Abstract
Background:
Multi-cancer early detection (MCED) blood tests can detect a cancer signal from circulating cell-free DNA (cfDNA). PATHFINDER was a prospective cohort study evaluating the feasibility of MCED testing for cancer screening.
Methods:
Between December 2019 and December 2020, a convenience sample of 6662 adults ≥50 years without signs or symptoms of cancer consented to MCED testing. Blood was collected, cfDNA analyzed, and results returned to participants’ physicians. If a cancer signal was detected, predicted cancer signal origins (CSOs) informed diagnostic evaluation. The primary outcome was time to, and extent of, diagnostic testing required to confirm the presence or absence of cancer.
Findings:
Cancer signal was detected in 92 (1.4%) of 6621 participants with analyzable results. There were 35/92 true positive (TP) and 57/92 false positive (FP) results. Excluding two participants whose diagnostic evaluations began before MCED test results were reported, median time to diagnostic resolution was 79 days (IQR 37–219): 57 (33–143) in TP and 162 (44–248) in FP participants. Most had both laboratory tests (26/33, 79% TP; 50/57, 88% FP) and imaging (30/33, 91% TP; 53/57, 93% FP). Fewer procedures were performed in FP (17/57, 30%) than TP (27/33, 82%) and few had surgery (one in FP; three in TP).
Interpretation:
This study supports the feasibility of MCED screening for cancer and underscores the need for further research evaluating its clinical utility.
Funding:
GRAIL, LLC, Menlo Park, CA, USA.
Keywords: early detection, multi-cancer, cancer, screening, prospective, cell-free DNA, tumor allele fraction
INTRODUCTION
Cancer is the second most common cause of death in Europe and the United States, and globally, more than 1.9 million new cases are expected to be diagnosed in 2023.1 Recommended cancer screening tests have reduced cancer-related mortality, but are only available for a few cancer types and represent less than 25% of cancer deaths in the U.S.2 The United States Preventive Services Task Force advises screening (Grade A/B) for breast, lung, colorectal, and cervical cancers. For many other cancer types, screening strategies either lack well-established net benefit or do not exist. As a result, many cancers are identified at late stages, when cure is less likely.3
Advances in genomics and machine learning have enabled development of blood tests for cancer screening, monitoring, and treatment selection.4–10 Multi-cancer early detection (MCED) blood tests have the potential to improve the efficiency and convenience of screening and do not carry the risks of whole-body imaging. However, experience is early and insight about how physicians and patients respond to a positive MCED test remains limited.7–10 The PATHFINDER study was designed to address this knowledge gap.
The MCED test investigated in this study detects cancer-specific DNA methylation patterns from cell-free DNA (cfDNA) shed by tumors into circulating blood. If a cancer signal is detected, the test also predicts the cancer signal origin (CSO; ie, the cancer or tissue type where the tumor arose).7, 8 This test was developed and validated in the Circulating Cell-Free Genome Atlas study (CCGA; NCT02889978, IDE# G190251). In CCGA, the test detected a signal for >50 distinct cancer types from a single blood draw and accurately predicted CSO in 89% of participants (1273/1435).7, 8 The PATHFINDER study (ClinicalTrials.gov, NCT04241796) evaluated feasibility of MCED testing in outpatient settings for adults over age 50 without symptoms of cancer.
METHODS
Study Design
MCED Test
The MCED test used in PATHFINDER uses next-generation sequencing to interrogate cell-free DNA (cfDNA) in peripheral blood to measure the extent and location of genomic DNA methylation patterns which are characteristic of cancers11 and specific to cancer type. A computational algorithm determines whether a cancer signal is present, and if so, predicts the most likely tumor type, labeled the cancer signal origin or CSO. This prediction may help to direct diagnostic evaluation.8, 12 CSO predictions were assigned from a set of 21 predetermined, categories that correspond to major tumor types described in Appendix Table A1.7, 8, 13 and are ranked in order of likelihood. CSO prediction accuracy was calculated as the number of patients with a correctly predicted tumor type, of which 113 (93.4%) were pathologically confirmed, among all patients with a confirmed cancer diagnosis following a positive MCED assay. Technical aspects of the assay and clinical validation have been described previously.7, 8
Study Sites and Participants
PATHFINDER assessed use of this test in a prospective cohort study conducted in oncology and primary care outpatient clinics at seven U.S. health networks, including hospitals and community-based clinics. Between December 12th, 2019 and December 4th, 2020, eligible participants, a non-consecutive convenience sample, were consented to MCED testing and one year of follow-up surveillance of their electronic health records. No specific diagnostic or screening evaluations were mandated. All study procedures were approved by a central or local Institutional Review Board (IRB). Additional details regarding study design have been previously published.13
Eligible participants were adults ≥50 years old, with or without additional cancer risk factors. Participants with additional risk met at least one of the following criteria: a history of smoking ≥100 cigarettes, cancer predisposition based on meeting criteria for germline testing from the National Comprehensive Cancer Network guidelines14 or having known hereditary cancer syndrome, or personal history of cancer with definitive treatment completed at least three years prior to enrollment.13 Participants were excluded if they were undergoing diagnostic evaluation for suspicion of cancer or cancer recurrence, had a history of untreated cancer, or had been treated for cancer within three years of enrollment.
Study Procedures
After informed consent, eligibility was confirmed, and participants contributed a blood specimen. Plasma isolation, followed by cfDNA extraction, was performed as previously described.7,8,13 Results of the MCED test were returned to the ordering physician within approximately 15 days and then shared with participants. The MCED test report provided a binary result (cancer signal detected or not detected), including prediction of the most likely CSOs if a signal was detected. If a cancer signal was detected, participants had diagnostic evaluations coordinated by and at the discretion of their physician. Physicians determined when the diagnostic workup was considered complete. Parameters for this diagnostic evaluation were not stipulated by the protocol, and the study sponsor supported all costs. This design enabled insight into the diagnostic pathways followed in response to MCED test results and CSO predictions. All participants, including those with no cancer signal detected, were advised to continue usual medical care and guideline-recommended screening.
An end of study cancer status assessment was conducted 12 months after enrollment and included electronic health record (EHR) review to confirm the presence or absence of cancer. Record review was supplemented with phone contact as needed. Cancer status assessment was considered complete if a cancer diagnosis was reported at any time during the follow-up period or there was no cancer diagnosis recorded in the EHR or through patient contact within 12 months of the MCED blood draw.
Outcomes
Extent of Diagnostic Testing
The primary objective was to determine the time required to achieve diagnostic resolution following receipt of a positive MCED test and to evaluate the extent of testing pursued. Time to diagnostic resolution for each participant was defined as the number of days between the date test results were available to the ordering physician through the reporting portal and the date of diagnostic resolution as determined by the ordering physician.
For each participant, the extent of diagnostic testing included all clinic visits, laboratory and imaging tests, and procedures recorded between the date a cancer signal detected test result was available in the portal and the date of diagnostic resolution or end of study, whichever came first. Procedures were categorized as either non-surgical (eg, endoscopy, bone marrow biopsy, core biopsy, fine needle aspiration, and pap smear) or surgical.
Cancer Diagnosis
A cancer diagnosis was established by pathologic, laboratory, and/or radiographic confirmation. Malignancies were categorized as either invasive solid tumors (excluding non-metastatic basal cell carcinoma and squamous cell carcinoma of the skin) or hematologic. Cancer diagnosis and staging for solid tumors was based on the American Joint Committee on Cancer (AJCC) Staging Manual version 8 and on AJCC or the Ann Arbor staging systems for hematologic malignancies.15 For participants with a cancer history of the same tumor type identified from MCED testing, staging was categorized as recurrent either locoregional or metastatic for solid tumors and as recurrent for hematologic malignancies. Premalignant conditions (eg, cervical dysplasia, monoclonal gammopathy of undetermined significance [MGUS]) were not considered cancer.
A 12-month follow-up period has traditionally been used to assess the performance characteristics of single cancer screening modalities, including mammography,16–18 low-dose computed tomography (LDCT) screening for lung cancer19–21 and ovarian cancer screening.22 The same interval was chosen for this study to facilitate evaluation of MCED test performance in juxtaposition to other tests. This arbitrary reference was not informed by biological considerations.
Within 12 months of enrollment participants with an MCED cancer signal detected and a clinically confirmed cancer were classified as true positive and those without confirmed cancer as false positive. Participants with no MCED cancer signal detected but who had a confirmed cancer diagnosis within 12 months were classified as false negative. False negatives were defined as those participants with cancers detected by means other than the diagnostic workup prompted by a positive MCED test. This included those detected by routine screening evaluations that occurred during the 12-month study period or those detected because the patient developed signs or symptoms. Screening was defined as standard if it was consistent with USPSTF grade A/B/C recommendations (breast, colorectal, cervical prostate, high risk lung).23 Cancers detected by non-standard screening included cancers of the ovary, testis, thyroid and skin that lack USPSTF recommendations and for breast, cervical, colorectal, prostate and lung cancers detected in individuals not covered by the USPSTF recommendations (for example at the extremes of age). Clinically detected cancers were defined as those diagnosed based on a patient’s presentation with clinical signs or symptoms such as pain, unexplained weight loss, or a palpable mass.
Safety
Safety assessments included a summary of adverse events (AEs) reported by site investigators at any time during the study, including at phlebotomy, results disclosure, and/or diagnostic evaluations in those with a cancer signal detected. AEs were reported to the study sponsor and the relevant IRB/Independent Ethics Committee. An independent Data Safety Monitoring Board provided oversight of the study conduct.
Test Performance
As a secondary objective, MCED test performance was evaluated with the following measures: resolution (true positive or false positive), positive and negative predictive value (PPV, NPV), specificity, and CSO accuracy. Participants with an indeterminate CSO were excluded from CSO accuracy calculations. Additional performance endpoints included the cancer yield rate defined as the number of TPs among all participants tested and the number needed to screen (NNTS) to detect one cancer.
Participant reported outcomes relating to anxiety, distress, uncertainty, and MCED test satisfaction were collected at the time of initial blood draw, when test results were returned, when diagnostic resolution was achieved, and at the end of the study and will be reported in a separate manuscript. A post hoc exploratory analysis of circulating tumor allele fraction (cTAF) was calculated for all cancers detected during the study. The detection threshold was defined as 3.1×10−4 cTAF at 99.3% specificity.24 All outcome measures are defined in Appendix Table A2.
Statistical Analyses
Analyses were descriptive with no pre-specified hypothesis testing. The analysis plan assumed that 70% of enrolled participants would have at least one additional cancer risk factor. Simulations estimated that a target enrollment of 6200 participants would generate 106–141 cancer signal detected results.13 The primary analysis was based on participants with a diagnostic evaluation triggered by a cancer signal detected result. Two-sided Wilcoxon 95% confidence intervals are reported for all test performance measures. Analyses were performed using R software, version 4.1.2.25 Protocol deviations occurred in a total of 1.6% (106/6662) of participants, including important protocol deviations in 0.8% (55/6662). Details are provided in Appendix Table A3.
Development of a Refined MCED Test
After PATHFINDER was launched, a refined MCED test version was developed, utilizing a larger training CCGA training dataset. All elements of this refined test were locked prior to sample processing, validation was performed in a new independent test set and the statistical analysis plan was finalized before evaluation. MCED test refinements included: 1) an increased specificity threshold for hematologic signals to reduce false positives from non-malignant hematologic conditions such as MGUS, and to increase the detection of solid cancers; 2) elimination of the ‘indeterminate’ label for cancer signal origin to generate a prediction for all test-positive samples; and 3) restriction of CSO predictions to the two most likely tumor types. Results of testing using the refined MCED test were not returned to either physicians or participants, and therefore did not influence diagnostic evaluations.
Role of the study sponsor
The study sponsor, Grail, contributed to the study design, data analysis, interpretation, drafting, review, and approval of the manuscript. The sponsor employed a medical writing agency to assist with manuscript preparation. However, the first and senior authors drafted and finalized the manuscript. All authors had access to the data, reviewed and approved the final version, and were responsible for the decision to submit for publication.
RESULTS
Participants
A total of 6662 participants were enrolled, 6625 were clinically evaluable, and 6621 had analyzable MCED results (Figure 1). Of these 6621 participants, 3681 (56%) were in the ‘with additional risk’ cohort. Participants were predominately white (91.7%) and highly educated (64.6% with college degrees), and fewer were current smokers (4.0%) compared to the general population (Table 1). Nearly a quarter of participants (24.5%) had a prior cancer history; demographic details and MCED test performance characteristics in this subset are listed in Appendix Table A4. At enrollment, most participants were up to date with cancer screening, with 92% reporting recent colorectal cancer screening and 80% of women reporting recent mammography (Table 1).
Figure 1:
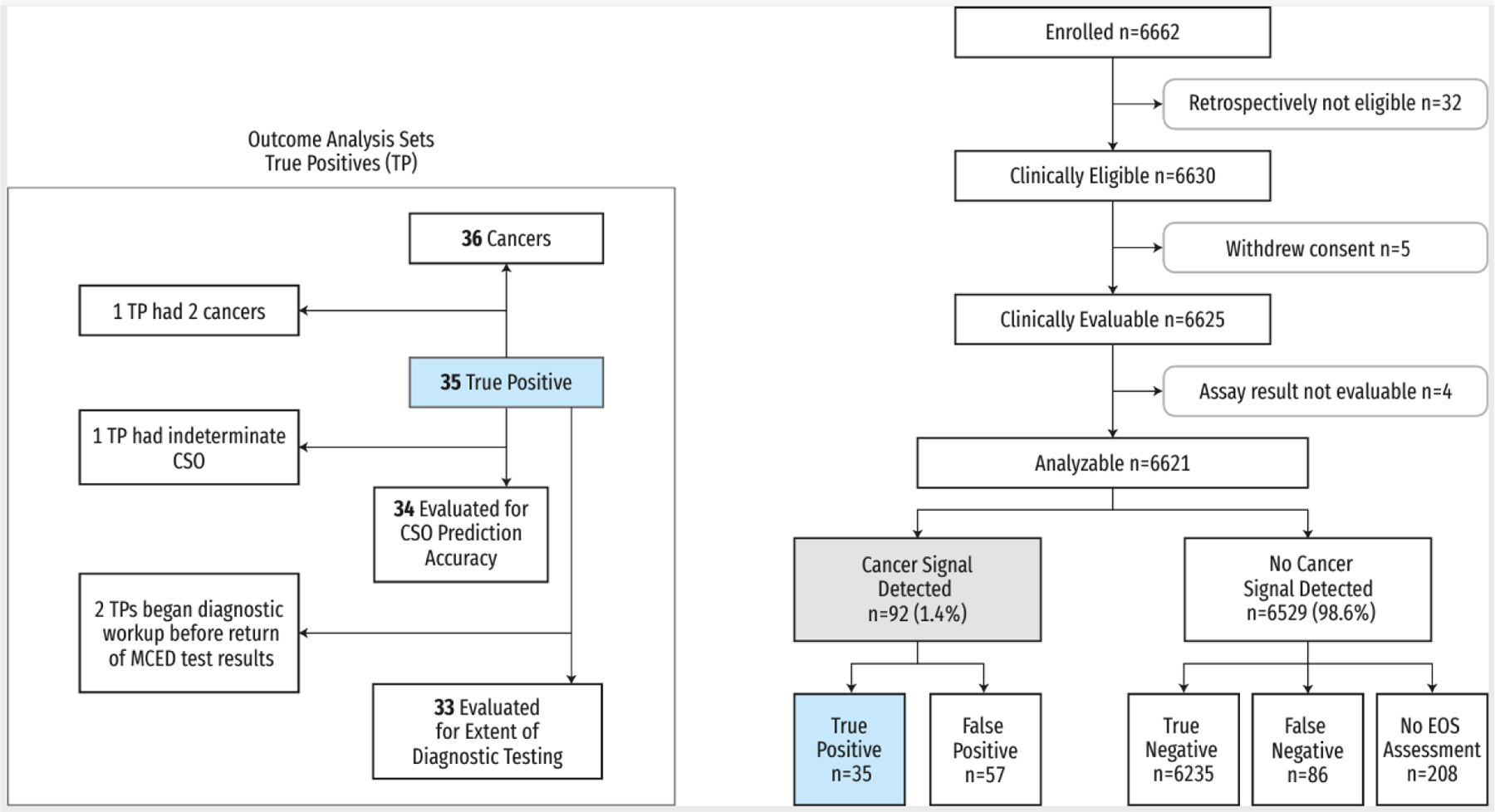
Trial profile
Participant flow diagram, including true positive analysis sets for outcomes with variable participant numbers to the left.
CSO=cancer signal origin. EOS=end of study. TP=true positives.
Table 1:
PATHFINDER participant demographics and baseline characteristics
| ≥50 y With Additional Risk n=3681 (56%) |
≥50 y Without Additional Risk n=2930 (44%) |
Total N=6621 |
|
|---|---|---|---|
| Agea, Median (Q1, Q3), y | 64.0 (58.0, 71.0) | 61.0 (55.0, 67.0) | 63.0 (56.0, 70.0) |
| Age Group, No. (%), y | |||
| 50–64 | 1858 (50.5) | 1933 (65.7) | 3791 (57.3) |
| 65–79 | 1637 (44.5) | 931 (31.7) | 2568 (38.8) |
| ≥80 | 186 (5.1) | 76 (2.6) | 262 (4.0) |
| Female, No. (%) | 2393 (65.0) | 1811 (61.6) | 4204 (63.5) |
| Race/ethnicity, No. (%) | |||
| Asianb | 39 (1.1) | 90 (3.1) | 129 (1.9) |
| Hispanic | 66 (1.8) | 68 (2.3) | 134 (2.0) |
| Non-Hispanic black | 44 (1.2) | 46 (1.6) | 90 (1.4) |
| Non-Hispanic white | 3441 (93.5) | 2630 (89.5) | 6071 (91.7) |
| Otherc | 28 (0.7) | 38 (1.3) | 66 (1.0) |
| Missing | 63 (1.7) | 68 (2.3) | 131 (2.0) |
| BMI Category, No. (%) | |||
| Underweight (<18.5 kg/m2) | 32 (0.9) | 18 (0.6) | 50 (0.8) |
| Normal (18.5 to <25 kg/m2) | 1045 (28.4) | 956 (32.5) | 2001 (30.2) |
| Overweight (25 to <30 kg/m2) | 1297 (35.2) | 1039 (35.3) | 2336 (35.3) |
| Obese (≥30 kg/m2) | 1267 (34.3) | 887 (30.2) | 2151 (32.5) |
| Other/Missing | 43 (1.2) | 40 (1.4) | 83 (1.3) |
| Education, No. (%) | |||
| <High school | 50 (1.4) | 15 (0.5) | 65 (1.0) |
| High school graduate | 345 (9.4) | 150 (5.1) | 495 (7.5) |
| Some college | 1060 (28.8) | 645 (21.9) | 1705 (25.8) |
| College graduate | 2176 (59.1) | 2100 (71.4) | 4276 (64.6) |
| Other/missing | 50 (1.4) | 30 (1.0) | 80 (1.2) |
| Smoking Status, No. (%) | |||
| Current Smoker | 268 (7.3) | 0 | 268 (4.0) |
| Former Smoker | 2229 (60.6) | 0 | 2229 (33.7) |
| Non-smoker | 1184 (32.3) | 2940 (100) | 4124 (62.3) |
| Eligible for Lung Cancer Screening,d No. (%) | 223 (6.1) | 0 | 223 (3.4) |
| Prior Cancer History, No. (%) | 1622 (44.1) | 0 | 1622 (24.5) |
| Cancer Predisposition, No. (%) | 425 (11.5) | 0 | 425 (6.4) |
| Up to Date With Standard Cancer Screening Prior to MCED Testing | |||
| Colorectal Cancere | 91% | 92% | 92% |
| Breast Cancerf | 78% | 83% | 80% |
Age was truncated at 85 years to protect confidentiality.
Non-Hispanic Asian, Native Hawaiian, or Pacific Islander.
Includes Unknown, American Indian, or Alaska Native.
Satisfy approved USPSTF criteria for lung cancer screening using LDCT.
Participants ≤75 years of age, up to date with USPSTF colorectal cancer screening recommendations (n=4888 total eligible with complete information).
Women 50–74 years of age up to date with breast cancer screening (USPSTF recommendations, MRI, or ultrasound; n=3547 total eligible with complete information).
BMI=body mass index. LDCT=low-dose computed tomography. MCED=multi-cancer early detection. MRI=magnetic resonance imaging. USPSTF=United States Preventive Services Task Force. y=years.
Cancer Signal Detection
A cancer signal was detected in 1.4% (92/6621) of participants (Figure 1), with a rate of 1.5% (56/3681) in those with additional risk and 1.2% (36/2940) in those without additional risk (Table 2). Of those with a cancer signal detected, 38% (35/92) were diagnosed with cancer (true positives) and 62% (57/92) had no cancer diagnosis (false positives; Figure 1). Most true positives (77%; 27/35) had a cTAF above 3.1×10−4, the MCED clinical detection threshold (Figure A1). The 92 positive MCED tests was lower than the projected 106–141 in the sample size calculations. The reasons for this discrepancy may stem from the lower than projected rate of enrollment of individuals with cancer risk factors, the healthy volunteer effect, and/or imprecise assumptions regarding cancer dwell times.
Table 2:
MCED test performance
| ≥50 y With Additional Risk n=3681 |
≥50 y Without Additional Risk n=2940 |
Total n=6621 |
|
|---|---|---|---|
| Resolution, n (%) | 56 (1.5) | 36 (1.2) | 92 (1.4) |
| True Positive | 24 (0.7) | 11 (0.4) | 35 (0.5) |
| False Positive | 32 (0.9) | 25 (0.9) | 57 (0.9) |
| PPV, n | 24/56 | 11/36 | 35/92 |
| % (95% CI) | 43 (30.8–55.9) | 31 (18.0–46.9) | 38 (28.8–48.3) |
| NPV, n | 3449/3502 | 2786/2819 | 6235/6321 |
| % (95% CI) | 98.5 (98.0–98.8) | 98.8 (98.4–99.2) | 98.6 (98.3–98.9) |
| Specificity, n | 3449/3480 | 2786/2810 | 6235/6290 |
| % (95% CI) | 99.1 (98.7–99.4) | 99.1 (98.7–99.4) | 99.1 (98.9–99.3) |
| Yield Rate, n | 24/3681 | 11/2940 | 35/6621 |
| % (95% CI) | 0.65 (0.41–0.92) | 0.37 (0.17–0.61) | 0.53 (0.36–0.71) |
| Number Needed to Screen, n | 3681/24 | 2940/11 | 6621/35 |
| N (95% CI) | 153 (108–245) | 267 (163–588) | 189 (141–276) |
| Predicted Origin Accuracy a | |||
| First CSO Correct, n | 20/23 | 9/11 | 29/34 |
| % (95% CI) | 87 (67.9, 95.5) | 82 (52.3, 94.9) | 85 (69.9, 93.6) |
| First or Second CSO Correct, n | 23/23 | 10/11 | 33/34 |
| % (95% CI) | 100 (85.7–100) | 91 (62.3–99.5) | 97 (85.1–99.8) |
Excludes one participant with indeterminate CSO from the true positive set.
CI=confidence interval. CSO=cancer signal origin. MCED=multi-cancer early detection. NPV=negative predictive value. PPV=positive predictive value.
Of those without a cancer signal detected, 95% (6235/6529) were true negatives, 1% (86/6529) were false negatives, and 3% (208/6529) did not have an end of study cancer status assessment. Ninety-five percent (82/86) of false negatives had a cTAF below 3.1×10−4 (Figure A1; Figures and Tables labeled A1, A2 etc. are contained in the Appendix).
Extent of Diagnostic Testing
Of the 92 participants with a cancer signal detected, two began their diagnostic evaluation based on symptoms before MCED test results were returned and are not included in the analysis of the extent of diagnostic testing shown in Table A5. Median numbers of clinic visits, lab tests, and imaging tests were similar for TP and FP participants, whereas TPs were more likely than FPs to undergo both nonsurgical and surgical procedures.
Time to Diagnostic Resolution
Median observed time to diagnostic resolution was 79 days (IQR 37–219). True positives had a shorter median time to resolution (57 days; IQR 33–143) compared with false positives (162 days; IQR 44–248; Figure 2). Most TPs (73%; 24/33) achieved diagnostic resolution within three months (Figure 2).
Figure 2:
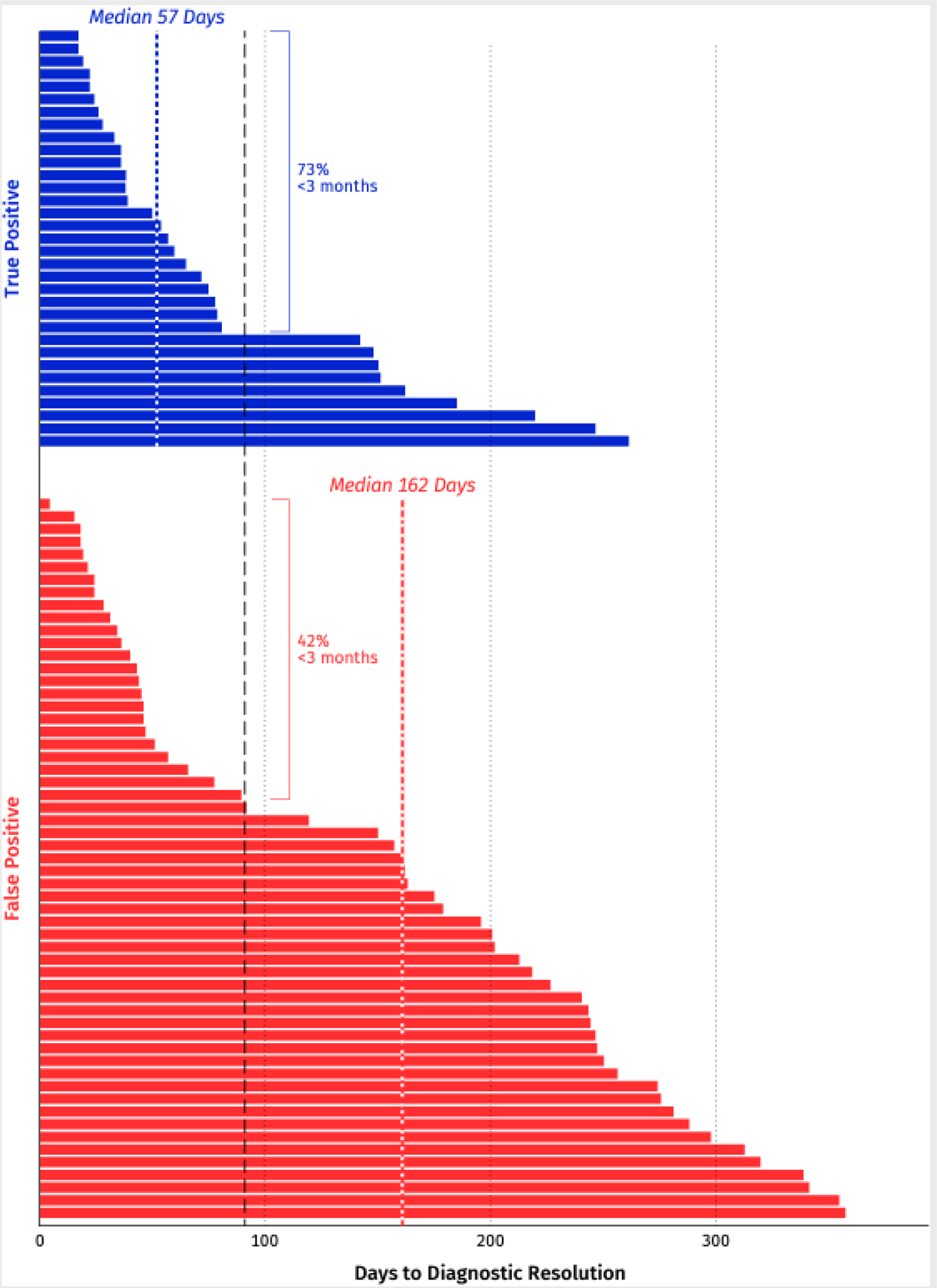
Time to diagnostic resolution for participants with cancer signal detected (n=90)
Lab and Imaging Tests
Most participants with positive MCED results (84%; 76/90) had lab tests (Figure 3). The most common lab tests (Table A6) were a metabolic panel (57%; 51/90), complete blood count with differential (53%; 48/90), and protein tumor markers (43%; 39/90).
Figure 3.
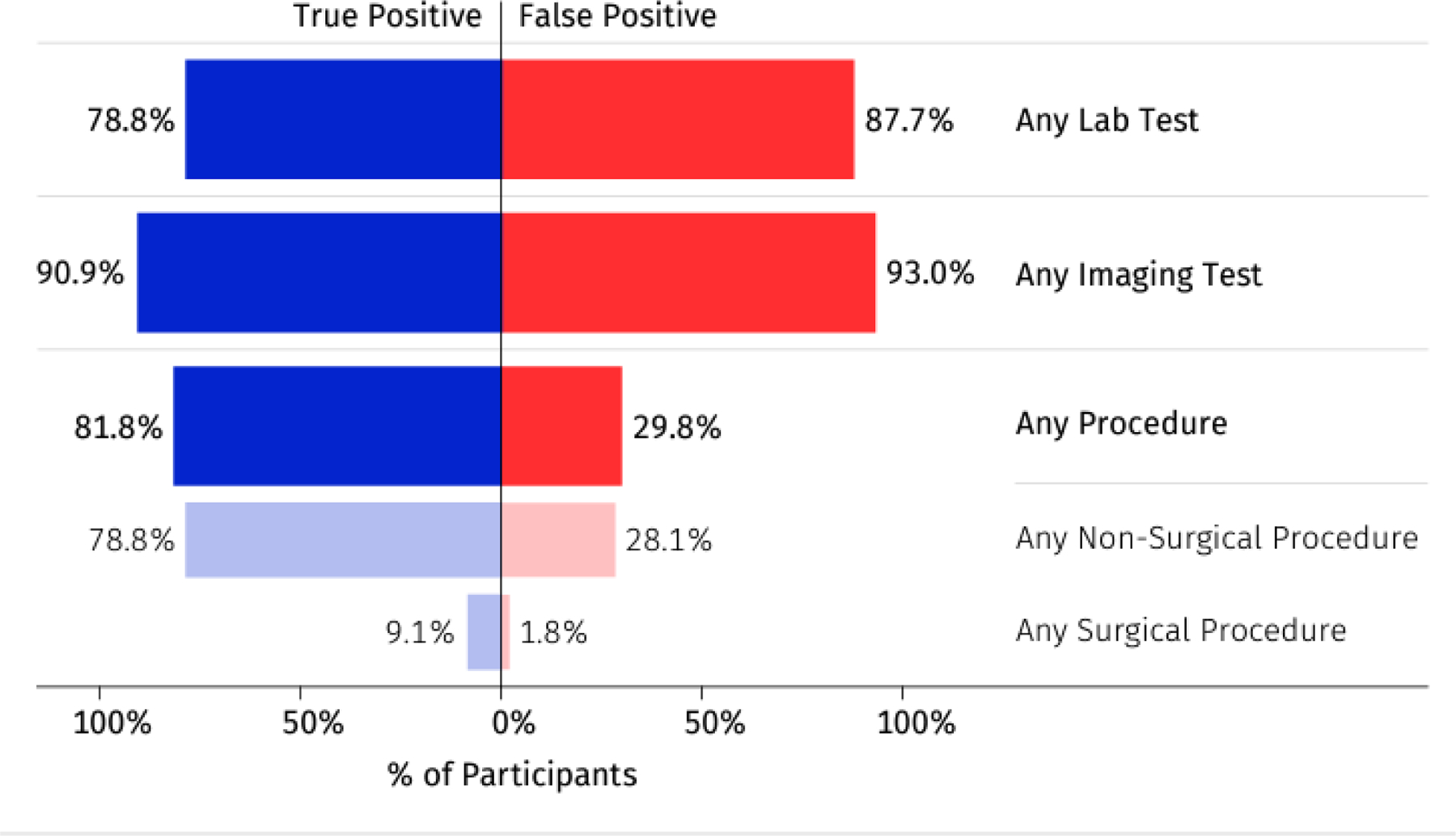
Extent of diagnostic testing in participants with cancer signal detected
At least one imaging test was performed in 92% (83/90) of participants with a cancer signal detected (Figure 3), and 53% of those (44/83) had more than one imaging study. PET-CT (61%; 55/90), CT (39%; 35/90), and MRI (21%; 19/90) were the most common tests (Table A6). Diagnostic tests performed in fewer than three participants are listed in Table A7. The majority of participants (73%; 32/44) with a hematologic CSO had a PET-CT as their first imaging test consistent with diagnostic guidelines.26
Procedures
Of the 90 participants with a diagnostic evaluation triggered by MCED testing, 49% (44/90) had at least one procedure (Figure 3 and Table A6). More true than false positive participants had procedures (82% vs 30%, respectively: Figure 3). In most FP participants, procedures were prompted by abnormal imaging. Three true positive participants had surgical procedures (lymph node mesenteric excision; mesenteric mass and mesenteric lymph node biopsy; base of tongue biopsy). Only one participant with a false positive result had a surgical procedure performed (inguinal orchiectomy), prompted by abnormal imaging.
Cancers Diagnosed After 12 Months
Twelve months after enrollment, 122 cancers were diagnosed in 121 participants (Tables A7a and A7b). Of these 121, 35 (29%) had an MCED cancer signal detected result and 86 (71%) did not. Of these 86, 38 were detected through screening – 29 in accordance with USPSTF guidelines and nine by nonstandard screening tests (Figure A2). The remainder were clinically detected. Of the 35 true positives, 69% (24/35) were in the additional risk cohort; 80% (28/35) had a new cancer, 17% (6/35) had recurrent cancer, and one had both, for a total of 36 cancers (19 solid tumors and 17 hematologic malignancies; Figure 4). In true positives with a new cancer diagnosis, 48% (14/29) of cancers were stage I-II (Figure 4 and Table A8a): six solid tumors and eight hematologic malignancies. A tumor type that does not currently have a USPSTF screening recommendation was identified in 74% (26/35) of participants (Figure 4 and Table A8a). Other reasons included: 1) routine screening (24%; 29/121) and 2) signs or symptoms of cancer (23%; 28/121; Figure A2 and Table A8b). Most cancers diagnosed in false negatives were stage I-II (73%; 55/75), excluding 11 recurrent cancers (Table A8b). Recurrent cancers were identified in 13% of participants (11/86) with false negative results. The histologies for cancers diagnosed in those with false negative MCED results are shown in Table A9.
Figure 4:
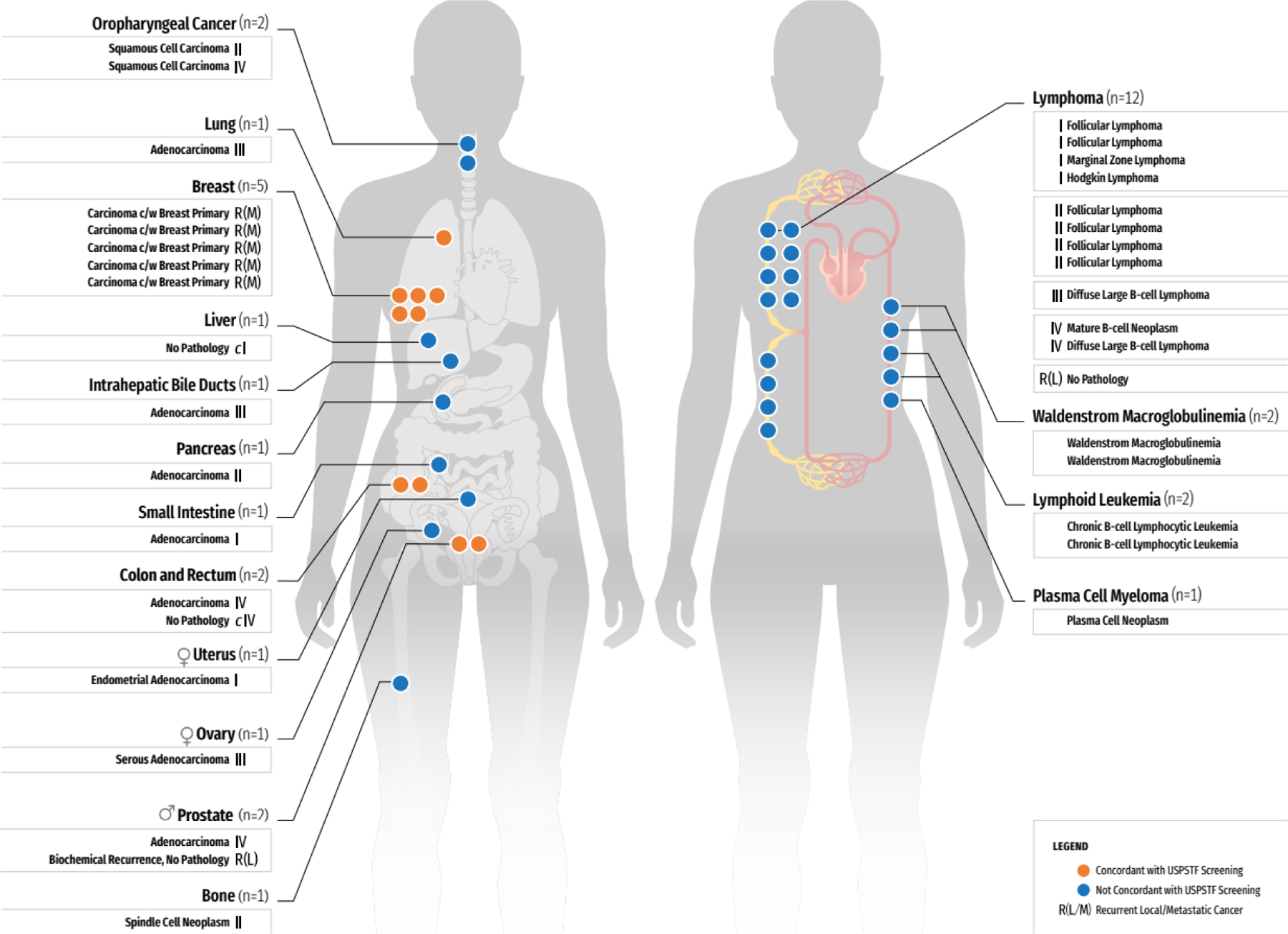
Cancer diagnosed after a positive MCED result
The 36 cancers diagnosed in the 35 true positive participants are depicted by a dot in the organ/system in which the cancer was found. Histology details are given when available. Of the 29 participants with new cancers, 14 (48%) were Stage I or II. Overall 74% cancers detected lack current USPSTF-recommendations for screening tests.
c=clinical staging/diagnosis. c/w=consistent with. MCED=multi-cancer early detection. R(L)=recurrent local cancer. R(M)=recurrent metastatic cancer. USPSTF=United States Preventive Services Task Force.
Adverse Events
Adverse events were reported for four participants, two with events related to phlebotomy (anxiety and bruising at the venipuncture site) and two with anxiety reported before MCED test results were returned. All events were mild in severity.
MCED Test Performance
Overall PPV was 38% (35/92), and was higher (43%, 24/56) in those with additional risk and lower (31%, 11/36) in those without additional risk (Table 2). NPV was 98.6% (6235/6321). Specificity was 99.1% (6235/6290). The cancer yield rate was 0.53% (35/6621), corresponding to a number needed to screen to detect one cancer of 189.
A correct first or second CSO prediction was returned for 97% (33/34) of true positives, excluding one participant with an indeterminate CSO (Table 2). The one participant with an inaccurate first (Colon/Rectum) and second (Upper Gastrointestinal Tract) CSO predictions was diagnosed with adenocarcinoma of the small intestine. Of the 57 false positives, 61% (35/57) had a hematologic CSO prediction; of these, 34% (12/35) had a hematologic precursor condition such as MGUS (appendix Table A10).
Results of the Refined MCED Test
Results of the refined MCED test are displayed in Table A12 and Figure A3. A total of 6578/6662 (98.7%) of participants had analyzable results for the refined MCED test. The refined test version improved specificity (99.5% [95% CI, 99.3–99.6]) and had PPV (43.1% [95% CI, 31.2–55.9]) similar to the study test version (Table 2 and Table A11). Characteristics of cancers detected with the refined MCED test are summarized in Table A13. Concordance between MCED the study and refined test versions is shown in Figure A4. The refined MCED test reduced the proportion of participants with a hematologic CSO prediction among false positives from 61% (35/57) to (12%; 4/33). With the refined MCED test version, 3 of the 4 participants with false positive results predicted to have a hematologic malignancy had a precursor condition such as MGUS.
DISCUSSION
The PATHFINDER study provides early evidence of the feasibility of blood testing to screen for multiple cancers with a single test, a departure from the prevailing paradigm requiring distinct screening tests for each cancer type. When the MCED assay was positive and cancer was present, the test accurately predicted the tumor origin, and half of all cancer diagnoses were made in less than two months. When cancer could not be confirmed, diagnostic evaluations, often with serial scans, continued for a longer duration typically extending for four months. Blood tests and advanced imaging studies were commonly used following a signal detected result.
Importantly, the MCED assay detected many cancer types that lack screening tests, including some found at early stages. For example, cancers of the bile duct, small intestine, pancreas, and a spindle cell neoplasm were detected at early stages amenable to surgical resection. These lethal malignancies are unlikely to have been identified upon routine physical examination or screening. Hematologic malignancies, particularly follicular lymphomas, were also detected at early stages, though the clinical significance of these more indolent neoplasms is less certain. Nearly half of the newly diagnosed cancers were identified at an early stage.
At the study’s inception, participants were highly adherent to established cancer screening and some received forms of screening that extend beyond USPSTF recommendations. Because this study represented a prevalence round of screening, indolent hematologic conditions were likely to have been overrepresented compared to what would be detected in later rounds of repeated screening. The high rate of screening participation in the study population may also explain both the number and types of cancers diagnosed in the absence of an MCED signal. For example, eight of the 11 total lung cancers diagnosed in this study arose in individuals who did not meet USPSTF 2021 criteria for low-dose CT screening.27 Most of these lung cancers were early stage (8/11 stage I and 2/11 stage II), and all had cTAF below the MCED detection threshold. By contrast, the one case of lung cancer diagnosed by MCED testing presented as stage III. The distribution of cancers in false negative participants did not have a clear pattern, other than the predictable outcome of identifying cancers that are commonly screen-detected such as breast and colorectal cancers. The majority of false negatives had cTAF below the MCED test limit of detection.24, 28–30 Because MCED testing technologies are still in early phases of development, rely on different approaches to cohort ascertainment and lack a consistent gold standard definition of a true positive it is infeasible to compare the sensitivity of different MCED test methods.
Both false positive results and the potential for overdiagnosis are inherent risks of all screening methods. In this study, fewer than 1% of participants had false positive results, which compares favorably to currently recommended single-cancer screening tests.18,31,32 In the CCGA study7, 33 we observed that cancers with detectable circulating tumor DNA were more likely to be lethal than those with undetectable levels of circulating tumor DNA. This suggests that MCED testing based on circulating tumor DNA technology may minimize the risk of overdiagnosis by preferentially detecting more lethal tumors. However, larger trials with longer-term follow-up are needed to quantify rates of overdiagnosis.
Study strengths include reliance on a validated fixed assay and algorithm to predict both cancer signal presence and CSO, and recruitment of subjects from a variety of healthcare settings in U.S. ambulatory care practice. The absence of protocol-mandated diagnostic algorithms allowed insight into physician judgment regarding clinical management of a positive MCED test result.
The study had several limitations. First, access to advanced imaging and laboratory testing is often limited by insurance coverage, but in this study was fully supported by the sponsor. Second, the generalizability of the cancer detection rate is constrained by the limited ethnic, racial, and socioeconomic diversity of the study cohort and the high rate of baseline adherence to cancer screening. Similarly, volunteer bias could have influenced participants’ risk or their preferences for undergoing comprehensive diagnostic evaluations. The number, types and stages of cancers detected reflect results of an initial screen of undetected cancers and may not be representative of the results of long-term screening. Third, the study was executed at the height of the COVID-19 pandemic, which hindered the ability to obtain diagnostic evaluations promptly and may have lengthened the diagnostic resolution intervals. Fourth, there was no structured evaluation system to elicit adverse event reports which may have led to underestimation. Fifth, the study was not designed to assess acceptability or cost effectiveness. Finally, the 12-month follow-up period was short, and rates of later cancer diagnoses are uncertain.
Although this study establishes clinical feasibility of MCED testing, forthcoming results of larger studies will be required to demonstrate clinical utility and effects on cancer mortality. Three currently active studies are evaluating the refined version of the MCED test developed in PATHFINDER in different populations: 1) The PATHFINDER2 (NCT05155605) cohort study, is evaluating test performance in a larger and more diverse US adult population; 2) The United Kingdom’s National Health Service NHS Galleri study (ISRCTN 91431511), is evaluating test performance in 140,000 adults randomized to usual cancer screening versus usual screening supplemented by annual MCED testing;34 and 3) the University of Oxford’s SYMPLIFY study (ISRCTN10226380) evaluates the use of MCED testing for individuals presenting to primary care physicians with nonspecific symptoms such as weight loss or fatigue.35 Results of these studies will contribute evidence regarding the utility of this MCED test in specific clinical contexts.
In conclusion, PATHFINDER establishes the feasibility of performing MCED testing in ambulatory practice and describes the diagnostic evaluations undertaken in response to receipt of a positive test suggesting a possible cancer diagnosis. It also provides preliminary estimates of test performance characteristics and the ultimate diagnostic yield of this emerging technology. This experience lays the foundation for larger scale studies to evaluate the safety, utility, and clinical effectiveness of MCED testing as a cancer screening strategy.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Prior evidence has established that cancers shed cell free DNA (cfDNA) into the bloodstream that is detectable before the onset of clinical signs and symptoms, creating a window of preclinical detectability. A PubMed search using the terms “multicancer early detection” OR “multicancer detection” OR “liquid biopsy AND cancer screening” returned 2,415 results. After excluding non-human studies, reviews, commentaries, health economics-focused articles, and single-cancer studies, we identified 12 analytic and/or case-control studies using a variety of technologies to detect cancer signals of multiple tumor types in blood. Collectively these studies have established the feasibility of and putative performance characteristics of genomic-based tools to detect cancers and predict their tissue of origin that could serve to establish a new blood-based screening paradigm. To date, there has been one published cohort study that evaluated the feasibility and safety of a blood-based assay for multicancer detection, but it was limited to women in a single health system and relied on whole body imaging rather than a blood test for tumor localization. There remains little evidence about the diagnostic evaluations that ensue following the use of this technology, the test performance characteristics or how patients and physicians cope with the results.
Added value of this study:
PATHFINDER assessed physician-guided diagnostic evaluations for adult participants who received positive results from a targeted-methylation blood-based multicancer early detection (MCED) test as well as the extent of imaging and diagnostic testing needed to reach diagnostic resolution. The study measured performance characteristics of this test applied among adults over age 50 with and without elevated cancer risk seen in outpatient medical practice. These findings add to emerging evidence supporting the feasibility of MCED testing to screen for multiple types of cancer simultaneously from a single blood specimen and characterize the diagnostic odysseys that follow receipt of a positive MCED test.
Implications of all the available evidence:
These results support further development of MCED testing for cancer screening and provide preliminary estimates of the consequences and diagnostic yield of this emerging technology. Further research is needed to determine the clinical utility of MCED testing to screen for cancer in diverse populations.
Acknowledgements
We acknowledge the contributions to this effort of the patients and their families and the research coordinators and data managers at the investigational sites. We would like to acknowledge Anne-Renee Hartman, MD and Jafi Lipson, MD for their contributions to the PATHFINDER study concept and design. We acknowledge Sana Raoof, MD for her contribution to the manuscript drafting. This study was sponsored by Grail, LLC. We acknowledge Sarah A. Prins, PhD (Grail, LLC) for manuscript editing and management. We acknowledge Jennifer Hepker, PhD (Prescott Medical Communications Group, Chicago, IL) and Neva West, PhD (NeuroWest Solutions, Seattle, WA) for medical writing and editorial and administrative support that was funded by GRAIL, LLC.
SUPPORT
Research support for the study was provided by GRAIL, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This trial is registered at ClinicalTrials.gov, NCT04241796.
PRIOR PRESENTATION
Presented in part at the 2022 ESMO annual meeting Paris, France, Sep 9–13, 2022 and the 2022 EDCC meeting, Portland, OR, USA, October 18–20, 2022.
CLINICAL TRIAL INFORMATION
Declaration of interests
Dr. Schrag reported an uncompensated advisory role and serving as a study investigator with research funding to Dana Farber Cancer Institute (GRAIL, LLC) and personal fees for editorial service (Journal of the American Medical Association).
Dr. Beer reported a consultant agreement with GRAIL, LLC, as well as AbbVie, Amgen, Arvinas, Inc, Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squib, Constellation, Dantari Pharmaceuticals, GlaxoSmithKline (GSK), Janssen, Myovant Sciences, Pfizer, Sanofi, and Sapience Therapeutics; he reports stock ownership in Arvinas, Inc, Salarius Pharmaceuticals, LLC, and Exact Sciences.
Dr. McDonnell reported a consultant agreement with GRAIL, LLC.
Dr. Nadauld reported stock ownership in Culmination Bio.
Dr. Dilaveri reported no disclosures.
Dr. Reid reported no disclosures.
Dr. Marinac reported no disclosures.
Dr. Chung reported stock ownership in Illumina, BMS, Gilead, Baxter, and Bayer and is an employee of GRAIL, LLC.
Dr. Fung and Ms. Lopatin reported stock ownership of Illumina and are employees of GRAIL, LLC.
Dr. Klein is an employee of GRAIL, LLC.
Data sharing
The datasets analyzed for this study are available upon reasonable request by email to the corresponding author.
REFERENCES
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Hackshaw A, Cohen SS, Reichert H, Kansal AR, Chung KC, Ofman JJ. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer. 2021;125(10):1432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke CA, Hubbell E, Kurian AW, Colditz GA, Hartman AR, Gomez SL. Projected Reductions in Absolute Cancer-Related Deaths from Diagnosing Cancers Before Metastasis, 2006–2015. Cancer Epidemiol Biomarkers Prev 2020;29(5):895–902. [DOI] [PubMed] [Google Scholar]
- 4.Beer TM. Novel blood-based early cancer detection: diagnostics in development. Am J Manag Care 2020;26(14 Suppl):S292–S9. [DOI] [PubMed] [Google Scholar]
- 5.Liu MC. Transforming the landscape of early cancer detection using blood tests-Commentary on current methodologies and future prospects. Br J Cancer. 2021;124(9):1475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackshaw A, Clarke CA, Hartman AR. New genomic technologies for multi-cancer early detection: Rethinking the scope of cancer screening. Cancer Cell. 2022;40(2):109–13. [DOI] [PubMed] [Google Scholar]
- 7.Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol 2021;32(9):1167–77. [DOI] [PubMed] [Google Scholar]
- 8.Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020;31(6):745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369(6499). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D Hallmarks of Cancer: New Dimensions. Cancer Discov 2022;12(1):31–46. [DOI] [PubMed] [Google Scholar]
- 12.Hubbell E, Venn O. Shared Cancer Signal: Evidence from Cross-Training. The Early Detection of Cancer Conference (EDCC) October 18–20; Portland, OR: 2022. [Google Scholar]
- 13.Nadauld LD, McDonnell CH 3rd, Beer TM, Liu MC, Klein EA, Hudnut A, et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers (Basel) 2021;13(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network® (NCCN®). NCCN Clinical Practice Guidelines, Detection, Prevention, and Risk Reduction [Available from: https://www.nccn.org/guidelines/category_2].
- 15.AJCC Cancer Staging Manual 8th ed: Springer Cham; 2017. XVII, 1032 p. [Google Scholar]
- 16.Chamberlain J, Clifford R, Nathan B, Price J, Burn I. Repeated screening for breast cancer. Journal of Epidemiology and Community Health. 1984;38:54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprague BL, Arao RF, Miglioretti DL, Henderson LM, Buist DS, Onega T, et al. National Performance Benchmarks for Modern Diagnostic Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman CD, Arao RF, Sprague BL, Lee JM, Buist DSM, Kerlikowske K, et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology.;283(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368(21):1980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers J-WJ, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. The Lancet Oncology. 2014;15(12):1342–50. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett EC, Silva M, Callister ME, Devaraj A. False-Negative Results in Lung Cancer Screening - Evidence and Controversies. Journal of Thoracic Oncology. 2021;16(6):912–21. [DOI] [PubMed] [Google Scholar]
- 22.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). The Lancet Oncology. 2009;10(4):327–40. [DOI] [PubMed] [Google Scholar]
- 23.United States Preventive Services Taskforce. Search Results: Published A,B,C Recommendations Cancer Screening 2023. [Available from: https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&grades%5B%5D=A&grades%5B%5D=B&grades%5B%5D=C&category%5B%5D=15&type%5B%5D=5&searchterm=].
- 24.Jamshidi A, Liu MC, Klein EA, Venn O, Hubbell E, Beausang JF, et al. Evaluation of cell-free DNA approaches for multi-cancer early detection. Cancer Cell. 2022;40(12):1537–49 e12. [DOI] [PubMed] [Google Scholar]
- 25.R Team. Core R: A Language and Environment for Statistical Computing [Available from: https://www.R-project.org/].
- 26.National Comprehensive Cancer Network® (NCCN®). NCCN Clinical Practice Guidelines, Treatment by Cancer Type [Available from: https://www.nccn.org/guidelines/category_1].
- 27.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325(10):962–70. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Dong Z, Hubbell E, Kurtzman KN, Oxnard GR, Venn O, et al. Prognostic Significance of Blood-Based Multi-cancer Detection in Plasma Cell-Free DNA. Clin Cancer Res 2021;27(15):4221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bredno J, Venn O, Chen X, Freese P, Ofman JJ. Circulating Tumor DNA Allele Fraction: A Candidate Biological Signal for Multicancer Early Detection Tests to Assess the Clinical Significance of Cancers. Am J Pathol 2022;192(10):1368–78. [DOI] [PubMed] [Google Scholar]
- 30.Bredno J, Lipson J, Venn O, Gross S, Fields AP, Beausang JF, et al. Tumor area and microscopic extent of invasion to determine circulating tumor DNA fraction in plasma and detectability of colorectal cancer (CRC). Journal of Clinical Oncology. 2020;38(4_suppl):243-. [Google Scholar]
- 31.Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162(7):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alsayid M, Singh MH, Issaka R, Laleau V, Day L, Lee J, et al. Yield of Colonoscopy After a Positive Result From a Fecal Immunochemical Test OC-Light. Clinical Gastroenterology and Hepatology. 2018;16(10):1593–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredno J, Lipson J, Venn O, Aravanis AM, Jamshidi A. Clinical correlates of circulating cell-free DNA tumor fraction. PLoS One. 2021;16(8):e0256436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neal RD, Johnson P, Clarke CA, Hamilton SA, Zhang N, Kumar H, et al. Cell-Free DNA-Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers (Basel) 2022;14(19):4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson BD, Oke J, Virdee PS, Harris DA, O’Doherty C, Park JE, et al. : Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study. Lancet Oncol 2023. Jun 20:S1470-2045(23)00277-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


