Funding acquisition (equal); supervision (equal); writing – review and editing (equal). Rajagopal Gayathri: Data curation (equal); methodology (equal). Natarajan Kalpana: Data curation (equal); formal analysis (equal); methodology (equal); software (equal). Mohan Anjana Ranjit: Validation (equal); writing – review and editing (equal).
FUNDING INFORMATION
This research was funded by Dr. Reddy's Laboratories Ltd.
CONFLICT OF INTEREST STATEMENT
None to report.
ACKNOWLEDGMENTS
We thank Dr. Soumik Kalita; Medical Research Division FamPhy, A‐12 502 Tulip Violet Sector 69, Gurugram, India 122101, and Dr. R Vaidya PhD for manuscript writing support.
Rachana, B. , Shobana, S. , Lalithya, P. V. , Sudha, V. , Vinita, S. , Gayathri, R. , Kalpana, N. , Ranjit, M. A. , & Viswanathan, M. (2023). Glycemic index of a nutritional supplement designed for people with chronic kidney disease. Food Science & Nutrition, 11, 5379–5387. 10.1002/fsn3.3495
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Altman, D. (1991). Practical statistics for medical research (1st ed.). Chapman and Hall. [Google Scholar]
- Anjana, R. M. , Sudha, V. , Lakshmipriya, N. S. S. , Subhashini, S. , Pradeepa, R. , Geetha, L. , Bai, M. R. , Gayathri, R. , Deepa, M. , Unnikrishnan, R. , Binu, V. S. , Kurpad, A. V. , & Mohan, V. (2015). Reliability and validity of a new physical activity questionnaire for India. The International Journal of Behavioral Nutrition and Physical Activity, 12(1), 40. 10.1186/s12966-015-0196-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin, L. S. , Kendall, C. W. , Jenkins, D. J. , Willett, W. C. , Astrup, A. , Barclay, A. W. , Björck, I. , Brand‐Miller, J. C. , Brighenti, F. , Buyken, A. E. , Ceriello, A. , La Vecchia, C. , Livesey, G. , Liu, S. , Riccardi, G. , Rizkalla, S. W. , Sievenpiper, J. L. , Trichopoulou, A. , Wolever, T. M. S. , … Poli, A. (2015). Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutrition, Metabolism, and Cardiovascular Diseases, 25(9), 795–815. [DOI] [PubMed] [Google Scholar]
- Bakris, G. , Coresh, J. , & Vassalotti, J. (2019). Prevalence and factors associated with undiagnosed chronic kidney disease in diabetes mellitus. American Journal of Kidney Diseases, 73(5), 653–654. 10.1053/j.ajkd.2019.03.048 [DOI] [Google Scholar]
- Benjamin, O. , & Lappin, S. (2021). End‐stage renal disease. StatPearls Publishing; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK499861/ [PubMed] [Google Scholar]
- Bikbov, B. , Purcell, C. , & Levey, A. (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet, 395(10225), 709–733. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns, F. , Bjorck, I. , Frayn, K. , Gibbs, A. L. , Lang, V. , Slama, G. , & Wolever, T. M. (2005). Glycaemic index methodology. Nutrition Research Reviews, 18(1), 145–171. 10.1079/NRR2005100 [DOI] [PubMed] [Google Scholar]
- Burrowes, J. D. , Larive, B. , Cockram, D. B. , & Burrowes, J. D. (2003). Effects of dietary intake, appetite, and eating habits on dialysis and non‐dialysis treatment days in hemodialysis patients: Cross‐sectional results from the HEMO study. Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 13(3), 191–198. [DOI] [PubMed] [Google Scholar]
- Cano, N. , Fiaccadori, E. , Tesinsky, P. , Toigo, G. , Druml, W. , DGEM (German Society for Nutritional Medicine) , Kuhlmann, M. , Mann, H. , Hörl, W. H. , & ESPEN (European Society for Parenteral and Enteral Nutrition) . (2006). ESPEN guidelines on enteral nutrition: Adult renal failure. Clinical Nutrition (Edinburgh, Scotland), 25, 295–310. 10.1016/j.clnu.2006.01.023 [DOI] [PubMed] [Google Scholar]
- Caramori, M. L. , Parks, A. , & Mauer, M. (2013). Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. Journal of the American Society of Nephrology: JASN, 24(7), 1175–1181. 10.1681/ASN.2012070739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavaroli, L. , Lee, D. , Ahmed, A. , Cheung, A. , Khan, T. A. , Blanco, S. , Mejia, M. A. , Jenkins, D. J. A. , Livesey, G. , Wolever, T. M. S. , Rahelić, D. , Kahleová, H. , Salas‐Salvadó, J. , Kendall, C. W. C. , & Sievenpiper, J. L. (2021). Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta‐analysis of randomised controlled trials. BMJ (Clinical Research Ed.), 374, n1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CTRI . (n.d.). Clinical trial of registry India . Retrieved from Clinical Trial of Registry India: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=59648
- Dharmarajan, S. H. , Bragg‐Gresham, J. L. , Morgenstern, H. , Gillespie, B. W. , Li, Y. , Powe, N. R. , Tuot, D. S. , Banerjee, T. , Burrows, N. R. , Rolka, D. B. , Saydah, S. H. , Saran, R. , & Centers for Disease Control and Prevention CKD Surveillance System . (2017). State‐level awareness of chronic kidney disease in the U.S. American Journal of Preventive Medicine, 53(3), 300–307. 10.1016/j.amepre.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth, W. , Abraira, C. , Moritz, T. , Reda, D. , Emanuele, N. , Reaven, P. D. , Zieve, F. J. , Marks, J. , Davis, S. N. , Hayward, R. , Warren, S. R. , Goldman, S. , McCarren, M. , Vitek, M. E. , Henderson, W. G. , Huang, G. D. , & VADT Investigators . (2009). Glucose control and vascular complications in veterans with type 2 diabetes. The New England Journal of Medicine, 360(2), 129–139. 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- Eleazu, O. (2016). The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. African Health Sciences, 16(2), 468–479. 10.4314/ahs.v16i2.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . (1998). Carbohydrates in human nutrition. Report of a joint FAO/WHO expert consultation. FAO Food Nutr. [PubMed] [Google Scholar]
- Fogelfeld, L. , Hart, P. , Miernik, J. , Ko, J. , Calvin, D. , Tahsin, B. , Adhami, A. , Mehrotra, R. , & Fogg, L. (2017). Combined diabetes‐renal multifactorial intervention in patients with advanced diabetic nephropathy: Proof‐of‐concept. Journal of Diabetes and its Complications, 31(3), 624–630. 10.1016/j.jdiacomp.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Fouque, D. , Kalantar‐Zadeh, K. , Kopple, J. , Cano, N. , Chauveau, P. , Cuppari, L. , Franch, H. , Guarnieri, G. , Ikizler, T. A. , Kaysen, G. , Lindholm, B. , Massy, Z. , Mitch, W. , Pineda, E. , Stenvinkel, P. , Treviño‐Becerra, A. , & Wanner, C. (2008). A proposed nomenclature and diagnostic criteria for protein‐energy wasting in acute and chronic kidney disease. Kidney International, 73(4), 391–398. 10.1038/sj.ki.5002585 [DOI] [PubMed] [Google Scholar]
- Fouque, D. , McKenzie, J. , de Mutsert, R. , Azar, R. , Teta, D. , Plauth, M. , Cano, N. , & Renilon Multicentre Trial Study Group . (2008). Use of a renal‐specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association – European Renal Association, 23(9), 2902–2910. [DOI] [PubMed] [Google Scholar]
- Fox, C. S. , Larson, M. G. , Leip, E. P. , Culleton, B. , Wilson, P. W. , & Levy, D. (2004). Predictors of new‐onset kidney disease in a community‐based population. JAMA, 291(7), 844–850. 10.1001/jama.291.7.844 [DOI] [PubMed] [Google Scholar]
- Gopinath, B. , Harris, D. C. , Flood, V. M. , Burlutsky, G. , Brand‐Miller, J. , & Mitchell, P. (2011). Carbohydrate nutrition is associated with the 5‐year incidence of chronic kidney disease. The Journal of Nutrition., 141(3), 433–439. 10.3945/jn.110.134304 [DOI] [PubMed] [Google Scholar]
- Henry, C. J. , Lightowler, H. J. , Newens, K. , Sudha, V. , Radhika, G. , Sathya, R. M. , & Mohan, V. (2008). Glycaemic index of common foods tested in the UK and India. The British Journal of Nutrition, 99(4), 840–845. 10.1017/S0007114507831801 [DOI] [PubMed] [Google Scholar]
- Hill, A. B. (1965). The environment and disease: Association or causation? Proceedings of the Royal Society of Medicine, 58(5), 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, S. , Habib, A. , & Najmi, A. K. (2019). Limited knowledge of chronic kidney disease among type 2 diabetes mellitus patients in India. International Journal of Environmental Research and Public Health, 16(8), 1443. 10.3390/ijerph16081443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikizler, T. A. , Burrowes, J. D. , Byham‐Gray, L. D. , Campbell, K. L. , Carrero, J. J. , Chan, W. , Fouque, D. , Friedman, A. N. , Ghaddar, S. , Goldstein‐Fuchs, D. J. , Kaysen, G. A. , Kopple, J. D. , Teta, D. , Yee‐Moon Wang, A. , & Cuppari, L. (2020). KDOQI clinical practice guideline for nutrition in CKD: 2020 update. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 76, S1–S107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- Ismail‐Beigi, F. , Craven, T. , Banerji, M. A. , Basile, J. , Calles, J. , Cohen, R. M. , Cuddihy, R. , Cushman, W. C. , Genuth, S. , Grimm, R. H., Jr. , Hamilton, B. P. , Hoogwerf, B. , Karl, D. , Katz, L. , Krikorian, A. , O'Connor, P. , Pop‐Busui, R. , Schubart, U. , Simmons, D. , … ACCORD trial group . (2010). Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet (London, England), 376, 419–430. 10.1016/S0140-6736(10)60576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO . (2010). Food products — Determination of the glycaemic index (GI) and recommendation for food classification. ISO 26642:2010. International Standards Organization. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:26642:ed‐1:v1:en [Google Scholar]
- Jenkins, D. , Wolever, T. , Collier, G. , Ocana, A. , Rao, V. B. , Buckley, G. , Lam, Y. , Mayer, A. , & Thompson, L. U. (1987). Metabolic effects of a low‐glycemic‐index diet. The American Journal of Clinical Nutrition., 46(6), 968–975. 10.1093/ajcn/46.6.968 [DOI] [PubMed] [Google Scholar]
- Jenkins, D. J. , Dehghan, M. , Mente, A. , Bangdiwala, S. I. , Rangarajan, S. , Srichaikul, K. , Mohan, V. , Avezum, A. , Díaz, R. , Rosengren, A. , Lanas, F. , Lopez‐Jaramillo, P. , Li, W. , Oguz, A. , Khatib, R. , Poirier, P. , Mohammadifard, N. , Pepe, A. , Alhabib, K. F. , … PURE Study Investigators . (2021). Glycemic index, glycemic load, and cardiovascular disease and mortality. The New England Journal of Medicine, 384(14), 1312–1322. 10.1056/NEJMoa2007123 [DOI] [PubMed] [Google Scholar]
- Jenkins, D. J. , Wolever, T. M. , Taylor, R. H. , Barker, H. , Fielden, H. , Baldwin, J. M. , Bowling, A. C. , Newman, H. C. , Jenkins, A. L. , & Goff, D. V. (1981). Glycemic index of foods: A physiological basis for carbohydrate exchange. The American Journal of Clinical Nutrition., 34(3), 362–366. 10.1093/ajcn/34.3.362 [DOI] [PubMed] [Google Scholar]
- Jensen, G. (2013). Oral nutritional supplementation. The American Journal of Managed Care, 19(2), 119–120. [PubMed] [Google Scholar]
- Kalantar‐Zadeh, K. , Cano, N. J. , Budde, K. , Chazot, C. , Kovesdy, C. P. , Mak, R. H. , Mehrotra, R. , Raj, D. S. , Sehgal, A. R. , Stenvinkel, P. , & Ikizler, T. A. (2011). Diets and enteral supplements for improving outcomes in chronic kidney disease. Nature Reviews. Nephrology, 7(7), 369–384. 10.1038/nrneph.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, H. (2019). Diet and chronic kidney disease. Advances in Nutrition, 10(Supplement_4), S367–S379. 10.1093/ADVANCES/NMZ011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey, G. , Taylor, R. , Livesey, H. F. , Buyken, A. E. , Jenkins, D. J. , Augustin, L. S. , Sievenpiper, J. L. , Barclay, A. W. , Liu, S. , Wolever, T. M. S. , Willett, W. C. , Brighenti, F. , Salas‐Salvadó, J. , Björck, I. , Rizkalla, S. W. , Riccardi, G. , Vecchia, C. L. , Ceriello, A. , Trichopoulou, A. , … Brand‐Miller, J. C. (2019). Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta‐analyses of prospective cohort studies. Nutrients, 11(6), 1280. 10.3390/nu11061280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah, J. Y. , Choy, S. W. , Roberts, M. A. , Desai, A. M. , Corken, M. , Gwini, S. M. , & McMahon, L. P. (2020). Oral protein‐based supplements versus placebo or no treatment for people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews, 5(5), CD012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgorzewicz, S. , Chmielewski, M. , Kaczkan, M. , Borek, P. , Lichodziejewska‐Niemierko, M. , & Rutkowski, B. (2016). Nutritional predictors of mortality in prevalent peritoneal dialysis patients. Acta Biochimica Polonica, 63(1), 111–115. 10.18388/abp.2015_1070 [DOI] [PubMed] [Google Scholar]
- Manish, M. (2015). Protein consumption in diet of adult Indians: A general consumer survey (PRODIGY). Indian Medical Gazette, 149(4), 149–150. [Google Scholar]
- Reutens, A. T. (2013). Epidemiology of diabetic kidney disease. The Medical Clinics of North America, 97(1), 1–18. 10.1016/j.mcna.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Saldana, T. M. , Basso, O. , Darden, R. , & Sandler, D. P. (2007). Carbonated beverages and chronic kidney disease. Epidemiology (Cambridge, Mass.), 18(4), 501–506. 10.1097/EDE.0b013e3180646338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, M. B. , Manson, J. E. , Ludwig, D. S. , Colditz, G. A. , Stampfer, M. J. , Willett, W. C. , & Hu, F. B. (2004). Sugar‐sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle‐aged women. Journal of the American Medical Association, 292, 927–934. 10.1001/jama.292.8.927 [DOI] [PubMed] [Google Scholar]
- Shoham, D. A. , Durazo‐Arvizu, R. , Kramer, H. , Luke, A. , Vupputuri, S. , Kshirsagar, A. , & Cooper, R. S. (2008). Sugary soda consumption and albuminuria: Results from the National Health and nutrition examination survey, 1999–2004. PLoS One, 3(10), e3431. 10.1371/journal.pone.0003431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, P. E. , & Levin, A. (2013). Evaluation and Management of Chronic Kidney Disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Annals of Internal Medicine, 158(11), 825–830. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- Sudha, V. , Radhika, G. , Sathya, R. M. , Ganesan, A. , & Mohan, V. (2006). Reproducibility and validity of an interviewer‐administered semi‐quantitative food frequency questionnaire to assess dietary intake of urban adults in southern India. International Journal of Food Sciences and Nutrition, 57(7–8), 481–493. 10.1080/09637480600969220 [DOI] [PubMed] [Google Scholar]
- Szczech, L. A. , Stewart, R. C. , Su, H. L. , DeLoskey, R. J. , Astor, B. C. , Fox, C. H. , McCullough, P. A. , & Vassalotti, J. A. (2014). Primary care detection of chronic kidney disease in adults with Type‐2 diabetes: The ADD‐CKD study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One, 9, e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. K. , Loh, Y. H. , Choong, H. L. , & Suhail, S. M. (2016). Subjective global assessment for nutritional assessment of hospitalized patients requiring haemodialysis: A prospective cohort study. Nephrology (Carlton, Vic.), 21(11), 944–949. 10.1111/nep.12707 [DOI] [PubMed] [Google Scholar]
- Thomas, D. , & Elliott, E. J. (2009). Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. The Cochrane Database of Systematic Reviews, 2009(1), CD006296. 10.1002/14651858.CD006296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko, E. J. , Kistler, B. M. , Fitschen, P. J. , & Wilund, K. R. (2015). Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 25(3), 276–283. 10.1053/j.jrn.2014.10.005 [DOI] [PubMed] [Google Scholar]
- United States Renal Data System . (2022). 2022 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda. Retrieved from https://adr.usrds.org/2022 [Google Scholar]
- USRDS . (2018). Chapter 4: Cardiovascular disease in patients with CKD. United States Renal Data Systems; Retrieved from https://www.usrds.org/media/1717/v1_c04_ckd_cvd_18_usrds.pdf [Google Scholar]
- Vega‐López, S. , Venn, B. J. , & Slavin, J. L. (2018). Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients, 10(10), 1361. 10.3390/nu10101361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Chen, X. , Song, Y. , Caballero, B. , & Cheskin, L. J. (2008). Association between obesity and kidney disease: A systematic review and meta‐analysis. Kidney International, 73(1), 19–33. 10.1038/sj.ki.5002586 [DOI] [PubMed] [Google Scholar]
- Webster, A. C. , Nagler, E. V. , Morton, R. L. , & Masson, P. (2017). Chronic kidney disease. Lancet (London, England), 389(10075), 1238–1252. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- Westland, G. J. , Grootendorst, D. C. , Halbesma, N. , Dekker, F. W. , & Verburgh, C. A. (2015). The nutritional status of patients starting specialized predialysis care. Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 25(3), 265–270. 10.1053/j.jrn.2014.10.004 [DOI] [PubMed] [Google Scholar]
- WHO . (n.d.). International clinical trials registry platform . Retrieved from International Clinical Trials Registry Platform. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/08/035929
- Wilson, B. , Fernandez‐Madrid, A. , Hayes, A. , Hermann, K. , Smith, J. , & Wassell, A. (2001). Comparison of the effects of two early intervention strategies on the health outcomes of malnourished hemodialysis patients. Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 11(3), 166–171. 10.1053/jren.2001.24364 [DOI] [PubMed] [Google Scholar]
- Wolever, T. M. (2006). The Glycaemic index: A physiological classification of dietary carbohydrate. CABI. 10.1079/9781845930516.0000 [DOI] [Google Scholar]
- Zha, Y. , & Qian, Q. (2017). Protein nutrition and malnutrition in CKD and ESRD. Nutrients, 9(3), 208. 10.3390/nu9030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


